Abstract
PURPOSE.
To determine the functional relationship between androgen receptor (AR) and PDGF D as it relates to the radiation response of PTEN-null prostate cancer (PCa) cells and the effect of enzalutamide on these interactions.
METHODS AND MATERIALS.
Using murine PTEN-null prostate epithelial cell line and human prostate carcinoma LNCaP (PTEN-mutant) models, nuclear and cytosolic AR levels were determined by immunoblot analysis and the transcriptional activity of nuclear AR was assessed by RT-PCR analysis of its target genes with or without irradiation. Cell survival was evaluated by clonogenic assay or sulforhodamine B (SRB) assay upon irradiation in the absence or presence of the AR antagonist enzalutamide.
RESULTS.
PTEN loss resulted in upregulation of AR expression in a PDGF-D dependent manner and irradiation selectively increased the nuclear AR protein level and its activity in a murine cell model. When the functional significance of AR in cell survival was tested, treatment with enzalutamide resulted in radiosensitization of human LNCaP cells. Similarly to the murine model, PDGF-D overexpression increased the nuclear AR level and its transcriptional activity in LNCaP cells. PDGF-D over-expression was associated with radioresistance and enzalutamide treatment effectively reversed PDGF-D-mediated radioresistance in LNCaP cells.
CONCLUSIONS.
We have demonstrated that AR, a target of the PTEN and PDGF D-downstream signaling program, contributes to radiation resistance in human PCa cells. In addition, this study suggests that anti-androgens such as enzalutamide may serve as radiation sensitizers for the treatment of PCa patients, particularly so in patients with loss of PTEN or overexpression of PDGF-D.
Keywords: PDGF D, radiation, PTEN, prostate cancer
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed non-cutaneous malignancy in men, with an expected incidence of over 230,000 cases in the United States in 2014 [1]. Outcomes following surgery or radiation for men with localized disease remain very favorable, with 5 year survival rates exceeding 95%. In those patients that present with unfavorable characteristics such as high grade (Gleason’s score ≥ 8) or locally advanced (≥T2c) disease, however, outcomes remain comparatively inferior [2,3]. A myriad of genetic alterations have been implicated in the development of PCa and have been associated with its prognosis [4]. Deletion of the phosphatase and tensin homolog (PTEN) gene, which functions as a critical tumor suppressor, is an event that is detectable in 20–68% of all primary PCa and has been correlated with more aggressive disease and poor outcomes in patients with PCa [5–7].
Previous work performed in our laboratory using murine prostate epithelial cell lines established from control and prostate specific PTEN-knockout mice demonstrated that loss of the PTEN gene results in a ligand switch from PDGF B to PDGF D, resulting in a selective increase of platelet-derived growth factor D (PDGF D) expression and its cognate receptor β-PDGFR [8]. The PDGF family of proteins is composed of four ligands (A-D) that are critical to the maintenance and homeostasis of normal tissues by regulating stromal interactions [9]. Aside from their role in normal tissue maintenance, the PDGF family has also been implicated in carcinogenesis and invasiveness, and has been shown to correlate with PCa stage [10]. We have previously shown that PDGF D expression induces an invasive phenotype of PCa cells and promotes intraosseous tumor growth [11,12]. Importantly, we have demonstrated that PTEN deletion results in increased tumorigenicity and radiation resistance that is PDGF D dependent [13].
In the present study, we tested our hypothesis that PTEN and PDGF D-mediated radioresistance involves modulation of the androgen receptor (AR) signaling system. AR is a nuclear steroid hormone receptor that binds to androgen and regulates gene expression critical for the development and maintenance of the prostate gland, as well as carcinogenesis and progression of PCa [14]. Our hypothesis is also based on a previous report demonstrating that loss of the PTEN gene is correlated with increased AR expression and castrate-resistance [15]. Thus, we aimed to assess the functional relationship between PDGF D and AR for the radiation resistant phenotype in PCa cells. Moreover, we wanted to investigate the effect of a second-generation anti-androgen (enzalutamide) on the radiation response of human PCa cells in the cellular context of PDGF D/AR signaling.
MATERIALS AND METHODS
Cell Lines and Culture
Establishment and the culture condition of PTEN−/− PCa and PTEN+/+ prostate epithelial cell lines were described previously [8]. Establishment and the culture condition of control and PDGF D overexpressing LNCaP lines were described previously [11].
