Abstract
Motor learning is characterized by patterns of cerebello‐striato‐cortical activations shifting in time, yet the early dynamic and function of these activations remains unclear. Five groups of subjects underwent either continuous or intermittent theta‐burst stimulation of one cerebellar hemisphere, or no stimulation just before learning a new motor sequence during fMRI scanning. We identified three phases during initial learning: one rapid, one slow, and one quasi‐asymptotic performance phase. These phases were not changed by left cerebellar stimulation. Right cerebellar inhibition, however, accelerated learning and enhanced brain activation in critical motor learning‐related areas during the first phase, continuing with reduced brain activation but high‐performance in late phase. Right cerebellar excitation did not affect the early learning process, but slowed learning significantly in late phase, along with increased brain activation. We conclude that the right cerebellum is a key factor coordinating other neuronal loops in the early acquisition of an explicit motor sequential skill. Hum Brain Mapp 38:1676–1691, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: basal ganglia, cerebellum, explicit sequence learning, functional MRI, theta‐burst stimulation
INTRODUCTION
Motor memory can be defined as “a representation of motor action in all of its forms, from skeletal movement to language, which is acquired through practice or experience” [Fuster, 1999]. Learning a new motor skill, and the acquisition of a novel sequence of movements in particular, is a highly complex phenomenon that involves multiple interconnected structures like the primary motor cortex (M1), supplementary motor area, basal ganglia, cerebellum, and hippocampus [Albouy et al., 2008, 2013a, 2013b; Coynel et al., 2009; Doyon and Benali, 2005; Doyon et al., 2003, 2009; Hikosaka et al., 2002; Karni et al., 1998; Lehéricy et al., 2005]. Learning of a new motor sequence typically follows several stages: (i) a “fast” learning period, that is, an initial, within‐session improvement period, followed by (ii) a period of consolidation lasting several hours, which assures the transition toward (iii) a “slow” learning period, consisting of delayed, incremental gains in performance emerging after continued practice [Karni et al., 1998]. Here, we have concentrated only on the initial, fast learning period.
Studies have shown that the cerebellum is particularly active during this fast learning phase [Hikosaka et al., 2002; Jenkins et al., 1994; Jueptner and Weiller, 1998; Middleton and Strick, 2000; Penhune and Doyon, 2005; Toni et al., 1998]—an activity that fades out with practice, only to become undetectable when the sequence of movements is well learned [Doyon et al., 2002, 2009; Penhune and Doyon, 2002; Toni et al., 1998]. Most motor learning studies report consistent changes within both cerebellar hemispheres, whatever the hand used for executing the task (for extensive literature review refer to [Lohse et al., 2014] and [Hardwick et al., 2013]), particularly in the very early stages of learning when decreased activation of the cerebellar cortex matches increased cerebellar nuclear activity [Doyon et al., 2003; Floyer‐Lea and Matthews, 2005]. At the same time, basal ganglia, particularly the putamen, are reported to be active throughout all learning stages. Yet, there seems to be a predominant activity shift within the basal ganglia during motor learning from the anterior, associative striatum to the posterior, sensorimotor striatum during learning of a motor sequence through repetition [Coynel et al., 2009; Lehéricy et al., 2005]. However, it is still unclear how the cortico‐striatal and cortico‐cerebellar loops interact during the acquisition of a new motor skill.
Here, we investigated the activity in these different loops with fMRI, after specifically interfering with the output of each cerebellar hemisphere separately in four groups of healthy right‐handed subjects engaged in learning a new explicit motor sequence. We took advantage of the capacity of the repetitive transcranial magnetic stimulation patterned as theta‐burst stimulation (TBS) to specifically alter the excitability of the cerebellar cortex over at least 30 minutes [Popa et al., 2010]. TBS of the cerebellum is also known to modulate the plasticity of remote structures with which the cerebellum is connected: intermittent TBS of the cerebellum (iTBSCB) blocks the remote heterosynaptic plastic response of M1, while continuous TBS of the cerebellum (cTBSCB) enhances it [Kishore et al., 2014a, 2014b; Popa et al., 2013]. Based on these findings and for the sake of simplicity of the discussion, we will assume that iTBSCB has a generally excitatory effect on the cerebellar output, while cTBSCB has an inhibitory effect on the cerebellar output. However, unless the exact mechanisms of action of cTBS or iTBS on the cerebellum are known at a cellular or microcircuit level, these generalizations should be considered only provisionally.
Another unresolved aspect is whether the effect of modulating the cerebellar output is purely anatomically lateralized (stimulation of one cerebellar hemisphere influences only the contralateral M1 and thus only the ipsilateral hand), functionally lateralized (stimulation of one cerebellar hemisphere impacts a particular lateralized function), or distributed over both hemispheres (stimulation of either hemisphere has the same effects). A recent meta‐analysis of the literature [Hardwick et al., 2013] suggested that motor sequence learning done with either hand involves nonexclusively the right cerebellar hemisphere. In the present study, we instructed participants to use their nondominant (left) hand, because this leads to greater changes than when the motor task is executed with the dominant hand. Thus, if the anatomical lateralization hypothesis were true, we would expect a major influence of the left cerebellar stimulation on learning using the left hand only, whereas if the functional lateralization hypothesis were true, we would expect a major influence of the right cerebellar stimulation.
By applying iTBSCB or cTBSCB, over the left or right posterior cerebellar hemispheres, in four age‐ and gender‐matched groups of subjects, we independently increased or decreased the contribution of each cerebellar hemisphere to the neural network involved in the motor sequence learning task. Any change should be particularly evident in the initial period of the motor learning, when the interactions between the motor cortices, the cerebellar structures, the basal ganglia, and the hippocampus are most prominent [Albouy et al., 2008, 2012, 2013a,2013b; Censor et al., 2012; Doyon and Benali, 2005; Doyon et al., 2009; Floyer‐Lea and Matthews, 2005; Hikosaka et al., 2002; Steele and Penhune, 2010]. Indeed, previous studies of explicit motor sequence learning have reported increase in performance reaching a plateau within approximately 15 min from the start of the motor practice [Albouy et al., 2012], enough to be covered by the maximum effect of TBS [Popa et al., 2010]. We scanned our subjects during this time window, while learning the motor sequence right after the TBS of the cerebellum. We hypothesized that if the cerebellum does influence motor learning in the same way it modulates the heterosynaptic plasticity of M1, then cerebellar excitation would prevent or slow down the acquisition of a new motor sequence, while cerebellar inhibition would enhance it when compared to the learning pattern of a nonstimulated, control group.
MATERIAL AND METHODS
Subjects
Seventy‐three right‐handed, healthy volunteers were initially recruited to participate in this study. A preliminary interview ascertained that they had no significant history of psychiatric or neurological disorders. Musicians and professional typists were excluded to avoid subjects with pre‐existing high finger coordination skills. All subjects gave written informed consent according to the guidelines of the ethical committee of the “Regroupement Neuroimagerie Québec” at the “Centre de Recherche de l'Institut Universitaire de Gériatrie de Montréal (CRIUGM),” Montreal, QC, Canada, and were compensated for their participation in the study. Participants were distributed into four groups, which randomly underwent continuous theta‐burst stimulation of the left (LcTBSCB group, N = 16) or right (RcTBSCB group, N = 19) cerebellar hemisphere, or intermittent theta‐burst stimulation of the left (LiTBSCB group, N = 16) or right (RiTBSCB group, N = 22) cerebellum. Results were compared with those of a control group of 55 right‐handed, healthy volunteers [from a previous study, see Albouy et al., 2015], who executed the exact same motor sequence learning task under identical fMRI scanning conditions, but without prior stimulation of the cerebellum. Data from nine subjects were excluded from the analyses for the following reasons: three participants were discarded from the RiTBSCB group due to technical difficulties (i.e., the motor threshold was too high to consider the stimulation efficient even at the maximum intensity allowed), while six additional participants (one from the RcTBSCB group, one from the LcTBSCB group, one from the LiTBSCB group, and three others from the control group) were considered outliers based on their motor learning performance (i.e., 2 standard deviations away from the average group changes in speed performance, see below). Data analyses were therefore carried out on the remaining 119 participants: 15 in the LiTBSCB group (mean age: 24.4 ± 3.1 years, 8 females), 15 in the LcTBSCB group (mean age: 24.9 ± 2.6 years, 8 females), 19 in the RiTBSCB group (mean age: 24.6 ± 2.3 years, 12 females), 18 in the RcTBSCB group (mean age: 23.3 ± 4 years, 10 females), and 52 in the control group (mean age: 24.2 ± 3.5 years, 33 females).
