Abstract
Background
Dental caries is one of the most common chronic diseases of childhood and is associated with adverse health and economic consequences for infants and their families. Socioeconomically disadvantaged children have a higher risk of early childhood caries (ECC).
Objectives
To assess the effects of interventions with pregnant women, new mothers or other primary caregivers of infants in the first year of life, for preventing ECC (from birth to six years of age).
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 14 January 2019), Cochrane Pregnancy and Childbirth Group's Trials Register (to 22 January 2019), Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Register of Studies, to 14 January 2019), MEDLINE Ovid (1946 to 14 January 2019), Embase Ovid (1980 to 14 January 2019) and CINAHL EBSCO (1937 to 14 January 2019). The US National Institutes of Health Trials Registry (ClinicalTrials.gov) and World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials. No restrictions were placed on language or publication status.
Selection criteria
Randomised controlled trials (RCTs) comparing one or more interventions with pregnant women, mothers, or other caregivers of infants in the first year of life (intervention types included clinical, oral health education/promotion such as hygiene education, breastfeeding and other dietary advice, and policy or health service), versus standard care or placebo or another intervention. For inclusion, trials had to report at least one caries outcome.
Data collection and analysis
Two review authors independently assessed trial eligibility, extracted data, assessed risk of bias, and assessed certainty of evidence using the GRADE approach.
Main results
We included 17 RCTs (4 cluster‐randomised), involving 23,732 caregivers (mainly mothers) and their children. Eleven RCTs assessed four oral health education/promotion interventions against standard care: child diet advice, child diet and feeding practice advice, breastfeeding promotion and support, and oral hygiene with child diet and feeding practice advice. Six trials assessed clinical interventions in mother's dentition, four trials chlorhexidine (CHX, a commonly prescribed antiseptic agent) or iodine‐NaF application and prophylaxis versus placebo, and two trials xylitol against CHX or CHX + xylitol. At most, three trials (maximum of 1148 children and 130 mothers) contributed data to any comparison. For many trials, risk of bias was judged unclear due to lack of methodological details reported, and there was high risk of attrition bias in some trials. None of the included trials indicated receiving funding that is likely to have influenced their results. The trials were performed in high‐, middle‐ and low‐income countries. In nine trials, participants were socioeconomically disadvantaged.
For child diet and feeding practice advice versus standard care, we observed a probable 15 per cent reduced risk of caries presence in primary teeth with the intervention (RR 0.85, 95% CI 0.75 to 0.97; 3 trials; 782 participants; moderate‐certainty evidence), and there may be a lower mean dmfs (decayed, missing, filled primary surfaces) score (MD ‐0.29, 95% CI ‐0.58 to 0; 2 trials; 757 participants; low‐certainty evidence); however, we are uncertain regarding the difference between the groups in mean dmft (decayed, missing, filled teeth) score (MD ‐0.90, 95% CI ‐1.85 to 0.05; 1 trial; 340 participants; very low‐certainty evidence).
For breastfeeding promotion and support versus standard care, we observed that there may be little or no difference between groups in the risk of caries presence in primary teeth (RR 0.96, 95% CI 0.89 to 1.03; 2 trials; 1148 participants; low‐certainty evidence), or mean dmft score (MD ‐0.12, 95% CI ‐0.59 to 0.36; 2 trials; 652 participants; low‐certainty evidence). Dmfs was not reported for this comparison.
We are uncertain whether child diet advice only compared with standard care reduces risk of caries presence in primary teeth (RR 1.08, 95% CI 0.34 to 3.37; 1 trial; 148 participants; very low‐certainty evidence). Dmfs and dmft were not reported for this comparison.
For oral hygiene, child diet and feeding practice advice versus standard care, we observed little or no reduced risk of caries presence in primary teeth (RR 0.91, 95% CI 0.75 to 1.10; 2 trials; 365 participants; low‐certainty evidence), and are uncertain regarding difference between the groups in mean dmfs score (MD ‐0.99, 95% CI ‐2.45 to 0.47; 1 trial; 187 participants; very low‐certainty evidence) and dmft score (MD ‐0.30, 95% CI ‐0.96 to 0.36; 1 trial; 187 participants; very low‐certainty evidence).
We observed there may be little or no difference in risk of caries presence in primary teeth between antimicrobial and placebo treatment in mother's dentition (RR 0.97, 95% CI 0.80 to 1.19; 3 trials; 479 participants; very low‐certainty evidence). No trials assessing this comparison reported dmfs or dmft.
For xylitol compared with CHX antimicrobial treatment, we observed there may be a lower mean dmft score with xylitol (MD ‐2.39; 95% CI ‐4.10 to ‐0.68; 1 trial, 113 participants; low‐certainty evidence); however, we are uncertain regarding the difference between groups in caries presence in primary teeth (RR 0.62, 95% CI 0.27 to 1.39; 1 trial, 96 participants; very low‐certainty evidence). Neither trial evaluating this comparison reported dmfs.
No trials assessed a health policy or service intervention.
Authors' conclusions
Moderate‐certainty evidence suggests that providing advice on diet and feeding to pregnant women, mothers or other caregivers with children up to the age of one year probably leads to a slightly reduced risk of early childhood caries (ECC). The remaining evidence is low to very low certainty and is insufficient for determining which, if any, other interventions types and features may be effective for preventing ECC.
Large, high‐quality RCTs of oral health education/promotion, clinical, and policy and service access interventions, are warranted to determine effects and relative effects of different interventions and inform practice. We have identified 12 studies currently in progress. Those designing future studies should describe the intervention components, setting and participants, consider if and how effects are modified by intervention features and participant characteristics, and adopt a consistent approach to measuring and reporting ECC.
Plain language summary
Interventions with pregnant women, new mothers and other primary caregivers for preventing tooth decay in young children
Question
Does providing pregnant women, new mothers and other primary caregivers of children in the first year of life with preventive dental care (other than fluorides) and information about healthy child diet and feeding practices prevent tooth decay in their children?
Background
Tooth decay in young children (early childhood caries or ECC) is very common, affecting billions of children worldwide, particularly poor children. Early childhood caries can have long‐lasting negative effects on health and it costs a lot to treat. It is well known that sugar and dental plaque (bacteria in the mouth) cause tooth decay. The attitudes, beliefs, and habits of pregnant women, mothers and other primary caregivers, influence the dental health of their children.
Study characteristics
We searched for evidence available up to 14 January 2019. We found 17 randomised controlled trials, which is the type of research that provides the most reliable results. The trials involved 23,732 caregivers (mainly mothers) and their children. The trials took place in a mix of high‐, middle‐, and low‐income countries. Participants were from low‐income communities in nine trials.
Eleven of the included trials evaluated oral health education and promotion interventions compared to usual care. We divided these into four subcategories: breastfeeding support (two trials), child diet advice only (one trial), child diet and feeding advice (three trials), or child diet and feeding advice combined with advice on keeping teeth clean (five trials).
Preventive dental care aimed at reducing bacteria in the mother’s mouth was evaluated in six trials: four compared putting a special varnish on the teeth compared with a 'placebo' (an inactive treatment that looked the same as the varnish), and two compared the use of chewing gum containing xylitol versus a chlorhexidine dental gel.
None of the included trials assessed programmes aimed at improving access to preventive dental services.
Main results
We found some evidence that children whose mothers (or other caregivers) received advice on healthy diet and feeding practice for infants and children were less likely to have tooth decay up to the age of six than those whose caregivers received the usual care.
The other oral health education interventions (breastfeeding support; advice about best child diet; advice about child diet, feeding and teeth cleaning) did not show that these interventions reduced the risk of tooth decay in young children compared with usual care. However, the findings of these studies were so uncertain that we cannot conclude these interventions do not work.
We found mixed evidence about treatments to reduce bacteria in mothers' mouths and cannot reach firm conclusions about whether or not these could potentially prevent early childhood caries.
None of the included trials indicated receiving funding that is likely to have influenced their results.
Authors' conclusions
Providing advice on diet and feeding to pregnant women, mothers or other caregivers with children up to the age of one year probably leads to a slightly reduced risk of tooth decay in their children during their early years. We need more high quality studies that have a large number of participants in order to find out if there are other interventions with caregivers that can help reduce early childhood tooth decay, and which features of interventions make them effective. We are aware of 12 studies currently in progress.
Summary of findings
Summary of findings for the main comparison. Summary of findings ‐ diet and feeding practice advice versus standard care.
| Diet and feeding practice advice for infants and young children compared with standard care for preventing caries in young children | ||||||
|
Population: for interventions, pregnant women and mothers or other caregivers of infants in the first year of life; for outcomes, children up to 6 years of age Settings: Brazil (2 RCTs), United Kingdom (1 RCT) Intervention: advice about a healthy diet (including breastfeeding promotion and sugar avoidance) and feeding practices (e.g. relating to use of bottle feeding and sleep), for infants and young children Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Diet and feeding practice advice | |||||
|
Caries presence in primary teeth (children 0 to 6 yrs) |
511 per 1000 | 440 per 1000 (383 to 501) | RR 0.85 (0.75 to 0.97) | 782 participants (3 studies) | ⊕⊕⊕⊝ moderate1 | |
|
dmfs index score (range 0 to 80, children 0 to 6 yrs) |
The mean dmfs index score in the standard care group ranged from 0.63 to 3.6 | The mean dmfs index score in the intervention group was 0.29 lower (0.58 lower to equal) | 757 participants (2 studies) | ⊕⊕⊝⊝ low2 | The dmfs index expresses the total number of decayed missing or filled surfaces in primary dentition (five per posterior tooth and four per anterior tooth) as a score (range 0‐80 surfaces, lower is better) | |
|
dmft index score (range 0‐20, children assessed at 4 yrs) |
The mean dmft index score in the standard care group was 4.15 | The mean dmft index score in the intervention group was 0.90 lower (1.85 lower to 0.05 higher) | 340 participants (1 study) | ⊕⊝⊝⊝ very low3 | The dmft index expresses the total number of teeth affected by tooth decay (missing or filled) in the primary dentition as a score (range 0‐20, lower is better) | |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; dmfs: decayed, missing and filled surfaces (in primary teeth of children); dmft: decayed, missing and filled teeth (primary, of children); RR: risk ratio; yrs: years | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 ROB (‐1): downgraded for unclear implications for risk of bias associated with high attrition in trials (not downgraded for lack of blinding of participants and personnel, which is a feature of all three included trials, as due to the objective outcome this is unlikely to have introduced bias)
2 ROB (‐1): downgraded for unclear implications for risk of bias associated with high attrition (not downgraded for lack of blinding due to objective outcome); imprecision (‐1): downgraded for confidence interval passing through line of no effect, signalling uncertainly about direction of intervention effect
3 ROB (‐1): downgraded for unclear implications for risk of bias associated with high attrition (not downgraded for lack of blinding due to objective outcome); imprecision (‐2): downgraded for confidence interval passing through line of no effect, and only 1 study with few participants
Summary of findings 2. Summary of findings ‐ breastfeeding promotion and support versus standard care.
| Breastfeeding promotion and support compared with standard care for preventing caries in young children | ||||||
|
Population: for interventions, pregnant and lactating women; for outcomes, young children up to 6 years of age Settings: Belarus (1 RCT), Uganda (1 RCT) Intervention: breastfeeding promotion and support (e.g. individual tailored home‐based peer counselling focused on providing information about the importance of breastfeeding and offering advice and support for healthy breastfeeding) Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Breastfeeding promotion and support | |||||
|
Caries presence in primary teeth (children 0 to 6 yrs) |
689 per 1000 |
661 per 1000 (613 to 709) |
RR 0.96 (0.89 to 1.03) | 1148 (2 studies) | ⊕⊕⊝⊝ low1 | |
|
dmfs index score (range 0 to 80) (children 0 to 6 yrs) |
Not assessed | The dmfs index expresses the total number of decayed missing or filled surfaces in primary dentition (five per posterior tooth and four per anterior tooth) as a score (range 0 to 80 surfaces, lower is better) | ||||
|
dmft index score (range 0 to 20) (children 0 to 6 yrs) |
The mean dmft index score in the standard care group ranged from 1.7 to 4.2 | The mean dmft index score in the intervention group was 0.12 lower (0.59 lower to 0.36 higher) | 652 (2 studies) | ⊕⊕⊝⊝ low1 | The dmft index expresses the total number of teeth affected by tooth decay (missing or filled) in the primary dentition as a score (range 0 to 20, lower is better) | |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; dmfs: decayed, missing and filled surfaces of primary teeth; dmft: decayed, missing and filled primary teeth; RR: risk ratio;yrs: years | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1ROB (‐1): downgraded for one of the two included trials at unclear risk of selection and detection bias, and with some attrition (this trial with 21% weight only in meta‐analysis) (not downgraded for lack of blinding of participants and personnel due to objective outcome); imprecision (‐1): downgraded for wide confidence interval passing through line of no effect
Summary of findings 3. Summary of findings ‐ dietary advice versus standard care.
| Dieatary advice for infants and young children compared with standard care for preventing caries in young children | ||||||
|
Population: for interventions, pregnant women and mothers or other caregivers of infants in the first year of life; for outcomes, children up to 6 years of age Setting: Finland (1 RCT) Intervention: advice about how to achieve a healthy diet for their infants (tailored advice focused on ensuring a diet low in saturated fat and cholesterol intake) Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Dietary advice | |||||
|
Caries presence in primary teeth (children 0 to 6 yrs) |
71 per 1000 | 77 per 1000 (25 to 241) | RR 1.08 (0.34 to 3.37) | 148 (1 RCT) | ⊕⊝⊝⊝ very low1 | |
|
dmfs index score (range 0 to 80) (children 0 to 6 yrs) |
Not assessed | The dmfs index expresses the total number of decayed missing or filled surfaces in primary dentition (five per posterior tooth and four per anterior tooth) as a score (range 0‐80 surfaces, lower is better) | ||||
|
dmft index score (range 0 to 20) (children 0 to 6 yrs) |
Not assessed | The dmft index expresses the total number of teeth affected by tooth decay (missing or filled) in the primary dentition as a score (range 0 to 20, lower is better) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; dmfs: decayed, missing and filled surfaces (in primary teeth of children); dmft: decayed, missing and filled teeth (primary, of children); NA: not applicable; RR: risk ratio; yrs: years | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1ROB (‐1): downgraded for risk of selection bias, and possible bias due to attrition (not downgraded for lack of blinding of participants and personnel due to objective outcome); imprecision (‐2): downgraded for wide confidence interval passing through line of no effect
Summary of findings 4. Summary of findings ‐ oral hygiene education combined with diet and feeding practice advice versus standard care.
| Oral hygiene education combined with diet and feeding practice advice for infants and young children compared with standard care for preventing caries in young children | ||||||
|
Population: for interventions, pregnant women and mothers or other caregivers of infants in the first year of life; for outcomes, children up to 6 years of age Settings: Australia (1 RCT), Canada (Cree communities, 1 RCT) Intervention: package of oral health education and promotion measures including oral hygiene advice for pregnant women, mothers infants and young children, and dietary and feeding practice advice focused on infants and young children Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Oral hygiene, dietary and feeding advice | |||||
|
Caries presence in primary teeth (children 0 to 6 yrs) |
537 per 1000 |
489 per 1000 (403 to 591) |
RR 0.91 (0.75 to 1.10) | 365 (2 studies) | ⊕⊕⊝⊝ low1 | |
|
dmfs index score (range 0 to 80) (children assessed at 6 yrs) |
The mean dmfs index score in the standard care group was 2.45 | The mean dmfs index score in the intervention group was 0.99 lower (2.45 lower to 0.47 higher) | 187 (1 study) |
⊕⊝⊝⊝ very low2 | The dmfs index expresses the total number of decayed missing or filled surfaces in primary dentition (five per posterior tooth and four per anterior tooth) as a score (range 0 to 80 surfaces, lower is better) | |
|
dmft index score (range 0 to 20) (children assessed at 6 yrs) |
The mean dmft index score in the standard care group was 1.29 | The mean dmft index score in the intervention group was 0.30 lower (0.96 lower to 0.36 higher) | 187 (1 study) |
⊕⊝⊝⊝ very low2 | The dmft index expresses the total number of decayed, missing or filled primary teeth as a score (range 0 to 20 teeth, lower is better) | |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;; dmfs: decayed, missing and filled surfaces (in primary teeth of children); dmft: decayed, missing and filled teeth (primary, of children); RR: risk ratio; yrs: years | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1ROB (‐1): downgraded for unclear risk of selection bias and unclear implications associated with loss of data. Imprecision (‐1): downgraded for confidence interval passing through line of no effect (signals uncertainty about direction of the intervention effect)
2 ROB (‐1): downgraded for unclear risk of selection bias, and uncertain risk of bias implications associated with attrition (not downgraded for lack of blinding due to objective outcome); Imprecision (‐2): downgraded for line passing through line of no effect and only one study in analysis
Summary of findings 5. Summary of findings ‐ antimicrobial treatment versus placebo.
| Antimicrobial treatment in pregnant women or new mothers compared with placebo for preventing caries in young children | ||||||
|
Population: for interventions, pregnant women and mothers of infants in the first year of life; for outcomes, children up to 6 years of age for outcome Settings: Brazil (1 RCT), USA (2 RCTs, one conducted in four American Indian communities in Oregon) Intervention: prophylaxis (teeth cleaning) and CHX or iodine‐NaF solution application in dentition of women Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo treatment | CHX or iodine‐NaF solution and prophylaxis treatment | |||||
|
Caries presence in primary teeth (children 0 to 6 yrs) |
436 per 1000 |
423 per 1000 (349 to 519) |
RR 0.97 (0.80 to 1.19) | 479 participants (3 studies) |
⊕⊝⊝⊝ very low1 | |
|
dmfs index score (range 0 to 80) (children 0 to 6 yrs) |
Not assessed | The dmfs index expresses the total number of decayed missing or filled surfaces in primary dentition (five per posterior tooth and four per anterior tooth) as a score (range 0 to 80 surfaces, lower is better) | ||||
|
dmft index score (range 0 to 20) (children assessed at 6 yrs) |
Not assessed | The dmft index expresses the total number of decayed, missing or filled primary teeth as a score (lower is better) | ||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CHX: chlorhexidine; CI: confidence interval; RR: risk ratio; yrs: years | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1ROB (‐1): downgraded for all three trials being at unclear risk of selection bias (not certain if random sequence generated and used to assign participants to groups) and attrition bias (loss of data) (not downgraded for lack of blinding due to objective outcome); inconsistency (‐1): downgraded for analysis indicating variation between the three included trials in the effect estimate (I2 = 52%); imprecision (‐1): downgraded for wide confidence intervention and confidence interval passing through the line of no effect (signalling uncertainty about the size and direction of intervention effect)
Summary of findings 6. Summary of findings ‐ xylitol chewing gum versus chlorhexidine (CHX) varnish or xylitol and CHX gum.
| Xylitol compared with CHX or CHX combined with xylitol antimicrobial treatment for preventing caries in children | ||||||
|
Population: for interventions, pregnant women and mothers of infants in the first year of life for the intervention; for outcomes, children up to 6 years of age Settings: Finland (1 RCT), Sweden (1 RCT) Intervention: consumption of xylitol chewing gum by women Comparison: consumption of CHX/xylitol gum by women or CHX varnish applied to women's dentition | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| CHX gum or varnish | Xylitol gum | |||||
|
Caries presence in primary teeth (children assessed at 4 yrs) |
250 per 1000 |
155 per 1000 (68 to 348) |
RR 0.62 (0.27 to 1.39) | 96 participants (1 study) |
⊕⊝⊝⊝ very low1 | |
|
dmfs index score (range 0 to 80) (children 0 to 6 yrs) |
Not assessed | The dmfs index expresses the total number of decayed missing or filled surfaces in primary dentition (five per posterior tooth and four per anterior tooth) as a score (range 0‐80 surfaces, lower is better) | ||||
|
dmft index score (range 0 to 20) (children assessed at 5 yrs) |
The mean dmft index score in the xylitol group was 3.22 | The mean dmft index score in the intervention group was 2.39 lower (4.10 to 0.68 lower) | 113 participants (1 study) | ⊕⊝⊝⊝ low2 | The dmft index expresses the total number of teeth affected by tooth decay (missing or filled) in the primary dentition as a score (range 0 to 20, lower is better) | |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; defs: decayed, extracted and filled surfaces; dmft: decayed, missing and filled teeth (primary, of children); RR: risk ratio; yrs: years | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1ROB (‐1): downgraded for unclear risk of selection and attrition bias (not downgraded for lack of blinding of participants and personal as objective outcome); Imprecision (‐2): wide confidence interval passing through line of no effect (uncertainly about direction and magnitude of intervention effect), and only one study
2ROB (‐1): downgraded for unclear risk of selection bias (uncertain whether participants randomly assigned to groups), and attrition bias (not downgraded for inability to blind participants or personnel as objective outcome); Imprecision (‐1): downgraded for moderately wide confidence interval and only one study
Background
Description of the condition
Dental caries is one of the most common chronic diseases of childhood, affecting between 30% to 50% of children in high‐income countries (AIHW 2016; Alsharif 2016; Dye 2015; Pitts 2015) and up to 90% in low‐ and middle‐income countries (Ayele 2013; Peltzer 2015) and other vulnerable populations (Calvasina 2015; Smith 2015). If left untreated, caries can cause pain, infection and sepsis (Nuttall 2006; Pine 2006; Tickle 2008). Severely affected children often require medical care including hospitalisation, systemic antibiotics, removal of teeth and general anaesthesia, all of which are associated with significant psychosocial and economic burdens to the child, their family and the community (Casamassimo 2009; Gilchrist 2015). At a population level, untreated caries in children is associated with poor growth outcomes (Alkarimi 2014), nutritional deficiencies (Schroth 2013; Schroth 2014), behavioural and sleep problems (Edelstein 2006), and compromised quality of life, school attendance and educational outcomes (Blumenshine 2008; Filstrup 2003; Moure‐Leite 2011). It is also recognised that caries in the primary dentition (arrangement of the baby teeth) is one of the main risk factors for caries in the permanent dentition (Colak 2013; Llena 2018; Peretz 2003). Therefore, preventing the development of dental caries in children is fundamental to improving long‐term oral and general health and well‐being.
The role of cariogenic (causing tooth decay) bacteria and fermentable carbohydrates (sugars) in the aetiology (causes) of caries is well recognised (Selwitz 2007; Tinanoff 2000). However, this understanding belies the fact that caries experience is a complex interplay between genetic, environmental and behavioural factors in which the traditional biological model is superimposed by child, family and community factors (Fisher‐Owens 2007). In very young children specifically, the influence of the attitudes, beliefs and practices of primary caregivers, generally mothers, is fundamental. Infants of mothers with dental caries are at increased risk of developing caries themselves (Harris 2004; Reisine 2008). Interventions targeted at mothers both during pregnancy and in the first year after birth have the potential to prevent the initiation and progression of caries in young children, and hence reduce the burden of this disease across the life‐course (Kohler 2012).
Description of the intervention
We assessed interventions intended to prevent tooth decay in young children (from birth to six years), provided to women during pregnancy or to new mothers and other primary caregivers of infants under 12 months. Interventions in the early days of a child's life to prevent early childhood caries (ECC), are underpinned by three mechanisms: optimising exposure of the infants to fluoride (through mother or other caregiver encouraging early exposure of their infant to fluoridated water and initiating use of age‐appropriate fluoride toothpaste); reducing the infant intra‐oral cariogenic bacterial load; and adopting dental health‐promoting practices including dietary changes, oral hygiene and routine use of dental services.
The approaches adopted can be divided into three broad categories.
Clinical interventions in pregnant women and new mothers of young infants (e.g. antimicrobial varnish applied to mothers' dentition, maternal use of chewing gums containing antimicrobial agents).
Oral health education/promotion targeted at pregnant women, new mothers or other caregivers of young infants (e.g. education on oral hygiene, which may include provision of equipment and demonstration of how to brush the teeth of young infants and children, dietary counselling focused on breastfeeding education and support, advice about a healthy diet and feeding practices for infants and young children).
Health service and policy interventions designed to modify access to oral health information and/or health services for pregnant women, new mothers or other caregivers, and their infants.
This review does not include fluoride supplementation, or clinical restorative and surgical treatment, which are evaluated in other Cochrane reviews (Takahashi 2015 and Iheozor‐Ejiofor 2017, respectively). Interventions that involve clinical treatment (including application of fluoride) to the infants themselves are also not included in this review.
How the intervention might work
1. Oral health education/promotion
Maternal oral health literacy, attitudes and behaviours are associated with infant caries risk (Divaris 2011; Finlayson 2007; Vann 2010). In traditional health education models, it is hypothesised that providing oral hygiene and/or dietary advice to mothers will improve oral health outcomes of children by changing behaviour (dietary choices and oral health hygiene practices), and interest in engaging with dental services (Yost 2008). This is based on the assumption that the mother is the primary carer and therefore she influences common risk factors through lifestyle changes within the family. For example, given the key role that sugar plays in the development of caries (Giacaman 2018), such oral health education interventions may aim to reduce sugar intake. Similarly, mothers improving their own oral hygiene practices may reduce caries in their offspring both by reducing maternal bacterial load and by modelling behaviour. Giving mothers information and/or providing them with free or low cost fluoridated toothpaste may optimise the exposure of their infant to fluoride, which is strongly associated with reduced caries risk (Davies 2003).
While there is significant evidence of the association between breastfeeding and general health, association with reducing dental caries is less clear (Peres 2018). A systematic review (Tham 2015) concluded breastfeeding to 12 months was associated with reduced dental caries, although some studies (Chaffee 2014; Feldens 2010; Yonezu 2006) found increased caries and the trend seemed to change with breastfeeding after 12 months. It has been hypothesized that any potential protective effect of breastfeeding is associated with the reduced sugar consumption and delayed use of the bottle (and consequently the substrate contained therein) among children who are breastfed (Peres 2018).
Education is a necessary but not a sufficient component of interventions that aim to change health behaviour, and the broader determinants of oral health should be addressed (Albino 2016). To ensure exposure, acceptability and effectiveness, consideration needs to be given to the timing, environment and format of health education and promotion interventions.
2. Clinical interventions
Colonisation of the oral cavity by cariogenic bacteria can occur even before teeth erupt in infants of mothers/other primary caregivers who themselves have poor oral health (dental caries, gingivitis (a common and mild form of gum disease that causes irritation, redness and swelling of the gingiva, the part of the gum around the base of the teeth) and periodontal disease (infections of the structures around the teeth, which include the gums, periodontal ligament and alveolar bone), and high counts of cariogenic bacteria (Teanpaisan 2007; Wan 2003). It is hypothesised that suppression of cariogenic oral flora in pregnant women and/or new mothers will inhibit such colonisation in their offspring and delay or prevent caries development. Strategies for reducing the oral microbial load in mothers/caregivers might include professional chemomechanical oral debridement (removal of damaged tissue or foreign objects from a wound) measures and/or topical or systemic antimicrobial agents.
Compromised maternal health, and in particular maternal vitamin D deficiency during pregnancy, predisposes children to developmental dental defects, specifically hypomineralised enamel (Schroth 2014). Teeth affected by hypomineralised enamel are more susceptible to colonisation by cariogenic bacteria and are often hypersensitive, making adequate oral hygiene difficult, hence increasing the risk of the child developing ECC (Hong 2009; Pascoe 1994; Schroth 2014). It is hypothesised that vitamin D supplementation of mothers during pregnancy will optimise dental development in their offspring and reduce the risk of caries development (Gyll 2018).
3. Access to services and/or policy
Inadequate access to preventive oral healthcare during pregnancy and in early childhood is associated with poor infant oral health outcomes (Yost 2008). Access to services is complex, and improving approachability, acceptability, availability, affordability and appropriateness promotes ongoing engagement with dental care (Levesque 2013). Public health policies optimising provision of access to culturally‐appropriate coordinated services for vulnerable populations of women of childbearing age may promote positive oral health outcomes during and in the first few years after pregnancy (Riggs 2016). It can be hypothesised that interventions such as models of interdisciplinary shared care, public‐private partnerships and community‐based collaborations promoting oral health and access to coordinated care will increase routine engagement with preventive dental health services, leading to improvements in maternal and child oral health.
Why it is important to do this review
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important ones to maintain on the Cochrane Library (Worthington 2015). This review was one of those identified as a priority by the dental public health expert panel (Cochrane Oral Health priority review portfolio).
There is evidence of a global increase in the prevalence of dental caries, particularly in young children (Alsharif 2016; Bagramian 2009). This is associated with substantial morbidity and cost to the individual, the family and society (Casamassimo 2009; Kassebaum 2017). Individuals from low socioeconomic, migrant, refugee and indigenous backgrounds, and those with special healthcare needs are disproportionately disadvantaged in this regard (Calvasina 2015; Riggs 2017a; Slack‐Smith 2011). Despite caries being considered almost entirely preventable, traditional approaches to prevention, based largely on individual responsibility, have been mostly unsuccessful in reducing the burden associated with this disease at a population level (Cohen 2017). While the influence of the primary caregiver (mainly mothers), given their own general and oral health and health literacy, on the oral health outcomes of their children is widely accepted (Saied‐Moallemi 2008), the effectiveness of interventions targeted at pregnant women and new mothers, for improving infant and young child oral health, is not.
This review will:
provide evidence of the effectiveness of interventions targeted at pregnant women and/or new mothers and other primary caregivers of infants in the first year of life in reducing dental caries in their children;
improve understanding of the mechanisms by which infant oral health may be influenced by mothers and other primary caregivers;
inform clinical and public health strategies to reduce the burden of dental caries in very young children.
Objectives
To assess the effects of interventions targeted at pregnant women, new mothers or other primary caregivers of infants in the first year of life, for preventing ECC (from birth to six years of age). Specifically, the intervention types include: 1) clinical interventions, 2) oral health education/promotion (such as infant and young child dietary advice (including relating to breastfeeding), child feeding practice advice, and oral hygiene advice for mothers and/or young children), and 3) policy and access to services.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and cluster‐RCTs were eligible for inclusion, whether published or unpublished. Abstracts were considered for inclusion.
Types of participants
Pregnant women and new mothers of young infants (up to 12 months) were the main participants in this review. Studies in which the intervention was provided to new mothers and other primary caregivers (e.g. fathers, grandmothers) of children in their first year of life were also considered. Studies involving new mothers or other primary caregivers of young children were only eligible if all the infants of randomised caregivers were younger than 12 months at baseline (i.e. just prior to when the intervention started).
For maternal outcomes, women of all ages were considered. For child outcomes, infants and children up to and including six years were eligible. There were no restrictions on maternal or child ethnicity, language spoken, gestation period, maternal or infant medical history or geographical location.
Types of interventions
Interventions with pregnant women, new mothers or other primary caregivers of infants in the first year of life, for preventing early childhood caries (ECC), including: 1) clinical treatments (e.g. application of antimicrobial agents), 2) oral health education and/or promotion, such as support for breastfeeding, dietary advice for infants and young children or oral hygiene education), and 3) health service and policy interventions designed to modify access to oral health information or services.
-
Comparison conditions:
placebo,
standard care, or
another intervention with pregnant women, new mothers or other primary caregivers of infants in the first year of life, for preventing ECC.
We considered the included interventions as standalone or combined interventions.
Fluoride supplementation interventions in mothers are evaluated in another Cochrane review (Takahashi 2015; Takahashi 2017). We excluded interventions that involved clinical treatment (including application of fluoride) to the infants themselves.
Types of outcome measures
Primary outcomes
The primary outcome was the clinical measure of dental caries in infants or children up to six years of age. This included:
caries presence in primary teeth (yes/no; including non‐cavitated (white spot lesion) and/or cavitated lesions);
dmft (decayed missing and filled teeth, lower case indicates deciduous teeth); and
dmfs (decayed missing and filled surfaces).
The d(e)fs and d(e)ft ('e' indicates an extracted tooth), variants of dmfs and dmft, were included as primary outcomes.
All included studies must have reported a primary outcome to be considered for inclusion. A range of tools can be used for caries diagnosis including both direct clinical assessment (e.g. WHO Guidelines, ICDAS (International Caries Detection and Assessment System)) and indirect methods such as radiographs and photographs. Any caries diagnostic tool was identified and reported.
Secondary outcomes
For the infant/child
Microbiological presence (for example, streptococcus mutans count);
Plaque;
Oral health behaviour;
Dental attendance.
For the mother
Caries, including presence (with/without), decayed, missing and filled teeth (DMFT), and decayed missing and filled surfaces (DMFS);
Plaque;
Microbiological presence (e.g. streptococcus mutans count);
Gingival health;
Change in self‐reported oral health behaviours (including diet) and attitudes.
We recorded any adverse events and additional outcomes that had not been prespecified.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials. There were no language, publication year or publication status restrictions.
Cochrane Oral Health's Trials Register (searched 14 January 2019) (Appendix 1).
Cochrane Pregnancy and Childbirth Group Trials Register (to 22 January 2019) (Appendix 2).
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 12) in the Cochrane Library (searched 14 January 2019) (Appendix 3).
MEDLINE Ovid (1946 to 14 January 2019) (Appendix 4).
Embase Ovid (1980 to 14 January 2019) (Appendix 5).
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 14 January 2019) (Appendix 6).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly‐sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6 (Lefebvre 2011).
Searching other resources
The following trial registries were searched for ongoing studies (see Appendix 7 for details of the search strategy).
US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (clinicaltrials.gov; searched 14 January 2019).
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 14 January 2019).
We sought unpublished trials by contacting experts in the field. We checked all references cited in the included papers for additional relevant studies. We included studies reported in English only, and plan to translate papers not published in English, where possible, in future updates.
We checked that none of the included studies in this review were retracted due to error or fraud.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
The methodology used for data collection and analysis is based on Chapter 22 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The authors complied with the Methodological Expectations of Cochrane Intervention Reviews (MECIR) (Chandler 2013).
Selection of studies
Two review authors independently assessed all of the titles and abstracts of the identified studies against the inclusion criteria for this review. The search was designed to be sensitive and included controlled clinical trials; these were filtered out early in the selection process if they were not randomised. For each study appearing to meet the inclusion criteria, or where there was insufficient information to make a clear decision, we obtained the full text of a potential study and two review authors independently assessed it to establish whether it met the inclusion criteria. Where agreement was not achieved, we consulted a third review author. After reading all of the retrieved full‐text articles, we discarded any that did not meet the inclusion criteria. We recorded details of those studies excluded at this stage, and reasons for exclusion, in a Characteristics of excluded studies table, as well as details of studies classified as ongoing and awaiting assessment.
Data extraction and management
Two review authors independently extracted the data from the studies using a predefined data extraction form (initially piloted on a small sample of studies). We resolved discrepancies through consultation with a third review author. If any information from the studies was unclear or missing, we contacted the authors of the original papers (where feasible) for further information.
For each study, we recorded the following data in Characteristics of included studies tables.
Year of publication, country of origin, source of study funding and conflicts of interest.
Details of the participants including population and participant criteria, demographic characteristics (age, socioeconomic status, ethnicity).
Details of type of intervention, intervention timing, comparator and co‐interventions.
Location, number of centres, recruitment period.
Details of the outcomes reported, including methods of assessment and time intervals.
Theory or model used as the basis of the intervention.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study using the Cochrane domain‐based, two‐part tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following domains.
Sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias, for example, baseline imbalance.
We resolved any disagreements through discussion, consulting a third review author to achieve consensus, when necessary, and consulting study authors to check missing information, where feasible. We completed a 'Risk of bias’ table for each included study, and collated the risk of bias results for all studies graphically. For each domain of risk of bias, we described what was reported to have happened and our rationale for assigning low, high or unclear risk of bias status for that domain. We provided summary assessments of the risk of bias for each important outcome (across domains) within and across studies (as per Table 8.7a in the Cochrane Handbook for Systematic Reviews of Interventions, Higgins 2011).
Measures of treatment effect
For the prespecified review outcomes, we extracted the raw data from the trial reports. For dichotomous outcomes, we calculated risk ratios (RRs) for the proportional difference between the intervention and comparison groups, along with 95% CIs. For continuous outcomes, we extracted and used the mean values and standard deviations (SD) reported in the studies in order to express the estimate of effect as a mean difference (MD) with 95% confidence interval (CI).
Unit of analysis issues
The unit of analysis for the primary outcome in this review was the child. For the secondary outcomes, the unit of analysis was the child or mother.
Cluster‐randomised trials
We adjusted the sample sizes and event rates of included cluster‐randomised trials using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using an estimate of the intra‐cluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we had used ICCs from other sources, we planned to report this, and conduct sensitivity analyses to investigate the effect of variation in the ICC. We included both cluster‐randomised trials and individually‐randomised trials in meta‐analysis following careful consideration of whether it was reasonable to combine the results. We acknowledged heterogeneity in the randomisation unit and performed a subgroup analysis to investigate the effects of the randomisation unit in the analysis that included cluster and individually randomised trials. We have detailed how we adjusted the data reported by each included cluster‐randomised trial for inclusion in the review analyses in an additional table (see Table 7).
