Abstract
The formation of a coherent and unified self‐concept represents a key developmental stage during adolescence. Imaging studies on self‐referential processing in adolescents are rare, and it is not clear whether neural structures involved in self‐reflection are also involved in reflections of familiar others. In the current study, 41 adolescents were asked to make judgments about trait adjectives during functional magnetic resonance imaging (fMRI): they had to indicate whether the word describes themselves, their friends, their teachers or politicians. Findings indicate a greater overlap in neural networks for responses to self‐ and friend‐related judgments compared to teachers and politicians. In particular, classic self‐reference structures such as the ventromedial prefrontal cortex and medial posterior parietal cortex also exhibited higher activation to judgments about friends. In contrast, brain responses towards judgments of teachers (familiar others) compared to politicians (unfamiliar others) did not significantly differ. Results support behavioral findings of a greater relevance of friends for the development of a self‐concept during adolescence and indicate underlying functional brain processes. Hum Brain Mapp 38:987–996, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: self‐concept, adolescence, vMPFC, dMPFC, mPPC
Abbreviations
- ANOVA
Analysis of variance
- EPI
Echo planar imaging
- FOV
Field of view
- FWE
Family wise error
- GLM
General linear model
- ROI
Regions of interest
- TE
Echo time
- TPJ
Temporoparietal junction
- TR
Repetition time
INTRODUCTION
Adolescence represents the key developmental stage for the formation of an abstract and differentiated self‐concept. Especially in mid‐adolescence, contradictions and incoherencies in the self‐image are frequently detected, while new cognitive developments enable the formation of more mature, inter‐contextual self‐descriptions [Harter, 2012]. The self is conceptualized as a structure that mediates intrapersonal (e.g., information processing, affect) as well as interpersonal processes (e.g., social perception, interaction strategies) [Heinz et al., 2012; Markus and Wurf, 1987]. During mid‐adolescence, cascades of hormonal and brain maturation changes associated with puberty coincide with critical social‐cognitive developments—intensifying social‐emotional experiences and increasing the sensitivity to the social environment, in particular to peers [Crone and Dahl, 2012; Nelson et al., 2005]. Also, studies on decision making processes underline the importance of the peer group; especially risky decisions are observed in adolescents in order to gain peer approval (for a review see [Blakemore and Robbins, 2012]. Many social experiences take place in school, where adolescents spend a lot of their time [Eccles and Roeser, 2011]. At school, along with peers, teachers are important attachment figures. The quality of the teacher‐student relationship is not only significant for academic outcomes, it is also a predictor of students' socio‐emotional well‐being and feeling of self‐esteem [Eccles and Roeser, 2011].
While the adult neural self‐network is well‐studied (for a meta‐analysis see Northoff et al., 2006 and Denny et al., 2012), researchers have only recently begun to analyze and compare key brain structures for adolescent self‐referential cognition [Pfeifer et al., 2007; Pfeifer et al., 2009; Pfeifer and Peake, 2012; Schneider et al., 2012]. In both adults and adolescents, the ventromedial prefrontal cortex (vMPFC) and the medial posterior parietal cortex (mPPC) are the paramount structures for self‐relevant processing [Denny et al., 2012; Heatherton et al., 2006; Lieberman, 2007; Northoff et al., 2006; Northoff et al., 2011; Pfeifer et al., 2009; Pfeifer and Peake, 2012; Schmitz and Johnson, 2007; van der Meer et al., 2010]. The vMPFC is known to be specifically involved when information relating to the self is processed, thereby coding for self‐relatedness of the processed content. That is, the closer, more familiar or more important the stimulus (e.g., another person) is to oneself, the higher the neural response of the vMPFC [Krienen et al., 2010; Murray et al., 2012]. This specificity of activation for self‐related material is less clear‐cut in case of the mPPC. Though most studies found higher activation for self‐related reflection compared to other‐related reflection, several researchers report the reversed pattern [Lombardo et al., 2010; Pfeifer and Blakemore, 2012], which could be caused by functional differences between different sub‐regions.
Beyond these regions, the temporoparietal junction (TPJ) and dorsomedial prefrontal cortex (dMPFC) also seem to be strongly involved in self‐related processing in adolescents relative to adults [Pfeifer et al., 2009; Pfeifer and Peake, 2012]. In contrast, these brain areas are generally found to be specialized on other‐directed cognition in adults, e.g., social perception, theory of mind and perspective taking [Bahnemann et al., 2010; Samson et al., 2004; Saxe and Kanwisher, 2003]. In detail, a meta‐analysis by Denny and colleagues (2012) revealed that dmPFC and TPJ (as well as cuneus) were more frequently activated by other‐related judgments. Altogether, the authors propose that ventral and dorsal mPFC lay at opposite ends of a functional gradient with increasingly ventral or dorsal mPFC activation for self‐ or other‐related judgments, respectively.