Motor Sequence Learning Task
Participants performed a sequential finger‐tapping task with their nondominant (left) hand [initially proposed by Karni et al., 1998]. They were instructed to tap continuously a five‐element finger sequence (4‐1‐3‐2‐4; 1 being the index finger) as fast as possible while making as few errors as possible using an MRI‐compatible response box. This experiment was realized using Cogent 2000 developed by the Cogent 2000 team at the FIL and the ICN, and Cogent Graphics developed by John Romaya at the LON at the Wellcome Department of Imaging Neuroscience. From the start, participants explicitly knew the sequence as they were told the sequence structure by the experimenter, but had to refrain from any practice before scanning. The task comprised 14 successive training blocks. Each training block consisted of a fixed number of key presses (i.e., 60 movements corresponding optimally to 12 repetitions of the sequence) to control for the number of finger movements (and hence, cerebral responses) per block. Each training block started with a green cross, displayed in the middle of the screen and indicating that participants had to produce the sequence. Once they had completed 60 key presses, the training block automatically switched to a 15‐s rest block (indicated by a red cross on the screen). Both the time and accuracy of the transition between two consecutive key presses were recorded. Given the explicit knowledge of an easy and short sequence, speed improvement rather than accuracy was expected to reflect learning.
Experimental Procedure
First, a high‐resolution anatomical T 1‐weighted scan was acquired using the 3T MRI system (see below for more details) installed in the “Unité de Neuroimagerie Fonctionelle” at the CRIUGM. The magnetic stimulations were then carried out with a Magstim Rapid2 magnetic stimulator and a figure‐of‐eight AirFilm coil with a 70‐mm loop diameter (Magstim Company, Withland, UK). The active motor threshold (AMT) of M1 was determined for each participant, using cortical representation of the first dorsal interosseus muscle. The AMT was the lowest stimulus intensity able to elicit a motor response of 200 μV above the background EMG level in at least 5 out of 10 trials, while the muscle was isometrically contracted at about 10% of the maximum voluntary contraction force. The repetitive stimulation was performed under neuronavigated control using a frameless stereotaxic system (Brainsight™2, Rogue Research, Montreal, Canada) at 80% AMT stimulation intensity. The proper location of the target, that is, lobule VIII [Popa et al., 2013], was identified for every participant based upon his or her own high‐resolution T 1‐weighted anatomical MRI (in our cohort of subjects, the target areas were centered around x = 28 ± 8, y = −79 ± 5, z = −53 ± 7 for the right cerebellar hemisphere, and x = −22 ± 9, y = −80 ± 4, z = −50 ± 8 for the left). The coil was positioned tangential to the skull over the back of the head with the handle pointing upwards [Ugawa et al., 1995], in such a way as to minimize the distance between the center of the coil and the cerebellar target. One of the following two stimulation protocols was then administered: (i) inhibition of the cerebellar cortex was obtained with cTBSCB, which consisted of 600 stimuli delivered in 3‐pulse bursts at 50 Hz repeated every 200 ms [Huang et al., 2005]; (ii) excitation of the cerebellar cortex was obtained with iTBSCB, which consisted of 600 stimuli delivered in 3‐pulse bursts at 50 Hz, repeated every 200 ms in 2‐s trains separated by 8‐s pauses. Importantly, the stimulation intensities used in this study were well below the maximum limit recommended by the guidelines for delivering TBS [Rossi et al., 2008].
The cerebellar stimulation was immediately followed by functional MRI acquisition during performance of the motor sequence‐learning task. The mean time between the end of stimulation and the start of the fMRI task did not differ between the four groups undergoing cerebellar conditioning (F(3,63)=1.05, P = 0.38): the LiTBSCB group (503 ± 55 s), the LcTBSCB group (528 ± 73 s), the RiTBSCB group (493 ± 100 s), and the RcTBSCB group (482 ± 65 s).
Behavioral Data Analyses
Motor sequence learning is generally defined as a reduction in response time (i.e., the transition time between two key presses) and number of errors across training [Doyon et al., 2009]. Accordingly, across all training blocks, we measured for each subject the changes in the number of correct key presses per block and the changes in mean transition time per block between two consecutive correct key presses. More specifically, as all groups demonstrated similar performance in the first block of trials in terms of accuracy (F(4,120)=0.45, P = 0.77) and speed (F(4,120)=1.08, P = 0.37), individual measures were normalized to the corresponding initial block using this formula: [100*(performanceblock_1 – performanceblock_i)/performanceblock_1]. Repeated measures analyses of variance (ANOVA) were then applied on these normalized data (accuracy and speed), with block as a within‐subjects factor and group as the between‐subjects factor. Greenhouse–Geisser corrections of P‐values were applied whenever the assumption of sphericity was violated (but all repeated measures effects are reported with the initial degrees of freedom).
Behavioral data were first analyzed including all 14 blocks and all five groups to determine group differences in motor sequence learning across the entire experimental task. In a second step, a sliding‐window method was used on the results of the control group to identify significantly different phases within the 14‐block series of nonmanipulated task performance. Bonferroni‐corrected t‐tests were used to correct for multiple testing within these sliding‐block analyses. First, one‐sample t‐tests (N = 13) were performed to determine the point beyond which the learning speed showed no further significant improvement from block to block (blockn − blockn −1). Next, we examined whether another significant change of performance was apparent by performing multiple pairwise t‐tests between block‐windows of variable length. Once these different phases in learning performance were defined, as a last step, repeated measures ANOVAs were performed (with block as a within‐subjects factor and group as the between‐subjects factor) to further specify the performance differences between all five groups for each phase separately. Whenever a repeated measures ANOVA revealed a significant difference in learning rate (i.e., a significant block × group interaction effect), a regression analysis for repeated measure designs [Lorch and Myers, 1990] was implemented: linear slope coefficients were computed for each subject based on the normalized data with training blocks as predictor [cfr. Gheysen et al., 2009]. If a significant group effect was found, pairwise comparisons (Bonferroni‐corrected) were then performed between the groups of interest: LiTBSCB versus Control, LcTBSCB versus Control, RiTBSCB versus Control, RcTBSCB versus Control, LiTBSCB versus LcTBSCB, and RiTBSCB versus RcTBSCB.
MRI Data Acquisition
Images were acquired with a whole body 3T Tim Trio scanner system (Siemens, Erlangen, Germany) equipped with a 32‐channel head coil. Head movements were minimized using foam padding within the head coil. Anatomical images were collected using a T 1‐weighted 3D magnetization‐prepared rapid acquisition with gradient echo (MPRAGE) sequence (TR = 2,300 ms, TE = 2.98 ms, TI = 900 ms, FA = 9°, 176 slices, FoV = 256 × 256 mm2, matrix size = 256 × 256 × 176, voxel size = 1 × 1 × 1 mm³). Multislice T 2*‐weighted functional MRI series were obtained with a gradient echo‐planar sequence using axial slice orientation (TR = 2,650 ms, TE = 30 ms, FA = 90°, 43 transverse slices, 3 mm slice thickness, 10% inter‐slice gap, FoV = 220 × 220 mm, matrix size = 64 × 64 × 43, voxel size = 3.4 × 3.4 × 3 mm). Slices were acquired along the z‐axis in ascending direction.
Functional MRI Data Analyses
Functional MRI data preprocessing and analyses were performed using MATLAB (The MathWorks, Inc., Natick, MA) and the SPM8 software (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Preprocessing steps included the realignment of functional time series (providing estimates of rigid head motion), the coregistration of functional and anatomical images, the spatial normalization to the Montreal Neurological Institute template, as well as a spatial smoothing using an isotropic 3D Gaussian kernel of 8 mm full‐width at half‐maximum.
Whole‐brain fMRI time‐series were analyzed to explore regional differences in motor learning‐related brain activation between the inhibitory, excitatory and no stimulation groups. First, a fixed‐effect general linear model (GLM) was defined for each participant including the motor sequence training blocks and the linear modulation by speed improvement (i.e., the individual normalized mean transition time between two consecutive correct key presses per block).
Consistent with behavioral analyses (see behavioral results section) we defined a separate regressor to account for the early (Blocks 1–3), mid (Blocks 4–9), and the late phase (Blocks 10–14) of motor sequence learning. The regressors were modeled as boxcars for each block and convolved with the canonical hemodynamic response function. Response errors and head motion parameters were entered into the GLM and modeled as covariates of no interest. High‐pass filtering with a cutoff of 128 s was implemented to remove low‐frequency drift. Serial correlations in fMRI signal were estimated using an autoregressive (Order 1) plus white noise model and a restricted maximum likelihood algorithm. Model parameters were estimated and used for single‐subject contrasts. Linear contrasts tested the effect of modulation of brain activity by performance, that is, regions where BOLD signal increased as speed improved for the early, mid, and late phase separately.
