Abstract
Objectives
Traumatic memories such as intrusions and flashbacks play a major role in the development and maintenance of post‐traumatic stress disorder (PTSD). A thorough understanding of the neural mechanisms underlying traumatic memories is indispensable for precise diagnosis, for personalized treatment and prevention. In particular, the identification of early neural predictor variables for intrusion development shortly after trauma exposure requires detailed investigation. Experimental design: Here, we examined the neural correlates of early experimental trauma memory retrieval in a traumatic film paradigm in 42 young healthy females, using both implicit and explicit retrieval tasks. Principal observations: We show that implicit experimental trauma retrieval specifically involved the retrosplenial cortex and the anterior cingulate cortex (ACC), while both retrieval tasks resulted in trauma‐related activity in the posterior cingulate cortex (PCC) and the precuneus. Importantly, neural activity early after experimental trauma exposure predicted later intrusion development, with independent contributions from activity in the retrosplenial cortex (implicit retrieval) and the PCC (explicit retrieval). Additional analyses revealed a stronger connectivity between the bilateral amygdala and the supplementary motor area, precentral and paracentral lobule for the control group compared to the experimental trauma group. Conclusions: Our study gives new insights in the neural correlates of experimental trauma memory retrieval and their predictive value for subsequent symptom development. Our results could provide the basis for personalized early treatment and prevention of PTSD. Hum Brain Mapp, 2017. © 2017 Wiley Periodicals, Inc. Hum Brain Mapp 38:3592–3602, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: trauma, retrieval, analogue study, intrusive memories, fMRI
INTRODUCTION
Traumatic memories are a crucial symptom of post‐traumatic stress disorder (PTSD) and play a major role in the development and maintenance of PTSD [APA, 2013; Elbert and Schauer, 2002; Michael et al., 2005]. Traumatic memories represent a major distress to patients as they are reactivated involuntarily after exposure to internal or external cues. They are rendered highly intrusive and disturbing [APA, 2013]. Theoretical approaches describe PTSD as a memory disorder. For example, Brewin et al. [1996] propose in their dual representation model that traumatic memories are associated with a situationally accessible memory system (SAM). The SAM system contains lower level perceptual processing and associated emotions, which are easily reactivated by memory cues and lead to intrusions, flashbacks and nightmares. In contrast, the verbally accessible memory (VAM) is a more complex system which enables voluntary verbal recall and interacts with autobiographical memories to achieve an integration of the trauma into personal context [Brewin, 2014; Brewin et al., 1996]. Along similar lines, Ehlers and Clark [2000] assume that trauma memories are inadequately integrated into context and other autobiographical memories. The authors propose that a traumatic memory is processed as if an individual would still be under current threat [Ehlers and Clark, 2000]. This leads to a strong implicit memory bias (perceptual priming) for stimuli associated with the trauma and a reduced threshold for trauma reactivation by traumatic memory cues. Also Elbert and Schauer [2002] hypothesize that the isolation and lack of integration of traumatic memories might be a major cause for the intrusive and involuntary nature of traumatic memories. They assume that autobiographical memories can be divided into cold (fact knowledge of life events) and hot (emotional and sensory) memories, which are typically interconnected for “healthy,” nontraumatic memories [Elbert and Schauer, 2002]. However, exposure to a traumatic event can induce unusually high arousal responses associated with strong physiological and neurophysiological responses such as stress hormone releases and activation of brain structures involved in emotional processing [DeQuervain et al., 2017; LeDoux, 2003; Ozer et al., 2003]. For example, exaggerated amygdala response during encoding of negative stimuli has been associated with increased hippocampal activity and PTSD symptom severity [Brohawn et al., 2010]. This “hyperactivity” during encoding of a traumatic event might lead to an “overconsolidation” of traumatic memories [Pitman, 1989]. During this process, memory‐related brain areas such as the amygdala and hippocampus can lose their interconnectivity [Elbert and Schauer, 2002], creating an isolated fear network of strong, integrated hot memories.
In sum, several theoretical accounts describe PTSD as a memory disorder [Brewin, 2014], mainly associated with a reduced integration of traumatic memories in the autobiographical memory network. PTSD patients typically exhibit an enhanced recall of trauma‐related stimuli, an altered retrieval of autobiographical memories in response to cue words and also show an enhanced implicit memory in complex tasks. [McNally, 1997]. Despite their intense vividness, traumatic memories have the capacity of changing their structure [McNally, 2005] They are rarely completely erased from a patient´s memory, but rather suppressed [McNally, 2005]. As memory processes are assumed to play a major role for PTSD, a thorough understanding of the neural mechanisms underlying the development of traumatic memories is indispensable and not yet achieved.
Previous research focusing on traumatic memory retrieval was mainly conducted in patients with full psychiatric manifestations of PTSD. It revealed altered neural correlates during the presentation of trauma‐related stimuli and trauma memory in a variety of brain regions. Most prominent are brain activations in the amygdala, ventromedial prefrontal cortex, anterior cingulate cortex (ACC) and insular cortex during trauma‐related stimuli [Pitman et al., 2012]. A recent meta‐analysis of trauma memory in PTSD revealed an increased activation in the retrosplenial cortex, precuneus, ACC and amygdala [Sartory et al., 2013]. Moreover, patients who implicitly processed trauma stimuli exhibited an increased activation in the amygdala, ventrolateral prefrontal cortex and fusiform gyrus [Morey et al., 2009] in a working memory task with trauma‐related distractors. In contrast, patients who explicitly focused on a trauma during a script‐driven imagery task exhibited increased brain activation in thalamus, ACC and medial frontal gyrus and enhanced connectivity in the posterior cingulate cortex (PCC), parietal lobe and occipital lobe, specifically in the right hemisphere [Lanius et al., 2001, 2004]. A recent study that directly compared conscious and subconscious processing of trauma reported that, while subconscious trauma processing resulted in an increased activity in the posterior cingulum, conscious trauma processing was correlated with an increased activity in the basal forebrain [Rabellino et al., 2016].
