Abstract
Reward deficits and associated striatal circuitry disturbances have been implicated in the onset and progression of major depressive disorder (MDD). However, no studies have been conducted to investigate how the striatal circuitry changes during standard antidepressant, which is important for development of novel and targeted treatments for MDD. We examined the seed‐to‐whole‐brain functional connectivity (FC) for six striatal subregions based on resting‐state fMRI data of 23 MDD patients before and after 8‐week duloxetine, a serotonin, and noradrenaline reuptake inhibitor. Twenty‐three healthy controls (HCs) were also scanned twice with an 8‐week interval. After the analysis of covariance, we observed significant group‐by‐time interaction on FC of the dorsal caudate (DC), ventral striatum (VS), and putamen seeds. Post hoc analyses revealed that the FC between several right striatal seeds and left superior frontal gyrus (SFG), between right DC and left precuneus, between right superior VS and left inferior parietal lobe, were significantly higher in MDD patients compared to HCs at baseline and were reduced after treatment. Conversely, the FC between right inferior VS and left cerebellum was lower in MDD patients and was increased after treatment. Patients with larger reduction in right superior VS—left SFG FC exhibited larger alleviation of rumination. These findings suggest that duloxetine modulates the striatal FC with dorsolateral prefrontal cortex, posterior default mode network, and cerebellum, and partly, these changes underlie symptomatic improvement. This study adds to our understanding of antidepressant mechanism and future therapeutic development might benefit from considering these striatal circuitry as potential targets.
Keywords: antidepressant, duloxetine, functional connectivity, major depressive disorder, resting‐state fMRI, striatum
1. INTRODUCTION
Major depressive disorder (MDD) is a very prevalent condition characterized by high recurrence, suicidality, and disability rates (Strawbridge, Young, & Cleare, 2017; Whiteford et al., 2013). Although various antidepressants available, there is still a considerable number of patients could not achieve remission, highlighting the need for developing new treatments (Iniesta et al., 2016). Researches examining the neural circuit of MDD, and how these circuits change with standard treatment will provide necessary knowledge to develop novel and targeted treatments (Strawbridge et al., 2017).
There is considerable evidence that the neural disturbances of MDD occur in a cortico‐striatal‐thalamic‐cortical (CSTC) circuit (Drevets, Price, & Furey, 2008). Within this circuit, the striatum receives information inputs from cortical and other subcortical areas and supports the reward function, which is fundamental to a variety of emotional and cognitive processes (Di Martino et al., 2008; Parent & Hazrati, 1995). This unique feature makes the striatum a critical entry point for investigating the neural circuit and treatment pathways of MDD. Many neuroimaging studies have implicated impaired striatal structure and function in depression symptomatology, such as lower volumes in the striatum (Koolschijn, van Haren, Lensvelt‐Mulders, Hulshoff Pol, & Kahn, 2009) and blunted striatal response to positive stimuli (Smoski et al., 2009; Stoy et al., 2012), which was particularly associated with the low motivation symptoms (i.e., anhedonia and energetic reduction) of MDD patients (Heller et al., 2013; Yang et al., 2017). Further, the striatal hyporesponsiveness has been suggested to normalize with successful antidepressant treatment (Stoy et al., 2012).
By measuring the temporal dependency of neural activation between anatomically separated regions, the resting‐state functional magnetic resonance imaging (R‐fMRI) provides a powerful tool for examining the neural circuitry of MDD from a connectivity perspective. Studies of R‐fMRI have demonstrated resting‐state functional connectivity (RSFC) changes in MDD patients between the striatum and a host of other structures within the CSTC circuit, frequently including the medial prefrontal cortex (PFC), anterior cingulate cortex (ACC), and default mode network (DMN; Admon et al., 2015; Furman, Hamilton, & Gotlib, 2011; Hwang et al., 2016; Leaver et al., 2016). A study (Kerestes et al., 2014) investigating whole‐brain connectivity of dorsal and ventral striatal seeds demonstrated higher RSFC between the bilateral dorsal caudate (DC) and ventrolateral PFC in MDD patients, which was associated with severer depressive symptoms. In the only published studies investigating antidepressant effect, researchers observed a downregulated RSFC between the striatum and dorsolateral PFC in healthy volunteers by noradrenergic antidepressant reboxetine (McCabe, Woffindale, Harmer, & Cowen, 2012) in MDD patients by acupuncture plus fluoxetine treatment (Wang, Wang, Liu, & Cowen, 2017).
Despite of increasing evidence suggesting the involvement of striatum and its projection areas in MDD, there has been a lack of investigations of how standard antidepressants alter striatal RSFC, and further, no studies have been conducted to examine antidepressant effects on the striatum at a subregion level. Of note, the striatum has a high anatomical and functional heterogeneity. Whereas the ventral striatum (VS) receives projections from the orbitofrontal cortex, ACC, and limbic structures involved in affective division, the dorsal striatum receives inputs from the dorsolateral PFC and primary sensorimotor cortex that support executive and motor functions (Di Martino et al., 2008; Parent & Hazrati, 1995). It is of great interest to examine therapeutic effects on seed‐to‐whole‐brain RSFC of striatal subregions as an important step in unraveling antidepressant mechanisms within the large‐scale circuit of CSTC.
Here, we aimed to investigate therapeutic effects of striatal connectivity changes after 8‐week duloxetine (a serotonin and noradrenaline reuptake inhibitor [SNRI] and first‐line antidepressant treatment) using R‐fMRI in a first‐episode, medication‐free, and non‐comorbid group with MDD. The healthy controls (HCs) were also scanned at baseline and 8 weeks later to confirm that the neural changes were due to medication versus test–retest effects. We examined the seed‐to‐whole‐brain RSFC for six predefined striatal subregions (including the DC, VS, and putamen). We hypothesized that duloxetine would modulate the RSFC between the striatal subregions and specific brain sites within the PFC and DMN based on their roles in emotional regulation and self‐reflection (Disner, Beevers, Haigh, & Beck, 2011) and their altered connectivity with the striatum observed in above‐mentioned functional imaging studies in MDD. The therapeutic effects in striatal RSFC might be also observed in other structures within the CSTC circuit.
