Abstract
Objective
Autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) are not only often comorbid but also overlapped in behavioral and cognitive abnormalities. Little is known about whether these shared phenotypes are based on common or different underlying neuropathologies. Therefore, this study aims to examine the disorder‐specific alterations in white matter (WM) structural property.
Method
The three comparison groups included 23 male adults with ASD (21.4 ± 3.1 years), 32 male adults with ADHD (23.4 ± 3.3 years), and 29 age‐matched healthy male controls (22.4 ± 3.3 years). After acquisition of the diffusion spectrum imaging (DSI), whole brain tractography was reconstructed by a tract‐based automatic analysis. Generalized fractional anisotropy (GFA) values were computed to indicate tract‐specific WM property with adjusted P value < 0.05 for false discovery rate correction.
Results
Post hoc analyses revealed that men with ASD exhibited significant lower GFA values than men with ADHD and male controls in six identified fiber tracts: the right arcuate fasciculus, right cingulum (hippocampal part), anterior commissure, and three callosal fibers (ventrolateral prefrontal cortex part, precentral part, superior temporal part). There was no significant difference in the GFA values of any of the fiber tracts between men with ADHD and controls. In men with ASD, the GFA values of the right arcuate fasciculus and right cingulum (hippocampal part) were negatively associated with autistic social‐deficit symptoms, and the anterior commissure GFA value was positively correlated with intelligence.
Conclusions
This study highlights the disorder‐specific alteration of the microstructural property of WM tracts in male adults with ASD. Hum Brain Mapp 38:384–395, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: autism spectrum disorder, attention deficit/hyperactivity disorder, diffusion spectrum imaging, tractography, white matter
INTRODUCTION
Autism spectrum disorder (ASD) and attention deficit/hyperactivity disorder (ADHD) are two common neurodevelopmental disorders [American Psychiatric Association, 2013]. ASD is characterized by impairment in social communication and interaction, alongside restricted, and repetitive behaviors/interests/activities. The core symptoms of ADHD include inattention, hyperactivity, and impulsivity. Despite the distinct diagnostic prototypes between ASD and ADHD, these two disorders also have some overlaps, in terms of clinical presentations [Leitner, 2014], neuropsychological functioning [Rommelse et al., 2011], and familial/genetic influences [Musser et al., 2014]. Yet, commonalities and distinctions of neuroimaging correlates between these two disorders have been infrequently studied and revealed inconclusive results [Brieber et al., 2007; Chantiluke et al., 2014; Christakou et al., 2013; Di Martino et al., 2013; Lim et al., 2015; Ray et al., 2014].
Parallel examinations of structural and functional neural substrates of ASD and ADHD highlight disrupted structural [Travers et al., 2012] and functional [Di Martino et al., 2013] brain connectivity of widespread circuitries in each condition, despite apparent inconsistencies across disorders and studies. Examining ASD and ADHD in the same study is warranted to determine any similar neural substrates in these two disorders [Christakou et al., 2013]. Limited studies directly comparing ASD and ADHD have only found some potential commonalities and noticeable disorder‐specificity in the neural correlates [Christakou et al., 2013; Di Martino et al., 2013]. For example, task‐based functional magnetic imaging (fMRI) studies demonstrated both common and disorder‐specific hypo‐/hyperactivations in task‐related brain regions during the sustained attention [Christakou et al., 2013] and temporal discounting task [Chantiluke et al., 2014]. Other studies also reported both shared and disorder‐specific abnormality in the degree of centrality derived from intrinsic functional connectivity [Di Martino et al., 2013] and structural changes in the regional volumes [Brieber et al., 2007; Lim et al., 2015]. Although these studies focused on regional abnormalities, the widely distributed patterns further support the notion of aberrant circuitries in both disorders [Ecker et al., 2015].
A recent diffusion MRI study, combining resting‐state fMRI, further suggests that ASD and ADHD exhibited distinct brain network organization in middle childhood [Ray et al., 2014]. Relative to typically developing children, children with ASD had over‐connectivity inside regions demonstrating rich‐club organization, that is, the hubs of a brain network tend to be more densely connected among themselves than nodes of a lower degree, whereas children with ADHD exhibited under‐connectivity in rich‐club areas [Ray et al., 2014]. Nonetheless, to our knowledge, only this study [Ray et al., 2014] used high angular resolution diffusion imaging to identify shared versus distinct white matter (WM) connectivity between ASD and ADHD, highlighting the pressing need to implement the state‐of‐the‐art technique to investigate microstructural connectivity atypicality among both disorders.
Overall, the current literature presented with heterogeneous findings, which may arise from the following reasons. Despite no overlap in the core diagnostic criteria, some levels of inattention and hyperactivity/impulsivity in ASD, whereas some levels of impaired social communication in ADHD, are noted [Rommelse et al., 2011], and ASD and ADHD co‐occur commonly. These have not been adequately accounted for in prior studies investigating these two disorders [Brieber et al., 2007; Chantiluke et al., 2014; Christakou et al., 2013; Di Martino et al., 2013; Lim et al., 2015; Ray et al., 2014], and may be partly addressed by the design of comparing participants with the pure ASD and ADHD without comorbidity. In addition, sex differences in clinical presentations and neuroanatomy of both disorders have been reported [Lai et al., 2015]. Among the previous imaging studies about ASD, most of the studies recruited all male participants [Brieber et al., 2007; Chantiluke et al., 2014; Christakou et al., 2013; Lim et al., 2015] or predominantly male participants [Di Martino et al., 2013], but most of the ADHD imaging studies included a substantial proportion of female participants [Chiang et al., 2015]. Lastly, neuroanatomical abnormalities in ASD and ADHD are substantially age‐dependent [Lin et al., 2015a; Shaw et al., 2007], introducing further heterogeneity in the literature. Intriguingly, none of the related literature has recruited adult participants to compare both disorders. To confine the studied age range to adulthood may better account for the different neurodevelopmental stages, that is, distinctly precocious or delayed maturation across disorders [Ecker et al., 2015], when comparing individuals with ASD, as well as those with ADHD, with typically developing controls.
This study, therefore, aimed to compare the whole‐brain WM microstructural property of male adults with ASD and those with ADHD as compared to healthy male adults, based on the state‐of‐art diffusion spectrum imaging (DSI) tractography, which may be advantageous as compared to Diffusion Tensor Imaging (DTI) in terms of resolutions of crossing fibers [Chen et al., 2015]. We hypothesized that men with ASD would have both similar and distinct WM microstructural properties with respect to men with ADHD and healthy controls. We did not hold specific hypotheses regarding the identified WM tracts given elusive and inconclusive evidence.