Results indicate the necessity of differentiating between different memory types in PTSD. Moreover, as the distress of traumatic memories is also due to involuntary intrusiveness, differences in neural correlates of implicit versus explicit retrieval attempts might also be functionally relevant for development and maintenance of the disorder.
The theories on trauma memory in PTSD mentioned before [Brewin et al., 1996; Ehlers and Clark, 2000; Elbert and Schauer, 2002; McNally, 1997] all assume that there are dysfunctions involved in different types of memories in PTSD. Presumably, these different memory systems also underlie diverse neural correlates in the brain [Brewin, 2001]. Implicit memory is induced by trauma cues and typical for the induction of intrusions. Explicit memory on the other hand involves a direct trauma retrieval and is used in trauma therapy to improve trauma memory integration.
Rabellino et al. [2016] innovatively combined different memory types in one study [Rabellino et al., 2016]. Their results revealed that processing of subconscious (subliminal) trauma‐related cues leads to fast defensive reactions of an innate alarm system that are not observed when conscious trauma memory stimuli are presented [Rabellino et al., 2016]. As a contrast, in the current study we evaluate neural responses to unintentionally processed conscious trauma cues (implicit memory). These are compared to intentional processing and imagination of conscious trauma scripts (explicit memory). To this extent, we used established paradigms on implicit [Morey et al., 2009; Oei et al., 2012] and explicit [Lanius et al., 2001, 2004] memories in one study. This will shed new light on memory processing in PTSD.
In addition, our study in healthy participants evaluates experimental trauma memory retrieval at a very early stage. Studies on patients with full psychiatric manifestations usually focused on traumatic memory retrieval in later stages of the disorder. However, neural correlates of trauma memory in early stages might have a crucial predictive value for later symptom development. Thus, in order to understand the neural mechanisms of the development of traumatic memories, an examination of the early period after trauma exposure is required. This can be achieved by an analogue study in healthy participants using the trauma‐film paradigm. The trauma‐film paradigm was first developed over 50 years ago [Horowitz, 1969; Lazarus, 1964] and has been validated in over 70 studies only in the past years [James et al., 2016]. It is an experimental medicine model that evaluates deviant behavior in non‐patient samples in order to analyze processes involved in the development of PTSD [James et al., 2016]. Responses comparable to real‐life trauma have been systematically observed in healthy subjects after an experimental trauma (i.e., trauma film) [James et al., 2016]. Responses include hallmark symptoms of PTSD [James et al., 2016]. Specifically, physiological arousal, intrusive memories, negative cognitions and mood have been reported [APA, 2013; Butler et al., 1995; Holmes et al., 2004; James et al., 2016; Weidmann et al., 2009]. However, in contrast to PTSD, symptoms after an experimental trauma only last for hours or days and finally decrease within one week [Holmes et al., 2004; James et al., 2016].
In our study, healthy participants watched a traumatic film consisting of a rape scene, which resembles witnessing a traumatic event. According to the diagnosis criteria for PTSD, both direct exposure to a traumatic event and witnessing trauma can result in PTSD [APA, 2013]. Witnessing trauma can result in behavioral impairments in both rats [Patki et al., 2014] and humans [Al‐Nuaimi et al., 2015; Atwoli et al., 2015]. Particularly witnessing sexual assaults have been associated with PTSD symptoms [Lueger‐Schuster et al., 2012; Witte et al., 2016].
Therefore, the trauma film paradigm used in the current study provides an optimal setting to bridge clinical and nonclinical samples in a highly controlled environment in order to examine PTSD symptoms [Clark and Mackay, 2015].
Concerning the neural correlates in analogue studies, there has only been one functional magnetic resonance imaging (fMRI) study focusing on the encoding of an experimental traumatic event itself [Bourne et al., 2013]. Neural correlates during encoding involved brain regions of the amygdala, striatum, thalamus and anterior cingulate cortex. These brain regions significantly predicted development of experimental trauma memory intrusions [Bourne et al., 2013].
In contrast, brain areas and processes involved in early retrieval attempts of traumatic memories and their relation to traumatic intrusion development as a predictor variable have not yet been identified. The present study aims at filling this gap by identifying neural correlates of experimental trauma memory retrieval shortly after exposure in an analogue paradigm with healthy participants. In particular, we directly compare neural correlates of implicit and explicit experimental trauma memory retrieval and their predictive values for the development of subsequent intrusive memories.
We hypothesize that different neural correlates are involved in implicit and explicit trauma film memory retrieval, both predicting later intrusive memories in a distinct way. We predict implicit memories to be associated with brain regions characteristic of emotional processing and emotional memory such as the ACC, amygdala and PCC. For the explicit memory task of trauma film retrieval, we predict both emotional‐memory associated brain regions and episodic/autobiographical memory regions such as the retrosplenial cortex, the PCC and the precuneus, as there is a deliberate focus on the trauma film memory itself.
METHODS AND MATERIAL
Participants
Forty‐five healthy, nonsmoking, right‐handed female participants underwent a general screening to exclude present and past medical and psychiatric disorders and for standard MRI exclusion criteria. Due to technical problems, three participants had to be discarded from the experiment (one participant fell asleep in the scanner and data of two participants could not be analyzed due to technical difficulties), leaving forty‐two healthy participants in the sample (mean age 23.3, range 19–33 years). Subjects provided their written informed consent after the study protocol had been fully explained. Additionally, subjects were reimbursed for their participation (25 CHF/h). The study protocol was in line with the Declaration of Helsinki and was approved by a local ethical review board.