2. METHODS
2.1. Subjects
The initial sample included 38 right‐handed, first‐episode, and medication‐free patients with MDD recruited from Outpatient Department of Peking University Institute of Mental Health. MDD diagnosis was confirmed by a psychiatrist using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998), a short structured interview developed according to the DSM‐IV criteria. Patients were required to have a current depressive episode with moderate‐to‐severe severity defined by a total score of 24‐item Hamilton rating scale for depression (HAMD; Hamilton, 1967) >22 points. Patients with a comorbid Axis I disorder except for anxiety disorders, Axis II personality disorder or intellectual disability, current smoking status (more than 10 cigarettes per day) were excluded. Among the initially recruited 38 patients, one had excessive head movement during scanning, five discontinued medication within 2 weeks of treatment due to significant side effects, seven refused to receive the follow‐up scans because of subjective conflicts with the fMRI scans or unsuitable personal time. These 13 patients withdrawn from the study. Patients who remained in this study did not differ significantly in age, gender, and baseline HAMD scores from those withdrew.
The HCs were required to be age‐ (±5 years) and gender‐matched with the patients, have no history of psychiatric illnesses in themselves and their first‐degree relatives, and have a HAMD score <7 points. Exclusion criteria for all subjects included a history of neurological illnesses or significant head trauma, substance dependence, or abuse within the last year, an experience of electroconvulsive therapy within the last 6 months, acute suicide, current pregnancy or breastfeeding, or any contraindications to magnetic resonance imaging (MRI) scan. The authors asserted that all procedures of this work complied with the ethical standards of relevant national and institutional committees on human research and with the Helsinki Declaration. Demographic and clinical data were provided in Table 1.
Table 1.
Sample characteristics
| MDD baseline (A) | MDD week 8 (B) | HCs baseline (C) | HCs week 8 (D) | p | |
|---|---|---|---|---|---|
| Gender (male/female) | 11/12 | 11/12 | 11/12 | 11/12 | >.99 |
| Age (years) | 29.7 ± 5.8 | 29.9 ± 5.8 | 29.3 ± 5.9 | 29.5 ± 5.9 | .821 |
| Education level (years) | 14.3 ± 3.2 | 14.3 ± 3.2 | 15.6 ± 1.8 | 15.6 ± 1.8 | .094 |
| Illness duration (months) | 7.5 ± 5.9 | 9.3 ± 6.1 | |||
| Total HAMD | 30.5 ± 5.4 | 6.3 ± 4.3 | 1.5 ± 1.7 | 1.2 ± 0.9 | <.001(A‐B; A‐C; B‐D) |
| Rumination | 13.6 ± 2.6 | 8.2 ± 2.5 | 5.9 ± 1.5 | 5.8 ± 1.2 | <.001(A‐B; A‐C; B‐D) |
Notes: Unless otherwise indicated, values shown are mean ± SD. The gender was analyzed using chi‐square test, while other variables were analyzed using independent‐sample t test.
Abbreviations: MDD, major depressive disorder; HAMD, Hamilton rating scale for depression; HCs, healthy controls.
2.2. Duloxetine administration and clinical assessment
We chose duloxetine as a therapeutic drug based on the following considerations: (a) Among the commonly used types of antidepressant, that is, selective serotonin reuptake inhibitor (SSRI), SNRI, and noradrenergic and specific serotonergic antidepressant (NaSSA), SSRI has been found to inhibit the reward processing (McCabe, Mishor, Cowen, & Harmer, 2010) and its efficacy in treating low‐motivation symptoms has been questioned. Moreover, we could see in clinical practice that some MDD patients using SSRI have complaint of emotional blunting experience. The NaSSA acts on multiple receptors and thus does not facilitate the observation of specific associations between the brain circuit changes and receptor regulation. In contrast, duloxetine has been shown to augment the neural activity in mesolimbic dopamine reward circuits of healthy volunteers (Ossewaarde et al., 2011) and induced striatal volume growth in MDD patients (Lai, 2011). (b) There is currently in clinical practice a lack of antidepressants directly acting on dopamine receptors that are specifically related to reward function, while clinically available dopamine‐modulating drugs mainly include atypical antipsychotics such as sulpiride and risperidone, which are not suitable for initial treatment of MDD.
Patients received oral treatment with duloxetine, administered in a daily dose of 20 mg starting as soon as possible after the baseline scans, increased to 60–90 mg/day within 2 weeks, and continued until their completion of the follow‐up scans. The dose was determined by the attending physicians based on symptomatic changes and side effects. The final doses of duloxetine were 90 mg/day (n = 16) or 60 mg/day (n = 7). During the 8‐week study period, we interviewed patients weekly to check their medication adherence within the last week. It has been confirmed that all of the 23 patients who completed the 8‐week treatment showed a good compliance. Patients received duloxetine without use of any other therapies during the study period. The HCs completed 8‐week study without medication. Besides the HAMD, the rumination scale of cognitive emotion regulation questionnaire (CERQ; Zhu et al., 2008) was also evaluated to reflect the negative rumination level of patients.
2.3. MRI data acquisition
The imaging scans were performed at a 3.0T Siemens MRI scanner. The resting‐state functional images were collected with a gradient‐echo echo‐planar imaging sequence: repetition time (TR) ms/echo time (TE) ms, 2,000/30; 90° flip angle; 64 × 64 matrix; field of view, 210 × 210 mm2; thickness/gap, 4/0.8 mm; 30 slices; 7 min acquisition time. For a registration purpose, T1‐weighted structural images were acquired with magnetization‐prepared rapidly acquired gradient‐echo sequence: TR ms/TE ms, 2,300/3.01; matrix, 256 × 256; spatial resolution, 1 × 1 × 1 mm3; 9° flip angle; 1 mm thickness; 176 slices. For the resting‐state scans, subjects were instructed to keep their eyes closed, remain still without head motion, not think of a specific thing consistently, and not fall asleep during the scan. All subjects reported a good adherence to these instructions through confirmation immediately after the fMRI scans.