METHODS
Participants
This work was approved by the Research Ethics Committee of National Taiwan University Hospital (201003087R, 201201006RIB), and all the participants provided their written informed consents after detailed explanations before study implementation. The sample consisted of 23 men with ASD, 32 men with ADHD, and 29 healthy male adults (controls), aged from 18 to 30 years old (Table 1). The ASD and ADHD groups were recruited consecutively at the Department of Psychiatry, National Taiwan University Hospital, Taipei, Taiwan. They were clinically diagnosed according to the DSM‐IV diagnostic criteria by the corresponding author (SSG). The healthy men, recruited via advertisements, were enrolled only if there was neither medical and neuropsychiatric illness, nor current or past history of using psychotropic agents. All the participants received the same evaluation procedures including the semi‐structured Conners' Adult ADHD Diagnostic Interview as described in the DSM‐IV (CAADID, Multi‐Health Systems) [Conners et al., 1999] for current ADHD, and the modified adult version of the ADHD supplement of the Chinese version of the Schedule for Affective Disorders and Schizophrenia–Epidemiological Version (K‐SADS‐E) for current and past ADHD [Ni et al., 2013] and SADS for other psychiatric disorders [Lin et al., 2015b], the Wechsler Adult Intelligence Scale‐Revised [Wechsler, 1981], and MRI assessments. In addition, parents of participants with a clinical diagnosis of ASD received the Autism Diagnostic Interview‐Revised (ADI‐R) to confirm ASD diagnosis [Gau et al., 2011]. Because none of participants had comorbidity of ASD and ADHD, only men with ASD reported on the Chinese version of the Autism Spectrum Quotient (AQ‐Chinese) [Lau et al., 2013], and their parents completed the Chinese version of the Social Responsiveness Scale (SRS‐Chinese) [Gau et al., 2013] for the autistic‐like social deficits of the participants. All the participants who had psychosis, mood disorders, learning disability, substance use, neurological disorders, or comorbid diagnosis of ASD (for the ADHD and control groups) and ADHD (for the ASD and control groups) were excluded from the study. In the ADHD group, 14 participants had ever been treated with methylphenidate for ADHD symptoms.
Table 1.
Demographic and clinical characteristics
| ASD | ADHD | Control | ANOVA | Post hoc | ||
|---|---|---|---|---|---|---|
| Mean ± SD | (n = 23) | (n = 32) | (n = 29) | F value | P‐value | analysisa |
| Age (range: 17.5–30 y/o) | 21.396 ± 3.05 | 23.359 ± 3.348 | 22.424 ± 3.328 | 2.44 | 0.094 | |
| Right‐handed (%) | 21 (91.3%) | 30 (93.8%) | 29 (100%) | 2.391b | 0.303 | |
| Intelligence quotient (IQ) | ||||||
| Verbal IQ | 98.27 ± 21.54 | 108 ± 10.05 | 113.93 ± 9.61 | 7.97 | <0.001 | ASD< ADHD, Control |
| Performance IQ | 97.27 ± 23.97 | 110.5 ± 13.32 | 112.93 ± 12.6 | 6.24 | 0.003 | ASD< ADHD, Control |
| Full‐scale IQ | 97.82 ± 22.51 | 109.69 ± 10.58 | 114.59 ± 11.06 | 8.27 | <0.001 | ASD< ADHD, Control |
| (Range of Full‐scale IQ) | 45–130 | 87–137 | 86–134 | |||
| The adult autism spectrum quotient | ||||||
| Socialness | 38.33 ± 5.59 | — | 24.93 ± 8.52 | 32.54 | <0.001 | |
| Mindreading | 23.29 ± 4.98 | — | 14.60 ± 1.84 | 41.27 | <0.001 | |
| Patterns | 12.24 ± 4.00 | — | 9.80 ± 2.26 | 4.52 | 0.041 | |
| Attention to details | 11.00 ± 1.95 | — | 9.73 ± 1.67 | 4.15 | 0.049 | |
| Attention switching | 17.33 ± 3.31 | — | 14.07 ± 3.20 | 8.78 | 0.006 | |
| Total score | 102.19 ± 12.74 | — | 73.15 ± 7.83 | <0.001 | ||
| Adult ADHD Self‐Report Scale | ||||||
| Inattention | — | 26.44 ± 4.74 | 12.12 ± 3.61 | 136.63 | <0.001 | |
| Hyperactivity/Impulsivity | — | 20.72 ± 5.33 | 7.97 ± 4.66 | 77.29 | <0.001 | |
| Head motionc (signal dropout count) | 40.30 ± 18.07 | 24.34 ± 17.56 | 32.83 ± 21.55 | 4.73 | 0.012 | ASD> ADHD |
Pairwise comparison by post hoc analysis with Scheffé Test.
Chi‐square test.
High signal dropout count imply more head motion.
Clinical Assessments
The adult ADHD supplement of the Chinese version of the K‐SADS‐E
In addition to clinical assessment, all participants were confirmed by the Chinese K‐SADS‐E interview to obtain the information on clinical symptoms and diagnoses of ADHD in childhood and adulthood according to the DSM‐IV diagnostic criteria. The K‐SADS‐E is a semi‐structured interview scale for the systematic assessment of both lifetime and current (past 6 months) diagnosis of mental disorders, including childhood and current diagnosis of ASD and ADHD. The Child Psychiatry Research Group in Taiwan developed the Chinese K‐SADS‐E via a two‐stage translation [Gau et al., 2005], and the ADHD supplement was modified into adult version by the corresponding author (SSG) [Chang et al., 2013]. The Chinese K‐SADS‐E and SADS are reliable and valid instruments to assess child [Chiang et al., 2015] and adult [Chang et al., 2013; Lin et al., 2016] psychiatric disorders in Taiwan. The details of the K‐SADS‐E interview training and best estimate of each DSM‐IV psychiatric diagnosis have been described elsewhere [Gau et al., 2010] and are provided upon request.
The Chinese version of the ADI‐R
The clinical diagnosis of ASD of participants with ASD was further confirmed by interviewing the parents using the Chinese version of the ADI‐R. The ADI‐R is a standardized, semi‐structured diagnostic interview scale to obtain data on past and current autistic symptoms, which is provided by parents or main caregivers. The algorithm focuses on reciprocal social interaction (cut‐off = 10), verbal communication (cut‐off = 8), nonverbal communication (cut‐off = 7), and restricted/repetitive/stereotyped patterns of behaviors (cut‐off = 3). The Chinese ADI‐R was approved by Western Psychological Services in 2007 [Gau et al., 2011].