Then, to allow for inference at a population level, single‐subject contrasts were entered in random‐effects analyses using one‐sample Student's t‐tests (to characterize separate group effects) and ANOVAs (to characterize between‐group differences). The resulting whole‐brain activation maps were first thresholded at P voxel < 0.005 (uncorrected for multiple comparisons) to ensure the full extent of brain regions. The statistical inferences were then carried out by means of Monte Carlo simulations (3dClustSim, http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) to control for false positive rate at the cluster level. We ran 10,000 Monte Carlo simulations taking into account the whole‐brain mask and the smoothness of the residuals of the second‐level models. For the initial P < 0.005 voxel‐wise threshold, the simulations determined the minimum cluster size (for each second‐level analysis) corresponding to a combined corrected threshold of P < 0.05. To note, when testing for pairwise group differences in parametric effects using the SPM8 software (e.g., [+ cTBSCB group] – [+ iTBSCB group] = BOLD responses positively modulated by performance but stronger for the cTBSCB group than for the iTBSCB group), results can be confounded by the reverse parametric effect of the second group in the comparison (e.g., it could reflect [+cTBSCB] + [−iTBSCB] = BOLD responses positively modulated by performance in the cTBSCB group and responses negatively modulated by performance in iTBSCB group). Therefore, to truly isolate the effects of interest, additional exclusive masks were applied on these group contrasts with the default P < 0.05 uncorrected voxel thresholds. These masks comprised the responses negatively modulated by performance resulting from the one‐sample t‐test of the second group in the respective group comparison.
RESULTS
Behavioral Results
Learning accuracy
As expected, accuracy rates were overall high (LiTBSCB group= 98.3 ± 2%; LcTBSCB group= 99 ± 0.9%; RiTBSCB group= 98.7 ± 1.2%; RcTBSCB group= 98.8 ± 1%; Control group= 97.8 ± 3.5%). Repeated‐measures ANOVA conducted on the normalized number of correct key presses, with block (14 levels) as within‐subjects factor and group (5 levels) as between‐subjects factor, revealed no significant changes in accuracy across blocks (F(13, 1,482)= 1.28; P = 0.22), groups (F(4,114)= 1.23; P = 0.3), nor any group × block interaction (F(52, 1,482)=0.64; P = 0.98).
Learning speed
Repeated‐measures ANOVA was performed on the normalized mean transition time, with block (14 levels) as the within‐subjects factor and group (5 levels) as the between‐subjects factor. The results revealed a significant increase in learning speed across blocks (main effect of block, F(13,1,482)= 94.26; P < 0.001), as well as a significant difference in average learning speed between groups (F(4,114)=2.9; P = 0.026). Moreover, when a quadratic‐trend (R 2 = 0.47) applied on the block factor was included in the repeated measures ANOVA, to take into account the nonlinear polynomial form of the learning curve across all blocks, the analysis yielded a significant block × group interaction (F(4, 114)=2.55; P = 0.04). This suggests that groups differed in their abilities to learn a new motor sequence and did so at different rates. As shown in Figure 1A, transitions between fast and slower rates of learning speed could be observed across the 14 blocks.
Figure 1.
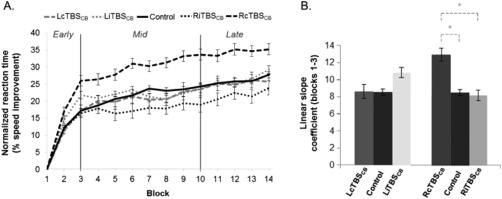
Behavioral results. (A) Mean normalized transition times per block and per group, (B) Mean linear slope coefficients of the normalized transition times per group across the early learning phase (Blocks 1–3); * P < 0.05 (Bonferroni corrected). Bars represent standard error of the mean (SEM).
To further investigate whether different types of cerebellar stimulation influenced differently each of these phases, we performed pairwise comparisons (Bonferroni corrected) between block windows of average learning performances (blockn − blockn −1). One‐sample t‐tests (Bonferroni‐corrected) showed significant increases in learning speed only from Blocks 1 to 3 in the control group (Blocks 1–2: t(51)=7.87, P < 0.0001; Blocks 2–3: t(51)=4.15, P = 0.0016). These results reveal a first point of transition in the learning process between Blocks 1–3 (first learning phase) and the remaining blocks (Fig. 1A).
Next, we investigated whether the learning curve had another significant phase change over longer periods. To that end, we performed multiple pairwise comparisons of speed improvement between block‐windows of variable length shifting progressively from Blocks 4 to 14 (e.g., a pairwise t‐test computed on the speed improvement on Blocks 4–6 vs. Blocks 7–14, then on Blocks 5–7 vs. Blocks 8–14, and so on). The performance was averaged for each window of blocks and normalized by its standard deviation to control for the size of the window (e.g., the average speed improvement on Blocks 4–6 divided by the standard deviation of speed improvement on Blocks 4–6). A significant difference in performance was found between Blocks 4–9 and Blocks 10–14, t(102)= −2.7, P = 0.008 (Fig. 1A). This separated the second phase (Blocks 4–9) from the third phase (Blocks 10–14) of the motor sequence learning.
First phase
Within this faster linear‐shaped learning phase, a 3 (block) × 5 (group) repeated‐measures ANOVA revealed significant changes in learning speed across the first three blocks (main effect of block, F(2,228)= 207.7; P < 0.001), with only a trend for the main effect of group (F(4,114)=2.17; P = 0.077). However, the repeated measures ANOVA with linear‐trend on the block factor (R 2 = 0.71) revealed a significant block × group interaction (F(4, 114)=2.77; P = 0.03), hence suggesting that, as expected, the rate of improvement differed between groups in the first phase of motor sequence learning. Subsequently, to determine which group was showing a different pattern of improvement, a regression analysis for repeated measures designs [Lorch and Myers, 1990] was implemented: linear slope coefficients were computed for each subject based on the normalized transition time data with training blocks as predictor [Gheysen et al., 2009] (Fig. 1B). During this first phase, the RcTBSCB group (slope= 12.98 ± 6.48) showed a significantly greater learning acceleration than the control group (slope= 8.57 ± 5.2, t(114)=2.94, P = 0.024) and the RiTBSCB group (slope= 8.23 ± 5.47, t(114)=2.70, P = 0.048), but there was no significant difference in learning slope between the RiTBSCB and control groups (t(114)= −0.23, P = 0.82). Interestingly, left cerebellar stimulation did not have any significant effect on early learning performance (LcTBSCB, slope= 8.62 ± 6.13, vs. control, t(114)=0.03, P = 1; LiTBSCB, slope= 10.87 ± 4.42, vs. control, t(114)=1.43, P = 0.93; LcTBSCB vs. LiTBSCB, t(114)=1.13, P = 1).
Second phase
A 6 (block) × 5 (group) repeated measures ANOVA over the second phase of motor sequence learning (Blocks 4–9) revealed significant changes in learning speed across blocks (main effect of block, F(5,570)= 6.22; P < 0.001), a significant difference in average learning speed between groups (F(4,114)=2.78; P = 0.03), but no block × group interaction effect (F(20,570)=0.83; P = 0.68). As expected from the results of the first phase, the average learning speed in the RcTBSCB group (30 ± 12.5%) was significantly higher than in the control group (learning speed = 21.9 ± 10.17, t(114)=4.16, P < 0.001) and the RiTBSCB group (learning speed = 17.7 ± 14.6%, t(114)=5.23, P < 0.001), but not between RiTBSCB and the control groups (t(114)= −2.16, P < 0.19). Again, the left cerebellar stimulation did not yield any significant effect in performance in the second learning phase (LcTBSCB, learning speed = 20.8 ± 14.7%, vs. control, t(114)= −0.5, P = 1; LiTBSCB, learning speed = 21.7 ± 6.42%, vs. control, t(114)= −0.07, P = 1; LcTBSCB vs. LiTBSCB, t(114)=0.34, P = 1).
Third phase
Finally, a 5 (block) by 5 (group) repeated measures ANOVA over the third phase of motor sequence learning showed significant changes in learning speed across blocks (main effect of block, F(4,456)= 6.41; P < 0.001) and differences in average learning speed between groups (F(4,114)=2.53; P = 0.044), but no block × group interaction (F(16,456)= 0.38; P = 0.98). The RcTBSCB group still showed an overall higher learning speed (34.4 ± 13.3%) than the control group (25.8 ± 11.13, t(114)=5.24, P < 0.001) and the RiTBSCB group (21.4 ± 16.3%, t(114)=6.54, P < 0.001). Importantly, in this third phase, performance of the RiTBSCB group in terms of average learning speed was now found to be significantly lower than that of the control group (t(114)= −2.68, P = 0.046). However, similar to the other two preceding phases, left cerebellar stimulation did not evoke any significant variation in learning speed during this phase (LcTBSCB = 25 ± 16.5%, vs. control, t(114)= −0.40, P = 1; LiTBSCB = 26 ± 8%, vs. control, t(114)=0.12, P = 1; LcTBSCB vs. LiTBSCB, t(114)=0.42, P = 1).
fMRI Results
Control group
Table 1 and Figure 2 present the clusters in which brain activity increased in proportion to performance improvement observed in the first, second, and third phases of motor sequence learning in the control group only (the single group effects for the other four groups can be found in Supporting Information Table 1).
