Abstract
Mosquitoes are the deadliest animals on earth and are the vectors of several neglected tropical diseases. Recently, essential oils have emerged as potential renewable, cost-effective, and environmentally benign alternatives to synthetic pesticides for control of mosquitoes. In this work, thirteen species of Piper were collected from different areas of central Vietnam. The essential oils were obtained by hydrodistillation and analyzed by gas chromatography–mass spectrometry. The essential oils were screened for mosquito larvicidal activity against Aedes aegypti. Four of the Piper essential oils showed outstanding larvicidal activity against Ae. aegypti, namely P. caninum, P. longum, P. montium, and P. mutabile, with LC50 and LC90 values less than 10 µg/mL. Multivariate analysis has correlated concentrations of β-caryophyllene, β-bisabolene, α-pinene, and β-pinene with mosquito larvicidal activity.
Keywords: piperaceae, mosquito-borne diseases, natural pest control
1. Introduction
The genus Piper is the largest in the Piperaceae and is made up of around 1050 species, many of which are important in traditional medicine and as culinary spices [1]. For example, P. nigrum (black pepper) fruit is the most consumed spice in the world, P. longum (long pepper) fruiting spikes are used similarly, P. methysticum (kava) root is used to produce an intoxicating herbal medicine, P. betle (betel) leaves are used to wrap areca (Areca catechu) nuts or tobacco (Nicotiana spp.) for chewing, and P. cubeba (cubeb) fruits are used to produce an essential oil [2]. The genus has shown high ethnobotanical utility worldwide and is of interest to a variety of fields and industries, including pharmaceutical botany, traditional medicine, aromatic industries, foods, and landscaping [3,4]. In Vietnam, there are about 45 species of Piper [5].
Mosquitoes have been and continue to be the deadliest animals on earth and are responsible for several of the world’s deadliest diseases, including malaria, yellow fever, dengue, filariasis, and many others [6]. Mosquito-borne infectious diseases have also been a constant problem in Vietnam. Dengue fever and dengue hemorrhagic fever are especially problematic and chikungunya fever is an emerging threat in the country [7,8]. Aedes aegypti (L.), the yellow fever mosquito, is an important vector of viral diseases including yellow fever [9], dengue [10], chikungunya [11], Zika [12], as well as others. Aedes albopictus (Skuse) (Diptera: Culicidae), the Asian tiger mosquito, is also an important vector of several viral pathogens, including dengue fever virus [13], yellow fever virus [14], chikungunya fever virus [15], and possibly Zika virus [16]. Culex quinquefasciatus Say (Diptera: Culicidae), the southern house mosquito, is a vector of lymphatic filariasis [17] as well as several arboviruses such as West Nile virus and St. Louis encephalitis virus [18], and possibly Zika virus [19].
There is a need for complementary vector control methods for controlling the spread of mosquito-borne diseases; resistance to synthetic insecticides is increasing worldwide [20,21,22,23,24], and the deleterious impacts of synthetic insecticides to the environment have been a major problem for many years [25,26]. Essential oils may provide environmentally safe and renewable alternatives to synthetic insecticides for mosquito control [27,28,29,30,31]. In this work, we present the essential oil compositions and mosquito larvicidal activities of several species of Piper growing wild in central Vietnam [32,33]:
Piper arboricola C. DC. (syn. Piper kadsura (Choisy) Ohwi, Piper futokadsura Siebold, Piper subglaucescens C. DC., local Vietnamese name Tiêu thượng mộc, is an understory liana native to China and Taiwan. In Vietnam, P. arboricola is found in Hà Tĩnh (Vũ Quang National Park), Thừa Thiên Huế Pro (Nam Đông District), and Lâm Đồng Province (Đà Lạt City: Đatanla).
Piper bavinum C. DC., local Vietnamese name Tiêu ba vì, is an understory climber found in China. In Vietnam, P. bavinum is found in Hà Nội City (Ba vì: Cốc Village) and Kon Tum Province.
Piper cambodianum P. Fourn. is found in Vietnam in Nghệ An, Đà Nẵng, and Ninh Thuận Provinces.
Piper caninum C. DC., Vietnamese name Tiêu chó, ranges from India, through Thailand, Malaysia, and Indonesia. In Vietnam, P. caninum is found in Kon Tum Province (Đác Tung).
Piper longum L., Vietnamese names Tiêu lá tím, Tất bạt, Tiêu dài, Tiêu lốt, and Trầu không dại, is an understory liana, which is distributed throughout India, China, Malaysia, Nepal, and Sri Lanka, as well as Vietnam.
Piper mekongense C. DC. (syn. Piper polysyphonum C. DC.), Vietnamese name Tiêu cửu long, is an understory shrub found in Laos, Cambodia, as well as Kon Tum province, Vietnam.
Piper montium C. DC., Vietnamese name Tiêu núi, is an understory climber. In Vietnam, the plant is found in Ninh Bình and Kon Tum provinces.
Piper mutabile C. DC., Vietnamese names Tiêu biến thể, Trầu biến thể, and Mâu linh, is an understory climber that typically grows on slopes in thickets along streams at elevations around 400–600 m. It is found in China as well as Quảng Ninh, Ninh Bình, Thanh Hóa, Nghệ An, and Kon Tum provinces of Vietnam.
Piper nigrum L., Vietnamese names Hồ tiêu and Tiêu, is a widespread liana, believed to have originated in India and Indonesia, but now cultivated throughout the tropics, including Vietnam provinces of Quảng Trị, Quảng Nam, Kon Tum, Đắc Lắc, Lâm Đồng, An Giang (Châu Đốc), and Kiên Giang (Phú Quốc).
Piper politifolium C. DC., Vietnamese name Tiêu lá láng, is native to the Neotropics, but introduced to Vietnam and found in the provinces Kon Tum (Đác Giây, Đác Môn), Gia Lai (An Khê, Kon Hà Nừng), Lâm Đồng (Bảo Lộc), and Đồng Nai.
Piper rubrum C. DC., Vietnamese name Tiêu đỏ, is an understory climber found in China as well as Vietnam, Phú Thọ (Tam Thanh), Ninh Bình, Quảng Nam.
Piper sarmentosum Roxb., Vietnamese names Lốt and Trầu già, is an herb growing in forests and wet places near villages, from sea level to 1000 m. The plant ranges from India and China through Thailand and Malaysia. In Vietnam, P. sarmentosum is found in the provinces of Nghệ An and Đà Nẵng.
Piper umbellatum L. (syn. Piper peltatum Ruiz & Pav., Pothomorphe peltata (L.) Miq., Pothomorphe umbellata (L.) Miq.) is an understory herb, native to the Neotropics, but introduced to Vietnam.
2. Results and Discussion
2.1. Plant Collection and Essential Oils
The plants materials (leaves and/or stems) from 13 Piper species were collected from various sites in Vietnam. The collection sites, plant materials, and essential oil yields for the Piper species are summarized in Table 1.
Table 1.
Collection sites and essential oil yields for Piper essential oils from central Vietnam.
| Piper Species | Voucher Numbers | Collection Site | Plant Parts | Essential Oil Yield (%) a |
|---|---|---|---|---|
| Piper arboricola C. DC. | LTH 105 | Ngoc Linh Nature Reserve-Quang Nam Province (15°50’16.0” N, 107°22’54.7” E, elev. 1341 m) | Leaves & stems | 0.31 |
| Piper arboricola C. DC. | LTH 105 | Ngoc Linh Nature Reserve-Quang Nam Province (15°50’16.0” N, 107°22’54.7” E, elev. 1341 m) | Leaves | 0.32 |
| Piper arboricola C. DC. | LTH 105 | Ngoc Linh Nature Reserve-Quang Nam Province (15°50’16.0” N, 107°22’54.7” E, elev. 1341 m) | Stems | 0.12 |
| Piper bavinum C. DC. | LTH 107 | Ngoc Linh Nature Reserve-Quang Nam Province (15°50’16.0” N, 107°22’54.7” E, elev. 1341 m) | Leaves & stems | 0.34 |
| Piper cambodianum P. Fourn. | LTH 108 | Ba Na-Nui Chua Nature Reserve (16°00′06.6″ N, 108°01′22.1″ E, elev. 791 m) |
Leaves & stems | 0.34 |
| Piper caninum C. DC. | LTH 112 | Chu Mom Ray National Park (14°25’33.5” N, 107°43’15.6” E, elev. 672 m) | Leaves & stems | 0.10 |
| Piper longum L. | LTH 109 | Chu Mom Ray National Park (14°25’33.5” N, 107°43’15.6” E, elev. 672 m) | Leaves & stems | 0.15 |
| Piper mekongense C. DC | LTH 110 | Chu Mom Ray National Park (14°25’33.5” N, 107°43’15.6” E, elev. 672 m) | Leaves & stems | 0.32 |
| Piper montium C. DC. | LTH 111 | Chu Mom Ray National Park (14°25’33.5” N, 107°43’15.6” E, elev. 672 m) | Leaves & stems | 0.10 |
| Piper mutabile C. DC. | LTH 118 | Chu Mom Ray National Park (14°25’33.5” N, 107°43’15.6” E, elev. 672 m) | Leaves & stems | 0.38 |
| Piper nigrum L. | LTH 113 | Hoa Vang district, Da Nang city (16°01’0.6” N, 108°4’25.6” E, elev. 28 m) | Leaves & stems | 0.39 |
| Piper politifolium C. DC. | LTH 114 | Chu Mom Ray National Park (14°25’33.5” N, 107°43’15.6” E, elev. 672 m) | Leaves & stems | 0.37 |
| Piper rubrum C. DC. | LTH 115 | Ngoc Linh Nature Reserve-Quang Nam Province (15°50’16.0” N, 107°22’54.7” E, elev. 1341 m) | Leaves | 0.41 |
| Piper rubrum C. DC. | LTH 115 | Ngoc Linh Nature Reserve-Quang Nam Province (15°50’16.0” N, 107°22’54.7” E, elev. 1341 m) | Stems | 0.38 |
| Piper sarmentosum Roxb. | LTH 116 | Hoa Vang district, Da Nang city (16°01’0.6” N, 108°4’25.6” E, elev. 28 m) | Leaves & stems | 0.29 |
| Piper umbellatum L. | LTH 117 | Chu Mom Ray National Park (14°25’33.5” N, 107°43’15.6” E, elev. 672 m) | Leaves & stems | 0.025 |
a Yields are based on mass of fresh plant material.
2.2. Essential Oil Compositions
The Vietnamese Piper species were analyzed by gas chromatography-mass spectrometry (GC-MS). A total of 272 compounds were identified in the Piper essential oils, which are compiled in Table 2. β-Caryophyllene was relatively abundant in all of the Piper essential oils and ranged from 4.0% (P. mekongense) to 44.8% (P. umbellatum). Also found in all of the essential oils were α-pinene (0.2–19.3%), β-pinene (0.1–26.9%), limonene (0.4–23.2%), α-copaene (0.2–13.7%), β-elemene (0.3–3.3%), and α-humulene (0.5–6.1%). β-Bisabolene was particularly abundant in P. sarmentosum (40.3%), P. montium (22.1%), and P. mutabile (12.9%), while decanal was an abundant component of P. rubrum stem essential oil (31.6%), and P. nigrum was rich in δ-elemene (20.4%).
Table 2.
Essential oil compositions (%) of Piper species from central Vietnam.