The Chinese version of the AQ (AQ‐Chinese)
The AQ [Baron‐Cohen et al., 2001] is a 50‐item adult self‐report measure of autistic traits with a 4‐point Likert scale ranging from 1 to 4. Higher score indicates the autistic end of the continuum. Exploratory and confirmatory factor analyses revealed that a 35‐item, 5‐dimensional factor solution of the AQ‐Chinese has favorable psychometric characteristics [Lau et al., 2013]. These five dimensions include the socialness (poor social skills and dislike social interaction), mindreading (difficulty in understanding other's intentions or feeling) and other three subscales (patterns, attention to details, and attention switching).
The Chinese version of the SRS
The SRS is a 65‐item rating scale measuring the severity of autism spectrum symptoms [Gau et al., 2013], rated on a 4‐point Likert scale from “0” (not true) to “3” (almost always true), and a higher score depicts more autistic features. Confirmatory factor analyses revealed that the Chinese version of the SRS, officially approved by Dr. Constantino in 2008, after removing 5 items, has a 4‐factor structure (i.e., socio‐communication, autism mannerisms, social awareness, and social emotion) [Gau et al., 2013].
MRI Assessments
Image acquisition
MRI was performed on a 3T MRI system (TIM Trio, Siemens, Erlangen, Germany) with a 32‐channel phased array head coil. All participants lied still on the table with head movement restricted by expandable foam cushions. T1‐weight images covering the whole head were acquired using a 3D magnetization‐prepared rapid gradient echo sequence: repetition time (TR) = 2,000 ms, echo time (TE) = 2.98 ms, inversion time = 900 ms, field of view (FOV) = 256 × 192 × 208 mm3, matrix size = 256 × 192 × 208, yielding an isotropic resolution of 1 × 1 × 1 mm3. DSI was acquired using a pulsed‐gradient spin‐echo EPI sequence with a twice‐refocused balanced echo [Reese et al., 2003]. Diffusion acquisition scheme with 102 diffusion‐weighted image volumes corresponding to the grid points within a half sphere of the 3D diffusion‐encoding space (q‐space) were applied with the maximum diffusion sensitivity value (bmax) of 4,000 s/mm2. The parameters were: TR/TE = 9,100 ms/142 ms, FOV = 200 mm × 200 mm, matrix size = 128 × 128, slice thickness = 2.5 mm without gap, and slice number = 54.
Substantial in‐scanner head motion may occur during DSI acquisitions due to relatively long scanning time. In the presence of strong diffusion‐sensitive gradients, especially those with high b‐values, jerky head motion induces signal loss in the diffusion‐weighted images. Our DSI datasets underwent a quality assurance procedure by counting the number of diffusion‐weighted images that had a significant signal dropout. All of the acquired DSI datasets (3,264 images per person) were scrutinized by calculating the signals in the central square (20 × 20 pixels) of each image. Data with more than 90 images of signal losses had significant reductions of generalized fractional anisotropy (GFA) values and was discarded [Chen et al., 2015]. The data used in the final analyses met the above‐mentioned criteria for quality control.
DSI image reconstruction
The diffusion probability density function (PDF) at each voxel was reconstructed based on the Fourier relationship between the PDF and q‐space signal [Callaghan et al., 1991]. Three‐dimensional Fourier transform was performed on the q‐space signal, applied with a Hanning filter of 17 units in width, to reconstruct the PDF. The orientation distribution function (ODF, ψ(u)) was determined by computing the second moment of the PDF along each of the 362 radial directions (sixfold tessellated icosahedron). The GFA value at each voxel was determined with the formula: SD(ψ)/RMS(ψ), where SD is the standard deviation and RMS is the root mean square of the ODF [Tuch, 2004]. The GFA corresponds to FA used in DTI studies, which may represent multiple dimensions regarding the microstructural property of the WM fiber tracts, including degree of myelination, fiber diameters, fiber density, or fiber coherence [Johansen‐Berg and Behrens, 2014].
Whole brain tract specific analysis
For the whole brain tract specific analysis, we used the tract‐based automatic analysis (TBAA) method developed by Chen et al. [2015] to enable efficient tract‐based analysis of the major fiber tracts over the entire brain. The TBAA method relies on a high quality DSI template [Hsu et al., 2015] and a list of tract coordinates on the template [Chen et al., 2015]. These two components allow us to sample the GFA values of each tract on each individual's diffusion dataset given known transformation between the DSI template and the individual DSI. The details of the construction of DSI template and tract coordinates are described elsewhere [Chen et al., 2015]. In brief, the DSI template, called NTU‐DSI‐122, is a DSI dataset averaged over 122 registered DSI datasets of healthy adults, and was built in the standard ICBM152 space. A total of 74 tracts were pre‐defined on the template by performing streamline‐based deterministic tractography with multiple regions of interests using in‐house software (DSI Studio: http://dsi-studio.labsolver.org). The coordinates of streamlines were aligned along the proceeding direction of each tract bundle, interpolated into 100 steps, and saved as the sampling coordinates of GFA.
We used the TBAA method as follows (Fig. 1). The DSI data of all 84 participants were registered to create a study‐specific template (SST), which was then registered to the NTU‐DSI‐122 template. Sampling coordinates of the pre‐defined 74 major WM tracts (as specified in Supporting Information Table S1) were transformed from the NTU‐DSI‐122 template to individual DSI via the transformation between the NTU‐DSI‐122 and SST, and the transformation between SST and individual DSI. GFA values were sampled in native DSI space using the transformed sampling coordinates from the NTU‐DSI‐122. In this study, the mean GFA value was calculated for each tract bundle in each participant.
Figure 1.
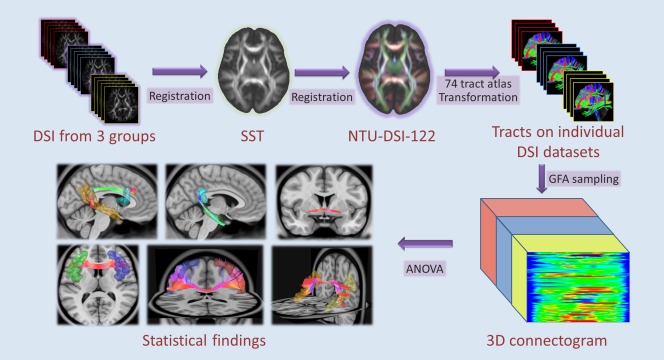
The analysis workflow of template‐based approach. Abbreviations: DSI, diffusion spectrum imaging; SST, study‐specific template; GFA, generalized fractional anisotropy. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Statistical Analyses
Data analysis was conducted by using SAS 9.2 version (SAS Institute, Cary, NC). The alpha value was preselected at the level of P < 0.05. The descriptive results were displayed as mean and SD for the continuous variables. The analyses of variance (ANOVAs) were used for continuous variables to compare the clinical measures and the mean GFA values of the whole‐brain WM tracts among the ASD, ADHD, and TD groups. To control for inflation of Type I error in multiple tests in 74 fiber tracts, a false discovery rate (FDR, q) correction was set at q < 0.05. To further discriminate the differences among the three study groups, post hoc pairwise analysis with Scheffé test was used. Pearson correlation (r) was used to correlate the autistic traits and microstructural property of the identified WM tracts with significant group difference in the ASD group. Multiple tests in the correlation analyses were also adjusted with FDR correction. Because in‐scanner head motion has been shown to be an important confounder in analyses of diffusion, we also tested whether the significant group differences in microstructural integrity remained using the signal dropout count as an additional nuisance covariate in statistical analyses [Koldewyn et al., 2014; Yendiki et al., 2013].