Table 1.
Brain areas where responses increased in proportion to learning speed in the early, mid, and late phase of motor skill learning, for the control group
| Area | Peak MNI coordinates (x,y,z) | Maximum Z‐score | Cluster size | ||
|---|---|---|---|---|---|
| Early‐phase: | |||||
| Right hippocampus | 28 | −12 | −15 | 3.99 | 197 |
| Left hippocampus | −34 | −12 | −19 | 3.54 | 159 |
| Mid‐phase: | no significant clusters | ||||
| Late‐phase: | |||||
| Right precentral gyrus | 42 | −24 | 65 | 4.22 | 1,102 |
| Left precentral gyrus | −26 | −14 | 73 | 4.60 | 644 |
| Left pallidum | −24 | −8 | −1 | 3.20 | 226 |
| Left putamen | −22 | 4 | 15 | 2.97 | |
| Left supplementary motor area | −10 | −12 | 55 | 3.35 | 196 |
| Left cerebellum crus II | −38 | −70 | −43 | 3.43 | 164 |
| Left cerebellum lobules IV–V | −14 | −46 | −19 | 3.52 | 141 |
| Right supplementary motor area | 4 | 4 | 71 | 3.50 | 71 |
Only clusters that surpassed the minimum volume threshold ensuring a false discovery rate of 5% are reported (number of voxels: k = 64 for early phase, k = 67 for mid phase, k = 65 for late phase). Peak coordinates within each cluster are labeled according to the AAL atlas [Anatomical Automatic Labeling, Tzourio‐Mazoyer et al., 2002],
Figure 2.
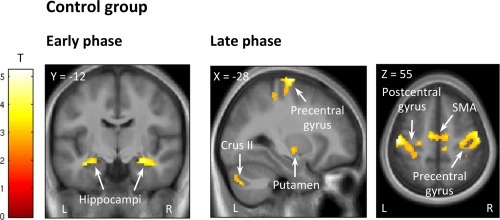
Functional imaging results of the nonstimulated control group. Cluster‐size Monte Carlo corrected maps (P < 0.05, minimum cluster size = 64 voxels) are displayed over the mean structural image of all subjects in the control group and show brain areas where responses increase in proportion to learning speed in the early phase (Blocks 1–3; left panel) and late phase (Blocks 10–14; right panel) of the motor sequence learning task. [Color figure can be viewed at http://wileyonlinelibrary.com]
As previously reported in the literature, the hippocampus, striatum, cerebellum, motor cortex were recruited during the learning of a new motor sequence over 14 blocks. In our study, however, we found that the hippocampus was already recruited in the very first blocks in control subjects.
Comparison between groups
Within the ANOVA including all five groups, a first F‐test explored any significant differences between any groups. Since this resulted in significant responses, two subsequent F‐tests were performed to evaluate the possible differences between the left cerebellar stimulation and the control groups, and between the right cerebellar stimulation and control groups. Whenever the latter F‐tests showed significant responses, follow‐up t‐tests were performed to specify the differential patterns of activations between pairs of groups. This procedure was used for the first, second, and third phases (Fig. 3).
Figure 3.
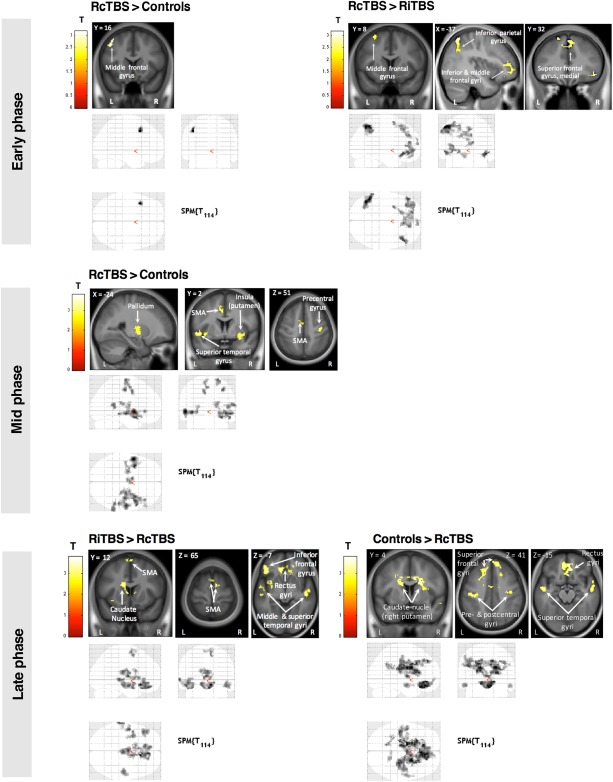
Functional imaging results of between‐group differences. Cluster‐size Monte Carlo corrected maps (P < 0.05, minimum cluster size = 66 voxels for early, 78 voxels for mid, and 80 voxels for late phase) are displayed over the mean structural image of all subjects and show brain areas where responses increase in proportion to learning speed in the early phase (Blocks 1–3), mid phase (Blocks 4–10), and late phase (Blocks 10–14) of the motor sequence learning task. [Color figure can be viewed at http://wileyonlinelibrary.com]
First phase of early motor sequence learning
This analysis identified brain regions where responses increased in proportion to performance speed in the first phase of motor sequence learning (i.e., the first three blocks). Significant main effects (F‐test) were observed in RcTBSCB and LiTBSCB groups in this phase (Supporting Information Table 1). Table 2 presents the different t‐tests performed to compare groups. Specifically, subjects undergoing RcTBSCB had higher activations in the left middle frontal gyrus than controls, and higher activations in the left inferior parietal gyrus, bilateral inferior, middle and superior frontal gyri, and anterior cingular cortex than subjects undergoing RiTBSCB.
Table 2.
Brain areas where responses increased in proportion to learning speed in one group compared to another, in the early phase of motor skill learning
| Area | Peak MNI coordinates (x,y,z) | Maximum Z‐score | Cluster size | ||
|---|---|---|---|---|---|
| RiTBSCB> Control: | no significant clusters | ||||
| RcTBSCB> Control: | |||||
| Left middle frontal gyrus | −40 | 16 | 45 | 3.10 | 66 |
| RcTBSCB > RiTBSCB: | |||||
| Left inferior parietal gyrus | −52 | −50 | 53 | 3.68 | 608 |
| Left inferior frontal gyrus, orbital | −52 | 40 | −5 | 3.44 | 335 |
| Left inferior frontal gyrus, triangular | −34 | 36 | 9 | 3.37 | |
| Left middle frontal gyrus, orbital | −34 | 50 | −1 | 3.29 | |
| Right superior frontal gyrus, medial | 6 | 30 | 47 | 3.26 | 321 |
| Left superior frontal gyrus, medial | −2 | 36 | 35 | 3.08 | |
| Left middle frontal gyrus | −30 | 8 | 63 | 3.21 | 154 |
| Left superior frontal gyrus | −18 | 24 | 49 | 3.15 | |
| Left rectus gyrus | −6 | 46 | −17 | 3.00 | 136 |
| Left medial frontal gyrus, orbital | −10 | 50 | −11 | 2.95 | |
| Right inferior frontal gyrus, orbital | 46 | 32 | −9 | 3.52 | 119 |
| Right anterior cingulum | 14 | 44 | 17 | 3.24 | 68 |
| Left anterior cingulum | −4 | 44 | 7 | 2.70 | |
| Right superior frontal gyrus, medial | 8 | 64 | 13 | 3.06 | 82 |
| Left superior frontal gyrus, medial | −4 | 60 | 27 | 2.65 | |
| RiTBSCB > RcTBSCB: | no significant clusters | ||||
| Control > RiTBSCB: | no significant clusters | ||||
| Control > RcTBSCB: | no significant clusters | ||||
Only clusters that surpassed the minimum volume threshold ensuring a false discovery rate of 5% are reported (number of voxels: k = 66). Peak coordinates within each cluster are labeled according to the AAL atlas.
Second phase of early motor sequence learning
This analysis identified brain regions where responses increased in proportion to performance speed in the second phase of motor sequence learning (i.e., Blocks 4–10). Significant main effects (F‐test) were observed in RcTBSCB and LiTBSCB groups in this phase (Supporting Information Table 1). Table 3 presents the different t‐tests performed to compare groups. Specifically, both RcTBSCB and RiTBSCB groups showed stronger activations than the control group. In particular, the RcTBSCB group exhibited stronger corticostriatal and frontal activity with respect to controls, while the RiTBSCB group exhibited stronger activity in the inferior occipital areas than controls. The LiTBSCB group also exhibited stronger activations than LcTBSCB and controls groups, but given that no significant behavioral differences were found, these results will not be discussed further.