Experimental Procedure and Design
Participants arrived at the imaging center and the procedure was explained. First, they were trained for the experimental paradigms (implicit and explicit memory task, see below). Afterwards one half of the subjects (n = 21) watched a trauma film (experimental trauma group) and the other half (n = 21) watched a neutral film (neutral group), according to a between subject design. 100 min after the film, participants performed first an implicit and then an explicit memory task. Meanwhile fMRI was performed. After the measurements, participants received an intrusion diary with a detailed explanation for the following seven days and were sent home (see Fig. 1A, for a summary of the procedure).
Figure 1.
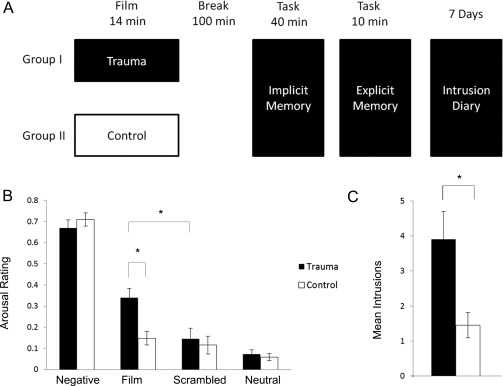
Study design and behavioral results: (A) Participants were randomly allocated to either a trauma film group or a control film group which was presented inside the MR scanner. After a 100 min break, experimental trauma memory retrieval was accessed by both an implicit and an explicit memory task. The implicit retrieval task consisted of a Sternberg task with film, negative, neutral and scrambled pictures as distractors. The explicit retrieval task was a script‐driven imagery task using an auditory representation of the traumatic film. During the following seven days participants filled in an intrusion diary. (B). Subjective ratings of the pictures used in the implicit memory tasks revealed a significantly higher arousal rating for participants in the trauma film group compared to the control group. Note that film pictures used in the implicit memory task were identical for both groups showing emotionally neutral film scenes. Thus, difference in arousal ratings can only be attributed to memories of the film. Arousal ratings of other picture categories did not differ significantly between the groups. (C) During the 7‐day period after the film presentation, participants in the trauma film group reported significantly more experimental trauma memory intrusions compared to the control group. *: P < 0.05; mean ± standard error are indicated.
Film Paradigm
According to the trauma film paradigm [Holmes and Bourne, 2008], participants either watched a trauma film or a control film (duration of both film clips: 14 min). In the trauma film, rape and further physical assault was depicted (film called “Irreversible”) [Gaspar Noe, 2002]. In the neutral film, a collection of neutral scenes with the same characters was displayed. Both films had the same duration. Participants had to rate feelings on an emotionality scale before and after the film.
Implicit Memory Recall: Sternberg Task with Emotional Distractors
For the implicit paradigm, we used a Sternberg working memory task with emotional distractors [Oei et al., 2012] (see Supporting Information for details). The emotional distractors were presented for 4 s and consisted of four different picture categories. Categories included 20 film pictures, 20 negative and 20 neutral pictures taken from the international affective pictures system [Lang et al., 2008] and 20 scrambled pictures. Both neutral and negative IAPS pictures were matched in brightness to the film pictures. Scrambled pictures consisted of a collage of all pictures and were blurred with a Gaussian filter. Notably, our film pictures consisted of emotionally neutral pictures taken from both films equally to prevent familiarity effects (trauma and neutral films). They included no action but rather served as a (initially neutral) reminder cue (e.g., tunnel where the protagonist was raped). Throughout all trials, participants were explicitly asked to focus on the Sternberg task and not on the distractors, so that the emotional distractors solely trigger implicit memories of the film watched before. After completion of the Sternberg task, all pictures were presented again and rated according to vividness and arousal on an 11‐point Likert scale.
In the explicit memory tasks, participants listened to an audiotape retelling the traumatic and the neutral film clip, according to a classical script‐driven imagery design [Pitman et al., 1987] (see Supporting Information for details).
Intrusion Diary
The intrusion diary consisted of a seven‐day diary which had to be filled in at home. Participants were educated about intrusive memories and asked to write down every intrusive memory that came to their mind. This included content, vividness, distress and intrusive characteristics for every memory that was experienced daily during this time. Intrusion diaries were analyzed and the sum of reported intrusions per week was used for further analyses. Scoring criteria for intrusions included ratings on distress, vividness and the sudden occurrence of the reported memories.
Functional MRI Data Acquisition and Analysis
Measurements were performed on a Philips Achieva 3.0 Tesla TX whole‐body magnetic resonance scanner (Philips Healthcare, Best, The Netherlands; see Supporting Information for details). All tasks and films were presented on MR compatible video goggles (Resonance Technology, Northridge). Furthermore, for good sound quality, headphones especially designed for MR measurements (MR Confon GmbH, Magdeburg, Germany) were used. Standard image data preparation, preprocessing and statistical analysis were performed with the software SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Functional data preprocessing included a correction for slice‐scan timing acquisition, realignment to the first volume using rigid body transformation and normalization to the EPI template in Montreal Neurological Institute (MNI) space. Images were subsequently resliced to a voxel resolution of 2 × 2 × 2 mm3 and smoothed using a 6 mm full width at half maximum Gaussian kernel. A general linear model was performed. In the first level analysis of the implicit memory task (Sternberg task), stimulus‐onsets and duration of each emotional distractor category (neutral film pictures, negative and neutral IAPS pictures and scrambled pictures) were included in the model. The main contrast of interest on the first level was the difference in activity for film pictures > scrambled pictures. In the explicit memory task (script driven imagery), first level fMRI‐models included stimulus‐onsets and duration of each audiotape (both trauma audiotape and control film audiotape). The main contrast of interest was the difference between the trauma audiotape and the neutral audiotape. In addition, six affine motion correction regressors were included as regressors of no interest in both analyses. On the second level, random effects analyses were performed for statistical inference on the group level. Statistical significance was set at p FWE.cluster < 0.05, with a cluster defining threshold (CDT) of P = 0.001 [Eklund et al., 2016].
