Abstract
This study investigated interactive exchange in lovers and the associated interpersonal brain synchronization (IBS) using functional near‐infrared spectroscopy (fNIRS)‐based hyperscanning. Three types of female‐male dyads, lovers, friends, and strangers, performed a cooperation task during which brain activity was recorded in right frontoparietal regions. We measured better cooperative behavior in lover dyads compared with friend and stranger dyads. Lover dyads demonstrated increased IBS in right superior frontal cortex, which also covaried with their task performance. Granger causality analyses in lover dyads revealed stronger directional synchronization from females to males than from males to females, suggesting different roles for females and males during cooperation. Our study refines the theoretical explanation of romantic interaction between lovers. Hum Brain Mapp 38:831–841, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: cooperation, lovers, interpersonal brain synchronization, fNIRS, hyperscanning
INTRODUCTION
Humans have a natural inclination to form bonds with others, which is more evidenced for romantic relationships (Acevedo et al., 2012; Fletcher et al., 2015). In this type of relationship, people of both sexes have a very large amount of social interactions, such as sharing positive life events and receiving quality feedback (Gable et al., 2003, 2004). Their interactions, with the same goals in most cases (Li et al., 2002; Li and Kenrick, 2006), set the stage for some degree of overlap in their behaviors. In the current study, we investigate how lovers interact with each other and any associcated synchronous brain activity between them.
According to the theory of attachment, individuals develop mental representations of their relations with parents, peers, lovers and so on (Furman et al., 2002, 2014). These representations are not fully congruent with each other. Romantic relationships, compared to other relationships, involve the integration of sexual, caregiving and affiliative behavioral systems (Furman et al., 2014). These behavioral systems provide rich opportunities for lovers to cooperate (Ackerman and Kenrick, 2009; Schneiderman et al., 2012), examples include mutual touch (Gallace and Spence, 2010), constructive problem‐solving (Assad et al., 2007), and emotion coordination (Randall et al., 2013). Neurobiological studies have even shown that when participants view pictures of a loved one, their oxytocin levels surged (de Boer et al., 2012; Zeki, 2007). Oxytocin is a hormone that plays a pivotal role in trust, empathy, and cooperation (Kosfeld et al., 2005; Schneiderman et al., 2012). These findings are suggestive of the cooperative nature of romantic relationships.
Gender differences in cooperation between lovers can be explained by established theories from psychology and evolutionary biology. The self‐construal theory suggests that females are interdependent and males are independent (Cross and Madson, 1997). Females are more sharing, affiliative, and emotionally supportive than males. They display greater levels of prosociality, such as empathy, selflessness, and positive emotions (e.g., Cross and Madson, 1997; Eckel and Grossman, 1998; Zakriski et al., 2005). It seems that, in general, females are more romantically submissive to males. However, there might be other possibilities. From an evolutionary biology perspective, females expend more physical and temporal resources in pregnancy and offspring care compared to males. The female tends to be relatively choosy regarding acceptable qualities in potential mates and to be actively restricted in their access to the opposite sex partners (Buss and Schmitt, 1993; Kenrick et al., 1990; Li et al., 2002; Perilloux et al., 2008). Males, on the other hand, may use more flexible tactics (e.g., flaunting) to demonstrate acceptable mate‐value (Geary et al., 2004; Griskevicius et al., 2006) and thus might be more capable of humoring females.
We asked two partners in a lover dyad to perform a cooperative task (Baker et al., 2016; Cheng et al., 2015; Cui et al., 2012). The lover dyad tried their best to simultaneously make quick responses to a stimulus. The quality of their performance was defined by the difference and variability between their response times. A small difference and less variability implied better cooperation (Funane et al., 2011; Vesper et al., 2011). During the task, we simultaneiously recorded brain activities from both partners, which is known as the “hyperscannning.” Hyperscanning can be used with fMRI (e.g., Chiu et al., 2008; Li et al., 2009), EEG (e.g., Lindenberger et al., 2009), and fNIRS (e.g., Cheng et al., 2015; Jiang et al., 2015; Tang et al., 2016). Previous studies using the hyperscanning technique during studies of cooperation found interpersonal brain synchronization (IBS) in right centroparietal regions (Dumas et al., 2010) and prefrontal cortex (McCabe et al., 2001).
Hyperscanning has been used to investigate neural links between lovers. Specifically, an fMRI study found that affective facial communication in romantic partners elicited activations in the same cerebral networks, such as anterior temporal, insular, and somato‐motor brain regions (Anders et al., 2011). A recent EEG study found that romantic kissing in lovers induced intra‐ and inter‐ brain synchronization in the theta‐alpha band (Müller and Lindenberger, 2014). We adopted the fNIRS‐based hyperscanning technique. The fNIRS device is more tolerant of motor artifacts and acheives temporal and spatial resolutions that are comparable to fMRI and EEG. A single fNIRS device can be divided for hyperscanning experiments by using half of the channels for each participant (Cheng et al., 2015; Cui et al., 2012). Therefore, the fNIRS set‐up can resolve the issue of variable sensitivities from different devices.
In sum, this study explored interactive exchange between lovers through fNIRS‐based hyperscanning. Based on the more interactive experiences and the cooperative nature of romantic relationships, we expected that lover dyads would outperform friend and stranger dyads on the cooperative task. Also, we anticipated that better performance would be accompanied by increased IBS between the two partners. Previous studies have found that right frontoparietal cortex is associated with cooperation and reciprocal interactions (Decety et al., 2004). Moreover, a right‐lateralized frontoparietal mirror‐neuron network was shown to be involved with social understanding (e.g., understand the emotions and actions of others, Gallese et al., 2004; Iacoboni et al., 2005) and was considered as the basis for bridging the self‐other gap during interaction (Uddin et al., 2007). Based on these studies, we defined the region‐of‐interest for the present study to be right frontoparietal area. Meanwhile, we examined gender differences at the level of behavior and brain activity, with the goal of providing evidence for either self‐construal theory or the evolutionary biological theory of heterosextual cooperation.