3. DATA ANALYSIS
3.1. Image preprocessing
The R‐fMRI images were processed with Data Processing Assistant for Resting‐State fMRI based on Statistical Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm)). After removal of the first 10 volumes, the remaining 200 volumes were corrected for different signal acquisition times. The volumes were motion‐corrected using a six‐parameter rigid‐body transformation. All subjects satisfied the criteria of spatial movement in any direction <2 mm or degree. Subjects showed no significant group differences in head‐motion parameters. Then, the nuisance signals including the Friston 24‐parameter model (Friston, Williams, Howard, Frackowiak, & Turner, 1996) of head motion, signals of cerebrospinal fluid, white matter, and whole brain were regressed from the data. Derived images were normalized to Montreal Neurological Institute (MNI) space and re‐sampled with 2 × 2 × 2 mm3 resolution using transformation parameters estimated by unified segmentation algorithm (Ashburner & Friston, 2005). The linear detrend and band‐pass filtering (0.01–0.1 Hz) were performed to reduce effects of low‐frequency drift and high‐frequency noise.
Considering a possible effect of micromovements on RSFC (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012), we calculated the framewise displacement (FD) values for each subject using the Jenkinson fomula (Jenkinson, Bannister, Brady, & Smith, 2002), which reflect the temporal derivative of micromovements (Power et al., 2012). Two patients with mean FD >0.2 mm were excluded, leaving data of 23 patients and 23 HCs for the final analysis.
3.2. Striatal RSFC analysis
We used a seed‐to‐whole‐brain analysis to compute the RSFC of striatal subregions. Specifically, the seeds were defined (MNI space) bilaterally in the DC (x = ± 13, y = 15, z = 9), superior VS (VSs; x = ± 10, y = 15, z = 0), inferior VS (VSi; x = ± 9, y = 9, z = −8), dorsal rostral putamen (DRP) (x = ± 25, y = 8, z = 6), dorsal caudal putamen (DCP; x = ± 28, y = 1, z = 3), and ventral rostral putamen (VRP; x = ± 20, y = 12, z = −3; Di Martino et al., 2008, Wang et al., 2018). Each seed covered 27 voxels in 2 mm3 space (radius = 3.5 mm). The mean time series of blood oxygenation level dependent signals were extracted from each seed. Then, Pearson's correlation coefficients were calculated between each seed's mean time series and each voxel's (excluding voxels contained within the seed) time series. In each subject, this generated 12 striatal RSFC images in total, representing one image per seed. The r‐value correlation images were z‐value converted, and then smoothed with a 6‐mm Gaussian kernel.
3.3. Statistical analysis
One‐sample t‐tests were performed to show within‐group striatal RSFC patterns, with a threshold of uncorrected p < .05. To determine treatment‐related changes, a repeated‐measured analysis of covariance (ANCOVA) and post hoc analyses were performed to obtain the group (MDD and HC)‐by‐time (Weeks 0 and 8) interaction, controlling for age, gender, education, and FD values. We restricted the ANCOVA results within a mask excluding the voxels showing significant RSFC changes within the HC group over time. This mask was generated by performing a paired t‐test on the RSFC images of the HC group between Weeks 0 and 8, thresholded with an uncorrected p < .05. The results were corrected for multiple comparisons with a cluster p < .05 and voxel p < .001 according to Gaussian random field theory. With this setup, we can control the family‐wise error rate under 5%, which satisfied the criteria recommended by Eklund, Nichols, & Knutsson, 2016. Given that we have used the strictest threshold for each seed and dividing 0.001 and 0.05 by six would result in very small p value and can be too conservative for our exploration, we chose to correct strictly for each seed but not at the seed level for these comparisons. Post hoc analyses (including 4 between‐group comparisons: MDDbaseline vs. HCbaseline, MDDbaseline vs. MDD8‐week, MDDbaseline vs. MDD8‐week, HCbaseline vs. HC8‐week) were performed by independent‐sample t‐tests for between‐group comparisons and paired t‐tests for within‐group comparisons.
Then, we performed correlation analyses between striatal RSFC changes (baseline minus Week 8) in clusters showing significant group‐by‐time interaction and changes (baseline minus Week 8) in symptomatic scores assessed by HAMD and CERQ‐rumination subscale, with age, gender, education, and FD values served as covariates. A bonferroni corrected threshold of p < .025 (0.05/2 [the number of scales used for correlation analysis]) was required for statistical significance.
4. RESULTS
4.1. Sample characteristics
There were no significant differences between MDD patients and HCs in terms of age, gender distribution, and educational level (Table 1). After 8 weeks of treatment, the scores of total HAMD and CERQ‐rumination subscale decreased (p < .001). Of the 23 patients, seven showed a clinical response to duloxetine defined as at least 50% decrease from baseline HAMD; others achieved remission defined as the HAMD score after 8‐week treatment <7 points.
4.2. Within‐group striatal RSFC
The striatal RSFC patterns (Figure S1) were compatible with those observed in other normal (Di Martino et al., 2008) and pathological (Di Martino et al., 2011; Gabbay et al., 2013) populations. The striatal RSFC obtained for MDD patients were largely similar to those of HCs. Significant positive RSFC was observed in all of the striatal subregions with their adjacent subcortical regions, in the DC and VS subregions with the medial PFC, and in the putamen subregions with the insula and inferior frontal gyrus.
4.3. Between‐group differences in striatal RSFC
Significant group‐by‐time interaction was observed in striatal RSFC with the PFC and DMN areas, and cerebellum (Figure 1, Table 2). Post hoc analysis revealed that duloxetine inhibited the abnormally high RSFC between the striatum and the brain areas within the PFC and posterior DMN (pDMN), but upregulated the abnormally low RSFC between the striatum and cerebellum (Figure 1, Table 2). Details for each striatal subregion were introduced below.
Figure 1.
