Abstract
Background:
The autonomic nervous system plays a key role in maintaining homeostasis and responding to external stimuli. In adults, exposure to fine particulate matter () has been associated with reduced heart rate variability (HRV), an indicator of cardiac autonomic control.
Objectives:
Our goal was to investigate the associations of exposure to fine particulate matter () with HRV as an indicator of cardiac autonomic control during early development.
Methods:
We studied 237 maternal–infant pairs in a Boston-based birth cohort. We estimated daily residential using satellite data in combination with land-use regression predictors. In infants at 6 months of age, we measured parasympathetic nervous system (PNS) activity using continuous electrocardiogram monitoring during the Repeated Still-Face Paradigm, an experimental protocol designed to elicit autonomic reactivity in response to maternal interaction and disengagement. We used multivariable linear regression to examine average exposure across pregnancy in relation to PNS withdrawal and activation, indexed by changes in respiration-corrected respiratory sinus arrhythmia ()—an established metric of HRV that reflects cardiac vagal tone. We examined interactions with infant sex using cross-product terms.
Results:
In adjusted models we found that a 1-unit increase in (in micrograms per cubic meter) was associated with a 3.53% decrease in baseline (95% CI: , 0.02). In models examining change between episodes, higher was generally associated with reduced PNS withdrawal during stress and reduced PNS activation during recovery; however, these associations were not statistically significant. We did not observe a significant interaction between and sex.
Discussion:
Prenatal exposure to may disrupt cardiac vagal tone during infancy. Future research is needed to replicate these preliminary findings. https://doi.org/10.1289/EHP4434
Introduction
Substantial evidence documents adverse cardiovascular effects of recent (hours to days) and chronic (weeks to years) ambient air pollution exposure in older children and adults (An et al. 2018; Chuang et al. 2007; Franklin et al. 2015; Pope et al. 2004), with mechanistic research highlighting the contribution of fine particulate matter (PM in aerodynamic diameter; ) to the development of adverse health outcomes (Nelin et al. 2012). For example, short- and long-term exposure to particulate pollution has been linked to reduced heart rate variability (HRV) (Chuang et al. 2007; Gold et al. 2000; Liao et al. 2004; Luttmann-Gibson et al. 2010; Park et al. 2005; Pieters et al. 2012), which in turn is associated with increased risk of myocardial infarction, arrhythmias, hypertension, metabolic syndrome, and corresponding morbidities (Buccelletti et al. 2009; Licht et al. 2010; Tentolouris et al. 2008; Thayer et al. 2010). A critical step in identifying individuals at risk for costly chronic cardiometabolic disorders is characterizing relevant exposures and mechanisms that lead to early predisposition. Although it is increasingly recognized that complex chronic diseases have their roots in early development (Hoffman et al. 2017; Thornburg 2015), little research has examined associations between exposure and autonomic nervous system (ANS) functioning in early life.
Critical components of the ANS develop and mature during gestation. For example, differentiation of the hypothalamic lateral zone and myelination of the vagus nerve typically occur by the end of the second trimester (Cheng et al. 2004; Koutcherov et al. 2003) and baroreflex responsivity increases throughout the remainder of gestation (Porges and Furman 2011). Clinical studies also support that parasympathetic control of cardiac rhythm is functional by the first half of pregnancy (Groome et al. 1994). Likewise, animal and human data show that programming of the infant ANS response begins in utero (Card et al. 2005; Igosheva et al. 2004; Jansson and Lambert 1999; Mukerjee et al. 2018), suggesting environmental exposures that alter the normal course of ANS maturation may have lifelong implications for mental and physical health (Abboud 2010; Ernst 2017; Rees 2014). During rest, the parasympathetic nervous system (PNS) maintains a state of internal homeostasis optimal for physical growth and development. When faced with external challenges, parasympathetic activity withdraws, enabling the sympathetic (SNS) branch to increase arousal and mobilize resources. For example, stress-induced withdrawal of inhibitory PNS inputs to pacemaker cells in the cardiac sinoatrial node (i.e., release of the vagal brake) results in increased heart rate and contraction force, leading to greater cardiac output and ultimately enabling an optimal response to external signals (Drew and Sinoway 2012).
Respiratory sinus arrhythmia (RSA) is a temporal pattern of HRV that reflects respiration-synchronized oscillations in PNS-SNS cycling and is widely used as a metric of cardiac vagal tone (Paton and Pickering 2012), including in young infants (Ritz et al. 2012). At rest, higher RSA indicates a greater potential to respond to stressful stimuli, whereas reduced RSA during stress reflects appropriate withdrawal of the PNS (Porges et al. 1996). Results from empirical research conducted in children suggest that higher resting RSA, as well as stress-induced reductions in RSA, are associated with positive behavioral, cognitive, and physical outcomes, including fewer externalizing problems (Calkins and Dedmon 2000; Calkins et al. 2007; El-Sheikh et al. 2007; Kahle et al. 2018), better emotion regulation (Butler et al. 2006; Oveis et al. 2009; Porges et al. 1996; Zhang et al. 2017), improved attention (Feldman 2009; Huffman et al. 1998; Suess et al. 1994), better working memory, higher cognitive efficiency (Staton et al. 2009), higher intelligence (Porges et al. 1994), better academic performance (Blair and Diamond 2008; Graziano et al. 2007; Staton et al. 2009), healthy body mass index (Graziano et al. 2011), and fewer sleep disruptions (El-Sheikh et al. 2007).
Fetal exposure to tobacco smoke is associated with lower resting RSA during infancy (Schuetze et al. 2011, 2013), suggesting prenatal programming of autonomic tone may be susceptible to disruption by environmental toxicants. No epidemiologic studies have investigated the relationship between prenatal exposure to and ANS functioning in early life. In the present study, we examined prenatal exposure to in relation to infant RSA measured during brief periods of emotional stress and recovery elicited by the Repeated Still-Face Paradigm (SFP-R). We hypothesized that higher prenatal exposure to in pregnancy would be associated with lower baseline cardiac vagal tone (i.e., lower resting RSA) and reduced PNS withdrawal during stress (i.e., attenuated decrease in RSA). We report the results of models examining prenatal exposure adjusted for postnatal among a subset of infants (86%) with available postnatal data; however, we do not investigate main effects of postnatal exposure given our focus on investigating prenatal programming effects.
Methods
Study Participants
Participants included maternal–infant pairs enrolled in the PRogramming of Intergenerational Stress Mechanisms (PRISM) study, an ethnically mixed pre-birth cohort that was designed to investigate the independent and joint effects of perinatal stress and environmental chemicals on child development (Brunst et al. 2014, 2017). Women were recruited from prenatal clinics in the Boston area at gestation [] between March 2011 and August 2012. Eligible women were English- or Spanish-speaking, of age, and pregnant with a singleton. Mothers who were or who reported alcoholic drinks/week prior to pregnancy or any alcohol during pregnancy were excluded. All study procedures were approved by the institutional review board at the Brigham and Women’s Hospital (BWH); Beth Israel Deaconess Medical Center relied on BWH for review and oversight of the protocol. Written informed consent was obtained in the mother’s primary language.
Exposure Assessment
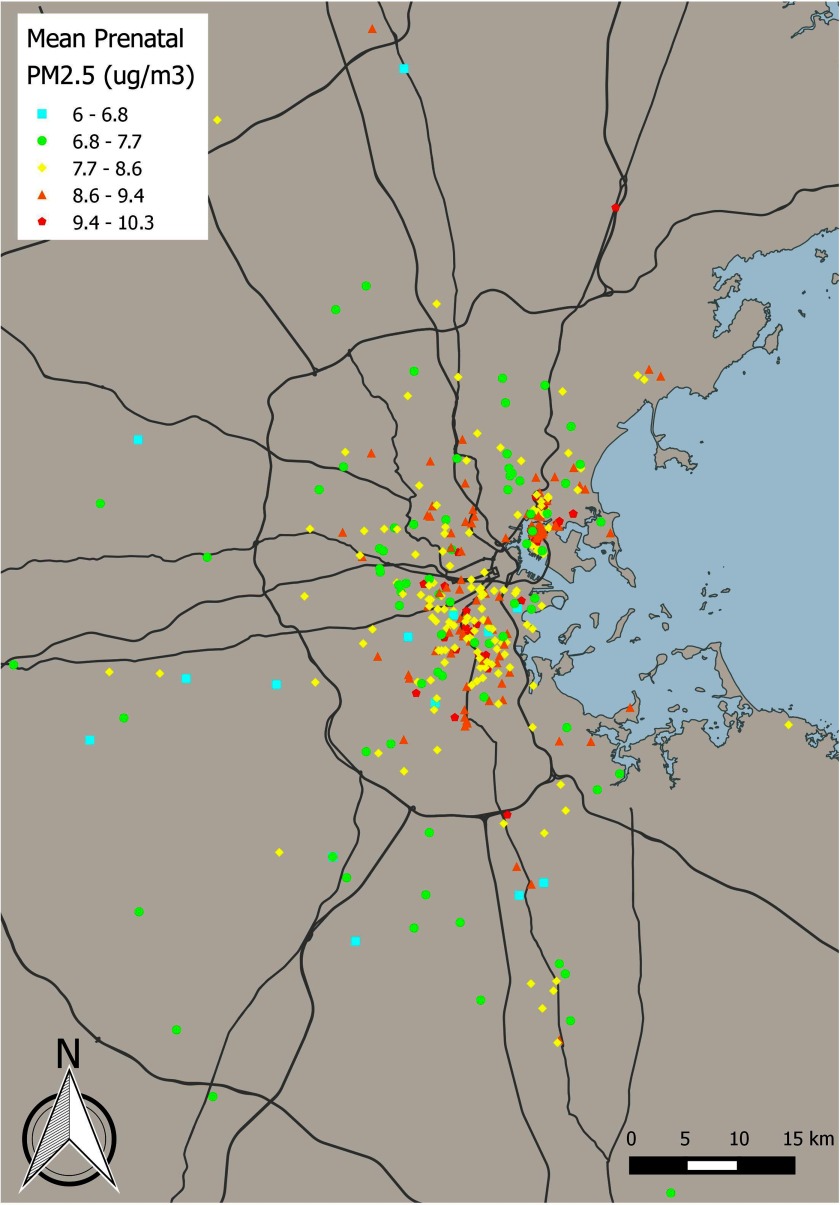
We geocoded maternal residential address during pregnancy using ESRI’s ArcGIS software, as previously described (Brunst et al. 2018). We estimated daily exposure for each study participant using a satellite-based hybrid model that combines spectral aerosol optical depth (AOD) estimates with monitoring data and spatiotemporal predictors (population density, elevation, traffic density, land use type, and point and area source emissions, air temperature, wind speed, daily visibility, sea land pressure, and relative humidity) (Kloog et al. 2014). AOD products were estimated from the Moderate Resolution Imaging Spectroradiometer (MODIS) satellite sensor at a spatial resolution using the Multi-Angle Implementation of Atmospheric Correction (MAIAC) algorithm (Kloog et al. 2014). Daily concentrations were obtained from the U.S. Environmental Protection Agency (EPA) Air Quality System (AQS) database and the Interagency Monitoring of Protected Visual Environments (IMPROVE) network. Data sources for temporal and spatial predictors have been previously described in detail (Kloog et al. 2014). Briefly, a mixed model was used to regress AOD values on monitoring data while adjusting for land use variables and allowing day-specific temporal factors to vary. These -calibrated AOD values were then used to predict concentrations in grid cells without monitoring data. In grid cells with no AOD value for a given day, was predicted based on in neighboring grids as well as the relationship between and AOD across the region and within grid cells. Finally, residuals from the final model were regressed using machine learning (support vector machine) against high-resolution () spatiotemporal predictors (traffic density, population density, elevation, percent urban, distance to major roads, distance to source emission points, and visibility) specific to a given ground monitor. We used 10-fold out-of-sample cross validation to evaluate model performance. We randomly divided the data into 90% and 10% subsets 10 times. For the data sets including 10% of values, we predicted concentrations using the model fit with the remaining 90% of the data; overall model performance was excellent (mean out-of-sample ). The spatial and temporal components of the out-of-sample results also demonstrated good fit to the withheld data (, , respectively). Our results revealed minimal bias in the predicted concentrations (slope of predictions vs. withheld ). We averaged predicted daily across pregnancy (estimated date of conception through delivery) for each study participant. Figure 1 presents a map of predicted, average exposure levels during pregnancy for mothers enrolled in PRISM. Among a subset of 204 infants (86%) with available postnatal data, we created a summary measure of postnatal exposure by averaging predicted daily values between birth and the day of SFP-R testing.