METHOD
Participants
Ninety‐eight adults (49 dyads, age: 21.07 ± 1.85 yrs) participated in the study. They were all male–female dyads, which included 17 lover dyads, 16 friend dyads, and 16 stranger dyads. Lover dyads as well as friend dyads had been acquainted before the experiment, and these two types of dyads did not differ in duration of acquaintance (21.01 ± 5.08 vs. 15.75 ± 3.41 months, t (31) = 1.23, P = 0.23, Cohen's d = 0.32). The lover dyads reported that they were intensely in love (duration of “being in love”: M = 15.15 months, range = 2–36 months). Each participant signed informed consent before the experiment and was paid ¥ 20 for participation. The University Committee on Human Research Protection of East China Normal University approved study procedure.
Tasks and Procedures
Two participants in a dyad sat side‐by‐side, separated by a board and in front of a shared computer display (Fig. 1A). The procedure consisted of Rest 1 (30 s), Task Block 1 (∼150 s), Rest 2 (30 s), Task Block 2 (∼150 s), and Rest 3 (30 s) (Fig. 1B). For each task block, participants needed to complete 20 trials. Within each trial (Fig. 1C), a hollow gray circle (0.6 – 1.5 s) was presented first, followed by a green signal. Upon seeing the signal, participants used their right index fingers to press the key simultaneously. The participant on the left (participant #1) used the “1” key on a keyboard, and the participant on the right (participant #2) used the “2” key on the other keyboard.
Figure 1.
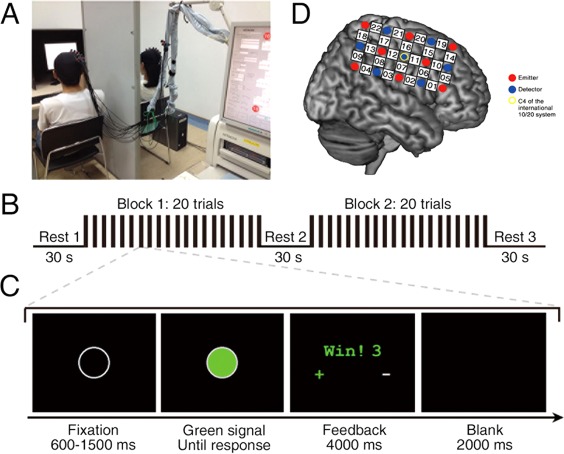
Experimental design. (A) Experimental setup. (B) Task design. There were two task blocks, each consisting of 20 trials. (C) Trial design. Events and time flow in a trial. (D) Optode probe set. The set was placed on the right frontoparietal cortices. [Color figure can be viewed at http://wileyonlinelibrary.com]
To maintain participants' attention and increase cooperative behaviors, we set a threshold (T) for a trial for which they would get a winning point:
where RT1 and RT2 were the response times of participant #1 and participant #2, respectively. The parameter 1/8 was chosen to maintain a reasonable difficulty level (Baker et al., 2016; Cheng et al., 2015; Cui et al., 2012). For each dyad, if the time difference was smaller than their threshold, both won one point; otherwise, they lost one point.
After key‐pressings, a feedback screen (4 s) appeared. The feedback consisted of the following information: (1) win or lose; (2) cumulative points; (3) who was faster (“+”) or slower (“−”). This information aided participants in adjusting their responses to maximize points. No verbal or physical communication was allowed. After the feedback, there was a blank screen (i.e., the intertrial interval) lasting 2 s.
Subjective Measurements
We collected each participant's attitudes towards her/his partner and the experiment, these information included: (1) trustworthiness for others in their daily life (pre‐experiment); (2) satisfaction for her/his own performance (post‐experiment); (3) cooperativeness during the cooperation (post‐experiment); (4) pleasantness while cooperating (post‐experiment); (5) concentration during tasks (post‐experiment); (6) favorability for the partner (pre‐ and post‐experiment). Participants used a 7‐point Likert scale, which ranged from 1 (“not very much”) to 7 (“very much”), for all ratings. No discussion was allowed while rating.
Data Acquisition
An ETG‐4000 optical topography system (Hitachi Medical Corporation, Japan) was used to record the oxyhemoglobin (HbO) and deoxyhemoglobin (HbR) concentrations for each dyad. The absorption of near infrared light (wavelengths: 695 and 830 nm) was measured at a sampling rate of 10 Hz. The optode probe set was placed over participants’ right frontoparietal regions (Fig. 1D), based on previous work showing cooperative exchange involved right frontal and parietal regions (Decety et al., 2004).
Two 3 × 5 optode probe sets (eight emitters and seven detectors, 3 cm optode separation) were used. Each set consists of 22 measurement channels (CHs). The center of the middle probe set row was placed at C4, according to the 10/20 international system. The middle probe set column was placed along the sagittal reference curve. The virtual registration method was used to determine the correspondence between the NIRS CHs and the measured points on the cerebral cortex (Singh et al., 2005; Tsuzuki et al., 2007).
Data Analysis
Behavior performance
For each participant dyad, response times (RTs), and winning trials were recorded. Because there were some outliers in the data, the median and median absolute deviation of response times (median‐RT, mad‐RT) were calculated among all trials in each participant. Outliers were identified by calculating an interval spanning over the mean ± 3 SD. We also analyzed the median and median absolute deviation of the difference of response times (median‐DRT and mad‐DRT) between the two participants in a dyad, similar to procedures used in previous studies (Funane et al., 2011; Vesper et al., 2011). To quantify participant performance and index the quality of cooperation, we calculated the percentage of winning trials (PWT; Cheng et al., 2015; Cui et al., 2012).
Nonparametric Wilcoxon tests were used on the median‐RT, mad‐RT, median‐DRT, and mad‐RT, because the data were not normally distributed. The PWT was distributed normally, so we applied a two‐way ANOVA, with factors of Group (lover, friend, stranger) by Task Block (blocks 1, 2). Finally, we used a Bonferroni correction to account for multiple comparisons.
