Abstract
Key evidence points toward alterations in the neurocircuitry of large‐scale networks among patients with posttraumatic stress disorder (PTSD). The pulvinar is a thalamic region displaying reciprocal connectivity with the cortex and has been shown to modulate alpha synchrony to facilitate network communication. During rest, the pulvinar displays functional connectivity with the posterior parietal cortex (PPC), a heteromodal network of brain areas underlying multisensory integration and socioaffective functions that are shown at deficit in PTSD. Accordingly, this study seeks to reveal the resting‐state functional connectivity (rsFC) patterns of individuals with PTSD, its dissociative subtype (PTSD + DS) and healthy controls. A whole‐brain rsFC analysis was conducted using SPM12 and PickAtlas. Connectivity was analyzed for the left and right pulvinar across groups of individuals with PTSD (n = 81), PTSD + DS (n = 49), and controls (n = 51). As compared to PTSD, controls displayed significantly greater pulvinar rsFC with the superior parietal lobule and precuneus. Moreover, as compared to PTSD + DS, controls showed increased pulvinar connectivity with the superior parietal lobule, inferior parietal lobule and the precuneus. PTSD groups did not display stronger connectivity with any region as compared to controls. Last, PTSD had greater rsFC in the supramarginal gyrus relative to PTSD + DS. Reduced connectivity between the pulvinar and PPC may explain impairments to autobiographical memory, self‐referential processing, and socioaffective domains in PTSD and PTSD + DS even at “rest.” Critically, these alterations appear to be exacerbated in individuals with PTSD + DS, which may have important implications for treatment.
Keywords: functional connectivity, posttraumatic stress disorder, psychopathology, pulvinar
1. INTRODUCTION
The pulvinar nuclei are high‐order thalamic nuclei that relay information to cortical and midbrain structures. Subcortically, the pulvinar innervates the superior colliculus and amygdala in a nonconscious, visual‐detection network that confers preferential processing to threatening stimuli (Bourne & Morrone, 2017; Liddell et al., 2005). Cortically, the pulvinar mediates transcortical communication and serves a role in the high‐order functions of attention (Saalmann, Pinsk, Wang, Li, & Kastner, 2012; Strumpf et al., 2013; Zhou, Schafer, & Desimone, 2016), emotion (Arend et al., 2015; Padmala, 2010), and spatial awareness (Karnath, Himmelbach, & Rorden, 2002). These functions rely on thalamic nuclei to bind dispersed cortical regions through synchronizing electrophysiological activity (Klimesch, 2012). Here, the pulvinar is thought to align excitability patterns within alpha frequencies to facilitate neuronal communication, known as alpha synchrony (Klimesch, 2012; Saalmann et al., 2012). Low‐frequency, alpha oscillations are believed to serve a direct and active role in network establishment and coordination (Buschman & Miller, 2007; Palva & Palva, 2007). After alpha synchrony is established, the cortex may project higher frequency waveforms throughout the network, resulting in cross‐frequency coupling (Canolty & Knight, 2010). This process is often referred to as cortical top–down modulation (Lakatos, O'connell, & Barczak, 2016). During cortical modulation, the pulvinar adopts the firing patterns of the gamma‐oscillating cortex (Zhou et al., 2016). This mechanism of cross‐frequency coupling, centralized on thalamocortical loops, is hypothesized to underlie, in part, functions of attention and consciousness (Llinas, Ribary, Contreras, & Pedroarena, 1998; Palva & Palva, 2007). Moreover, pulvinar deactivation has been shown to reduce visual responsiveness and the influence of attention over the cortex, suggesting the pulvinar acts to enable cortical function within large‐scale networks, alterations in which may contribute to cognitive and affective dysfunctions seen across psychopathologies (Lakatos et al., 2016).
Proposed initially by Menon (2011), intrinsic connectivity networks (ICNs) are large‐scale neurocognitive networks required to interface with the world. The default‐mode network (DMN) is a task‐negative ICN activated during internal cognition, self‐referential processing, and autobiographical memory (Menon & Uddin, 2010; Rabellino et al., 2015). Critically, ICNs function in opposition, where the engagement of one network results in the inhibition of the others (Menon & Uddin, 2010). The ability to coordinate activation within these networks is crucial, appearing to rely on pulvinar connectivity. Emerging evidence suggests that the position and functional organization of the pulvinar make it an apt structure to serve as “coordinator” between large‐scale networks (Chen et al., 2013; Hamilton et al., 2012). First, studies employing functional magnetic resonance imaging (fMRI) concurrent with magnetoencephalography reveal that the pulvinar displays bidirectional connectivity with ICN nodes (Barron, Eickhoff, Clos, & Fox, 2015; Stein et al., 2000). Second, the pulvinar serves a fundamental role in binding networks through alpha synchrony and subsequently engaging in gamma oscillations during high‐order processing, such as attention (Klimesch, 2012; Zhou et al., 2016). Third, the pulvinar mediates transmission in both stimulus‐driven and goal‐directed attention networks (Baluch & Itti, 2011; Barron et al., 2015; Itti, 2005). Indeed, this latter finding led Barron et al. (2015) to posit that the pulvinar may serve as a meta‐controller of attention, where it organizes the switching between bottom–up and top–down modes of attention. Taken together, the organization of large‐scale networks may be centralized and regulated through pulvinar connectivity.
Posttraumatic stress disorder (PTSD) is associated with altered neurocircuitry of ICNs that is expressed in symptomatically distinct phenotypes (Koch et al., 2016). Dysfunction of these large‐scale networks relate directly to observed impairments in PTSD, which include alterations in attention (Block et al., 2017; Hart et al., 2017; Shalev et al., 1998), awareness (Craig, 2009), and emotion regulation (Etkin & Wagner, 2007; Lanius et al., 2010). In general, PTSD is associated with an increase in salience network connectivity and a decrease in DMN connectivity (Bluhm et al., 2009; Patel, Spreng, Shin, & Girard, 2012; Sripada et al., 2012). Moreover, alterations have been reported across all ICNs in PTSD and, critically, these alterations correlate with symptom severity (Cisler, Scott Steele, Smitherman, Lenow, & Kilts, 2013; Rabellino et al., 2015; Tursich et al., 2015). Key regions typically displaying reduced functional connectivity at rest in PTSD include the precuneus, superior parietal lobule, inferior parietal lobule, and angular gyrus (Bluhm et al., 2009; Cisler et al., 2013; Di Gangi et al., 2016; Dunkley et al., 2015; Hart et al., 2017; Sripada et al., 2012). Early trauma and its resulting perturbation of the developmental trajectory of the networks may result in alterations in the connectivity and operation of these large‐scale networks (Daniels, Frewen, McKinnon, & Lanius, 2011). As noted, the pulvinar may coordinate each of these networks and has been shown to be involved in the posttraumatic response (Chen et al., 2013; Lanius, Frewen, Tursich, Jetly, & McKinnon, 2015).
Despite such knowledge, to date, no study has sought specifically to examine resting‐state functional connectivity (rsFC) of the pulvinar nuclei in PTSD. The pulvinar is a structure with optimal position and organization to coordinate networks whose interplay give rise to high‐order functions. In PTSD, thalamic nuclei show less activation when compared to trauma‐exposed controls (Lanius et al., 2001), a finding confirmed in a meta‐analysis indicating thalamic hypoactivation in PTSD (Etkin & Wagner, 2007). Accordingly, the objective of this study was to examine pulvinar rsFC across a sample of PTSD patients with (+DS) and without the dissociative subtype as compared to healthy controls. Here, we hypothesized that PTSD patients would show reduced pulvinar connectivity with regions underlying internal cognition and self‐referential processing, a hypothesis driven by previous observations of reductions in DMN interconnectivity in PTSD (Bluhm et al., 2009). Given that individuals with PTSD + DS generally show greater deficits in socio‐cognitive functioning, we predict that reductions in pulvinar rsFC will be exacerbated in this group (Lanius, Bluhm, & Frewen, 2011).
2. METHODS
2.1. Participants and procedure
This study was approved by the Western University's Research Ethics Board and adhered to the standards and regulations set forth by the Tri‐Council Policy. In total, 181 participants were involved in the study, including 130 patients with PTSD (PTSD: n = 81; PTSD + DS: n = 49) and 51 controls. All participants generated written and informed consent for their involvement. Participants were recruited between 2009 and 2017 by the London Health Services Centre through referrals by physicians, mental health professionals, community clinics, and advertisements within the London, Ontario area. Data collected for these participants have been analyzed separately and reported in previous studies within our laboratory (see Harricharan et al., 2016; Nicholson et al., 2016; Olivé et al., 2018).