| RI a | Compound | P. arboricola | P. arboricola | P. arboricola | P. bavinum | P. cambodianum | P. caninum | P. longum | P. mekongense |
| Leaves & Stems | Leaves b | Stems b | Leaves & Stems | Leaves & Stems b | Leaves & Stems b | Leaves & Stems b | Leaves & Stems b | ||
| 900 | Nonane | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 919 | 5,5-Dimethyl-1-vinylbicyclo[2.1.1]hexane | --- | --- | --- | --- | Tr c | --- | --- | --- |
| 920 | Tricyclene | --- | --- | --- | --- | --- | --- | --- | tr |
| 923 | α-Thujene | 0.1 | tr | 0.1 | tr | tr | 0.6 | 0.3 | tr |
| 930 | α-Pinene | 0.5 | 6.4 d | 19.3 | 3.3 | 4.1 | 6.2 | 5.1 | 3.8 |
| 945 | α-Fenchene | --- | --- | --- | --- | --- | --- | --- | tr |
| 947 | Camphene | 0.6 | 0.6 | 0.7 | 0.3 | 0.1 | 1.1 | 1.0 | 1.4 |
| 970 | Sabinene | 0.7 | tr | 0.6 | 0.1 | 0.2 | 1.8 | 0.2 | 0.1 |
| 975 | β-Pinene | 0.1 | 0.8 | 26.9 | 3.9 | 4.6 | 6.8 | 10.6 | 5.3 |
| 982 | 6-Methylhept-5-en-2-one | --- | --- | --- | --- | --- | --- | --- | --- |
| 986 | Myrcene | 6.6 | 0.5 | 1.1 | 0.2 | 2.7 | 0.7 | 0.8 | 0.2 |
| 997 | δ-2-Carene | tr | --- | --- | --- | --- | --- | --- | tr |
| 1002 | p-Mentha-1(7),8-diene | --- | --- | --- | --- | tr | --- | --- | tr |
| 1003 | Octanal | --- | --- | --- | --- | --- | --- | --- | --- |
| 1004 | α-Phellandrene | tr | 0.1 | 0.1 | 0.3 | tr | 0.1 | 0.1 | tr |
| 1007 | δ-3-Carene | --- | --- | --- | --- | tr | --- | 0.1 | tr |
| 1010 | Hexyl acetate | --- | tr | --- | --- | --- | --- | --- | --- |
| 1015 | α-Terpinene | --- | --- | 0.1 | --- | tr | 0.4 | 0.2 | --- |
| 1018 | m-Cymene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1022 | p-Cymene | 0.2 | 1.3 | 0.5 | tr | tr | 0.6 | 0.6 | 0.2 |
| 1027 | Limonene | 23.2 | 2.4 | 6.8 | 2.2 | 7.9 | 1.6 | 1.4 | 0.8 |
| 1028 | β-Phellandrene | 0.1 | 0.2 | 0.4 | 0.2 | 0.2 | 3.4 | 0.1 | 0.1 |
| 1030 | 1,8-Cineole | 0.1 | tr | 0.2 | --- | tr | 0.4 | 0.2 | 0.1 |
| 1033 | (Z)-β-Ocimene | 0.3 | 0.1 | --- | 0.3 | tr | tr | 0.1 | tr |
| 1035 | 2-Heptyl acetate | --- | --- | --- | --- | --- | --- | --- | 0.1 |
| 1043 | (E)-β-Ocimene | 0.1 | tr | --- | 0.2 | tr | 0.2 | 1.5 | 0.1 |
| 1047 | 2,3,6-Trimethylhepta-1,5-diene | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1055 | γ-Terpinene | --- | tr | 0.2 | 0.1 | 0.2 | 1.5 | 1.4 | --- |
| 1057 | (2E)-Octenal | --- | --- | --- | --- | --- | --- | --- | --- |
| 1067 | cis-Sabinene hydrate | --- | --- | --- | --- | --- | 1.2 | --- | --- |
| 1079 | p-Mentha-2,4(8)-diene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1083 | Terpinolene | 0.4 | 0.1 | 0.3 | 0.1 | 0.1 | 0.3 | 0.1 | tr |
| 1088 | 2-Nonanone | 0.2 | --- | --- | --- | --- | --- | 0.1 | tr |
| 1090 | Rosefuran | --- | --- | --- | --- | --- | --- | --- | --- |
| 1093 | α-Pinene oxide | --- | --- | --- | --- | --- | --- | --- | --- |
| 1097 | Linalool | 0.8 | 1.1 | 0.8 | 0.3 | 0.3 | 10.1 | 3.4 | 0.2 |
| 1098 | trans-Sabinene hydrate | --- | --- | --- | --- | --- | 1.2 | --- | --- |
| 1098 | 2-Nonanol | tr | --- | --- | --- | --- | --- | --- | 0.1 |
| 1101 | 6-Methyl-3,5-heptadien-2-one | --- | --- | --- | --- | --- | --- | --- | --- |
| 1102 | Nonanal | 0.2 | --- | --- | --- | --- | --- | --- | --- |
| 1103 | 1-Octen-3-yl acetate | tr | --- | --- | --- | --- | --- | --- | 0.2 |
| 1105 | p-Mentha-2-8-dien-1-ol | --- | --- | --- | --- | --- | --- | --- | tr |
| 1111 | 4,8-Dimethylnona-1,3,7-triene | 0.3 | tr | --- | --- | 0.3 | --- | --- | tr |
| 1115 | 3-Octyl acetate | --- | --- | --- | --- | --- | --- | --- | tr |
| 1117 | trans-p-Mentha-2,8-dien-1-ol | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1122 | cis-p-Menth-2-en-1-ol | --- | --- | --- | --- | --- | 0.4 | --- | --- |
| 1126 | allo-Ocimene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1127 | (Z)-Myroxide | --- | --- | --- | --- | --- | --- | --- | --- |
| 1128 | cis-Limonene oxide | tr | --- | --- | --- | --- | --- | --- | --- |
| 1129 | Limona ketone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1132 | cis-p-Mentha-2,8-dien-1-ol | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1133 | trans-Limonene oxide | tr | --- | --- | --- | --- | --- | --- | --- |
| 1135 | Nopinone | --- | --- | --- | --- | --- | --- | --- | tr |
| 1137 | (E)-Myroxide | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1138 | trans-Pinocarveol | --- | --- | --- | --- | --- | --- | --- | 0.1 |
| 1140 | trans-p-Menth-2-en-1-ol | --- | --- | --- | --- | --- | 0.3 | --- | --- |
| 1143 | trans-Verbenol | --- | --- | --- | --- | --- | --- | --- | tr |
| 1144 | Camphor | tr | 0.1 | --- | --- | --- | 0.8 | 0.4 | tr |
| 1152 | Camphene hydrate | --- | --- | --- | --- | --- | --- | tr | tr |
| 1153 | Sabina ketone | --- | --- | --- | --- | --- | --- | --- | tr |
| 1158 | Isoborneol | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1159 | Pinocarvone | --- | --- | --- | --- | --- | --- | --- | tr |
| 1167 | Rosefuran epoxide | --- | --- | --- | --- | --- | --- | --- | --- |
| 1167 | p-Mentha-1,5-dien-8-ol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1168 | 1-Nonanol | 0.3 | --- | --- | --- | --- | --- | --- | tr |
| 1169 | Borneol | --- | tr | --- | --- | --- | 0.1 | tr | tr |
| 1171 | (3E,5E)-Undeca-1,3,5-triene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1174 | Isopinocamphone | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1177 | 2-Isopropenyl-5-methyl-4-hexenal | --- | --- | --- | --- | --- | --- | tr | --- |
| 1178 | Terpinen-4-ol | 0.1 | --- | 0.1 | --- | 0.1 | 7.7 | 0.2 | --- |
| 1182 | Naphthalene | --- | 0.2 | --- | --- | --- | 1.2 | 0.3 | --- |
| 1184 | p-Cymen-8-ol | 0.4 | --- | --- | --- | --- | --- | --- | 0.1 |
| 1185 | Cryptone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1193 | Myrtenal | --- | --- | --- | --- | --- | --- | --- | 0.1 |
| 1193 | α-Terpineol | --- | tr | --- | 0.1 | 0.1 | 0.4 | 0.2 | --- |
| 1199 | (3Z)-Octenyl acetate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1201 | cis-Sabinol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1203 | Decanal | 6.2 | 0.6 | 3.5 | 0.2 | --- | --- | --- | --- |
| 1206 | (3E)-Octenyl acetate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1215 | trans-Carveol | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1216 | endo-Fenchyl acetate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1221 | 2-Hydroxycineole | --- | --- | --- | --- | --- | --- | --- | --- |
| 1222 | Nerol | --- | --- | --- | --- | tr | --- | --- | --- |
| 1224 | Citronellol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1225 | Isobornyl formate | --- | --- | --- | --- | --- | --- | --- | 0.2 |
| 1228 | cis-Carveol | tr | --- | --- | --- | --- | --- | --- | --- |
| 1228 | 2-Nonyl acetate | --- | --- | --- | --- | --- | --- | --- | 0.1 |
| 1233 | Bornyl formate | --- | --- | --- | --- | --- | --- | --- | 1.6 |
| 1237 | Ascaridole | --- | --- | --- | --- | --- | 0.7 | 0.1 | --- |
| 1240 | Carvone | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1248 | Geraniol | --- | --- | --- | --- | tr | --- | --- | --- |
| 1248 | Linalyl acetate | --- | 0.1 | --- | --- | --- | --- | --- | --- |
| 1256 | Methyl citronellate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1269 | 1-Decanol | 1.1 | tr | --- | --- | --- | --- | --- | --- |
| 1271 | Methyl hydrocinnamate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1277 | 9-Decen-1-ol | --- | --- | --- | --- | --- | --- | --- | 0.1 |
| 1281 | Isobornyl acetate | --- | --- | 0.2 | --- | --- | --- | 0.1 | 0.1 |
| 1282 | Bornyl acetate | --- | 1.3 | --- | 0.1 | --- | 0.7 | 0.3 | 0.2 |
| 1288 | Safrole | --- | 0.1 | --- | 0.5 | --- | --- | --- | --- |
| 1290 | 2-Undecanone | 0.8 | 0.1 | --- | --- | tr | 0.4 | 1.7 | 0.2 |
| 1293 | Methyl myrtenate | --- | --- | --- | --- | --- | --- | tr | --- |
| 1299 | 2-Undecanol | --- | --- | --- | --- | tr | --- | tr | 0.5 |
| 1300 | Tridecane | --- | 0.1 | 0.4 | --- | --- | 1.2 | 0.1 | --- |
| 1303 | Undecanal | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1304 | Isoascaridole | --- | --- | --- | --- | --- | 0.5 | 0.1 | --- |
| 1319 | Methyl geranate | --- | --- | --- | --- | --- | --- | tr | --- |
| 1328 | Myrtenyl acetate | --- | tr | --- | --- | --- | --- | --- | --- |
| 1328 | Bicycloelemene | 0.8 | 0.3 | --- | 0.3 | 0.3 | --- | tr | 0.3 |
| 1332 | δ-Elemene | 0.2 | 0.1 | --- | 0.2 | 0.2 | --- | tr | 0.7 |
| 1343 | α-Cubebene | 0.1 | 3.0 | 0.8 | 0.5 | 0.4 | 0.3 | 0.1 | 0.2 |
| 1344 | α-Terpinyl acetate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1347 | Citronellyl acetate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1358 | Decanoic acid | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1364 | Cyclosativene | --- | 0.3 | 0.1 | 0.1 | tr | --- | 0.1 | 0.2 |
| 1365 | Hydrocinnamyl acetate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1366 | α-Ylangene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1368 | Isoledene | --- | tr | --- | 0.2 | tr | --- | tr | tr |
| 1372 | α-Copaene | 0.8 | 13.7 | 4.2 | 3.2 | 2.1 | 0.4 | 0.3 | 0.7 |
| 1375 | Geranyl acetate | --- | 0.1 | --- | --- | --- | 0.2 | --- | --- |
| 1376 | (E)-β-Damascenone | --- | --- | --- | --- | --- | --- | tr | --- |
| 1380 | β-Bourbonene | 0.3 | 0.2 | --- | --- | 0.2 | 0.1 | tr | 0.1 |
| 1384 | β-Cubebene | 0.2 | 1.7 | 0.6 | 1.6 | 0.2 | --- | 0.1 | 0.5 |
| 1385 | 7-epi-Sesquithujene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1386 | β-Elemene | 1.6 | 0.7 | 0.4 | 2.0 | 1.2 | 0.4 | 0.3 | 2.1 |
| 1398 | Sesquithujene | --- | tr | 0.1 | --- | --- | --- | --- | --- |
| 1402 | Italicene | --- | 0.1 | --- | --- | --- | --- | --- | --- |
| 1402 | (Z)-Caryophyllene | --- | --- | --- | --- | 0.4 | --- | --- | --- |
| 1403 | α-Gurjunene | 0.1 | 0.3 | 0.2 | 1.1 | 0.2 | --- | 0.1 | 0.2 |
| 1406 | Dodecanal | 0.6 | 0.2 | 0.3 | --- | --- | 0.2 | tr | --- |
| 1408 | cis-5-Hydroxy-p-menth-6-en-2-one | --- | --- | --- | --- | --- | --- | --- | --- |
| 1408 | β-Maaliene | --- | --- | --- | --- | --- | --- | --- | 0.2 |
| 1409 | cis-α-Bergamotene | --- | 0.3 | 1.0 | 0.4 | --- | 0.2 | tr | --- |
| 1416 | β-Caryophyllene | 14.1 | 9.4 | 4.9 | 6.2 | 19.2 | 10.3 | 7.9 | 4.0 |
| 1419 | (E)-α-Ionone | --- | tr | --- | --- | --- | --- | --- | --- |
| 1422 | γ-Maaliene | tr | --- | --- | --- | tr | --- | --- | --- |
| 1423 | γ-Elemene | --- | --- | --- | --- | --- | --- | --- | 0.9 |
| 1424 | trans-5-Hydroxy-p-menth-6-en-2-one | --- | --- | --- | --- | --- | --- | --- | --- |
| 1424 | β-Gurjunene | --- | --- | --- | --- | --- | --- | --- | 2.1 |
| 1426 | β-Copaene | 0.3 | 0.7 | 0.8 | 0.4 | 0.2 | 0.4 | 0.1 | --- |
| 1430 | trans-α-Bergamotene | --- | 0.1 | 0.1 | 0.2 | tr | 0.1 | tr | --- |
| 1430 | α-Maaliene | 0.1 | --- | --- | --- | --- | --- | tr | 0.1 |
| 1431 | α-Guaiene | --- | 0.7 | 0.1 | 0.5 | 0.3 | --- | --- | --- |
| 1435 | Aromadendrene | 0.6 | 0.2 | --- | 0.1 | 0.3 | 0.3 | 0.1 | 0.2 |
| 1438 | (Z)-β-Farnesene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1439 | 6,9-Guaiadiene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1442 | Myltayl-4(12)-ene | --- | --- | --- | --- | --- | --- | --- | 0.2 |
| 1443 | Selina-5,11-diene | --- | --- | --- | --- | --- | --- | tr | --- |
| 1445 | cis-Muurola-3,5-diene | --- | 0.9 | 0.3 | --- | 0.1 | --- | tr | --- |
| 1445 | Geranyl acetone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1446 | trans-Muurola-3,5-diene | --- | --- | --- | 1.6 | --- | --- | --- | --- |
| 1449 | (E)-β-Farnesene | --- | 0.2 | 0.3 | 0.5 | tr | 1.0 | 0.4 | --- |