1. Details on adjustments made for cluster‐randomised trials.
| Study | Average cluster size (M) used to compute design effect 1 | ICC used to compute design effect | Design effect factor used in review analyses |
| Birungi 2015 | Not applicable | Not applicable | Not applicable; adjusted results included in the review meta‐analyses |
| Chaffee 2013 | Intervention: 26, the median cluster size reported by trial authors (range 10 to 36) Control: 17, the median cluster size reported by trial authors (range 5 to 34) |
For caries incidence outcome 0.014 and dmfs index outcome, 0.010. These were the ICCs reported by trial authors as used in adjusted analyses. | Caries incidence outcome: intervention group 1.35; control group 1.22 dmfs index outcome: intervention group 1.25; control group 1.16 |
| Harrison 2012 | Not applicable | Not applicable | 1.35 (the design effect reported as used by authors in their adjusted analysis, for all outcomes and groups) |
| Kramer 2001 | 448, computed by dividing the total number of children included in the caries assessment at 6 years (n = 13, 883) by 31, the number of clusters (hospitals/polyclinics) randomised | For caries incidence, 0.04, the ICC reported by trial authors as used in the adjusted analysis for this outcome For dmft, 0.13, the ICC reported by trial authors as used in the adjusted analysis for this outcome |
19 for caries incidence outcome (both groups) 59 for dmft index outcome (both groups) |
| Muhoozi 2017 | 51, the mean cluster size reported by trial authors | 0.01, the ICC trial authors reported as used in their adjusted analyses | 1.5 for all outcomes reported, and both groups |
dmfs: decayed missing filled primary surfaces; dmft: decayed missing filled primary teeth; ICC: intra cluster correlation coefficient; M: average cluster size
1 Design effect = 1 + (M‐1) * ICC
Cross‐over trials
Not eligible in this review.
Multi‐arm trials
For included multi‐arm trials, we used methods described in the Cochrane Handbook for Systematic Reviews of Interventions to overcome possible unit‐of analysis errors (Higgins 2011), by including only relevant groups (that met the intervention eligibility criteria), combining groups to make a single pairwise comparison (where appropriate), or by splitting the 'shared' group into two (or more) groups with smaller sample sizes, and including the two (or more) comparisons (see Included studies text for details of how this was done for each of the two multi‐arm trials we included).
Dealing with missing data
Where feasible, we attempted to contact the author(s) of included studies for clarification or details of missing data. We planned to use the methods described in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions to estimate missing standard deviations (Higgins 2011). We did not use any other statistical methods or perform any further imputation to account for missing data.
Assessment of heterogeneity
This review includes diverse interventions and we expected heterogeneity of intervention content, outcomes and outcome measures. We therefore planned to consider the feasibility of performing meta‐analysis on a subgroup of the studies once the data were extracted and the 'Risk of bias' assessment had been completed. We planned to test for heterogeneity using a Chi2 test where P < 0.1 gives an indication of the presence of heterogeneity, with inconsistency quantified and represented by the I2 statistic. The thresholds for interpretation were as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%; may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Where heterogeneity was detected (if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity), we planned to investigate possible causes and address them using methods described in Higgins 2011).
Assessment of reporting biases
Where possible, we planned to use multiple sources of data, including data from unpublished trials, if available, to assess reporting biases. For meta‐analysis including more than 10 studies, we planned to generate funnel plots and assess publication bias according to the recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We carried out statistical analysis using Review Manager 5 software (Review Manager 2014). We combined mean differences (using standardised mean differences where studies used different scales) for continuous outcomes, and combined relative risks for dichotomous outcomes, using a fixed‐effect model (as there were only two or three studies in each analysis). We planned to use a random‐effects model if there were four or more studies.
We performed meta‐analysis combining outcomes data only from studies evaluating similar included interventions (as standalone or combined interventions) against placebo or standard care. For child dental attendance and the oral behavioural outcomes, we tabulated the results as, due to variation across studies in the definition of measures, the data were not suitable for inclusion in a meta‐analysis.
As specified in the protocol, we included any adverse effects reported by studies in a table.
Subgroup analysis and investigation of heterogeneity
For the primary outcome of this review, we planned to carry out the following subgroup analysis to investigate the influence of possible effect modifiers on measures of effect. We planned to assess subgroup differences by interaction tests available within RevMan (Review Manager 2014) and report the results of these analyses quoting the Chi² statistic, P and interaction test I² values.
Intervention start time points: prenatal versus postnatal;
Intervention duration: ≤ 6 months versus > 6 months versus unspecified;
Child participant age at caries assessment: 3 years or less versus > 3 to 6 years;
Participant socioeconomic status: low (specified by author(s)) versus mixed or any (specified or unclear/not reported);
Unit of randomisation: cluster‐randomised trials versus individually‐randomised trials.
Sensitivity analysis
For all primary outcome meta‐analyses, we undertook sensitivity analyses, where relevant, to assess the robustness of the results by excluding studies assessed as high risk of bias for two or more domains.
Summary of findings and assessment of certainty of evidence using GRADE
We evaluated the certainty of the evidence for the primary outcome measures, caries presence in primary teeth, dmfs index, and dmft index, using the GRADE approach as outlined in the GRADE handbook (GRADE 2004). The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. In RCTs, the evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias (study limitations), indirectness of evidence, inconsistency, imprecision of effect estimates or potential publication bias. We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 in order to create 'Summary of findings' tables for these outcomes (GRADE 2004; GRADEpro Guideline Development Tool).
Results
Description of studies
Results of the search
Searches of databases retrieved a total of 1042 records, from which 436 duplicates were removed, leaving 606 unique records. We identified nine additional records through searching other sources. Therefore, 615 unique title and abstract records were screened. We rejected 464 of these as irrelevant, and assessed 151 full texts for eligibility. We included 17 studies reported in 52 papers (Birungi 2015; Chaffee 2013; Dasanayake 1993; Dasanayake 2002; Feldens 2007; Hallas 2015; Harrison 2012; Kramer 2001; Lapinleimu 1995; Muhoozi 2017; Veronneau 2010; Plutzer 2008; Robertson 2013; Soderling 2000; Thorild 2003; Watt 2009; Zanata 2003). We excluded 80 records reporting on 49 studies, noting reasons for the exclusions (see Characteristics of excluded studies tables). Eight studies (10 records) are ongoing (see Characteristics of ongoing studies) and four studies (nine records) are awaiting further classification, pending availability of data on caries in infants and children (Batra 2018; Jamieson 2012) or translation of full texts into English (Klastersky Genot 1970; Ratte 1969) (see Characteristics of studies awaiting classification). See Figure 1.
1.
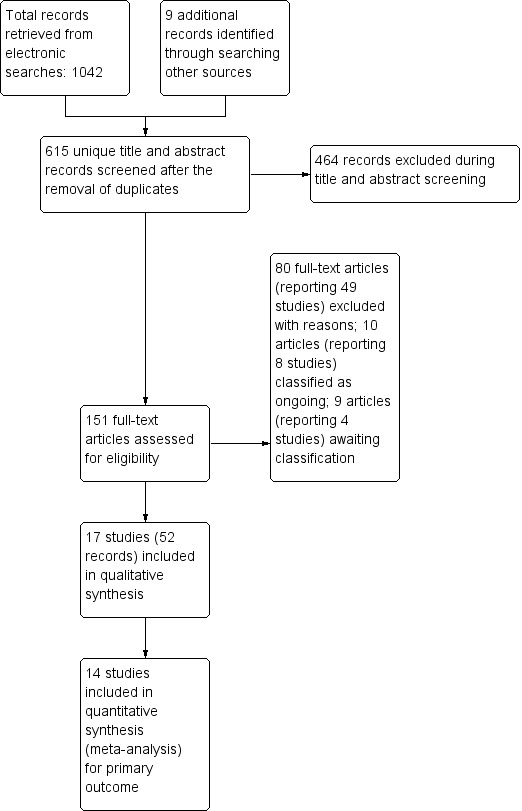
Results of search and study selection for inclusion in the review
Included studies
Following application of the review eligibility criteria, we included 17 randomised controlled trials (RCTs) in this review (Birungi 2015; Chaffee 2013; Dasanayake 1993; Dasanayake 2002; Feldens 2007Hallas 2015; Harrison 2012; Kramer 2001; Lapinleimu 1995; Muhoozi 2017; Plutzer 2008; Robertson 2013; Soderling 2000; Thorild 2003; Veronneau 2010; Watt 2009; Zanata 2003). Five trials were cluster‐randomised: three randomising community units (Birungi 2015; Harrison 2012; Muhoozi 2017) and two randomising health service units (Chaffee 2013; Kramer 2001). Three of the included trials were multi‐arm trials (Plutzer 2008; Soderling 2000; Thorild 2003).
A total of 23,732 caregivers and their foetuses or infants were randomised in the 17 included trials. In 15 of the included trials (Birungi 2015; Chaffee 2013; Dasanayake 1993; Dasanayake 2002; Feldens 2007; Hallas 2015; Harrison 2012; Kramer 2001; Plutzer 2008; Robertson 2013; Soderling 2000; Thorild 2003; Veronneau 2010; Watt 2009; Zanata 2003), 22,167 pregnant women and/or new mothers and their foetuses/infants were randomised, and the intervention(s) for preventing caries in children was delivered to the pregnant women and/or new mothers. In the Lapinleimu 1995 trial, families of young infants were randomised, and the intervention was delivered to parents (1054 mothers and fathers). In the Muhoozi 2017 trial, 511 mother and grandmother caregivers of young children were randomised to receive the intervention or standard care. In Lapinleimu 1995 and Muhoozi 2017, it was not possible to differentiate between mothers, fathers or other primary caregivers.
Nine of the included trials specified that only singleton foetuses/infants were eligible for inclusion (Birungi 2015; Feldens 2007; Hallas 2015; Kramer 2001; Plutzer 2008; Robertson 2013; Soderling 2000; Veronneau 2010; Watt 2009). Lapinleimu 1995 included eight twin pairs. The remaining seven trials provided no information about whether only singletons or singletons and multiples were included (Chaffee 2013; Dasanayake 1993; Dasanayake 2002; Harrison 2012; Lapinleimu 1995; Muhoozi 2017; Thorild 2003; Veronneau 2010; Zanata 2003). Therefore, we cannot provide an accurate number for the total number of foetuses/infants randomised in the included trials.
There is wide variation across the included trials in the number of included participants. Kramer 2001, a cluster‐randomised trial, randomised 17,046 women, following up 13,889 infants of these mothers for caries assessment (at six years of age). Plutzer 2008 and Feldens 2007 are the largest individually randomised included trials, randomising 649 and 500 mother‐infant pairs, respectively. Hallas 2015 and Dasanayake 1993 are the smallest trials included, randomising 94 and 62 mothers and their infants, respectively.
Substantially fewer women and children were included in the analyses for our primary and secondary outcomes than were randomised, with a maximum of 1148 children and 130 mothers included in any of our meta‐analyses.
Settings
The 17 included trials were conducted in a mix of high‐, middle‐ and low‐income countries. Three were conducted in the USA (Dasanayake 1993; Hallas 2015; Robertson 2013); three in Brazil (Chaffee 2013; Feldens 2007; Zanata 2003); two in Canada (Harrison 2012; Veronneau 2010), two in Finland (Lapinleimu 1995; Soderling 2000); two in Uganda (Birungi 2015; Muhoozi 2017) and one each in Australia (Plutzer 2008), Belarus (Kramer 2001), Sweden (Thorild 2003), and UK (Watt 2009). Country location was not reported in one trial (Dasanayake 2002).
Participants
In 15 of the 17 included trials, interventions were with pregnant women and/or new mothers of children younger than one year (at intervention start) (Birungi 2015; Chaffee 2013; Dasanayake 1993; Dasanayake 2002; Feldens 2007; Hallas 2015; Harrison 2012; Kramer 2001; Plutzer 2008; Robertson 2013; Soderling 2000; Thorild 2003; Veronneau 2010; Watt 2009; Zanata 2003). In one study, Lapinleimu 1995, the intervention was with new mothers and fathers of infants younger than one year, and in Muhoozi 2017 some of the primary caregivers who received the intervention were grandmothers (as due to absent mothers and fathers, they were the primary caregivers of the included infants).
Characteristics of the included participants are summarised below, and in additional tables (access to fluoridated water Table 8, age Table 9, socioeconomic status Table 10 and ethnicity Table 11).
2. Participant access to fluoridated water.
| Study ID | Intervention group | Control group |
| Birungi 2015 | Quote: "The fluoride concentration in drinking water is not monitored and may vary across the different geographical regions". | |
| Chaffee 2013 | Quote: "Residents in the city where the study was conducted were supplied with fluoridated water 0.7 ppm". | |
| Dasanayake 1993 | Quote: "Eligible mothers resided in a fluoridated community with their spouse or significant other". | |
| Dasanayake 2002 | Not reported | |
| Feldens 2007 | Quote: "almost all households within the study area (Sao Leopoldo, Brazil), had access to public water supply with fluoride level 0.7 ppm". | |
| Hallas 2015 | Not reported | |
| Harrison 2012 | Quote: "Eeyou Istchee community water supplies have no added fluoride". | |
| Kramer 2001 | Quote: "Drinking water is not fluoridated in Belarus" (where the study was conducted). Fluoride concentrations in drinking water are not monitored and may vary across geographic regions". | |
| Lapinleimu 1995 | Not reported | |
| Muhoozi 2017 | Quote: "The overall mean (SD) fluoride concentration in water in the study area (both study groups combined) was below the levels of caries prevention effect. Generally, most households (89.5%, both study groups combined) used water that was low in fluoride (< 0.70 mg/L). There was no difference in the concentration of fluoride in water between the two groups (P = 0.39)". | |
| Plutzer 2008 | Not reported | |
| Robertson 2013 | Authors reported that all participants were from American Indian communities in Oregon, Washington, and Arizona with fluoridated water systems; no further details. | |
| Soderling 2000 | Not reported | |
| Thorild 2003 | Not reported | |
| Veronneau 2010 | Not reported | |
| Watt 2009 | Not reported | |
| Zanata 2003 | Not reported | |
ppm: parts per million; SD: standard deviation
3. Participant socioeconomic status.
| Study ID | Intervention group | Control group |
| Birungi 2015 | Socioeconomically disadvantaged women and their infants/children; 64.7% and 35.3% of participants followed up for 5‐year outcomes reported by authors to be "poor" and "less poor", respectively. | |
| Chaffee 2013 | Social class by ABIPEME index (includes material possessions and education, A highest, E lowest status), n (%): A1: 0 (0) A2: 0 (0) B1: 8 (3.4) B2: 46 (19.5) C: 137 (58.1) D: 40 (17.0) E: 5 (2.1) |
Social class by ABIPEME index (includes material possessions and education, A highest, E lowest status), n (%): A1: 0 (0) A2: 0 (0) B1: 7 (3.2) B2: 38 (17.2) C: 136 (61.1) D: 39 (17.2) E: 2 (0.9) |
| Dasanayake 1993 | Not reported | |
| Dasanayake 2002 | Not reported | |
| Feldens 2007 | Quote: "The income was low for most of the families". Household income below one minimum wage of the national salary: 10.7% Household income between 1 and 3 minimum wages: 63.5% |
Quote: "The income was low for most of the families". Household income below one minimum wage of the national salary: 11. 1% Household income between 1 and 3 minimum wages: 58.7% |
| Hallas 2015 | Socioeconomically disadvantaged women and their infants/children | |
| Harrison 2012 | Socioeconomically disadvantaged women and their infants/children | |
| Kramer 2001 | Not reported | |
| Lapinleimu 1995 | Not reported | |
| Muhoozi 2017 | Socioeconomically disadvantaged women and their infants/children; about 84.4%, 83.8% and 76.4% of the households in the intervention group suffered mild to severe household food insecurity at baseline, at 12 to 16 months, and at 20 to 24 months, respectively. Maternal education: mean 4.9, SD 2.8 yrs |
Socioeconomically disadvantaged women and infants/children; about 85.9%, 89.3%, and 80.0% of the households in the control group suffered mild to severe household food insecurity at baseline, at 12 to 16 months, and at 20 to 24 months, respectively. Maternal education: mean 4.9, SD 2.8 yrs |
| Plutzer 2008 | Mixed socioeconomic status Quote: participant "residences were distributed over 151 postcodes across Adelaide and its suburbs"; no further details provided. |
|
| Robertson 2013 | Socioeconomically disadvantaged women and their infants/children | |
| Soderling 2000 | Not reported | |
| Thorild 2003 | Not reported | |
| Veronneau 2010 | Not reported | |
| Watt 2009 | Quote: "Overall, the sample was relatively disadvantaged with 28% being lone parents, 57% living in social housing and 33% receiving income support/job seekers allowance". | |
| Zanata 2003 | Socioeconomically disadvantaged | |
ABIPEME index: Associação Brasileira dos Institutos de Pesquisa de Mercado fundada em ‐ the Brazilian Association of Market Survey Institutes categorization of Brazilian socioeconomic class; SD: standard deviation; yrs: years
4. Participant age at recruitment or baseline.
| Study ID | Intervention group | Control group |
| Birungi 2015 | Mothers: mean 25 (IQR 20 to 30) yrs Infants: in utero (from 28 to 32 wks GA) |
Mothers: mean 24 (IQR 20 to 30) yrs Infants: in utero (from 28 to 32 wks GA) |
| Chaffee 2013 | Mothers: mean 27.1, SD 6.7 yrs Infants: newborn |
Mothers: mean 25.7, SD 6.6 yrs Infants: newborn |
| Dasanayake 1993 | Mothers: mean 24.0, SD 4.2 yrs Infants: in utero (from 28 to 40 wks GA) |
Mothers: mean 22.8, SD 3.0 yrs Infants: in utero (from 28 to 40 wks GA) |
| Dasanayake 2002 | Mothers: mean 20.1, SD 3.1 yrs Infants: in utero (mean 39.4 SD 1.7 wks GA) |
Mothers: mean 19.8, SD 2.7 yrs Infants: in utero (mean 39.5 SD 1.1 wks GA) |
| Feldens 2007 | Mothers: mean 25.7, SD 6.6 yrs at enrolment; mother teenager at child's birth: intervention group 17.8% and control group 19.7% Infants: newborn |
|
| Hallas 2015 | Mothers: not reported Infants: between 1 and 5 days |
|
| Harrison 2012 | Mothers: mean 25.5, SD 6.4 yrs; range 15 to 44 yrs Infants: in utero (12 to 34 wks GA) or newborn |
Mothers: mean 25.6, SD 5.8 yrs; range 15 to 39 yrs Infants: in utero (12 to 34 wks GA) or newborn |
| Kramer 2001 | Mothers: < 20 yrs 14.1%; 20 to 34 yrs 81.4%; > 35 yrs 4.2% Infants: newborn |
Mothers: < 20 13.5%; 20 to 34 82.3%; > 35 4.2% Infants: newborn |
| Lapinleimu 1995 | Parents: (46.8% mothers): mean 34.2, (range 23 to 61) Infants: from 7 to 13 mths |
|
| Muhoozi 2017 | Mothers: mean 26.1, SD 5.8 years Infants: mean 7.4 , SD 0.8 mths |
Mothers: mean 26.8, SD 6.3 yrs Infants: mean 7.3, SD 0.9 mths |
| Plutzer 2008 | Mothers: mean 25.4, SD 4.6 yrs Infants: in utero (ranged from 18 to 32 wks GA) |
|
| Robertson 2013 | Mothers: mean 26.8, SD 6.4 yrs Infants: mean 5.26, SD 0.64 mths; 2 years at caries assessment |
|
| Soderling 2000 | Mothers: xylitol group mean 29.3 yrs, 95% CI 28.3 to 30.3; CHX group 28.8, 95% CI 27.2 to 30.4 at enrolment Infants: 6 mths |
Mothers mean: 31.6, 95% CI 29.9 to 33.3 yrs at enrolment Infants: 6 mths |
| Thorild 2003 | Mothers: mean 30.1, range 17 to 44 yrs Infants: from 6 to 18 mths |
|
| Veronneau 2010 | Mothers: not reported Infants: 6 mths |
|
| Watt 2009 | Mothers: mean age 30 yrs 10 wks Infants: mean age 10 wks |
|
| Zanata 2003 | Mothers: not reported Infants: in utero (from 28 to 40 wks GA) |
|
CHX: chlorhexidine; CI: confidence interval; GA: gestational age; IQR: interquartile range; mths: months; SD: standard deviation; wks: weeks; yrs: years
5. Participant ethnicity.
| Study ID | Intervention group (%) | Control group (%) |
| Birungi 2015 | Not reported | |
| Chaffee 2013 | Black, mixed or other: 39.2 White: 60.8 |
Black, mixed or other: 49.3 White: 50.7 |
| Dasanayake 1993 | Black: 52 White: 48 |
Black: 68 White: 32 |
| Dasanayake 2002 | Black 84 White 11 Other: 5 |
Black: 97 White: 3 Other. 0 |
| Feldens 2007 | All participants were Portuguese‐speaking. | |
| Hallas 2015 | Quote: "Families from diverse ethnic backgrounds...Forty‐nine mothers were Spanish speaking; 10 of these mothers also spoke English. Forty‐five mothers spoke English but also spoke their native languages, which included Chinese (N = 1), Bengali (N = 5), Russian (N = 2), and Turkish (N = 1)”. | |
| Harrison 2012 | All participants First Nations people living in Cree communities, Quebec, Canada | |
| Kramer 2001 | Not reported | |
| Lapinleimu 1995 | Not reported | |
| Muhoozi 2017 | Not reported | |
| Plutzer 2008 | Not reported | |
| Robertson 2013 | All participants were American Indians or Alaskan Natives living in USA. | |
| Soderling 2000 | Not reported | |
| Thorild 2003 | Not reported | |
| Veronneau 2010 | Not reported | |
| Watt 2009 | Authors reported that 50% of participants were white; no further details. | |
| Zanata 2003 | Not reported | |
Access to fluoridated water
In eight of the included trials (Birungi 2015; Chaffee 2013; Dasanayake 1993; Feldens 2007; Harrison 2012; Kramer 2001; Muhoozi 2017; Robertson 2013), authors reported on the status of community water fluoridation where the study was located: Chaffee 2013, Feldens 2007 and Muhoozi 2017 reported specific fluoride concentrations; Birungi 2015, Dasanayake 1993 and Robertson 2013 reported that water was fluoridated without concentration levels; authors of the remaining two trials reported that the water supplied to participants in the study communities had no fluoride added (Harrison 2012; Kramer 2001). In nine trials, community water fluoridation status was not reported (Dasanayake 2002; Hallas 2015; Lapinleimu 1995; Plutzer 2008; Soderling 2000; Thorild 2003; Veronneau 2010; Watt 2009; Zanata 2003) (see Table 8).
Socioeconomic status
In nine of the 17 included trials (Birungi 2015; Chaffee 2013; Feldens 2007; Hallas 2015; Harrison 2012; Muhoozi 2017; Robertson 2013; Watt 2009; Zanata 2003), data reported on socioeconomic status suggested participants were socioeconomically disadvantaged. Plutzer 2008 included participants of mixed socioeconomic status. In the remaining seven trials (Dasanayake 1993; Dasanayake 2002; Kramer 2001; Lapinleimu 1995; Soderling 2000; Thorild 2003; Veronneau 2010), we were unable to determine participant socioeconomic status with the information provided on participant characteristics (see Table 9).
Age
The mean age of mothers at recruitment or at baseline was 26.9 years and ranged from 17 to 44 years old, reported by 12 trials (Birungi 2015; Chaffee 2013; Dasanayake 1993; Dasanayake 2002; Feldens 2007; Harrison 2012; Lapinleimu 1995; Muhoozi 2017; Plutzer 2008; Robertson 2013; Soderling 2000; Thorild 2003; Watt 2009); one trial reported maternal age range as a proportion (Kramer 2001). Three studies did not report maternal age (Hallas 2015; Veronneau 2010; Zanata 2003). Regarding infants, seven trials began with infants in utero (Birungi 2015; Dasanayake 1993; Dasanayake 2002; Harrison 2012; Plutzer 2008; Zanata 2003); three trials reported infants as newborns (not further defined) (Chaffee 2013; Feldens 2007; Kramer 2001), Hallas 2015 recruited infants one to five days old and Watt 2009 when infants were 10 weeks old. The remaining trials started when infants were between 5 to 18 months old (Lapinleimu 1995; Muhoozi 2017; Robertson 2013; Soderling 2000; Thorild 2003; Veronneau 2010). Maternal and infant age across the included studies is summarised further in Table 10.
Ethnicity
Half of the trials did not report the ethnic or racial background of participants (Birungi 2015; Kramer 2001; Lapinleimu 1995; Muhoozi 2017; Plutzer 2008; Soderling 2000; Thorild 2003; Veronneau 2010; Zanata 2003). Three trials reported the proportion of black/white participants (Chaffee 2013; intervention group: white 144 (60.8%), black, mixed or other, 99 (39.2%); control group: white 112 (50.7%), black, mixed or other 109 (49.3%); Dasanayake 1993; intervention group: 12/23 (52%) black, 11/23 (48%) white, control group: 17/25 (68 %) black, 8/25 (32 %) white; Dasanayake 2002 intervention group: black (84%), white (11%), other (5%); control group: black (97%), white (3%), other (0)); two trials reported the Indigenous background of participants (Harrison 2012: First Nations people (100%); Robertson 2013 American Indians or Alaskan Natives (100%)); two trials reported specific ethnicity/language of participants (Feldens 2007: Portuguese‐speaking Brazilians (100%); Hallas 2015: forty‐nine mothers were Spanish‐speaking; 10 of these mothers also spoke English. Forty‐five mothers spoke English but also spoke their native languages, which included Chinese (N = 1), Bengali (N = 5), Russian (N = 2), and Turkish (N = 1); and Watt 2009 reported the proportion of white participants, (intervention group: white (50%), control group (50%)) (See Table 11).
Diagnosis of dental caries in children
As expected, definitions of caries used in the assessment of children aged up to six years as with (without) caries differed across the included trials, with various levels of decay required for a caries diagnosis. Whilst some trials included white spots in the definition of decay, others required a carious lesion to be present for a caries diagnosis. Child age at the time of the assessment also varied across the trials. Definitions of caries used to assess children with caries in the trials, and ages of the included children at caries assessment are provided in Table 12.
6. Diagnosis of caries presence in primary teeth.
| Study ID | Diagnosis | Assessment age |
| Birungi 2015 | dmft > 0 Quote: "decayed, missing, and filled teeth index (dmft) defined in accordance with the WHO guidelines. A tooth was recorded as decayed if it was visually cavitated using a disposable mirror and dental explorer (Double ended No.23). A missing tooth was qualified as missing if extracted due to caries, as confirmed by the caregiver". |
5 yrs |
| Chaffee 2013 | dmfs ≥ 1 Quote: "Evaluations were visual, following WHO protocol. Non‐cavitated (white‐spot) lesions were also reported". |
3 yrs |
| Dasanayake 1993 | Quote: "One or more carious teeth". No further details | 3 yrs |
| Dasanayake 2002 | Not reported | Not applicable |
| Feldens 2007 | One or more cavitated, missing, or filled smooth surfaces in primary maxillary anterior teeth (d1 + mft ≥ 1). | 4 yrs |
| Hallas 2015 | Quote: "Any cavitated or white spots (demineralization of tooth appears as a white spot on the tooth surface) in primary teeth"; narrative outcome report included in this review only. | 6 and 12 mths |
| Harrison 2012 | Quote: "Criteria for caries detection were similar to those described by Pitts and co‐workers (Pitts 2001). Enamel caries (d2 = substance loss), dentinal caries (d3), pulpal caries (d4), restorations (f), and absence due to caries (e) were recorded". We included the d2 (enamel caries, substance loss in primary teeth) measure in the review meta‐analysis for any caries presence in primary teeth, as this was the primary caries outcome specified for the Harrison 2012 study. |
At least 30 mths |
| Kramer 2001 | DMFT (deciduous or permanent) ≥ 1; DMFT was defined as deciduous or permanent teeth that are carious, filled, extracted because of caries, or unerupted. Pre‐carious stages of decay were not included. | 6 yrs (mean 6.6, SD + 0.3 yrs) |
| Lapinleimu 1995 | "Any carious teeth", recorded according to the WHO criteria (World Health Organization 1979); only lesions with clear cavitations included | 3 yrs |
| Muhoozi 2017 | Occurrence of carious lesions in primary teeth, registered as unmistakable cavities progressing into the dentine as recommended by WHO were counted only; the diagnosis was limited to the upper front four primary teeth. Quote: "The photographs were taken with a Canon EOS 1100D Camera (Canon Inc., Taiwan) with a 60 mm macro‐lens and a macro‐ring flash. We aimed at an aperture of F stop 22 for the sharpness of the picture. ECC is defined as the occurrence of any signs of dental caries on any tooth surface during the first 3 years of life [33]. However, as the early stages of dental caries are not possible to identify on photographs, only obvious, cavitated lesions into the dentine were registered as caries. The photographs of the upper front teeth were evaluated by two experienced dentists (ABS and TW) who were blinded to the children’s group allocation. Interexaminer agreement measured by kappa was 0.97. In case of disagreement, the tooth was scored as sound". |
3 yrs |
| Plutzer 2008 | d3mft > 0 Quote: "To avoid variation in assessment, we did not consider dental examinations conducted by private practitioners, as these were not calibrated. For the same reason and to concentrate on substantive outcomes, we disregarded noncavitated (enamel) lesions, considering only dentine lesions (i.e. d3 lesions) as unequivocal evidence of decay. Trialists reported that dental examinations conducted by private practitioners, were not considered as these were not calibrated." |
6 to 7 yrs |
| Robertson 2013 | Any non‐cavitated lesions (d1), lesions where the cavitation extends into, but not through, the enamel (d2), or cavitated lesions that involve the dentine (d3). | 2 yrs |
| Soderling 2000 | dmft > 0 Quote: "Caries was recorded as decayed, missing and filled teeth...Dental caries was registered according to the WHO criteria (World Health Organization 1979), and the teeth were examined by means of a sharp explorer, fiber optic transillumination (FOTI), and mouth mirror. For the analyses, only lesions extending to the dentin, and fillings, were included.” |
2 yrs |
| Thorild 2003 | defs > 0 | 4 yrs |
| Veronneau 2010 | Not specified; only narrative outcome report included in this review. | not specified |
| Watt 2009 | dmft > 0 Quote: "The outcome measure for dental status was the dmft index (decayed, missing and filled deciduous teeth). Children were examined while standing in front of the sitting examiner. The diagnosis was visual using a sterilized plane mouth mirror and a MAG‐LED™ Mini Maglite® torch (MAG Instrument Inc, Ontario, California, USA). Data were recorded by tooth. Teeth were coded as decayed, filled or missing according to BASCD...The data collector (AS) was a trained and registered dentist". |
4 to 5 yrs (mean 4.7) |
| Zanata 2003 | Any carious lesions in primary teeth, including demineralisation areas or white spot lesions | 2 yrs |
BASCD: Brtish Association for the Study of Community Dentistry; d2: enamel caries in primary teeth (substance loss); d3: dentinal caries in primary teeth; d4: pulpal caries; defs: decayed extracted and filled surfaces; dfs: decayed and filled surfaces; dft: decayed and filled teeth; dmfs: decayed, missing and filled surfaces (primary); dmft: decayed, missing and filled teeth (primary); DMFT: decayed, missing and filled teeth (primary and permanent); e: primary teeth absence due to caries; ECC: early childhood caries; f: restorations in primary teeth; FOTI: fibre optic transillumination; fs: filled surfaces in primary teeth; mfs: missing and filled surfaces (primary); mths: months; SD: standard deviation; WHO: World Health Organisation; yrs: years
Interventions
Oral health education/promotion
-
Diet and feeding practice advice for infants and young children versus standard care:
Chaffee 2013: assessed dietary advice relating to breastfeeding, timing of weaning and a healthy weaning diet (e.g. low in sugar) delivered by healthcare workers who were trained in Brazilian infant feeding guidelines for children under two years of age and provided when mothers attended clinics for pre and postnatal visits. While the advice included good hygiene practices in food preparation and handling, and recommendations relating to infant sleeping and feeding practices (such as bottle use), no specific oral hygiene message was included in the intervention evaluated.
Feldens 2007: assessed a home visit dietary intervention delivered by trained field workers who counselled the mothers about breastfeeding and healthy weaning. The intervention was based on the WHO recommendations known as the ‘Ten Steps for Healthy Feeding of Children Younger than 2 Years’ and included: breastfeeding promotion and support, advice about a healthy weaning diet, provision of recipes for a healthy young child diet which were informed by affordable traditional food sources in the region, and advice about healthy feeding practices (e.g. recommendation that infants do not sleep with a bottle). No specific advice about oral hygiene was provided.
Watt 2009: assessed a feeding intervention delivered by local volunteers who were trained to provide home‐based non judgemental support and practical assistance on infant feeding, in particular, relating to weaning. On average, each mother received five home visits (mean length 60 minutes per visit).The intervention infant nutrition education assessed was designed to empower the women to follow current guidance on the later stages of infant feeding practices, in particular, when to introduce solids, the types of foods and drinks to give a child with emphasis on the importance of fruit and vegetables, and when to stop using a feeding bottle.
-
Breastfeeding promotion and support versus standard care:
Birungi 2015: assessed individual tailored home‐based peer counselling designed to promote exclusive breastfeeding in the immediate postpartum period provided to new mothers. The intervention was delivered by trained workers from the local community and started during pregnancy, with one visit during late pregnancy, and four visits through weeks one to ten after birth.
Kramer 2001: assessed a breastfeeding promotion intervention based on the WHO/UNICEF Baby‐Friendly Hospital Initiative, which emphasises healthcare worker assistance with initiating and maintaining breastfeeding and lactation and postnatal breastfeeding support.
-
Dieatary advice versus standard care:
Lapinleimu 1995: assessed an infant diet low in saturated fat and cholesterol. Every one to three months, parents in the intervention group received dietary advice aimed at adequate energy supply, with low fat intake. The intervention began when children were seven months old and was provided until they were 13 months. No specific oral hygiene, breastfeeding or feeding practice messages were included in the intervention assessed by this trial.
-
Oral hygiene, dietary and feeding practice advice versus standard care (*no data from trial in the review meta‐analysis):
Hallas 2015*: assessed a package of newborn oral healthcare education messages including advice about oral hygiene for mothers and infants and healthy feeding, and dietary practices for infants and young children. The education was provided via an eight‐minute video, delivered at the bedside of mothers during their postnatal hospital stay.
Harrison 2012: evaluated a programme of oral health education provided to mothers that started during pregnancy (one counselling session), with six additional sessions after birth, up to the child's second birthday, delivered at the time of routine infant wellness clinic visits. Advice included general oral hygiene messages for the mother and child, demonstration of how to clean infants' teeth, and advice about healthy infant feeding. Individuals from the local communities who had been trained by the study personnel led the intervention delivery, provided using the motivational interviewing technique.
Muhoozi 2017: assessed a package of health promotion measures that included oral hygiene education targeted at mothers and infants, and information to support a healthy infant diet and feeding practices. The measures included demonstration of how to cook meals. Caregivers of the included children were encouraged to have a kitchen garden with vegetables and domestic animals (chicken/rabbits), to provide cheap animal protein. The intervention started when infants were between six and eight months of age and was implemented for six months.
Plutzer 2008: evaluated a package of measures consisting of oral health and nutrition advice during pregnancy (targeted at mothers' health), infant oral health education, and advice about a healthy diet and feeding practices for very young children. The intervention was started with women during their pregnancy (one session), and continued until infants were one year of age.
Veronneau 2010*: assessed information about oral health provided to mothers by dental hygienists during four sessions at six‐month intervals starting in the early postpartum period (no further details provided in the conference abstract reporting this study).
Clinical
-
Antimicrobial treatment (CHX or iodine‐NaF solution and prophylaxis) in mother dentition versus placebo:
Dasanayake 1993: assessed application of an iodine‐NaF solution (after a brief prophylaxis/teeth clean) in mothers' dentition (six applications, started around the time of the infant's first tooth emergence) compared with a placebo varnish.
Dasanayake 2002: assessed a 10% CHX varnish applied to the dentition of mothers (four treatments, one per week over four weeks, started when babies were about six months, i.e. around the time of first tooth emergence), compared with a placebo varnish.
Robertson 2013: assessed a 10% CHX dental varnish applied to the dentition of mothers after a brief prophylaxis treatment (six treatments, started when infant was six months) compared with placebo varnish (alcohol).
Zanata 2003: assessed a topical application of a NaF and iodine solution immediately after prophylaxis and three and five days later combined with restorative care compared with placebo treatment.
-
Xylitol versus CHX or CHX + xylitol antimicrobial treatment in dentition of mothers:
Soderling 2000: assessed maternal consumption of xylitol chewing gum two of three times per day continuing until the child was three years of age versus CHX varnish applied to the dentition of mothers at 6, 12 and 18 months after the birth of the child.
Thorild 2003: assessed maternal consumption of xylitol chewing gum versus CHX/xylitol chewing gum. Mothers in both groups chewed one piece of the gum for five minutes, three times a day, starting at six months postpartum, up to 18 months postpartum.
Access to services and/or policy
Not reported by the included trials.
For additional details on the interventions evaluated, see Characteristics of included studies.
Multi‐arm trials
Three trials had multiple arms (Plutzer 2008; Soderling 2000; Thorild 2003). We either combined relevant groups or included only two relevant groups in the meta‐analyses as follows:
Plutzer 2008: included three groups of women, mothers who received oral health and dietary advice via printed information and via telephonic interview (high‐intensity intervention group); mothers who received the same advice but only in printed form (low‐intensity intervention group); and a standard care group. We combined the low‐ and high‐intensity intervention groups and compared this group with the standard care group for inclusion in the review analyses.
Soderling 2000: included three groups of women, a xylitol chewing gum group, a CHX varnish group and a fluoride varnish group. We included the first two groups as a pairwise comparison, as fluoride treatment in mothers is excluded from this review.
Thorild 2003: included three groups of women, a xylitol chewing gum group, a chlorhexidine/xylitol chewing gum group, and a xylitol fluoride chewing gum group. We included the first two groups as a pairwise comparison (due to the exclusion of fluoride treatment in mothers).
