Abstract
Nowadays, epoxy composites are elements of engineering materials and systems. Although they are known as versatile materials, epoxy resins suffer from high flammability. In this sense, flame retardancy analysis has been recognized as an undeniable requirement for developing future generations of epoxy-based systems. A considerable proportion of the literature on epoxy composites has been devoted to the use of phosphorus-based additives. Nevertheless, innovative flame retardants have coincidentally been under investigation to meet market requirements. This review paper attempts to give an overview of the research on flame retardant epoxy composites by classification of literature in terms of phosphorus (P), non-phosphorus (NP), and combinations of P/NP additives. A comprehensive set of data on cone calorimetry measurements applied on P-, NP-, and P/NP-incorporated epoxy systems was collected and treated. The performance of epoxy composites was qualitatively discussed as Poor, Good, and Excellent cases identified and distinguished by the use of the universal Flame Retardancy Index (FRI). Moreover, evaluations were rechecked by considering the UL-94 test data in four groups as V0, V1, V2, and nonrated (NR). The dimensionless FRI allowed for comparison between flame retardancy performances of epoxy composites. The results of this survey can pave the way for future innovations in developing flame-retardant additives for epoxy.
Keywords: epoxy, Flame Retardancy Index (FRI), fire retardancy, cone calorimetry
1. Introduction
Innovations are mainly born in a very disciplined manner, but sometimes they arise from serendipity. Regardless of the origin of innovative materials and systems, the identification and classification of systems in terms of explanatory variables requires the use of universal, well-accepted criteria. Nowadays, epoxy-based composites are elements of advanced systems [1,2,3]. There has been continued interest in the use of epoxy for developing a wide variety of general- and specific-purpose products such as adhesives, coatings, and medical devices thanks to the versatility of this thermosetting material [4,5,6,7]. Nevertheless, research outcomes reveal that epoxy is highly flammable, and one principally requires flame retardant materials for applications where epoxy should stand against fire [8,9,10,11,12]. In general, it has been understood that careful selection of additives is the first step in development of flame retardant polymer composites, but the performance of the material may additionally depend on the type and the amount of additives used individually or simultaneously [13,14]. Particularly, flame retardant epoxy composites consisting of phosphorus flame-retardant additives were the subject of different reports [15,16]. Moreover, combination of phosphorus and nonphosphorus additives was considered in the quest of higher flame retardancy performance [17,18,19]. In almost all reports, however, there was a lack of a correlation between the crosslinking state of resin in the presence of additives and flame retardancy.
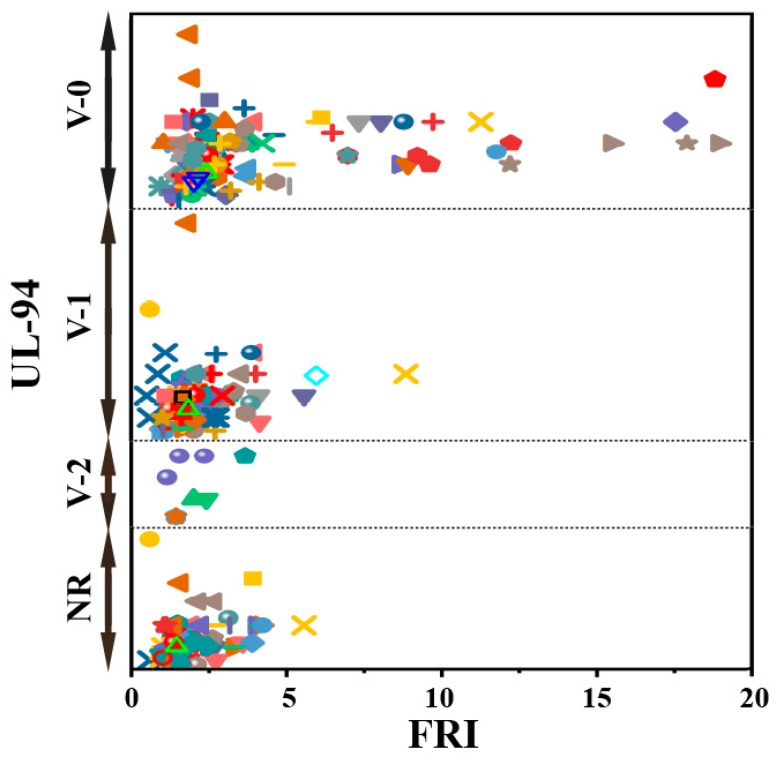
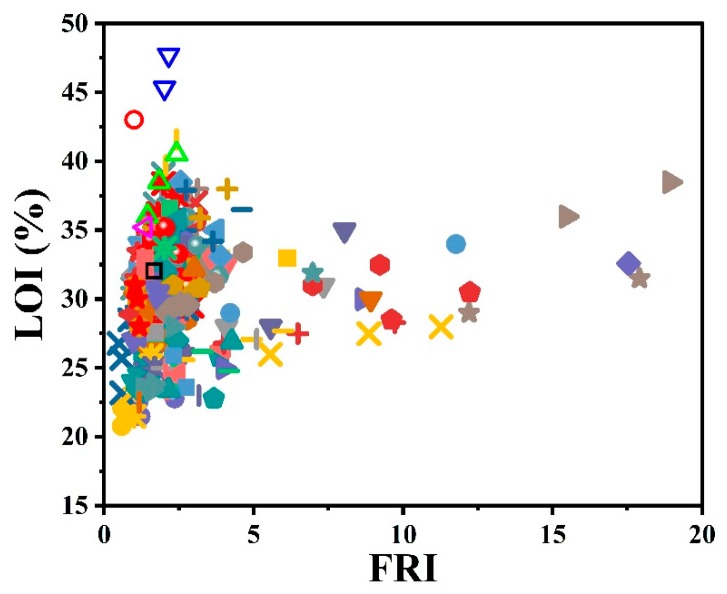
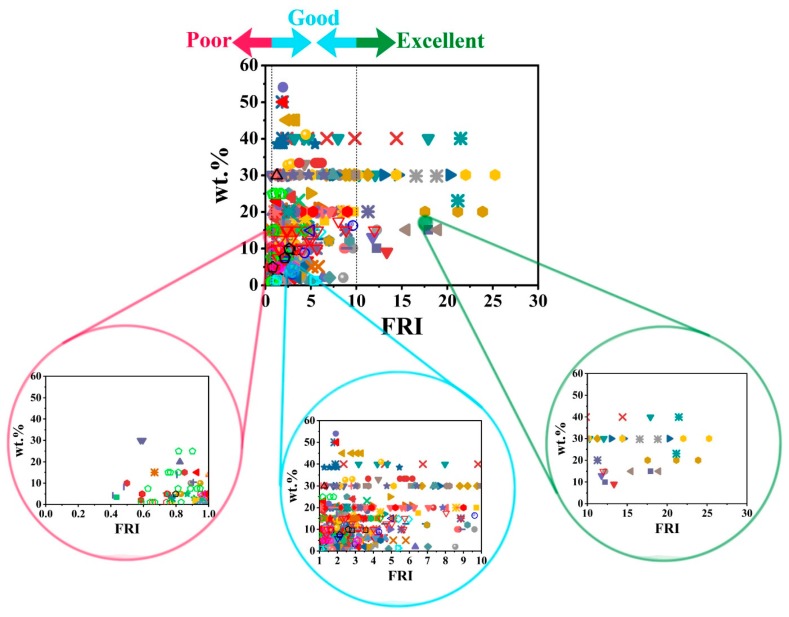
In a previous work, we used two dimensionless indexes to correlate cure state with corrosion inhibition and flame-retardant properties of epoxy/Fe3O4 nanocomposites [20]. By the use of dimensionless Cure Index [21] and dimensionless Flame Retardancy Index (FRI) [22], it was demonstrated that the quality of cure in epoxy composites (Poor, Good, or Excellent) can be correlated to the performance of flame retardancy (Poor, Good, or Excellent). The FRI was also powerful in exploring the complementary actions of mineral and organic additives in polymer systems in terms of the peak of HRR (pHRR), the total heat release (THR), and the time to ignition (TTI) of neat polymer and polymer composites [23]. In this work, with the aim of recognizing the future ahead of innovations in flame-retardant epoxy composites, reports on flame-retardant epoxy composites were comprehensively reviewed and then classified as a function of their flame retardancy performance by the use of the FRI criterion. Classification was performed on account of phosphorus (P)-, nonphosphorus (NP)-, and combined P/NP-incorporated epoxy composites. In each class, comprehensive master tables were provided in which the polymer matrix, the additives, the content of additives, and cone calorimetry data including TTI, THR, and pHRR and the calculated FRI values were summarized. Moreover, the available UL-94 test data were provided and plotted similar to the FRI curves, but in four groups of V0, V1, V2, and nonrated (NR).
2. Epoxy Resins Containing Phosphorus-Based Flame Retardants
According to the literature, a variety of phosphorus-based flame retardants have been used in epoxy resins. Table 1 summarizes pHRR, THR, and TTI and the FRI values of epoxy/P systems. The percentage of incorporated flame retardant (FR) as well as the results of limiting oxygen index (LOI) and UL-94 test are given.
Table 1.
The flame retardancy performance of epoxy containing phosphorus-based (P) flame retardants in terms of FRI (* the name and percentage of incorporated flame retardant is given after each epoxy resin). Notes a to l on the bottom of the table are representative of composite systems containing woven or nonwoven fibers.
| Epoxy Resins and Incorporated Phosphorus FR * | wt.% | TTI (s) | pHRR (kW·m−2) | THR (MJ·m−2) | FRI | LOI | UL94 | Ref. |
|---|---|---|---|---|---|---|---|---|
| 0 | 49 | 1477 | 118 | — | 27 | NR | [24] | |
| N, N′-diallyl-p-phenylphosphonicdiamide (FP1) | 4 | 46 | 831 | 106 | 1.85 | 33 | NR | [24] |
| N, N′-diallyl-p-phenylphosphonicdiamide (FP1) | 6 | 42 | 500 | 115 | 2.59 | 36 | V-1 | [24] |
| N, N′-diallyl-p-phenylphosphonicdiamide (FP1) | 8 | 40 | 587 | 109 | 2.22 | 38 | V-0 | [24] |
| 0 | 31 | 1068 | 76 | — | 23.7 | NR | [25] | |
| (bis(4- hydroxyphenyl) methyl) diphenylphosphine oxide (DPO-PHE) | 11.68 | 41 | 657 | 59 | 2.76 | 32.1 | V-0 | [25] |
| 1-(bis(4-hydroxyphenyl)methyl)-9,10-dihydro-9- oxa-10-phosphaphenan-threne-10-oxide (DOPO-PHE) | 12.03 | 39 | 956 | 57 | 1.87 | 30.5 | V-0 | [25] |
| 0 | 47 | 1208 | 80 | — | 22.5 | NR | [26] | |
| Reaction between 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & cyanuric chloride (DOPO-T) | 2.34 | 38 | 836 | 69 | 1.35 | 32.5 | NR | [26] |
| reaction between 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & cyanuric chloride (DOPO-T) | 4.67 | 36 | 727 | 62 | 1.64 | 34.6 | V-1 | [26] |
| reaction between 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & cyanuric chloride (DOPO-T) | 6.99 | 32 | 629 | 56 | 1.86 | 36.2 | V-1 | [26] |
| Reaction between 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & cyanuric chloride (DOPO-T) | 9.34 | 30 | 613 | 54 | 1.86 | 33.4 | V-0 | [26] |
| 0 | 131 | 495 | 179 | — | 21.3 | V-2 | [27] | |
| Aluminum ethylphenylphosphinate (AEPP) | 5 | 119 | 254 | 131 | 2.41 | 23.3 | V-2 | [27] |
| aluminum ethylphenylphosphinate (AEPP) | 10 | 105 | 241 | 124 | 2.37 | 25.7 | V-1 | [27] |
| aluminum ethylphenylphosphinate (AEPP) | 15 | 91 | 223 | 119 | 2.31 | 28.2 | V-0 | [27] |
| 0 | 32 | 827 | 116 | — | 21.8 | NR | [28] | |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 3 | 41 | 387 | 104 | 3.05 | 32.7 | V-0 | [28] |
| 0 | 32 | 781 | 107 | — | 21.8 | NR | [29] | |
| Phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 10 | 38 | 508 | 83 | 2.35 | 38 | V-0 | [29] |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 11 | 43 | 441 | 96 | 2.65 | 37.4 | V-0 | [29] |
| 0 | 32 | 781 | 107 | — | 21.8 | NR | [30] | |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 7 | 36 | 491 | 80 | 2.39 | 35.7 | V-0 | [30] |
| 0 | 32 | 781 | 107 | — | 21.8 | NR | [31] | |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 1 | 33 | 516 | 116 | 1.43 | 24.1 | V-2 | [31] |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative(DiDOPO) | 5 | 35 | 491 | 81 | 2.29 | 35.8 | V-0 | [31] |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 10 | 38 | 508 | 83 | 2.35 | 38 | V-0 | [31] |
| 0 | 32 | 781 | 107 | — | 21.8 | NR | [32] | |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 1 | 33 | 516 | 116 | 1.43 | 24.1 | V-2 | [32] |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 5 | 35 | 491 | 81 | 2.29 | 35.7 | V-0 | [32] |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 10 | 38 | 508 | 83 | 2.35 | 38 | V-0 | [32] |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 15 | 41 | 436 | 72 | 3.41 | 33.6 | V-0 | [32] |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative (DiDOPO) | 20 | 16 | 298 | 68 | 2.06 | 27.5 | V-0 | [32] |
| 0 | 19 | 1324.6 | 95.7 | — | 19.2 | HB | [33] | |
| pentaerythritol phosphate melamine salt (PPMS) | 15 | 20 | 491.6 | 74 | 3.66 | 22.8 | V-2 | [33] |
| pentaerythritol phosphate melamine salt functionalized Expandable graphite (PPMS-EG) | 15 | 16 | 414.3 | 66.7 | 3.86 | 25.8 | V-1 | [33] |
| 0 | 15 | 1334.6 | 100.1 | — | 19.3 | HB | [34] | |
| Pentaerythritol phosphate melamine salt-functionalized Multiwalled carbon nanotube (PPMS-MWCNT) | 5 | 13 | 1013.4 | 93.7 | 1.21 | 21.5 | HB | [34] |
| Pentaerythritol phosphate melamine salt-functionalized Multiwalled carbon nanotube (PPMS-MWCNT) | 10 | 8 | 680.7 | 90.7 | 1.15 | 22.6 | V-2 | [34] |
| Pentaerythritol phosphate melamine salt-functionalized Multiwalled carbon nanotube (PPMS-MWCNT) | 15 | 6 | 444.6 | 77.6 | 1.54 | 24.5 | V-2 | [34] |
| pentaerythritol phosphate melamine salt (PPMS) | 15 | 11 | 489.5 | 85.2 | 2.34 | 22.8 | V-2 | [34] |
| 0 | 66 | 793.5 | 86.3 | — | 21 | NR | [35] | |
| diphenyl 1H-imidazol-1-ylphosphonate (DPIPP) | 7.5 | 56 | 535.2 | 61.3 | 1.77 | 27.5 | NR | [35] |
| diphenyl 1H-imidazol-1-ylphosphonate (DPIPP) | 15 | 59 | 427.5 | 53.7 | 2.66 | 31.5 | V-0 | [35] |
| 1-(diphenylphosphinyl)-1H-imidazole oxide (DPPIO) | 7.5 | 62 | 583.1 | 60 | 1.83 | 33 | NR | [35] |
| 1-(diphenylphosphinyl)-1H-imidazole oxide (DPPIO) | 15 | 63 | 432.9 | 48.4 | 3.11 | 38 | V-0 | [35] |
| 0 | 57 | 770.1 | 82.6 | — | 20.5 | NR | [36] | |
| imidazolium dibenzo[c,e[1,2]oxaphosphate (IDOP) | 5 | 65 | 617.5 | 65.8 | 1.78 | 27 | NR | [36] |
| imidazolium dibenzo[c,e [1,2]oxaphosphate (IDOP) | 10 | 67 | 586.5 | 64.2 | 1.98 | 34.5 | V-1 | [36] |
| imidazolium dibenzo[c,e [1,2]oxaphosphate (IDOP) | 15 | 68 | 485.6 | 51.2 | 3.05 | 37 | V-0 | [36] |
| 0 | 63 | 731.2 | 103.2 | — | 21.1 | NR | [37] | |
| polyphosphoric acid piperazine (PPAP) | 5 | 38 | 511.9 | 92.5 | 0.96 | 30.8 | V-0 | [37] |
| diglycidyl ether of bisphenol A epoxy resin epoxy/hollow glass microspheres(foam) | 0 | 17 | 444.92 | 138.2 | — | 21.5 | NR | [38] |
| aluminum diisobutylphosphinate (AlPBu) | 10 | 17 | 272.28 | 113.2 | 1.99 | 26.5 | NR | [38] |
| aluminum diisobutylphosphinate (AlPBu) | 12.5 | 17 | 264.98 | 110.8 | 2.09 | 27.8 | V-1 | [38] |
| Aluminum diisobutylphosphinate (AlPBu) | 15 | 17 | 260.77 | 109.3 | 2.15 | 29 | V-0 | [38] |
| 0 | 53 | 1484 | 86.4 | — | 26 | NR | [39] | |
| 6-morpholino-6Hdibenzo[c,e][1,2]oxaphosphinine 6-oxide (MPL-DOPO) | 2.5 | 46 | 1296 | 74.3 | 1.15 | 29.5 | V-1 | [39] |
| 6-morpholino-6Hdibenzo[c,e][1,2]oxaphosphinine 6-oxide (MPL-DOPO) | 5 | 45 | 1145 | 67.1 | 1.41 | 30.5 | V-0 | [39] |
| 6,6′-((methylenebis(4,1 phenylene))bis(azanediyl))bis(6Hdibenzo[c,e][1,2]oxaphosphinine 6-oxide) (DDM-DOPO) | 2.5 | 51 | 1236 | 76.5 | 1.30 | 30 | V-0 | [39] |
| 6,6′-((methylenebis(4,1 phenylene))bis(azanediyl))bis(6Hdibenzo[c,e][1,2]oxaphosphinine 6-oxide) (DDM-DOPO) | 5 | 48 | 999 | 69.7 | 1.66 | 31.5 | V-0 | [39] |
| 0 | 71 | 654.3 | 100.3 | — | 25.7 | NR | [40] | |
| 6-(((1H-tetrazol-5-yl)amino)(4hydroxyphenyl)methyl)dibenzo[c,e][1,2]oxaphosphinine 6-oxide (ATZ) | 6 | 81 | 482.5 | 83.9 | 1.84 | 33.7 | V-0 | [40] |
| Waterborne EP resin | 0 | 25 | 343.7 | 18.3 | — | 19.3 | NR | [41] |
| phosphated K-carrageenan (P-KC) | 30 | 14 | 313.7 | 19.3 | 0.58 | 20.8 | NR | [41] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 30 | 10 | 279.6 | 15.1 | 0.59 | 22.1 | V-1 | [41] |
| 0 | 39 | 1162 | 104 | — | 26.8 | NR | [42] | |
| Tris(Bis(4((Diphenoxyphosphoryl)Oxy)Phenyl)Methyl)Benzene-1,3,5-Tricarboxylate (DHPP-OH-BAC) | 5 | 50 | 796 | 97 | 2.00 | 31.2 | V-2 | [42] |
| Tris(Bis(4((Diphenoxyphosphoryl)Oxy)Phenyl)Methyl)Benzene-1,3,5-Tricarboxylate (DHPP-OH-BAC) | 10 | 58 | 643 | 91 | 3.07 | 32.4 | V-1 | [42] |
| Tris(Bis(4((Diphenoxyphosphoryl)Oxy)Phenyl)Methyl)Benzene-1,3,5-Tricarboxylate (DHPP-OH-BAC) | 15 | 62 | 610 | 88 | 3.57 | 33.6 | V-0 | [42] |
| 0 | 40 | 1511.7 | 115.8 | — | 19 | NR | [43] | |
| poly(pentaerythritol phosphate phosphinic acyl piperazine) (PPAP) | 5 | 38 | 838.1 | 75.4 | 2.63 | 26 | NR | [43] |
| poly(pentaerythritol phosphate phosphinic acyl piperazine) (PPAP) | 10 | 36 | 522 | 54.2 | 5.56 | 28 | V-1 | [43] |
| poly(pentaerythritol phosphate phosphinic acyl piperazine) (PPAP) | 20 | 34 | 416 | 44.5 | 8.03 | 35 | V-0 | [43] |
| 0 | 61 | 1125.8 | 66.2 | — | 26.5 | NR | [44] | |
| 1-methyl-3-((6-oxidodibenzo[c,e][1,2]oxaphosphinin 6-yl)methyl)-1H-imidazol-3-ium 4 methylbenzenesulfonate ([Dmim]Tos) | 2.4 | 51 | 947.6 | 67.3 | 0.97 | 31.7 | V-1 | [44] |
| 1-methyl-3-((6-oxidodibenzo[c,e][1,2]oxaphosphinin 6-yl)methyl)-1H-imidazol-3-ium 4 methylbenzenesulfonate ([Dmim]Tos) | 4 | 57 | 705.4 | 57.6 | 1.71 | 32.5 | V-0 | [44] |
| 1-methyl-3-((6-oxidodibenzo[c,e][1,2]oxaphosphinin 6-yl)methyl)-1H-imidazol-3-ium 4 methylbenzenesulfonate ([Dmim]Tos) | 7.5 | 51 | 767 | 56.2 | 1.44 | 33.9 | V-0 | [44] |
| 0 | 32 | 1111 | 18.2 | — | 20.5 | NR | [45] | |
| melamine phenylphosphate (MPhP) | 10 | 38 | 1008 | 12.4 | 1.92 | 23.5 | NR | [45] |
| melamine phenylphosphate (MPhP) | 15 | 40 | 846 | 12.2 | 2.44 | 24.5 | V-1 | [45] |
| melamine phenylphosphate (MPhP) | 20 | 41 | 545 | 12 | 3.96 | 26.5 | V-0 | [45] |
| 0 | 74 | 1205.4 | 77.1 | — | 26.4 | NR | [46] | |
| melamine-organophosphinic acid salt (MDOP) | 0.96 | 79 | 1426.4 | 75.4 | 0.92 | 31 | V-1 | [46] |
| melamine-organophosphinic acid salt (MDOP) | 1.9 | 76 | 1209.5 | 74.2 | 1.06 | 32 | V-1 | [46] |
| melamine-organophosphinic acid salt (MDOP) | 3.75 | 78 | 915.3 | 67.1 | 1.59 | 35.6 | V-0 | [46] |
| melamine-organophosphinic acid salt (MDOP) | 7.24 | 67 | 660.7 | 60.2 | 2.11 | 38 | V-0 | [46] |
| 0 | 70 | 1491 | 81 | — | 19 | NR | [47] | |
| aluminum diethyl phosphinate (AlPi) | 7 | 58 | 572 | 63 | 2.77 | 28.5 | V-0 | [47] |
| Melamine polyphosphate (MPP) | 7 | 75 | 479 | 68 | 3.97 | — | — | [47] |
| 0 | 70 | 1000.5 | 95.2 | — | 22.6 | NR | [48] | |
| bisphenol-A bridged penta(phenoxy)cyclotriphosphazene (A-BP) | 9 | 62 | 783 | 55.9 | 1.92 | 33.9 | V-0 | [48] |
| 0 | 60 | 1285 | 83.5 | — | 25.5 | NR | [19] | |
| cage–ladder-structure, phosphorus-containing polyhedral oligomeric silsesquinoxane (CLEP–DOPO–POSS) via the hydrolytic condensation of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO)–vinyl trimethoxysilane (VTMS)with 2-(3,4-epoxycyclohexyl) ethyl trimethoxysilane (CLEP–DOPO–POSS) | 2.91 | 62 | 961 | 84.9 | 1.35 | 31.9 | V-0 | [19] |
| 0 | 95 | 939 | 98 | — | 23 | NR | [49] | |
| copper phenylphosphate nanoplate (CuPP) | 1 | 103 | 511 | 93 | 2.09 | 32.4 | NR | [49] |
| copper phenylphosphate nanoplate (CuPP) | 2 | 80 | 466 | 83 | 2.00 | 35.5 | V-1 | [49] |
| copper phenylphosphate nanoplate (CuPP) | 4 | 88 | 454 | 82 | 2.28 | 38.2 | V-1 | [49] |
| copper phenylphosphate nanoplate (CuPP) | 6 | 88 | 448 | 72 | 2.64 | 37.8 | V-1 | [49] |
| copper phenylphosphate nanoplate (CuPP) | 8 | 86 | 401 | 73 | 2.84 | 34.6 | V-1 | [49] |
| 0 | 69 | 1139.7 | 75.7 | — | 25.2 | NR | [50] | |
| reaction of 2-chloro-5,5-dimethyl-1,3,2-dioxaphosphinane-2-oxide & 2-aminobenzothiazole (DOP-ABZ) | 15 | 66 | 327.2 | 63 | 4.00 | 26.8 | V-1 | [50] |
| reaction of 2-chloro-5,5-dimethyl-1,3,2-dioxaphosphinane-2-oxide & 2-aminobenzothiazole (DOP-ABZ) | 17.5 | 65 | 308.9 | 40.6 | 6.48 | 27.5 | V-0 | [50] |
| reaction of 2-chloro-5,5-dimethyl-1,3,2-dioxaphosphinane-2-oxide & 2-aminobenzothiazole (DOP-ABZ) | 20 | 52 | 238.9 | 28 | 9.72 | 28.3 | V-0 | [50] |
| 0 | 36 | 1558 | 93 | — | 24.2 | NR | [51] | |
| 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 7.11 | 33 | 1301 | 64.6 | 1.58 | 35.1 | V-1 | [51] |
| reaction between 1,4-Phthalaldehyde & 2-benzothiazolamine & 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (BPD) | 3.38 | 34 | 1313 | 78.9 | 1.32 | 32.8 | V-1 | [51] |
| reaction between 1,4-Phthalaldehyde & 2-benzothiazolamine & 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (BPD) | 6.71 | 32 | 1273 | 69.8 | 1.44 | 34.3 | V-1 | [51] |
| reaction between 1,4-Phthalaldehyde & 2-benzothiazolamine & 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (BPD) | 10.04 | 33 | 1220 | 63.8 | 1.70 | 36.9 | V-0 | [51] |
| reaction between 1,4-Phthalaldehyde & 2-benzothiazolamine & 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (BPD) | 13.41 | 31 | 1071 | 59.1 | 1.97 | 39.1 | V-0 | [51] |
| 0 | 61 | 1208 | 77.3 | — | 22.5 | NR | [52] | |
| 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 7.7 | 56 | 828 | 61.6 | 1.68 | 34.5 | V-1 | [52] |
| hexa-phenoxy-cyclotriphosphazene (HPCP) | 8.2 | 52 | 510 | 63.1 | 2.47 | 32.5 | V-1 | [52] |
| 78 | 1934.2 | 103.3 | — | 23.5 | NR | [53] | ||
| reaction between 4-(hydroxymethyl)-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane 1-oxide & 6-(2,5-dihydroxyphenyl)-6H-dibenzo[c,e][1,2]oxaphosphinine 6-oxide (DOPO-TPMP) | 2.5 | 76 | 1683.9 | 91.1 | 1.26 | 28.2 | V-1 | [53] |
| reaction between 4-(hydroxymethyl)-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane 1-oxide & 6-(2,5-dihydroxyphenyl)-6H-dibenzo[c,e] [1,2]oxaphosphinine 6-oxide (DOPO-TPMP) | 5 | 72 | 1544.8 | 82.9 | 1.44 | 34.8 | V-1 | [53] |
| reaction between 4-(hydroxymethyl)-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane 1-oxide & 6-(2,5-dihydroxyphenyl)-6H-dibenzo[c,e][1,2]oxaphosphinine 6-oxide (DOPO-TPMP) | 7.5 | 72 | 1483.6 | 75.7 | 1.64 | 35.6 | V-0 | [53] |
| reaction between 4-(hydroxymethyl)-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane 1-oxide & 6-(2,5-dihydroxyphenyl)-6H-dibenzo[c,e][1,2]oxaphosphinine 6-oxide (DOPO-TPMP) | 10 | 63 | 819.3 | 69.2 | 2.84 | 36.1 | V-0 | [53] |
| 54 | 880 | 187 | — | 24.1 | NR | [54] | ||
| 10-(hydroxy(4-hydroxyphenyl)methyl)-5,10-dihydrophenophosphazinine-10-oxide (HB-DPPA) | 2 | 65 | 800 | 162 | 1.52 | 29.3 | V-0 | [54] |
| 53 | 1121 | 102 | — | 20 | NR | [55] | ||
| ammonium polyphosphate (APP) | 21 | 57 | 594 | 53 | 3.90 | 33 | NR | [55] |
| ethanediamine-modified ammonium polyphosphate (EDA-APP) | 21 | 61 | 398 | 54 | 6.12 | 33 | V-0 | [55] |
| 45 | 1091 | 83 | — | 22.8 | NR | [56] | ||
| hexakis(4-boronic acid-phenoxy)-cyclophosphazene (CP-6B) | 3 | 42 | 608 | 71 | 1.95 | 30.8 | V-0 | [56] |
| 57 | 1108 | 96.2 | — | 22 | NR | [57] | ||
| N,N′-diamyl-p-phenylphosphonicdiamide (PM) | 2 | 56 | 970 | 84.2 | 1.28 | 24.5 | NR | [57] |
| N,N′-diamyl-p-phenylphosphonicdiamide (PM) | 6 | 54 | 840 | 78.5 | 1.53 | 25.5 | NR | [57] |
| IC: inclusion complex β-cyclodextrin & N,N′-diamyl-p-phenylphosphonicdiamide (PM-βCD) | 2 | 55 | 905 | 73 | 1.55 | 26.5 | NR | [57] |
| IC: inclusion complex β-cyclodextrin & N,N′-diamyl-p-phenylphosphonicdiamide (PM-βCD) | 6 | 50 | 541 | 68.8 | 2.51 | 26.8 | NR | [57] |
| 43 | 469 | 66.2 | — | 24.7 | NR | [58] | ||
| poly(4,40-diamino diphenyl sulfone 2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane-4-methanol-substituted phosphoramide) (PSA) | 10 | 28 | 149 | 33.2 | 4.08 | 28 | V-1 | [58] |
| poly(4,40-diamino diphenyl sulfone 2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane-4-methanol-substituted phosphoramide) (PSA) | 20 | 26 | 118 | 21.7 | 7.33 | 31 | V-0 | [58] |
| 82 | 1148 | 88.4 | — | 21 | NR | [59] | ||
| bisphenol A bridged penta(anilino) cyclotriphosphazene (BPA-BPP) | 9 | 72 | 457 | 78.4 | 2.48 | 28.7 | V-1 | [59] |
| 46 | 1291 | 87.2 | — | 23 | NR | [60] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 9.1 | 26 | 893 | 59.6 | 1.19 | 29 | NR | [60] |
| 1-oxo-4-hydroxymethyl-2,6,7-trioxa-l phosphabicyclo[2.2.2] octane (PEPA) | 9.1 | 40 | 847 | 59.5 | 1.94 | 28 | NR | [60] |
| reaction between 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-1-oxo-4-hydroxymethyl-2,6,7-trioxa-l phosphabicyclo[2.2.2] octane (DOPO-PEPA) | 5.7 | 44 | 873 | 60.9 | 2.02 | 30 | V-0 | [60] |
| reaction between 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-1-oxo-4-hydroxymethyl-2,6,7-trioxa-l phosphabicyclo[2.2.2] octane (DOPO-PEPA) | 7.4 | 48 | 683 | 46.3 | 3.71 | 35 | V-0 | [60] |
| reaction between 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-1-oxo-4-hydroxymethyl-2,6,7-trioxa-l phosphabicyclo[2.2.2] octane (DOPO-PEPA) | 9.1 | 42 | 595 | 45.9 | 3.76 | 35 | V-0 | [60] |
| 58 | 839 | 129 | — | — | NR | [61] | ||
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10 phosphaphenanthrene-10-oxide (DOPO-POSS) | 2.5 | 58 | 631 | 104 | 1.64 | 27.1 | V-1 | [61] |
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10 phosphaphenanthrene-10-oxide (DOPO-POSS) | 5 | 62 | 404 | 87 | 3.29 | — | NR | [61] |
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10 phosphaphenanthrene-10-oxide (DOPO-POSS) | 10 | 61 | 346 | 79 | 4.16 | — | NR | [61] |
| 53 | 1034 | 114 | — | 24.2 | NR | [62] | ||
| Hexaphenoxycyclotriphosphazene (HPCTP) | 7.46 | 56 | 918 | 94 | 1.44 | 26.2 | V-1 | [62] |
| Hexaphenoxycyclotriphosphazene (HPCTP) | 11.19 | 53 | 796 | 83 | 1.78 | 28 | V-0 | [62] |
| Hexaphenoxycyclotriphosphazene (HPCTP) | 14.92 | 54 | 840 | 78 | 1.83 | 28.6 | V-0 | [62] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 6.97 | 51 | 947 | 92 | 1.30 | 25.9 | NR | [62] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 10.46 | 50 | 850 | 88 | 1.48 | 27.4 | NR | [62] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 13.94 | 46 | 785 | 81 | 1.60 | 27.8 | V-1 | [62] |
| 60 | 872.8 | 88.5 | — | 22.5 | NR | [63] | ||
| 2-(hydroxy(phenyl)methyl)-5,5-dimethyl-1,3,2-dioxaphosphinane 2-oxide (TP) | 12.42 | 23 | 312.6 | 59 | 1.60 | 31.8 | V-1 | [63] |
| [4-(2,4,6-Tris[24] dioxaphosphinan-2-yl) hydroxymety] phenoxy]-(1,3,5)-triazine (TNTP) | 14.36 | 34 | 253 | 65.8 | 2.62 | 32.4 | V-0 | [63] |
| 47 | 1208 | 81 | — | 22.5 | NR | [64] | ||
| 9,10-dihydro-9-oxa-10 phosphaphenanthrene-10-oxide (DOPO) | 7 | 32 | 853 | 64 | 1.22 | 34 | V-1 | [64] |
| reaction between triglycidyl isocyanurate, 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & phenylboronic acid (BNP) | 7 | 38 | 505 | 60 | 2.61 | 29.5 | NR | [64] |
| reaction between triglycidyl isocyanurate, 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & phenylboronic acid (BNP) | 11 | 35 | 425 | 52 | 3.29 | 32 | V-1 | [64] |
| reaction between triglycidyl isocyanurate, 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & phenylboronic acid (BNP) | 14.7 | 34 | 410 | 50 | 3.45 | 32.5 | V-0 | [64] |
| reaction between triglycidyl isocyanurate, 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & phenylboronic acid (BNP) | 18.4 | 33 | 400 | 47 | 3.65 | 33.3 | V-0 | [64] |
| 47 | 1208 | 81 | — | 22.5 | NR | [65] | ||
| 9,10-dihydro-9-oxa-10 phosphaphenanthrene-10-oxide (DOPO) | 7 | 32 | 853 | 64 | 1.22 | 34 | V-1 | [65] |
| reaction between triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10 phosphaphenanthrene-10-oxide & boric acid (DTB) | 7 | 32 | 556 | 61 | 1.96 | 31.5 | NR | [65] |
| reaction between triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10 phosphaphenanthrene-10-oxide & boric acid (DTB) | 10 | 33 | 453 | 55 | 2.75 | 33.2 | V-1 | [65] |
| reaction between triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10 phosphaphenanthrene-10-oxide & boric acid (DTB) | 15 | 34 | 425 | 54 | 3.08 | 35.6 | V-0 | [65] |
| reaction between triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10 phosphaphenanthrene-10-oxide & boric acid (DTB) | 20 | 31 | 461 | 57 | 2.45 | 35.2 | V-0 | [65] |
| 58 | 1208 | 80.6 | — | 22.5 | NR | [66] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 7.7 | 58 | 828 | 63.7 | 1.84 | 34.5 | V-1 | [66] |
| hexa-phenoxy-cyclotriphosphazene (HPCP) | 8.2 | 49 | 510 | 64 | 2.52 | 32.5 | V-1 | [66] |
| 57 | 1557 | 94.5 | — | 24.5 | NR | [67] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 7.1 | 52 | 1301 | 65 | 1.58 | 35.2 | V-1 | [67] |
| 61 | 1208 | 80.6 | — | 22.5 | NR | [68] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 7 | 58 | 833 | 66.3 | 1.67 | 34 | V-1 | [68] |
| tri(phosphaphenanthrene-maleimide-phenoxyl)-triazine (DOPO-TMT) | 7 | 56 | 919 | 71.2 | 1.36 | 29.5 | NR | [68] |
| tri(phosphaphenanthrene-maleimide-phenoxyl)-triazine (DOPO-TMT) | 10.4 | 56 | 694 | 63.7 | 2.02 | 33 | V-1 | [68] |
| tri(phosphaphenanthrene-maleimide-phenoxyl)-triazine (DOPO-TMT) | 13.9 | 53 | 776 | 60.6 | 1.79 | 36.2 | V-0 | [68] |
| tri(phosphaphenanthrene-maleimide-phenoxyl)-triazine (DOPO-TMT) | 17.3 | 48 | 556 | 56.5 | 2.43 | 37.5 | V-0 | [68] |
| tri(phosphaphenanthrene-maleimide-phenoxyl)-triazine (DOPO-TMT) | 20.8 | 50 | 674 | 59.6 | 1.98 | 38.4 | V-0 | [68] |
| 47 | 1208 | 80.6 | — | 22.5 | NR | [69] | ||