| 1450 | Valerena-4,7(11)-diene | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1452 | α-Humulene | 1.1 | 1.7 | 0.5 | 6.1 | 2.0 | 1.4 | 1.4 | 1.9 |
| 1453 | Sesquisabinene | --- | tr | 0.2 | 0.1 | --- | --- | --- | --- |
| 1456 | allo-Aromadendrene | 0.1 | 0.1 | --- | 0.2 | 0.4 | 0.1 | --- | --- |
| 1457 | Eudesma-1,4(15),11-triene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1459 | cis-Muurola-4(14),5-diene | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1462 | cis-Cadina-1(6),4-diene | --- | --- | --- | --- | tr | --- | --- | --- |
| 1464 | Dehydroisolongifolenene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1467 | Selina-4,11-diene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1467 | (2E)-Undecenyl acetate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1468 | trans-Cadina-1(6),4-diene | --- | 1.1 | 0.5 | --- | 0.3 | --- | 0.1 | --- |
| 1470 | γ-Gurjunene | --- | --- | --- | 1.7 | --- | --- | --- | --- |
| 1470 | Amorpha-4,7(11)-diene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1471 | γ-Muurolene | 0.2 | 0.4 | 0.2 | 0.3 | 9.6 | 0.5 | 0.4 | 2.2 |
| 1472 | β-Acoradiene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1473 | γ-Curcumene | --- | 0.2 | 2.3 | 5.8 | --- | --- | --- | --- |
| 1475 | cis-4,10-epoxy-Amorphane | --- | --- | --- | --- | 0.6 | --- | --- | --- |
| 1476 | α-Amorphene | --- | tr | --- | --- | --- | --- | --- | --- |
| 1477 | Germacrene D | 2.8 | --- | --- | 4.7 | 1.5 | --- | 1.2 | 5.2 |
| 1477 | ar-Curcumene | --- | 5.5 | 9.6 | --- | --- | 4.9 | --- | --- |
| 1480 | (Z,Z)-α-Farnesene | --- | 0.3 | 0.5 | 0.3 | --- | 0.3 | tr | --- |
| 1480 | β-Chamigrene | --- | --- | --- | --- | 0.9 | --- | --- | 1.3 |
| 1480 | 1-Dodecanol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1484 | δ-Selinene | --- | tr | --- | --- | --- | --- | --- | --- |
| 1486 | trans-Muurola-4(14),5-diene | --- | 1.6 | 0.6 | 1.4 | --- | --- | 0.3 | --- |
| 1486 | β-Selinene | 0.1 | 0.2 | 1.1 | 1.6 | 0.6 | 1.4 | tr | 1.0 |
| 1487 | Viridiflorene (=Ledene) | 0.4 | --- | --- | --- | 0.6 | --- | --- | 0.3 |
| 1488 | Curzerene | --- | --- | --- | --- | --- | --- | --- | 0.2 |
| 1488 | α-Zingiberene | --- | --- | --- | --- | --- | 0.8 | --- | --- |
| 1490 | Unidentified sesquiterpenoide | --- | --- | --- | --- | --- | --- | 2.3 | --- |
| 1492 | Asaricin | --- | --- | --- | --- | --- | --- | --- | --- |
| 1492 | Bicyclogermacrene | 11.3 | 7.9 | --- | 8.9 | 3.7 | --- | 1.5 | 5.6 |
| 1492 | α-Selinene | --- | --- | --- | --- | --- | 1.1 | --- | --- |
| 1495 | α-Muurolene | 0.1 | 0.9 | 0.5 | 0.8 | 1.3 | 0.5 | 0.8 | 1.1 |
| 1495 | cis-β-Guaiene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1497 | (Z)-α-Bisabolene | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1499 | α-Bulnesene | --- | 2.5 | 0.7 | 3.0 | 1.2 | --- | --- | --- |
| 1500 | δ-Amorphene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1500 | Pentadecane | --- | --- | 0.5 | --- | --- | --- | --- | --- |
| 1501 | (E,E)-α-Farnesene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1504 | β-Bisabolene | --- | 0.7 | 0.7 | 0.7 | 0.1 | 8.7 | 4.5 | 0.2 |
| 1505 | β-Curcumene | --- | 0.1 | 0.3 | 1.5 | --- | --- | --- | --- |
| 1509 | γ-Cadinene | 0.1 | 0.2 | --- | 0.2 | 0.5 | 0.5 | 1.3 | 0.6 |
| 1511 | Cubebol | 0.1 | 1.7 | --- | 0.7 | 1.0 | 0.1 | 0.3 | 1.0 |
| 1514 | δ-Cadinene | 0.3 | 6.0 | 1.7 | 4.8 | 6.1 | 1.6 | 3.5 | 1.8 |
| 1515 | 7-epi-α-Selinene | --- | --- | --- | --- | --- | --- | --- | 1.4 |
| 1518 | trans-Calamenene | 0.1 | 8.9 | 2.4 | 1.1 | 0.2 | --- | --- | 0.2 |
| 1519 | β-Sesquiphellandrene | --- | --- | 0.2 | 0.2 | --- | 1.2 | --- | --- |
| 1520 | Zonarene | --- | --- | --- | 0.4 | 0.2 | --- | --- | --- |
| 1523 | (E)-γ-Bisabolene | --- | --- | --- | 0.2 | --- | --- | --- | --- |
| 1526 | Kessane | --- | --- | --- | --- | 0.2 | --- | --- | --- |
| 1527 | Nootkatene | --- | 0.1 | --- | --- | --- | --- | --- | --- |
| 1529 | trans-Cadina-1,4-diene | --- | 0.8 | 0.3 | 0.9 | 0.7 | --- | 0.1 | --- |
| 1533 | α-Cadinene | --- | tr | --- | --- | 0.1 | --- | 0.3 | 0.2 |
| 1537 | cis-Cadinene ether | --- | tr | --- | --- | --- | --- | --- | --- |
| 1538 | (E)-α-Bisabolene | --- | --- | --- | --- | --- | --- | 0.2 | --- |
| 1538 | α-Calacorene | --- | 0.4 | --- | --- | 0.1 | --- | --- | 0.2 |
| 1542 | trans-Cadinene ether | --- | 0.5 | --- | --- | --- | --- | 0.2 | --- |
| 1544 | Unidentified sesquiterpenoid f | 0.3 | --- | --- | --- | --- | --- | 1.3 | --- |
| 1545 | α-Elemol | --- | --- | --- | --- | 0.1 | --- | --- | 0.3 |
| 1554 | Germacrene B | 0.1 | --- | --- | --- | --- | --- | --- | 2.0 |
| 1557 | (E)-Nerolidol | 1.7 | 0.4 | --- | 0.2 | 1.6 | 2.3 | 8.1 | 0.9 |
| 1558 | β-Calacorene | --- | 0.1 | --- | --- | --- | --- | --- | 0.2 |
| 1565 | Maaliol | --- | --- | --- | --- | --- | --- | --- | 13.8 |
| 1565 | Palustrol | --- | --- | --- | 1.1 | 0.1 | --- | --- | --- |
| 1566 | Dendrolasin | --- | --- | --- | --- | --- | --- | --- | --- |
| 1573 | Spathulenol | 8.3 | 0.6 | --- | 0.4 | 1.3 | 1.2 | 0.7 | 6.4 |
| 1574 | Germacra-1(10),5-dien-4β-ol | --- | --- | --- | --- | --- | --- | 0.6 | --- |
| 1578 | Caryophyllene oxide | 3.3 | 0.4 | --- | 0.2 | 1.6 | 3.1 | 0.8 | 1.3 |
| 1582 | Globulol | 0.6 | 0.4 | --- | 0.8 | 0.3 | 0.5 | --- | 2.2 |
| 1583 | Gleenol | --- | 0.3 | --- | --- | --- | --- | --- | --- |
| 1586 | Unidentified sesquiterpenoid g | --- | 0.1 | --- | --- | 0.1 | --- | --- | --- |
| 1590 | Viridiflorol | 1.2 | 0.5 | --- | 1.0 | 6.5 | 0.5 | --- | 1.4 |
| 1593 | Cubeban-11-ol | --- | 0.1 | --- | 0.2 | --- | --- | --- | --- |
| 1595 | Humulene epoxide I | --- | --- | --- | --- | --- | --- | --- | --- |
| 1597 | Curzerenone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1600 | Rosifoliol | --- | --- | --- | 0.1 | --- | --- | --- | 0.1 |
| 1600 | Guaiol | --- | --- | --- | --- | 0.8 | --- | --- | --- |
| 1601 | Ledol | --- | 0.1 | --- | 0.9 | 0.3 | --- | --- | --- |
| 1601 | β-Oplopenone | --- | --- | --- | --- | --- | --- | 0.5 | --- |
| 1605 | β-Atlantol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1606 | Humulene epoxide II | 0.2 | 0.1 | --- | --- | 0.1 | 0.3 | --- | 0.3 |
| 1606 | Tetradecanal | --- | --- | --- | --- | --- | --- | --- | --- |
| 1609 | Unidentified sesquiterpenoid h | --- | --- | --- | --- | --- | --- | --- | --- |
| 1612 | 1,10-di-epi-Cubenol | --- | --- | --- | --- | 0.1 | --- | 0.1 | --- |
| 1613 | α-Corocalene | --- | 0.1 | --- | --- | --- | --- | --- | --- |
| 1616 | Junenol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1623 | Selina-6-en-4β-ol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1624 | 1-epi-Cubenol | 0.1 | 2.1 | 0.3 | 2.6 | 0.7 | --- | 0.3 | --- |
| 1626 | iso-Spathulenol | 0.7 | --- | --- | --- | 0.3 | --- | --- | 1.3 |
| 1628 | Caryophylla-4(12),8(13)-dien-5α-ol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1633 | Caryophylla-4(12),8(13)-dien-5β-ol | --- | --- | --- | --- | 0.2 | --- | --- | --- |
| 1636 | allo-Aromadendrene epoxide | --- | --- | --- | --- | --- | --- | --- | --- |
| 1638 | τ-Cadinol | --- | --- | --- | --- | 0.9 | 0.4 | 2.2 | 1.1 |
| 1640 | τ-Muurolol | 0.2 | --- | --- | 0.6 | 0.6 | 0.5 | 2.0 | 2.9 |
| 1640 | Cubenol | --- | 1.4 | --- | 1.7 | --- | --- | --- | --- |
| 1643 | α-Muurolol (= δ-Cadinol) | 0.1 | 0.4 | --- | 0.7 | 2.4 | 0.5 | 1.2 | 0.8 |
| 1648 | Unidentified sesquiterpenoid i | --- | --- | --- | 1.6 | --- | --- | --- | 1.3 |
| 1651 | α-Eudesmol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1652 | α-Cadinol | --- | 0.2 | --- | 1.2 | 1.8 | 0.8 | 4.9 | 3.6 |
| 1654 | Selin-11-en-4α-ol | --- | --- | --- | 0.4 | --- | --- | --- | 0.9 |
| 1655 | Pogostol | --- | 0.4 | --- | 0.4 | 0.2 | --- | --- | --- |
| 1656 | Unidentified fatty acid derivative j | 0.2 | --- | --- | --- | --- | --- | --- | --- |
| 1658 | ar-Turmerone | --- | 0.2 | --- | --- | --- | --- | --- | --- |
| 1658 | 9-Methoxycalamenene | --- | --- | --- | --- | --- | --- | --- | 0.3 |
| 1661 | Intermedeol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1661 | Bulnesol | --- | --- | --- | --- | 0.3 | --- | --- | --- |
| 1663 | (6Z)-Pentadecen-2-one | --- | --- | --- | --- | --- | --- | --- | --- |
| 1664 | (2E,6Z)-Farnesol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1666 | 14-Hydroxy-9-epi-(E)-caryophyllene | --- | --- | --- | --- | 0.1 | --- | --- | --- |
| 1670 | Cadalene | --- | 0.1 | --- | --- | --- | --- | --- | --- |
| 1680 | Unidentified sesquiterpenoid k | --- | --- | --- | --- | --- | --- | 5.7 | --- |
| 1680 | Germacra-4(15),5,10(14)-trien-1α-ol | --- | --- | --- | --- | tr | --- | --- | --- |
| 1681 | epi-α-Bisabolol | --- | --- | --- | --- | --- | 2.4 | ||
| 1684 | α-Bisabolol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1686 | (2Z,6Z)-Farnesol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1690 | Shyobunol | --- | --- | --- | --- | 1.5 | --- | --- | --- |
| 1690 | 2-Pentadecanone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1700 | Heptadecane | --- | --- | --- | --- | --- | --- | --- | --- |
| 1710 | Unidentified sesquiterpenoidl | --- | --- | --- | --- | --- | --- | 11.9 | --- |
| 1711 | (2E,6Z)-Farnesol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1722 | Cryptomerione | --- | --- | --- | --- | --- | --- | --- | --- |
| 1725 | Zerumbone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1747 | Cyclocolorenone | 0.5 | 0.1 | 0.1 | 7.9 | --- | --- | --- | --- |
| 1763 | Benzyl benzoate | --- | --- | --- | --- | 0.1 | --- | --- | --- |
| 1798 | Curzerenone B | --- | --- | --- | --- | --- | --- | --- | --- |
| 1809 | Hexadecanal | --- | --- | --- | --- | --- | --- | --- | --- |
| 1828 | (2Z,6E)-Farnesyl acetate | --- | --- | --- | --- | --- | --- | --- | --- |
| 2017 | (E,E)-Geranyl linalool | --- | --- | --- | --- | --- | --- | --- | --- |
| 2103 | (E)-Phytol | --- | --- | --- | --- | --- | --- | --- | --- |
| Monoterpene hydrocarbons | 33.0 | 12.5 | 57.2 | 11.0 | 20.0 | 25.3 | 23.7 | 11.9 | |
| Oxygenated monoterpenoids | 1.8 | 2.7 | 1.3 | 0.5 | 0.4 | 24.6 | 5.1 | 2.6 | |
| Sesquiterpene hydrocarbons | 35.9 | 73.0 | 36.3 | 64.1 | 55.2 | 36.5 | 25.3 | 38.4 | |
| Oxygenated sesquiterpenoids | 17.0 | 9.7 | 0.4 | 20.9 | 23.4 | 10.1 | 21.9 | 41.0 | |
| Diterpenoids | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Benzenoids | 0.0 | 0.1 | 0.0 | 0.5 | 0.1 | 0.0 | 0.0 | 0.0 | |
| Others | 9.9 | 1.1 | 4.8 | 0.2 | 0.3 | 3.0 | 2.1 | 1.3 | |
| Total identified | 97.6 | 99.0 | 100.0 | 97.1 | 99.5 | 99.4 | 78.2 | 95.2 | |
| RI a | Compound | P. montium | P. mutabile | P. nigrum | P. politifolium | P. rubrum | P. rubrum | P. sarmentosum | P. umbellatum |
| Leaves & Stems b | Leaves & Stems b | Leaves & Stems b | Leaves & Stems | Leaves b | Stems b | Leaves & Stems b | Leaves & Stems | ||
| 900 | Nonane | --- | --- | --- | --- | --- | --- | --- | --- |
| 919 | 5,5-Dimethyl-1-vinylbicyclo[2.1.1]hexane | --- | --- | --- | --- | --- | --- | --- | --- |
| 920 | Tricyclene | --- | --- | --- | tr c | --- | --- | tr | --- |
| 923 | α-Thujene | tr | --- | tr | 0.8 | tr | 0.1 | 0.1 | --- |
| 930 | α-Pinene | 0.2 | 3.1 | 0.8 | 12.9 d | 0.9 | 0.5 | 2.3 | 0.4 |
| 945 | α-Fenchene | --- | --- | tr | --- | --- | --- | tr | --- |
| 947 | Camphene | tr | 1.0 | tr | 3.2 | 0.2 | 0.8 | 2.0 | 0.1 |
| 970 | Sabinene | --- | --- | tr | 1.7 | 0.2 | 2.1 | 0.1 | 0.1 |
| 975 | β-Pinene | 0.2 | 5.5 | 1.3 | 7.1 | 0.1 | 0.3 | 5.5 | 0.8 |