Comparisons
Oral health education/promotion
Diet and feeding practice advice for infants and young children versus standard care: three trials (Chaffee 2013; Feldens 2007; Watt 2009), all with data in the review meta‐analysis. The studies included in this comparison assessed the effects of dietary advice (including relating to breastfeeding, but also about healthy weaning) plus feeding practice advice for infants and young children, compared to standard care. We pooled the data from these three trials as they assessed the same range of interventions (education to change/improve children's diet including breastfeeding promotion, ensuring introduction of healthy first foods/solids when weaning, and advice about healthy feeding practices, e.g. not allowing children to drink sugary drinks in bottles, not allowing children to sleep with bottles), and therefore the mechanism working to reduce risk of caries/tooth decay in children (reducing antimicrobial load in the mouths of children) was similar in the studies.
Breastfeeding education and support versus standard care: two trials (Birungi 2015; Kramer 2001), both included in the review meta‐analysis. These two studies were pooled for analysis as the mechanism working on tooth decay was the same as the first set of studies (reduction in antimicrobial load in the mouths of children), however the health promotion intervention assessed was narrower.
Dietary advice for infants and young children versus standard care: one trial, Lapinleimu 1995. We included the data from this trial in a separate comparison, as it assessed dietary advice only, without any advice about breastfeeding (unlike the trials in the first two comparisons) or education about healthy child feeding practices.
Oral hygiene, dietary, and feeding practice advice versus standard care: five trials (Hallas 2015; Harrison 2012; Muhoozi 2017; Plutzer 2008; Veronneau 2010), three with data in the review meta‐analysis. These studies were combined for analysis as each assessed a holistic package of measures, including oral hygiene advice for mothers and diet, plus education focused on a health diet and feeding practices for infants and young children, compared with standard care. It makes sense to include these in a separate comparison, as there is an additional mechanism of action working on caries in the interventions assessed in these studies, namely the change in microbial load in the mother's mouth. Also, the range of interventions assessed by these studies is wider than in the other included oral health education/promotion comparisons above.
Clinical
Antimicrobial treatment (CHX or iodine‐NaF and prophylaxis) in mother dentition versus placebo: four trials (Dasanayake 1993; Dasanayake 2002; Robertson 2013; Zanata 2003), all contributing data to the review meta‐analysis.
Xylitol versus CHX or CHX + xylitol antimicrobial treatment in dentition of mothers: two trials (Soderling 2000; Thorild 2003), both providing data for analysis.
Outcomes
Outcomes for meta‐analysis were reported for the primary review outcome, caries in infants up to six years of age, by 14 trials (Birungi 2015; Chaffee 2013; Dasanayake 1993; Feldens 2007; Harrison 2012; Kramer 2001; Lapinleimu 1995; Muhoozi 2017; Plutzer 2008; Robertson 2013; Soderling 2000; Thorild 2003; Watt 2009; Zanata 2003).
Diet and feeding practice advice for infants and young children versus standard care
For this comparison, for the primary outcome, we included data from three studies (Chaffee 2013; Feldens 2007; Watt 2009) in the review meta‐analysis, reporting four measures: caries presence in primary teeth, three studies (Chaffee 2013; Feldens 2007; Watt 2009;); dmfs index, two studies (Chaffee 2013; Feldens 2007); dmft index, one study (Feldens 2007); and dm1 + mfs ≥ 5, one study (Feldens 2007).
For all the infant/child secondary outcomes, including microbiological presence (e.g. streptococcus mutans count, plaque and dental attendance), we were unable to include data from any study in the review meta‐analysis; however, we were able to include oral health behaviours from two trials (Feldens 2007; Watt 2009) as other data.
Considering the secondary outcomes for the mother, for caries, we were unable to include any data in the review analysis. We included change in self‐reported oral health behaviours from one trial (Watt 2009) as other data, and one trial only (Feldens 2007) provided any information on adverse events for mother or child.
Breastfeeding education and support versus standard care
We included data from two trials (Birungi 2015; Kramer 2001) reporting the two primary outcome measures: caries presence in primary teeth and dmft index. We were unable to include any other data or narrative outcomes for this comparison. Neither of the included trials provided any information on adverse events.
Dietary advice for infants and young children versus standard care
Only one study, Lapinleimu 1995, provided data for the primary outcome, caries presence in primary teeth. No other data were provided by this or any other trial for the primary outcomes, or child secondary outcomes. However, Lapinleimu 1995 provided data on child oral health behaviours that we included as other data.
Lapinleimu 1995 provided data for the mother secondary outcomes: plaque (assessed as presence of sub‐and supragingival calculus), gingival health in mothers (assessed as presence of mild or moderate bone loss), and information on change in self‐reported oral health behaviours that we were able to include as other data. Lapinleimu 1995, the only trial reporting this comparison, provided no information on adverse events.
Oral hygiene, diet and feeding practice advice versus standard care
For the primary outcome, we included data from two studies (Harrison 2012; Plutzer 2008) in the review meta‐analysis reporting caries presence in any primary teeth of children, and data from one study (Muhoozi 2017) reporting on caries presence in the top front four teeth of children. Additionally, for dmft index and SiC30 index (SiC30 index is the mean dmft among the 30% of children with the highest caries score), we included data from one study (Plutzer 2008). We included narrative outcome reports from two trials (Hallas 2015; Veronneau 2010) for caries presence in primary teeth, and from one trial (Veronneau 2010) for d1‐4efs.
For the secondary outcomes for the infant/child, we were unable to include any data in meta‐analysis. We were able to include dental attendance as other data from two studies (Harrison 2012; Plutzer 2008), and other data on oral health behaviours from one trial (Muhoozi 2017). Infant/child microbiological presence, and plaque, were not reported by any of the trials evaluating oral hygiene advice combined with infant diet and feeding practice advice against standard care.
Considering the secondary outcomes for the mother, for caries, we were unable to include data in the analysis. Change in self‐reported oral health behaviours from one study (Plutzer 2008) were included as data. Only one study (Harrison 2012) provided any information on adverse events for mother or child.
Antimicrobial treatment (CHX or iodine‐NaF and prophylaxis) in mother dentition versus placebo
For the primary outcome, caries presence in primary teeth, we included data from three studies (Dasanayake 1993; Robertson 2013; Zanata 2003) in the review meta‐analysis.
For the secondary outcomes for the infant/child, we were unable to include data from any trials in the review; however, for microbiological presence, more specifically, mutans streptococcus colonisation (any presence), we included narrative outcomes from two trials (Dasanayake 1993; Dasanayake 2002).
Considering the secondary outcomes for the mother, for caries, we were able to include data in the analysis for DMFT: increment, one trial (Dasanayake 2002) and DMFS: increment, two trials (Dasanayake 2002; Zanata 2003). No trial provided data for plaque, mother gingival health, microbiological presence or change in self‐reported oral health behaviours. The Dasanayake 1993 and Dasanayake 2002 trials provided narrative outcomes for microbiological presence in mothers, and adverse events for mother or child.
Xylitol versus CHX or CHX + xylitol antimicrobial treatment in dentition of mothers
For the primary outcome, we included data from two studies (Soderling 2000; Thorild 2003) in the review analysis, reporting four measures: caries presence in primary teeth, one study (Thorild 2003); dmft index, one study (Soderling 2000); defs score, one study (Thorild 2003); and defs categories (1‐3; 3‐4; ≥ 5), one study (Thorild 2003). Additionally, for caries presence in primary teeth, we included a narrative outcome from one study (Soderling 2000).
For the secondary outcomes for the infant/child, for child microbiological presence: mutans streptococci colonisation (any), we included data from two trials (Soderling 2000; Thorild 2003) in the meta‐analysis, and for mutans streptococci score (categories, including 0, 1, 2, 3), we included data from one trial (Thorild 2003). Narrative outcomes on microbiological presence: mutans streptococci colonisation (score) was also provided by two trials ((Dasanayake 1993; Dasanayake 2002). No other secondary review outcomes for the infant/child were reported.
Considering the secondary outcomes for the mother, for caries, we were able to include data in the analysis for DMFT: increment, one trial (Dasanayake 2002) and DMFS: increment, two trials (Dasanayake 2002; Zanata 2003). No trial provided data for plaque. One trial (Thorild 2003) provided data for inclusion in the review analysis on microbiological presence: mutans streptococci colonisation (level, CFU/mL), and we were able to include narrative reports for this outcome, from two trials (Dasanayake 1993, Dasanayake 2002). We also included adverse events for mother or child from the Dasanayake 1993 and Dasanayake 2002 studies.
Subgroups
The small number of trials included in the review meta‐analysis precluded investigating the influence of potential effect modifiers via subgroup analysis. The subgroup classifications for included studies are provided below, to facilitate such analysis in future.
Healthy diet and feeding practice advice for infants and young children versus standard care:
Intervention start: prenatal, one trial (Chaffee 2013); postnatal, two trials (Feldens 2007; Lapinleimu 1995; Watt 2009);
Intervention duration: ≤ 6 months, no trials; > 6 months intervention duration, three trials (Chaffee 2013; Feldens 2007; Watt 2009);
Child age at caries assessment: ≤ 3 years at caries assessment, Chaffee 2013; > 3 ≤ 6 years at caries assessment, Feldens 2007; Watt 2009;
Socioeconomic status: low, Chaffee 2013, Feldens 2007, Watt 2009; any or mixed, no trials;
Unit of randomisation: individually‐randomised trials, Feldens 2007, Watt 2009; cluster‐randomised trials, Chaffee 2013.
Breastfeeding promotion and support versus standard care:
Intervention start: prenatal, Birungi 2015; postnatal, Kramer 2001;
Intervention duration: ≤ 6 months, Birungi 2015; > 6 months, Kramer 2001;
Child age at caries assessment: ≤ 3 years, no trials; > 3 ≤ 6 years, two trials, Birungi 2015 and Kramer 2001;
Socioeconomic status: low, Birungi 2015; any or mixed, Kramer 2001;
Unit of randomisation: individually randomised, no trials; cluster‐randomised, two trials, Birungi 2015 and Kramer 2001.
Dietary advice for infants and young children versus standard care:
Intervention start: prenatal, no trial, postnatal, Lapinleimu 1995;
Intervention duration: ≤ 6 months, no trials; > 6 months intervention duration, Lapinleimu 1995;
Child age at caries assessment: ≤ 3 years at caries assessment, Lapinleimu 1995; > 3 ≤ 6 years at caries assessment, no trials;
Socioeconomic status: low, no trials; any or mixed, Lapinleimu 1995;
Unit of randomisation: individually‐randomised, Lapinleimu 1995; cluster‐randomised trials, no trials.
Oral hygiene education, dietary and feeding practice advice versus standard care:
Intervention start: prenatal, Harrison 2012; postnatal, Hallas 2015; Muhoozi 2017; Plutzer 2008; Veronneau 2010;
Intervention duration: ≤ 6 months intervention duration, Hallas 2015; Muhoozi 2017; Plutzer 2008; > 6 months intervention duration, Harrison 2012; not reported, Veronneau 2010;
Child age at caries assessment: ≤ 3 years at caries assessment, Hallas 2015; Harrison 2012; Muhoozi 2017; > 3 ≤ 6 years at caries assessment, Plutzer 2008; not reported, Veronneau 2010;
Socioeconomic status: low, Hallas 2015; Harrison 2012; Muhoozi 2017; any or mixed, Plutzer 2008; Veronneau 2010;
Unit of randomisation: individually‐randomised trials, Hallas 2015; Plutzer 2008; Veronneau 2010; cluster‐randomised trials, Harrison 2012, Muhoozi 2017.
Antimicrobial treatment (CHX or iodine‐NaF and prophylaxis) in mother dentition versus placebo:
Intervention start: prenatal, Zanata 2003; postnatal, Dasanayake 1993; Dasanayake 2002; Robertson 2013;
Intervention duration: ≤ 6 months intervention duration, Dasanayake 2002; Zanata 2003; > 6 months intervention duration (Dasanayake 1993; Robertson 2013);
Child age at caries assessment: ≤ 3 years at caries assessment, Dasanayake 1993; Dasanayake 2002; Robertson 2013; Zanata 2003;
Socioeconomic status: low, Robertson 2013; Zanata 2003; any or mixed, Dasanayake 1993; Dasanayake 2002;
Unit of randomisation: individually‐randomised trials, Dasanayake 1993; Dasanayake 2002; Robertson 2013; Zanata 2003.
CHX versus xylitol antimicrobial agent in dentition of mothers:
Intervention start: prenatal, no trials; postnatal, two trials, Soderling 2000; Thorild 2003;
Intervention duration: > 6 months intervention duration, Soderling 2000; Thorild 2003:
Child age at caries assessment: > 3 ≤ 6 years at caries assessment, Soderling 2000; Thorild 2003;
Socioeconomic status: any or mixed, Soderling 2000; Thorild 2003;
Unit of randomisation: individually‐randomised trials, Soderling 2000; Thorild 2003.
Funding
Funding sources were reported by all 17 included trials (Birungi 2015; Chaffee 2013; Dasanayake 1993; Dasanayake 2002; Feldens 2007; Hallas 2015; Harrison 2012; Kramer 2001; Lapinleimu 1995; Plutzer 2008; Robertson 2013; Soderling 2000; Thorild 2003; Veronneau 2010; Watt 2009; Zanata 2003). Funding bodies listed by the trials were noncommercial organisations (e.g. government funding bodies, health services or other not‐for‐profit foundations) in 15 of the trials. For two trials, commercial organisations provided some or all of the funding: Oralife Inc. in Toronto Canada, provided therapeutic agents and partial funding for the Dasanayake 2002 study; Colgate Oral Care and Johnson & Johnson Pacific Company provided some funding for the Plutzer 2008 trial.
Declarations of interest
Seven of the trials (Birungi 2015; Chaffee 2013; Hallas 2015; Harrison 2012; Plutzer 2008;Thorild 2003; Watt 2009) reported that there were no conflicts of interests for any of the authors. Eight trials (Dasanayake 1993; Dasanayake 2002; Feldens 2007; Lapinleimu 1995; Muhoozi 2017; Veronneau 2010; Robertson 2013; Soderling 2000) did not report any information regarding declarations of interest. One trial (Kramer 2001) reported information related to potential conflicts of interest for the trial authors. The declarations and potential conflicts of interest relating to them were unclear in the remaining trial (Zanata 2003), as they were not reported in English.
Excluded studies
We excluded 49 studies (Abanto 2012; Adams 2017; Alamoudi 2012; Al Khamis 2017; Bahri 2015; Bergel 2010; Brambilla 1998; Cardoso 2018; Rivas Castillo 2014; Cibulka 2011; Cockburn 1980; Curnow 2002; Kowash 2000; Plonka 2013; NCT02578966; Geisinger 2014; George 2018; Gomez 2001; Harjunmaa 2016; Hillman 1962; Holt 1985; Jiang 2015; Joury 2016; Karanja 2012; Kohler 1983; Kraivaphan 2007; Leverett 1997; Lopez 2002; Ma 2017; Macones 2010; Mohebbi 2009; Nakai 2010; NCT00719238; NCT01652300; NCT01763138; NCT02436811; NCT03273725; NCT03478748; NCT03529500; NCT03598972; NCT03693443; Olak 2012; Ramos‐Gomez 2012; Stensson 2014; Tenovuo 1992; Turksel 2004; Weber‐Gasparoni 2013; Weinstein 2004; Zhan 2012).
Eleven studies included pregnant women only (Al Khamis 2017; Bahri 2015; Rivas Castillo 2014; Cibulka 2011; Geisinger 2014; Harjunmaa 2016; Hillman 1962; Jiang 2015; Kraivaphan 2007; NCT01652300);
In ten studies, dental caries in children was not included as a study outcome (Abanto 2012; Brambilla 1998; Cockburn 1980; George 2018; Kohler 1983; Lopez 2002; Macones 2010; Nakai 2010; NCT00719238; NCT02436811);
In two studies, child caries was assessed when children were older than six years of age (Bergel 2010; Stensson 2014);
In 11 studies, the intervention was delivered to mothers who were not all mothers of children younger than 12 months at baseline (when the intervention started) (Alamoudi 2012; Cardoso 2018; Holt 1985; Joury 2016; Mohebbi 2009; NCT01763138; NCT03478748; Tenovuo 1992; Turksel 2004; Weber‐Gasparoni 2013; Weinstein 2004);
Six studies assessed interventions targeted at young children, not pregnant women or mothers of infants up to the age of 12 months (Ma 2017; Karanja 2012; Curnow 2002; Kowash 2000; Plonka 2013; Zhan 2012);
In the Leverett 1997 trial, the intervention targeted at pregnant women was a fluoride intervention, an intervention type excluded from this review as it is being evaluated in another Cochrane Review;
Seven of the studies were excluded on the basis of design, as they were observational studies or nonrandomised controlled trials (Adams 2017; Gomez 2001; Olak 2012; NCT03273725; NCT03529500; NCT03598972; NCT03693443).
Risk of bias in included studies
For a summary of the risk of bias across the included trials, see Figure 2 and Figure 3.
2.
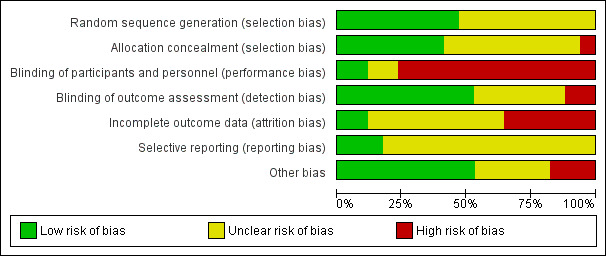
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged eight of the included trials as reporting some form of adequate random sequencing such as a computer‐generated sequence of random numbers and, therefore, as at low risk of bias associated with sequence generation (Birungi 2015; Chaffee 2013; Feldens 2007; Kramer 2001; Lapinleimu 1995; Muhoozi 2017; Plutzer 2008; Watt 2009). In the remaining nine trials (Dasanayake 1993; Dasanayake 2002; Hallas 2015; Harrison 2012; Robertson 2013; Soderling 2000; Thorild 2003; Veronneau 2010; Zanata 2003), we assessed the risk of selection bias associated with sequence generation as unclear, with insufficient information provided.
We assessed six of the trials as reporting a method of allocation concealment that was likely to have been effective, and hence as low risk of selection bias associated with allocation concealment (Chaffee 2013; Feldens 2007; Harrison 2012; Kramer 2001; Robertson 2013; Watt 2009). For 10 trials (Birungi 2015; Dasanayake 1993; Dasanayake 2002; Hallas 2015; Lapinleimu 1995; Muhoozi 2017; Soderling 2000; Thorild 2003; Veronneau 2010; Zanata 2003), we judged the risk of bias due to allocation concealment as unclear, due to lack of information provided on the methods used to conceal allocation during randomisation. For the remaining trial (Plutzer 2008), we judged the risk of selection bias associated with allocation concealment as high.
Therefore, considering risk of selection bias overall, we assessed four trials as being at low risk: Chaffee 2013; Feldens 2007; Kramer 2001; Watt 2009.
Blinding
Performance bias
We judged two trials to have low risk of performance bias (Dasanayake 1993; Robertson 2013), with adequate methods for blinding participants and study personnel reported. We judged one trial, Dasanayake 2002, to be at unclear risk of performance bias, with adequate methods of blinding mothers receiving the clinical treatment/placebo reported, but no information provided on blinding of study personnel. In 14 trials, the risk of performance bias, due to inadequate blinding of participant mothers and/or trial personnel, was judged to be high (Birungi 2015; Chaffee 2013; Feldens 2007; Hallas 2015; Harrison 2012; Kramer 2001; Lapinleimu 1995; Muhoozi 2017; Plutzer 2008; Soderling 2000; Thorild 2003; Veronneau 2010; Zanata 2003). While for some of the trials, lack of blinding was specifically stated, for others, no information was provided, and considering the nature of the intervention assessed, we judged effective blinding was unlikely.
Detection bias
Considering blinding of outcome assessors, nine trials clearly indicated that blinded trial personnel performed the outcome assessment or data collection, and we judged them to be at low risk of detection bias (Birungi 2015; Dasanayake 2002; Feldens 2007; Harrison 2012; Kramer 2001; Muhoozi 2017; Plutzer 2008; Thorild 2003; Veronneau 2010). For six trials (Chaffee 2013; Dasanayake 1993; Hallas 2015; Lapinleimu 1995; Robertson 2013; Watt 2009), we judged the risk of detection bias to be unclear, with trials not indicating clearly how outcome assessors were blinded. We judged the remaining two trials to be at high risk of detection bias as the authors reported that clinical assessors were not blind (for caries assessment) (Soderling 2000; Zanata 2003).
Incomplete outcome data
We judged two trials to be at a low risk of attrition bias, with minimal to moderate losses to follow‐up, and similar numbers/reasons for losses between groups (Dasanayake 2002; Kramer 2001). In the Kramer 2001 trial, a large cluster‐randomised trial, whilst losses before child caries assessment were moderate (nearly 20%), they were not judged to constitute a high risk of bias, due to the similarity in the level of attrition across groups.
We judged nine trials to be at unclear risk of attrition bias (Chaffee 2013; Dasanayake 1993; Feldens 2007; Harrison 2012; Robertson 2013; Soderling 2000; Thorild 2003; Veronneau 2010; Zanata 2003). In one of the trials (Dasanayake 1993), attrition was 22% overall before child caries assessment, and we were unable to determine differences in attrition rates and reasons across groups due to absence of data. In the Harrison 2012 trial, caries outcome data was provided for 110/131 (84%) and 131/141 (92%) infants of mothers randomised to the intervention and control groups, respectively. In Veronneau 2010, limited information provided in the conference abstract report precluded confident assessment as high or low risk of bias. Ramos‐Gomez 2012a reported losses before caries assessment of 32 and 34 percent in the intervention and control group, respectively; however, without reasons for losses reported by group, we were unable to confidently assess risk of attrition bias as high or low. In Robertson 2013, the attrition rates were relatively low in each group, but with some differences in the level of attrition across groups, causing uncertain implications for attrition bias. Soderling 2000 reported insufficient detail to assess attrition bias as high or low risk. In Thorild 2003, the levels of attrition at the caries assessment time points were moderate in the two groups included in this review, with small differences in the level of attrition across groups and uncertain implications for risk of bias. The losses prior to caries assessment in the Feldens 2007 trial were almost 30% in the two groups at the 4‐year caries assessment, however, there was marginal difference between the groups in the attrition rate, and reasons provided for losses were similar. In Chaffee 2013, a large cluster‐randomised trial, dental caries data were only available for 64.1% (458/715) of the initial sample; however, the trial authors reported that losses were principally due to withdrawal from the study or inability to locate and did not differ significantly by allocation status. Zanata 2003 also reported moderate attrition before the caries assessment; however, with very small differences between the groups in the level of attrition.
We considered six trials to be at high risk of attrition bias (Birungi 2015; Hallas 2015; Lapinleimu 1995; Muhoozi 2017; Plutzer 2008; Watt 2009). In Birungi 2015, attrition rates were high, over 40% before caries assessment, similar across groups, and authors reported significant differences between groups in the sample after attrition. In the Hallas 2015 trial, only 10/84 of the infants of mothers randomised were available for caries assessment and there was no reporting of their group status. In Lapinleimu 1995, only 78/540 (14%) and 70/522 (15%) of the infants of parents randomised to the intervention and control group, respectively, were included in the follow‐up dental study assessing caries in children at three years, of which 72/78 (92%) and 65/70 (93%), respectively, completed the three‐year substudy. In the Muhoozi 2017 trial, 170/263 (64.6%) of infants randomised to the intervention group, and 169/248 (68.1%) of infants randomised to the control group were available for caries assessment at 36 months of age. In Plutzer 2008, 75.4% of the intervention group infants and 66.8% of control group infants were available for the caries assessment at 20 months of age; and 29% and 28% of infants of mothers randomised to the intervention and control groups, respectively, were available for the caries assessment at age six to seven years. In the Watt 2009 trial, only 44/157 and 41/155 of infants of mothers randomised to intervention and control groups, respectively, were available for the child caries outcome assessment at four years.
Judgements regarding risk of attrition bias were primarily made considering the assessment of child caries, the primary outcome of this review.
Selective reporting
We judged only three trials to be at low risk of selection bias (Dasanayake 2002; Feldens 2007; Kramer 2001), providing data for prespecified and/or expected outcomes (including from the published protocols). The remaining 14 trials were judged to be at unclear risk of reporting bias (Birungi 2015; Chaffee 2013; Dasanayake 1993; Hallas 2015; Harrison 2012; Lapinleimu 1995; Muhoozi 2017; Plutzer 2008; Robertson 2013; Soderling 2000; Thorild 2003; Veronneau 2010; Watt 2009; Zanata 2003). For most of these trials, there was insufficient information to confidently assess selective reporting. Four of the trials were judged to be at unclear risk of reporting bias due to caries in infants/children not being included as a specified outcome in the study protocol (Chaffee 2013; Lapinleimu 1995; Muhoozi 2017; Watt 2009).
Other potential sources of bias
We judged nine trials to be at a low risk of other bias (Chaffee 2013; Dasanayake 1993; Dasanayake 2002; Feldens 2007; Lapinleimu 1995; Muhoozi 2017; Plutzer 2008; Thorild 2003; Watt 2009). One trial was judged as at high risk of other potential sources of bias (Birungi 2015), as authors reported that there was a high risk of a difference between the groups assessed for caries, more specifically, in socioeconomic status. For the remaining seven trials (Hallas 2015; Harrison 2012; Kramer 2001; Robertson 2013; Soderling 2000; Veronneau 2010; Zanata 2003), the risk of other bias was judged to be unclear, due to: failure to possible lack of standardisation of caries assessment across groups (Kramer 2001); possible baseline imbalances between groups (Harrison 2012; Soderling 2000); insufficient information on methods to confidently assess other sources of bias (Hallas 2015; Veronneau 2010; Zanata 2003); or possible intervention infidelity and insufficient information on methods to adequately assess other potential sources of bias (Robertson 2013).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
See Table 1, Table 2; Table 3; Table 4; Table 5; Table 6.
Diet and feeding practice advice for infants and young children versus standard care
Three trials evaluated this comparison (Chaffee 2013; Feldens 2007; Watt 2009).
Primary outcome
Caries presence in primary teeth
We observed a 15% reduced risk of caries presence in the primary teeth of children of mothers who received the diet and feeding advice intervention compared with the standard care group (RR 0.85, 95% CI 0.75 to 0.97; 3 trials, 782 participants; moderate‐certainty evidence; Analysis 1.1).The two trials assessed as not at high risk of bias for more than one risk of bias domain, were included in sensitivity analyses (Chaffee 2013; Feldens 2007). There was still evidence of a reduced risk of any caries presence in primary teeth between the intervention and standard care groups, though there was a marginally larger reduction in risk (RR 0.84, 95% CI 0.74 to 0.96; 2 trials; 697 participants).
1.1. Analysis.
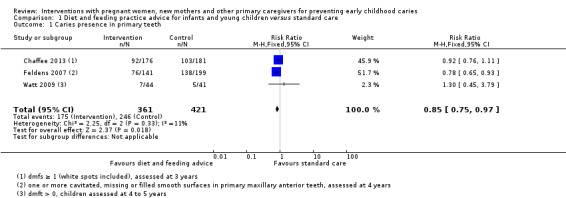
Comparison 1 Diet and feeding practice advice for infants and young children versus standard care, Outcome 1 Caries presence in primary teeth.
dmfs index score
There was a possible lower mean dmfs index score in the diet and feeding practice advice intervention group compared with the standard care group (MD ‐0.29, 95% CI ‐0.58 to 0.00; 3 trials, 757 participants; low‐certainty evidence; Analysis 1.2).The sensitivity analysis supported the main analyses by showing a possible lower mean score for children in the intervention compared with the standard care group (MD ‐0.29, 95% CI ‐0.58 to 0.00; 2 studies; 757 participants).
1.2. Analysis.

Comparison 1 Diet and feeding practice advice for infants and young children versus standard care, Outcome 2 dmfs index.
dmft index score
There was a possible lower mean dmft index score in the diet and feeding practice intervention group compared with the standard care group (MD ‐0.90, 95% CI ‐1.85 to 0.05; 1 trial, 340 participants; very low‐certainty evidence; Analysis 1.3).The sensitivity analysis results for dmft index score (MD ‐0.38, 95% CI ‐1.03 to 0.28, 2 studies; 575 participants) supported the finding of the main analysis, showing no evidence of a difference between the groups.
1.3. Analysis.
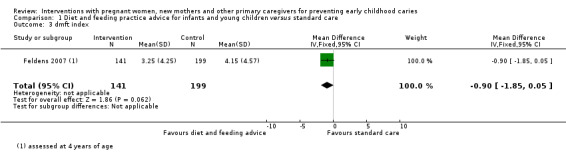
Comparison 1 Diet and feeding practice advice for infants and young children versus standard care, Outcome 3 dmft index.
d1 + mfs ≥ 5
One trial, Feldens 2007 reported caries severity assessed as d1 + mfs ≥ 5, and showed a lower risk in the diet and feeding practice advice intervention group compared with the standard care group (RR 0.68, 95% CI 0.50 to 0.92; 1 trial; 340 participants Analysis 1.4).
1.4. Analysis.
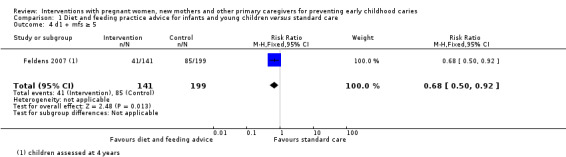
Comparison 1 Diet and feeding practice advice for infants and young children versus standard care, Outcome 4 d1 + mfs ≥ 5.
Secondary outcomes
For the infant/child
Microbiological presence
Not reported.
Plaque
Not reported.
Dental attendance
Not reported.
Dental general anaesthetics
Not reported.
Oral health behaviours
Two trials (Feldens 2007; Watt 2009) provided information related to infant/child oral health behaviours, which (given the variation in reporting) was not considered suitable for meta‐analysis. We have summarised the findings from the trials in Analysis 1.5. Both trials observed some evidence of benefit in favour of the diet and feeding practice intervention group compared with the standard care group.
1.5. Analysis.
Comparison 1 Diet and feeding practice advice for infants and young children versus standard care, Outcome 5 Child oral health behaviours.
| Child oral health behaviours | ||||
|---|---|---|---|---|
| Study | Behaviour changes associated with the intervention | Assessment time period | Benefit in favour of intervention | Benefit in favour of control |
| Feldens 2007 | "The intervention group had significantly longer duration of exclusive breast feeding (P ¼ 0.000), later introduction of sugar (P = 0.005), and smaller probability of ever having eaten biscuits (P = 0.000), honey (P = 0.003), soft drinks (P = 0.02), fromage‐frais (P = 0.001), chocolate and sweets (P = 0.001)" (Feldens 2007, p.215). | Four‐year follow‐up, children 4‐5 years of age | Some | No |
| Watt 2009 | "Frequency of consumption for milk and water was similar in both groups. More intervention group children drank pure, unsweetened fruit juice on a daily basis compared with the control group (RR = 1.57; 95% CI 0.99, 2.49). It was also more likely for intervention group children never to be given squash (RR = 1.76; 95% CI 1.20, 2.58). Daily consumption of tea, fizzy drinks or ready‐to‐drink soft drinks was rare in both groups (results not shown). Outcomes relating to drinking utensils and habits were consistently more favourable among intervention group children, although the differences were not statistically significant. Fewer intervention group children used feeder beakers with a spout as their main drinking utensil, used a baby bottle after their 4th birthday or usually took a bottle into bed. No difference was found in the consumption of bedtime drinks other than water (results not shown).Confectionary consumption was similar in both groups" (Scheiwe 2010, pg. 328). | Four‐year follow‐up, when children 4‐5 years of age | Some | |
For the mother
Plaque
Not reported.
Microbiological presence
Not reported.
Gingival health
Not reported.
Oral health behaviour
One trial (Watt 2009) provided information on mother self‐reported oral health behaviours, which we included as other data, and which showed some benefit in favour of the diet and feeding practice intervention compared with the standard care group (see Analysis 1.6),
1.6. Analysis.
Comparison 1 Diet and feeding practice advice for infants and young children versus standard care, Outcome 6 Change in mother self‐reported oral health behaviours (including diet) and attitudes.
| Change in mother self‐reported oral health behaviours (including diet) and attitudes | ||||
|---|---|---|---|---|
| Study | Behaviour changes associated with the intervention | Assessment time period | Benefit in favour of intervention | Benefit in favour of control |
| Watt 2009 | Women who reported that they "felt very confident to know what foods are good for child", % intervention group: 69; control group 43. Quote: "mothers from the intervention group had better nutritional knowledge and confidence". |
4‐year follow‐up | Some | |
Adverse events for mother or child
One trial only (Feldens 2007) provided information on adverse events for the comparison, infant and young child diet and feeding practice intervention versus standard care, and reported no events (Analysis 1.7).
1.7. Analysis.
Comparison 1 Diet and feeding practice advice for infants and young children versus standard care, Outcome 7 Adverse events for mother or child.
| Adverse events for mother or child | |
|---|---|
| Study | |
| Feldens 2007 | Quote: "none reported" |
Breastfeeding promotion and support versus standard care
Primary outcome
Any caries presence in primary teeth
There was no evidence of a difference in the risk of caries presence between the breastfeeding support and standard care groups (RR 0.96, 95% CI 0.89 to 1.03; 2 trials; 1148 participants; low‐certainty evidence; Analysis 2.1). Senstivity analysis, including only the one trial (Kramer 2001), assessed as not high risk of bias for more than one domain, similarly showed no evidence of a difference between the groups (RR 0.97, 95% CI 0.91 to 1.03; 1 trial; 731 participants).
2.1. Analysis.
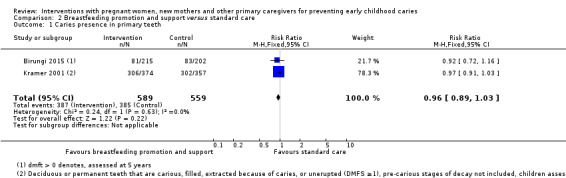
Comparison 2 Breastfeeding promotion and support versus standard care, Outcome 1 Caries presence in primary teeth.
dmfs index score
Not reported.
dmft index score
We observed no evidence of a difference between the breastfeeding intervention and standard care groups in the mean dmft index score (MD ‐0.12, 95% CI ‐0.59 to 0.36; 2 trials; 652 participants; low‐certainty evidence; Analysis 2.2). Sensitivity analysis, including the one trial (Kramer 2001) assessed as not high risk of bias for more than one domain similarly, showed no difference between the groups (MD 0.10, 95% CI ‐0.81 to 1.01; 1 trial; 235 participants).
2.2. Analysis.
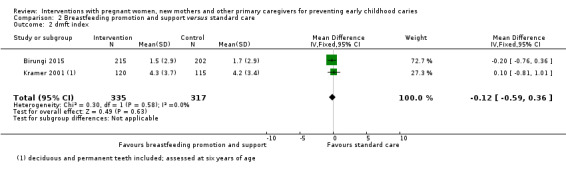
Comparison 2 Breastfeeding promotion and support versus standard care, Outcome 2 dmft index.
Secondary outcomes
Not reported.
Dietary advice for infants and young children versus standard care
One trial evaluated this comparison (Lapinleimu 1995).
Primary outcome
Any caries presence in primary teeth
We observed no evidence of a difference in caries presence in primary teeth between the dietary advice for infants and young children and standard care groups (RR 1.08, 95% CI 0.34 to 3.37; 1 trial; 148 participants; very low‐certainty evidence; Analysis 3.1).
3.1. Analysis.
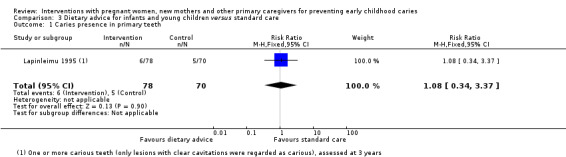
Comparison 3 Dietary advice for infants and young children versus standard care, Outcome 1 Caries presence in primary teeth.
Secondary outcomes
For the infant/child
Oral health behaviour
Data on oral health behaviours from Lapinleimu 1995, which we included as other data, showed no evidence of a difference between groups (see Analysis 3.2).
3.2. Analysis.
Comparison 3 Dietary advice for infants and young children versus standard care, Outcome 2 Child oral health behaviours.
| Child oral health behaviours | ||||
|---|---|---|---|---|
| Study | Behaviour change assocaited with intervention | Assessment time period | Benefit in favour of intervention | Benefit in favour of control |
| Lapinleimu 1995 | "The dental health of the intervention children and control children showed no differences. Only 24% of the intervention children and 39% of the control children brushed their teeth without parental assistance (p < 0.05). Flouridated toothpaste and fluoride tablets were used daily by by 62% and 52% of the children, respectively, and no differences were found between the intervention and control children in this respect" (Karjlainen 1997, p.182). | Three‐year follow‐up, children 3 years of age | No | No |
For the mother
Plaque
Lapinleimu 1995 reported presence of plaque in mother dentition assessed as sub and supragingival calculus, and we observed no evidence of a difference in risk between the dietary advice and standard care groups (RR 0.92, 95% CI 0.62 to 1.37; 1 trial; 133 participants; Analysis 3.3).
3.3. Analysis.
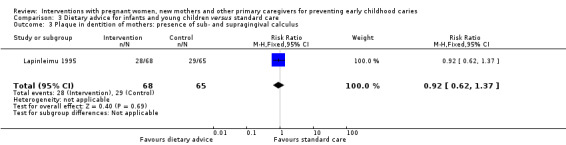
Comparison 3 Dietary advice for infants and young children versus standard care, Outcome 3 Plaque in dentition of mothers: presence of sub‐ and supragingival calculus.