When comparing trait words referring to oneself against trait words referring to close friends, highly overlapping neural activation patterns have been observed in adolescents [Schneider et al., 2012]. Also, when adolescents have to take their own perspective or the perspective of their best friend regarding self‐related trait words, it has been shown that peer evaluations are highly salient and self‐relevant in adolescents [Jankowski et al., 2014]. Yet, it is not clear whether these findings are a mere familiarity effect since especially during adolescence, the peer group is highly salient and very well known [Brown and Larson, 2009; Parker et al., 2006]. By adding other reference persons from a similar social context such as school to the study design, it might be possible to find a graduation in brain responses. Results comparing different reference persons could also strengthen previous findings regarding a greater relevance of friends for the self‐concept during adolescence [Jankowski et al., 2014].
Focusing on the social context of schools as being crucial for social experiences in adolescence, the current study explores whether adolescent neural self‐ and social brain regions show distinguished processing patterns for familiar others such as friends and teachers as compared to the self as well as to a more distant social group (politicians). We thus aimed to investigate whether the importance of friends for the self‐concept can be supported by neural data. During functional magnetic resonance imaging (fMRI), we asked adolescents to judge themselves, their friends and teachers as well as politicians on several personality traits and analyzed the neural response of classical self‐network areas (vMPFC and mPPC) as well as dMPFC and TPJ whether it is related to adolescent self‐processing. In general, we expected the highest activation during self‐judgments in our regions of interest (ROI) vMPFC and mPPC as well as an additional involvement of the dMPFC and TPJ. Moreover, we hypothesized that these self‐relevant brain regions are significantly more activated during friends‐ compared to teacher‐ and politician‐judgments. With respect to neural processing of teacher‐judgments, we expected teacher‐judgments to elicit a higher neural activation than politician‐judgments but less than friend‐judgments.
MATERIALS AND METHODS
Participants
The study was performed in accordance with the Declaration of Helsinki and approved by the ethics committee of the German Psychological Society. All participants as well as legal guardians provided written informed consent. 47 participants were screened for the following exclusion criteria: (1) adverse health conditions, neurological or psychological disorders, (2) use of medications that influence central nervous system function, (3) non‐removable ferromagnetic material; participants with more than 3 mm translation and 3° rotation of head movement during the fMRI paradigm were also excluded. This led to the exclusion of six participants: five due to excessive head movements and one due to neurological abnormalities. The final sample consisted of 41 healthy adolescents (27 females) between 14 to 16 years (M = 15.2 years, SD = 0.45). One out of 41 participants was left‐handed, as verified by an Edinburgh Handedness Inventory Score [Oldfield, 1971] of −100, while the remaining were all right‐handed (M = 82.21, SD = 14.1). Since data analysis without the left‐handed participant yielded equivalent results (data not shown), results including the left‐handed person are reported.
Self‐Reference Paradigm
Each trial of the task consisted of a cue word (i.e., “you,” “friends,” “teachers,” “politicians,” or “syllables”) presented above a central fixation cross and one out of 30 personality trait adjectives (e.g., “creative” or “smart”) presented below. Depending on the cue word presented, participants were instructed to decide whether or not the word described themselves (self‐reference), their friends, their teachers (familiar others) or politicians (distant others). Please note that for the subsequent analysis, the actual answer was not investigated. In control trials, participants had to indicate whether or not the adjectives had exactly two syllables. Each adjective was presented in all five different judgment conditions. Responses were given with the left (if they agreed that the word describes the respective person) and right thumb (if they did not agree that the word describes the respective person). Prior to scanning, the task was practiced until participants felt thoroughly comfortable with all parts of it. Stimuli were drawn from the Berlin Affective Word List [Vo et al., 2009] and were counter‐balanced based on emotional valence and arousal of the words: words with medium arousal (between 2 and 4 on a 5‐point scale) were chosen, out of which 10 words with a positive valence, 10 with a neutral and 10 with a negative valence were then selected in a second step.
Imaging Data Acquisition
The trait adjectives were presented pseudo‐randomly and separated by a jittered inter‐stimulus interval of 1,500–9,500 ms, during which participants viewed a fixation cross. The jitter distribution had an exponential slope with fewer long jitter times and a mean of 3,400 ms. The adjectives were presented until the subject responded with a maximum event duration of 2,500 ms. The complete task consisted of 150 trials (30 trials per condition) and lasted for a maximum of 15:04 minutes.
FMRI data were collected with a 3 Tesla Magnetom Trio scanner system (Siemens Medical Systems, Erlangen, Germany) using a 12‐channel radiofrequency head coil. Stimuli were projected onto a screen at the end of the magnet bore by a video projector (NEC GT950 NEC Corporation, Itasca, IL, USA, resolution 1,024 × 768 pixels), which participants viewed via a mirror on the head coil.
First, a T1‐weighted 3D MPRAGE (magnetization‐prepared rapid gradient‐echo) sequence with 192 continuous sagittal slices [image matrix = 256 × 256, repetition time (TR) = 1,900 ms, echo time (TE) = 2.52 ms, flip angle = 9°, field of view (FOV) = 256 × 256, voxel size = 1 × 1 × 1 mm3] was conducted, followed by a T2*‐weighted gradient echo planar imaging (EPI) sequence sensitive to BOLD contrast (33 transversal slices, image matrix = 64 × 64, TR = 2,000 ms, TE = 30 ms, flip angle = 78°, FOV = 192 × 192, voxel size = 3 × 3 × 3 mm3, interslice gap = 0.8 mm). The self‐reference task comprised 150 functional runs of 450 volume acquisitions each axial aligned.