| 982 | 6-Methylhept-5-en-2-one | --- | --- | --- | --- | --- | --- | tr | --- |
| 986 | Myrcene | tr | 0.2 | 0.5 | 1.6 | 4.1 | 0.9 | 0.4 | 0.3 |
| 997 | δ-2-Carene | --- | --- | --- | --- | tr | --- | --- | --- |
| 1002 | p-Mentha-1(7),8-diene | --- | --- | --- | --- | --- | --- | --- | tr |
| 1003 | Octanal | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1004 | α-Phellandrene | 0.2 | --- | 1.0 | 0.4 | --- | 0.1 | --- | tr |
| 1007 | δ-3-Carene | --- | 0.2 | 4.6 | 2.3 | --- | --- | --- | tr |
| 1010 | Hexyl acetate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1015 | α-Terpinene | tr | --- | tr | 0.4 | --- | 0.1 | --- | --- |
| 1018 | m-Cymene | --- | --- | tr | --- | --- | --- | --- | --- |
| 1022 | p-Cymene | 0.2 | --- | 0.1 | 0.3 | 0.2 | 0.2 | 0.1 | tr |
| 1027 | Limonene | 0.4 | 3.8 | 2.4 | 2.6 | 7.0 | 9.3 | 1.3 | 0.9 |
| 1028 | β-Phellandrene | 0.2 | 0.4 | 0.1 | 1.4 | tr | 0.8 | tr | 0.5 |
| 1030 | 1,8-Cineole | 0.2 | 0.2 | --- | --- | --- | --- | 0.5 | --- |
| 1033 | (Z)-β-Ocimene | tr | --- | tr | 0.4 | 0.2 | tr | 0.3 | 4.3 |
| 1035 | 2-Heptyl acetate | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1043 | (E)-β-Ocimene | tr | 0.2 | 0.2 | 2.8 | tr | 0.1 | 1.3 | 1.0 |
| 1047 | 2,3,6-Trimethylhepta-1,5-diene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1055 | γ-Terpinene | 0.1 | --- | 0.1 | 0.6 | --- | 0.1 | --- | tr |
| 1057 | (2E)-Octenal | 0.2 | --- | --- | --- | --- | --- | --- | --- |
| 1067 | cis-Sabinene hydrate | tr | --- | --- | --- | --- | --- | --- | --- |
| 1079 | p-Mentha-2,4(8)-diene | --- | --- | 0.1 | --- | --- | --- | --- | --- |
| 1083 | Terpinolene | tr | 0.2 | 0.2 | 5.7 | 0.1 | 0.6 | --- | tr |
| 1088 | 2-Nonanone | --- | --- | --- | --- | --- | 0.2 | 0.1 | --- |
| 1090 | Rosefuran | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1093 | α-Pinene oxide | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1097 | Linalool | 2.1 | 3.7 | 0.8 | 0.8 | 0.4 | 0.3 | 0.4 | 0.5 |
| 1098 | trans-Sabinene hydrate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1098 | 2-Nonanol | --- | --- | --- | --- | --- | 0.1 | --- | --- |
| 1101 | 6-Methyl-3,5-heptadien-2-one | --- | --- | --- | --- | --- | --- | 0.2 | --- |
| 1102 | Nonanal | --- | --- | --- | --- | 0.1 | 0.3 | --- | --- |
| 1103 | 1-Octen-3-yl acetate | --- | --- | --- | --- | --- | --- | --- | 0.4 |
| 1105 | p-Mentha-2-8-dien-1-ol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1111 | 4,8-Dimethylnona-1,3,7-triene | --- | --- | --- | --- | 0.7 | 0.5 | tr | tr |
| 1115 | 3-Octyl acetate | --- | --- | --- | --- | --- | --- | --- | 0.1 |
| 1117 | trans-p-Mentha-2,8-dien-1-ol | --- | --- | --- | --- | --- | --- | --- | tr |
| 1122 | cis-p-Menth-2-en-1-ol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1126 | allo-Ocimene | --- | --- | --- | --- | --- | --- | tr | 0.2 |
| 1127 | (Z)-Myroxide | --- | --- | --- | --- | --- | --- | tr | --- |
| 1128 | cis-Limonene oxide | --- | --- | --- | --- | --- | --- | --- | --- |
| 1129 | Limona ketone | --- | --- | --- | --- | --- | --- | tr | --- |
| 1132 | cis-p-Mentha-2,8-dien-1-ol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1133 | trans-Limonene oxide | --- | --- | --- | --- | --- | --- | --- | --- |
| 1135 | Nopinone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1137 | (E)-Myroxide | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1138 | trans-Pinocarveol | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1140 | trans-p-Menth-2-en-1-ol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1143 | trans-Verbenol | --- | --- | --- | --- | --- | --- | tr | --- |
| 1144 | Camphor | tr | 1.0 | --- | --- | --- | --- | tr | --- |
| 1152 | Camphene hydrate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1153 | Sabina ketone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1158 | Isoborneol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1159 | Pinocarvone | --- | --- | --- | --- | --- | --- | tr | --- |
| 1167 | Rosefuran epoxide | --- | --- | --- | --- | --- | --- | tr | --- |
| 1167 | p-Mentha-1,5-dien-8-ol | --- | --- | --- | --- | --- | --- | --- | tr |
| 1168 | 1-Nonanol | --- | --- | --- | --- | 0.1 | 0.2 | --- | --- |
| 1169 | Borneol | --- | 0.1 | --- | --- | --- | --- | 0.1 | --- |
| 1171 | (3E,5E)-Undeca-1,3,5-triene | --- | --- | --- | --- | --- | --- | --- | tr |
| 1174 | Isopinocamphone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1177 | 2-Isopropenyl-5-methyl-4-hexenal | --- | 0.2 | --- | --- | --- | --- | --- | --- |
| 1178 | Terpinen-4-ol | 0.8 | 0.3 | --- | 0.4 | --- | 0.1 | tr | --- |
| 1182 | Naphthalene | 0.8 | 2.3 | --- | --- | --- | 0.3 | --- | 0.2 |
| 1184 | p-Cymen-8-ol | --- | --- | --- | 0.1 | 0.2 | 0.2 | tr | --- |
| 1185 | Cryptone | --- | --- | --- | --- | --- | --- | tr | --- |
| 1193 | Myrtenal | --- | --- | --- | --- | --- | --- | 0.2 | --- |
| 1193 | α-Terpineol | 0.1 | 0.3 | tr | 0.1 | --- | --- | --- | --- |
| 1199 | (3Z)-Octenyl acetate | --- | --- | --- | --- | --- | --- | tr | 0.1 |
| 1201 | cis-Sabinol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1203 | Decanal | --- | --- | --- | --- | 1.3 | 31.6 | --- | --- |
| 1206 | (3E)-Octenyl acetate | --- | --- | --- | --- | --- | --- | 0.1 | 0.1 |
| 1215 | trans-Carveol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1216 | endo-Fenchyl acetate | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1221 | 2-Hydroxycineole | --- | --- | --- | --- | --- | --- | --- | --- |
| 1222 | Nerol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1224 | Citronellol | --- | --- | tr | --- | --- | --- | --- | --- |
| 1225 | Isobornyl formate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1228 | cis-Carveol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1228 | 2-Nonyl acetate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1233 | Bornyl formate | --- | --- | --- | --- | --- | --- | --- | tr |
| 1237 | Ascaridole | --- | --- | --- | --- | --- | --- | --- | --- |
| 1240 | Carvone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1248 | Geraniol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1248 | Linalyl acetate | --- | --- | 0.1 | --- | --- | --- | --- | --- |
| 1256 | Methyl citronellate | --- | --- | 0.5 | --- | --- | --- | --- | --- |
| 1269 | 1-Decanol | 0.2 | --- | --- | --- | 0.1 | 3.4 | --- | --- |
| 1271 | Methyl hydrocinnamate | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1277 | 9-Decen-1-ol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1281 | Isobornyl acetate | 0.1 | --- | --- | --- | --- | --- | 1.6 | 0.2 |
| 1282 | Bornyl acetate | --- | --- | --- | 0.1 | 0.1 | --- | 0.1 | tr |
| 1288 | Safrole | --- | --- | --- | 2.7 | --- | --- | --- | 0.1 |
| 1290 | 2-Undecanone | 0.1 | --- | 0.2 | 1.6 | 0.2 | 2.3 | 0.3 | tr |
| 1293 | Methyl myrtenate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1299 | 2-Undecanol | --- | --- | tr | --- | --- | 0.1 | --- | tr |
| 1300 | Tridecane | 2.8 | 1.9 | --- | --- | --- | 0.5 | --- | --- |
| 1303 | Undecanal | --- | --- | --- | --- | --- | 0.1 | --- | --- |
| 1304 | Isoascaridole | --- | --- | --- | --- | --- | --- | --- | --- |
| 1319 | Methyl geranate | --- | --- | 0.1 | --- | --- | --- | --- | --- |
| 1328 | Myrtenyl acetate | 0.1 | --- | --- | --- | --- | --- | --- | --- |
| 1328 | Bicycloelemene | --- | --- | 1.6 | 0.4 | 1.1 | 0.3 | --- | 0.9 |
| 1332 | δ-Elemene | 0.1 | 0.2 | 20.4 | 0.2 | 0.1 | 0.1 | 0.4 | 9.2 |
| 1343 | α-Cubebene | 0.2 | 0.1 | 1.1 | 0.1 | 0.4 | 0.1 | --- | 0.1 |
| 1344 | α-Terpinyl acetate | --- | --- | --- | --- | --- | --- | 0.3 | --- |
| 1347 | Citronellyl acetate | --- | --- | 0.1 | --- | --- | --- | --- | --- |
| 1358 | Decanoic acid | --- | --- | --- | --- | --- | 0.4 | --- | --- |
| 1364 | Cyclosativene | 0.2 | 0.3 | 0.1 | --- | 0.1 | --- | --- | tr |
| 1365 | Hydrocinnamyl acetate | --- | --- | --- | --- | --- | --- | 0.5 | --- |
| 1366 | α-Ylangene | 0.1 | --- | 0.1 | --- | --- | --- | --- | --- |
| 1368 | Isoledene | --- | --- | 0.3 | --- | 0.1 | --- | --- | --- |
| 1372 | α-Copaene | 0.7 | 1.3 | 3.5 | 1.1 | 7.1 | 1.7 | 0.2 | 0.4 |
| 1375 | Geranyl acetate | 0.2 | --- | --- | --- | --- | --- | --- | --- |
| 1376 | (E)-β-Damascenone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1380 | β-Bourbonene | 0.1 | --- | 0.1 | 0.1 | 0.4 | 0.1 | 0.1 | tr |
| 1384 | β-Cubebene | --- | --- | --- | 0.2 | 0.4 | 0.2 | --- | 0.1 |
| 1385 | 7-epi-Sesquithujene | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1386 | β-Elemene | 1.2 | 0.4 | 3.3 | 0.6 | 2.8 | 0.5 | 2.0 | 0.6 |
| 1398 | Sesquithujene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1402 | Italicene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1402 | (Z)-Caryophyllene | --- | --- | tr | --- | --- | --- | --- | tr |
| 1403 | α-Gurjunene | tr | 0.2 | 2.2 | 0.1 | 0.2 | 0.1 | --- | 0.1 |
| 1406 | Dodecanal | 0.1 | --- | --- | --- | 0.3 | 5.0 | --- | --- |
| 1408 | cis-5-Hydroxy-p-menth-6-en-2-one | --- | --- | --- | --- | --- | --- | --- | --- |
| 1408 | β-Maaliene | --- | --- | 0.4 | --- | --- | --- | --- | --- |
| 1409 | cis-α-Bergamotene | 0.4 | 0.2 | --- | --- | 0.1 | --- | 0.1 | --- |
| 1416 | β-Caryophyllene | 19.2 | 16.5 | 7.7 | 6.5 | 22.9 | 9.7 | 5.5 | 44.8 |
| 1419 | (E)-α-Ionone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1422 | γ-Maaliene | --- | --- | tr | --- | 0.1 | --- | --- | tr |
| 1423 | γ-Elemene | --- | --- | 0.2 | --- | --- | --- | --- | 0.2 |
| 1424 | trans-5-Hydroxy-p-menth-6-en-2-one | --- | --- | --- | --- | --- | --- | --- | --- |
| 1424 | β-Gurjunene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1426 | β-Copaene | 0.6 | 0.9 | --- | 0.2 | 0.5 | 0.2 | 0.1 | 0.3 |
| 1430 | trans-α-Bergamotene | 0.2 | 0.2 | --- | 0.1 | --- | --- | 0.1 | tr |
| 1430 | α-Maaliene | --- | --- | --- | --- | 0.1 | --- | --- | tr |
| 1431 | α-Guaiene | --- | --- | --- | 0.2 | --- | --- | --- | --- |
| 1435 | Aromadendrene | 0.2 | 0.4 | 0.9 | 0.1 | 1.3 | 0.2 | 0.1 | 0.1 |
| 1438 | (Z)-β-Farnesene | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1439 | 6,9-Guaiadiene | --- | --- | 0.5 | --- | --- | --- | --- | 0.2 |
| 1442 | Myltayl-4(12)-ene | --- | 0.1 | --- | --- | 0.1 | --- | --- | tr |
| 1443 | Selina-5,11-diene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1445 | cis-Muurola-3,5-diene | 0.1 | 0.1 | 0.1 | --- | --- | --- | --- | 0.2 |
| 1445 | Geranyl acetone | --- | --- | 0.4 | --- | --- | --- | --- | 0.4 |
| 1446 | trans-Muurola-3,5-diene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1449 | (E)-β-Farnesene | 1.6 | 1.1 | --- | --- | --- | --- | 2.2 | 0.1 |
| 1450 | Valerena-4,7(11)-diene | --- | --- | tr | --- | 0.1 | --- | --- | --- |
| 1452 | α-Humulene | 1.6 | 2.1 | 2.7 | 3.2 | 1.9 | 0.8 | 1.2 | 3.8 |
| 1453 | Sesquisabinene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1456 | allo-Aromadendrene | 0.1 | --- | --- | --- | 0.2 | --- | --- | 0.1 |
| 1457 | Eudesma-1,4(15),11-triene | --- | --- | 0.6 | --- | --- | --- | --- | --- |
| 1459 | cis-Muurola-4(14),5-diene | --- | --- | --- | --- | --- | --- | --- | 0.1 |
| 1462 | cis-Cadina-1(6),4-diene | --- | --- | 0.4 | --- | --- | --- | --- | --- |
| 1464 | Dehydroisolongifolenene | --- | --- | --- | --- | --- | --- | --- | 0.1 |
| 1467 | Selina-4,11-diene | --- | --- | --- | --- | --- | --- | --- | 0.1 |
| 1467 | (2E)-Undecenyl acetate | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1468 | trans-Cadina-1(6),4-diene | 0.2 | --- | --- | --- | --- | --- | --- | --- |
