Abstract
Background:
Pregnant women and children are especially vulnerable to exposures to food contaminants, and a balanced diet during these periods is critical for optimal nutritional status.
Objectives:
Our objective was to study the association between diet and measured blood and urinary levels of environmental contaminants in mother–child pairs from six European birth cohorts ( mothers and 1,288 children).
Methods:
We assessed the consumption of seven food groups and the blood levels of organochlorine pesticides, polybrominated diphenyl ethers, polychlorinated biphenyls (PCBs), per- and polyfluoroalkyl substances (PFAS), and heavy metals and urinary levels of phthalate metabolites, phenolic compounds, and organophosphate pesticide (OP) metabolites. Organic food consumption during childhood was also studied. We applied multivariable linear regressions and targeted maximum likelihood based estimation (TMLE).
Results:
Maternal high () versus low () fish consumption was associated with 15% higher PCBs [geometric mean (GM) ; 95% confidence interval (CI): 1.02, 1.29], 42% higher perfluoroundecanoate (PFUnDA) (; 95% CI: 1.20, 1.68), 89% higher mercury (Hg) (; 95% CI: 1.47, 2.41) and a 487% increase in arsenic (As) (; 95% CI: 2.57, 9.23) levels. In children, high () versus low () fish consumption was associated with 23% higher perfluorononanoate (PFNA) (; 95% CI: 1.08, 1.40), 36% higher PFUnDA (; 95% CI: 1.12, 1.64), 37% higher perfluorooctane sulfonate (PFOS) (; 95% CI: 1.22, 1.54), and higher Hg and As [ (95% CI: 1.91, 4.31) and (95% CI: 2.23, 3.21)] concentrations. Using TMLE analysis, we estimated that fish consumption within the recommended 2–3 times/week resulted in lower PFAS, Hg, and As compared with higher consumption. Fruit consumption was positively associated with OP metabolites. Organic food consumption was negatively associated with OP metabolites.
Discussion:
Fish consumption is related to higher PFAS, Hg, and As exposures. In addition, fruit consumption is a source of exposure to OPs. https://doi.org/10.1289/EHP5324
Introduction
During gestation and early postnatal development, the fetus and the child, respectively, are vulnerable to the effects of environmental chemicals due to rapid cellular differentiation and tissue development and the incomplete development of protective mechanisms (Heindel et al. 2015a). More specifically, developmental low-dose exposure to persistent organic pollutants, such as organochlorine compounds and polybrominated diphenyl ethers (PBDEs), has been consistently linked to restricted fetal growth, neurotoxicity, immunotoxicity, and reduced fertility, and robust evidence is emerging on their obesogenic effects (Heindel et al. 2015b; Johansson et al. 2017; Linares et al. 2015; Vrijheid et al. 2016; Vuong et al. 2018). In addition, early life exposure to per- and polyfluoroalkyl substances (PFAS), commonly used in a wide variety of products, can induce carcinogenic, hepatotoxic, and immunotoxic effects, as well as adverse birth outcomes, childhood obesity, and type 2 diabetes (Alderete et al. 2019; Braun 2017; Heindel et al. 2015b; Johansson et al. 2017; Liew et al. 2018; Lim 2019). Prenatal exposure to toxic metals, such as mercury (Hg), cadmium (Cd), and lead (Pb) in early life, can disrupt the development of the nervous system, and such exposures can affect the child’s cognitive, motor, and behavioral development. In addition, evidence is building on the immunotoxic effects of metal exposure (Claus Henn et al. 2014; Farzan et al. 2016; Valeri et al. 2017). On the other hand, early life exposure to nonpersistent pollutants such as phthalates, environmental phenols, and organophosphate pesticides (OPs) has been also linked to adverse neurobehavioral outcomes, obesity, and asthma (Braun 2017; Heindel et al. 2015b; Hertz-Picciotto et al. 2018; Philippat et al. 2017; Philips et al. 2017; Raanan et al. 2015). In summary, early life exposure to several environmental chemical hazards has been linked to adverse health outcomes, and even though acute high exposures can induce such effects, continuous low-dose exposures have been consistently linked to toxic effects (Myers et al. 2009; Taylor et al. 2013; Vandenberg et al. 2012). Hence, determining the sources of exposure and eliminating exposure to environmental hazards at critical developmental stages is a major public health issue.
Diet is a major source of exposure to many hazardous environmental factors. Food can be contaminated via transfer of substances from contaminated marine and/or agricultural food chains, during food production or food processing, or as a result of leakage from food packaging (Domingo and Nadal 2017; Ng and von Goetz 2017). Dietary exposure to persistent organic pollutants (POPs), including polychlorinated dioxins/furans and biphenyls (PCDDs/PCDFs and PCBs), dichlorodiphenyldichloroethylene (DDE), hexachlorobenzene (HCB), PBDEs and PFASs is driven mainly by consumption of foods of marine or terrestrial animal origin (Domingo and Nadal 2017; EFSA 2006a, 2006b, 2012b). Such contaminants are found in biological samples from pregnant women worldwide, and positive associations between blood concentrations of POPs and consumption of fish and seafood, meat, and dairy products have been consistently reported (Brantsæter et al. 2013; Cao et al. 2011; Cequier et al. 2015; Llop et al. 2010; Manzano-Salgado et al. 2016; Miyashita et al. 2015; Papadopoulou et al. 2014). Similarly, seafood and other foods of animal origin have been identified as sources of exposure to POPs for children although fewer studies have focused on this age group (Caspersen et al. 2016; González-Alzaga et al. 2018; Jain 2018; Wu et al. 2015).
Fish and seafood are an established dietary source of Hg and arsenic (As). Dietary determinants of other toxic elements, such as Cd and Pb are more diverse (Baeyens et al. 2014; Berglund et al. 2015; Castaño et al. 2015; Li et al. 2014; Llop et al. 2014; Miyashita et al. 2015). Regarding exposure to nonpersistent contaminants, consumption of canned food has been related to increased urinary levels of bisphenol A (BPA) (Casas et al. 2013; Covaci et al. 2015), and consumption of food stored in plastic packaging has been related to higher urinary levels of phthalate metabolites (Giovanoulis et al. 2016). For other nonpersistent compounds, such as parabens and triclosan (TCS), the dietary sources are unclear (Larsson et al. 2014; Sakhi et al. 2018). For nonoccupationally exposed populations, contaminated fruit and vegetable consumption has been identified as a major contributor to OP exposure, monitored as increased concentrations of urinary nonspecific OP metabolites (Cequier et al. 2017; Katsikantami et al. 2019; van den Dries et al. 2018).
Most earlier studies have focused on the dietary determinants of exposure to single contaminants, even though several contaminants frequently share common sources of exposure. Few studies have reported contaminants’ patterns and their association with nutrients and the consumption of specific foods (Birgisdottir et al. 2013; Dassuncao et al. 2018; Hu et al. 2018; Veyhe et al. 2015). The identification of such common dietary sources of exposure can assist in designing and delivering effective dietary advice. Dietary recommendations must be balanced between the health risks related to dietary exposure to environmental contaminants and the health benefits of important nutrients.
In the present analyses within a multicenter study, we aimed to systematically investigate and identify the dietary determinants of blood and urinary biomarkers of a wide range of ubiquitous environmental contaminants in pregnant mothers and their children. We further evaluated the effect of dietary recommendations on the concentrations of several environmental contaminants using targeted maximum likelihood based estimation (TMLE).
Methods
Study Population
This study was conducted within the Human Early Life Exposome (HELIX) project (Maitre et al. 2018; Vrijheid et al. 2014), which draws resources from six existing European longitudinal population-based birth cohorts: Born in Bradford (United Kingdom; BiB) (Wright et al. 2013); Étude des Déterminants pré et postnatals du développement et de la santé de l’ENfant (France; EDEN) (Drouillet et al. 2009); INfancia y Medio Ambiente (Spain; INMA) (Guxens et al. 2012); Kaunas Cohort (Lithuania; KANC) (Grazuleviciene et al. 2009); Norwegian Mother and Child Cohort Study (Norway; MoBa) (Magnus et al. 2016), and Rhea (Greece) (Chatzi et al. 2017). Our study consisted of 818 mothers and 1,288 children and included information on maternal diet, child’s diet, and measured levels of environmental contaminants during pregnancy and childhood. This subsample, called the HELIX subcohort, is nested within the entire HELIX cohort ( mother–child pairs) by inclusion of approximately 200 pairs from each cohort (Maitre et al. 2018). The detailed description of the study design and population is provided by Maitre et al. (2018). In brief, the included children were between 6 and 11 y of age, and the data and biological sample collection was conducted from 2013 to 2015. Eligibility criteria for inclusion in the subcohort were a) age 6–11 y at the time of the visit, with a preference for ages 7–9 y if possible; b) sufficient stored pregnancy blood and urine samples available for analysis of prenatal exposure biomarkers; c) complete address history available from first to last follow-up point; and d) no serious health problems that may affect the performance of the clinical testing or impact the volunteer’s safety (e.g., acute respiratory infection). Each cohort selected participants at random from the eligible pool within the entire cohort and invited them to participate in this subcohort until the required number of participants was reached.
Dietary Assessment
Information on maternal diet during pregnancy was collected using validated semiquantitative cohort-specific food frequency questionnaires (FFQs) (Brantsæter et al. 2008; Chatzi et al. 2011; Deschamps et al. 2009; Raynor and Born in Bradford Collaborative Group 2008; Vioque et al. 2013). Information about maternal diet during pregnancy was not available for the KANC cohort. For the BiB cohort, the collected information on maternal diet was via a short questionnaire and was limited to some but not all food groups (the missing food groups were fruits, vegetables, pulses, and dairy products). Hence, women from the KANC and BiB cohort were not included in our analysis. Detailed information on each FFQ is presented in Excel Table S1 for the included cohorts. Information about the children’s diet was collected during the HELIX follow-up, when children were called to attend detailed examinations (Maitre et al. 2018) via a semiquantitative FFQ covering the child’s habitual diet, which was filled in by the parent attending the examination appointment. The FFQ was developed by the HELIX research group and translated and was applied to all cohorts. We defined weekly consumption frequency of seven food groups: three foods of animal origin (meat and meat products, fish and seafood, and dairy products) and four foods of plant origin (vegetables, fruits, bread and cereals, and pulses such as dry beans, dry peas, chickpeas, and lentils). In addition, the children’s organic food consumption was assessed by one question asking the parent to report their child’s frequency of eating organic food. The answers were aggregated into a three-category variable: no organic consumption, once a week or less, and more than once a week.
Chemical Analysis
The concentrations of several environmental contaminants were measured in maternal biological samples collected prenatally or at birth and in children’s biological samples collected during the HELIX follow-up. A detailed description of the analytical methods, the quality assurance and quality control, and the measured maternal and child concentrations have been published previously (Haug et al. 2018).