Image Processing and Data Analysis
In order to account for T1 saturation, the first three scans of each EPI scanning session were excluded. We used Statistical Parametric Mapping program (SPM 8; Wellcome Trust Center for Neuroimaging, London, UK) for image processing and analysis. Data was submitted to the following pre‐processing steps:
First, EPI‐images were slice‐timing corrected and realigned correcting for head movement (six‐parameter rigid body transformation). The anatomical MPRAGE was coregistered to the mean EPI and in a second step segmented into grey matter, white matter and cerebrospinal fluid. The acquired segmentation matrix was further used for the normalization of functional (voxel size = 3 × 3 × 3 mm3) and structural images (voxel size = 1 × 1 × 1 mm3) to the MNI template (Montreal Neurological Institute). In a last step, data were spatially smoothed utilizing a 7 mm (full width at half maximum) Gaussian kernel.
Effects on the first level were estimated with an event‐related general linear model (GLM) convolving each trial with a hemodynamic response function and a high‐pass filter of 128 seconds was applied. ‘Type of judgment’ (self, friends, teachers, politicians or syllables) represented the regressors of interest, whereas button press (left or right) and the six movement parameters were included as regressors of no interest in order to account for any residual movement‐related effects. Using the resulting parameters, differential contrast images in which the control condition (syllables) was subtracted from every judgment condition (self, friends, teachers, politicians) were created.
At the group level, individual contrast images were entered into a mixed effects analysis of variance (ANOVA) with the between subject factor “reference person” (self, friends, teachers, politicians) and the within factor “subject”.
Separate probabilistic ROIs were created for the bilateral vMPFC and dMPFC as well as left and right mPPC and TPJ. Here, coordinates for the different areas were drawn from neuroimaging studies that used a comparable self‐reference paradigm from the contrast self > others, pooled and—if necessary—transformed to the MNI space by the affine algorithm proposed by [Brett et al., 2001] (see Supporting Information S1). The procedure is described in more detail in Lorenz et al. [2014] (a figure can be found in Supporting Information S2).
Reaction Times (RT)
Normality assumption was confirmed by means of normality plots as well as the Shapiro‐Wilk‐Test. Repeated measures ANOVA was computed in IBM SPSS Statistics 20 for Windows (SPSS Inc., Chicago, IL, USA) to analyze the effect of condition (five levels: 4 different judgment conditions + syllables condition) on reaction time. Post‐hoc, we compared all conditions by means of paired samples t‐tests, which were Bonferroni corrected.
RESULTS
Behavioral Results
A repeated‐measures ANOVA with the factor condition (five levels) and the dependent variable reaction time was conducted. As Mauchly's test of sphericity indicated that the assumption of sphericity had been violated, we used Greenhouse‐Geisser correction before evaluating significance. The corrected repeated measures ANOVA showed a significant main effect of condition with F(4,160) = 60.13, P < 0.001. Bonferroni corrected paired samples t‐tests revealed that participants responded fastest when they judged either themselves or their friends and took significantly longer when they were to judge either their teachers or politicians (see Fig. 1). Mean reaction time for syllables was significantly longer compared to all other conditions. For details on mean reaction times and standard deviations see Supporting Information S3a and for details on test statistics and P‐values of the paired samples t‐tests refer to Supporting Information S3b.
Figure 1.
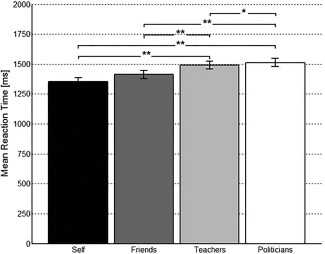
Mean reaction time for the judgment of themselves, their friends, their teachers or politicians. ** = P < 0.001; * = P < 0.05; error bars represent standard error; for clarity reasons, we did not include a bar representing mean reaction time for the syllables condition. Mean reaction time for syllables was significantly longer compared to all other conditions (P < 0.001).
Moreover, we analyzed endorsement to positive and negative trait words for all reference persons (see Table 1). Interestingly, in the self‐ as well as the friends condition, positive trait words were more frequently endorsed than negative trait words.
Table 1.
Endorsement to positive and negative trait words for all conditions separately (i.e., reference persons)
| Reference person | Positive traits | Negative traits |
|---|---|---|
| Mean endorsement (SD) in % | Mean endorsement (SD) in % | |
| Self | 78.78 (13.31) | 34.52 (19.28) |
| Friends | 79.82 (12.46) | 34.56 (17.2) |
| Teachers | 54.06 (21.37) | 40.53 (14.66) |
| Politicians | 43.01 (21.66) | 36.55 (17.08) |
Note. SD = standard deviation.