Reagents
Antibodies were purchased: AR (Santa Cruz Biotechnology; Santa Cruz, CA); Akt and PTEN (Cell Signaling Technology; Danvers, MA); β-actin (Sigma; St. Louis, MO); and Histone H1 (Millipore; Billerica, MA). Inhibitors were purchased: Cyclohexamide, MG132, and PD98059 (Sigma); JNK Inhibitor II (Fisher Scientific, Waltham, MA); BAY 11–7085 (Santa Cruz Biotechnology); Enzalutamide (Selleck Chemicals, Houston, TX).
Immunoblot Analysis
Cells were grown to confluence, washed twice with PBS then serum starved for the specified time points. Cells were then harvested in cold PBS and lysed as described by Najy A. et al. [16]. Briefly, cells were lysed in radioimmunoprecipitation assay lysis buffer (Millipore) containing 1 mmol/L phenylmethylsulfonylfluoride, 2 mmol/L sodium orthovanidate, 1 mmol/L sodium fluoride, and a complete protease inhibitor cocktail (Roche) for 30min, then centrifuged for another 20min. Lysate was quantified using the BCA protein assay kit (Pierce Biotechnology, Grand Island, NY) and 30 μg was used for immunoblot analysis.
For the analysis of PDGF D expression, vector control (Neo) and PDGF D over-expressing LNCaP cells were grown to confluence, washed twice with PBS then serum starved for 16 hr. Conditioned media was cleared of cell debris via centrifugation and subjected to immunoblot anlaysis using a custom PDGF D antibody against the PDGF D growth factor domain [11].
Radiation Delivery
Cells were grown on 100 mm dishes until reaching 90% confluence, and then changed into phenol-red free advanced DMEM or RPMI for PTEN−/− and LNCaP cells, respectively. Enzalutamide-treated parental LNCaP cells were supplemented with 1% charcoal-stripped serum (CSS) and 10 nM of the synthetic androgen, R1881 (Perkin Elmer, Norton, OH). Cells were then irradiated to 0 (sham), 2 or 6Gy utilizing a Co-60 source with a dose rate of approximately 150 cGy per min.
Subcellular Fractionation
Cells were harvested in 1M HEPES, 1M KCl, 0.5 M EDTA, 0.1 M EGTA, and 10% NP-40 on ice at 1 and 4 hr following irradiation. Cell lysates were spun at 13,000 rpm and cytosolic supernatant was obtained. The remaining pellet was suspended with nuclear extraction buffer consisting of 1M HEPES, 5M NaCl, 0.5 M EDTA, and 0.1 M EGTA on ice for 30 min, followed by centrifugation at 13,000 rpm. Protein concentration and SDS-PAGE was performed as described above. In an experiment to address the AR protein stabilization, parental LNCaP cells were treated with 2 μg/ml cyclohexamide in phenol-red free RPMI for 2 hr to prevent new protein synthesis. Cyclohexamide was removed and cells were treated with vehicle (DMSO) or 50 μM MG132 prior to radiation. Cells were then harvested 1 hr post radiation and fractionated into cytosolic and nuclear fractions.
Inhibition of PDGF D Downstream Signaling Pathways
PDGF D LNCaP cells were grown in normal growth media then changed into phenol-red free, serum free RPMI containing vehicle, 25 μM PD98059, 25 μM JNK II Inhibitor or 10 μM BAY 11–7095 for 8hr. Cell lysates were collected and subjected to immunoblot and PCR analyses.
RT-PCR
mRNA was purified using the RNEasy kit (Qiagen, Valencia, CA). cDNA synthesis was performed using the iScript cDNA synthesis kit (BioRad, Hercules, CA), and PCR was performed using the GoTaq DNA Polymerase kit (Promega, Madison, WI) using the following primers: human/murine AR: forward ACAT-CAAGGAACTCGATCGTATCATTGC, reverse TTGGG CACTTGCACAGAGAT, murine Nkx 3.1: forward TAGGGTAGCGGAGCCCCGAG, reverse GCGACCCC CTGCTTTGTCGG, human PSA: forward GGCCAGG-TATTTCAGGTCAG, reverse CCACGATGGTGTCCTT-GAC, human TMPRSS2: forward AATCGGTGTGTTCG CCTCTAC, reverse GCGGCTGTCACGATCC and β-actin: forward CTCACCGAGCGCGGCTACA, reverse CTCCTGCTTGCTGATCCACAT.
Quantitative PCR
qPCR was performed on an Eppendorf Master-cycler ep realplex2 using the ABsolute QPCR SYBR Green Mix Plus ROX Vial (Thermo Scientific). Gene quantification was performed using the formula 2^-ΔΔCT where ΔΔCT represents the threshold cycle difference normalized to β-actin mRNA levels. Data were expressed as fold change relative to the experimental control. Assays were set up in triplicate and repeated at least three independent times.