Table 3.
Brain areas where responses increased in proportion to learning speed in one group compared to another, in the mid‐phase of motor skill learning
| Area | Peak MNI coordinates (x,y,z) | Maximum Z‐score | Cluster size | ||
|---|---|---|---|---|---|
| RcTBSCB > Control: | |||||
| Right superior temporal gyrus | 54 | −12 | −7 | 3.56 | 869 |
| Right insula | 44 | 10 | −5 | 3.49 | |
| Right superior temporal pole | 54 | 12 | −15 | 3.48 | |
| Left superior temporal gyrus | −52 | 4 | −3 | 3.76 | 633 |
| Left pallidum | −24 | −8 | −1 | 3.31 | |
| Left supplementary motor area | −8 | 2 | 45 | 3.33 | 172 |
| Right superior temporal gyrus | 50 | −40 | 11 | 3.33 | 169 |
| Right precentral gyrus | 38 | −16 | 51 | 3.47 | 89 |
| Right inferior frontal gyrus, triangular | 40 | 32 | −1 | 3.22 | 80 |
| Right precentral gyrus | 26 | −14 | 73 | 3.32 | 119 |
| RiTBSCB > Control: | |||||
| Right fusiform gyrus | 24 | −80 | −3 | 3.40 | 283 |
| Right inferior occipital gyrus | 34 | −76 | −7 | 3.30 | |
| Right calcarine sulcus | 26 | −72 | 7 | 3.25 | |
| RiTBSCB > RcTBSCB | no significant clusters | ||||
| LcTBSCB > Control | no significant clusters | ||||
| LcTBSCB > LiTBSCB: | |||||
| Right hippocampus | 36 | −32 | −5 | 3.75 | 78 |
| LiTBSCB > Control: | |||||
| Right medial frontal gyrus, orbital | 12 | 50 | −15 | 4.91 | 1,255 |
| Left rectus gyrus | −10 | 22 | −11 | 4.79 | |
| Left insula | −36 | 10 | −11 | 4.04 | 683 |
| Left middle temporal gyrus | −52 | −24 | −11 | 3.76 | |
| Left inferior frontal gyrus, orbital | −46 | 38 | −5 | 4.26 | 269 |
| Left inferior frontal gyrus, triangular | −54 | 34 | −1 | 3.82 | |
| Right inferior frontal gyrus, orbital | 46 | 36 | −7 | 3.37 | 179 |
| Right middle temporal gyrus | 62 | −22 | −7 | 3.31 | 125 |
| LiTBSCB > LcTBSCB: | |||||
| Right medial frontal gyrus, orbital | 10 | 52 | −15 | 4.50 | 1,329 |
| Right rectus gyrus | 8 | 30 | −19 | 4.16 | |
| Left superior temporal gyrus | −48 | −8 | −15 | 4.24 | 858 |
| Left insula | −34 | 12 | −13 | 3.95 | |
| Right middle temporal gyrus | 58 | −12 | −21 | 3.85 | 288 |
| Left superior frontal gyrus, medial | −6 | 50 | 47 | 3.68 | 163 |
| Left superior frontal gyrus | −12 | 44 | 53 | 3.66 | |
| Right inferior frontal gyrus, orbital | 48 | 36 | −7 | 4.03 | 108 |
| Control > RcTBSCB | no significant clusters | ||||
| Control > RiTBSCB | no significant clusters | ||||
| Control > LcTBSCB | no significant clusters | ||||
| Control > LiTBSCB | no significant clusters | ||||
Only clusters that surpassed the minimum volume threshold ensuring a false discovery rate of 5% are reported (number of voxels: k = 78). Peak coordinates within each cluster are labeled according to the AAL atlas.
Third phase of early motor sequence learning
This analysis identified brain regions where responses increased in proportion to performance speed in the third phase of motor sequence learning (i.e., Blocks 11–14). Significant main effects (F‐test) were observed in RiTBSCB, LiTBSCB, and LcTBSCB groups in this phase (Supporting Information Table 1). Table 4 presents the different t‐tests performed to compare groups. Specifically, no activation differences between the control group and the RiTBSCB group were observed. In this phase however, the RcTBSCB group showed bilateral lower brain activity than the control group in the caudate nuclei, superior temporal gyri, and middle cingular cortex, and lower brain activity than the RiTBSCB group in the rectus gyri, temporal lobes, and supplementary motor areas.
Table 4.
Brain areas where responses increased in proportion to learning speed in one group compared to another, in the late‐phase of motor skill learning
| Area | Peak MNI coordinates (x,y,z) | Maximum Z‐score | Cluster size | ||
|---|---|---|---|---|---|
| RcTBSCB> Control: | no significant clusters | ||||
| RcTBSCB> RiTBSCB: | no significant clusters | ||||
| RiTBSCB> Control: | no significant clusters | ||||
| RiTBSCB> RcTBSCB: | |||||
| Right rectus gyrus | 6 | 42 | −17 | 3.37 | 468 |
| Right middle temporal gyrus | 54 | −14 | −17 | 2.97 | 280 |
| Right superior temporal gyrus | 50 | −22 | −3 | 2.90 | |
| Left inferior frontal gyrus, orbital | −44 | 30 | −7 | 3.20 | 278 |
| Left inferior frontal gyrus, triangular | −40 | 30 | 1 | 3.00 | |
| Left superior temporal pole | −44 | 6 | −19 | 3.10 | 179 |
| Left caudate nucleus | −6 | 18 | 5 | 3.18 | 120 |
| Left middle temporal gyrus | −66 | −28 | 1 | 3.27 | 113 |
| Left superior temporal gyrus | −50 | −4 | −13 | 3.27 | 101 |
| Right supplementary motor area | 10 | 2 | 65 | 3.14 | 101 |
| Left supplementary motor area | −6 | 12 | 67 | 2.83 | |
| Control > RiTBSCB | no significant clusters | ||||
| Control > RcTBSCB | |||||
| Left caudate nucleus | −16 | −4 | 25 | 3.54 | 2,017 |
| Right caudate nucleus | 20 | 4 | 25 | 3.53 | |
| Right rectus gyrus | 8 | 38 | −15 | 3.47 | 826 |
| Left postcentral gyrus | −44 | −20 | 37 | 3.14 | 289 |
| Right superior frontal gyrus | 14 | 42 | 41 | 3.37 | 156 |
| Right superior frontal gyrus, medial | 4 | 50 | 41 | 2.74 | |
| Right superior temporal gyrus | 54 | −10 | −15 | 3.01 | 114 |
| Right middle cingulum | 6 | −44 | 31 | 2.82 | 100 |
| Left precuneus gyrus | −14 | −56 | 33 | 3.17 | 96 |
| Left superior temporal gyrus | −50 | −6 | −13 | 3.17 | 92 |
| Left middle temporal gyrus | −48 | −14 | −11 | ||
Only clusters that surpassed the minimum volume threshold ensuring a false discovery rate of 5% are reported (number of voxels: k = 80). Peak coordinates within each cluster are labeled according to the AAL atlas.
DISCUSSION
In this study, we found that inhibition of the right cerebellar cortex enhanced the speed of acquisition of a new motor sequence performed with the left hand at the very beginning of the motor sequence learning, when compared to the control group. By contrast, excitation of the right cerebellar cortex lowered the speed of acquisition of a new motor sequence, especially in the third phase of the early motor sequence learning. Interestingly, only the right, and not the left cerebellar stimulation influenced the learning speed, despite the fact that the task was performed with the left hand in all instances, that is, predominantly with the right motor cortex, which receives projections from the left cerebellum. This means that if stimulation of the cerebellum would have acted purely on the motor component of the sequence execution [Seidler et al., 2002], then the left but not the right cerebellar stimulation should have had the biggest impact on performance. But this was not the case. It suggests that, in our experimental setup, the cerebellar stimulation interfered only with those circuits responsible for sequence acquisition and not motor execution per se. The finding is consistent with previous work in our laboratory, which has shown that these circuits may be distinct, and only partially overlapping [Orban et al., 2010].
Previous studies using both inhibitory and excitatory stimulation of the cerebellar hemisphere reported impaired motor execution during nonrepetitive, adaptation tasks [Cantarero et al., 2015; Galea et al., 2011; Herzfeld et al., 2014; Jayaram et al., 2011; Li Voti et al., 2014; Miall and Christensen, 2004; Miall et al., 2007]. This is not in contradiction with our own pattern of results, since different circuits might carry out the sensorimotor adaptation (in which online error monitoring is important) and the explicit sequence learning (in which extraction of the pattern is key). In fact, in our experiment, the error rate was close to zero and similar between groups, suggesting that the cerebellar stimulation did influence a more subtle mechanism of the sequence learning than just motor adjustment through correction of motor errors [Imamizu et al., 2000; van Mier et al., 2004]. The present results are also in line with previous findings that parts of the right cerebellum might be specifically involved in the left‐hand sequence learning process per se [Orban et al., 2010].