Figure 1.
Map of predicted levels for PRISM study participants during pregnancy. Each point represents residential () averaged across pregnancy. , fine particulate matter; PRISM, PRogramming of Intergenerational Stress Mechanisms.
Repeated Still-Face Paradigm
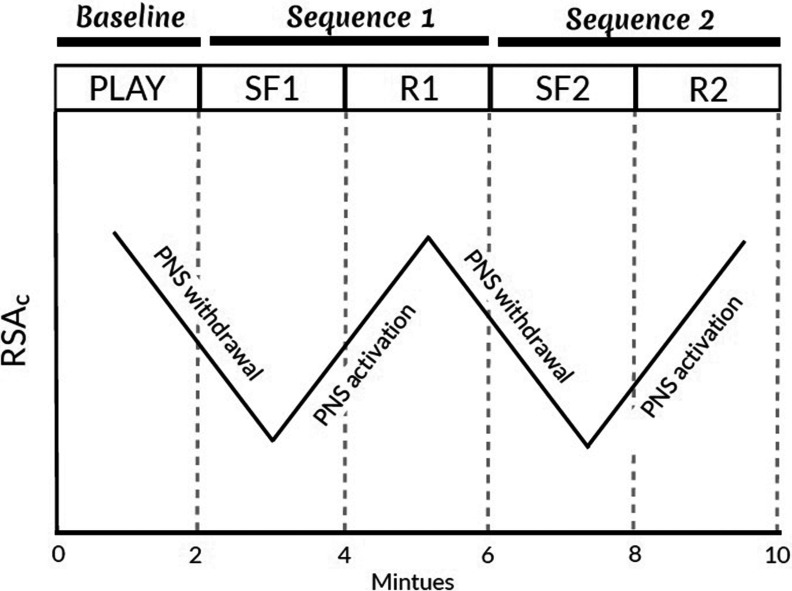
Mother–infant pairs completed the SFP-R during a follow-up visit to our laboratory when the infant was approximately 6 months of age. The SFP-R is a 10-min observational procedure designed to assess infant reactivity to and recovery from brief, moderate levels of stress induced by maternal disengagement (Tronick et al. 1978). The procedure is considered the gold-standard protocol for evaluating infant reactivity to moderate stress and is commonly coupled with measurement of RSA and other physiological indicators (Adamson and Frick 2003; Mesman et al. 2009). During the SFP-R, the infant was positioned in a car seat facing the mother, who was instructed to play with her infant as she normally would (Play). After 2 min, the mother transitioned to the first Still-Face episode (), during which she maintained a neutral facial expression and avoided touching or vocalizing with her infant. Following 2 min of disengagement, the mother resumed interacting with her infant (Reunion 1; ). The 4-min Still-Face-Reunion sequence was then repeated, with the mother transitioning directly to the second Still-Face episode () following the first Reunion episode () and to the second Reunion episode () following the second Still-Face episode (Figure 2 provides a stylized representation of a typical RSA response profile during the SFP-R procedure). The procedure was stopped if the infant remained distressed by the end of the first Reunion episode. Likewise, Still-Face episodes were terminated early () if the infant engaged in 1 min of continuous fussing or 30 s of hard crying. Trained research assistants recorded whether a maternal behavioral violation occurred (yes/no) during the Still-Face episodes based on video recordings of the session. Violations included the following: maternal touching, verbalizing with, laughing at, turning from, making faces at, or otherwise interacting with the infant in any way, including giving the infant an object or displaying clear signs of negativity. When administering the SFP-R, study staff followed a standardized protocol, including explicit written instructions detailing protocol set-up (e.g., where to seat the mother relative to the infant), use of video recording equipment (e.g., where to focus the camera to allow proper coding), physiology data acquisition (e.g., where and how to place electrodes), and interactions with the study participants (e.g., a verbal script that is administered to the mother at the start of the procedure). In addition, factors related to administration of the SFP-R were kept as constant as possible between laboratory visits. For example, the infant seat used during the SFP-R was strapped to a table so that it could not be moved and thus was always positioned in the same location for all SFP-R administrations. Before administering the SFP-R, study staff were trained to follow all procedures and materials by a developmental psychologist. As part of this training, staff watched video recordings of previously recorded SFP-R sessions from other studies and practiced administering the protocol under supervision. A developmental psychologist also reviewed live sessions and video recordings of sessions for quality control and met regularly with staff to discuss any concerns regarding protocol administration. Because all sessions were video recorded, including provision of instructions to the mother and all video sessions were coded for participant behaviors, any anomalies in administration were observable and noted during coding. Notably, anomalies were rare, and the SFP-R is robust to minor anomalies in administration.
Figure 2.
Stylized representation of a typical response during the Repeated Still-Face Paradigm (SFP-R). The SFP-R is a 10 min, 5-episode procedure (, , , , ) that is designed to induce moderate stress in the infant via maternal disengagement and reunion. During the Play and Reunion episodes, the mother is instructed to interact with her infant, who is seated in a car seat across from her, as she normally would. During the Still-Face episodes, the mother maintains a neutral facial expression and avoids touching or vocalizing with her infant. PNS, parasympathetic nervous system; , first Reunion episode; , second Reunion episode; , respiratory sinus arrhythmia adjusted for total respiratory cycle time (); , first Still-Face episode; , second Still-Face episode.
We measured infant respiration and cardiac activity during the SFP-R using a noninvasive ambulatory respiratory inductance plethysmography device (BioRadio) that continuously records and stores raw signals from two inductance bands and a three-lead electrocardiogram (ECG). We calibrated inductance band output by volume using the qualitative diagnostic calibration procedure and processed respiration and heart rate data using VivoSense software (Vivonoetics), which is designed for analysis of complex waveforms, detection of artifacts, and calibration of respiratory parameters (Ritz et al. 2012; Sackner et al. 1989). We calculated tidal volume-normalized RSA within each breathing cycle as the difference between the longest (peak) and shortest (valley) cardiac interbeat interval (IBI; in milliseconds) divided by tidal volume (; in milliliters) (Bosquet Enlow et al. 2014; Schulz et al. 2009). To correct for inter-individual variation in respiration, which has been shown to improve estimation of cardiac vagal activity (Grossman et al. 1991; Ritz et al. 2001; Saul et al. 1989), we performed a within-individual regression of natural-log transformed tidal volume-normalized RSA (in milliseconds per milliliter) on total respiratory cycle time (; in seconds) during a 5-min period of natural breathing, as previously described (Enlow et al. 2009), and added the resulting residual value for each infant to the mean across all infants in the sample (; in milliseconds per milliliter) (Ritz et al. 2012). We natural-log transformed tidal volume-normalized RSA before residualizing against to meet normality assumptions of linear regression.
We quantified infant movement by coding activity in 10-s intervals from videos of the SFP-R using a four-point scale (: no movement other than slow moving of fingers; movements: slow bending but not lifting of limbs; movements: slow lifting of limbs; movements: forceful lifting of limbs (scale modified from Bazhenova et al. 2007). We averaged all 10-s interval scores within an episode and multiplied this value by 100 to create a possible activity score for each episode ranging from 0 to 360. Infant activity level was scored by three independent research assistants, with every fourth infant coded by two research assistants; the inter-rater reliability scores across coders indicated a high degree of consistency ().