| hexa(4-maleimido-phenoxyl) cyclotriphosphazene (HMCP) | 3.4 | 39 | 751 | 77 | 1.39 | 27 | NR | [69] |
| hexa(4-maleimido-phenoxyl) cyclotriphosphazene (HMCP) | 6.8 | 38 | 469 | 66.5 | 2.52 | 29 | V-1 | [69] |
| hexa(4-maleimido-phenoxyl) cyclotriphosphazene (HMCP) | 10.2 | 36 | 506 | 63 | 2.33 | 33.4 | V-0 | [69] |
| hexa(4-maleimido-phenoxyl) cyclotriphosphazene (HMCP) | 13.6 | 36 | 467 | 58 | 2.75 | 35 | V-0 | [69] |
| hexa(4-maleimido-phenoxyl) cyclotriphosphazene (HMCP) | 17 | 39 | 351 | 50 | 4.60 | 36.5 | V-0 | [69] |
| 53 | 939.2 | 227.4 | — | 24.2 | NR | [70] | ||
| addition reaction between DOPO and Schiff-base obtained in advance by the condensation of 4,4′-diaminodiphenyl methane & 4-hydroxybenzaldehyde (DOPO-bp) | 3.4 | 48 | 757.1 | 154.1 | 1.65 | 30.5 | V-1 | [70] |
| addition reaction between DOPO and Schiff-base obtained in advance by the condensation of 4,4′-diaminodiphenyl methane & 4-hydroxybenzaldehyde (DOPO-bp) | 6.7 | 47 | 633.9 | 145.2 | 2.05 | 39.7 | V-0 | [70] |
| addition reaction between DOPO and Schiff-base obtained in advance by the condensation of 4,4′-diaminodiphenyl methane & 4-hydroxybenzaldehyde (DOPO-bp) | 13.5 | 39 | 535.1 | 121.9 | 2.40 | 41.6 | V-0 | [70] |
| 63 | 619.9 | 77.6 | — | 21.7 | NR | [71] | ||
| hexa-[4-(phydroxyanilino- phosphaphenanthrene methyl)-phenoxyl]-cyclotriphosphazene (CTP-DOPO) | 10.6 | 52 | 349.9 | 51.7 | 2.19 | 36.6 | V-0 | [71] |
| 63 | 731.2 | 103.2 | — | 20.3 | NR | [72] | ||
| polymelamine tetramethylene phosphonium sulfate (PMTMPS) | 11 | 59 | 489.9 | 80.9 | 1.78 | 32.5 | V-0 | [72] |
| 63 | 731.4 | 103.2 | — | 20.3 | NR | [73] | ||
| poly(urea tetramethylene phosphonium sulfate) (PUTMPS) | 12 | 57 | 525.8 | 79.2 | 1.63 | 31.3 | V-0 | [73] |
| 56 | 1420 | 144 | — | 26.2 | NR | [74] | ||
| aluminum poly-hexamethylenephosphinate (APHP) | 2 | 54 | 742 | 98 | 2.71 | 29.3 | NR | [74] |
| aluminum poly-hexamethylenephosphinate (APHP) | 4 | 58 | 540 | 95 | 4.12 | 32.7 | V-1 | [74] |
| aluminum poly-hexamethylenephosphinate (APHP) | 6 | 55 | 603 | 93 | 3.58 | 33.1 | NR | [74] |
| 56 | 1420 | 116 | — | 26.2 | NR | [75] | ||
| aluminum poly-hexamethylenephosphinate (APHP) | 6 | 55 | 603 | 69 | 3.88 | 33.1 | NR | [75] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide (DOPO) | 6 | 44 | 725 | 70 | 2.55 | 38.5 | V-1 | [75] |
| 101 | 685 | 106 | — | 19 | NR | [76] | ||
| α,ω-dicarboxyl aromatic polyphosphonate (HP-1001-COOH) | 10 | 72 | 454 | 84 | 1.35 | 26.6 | NR | [76] |
| α,ω-dicarboxyl aromatic polyphosphonate (HP-1001-COOH) | 20 | 68 | 393 | 79 | 1.57 | 30.9 | NR | [76] |
| α,ω-dicarboxyl aromatic polyphosphonate (HP-1001-COOH) | 30 | 66 | 324 | 75 | 1.95 | 32.4 | V-0 | [76] |
| α,ω-dicarboxyl aromatic polyphosphonate (HP-1001-COOH) | 40 | 68 | 351 | 74 | 1.88 | 30.3 | V-0 | [76] |
| α,ω-dicarboxyl aromatic polyphosphonate (HP-1001-COOH) | 50 | 76 | 351 | 85 | 1.83 | 27 | V-1 | [76] |
| 56 | 1420 | 140 | — | 26 | NR | [77] | ||
| reaction between triallyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TAD) | 4 | 46 | 1106 | 82 | 1.80 | 33.6 | V-1 | [77] |
| 69 | 966 | 93.9 | — | 22.5 | NR | [78] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 10 | 50 | 463 | 64.8 | 2.19 | 30.6 | V-1 | [78] |
| reaction between triallyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TAD) | 6 | 51 | 691 | 60.8 | 1.59 | 32.4 | NR | [78] |
| reaction between triallyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TAD) | 8 | 56 | 590 | 53.7 | 2.32 | 32.6 | V-1 | [78] |
| reaction between triallyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TAD) | 10 | 54 | 452 | 57.7 | 2.72 | 34.2 | V-1 | [78] |
| reaction between triallyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TAD) | 12 | 55 | 641 | 55.7 | 2.02 | 33.5 | V-0 | [78] |
| 52 | 1334.3 | 58.8 | — | 22.2 | NR | [79] | ||
| piperazine-modified ammonium polyphosphate (PAz-APP) | 10 | 33 | 261.5 | 15.6 | 12.20 | 29 | V-0 | [79] |
| piperazine-modified ammonium polyphosphate (PAz-APP) | 15 | 33 | 246.1 | 11.3 | 17.90 | 31.5 | V-0 | [79] |
| 40 | 980.4 | 55.2 | — | 21.5 | NR | [80] | ||
| diethylenetriamine-modified ammonium polyphosphate (DETA-APP) | 10 | 35 | 388 | 12.7 | 9.60 | 28.5 | V-0 | [80] |
| diethylenetriamine-modified ammonium polyphosphate (DETA-APP) | 15 | 32 | 310.5 | 11.4 | 12.23 | 30.5 | V-0 | [80] |
| 52 | 995 | 93.3 | — | 22.5 | NR | [81] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 8.3 | 57 | 437.2 | 60.6 | 3.84 | 31.7 | V-1 | [81] |
| tri-(phosphaphenanthrene-(hydroxyl-methylene)-phenoxyl)-1, 3, 5-triazine (Trif-DOPO) | 11.7 | 48 | 390.8 | 70.4 | 3.11 | 33.9 | NR | [81] |
| tri-(phosphaphenanthrene-(hydroxyl-methylene)-phenoxyl)-1, 3, 5-triazine (Trif-DOPO) | 14 | 44 | 420.7 | 67.9 | 2.74 | 36 | V-0 | [81] |
| 61 | 1420 | 144 | — | 26.4 | NR | [82] | ||
| addition reaction of 1,3,5-triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & 10-(2,5-dihydroxyphenyl)-10-H-9-oxa-10-phosphaphenanthrene-10-oxide (TOD) | 2 | 61 | 852 | 89 | 2.69 | 32.8 | V-1 | [82] |
| addition reaction of 1,3,5-triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & 10-(2,5-dihydroxyphenyl)-10-H-9-oxa-10-phosphaphenanthrene-10-oxide (TOD) | 4 | 61 | 830 | 77 | 3.19 | 35.9 | V-0 | [82] |
| addition reaction of 1,3,5-triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & 10-(2,5-dihydroxyphenyl)-10-H-9-oxa-10-phosphaphenanthrene-10-oxide (TOD) | 6 | 61 | 720 | 69 | 4.11 | 38 | V-0 | [82] |
| 68 | 1730 | 110 | — | 23 | NR | [83] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-4,4-diaminodiphenyl methane (DOPO-DDM) | 10 | 76 | 1480 | 49 | 2.93 | 29.5 | V-1 | [83] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-4,4-diaminodiphenyl sulfone (DOPO-DDE) | 10 | 78 | 1370 | 56 | 2.84 | 31.5 | V-0 | [83] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-4,40-diaminodiphenyl ether (DOPO-DDS) | 10 | 74 | 1190 | 60 | 2.90 | 31 | V-0 | [83] |
| 61 | 893 | 112 | — | 23 | NR | [84] | ||
| diphenylphosphine containing polyhedral oligomeric silsesquioxanes (DPP-POSS) | 5 | 65 | 489 | 94.1 | 2.31 | 33.2 | V-0 | [84] |
| diphenylphosphine oxide containing polyhedral oligomeric silsesquioxanes (DPOP-POSS) | 5 | 62 | 419 | 87.8 | 2.76 | 29.3 | V-1 | [84] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide containing polyhedral oligomeric silsesquioxanes (DOPO-POSS) | 5 | 64 | 433 | 91.1 | 2.66 | 30 | V-1 | [84] |
| 69 | 961 | 96 | — | 20 | NR | [85] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide modified Aluminum hydroxide (ATH-DOPO) | 10 | 75 | 586 | 64 | 2.67 | 25.6 | NR | [85] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide modified Aluminum hydroxide (ATH-DOPO) | 20 | 87 | 341 | 57 | 5.98 | 27.7 | V-0 | [85] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide modified honeycomb-like mesoporous aluminum hydroxide (pATH-DOPO) | 10 | 75 | 391 | 52 | 4.93 | 27.1 | V-0 | [85] |
| 70 | 1000 | 89 | — | 21.5 | NR | [86] | ||
| bisphenol-S bridged penta(anilino)cyclotriphosphazene (BPS-BPP) | 9 | 62 | 537 | 76 | 1.93 | 29.7 | V-1 | [86] |
| 62 | 688 | 106 | — | 21 | NR | [87] | ||
| 1,3,5-tris(3-(diphenylphosphoryl)propyl)-1,3,5-triazinane-2,4,6-trione (PN) | 15 | 55 | 567 | 82 | 1.39 | 33.5 | V-0 | [87] |
| [(1,1,3,3-tetramethyl-1,3-disiloxanediyl)-di-2,1-ethanediyl]-bis(diphenylphosphine oxide) (PSi) | 25 | 49 | 309 | 74 | 2.52 | 34 | V-0 | [87] |
| 75 | 685 | 95 | — | 20.3 | NR | [88] | ||
| bis(2,6-dimethyphenyl) phenylphosphonate (BDMPP) | 14 | 65 | 528 | 68 | 1.57 | 33.8 | V-0 | [88] |
| 62 | 840 | 84 | — | 23 | V-1 | [89] | ||
| amine-terminated cyclophosphazene (ATCP) | 15 | 66 | 658 | 62 | 1.84 | 35 | V-0 | [89] |
| 57 | 713 | 64 | — | 28 | V-1 | [90] | ||
| amine-terminated cyclophosphazene (ATCP) | 15 | 52 | 610 | 58 | 1.17 | 34 | V-0 | [90] |
| 63 | 1068 | 76 | — | 26 | NR | [91] | ||
| 9, 10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 4.5 | 83 | 724 | 73 | 2.02 | 31.5 | V-1 | [91] |
| reaction between 9, 10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & 2-aminobenzothiazole (DOPO-ABZ) | 7.5 | 71 | 652 | 72 | 1.94 | 33.5 | V-0 | [91] |
| reaction between 9, 10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide & 2-aminobenzothiazole (DOPO-ABZ) | 10 | 66 | 609 | 67 | 2.08 | 33.5 | V-0 | [91] |
| 47 | 1208 | 81 | — | 22.5 | NR | [92] | ||
| reaction between maleimide & phosphaphenanthrene & triazine-trione (DMT) | 3.3 | 39 | 837 | 67 | 1.44 | 31.2 | NR | [92] |
| reaction between maleimide & phosphaphenanthrene & triazine-trione (DMT) | 6.6 | 35 | 685 | 63 | 1.68 | 32.8 | NR | [92] |
| reaction between maleimide & phosphaphenanthrene & triazine-trione (DMT) | 10 | 37 | 544 | 62 | 2.28 | 34.4 | V-1 | [92] |
| reaction between maleimide & phosphaphenanthrene & triazine-trione (DMT) | 13.5 | 36 | 506 | 60 | 2.46 | 35.8 | V-0 | [92] |
| reaction between maleimide & phosphaphenanthrene & triazine-trione (DMT) | 17 | 34 | 491 | 58 | 2.48 | 33 | V-0 | [92] |
| 50 | 860 | 112 | — | 23 | NR | [93] | ||
| Ammonium polyphosphate (APP) | 10 | 59 | 458 | 62 | 4.00 | 25 | NR | [93] |
| Ammonium polyphosphate–montmorillonite (APP-MMT) | 10 | 60 | 393 | 34 | 8.65 | 30 | V-0 | [93] |
| 50 | 860 | 133 | — | 23 | NR | [94] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 6 | 64 | 502 | 79 | 3.69 | 31.2 | V-1 | [94] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-Montmorillonite (DOPO-MMT) | 6 | 59 | 398 | 73 | 4.64 | 33.4 | V-0 | [94] |
| 65 | 966 | 96 | — | 22.5 | NR | [95] | ||
| aluminum poly-hexamethylenephosphinate (APHP) | 10 | 56 | 855 | 90 | 1.03 | 31.5 | NR | [95] |
| bisphenol-A bis(diphenyl phosphate) (BDP) | 10 | 50 | 746 | 86 | 1.11 | 33.4 | NR | [95] |
| 56 | 722.7 | 86.7 | — | 20.5 | NR | [96] | ||
| isopropylphenyl phosphate (FIPF) | 20 | 47 | 363.1 | 61 | 2.37 | 33 | V-0 | [96] |
| tertbutylphenyl phosphate (FTBF) | 20 | 50 | 361.8 | 61.4 | 2.51 | 30.3 | V-0 | [96] |
| 47 | 955 | 59.7 | — | 22.5 | NR | [97] | ||
| phenylphosphonic di-benzothiazolyl amide (PPDAB) | 10 | 65 | 611 | 46.4 | 2.78 | 31 | V-0 | [97] |
| 48 | 1227 | 111 | — | 26.8 | NR | [98] | ||
| boron phosphate (BP) | 5 | 46 | 892 | 91 | 1.60 | 28.3 | V-1 | [98] |
| boron phosphate (BP) | 9 | 47 | 805 | 89 | 1.86 | 29.2 | V-1 | [98] |
| boron phosphate (BP) | 15 | 46 | 602 | 84 | 2.58 | 31.5 | V-1 | [98] |
| 40 | 1163.1 | 90.3 | — | 22 | NR | [99] | ||
| polystyrene encapsulating ammonium polyphosphate (PS-APP) | 2 | 21 | 1092.2 | 86.4 | 0.58 | 23.2 | NR | [99] |
| polystyrene encapsulating ammonium polyphosphate (PS-APP) | 5 | 20 | 959.5 | 92.6 | 0.59 | 25.7 | V-1 | [99] |
| polystyrene encapsulating ammonium polyphosphate (PS-APP) | 10 | 10 | 614.2 | 85.8 | 0.49 | 26.8 | V-1 | [99] |
| polystyrene encapsulating ammonium polyphosphate (PS-APP) | 15 | 8 | 375.4 | 65.7 | 0.85 | 28.5 | V-1 | [99] |
| polystyrene encapsulating ammonium polyphosphate (PS-APP) | 20 | 25 | 733.7 | 81.7 | 1.09 | 28.7 | V-1 | [99] |
| 46 | 892 | 137 | — | 20 | NR | [100] | ||
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO-POSS) | 2.5 | 46 | 963 | 129 | 0.98 | 21.5 | NR | [100] |
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO-POSS) | 5 | 47 | 937 | 128 | 1.04 | 23.5 | NR | [100] |
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO-POSS) | 10 | 46 | 690 | 113 | 1.56 | 25.9 | V-1 | [100] |
| 58 | 839 | 129 | — | 22 | NR | [100] | ||
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO-POSS) | 2.5 | 58 | 631 | 104 | 1.64 | 27.1 | V-1 | [100] |
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO-POSS) | 5 | 62 | 404 | 87 | 3.29 | 26.2 | NR | [100] |
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO-POSS) | 10 | 61 | 346 | 79 | 4.16 | 24.8 | NR | [100] |
| 45 | 855 | 112 | — | 25 | NR | [101] | ||
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO-POSS) | 2.5 | 48 | 969 | 103 | 1.02 | 30.2 | V-1 | [101] |
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO-POSS) | 5 | 58 | 588 | 92 | 2.28 | 28.5 | NR | [101] |
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO-POSS) | 10 | 61 | 483 | 85 | 3.16 | 23 | NR | [101] |
| 45 | 855 | 112 | — | 25 | NR | [102] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 5 | 54 | 731 | 93 | 1.69 | 27.6 | NR | [102] |
| 45 | 855 | 112 | — | 25 | NR | [103] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 6.3 | 54 | 686 | 96 | 1.74 | 30.5 | NR | [103] |
| 45 | 855 | 112 | — | 25 | NR | [104] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 6.3 | 54 | 686 | 96 | 1.74 | 30.5 | NR | [104] |
| 50 | 860 | 112 | — | 23 | NR | [105] | ||
| ammonium polyphosphate montmorillonite nanocomposite (APP-MMT) | 10 | 60 | 393 | 33 | 8.91 | 30 | V-0 | [105] |
| 50 | 860 | 112 | — | 23 | NR | [106] | ||
| 1-oxo-4-hydroxymethyl-2,6,7-trioxa-l-phosphabicyclo[2.2.2] octane (PEPA) | 5.2 | 53 | 538 | 78 | 2.43 | 27 | NR | [106] |
| Ammonium polyphosphate (APP) | 2.9 | 61 | 1087 | 96 | 1.12 | 23.5 | NR | [106] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 6.3 | 55 | 684 | 76 | 2.03 | 32 | NR | [106] |
| 76 | 1160.9 | 135 | — | 22.5 | NR | [107] | ||
| poly(4,4-dihydroxy-1-methyl-ethyl diphenol-o-bicyclic pentaerythritol phosphatephosphate) (PCPBO) | 5 | 65 | 882.8 | 132.1 | 1.14 | 27.3 | NR | [107] |
| poly(4,4-dihydroxy-1-methyl-ethyl diphenol-o-bicyclic pentaerythritol phosphatephosphate) (PCPBO) | 10 | 61 | 460.5 | 122.3 | 2.23 | 28.8 | NR | [107] |
| poly(4,4-dihydroxy-1-methyl-ethyl diphenol-o-bicyclic pentaerythritol phosphatephosphate) (PCPBO) | 15 | 44 | 375.4 | 119.8 | 2.017 | 30.3 | V-1 | [107] |
| poly(4,4-dihydroxy-1-methyl-ethyl diphenol-o-bicyclic pentaerythritol phosphatephosphate) (PCPBO) | 20 | 31 | 337.1 | 117.3 | 1.616 | 31.2 | V-0 | [107] |
| 57 | 1730.27 | 114.16 | — | 21.5 | NR | [108] | ||
| ammonium polyphosphate (APP) | 15 | 63 | 397.89 | 35.49 | 15.46 | 36 | V-0 | [108] |
| glycidyl methacrylate microencapsulated ammonium polyphosphate (GMA-APP) | 15 | 68 | 283.09 | 44 | 18.91 | 38.5 | V-0 | [108] |
| 62 | 1192 | 184 | — | 20.9 | NR | [109] | ||
| ammonium polyphosphate(APP) | 12 | 41 | 200 | 104 | 6.97 | 31 | V-0 | [109] |
| modified ammonium polyphosphate(MAPP) | 12 | 47 | 184 | 98 | 9.22 | 32.5 | V-0 | [109] |
| 62 | 1192 | 184 | — | 20.9 | NR | [110] | ||
| ammonium polyphosphate(APP) | 12 | 41 | 200 | 104 | 6.97 | 31.9 | V-0 | [110] |
| 66 | 893 | 68 | — | 22.5 | NR | [111] | ||
| hexa-(phosphaphenanthrene -hydroxyl-methyl-phenoxyl)-cyclotriphosphazene(HAP-DOPO) | 9.3 | 51 | 383 | 53 | 2.31 | 31 | V-0 | [111] |
| hexa-(phosphaphenanthrene -hydroxyl-methyl-phenoxyl)-cyclotriphosphazene(HAP-DOPO) | 15.47 | 43 | 303 | 41 | 3.18 | 30.8 | V-0 | [111] |
| 65 | 966 | 102 | — | 22.5 | NR | [112] | ||
| ring-opening addition reaction between 1,3,5-triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TGIC-DOPO) | 6.1 | 54 | 800 | 75 | 1.36 | 33.3 | NR | [112] |
| ring-opening addition reaction between 1,3,5-triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TGIC-DOPO) | 8.1 | 54 | 680 | 76 | 1.58 | 34.3 | V-1 | [112] |
| ring-opening addition reaction between 1,3,5-triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TGIC-DOPO) | 10.2 | 50 | 520 | 71 | 2.05 | 35.2 | V-1 | [112] |
| ring-opening addition reaction between 1,3,5-triglycidyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TGIC-DOPO) | 12.2 | 48 | 481 | 61 | 2.47 | 33.3 | V-0 | [112] |
| 35 | 1719 | 74.2 | — | 25 | HB | [113] | ||
| 9,10-dihydro-9-oxy-10-phosphaphenanthrene-10-oxide units linked to the star-shaped aliphatic ground body tetra-[(acryloyloxy)ethyl] pentarythrit (DOPP) | 19.6 | 40 | 1191 | 44.8 | 2.73 | 37.9 | V-1 | [113] |
| 9,10-dihydro-9-oxy-10-phosphaphenanthrene-10-oxide units linked to the star-shaped aliphatic ground body heterocyclic tris-[(acryloyloxy)ethyl] isocyanurate (DOPI) | 23.1 | 36 | 869 | 41.5 | 3.63 | 34.2 | V-0 | [113] |
| 49 | 781 | 76 | — | 20.5 | NR | [114] | ||
| poly(melamine-ethoxyphosphinyl-diisocyanate) (PMPC) | 10 | 59 | 390 | 33 | 5.55 | 26 | NR | [114] |
| poly(melamine-ethoxyphosphinyl-diisocyanate) (PMPC) | 15 | 64 | 292 | 30 | 8.85 | 27.5 | V-1 | [114] |
| poly(melamine-ethoxyphosphinyl-diisocyanate) (PMPC) | 20 | 59 | 235 | 27 | 11.26 | 28 | V-0 | [114] |
| 64 | 821 | 94 | — | 23.2 | NR | [115] | ||
| 9, 10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) | 5 | 54 | 461 | 70 | 2.01 | 33.7 | V-1 | [115] |
| 60 | 920 | 90.5 | — | 22.7 | NR | [116] | ||
| ((1,1,3,3-tetramethyldisiloxane-1,3-diyl)bis(propane-3,1-diyl))bis(2-methoxy-4,1-phenylene)bis(phenylphosphonochloridate) modified Magnesium-Aluminum layered double hydroxide (SIEPDP-Mg-Al LDH) | 4 | 55 | 658 | 86.9 | 1.33 | 25.3 | V-1 | [116] |
| 64 | 939 | 179 | — | 19.6 | NR | [117] | ||
| ammonium polyphosphate (APP) | 5 | 61 | 283 | 111 | 5.09 | 27.1 | V-0 | [117] |
| 53 | 1262 | 84.7 | — | 25 | NR | [118] | ||
| cardanol derived benzoxazine monomer (CBz) | 10 | 49 | 1119 | 80.5 | 1.09 | 31 | V-1 | [118] |
| cardanol derived benzoxazine monomer (CBz) | 15 | 50 | 920 | 79.4 | 1.38 | 32 | V-0 | [118] |
| cardanol derived benzoxazine monomer (CBz) | 20 | 50 | 962 | 77.2 | 1.35 | 33 | V-0 | [118] |
| 59 | 1063 | 76.1 | — | 25.8 | NR | [119] | ||
| poly (piperazine phosphaphenanthrene) (DOPMPA) | 10 | 68 | 393 | 56.3 | 4.21 | 29 | NR | [119] |
| poly (piperazine phosphaphenanthrene) (DOPMPA) | 13 | 67 | 285 | 27.4 | 11.76 | 34 | V-0 | [119] |
| 27 | 673.7 | 56 | — | 22.3 | NR | [9] | ||
| reaction of spirocyclic pentaerythritol bisphosphorate disphosphoryl chloride & 2,4-dihydroxybenzophenone (MFR) | 10 | 26 | 402.3 | 53.3 | 1.69 | 29.6 | V-1 | [9] |
| reaction of spirocyclic pentaerythritol bisphosphorate disphosphoryl chloride & 2,4-dihydroxybenzophenone (MFR) | 15 | 17 | 479.7 | 47.8 | 1.03 | 30.8 | V-0 | [9] |
| reaction of spirocyclic pentaerythritol bisphosphorate disphosphoryl chloride & 2,4-dihydroxybenzophenone (MFR) | 20 | 22 | 241.6 | 42.3 | 3.00 | 32.2 | V-0 | [9] |
| 58 | 1369 | 135.6 | — | 23.5 | NR | [17] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-covalent organic frameworksnanosheets(reaction between melamine & o-phthalaldehyde) (DOPO-COFs) | 0.4 | 70.2 | 1295 | 133.4 | 1.30 | 23.5 | NR | [17] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-covalent organic frameworksnanosheets(reaction between melamine & o-phthalaldehyde) (DOPO-COFs) | 0.8 | 64 | 1086 | 125.3 | 1.50 | 24 | NR | [17] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-covalent organic frameworksnanosheets(reaction between melamine & o-phthalaldehyde) (DOPO-COFs) | 1.6 | 58.6 | 1227 | 131.5 | 1.16 | 24.5 | NR | [17] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-covalent organic frameworksnanosheets(reaction between melamine & o-phthalaldehyde) (DOPO-COFs) | 3.2 | 60.7 | 1117 | 110.5 | 1.57 | 25 | NR | [17] |
| COFs: covalent organic frameworksnanosheets(reaction between melamine & o-phthalaldehyde) (COFs) | 3.2 | 55 | 1295 | 140.4 | 0.96 | 24 | NR | [17] |
| 21 | 1910 | 84.4 | — | 22.1 | NR | [120] | ||
| melamine coated ammonium polyphosphate (Mel-APP) | 20 | 22 | 312.6 | 30.8 | 17.54 | 32.6 | V-0 | [120] |
| 0 | 51 | 1914 | 81.9 | — | 22 | NR | [121] | |
| phosphorus and nitrogen-containing flame retardant (FR) | 1 | 43 | 1631 | 69.6 | 1.16 | 22.5 | NR | [121] |
| 0 | 50 | 1712 | 83.7 | — | — | NR | [122] | |
| poly(4,4′-diamino diphenyl sulfone phenyl phosphonamide) (ArPN2) | 15 | 29 | 847 | 61.5 | 1.59 | — | V-0 | [122] |
| poly(bisphenol sulfone phenyl phosphonate) (ArPO2) | 15 | 32 | 608 | 42.7 | 3.53 | — | V-1 | [122] |
| poly(4,4′-dia-minodiphenyl sulfone phenyl dichlorophosphate) (ArOPN2) | 15.6 | 30 | 546 | 59.4 | 2.65 | — | NR | [122] |
| poly(bisphenol sulfone phenoxy phosphate) (ArOPO2) | 15.6 | 30 | 726 | 55.3 | 2.14 | — | NR | [122] |
| 0 | 75 | 977 | 100 | — | — | NR | [123] | |
| ionic liquid-based metal–organic hybrid = Phosphomolybdic acid hydrate:PMA & 1-ethyl 3-(diethoxyphosphoryl)-propylimidazolium bromide:IL (PMAIL) | 6 | 85 | 674.4 | 99 | 1.65 | — | V-0 | [123] |
| epoxy novolac resin | 0 | 51 | 682 | 110 | — | — | NR | [124] |
| oligo[DOPAc-2-tris(acryloyloxy)ethyl isocyanurate] (oDOPI) | 13.81 | 52 | 426 | 86 | 2.08 | — | V-0 | [124] |
| Phosphazene (PZ) | 10.8 | 50 | 466 | 80 | 1.97 | — | V-0 | [124] |
| melamine polyphosphate(MPP) | 15 | 45 | 370 | 86 | 2.08 | — | V-1 | [124] |
| 0 | 50 | 985.7 | 91 | — | — | NR | [125] | |
| aluminum hypophosphite (AHP) | 5 | 48 | 970.2 | 89 | 0.99 | — | V-1 | [125] |
| 23 | 1910 | 61 | — | — | NR | [126] | ||
| Melamine coated ammonium polyphosphate (Mel-APP) | 29.7 | 24 | 281 | 23 | 18.81 | — | V-0 | [126] |
| 54 | 1068 | 75.8 | — | — | HB | [127] | ||
| Melamine poly(aluminum phosphate) (MPAlP) | 20 | 40 | 540 | 60 | 1.85 | — | HB | [127] |
| melamine poly(zinc phosphate) (MPZnP) | 20 | 43 | 312 | 60 | 3.44 | — | HB | [127] |
| melamine poly(magnesium phosphate) (MPMgP) | 20 | 44 | 298 | 57.3 | 3.86 | — | V-1 | [127] |
| melamine polyphosphate (MPP) | 20 | 38 | 244 | 26.6 | 8.77 | — | V-0 | [127] |
| diethyl aluminum phosphinate (AlPi-Et) | 20 | 41 | 492 | 55.8 | 2.23 | — | V-0 | [127] |
| 6H-dibenz[c,e][1,2] oxaphosphorin-6-propanoic acid, butyl ester, 6-oxide (DOPAc-Bu) | 20 | 44 | 624 | 50.2 | 2.10 | — | HB | [127] |
| 53 | 1084 | 115 | — | — | NR | [128] | ||
| hexaphenoxycyclotriphosphazene (HPCTP) | 5 | 58 | 807 | 96 | 1.76 | — | V-0 | [128] |
| hexaphenoxycyclotriphosphazene (HPCTP) | 10 | 60 | 566 | 93 | 2.68 | — | V-0 | [128] |
| hexaphenoxycyclotriphosphazene (HPCTP) | 15 | 51 | 513 | 82 | 2.85 | — | V-0 | [128] |
| 63 | 1321 | 157 | — | — | NR | [129] | ||
| Hexaphenoxycyclotriphosphazene (HPCTP) | 15 | 54 | 513 | 82 | 4.22 | — | V-0 | [129] |
| 100 | 733 | 141 | — | 21 | HB | [130] | ||
| Tetraphenylphosphonium modified montmorillonite (TPP-MMT) | 5 | 110 | 482 | 140 | 1.68 | 25 | HB | [130] |
| 47 | 891 | 151 | — | 21 | HB | [130] | ||
| Tetraphenylphosphonium modified montmorillonite (TPP-MMT) | 5 | 53 | 571 | 138 | 1.92 | 25 | HB | [130] |
| 22 | 1196 | 147 | — | 21 | HB | [130] | ||
| Tetraphenylphosphonium modified montmorillonite (TPP-MMT) | 5 | 25 | 694 | 140 | 2.05 | 25 | HB | [130] |
| 49 | 904 | 95 | — | 21 | NR | [131] | ||
| hyperbranched poly(phosphoester) (hbPPE) | 10 | 49 | 506 | 62 | 2.73 | 23.6 | HB | [131] |
| hyperbranched poly(phosphoester) (hbPPE) | 20 | 49 | 699 | 53 | 2.31 | 25.9 | HB | [131] |
| 0 | 58 | 1126.3 | 100.36 | — | 26.1 | — | [132] | |
| poly(cyclotriphosphazeneco-4,4′-sulfonyldiphenol) (PZS) | 3 | 61 | 986.5 | 91.89 | 1.31 | 28.6 | — | [132] |
| hybrid poly(cyclotriphosphazeneco-4,4′-sulfonyldiphenol)-strontium hydroxystannate nanorod (PZS@SrSn(OH)6) | 3 | 60 | 801.2 | 88.96 | 1.64 | 29.5 | — | [132] |
| 0 | 36.6 | 970.9 | 59.1 | — | 19.8 | — | [133] | |
| 1-oxo-4-hydroxymethyl-2,6,7-trioxa-l-phosphabicyclo [2.2.2] octane modified trimellitic anhydride chloride (PEPA-TMAC) | 16.5 | 30.1 | 523.7 | 42 | 2.14 | 23.4 | — | [133] |
| 1-oxo-4-hydroxymethyl-2,6,7-trioxa-l-phosphabicyclo [2.2.2] octane modified trimellitic anhydride chloride (PEPA-TMAC) | 33 | 33.9 | 337.2 | 36.9 | 4.27 | 26.9 | — | [133] |
| 50 | 986 | 91.1 | — | 25.9 | — | [134] | ||
| poly(cyclotriphosphazene-c-sulfonyldiphenol) (PCPS) | 1 | 49 | 979 | 92.1 | 0.97 | 27 | — | [134] |
| poly(cyclotriphosphazene-c-sulfonyldiphenol) (PCPS) | 3 | 44 | 500 | 85.8 | 1.84 | 29.8 | — | [134] |
| poly(cyclotriphosphazene-c-sulfonyldiphenol) (PCPS) | 5 | 43 | 542 | 78.7 | 1.81 | 30.5 | — | [134] |
| 60 | 1146 | 56 | — | 26.5 | — | [135] | ||
| Boron phosphate: reaction between boric acid & phosphoric acid by calcining at 300 ˚C (BP1) | 5 | 53 | 652 | 31 | 2.80 | 29.6 | — | [135] |
| Boron phosphate: reaction between boric acid & phosphoric acid by calcining at 400 ˚C (BP2) | 5 | 53 | 654 | 34 | 2.54 | 29.7 | — | [135] |
| Boron phosphate: reaction between boric acid & phosphoric acid by calcining at 500 ˚C (BP3) | 5 | 54 | 681 | 33 | 2.57 | 29.6 | — | [135] |
| Boron phosphate: reaction between boric acid & phosphoric acid by calcining at 600 ˚C (BP4) | 5 | 56 | 710 | 38 | 2.22 | 29.3 | — | [135] |
| Boron phosphate: reaction between boric acid & phosphoric acid by calcining at 700 ˚C (BP5) | 5 | 56 | 754 | 38 | 2.09 | 29 | — | [135] |
| 86 | 1650 | 213 | — | 20.2 | — | [136] | ||
| 3-((Methoxydiphenylsilyl) oxy)-9-methyl-2, 4, 8, 10-tetraoxa-3, 9-diphosphaspiro [5. 5] undecane 3, 9-dioxide (SDPS) | 10.4 | 62 | 1378 | 203 | 0.90 | 28.9 | — | [136] |
| 48 | 1023 | 109 | — | 22.2 | — | [137] | ||
| dibenzylphosphinic acid modified aluminum hydroxide (AOPH-NR) | 4.25 | 79 | 789 | 101 | 2.30 | 28 | — | [137] |
| diallylphosphinic acid modified aluminum hydroxide (AOPH-C1) | 4.25 | 80 | 1092 | 107 | 1.59 | 23.4 | — | [137] |
| bis(3-methoxy-3-oxopropyl)phosphinic acid modified aluminum hydroxide (AOPH-C2) | 4.25 | 58 | 1063 | 99 | 1.28 | 23.6 | — | [137] |
| bis(2-cyanoethyl)phosphinic acid modified aluminum hydroxide (AOPH-C3) | 4.25 | 78 | 1024 | 106 | 1.66 | 23.8 | — | [137] |
| epoxy acrylate | 41 | 889 | 28.3 | — | 21 | — | [138] | |
| N,N-bis(2-hydroxyethyl acrylate) aminomethyl phosphonic acid diethylester (BHAAPE) | 5 | 35 | 719 | 25.3 | 1.18 | 28 | — | [138] |