RESULTS
Sample Characteristics
There were no significant differences in age and handedness among the ASD, ADHD, and control groups (Table 1). Men with ASD had lower IQ profiles than men with ADHD and controls, with no difference between the latter two groups. Compared with controls, men with ASD showed significantly higher autism spectrum symptoms, and men with ADHD demonstrated significantly higher ADHD symptoms as expected. We identified that the ASD group had significant higher levels of in‐scanner head motion, in terms of DSI signal dropout counts, as compared to the ADHD group (Table 1).
Microstructural Integrity of the WM Tracts
Among 74 WM tracts over the entire brain, ANOVA with FDR correction identified six tracts with significant group differences in GFA values (q‐value < 0.05) (Table 2 and Supporting Information Table S1). These six tracts included the right arcuate fasciculus, right cingulum hippocampal part, anterior commissure, and three callosal fibers (VLPFC part, precentral part, superior temporal part) (Fig. 2). The left arcuate fasciculus (q = 0.063) and left medial lemniscus (q = 0.063) exhibited trend‐level significance in group comparisons (Supporting Information Table S1). Post hoc pairwise comparisons revealed that men with ASD had significantly lower mean GFA values in the right arcuate fasciculus and three callosal fibers (VLPFC part, precentral part, superior temporal part) than men with ADHD and controls; and in the right hippocampal cingulum and anterior commissure than men with ADHD (Table 2). After controlling for head motion level according to the signal dropout count, the statistical significance of microstructural property among the three groups did not have considerable differences (Supporting Information Table S4). The only significant difference was that men with ASD had lower mean GFA values in the corpus callosum, precentral part, than men with ADHD, and controls in the Post hoc comparisons.
Table 2.
Comparisons of the mean GFA values of the WM tracts
| Post hoc analysisa | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ASD (n = 23) | ADHD (n = 32) | Control (n = 29) | Three group comparison | ASD versus ADHD | ASD versus Control | ADHD versus Control | |||
| Fiber tract | GFA value | GFA value | GFA value | Uncorrected P‐valueb | q‐valuec | P‐value | P‐value | P‐value | Directions |
| Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| Whole‐brain | 0.289 ± 0.006 | 0.291 ± 0.004 | 0.289 ± 0.005 | 0.233 | 0.231 | 0.856 | 0.469 | — | |
| Rt arcuate fasciculus | 0.267 ± 0.012 | 0.278 ± 0.014 | 0.280 ± 0.01 | 0.001 | 0.017 | 0.006 | 0.001 | 0.788 | ASD<ADHD,Control |
| Rt cingulum (hippocampus) | 0.229 ± 0.022 | 0.249 ± 0.018 | 0.242 ± 0.02 | 0.002 | 0.019 | 0.002 | 0.057 | 0.499 | ASD<ADHD |
| Anterior commissure | 0.154 ± 0.014 | 0.168 ± 0.015 | 0.160 ± 0.015 | 0.002 | 0.020 | 0.003 | 0.327 | 0.099 | ASD<ADHD |
| CC (VLPFC part) | 0.274 ± 0.023 | 0.297 ± 0.015 | 0.291 ± 0.018 | <0.001 | 0.003 | <0.001 | 0.005 | 0.547 | ASD<ADHD,Control |
| CC (precentral part) | 0.326 ± 0.023 | 0.342 ± 0.008 | 0.337 ± 0.014 | 0.001 | 0.015 | 0.001 | 0.026 | 0.539 | ASD<ADHD,Control |
| CC (superior temporal part) | 0.321 ± 0.012 | 0.332 ± 0.009 | 0.331 ± 0.013 | 0.001 | 0.017 | 0.001 | 0.009 | 0.842 | ASD<ADHD,Control |
Pairwise comparison by post hoc analysis with Scheffé Test.
Statistical comparison among three groups were tested by ANOVA.
Adjusted P‐value with false discovery rate correction for multiple comparisons.
Abbreviations: Rt, right; CC, corpus callosum; VLPFC, ventrolateral prefrontal cortex.
Figure 2.
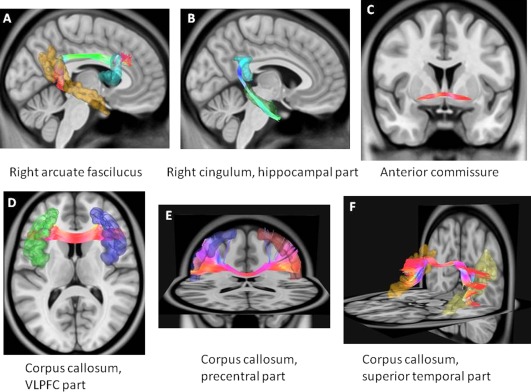
Reconstruction of the white matter tracts with significant differences between autism spectrum disorder and ADHD. These tracts include A) the right arcuate fasciculus, B) the right hippocampal cingulum, C) the anterior commissure, D) the corpus callosum, connecting bilateral ventrolateral prefrontal cortices, E) the corpus callosum connecting bilateral precentral gyri, and F) the corpus callosum connecting bilateral superior temporal parts. [Color figure can be viewed at http://wileyonlinelibrary.com.]
A wide range of cognitive abilities, from intellectual disability to superior intelligence, was also noted in our participants with ASD (full‐scale IQ 45‐130). Therefore, we conducted a supplementary analysis to minimize the effect of low IQ on the WM microstructural property in ASD. We excluded four patients with ASD whose full‐scale IQ was lower than 70, and identified the similar findings in terms of WM property. As shown in Supporting Information Table 2, men with high‐functioning ASD (n = 19) still had significantly lower GFA in all these six WM tracts than men with ADHD (P < 0.05). But when compared to controls, they only showed significantly lower GFA in the right arcuate fasciculus and two callosal fibers (VLPFC part and superior temporal part).
