Abstract
Reexperiencing symptoms in adolescent Post‐Traumatic Stress Disorder (PTSD) are characterized by the apparition of vivid intrusive images of the traumatic event. The emergence of these intrusions is thought to be related to a deficiency in context processing and could then be related to hippocampal alterations. The hippocampus is a complex structure which can be divided into several subfields, namely, the Cornu Ammonis (CA1, CA2, and CA3), the subiculum, and the dentate gyrus (DG). As each subfield presents different histological characteristics and functions, it appears more relevant to consider hippocampal subfields, instead of only assessing the whole hippocampus, to understand the neurobiology of PTSD. Hence, this study presents the first investigation of structural alterations within hippocampal subfields and their links to reexperiencing symptoms in adolescent PTSD. Hippocampal subfields were manually delineated on high‐resolution MRI images in 15 adolescents (13–18 years old) with PTSD and 24 age‐matched healthy controls. The volume of the region CA2‐3/DG region was significantly smaller in the PTSD group compared to controls in both hemispheres. No other significant difference was found for other subfields. Moreover, the volume of the left CA2‐3/DG was negatively correlated with the intrusion score (as measured by the Impact of Events Scale‐Revised) in the PTSD group. To conclude, an alteration in the hippocampal subregion CA2‐3/DG, known to resolve interferences between new and similar stored memories, could participate in the apparition of intrusive trauma memories in adolescents with PTSD.
Keywords: adolescents, hippocampal subfields, MRI, post‐traumatic stress disorder
1. INTRODUCTION
Individuals who lived a traumatic experience are at risk of developing a post‐traumatic stress disorder (PTSD). This psychiatric disorder is associated with reexperiencing symptoms, which are characterized by the intrusions of vivid and involuntary memories of the traumatic event. PTSD symptoms also encompass avoidance of trauma‐reminders, excessive physiological arousal, and negative alteration in cognition and mood (American Psychiatric Association, 2013). Deficient context processing could be at the core of PTSD pathophysiology (Liberzon & Abelson, 2016). Processing contextual information is crucial to disambiguate cues associated with safety and threat and, consequently, adapt our behavior to the environment (Liberzon & Abelson, 2016; Maren, Phan, & Liberzon, 2013). Hence, deficient context processing could participate in the emergence of recurrent intrusive memories and the overgeneralization of fear responses in safe environments (Liberzon & Abelson, 2016). These intrusions could be related to an alteration of the hippocampal structure, known to be involved in contextual memory (Maren et al., 2013). Indeed, several neuroimaging studies reported smaller hippocampal volumes in adults suffering from PTSD compared to people who lived a traumatic experience without PTSD and compared to healthy controls (Logue et al., 2018; Pitman et al., 2012).
The hippocampus is a complex structure which can be divided into several subfields, namely, the Cornu Ammonis (CA1, CA2, and CA3), the subiculum, and the dentate gyrus (Duvernoy, 2005). These hippocampal subfields have distinct histological characteristics and present, thereby, differential vulnerability to pathological conditions such as Alzheimer's disease, schizophrenia, and major depression (Cao et al., 2017; de Flores et al., 2015; Ho et al., 2017; Tannous et al., 2018) and to other factors like stress (Gould, 2007). Furthermore, hippocampal subfields are involved in different memory processes. It has been shown that CA1 is involved in pattern completion (ability to recall a memory based on a partial cue; Carr, Rissman, & Wagner, 2010). The dentate gyrus (DG) plays a role in pattern separation (ability to diminish the similarity between two resembling memories) and CA3 is thought to be involved in both processes. Hence, investigating hippocampal subregions, instead of the hippocampus as a single entity, appears more appropriate for the understanding of the neurobiology of PTSD and the mechanisms underlying the reexperiencing symptoms.
Alterations within hippocampal subregions can be examined in volumetric studies using high‐resolution Magnetic Resonance Imaging (MRI). Wang et al. (2010) manually segmented a part of the hippocampal body and found that only the CA3/DG volume was significantly smaller in veterans with PTSD compared to veterans without PTSD. Although they did not investigate subfield volumes of the entire hippocampus, they suggested that PTSD was not related to a global atrophy of the hippocampus but to alterations of these specific subfields (Wang et al., 2010). These results are in line with theoretical models which propose that reexperiencing symptoms could be related to a pattern separation dysfunction resulting from a reduced neurogenesis in the dentate gyrus (Besnard & Sahay, 2016; Liberzon & Abelson, 2016). To our knowledge, there is no existing investigation of hippocampal subfield alterations in adolescent PTSD. This could result from the consistent finding that the hippocampus is not impaired in children and adolescents with PTSD. Unlike work in adults, most pediatric neuroimaging studies do not report any significant difference in hippocampal volume in children and adolescents with PTSD compared to controls (Morey, Haswell, Hooper, & De Bellis, 2016; Woon & Hedges, 2008) (but see (Mutluer et al., 2017)). Nonetheless, these studies may have failed to detect any alteration because of the use of low‐resolution structural MRI images or processing techniques not adapted to pediatric populations (Keding & Herringa, 2015). For instance, the automatic segmentation method Freesurfer is known to over‐estimate hippocampal volumes in children (Schoemaker et al., 2016). In addition, even if there are no global changes detected on the hippocampus, it does not mean that there are no local effects on hippocampal subfields. For example, one study (Gogtay et al., 2006) showed that the volume of the whole hippocampus in 4 years old was similar to young adults, but recent volumetric studies revealed differential ongoing maturation of the hippocampal subfields during childhood and adolescence (Daugherty, Flinn, & Ofen, 2017; Lee, Ekstrom, & Ghetti, 2014). Hence, even if there is no alteration of the volume of the whole hippocampus, PTSD symptomatology in adolescents may be associated with alterations of specific hippocampal subfields.