Psychophysiological Interaction Effects and Brain Behavioral Correlates
For psychophysiological interaction (PPI) analyses and the brain‐behavior correlates, we extracted seeds from second level group results for the implicit (ACC, PCC and retrosplenial cortex) and explicit memory task (precuneus, PCC) using a 6 mm sphere around the center coordinates of the second level cluster peaks (see Supporting Information).
Additionally for the PPI, we used the hippocampus and amygdala as predefined regions of interest (ROIs) from the Wake Forest University (WFU) Pickatlas. Statistical significance was set at p FWE.cluster < 0.05, with a cluster defining threshold (CDT) of P = 0.001 [Eklund et al., 2016].
For the brain behavior correlates a stepwise linear regression was performed with the sum of intrusions as a dependent variable and peak levels (6 mm sphere) of brain regions found in the second level analysis of both implicit and explicit tasks as independent variables (please see below). Stepwise selection criteria for inclusion: probability of F ≤ 0.05 an exclusion: probability of F ≤ 0.10.
RESULTS
Behavioral Results
As expected, participants who had seen the traumatic film (experimental trauma group) rated the film pictures as more arousing and negatively valenced as the neutral film group (t (40) = 3.5 P = 0.001), see Figure 1B, for ratings and demographic data see Supporting Information Table I and II. Note that all film pictures showed neutral scenes from both the trauma and the neutral films, so no explicit traumatic action was shown. Thus, participants from the experimental trauma group rated the same (initially neutral) film pictures as more negative and more arousing than the neutral group due to watching the traumatic film before. In addition, participants from the experimental trauma group rated the film pictures as more negative and more arousing than neutral IAPS pictures (t 20 = 6.6, P < 0.001), while participants from the control group did not do that. Subjective valence and arousal ratings did not differ significantly between the experimental trauma and the control group for negative, neutral and scrambled pictures (all P > 0.10). The pattern was substantiated by a significant interaction effect in the between‐subject factor of film type (experimental trauma vs. neutral) and picture type (negative, neutral film and scrambled pictures; valence F 3,120 = 7.80, P = 0.003 and arousal F 3,120 = 5.44, P < 0.001). Performances in the Sternberg task did not differ significantly, neither between the two experimental groups nor between pictures types (all P > 0.10).
Main Effects of Brain Activity Underlying Experimental Trauma Memory Retrieval
In accordance with previous studies in PTSD patients, implicit retrieval of experimental trauma memories was associated with robustly increased brain activity in the retrosplenial cortex/precuneus, PCC and anterior cingulate cortex (ACC, all P < 0.05, family‐wise error corrected for multiple comparisons for the whole brain on cluster level; see Fig. 2A,B and Table 1).
Figure 2.
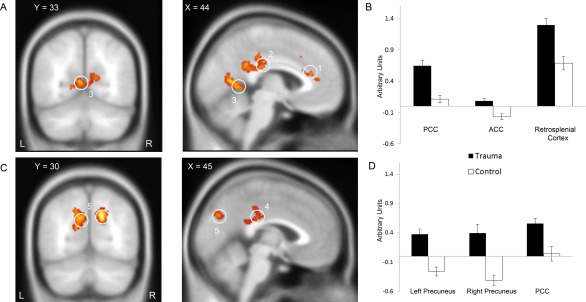
Implicit memory task (2A and 2B) and explicit memory task (2C and 2D). Neural correlates during the implicit memory task revealed activation the ACC (1), PCC (2) and retrosplenial cortex (3). During the explicit memory task activations were found in the PCC (4) and precuneus (5). Coronal and sagittal slices are shown with a threshold of p FWE.cluster = 0.05, superimposed on a canonical normalized image of SPM8 (mean t1 image, avg152T1.nii).
Table 1.
Clusters found during the implicit memory task for both the experimental trauma > control group and control > experimental trauma group
| M N I | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | No of voxels | L/R | BA | X | Y | Z | t (peak) | p FWE.cluster |
|
Implicit memory retrieval: Trauma > control group |
||||||||
| Cingulate gyrus, cerebellum | 827 | R | 31 | 4 | −40 | 32 | 5.91 | 0.000 |
| L | 31 | −2 | −32 | 36 | 4.53 | |||
| R | 24 | 6 | −16 | 36 | 4.53 | |||
| Anterior cingulate | 170 | R/L | 32 | 0 | 40 | 12 | 5.30 | 0.007 |
| L | 24 | −4 | 32 | 18 | 4.25 | |||
| L | 33 | −2 | 22 | 20 | 4.06 | |||
| Retrosplenial cortex, posterior cingulate, left cerebellum | 480 | L | −6 | −50 | 0 | 4.21 | 0.000 | |
| L | 30 | −4 | −64 | 10 | 4.17 | |||
| R | 30 | 10 | −58 | 6 | 3.77 | |||
| Control > trauma group | No suprathreshold voxels | |||||||
Figure 3.