| N,N-bis(2-hydroxyethyl acrylate) aminomethyl phosphonic acid diethylester (BHAAPE) | 10 | 25 | 590 | 23.7 | 1.09 | 30 | — | [138] |
| N,N-bis(2-hydroxyethyl acrylate) aminomethyl phosphonic acid diethylester (BHAAPE) | 20 | 19 | 508 | 22.3 | 1.02 | 31 | — | [138] |
| 0 | 25 | 1113 | 222.9 | — | — | — | [139] | |
| ammonium polyphosphate (APP) | 10 | 35 | 685.9 | 127.4 | 3.97 | — | — | [139] |
| 0 | 60 | 2187 | 124 | — | — | — | [140] | |
| poly (cyclotriphosphazene-co-4,4′-sulfonyldiphenol) (PZS) | 2 | 57 | 1871 | 101 | 1.36 | — | — | [140] |
| poly (cyclotriphosphazene-co-4,4′-sulfonyldiphenol)@molybdenum disulfide nanoflower (PZS@MoS2) | 2 | 52 | 1335 | 91 | 1.93 | — | — | [140] |
| poly (cyclotriphosphazene-co-4,4′-sulfonyldiphenol)@molybdenum disulfide nanoflower (PZS@MoS2) | 3 | 56 | 1251 | 85 | 2.38 | — | — | [140] |
| 0 | 19 | 980 | 81 | — | — | — | [141] | |
| N,N′-dibutyl-phosphate diamide assembled into the cavity of β-cyclodextrin (DBPDA-βCD) | 3 | 19 | 756 | 75 | 1.40 | — | — | [141] |
| 0 | 78 | 2116 | 167.1 | — | — | — | [142] | |
| Polyphosphazene functionalized black phosphorus nanosheets (BP-PZN) | 0.5 | 78 | 1613.7 | 119.8 | 1.82 | — | — | [142] |
| Polyphosphazene functionalized black phosphorus nanosheets (BP-PZN) | 1 | 85 | 1082.1 | 73.5 | 4.84 | — | — | [142] |
| Polyphosphazene functionalized black phosphorus nanosheets (BP-PZN) | 2 | 81 | 859.5 | 60.8 | 7.02 | — | — | [142] |
| black phosphorus bulk nanosheets (BP-Bulk) | 2 | 87 | 1082.3 | 94.3 | 3.86 | — | — | [142] |
| 63 | 1396.9 | 81.3 | — | — | — | [143] | ||
| ene-terminated hyperbranched polyphosphate acrylate (HPPA) | 2 | 57 | 1096.9 | 75.4 | 1.24 | — | — | [143] |
| ene-terminated hyperbranched polyphosphate acrylate-thiol-functionalized mesoporous silica (HPPA-SH-mSiO2) | 2 | 62 | 995.3 | 68.3 | 1.64 | — | — | [143] |
| 76 | 850 | 88 | — | — | — | [144] | ||
| phosphorous metal-organic framework (P-MOF) | 0.5 | 75 | 766 | 84 | 1.14 | — | — | [144] |
| phosphorous metal-organic framework (P-MOF) | 1 | 79 | 728 | 71 | 1.50 | — | — | [144] |
| phosphorous metal-organic framework (P-MOF) | 2 | 70 | 615 | 69 | 1.62 | — | — | [144] |
| 53 | 1484 | 86.3 | — | — | — | [145] | ||
| cardanol-derived zirconium phosphate (CZrP) | 2 | 56 | 1122 | 76.1 | 1.58 | — | — | [145] |
| cardanol-derived zirconium phosphate (CZrP) | 4 | 50 | 970 | 73.2 | 1.70 | — | — | [145] |
| cardanol-derived zirconium phosphate (CZrP) | 6 | 54 | 858 | 67.8 | 2.24 | — | — | [145] |
| zirconium phosphate (ZrP) | 6 | 51 | 1248 | 85.5 | 1.15 | — | — | [145] |
| 24 | 1002.4 | 104.1 | — | — | — | [146] | ||
| Dimethyl methylphosphonate loaded halloysite nanotube (DMMP-HNT) | 20 | 24 | 578.1 | 73.8 | 2.44 | — | — | [146] |
| 54 | 1068 | 76 | — | 21 | — | [147] | ||
| melamine poly(magnesium phosphate) (S600) | 20 | 44 | 298 | 57 | 3.89 | — | — | [147] |
| aluminium diethylphosphinate (AlPi) | 20 | 41 | 492 | 56 | 2.23 | — | — | [147] |
| melamine polyphosphate (MPP) | 20 | 38 | 244 | 26 | 9.00 | — | — | [147] |
| 74 | 1915.3 | 107.6 | — | — | — | [148] | ||
| poly-(cyclotriphos pazene-co-4,40-diaminodiphenyl ether) surface modified silica nanospheres (SiO2@PZM) | 1 | 80 | 1363.4 | 86.8 | 1.88 | — | — | [148] |
| poly-(cyclotriphos pazene-co-4,40-diaminodiphenyl ether) surface modified silica nanospheres-cuprous (SiO2@PZM@Cu) | 1 | 74 | 1289.3 | 78 | 2.04 | — | — | [148] |
| poly-(cyclotriphos pazene-co-4,40-diaminodiphenyl ether) surface modified silica nanospheres-cuprous (SiO2@PZM@Cu) | 2 | 80 | 1188.8 | 73.9 | 2.53 | — | — | [148] |
| 82 | 1820.7 | 99.3 | — | — | — | [149] | ||
| functionalized polyphosphazene nanotubes wrapped with a cross-linked DOPO-based flame retardant (FR@PZS) | 0.5 | 82 | 1584.2 | 87 | 1.31 | — | — | [149] |
| functionalized polyphosphazene nanotubes wrapped with a cross-linked DOPO-based flame retardant (FR@PZS) | 1 | 82 | 1298.2 | 80.8 | 1.72 | — | — | [149] |
| functionalized polyphosphazene nanotubes wrapped with a cross-linked DOPO-based flame retardant (FR@PZS) | 3 | 82 | 982.6 | 72.4 | 2.54 | — | — | [149] |
| polyphosphazene nanotube (PZS) | 3 | 82 | 1152.5 | 83.9 | 1.86 | — | — | [149] |
| 38 | 943 | 60.3 | — | — | — | [150] | ||
| ammonium polyphosphate (APP) | 5 | 36 | 543 | 58.8 | 1.68 | — | — | [150] |
| 45 | 855 | 118 | — | — | — | [151] | ||
| polyhedral oligomeric silsesquioxane containing 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO-POSS) | 20 | 57 | 431 | 91 | 3.25 | — | — | [151] |
| Epoxy acrylic | 32 | 223.4 | 30.8 | — | — | — | [152] | |
| ammonium polyphosphate (APP) | 30 | 35 | 225.2 | 30.7 | 1.08 | — | — | [152] |
| Co-microencapsulated ammonium polyphosphate and pentaerythritol (M(APP & PER)) | 30 | 58 | 233.2 | 27.3 | 1.95 | — | — | [152] |
| 29 | 2467 | 164 | — | — | — | [153] | ||
| Triphenylphosphite (TPPi) | 15 | 21 | 504 | 114 | 5.09 | — | — | [153] |
| Triphenylphosphate (TPPa) | 15 | 12 | 1959 | 128 | 0.66 | — | — | [153] |
| triphenylphosphine oxide (TPPO) | 15 | 34 | 1310 | 126 | 2.87 | — | — | [153] |
| — | 32 | 2572 | 184 | — | — | — | [154] | |
| poly(m-phenylene methyl 1phosphonate) (PMP) | 11.4 | 12 | 724 | 102 | 2.40 | — | — | [154] |
| 9,10-dihydro-9-oxa-10phosphaphenanthrene-10-oxide (DOPO) | 13.9 | 7 | 1286 | 100 | 0.80 | — | — | [154] |
| red phosphorus (RP) | 4.3 | 7 | 1614 | 156 | 0.41 | — | — | [154] |
| aluminum diethylphosphinate (OP) | 8.3 | 7 | 1480 | 146 | 0.47 | — | — | [154] |
| 33 | 910 | 97.54 | — | — | — | [155] | ||
| IFR: reaction between phosphorus acid & melamine & pentaerythritol with the molar ratio of 1:1:2.12 (IFR) | 30 | 38 | 357 | 80.35 | 3.56 | — | — | [155] |
| IFR: reaction between phosphorus acid & melamine & pentaerythritol with the molar ratio of 1:1:2.12 (IFR) | 30 | 65 | 350 | 82.28 | 6.07 | — | — | [155] |
| IFR: reaction between phosphorus acid & melamine & pentaerythritol with the molar ratio of 1:1:2.12 (IFR) | 30 | 71 | 263 | 73.25 | 9.91 | — | — | [155] |
| 67 | 979.7 | 128 | — | — | — | [156] | ||
| Butyl phosphate ester (EPE) | 33.3 | 35 | 203.3 | 87 | 3.70 | — | — | [156] |
| Ethylphosphonate ester (EPE) | 33.3 | 76 | 304.8 | 80 | 5.83 | — | — | [156] |
| Butanediol and butanol mixed phosphate ester (BBPE) | 33.3 | 76 | 300.4 | 83 | 5.70 | — | — | [156] |
| Butanediol and octanol mixed phosphate ester (BOPE) | 33.3 | 79 | 296.9 | 91 | 5.47 | — | — | [156] |
| Hexanediol and butanol mixed phosphate ester (HBPE) | 33.3 | 82 | 283.1 | 88 | 6.16 | — | — | [156] |
| 32 | 910 | 98 | — | — | — | [157] | ||
| IFR: reaction between phosphorus acid & melamine & pentaerythritol with the molar ratio of 1:1:2 (IFR) | 30 | 61 | 341 | 68 | 7.33 | — | — | [157] |
| IFR: reaction between phosphorus acid & melamine & pentaerythritol with the molar ratio of 1:1:2 (IFR) | 30 | 41 | 248 | 73 | 6.31 | — | — | [157] |
| IFR: reaction between phosphorus acid & melamine & pentaerythritol with the molar ratio of 1:1:2 (IFR) | 30 | 41 | 268 | 68 | 6.26 | — | — | [157] |
| IFR: reaction between phosphorus acid & melamine & pentaerythritol with the molar ratio of 1:1:2 (IFR) | 30 | 45 | 237 | 71 | 7.45 | — | — | [157] |
| 94 | 1097.2 | 119 | — | — | — | [158] | ||
| phosphorus oxychloride & pentaerythritol (POCl3 & PER) modified expandable graphite (EGM) | 5 | 76 | 276.2 | 136 | 2.81 | — | — | [158] |
| phosphorus oxychloride & pentaerythritol (POCl3 & PER) modified expandable graphite (EGM) | 15 | 45 | 184.1 | 88 | 3.85 | — | — | [158] |
| 54 | 1327 | 99.1 | — | — | — | [159] | ||
| Phosphorylated chitosan modified montmorillonite intercalation iron compounds (PCTS-Fe-OMMT) | 1 | 51 | 1071 | 88.3 | 1.31 | — | — | [159] |
| Phosphorylated chitosan modified montmorillonite intercalation iron compounds (PCTS-Fe-OMMT) | 3 | 48 | 917 | 86.8 | 1.46 | — | — | [159] |
| Phosphorylated chitosan modified montmorillonite intercalation iron compounds (PCTS-Fe-OMMT) | 5 | 44 | 794 | 82.2 | 1.64 | — | — | [159] |
| 41 | 1222 | 159 | — | — | — | [160] | ||
| ammonium polyphosphate (APP) | 20 | 49 | 879 | 105 | 2.51 | — | — | [160] |
| ammonium polyphosphate (APP) | 40 | 56 | 225 | 55 | 21.44 | — | — | [160] |
| 0 | 47 | 1630 | 82.3 | — | — | — | [161] | |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-phosphonamidate functionalized reduced graphene oxide(DOPOph-RGNO) | 1 | 49 | 1268 | 62.3 | 1.77 | — | — | [161] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-phosphonamidate functionalized reduced graphene oxide(DOPOph-RGNO) | 2 | 43 | 1248 | 55 | 1.78 | — | — | [161] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-phosphonamidate functionalized reduced graphene oxide(DOPOph-RGNO) | 3 | 45 | 1117 | 54 | 2.12 | — | — | [161] |
| 0 | 21 | 453.5 | 36.2 | — | 22.1 | NR | [120] | |
| melamine coated ammonium polyphosphate (Mel-APP) a | 9.59 | 20 | 290.4 | 32.2 | 1.67 | 32 | V-1 | [120] |
| 0 | 53 | 387 | 24.3 | — | 31 | NR | [24] | |
| N, N′-diallyl-p-phenylphosphonicdiamide (FP1) b | 2.6 | 49 | 423 | 20.4 | 1.00 | 43 | NR | [24] |
| 0 | 54 | 508.3 | 47.8 | — | 31 | NR | [162] | |
| polyelectrolyte complexes consisting of chitosan & ammonium polyphosphate (PEC) c | 5.2 | 51 | 358 | 44 | 1.45 | 36 | NR | [162] |
| polyelectrolyte complexes consisting of chitosan & ammonium polyphosphate (PEC) c | 6.9 | 50 | 307.5 | 39.6 | 1.84 | 38.5 | V-1 | [162] |
| polyelectrolyte complexes consisting of chitosan & ammonium polyphosphate (PEC) c | 8.1 | 49 | 255.9 | 35.5 | 2.42 | 40.5 | V-0 | [162] |
| 51 | 347 | 26.2 | — | 33.2 | HB | [113] | ||
| 9,10-dihydro-9-oxy-10-phosphaphenanthrene-10-oxide units linked to the star-shaped aliphatic ground body tetra-[(acryloyloxy)ethyl] pentarythrit (DOPP) d | 5.9 | 56 | 248 | 19.9 | 2.02 | 45.3 | V-0 | [113] |
| 9,10-dihydro-9-oxy-10-phosphaphenanthrene-10-oxide units linked to the star-shaped aliphatic ground body heterocyclic tris-[(acryloyloxy)ethyl] isocyanurate (DOPI) d | 6.9 | 60 | 247 | 20 | 2.16 | 47.7 | V-0 | [113] |
| 24 | 451 | 37 | — | — | NR | [126] | ||
| Melamine coated ammonium polyphosphate (Mel-APP) e | 14.6 | 22 | 233 | 11 | 5.96 | — | V-1 | [126] |
| — | 42 | 385 | 21.8 | — | 27.5 | — | [163,164] | |
| IFR: contains melamine phosphate (IFR) f | 4.7 | 35 | 278 | 18.3 | 1.37 | 35.2 | — | [163,164] |
| 28 | 349 | 20.4 | — | — | — | [150] | ||
| ammonium polyphosphate (APP) g | 5 | 24 | 345 | 18.6 | 0.95 | — | — | [150] |
| 21.2 | 720.5 | 68 | — | — | — | [165] | ||
| ammonium polyphosphate (APP) h | 3.15 | 20.3 | 375.3 | 42 | 2.97 | — | — | [165] |
| ammonium polyphosphate (APP) h | 8.88 | 18.1 | 293.8 | 33 | 4.31 | — | — | [165] |
| ammonium polyphosphate (APP) h | 16.32 | 21 | 186.7 | 27 | 9.62 | — | — | [165] |
| 44 | 853 | 51.9 | — | — | — | [166] | ||
| melamine phosphate (MP) i | 5 | 38 | 528 | 48.8 | 1.48 | — | — | [166] |
| 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) i | 5 | 34 | 624 | 41.3 | 1.32 | — | — | [166] |
| 0 | 39 | 456 | 38 | — | — | — | [167] | |
| IFR: contains melamine phosphate (IFR) j | 5 | 35 | 374 | 28.8 | 1.44 | — | — | [167] |
| IFR: contains melamine phosphate (IFR) j | 10 | 50 | 226 | 17.3 | 5.68 | — | — | [167] |
| IFR: contains melamine phosphate (IFR) j | 15 | 94 | 253 | 18.6 | 8.87 | — | — | [167] |
| 55 | 754 | 61.3 | — | — | — | [168] | ||
| ammonium polyphosphate (APP) k | 15 | 46 | 259 | 34.4 | 4.33 | — | — | [168] |
| 39 | 642 | 64.2 | — | — | — | [168] | ||
| ammonium polyphosphate (APP) l | 15 | 44 | 232 | 40.1 | 4.99 | — | — | [168] |
a Matrix: eight layers of Woven E-glass fabric reinforced epoxy; b Matrix: six layers of dry carbon fiber fabric reinforced RTM6 epoxy; c Matrix: Unidirectional carbon fiber reinforced epoxy resin; d Matrix: Carbon fibers reinforced epoxy; e Matrix: eight layers of Woven E-glass fabric reinforced epoxy; f Matrix: eight layers of woven E-glass reinforced film of multifunctional epoxy resin; g Matrix: carbon fiber reinforced epoxy resin; h Matrix: four fabric layers of unidirectional hemp fabric reinforced epoxy; I Matrix: eight layers of woven roving glass fabric reinforced epoxy phenol novolak resin blend; j Matrix: eight layers of woven E-glass reinforced epoxy; k Matrix: six layers of plain weave hemp fabric-reinforced epoxy; l Matrix: six layers of plain weave Hemp fabrics treated with water glass-reinforced epoxy.
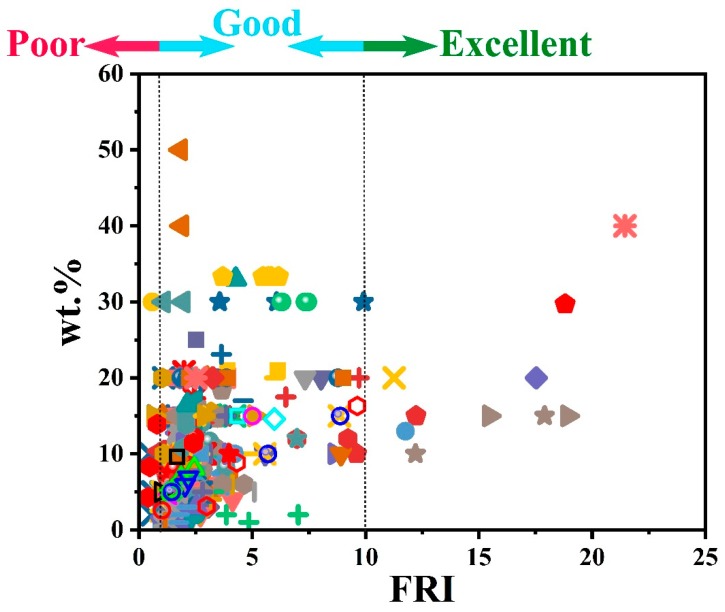
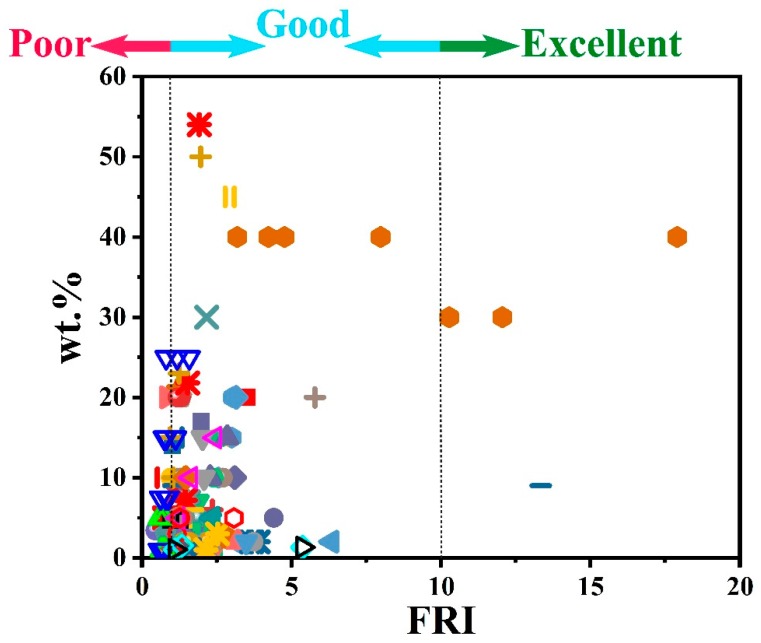
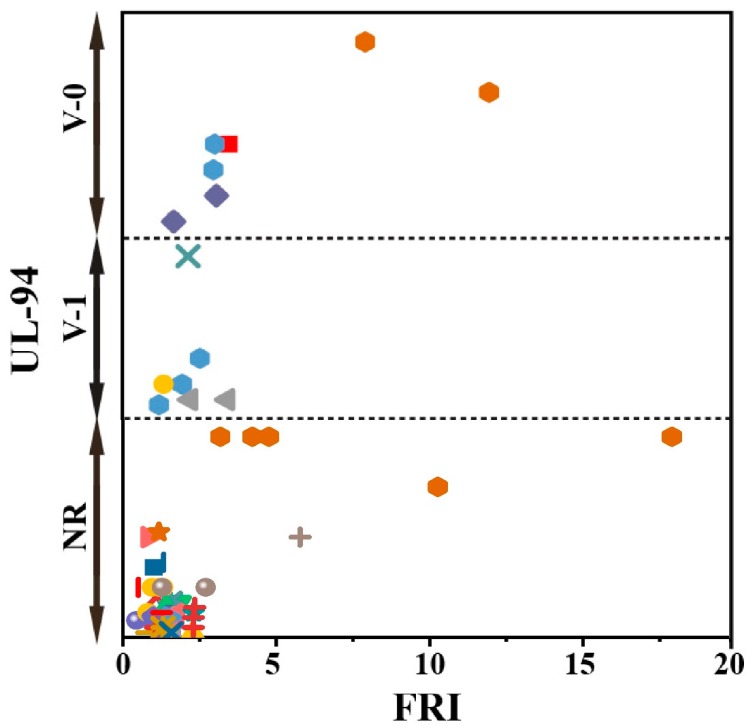
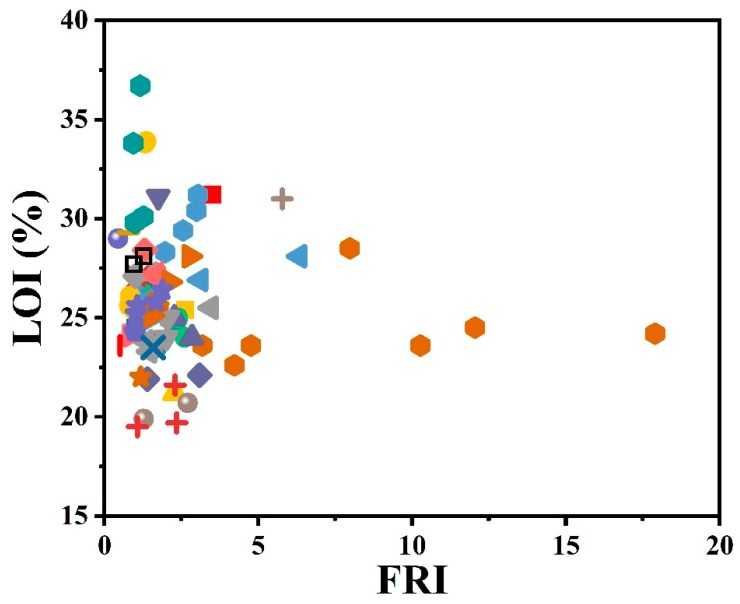
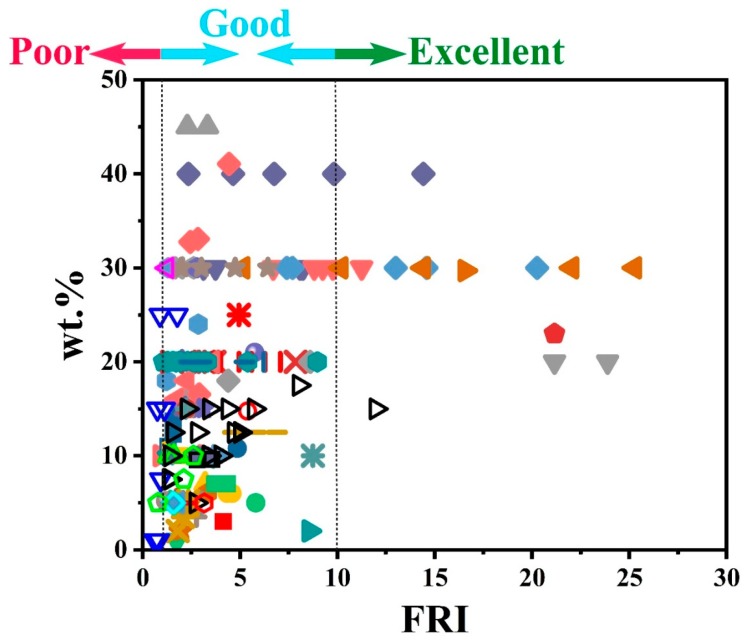
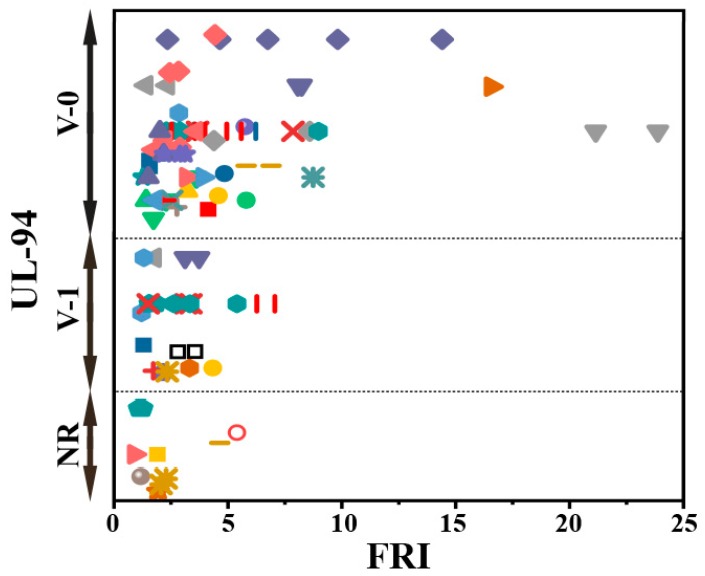
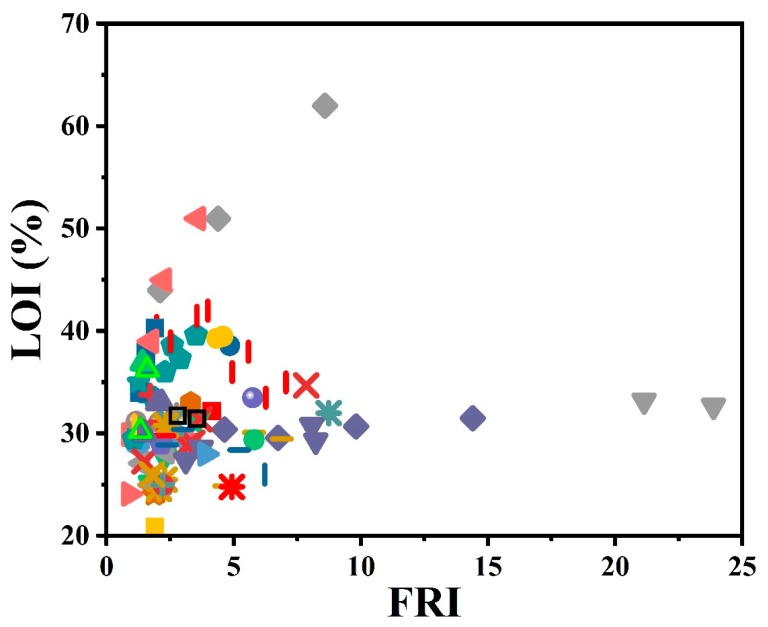
A brief yet informative view of the effect of the used P family of FRs on the flame retardancy performance of epoxy resins is given in Figure 1. It is apparent from the figure that all sorts of behavior, including Poor, Good, and Excellent flame-retardant performance, are achieved. This is the characteristic of dependency of flame retardancy performance on both the type and the content of the P type of FR. It can be observed that the majority of epoxy systems contains less than 20 wt.% of phosphorus flame retardants. For instance, a compromise between FRI and FR loading percentage was achieved by incorporation of encapsulated ammonium polyphosphate (APP- ) at 15 wt.% with an FRI value of 19. Detailed information about the type of phosphorus flame retardants was provided to the reader in the caption of Figure 1. Thus, innovations in design and manufacture of P type FR for epoxy should carefully meet the requirements based on the lesson learned from the multivariable behavior of flame retardancy brought about by P-type FR additives. Precise detection of the performance of each class of P-type FR in this table from one side and the chemical structure of the used FR from the other side should be balanced towards a high-performance FR for developing flame-retardant epoxy composites.
) at 15 wt.% with an FRI value of 19. Detailed information about the type of phosphorus flame retardants was provided to the reader in the caption of Figure 1. Thus, innovations in design and manufacture of P type FR for epoxy should carefully meet the requirements based on the lesson learned from the multivariable behavior of flame retardancy brought about by P-type FR additives. Precise detection of the performance of each class of P-type FR in this table from one side and the chemical structure of the used FR from the other side should be balanced towards a high-performance FR for developing flame-retardant epoxy composites.
Figure 1.
Flame retardancy analysis of epoxy resins containing phosphorus flame retardants in terms of the FRI values as a function of P type and content. Symbols are indicative of different types of phosphorus flame retardant used. Hollow symbols are indicative of fiber-incorporated composites with details earlier given in the bottom of Table 1 as a to l notes. Here:  FP1-4, FP1-6, FP1-8 [24],
FP1-4, FP1-6, FP1-8 [24],  DPO-PHE-11.68, DOPO-PHE-12.03 [25],
DPO-PHE-11.68, DOPO-PHE-12.03 [25],  DOPO-T-2.34, DOPO-T-4.67, DOPO-T-6.99, DOPO-T-9.34 [26],
DOPO-T-2.34, DOPO-T-4.67, DOPO-T-6.99, DOPO-T-9.34 [26],  AEPP-5, AEPP-10, AEPP-15 [27],
AEPP-5, AEPP-10, AEPP-15 [27],  DiDOPO-3 [28],
DiDOPO-3 [28],  DiDOPO-10, DiDOPO-11 [29],
DiDOPO-10, DiDOPO-11 [29],  DiDOPO-7 [30],
DiDOPO-7 [30],  DiDOPO-1, DiDOPO-5, DiDOPO-10 [31],
DiDOPO-1, DiDOPO-5, DiDOPO-10 [31],  DiDOPO-1, DiDOPO-5, DiDOPO-10, DiDOPO-15, DiDOPO-20 [32],
DiDOPO-1, DiDOPO-5, DiDOPO-10, DiDOPO-15, DiDOPO-20 [32],  PPMS-15, PPMS-EG-15 [33],
PPMS-15, PPMS-EG-15 [33],  PPMS-MWCNT-5, PPMS-MWCNT-10, PPMS-MWCNT-15, PPMS-15 [34],
PPMS-MWCNT-5, PPMS-MWCNT-10, PPMS-MWCNT-15, PPMS-15 [34],  DPIPP-7.5, DPIPP-15, DPPIO-7.5, DPPIO-15 [35],
DPIPP-7.5, DPIPP-15, DPPIO-7.5, DPPIO-15 [35],  IDOP-5, IDOP-10, IDOP-15 [36],
IDOP-5, IDOP-10, IDOP-15 [36],  PPAP-5 [37],
PPAP-5 [37],  AlPBu-10, AlPBu-11, AlPBu-12 [38],
AlPBu-10, AlPBu-11, AlPBu-12 [38],  MPL-DOPO-2.5, MPL-DOPO-5, DDM-DOPO-2.5, DDM-DOPO-5 [39],
MPL-DOPO-2.5, MPL-DOPO-5, DDM-DOPO-2.5, DDM-DOPO-5 [39],  ATZ-6 [40],
ATZ-6 [40],  P-KC-30, DOPO-30 [41],
P-KC-30, DOPO-30 [41],  DHPP-OH-BAC-5, DHPP-OH-BAC-10, DHPP-OH-BAC-15 [42],
DHPP-OH-BAC-5, DHPP-OH-BAC-10, DHPP-OH-BAC-15 [42],  PPAP-5, PPAP-10, PPAP-20 [43],
PPAP-5, PPAP-10, PPAP-20 [43],  [Dmim]Tos-2.4, [Dmim]Tos-4, [Dmim]Tos-7.5 [44],
[Dmim]Tos-2.4, [Dmim]Tos-4, [Dmim]Tos-7.5 [44],  MPhP-10, MPhP-15, MPhP-20 [45],
MPhP-10, MPhP-15, MPhP-20 [45],  MDOP-0.96, MDOP-1.9, MDOP-3.75, MDOP-7.24 [46],
MDOP-0.96, MDOP-1.9, MDOP-3.75, MDOP-7.24 [46],  AlPi-7, MPP-7 [47],
AlPi-7, MPP-7 [47],  A-BP-9 [48],
A-BP-9 [48],  CLEP–DOPO–POSS-2.91 [19],
CLEP–DOPO–POSS-2.91 [19],  CuPP-1, CuPP-2, CuPP-4, CuPP-6, CuPP-8 [49],
CuPP-1, CuPP-2, CuPP-4, CuPP-6, CuPP-8 [49],  DOP-ABZ-15, DOP-ABZ-17.5, DOP-ABZ-20 [50],
DOP-ABZ-15, DOP-ABZ-17.5, DOP-ABZ-20 [50],  DOPO-7.11, BPD-3.38, BPD-6.71, BPD-10.04, BPD-13.41 [51],
DOPO-7.11, BPD-3.38, BPD-6.71, BPD-10.04, BPD-13.41 [51],  DOPO-7.7, HPCP-8.2 [52],
DOPO-7.7, HPCP-8.2 [52],  DOPO-TPMP-2.5, DOPO-TPMP-5, DOPO-TPMP-7.5, DOPO-TPMP-10 [53],
DOPO-TPMP-2.5, DOPO-TPMP-5, DOPO-TPMP-7.5, DOPO-TPMP-10 [53],  HB-DPPA-2 [54],
HB-DPPA-2 [54],  APP-21, EDA-APP-21 [55],
APP-21, EDA-APP-21 [55],  CP-6B-3 [56],
CP-6B-3 [56],  PM-2, PM-6, PM-βCD-2, PM-βCD-6 [57],
PM-2, PM-6, PM-βCD-2, PM-βCD-6 [57],  PSA-10, PSA-20 [58],
PSA-10, PSA-20 [58],  BPA-BPP-9 [59],
BPA-BPP-9 [59],  DOPO-9.1, PEPA-9.1, DOPO-PEPA-5.7, DOPO-PEPA-7.4, DOPO-PEPA-9.1 [60],
DOPO-9.1, PEPA-9.1, DOPO-PEPA-5.7, DOPO-PEPA-7.4, DOPO-PEPA-9.1 [60],  DOPO-POSS-2.5, DOPO-POSS-5, DOPO-POSS-10 [61],
DOPO-POSS-2.5, DOPO-POSS-5, DOPO-POSS-10 [61],  HPCTP-7.46, HPCTP-11.19, HPCTP-14.92, DOPO-6.97, DOPO-10.46, DOPO-13.94 [62],
HPCTP-7.46, HPCTP-11.19, HPCTP-14.92, DOPO-6.97, DOPO-10.46, DOPO-13.94 [62],  TP-12.42, TNTP-14.36 [63],
TP-12.42, TNTP-14.36 [63],  DOPO-7, BNP-7, BNP-11, BNP-14.7, BNP-18.4 [64],
DOPO-7, BNP-7, BNP-11, BNP-14.7, BNP-18.4 [64],  DOPO-7, DTB-7, DTB-10, DTB-15, DTB-20 [65],
DOPO-7, DTB-7, DTB-10, DTB-15, DTB-20 [65],  DOPO-7.7, HPCP-8.2 [66],
DOPO-7.7, HPCP-8.2 [66],  DOPO-7.1 [67],
DOPO-7.1 [67],  DOPO-7, DOPO-TMT-7, DOPO-TMT-10.4, DOPO-TMT-13.9, DOPO-TMT-17.3, DOPO-TMT-20.8 [68],
DOPO-7, DOPO-TMT-7, DOPO-TMT-10.4, DOPO-TMT-13.9, DOPO-TMT-17.3, DOPO-TMT-20.8 [68],  HMCP-3.4, HMCP-6.8, HMCP-10.2, HMCP-13.6, HMCP-17 [69],
HMCP-3.4, HMCP-6.8, HMCP-10.2, HMCP-13.6, HMCP-17 [69],  DOPO-bp-3.4, DOPO-bp-6.7, DOPO-bp-13.5 [70],
DOPO-bp-3.4, DOPO-bp-6.7, DOPO-bp-13.5 [70],  CTP-DOPO-10.6 [71],
CTP-DOPO-10.6 [71],  PMTMPS-11 [72],
PMTMPS-11 [72],  PUTMPS-12, [73],
PUTMPS-12, [73],  APHP-2, APHP-4, APHP-6 [74],
APHP-2, APHP-4, APHP-6 [74],  APHP-6, DOPO-6 [75],
APHP-6, DOPO-6 [75],  HP-1001-COOH-10, HP-1001-COOH-20, HP-1001-COOH-30, HP-1001-COOH-40, HP-1001-COOH-50 [76],
HP-1001-COOH-10, HP-1001-COOH-20, HP-1001-COOH-30, HP-1001-COOH-40, HP-1001-COOH-50 [76],  TAD-4 [77],
TAD-4 [77],  DOPO-10, TAD-6, TAD-8, TAD-10, TAD-12 [78],
DOPO-10, TAD-6, TAD-8, TAD-10, TAD-12 [78],  PAz-APP-10, PAz-APP-15 [79],
PAz-APP-10, PAz-APP-15 [79],  DETA-APP-10, DETA-APP-15 [80],
DETA-APP-10, DETA-APP-15 [80],  DOPO-8.3, Trif-DOPO-11.7, Trif-DOPO-14 [81],
DOPO-8.3, Trif-DOPO-11.7, Trif-DOPO-14 [81],  TOD-2, TOD-4, TOD-6 [82],
TOD-2, TOD-4, TOD-6 [82],  DOPO-DDM-10, DOPO-DDE-10, DOPO-DDS-10 [83],
DOPO-DDM-10, DOPO-DDE-10, DOPO-DDS-10 [83],  DPP-POSS-5, DPOP-POSS-5, DOPO-POSS-5 [84],
DPP-POSS-5, DPOP-POSS-5, DOPO-POSS-5 [84],  ATH-DOPO-10, ATH-DOPO-20, pATH-DOPO-10 [85],
ATH-DOPO-10, ATH-DOPO-20, pATH-DOPO-10 [85],  BPS-BPP-9 [86],
BPS-BPP-9 [86],  PN-15, PSi-25 [87],
PN-15, PSi-25 [87],  BDMPP-14 [88],
BDMPP-14 [88],  ATCP-15 [89],
ATCP-15 [89],  ATCP-15 [90],
ATCP-15 [90],  DOPO-4.5, DOPO-ABZ-7.5, DOPO-ABZ-10 [91],
DOPO-4.5, DOPO-ABZ-7.5, DOPO-ABZ-10 [91],  DMT-3.3, DMT-6.6, DMT-10, DMT-13.5, DMT-17 [92],
DMT-3.3, DMT-6.6, DMT-10, DMT-13.5, DMT-17 [92],  APP-10, APP-MMT-10 [93],
APP-10, APP-MMT-10 [93],  DOPO-6, DOPO-MMT-6 [94],
DOPO-6, DOPO-MMT-6 [94],  APHP-10, BDP-10 [95],
APHP-10, BDP-10 [95],  FIPF-20, FTBF-20 [96],
FIPF-20, FTBF-20 [96],  PPDAB-10 [97],
PPDAB-10 [97],  BP-5, BP-9, BP-15 [98],
BP-5, BP-9, BP-15 [98],  PS-APP-2, PS-APP-5, PS-APP-10, PS-APP-15, PS-APP-20 [99],
PS-APP-2, PS-APP-5, PS-APP-10, PS-APP-15, PS-APP-20 [99],  DOPO-POSS-2.5, DOPO-POSS-5, DOPO-POSS-10 [100],
DOPO-POSS-2.5, DOPO-POSS-5, DOPO-POSS-10 [100],  DOPO-POSS-2.5, DOPO-POSS-5, DOPO-POSS-10 [100],
DOPO-POSS-2.5, DOPO-POSS-5, DOPO-POSS-10 [100],  DOPO-POSS-2.5, DOPO-POSS-5, DOPO-POSS-10 [101],
DOPO-POSS-2.5, DOPO-POSS-5, DOPO-POSS-10 [101],  DOPO-5 [102],
DOPO-5 [102],  DOPO-6.3 [103],
DOPO-6.3 [103],  DOPO-6.3 [104],
DOPO-6.3 [104],  APP-MMT-10 [105],
APP-MMT-10 [105],  PEPA-5.2, APP-2.9, DOPO-6.3 [106],
PEPA-5.2, APP-2.9, DOPO-6.3 [106],  PCPBO-5, PCPBO-10, PCPBO-15, PCPBO-20 [107],
PCPBO-5, PCPBO-10, PCPBO-15, PCPBO-20 [107],  APP-15, GMA-APP-15 [108],
APP-15, GMA-APP-15 [108],  APP-12, MAPP-12 [109],
APP-12, MAPP-12 [109],  APP-12 [110],
APP-12 [110],  HAP-DOPO-9.3, HAP-DOPO-15.47 [111],
HAP-DOPO-9.3, HAP-DOPO-15.47 [111],  TGIC-DOPO-6.1, TGIC-DOPO-8.1, TGIC-DOPO-10.2, TGIC-DOPO-12.2 [112],
TGIC-DOPO-6.1, TGIC-DOPO-8.1, TGIC-DOPO-10.2, TGIC-DOPO-12.2 [112],  DOPP-19.6, DOPI-23.1 [113],
DOPP-19.6, DOPI-23.1 [113],  PMPC-10, PMPC-15, PMPC-20 [114],
PMPC-10, PMPC-15, PMPC-20 [114],  DOPO-5 [115],
DOPO-5 [115],  SIEPDP-Mg-Al LDH-4 [116],
SIEPDP-Mg-Al LDH-4 [116],  CBz-10, CBz-15, CBz-20 [118],
CBz-10, CBz-15, CBz-20 [118],  APP-5 [117],
APP-5 [117],  DOPMPA-10, DOPMPA-13 [119],
DOPMPA-10, DOPMPA-13 [119],  MFR-10, MFR-15, MFR-20 [9],
MFR-10, MFR-15, MFR-20 [9],  DOPO-COFs-0.4, DOPO-COFs-0.8, DOPO-COFs-1.6, DOPO-COFs-3.2, COFs-3.2 [17],
DOPO-COFs-0.4, DOPO-COFs-0.8, DOPO-COFs-1.6, DOPO-COFs-3.2, COFs-3.2 [17],  Mel-APP-20 [120],
Mel-APP-20 [120],  FR-1 [121],
FR-1 [121],  ArPN2-15, ArPO2-15, ArOPN2-15.6, ArOPO2-15.6 [122],
ArPN2-15, ArPO2-15, ArOPN2-15.6, ArOPO2-15.6 [122],  PMAIL-6 [123],
PMAIL-6 [123],  oDOPI-13.81, PZ -10.8, MPP-15 [124],
oDOPI-13.81, PZ -10.8, MPP-15 [124],  AHP-5 [125],
AHP-5 [125],  Mel-APP-29.7 [126],
Mel-APP-29.7 [126],  MPAlP-20, MPZnP-20, MPMgP-20, MPP-20, AlPi-Et-20, DOPAc-Bu-20 [127],
MPAlP-20, MPZnP-20, MPMgP-20, MPP-20, AlPi-Et-20, DOPAc-Bu-20 [127],  HPCTP-5, HPCTP-10, HPCTP-15 [128],
HPCTP-5, HPCTP-10, HPCTP-15 [128],  HPCTP-15 [129],
HPCTP-15 [129],  TPP-MMT-5 [130],
TPP-MMT-5 [130],  TPP-MMT-5 [130],
TPP-MMT-5 [130],  TPP-MMT-5 [130],
TPP-MMT-5 [130],  hbPPE-10, hbPPE-20 [131],
hbPPE-10, hbPPE-20 [131],  PZS-3, PZS@SrSn(OH)6-3 [132],
PZS-3, PZS@SrSn(OH)6-3 [132],  PEPA-TMAC-16.5, PEPA-TMAC-33 [133],
PEPA-TMAC-16.5, PEPA-TMAC-33 [133],  PCPS-1, PCPS-3, PCPS-5 [134],
PCPS-1, PCPS-3, PCPS-5 [134],  BP1-5, BP2-5, BP3-5, BP4-5, BP5-5 [135],
BP1-5, BP2-5, BP3-5, BP4-5, BP5-5 [135],  SDPS-10.4 [136],
SDPS-10.4 [136],  AOPH-NR-4.25, AOPH-C1-4.25, AOPH-C2-4.25, AOPH-C3-4.25 [137],
AOPH-NR-4.25, AOPH-C1-4.25, AOPH-C2-4.25, AOPH-C3-4.25 [137],  BHAAPE-5, BHAAPE-10, BHAAPE-20 [138],
BHAAPE-5, BHAAPE-10, BHAAPE-20 [138],  APP-10 [139],
APP-10 [139],  PZS-2, PZS@MoS2-2, PZS@MoS2-3 [140],
PZS-2, PZS@MoS2-2, PZS@MoS2-3 [140],  DBPDA-βCD-3 [141],
DBPDA-βCD-3 [141],  BP-PZN-0.5, BP-PZN-1, BP-PZN-2, BP-Bulk-2 [142],
BP-PZN-0.5, BP-PZN-1, BP-PZN-2, BP-Bulk-2 [142],  HPPA-2, HPPA-SH-mSiO2-2 [143],
HPPA-2, HPPA-SH-mSiO2-2 [143],  P-MOF-0.5, P-MOF-1, P-MOF-2 [144],
P-MOF-0.5, P-MOF-1, P-MOF-2 [144],  CZrP-2, CZrP-4, CZrP-6, ZrP-6 [145],
CZrP-2, CZrP-4, CZrP-6, ZrP-6 [145],  DMMP-HNT-20 [146],
DMMP-HNT-20 [146],  S600-20, AlPi-20, MPP-20 [147],
S600-20, AlPi-20, MPP-20 [147],  SiO2@PZM-1, SiO2@PZM@Cu-1, SiO2@PZM@Cu-2 [148],
SiO2@PZM-1, SiO2@PZM@Cu-1, SiO2@PZM@Cu-2 [148],  FR@PZS-0.5, FR@PZS-1, FR@PZS-3, PZS-3 [149],
FR@PZS-0.5, FR@PZS-1, FR@PZS-3, PZS-3 [149],  APP-5 [150],
APP-5 [150],  DOPO-POSS [151],
DOPO-POSS [151],  APP-30, M(APP & PER)-30 [152],
APP-30, M(APP & PER)-30 [152],  TPPi-15, TPPa-15, TPPO-15 [153],
TPPi-15, TPPa-15, TPPO-15 [153],  PMP-11.4, DOPO-13.9, RP-4.3, OP-8.3 [154],
PMP-11.4, DOPO-13.9, RP-4.3, OP-8.3 [154],  IFR-30, IFR-30, IFR-30 [155],
IFR-30, IFR-30, IFR-30 [155],  BPE-33.3, EPE-33.3, BBPE-33.3, BOPE-33.3, HBPE-33.3 [156],
BPE-33.3, EPE-33.3, BBPE-33.3, BOPE-33.3, HBPE-33.3 [156],  IFR-30, IFR-30, IFR-30, IFR-30 [157],
IFR-30, IFR-30, IFR-30, IFR-30 [157],  EGM-5, EGM-15 [158],
EGM-5, EGM-15 [158],  PCTS-Fe-OMMT-1, PCTS-Fe-OMMT-3, PCTS-Fe-OMMT-5 [159],
PCTS-Fe-OMMT-1, PCTS-Fe-OMMT-3, PCTS-Fe-OMMT-5 [159],  APP-20, APP-40 [160],
APP-20, APP-40 [160],  DOPOph-RGNO-1, DOPOph-RGNO-2, DOPOph-RGNO-3 [161],
DOPOph-RGNO-1, DOPOph-RGNO-2, DOPOph-RGNO-3 [161],  Mel-APP-9.59 [120],
Mel-APP-9.59 [120],  FP1-2.6 [24],
FP1-2.6 [24],  PEC-5.2, PEC-6.9, PEC-8.1 [162],
PEC-5.2, PEC-6.9, PEC-8.1 [162],  DOPP-5.9, DOPI-6.9 [113],
DOPP-5.9, DOPI-6.9 [113],  Mel-APP-14.6 [126],
Mel-APP-14.6 [126],  IFR-4.7 [163,164],
IFR-4.7 [163,164],  APP-5 [150],
APP-5 [150],  APP-3.15, APP-8.88, APP-16.32 [165],
APP-3.15, APP-8.88, APP-16.32 [165],  MP-5, DOPO-5 [166],
MP-5, DOPO-5 [166],  IFR-5, IFR-10, IFR-15 [167],
IFR-5, IFR-10, IFR-15 [167],  APP-15 [168],
APP-15 [168],  APP-15 [168].