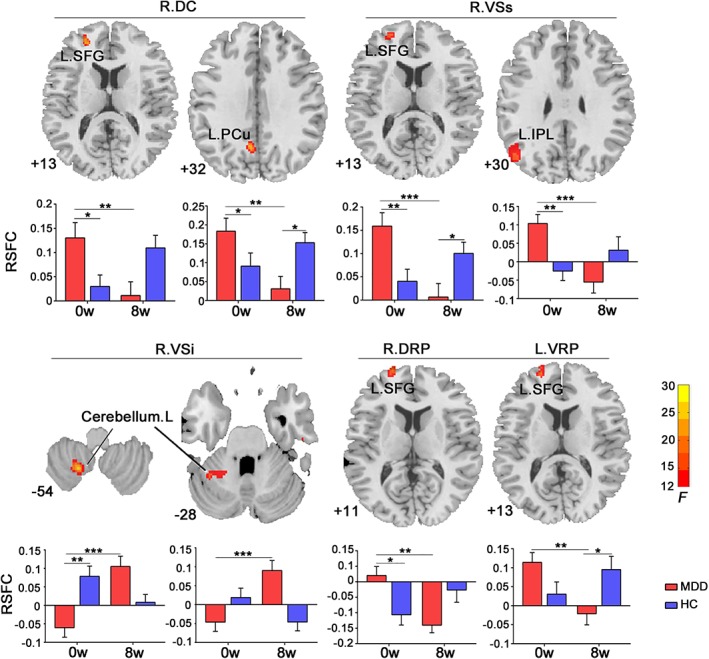
Striatal RSFC changes following treatment. The axial images show significant group‐by‐time interaction on resting‐state functional connectivity (RSFC) between right dorsal caudate (DC) and left superior frontal gyrus (SFG), left precuneus (PCu); between right VSs and left SFG, left inferior parietal lobe (IPL); between right VSi and left cerebellum; between both putamen subregions (including right dorsal rostral putamen (DRP) and left ventral rostral putamen [VRP]) and left SFG. The numbers at the lower‐left corner of axial image refer to MNI z‐coordinate. The bar maps show within‐ and between‐group differences in striatal RSFC. The data were expressed as mean value ± SE. *p < .05; **p < .005; ***p < .001. VSs, superior ventral striatum
Table 2.
Significant group‐by‐time interaction in striatal RSFC
| Subregion | Cluster size (voxels) | MNI coordinates (x, y, z) | F | |
|---|---|---|---|---|
| Right DC | ||||
| Left superior frontal gyrus | 97 | −24, 50, 14 | 17.426 | |
| Left precuneus | 74 | −6, −58, 34 | 17.398 | |
| Right VSs | ||||
| Left superior frontal gyrus | 248 | −20, 52, 6 | 28.973 | |
| Left inferior parietal lobe | 154 | −48, −70, 30 | 16.230 | |
| Right VSi | ||||
| Left cerebellum | 96/66 | −20, −58, −54/−36, −50, −30 | 30.029/17.759 | |
| Right DRP | ||||
| Left superior frontal gyrus | 59 | −20, 68, 12 | 17.004 | |
| Left VRP | ||||
| Left superior frontal gyrus | 146 | −20, 54, 8 | 13.957 |
Abbreviations: DC, dorsal caudate; DRP, dorsal rostral putamen; F, statistical value of the peak voxel; MNI, Montreal Neurological Institute; RSFC, resting‐state functional connectivity; VRP, ventral rostral putamen; VSi, inferior ventral striatum; VSs, superior ventral striatum; x, y, z, coordinate of the peak voxel in the MNI space.
4.3.1. DC seeds
We observed significant group‐by‐time interaction in the RSFC between the right DC seed and the left SFG, the left precuneus (PCu). The post hoc analysis showed that the RSFC were significantly higher or showed a higher tendency in MDD patients compared with HCs at baseline (left PCu: t 44 = 2.556, p = .014; left SFG: t 44 = 1.894, p = .065), but were reduced following treatment (left PCu: t 22 = −2.713, p = .013; left SFG: t 22 = −4.001, p = .001). After 8 weeks, the RSFC in the left PCu was lower in MDD patients compared to HCs (left PCu: t 44 = −2.551, p = .014).
4.3.2. VS seeds (VSs, VSi)
Significant group‐by‐time interaction was observed for both the VSs and VSi seeds. The right VSs showed higher RSFC with the left SFG and left inferior parietal lobe (IPL) in MDD patients compared with HCs at baseline (left SFG: t 44 = 3.068, p = .004; left IPL: t 44 = 3.644, p = .001), but was reduced following treatment (left SFG: t 22 = −5.739, p < .001; left IPL: t 22 = −4.174, p < .001). After 8 weeks, the RSFC between the right VSs and the left SFG was lower in MDD patients compared to HCs (t 44 = −2.495, p = .016). Conversely, the right VSi seed showed lower RSFC with two clusters within the left cerebellum in MDD patients compared with the HCs at baseline (t 44 = −3.693, p = .001; t 44 = −0.046, p = .069) and was increased following treatment (t 22 = 3.693, p = .001; t 22 = 4.207, p < .001).
4.3.3. Putamen seeds (DRP, DCP, and VRP)
Significant group‐by‐time interaction was observed in the RSFC between both the right DRP and left VRP seeds and the left SFG. These RSFCs were higher in MDD patients compared with HCs at baseline (DRP‐SFG: t 44 = 2.881, p = .006; VRP‐SFG: t 44 = 2.046, p = .047), but were reduced following treatment (DRP‐SFG: t 22 = −3.594, p = .002; VRP‐SFG: t 22 = −3.841, p = .001). Patients with larger reduction in right VSs‐left SFG RSFC showed larger reduction in CERQ rumination scores (Figure 2).
Figure 2.