| 1470 | γ-Gurjunene | --- | --- | 0.6 | --- | 0.1 | --- | --- | --- |
| 1470 | Amorpha-4,7(11)-diene | --- | --- | --- | --- | --- | --- | --- | 1.7 |
| 1471 | γ-Muurolene | 1.0 | 1.0 | 3.6 | 0.1 | 0.2 | 0.3 | --- | 0.4 |
| 1472 | β-Acoradiene | --- | --- | --- | --- | --- | --- | 0.7 | --- |
| 1473 | γ-Curcumene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1475 | cis-4,10-epoxy-Amorphane | --- | --- | --- | --- | --- | --- | --- | --- |
| 1476 | α-Amorphene | --- | 0.2 | 0.2 | --- | --- | --- | --- | --- |
| 1477 | Germacrene D | --- | --- | 1.1 | 5.1 | 2.9 | 1.8 | --- | 1.8 |
| 1477 | ar-Curcumene | 15.1 | 8.2 | --- | --- | --- | --- | 1.8 | --- |
| 1480 | (Z,Z)-α-Farnesene | 0.8 | 0.7 | --- | --- | --- | --- | 0.5 | --- |
| 1480 | β-Chamigrene | --- | --- | --- | --- | 0.1 | --- | --- | --- |
| 1480 | 1-Dodecanol | --- | --- | --- | --- | --- | 0.5 | --- | --- |
| 1484 | δ-Selinene | --- | --- | 2.2 | --- | --- | --- | --- | 1.3 |
| 1486 | trans-Muurola-4(14),5-diene | 0.4 | 0.3 | 2.0 | --- | --- | --- | --- | 0.2 |
| 1486 | β-Selinene | 1.4 | 0.7 | 5.1 | 0.6 | 0.3 | 0.1 | 4.4 | 0.3 |
| 1487 | Viridiflorene (=Ledene) | --- | --- | 2.5 | 0.1 | 0.9 | 0.3 | --- | --- |
| 1488 | Curzerene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1488 | α-Zingiberene | 3.1 | 2.2 | --- | --- | --- | --- | --- | --- |
| 1490 | Unidentified sesquiterpenoid e | --- | --- | --- | --- | --- | --- | --- | --- |
| 1492 | Asaricin | --- | 3.2 | --- | 18.7 | --- | --- | --- | --- |
| 1492 | Bicyclogermacrene | --- | --- | --- | --- | 15.7 | 3.5 | --- | 1.6 |
| 1492 | α-Selinene | 0.9 | --- | 4.3 | --- | --- | --- | 3.2 | --- |
| 1495 | α-Muurolene | 1.5 | 1.1 | 0.2 | 0.3 | 0.3 | 0.1 | 0.1 | 0.3 |
| 1495 | cis-β-Guaiene | --- | --- | --- | --- | --- | --- | --- | 0.2 |
| 1497 | (Z)-α-Bisabolene | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1499 | α-Bulnesene | --- | --- | --- | --- | 0.1 | --- | --- | --- |
| 1500 | δ-Amorphene | --- | --- | 0.2 | --- | --- | --- | --- | --- |
| 1500 | Pentadecane | --- | --- | --- | --- | 0.2 | 5.3 | --- | --- |
| 1501 | (E,E)-α-Farnesene | --- | --- | 0.4 | 0.1 | --- | --- | --- | --- |
| 1504 | β-Bisabolene | 22.1 | 12.9 | 0.3 | 0.1 | 0.1 | --- | 40.3 | 0.6 |
| 1505 | β-Curcumene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1509 | γ-Cadinene | 0.6 | 0.7 | 0.2 | 0.1 | 0.1 | --- | --- | 0.4 |
| 1511 | Cubebol | 0.1 | --- | 0.2 | 0.1 | 0.1 | 0.1 | --- | 0.2 |
| 1514 | δ-Cadinene | 2.1 | 2.8 | 1.4 | 1.5 | 0.9 | 0.5 | --- | 1.2 |
| 1515 | 7-epi-α-Selinene | --- | --- | --- | --- | --- | --- | 0.2 | --- |
| 1518 | trans-Calamenene | 0.3 | 0.4 | 0.2 | tr | 1.1 | 0.2 | --- | 0.5 |
| 1519 | β-Sesquiphellandrene | 2.9 | 2.1 | --- | 0.1 | --- | --- | 0.2 | 0.1 |
| 1520 | Zonarene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1523 | (E)-γ-Bisabolene | 0.2 | 0.1 | --- | --- | --- | --- | --- | --- |
| 1526 | Kessane | --- | --- | --- | --- | --- | --- | --- | --- |
| 1527 | Nootkatene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1529 | trans-Cadina-1,4-diene | 0.2 | 0.2 | 0.7 | --- | --- | --- | --- | 0.1 |
| 1533 | α-Cadinene | 0.2 | 0.2 | 0.1 | --- | --- | --- | --- | 0.2 |
| 1537 | cis-Cadinene ether | --- | --- | --- | --- | --- | --- | --- | --- |
| 1538 | (E)-α-Bisabolene | --- | --- | --- | --- | --- | --- | 0.5 | --- |
| 1538 | α-Calacorene | 0.3 | 0.3 | 0.1 | --- | --- | --- | --- | 0.2 |
| 1542 | trans-Cadinene ether | --- | --- | --- | --- | --- | --- | --- | --- |
| 1544 | Unidentified sesquiterpenoid f | --- | --- | --- | --- | --- | --- | --- | --- |
| 1545 | α-Elemol | 0.1 | --- | 0.1 | --- | --- | --- | 0.2 | --- |
| 1554 | Germacrene B | --- | --- | 0.2 | 0.1 | --- | --- | --- | 0.2 |
| 1557 | (E)-Nerolidol | --- | --- | 0.2 | 0.7 | 2.6 | 4.7 | 0.7 | 4.1 |
| 1558 | β-Calacorene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1565 | Maaliol | --- | 0.3 | --- | 0.2 | --- | --- | --- | 1.0 |
| 1565 | Palustrol | --- | --- | 0.1 | 0.1 | --- | --- | --- | --- |
| 1566 | Dendrolasin | --- | --- | --- | --- | --- | --- | tr | --- |
| 1573 | Spathulenol | 0.5 | 1.0 | 0.3 | 1.0 | 8.6 | 1.1 | 1.3 | --- |
| 1574 | Germacra-1(10),5-dien-4β-ol | --- | --- | --- | --- | --- | --- | --- | 0.9 |
| 1578 | Caryophyllene oxide | 3.1 | 3.5 | 0.2 | 0.5 | 3.8 | 1.3 | 5.1 | 2.5 |
| 1582 | Globulol | 0.2 | 0.4 | 0.3 | 0.2 | 0.5 | --- | 0.1 | 0.3 |
| 1583 | Gleenol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1586 | Unidentified sesquiterpenoid g | --- | --- | 3.2 | --- | --- | --- | --- | 0.1 |
| 1590 | Viridiflorol | 0.2 | 0.2 | 0.1 | 0.2 | 1.0 | 0.2 | tr | 0.2 |
| 1593 | Cubeban-11-ol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1595 | Humulene epoxide I | --- | --- | --- | --- | --- | --- | tr | --- |
| 1597 | Curzerenone | 0.4 | --- | --- | --- | --- | --- | --- | --- |
| 1600 | Rosifoliol | --- | --- | --- | tr | --- | --- | --- | tr |
| 1600 | Guaiol | --- | --- | 0.1 | --- | --- | --- | --- | tr |
| 1601 | Ledol | --- | --- | --- | 0.1 | --- | --- | --- | --- |
| 1601 | β-Oplopenone | --- | --- | --- | --- | --- | --- | --- | --- |
| 1605 | β-Atlantol | --- | --- | 0.5 | --- | --- | --- | --- | --- |
| 1606 | Humulene epoxide II | 0.2 | 0.2 | --- | 0.1 | 0.2 | 0.1 | 0.8 | 0.1 |
| 1606 | Tetradecanal | --- | --- | --- | --- | --- | 0.4 | --- | --- |
| 1609 | Unidentified sesquiterpenoid h | --- | 2.8 | --- | --- | --- | --- | --- | --- |
| 1612 | 1,10-di-epi-Cubenol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1613 | α-Corocalene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1616 | Junenol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1623 | Selina-6-en-4β-ol | --- | --- | 0.7 | --- | --- | --- | --- | 0.9 |
| 1624 | 1-epi-Cubenol | 0.5 | 0.4 | --- | 0.1 | 0.2 | 0.3 | --- | --- |
| 1626 | iso-Spathulenol | --- | --- | 3.9 | --- | 0.5 | 0.1 | 0.5 | 2.5 |
| 1628 | Caryophylla-4(12),8(13)-dien-5α-ol | --- | --- | --- | --- | --- | --- | 0.4 | --- |
| 1633 | Caryophylla-4(12),8(13)-dien-5β-ol | --- | --- | --- | --- | --- | --- | 0.2 | 0.2 |
| 1636 | allo-Aromadendrene epoxide | --- | --- | --- | --- | --- | --- | --- | --- |
| 1638 | τ-Cadinol | 0.4 | 0.3 | --- | 0.1 | --- | 0.2 | --- | 0.4 |
| 1640 | τ-Muurolol | 0.8 | 1.2 | --- | 0.1 | --- | 0.1 | --- | 0.5 |
| 1640 | Cubenol | --- | --- | --- | --- | 0.3 | --- | --- | --- |
| 1643 | α-Muurolol (= δ-Cadinol) | 0.9 | 1.3 | --- | 0.2 | 0.2 | 0.3 | 0.2 | 0.1 |
| 1648 | Unidentified sesquiterpenoid i | --- | --- | --- | 0.1 | --- | --- | --- | --- |
| 1651 | α-Eudesmol | --- | --- | --- | --- | --- | --- | 0.2 | --- |
| 1652 | α-Cadinol | 1.6 | 1.2 | 0.3 | 0.3 | --- | 0.3 | --- | 0.7 |
| 1654 | Selin-11-en-4α-ol | --- | --- | 0.1 | --- | --- | 0.3 | --- | --- |
| 1655 | Pogostol | 0.5 | --- | --- | --- | --- | --- | 0.3 | --- |
| 1656 | Unidentified fatty acid derivativej | --- | 1.0 | --- | 3.9 | --- | --- | --- | --- |
| 1658 | ar-Turmerone | --- | --- | --- | --- | --- | 0.1 | --- | --- |
| 1658 | 9-Methoxycalamenene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1661 | Intermedeol | --- | --- | tr | --- | --- | --- | 0.2 | --- |
| 1661 | Bulnesol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1663 | (6Z)-Pentadecen-2-one | --- | --- | --- | 0.2 | --- | 0.4 | --- | --- |
| 1664 | (2E,6Z)-Farnesol | --- | --- | --- | --- | --- | --- | --- | 0.2 |
| 1666 | 14-Hydroxy-9-epi-(E)-caryophyllene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1670 | Cadalene | --- | --- | --- | --- | --- | --- | --- | --- |
| 1680 | Unidentified sesquiterpenoid k | --- | --- | --- | --- | 0.2 | --- | --- | --- |
| 1680 | Germacra-4(15),5,10(14)-trien-1α-ol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1681 | epi-α-Bisabolol | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1684 | α-Bisabolol | --- | --- | --- | --- | --- | --- | 0.2 | 0.2 |
| 1686 | (2Z,6Z)-Farnesol | --- | --- | --- | --- | --- | --- | 0.3 | --- |
| 1690 | Shyobunol | --- | --- | --- | --- | --- | --- | --- | --- |
| 1690 | 2-Pentadecanone | --- | --- | --- | 0.1 | --- | 0.3 | --- | --- |
| 1700 | Heptadecane | --- | --- | --- | --- | --- | 0.3 | --- | --- |
| 1710 | Unidentified sesquiterpenoid l | --- | --- | --- | --- | --- | --- | --- | --- |
| 1711 | (2E,6Z)-Farnesol | --- | --- | 0.2 | --- | --- | --- | 0.2 | --- |
| 1722 | Cryptomerione | --- | --- | --- | --- | --- | --- | 0.3 | --- |
| 1725 | Zerumbone | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 1747 | Cyclocolorenone | --- | --- | --- | 0.5 | tr | 0.1 | --- | --- |
| 1763 | Benzyl benzoate | --- | --- | --- | --- | --- | --- | --- | --- |
| 1798 | Curzerenone B | 0.3 | --- | --- | --- | --- | --- | --- | --- |
| 1809 | Hexadecanal | --- | --- | --- | --- | --- | 0.2 | --- | --- |
| 1828 | (2Z,6E)-Farnesyl acetate | --- | --- | 0.1 | --- | --- | --- | 0.1 | --- |
| 2017 | (E,E)-Geranyl linalool | --- | --- | --- | --- | --- | --- | 0.1 | --- |
| 2103 | (E)-Phytol | --- | --- | 0.1 | --- | --- | --- | 0.3 | 0.9 |
| Monoterpene hydrocarbons | 1.5 | 14.6 | 11.4 | 44.3 | 13.0 | 15.9 | 13.2 | 8.5 | |
| Oxygenated monoterenoids | 3.5 | 5.9 | 1.5 | 1.4 | 0.6 | 0.6 | 3.6 | 0.7 | |
| Sesquiterpene hydrocarbons | 79.9 | 58.2 | 71.9 | 21.4 | 62.9 | 20.9 | 64.1 | 72.6 | |
| Oxygenated sesquiterpenoids | 10.0 | 10.1 | 7.4 | 4.5 | 18.1 | 9.3 | 11.3 | 15.0 | |
| Diterpenoids | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.4 | 0.9 | |
| Benzenoids | 0.0 | 3.2 | 0.0 | 21.4 | 0.0 | 0.0 | 0.6 | 0.1 | |
| Others | 4.3 | 4.2 | 0.5 | 1.9 | 2.9 | 52.4 | 0.9 | 1.3 | |
| Total identified | 99.2 | 96.2 | 92.8 | 94.8 | 97.5 | 99.0 | 94.2 | 99.0 |
a RI = “Retention Index” determined in reference to a homologous series of n-alkanes. b Essential oil was used for larvicidal activity screening. c tr = “trace” (<0.05%). d Major essential oil components (>5%) are highlighted in blue bold font. e MS(EI): 202(45%), 187(18%), 159(70%), 145(47%), 131(58%), 119(60%), 117(60%), 105(71%), 91(100%), 81(55%), 43(100%). f MS(EI): 218(8%), 176(13%), 161(17%), 134(32%), 119(100%), 105(48%), 91(38%), 77(18%), 55(16%), 41(27%). g MS(EI): 220(11%), 205(10%), 187(9%), 162(63%), 147(65%), 134(41%), 119(100%), 107(42%), 105(53%), 93(67%), 91(58%), 79(29%), 69(18%), 55(21%), 43(100%), 41(28%). h MS(EI): 204(56%), 161(30%), 148(18%), 135(25%), 131(100%), 122(14%), 117(18%), 115(19%), 103(34%), 91(11%), 77(23%), 51(11%), 41(7%). i MS(EI): 223(8%), 162(97%), 147(67%), 133(65%), 119(60%), 105(100%), 94(81%), 91(68%), 81(52%), 67(25%), 59(88%), 43(34%), 41(33%). j MS(EI): 164(13%), 95(20%), 94(21%), 93(28%), 91(21%), 81(38%), 80(65%), 79(100%), 67(56%), 55(27%), 54(18%), 43(98%), 41(38%). k MS(EI): 218(51%), 190(14%), 175(30%), 161(79%), 147(49%), 134(87%), 119(100%), 105(53%), 91(49%), 77(21%), 55(17%), 41(34%). l MS(EI): 218(6%), 203(8%), 176(40%), 161(100%), 147(28%), 124(65%), 121(26%), 119(27%), 105(31%), 95(44%), 91(30%), 77(20%), 67(31%), 55(17%), 41(37%).
Interestingly, many Piper essential oils are rich in phenylpropanoids [2], particularly Pipers from the Neotropics [34,35]. In this current study, however, phenylpropanoids were generally found to be lacking, with the exception of P. politifolium, which had asaricin (18.7%) and safrole (2.7%). As far as we are aware, there have been no previous studies on the essential oils of P. arboricola, P. cambodianum, P. caninum, P. mekongense, P. montium, P. mutabile, P. politifolium, or P. rubrum.
