Abstract
Mind wandering (MW) has become a prominent topic of neuroscientific investigation due to the importance of understanding attentional processes in our day‐to‐day experiences. Emerging evidence suggests a critical role for three large‐scale brain networks in MW: the default network (DN), the central executive network (CEN), and the salience network (SN). Advances in analytical methods for neuroimaging data (i.e., dynamic functional connectivity, DFC) demonstrate that the interactions between these networks are not static but dynamically fluctuate over time (Chang & Glover, 2010, NeuroImage, 50(1), 81–98). While the bulk of the evidence comes from studies involving resting‐state functional MRI, a few studies have investigated DFC during a task. Direct comparison of DFC during rest and task with frequent MW is scarce. The present study applies the DFC method to neuroimaging data collected from 30 participants who completed a resting‐state run followed by two runs of sustained attention to response task (SART) with embedded probes indicating a high prevalence of MW. The analysis identified five DFC states. Differences between rest and task were noted in the frequency of three DFC states. One DFC state characterized by negative DN–CEN/SN connectivity along with positive CEN–SN connectivity was more frequently observed during task vs. rest. Two DFC states, one of which was characterized by weaker connectivity between networks, were more frequently observed during rest than task. These findings suggest that the dynamic relationships between brain networks may vary as a function of whether ongoing cognitive activity unfolds in an “unconstrained” manner during rest or is “constrained” by task demands.
Keywords: dynamic functional connectivity, self‐generated thoughts, sustained attention, unconstrained mind wandering
1. INTRODUCTION
Mind wandering (MW) is a complex and multifaceted construct (Seli et al., 2018; Wang, Poerio, et al., 2018) that encompasses self‐generated thoughts and various cognitive states related to an “inner life.” MW has solicited immense research interest, particularly in the area of attention, due to its potential to explain attentional lapses in daily activities (Thomson, Besner, & Smilek, 2015). The mind can start to wander away from the immediate external environment during periods of wakeful rest (akin to task‐free, resting‐state) as well as during mental activities with externally imposed constraints (akin to experimental task settings). Indeed, the neural underpinnings of MW have been investigated separately in resting‐state studies (Doucet et al., 2012; Godwin et al., 2017; Gorgolewski et al., 2014; Poerio et al., 2017; Turnbull et al., 2019; Van Calster, D'Argembeau, Salmon, Peters, & Majerus, 2017; Wang, Bzdok, et al., 2018; Wang et al., 2009) and in task‐based studies (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Hasenkamp, Wilson‐Mendenhall, Duncan, & Barsalou, 2012; Sormaz et al., 2018; Stawarczyk, Majerus, Maquet, & D'Argembeau, 2011). Available evidence suggests that, broadly, MW is associated with specific patterns of activation and connectivity of the brain regions that are part of three prominent neurocognitive networks: the default network (DN), the salience network (SN), and the central executive network (CEN; Christoff, Irving, Fox, Spreng, & Andrews‐Hanna, 2016; Fox, Spreng, Ellamil, Andrews‐Hanna, & Christoff, 2015).
The DN includes a set of core brain regions, the medial prefrontal cortex (mPFC) and the posterior cingulate cortex (PCC; Andrews‐Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Buckner, 2012), which demonstrate intrinsic functional coupling with one another during the resting state (Di & Biswal, 2014a; Greicius, Krasnow, Reiss, & Menon, 2003; Uddin, Kelly, Biswal, Castellanos, & Milham, 2009). The DN is traditionally thought to play a critical role in internally directed cognition versus externally directed cognition (Uddin, Iacoboni, Lange, & Keenan, 2007). Externally directed cognition is proposed to involve the CEN, which comprises the dorsolateral prefrontal cortex (dlPFC) and the posterior parietal cortex (PPC; Corbetta & Shulman, 2002). The switching and coordination between DN and CEN are suggested to be facilitated by the SN, comprising key components such as the frontoinsular cortex (FIC) and the dorsal anterior cingulate cortex (dACC) (Goulden et al., 2014; Menon & Uddin, 2010; Sridharan, Levitin, & Menon, 2008; Uddin, 2015). Emerging evidence suggests a more complex, dynamic relationship than initially suggested between these networks (Ciric, Nomi, Uddin, & Satpute, 2017; Najafi, McMenamin, Simon, & Pessoa, 2016).
Indeed, brain networks dynamically interact over time, as revealed by recent advances in analytical methods for functional neuroimaging data (i.e., time‐varying functional connectivity, also referred to as dynamic functional connectivity, DFC; Allen et al., 2014; Chang & Glover, 2010). This analytical method has been applied in both resting‐state and task‐based fMRI investigations and has revealed reoccurring patterns of DFC between networks, referred to as brain states (Allen et al., 2014; Marusak et al., 2017; Nomi, Vij, et al., 2017; Rashid, Damaraju, Pearlson, & Calhoun, 2014). Emerging evidence has suggested a correspondence between DFC states and mental states (e.g., Gonzalez‐Castillo et al., 2015). The emphasis on brain dynamics as a promising new approach (Calhoun et al., 2014; Cohen, 2017; Hutchison, Womelsdorf, Gati, Everling, & Menon, 2013; Williams & Henson, 2018) goes along with an increased interest in better delineating the brain dynamics related to MW during rest and task (Christoff et al., 2016; Kucyi, 2017; Zabelina & Andrews‐Hanna, 2016).
Time‐varying functional connectivity analyses have been used in few resting‐state and/or task‐based studies related to MW (Karapanagiotidis et al., 2018; Kucyi & Davis, 2014; Mooneyham et al., 2017). For example, Kucyi and Davis (2014) investigated both static and dynamic functional connectivity of the DN during rest and a painful stimulation task with MW probes. They found that dynamic, but not static, functional connectivity within the DN was related to MW reported during the task. In addition, Mooneyham et al. (2017) examined the DFC between DN, SN, and CEN during an attention‐to‐the‐breath task, which is characterized by fluctuations between focused attention and MW (Hasenkamp et al., 2012). In this study, the DFC state that was characterized by decreased DN–SN/CEN connectivity and increased CEN–SN positive connectivity was associated with greater dispositional mindfulness and proposed to reflect a “focused attention” state, while the DFC state that was characterized by positive connectivity between and within the three networks was inferred to reflect a “MW” state. As such, there is initial evidence of the utility of examining time‐varying functional connectivity in the context of attention tasks during which MW is likely to occur.
While emerging evidence highlights the need to consider brain dynamics in both resting‐state and task‐based fMRI studies, direct rest‐task comparisons of DFC involving an attention task to assess MW are scarce. Emerging research suggests that resting‐state FC profiles may shape task FC profiles, while there can be meaningful differences in the FC patterns between rest and task (Bellana, Liu, Diamond, Grady, & Moscovitch, 2017; Bolt, Nomi, Rubinov, & Uddin, 2017; Warren et al., 2018). DFC investigations comparing rest and task states are warranted for a more comprehensive understanding of dynamic brain network (re)configurations as a function of various cognitive states (Geerligs, Rubinov, Cam, & Henson, 2015), especially as they are related to the performance on attention tasks (Fong et al., 2019).
Here, we aim to compare DFC states between rest and an attention task (i.e., sustained attention to response task, SART) that has been widely used to investigate MW at both the behavioral and neural levels (e.g., Christoff et al., 2009; Denkova, Brudner, Zayan, Dunn, & Jha, 2018; Smallwood et al., 2004; Smallwood, Beach, Schooler, & Handy, 2008; Smilek, Carriere, & Cheyne, 2010). Because of its monotonous and repetitive nature, SART has been proposed to promote MW. MW during SART has typically been explored via experience‐sampling probes, which reveal frequent self‐reported MW during this task (Christoff et al., 2009; Denkova et al., 2018; Seli, 2016). In the present study, participants completed a 6‐min resting‐state scan followed by 30 min of the SART. Based on prior investigations involving static FC, we expected to observe brain states common to both rest and task, as well as states that differentiate between rest and task. Furthermore, based on prior work (Mooneyham et al., 2017), we predicted that states characterized by DN–CEN and DN–SN anticorrelation would occur more often during the attention task, as an indication of task‐imposed constraints.
2. MATERIALS AND METHODS
2.1. Participants
Forty‐six healthy adults participated in the study (30 women; M age = 31.22, SD = 11.51).1 Participants had normal or corrected‐to‐normal vision and did not report history of neurological or psychiatric illness. The experimental protocol was approved by the University of Miami Institutional Research Board, and all participants provided written informed consent and received monetary compensation for their participation.