To account for the possible medication effects on WM property, we conducted another supplementary analysis excluding 14 participants with ADHD who had taken methylphenidate (Supporting Information Table 3). The results were similar with those shown in Table 2. Drug‐naive men with ADHD (n = 18) still had significantly higher mean GFA in all these six WM tracts than men with ASD (P < 0.05), and they did not show difference in the mean GFA values in these six WM tracts compared to controls.
Correlations with Clinical Measures in ASD
We correlated the GFA values of 6 identified tracts with autistic symptoms, assessed by self‐reports on the AQ‐Chinese, and parents' reports on the SRS‐Chinese in the ASD group. Although we did not find associations between the property of WM tracts and the total score of the AQ and the SRS, we did find significant associations with scores of some subscales evaluating social deficits. We found that the right arcuate fasciculus GFA value was negatively correlated with the mindreading subscale of the AQ‐Chinese (r = −0.595, q = 0.01, Fig. 3A), meaning that decreasing microstructural integrity of the right arcuate fasciculus was associated with increasing difficulty in understanding other's intentions or feelings; and the relationship between the right hippocampal cingulum and the socialness subscale in the AQ‐Chinese reached nominal significance (r = −0.469, uncorrected P = 0.032). Moreover, the right hippocampal cingulum exhibited significant negative correlations with the social awareness subscale of the SRS (r = −0.593, q = 0.013, see Fig. 3B). In general, the lower the mean GFA of these tracts exhibited, the more severe social deficits were shown.
Figure 3.
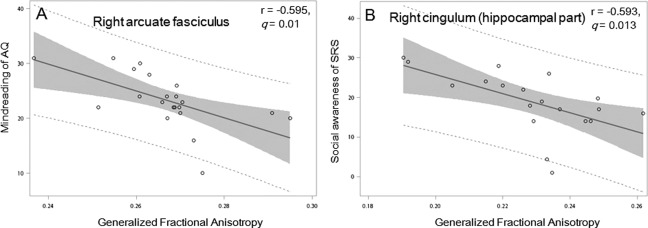
Correlation between the mean generalized fractional anisotropy values of the white matter tracts and social deficit and intelligence in men with autism spectrum disorder. We found negative associations between A) the right arcuate fasciculus GFA value and the mindreading subscale of the AQ‐Chinese, B) the right hippocampal cingulum and the socialness subscale in the AQ‐Chinese, and C) the right hippocampal cingulum and the social awareness subscale of the SRS. Abbreviations: AQ, the Chinese version of the Autism Spectrum Quotient; SRS, the Chinese version of the Social Responsiveness Scale.
DISCUSSION
To the best of our knowledge, this is the first study to directly compare the WM microstructural property of the whole‐brain fiber tracts in men with ASD and men with ADHD using DSI tractography. This study had some novel findings. First, men with ASD had distinctly lower mean GFA values than men with ADHD in the right arcuate fasciculus, right hippocampal cingulum, anterior commissure, and three callosal fibers (VLPFC part, precentral part, superior temporal part). Second, mean GFA values of the right arcuate fasciculus and right hippocampal cingulum were negatively associated with autistic social‐deficit symptoms.
In accordance with earlier literature [Brieber et al., 2007; Chantiluke et al., 2014; Christakou et al., 2013; Di Martino et al., 2013; Lim et al., 2015; Ray et al., 2014], our study examining whole brain fiber tracts microstructural property among ASD and ADHD provides evidence to support disease‐specific abnormalities in ASD, despite involvement of different brain regions. Inconsistency between our results and others with regards to specific brain regions associated with ASD‐specific abnormalities may result from heterogeneity on the spectrum [Lai et al., 2015], varied imaging modalities and methodologies, different sample sizes, and differences in age investigated [Ecker et al., 2015; Rommelse et al., 2011]. In contrast to previous functional and structural imaging studies focusing on children and adolescents [Brieber et al., 2007; Chantiluke et al., 2014; Christakou et al., 2013; Di Martino et al., 2013; Lim et al., 2015; Ray et al., 2014], this study is the first to examine diffusion imaging in adult male populations. Age appears to be a significant factor influencing the variability across imaging studies [Ecker et al., 2015; Rommelse et al., 2011]. Our approach of restricting sample to adults may better disentangle specific diagnostic effects, as the altered WM integrity during different neurodevelopmental stages among these two disorders diminish with maturation [Ecker et al., 2015]. Conversely, despite elusive conclusions, we should bear in mind that environment and experiences would moderate myelination, related to GFA values, in an inter‐individually variable way [Tymofiyeva et al., 2014]. This caveat may confound findings in WM property in adult cohort to some extent, warranting further investigation.
Our findings in the ASD group grossly concur with earlier DTI studies, which showed altered microstructural property in the arcuate fasciculus [Travers et al., 2012], corpus callosum [Onnink et al., 2015; Travers et al., 2015], and cingulum bundle [Ikuta et al., 2014; Travers et al., 2012] in ASD, and with our recent DSI study, which identified altered microstructural integrity in the corpus callosum in adolescents with ASD [Lo et al., 2011]. Such decreased corpus callosal fractional anisotropy (FA) values, assessed by DTI, persists into adulthood [Travers et al., 2015]. Regarding the sub‐parts of the corpus callosum, the VLPFC is responsible for top‐down attentional and inhibitory control, and the VLPFC part of the corpus callosum may facilitate integration of processing verbal and visual stimuli [Levy and Wagner, 2011]. The precentral gyrus is implicated in planning and executing movements [Mahajan et al., 2016]. The superior temporal part of the corpus callosum may be important in integrating auditory language comprehension involving the left hemisphere, and processing emotional information from the face and voice relying on the right superior temporal sulcus [Watson et al., 2014]. Moreover, the VLPFC and the superior temporal sub‐parts of the corpus callosum may interconnect the key regions within the mirror neuron system, which is hypothesized as one of core systems involved in ASD [Perkins et al., 2015]. Communication between bilateral hemispheres in these regions via corpus callosum may help to integrate verbal and visual information and facilitate top‐down control in ASD [Levy and Wagner, 2011; Watson et al., 2014]. Overall, our findings regarding the corpus callosum could further support inter‐hemispheric dysconnectivity in ASD [Travers et al., 2012].