The analysis of the behavioral data in the control group revealed two major inflexion points in the learning curve: one after the third block and one after the 10th block. Particularly, the performance drastically increased during the first three blocks, only to turn to a much slower increase in Blocks 4 through 10, before reaching an asymptotic level in the last four blocks. Most of the motor learning studies have investigated the acquisition and consolidation of a new sequence over large periods of time ranging from hours to days and even months [Doyon and Benali, 2005; Lehéricy et al., 2005; Lungu et al., 2014; for a review see Hardwick et al., 2013]. The 14 blocks used in our study can be considered as part of the initial motor sequence learning. Thus, to our knowledge, this is the first study to take a closer look at the different phases within this initial stage of motor sequence learning. Moreover, in the control group, the learning speed positively correlated with the BOLD signal in the two hippocampi in the first phase, only to shift to the precentral gyri bilaterally, left postcentral gyrus, left pallidus, left putamen, bilateral SMA, and left cerebellum in the third phase. These results are in line with our previous work showing an antagonistic pattern of activation between hippocampo‐ and striato‐cortical networks in which the hippocampus is recruited early on during training, whereas activity in the striatum increases as a function of practice [see Albouy et al., 2013a for a review]. This suggests that complex plastic phenomena occur within the first 14 blocks of acquisition of an explicit motor task that may condition the way in which the task is subsequently internalized and consolidated. Our results show that the hippocampus is significantly recruited already in the first three blocks (corresponding on average to the first 2 min of task performance, just before an abrupt slowing of the performance), which suggests that it might be responsible for the monitoring and labeling of a repeated movement sequence as a potentially meaningful program to be transferred to other structures for storage later on [Albouy et al., 2013a; Davachi and DuBrow, 2015; Gheysen et al., 2010; Lohse et al., 2014]. It was also proposed that the hippocampus might be in competition with the striatum [Albouy et al., 2008, 2015; Lungu et al., 2014] and that both the early recruitment of this structure, as well as the strength of its competitive interaction with the striatum influence subsequent motor memory consolidation [Albouy et al., 2008, 2013a; Steele and Penhune, 2010]. Indeed, significant BOLD signal associated with the error‐free repetition of the same motor sequence moved to the cerebello‐cortical and striato‐cortical circuits toward the end of the practice period, in the third phase. This transfer is consistent with the fact that the striato‐cortical circuit is involved in acquiring and maintaining the new motor skill [Lehéricy et al., 2005; Ma et al., 2010; Penhune and Doyon, 2002, 2005]. This relatively rapid transfer from the hippocampus toward the other cortico‐subcortical loops opens the possibility of modulating their interaction by interfering with one or more nodes of the network—which we did by artificially modulating the cerebellar output.
First Phase of Early Motor Sequence Learning
The improvement of performance speed was significantly higher after inhibitory stimulation of the right cerebellar cortex in the first phase. When comparing the fMRI BOLD contrasts between groups, this translated into a higher activation in the left middle frontal gyrus with respect to controls, and to a higher activation in the mainly left superior, middle and inferior frontal gyri, inferior parietal, and anterior cingulum with respect to the group that underwent right cerebellar excitation. In the light of previous literature, it is plausible that right cerebellar inhibition reduced the inhibitory control of the Purkinje cells on the cerebellar nuclei, and hence disinhibited the glutamatergic projections toward the thalamic relays. Since the cerebellum projects massively through the thalamus toward the frontal areas [see Middleton and Strick, 2000 for review], this could have enhanced thalamic output toward areas sustaining the learning of a sequence and boosted their activation.
The middle frontal areas that appear more activated after right cTBSCB in our study are the same areas that have been described to be associated with verbal fluency and sentence processing [Homae et al., 2003; McAvoy et al., 2016; Tate et al., 2014] as well as constructive episodic memory [Addis et al., 2009]. Indeed, during the post‐scan debriefing, the subjects reported almost always utilizing a kind of internal‐speech for guiding and remembering the sequence, especially when starting to practice (singing, counting, internal‐speaking, etc.). These brain responses could thus be interpreted as a more efficient use of the sequencing circuits shared with speech production, at least during the first phase of initial motor sequence learning. This model is in line with a recently formulated perspective on the evolution of human language, which advocates for a “high‐jacking” of more basic motor circuits by complex processes under the pressure of evolution [Corballis, 2015]. In support of this hypothesis comes the observation that the circuits involved in spoken and in sign languages are strikingly similar, despite the evident differences in both input and output [Horwitz et al., 2003; Sandler and Lillo‐Martin, 2006; van der Lely and Pinker, 2014]. Also, mentally imagining the repetition of the motor sequence or verbally repeating the sequence seems to have similar positive effects on motor execution [Hétu et al., 2013; Orban et al., 2008; Saimpont et al., 2013]. From this perspective, the processes of learning a motor sequence with the fingers or a verbal sequence would share the same circuits for internalizing and retaining the sequence. It is, therefore, reasonable to consider that at least some components of the motor sequence learning would use the same lateralization as the production of speech [Arasanz et al., 2012; Hardwick et al., 2013; Murdoch, 2010]. Since the cerebello‐cortical connections are strongly lateralized [McAvoy et al., 2016; Simonyan and Fuertinger, 2015; Wang et al., 2013], it is possible that those same components associated with speech production and shared with the acquisition of a motor sequence would have an equally lateralized representation in the cerebellum [Kuper et al., 2011]. Indeed, we found significant alterations of the learning speed only after the right and not left cerebellar stimulation, which suggests that the learning of an explicit motor sequence depends on a dedicated circuit, rather than on pure ipsilateral motor monitoring from cerebellar lobules IV–VI. Moreover, the right inferior posterior cerebellar lobules VIIb and VIII, which were our target for TBS, show task‐related activity when items are maintained in mind over a delay and coactivate with inferior parietal regions implicated in storage‐related processing [Chen and Desmond, 2005]. These findings lead to the assumption that the inferior‐lateral cerebellum contributes to sequence storage.
Second Phase of Early Motor Sequence Learning
The improvement of speed performance underwent a sudden slowing down during the fourth block in all groups, with or without cerebellar stimulation. It can be speculated that around this “bending point” the neuronal circuits executing and monitoring the repetitions deem the repeated action as worthy of retention, and other, more complex monitoring mechanisms take over. The number of repetitions itself might be the trigger for change: the monitoring of a repeated action switches to retention beyond a given threshold. Despite the significant differences in performance with respect to the other two phases, fMRI did not detect any significant clusters in controls in the second phase, probably due to the unstable and highly volatile nature of the transition during which strategies are rapidly switched to select the optimal one [Adi‐Japha et al., 2008; Albouy et al., 2012]. There were, however, significant differences in activation between different stimulation groups and controls. In particular, the RcTBSCB group exhibited stronger corticostriatal and frontal activity—probably a cascading effect of the boosted activity in areas responsible for monitoring the repeated pattern in the first phase.
It is worth noting that the higher performance level reached by the RcTBSCB group before the first bending point was maintained throughout the remaining blocks, but the further increase of the speed performance did not differ with respect to the other groups as no significant interaction was found between block and group.
Third Phase of Early Motor Sequence Learning
A second “bending point” of the performance curve seemed to emerge around the 10th block. From this point on, the performance of the RiTBSCB group became significantly lower not only with respect to the RcTBSCB group, but also to controls. This marks a further significant slowing in acquisition of the repeated sequence. Following the same reasoning as for the transition between the first and second phase of motor sequence learning, each new “bending point” might represent a significant change in the way the neuronal circuits process a repeated action. The fact that RiTBSCB induces this tardive slowing of performance suggests that the stimulation before the motor learning task interfered with and conditioned the natural encoding of the motor information in a way that becomes evident at later stages of learning. Since RiTBSCB is able to prevent associative changes in the motor cortex by gating the proprioceptive information [Kishore et al., 2014a], it is possible that the repetition of the motor sequence after RiTBSCB lacked part of the proprioceptive information necessary for a proper retention. In addition, RiTBSCB can induce diminished output from the dentate nucleus, and subsequently from its thalamic relay neurons toward the striatum [Bostan et al., 2010; Hoshi et al., 2005; Ichinohe et al., 2000; Pelzer et al., 2013]. This diminished drive would hinder an efficient transmission of the refined motor sequence from the anterior, “associative” striatum to the posterior, “sensorimotor” striatum [Lehéricy et al., 2005] in a timely and efficient manner [Chen et al., 2014; Rossi et al., 2008]. This would require more resources for sustaining the repetition of the motor sequence in the third phase after RiTBSCB than after RcTBSCB, or, in other words, more effort from the anterior striatum is needed to “push through” a less efficient program. Interestingly, the RcTBSCB group seemed to need even less resources than the controls bilaterally in the caudate, superior prefrontal and superior temporal areas in the third phase. This suggests that the increased information flow allowed in the first phase by the inhibition of the cerebellar cortex is also benefiting the later stages of motor sequence acquisition.