This present study aims to examine the volume of the hippocampus as a whole and hippocampal subfields in adolescent PTSD. The hippocampus and its subfields were manually segmented on high‐resolution 3 T MRI images and, then, their volumes were extracted. We predicted smaller volumes of the CA3 and DG subfields in adolescents with PTSD compared to controls. We also hypothesized that these alterations would be related to reexperiencing symptoms.
2. METHODS AND MATERIALS
2.1. Participants
Fifteen adolescents with PTSD, aged 13–18 years (13 females), were recruited through the departments of child and adolescent psychiatry of three French University Hospitals (Caen, Rennes, Rouen). PTSD adolescents received no psychotropic medication during the previous week and were free from other mental disorders including major depression. Twenty‐five typically developing adolescents (12 females) with no history of trauma were recruited by prospecting in several junior high schools of the region (Normandy, France). One subject was excluded due to severe motion artifacts on the MRI data.
Hence, 15 PTSD patients and 24 controls were included in the analyses (see Table 1). Some participants for this study took part in a larger project investigating self‐reference processing (Dégeilh et al., 2017). All were right‐handed and French native speakers. None of them reported any prior or neurological or learning disabilities, head trauma, and MRI contra‐indications. The study was approved by the local Ethics Committee (CPP Nord Ouest III). All adolescents and their parents signed informed consent after a comprehensive description of the study.
Table 1.
Demographic and psychopathological measures, depicting means and standard deviations (SD), for adolescents with PTSD and healthy controls
| PTSD | Controls | t | p value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Number (F/M) | 15 (13/2) | 24 (12/12) | – | – |
| Age (months) | 187.20 ± 18.46 | 196.92 ± 21.08 | 1.46 | .151 |
| IQ (WISC‐IV)a | 97.43 ± 19.48 | 109.04 ± 17.24 | 1.91 | .064 |
| Index trauma (n) |
Sexual abuse (11) Witness of suicide (2) Car accident (1) Loss of loved one (1) |
– | – | – |
| PTSD duration (months) | 25.27 ± 24.58 | – | – | – |
| Age of onset (years) | 13.33 ± 1.72 | – | – | – |
| IES‐R | ||||
| Intrusion | 20.40 ± 8.58 | 8.16 ± 7.29 | 4.76 | <.001 |
| Hyperarousal | 13.60 ± 5.37 | 3.21 ± 4.83 | 6.26 | <.001 |
| Avoidance | 18.07 ± 7.10 | 8.63 ± 6.99 | 4.07 | <.001 |
| CDI | 19.73 ± 9.58 | 9.17 ± 4.85 | 4.57 | <.001 |
Missing data of one patient.
2.2. Assessment
A board‐certified child and adolescent psychiatrist interviewed and screened all participants. Psychiatric diagnoses were assessed using the Structured Clinical Interview‐Clinician Version (SCID‐CV) (First, Spitzer, Gibbon, & Williams, 1996; Lobbestael, Leurgans, & Arntz, 2011). PTSD severity was additionally examined with the French version of the Impact of Event Scale Revised (IES‐R) (Brunet, St‐Hilaire, Jehel, & King, 2003; Weiss & Marma, 1997). This scale assessed intrusions, hyperarousal, and avoidance symptoms. Major depression was categorically screened using the SCID‐CV (First et al., 1996; Lobbestael et al., 2011) and dimensionally measured with the French version of the Children Depression Inventory (CDI; Dugas & Bouvard, 1996; Kovacs, 1981).
2.3. Neuroimaging data acquisition
All participants were scanned with a 3 T Philips MRI scanner at the Cyceron Center (Caen, France). T1‐weighted anatomical volumes were acquired using a three‐dimensional fast‐field echo sequence (3D‐T1‐FFE sagittal; Repetition Time (RT) = 20 ms; Echo Time (ET) = 4.6 ms; flip angle = 10°; 180 slices; slice thickness = 1 mm; field of view = 256 × 256 mm2, in‐plane resolution = 1 × 1 mm2; acquisition time = 9 min 41 s). To segment the hippocampal subfields, a high‐resolution proton density‐weighted sequence was acquired perpendicularly to the long axis of the hippocampus (Repetition Time [RT] = 3,500 ms; Echo Time [ET] = 19 ms; flip angle = 90°; 13 slices; slice thickness = 2 mm; interslice gap = 2 mm; in‐plane resolution = 0.375 × 0.375 mm2; acquisition time = 7 min 38 s).
2.4. Manual segmentation of hippocampal subfields
Hippocampal subfields were manually demarcated following the procedure developed in our laboratory (Figure 1) (de Flores et al., 2015; La Joie et al., 2010). From the most anterior part of the hippocampus to the apparition of colliculi, the hippocampus was segmented into three areas: (1) Subiculum; (2) CA1; and (3) a region combining CA2, CA3, and the DG (“CA2‐3/DG”). Because it is particularly difficult to differentiate the subfields in the tail of the hippocampus, the most posterior part of the hippocampus (posterior to the colliculi) was segmented into one single region called “tail.” The volume of the whole hippocampus corresponded to the sum of the four segmented subregions (subiculum, CA1, CA2‐3/DG, tail). Manual delineations were all performed by the same rater (CP), blind to any information concerning the participants. Then, a second rater (CA), also blind to the identity of the participants, visually checked and validated all the delineations. Before segmenting the data, CP was trained during several months by two expert raters (RDF and CA). The formation was validated by a high interrater reliability between CP and CA on an independent sample of 20 healthy subjects (Intraclass Correlation Coefficients: ICC(2;1) = 0.94, 0.91, and 0.91 for CA1, Subiculum, and CA2‐3/DG, respectively).