Clonogenic Cell Survival Assay
Cells at 90% confluence were trypsinized, counted by the trypan blue exclusion assay and re-seeded onto 6-well plates in triplicate. Cells were cultured over-night in the presence of 10 μM enzalutamide or vehicle (dimethyl sulfoxide, DMSO). Subsequently, cells were irradiated with 0, 2, 4, and 6 Gy at room temperature under atmospheric oxygen conditions and maintained in culture until colonies had grown to at least 50 cells (10 days post-radiation). Colonies were washed, fixed with ice-cold 70% EtOH, and stained with 1% Crystal Violet. Survival curves were generated after normalizing the number of colonies against sham radiation (0 Gy). Two-tailed t-test was used to determine differences in the fraction of surviving colonies between the two groups.
In Vitro Toxicology Assay
Cells were plated in media containing 2% FBS ± 10 μM enzalutamide in 96-well plates. Twenty four hours post plating, cells were radiated at 0 (sham), 2, 4, and 6 Gy. Cells were fixed with 50% Trichloroacetic Acid, washed with water then stained with a Sulforhodamine B Solution according to the Sulforhodamine B based In Vitro Toxicology Assay (Sigma) kit protocol. SRB was measured by absorbance at 565 nm wavelength using a BioRad Micro-plate reader. Assay was performed in triplicate and repeated at least three independent times.
RESULTS
PDGF D Signaling Regulates AR Expression and Activity in PTEN Null Murine Prostate Cells
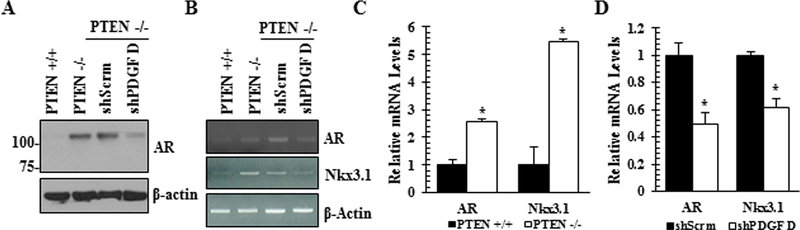
Previously we have reported that loss of PTEN leads to a PDGF ligand switch from PDGF B to PDGF D [8]. These PTEN null cells are radiation resistant and this phenotype is dependent upon PDGF D expression [13]. Since androgen receptor (AR), known to mediate survival signaling, is upregulated upon PTEN loss [15], we wished to test if AR signaling contributes to the PDGF D-dependent radiation resistance of PTEN−/− cells. In agreement with the previous report [15], AR expression is upregulated in PTEN−/− cells at both protein and RNA levels, resulting in upregulation of the AR response gene Nkx3.1 (Fig. 1). In response to PDGF D downregulation, AR expression and its activity were greatly reduced in PTEN null cells, demonstrating that PDGF D is an essential signaling component for AR expression upon PTEN loss in prostate epithelial cells.
Fig. 1.
PDGF D regulates androgen receptor (AR) expression. Parental murine PTEN wildtype (PTEN+/+), PTEN knockout (PTEN−/−), and PTEN−/− expressing scrambled (shScrm) or PDGF D shRNA (shPDGF D) prostate epithelial cells were subjected to immunoblot (A), qualitative RT-PCR (B) and quantitative RT-PCR (C-D) analyses to determine the levels of AR and its target gene NKx13.l.