Paired samples t‐tests did not yield any significant differences between the self and friends‐condition for endorsement of positive as well as negative trait words (see Table 2). On the contrary, positive trait words for teachers and politicians were endorsed differently compared to the self and friends condition. For the latter, also negative trait words differed significantly from teachers. For comparison of teachers and politicians, endorsement of positive trait words differed significantly.
Table 2.
Paired t‐tests comparing endorsement to positive and negative trait words between conditions (i.e., reference persons)
| T‐Test | Positive traits | Negative traits | |
|---|---|---|---|
| P | P | ||
| Self vs. | Friends | .57 | .989 |
| Teachers | <0.001* | .097 | |
| Politicians | <0.001* | .637 | |
| Friends vs. | Teachers | <0.001* | <0.001* |
| Politicians | <0.001* | .563 | |
| Teachers vs. | Politicians | <0.001* | .121 |
Note. * asterisks mark comparisons that survive Bonferroni correction at P < . 0.004.
Regions of Interest Analyses
As described in the methods section for the imaging analyses, we conducted an ANOVA with the factor reference person (self, friends, teachers, politicians). Results are reported within our a priori defined literature‐based ROIs (vMPFC, dMPFC, mPPC, TPJ) using small volume correction with a significance level set at P < 0.05 family wise error (FWE) corrected. Results surviving a Bonferroni correction for the 12 post‐hoc t‐tests within our 6 ROI (P < 0.0007) are indicated in Table 4.
Table 4.
Comparisons between all reference conditions within a‐priori ROI
| t‐contrast | Region | H | Cluster size (voxels) | MNI coordinates (peak) | t (peak) | P (FWE) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| self > friends | vMPFC | bil | 568 | −3 | 53 | 13 | 8.39 | <0.001* |
| dMPFC | bil | 295 | −6 | 47 | 28 | 6.91 | <0.001* | |
| mPPC | L | 42 | −3 | −46 | 40 | 4.15 | .003 | |
| TPJ | L | 84 | −57 | −52 | 34 | 6.71 | <0.001* | |
| friends > self | – | – | – | – | – | – | – | – |
| self > teachers | vMPFC | bil | 598 | −9 | 50 | 4 | 8.63 | <0.001* |
| dMPFC | bil | 202 | −6 | 50 | 28 | 4.97 | <0.001* | |
| mPPC | L | 66 | −3 | −49 | 43 | 5.07 | <0.001* | |
| mPPC | R | 25 | 3 | −52 | 43 | 3.62 | .013 | |
| TPJ | L | 79 | −57 | −52 | 34 | 6.14 | <0.001* | |
| TPJ | R | 81 | 57 | −49 | 31 | 4.67 | <0.001* | |
| teachers > self | mPPC | L | 42 | 0 | −55 | 19 | 4.55 | .001 |
| mPPC | R | 37 | 9 | −52 | 19 | 4.59 | .001 | |
| self > politicians | vMPFC | bil | 620 | −9 | 50 | 1 | 10.28 | <0.001* |
| dMPFC | bil | 83 | −6 | 47 | 28 | 4.43 | .002 | |
| mPPC | L | 97 | −3 | −52 | 43 | 6.61 | <0.001* | |
| mPPC | R | 41 | 3 | −52 | 43 | 4.64 | <0.001* | |
| TPJ | L | 72 | −51 | −52 | 19 | 6.66 | <0.001* | |
| TPJ | R | 56 | 51 | −46 | 28 | 4.15 | .002 | |
| politicians > self | – | – | – | – | – | – | – | <0.001* |
| friends > teachers | vMPFC | bil | 296 | −15 | 44 | −2 | 3.58 | .050 |
| mPPC | R | 18 | 6 | −55 | 43 | 3.81 | .008 | |
| teachers > friends | dMPFC | bil | 140 | −9 | 53 | 37 | 4.14 | .007 |
| mPPC | L | 41 | −3 | −55 | 16 | 3.77 | .009 | |
| friends > politicians | vMPFC | bil | 425 | −12 | 44 | −2 | 5.65 | <0.001* |
| mPPC | L | 67 | −6 | −55 | 43 | 5.96 | <0.001* | |
| politicians > friends | dMPFC | bil | 265 | −9 | 53 | 37 | 4.95 | <0.001* |
| teachers > politicians | – | – | – | – | – | – | – | – |
| politicians > teachers | − | – | – | – | – | – | – | – |
Note. Peak voxel coordinates and t‐values for activation clusters in all a‐priori defined ROI, alpha‐error adjusted for respective ROI‐volume with P < .05; * activations marked with an asterisk also survive Bonferroni correction at P < . 0.0007; vMPFC = ventromedial prefrontal cortex; dMPFC = dorsomedial prefrontal cortex; mPPC = medial posterior parietal cortex; TPJ = temporoparietal junction; H = hemisphere; MNI = Montreal Neurological Institute; bil = bilateral; L = left; R = right.
We observed a significant main effect of condition, i.e., reference person, within all ROIs (Table 3).
Table 3.