Covariates
During the prenatal and early postnatal periods, trained research assistants collected extensive information on maternal sociodemographic characteristics and lifestyle factors using questionnaires. We assessed prenatal exposure to cigarette smoke based on maternal self-report of smoking during pregnancy (ever vs. never), as well as maternal report of exposure to environmental tobacco smoke (ETS) during pregnancy ( vs. ). Likewise, we assessed postnatal exposure to ETS based on the mother’s report of infant exposure to cigarette smoke for 1 h/week or more at 2 and 6 months after birth. We evaluated material hardship based on the mother’s response (not at all likely vs. somewhat likely vs. likely to extremely likely) to the question: “In the next two months, how likely is it that you and your family will experience hardships such as inadequate housing, food, or medical attention?” We evaluated maternal stress during pregnancy using the Life Stressor Checklist–Revised (LSC-R), which is an established self-report instrument for assessing traumatic and stressful life events (Wolfe and Kimerling 1997). The mother was asked whether she had ever experienced a series () of stressful life events and the degree [not at all (1) to extremely (5)] to which the event affected her life in the previous year. We summed scores across the 30 items such that final scores (possible range 0–150) reflect a mother’s subjective rating of how a stressor affected her life in the previous year (i.e., through the majority of pregnancy and the entire postpartum period).
Statistical Analysis
We used multivariable linear regression to model pregnancy-averaged exposure treated as a continuous variable in relation to baseline (Play) (Model 1). We additionally modeled associations between continuous, pregnancy-averaged and PNS withdrawal and activation, indexed by changes in back-transformed between sequential episodes (Model 2: Play to ; Model 3: to ; Model 4: to ; Model 5: : to ). Associations with categorized into quartiles supported the use of linear models (see Figure S1) and histograms confirmed normality of model residuals. We used t-tests and chi-square tests of independence to examine differences between infants included and excluded from the analysis as appropriate. To account for noise in signals attributable to infant motor activity and jostling of ECG wires, we a priori adjusted models for infant activity level, which often accompanies distress on autonomic measures and was higher during Still-Face (: ) compared with Play () and Reunion () episodes (see Table S1) (Bazhenova et al. 2007). For models examining change between episodes, we adjusted for the difference in infant activity between the two episodes. We considered several sociodemographic, lifestyle, and perinatal factors as potential confounders (maternal race/ethnicity, age, education, marital status, household material hardship, life stress, depressive symptoms, smoking during pregnancy, exposure to ETS during pregnancy, parity, and infant gestational age and birthweight) and used directed acyclic graph (DAG) theory to select covariates for inclusion in adjusted models based on previous research and substantive knowledge (see Figure S2) (Textor et al. 2011, 2016). In addition to infant activity, we adjusted final models for race/ethnicity (black vs. Hispanic vs. white vs. other), material hardship (not likely vs. somewhat likely vs. likely to extremely likely), and maternal stress (continuous LSC-R score). These variables were sufficient to close biasing paths based on the conditional dependencies encoded by our proposed causal diagram, with the exception of potential confounding by postnatal exposure. As described below, in sensitivity analyses we examined models also adjusting for postnatal exposure among a subset of infants with available data. Differences in physiologic stress reactivity by sex are well established (Aults et al. 2015; Tibu et al. 2014; Vidal-Ribas et al. 2017; Wilhelm et al. 2017), and previous research suggests boys and girls have differential susceptibility to prenatal air pollution exposure (Bertin et al. 2015; Brunst et al. 2018; YH Chiu et al. 2016; YM Chiu et al. 2017; Lee et al. 2018a, 2018b); therefore, we explored effect modification by infant sex using cross-product terms and stratified analyses.
We conducted several sensitivity analyses to evaluate the robustness of our findings. First, we investigated whether children who failed to complete the full SFP-R protocol differed from those who completed the protocol by analyzing final models excluding children who did not complete the second sequence. Second, we analyzed models excluding 14 infants diagnosed with a chronic medical condition (atopic disorder, acid reflux, hypothyroidism, heart murmur, dysphagia, or hydronymphosis). Third, we dropped maternal–infant pairs with one or more Still-Face violation. Fourth, we analyzed models excluding infants whose state was not “alert, rested, and feeling good” at the time of the laboratory visit as reported by the mother. Fifth, we analyzed models excluding infants exposed to cigarette exposure for per week during the postnatal period based on maternal report (). To examine the potential impact of short-term and long-term postnatal exposure, we examined models additionally adjusting for a) residential levels on the day of testing treated as a continuous variable, and b) average exposure between birth and the date of SFP-R testing treated as a continuous variable. Postnatal was estimated using the same satellite-based hybrid model and methodology as was used to estimate prenatal exposure; geocoding of residential addresses was updated to account for any moves between the prenatal and postnatal periods. We also evaluated the potential impact of several variables related to the day of SFP-R testing, including ambient temperature, humidity, and barometric pressure in the laboratory as well as participant travel time to the laboratory. Finally, we evaluated models adjusting for LSC-R scores treated as a dichotomous variable stratified at the fourth quartile ( vs. ) to evaluate the degree to which our assumption of linearity impacted results. All analyses were conducted using SAS (version 9.4; SAS Institute Inc.) or RStudio (version 1.1.447; RStudio).
Results
Characteristics of Study Participants
At the prenatal visit, 417 mothers were eligible and enrolled into the Boston-based PRISM study. At the 6-month follow-up visit, 244 infants completed the SFP-R and had acceptable ECG data for extraction of RSA. We excluded 2 infants with missing data and 5 infants with missing maternal stress data, resulting in a final sample size of 237 mother–infant pairs (57% of those enrolled) (see Figure S3). Table 1 presents characteristics of included participants stratified by quartile of exposure. The majority of mothers were racial/ethnic minorities (26% black, 32% Hispanic, 8% mixed/other), 23% had less than a high school education, and 31% reported it was somewhat to extremely likely that her family would experience material hardship within the next 2 months. The and LSC-R scores were and , respectively, and the range was 0–96. These scores are higher than those reported by a sample of 179 active-duty U.S. military service members (, range: 0–35) (Bakalar et al. 2018), but lower than a clinical sample of new mothers enrolled in substance-abuse treatment program (, , range: 17–104) and their demographically matched controls (, , range: 2–58) (Goldman Fraser et al. 2010). The mean gestational age of infants was 39 weeks, with 9% of the sample born premature (). Estimated average prenatal exposure across pregnancy was normally distributed with a mean of and range of .
Table 1.
Characteristics of mother–infant pairs enrolled in the Boston-based PRISM study () stratified by quartile of exposure during pregnancy [ or (%)].
| Characteristic | Overall () | Q1 () | Q2 () | Q3 () | Q4 () |
|---|---|---|---|---|---|
| Prenatal () | |||||
| Maternal age at enrollment (y) | |||||
| Maternal race/ethnicity | |||||
| White | 80 (34) | 32 (54) | 27 (47) | 15 (25) | 6 (10) |
| Black | 61 (26) | 15 (25) | 17 (29) | 17 (28) | 12 (20) |
| Hispanic | 77 (32) | 10 (17) | 9 (16) | 18 (30) | 40 (67) |
| Other/mixed | 19 (8) | 2 (3) | 5 (9) | 10 (17) | 2 (3) |
| Maternal high school degree | |||||
| No | 54 (23) | 9 (16) | 10 (17) | 13 (22) | 22 (37) |
| Yes | 180 (77) | 48 (84) | 48 (83) | 46 (78) | 38 (63) |
| Material hardship | |||||
| Not likely | 163 (69) | 45 (76) | 45 (78) | 37 (62) | 36 (60) |
| Somewhat likely | 48 (20) | 8 (13) | 9 (16) | 16 (27) | 15 (25) |
| Likely, very likely, or extremely likely | 26 (11) | 6 (10) | 4 (7) | 7 (12) | 9 (15) |
| Maternal Life Stressor Checklist–Reviseda | |||||
| Tobacco smoke exposure during pregnancyb | |||||
| No | 200 (84) | 52 (88) | 48 (83) | 48 (62) | 52 (87) |
| Yes | 37 (16) | 7 (12) | 10 (17) | 12 (20) | 8 (13) |
| Tobacco smoke exposure after birthc | |||||
| No | 215 (94) | 55 (98) | 52 (93) | 53 (93) | 55 (92) |
| Yes | 14 (6) | 1 (2) | 4 (7) | 4 (7) | 5 (8) |
| Infant sex | |||||
| Male | 129 (54) | 33 (56) | 31 (53) | 37 (62) | 28 (47) |
| Female | 108 (46) | 26 (44) | 27 (47) | 23 (38) | 32 (53) |
| Gestational age (weeks) | |||||
| Birthweight (kg) | |||||
| Infant alert, rested, and feeling good at SFP-R | |||||
| No | 30 (19) | 6 (13) | 4 (10) | 9 (21) | 11 (35) |
| Yes | 129 (81) | 39 (87) | 37 (90) | 33 (79) | 20 (65) |
Note: There are no missing covariate data with the exception of maternal high school degree ( missing). ETS, environmental tobacco smoke; , fine particulate matter; PRISM, Programming of Intergenerational Stress Mechanisms; Q, quartile; SFP-R, Still-Face Paradigm–Repeated.
Assessed with the Life Stressor Checklist–Revised, possible range: 0–150.
Maternal self-report of smoking or exposure to ETS for during pregnancy.
Maternal report of infant exposure to ETS for assessed at ages 2 and 6 months, .
We did not detect significant differences between enrolled participants included and excluded from the analysis except that fewer mothers included in the analysis had a high school degree (77%) compared with those excluded (86%, ) (see Tables S2 and S3). Similarly, infants that completed both Still-Face sequences did not significantly differ from those completing only one sequence except that they were less likely to have been prenatally exposed to tobacco smoke (14% vs. 25%, ) (see Tables S2 and S3).
Change in across Episodes
scores within each SFP-R episode followed a natural log-normal distribution. We found an average decrease in during stress sequences [Play to : [95% confidence interval (CI): , ]; to : (95% CI: , )] and an average increase in during recovery sequences [ to : (95% CI: 5.40, 8.60); to : (95% CI: 4.26, 8.51)], independent of exposure, which is consistent with the expected pattern of PNS withdrawal and activation across the SFP-R (Table 2). At baseline, girls [: ] had significantly lower compared with boys () and showed less change in between sequential episodes (Table 3).
Table 2.