Interpersonal brain synchronization (IBS)
Because the HbO signal has been demonstrated to be most sensitive to changes in cerebral blood flow during fNIRS measurements (Cui et al., 2012; Hoshi, 2007), we focused on the HbO time series. However, HbR time series were also analyzed (see Supporting Information).
During preprocessing, the initial and ending periods (Rest 1 and Rest 3, respectively) of the task were removed. A line of evidence mentioned that the fNIRS can measure both global (i.e., systemic components which are not task‐specific activity) and cortical blood oxygen level dependent (BOLD) activities (Ferrari and Quaresima, 2012; Kirilina et al., 2012), Therefore, we first used a principal component spatial filter algorithm (i.e., PCA approach using Gaussian spatial filtering) to remove the global components (Zhang et al., 2016). Wavelet transform coherence (WTC) was then used to assess the relationship between HbO time series for each dyad (for more details see Grinsted et al., 2004). Based on WTC analyses of two time series generated by each participant dyad, we focused on the frequency band between 3.2 s and 12.8 s (i.e., 0.08 – 0.31 Hz) that was more sensitive to our task (Fig. 2). Specifically, this frequency band includes the period of the single trial (∼7 s) in the cooperation task indicating that this coherence increase is task‐related. The selection of frequency band was also in accordance with previous studies using the same paradigm (Baker et al., 2016; Cheng et al., 2015; Cui et al., 2012). Focusing on this frequency band enabled removal of high‐ and low‐frequency artificial noises, such as those related to cardiac pulsation (∼1 Hz).
Figure 2.
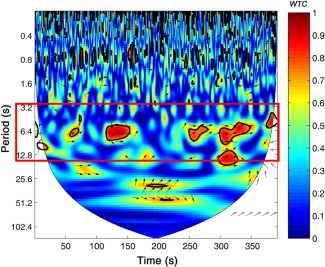
Frequency band of interest. Interpersonal brain synchronization (IBS) indicated by wavelet transform coherence (WTC). The coherence based on raw HbO signal from channel 20 (CH20) in a representative pair of lovers. The red border represents the frequency band of interest (3.2 s – 12.8 s), indicating when the task was carried out. The color bar denotes the value of WTC (1 = highest coherence, 0 = lowest coherence). [Color figure can be viewed at http://wileyonlinelibrary.com]
For each pair of CHs between two participants, the average cross‐brain coherence, or IBS, was calculated in task blocks and resting period. We defined IBS as the mean of coherence in two task blocks minus the coherence during the inter‐block rest period (Rest 2):
We converted IBS values to Fisher z‐statistics and then calculated a one‐sample t‐test with false discovery rate (FDR) correction across all CHs (P < 0.05). We generated a t‐map of IBS and smoothed it using the spline method. If a channel was found with significant IBS, a one‐way ANOVA was conducted on that IBS with the factor of Group. Bonferroni correction was used to account for post hoc multiple comparisons.
Directional coupling
To estimate the direction of synchronization for CHs that exhibited significant IBS, Granger causality analyses (GCA) were conducted (see more details in Barnett and Seth, 2014; Im et al., 2010). GCA uses vector autoregressive models to measure the causal relationship between time series in brain data. Our GCA was based on PCA‐corrected signals during the task periods. The pairwise‐conditional Granger‐causality (G‐causality) of both participant directions (i.e., from females to males; from males to females) was calculated. Then, one‐sample t‐tests were used to examine whether each direction differed from zero, and two‐sample t‐tests were used to compare differences between two directions.
Subjective measurements
A series of two‐way ANOVAs were conducted to estimate the effects of Group and Gender on participants’ ratings of their (1) trustworthiness, (2) satisfaction, (3) cooperativeness, (4) pleasantness, and (5) concentration. One three‐way ANOVA was carried out on partner favorability with the factors of Group, Gender, and Time (pre‐ vs. post‐experiment). We conducted follow‐up t‐tests with Bonferroni corrections when necessary.
RESULTS
Behavioral Performance
For individual behavioral data, Wilcoxon tests on median‐RT showed that lover dyads responded faster than stranger dyads (Bonferroni corrected, P < 0.05; see Supporting Information Table S1). Similar analyses on mad‐RT revealed that responses from lover and friend dyads were less variable than stranger dyads responses (Bonferroni corrected, Ps < 0.05; see Supporting Information Table S2). Additionally, we explored gender differences on median‐RT and mad‐RT between these three groups. Wilcoxon tests on median‐RT showed that males responded slower than females in the lover dyads (P < 0.05), but not in friend and stranger dyads (Ps > 0.05; see Supporting Information, Table S1). No other significant effect was found.
We tested for joint cooperation by using Wilcoxon tests on median‐DRT. These tests showed significant group differences between lover and friend dyads (P < 0.05), and between lover and stranger dyads (P < 0.05; Fig. 3A). These differences indicate that median‐DRT in lover dyads was smaller than friend and stranger dyads. Similar analyses on mad‐DRT confirmed that mad‐DRT in lover dyads was smaller than friend and stranger dyads (Ps < 0.05; Fig. 3B).
Figure 3.
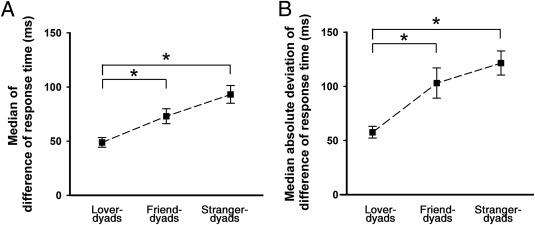
Cooperation performance. (A) The median of difference of response time (median‐DRT). (B) The median absolute deviation of difference of response time (mad‐DRT). * P < 0.05, after correction. Error bars represent standard errors.