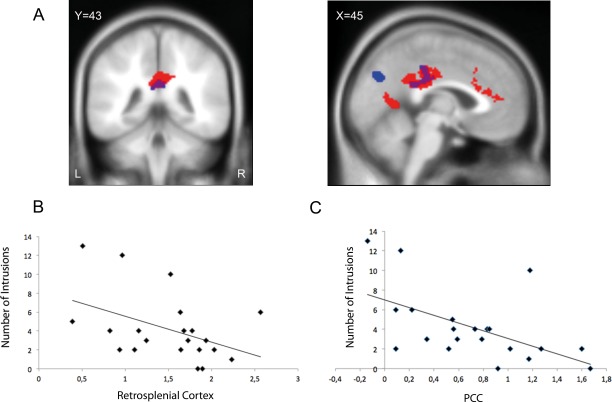
(A) Conjunction analyses of both the implicit (red) and the explicit memory task (blue) revealed an overlap in the posterior cingulate cortex (PCC) (violet) associated with memory retrieval of the traumatic film. Coronal (left) and sagittal (right) slices are shown thresholded at p FWE.cluster = 0.05, superimposed on a canonical normalized image of SPM8 (mean t1 image, avg152T1.nii). (B) Data extraction from the three clusters of activity observed in the implicit task (PCC, ACC and retrosplenial cortex) revealed a significant prediction of the number of intrusive memories only for the retrosplecial cortex. (C) For the explicit retrieval task, only activity in PCC predicted later intrusion development. These predictions were independent of each other as confirmed by partial correlation analyses (retrosplenial cortex (implicit retrieval task) and number of intrusions r = −0.467 r 2 (controlled for PCC); PCC (explicit retrieval task) and number of intrusions: r = −0.570, P < 0.05, controlled for retrosplenial cortex, both P < 0.05).
When looking at experimental trauma > control group and specifically at the film pictures > negative IAPS pictures contrast, the retrosplenial cortex and the precuneus were still significantly different. However, the PCC and ACC were not found in this contrast, indicating their involvement mainly in an overall heightened response to negative stimuli (see Supporting Information Fig. 3).
Interestingly, also in the explicit memory retrieval task (script‐driven imagery), brain activation was increased in the experimental trauma group compared to the neutral group. Importantly, brain regions were partly similar to the implicit retrieval task, including the left and right precuneus and the PCC (P < 0.05, family‐wise error corrected for multiple comparisons on the cluster level (CDT of P = 0.001), see Fig. 2C,D and Table 2). No increases in brain activity were observed in the control group compared to the experimental trauma group, neither in the implicit nor the explicit retrieval task. Note that between groups (experimental trauma and control) exactly the same stimuli were presented in both retrieval tasks (i.e., implicit: film pictures; explicit: trauma scripts). Thus, any difference in brain activity between the experimental trauma and the control group can only be explained by the fact that the experimental trauma group previously saw the trauma film and retrieved memories of this film during the implicit and explicit retrieval tasks.
Table 2.
Clusters found during the explicit memory task for both the experimental trauma > control group and control > experimental trauma group
| M N I | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | No of voxels | L/R | BA | X | Y | Z | t (peak) | p fwE.cluster |
|
Explicit memory retrieval: Trauma > control group |
||||||||
| Posterior cingulate, cuneus | 388 | R | 7 | 14 | −72 | 34 | 6.20 | 0.000 |
| R | 31 | 24 | −64 | 16 | 3.69 | |||
| Precuneus, cuneus | 659 | L | 7 | −6 | −72 | 32 | 5.34 | 0.000 |
| L | 31 | −14 | −64 | 22 | 4.78 | |||
| L | 7 | −14 | −72 | 38 | 4.62 | |||
| Cingulate gyrus, posterior cingulate | 180 | L | 23 | −4 | −28 | 32 | 3.97 | 0.016 |
| R | 23 | 2 | −42 | 24 | 3.87 | |||
| R/L | 31 | 0 | −32 | 40 | 3.68 | |||
| Control > trauma group | No suprathreshold voxels | |||||||
Psychophysiological Interaction Analyses of Experimental Trauma Memory Retrieval
In the implicit retrieval task, PPI analyses with a bilateral amygdala seed revealed a higher connectivity with the supplementary motor area, precentral and paracentral lobule for film pictures > scrambled pictures (p FWE.cluster < 0.05, see Supporting Information Fig. 1), that was significantly stronger in the control group compared to the experimental trauma group. Interestingly, the higher connectivity with the supplementary motor area and precentral and paracentral lobule was found also with a bilateral hippocampus seed, an ACC and a PCC seed. There were no significant connectivities with any of the reported seeds that were stronger for the experimental trauma compared to the control group.
In the explicit retrieval task, the PPIs with a bilateral amygdala and hippocampus seed revealed no significant differences in connectivity. However, a PCC seed revealed significant higher connectivity in the left and right superior‐, middle‐ and inferior‐temporal lobe and supramarginal gyrus (p FWE.cluster < 0.05). This result was found in the trauma audio file > control audio file for the control group compared to the experimental trauma group. There were no significant PPI results in any of these seeds in the experimental trauma group > control group contrast.