Exclusion criteria for the study included nonconformity with 3.0 T functional scanning requirements, pregnancy, history of neurological or developmental disorders, diagnosis of schizophrenia or bipolar disorder, alcohol or drug dependence disorder within six months prior to participation, and a history of head trauma. Inclusion for PTSD and PTSD + DS was based on a Structured Clinical Interview for DSM‐IV Axis‐I disorders (SCID) as well as a Clinician Administered PTSD Scale (CAPS) [CAPS version IV: n = 136 (cutoff score > 50 meets PTSD threshold); CAPS version 5: n = 45 (uses different scoring system with no definitive cutoff) (Blake et al., 1995)] to assess the severity and frequency of symptoms. In addition, the Childhood Trauma Questionnaire (CTQ), Multiscale Dissociation Inventory (MDI), and Beck's Depression Inventory (BDI) were administered. The CTQ is a screening tool for maltreatment histories that can be applied to both clinical and non‐referred groups (Bernstein et al., 2003). The MDI analyzes the dimensionality of dissociative experiences, with focus on five symptom clusters, including disengagement, identity dissociation, emotional constriction, memory disturbance, and depersonalization/derealization (Briere, Weathers, & Runtz, 2005). In our analyses, we placed predominant interest in the latter cluster of the MDI. Patients meeting criteria for the dissociative subtype scored at or above two in frequency and intensity for depersonalization/derealization symptoms as per standard methods (Harricharan et al., 2016; Nicholson et al., 2016; Steuwe et al., 2014). Finally, the BDI is a seven‐item, self‐report screening instrument used to assess cognitive and affective symptoms related to a major depression (Beck, Guth, Steer, & Ball, 1997). Statistical analysis carried out for clinical and demographic measures were computed using SPSS (Version 24, IBM SPSS Statistics for Windows, Armonk, NY) and are included in Table 1.
Table 1.
Clinical and demographic differences between groups
| Measure | PTSD + DS (N = 49), M ± SD (n of received scores) | PTSD (N = 81), M ± SD (n of received scores) | Healthy controls (N = 51), M ± SD (n of received scores) |
|---|---|---|---|
| Years of age | 40 ± 13.5 | 39 ± 11.8 | 35 ± 11.6 |
| Sex | Male = 10, female = 38 | Male = 33, female = 46 | Male = 17, female = 34 |
| CAPS total | 81.6 ± 12.9 (n = 29) | 66.6 ± 14.9 (n = 52) | 0.6 ± 2.7 (n = 51) |
| Caps 5 | 41.4 ± 7.7 (n = 19) | 36.6 ± 9.2 (n = 26) | NA |
| CTQ | 69.7 ± 19.4 (n = 46) | 56.6 ± 23.1 (n = 74) | 32.3 ± 9.2 (n = 49) |
| BDI | 35.1 ± 11.7 (n = 46) | 23.2 ± 8.3 (n = 70) | 1.1 ± 1.9 (n = 49) |
| MDI total | 80.9 ± 22.2 (n = 45) | 53.6 ± 14.8 (n = 74) | 34.0 ± 3.9 (n = 50) |
| MDI‐Dep | 12.9 ± 5.6 (n = 45) | 6.7 ± 2.6 (n = 74) | 5.2 ± 0.6 (n = 50) |
| MDI‐Der | 13.3 ± 4.4 (n = 45) | 8.5 ± 3.2 (n = 74) | 5.2 ± 0.6 (n = 50) |
| MDI‐Dep/Der | 12.9 ± 4.6 (n = 37) | 7.7 ± 2.7 (n = 64) | 5.2 ± 0.5 (n = 50) |
| RSDI‐Diss | 4.9 ± 2.0 (n = 27) | 3.6 ± 1.3 (n = 63) | 2.7 ± 0.5 (n = 49) |
| STAI | 6.2 ± 2.5 (n = 27) | 5.8 ± 2.1 (n = 63) | 3.6 ± 1.2 (n = 49) |
| CADSS | 4.7 ± 2.7 (n = 27) | 3.7 ± 1.2 (n = 63) | 3.2 ± 0.6 (n = 49) |
| MDD status | N = 22 | N = 12 | N = 0 |
Note. Abbreviations: BDI = Beck Depression Inventory; CADSS = Clinician‐Administered Dissociative States Scale; CAPS = Clinician Administered PTSD Scale; CTQ = Childhood Trauma Questionnaire; MDD = Major Depression Disorder; MDI = Multiscale Dissociation Inventory (Dep = Depersonalization Subscale; Der = Derealization Subscale; Dep/Der = Depersonalization and Derealization Subscales Averaged); RSDI‐Diss = Responses to Script Driven Imagery Scale – Dissociation Subscale; STAI = State‐Trait Anxiety Inventory.
Age, sex, and trait scores (CAPS total, Caps 5, CTQ, BDI, MDI (total, Dep, Der, Dep/Der)), state scores (RSDI‐Diss, STAI, CADSS), and MDD status reported for all sample groups (PTSD + DS, PTSD, healthy controls) as mean values plus/minus standard deviations.
The study procedure involved a 6‐min resting‐state scan during which participants were instructed to relax and let their minds wander. Participants completed the State‐Trait Anxiety Inventory (STAI) (Spielberger, 2010), the Responses to Strict‐Driven Imagery Scale (RSDI) (Hopper, Frewen, van der Kolk, & Lanius, 2007), and the Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998) following the scan to identify state anxiety, PTSD, and dissociative symptoms, respectively, during the resting scan.
2.2. Image acquisition
During functional magnetic resonance imaging (fMRI), blood oxygen‐level dependent (BOLD) signals were collected across the whole brain for participants. Scanning was conducted on a 3.0 T scanner at either Robarts Research Institute (Siemens MAGNETOM Fit Whole‐Body) or at Lawson Health Research Institute (Siemens Biograph mMR, Siemens Medical Solutions, Erlangen, Germany). Functional data were collected on a 32‐channel phased array head coil using the manufacturer's standard gradient‐echo EPI pulse sequence (single‐shot, blipped EPI) with interleaved slice acquisition order and tridimensional perspective correction and an isotropic resolution of 2 mm [(FOV = 192 mm × 192 mm × 128 mm (94 × 94 matrix, 64 slices), TR/TE = 3,000 ms/20 ms, FA = 90° (FOV = field of view; TR = time resolution; TE = echo time; FA = flip angle)].
2.3. Preprocessing
Data preprocessing was conducted on Statistical Parametric Mapping (SPM12, Wellcome Trust Centre for Neuroimaging, London, UK: http://www.fil.ion.ucl.ac.uk/spm) within MATLAB 9.2 (R2017a, Mathworks Inc., MA). Functional images for each participant were realigned to the first volume of each session to correct for movement in the scanner. From this, a mean functional image was created for each subject, which was then co‐registered to the T1‐weighted anatomical image to realign BOLD signals for the participant within their anatomical space. All volumes in a series were registered to an EPI template in MNI space using a deformation matrix. An ART regressor was calculated for each subject and added as a covariate to account for effects of movement and global signal correction (version 2015‐10; Gabrieli Lab, McGovern Institute for Brain Research, Cambridge, MA). Factory default thresholds for outliers in the ART regressor were selected (global signal threshold = 9.0 mm, absolute subject motion threshold = 2.0 mm, rotational threshold = .05 mm, scan‐to‐scan subject motion = 2.0 mm, and scan‐to‐scan subject rotation = .02 mm) and employed within the first‐level (within‐subject) of analysis. A three‐dimensional isotropic 6 mm full‐width at half‐maximum Gaussian kernel was applied to each set of volumes to smooth the functional data. Band‐pass filtering was conducted using a high‐pass (0.012 Hz) and low‐pass (0.1 Hz) filter to denoise the data from physiological effects and also to target frequencies of interest for resting‐state; for example, the amplitude of low‐frequency fluctuation that makes up the resting signal.
2.4. Statistical analysis
2.4.1. Within‐subject analysis
A linear model with a mean signal intensity time course and an ART regressor were generated for each subject during the resting scan. The rsFC patterns were analyzed with the left and right pulvinar seeded. The pulvinar seeds were adopted from the TD broadmann+ atlas from the PickAtlas software (WFU Pickatlas, version 2.5.2. A mean signal intensity time course was extracted from the left and right pulvinar for subject's functional volumes using in‐house software developed by co‐author Jean Théberge. Positive correlation maps between pulvinar signal intensity time course and whole brain voxels were created using SPM12.
Figure 1.