Main effect of condition (i.e., reference person) within a‐priori ROI
| F‐contrast | Region | H | Cluster size (voxels) | MNI coordinates (peak) | F (peak) | P (FWE) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| ME condition | vMPFC | bil | 618 | −9 | 50 | 1 | 40.24 | <0.001 |
| (reference person) | dMPFC | bil | 262 | −6 | 47 | 28 | 17.49 | <0.001 |
| mPPC | L | 118 | −6 | −55 | 43 | 17.50 | <0.001 | |
| mPPC | R | 69 | 3 | −55 | 40 | 10.35 | <0.001 | |
| TPJ | L | 76 | −57 | −52 | 34 | 19.71 | <0.001 | |
| TPJ | R | 52 | 57 | −49 | 31 | 8.01 | .006 | |
Note. Peak voxel coordinates and F‐values for activation clusters in all a‐priori ROI, alpha‐error adjusted for respective ROI‐volume with P < .05 ME = main effect; vMPFC = ventromedial prefrontal cortex; dMPFC = dorsomedial prefrontal cortex; mPPC = medial posterior parietal cortex; TPJ = temporoparietal junction; H = hemisphere; MNI = Montreal Neurological Institute; bil = bilateral; L = left; R = right.
Post‐hoc t‐tests are being reported for the respective ROIs separately, an overview can be found in Table 4 and Figure 2.
Figure 2.
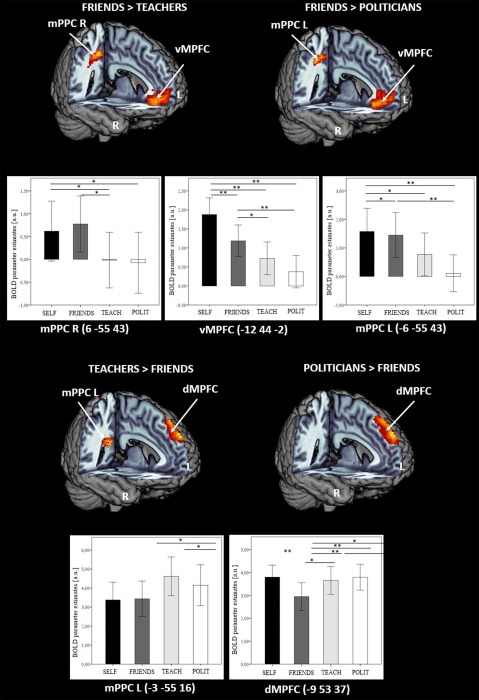
T‐contrast images for friends > teachers and friends > politicians as well as for teachers > friends and politicians > friends in ROI‐based analyses. Images were masked with a priori defined ROI‐volumes (vMPFC, dMPFC, mPPC) and thresholded at P < 0.05 uncorrected with a voxel extend of 10. Bar graphs: x‐axis represents bar graphs for each judgment condition within significant ROI, y‐axis represents the mean BOLD parameter estimates of the peak voxel within each ROI in arbitrary units; ** = P < 0.001; * = P < 0.05; error bars represent standard error; numbers in brackets indicate MNI coordinates; Abbreviations: vMPFC = ventromedial prefrontal cortex; dMPFC = dorsomedial prefrontal cortex; mPPC = medial posterior parietal cortex; L = left, R = right; SELF = judgment of themselves, FRIENDS = judgment for their friends, TEACH = judgment for their teachers, POLIT = judgment for politicians, BOLD = blood oxygen level dependent; a.u. = arbitrary units. [Color figure can be viewed at http://wileyonlinelibrary.com]
vMPFC
Higher neural activation within vMPFC was observed for self‐reference compared to all other reference persons (self > friends, self > teachers, self > politicians), as well as for friends compared to teachers and politicians (friends > teachers, friends > politicians).
dMPFC
Post‐hoc t‐tests revealed higher activation within the dMPFC for the self compared to the all other reference persons (self > friends, self > teachers, self > politicians). Moreover, the comparison of teachers > friends as well as politicians > friends revealed a higher activation of the dMPFC.
mPPC
Higher activation in bilateral mPPC for the self‐reference condition was observed compared to teachers and politicians (self > teachers, self > politicians), respectively, as well as compared to friends in left mPPC (self > friends). Friends‐reference compared to teachers as well as politicians (friends > teachers; friends > politicians) activated the same clusters, located in an anterior superior part of the precuneus.
Notably, comparing teachers and the self‐condition (teachers > self) as well as teachers and the friends‐condition (teachers > friends), we found a significantly higher activation in bilateral and left mPPC, respectively; these clusters were located in the inferior posterior cingulate cortex.
TPJ
For the self‐reference condition compared to teachers and politicians (self > teachers, self > politicians), higher activation within bilateral TPJ has been found. For self > friends, only within left TPJ, the self‐condition differed significantly from friends showing increased TPJ activation for self vs. friends.
In further exploratory analyses, activations outside the predefined ROI are reported at P < 0.05 FWE‐corrected, which can be found in the Supporting Information S4.