Brain Activity during Experimental Trauma Memory Retrieval Predicts Later Intrusions
Finally, we examined the important question whether brain activity identified during implicit and explicit retrieval early after experimental trauma exposure has any predictive value for later development of intrusive memories. First, eigenvariates were extracted from the three activity clusters identified in implicit memory retrieval task (ACC, retrosplenial cortex and PCC). In addition, data was also extracted from the two activity clusters identified in the explicit memory retrieval task (PCC, precuneus). All five variables were entered as predictors in a step‐wise linear regression analysis. This analysis revealed a significant prediction of the number of intrusive memories by activity in the retrosplenial cortex in the implicit retrieval task (R2 = 0.263) (P < 0.05) and by activity in the PCC in the explicit memory task (R 2 = 0.152; P < 0.05). The combined prediction was significantly more robust compared to the predictive value of each of the two predictors alone (R2 = 0.452). Subsequent partial correlation analyses confirmed that both predictors independently contributed to the prediction of the number of intrusions when controlling for the other predictor, respectively (retrosplenial cortex (implicit retrieval task) and number of intrusions r = −0.467 (controlled for PCC); PCC (explicit retrieval task) and number of intrusions: r = −0.570, P < 0.05, controlled for retrosplenial cortex, both P < 0.05), see Figure 3. Thus, both brain activities during implicit and explicit experimental trauma memory retrieval significantly predict later intrusion development, but they explain distinct aspects of the variance. Further variables such as anxiety ratings, and arousal and valence ratings did not contribute to the model.
As the film pictures > negative IAPS pictures revealed a trauma‐related significant activation only in the precuneus and retrosplenial cortex, we have also controlled whether these activations predicted intrusions development. Stepwise linear regression analyses revealed that also the slightly altered peak of the retrosplenial cortex ([X Y Z] = −6 −56 4) predicted later intrusion development (R 2 = 0.207) (P< 0.05).
Intrusions taken as a covariate during the explicit memory task revealed additional correlations with neural activity in the precentral lobe, cerebellum, vermis (p FWE.cluster < 0.05) and orbitofrontal cortex (p FWE.cluster < 0.10), see Supporting Information Figure 2. No additional brain‐behavior correlations were found when intrusions were taken as a covariate in the implicit memory task.
DISCUSSION
Our findings reveal a highly specific network of increased activation in the cingulate cortex, retrosplenial cortex and precuneus during retrieval of memories associated with a traumatic film. This network was specific for retrieval of experimental trauma memories, as the activation in this network critically depended on the existence of memory representation of the traumatic film: The activation only occurred in participants that saw and witnessed traumatic scenes compared to a control group that was presented with neutral scenes from the same film and thus did not witness a traumatic event. In addition, this experimental trauma memory retrieval network also depended on the type of retrieval access: while implicit and automatic retrieval activated the retrosplenial cortex, precuneus and the ACC as well as PCC, explicit and intentional retrieval activated the PCC and the precuneus. Interestingly, the retrosplenial cortex and precuneus seem to be specific for the experimental trauma material, whereas the ACC and PCC were rather associated with overall heightened responses to negative stimuli during the implicit memory task.
Our results are consistent with theories on PTSD [Brewin et al., 1996; Ehlers and Clark, 2000; Elbert and Schauer, 2002; McNally, 1997] indicating that different types of memory are altered after trauma. Indeed, the different memory types underlie distinct neural pathways in our study [Brewin, 2001]. However, there was also an overlap between implicit and explicit memory. The strongest overlap of both types of memory retrieval access was observed in the PCC. Particularly the retrosplenial cortex and the PCC have been strongly implicated in self‐referential processing as well as in episodic memory encoding and retrieval, more precisely with respect to autobiographical memories [Foster et al., 2013; Maddock et al., 2001]. The PCC is hypothesized to be of high importance in successful memory retrieval. Furthermore, the PCC has been reported to be implicated in the processing of emotional salience [Maddock et al., 2003]. Both the PCC and the retrosplenial cortex have been proposed as mediators for the interaction between emotions and memory [Maddock, 1999; Maddock et al., 2003].
Interestingly, the ACC has been associated with detection of unfavorable outcomes, response errors, response conflict and decision uncertainty [Ridderinkhof et al., 2004], which might explain the additional activation in this region during implicit and involuntary retrieval cues by the distractor pictures presented during the Sternberg task. Importantly, all of the identified brain regions have been previously implicated also in retrieval of trauma memories in patients suffering from PTSD: A recent meta‐analysis of trauma memory in PTSD patients with full psychiatric manifestation reported also activation in the retrosplenial cortex, precuneus, and ACC [Sartory et al., 2013]. Thus, neural correlates in healthy subjects at an early stage after a trauma film resemble neural correlates found in PTSD patients with full psychiatric manifestation.
Interestingly, here we did not observe any increased amygdala activation during retrieval of experimental trauma memories. This accounts for both implicit and explicit retrieval, which might point to a stronger emotional reactivation during exposure to traumatic cues in patients compared to healthy females. However, we observed a significant decrease in connectivity between the amygdala and the supplementary motor area during implicit retrieval of experimental trauma memories. The supplementary motor area has been associated with readiness for action in previous literature [Cunnington et al., 2005]. In case of an inability to either fight or flee, less activity in the supplementary motor area can be seen as a consequence of this inability. The displayed phenomenon can be observed in PTSD patients, where freezing or fainting occurs during trauma exposure and reliving, as suggested by dissociations theories in PTSD [Schauer and Elbert, 2010]. However, this interpretation cannot be proven in our study as we have not measured symptoms on dissociation scales to support this claim. Moreover, the decrease in connectivity has only been observed in the control > experimental trauma contrast and therefore cannot be uniquely ascribed to the effects of the trauma film. However, the decreased connectivity between the amygdala and the supplementary motor area in our data again points to a similar neural mechanism as in PTSD patients already early after experimental trauma exposure. This might be particularly important during implicit and involuntary retrieval conditions.