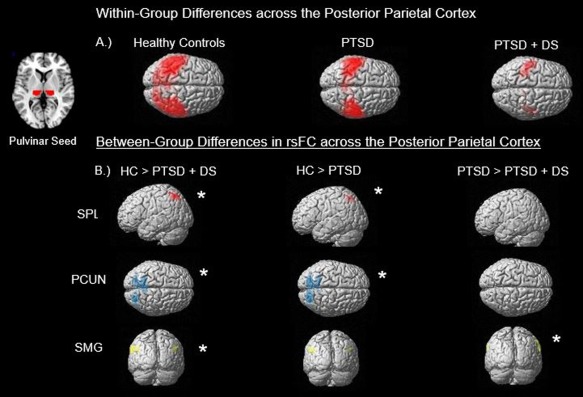
This illustration summarizes the main findings from the (a) within‐group and (b) between‐group analyses. Section (a) demonstrates the resting‐state functional connectivity (rsFC) across all subjects within their respective group restricted to the posterior parietal cortex. Also included is a generated mask of the pulvinar which was used as the seed to which whole‐brain voxel correlations were conducted against. As seen, healthy controls (HC) demonstrate the greatest pulvinar rsFC and PTSD + DS had the least, with PTSD showing an intermediary of the two. Section (b) breaks down the findings of the between‐group contrasts to three regions, the superior parietal lobule (SPL), displayed in red, precuneus (PCUN), displayed in blue, and supramarginal gyrus (SMG), which is contained within the inferior parietal lobule (IPL), displayed in yellow. Findings were restricted to the left SPL, as it was the only hemisphere displaying any results. Additionally, significant results for the PCUN were generated for both the left and right hemisphere in HC > PTSD + DS, and only the left hemisphere in HC > PTSD. Last, significance for PTSD > PTSD + DS was found within the right SMG. Asterisks indicate significance at pFWE < .05, k = 10 [Color figure can be viewed at http://wileyonlinelibrary.com]
2.4.2. Full factorial
A full‐factorial analysis of variance (ANOVA) was conducted on these data to examine the 3 × 2 interaction between group (Control, PTSD, PTSD + DS) and seed region (left, right). The scanner site location was added to the analysis as a covariate at the level of group. Within‐group and between‐group rsFC patterns were explored using random‐field theory as constructed by SPM12 (p‐FWE corrected < .05, k = 10). A mask was applied to preclude the effects of white matter and cerebral spinal fluid differences. Variances were set to unequal to account for differences in group size. Brain regions for the analyses were identified using the neuromorphometrics atlas included in SPM12 and cross‐referenced with the Talairach Client application through Talairach Daemon (version 2.4.3, Research Imaging Institute, TX) using the MNI2Tal tool ad‐on in MATLAB. All reported results were whole‐brain, peak‐corrected at a threshold of p‐FWE < .05, k = 10.
2.4.3. Clinical correlations
Subsequently, clinical correlations between patterns of rsFC within groups and clinical scores were conducted for both trait measures (CAPS, CTQ, MDI, and BDI) and postscan state measures (STAI, CADSS, and RSDI).
3. RESULTS
3.1. Overview of imaging findings
Overall, this study revealed unique rsFC profiles for the different groups. Here, connectivity between the pulvinar and the cortex was strongest in healthy controls, followed by PTSD, and significantly weaker in PTSD + DS. A full‐factorial analysis revealed that between‐group differences were driven largely by connectivity with the posterior parietal cortex, as well as the posterior DMN, specifically the superior parietal lobule, inferior parietal lobule, and precuneus (Figure 1). Compared to both PTSD groups, healthy controls displayed greater connectivity between the pulvinar and the left superior parietal lobule, right inferior parietal lobule, left and right precuneus, and other parietal regions. By contrast, the PTSD group showed significantly greater connectivity than PTSD + DS between the right pulvinar and the right supramarginal and right precentral gyri. When compared to controls, neither of the PTSD groups showed greater connectivity between the pulvinar and any brain region. Finally, among the PTSD groups, derealization scores from the MDI correlated positively with connectivity between the left pulvinar and the left lingual and fusiform gyri. In addition, state‐dissociative scores measured by the RSDI correlated negatively with left pulvinar and right superior parietal lobule connectivity.
3.2. Clinical and demographic results
An ANOVA conducted across the three groups failed to reveal a significant difference of age (p = .065, df = 2). In addition, a Pearson's chi‐square test failed to demonstrate a significant effect of sex between the groups (p = .053, df = 2). Kruskal–Wallis ANOVAs were carried out for psychological measures with significant effects found for all trait‐score tests, including the CTQ, MDI, and BDI. Similarly, all state‐anxiety clinical scores (e.g., RSDI, STAI, CADSS) measured at rest reached significance across PTSD groups (all p < .001). Consequently, post‐hoc Games–Howell comparisons were conducted for all measures between PTSD and PTSD + DS. Results indicated that PTSD + DS scored significantly higher on the trait‐scores of CTQ, MDI, and BDI (p < .001). For resting‐state clinical scores, only the STAI failed to reveal significant differences between PTSD and PTSD + DS (p = .401). Both CADSS and the RSDI reported greater scores for PTSD + DS as compared to PTSD in the Games–Howell tests (p < .05) (Table 1). As mentioned, the state‐anxiety scores are used to identify the PTSD + DS group from that of PTSD, wherein higher scores on these measures is indicative of subtype identity.
3.3. Full‐factorial ANOVA (3 × 2)
A full‐factorial ANOVA conducted on these data revealed significant main effects of condition (left, right pulvinar) and group (Control, PTSD, PTSD + DS). A full breakdown of the factorial results is tabled in the Supporting Information (See Supplemental Table 1). There were no significant interactions found between the variables.
3.4. Within‐group connectivity differences
A full breakdown of within‐group connectivity differences is included in the Supporting Information (See Supplemental Table 2).
3.5. Between‐group connectivity differences
3.5.1. Control > PTSD + DS
Healthy controls demonstrated significantly greater connectivity between the left pulvinar and the left superior parietal lobule (BA 7), the left middle temporal gyrus (BA 37), and the right postcentral gyrus (BA 3). Moreover, as compared to PTSD + DS, controls showed greater connectivity between the right pulvinar and the left superior frontal gyrus (BA 6), the left superior parietal lobule (BA 7), the left and right precuneus (BA 7), the right inferior parietal lobule (BA 40), and the right precentral gyrus medial segment (BA 6). A comprehensive summary of all comparisons can be found in Table 2.
Table 2.
Between‐group differences in left and right pulvinar connectivity
| Contrast | LR | BA | Region | k | p(FWE‐cor) | z | MNI coordinates | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Control > PTSD + DS (L) | L | 37 | Middle temporal gyrus | 298 | .016 | 4.78 | −62 | −56 | −4 |
| L | 7 | Superior parietal lobule | 632 | .029 | 4.70 | −22 | −58 | 60 | |
| R | 3 | Postcentral gyrus | 66 | .040 | 4.62 | 32 | −36 | 52 | |
| Control > PTSD + DS (R) | L | 6 | Superior frontal gyrus | 303 | .005 | 5.13 | −24 | 0 | 72 |
| L | 7 | Superior parietal lobule | 837 | .006 | 5.10 | −22 | −58 | 60 | |
| L | 7 | Precuneus | Of 837 | .007 | 5.05 | −26 | −52 | 48 | |
| R | 7 | Precuneus | Of 837 | .008 | 5.02 | 6 | −54 | 48 | |
| R | 40 | Inferior parietal lobule | 477 | .030 | 4.69 | 38 | −42 | 54 | |
| R | 6 | Precentral gyrus medial segment | 65 | .040 | 4.62 | 12 | −26 | 54 | |
| Control > PTSD (L) | L | 37 | Fusiform gyrus | 45 | .004 | 5.17 | −46 | −52 | −16 |
| L | 7 | Superior parietal lobule | 683 | .008 | 5.03 | −32 | −66 | 54 | |
| L | 7 | Superior parietal lobule | Of 683 | .015 | 4.86 | −22 | −58 | 60 | |
| L | 7 | Superior parietal lobule | Of 683 | .027 | 4.72 | −38 | −52 | 62 | |
| Control > PTSD (R) | L | 7 | Superior parietal lobule | 1211 | .005 | 5.11 | −20 | −62 | 54 |
| L | 19 | Precuneus | Of 1211 | .017 | 4.84 | −22 | −82 | 40 | |
| L | 7 | Superior parietal lobule | Of 1211 | .018 | 4.82 | −24 | −56 | 48 | |
| PTSD + DS > Control (L) | None | ||||||||
| PTSD + DS > Control (R) | None | ||||||||
| PTSD +DS > PTSD (L) | None | ||||||||
| PTSD + DS > PTSD (R) | None | ||||||||
| PTSD > Control (L) | None | ||||||||
| PTSD > Control (R) | None | ||||||||
| PTSD > PTSD + DS (L) | R | 40 | Supramarginal gyrus | 497 | .015 | 4.87 | 66 | −26 | 28 |
| PTSD > PTSD + DS (R) | R | 40 | Supramarginal gyrus | 773 | .002 | 5.32 | 66 | −24 | 26 |
| R | 6 | Precentral gyrus | Of 773 | .016 | 4.78 | 62 | 2 | 26 | |
Note. Group differences in functional connectivity between PTSD, PTSD + DS, and healthy controls from each pulvinar seed region (left or right) throughout the cortex. All reported results are whole‐brain, peak‐corrected at a threshold of p‐FWE <.05, k = 10. The contrast column lists the specific comparison of functional connectivity and the seeded pulvinar areas in brackets. The hemisphere of the region (LR), corresponding Broadmann Area (BA), region, cluster size (k), significance (p(FWE)‐cor), z‐score (z), and MNI coordinates (x, y, z) of the peak are included as columns.
3.5.2. Control > PTSD
As compared to PTSD, healthy controls showed significantly greater connectivity between the left pulvinar and the left superior parietal lobule (BA 7) and the left fusiform gyrus (BA 37). Moreover, as compared to PTSD, controls showed an increase in right pulvinar connectivity with the left superior parietal lobule (BA 7) and the left precuneus (BA 19).