Microbiological presence
Not reported.
Gingival health
Data from Lapinleimu 1995 on mother gingival health, assessed as presence of mild or moderate bone loss, showed no evidence of a difference in risk between the dietary intervention and standard care groups (RR 1.43, 95% CI 0.42 to 4.85; 1 trial; 133 participants; Analysis 3.4).
3.4. Analysis.
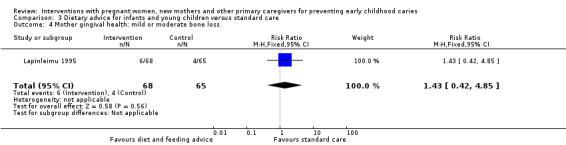
Comparison 3 Dietary advice for infants and young children versus standard care, Outcome 4 Mother gingival health: mild or moderate bone loss.
Oral health behaviour
Data from Lapinleimu 1995 on mother oral health behaviour, which we summarised as other data (see Analysis 3.5), showed some evidence of benefit in favour of the intervention compared with standard care.
3.5. Analysis.
Comparison 3 Dietary advice for infants and young children versus standard care, Outcome 5 Change in mother self‐reported oral health behaviours (including diet) and attitudes.
| Change in mother self‐reported oral health behaviours (including diet) and attitudes | ||||
|---|---|---|---|---|
| Study | Behaviour change associated with intervention | Assessment time period | Benefit in favour of intervention | Benefit in favour of control |
| Lapinleimu 1995 | ≥ 3 years since the previous dental examination or treatment, %: intervention mothers 4; control mothers 9 |
4‐year follow‐up | Some | |
Adverse events for mother or child
Not reported.
Oral hygiene, diet and feeding advice versus standard care
Primary outcome
Any caries presence in primary teeth
Two trials reported on any caries presence in primary teeth, showing no evidence of a difference between the intervention and standard care groups (RR 0.91, 95% CI 0.75 to 1.10; 2 trials; 365 participants; low‐certainty evidence). One trial, that included support to enable caregivers to provide children with a higher protein diet as well as provision of recipes to improve the type of meals provided to children, reported on caries presence in the top front four teeth of children only; it similarly showed no evidence of a difference between the groups (RR 0.68, 95% CI 0.42 to 1.10; 1 trial; 226 participants; very low‐certainty evidence) (Analysis 4.1). Sensitivity analysis excluding the two trials assessed as high risk of bias for more than two domains confirmed the main analysis result, by showing no evidence of a difference between the groups (RR 0.88, 95% CI 0.72 to 1.07; 1 trial; 178 participants).
4.1. Analysis.
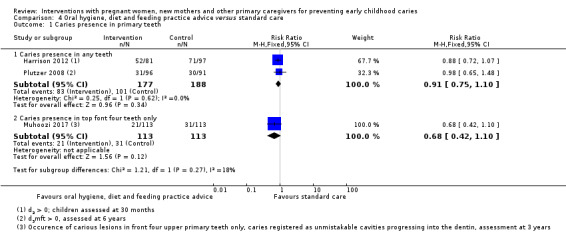
Comparison 4 Oral hygiene, diet and feeding practice advice versus standard care, Outcome 1 Caries presence in primary teeth.
Hallas 2015 reported that assessment of the 10/94 infants who returned for the 6‐month and 12‐month visits revealed no white spot lesions on any teeth, and that all infants were determined to be cavity‐free at both visits. The authors of the Veronneau 2010 trial reported that: "at 30 months, 86.8% and 86.9% of test and control groups respectively were caries free. However, at 5‐6 yrs old...40% of the test group and 31.7% of the control group was caries free (p = 0.09)".
dmfs index score
Only one trial (Plutzer 2008) reported dmfs index, and showed no evidence of a difference between the oral hygiene, diet and feeding advice intervention and standard care groups in the mean dmfs index score (MD ‐0.99, 95% ‐2.45 to 0.47; 1 trial, 187 participants; very low‐certainty evidence; Analysis 4.2).
4.2. Analysis.
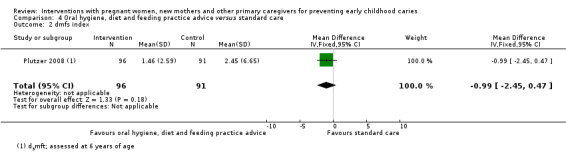
Comparison 4 Oral hygiene, diet and feeding practice advice versus standard care, Outcome 2 dmfs index.
dmft index score
Plutzer 2008 was the only trial to report on dmft index, and showed no evidence of difference between the oral hygiene, diet and feeding advice intervention and standard care groups in the mean dmft index score (MD ‐0.30, MD ‐0.96 to 0.36; 1 trial; 187 participants; very low‐certainty evidence; Analysis 4.3).
4.3. Analysis.
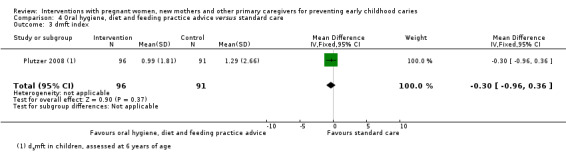
Comparison 4 Oral hygiene, diet and feeding practice advice versus standard care, Outcome 3 dmft index.
SiC30 index score
One trial only, Plutzer 2008, reported mean SiC30 index score and showed a result favouring oral hygiene, diet and feeding advice intervention over standard care (MD ‐0.93, 95% CI ‐1.73 to ‐0.13; 1 trial; 187 participants; Analysis 4.4).
4.4. Analysis.

Comparison 4 Oral hygiene, diet and feeding practice advice versus standard care, Outcome 4 SiC30 index.
d1‐4 efs
Veronneau 2010 reported that: "At 30 months, both groups had mean d1‐4efs scores of 0.7...However, at 5‐6 yrs old, the mean d1‐4 efs scores were 4.9 for the test group and 6.8 for the control group (p < 0.001 for the difference)".
Secondary outcomes
For the infant/child
Microbiological presence
Not reported.
Plaque
Not reported.
Oral health behaviours
One study (Muhoozi 2017) reported changes in child oral health behaviours associated with the intervention, included as other data, which showed some benefit in favour of oral hygiene, diet and feeding practice advice (Analysis 4.5 ).
4.5. Analysis.
Comparison 4 Oral hygiene, diet and feeding practice advice versus standard care, Outcome 5 Child oral health behaviours.
| Child oral health behaviours | ||||
|---|---|---|---|---|
| Study | Behaviour changes associated with the intervention | Assessment time period | Benefit in favour of intervention | Benefit in favour of control |
| Muhoozi 2017 | Quote: "The frequency of cleaning of the child's teeth at 36 months was about twice as high in the intervention as in the control group (84.3% vs 46.6%; P = 0.0001...The materials which were reportedly used in the cleaning of the child's oral cavity included toothbrush with water, clean cloth and water, stick or herbs and a finger and water. The use of toothbrush and water was reported significantly more common in the intervention group than control group...The proportion of mothers who reported giving night feeds to the children was higher in the control group than the intervention group". | Intervention started when children between 6 and 8 months; children assessed at 36 months | Some | |
Dental attendance
Plutzer 2008 reported dental attendance, as cumulative categories of child visits from birth to six years of age (including 1 to 2 visits, 3 to 4 visits and ≥ 5 visits), which we have included as other data (see Analysis 4.6). For 1 to 2 visits, children in the oral hygiene, diet and feeding advice intervention group were more likely to attend than those in the standard care group; for 3 to 4 visits there was no difference between the groups, and for ≥ 5 visits, children in the intervention group were less likely than those in the standard care group to attend services.
4.6. Analysis.
Comparison 4 Oral hygiene, diet and feeding practice advice versus standard care, Outcome 6 Child dental attendance.
| Child dental attendance | |||||
|---|---|---|---|---|---|
| Study | Measure | Intervention group # events/participants | Control group # events / participants | Effect estimate Risk Ratio (M‐H, Fixed, 95% CI) | Favoured group |
| Harrison 2012 | Saw dentist due to tooth pain | 13/110 | 22/131 | 0.70 (0.37, 1.33) | Neither |
| Plutzer 2008 | Cumulative categories of visits, from birth to 6 years 2 visits 3‐4 visits ≥ 5 visits |
85/117 28/117 4/117 |
72/113 29/113 12/113 |
1.14 (0.95, 1.36) 0.93 (0.59, 1.46) 0.32 (0.11, 0.97) |
Neither Neither Oral hygiene, diet and feeding advice |
For the mother
Plaque
Not reported.
Microbiological presence
Not reported.
Gingival health
Not reported
Oral health behaviour
One trial (Plutzer 2008) provided information on changes in mother behaviours related to oral health, which we summarised as other data in Analysis 4.7. The findings from this trial showed some benefit in favour of oral hygiene, diet and feeding practice intervention compared with standard care.
4.7. Analysis.
Comparison 4 Oral hygiene, diet and feeding practice advice versus standard care, Outcome 7 Change in mother self‐reported oral health behaviours (including diet) and attitudes.
| Change in mother self‐reported oral health behaviours (including diet) and attitudes | ||||
|---|---|---|---|---|
| Study | Behaviour changes associated with the intervention | Assessment time period | Benefit in favour of intervention | Benefit in favour of control |
| Plutzer 2008 | Remedies used to by mothers to alleviate teething problems in infants, %:
Quote: "Providing mothers with information on how to address teething symptoms markedly reduced the use of medications for symptom relief. There is still need for better evidence, first, on what symptoms can or cannot be attributed to teething and, second, on what is effective in alleviating them." |
Intervention delivered to mothers at 6 and 12 months postpartum; behaviour change assessed at infant age 20 months. | Some | |
Adverse events for mother or child
One trial (Harrison 2012) reported observing no adverse events. None of the other trials assessing oral hygiene, diet and feeding advice compared with standard care provided information relating to adverse events (Analysis 4.8).
4.8. Analysis.
Comparison 4 Oral hygiene, diet and feeding practice advice versus standard care, Outcome 8 Adverse events for mother or child.
| Adverse events for mother or child | |
|---|---|
| Study | |
| Harrison 2012 | Quote: "no adverse events were reported". |
Antimicrobial treatment (CHX or iodine‐NaF and prophylaxis) in mother dentition versus placebo
Four trials evaluated this comparison (Dasanayake 1993; Dasanayake 2002; Robertson 2013; Zanata 2003).
Primary outcome
Any caries presence in primary teeth
There was no evidence of a difference in the risk of caries presence in primary teeth between the antimicrobial treatment (CHX or idodine‐NaF) intervention and placebo or no antimicrobial treatment groups (RR 0.97, 95% CI 0.80 to 1.19; 3 trials; 479 participants; very low‐certainty evidence; Analysis 5.1). For this analysis, we observed moderate statistical heterogeneity (Chi² = 4.14, P = 0.13, I² = 52%). Two trials assessed as not at high risk of bias for more than one risk of bias domain, were included in sensitivity analyses (Dasanayake 1993; Robertson 2013). The result was similar to the main analysis: we observed no evidence of a difference between treatment and placebo groups in risk of caries presence in primary teeth (RR 1.03, 95% CI 0.84 to 1.27; 2 trials; 415 participants).
5.1. Analysis.
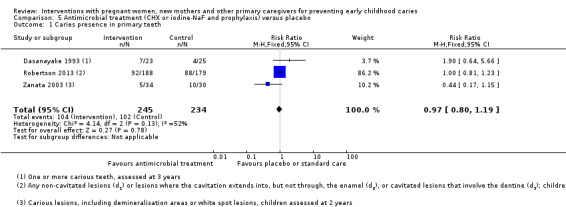
Comparison 5 Antimicrobial treatment (CHX or iodine‐NaF and prophylaxis) versus placebo, Outcome 1 Caries presence in primary teeth.
Secondary outcomes
For the infant/child
Microbiological presence
Dasanayake 1993 reported that "crude overall incidence of MS acquisition in children of the treatment mothers was 36% greater than that of the control children; however, the difference was not statistically significant. The incidence estimates were adjusted for race and gender. After the adjustment, there was no significant excess of MS incidence in either group." In this study, a child was defined as colonised with MS (mutans streptococci) if any two consecutive samples among all saliva, plaque, or swab samples were positive for MS. This study also reported that "the median time of colonisation of the treatment group infants was 26 months compared with 32 months for the control group. However, these mean times were not significantly different from each other since their two survival curves, indicating the time until colonization, were homogeneous".
Dasanayake 2002 reported that: "There were no significant differences in the percentage of children with detectable levels of S. mutans in plaque during the study period or in the mean times to oral colonization of S. mutans". Children in this trial had their first positive sample for S. mutans between 19 to 29 months (median age 24 to 27 months).
Plaque
Not reported.
Oral health behaviours
Not reported.
Dental attendance
Not reported.
Dental general anaesthetics
Not reported.
For the mother
Caries
Two trials (Dasanayake 2002; Zanata 2003) reported dmfs increment (change in dmfs score) in mothers, and we observed no evidence of a difference between the antimicrobial treatment group and placebo or no antimicrobial treatment groups (MD ‐0.21, 95% CI ‐2.22 to 1.79; 2 trials; 130 participants; Analysis 5.2). One trial (Dasanayake 2002) reported DMFT increment (change in DMFT score) and similarly showed no evidence of a difference between the groups (MD ‐0.30, 95% CI ‐1.86 to 1.26; 1 trial; 66 participants; Analysis 5.3).
5.2. Analysis.
Comparison 5 Antimicrobial treatment (CHX or iodine‐NaF and prophylaxis) versus placebo, Outcome 2 Mother DMFS increment.
5.3. Analysis.
Comparison 5 Antimicrobial treatment (CHX or iodine‐NaF and prophylaxis) versus placebo, Outcome 3 Mother DMFT increment.
Microbiological presence
Dasanayake 1993 reported that: “Immediately following the treatment period, there was a significant reduction of MS by 70% (P = 0.04), a 45% decline in lactobacilli (P = 0.04), a 46% decline in total streptococci (P = 0.002) and a 42% decline in total cultivable bacteria (P = 0.004) in the treatment group. S. sattguis increased significantly (32%; P = 0.01) in the control group. None of the post‐treatment values in the treatment group was significantly different from the corresponding values in the control group as indicated by the repeated measures of analysis of variance. However, the post‐treatment values for this group were consistently lower than the control group."
Dasanayake 2002 reported that: "The effect of the chlorhexidine varnish on the maternal S. mutans levels is shown in figure 1. The treatment group exhibited a significant reduction in the S. mutans levels in stimulated saliva compared to the control group. This reduction began after the 2nd of the first 4 applications given between six and seven months after delivery and remained significant for about 12 months. Repeated‐measures Anova that included the number of antibiotic episodes as an independent variable indicated that this treatment effect over time was statistically significant (p = 0.0002 for the group vs time interaction term in the mixed model)".
Gingival health
Not reported.
Change in self‐reported oral health behaviours
Not reported.
Adverse events for mother or child
Two trials (Dasanayake 1993; Dasanayake 2002) reported information relating to adverse events for mother or child, which we have recorded as other data (see Analysis 5.4). In both trials, adverse events were reported related to the topical application of treatment solutions.
5.4. Analysis.
Comparison 5 Antimicrobial treatment (CHX or iodine‐NaF and prophylaxis) versus placebo, Outcome 4 Adverse events for mother or child.
| Adverse events for mother or child | |
|---|---|
| Study | |
| Dasanayake 1993 | Quote: "Eight mothers in the treatment group and three in the control group reported adverse effects from the topical application of treatment solutions, but the difference was not statistically significant (P = 0.09)." |
| Dasanayake 2002 | Quote: "Twenty adverse events were recorded for 14 women (9 in the treatment group and 5 in the control group): staining of teeth (8); minor ulcers (4); nausea (1), and gingival irritation or burning sensation during application (7). None of these events were classified as serious by FDA criteria, and all subjects have recovered uneventfully. There were more events in the treatment group (n = 13) compared to the control group (n = 7), but the difference was not statistically significant (p = 0.13), nor was the difference in the number of women experiencing adverse events (p = 0.25)." |
Xylitol versus CHX or CHX + xylitol antimicrobial treatment
Primary outcome
Any caries presence in primary teeth
Data from one trial (Thorild 2003) showed no evidence of a difference in the risk of caries presence in primary teeth between the xylitol antimicrobial antimicrobial intervention and CHX intervention groups (RR 0.62, 95% CI 0.27 to 1.39; 1 trial; 96 participants; very low‐certainty evidence; Analysis 6.1).
6.1. Analysis.

Comparison 6 Xylitol versus CHX or CHX + xylitol antimicrobial treatment, Outcome 1 Caries presence in primary teeth.
Soderling 2000 reported that "the differences in risk (at the age of 2 years) between the chlorhexidine and the xylitol groups (RR = 1.39; 95% CI, 0.69‐2.79)...were not statistically significant”. The dmft index was used in this study to assess caries in dentition of children, with only lesions extending to the dentin, and fillings, included in the diagnosis of caries presence.
dmft index (score)
Data from Soderling 2000 showed a lower mean dmft in the xylitol intervention group compared with the CHX intervention group at 5‐year assessment (MD ‐2.39, 95% CI ‐4.10 to ‐0.68; 113 participants; low‐certainty evidence; Analysis 6.2).
6.2. Analysis.

Comparison 6 Xylitol versus CHX or CHX + xylitol antimicrobial treatment, Outcome 2 dmft index.
defs index (score)
Thorild 2003 reported mean dmfs index score, and showed no evidence of a difference between the xylitol and CHXl intervention groups (MD ‐0.28, 95% ‐0.83 to 0.27; 1 trial, 96 participants; very low‐certainty evidence; Analysis 6.3).
6.3. Analysis.
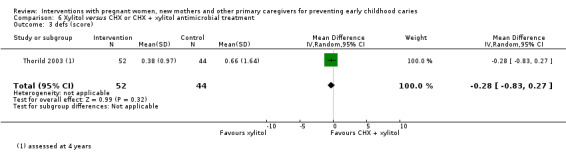
Comparison 6 Xylitol versus CHX or CHX + xylitol antimicrobial treatment, Outcome 3 defs (score).
defs index (score categories)
Thorild 2003 also reported defs score categories and we similarly observed no evidence of a difference between the xylitol intervention and CHX/xylitol groups: 1 to 3 defs (RR 0.48, 95% CI 0.15 to 1.54; 1 trial; 96 participants), 3 to 4 defs (RR 0.85, 95% CI 0.18 to 3.98; 1 trial; 96 participants), ≥ 5 defs (RR 0.28, 95% CI 0.01 to 6.78) (very low‐certainty evidence; Analysis 6.4).
6.4. Analysis.
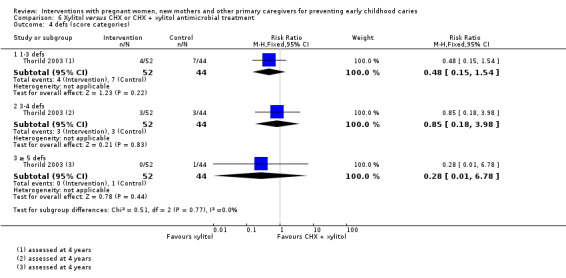
Comparison 6 Xylitol versus CHX or CHX + xylitol antimicrobial treatment, Outcome 4 defs (score categories).
Secondary outcomes
For the infant/child
Microbiological presence
Two trials (Soderling 2000; Thorild 2003) reported any mutans streptococci colonisation and showed a lower risk of any mutans streptococci colonisation in the children of mothers who were in the xylitol intervention compared with CHX or CHX combined with xylitol intervention group (RR 0.60; 95% CI 0.45 to 0.81; Analysis 6.5).
6.5. Analysis.
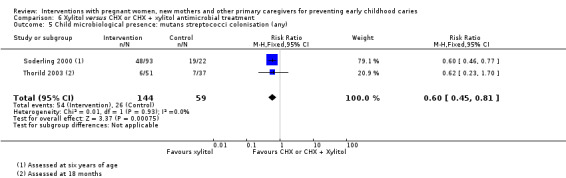
Comparison 6 Xylitol versus CHX or CHX + xylitol antimicrobial treatment, Outcome 5 Child microbiological presence: mutans streptococci colonisation (any).
Thorild 2003 reported mutans streptococci colonisation score categories, and we observed no evidence of a difference in risk between the xylitol intervention and xylitol combined with CHX intervention group, for any of four categories: score 0 (RR 1.12, 95% CI 0.88 to 1.41; 1 trial; 100 participants); score 1 (RR 0.65, 95% CI 0.21 to 2.01; 1 trial; 100 participants); score 2 (RR 0.39, 95% CI 0.08 to 2.05; 1 trial; 100 participants); score 3 (RR 1.31, 95% CI 0.33 to 5.18; 1 trial; 100 participants) (Analysis 6.6).
6.6. Analysis.
Comparison 6 Xylitol versus CHX or CHX + xylitol antimicrobial treatment, Outcome 6 Child microbiological presence: mutans streptococci (score categories).
Plaque
Not reported.
Oral health behaviours
Not reported.
Dental attendance
Not reported.
Dental general anaesthetics
Not reported.
For the mother
Plaque
Not reported.
Microbiological presence
One trial (Soderling 2000) reported mutans streptococci colonisation level in mothers (CFU/mL), assessed at the three‐year child caries assessment time point, and we observed a lower level of colonisation in the xylitol intervention compared with the CHX intervention group (MD 0.50, 95% CI 0.15 to 0.85; 1 trial, 126 participants; Analysis 6.7).
6.7. Analysis.
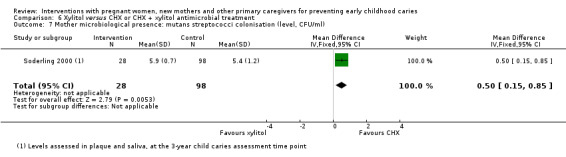
Comparison 6 Xylitol versus CHX or CHX + xylitol antimicrobial treatment, Outcome 7 Mother microbiological presence: mutans streptococci colonisation (level, CFU/ml).
Gingival health
Not reported.
Oral health behaviours
Not reported.
Adverse events for mother or child
Not reported.
Discussion
Summary of main results
In this Cochrane review, we included 17 RCTs (four cluster‐randomised), assessing a clinical, or oral health education and/or promotion intervention, with women during pregnancy and mothers or other caregivers of infants in the first year of life, and reporting at least one measure of caries in children (at up to six years). The 17 trials randomised 23,732 caregivers (most were pregnant women and new mothers, though a small number were grandmothers and fathers), and their children. Three trials assessed diet and feeding practice advice for infants and young children against standard care; two assessed breastfeeding promotion and support versus standard care; one assessed dietary advice for infants and young children against standard care; five assessed oral hygiene, diet and feeding practice advice versus standard care; four assessed antimicrobial treatment (including chlorhexidine or iodine‐NaF application and prophylaxis) in dentition of women versus placebo; and two assessed xylitol against CHX or CHX combined with xylitol antimicrobial treatment in dentition of women. The studies were performed in a mix of high‐ middle‐ and low‐income countries. In nine of the included trials, participants were socioeconomically disadvantaged.
Considering the oral health education or promotion interventions, for the primary outcome caries presence in primary teeth, we observed a 15 percent reduced risk in children of caregivers who received an infant and young child diet and feeding practice intervention compared with standard care (RR 0.85, 95% CI 0.75 to 0.97; 3 trials; 782 participants; moderate‐certainty evidence) and the mean dmfs score was possibly lower in the intervention compared with standard care group (low‐certainty evidence). However, no clear difference was observed between these groups in mean dmft (very low‐certainty evidence). We observed a possible reduced risk of caries presence in primary teeth in the breastfeeding promotion and support intervention compared with the standard care group (low‐certainty evidence); however, there was no evidence of a difference between these two groups in mean dmft score (low‐certainty evidence). We observed no evidence of a difference in risk of caries presence in primary teeth between children of caregivers who received infant/young child dietary advice only, compared with standard care (very low‐certainty evidence). No evidence of difference was seen between children of caregivers who received oral hygiene, diet and feeding practice advice compared with standard care in any caries presence in primary teeth (low‐certainty evidence), or in mean dmfs and dmft scores (very low‐certainty evidence).
Considering the two clinical intervention comparisons, for antimicrobial treatment versus placebo, we observed no evidence of a difference between groups in caries presence in primary teeth (very low‐certainty evidence), the only primary outcome measure reported. There was a lower mean dmft in children of mothers who received xylitol compared with the CHX antimicrobial intervention group (low‐certainty evidence), but no evidence of a difference between these two groups in caries presence in primary teeth (very low‐certainty evidence).
No adverse events for mother or child were reported by trials in the health education/promotion comparisons. Two studies assessing antimicrobial treatments reported adverse events.
No subgroup analyses (based on intervention start, intervention duration, child age at caries assessment, participant socioeconomic status, and trial design (unit of randomisation)) were performed due to paucity of data. Sensitivity analyses (restricted to the trials not assessed as being 'high risk' in two or more risk of bias domains) largely supported findings observed in the main analyses.
Overall completeness and applicability of evidence
The evidence for interventions with pregnant women, new mothers and other caregivers of children in the first year of life for preventing ECC is insufficient for drawing robust conclusions. Though we were able to include 17 trials involving 23,732 caregivers (mainly mothers) and their children, only 15 provided data for inclusion in the review analysis, and they assessed six interventions. All analyses included few studies (between one and three) and participant numbers were low; additionally, many of the included trials reported on few outcomes of relevance to this review. None of the included trials assessed a health service and/or policy intervention designed to modify access to oral health information or services, and as four of the interventions assessed were against placebo or standard care, our assessment of the relative effect of different intervention types was limited to one pairwise comparison.
Considering the comparison, infant and young child diet and feeding practice advice versus standard care, and the primary outcome, three trials with 782 child participants provided data for meta‐analyses on caries presence in primary teeth; two trials with 747 participants contributed data for dmfs index score; and one trial, with 340 participants, contributed data for both dmft index score and d1 + mfs ≥ 5. None of the included trials provided data for the child secondary outcomes included in this review. For mother secondary outcomes, evidence was limited to one trial providing data for few participants on plaque and gingival health.
For breastfeeding promotion and support versus standard care, two trials only were included, and evidence was limited to data on two caries outcomes: caries presence in primary teeth (1148 participants), and dmft index score (652 participants).
For the evaluation of dietary advice for infants and young child compared with standard care, data were available from only one trial, for the primary outcome, caries presence in primary teeth (148 participants), and secondary mother outcomes, plaque and gingival health (133 participants).
For oral hygiene advice combined with diet and feeding practice advice for infants and young children versus standard care, and the primary outcome, the evidence included only three trials with 591 participants reporting on caries presence in primary teeth, and one trial with 187 participants reporting the dmfs, dmft, and SiC30 indexes. Additionally, narrative caries outcomes were reported by two studies. Regarding the secondary outcomes: for the child, two studies with 208 participants reported on dental anaesthetics; no data were included in analysis for any of the other outcomes; and we were able to include outcomes as other data on child oral health behaviours from one study, and dental attendance from two studies. For the secondary outcomes relating to mothers, we were only able to include other data on change in mother self‐reported oral health behaviours (including diet) and attitudes, from one study.
Considering the two clinical intervention comparisons, for antimicrobial treatment in mothers versus placebo, evidence for the primary outcome was limited to three studies with 479 participants reporting on caries presence in primary teeth. No data were available for inclusion in analysis for the child secondary outcomes; narrative outcomes were included from two trials on microbiological presence. We were able to include data in analysis only for two mother secondary outcomes; DMFS increment (2 trials, 130 participants) and DMFT increment (one trial, 66 participants); and the same two trials reported narrative outcomes on microbiological presence in mother dentition. For the pairwise comparison of the two types of antimicrobial treatment, we were able to include data in analysis from only two studies, on four primary outcome measures: caries presence in primary teeth (96 participants), dmft index score (113 participants), defs index score (96 participants) and defs score ≥ 5 (96 participants).
Whilst we planned to explore variation in effects due to difference in intervention features and characteristics of participants through subgroup analysis (including intervention start: prenatal versus postnatal; intervention duration, ≤ 6 months versus > 6 months; child age at caries assessment; ≤ 3 years versus > 3 years; socioeconomic status, low versus mixed or any), we were unable to perform these analyses for any of the six comparisons due to the small number of studies included in analysis. Further, the included trials used a variety of definitions of outcomes including the definition/diagnosis of caries, and different assessment time points, which further complicates interpretation of the data, and may limit the applicability of the results.
Quality of the evidence
Risk of bias in the included studies was mixed. Across the included trials, there was a general lack of methodological detail provided to assess specific aspects of risk of bias, leading to many 'unclear' judgements. In most of the included trials, blinding of participants and personnel was not possible due to the nature of the intervention assessed, which is a concern for the subjective outcomes, but is less likely to have introduced bias for objective outcomes including caries. Most of the included trials were judged at high or unclear risk of attrition bias, due to moderate or high numbers of infants not being available for the caries assessments and differences in the proportions of infants 'lost to follow‐up' across the groups compared.
We were able to include seven of the 15 trials contributing data for analysis, that were judged at high risk of bias for no more than one of the risk of bias assessment items, in sensitivity analyses, which mostly supported findings from the main analyses.
For the primary outcomes, caries presence in primary teeth, dmfs and dmft scores, we assessed the certainty of the evidence using the GRADE approach. The certainty of the evidence available varied across the six comparisons evaluated in the review, as follows: infant and young child diet and feeding practice advice compared to standard care, moderate to very low‐certainty evidence; breastfeeding promotion and support versus standard care, low‐certainty evidence; dietary advice compared with standard care, very low‐certainty evidence; oral hygiene, diet and feeding practice advice versus standard care, low to very low‐certainty evidence; antimicrobial treatment in dentition of mothers versus placebo, very low‐certainty evidence; xylitol chewing gum versus CHX varnish antimicrobial treatment in dentition of mothers, low to very low‐certainty evidence. For all the comparisons, evidence was predominantly downgraded due to design limitations (risk of bias), and imprecision (uncertain effect estimates, and at times small sample sizes and low event rates).
Potential biases in the review process
The search for trials in this area was performed using Cochrane Oral Health's and Cochrane Pregnancy and Childbirth's Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) and leading electronic databases indexing relevant research. It is unlikely that trials that have been conducted have been missed; however, unpublished trials, or ongoing trials not registered in clinical trial registries could be missing. Should such trials be identified, we will include them in future updates of the review.
We aimed to reduce bias wherever possible by having at least two review authors independently working on trial selection, data extraction, risk of bias judgements, and GRADE assessments.
Agreements and disagreements with other studies or reviews
A review by Chen 2019 affirmed that ECC remains a global public health issue. However, despite the obvious potential opportunity, there have been relatively few studies undertaken exploring the effects of interventions targeted at pregnant and new mothers and/or carers to address this issue.
One Cochrane review has assessed the RCT evidence on fluoride supplementation (with tablets, drops, lozenges or chewing gum) in pregnant women compared with no fluoride supplementation during pregnancy for preventing dental caries in the primary dentition of children (Takahashi 2017). Takahashi 2017 included one RCT providing a maximum of 938 participants with data for analysis and found no evidence of a difference between the intervention and no treatment groups in caries presence, assessed at two time points, three and five years (low‐quality evidence). The authors concluded that there was no evidence that fluoride supplements taken by women during pregnancy are effective in preventing dental caries in their offspring, which is unsurprising given contemporary understanding of the primarily topical (as opposed to systemic) mode of action of fluoride in preventing dental caries.
Intending to explore the hypothesis that reducing maternal oral cariogenic microbial load will reduce the risk of their offspring developing caries, another Cochrane review assessed the RCT evidence on the effects of treating periodontal disease in pregnant women on perinatal and maternal morbidity and mortality outcomes (Iheozor‐Ejiofor 2017). This review included no caries outcomes.
Several other systematic and nonsystematic reviews have assessed evidence to determine effects of interventions with pregnant women and/or new mothers/other caregivers of infants during the first year of life on ECCs. A review of RCTs only, by Muthu and colleagues aimed to evaluate use of prenatal fluoride, chlorhexidine mouth rinses, and xylitol (labelled pharmacological interventions) in altering the mutans streptococci levels and reducing caries in children (Muthu 2015). Two trials were included in this review, neither of which provided any data on caries outcomes. Similar to our findings relating to the antimicrobial treatments in pregnant women and mothers, Muthu 2015 concluded that there was a dearth of evidence supporting the use of pharmacological interventions for expectant mothers for altering the mutans streptococci levels in their children, and further noted the lack of evidence that any such change in microbial load was actually associated with a reduction in caries.
Henry 2017 reviewed the evidence for the efficacy of oral health education programmes provided to pregnant mothers in preventing ECC and attempted to determine the most effective programme. Whilst the review, which included RCTs only, was published in 2017, the database search strategy included studies published up to 26 August 2013 only. Four RCTs were included, one of which (Weinstein 2004) we had excluded as some of the infant participants were older than one year of age at the start of intervention delivery. The other three studies are included in our review. Whilst no meta‐analysis was reported, risk ratios were calculated and interpreted. Henry 2017 concluded that there was some evidence to suggest that oral health education in pregnant women may have a positive impact in preventing ECC in their children, but noted that this recommendation was 'weak' (no GRADE assessment of evidence certainty was performed). These reviewers concluded that the most effective intervention cannot be ascertained due to variations in and the limited number of interventions assessed. This supports our conclusions that, whilst there is some evidence suggesting health education and promotion interventions with pregnant women and caregivers in the first year of life (e.g. dietary and feeding practice advice, and oral hygiene instruction) may be effective for reducing risk of ECC, the accumulated RCT evidence is limited and uncertain, and does not allow for the determination of the most effective intervention(s) in pregnant women (and new mothers and other caregivers) for preventing ECC.
Moynihan 2019 systematically reviewed evidence on the impact of modifiable risk factors for preventing ECC. Twelve questions relating to infant feeding, diet, oral hygiene, and fluoride, defined by a WHO expert panel, were addressed in this review. The review was commissioned to inform recommendations in a World Health Organisation (WHO) manual on ECC prevention. The Moynihan 2019 review included a range of evidence types (RCTs, cohort studies, case control studies, cross‐sectional studies). The best available (highest level) were synthesised for each question, where possible, using meta‐analysis. Questions relating to the use of fluoride toothpaste were excluded from the review due to proven efficacy. The population inclusion criteria were different to those in our review. For instance, Moynihan 2019 included children < 72 months and their caregivers, whereas we included studies involving pregnant women and infants up to the age of one (inclusive) at the start of intervention delivery/at baseline). The dental caries outcome measures included in this review were similar to those in our review, and GRADE was used to assess evidence quality in the Moynihan 2019 review. Only one of the questions addressed by the Moynihan 2019 review is relevant to our review: 'is oral health education for caregivers effective for preventing ECC?' For this question, six RCTs were identified and synthesised, and two meta‐analyses were performed, both including three trials only. The conclusions were ambiguous, with one meta‐analysis showing no evidence of a difference in dmft: (standardised mean difference ‐0.15, 95% ‐0.34 to 0.05; P = 0.14; moderate‐quality evidence) between the group of children whose mothers received oral health education compared to the group whose mothers received no oral health education. The second meta‐analysis showed that children of caregivers who received oral health education had a reduced risk of ECC (where the outcome of interest was 'caries present') compared with those of caregivers who had never received oral health education (OR 0.39; 95% CI 0.19 to 0.79; moderate‐quality evidence). The ambiguous findings about the effects of oral health education for caregivers on ECC of the Moynihan 2019 review, are in agreement with the findings of the assessment of health education and promotion interventions in this review, and support the conclusion that the evidence is insufficient to guide practice. Unlike our review, in which we defined the age at time of caries assessment (between birth and six years of age), Moynihan 2019 did not specify the age of caries assessment. In addition, Moynihan 2019 included two cohort and six quasi‐experimental studies that could not contribute further to informing the questions remaining around the effect of oral health education provided to caregivers of young children (age < 72 months) in preventing ECC.
Xiao 2019 systematically reviewed the evidence relating to the association between prenatal oral healthcare, ECC, and streptococcus mutans carriage in children, considering RCT and observational evidence. Three RCTs, one prospective cohort study, and one nested case‐control study, were included in this review. The types of prenatal oral healthcare tested in these five studies were: provision of fluoride supplements, oral examinations/cleanings, oral health education provided to pregnant women, referrals for dental care, and xylitol gum chewing for pregnant women. Data from four studies on caries incidence (presence) were included in a meta‐analysis. The results reported suggested a beneficial effect of prenatal oral healthcare against ECC: at one year, OR 0.12, 95% CI 0.02 to 0.77; at two years, OR 0.18, 95% CI 0.05 to 0.63; at three years, 0R 0.25, 95% CI 0.09 to 0.64; and at four years, OR 0.35, 95% CI 0.12 to 1.00. The authors of the Xiao 2019 review recommended prioritising the evaluation of interventions that restore an expectant mother's oral health to a disease‐free state in future research.
Although a small body of evidence is evolving to support the benefit of interventions targeted at pregnant women and/or new mothers/other caregivers of young infants for preventing ECC, the quantity and quality of that evidence remains limited. Uncertainty remains, particularly in relation to the types of interventions that are the most effective (and their specific features), and the groups of women and infants in whom such interventions are likely to be beneficial.
Authors' conclusions
Implications for practice.
Moderate‐certainty evidence suggests that providing pregnant women, new mothers or other caregivers with diet and feeding practice advice for infants and young children probably leads to a slightly reduced risk of caries; however, the evidence available for other types of interventions is uncertain and we are unable to draw any reliable conclusions. The current evidence is insufficient to evaluate which intervention features are effective, and most effective, for preventing early childhood caries.