Adjusted change [ (95% CI)] in at baseline (play) and between sequential SFP-R episodes () and for a 1-unit increase in ().
| SFP-R episode | Typical PNS response | Adjusted change in across the SFP-R () | Adjusted change in across the SFP-R per a 1-unit increase in () |
|---|---|---|---|
| Model 1: Play | Baseline | 16.73 (15.45, 18.11)a | (, 0.02)b |
| Model 2: Play to | Withdrawal | (, ) | 0.74 (, 1.60) |
| Model 3: to | Activation | 7.00 (5.40, 8.60) | 0.04 (, 0.92) |
| Model 4: to | Withdrawal | (, )c | 0.61 (, 1.66)c |
| Model 5: to | Activation | 6.39 (4.26, 8.51)c | (, 0.15)c |
Note: The models were adjusted for infant activity, maternal race/ethnicity, maternal stress and material hardship. CI, confidence interval; , fine particulate matter; , respiratory sinus arrhythmia [in ms/mL corrected for total respiratory cycle time ()]; R, Reunion; SF, Still-Face, SFP-R, Repeated Still-Face Paradigm.
Adjusted geometric mean.
during Play is natural-log transformed; therefore, the estimate is interpreted as the percentage change in for a 1-unit increase in .
.
Table 3.
Sex-stratified adjusted change [ (95% CI)] in at baseline (play) and between sequential SFP-R episodes () and for a 1-unit increase in ().
| SFP-R episode | Typical PNS response | Girls () | Boys () | Sex p-Value | ||
|---|---|---|---|---|---|---|
| Adjusted change in across the SFP-R | Adjusted change in across the SFP-R per a 1-unit increase in | Adjusted change in across the SFP-R | Adjusted change in across the SFP-R per a 1-unit increase in | |||
| Model 1: Play | Baseline | 15.04 (13.40, 16.87)a | (, 1.82)b | 18.35 (16.49, 20.42)a | (, 0.47)b | 0.66 |
| Model 2: Play to | Withdrawal | (, ) | 0.88 (, 2.11) | (, ) | 0.77 (, 1.98) | 0.90 |
| Model 3: to | Activation | 6.36 (3.87, 8.86) | (, 0.61) | 7.33 (5.27, 9.39) | 0.67 (, 1.83) | 0.30 |
| Model 4: to | Withdrawal | (, )c | 1.63 (, 3.31)c | (, )d | 0.15 (, 1.53)d | 0.35 |
| Model 5: to | Activation | 7.05 (3.78, 10.33)c | (, 0.11)c | 5.11 (2.42, 7.80)d | 0.72 (, 2.27)d | 0.05 |
Note: The models were adjusted for infant activity, maternal race/ethnicity, maternal stress and material hardship. CI, confidence interval; , fine particulate matter; PNS, parasympathetic nervous system; , respiratory sinus arrhythmia [in ms/mL corrected for total respiratory cycle time ()]; R, Reunion; SF, Still-Face; SFP-R, Repeated Still-Face Paradigm.
Adjusted geometric mean.
during Play is natural-log transformed; therefore, the estimate is interpreted as the percentage change in for a 1-unit increase in .
.
.
Associations between and
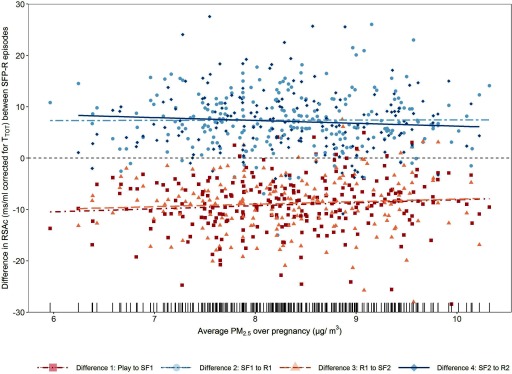
In adjusted models we found that at baseline decreased with increasing prenatal exposure to [3.53% decrease in for a 1-unit () increase in (95% CI: , 0.02)] (Table 2). When examining change between episodes, we found that for every 1-unit increase in exposure, the average decrease in during the first stress sequence (Play to ) was attenuated by 0.74 units (95% CI: , 1.60), and the average decrease in during the second stress sequence ( to ) was attenuated by 0.61 units (95% CI: , 1.66). Although these associations were not statistically significant, they suggest a possible inhibitory effect of on PNS withdrawal during stress (Table 2; Figure 3). In models examining change in between stress and recovery, for which we expect to increase with PNS activation, was associated with an attenuated increase in during the second [ to : (95% CI: , 0.15)], but not first [ to : (95% CI: , 0.92)] sequence. Overall, these findings suggest that higher maternal exposure during pregnancy may be associated with less infant PNS withdrawal during stress and less infant PNS activation during recovery, resulting in an overall flattened response (Table 2; Figure 3). We did not observe a statistically significant interaction between and sex in any model (Table 3); however, with increasing , girls showed a trend toward a reduced ability to recover following stress, as indicated by negative effect estimates between Still-Face and Reunion episodes (Table 3). In contrast, among boys we observed positive, yet statistically insignificant, changes in between stress and recovery per 1-unit increase in , suggesting a potential improved ability to recover.
Figure 3.
Scatter plot of difference in () between sequential SFP-R episodes by () among infants enrolled in the Boston-based PRISM study (). Plotted values are residuals from linear regression models between predictors (, infant activity, race/ethnicity, maternal stress, material hardship) and added to the grand mean for each episode. The lines correspond to the estimated coefficients in Table 2 and the rug on the x-axis represents the distribution of concentrations. , fine particulate matter; PRISM, PRogramming of Intergenerational Stress Mechanisms; R, Reunion; , respiratory sinus arrhythmia [in ms/mL corrected for total respiratory cycle time ()]; SF, Still-Face; SFP-R, Repeated Still-Face Paradigm.
Sensitivity Analyses
Results from models excluding infants who a) experienced one or more Still-Face violation (), b) were reported to be not alert or rested at the time of testing (), c) had a chronic medical condition (), or d) were postnatally exposed to ETS () did not substantially deviate from overall findings (see Table S4). Consistent with models including all infants [, at Play: (95% CI: , 0.02)] baseline PNS levels also decreased with increasing among the subset of infants that completed both Still-Face episodes [, Play: (95% CI: , 0.98)]. Likewise, results from models examining PNS withdrawal and activation among the subset of infants that completed both Still-Face episodes were not substantially different from models including all infants (see Table S4). Among the 204 participants with available postnatal data, residential concentrations between birth and the 6-month SFP-R visit were approximately normally distributed with a of and range of 6.12–. Predicted concentrations on the day of testing had a small right tail with a of and range of 2.10–. Findings from models adjusting for these chronic and short-term postnatal exposure metrics did not substantially vary from main results (see Table S5). The (range) laboratory temperature (degrees Fahrenheit), humidity (percentage), and barometric pressure [milliliters mercury (mmHg)] on the day of testing among 142 participants for which this information was collected was: (65.9–77.7), (20.0–65.0), and (19.9–39.8), respectively. Participant travel time to the laboratory was on average (range: 5–120 min). Models adjusting for these laboratory conditions were similar to results from main models (see Table S6). Finally, models treating maternal LSC-R scores as a dichotomous (quartile 4 vs. quartiles 1, 2, and 3 collapsed) variable were similar in magnitude and direction to final results presented in the manuscript (see Table S7).
Discussion
To our knowledge this is the first study to suggest that higher maternal exposure to during pregnancy may be associated with decreased resting vagal tone among infants. We additionally detected a trend toward reduced PNS withdrawal during stress, a pattern that may indicate reduced autonomic flexibility. Although significant differences in exposure pathways and putative underlying biological mechanisms exist when considering prenatal versus postnatal exposure, we note that our findings are generally consistent with previous observational research conducted in adults that show exposure to particulate air pollution is inversely related to HRV (Devlin et al. 2003; Gold et al. 2000; Pope et al. 2004). Consistent with findings among older children and adolescents (5–19 years of age), baseline levels were significantly lower among girls compared with boys (Koenig et al. 2017). However, we did not detect differences between boys and girls in relation to exposure, which may in part reflect our relatively small sample size.
Although the physiological significance and precise mechanisms underlying the respiratory–cardiac coupling characteristic of RSA remain incompletely understood (Yasuma and Hayano 2004), substantial observational and clinical research has linked lower resting vagal tone with increased psychological, behavioral, and somatic disease risk across the life span. For example, low vagal tone during infancy has been associated with an increased incidence of sudden infant death syndrome (Peirano et al. 1992). Among children, reduced vagal withdrawal during emotionally or cognitively challenging tests has been shown to predict obesity (Graziano et al. 2011) and high blood pressure (Gangel et al. 2017) during early to late childhood. Likewise, a recent meta-analysis of 44 studies ( children) found greater RSA withdrawal in response to stress was associated with fewer externalizing, internalizing, and cognitive/academic problems (Graziano and Derefinko 2013). In adults, lower RSA is an established risk factor for cardiovascular disease (Dekker et al. 2000)—the leading cause of death worldwide (GBD 2016 Causes of Death Collaborators 2017)—as well as for other chronic diseases [diabetes, hypertension, and obesity (Masi et al. 2007)] and psychopathologies [depression, anxiety disorders, borderline personality disorder, and schizophrenia (Bylsma et al. 2014; Clamor et al. 2016; Jurysta et al. 2010; Kemp et al. 2010; Koenig et al. 2016)]. Owing to differences in study design and catchment populations, as well as variation in the approach to quantifying and reporting RSA across research labs and publications, it is not possible to directly compare the magnitude of our observed associations to previous studies examining RSA in relation to health outcomes or to determine the physiological significance of our findings. However, the direction of observed associations is consistent with research demonstrating adverse effects of on cardiovascular and autonomic health. In addition, several observational studies in children have found inverse associations between prenatal exposure to air pollution and neurocognitive and behavioral outcomes (Chiu et al. 2016; Cowell et al. 2015; Jedrychowski et al. 2015; Yorifuji et al. 2016). It is plausible that these associations are partially mediated by -related disruption of cardiac vagal tone, which has previously been linked with poor emotional, behavioral, and cognitive outcomes in children.