3.5.3. PTSD > PTSD + DS
As compared to PTSD + DS, the PTSD group displayed significantly greater connectivity between the left pulvinar and right supramarginal gyrus (BA 40). In addition, the right pulvinar demonstrated greater connectivity with the right supramarginal gyrus (BA 40) and right precentral gyrus (BA 6) in PTSD as compared to PTSD + DS.
3.5.4. PTSD or PTSD + DS > control
As compared to healthy controls, neither PTSD nor PTSD + DS showed significantly increased connectivity between the left or right pulvinar with any brain region included in the analyses.
3.6. Clinical measure correlations and functional connectivity
CAPS total scores displayed a negative correlation between the left pulvinar and the right precentral gyrus (BA 6) [(x: 58, y: 2, z: 40), k = 29, pFWE = .016] across all PTSD patients. Specifically, as symptom severity scores increased, the rsFC between the left pulvinar and right precentral gyrus decreased. CADSS state dissociation scores across PTSD patients correlated negatively with connectivity between the right pulvinar and the right postcentral gyrus (BA 1) [(x: 34, y: −30, z: 44), k = 319, pFWE = .032]. Furthermore, a significant negative correlation was detected between the RSDI state dissociation scores and connectivity between the left pulvinar and the right postcentral (BA 1) [(x: 36, y: −32, z: 44), k = 1,405, pFWE = .017], the right superior parietal lobule (BA 40) [(x: 40, y: −36, z: 56), k = 1,405, pFWE = .023], and the left middle temporal gyrus (BA 21) [(x: −64, y: −38, z: −14), k = 1,541, pFWE = .019]. Finally, positive correlations emerged between MDI trait derealization scores and connectivity between the left pulvinar and the left lingual (BA 19) [(x: −24, y: −52, z: −8), k = 458, pFWE = .022], and the left fusiform gyrus (BA 19) [(x: 20, y: −64 z: −4), k = 458, pFWE = .037]. Here, an increase in trait derealization scores was accompanied by a similar increase in the rsFC between the left pulvinar and these two temporal regions. No significant correlations, however, emerged for scores on the BDI, CTQ, or on the state‐anxiety measure of STAI. All clinical correlations are displayed in detail in Table 3.
Table 3.
Correlations of within‐group clinical scores and left and right pulvinar connectivity
| Correlation | +/− | LR | BA | Region | k | p(FWE‐cor) | z | MNI coordinates | ||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| CADSS (L) | +/− | None | ||||||||
| CADSS (R) | + | None | ||||||||
| − | R | 1 | Postcentral gyrus | 319 | .032 | 4.41 | 34 | −30 | 44 | |
| CAPS total (L) | + | None | ||||||||
| − | R | 6 | Precentral gyrus | 29 | .016 | 4.74 | 58 | 2 | 40 | |
| − | R | 4/6 | Postcentral gyrus | Of 29 | .024 | 4.63 | 58 | −6 | 44 | |
| CAPS total (R) | +/− | None | ||||||||
| MDI derealization (L) | + | L | 19 | Lingual gyrus | 458 | .022 | 4.59 | −24 | −52 | −8 |
| + | L | 19 | Occipital fusiform | Of 458 | .037 | 4.47 | −20 | −64 | −4 | |
| − | None | |||||||||
| MDI derealization (R) | +/− | None | ||||||||
| RSDI dissociative (L) | + | None | ||||||||
| − | R | 1 | Postcentral gyrus | 1405 | .017 | 4.59 | 36 | −32 | 44 | |
| − | R | 40 | Superior Parietal Lobe | Of 1405 | .023 | 4.49 | 40 | −36 | 56 | |
| − | L | 21 | Middle temporal | 1541 | .019 | 4.55 | −64 | −38 | −14 | |
| − | L | 1 | Postcentral | Of 1541 | .035 | 4.39 | −42 | −32 | 44 | |
| − | R | 23 | Posterior cingulate | 1489 | .029 | 4.43 | 2 | −44 | 36 | |
| − | R | 21 | Middle temporal | 250 | .044 | 4.33 | 66 | −40 | −8 | |
| RSDI dissociative (R) | +/− | None | ||||||||
Note. Abbreviations: BDI = Beck Depression Inventory; CADSS = Clinician‐Administered Dissociative States Scale; CAPS = Clinician Administered PTSD Scale; CTQ = Childhood Trauma Questionnaire; MDI = Multiscale Dissociation Inventory (Dep = Depersonalization Subscale; Der = Derealization Subscale; Dep/Der = Depersonalization and Derealization Subscales Averaged); RSDI‐Diss = Responses to Script Driven Imagery Scale – Dissociation Subscale; STAI = State‐Trait Anxiety Inventory.
Clinical and state‐dissociative score correlations conducted with left or right pulvinar connectivity across the whole‐brain. All reported results are whole‐brain, peak‐corrected at a threshold of p‐FWE <.05, k = 10. The rows represent within‐group (PTSD, PTSD + DS) correlations between the seeded left and right pulvinar and clinical scores that survived correction. The columns include the direction of the correlation (positive or negative), hemisphere of the region (LR), corresponding Broadmann Area (BA), region, cluster size (k), significance (p(FWE)‐cor)), z‐score (z), and MNI coordinates (x, y, z) of the peak are included as columns.
4. DISCUSSION
4.1. Overview
To date, no study has examined the resting‐state functional connectivity (rsFC) profiles of the pulvinar within PTSD and PTSD + DS despite key proposals that the pulvinar may serve as a candidate locus in the regulation of large‐scale networks underlying many high‐level functions. Accordingly, this study sought to examine differences in pulvinar connectivity between PTSD and PTSD + DS in an attempt to improve pathophysiological understanding of the disorder. As predicted, our analyses revealed greater rsFC in controls with regions contained within the posterior parietal cortex, including the precuneus, supramarginal gyrus, and the superior and inferior parietal lobules. As compared to controls, neither of the PTSD groups exhibited greater pulvinar connectivity with any brain region. Importantly, the reductions in rsFC displayed by PTSD and PTSD + DS are not representative of a global decrease in functional connectivity across the pathological brain. Here, the functional data for our groups have been reported elsewhere in separate analyses that demonstrate increases in patient rsFC in the periaqueductal grey (Harricharan et al., 2016) and amygdala (Nicholson et al., 2016) with the cortex. Despite these increases, we found decreases in pulvinar connectivity in PTSD and PTSD + DS with parietal regions involved in sensory processing and exteroception as compared to controls. Critically, correlations performed on clinical and dissociation scores validated our prediction that symptom severity is inversely associated with pulvinar connectivity with the postcentral gyrus, precentral gyrus, and superior parietal lobule. We discuss these results in turn.
4.2. Pulvinar connectivity with pre‐ and post‐central gyrus
The analysis revealed greater connectivity between the left pulvinar and the right postcentral gyrus (BA 3) in controls relative to PTSD + DS but not as compared to PTSD. The postcentral gyrus contains the somatosensory cortex, a somatotopically organized area wherein thalamocortical projections relay tactile stimuli for processing. Notably, this area is activated during relaxation or when performing meditation (Lazar et al., 2000). Mindfulness‐based stress reduction programs report increased interconnectivity of ICNs following training, including the postcentral gyrus (Kilpatrick et al., 2011). Here, Kilpatrick et al. (2011) suggest increased connectivity with the postcentral gyrus reflects enhanced sensory processing and awareness of experience. Crucially, PTSD + DS is often comorbid not only with altered DMN connectivity but also with alexithymia, or a deficit in the ability to identify and to respond to experience (Frewen, Pain, Dozois, & Lanius, 2006; Rabellino et al., 2015; Sripada et al., 2012). These alterations of PTSD + DS neurocircuitry may present difficulties in the ability to achieve a restful state, as reflected in decreased connectivity with the postcentral gyrus. In support of this observation, state RSDI dissociative symptoms during the resting scan correlated negatively with pulvinar–postcentral gyrus connectivity. This dissociative subscale measures the subjective sense of being detached from the body and the surroundings (Hopper et al., 2007), where disconnection from one's body may be supported by altered pulvinar–somatosensory connectivity (Jones, 2001).
In addition, our results revealed greater connectivity between the right pulvinar and the right medial segment of the precentral gyrus (BA 6) in controls as compared to PTSD + DS. The precentral gyrus is composed largely of the premotor and supplementary motor cortices. These regions coordinate the execution of voluntary movements by translating commands to the motor cortex (BA 4). Decreased connectivity of the precentral gyrus and the anterior cingulate cortex has been reported at rest in PTSD (Kennis, Rademaker, van Rooij, Kahn, & Geuze, 2015). Moreover, a recent meta‐analysis found that precentral gyrus activation is reduced consistently in PTSD as compared to trauma‐exposed controls (Patel et al., 2012). Other studies, however, have found increases in rsFC of the precentral gyrus with key central autonomic network areas in PTSD (Thome et al., 2017). We hypothesize that sympathetic overactivation in PTSD, which can coalesce with motor agitation and defensive posturing at rest (Kozlowska, Walker, McLean, & Carrive, 2015), is mediated by pulvinar‐independent circuitry to the motor cortex. Here, whereas greater pulvinar–precentral connectivity in controls may indicate higher transmission of voluntary motor commands, the increased precentral and autonomic network connectivity described by Thome et al. (2017) may describe greater subcortically driven, involuntary motor projections. Additionally, in this study, CAPS total scores and pulvinar–precentral connectivity correlated negatively, where patients with the highest symptom severity displayed the lowest connectivity between the right pulvinar and the precentral gyrus. As higher symptom scores are indicative of the PTSD + DS diagnoses, it may be that lowered connectivity with the precentral gyrus represents a reduced transmission of voluntary movement commands, as PTSD + DS is more characteristic of sympathetic‐mediated defensive posturing and immobility which may arise via autonomic nervous commands.