2.2. MRI data acquisition and procedure
MRI data were collected using a 3‐Tesla General Electric scanner. The functional images consisted of series of images acquired in oblique axial fashion using an echoplanar imaging sequence (TR = 2000 ms, TE = 30 ms, field of view = 220 mm, matrix size 64 × 64, flip angle = 75, number of slices = 41, slice thickness = 3.4 mm, voxel size = 3.4 × 3.4 × 3.4 mm), thus allowing for full‐brain coverage. Anatomical images were acquired using on a three‐dimensional (3D) Bravo sequence (TR = 9.2 ms, TE = 3.7 ms, field of view = 256 mm, matrix size = 256 × 256, slice thickness = 1 mm, voxel size 1 × 1 × 1 mm).
2.2.1. Resting state fMRI
At the beginning of the fMRI session, participants completed a 6‐min resting‐state run consisting of 180 volumes. They were instructed to lie still with their eyes open and to allow their thoughts to flow without focusing on any particular structured mental activity, such as mental calculation or counting.
2.2.2. Sustained attention to response task fMRI
Then, participants performed a modified version of the Sustained Attention to Response Task (SART, Robertson et al., 1997), which was previously used to investigate the neural correlates of MW (Christoff et al., 2009). The task consisted of a continuous array of single digits (0 through 9) presented visually. Each digit was displayed for 500 ms followed by a fixation cross displayed for 1,500 ms (see Figure 1). Participants were instructed to withhold their responses (i.e., not pressing any button) to the digit 3 (target) and to respond by a button press to all other digits (nontargets). Responses were accepted during the stimulus display as well as during the fixation cross that followed stimulus offset. Based on previous studies suggesting that low target occurrence can increase the probability of MW (Smallwood et al., 2004), targets were presented on ~ 5% of the trials. Hence, 30 target trials were presented throughout the experiment and always separated by at least six nontarget trials. The average interval between target trials was 48 s.
Figure 1.
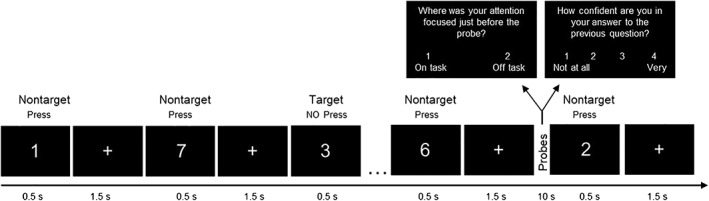
Diagram of the task. Participants completed a modified version of the sustained attention to response task (SART) during which they were presented with a continuous array of single digits and were instructed to respond by a button press during presentation of all digits other than 3 (nontargets) and to withhold responses to 3 (targets). Intermittently, they were probed about their mind wandering (probe 1) and confidence (probe 2)
On occasion, and in a pseudorandom fashion to limit participant expectation, two probe questions related to MW and confidence were presented in succession. The first probe question (Probe 1) assessed task engagement and MW by asking “Where was your attention focused just before the probe?” Participants responded by choosing between (a) “on task” or (b) “off task” response. “On task” was described to the participants as being fully focused on performing the task‐at‐hand. “Off task” was described to the participants as thinking of anything unrelated to the task, such as plans for the weekend, an earlier dispute, or any personal experiences. The second probe question (Probe 2) assessed the participant's confidence regarding the Probe 1 response; this procedure was akin to confidence judgments used to assess meta‐cognition in the perception or memory domain in prior studies (e.g., Fleming & Dolan, 2012). The question asked, “How confident are you in your answer to the previous question” and participants rated their confidence on a 4‐point scale ranging from 1 being “not all” to 4 being “very confident”. Each probe question was presented on the screen for 4 s. After the second question, a fixation cross was presented for 2 s after which the presentation of digits was resumed. Thirty probe trials were presented throughout the experiment and always separated by at least six nontarget trials. Based on previous studies suggesting that a 1‐min interval between probes led to ~50% mind wandering reports during a task (Seli, Carriere, Levene, & Smilek, 2013), the average interval between probe trials was 50 s.
The task consisted of two runs, each lasting approximately 15 min. Each run consisted of 296 nontargets, 15 targets, and 15 probes. During the last 60 s of each run, participants performed a letter task requiring a button press every time a letter was presented on the screen. Each letter was displayed for 500 ms followed by a fixation cross displayed for 1,500 ms; thus, a total of 30 letters were presented at the end of each task run. The latter task was designed to control for low level perceptual and motor demands (Forster, Nunez Elizalde, Castle, & Bishop, 2013).
Before performing the two SART runs in the fMRI scanner, participants were given detailed instructions and performed an 8‐min practice in a mock scanner in order to familiarize themselves with the task and environment, and to ensure that they understood the instructions regarding the probe questions.
E‐Prime 2.0 software (Psychology Software Tools Inc., Sharpsburg, PA) was used for stimulus presentation and collection of behavioral responses. All stimuli were white and centred on‐screen with a black background. All responses were made on a four‐button MRI‐compatible response box placed under the subject's right hand.
In addition, participants completed an 8‐min n‐back task at the end of the scanning session. These data are outside of the scope of the present report.
2.3. Data analyses
The main goal of the present study was to perform time‐varying analyses on the resting‐state run and the two SART task runs. We collected neuroimaging data from 46 participants. However, the final DFC analyses included only 30 participants. Participants' data were excluded due to incidental findings (n = 2), not complying with task instructions (n = 1), incomplete resting‐state run (n = 1), and excessive motion (n = 12 with motion greater than 3 mm in any direction and mean framewise displacement [FD] greater than 0.2 mm at any of the three runs, Power et al., 2014). The greater proportion of participants' data excluded for excessive motion could be due to (a) the conservative motion cutoff criteria; (b) the long length of each task run (~15 min), and (c) the exclusion from the analyses for excessive motion in any of the three runs. Indeed, to avoid motion confound, we adopted stringent motion exclusion criteria (Parkes, Fulcher, Yucel, & Fornito, 2018) and checked for potential differences in mean FD across runs as well as potential correlations between mean FD and brain states metrics (see section 2.3.3. Exploratory follow‐up and control analyses). Time‐varying analyses were based on 30 participants' data (20 women; M age = 29.17, SD = 11.58).
2.3.1. Behavioral task analyses
While SART can yield various outcomes, the present study focused on three main outcomes related to (a) accuracy as indexed by A prime, which is a nonparametric measure of sensitivity considering the rate of correct target trials and incorrect nontarget trials (Stanislaw & Todorov, 1999), (b) RT variability as indexed by the intra‐individual coefficient of variation (ICV), which is calculated by dividing the standard deviation RT of correct nontarget trials by the mean RT of correct nontarget trials (i.e., standard deviation RT/mean RT; Bastian & Sackur, 2013), and (c) subjective reports of mind wandering calculated as the percentage of “off task” reports throughout each task run. The first two outcomes are typically considered objective SART metrics and the third is considered a subjective outcome, since it pertains to self‐reports. Paired t tests were used to compare the two task runs on these outcomes.
2.3.2. Time‐varying resting‐state and task fMRI analyses
Preprocessing and ROI signal extraction
Preprocessing steps were separately performed for the resting‐state run (rest run) and task runs (task run 1 and task run 2) in the same order: realignment, brain extraction, coregistration, normalization to the EPI template (2 × 2 × 2), and smoothing (6‐mm full‐width half‐maximum isotropic Kernel). Before applying smoothing, fMRI data were detrended, regression of Friston's 24 motion parameters (6 rigid‐parameter time series, their temporal derivatives, plus all 12 regressors squared) and band‐pass filtering (0.01–0.1 Hz) were applied. All steps were conducted using the Data Processing and Analysis for Brain Imaging (DPABI) toolbox (Yan, Wang, Zuo, & Zang, 2016).
After pre‐processing, time series were extracted for the core regions of the DN, CEN, and SN.2 This procedure is akin to the one used by Mooneyham et al. (2017) and based on the ROIs originally provided by Sridharan et al. (2008). The regions included the mPFC and PCC for the DN, the FIC and dACC for SN, and the dlPFC and PPC for the CEN (see Table 1, see also Figure S1). Time courses were extracted from the 6‐mm radius sphere around the coordinates provided in Table 1 using the DPABI toolbox.
Table 1.