Little evidence supports the passage of across the placental barrier, suggesting that the mechanisms through which maternal exposure to during pregnancy disrupt fetal development involve changes to the maternal or placental systems or to both. Putative mechanisms based on prior research include altered placental gene expression, increased oxidative stress, and changes to maternal immune system status. Experiments using a transgenic mouse model have shown insufficient levels of brain-derived neurotrophic factor (BDNF), which promotes survival of parasympathetic (i.e., cholinergic) neurons during embryonic development, results in reduced cardioinhibitory vagal activity in the brainstem and leads to decreased parasympathetic tone (Wan et al. 2014). Prenatal exposure to has been associated with changes in expression of placental genes key to fetal neurodevelopment, including those involved in BDNF signaling pathways (Saenen et al. 2015), suggesting has the potential to disrupt central control of PNS activity. Further, although we are not aware of studies that have investigated prenatal exposure to in relation to anatomical changes in fetal brain structure, prenatal exposure to cigarette smoke has been associated with hypodevelopment of brainstem nuclei involved in autonomic control (Lavezzi et al. 2016) and reduced RSA modulation in infancy (Schuetze et al. 2013). Alternatively, it is plausible that targets overlapping pathways between the autonomic and stress response systems. A recent randomized, double-blind crossover trial in humans found higher exposure to was associated with significantly increased levels of cortisol, epinephrine, and norepinephrine (Li et al. 2017), which are end products of hypothalamic–pituitary–adrenal (HPA) axis activation. Maternal cortisol crosses the placenta leading to elevated levels in fetal circulation (Benediktsson et al. 1997; Hennessy et al. 1982) and animal models have demonstrated maternal catecholamines reduce uterine blood flow (Barton et al. 1974) and placental perfusion (Salomon et al. 2006), resulting in a sustained increase in fetal catecholamine production (Gu et al. 1985). In sheep, elevated prenatal exposure to these hormones has been associated with lower fetal blood pressure and heart rate, as well as with significantly shortened increases in fetal blood pressure during acute maternal stress, providing evidence that cardiovascular responses to stress may begin in utero (Dreiling et al. 2018).
Particulate air pollution is known to induce cellular oxidative stress in the peripheral circulation via several pathways (Lodovici and Bigagli 2011; Miller et al. 2012; Risom et al. 2005; Rossner et al. 2007). Recently, exposure during pregnancy has been associated with elevated oxidative stress at the maternal–fetal interface, as indicated by significantly increased levels of 3-nitrotyrosine in the placenta (Saenen et al. 2016). Likewise, cigarette smoke during pregnancy has been associated with elevated markers of oxidative stress not only in maternal blood but also in placental tissue and cord blood (Aycicek and Ipek 2008; Aycicek et al. 2011). These findings suggest environmentally induced changes in the maternal milieu may translate to the intra-uterine environment. In adults, reactive oxygen species have been shown to play a role in development of autonomic dysfunction, including disruption to central cardiovascular regulation (Hirooka et al. 2010). During fetal development, correlates of oxidative stress have been linked to programming of ANS dysfunction in rodent (Danson and Paterson 2006; YC Wu et al. 2011) and primate (Duncan et al. 2009; Slotkin et al. 2011) models. Taken together, these findings support that increased oxidative stress levels may be one putative mechanism through which particulate pollution alters early life programming of autonomic balance.
The PNS plays a key role in anti-inflammatory responses (Czura and Tracey 2005; Tracey 2002), with multiple studies demonstrating inverse correlations between C-reactive protein (CRP), a marker of acute systemic inflammation, and vagal tone in adults (Carney et al. 2007; Frasure-Smith et al. 2009; Haensel et al. 2008; Lampert et al. 2008). In murine models, exposure to or diesel exhaust particles has been shown to upregulate pro-inflammatory markers in the placenta [interleukin-6 (IL-6), tumor necrosis factor-alpha (), ] (Auten et al. 2009, 2012; Fujimoto et al. 2005) and fetal brain (Bolton et al. 2012). In humans, exposure to PM has been associated with elevated in cord blood (Latzin et al. 2011) and increased levels of maternal (Lee et al. 2011) and fetal (van den Hooven et al. 2012) CRP. Based on these findings, it is plausible that prenatal exposure to triggers activation of anti-inflammatory neural circuits, with potential downstream consequences for programming of other PNS-controlled pathways (i.e., parasympathetic outflow to the heart).
We are not aware of experimental animal research that has directly investigated the effects of exposure during pregnancy on offspring autonomic parameters. However, several studies using rodent models have suggested that maternal exposure to during pregnancy can program other key physiological systems, including cardiovascular, renal, and metabolic systems, independent of offspring exposure during postnatal life. For example, adult offspring of dams exposed to throughout pregnancy show evidence of altered cardiac volume, cardiac inflammation, electrical remodeling, and significant cardiac dysfunction (Gorr et al. 2014; Stapleton et al. 2018; Tanwar et al. 2017). Likewise, murine research has linked later life metabolic dysfunction with gestational exposure. Specifically, research has shown that adult offspring prenatally exposed to present with significantly altered cell function and morphology and impaired glucose tolerance (Chen et al. 2018). These offspring also show significantly decreased birthweight, but increased adiposity and body weight among males (Chen et al. 2017). Evidence of prenatal reprogramming by also extends to the renal system; research in rats suggests that adult offspring of dams exposed to via oropharyngear drip during pregnancy have impaired renal dopamine D1 receptor-mediated sodium excretion and increased blood pressure (Ye et al. 2018). Although these studies do not provide direct experimental evidence to support our findings or hypothesis of a prenatal programming mechanism, they suggest that maternal exposure to during pregnancy has the potential to disrupt offspring physiological systems independent of direct postnatal exposure. Given putative differences in exposure pathways and biological mechanisms between the prenatal and postnatal periods, including little evidence of a correlation between direct infant exposure and biologically relevant effects of maternal exposure, we do not conceptualize infant exposure as a confounder of prenatal –infant RSA associations. The results of sensitivity analyses examining models additionally controlling for postnatal exposure a) on the day of RSA testing and b) averaged between birth and RSA testing further support that postnatal exposure does not confound the prenatal association that we observed in the PRISM sample.
This study is one of the first to examine prenatal exposure to ambient in relation to early life autonomic function, which we assessed in a controlled laboratory environment. We measured RSA during the SFP-R, which is considered the gold-standard protocol for eliciting vagal withdrawal in infants and is commonly coupled with assessment of physiological measures (Adamson and Frick 2003; Mesman et al. 2009). Assessing HRV using an electrophysiological approach parallel to adult studies is challenging in young infants given their rapid underlying heart and respiration rates. We were able to improve precision of RSA values by correcting for respiration using our previously validated approach (Ritz et al. 2012), as well as adjusting for infant activity, which we coded at a high temporal resolution (10-s intervals). As is typical given challenges with obtaining valid cardiac data from young children, we were unable to use data from 53 infants due to excessive respiratory artifacts () or technical problems with the equipment (), which reduced our power to detect associations with .
The high predictive accuracy of our –resolution spatiotemporal land-use regression model for estimating exposure is expected to minimize measurement error and consequent downward biases in effect estimates. However, our use of maternal residential location to predict individual exposure may have introduced misclassification among mothers who spent substantial time away from the home. It is possible that maternal time spent away from home could be positively (i.e., maternal employment outside the home leading to better socioeconomic circumstances and more household resources) or negatively (less bonding and direct interaction with baby during early life) related to infant autonomic reactivity to stress. Given these uncertainties, we are unable to speculate how potential misclassification would affect observed associations.
In the PRISM cohort, pregnancy-averaged concentrations (maximum: ) are lower than the U.S. EPA National Ambient Air Quality Standards annual limit (i.e., ), suggesting exposure reflects that of the general U.S. population; however, our findings may not be generalizable to regions with higher ambient levels. In addition, although we view the sociodemographic diversity of the sample, including a large representation of minority and lower income families, as a strength of this research, our findings may not be generalizable to more racially homogenous, rural communities. Likewise, given that we examined autonomic tone only once, at approximately 6 months of age, it remains unknown whether our findings will extend to later childhood.
Previous research in adults has documented associations between recent (hours) exposure to and changes in resting HRV (Breitner et al. 2019; He et al. 2011; S Wu et al. 2011; Zanobetti et al. 2010). Unfortunately, we were not able to monitor levels in the testing room during the SFP-R. However, all laboratory procedures took place in the same climate-controlled, indoor testing room with no windows open to the ambient environment, reducing the likelihood of fluctuations in indoor levels. In addition, in sensitivity analyses, adjusting for short-term postnatal exposure did not attenuate associations between prenatal and measures of . Likewise, including average postnatal exposure during the first 6 months of life as a covariate did not substantially alter associations between prenatal and . Importantly, if there is misclassification in our measure of postnatal (e.g., due to variability in the amount of time infants spend outdoors), it is possible that uncontrolled, residual confounding by postnatal exposure remains. In addition, because the precise biological mechanisms linking maternal exposure to during pregnancy with changes in infant RSA are unknown, it is possible fetal exposure to the biologically relevant consequences of maternal exposure is misclassified due to inter-individual variability in maternal biology. As with all observational studies, there is also a possibility for residual confounding by unmeasured factors related to both and RSA. For example, it is plausible that cultural differences not captured by race/ethnicity could influence where a mother resides and may also relate to parenting styles, which in turn could be linked to infant autonomic reactivity during the SFP-R. We were not able to investigate associations between RSA and other air pollutants (e.g., ozone, carbon monoxide, nitrogen oxides, sulfur dioxide, elemental carbon) that may co-vary or interact with (Buteau and Goldberg 2016). Future research that is able to investigate combined exposures to these pollutants is needed to better understand how mixtures of ambient air pollutants impact the developing autonomic and cardiovascular systems. In addition, although we had information on postnatal exposure for a subset of infants, we focused analyses on gestational exposure given our interests in understanding prenatal programming effects. Future research investigating exposure during infancy and studies evaluating whether the mechanisms underlying associations differ depending on the timing of exposure (e.g., prenatal vs. postnatal) will contribute to our understanding of how early life exposure impacts autonomic system development and function.