4.3. Superior parietal lobule
Relative to both PTSD and PTSD + DS, healthy controls showed stronger left and right pulvinar connectivity with the left superior parietal lobule. The superior parietal lobule (BA 5, 7) lies above the intraparietal sulcus within the posterior parietal cortex, a heteromodal association cortex integrating sensory information to construct an awareness of one's internal state, body schema, and relation to external space (Pearson, 2009). The superior parietal lobule functions during visuo‐motor coordination, working memory processing, and while exerting top–down control of attention (Behrmann, Geng, & Shomstein, 2004; Koenigs, Barbey, Postle, & Grafman, 2009; Wolbers, Weiller, & Büchel, 2003). Reduced connectivity of the superior parietal lobule at rest has been reported in veterans and in trauma‐exposed adolescents with and without PTSD (Di Gangi et al., 2016; Pan et al., 2016). Moreover, Dunkley et al. (2015) have shown atypical coordination of neural synchronization within the right superior parietal lobule in PTSD during a high‐demand attention task. In this study, as compared to controls, the PTSD group displayed higher‐frequency synchrony between the right superior parietal lobule and other central executive network regions. The authors interpreted this as compensatory, where, as attentional demands increased, higher oscillations were required for PTSD patients to perform at a level comparable to controls. As we employed a resting‐state paradigm in this study, reduced pulvinar connectivity with the superior parietal lobule in PTSD would be predicted given the deficits PTSD displays in working memory and top–down attention (Blair et al., 2013; Schweizer & Dalgleish, 2011; Vasterling et al., 2002). Moreover, state dissociative scores during the resting scan for the RSDI dissociative subscale correlated negatively with left pulvinar connectivity with the right superior parietal lobule. The superior parietal lobule is also involved in body–part localization, as evidenced by studies employing the rubber hand illusion (RHI), a multisensory integration paradigm that applies visuo‐tactile stimulation to produce alterations in body ownership (Felician et al., 2004; Tsakiris, Hesse, Boy, Haggard, & Fink, 2007). Superior parietal lobule activation increases as controls adopt the rubber hand as their own. Interestingly, a pilot study investigated the effects of the RHI in PTSD + DS and found that patients were more easily manipulated toward alterations in body ownership (Rabellino et al., 2016). These results corroborate our findings as PTSD + DS displayed the weakest pulvinar–superior parietal connectivity at rest, a pattern that may render them more likely to experience alterations of body ownership.
4.4. Precuneus
Our analyses revealed a decrease in right pulvinar connectivity with the left precuneus in PTSD and with the left and right precuneus in PTSD + DS as compared to controls. The precuneus is involved in visual imagery processes during episodic memory retrieval and self‐referential processing (Cavanna & Trimble, 2006; Fletcher et al., 1995). Recent studies indicate that PTSD is associated with increased precuneus activation during symptom provocation paradigms (Patel et al., 2012; Sartory et al., 2013). During resting state, whereas reductions in connectivity between the precuneus have been reported for other regions, including the posterior cingulate cortex (PCC) (Bluhm et al., 2009) and the vestibular nuclei (Harricharan et al., 2017), increases in precuneus connectivity have been found with the amygdala (Nicholson et al., 2016). Increases in amygdala–precuneus connectivity may affect autobiographical memory retrieval of traumatic memories (Lanius et al., 2004), where PTSD is characterized by the re‐experiencing of traumatic memories that emerge from a network including the precuneus (Cabeza & St Jacques, 2007; Svoboda, McKinnon, & Levine, 2006). However, the emotionally intense component of traumatic memories is thought to be driven by the excitation of sympathetic circuitry, which is largely coordinated by amygdala activation (Patel et al., 2012; Yehuda & LeDoux, 2007). This co‐occurrence of precuneus and amygdala activation during trauma recall may explain their heightened connectivity at rest. Alternatively, decreased connectivity between the precuneus and DMN regions (medial prefrontal, pulvinar, PCC) lends support to the dysfunction of the DMN and impairments in self‐referential processing which are characteristic of PTSD + DS (Frewen et al., 2011).
4.5. Inferior parietal lobule
Our results demonstrated an increase in right pulvinar connectivity with the right inferior parietal lobule when comparing controls as to PTSD + DS. The inferior parietal lobule lies beneath the intraparietal sulcus and is divided into the supramarginal and angular gyri, the latter overlapping with the temporoparietal junction. The inferior parietal lobule serves a role in high‐level social‐cognitive processes, including theory of mind, sense of agency, and perspective‐taking. Perspective‐taking relies on the ability to make self‐other distinctions and to appraise a situation from another's viewpoint. Lamm, Batson, and Decety (2007) have demonstrated that changing perspectives from that of the self to that of another while watching a video of someone being afflicted with pain results in reductions of affective response toward the video that is associated with the recruitment of inferior parietal lobule activation. In addition to its role in high‐level processing, the inferior parietal lobule, along with the insular cortex, is involved in attentional reorienting—a stimulus‐driven process of directing attention to relevant and salient stimuli (Behrmann, Geng, & Shomstein, 2004; Decety & Lamm, 2007). Both perspectives contribute to our understanding of the present findings in relation to PTSD + DS. First, reductions in inferior parietal lobule connectivity with the pulvinar may underlie, in part, PTSD + DS patients’ common engagement in dissociative experiences that are associated with deficits to theory of mind and perspective‐taking (Burack et al., 2006; Nazarov et al., 2014). Alternatively, PTSD + DS is characterized by an overmodulation of affect and the sensation of being detached from one's surroundings (Hopper et al., 2007; Lanius et al., 2010), which may be contributed to by weakened connectivity between the pulvinar and neural regions involved in stimulus‐driven attentional reorienting.
4.6. Supramarginal gyrus
Our analysis revealed significantly greater rsFC between the right pulvinar with the left supramarginal gyrus (BA 40) in healthy participants compared to PTSD + DS. In addition, we found greater left and right pulvinar rsFC with the right supramarginal gyrus when comparing PTSD to PTSD + DS. Wernicke's area, a region involved in the comprehension of language, is localized to the posterior portion of the supramarginal gyrus in the hand‐dominated hemisphere (Parker et al., 2005). The opposite hemisphere, generally the right supramarginal gyrus, has been implicated in self‐other distinctions (Morishima, Schunk, Bruhin, Ruff, & Fehr, 2012; Santiesteban, Banissy, Catmur, & Bird, 2012; Silani, Lamm, Ruff, & Singer, 2013). The right supramarginal gyrus displays strong connectivity with the medial cingulate and insular cortices, regions involved in the salience network and affect regulation (Mars et al., 2012). Moreover, damage to the right supramarginal gyrus produces distortions of bodily knowledge and self‐awareness while stimulation can induce out‐of‐body experiences (Berlucchi & Aglioti, 1997; Ionta, Gassert, & Blanke, 2011). These converging findings suggest the right supramarginal gyrus may serve to integrate exteroceptive and interoceptive information, processed in the temporoparietal junction and insular cortex, respectively (Craig, 2009). Dysfunction of the right supramarginal gyrus may result in a mismatch of external and internal signals, resulting in impairments to self‐other distinctions (Silani et al., 2013). The functions underpinned in the right supramarginal gyrus overlap with the symptoms of depersonalization and derealization that characterize PTSD + DS (Steuwe et al., 2014; Wolf et al., 2012). Distortions in bodily‐ and self‐awareness are not features of PTSD and, hence, can explain the greater connectivity found between the pulvinar and right supramarginal gyrus in PTSD relative to PTSD + DS. Moreover, the dense projections received by the pulvinar from the cingulate, insular, and sensory cortices make it an ideal candidate to route this information to the right supramarginal gyrus for processing. In terms of PTSD + DS, reduced pulvinar‐supramarginal connectivity may be conceptualized as impairing the integration of stimuli originating from the environment and the body (Behrmann, Geng, & Shomstein, 2004).