Only a few studies have explored relationships between alterations in the WM and clinical and neuropsychological performances in patients with ASD [Ikuta et al., 2014], limiting our understanding on the clinical significance of alterations in the WM tracts. The finding of reducing the right arcuate fasciculus GFA values in men with ASD is consistent with the earlier literature which shows aberrant connectivity of the arcuate fasciculus in children [Poustka et al., 2012] and adolescents [Fletcher et al., 2010] with ASD. In contrast, young children with ASD showed widespread increase of FA in major WM tracts, including the arcuate fasciculus [Billeci et al., 2012]. This is possibly explained by age range, different fiber tracking algorithms or different metric definition of diffusion anisotropy between DSI and DTI. Lewis et al. suggested that impaired microstructure in the arcuate fasciculus of the patients with tuberous sclerosis complex may indicate an increased risk of ASD, because their DTI study revealed that tuberous sclerosis complex patients with ASD had lower FA than those without ASD [Lewis et al., 2013]. Although the left arcuate fasciculus is well known to be responsible for language function [Catani and Mesulam, 2008], the right hemisphere is better able to process speech with emotional prosody [Godfrey and Grimshaw, 2015]. FA of the right arcuate fasciculus has been reported to be negatively correlated with the severity of communication symptoms in children with ASD [Poustka et al., 2012], and negatively associated with the pitch discrimination and pitch‐related learning abilities in healthy adults [Loui et al., 2011]. Our result of the associations between the right arcuate fasciculus and the ability to understand others' intentions/feelings is consistent with the previous literature and may contribute to new clinical implications.
Regarding the hippocampal cingulum, the microstructural WM property is suggested to be related to the ability of behavioral regulation in adolescents and young adults with ASD [Ikuta et al., 2014], affective flattening in patients with schizophrenia [Whitford et al., 2014], and visual memory in patients with early Alzheimer's disease [Lin et al., 2014b]. Our work provides new evidence to suggest the relationship between the microstructural property of the hippocampal cingulum and social awareness/social skills in ASD. This may partially correspond with behavioral and emotional regulation in previous studies [Ikuta et al., 2014].
Beyond our anticipation, the present study only identified disorder‐specific abnormalities in the WM microstructural property in ASD, but neither ADHD‐specific nor common atypicality was found. The negative finding is inconsistent not only with studies comparing ADHD and ASD [Brieber et al., 2007; Chantiluke et al., 2014; Christakou et al., 2013; Di Martino et al., 2013; Lim et al., 2015; Ray et al., 2014] but also with abnormality in the WM tracts reported in studies comparing only ADHD and controls [Onnink et al., 2015]. However, much less investigation has been conducted on adults with ADHD, compared to child population [Onnink et al., 2015]. An earlier meta‐analysis suggests that adults with ADHD have less extensive regions of brain structural abnormality than ADHD children [Frodl and Skokauskas, 2012]. A recent DTI study also reported the lack of significant regional WM differences, in terms of the FA values, in both children and adults with ADHD [Yoncheva et al., 2016], supporting the present negative results. Other explanations of this negative finding may involve sex/gender impacts [Villemonteix et al., 2015], in‐scanner head motion [Koldewyn et al., 2014], sample size, and medication effects [Lin et al., 2014a]. We boldly speculate that the inconsistency may partly arise from the sex/gender‐based brain differences in ADHD [Fan et al., in prep; Villemonteix et al., 2015]. Sex/gender‐specific differences in grey matter volume [Villemonteix et al., 2015] and surface area [Dirlikov et al., 2015] have been reported between boys and girls with ADHD. Our recent DSI study [Fan et al., in prep, in preparation] examining sex/gender differences in the WM tracts in adults with ADHD showed that as compared to the same‐gender healthy adults, women with ADHD, relative to men with ADHD, had more diffuse WM tracts showing altered microstructural property. These female‐specific differences also overlapped with the findings from comparisons between men and women with ADHD [Fan et al., in prep], suggesting less marked alterations in the WM property in men with ADHD. Moreover, recruitment of pure male participants in the present study may also reduce sensitivity to detect brain differences in adults with ADHD. This unique feature of the participants might also in part explain the distinct findings from previous studies [Onnink et al., 2015; Ray et al., 2014]. Additionally, varied levels of in‐scanner head motion may also partly contribute to conflicting results across studies, as motion would introduce spurious group differences [Koldewyn et al., 2014; Yendiki et al., 2013], and worryingly impact the interpretations of earlier literature without reporting motion extents. Following the published method [Chen et al., 2015; Hsu et al., 2015], we visually scrutinized every axial slice in DSI datasets to ensure restricted in‐scanner head motion in each participant in the present study. Moreover, our sample size is smaller than some of large‐scale studies [Onnink et al., 2015], probably resulting in insufficient statistical power to detect significant difference after correcting for multiple comparisons in the ADHD group. Additionally, although there has been no study reporting the effect of ADHD medications on the development and WM tracts in human, the potential effect of ADHD medications on the development and WM tracts needed to be addressed, despite our findings of similar patterns in those treatment‐naïve participants with ADHD. Replications of the present findings await future studies accounting for these varied issues in methodology.
LIMITATIONS
Despite the strengths of using DSI tractography analysis to comprehensively investigate whole‐brain fiber tracts [Chen et al., 2015; Hsu et al., 2015], a relatively larger sample size than most of previous investigations [Brieber et al., 2007; Chantiluke et al., 2014; Christakou et al., 2013; Ray et al., 2014], as well as adult population with pure male participants and narrow age range to minimize the confounding effects of sex and age, the present study still had two methodological limitations. First, sample restriction to all male adults enhances the homogeneity but limits the generalizability of our results to child and adolescent patients and females with the disorders. Second, the ASD group had a wide range of intellectual functions. Our clinical reports revealed that ASD participants with low intelligence had acceptable adaptive functioning in daily life, consistent with the speculation that autism spectrum intelligence is distinctly uneven and easily under‐estimated [Dawson et al., 2007]. Lastly, a lack of data on assessments of co‐occurring symptoms of ASD or ADHD (i.e., no ADHD symptoms severity assessed in the ASD group, and no autistic level assessed in the ADHD group), alongside a lack of samples with co‐occurring ASD and ADHD limit the clinical implication for currently‐investigated brain‐behavior relationships. Also, this unavailability of dimensional behavioral data, together with no inclusion of participants with co‐occurring ASD and ADHD, limit complete delineation of overlapping neural profiles of these two disorders. This should be judiciously accounted for in the future study.
CONCLUSIONS
This study provides strong evidence to support disorder‐specific alterations of the WM microstructure in men with ASD. Clinical implications for these disorder‐specific alterations are further endorsed by brain‐behavior associations. To further advance understanding of disorder‐specific and common biomarkers, we suggest future studies using a longitudinal study design, multi‐modal imaging methodology, a wide range of age groups, and the four group comparison (adding the comorbid ASD and ADHD group, and assessing comorbid symptoms of ASD and ADHD in all participants).
Supporting information
Supporting Information 1
Supporting Information 2
ACKNOWLEDGMENT
All the authors report no financial relationships with commercial interests.
http://ClinicalTrials.gov number, NCT01582256, NCT01247610.