Figure 1.

Example of segmentation of the hippocampus into the following subfields: CA1 (blue), CA2‐3/DG (yellow), and subiculum (green). These hippocampal subregions were delineated on 9 slices on average. However, for the purpose and ease of illustration, segmentation is displayed on three representative slices along the anterior (ant) – posterior (post) axis of the hippocampus. For a complete slice‐by‐slice example of subfields segmentation, see (La Joie et al., 2010) [Color figure can be viewed at http://wileyonlinelibrary.com]
2.5. Statistical analyses
Statistical analyses were performed using Statistica 13 (Statsoft, Tulsa, OK). First, raw volumes were normalized to the total intracranial volume (TIV) in order to correct for head‐size differences (de Flores et al., 2015; La Joie et al., 2010). TIV values were extracted from the T1‐weighted images with the Computational Anatomy Toolbox (CAT12). To evaluate group differences, we performed general linear models (GLMs) with the TIV‐adjusted volumes as dependent variables, diagnosis as independent variable, and sex as nuisance covariate. As several studies reported age‐related differences in hippocampal subfields volumes across lifespan, we also considered controlling our analyses for age (Daugherty, Bender, Raz, & Ofen, 2016) despite this variable not differing significantly between our groups. To choose the best statistical model, with or without age as a covariate, we conducted F‐tests and AICc (Akaike Information Criterion corrected for small sample) prior to the statistical evaluation (Burnham, Anderson, & Huyvaert, 2011; R Core Team, 2018).
Levene tests were performed to check the homogeneity of variance: p values were superior to .05 for all volumes except for the tail region. However, this tail region has been disregarded a priori within this study as the anatomy of the hippocampal tail is complex and subfields cannot be distinguished in this area. Still, we reported the measurements for the tail region for the sake of completeness of our data.
Then, when the hippocampal subfields volumes were significantly different between groups, we conducted partial correlations between the TIV‐adjusted volumes and the three scores (intrusion, hyperarousal, and avoidance) of the IES‐R scale assessing for symptom severity. We also performed partial correlations with PTSD duration and age of onset. To adjust for multiple comparisons, we applied a FDR correction (False Discovery Rate, (Benjamini & Hochberg, 1995)), using the function “p.adjust()” of the package “stats” in R (R Core Team, 2018).
3. RESULTS
3.1. Participant characteristics
Participant characteristics are displayed in Table 1. There were no significant differences between groups for age and IQ. PTSD patients showed significantly higher levels of symptom severity on the three scores of the IES‐R scale (intrusion: t = 4.76, p < .001; hyperarousal: t = 6.26, p < .001; and avoidance: t = 4.07, p < .001) compared to controls. They also had a higher score on the depression scale (CDI score: t = 4.57, p < .001) compared to controls.
3.2. Hippocampal alterations in PTSD
TIV‐adjusted volumes (mean ± SD) of the hippocampal subfields and GLMs results are summarized in Table 2. Concerning the whole hippocampus, the volumes of left and right hippocampi were significantly smaller in the PTSD group compared to controls. Concerning hippocampal subfields, no significant differences were found between the groups for the subiculum and CA1 volumes. However, the CA2‐3/DG volumes were smaller in the PTSD group compared to controls on both (left and right) sides (Figure 2).
Table 2.
TIV‐adjusted hippocampal volumes in adolescents with PTSD and healthy controls
| TIV‐adjusted volumes (mm3) | Group | Sex | Age | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PTSD | Controls | F | p | p FDR | F | p | F | p | |
| Hippocampus | |||||||||
| L | 3,011 ± 413 | 3,511 ± 379 | 10.14 | .003 | 0.008 | 1.301 | .262 | – | – |
| R | 3,001 ± 373 | 3,573 ± 342 | 16.99 | .0002 | 0.002 | 1.696 | .201 | – | – |
| CA1 | |||||||||
| L | 878 ± 152 | 966 ± 112 | 0.533 | .470 | 0.470 | 4.257 | .047 | 6.45 | .016 |
| R | 877 ± 114 | 1,001 ± 153 | 1.835 | .184 | 0.294 | 4.711 | .037 | 4.17 | .049 |
| CA2‐3/DG | |||||||||
| L | 857 ± 162 | 1,083 ± 153 | 10.43 | .003 | 0.008 | 1.105 | .300 | 4.69 | .037 |
| R | 890 ± 180 | 1,068 ± 162 | 7.341 | .010 | 0.020 | 0.371 | .546 | – | – |
| Subiculum | |||||||||
| L | 773 ± 164 | 841 ± 111 | 0.805 | .376 | 0.429 | 2.271 | .141 | – | – |
| R | 830 ± 156 | 899 ± 112 | 1.105 | .300 | 0.400 | 1.561 | .219 | – | – |
| Tail | |||||||||
| L | 503 ± 77 | 621 ± 250 | – | – | – | – | – | – | – |
| R | 404 ± 133 | 605 ± 228 | – | – | – | – | – | – | – |
Abbreviations: L = left; R = right
Figure 2.