5. LIMITATIONS AND FUTURE DIRECTIONS
There are several limitations of this study that may be addressed by future research. First, the lifetime prevalence of PTSD among men and women is 3.6% and 9.7%, respectively (Kessler et al., 2005). Future research should seek to identify sex differences, as the causes, developmental trajectory, and mechanism of disorder may vary between the sexes (Tolin & Foa, 2006). Second, BOLD recordings have a higher affinity for cortical functional changes when compared to the midbrain. The cortex contains more cells than the midbrain and the organization of grey and white matter within the midbrain is not as easily delineated as in the cortex, resulting in the potential for grey matter to be incorrectly identified. This may explain why regions such as the superior colliculus and amygdala did not appear in the analysis despite their association with the pulvinar. Finally, fMRI has the disadvantage of poor temporal resolution where resolving the directionality problem would require a temporal dissection of the modulatory effect at the level of the pulvinar and its subsequent effect on network establishment throughout the cortex.
6. CONCLUSION
The pulvinar nuclei and the thalamus, more generally, have long been proposed as central enablers of high‐level functions, such as consciousness (Llinas et al., 1998; Steriade & Llinás, 1988). As our understanding of the functional organization of thalamic nuclei expands, the influence of these regions on network coordination becomes increasingly apparent. Here, we demonstrate whole‐brain corrected reductions in the resting‐state functional connectivity of the pulvinar nuclei with parietal regions underlying multimodal sensory integration and socioaffective functions in PTSD and PTSD + DS. The precuneus, supramarginal gyrus, superior, and inferior parietal lobules are contained within the posterior parietal cortex and together construct a working model of the world by fusing multiple senses in real time (Akrami, Kopec, Diamond, & Brody, 2018; Nikbakht, Tafreshiha, Zoccolan, & Diamond, 2018). The fusing of these senses relies on thalamic nuclei to bind dispersed cortex through thalamocortical loops and this process is recursive where our working model must be continually updated as new sensations are experienced (Wolpert, Goodbody, & Husain, 1998). Crucially, this system displays reduced connectivity with the pulvinar in PTSD that may contribute to the altered exteroceptive capacity of these patients (Harricharan et al., 2017; Pearson, 2009). Here, it is likely that observed deficits in socioaffective functions in PTSD are associated with alterations in the pulvinar‐orchestrated functions of alpha synchrony and thalamocortical binding. Accordingly, additional research is urged to identify the effects of trauma on the binding properties and ability of the pulvinar to modulate alpha, as these activities may be central to the restoration of large‐scale networks in PTSD and may inform further novel adjunctive treatments for PTSD, such as neurofeedback (Lanius et al., 2015). In addition, it is critical that future research investigates the connectivity of additional high‐order thalamic nuclei to determine whether rsFC reductions are a global thalamic phenomenon in PTSD or are specific to the pulvinar nuclei. Here, the mediodorsal nucleus presents as an interesting thalamic structure given its reciprocal connectivity with regions underlying executive functions (Golden, Graff‐Radford, Jones, & Benarroch, 2017), memory (Cona, Laera, Edelstyn, & Bisiacchi, 2018), and emotion—all factors involved in the posttraumatic response (Metzger, 2010).
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Table 1
Supporting Information Table 2
Terpou BA, Densmore M, Théberge J, Frewen P, McKinnon MC, Lanius RA. Resting‐state pulvinar‐posterior parietal decoupling in PTSD and its dissociative subtype. Hum Brain Mapp. 2018;39:4228–4240. 10.1002/hbm.24242
Footnotes
Funding information Canadian Institutes of Health Research, Grant/Award Numbers: 137150, 97914
REFERENCES
- Arend, I. , Henik, A. , & Okon‐Singer, H. (2015). Dissociating emotion and attention functions in the pulvinar nucleus of the thalamus. Neuropsychology, 29(2), 191–196. [DOI] [PubMed] [Google Scholar]
- Akrami, A. , Kopec, C., D. , Diamond, M., E. , & Brody, C., D. (2018). Posterior parietal cortex represents sensory history and mediates its effects on behavior. bioRxiv, 182246. [DOI] [PubMed] [Google Scholar]
- Barron, D. S. , Eickhoff, S. B. , Clos, M. , & Fox, P. T. (2015). Human pulvinar functional organization and connectivity. Human Brain Mapping, 36(7), 2417–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluch, F. , & Itti, L. (2011). Mechanisms of top‐down attention. Trends in Neurosciences, 34(4), 210–224. [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Guth, D. , Steer, R. A. , & Ball, R. (1997). Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behaviour Research and Therapy, 35(8), 785–791. [DOI] [PubMed] [Google Scholar]
- Behrmann, M. , Geng, J. J. , & Shomstein, S. (2004). Parietal cortex and attention. Current Opinion in Neurobiology, 14(2), 212–217. [DOI] [PubMed] [Google Scholar]
- Berlucchi, G. , & Aglioti, S. (1997). The body in the brain: Neural bases of corporeal awareness. Trends in Neurosciences, 20(12), 560–564. [DOI] [PubMed] [Google Scholar]
- Bernstein, D. P. , Stein, J. A. , Newcomb, M. D. , Walker, E. , Pogge, D. , Ahluvalia, T. , … Zule, W. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect, 27(2), 169–190. [DOI] [PubMed] [Google Scholar]
- Blair, K. S. , Vythilingam, M. , Crowe, S. L. , McCaffrey, D. E. , Ng, P. , Wu, C. C. , … Blair, R. J. R. (2013). Cognitive control of attention is differentially affected in trauma‐exposed individuals with and without post‐traumatic stress disorder. Psychological Medicine, 43(01), 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, D. D. , Weathers, F. W. , Nagy, L. M. , Kaloupek, D. G. , Gusman, F. D. , Charney, D. S. , & Keane, T. M. (1995). The development of a Clinician‐Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Block, S. , King, A. P. , Sripada, R. K. , Weissman, D. H. , Welsh, R. , & Liberzon, I. (2017). Behavioral and neural correlates of disrupted orienting attention in posttraumatic stress disorder. Cognitive, Affective, & Behavioral Neuroscience, 17(2), 422–436. [DOI] [PubMed] [Google Scholar]
- Bluhm, R. L. , Williamson, P. C. , Osuch, E. A. , Frewen, P. A. , Stevens, T. K. , Boksman, K. , … Lanius, R. A. (2009). Alterations in default network connectivity in posttraumatic stress disorder related to early‐life trauma. Journal of Psychiatry & Neuroscience, 34(3), 187–194. [PMC free article] [PubMed] [Google Scholar]
- Bourne, J. A. , & Morrone, M. C. (2017). Plasticity of visual pathways and function in the developing brain: Is the pulvinar a crucial player? Frontiers in Systems Neuroscience, 11, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner, J. D. , Krystal, J. H. , Putnam, F. W. , Southwick, S. M. , Marmar, C. , Charney, D. S. , & Mazure, C. M. (1998). Measurement of dissociative states with the clinician‐administered dissociative states scale (CADSS). Journal of Traumatic Stress, 11(1), 125–136. [DOI] [PubMed] [Google Scholar]
- Briere, J. , Weathers, F. W. , & Runtz, M. (2005). Is dissociation a multidimensional construct? Data from the Multiscale Dissociation Inventory. Journal of Traumatic Stress, 18(3), 221–231. [DOI] [PubMed] [Google Scholar]
- Burack, J. A. , Flanagan, T. , Peled, T. , Sutton, H. M. , Zygmuntowicz, C. , & Manly, J. T. (2006). Social perspective‐taking skills in maltreated children and adolescents. Developmental Psychology, 42(2), 207–217. [DOI] [PubMed] [Google Scholar]
- Buschman, T. J. , & Miller, E. K. (2007). Top‐down versus bottom‐up control of attention in the prefrontal and posterior parietal cortices. Science, 315(5820), 1860–1864. [DOI] [PubMed] [Google Scholar]
- Cabeza, R. , & St Jacques, P. (2007). Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences, 11(5), 219–227. [DOI] [PubMed] [Google Scholar]
- Canolty, R. T. , & Knight, R. T. (2010). The functional role of cross‐frequency coupling. Trends in Cognitive Sciences, 14(11), 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(3), 564–583. [DOI] [PubMed] [Google Scholar]
- Chen, A. C. , Oathes, D. J. , Chang, C. , Bradley, T. , Zhou, Z.‐W. , Williams, L. M. , … Etkin, A. (2013). Causal interactions between fronto‐parietal central executive and default‐mode networks in humans. Proceedings of the National Academy of Sciences, 110(49), 19944–19949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler, J. M. , Scott Steele, J. , Smitherman, S. , Lenow, J. K. , & Kilts, C. D. (2013). Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: A network‐level analysis among adolescent girls. Psychiatry Research ‐ Neuroimaging, 214(3), 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona, G. , Laera, G. , Edelstyn, N. , & Bisiacchi, P., S. (2018). Deficits in prospective memory following damage to the medial subdivision of the mediodorsal thalamic nucleus. Journal of Neuropsychology. [DOI] [PubMed] [Google Scholar]