ROIs used in the present study
| Network | Region | Hemisphere | MNI coordinates |
|---|---|---|---|
| DMN | mPFC | L | −2, 36, −10 |
| DMN | PCC | L | −7, −43, 33 |
| SN | FIC | R | 37, 25, −4 |
| SN | FIC | L | −32, 24, −6 |
| SN | ACC | R | 4, 30, 30 |
| CEN | dlPFC | R | 45, 16, 45 |
| CEN | dlPFC | L | −45, 16, 45 |
| CEN | PPC | R | 54, −50, 50 |
| CEN | PPC | L | −38, −53, 45 |
Dynamic functional connectivity analyses
Rest and task run time series of the nine ROIs were concatenated following the procedure for concatenating rest and task data for DFC analysis previously reported (Hutchison & Morton, 2015). Concatenated data were submitted to DFC analysis using a sliding window approach via the GIFT toolbox (http://mialab.mrn.org/software/gift/). The choice of window length of 44 s with a step size of 1 TR was based on previous research also utilizing window sizes between 30 and 60 s (Allen et al., 2014; Ciric et al., 2017; Hutchison & Morton, 2015; Nomi, Bolt, Ezie, Uddin, & Heller, 2017; Nomi et al., 2016; Nomi, Vij, et al., 2017; Steimke et al., 2017; Yang, Craddock, Margulies, Yan, & Milham, 2014), as well as research demonstrating that such window sizes capture variability not found in longer windows (Allen et al., 2014; Hutchison et al., 2013). However, DFC analyses were also repeated with a window length of 66 s. The temporal structure of each task run was preserved without isolating or deleting probe periods because probes are an integral part of the task, and the sliding window analysis focuses on uninterrupted continuous temporal dynamics. This approach also follows previous work exploring DFC during rest and task, including the entire task (Hutchison & Morton, 2015). Only the windows of overlap between the rest run and task run 1 and between task run 1 and task run 2 were deleted. This produced a correlation matrix that was 958 (sliding windows) × 36 (paired connections) per subject. There were 158 windows for the rest run, and 400 windows for each of the task runs. Individual correlation matrices were concatenated across subjects and submitted to k‐means clustering analysis to assess the frequency and structure of dynamically reoccurring DFC states, also referred to as brain states. The optimal number of clusters (k) was estimated by applying the elbow criterion, which is computed as the ratio between within cluster distance to between‐cluster distance (Allen et al., 2014). After determining the optimal number of brain states, DFC metrics were calculated separately for each of the three runs (rest run, task run 1 and task run 2) for each participant. These metrics consisted of (a) frequency of occurrence, calculated as the percent that a brain state occurred throughout the duration of each run, and (b) dwell time, calculated as the average length of time, measured in sliding windows, that a participant stayed in a given brain state.
To examine differences between rest and task for each brain state, a series of repeated measures ANOVAs with run type as within‐subject factor with three levels (rest run, task run 1, and task run 2) were performed separately for frequency of occurrence and dwell time using the Statistical Package for the Social Sciences (SPSS). Because the effect of run type was investigated for each state separately using five separate ANOVAs for frequency of occurrence and five separate ANOVAs for dwell time, results were considered significant after Bonferroni correction for multiple comparisons (.05/10 = .005). Effects that violated assumptions of sphericity were adjusted using the Greenhouse–Geisser or Huynh (if ε > 0.75), and adjusted degrees of freedom are reported. Significant main effects of run type were followed by post hoc comparisons with Bonferroni adjusted p values, and confidence intervals around the mean difference are reported. Effect sizes are reported as partial eta‐squared (η2 p) for F tests and as Hedges g av for paired tests (Lakens, 2013).
2.3.3. Exploratory follow‐up and control analyses
Correlation between objective and subjective SART outcomes and brain state metrics
For exploratory purposes, the relationship between SART outcomes and brain state metrics was examined by calculating Pearson correlations between the three SART outcomes of interest (A prime, ICV, and % of “off task” reports) and frequency of occurrence and dwell time of the states showing significant rest‐task differences. Because the number of participants included in the exploratory correlation analyses (n = 30) may not be sufficient to establish reliable effects between task outcomes and brain state metrics, the brain‐behavior results should be considered as preliminary, and are provided for completeness as a guide for future investigations targeting larger sample sizes.
Task runs split into equal halves
Because task runs were longer than the rest run, we checked if differences between rest and task DFC metrics could be driven by either the first or second half of the task runs. For this purpose, a series of paired t tests were performed to compare the frequency and dwell time of each state for the first half and second half of the task runs.
Head motion
To check for potential differences in head motion between rest and task, we performed an ANOVA using the mean FD for rest and each task run. In order to check for potential relationships between head motion and brain state metrics, we performed a series of Pearson correlations between the mean FD for each run with the frequency and dwell time of each brain state.
Window sizes
To assess the robustness of the effects across window lengths, additional supplementary analyses were conducted using a different window size (66 s).
2.3.4. Univariate task analyses
For completeness, we also performed univariate analyses on the SART neuroimaging data using a procedure similar to prior investigations examining MW during the SART (Christoff et al., 2009; Stawarczyk et al., 2011).
Participants
Twenty‐seven participants were included in this analysis. Two participants included in the time‐varying analysis did not have enough mind wandering reports (<3 mind wandering reports) to allow valid comparison between “off task” and “on task”, and one had corrupted E‐Prime timing data.
Preprocessing
Standard task fMRI preprocessing was performed with steps including realignment, brain extraction, coregistration, normalization to the EPI template, and smoothing (6 mm full‐width half‐maximum isotropic Kernel). All steps were conducted using the Data Processing and Analysis for Brain Imaging (DPABI) toolbox (Yan et al., 2016) using SPM12.
First‐level analyses
At the individual level, we used a modeling procedure akin to the one used in prior studies investigating self‐reported mind wandering during an attention task (Christoff et al., 2009; Stawarczyk et al., 2011). Using the general linear model (GLM) in SPM 12, the 10‐s periods preceding the probes were modeled as two types of “probe” epochs according to participants' subjective responses to the first probe question (i.e., “on task” and “off task”). Specifically, the 10‐s preprobe epochs began at the onset of the fifth nontarget trial preceding the probe and ended at the onset of the probe. For completeness, the same procedure was followed for the 10‐s periods preceding the targets by modeling two types of “target” epochs for the participants' objective performance on the target trials (i.e., “correct withhold” and “error of commission”), respectively. Confidence ratings were entered as parametric modulation regressors separately for the “on task” and “off task” probe epochs. In addition, the probe intervals were modeled as 10‐s epochs beginning at the onset of Probe; target and nontarget trials (other than those included in the epochs preceding targets and probes) were modeled as separate events. The letter task at the end of each SART run was modeled as a 60‐s epoch. The canonical hemodynamic response function (hrf) was used to model each type of event and epoch. Motion parameters calculated during realignment were included as covariates of no interest to control for movement artifacts. At the subject level, contrasts of interests were identified to compare “on task” and “off task” intervals.
Group‐level analyses
Individual contrast images (“off task” minus “on task”, “on task” minus “off task”) were entered into second‐level random effects analyses using one‐sample t tests (p < .05 FWE).
3. RESULTS
3.1. Behavioral SART results
A prime for task run 1 (M = 0.91, SD = 0.07) was significantly greater than that for task run 2 (M = 0.85, SD = 0.08; t(29) = 4.74, p < .001, 95% CI [0.031, 0.079], Hedges g av = 0.672) even at Bonferroni‐corrected threshold of p < .02 (.05/3 t tests = .02). However, ICV for task run 1 (M = 0.33, SD = 0.15) was not significantly different from that for task run 2 (M = 0.37, SD = 0.15; t(29) = −1.44, p = .16, 95% CI [−0.084, 0.015], Hedges g av = 0.227) and the percentage of “off task” reports for task run 1 (M = 50.83%, SD = 19.44) was not significantly different from that for task run 2 (M = 53.35%, SD = 22.62; t(29) = −0.719, p = .478, 95% CI [−9.680, 4.643], Hedges g av = 0.116).
3.2. Time‐varying resting‐state and task fMRI results
DFC analyses revealed five brain states that dynamically reoccurred during rest and task runs (see Figure 2). State 1 was characterized by slightly negative connectivity between DN and SN, and DN and CEN, particularly in the case of the mPFC, and positive connectivity between SN and CEN. States 2 was characterized by slightly negative connectivity between SN and CEN, particularly for FIC bilaterally, and connectivity going in opposite directions between PCC and SN (negative), and PCC and CEN (positive). State 3 was characterized by an overall weaker connectivity between networks and slightly positive connectivity within network. State 4 and State 5 were characterized by overall positive connectivity between and within networks, particularly stronger for State 5. Below, we report the results from the analyses comparing rest and task runs for the frequency of occurrence and dwell time of the five states. Of note, the two metrics were overall highly correlated with each other [all rs > .67 and ps < .001 surviving Bonferroni correction of p < .003 (.05/15 correlations [5 states for rest, 5 for task run 1, 5 for task run 2] = .003), except for the correlation between frequency and dwell time of State 4 with r = .389, p = .034].
Figure 2.