APP-15 [168].
Although variation of FRI values according to the composition reflects the flame retardancy of epoxy composites from cone calorimetry angle (the most reliable test among those normally used for analysis of performance of flame retardants), other types of flame tests would give more insights into the real effect of one or complementary actions of two or more P type FR additives in epoxy. Based on available data, a brief view of the effect of the used P-based FRs on the flame retardancy performance of epoxy resins as a function of UL94 results is given in Figure 2. The distribution of data in this figure gives useful information about the efficiency of the FR system in harsh conditions. For instance, this figure suggests that V-0 performance in UL94 can be achieved even at the Poor category of flame retardancy performance in terms of FRI. It appears that it is not possible to roughly correlate the obtained results in UL94 to those obtained in cone calorimetry tests.
Figure 2.
Flame retardancy analysis of epoxy resins containing phosphorus flame retardants in terms of the FRI values as a function of UL-94 test results. Symbols are indicative of different types of phosphorus flame retardant used. Hollow symbols are indicative of fiber-incorporated composites with details earlier given in the bottom of Table 1 as a to l notes. The vertical variation in each category, i.e., V-0, V-1, V-2, and NR, is schematically representative of the amount of additive used. For example, among two data distinguished by different symbols having the same or very close FRI values (horizontal quantity) in a given category (e.g., V-1), which have different vertical quantity both revealed V-1 behavior in UL-94 test, but the upper was an FR used in more quantity in preparation of epoxy composites.
Another test of importance is the limiting oxygen index (LOI), which is demonstrative of flammability. A self-extinguishing behavior is expected when the LOI value is higher than 28. A brief overview of the effect of the used phosphorus-type flame retardants on the flame retardancy performance of epoxy resins as a function of LOI results is given in Figure 3. Surprisingly, the highest value obtained in LOI testing is located in the Good zone of FRI. The collection of data with FRI values below 5, where LOI% varies depending on the type of phosphorus additive and undoubtedly the content, is hidden behind these symbols.
Figure 3.
Flame retardancy analysis of epoxy resins containing phosphorus flame retardants in terms of the FRI values as a function of LOI test results. Symbols are indicative of different types of phosphorus flame retardant used. Hollow symbols are indicative of fiber-incorporated composites with details earlier given in the bottom of Table 1 as a to l notes.
3. Epoxy Resins Containing Nonphosphorus Flame Retardants
According to the literature, a variety of nonphosphorus FRs have been used in epoxy resins. Table 2 summarizes pHRR, THR, and TTI and the FRI values of epoxy/NP systems. The percentage of incorporated FR as well as the results of LOI and UL-94 test are also given for comprehensive determination of the behavior of this family of epoxy composites.
Table 2.
The state of flame retardancy performance of epoxy resins containing nonphosphorus flame retardants in terms of FRI (* the name and percentage of incorporated flame retardant is given after each epoxy resin). The notes a to h on the bottom of the table are representative of composite systems containing woven or nonwoven fibers.
| Epoxy Resins and Incorporated Non Phosphorus FR * | wt.% | TTI (s) | pHRR (kW.m−2) | THR (MJ·m−2) | FRI | LOI | UL94 | Ref. |
|---|---|---|---|---|---|---|---|---|
| 0 | 11 | 781 | 142 | — | 21.8 | [169] | ||
| (2,4,6-tris(4-boronic-2-thiophene)-1,3,5-triazine (3TT-3BA) | 20 | 17 | 454 | 108 | 3.49 | 31.2 | V-0 | [169] |
| 0 | 32 | 827 | 116 | — | 21.8 | NR | [28] | |
| graphene nanosheet (GN) | 3 | 35 | 560 | 113 | 1.65 | 26.7 | NR | [28] |
| 0 | 32 | 781 | 107 | — | 21.8 | NR | [29] | |
| multiwalled carbon nanotube (MWCNT) | 0.8 | 40 | 473 | 97 | 2.27 | 21.2 | NR | [29] |
| 0 | 32 | 781 | 107 | — | 21.8 | NR | [30] | |
| Organically modified montmorillonite (DK4:two longchain alkyl ammonium modified montmorillonite) (OMMT) | 7 | 40 | 576 | 98 | 1.85 | 23.7 | NR | [30] |
| 0 | 32 | 781 | 107 | — | 21.8 | NR | [31] | |
| organomodified magnesium aluminium layered double hydroxide (OLDH) | 1 | 35 | 543 | 121 | 1.39 | 21.9 | NR | [31] |
| organomodified magnesium aluminium layered double hydroxide (OLDH) | 5 | 35 | 521 | 104 | 1.68 | 23.6 | V-0 | [31] |
| organomodified magnesium aluminium layered double hydroxide (OLDH) | 10 | 49 | 391 | 106 | 3.08 | 22.1 | V-0 | [31] |
| 0 | 71 | 1146 | 56 | — | 21.2 | NR | [170] | |
| magnesium aluminium layered double hydroxide (MgAl-LDH) | 2 | 63 | 865 | 49 | 1.34 | 23.8 | NR | [170] |
| zeolitic imidazolate framework8 (ZIF8) | 2 | 58 | 886 | 41 | 1.44 | 23.3 | NR | [170] |
| zeolitic imidazolate framework8 decorated magnesium aluminium layered double hydroxide (ZIF8@MgAl-LDH) | 2 | 54 | 562 | 39 | 2.22 | 24.7 | V-1 | [170] |
| zeolitic imidazolate framework67 (ZIF67) | 2 | 62 | 817 | 42 | 1.63 | 23.6 | NR | [170] |
| zeolitic imidazolate framework67 decorated MgAl-layered double hydroxide (ZIF67@MgAl-LDH) | 2 | 56 | 432 | 34 | 3.44 | 25.5 | V-1 | [170] |
| 0 | 61 | 1208 | 77.3 | — | 22.5 | NR | [52] | |
| triazine-based flame retardant (TAT) | 20 | 42 | 1030 | 75.8 | 0.82 | 24.1 | NR | [52] |
| 35 | 1065 | 80.3 | — | 22.9 | NR | [171] | ||
| 2,4,6-tris-(4-boronphenoxy)-(1,3,5)-triazine (TNB) | 1 | 23 | 686 | 68.1 | 1.20 | 26.1 | V-1 | [171] |
| 2,4,6-tris-(4-boronphenoxy)-(1,3,5)-triazine (TNB) | 5 | 22 | 427 | 64.1 | 1.96 | 28.3 | V-1 | [171] |
| 2,4,6-tris-(4-boronphenoxy)-(1,3,5)-triazine (TNB) | 10 | 20 | 324 | 59.3 | 2.54 | 29.4 | V-1 | [171] |
| 2,4,6-tris-(4-boronphenoxy)-(1,3,5)-triazine (TNB) | 15 | 22 | 309 | 58.3 | 2.98 | 30.4 | V-0 | [171] |
| 2,4,6-tris-(4-boronphenoxy)-(1,3,5)-triazine (TNB) | 20 | 22 | 305 | 58 | 3.03 | 31.2 | V-0 | [171] |
| 53 | 1121 | 102 | — | 20 | NR | [55] | ||
| Cuprous oxide (Cu2O) | 21 | 47 | 1007 | 86 | 1.17 | 22 | NR | [55] |
| 45 | 1091 | 83 | — | 22.8 | NR | [56] | ||
| magnesium hydroxide (MH) | 3 | 38 | 751 | 80 | 1.27 | 25.2 | NR | [56] |
| 60 | 873 | 88.5 | — | 22.5 | NR | [63] | ||
| 2,4,6-triphenoxy-1,3,5-triazine (TN) | 3.42 | 25 | 943 | 78.4 | 0.43 | 29 | NR | [63] |
| 58 | 1208 | 80.6 | — | 22.5 | NR | [66] | ||
| expandable graphite (EG) | 20 | 49 | 225 | 63.3 | 5.77 | 31 | NR | [66] |
| 57 | 1557 | 94.5 | — | 24.5 | NR | [67] | ||
| nucleophilic substitution reaction between N-(4-hydroxyphenyl) maleimide & cyanuric chloride (TMT) | 8 | 52 | 1395 | 88.4 | 1.08 | 27 | NR | [67] |
| 61 | 1208 | 80.6 | — | 22.5 | NR | [68] | ||
| nucleophilic substitution reaction between N-(4-hydroxyphenyl) maleimide & cyanuric chloride (TMT) | 7 | 61 | 858 | 73.5 | 1.54 | 25.5 | NR | [68] |
| 56 | 1420 | 140 | — | 26 | NR | [77] | ||
| organically modified montmorillonite (OMMT) | 1 | 39 | 1540 | 116 | 0.77 | 29.3 | NR | [77] |
| 69 | 966 | 93.9 | — | 22.5 | NR | [78] | ||
| triallyl isocyanurate (TAIC) | 10 | 61 | 1306 | 123 | 0.50 | 23.6 | NR | [78] |
| 52 | 995 | 93.3 | — | 22.5 | NR | [81] | ||
| Triphenoxy-1,3,5-triazine (TPT) | 14 | 48 | 964 | 88.7 | 1.00 | 24.5 | NR | [81] |
| 67 | 950 | 98 | — | 24.1 | NR | [172] | ||
| Halloysite nanotube (HNT) | 5 | 65 | 1170 | 93 | 0.83 | 26.1 | NR | [172] |
| Halloysite nanotube (HNT) | 10 | 65 | 1002 | 95 | 0.94 | 25.4 | NR | [172] |
| biomimetic polydopamine nanocoating functionalized Halloysite nanotube (HNT@PDA) | 5 | 65 | 1088 | 104 | 0.79 | 25.6 | NR | [172] |
| biomimetic polydopamine nanocoating functionalized Halloysite nanotube (HNT@PDA) | 10 | 67 | 881 | 91 | 1.16 | 25.6 | NR | [172] |
| biomimetic polydopamine nanocoating functionalized Halloysite nanotube and ultrafine Fe(OH)3 nanoparticles (HNT@PDA@Fe(OH)3) | 5 | 61 | 695 | 90 | 1.35 | 33.9 | V-1 | [172] |
| biomimetic polydopamine nanocoating functionalized Halloysite nanotube and ultrafine Fe(OH)3 nanoparticles (HNT@PDA@Fe(OH)3) | 10 | 58 | 698 | 88 | 1.31 | 33.8 | NR | [172] |
| 50 | 860 | 133 | — | 23 | NR | [94] | ||
| Montmorillonite (MMT) | 6 | 49 | 792 | 100 | 1.41 | 26 | NR | [94] |
| 45 | 855 | 112 | — | 25 | NR | [102] | ||
| octaphenyl polyhedral oligomeric silsesquioxane (OPS) | 5 | 60 | 712 | 103 | 1.74 | 31.1 | NR | [102] |
| 45 | 855 | 112 | — | 25 | NR | [103] | ||
| Octaphenyl silsesquioxane (OPS) | 4.1 | 55 | 626 | 112 | 1.66 | 27.2 | NR | [103] |
| Polyphenyl silsesquioxane (PPSQ) | 4.1 | 50 | 925 | 116 | 0.99 | 27.1 | NR | [103] |
| 45 | 855 | 112 | — | 25 | NR | [104] | ||
| Octaphenyl silsesquioxane (OPS) | 4.1 | 55 | 626 | 112 | 1.66 | 27.2 | NR | [104] |
| Octaaminophenylsilsesquioxane (OAPS) | 4.6 | 57 | 635 | 110 | 1.73 | 27 | NR | [104] |
| 50 | 860 | 112 | — | 23 | NR | [106] | ||
| Octaphenyl polyhedral oligomeric silsesquioxane (OPS) | 4.1 | 55 | 626 | 112 | 1.51 | 25 | NR | [106] |
| 57 | 459 | 55.2 | — | 19.5 | NR | [173] | ||
| aluminum trihydroxide (ATH) | 40 | 68 | 231 | 41.2 | 3.17 | 23.6 | NR | [173] |
| Colemanite (C) | 40 | 58 | 158 | 34.3 | 4.75 | 23.6 | NR | [173] |
| Ulexite (U) | 40 | 62 | 171 | 38.2 | 4.21 | 22.6 | NR | [173] |
| boric acid (BA) | 40 | 76 | 132 | 32.1 | 7.97 | 28.5 | V-0 | [173] |
| boric oxide (BO) | 40 | 68 | 82 | 20.6 | 17.89 | 24.2 | NR | [173] |
| melamine borate (MB) | 30 | 78 | 107 | 26.9 | 12.05 | 24.5 | V-0 | [173] |
| guanidinium nonaborate (GB) | 30 | 65 | 105 | 26.8 | 10.27 | 23.6 | NR | [173] |
| 64 | 821 | 94 | — | 23.2 | NR | [115] | ||
| polyhedral oligomeric octadiphenylsulfonylsilsesquioxane (ODPSS) | 5 | 59 | 417 | 74 | 2.30 | 24.3 | NR | [115] |
| 60 | 920 | 90.5 | — | 22.7 | NR | [116] | ||
| Magnesium-Aluminum layered double hydroxide (Mg-Al LDH) | 4 | 53 | 835 | 89.6 | 0.98 | 24.3 | NR | [116] |
| 108 | 1634 | 78 | — | 19.8 | NR | [174] | ||
| Trisilanolisobutyl Polyhedral oligomeric silsesquioxane (T8POSS) | 10 | 99 | 774 | 56 | 2.69 | 20.7 | NR | [174] |
| triglycidyl isocyanurate (TGIC) | 10 | 86 | 1190 | 67 | 1.27 | 19.9 | NR | [174] |
| 0 | 51 | 1914 | 81.9 | — | 22 | NR | [121] | |
| reduced graphene oxide (RGO) | 1 | 47 | 1356 | 67.6 | 1.57 | 23.5 | NR | [121] |
| 21 | 1910 | 84.4 | — | 22.1 | NR | [120] | ||
| halloysite nano-tube (HNT) | 2 | 20 | 1591 | 90.7 | 1.06 | 19.5 | NR | [120] |
| layered double hydroxide (LDH) | 2 | 21 | 803 | 87.5 | 2.29 | 21.6 | NR | [120] |
| layered double hydroxide (LDH) | 4 | 22 | 861 | 85.4 | 2.29 | 20. 6 | NR | [120] |
| layered double hydroxide (LDH) | 6 | 20 | 791 | 82.9 | 2.34 | 19.7 | NR | [120] |
| epoxy novolac resin | 0 | 51 | 682 | 110 | — | — | NR | [124] |
| Boehmite (AlO(OH)) | 30 | 69 | 535 | 88 | 2.15 | — | V-1 | [124] |
| 0 | 50 | 992 | 91 | — | — | NR | [125] | |
| activated carbon spheres (ACS) | 2 | 56 | 898 | 91 | 1.23 | — | — | [125] |
| activated carbon spheres@SnO2 hybrid (ACS@SnO2) | 2 | 50 | 761 | 98 | 1.21 | — | — | [125] |
| activated carbon spheres@SnO2@NiO hybrid (ACS@SnO2@NiO) | 2 | 56 | 839 | 92 | 1.31 | — | NR | [125] |
| 0 | 50 | 986 | 91 | — | — | NR | [125] | |
| activated carbon spheres@SnO2@NiO hybrid (ACS@SnO2@NiO) | 5 | 51 | 823 | 88 | 1.26 | — | NR | [125] |
| 63 | 1321 | 157 | — | — | NR | [129] | ||
| octapropylglycidylether polyhedral oligomeric silsesquioxane (OGPOSS) | 15 | 60 | 1026 | 145 | 1.32 | — | NR | [129] |
| 0 | 19 | 1325 | 95.7 | — | 19.2 | HB | [33] | |
| Expandable graphite (EG) | 15 | 34 | 1015 | 85.3 | 2.61 | 25.4 | HB | [33] |
| 100 | 733 | 141 | — | 21 | HB | [130] | ||
| Silicate glass (CP) | 10 | 101 | 315 | 139 | 2.38 | 25 | HB | [130] |
| Silicate glass (CP) | 15 | 89 | 268 | 132 | 2.60 | 24 | HB | [130] |
| 47 | 891 | 151 | — | 21 | HB | [130] | ||
| Silicate glass (CP) | 10 | 44 | 408 | 136 | 2.27 | 25 | HB | [130] |
| Silicate glass (CP) | 15 | 46 | 346 | 134 | 2.84 | 24 | HB | [130] |
| 22 | 1196 | 147 | — | 21 | HB | [130] | ||
| Silicate glass (CP) | 10 | 20 | 565 | 137 | 2.06 | 25 | HB | [130] |
| Silicate glass (CP) | 15 | 19 | 585 | 129 | 2.01 | 24 | HB | [130] |
| 0 | 58 | 1126 | 100 | — | 26.1 | — | [132] | |
| strontium hydroxystannate nanorod (SrSn(OH)6) | 3 | 55 | 889 | 92.6 | 1.30 | 28.4 | — | [132] |
| 0 | 73 | 1054 | 39.1 | — | 22.4 | — | [175] | |
| silica nanoparticles (SiO2) | 2 | 65 | 727 | 34.4 | 1.46 | 26 | — | [175] |
| Zeolitic imidazolate framework-8 nanocrystals (ZIF8) | 2 | 60 | 431 | 25.3 | 3.10 | 26.9 | — | [175] |
| Zeolitic imidazolate framework-8 coated with SiO2 (ZIF8@SiO2) | 2 | 68 | 254 | 23.9 | 6.32 | 28.1 | — | [175] |
| 0 | 69 | 1150 | 54.7 | — | 22 | — | [176] | |
| molybdenum disulfide (MoS2) | 2 | 65 | 854 | 41.7 | 1.66 | 25.7 | — | [176] |
| titanium dioxide nanotube (TNT) | 2 | 58 | 815 | 39.5 | 1.64 | 25.5 | — | [176] |
| molybdenum disulfide decorated titanium dioxide nanotube (MoS2-TNT) | 1 | 63 | 859 | 43.7 | 1.53 | 25.1 | — | [176] |
| molybdenum disulfide decorated titanium dioxide nanotube (MoS2-TNT) | 2 | 60 | 701 | 37.1 | 2.10 | 26.8 | — | [176] |
| molybdenum disulfide decorated titanium dioxide nanotube (MoS2-TNT) | 3 | 61 | 627 | 32.1 | 2.76 | 28.1 | — | [176] |
| 45 | 1193 | 76 | — | 23.8 | — | [177] | ||
| Sepiolite (Sep) | 2 | 49 | 1288 | 78 | 0.98 | 29.8 | — | [177] |
| Sepiolite (Sep) | 4 | 61 | 963 | 101 | 1.26 | 30.1 | — | [177] |
| Fe3O4-doped sepiolite (Fe3o4–Sep) | 2 | 42 | 1093 | 83 | 0.93 | 33.8 | — | [177] |
| Fe3O4-doped sepiolite (Fe3o4–Sep) | 4 | 45 | 883 | 89 | 1.15 | 36.7 | — | [177] |
| 45 | 1193 | 76 | — | 23.8 | — | [178] | ||
| oxidized graphene nanoplatelets (GNO) | 1 | 49 | 1204 | 81 | 1.01 | 25.2 | — | [178] |
| oxidized graphene nanoplatelets (GNO) | 3 | 47 | 1244 | 72 | 1.05 | 25.6 | — | [178] |
| Cu-doped graphene (GN-Cu) | 1 | 45 | 825 | 66 | 1.66 | 25.8 | — | [178] |
| Cu-doped graphene (GN-Cu) | 3 | 47 | 786 | 64 | 1.88 | 26.4 | — | [178] |
| 0 | 54 | 1068 | 76 | — | 21 | — | [147] | |
| Boehmite (AlO(OH)) | 20 | 49 | 870 | 65 | 1.30 | — | — | [147] |
| 54 | 1068 | 75.8 | — | — | HB | [127] | ||
| Boehmite (AlO(OH)) | 20 | 49 | 870 | 65.5 | 1.28 | — | HB | [127] |
| amorphous silicon dioxide (SiO2) | 20 | 41 | 907 | 57.6 | 1.17 | — | HB | [127] |
| Bisphenol-A | 0 | 22 | 1680 | 79 | — | — | — | [179] |
| α-Manganese dioxide nanosheets (α-MnO2) | 0.5 | 25 | 1701 | 77 | 1.15 | — | — | [179] |
| α-Manganese dioxide nanosheets (α-MnO2) | 1 | 24 | 1480 | 73 | 1.34 | — | — | [179] |
| α-Manganese dioxide nanosheets (α-MnO2) | 2 | 23 | 1400 | 67 | 1.47 | — | — | [179] |
| δ-Manganese dioxide nanosheets (δ-MnO2) | 0.5 | 25 | 1617 | 74 | 1.26 | — | — | [179] |
| δ-Manganese dioxide nanosheets (δ-MnO2) | 1 | 26 | 1547 | 74 | 1.37 | — | — | [179] |
| δ-Manganese dioxide nanosheets (δ-MnO2) | 2 | 27 | 1358 | 64 | 1.87 | — | — | [179] |
| 0 | 60 | 2187 | 124 | — | — | — | [140] | |
| molybdenum disulfide nanoflower (MoS2) | 2 | 49 | 1457 | 98 | 1.55 | — | — | [140] |
| 0 | 47.7 | 1308 | 86.8 | — | — | — | [180] | |
| Aminopropylisobutyl polyhedral oligomeric silsesquioxane (AI-POSS) | 7.2 | 44.3 | 880 | 83.6 | 1.43 | — | — | [180] |
| Aminopropylisobutyl polyhedral oligomeric silsesquioxane (AI-POSS) | 21.8 | 36.3 | 585 | 97.7 | 1.51 | — | — | [180] |
| Aminopropylisobutyl polyhedral oligomeric silsesquioxane (AI-POSS) | 54 | 32.2 | 616 | 65.3 | 1.90 | — | — | [180] |
| 0 | 5 | 986 | 113 | — | — | — | [181] | |
| Expandable graphite (EG) | 9 | 10 | 152 | 110 | 13.33 | — | — | [181] |
| halloysite nanotube (HNT) | 9 | 5 | 969 | 110 | 1.04 | — | — | [181] |
| 0 | 117 | 1184 | 95.3 | — | — | — | [182] | |
| Boron Nitride with D50 = 2 μm (BN 2 μm) | 45 | 175 | 767 | 71.5 | 3.07 | — | — | [182] |
| Boehmite with D50 = 2 μm (BT 2 μm) | 45 | 140 | 674 | 72.2 | 2.77 | — | — | [182] |
| 0 | 22 | 1650 | 80 | — | — | — | [183] | |
| Manganese dioxide (MnO2) | 2 | 27 | 1443 | 71 | 1.58 | — | — | [183] |
| Manganese dioxide@zinc hydroxystannate binary hybrid (MnO2@ZHS) | 0.5 | 24 | 1487 | 56 | 1.72 | — | — | [183] |
| Manganese dioxide@zinc hydroxystannate binary hybrid (MnO2@ZHS) | 1 | 25 | 1275 | 49 | 2.40 | — | — | [183] |
| Manganese dioxide@zinc hydroxystannate binary hybrid (MnO2@ZHS) | 2 | 23 | 989 | 61 | 2.28 | — | — | [183] |
| Diglycidyl ether of bisphenol-F epoxy | 0 | 66 | 1197 | 82.7 | — | — | — | [184] |
| ionic liquid flame retardant (ILFR) | 5 | 55 | 753 | 62.5 | 1.75 | — | — | [184] |
| boron nitride nanosheets (BN) | 5 | 70 | 813 | 68.2 | 1.89 | — | — | [184] |
| ionic liquid flame retardant functionalized boron nitride nanosheets (ILFR-fBN) | 5 | 104 | 689 | 51.5 | 4.39 | — | — | [184] |
| 63 | 1397 | 81.3 | — | — | — | [143] | ||
| thiol-functionalized mesoporous silica (SH-mSiO2) | 2 | 65 | 1117 | 77.8 | 1.34 | — | — | [143] |
| 52 | 972 | 99 | — | — | — | [185] | ||
| short carbon fiber (SCF) | 0.5 | 69 | 793 | 92 | 1.75 | — | — | [185] |
| short carbon fiber (SCF) | 0.7 | 80 | 723 | 88 | 2.32 | — | — | [185] |
| short carbon fiber (SCF) | 1 | 62 | 840 | 89 | 1.53 | — | — | [185] |
| short carbon fiber (SCF) | 1.5 | 98 | 793 | 101 | 2.26 | — | — | [185] |
| 24 | 1002 | 104 | — | — | — | [146] | ||
| halloysite nanotube (HNT) | 20 | 43 | 790 | 75.2 | 3.14 | — | — | [146] |
| 38 | 1542 | 76.2 | — | — | — | [186] | ||
| nanomer I.28E organoclay (m-Clay) | 2.5 | 58 | 1298 | 56.6 | 2.44 | — | — | [186] |
| Deoxyribonucleic Acid modified clay (d-Clay) | 2.5 | 55 | 1220 | 52.4 | 2.66 | — | — | [186] |
| 22 | 1032 | 49.2 | — | — | — | [187] | ||
| Layered double hydroxide (LDH) | 3 | 27 | 968 | 49.6 | 1.29 | — | — | [187] |
| β-Iron oxyhydroxide (β-FeOOH) | 3 | 25 | 857 | 48 | 1.40 | — | — | [187] |
| Layered double hydroxide nanosheet-wrapped β-Iron oxyhydroxide rod hybrid (LDH-β-FeOOH) | 3 | 20 | 736 | 44.8 | 1.40 | — | — | [187] |
| 47 | 1083 | 45.7 | — | — | — | [188] | ||
| amorphous hydrous TiO2 solid spheres (AHTSS) | 0.5 | 52 | 1125 | 46 | 1.05 | — | — | [188] |
| amorphous hydrous TiO2 solid spheres (AHTSS) | 2 | 53 | 951 | 43.6 | 1.34 | — | — | [188] |
| urchin-like mesoporous TiO2 hollow spheres (UMTHS) | 0.5 | 52 | 827 | 43.3 | 1.52 | — | — | [188] |
| urchin-like mesoporous TiO2 hollow spheres (UMTHS-2) | 2 | 52 | 706 | 38.5 | 2.01 | — | — | [188] |
| 65 | 1592 | 39.7 | — | — | — | [189] | ||
| chitosan-modified molybdenum disulfide nanosheets (CS-MoS2) | 0.5 | 71 | 1243 | 35.9 | 1.54 | — | — | [189] |
| chitosan-modified molybdenum disulfide nanosheets (CS-MoS2) | 1 | 74 | 1107 | 28.6 | 2.27 | — | — | [189] |
| chitosan-modified molybdenum disulfide nanosheets (CS-MoS2) | 2 | 75 | 902 | 33.9 | 2.38 | — | — | [189] |
| molybdenum disulfide nanosheets (MoS2) | 2 | 72 | 1178 | 40.1 | 1.48 | — | — | [189] |
| 74 | 1915 | 108 | — | — | — | [148] | ||
| silica nanospheres (SiO2) | 1 | 74 | 1777 | 95.6 | 1.21 | — | — | [148] |
| 38 | 943 | 60.3 | — | — | — | [150] | ||
| carbon nanotube (CNT) | 1 | 26 | 673 | 53.8 | 1.07 | — | — | [150] |
| chemical treatment carbon nanotube (CCNT) | 1 | 32 | 837 | 57.4 | 0.99 | — | — | [150] |
| thermal treatment carbon nanotube (TCNT) | 1 | 25 | 585 | 56.6 | 1.13 | — | — | [150] |
| layered double hydroxide (LDH) | 5 | 35 | 578 | 58.4 | 1.55 | — | — | [150] |
| Hydrogenated fatty acid modified layered double hydroxide (OLDH) | 5 | 38 | 453 | 66.5 | 1.88 | — | — | [150] |
| Montmorillonite (MMT) | 5 | 38 | 717 | 58.6 | 1.35 | — | — | [150] |
| Quaternary ammonium salt modified montmorillonite (OMMT) | 5 | 33 | 823 | 61.7 | 0.97 | — | — | [150] |
| aluminium trihydroxide (ATH) | 5 | 35 | 617 | 59.2 | 1.43 | — | — | [150] |
| 65 | 993 | 141 | — | — | — | [190] | ||
| Expanded graphite (EG) | 5 | 68 | 1188 | 125 | 0.98 | — | — | [190] |
| Expanded graphite (EG) | 10 | 80 | 1487 | 113 | 1.02 | — | — | [190] |
| Expanded graphite (EG) | 15 | 102 | 1911 | 124 | 0.92 | — | — | [190] |
| Expanded graphite (EG) | 23 | 116 | 1992 | 102 | 1.23 | — | — | [190] |
| Expanded graphite (EG) | 50 | 132 | 1800 | 81 | 1.95 | — | — | [190] |
| 141 | 932 | 74.3 | — | — | — | [191,192] | ||
| Bentonite (BT) | 3 | 150 | 1094 | 74 | 0.91 | — | — | [191,192] |
| Bentonite (BT) | 5 | 158 | 1192 | 88.1 | 0.73 | — | — | [191,192] |
| 6-(4-butylphenyl)21,3,5-triazine-2,4-diamine modified bentonite (BFTDA-BT) | 3 | 140 | 966 | 74.1 | 0.96 | — | — | [191,192] |
| 6-(4-butylphenyl)21,3,5-triazine-2,4-diamine modified bentonite (BFTDA-BT) | 5 | 145 | 998 | 82.2 | 0.86 | — | — | [191,192] |
| 11-amino-N-(pyridine-2yl)undecanamide modified bentonite (APUA-BT) | 3 | 138 | 772 | 74.7 | 1.17 | — | — | [191,192] |
| 11-amino-N-(pyridine-2yl)undecanamide modified bentonite (APUA-BT) | 5 | 139 | 814 | 74.2 | 1.13 | — | — | [191,192] |
| 68 | 1730 | 113 | — | — | — | [193] | ||
| graphene nanosheets (GN) | 2 | 86 | 980 | 65.1 | 3.87 | — | — | [193] |
| Ni–Fe layered double hydroxide (Ni–Fe LDH) | 2 | 80 | 1070 | 58.9 | 3.65 | — | — | [193] |
| 49 | 1261 | 114 | — | — | — | [194] | ||
| octaammonium polyhedral oligomeric silsesquioxane-modified montmorillonite (OAPOSS-MMT) | 2 | 42 | 1207 | 103 | 0.99 | — | — | [194] |
| octaammonium polyhedral oligomeric silsesquioxane-modified montmorillonite (OAPOSS-MMT) | 4 | 48 | 1095 | 94 | 1.36 | — | — | [194] |
| octaammonium polyhedral oligomeric silsesquioxane-modified montmorillonite (OAPOSS-MMT) | 6 | 50 | 982 | 88 | 1.69 | — | — | [194] |