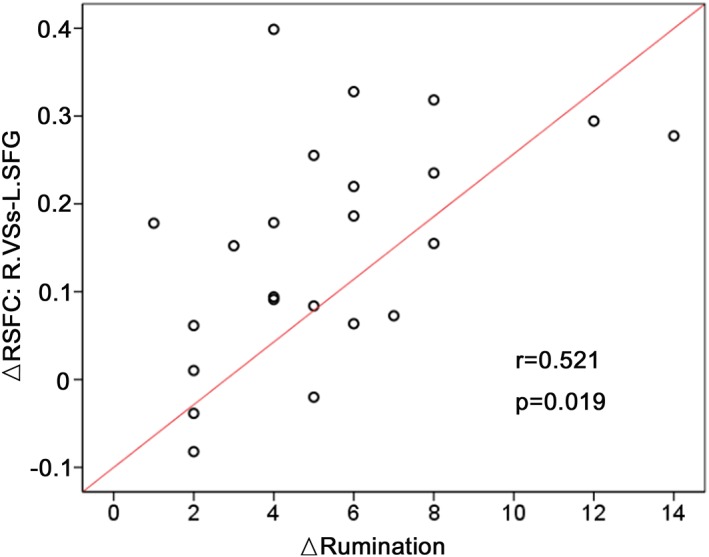
Relationships between striatal RSFC changes and rumination following treatment. The scatter map shows correlation in RSFC reduction between the right superior ventral striatum (VSs) and left superior frontal gyrus (SFG) with rumination scores following treatment. ▵ baseline‐week 8. RSFC, resting‐state functional connectivity
5. DISCUSSION
Our study is the first to address whether antidepressant impacts seed‐to‐whole‐brain RSFC for striatal subregions. Basically consistent with our prior hypothesis, the results indicate that duloxetine could downregulate abnormally high RSFC in the DC and putamen subregions with the SFG and pDMN areas. The therapeutic effect was also associated with a reversal of abnormally low RSFC between the VS and cerebellum. Treatment‐related decrease in VSs‐SFG RSFC might be especially related to the relief of rumination symptoms.
The normalized effect of duloxetine on RSFC between the left SFG and 3‐part striatal subregion (including the DC, VS, and putamen) is the first important finding of our study. The dorsolateral PFC (dlPFC) is a central region within depressive CSTC circuit and supports a series of cognitive processes, such as executive planning, self‐monitoring, and error detection (Kim, Chung, & Kim, 2013; Kim, Johnson, & Gold, 2014). Researchers found higher dlPFC activation in acutely depressed patients during the anticipation and receipt of reward stimuli (Forbes et al., 2009). Higher PFC input to striatum has been observed in MDD patients in response to loss than reward outcome, and stronger striatal coupling to the superior and middle frontal gyrus was linked to suicidality (Quevedo et al., 2017). Even during resting state, MDD patients also displayed increased RSFC between the DC and dorsolateral PFC, which significantly correlated with depressive severity (Furman et al., 2011). We could thus speculate that increased striatal‐SFG RSFC may underlie cognitive conflicting symptoms (i.e., cognitive blunting, rumination, difficulties in decision making) torturing the patients who are in a depressive episode (Disner et al., 2011). Inhibiting this RSFC may lead to a beneficial impact on cognitive performances in MDD patients. Further, we found that a larger decrease in the VSs‐SFG RSFC after duloxetine was associated with more significant alleviation of rumination. This result is compatible with two repetitive transcranial magnetic stimulation, (rTMS) studies (Avissar et al., 2017; Du et al., 2018) indicating that the RSFC of stimulated DLPFC‐VS/NAcc pathway predicted the antidepression effects of rTMS. Combined, these results strongly suggest a potential of the striatal‐DLPFC circuit as a target pathway for MDD treatment, and also, an objective indicator for the evaluation of clinical response to SNRI.
Duloxetine also demonstrated a normalized effect on abnormally high RSFC between the DC and putamen subregions and the pDMN areas. The DMN is highly active at rest but deactivated during the goal‐directed behaviors, and plays a central role in self‐related cognitive activities (Andrews‐Hanna, 2012). The pDMN, with direct connections to the basal ganglia and other components within the CSTC circuit (Vatansever, Manktelow, Sahakian, Menon, & Stamatakis, 2016), sustains episodic memory and self‐consciousness (Andrews‐Hanna, 2012). Higher striatal coupling to the PCu observed during loss versus reward stimuli was found to predict severer anhedonia and depressive symptoms (Quevedo et al., 2017). Higher resting‐state activity and FC were also observed in the DMN areas of MDD patients, which was associated with negative self‐attribution and rumination (Hamilton, Farmer, Fogelman, & Gotlib, 2015; Hwang et al., 2016). Because of extensive involvement in negative emotional and self‐reflective processing, the pDMN is considered important in treatment of mood disorders. As indicated, abnormally high RSFC in the pDMN has been normalized after successful antidepressant treatment (Li et al., 2013). Using striatal RSFC as an indicator, our study provides direct evidence that clinical response to duloxetine in MDD patients was associated with an inhibition of the pDMN‐caudate coupling, suggesting that antidepressant mechanism of duloxetine may involve pathway from the pDMN to striatum. Such a connectivity reduction likely contributes to disengagement of persistent and pervasive negative self‐cognition and rumination seen in MDD patients.
Another important finding is lower RSFC between the right VSi and left cerebellum observed in MDD patients compared to HCs and its subsequent reversal following duloxetine. The striatum receives cortical input via the thalamus and projects to the PFC, supplementary motor area, and cerebellum (Parent & Hazrati, 1995). These brain areas and circuits are involved in motor planning and execution (Herrero, Barcia, & Navarro, 2002; Parent & Hazrati, 1995). The cerebellum is also thought to be involved in cognitive function (Schmahmann & Caplan, 2006). Relevantly, MDD patients can manifest a series of cognition‐related psychomotor symptoms, such as less words, slow thinking, and reduced activity (Buyukdura, McClintock, & Croarkin, 2011). The brain imaging studies (Rentería et al., 2017; Naismith et al., 2002; Steffens & Krishnan, 1998) have demonstrated structural and functional abnormalities in the striatum of MDD patients, such as white matter changes, lower gray matter volumes and blood flow, which were more obvious in patients with significant psychomotor stagnation (Hickie et al., 1996; Naismith et al., 2002; Steffens & Krishnan, 1998). In the cerebellum, researchers also observed lower resting‐state activity in MDD patients (Guo et al., 2011; Liu et al., 2010) and a high‐risk group of MDD (Liu et al., 2010). Distinct from the examination of local regional neural activities in these previous studies, this study revealed from connectivity or circuitry perspective that MDD patients existed a dys‐connectivity between the VSi and cerebellar areas, a restoration of this connectivity might represent another target pathway for antidepressant treatment, which might contribute to the improvement of psychomotor activation in MDD.