Functional Interplay between Cerebello‐Cortical and Striato‐Cortical Systems
The early boost in the motor sequence acquisition speed for the RcTBSCB group led to a higher performance at the first inflexion point of the learning pattern. The significantly higher learning speed was subsequently maintained throughout the rest of the task. The BOLD contrasts between the right cerebellar inhibition group on the one hand, and the control or right cerebellar excitation group on the other hand revealed significant activations in striatal regions in the second and third phase of motor sequence learning. While in the first blocks, the right cerebellar inhibition group showed increased left frontal activity when compared to the controls, in the last blocks, it showed bilateral lower activity than controls over extensive territories, ranging from the caudate nuclei to the frontal and temporal lobes. Could this be explained by the lower effort needed to maintain the execution speed of the learned sequence after the initial boost? Additional experiments with more challenging sequences/paradigms will be needed to elucidate this point.
The fact that cerebellar stimulation entrained complex shifts in cortical and subcortical activations underlying changes in motor performance supports the tight functional link between these structures. The cerebellum is only apparently in opposition with the rest of the brain due to the inhibitory projection from its cortex onto its nuclei [Ito, 2006]. It actually plays a pivotal role in balancing the interaction between the basal ganglia and the cortical areas responsible for processing motor sequences. One further argument in favor of this point is the mismatch between the time points at which the effects of each stimulation become clear. If the stimulated cerebellar areas were directly involved just in the motor control, the behavioral outcome and supporting brain activity would have diverged at the same delay postintervention. But they did not, which suggests a complex phenomenon in which the cerebellum controls nonlinear interactions between other structures involved in acquisition and storing of motor sequences.
It can be argued that one confounding factor in our study could be the use of the nondominant hand. Left‐hand movements in right‐handed subjects are typically characterized by more bilateral activation patterns than right‐hand movements due to the known specialization of the left hemisphere in motor control [Serrien et al., 2006]. This might reduce or mask some expected asymmetries in cortical activation, hence possibly limiting the interpretation of our results to this effector only. At the same time, the motor cortices controlling each hand (and the associated learning strategies) might use intrinsically different models. A clear answer to this question would require performing the same set of recordings on right‐handed subjects performing the task with their right hand.
CONCLUSION
In conclusion, our results support the view that functions lateralized in the brain also have lateralized counterparts in the cerebellum. We demonstrate that certain types of motor learning (like explicit motor sequence learning) can be bidirectionally modulated with noninvasive brain stimulation by changing the weight of the cerebellar control over the rest of the neural network: diminishing the output of the right inferior cerebellar cortex facilitates the rapid execution and identification of the motor sequence to be retained by the cortical structures; enhancing the output of the right inferior cerebellar cortex interferes with the transfer of the newly acquired motor sequence within the striatal and cortical circuits. Combining this kind of cerebellar modulation with the previously suggested incremental strategy of motor sequence learning [Lungu et al., 2014] might enhance the efficiency of clinical rehabilitation protocols.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank Ovidiu Lungu, Philippe Saucier, and Kristina Aurousseau for their assistance with the MRI scanning, and also Arnaud Boré and Francine Bélanger for their invaluable assistance in running this project. The Franco‐Canadian co‐operation for this study was possible through the International Laboratory for Neuroimaging and Modeling/Laboratoire International de Neuroimagerie et Modélisation (LINeM)—a collaborative initiative between the University of Montréal and the Institut National de la Santé et de la Recherche Médicale (Inserm).
Freja Gheysen and Gabriel Lasne contributed equally to this work
REFERENCES
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL (2009): Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia 47:2222–2238. [DOI] [PubMed] [Google Scholar]
- Adi‐Japha E, Karni A, Parnes A, Loewenschuss I, Vakil E (2008): A shift in task routines during the learning of a motor skill: Group‐averaged data may mask critical phases in the individuals' acquisition of skilled performance. J Exp Psychol Learn Mem Cogn 34:1544–1551. [DOI] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang‐Vu T, Darsaud A, Ruby P, Luppi P, Degueldre C, Peigneux P, Luxen A, Maquet P (2008): Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron 58:261–272. [DOI] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Vandewalle G, Darsaud A, Gais S, Rauchs G, Desseilles M, Boly M, Dang‐Vu T, Balteau E, Degueldre C, Phillips C, Luxen A, Maquet P (2012): Neural correlates of performance variability during motor sequence acquisition. Neuroimage 60:324–331. [DOI] [PubMed] [Google Scholar]
- Albouy G, King BR, Maquet P, Doyon J (2013a): Hippocampus and striatum: Dynamics and interaction during acquisition and sleep‐related motor sequence memory consolidation. Hippocampus 23:985–1004. [DOI] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Vandewalle G, Darsaud A, Gais S, Rauchs G, Desseilles M, Boly M, Dang‐Vu T, Balteau E, Degueldre C, Phillips C, Luxen A, Maquet P (2013b): Interaction between hippocampal and striatal systems predicts subsequent consolidation of motor sequence memory. PLoS One 8:e59490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Fogel S, King BR, Laventure S, Benali H, Karni A, Carrier J, Robertson EM, Doyon J (2015): Maintaining vs. enhancing motor sequence memories: Respective roles of striatal and hippocampal systems. Neuroimage 108:423–434. [DOI] [PubMed] [Google Scholar]
- Arasanz CP, Staines WR, Roy EA, Schweizer TA (2012): The cerebellum and its role in word generation: A cTBS study. Cortex 48:718–724. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL (2010): The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero G, Spampinato D, Reis J, Ajagbe L, Thompson T, Kulkarni K, Celnik P (2015): Cerebellar direct current stimulation enhances on‐line motor skill acquisition through an effect on accuracy. J Neurosci 35:3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Sagi D, Cohen LG (2012): Common mechanisms of human perceptual and motor learning. Nat Rev Neurosci 3:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fremont R, Arteaga‐Bracho EE, Khodakhah K (2014): Short latency cerebellar modulation of the basal ganglia. Nat Neurosci 17:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Desmond JE (2005): Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage 24:332–338. [DOI] [PubMed] [Google Scholar]
- Corballis M (2015): What's left in language? Beyond the classical model. Ann N Y Acad Sci 1359:14–29. [DOI] [PubMed] [Google Scholar]
- Coynel D, Marrelec G, Perlbarg V, Pélégrini‐Issac M, de Moortele PF, Ugurbil K, Doyon J, Benali H, Lehéricy S (2009): Dynamics of motor‐related functional integration during motor sequence learning. NeuroImage 49:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, DuBrow S (2015): How the hippocampus preserves order: The role of prediction and context. Trends Cogn Sci 19:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Benali H (2005): Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15:161–167. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG (2002): Experience‐dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99:1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG (2003): Distinct contribution of the cortico‐striatal and cortico‐cerebellar systems to motor skill learning. Neuropsychologia 41:252–262. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H (2009): Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 199:61–75. [DOI] [PubMed] [Google Scholar]
- Floyer‐Lea A, Matthews PM (2005): Distinguishable brain activation networks for short‐ and long‐term motor skill learning. J Neurophysiol 94:512–518. [DOI] [PubMed] [Google Scholar]
- Fuster JM ( 1999): Memory in the Cerebral Cortex: An Empirical Approach to Neural Networks in the Human and Nonhuman Primate. Cambridge: MIT Press. [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry J‐JOJ, Celnik P (2011): Dissociating the roles of the cerebellum and motor cortex during adaptive learning: The motor cortex retains what the cerebellum learns. Cereb Cortex 21:1761–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen F, Gevers W, De Schutter E, Van Waelvelde H, Fias W (2009): Disentangling perceptual from motor implicit sequence learning with a serial color‐matching task. Exp Brain Res 197:163–174. [DOI] [PubMed] [Google Scholar]