| 31 | 1933 | 146 | — | — | — | [195] | ||
| Sodium magadiite (Na-magadiite) | 3 | 39 | 1283 | 116 | 2.38 | — | — | [195] |
| Sodium magadiite reaction with silane coupling agent (S-Na-magadiite) | 3 | 38 | 1641 | 120 | 1.75 | — | — | [195] |
| protonated magadiite reaction with silane coupling agent (S-H-magadiite) | 3 | 38 | 1416 | 114 | 2.14 | — | — | [195] |
| organo-modified magadiite (OM-magadiite) | 3 | 29 | 1332 | 105 | 1.88 | — | — | [195] |
| silane grafting organo modified magadiite (S-OM-magadiite) | 3 | 34 | 1273 | 103 | 2.36 | — | — | [195] |
| — | 32 | 2572 | 184 | — | — | — | [154] | |
| tetrabromobisphenol-A (TBBA) | 17 | 17 | 1390 | 92 | 1.96 | — | — | [154] |
| 90 | 1653 | 130 | — | — | — | [196] | ||
| graphene sheet (GN) | 2 | 84 | 1156 | 108 | 1.60 | — | — | [196] |
| Ce-doped MnO2 (Ce–MnO2) | 2 | 79 | 920 | 96.7 | 2.11 | — | — | [196] |
| Ce-doped MnO2 decorated graphene sheets (Ce–MnO2–GN) | 2 | 100 | 765 | 83.8 | 3.72 | — | — | [196] |
| 89 | 1473 | 87.8 | — | — | — | [197] | ||
| mesoporous silica (m-SiO2) | 2 | 107 | 1191 | 96.5 | 1.35 | — | — | [197] |
| Co−Al layered double hydroxide (Co−Al LDH) | 2 | 103 | 1188 | 84.3 | 1.49 | — | — | [197] |
| mesoporous silica@Co−Al layered double hydroxide (m-SiO2@Co−Al LDH) | 2 | 110 | 894 | 56 | 3.19 | — | — | [197] |
| 65 | 1653 | 130 | — | — | — | [198] | ||
| Zinc sulfide (ZnS) | 2 | 88 | 1213 | 119 | 2.00 | — | — | [198] |
| graphene sheet (GN) | 2 | 70 | 1141 | 108 | 1.88 | — | — | [198] |
| Zinc sulfide decorated Graphene sheets (ZnS-GN) | 2 | 87 | 879 | 94.2 | 3.47 | — | — | [198] |
| 55 | 1298 | 97.6 | — | — | — | [199] | ||
| hydrated pre-treated sepiolite (sep idra) | 2 | 55 | 1370 | 101 | 0.91 | — | — | [199] |
| hydrated pre-treated sepiolite (sep idra) | 5 | 65 | 1157 | 99.5 | 1.30 | — | — | [199] |
| hydrated pre-treated sepiolite (sep idra) | 10 | 65 | 1072 | 95.7 | 1.45 | — | — | [199] |
| dehydrated pre-treated sepiolite (sep anidra) | 2 | 55 | 1129 | 97 | 1.157 | — | — | [199] |
| dehydrated pre-treated sepiolite (sep anidra) | 5 | 65 | 1114 | 107 | 1.26 | — | — | [199] |
| dehydrated pre-treated sepiolite (sep anidra) | 10 | 65 | 958 | 108 | 1.45 | — | — | [199] |
| 94 | 1097 | 119 | — | — | — | [158] | ||
| expandable graphite (EG) | 5 | 111 | 463 | 142 | 2.34 | — | — | [158] |
| 54 | 1327 | 99.1 | — | — | — | [159] | ||
| chitosan modified montmorillonite intercalation iron compounds (CTS-Fe-OMMT) | 3 | 55 | 1168 | 91.4 | 1.25 | — | — | [159] |
| cetyltrimethylammoniumbromide modified montmorillonite intercalation iron compounds (CTAB-Fe-OMMT) | 3 | 47 | 975 | 89.2 | 1.31 | — | — | [159] |
| 80.4 | 1111 | 140 | — | — | — | [200] | ||
| aminated multiwalled carbon nanotubes supplied by the Polish company (A-MWCNT(Polish)) | 0.05 | 72.8 | 1161 | 93.6 | 1.29 | — | — | [200] |
| aminated multiwalled carbon nanotubes supplied by the Polish company (A-MWCNT(Polish)) | 0.1 | 68.8 | 992 | 93.6 | 1.43 | — | — | [200] |
| aminated multiwalled carbon nanotubes supplied by the Polish company (A-MWCNT(Polish)) | 0.5 | 74 | 926 | 96.9 | 1.59 | — | — | [200] |
| aminated multiwalled carbon nanotubes supplied by the Polish company (A-MWCNT(Polish)) | 1 | 71.9 | 875 | 92.6 | 1.72 | — | — | [200] |
| aminated multiwalled carbon nanotubes supplied by the Polish company (A-MWCNT(Polish)) | 5 | 78.3 | 1141 | 98.9 | 1.34 | — | — | [200] |
| carboxylated multiwalled carbon nanotubes supplied by the Polish company (C-MWCNT(Polish)) | 0.05 | 78.7 | 1080 | 101 | 1.40 | — | — | [200] |
| carboxylated multiwalled carbon nanotubes supplied by the Polish company (C-MWCNT(Polish)) | 0.1 | 72.6 | 1250 | 100 | 1.12 | — | — | [200] |
| carboxylated multiwalled carbon nanotubes supplied by the Polish company (C-MWCNT(Polish)) | 0.5 | 80.2 | 1163 | 98.8 | 1.35 | — | — | [200] |
| carboxylated multiwalled carbon nanotubes supplied by the Polish company (C-MWCNT(Polish)) | 1 | 81.2 | 945 | 102 | 1.63 | — | — | [200] |
| carboxylated multiwalled carbon nanotubes supplied by the Belgian company (C-MWCNT(Belgian)) | 0.05 | 76.2 | 919 | 96.3 | 1.66 | — | — | [200] |
| carboxylated multiwalled carbon nanotubes supplied by the Belgian company (C-MWCNT(Belgian)) | 0.5 | 67.4 | 1110 | 99 | 1.19 | — | — | [200] |
| carboxyammonium multiwalled carbon nanotubes supplied by the Polish company (CA-MWCNT(Polish)) | 0.05 | 83.9 | 1240 | 104 | 1.26 | — | — | [200] |
| carboxyammonium multiwalled carbon nanotubes supplied by the Polish company (CA-MWCNT(Polish)) | 0.1 | 73.8 | 1162 | 99 | 1.24 | — | — | [200] |
| carboxyammonium multiwalled carbon nanotubes supplied by the Polish company (CA-MWCNT(Polish)) | 0.5 | 76 | 1095 | 99.5 | 1.35 | — | — | [200] |
| carboxyammonium multiwalled carbon nanotubes supplied by the Polish company (CA-MWCNT(Polish)) | 1 | 67.3 | 1192 | 97.8 | 1.12 | — | — | [200] |
| carboxyammonium multiwalled carbon nanotubes supplied by the Polish company (CA-MWCNT(Polish)) | 5 | 69.7 | 1198 | 100 | 1.12 | — | — | [200] |
| aminated multiwalled carbon nanotubes supplied by the Belgian company (A-MWCNT(Belgian)) | 0.05 | 77.4 | 1314 | 98.3 | 1.16 | — | — | [200] |
| aminated multiwalled carbon nanotubes supplied by the Belgian company (A-MWCNT(Belgian)) | 0.1 | 80.2 | 1225 | 98.6 | 1.28 | — | — | [200] |
| aminated multiwalled carbon nanotubes supplied by the Belgian company (A-MWCNT(Belgian)) | 0.5 | 56.6 | 1005 | 62.4 | 1.75 | — | — | [200] |
| 66 | 934 | 95 | — | — | — | [201] | ||
| graphene oxide (GNO) | 1 | 76 | 811 | 133 | 0.94 | — | — | [201] |
| 41 | 1222 | 159 | — | — | — | [160] | ||
| onium ion modified nanoclay (I.30E) | 3 | 32 | 1274 | 154 | 0.77 | — | — | [160] |
| 0 | 101 | 1348 | 87.1 | — | — | — | [202] | |
| molybdenum disulfide (MoS2) | 2 | 96 | 1076 | 75.7 | 1.37 | — | — | [202] |
| graphene (GN) | 2 | 92 | 965 | 70.1 | 1.58 | — | — | [202] |
| molybdenum disulfide modified graphene (MoS2-GN) | 2 | 90 | 730 | 65.1 | 2.20 | — | — | [202] |
| 0 | 47 | 1630 | 82.3 | — | — | — | [161] | |
| graphene oxide(GNO) | 1 | 41 | 1426 | 76.8 | 1.06 | — | — | [161] |
| epoxy resin modified with (3-isocyanatopropyl)-triethoxysilane | 0 | 93 | 1331 | 63.8 | — | — | — | [203] |
| hydroxylated hexagonal boron nitride (BNO) | 1 | 113 | 860 | 56.3 | 2.13 | — | — | [203] |
| hydroxylated hexagonal boron nitride (BNO) | 3 | 117 | 765 | 55.5 | 2.51 | — | — | [203] |
| — | 42 | 385 | 21.8 | — | 27.5 | — | [163,164] | |
| cellulosic fibre containing polysilicic acid (Vis) a | 4.7 | 41 | 329 | 19.4 | 1.28 | 28.1 | — | [163,164] |
| phenol–formaldehyde fibers (Ky) a | 4.7 | 51 | 367 | 28.8 | 0.96 | 27.7 | — | [163,164] |
| 44 | 818 | 28.8 | — | — | — | [204] | ||
| Nanoclay (clay) b | 1 | 32 | 558 | 26.4 | 1.16 | — | — | [204] |
| Nanoclay (clay) b | 3 | 32 | 570 | 25.5 | 1.18 | — | — | [204] |
| Nanoclay (clay) b | 5 | 32 | 533 | 24.8 | 1.29 | — | — | [204] |
| 28 | 349 | 20.4 | — | — | — | [150] | ||
| layered double hydroxide (LDH) c | 5 | 22 | 343 | 21.9 | 0.74 | — | — | [150] |
| Hydrogenated fatty acid modified layered double hydroxide (OLDH) c | 5 | 21 | 310 | 23 | 0.74 | — | — | [150] |
| carbon nanotube (CNT) c | 1 | 27 | 396 | 22.7 | 0.76 | — | — | [150] |
| chemical treatment carbon nanotube (CCNT) c | 1 | 26 | 411 | 21.7 | 0.74 | — | — | [150] |
| thermal treatment carbon nanotube (TCNT) c | 1 | 27 | 471 | 22.2 | 0.65 | — | — | [150] |
| aluminium trihydroxide (ATH) c | 5 | 22 | 417 | 22.6 | 0.59 | — | — | [150] |
| 0 | 33 | 520 | 29.4 | — | — | — | [205] | |
| magnesium hydroxide (Mg(OH)2) d | 1 | 28 | 518 | 37.4 | 0.67 | — | — | [205] |
| magnesium hydroxide (Mg(OH)2) d | 7.5 | 30 | 550 | 28.4 | 0.89 | — | — | [205] |
| magnesium hydroxide (Mg(OH)2) d | 15 | 30 | 392 | 31.2 | 1.13 | — | — | [205] |
| magnesium hydroxide (Mg(OH)2) d | 25 | 35 | 476 | 41.7 | 0.81 | — | — | [205] |
| aluminum hydroxide (Al(OH)3) d | 1 | 28 | 456 | 37.3 | 0.76 | — | — | [205] |
| aluminum hydroxide (Al(OH)3) d | 7.5 | 28 | 585 | 35.3 | 0.62 | — | — | [205] |
| aluminum hydroxide (Al(OH)3) d | 15 | 26 | 451 | 32.8 | 0.81 | — | — | [205] |
| aluminum hydroxide (Al(OH)3) d | 25 | 32 | 396 | 31.6 | 1.18 | — | — | [205] |
| Zinc borate (ZB) d | 1 | 26 | 572 | 35.9 | 0.58 | — | — | [205] |
| Zinc borate (ZB) d | 7.5 | 32 | 427 | 42.7 | 0.81 | — | — | [205] |
| Zinc borate (ZB) d | 15 | 27 | 458 | 36.3 | 0.75 | — | — | [205] |
| Zinc borate (ZB) d | 25 | 37 | 352 | 30.6 | 1.59 | — | — | [205] |
| 46 | 568 | 23.2 | — | — | — | [206] | ||
| Single-walled carbon nanotube Buckypaper (SWCNT-BP) e | 1.06 | 50 | 526 | 24.5 | 1.11 | — | — | [206] |
| multiwalled carbon nanotube Buckypaper (MWCNT-BP) e | 1.34 | 64 | 258 | 13.2 | 5.38 | — | — | [206] |
| carbon nanofiber (CNF) e | 1.57 | 59 | 508 | 24.8 | 1.34 | — | — | [206] |
| 0 | 39 | 456 | 38 | — | — | — | [167] | |
| cellulosic fibre containing polysilicic acid (Vis) f | 5 | 46 | 451 | 37.2 | 1.21 | — | — | [167] |
| cellulosic fibre containing polysilicic acid (Vis) f | 10 | 58 | 434 | 36.3 | 1.63 | — | — | [167] |
| cellulosic fibre containing polysilicic acid (Vis) f | 15 | 55 | 321 | 31.1 | 2.44 | — | — | [167] |
| 46 | 568 | 23.2 | — | — | — | [207] | ||
| Single-walled carbon nanotube Buckypaper (SWCNT-BP) g | 1.06 | 50 | 526 | 24.5 | 1.11 | — | — | [207] |
| multiwalled carbon nanotube Buckypaper (MWCNT-BP) g | 1.34 | 64 | 258 | 13.2 | 5.38 | — | — | [207] |
| 125 | 857 | 50 | — | — | — | [174] | ||
| Trisilanolisobutyl Polyhedral oligomeric silsesquioxane (T8POSS) h | 5 | 121 | 420 | 32 | 3.08 | — | — | [174] |
| triglycidyl isocyanurate (TGIC) h | 5 | 108 | 620 | 47 | 1.27 | — | — | [174] |
a Matrix: eight layers of woven E-glass reinforced film of multifunctional epoxy resin; b Matrix: six layers of biaxial E-glass fabric reinforced epoxy; c Matrix: carbon fiber reinforced epoxy resin; d Matrix: eight plies of carbon fiber reinforced system HexFlow RTM6 (matrix) and HexForce G0939 (fabric); e Matrix: six layers of IM-7 carbon fiber fabrics reinforced epoxy; f Matrix: eight layers of woven E-glass reinforced epoxy; g Matrix: six layers of IM-7 carbon fiber fabrics reinforced epoxy; h Matrix: eight layers of woven glass Fiber Reinforced epoxy.
From the comparison between Table 1 and Table 2, one can simply infer that the NP family is less effective in terms of the flame retardancy of the composite epoxy with respect to the P family of FR. The effect of the used NP-type FR on the flame retardancy performance of epoxy resins can be visually assessed in Figure 4. Moreover, detailed information about the type of NP additives is provided to the reader in the caption of Figure 4. The quality of epoxy composites containing NP additives suggests that even at high loading levels it is difficult to attain very high efficiencies. As an informative case, alumina Trihydrate (ATH,  ) has been used in a wide range of content in development of flame-retardant epoxy nanocomposites. It can be seen that at high loading rate (up to 30 wt.%), it gives the best results, Excellent in terms of FRI. It can be concluded that the NP class of additives are not individually responsible for high fire resistance of epoxy.
) has been used in a wide range of content in development of flame-retardant epoxy nanocomposites. It can be seen that at high loading rate (up to 30 wt.%), it gives the best results, Excellent in terms of FRI. It can be concluded that the NP class of additives are not individually responsible for high fire resistance of epoxy.
Figure 4.
Flame retardancy analysis of epoxy resins containing nonphosphorus flame retardants in terms of the FRI values as a function of NP type and content. Symbols are indicative of different types of NP type of FR used. Hollow symbols are indicative of fiber-incorporated composites with details earlier given in the bottom of Table 1 as notes a to h. Here:  3TT-3BA-20 [169],
3TT-3BA-20 [169],  GN-3 [28],
GN-3 [28],  MWCNT-0.8 [29],
MWCNT-0.8 [29],  OMMT-7 [30],
OMMT-7 [30],  OLDH-1, OLDH-5, OLDH-10 [31],
OLDH-1, OLDH-5, OLDH-10 [31],  MgAl-LDH-2, ZIF8-2, ZIF8@MgAl-LDH-2, ZIF67-2, ZIF67@MgAl-LDH-2 [170],
MgAl-LDH-2, ZIF8-2, ZIF8@MgAl-LDH-2, ZIF67-2, ZIF67@MgAl-LDH-2 [170],  TAT-20 [52],
TAT-20 [52],  TNB-1, TNB-5, TNB-10, TNB-15, TNB-20 [171],
TNB-1, TNB-5, TNB-10, TNB-15, TNB-20 [171],  Cu2O-21 [55],
Cu2O-21 [55],  MH-3, [56],
MH-3, [56],  TN-3.42 [63],
TN-3.42 [63],  EG-20 [66],
EG-20 [66],  TMT-8 [67],
TMT-8 [67],  TMT-7 [68],
TMT-7 [68],  OMMT-1 [77],
OMMT-1 [77],  TAIC-10 [78],
TAIC-10 [78],  TPT-14 [81],
TPT-14 [81],  HNT-5, HNT-10, HNT@PDA-5, HNT@PDA-10, HNT@PDA@Fe(OH)3-5, HNT@PDA@Fe(OH)3-10 [172],
HNT-5, HNT-10, HNT@PDA-5, HNT@PDA-10, HNT@PDA@Fe(OH)3-5, HNT@PDA@Fe(OH)3-10 [172],  MMT-6 [94],
MMT-6 [94],  OPS-5 [102],
OPS-5 [102],  OPS-4.1, PPSQ-4.1 [103],
OPS-4.1, PPSQ-4.1 [103],  OPS-4.1, OAPS-4.6 [104],
OPS-4.1, OAPS-4.6 [104],  OPS-4.1 [106],
OPS-4.1 [106],  ATH-40, C-40, U-40, BA-40, BO-40, MB-30, GB-30 [173],
ATH-40, C-40, U-40, BA-40, BO-40, MB-30, GB-30 [173],  ODPSS-5 [115],
ODPSS-5 [115],  Mg-Al LDH-4 [116],
Mg-Al LDH-4 [116],  T8POSS-10, TGIC-10 [174],
T8POSS-10, TGIC-10 [174],  RGO-1 [121],
RGO-1 [121],  HNT-2, LDH-2, LDH-4, LDH-6 [120],
HNT-2, LDH-2, LDH-4, LDH-6 [120],  AlO(OH)-30 [124],
AlO(OH)-30 [124],  ACS-2, ACS@SnO2-2, ACS@SnO2@NiO-2 [125],
ACS-2, ACS@SnO2-2, ACS@SnO2@NiO-2 [125],  ACS@SnO2@NiO-5 [125],
ACS@SnO2@NiO-5 [125],  OGPOSS-15 [129],
OGPOSS-15 [129],  EG-15 [33],
EG-15 [33],  CP-10, CP-15 [130],
CP-10, CP-15 [130],  CP-10, CP-15 [130],
CP-10, CP-15 [130],  CP-10, CP-15 [130],
CP-10, CP-15 [130],  SrSn(OH)6-3 [132],
SrSn(OH)6-3 [132],  SiO2-2, ZIF8-2, ZIF8@SiO2-2 [175],
SiO2-2, ZIF8-2, ZIF8@SiO2-2 [175],  MoS2-2, TNT-2, MoS2-TNT-1, MoS2-TNT-2, MoS2-TNT-3 [176],
MoS2-2, TNT-2, MoS2-TNT-1, MoS2-TNT-2, MoS2-TNT-3 [176],  Sep-2, Sep-4, Fe3o4–Sep-2, Fe3o4–Sep-4 [177],
Sep-2, Sep-4, Fe3o4–Sep-2, Fe3o4–Sep-4 [177],  GNO-1, GNO-3, GN-Cu-1, GN-Cu-3 [178],
GNO-1, GNO-3, GN-Cu-1, GN-Cu-3 [178],  AlO(OH)-20 [147],
AlO(OH)-20 [147],  AlO(OH)-20, SiO2-20 [127],
AlO(OH)-20, SiO2-20 [127],  α-MnO2-0.5, α-MnO2-1, α-MnO2-2, δ-MnO2-0.5, δ-MnO2-1, δ-MnO2-2 [179],
α-MnO2-0.5, α-MnO2-1, α-MnO2-2, δ-MnO2-0.5, δ-MnO2-1, δ-MnO2-2 [179],  MoS2-2 [140],
MoS2-2 [140],  AI-POSS-7.2, AI-POSS-21.8, AI-POSS-54 [180],
AI-POSS-7.2, AI-POSS-21.8, AI-POSS-54 [180],  EG-9, HNT-9 [181],
EG-9, HNT-9 [181],  BN 2 μm-45, BT 2 μm-45 [182],
BN 2 μm-45, BT 2 μm-45 [182],  MnO2-2, MnO2@ZHS-0.5, MnO2@ZHS-1, MnO2@ZHS-2 [183],
MnO2-2, MnO2@ZHS-0.5, MnO2@ZHS-1, MnO2@ZHS-2 [183],  ILFR-5, BN-5, ILFR-fBN-5 [184],
ILFR-5, BN-5, ILFR-fBN-5 [184],  SH-mSiO2-2 [143],
SH-mSiO2-2 [143],  SCF-0.5, SCF-0.7, SCF-1, SCF-1.5 [185],
SCF-0.5, SCF-0.7, SCF-1, SCF-1.5 [185],  HNT-20 [146],
HNT-20 [146],  m-Clay-2.5, d-Clay-2.5 [186],
m-Clay-2.5, d-Clay-2.5 [186],  LDH-3, β-FeOOH-3, LDH-β-FeOOH-3 [187],
LDH-3, β-FeOOH-3, LDH-β-FeOOH-3 [187],  AHTSS-0.5, AHTSS-2, UMTHS-0.5, UMTHS-2 [188],
AHTSS-0.5, AHTSS-2, UMTHS-0.5, UMTHS-2 [188],  CS-MoS2-0.5, CS-MoS2-1, CS-MoS2-2, MoS2-2 [189],
CS-MoS2-0.5, CS-MoS2-1, CS-MoS2-2, MoS2-2 [189],  SiO2-1 [148],
SiO2-1 [148],  CNT-1, CCNT-1, TCNT-1, LDH-5, OLDH-5, MMT-5, OMMT-5, ATH-5 [150],
CNT-1, CCNT-1, TCNT-1, LDH-5, OLDH-5, MMT-5, OMMT-5, ATH-5 [150],  EG-5, EG-10, EG-15, EG-23, EG-50 [190],
EG-5, EG-10, EG-15, EG-23, EG-50 [190],  BT-3, BT-5, BFTDA-BT-3, BFTDA-BT-5, APUA-BT-3, APUA-BT-5 [191,192],
BT-3, BT-5, BFTDA-BT-3, BFTDA-BT-5, APUA-BT-3, APUA-BT-5 [191,192],  GN-2, Ni–Fe LDH-2 [193],
GN-2, Ni–Fe LDH-2 [193],  OAPOSS-MMT-2, OAPOSS-MMT-4, OAPOSS-MMT-6 [194],
OAPOSS-MMT-2, OAPOSS-MMT-4, OAPOSS-MMT-6 [194],  Na-magadiite-3, S-Na-magadiite-3, S-H-magadiite-3, OM-magadiite-3, S-OM-magadiite-3 [195],
Na-magadiite-3, S-Na-magadiite-3, S-H-magadiite-3, OM-magadiite-3, S-OM-magadiite-3 [195],  TBBA-17 [154],
TBBA-17 [154],  GN-2, Ce–MnO2-2, Ce–MnO2–GN-2 [196],
GN-2, Ce–MnO2-2, Ce–MnO2–GN-2 [196],  m-SiO2-2, Co−Al LDH-2, m-SiO2@Co−Al LDH-2 [197],
m-SiO2-2, Co−Al LDH-2, m-SiO2@Co−Al LDH-2 [197],  ZnS-2, GN-2, ZnS-GN-2 [198],
ZnS-2, GN-2, ZnS-GN-2 [198],  sep idra-2, sep idra-5, sep idra-10, sep anidra-2, sep anidra-5, sep anidra-10 [199],
sep idra-2, sep idra-5, sep idra-10, sep anidra-2, sep anidra-5, sep anidra-10 [199],  EG-5 [158],
EG-5 [158],  CTS-Fe-OMMT-3, CTAB-Fe-OMMT-3 [159],
CTS-Fe-OMMT-3, CTAB-Fe-OMMT-3 [159],  A-MWCNT(Polish)-0.05, A-MWCNT(Polish)-0.1, A-MWCNT(Polish)-0.5, A-MWCNT(Polish)-1, A-MWCNT(Polish)-5, C-MWCNT(Polish)-0.05, C-MWCNT(Polish)-0.1, C-MWCNT(Polish)-0.5, C-MWCNT(Polish)-1, C-MWCNT(Belgian)-0.05, C-MWCNT(Belgian)-0.5, CA-MWCNT(Polish)-0.05, CA-MWCNT(Polish)-0.1, CA-MWCNT(Polish)-0.5, CA-MWCNT(Polish)-1, CA-MWCNT(Polish)-5, A-MWCNT(Belgian)-0.05, A-MWCNT(Belgian)-0.1, A-MWCNT(Belgian)-0.5 [200],
A-MWCNT(Polish)-0.05, A-MWCNT(Polish)-0.1, A-MWCNT(Polish)-0.5, A-MWCNT(Polish)-1, A-MWCNT(Polish)-5, C-MWCNT(Polish)-0.05, C-MWCNT(Polish)-0.1, C-MWCNT(Polish)-0.5, C-MWCNT(Polish)-1, C-MWCNT(Belgian)-0.05, C-MWCNT(Belgian)-0.5, CA-MWCNT(Polish)-0.05, CA-MWCNT(Polish)-0.1, CA-MWCNT(Polish)-0.5, CA-MWCNT(Polish)-1, CA-MWCNT(Polish)-5, A-MWCNT(Belgian)-0.05, A-MWCNT(Belgian)-0.1, A-MWCNT(Belgian)-0.5 [200],  GNO-1 [201],
GNO-1 [201],  I.30E-3 [160],
I.30E-3 [160],  MoS2-2,GN-2, MoS2-GN-2 [202],
MoS2-2,GN-2, MoS2-GN-2 [202],  GNO-1 [161],
GNO-1 [161],  BNO-1, BNO-3 [203],
BNO-1, BNO-3 [203],  Vis-4.7, Ky-4.7 [163,164],
Vis-4.7, Ky-4.7 [163,164],  clay-1, clay-3, clay-5 [204],
clay-1, clay-3, clay-5 [204],  LDH-5, OLDH-1, CNT-1, CCNT-1, TCNT-1, ATH-5 [150],
LDH-5, OLDH-1, CNT-1, CCNT-1, TCNT-1, ATH-5 [150],  Mg(OH)2-1, Mg(OH)2-7.5, Mg(OH)2-15, Mg(OH)2-25, Al(OH)3-1, Al(OH)3-7.5, Al(OH)3-15, Al(OH)3-25, ZB-1, ZB-7.5, ZB-15, ZB-25 [205],
Mg(OH)2-1, Mg(OH)2-7.5, Mg(OH)2-15, Mg(OH)2-25, Al(OH)3-1, Al(OH)3-7.5, Al(OH)3-15, Al(OH)3-25, ZB-1, ZB-7.5, ZB-15, ZB-25 [205],  SWCNT-BP-1.06, MWCNT-BP-1.34, CNF-1.57 [206],
SWCNT-BP-1.06, MWCNT-BP-1.34, CNF-1.57 [206],  Vis-5, Vis-10, Vis-15 [167],
Vis-5, Vis-10, Vis-15 [167],  SWCNT-BP-1.06, MWCNT-BP-1.34 [207],
SWCNT-BP-1.06, MWCNT-BP-1.34 [207],  T8POSS-5, TGIC-5 [174].
T8POSS-5, TGIC-5 [174].
A brief overview of the effect of the NP used as FR in epoxy composite preparation and on the flame retardancy performance of epoxy resins as a function of UL-94 results is given in Figure 5. Since data are limited and spread over the plot, there is no conclusion about the relationship between FRI (cone calorimetry) and UL-94 analysis to be highlighted. Nevertheless, all sorts of behavior can be seen in the plot, depending on the type and content of NP type of FRs. It is worthy of note that the NR category of UL-94 constitutes a high proportion of the results.
Figure 5.
Flame retardancy analysis of epoxy resins containing nonphosphorus flame retardants in terms of the FRI values as a function of UL-94 test results. Symbols are indicative of different types of NP type of FR used in this figure. Hollow symbols are indicative of fiber-incorporated composites with details given in the bottom of Table 2 as notes a to h. The vertical variation in each category, i.e., V-0, V-1, V-2, and NR, is schematically representative of the amount of additive used. For example, among two data distinguished by different symbols having the same or very close FRI values (horizontal quantity) in a given category (e.g., V-1), which have different vertical quantity both revealed V-1 behavior in UL-94 test, but the upper was an FR used in greater quantity in preparation of epoxy composites.
A brief overview of the effect of NP-type FR on the flame retardancy performance of epoxy resins as a function of LOI results is given in Figure 6. Surprisingly, the highest value obtained in LOI testing is located in Poor zone of FRI. On the other hand, Excellent flame retardancy seen at high FRI values has LOI of about 22%. From this perspective, it can be concluded that cone calorimetry is not monotonically representative of the character of FR when used in epoxy.
Figure 6.
Flame retardancy analysis of epoxy resins containing nonphosphorus flame retardants in terms of the FRI values as a function of LOI test results. Symbols are indicative of different types of NP flame retardant used. Hollow symbols are indicative of fiber-incorporated composites with details given in the bottom of Table 2 as notes a to h.
4. Epoxy Resins Containing Combinatorial Flame Retardant Systems
Assessing the flame retardancy performance of P- and NP-incorporated epoxy systems unraveled the inadequacy of using one FR additive alone when a high performance is required. The antagonism or synergism may be the result of using two or more FR systems in a given polymer matrix. In the case of epoxy, there have been some attempts towards combinatorial use of P and NP additives for the sake of higher performance. Table 3 summarizes pHRR, THR, TTI, and FRI values of epoxy/P/NP combinatorial flame-retardant systems. The percentage of incorporated FR as well as the results of LOI and UL-94 tests are also given.
Table 3.
The flame retardancy performance of epoxy containing combinatory flame retardants in terms of FRI (* the name and percentage of incorporated flame retardant is given after each epoxy resin). Notes a to i on the bottom of the table are representative of composite systems containing woven or nonwoven fibers.