The essential oil from the leaves and stems of P. bavinum, collected in Hainan, China, was found to be rich in spathulenol (8.0%), caryophyllene oxide (7.8%), propiopiperone (7.8%), and 2,4,6-trimethylbenzenepropanol (26.9%) [36]. The identification of 2,4,6-trimethylbenzenepropanol is doubtful, however; the identification was based only on the mass spectrum (retention indices not reported) and the compound is not found in the Dictionary of Natural Products [37]. In contrast, the essential oil of P. bavinum from Huong Son, Ha Tinh Province, Vietnam, was composed largely of bicyclogermacrene (10.6%), globulol (5.7%), viridiflorene (5.1%), α-pinene (4.4%), viridiflorol (3.5%), terpinen-4-ol (3.2%), and α-gurjunene (3.0%) [38]. The major components in P. bavinum essential oil in this study were bicyclogermacrene (8.9%), cyclocolorenone (7.9%), β-caryophyllene (6.2%), α-humulene (6.1%), γ-curcumene (5.8%), as well as α-pinene (3.3%), α-gurjunene (1.1%), and viridiflorol (1.0%). Thus, there are qualitative similarities between the two Vietnamese P. bavinum samples.
The phytochemistry of P. longum has been reviewed [2,39,40]. The leaf essential oil of P. longum from the Western Ghats region of Kerala, India, had elemol (22.5%), β-caryophyllene (16.8%), and α-humulene (5.8%) as the major components, while the stem essential oil was dominated by β-pinene (34.8%), α-pinene (14.0%), limonene (10.3%), and β-caryophyllene (9.3%) [41]. A report on the leaf essential oil of P. longum from Nghệ An Province, Vietnam showed the major components to be fonenol (40.5%) and elemol (8.2%) [42], and is, therefore, remarkably different from the P. longum essential oil in this present study, which was composed largely of α-pinene (5.1%), β-pinene (10.6%), β-caryophyllene (7.9%), (E)-nerolidol (8.1%), and two unidentified sesquiterpenoids (5.7% and 11.9%).
The major components in the essential oil from the leaves and stems of P. nigrum from Vietnam in this study were δ-elemene (20.4%), β-caryophyllene (7.7%), and β-selinene (5.1%). There have been numerous studies on the essential oils of P. nigrum, mostly on the fruit essential oils, but the leaves and stems have also been studied [2]. There are large variations in the leaf essential oil compositions of P. nigrum from India depending on the geographical location [43] and the particular cultivar [44]. Indian P. nigrum leaf oils are generally dominated by (E)-nerolidol, β-caryophyllene, germacrene D, and elemol, but show wide variation in concentrations [43,44]. In contrast, the leaf essential oil of P. nigrum “Bragantina”, cultivated in Belém, Pará State, Brazil, showed cubenol (14.6–21.5%), δ-cadinene (2.7–5.5%), bicyclogermacrene (6.4–8.2%), β-selinene (4.1–6.2%), α-copaene (3.5–5.2%), and α-gurjunene (3.5–5.1%) as the major components [45], while the essential oil from Hainan, China, was rich in β-caryophyllene (13.8%), spathulenol (6.2%), and caryophyllene oxide (6.0%) [36].
The phytochemistry of P. sarmentosum has also been reviewed [2,46]. The essential oils of P. sarmentosum have shown great variation, depending on geographical location. The essential oil from the leaves and stems of P. sarmentosum collected in Guangzhou, China, had asaricin (54.5%) and palmitic acid (8.2%) as major components [36]. On the other hand, the leaf essential oil of P. sarmentosum from Kuching, Sarawak, Malaysia, was composed largely of spathulenol (21.0%), myristicin (18.8%), β-caryophyllene (18.2%), and (E,E)-farnesol (10.5%) [47]. Furthermore, the essential oil from leaves and stems of P. sarmentosum from China (location not provided) was dominated by myristicin (65.2%) and β-caryophyllene (13.9%) [48]. The leaf essential oil of P. sarmentosum from Bạch Mã National Park, central Vietnam, was made up largely of benzenoids, benzyl benzoate (49.1%), benzyl alcohol (17.9%), benzyl salicylate (10.0%), and (2E)-butenylbenzene [49]. In complete contrast to these previous studies, the essential oil composition of P. sarmentosum essential oil from Da Nang, Vietnam, in this study, was composed of β-bisabolene (40.3%), β-pinene (5.5%), β-caryophyllene (5.5%), and caryophyllene oxide (5.1%) as the major components; neither asaricin, palmitic acid, myristicin, nor benzyl derivatives were observed in the present Vietnamese sample.
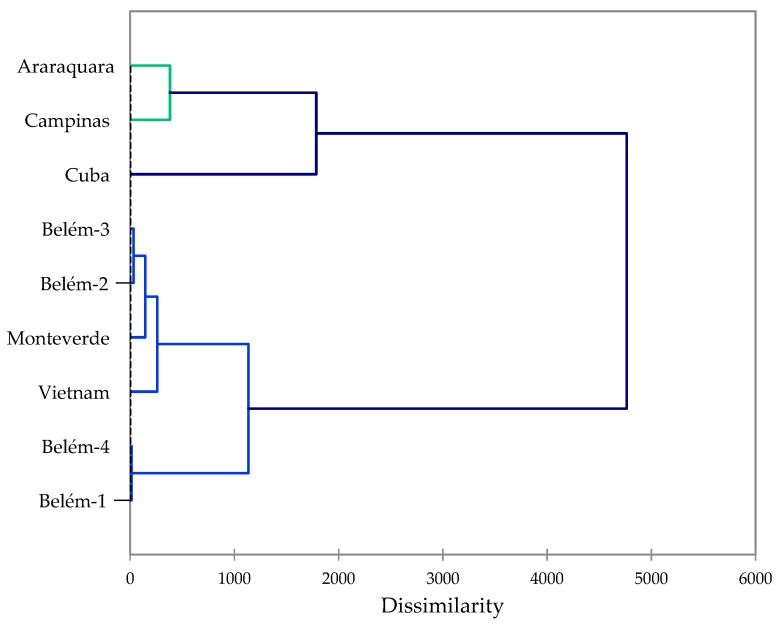
P. umbellatum, native to the Neotropics, has been investigated from several geographical locations, including Belém, Pará State, Brazil [50]; Campinas, São Paulo State, Brazil [51]; Araraquara, São Paulo State, Brazil [52]; Monteverde, Costa Rica [53]; and Topes de Collantes, Cuba [54]. The major components of P. umbellatum essential oils are summarized in Table 3. A cluster analysis based on the concentrations of the major components reveals two clearly defined groups. The P. umbellatum essential oil fits into a cluster along with essential oils from Monteverde, Costa Rica, and Belém, Brazil (Figure 1).
Table 3.
Major essential oil components (%) of Piper umbellatum.
| Vietnam | Belém-1 | Belém-2 | Belém-3 | Belém-4 | Campinas | Monteverde | Cuba | Araraquara | |
|---|---|---|---|---|---|---|---|---|---|
| Compound | This work | [50] | [50] | [50] | [50] | [51] | [53] | [54] | [52] |
| Camphor | 0.0 | 0.0 | 0.3 | 4.2 | 0.0 | 0.0 | 0.0 | 9.6 | 0.0 |
| Safrole | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 26.4 | 0.0 |
| δ-Elemene | 9.2 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 1.4 | 0.0 | 0.0 |
| β-Elemene | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6.9 | 0.0 | 0.0 |
| β-Caryophyllene | 44.8 | 68.0 | 39.1 | 39.5 | 64.8 | 6.3 | 28.3 | 6.6 | 3.0 |
| α-Humulene | 3.8 | 5.9 | 4.0 | 2.1 | 6.5 | 0.7 | 2.2 | 0.0 | 1.1 |
| γ-Muurolene | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.5 | 8.9 |
| Germacrene D | 1.8 | 6.2 | 13.3 | 12.7 | 8.7 | 55.8 | 16.7 | 3.7 | 34.2 |
| Bicyclogermacrene | 1.6 | 2.2 | 5.8 | 4.3 | 3.2 | 11.8 | 6.6 | 0.5 | 9.0 |
| γ-Cadinene | 0.4 | 0.4 | 1.4 | 2.1 | 0.0 | 1.4 | 0.0 | 0.0 | 5.9 |
| δ-Cadinene | 1.2 | 1.0 | 3.8 | 2.2 | 1.0 | 2.0 | 0.2 | 2.0 | 15.0 |
| (E)-Nerolidol | 4.1 | 4.9 | 4.8 | 11.1 | 7.5 | 0.4 | 4.4 | 4.9 | 4.4 |
Figure 1.
Agglomerative hierarchical cluster analysis based on the major components in Piper umbellatum essential oils.
2.3. Larvicidal Activities
Several of the Piper essential oils, depending on essential oil availability and availability of mosquito larvae, were screened for larvicidal activity against Aedes aegypti, and, when available, Aedes albopictus and Culex quinquefasciatus. The larvicidal activities are summarized in Table 4. According to a review by Dias and co-authors, plant essential oils are considered to have Ae. aegypti larvicidal activity if the LC50 values are less than 100 µg/mL [55]. Based on this criterion, all of the Piper essential oils in this investigation are active against larvae of this mosquito. Furthermore, four of the Piper essential oils showed outstanding larvicidal activity against Ae. aegypti, namely P. caninum, P. longum, P. montium, and P. mutabile, which had LC50 and LC90 values less than 10 µg/mL. In addition, P. nigrum essential oil had LC50 < 10 µg/mL and LC90 < 20 µg/mL. Although P. cambodianum had LC50 less than 10 µg/mL, the LC90 values were greater than 30 µg/mL.
Table 4.
Larvicidal activities (LC50 and LC90, μg/mL) of Piper essential oils from central Vietnam.
| Mosquito Species | Piper species | ||||||
| P. arboricola | P. arboricola | P. cambodianum | P. caninum | P. longum | P. mekongense | P. montium | |
| Leaves | Stems | Leaves & Stems | Leaves & Stems | Leaves & Stems | Leaves & Stems | Leaves & Stems | |
| Aedes aegypti | |||||||
| 24-h LC50 (95% confidence) | 26.85 (24.55–29.52) | 28.45 (26.54–30.77) | 8.861 (7.012–10.650) | 1.376 (1.252–1.513) | 5.342 (4.950–5.757) | 40.16 (37.09–42.88) | 1.964 (1.778–2.169) |
| 24-h LC90 (95% confidence) | 47.83 (37.83–71.41) | 41.88 (37.37–49.09) | 36.42 (29.16–49.60) | 2.419 (2.126–2.887) | 7.730 (7.006–8.955) | 52.66 (49.13–57.71) | 3.743 (3.273–4.472) |
| 48-h LC50 (95% confidence) | 15.59 (12.46–18.32) | 26.60 (24.51–28.97) | 6.773 (4.957–8.467) | 1.304 (1.183–1.437) | 4.979 (4.608–5.377) | 36.91 (34.35–39.56) | 1.533 (1.376–1.703) |
| 48-h LC90 (95% confidence) | 46.24 (40.60–55.31) | 39.79 (35.89–46.73) | 30.62 (24.39–42.18) | 2.351 (2.060–2.822) | 7.354 (6.659–8.469) | 50.00 (46.18–55.42) | 3.127 (2.714–3.773) |
| Aedes albopictus | |||||||
| 24-h LC50 (95% confidence) | N.T. | N.T. | 34.20 (31.90–36.73) | N.T. | N.T. | N.T. | N.T. |
| 24-h LC90 (95% confidence) | N.T. | N.T. | 45.98 (42.28–51.38) | N.T. | N.T. | N.T. | N.T. |
| 48-h LC50 (95% confidence) | N.T. | N.T. | 31.13 (29.15–33.70) | N.T. | N.T. | N.T. | N.T. |
| 48-h LC90 (95% confidence) | N.T. | N.T. | 40.82 (37.09–47.24) | N.T. | N.T. | N.T. | N.T. |
| Culex quinquefasciatus | |||||||
| 24-h LC50 (95% confidence) | N.T. | N.T. | 56.16 (51.10–61.54) | N.T. | N.T. | N.T. | N.T. |
| 24-h LC90 (95% confidence) | N.T. | N.T. | 104.7 (93.0–121.7) | N.T. | N.T. | N.T. | N.T. |
| 48-h LC50 (95% confidence) | N.T. | N.T. | 51.21 (46.52–56.27) | N.T. | N.T. | N.T. | N.T. |
| 48-h LC90 (95% confidence) | N.T. | N.T. | 97.91 (86.55–114.54) | N.T. | N.T. | N.T. | N.T. |
| Mosquito Species | Piper species | Permethrin | |||||
| P. montium | P. mutabile | P. nigrum | P. rubrum | P. rubrum | P. sarmentosum | ||
| Leaves & Stems | Leaves & Stems | Leaves & Stems | Leaves | Stems | Leaves & Stems | ||
| Aedes aegypti | |||||||
| 24-h LC50 (95% confidence) | 1.925 (1.771–2.092) | 1.850 (1.715–1.997) | 8.805 (7.905–9.811) | 24.98 (22.74–27.48) | 30.87 (28.66–33.32) | 22.82 (17.73–27.91) | 6.4 × 10−4 (5.5 × 10−4–7.5 × 10−4) |
| 24-h LC90 (95% confidence) | 3.180 (2.784–3.793) | 2.696 (2.442–3.108) | 18.83 (16.15–23.13) | 43.70 (38.35–52.39) | 45.54 (40.14–54.01) | 130.6 (88.7–256.8) | 2.5 × 10−3 (1.9 × 10−3–3.44 × 10−3) |
| 48-h LC50 (95% confidence) | 1.691 (1.531–1.867) | 1.695 (1.544–1.842) | 6.909 (6.149–7.720) | 22.15 (20.19–24.25) | 26.09 (23.83–28.63) | 18.42 (12.97–23.48) | N.T. |
| 48-h LC90 (95% confidence) | 3.024 (2.714–3.509) | 2.641 (2.367–3.122) | 15.49 (13.28–19.03) | 37.18 (32.97–43.97) | 44.80 (40.63–51.40) | 126.8 (90.1–220.9) | N.T. |
| Aedes albopictus | |||||||
| 24-h LC50 (95% confidence) | N.T. | N.T. | 43.92 (40.18–47.95) | N.T. | N.T. | 49.24 (44.72–53.99) | 2.3 × 10−3 (2.1 × 10−3–2.6 × 10−3) |
| 24-h LC90 (95% confidence) | N.T. | N.T. | 74.25 (66.15–86.73) | N.T. | N.T. | 90.30 (80.19–105.31) | 4.2 × 10−3 (3.8 × 10−3–4.9 × 10−3) |
| 48-h LC50 (95% confidence) | N.T. | N.T. | 39.19 (35.75–42.86) | N.T. | N.T. | 38.33 (33.62–42.97) | N.T. |
| 48-h LC90 (95% confidence) | N.T. | N.T. | 67.14 (59.57–79.16) | N.T. | N.T. | 86.32 (74.63–104.92) | N.T. |
| Culex quinquefasciatus | |||||||
| 24-h LC50 (95% confidence) | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | 1.7 × 10−2 (1.5 × 10−2–1.8 × 10−2) |
| 24-h LC90 (95% confidence) | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | 2.9 × 10−2 (2.7 × 10−2–3.3 × 10−2) |
| 48-h LC50 (95% confidence) | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. |
| 48-h LC90 (95% confidence) | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. |
N.T. = Not tested.