REFERENCES
- American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association; [Google Scholar]
- Baron‐Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E (2001): The autism‐spectrum quotient (AQ): Evidence from Asperger syndrome/high‐functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 31:5–17. [DOI] [PubMed] [Google Scholar]
- Billeci L, Calderoni S, Tosetti M, Catani M, Muratori F (2012): White matter connectivity in children with autism spectrum disorders: A tract‐based spatial statistics study. BMC Neurol 12:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieber S, Neufang S, Bruning N, Kamp‐Becker I, Remschmidt H, Herpertz‐Dahlmann B, Fink GR, Konrad K (2007): Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry 48:1251–1258. [DOI] [PubMed] [Google Scholar]
- Callaghan PT, Coy A, Macgowan D, Packer KJ, Zelaya FO (1991): Diffraction‐like effects in {NMR} diffusion studies of fluids in porous solids. Nature 351:467–469. [Google Scholar]
- Catani M, Mesulam M (2008): The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 44:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LR, Chiu YN, Wu YY, Gau SS (2013): Father's parenting and father‐child relationship among children and adolescents with attention‐deficit/hyperactivity disorder. Compr Psychiatry 54:128–140. [DOI] [PubMed] [Google Scholar]
- Chantiluke K, Christakou A, Murphy CM, Giampietro V, Daly EM, Ecker C, Brammer M, Murphy DG, Consortium MA, Rubia K (2014): Disorder‐specific functional abnormalities during temporal discounting in youth with Attention Deficit Hyperactivity Disorder (ADHD), Autism and comorbid ADHD and Autism. Psychiatry Res 223:113–120. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Lo YC, Hsu YC, Fan CC, Hwang TJ, Liu CM, Chien YL, Hsieh MH, Liu CC, Hwu HG, Tseng WY (2015): Automatic whole brain tract‐based analysis using predefined tracts in a diffusion spectrum imaging template and an accurate registration strategy. Hum Brain Mapp 36:3441–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang HL, Chen YJ, Lo YC, Tseng WY, Gau SS (2015): Altered white matter tract property related to impaired focused attention, sustained attention, cognitive impulsivity and vigilance in attention‐deficit/hyperactivity disorder. J Psychiatry Neurosci 40:140106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Murphy CM, Chantiluke K, Cubillo AI, Smith AB, Giampietro V, Daly E, Ecker C, Robertson D, MRC AIMS consortium , Murphy DG, Rubia K (2013): Disorder‐specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with autism. Mol Psychiatry 18:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Sparrow E ( 1999): Conners' Adult ADHD Rating Scales (CAARS). New York: MHS. [Google Scholar]
- Dawson M, Soulieres I, Gernsbacher MA, Mottron L (2007): The level and nature of autistic intelligence. Psychol Sci 18:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Zuo XN, Kelly C, Grzadzinski R, Mennes M, Schvarcz A, Rodman J, Lord C, Castellanos FX, Milham MP (2013): Shared and distinct intrinsic functional network centrality in autism and attention‐deficit/hyperactivity disorder. Biol Psychiatry 74:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirlikov B, Shiels Rosch K, Crocetti D, Denckla MB, Mahone EM, Mostofsky SH (2015): Distinct frontal lobe morphology in girls and boys with ADHD. Neuroimage Clin 7:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Bookheimer SY, Murphy DG (2015): Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. Lancet Neurol 14:1121–1134. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, Alexander AL, Bigler ED, Lange N, Lainhart JE (2010): Microstructural connectivity of the arcuate fasciculus in adolescents with high‐functioning autism. Neuroimage 51:1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Skokauskas N (2012): Meta‐analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand 125:114–126. [DOI] [PubMed] [Google Scholar]
- Gau SS, Chong MY, Chen TH, Cheng AT (2005): A 3‐year panel study of mental disorders among adolescents in Taiwan. Am J Psychiatry 162:1344–1350. [DOI] [PubMed] [Google Scholar]
- Gau SS, Lin YJ, Cheng AT, Chiu YN, Tsai WC, Soong WT (2010): Psychopathology and symptom remission at adolescence among children with attention‐deficit/hyperactivity disorder in Taiwan. Aust N Z J Psychiatry 44:323–332. [DOI] [PubMed] [Google Scholar]
- Gau SSF, Lee CM, Lai MC, Chiu YN, Huang YF, Kao JD, Wu YY (2011): Psychometric properties of the Chinese version of the Social Communication Questionnaire. Res Autism Spectr Disord 5:809–818. [Google Scholar]
- Gau SS, Liu LT, Wu YY, Chiu YN, Tsai WC (2013): Psychometric properties of the Chinese version of the social responsiveness scale. Res. Autism Spectr Disord 7:349–360. [Google Scholar]
- Godfrey HK, Grimshaw GM (2015): Emotional language is all right: Emotional prosody reduces hemispheric asymmetry for linguistic processing. Laterality 1–17. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Lo YC, Chen YJ, Wedeen VJ, Isaac Tseng WY (2015): NTU‐DSI‐122: A diffusion spectrum imaging template with high anatomical matching to the ICBM‐152 space. Hum Brain Mapp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T, Shafritz KM, Bregman J, Peters BD, Gruner P, Malhotra AK, Szeszko PR (2014): Abnormal cingulum bundle development in autism: A probabilistic tractography study. Psychiatry Res 221:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H, Behrens TEJ ( 2014): Diffusion MRI: From Quantitative Measurement to In‐Vivo Neuroanatomy, Vol. xii London, UK; Waltham, MA: Elsevier/Academic Press; 614 p. [Google Scholar]
- Koldewyn K, Yendiki A, Weigelt S, Gweon H, Julian J, Richardson H, Malloy C, Saxe R, Fischl B, Kanwisher N (2014): Differences in the right inferior longitudinal fasciculus but no general disruption of white matter tracts in children with autism spectrum disorder. Proc Natl Acad Sci USA 111:1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron‐Cohen S (2015): Sex/gender differences and autism: Setting the scene for future research. J Am Acad Child Adolesc Psychiatry 54:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WY, Gau SS, Chiu YN, Wu YY, Chou WJ, Liu SK, Chou MC (2013): Psychometric properties of the Chinese version of the Autism Spectrum Quotient (AQ). Res Dev Disabil 34:294–305. [DOI] [PubMed] [Google Scholar]
- Leitner Y (2014): The co‐occurrence of autism and attention deficit hyperactivity disorder in children—What do we know? Front Hum Neurosci 8:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD (2011): Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci 1224:40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis WW, Sahin M, Scherrer B, Peters JM, Suarez RO, Vogel‐Farley VK, Jeste SS, Gregas MC, Prabhu SP, Nelson CA, III , Warfield SK (2013): Impaired language pathways in tuberous sclerosis complex patients with autism spectrum disorders. Cereb Cortex 23:1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Chantiluke K, Cubillo AI, Smith AB, Simmons A, Mehta MA, Rubia K (2015): Disorder‐specific grey matter deficits in attention deficit hyperactivity disorder relative to autism spectrum disorder. Psychol Med 45:965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Gau SS, Huang‐Gu SL, Shang CY, Wu YH, Tseng WY (2014a): Neural substrates of behavioral variability in attention deficit hyperactivity disorder: Based on ex‐Gaussian reaction time distribution and diffusion spectrum imaging tractography. Psychol Med 44:1751–1764. [DOI] [PubMed] [Google Scholar]