Finally, we show that activation in the experimental trauma retrieval network predicted later intrusion development in a highly specific manner: While activation in the retrosplenial cortex predicted intrusions when experimental trauma memory retrieval was implicit, activation in the PCC independently predicted intrusive memories under explicit retrieval conditions. Our results confirm that there is a high predictive potential of neural correlates for the development of experimental trauma memories, together explaining almost 40% of the variance of the number of experimental trauma memory intrusions during seven days after experimental trauma exposure. As intrusive memories are a critical risk factor for the development and maintenance of PTSD [Michael et al., 2005], a possible change in activity in these brain regions might be highly relevant for early intervention strategies. Interestingly, the predictions were negative in both the implicit and explicit retrieval conditions: Higher activation in the regions predicted a smaller number of intrusions during the subsequent seven days. Thus, activation in these regions might be indicative for successful memory integration within autobiographical networks (including the appropriate contextual information) rather than “overconsolidation” of traumatic memories. Our findings suggest that facilitating proper memory integration in the regions of the retrosplenial cortex and PCC early after experimental trauma exposure might support the formation of a controllable memory representation of the traumatic event in appropriate autobiographical contexts. Increased participation of the brain regions involved in long‐term memory storage might thereby avoid or at least reduce the chance of distinct “overconsolidation” and “decontextualisation,” which might underlie the intrusive and uncontrollable character of traumatic memories.
A clear strength of the presented study is that we gained new insights into neural processing early after experimental trauma exposure due to our elaborated study design. Longitudinal studies in patients are hard to conduct but necessary for identifying predictor variables. Additional high heterogeneity in the type of trauma makes it difficult to find key predictor variables in PTSD patient studies. In our study with healthy participants and an equal “trauma exposure,” implicit and explicit memory recall was distinguished and intrusions could be predicted in a unique way. Moreover, we directly used stimuli of the “trauma” itself, which served as cues for memory recall. So far there has been no study directly using exactly the same visual stimuli in a retrieval task as during the experimental trauma itself. Results of our experimental trauma‐specific neural correlates and their prediction value, therefore, give a new relevant perspective for the field of neuroscience and PTSD.
A major limitation of this study is that we only evaluated healthy participants. Moreover, the experimental trauma rather includes features of witnessing a trauma. Patient samples with PTSD will reveal additional and stronger neural correlates during “real” trauma memory exposure. Therefore, a replication of our findings has to be made in a longitudinal perspective after trauma exposure until the development of full PTSD psychiatric manifestation. Moreover, both functional and structural neural correlates of PTSD patients might differ already before traumatization and this may lead to altered processing of a traumatic event. As we were not able to address this topic in our study, this further limits the relevance of our findings. Future studies should focus more on predefined vulnerability factors such as hippocampal atrophy [Gilbertson et al., 2002] before traumatization in order to further specify predictive factors and potential preventive treatments in PTSD.
Furthermore, also differences in encoding during experimental trauma exposure might alter traumatic memory processing and subsequently PTSD symptoms. Therefore, our findings are also limited in respect to predefined differences in encoding. Future studies should also focus on pretraumatic and peritraumatic differences in encoding and their prediction toward altered memory processing and PTSD symptoms.
Our study gives new insights in the neural correlates of experimental trauma memory and their predictive value for symptom development. With the identification of the relevance of PCC and retrosplenial cortex, preventive and targeted treatment can be developed. Future research should focus on interventions that might support memory integration to diminish these trauma‐specific neural correlates and therefore reduce the occurrence of later symptom development. A possible intervention that targets memory integration can be potentially found by enhancing memory consolidation and integration during sleep [Kleim et al., 2016; Stickgold, 2002].
FINANCIAL DISCLOSURES
The authors have no conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENT
We thank Sarah Schoch, Julia Frey, Anja Betschart and Marco Bigica for assistance in data collection.
REFERENCES
- Al‐Nuaimi MA, Hamad RA, Lafta RK (2015): Effects of witnessing or exposure to community violence on mental health of Iraqi men. Qatar Med J 2015:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM‐5). American Psychiatric Association Publishing Washington, DC.
- Atwoli L, Platt J, Williams DR, Stein DJ, Koenen KC (2015): Association between witnessing traumatic events and psychopathology in the South African Stress and Health Study. Soc Psychiatry Psychiatr Epidemiol 50:1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne C, Mackay CE, Holmes EA (2013): The neural basis of flashback formation: The impact of viewing trauma. Psychol Med 43:1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR (2001): A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behav Res Ther 39:373–393. [DOI] [PubMed] [Google Scholar]
- Brewin CR (2014): Episodic memory, perceptual memory, and their interaction: Foundations for a theory of posttraumatic stress disorder. Psychol Bull 140:69–97. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Dalgleish T, Joseph S (1996): A dual representation theory of posttraumatic stress disorder. Psychol Rev 103:670–686. [DOI] [PubMed] [Google Scholar]
- Brohawn KH, Offringa R, Pfaff DL, Hughes KC, Shin LM (2010): The neural correlates of emotional memory in posttraumatic stress disorder. Biol Psychiatry 68:1023–1030. [DOI] [PubMed] [Google Scholar]
- Butler G, Wells A, Dewick H (1995): Differential effects of worry and imagery after exposure to a stressful stimulus: A pilot study. Behav Cogn Psychother 23:45–56. [Google Scholar]