DISCUSSION
To the best of our knowledge, this is the first study that examines the neural basis of self‐judgments in adolescence in comparison to judgments of familiar others (i.e., friends and teachers) and unfamiliar persons (i.e., politicians) in a rather large sample. We observed that brain structures, which have been associated with self‐referential processes in previous studies (vMPFC, mPPC), distinguished processing of characteristics attributed to friends compared to teachers and unknown others (politicians). Moreover, self‐ and friend‐judgments were associated with a greater overlap of activation in vMPFC as well as mPPC compared to the other conditions.
The Neural “Self‐Network” in Adolescents
As suggested in previous studies, we were able to confirm findings regarding the involvement of cortical midline structures during self‐related processes within children as well as adolescents [Pfeifer et al., 2007, 2009]. Especially the vMPFC has repeatedly been shown to be involved in self‐referential processing [for meta‐analyses see Denny et al., 2012; Northoff et al., 2006; van der Meer et al., 2010]. Interestingly, previous studies in adults indicate a stronger vMPFC involvement for stimuli that are closer or more important to oneself [Krienen et al., 2010; Murray et al., 2012; van der Meer et al., 2010]. Moreover, former studies indicate that the vMPFC activates preferentially for the self as well as for those others that are perceived as similar to the self [Benoit et al., 2010; Mitchell et al., 2006]. Regarding the dMPFC, our result of a higher dMPFC engagement during self‐appraisal is in line with Pfeifer's observation in children: possibly, adolescents show a similar pattern as children where greater similarities between reflections on the self and other persons have been found in the dMPFC [Pfeifer et al., 2007]. Therefore, it could be hypothesized that adolescents have to activate some brain areas such as the dMPFC when processing self‐related information. Later in life, such brain areas might develop into regions mainly providing the perspective of “others” as indicated by findings in adults showing higher involvement of the dMPFC in judgments about others [Mitchell et al., 2005].
Furthermore, we observed that not only the MPFC but also the mPPC and the TPJ yielded the strongest activation for the self‐referential condition. The mPPC, specifically the anterior part, has frequently been reported to be involved in self‐related imagery [Cavanna and Trimble, 2006]. Our results underline the importance of considering subdivisions of the mPPC, with the anterior part possibly being involved in self‐referential processing in adolescents. The TPJ has previously been implicated in the self‐referential network in adolescents [Pfeifer et al., 2009]. Studies in adults, on the other hand, highlight the TPJ as one of the most important brain areas for social cognition [Samson et al., 2004; Young et al., 2010] and indicate an engagement of the TPJ in mentalizing activities related to others compared to the self [Saxe et al., 2006]. Importantly, our results not only suggest that the TPJ is also involved in self‐processing in adolescents, it also indicates that the TPJ is even more engaged in cognitions related to the self than to others in adolescents. One possible interpretation is that adolescence represents a “self‐discovery” phase where adolescents struggle to build a coherent self‐concept, therefore relying more heavily on mentalizing processes involving several brain areas later more strongly dedicated to social cognition. To confirm that the TPJ plays an important role in self‐referential processing in adolescents, research directly comparing adolescents and adults is needed.
The Neural “Social Network” in Adolescents
The direct comparison of the social conditions revealed that vMPFC activation as well as activation in mPPC was greater for friends‐ compared to teacher‐ or politician‐judgments, pointing to an overlap in brain networks for self‐ and friend‐conditions independent of familiarity with the “other.” This is in line with our hypothesis and it extends findings by Schneider et al. [2012] where “self” and the “best friend” conditions did not differ significantly with respect to activated brain areas. Yet, in the study of Schneider et al. [2012], a heterogeneous sample of 12 to 20 year old subjects was investigated, while we focused on subjects in mid‐adolescence. Especially during adolescence, friends are of great importance for the development of a self‐concept [Harter, 2012] and close others are more likely to be included in this self‐concept [Aron et al., 2004]. Also our behavioral findings regarding similar endorsement for self‐ and friend‐related trait judgments, point to this similarity in self‐ and friend‐cognition. This seems to be a specific finding beyond mere familiarity effects since it can be clearly distinguished from judgments regarding either familiar teachers or unfamiliar politicians. Greater vMPFC activation for the friends‐condition could therefore function as a neural correlation of these behavioral findings. In this context, a heightened activity in the mPPC in the friends‐ compared to the teachers‐ or politicians‐condition may suggest that adolescents rely increasingly on self‐related mental imagery when judging their friends.
Interestingly, in the dMPFC we found a different pattern, with the lowest activation in the friends‐condition and similarly high activation for teachers and politicians. Some studies in adults indicate a strong activation of the dMPFC in making judgments about others, in particular people dissimilar to oneself [Mitchell et al., 2004, 2005, 2006]. Regarding other persons, neural activation patterns appear to be similar in adults and adolescents. The dMPFC is, in contrast to vMPFC, believed to support externally‐focused processes and effortful social processing [Lieberman, 2007] with an essential role in cognitive control during negative value coding (such as social conflict, evaluation of risk etc.) (O'Reilly, 2010) Possibly, judging persons that are dissimilar to oneself (such as teachers and politicians) represents a more effortful cognitive process than judging oneself or similar persons.