Implications for research.
Additional adequately‐powered, well‐designed RCTs, are needed to assess the effects of interventions with mothers and other primary caregivers during pregnancy and/or the first year of a child's life for preventing early childhood caries. Future studies should assess not only emerging oral health education/promotion interventions and clinical interventions, but also health service and/or policy intervention(s) designed to modify access to oral health information or services for pregnant women and/or mothers/other caregivers of young children. Careful consideration should be given in future trials to collecting and reporting data on relevant participant characteristics (e.g. socioeconomic status, access to and level of fluoride in water), and to specific features associated with each intervention, to enable assessment of variation in intervention effects, and to determine what interventions work best, particularly for vulnerable populations.
This review has highlighted a paucity of data, and wider challenge in oral health research: the lack of consistency in recording and reporting caries outcomes, which makes inter‐study comparisons difficult. In part, this is the result of lack of agreement amongst researchers as to what makes a good caries outcome measure, but is also a reflection of the disproportionate cost of embedding a comprehensive rigorous dental evaluation in any community‐based complex intervention. Future research efforts could be invested in exploring consistent collection of oral health data using consistent clinically relevant outcomes measured at key time points, in an efficient and cost‐effective manner at a population level.
We have identified eight planned or ongoing studies and four are awaiting classification (pending the reporting of data on caries in primary dentition of children). We will consider these in the first review update.
What's new
| Date | Event | Description |
|---|---|---|
| 6 April 2020 | Amended | Minor edit to description of GRADE in 'Summary of findings' tables |
History
Protocol first published: Issue 4, 2016 Review first published: Issue 11, 2019
| Date | Event | Description |
|---|---|---|
| 22 November 2019 | Amended | Correcting typo and acknowledgment |
Acknowledgements
We thank Cochrane Oral Health editorial team members for their help in preparing this systematic review, particularly Helen Worthington, Jan Clarkson, Valeria Marinho, Anne Littlewood, Ruth Floate and Laura MacDonald. We also thank Nuala Livingstone, Kate Morgaine and Annetta Kit Lam Tsang for their comments and Anne Lethaby for final copy editing.
We thank Philippa Middleton and Dannielle Vanpraag for their valuable contributions in completing this review. Jane Yelland is supported by a National Health and Medical Research Council Translating Research into Practice Fellowship (2018‐2019). The Murdoch Children's Research Institute acknowledges the support of the Victorian Government’s Operational Infrastructure Support Program.
Appendices
Appendix 1. Cochrane Oral Health’s Trials Register search strategy
Cochrane Oral Health’s Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see https://oralhealth.cochrane.org/trials
1 (teeth and (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)):ti,ab 2 (tooth and (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)):ti,ab 3 (dental and (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)):ti,ab 4 (enamel and (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)):ti,ab 5 (dentin and (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)):ti,ab 6 ((dental or tooth or teeth) and plaque):ti,ab 7 "early childhood caries":ti,ab 8 #1 or #2 or #3 or #4 or #5 or #6 or #7 9 pregnan*:ti,ab 10 (expect* and mother*):ti,ab 11 (baby or babies or infant*):ti,ab 12 ((primary or deciduous or milk or natal) and (tooth or teeth or dentition)):ti,ab 13 (mother* or maternal* or maternity or mum* or mom*):ti,ab 14 #9 or #10 or #11 or #12 15 (#8 and #13 and #14) AND (INREGISTER)
Appendix 2. The Cochrane Pregnancy and Childbirth Group Trials Register search strategy
For information on how the Cochrane Pregnancy and Childbirth Group Trials Register is compiled, see https://pregnancy.cochrane.org/pregnancy‐and‐childbirth‐groups‐trials‐register
(dental OR dentin OR teeth OR tooth) AND (decay OR deminerali* OR reminerali* OR lesion* OR caviti* OR education OR treat* OR prevent*) OR "oral health" OR "oral care" OR caries OR carious OR plaque OR enamel OR dentition
Appendix 3. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 [mh "tooth demineralization"] #2 (teeth near/5 (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)):ti,ab #3 (tooth near/5 (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)):ti,ab #4 (dental near/5 (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)):ti,ab #5 (enamel near/5 (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)):ti,ab #6 (dentin near/5 (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)):ti,ab #7 [mh ^"DMF index"] #8 [mh ^"dental plaque"] #9 ((dental or tooth or teeth) near/4 plaque):ti,ab #10 "early childhood caries":ti,ab #11 {or #1‐#10} #12 [mh infant] #13 [mh pregnancy] #14 [mh "prenatal exposure delayed effects"] #15 pregnan*:ti,ab #16 (expect* near/3 mother*):ti,ab #17 (baby or babies or infant*):ti,ab #18 [mh "tooth, deciduous"] #19 ((primary or deciduous or milk or natal) near/5 (tooth or teeth or dentition)):ti,ab #20 [mh mothers] #21 [mh "maternal behavior"] #22 (mother* or maternal* or maternity or mum* or mom*):ti,ab #23 {or #12‐#19} #24 {or #20‐#22} #25 #11 and #23 and #24
Appendix 4. MEDLINE Ovid search strategy
1. exp Tooth demineralization/ 2. (teeth adj5 (cavit$ or caries or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 3. (tooth adj5 (cavit$ or caries or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 4. (dental adj5 (cavit$ or caries or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 5. (enamel adj5 (cavit$ or caries or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 6. (dentin adj5 (cavit$ or caries or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 7. DMF Index/ 8. Dental plaque/ 9. ((dental or tooth or teeth) adj4 plaque).mp. 10. "early childhood caries".mp. 11. or/1‐10 12. Pregnancy/ 13. Prenatal exposure/ 14. exp Infant/ 15. pregnan$.mp. 16. (expect$ adj3 mother$).mp. 17. (baby or babies or infant$).mp. 18. Tooth, deciduous/ 19. ((primary or deciduous or milk or natal) adj5 (tooth or teeth or dentition)).mp. 20. or/12‐19 21. Mothers/ 22. Maternal behavior/ 23. (mother$ or maternal$ or maternity or mum$ or mom$).mp. 24. or/21‐23 25. 11 and 20 and 24
This subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity‐ maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 5. Embase Ovid search strategy
1. Dental caries/ 2. (teeth adj5 (cavit$ or caries or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 3. (tooth adj5 (cavit$ or caries or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 4. (dental adj5 (cavit$ or caries or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 5. (enamel adj5 (cavit$ or caries or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 6. (dentin adj5 (cavit$ or caries or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 7. Tooth plaque/ 8. ((dental or tooth or teeth) adj4 plaque).mp. 9. "early childhood caries".mp. 10. or/1‐9 11. Pregnancy/ 12. Prenatal exposure/ 13. exp Infant/ 14. pregnan$.mp. 15. (expect$ adj3 mother$).mp. 16. (baby or babies or infant$).mp. 17. Tooth, deciduous/ 18. ((primary or deciduous or milk or natal) adj5 (tooth or teeth or dentition)).mp. 19. or/11‐18 20. Mother/ 21. Maternal behavior/ 22. (mother$ or maternal$ or maternity or mum$ or mom$).mp. 23. or/20‐22 24. 10 and 19 and 23
This subject search was linked to Cochrane Oral Health’s filter for identifying RCTs in Embase Ovid:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 6. CINAHL EBSCO search strategy
S24 S10 and S19 and S23 S23 S20 or S21 or S22 S22 (mother* or maternal* or maternity or mum* or mom*) S21 (MH maternal behavior) S20 (MH Mothers+) S19 S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S18 ((primary or deciduous or milk or natal) N5 (tooth or teeth or dentition)) S17 (MH "Tooth, Deciduous") S16 (baby or babies or infant*) S15 (expect* N3 mother*) S14 pregnan* S13 (MH infant+) S12 (MH "Prenatal Exposure Delayed Effects") S11 (MH Pregnancy+) S10 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 S9 "early childhood caries" S8 ((dental or tooth or teeth) N4 plaque) S7 (MH dental plaque) S6 (tooth N5 (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)) S5 (dentin N5 (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)) S4 (enamel N5 (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)) S3 (dental N5 (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)) S2 (teeth N5 (cavit* or caries or carious or decay* or lesion* or deminerali* or reminerali*)) S1 (MH Tooth demineralization+)
This subject search was linked to Cochrane Oral Health’s filter for CINAHL EBSCO:
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S3 TI random* or AB random* S4 AB "latin square" or TI "latin square" S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S6 MH Placebos S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S8 TI blind* or AB mask* or AB blind* or TI mask* S9 S7 and S8 S10 TI Placebo* or AB Placebo* or SU Placebo* S11 MH Clinical Trials S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 7. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and World Health Organization International Clinical Trials Registry Platform search strategy
caries and pregnancy
caries and mother
Data and analyses
Comparison 1. Diet and feeding practice advice for infants and young children versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caries presence in primary teeth | 3 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.97] |
| 2 dmfs index | 2 | 757 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.58, 0.00] |
| 3 dmft index | 1 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.85, 0.05] |
| 4 d1 + mfs ≥ 5 | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.92] |
| 5 Child oral health behaviours | Other data | No numeric data | ||
| 6 Change in mother self‐reported oral health behaviours (including diet) and attitudes | Other data | No numeric data | ||
| 7 Adverse events for mother or child | Other data | No numeric data |
Comparison 2. Breastfeeding promotion and support versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caries presence in primary teeth | 2 | 1148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.03] |
| 2 dmft index | 2 | 652 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.59, 0.36] |
Comparison 3. Dietary advice for infants and young children versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caries presence in primary teeth | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.34, 3.37] |
| 2 Child oral health behaviours | Other data | No numeric data | ||
| 3 Plaque in dentition of mothers: presence of sub‐ and supragingival calculus | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.37] |
| 4 Mother gingival health: mild or moderate bone loss | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.42, 4.85] |
| 5 Change in mother self‐reported oral health behaviours (including diet) and attitudes | Other data | No numeric data |
Comparison 4. Oral hygiene, diet and feeding practice advice versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caries presence in primary teeth | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Caries presence in any teeth | 2 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.10] |
| 1.2 Caries presence in top font four teeth only | 1 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.42, 1.10] |
| 2 dmfs index | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐2.45, 0.47] |
| 3 dmft index | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.96, 0.36] |
| 4 SiC30 index | 1 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.73, ‐0.13] |
| 5 Child oral health behaviours | Other data | No numeric data | ||
| 6 Child dental attendance | Other data | No numeric data | ||
| 7 Change in mother self‐reported oral health behaviours (including diet) and attitudes | Other data | No numeric data | ||
| 8 Adverse events for mother or child | Other data | No numeric data |
Comparison 5. Antimicrobial treatment (CHX or iodine‐NaF and prophylaxis) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caries presence in primary teeth | 3 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.80, 1.19] |
| 2 Mother DMFS increment | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐2.22, 1.79] |
| 3 Mother DMFT increment | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.86, 1.26] |
| 4 Adverse events for mother or child | Other data | No numeric data |
Comparison 6. Xylitol versus CHX or CHX + xylitol antimicrobial treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caries presence in primary teeth | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.39] |
| 2 dmft index | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐2.39 [‐4.10, ‐0.68] |
| 3 defs (score) | 1 | 96 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.83, 0.27] |
| 4 defs (score categories) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 1‐3 defs | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.15, 1.54] |
| 4.2 3‐4 defs | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.18, 3.98] |
| 4.3 ≥ 5 defs | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.78] |
| 5 Child microbiological presence: mutans streptococci colonisation (any) | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.45, 0.81] |
| 6 Child microbiological presence: mutans streptococci (score categories) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 0 | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.88, 1.41] |
| 6.2 1 | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.21, 2.01] |
| 6.3 2 | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 2.05] |
| 6.4 3 | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.33, 5.18] |
| 7 Mother microbiological presence: mutans streptococci colonisation (level, CFU/ml) | 1 | 126 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.15, 0.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Birungi 2015.
| Methods | Cluster‐RCT (randomisation by community unit): NCT00397150 (PROMISE‐EBF trial, Ugandan site), with follow‐up study of infants at 5 years | |
| Participants | 765 pregnant women and their fetuses from 24 community clusters were randomised. Inclusion criteria: women residing in a selected cluster, ≥ 6 months pregnant, with no plans to move outside of the cluster within 1 year Exclusion criteria: reduced ability to collaborate for psychological/mental reasons, severely ill, given birth more than 7 days ago, multiple birth, newborn with severe malformation Setting: Mbale district, Eastern Uganda (data collection, including for the follow‐up study, from 2006 to 2011) |
|
| Interventions |
Group 1 (n = 396 pregnant women from 12 clusters randomised) Women received individual tailored home‐based peer counselling focused on promoting exclusive breastfeeding. The intervention was delivered by workers from the community who were trained in the intervention protocol. The counselling included one prenatal visit followed by four postpartum visits. Group 2 (n = 369 pregnant from 12 clusters randomised) Women received the standard care delivered by public health services. Timing: commenced towards the end of pregnancy, and continued through weeks 1 to 10 after birth (≤ 6 months intervention duration) Theory or model used as a basis for intervention: not reported |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: caries in primary teeth, dmft index; secondary outcomes: none Narrative text for: none Tabulated data for: none Additional outcomes that had not been prespecified: Child: none reported. Mother: breastfeeding |
|
| Notes |
Funding: Quote: "This work was supported by European Union Sixth Framework International Cooperation–Developing Countries, Research Council of Norway, Swedish International Development Cooperation Agency, Norwegian Programme for Development, Research and Education, South African National Research Foundation, and Rockefeller Brothers Foundation". Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A total of 24 clusters were stratified into urban and rural and allocated at random (computer generated with an allocation ration 1:1)". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely participants and personnel were blinded considering the nature of the intervention assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The interviewers and dentists were aware of the children’s involvement in the PROMISE EBF trial but were blinded with respect to their group allocation”. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall, 417/765 (55%) children of the mothers randomised were available for caries and other outcome assessment at 5 years, of which 215/396 (54.3%) were children of mothers randomised to the intervention group, and 202/369 (54.7%) were children of mothers randomised to the control group. Therefore, very high loss to follow‐up. Though the losses were relatively evenly distributed across the two groups, the authors reported differences in the characteristics of the children in the two groups at the 5‐year assessment (see other bias below). |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes reported, but caries in infants/children not included as specified outcome in the study protocol. |
| Other bias | High risk | The authors reported that the intervention and control group child cohorts from the PROMISE‐EBF study, used for the included follow‐up study, "differed substantially with respect to the prevalence of EBF at 24 weeks of infant's age (59% versus 12%). Additionally, they reported significant differences between the groups at the 5‐year follow‐up data collection point (when caries were assessed), in socioeconomic status". |
Chaffee 2013.
| Methods | Cluster‐RCT (randomisation by health service unit): NCT00635453 (Porto Alegra Early Life Nutrition and Health Study), with 3‐year follow‐up of infants | |
| Participants | 715 mothers and pregnant women and their fetuses/infants from 20 health service clusters were randomised. Inclusion criteria: all pregnant women with scheduled clinic visits from April to December 2008 (and their foetuses/infants) in the selected study services (births occurred from May 2008 to February 2009) Exclusion criteria: for health centres, ≤ 100 infant patient visits in 2006; staff‐sharing among clinics or participation in a contemporaneous community‐based dietary programme; for participants: HIV+ mothers; and infants with congenital malformations Setting: Health units in Porto Alegra, Rio Grande do Sul, Brazil (women were enrolled from April to December 2008, child caries assessments occurred from August 2011 to June 2012). |
|
| Interventions |
Group 1 (n = 360 pregnant women from 9 clusters randomised) Women received dietary advice from healthcare workers who were trained in infant feeding guidelines, namely the "Ten steps of Healthy Diet for Brazillian Children under Two Years of Age", plus written material relating to the dietary advice. The recommendations in these guidelines included: (1) exclusive breastfeeding to 6 months of age; (2) continued breastfeeding to 2 yrs of age, with gradual introduction of complementary foods; (3) at 6 months, start complementary feeding (grains, meat, fruits) 3 times daily while continuing breastfeeding; (4) mealtimes at regular intervals, adjusted to the child’s internal hunger cues; (5) new foods should gradually get thicker until the child is able to eat a family meal, but foods should never be liquefied; (6) provision of a variety of healthy foods every day; (7) daily intake of different fruits and vegetables; (8) avoidance of sugar, sweets, soft drinks, salty snacks, and processed and fried foods; (9) implementation of good hygiene practices in food preparation and handling; and (10) adequate, responsive feeding during illness. The guidelines contained no specific oral heath messages. Group 2 (n = 355 pregnant women from 11 clusters randomised) Women received standard care. Timing: counselling was provided when mothers attended clinics for pre and postnatal visits; no further details on timing of intervention were provided (> 6 months intervention duration). Theory or model used as a basis for intervention: clinical guidelines for early infant feeding, more specifically the “Ten steps for healthy feeding of children younger than two years” |
|
| Outcomes |
Data in meta‐analysis for: primary: caries presence in primary teeth, dmfs index; secondary outcomes: none Narrative text for: none Tabulated data for: none Additional outcomes that had not been prespecified: Child: none reported. Mother: none reported |
|
| Notes |
Funding: "The Brazilian Ministry of Health, the Rio Grande do Sul Research Support Foundation (FAPERGS), and NIH‐NIDCR grant F30DE022208 (to BWC) supported this research". Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Of the 31 eligible health centres, 16 were initially selected via a witnessed drawing, by the principal investigator, of labelled markers from an opaque container, such that 2 health centres would be included from each of the city’s 8 geo‐administrative districts. Following a stratified randomisation scheme, health centres were block‐randomised by district, with one health centre per district allocated to the intervention and another to the control. To increase statistical power, 4 additional health centres from the original 31, regardless of district, were randomly drawn. Health centre size differed, and thus, to maintain a balanced number of births by group, these additional 4 health centres were block‐randomised at a 1:3 ratio. This yielded 9 intervention and 11 control group health centres". |
| Allocation concealment (selection bias) | Low risk | Opaque container used to ensure allocation concealment during randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the health centers were invited to participate without disclosure of allocation status". It is likely that participants and study personnel were aware of their group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Dental outcomes were available for 64.1% (458/715) of the initial sample. Losses were principally due to withdrawal from the study or inability to locate and did not differ significantly by allocation status...Children available for analysis differed statistically significantly from those lacking dental information for 3 variables: mean maternal age (26.4 yrs intervention vs 25.2 yrs control), proportion having fathers with ≤ 8 yrs of education (49.9% vs 43.3%), and proportion low social class (78.3% vs 82.4%)". |
| Selective reporting (reporting bias) | Unclear risk | Caries in infants/children was not prespecified as an outcome in the study protocol. Quote: "mother‐child pairs were enrolled at baseline, prior to the decision that dental outcomes would be assessed". |
| Other bias | Low risk | No signs of other bias |
Dasanayake 1993.
| Methods | RCT | |
| Participants | 62 pregnant women and their foetuses were randomised. Inclusion criteria: women in their third trimester of pregnancy attending the Jefferson County Health Department in Birmingham residing in a fluoridated community with their spouse or significant other as the only other adult with at most one other child at home who: did not plan to breast feed their infant; did not receive any form of anti‐bacterial therapy 3 months prior to enrolment; and who had 2.5 x 104 or more colony forming units (CFU) of MS per mL of unstimulated saliva on at least two of three screening samples obtained during consecutive visits Exclusion criteria: not reported Setting: maternal and infant care program (MIC) of the Jefferson County Health Department in Birmingham, Alabama, USA (study dates not reported) Important health characteristics reported: all included women had 2.5 x 104 or more colony forming units (CFU) of MS per mL of unstimulated saliva on at least two of three screening samples obtained during consecutive visits. |
|
| Interventions |
Group 1 (n randomised not reported, 23 children were included in the caries assessment at 3 years) Women received L‐NaF antimicrobial solutions to their dentition. The sealant applications (Delton clear shade; Johnson and Johnson, East Windsor, NJ) were applied to all nonrestored occlusal fissures, by a clinician. Around the time of the emergence of the infant's first tooth, a dental hygienist, who was masked to the treatment/control status of the participants, performed a dental prophylaxis consisting of a supragingival scaling, polishing with a rubber cup, water, and pumice, and flossing on the mother. Next, the hygienist applied either the treatment or placebo. The active treatment solution consisted of I2‐NaF solution [1.0 g I2 (USP grade), 1.0 g Kl (USP grade), 53.0 mL glycerin (USP grade), 1.2 g NaF (USP grade) and water to make 100 mL]. The solution was then adjusted to pH 4.5 with 85% H3PO4. consisting of a red disclosing solution [47.0 mL erythrocin dye (Butler Dental Co.), 53.0 mL glycerin, and 47.0 mL water adjusted to pH 4.5 with 85% H3PO4]. All agents were prepared within 2 weeks of application by a pharmacist and stored under refrigeration in coded amber glass syringes to maintain the blindness. Two mL of the agent were administered to the mothers for 5 min every other day over a period of 2 weeks, according to the method described previously. The iodine content of each batch of active agent was confirmed by titration with sodium thiosulfate (30). After application of the last treatment, all mothers were examined by an oral pathologist to evaluate any potential harmful effects of treatment/placebo applications. Number of participants randomised not reported, 23 mother‐child pairs included data outcome for study outcomes. Group 2 (n randomised not reported, 25 children were included in the caries assessment at 3 years) Women received a placebo treatment agent consisting of a red disclosing solution (47.0 mL erythrocin dye (Butler Dental Co.), 53.0 mL glycerin, and 47.0 mL water adjusted to pH 4.5 with 85% H3PO4). Intervention timing: intervention started 6 months after birth (around the time of the emergence of first teeth), and lasted for 2 weeks, with application of the agent for 5 minutes every other day (≤ 6 months intervention duration). Theory or model used as a basis for intervention: not reported All participants: mothers received complete restorative treatment prior to receiving the intervention or placebo treatment. |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: caries presence in primary teeth; secondary outcome: adverse events for mother or child Narrative text for: child microbiological presence; mother microbiological presence Tabulated data for: adverse effects for mother or child Additional outcomes that had not been prespecified: Child: none reported. Mother: none reported |
|
| Notes |
Funding: Quote: "This study was supported by contract //NolDE‐42552 from the National Institute of Dental Research". Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “mothers were randomly assigned to either the treatment or control group”. |
| Allocation concealment (selection bias) | Low risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | For blinding of participants: ensured by use of placebo comparator; for personnel blinding, quote: “Sealants were applied by a clinician not involved in any other aspect of the study in an attempt to maintain the masking...All agents were prepared within 2 weeks of application by a pharmacist and stored under refrigeration in coded amber glass syringes to maintain the blindness." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors reported that 48/62 of the women and infant pairs randomised completed the study, of which 23 and 25 pairs, respectively, were randomised to the treatment and control groups. The numbers randomised to each group initially was not reported (therefore not possible to compare attrition rates across groups and confidently assess whether this domain was at high or low risk of attrition bias). |
| Selective reporting (reporting bias) | Unclear risk | Without access to the study protocol, we were unable to confidently assess reporting bias. |
| Other bias | Low risk | Authors reported that the control and treatment groups were homogenous with respect to age, race, baseline caries experience, and oral bacterial levels; data to demonstrate similarity between the two groups provided. No clear signs of other bias |
Dasanayake 2002.
| Methods | RCT | |
| Participants | 75 pregnant women and their foetuses were randomised. Inclusion criteria: women between 16 and 45 years of age attending a County Deparment of Health Maternal and Child Health Clinic during their second trimester of pregnancy, who planned to live in the study area for the next 4 years, and who had at least 10 teeth with no open cavities. Exclusion criteria: known to be HIV positive, no commitment to use the County Health Department Pediatric Clinic during the course of the study, plans to move out of the study area in the next 4 years, planned to breastfeed, or became pregnant during the trial Setting: a County Department of Health Maternal and Child Health Clinic (country and study dates not reported) Important health characteristics reported: women in the intervention group had a mean 51 (SD 1.0) log10S. mutans count; women in the control group had a mean 4.6 (SD 1.8) log10S. mutans count; none of the included women were known to be HIV+. |
|
| Interventions |
Group 1 (n = 38 women randomised) Mothers received a 10% chlorhexidine varnish treatment, applied by trained dental hygienists. The varnish was applied to each subject 6 months after delivery, every four weeks. The first of these 4 applications coincided approximately with the eruption of the first tooth. Subsequent to the first set of 4 applications, a single application was given every 6 months. Group 2 (n = 37 women randomised) Mothers received a placebo varnish containing 1% hydroxypropyl cellulose, 0.2% quinine hydrocholoride and food colouring, at the same time intervals as the treatment group. Since there was a concern that polyurethane alone can reduce the S. mutans levels, normal saline was used as stage 2 for the control group. Intervention timing: varnish applied at 12, 18, 24, 30 and 36 months postpartum (intervention duration > 6 months) Theory or model used as a basis for intervention: not reported All participants: mothers received emergency restorative care and prophylaxis prior to receiving the start of the trial. Participants who developed new caries lesions during the study were referred to the County Department of Health for free restorative treatment. |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: none; secondary outcomes: mother DMFS index, mother DMFT index, adverse events for mother or child Narrative text for: child microbiological presence; mother microbiological presence Tabulated data for: adverse events for mother or child Additional outcomes that had not been prespecified: Child: none reported. Mother: none reported |
|
| Notes |
Funding: Quote: “Oralife Inc. in Toronto Canada provided the therapeutic agents and partial funding for the study”. Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants was achieved by use of the placebo varnish comparator, and addition of quinine and food colouring to the placebo vanish which made it similar to the chlorhexidine varnish in taste and appearance, ensured blinding of participants. Considering study personnel, not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A calibrated and fully blinded examiner performed dental examinations”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/38 (13%) and 4/37 (11%) of the women randomised to the control group and their infants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as described initially |
| Other bias | Low risk | Quote: “Subsequent to randomization, the two groups of mothers were comparable in terms of age, race, baseline differences in infants’ gender, method of delivery, birth weight or the length of gestation (table 1)". |
Feldens 2007.
| Methods | RCT: NCT00629629. | |
| Participants | 500 women (new mothers) and their infants were randomised. Inclusion criteria: women who had given birth to an apparently normal, single, full term (≥ 37 weeks) baby with birthweight equal to or greater than 2500 g and without an impediment to breastfeeding (e.g. HIV/AIDS) Exclusion criteria: HIV‐positive mothers; infants with congenital malformation(s), infants referred to intensive care unit, multiple pregnancy Setting: Sao Leopoldo Brazil (mother‐child pairs recruited from the town's only publicly funded hospital, which mainly serves low‐income population, from October 2001 to June 2002) |
|
| Interventions |
Group 1 (n = 200 women randomised) Mothers received a home visit dietary intervention known as the 'Ten Steps to Healthy Feeding of Younger Children' intervention. The dietary advice was given by 12 trained field workers who counselled the mothers about breast feeding and healthy weaning, based on the WHO recommendations known as the ‘Ten Steps for Healthy Feeding Children Younger than 2 Years’. The advice was provided in an informal manner and considered the mother’s opinions and concerns about child rearing and child diet, as well as the cultural and economic aspects of feeding practices in Southern Brazil. Particularly, the dietary advice aimed at exclusive breastfeeding up to 6 months; after 6 months, breastfeeding on demand was discouraged and the importance of a reasonable meal interval (about 3 h) for the child to be hungry was emphasised. The mothers of breastfed babies who were older than 6 months were encouraged to continue breastfeeding but it was also recommended that they should gradually substitute three breastfeeding meals by a 3‐times‐a‐day solid diet including a variety of fruits, cooked vegetables, meat and cereals, as to meet the family meals at the age of 1 year. The mothers of the bottle‐fed babies who were older than 6 months were encouraged to gradually substitute all bottles by a 5‐a‐day solid diet rich in nutrients maintaining reasonable intervals between meals. All mothers were advised not to use bottle or breastfeeding as pacifiers and they were encouraged to gradually restrict either bottle or breastfeeding during the night. The mothers were also advised against the addition of sugars (sugar cane, honey) in fruits, porridge, juices, milk or other liquids and against the provision of soft drinks, sweets and savoury snacks; they were encouraged to avoid fried food and to use salt in moderation. Advice on hygiene practices in food preparation and handling was also provided. A leaflet was used to guide the advice and was handed to the mother as a reminder. The mothers also received verbal and written information about preparation of complementary food and recipes of healthy food for the child’s age, traditionally used by families in this region. No specific advice about oral hygiene was provided. Group 2 (n = 300 women randomised) The control group received routine assistance/standard care delivered by their paediatricians in the health service. Intervention timing: monthly advice from birth up to six months, thereafter advice at 8, 10 and 12 months postpartum (> 6 months intervention duration) Theory or model used as a basis for intervention: World Health Organization recommendations for feeding young children |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: caries presence in primary teeth, dmfs index, dmft index, d1 + mfs > 5; secondary outcomes: none Narrative text for: none Tabulated data for: child oral health behaviours; adverse events for mother or child Additional outcomes that had not been prespecified: Child: none reported. Mother: breastfeeding |
|
| Notes |
Funding: "This project was supported by the Brazilian National Counsel for Scientific and Technological Development (CNPq). Manuscript writing was also supported by the National Institute of Science and Technology for Health Technology Assessment (IATS)". Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...assignments of two fifths of the mothers to the intervention group (n ¼ 200) and the others to the control group (n ¼ 300). Blocked randomisation was used to avoid imbalance at any point of the randomisation process. The mothers who had agreed to participate were sequentially included in a list based on time of delivery and then grouped in blocks of five. Two mothers from each block were randomly assigned to the intervention group, with the process being repeated for consecutive blocks. A larger control group was chosen to increase the study power with a reasonably small increase in the costs of the study". |
| Allocation concealment (selection bias) | Low risk | Quote: "A researcher not directly involved in the selection process (MRV) conducted the randomisation". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely considering the type of intervention evaluated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors reported that the dentists who assessed infant/child teeth for caries were blind to group allocation, at the 1‐ and 4‐year time points. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Moderately high attrition, with the rate slightly higher in the control than intervention group. Quote: "Losses comprised 122 children (intervention: n = 42⁄ 200, 21%; controls: n = 80⁄ 300, 27%) at the first year dental examination and 38 additional children (intervention: n = 17, 8.5%; controls: n = 21, 7.0%) at age 4 years. The main reason for losses up to the 1‐year assessment, as shown in the Figure, was inability to locate the child’s home, usually because of the family having moved to another city. Losses between the assessment at 1 and 4 years of age were again mainly caused by family relocation (intervention n = 11; controls n = 13); other causes being inability to locate the address (intervention n = 4; controls n = 6) and refusal (intervention n = 2; controls n = 2)". |
| Selective reporting (reporting bias) | Low risk | Dental health in children a prespecified outcome in the study protocol. Additionally, most outcomes specified in the protocol reported |
| Other bias | Low risk | Data presented showed no evidence of any significant imbalance between groups on key characteristics, even with the relatively high attrition rate, and uneven attrition across the groups. |
Hallas 2015.
| Methods | RCT | |
| Participants | 94 women (new mothers) and their infants were randomised. Inclusion criteria: all mothers who delivered a healthy full‐term infant at Bellevue Hospital were eligible to participate in the study while they were on the postpartum unit and to participate in the 6‐month and 12‐month follow‐up program for evaluation of the infant’s oral health status. Exclusion criteria: not reported Setting: Bellevue Hospital, a major urban academic teaching hospital in Manhattan, New York, USA (study conducted from 4 January 2010 to 4 January 2011) Important health characteristics reported: Cree children in the included communities known to be at a higher risk of dental disease, including early childhood caries (ECC), than nonIndigenous children in Canada |
|
| Interventions |
Group 1 (n = 47 women randomised) Mothers viewed an 8‐minute newborn oral health educational digital versatile disc (DVD) at the bedside, designed by the principal investigator and co‐investigators based on the best available evidence for oral healthcare for infants and young children to prevent formation of white spots, demineralisation, and dental caries in the first few years of life. The content of the video included: goals for growing up cavity free; definition of early childhood caries; pattern of tooth eruption; how to keep baby teeth healthy; newborn and infant oral care by parents; when to start brushing baby teeth; when to use toothpaste with fluoride; sleep time habits; teething: dispelling myths; teething symptoms; bacterial transmission from mother to baby; importance of mother caring for her own teeth; infant diet: avoiding sugary foods; diet and health; bad eating habits; establishing the dental home; the first dental home; fluoride varnish Group 2 (n = 37 women randomised) Mothers randomised to the control group viewed a standardised 8‐minute DVD on nutrition for newborns and infants. Intervention timing: delivered in the immediate postpartum period, during mothers' postnatal hospital stay (intervention duration < 6 months) Theory or model used as a basis for intervention: authors stated that the intervention was informed by evidence on best practice for infant oral health. All participants: mothers in both groups received routine newborn education by nurses, the lactation consultant, physicians, and residents. This included information on feeding and bathing the infant and identification of signs of illness but did not include any oral health education or instruction. |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: none; secondary outcomes: none Narrative text for: any caries presence in primary teeth Tabulated data for: none Additional outcomes that had not been prespecified: Child: none reported. Mother: none reported |
|
| Notes |
Funding: grant from the American Dental Association and the Samuel D. Harris Fund for Children’s Dental Health Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A computer was used to randomly assign 47 mothers to the treatment group and 47 mothers to the control group". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not reported and unlikely that participants or personnel were blind to group assignment considering the type of intervention assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors stated that "assessments were conducted at 2 clinics by non‐study staff"; no other details provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Due to significant no show, child caries were not reported by group, nor was the second study outcome for inclusion in this review, mothers self‐reported oral health behaviour reported by group. Quote: "Despite numerous efforts to contact each mother who enrolled in the study to remind her to return with her infant for the 6‐month and 12‐month oral health assessment follow‐up visits at either the Bellevue or NYU paediatric dental clinic, only 10 mothers returned for both the 6‐month and 12‐month infant follow‐up visits. Therefore, data analysis for statistical significance for follow‐up visits could not be conducted as planned”. |
| Selective reporting (reporting bias) | Unclear risk | Without access to the study protocol, it was not possible to confidently assess this trial as being at high risk or low risk of selective reporting. |
| Other bias | Unclear risk | Limited data comparing key characteristics of participants provided and therefore not clear how similar the groups were at baseline and caries assessment |
Harrison 2012.
| Methods | Cluster‐randomised controlled trial (randomisation by community unit): ISRCTN41467632; NCT00175318 | |
| Participants | 272 women (pregnant women or new mothers) and their infants from 9 communities were randomised. Inclusion criteria: Cree woman residing in a community selected for the study, recently having given birth or between the 12th and 34th weeks of pregnancy, healthy infants or with a medical condition (e.g. congenital abnormality) included, family consented to participation Exclusion criteria: woman who had knowledge of an impending permanent move out of her current community. Setting: Cree communities located to the east and southeast of James Bay, in Quebec, Canada (recruitment January 2005 to October 2007) Important health characteristics: Cree children in the communities from which the participants were drawn known to be at a higher risk of dental disease, including caries, than nonIndigenous children in Canada |
|
| Interventions |
Group 1 (n = 131 women from 5 communities randomised) Women in the intervention communities received a one‐on‐one counselling intervention during pregnancy and up to 6 more sessions before the child's second birthday. The oral health education was delivered by Aboriginal women living in the study communities who were trained in the motivational interviewing (MI) technique. Mothers received resources at each MI visit to enable them to implement selected behaviours including infant toothbrushes, toothpaste and sippy cups. Group 2 (n = 141 women from 4 communities randomised) Women randomised to the control group received standard health education and promotion provided by local health clinics. More specifically, women received a culturally‐appropriate educational pamphlet describing healthy dental care practices for young children. Pamphlets were mailed to mothers when their child was 6 months of age and again at 18 months of age. The pamphlet titled "Protect Baby Teeth: Circle of Smiles" had been previously produced in 2000 by the Nursing Caries Committee of the St. Theresa Point First Nation of Manitoba, Canada and is available from them on request. Intervention timing: started during pregnancy (1 counselling session), with six additional sessions delivered after birth, up to the child's second birthday, at the time of routine infant wellness clinic visits (> 6 months intervention duration) Theory or model used as a basis for intervention; the MI‐style scripts were based on scripts from a previous trial (Weinstein 2004), with one type of script used for mothers whose child had experienced the first tooth eruption, and another for new mothers. All participants: at one year of age, all infants received fluoride varnish, provided at local clinics. |
|
| Outcomes |
Data in meta‐analysis: primary outcome: caries presence in primary teeth; secondary outcomes: none Additional narrative text for: none Tabulated data for: dental attendance, adverse events for mother or child Additional outcomes that had not been prespecified: Child: child receipt of anaesthetic for dental treatment, parent report of ‘dental‐caries related’ child quality of life. Mother: none reported |
|
| Notes |
Funding: "This research was supported by the Canadian Institute of Health Research (grant #FRN 67817)." Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was done over community radio with two “rounds” of a constrained randomisation process. Two baskets contained envelopes marked “test” or “control”: one basket for large communities (2 envelopes: 1 test, 1 control) and another for smaller communities (7 envelopes: 4 test, 3 control). Communities were randomised in each round by alphabetical ordering of the communities’ names. For example, for each round, the first name on the alphabetical list of communities was announced, followed by the drawing of an envelope from the basket; the next name was announced, followed by another draw until all envelopes were allocated. Of the 9 communities, 5 were allocated to test and 4 to control conditions". |
| Allocation concealment (selection bias) | Low risk | Envelopes and community radio |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Mothers and interveners were aware of their community’s allocation”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "when each child was at least 30 month old, clinical data were collected by calibrated examiners, masked to the community's assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "assessment rates in the villages ranged from 73‐100%"; caries outcome data was provided for 110/131 (84%) and 131/141 (92%) infants of mothers randomised to the intervention and control groups respectively. |
| Selective reporting (reporting bias) | Unclear risk | Child caries specified as a study outcome (secondary) in the protocol. The authors stated in the protocol that data on mothers dental health knowledge, oral home care practices, child‐feeding and comforting practices will be collected at 30 months and compared between test and control communities, however no results have been presented for these secondary outcomes of the study. |
| Other bias | Unclear risk | Baseline characteristics were comparable but not equivalent for both groups: Quote: "We compared demographic and behavioral characteristics at baseline to assess the success of randomization. The distributions of most variables were very similar for the two randomised groups of mothers specifically, for age, dental knowledge score, other children, toothbrushing, and recent dental visit (Harrison 2010). However, despite the random assignment of communities, fewer test mothers had already delivered at time of enrolment (19.2% vs 40.0%), had visited a dentist for toothache (35.5% vs 50.0%), and had other children with a previous tooth extraction (34.1% vs 48.9%). Therefore, secondary analyses of outcomes were done with regression adjustment to control for baseline differences. We compared the results of the adjusted and unadjusted analyses to determine the impact of these differences". |
Kramer 2001.