Exposure to Ionizing Radiation Increases the Level of Nuclear AR Protein in Murine and Human Prostate Cancer Cells Without Wild-Type PTEN
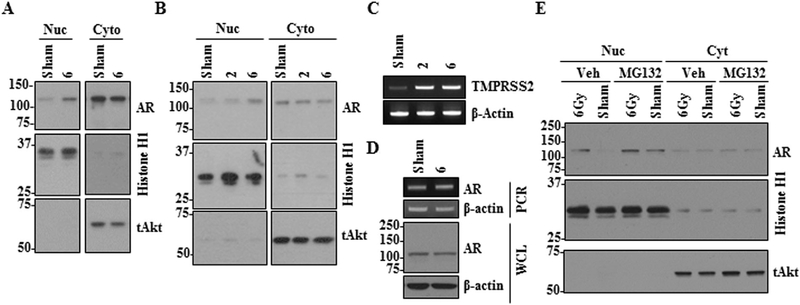
Next, we sought to determine the effect of radiation on subcellular localization of AR. To this end, murine PTEN−/− cells were exposed to 0Gy (sham treatment) or 6 Gy followed by fractionation into nuclear extract and cytosolic proteins at the indicated time points. As shown in Figure 2A, there were marked increases in the levels of nuclear AR post-irradiation. To examine if this radiation effect on AR was reproducible in human PCa cells, we utilized LNCaP cells. LNCaP was chosen because it is a well-characterized PCa cell line that carries a functional AR system and a non-functional PTEN gene, making this cell line most compatible with our murine PTEN−/− line. Consistently, irradiation effectively increased the level of nuclear AR and AR activity in LNCaP cells (Fig. 2B and C). Next we examined if enhanced nuclear AR is due to increased AR expression or protein stabilization. Exposure of parental LNCaP cells to radiation doses as high as 6 Gy had little effect on AR RNA or total protein levels (Fig. 2D). However, treatment of LNCaP cells with the proteasome inhibitor MG132 prior to radiation markedly increased AR nuclear localization in sham and 6 Gy radiated cells, suggesting that nuclear AR protein is unstable and that radiation may enhance AR stabilization leading to accumulation of AR protein in the nucleus (Fig. 2E).
Fig. 2.
Exposure to ionizing radiation increases the level of nuclear AR expression. AR levels in the nucleus and cytoplasm of murine PTEN−/− (A) and human (LNCaP) cells (B) were determined by immunoblot analysis 1 hr following exposure to 0, 2, or 6 Gy. (C) TMPRSS2 expression in LNCaP cells was examined by PCR analysis 1 hr following irradiation. (D) Expression levels of AR RNA and protein in LNCaP cells 1 hr post exposure to 0 or 6 Gy radiation were determined by PCR and immunoblot analyses using whole cell lysate (WCL), respectively. (E) Parental LNCaP cells were treated with vehicle (Veh) or MGI32 prior to exposure to 0 or 6 Gy radiation and AR levels in the nucleus and cytoplasm I hr post radiation were determined by immunoblot analysis. Histone HI and total Akt proteins were used as loading controls for nuclear and cytoplasmic fractions, respectively.
Selective Inhibition of AR Results in Radiosensitization of Human Prostate Cancer Cells
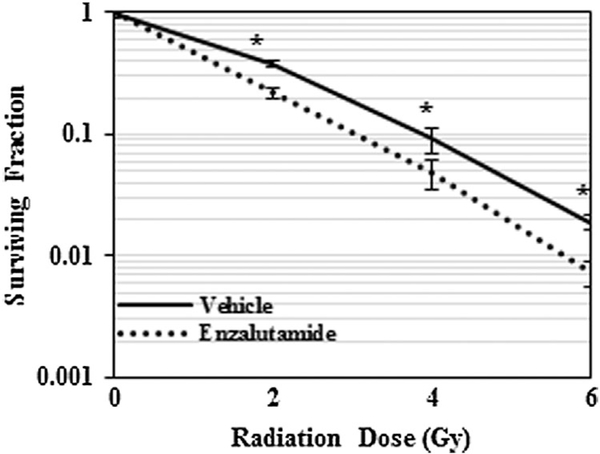
Following the analysis showing an increase in the level of nuclear AR in response to radiation, we postulated that AR contributes to radio-resistance of prostate cancer cells. To investigate this, we performed a clonogenic cell survival assay following irradiation in the presence or absence of pre-treatment with a second-generation anti-androgen, enzalutamide. We selected enzalutamide due to its increased affinity for binding to AR, as well as its demonstrated ability to prevent translocation of AR to the nucleus and subsequent activation of its downstream signaling program, an effect that classic anti-androgens such as bicalutamide do not exert [17]. As shown in Figure 3, treatment with enzalutamide resulted in a marked radiosensitization of human PCa cells to irradiation at all radiation doses (2,4, and 6 Gy).
Fig. 3.
AR inhibition sensitizes LNCaP cells to radiation. Graphic representation of clonogenic cell survival assay of LNCaP cells exposed to 0, 2, 4, 6 Gy with or without enzalutamide treatment. *P < 0.05.