| Epoxy Resins and Incorporated P/NP FR * | wt.% | TTI (s) | pHRR (kW·m−2) | THR (MJ·m−2) | FRI | LOI | UL94 | Ref. |
|---|---|---|---|---|---|---|---|---|
| 0 | 32 | 827 | 116 | — | 21.8 | NR | [28] | |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative/graphene nanosheet (DiDOPO/GN) | 3 | 51 | 374 | 99 | 4.13 | 32.2 | V-0 | [28] |
| 0 | 32 | 781 | 107 | — | 21.8 | NR | [29] | |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative/multiwalled carbon nanotube (DiDOPO/MWCNT) | 10.8 | 47 | 352 | 72 | 4.84 | 38.6 | V-0 | [29] |
| 0 | 32 | 781 | 107 | — | 21.8 | NR | [30] | |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative/Organically modified montmorillonite (DiDOPO/OMMT) | 7 | 46 | 396 | 95 | 3.19 | 32.2 | V-0 | [30] |
| 0 | 32 | 781 | 107 | — | 21.8 | NR | [31] | |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative/organomodified magnesium aluminium layered double hydroxide (DiDOPO/OLDH) | 1 | 41 | 437 | 142 | 1.73 | 25.2 | V-0 | [31] |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative/organomodified magnesium aluminium layered double hydroxide (DiDOPO/OLDH) | 5 | 44 | 420 | 120 | 2.28 | 27.8 | V-0 | [31] |
| phenethyl-bridged 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivative/organomodified magnesium aluminium layered double hydroxide (DiDOPO/OLDH) | 10 | 46 | 406 | 82 | 3.61 | 31.5 | V-0 | [31] |
| 0 | 30 | 1293 | 86.9 | — | 19.2 | HB | [208] | |
| IFR: Ammonium polyphosphate & pentaerythritol & melamine(APP & PER & MEL/5:3:2) (IFR) | 40 | 10 | 314 | 51 | 2.34 | 29.1 | V-0 | [208] |
| IFR: Ammonium polyphosphate & pentaerythritol & melamine(APP & PER & MEL/5:3:2)/Chicken eggshell (IFR/CES) | 40 | 22 | 266 | 45.9 | 6.75 | 29.6 | V-0 | [208] |
| IFR: Ammonium polyphosphate & pentaerythritol & melamine(APP & PER & MEL/5:3:2)/Chicken eggshell (IFR/CES) | 40 | 12 | 235 | 41.3 | 4.63 | 30.4 | V-0 | [208] |
| IFR: Ammonium polyphosphate & pentaerythritol & melamine(APP & PER & MEL/5:3:2)/Chicken eggshell (IFR/CES) | 40 | 23 | 181 | 33 | 14.4 | 31.5 | V-0 | [208] |
| IFR: Ammonium polyphosphate & pentaerythritol & melamine(APP & PER & MEL/5:3:2)/Chicken eggshell (IFR/CES) | 40 | 20 | 201 | 38 | 9.81 | 30.7 | V-0 | [208] |
| Waterborne EP resin | 0 | 25 | 344 | 18.3 | — | 19.3 | NR | [41] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide/phosphated K-carrageenan (DOPO/P-KC) | 30 | 13 | 176 | 13.3 | 1.39 | 27.1 | V-0 | [41] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide/phosphated K-carrageenan (DOPO/P-KC) | 30 | 15 | 131 | 12.3 | 2.34 | 28.2 | V-0 | [41] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide/phosphated K-carrageenan (DOPO/P-KC) | 30 | 20 | 197 | 14.5 | 1.76 | 25 | V-1 | [41] |
| 0 | 49 | 1247 | 49.8 | — | 22.5 | NR | [209] | |
| microencapsulated ammonium polyphosphate/pentaerythritol (mAPP/PER) | 10 | 27 | 961 | 39.9 | 0.89 | 29.9 | NR | [209] |
| microencapsulated ammonium polyphosphate/regenerated cotton cellulose (mAPP/RCC) | 10 | 30 | 1055 | 40.5 | 0.89 | 24.1 | NR | [209] |
| microencapsulated ammonium polyphosphate/oxidized regenerated cotton cellulose (mAPP/ORCC) | 10 | 29 | 554 | 20.9 | 3.17 | 29.5 | V-0 | [209] |
| 0 | 21 | 490 | 103 | — | 18.3 | NR | [210] | |
| 2,6,7-trioxa-1-phosphabicyclo-[2.2.2]-octane-4-methanol-trimellitic anhydride/melamine cyanurate (PEPA–TMA/MCA) | 18 | 17 | 378 | 90.4 | 1.20 | 28.9 | V-1 | [210] |
| 2,6,7-trioxa-1-phosphabicyclo-[2.2.2]-octane-4-methanol-trimellitic anhydride/melamine cyanurate (PEPA–TMA/MCA) | 24 | 15 | 221 | 57.6 | 2.84 | 29.8 | V-0 | [210] |
| 2,6,7-trioxa-1-phosphabicyclo-[2.2.2]-octane-4-methanol-trimellitic anhydride/melamine cyanurate (PEPA–TMA/MCA) | 30 | 12 | 296 | 74.8 | 1.31 | 29.1 | V-1 | [210] |
| 0 | 71 | 1146 | 56 | — | 21.2 | NR | [170] | |
| zeolitic imidazolate framework8/MgAl-layered double hydroxide (ZIF8/MgAl-LDH) | 2 | 64 | 742 | 42 | 1.86 | 24 | NR | [170] |
| zeolitic imidazolate framework67/MgAl-layered double hydroxide (ZIF67/MgAl-LDH) | 2 | 65 | 719 | 41 | 1.99 | 24.2 | NR | [170] |
| 0 | 61 | 1208 | 77.3 | — | 22.5 | NR | [52] | |
| triazine-based flame retardant/9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TAT/DOPO) | 20 | 44 | 849 | 74.3 | 1.07 | 29.5 | NR | [52] |
| triazine-based flame retardant/9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TAT/DOPO) | 20 | 44 | 682 | 64.5 | 1.53 | 34 | V-1 | [52] |
| triazine-based flame retardant/9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TAT/DOPO) | 20 | 47 | 558 | 56.3 | 2.29 | 36 | V-0 | [52] |
| triazine-based flame retardant/9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TAT/DOPO) | 20 | 41 | 500 | 48.5 | 2.59 | 38.6 | V-0 | [52] |
| triazine-based flame retardant/hexa-phenoxy-cyclotriphosphazene (TAT/HPCP) | 20 | 46 | 774 | 72.3 | 1.26 | 30.1 | NR | [52] |
| triazine-based flame retardant/hexa-phenoxy-cyclotriphosphazene (TAT/HPCP) | 20 | 43 | 598 | 59.3 | 1.86 | 33.5 | V-1 | [52] |
| triazine-based flame retardant/hexa-phenoxy-cyclotriphosphazene (TAT/HPCP) | 20 | 48 | 484 | 52.6 | 2.89 | 37.3 | V-0 | [52] |
| triazine-based flame retardant/hexa-phenoxy-cyclotriphosphazene (TAT/HPCP) | 20 | 48 | 437 | 47.8 | 3.52 | 39.6 | V-0 | [52] |
| 53 | 1121 | 102 | — | 20 | NR | [55] | ||
| ethanediamine-modified ammonium polyphosphate/Cuprous oxide (EDA-APP/Cu2O) | 21 | 62 | 364 | 64 | 5.74 | 33.5 | V-0 | [55] |
| 45 | 1091 | 83 | — | 22.8 | NR | [56] | ||
| hexakis(4-boronic acid-phenoxy)-cyclophosphazene/magnesium hydroxide (CP-6B/MH) | 3.5 | 49 | 535 | 67 | 2.75 | 31.9 | V-0 | [56] |
| 93.6 | 851 | 91.7 | — | 19.7 | NR | [211] | ||
| IFR:ammonium polyphosphate & pentaerythritol(APP & PER/3:1) (IFR) | 20 | 42.8 | 266 | 89.7 | 1.50 | 27.3 | V-1 | [211] |
| IFR:ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/Hollow glass microsphere (IFR/HGM) | 20 | 55.4 | 246 | 59.7 | 3.15 | 28.8 | V-1 | [211] |
| IFR:ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/Hollow glass microsphere (IFR/HGM) | 20 | 50.6 | 210 | 59.6 | 3.36 | 29.1 | V-1 | [211] |
| IFR:ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/Hollow glass microsphere (IFR/HGM) | 20 | 74.9 | 178 | 44.8 | 7.85 | 34.7 | V-0 | [211] |
| IFR:ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/Hollow glass microsphere (IFR/HGM) | 20 | 51.2 | 215 | 54.3 | 3.67 | 31.4 | V-0 | [211] |
| 43 | 469 | 66.2 | — | 24.7 | NR | [58] | ||
| Ammonium polyphosphate/poly(4,40-diamino diphenyl sulfone 2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane-4-methanol-substituted phosphoramide) (APP/PSA) | 10 | 34 | 132 | 21.3 | 8.73 | 32 | V-0 | [58] |
| 29 | 1340 | 36.3 | — | 22.5 | NR | [212] | ||
| microencapsulated ammonium polyphosphate/pentaerythritol (MFAPP/PER) | 12.5 | 24 | 422 | 20.6 | 4.63 | 24.9 | NR | [212] |
| microencapsulated ammonium polyphosphate/corn starch (MFAPP/ST) | 12.5 | 24 | 457 | 15.2 | 5.80 | 30.1 | V-0 | [212] |
| microencapsulated ammonium polyphosphate/oxidized corn starch (MFAPP/OST) | 12.5 | 22 | 400 | 13.4 | 6.88 | 29.5 | V-0 | [212] |
| 58 | 1208 | 80.6 | — | 22.5 | NR | [66] | ||
| expandable graphite/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (EG/DOPO) | 20 | 48 | 236 | 48.4 | 7.05 | 35 | V-1 | [66] |
| expandable graphite/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (EG/DOPO) | 20 | 48 | 296 | 48.8 | 5.58 | 38 | V-0 | [66] |
| expandable graphite/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (EG/DOPO) | 20 | 48 | 405 | 50 | 3.98 | 42 | V-0 | [66] |
| expandable graphite/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (EG/DOPO) | 20 | 48 | 442 | 51.4 | 3.55 | 41.5 | V-0 | [66] |
| expandable graphite/hexa-phenoxy-cyclotriphosphazene (EG/HPCP) | 20 | 48 | 259 | 49.7 | 6.26 | 33.5 | V-1 | [66] |
| expandable graphite/hexa-phenoxy-cyclotriphosphazene (EG/HPCP) | 20 | 48 | 340 | 48 | 4.94 | 36 | V-0 | [66] |
| expandable graphite/hexa-phenoxy-cyclotriphosphazene (EG/HPCP) | 20 | 48 | 809 | 50.6 | 1.97 | 40.5 | V-0 | [66] |
| expandable graphite/hexa-phenoxy-cyclotriphosphazene (EG/HPCP) | 20 | 48 | 760 | 42.2 | 2.51 | 39 | V-0 | [66] |
| 57 | 1557 | 94.5 | — | 24.5 | NR | [67] | ||
| nucleophilic substitution reaction between N-(4-hydroxyphenyl) maleimide & cyanuric chloride/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TMT/DOPO) | 11 | 45 | 1210 | 74.7 | 1.29 | 34 | V-1 | [67] |
| nucleophilic substitution reaction between N-(4-hydroxyphenyl) maleimide & cyanuric chloride/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TMT/DOPO) | 12.3 | 46 | 1085 | 70.3 | 1.56 | 36.5 | V-0 | [67] |
| nucleophilic substitution reaction between N-(4-hydroxyphenyl) maleimide & cyanuric chloride/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TMT/DOPO) | 13.7 | 47 | 1105 | 70.8 | 1.55 | 38 | V-0 | [67] |
| nucleophilic substitution reaction between N-(4-hydroxyphenyl) maleimide & cyanuric chloride/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (TMT/DOPO) | 15 | 44 | 980 | 61 | 1.90 | 40.3 | V-0 | [67] |
| 56 | 1420 | 116 | — | 26.2 | NR | [75] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide/aluminum poly-hexamethylenephosphinate (DOPO/APHP) | 6 | 50 | 539 | 63 | 4.33 | 39.3 | V-1 | [75] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide/aluminum poly-hexamethylenephosphinate (DOPO/APHP) | 6 | 46 | 510 | 58 | 4.57 | 39.5 | V-0 | [75] |
| 56 | 1420 | 140 | — | 26 | NR | [77] | ||
| reaction between triallyl isocyanurate & 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide/organically modified montmorillonite (TAD/OMMT) | 5 | 41 | 961 | 108 | 1.40 | 36.9 | V-0 | [77] |
| 82 | 685 | 145 | — | 21.3 | NR | [213] | ||
| flame retardant containing phosphorus & 4-tert-butylcalix[4]arene/ammonium polyphosphate (FR/APP) | 30 | 92 | 332 | 108 | 3.11 | 27.4 | V-1 | [213] |
| flame retardant containing phosphorus & 4-tert-butylcalix[4]arene/ammonium polyphosphate (FR/APP) | 30 | 91 | 361 | 82 | 3.73 | 28.6 | V-1 | [213] |
| flame retardant containing phosphorus & 4-tert-butylcalix[4]arene/ammonium polyphosphate (FR/APP) | 30 | 115 | 229 | 74 | 8.22 | 29.3 | V-0 | [213] |
| flame retardant containing phosphorus & 4-tert-butylcalix[4]arene/ammonium polyphosphate (FR/APP) | 30 | 100 | 203 | 74 | 8.07 | 30.8 | V-0 | [213] |
| 62 | 840 | 84 | — | 23 | V-1 | [89] | ||
| amine-terminated cyclophosphazene/3-aminopropyltrimethoxy silane-functionalized rice husk ash (ATCP/FRHA) | 16 | 56 | 542 | 56 | 2.10 | 44 | V-0 | [89] |
| amine-terminated cyclophosphazene/3-aminopropyltrimethoxy silane-functionalized rice husk ash (ATCP/FRHA) | 18 | 69 | 427 | 42 | 4.38 | 51 | V-0 | [89] |
| amine-terminated cyclophosphazene/3-aminopropyltrimethoxy silane-functionalized rice husk ash (ATCP/FRHA) | 20 | 77 | 340 | 30 | 8.59 | 62 | V-0 | [89] |
| 57 | 713 | 64 | — | — | — | [90] | ||
| amine-terminated cyclophosphazene/3-aminopropyltrimethoxy silane-functionalized rice husk ash (ATCP/FRHA) | 16 | 48 | 435 | 51 | 1.73 | 39 | V-0 | [90] |
| amine-terminated cyclophosphazene/3-aminopropyltrimethoxy silane-functionalized rice husk ash (ATCP/FRHA) | 18 | 45 | 374 | 43 | 2.24 | 45 | V-0 | [90] |
| amine-terminated cyclophosphazene/3-aminopropyltrimethoxy silane-functionalized rice husk ash (ATCP/FRHA) | 20 | 40 | 289 | 31 | 3.57 | 51 | V-0 | [90] |
| 50 | 860 | 112 | — | 23 | NR | [93] | ||
| Ammonium polyphosphate/montmorillonite (APP/MMT) | 10 | 53 | 524 | 50 | 3.90 | 28 | V-0 | [93] |
| 50 | 860 | 133 | — | 23 | NR | [94] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide/Montmorillonite (DOPO/MMT) | 6 | 52 | 473 | 76 | 3.31 | 33 | V-1 | [94] |
| 65 | 966 | 96 | — | 22.5 | NR | [95] | ||
| bisphenol-A bis(diphenyl phosphate)/aluminum poly-hexamethylenephosphinate (BDP/PHP) | 10 | 51 | 672 | 86 | 1.26 | 35 | V-0 | [95] |
| 45 | 855 | 112 | — | 3 | 3.2 | [102] | ||
| octaphenyl polyhedral oligomeric silsesquioxane/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (OPS/DOPO) | 5 | 54 | 603 | 89 | 2.14 | 29 | V-1 | [102] |
| 45 | 855 | 112 | — | 25 | NR | [103] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide/Octaphenyl silsesquioxane (DOPO/OPS) | 5.2 | 51 | 557 | 95 | 2.05 | 31.1 | V-0 | [103] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide/Polyphenyl silsesquioxane (DOPO/PPSQ) | 5.2 | 49 | 895 | 100 | 1.17 | 31.2 | NR | [103] |
| 45 | 855 | 112 | — | 25 | NR | [104] | ||
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide/Octaphenyl silsesquioxane (DOPO/OPS) | 5.2 | 51 | 557 | 95 | 2.05 | 31.1 | V-0 | [104] |
| 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide/Octaaminophenylsilsesquioxane (DOPO/OAPS) | 5.4 | 53 | 645 | 102 | 1.71 | 33.8 | V-1 | [104] |
| 50 | 860 | 112 | — | 3 | 3.2 | [105] | ||
| octaphenyl polyhedral oligomeric silsesquioxane/ 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (OPS/DOPO) | 5 | 58 | 540 | 82 | 2.52 | 31 | V-0 | [105] |
| 50 | 860 | 112 | — | — | — | [106] | ||
| Octaphenyl polyhedral oligomeric silsesquioxane/1-oxo-4-hydroxymethyl-2,6,7-trioxa-l-phosphabicyclo[2.2.2] octane (OPS/PEPA) | 4.7 | 52 | 524 | 84 | 2.28 | 25.5 | NR | [106] |
| Octaphenyl polyhedral oligomeric silsesquioxane/Ammonium polyphosphate (OPS/APP) | 3.5 | 63 | 584 | 101 | 2.06 | 24.6 | NR | [106] |
| Octaphenyl polyhedral oligomeric silsesquioxane/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (OPS/DOPO) | 5.2 | 55 | 548 | 83 | 2.33 | 30.8 | V-1 | [106] |
| 64 | 821 | 94 | — | 23.2 | NR | [115] | ||
| polyhedral oligomeric octadiphenylsulfonylsilsesquioxane/9, 10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (ODPSS/DOPO) | 5 | 57 | 438 | 69 | 2.27 | 29.8 | V-0 | [115] |
| 20 | 662 | 88.6 | — | 20.5 | NR | [214] | ||
| bis(diphenyl phosphate) oligomer/polyphosphoric acid (BBO/PPA) | 20 | 30 | 224 | 63.2 | 6.21 | 26 | V-0 | [214] |
| 108 | 1634 | 78 | — | 19.8 | NR | [174] | ||
| Trisilanolisobutyl Polyhedral oligomeric silsesquioxane/triglycidyl isocyanurate (T8POSS/TGIC) | 10 | 88 | 944 | 58 | 1.90 | 20.9 | NR | [174] |
| 64 | 939 | 179 | — | 19.6 | NR | [117] | ||
| ammonium polyphosphate/metal compounds (APP/CoSA) | 5 | 65 | 310 | 95 | 5.80 | 29.4 | V-0 | [117] |
| 53 | 1262 | 84.7 | — | 25 | NR | [118] | ||
| cardanol derived benzoxazine monomer/boron-doped graphene (CBz/BGN) | 10 | 49 | 870 | 75.9 | 1.50 | 30 | V-0 | [118] |
| cardanol derived benzoxazine monomer/boron-doped graphene (CBz/BGN) | 15 | 52 | 650 | 74.4 | 2.17 | 33 | V-0 | [118] |
| cardanol derived benzoxazine monomer/boron-doped graphene (CBz/BGN) | 20 | 56 | 716 | 78.7 | 2.00 | 33 | V-0 | [118] |
| 21 | 1910 | 84.4 | — | 22.1 | NR | [120] | ||
| melamine coated ammonium polyphosphate/layered double hydroxide (Mel-APP/LDH) | 20 | 20 | 240 | 30.3 | 21.10 | 33.2 | V-0 | [120] |
| melamine coated ammonium polyphosphate/halloysite nano-tube (Mel-APP/HNT) | 20 | 20 | 246 | 26.2 | 23.90 | 32.7 | V-0 | [120] |
| epoxy novolac resin | 0 | 51 | 682 | 110 | — | — | NR | [124] |
| oligo[DOPAc-2-tris(acryloyloxy)ethyl isocyanurate] /melamine polyphosphate (oDOPI/MPP) | 32.8 | 48 | 341 | 85 | 2.44 | — | V-0 | [124] |
| boehmite/oligo[DOPAc-2-tris(acryloyloxy)ethyl isocyanurate] (AlO(OH)/oDOPI) | 41.1 | 71 | 319 | 74 | 4.42 | — | V-0 | [124] |
| melamine polyphosphate/phosphazene (MPP/PZ) | 16.5 | 50 | 310 | 82 | 2.89 | — | V-0 | [124] |
| boehmite/phosphazene (AlO(OH)/PZ) | 33.1 | 66 | 435 | 79 | 2.83 | — | V-0 | [124] |
| 0 | 50 | 986 | 91 | — | — | NR | [125] | |
| aluminum hypophosphite/activated carbon spheres@SnO2@NiO hybrid (AHP/ACS@SnO2@NiO) | 5 | 54 | 714 | 76 | 1.78 | — | V-0 | [125] |
| 23 | 1910 | 61 | — | — | NR | [126] | ||
| Melamine coated ammonium polyphosphate/Talc (Mel-APP/Talc) | 29.7 | 28 | 357 | 24 | 16.60 | — | V-0 | [126] |
| 54 | 1068 | 75.8 | — | — | HB | [127] | ||
| melamine polyphosphate/melamine poly(zinc phosphate) (MPP/MPZnP) | 20 | 38 | 207 | 51.1 | 5.39 | — | V-1 | [127] |
| diethyl aluminum phosphinate/melamine poly(zinc phosphate) (AlPi-Et/MPZnP) | 20 | 43 | 405 | 51.2 | 3.11 | — | HB | [127] |
| 6H-dibenz[c,e][1,2] oxaphosphorin-6-propanoic acid, butyl ester, 6-oxide/melamine poly(zinc phosphate) (DOPAc-Bu/MPZnP) | 20 | 42 | 329 | 57.6 | 3.32 | — | V-1 | [127] |
| boehmite/melamine poly(zinc phosphate) (AlO(OH)/MPZnP) | 20 | 43 | 438 | 57.2 | 2.57 | — | HB | [127] |
| amorphous silicon dioxide/melamine poly(zinc phosphate) (MPZnP/SiO2) | 20 | 37 | 525 | 62.4 | 1.69 | — | HB | [127] |
| melamine polyphosphate/melamine poly(zinc phosphate) (MPP/MPZnP) | 20 | 41 | 211 | 32.5 | 8.96 | — | V-0 | [127] |
| diethyl aluminum phosphinate/melamine poly(zinc phosphate) (AlPi-Et/MPZnP) | 20 | 41 | 435 | 53.8 | 2.63 | — | V-1 | [127] |
| 6H-dibenz[c,e][1,2] oxaphosphorin-6-propanoic acid, butyl ester, 6-oxide/melamine poly(zinc phosphate) (DOPAc-Bu/MPZnP) | 20 | 41 | 412 | 52.1 | 2.86 | — | HB | [127] |
| boehmite/melamine poly(zinc phosphate) (AlO(OH)/MPZnP) | 20 | 43 | 575 | 57.9 | 1.94 | — | HB | [127] |
| amorphous silicon dioxide/melamine poly(zinc phosphate) (SiO2/MPZnP) | 20 | 37 | 681 | 65.6 | 1.24 | — | HB | [127] |
| 63 | 1321 | 157 | — | — | NR | [129] | ||
| hexaphenoxycyclotriphosphazene/octapropylglycidylether polyhedral oligomeric silsesquioxane (HPCTP/OGPOSS) | 15 | 58 | 707 | 123 | 2.20 | — | V-0 | [129] |
| hexaphenoxycyclotriphosphazene/octapropylglycidylether polyhedral oligomeric silsesquioxane (HPCTP/OGPOSS) | 15 | 56 | 581 | 110 | 2.88 | — | V-0 | [129] |
| hexaphenoxycyclotriphosphazene/octapropylglycidylether polyhedral oligomeric silsesquioxane (HPCTP/OGPOSS) | 15 | 56 | 560 | 105 | 3.14 | — | V-0 | [129] |
| 100 | 733 | 141 | — | 21 | HB | [130] | ||
| Tetraphenylphosphonium modified montmorillonite/Silicate glass (CP/TPP-MMT) | 15 | 101 | 353 | 131 | 2.26 | 25 | HB | [130] |
| 47 | 891 | 151 | — | 21 | HB | [130] | ||
| Tetraphenylphosphonium modified montmorillonite/Silicate glass (CP/TPP-MMT) | 15 | 48 | 474 | 130 | 2.23 | 25 | HB | [130] |
| 22 | 1196 | 147 | — | 21 | HB | [130] | ||
| Tetraphenylphosphonium modified montmorillonite/Silicate glass (CP/TPP-MMT) | 15 | 22 | 617 | 130 | 2.19 | 25 | HB | [130] |
| 0 | 69 | 1150 | 54.7 | — | 22 | — | [176] | |
| molybdenum disulfide/titanium dioxide nanotube (MoS2/TNT) | 2 | 56 | 742 | 38.6 | 1.78 | 26 | — | [176] |
| 24 | 1002 | 104 | — | 18 | — | [215] | ||
| Ammonium polyphosphate/Pentaerythritol modified halloysite tube (APP/PER-HNT) | 25 | 33 | 562 | 51.8 | 4.93 | 24.8 | — | [215] |
| 54 | 1068 | 76 | — | 21 | — | [147] | ||
| melamine poly(magnesium phosphate)/aluminium diethylphosphinate (S600/AlPi) | 20 | 44 | 479 | 46 | 3.00 | 30.4 | — | [147] |
| melamine poly(magnesium phosphate)/boehmite (S600/AlO(OH)) | 20 | 38 | 437 | 55 | 2.38 | 28.9 | — | [147] |
| melamine poly(magnesium phosphate)/melamine polyphosphate (S600/MPP) | 20 | 39 | 208 | 54 | 5.22 | 28.4 | — | [147] |
| 86 | 1650 | 213 | — | 20.2 | — | [136] | ||
| 3-((Methoxydiphenylsilyl) oxy)-9-methyl-2, 4, 8, 10-tetraoxa-3, 9-diphosphaspiro [5. 5] undecane 3, 9-dioxide/Mono (4, 6-diamino-1, 3, 5-triazin-2-aminium) (2, 4, 8, 10-tetraoxa-3, 9-diphosphaspiro [5. 5] undecane-3, 9-bis (olate) 3, 9-dioxide) (SDPS/SPDM) | 10.4 | 62 | 1122 | 207 | 1.09 | 30.8 | — | [136] |
| 0 | 70 | 1491 | 81 | — | 19 | NR | [47] | |
| aluminum diethyl phosphinate/Melamine polyphosphate (AlPi/MPP) | 7 | 61 | 505 | 48 | 4.34 | — | — | [47] |
| aluminum diethyl phosphinate/Melamine polyphosphate/aluminum oxide (AlPi/MPP/Al2O3) | 7 | 66 | 533 | 58 | 3.68 | — | — | [47] |
| 0 | 25 | 1113 | 223 | — | — | — | [139] | |
| ammonium polyphosphate/char sulfonic acid (APP/CSA) | 10 | 24 | 672 | 127 | 2.78 | — | — | [139] |
| ammonium polyphosphate/char sulfonic acid (APP/CSA) | 10 | 23 | 665 | 107 | 3.21 | — | — | [139] |
| ammonium polyphosphate/char sulfonic acid (APP/CSA) | 10 | 27 | 698 | 137 | 2.81 | — | — | [139] |
| 0 | 117 | 1184 | 95.3 | — | — | — | [182] | |
| Boron Nitride with D50 = 12 μm/Boron Nitride with D50 = 2 μm (BN 12 μm/BN 2 μm) | 45 | 164 | 918 | 75.7 | 2.28 | — | — | [182] |
| Boron Nitride with D50 = 12 μm/Boehmite with D50 = 2 μm (BN 12 μm/BT 2 μm) | 45 | 163 | 729 | 65.1 | 3.31 | — | — | [182] |
| 60 | 923 | 124 | — | — | — | [216] | ||
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1) (IFR) | 30 | 64 | 285 | 64.1 | 6.69 | — | — | [216] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/ferric phosphate (IFR/FeP) | 30 | 46 | 170 | 56 | 9.23 | — | — | [216] |
| IFR:ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/ferric phosphate (IFR/FeP) | 30 | 42 | 185 | 49.3 | 8.80 | — | — | [216] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/ferric phosphate (IFR/FeP) | 30 | 39 | 167 | 39.7 | 11.20 | — | — | [216] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/ferric phosphate (IFR/FeP) | 30 | 41 | 180 | 44.6 | 9.76 | — | — | [216] |
| 62 | 913 | 155 | — | — | — | [217] | ||
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1) (IFR) | 30 | 49 | 260 | 56 | 7.68 | — | — | [217] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/ferrite yellow: goethite (IFR/αFeOOH) | 30 | 46 | 172 | 47 | 13.00 | — | — | [217] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/ferrite yellow: goethite (IFR/αFeOOH) | 30 | 53 | 166 | 36 | 20.20 | — | — | [217] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/ferrite yellow: goethite (IFR/αFeOOH) | 30 | 50 | 196 | 40 | 14.60 | — | — | [217] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/ferrite yellow: goethite (IFR/αFeOOH) | 30 | 52 | 217 | 74 | 7.39 | — | — | [217] |
| 60 | 923 | 124 | — | — | — | [218] | ||
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1) (IFR) | 30 | 49 | 285 | 64.1 | 5.12 | — | — | [218] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/iron oxide brown (IFR/iron oxide brown) | 30 | 34 | 167 | 38.3 | 10.20 | — | — | [218] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/iron oxide brown (IFR/iron oxide brown) | 30 | 45 | 126 | 31 | 22.00 | — | — | [218] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/iron oxide brown (IFR/iron oxide brown) | 30 | 48 | 124 | 29.3 | 25.20 | — | — | [218] |
| IFR: ammonium polyphosphate & pentaerythritol(APP & PER/3:1)/iron oxide brown (IFR/iron oxide brown) | 30 | 53 | 163 | 43.2 | 14.40 | — | — | [218] |
| 68 | 1730 | 113 | — | — | — | [193] | ||
| Ni–Fe layered double hydroxide/graphene nanosheets (Ni–Fe LDH/GN) | 2 | 89 | 678 | 44.2 | 8.55 | — | — | [193] |
| Epoxy acrylic | 32 | 223 | 30.8 | — | — | — | [152] | |
| ammonium polyphosphate/pentaerythritol (APP/PER) | 30 | 61 | 188 | 25.2 | 2.77 | — | — | [152] |
| 70 | 934 | 124 | — | — | — | [219] | ||
| IFR: ammonium polyphosphate & pentaerythrite(APP & PER/3:1) (IFR) | 30 | 70 | 282 | 64 | 6.42 | — | — | [219] |
| IFR: ammonium polyphosphate & pentaerythrite(APP & PER/3:1)/organic-modified iron–montmorillonite (IFR/Fe-OMMT) | 30 | 20 | 243 | 70 | 1.95 | — | — | [219] |
| IFR: ammonium polyphosphate & pentaerythrite(APP & PER/3:1)/organic-modified iron–montmorillonite (IFR/Fe-OMMT) | 30 | 15 | 153 | 54 | 3.00 | — | — | [219] |
| IFR: ammonium polyphosphate & pentaerythrite(APP & PER/3:1)/organic-modified iron–montmorillonite (IFR/Fe-OMMT) | 30 | 30 | 154 | 68 | 4.74 | — | — | [219] |
| IFR: ammonium polyphosphate & pentaerythrite(APP & PER/3:1)/organic-modified iron–montmorillonite (IFR/Fe-OMMT) | 30 | 15 | 194 | 65 | 1.97 | — | — | [219] |
| 41 | 1222 | 159 | — | — | — | [160] | ||
| ammonium polyphosphate/onium ion modified nanoclay (APP/I.30E) | 23 | 149 | 363 | 92 | 21.10 | — | — | [160] |
| 0 | 21 | 454 | 36.2 | — | 22.1 | NR | [120] | |
| melamine coated ammonium polyphosphate/layered double hydroxide (Mel-APP/LDH) a | 9.55 | 21 | 259 | 22.6 | 2.81 | 31.7 | V-1 | [120] |
| melamine coated ammonium polyphosphate/halloysite nano-tube (Mel-APP/HNT) a | 9.61 | 22 | 262 | 18.4 | 3.57 | 31.4 | V-1 | [120] |
| 24 | 451 | 37 | — | — | NR | [126] | ||
| Melamine coated ammonium polyphosphate/Talc (Mel-APP/Talc) b | 14.8 | 21 | 169 | 16 | 5.40 | — | NR | [126] |
| — | 42 | 385 | 21.8 | — | 27.5 | — | [163,164] | |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) c | 10 | 38 | 262 | 17.9 | 1.62 | 36.2 | — | [163,164] |
| IFR contains melamine phosphate/phenol–formaldehyde fibers (Ky/IFR) c | 10 | 55 | 354 | 23.2 | 1.34 | 30.2 | — | [163,164] |
| 0 | 33 | 520 | 29.4 | — | — | — | [205] | |
| Zinc borate/magnesium hydroxide (ZB/Mg(OH)2) d | 1 | 32 | 552 | 41.8 | 0.64 | — | — | [205] |
| Zinc borate/magnesium hydroxide (ZB/Mg(OH)2) d | 7.5 | 37 | 483 | 37.4 | 0.95 | — | — | [205] |
| Zinc borate/magnesium hydroxide (ZB/Mg(OH)2) d | 15 | 38 | 439 | 35.4 | 1.13 | — | — | [205] |
| Zinc borate/magnesium hydroxide (ZB/Mg(OH)2) d | 25 | 40 | 380 | 27.2 | 1.79 | — | — | [205] |
| Zinc borate/aluminum hydroxide (ZB/Al(OH)3) d | 1 | 33 | 525 | 35 | 0.83 | — | — | [205] |
| Zinc borate/aluminum hydroxide (ZB/Al(OH)3) d | 7.5 | 36 | 480 | 37.4 | 0.93 | — | — | [205] |
| Zinc borate/aluminum hydroxide (ZB/Al(OH)3) d | 15 | 27 | 439 | 37.2 | 0.77 | — | — | [205] |
| Zinc borate/aluminum hydroxide (ZB/Al(OH)3) d | 25 | 30 | 409 | 37.7 | 0.90 | — | — | [205] |
| 44 | 853 | 51.9 | — | — | — | [166] | ||
| melamine phosphate/Graphene (MP/GN) e | 5 | 36 | 483 | 47.9 | 1.57 | — | — | [166] |
| 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide/Graphene (DOPO/GN) e | 5 | 32 | 538 | 36.5 | 1.64 | — | — | [166] |
| 119 | 294 | 114 | — | — | — | [220] | ||
| organic phosphinate/Zinc borate (PFR/ZB) f | 30 | 116 | 209 | 123 | 1.27 | — | — | [220] |
| 0 | 39 | 456 | 38 | — | — | — | [167] | |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 5 | 49 | 391 | 20.3 | 2.74 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 7.5 | 45 | 433 | 34 | 1.36 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 10 | 52 | 488 | 33.2 | 1.43 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 12.5 | 54 | 488 | 31.3 | 1.57 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 15 | 66 | 451 | 28.4 | 2.29 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 7.5 | 39 | 379 | 32.2 | 1.42 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 10 | 80 | 408 | 25.5 | 3.42 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 12.5 | 59 | 379 | 24.5 | 2.82 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 15 | 77 | 434 | 22.9 | 3.44 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 10 | 76 | 346 | 24.3 | 4.02 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 12.5 | 89 | 342 | 23 | 5.03 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 15 | 90 | 442 | 20.6 | 4.39 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 12.5 | 67 | 277 | 22.8 | 4.71 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 15 | 89 | 339 | 20.3 | 5.75 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 15 | 97 | 226 | 15.9 | 12.00 | — | — | [167] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) g | 17.5 | 100 | 236 | 23.4 | 8.05 | — | — | [167] |
| 125 | 857 | 50 | — | — | — | [174] | ||
| Trisilanolisobutyl Polyhedral oligomeric silsesquioxane/triglycidyl isocyanurate (T8POSS/TGIC) h | 5 | 114 | 385 | 32 | 3.17 | — | — | [174] |
| 40 | 525 | 62 | — | — | — | [221] | ||
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) i | 5 | 24 | 365 | 67 | 0.80 | — | — | [221] |
| IFR contains melamine phosphate/cellulosic fibre containing polysilicic acid (IFR/Vis) i | 7.5 | 31 | 290 | 41 | 2.12 | — | — | [221] |
| IFR contains melamine phosphate/cellulosic fiber containing polysilicic acid (IFR/Vis) i | 10 | 28 | 242 | 36 | 2.62 | — | — | [221] |
a Matrix: eight layers of woven E-glass fabric reinforced epoxy; b Matrix: eight layers of woven E-glass fabric reinforced epoxy; c Matrix: eight layers of woven E-glass reinforced film of multifunctional epoxy resin; d Matrix: eight plies of carbon fiber reinforced system HexFlow RTM6 (matrix) and HexForce G0939 (fabric); e Matrix: eight layers of woven roving glass fabric reinforced epoxy phenol novolak resin blend; f Matrix: epoxy fiber S2-glass panels; g Matrix: eight layers of woven E-glass reinforced epoxy; h Matrix: eight layers of woven glass Fiber Reinforced epoxy; i Matrix: eight ply woven roving E-glass fiber-reinforced epoxy.
To give a more meaningful overview of the effect of combined P and NP additives on flame retardancy performance of epoxy, FRI values are calculated by using calorimetric data given in Table 3 and plotted in Figure 7. In this figure, the vertical axis shows the amount of additive system used in preparation of epoxy composites. The plot also reveals that three types of flame retardancy performances are observed, depending on the type of combinatorial systems as well as the amount of FR additives used. Attention should be paid to the fact that even at lower loading levels, careful coupling of one or more P and NP additives could lead to superiority of the FR system used, and there was a possibility for attaining higher performances compared to highly-filled systems (FR content ≥ 40). Thus, careful selection of complementary additives with disciplined loading can result in high flame retardancy performance.
Figure 7.
Flame retardancy analysis of epoxy resins containing combinatorial flame retardant systems in terms of the FRI values as a function of combinatorial flame retardants systems retardant type and content. Symbols are indicative of different types of combinatorial flame retardants systems used. Hollow symbols are indicative of fiber-incorporated composites with details earlier given in the bottom of Table 1 as notes a to i. Here:  DiDOPO-1.5/GN-1.5 [28],
DiDOPO-1.5/GN-1.5 [28],  DiDOPO-10/MWCNT-0.8 [29],
DiDOPO-10/MWCNT-0.8 [29],  DiDOPO-3.5/OMMT-3.5 [30],
DiDOPO-3.5/OMMT-3.5 [30],  DiDOPO-0.5/OLDH-0.5, DiDOPO-2.5/OLDH-2.5, DiDOPO-5/OLDH-5 [31],
DiDOPO-0.5/OLDH-0.5, DiDOPO-2.5/OLDH-2.5, DiDOPO-5/OLDH-5 [31],  IFR-40, IFR-39/CES-1, IFR-38/CES-2, IFR-37/CES-3, IFR-35/CES-5 [208],
IFR-40, IFR-39/CES-1, IFR-38/CES-2, IFR-37/CES-3, IFR-35/CES-5 [208],  DOPO-15/P-KC-15, DOPO-20/P-KC-10, DOPO-25/P-KC-5 [41],
DOPO-15/P-KC-15, DOPO-20/P-KC-10, DOPO-25/P-KC-5 [41],  mAPP-5/PER-5, mAPP-5/RCC-5, mAPP-5/ORCC-5 [209],
mAPP-5/PER-5, mAPP-5/RCC-5, mAPP-5/ORCC-5 [209],  PEPA–TMA-12/MCA-6, PEPA–TMA-16/MCA-8, PEPA–TMA-20/MCA-10 [210],
PEPA–TMA-12/MCA-6, PEPA–TMA-16/MCA-8, PEPA–TMA-20/MCA-10 [210],  ZIF8-1/MgAl-LDH-1, ZIF67-1/MgAl-LDH-1 [170],
ZIF8-1/MgAl-LDH-1, ZIF67-1/MgAl-LDH-1 [170],  TAT-18/DOPO-2, TAT-16/DOPO-4, TAT-14/DOPO-6, TAT-12/DOPO-8, TAT-18/HPCP-2, TAT-16/HPCP-4, TAT-14/HPCP-6, TAT-12/HPCP-8 [52],
TAT-18/DOPO-2, TAT-16/DOPO-4, TAT-14/DOPO-6, TAT-12/DOPO-8, TAT-18/HPCP-2, TAT-16/HPCP-4, TAT-14/HPCP-6, TAT-12/HPCP-8 [52],  EDA-APP-19/Cu2O-2 [55],
EDA-APP-19/Cu2O-2 [55],  CP-6B-3/MH-0.5 [56],
CP-6B-3/MH-0.5 [56],  IFR-20, IFR-19.5/HGM-0.5, IFR-19/HGM-1, IFR-18/HGM-2, IFR-16/HGM-4 [211],
IFR-20, IFR-19.5/HGM-0.5, IFR-19/HGM-1, IFR-18/HGM-2, IFR-16/HGM-4 [211],  APP-5/PSA-5 [58],
APP-5/PSA-5 [58],  MFAPP-6.25/PER-6.25, MFAPP-6.25/ST-6.25, MFAPP-6.25/OST-6.25 [212],
MFAPP-6.25/PER-6.25, MFAPP-6.25/ST-6.25, MFAPP-6.25/OST-6.25 [212],  EG-16/DOPO-4, EG-14/DOPO-6, EG-12/DOPO-8, EG-10/DOPO-10, EG-16/HPCP-4, EG-14/HPCP-6, EG-12/HPCP-8, EG-10/HPCP-10 [66],
EG-16/DOPO-4, EG-14/DOPO-6, EG-12/DOPO-8, EG-10/DOPO-10, EG-16/HPCP-4, EG-14/HPCP-6, EG-12/HPCP-8, EG-10/HPCP-10 [66],  TMT-8.3/DOPO-2.7, TMT-8.2/DOPO-4.1, TMT-8.1/DOPO-5.6, TMT-8/DOPO-7 [67],
TMT-8.3/DOPO-2.7, TMT-8.2/DOPO-4.1, TMT-8.1/DOPO-5.6, TMT-8/DOPO-7 [67],  DOPO-3/APHP-3, DOPO-4/APHP-2 [75],
DOPO-3/APHP-3, DOPO-4/APHP-2 [75],  TAD-4/OMMT-1 [77],
TAD-4/OMMT-1 [77],  FR-20/APP-10, FR-15/APP-15, FR-12/APP-18, FR-10/APP-20 [213],
FR-20/APP-10, FR-15/APP-15, FR-12/APP-18, FR-10/APP-20 [213],  ATCP-15/FRHA-1, ATCP-15/FRHA-3, ATCP-15/FRHA-5 [89],
ATCP-15/FRHA-1, ATCP-15/FRHA-3, ATCP-15/FRHA-5 [89],  ATCP-15/FRHA-1, ATCP-15/FRHA-3, ATCP-15/FRHA-5 [90],
ATCP-15/FRHA-1, ATCP-15/FRHA-3, ATCP-15/FRHA-5 [90],  APP-4/MMT-6 [93],
APP-4/MMT-6 [93],  DOPO-5/MMT-1 [94],
DOPO-5/MMT-1 [94],  BDP-6.7/PHP-3.3 [95],
BDP-6.7/PHP-3.3 [95],  OPS-2.5/DOPO-2.5 [102],
OPS-2.5/DOPO-2.5 [102],  DOPO-3.1/OPS-2.1, DOPO-3.1/PPSQ-2.1 [103],
DOPO-3.1/OPS-2.1, DOPO-3.1/PPSQ-2.1 [103],  DOPO-3.1/OPS-2.1, DOPO-3.1/OAPS-2.3 [104],
DOPO-3.1/OPS-2.1, DOPO-3.1/OAPS-2.3 [104],  OPS-2.5/DOPO-2.5 [105],
OPS-2.5/DOPO-2.5 [105],  OPS-2.1/PEPA-2.6, OPS-2.1/APP-1.4, OPS-2.1/DOPO-3.1 [106],
OPS-2.1/PEPA-2.6, OPS-2.1/APP-1.4, OPS-2.1/DOPO-3.1 [106],  ODPSS-2.5/DOPO-2.5 [115],
ODPSS-2.5/DOPO-2.5 [115],  BBO-10/PPA-10 [214],
BBO-10/PPA-10 [214],  T8POSS-5/TGIC-5 [174],
T8POSS-5/TGIC-5 [174],  APP-4.83/CoSA-0.17 [117],
APP-4.83/CoSA-0.17 [117],  CBz-8/BGN-2, CBz-13/BGN-2, CBz-18/BGN-2 [118],
CBz-8/BGN-2, CBz-13/BGN-2, CBz-18/BGN-2 [118],  Mel-APP-18/LDH-2, Mel-APP-18/HNT-2 [120],
Mel-APP-18/LDH-2, Mel-APP-18/HNT-2 [120],  oDOPI-17.76/MPP-15, AlO(OH)-30/oDOPI-11.05, MPP-15/PZ-1.54, AlO(OH)-30/PZ-3.08 [124],
oDOPI-17.76/MPP-15, AlO(OH)-30/oDOPI-11.05, MPP-15/PZ-1.54, AlO(OH)-30/PZ-3.08 [124],  AHP-4.5/ACS@SnO2@NiO-0.5 [125],
AHP-4.5/ACS@SnO2@NiO-0.5 [125],  Mel-APP-19.97/Talc-9.73 [126],
Mel-APP-19.97/Talc-9.73 [126],  MPP-10/MPZnP-10, AlPi-Et-10/MPZnP-10, DOPAc-Bu-10/MPZnP-10, AlO(OH)-10/MPZnP-10, MPZnP-10/SiO2-10, MPP-13.4/MPZnP-6.6, AlPi-Et-13.4/MPZnP-6.6, DOPAc-Bu-13.4/MPZnP-6.6, AlO(OH)-13.4/MPZnP-6.6, SiO2-13.4/MPZnP-6.6 [127],
MPP-10/MPZnP-10, AlPi-Et-10/MPZnP-10, DOPAc-Bu-10/MPZnP-10, AlO(OH)-10/MPZnP-10, MPZnP-10/SiO2-10, MPP-13.4/MPZnP-6.6, AlPi-Et-13.4/MPZnP-6.6, DOPAc-Bu-13.4/MPZnP-6.6, AlO(OH)-13.4/MPZnP-6.6, SiO2-13.4/MPZnP-6.6 [127],  HPCTP-10/OGPOSS-5, HPCTP-7.5/OGPOSS-7.5, HPCTP-5/OGPOSS-10 [129],
HPCTP-10/OGPOSS-5, HPCTP-7.5/OGPOSS-7.5, HPCTP-5/OGPOSS-10 [129],  CP-10/TPP-MMT-5 [130],
CP-10/TPP-MMT-5 [130],  CP-10/TPP-MMT-5 [130],
CP-10/TPP-MMT-5 [130],  CP-10/TPP-MMT-5 [130],
CP-10/TPP-MMT-5 [130],  MoS2-1/TNT-1 [176],
MoS2-1/TNT-1 [176],  APP-15/PER-HNT-10 [215],
APP-15/PER-HNT-10 [215],  S600-10/AlPi-10, S600-10/AlO(OH)-10, S600-10/MPP-10 [147],
S600-10/AlPi-10, S600-10/AlO(OH)-10, S600-10/MPP-10 [147],  SDPS-5.2/SPDM-5.2 [136],
SDPS-5.2/SPDM-5.2 [136],  AlPi-4.7/MPP-2.3, AlPi-4.5/MPP-2.25/Al2O3-0.25 [47],
AlPi-4.7/MPP-2.3, AlPi-4.5/MPP-2.25/Al2O3-0.25 [47],  APP-8/CSA-2, APP-7.5/CSA-2.5, APP-6.7/CSA-3.3 [139],
APP-8/CSA-2, APP-7.5/CSA-2.5, APP-6.7/CSA-3.3 [139],  BN 12 μm-33.75/BN 2 μm-11.25, BN 12 μm-33.75/BT 2 μm-11.25 [182],
BN 12 μm-33.75/BN 2 μm-11.25, BN 12 μm-33.75/BT 2 μm-11.25 [182],  IFR-30, IFR-29.5/FeP-0.5, IFR-29/FeP-1, IFR-28/FeP-2, IFR-27/FeP-3 [216],
IFR-30, IFR-29.5/FeP-0.5, IFR-29/FeP-1, IFR-28/FeP-2, IFR-27/FeP-3 [216],  IFR-30, IFR-29.5/αFeOOH-0.5, IFR-29/αFeOOH-1, IFR-28/αFeOOH-2, IFR-27/αFeOOH-3 [217],
IFR-30, IFR-29.5/αFeOOH-0.5, IFR-29/αFeOOH-1, IFR-28/αFeOOH-2, IFR-27/αFeOOH-3 [217],  IFR-30, IFR-29.5/iron oxide brown-0.5, IFR-29/iron oxide brown-1, IFR-28/iron oxide brown-2, IFR-27/iron oxide brown-3 [218],
IFR-30, IFR-29.5/iron oxide brown-0.5, IFR-29/iron oxide brown-1, IFR-28/iron oxide brown-2, IFR-27/iron oxide brown-3 [218],  Ni–Fe LDH-2/GN-2 [193],
Ni–Fe LDH-2/GN-2 [193],  APP-22.5/PER-7.5 [152],
APP-22.5/PER-7.5 [152],  IFR-30, IFR29.5/Fe-OMMT-0.5, IFR-29/Fe-OMMT-1, IFR-28/Fe-OMMT-2, IFR-27/Fe-OMMT-3 [219],
IFR-30, IFR29.5/Fe-OMMT-0.5, IFR-29/Fe-OMMT-1, IFR-28/Fe-OMMT-2, IFR-27/Fe-OMMT-3 [219],  APP-20/I.30E-3 [160],
APP-20/I.30E-3 [160],  Mel-APP-8.59/LDH-0.96, Mel-APP-8.65/HNT-0.96 [120],
Mel-APP-8.59/LDH-0.96, Mel-APP-8.65/HNT-0.96 [120],  Mel-APP-9.93/Talc-4.84 [126],
Mel-APP-9.93/Talc-4.84 [126],  , IFR-5/Vis-5, Ky-5/IFR-5 [163,164],
, IFR-5/Vis-5, Ky-5/IFR-5 [163,164],  ZB-0.5/Mg(OH)2-0.5, ZB-3.75/Mg(OH)2-3.75, ZB-7.5/Mg(OH)2-7.5, ZB-12.5/Mg(OH)2-12.5, ZB-0.5/Al(OH)3-0.5, ZB-3.75/Al(OH)3-3.75, ZB-7.5/Al(OH)3-7.5, ZB-12.5/Al(OH)3-12.5 [205],
ZB-0.5/Mg(OH)2-0.5, ZB-3.75/Mg(OH)2-3.75, ZB-7.5/Mg(OH)2-7.5, ZB-12.5/Mg(OH)2-12.5, ZB-0.5/Al(OH)3-0.5, ZB-3.75/Al(OH)3-3.75, ZB-7.5/Al(OH)3-7.5, ZB-12.5/Al(OH)3-12.5 [205],  MP-4.5/GN-0.5, DOPO-4.5/GN-0.5 [166],
MP-4.5/GN-0.5, DOPO-4.5/GN-0.5 [166],  PFR-25/ZB-5 [220],
PFR-25/ZB-5 [220],  IFR-2.5/Vis-2.5, IFR-2.5/Vis-5, IFR-2.5/Vis-7.5, IFR-2.5/Vis-10, IFR-2.5/Vis-12.5, IFR-5/Vis-2.5, IFR-5/Vis-5, IFR-5/Vis-7.5, IFR-5/Vis-10, IFR-7.5/Vis-2.5, IFR-7.5/Vis-5, IFR-7.5/Vis-7.5, IFR-10/Vis-2.5, IFR-10/Vis-5, IFR-12.5/Vis-2.5, IFR-15/Vis-2.5 [167],
IFR-2.5/Vis-2.5, IFR-2.5/Vis-5, IFR-2.5/Vis-7.5, IFR-2.5/Vis-10, IFR-2.5/Vis-12.5, IFR-5/Vis-2.5, IFR-5/Vis-5, IFR-5/Vis-7.5, IFR-5/Vis-10, IFR-7.5/Vis-2.5, IFR-7.5/Vis-5, IFR-7.5/Vis-7.5, IFR-10/Vis-2.5, IFR-10/Vis-5, IFR-12.5/Vis-2.5, IFR-15/Vis-2.5 [167],  T8POSS-2.5/TGIC-2.5 [174],
T8POSS-2.5/TGIC-2.5 [174],  IFR-2.5/Vis-2.5, IFR-3.75/Vis-3.75, IFR-7.5/Vis-2.5 [221].