Strengths of this study include that all patients were drug‐naive and in their first depressive episode. The HCs were scanned twice to avoid effects of natural brain changes over time. However, several issues need to be further addressed. We cannot determine exactly whether the RSFC changes following treatment was due to a pharmacological effect, natural course of the illness, a placebo effect, or some combinations of these possibilities. The ideal controls would be groups of untreated or placebo‐controlled patients. However, its should be noted that precious double‐blind, randomized, placebo‐controlled studies (McCabe & Mishor, 2011; Norbury et al., 2009) have confirmed a specific action of SSRI citalopram and SNRI reboxetine on brain function, which might support our proposal that symptom severity and duloxetine action work by targeting RSFC in specific regions. Moreover, although we recruited a healthy volunteer sample to control for test–retest effects, the repeat testing/time may be also an influencing factor for brain functional observation. Another thing to emphasize is that a few of the patients discontinued medication due to a poor response to duloxetine and therefore were withdrew from this study, which lead to the patients who completed the follow‐up scans were all duloxetine responders. Future studies should attempt to include both patients responsive and nonresponsive to duloxetine to enable the identification of striatal RSFC changes related to treatment nonresponsiveness. Finally, the specificity of striatal RSFC changes to duloxetine should be confirmed by randomizing MDD patients to duloxetine versus another antidepressant (both pharmacological and psychological) with a different hypothesized acting mechanism.
In conclusion, our data suggests that therapeutic effect of duloxetine is associated with a normalizing effect on striatal RSFC with the dorsolateral PFC, pDMN, and cerebellum. This study elucidates the striatal circuitry involved in SNRI treatment (i.e., CSTC and cerebellum) and suggests important biological pathways by which antidepressants may treat MDD. Future researches that integrate resting and reward tasks as well as the psychological tests will help to verify behavioral implication of treatment‐related brain changes. Finally, future researches for therapeutic development of MDD might benefit from considering this striatal circuitry as potential intervention targets.
CONFLICT OF INTEREST
None.
Supporting information
Figure S1 Within‐group patterns of striatal RSFC. DC, dorsal caudate; VSs, superior ventral striatum; VSi, inferior ventral striatum; DRP, dorsal rostral putamen; DCP, dorsal caudal putamen; VRP, ventral rostral putamen.
Wang L, An J, Gao H‐M, et al. Duloxetine effects on striatal resting‐state functional connectivity in patients with major depressive disorder. Hum Brain Mapp. 2019;40:3338–3346. 10.1002/hbm.24601
Funding information National key research and development program, Grant/Award Number: T.M.S., 2016YFC1307103; Beijing Municipal Science and Technology Project, Grant/Award Number: T.M.S., Z171100000117016; National Key Technology R&D Program, Grant/Award Number: T.M.S., 2015BAI13B01; National Natural Science Foundation of China, Grant/Award Number: W.L, 81701779; T.M.S., 81630031
REFERENCES
- Admon, R. , Nickerson, L. D. , Dillon, D. G. , Holmes, A. J. , Bogdan, R. , Kumar, P. , … Pizzagalli, D. A. (2015). Dissociable cortico‐striatal connectivity abnormalities in major depression in response to monetary gains and penalties. Psychological Medicine, 45(1), 121–131. 10.1017/S0033291714001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. (2012). The brain's default network and its adaptive role in internal mentation. The Neuroscientist, 18(3), 251–270. 10.1177/1073858411403316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. [DOI] [PubMed] [Google Scholar]
- Avissar, M. , Powell, F. , Ilieva, I. , Respino, M. , Gunning, F. M. , Liston, C. , & Dubin, M. J. (2017). Functional connectivity of the left DLPFC to striatum predicts treatment response of depression to TMS. Brain Stimulation, 10(5), 919–925. 10.1016/j.brs.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukdura, J. S. , McClintock, S. M. , & Croarkin, P. E. (2011). Psychomotor retardation in depression: Biological underpinnings, measurement, and treatment. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 35(2), 395–409. 10.1016/j.pnpbp.2010.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino, A. , Kelly, C. , Grzadzinski, R. , Zuo, X. N. , Mennes, M. , Mairena, M. A. , & Milham, M. P. (2011). Aberrant striatal functional connectivity in children with autism. Biological Psychiatry, 69(9), 847–856. 10.1016/j.biopsych.2010.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino, A. , Scheres, A. , Margulies, D. S. , Kelly, A. M. , Uddin, L. Q. , Shehzad, Z. , … Milham, M. P. (2008). Functional connectivity of human striatum: A resting state FMRI study. Cerebral Cortex, 18(12), 2735–2747. 10.1093/cercor/bhn041 [DOI] [PubMed] [Google Scholar]
- Disner, S. G. , Beevers, C. G. , Haigh, E. A. , & Beck, A. T. (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews. Neuroscience, 12(8), 467–477. 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Drevets, W. C. , Price, J. L. , & Furey, M. L. (2008). Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Structure & Function, 213(1–2), 93–118. 10.1007/s00429-008-0189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, L. , Liu, H. , Du, W. , Chao, F. , Zhang, L. , Wang, K. , … Tang, Y. (2018). Stimulated left DLPFC‐nucleus accumbens functional connectivity predicts the anti‐depression and anti‐anxiety effects of rTMS for depression. Translational Psychiatry, 7(11), 3 10.1038/s41398-017-0005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund, A. , Nichols, T. E. , & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, E. E. , Hariri, A. R. , Martin, S. L. , Silk, J. S. , Moyles, D. L. , Fisher, P. M. , … Dahl, R. E. (2009). Altered striatal activation predicting real‐world positive affect in adolescent major depressive disorder. The American Journal of Psychiatry, 166(1), 64–73. 10.1176/appi.ajp.2008.07081336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. , Williams, S. , Howard, R. , Frackowiak, R. S. , & Turner, R. (1996). Movement‐related effects infMRI time‐series. Magnetic Resonance in Medicine, 35(3), 346–355. [DOI] [PubMed] [Google Scholar]