| Methods | Cluster‐randomised controlled trial (randomisation by maternal hospital/clinic): ISRCTN37687716, NCT01561612 (PROmotion of Breastfeeding Trial) with 6‐year follow‐up of children | |
| Participants | 17,046 women (new mothers) and their infants from 31 hospitals/clinics were randomised, 13,889 were involved in the follow‐up study. Inclusion criteria: women who expressed an intention to breastfeed on admission to the postpartum ward and who had given birth to a healthy, singleton infant of 37 weeks or more gestation with a birth weight of ≥ 2500 g and Apgar score 5 or higher at 5 minutes Exclusion criteria: illness that would contraindicate breastfeeding or severely compromise its success Setting: maternity hospitals and polyclinics in Belarus (participants recruited for the RCT June 1996 ‐ December 1997, and for the follow‐up study included in this review December 2002‐April 2005) |
|
| Interventions |
Group 1 (n = 8865 women from 16 clusters randomised) Women received a breastfeeding promotion intervention based on the WHO/UNICEF Baby‐Friendly Hospital Initiative, which emphasised healthcare worker assistance with initiating and maintaining breastfeeding, and also provided lactation and postnatal breastfeeding support. Group 2 (n = 8181 women from 15 clusters were randomised). Standard care (i.e. usual infant feeding practices and policies) Intervention timing: started during labour, continued through the immediate postpartum period (hospital stay and postnatal visits to poly clinics) (intervention duration > 6 months) Theory or model used as basis for intervention: BFHI – Baby friendly hospital initiative (WHO and UNICEF) |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: caries presence in primary teeth, dmft index; secondary outcomes: none Additional narrative text for: none Tabulated data for: none Additional outcomes that had not been prespecified: Child: gastrointestinal tract infection, respiratory tract infection (including upper respiratory tract infections, otitis media, croup, wheezing, or pneumonia), atopic eczema, anthropometric and blood pressure. Mother: breastfeeding |
|
| Notes |
Funding: Canadian Institutes of Health Research Declarations of interest: quote: "Dr. Kramer is a Senior Investigator of CIHR. Dr. Platt is a Monat‐McPherson Career Investigator of McGill University and a career investigator (chercheur‐boursier) of the Fonds de la recherche en santé du Québec". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Coin flip during community meetings |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | How participants and personnel blinded not reported; unlikely considering the type of intervention assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Dentists performing these evaluations ..were unaware of the fact that the children examined had participated in PROBIT and, in particular, of the experimental vs control treatment allocation of each polyclinic". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 13,889 PROBIT children were seen in follow‐up, representing 81.5% of the 17,046 originally randomised. Of the 3,157 (17,046 – 13,889) children randomised but not followed up, 88 had died, 2,938 were lost to follow‐up, and 131 were unable/unwilling to come for their follow‐up visit. Follow‐up rates were similar in the experimental (80.2%) and control (82.9%) polyclinics but varied considerably by polyclinic: from 56.1% at one of the Minsk polyclinics to 94.6% at Klimovichi, a small rural‐based polyclinic." 13, 883 children are included in the reporting of caries outcomes, therefore, there were six missing children in the caries outcomes report, with no explanation of why or from which groups. Whilst the reason for the missing children was unclear, it was too small to constitute a risk of bias concern. |
| Selective reporting (reporting bias) | Low risk | Dental health of children was prespecified in the study protocol as a secondary outcome, and a comprehensive range of prespecified outcomes reported. |
| Other bias | Unclear risk | Quote: "Dentists performing these evaluations had no specific training to standardize their examinations...One potential limitation of our study is that the caries data are based on routine examinations by a large number of un calibrated public health dentists. Such unstandardized examinations could lead to non differential (by treatment) misclassification of caries and thus bias any true treatment effects towards the null, although Hausen 2001 have reported similar caries diagnoses recorded by trained, calibrated dentists and public dental clinics in Finland". |
Lapinleimu 1995.
| Methods | Randomised controlled trial (STRIP baby project), with dental substudy following children to 3 years | |
| Participants | 1054 families (including mother and/or father primary caregivers) with 1062 infants aged 7 months were randomised. Inclusion criteria: healthy infants between the ages of 7 and 13 months (every fifth child of the main study was invited to participate in the dental study) Exclusion criteria: not reported Setting: well‐baby clinics in the city of Turku, Finland (patients recruited for main study March 1990 to May 1992) |
|
| Interventions |
Group 1 (n = 537 families, n= 540 infants randomised) Parents assigned to this group received dietary advice aimed at achieving a healthy diet for their infants, low in saturated fat and cholesterol. Every 1 ‐ 3 months, families in this group received dietary advice focused on how to ensure an adequate energy supply. The best diet for the child was defined as one that contained energy according to the child's hunger, with 30‐35% of energy derived from fat, proportions of polyunsaturated / monounsaturated / saturated fatty acids (P/M/S ratio) of 1/1/1, daily cholesterol intake of less than 200 mg, 15% of energy from proteins, and 55% from carbohydrates. Based on the dietary histories of the children and their parents, individually tailored instructions were given to adjust fat intake of the children to be 30 ‐ 35% of energy intake after the age of 7 ‐ 8 months and to reduce intake of SAFA and cholesterol. The intervention group mothers were encouraged to continue breastfeeding as long as they found it feasible. The dietitians advice sessions lasted 20‐25 minutes and occurred at every visit (7, 8, 10, and 13 months). Group 2 (n = 517 families, n = 522 infants randomised) Standard care/diet. More specifically, the parent(s) assigned to the control group received written information of a well‐balanced and healthy diet for infants, available also at well‐baby clinics in Finland. The control group families were also advised to continue breastfeeding or formula feeding until the child was 1 year old; thereafter, cows' milk with at least 1.9% fat was suggested. No individualised dietary counselling was given and diet‐related topics were discussed only briefly. Control group families were met twice, when the child was 7 months and 13 months. Intervention timing: intervention started when infants were 7 months; completed when infants were 13 months (intervention duration > 6 months). Theory or model used as a basis for intervention: not reported |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: caries presence in primary teeth; secondary outcomes: plaque in dentition of mothers: presence of sub and supragingival calculus; mother gingival health: mild or moderate bone loss Additional narrative text for: none Tabulated data for: child oral health behaviours; mother self‐reported oral health behaviours (including diet) and attitudes Additional outcomes that had not been prespecified: Child: anthropometric; cholesterol, high‐density‐lipoprotein (HDL)‐cholesterol apolipoproteins A1 and B, energy (KJ) carbohydrates, fats, polyunsaturated fats, monounsaturated fats, saturated fats. Mother: breastfeeding |
|
| Notes |
Funding: This study was supported by grants from the Mannerheim League for Child Welfare, the Finnish Cardiac Research Foundation, the Medical Council of the Academy of Finland, the Yrjo Jahnsson Foundation, the Foundation for Paediatric Research, Finland, Piltti Foundation, the Juho Vainio Foundation, the Turku University Foundation, and Van den Bergh Foods Company and the substudy was "financially supported by the Yrjo Jahnsson Foundation". Declarations of interest: not reported Three twin pairs were allocated to the intervention group, and five twin pairs to the control group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The study population consisted of 1054 families with 1062 infants, who were allocated to intervention (n = 540) and control (n = 522) groups by random numbers at the 7‐month visit". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; not likely considering nature of intervention (education relating to diet) assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors reported that one‐fifth of the parents who participated in the initial study were invited to participate in the follow‐up study. Only 78/540 infants randomised to the intervention group, and 70/522 randomised to the control group were included in the follow‐up study, of which 72/78 (92%) and 65/70 (93%) in the two groups respectively completed the 3‐year dental substudy. |
| Selective reporting (reporting bias) | Unclear risk | The dental study (caries assessment) was added as a substudy; therefore, caries in infants/children was not a prespecified outcome in the initial protocol for the RCT. |
| Other bias | Low risk | No sign of other bias |
Muhoozi 2017.
| Methods | Randomised controlled trial (cluster randomisation, by community unit/sub counties); NCT02098031, with 3‐year follow‐up of infants | |
| Participants | 511 caregivers (mothers or grandmothers) and infants randomised Inclusion criteria: all consenting households with infants aged 6‐8 months within a participating village; children who did not have a mother as a caregiver were included and recruited with a grandmother. Exclusion criteria: households were excluded if the child had, 1) congenital malformation(s), 2) a physical disorder that would influence growth or preclude anthropometric measurements or influence nutrient intake, 3) been diagnosed with a mental or brain illness as reported by the mother or a health worker, 4) if the household was likely to migrate within the study period, or 5) if the mother was unable to provide information or unwilling to participate in the study. Town centres within the included districts were excluded to minimise differences in socioeconomic status, oral hygiene and feeding practices. Setting: Kabale and Kisoro districts in South‐Western Uganda (RCT conducted between October 2013 and January 2015) Important health characteristics reported: 35.7% and 28.6% of infants in the intervention and control groups, respectively, had a reported illness at baseline. |
|
| Interventions |
Group 1 (n = 263 caregivers randomised) Women (mothers or grandmothers) received nutrition and hygiene education, including oral healthcare for mothers of new babies, delivered in three main sessions, each lasting 6‐8 hours, over a six‐month period. Nutrition education: included provision of formulated recipes and demonstration of how to cook using locally available foods, including good quality protein. The mothers were encouraged to have a kitchen garden with vegetables and domestic animals (chicken/rabbits), to provide cheap animal protein. Hygeine education: the intervention focused on oral hygiene, which included demonstration of how to brush infant's teeth, handwashing before feeding, strategies to avoid cross contamination (e.g. not sharing utensils), and use of clean utensils during food preparation. Play therapy, and other interventions to support infant development were also included. Group 2 (n = 248 caregivers randomised) Standard care Intervention timing: intervention started when children were aged between six and eight months, and lasted for six months. Theory or model used as basis for intervention: nutrition education component was based on the WHO 10 guiding principles of complementary feeding of breastfed children (PAHO/WHO 2003). |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: caries presence in primary teeth (top front four teeth only); secondary outcomes: none Additional narrative text for: none Tabulated data for: child oral health behaviours Additional outcomes that had not been prespecified: Child: nutritional status,anthropometric measures, child development. Mother: none reported |
|
| Notes |
Funding: Thorne Holst Foundation and University of Oslo Declarations of interest: author declarations not reported; reported that "the funders had no role its design or conduct" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a three stage procedure to obtain households for the trial. First, by simple random sampling, sub counties in both districts were allocated to the intervention or control group. Second, all the villages in each participating sub county (intervention or control) were listed alphabetically and assigned numbers in ascending order. By use of computer‐ generated random numbers, villages whose position matched with the random numbers were identified eligible. Third, by complete enumeration, all consenting households with children aged 6–8 months within a participating village were recruited to the study by simple random sampling, sub counties in both districts were allocated to the intervention or control group". |
| Allocation concealment (selection bias) | Unclear risk | Method to conceal allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Authors reported that this was an open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " The study personnel collecting the data and analysing the study data and analysing the study outcomes was blinded to group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 170/263 (64.6%) of infants randomised to the intervention group, and 169/248 (68.1%) of infants randomised to the control group, were available for caries assessment at 36 months of age. |
| Selective reporting (reporting bias) | Unclear risk | Comprensive reporting of all outcomes prespecified in protocol; however caries, assessed in a follow‐up study, was not an outcome prespecified in the study protocol. |
| Other bias | Low risk | Data comparing study population characterisations for the two groups, at baseline (main RCT study) and in the follow‐up study, suggested that the only significant difference between the groups was breastfeeding frequency, with a higher proportion of mothers in the control group (75.4%) reporting feeding ≥ 8 times a day than mothers in the intervention group (64.5%). |
Plutzer 2008.
| Methods | Randomised controlled trial (Cavity Free Children Trial; multi‐arm trial with 3 arms; low and high intensity group combined for inclusion in review meta‐analysis) | |
| Participants | 649 pregnant women and their fetuses were randomised (all included in this review). Inclusion criteria: nulliparous women pregnant women attending regular antenatal visits at the participating teaching (public) hospitals (most women were in their 5th to 7th months of pregnancy) Exclusion criteria: high risk and multiple pregnancies; improperly completed questionnaires and mother's inability to comprehend written English Setting: Teaching (public) hospitals in Adelaide, South Australia (participants recruited in 2002) |
|
| Interventions |
Group 1 (n = 165 women randomised) Women received printed information about oral health applied in the form of anticipatory guidance at enrolment into the study. The information included messages focused on their own oral health and nutrition during pregnancy, healthy diet advice for young infants and children, and information about healthy feeding practices (e.g. pacifiers, infant sleep and importance of primary teeth for infants). At 6 months postpartum they received anticipatory guidance about oral health for infants via mail, reinforced during a scripted telephone interview which also included consultation on issues arising in the interview. Women received a third round of guidance, focused on oral health of 12‐month children, at 12 months postpartum. Group 2 (n = 162 women randomised) Women received the same interventions as women in Group 1, however no structured telephone interview/advice; n = 156 women, randomly assigned after second round advice was provided. We combined these two groups for inclusion in this review. Group 3 (n = 322 women randomised) Women received standard care. Intervention timing: one session delivered during pregnancy (5 to 7 months); subsequent sessions at 6 and 12 months postpartum (> 6 months intervention duration) Theory or model used as a basis for intervention: Nowak 1995 model of anticipatory guidance delivered by paediatricians and family physicians in well childcare clinics in the early years to improve oral health in young children |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: caries presence in primary teeth; dmfs index; dmft index; SiC30 index; secondary outcomes: none Additional narrative text for: none Tabulated data for: child dental attendance; change in mother self‐reported oral health behaviours (including diet) and attitudes; adverse events for mother or child Additional outcomes that had not been prespecified: Child: type of dental provider, child receipt of anaesthetic for dental treatment, infant health at birth (gestation at birth, birth weight, sex, race, congenital abnormalities, Apgar score and resuscitation at birth). Mother: access to oral health information; maternal health at birth of study child (gestation at first visit, blood pressure, hospitalisation during pregnancy, onset of labour, postnatal hospital stay, some laboratory results not further specified) |
|
| Notes |
Funding: Chanel 7 Children's Research Foundation of South Australia, Colgate Oral Care, Johnson & Johnson Pacific Company and The University of Adelaide Declarations of interest: none declared Groups 1 and 2 combined for inclusion in the review analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used to allocate women into test or control groups (before their consent was sought as Zelin RCT design). |
| Allocation concealment (selection bias) | High risk | Zelin RCT design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Zelin RCT design Quote: "The potential participants were randomly allocated to the test and control groups, then approached about the aims of the study and their group allocation. They had the opportunity to accept or refuse the group to which they were randomly allocated. Lack of blinding and potential loss of statistical power (if many participants refuse the allocated group) are the main disadvantages of the design". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To assist with blinding the examiner from knowing the characteristics of the child (test/control group), the examinations were organized through a dental receptionist who received the examination schedules". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 75.4% of the intervention group infants and 66.8% of control group infants were available for the caries assessment at 20 months of age. 96/327 (29%) and 91/322 (28%) of infants of mothers randomised to the intervention and control groups respectively were available for the caries assessment at age 6‐7 years; 117/327 (35%) and 113/322 (35%) were available for the dental visit outcome assessment. |
| Selective reporting (reporting bias) | Unclear risk | Without access to a study protocol, we were unable to confidently assess reporting bias. |
| Other bias | Low risk | The authors reported that after being told their group assignment (Zelin design), very few participants switched groups. Considering group imbalance, the authors reported that: "The only significant differences between the groups at baseline were in the use of dental floss (31.6% in the test group used versus 22.6% women in the control group; chi square P < 0.01) and in the use of alcoholic drinks during the pregnancy (12.4% in the test group compared with 7.4% in the control group; chi square P < 0.05)". Additionally, analysis comparing participants in the intervention and control groups included in the 6‐7 year outcome assessment showed no significant difference between the groups in key characteristics. |
Robertson 2013.
| Methods | Randomised controlled trial | |
| Participants | 414 women (new mothers) and their infants were randomised. Inclusion criteria: woman located in the selected study communities, able to provide informed consent, with at least 20 natural teeth and unrestored caries or a previous child with documented early childhood caries; and with a child between 4.5 and 6.0 months of age (with or without teeth) Exclusion criteria: presence of orthodontic appliance and pregnancy Setting: local Indigenous Health Service (IHS) or tribally operated community dental clinics in four different American Indian communities in Oregon, Washington and Arizona USA (recruitment and study dates not reported) Important health characteristics reported: high prevalence, severity, and morbidity from ECC in the included. AI/AN communities |
|
| Interventions |
Group 1 (n = 204 women randomised) Women received a 10% chlorhexidine (CHX) dental vanish treatment. Treatments (six) were applied by a trained hygienist or dental assistant after a brief rubber cup prophylaxis. They were applied in two stages: Stage 1 contained 10% CHX diacetate w/v suspended in a solution of Sumatra benzoin and alcohol. Stage 2 was a proprietary aqueous dispersion of inert methacrylate approved for use by the FDA under license K023671. The stage 2 coating was designed to prolong the contact time between the CHX and the tooth. The mean dose of CHX at each application visit was 37.4 mg (14); the cumulative mean dose was 224 mg. Group 2 (n = 210 women randomised) Women received placebo treatment which consisted of Sumatra benzoin and alcohol solution, delivered by the same providers and in the same setting as the active treatment. Intervention timing: intervention started between 5.5 to 6.0 months after birth (4 weekly treatments), with two further treatments one year and 18 months later (> 6 months intervention duration). Theory or model used as a basis for intervention: authors claimed: "extensive literature on the use of CHX‐containing products in different vehicles and concentrations to prevent caries"; no specific theory or model reported All participants: mothers' caries restored at enrolment |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: caries presence in primary teeth; secondary outcomes: none Additional narrative text for: none Tabulated data for: none Additional outcomes that had not been prespecified: Child: none reported. Mother: none reported |
|
| Notes |
Funding: HRSA grant R40MC03621. CHXTechnologies, Inc., Toronto, Canada, provided the study products (Prevora® and placebo) without charge plus initial training for study staff. Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study sites received consecutively numbered boxes of the study product which were numbered by the research pharmacist prior to shipment. Each box contained separate vials for each study visit. As participants were enrolled, they were assigned the next numbered product box". |
| Allocation concealment (selection bias) | Low risk | Boxes with study product and group assignment were numbered by the research pharmacist prior to shipment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Because the active and placebo study products were identical in colour, smell, taste, and viscosity, neither the participants nor study staff knew whether the product was active or placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 188/204 (92%) infants of mothers randomised to the treatment group, and 179/210 (85%) infants of mothers randomised to the control group, had a post‐baseline caries assessment. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess this domain without access to the study protocol |
| Other bias | High risk | No data showing similarity of groups on key characteristics provided. Additionally, possible intervention infidelity as the authors reported unequal application of intervention treatment across the groups. |
Soderling 2000.
| Methods | Randomised controlled trial (multi‐arm trial, with 3 arms) | |
| Participants | 188 women (new mothers) and their children were randomised (159 women to the two groups included in this review). Inclusion criteria: woman located in the selected study communities; able to provide informed consent; with at least 20 natural teeth and unrestored caries or a previous child with documented ECC; and with a child between 4.5 and 6.0 moths of age with or without teeth Exclusion criteria: not reported Setting: Ylivieska, Alavieska and Sievi Health Centers in the central part of Finland Important health characteristics reported: included mothers had high counts of salivary mutans streptococci during pregnancy, otherwise healthy. |
|
| Interventions |
Group 1: xylitol gum (n = 127 women randomised) Women were requested to chew xylitol chewing gum three months after the birth of the baby, the chewing gum contained xylitol as the only sweetener (65% w/w), average daily dose of xylitol 6 to 7 g, average consumption frequency four times per day. Group 2: chlorhexidine varnish (n = 32 women randomised) Women received a total of three chlorhexidine varnish ((EC40®, Certichem, Nijmegen, The Netherlands) treatments. Group 3: Fluoride varnish (n = 36 women randomised) Women randomised to this group received fluoride varnish (Duraphat®, Rhone‐Poulenc Rorer, GmbH, K6ln, Germany) treatment (not included in this review analyses, see notes below for reason). Intervention timing: mothers started using xylitol chewing gum three months after birth and the use of xylitol was discontinued 24 months after delivery; CHX varnish was applied to the dentition of mothers at 6, 12 and 18 months postpartum (> 6 months intervention duration). Theory or model used as a basis for intervention: not reported All participants: all children, regardless of the study group to which they were assigned, and the possible caries risk, received an oral healthcare program which was routinely given to children under 5 years of age in the Finish public healthcare system, and all childrenwere "not treated with any prophylactic measure before the age of 2 years". |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: dmft index; secondary outcomes: child microbiological presence: mutans streptococci colonisation (any); mother microbiological presence: mutans streptococci colonisation (level, CFU/mL) Additional narrative text for: caries presence in primary teeth Tabulated data for: none Additional outcomes that had not been prespecified: Child: none reported. Mother: none reported |
|
| Notes |
Funding: "This study was supported in part by the Academy of Finland; Cultor, Finland; and Xyrofin, UK. Leaf, Finland, manufactured and donated the chewing gums used in the study. The chlorhexidine varnish was a kind gift from Dr. Thijs Schaeken, Nijmegen, The Netherlands". Declarations of interest: not reported The fluoride varnish group was not included as fluoride treatment in mothers was an excluded intervention in this review due it being included in another Cochrane review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the subjects were randomly divided into three study groups". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | For study personnel not reported, and not feasible for study participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | For microbiological outcomes, blinding: "The persons involved in the collection and analysis of the microbiological samples were blinded as to the study design and group". For caries, no blinding: "The examiners were not blinded as to the mother's group during the first two annual examinations when the children were 1 and 2 years of age but were blinded during the clinical examinations of the child at the ages of 3, 4, and 5 years". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "loss to follow‐up was 19% at 3 years and 25% at 6 years". |
| Selective reporting (reporting bias) | Unclear risk | Unable to be confidently assessed without access to study protocol |
| Other bias | High risk | Substantially more mothers (120) were assigned to the xylitol than the CHX group (32), and few data provided to show similarity of participants in the groups at baseline. Additionally, possible intervention infidelity, as the authors reported that: "by the age of 6 years, around one‐third of the children in each of the groups chewed xylitol gum themselves". |
Thorild 2003.
| Methods | Randomised controlled trial (multi‐arm trial, with 3 arms) | |
| Participants | 173 women (new mothers) and their infants were randomised (116 to the groups included in this review). Inclusion criteria: healthy mothers with high counts of salivary mutans streptococci (MS) (> 150 CFU) and their three‐month old infants residing in the study area Exclusion criteria: not reported Setting: city of Varberg, a mid‐sized community in south‐west Sweden Important health characteristics of mothers reported: included women had high counts of salivary mutans streptococci at 3 months postpartum, otherwise healthy. |
|
| Interventions |
Group 1: xylitol gum (n = 61 randomised) Women received gum containing 650 mg xylitol (Xylitol, Leaf, Finland), total weight/gum 1050 mg; requested to chew three pieces/day, for five minutes, in the morning, at noon, and in the evening. Group 2: chlorhexidine/xylitol gum (n = 55 randomised) Women received gum containing 532.5 mg xylitol, 5.0 mg chlorhexidine, and 141.9 mg sodium fluoride for a total weight/gum 1120.1 mg; they were requested to chew three pieces daily for five minutes, in the morning, at noon and in the evening. Group 3: sodium fluoride/xylitol gum (n = 57 randomised) Women received gum containing 288.5 mg xylitol, 188.8 mg sorbitol and 0.55 mg sodium fluoride, total weight/gum 870 mg; they were requested to chew 3 pieces daily, for five minutes, in the morning, at noon and in the evening. Intervention timing: intervention delivered 6 though 18 months postpartum (> 6 months intervention duration) Theory or model used as a basis for intervention: not reported |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: caries presence in primary teeth; defs score; defs score categories; secondary outcomes: child microbiological presence: mutans streptococci colonisation (any); child microbiological presence; mutans streptococci score categories Additional narrative text for: none Tabulated data for: none Additional outcomes that had not been prespecified: Child: none reported. Mother: none reported |
|
| Notes |
Funding: "The study was supported by grants from the Swedish Dental Society, the Swedish Patent Revenue Fund and the County Councils of Halland and Västerbotten". Declarations of interest: none declared As fluoride is not an eligible intervention in this review, we have excluded the sodium fluoride/xylitol/sorbitol gum group, and included this trial in the comparison of xylitol versus CHX combined with xylitol. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Three experimental groups and one reference group were formed on the basis of the maternal MS counts. The mothers with high counts of salivary MS (≥ 150 colony forming units CFU) were randomly assigned to three experimental groups". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely considering the type of intervention assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all children were examined in a dental chair by one trained examiner (IT) blind to which group the child belonged". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At the 3‐year time point: 56/61 (91%) and 44/55 (80%) of infants were available for assessment in the xylitol and CHX/ and fluoride groups respectively. At the 4‐year data collection time point: 52/61 (85%) and 44/55 (80%) infants of mothers randomised to the xylitol and CHX/xylitol groups, respectively, were available for assessment. |
| Selective reporting (reporting bias) | Unclear risk | Without access to study protocol, cannot assess confidently as high or low risk |
| Other bias | Low risk | There were no signs of other bias in the study reporting. |
Veronneau 2010.
| Methods | Randomised controlled trial | |
| Participants | 821 women and their infants were randomised. Inclusion criteria: not reported Exclusion criteria: not reported Setting: 11 community health centres throughout Quebec, Canada |
|
| Interventions |
Group 1 (n for women randomised not reported) Women received a "community‐based health education intervention aimed at preventing caries in young children...delivered by dental hygienists during four sessions" (no further details provided in conference abstracts reporting this study). Group 2 (n for women randomised not reported) Women received standard care. Intervention timing: 4 sessions at six month intervals postpartum (start and end date not reported) (> 6 months intervention duration) Theory or model used as basis for intervention: not reported |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: none; secondary outcomes: none Additional narrative text for: any caries present in primary teeth; dmfs index Tabulated data for: none Additional outcomes that had not been prespecified: Child: none reported. Mother: none reported |
|
| Notes |
Funding: Canandian Institutes of Health Research Declarations of interest: not reported Conference abstract report available for inclusion in this review only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The dyads were randomised to either an educational intervention....or to a normal care control group". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Authors reported that this was a single blinded trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evaluation was carried out by dental hygienists blinded to the test/control status of the children, in a school class room". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors reported that outcomes were assessed in 749 infants at the 30‐month old data collection time point, and in 377 children at the 5‐6 year data collection time point; no further details, including on attrition group for the caries outcome. |
| Selective reporting (reporting bias) | Unclear risk | Without access to the study protocol, we were unable to assess confidently whether the study was at high or low risk of bias. |
| Other bias | Unclear risk | Insufficient information on study methods to assess this trial confidently as at 'high' or 'low risk' of other bias |
Watt 2009.
| Methods | Randomised controlled trial: ISRCTN55500035, with 4‐year follow‐up of infants | |
| Participants | 312 new mothers and their infants were randomised. Inclusion criteria: women from Registrar General occupational classes II–V (non‐professional), singleton babies born > 37 weeks, with birth weight above 2500 g, able to understand written and spoken English, resident in study area. Originally, the intention was to restrict the sample to first‐time mothers only. However, major difficulties were encountered in recruiting sufficient numbers of first‐time mothers over the initial 12 weeks of the recruitment period. The inclusion criteria were therefore changed to include all new mothers. Exclusion criteria: women < 17 years, infants diagnosed with a serious medical condition or who were on special diets due to medical problems, infants > 12 weeks, women/infants from professional households from social class I, women unable to communicate effectively in English Setting: baby clinics in two relatively socioeconomically disadvantaged inner‐city London boroughs in the United Kingdom (women recruited December 2002 to February 2004) Important health characteristics reported: all included women were without a serious medical condition; 43% reported consuming > five portions of fruit and vegetables a day. |
|
| Interventions |
Group 1 (n = 157 women randomised) Women assigned to this group received a peer‐led infant feeding intervention delivered by local volunteers who were trained to provide home‐based nonjudgemental support and practical assistance on infant feeding, in particular, relating to weaning. On average, each mother received 5 home visits (mean length 60 minutes per visit).The intervention adopted a holistic approach to infant nutrition designed to empower the women to follow current guidance on the later stages of infant feeding practices, in particular, when to introduce solids, the types of foods and drinks to give a child with emphasis on the importance of fruit and vegetables, and when to stop using a feeding bottle. Group 2 (n = 155 women randomised) Women received standard care (professional support from health visitors and GPs). Timing: delivered during the first year of life, over a nine‐month period (> 6 months intervention duration) Theory or model used as a basis for intervention: authors reported that the approach was based on the Community Mothers Programme (Johnson 1993) and evidence about effective peer support for breastfeeding practices. |
|
| Outcomes |
Data in meta‐analysis for: primary outcome: caries presence in primary teeth; secondary outcomes: none Tabulated data for: child oral health behaviours; change in mother self‐reported oral health behaviours (including diet) and attitudes Additional narrative text for: none Additional outcomes that had not been prespecified: Child: supine length and weight, BMI, general health, health problems. Mother: none reported |
|
| Notes |
Funding: Four‐year follow‐up study "was funded by UCL... The original study was funded by the UK Food Standards Agency". Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using random digit computer tables” |
| Allocation concealment (selection bias) | Low risk | Quote: "The study administrator was responsible for the randomisation process". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely considering the type of intervention assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors claimed that those responsible for recruiting and assessing outcomes were all masked to group assignment. However, it was not specifically stated that the dentists who assessed child caries were blind to group assignment, and with respect to the secondary outcome 'mother's health behaviours and attitudes', the assessors were clearly not blinded (self report). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 44/157 and 41/155 of infants of mothers randomised to intervention and control groups respectively were available for the child caries outcome assessment at the 4 years follow‐up time point (relevant to the caries data included in this review). |
| Selective reporting (reporting bias) | Unclear risk | Caries not specified as an outcome in the study protocol |
| Other bias | Low risk | No indication of other bias |
Zanata 2003.
| Methods | Randomised controlled trial | |
| Participants | 81 pregnant women and their fetuses were randomised. Inclusion criteria: pregnant women (in the second or third trimester), without any medical recommendations that could make dental treatment inadvisable, and presenting three or more active carious lesions (cavities) in smooth dental surfaces (proximal, buccal or lingual) Exclusion criteria: not reported Setting: nine Basic Health Units in the suburbs of Bauru, Brazil (recruitment and study dates not reported) Important health characteristics reported: all included women had three or more active carious lesions (cavities) in smooth dental surfaces (proximal, buccal or lingual). |
|
| Interventions |
Group 1 (n = 43 women randomised) Women received primary care intervention and topical application of antimicrobial agents at baseline. The primary care intervention comprised elimination of infection sites through tooth extraction,endodontic dressings, root scaling and sealing of cavities with glass ionomer cement Fuji IX (GC Dental Co., Japan). The topical application of NaF and iodine solution was carried out in 3 sessions: the first immediately after prophylaxis, and the second and third applications after 3 and 5 days, respectively, without prophylaxis, after dental care of the patient, as suggested by Caufield 1979. The composition of this solution was 1.0 g KI, 1.2 g NaF, 53.0 mL glycerin, H2O to complete 100 mL, solution adjusted to pH 4.5 using 85% H3PO4 according to the recommendations of Dasanayake 1993. At six months follow‐up, the antimicrobial solution and topical fluoride were reapplied in the experimental group mothers. At 12 months follow‐up: prophylaxis, fluoride therapy and decontamination with iodine solution treatment and all new cavities were excavated and sealed and defective restorations were repaired. Group 2 (n = 38 women randomised) At baseline/enrolment, cavities in posterior teeth were filled with the zinc oxide‐eugenol cement IRM (Dentsply Ltd., Petropolis, RJ, Brazil), whereas the anterior teeth were restored with the composite Fill Magic (Vigodent, Rio de Janeiro, RJ, Brazil). The first intention was to restore all cavities with zinc oxideeugenol cement, which is the intermediate restorative material used by public health services in Bauru. However, because of immediate failure of this material in a number of class III, IV and V preparations and its unpleasant appearance that led to rejection by the patients, the composite was used. At 6 and 12 months, clinical intervention received by women in this group included treatment for emergency procedures only. Timing: initial intervention during second or third trimester of pregnancy, with follow‐up interventions at 6 and 12 months (> 6 months intervention duration) Theory or model used as a basis for intervention: the composition of the antimicrobial agent applied to the teeth of women was based on the recommendations of Dasanayake 1993. All participants: received oral health education, targeted at mother and child, at baseline and follow‐up (six and 12 months) |
|
| Outcomes |
Data in meta‐analysis for:primary outcome: caries presence in primary teeth; secondary outcomes: mother DMFS increment Tabulated data for: none Additional narrative text for: none Additional outcomes that had not been prespecified: Child: none reported. Mother: none reported |
|
| Notes |
Funding: Quote: "We thank FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for the technical, scientific and financial support that were fundamental for the accomplishment of this study". Declarations of interest: unclear; not reported in English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "these subjects were randomly divided into two groups, experimental and control". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported; unlikely considering intervention and comparator assessed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "clinical assessments (not blind)" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High loss to follow‐up, with 30/38 (78%) of the women randomised to the control group and 34/43 (79%) of the women randomised to the experimental group completing the study; however marginal difference in attrition rates across groups |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol and therefore could not confidently assess this domain as at high or low risk of bias |
| Other bias | Unclear risk | Insufficient information to assess confidently, e.g. lack of adequate data comparing group participants on key characteristics |
AI: American Indian; AIDS: aquired immune deficiency syndrome; AN: Alaskan native; BFHI: baby friendly hospital initiative; BMI: body mass index; CFU: colony‐forming unit; CHX: chlorhexidine; defs: decayed, extracted and filled surfaces; dmfs: decayed, missing and filled services in primary teeth; dmft: decayed, missing and filled primary teeth; DVD: digital versatile disk; EBF: exclusively breastfed; ECC: early childhood caries; FAPESP: Fundação de Amparo à Pesquisa do Estado de São Paulo; FDA: Food and Drug Administration; H2O: dihydrogen monoxide (water); H3PO4: phosphoric acid; HDL: high density lipoprotein; HS: Indigenous Health Service; IRM: intermediate restorative material; KI: potassium Iodine; KJ: kilojules; MI: motivational interviewing; MIC: maternal and infant care; MS: mutans streptococci; NaF: sodium fluoride; pH: power of hydrogen; P/M/S: polyunsaturated / monounstaturated / saturated fatty acids; RCT: randomised controlled trial; SAFA: saturated fatty acid; STRIP: Special Turku Coronary Risk Factor Intervention Project for Babies; UNICEF: United Natiions International Children's Emergency Fund; USP: United States Pharmacopeia; w/v: weight / volume; w/w: weight/weight; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abanto 2012 | Infant/child caries not a study outcome |
| Adams 2017 | Design: nonrandomised trial and in addition included only women, therefore dental caries in children not a study outcome |
| Al Khamis 2017 | Study population: no infants included (pregnant women only) |
| Alamoudi 2012 | Study population: infants not all younger than age 1 year at recruitment |
| Bahri 2015 | Study population: no infants included (pregnant women only) |
| Bergel 2010 | Outcomes and child age: caries assessed when infants of women who received maternal calcium during pregnancy were 12 years of age |
| Brambilla 1998 | Infant/child caries not a study outcome |
| Cardoso 2018 | Population and outcomes: infants aged 6 to 36 months at baseline, and caries in infants/children not a study outcome |
| Cibulka 2011 | Population: pregnant women only included |
| Cockburn 1980 | Outcomes: caries in children aged 0 to 6 years not a study outcome |
| Curnow 2002 | Population: intervention (supervised toothbrushing), targeted at children in their first year of school |
| Geisinger 2014 | Population and outcomes: pregnant women only included in trial, and therefore the primary outcome of this review, caries in infants/children, not a study outcome |
| George 2018 | Outcomes: caries in infants/children not an outcome (study outcomes included gestational age a birth, premature birth and birth weight) |
| Gomez 2001 | Design: observational study |
| Harjunmaa 2016 | Population and outcomes: study assessed the impact of different micronutrient supplements on oral health status of pregnant women; no infants included |
| Hillman 1962 | Population and outcomes: intervention evaluated targeted at pregnant women and no infants included in study |
| Holt 1985 | Population: children aged 2 years at baseline |
| Jiang 2015 | Population: protocol for an RCT to assess the effects of a nonalcoholic antimicrobial mouth rinse and oral health education in pregnant women |
| Joury 2016 | Population: infants 1 year at baseline |
| Karanja 2012 | Population and outcomes: this RCT (PTOTS) evaluated oral health education targeted at toddlers aged 0 to 2 years, and caries in children was not a study outcome |
| Kohler 1983 | Outcomes: infant/child caries not a study outcome |
| Kowash 2000 | Population: oral health interventions assessed and delivered when children between 2 and 3 years of age |
| Kraivaphan 2007 | Population and outcomes: participants pregnant women, caries in children age 0 to 6 years not a reported outcome |
| Leverett 1997 | Intervention: this trial evaluated a fluoride supplementation intervention targeted at pregnant women, an intervention type excluded from this review as it is being evaluated in a Cochrane review currently in process (Takahashi 2015). |
| Lopez 2002 | Outcomes: caries in infants/children not a study outcome |
| Ma 2017 | Intervention and population: this trial evaluated the effectiveness of a clinical intervention targeted at children who were older than 1 year at baseline. |
| Macones 2010 | Outcomes: caries in infants/children not a study outcome |
| Mohebbi 2009 | Population: child aged 12 to 15 months at baseline |
| Nakai 2010 | Outcomes: infant/child caries not a study outcome of this trial |
| NCT00719238 | Outcomes: caries in infants/children not a study outcome |
| NCT01652300 | Population: pregnant women included only, no infants/children, and caries in children not a study outcome |
| NCT01763138 | Population and outcomes: intervention delivered to mothers of infants aged 9 to 15 months and child caries not a study outcome |
| NCT02436811 | Outcomes: caries in infants/children not a study outcome |
| NCT02578966 | Population: children in the RCT assessing oral health education provided using the motivational interviewing technique versus traditional technique were 12 to 14 months at recruitment. |
| NCT03273725 | Study design and outcomes: observational study that used data from the Training in Pregnancy (TRIP), an RCT that evaluated effect of exercise during pregnancy on pregnancy‐related illnesses, to assess associations between maternal pre and postnatal Vitamin D levels and dental caries in children aged 7 to 9 years |
| NCT03478748 | Population: children 2 to 3 years of age at baseline |
| NCT03529500 | Study design and population: observational (cross‐sectional) study that evaluated effects of chronic malnutrition on the oral health of children aged one to five years |
| NCT03598972 | Study design: not an RCT or quasi‐experimental study |
| NCT03693443 | Study design: cross‐sectional study evaluating the evaluation of the oral health beliefs, knowledge, and behavioural attitudes towards early childhood caries |
| Olak 2012 | Study design: not an RCT or quasi‐experimental study |
| Plonka 2013 | Intervention: preventive products (CPP‐ACP, fluoride varnish or chlorhexidine) applied directly to the infants’ teeth (child the target, not the mother, who was the vehicle by which the substrate was applied) |
| Ramos‐Gomez 2012 | Intervention: main intervention preventive fluoride application in teeth of infants |
| Rivas Castillo 2014 | Population: protocol for a trial including pregnant women and evaluating a relevant intervention delivered to pregnant women on their oral health status |
| Stensson 2014 | Participant age at which outcomes assessed: caries assessment at 9 years of age |
| Tenovuo 1992 | Population: children randomised at age 1 year |
| Turksel 2004 | Population: infants were between 2 and 18 months old at recruitment. |
| Weber‐Gasparoni 2013 | Population: infants were between 2 and 18 months old at recruitment. |
| Weinstein 2004 | Population: 240 infants aged 6 to 18 included in the study (i.e. not all younger than one year during intervention delivery); trial which evaluated motivational interviewing counselling treatment compared with traditional health education delivered to parents of young children at high risk of developing dental caries, otherwise met all review inclusion criteria |
| Zhan 2012 | Population and intervention: intervention evaluated in this trial was xylitol‐containing tooth‐wipes applied to teeth of children (i.e. intervention not targeted at mothers), and children older than one years (aged 6 to 35 months) included |
CPP‐ACP: Casein phosphopeptide‐amorphous calcium phosphate; PTOTS: The Prevention of Toddler Obesity and Teeth Health Study; RCT: randomised controlled trial; TRIP: training in pregnancy
Characteristics of studies awaiting assessment [ordered by study ID]
Batra 2018.