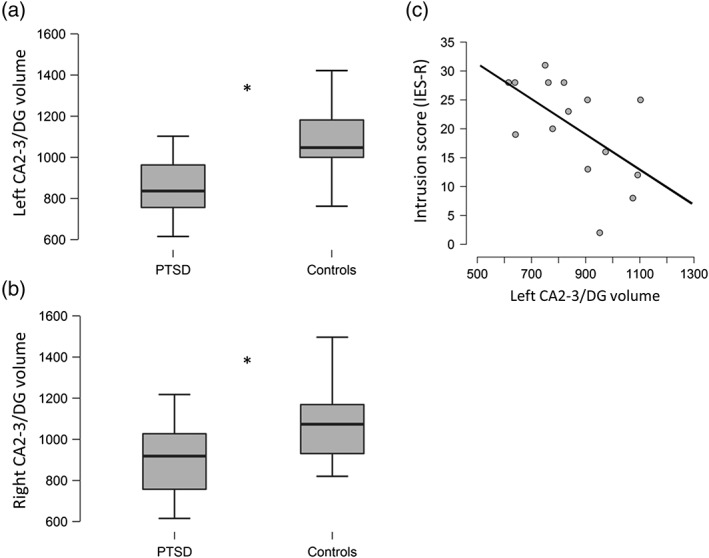
(a, b) Significant differences (*p FDR < 0.05) in TIV‐adjusted CA2‐3/DG volumes (mm3) between the PTSD group and the healthy controls; (c) Negative correlation between the TIV‐adjusted left CA2‐3/DG volume (mm3) and the intrusion score (IES‐R) in the PTSD group
3.3. Hippocampal alterations in PTSD controlling for depression symptoms
Although screening excluded patients with major depression, the PTSD group presented a higher score on the depression scale (CDI) compared to controls. As depression could be related to structural changes in hippocampal subfields (Huang et al., 2013), we added the depression score (CDI) as nuisance covariate in our analyses. The significant results remained unchanged: we found smaller hippocampal and CA2‐3/DG volumes in the PTSD group (See Table 3).
Table 3.
Hippocampal alterations in adolescent PTSD controlling for depression symptoms
| Group | Sex | Depression | Age | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | p FDR | F | p | F | p | F | p | |
| Hippocampus | |||||||||
| L | 8.853 | .0053 | 0.016 | 1.346 | .254 | 0.420 | .521 | – | – |
| R | 16.518 | .0003 | 0.002 | 1.847 | .183 | 1.306 | .261 | – | – |
| CA1 | |||||||||
| L | 2.251 | .1427 | 0.169 | 4.812 | .035 | 2.609 | .116 | 7.155 | .011 |
| R | 3.948 | .0550 | 0.088 | 5.245 | .028 | 2.336 | .136 | 4.648 | .038 |
| CA2‐3/DG | |||||||||
| L | 7.902 | .0081 | 0.017 | 1.099 | .302 | 0.057 | .812 | 4.607 | .039 |
| R | 7.684 | .0084 | 0.017 | 0.425 | .519 | 0.905 | .348 | – | – |
| Subiculum | |||||||||
| L | 1.364 | .2507 | 0.251 | 2.347 | .135 | 0.585 | .449 | – | – |
| R | 2.185 | .1483 | 0.169 | 1.691 | .202 | 1.163 | .288 | – | – |
Abbreviations: L = left; R = right
3.4. Correlations with symptom severity
The relation between CA2‐3/DG volumes and symptom severity were explored using partial correlations (controlling for sex, age, and depression). The left CA2‐3/DG was negatively correlated with the intrusion score (r = −0.74, p = .006, p FDR = 0.036; Figure 2), but was not correlated with the hyperarousal score (r = −0.50, p = .099, p FDR = 0.297), nor with the avoidance score (r = −0.19, p = .55, p FDR = 0.66). The right CA2‐3/DG was not correlated with any of the three IES‐R scores (intrusion score: r = −0.36, p = .26, p FDR = 0.52; hyperarousal score: r = −0.14, p = .66, p FDR = 0.66; avoidance score: r = 0.24, p = .46, p FDR = 0.66).
3.5. Correlations with PTSD duration and age of onset
Partial correlations (controlling for sex, age, and depression) were also conducted between PTSD duration, age of onset and CA2‐3/DG volumes. However, none of these correlations were significant (p > .05).
3.6. Supplementary analyses
The significant results remained unchanged if we only considered the female subjects (nPTSD = 13, nControls = 12). The hippocampal volumes were significantly smaller in adolescents with PTSD compared to controls (left hippocampus: F = 11.70, p = .003; right hippocampus: F = 18.25, p = .003). The CA2‐3/DG regions were significantly smaller in the PTSD group compared to controls (left CA2‐3/DG: F = 9.18, p = .006; right CA2‐3/DG: F = 13.68, p = .001). And the left CA2‐3/DG volume was negatively correlated with the intrusion score (r = −0.74, p = .008).
4. DISCUSSION
This study presents the first investigation of alterations within hippocampal subfields and their links to reexperiencing symptoms in adolescent PTSD. Using high resolution MRI and manual segmentation, we show that the hippocampal volumes are smaller in adolescents with PTSD compared to controls and that these alterations are specific to the CA2‐3/DG region. Noteworthy, these differences between groups are still significant after controlling for depression symptoms. In addition, we report a negative correlation between the intrusions score and the left CA2‐3/DG volume in the PTSD group. Our results suggest that an alteration in the hippocampal subregion CA2‐3/DG could participate in the emergence of reexperiencing symptoms in adolescents with PTSD.