The analysis of PWT (ANOVA with factors of Group and Task Block) yielded statistical significance only for Task Block (F (1,46) = 24.35, P < 0.001, = 0.35) with the higher PWT in block 2 among all groups (Ps < 0.05; see Supporting Information, Table S3 and Fig. S1). These findings confirm that all participants performed the cooperation task as instructed.
Interpersonal Brain Synchronization (IBS)
A series of one‐sample t‐test analyses of IBS in lover dyads found significant IBS at CH19 (t (16) = 2.40, P < 0.05, Cohen's d = 0.82) and CH20 (t (16) = 4.25, P < 0.001, Cohen's d = 1.46). However, only IBS at CH20 survived after FDR correction (P < 0.05; Fig. 4A). CH20 was roughly located in the right superior frontal cortex (r‐SFC; Tzourio‐Mazoyer et al., 2002). In contrast, no significant IBS was found in friend or stranger dyads. A one‐way ANOVA analysis demonstrated that IBS at CH20 was affected by Group, (F (2,46) = 3.91, P < 0.05, = 0.14). The post hoc tests revealed that IBS in lovers was significantly greater than that in friends and strangers (P < 0.05; Fig. 4B).
Figure 4.
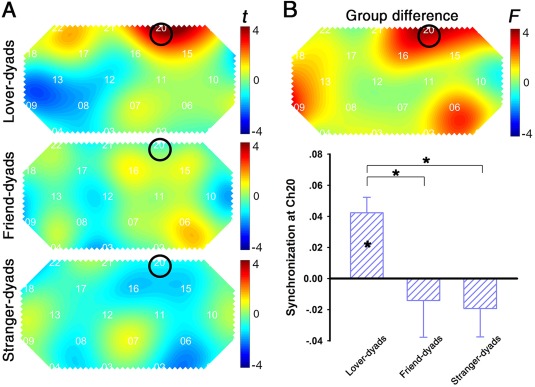
Interpersonal brain synchronization (IBS). (A) One‐sample t‐test map of IBS. (B) Upper graph: one‐way ANOVA results of the IBS to identify group differences. Lower graph: The amplitude of synchronization at channel 20 (CH20). Note that only in lover dyads, a significant IBS at CH20 was found after FDR correction. Synchronization in lover dyads is higher than that in other dyads. * P < 0.05, after correction. Error bars represent standard errors. [Color figure can be viewed at http://wileyonlinelibrary.com]
The IBS‐Behavior Relation
Pearson correlation analyses were conducted on the significant IBS at CH20 and behavior indices (e.g., DRT, PWT). IBS and median‐DRT were correlated (Fig. 5A, r = −0.50, P < 0.05), indicating that the IBS found in lover dyads was associated with their cooperative performance. However, similar correlations were not statistically significant in friends or strangers (Ps > 0.1).
Figure 5.
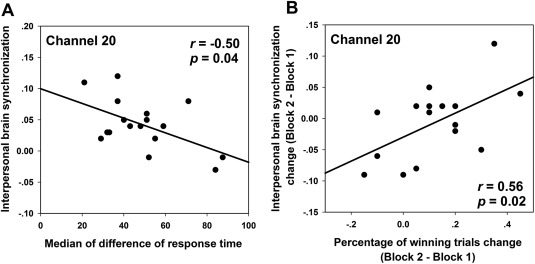
IBS‐behavior correlation in lover dyads. Correlations (A) between the IBS at CH20 and median‐DRT, (B) between IBS change at CH20 (IBSblock 2 – block 1) and percentage of winning trials change (PWTblock 2 – block 1).
We also calculated the change in IBS between task blocks by subtracting the coherence value in task block 1 from that in task block 2, IBSblock 2 – block 1, based on previous work (Cheng et al., 2015). The correlation between IBSblock 2 – block 1 and the increase of winning trials from block 1 to block 2, PWTblock 2 – block 1, was significant (Fig. 5B, r = 0.56, P < 0.05). In contrast, this relationship was not significant in friend and stranger dyads (Ps > 0.1).
Directional Coupling
To determine the direction of IBS at CH20, we conducted one‐sample t‐tests on the mean G‐causality. For lover dyads, both directions demonstrated significant increases in the mean G‐causality relative to zero: from females to males (t (16) = 7.02, P < 0.001, Cohen's d = 2.41) and from male to female (t (16) = 4.23, P < 0.001, Cohen's d = 1.45; Fig. 6A). A two‐sample t‐test revealed that mean G‐causality from females to males was significantly larger than from males to females (t (16) = 2.97, P < 0.01, Cohen's d = 0.91; Fig. 6B). In contrast, no significant G‐causality in either direction or difference between the two directions was found for the other dyads (Ps > 0.05).
Figure 6.
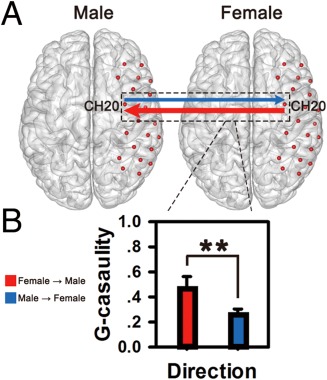
Directional coupling in lover dyads. (A) The G‐causality was analyzed on two directions (from males to females, from females to males). Significant G‐causality was indicated by the solid‐lines (thresholded at P < 0.001). (B) Mean G‐causalities in both directions were illustrated in the bar graphs. The mean causality from females to males (indicated by red color) was significantly higher than that from male to female (indicated by blue color). **P < 0.01. Error bars represent standard errors. [Color figure can be viewed at http://wileyonlinelibrary.com]
Subjective Measurements
The results of two‐way ANOVAs showed no significant effect of Group in evaluations of trustworthiness and concentration. However, the effect of Group was significant on self‐rated pleasantness (F (2,95) = 8.62, P < 0.001, = 0.08), satisfaction (F (2,95) = 5.71, P < 0.01, = 0.06), and cooperativeness (F (2,95) = 10.20, P < 0.001, = 0.10). Post hoc tests demonstrated that lover dyads felt more pleasant and more satisfied than stranger dyads did (Ps < 0.05), but not than the friend dyads did (Ps > 0.05). Lovers felt more cooperative than friend and stranger dyads (Ps < 0.05). To address the potential contribution of these subjective experiences to IBS, we examined whether the self‐rated pleasantness, satisfaction or cooperativeness correlated with IBS. None of these correlations survived (Ps > 0.1).