| Methods | RCT (pilot study) |
| Participants | 60 women and their infants aged 8 to 12 months were randomised, in India (recruited May to July 2014) |
| Interventions | 1) oral health education provided using motivational interviewing 2) oral health education provided using traditional techniques 3) standard care |
| Outcomes | Dental caries in children (present/absent); plaque in children (present/absent); mother self‐reported oral health knowledge and behaviour |
| Notes | Baseline data on caries and other outcomes for children in all groups reported only; additional data may be reported by a follow‐up of this study in future, including for our primary review outcome. |
Jamieson 2012.
| Methods | RCT (Baby Teeth Talk project, ACTRN12611000111976) |
| Participants | 450 Aboriginal pregnant women and their infants were randomised in South Australia, and 223 Maori pregnant women and their infants in New Zealand were randomised; (date of first participant enrolment 31 January 2011). |
| Interventions | 1) provision of dental care to mother during pregnancy (including extractions, restorations, scaling and prophylaxis); fluoride varnish application to teeth of children (at 6, 12 and 18 months, children in delayed intervention group at 24, 30 and 36 months); motivational interviewing; and anticipatory guidance. Dental treatment was delivered as a standalone intervention, but the motivational interviewing and anticipatory guidance was conducted during the same sessions as the fluoride varnish. 2) standard care |
| Outcomes |
Primary outcome: prevalence of dental caries in children, assessed at 2 and 3 years Secondary outcomes: carer self‐reported health knowledge and oral self care (assessed at child age two and three years); carer dental health service utilisation (assessed at child age two and three years); carer oral health‐related self‐efficacy (assessed at child age two and three years); carer oral health literacy (assessed at child age two and three years); average daily energy intake (assessed by 3 X 24‐hour diet recalls, at child age two years); food and nutrient intake (assessed by Food Frequency Questionnaires, at child age three and five years) |
| Notes | Dental caries in children not reported yet. Anticipated date of last data collection 15 December 2017. |
Klastersky Genot 1970.
| Methods | RCT |
| Participants | |
| Interventions | Tetracycline, administered during pregnancy to affect deciduous teeth |
| Outcomes | |
| Notes | Full text not accessible in English (yet) |
Ratte 1969.
| Methods | Long‐term trial (no further details on design) |
| Participants | |
| Interventions | Intrauterine dental caries prevention, no further details provided in title and abstract for this citation. |
| Outcomes | |
| Notes | Citation available only, no full‐text access; citation indicates article written in German. |
Characteristics of ongoing studies [ordered by study ID]
Arrow 2013.
| Trial name or title | Reducing disease burden and health inequalities arising from chronic dental disease among Indigenous children: an early childhood caries intervention |
| Methods | RCT: ANCTRN12611000111976 |
| Participants | Recruitment target: 1028 Inclusion criteria: Child/parent newborn dyads attending the all child/community health clinics in metropolitan Perth and Bunbury/Bussleton Exclusion criteria: not reported Setting: Perth, Bunbury and Busselton, Australia |
| Interventions |
Intervention group: mothers provided with tailored oral health counselling by oral health consultants trained in motivational interviewing and anticipatory guidance Control group: standard care (early oral health screening program, "lift the lip" program, available through Western Australia since 2011) |
| Outcomes |
Primary outcome: incidence of dental decay in primary teeth of children, counts of dmft/s (including non‐cavitated lesions), assessed at 24 and 36 months; prevalence of obesity in children, measured using child height/length and weight and BMI, at 24 and 36 months Other outcomes: changes in knowledge, attitude, behaviour and self‐efficacy of parents towards the oral health of their child (various measures); nutritional and dietary patterns (various measures); dental decay in children at 5 years of age; referral for care under general anaesthesia, cumulative, assessed at 5 years of age |
| Starting date | Anticipated start date 1/08/2011 |
| Contact information | Peter Arrow, email: parrow@ozemail.com.au |
| Notes |
Batliner 2014.
| Trial name or title | Promoting behavioural change for oral health in American Indian mothers and children |
| Methods | RCT: NCT01116726 |
| Participants | Recruitment target: 1134 participants Inclusion criteria: American Indian, as defined by the tribe; mothers or caregivers of newborn children; at least 15 to 44 years of age (minors who are 15 to 17 years of age must get consent from a parent or legal guardian according to Tribal, State and IHS rules and regulations); able to read, understand and sign a consent/assent form; be willing and able to follow study procedures and instructions. Trialists noted that, although expected to be rare, if the father is a sole caregiver, he and his child will be eligible for the study. Exclusion criteria: none declared Setting: United States |
| Interventions |
Intervention group: motivational interviewing and enhanced community services. Motivational interviewing involves home visits, concentrating on the mitigation of behavioural risk factors for early childhood caries. These take place shortly after childbirth and at months 6. 12, and 18. Enhanced community services involve the development of culturally appropriate messages related to the mitigation of behavioural risk factors for early childhood caries through public service announcement and brochures. Control group: enhanced community services, as per the intervention group |
| Outcomes |
Primary outcome: decayed, missing and filled tooth surfaces (dmfs), assessed over 3 years Other outcomes: dental knowledge, attitudes, and behaviours of mothers, assessed over 3 years; dental caries patterns of children, assessed over 3 years; costs of dental care, assessed up to 3 years after randomisation; other decayed, missing and filled tooth surfaces measures, assessed over 3 years |
| Starting date | August 2011 |
| Contact information | Terry Batliner, email: not reported |
| Notes | Last updated post on Clinical Trials.gov site: 18 January 2018 |
NCT00066040.
| Trial name or title | Prevention of transmission of bacteria that cause cavities from mothers to their children |
| Methods | RCT |
| Participants | Recruitment target: 280 participants Inclusion criteria: first‐time medically healthy mother ≤ 35 years, with at least 20 teeth and high levels of mutans streptococci and their infants ≥ 2 months of age Exclusion criteria: no fluoride exposure in the previous 6 months; no cognitive impairment; < 2 months Setting: Brazil |
| Interventions | 1) Cervite chlorhexidine varnish applied to dentition of mothers 2) Duraphat fluoride varnish applied to dentition of mothers 3) Maternal consumption of xylitol gum (from Fennbon, Finland) |
| Outcomes | Outcome(s): mutans streptococci in mothers and infants; dental caries in children (no further details) |
| Starting date | January 2001 |
| Contact information | Walter Bretz, email: not reported |
| Notes | Study completed in January 2006 |
NCT00067340.
| Trial name or title | Caries transmission prevention in Alaska native Infants |
| Methods | RCT: NCT00067340 |
| Participants | Recruitment target: 250 participants Inclusion criteria: primiparous or multiparous Alaska native mothers of all ages; in the last month of pregnancy; reside in the health service delivery area of the native health corporation, in one of the communities with the highest birth counts from 2002; eligible for obstetric care from the health corporation; plan to give birth to their infant in a specified city of Alaska Exclusion criteria: not reported Setting: North West Alaska, USA |
| Interventions |
Intervention group: maternal chlorhexidine mouthwash prior to delivery (twice daily, over a two‐week period) followed by a subsequent two‐year period of maternal xylitol gum use Control group: standard care |
| Outcomes |
Primary outcome: caries in children, assessed at 12 and 24 months Other outcomes: mother and child mutans streptococci counts, assessed at 12 and 24 months |
| Starting date | April 2003 |
| Contact information | David Grossmman, email: not reported |
| Notes | Address of Principal Investigator/contact person: University of Washington, no further details provided |
NCT01038479.
| Trial name or title | Maternal consumption of xylitol to reduce early childhood decay (MaXED Study) (MaXED) |
| Methods | RCT: NCT01038479 |
| Participants | Recruitment target: 1064 participants Inclusion criteria: mother with high counts of mutans streptococci (equal or higher than log 5); child less than 3 months of age; mother who has a close relationship with Fife (e.g. lives or works in Fife); child seen by health visitor; mother is the main carer of her child(ren) Exclusion criteria: mother with low or no mutans streptococci; child older than 3 months of age; child not seen by health visitor; no close relationship with Fife (e.g. doesn't live or work in Fife); mother is not the main carer of child(ren) Setting: UK (Scottish population) |
| Interventions |
Intervention group: maternal consumption of 5 grams of xylitol per day plus Childsmile preventative programme (www.child‐smile.org) Control group: Childsmile preventative programme |
| Outcomes |
Primary outcome: caries occurrence in children, assessed at age 3 and 5 years; oral microbial colonisation in children, assessed at 2 years Other outcomes: mother acceptability of the invention, qualitative assessment using periodic questionnaires, assessed at 2 years |
| Starting date | December 2009 |
| Contact information | Brett Duane, email: not reported |
| Notes | Address of principal investigator: Leven, Fife, United Kingdom, KY8 5RR |
NCT01502566.
| Trial name or title | A cluster‐randomized trial of the effectiveness of an educational intervention in preventing early childhood caries |
| Methods | RCT (cluster‐randomised) |
| Participants | Recruitment target: 500 participants, 24 public health centres (clusters) Inclusion criteria: children ≤ 12 months of age and their mothers in Pelotas, Brazil (no further details) Exclusion criteria: not reported Setting: Brazil |
| Interventions |
Intervention group: on the Brazillian National Vaccination Day, mothers/children allocated to this arm receive a pamphlet containing key information on dental caries prevention, together with oral instructions about how to avoid dental caries in children. Control group: standard care |
| Outcomes |
Primary outcome: child caries status (dmfs), assessed at 12 months of age Other outcomes: child dental plaque index, assessed at 12 months of age |
| Starting date | June 2010 |
| Contact information | Maximiliano S Censi, email: not reported |
| Notes | Address of investigators: Fenderal University of Pelotas ‐ School of Dentisty, Pelotas RS 96015560, Brazil |
NCT02937194.
| Trial name or title | Family‐centered oral health promotion for new parents |
| Methods | RCT: NCT02937194 |
| Participants | Recruitment target: 584 participants Inclusion criteria: first time pregnancy; of Chinese ethnicity; ability to speak Cantonese and read traditional Chinese Exclusion criteria: women with any communication difficulties noted; informed consent not obtained Setting: Hong Kong, China |
| Interventions |
Intervention group: personal oral health instruction combined with oral health education provided through pamphlet distribution Control group: oral health education provided through pamphlet distribution |
| Outcomes |
Primary outcome: prevalence of caries, assessed at 36 months; proportion of parents who brush their infants' teeth regularly, assessed at 12 months Other outcomes: Infants' feeding and dietary habits at 12 months; mothers', fathers' and infants' oral hygiene status, assessed at 12 and 36 months; caries increment in children, assessed at 24 and 36 months; mutans streptococci in mothers and children, assessed at 12, 24 and 36 months; mothers' and fathers' change in periodontal condition, assessed at 12 and 24 months |
| Starting date | January 2014 |
| Contact information | May Chun Mei Wong, email: mcmwong@hku.hk |
| Notes |
NCT03077425.
| Trial name or title | Obesity and caries in young South Asian children: a common risk factor approach (CHALO) |
| Methods | RCT: NCT03077425 |
| Participants | Recruitment target: 377 participants Inclusion criteria: child < 6 months of age at time of recruitment; child is enrolled in either Medicaid or CHIP; mother was born in India, Pakistan or Bangladesh; mother speaks standard Bengali, English or Hindi/Urdu; mother is index child's primary caretaker Exclusion criteria: inability to provide informed consent; plans to travel for > 1 month during follow‐up; child health condition barring participation (per paediatrician review of recruitment lists) Setting: participants recruited from New York City (n = 3) and New Jersy (n = 2) paediatric practices, USA |
| Interventions |
Intervention group: community health worker‐led intervention comprised of: a) home visits with mothers/families (6 visits over 1 year) and follow‐up telephone support; b) patient navigation to make/keep timely dental visits (2 visits by 18 months). Intervention includes provision of pamphlet with information relating to oral health and dental referral list. Control group: enhanced usual care consisting of community healthcare workers providing mothers with a pamphlet and dental referral list (same as provided to intervention group mothers) |
| Outcomes |
Primary outcome: number and amount of sippy cup and/or bottles consumed/day by child, assessed at 18 months of age Other outcomes: number of sweeteners and/or solids/day added to child's sippy sups/bottles, assessed at 18 months of age; fruit & vegetable servings/day for child, assessed at 18 months of age; juice & sweet drinks servings/day for child, assessed at 18 months of age; frequency of child drinking from a bottle or sippy cup/day when put to bed or nap, assessed using MySmileBuddy at 18 months of age; frequency of sweet & salty snacks consumed by child, assessed 18 months of age; time spent in active play by child, assessed at 18 months of age; child screen time (various measures, including time spent in front of TV) assessed at 18 months of age; frequency of parent wiping/brushing teeth, assessed at 18 months of age; number of dental visits, assessed at 18 months of age; visible child caries, assessed by intra‐oral camera at 18 months of age; caries severity, assessed by dfs index at 18 months of age; weight for length measures, assessed at 18 months of age; change in Weight Velocity Z Scores, 6 months to 12 months, and 12 months to 18 months |
| Starting date | December 2017 |
| Contact information | Karen Bonuck, email: karen.bonuck@einstein.yu.edu |
| Notes | Principal Investigator: Alison Karasz, Albert Einstein College of Medicine, Inc. |
BMI: Body mass index; CHALO: Child Health Action to Lower Oral Health and Obesity; CHIP: Children's Health Insurance Program; dfs: decayed and filled surface (primary); dmfs:decayed missing and filled surfaces (primary); dmft: decayed missing and filled teeth (primary); IHS: Indigenous Health Service; MaXED: Maternal Consumption of Xylitol to Reduce Early Childhood Decay
Differences between protocol and review
We have extended the participant inclusion criteria review beyond 'new mothers' only, to include primary carers (e.g. fathers and grandmothers) in the first year of life. The decision was made to ensure the review was relevant and inclusive of all primary carers. We did not search specific congress websites (e.g. the American Association for Dental Research (AADR) and International Association for Dental Research (IADR)). We did not include one of the infant/child secondary outcomes specified in the protocol, dental general anaesthetics, in the review, as receipt of general anaesthetics for caries is indistinguishable from receipt of general anaesthetics for other types of dental treatment (e.g. for trauma).
Contributions of authors
For this review, Elisha Riggs and Judith Gomersall assessed the citations and studies found for inclusion, extracted data and assessed risk of bias and certainty of evidence using the GRADE approach. Judith Gomersall led the data analysis, with Elisha Riggs checking data entry in RevMan. Judith Gomersall and Elisha Riggs wrote the first draft of the review. Nicky Kilpatrick assisted when a third assessor was required and provided clinical oversight. All other review authors (Linda Slack‐Smith, Barbara Chadwick, Muthu Murugan and Jane Yelland) assisted with data interpretation and edited and commented on the draft review.
Sources of support
Internal sources
School of Dentistry, The University of Manchester, UK.
External sources
-
National Insitute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Oral Health Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and the Swiss Society for Endodontology, Switzerland
Declarations of interest
Elisha Riggs: none to declare Nicky Kilpatrick: none to declare Linda Slack‐Smith: none to declare Barbara Chadwick: none to declare Jane Yelland: none to declare Murugan Muthu: none to declare Judith Gomersall: none to declare
Edited (no change to conclusions)
References
References to studies included in this review
Birungi 2015 {published data only}
- Birungi N, Fadnes L, Kasangaki A, Nankabirwa V, Okullo I, Lie S, et al. the PROMISE‐EBF study group. Assessing casual effects of early life‐course factors on early caries in 5‐year‐old Ugandan children using directed acyclic graphs (DAGs): a prospective cohort study. Community Dentistry and Oral Epidemiology 2017;45:512‐21. [DOI] [PubMed] [Google Scholar]
- Birungi N, Fadnes LT, Okullo I, Kasangaki A, Nankabirwa V, Ndeezi G, et al. Effect of breastfeeding promotion on early childhood caries and breastfeeding duration among 5 year old children in Eastern Uganda: a cluster randomized trial. PlOS One 2015;10:e0125352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00397150. PROMISE EBF: safety and efficacy of exclusive breastfeeding in the ERA of HIV in Sub‐Saharan Africa. clinicaltrials.gov/ct2/show/NCT00397150 (first received 8 November 2006).
Chaffee 2013 {published data only}
- Chaffee BW, Feldens CA, Vitolo MR. Caries prevention through healthcare worker training: a randomized controlled trial [abstract]. General Session of the International Association for Dental Research; 2012, Jun 20‐23; Iguacu Falls, Brazil. 2012:Abstract no: 2988.
- Chaffee BW, Feldens CA, Vitolo MR. Cluster‐randomized trial of infant nutrition training for caries prevention. Journal of Dental Research 2013;92:29S‐36S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee BW, Vitolo MR, Feldens CA. The Porto Alegre early life nutrition and health study. Revista brasileira de epidemiologia [Brazilian journal of epidemiology] 2014;17:1015‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00635453. Impact of the "Ten steps for healthy feeding of children younger than two years" in health centres. clinicaltrials.gov/ct2/show/NCT00635453 (first received 13 March 2008).
Dasanayake 1993 {published data only}
- Dasanayake AP, Caufield PW, Cutter GR, Stiles HM. Transmission of mutans streptococci to infants following short term application of an iodine‐NaF solution to mothers' dentition. Community Dentistry and Oral Epidemiology 1993;21:136‐42. [DOI] [PubMed] [Google Scholar]
Dasanayake 2002 {published data only}
- Dasanayake AP, Wiener HW, Li Y, Vermund SH, Caufield PW. Lack of effect of chlorhexidine varnish on Streptococcus mutans transmission and caries in mothers and children. Caries Research 2002;36:288‐93 Erratum in Caries Research 2010;44(5):508 [Note: Vermund, S V [corrected to Vermund, S H]]. [DOI] [PubMed] [Google Scholar]
Feldens 2007 {published data only}
- Feldens CA, Giugliani ER, Duncan BB, Drachler Mde L, Vitolo MR. Long‐term effectiveness of a nutritional program in reducing early childhood caries: a randomized trial. Community Dentistry and Oral Epidemiology 2010;38:324‐32. [DOI] [PubMed] [Google Scholar]
- Feldens CA, Vitolo MR, Drachler Mde L. A randomized trial of the effectiveness of home visits in preventing early childhood caries. Community Dentistry and Oral Epidemiology 2007;35:215‐23. [DOI] [PubMed] [Google Scholar]
- John J. Home visits for dietary advice reduce caries. Evidence‐Based Dentistry 2008;9:11. [DOI] [PubMed] [Google Scholar]
- NCT00629629. Impacts of the 10 steps for healthy feeding in infants: a randomized field trial. clinicaltrials.gov/show/NCT00629629 (first received 6 March 2008).
- Vitolo MR, Bortolini GA, Feldens CA, Drachler Mde L. Impacts of the 10 steps to healthy feeding in infants: a randomized field trial. Cadernos de Saude Publica 2005;21:1448‐57. [DOI] [PubMed] [Google Scholar]
Hallas 2015 {published data only}
- Hallas D, Fernandez JB, Lim LJ, Catapano P, Dickson SK, Blouin KR, et al. OHEP: an oral health education program for mothers of newborns. Journal of Pediatric Healthcare 2015;29:181‐90. [DOI] [PubMed] [Google Scholar]
Harrison 2012 {published data only}
- Harrison R, Veronneau J, Leroux B. Design and implementation of a dental caries prevention trial in remote Canadian Aboriginal communities. Trials 2010;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RL, Veronneau J, Leroux B. Effectiveness of maternal counselling in reducing caries in Cree children. Journal of Dental Research 2012;91:1032‐7. [DOI] [PubMed] [Google Scholar]
- ISRCTN41467632. Dental caries prevention program for Cree mothers and infants. isrctn.com/ISRCTN41467632 (first received 29 June 2004).
- NCT00175318. Testing a tooth decay prevention program with Cree mothers and infants. clinicaltrials.gov/ct2/show/NCT00175318 (first received 15 September 2005).
- Veronneau J, Harrison R, Leroux B. Caries status of children in a randomized prevention trial [abstract]. 89th General Session of the International Association for Dental Research; 2011, Mar 16‐19; San Diego, California, United States. 2011:Abstract no: 352.
Kramer 2001 {published data only}
- ISRCTN37687716. PROmotion of breastfeeding intervention trial. www.isrctn.com/ISRCTN37687716 (first received 25 February 2005).
- Kramer M, Chalmers B, Hodnett E, Sevkovskaya H, Dzikovich I, Shapiro S, et al. PROmotion of Breastfeeding Intervention Trial (PROBIT): a randomised trial in the Republic of Belarus. Journal of the American Medical Association 2001;285(4):413‐9. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Vanilovich I, Matush L, Bogdanovich N, Zhang X, Shishko G, et al. The effect of prolonged and exclusive breast‐feeding on dental caries in early school‐age children. new evidence from a large randomized trial. Caries Research 2007;41:484‐8. [DOI] [PubMed] [Google Scholar]
Lapinleimu 1995 {published data only}
- Karjalainen S, Sewon L, Soderling E, Lapinleimu H, Seppanen R, Simell O. Oral health of 3‐year‐old children and their parents after 29 months of child‐focused antiatherosclerotic dietary intervention in a prospective randomized trial. Caries Research 1997;31:180‐5. [DOI] [PubMed] [Google Scholar]
- Lapinleimu H, Viikari J, Joinen E, Salo P, Routi T, Leino A, et al. Prospective randomised trial in 1062 infants of diet low in saturated fat and cholesterol. Lancet 1995;345:471‐6. [DOI] [PubMed] [Google Scholar]
Muhoozi 2017 {published data only}
- Muhoozi G, Atukunda P, Diep L, Mwadime R, Kaaya A, Skaare A, et al. Nutrition, hygiene, and stimulation education to improve growth, cognitive, language, and motor development among infants in Uganda: a cluster randomised trial. Maternal and Child Nutrition 2017;14e12527:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhoozi G, Atukunda P, Skaare A, Willumsen T, Diep L, Westerberg A, et al. Effects of nutrition and hygiene education on oral health and growth among toddlers in rural Uganda: follow‐up of a cluster randomised controlled trial. Tropical Medicine and International Health 2018;23(4):391‐404. [DOI] [PubMed] [Google Scholar]
- NCT02098031. Improving the nutrition status of infants in south‐western Uganda. clinicaltrials.gov/ct2/show/NCT02098031 (first received 27 March 2014).
Plutzer 2008 {published data only}
- Lucey SM. Oral health promotion initiated during pregnancy successful in reducing early childhood caries. Evidence‐based Dentistry 2009;10:100‐1. [DOI] [PubMed] [Google Scholar]
- Plutzer K. Long‐term effect of randomised trial to prevent childhood caries [abstract]. 89th General Session of the International Association for Dental Research; 2011, Mar 16‐19; San Diego, California, United States. 2011:Abstract no: 1788.
- Plutzer K, Keirse MJ. Incidence and prevention of early childhood caries in one‐ and two‐parent families. Child 2011;37:5‐10. [DOI] [PubMed] [Google Scholar]
- Plutzer K, Keirse MJ. Influence of an intervention to prevent early childhood caries initiated before birth on children's use of dental services up to 7 years of age. Open Dentistry Journal 2014;8:104‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutzer K, Spencer AJ. Efficacy of an oral health promotion intervention in the prevention of early childhood caries. Community Dentistry and Oral Epidemiology 2008;36:335‐46. [DOI] [PubMed] [Google Scholar]
- Plutzer K, Spencer AJ, Keirse MJ. How first‐time mothers perceive and deal with teething symptoms: a randomized controlled trial. Child 2012;38:292‐9. [DOI] [PubMed] [Google Scholar]
- Plutzer K, Spencer AJ, Keirse MJ. Reassessment at 6‐7 years of age of a randomized controlled trial initiated before birth to prevent early childhood caries. Community Dentistry and Oral Epidemiology 2012;40:116‐24. [DOI] [PubMed] [Google Scholar]
- Ramos‐Gomez F. Early maternal exposure to children’s oral health may be correlated with lower early childhood caries prevalence in their children [Discussion of Plutzer K, Spencer AJ, Keirse M. Reassessment at 6‐7 years of age of a randomized controlled trial initiated before birth to prevent early childhood caries. Community Dentistry and Oral Epidemiology 2011 Oct 24]. Journal of Evidence Based Dental Practice 2012;12(2):113‐5. [Google Scholar]
Robertson 2013 {published data only}
- Robertson LD, Phipps KR, Oh J, Loesche WJ, Kaciroti N, Symington JM. Using chlorhexidine varnish to prevent early childhood caries in American Indian children. Journal of Public Health Dentistry 2013;73:24‐31. [DOI] [PubMed] [Google Scholar]
Soderling 2000 {published data only}
- Chestnutt I. Does chewing xylitol gum result in suppression of mutans streptococci?. Evidence‐based Dentistry 2003;4:8. [Google Scholar]
- Isokangas P, Soderling E, Pienihakkinen K, Alanen P. Occurence of dental decay in children after maternal consumption of xylitol chewing gum, a follow‐up from 0 to 5 years of age. Journal of Dental Research 2000;79(11):1885‐9. [DOI] [PubMed] [Google Scholar]
- Soderling E, Isokangas P, Pienihakkinen K, Tenovuo J. Infuence of maternal xylitol consumption on acquisition of mutans streptococci by infants. Journal of Dental Research 2000;79(3):882‐7. [DOI] [PubMed] [Google Scholar]
- Söderling EP, Isokangas P, Pienihäkkinen KJ, Tenovuo J. Influence of maternal xylitol consumption on mother‐child transmission of mutans streptococci: 6‐year follow‐up. Caries Research 2001;35:173‐7. [DOI] [PubMed] [Google Scholar]
Thorild 2003 {published data only}
- Thorild I, Lindau B, Twetman S. Caries in 4‐year‐old children after maternal chewing of gums containing combinations of xylitol, sorbitol, chlorhexidine and fluoride. European Archives of Paediatric Dentistry 2006;7:241‐5. [DOI] [PubMed] [Google Scholar]
- Thorild I, Lindau B, Twetman S. Long‐term effect of maternal xylitol exposure on their children's caries prevalence. European Archives of Paediatric Dentistry 2012;13:305‐7. [DOI] [PubMed] [Google Scholar]
- Thorlid I, Lindau B, Twetman S. Effect of maternal use of chewing gums containing xylitol, chlorhexidine or fluoride on mutans streptococci colonization in the mothers' infant children. Oral Health and Preventative Dentistry 2003;1:53‐7. [PubMed] [Google Scholar]
- Thorlid I, Lindau B, Twetman S. Salivary mutans streptococci and dental caries in three‐year‐old children after maternal exposure to chewing gums containing combinations of xylitol, sorbitol, chlorhexidine, and fluoride. Acta Odontological Scandinavica 2004;62:245‐50. [`] [DOI] [PubMed] [Google Scholar]
Veronneau 2010 {published data only}
- Allison PJ, Veronneau J, Shapiro S, Platt RW. Caries risk reduction through an early childhood caries‐prevention program. IADR General Session; 2010, Jul 14‐17; Barcelona, Spain. 2010:1550.
- Veronneau J, Allison PJ, Shapiro S, Platt RW. Long‐term effect of a caries‐prevention program delivered in early childhood. IADR General Session; 2010, July 14‐17; Barcelona, Spain. 2010:181.
Watt 2009 {published data only}
- ISRCTN5550035. Promoting recommended infant feeding practices in a low income sample ‐ randomised controlled trial of a peer support intervention. https://doi.org/10.1186/ISRCTN55500035 (first received 9 July 2007).
- Scheiwe A, Hardy R, Watt RG. Four‐year follow‐up of a randomized controlled trial of a social support intervention on infant feeding practices. Maternal & Child Nutrition 2010;6:328‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt RG, McGlone P, Russell JJ, Tull KI, Dowler E. The process of establishing, implementing, and maintaining a social support feeding programme. Public Health Nutrition 2006;9(6):714‐21. [DOI] [PubMed] [Google Scholar]
- Watt RG, Tull KI, Hardy R, Wiggins M, Kelly Y, Molloy B, et al. Effectiveness of a social support intervention on infant feeding practices: randomised controlled trial. Journal of Epidemiology and Community Health 2009;63(2):156‐62. [DOI] [PubMed] [Google Scholar]
Zanata 2003 {published data only}
- Zanata RL, Navarro MF, Pereira JC, Franco EB, Lauris JR, Barbosa SH. Effect of caries preventive measures directed to expectant mothers on caries experience in their children. Brazilian Dental Journal 2003;14:75‐81. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abanto 2012 {published data only}
- Abanto J, Rezende KM, Carvalho TS, Correa FN, Vilela T, Bonecker M, et al. Effectiveness of tooth wipes in removing babies' dental biofilm. Oral Health & Preventive Dentistry 2012;10:319‐26. [PubMed] [Google Scholar]
Adams 2017 {published data only}
- Adams SH, Gregorich SE, Rising SS, Hutchison M, Chung LH. Integrating a nurse‐midwife‐led oral health intervention into Centering Pregnancy prenatal care: results of a pilot study. Journal of Midwifery & Women's Health 2017;62(4):463‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alamoudi 2012 {published data only}
- Alamoudi NM, Hanno AG, Almushayt AS, Masoud MI, Ashiry EA, Derwi DA. Early prevention of childhood caries with maternal xylitol consumption. Saudi Medical Journal 2014;35:592‐7. [PubMed] [Google Scholar]
- Alamoudi NM, Hanno AG, Sabbagh HJ, Masoud MI, Almushayt AS, Derwi DA. Impact of maternal xylitol consumption on mutans streptococci, plaque and caries levels in children. Journal of Clinical Pediatric Dentistry 2012;37:163‐6. [DOI] [PubMed] [Google Scholar]
- NCT02036151. Impact of maternal xylitol consumption on mutans streptococci, plaque and caries levels in children. clinicaltrials.gov/show/NCT02036151 (first received 14 January 2014).
Al Khamis 2017 {published data only}
- Al Khamis S, Asimakopoulou K, Newton T, Daly B. The effect of dental health education on pregnant women's adherence with toothbrushing and flossing ‐ a randomized control trial. Community Dentistry and Oral Epidemiology 2017;45:469‐77. [DOI] [PubMed] [Google Scholar]
- Al Khamis SS, Asimakopoulou K, Newton T, Daly B. Dental health education for pregnant women: randomised controlled trial [abstract]. 6th General Session of the Pan European Region of the IADR; 2012, Sep 12‐15; Helsinki, Finland. 2012:Abstract no: 199.
Bahri 2015 {published data only}
- Bahri N, Tohidinik HR, Bahri N, Iliati HR, Moshki M, Darabi F. Educational intervention to improve oral health beliefs and behaviors during pregnancy: a randomized‐controlled trial. Journal of the Egyptian Public Health Association 2015;90:41‐5. [DOI] [PubMed] [Google Scholar]
Bergel 2010 {published data only}
- Bergel E, Gibbons L, Rasines MG, Luetich A, Belizan JM. Maternal calcium supplementation during pregnancy and dental caries of children at 12 years of age: follow‐up of a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2010;89:1396‐402. [DOI] [PubMed] [Google Scholar]
- Bergel E, Gibbons L, Rasines MG, Luetich A, Belizan JM. Maternal calcium supplementation during pregnancy and dental caries of children at 12 years of age: follow‐up of a randomized controlled trial. Obstetrical & Gynecological Survey 2011;66:130‐1. [DOI] [PubMed] [Google Scholar]
Brambilla 1998 {published data only}
- Brambilla E, Felloni A, Gagliani M, Malerba A, Garcia‐Godoy F, Strohmenger L. Caries prevention during pregnancy: results of a 30‐month study. Journal of the American Dental Association 1998;129:871‐7. [DOI] [PubMed] [Google Scholar]
Cardoso 2018 {published data only}
- Cardoso CAB, Santos NM, Fracasso MLC, Provenzano MGA, Oliveira TM, Rios D. Dental plaque disclosure as an auxiliary method for infants' oral hygiene. European Archives of Paediatric Dentistry 2018;19(3):139‐45. [DOI] [PubMed] [Google Scholar]
Cibulka 2011 {published data only}
- Cibulka NJ, Forney S, Goodwin K, Lazaroff P, Sarabia R. Improving oral health in low‐income pregnant women with a nurse practitioner‐directed oral care program. Journal of the American Academy of Nurse Practitioners 2011;23:249‐57. [DOI] [PubMed] [Google Scholar]
Cockburn 1980 {published data only}
- Cockburn F, Belton NR, Purvis RJ, Giles MM, Brown JK, Turner TL, et al. Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. British Medical Journal 1980;281:11‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curnow 2002 {published data only}
- Curnow MM, Pine CM, Burnside G, Nicholson JA, Chesters RK, Huntington E. A randomised controlled trial of the efficacy of supervised toothbrushing in high‐caries‐risk children. Caries Research 2002;36:294‐300. [DOI] [PubMed] [Google Scholar]
Geisinger 2014 {published data only}
- Geisinger ML, Geurs NC, Bain JL, Kaur M, Vassilopoulos PJ, Cliver SP, et al. Oral health education and therapy reduces gingivitis during pregnancy. Journal of Clinical Periodontology 2014;41:141‐8. [DOI] [PubMed] [Google Scholar]
George 2018 {published data only}
- ACTRN12001271897. Improving maternal and infant outcomes: a multicentre randomised controlled trial of midwifery and dental intervention. www.anzctr.org.au/ACTRN12612001271897.aspx (first received 29 November 2012).