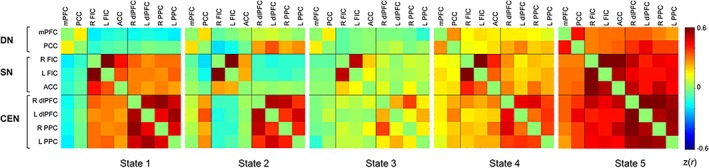
Five brain states reoccurred during resting‐state run and task runs. CEN, central executive network; DN, default network; SN, salience network [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2.1. Frequency of occurrence
Repeated‐measures ANOVA revealed a significant main effect of run type for the frequency of occurrence of State 1 [F(2, 58) = 15.779, p < .001, η2 p = 0.352], with lower frequency for rest run compared to task run 1 (p < .001, 95% CI [−24.821, −8.552], Hedges g av = 1.383) and to task run 2 (p = .004, 95% CI [−21.105, −3.384], Hedges g av = 0.880, see Figure 3); no significant difference between task run 1 and task run 2 was noted (p = .240, 95% CI [−1.776, 10.660], Hedges g av = 0.290). There was also a significant main effect of run type for the frequency of occurrence of State 2 [F(1.471, 42.657) = 10.457, p = .001, η2 p = 0.265], with higher frequency for rest run compared to task run 1 (p = .001, 95% CI [4.720, 21.977], Hedges g av = 0.802) and to task run 2 (p = .017, 95% CI [1.616, 20.331], Hedges g av = 0.621); no significant difference between task run 1 and task run 2 was noted (p = .734, 95% CI [−7.458, 2.708]; Hedges g av = 0.215). In addition, there was a significant main effect of run type for the frequency of occurrence of State 3 [F(1.701, 49.334) = 7.033, p = .003, η2 p = 0.195], with higher frequency for rest run compared to task run 1 (p = .050, 95% CI [0.015, 19.453], Hedges g av = 0.490) and to task run 2 (p = .006, 95% CI [2.746, 19.555], Hedges g av = 0.549); no significant difference between task run 1 and task run 2 was noted (p = 1.000, 95% CI [−4.738, 7.571], Hedges g av = 0.085). There was no significant main effect of run type for the frequency of occurrence of State 4 [F(1.715, 49.729) = 0.632, p = .512, η2 p = 0.021] or State 5 [F(1.284, 37.232) = 2.370, p = .126, η2 p = 0.076]. Because the absence of statistical significance does not imply the absence of an effect, and may simply result from a lack of power, we further calculated and reported effect sizes for all the paired comparisons including those for States 4 and 5 (see Table S1). Note that the effect sizes for all paired comparisons for State 4 were small (Hedges g av < 0.2).
Figure 3.
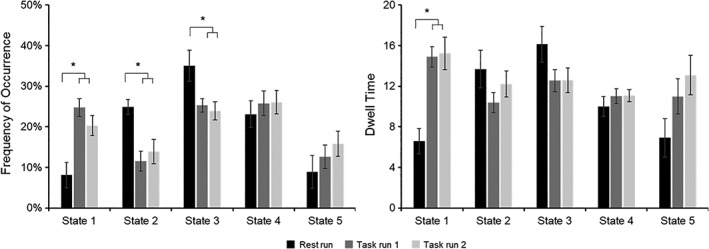
Frequency of occurrence and dwell time for each state for resting state and task, with the first three states showing a significant difference in frequency between rest run and task runs and State 1 showing also a significant difference in dwell time between rest run and task runs. Error bars present standard error of the mean
3.2.2. Dwell time
Repeated‐measures ANOVA revealed a significant main effect of run type for dwell time of State 1 [F(2, 58) = 15.956, p < .001, η2 p = 0.355], with lower dwell time for rest run compared to task run 1 (p < .001, 95% CI [−12.171, −4.467], Hedges g av = 1.321) and to task run 2 (p < .001, 95% CI [−13.719, −3.595], Hedges g av = 1.079, see Figure 3); no significant difference between task run 1 and task run 2 was noted (p = 1.000, 95% CI [−4.570, 3.894], Hedges g av = 0.045). No significant main effect of run type was observed for dwell time of State 2 [F(1.711, 49.610) = 1.642, p = .206, η2 p = 0.054], State 3 [F(1.403, 40.683) = 3.123, p = .071, η2 p = 0.097], State 4 [F(2, 58) = 0.609, p = .547, η2 p = 0.021], and State 5 [F(2, 58) = 4.848, p = .011, η2 p = 0.143] considering Bonferroni‐corrected threshold of p = .005 for significance. For completeness, we further reported all effect sizes for the paired comparisons (see Table S1).
3.3. Results for exploratory follow‐up and control analyses
3.3.1. Correlation between objective and subjective SART outcomes and brain state metrics
While a higher frequency for State 1 during run 1 was linked to better task performance as indexed by A prime, this correlation did not survive Bonferroni correction (see Table S2). No other correlations between task outcomes and brain metrics were noted. Nevertheless, these brain‐behavior correlations should be considered with caution because of the small sample size; they are provided for completeness and as a guide for future investigations targeting larger sample sizes.
3.3.2. Task runs split into equal halves
Paired t tests comparing the first half to the second half of each task run revealed some differences for task run 1, with the frequency of State 2 being significantly higher for the first half vs. second half after Bonferroni correction (see Table S3). To check if this may have had affected the rest‐task differences reported above, we ran a repeated‐measures ANOVA with split task runs (five levels: rest run, task run 1 first half, task run 1 second half, task run 2 first half, and task run 2 first half) for frequency of occurrence. The results revealed similar results to the ANOVA without split task runs (see Supporting Information section 3.2, Task runs split into equal halves). Hence, a difference in State 2 frequency between the first and second half of the task run 1 could not explain the observed rest‐task differences for the frequency of State 2.
3.3.3. Motion consideration
There was no significant difference in the mean FD, F(1.362, 39.489) = 1.428, p = .248, across the three runs [M rest run = 0.105 (SD = 0.040), M task run 1 = 0.104 (SD = 0.036), and M task run 2 = 0.113 (SD = 0.043)]. In addition, there were no significant relationships between the mean FD and the frequency or dwell time of the three states (States 1, 2, and 3) that showed significant differences between rest and task (see Table S4). These results suggest that it is very unlikely that our main findings showing rest‐task differences were driven by motion confounds.
3.3.4. Window size of 66 s
Similar results were observed for the additional analyses conducted with a window size of 66 s (see details in Supporting Information section 3.4, Window size and Figure S2).
Consistent with the 44‐s window size analyses, repeated‐measures ANOVAs for frequency of occurrence revealed a significant main effect of run type for State 1 [F(2, 58) = 14.003, p < .001, η2 p = 0.326], and State 2 [F(1.311, 38.016) = 13.848, p < .001, η2 p = 0.323]. The main effect of run type for State 3 [F(1.601, 46.416) = 4.123, p = .030, η2 p = 0.124] did not reach Bonferroni‐corrected threshold of p = .005 for significance. There was no significant main effect of run type for the frequency of occurrence of State 4 (p = .460) or State 5 (p = .068 ). In addition, consistent with the 44‐s window size analyses, repeated‐measures ANOVAs for dwell time revealed a significant main effect of run type only for State 1 [F(2, 58) = 21.255, p < .001, η2 p = 0.423], but not for the other states.
3.4. Univariate results
Correction for multiple comparisons (p = .05 FWE) did not reveal significant activations for the “off task” vs. “on task” contrast, nor for the “on task” vs. “off task” contrast. Adopting a less conservative threshold of p < .001 (uncorrected) solely for the purpose of comparing the current results with prior MW studies using an uncorrected threshold (e.g., Christoff et al., 2009), revealed that the “off task” vs. “on task” contrast yielded increased activity in the right inferior parietal lobule (BA40; 50 −43 36, z = 3.33, k = 9) and fusiform gyrus (BA37; 46 −39 −7, z = 3.31, k = 5). No significant activations were noted for the “on task” vs. “off task” contrast (see Figure S3).
4. DISCUSSION
The primary aim of the current study was to investigate dynamic interactions between and within three core neurocognitive brain networks in the context of rest and an attention task with frequent self‐reported MW. The results revealed five brain states reoccurring across rest and task. Differences between rest and task were revealed by differences in the frequency of occurrence of three states.
State 1 occurred more frequently during task (~20–25%) than rest (~8%) and participants had longer dwell times in State 1 during task (~15 windows) than rest (~7 windows). This state was characterized by negative DN–CEN and DN–SN functional connectivity and positive SN–CEN functional connectivity. We note that the negative connectivity pattern was prominent for the mPFC but not the PCC component of DN. This finding is consistent with the anterior–posterior dissociation of the DN previously reported in resting‐state and task fMRI literatures (Goodman et al., 2017; Johnson et al., 2006; Qin et al., 2012; Sestieri, Corbetta, Romani, & Shulman, 2011; Uddin et al., 2009).