Hippocampal volumes were smaller in adolescents with PTSD compared to controls. PTSD has been previously associated with hippocampal alterations in adults. Indeed, the ENIGMA consortium reported smaller hippocampal volumes on a large cohort (1,868 subjects) in civilians and veterans with PTSD compared to controls and, a negative correlation between hippocampal volumes and PTSD severity (Logue et al., 2018). In contrast, most of pediatric studies failed to report any hippocampal alterations in children and adolescents with PTSD compared to healthy subjects (Morey et al., 2016; Woon & Hedges, 2008) (but see (Mutluer et al., 2017)). Woon and Hedges (2008) hypothesized that the trauma and the development of PTSD could have a subsequent effect on hippocampal maturation and thus, the atrophy could only be detectable in adults. However, these studies could have failed to detect any alteration because of the use of low resolution structural MRI images or segmentation techniques that may not have been adapted to pediatric populations. Indeed, using high‐resolution MRI images and manual segmentation, we found smaller hippocampal volumes in adolescents with PTSD, in accordance with Mutluer et al. (2017) who also manually segmented the whole hippocampus in adolescents with PTSD.
Furthermore, our results showed that hippocampal alterations in PTSD adolescents are specific to the CA2‐3/DG region. These findings are in line with Wang et al. (2010) who found smaller CA3/DG volumes in veterans with PTSD compared to veterans without PTSD. Hippocampal subfields have distinctive histological characteristics. Interestingly, neurogenesis in the dentate gyrus is thought to be present during the entire life span (Boldrini et al., 2018; Eriksson et al., 1998; Spalding et al., 2013), but this process can be negatively affected in diverse pathological conditions. Hippocampal differences described in PTSD populations could then be related to the development of comorbidities, such as major depression (see (Huang et al., 2013)). In our study, although screening excluded patients with major depression, they presented a higher score on the depression scale (CDI) compared to controls. Depression was thus added as a nuisance covariate in all our analyses. Even controlling for depression, we found smaller volumes of the CA2‐3/DG region in the PTSD group. This result suggests that hippocampal alterations are related to PTSD and are not exclusively associated with other comorbidities, like depression.
Mechanisms underlying hippocampal alterations in PTSD still remain elusive: the smaller hippocampal volumes reported in patients could result from trauma exposure or be a preexisting condition and, thus, be a vulnerability factor for the development of PTSD (Gilbertson et al., 2002; Pitman et al., 2012). First, hippocampal alterations could be caused by the massive amount of stress generated during the traumatic event. Indeed, the hippocampus comprises many glucocorticoid receptors and pathological stress is known to reduce dendritic branching in the Cornu Ammonis and to negatively affect neurogenesis (Gould, 2007; Schoenfeld, McCausland, Morris, Padmanaban, & Cameron, 2017). Nonetheless, CA3/DG modifications are thought to be reversible after a certain time without stress exposure (Gould, 2007; Heine, Maslam, Zareno, Joëls, & Lucassen, 2004). Hence, the small volumes of CA2‐3/DG reported in the PTSD group could reflect a deficient process of recovery from trauma exposure.
However, one study, realized in identical twins discordant for trauma exposure, suggests that PTSD patients could already have smaller hippocampi before the trauma (Gilbertson et al., 2002). They found that the veterans with PTSD and their unexposed co‐twins had similar hippocampal volumes and these two groups had smaller hippocampal volumes compared to the veterans without PTSD and the unexposed non‐PTSD co‐twins. Hence, having small hippocampal volumes, and especially small CA2‐3/DG volumes, could be considered as a risk factor to develop PTSD. According to Kempermann's hypothesis (Kempermann, 2008), the newborn hippocampal neurons (in the dentate gyrus) constitute a “neurogenic reserve” and could be considered as an adaptive advantage to face novelty and complex situations. This neural reserve is also thought to have a compensatory potential to maintain the hippocampal function in pathological conditions. Hence, the initial neurogenesis capacity in the dentate gyrus (before the trauma) could influence the way a person will face the traumatic event and overcome the potential consequences. In line with this hypothesis, Hill, Sahay, and Hen (2015) showed that mice with initial increased neurogenesis developed less anxiety‐related behaviors compared to controls during a chronic stress paradigm. Further work is required to understand to which extent an enhanced neurogenesis capacity in the dentate gyrus (before the trauma) could be protective, help to overcome the alterations caused by the stress of the traumatic event and, thus, prevent the development of PTSD.
It is widely acknowledged that the hippocampus and its different subfields play a crucial role in memory processes (Horner & Doeller, 2017; Rolls, 2016). The dentate gyrus is thought to underlie pattern separation (Berron et al., 2016) and thus, reduces interferences between new information and similar stored memories (Besnard & Sahay, 2016; Kheirbek, Klemenhagen, Sahay, & Hen, 2012). This function is crucial to encode precise episodic memories and to further discriminate similar contextual representations. This mechanism permits to generate adaptive behaviors to our environment by allowing, for instance, the discrimination of contextual information previously associated with safety or danger. In animal studies, a reduced production of neurons in the dentate gyrus has been associated with pattern separation impairment (Clelland et al., 2009; Tronel et al., 2012). Hence, the smaller dentate gyrus volumes reported in the PTSD group could reflect reduced pattern separation capacities compared to controls. Although we did not assess pattern separation abilities in this study, this hypothesis is consistent with several studies reporting impoverished autobiographical memories in adults with PTSD (Lapidow & Brown, 2015). Indeed, this difficulty to recall specific and detailed episodes of their past (reported as overgeneralized memory) could be partly caused by a low capacity to reduce the overlap between the memory traces of two similar experiences.