As presented in Excel Table S2, the environmental contaminants measured in blood were organochlorine compounds including PCBs (CB 118, CB 138, CB 153, CB 170, CB 180), dichlorodiphenyl trichloroethane (DDT), DDE, HCB, PBDEs (BDE 47, BDE 153), and PFASs [perfluorohexane sulfonate (PFHxS), perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), perfluorononanoate (PFNA), perfluoroundecanoate (PFUnDA)] as well as the toxic elements Hg, Cd, Pb, and As. Notably, only concentrations of PFASs and toxic elements were available for mothers in the KANC cohort, with PFAS measured in whole blood rather than in serum or plasma as in the other cohorts. In addition, maternal toxic elements in the INMA cohort were measured in cord blood samples, rather than in maternal perinatal samples as in the other cohorts. The contaminants measured in urine were phthalate metabolites [Monoethyl phthalate (MEP), Mono-isobutyl phthalate (MiBP), Mono-n-butyl phthalate (MnBP), Mono benzyl phthalate (MBzP), Mono-2-ethylhexyl phthalate (MEHP), Mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), Mono-2-ethyl-5-oxohexyl phthalate (MEOHP), Mono-2-ethyl 5-carboxypentyl phthalate (MECPP), Mono-4-methyl-7-hydroxyoctyl phthalate (oh-MiNP), Mono-4-methyl-7-oxooctyl phthalate (oxo-MiNP)], phenols [MEPA, ETPA, propylparaben (PRPA), butylparaben (BUPA) BPA, OXBE, TCS] and OP metabolites [dimethyl phosphate (DMP), dimethyl thiophosphate (DMTP), dimethyl dithiophosphate (DMDTP), diethyl phosphate (DEP), thiophosphate (DETP), diethyl dithiophosphate (DEDTP)]. Urinary creatinine and plasma or serum lipids concentrations were measured to facilitate adjustment for the urinary metabolites of phthalates, phenols, and OPs and the lipophilic compounds (PCBs, DDT, DDE, HCB, PBDEs), respectively. The maternal urinary metabolite concentrations for phthalates, phenols, and OPs as well as the maternal concentrations of the lipophilic compounds were not available in the KANC cohort. Values below the limit of quantitation were replaced with the observed values whenever available (Haug et al. 2018). For nonobserved concentrations, singly imputed values were obtained using a quantile regression approach. The detailed numbers of measured, quantified and included samples, overall and by cohort, are presented in Excel Tables S3 and S4 for maternal and child samples, respectively. The OP metabolites, DMDTP and DEDTP were not included in our analysis due to low detection frequencies in maternal and children samples. In addition, the individual concentrations of the four nonspecific OP metabolites were converted to their molar concentrations (nanomoles/gram of creatinine) and summarized to the total dialkylphosphate metabolites (sumDAPs; sum of DMP, DMTP, DEP, DETP), total dimethylphosphate metabolites (sumDM; sum of DMP and DMTP), and total diethylphosphate metabolites (sumDE; sum of DEP and DETP). Last, because common food sources were suspected sources, PCBs were analyzed as a summarized concentration (sumPCBs) rather than single congeners, and the di(2-ethylhexyl) phthalate (DEHP) metabolites, MEHP, MEHHP, MEOHP, and MECPP, were also included as a summed molar concentration (i.e., sumDEHP metabolites).
Statistical Analysis
To identify dietary determinants of measured concentrations of environmental contaminants, we conducted complete case linear regression analysis for single contaminants adjusted for covariates. Based on a priori knowledge on covariates suspected to be related to both dietary habits and maternal levels of contaminants, the following variables were included in the pregnancy models: cohort, maternal age (in years), educational level (low/middle/high), parity (nulliparous/multiparous), prepregnancy body mass index (in kilograms per meter squared), gestational weight gain (in kilograms), active smoking during pregnancy (yes/no), year of delivery [minimum–maximum (]. Finally, child’s ethnicity (white European/other) was used as a proxy for maternal ethnicity, as the later was not available (see Excel Table S5). The associations reported for pregnant women did not include women from the KANC and BiB cohorts because of missing maternal dietary information. For associations between child’s diet and measured levels of environmental contaminants, the following a priori covariates were included in the multivariable models: cohort, age (years), body mass index (in kilograms per meter squared), Family Affluence Score (low/middle/high), year of birth () and firstborn (yes/no).
In the multivariate regression analysis, blood and urinary concentrations of environmental contaminants were the dependent variables and were log-transformed to approach normal distribution. Food intakes were the independent variables and were categorized into tertiles. Hence, beta coefficients from all models were exponentiated (base 10) to produce the ratio of the geometric mean (GM) of contaminant concentrations of each category in respect to the GM of the reference category. Because food group intakes tend to correlate, the variance inflation factor (VIF) was used to assess multicollinearity, and for all the models, an was observed. Hence, the final models presented for pregnant women as well as for children were mutually adjusted for all food groups. Because multiple testing can be an issue, we have also reported the observed associations after correction for multiple comparisons with a false discovery rate controlled at (Benjamini and Yekutieli 2005).
Because smoking is a major nondietary source of exposure to Cd, dietary determinants of Cd were explored in the whole population as well as in nonsmokers. As a secondary analysis and in order to explore the effect of prenatal exposure to contaminants on the postnatal dietary exposure, we adjusted the children’s models for the corresponding contaminant concentration in the mother’s sample during pregnancy.
For children, the associations between the frequency of organic food consumption with the measured environmental contaminants in blood and urine were also examined in multivariable linear regression models, adjusted for the same covariates as the analysis of child’s diet, including all the food groups.
Finally, we performed TMLE analysis (Schuler and Rose 2017), aiming to estimate the marginal GM difference in environmental contaminants levels (Y) that were previously identified to be related with fish and fruit consumption if all HELIX mothers and children were given a hypothetical intervention () relative to a scenario in which none of the mothers and children were given that intervention (): , where W is a matrix of covariates. To estimate [E(Y|A,W)] and E(A|W), we used the Super Learner algorithm, an ensemble machine learning algorithm that uses a weighted combination of algorithms to return a prediction function that minimizes cross-validated mean squared error (van der Laan et al. 2007). Covariates used in the TMLE analysis were the same as those included in the regression analyses and are described above. The potential interventions of fish and fruit intake evaluated were based on international dietary recommendations (AHA/ASA 2017; WHO 2006): for fish, consuming fish according to the dietary recommendations (mothers: up to 3 meals/week, children: up to 2 meals/week) versus exceeding the dietary recommendation, and for fruit, consuming fruits according to the recommendation (both mothers and children: at least 2 servings/d) versus not reaching the recommendation for daily fruit intake.
Hence, the marginal GM difference of PCBs, PFASs, As, and Hg concentrations for the different fish intakes and of OPs metabolites for the different fruit intakes were evaluated in separate TMLE models. These chemicals were chosen based on statistical significant and dose–response associations derived from the linear regression analysis and were consistent in mothers and children.
Statistical analyses were performed using Stata statistical software (release 14; Stata Corporation) and R (version 3.2.2; R Development Core Team). We used as the level of significance in our analyses.
Results
Maternal and Child Diet in the HELIX Subcohort
The median intake of meat and fish during pregnancy was 8 and 3 times/week, respectively (Table 1). The median intake of meat and fish in children was 8 and 2 times/week, respectively. Both mothers and children consumed vegetables, fruits, dairy, and cereals much more frequently ().
Table 1.
Weekly consumption of major food groups (in times per week) in women during pregnancy and children as assessed at 5–12 y of age, at the HELIX follow-up.
| Food groups (times/week) | N | Non-consumers (%) | Median | P25 | P75 |
|---|---|---|---|---|---|
| Maternal dieta | |||||
| Meat | 929 | 0.5 | 8 | 6 | 12 |
| Fish and seafood | 940 | 2 | 3 | 2 | 5 |
| Vegetablesb | 827 | 0.2 | 12 | 8 | 20 |
| Fruitsb | 832 | 0.6 | 14 | 8 | 21 |
| Dairy productsb | 829 | 0.1 | 21 | 15 | 31 |
| Bread and cereals | 944 | 0 | 17 | 6 | 33 |
| Pulsesb | 832 | 25 | 1 | 0 | 3 |
| Child diet at 5–12 y of agec | |||||
| Meat | 1,291 | 0.2 | 8 | 5 | 10 |
| Fish and seafood | 1,291 | 4 | 2 | 1 | 4 |
| Vegetables | 1,290 | 1 | 7 | 4 | 10 |
| Fruits | 1,289 | 0.3 | 9 | 6 | 18 |
| Dairy products | 1,291 | 0.3 | 20 | 13 | 28 |
| Bread and cereals | 1,289 | 0 | 19 | 13 | 26 |
| Pulses | 1,290 | 11 | 1 | 1 | 3 |
| Organic food consumption | N | % | |||
| No | 427 | 33 | — | — | — |
| Once a week | 534 | 42 | — | — | — |
| More than once a week | 325 | 25 | — | — | — |
Note: —, Not applicable; BiB, Born in Bradford; EDEN, Étude des Déterminants pré et postnatals du développement et de la santé de l’ENfant; France; HELIX, Human Early-Life Exposome; INMA, INfancia y Medio Ambiente; KANC, Kaunas Cohort; MoBa, Norwegian Mother and Child Cohort Study; P, percentile.
No information for KANC cohort.
No information for the BiB cohort.
from BiB, from EDEN, from KANC, from MoBa, from Rhea, from INMA.
By cohort, the women from the Norwegian and British cohorts reported the highest median intake of meat, and those from the Norwegian and Spanish cohort the highest median intake of fish (See Excel Table S6). Among the four cohorts with available dietary information, the women from the French cohort reported the lowest median intake of fruits and vegetables, but they had the highest median intake of pulses. Regarding the children, British and Norwegian children had higher median fruit consumption than the other cohorts, whereas Lithuanian children had a median intake of dairy products at half of the overall median (see Excel Table S6). The frequency of consumption differed by cohort for all food groups (p , Kruskal-Wallis test). Higher maternal education was related to higher maternal dairy and cereal intake and higher child vegetable intake (see Excel Table S7). Women with medium education had lower fish intake than low- and high-educated women.
In addition, 33% of the children reported no consumption of organic food, and 25% of the children were consuming organic food more than 1 time/week (Table 1). Organic food consumption was much more frequent in children of high-educated mothers than of low-educated mothers (see Excel Table S7).
Food Consumption and Measured Environmental Contaminants in Blood during Pregnancy and Childhood
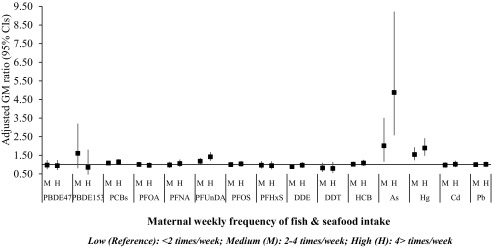
Fish consumption during pregnancy was positively associated with maternal concentrations of PCBs, PFUnDA, As, and Hg in a dose–response manner (Figure 1; see also Excel Table S8). More specifically, high fish intake () was associated with 15% higher sumPCBs [; 95% confidence interval (CI): 1.02, 1.29], 42% higher PFUnDA and 89% higher Hg blood concentrations compared with the lowest intake (). The largest difference was observed for As, with a 201% increase in the blood concentration for medium fish intake (2–4 times/week) and 487% increase for high fish intake. These results remained significant after controlling for multiple comparisons.
Figure 1.