In summary, our findings support previous research conducted in older children and adults that has found is inversely associated with HRV and suggest that environmentally induced disruption of autonomic tone may extend to the prenatal period. Putative mechanisms include altered immune function, endocrine signaling, enhanced oxidative stress, or disruption of central control of PNS activity at the level of the brainstem or a combination of these mechanisms potentially via altered placenta gene signaling pathways. Although it is difficult to interpret the physiological relevance associated with the degree of change we observed, previous research has linked lower RSA and reduced PNS withdrawal with a number of chronic diseases and psychopathologies (Bylsma et al. 2014; Clamor et al. 2016; Dekker et al. 2000; Gangel et al. 2017; Graziano et al. 2011; Jurysta et al. 2010; Kemp et al. 2010; Koenig et al. 2016; Masi et al. 2007). These findings, in combination with increasing worldwide exposure to , emphasize the importance of examining early life exposure to in relation to autonomic outcomes. However, until these preliminary findings are replicated by future studies, it is critical they be interpreted with caution.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health(NIH)/National Heart, Lung, and Blood Institute (R01 HL095606 and R01 HL114396) and NIH/National Institute of Environmental Health Sciences (NIEHS; R21 ES021318, and P30 ES023515). During preparation of this manuscript, W.J.C. was supported by NIH/National Institute of Child Health and Human Development (T32 HD049311), K.J.B. was supported by the NIH/NIEHS (R00 ES024116 and P30 ES006096), and M.B.E. was supported by the Program for Behavioral Science in the Department of Psychiatry at Boston Children’s Hospital.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4434).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Abboud FM. 2010. The Walter B. Cannon Memorial Award Lecture, 2009. Physiology in perspective: the wisdom of the body. In search of autonomic balance: the good, the bad, and the ugly. Am J Physiol Regul Integr Comp Physiol 298(6):R1449–1467, PMID: 20219871, 10.1152/ajpregu.00130.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson LB, Frick JE. 2003. The still-face: a history of a shared experimental paradigm. Infancy 4(4):451–473, 10.1207/S15327078IN0404_01. [DOI] [Google Scholar]
- An Z, Jin Y, Li J, Li W, Wu W. 2018. Impact of particulate air pollution on cardiovascular health. Curr Allergy Asthma Rep 18(3):15, PMID: 29470659, 10.1007/s11882-018-0768-8. [DOI] [PubMed] [Google Scholar]
- Aults CD, Cooper PJ, Pauletti RE, Jones NA, Perry DG. 2015. Child sex and respiratory sinus arrhythmia reactivity as moderators of the relation between internalizing symptoms and aggression. Appl Psychophysiol Biofeedback 40(4):269–276, PMID: 26159768, 10.1007/s10484-015-9294-9. [DOI] [PubMed] [Google Scholar]
- Auten RL, Gilmour MI, Krantz QT, Potts EN, Mason SN, Foster WM. 2012. Maternal diesel inhalation increases airway hyperreactivity in ozone-exposed offspring. Am J Respir Cell Mol Biol 46(4):454–460, PMID: 22052876, 10.1165/rcmb.2011-0256OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Auten RL, Potts EN, Mason SN, Fischer B, Huang Y, Foster WM. 2009. Maternal exposure to particulate matter increases postnatal ozone-induced airway hyperreactivity in juvenile mice. Am J Respir Crit Care Med 180(12):1218–1226, PMID: 19762564, 10.1164/rccm.200901-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Aycicek A, Ipek A. 2008. Maternal active or passive smoking causes oxidative stress in cord blood. Eur J Pediatr 167(1):81–85, PMID: 17297611, 10.1007/s00431-007-0433-z. [DOI] [PubMed] [Google Scholar]
- Aycicek A, Varma M, Ahmet K, Abdurrahim K, Erel O. 2011. Maternal active or passive smoking causes oxidative stress in placental tissue. Eur J Pediatr 170(5):645–651, PMID: 20981440, 10.1007/s00431-010-1338-9. [DOI] [PubMed] [Google Scholar]
- Bakalar JL, Barmine M, Druskin L, Olsen CH, Quinlan J, Sbrocco T, et al. . 2018. Childhood adverse life events, disordered eating, and body mass index in US military service members. Int J Eat Disord 51(5):465–469, PMID: 29500835, 10.1002/eat.22851. [DOI] [PubMed] [Google Scholar]
- Barton MD, Killam AP, Meschia G. 1974. Response of ovine uterine blood flow to epinephrine and norepinephrine. Proc Soc Exp Biol Med 145(3):996–1003, PMID: 4818619, 10.3181/00379727-145-37941. [DOI] [PubMed] [Google Scholar]
- Bazhenova OV, Stroganova TA, Doussard-Roosevelt JA, Posikera IA, Porges SW. 2007. Physiological responses of 5-month-old infants to smiling and blank faces. Int J Psychophysiol 63(1):64–76, PMID: 17056142, 10.1016/j.ijpsycho.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benediktsson R, Calder AA, Edwards CR, Seckl JR. 1997. Placental 11β-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 46(2):161–166, PMID: 9135697, 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- Bertin M, Chevrier C, Serrano T, Monfort C, Cordier S, Viel JF. 2015. Sex-specific differences in fetal growth in newborns exposed prenatally to traffic-related air pollution in the PELAGIE mother–child cohort (Brittany, France). Environ Res 142:680–687, PMID: 26378737, 10.1016/j.envres.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Blair C, Diamond A. 2008. Biological processes in prevention and intervention: the promotion of self-regulation as a means of preventing school failure. Dev Psychopathol 20(3):899–911, PMID: 18606037, 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, et al. . 2012. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J 26(11):4743–4754, PMID: 22815382, 10.1096/fj.12-210989. [DOI] [PubMed] [Google Scholar]
- Bosquet Enlow M, King L, Schreier HM, Howard JM, Rosenfield D, Ritz T, et al. . 2014. Maternal sensitivity and infant autonomic and endocrine stress responses. Early Hum Dev 90(7):377–385, PMID: 24794304, 10.1016/j.earlhumdev.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner S, Peters A, Zareba W, Hampel R, Oakes D, Wiltshire J, et al. . 2019. Ambient and controlled exposures to particulate air pollution and acute changes in heart rate variability and repolarization. Sci Rep 9(1):1946, PMID: 30760868, 10.1038/s41598-019-38531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, Sanchez-Guerra M, Chiu YM, Wilson A, Coull BA, Kloog I, et al. . 2018. Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: effect modification by maternal lifetime trauma and child sex. Environ Int 112:49–58, PMID: 29248865, 10.1016/j.envint.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, Sanchez Guerra M, Gennings C, Hacker M, Jara C, Bosquet Enlow M, et al. . 2017. Maternal lifetime stress and prenatal psychological functioning and decreased placental mitochondrial DNA copy number in the PRISM study. Am J Epidemiol 186(11):1227–1236, PMID: 28595325, 10.1093/aje/kwx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, Wright RO, DiGioia K, Enlow MB, Fernandez H, Wright RJ, et al. . 2014. Racial/ethnic and sociodemographic factors associated with micronutrient intakes and inadequacies among pregnant women in an urban US population. Public Health Nutr 17(9):1960–1970, PMID: 24476840, 10.1017/S1368980013003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccelletti E, Gilardi E, Scaini E, Galiuto L, Persiani R, Biondi A, et al. . 2009. Heart rate variability and myocardial infarction: systematic literature review and metanalysis. Eur Rev Med Pharmacol Sci 13(4):299–307, PMID: 19694345. [PubMed] [Google Scholar]
- Buteau S, Goldberg MS. 2016. A structured review of panel studies used to investigate associations between ambient air pollution and heart rate variability. Environ Res 148:207–247, PMID: 27085495, 10.1016/j.envres.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. 2006. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology 43(6):612–622, PMID: 17076818, 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Salomon K, Taylor-Clift A, Morris BH, Rottenberg J. 2014. Respiratory sinus arrhythmia reactivity in current and remitted major depressive disorder. Psychosom Med 76(1):66–73, PMID: 24367127, 10.1097/PSY.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Blandon AY, Williford AP, Keane SP. 2007. Biological, behavioral, and relational levels of resilience in the context of risk for early childhood behavior problems. Dev Psychopathol 19(3):675–700, PMID: 17705898, 10.1017/S095457940700034X. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE. 2000. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. J Abnorm Child Psychol 28(2):103–118, PMID: 10834764, 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Card JP, Levitt P, Gluhovsky M, Rinaman L. 2005. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J Neurosci 25(40):9102–9111, PMID: 16207869, 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Stein PK, Miller GE, Steinmeyer B, Rich MW, et al. . 2007. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosom Res 62(4):463–467, PMID: 17383498, 10.1016/j.jpsychores.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Liang S, Qin X, Zhang L, Qiu L, Chen S, et al. . 2018. Prenatal exposure to diesel exhaust PM2.5 causes offspring β cell dysfunction in adulthood. Am J Physiol Endocrinol Metab 315(1):E72–E80, PMID: 29351483, 10.1152/ajpendo.00336.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wang X, Hu Z, Zhou H, Xu Y, Qiu L, et al. . 2017. Programming of mouse obesity by maternal exposure to concentrated ambient fine particles. Part Fibre Toxicol 14(1):20, PMID: 28645299, 10.1186/s12989-017-0201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Zhou X, Qu J, Ashwell KW, Paxinos G. 2004. Central vagal sensory and motor connections: human embryonic and fetal development. Auton Neurosci 114(1–2):83–96, PMID: 15331048, 10.1016/j.autneu.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Hsu HH, Coull BA, Bellinger DC, Kloog I, Schwartz J, et al. . 2016. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ Int 87:56–65, PMID: 26641520, 10.1016/j.envint.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YM, Hsu HL, Wilson A, Coull BA, Pendo MP, Baccarelli A, et al. . 2017. Prenatal particulate air pollution exposure and body composition in urban preschool children: examining sensitive windows and sex-specific associations. Environ Res 158:798–805, PMID: 28759881, 10.1016/j.envres.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. 2007. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med 176(4):370–376, PMID: 17463411, 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- Clamor A, Lincoln TM, Thayer JF, Koenig J. 2016. Resting vagal activity in schizophrenia: meta-analysis of heart rate variability as a potential endophenotype. Br J Psychiatry 208(1):9–16, PMID: 26729841, 10.1192/bjp.bp.114.160762. [DOI] [PubMed] [Google Scholar]