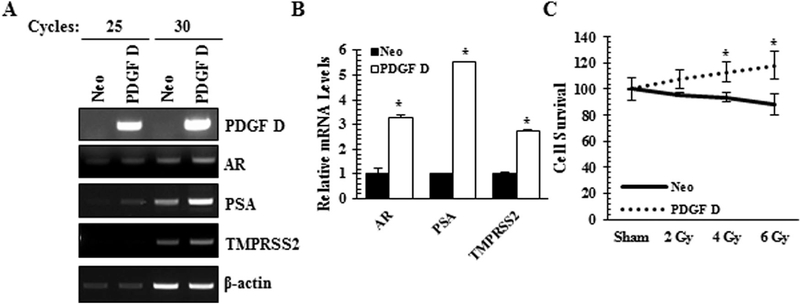
PDGF D Overexpression in Human Prostate Cancer Cells Enhances AR Expression and Its Activity
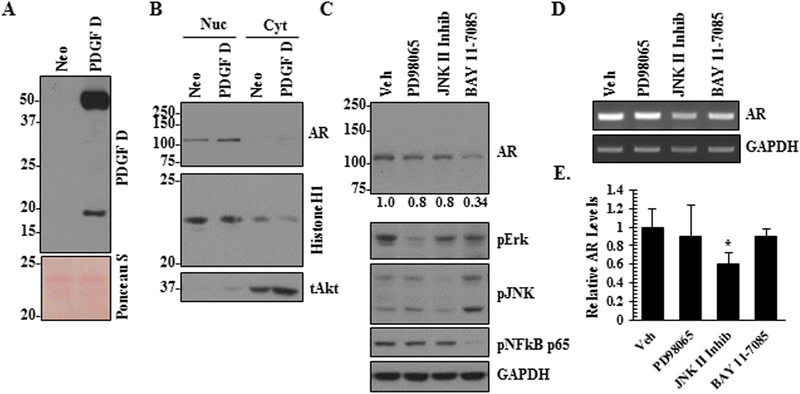
To complement the PDGF D knockdown approach in the murine PCa cells to study the PDGF D/AR axis, we overexpressed PDGF D in LNCaP cells. PDGF D is secreted into the extracellular milieu as an inactive 50 kDa protein which can be activated into an 18kDa growth factor domain by serine proteases [10,18]. In our overexpression model, we confirmed high levels of both pro- and active forms of PDGF D secreted by PDGF D LNCaP cells as compared to vector control (Neo) cells (Fig. 4A). When we examined whether PDGF D overexpression affects AR expression, subcellular fractionation revealed increases in the levels of nuclear AR protein (Fig. 4B) and AR mRNA (Fig. 5A and B). Next we made an attempt to determine which of the PDGF D downstream signaling molecule(s) play(s) a critical role for the induction of AR expression in PCa cells. Since PDGF signaling were known to activate the MAPK and NFκB pathways [16,19], we treated PDGF D LNCaP cells with Erk (PD98065), JNK (JNK II Inhibitor) and NFκB (BAY 11–7095) inhibitors and monitored AR levels. As shown in Figure 4C, the specificity of these inhibitors was confirmed by immunoblot analyses using antibodies that recognize active forms of these signaling molecules. Inhibition of NFκB markedly reversed PDGF D-induced AR expression at the protein level (Fig. 4C), while JNK inhibition significantly reduced AR expression at the RNA level (Figs. 4D and E). These results support roles of JNK and NFκB in the PDGF D-specific regulation of AR expression.
Fig. 4.
PDGF D-mediated AR regulation is JNK and NFκB-dependent. Immunoblot analyses of PDGF D in conditioned medium (A) and AR in nucleus and cytoplasm (B) of control (Neo) and PDGF D overexpressing LNCaP cells. Ponceau S was used as a loading control of conditioned media, while histone HI and total Akt proteins were used as loading controls for nuclear and cytoplasmic fractions, respectively. PDGF D LNCaP cells were treated with 25 μM PD98065, 25 Μm JNK II Inhibitor and 10 μM BAY 11–7095 for 8 hr, followed by immunoblot analysis of AR protein (C) and qualitative RT-PCR (D) and quantitative real-time RT-PCR analyses (E) of AR RNA.
Fig. 5.
PDGF D upregulates AR expression and its activity as well as radiation resistance. Qualitative RT-PCR (A) and quantitative RT-PCR (B) analyses of AR, its target genes (PSA and TMPRSS2) and β-actin as a control. (C) Radiation resistance was determined using the Sulforhodamine B based In Vitro Toxicology Assay 48 hrs post radiation. *P < 0.05.
To determine the transcriptional activity of AR, we performed qualitative and quantitative RT-PCR analyses of AR response genes such as PSA and TMPRSS2. Similarly to murine PCa cells, the PDGF D/AR signaling axis upregulated the expression levels of AR response genes (Fig. 5A and B).