Considering the teachers‐condition in more detail, the ventral‐anterior area of the mPPC yielded a significant activation difference when compared to the self‐ or friends‐condition. This part of the PPC has been found to be engaged in episodic memory retrieval [Gainotti et al., 1998; Vann et al., 2009]. Retrieving the necessary content from episodic memory to judge teachers may be more difficult than retrieving it for self and friends. A review by Lieberman [2007] proposes that the medial parietal cortex (like the dMPFC) is involved in “controlled social cognition,” e.g., reflected appraisals, while the vMPFC is involved in “automatic social cognition”. Judging teachers, which might represent a more heterogeneous group more dissimilar to oneself could therefore require more contemplation on the presented trait words compared to the self‐ and friends‐conditions. This might also be reflected in the behavioral reaction times pointing to a higher cognitive load with significantly longer reaction times when adolescents were to judge either teachers or politicians compared to friends. This hypothesis is further supported by our finding of higher dMPFC activity in teacher‐ compared to friend‐judgments, also pointing to effortful social processing [Lieberman, 2007].
In general, brain activation clusters for politician‐judgments did not differ from those of the teacher‐judgments. Possibly, since academic motivation usually decreases during mid‐adolescence [Eccles et al., 1998; Zusho and Pintrich, 2001], teachers' influence on adolescents' self‐concept also decreases. Therefore, our hypothesis regarding a graduation of other person judgments (from friends to teachers to politicians) was not confirmed and this observation suggests that other factors than mere familiarity need to be considered and investigated in future studies.
Limitations
When interpreting the present results, we often relied on data from studies in adults since information about children and adolescents is sparse or even absent. Though our findings indicate a difference in the neural networks of adolescents versus adults when processing self‐ and other‐related stimuli, this assumption has to be explored in future studies considering direct comparisons of adolescents and adults. Furthermore, the development from middle to late adolescence is of interest in order to study the suggested functional distinction of the dMPFC as well as TPJ during maturation and the development of the self‐concept in adolescence. Here, longitudinal data is strongly needed. Future studies should also investigate the peer group in more detail, e.g., whether there are differences between close friends and unfamiliar peers.
Conclusion
Altogether, our results indicate that the vMPFC and dMPFC as well as TPJ and mPPC are crucial brain structures within the adolescent neural self‐network. Moreover, in adolescents, self‐judgment and the judgment of close others, i.e., friends, were being processed in overlapping neural networks, pointing to potentially related neural processes. These processes can be distinguished from familiar others such as teachers, where the activated neural networks mostly resemble those of unfamiliar persons such as politicians. For the self‐concept of adolescents, the perceived similarity between oneself and friends was localized in similar neural structures and these findings may help to explain behavioral findings of a reliance of adolescents' self‐concept on relevant others.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Eva Flemming for help with fMRI data acquisition. In addition, the authors thank the principals, teachers and students as well as parents for their cooperation in making this study possible.
The authors of this article declare no financial or other conflicts of interest. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- Aron A, McLaughlin‐Volpe T, Mashek D, Lewandowski G, Wright SC, Aron EN (2004): Including others in the self. Eur Rev Soc Psychol 15:101–132. [Google Scholar]
- Bahnemann M, Dziobek I, Prehn K, Wolf I, Heekeren HR (2010): Sociotopy in the temporoparietal cortex: Common versus distinct processes. Soc Cogn Affect Neurosci 5:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Volle E, Burgess PW (2010): When I think about me and simulate you: Medial rostral prefrontal cortex and self‐referential processes. NeuroImage 50:1340–1349. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Robbins TW (2012): Decision‐making in the adolescent brain. Nat Neurosci 15:1184–1191. [DOI] [PubMed] [Google Scholar]
- Brett M, Christoff K, Cusack R, Lancaster J (2001): Using the Talairach atlas with the MNI template. NeuroImage 13:85. [Google Scholar]
- Brown BB, Larson J. (2009): Peer relationships in adolescence In: Lerner RM. & Steinberg L, editors. Handbook of Adolescent Psychology, 3rd ed., Vol. 2 Hoboken, NJ, US: John Wiley & Sons Inc; pp. 74–103. [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129:564–583. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE (2012): Understanding adolescence as a period of social‐affective engagement and goal flexibility. Nat Rev Neurosci 13:636–650. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN (2012): A meta‐analysis of functional neuroimaging studies of self‐ and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci 24:1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JS, Roeser RW (2011): Schools as developmental contexts during adolescence. J Res Adolescence 21:225–241. [Google Scholar]
- Eccles J, Wigfield A, Schiefele A (1998): Motivation to succeed In: Damon W, Eisenberg N, editors. Handbook of Child Psychology. New York, NY: John Wiley & Sons; pp 1017–1095. [Google Scholar]
- Gainotti G, Almonti S, Di Betta AM, Silveri MC (1998): Retrograde amnesia in a patient with retrosplenial tumour. Neurocase: The Neural Basis of Cognition 4:519–526. [Google Scholar]