Finally, we conducted a three‐way ANOVA on partner favorability that showed effects of Time (F (1,95) = 46.29, P < 0.001, = 0.21) and an interaction between Time and Group (F (2,95) = 8.11, P < 0.001, = 0.08). Further analyses showed that friend and stranger dyads developed more favorable impressions of their partners from the beginning to the end of the experiment (Ps < 0.01), while lover dyads did not (P > 0.1). The descriptive statistics can be found in Supporting Information, Table S4.
DISCUSSION
To our knowledge, this is the first fNIRS‐based hyperscanning study exploring interactive exchange in lovers. Behavioral results showed that response time differences between lover dyads were smaller and less variable than friend and stranger dyads, indicating better cooperation among lovers. More importantly, fNIRS results showed interpersonal brain synchronization (IBS) only for lover dyads. The IBS obtained in lover dyads was also found to correlate with their task performance.
We found IBS in lover dyads using a task involving interactive exchange in the form of simultaneously pressing keys to maximize joint performance. Our results are consistent with IBS findings in other interactive activities, such as game playing (Liu et al., 2016), button pressing (Funane et al., 2011), and jointly acting (Dommer et al., 2012). IBS might be accounted for by the familiarity lovers have with each other or by their emotional involvement with each other. However, we think these factors are unlikely based on the following evidence. First, we observed no significant IBS in friend dyads, who had a similar duration of acquaintance as lover dyads. Second, although lover‐dyads reported more pleasantness during the interactive exchange, our analyses suggested that it might not affect the IBS (see Subjective Measurements in the Results part). Thus, we argue that IBS found in lover dyads arises from working together on the task, echoing the proposal that “significant others” trigger response facilitation and therefore lead to two interactive partners “being in tune” (Byrne, 2005). The cooperative task might also recruit other cognitive processes, such as shared emotions and social intentions, in addition to joint actions. Whether our findings can generalize to these modalities is a direction for future research.
We found IBS in the right superior frontal cortex (r‐SFC). Previous functional brain imaging studies reported the engagement of superior frontal gyrus/intraparietal sulcus while viewing a lover's photograph (Bartels and Zeki, 2000, 2004; Xu et al., 2011) and medial orbitofrontal cortex/parietal lobes while thinking of their romantic partner (Xu et al., 2012). A recent study even found that functional resting‐state connectivity in the frontoparietal network was significantly enhanced for the “in‐love” group (Song et al., 2015). The function of r‐SFC has been intensely discussed in the literature: it has been implicated in implicit understanding of others’ action (Hari and Kujala, 2009), response inhibition (Dziobek et al., 2012), working memory (du Boisgueheneuc et al., 2006), and self‐awareness (Goldberg et al., 2006). The role of r‐SFC identified here offers support for the theory of mind hypothesis (Cui et al., 2012; Yang et al., 2015), as our cooperation game required understanding, modeling, and predicting partner behavior. Certainly, the exact meaning of the IBS at superior frontal cortex (SFC) is a direction for future research.
We observed similarities and differences between our findings and two recent studies using fNIRS‐based hyperscanning. Cui et al. (2012) found IBS at the r‐SFC for the cooperation of 11 participant‐dyads, but the gender of the two partners was not under consideration. In a recent study that controlled for gender, Cheng et al. (2015) revealed IBS at the frontopolar cortex, orbitofrontal cortex, and left dorsolateral prefrontal cortex for female‐male dyads, but not for female‐female dyads and male‐male dyads. The fNIRS optode patch was placed mostly over the prefrontal cortex, and the two partners were unacquainted with each other before the experiment. By contrast, our study relied on the a priori hypothesis that right frontoparietal brain areas likely underlay social understanding and interactive exchange between partners.
We found gender differences in lover dyads: males tended to respond more slowly to cues. The slower response suggests that males needed extra time to adapt their responses to females’ responses. In agreement with our finding, a recent study found that when judging the degree of romance in sentences, males responded more slowly compared to females. The authors posit that males may use more cognitive effort to perceive romance (Yin et al., 2013). These findings support different roles for females and males in romantic relationships.
Our GCA results for lover dyads further showed that the direction of IBS from females to males was stronger than from males to females, implying that the primary information flow was from females to males. Previous studies showed IBS directional differences for partners in relationships as gesturer‐guesser (Schippers et al., 2010), model‐imitator (Holper et al., 2012), and leader‐follower (Jiang et al., 2015). Specifically, the brain activity in the gesturer predicted that of the guesser (Schippers et al., 2010), the brain activity of the model caused that of the imitator (Holper et al., 2012), and there was a more important role for the leader compared to the follower (Jiang et al., 2015). In our study, females might “lead” the activity and “send” information to males, who “receive” the information and play the “follower” role. Results from Granger causality analyses highlight the important role of dynamic social interactions in lovers’ cooperation (Schilbach et al., 2013).
Gender differences found for lover dyads tends to support the evolutionary biological perspective that the male is more capable of going along with females’ behavior (Griskevicius et al., 2006; Hyde, 2014). We note that our participants are mostly undergraduate university students. They are in the early stages of the romantic relationship, during which males exert themselves, display acceptable attributes, and demonstrate the ability to achieve sexual access. As such, the examination of gender differences in lovers should consider more factors, such as relationship stage (Kenrick et al., 1990), attachment style (Furman et al., 2002), and affiliation (Furman et al., 2014), to name a few. In addition, studies have shown that in situations involving prosocial behaviors, males are expected to undertake the roles of high status and power, and thus were more agentic and dominant than females (e.g., Eagly, 2009). Further investigation is needed to elaborate the gender differences in romantic lovers during interactive exchange.