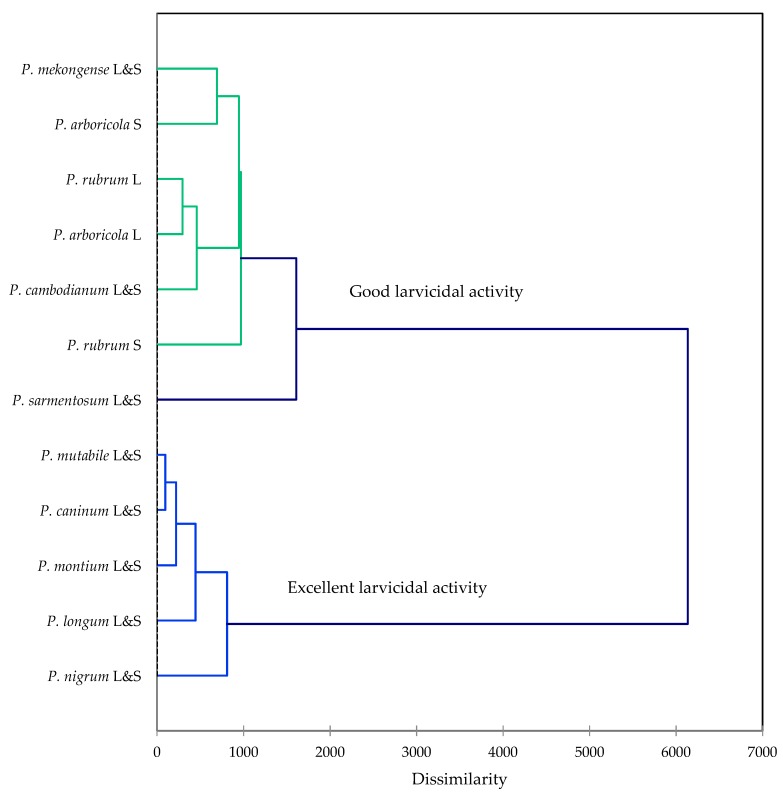
In order to provide insight into the components responsible for the larvicidal activity, a multivariate analysis, agglomerative hierarchical cluster (AHC), and principal component analysis (PCA) were carried out to correlate composition with activity. The cluster analysis revealed two clearly defined clusters, one cluster with excellent larvicidal activity (P. mutabile, P. caninum, P. montium, P. longum, and P. nigrum) and another cluster of Piper essential oils with good larvicidal activity (Figure 2).
Figure 2.
Agglomerative hierarchical cluster analysis based on the major components of the Piper essential oils from central Vietnam along with larvicidal activities (LC50 and LC90) against Aedes aegypti.
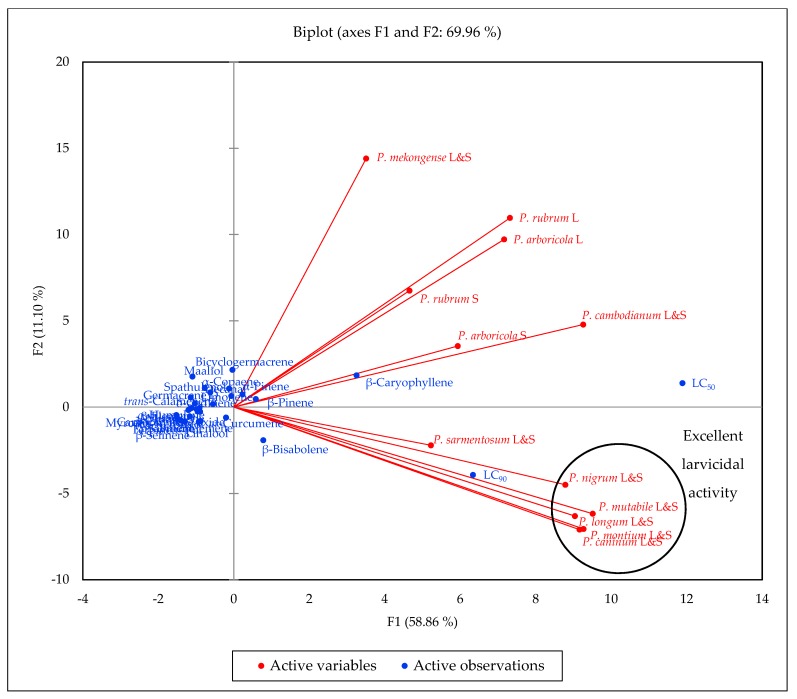
The principal component analysis indicates a positive correlation between Ae. aegypti larvicidal activity (LC50 and LC90) and concentrations of β-caryophyllene, β-bisabolene, α-pinene, and β-pinene (Figure 3). In addition, P. caninum, P. montium, and P. mutabile essential oils had relatively high concentrations of ar-curcumene; P. caninum, P. longum, P. montium, and P. mutabile had relatively high concentrations of linalool; and P. nigrum essential oil was rich in δ-elemene.
Figure 3.
Principal component biplot of PC1 and PC2 scores and loadings indicating the correlation of chemical components of Piper essential oils from central Vietnam and Aedes aegypti larvicidal activity.
Several Piper essential oils had previously demonstrated larvicidal activity against Ae. aegypti [55,56,57]. The fruit essential oil of P. aduncum from northeastern Brazil was found to be rich in both β-pinene (32.7%) and β-caryophyllene (17.1%) and showed larvicidal activity with LC50 of 30.2 µg/mL [58]. Similarly, P. nigrum fruit essential oil with β-caryophyllene (24.2%) and β-pinene (5.4%) had LC50 of 75.8 µg/mL [58]. The phenylpropanoid-rich essential oils of P. permucronatum and P. hostmanianum showed larvicidal activity against Ae. aegypti of 36 and 54 µg/mL, respectively [56]. Furthermore, P. sarmentosum essential oil from Thailand, dominated by croweacin (71.0%) and β-caryophyllene (7.4%), showed very good mosquito larvicidal activity against Anopheles cracens [59]. In addition, α-pinene, β-pinene [60], linalool [61], and β-caryophyllene [62] had previously shown Ae. aegypti larvicidal activities with LC50 of 15.4, 12.1, 38.6, and 88.3 µg/mL, respectively.
3. Materials and Methods
3.1. Plant Material
Piper plant material (leaves and stems) was collected from several different locations in central Vietnam (Table 1). The plants were identified by Dr. Do Ngoc Dai, and voucher specimens (see Table 1) have been deposited in the Pedagogical Institute of Science, Vinh University. The fresh plant materials (2.0 kg each) were shredded and hydrodistilled for 4 h using a Clevenger type apparatus (Witeg Labortechnik, Wertheim, Germany). Essential oil yields are summarized in Table 1.
3.2. Gas Chromatographic-Mass Spectral Analysis
Each of the Piper essential oils was analyzed by gas chromatography–mass spectrometry (GC-MS) as previously described [63]: Shimadzu GCMS-QP2010 Ultra (Shimadzu Scientific Instruments, Columbia, MD, USA), electron impact (EI) mode (electron energy = 70 eV), scan range = 40–400 atomic mass units, scan rate = 3.0 scans/s, and GC–MS solution software. The GC column was a ZB-5 fused silica capillary column (30 m length × 0.25 mm internal diameter) with a (5% phenyl)-polymethylsiloxane stationary phase and a film thickness of 0.25 μm; He carrier gas with a column head pressure of 552 kPa and flow rate of 1.37 mL/min; injector temperature = 250 °C, ion source temperature = 200 °C. The GC oven temperature program was programmed to have an initial temperature of 50 °C, and the temperature increased at a rate of 2 °C/min to 260 °C. A 5% w/v solution of the sample in CH2Cl2 was prepared, and 0.1 μL was injected with a splitting mode (30:1). Identification of the oil components was based on their retention indices determined by reference to a homologous series of n-alkanes, and by comparison of their mass spectral fragmentation patterns with those reported in the databases [64,65,66,67]. The percentages of each component in the essential oils are reported as raw percentages based on total ion current without standardization. Gas chromatograms for the Piper essential oils are available as supplementary material.
3.3. Mosquito Larvicidal Assay
Eggs of Ae. aegypti were purchased from Institute of Biotechnology, Vietnam Academy of Science and Technology, and maintained at the Laboratory of Department of Pharmacy of Duy Tan University, Da Nang, Vietnam. For the assay, aliquots of the essential oils of Piper species, dissolved in DMSO (1% stock solution), was placed in a 500-mL beaker and added to water that contained 20 larvae (third and early fourth instar). With each experiment, a set of controls using DMSO was also run for comparison. Mortality was recorded after 24 h and again after 48 h of exposure during which no nutritional supplement was added. The experiments were carried out 25 ± 2 °C. Each test was conducted with four replicates with several concentrations (100, 50, 25, 12.5, 6.0, 3.0, 1.5, and 0.7 μg/mL). Larvicidal activities against Ae. albopictus and Culex quinquefasciatus (reared from wild populations) were determined similarly with concentrations of 150, 100, 50, 25, and 12.5 μg/mL. Permethrin was used as a positive control.
3.4. Statistical Analysis
Mosquito larvicidal activities (LC50 and LC90) were determined by log-probit analysis using XLSTAT v. 2018.5 (Addinsoft, Paris, France). The chemical compositions of the Piper essential oils were used in the multivariate analyses. The essential oil compositions were treated as operational taxonomic units (OTUs), and the concentrations (percentages) of 29 major essential oil components and the 24-h LC50 and LC90 larvicidal activity data were used to determine the associations between the Piper essential oils using agglomerative hierarchical cluster (AHC) analysis using XLSTAT Premium, version 2018.5 (Addinsoft, Paris, France). Dissimilarity was determined using Euclidean distance, and clustering was defined using Ward’s method. For the principal component analysis (PCA), the 29 major components and the larvicidal data were taken as variables using a Pearson correlation matrix using XLSTAT Premium, version 2018.5 (Addinsoft, Paris, France). A total of 414 data (31 variables × 12 samples) were used for the PCA.
4. Conclusions
Plant materials from 13 species of Piper, growing in central Vietnam were collected and the essential oils obtained and chemically analyzed. Larvicidal activity assays indicated that five Piper species (P. nigrum, P. mutabile, P. longum, P. montium, and P. caninum) showed excellent larvicidal activities with LC50 and LC90 values < 10 μg/mL, and that the larvicidal activities are correlated with concentrations of β-caryophyllene, β-bisabolene, β-pinene, and α-pinene. These Piper essential oils deserve consideration for future applications as natural, sustainable alternatives to synthetic pesticides for mosquito control.
Acknowledgments
P.S. and W.N.S. participated in this work as part of the activities of the Aromatic Plant Research Center (APRC, https://aromaticplant.org/).
Supplementary Materials
The following are available, Figures S1–S16: Total Ion Chromatograms of Essential Oils of Piper Species from Central Vietnam.
Author Contributions
Conceptualization, L.T.H. and P.S.; Data curation, W.N.S.; Formal analysis, W.N.S.; Funding acquisition, N.H.H.; Investigation, L.T.H., N.H.H., D.N.D., T.A.T., and V.T.H.; Methodology, L.T.H., N.H.H., and P.S.; Project administration, N.H.H.; Resources, L.T.H., N.H.H., D.N.D., and P.S.; Software, P.S.; Supervision, N.H.H. and W.N.S.; Validation, L.T.H., N.H.H., P.S., and W.N.S.; Visualization, W.N.S.; Writing—original draft, W.N.S.; Writing—review and editing, L.T.H., N.H.H., D.N.D., P.S., and W.N.S.
Funding
This research was funded by Duy Tan University.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Essential oil samples are not currently available.
References
- 1.Mabberley D.J. Mabberley’s Plant-Book. 3rd ed. Cambridge University Press; Cambridge, UK: 2008. [Google Scholar]
- 2.Salehi B., Zakaria Z.A., Gyawali R., Ibrahim S.A., Rajkovic J., Shinwari Z.K., Khan T., Sharifi-Rad J., Ozleyen A., Turkdonmez E., et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules. 2019;24:1364. doi: 10.3390/molecules24071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaveerach A., Mokkamul P., Sudmoon R., Tanee T. Ethnobotany of the Genus Piper (Piperaceae) in Thailand. Ethnobot. Res. Appl. 2006;4:223–231. doi: 10.17348/era.4.0.223-231. [DOI] [Google Scholar]
- 4.Vo V.C. The Dictionary of Medicinal Plants of Vietnam 1. Medical Publishing House; Hanoi, Vietnam: 2012. [Google Scholar]
- 5.Van Chinh H., Hoi T.M. Bổ A new record of Piper minutistigmum C. DC. (Piperaceae) for flora in Vietnam. VNU J. Sci. Nat. Sci. Technol. 2019;35:32–35. [Google Scholar]
- 6.AMCA Mosquito-Borne Diseases. [(accessed on 2 October 2019)]; Available online: https://www.mosquito.org/page/diseases.