- Harter, S. (2012) The Construction of the Self: Developmental and Sociocultural Foundations. New York, NY: Guilford Press. [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM (2006): Medial prefrontal activity differentiates self from close others. Soc Cogn Affect Neurosci 1:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Bermpohl F, Frank M (2012): Construction and interpretation of self‐related function and dysfunction in Intercultural Psychiatry. Eur Psychiatry 27 Suppl 2:S32–S43. [DOI] [PubMed] [Google Scholar]
- Jankowski KF, Moore WE, Merchant JS, Kahn LE, Pfeifer JH (2014): But do you think I'm cool? Developmental differences in striatal recruitment during direct and reflected social self‐evaluations. Dev Cogn Neurosci 8:40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Tu PC, Buckner RL (2010): Clan mentality: Evidence that the medial prefrontal cortex responds to close others. J Neurosci 30:13906–13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD (2007): Social cognitive neuroscience: A review of core processes. Annu Rev Psychol 58:259–289. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, Consortium MA, Baron‐Cohen S (2010): Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci 22:1623–1635. [DOI] [PubMed] [Google Scholar]
- Lorenz RC, Gleich T, Beck A, Pöhland L, Raufelder D, Sommer W, Rapp MA, Kühn S, Gallinat J (2014): Reward anticipation in the adolescent and aging brain. Hum Brain Mapp 35:5153–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H, Wurf E (1987): The dynamic self‐concept: A social psychological perspective. Annu Rev Psychol 38:299–337. [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR (2004): Encoding‐specific effects of social cognition on the neural correlates of subsequent memory. J Neurosci 24:4912–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN (2005): The link between social cognition and self‐referential thought in the medial prefrontal cortex. J Cogn Neurosci 17:1306–1315. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR (2006): Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 50:655–663. [DOI] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbane M (2012): Degrees of separation: A quantitative neuroimaging meta‐analysis investigating self‐specificity and shared neural activation between self‐ and other‐reflection. Neurosci Biobehav Rev 36:1043–1059. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS (2005): The social re‐orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychol Med 35:163–174. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J (2006): Self‐referential processing in our brain–a meta‐analysis of imaging studies on the self. NeuroImage 31:440–457. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, Feinberg TE (2011): Brain imaging of the self–conceptual, anatomical and methodological issues. Conscious Cogn 20:52–63. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC (2010): The What and How of prefrontal cortical organization. Trends Neurosci 33:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Parker JG, Rubin KH, Erath SA, Wojslawowicz JC, Buskirk AA (2006): Peer relationships, child development, and adjustment: A developmental psychopathology perspective In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology, Theory and Method, 2nd ed., Vol. 1 Hoboken, NJ, US: John Wiley & Sons Inc; pp. 419–493. [Google Scholar]
- Pfeifer JH, Blakemore SJ (2012): Adolescent social cognitive and affective neuroscience: Past, present, and future. Soc Cogn Affect Neurol 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Peake SJ (2012): Self‐development: Integrating cognitive, socioemotional, and neuroimaging perspectives. Dev Cogn Neurosci 2:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M (2007): “I know you are but what am I?”: Neural bases of self‐ and social knowledge retrieval in children and adults. J Cogn Neurosci 19:1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD (2009): Neural correlates of direct and reflected self‐appraisals in adolescents and adults: When social perspective‐taking informs self‐perception. Child Dev 80:1016–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW (2004): Left temporoparietal junction is necessary for representing someone else's belief. Nat Neurosci 7:499–500. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N (2003): People thinking about thinking people. The role of the temporo‐parietal junction in “theory of mind”. NeuroImage 19:1835–1842. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J (2006): Overlapping and non‐overlapping brain regions for theory of mind and self reflection in individual subjects. Soc Cogn Affect Neurosci 1:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC (2007): Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev 31:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Debbane M, Lagioia A, Salomon R, d'Argembeau A, Eliez S (2012): Comparing the neural bases of self‐referential processing in typically developing and 22q11.2 adolescents. Dev Cogn Neurosci 2:277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS (2010): Self‐reflection and the brain: A theoretical review and meta‐analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev 34:935–946. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA (2009): What does the retrosplenial cortex do?. Nat Rev Neurosci 10:792–802. [DOI] [PubMed] [Google Scholar]
- Vo ML, Conrad M, Kuchinke L, Urton K, Hofmann MJ, Jacobs AM (2009): The Berlin affective word list reloaded (BAWL‐R). Behav Res Methods 41:534–538. [DOI] [PubMed] [Google Scholar]
- Young L, Dodell‐Feder D, Saxe R (2010): What gets the attention of the temporo‐parietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia 48:2658–2664. [DOI] [PubMed] [Google Scholar]
- Zusho A, Pintrich PR (2001): Motivation in the second decade of life In: Urdan T, Pajares F, editors. Adolescents and Education: General Issues in the Education of Adolescents. Greenwich, CT: Information Age Publishing; pp 163–200. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