Investigation of IBS using two‐person interactions is a promising yet nascent field, and it could provide insight into higher cognitive functions, such as cooperation and communication. However, fNIRS faces some analytic challenges. First, the spontaneous oscillations of blood flow (i.e., Mayer waves, ∼0.1 Hz), which might contaminate the task‐related brain activities (Lloyd‐Fox et al., 2010; Scholkmann et al., 2014), were included in our frequency of interest (0.08–0.31 Hz). Consequently, we carried out a parallel IBS analysis in the frequency band of 0.09–0.11 Hz, but no significant group difference was detected (see Supporting Information, Fig. S2). Such physiological process therefore should have equal impact on fNIRS responses among all groups and cannot explain our finding of increased IBS only in the lover dyads. Second, fNIRS signals were contaminated with global systemic components, which are not task‐specific, such as the scalp blood flow and changes in blood pressure. Therefore, we used a PCA approach that included Gaussian spatial filtering to remove the global systemic components (Zhang et al., 2016), although such signals cannot be filtered out completely. Third, we detected the primary components of IBS based on the HbO measurement, and we cannot exclude the possibility of additional useful information in the HbR signal. Previous studies have shown that HbO is more influenced by scalp blood flow and HbR is more related to neurovascular coupling (Ou et al., 2009; Zhang et al., 2016). Therefore, we also analyzed the HbR time series and found a similar effect at the r‐SFC (see Supporting Information, Fig. S3). Because fNIRS signals might suffer from various confounding contaminants such as physiological processes and global systemic effects, future studies could (1) collect physiological responses, and (2) analyze both HbO and HbR concentrations to get a full picture about measured brain activity.
Limitations of this study are important to note. First, the optode probe set of NIRS only covered the right frontoparietal regions of the brain, leaving other regions unexplored. In a previous study of cooperative performance, IBS was also found at the left inferior parietal lobule and the occipital cortex (Egetemeir et al., 2011). The roles of these brain regions could be examined by measuring from the entire brain. Second, close personal relationships are found to be closely related to motivation and reward brain systems (Acevedo et al., 2012; Aron et al., 2005), and the relevant brain structures are mostly subcortical. These subcortical structures could be investigated by using fMRI‐based techniques. Third, we cannot dismiss the potential effects of motivational state on the establishment of IBS, since motivation plays an important role during the social interactions (Canessa et al., 2012). Future studies could manipulate or control participant's motivational state and explore the relationship between motivation and brain activity.
In summary, we demonstrated that cooperative behavior between lover dyads was better than friend and stranger pairs and identified IBS in the right superior frontal cortex. Our findings support the theory that there are distinctive mental representations developed for individuals in relationships with others. Performance differences between partners and the direction of IBS suggest a leading/affiliative role for females/males in romantic relationships, primarily supporting theories from evolutionary biology. Our study advances the understanding of the unique brain signature for lovers’ interactions and potentially provides insights in the evolution of human love.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Authors thank Dr. Kimberlee D'Ardenne from the Department of Psychology at Arizona State University for her wonderful suggestions and grammar corrections. Authors thank Yue Yang and Guowei Chen for their technical assistance with the fNIRS hyperscanning. Authors thank Qun Ye and Lili Zheng for their assistance in data acquisition and entry.
Contributor Information
Xianchun Li, Email: xcli@psy.ecnu.edu.cn.
Yi Hu, Email: yhu@psy.ecnu.edu.cn.
REFRENCES
- Acevedo BP, Aron A, Fisher HE, Brown LL (2012): Neural correlates of long‐term intense romantic love. Soc Cogn Affect Neurosci 7:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman JM, Kenrick DT (2009): Cooperative courtship: Helping friends raise and raze relationship barriers. Pers Soc Psychol B 35:1285–1300. [DOI] [PubMed] [Google Scholar]
- Anders S, Heinzle J, Weiskopf N, Ethofer T, Haynes JD (2011): Flow of affective information between communicating brains. Neuroimage 54:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li HF, Brown LL (2005): Reward, motivation, and emotion systems associated with early‐stage intense romantic love. J Neurophysiol 94:327–337. [DOI] [PubMed] [Google Scholar]
- Assad KK, Donnellan MB, Conger RD (2007): Optimism: An enduring resource for romantic relationships. J Pers Soc Psychol 93:285–297. [DOI] [PubMed] [Google Scholar]
- Baker JM, Liu N, Cui X, Vrticka P, Saggar M, Hosseini SM, Reiss AL (2016): Sex differences in neural and behavioral signatures of cooperation revealed by fNIRS hyperscanning. Sci Rep 6:26492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett L, Seth AK (2014): The MVGC multivariate Granger causality toolbox: A new approach to Granger‐causal inference. J Neurosci Methods 223:50–68. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S (2000): The neural basis of romantic love. Neuroreport 11:3829–3834. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S (2004): The neural correlates of maternal and romantic love. Neuroimage 21:1155–1166. [DOI] [PubMed] [Google Scholar]
- Buss DM, Schmitt DP (1993): Sexual strategies theory—An evolutionary perspective on human mating. Psychol Rev 100:204–232. [DOI] [PubMed] [Google Scholar]
- Byrne RW (2005): Social cognition: imitation, imitation, imitation. Curr Biol 15:498–500. [DOI] [PubMed] [Google Scholar]