IFR-2.5/Vis-2.5, IFR-3.75/Vis-3.75, IFR-7.5/Vis-2.5 [221].
When looking at the UL-94 test results (considering the fact that there were some data in Table 3 for some systems to be plotted and discussed in Figure 8), it can be seen that, except for some data, the whole systems take Poor and Good labels based on FRI values. It is also interesting to note that for a given category, e.g., V-0, the amount of additive changes the FRI, and UL-94 testing does not make sense of such variations.
Figure 8.
Flame retardancy analysis of epoxy resins containing combinatorial flame retardants in terms of the FRI values as a function of UL-94 test results. Symbols are indicative of different types of combinatorial flame retardants used. Hollow symbols are indicative of fiber-incorporated composites with details given in the bottom of Table 1 as a to i notes. The vertical variation in each category, i.e., V-0, V-1, and NR, is schematically representative of the amount of additive used. For example, two data distinguished by different symbols have the same or very close FRI values (horizontal quantity) in a given category (e.g., V-1), but higher V-1 behavior in UL-94 testing means the FR was used in greater quantity.
The more interesting outcome of this work is that LOI percent similarly detects Poor and Good behaviors, not principally Excellent performance (Figure 9). This suggests that development of innovative FR additives by combination of P and NP and using highly efficient synthesis routes is the essential step to be taken in the near future for developing flame retardant epoxy composites.
Figure 9.
Flame retardancy analysis of epoxy resins containing combinatorial flame-retardant systems in terms of the FRI values as a function of LOI test results. Symbols are indicative of different types of combinatorial flame-retardant systems used. Hollow symbols are indicative of fiber-incorporated composites with details given in the bottom of Table 1 as notes a to i.
5. Concluding Remarks and Future Perspective
In previous sections, we categorized the flame-retardant properties of epoxy resins in terms of the universal FRI criterion and the content of flame retardants of three families. We also attempted to find possible correlations between cone calorimetry (reflected in FRI variations), UL-94, and LOI analyses. Since cone calorimetry is the best way to simulate real state combustion of polymers, here, we give a general picture of flame retardancy of epoxy resins (Figure 10). The Poor, Good, or Excellent flame retardancy cases are the result of the P, NP, or P/NP types of flame retardants used in preparation of epoxy composites as well as the FR loading. Each kind of behavior can be visualized by providing a full snapshot of the Poor, Good, and Excellent regions of the FRI to see how closely the data are collected in each zone. Overall, it can be seen that Poor and Good are the cases for majority of data, while the Excellent zone contains limited data. This highlights the difficulty of achieving high flame-retardant efficiency in epoxy composites when merely using flame retardants. Thus, development of innovative flame retardants through blending different FR families and making them reactive towards epoxy may result in a fully cured 3D network with high flame resistance. This requires the knowledge and experience of chemists and engineers who can adjust the performance of the system in a very disciplined manner. Moreover, using bio-based epoxy resins with limited environmental threats would be another solution to the question of “which FR additive(s) meet the requirements of highly flame-retardant epoxy composites?”.
Figure 10.
Overall flame retardancy behavior of epoxy resins regardless of the type of flame retardant. Poor, Good, and Excellent efficiencies are magnified to give a close-up of the data distribution.
Author Contributions
Conceptualization, H.V. and M.R.S.; methodology, H.V. and M.R.S.; validation, H.V. and M.R.S.; investigation, E.M., H.V. and M.R.S.; data curation, E.M.; writing—original draft preparation, H.V. and M.R.S.; writing—review and editing H.V., S.T. and M.R.S.; visualization, H.V., S.T. and M.R.S.; supervision, H.V., S.T. and M.R.S.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jin F.-L., Li X., Park S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015;29:1–11. doi: 10.1016/j.jiec.2015.03.026. [DOI] [Google Scholar]
- 2.Tan S., Chow W. Biobased epoxidized vegetable oils and its greener epoxy blends: A review. Polym. Plast. Technol. Eng. 2010;49:1581–1590. doi: 10.1080/03602559.2010.512338. [DOI] [Google Scholar]
- 3.Wang X., Hu Y., Song L., Xing W., Lu H., Lv P., Jie G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer. 2010;51:2435–2445. doi: 10.1016/j.polymer.2010.03.053. [DOI] [Google Scholar]
- 4.Ahmadi Z. Epoxy in nanotechnology: A short review. Prog. Org. Coat. 2019;132:445–448. doi: 10.1016/j.porgcoat.2019.04.003. [DOI] [Google Scholar]
- 5.Ahmadi Z. Nanostructured epoxy adhesives: A review. Prog. Org. Coat. 2019;135:449–453. doi: 10.1016/j.porgcoat.2019.06.028. [DOI] [Google Scholar]
- 6.Manouchehri S., Bagheri B., Rad S.H., Nezhad M.N., Kim Y.C., Park O.O., Farokhi M., Jouyandeh M., Ganjali M.R., Yazdi M.K., et al. Electroactive bio-epoxy incorporated chitosan-oligoaniline as an advanced hydrogel coating for neural interfaces. Prog. Org. Coat. 2019;131:389–396. doi: 10.1016/j.porgcoat.2019.03.022. [DOI] [Google Scholar]
- 7.Wu K., Song L., Hu Y., Lu H., Kandola B.K., Kandare E. Synthesis and characterization of a functional polyhedral oligomeric silsesquioxane and its flame retardancy in epoxy resin. Prog. Org. Coat. 2009;65:490–497. doi: 10.1016/j.porgcoat.2009.04.008. [DOI] [Google Scholar]
- 8.Levchik S.V., Weil E.D. Thermal decomposition, combustion and flame-retardancy of epoxy resins—A review of the recent literature. Polym. Int. 2004;53:1901–1929. doi: 10.1002/pi.1473. [DOI] [Google Scholar]
- 9.Liu S., Yu B., Feng Y., Yang Z., Yin B. Synthesis of a multifunctional bisphosphate and its flame retardant application in epoxy resin. Polym. Degrad. Stab. 2019;165:92–100. doi: 10.1016/j.polymdegradstab.2019.04.022. [DOI] [Google Scholar]
- 10.Weil E.D., Levchik S. A review of current flame retardant systems for epoxy resins. J. Fire Sci. 2004;22:25–40. doi: 10.1177/0734904104038107. [DOI] [Google Scholar]
- 11.Rad E.R., Vahabi H., de Anda A.R., Saeb M.R., Thomas S. Bio-epoxy resins with inherent flame retardancy. Prog. Org. Coat. 2019;135:608–612. doi: 10.1016/j.porgcoat.2019.05.046. [DOI] [Google Scholar]
- 12.Wang X., Xing W., Song L., Yu B., Shi Y., Yang W., Hu Y. Flame retardancy and thermal properties of novel UV-curing epoxy acrylate coatings modified by phosphorus-containing hyperbranched macromonomer. J. Polym. Res. 2013;20:165. doi: 10.1007/s10965-013-0165-x. [DOI] [Google Scholar]
- 13.Vahabi H., Saeb M.R., Formela K., Cuesta J.-M.L. Flame retardant epoxy/halloysite nanotubes nanocomposite coatings: Exploring low-concentration threshold for flammability compared to expandable graphite as superior fire retardant. Prog. Org. Coat. 2018;119:8–14. doi: 10.1016/j.porgcoat.2018.02.005. [DOI] [Google Scholar]
- 14.Vahabi H., Raveshtian A., Fasihi M., Sonnier R., Saeb M.R., Dumazert L., Kandola B.K. Competitiveness and synergy between three flame retardants in poly (ethylene-co-vinyl acetate) Polym. Degrad. Stab. 2017;143:164–175. doi: 10.1016/j.polymdegradstab.2017.07.005. [DOI] [Google Scholar]
- 15.Battig A., Markwart J.C., Wurm F.R., Schartel B. Hyperbranched phosphorus flame retardants: Multifunctional additives for epoxy resins. Polym. Chem. 2019;10:4346–4358. doi: 10.1039/C9PY00737G. [DOI] [Google Scholar]
- 16.Zou B., Qiu S., Ren X., Zhou Y., Zhou F., Xu Z., Zhao Z., Song L., Hu Y., Gong X. Combination of black phosphorus nanosheets and MCNTs via phosphoruscarbon bonds for reducing the flammability of air stable epoxy resin nanocomposites. J. Hazard. Mater. 2019;383:121069. doi: 10.1016/j.jhazmat.2019.121069. [DOI] [PubMed] [Google Scholar]
- 17.Mu X., Wang D., Pan Y., Cai W., Song L., Hu Y. A facile approach to prepare phosphorus and nitrogen containing macromolecular covalent organic nanosheets for enhancing flame retardancy and mechanical property of epoxy resin. Compos. Part B Eng. 2019;164:390–399. doi: 10.1016/j.compositesb.2018.12.036. [DOI] [Google Scholar]
- 18.Duan H., Chen Y., Ji S., Hu R., Ma H. A novel phosphorus/nitrogen-containing polycarboxylic acid endowing epoxy resin with excellent flame retardance and mechanical properties. Chem. Eng. J. 2019;375:121916. doi: 10.1016/j.cej.2019.121916. [DOI] [Google Scholar]
- 19.Li S., Zhao X., Liu X., Yang X., Yu R., Zhang Y., Huang W., Deng K. Cage–ladder-structure, phosphorus-containing polyhedral oligomeric silsesquinoxanes as promising reactive-type flame retardants for epoxy resin. J. Appl. Polym. Sci. 2019;136:47607. doi: 10.1002/app.47607. [DOI] [Google Scholar]
- 20.Jouyandeh M., Rahmati N., Movahedifar E., Hadavand B.S., Karami Z., Ghaffari M., Taheri P., Bakhshandeh E., Vahabi H., Ganjali M.R., et al. Properties of nano-Fe3O4 incorporated epoxy coatings from Cure Index perspective. Prog. Org. Coat. 2019;133:220–228. doi: 10.1016/j.porgcoat.2019.04.034. [DOI] [Google Scholar]
- 21.Jouyandeh M., Paran S.M.R., Jannesari A., Saeb M.R. ‘Cure Index’ for thermoset composites. Prog. Org. Coat. 2019;127:429–434. doi: 10.1016/j.porgcoat.2018.11.025. [DOI] [Google Scholar]
- 22.Vahabi H., Kandola B.K., Saeb M.R. Flame retardancy index for thermoplastic composites. Polymers. 2019;11:407. doi: 10.3390/polym11030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vahabi H., Laoutid F., Movahedifar E., Khalili R., Rahmati N., Vagner C., Cochez M., Brison L., Ducos F., Ganjali M.R. Description of complementary actions of mineral and organic additives in thermoplastic polymer composites by Flame Retardancy Index. Polym. Adv. Technol. 2019;30:2056–2066. doi: 10.1002/pat.4638. [DOI] [Google Scholar]
- 24.Zhao X., Zhang L., Alonso J.P., Delgado S., Martínez-Miranda M., Wang D.-Y. Influence of phenylphosphonic amide on rheological, mechanical and flammable properties of carbon fiber/RTM6 composites. Compos. Part B Eng. 2018;149:74–81. doi: 10.1016/j.compositesb.2018.05.018. [DOI] [Google Scholar]
- 25.Zhao J., Dong X., Huang S., Tian X., Song L., Yu Q., Wang Z. Performance comparison of flame retardant epoxy resins modified by DPO–PHE and DOPO–PHE. Polym. Degrad. Stab. 2018;156:89–99. doi: 10.1016/j.polymdegradstab.2018.08.007. [DOI] [Google Scholar]
- 26.Yang S., Hu Y., Zhang Q. Synthesis of a phosphorus–nitrogen-containing flame retardant and its application in epoxy resin. High Perform. Polym. 2019;31:186–196. doi: 10.1177/0954008318756496. [DOI] [Google Scholar]
- 27.Yang J.W., Wang Z.Z. Synthesis of aluminum ethylphenylphosphinate flame retardant and its application in epoxy resin. Fire Mater. 2018;42:638–644. doi: 10.1002/fam.2518. [DOI] [Google Scholar]
- 28.Yan W., Zhang M.-Q., Yu J., Nie S.-Q., Zhang D.-Q., Qin S.-H. Synergistic flame-retardant effect of epoxy resin combined with phenethyl-bridged DOPO derivative and graphene nanosheets. Chin. J. Polym. Sci. 2019;37:79–88. doi: 10.1007/s10118-019-2175-6. [DOI] [Google Scholar]
- 29.Yan W., Yu J., Zhang M., Wang T., Wen C., Qin S., Huang W. Effect of multiwalled carbon nanotubes and phenethyl-bridged DOPO derivative on flame retardancy of epoxy resin. J. Polym. Res. 2018;25:72. doi: 10.1007/s10965-018-1472-z. [DOI] [Google Scholar]
- 30.Yan W., Yu J., Zhang M., Wang T., Li W., Qin S., Long L. Enhanced flame retardancy of epoxy resin containing a phenethyl-bridged DOPO derivative/montmorillonite compound. J. Fire Sci. 2018;36:47–62. doi: 10.1177/0734904117740820. [DOI] [Google Scholar]
- 31.Yan W., Yu J., Zhang M., Qin S., Wang T., Huang W., Long L. Flame-retardant effect of a phenethyl-bridged DOPO derivative and layered double hydroxides for epoxy resin. RSC Adv. 2017;7:46236–46245. doi: 10.1039/C7RA08173A. [DOI] [Google Scholar]
- 32.Yan W., Yu J., Zhang M., Long L., Wang T., Qin S., Huang W. Novel flame retardancy effect of phenethyl-bridged DOPO derivative on epoxy resin. High Perform. Polym. 2018;30:667–676. doi: 10.1177/0954008317716525. [DOI] [Google Scholar]
- 33.Yan L., Xu Z., Wang X., Deng N., Chu Z. Preparation of a novel mono-component intumescent flame retardant for enhancing the flame retardancy and smoke suppression properties of epoxy resin. J. Therm. Anal. Calorim. 2018;134:1505–1519. doi: 10.1007/s10973-018-7810-x. [DOI] [Google Scholar]
- 34.Xu Z., Deng N., Yan L., Chu Z. Functionalized multiwalled carbon nanotubes with monocomponent intumescent flame retardant for reducing the flammability and smoke emission characteristics of epoxy resins. Polym. Adv. Technol. 2018;29:3002–3013. doi: 10.1002/pat.4420. [DOI] [Google Scholar]
- 35.Xu Y.-J., Wang J., Tan Y., Qi M., Chen L., Wang Y.-Z. A novel and feasible approach for one-pack flame-retardant epoxy resin with long pot life and fast curing. Chem. Eng. J. 2018;337:30–39. doi: 10.1016/j.cej.2017.12.086. [DOI] [Google Scholar]
- 36.Xu Y.-J., Chen L., Rao W.-H., Qi M., Guo D.-M., Liao W., Wang Y.-Z. Latent curing epoxy system with excellent thermal stability, flame retardance and dielectric property. Chem. Eng. J. 2018;347:223–232. doi: 10.1016/j.cej.2018.04.097. [DOI] [Google Scholar]
- 37.Xu M.-J., Xia S.-Y., Liu C., Li B. Preparation of poly (phosphoric acid piperazine) and its application as an effective flame retardant for epoxy resin. Chin. J. Polym. Sci. 2018;36:655–664. doi: 10.1007/s10118-018-2036-8. [DOI] [Google Scholar]
- 38.Xiang Y., Wang L., Yang Z., Gao P., Qin S., Yu J. Effect of aluminum phosphinate on the flame-retardant properties of epoxy syntactic foams. J. Therm. Anal. Calorim. 2019;137:1645–1656. doi: 10.1007/s10973-019-08051-9. [DOI] [Google Scholar]
- 39.Wang P., Fu X., Kan Y., Wang X., Hu Y. Two high-efficient DOPO-based phosphonamidate flame retardants for transparent epoxy resin. High Perform. Polym. 2019;31:249–260. doi: 10.1177/0954008318762037. [DOI] [Google Scholar]
- 40.Wang P., Chen L., Xiao H. Flame retardant effect and mechanism of a novel DOPO based tetrazole derivative on epoxy resin. J. Anal. Appl. Pyrolysis. 2019;139:104–113. doi: 10.1016/j.jaap.2019.01.015. [DOI] [Google Scholar]
- 41.Wang N., Teng H., Li L., Zhang J., Kang P. Synthesis of phosphated K-carrageenan and Its application for flame-retardant waterborne epoxy. Polymers. 2018;10:1268. doi: 10.3390/polym10111268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian L., Li X., Wang L., Zhang Y., Cui J., Guo J., Yang B. Synthesis and characterization of an efficient flame retardant based on aromatic ring and phosphate ester for epoxy resin. Polym. Eng. Sci. 2019;59:E406–E413. doi: 10.1002/pen.25072. [DOI] [Google Scholar]
- 43.Sun Z., Hou Y., Hu Y., Hu W. Effect of additive phosphorus-nitrogen containing flame retardant on char formation and flame retardancy of epoxy resin. Mater. Chem. Phys. 2018;214:154–164. doi: 10.1016/j.matchemphys.2018.04.065. [DOI] [Google Scholar]
- 44.Shi Y.-Q., Fu T., Xu Y.-J., Li D.-F., Wang X.-L., Wang Y.-Z. Novel phosphorus-containing halogen-free ionic liquid toward fire safety epoxy resin with well-balanced comprehensive performance. Chem. Eng. J. 2018;354:208–219. doi: 10.1016/j.cej.2018.08.023. [DOI] [Google Scholar]
- 45.Shi Y., Wang Z., Zhou J.-a. Facile synthesis of a flame retardant melamine phenylphosphate and its epoxy resin composites with simultaneously improved flame retardancy, smoke suppression and water resistance. RSC Adv. 2018;8:39214–39221. doi: 10.1039/C8RA08696F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen D., Xu Y.-J., Long J.-W., Shi X.-H., Chen L., Wang Y.-Z. Epoxy resin flame-retarded via a novel melamine-organophosphinic acid salt: Thermal stability, flame retardance and pyrolysis behavior. J. Anal. Appl. Pyrolysis. 2017;128:54–63. doi: 10.1016/j.jaap.2017.10.025. [DOI] [Google Scholar]
- 47.Zhong L., Zhang K.-X., Wang X., Chen M.-J., Xin F., Liu Z.-G. Synergistic effects and flame-retardant mechanism of aluminum diethyl phosphinate in combination with melamine polyphosphate and aluminum oxide in epoxy resin. J. Therm. Anal. Calorim. 2018;134:1637–1646. doi: 10.1007/s10973-018-7699-4. [DOI] [Google Scholar]
- 48.Liang W.-J., Zhao B., Zhang C.-Y., Jian R.-K., Liu D.-Y., Liu Y.-Q. Enhanced flame retardancy of DGEBA epoxy resin with a novel bisphenol-A bridged cyclotriphosphazene. Polym. Degrad. Stab. 2017;144:292–303. doi: 10.1016/j.polymdegradstab.2017.08.027. [DOI] [Google Scholar]
- 49.Kong Q., Wu T., Zhang J., Wang D.-Y. Simultaneously improving flame retardancy and dynamic mechanical properties of epoxy resin nanocomposites through layered copper phenylphosphate. Compos. Sci. Technol. 2018;154:136–144. doi: 10.1016/j.compscitech.2017.10.013. [DOI] [Google Scholar]
- 50.Jian R.-K., Ai Y.-F., Xia L., Zhao L.-J., Zhao H.-B. Single component phosphamide-based intumescent flame retardant with potential reactivity towards low flammability and smoke epoxy resins. J. Hazard. Mater. 2019;371:529–539. doi: 10.1016/j.jhazmat.2019.03.045. [DOI] [PubMed] [Google Scholar]
- 51.Huo S., Wang J., Yang S., Chen X., Zhang B., Wu Q., Zhang B. Flame-retardant performance and mechanism of epoxy thermosets modified with a novel reactive flame retardant containing phosphorus, nitrogen, and sulfur. Polym. Adv. Technol. 2018;29:497–506. doi: 10.1002/pat.4145. [DOI] [Google Scholar]
- 52.Huo S., Wang J., Yang S., Cai H., Zhang B., Chen X., Wu Q., Yang L. Synergistic effect between a novel triazine-based flame retardant and DOPO/HPCP on epoxy resin. Polym. Adv. Technol. 2018;29:2774–2783. doi: 10.1002/pat.4400. [DOI] [Google Scholar]
- 53.Gangireddy C.S.R., Wang X., Kan Y., Song L., Hu Y. Synthesis of a novel DOPO-based polyphosphoramide with high char yield and its application in flame-retardant epoxy resins. Polym. Int. 2019;68:936–945. doi: 10.1002/pi.5784. [DOI] [Google Scholar]
- 54.Dong C., Wirasaputra A., Luo Q., Liu S., Yuan Y., Zhao J., Fu Y. Intrinsic flame-retardant and thermally stable epoxy endowed by a highly efficient, multifunctional curing agent. Materials. 2016;9:1008. doi: 10.3390/ma9121008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen M.-J., Wang X., Li X.-L., Liu X.-Y., Zhong L., Wang H.-Z., Liu Z.-G. The synergistic effect of cuprous oxide on an intumescent flame-retardant epoxy resin system. RSC Adv. 2017;7:35619–35628. doi: 10.1039/C7RA05482C. [DOI] [Google Scholar]
- 56.Ai L., Chen S., Zeng J., Yang L., Liu P. Synergistic flame retardant effect of an intumescent flame retardant containing boron and magnesium hydroxide. ACS Omega. 2019;4:3314–3321. doi: 10.1021/acsomega.8b03333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao X., Xiao D., Alonso J.P., Wang D.-Y. Inclusion complex between beta-cyclodextrin and phenylphosphonicdiamide as novel bio-based flame retardant to epoxy: Inclusion behavior, characterization and flammability. Mater. Des. 2017;114:623–632. doi: 10.1016/j.matdes.2016.11.093. [DOI] [Google Scholar]
- 58.Zhao W., Liu J., Peng H., Liao J., Wang X. Synthesis of a novel PEPA-substituted polyphosphoramide with high char residues and its performance as an intumescent flame retardant for epoxy resins. Polym. Degrad. Stab. 2015;118:120–129. doi: 10.1016/j.polymdegradstab.2015.04.023. [DOI] [Google Scholar]
- 59.Zhao B., Liang W.-J., Wang J.-S., Li F., Liu Y.-Q. Synthesis of a novel bridged-cyclotriphosphazene flame retardant and its application in epoxy resin. Polym. Degrad. Stab. 2016;133:162–173. doi: 10.1016/j.polymdegradstab.2016.08.013. [DOI] [Google Scholar]
- 60.Zhang Y., Yu B., Wang B., Liew K.M., Song L., Wang C., Hu Y. Highly effective P–P synergy of a novel DOPO-based flame retardant for epoxy resin. Ind. Eng. Chem. Res. 2017;56:1245–1255. doi: 10.1021/acs.iecr.6b04292. [DOI] [Google Scholar]
- 61.Zhang W., Fina A., Cuttica F., Camino G., Yang R. Blowing-out effect in flame retarding epoxy resins: Insight by temperature measurements during forced combustion. Polym. Degrad. Stab. 2016;131:82–90. doi: 10.1016/j.polymdegradstab.2016.07.002. [DOI] [Google Scholar]
- 62.Zhang C., Pan M., Qu L., Sun G. Effect of phosphorus-containing flame retardants on flame retardancy and thermal stability of tetrafunctional epoxy resin. Polym. Adv. Technol. 2015;26:1531–1536. doi: 10.1002/pat.3576. [DOI] [Google Scholar]
- 63.You G., Cheng Z., Tang Y., He H. Functional group effect on char formation, flame retardancy and mechanical properties of phosphonate–triazine-based compound as flame retardant in epoxy resin. Ind. Eng. Chem. Res. 2015;54:7309–7319. doi: 10.1021/acs.iecr.5b00315. [DOI] [Google Scholar]
- 64.Yang S., Zhang Q., Hu Y. Preparation and investigation of flame-retardant epoxy resin modified with a novel halogen-free flame retardant containing phosphaphenanthrene, triazine-trione, and organoboron units. J. Appl. Polym. Sci. 2017;134:45291. doi: 10.1002/app.45291. [DOI] [Google Scholar]
- 65.Yang S., Zhang Q., Hu Y. Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym. Degrad. Stab. 2016;133:358–366. doi: 10.1016/j.polymdegradstab.2016.09.023. [DOI] [Google Scholar]
- 66.Yang S., Wang J., Huo S., Wang M., Wang J., Zhang B. Synergistic flame-retardant effect of expandable graphite and phosphorus-containing compounds for epoxy resin: Strong bonding of different carbon residues. Polym. Degrad. Stab. 2016;128:89–98. doi: 10.1016/j.polymdegradstab.2016.03.017. [DOI] [Google Scholar]
- 67.Yang S., Wang J., Huo S., Wang M., Wang J. Preparation and flame retardancy of a compounded epoxy resin system composed of phosphorus/nitrogen-containing active compounds. Polym. Degrad. Stab. 2015;121:398–406. doi: 10.1016/j.polymdegradstab.2015.10.006. [DOI] [Google Scholar]
- 68.Yang S., Wang J., Huo S., Wang M., Cheng L. Synthesis of a phosphorus/nitrogen-containing additive with multifunctional groups and its flame-retardant effect in epoxy resin. Ind. Eng. Chem. Res. 2015;54:7777–7786. doi: 10.1021/acs.iecr.5b02026. [DOI] [Google Scholar]
- 69.Yang S., Wang J., Huo S., Wang J., Tang Y. Synthesis of a phosphorus/nitrogen-containing compound based on maleimide and cyclotriphosphazene and its flame-retardant mechanism on epoxy resin. Polym. Degrad. Stab. 2016;126:9–16. doi: 10.1016/j.polymdegradstab.2016.01.011. [DOI] [Google Scholar]
- 70.Xu W., Wirasaputra A., Liu S., Yuan Y., Zhao J. Highly effective flame retarded epoxy resin cured by DOPO-based co-curing agent. Polym. Degrad. Stab. 2015;122:44–51. doi: 10.1016/j.polymdegradstab.2015.10.012. [DOI] [Google Scholar]
- 71.Xu M.-J., Xu G.-R., Leng Y., Li B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016;123:105–114. doi: 10.1016/j.polymdegradstab.2015.11.018. [DOI] [Google Scholar]
- 72.Xu M.-J., Ma Y., Hou M.-J., Li B. Synthesis of a cross-linked triazine phosphine polymer and its effect on fire retardancy, thermal degradation and moisture resistance of epoxy resins. Polym. Degrad. Stab. 2015;119:14–22. doi: 10.1016/j.polymdegradstab.2015.04.027. [DOI] [Google Scholar]
- 73.Xu M., Li X., Li B. Synthesis of a novel cross-linked organophosphorus-nitrogen containing polymer and its application in flame retardant epoxy resins. Fire Mater. 2016;40:848–860. doi: 10.1002/fam.2349. [DOI] [Google Scholar]
- 74.Wang J., Qian L., Xu B., Xi W., Liu X. Synthesis and characterization of aluminum poly-hexamethylenephosphinate and its flame-retardant application in epoxy resin. Polym. Degrad. Stab. 2015;122:8–17. doi: 10.1016/j.polymdegradstab.2015.10.011. [DOI] [Google Scholar]
- 75.Wang J., Qian L., Huang Z., Fang Y., Qiu Y. Synergistic flame-retardant behavior and mechanisms of aluminum poly-hexamethylenephosphinate and phosphaphenanthrene in epoxy resin. Polym. Degrad. Stab. 2016;130:173–181. doi: 10.1016/j.polymdegradstab.2016.06.010. [DOI] [Google Scholar]
- 76.Wang G., Nie Z. Synthesis of a novel phosphorus-containing epoxy curing agent and the thermal, mechanical and flame-retardant properties of the cured products. Polym. Degrad. Stab. 2016;130:143–154. doi: 10.1016/j.polymdegradstab.2016.06.002. [DOI] [Google Scholar]
- 77.Tang S., Wachtendorf V., Klack P., Qian L., Dong Y., Schartel B. Enhanced flame-retardant effect of a montmorillonite/phosphaphenanthrene compound in an epoxy thermoset. RSC Adv. 2017;7:720–728. doi: 10.1039/C6RA25070J. [DOI] [Google Scholar]
- 78.Tang S., Qian L., Liu X., Dong Y. Gas-phase flame-retardant effects of a bi-group compound based on phosphaphenanthrene and triazine-trione groups in epoxy resin. Polym. Degrad. Stab. 2016;133:350–357. doi: 10.1016/j.polymdegradstab.2016.09.014. [DOI] [Google Scholar]
- 79.Tan Y., Shao Z.-B., Yu L.-X., Long J.-W., Qi M., Chen L., Wang Y.-Z. Piperazine-modified ammonium polyphosphate as monocomponent flame-retardant hardener for epoxy resin: Flame retardance, curing behavior and mechanical property. Polym. Chem. 2016;7:3003–3012. doi: 10.1039/C6PY00434B. [DOI] [Google Scholar]
- 80.Tan Y., Shao Z.-B., Chen X.-F., Long J.-W., Chen L., Wang Y.-Z. Novel multifunctional organic–inorganic hybrid curing agent with high flame-retardant efficiency for epoxy resin. ACS Appl. Mater. Interfaces. 2015;7:17919–17928. doi: 10.1021/acsami.5b04570. [DOI] [PubMed] [Google Scholar]
- 81.Qian L., Qiu Y., Liu J., Xin F., Chen Y. The flame retardant group-synergistic-effect of a phosphaphenanthrene and triazine double-group compound in epoxy resin. J. Appl. Polym. Sci. 2014;131:39709. doi: 10.1002/app.39709. [DOI] [Google Scholar]
- 82.Qiu Y., Qian L., Xi W. Flame-retardant effect of a novel phosphaphenanthrene/triazine-trione bi-group compound on an epoxy thermoset and its pyrolysis behaviour. RSC Adv. 2016;6:56018–56027. doi: 10.1039/C6RA10752D. [DOI] [Google Scholar]
- 83.Qian X., Song L., Hu Y., Jiang S. Novel DOPO-based epoxy curing agents. J. Therm. Anal. Calorim. 2016;126:1339–1348. doi: 10.1007/s10973-016-5604-6. [DOI] [Google Scholar]
- 84.Qi Z., Zhang W., He X., Yang R. High-efficiency flame retardency of epoxy resin composites with perfect T8 caged phosphorus containing polyhedral oligomeric silsesquioxanes (P-POSSs) Compos. Sci. Technol. 2016;127:8–19. doi: 10.1016/j.compscitech.2016.02.026. [DOI] [Google Scholar]
- 85.Pan Y.-T., Zhang L., Zhao X., Wang D.-Y. Interfacial engineering of renewable metal organic framework derived honeycomb-like nanoporous aluminum hydroxide with tunable porosity. Chem. Sci. 2017;8:3399–3409. doi: 10.1039/C6SC05695D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang W.-j., Zhao B., Zhao P.-h., Zhang C.-y., Liu Y.-q. Bisphenol-S bridged penta (anilino) cyclotriphosphazene and its application in epoxy resins: Synthesis, thermal degradation, and flame retardancy. Polym. Degrad. Stab. 2017;135:140–151. doi: 10.1016/j.polymdegradstab.2016.11.023. [DOI] [Google Scholar]
- 87.Li Z., Song T., Liu J., Yan Y. Thermal and combustion behavior of phosphorus–nitrogen and phosphorus–silicon retarded epoxy. Iran. Polym. J. 2017;26:21–30. doi: 10.1007/s13726-016-0495-8. [DOI] [Google Scholar]
- 88.Li Y., Zheng H., Xu M., Li B., Lai T. Synthesis of a novel phosphonate flame retardant and its application in epoxy resins. J. Appl. Polym. Sci. 2015;132:42765. doi: 10.1002/app.42765. [DOI] [Google Scholar]
- 89.Krishnadevi K., Selvaraj V. Development of cyclophosphazene and rice husk ash incorporated epoxy composites for high performance applications. Polym. Bull. 2017;74:1791–1815. doi: 10.1007/s00289-016-1805-1. [DOI] [Google Scholar]
- 90.Krishnadevi K., Selvaraj V. Development of halogen-free flame retardant phosphazene and rice husk ash incorporated benzoxazine blended epoxy composites for microelectronic applications. New J. Chem. 2015;39:6555–6567. doi: 10.1039/C5NJ00364D. [DOI] [Google Scholar]
- 91.Jian R., Wang P., Duan W., Wang J., Zheng X., Weng J. Synthesis of a novel P/N/S-containing flame retardant and its application in epoxy resin: Thermal property, flame retardance, and pyrolysis behavior. Ind. Eng. Chem. Res. 2016;55:11520–11527. doi: 10.1021/acs.iecr.6b03416. [DOI] [Google Scholar]
- 92.Huo S., Wang J., Yang S., Wang J., Zhang B., Zhang B., Chen X., Tang Y. Synthesis of a novel phosphorus-nitrogen type flame retardant composed of maleimide, triazine-trione, and phosphaphenanthrene and its flame retardant effect on epoxy resin. Polym. Degrad. Stab. 2016;131:106–113. doi: 10.1016/j.polymdegradstab.2016.07.013. [DOI] [Google Scholar]
- 93.He X., Zhang W., Yi D., Yang R. Flame retardancy of ammonium polyphosphate–montmorillonite nanocompounds on epoxy resin. J. Fire Sci. 2016;34:212–225. doi: 10.1177/0734904116637213. [DOI] [Google Scholar]
- 94.He X., Zhang W., Yang R. The characterization of DOPO/MMT nanocompound and its effect on flame retardancy of epoxy resin. Compos. Part A Appl. Sci. Manuf. 2017;98:124–135. doi: 10.1016/j.compositesa.2017.03.020. [DOI] [Google Scholar]
- 95.Fang Y., Qian L., Huang Z. Synergistic barrier flame-retardant effect of aluminium poly-hexamethylenephosphinate and bisphenol-A bis (diphenyl phosphate) in epoxy resin. Polym. Int. 2017;66:719–725. doi: 10.1002/pi.5320. [DOI] [Google Scholar]
- 96.Bereska A., Kafarski P., Bereska B., Tkacz B., Iłowska J., Lenża J. The application of organophosphorus flame-retardants in epoxy resin. J. Vinyl Addit. Technol. 2017;23:142–151. doi: 10.1002/vnl.21492. [DOI] [Google Scholar]
- 97.Jian R., Wang P., Duan W., Xia L., Zheng X. A facile method to flame-retard epoxy resin with maintained mechanical properties through a novel P/N/S-containing flame retardant of tautomerization. Mater. Lett. 2017;204:77–80. doi: 10.1016/j.matlet.2017.05.135. [DOI] [Google Scholar]
- 98.Zhou Y., Feng J., Peng H., Qu H., Hao J. Catalytic pyrolysis and flame retardancy of epoxy resins with solid acid boron phosphate. Polym. Degrad. Stab. 2014;110:395–404. doi: 10.1016/j.polymdegradstab.2014.10.009. [DOI] [Google Scholar]
- 99.Zhao C., Li Y., Xing Y., He D., Yue J. Flame retardant and mechanical properties of epoxy composites containing APP− PSt core− shell microspheres. J. Appl. Polym. Sci. 2014;131:40218. doi: 10.1002/app.40218. [DOI] [Google Scholar]
- 100.Zhang W., Li X., Yang R. Blowing-out effect in epoxy composites flame retarded by DOPO-POSS and its correlation with amide curing agents. Polym. Degrad. Stab. 2012;97:1314–1324. doi: 10.1016/j.polymdegradstab.2012.05.020. [DOI] [Google Scholar]
- 101.Zhang W., Li X., Yang R. Novel flame retardancy effects of DOPO-POSS on epoxy resins. Polym. Degrad. Stab. 2011;96:2167–2173. doi: 10.1016/j.polymdegradstab.2011.09.016. [DOI] [Google Scholar]
- 102.Zhang W., Li X., Li L., Yang R. Study of the synergistic effect of silicon and phosphorus on the blowing-out effect of epoxy resin composites. Polym. Degrad. Stab. 2012;97:1041–1048. doi: 10.1016/j.polymdegradstab.2012.03.008. [DOI] [Google Scholar]
- 103.Zhang W., Li X., Jiang Y., Yang R. Investigations of epoxy resins flame-retarded by phenyl silsesquioxanes of cage and ladder structures. Polym. Degrad. Stab. 2013;98:246–254. doi: 10.1016/j.polymdegradstab.2012.10.005. [DOI] [Google Scholar]
- 104.Zhang W., Li X., Fan H., Yang R. Study on mechanism of phosphorus–silicon synergistic flame retardancy on epoxy resins. Polym. Degrad. Stab. 2012;97:2241–2248. doi: 10.1016/j.polymdegradstab.2012.08.002. [DOI] [Google Scholar]
- 105.Zhang W., He X., Song T., Jiao Q., Yang R. Comparison of intumescence mechanism and blowing-out effect in flame-retarded epoxy resins. Polym. Degrad. Stab. 2015;112:43–51. doi: 10.1016/j.polymdegradstab.2014.11.017. [DOI] [Google Scholar]