- Craig, A. D. (2009). How do you feel now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Daniels, J. K. , Frewen, P. , McKinnon, M. C. , & Lanius, R. A. (2011). Default mode alterations in posttraumatic stress disorder related to early‐life trauma: A developmental perspective. Journal of Psychiatry & Neuroscience, 36(1), 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety, J. , & Lamm, C. (2007). The role of the right temporoparietal junction in social interaction: How low‐level computational processes contribute to meta‐cognition. Neuroscientist, 13(6), 580–593. [DOI] [PubMed] [Google Scholar]
- Di Gangi, J. A. , Tadayyon, A. , Fitzgerald, D. A. , Rabinak, C. A. , Kennedy, A. , Klumpp, H. , … Phan, K. L. (2016). Reduced default mode network connectivity following combat trauma. Neuroscience Letters, 615, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley, B. T. , Sedge, P. A. , Doesburg, S. M. , Grodecki, R. J. , Jetly, R. , Shek, P. N. , … Pang, E. W. (2015). Theta, mental flexibility, and post‐traumatic stress disorder: Connecting in the parietal cortex. PLoS ONE, 10(4), e0123541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , & Wagner, T. D. (2007). Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felician, O. , Romaiguère, P. , Anton, J.‐L. , Nazarian, B. , Roth, M. , Poncet, M. , & Roll, J.‐P. (2004). The role of human left superior parietal lobule in body part localization. Annals of Neurology, 55(5), 749–751. [DOI] [PubMed] [Google Scholar]
- Fletcher, P. C. , Happé, F. , Frith, U. , Baker, S. C. , Dolan, R. J. , Frackowiak, R. S. J. , & Frith, C. D. (1995). Other minds in the brain: A functional imaging study of “theory of mind” in story comprehension. Cognition, 57(2), 109–128. [DOI] [PubMed] [Google Scholar]
- Frewen, P. A. , Dozois, D. J. A. , Neufeld, R. W. J. , Densmore, M. , Stevens, T. K. , & Lanius, R. A. (2011). Self‐referential processing in women with PTSD: Affective and neural response. Psychological Trauma: Theory, Research, Practice, and Policy, 3(4), 318–328. [Google Scholar]
- Frewen, P. A. , Pain, C. , Dozois, D. J. A. , & Lanius, R. A. (2006). Alexithymia in PTSD: Psychometric and fMRI studies. Annals of the New York Academy of Sciences, 1071(1), 397–400. [DOI] [PubMed] [Google Scholar]
- Golden, E. C. , Graff‐Radford, J. , Jones, D. T. , & Benarroch, E. E. (2017). Author response: Mediodorsal nucleus and its multiple cognitive functions. Neurology, 89(1), 107. [DOI] [PubMed] [Google Scholar]
- Hamilton, J. P. , Etkin, A. , Furman, D. J. , Lemus, M. G. , Johnson, R. F. , & Gotlib, I. H. (2012). Functional neuroimaging of major depressive disorder: A meta‐analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry, 169(7), 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harricharan, S., Rabellino, D., Frewen, P. A., Densmore, M., Théberge, J., McKinnon, M. C., … Lanius, R. A. (2016). fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain and Behavior, 6(12), e00579. [DOI] [PMC free article] [PubMed]
- Harricharan, S. , Nicholson, A. A. , Densmore, M. , Théberge, J. , McKinnon, M. C. , Neufeld, R. W. J. , & Lanius, R. A. (2017). Sensory overload and imbalance: Resting‐state vestibular connectivity in PTSD and its dissociative subtype. Neuropsychologia, 106, 169–178. [DOI] [PubMed] [Google Scholar]
- Hart, H. , Lim, L. , Mehta, M. A. , Chatzieffraimidou, A. , Curtis, C. , Xu, X. , … Rubia, K. (2017). Reduced functional connectivity of fronto‐parietal sustained attention networks in severe childhood abuse. PLoS ONE, 12(11), e0188744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper, J. W. , Frewen, P. A. , van der Kolk, B. A. , & Lanius, R. A. (2007). Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script‐driven trauma imagery. Journal of Traumatic Stress, 20(5), 713–725. [DOI] [PubMed] [Google Scholar]
- Ionta, S. , Gassert, R. , & Blanke, O. (2011). Multi‐sensory and sensorimotor foundation of bodily self‐consciousness ‐ an interdisciplinary approach. Frontiers in Psychology, 2, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti, L. (2005). Models of bottom‐up attention and saliency. Neurobiology of Attention, 576–582. [Google Scholar]
- Jones, E. G. (2001). The thalamic matrix and thalamocortical synchrony. Trends in Neurosciences, 24(10), 595–601. [DOI] [PubMed] [Google Scholar]
- Karnath, H. , Himmelbach, M. , & Rorden, C. (2002). The subcortical anatomy of human spatial neglect: Putamen, caudate nucleus and pulvinar. Brain, 125(2), 350–360. [DOI] [PubMed] [Google Scholar]
- Kennis, M. , Rademaker, A. R. , van Rooij, S. J. H. , Kahn, R. S. , & Geuze, E. (2015). Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post‐traumatic stress disorder. Human Brain Mapping, 36(1), 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C. , Berglund, P. , Demler, O. , Jin, R. , Merikangas, K. R. , & Walters, E. E. (2005). Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Kilpatrick, L. A. , Suyenobu, B. Y. , Smith, S. R. , Bueller, J. A. , Goodman, T. , Creswell, J. D. , … Naliboff, B. D. (2011). Impact of mindfulness‐based stress reduction training on intrinsic brain connectivity. NeuroImage, 56(1), 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch, W. (2012). Alpha‐band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16(12), 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, S. B. J. , van Zuiden, M. , Nawijn, L. , Frijling, J. L. , Veltman, D. J. , & Olff, M. (2016). Aberrant resting‐state brain activity in posttraumatic stress disorder: A meta‐analysis and systematic review. Depression and Anxiety, 33(7), 592–605. [DOI] [PubMed] [Google Scholar]
- Koenigs, M. , Barbey, A. K. , Postle, B. R. , & Grafman, J. (2009). Superior parietal cortex is critical for the manipulation of information in working memory. Journal of Neuroscience, 29(47), 14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowska, K. , Walker, P. , McLean, L. , & Carrive, P. (2015). Fear and the defense cascade. Harvard Review of Psychiatry, 23(4), 263–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm, C. , Batson, C. D. , & Decety, J. (2007). The neural substrate of human empathy: Effects of perspective‐taking and cognitive appraisal. Journal of Cognitive Neuroscience, 19(1), 42–58. [DOI] [PubMed] [Google Scholar]
- Lanius, R. A. , Bluhm, R. L. , & Frewen, P. A. (2011). How understanding the neurobiology of complex post‐traumatic stress disorder can inform clinical practice: A social cognitive and affective neuroscience approach. Acta Psychiatrica Scandinavica, 124(5), 331–348. [DOI] [PubMed] [Google Scholar]
- Lanius, R. A. , Frewen, P. A. , Tursich, M. , Jetly, R. , & McKinnon, M. C. (2015). Restoring large‐scale brain networks in PTSD and related disorders: A proposal for neuroscientifically‐informed treatment interventions. European Journal of Psychotraumatology, 6(1), 27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A. , Vermetten, E. , Loewenstein, R. J. , Brand, B. , Schmahl, C. , Bremner, J. D. , & Spiegel, D. (2010). Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry, 167(6), 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A. , Williamson, P. C. , Densmore, M. , Boksman, K. , Neufeld, R. W. , Gati, J. S. , & Menon, R. S. (2004). The nature of traumatic memories: A 4‐T fMRI functional connectivity analysis. American Journal of Psychiatry, 161(1), 36–44. [DOI] [PubMed] [Google Scholar]
- Lanius, R. A. , Williamson, P. C. , Densmore, M. , Boksman, K. , Gupta, M. A. , Neufeld, R. W. , … Menon, R. S. (2001). Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. American Journal of Psychiatry, 158(11), 1920–1922. [DOI] [PubMed] [Google Scholar]
- Lakatos, P. , O'connell, M. N. , & Barczak, A. (2016). Pondering the pulvinar. Neuron, 89(1), 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar, S. W. , Bush, G. , Gollub, R. L. , Fricchione, G. L. , Khalsa, G. , & Benson, H. (2000). Functional brain mapping of the relaxation response and meditation. NeuroReport, 11(7), 1581–1585. [PubMed] [Google Scholar]
- Liddell, B. J. , Brown, K. J. , Kemp, A. H. , Barton, M. J. , Das, P. , Peduto, A. , … Williams, L. M. (2005). A direct brainstem‐amygdala‐cortical “alarm” system for subliminal signals of fear. NeuroImage, 24(1), 235–243. [DOI] [PubMed] [Google Scholar]
- Llinas, R. , Ribary, U. , Contreras, D. , & Pedroarena, C. (1998). The neuronal basis for consciousness. Philosophical Transactions of the Royal Society B: Biological Sciences, 353(1377), 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars, R. B. , Sallet, J. , Schüffelgen, U. , Jbabdi, S. , Toni, I. , & Rushworth, M. F. S. (2012). Connectivity‐based subdivisions of the human right “temporoparietal junction area”: Evidence for different areas participating in different cortical networks. Cerebral Cortex (New York, N.Y. : 1991), 22(8), 1894–1903. [DOI] [PubMed] [Google Scholar]