- Canessa N, Alemanno F, Riva F, Zani A, Proverbio AM, Mannara N, Perani D, Cappa SF (2012): The neural bases of social intention understanding: The role of interaction goals. PLoS One 7:e42347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XJ, Li XC, Hu Y (2015): Synchronous brain activity during cooperative exchange depends on gender of partner: A fNIRS‐based hyperscanning study. Hum Brain Mapp 36:2039–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, Montague PR (2008): Self responses along cingulate cortex reveal quantitative neural phenotype for high‐functioning autism. Neuron 57:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SE, Madson L (1997): Models of the self: Self‐construals and gender. Psychol Bull 122:5–37. [DOI] [PubMed] [Google Scholar]
- Cui X, Bryant DM, Reiss AL (2012): NIRS‐based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage 59:2430–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer A, van Buel EM, Ter Horst GJ (2012): Love is more than just a kiss: A neurobiological perspective on love and affection. Neuroscience 201:114–124. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN (2004): The neural bases of cooperation and competition: An fMRI investigation. Neuroimage 23:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommer L, Jager N, Scholkmann F, Wolf M, Holper L (2012): Between‐brain coherence during joint n‐back task performance: A two‐person functional near‐infrared spectroscopy study. Behav Brain Res 234:212–222. [DOI] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B (2006): Functions of the left superior frontal gyrus in humans: A lesion study. Brain 129:3315–3328. [DOI] [PubMed] [Google Scholar]
- Dumas G, Nadel J, Soussignan R, Martinerie J, Garnero L (2010): Inter‐brain synchronization during social interaction. PLoS One 5:e12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziobek I, Preissler S, Grozdanovic Z, Heuser I, Heekeren HR, Roepke S (2012): Neuronal correlates of altered empathy and social cognition in borderline personality disorder. Neuroimage 57:539–548. [DOI] [PubMed] [Google Scholar]
- Eagly AH (2009): The his and hers of prosocial behavior: An examination of the social psychology of gender. Am Psychol 64:644–658. [DOI] [PubMed] [Google Scholar]
- Eckel CC, Grossman PJ (1998): Are women less selfish than men? Evidence from dictator experiments. Econ J 108:726–735. [Google Scholar]
- Ferrari M, Quaresima V (2012): A brief review on the history of human functional near‐infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 63:921–935. [DOI] [PubMed] [Google Scholar]
- Egetemeir J, Stenneken P, Koehler S, Fallgatter AJ, Herrmann MJ (2011): Exploring the neural basis of real‐life joint action: Measuring brain activation during joint table setting with functional near‐infrared spectroscopy. Front Hum Neurosci 5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GJO, Simpson JA, Campbell L, Overall NC (2015): Pair‐bonding, romantic love, and evolution: The curious case of homo sapiens. Perspect Psychol Sci 10:20–36. [DOI] [PubMed] [Google Scholar]
- Funane T, Kiguchi M, Atsumori H, Sato H, Kubota K, Koizumi H (2011): Synchronous activity of two people's prefrontal cortices during a cooperative task measured by simultaneous near‐infrared spectroscopy. J Biomed Opt 16:077011. [DOI] [PubMed] [Google Scholar]
- Furman W, Simon VA, Shaffer L, Bouchey HA (2002): Adolescents' working models and styles for relationships with parents, friends, and romantic partners. Child Dev 73:241–255. [DOI] [PubMed] [Google Scholar]
- Furman W, Stephenson JC, Rhoades GK (2014): Positive interactions and avoidant and anxious representations in relationships with parents, friends, and romantic partners. J Res Adolesc 24:615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable SL, Reis HT, Elliot AJ (2003): Evidence for bivariate systems: An empirical test of appetition and aversion across domains. J Res Pers 37:349–372. [Google Scholar]
- Gable SL, Reis HT, Impett EA, Asher ER (2004): What do you do when things go right? The intrapersonal and interpersonal benefits of sharing positive events. J Pers Soc Psychol 87:228–245. [DOI] [PubMed] [Google Scholar]
- Gallace A, Spence C (2010): The science of interpersonal touch: An overview. Neurosci Biobehav Rev 34:246–259. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G (2004): A unifying view of the basis of social cognition. Trends Cogn Sci 8:396–403. [DOI] [PubMed] [Google Scholar]
- Geary DC, Vigil J, Byrd‐Craven J (2004): Evolution of human mate choice. J Sex Res 41:27–42. [DOI] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R (2006): When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron 50:329–339. [DOI] [PubMed] [Google Scholar]
- Grinsted A, Moore JC, Jevrejeva S (2004): Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Proc Geoph 11:561–566. [Google Scholar]
- Griskevicius V, Cialdini RB, Kenrick DT (2006): Peacocks, Picasso, and parental investment: The effects of romantic motives on creativity. J Pers Soc Psychol 91:63–76. [DOI] [PubMed] [Google Scholar]
- Hari R, Kujala MV (2009): Brain basis of human social interaction: From concepts to brain imaging. Physiol Rev 89:453–479. [DOI] [PubMed] [Google Scholar]
- Holper L, Scholkmann F, Wolf M (2012): Between‐brain connectivity during imitation measured by fNIRS. Neuroimage 63:212–222. [DOI] [PubMed] [Google Scholar]
- Hoshi Y (2007): Functional near‐infrared spectroscopy: Current status and future prospects. J Biomed Opt 12:062106. [DOI] [PubMed] [Google Scholar]
- Hyde JS (2014): Gender similarities and differences. Annu Rev Psychol 65:373–398. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar‐Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G (2005): Grasping the intentions of others with one's own mirror neuron system. PLoS Biol 3:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im CH, Jung YJ, Lee S, Koh D, Kim DW, Kim BM (2010): Estimation of directional coupling between cortical areas using near‐infrared spectroscopy (NIRS). Opt Express 18:5730–5739. [DOI] [PubMed] [Google Scholar]
- Jiang J, Chen CS, Dai BH, Shi G, Ding GS, Liu L, Lu CM (2015): Leader emergence through interpersonal neural synchronization. Proc Natl Acad Sci USA 112:4274–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenrick DT, Sadalla EK, Groth G, Trost MR (1990): Evolution, traits, and the stages of human courtship: Qualifying the parental investment model. J Pers 58:97–116. [DOI] [PubMed] [Google Scholar]