Overall, the State 1 pattern of negative mPFC‐CEN/SN connectivity and positive CEN–SN connectivity is in line with prior literature suggesting a role for the SN in switching between the DN and CEN to guide task‐relevant behavior (Goulden et al., 2014; Menon & Uddin, 2010; Sridharan et al., 2008), while “inhibiting” task‐irrelevant self‐directed thoughts. A brain state with a similar pattern of connectivity was reported as one of the three DFC states during the attention‐to‐the‐breath task (Mooneyham et al., 2017). This brain state was interpreted as reflecting the focused attention state. The present findings enrich prior research by showing that while present during both rest and task conditions, the brain network configuration characterizing State 1 occurs more frequently during task than rest, and is the least frequent state occurring during rest. Hence, State 1 may be a network configuration prominent during task that enables attention to be deployed toward goal‐relevant behavior despite instances of off‐task thoughts.
In contrast to State 1, State 2 occurred more frequently during rest than task. State 2 points to the anterior–posterior dissociation of the DN and further reveals a dissociation in the direction of functional connectivity between PCC and SN (negative), and PCC and CEN (positive). On the one hand, a negative functional connectivity between PCC and insula was previously reported for “off task” episodes during the SART (Christoff, 2012). On the other hand, a strong coupling between PCC and dlPFC has been observed during elaboration of past autobiographical memories (Inman, James, Vytal, & Hamann, 2018), which is also consistent with a stronger DN–CEN coupling during planning of autobiographical events (Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010). MW may share some characteristics with autobiographical memory (Maillet & Schacter, 2016; Seli et al., 2018) and some forms of MW may involve deliberate elaboration processes similar to those occurring during autobiographical recollection or planning. Relatedly, deliberate MW has been associated with a stronger coupling between DN and CEN nodes (Golchert et al., 2017). As such, engaging in elaborative and detailed MW that is uninterrupted by external constraints or occurs in a more deliberate manner could be related to the more frequent occurrence of State 2 during rest than during task.
State 3 is the most frequently occurring state during rest (35%) and is characterized by overall weaker connectivity between and within networks. This finding is in line with prior evidence revealing that the most frequent DFC states during rest are characterized by attenuated between and within network connectivity (Allen et al., 2014; Nomi, Bolt, et al., 2017; Nomi, Vij, et al., 2017). These states have been referred to as “metastable brain states” (Nomi, Vij, et al., 2017), that are in the middle of a continuum providing the balance between extremely focused (ordered) and extreme unfocused (disordered) states (Hellyer, Scott, Shanahan, Sharp, & Leech, 2015; Tognoli & Kelso, 2014). From this perspective, relatively greater frequency of State 3 during rest versus task could reflect the nature of the resting state, which is unconstrained by specific attentional demands and therefore, may involve more frequent states of flexibility and readiness for appropriate responding to a broad range of processes.
In contrast to the first three states, State 4, characterized by synchrony between and within networks, was not significantly different between rest and task runs (in addition to exhibiting very small effect sizes for the paired comparisons). Engaging in self‐generated thoughts regardless of whether they occur during rest or task, is likely to require cognitive resources (Thomson et al., 2015) and hence, correspondingly some level of synchrony between networks. As such, one possible interpretation could be that State 4 may be related to the emergence of such thoughts regardless of the context (rest or task) and whether they will be further elaborated in detail or not.
Thus, so far, we have presented the DFC states revealed in the present study by discussing the relationship between core network components as they relate to task and rest. An alternative approach is to adopt a more global or holistic view of brain function that does not necessarily subscribe to dichotomies such as task versus rest, task‐evoked versus intrinsic, task‐positive brain network versus task‐negative brain network, CEN versus DN (Bolt, Anderson, & Uddin, 2018). Following a more holistic perspective, brain states can be seen as the repertoire of more or less flexible brain network configurations that emerge dynamically to enable context‐appropriate behavior based on the skillful interchange between external and internal needs. This perspective also aligns well with the brainweb vision proposed by Varela et al. (Varela, Lachaux, Rodriguez, & Martinerie, 2001) in which the “brain appears as a resourceful complex system that satisfies simultaneously the exogenous and endogenous constraints that arise at each moment by transiently settling in a globally consistent state” (Varela et al., 2001, p. 237). Accordingly, the observed relative differences between task and rest in the frequency of some, but not all brain states, could reflect the enactment of attentional processes that can be described on a continuum from externally driven to internally driven mental activities.
A general consideration of the rest‐task comparison concerns the potentially confounding effect of task activations on task functional connectivity. Indeed, Cole et al. (2018) recently demonstrated the problematic impact of task‐related activations on functional connectivity in the context of cognitive tasks with block designs and reported efficient correction methods. However, as the authors mentioned, these issues remain ambiguous and understudied in the context of cognitive tasks involving continuous events (such as SART), rather than experimentally manipulated and alternating blocks of rest and task. Indeed, there is a need for more research related to the appropriate methods of correction in the case of continuous performance tasks. In SART, there is also an additional complexity arising from the occurrence of internally generated events and their interaction with the processing of external task‐relevant events. Further research is needed to systematically evaluate these issues in tasks using a continuous design like the SART and provide appropriate methods of correction.
Finally, while the univariate analyses were not the main focus herein, they were provided for completeness. These analyses did not reveal significant activations in the DN core regions, such as mPFC and PCC, which were reported in some prior SART studies (Christoff et al., 2009; Stawarczyk et al., 2011). The difference in results could be due at least to some degree to methodological differences. For example, the duration of the task was shorter in the present study compared to Christoff et al.'s study (~30 min vs. ~60 min). However, the present study did include more participants (27 for the univariate analyses) but this may have led to greater variability. Indeed, MW is a multifaceted phenomenon (Seli et al., 2018) and hence, greater inter‐subject variability in the content as well as in the duration of the MW episode may preclude a consistent expression of the same features across all subject. Relatedly, asking participants to choose between being on task and off task is an imperfect and insufficient method to capture the variety of self‐generated thoughts arising during a cognitive task (Robison, Miller, & Unsworth, 2019). While the distinction between on task and off task is more prominent at early perceptual stages reflected in the attenuated neural processing of external stimuli during MW (e.g., Denkova et al., 2018), there may be more heterogeneity in the level of detail and the type of MW (e.g., spontaneous vs. deliberate) after perceptual disengagement from the task. Indeed, MW appears to be a multifaceted construct related to a variety of self‐generated thoughts (Seli et al., 2018). Self‐generated thoughts that are elaborated in detail and relying on autobiographical memory or deliberate MW processes may be more prominently associated with DN, as revealed by emerging research (Golchert et al., 2017; Murphy, Wang, et al., 2019; Sormaz et al., 2018; Spreng et al., 2014; Spreng et al., 2010). Indeed, accumulating evidence has suggested that the link between DN and MW is more complex than initially conceived (e.g., Murphy, Poerio, et al., 2019; Sormaz et al., 2018; Turnbull et al., 2019) and “patterns of activity within the DMN are neither necessary nor sufficient to determine if attention is directed away from the task” (Sormaz et al., 2018, p. 9321).
4.1. Limitations
There are several important caveats to the present study. First, we focused on the three core neurocognitive networks and their dynamic interactions. This was based on prior research highlighting the role of these networks in the fluctuations between attention and MW. Future studies should examine additional large‐scale brain networks to achieve a better understanding of rest‐task dynamics. Second, we did not track MW during rest in a similar manner to the attention task. One potential way to address this in future neuroimaging investigations would be to collect and directly compare retrospective reports of the phenomenology of MW episodes during rest (Gorgolewski et al., 2014) as well as during task (Sormaz et al., 2018) in relation to brain dynamics. Third, MW probe intervals were included in the DFC analyses because censuring them would distort the temporal dynamics that are the focus of the present study. While the probes are part of the task and an indication of whether the participant is paying attention, we acknowledge that including them in the analyses may have added some additional confounding elements related to memory and decision‐making processes. Finally, because of the general limitations of the dichotomous nature of collecting MW reports used herein, specific investigation of the brain mechanisms of MW as a function of the content of “off task” reports is beyond the scope or capability of this methodology (Karapanagiotidis et al., 2018; Sormaz et al., 2018 for focus on the content of MW).
5. CONCLUSIONS
The present study investigated brain network configurations concurrently during rest and a task characterized by the interplay between focused attention and MW episodes. The present findings suggest that while rest and task conditions may involve a common set of brain states, meaningful differences in features of some of the states can be observed between them. Namely, the task condition was associated with a more frequent occurrence of the brain state characterized by negative mPFC‐CEN and mPFC‐SN connectivity along with positive CEN–SN connectivity, while rest was associated with a more frequent occurrence of a brain state characterized by weak between‐ and within‐connectivity, which is typically associated with greater potential for reorganization. Taken together, these findings highlight the need to consider task and rest states concurrently, from a DFC perspective, in order to be able to achieve greater insight into brain network reconfigurations emerging during various situations to enable cognitive states that can be more or less constrained by external task demands.