Adjusted linear regression associations between weekly consumption of fish and seafood and measured levels of contaminants in maternal blood collected during pregnancy (). Models are mutually adjusted for all food groups and for cohort, maternal age, educational level, parity, prepregnancy body mass index, gestational weight gain, active smoking during pregnancy, and year of delivery. Women from KANC and BiB cohorts are not included. Corresponding numeric data are reported in Excel Table S8. As, arsenic; BiB, Born in Bradford cohort; Cd, cadmium; CI, confidence interval; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; GM, geometric mean; HCB, hexachlorobenzene; Hg, mercury; KANC, Kaunas Cohort; Pb, lead; PBDE, polybrominated diphenyl ether; PCB, polychlorinated biphenyl; PFHxs, perfluorohexane sulfonate; PFNA, Perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoate.
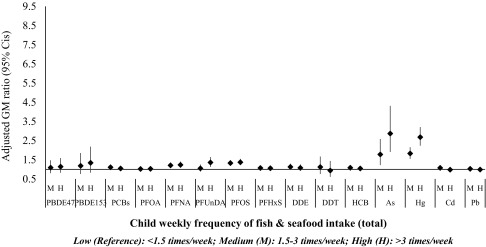
In children, medium fish intake (1.5–3 times/week) was associated with 11% higher PCBs, 21% higher PFNA, 33% higher PFOS, 8% higher HCB, 77% higher As, and 82% higher Hg. High fish intake () was associated with 23% higher PFNA, 36% higher PFUnDA, 37% higher PFOS, and higher As and Hg concentrations in child’s blood. These results remained significant after controlling for multiple comparisons (Figure 2; see also Excel Table S9).
Figure 2.
Adjusted linear regression associations between weekly consumption of fish and measured levels of contaminants in child blood (). Models are mutually adjusted for all food groups and for cohort, age, body mass index, Family Affluence Score, year of birth, and firstborn. Corresponding numeric data are reported in Excel Table S9. As, arsenic; Cd, cadmium; CI, confidence interval; DDE, dichlorodiphenyl dichloroethylene; DDT, dichlorodiphenyl trichloroethane; GM, geometric mean; HCB, hexachlorobenzene; Hg, mercury; Pb, lead; PBDE, polybrominated diphenyl ether; PCB, polychlorinated biphenyl; PFHxs, perfluorohexane sulfonate; PFNA, Perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoate.
In a secondary analysis, after adjustment for the prenatal exposure to the respective environmental contaminants (by including maternal levels measured in pregnancy), similar results were obtained for high fish intake in childhood and PFAS, As, and Hg levels (see Excel Table S11).
In children, although we had detailed information of consumption of different types of seafood, this information was not available for mothers. Most children reported consumption of white fish more than 1 time/week (64%), of oily fish more than 1 time/week (37%), and of any shellfish (crustaceans and bivalves) (53%). Any shellfish consumption was associated with higher blood levels of PCBs, all PFASs, As, and Hg (Figure 3; see also Excel Table S12). Consumption of oily fish more than once a week was associated with higher blood levels of PCBs, PFNA, PFUnDA, PFOS, As, and Hg, and similar results were found for white fish consumption of more than 1 time/week, but not for PFUnDA and PCBs.
Figure 3.
Adjusted linear regression associations between consumption of different seafood types and blood levels of PFAS, As, and Hg in childhood (). Models are mutually adjusted for all food groups and for cohort, age, body mass index, Family Affluence Score, year of birth, and firstborn. Corresponding numeric data are reported in Excel Table S12. As, arsenic; CI, confidence interval; GM, geometric mean; Hg, mercury; PFHxS, perfluorohexane sulfonate; PFNA, Perfluorononanoate; PFOA, perfluorooctanoate; PFUnDA, perfluoroundecanoate; sumPCB, summarized concentration of polychlorinated biphenyls.
Regarding other foods of animal origin, high meat intake during pregnancy () was associated with lower prenatal levels of DDE and DDT, whereas high meat intake in childhood () was associated with lower blood Hg (see Excel Tables S8 and S9). In nonsmoking mothers and all children, high meat intake was associated with lower Cd levels in blood [ (95% CI: 0.66, 0.96) and (95% CI: 0.77, 0.98), respectively] (see Excel Tables S9 and S10). High dairy intake was associated with lower PFUnDA in maternal blood and lower PCBs, DDT, and As in children’s blood. Consistently for both populations, high dairy intake was associated with approximately 20% lower Hg levels and 11–14% lower Pb levels. After controlling for multiple comparisons, only the association between dairy and lower Pb in children remained significant.
When exploring the associations between consumption of foods of plant origin—namely vegetables, fruits, cereals, and pulses—much fewer and less consistent associations were observed than those for foods of animal origin (see Excel Tables S13 and S14). High vegetable intake in pregnancy was associated with higher maternal PFOA levels, whereas high consumption of fruit and pulses in children was associated with higher PFUnDA levels. These associations were not significant after controlling for multiple comparisons.
TMLE Analysis—Fish Recommendations and Levels of Environmental Contaminants
Using TMLE analysis, we estimated that a population of pregnant women in which everyone was consuming fish in an amount equal to or lower than the dietary recommendation (up to 3 servings/week) would have marginal GM PFNA, As, and Hg blood concentrations of (95% CI: , ), (95% CI: , ), and (95% CI: , ) lower than in a population of pregnant women where everyone was consuming fish (Table 2). Regarding children, if the whole population were consuming fish equal to or lower than the dietary recommendation (up to 2 servings/week) they would have and lower PFUnDA and PFOS blood levels and and lower As and Hg levels than if the whole population were consuming more fish meals per week. Nonsignificant associations were observed for other PFAS.
Table 2.
Estimation of the marginal geometric mean difference in sumPCBs, PFAS, As, and Hg concentrations in mothers and children from a hypothetical intervention scenario of weekly fish consumption.
| Pollutants | Exposed/total (/) | Intervention scenario | |
|---|---|---|---|
| 95% CI | |||
| Women (pregnancy)a | |||
| SumPCBs (ng/g lipid) | 347/516 | 1.13 | , 17.46 |
| PFOA () | 377/722 | , 0.17 | |
| PFNA () | 377/722 | , | |
| PFUnDA () | 340/524 | , 0.00 | |
| PFOS () | 377/722 | 0.83 | , 1.68 |
| PFHxS () | 377/722 | 0.04 | , 0.15 |
| As () | 256/409 | , | |
| Hg () | 289/590 | , | |
| Children (5–12 y of age)b | |||
| SumPCBs (ng/g lipid) | 817/1,265 | 1.63 | , 4.36 |
| PFOA () | 835/1,286 | , 0.04 | |
| PFNA () | 835/1,286 | , 0.03 | |
| PFUnDA () | 835/1,286 | , | |
| PFOS () | 835/1,286 | , | |
| PFHxS () | 835/1,286 | 0.05 | , 0.30 |
| As () | 833/1,283 | , | |
| Hg () | 833/1,283 | , | |
Intervention scenario: : following the dietary recommendation for weekly fish consumption (women: up to 3 meals/week and children: up to 2 meals/week); : exceeding the dietary recommendation for weekly fish consumption. As, arsenic; BiB, Born in Bradford cohort; CI, confidence interval; Hg, mercury; KANC, Kaunas Cohort; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoate; SumPCBs, summarized concentration of polychlorinated biphenyls; : marginal geometric mean difference between exposed and unexposed.
Models are adjusted for all food groups and for cohort, maternal age, educational level, parity, prepregnancy body mass index, gestational weight gain, active smoking during pregnancy, and year of delivery. Women from KANC and BiB are not included.
Models are adjusted for all food groups and for cohort, age, body mass index, Family Affluence Score, year of birth, and firstborn.
Food Consumption and Measured Environmental Contaminants in Urine during Pregnancy and Childhood
During pregnancy, consumption of meat was associated with higher levels of BPA and lower levels of oxo-MiNP, and high consumption of fish was associated with higher levels of PRPA and BUPA (see Excel Table S8). Medium fish intake in childhood (1.5–3 times/week) was associated with higher urinary BPA, whereas higher fish intake () was associated with lower OXBE levels. Finally, dairy consumption in the medium tertile (15–26 times/week) was associated with higher urinary BUPA levels. Only the associations between maternal meat intake and BPA remained significant after controlling for multiple comparisons.
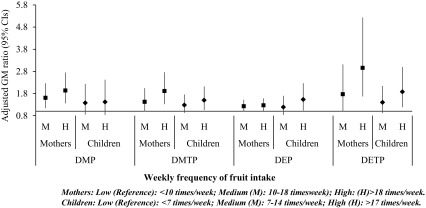
Regarding foods of plant origin, fruit intake was consistently associated with higher levels of urinary OP metabolites in both populations, in a dose–response manner (Figure 4; see also Excel Tables S13 and S14). More specifically, high fruit intake during pregnancy () was associated with more than 90% higher DMP and DMTP, 26% higher DEP, and a 295% increase in DETP urinary levels in pregnancy. Only associations with DEP did not remained significant after accounting for multiple comparisons. Although similar results were obtained when using the summarized OP concentrations as sumDAPs, sumDMs, and sumDEs, the effect estimates were attenuated (see Excel Table S15).
Figure 4.
Adjusted linear regression associations between weekly consumption of fruit and measured levels of organophosphate pesticide (OP) metabolites in maternal () and child urine (). Maternal models are mutually adjusted for all food groups and for cohort, maternal age, educational level, parity, prepregnancy body mass index, gestational weight gain, active smoking during pregnancy, and year of delivery. Child models are mutually adjusted for all food groups and for cohort, age, body mass index, Family Affluence Score, year of birth, and firstborn. Women from KANC and BiB cohorts are not included. Corresponding numeric data are reported in Excel Tables S13 and S14. BiB, Born in Bradford cohort; CI, confidence interval; DEP, diethyl phosphate; DETP; diethyl thiophosphate; DMP, dimethyl phosphate; DMTP, dimethyl thiophosphate; GM, geometric mean; KANC, Kaunas Cohort.
In children, only the highest fruit intake () was associated with increased urinary levels of OP metabolites (Figure 4; see also Excel Table S14). The highest increase, by 88%, was observed for DETP, as for the pregnant women, followed by approximately 50% increase in DMTP and DEP. When OP metabolized were analyzed in sums, the effect estimates were significant although some were attenuated (see Excel Table S15).
Regarding other foods of plant origin, vegetable consumption was positively associated with PRPA and MEP urinary levels in pregnancy, as well as with BPA and TCS levels in childhood (see Excel Tables S13 and S14). Cereal consumption was negatively associated with BPA, DEHP metabolites, and OH-MiNP in pregnancy. In children, fruit intake was negatively associated with BPA, whereas cereal intake was positively associated with MBzP and negatively associated with OH-MiNP levels. None of these associations remained significant after controlling for multiple comparisons.
TMLE Analysis—Fruit Recommendations and Levels of Environmental Contaminants
Using TMLE analysis, we estimated that if the whole population of pregnant women were following the recommendation of consuming at least two servings of any fruit per day (2 fruits/d), it would result in creatinine higher DMP levels, compared with women consuming (Table 3). Under a similar scenario, maternal DAPs would be creatinine higher, and maternal DMs, creatinine higher, than the levels of women consuming . Regarding the children, we found no significant increase of urinary OP metabolites related to the recommended fruit consumption.