- Kirilina E, Jelzow A, Heine A, Niessing M, Wabnitz H, Bruhl R, Ittermann B, Jacobs AM, Tachtsidis I (2012): The physiological origin of task‐evoked systemic artefacts in functional near infrared spectroscopy. Neuroimage 61:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005): Oxytocin increases trust in humans. Nature 435:673–676. [DOI] [PubMed] [Google Scholar]
- Li J, Xiao E, Houser D, Montague PR (2009): Neural responses to sanction threats in two‐party economic exchange. Proc Natl Acad Sci USA 106:16835–16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li NP, Bailey JM, Kenrick DT, Linsenmeier JAW (2002): The necessities and luxuries of mate preferences: Testing the tradeoffs. J Pers Soc Psychol 82:947–955. [PubMed] [Google Scholar]
- Li NP, Kenrick DT (2006): Sex similarities and differences in preferences for short‐term mates: What, whether, and why. J Pers Soc Psychol 90:468–489. [DOI] [PubMed] [Google Scholar]
- Liu N, Mok C, Witt E, Pradhan AH, Chen JE, Reiss AL (2016): NIRS‐based hyperscanning reveals inter‐brain neural synchronization during cooperative Jenga game with face‐to‐face communication. Front Hum Neurosci 10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Li SC, Gruber W, Muller V (2009): Brains swinging in concert: Cortical phase synchronization while playing guitar. BMC Neurosci 10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd‐Fox S, Blasi A, Elwell CE (2010): Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev 34:269–284. [DOI] [PubMed] [Google Scholar]
- McCabe K, Houser D, Ryan L, Smith V, Trouard T (2001): A functional imaging study of cooperation in two‐person reciprocal exchange. Proc Natl Acad Sci USA 98:11832–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V, Lindenberger U (2014): Hyper‐brain networks support romantic kissing in humans. PLoS One 9:e112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou W, Nissila I, Radhakrishnan H, Boas DA, Hamalainen MS, Franceschini MA (2009): Study of neurovascular coupling in humans via simultaneous magnetoencephalography and diffuse optical imaging acquisition. Neuroimage 46:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perilloux C, Fleischman DS, Buss DM (2008): The daughter‐guarding hypothesis: Parental influence on, and emotional reactions to, offspring's mating behavior. Evol Psychol 6:217–233. [Google Scholar]
- Randall AK, Post JH, Reed RG, Butler EA (2013): Cooperating with your romantic partner: Associations with interpersonal emotion coordination. J Soc Pers Relat 30:1072–1095. [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K (2013): Toward a second‐person neuroscience. Behav Brain Sci 36:393–414. [DOI] [PubMed] [Google Scholar]
- Schippers MB, Roebroeck A, Renken R, Nanetti L, Keysers C (2010): Mapping the information flow from one brain to another during gestural communication. Proc Natl Acad Sci USA 107:9388–9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory‐Sharon O, Leckman JF, Feldman R (2012): Oxytocin during the initial stages of romantic attachment: Relations to couples' interactive reciprocity. Psychoneuroendocrinology 37:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Pavia JM, Wolf U, Wolf M (2014): A review on continuous wave functional near‐infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85:6–27. [DOI] [PubMed] [Google Scholar]
- Singh AK, Okamoto M, Dan H, Jurcak V, Dan I (2005): Spatial registration of multichannel multi‐subject fNIRS data to MNI space without MRI. Neuroimage 27:842–851. [DOI] [PubMed] [Google Scholar]
- Song H, Zou Z, Kou J, Liu Y, Yang L, Zilverstand A, d'Oleire Uquillas F, Zhang X (2015): Love‐related changes in the brain: A resting‐state functional magnetic resonance imaging study. Front Hum Neurosci 9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Mai X, Wang S, Zhu C, Krueger F, Liu C (2016): Interpersonal brain synchronization in the right temporo‐parietal junction during face‐to‐face economic exchange. Soc Cogn Affect Neurosci 11:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki D, Jurcak V, Singh AK, Okamoto M, Watanabe E, Dan I (2007): Virtual spatial registration of stand‐alone fNIRS data to MNI space. Neuroimage 34:1506–1518. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP (2007): The self and social cognition: The role of cortical midline structures and mirror neurons. Trends Cogn Sci 11:153–157. [DOI] [PubMed] [Google Scholar]
- Vesper C, van der Wel RPRD, Knoblich G, Sebanz N (2011): Making oneself predictable: Reduced temporal variability facilitates joint action coordination. Exp Brain Res 211:517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Aron A, Brown L, Cao GK, Feng TY, Weng XC (2011): Reward and motivation systems: A brain mapping study of early–stage intense romantic love in Chinese participants. Hum Brain Mapp 32:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Brown L, Aron A, Cao GK, Feng TY, Acevedo B, Weng XC (2012): Regional brain activity during early‐stage intense romantic love predicted relationship outcomes after 40 months: An fMRI assessment. Neurosci Lett 526:33–38. [DOI] [PubMed] [Google Scholar]
- Yang DY, Rosenblau G, Keifer C, Pelphrey KA (2015): An integrative neural model of social perception, action observation, and theory of mind. Neurosci Biobehav Rev 51:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Zhang JX, Xie J, Zou ZL, Huang XT (2013): Gender differences in perception of romance in Chinese college students. PLoS One 8:e76294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakriski AL, Wright JC, Underwood MK (2005): Gender similarities and differences in children's social behavior: Finding personality in contextualized patterns of adaptation. J Pers Soc Psychol 88:844–855. [DOI] [PubMed] [Google Scholar]
- Zeki S (2007): The neurobiology of love. FEBS Lett 581:2575–2579. [DOI] [PubMed] [Google Scholar]
- Zhang X, Noah JA, Hirsch J (2016): Separation of the global and local components in functional near‐infrared spectroscopy signals using principal component spatial filtering. Neurophotonics 3:015004‐015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