DATA AVAILABILITY STATEMENT
The raw data that support the findings of this study are available from the first author upon reasonable request.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
This work was supported by the DOD Award Number WX81XWH‐11‐2‐0044 to APJ and the National Institute of Mental Health (R01MH107549) to LQU. Special thanks to Dr. Shruti Gopal Vij for fruitful discussions on the analysis strategies and Dr Anthony Zanesco for comments on an earlier version of the manuscript. We wish to thank Dr. Pattany for MRI parameters set‐up and Ms. Reyes for technical assistance with MRI data collection.
Denkova E, Nomi JS, Uddin LQ, Jha AP. Dynamic brain network configurations during rest and an attention task with frequent occurrence of mind wandering. Hum Brain Mapp. 2019;40:4564–4576. 10.1002/hbm.24721
Funding information National Institute of Mental Health, Grant/Award Number: R01MH107549; U.S. Department of Defense, Grant/Award Number: WX81XWH‐11‐2‐0044
Footnotes
While handedness was not formally evaluated, three subjects self‐reported being left‐handed. This is consistent with the recent recommendation to include left‐handed individuals in neuroimaging studies in a proportion similar to the 10% left‐handed population frequency (Willems, Van der Haegen, Fisher, & Francks, 2014). It is possible that inclusion of left‐handed participants may eventually lead to increased variance between subjects and hence reduced statistical sensitivity in the present study. While recent evidence suggests that functional connectivity in motor areas but not in nonmotor areas during the resting‐state may show differences between right‐ and left‐handed participants (Pool, Rehme, Eickhoff, Fink, & Grefkes, 2015), future studies should consider a formal investigation of the impact of handedness on DFC patterns.
The CEN has been also referred to as the task‐positive network or frontoparietal network (Chang & Glover, 2010; M. D. Fox et al., 2005; Power et al., 2011). Because the present study was based on prior resting‐state and task‐based studies that focused on the core neurocognitive networks referred to as DN, CEN, and SN (Di & Biswal, 2014b; Goulden et al., 2014; Hasenkamp et al., 2012; Mooneyham et al., 2017; Sridharan et al., 2008; Uddin, 2011), we use the CEN term rather than task‐positive network or frontoparietal network.
REFERENCES
- Allen, E. A. , Damaraju, E. , Plis, S. M. , Erhardt, E. B. , Eichele, T. , & Calhoun, V. D. (2014). Tracking whole‐brain connectivity dynamics in the resting state. Cerebral Cortex, 24(3), 663–676. 10.1093/cercor/bhs352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. , Reidler, J. S. , Sepulcre, J. , Poulin, R. , & Buckner, R. L. (2010). Functional‐anatomic fractionation of the brain's default network. Neuron, 65(4), 550–562. 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian, M. , & Sackur, J. (2013). Mind wandering at the fingertips: Automatic parsing of subjective states based on response time variability. Frontiers in Psychology, 4, 573 10.3389/fpsyg.2013.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellana, B. , Liu, Z. X. , Diamond, N. B. , Grady, C. L. , & Moscovitch, M. (2017). Similarities and differences in the default mode network across rest, retrieval, and future imagining. Human Brain Mapping, 38(3), 1155–1171. 10.1002/hbm.23445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt, T. , Anderson, M. L. , & Uddin, L. Q. (2018). Beyond the evoked/intrinsic neural process dichotomy. Network Neurosciences, 2(1), 1–22. 10.1162/NETN_a_00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt, T. , Nomi, J. S. , Rubinov, M. , & Uddin, L. Q. (2017). Correspondence between evoked and intrinsic functional brain network configurations. Human Brain Mapping, 38(4), 1992–2007. 10.1002/hbm.23500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. (2012). The serendipitous discovery of the brain's default network. NeuroImage, 62(2), 1137–1145. 10.1016/j.neuroimage.2011.10.035 [DOI] [PubMed] [Google Scholar]
- Calhoun, V. D. , Miller, R. , Pearlson, G. , & Adali, T. (2014). The chronnectome: time‐varying connectivity networks as the next frontier in fMRI data discovery. Neuron, 84(2), 262–274. 10.1016/j.neuron.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. , & Glover, G. H. (2010). Time‐frequency dynamics of resting‐state brain connectivity measured with fMRI. NeuroImage, 50(1), 81–98. 10.1016/j.neuroimage.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff, K. (2012). Undirected thought: Neural determinants and correlates. Brain Research, 1428, 51–59. 10.1016/j.brainres.2011.09.060 [DOI] [PubMed] [Google Scholar]
- Christoff, K. , Gordon, A. M. , Smallwood, J. , Smith, R. , & Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff, K. , Irving, Z. C. , Fox, K. C. , Spreng, R. N. , & Andrews‐Hanna, J. R. (2016). Mind‐wandering as spontaneous thought: A dynamic framework. Nature Reviews. Neuroscience, 17(11), 718–731. 10.1038/nrn.2016.113 [DOI] [PubMed] [Google Scholar]
- Ciric, R. , Nomi, J. S. , Uddin, L. Q. , & Satpute, A. B. (2017). Contextual connectivity: A framework for understanding the intrinsic dynamic architecture of large‐scale functional brain networks. Scientific Reports, 7(1), 6537 10.1038/s41598-017-06866-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. R. (2017). The behavioral and cognitive relevance of time‐varying, dynamic changes in functional connectivity. Neuroimage, 80, 515–525. 10.1016/j.neuroimage.2017.09.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W. , Ito, T. , Schultz, D. , Mill, R. , Chen, R. , & Cocuzza, C. (2018). Task activations produce spurious but systematic inflation of task functional connectivity estimates. Neuroimage, 189, 1–18. 10.1016/j.neuroimage.2018.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews. Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Denkova, E. , Brudner, E. G. , Zayan, K. , Dunn, J. , & Jha, A. P. (2018). Attenuated face processing during mind wandering. Journal of Cognitive Neuroscience, 30(11), 1691–1703. 10.1162/jocn_a_01312 [DOI] [PubMed] [Google Scholar]
- Di, X. , & Biswal, B. B. (2014a). Identifying the default mode network structure using dynamic causal modeling on resting‐state functional magnetic resonance imaging. NeuroImage, 86, 53–59. 10.1016/j.neuroimage.2013.07.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di, X. , & Biswal, B. B. (2014b). Modulatory interactions between the default mode network and task positive networks in resting‐state. PeerJ, 2, e367 10.7717/peerj.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet, G. , Naveau, M. , Petit, L. , Zago, L. , Crivello, F. , Jobard, G. , … Joliot, M. (2012). Patterns of hemodynamic low‐frequency oscillations in the brain are modulated by the nature of free thought during rest. NeuroImage, 59(4), 3194–3200. 10.1016/j.neuroimage.2011.11.059 [DOI] [PubMed] [Google Scholar]
- Fleming, S. M. , & Dolan, R. J. (2012). The neural basis of metacognitive ability. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1594), 1338–1349. 10.1098/rstb.2011.0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, A. H. C. , Yoo, K. , Rosenberg, M. D. , Zhang, S. , Li, C. R. , Scheinost, D. , … Chun, M. M. (2019). Dynamic functional connectivity during task performance and rest predicts individual differences in attention across studies. NeuroImage, 188, 14–25. 10.1016/j.neuroimage.2018.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster, S. , Nunez Elizalde, A. O. , Castle, E. , & Bishop, S. J. (2013). Unraveling the anxious mind: Anxiety, worry, and frontal engagement in sustained attention versus off‐task processing. Cerebral Cortex, 25, 609–618. 10.1093/cercor/bht248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, K. C. , Spreng, R. N. , Ellamil, M. , Andrews‐Hanna, J. R. , & Christoff, K. (2015). The wandering brain: Meta‐analysis of functional neuroimaging studies of mind‐wandering and related spontaneous thought processes. NeuroImage, 111, 611–621. 10.1016/j.neuroimage.2015.02.039 [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs, L. , Rubinov, M. , Cam, C. , & Henson, R. N. (2015). State and trait components of functional connectivity: Individual differences vary with mental state. The Journal of Neuroscience, 35(41), 13949–13961. 10.1523/JNEUROSCI.1324-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin, C. A. , Hunter, M. A. , Bezdek, M. A. , Lieberman, G. , Elkin‐Frankston, S. , Romero, V. L. , … Schumacher, E. H. (2017). Functional connectivity within and between intrinsic brain networks correlates with trait mind wandering. Neuropsychologia, 103, 140–153. 10.1016/j.neuropsychologia.