Upregulation of PDGF D Leads to Radiation Resistance of LNCap PCa Cells in an AR Dependent Manner
Our previous findings demonstrated a role for PDGF D in radiation resistance of murine PCa cells [13]. We next sought to assess this phenomenon in our human PCa model. Because clonogenic assay was not possible due to the highly migratory phenotype of PDGF D LNCaP cells, alternatively the sulforhodamine B (SRB) assay) was performed to assess cell survival upon irradiation [20]. As shown in Figure 5C, PDGF D LNCaP cells were resistant to irradiation while control LNCaP cells demonstrated a significant reduction in cell survival. Interestingly, radiation promoted cell proliferation of LNCaP cells when PDGF D signaling is constitutively active in PDGF D LNCaP cells.
Next, we examined whether AR, a PDGF-D downstream signaling molecule in PCa cells, contributes to PDGF D-mediated radiation resistance. As expected, treatment with enzalutamide drastically reduced the level of nuclear AR in PDGF D LNCaP cells (Fig. 6A), accompanied with reduced AR activity as determined by quantitative RT-PCR analysis of the AR response gene PSA (Fig. 6B). Importantly, enzalutamide sensitized PDGF D LNCaP cells to radiation, effectively reversing PDGF D-mediated radiation resistant phenotype (Fig. 6C).
Fig. 6.
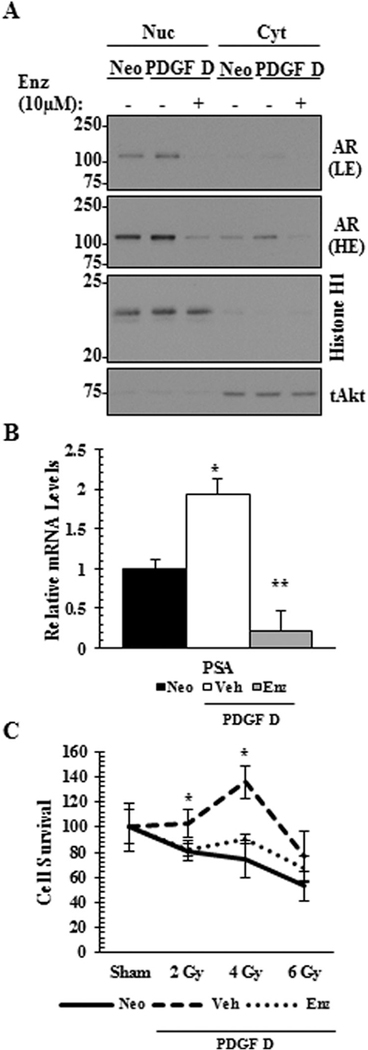
AR inhibition reverses PDGF D-mediated radiation resistance in LNCaP cells. PDGF D LNCaP cells treated with vehicle or 10 μMenzalutamide (Enz) for 16 hr were subjected to immunoblot analysis (A) to determine the levels of nuclear or cytoplasmic AR localization and to quantitative RT-PCR analysis (B) to determine the RNA level of AR target gene PSA. LE, light exposure; HE, high exposure. Radiation resistance in the presence of enzalutamide was monitored 4-days post radiation (C) using Sulforhodamine B based In Vitro Toxicology Assay. *P < 0.05.
DISCUSSION
PTEN is a well-characterized tumor suppressor gene that is responsible for regulation of the PI3K/ Akt/m-TOR signaling pathway [21]. Loss of the PTEN gene has been correlated with the development of numerous malignancies, including prostate, breast, endometrial, and primary central nervous system tumors [22]. In patients with prostate cancer who underwent surgical resection, loss of the PTEN gene on pathologic specimens was correlated with higher Gleason score, advanced tumor stage, lymph node involvement, and castrate-resistance [7]. Because of its direct inhibition of the PI3K/Akt pathway, a logical assumption is that dysregulation of this signaling axis is the primary oncogenic event in patients with loss of the PTEN gene suppressor. Indeed, multiple single institutional trials have investigated the use of mTOR inhibitors in the treatment of patients with castrate-resistant prostate cancer, mainly in the setting of metastatic disease, but have reported disappointing results thus far [23–25].
One potential mechanism by which PTEN loss may affect cell survival and oncogenesis specific to prostate cancer involves a potential interaction between androgen receptor and PTEN. The androgen receptor system plays a critical role in prostate carcinogenesis and previous work has correlated PTEN loss with increased AR expression [15]. In our study, we have confirmed the finding that AR expression is increased in PTEN−/− prostate cancer. Moreover, we have demonstrated that there is a selective increase in nuclear AR in response to radiation exposure in the murine PTEN−/− cell line and we confirmed this effect a human prostate cancer cell line (LNCaP). To our knowledge, this is the first study to investigate the effect of radiation on the expression and signaling of the AR system and our work suggests that AR plays a role in cell survival in response to radiation exposure.