- 7.Lien P.T.K., Briant L., Tang T.B., Trang B.M., Gavotte L., Cornillot E., Duoc V.T., Duong T.N., Frutos R., Nga P.T. Surveillance of dengue and chikungunya infection in Dong Thap, Vietnam: A 13-month study. Asian Pac. J. Trop. Med. 2016;9:39–43. doi: 10.1016/j.apjtm.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Pham Thi K.L., Briant L., Gavotte L., Labbe P., Perriat-Sanguinet M., Cornillot E., Vu T.D., Nguyen T.Y., Tran V.P., Nguyen V.S., et al. Incidence of dengue and chikungunya viruses in mosquitoes and human patients in border provinces of Vietnam. Parasites Vectors. 2017;10:556. doi: 10.1186/s13071-017-2422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett A.D.T., Higgs S. Yellow fever: A disease that has yet to be conquered. Annu. Rev. Entomol. 2007;52:209–229. doi: 10.1146/annurev.ento.52.110405.091454. [DOI] [PubMed] [Google Scholar]
- 10.Gubler D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998;11:480–496. doi: 10.1128/CMR.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhimal M., Gautam I., Joshi H.D., Hara R.B.O. Risk factors for the presence of Chikungunya and dengue vectors (Aedes aegypti and Aedes albopictus), their altitudinal distribution and climatic determinants of their abundance in central Nepal. PLoS Negl. Trop. Dis. 2015;9:e0003545. doi: 10.1371/journal.pntd.0003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benelli G., Mehlhorn H. Declining malaria, rising of dengue and Zika virus: Insights for mosquito vector control. Parasitol. Res. 2016;115:1747–1754. doi: 10.1007/s00436-016-4971-z. [DOI] [PubMed] [Google Scholar]
- 13.Lambrechts L., Scott T.W., Gubler D.J. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 2010;4:e646. doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lourenço de Oliveira R., Vazeille M., de Filippis A.M.B., Failloux A.B. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. Am. J. Trop. Med. Hyg. 2003;69:105–114. doi: 10.4269/ajtmh.2003.69.105. [DOI] [PubMed] [Google Scholar]
- 15.Vazeille M., Moutailler S., Coudrier D., Rousseaux C., Khun H., Huerre M., Thiria J., Dehecq J.S., Fontenille D., Schuffenecker I., et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE. 2007;2:e1168. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong P.-S.J., Li M.I., Chong C.-S., Ng L.-C., Tan C.-H. Aedes (Stegomyia) albopictus (Skuse): A potential vector of Zika virus in Singapore. PLoS Negl. Trop. Dis. 2013;7:e2348. doi: 10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albuquerque C.M.R., Cavalcanti V.M.S., Melo M.A.V., Verçosa P., Regis L.N., Hurd H. Bloodmeal microfilariae density and the uptake and establishment of Wuchereria bancrofti infections in Culex quinquefasciatus and Aedes aegypti. Mem. Inst. Oswaldo Cruz. 1999;94:591–596. doi: 10.1590/S0074-02761999000500005. [DOI] [PubMed] [Google Scholar]
- 18.Turell M.J. Members of the Culex pipiens complex as vectors of viruses. J. Am. Mosq. Control Assoc. 2012;28:123–127. doi: 10.2987/8756-971X-28.4.123. [DOI] [PubMed] [Google Scholar]
- 19.van den Hurk A.F., Hall-Mendelin S., Jansen C.C., Higgs S. Zika virus and Culex quinquefasciatus mosquitoes: A tenuous link. Lancet Infect. Dis. 2017;17:1014–1016. doi: 10.1016/S1473-3099(17)30518-2. [DOI] [PubMed] [Google Scholar]
- 20.Hemingway J., Ranson H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 21.Naqqash M.N., Gökçe A., Bakhsh A., Salim M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 2016;115:1363–1373. doi: 10.1007/s00436-015-4898-9. [DOI] [PubMed] [Google Scholar]
- 22.Vontas J., Kioulos E., Pavlidi N., Morou E., della Torre A., Ranson H. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic. Biochem. Physiol. 2012;104:126–131. doi: 10.1016/j.pestbp.2012.05.008. [DOI] [Google Scholar]
- 23.Liu N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015;60:537–559. doi: 10.1146/annurev-ento-010814-020828. [DOI] [PubMed] [Google Scholar]
- 24.Smith L.B., Kasai S., Scott J.G. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 2016;133:1–12. doi: 10.1016/j.pestbp.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Kamrin M.A. Pesticide Profiles: Toxicity, Environmental Impact, and Fate. CRC Press; Boca Raton, FL, USA: 1997. [Google Scholar]
- 26.Goulson D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013;50:977–987. doi: 10.1111/1365-2664.12111. [DOI] [Google Scholar]
- 27.Silva W.J., Dória G.A.A., Maia R.T., Nunes R.S., Carvalho G.A., Blank A.F., Alves P.B., Marçal R.M., Cavalcanti S.C.H. Effects of essential oils on Aedes aegypti larvae: Alternatives to environmentally safe insecticides. Bioresour. Technol. 2008;99:3251–3255. doi: 10.1016/j.biortech.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 28.Benelli G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015;114:2801–2805. doi: 10.1007/s00436-015-4586-9. [DOI] [PubMed] [Google Scholar]
- 29.Masetti A. The potential use of essential oils against mosquito larvae: A short review. Bull. Insectol. 2016;69:307–310. [Google Scholar]
- 30.Pavela R., Benelli G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016;21:1000–1007. doi: 10.1016/j.tplants.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Andrade-Ochoa S., Sánchez-Torres L.E., Nevárez-Moorillón G.V., Camacho A.D., Nogueda-Torres B. Aceites esenciales y sus constituyentes como una alternativa en el control de mosquitos vectores de enfermedades. Biomedica. 2017;37:224–243. doi: 10.7705/biomedica.v37i0.3475. [DOI] [PubMed] [Google Scholar]
- 32.Pham H.H. An Illustrated Flora of Vietnam, Vol. 1. Young Publishing House; Ho Chi Minh City, Vietnam: 1999. [Google Scholar]
- 33.Missouri Botanical Garden Tropicos.org. [(accessed on 27 October 2019)]; Available online: www.tropicos.org.
- 34.da Silva J.K.R., Silva N.N.S., Santana J.F.S., Andrade E.H.A., Maia J.G.S., Setzer W.N. Phenylpropanoid-rich essential oils of Piper species from the Amazon and their antifungal and anti-cholinesterase activities. Nat. Prod. Commun. 2016;11:1907–1911. doi: 10.1177/1934578X1601101233. [DOI] [PubMed] [Google Scholar]
- 35.da Silva J.K., da Trindade R., Alves N.S., Figueiredo P.L., Maia J.G.S., Setzer W.N. Essential oils from Neotropical Piper species and their biological activities. Int. J. Mol. Sci. 2017;18:2571. doi: 10.3390/ijms18122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiang C.P., Han J.X., Li X.C., Li Y.H., Zhang Y., Chen L., Qu Y., Hao C.Y., Li H.Z., Yang C.R., et al. Chemical composition and acetylcholinesterase inhibitory activity of essential oils from Piper ppecies. J. Agric. Food Chem. 2017;65:3702–3710. doi: 10.1021/acs.jafc.7b01350. [DOI] [PubMed] [Google Scholar]
- 37.Dictionary of Natural Products Dictionary of Natural Products on DVD. [(accessed on 27 October 2019)];2019 Available online: https://www.bookdepository.com/Dictionary-Natural-Products-on-DVD-John-Buckingham/9780412491504.
- 38.Lesueur D., Bighelli A., Casanova J., Hoi T.M., Thai T.H. Composition of the essential oil of Piper bavinum C. DC. from Vietnam. J. Essent. Oil Res. 2009;21:16–18. doi: 10.1080/10412905.2009.9700095. [DOI] [Google Scholar]
- 39.Zaveri M., Khandhar A., Patel S., Patel A. Chemistry and pharmacology of Piper longum L. Int. J. Pharm. Sci. Rev. Res. 2010;5:67–76. [Google Scholar]
- 40.Khushbu C., Roshni S., Anar P., Carol M., Mayuree P. Phytochemical and therapeutic potential of Piper longum Linn: A review. Int. J. Res. Ayurveda Pharm. 2011;2:157–161. [Google Scholar]
- 41.Varughese T., Unnikrishnan P.K., Deepak M., Balachandran I., Rema Shree A.B. Chemical composition of the essential oils from stem, root, fruit and leaf of Piper longum Linn. J. Essent. Oil-Bearing Plants. 2016;19:52–58. doi: 10.1080/0972060X.2015.1119065. [DOI] [Google Scholar]
- 42.Hieu L.D., Hoi T.M., Thang T.D., Eresanya O.I., Ogunwande I.A. Fonenol, the main constituent of the essential oil of the leaf of Piper longum L. Am. J. Essent. Oils Nat. Prod. 2018;6:16–19. [Google Scholar]
- 43.Parthasarathy U., Asish G.R., Zachariah T.J., Saji K.V., George J.K., Jayarajan K., Mathew P.A., Parthasarathy V.A. Spatial influence on the important volatile oils of Piper nigrum leaves. Curr. Sci. 2008;94:1632–1635. [Google Scholar]
- 44.Zachariah T.J., Safeer A.L., Jayarajan K., Leela N.K., Vipin T.M., Saji K.V., Shiva K.N., Parthasarathy V.A., Mammootty K.P. Correlation of metabolites in the leaf and berries of selected black pepper varieties. Sci. Hortic. (Amsterdam) 2010;123:418–422. doi: 10.1016/j.scienta.2009.09.017. [DOI] [Google Scholar]
- 45.da Trindade R., Almeida L., Xavier L., Lins A.L., Andrade E.H., Maia J.G., Mello A., Setzer W.N., Ramos A., da Silva J.K. Arbuscular mycorrhizal fungi colonization promotes changes in the volatile compounds and enzymatic activity of lipoxygenase and phenylalanine ammonia lyase in Piper nigrum L. ‘Bragantina.’ Plants. 2019;8:442. doi: 10.3390/plants8110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ab Rahman S.F., Sijam K., Omar D. Piper sarmentosum Roxb.: A mini review of ethnobotany, phytochemistry and pharmacology. J. Anal. Pharm. Res. 2016;2:31. [Google Scholar]
- 47.Chieng T.C., Assim Z.B., Fasihuddin B.A. Toxicity and antitermite activities of the essential oils from Piper sarmentosum. Malaysian J. Anal. Sci. 2008;12:234–239. [Google Scholar]
- 48.Qin W., Huang S., Li C., Chen S., Peng Z. Biological activity of the essential oil from the leaves of Piper sarmentosum Roxb. (Piperaceae) and its chemical constituents on Brontispa longissima (Gestro) (Coleoptera: Hispidae) Pestic. Biochem. Physiol. 2010;96:132–139. doi: 10.1016/j.pestbp.2009.10.006. [DOI] [Google Scholar]
- 49.Hieu L.D., Thang T.D., Hoi T.M., Ogunwande I.A. Chemical composition of essential oils from four Vietnamese species of Piper (Piperaceae) J. Oleo Sci. 2014;63:211–217. doi: 10.5650/jos.ess13175. [DOI] [PubMed] [Google Scholar]
- 50.Moraes M.S., Machado S.R., Marques M.O.M. Essential oil of the Pothomorphe peltata (L.) Miq. J. Essent. Oil Res. 2004;16:15–16. doi: 10.1080/10412905.2004.9698637. [DOI] [Google Scholar]
- 51.Perigo C.V., Torres R.B., Bernacci L.C., Guimarães E.F., Haber L.L., Facanali R., Vieira M.A.R., Quecini V., Marques M.O.M. The chemical composition and antibacterial activity of eleven Piper species from distinct rainforest areas in Southeastern Brazil. Ind. Crops Prod. 2016;94:528–539. doi: 10.1016/j.indcrop.2016.09.028. [DOI] [Google Scholar]
- 52.Morandim-Giannetti A.A., Pin A.R., Pietro N.A.S., de Oliveira H.C., Mendes-Giannini M.J.S., Alecio A.C., Kato M.J., de Oliveira J.E., Furlan M. Composition and antifungal activity against Candida albicans, Candida parapsilosis, Candida krusei and Cryptococcus neoformans of essential oils from leaves of Piper and Peperomia species. J. Med. Plants Res. 2010;4:1810–1814. [Google Scholar]
- 53.Vogler B., Noletto J.A., Haber W.A., Setzer W.N. Chemical constituents of the essential oils of three Piper species from Monteverde, Costa Rica. J. Essent. Oil Bear. Plants. 2006;9:230–238. doi: 10.1080/0972060X.2006.10643496. [DOI] [Google Scholar]
- 54.Rodríguez E.J., Saucedo-Hernández Y., Vander Heyden Y., Simó-Alfonso E.F., Ramis-Ramo G., Lerma-García M.J., Monteagudo U., Bravo L., Medinilla M., de Armas Y., et al. Chemical analysis and antioxidant activity of the essential oils of three Piperaceae species growing in the central region of Cuba. Nat. Prod. Commun. 2013;8:1325–1328. doi: 10.1177/1934578X1300800935. [DOI] [PubMed] [Google Scholar]
- 55.Dias C.N., Fernandes D., Moraes C. Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicide: Review. Parasitol. Res. 2014;113:565–592. doi: 10.1007/s00436-013-3687-6. [DOI] [PubMed] [Google Scholar]
- 56.de Morais S.M., Facundo V.A., Bertini L.M., Cavalcanti E.S.B., dos Anjos Júnior J.F., Ferreira S.A., de Brito E.S., Neto M.A. Chemical composition and larvicidal activity of essential oils from Piper species. Biochem. Syst. Ecol. 2007;35:670–675. doi: 10.1016/j.bse.2007.05.002. [DOI] [Google Scholar]
- 57.Marques A.M., Kaplan M.A.C. Active metabolites of the genus Piper against Aedes aegypti: Natural alternative sources for dengue vector control. Univ. Sci. 2015;20:61–82. doi: 10.11144/Javeriana.SC20-1.amgp. [DOI] [Google Scholar]
- 58.da Costa J.G.M., dos Santos P.F., Brito S.A., Rodrigues F.F.G., Coutinho H.D.M., Botelho M.A., de Lima S.G. Composição química e toxicidade de óleos essenciais de espécies de Piper frente a larvas de Aedes aegypti L. (Diptera: Culicidae) Lat. Am. J. Pharm. 2010;29:463–467. [Google Scholar]
- 59.Intirach J., Junkum A., Tuetun B., Choochote W., Chaithong U., Jitpakdi A., Riyong D., Champakaew D., Pitasawat B. Chemical constituents and combined larvicidal effects of selected essential oils against Anopheles cracens (Diptera: Culicidae) Psyche (Stuttg). 2012;2012:591616. doi: 10.1155/2012/591616. [DOI] [Google Scholar]
- 60.Lucia A., Gonzalez Audino P., Seccacini E., Licastro S., Zerba E., Masuh H. Larvicidal effect of Eucalyptus grandis essential oil and turpentine and their major components on Aedes aegypti larvae. J. Am. Mosq. Control Assoc. 2007;23:299–303. doi: 10.2987/8756-971X(2007)23[299:LEOEGE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 61.Govindarajan M. Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (Willd.) Hook. f. ex Benth (Rutaceae) against three mosquito species. Asian Pac. J. Trop. Med. 2010;3:874–877. doi: 10.1016/S1995-7645(10)60210-6. [DOI] [Google Scholar]
- 62.Perumalsamy H., Kim N.-J., Ahn Y.-J. Larvicidal activity of compounds isolated from Asarum heterotropoides against Culex pipiens pallens, Aedes aegypti, and Ochlerotatus togoi (Diptera: Culicidae) J. Med. Entomol. 2009;46:1420–1423. doi: 10.1603/033.046.0624. [DOI] [PubMed] [Google Scholar]
- 63.Hung N.H., Satyal P., Hieu H.V., Chuong N.T.H., Dai D.N., Huong L.T., Tai T.A., Setzer W.N. Mosquito larvicidal activity of the essential oils of Erechtites species growing wild in Vietnam. Insects. 2019;10:47. doi: 10.3390/insects10020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 65.Mondello L. FFNSC 3. Shimadzu Scientific Instruments; Columbia, ML, USA: 2016. [Google Scholar]
- 66.NIST17 . NIST17. National Institute of Standards and Technology; Gaithersburg, ML, USA: 2017. [Google Scholar]
- 67.Satyal P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils, University of Alabama in Huntsville. [(accessed on 7 October 2019)];2015 Available online: https://cdm16608.contentdm.oclc.org/digital/collection/p16608coll23/id/32103/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.