In addition to their implication in memory deficits, the CA2‐3/DG alterations could be at the core of some psychopathological symptoms. Our study provides, for the first time in humans, evidence of a relation between specific hippocampal subfields alterations and intrusions in PTSD. Our results showed a negative correlation between the volume of the left CA2‐3/DG region and the intrusion score in the PTSD group. As discussed previously, a reduced dentate gyrus volume could reflect low pattern separation capacities in the PTSD group. According to the theoretical model proposed by Besnard and Sahay (2016), if the interferences between a new experience and the memory of the traumatic event are not resolved, nonrelated trauma cues could be recruited and associated with the memory trace of the traumatic event. This incorporation of nonrelated trauma cues could then lead to higher probabilities to reactivate the trauma memory in safe environments, and promote overgeneralization of fear (Besnard & Sahay, 2016). Therefore, an alteration in the hippocampal subregion CA2‐3/DG, known to be involved in pattern separation, could participate in the apparition of reexperiencing symptoms in adolescents with PTSD.
4.1. Methodological considerations and future directions
This study presents some methodological limitations. First, we included a small sample because (1) it is very difficult to recruit teenagers with post‐traumatic stress disorder without any other comorbidity and (2) manual segmentation is a laborious and very time consuming methodology. Even though the results of this study corroborate adult literature (Wang et al., 2010) and resonate with several theoretical models (Besnard & Sahay, 2016; Liberzon & Abelson, 2016), further studies with larger sample are needed to confirm these results. Second, because of the cross‐sectional design, we were not able to investigate the effects of trauma exposure and the development of PTSD on hippocampal maturation. The brain, and more precisely the hippocampus, undergoes several structural changes during adolescence. This plasticity is thought to be an adaptive response which allows adolescents to learn how to face new experiences without the care of their parents and, thus to be prepared for adulthood (Curlik, DiFeo, & Shors, 2014). However, despite this plasticity, some studies consider that the adolescent brain is more vulnerable to the effects of stress exposure than adults (Holder & Blaustein, 2014; Romeo, 2017). In addition, a stress perceived during adolescence could negatively affect hippocampal maturation and cause negative long‐term effects which could last into adulthood (Holder & Blaustein, 2014; Hueston, Cryan, & Nolan, 2017). Therefore, further studies are required to: (1) understand if the vulnerability to the effects of stress exposure on the brain during adolescence can be a risk factor to develop PTSD; and (2) investigate the effects of the development of PTSD on the maturation of hippocampal subfields. Third, we had a majority of adolescent females in the PTSD group. This can be explained by the fact that the prevalence of developing PTSD is higher in females than males (McLaughlin et al., 2013). As a consequence, we were unable to assess if there was an effect of sex on hippocampal subfield alterations in adolescent PTSD. Nonetheless, studies in adults reported no significant hippocampal differences between females and males suffering from PTSD and thus, suggest that the hippocampal alterations are independent of sex (Woon & Hedges, 2011). In addition, we conducted analyses on females only and we did not observe any difference with previous analyses performed on the whole group.
5. CONCLUSIONS
Adolescent PTSD was associated with hippocampal alterations. Using high‐resolution images and manual segmentation, we found smaller volumes of the whole hippocampus (in both hemispheres) in the PTSD group. This first result highlights the importance of using segmentation methods adapted to an adolescent population to characterize brain differences in PTSD. Moreover, the hippocampal alterations were specific to CA2‐3/DG and, the volume of this region was negatively correlated with intrusion symptoms. Hence, our study provides, for the first time in humans, evidence of a relation between specific hippocampal subfields alterations and reexperiencing symptoms in PTSD. These findings resonate with recent theoretical models (Besnard & Sahay, 2016; Liberzon & Abelson, 2016) which propose that a reduced pattern separation (function subtended by CA3 and the dentate gyrus) could be at the core of the intrusions symptoms.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by the University Hospital of Caen, the Caen school district, and the Mutuelle Générale de l'Education Nationale (insurance company). We would like to thank C. Lebouleux, M.H. Noël, and M.C. Onfroy for their help in data acquisition, F. Dégeilh, S. Egret and F. Mézenge for assistance in testing participants, and S. Segobin for statistical advice and proofreading of the article. We are also thankful to the adolescents and institutions that took part in our research.