- Menon, V. (2011). Large‐scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214(5–6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger, C. D. (2010). High field fMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Frontiers in Neuroanatomy, 4, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima, Y. , Schunk, D. , Bruhin, A. , Ruff, C. C. , & Fehr, E. (2012). Linking brain structure and activation in temporoparietal junction to explain the neurobiology of human altruism. Neuron, 75(1), 73–79. [DOI] [PubMed] [Google Scholar]
- Nazarov, A. , Frewen, P. , Parlar, M. , Oremus, C. , Macqueen, G. , Mckinnon, M. , & Lanius, R. (2014). Theory of mind performance in women with posttraumatic stress disorder related to childhood abuse. Acta Psychiatrica Scandinavica, 129(3), 193–201. [DOI] [PubMed] [Google Scholar]
- Nicholson, A. A. , Sapru, I. , Densmore, M. , Frewen, P. A. , Neufeld, R. W. J. , Théberge, J. , … Lanius, R. A. (2016). Unique insula subregion resting‐state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Research ‐ Neuroimaging, 250, 61–72. [DOI] [PubMed] [Google Scholar]
- Nikbakht, N. , Tafreshiha, A. , Zoccolan, D. , & Diamond, M. E. (2018). Supralinear and supramodal integration of visual and tactile signals in rats: Psychophysics and neuronal mechanisms. Neuron, 97(3), 626–639.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivé, I. , Densmore, M. , Harricharan, S. , Théberge, J. , McKinnon, M. C. , & Lanius, R. (2018). Superior colliculus resting state networks in post‐traumatic stress disorder and its dissociative subtype. Human Brain Mapping, 39(1), 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala, S. (2010). Pulvinar and affective significance: Responses track moment‐to‐moment stimulus visibility. Frontiers in Human Neuroscience, 4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva, S. , & Palva, J. M. (2007). New vistas for α‐frequency band oscillations. Trends in Neurosciences, 30(4), 150–158. [DOI] [PubMed] [Google Scholar]
- Pan, L. , Zimmer, T. M. , Segreti, A. M. , Martin, P. C. , & Klawson, E. K. (2016). Superior parietal volume in adolescents with a history of trauma. Journal of Child and Adolescent Behavior, 4, 303. [Google Scholar]
- Parker, G. J. M. , Luzzi, S. , Alexander, D. C. , Wheeler‐Kingshott, C. A. M. , Ciccarelli, O. , & Lambon Ralph, M. A. (2005). Lateralization of ventral and dorsal auditory‐language pathways in the human brain. NeuroImage, 24(3), 656–666. [DOI] [PubMed] [Google Scholar]
- Patel, R. , Spreng, R. N. , Shin, L. M. , & Girard, T. A. (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: A meta‐analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 36(9), 2130–2142. [DOI] [PubMed] [Google Scholar]
- Pearson, H. J. (2009). Present and accounted for: Sensory stimulation and parietal neuroplasticity. Journal of EMDR Practice and Research, 3(1), 39–49. [Google Scholar]
- Rabellino, D., Harricharan, S., Frewen, P. A., Burin, D., McKinnon, M. C., & Lanius, R. A. (2016). “I can't tell whether it's my hand”: A pilot study of the neurophenomenology of body representation during the rubber hand illusion in trauma‐related disorders. European Journal of Psychotraumatology, 7(1), 32918. [DOI] [PMC free article] [PubMed]
- Rabellino, D. , Tursich, M. , Frewen, P. A. , Daniels, J. K. , Densmore, M. , Théberge, J. , & Lanius, R. A. (2015). Intrinsic connectivity networks in post‐traumatic stress disorder during sub‐ and supraliminal processing of threat‐related stimuli. Acta Psychiatrica Scandinavica, 132(5), 365–378. [DOI] [PubMed] [Google Scholar]
- Saalmann, Y. B. , Pinsk, M. A. , Wang, L. , Li, X. , & Kastner, S. (2012). The pulvinar regulates information transmission between cortical areas based on attention demands. Science, 337(6095), 753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiesteban, I. , Banissy, M. J. , Catmur, C. , & Bird, G. (2012). Enhancing social ability by stimulating right temporoparietal junction. Current Biology, 22(23), 2274–2277. [DOI] [PubMed] [Google Scholar]
- Sartory, G. , Cwik, J. , Knuppertz, H. , Schürholt, B. , Lebens, M. , Seitz, R. J. , & Schulze, R. (2013). In search of the trauma memory: A meta‐analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD). PLoS ONE, 8(3), e58150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer, S. , & Dalgleish, T. (2011). Emotional working memory capacity in posttraumatic stress disorder (PTSD). Behaviour Research and Therapy, 49(8), 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev, A. Y. , Freedman, S. , Peri, T. , Brandes, D. , Sahar, T. , Orr, S. P. , & Pitman, R. K. (1998). Prospective study of posttraumatic stress disorder and depression following trauma. American Journal of Psychiatry, 155(5), 630–637. [DOI] [PubMed] [Google Scholar]
- Silani, G. , Lamm, C. , Ruff, C. C. , & Singer, T. (2013). Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. Journal of Neuroscience, 33(39), 15466–15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. D. (2010). State‐trait anxiety inventory. The Corsini encyclopedia of psychology. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- Sripada, R. K. , King, A. P. , Welsh, R. C. , Garfinkel, S. N. , Wang, X. , Sripada, C. S. , & Liberzon, I. (2012). Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine, 74(9), 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, T. , Moritz, C. , Quigley, M. , Cordes, D. , Haughton, V. , & Meyerand, E. (2000). Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. American Journal of Neuroradiology, 21(8), 1397–1401. [PMC free article] [PubMed] [Google Scholar]
- Steriade, M. , & Llinás, R. R. (1988). The functional states of the thalamus and the associated neuronal interplay. Physiological Reviews, 68(3), 649–742. [DOI] [PubMed] [Google Scholar]
- Steuwe, C. , Daniels, J. K. , Frewen, P. A. , Densmore, M. , Pannasch, S. , Beblo, T. , … Lanius, R. A. (2014). Effect of direct eye contact in PTSD related to interpersonal trauma: An fMRI study of activation of an innate alarm system. Social Cognitive and Affective Neuroscience, 9(1), 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumpf, H. , Mangun, G. R. , Boehler, C. N. , Stoppel, C. , Schoenfeld, M. A. , Heinze, H. J. , & Hopf, J. M. (2013). The role of the pulvinar in distractor processing and visual search. Human Brain Mapping, 34(5), 1115–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda, E. , McKinnon, M. C. , & Levine, B. (2006). The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia, 44(12), 2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome, J. , Densmore, M. , Frewen, P. A. , McKinnon, M. C. , Théberge, J. , Nicholson, A. A. , … Lanius, R. A. (2017). Desynchronization of autonomic response and central autonomic network connectivity in posttraumatic stress disorder. Human Brain Mapping, 38(1), 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin, D. F. , & Foa, E. B. (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132(6), 959–992. [DOI] [PubMed] [Google Scholar]
- Tsakiris, M. , Hesse, M. D. , Boy, C. , Haggard, P. , & Fink, G. R. (2007). Neural signatures of body ownership: A sensory network for bodily self‐consciousness. Cerebral Cortex (New York, N.Y. : 1991), 17(10), 2235–2244. [DOI] [PubMed] [Google Scholar]
- Tursich, M. , Ros, T. , Frewen, P. A. , Kluetsch, R. C. , Calhoun, V. D. , & Lanius, R. A. (2015). Distinct intrinsic network connectivity patterns of post‐traumatic stress disorder symptom clusters. Acta Psychiatrica Scandinavica, 132(1), 29–38. [DOI] [PubMed] [Google Scholar]
- Vasterling, J. J. , Duke, L. M. , Brailey, K. , Constans, J. I. , Allain, A. N. , & Sutker, P. B. (2002). Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology, 16(1), 5–14. [DOI] [PubMed] [Google Scholar]
- Wolbers, T. , Weiller, C. , & Büchel, C. (2003). Contralateral coding of imagined body parts in the superior parietal lobe. Cerebral Cortex (New York, N.Y. : 1991), 13(4), 392–399. [DOI] [PubMed] [Google Scholar]
- Wolf, E. J. , Miller, M. W. , Reardon, A. F. , Ryabchenko, K. A. , Castillo, D. , & Freund, R. (2012). A latent class analysis of dissociation and posttraumatic stress disorder: Evidence for a dissociative subtype. Archives of General Psychiatry, 69(7), 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert, D. M. , Goodbody, S. J. , & Husain, M. (1998). Maintaining internal representations: The role of the human superior parietal lobe. Nature Neuroscience, 1(6), 529–533. [DOI] [PubMed] [Google Scholar]
- Yehuda, R. , & LeDoux, J. (2007). Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron, 56(1), 19–32. [DOI] [PubMed] [Google Scholar]
- Zhou, H. , Schafer, R. J. , & Desimone, R. (2016). Pulvinar‐cortex interactions in vision and attention. Neuron, 89(1), 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Table 1
Supporting Information Table 2