Androgen deprivation therapy (ADT) has long been a mainstay in the treatment of prostate cancer, both in the setting of localized and metastatic disease. A number of large trials have demonstrated the benefits of ADT when given in concert with definitive radiotherapy in men with intermediate and high risk prostate cancer [26–28]. More recently, the development of the second-generation anti-androgen enzalutamide has generated enthusiasm for its use after the results of a large phase III trial demonstrating an impressive progression-free and overall survival benefit in men with castrate-resistant metastatic disease [29]. This drug is thought to be superior to classic anti-androgens, such as bicalutamide, due to its stronger binding affinity for AR, as well as its demonstrated ability to prevent nuclear translocation of AR [17]. Multiple prospective trials are currently looking at the use of enzalutamide as a potential radiosensitizer, both in the intact and post-operative setting. In this study, we have shown that treatment with enzalutamide results in significant radiosensitization of human LNCaP cells at all observed dose levels (2,4, and 6 Gy).
In an attempt to further characterize the relationship between AR and PTEN as it relates to radiation resistance, we sought to characterize the role of PDGF D in this interaction. We have previously shown that PDGF D expression results in increased tumor growth and an invasive phenotype of PCa cells. Additionally, we have demonstrated that PTEN−/− murine prostate epithelial cells are radioresistant compared with PTEN+/+ counterparts in a PDGF D-dependent manner [13]. In the present study, we demonstrate that AR is a downstream mediator of PDGF D signaling in response to irradiation in both murine and human PCa cells.
CONCLUSIONS
The present study demonstrates that AR-mediated cell survival signaling contributes to radiation resistance in human prostate cancers cells. In addition, our work suggests that anti-androgens such as enzalutamide may serve as radiation sensitizers for the treatment of PCa patients, particularly so in patients with loss of PTEN or overexpression of PDGF D. Future translational work is needed to determine the molecular profile of patients who would receive the greatest benefit from androgen deprivation therapy administered concomitantly with definitive radiotherapy.
ACKNOWLEDGMENTS
This work was supported by the NIH/National Cancer Institute Grants CA123362 (to H-R. C. K.).
Grant sponsor: NIH/National Cancer Institute Grant; Grant number: RO1CA123362.
REFERENCES
- 1.Howlader N NA, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute; Bethesda, MD; 2014. p Based on November 2013 SEER data submission, posted to the SEER web site, April 2014. [Google Scholar]
- 2.Nguyen QN, Levy LB, Lee AK, Choi SS, Frank SJ, Pugh TJ, McGuire S, Hoffman K, Kuban DA. Long-term outcomes for men with high-risk prostate cancer treated definitively with external beam radiotherapy with or without androgen deprivation. Cancer 2013;119(18):3265–3271. [DOI] [PubMed] [Google Scholar]
- 3.Berg A, Lilleby W, Bruland OS, Fossa SD. 10-year survival and quality of life in patients with high-risk pN0 prostate cancer following definitive radiotherapy. Int J Radiat Oncol Biol Phys 2007;69(4):1074–1083. [DOI] [PubMed] [Google Scholar]
- 4.Institute NC. PDQ® Genetics of Prostate Cancer. Bethesda, MD: National Cancer Institute 2015. [Google Scholar]
- 5.Yoshimoto M, Cutz JC, Nuin PA, Joshua AM, Bayani J, Evans AJ, Zielenska M, Squire JA. Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet Cytogenet 2006;169(2): 128–137. [DOI] [PubMed] [Google Scholar]
- 6.McCall P, Witton CJ, Grimsley S, Nielsen KV, Edwards J. Is PTEN loss associated with clinical outcome measures in human prostate cancer? Br J cancer 2008;99(8):1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krohn A, Diedler T, Burkhardt L, Mayer PS, De Silva C, Meyer-Kornblum M, Kotschau D, Tennstedt P, Huang J, Gerhauser C, Mader M, Kurtz S, Sirma H, Saad F, Steuber T, Graefen M, Plass C, Sauter G, Simon R, Minner S, Schlomm T. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am J pathol 2012;181(2):401–412. [DOI] [PubMed] [Google Scholar]