- 106.Zhang W., He X., Song T., Jiao Q., Yang R. The influence of the phosphorus-based flame retardant on the flame retardancy of the epoxy resins. Polym. Degrad. Stab. 2014;109:209–217. doi: 10.1016/j.polymdegradstab.2014.07.023. [DOI] [Google Scholar]
- 107.Tian N., Gong J., Wen X., Yao K., Tang T. Synthesis and characterization of a novel organophosphorus oligomer and its application in improving flame retardancy of epoxy resin. RSC Adv. 2014;4:17607–17614. doi: 10.1039/C4RA01525H. [DOI] [Google Scholar]
- 108.Tang Q., Wang B., Shi Y., Song L., Hu Y. Microencapsulated ammonium polyphosphate with glycidyl methacrylate shell: Application to flame retardant epoxy resin. Ind. Eng. Chem. Res. 2013;52:5640–5647. doi: 10.1021/ie302591r. [DOI] [Google Scholar]
- 109.Qu H., Wu W., Hao J., Wang C., Xu J. Inorganic–organic hybrid coating-encapsulated ammonium polyphosphate and its flame retardancy and water resistance in epoxy resin. Fire Mater. 2014;38:312–322. doi: 10.1002/fam.2182. [DOI] [Google Scholar]
- 110.Hongqiang Q., Weihong W., Jianwei H., Jianhong S., Jianzhong X. Intumescent flame retardancy and thermal degradation of epoxy resin filled with ammonium polyphosphate using thermogravimetric analysis–fourier transform infrared spectroscopy and thermogravimetric analysis–mass spectrometry. J. Macromol. Sci. Part B. 2014;53:278–295. doi: 10.1080/00222348.2013.810101. [DOI] [Google Scholar]
- 111.Qian L.-J., Ye L.-J., Xu G.-Z., Liu J., Guo J.-Q. The non-halogen flame retardant epoxy resin based on a novel compound with phosphaphenanthrene and cyclotriphosphazene double functional groups. Polym. Degrad. Stab. 2011;96:1118–1124. doi: 10.1016/j.polymdegradstab.2011.03.001. [DOI] [Google Scholar]
- 112.Qian L., Qiu Y., Sun N., Xu M., Xu G., Xin F., Chen Y. Pyrolysis route of a novel flame retardant constructed by phosphaphenanthrene and triazine-trione groups and its flame-retardant effect on epoxy resin. Polym. Degrad. Stab. 2014;107:98–105. doi: 10.1016/j.polymdegradstab.2014.05.007. [DOI] [Google Scholar]
- 113.Perret B., Schartel B., Stöß K., Ciesielski M., Diederichs J., Döring M., Krämer J., Altstädt V. Novel DOPO-based flame retardants in high-performance carbon fibre epoxy composites for aviation. Eur. Polym. J. 2011;47:1081–1089. doi: 10.1016/j.eurpolymj.2011.02.008. [DOI] [Google Scholar]
- 114.Lv Q., Huang J.-Q., Chen M.-J., Zhao J., Tan Y., Chen L., Wang Y.-Z. An effective flame retardant and smoke suppression oligomer for epoxy resin. Ind. Eng. Chem. Res. 2013;52:9397–9404. doi: 10.1021/ie400911r. [DOI] [Google Scholar]
- 115.Li Z., Yang R. Study of the synergistic effect of polyhedral oligomeric octadiphenylsulfonylsilsesquioxane and 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide on flame-retarded epoxy resins. Polym. Degrad. Stab. 2014;109:233–239. doi: 10.1016/j.polymdegradstab.2014.07.024. [DOI] [Google Scholar]
- 116.Li C., Wan J., Kalali E.N., Fan H., Wang D.-Y. Synthesis and characterization of functional eugenol derivative based layered double hydroxide and its use as a nanoflame-retardant in epoxy resin. J. Mater. Chem. A. 2015;3:3471–3479. doi: 10.1039/C4TA05740F. [DOI] [Google Scholar]
- 117.Wang J.-S., Liu Y., Zhao H.-B., Liu J., Wang D.-Y., Song Y.-P., Wang Y.-Z. Metal compound-enhanced flame retardancy of intumescent epoxy resins containing ammonium polyphosphate. Polym. Degrad. Stab. 2009;94:625–631. doi: 10.1016/j.polymdegradstab.2009.01.006. [DOI] [Google Scholar]
- 118.Guo W., Wang X., Gangireddy C.S.R., Wang J., Pan Y., Xing W., Song L., Hu Y. Cardanol derived benzoxazine in combination with boron-doped graphene toward simultaneously improved toughening and flame retardant epoxy composites. Compos. Part A Appl. Sci. Manuf. 2019;116:13–23. doi: 10.1016/j.compositesa.2018.10.010. [DOI] [Google Scholar]
- 119.Chen R., Hu K., Tang H., Wang J., Zhu F., Zhou H. A novel flame retardant derived from DOPO and piperazine and its application in epoxy resin: Flame retardance, thermal stability and pyrolysis behavior. Polym. Degrad. Stab. 2019;166:334–343. doi: 10.1016/j.polymdegradstab.2019.06.011. [DOI] [Google Scholar]
- 120.Rajaei M., Kim N., Bickerton S., Bhattacharyya D. A comparative study on effects of natural and synthesised nano-clays on the fire and mechanical properties of epoxy composites. Compos. Part B Eng. 2019;165:65–74. doi: 10.1016/j.compositesb.2018.11.089. [DOI] [Google Scholar]
- 121.Yu B., Shi Y., Yuan B., Qiu S., Xing W., Hu W., Song L., Lo S., Hu Y. Enhanced thermal and flame retardant properties of flame-retardant-wrapped graphene/epoxy resin nanocomposites. J. Mater. Chem. A. 2015;3:8034–8044. doi: 10.1039/C4TA06613H. [DOI] [Google Scholar]
- 122.Zhao W., Li Y., Li Q., Wang Y., Wang G. Investigation of the structure-property effect of phosphorus-containing polysulfone on decomposition and flame retardant epoxy resin composites. Polymers. 2019;11:380. doi: 10.3390/polym11020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xiao F., Wu K., Luo F., Yao S., Lv M., Zou H., Lu M. Influence of ionic liquid-based metal–organic hybrid on thermal degradation, flame retardancy, and smoke suppression properties of epoxy resin composites. J. Mater. Sci. 2018;53:10135–10146. doi: 10.1007/s10853-018-2318-0. [DOI] [Google Scholar]
- 124.Schmidt C., Ciesielski M., Greiner L., Döring M. Novel organophosphorus flame retardants and their synergistic application in novolac epoxy resin. Polym. Degrad. Stab. 2018;158:190–201. doi: 10.1016/j.polymdegradstab.2018.09.001. [DOI] [Google Scholar]
- 125.Liu H., Wu W., He S., Song Q., Li W., Zhang J., Qu H., Xu J. Activated carbon spheres (ACS)@ SnO2@ NiO with a 3D nanospherical structure and its synergistic effect with AHP on improving the flame retardancy of epoxy resin. Polym. Adv. Technol. 2019;30:951–962. doi: 10.1002/pat.4529. [DOI] [Google Scholar]
- 126.Rajaei M., Wang D.-Y., Bhattacharyya D. Combined effects of ammonium polyphosphate and talc on the fire and mechanical properties of epoxy/glass fabric composites. Compos. Part B Eng. 2017;113:381–390. doi: 10.1016/j.compositesb.2017.01.039. [DOI] [Google Scholar]
- 127.Müller P., Schartel B. Melamine poly (metal phosphates) as flame retardant in epoxy resin: Performance, modes of action, and synergy. J. Appl. Polym. Sci. 2016;133:43549. doi: 10.1002/app.43549. [DOI] [Google Scholar]
- 128.Pan M., Huang R., Wang T., Huang D., Mu J., Zhang C. Preparation and properties of epoxy resin composites containing hexaphenoxycyclotriphosphazene. High Perform. Polym. 2014;26:114–121. doi: 10.1177/0954008313501008. [DOI] [Google Scholar]
- 129.Pan M., Zhang C., Zhai X., Qu L., Mu J. Effect of hexaphenoxycyclotriphosphazene combined with octapropylglycidylether polyhedral oligomeric silsesquioxane on thermal stability and flame retardancy of epoxy resin. High Perform. Polym. 2014;26:744–752. doi: 10.1177/0954008314528227. [DOI] [Google Scholar]
- 130.Wu G.M., Schartel B., Kleemeier M., Hartwig A. Flammability of layered silicate epoxy nanocomposites combined with low-melting inorganic ceepree glass. Polym. Eng. Sci. 2012;52:507–517. doi: 10.1002/pen.22111. [DOI] [Google Scholar]
- 131.Täuber K., Marsico F., Wurm F.R., Schartel B. Hyperbranched poly (phosphoester) s as flame retardants for technical and high performance polymers. Polym. Chem. 2014;5:7042–7053. doi: 10.1039/C4PY00830H. [DOI] [Google Scholar]
- 132.Zhang C., Guo X., Ma S., Liu X., Xu J., Ma H. Synthesis of an organic-inorganic hybrid strontium hydroxystannate nanorod and application as novel flame retardant. Mater. Lett. 2018;229:297–300. doi: 10.1016/j.matlet.2018.07.047. [DOI] [Google Scholar]
- 133.Ma T., Li L., Liu T., Guo C. Synthesis of a caged bicyclic phosphates derived anhydride and its performance as a flame-retardant curing agent for epoxy resins. Polym. Adv. Technol. 2019;30:1314–1324. doi: 10.1002/pat.4565. [DOI] [Google Scholar]
- 134.Guo X., Wang X., Liu X., Zheng Y., Xu J., Ma H. Synthesis and application of a dual functional P/N/S-containing microsphere with enhanced flame retardancy and mechanical strength on EP resin. Polym. Adv. Technol. 2018;29:2665–2673. doi: 10.1002/pat.4380. [DOI] [Google Scholar]
- 135.Li Y.-Y., Wang Y.-L., Yang X.-M., Liu X., Yang Y.-G., Hao J.-W. Acidity regulation of boron phosphate flame retardant and its catalyzing carbonization mechanism in epoxy resin. J. Therm. Anal. Calorim. 2017;129:1481–1494. doi: 10.1007/s10973-017-6311-7. [DOI] [Google Scholar]
- 136.Song S., Ma J., Cao K., Chang G., Huang Y., Yang J. Synthesis of a novel dicyclic silicon-/phosphorus hybrid and its performance on flame retardancy of epoxy resin. Polym. Degrad. Stab. 2014;99:43–52. doi: 10.1016/j.polymdegradstab.2013.12.013. [DOI] [Google Scholar]
- 137.Jiajun M., Junxiao Y., Yawen H., Ke C. Aluminum–organophosphorus hybrid nanorods for simultaneously enhancing the flame retardancy and mechanical properties of epoxy resin. J. Mater. Chem. 2012;22:2007–2017. doi: 10.1039/C1JM13332B. [DOI] [Google Scholar]
- 138.Jiang S., Shi Y., Qian X., Zhou K., Xu H., Lo S., Gui Z., Hu Y. Synthesis of a novel phosphorus-and nitrogen-containing acrylate and its performance as an intumescent flame retardant for epoxy acrylate. Ind. Eng. Chem. Res. 2013;52:17442–17450. doi: 10.1021/ie4028439. [DOI] [Google Scholar]
- 139.Yuan L., Feng S., Hu Y., Fan Y. Effect of char sulfonic acid and ammonium polyphosphate on flame retardancy and thermal properties of epoxy resin and polyamide composites. J. Fire Sci. 2017;35:521–534. doi: 10.1177/0734904117720121. [DOI] [Google Scholar]
- 140.Zhou X., Qiu S., Xing W., Gangireddy C.S.R., Gui Z., Hu Y. Hierarchical polyphosphazene@ molybdenum disulfide hybrid structure for enhancing the flame retardancy and mechanical property of epoxy resins. ACS Appl. Mater. Interfaces. 2017;9:29147–29156. doi: 10.1021/acsami.7b08878. [DOI] [PubMed] [Google Scholar]
- 141.Shan X., Jiang K., Li J., Song Y., Han J., Hu Y. Preparation of β-cyclodextrin inclusion complex and its application as an intumescent flame retardant for epoxy. Polymers. 2019;11:71. doi: 10.3390/polym11010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qiu S., Zhou Y., Zhou X., Zhang T., Wang C., Yuen R.K., Hu W., Hu Y. Air-stable polyphosphazene-functionalized few-layer black phosphorene for flame retardancy of epoxy resins. Small. 2019;15:1805175. doi: 10.1002/smll.201805175. [DOI] [PubMed] [Google Scholar]
- 143.Huang Z., Wang D., Zhu Y., Zeng W., Hu Y. The influence of mesoporous silica modified with phosphorus and nitrogen-containing hyperbranched molecules on thermal stability, combustion behavior, and toxic volatiles of epoxy resin. Polym. Adv. Technol. 2018;29:372–383. doi: 10.1002/pat.4124. [DOI] [Google Scholar]
- 144.Hou Y., Hu W., Gui Z., Hu Y. A novel Co (Ⅱ)–based metal-organic framework with phosphorus-containing structure: Build for enhancing fire safety of epoxy. Compos. Sci. Technol. 2017;152:231–242. doi: 10.1016/j.compscitech.2017.08.032. [DOI] [Google Scholar]
- 145.Fu X.-L., Wang X., Xing W., Zhang P., Song L., Hu Y. Two-dimensional cardanol-derived zirconium phosphate hybrid as flame retardant and smoke suppressant for epoxy resin. Polym. Degrad. Stab. 2018;151:172–180. doi: 10.1016/j.polymdegradstab.2018.03.006. [DOI] [Google Scholar]
- 146.Zheng T., Ni X. Loading an organophosphorous flame retardant into halloysite nanotubes for modifying UV-curable epoxy resin. RSC Adv. 2016;6:57122–57130. doi: 10.1039/C6RA08178A. [DOI] [Google Scholar]
- 147.Sut A., Greiser S., Jäger C., Schartel B. Synergy in flame-retarded epoxy resin: Identification of chemical interactions by solid-state NMR. J. Therm. Anal. Calorim. 2016;128:141–153. doi: 10.1007/s10973-016-5934-4. [DOI] [Google Scholar]
- 148.Qiu S., Xing W., Feng X., Yu B., Mu X., Yuen R.K., Hu Y. Self-standing cuprous oxide nanoparticles on silica@ polyphosphazene nanospheres: 3D nanostructure for enhancing the flame retardancy and toxic effluents elimination of epoxy resins via synergistic catalytic effect. Chem. Eng. J. 2017;309:802–814. doi: 10.1016/j.cej.2016.10.100. [DOI] [Google Scholar]
- 149.Qiu S., Wang X., Yu B., Feng X., Mu X., Yuen R.K., Hu Y. Flame-retardant-wrapped polyphosphazene nanotubes: A novel strategy for enhancing the flame retardancy and smoke toxicity suppression of epoxy resins. J. Hazard. Mater. 2017;325:327–339. doi: 10.1016/j.jhazmat.2016.11.057. [DOI] [PubMed] [Google Scholar]
- 150.Martins M.S., Schartel B., Magalhães F.D., Pereira C. The effect of traditional flame retardants, nanoclays and carbon nanotubes in the fire performance of epoxy resin composites. Fire Mater. 2017;41:111–130. doi: 10.1002/fam.2370. [DOI] [Google Scholar]
- 151.Zhang W., Li X., Yang R. Pyrolysis and fire behaviour of epoxy resin composites based on a phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS) Polym. Degrad. Stab. 2011;96:1821–1832. doi: 10.1016/j.polymdegradstab.2011.07.014. [DOI] [Google Scholar]
- 152.Sun L., Qu Y., Li S. Co-microencapsulate of ammonium polyphosphate and pentaerythritol in intumescent flame-retardant coatings. J. Therm. Anal. Calorim. 2013;111:1099–1106. doi: 10.1007/s10973-012-2494-0. [DOI] [Google Scholar]
- 153.Mariappan T., Zhou Y., Hao J., Wilkie C.A. Influence of oxidation state of phosphorus on the thermal and flammability of polyurea and epoxy resin. Eur. Polym. J. 2013;49:3171–3180. doi: 10.1016/j.eurpolymj.2013.06.009. [DOI] [Google Scholar]
- 154.Mariappan T., Wilkie C.A. Flame retardant epoxy resin for electrical and electronic applications. Fire Mater. 2014;38:588–598. doi: 10.1002/fam.2199. [DOI] [Google Scholar]
- 155.Jiao C., Dong J., Yu J., Chen X. Effect of toughener on combustion and thermal degradation of flame retardant epoxy composites. Plast. Rubber Compos. 2014;43:105–110. doi: 10.1179/1743289814Y.0000000078. [DOI] [Google Scholar]
- 156.Jiao C., Dong J., Zhang C., Zhuo J., Chen X. Synthesis and properties of a phosphate ester as curing agent in an epoxy resin system. Iran. Polym. J. 2014;23:591–598. doi: 10.1007/s13726-014-0253-8. [DOI] [Google Scholar]
- 157.Jiao C., Dong J., Chen X., Li S. Influence of T31 content on combustion and thermal degradation behaviors on flame-retardant epoxy composites. J. Therm. Anal. Calorim. 2013;114:1201–1206. doi: 10.1007/s10973-013-3057-8. [DOI] [Google Scholar]
- 158.Jiao C., Zhang C., Dong J., Chen X., Qian Y., Li S. Combustion behavior and thermal pyrolysis kinetics of flame-retardant epoxy composites based on organic–inorganic intumescent flame retardant. J. Therm. Anal. Calorim. 2015;119:1759–1767. doi: 10.1007/s10973-014-4379-x. [DOI] [Google Scholar]
- 159.Kong Q., Zhang Y., Zhang X., Xiang B., Yi Y., Zhu J., Zhang F., Zhu J., Zhang J. Functionalized montmorillonite intercalation iron compounds for improving flame retardancy of epoxy resin nanocomposites. J. Nanosci. Nanotechnol. 2019;19:5803–5809. doi: 10.1166/jnn.2019.16540. [DOI] [PubMed] [Google Scholar]
- 160.Suihkonen R., Nevalainen K., Vuorinen J. The effect of ammonium polyphosphate and nanoclay on the rheological, thermal, and flame retardant properties of epoxy. Annu. Trans. Nord. Rheol. Soc. 2009;17:263–268. [Google Scholar]
- 161.Shi Y., Yu B., Zheng Y., Yang J., Duan Z., Hu Y. Design of reduced graphene oxide decorated with DOPO-phosphanomidate for enhanced fire safety of epoxy resin. J. Colloid Interface Sci. 2018;521:160–171. doi: 10.1016/j.jcis.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 162.Shi X.-H., Chen L., Liu B.-W., Long J.-W., Xu Y.-J., Wang Y.-Z. Carbon fibers decorated by polyelectrolyte complexes toward their epoxy resin composites with high fire safety. Chin. J. Polym. Sci. 2018;36:1375–1384. doi: 10.1007/s10118-018-2164-1. [DOI] [Google Scholar]
- 163.Kandola B.K., Horrocks A.R., Myler P., Blair D. New developments in flame retardancy of glass-reinforced epoxy composites. J. Appl. Polym. Sci. 2003;88:2511–2521. doi: 10.1002/app.11909. [DOI] [Google Scholar]
- 164.Kandola B., Horrocks A., Myler P., Blair D. Mechanical performance of heat/fire damaged novel flame retardant glass-reinforced epoxy composites. Compos. Part A Appl. Sci. Manuf. 2003;34:863–873. doi: 10.1016/S1359-835X(03)00156-8. [DOI] [Google Scholar]
- 165.Boccarusso L., Carrino L., Durante M., Formisano A., Langella A., Minutolo F.M.C. Hemp fabric/epoxy composites manufactured by infusion process: Improvement of fire properties promoted by ammonium polyphosphate. Compos. Part B Eng. 2016;89:117–126. doi: 10.1016/j.compositesb.2015.10.045. [DOI] [Google Scholar]
- 166.Wang X., Song L., Pornwannchai W., Hu Y., Kandola B. The effect of graphene presence in flame retarded epoxy resin matrix on the mechanical and flammability properties of glass fiber-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2013;53:88–96. doi: 10.1016/j.compositesa.2013.05.017. [DOI] [Google Scholar]
- 167.Kandola B., Myler P., Horrocks A., El-Hadidi M., Blair D. Empirical and numerical approach for optimisation of fire and mechanical performance in fire-retardant glass-reinforced epoxy composites. Fire Saf. J. 2008;43:11–23. doi: 10.1016/j.firesaf.2007.04.001. [DOI] [Google Scholar]
- 168.Branda F., Malucelli G., Durante M., Piccolo A., Mazzei P., Costantini A., Silvestri B., Pennetta M., Bifulco A. Silica treatments: A fire retardant strategy for hemp fabric/epoxy composites. Polymers. 2016;8:313. doi: 10.3390/polym8080313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang T., Liu W., Wang M., Liu P., Pan Y., Liu D. Synthesis of a boron/nitrogen-containing compound based on triazine and boronic acid and its flame retardant effect on epoxy resin. High Perform. Polym. 2017;29:513–523. doi: 10.1177/0954008316650929. [DOI] [Google Scholar]
- 170.Li A., Xu W., Chen R., Liu Y., Li W. Fabrication of zeolitic imidazolate frameworks on layered double hydroxide nanosheets to improve the fire safety of epoxy resin. Compos. Part A Appl. Sci. Manuf. 2018;112:558–571. doi: 10.1016/j.compositesa.2018.07.001. [DOI] [Google Scholar]
- 171.Chen S., Ai L., Zhang T., Liu P., Liu W., Pan Y., Liu D. Synthesis and application of a triazine derivative containing boron as flame retardant in epoxy resins. Arab. J. Chem. 2018 doi: 10.1016/j.arabjc.2018.08.007. [DOI] [Google Scholar]
- 172.Li Z., Liu L., González A.J., Wang D.-Y. Bioinspired polydopamine-induced assembly of ultrafine Fe (OH) 3 nanoparticles on halloysite toward highly efficient fire retardancy of epoxy resin via an action of interfacial catalysis. Polym. Chem. 2017;8:3926–3936. doi: 10.1039/C7PY00660H. [DOI] [Google Scholar]
- 173.Unlu S.M., Dogan S.D., Dogan M. Comparative study of boron compounds and aluminum trihydroxide as flame retardant additives in epoxy resin. Polym. Adv. Technol. 2014;25:769–776. doi: 10.1002/pat.3274. [DOI] [Google Scholar]
- 174.Wu K., Kandola B.K., Kandare E., Hu Y. Flame retardant effect of polyhedral oligomeric silsesquioxane and triglycidyl isocyanurate on glass fibre-reinforced epoxy composites. Polym. Compos. 2011;32:378–389. doi: 10.1002/pc.21052. [DOI] [Google Scholar]
- 175.Xu W., Wang G., Liu Y., Chen R., Li W. Zeolitic imidazolate framework-8 was coated with silica and investigated as a flame retardant to improve the flame retardancy and smoke suppression of epoxy resin. RSC Adv. 2018;8:2575–2585. doi: 10.1039/C7RA12816A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li A., Xu W., Wang G., Wang X. Novel strategy for molybdenum disulfide nanosheets grown on titanate nanotubes for enhancing the flame retardancy and smoke suppression of epoxy resin. J. Appl. Polym. Sci. 2018;135:46064. doi: 10.1002/app.46064. [DOI] [Google Scholar]
- 177.Liu Y., Kong Q.H., Zhao X.M., Zhu P., Zhao J., Esteban-Cubillo A., Santarén J., Wang D.Y. Effect of Fe3O4-doped sepiolite on the flammability and thermal degradation properties of epoxy composites. Polym. Adv. Technol. 2017;28:971–978. doi: 10.1002/pat.3715. [DOI] [Google Scholar]
- 178.Liu Y., Babu H.V., Zhao J., Goñi-Urtiaga A., Sainz R., Ferritto R., Pita M., Wang D.-Y. Effect of Cu-doped graphene on the flammability and thermal properties of epoxy composites. Compos. Part B Eng. 2016;89:108–116. doi: 10.1016/j.compositesb.2015.11.035. [DOI] [Google Scholar]
- 179.Wang W., Kan Y., Liew K.M., Song L., Hu Y. Comparative investigation on combustion property and smoke toxicity of epoxy resin filled with α-and δ-MnO2 nanosheets. Compos. Part A Appl. Sci. Manuf. 2018;107:39–46. doi: 10.1016/j.compositesa.2017.12.021. [DOI] [Google Scholar]
- 180.Sharma A.K., Sloan R., Ramakrishnan R., Nazarenko S.I., Wiggins J.S. Structure-property relationships in epoxy hybrid networks based on high mass fraction pendant POSS incorporated at molecular level. Polymer. 2018;139:201–212. doi: 10.1016/j.polymer.2018.02.014. [DOI] [Google Scholar]
- 181.Saeb M., Vahabi H., Jouyandeh M., Movahedifar E., Khalili R. Epoxy-based flame retardant nanocomposite coatings: Comparison between functions of expandable graphite and halloysite nanotubes. Prog. Color Colorants Coat. 2017;10:245–252. [Google Scholar]
- 182.Pawelski C., Kang E., Bakis G., Altstädt V. Effect of filler type and particle size distribution on thermal properties of bimodal and hybrid–BN/Boehmite-filled EP-Novolac composites. AIP Conference Proceedings. 2019;2055:050007. doi: 10.1063/1.5084826. [DOI] [Google Scholar]
- 183.Liu L., Wang W., Shi Y., Fu L., Xu L., Yu B. Electrostatic-interaction-driven assembly of binary hybrids towards fire-safe epoxy resin nanocomposites. Polymers. 2019;11:229. doi: 10.3390/polym11020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li X., Feng Y., Chen C., Ye Y., Zeng H., Qu H., Liu J., Zhou X., Long S., Xie X. Highly thermally conductive flame retardant epoxy nanocomposites with multifunctional ionic liquid flame retardant-functionalized boron nitride nanosheets. J. Mater. Chem. A. 2018;6:20500–20512. doi: 10.1039/C8TA08008A. [DOI] [Google Scholar]
- 185.Chai G.-q., Wang Z., Zhang X. Study of the flame retardant properties of short carbon fiber–reinforced epoxy composites. High Perform. Polym. 2018;30:1027–1035. doi: 10.1177/0954008317736374. [DOI] [Google Scholar]
- 186.Zabihi O., Ahmadi M., Khayyam H., Naebe M. Fish DNA-modified clays: Towards highly flame retardant polymer nanocomposite with improved interfacial and mechanical performance. Sci. Rep. 2016;6:38194. doi: 10.1038/srep38194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang W., Pan H., Shi Y., Pan Y., Yang W., Liew K., Song L., Hu Y. Fabrication of LDH nanosheets on β-FeOOH rods and applications for improving the fire safety of epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016;80:259–269. doi: 10.1016/j.compositesa.2015.10.031. [DOI] [Google Scholar]
- 188.Wang W., Kan Y., Pan Y., Yuan Y., Liew K.M., Hu Y. Urchinlike shells of TiO2 hollow spheres for improving the fire safety of epoxy resin. Ind. Eng. Chem. Res. 2017;56:1341–1348. doi: 10.1021/acs.iecr.6b03979. [DOI] [Google Scholar]
- 189.Wang D., Song L., Zhou K., Yu X., Hu Y., Wang J. Anomalous nano-barrier effects of ultrathin molybdenum disulfide nanosheets for improving the flame retardance of polymer nanocomposites. J. Mater. Chem. A. 2015;3:14307–14317. doi: 10.1039/C5TA01720C. [DOI] [Google Scholar]
- 190.Laachachi A., Burger N., Apaydin K., Sonnier R., Ferriol M. Is expanded graphite acting as flame retardant in epoxy resin? Polym. Degrad. Stab. 2015;117:22–29. doi: 10.1016/j.polymdegradstab.2015.03.016. [DOI] [Google Scholar]
- 191.Benelli T., Mazzocchetti L., D’Angelo E., Lanzi M., Saraga F., Sambri L., Franchini M.C., Giorgini L. New nitrogen-rich heterocycles for organo-modified bentonites as flame retardant fillers in epoxy resin nanocomposites. Polym. Eng. Sci. 2017;57:621–630. doi: 10.1002/pen.24565. [DOI] [Google Scholar]
- 192.Benelli T., D’Angelo E., Mazzocchetti L., Saraga F., Sambri L., Franchini M.C., Giorgini L. Organo-modified bentonites as new flame retardant fillers in epoxy resin nanocomposites. Aip Conf. Proc. 2016;1736:020142. doi: 10.1063/1.4949717. [DOI] [Google Scholar]
- 193.Wang X., Zhou S., Xing W., Yu B., Feng X., Song L., Hu Y. Self-assembly of Ni–Fe layered double hydroxide/graphene hybrids for reducing fire hazard in epoxy composites. J. Mater. Chem. A. 2013;1:4383–4390. doi: 10.1039/c3ta00035d. [DOI] [Google Scholar]
- 194.Pan M., Guan D., Wang T., Huang R., Mu J., Zhang C. Morphology, thermal properties, and fire behavior of epoxy resin nanocomposites containing octaammonium polyhedral oligomeric silsesquioxane-modified montmorillonite. High Perform. Polym. 2013;25:992–999. doi: 10.1177/0954008313493101. [DOI] [Google Scholar]
- 195.Mariappan T., Yi D., Chakraborty A., Singha N.K., Wilkie C.A. Thermal stability and fire retardancy of polyurea and epoxy nanocomposites using organically modified magadiite. J. Fire Sci. 2014;32:346–361. doi: 10.1177/0734904113516268. [DOI] [Google Scholar]
- 196.Jiang S.-D., Bai Z.-M., Tang G., Song L., Stec A.A., Hull T.R., Zhan J., Hu Y. Fabrication of Ce-doped MnO 2 decorated graphene sheets for fire safety applications of epoxy composites: Flame retardancy, smoke suppression and mechanism. J. Mater. Chem. A. 2014;2:17341–17351. doi: 10.1039/C4TA02882A. [DOI] [Google Scholar]
- 197.Jiang S.-D., Bai Z.-M., Tang G., Song L., Stec A.A., Hull T.R., Hu Y., Hu W.-Z. Synthesis of mesoporous silica@ Co–Al layered double hydroxide spheres: Layer-by-layer method and their effects on the flame retardancy of epoxy resins. ACS Appl. Mater. Interfaces. 2014;6:14076–14086. doi: 10.1021/am503412y. [DOI] [PubMed] [Google Scholar]
- 198.Jiang S.-D., Bai Z.-M., Tang G., Hu Y., Song L. Synthesis of ZnS decorated graphene sheets for reducing fire hazards of epoxy composites. Ind. Eng. Chem. Res. 2014;53:6708–6717. doi: 10.1021/ie500023w. [DOI] [Google Scholar]
- 199.Zotti A., Borriello A., Ricciardi M., Antonucci V., Giordano M., Zarrelli M. Effects of sepiolite clay on degradation and fire behaviour of a bisphenol A-based epoxy. Compos. Part B Eng. 2015;73:139–148. doi: 10.1016/j.compositesb.2014.12.019. [DOI] [Google Scholar]
- 200.Wladyka-Przybylak M., Wesolek D., Gieparda W., Boczkowska A., Ciecierska E. Functionalization effect on physico-mechanical properties of multi-walled carbon nanotubes/epoxy composites. Polym. Adv. Technol. 2011;22:48–59. doi: 10.1002/pat.1768. [DOI] [Google Scholar]
- 201.Wang Z., Tang X.-Z., Yu Z.-Z., Guo P., Song H.-H., Duc X.-S. Dispersion of graphene oxide and its flame retardancy effect on epoxy nanocomposites. Chin. J. Polym. Sci. 2011;29:368–376. doi: 10.1007/s10118-011-1037-7. [DOI] [Google Scholar]
- 202.Wang D., Zhou K., Yang W., Xing W., Hu Y., Gong X. Surface modification of graphene with layered molybdenum disulfide and their synergistic reinforcement on reducing fire hazards of epoxy resins. Ind. Eng. Chem. Res. 2013;52:17882–17890. doi: 10.1021/ie402441g. [DOI] [Google Scholar]
- 203.Yu B., Xing W., Guo W., Qiu S., Wang X., Lo S., Hu Y. Thermal exfoliation of hexagonal boron nitride for effective enhancements on thermal stability, flame retardancy and smoke suppression of epoxy resin nanocomposites via sol–gel process. J. Mater. Chem. A. 2016;4:7330–7340. doi: 10.1039/C6TA01565D. [DOI] [Google Scholar]
- 204.Ngo T., Nguyen Q., Nguyen T., Tran P. Effect of nanoclay on thermomechanical properties of epoxy/glass fibre composites. Arab. J. Sci. Eng. 2016;41:1251–1261. doi: 10.1007/s13369-015-1898-0. [DOI] [Google Scholar]
- 205.Eibl S. Potential for the formation of respirable fibers in carbon fiber reinforced plastic materials after combustion. Fire Mater. 2017;41:808–816. doi: 10.1002/fam.2423. [DOI] [Google Scholar]
- 206.Wu Q., Zhu W., Zhang C., Liang Z., Wang B. Study of fire retardant behavior of carbon nanotube membranes and carbon nanofiber paper in carbon fiber reinforced epoxy composites. Carbon. 2010;48:1799–1806. doi: 10.1016/j.carbon.2010.01.023. [DOI] [Google Scholar]
- 207.Wu Q., Bao J., Zhang C., Liang R., Wang B. The effect of thermal stability of carbon nanotubes on the flame retardancy of epoxy and bismaleimide/carbon fiber/buckypaper composites. J. Therm. Anal. Calorim. 2010;103:237–242. doi: 10.1007/s10973-010-0960-0. [DOI] [Google Scholar]
- 208.Xu Z., Chu Z., Yan L., Chen H., Jia H., Tang W. Effect of chicken eggshell on the flame-retardant and smoke suppression properties of an epoxy-based traditional APP-PER-MEL system. Polym. Compos. 2019;40:2712–2723. doi: 10.1002/pc.25077. [DOI] [Google Scholar]
- 209.Peng H., Zhang S., Yin Y., Jiang S., Mo W. Fabrication of c-6 position carboxyl regenerated cotton cellulose by H2O2 and its promotion in flame retardency of epoxy resin. Polym. Degrad. Stab. 2017;142:150–159. doi: 10.1016/j.polymdegradstab.2017.05.026. [DOI] [Google Scholar]
- 210.Ma T., Li L., Wang Q., Guo C. High-performance flame retarded paraffin/epoxy resin form-stable phase change material. J. Mater. Sci. 2019;54:875–885. doi: 10.1007/s10853-018-2846-7. [DOI] [Google Scholar]
- 211.Zhuo J., Xie L., Liu G., Chen X., Wang Y. The synergistic effect of hollow glass microsphere in intumescent flame-retardant epoxy resin. J. Therm. Anal. Calorim. 2017;129:357–366. doi: 10.1007/s10973-017-6142-6. [DOI] [Google Scholar]
- 212.Zhang S., Liu F., Peng H., Peng X., Jiang S., Wang J. Preparation of novel C-6 position carboxyl corn starch by a green method and its application in flame retardance of epoxy resin. Ind. Eng. Chem. Res. 2015;54:11944–11952. doi: 10.1021/acs.iecr.5b03266. [DOI] [Google Scholar]
- 213.Lu L., Qian X., Zeng Z., Yang S., Shao G., Wang H., Jin J., Xu X. Novel phosphorus-based flame retardants containing 4-tert-butylcalix [4] arene: Preparation and application for the fire safety of epoxy resins. J. Appl. Polym. Sci. 2017;134:45105. doi: 10.1002/app.45105. [DOI] [Google Scholar]
- 214.Gao M., Sun Y. Flame retardancy and thermal degradation behaviors of epoxy resins containing bisphenol a bis (diphenyl phosphate) oligomer. Polym. Eng. Sci. 2013;53:1125–1130. doi: 10.1002/pen.23360. [DOI] [Google Scholar]
- 215.Zheng T., Ni X. Loading the polyol carbonization agent into clay nanotubes for the preparation of environmentally stable UV-cured epoxy materials. J. Appl. Polym. Sci. 2017;134:45045. doi: 10.1002/app.45045. [DOI] [Google Scholar]
- 216.Liu L., Chen X., Jiao C. Influence of ferric phosphate on smoke suppression properties and combustion behavior of intumescent flame retardant epoxy composites. Iran. Polym. J. 2015;24:337–347. doi: 10.1007/s13726-015-0327-2. [DOI] [Google Scholar]
- 217.Chen X., Liu L., Jiao C., Qian Y., Li S. Influence of ferrite yellow on combustion and smoke suppression properties in intumescent flame-retardant epoxy composites. High Perform. Polym. 2015;27:412–425. doi: 10.1177/0954008314553644. [DOI] [Google Scholar]
- 218.Chen X., Liu L., Jiao C. Influence of iron oxide brown on smoke-suppression properties and combustion behavior of intumescent flame-retardant epoxy composites. Adv. Polym. Technol. 2015;34:21516. doi: 10.1002/adv.21516. [DOI] [Google Scholar]
- 219.Chen X., Liu L., Zhuo J., Jiao C., Qian Y. Influence of organic-modified iron–montmorillonite on smoke-suppression properties and combustion behavior of intumescent flame-retardant epoxy composites. High Perform. Polym. 2015;27:233–246. doi: 10.1177/0954008314544341. [DOI] [Google Scholar]
- 220.Morgan A.B., Galaska M. Microcombustion calorimetry as a tool for screening flame retardancy in epoxy. Polym. Adv. Technol. 2008;19:530–546. doi: 10.1002/pat.1100. [DOI] [Google Scholar]
- 221.Kandare E., Kandola B.K., Myler P., Edwards G. Thermo-mechanical responses of fiber-reinforced epoxy composites exposed to high temperature environments. Part I: Experimental data acquisition. J. Compos. Mater. 2010;44:3093–3114. doi: 10.1177/0021998310373511. [DOI] [Google Scholar]