- George A, Dahlen HG, Blinkhorn A, Ajwani S, Bhole S, Ellis S, et al. Evaluation of a midwifery initiated oral health‐dental service program to improve oral health and birth outcomes for pregnant women: a multi‐centre randomised controlled trial. International Journal of Nursing Studies 2018;82:49‐57. [DOI] [PubMed] [Google Scholar]
- George A, Duff M, Johnson M, Dahlen H, Blinkhorn A, Ellis S, et al. Piloting of an oral health education programme and knowledge test for midwives. Contemporary Nurse 2014;46(2):180‐6. [DOI] [PubMed] [Google Scholar]
- Johnson MGA, Dahlen H, Ajwani S, Bhole S, Blinkhorn A, Ellis S, et al. The midwifery initiated oral health‐dental service protocol: an intervention to improve oral health outcomes for pregnant women. BMC Oral Health 2015;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomez 2001 {published data only}
- Gomez SS, Emilson CG, Weber AA, Uribe S. Prolonged effect of a mother‐child caries preventive program on dental caries in the permanent 1st molars in 9 to 10‐year‐old children. Acta Odontologica Scandinavica 2007;65:271‐4. [DOI] [PubMed] [Google Scholar]
- Gomez SS, Weber AA. Effectiveness of a caries preventive program in pregnant women and new mothers on their offspring. International Journal of Paediatric Dentistry 2001;11:117‐22. [DOI] [PubMed] [Google Scholar]
Harjunmaa 2016 {published data only}
- Harjunmaa U, Järnstedt J, Dewey KG, Ashorn U, Maleta K, Vosti SA, et al. Nutrient supplementation may adversely affect maternal oral health ‐‐ a randomised controlled trial in rural Malawi. Maternal & Child Nutrition 2016;12:99‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hillman 1962 {published data only}
- Hillman RW, Cabaud PG, Schenone RA. The effects of pyridoxine supplements on the dental caries experience of pregnant women. American Journal of Clinical Nutrition 1962;10:512‐5. [DOI] [PubMed] [Google Scholar]
Holt 1985 {published data only}
- Holt R, Winter G, Fox B, Askew R, Lo G. Dental health education through home visits to mothers with young children. Community Dentistry and Oral Epidemiology 1983;11:98‐101. [DOI] [PubMed] [Google Scholar]
- Holt RD, Winter GB, Fox B, Askew R. Effects of dental health education for mothers with young children in London. Community Dentistry and Oral Epidemiology 1985;13:148‐51. [DOI] [PubMed] [Google Scholar]
- Holt RD, Winter GB, Fox B, Askew R. Second assessment of London children involved in a scheme of dental health education in infancy. Community Dentistry and Oral Epidemiology 1989;17:180‐2. [DOI] [PubMed] [Google Scholar]
Jiang 2015 {published data only}
- Jiang H, Xiong X, Buekens P, Su Y, Qian X. Use of mouth rinse during pregnancy to improve birth and neonatal outcomes: a randomized controlled trial. BMC Pregnancy and Childbirth 2015;15:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joury 2016 {published data only}
- Joury E, Alghadban M, Elias K, Bedi R. Impact of providing free preventive dental products without health workers' counselling on infants' tooth‐brushing and bottle‐feeding termination practices: a randomised controlled trial. Community Dental Health 2016;33:213‐7. [DOI] [PubMed] [Google Scholar]
Karanja 2012 {published data only}
- Karanja N, Aickin M, Lutz T, Mist S, Jobe JB, Maupomé G, Ritenbaugh C. A community‐based intervention to prevent obesity beginning at birth among American Indian children: study design and rationale for the PTOTS study. Journal of Primary Prevention 2012;33(4):161‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohler 1983 {published data only}
- Kohler B, Bratthall D, Krasse B. Preventive measures in mothers influence the establishment of the bacterium Streptococcus mutans in their infants. Archives of Oral Biology 1983;28:225‐31. [DOI] [PubMed] [Google Scholar]
Kowash 2000 {published data only}
- Kowash MB, Pinfield A, Smith J, Curzon ME. Effectiveness on oral health of a long‐term health education programme for mothers with young children. British Dental Journal 2000;188:201‐5. [DOI] [PubMed] [Google Scholar]
- Kowash MB, Toumba KJ, Curzon ME. Cost‐effectiveness of a long‐term dental health education program for the prevention of early childhood caries. European Archives of Paediatric Dentistry 2006;7:130‐5. [DOI] [PubMed] [Google Scholar]
- Stillman‐Lowe C. Oral health – educating mothers with young children. British Dental Journal 2000;188:199. [Google Scholar]
Kraivaphan 2007 {published data only}
- Kraivaphan P, Amornchat C, Triratana T. Effects of a triclosan dentifrice on plaque formation, gingivitis and gingival bleeding in pregnant women: five‐month clinical results. Southeast Asian Journal of Tropical Medicine and Public Health 2007;38:594‐7. [PubMed] [Google Scholar]
- Kraivaphan P, Amornchat C, Triratana T, Leethochawalit U. Clinical effect of a triclosan containing dentifrice on gingivitis during pregnancy and post‐partum. Southeast Asian Journal of Tropical Medicine and Public Health 2006;37:820‐5. [PubMed] [Google Scholar]
Leverett 1997 {published data only}
- Leverett DH, Adair SM, Vaughan BW, Proskin HM, Moss ME. Randomized clinical trial of the effect of prenatal fluoride supplements in preventing dental caries. Caries Research 1997;31:174‐9. [DOI] [PubMed] [Google Scholar]
Lopez 2002 {published data only}
- López NJ, Silva I, Ipinza J, Gutiérrez J. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy‐associated gingivitis. Journal of Periodontology 2005;76:2144‐53. [DOI] [PubMed] [Google Scholar]
- López NJ, Smith P, Gutierrez J. Periodontal therapy reduces the risk of preterm low birth weight (AADR Abstract Annual Meeting March 7‐10 2001). Journal of Dental Research 2001;80:188, Abstract no: 1223. [Google Scholar]
- López NJ, Smith PC, Gutierrez J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. Journal of Periodontology 2002;73:911‐24. [DOI] [PubMed] [Google Scholar]
Ma 2017 {published data only}
- Ma XH, Xie NN. Application of different biomaterials in the preservation of vital pulp in carious deciduous teeth: a prospective, single‐center, randomized, controlled clinical trial. Chinese Journal of Tissue Engineering Research 2017;21:3494‐500. [Google Scholar]
Macones 2010 {published data only}
- Macones GA, Parry S, Nelson DB, Strauss JF, Ludmir J, Cohen AW, et al. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS). American Journal of Obstetrics and Gynecology 2010;202:147.e1‐8. [DOI] [PubMed] [Google Scholar]
Mohebbi 2009 {published data only}
- Mohebbi SZ, Virtanen JI, Vahid‐Golpayegani M, Vehkalahti MM. A cluster randomised trial of effectiveness of educational intervention in primary health care on early childhood caries. Caries Research 2009;43:110‐8. [DOI] [PubMed] [Google Scholar]
Nakai 2010 {published data only}
- Nakai Y, Shinga‐Ishihara C, Kaji M, Moriya K, Murakami‐Yamanaka K, Takimura M. Xylitol gum and maternal transmission of Mutans Streptococci. Journal of Dental Research 2010;89(1):56‐60. [DOI] [PubMed] [Google Scholar]
NCT00719238 {published data only}
- NCT00719238. A pilot study to promote maternal and infant oral health. clinicaltrials.gov/show/NCT00719238 (first received 21 July 2008).
NCT01652300 {published data only}
- NCT01652300. The effect of oral health education in pregnancy. clinicaltrials.gov/ct2/show/NCT01652300 (first received 30 July 2012).
NCT01763138 {published data only}
- NCT01763138. Effect of educational intervention in mothers for prevention of caries in their children, a randomized controlled trial. clinicaltrials.gov/show/NCT01763138 (first received 8 January 2013).
NCT02436811 {published data only}
- NCT02436811. Oral health literacy and oral education. clinicaltrials.gov/ct2/show/NCT02436811 (first received 7 May 2015).
NCT02578966 {published data only}
- NCT02578966. Cohort zero caries (CZC): impact of preventive child oral health programs in primary health care. clinicaltrials.gov/show/NCT02578966 (first received 19 October 2015).
NCT03273725 {published data only}
- NCT03273725. Maternal vitamin D levels in pregnancy and dental caries in children. clinicaltrials.gov/show/NCT03273725 (first received 6 September 2017).
NCT03478748 {published data only}
- NCT03478748. The impact of anticipatory guidance on early childhood caries: a quasi‐experimental study. clinicaltrials.gov/show/NCT03478748 (first received 27 March 2018). [DOI] [PMC free article] [PubMed]
NCT03529500 {published data only}
- NCT03529500. Chronic malnutrition and oral health status in children aged one to five years. clinicaltrials.gov/show/NCT03529500 (first received 18 May 2018).
NCT03598972 {published data only}
- NCT03598972. The effect of prenatal vitamins on the tooth structure. clinicaltrials.gov/show/NCT03598972 (first received 25 July 2018).
NCT03693443 {published data only}
- NCT03693443. Knowledge and behaviour toward early childhood caries. clinicaltrials.gov/ct2/show/NCT03693443 (first received 3 October 2018).
Olak 2012 {published data only}
- Olak J, Saag M, Vahlberg T, Soderling E, Karjalainen S. Caries prevention with xylitol lozenges in children related to maternal anxiety. A demonstration project. European Archives of Paediatric Dentistry 2012;13:64‐9. [DOI] [PubMed] [Google Scholar]
Plonka 2013 {published data only}
- Plonka KA, Pukallus ML, Holcombe TF, Barnett AG, Walsh LJ, Seow WK. Randomized controlled trial: a randomized controlled clinical trial comparing a remineralizing paste with an antibacterial gel to prevent early childhood caries. Pediatric Dentistry 2013;35:8‐12. [PubMed] [Google Scholar]
- Pukallus ML, Plonka KA, Barnett AG, Walsh LJ, Holcombe TF, Seow WK. A randomised, controlled clinical trial comparing chlorhexidine gel and low‐dose fluoride toothpaste to prevent early childhood caries. International Journal of Paediatric Dentistry 2013;23:216‐24 [Erratum in International Journal of Paediatric Dentistry 2013, 23(5):318]. [DOI] [PubMed] [Google Scholar]
- Pukallus ML, Plonka KA, Holcombe TF, Barnett AG, Walsh LJ, Seow WK. A randomized controlled trial of a 10 percent CPP‐ACP cream to reduce mutans streptococci colonization. Pediatric Dentistry 2013;35:550‐5. [PubMed] [Google Scholar]
Ramos‐Gomez 2012 {published data only}
- Kopycka‐Kedzierawski Dorota T. Maternal salivary bacterial challenge is associated with oral infection among children and predicts early childhood caries (ECC) incidence in a high‐risk cohort of 36‐month‐old children. Journal of Evidence‐based Dental Practice 2014;14:147‐148 2. [DOI] [PubMed] [Google Scholar]
- NCT00066950. Prevention management model for early childhood caries (MAYA project). https://clinicaltrials.gov/ct2/show/NCT00066950 (first received 8 August 2003).
- Ramos‐Gomez F, Chung LH, Gonzalez Beristain R, Santo W, Jue B, Weintraub J, et al. Recruiting and retaining pregnant women from a community health center at the US‐Mexico border for the Mothers and Youth Access clinical trial. Clinical Trials 2008;5:336‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos‐Gomez F, Gansky S, Santo W, Featherstone JD, Weintraub JA. "MAYA" ‐ randomized clinical trial to prevent early childhood caries [abstract]. 39th Annual Meeting of the American Association for Dental Research and the 33rd Annual Meeting of the Canadian Association for Dental Research; 2010, Mar 3‐6; Washington DC, United States. 2010:Abstract no: 847.
- Ramos‐Gomez FJ, Gansky SA, Featherstone JD, Jue B, Gonzalez‐Beristain R, Santo W, et al. Mother and youth access (MAYA) maternal chlorhexidine, counselling and paediatric fluoride varnish randomized clinical trial to prevent early childhood caries. International Journal of Paediatric Dentistry 2012;22:169‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rivas Castillo 2014 {published data only}
- Rivas Castillo MT, Romero Sanchez M, Rosa Varez Z. Impact on quality control measures oral health in pregnant women [Impacto de medidas preventivas sobre la calidad de la salud bucodental en la gestante]. Nure Investigación 2014;11:1‐18. [Google Scholar]
Stensson 2014 {published data only}
- Stensson M, Koch G, Coric S, Abrahamsson TR, Jenmalm MC, Birkhed D, et al. Oral administration of lactobacillus reuteri during the first year of life reduces caries prevalence in the primary dentition at 9 years of age. Caries Research 2014;48:111‐7. [DOI] [PubMed] [Google Scholar]
Tenovuo 1992 {published data only}
- Tenovuo J, Hakkinen P, Paunio P, Emilson CG. Effects of chlorhexidine‐fluoride gel treatments in mothers on the establishment of mutans streptococci in primary teeth and the development of dental caries in children. Caries Research 1992;26:275‐80. [DOI] [PubMed] [Google Scholar]
Turksel 2004 {published data only}
- Turksel Dulgergil C, Satici O, Yildirim I, Yavuz I. Prevention of caries in children by preventive and operative dental care for mothers in rural Anatolia, Turkey. Acta Odontologica Scandinavica 2004;62:251‐7. [DOI] [PubMed] [Google Scholar]
Weber‐Gasparoni 2013 {published data only}
- Weber‐Gasparoni K, Reeve J, Ghosheh N, Warren J, Drake D, Kramer K, et al. An effective psycho educational intervention for early childhood caries prevention: part I. Pediatric Dentisty Vol. 35, issue 3:241‐6. [PMC free article] [PubMed]
- Weber‐Gasparoni K, Warren JJ, Reeve J, Drake D, Kramer K, Marshall T, et al. An effective psycho educational intervention for early childhood caries prevention: part II. Pediatric Dentisty 2013; Vol. 3, issue 35:247‐51.. [PMC free article] [PubMed]
Weinstein 2004 {published data only}
- Harrison R, Benton T, Everson‐Stewart S, Weinstein P. Effect of motivational interviewing on rates of early childhood caries: a randomized trial. Pediatric Dentistry 2007;29:16‐22. [PubMed] [Google Scholar]
- Milgrom P, Riedy CA, Weinstein P, Mancl LA, Garson G, Huebner CE, et al. Design of a community‐based intergenerational oral health study: "Baby Smiles". BMC Oral Health 2013;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein P, Harrison R, Benton T. Motivating mothers to prevent caries: confirming the beneficial effect of counseling. Journal of the American Dental Association 2006;137:789‐93. [DOI] [PubMed] [Google Scholar]
- Weinstein P, Harrison R, Benton T. Motivating parents to prevent caries in their young children: one‐year findings. Journal of the American Dental Association 2004;135:731‐8. [DOI] [PubMed] [Google Scholar]
- Weinstein P, Milgrom P, Riedy CA, Mancl LA, Garson G, Huebner CE, et al. Treatment fidelity of brief motivational interviewing and health education in a randomized clinical trial to promote dental attendance of low‐income mothers and children: community‐based intergenerational oral health study "Baby Smiles". BMC Oral Health 2014;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhan 2012 {published data only}
- NCT01468727. Study on use of xylitol‐wipes to prevent dental caries (WIPE). clinicaltrials.gov/show/NCT01468727 (first received 9 November 2011).
- Zhan L, Cheng J, Chang P, Ngo M, Denbesten PK, Hoover CI, et al. Effects of xylitol wipes on cariogenic bacteria and caries in young children. Journal of Dental Research 2012;91:85S‐90S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Ngo M, Hoover CI, Besten P, Featherstone JD. Effectiveness of xylitol wipes on cariogenic bacterial transmission in infants. 87th General Session and Exhibition of IADR/AADR/CADR; 2009, Apr 1‐4; Miami, Florida, United States. 2016:674.
References to studies awaiting assessment
Batra 2018 {published data only}
- Batra M, Shah A, Virtanen J. Integration of oral health in primary health care through motivational interviewing for mothers of young children: a pilot study. Journal of Indian Society of Pedodontics and Preventive Dentisry 2018;36(1):86‐92. [DOI] [PubMed] [Google Scholar]
Jamieson 2012 {published data only}
- ACTRN12611000111976. Reducing disease burden and health inequalities arising from chronic dental disease among Indigenous children: an early childhood caries intervention. anzctr.org.au/ACTRN12611000111976.aspx (first received 20 December 2010).
- Broughton JR, Maipi JT, Person M, Thomson WM, Morgaine KC, Tiakiwai S, et al. Reducing disease burden and health inequalities arising from chronic disease among indigenous children: an early childhood caries intervention in Aotearoa/New Zealand. BMC Public Health 2013; Vol. 13:1177. [DOI] [PMC free article] [PubMed]
- Broughton JR, Person M, Maipi JT, Cooper‐Te KR, Smith‐Wilkinson A, Tiakiwai S, et al. Ukaipo niho: the place of nurturing for oral health. New Zealand Dental Journal 2014:18‐23. [PubMed]
- Jamieson L, Bradshaw J, Lawrence H, Broughton J, Venner K. Fidelity of motivational Interviewing in an early childhood caries intervention Involving Indigenous Australian mothers. Journal of Health Care for the Poor and Underserved 2016; Vol. 27:125–38.. [DOI] [PubMed]
- NCT02151916. Preventing early childhood caries in indigenous children: the Baby Teeth Talk study (BTT). clinicaltrials.gov/show/NCT02151916 (first received 2 June 2014).
- Smithers LG, Lynch J, Hedges J, Jamieson L. Diet and anthropometry at 2 years of age following an oral health promotion programme for Australian Aboriginal children and their carers: a randomised controlled trial. British Journal of Nutrition 2017;118:1061‐9. [DOI] [PubMed] [Google Scholar]
Klastersky Genot 1970 {published data only}
- Klastersky‐Genot MT. Effects of tetracycline, administered during pregnancy, on the deciduous teeth. A double blind controlled study. Acta Stomatological Belgica 1970; Vol. 67, issue 1:107‐24. [PubMed]
Ratte 1969 {published data only}
- Ratte H, Uhlig H. Results of a long‐term trial for intrauterine dental caries prevention. Die Medizinische Welt 1969;36:1987‐8. [PubMed] [Google Scholar]
References to ongoing studies
Arrow 2013 {published data only}
- ACTRN126110009997954. Early childhood oral health promotion: brief motivational interviewing intervention among parents of new‐born children to reduce early childhood caries. www.anzctr.org.au/ACTRN12611000997954.aspx (first received 16 September 2011).
- Arrow P, Raheb J, Miller M. Brief oral health promotion intervention among parents of young children to reduce early childhood dental decay. BMC Public Health 2013;13:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batliner 2014 {published data only}
- Batliner T, Fehringer KA, Tiwari T, Henderson WG, Wilson A, Brega AG, et al. Motivational interviewing with American Indian mothers to prevent early childhood caries: study design and methodology of a randomized control trial. Trials 2014;15:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01116726. Promoting behavioral change for oral health in American Indian mothers and children. clinicaltrials.gov/show/NCT01116726 (first received 5 May 2010).
NCT00066040 {published data only}
- NCT00066040. Prevention of transmission of bacteria that cause cavities from mothers to their children. clinicaltrials.gov/show/NCT00066040 (first received 5 August 2003).
NCT00067340 {published data only}
- NCT00067340. Northwest Alaska Center to Reduce Oral Health Disparity project 2: caries transmission prevention in Alaska native infants. clinicaltrials.gov/show/NCT00067340 (first received 20 August 2003).
NCT01038479 {published data only}
- NCT01038479. Maternal consumption of xylitol to reduce early childhood decay (MaXED study). clinicaltrials.gov/show/NCT01038479 (first received 24 December 2009).
NCT01502566 {published data only}
- NCT01502566. A cluster‐randomized trial of the effectiveness of an educational intervention in preventing early childhood caries. clinicaltrials.gov/show/NCT01502566 (first received 30 December 2011).
NCT02937194 {published data only}
- NCT02937194. Family‐centered oral health promotion for new parents. ClinicalTrials.gov/show/NCT02937194 (first received 18 October 2016).
NCT03077425 {published data only}
- NCT03077425. Obesity and caries in young South Asian children: a common risk factor approach (CHALO). ClinicalTrials.gov/show/NCT03077425 (first received 13 March 2017).
Additional references
AIHW 2016
- Australian Institute of Health and Welfare. Oral health and dental care in Australia. Key facts and figures 2015. Canberra: AIHW; 2016, Dental Statistics and Research Series. Cat. no. DEN 229 2016.
Albino 2016
- Albino J, Tiwari T. Preventing childhood caries: a review of recent behavioral research. Journal of Dental Research 2016;95(1):35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alkarimi 2014
- Alkarimi HA, Watt RG, Pikhart H, Sheiham A, Tsakos G. Dental caries and growth in school‐age children. Pediatrics 2014;133(3):e616‐23. [DOI] [PubMed] [Google Scholar]
Alsharif 2016
Ayele 2013
- Ayele FA, Taye BW, Ayele TA, Gelaye KA. Predictors of dental caries among children 7‐14 years old in Northwest Ethiopia: a community based cross‐sectional study. BMC Oral Health 2013;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bagramian 2009
- Bagramian RA, Garcia‐Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. American Journal of Dentistry 2009;22(1):3‐8. [PubMed] [Google Scholar]
Blumenshine 2008
- Blumenshine SL, Vann WFJ, Gizlice Z, Lee JY. Children's school performance: impact of general and oral health. Journal of Public Health Dentistry 2008;68(2):82‐7. [DOI] [PubMed] [Google Scholar]
Calvasina 2015
- Calvasina P, Muntaner C, Quinonez C. The deterioration of Canadian immigrants' oral health: analysis of the Longitudinal Survey of Immigrants to Canada. Community Dentistry and Oral Epidemiology 2015;43:424‐32. [DOI] [PubMed] [Google Scholar]
Casamassimo 2009
- Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E. Beyond the dmft: the human and economic cost of early childhood caries. Journal of the American Dental Assocication 2009;140(6):650‐7. [DOI] [PubMed] [Google Scholar]
Caufield 1979
- Caufield PW, Gibbons RJ. Suppression of Streptococcus mutans in the mouths of humans by a dental prophylaxis and tropically applied iodine. Journal of Dental Research 1979;58:1317‐26. [DOI] [PubMed] [Google Scholar]
Chaffee 2014
- Chaffee BW, Feldens CA, Vitolo MR. Association of long duration breastfeeding and dental caries estimated with marginal structural models. Annals of Epidemiology 2014;24:448‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chandler 2013
- Chandler J, Churchill R, Higgins J, Lasserson T, Tovey D. Methodological standards for the conduct of new Cochrane Intervention Reviews. Version 2.3. editorial‐unit.cochrane.org/mecir 2013.
Chen 2019
- Chen, KJ, Gao SS, Duangthip D, Lo ECM, Chu CH. Prevalence of early childhood caries among 5‐year‐old children: a systematic review. Journal of Investigative and Clinical Dentistry 2019;10(1):e12376. [DOI] [PubMed] [Google Scholar]
Cohen 2017
- Cohen LC, Dahlen G, Escobar A, Fejerskov O, Johnson NW, Manji F. Dentistry in crisis: time to change. La Cascada declaration. Australian Dental Journal 2017;62(3):258‐60. [PUBMED: 28793371] [DOI] [PubMed] [Google Scholar]
Colak 2013
- Colak H, Coruh T, Dulgergil MD, Hamidi MM. Early childhood caries update: a review of causes, diagnoses and treatments. Journal of Natural Science, Biology and Medicine 2013;4:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2003
- Davies GM, Worthingon HV, Ellwood RP, Blinkhorn AS, Taylor GO, Davies RM, et al. An assessment of the cost effectiveness of a postal toothpaste programme to prevent caries among five‐year‐old children in the North West of England. Community Dental Health 2003;20:207‐10. [PubMed] [Google Scholar]
Divaris 2011
- Divaris K, Lee JY, Baker AD, Vann WF. Caregivers' oral health literacy and their young children's oral health‐related quality‐of‐life. Acta Odontologica Scandinavica 2011;70(5):390‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dye 2015
- Dye B, Thornton‐Evans G, Li X, Iafolla T. Dental caries and sealant prevalence in children and adolescents in the United States, 2011–2012. www.cdc.gov/nchs/products/databriefs/db191.htm (accessed prior to 3 October 2019).
Edelstein 2006
- Edelstein BL. The dental caries pandemic and disparities problem. BMC Oral Health 2006;6 Suppl 1:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldens 2010
- Feldens CA, Giugliani ERJ, Vigo A, Vitolo MR. Early feeding practices and severe early childhood caries in four‐year‐old children from southern Brazil: a birth cohort study. Caries Research 2010;44:445‐52. [DOI] [PubMed] [Google Scholar]
Filstrup 2003
- Filstrup SL, Briskie D, Fonseca M, Lawrence L, Wandera A, Inglehart MR. Early childhood caries and quality of life: child and parent perspectives. Pediatric Dentistry 2003;25(5):431‐40. [PubMed] [Google Scholar]
Finlayson 2007
- Finlayson TL, Siefert K, Ismail AI, Sohn W. Maternal self‐efficacy and 1‐5‐year‐old children's brushing habits. Community Dentistry and Oral Epidemiology 2007;35(4):272‐81. [DOI] [PubMed] [Google Scholar]
Fisher‐Owens 2007
- Fisher‐Owens SA, Gansky SA, Platt LJ, Weintraub JA, Soobader MJ, Bramlett MD, et al. Influences on children's oral health: a conceptual model. Pediatrics 2007;120(3):e510‐20. [DOI] [PubMed] [Google Scholar]
Giacaman 2018
- Giacaman R. Sugars and beyond. The role of sugars and the other nutrients and their potential impact on caries. Oral Diseases 2018;24(7):1185‐97. [DOI] [PubMed] [Google Scholar]
Gilchrist 2015
- Gilchrist F, Marshman Z, Deery C, Rodd HD. The impact of dental caries on children and young people: what they have to say?. International Journal of Paediatric Dentistry 2015;25(5):327‐38. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gyll 2018
- Gyllo J, Ridell K, Ohlund I, Akeson PK, Johansson I, Holgerson PL. Vitamin D status and dental caries in healthy Swedish children. Nutrition Journal 2018;17(11):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2004
- Harris R, Nicoll AD, Adair PM, Pine CM. Risk factors for dental caries in young children: a systematic review of the literature. Community Dental Health 2004;21:71‐85. [PubMed] [Google Scholar]
Harrison 2010
- Harrison R, Véronneau J, Leroux B. Design and implementation of a dental caries prevention trial in remote Canadian Aboriginal communities. Trials 2010;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hausen 2001
- Hausen H, Kärkkäinen S, Seppä L. Caries data collected from public health records compared with data based on examinations by trained examiners. Caries Research 2001;35:360–5. [DOI] [PubMed] [Google Scholar]
Henry 2017
- Henry JA, Muthu MS, Swaminathan K, Kirubakaran R. Do oral health educational programmes for expectant mothers prevent early childhood caries? A systematic review. Oral Health and Preventive Dentistry 2017;15(3):215‐21. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins J, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hong 2009
- Hong L, Levy SM, Warren JJ, Broffitt B. Association between enamel hypoplasia and dental caries in primary second molars: a cohort study. Caries Research 2009;43(5):345‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iheozor‐Ejiofor 2017
- Iheozor‐Ejiofor Z, Middleton P, Esposito M, Glenny AM. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD005297.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1993
- Johnson Z, Howell F, Molloy B. Community mothers programme: randomised controlled trial of a non‐professional intervention in parenting. British Medical Journal 1993;306:1449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassebaum 2017
- Kassebaum NJ, Smith AGC, Bernabe E, Fleming TD, Reynolds AE, Vos T, et al. Global, regional, and national prevalence, incidence, and disability‐adjusted life years for oral conditions for 195 countries, 1990‐2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. Journal of Dental Research 2017;96(4):380‐7. [PUBMED: 28792274] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohler 2012
- Kohler B, Andreen I. Mutans streptococci and caries prevalence in children after early maternal caries prevention: a follow‐up at 19 years of age. Caries Research 2012;46(5):474‐80. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Levesque 2013
- Levesque JF, Harris MF, Russell G. Patient‐centred access to health care: conceptualising access at the interface of health systems and populations. International Journal for Equity in Health 2013;12(18):1‐9. [DOI: 10.1186/1475-9276-12-18] [DOI] [PMC free article] [PubMed] [Google Scholar]
Llena 2018
- Llena C, Calabuig E. Risk factors associated with new caries lesions in permanent first molars in children: a 5‐year historical cohort follow‐up study. Clinical Oral Investigations 2018;22(3):1579‐86. [DOI] [PubMed] [Google Scholar]
Moure‐Leite 2011
- Moure‐Leite FR, Ramos‐Jorge J, Ramos‐Jorge ML, Paiva SM, Vale MP, Pordeus IA. Impact of dental pain on daily living of five‐year‐old Brazilian preschool children: prevalence and associated factors. European Archives of Paediatric Dentistry 2011;12(6):293‐7. [DOI] [PubMed] [Google Scholar]
Moynihan 2019
- Moynihan P, Tanner LM, Holmes RD, Hillier‐Brown F, Mashayekhi A, Kelly SAM, et al. Systematic review of evidence pertaining to risk factors that modify risk of early childhood caries. Journal of Dental Research Clinical & Translational Research 2019 [Epub ahead of print]. [2380084418824262] [DOI] [PubMed]
Muthu 2015
- Muthu MS, Ankita S, Renugalakshmi A, Richard K. Review. Impact of pharmacological interventions in expectant mothers resulting in altered mutans streptococci levels in their children. Pediatric Dentistry 2015;37(5):422‐8. [PubMed] [Google Scholar]
Nowak 1995
- Nowak AJ, Casamassimo PS. Using anticipatory guidance to provide early dental intervention. Journal of the American Dental Association 1995;126:1156–63. [DOI] [PubMed] [Google Scholar]
Nuttall 2006
- Nuttall NM, Steele JG, Evans D, Chadwick B, Morris AJ, Hill K. The reported impact of oral condition on children in the United Kingdom, 2003. British Dental Journal 2006;200(10):551‐5. [DOI] [PubMed] [Google Scholar]
PAHO/WHO 2003
- PAHO/WHO 2003. Guiding principles for complementary feeding of the breastfed child. Washington D.C.: PAHO/WHO Division of health Promotion and Protection, 2003. [Google Scholar]
Pascoe 1994
- Pascoe L, Seow WK. Enamel hypoplasia and dental caries in Australian aboriginal children: prevalence and correlation between the two diseases. Pediatric Dentistry 1994;16(3):193‐9. [PubMed] [Google Scholar]
Peltzer 2015
- Peltzer K, Mongkolchati A. Severe early childhood caries and social determinants in three‐year‐old children from Northern Thailand: a birth cohort study. BMC Oral Health 2015;15:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peres 2018
- Peres KG, Chaffee BW, Feldens CA, Flores‐Mir C, Moynihan P, Rugg‐Gunn A. Breastfeeding and oral health: evidence and methodological challenges. Journal of Dental Research 2018;97(3):251‐8. [DOI] [PubMed] [Google Scholar]
Peretz 2003
- Peretz B, Ram D, Azo E, Efrat Y. Preschool caries as an indicator of future caries: a longitudinal study. Pediatric Dentistry 2003;25(2):114‐8. [PubMed] [Google Scholar]
Pine 2006
- Pine CM, Harris RV, Burnside G, Merrett MC. An investigation of the relationship between untreated decayed teeth and dental sepsis in 5‐year‐old children. British Dental Journal 2006;200(1):45‐7; discussion 29. [DOI] [PubMed] [Google Scholar]
Pitts 2001
- Pitts NB, Evans DJ, Nugent ZJ. The dental caries experience of 5‐year‐old children in Great Britain. Surveys coordinated by the British Association for the Study of Community Dentistry in 1999/2000. Community Dental Health 2001;18:49‐55. [PubMed] [Google Scholar]
Pitts 2015
- Pitts N, Chadwick B, Anderson T, Health and Social Care Information Centre. Dental disease and damage in children: England, Wales and Northern Ireland. Children’s Dental Health Survey 2013. Report 2 2015.
Reisine 2008
- Reisine S, Tellez M, Willem J, Sohn W, Ismail A. Relationship between caregiver's and child's caries prevalence among disadvantaged African Americans. Community Dentistry and Oral Epidemiology 2008;36(3):191‐200. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhangen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riggs 2016
- Riggs E, Yelland J, Shankumar R, Kilpatrick N. 'We are all scared for the baby': promoting access to dental services for refugee background women during pregnancy. BMC Pregnancy and Childbirth 2016;16:12. [PUBMED: 26794243] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riggs 2017a
- Riggs E, Rajan S, Casey S, Kilpatrick N. Refugee child oral health. Oral Diseases 2017;23(3):292‐9. [PUBMED: 27385659] [DOI] [PubMed] [Google Scholar]
Saied‐Moallemi 2008
- Saied‐Moallemi Z, Virtanen JI, Ghofranipour F, Murtomaa H. Influence of mothers' oral health knowledge and attitudes on their children's dental health. European Archives of Paediatric Dentistry 2008;9(2):79‐83. [PUBMED: 18534175] [DOI] [PubMed] [Google Scholar]
Schroth 2013
- Schroth RJ, Levi J, Kliewer E, Friel J, Moffatt ME. Association between iron status, iron deficiency anaemia, and severe early childhood caries: a case‐control study. BMC Pediatrics 2013;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroth 2014
- Schroth RJ, Lavelle C, Tate R, Bruce S, Billings RJ, Moffatt ME. Prenatal vitamin D and dental caries in infants. Pediatrics 2014;133(5):e1277‐e84. [DOI] [PubMed] [Google Scholar]
Selwitz 2007
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369(9555):51‐9. [DOI] [PubMed] [Google Scholar]
Slack‐Smith 2011
- Slack‐Smith LM, Read AW, Colvin LJ, Leonard H, Kilpatrick N, McAullay D, et al. Total population investigation of dental hospitalizations in indigenous children under five years in Western Australia using linked data. Australian Dental Journal 2011;56(4):358‐64. [PUBMED: 22126344] [DOI] [PubMed] [Google Scholar]
Smith 2015
- Smith L, Blinkhorn A, Moir R, Brown N, Blinkhorn F. An assessment of dental caries among young Aboriginal children in New South Wales, Australia: a cross‐sectional study. BMC Public Health 2015;15(1):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takahashi 2015
- Takahashi R, Ota E, Hoshi K, Naito T, Toyoshima Y, Yuasa H, et al. Fluoride supplementation in pregnant women for preventing dental caries in the primary teeth of their children. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011850] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takahashi 2017
- Takahashi R, Ota E, Hoshi K, Naito T, Toyoshima Y, Yuasa H, et al. Fluoride supplementation (with tablets, drops, lozenges or chewing gum) in pregnant women for preventing dental caries in the primary teeth of their children. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD011850.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teanpaisan 2007
- Teanpaisan R, Thitasomakul S, Piwat S, Thearmontree A, Pithpornchaiyakul W, Chankanka O. Longitudinal study of the presence of mutans streptococci and lactobacilli in relation to dental caries development in 3‐24 month old Thai children. International Dental Journal 2007;57(6):445‐51. [DOI] [PubMed] [Google Scholar]
Tham 2015
- Tham R, Bowatte G, Dharmage SC, Tan DJ, Lau MXN, Dai X, et al. Breastfeeding and the risk of dental caries: a systematic review and meta‐analysis. Acta Paediatrica 2015;104:62‐84. [DOI] [PubMed] [Google Scholar]
Tickle 2008
- Tickle M, Blinkhorn AS, Milsom KM. The occurrence of dental pain and extractions over a 3‐year period in a cohort of children aged 3‐6 years. Journal of Public Health Dentistry 2008;68(2):63‐9. [DOI] [PubMed] [Google Scholar]
Tinanoff 2000
- Tinanoff N, Palmer CA. Dietary determinants of dental caries and dietary recommendations for preschool children. Journal of Public Health Dentistry 2000;60:197‐206. [DOI] [PubMed] [Google Scholar]
Vann 2010
- Vann W, Lee J, Baker D, Divaris K. Oral health literacy among female caregivers impact on oral health outcomes in early childhood. Journal of Dental Research 2010;89(12):1395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2003
- Wan AK, Seow WK, Purdie DM, Bird PS, Walsh LJ, Tudehope DI. A longitudinal study of Streptococcus mutans colonization in infants after tooth eruption. Journal of Dental Research 2003;82(7):504‐8. [DOI] [PubMed] [Google Scholar]
World Health Organization 1979
- World Health Organization. A Guide to Oral Health. Epidemiological Investigations. Geneva: World Health Organization, 1979. [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision‐making. Evidence‐based Dentistry 2015;16(3):69‐71. [DOI] [PubMed] [Google Scholar]
Xiao 2019
- Xiao J, Alkhers N, Kopycka‐Kedzierawski DT, Billings RJ, Wu TT, Castillo DA, et al. Prenatal oral health care and early childhood caries prevention: a systematic review and meta‐analysis. Caries Research 2019 [Epub ahead of print]:1‐11. [DOI] [PMC free article] [PubMed]
Yonezu 2006
- Yonezu T, Ushida N, Yakushiji M. Longitudinal study of prolonged breast‐ or bottle‐feeding on dental caries in Japanese children. Bulletin of Tokyo Dental College 2006;47:157–60. [DOI] [PubMed] [Google Scholar]
Yost 2008
- Yost J, Li Y. Promoting oral health from birth through childhood: prevention of early childhood caries. American Journal of Maternal Child Nursing 2008;33(1):17‐23. [DOI] [PubMed] [Google Scholar]