Table 3.
Estimation of the marginal geometric mean difference in urinary organophosphate pesticide metabolites concentrations in mothers and children from a hypothetical intervention scenario of weekly fruit consumption.
| Pollutants | Exposed/total (/) | Intervention scenario | |
|---|---|---|---|
| 95% CI | |||
| Women (pregnancy)a | |||
| DMP ( creatinine) | 182/745 | 4.51 | 0.77, 8.26 |
| DMTP ( creatinine) | 184/747 | 3.96 | , 8.15 |
| DEP ( creatinine) | 184/745 | 1.16 | , 2.88 |
| DETP ( creatinine) | 178/712 | 0.88 | , 1.77 |
| sumDAPs (nmol/g creatinine) | 176/708 | 77.5 | 18.5,136.5 |
| sumDM (nmol/g creatinine) | 176/708 | 63.0 | 9.1,116.8 |
| sumDE (nmol/g creatinine) | 176/708 | 12.7 | , 27.6 |
| Children (5–12 y of age)b | |||
| DMP ( creatinine) | 184/1,286 | 0.55 | , 1.52 |
| DMTP ( creatinine) | 184/1,286 | 0.43 | , 2.49 |
| DEP ( creatinine) | 184/1,286 | 3.97 | , 10.69 |
| DETP ( creatinine) | 184/1,286 | 0.56 | , 1.14 |
| sumDAPs (nmol/g creatinine) | 184/1,286 | 36.4 | , 84.7 |
| sumDM (nmol/g creatinine) | 184/1,286 | 7.5 | , 26.7 |
| sumDE (nmol/g creatinine) | 184/1,286 | 29.2 | , 75.3 |
Intervention scenario: : following the dietary recommendation for daily fruit consumption (women and children: at least 2 servings of fruits/day); : not reaching the dietary recommendation for daily fruit consumption. BIB, Born in Bradford cohort; CI, confidence interval; DEP, diethyl phosphate; DETP; diethyl thiophosphate; DMP, dimethyl phosphate; DMTP, dimethyl thiophosphate; KANC, Kaunas Cohort; sumDAPs, total dialkylphosphate metabolites (DMP, DMTP, DEP, DETP); sumDE, total diethylphosphate metabolites (DEP and DETP); sumDM, total dimethylphosphate metabolites (DMP and DMTP); : marginal geometric mean difference between exposed and unexposed.
Models are adjusted for all food groups and for cohort, maternal age, educational level, parity, prepregnancy body mass index, gestational weight gain, active smoking during pregnancy, and year of delivery. Women from KANC and BiB are not included.
Models are adjusted for all food groups and for cohort, age, body mass index, Family Affluence Score, year of birth, and firstborn.
Organic Food Consumption in Childhood
Organic food consumption in childhood was associated with blood and urine levels of several environmental contaminants in children samples, in a dose-related manner (see Excel Table S16). Regarding positive associations, the largest increase was found for PBDEs, with 50% and 80% higher PBDE47 and PBDE153 levels associated with organic food consumption more than once per week, compared with no organic food consumption. Similarly, 28% higher PCBs and 9% higher PFOA levels were found. Regarding negative associations, the largest effect estimate was found for DEP, with 38% lower levels reported for consumers of organic food of compared with no organic food consumers. In addition, organic food consumption was associated with 8% lower Pb, 26% lower MEP, and 11% lower DEHP metabolites.
Discussion
In this international, multicenter study, we systematically analyzed the dietary predictors of exposure to a wide range of environmental contaminants in pregnancy and childhood. We found that fish consumption was positively associated with exposure to multiple contaminants, including PFASs, As, and Hg. Using TMLE analysis, we estimated that adherence to the dietary recommendations (pregnant women: , children ) for fish intake would result in lower exposure to PFASs, As, and Hg compared with those exceeding these recommendation. Fruit consumption was associated with increased levels of urinary OP metabolites concentrations in both pregnant women and children. Using TMLE analysis, we found that consuming more than 2 fruits/d could increase the exposure of pregnant women to OPs, compared with lower fruit intake.
Previous research has showed that fish consumption is a major source of exposure to PFASs for several adult populations worldwide (Christensen et al. 2017; Domingo and Nadal 2017; Ericson et al. 2008; Haug et al. 2010), including pregnant women and women of reproductive age (Berg et al. 2014; Brantsæter et al. 2013; Jain 2013; Manzano-Salgado et al. 2016). Nevertheless, studies in children have failed to find an association between fish intake and blood PFASs, attributing the absence of association to lower fish intake in children than in adults (Jain 2018; Wu et al. 2015). In the present study, fish consumption was positively associated with maternal PFUnDA levels, in a dose–response manner, whereas in children only high fish intake () were significantly associated with increased PFUnDA. In addition, we found that the association between oily fish consumption and PFUnDA in children was stronger than for white fish. Fish consumption was identified as a major contributor to PFUnDA exposure in a study analyzing foods from European markets (Klenow et al. 2013). The higher bioaccumulation potential in the aquatic food webs of long-chain PFASs (C9–C12), like PFUnDA, compared with the short-chain PFASs, as well as the nondecreasing environmental levels of PFUnDA, might explain why mainly PFUnDA was consistently associated with fish intake in both pregnant women and children (Bjerregaard-Olesen et al. 2016; Ng and Hungerbühler 2014). PFNA and PFOS levels were also positively associated with fish intake in children, whereas a similar increase was observed for medium and high intake. This might be explained by differences in the choices of specific fish species, meaning that the children in the high fish group chose fish that did not further contribute to PFOS and PFNA exposure. However, the analysis of oily and white fish consumption showed similar associations for FPNA and PFOS.
The strongest associations were found between fish consumption and blood levels of Hg and As in both pregnant women and children. Intake in the highest tertile ( for pregnant women and for children) were associated with two- and five-times higher blood Hg and As levels. Fish consumption is an established source of human exposure to these toxic elements (Awata et al. 2017; Birgisdottir et al. 2013; Brantsæter et al. 2010; EFSA 2012a; Jin et al. 2014; Miklavčič et al. 2013; Outzen et al. 2015; Veyhe et al. 2015), even in infants (Choi et al. 2017). Although fish and other seafood can be high in total As, the contribution of the more toxic inorganic As is only 2–3.5% of the total As (EFSA 2009, 2014). Nevertheless, there are large knowledge gaps regarding the toxicological potential of individual organic As species, such as arsenosugars and arsenolipids, for which seafood is a major source of exposure (Molin et al. 2017; Taylor et al. 2017) Unfortunately, the exposure levels to As species, within the organic or inorganic fraction, was not available in our study. On the other hand, the more toxic methyl mercury contributes up to 100% of the total Hg in fish (EFSA 2015a).
By applying TMLE analysis, we found that diets that did not exceed the recommended fish intake were associated with significantly lower PFNA, As, and Hg exposure in pregnant women and lower PFUnDA, PFOS, As, and Hg exposure in children. Overall, the exposure levels of the assessed environmental contaminants in our study population were lower or within the previously reported ranges for European and American populations (Haug et al. 2018). Haug et al. (2018) assessed the exposure levels of the HELIX population by human biomonitoring (HBM) cutoffs based on human health risk (The Human Biomonitoring Commission, Germany, 2017). For blood Hg, 72 women and 31 children exceeded the cutoff (), indicating some health risk related to the exposure. Similar cutoffs have been used to assess levels of concern in the National Health and Nutrition Examination Survey (NHANES) population () (Mahaffey et al. 2009). According to our TMLE findings, fish consumption within the recommended levels, one fourth of the women and one third of the children who were above the cutoff will be below the cutoff. Not exceeding the recommended fish consumption could result in a reduction of maternal serum Hg and PFNA and child Hg concentrations in levels below the 25th percentile of the NHANES survey levels for the respective population groups (Jain and Ducatman 2019; Schober et al. 2003). Nevertheless, we note that these interpretations and extrapolations of our effect estimates need to be interpreted with caution. Our study population reported more frequent fish consumption than what has been previously reported in large population-based studies (Garaulet et al. 2011; Papanikolaou et al. 2014; Stratakis et al. 2017; Tognon et al. 2014). More than half of the included pregnant women and children were consuming fish up to the recommendation (see Excel Table S17). Overall, then, in a population of women of reproductive age and young children with relatively high fish intake, not exceeding the recommended weekly servings could effectively reduce their exposure as well as their child’s prenatal and postnatal exposure to As, Hg, and some PFASs, without losing the health benefits associated with fish consumption (EFSA 2015a).
We found a positive association between fruit intake and concentrations of OP metabolites in urine for both pregnant women and children. Pesticides commonly used all over Europe and in other countries worldwide, such as chlorpyrifos, can contribute to such exposures, monitored as increased urinary levels of dialkyl phosphates metabolites (Cequier et al. 2017). Fruit consumption has been consistently related to higher exposure to OPs in adults and children (Berman et al. 2013; Cequier et al. 2017; Lewis et al. 2015; McKelvey et al. 2013; Muñoz-Quezada et al. 2012; Osaka et al. 2016; Sokoloff et al. 2016). In addition, organic food consumption in childhood was associated with lower levels of OP metabolites (i.e., DEP). This is line with several current studies, including an intervention study in Californian children and a randomized cross-over study in Australian adults (Berman et al. 2016; Bradman et al. 2015; Oates et al. 2014). Given the lower levels of pesticide residues in organic food compared with conventionally grown food (EFSA 2015b), organic food consumption may be an effective way to reduce the dietary exposure to OPs in European populations.
On the other hand, our TMLE analysis showed that adherence to the recommended fruit intake would result in higher OP exposure and that was significant for pregnant women only. In our study population, a fourth of the included women and 14% of the included children adhered to the fruit recommendation (see Excel Table S17) (WHO 2000). Hence, the diet quality of women of reproductive age and children can be improved by increasing the intake of fruit and vegetables and this is advisable. Nevertheless, a future intervention aiming to increase fruit intake in these population groups, could result in increased exposure to OPs, especially in women, consequently exposing the developing fetus to these chemicals. High prenatal OP exposure is of concern because it has been linked to restricted fetal growth and reduced neurodevelopment in children, including autism spectrum disorders (González-Alzaga et al. 2014; Harley et al. 2011; Naksen et al. 2015; Rauch et al. 2012; Sagiv et al. 2018). However, supporting the choice of organically over conventionally grown fruits might be an effective way to increase the dietary quality of a population and the related beneficial health effects without increasing the possible risks related to pesticide exposure.