Postel C, Viard A, André C, et al. Hippocampal subfields alterations in adolescents with post‐traumatic stress disorder. Hum Brain Mapp. 2019;40:1244–1252. 10.1002/hbm.24443
Funding information Mutuelle Générale de l'Education Nationale; University Hospital of Caen
REFERENCES
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, D.C.: American Psychiatric Publishing. [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B: Methodological, 57, 289–300. [Google Scholar]
- Berron, D. , Schütze, H. , Maass, A. , Cardenas‐Blanco, A. , Kuijf, H. J. , Kumaran, D. , & Düzel, E. (2016). Strong evidence for pattern separation in human dentate gyrus. The Journal of Neuroscience, 36, 7569–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard, A. , & Sahay, A. (2016). Adult hippocampal neurogenesis, fear generalization, and stress. Neuropsychopharmacology, 41, 24–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini, M. , Fulmore, C. A. , Tartt, A. N. , Simeon, L. R. , Pavlova, I. , Poposka, V. , … Mann, J. J. (2018). Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell, 22, 589–599.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet, A. , St‐Hilaire, A. , Jehel, L. , & King, S. (2003). Validation of a French version of the impact of event scale‐revised. Canadian Journal of Psychiatry, 48, 56–61. [DOI] [PubMed] [Google Scholar]
- Burnham, K. P. , Anderson, D. R. , & Huyvaert, K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behavioral Ecology and Sociobiology, 65, 23–35. [Google Scholar]
- Cao, B. , Passos, I. C. , Mwangi, B. , Amaral‐Silva, H. , Tannous, J. , Wu, M.‐J. , … Soares, J. C. (2017). Hippocampal subfield volumes in mood disorders. Molecular Psychiatry, 22, 1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, V. A. , Rissman, J. , & Wagner, A. D. (2010). Imaging the human medial temporal lobe with high‐resolution fMRI. Neuron, 65, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland, C. , Choi, M. , Romberg, C. , Clemenson, G. , Fragniere, A. , Tyers, P. , … Bussey, T. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science, 325, 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curlik, D. M. , DiFeo, G. , & Shors, T. J. (2014). Preparing for adulthood: Thousands upon thousands of new cells are born in the hippocampus during puberty, and most survive with effortful learning. Frontiers in Neuroscience, 8, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty, A. M. , Bender, A. R. , Raz, N. , & Ofen, N. (2016). Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus, 26, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty, A. M. , Flinn, R. , & Ofen, N. (2017). Hippocampal CA3‐dentate gyrus volume uniquely linked to improvement in associative memory from childhood to adulthood. NeuroImage, 153, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Flores, R. , la Joie, R. , Landeau, B. , Perrotin, A. , Mézenge, F. , de la Sayette, V. , … Chételat, G. (2015). Effects of age and Alzheimer's disease on hippocampal subfields. Human Brain Mapping, 36, 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dégeilh, F. , Viard, A. , Guénolé, F. , Gaubert, M. , Egler, P.‐J. , Egret, S. , … Guillery‐Girard, B. (2017). Functional brain alterations during self‐reference processing in adolescents with sexual abuse‐related post‐traumatic stress disorder: A preliminary report. Neurocase, 23, 52–59. [DOI] [PubMed] [Google Scholar]
- Dugas, M. , & Bouvard, M. (1996). French version of the children depression inventory In Guelfi J. (Ed.), L'évaluation clinique standardisée en psychiatrie (pp. 543–548). Boulogne: Pierre Fabre. [Google Scholar]
- Duvernoy, H. (2005). The human hippocampus: Functional anatomy, vascularization and serial sections with MRI. Berlin: Springer‐Verlag. [Google Scholar]
- Eriksson, P. S. , Perfilieva, E. , Björk‐Eriksson, T. , Alborn, A.‐M. , Nordborg, C. , Peterson, D. A. , & Gage, F. H. (1998). Neurogenesis in the adult human hippocampus. Nature Medicine, 4, 1313–1317. [DOI] [PubMed] [Google Scholar]
- First, M. , Spitzer, R. , Gibbon, M. , & Williams, J. (1996). Structured clinical interview for DSM‐IV Axis I disorders, clinician version (SCID‐CV). Washington, D.C.: American Psychiatric Press, Inc. [Google Scholar]
- Gilbertson, M. W. , Shenton, M. E. , Ciszewski, A. , Kasai, K. , Lasko, N. B. , Orr, S. P. , & Pitman, R. K. (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience, 5, 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay, N. , Nugent, T. F. , Herman, D. H. , Ordonez, A. , Greenstein, D. , Hayashi, K. M. , … Thompson, P. M. (2006). Dynamic mapping of normal human hippocampal development. Hippocampus, 16, 664–672. [DOI] [PubMed] [Google Scholar]
- Gould, E. (2007). Structural plasticity In Andersen P., Morris R., Amaral D., Bliss T., & O'Keefe J. (Eds.), The hippocampus book (pp. 321–341). New York: Oxford University Press. [Google Scholar]
- Heine, V. M. , Maslam, S. , Zareno, J. , Joëls, M. , & Lucassen, P. J. (2004). Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. The European Journal of Neuroscience, 19, 131–144. [DOI] [PubMed] [Google Scholar]
- Hill, A. S. , Sahay, A. , & Hen, R. (2015). Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression‐like behaviors. Neuropsychopharmacology, 40, 2368–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, N. F. , Iglesias, J. E. , Sum, M. Y. , Kuswanto, C. N. , Sitoh, Y. Y. , De Souza, J. , … Holt, D. J. (2017). Progression from selective to general involvement of hippocampal subfields in schizophrenia. Molecular Psychiatry, 22, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder, M. K. , & Blaustein, J. D. (2014). Puberty and adolescence as a time of vulnerability to stressors that Alter neurobehavioral processes. Frontiers in Neuroendocrinology, 35, 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner, A. J. , & Doeller, C. F. (2017). Plasticity of hippocampal memories in humans. Current Opinion in Neurobiology, 43, 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Coupland, N. J. , Lebel, R. M. , Carter, R. , Seres, P. , Wilman, A. H. , & Malykhin, N. V. (2013). Structural changes in hippocampal subfields in major depressive disorder: A high‐field magnetic resonance imaging study. Biol psychiatry, 74, 62–68. [DOI] [PubMed] [Google Scholar]