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Golchert, J. , Smallwood, J. , Jefferies, E. , Seli, P. , Huntenburg, J. M. , Liem, F. , … Margulies, D. S. (2017). Individual variation in intentionality in the mind‐wandering state is reflected in the integration of the default‐mode, fronto‐parietal, and limbic networks. NeuroImage, 146, 226–235. 10.1016/j.neuroimage.2016.11.025 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Castillo, J. , Hoy, C. W. , Handwerker, D. A. , Robinson, M. E. , Buchanan, L. C. , Saad, Z. S. , & Bandettini, P. A. (2015). Tracking ongoing cognition in individuals using brief, whole‐brain functional connectivity patterns. Proceedings of the National Academy of Sciences of the United States of America, 112(28), 8762–8767. 10.1073/pnas.1501242112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, A. M. , Wang, Y. , Kwon, W. S. , Byun, S. E. , Katz, J. S. , & Deshpande, G. (2017). Neural correlates of consumer buying motivations: A 7T functional magnetic resonance imaging (fMRI) study. Frontiers in Neuroscience, 11, 512 10.3389/fnins.2017.00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski, K. J. , Lurie, D. , Urchs, S. , Kipping, J. A. , Craddock, R. C. , Milham, M. P. , … Smallwood, J. (2014). A correspondence between individual differences in the brain's intrinsic functional architecture and the content and form of self‐generated thoughts. PLoS One, 9(5), e97176 10.1371/journal.pone.0097176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden, N. , Khusnulina, A. , Davis, N. J. , Bracewell, R. M. , Bokde, A. L. , McNulty, J. P. , & Mullins, P. G. (2014). The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. NeuroImage, 99, 180–190. 10.1016/j.neuroimage.2014.05.052 [DOI] [PubMed] [Google Scholar]
- Greicius, M. D. , Krasnow, B. , Reiss, A. L. , & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258. 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp, W. , Wilson‐Mendenhall, C. D. , Duncan, E. , & Barsalou, L. W. (2012). Mind wandering and attention during focused meditation: A fine‐grained temporal analysis of fluctuating cognitive states. NeuroImage, 59(1), 750–760. 10.1016/j.neuroimage.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Hellyer, P. J. , Scott, G. , Shanahan, M. , Sharp, D. J. , & Leech, R. (2015). Cognitive flexibility through metastable neural dynamics is disrupted by damage to the structural Connectome. The Journal of Neuroscience, 35(24), 9050–9063. 10.1523/JNEUROSCI.4648-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison, R. M. , & Morton, J. B. (2015). Tracking the Brain's functional coupling dynamics over development. The Journal of Neuroscience, 35(17), 6849–6859. 10.1523/JNEUROSCI.4638-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison, R. M. , Womelsdorf, T. , Gati, J. S. , Everling, S. , & Menon, R. S. (2013). Resting‐state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Human Brain Mapping, 34(9), 2154–2177. 10.1002/hbm.22058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman, C. S. , James, G. A. , Vytal, K. , & Hamann, S. (2018). Dynamic changes in large‐scale functional network organization during autobiographical memory retrieval. Neuropsychologia, 110, 208–224. 10.1016/j.neuropsychologia.2017.09.020 [DOI] [PubMed] [Google Scholar]
- Johnson, M. K. , Raye, C. L. , Mitchell, K. J. , Touryan, S. R. , Greene, E. J. , & Nolen‐Hoeksema, S. (2006). Dissociating medial frontal and posterior cingulate activity during self‐reflection. Social Cognitive and Affective Neuroscience, 1(1), 56–64. 10.1093/scan/nsl004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapanagiotidis, T. , Vidaurre, D. , Quinn, A. J. , Vatansever, D. , Poerio, G. L. , Jefferies, E. , … Smallwood, J. (2018). Neural dynamics at rest associated with patterns of ongoing thought. BioRxiv. 10.1101/454371 [DOI] [Google Scholar]
- Kucyi, A. (2017). Just a thought: How mind‐wandering is represented in dynamic brain connectivity. NeuroImage, 180, 505–514. 10.1016/j.neuroimage.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Kucyi, A. , & Davis, K. D. (2014). Dynamic functional connectivity of the default mode network tracks daydreaming. NeuroImage, 100, 471–480. 10.1016/j.neuroimage.2014.06.044 [DOI] [PubMed] [Google Scholar]
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t‐tests and ANOVAs. Frontiers in Psychology, 4, 863 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet, D. , & Schacter, D. L. (2016). From mind wandering to involuntary retrieval: Age‐related differences in spontaneous cognitive processes. Neuropsychologia, 80, 142–156. 10.1016/j.neuropsychologia.2015.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak, H. A. , Calhoun, V. D. , Brown, S. , Crespo, L. M. , Sala‐Hamrick, K. , Gotlib, I. H. , & Thomason, M. E. (2017). Dynamic functional connectivity of neurocognitive networks in children. Human Brain Mapping, 38(1), 97–108. 10.1002/hbm.23346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5–6), 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooneyham, B. W. , Mrazek, M. D. , Mrazek, A. J. , Mrazek, K. L. , Phillips, D. T. , & Schooler, J. W. (2017). States of mind: Characterizing the neural bases of focus and mind‐wandering through dynamic functional connectivity. Journal of Cognitive Neuroscience, 29(3), 495–506. 10.1162/jocn_a_01066 [DOI] [PubMed] [Google Scholar]
- Murphy, C. , Poerio, G. , Sormaz, M. , Wang, H. T. , Vatansever, D. , Allen, M. , … Smallwood, J. (2019). Hello, is that me you are looking for? A re‐examination of the role of the DMN in off‐task thought. BioRxiv. 10.1101/612465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, C. , Wang, H. T. , Konu, D. , Lowndes, R. , Margulies, D. S. , Jefferies, E. , & Smallwood, J. (2019). Modes of operation: A topographic neural gradient supporting stimulus dependent and independent cognition. NeuroImage, 186, 487–496. 10.1016/j.neuroimage.2018.11.009 [DOI] [PubMed] [Google Scholar]
- Najafi, M. , McMenamin, B. W. , Simon, J. Z. , & Pessoa, L. (2016). Overlapping communities reveal rich structure in large‐scale brain networks during rest and task conditions. NeuroImage, 135, 92–106. 10.1016/j.neuroimage.2016.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi, J. S. , Bolt, T. S. , Ezie, C. E. C. , Uddin, L. Q. , & Heller, A. S. (2017). Moment‐to‐moment BOLD signal variability reflects regional changes in neural flexibility across the lifespan. The Journal of Neuroscience, 37(22), 5539–5548. 10.1523/JNEUROSCI.3408-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi, J. S. , Farrant, K. , Damaraju, E. , Rachakonda, S. , Calhoun, V. D. , & Uddin, L. Q. (2016). Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Human Brain Mapping, 37(5), 1770–1787. 10.1002/hbm.23135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi, J. S. , Vij, S. G. , Dajani, D. R. , Steimke, R. , Damaraju, E. , Rachakonda, S. , … Uddin, L. Q. (2017). Chronnectomic patterns and neural flexibility underlie executive function. NeuroImage, 147, 861–871. 10.1016/j.neuroimage.2016.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes, L. , Fulcher, B. , Yucel, M. , & Fornito, A. (2018). An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting‐state functional MRI. NeuroImage, 171, 415–436. 10.1016/j.neuroimage.2017.12.073 [DOI] [PubMed] [Google Scholar]
- Poerio, G. L. , Sormaz, M. , Wang, H. T. , Margulies, D. , Jefferies, E. , & Smallwood, J. (2017). The role of the default mode network in component processes underlying the wandering mind. Social Cognitive and Affective Neuroscience, 12(7), 1047–1062. 10.1093/scan/nsx041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool, E. M. , Rehme, A. K. , Eickhoff, S. B. , Fink, G. R. , & Grefkes, C. (2015). Functional resting‐state connectivity of the human motor network: Differences between right‐ and left‐handers. NeuroImage, 109, 298–306. 10.1016/j.neuroimage.2015.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Cohen, A. L. , Nelson, S. M. , Wig, G. S. , Barnes, K. A. , Church, J. A. , … Petersen, S. E. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Mitra, A. , Laumann, T. O. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84, 320–341. 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, P. , Liu, Y. , Shi, J. , Wang, Y. , Duncan, N. , Gong, Q. , … Northoff, G. (2012). Dissociation between anterior and posterior cortical regions during self‐specificity and familiarity: A combined fMRI‐meta‐analytic study. Human Brain Mapping, 33(1), 154–164. 10.1002/hbm.21201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid, B. , Damaraju, E. , Pearlson, G. D. , & Calhoun, V. D. (2014). Dynamic connectivity states estimated from resting fMRI identify differences among schizophrenia, bipolar disorder, and healthy control subjects. Frontiers in Human Neuroscience, 8, 897 10.3389/fnhum.2014.00897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, I. H. , Manly, T. , Andrade, J. , Baddeley, B. T. , & Yiend, J. (1997). Oops!': performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia, 35(6), 747–758. [DOI] [PubMed] [Google Scholar]