Negative associations between food intake and levels of environmental contaminants are more challenging to interpret and are scarcely reported. Nevertheless, some associations may be explained by well-known biological interactions between essential nutrients and toxic elements. High meat intake was associated with a decrease in blood Cd levels in nonsmoking women and all children. A similar finding was reported in nonsmoking women from 16 European countries (Berglund et al. 2015). In a status of low iron, the uptake and absorption of divalent metals, including Cd, is higher, resulting in increased Cd blood levels (Meltzer et al. 2010). Hence, meat eaters might have better iron status, resulting in lower uptake of Cd and lower concentrations in blood. Unfortunately, we could not explore this in more details because iron status assessment was not available in our study. In addition, we observed that dairy consumption was associated with lower Pb and Hg levels in both pregnant women and children. Pb absorption is enhanced under calcium (Ca) deficiency, whereas Pb can interfere the metabolism of Ca and disrupt the intercellular Ca homeostasis and functions (Kwong et al. 2004; Talpur et al. 2018). Hence, high dairy intake, resulting in high Ca intake, might reduce Pb absorption and its related neurotoxic effects (Gomes et al. 2017). Even though the Ca-Pb interaction is one of the most-studied essential nutrient–toxic element interactions, little evidence exists between dairy intake and Pb blood levels, especially in children. To our knowledge, only one study, of 5- to 8-y-old children () from Uruguay, found a negative trend between dairy consumption, mainly yogurt, and blood Pb concentrations, and between estimated Ca intake from diet and blood Pb (Kordas et al. 2018). Calcium homeostasis is also a target of Hg, along with Cd and Pb, and in vitro studies have shown that the toxic metals can disturb the intracellular Ca homeostasis and act on Ca-mediated cell functions (Hechtenberg and Beyersmann 1991; Kirk et al. 2003).
Fewer and less consistent associations were observed between food intake and urinary levels of nonpersistent contaminants. Fish and vegetable intake were identified as potential emerging dietary sources OXBE, TCS, and parabens. Some parabens are permitted to be applied on food in the European Union, whereas besides such uses, parabens, along with OXBE and TCS, are widely used in cosmetics, personal care products, and pharmaceuticals as antimicrobial preservatives (Andersen 2008; Dann and Hontela 2011; DiNardo and Downs 2018; EFSA 2004; Sakhi et al. 2018). Common to all the abovementioned environmental phenols, no dietary exposure sources have been identified (Sakhi et al. 2018), whereas their widespread use could result to the contamination of the aquatic biota and crops via wastewater and sewage effluent contamination (Dann and Hontela 2011; van Wijnen et al. 2018). For example, OXBE has been found globally in water, soil, sediments, sludge, and biota and can bioaccumulate in fish (Balmer et al. 2005; Brausch and Rand 2011; Gago-Ferrero et al. 2012). Taken together, our results suggest that vegetables and fish consumption might be an emerging source of human dietary exposure to environmental phenols commonly used in personal care products including parabens, OXBE and TCS. We acknowledge that the large contribution of nondietary exposure sources to several nonpersistent compounds might explain the weaker and fewer associations observed. In addition, several co-exposures from personal care products, rather than diet itself, might explain our findings.
Strengths and Limitations
One of the main strengths of our study is the inclusion of biomarkers for a large number of contaminants from diverse groups of chemicals, including persistent and nonpersistent environmental chemicals. The large sample size as well as the focus on vulnerable groups, namely pregnant women and young children, are strengths of our study. Single-country studies with similar aims as ours have generated inconsistent results, even for well-known dietary exposures, resulting from many factors, including sample size and low variability in food intake. Small differences in the predictor variables across the population (food intake) might contribute to a lower explained variation of the outcome (biomarkers of exposure to contaminants), even though diet still would be the main contributor to exposure (Pearce 2011). Hence, a multicountry design, with a wide span from north to south Europe, can increase the variation in both food intake and measured biomarkers, as well as the generalizability of the findings across Europe. Nevertheless, we acknowledge that by pooling data from different countries, country-specific dietary habits and differences in food contamination levels between countries are not fully acknowledged. Hence, the extrapolation of our findings for each country needs careful interpretation. In addition, the use of TMLE to test the effect on dietary recommendations on exposure to environmental chemicals is a strength of our study. Such methodologies can be used to better inform future public health policies.
On the other hand, a limitation related to this design is the different FFQs used for the assessment of maternal diet. However, in children, an identical FFQ was applied in all centers. The use of FFQs might not be an appropriate dietary assessment method, especially for exposure to contaminants with short half-lives, given that FFQs are made to capture long-term dietary habits that most probably do not reflect day-to-day variations (Papadopoulou et al. 2016). Estimated energy intake was not available in our study and is not included as a covariate in our models given that it is widely recommended for studies exploring diet–health associations (Rhee et al. 2014). Due to the multicountry design, we considered that energy calculations based on country-specific nutrition composition tables would induce bias in our estimates, rather than reduce it. On the other hand, body weight is an independent and objective marker that is strongly associated with energy expenditure and it has been taken into account in our analyses. Although in some respect, the HELIX cohorts were ideally suited for pooling because of similar data collection tools, there was also variability in confounders among the six cohorts, described in detail by Maitre et al. (2018). Thus, although our models are adjusted for cohort, maternal education, and family affluence, we cannot rule out the possibility of bias in our effect estimates due to residual confounding due to socioeconomic position. Montazeri et al. (2019) have reported and discussed the socioeconomic disparities in exposure to environmental contaminants in the HELIX population and in light of our results, such socioeconomic disparities can be partially explained by dietary behavior. Finally, for the TMLE analysis, although we used clinically based international dietary advice to estimate effects on blood and urinary biomarkers of contaminants, we acknowledge that country-specific dietary advice may exist, including detailed consumption advice for specific fish species (Oken et al. 2003; Oken 2015).
Conclusion
In conclusion, high fish consumption during the critical developmental periods of pregnancy and early childhood was associated with higher concentrations of PFASs, As, and Hg in biological samples collected from pregnant women and children. These compounds have certain or probable health effects following early life exposures. Our findings suggest that exposure to these persistent compounds could be reduced by changes in dietary behaviors, such as not exceeding the current dietary recommendation for fish. In addition, high fruit consumption was related to increased exposure to pesticides, whereas the choice of organic over conventionally grown fruits contributed to lower exposures of pesticide residues. Overall, given that foods are a vessel of essential nutrients as well as toxic environmental contaminants, potential dietary interventions, focused on fruit and fish intake, may consider also accounting for the simultaneous increase in PFAS, As, and Hg exposure and balance the benefits of a healthy diet with the harms of exposure to toxic environmental chemicals.
Supplementary Material
Acknowledgments
We are grateful to all the participating children, parents, practitioners and researchers in the six cohorts who took part in this study. Born in Bradford (BiB) is only possible because of the enthusiasm and commitment of the Children and Parents in BiB. We are grateful to all the participants, health professionals and researchers who have made Born in Bradford happen.
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 308,333, the Human Early Life Exposome (HELIX) project. The Environment and Childhood (INMA) Sabadell cohort and biomarker measurements were funded by grants from Instituto de Salud Carlos III [Red INMA G03/176; CB06/02/0041; PI041436; PI081151 including Federación Española de Enfermedades Raras (FEDER) funds; PI12/01,890 including FEDER funds; CP13/00,054 including FEDER funds], CIBERESP, Generalitat de Catalunya-Consell Interdepartamental de Recerca i Innovació Tecnològica (CIRIT) 1999SGR 00,241, Generalitat de Catalunya-Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) (2009 SGR 501, 2014 SGR 822), Fundació La marató de TV3 (090,430), Spanish Ministry of Economy and Competitiveness (SAF2012-32,991 including FEDER funds). The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, National Institutes of Health, National Institute of Neurological Disorders and Stroke (NIH/NINDS; grants UO1 NS 047,537-01 and UO1 NS 047,537-06A1). The Rhea cohort was financially supported by European projects [EU FP6-2003-Food-3-NewGeneris, EU FP6 STREP Hiwate, EU FP7 ENV.2007.1.2.2.2. (project no. 211,250 Escape), EU FP7-2008-ENV-1.2.1.4 Envirogenomarkers, EU FP7-HEALTH-2009-single stage CHICOS, EU FP7 ENV.2008.1.2.1.6. (proposal no. 226,285 ENRIECO), EU- FP7-HEALTH-2012 (proposal no. 308,333 HELIX)] and by the Greek Ministry of Health (Program of Prevention of Obesity and Neurodevelopmental Disorders in Preschool Children, in Heraklion District, Crete, Greece: 2011–2014; “Rhea Plus”: Primary Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012–15). The Lithuanian Kaunas Cohort (KANC) supported by the Lithuanian Agency for Science Innovation and Technology (No. 31V-77). M.C. received funding from Instituto de Salud Carlos III (Ministry of Economy and Competitiveness) (MS16/00,128). E.P. and A.L.B. received funding from the Norwegian Research Council (Project No. 268,465). R.R.C.M. and J.W. were supported by the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care (CLAHRC) Yorkshire and Humber (IS-CLA-0113-10,020; http://www.clahrc-yh.nihr.ac.uk). L.C. received funding from the NIH (P30ES007048) and National Institute of Environmental Health Sciences (NIEHS R21ES02890).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5324).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- AHA/ASA (American Heart Association, American Stroke Association). 2017. The American Heart Association’s diet and lifestyle recommendations. http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/Nutrition/The-American-Heart-Associations-Diet-and-Lifestyle-Recommendations_UCM_305855_Article.jsp#.WkuCuNCnFaQ [accessed 1 February 2018].