- GBD 2016 Causes of Death Collaborators. 2017. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100):1151–1210, PMID: 28919116, 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Bellinger DC, Coull BA, Gennings C, Wright RO, Wright RJ. 2015. Associations between prenatal exposure to black carbon and memory domains in urban children: modification by sex and prenatal stress. PLoS One 10(11):e0142492, PMID: 26544967, 10.1371/journal.pone.0142492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czura CJ, Tracey KJ. 2005. Autonomic neural regulation of immunity. J Intern Med 257(2):156–166, PMID: 15656874, 10.1111/j.1365-2796.2004.01442.x. [DOI] [PubMed] [Google Scholar]
- Danson EJ, Paterson DJ. 2006. Reactive oxygen species and autonomic regulation of cardiac excitability. J Cardiovasc Electrophysiol 17(Suppl 1):S104–S112, PMID: 16686664, 10.1111/j.1540-8167.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, et al. . 2000. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk in Communities. Circulation 102(11):1239–1244, PMID: 10982537, 10.1161/01.CIR.102.11.1239. [DOI] [PubMed] [Google Scholar]
- Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. 2003. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J Suppl 40:76s–80s, PMID: 12762579, 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- Dreiling M, Schiffner R, Bischoff S, Rupprecht S, Kroegel N, Schubert H, et al. . 2018. Impact of chronic maternal stress during early gestation on maternal-fetal stress transfer and fetal stress sensitivity in sheep. Stress 21(1):1–10, PMID: 29041862, 10.1080/10253890.2017.1387534. [DOI] [PubMed] [Google Scholar]
- Drew RC, Sinoway LI. 2012. Autonomic control of the heart. In: Primer on the Autonomic Nervous System. 3rd ed Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, eds. Amsterdam; Netherlands: Elsevier/Academic Press, 177–180. [Google Scholar]
- Duncan JR, Garland M, Myers MM, Fifer WP, Yang M, Kinney HC, et al. . 2009. Prenatal nicotine-exposure alters fetal autonomic activity and medullary neurotransmitter receptors: implications for sudden infant death syndrome. J Appl Physiol (1985) 107(5):1579–1590, PMID: 19729586, 10.1152/japplphysiol.91629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Keller PS. 2007. Children’s sleep and adjustment: the moderating role of vagal regulation. J Sleep Res 16(4):396–405, PMID: 18036085, 10.1111/j.1365-2869.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- Enlow MB, Kullowatz A, Staudenmayer J, Spasojevic J, Ritz T, Wright RJ. 2009. Associations of maternal lifetime trauma and perinatal traumatic stress symptoms with infant cardiorespiratory reactivity to psychological challenge. Psychosom Med 71(6):607–614, PMID: 19553287, 10.1097/PSY.0b013e3181ad1c8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst G. 2017. Heart-rate variability—more than heart beats? Front Public Health 5:240, PMID: 28955705, 10.3389/fpubh.2017.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. 2009. The development of regulatory functions from birth to 5 years: insights from premature infants. Child Dev 80(2):544–561, PMID: 19467010, 10.1111/j.1467-8624.2009.01278.x. [DOI] [PubMed] [Google Scholar]
- Franklin BA, Brook R, Pope CA III. 2015. Air pollution and cardiovascular disease. Curr Probl Cardiol 40(5):207–238, PMID: 25882781, 10.1016/j.cpcardiol.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F, Irwin MR, Talajic M, Pollock BG. 2009. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. Brain Behav Immun 23(8):1140–1147, PMID: 19635552, 10.1016/j.bbi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Fujimoto A, Tsukue N, Watanabe M, Sugawara I, Yanagisawa R, Takano H, et al. . 2005. Diesel exhaust affects immunological action in the placentas of mice. Environ Toxicol 20(4):431–440, PMID: 16007645, 10.1002/tox.20129. [DOI] [PubMed] [Google Scholar]
- Gangel MJ, Shanahan L, Kolacz J, Janssen JA, Brown A, Calkins SD, et al. . 2017. Vagal regulation of cardiac function in early childhood and cardiovascular risk in adolescence. Psychosom Med 79(6):614–621, PMID: 28207613, 10.1097/PSY.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, et al. . 2000. Ambient pollution and heart rate variability. Circulation 101(11):1267–1273, PMID: 10725286, 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Goldman Fraser J, Harris-Britt A, Leone Thakkallapalli E, Kurtz-Costes B, Martin S. 2010. Emotional availability and psychosocial correlates among mothers in substance-abuse treatment and their young infants. Infant Ment Health J 31(1):1–15, PMID: 28543592, 10.1002/imhj.20239. [DOI] [PubMed] [Google Scholar]
- Gorr MW, Velten M, Nelin TD, Youtz DJ, Sun Q, Wold LE. 2014. Early life exposure to air pollution induces adult cardiac dysfunction. Am J Physiol Heart Circ Physiol 307(9):H1353–H1360, PMID: 25172901, 10.1152/ajpheart.00526.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano PA, Calkins SD, Keane SP, O’Brien M. 2011. Cardiovascular regulation profile predicts developmental trajectory of BMI and pediatric obesity. Obesity (Silver Spring) 19(9):1818–1825, PMID: 21546929, 10.1038/oby.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P, Derefinko K. 2013. Cardiac vagal control and children’s adaptive functioning: a meta-analysis. Biol Psychol 94(1):22–37, PMID: 23648264, 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano PA, Reavis RD, Keane SP, Calkins SD. 2007. The role of emotion regulation and children’s early academic success. J Sch Psychol 45(1):3–19, PMID: 21179384, 10.1016/j.jsp.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groome LJ, Mooney DM, Bentz LS, Wilson JD. 1994. Vagal tone during quiet sleep in normal human term fetuses. Dev Psychobiol 27(7):453–466, PMID: 7843499, 10.1002/dev.420270704. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. 1991. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology 28(2):201–216, PMID: 1946886, 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Gu W, Jones CT, Parer JT. 1985. Metabolic and cardiovascular effects on fetal sheep of sustained reduction of uterine blood flow. J Physiol 368(1):109–129, PMID: 4078738, 10.1113/jphysiol.1985.sp015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. 2008. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 33(10):1305–1312, PMID: 18819754, 10.1016/j.psyneuen.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Shaffer ML, Li X, Rodriguez-Colon S, Wolbrette DL, Williams R, et al. . 2011. Individual-level PM2.5 exposure and the time course of impaired heart rate variability: the APACR Study. J Expo Sci Environ Epidemiol 21(1):65–73, PMID: 20372190, 10.1038/jes.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy DP, Coghlan JP, Hardy KJ, Scoggins BA, Wintour EM. 1982. The origin of cortisol in the blood of fetal sheep. J Endocrinol 95(1):71–79, PMID: 7130892, 10.1677/joe.0.0950071. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Sagara Y, Kishi T, Sunagawa K. 2010. Oxidative stress and central cardiovascular regulation. Pathogenesis of hypertension and therapeutic aspects. Circ J 74(5):827–835, PMID: 20424336, 10.1253/circj.cj-10-0153. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Reynolds RM, Hardy DB. 2017. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr Rev 75(12):951–970, PMID: 29186623, 10.1093/nutrit/nux053. [DOI] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Forges SW. 1998. Infant temperament and cardiac vagal tone: assessments at twelve weeks of age. Child Dev 69(3):624–635, PMID: 9680676, 10.1111/j.1467-8624.1998.tb06233.x. [DOI] [PubMed] [Google Scholar]
- Igosheva N, Klimova O, Anishchenko T, Glover V. 2004. Prenatal stress alters cardiovascular responses in adult rats. J Physiol 557(1):273–285, PMID: 15034122, 10.1113/jphysiol.2003.056911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T, Lambert GW. 1999. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3–4 months of age. J Hypertens 17(9):1239–1248, PMID: 10489100, 10.1097/00004872-199917090-00002. [DOI] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Camann D, Spengler J, Butscher M, Mroz E, et al. . 2015. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ Sci Pollut Res Int 22(5):3631–3639, PMID: 25253062, 10.1007/s11356-014-3627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurysta F, Kempenaers C, Lancini J, Lanquart JP, van de Borne P, Linkowski P. 2010. Altered interaction between cardiac vagal influence and delta sleep EEG suggests an altered neuroplasticity in patients suffering from major depressive disorder. Acta Psychiatr Scand 121(3):236–239, PMID: 19764928, 10.1111/j.1600-0447.2009.01475.x. [DOI] [PubMed] [Google Scholar]