- Furman, D. J. , Hamilton, J. P. , & Gotlib, I. H. (2011). Frontostriatal functional connectivity in major depressive disorder. Biology of Mood & Anxiety Disordorders, 1(1), 11 10.1186/2045-5380-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay, V. , Ely, B. A. , Li, Q. , Bangaru, S. D. , Panzer, A. M. , Alonso, C. M. , … Milham, M. P. (2013). Striatum‐based circuitry of adolescent depression and anhedonia. Journal of the American Academy of Child and Adolescent Psychiatry, 52(6), 628–641. 10.1016/j.jaac.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W. B. , Sun, X. L. , Liu, L. , Wang, Y. , Zhao, B. , Lv, Y. , … Du, C. (2011). Disrupted regional homogeneity in treatment‐resistant depression: A resting‐state fMRI study. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 35(5), 1297–1302. 10.1016/j.pnpbp.2011.02.006 [DOI] [PubMed] [Google Scholar]
- Hamilton, J. P. , Farmer, M. , Fogelman, P. , & Gotlib, I. H. (2015). Depressive rumination, the default‐mode network, and the dark matter of clinical neuroscience. Biological Psychiatry, 78(4), 224–230. 10.1016/j.biopsych.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, M. (1967). Development of a rating scale for primary depressive illness. The British Journal of Social and Clinical Psychology, 6(4), 278–296. [DOI] [PubMed] [Google Scholar]
- Heller, A. S. , Johnstone, T. , Light, S. N. , Peterson, M. J. , Kolden, G. G. , Kalin, N. H. , & Davidson, R. J. (2013). Relationships between changes in sustained fronto‐striatal connectivity and positive affect in major depression resulting from antidepressant treatment. The American Journal of Psychiatry, 170(2), 197–206. 10.1176/appi.ajp.2012.12010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero, M. T. , Barcia, C. , & Navarro, J. M. (2002). Functional anatomy of thalamus and basal ganglia. Child's Nervous System, 18(8), 386–404. 10.1007/s00381-002-0604-1 [DOI] [PubMed] [Google Scholar]
- Hwang, J. W. , Xin, S. C. , Ou, Y. M. , Zhang, W. Y. , Liang, Y. L. , Chen, J. , … Kong, J. (2016). Enhanced default mode network connectivity with ventral striatum in subthreshold depression individuals. Journal of Psychiatric Research, 76, 111–120. 10.1016/j.jpsychires.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniesta, R. , Malki, K. , Maier, W. , Rietschel, M. , Mors, O. , Hauser, J. , … Uher, R. (2016). Combining clinical variables to optimize prediction of antidepressant treatment outcomes. Journal of Psychiatric Research, 78, 94–102. 10.1016/j.jpsychires.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , Bannister, P. , Brady, M. , & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Kerestes, R. , Harrison, B. J. , Dandash, O. , Stephanou, K. , Whittle, S. , Pujol, J. , & Davey, C. G. (2014). Specific functional connectivity alterations of the dorsal striatum in young people with depression. Neuroimage: Clinical, 7, 266–272. 10.1016/j.nicl.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C. , Chung, C. , & Kim, J. (2013). Task‐dependent response conflict monitoring and cognitive control in anterior cingulate and dorsolateral prefrontal cortices. Brain Research, 1537, 216–223. 10.1016/j.brainres.2013.08.055 [DOI] [PubMed] [Google Scholar]
- Kim, C. , Johnson, N. F. , & Gold, B. T. (2014). Conflict adaptation in prefrontal cortex: Now you see it, now you don't. Cortex, 50, 76–85. 10.1016/j.cortex.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn, P. C. , van Haren, N. E. , Lensvelt‐Mulders, G. J. , Hulshoff Pol, H. E. , & Kahn, R. S. (2009). Brain volume abnormalities in major depressive disorder: A meta‐analysis of magnetic resonance imaging studies. Human Brain Mapping, 30(11), 3719–3735. 10.1002/hbm.20801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, C. H. (2011). Duloxetine‐related growth of putamen and brainstem in first‐onset drug‐naive major depressive disorder with panic disorder: A case series. The Journal of Neuropsychiatry and Clinical Neurosciences, 23(2), E40–E41. 10.1176/appi.neuropsych.23.2.E40 [DOI] [PubMed] [Google Scholar]
- Leaver, A. M. , Espinoza, R. , Joshi, S. H. , Vasavada, M. , Njau, S. , Woods, R. P. , & Narr, K. L. (2016). Desynchronization and plasticity of Striato‐frontal connectivity in major depressive disorder. Cerebral Cortex, 26(11), 4337–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, B. , Liu, L. , Friston, K. J. , Shen, H. , Wang, L. , Zeng, L. L. , & Hu, D. (2013). A treatment‐resistant default mode subnetwork in major depression. Biological Psychiatry, 74(1), 48–54. 10.1016/j.biopsych.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Xu, C. , Xu, Y. , Wang, Y. , Zhao, B. , Lv, Y. , … Du, C. (2010). Decreased regional homogeneity in insula and cerebellum: A resting‐state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Research, 182(3), 211–215. 10.1016/j.pscychresns.2010.03.004 [DOI] [PubMed] [Google Scholar]