- Alderete TL, Jin R, Walker DI, Valvi D, Chen Z, Jones DP. 2019. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: a proof-of-concept analysis. Environ Int 126:445–453, PMID: 30844580, 10.1016/j.envint.2019.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen FA. 2008. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol 27(Suppl 4):1–82, PMID: 19101832, 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- Awata H, Linder S, Mitchell LE, Delclos GL. 2017. Association of dietary intake and biomarker levels of arsenic, cadmium, lead, and mercury among Asian populations in the United States: NHANES 2011–2012. Environ Health Perspect 125(3):314–323, PMID: 27586241, 10.1289/EHP28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens W, Vrijens J, Gao Y, Croes K, Schoeters G, Den Hond E. 2014. Trace metals in blood and urine of newborn/mother pairs, adolescents and adults of the Flemish population (2007–2011). Int J Hyg Environ Health 217(8):878–890, PMID: 25041848, 10.1016/j.ijheh.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Balmer ME, Buser HR, Muller MD, Poiger T. 2005. Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from Swiss lakes. Environ Sci Technol 39(4):953–962, PMID: 15773466, 10.1021/es040055r. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. 2005. Quantitative trait loci analysis using the false discovery rate. Genetics 171(2):783–790, PMID: 15956674, 10.1534/genetics.104.036699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg V, Nøst TH, Huber S, Rylander C, Hansen S, Veyhe AS, et al. . 2014. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ Int 69:58–66, PMID: 24815340, 10.1016/j.envint.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Berglund M, Larsson K, Grandér M, Casteleyn L, Kolossa-Gehring M, Schwedler G, et al. . 2015. Exposure determinants of cadmium in European mothers and their children. Environ Res 141:69–76, PMID: 25465922, 10.1016/j.envres.2014.09.042. [DOI] [PubMed] [Google Scholar]
- Berman T, Göen T, Novack L, Beacher L, Grinshpan L, Segev D, et al. . 2016. Urinary concentrations of organophosphate and carbamate pesticides in residents of a vegetarian community. Environ Int 96:34–40, PMID: 27588700, 10.1016/j.envint.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Berman T, Goldsmith R, Göen T, Spungen J, Novack L, Levine H, et al. . 2013. Urinary concentrations of organophosphate pesticide metabolites in adults in Israel: demographic and dietary predictors. Environ Int 60:183–189, PMID: 24064379, 10.1016/j.envint.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Birgisdottir BE, Knutsen HK, Haugen M, Gjelstad IM, Jenssen MTS, Ellingsen DG, et al. . 2013. Essential and toxic element concentrations in blood and urine and their associations with diet: results from a Norwegian population study including high-consumers of seafood and game. Sci Total Environ 463–464:836–844, PMID: 23867847, 10.1016/j.scitotenv.2013.06.078. [DOI] [PubMed] [Google Scholar]
- Bjerregaard-Olesen C, Bach CC, Long M, Ghisari M, Bossi R, Bech BH, et al. . 2016. Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008–2013. Environ Int 91:14–21, PMID: 26891270, 10.1016/j.envint.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Bradman A, Quirós-Alcalá L, Castorina R, Aguilar Schall R, Camacho J, Holland NT, et al. . 2015. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ Health Perspect 123(10):1086–1093, PMID: 25861095, 10.1289/ehp.1408660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsæter AL, Haugen M, Alexander J, Meltzer HM. 2008. Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Matern Child Nutr 4(1):28–43, PMID: 18171405, 10.1111/j.1740-8709.2007.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsæter AL, Haugen M, Thomassen Y, Ellingsen DG, Ydersbond TA, Hagve T-A, et al. . 2010. Exploration of biomarkers for total fish intake in pregnant Norwegian women. Public Health Nutr 13(1):54–62, PMID: 19490733, 10.1017/S1368980009005904. [DOI] [PubMed] [Google Scholar]
- Brantsæter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, et al. . 2013. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int 54:74–84, PMID: 23419425, 10.1016/j.envint.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM. 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13(3):161–173, PMID: 27857130, 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brausch JM, Rand GM. 2011. A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82(11):1518–1532, PMID: 21185057, 10.1016/j.chemosphere.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Cao LL, Yan CH, Yu XD, Tian Y, Zhao L, Liu JX, et al. . 2011. Relationship between serum concentrations of polychlorinated biphenyls and organochlorine pesticides and dietary habits of pregnant women in Shanghai. Sci Total Environ 409(16):2997–3002, PMID: 21665017, 10.1016/j.scitotenv.2011.04.040. [DOI] [PubMed] [Google Scholar]
- Casas M, Valvi D, Luque N, Ballesteros-Gomez A, Carsin AE, Fernandez MF, et al. . 2013. Dietary and sociodemographic determinants of bisphenol A urine concentrations in pregnant women and children. Environ Int 56:10–18, PMID: 23542682, 10.1016/j.envint.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Caspersen IH, Kvalem HE, Haugen M, Brantsæter AL, Meltzer HM, Alexander J, et al. . 2016. Determinants of plasma PCB, brominated flame retardants, and organochlorine pesticides in pregnant women and 3 year old children in the Norwegian Mother and Child Cohort Study. Environ Res 146:136–144, PMID: 26749444, 10.1016/j.envres.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Castaño A, Cutanda F, Esteban M, Pärt P, Navarro C, Gómez S, et al. . 2015. Fish consumption patterns and hair mercury levels in children and their mothers in 17 EU countries. Environ Res 141:58–68, PMID: 25667172, 10.1016/j.envres.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Cequier E, Marcé RM, Becher G, Thomsen C. 2015. Comparing human exposure to emerging and legacy flame retardants from the indoor environment and diet with concentrations measured in serum. Environ Int 74:54–59, PMID: 25454220, 10.1016/j.envint.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Haug LS, Thomsen C. 2017. Exposure to organophosphorus pesticides in Norwegian mothers and their children: diurnal variability in concentrations of their biomarkers and associations with food consumption. Sci Total Environ 590–591:655–662, PMID: 28284640, 10.1016/j.scitotenv.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Chatzi L, Leventakou V, Vafeiadi M, Koutra K, Roumeliotaki T, Chalkiadaki G, et al. . 2017. Cohort profile: the mother–child cohort in Crete, Greece (Rhea study). Int J Epidemiol 46(5):1392–1393k, PMID: 29040580, 10.1093/ije/dyx084. [DOI] [PubMed] [Google Scholar]
- Chatzi L, Melaki V, Sarri K, Apostolaki I, Roumeliotaki T, Georgiou V, et al. . 2011. Dietary patterns during pregnancy and the risk of postpartum depression: the mother-child ‘Rhea’ cohort in Crete, Greece. Public Health Nutr 14(9):1663–1670, PMID: 21477412, 10.1017/S1368980010003629. [DOI] [PubMed] [Google Scholar]
- Choi J, Chang JY, Hong J, Shin S, Park JS, Oh S. 2017. Low-level toxic metal exposure in healthy weaning-age infants: association with growth, dietary intake, and iron deficiency. Int J Environ Res Public Health 14(4):E388, PMID: 28383506, 10.3390/ijerph14040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KY, Raymond M, Blackowicz M, Liu Y, Thompson BA, Anderson HA, et al. . 2017. Perfluoroalkyl substances and fish consumption. Environ Res 154:145–151, PMID: 28073048, 10.1016/j.envres.2016.12.032. [DOI] [PubMed] [Google Scholar]
- Claus Henn B, Coull BA, Wright RO. 2014. Chemical mixtures and children’s health. Curr Opin Pediatr 26(2):223–229, PMID: 24535499, 10.1097/MOP.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaci A, Den Hond E, Geens T, Govarts E, Koppen G, Frederiksen H, et al. . 2015. Urinary BPA measurements in children and mothers from six European member states: overall results and determinants of exposure. Environ Res 141:77–85, PMID: 25440295, 10.1016/j.envres.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Dann AB, Hontela A. 2011. Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol 31(4):285–311, PMID: 21462230, 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- Dassuncao C, Hu XC, Nielsen F, Weihe P, Grandjean P, Sunderland EM. 2018. Shifting global exposures to poly- and perfluoroalkyl substances (PFASs) evident in longitudinal birth cohorts from a seafood-consuming population. Environ Sci Technol, PMID: 29516726, 10.1021/acs.est.7b06044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps V, de Lauzon-Guillain B, Lafay L, Borys JM, Charles MA, Romon M. 2009. Reproducibility and relative validity of a food-frequency questionnaire among French adults and adolescents. Eur J Clin Nutr 63(2):282–291, PMID: 17882132, 10.1038/sj.ejcn.1602914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo JC, Downs CA. 2018. Dermatological and environmental toxicological impact of the sunscreen ingredient oxybenzone/benzophenone-3. J Cosmet Dermatol 17(1):15–19, PMID: 29086472, 10.1111/jocd.12449. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Nadal M. 2017. Per- and polyfluoro alkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. J Agric Food Chem 65(3):533–543, PMID: 28052194, 10.1021/acs.jafc.6b04683. [DOI] [PubMed] [Google Scholar]
- Drouillet P, Kaminski M, De Lauzon-Guillain B, Forhan A, Ducimetière P, Schweitzer M, et al. . 2009. Association between maternal seafood consumption before pregnancy and fetal growth: evidence for an association in overweight women. the EDEN mother-child cohort. Paediatr Perinat Epidemiol 23(1):76–86, PMID: 19228317, 10.1111/j.1365-3016.2008.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA. 2004. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to para hydroxybenzoates (E 214-219). EFSA J 83:1–26, 10.2903/j.efsa.2004.83. [DOI] [Google Scholar]
- EFSA. 2006a. Opinion of the Scientific Panel on Contaminants in the Food Chain [CONTAM] related to DDT as an undesirable substance in animal feed. EFSA J 433:1–69, 10.2903/j.efsa.2006.433. [DOI] [Google Scholar]
- EFSA. 2006b. Opinion of the Scientific Panel on Contaminants in the Food Chain [CONTAM] related to hexachlorobenzene as undesirable substance in animal feed. EFSA J 402:1–49, 10.2903/j.efsa.2006.402. [DOI] [Google Scholar]
- EFSA. 2009. Scientific opinion on arsenic in food. EFSA J 7(10):1351, 10.2903/j.efsa.2009.1351. [DOI] [Google Scholar]
- EFSA. 2012a. Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J 10(12):2985, 10.2903/j.efsa.2012.2985. [DOI] [Google Scholar]
- EFSA. 2012b. Update of the monitoring of levels of dioxins and PCBs in food and feed. EFSA J 10(7):2832, 10.2903/j.efsa.2012.2832. [DOI] [Google Scholar]
- EFSA. 2014. Dietary exposure to inorganic arsenic in the European population. EFSA J 12(3):3597, 10.2903/j.efsa.2014.3597. [DOI] [Google Scholar]
- EFSA. 2015a. Statement on the benefits of fish/seafood consumption compared to the risks of methylmercury in fish/seafood. EFSA J 13(1):3982, 10.2903/j.efsa.2015.3982. [DOI] [Google Scholar]
- EFSA. 2015b. The 2013 European Union report on pesticide residues in food. EFSA J 13(3):4038, 10.2903/j.efsa.2015.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson I, Martí-Cid R, Nadal M, Van Bavel B, Lindström G, Domingo JL. 2008. Human exposure to perfluorinated chemicals through the diet: intake of perfluorinated compounds in foods from the Catalan (Spain) market. J Agric Food Chem 56(5):1787–1794, PMID: 18251500, 10.1021/jf0732408. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Li Z, Korrick SA, Spiegelman D, Enelow R, Nadeau K, et al. . 2016. Infant infections and respiratory symptoms in relation to in utero arsenic exposure in a U.S. cohort. Environ Health Perspect 124(6):840–847, PMID: 26359651, 10.1289/ehp.1409282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago-Ferrero P, Díaz-Cruz MS, Barceló D. 2012. An overview of UV-absorbing compounds (organic UV filters) in aquatic biota. Anal Bioanal Chem 404(9):2597–2610, PMID: 22669305, 10.1007/s00216-012-6067-7. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Ortega FB, Ruiz JR, Rey-López JP, Béghin L, Manios Y, et al. . 2011. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. the HELENA study. Int J Obes (Lond) 35(10):1308–1317, PMID: 21792170, 10.1038/ijo.2011.149. [DOI] [PubMed] [Google Scholar]