- 8.Conley-LaComb MK, Huang W, Wang S, Shi D, Jung YS, Najy A, Fridman R, Bonfil RD, Cher ML, Chen YQ, Kim HR. PTEN regulates PDGF ligand switch for beta-PDGFR signaling in prostate cancer. Am J pathol 2012;180(3):1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008;22(10): 1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ustach CV, Huang W, Conley-LaComb MK, Lin CY, Che M, Abrams J, Kim HR. A novel signaling axis of matriptase/PDGF-D/ss-PDGFR in human prostate cancer. Cancer Res 2010;70(23):9631–9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ustach CV, Taube ME, Hurst NJ Jr, Bhagat S, Bonfil RD, Cher ML, Schuger L, Kim HR. A potential oncogenic activity of platelet-derived growth factor d in prostate cancer progression. Cancer Res 2004;64(5):1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W, Fridman Y, Bonfil RD, Ustach CV, Conley-LaComb MK, Wiesner C, Saliganan A, Cher ML, Kim HR. A novel function for platelet-derived growth factor D: Induction of osteoclastic differentiation for intraosseous tumor growth. Oncogene 2012;31(42):4527–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen M, Najy AJ, Snyder M, Movilla LS, Kim HR. A critical role of the PTEN/PDGF signaling network for the regulation of radiosensitivity in adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 2014;88(1):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinog 2011;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Wu J, Suburu J, Gu Z, Cai J, Axanova LS, Cramer SD, Thomas MJ, Perry DL, Edwards IJ, Mucci LA, Sinnott JA, Loda MF, Sui G, Berquin IM, Chen YQ. Effect of dietary polyunsaturated fatty acids on castration-resistant Pten-null prostate cancer. Carcinogenesis 2012;33(2):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Najy AJ, Won JJ, Movilla LS, Kim HR. Differential tumorigenic potential and matriptase activation between PDGF B versus PDGF D in prostate cancer. Mol Cancer Res 2012;10(8): 1087–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009;324(5928):787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ustach CV, Kim HR. Platelet-derived growth factor D is activated by urokinase plasminogen activator in prostate carcinoma cells. Mol Cell Biol 2005;25(14):6279–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eger G, Papadopoulos N, Lennartsson J, Heldin CH. NR4A1 promotes PDGF-BB-induced cell colony formation in soft agar. PLoS ONE 2014;9(9):e109047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HE, Krug MA, Han I, Ensley J, Yoo GH, Forman JD, Kim HR. Neutron radiation enhances cisplatin cytotoxicity independently of apoptosis in human head and neck carcinoma cells. Clin Cancer Res 2000;6(10):4142–4147. [PubMed] [Google Scholar]
- 21.Dillon LM, Miller TW. Therapeutic targeting of cancers with loss of PTEN function. Curr Drug Targets 2014;15(1):65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer 2011;11(4):289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Templeton AJ, Dutoit V, Cathomas R, Rothermundt C, Bartschi D, Droge C, Gautschi O, Borner M, Fechter E, Stenner F, Winterhalder R, Muller B, Schiess R, Wild PJ, Ruschoff JH, Thalmann G, Dietrich PY, Aebersold R, Klingbiel D, Gillessen S. Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08). Eur Urol 2013;64(1):150–158. [DOI] [PubMed] [Google Scholar]
- 24.Kruczek K, Ratterman M, Tolzien K, Sulo S, Lestingi TM, Nabhan C. A phase II study evaluating the toxicity and efficacy of single-agent temsirolimus in chemotherapy-naive castration-resistant prostate cancer. Br J cancer 2013;109(7):1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong AJ, Shen T, Halabi S, Kemeny G, Bitting RL, Kartcheske P, Embree E, Morris K, Winters C, Jaffe T, Fleming M, George DJ. A phase II trial of temsirolimus in men with castration-resistant metastatic prostate cancer. Clin Genitourin Cancer 2013;11(4):397–406. [DOI] [PubMed] [Google Scholar]
- 26.Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, Hug EB, Asbell SO, Grignon D. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma-long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys 2005;61(5):1285–1290. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, Venkatesan V, Lawton CA, Rosenthal SA, Sandler HM, Shipley WU. Ten-year follow-up of radiation therapy oncology group protocol 92–02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 2008;26(15):2497–2504. [DOI] [PubMed] [Google Scholar]
- 28.Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Billiet I, Torecilla JL, Pfeffer R, Cutajar CL, Van der Kwast T, Collette L. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 2010;11(11): 1066–1073. [DOI] [PubMed] [Google Scholar]
- 29.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371(5):424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]