- Gheysen F, Van Opstal F, Roggeman C, Van Waelvelde H, Fias W (2010): Hippocampal contribution to early and later stages of implicit motor sequence learning. Exp Brain Res 202:795–807. [DOI] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB (2013): A quantitative meta‐analysis and review of motor learning in the human brain. Neuroimage 67:283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld DJ, Pastor D, Haith AM, Rossetti Y, Shadmehr R, O'Shea J (2014): Contributions of the cerebellum and the motor cortex to acquisition and retention of motor memories. Neuroimage 98:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hétu S, Grégoire M, Saimpont A, Coll M‐PP, Eugène F, Michon P‐EE, Jackson PL (2013): The neural network of motor imagery: An ALE meta‐analysis. Neurosci Biobehav Rev 37:930–949. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H (2002): Central mechanisms of motor skill learning. Curr Opin Neurobiol 12:217–222. [DOI] [PubMed] [Google Scholar]
- Homae F, Yahata N, Sakai K (2003): Selective enhancement of functional connectivity in the left prefrontal cortex during sentence processing. Neuroimage 20:578–586. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Amunts K, Bhattacharyya R, Patkin D, Jeffries K, Zilles K, Braun AR (2003): Activation of Broca's area during the production of spoken and signed language: A combined cytoarchitectonic mapping and PET analysis. Neuropsychologia 41:1868–1876. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL (2005): The cerebellum communicates with the basal ganglia. Nat Neurosci 8:1491–1493. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC (2005): Theta burst stimulation of the human motor cortex. Neuron 45:201–206. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Mori F, Shoumura K (2000): A di‐synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res 880:191–197. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Pütz B, Yoshioka T, Kawato M (2000): Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403:192–195. [DOI] [PubMed] [Google Scholar]
- Ito M (2006): Cerebellar circuitry as a neuronal machine. Prog Neurobiol 78:272–303. [DOI] [PubMed] [Google Scholar]
- Jayaram G, Galea JM, Bastian AJ, Celnik P (2011): Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex 21:1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE (1994): Motor sequence learning: A study with positron emission tomography. J Neurosci 14:3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueptner M, Weiller C (1998): A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain 121:1437–1449. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey‐Hipolito C (1998): The acquisition of skilled motor performance: Fast and slow experience‐driven changes in primary motor cortex. Proc Natl Acad Sci USA 95:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore A, Popa T, Balachandran A, Chandran S, Pradeep S, Backer F, Krishnan S, Meunier S (2014a): Cerebellar sensory processing alterations impact motor cortical plasticity in parkinson's disease: Clues from dyskinetic patients. Cereb Cortex 8:2055–2067. [DOI] [PubMed] [Google Scholar]
- Kishore A, Popa T, James P, Yahia‐Cherif L, Backer F, Varughese Chacko L, Govind P, Pradeep S, Meunier S (2014b): Age‐related decline in the responsiveness of motor cortex to plastic forces reverses with levodopa or cerebellar stimulation. Neurobiol Aging 35:2541–2551. [DOI] [PubMed] [Google Scholar]
- Kuper M, Dimitrova A, Thurling M, Maderwald S, Roths J, Elles HG, Gizewski ER, Ladd ME, Diedrichsen J, Timmann D (2011): Evidence for a motor and a non‐motor domain in the human dentate nucleus—An fMRI study. Neuroimage 54:2612–2622. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele PF, Pélégrini‐Issac M, Waechter T, Ugurbil K, Doyon J (2005): Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA 102:12566–12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Voti P, Conte A, Rocchi L, Bologna M, Khan N, Leodori G, Berardelli A (2014): Cerebellar continuous theta‐burst stimulation affects motor learning of voluntary arm movements in humans. Eur J Neurosci 39:124–131. [DOI] [PubMed] [Google Scholar]
- Lohse KR, Wadden KK, Boyd LA, Hodges NJ (2014): Motor skill acquisition across short and long time scales: A meta‐analysis of neuroimaging data. Neuropsychologia 59:130–141. [DOI] [PubMed] [Google Scholar]
- Lorch RF, Myers JL (1990): Regression analyses of repeated measures data in cognitive research. J Exp Psychol Learn Mem Cogn 16:149–157. [DOI] [PubMed] [Google Scholar]
- Lungu O, Monchi O, Albouy G, Jubault T, Ballarin E, Burnod Y, Doyon J (2014): Striatal and hippocampal involvement in motor sequence chunking depends on the learning strategy. PLoS One 9:e103885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wang B, Narayana S, Hazeltine E, Chen X, Robin DA, Fox PT, Xiong J (2010): Changes in regional activity are accompanied with changes in inter‐regional connectivity during 4 weeks motor learning. Brain Res 1318:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy M, Mitra A, Coalson RS, d'Avossa G, Keidel JL, Petersen SE, Raichle ME (2016): Unmasking language lateralization in human brain intrinsic activity. Cereb Cortex 26:1733–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Christensen LO (2004): The effect of rTMS over the cerebellum in normal human volunteers on peg‐board movement performance. Neurosci Lett 371:185–189. [DOI] [PubMed] [Google Scholar]
- Miall RC, Christensen L, Cain O, Stanley J (2007): Disruption of state estimation in the human lateral cerebellum. PLoS Biol 5:e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick P (2000): Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Rev 31:236–250. [DOI] [PubMed] [Google Scholar]
- Murdoch BE (2010): The cerebellum and language: Historical perspective and review. Cortex 46:858–868. [DOI] [PubMed] [Google Scholar]
- Orban P, Lungu O, Doyon J (2008): Motor sequence learning and developmental dyslexia. Ann N Y Acad Sci 1145:151–172. [DOI] [PubMed] [Google Scholar]
- Orban P, Peigneux P, Lungu O, Albouy G, Breton E, Laberenne F, Benali H, Maquet P, Doyon J (2010): The multifaceted nature of the relationship between performance and brain activity in motor sequence learning. NeuroImage 49:694–702. [DOI] [PubMed] [Google Scholar]
- Pelzer E, Hintzen A, Goldau M, von Cramon D, Timmermann L, Tittgemeyer M (2013): Cerebellar networks with basal ganglia: Feasibility for tracking cerebello‐pallidal and subthalamo‐cerebellar projections in the human brain. Eur J Neurosci 38:3106–3114. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Doyon J (2002): Dynamic cortical and subcortical networks in learning and delayed recall of timed motor sequences. J Neurosci 22:1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB, Doyon J (2005): Cerebellum and M1 interaction during early learning of timed motor sequences. Neuroimage 26:801–812. [DOI] [PubMed] [Google Scholar]
- Popa T, Russo M, Meunier S (2010): Long‐lasting inhibition of cerebellar output. Brain Stimul 3:161–169. [DOI] [PubMed] [Google Scholar]
- Popa T, Velayudhan B, Hubsch C, Pradeep S, Roze E, Vidailhet M, Meunier S, Kishore A (2013): Cerebellar processing of sensory inputs primes motor cortex plasticity. Cereb Cortex 23:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Mataluni G, De Bartolo P, Prosperetti C, Foti F, De Chiara V, Musella A, Mandolesi L, Bernardi G, Centonze D, Petrosini L (2008): Cerebellar control of cortico‐striatal LTD. Restor Neurol Neurosci 26:475–480. [PubMed] [Google Scholar]
- Saimpont A, Lafleur MF, Malouin F, Richards CL, Doyon J, Jackson PL (2013): The comparison between motor imagery and verbal rehearsal on the learning of sequential movements. Front Hum Neurosci 7:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler W, Lillo‐Martin D (2006): Sign Language and Linguistic Universals. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Seidler RD, Purushotham A, Kim SG, Uğurbil K, Willingham D, Ashe J (2002): Cerebellum Activation Associated with Performance Change but Not Motor Learning. Science 296:2043–2046. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Ivry RB, Swinnen SP (2006): Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci 7:160–166. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Fuertinger S (2015): Speech networks at rest and in action: Interactions between functional brain networks controlling speech production. J Neurophysiol 113:2967–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C, Penhune V (2010): Specific increases within global decreases: A functional magnetic resonance imaging investigation of five days of motor sequence learning. J Neurosci 30:8332–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate MC, Herbet G, Moritz‐Gasser S, Tate JE, Duffau H (2014): Probabilistic map of critical functional regions of the human cerebral cortex: Broca's area revisited. Brain 137:2773–2782. [DOI] [PubMed] [Google Scholar]
- Toni I, Krams M, Turner R, Passingham RE (1998): The time course of changes during motor sequence learning: A whole‐brain fMRI study. Neuroimage 8:50–61. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I (1995): Magnetic stimulation over the cerebellum in humans. Ann Neurol 37:703–713. [DOI] [PubMed] [Google Scholar]
- Van der Lely HK, Pinker S (2014): The biological basis of language: Insight from developmental grammatical impairments. Trends Cogn Sci 18:586–595. [DOI] [PubMed] [Google Scholar]
- Van Mier HI, Perlmutter JS, Petersen SE (2004): Functional changes in brain activity during acquisition and practice of movement sequences. Motor Control 8:500–520. [DOI] [PubMed] [Google Scholar]
- Wang D, Buckner RL, Liu H (2013): Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J Neurophysiol 109:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