- Giovanoulis G, Alves A, Papadopoulou E, Cousins AP, Schütze A, Koch HM, et al. . 2016. Evaluation of exposure to phthalate esters and DINCH in urine and nails from a Norwegian study population. Environ Res 151:80–90, PMID: 27466754, 10.1016/j.envres.2016.07.025. [DOI] [PubMed] [Google Scholar]
- Gomes WR, Devóz PP, Araújo ML, Batista BL, Barbosa F Jr, Barcelos GRM. 2017. Milk and dairy products intake is associated with low levels of lead (Pb) in workers highly exposed to the metal. Biol Trace Elem Res 178(1):29–35, PMID: 27988825, 10.1007/s12011-016-0913-y. [DOI] [PubMed] [Google Scholar]
- González-Alzaga B, Lacasaña M, Aguilar-Garduño C, Rodríguez-Barranco M, Ballester F, Rebagliato M, et al. . 2014. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett 230(2):104–121, PMID: 24291036, 10.1016/j.toxlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- González-Alzaga B, Lacasaña M, Hernández AF, Arrebola JP, López-Flores I, Artacho-Cordón F, et al. . 2018. Serum concentrations of organochlorine compounds and predictors of exposure in children living in agricultural communities from South-Eastern Spain. Environ Pollut 237:685–694, PMID: 29129429, 10.1016/j.envpol.2017.10.109. [DOI] [PubMed] [Google Scholar]
- Grazuleviciene R, Danileviciute A, Nadisauskiene R, Vencloviene J. 2009. Maternal smoking, GSTM1 and GSTT1 polymorphism and susceptibility to adverse pregnancy outcomes. Int J Environ Res Public Health 6(3):1282–1297, PMID: 19440446, 10.3390/ijerph6031282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Ballester F, Espada M, Fernández MF, Grimalt JO, Ibarluzea J, et al. . 2012. Cohort profile: the INMA—INfancia y Medio Ambiente–(Environment and Childhood) Project. Int J Epidemiol 41(4):930–940, PMID: 21471022, 10.1093/ije/dyr054. [DOI] [PubMed] [Google Scholar]
- Harley KG, Huen K, Aguilar Schall R, Holland NT, Bradman A, Barr DB, et al. . 2011. Association of organophosphate pesticide exposure and paraoxonase with birth outcome in Mexican-American women. PloS One 6(8):e23923, PMID: 21904599, 10.1371/journal.pone.0023923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug LS, Sakhi AK, Cequier E, Casas M, Maitre L, Basagaña X, et al. . 2018. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ Int 121(Pt 1):751–763, PMID: 30326459, 10.1016/j.envint.2018.09.056. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Brantsæter AL, Kvalem HE, Haugen M, Becher G, et al. . 2010. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ Int 36(7):772–778, PMID: 20579735, 10.1016/j.envint.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Hechtenberg S, Beyersmann D. 1991. Inhibition of sarcoplasmic reticulum Ca2+-ATPase activity by cadmium, lead and mercury. Enzyme 45(3):109–115, PMID: 1840035, 10.1159/000468875. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, et al. . 2015a. Developmental origins of health and disease: integrating environmental influences. Endocrinology 156(10):3416–3421, PMID: 26241070, 10.1210/en.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Newbold R, Schug TT. 2015b. Endocrine disruptors and obesity. Nat Rev Endocrinol 11(11):653–661, PMID: 26391979, 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Sass JB, Engel S, Bennett DH, Bradman A, Eskenazi B, et al. . 2018. Organophosphate exposures during pregnancy and child neurodevelopment: recommendations for essential policy reforms. PLoS Med 15(10):e1002671, PMID: 30356230, 10.1371/journal.pmed.1002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XC, Dassuncao C, Zhang X, Grandjean P, Weihe P, Webster GM, et al. . 2018. Can profiles of poly- and perfluoroalkyl substances (PFASs) in human serum provide information on major exposure sources? Environ Health 17(1):11, PMID: 29391068, 10.1186/s12940-018-0355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB. 2013. Effect of pregnancy on the levels of selected perfluoroalkyl compounds for females aged 17–39 years: data from National Health and Nutrition Examination Survey 2003–2008. J Toxicol Environ Health A 76(7):409–421, PMID: 23611181, 10.1080/15287394.2013.771547. [DOI] [PubMed] [Google Scholar]
- Jain RB. 2018. Contribution of diet and other factors to the observed levels of selected perfluoroalkyl acids in serum among US children aged 3-11 years. Environ Res 161:268–275, PMID: 29169101, 10.1016/j.envres.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Jain RB, Ducatman A. 2019. Roles of gender and obesity in defining correlations between perfluoroalkyl substances and lipid/lipoproteins. Sci Total Environ 653:74–81, PMID: 30408670, 10.1016/j.scitotenv.2018.10.362. [DOI] [PubMed] [Google Scholar]
- Jin L, Liu J, Ye B, Ren A. 2014. Concentrations of selected heavy metals in maternal blood and associated factors in rural areas in Shanxi Province, China. Environ Int 66:157–164, PMID: 24584080, 10.1016/j.envint.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Johansson HKL, Svingen T, Fowler PA, Vinggaard AM, Boberg J. 2017. Environmental influences on ovarian dysgenesis—developmental windows sensitive to chemical exposures. Nat Rev Endocrinol 13(7):400–414, PMID: 28450750, 10.1038/nrendo.2017.36. [DOI] [PubMed] [Google Scholar]
- Katsikantami I, Colosio C, Alegakis A, Tzatzarakis MN, Vakonaki E, Rizos AK, et al. . 2019. Estimation of daily intake and risk assessment of organophosphorus pesticides based on biomonitoring data—the internal exposure approach. Food Chem Toxicol 123:57–71, PMID: 30352298, 10.1016/j.fct.2018.10.047. [DOI] [PubMed] [Google Scholar]
- Kirk CJ, Bottomley L, Minican N, Carpenter H, Shaw S, Kohli N, et al. . 2003. Environmental endocrine disrupters dysregulate estrogen metabolism and Ca2+ homeostasis in fish and mammals via receptor-independent mechanisms. Comp Biochem Physiol A Mol Integr Physiol 135(1):1–8, PMID: 12727545, 10.1016/S1095-6433(02)00366-5. [DOI] [PubMed] [Google Scholar]
- Klenow S, Heinemeyer G, Brambilla G, Dellatte E, Herzke D, de Voogt P. 2013. Dietary exposure to selected perfluoroalkyl acids (PFAAs) in four European regions. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 30(12):2141–2151, PMID: 24279394, 10.1080/19440049.2013.849006. [DOI] [PubMed] [Google Scholar]
- Kordas K, Burganowski R, Roy A, Peregalli F, Baccino V, Barcia E, et al. . 2018. Nutritional status and diet as predictors of children’s lead concentrations in blood and urine. Environ Int 111:43–51, PMID: 29172090, 10.1016/j.envint.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong WT, Friello P, Semba RD. 2004. Interactions between iron deficiency and lead poisoning: epidemiology and pathogenesis. Sci Total Environ 330(1–3):21–37, PMID: 15325155, 10.1016/j.scitotenv.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Larsson K, Ljung Björklund K, Palm B, Wennberg M, Kaj L, Lindh CH, et al. . 2014. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ Int 73:323–333, PMID: 25216151, 10.1016/j.envint.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RC, Cantonwine DE, Anzalota Del Toro LV, Calafat AM, Valentin-Blasini L, Davis MD, et al. . 2015. Distribution and determinants of urinary biomarkers of exposure to organophosphate insecticides in Puerto Rican pregnant women. Sci Total Environ 512–513:337–344, PMID: 25634738, 10.1016/j.scitotenv.2015.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MM, Wu MQ, Xu J, Du J, Yan CH. 2014. Body burden of Hg in different bio-samples of mothers in Shenyang city, China. PloS One 9(5):e98121, PMID: 24858815, 10.1371/journal.pone.0098121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Goudarzi H, Oulhote Y. 2018. Developmental exposures to perfluoroalkyl substances (PFASs): an update of associated health outcomes. Curr Environ Health Rep 5(1):1–19, PMID: 29556975, 10.1007/s40572-018-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X. 2019. Tainted water: the scientists tracing thousands of fluorinated chemicals in our environment. Nature 566(7742):26–29, PMID: 30728524, 10.1038/d41586-019-00441-1. [DOI] [PubMed] [Google Scholar]
- Linares V, Bellés M, Domingo JL. 2015. Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol 89(3):335–356, PMID: 25637414, 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- Llop S, Ballester F, Vizcaino E, Murcia M, Lopez-Espinosa MJ, Rebagliato M, et al. . 2010. Concentrations and determinants of organochlorine levels among pregnant women in Eastern Spain. Sci Total Environ 408(23):5758–5767, PMID: 20832846, 10.1016/j.scitotenv.2010.07.085. [DOI] [PubMed] [Google Scholar]
- Llop S, Murcia M, Aguinagalde X, Vioque J, Rebagliato M, Cases A, et al. . 2014. Exposure to mercury among Spanish preschool children: trend from birth to age four. Environ Res 132:83–92, PMID: 24747554, 10.1016/j.envres.2014.03.023. [DOI] [PubMed] [Google Scholar]
- Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, et al. . 2016. Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 45(2):382–388, PMID: 27063603, 10.1093/ije/dyw029. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Jeffries RA. 2009. Adult women’s blood mercury concentrations vary regionally in the United States: association with patterns of fish consumption (NHANES 1999–2004). Environ Health Perspect 117(1):47–53, PMID: 19165386, 10.1289/ehp.11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre L, de Bont J, Casas M, Robinson O, Aasvang GM, Agier L, et al. . 2018. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open 8(9):e021311, PMID: 30206078, 10.1136/bmjopen-2017-021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Martinez D, Ibarluzea J, et al. . 2016. Variability of perfluoroalkyl substance concentrations in pregnant women by socio-demographic and dietary factors in a Spanish birth cohort. Environ Int 92–93:357–365, PMID: 27132161, 10.1016/j.envint.2016.04.004. [DOI] [PubMed] [Google Scholar]
- McKelvey W, Jacobson JB, Kass D, Barr DB, Davis M, Calafat AM, et al. . 2013. Population-based biomonitoring of exposure to organophosphate and pyrethroid pesticides in New York City. Environ Health Perspect 121(11–12):1349–1356, PMID: 24076605, 10.1289/ehp.1206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HM, Brantsæter AL, Borch-Iohnsen B, Ellingsen DG, Alexander J, Thomassen Y, et al. . 2010. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ Res 110(5):497–504, PMID: 20381026, 10.1016/j.envres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Miklavčič A, Casetta A, Snoj Tratnik J, Mazej D, Krsnik M, Mariuz M, et al. . 2013. Mercury, arsenic and selenium exposure levels in relation to fish consumption in the Mediterranean area. Environ Res 120:7–17, PMID: 22999706, 10.1016/j.envres.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Miyashita C, Sasaki S, Saijo Y, Okada E, Kobayashi S, Baba T, et al. . 2015. Demographic, behavioral, dietary, and socioeconomic characteristics related to persistent organic pollutants and mercury levels in pregnant women in Japan. Chemosphere 133:13–21, PMID: 25829055, 10.1016/j.chemosphere.2015.02.062. [DOI] [PubMed] [Google Scholar]