- Robison, M. K. , Miller, A. L. , & Unsworth, N. (2019). Examining the effects of probe frequency, response options, and framing within the thought‐probe method. Behavior Research Methods, 51(1), 398–408. 10.3758/s13428-019-01212-6 [DOI] [PubMed] [Google Scholar]
- Seli, P. (2016). The attention‐lapse and motor decoupling accounts of SART performance are not mutually exclusive. Consciousness and Cognition, 41, 189–198. 10.1016/j.concog.2016.02.017 [DOI] [PubMed] [Google Scholar]
- Seli, P. , Carriere, J. S. , Levene, M. , & Smilek, D. (2013). How few and far between? Examining the effects of probe rate on self‐reported mind wandering. Frontiers in Psychology, 4, 430 10.3389/fpsyg.2013.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli, P. , Kane, M. J. , Smallwood, J. , Schacter, D. L. , Maillet, D. , Schooler, J. W. , & Smilek, D. (2018). Mind‐wandering as a natural kind: A family‐resemblances view. Trends in Cognitive Sciences, 22(6), 479–490. 10.1016/j.tics.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri, C. , Corbetta, M. , Romani, G. L. , & Shulman, G. L. (2011). Episodic memory retrieval, parietal cortex, and the default mode network: Functional and topographic analyses. The Journal of Neuroscience, 31(12), 4407–4420. 10.1523/JNEUROSCI.3335-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood, J. , Beach, E. , Schooler, J. W. , & Handy, T. C. (2008). Going AWOL in the brain: Mind wandering reduces cortical analysis of external events. Journal of Cognitive Neuroscience, 20(3), 458–469. 10.1162/jocn.2008.20037 [DOI] [PubMed] [Google Scholar]
- Smallwood, J. , Davies, J. B. , Heim, D. , Finnigan, F. , Sudberry, M. , O'Connor, R. , & Obonsawin, M. (2004). Subjective experience and the attentional lapse: Task engagement and disengagement during sustained attention. Consciousness and Cognition, 13(4), 657–690. 10.1016/j.concog.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Smilek, D. , Carriere, J. S. , & Cheyne, J. A. (2010). Failures of sustained attention in life, lab, and brain: Ecological validity of the SART. Neuropsychologia, 48(9), 2564–2570. 10.1016/j.neuropsychologia.2010.05.002 [DOI] [PubMed] [Google Scholar]
- Sormaz, M. , Murphy, C. , Wang, H. T. , Hymers, M. , Karapanagiotidis, T. , Poerio, G. , … Smallwood, J. (2018). Default mode network can support the level of detail in experience during active task states. Proceedings of the National Academy of Sciences of the United States of America, 115(37), 9318–9323. 10.1073/pnas.1721259115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. , DuPre, E. , Selarka, D. , Garcia, J. , Gojkovic, S. , Mildner, J. , … Turner, G. R. (2014). Goal‐congruent default network activity facilitates cognitive control. The Journal of Neuroscience, 34(42), 14108–14114. 10.1523/JNEUROSCI.2815-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. , Stevens, W. D. , Chamberlain, J. P. , Gilmore, A. W. , & Schacter, D. L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal‐directed cognition. NeuroImage, 53(1), 303–317. 10.1016/j.neuroimage.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan, D. , Levitin, D. J. , & Menon, V. (2008). A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12569–12574. 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw, H. , & Todorov, N. (1999). Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers, 31(1), 137–149. [DOI] [PubMed] [Google Scholar]
- Stawarczyk, D. , Majerus, S. , Maquet, P. , & D'Argembeau, A. (2011). Neural correlates of ongoing conscious experience: Both task‐unrelatedness and stimulus‐independence are related to default network activity. PLoS One, 6(2), e16997 10.1371/journal.pone.0016997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimke, R. , Nomi, J. S. , Calhoun, V. D. , Stelzel, C. , Paschke, L. M. , Gaschler, R. , … Uddin, L. Q. (2017). Salience network dynamics underlying successful resistance of temptation. Social Cognitive and Affective Neuroscience, 12(12), 1928–1939. 10.1093/scan/nsx123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson, D. R. , Besner, D. , & Smilek, D. (2015). A resource‐control account of sustained attention: Evidence from mind‐wandering and vigilance paradigms. Perspectives on Psychological Science, 10(1), 82–96. 10.1177/1745691614556681 [DOI] [PubMed] [Google Scholar]
- Tognoli, E. , & Kelso, J. A. (2014). The metastable brain. Neuron, 81(1), 35–48. 10.1016/j.neuron.2013.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull, A. , Wang, H. T. , Schooler, J. W. , Jefferies, E. , Margulies, D. S. , & Smallwood, J. (2019). The ebb and flow of attention: Between‐subject variation in intrinsic connectivity and cognition associated with the dynamics of ongoing experience. NeuroImage, 185, 286–299. 10.1016/j.neuroimage.2018.09.069 [DOI] [PubMed] [Google Scholar]
- Uddin, L. Q. (2011). Resting‐state FMRI and developmental systems neuroscience. Frontiers in Neuroscience, 5, 14 10.3389/fnins.2011.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews. Neuroscience, 16(1), 55–61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Uddin, L. Q. , Iacoboni, M. , Lange, C. , & Keenan, J. P. (2007). The self and social cognition: The role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences, 11(4), 153–157. 10.1016/j.tics.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Uddin, L. Q. , Kelly, A. M. , Biswal, B. B. , Castellanos, F. X. , & Milham, M. P. (2009). Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping, 30(2), 625–637. 10.1002/hbm.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Calster, L. , D'Argembeau, A. , Salmon, E. , Peters, F. , & Majerus, S. (2017). Fluctuations of Attentional networks and default mode network during the resting state reflect variations in cognitive states: Evidence from a novel resting‐state experience sampling method. Journal of Cognitive Neuroscience, 29(1), 95–113. 10.1162/jocn_a_01025 [DOI] [PubMed] [Google Scholar]
- Varela, F. , Lachaux, J. P. , Rodriguez, E. , & Martinerie, J. (2001). The brainweb: Phase synchronization and large‐scale integration. Nature Reviews. Neuroscience, 2(4), 229–239. 10.1038/35067550 [DOI] [PubMed] [Google Scholar]
- Wang, H. T. , Bzdok, D. , Margulies, D. , Craddock, C. , Milham, M. , Jefferies, E. , & Smallwood, J. (2018). Patterns of thought: Population variation in the associations between large‐scale network organisation and self‐reported experiences at rest. NeuroImage, 176, 518–527. 10.1016/j.neuroimage.2018.04.064 [DOI] [PubMed] [Google Scholar]
- Wang, H. T. , Poerio, G. , Murphy, C. , Bzdok, D. , Jefferies, E. , & Smallwood, J. (2018). Dimensions of experience: Exploring the heterogeneity of the wandering mind. Psychological Science, 29(1), 56–71. 10.1177/0956797617728727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Yu, C. , Xu, L. , Qin, W. , Li, K. , Xu, L. , & Jiang, T. (2009). Offline memory reprocessing: Involvement of the brain's default network in spontaneous thought processes. PLoS One, 4(3), e4867 10.1371/journal.pone.0004867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, K. N. , Hermiller, M. S. , Nilakantan, A. S. , O'Neil, J. , Palumbo, R. T. , & Voss, J. L. (2018). Increased fMRI activity correlations in autobiographical memory versus resting states. Human Brain Mapping, 39, 4312–4321. 10.1002/hbm.24248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, N. , & Henson, R. N. (2018). Recent advances in functional neuroimaging analysis for cognitive neuroscience. Brain and Neuroscience Advances, 2, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems, R. M. , Van der Haegen, L. , Fisher, S. E. , & Francks, C. (2014). On the other hand: Including left‐handers in cognitive neuroscience and neurogenetics. Nature Reviews. Neuroscience, 15(3), 193–201. 10.1038/nrn3679 [DOI] [PubMed] [Google Scholar]
- Yan, C. G. , Wang, X. D. , Zuo, X. N. , & Zang, Y. F. (2016). DPABI: Data Processing & Analysis for (resting‐state) brain imaging. Neuroinformatics, 14(3), 339–351. 10.1007/s12021-016-9299-4 [DOI] [PubMed] [Google Scholar]
- Yang, Z. , Craddock, R. C. , Margulies, D. S. , Yan, C. G. , & Milham, M. P. (2014). Common intrinsic connectivity states among posteromedial cortex subdivisions: Insights from analysis of temporal dynamics. NeuroImage, 93(Pt 1), 124–137. 10.1016/j.neuroimage.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabelina, D. L. , & Andrews‐Hanna, J. R. (2016). Dynamic network interactions supporting internally‐oriented cognition. Current Opinion in Neurobiology, 40, 86–93. 10.1016/j.conb.2016.06.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The raw data that support the findings of this study are available from the first author upon reasonable request.