- Lin YC, Shih YC, Tseng WY, Chu YH, Wu MT, Chen TF, Tang PF, Chiu MJ (2014b): Cingulum correlates of cognitive functions in patients with mild cognitive impairment and early Alzheimer's disease: A diffusion spectrum imaging study. Brain Topogr 27:393–402. [DOI] [PubMed] [Google Scholar]
- Lin HY, Ni HC, Lai MC, Tseng WY, Gau SS (2015a): Regional brain volume differences between males with and without autism spectrum disorder are highly age‐dependent. Mol Autism 6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Lo KW, Yang LK, Gau SS (2015b): Validation of DSM‐5 age‐of‐onset criterion of attention deficit/hyperactivity disorder (ADHD): Comparison of life quality, social impairment, and family function. Res Dev Disabil 47:48–60. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Yang LK, Gau SS (2016): Psychiatric comorbidities of adults with early‐ and late‐onset attention‐deficit/hyperactivity disorder. Aust N Z J Psychiatry 50:548–556. [DOI] [PubMed] [Google Scholar]
- Lo YC, Soong WT, Gau SS, Wu YY, Lai MC, Yeh FC, Chiang WY, Kuo LW, Jaw FS, Tseng WY (2011): The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: A study using diffusion spectrum imaging tractography. Psychiatry Res 192:60–66. [DOI] [PubMed] [Google Scholar]
- Loui P, Li HC, Schlaug G (2011): White matter integrity in right hemisphere predicts pitch‐related grammar learning. Neuroimage 55:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan R, Dirlikov B, Crocetti D, Mostofsky SH (2016): Motor circuit anatomy in children with autism spectrum disorder with or without attention deficit hyperactivity disorder. Autism Res 9:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Hawkey E, Kachan‐Liu SS, Lees P, Roullet JB, Goddard K, Steiner RD, Nigg JT (2014): Shared familial transmission of autism spectrum and attention‐deficit/hyperactivity disorders. J Child Psychol Psychiatry 55:819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HC, Shang CY, Gau SS, Lin YJ, Huang HC, Yang LK (2013): A head‐to‐head randomized clinical trial of methylphenidate and atomoxetine treatment for executive function in adults with attention‐deficit hyperactivity disorder. Int J Neuropsychopharmacol 16:1959–1973. [DOI] [PubMed] [Google Scholar]
- Onnink AM, Zwiers MP, Hoogman M, Mostert JC, Dammers J, Kan CC, Vasquez AA, Schene AH, Buitelaar J, Franke B (2015): Deviant white matter structure in adults with attention‐deficit/hyperactivity disorder points to aberrant myelination and affects neuropsychological performance. Prog Neuropsychopharmacol Biol Psychiatry 63:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins TJ, Bittar RG, McGillivray JA, Cox II, Stokes MA (2015): Increased premotor cortex activation in high functioning autism during action observation. J Clin Neurosci 22:664–669. [DOI] [PubMed] [Google Scholar]
- Poustka L, Jennen‐Steinmetz C, Henze R, Vomstein K, Haffner J, Sieltjes B (2012): Fronto‐temporal disconnectivity and symptom severity in children with autism spectrum disorder. World J Biol Psychiatry 13:269–280. [DOI] [PubMed] [Google Scholar]
- Ray S, Miller M, Karalunas S, Robertson C, Grayson DS, Cary RP, Hawkey E, Painter JG, Kriz D, Fombonne E, Nigg JT, Fair DA(2014): Structural and functional connectivity of the human brain in autism spectrum disorders and attention‐deficit/hyperactivity disorder: A rich club‐organization study. Hum Brain Mapp 35:6032–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ (2003): Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magn Reson Med 49:177–182. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Geurts HM, Franke B, Buitelaar JK, Hartman CA (2011): A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention‐deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci Biobehav Rev 35:1363–1396. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL (2007): Attention‐deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA 104:19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp do PM, Destiche D, Doran S, Bigler ED, Lange N, Lainhart JE, Alexander AL (2012): Diffusion tensor imaging in autism spectrum disorder: A review. Autism Res 5:289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Tromp do PM, Adluru N, Lange N, Destiche D, Ennis C, Nielsen JA, Froehlich AL, Prigge MB, Fletcher PT, Anderson JS, Zielinski BA, Bigler ED, Lainhart JE, Alexander AL (2015): Atypical development of white matter microstructure of the corpus callosum in males with autism: A longitudinal investigation. Mol Autism 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS (2004): Q‐ball imaging. Magn Reson Med 52:1358–1372. [DOI] [PubMed] [Google Scholar]
- Tymofiyeva O, Hess CP, Xu D, Barkovich AJ (2014): Structural MRI connectome in development: Challenges of the changing brain. Br J Radiol 87:20140086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemonteix T, De Brito SA, Slama H, Kavec M, Baleriaux D, Metens T, Baijot S, Mary A, Peigneux P, Massat I (2015): Grey matter volume differences associated with gender in children with attention‐deficit/hyperactivity disorder: A voxel‐based morphometry study. Dev Cogn Neurosci 14:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R, Latinus M, Noguchi T, Garrod O, Crabbe F, Belin P (2014): Crossmodal adaptation in right posterior superior temporal sulcus during face‐voice emotional integration. J Neurosci 34:6813–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D ( 1981): Manual for the Wechsler Adult Intelligence Scale—Revised. New York: Psychological Corporation. [Google Scholar]
- Whitford TJ, Lee SW, Oh JS, de Luis‐Garcia R, Savadjiev P, Alvarado JL, Westin CF, Niznikiewicz M, Nestor PG, McCarley RW, Kubicki M, Shenton ME (2014): Localized abnormalities in the cingulum bundle in patients with schizophrenia: A Diffusion Tensor tractography study. Neuroimage Clin 5:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B (2013): Spurious group differences due to head motion in a diffusion MRI study. Neuroimage 88C:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoncheva YN, Somandepalli K, Reiss PT, Kelly C, Di Martino A, Lazar M, Zhou J, Milham MP, Castellanos FX (2016): Mode of Anisotropy Reveals Global Diffusion Alterations in Attention‐Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry 55:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Supporting Information 2