- McCabe, C. , & Mishor, Z. (2011). Antidepressant medications reduce subcortical‐cortical resting‐state functional connectivity in healthy volunteers. NeuroImage, 57(4), 1317–1323. 10.1016/j.neuroimage.2011.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe, C. , Mishor, Z. , Cowen, P. J. , & Harmer, C. J. (2010). Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biological Psychiatry, 67(5), 439–445. 10.1016/j.biopsych.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe, C. , Woffindale, C. , Harmer, C. J. , & Cowen, P. J. (2012). Neural processing of reward and punishment in young people at increased familial risk of depression. Biological Psychiatry, 72(7), 588–594. 10.1016/j.biopsych.2012.04.034 [DOI] [PubMed] [Google Scholar]
- Naismith, S. , Hickie, I. , Ward, P. B. , Turner, K. , Scott, E. , Little, C. , … Parker, G. (2002). Caudate nucleus volumes and genetic determinants of homocysteine metabolism in the prediction of psychomotor speed in older persons with depression. The American Journal of Psychiatry, 159(12), 2096–2098. 10.1176/appi.ajp.159.12.2096 [DOI] [PubMed] [Google Scholar]
- Norbury, R. , Taylor, M. J. , Selvaraj, S. , Murphy, S. E. , Harmer, C. J. , & Cowen, P. J. (2009). Short‐term antidepressant treatment modulates amygdala response to happy faces. Psychopharmacology, 206(2), 197–204. 10.1007/s00213-009-1597-1 [DOI] [PubMed] [Google Scholar]
- Ossewaarde, L. , Verkes, R. J. , Hermans, E. J. , Kooijman, S. C. , Urner, M. , Tendolkar, I. , … Fernández, G. (2011). Two‐week administration of the combined serotonin‐noradrenaline reuptake inhibitor duloxetine augments functioning of mesolimbic incentive processing circuits. Biological Psychiatry, 70(6), 568–574. 10.1016/j.biopsych.2011.03.041 [DOI] [PubMed] [Google Scholar]
- Parent, A. , & Hazrati, L. N. (1995). Functional anatomy of the basal ganglia. I. The cortico‐basal ganglia‐thalamo‐cortical loop. Brain Research. Brain Research Reviews, 20(1), 91–127. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, J. L. , & Drevets, W. C. (2012). Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences, 16(1), 61–71. 10.1016/j.tics.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Quevedo, K. , Ng, R. , Scott, H. , Kodavaganti, S. , Smyda, G. , Diwadkar, V. , & Phillips, M. (2017). Ventral striatum functional connectivity during rewards and losses and symptomatology in depressed patients. Biological Psychology, 123, 62–73. 10.1016/j.biopsycho.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentería, M. E. , Schmaal, L. , Hibar, D. P. , Couvy‐Duchesne, B. , Strike, L. T. , Mills, N. T. , … Hickie, I. B. (2017). Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta‐analysis from the ENIGMA‐MDD working group. Transl Psychiatry, 7(5), e1116 10.1038/tp.2017.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann, J. D. , & Caplan, D. (2006). Cognition, emotion and the cerebellum. Brain, 129(Pt 2), 290–292. 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , Weiller, E. , … Dunbar, G. C. (1998). The mini‐international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. The Journal of Clinical Psychiatry, 20, 22–33. [PubMed] [Google Scholar]
- Smoski, M. J. , Felder, J. , Bizzell, J. , Green, S. R. , Ernst, M. , Lynch, T. R. , & Dichter, G. S. (2009). fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders, 118(1–3), 69–78. 10.1016/j.jad.2009.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens, D. C. , & Krishnan, K. R. (1998). Structural neuroimaging and mood disorders: Recent findings, implications for classification, and future directions. Biological Psychiatry, 43(10), 705–712. [DOI] [PubMed] [Google Scholar]
- Stoy, M. , Schlagenhauf, F. , Sterzer, P. , Bermpohl, F. , Hägele, C. , Suchotzki, K. , … Ströhle, A. (2012). Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. Journal of Psychopharmacology, 26(5), 677–688. 10.1177/0269881111416686 [DOI] [PubMed] [Google Scholar]
- Strawbridge, R. , Young, A. H. , & Cleare, A. J. (2017). Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatric Disease and Treatment, 13, 1245–1262. 10.2147/NDT.S114542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever, D. , Manktelow, A. E. , Sahakian, B. J. , Menon, D. K. , & Stamatakis, E. A. (2016). Cognitive flexibility: A default network and basal ganglia connectivity perspective. Brain Connectivity, 6(3), 201–207. 10.1089/brain.2015.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Wang, K. , Liu, J. H. , Wang, Y. P. (2018). Altered Default Mode and Sensorimotor Network Connectivity With Striatal Subregions in Primary Insomnia: A Resting‐State Multi‐Band fMRI Study. Front Neurosci, 12, 917 10.3389/fnins.2018.00917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Wang, X. , Liu, J. , & Cowen, P. J. (2017). Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. Journal of Psychiatric Research, 84, 18–26. 10.1016/j.jpsychires.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford, H. A. , Degenhardt, L. , Rehm, J. , Baxter, A. J. , Ferrari, A. J. , Erskine, H. E. , & Vos, T. (2013). Global burden of disease attributable to mental and substance use disorders: Findings from the global burden of disease study 2010. Lancet, 382(9904), 1575–1786. 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- Yang, X. H. , Tian, K. , Wang, D. F. , Wang, Y. , Cheung, E. F. C. , Xie, G. R. , & Chan, R. C. K. (2017). Anhedonia correlates with abnormal functional connectivity of the superior temporal gyrus and the caudate nucleus in patients with first‐episode drug‐naive major depressive disorder. Journal of Affective Disorders, 218, 284–290. 10.1016/j.jad.2017.04.053 [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Auerbach, R. P. , Yao, S. , Abela, J. R. Z. , Xiao, J. , & Tong, X. (2008). Psychometric properties of the cognitive emotion regulation questionnaire: Chinese version. Cognition & Emotion, 22, 288–307. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Within‐group patterns of striatal RSFC. DC, dorsal caudate; VSs, superior ventral striatum; VSi, inferior ventral striatum; DRP, dorsal rostral putamen; DCP, dorsal caudal putamen; VRP, ventral rostral putamen.