- Clark IA, Mackay CE (2015): Mental imagery and post‐traumatic stress disorder: A neuroimaging and experimental psychopathology approach to intrusive memories of trauma. Front Psychiatry 6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Moser E (2005): Premovement activity of the pre‐supplementary motor area and the readiness for action: Studies of time‐resolved event‐related functional MRI. Hum Mov Sci 24:644–656. [DOI] [PubMed] [Google Scholar]
- de Quervain Schwabe L, Roozendaal B (2017): Stress, glucocorticoids and memory: Implications for treating fear‐related disorders. Nat Rev Neurosci 18:7–19. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Clark DM (2000): A cognitive model of posttraumatic stress disorder. Behav Res Ther 38:319–345. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proc Natl Acad Sci USA 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Schauer M (2002): Burnt into memory. Nature 419:883. [DOI] [PubMed] [Google Scholar]
- Foster BL, Kaveh A, Dastjerdi M, Miller KJ, Parvizi J (2013): Human retrosplenial cortex displays transient theta phase locking with medial temporal cortex prior to activation during autobiographical memory retrieval. J Neurosci 33:10439–10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK (2002): Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 5:1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes EA, Bourne C (2008): Inducing and modulating intrusive emotional memories: A review of the trauma film paradigm. Acta Psychol 127:553–566. [DOI] [PubMed] [Google Scholar]
- Holmes EA, Brewin CR, Hennessy RG (2004): Trauma films, information processing, and intrusive memory development. J Exp Psychol Gen 133:3–22. [DOI] [PubMed] [Google Scholar]
- Horowitz MJ (1969): Psychic trauma. Return of images after a stress film. Arch Gen Psychiatry 20:552–559. [DOI] [PubMed] [Google Scholar]
- James EL, Lau‐Zhu A, Clark IA, Visser RM, Hagenaars MA, Holmes EA (2016): The trauma film paradigm as an experimental psychopathology model of psychological trauma: Intrusive memories and beyond. Clin Psychol Rev 47:106–142. [DOI] [PubMed] [Google Scholar]
- Kleim B, Wysokowsky J, Schmid N, Seifritz E, Rasch B (2016): Effects of sleep after experimental trauma on intrusive emotional memories. Sleep 39:2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (2008): International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A‐8. University of Florida, Gainesville, FL.
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS (2001): Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. Am J Psychiatry 158:1920–1922. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS (2004): The nature of traumatic memories: A 4‐T FMRI functional connectivity analysis. Am J Psychiatry 161:36–44. [DOI] [PubMed] [Google Scholar]
- Lazarus RS (1964): A laboratory approach to the dynamics of psychological stress. Am Psychol 19:400–411. [Google Scholar]
- LeDoux J (2003): The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueger‐Schuster B, Gluck TM, Tran US, Zeilinger EL (2012): Sexual violence by occupational forces during and after World War II: Influence of experiencing and witnessing of sexual violence on current mental health in a sample of elderly Austrians. Int Psychogeriatr 24:1354–1358. [DOI] [PubMed] [Google Scholar]
- Maddock RJ (1999): The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends Neurosci 22:310–316. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2001): Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104:667–676. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2003): Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ (1997): Implicit and explicit memory for trauma‐related information in PTSD. Ann N Y Acad Sci 821:219–224. [DOI] [PubMed] [Google Scholar]
- McNally RJ (2005): Debunking myths about trauma and memory. Can J Psychiatry 50:817–822. [DOI] [PubMed] [Google Scholar]
- Michael T, Ehlers A, Halligan SL, Clark DM (2005): Unwanted memories of assault: What intrusion characteristics are associated with PTSD? Behav Res Ther 43:613–628. [DOI] [PubMed] [Google Scholar]
- Morey RA, Dolcos F, Petty CM, Cooper DA, Hayes JP, LaBar KS, McCarthy G (2009): The role of trauma‐related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J Psychiatr Res 43:809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe G, Rossignon C (2002): Irreversible [Motion Picture]. France: Mars Films.
- Oei NY, Veer IM, Wolf OT, Spinhoven P, Rombouts SA, Elzinga BM (2012): Stress shifts brain activation towards ventral 'affective' areas during emotional distraction. Soc Cogn Affect Neurosci 7:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DS (2003): Predictors of posttraumatic stress disorder and symptoms in adults: A meta‐analysis. Psychol Bull 129:52–73. [DOI] [PubMed] [Google Scholar]
- Patki G, Solanki N, Salim S (2014): Witnessing traumatic events causes severe behavioral impairments in rats. Int J Neuropsychopharmacol 17:2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK (1989): Post‐traumatic stress disorder, hormones, and memory. Biol Psychiatry 26:221–223. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM (1987): Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry 44:970–975. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I (2012): Biological studies of post‐traumatic stress disorder. Nat Rev Neurosci 13:769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D, Densmore M, Frewen PA, Theberge J, Lanius RA (2016): The innate alarm circuit in post‐traumatic stress disorder: Conscious and subconscious processing of fear‐ and trauma‐related cues. Psychiatry Res 248:142–150. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004): The role of the medial frontal cortex in cognitive control. Science 306:443–447. [DOI] [PubMed] [Google Scholar]
- Sartory G, Cwik J, Knuppertz H, Schurholt B, Lebens M, Seitz RJ, Schulze R (2013): In search of the trauma memory: A meta‐analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD). PLoS One 8:e58150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer M, Elbert T (2010): Dissociation following traumatic stress. J Psychol 218:109–127. [Google Scholar]
- Stickgold R (2002): EMDR: A putative neurobiological mechanism of action. J Clin Psychol 58:61–75. [DOI] [PubMed] [Google Scholar]
- Weidmann A, Conradi A, Groger K, Fehm L, Fydrich T (2009): Using stressful films to analyze risk factors for PTSD in analogue experimental studies–which film works best? Anxiety Stress Coping 22:549–569. [DOI] [PubMed] [Google Scholar]
- Witte TH, Casper DM, Hackman CL, Mulla MM (2016): Bystander interventions for sexual assault and dating violence on college campuses: Are we putting bystanders in harm's way? J Am Coll Health 65:149–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