- Hueston, C. M. , Cryan, J. F. , & Nolan, Y. M. (2017). Stress and adolescent hippocampal neurogenesis: Diet and exercise as cognitive modulators. Translational Psychiatry, 7, e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keding, T. J. , & Herringa, R. J. (2015). Abnormal structure of fear circuitry in pediatric post‐traumatic stress disorder. Neuropsychopharmacology, 40, 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann, G. (2008). The neurogenic reserve hypothesis: What is adult hippocampal neurogenesis good for? Trends in Neurosciences, 31, 163–169. [DOI] [PubMed] [Google Scholar]
- Kheirbek, M. A. , Klemenhagen, K. C. , Sahay, A. , & Hen, R. (2012). Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nature Neuroscience, 15, 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs, M. (1981). Rating scales to assess depression in school‐aged children. Acta Paedopsychiatrica, 46, 305–315. [PubMed] [Google Scholar]
- La Joie, R. , Fouquet, M. , Mézenge, F. , Landeau, B. , Villain, N. , Mevel, K. , … Chételat, G. (2010). Differential effect of age on hippocampal subfields assessed using a new high‐resolution 3T MR sequence. NeuroImage, 53, 506–514. [DOI] [PubMed] [Google Scholar]
- Lapidow, E. , & Brown, A. (2015). Autobiographical memories and PTSD In Martin C., Preedy V., & Patel V. (Eds.), Comprehensive guide to post‐traumatic stress disorders (pp. 1–13). Basel: Springer. [Google Scholar]
- Lee, J. K. , Ekstrom, A. D. , & Ghetti, S. (2014). Volume of hippocampal subfields and episodic memory in childhood and adolescence. NeuroImage, 94, 162–171. [DOI] [PubMed] [Google Scholar]
- Liberzon, I. , & Abelson, J. L. (2016). Context processing and the neurobiology of post‐traumatic stress disorder. Neuron, 92, 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbestael, J. , Leurgans, M. , & Arntz, A. (2011). Inter‐rater reliability of the structured clinical interview for DSM‐IV Axis I disorders (SCID I) and Axis II disorders (SCID II). Clinical Psychology & Psychotherapy, 18, 75–79. [DOI] [PubMed] [Google Scholar]
- Logue, M. W. , van Rooij, S. J. H. , Dennis, E. L. , Davis, S. L. , Hayes, J. P. , Stevens, J. S. , … Morey, R. A. (2018). Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA‐PGC study: Subcortical Volumetry results from posttraumatic stress disorder consortia. Biological Psychiatry, 83, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren, S. , Phan, K. L. , & Liberzon, I. (2013). The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nature Reviews. Neuroscience, 14, 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. A. , Koenen, K. C. , Hill, E. D. , Petukhova, M. , Sampson, N. A. , Zaslavsky, A. M. , & Kessler, R. C. (2013). Trauma exposure and posttraumatic stress disorder in a National Sample of adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 52, 815–830.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey, R. A. , Haswell, C. C. , Hooper, S. R. , & De Bellis, M. D. (2016). Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology, 41, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutluer, T. , Şar, V. , Kose‐Demiray, Ç. , Arslan, H. , Tamer, S. , Inal, S. , & Kaçar, A. Ş. (2017). Lateralization of neurobiological response in adolescents with post‐traumatic stress disorder related to severe childhood sexual abuse: The tri‐modal reaction (T‐MR) model of protection. Journal of Trauma & Dissociation, 19, 108–125. [DOI] [PubMed] [Google Scholar]
- Pitman, R. K. , Rasmusson, A. M. , Koenen, K. C. , Shin, L. M. , Orr, S. P. , Gilbertson, M. W. , … Liberzon, I. (2012). Biological studies of posttraumatic stress disorder. Nature Reviews. Neuroscience, 13, 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.r-project.org/ [Google Scholar]
- Rolls, E. T. (2016). Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiol learn mem, 129, 4–28. [DOI] [PubMed] [Google Scholar]
- Romeo, R. D. (2017). The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. Brain res, 1654, 185–191. [DOI] [PubMed] [Google Scholar]
- Schoemaker, D. , Buss, C. , Head, K. , Sandman, C. A. , Davis, E. P. , Chakravarty, M. M. , … Pruessner, J. C. (2016). Hippocampus and amygdala volumes from magnetic resonance images in children: Assessing accuracy of FreeSurfer and FSL against manual segmentation. NeuroImage, 129, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld, T. J. , McCausland, H. C. , Morris, H. D. , Padmanaban, V. , & Cameron, H. A. (2017). Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol psychiatry, 82, 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding, K. L. , Bergmann, O. , Alkass, K. , Bernard, S. , Salehpour, M. , Huttner, H. B. , … Frisén, J. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell, 153, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous, J. , Amaral‐Silva, H. , Cao, B. , Wu, M.‐J. , Zunta‐Soares, G. B. , Kazimi, I. , … Soares, J. C. (2018). Hippocampal subfield volumes in children and adolescents with mood disorders. Journal of Psychiatric Research, 101, 57–62. [DOI] [PubMed] [Google Scholar]
- Tronel, S. , Belnoue, L. , Grosjean, N. , Revest, J.‐M. , Piazza, P.‐V. , Koehl, M. , & Abrous, D. N. (2012). Adult‐born neurons are necessary for extended contextual discrimination. Hippocampus, 22, 292–298. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Neylan, T. C. , Mueller, S. G. , Lenoci, M. , Truran, D. , Marmar, C. R. , … Schuff, N. (2010). Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Archives of General Psychiatry, 67, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, D. , & Marma, C. (1997). The impact of event scale‐revised In Wilson J. & Keane T. (Eds.), Assessing psychological trauma and PTSD: A practitioner's handbook (pp. 399–411). New York City: Guilford Press. [Google Scholar]
- Woon, F. , & Hedges, D. W. (2011). Gender does not moderate hippocampal volume deficits in adults with posttraumatic stress disorder: A meta‐analysis. Hippocampus, 21, 243–252. [DOI] [PubMed] [Google Scholar]
- Woon, F. L. , & Hedges, D. W. (2008). Hippocampal and amygdala volumes in children and adults with childhood maltreatment‐related posttraumatic stress disorder: A meta‐analysis. Hippocampus, 18, 729–736. [DOI] [PubMed] [Google Scholar]