- Molin M, Ulven SM, Dahl L, Lundebye AK, Holck M, Alexander J, et al. . 2017. Arsenic in seafood is associated with increased thyroid-stimulating hormone (TSH) in healthy volunteers—a randomized controlled trial. J Trace Elem Med Biol 44:1–7, PMID: 28965562, 10.1016/j.jtemb.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Montazeri P, Thomsen C, Casas M, de Bont J, Haug LS, Maitre L, et al. . 2019. Socioeconomic position and exposure to multiple environmental chemical contaminants in six European mother-child cohorts. Int J Hyg Environ Health 222(5):864–872, PMID: 31010791, 10.1016/j.ijheh.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Quezada MT, Iglesias V, Lucero B, Steenland K, Barr DB, Levy K, et al. . 2012. Predictors of exposure to organophosphate pesticides in schoolchildren in the province of Talca, Chile. Environ Int 47:28–36, PMID: 22732215, 10.1016/j.envint.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JP, Zoeller RT, vom Saal FS. 2009. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ Health Perspect 117(11):1652–1655, PMID: 20049113, 10.1289/ehp.0900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naksen W, Prapamontol T, Mangklabruks A, Chantara S, Thavornyutikarn P, Srinual N, et al. . 2015. Associations of maternal organophosphate pesticide exposure and PON1 activity with birth outcomes in SAWASDEE birth cohort, Thailand. Environ Res 142:288–296, PMID: 26186137, 10.1016/j.envres.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CA, Hungerbühler K. 2014. Bioaccumulation of perfluorinated alkyl acids: observations and models. Environ Sci Technol 48(9):4637–4648, PMID: 24762048, 10.1021/es404008g. [DOI] [PubMed] [Google Scholar]
- Ng CA, von Goetz N. 2017. The global food system as a transport pathway for hazardous chemicals: the missing link between emissions and exposure. Environ Health Perspect 125(1):1–7, PMID: 27384039, 10.1289/EHP168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates L, Cohen M, Braun L, Schembri A, Taskova R. 2014. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ Res 132:105–111, PMID: 24769399, 10.1016/j.envres.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Oken E. 2015. Consumption of fish and long-chain n-3 polyunsaturated fatty acids during pregnancy: has the tide turned? Paediatr Perinat Epidemiol 29(5):388–390, PMID: 26264568, 10.1111/ppe.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Berland WE, Simon SR, Rich-Edwards JW, Gillman MW. 2003. Decline in fish consumption among pregnant women after a national mercury advisory. Obstet Gynecol 102(2):346–351, PMID: 12907111, 10.1016/s0029-7844(03)00484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka A, Ueyama J, Kondo T, Nomura H, Sugiura Y, Saito I, et al. . 2016. Exposure characterization of three major insecticide lines in urine of young children in Japan—neonicotinoids, organophosphates, and pyrethroids. Environ Res 147:89–96, PMID: 26855126, 10.1016/j.envres.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Outzen M, Tjønneland A, Larsen EH, Hansen M, Andersen KK, Christensen J, et al. . 2015. Effect of increased intake of fish and mussels on exposure to toxic trace elements in a healthy, middle-aged population. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32(11):1858–1866, PMID: 26284299, 10.1080/19440049.2015.1072878. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, Kogevinas M, Botsivali M, Pedersen M, Besselink H, Mendez MA, et al. . 2014. Maternal diet, prenatal exposure to dioxin-like compounds and birth outcomes in a European prospective mother–child study (NewGeneris). Sci Total Environ 484:121–128, PMID: 24691212, 10.1016/j.scitotenv.2014.03.047. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, Padilla-Sanchez JA, Collins CD, Cousins IT, Covaci A, de Wit CA, et al. . 2016. Sampling strategy for estimating human exposure pathways to consumer chemicals. Emerg Contam 2(1):26–36, 10.1016/j.emcon.2015.12.002. [DOI] [Google Scholar]
- Papanikolaou Y, Brooks J, Reider C, Fulgoni VL III.. 2014. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutr J 13(1):31, PMID: 24694001, 10.1186/1475-2891-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce N. 2011. Epidemiology in a changing world: variation, causation and ubiquitous risk factors. Int J Epidemiol 40(2):503–512, PMID: 21247886, 10.1093/ije/dyq257. [DOI] [PubMed] [Google Scholar]
- Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, et al. . 2017. Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 years. Environ Health Perspect 125(9):097014, PMID: 28937960, 10.1289/EHP1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Trasande L. 2017. Effects of early exposure to phthalates and bisphenols on cardiometabolic outcomes in pregnancy and childhood. Reprod Toxicol 68:105–118, PMID: 27596818, 10.1016/j.reprotox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raanan R, Harley KG, Balmes JR, Bradman A, Lipsett M, Eskenazi B. 2015. Early-life exposure to organophosphate pesticides and pediatric respiratory symptoms in the CHAMACOS cohort. Environ Health Perspect 123(2):179–185, PMID: 25369257, 10.1289/ehp.1408235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SA, Braun JM, Barr DB, Calafat AM, Khoury J, Montesano AM, et al. . 2012. Associations of prenatal exposure to organophosphate pesticide metabolites with gestational age and birth weight. Environ Health Perspect 120(7):1055–1060, PMID: 22476135, 10.1289/ehp.1104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor P, Born in Bradford Collaborative Group. 2008. Born in Bradford, a cohort study of babies born in Bradford, and their parents: protocol for the recruitment phase. BMC Public Health 8:327, PMID: 18811926, 10.1186/1471-2458-8-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee JJ, Cho E, Willett WC. 2014. Energy adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiological analyses. Public Health Nutr 17(5):1054–1060, PMID: 23701939, 10.1017/S1368980013001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Harris MH, Gunier RB, Kogut KR, Harley KG, Deardorff J, et al. . 2018. Prenatal organophosphate pesticide exposure and traits related to autism spectrum disorders in a population living in proximity to agriculture. Environ Health Perspect 126(4):047012, PMID: 29701446, 10.1289/EHP2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi AK, Sabaredzovic A, Papadopoulou E, Cequier E, Thomsen C. 2018. Levels, variability and determinants of environmental phenols in pairs of Norwegian mothers and children. Environ Int 114:242–251, PMID: 29524920, 10.1016/j.envint.2018.02.037. [DOI] [PubMed] [Google Scholar]
- Schober SE, Sinks TH, Jones RL, Bolger PM, McDowell M, Osterloh J, et al. . 2003. Blood mercury levels in US children and women of childbearing age, 1999–2000. JAMA 289(13):1667–1674, PMID: 12672735, 10.1001/jama.289.13.1667. [DOI] [PubMed] [Google Scholar]
- Schuler MS, Rose S. 2017. Targeted maximum likelihood estimation for causal inference in observational studies. Am J Epidemiol 185(1):65–73, PMID: 27941068, 10.1093/aje/kww165. [DOI] [PubMed] [Google Scholar]
- Sokoloff K, Fraser W, Arbuckle TE, Fisher M, Gaudreau E, LeBlanc A, et al. . 2016. Determinants of urinary concentrations of dialkyl phosphates among pregnant women in Canada—results from the MIREC study. Environ Int 94:133–140, PMID: 27243443, 10.1016/j.envint.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Stratakis N, Roumeliotaki T, Oken E, Ballester F, Barros H, Basterrechea M, et al. . 2017. Fish and seafood consumption during pregnancy and the risk of asthma and allergic rhinitis in childhood: a pooled analysis of 18 European and US birth cohorts. Int J Epidemiol 46(5):1465–1477, PMID: 28338907, 10.1093/ije/dyx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpur S, Afridi HI, Kazi TG, Talpur FN. 2018. Interaction of lead with calcium, iron, and zinc in the biological samples of malnourished children. Biol Trace Elem Res, PMID: 28861860, 10.1007/s12011-017-1141-9. [DOI] [PubMed] [Google Scholar]
- Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, et al. . 2013. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a National Toxicology Program workshop review. Environ Health Perspect 121(7):774–783, PMID: 23651634, 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, et al. . 2017. Human exposure to organic arsenic species from seafood. Sci Total Environ 580:266–282, PMID: 28024743, 10.1016/j.scitotenv.2016.12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Human Biomonitoring Commission, Germany. 2017. https://www.umweltbundesamt.de/en/topics/health/commissions-working-groups/humanbiomonitoring-commission/reference-hbm-values [accessed 8 February 2019].
- Tognon G, Moreno LA, Mouratidou T, Veidebaum T, Molnár D, Russo P, et al. . 2014. Adherence to a Mediterranean-like dietary pattern in children from eight European countries. the IDEFICS study. Int J Obes (Lond) 38(Suppl 2):S108–S114, PMID: 25219407, 10.1038/ijo.2014.141. [DOI] [PubMed] [Google Scholar]
- Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, et al. . 2017. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: evidence from rural Bangladesh. Environ Health Perspect 125(6):067015, PMID: 28669934, 10.1289/EHP614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Dries MA, Pronk A, Guxens M, Spaan S, Voortman T, Jaddoe VW, et al. . 2018. Determinants of organophosphate pesticide exposure in pregnant women: a population-based cohort study in the Netherlands. Int J Hyg Environ Health 221(3):489–501, PMID: 29499913, 10.1016/j.ijheh.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan MJ, Polley EC, Hubbard AE. 2007. Super learner. Stat Appl Genet Mol Biol 6(1):Article25, PMID: 17910531, 10.2202/1544-6115.1309. [DOI] [PubMed] [Google Scholar]
- van Wijnen J, Ragas AMJ, Kroeze C. 2018. River export of triclosan from land to sea: a global modelling approach. Sci Total Environ 621:1280–1288, PMID: 29079081, 10.1016/j.scitotenv.2017.10.100. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al. . 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33(3):378–455, PMID: 22419778, 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyhe AS, Hofoss D, Hansen S, Thomassen Y, Sandanger TM, Odland JØ, et al. . 2015. The Northern Norway Mother-and-Child Contaminant Cohort (MISA) Study: PCA analyses of environmental contaminants in maternal sera and dietary intake in early pregnancy. Int J Hyg Environ Health 218(2):254–264, PMID: 25556042, 10.1016/j.ijheh.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Vioque J, Navarrete-Muñoz EM, Gimenez-Monzó D, García-de-la-Hera M, Granado F, Young IS, et al. . 2013. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J 12:26, PMID: 23421854, 10.1186/1475-2891-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. 2016. Environmental pollutants and child health—a review of recent concerns. Int J Hyg Environ Health 219(4–5):331–342, PMID: 27216159, 10.1016/j.ijheh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Slama R, Robinson O, Chatzi L, Coen M, van den Hazel P, et al. . 2014. The Human Early-Life Exposome (HELIX): project rationale and design. Environ Health Perspect 122(6):535–544, PMID: 24610234, 10.1289/ehp.1307204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A. 2018. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: current findings and future directions. Horm Behav 101:94–104, PMID: 29137973, 10.1016/j.yhbeh.2017.11.008. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2000. CINDI Dietary Guide. Copenhagen, Denmark: WHO. [Google Scholar]
- WHO. 2006. Food and Nutrition Policy for schools: A tool for the Development of School Nutrition Programmes in the WHO European Region. Copenhagen, Denmark: WHO, Programme for Nutrition and Food Security; http://www.euro.who.int/__data/assets/pdf_file/0019/152218/E89501.pdf?ua=1 [accessed 29 September 2019]. [Google Scholar]
- Wright J, Small N, Raynor P, Tuffnell D, Bhopal R, Cameron N, et al. . 2013. Cohort profile: the Born in Bradford multi-ethnic family cohort study. Int J Epidemiol 42(4):978–991, PMID: 23064411, 10.1093/ije/dys112. [DOI] [PubMed] [Google Scholar]
- Wu XM, Bennett DH, Calafat AM, Kato K, Strynar M, Andersen E, et al. . 2015. Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and adults in California. Environ Res 136:264–273, PMID: 25460645, 10.1016/j.envres.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.