- Kahle S, Utendale WT, Widaman KF, Hastings PD. 2018. Parasympathetic regulation and inhibitory control predict the development of externalizing problems in early childhood. J Abnorm Child Psychol 46(2):237–249, PMID: 28493111, 10.1007/s10802-017-0305-6. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. 2010. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry 67(11):1067–1074, PMID: 20138254, 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, et al. . 2014. A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos Environ 95:581–590, PMID: 28966552, 10.1016/j.atmosenv.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J, Kemp AH, Feeling NR, Thayer JF, Kaess M. 2016. Resting state vagal tone in borderline personality disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 64:18–26, PMID: 26169575, 10.1016/j.pnpbp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Koenig J, Rash JA, Campbell TS, Thayer JF, Kaess M. 2017. A meta-analysis on sex differences in resting-state vagal activity in children and adolescents. Front Physiol 8:582, PMID: 28883794, 10.3389/fphys.2017.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutcherov Y, Mai JK, Paxinos G. 2003. Hypothalamus of the human fetus. J Chem Neuroanat 26(4):253–270, PMID: 14729128, 10.1016/j.jchemneu.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Lampert R, Bremner JD, Su S, Miller A, Lee F, Cheema F, et al. . 2008. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J 156(4):759.e1–759.e7, PMID: 18926158, 10.1016/j.ahj.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Röösli M, et al. . 2011. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PLoS One 6(8):e23130, PMID: 21826232, 10.1371/journal.pone.0023130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi AM, Ferrero S, Matturri L, Roncati L, Pusiol T. 2016. Developmental neuropathology of brainstem respiratory centers in unexplained stillbirth: what’s the meaning? Int J Dev Neurosci 53:99–106, PMID: 27477774, 10.1016/j.ijdevneu.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Lee A, Hsu H-HL, Chiu Y-HM, Bose S, Rosa MJ, Kloog I, et al. . 2018a. Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J Allergy Clin Immunol 141(5):1880–1886, PMID: 28801196, 10.1016/j.jaci.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Le Grand B, Hsu H-HL, Chiu Y-HM, Brennan KJ, Bose S, et al. . 2018b. Prenatal fine particulate exposure associated with reduced childhood lung function and nasal epithelia GSTP1 hypermethylation: sex-specific effects. Respir Res 19(1):76, PMID: 29703190, 10.1186/s12931-018-0774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P-C, Talbott EO, Roberts JM, Catov JM, Sharma RK, Ritz B. 2011. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology 22(4):524–531, PMID: 21516040, 10.1097/EDE.0b013e31821c6c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, et al. . 2017. Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation 136(7):618–627, PMID: 28808144, 10.1161/CIRCULATIONAHA.116.026796. [DOI] [PubMed] [Google Scholar]
- Liao D, Duan Y, Whitsel EA, Zheng ZJ, Heiss G, Chinchilli VM, et al. . 2004. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol 159(8):768–777, PMID: 15051586, 10.1093/aje/kwh109. [DOI] [PubMed] [Google Scholar]
- Licht CMM, Vreeburg SA, van Reedt Dortland AK, Giltay EJ, Hoogendijk WJ, DeRijk RH, et al. . 2010. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. J Clin Endocrinol Metab 95(5):2458–2466, PMID: 20237163, 10.1210/jc.2009-2801. [DOI] [PubMed] [Google Scholar]
- Lodovici M, Bigagli E. 2011. Oxidative stress and air pollution exposure. J Toxicol 2011:487074, PMID: 21860622, 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttmann-Gibson H, Suh HH, Coull BA, Dockery DW, Sarnat SE, Schwartz J, et al. . 2010. Systemic inflammation, heart rate variability and air pollution in a cohort of senior adults. Occup Environ Med 67(9):625–630, PMID: 20519749, 10.1136/oem.2009.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Rickett EM, Cacioppo JT. 2007. Respiratory sinus arrhythmia and diseases of aging: obesity, diabetes mellitus, and hypertension. Biol Psychol 74(2):212–223, PMID: 17034928, 10.1016/j.biopsycho.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesman J, van Ijzendoorn MH, Bakermans-Kranenburg MJ. 2009. The many faces of the Still-Face Paradigm: a review and meta-analysis. Dev Rev 29(2):120–162, 10.1016/j.dr.2009.02.001. [DOI] [Google Scholar]
- Miller MR, Shaw CA, Langrish JP. 2012. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol 8(4):577–602, PMID: 22871197, 10.2217/fca.12.43. [DOI] [PubMed] [Google Scholar]
- Mukerjee S, Zhu Y, Zsombok A, Mauvais-Jarvis F, Zhao J, Lazartigues E. 2018. Perinatal exposure to Western diet programs autonomic dysfunction in the male offspring. Cell Mol Neurobiol 38(1):233–242, PMID: 28478572, 10.1007/s10571-017-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelin TD, Joseph AM, Gorr MW, Wold LE. 2012. Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol Lett 208(3):293–299, PMID: 22119171, 10.1016/j.toxlet.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D. 2009. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion 9(2):265–270, PMID: 19348538, 10.1037/a0015383. [DOI] [PubMed] [Google Scholar]
- Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J. 2005. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ Health Perspect 113(3):304–309, PMID: 15743719, 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR, Pickering AE. 2012. Cross-talk between body systems: respiratory-cardiovascular coupling in health and disease. In: Primer on the Autonomic Nervous System. 3rd ed Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, eds. Amsterdam; Netherlands: Elsevier/Academic Press, 151–155. [Google Scholar]
- Peirano P, Cauchemez B, Monod N. 1992. Heart rate and its variability in sudden infant death syndrome (SIDS) victims. Pediatr Res 32(6):739, 10.1203/00006450-199212000-00035. [DOI] [Google Scholar]
- Pieters N, Plusquin M, Cox B, Kicinski M, Vangronsveld J, Nawrot TS. 2012. An epidemiological appraisal of the association between heart rate variability and particulate air pollution: a meta-analysis. Heart 98(15):1127–1135, PMID: 22628541, 10.1136/heartjnl-2011-301505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA III, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, et al. . 2004. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect 112(3):339–345, PMID: 14998750, 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. 1996. Infant regulation of the vagal “brake” predicts child behavior problems: a psychobiological model of social behavior. Dev Psychobiol 29(8):697–712, PMID: 8958482, . [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Suess PE. 1994. Cardiac vagal tone: stability and relation to difficultness in infants and 3-year-olds. Dev Psychobiol 27(5):289–300, PMID: 7926281, 10.1002/dev.420270504. [DOI] [PubMed] [Google Scholar]
- Porges SW, Furman SA. 2011. The early development of the autonomic nervous system provides a neural platform for social behavior: a polyvagal perspective. Infant Child Dev 20(1):106–118, PMID: 21516219, 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees CA. 2014. Lost among the trees? The autonomic nervous system and paediatrics. Arch Dis Child 99(6):552–562, PMID: 24573884, 10.1136/archdischild-2012-301863. [DOI] [PubMed] [Google Scholar]
- Risom L, Møller P, Loft S. 2005. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res 592(1–2):119–137, PMID: 16085126, 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Ritz T, Bosquet Enlow M, Schulz SM, Kitts R, Staudenmayer J, Wright RJ. 2012. Respiratory sinus arrhythmia as an index of vagal activity during stress in infants: respiratory influences and their control. PLoS One 7(12):e52729, PMID: 23300753, 10.1371/journal.pone.0052729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T, Thöns M, Dahme B. 2001. Modulation of respiratory sinus arrhythmia by respiration rate and volume: stability across posture and volume variations. Psychophysiology 38(5):858–862, PMID: 11577909, 10.1111/1469-8986.3850858. [DOI] [PubMed] [Google Scholar]
- Rossner P Jr, Svecova V, Milcova A, Lnenickova Z, Solansky I, Santella RM, et al. . 2007. Oxidative and nitrosative stress markers in bus drivers. Mutat Res 617(1–2):23–32, PMID: 17328930, 10.1016/j.mrfmmm.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Sackner MA, Watson H, Belsito AS, Feinerman D, Suarez M, Gonzalez G, et al. . 1989. Calibration of respiratory inductive plethysmograph during natural breathing. J Appl Physiol (1985) 66(1):410–420, PMID: 2917945, 10.1152/jappl.1989.66.1.410. [DOI] [PubMed] [Google Scholar]
- Saenen ND, Plusquin M, Bijnens E, Janssen BG, Gyselaers W, Cox B, et al. . 2015. In utero fine particle air pollution and placental expression of genes in the brain-derived neurotrophic factor signaling pathway: an ENVIRONAGE birth cohort study. Environ Health Perspect 123(8):834–840, PMID: 25816123, 10.1289/ehp.1408549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenen ND, Vrijens K, Janssen BG, Madhloum N, Peusens M, Gyselaers W, et al. . 2016. Placental nitrosative stress and exposure to ambient air pollution during gestation: a population study. Am J Epidemiol 184(6):442–449, PMID: 27601048, 10.1093/aje/kww007. [DOI] [PubMed] [Google Scholar]
- Salomon LJ, Siauve N, Taillieu F, Balvay D, Vayssettes C, Frija G, et al. . 2006. In vivo dynamic MRI measurement of the noradrenaline-induced reduction in placental blood flow in mice. Placenta 27(9–10):1007–1013, PMID: 16316684, 10.1016/j.placenta.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Saul JP, Berger RD, Chen MH, Cohen RJ. 1989. Transfer function analysis of autonomic regulation. II. Respiratory sinus arrhythmia. Am J Physiol 256(1 Pt 2):H153–H161, PMID: 2912177, 10.1152/ajpheart.1989.256.1.H153. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Colder CR, Gray TR, Huestis MA. 2011. Physiological regulation in cigarette exposed infants: an examination of potential moderators. Neurotoxicol Teratol 33(5):567–574, PMID: 21798345, 10.1016/j.ntt.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Colder CR, Gray TR, Huestis MA. 2013. Physiological regulation at 9 months of age in infants prenatally exposed to cigarettes. Infancy 18(2):233–255, PMID: 23646002, 10.1111/j.1532-7078.2012.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz SM, Ayala E, Dahme B, Ritz T. 2009. A MATLAB toolbox for correcting within-individual effects of respiration rate and tidal volume on respiratory sinus arrhythmia during variable breathing. Behav Res Methods 41(4):1121–1126, PMID: 19897819, 10.3758/BRM.41.4.1121. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Spindel ER. 2011. Prenatal nicotine exposure in rhesus monkeys compromises development of brainstem and cardiac monoamine pathways involved in perinatal adaptation and sudden infant death syndrome: amelioration by vitamin C. Neurotoxicol Teratol 33(3):431–434, PMID: 21320590, 10.1016/j.ntt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton PA, Wingard CJ, Nurkiewicz TR, Holloway AC, Zelikoff JT, Knudsen TB, et al. . 2018. Cardiopulmonary consequences of gestational toxicant exposure: symposium overview at the 56th annual SOT meeting, Baltimore, MD. Reprod Toxicol 79:16–20, PMID: 29709519, 10.1016/j.reprotox.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton L, El-Sheikh M, Buckhalt JA. 2009. Respiratory sinus arrhythmia and cognitive functioning in children. Dev Psychobiol 51(3):249–258, PMID: 19107730, 10.1002/dev.20361. [DOI] [PubMed] [Google Scholar]
- Suess PE, Porges SW, Plude DJ. 1994. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology 31(1):17–22, PMID: 8146250, 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Tanwar V, Gorr MW, Velten M, Eichenseer CM, Long VP III, Bonilla IM, et al. . 2017. In utero particulate matter exposure produces heart failure, electrical remodeling, and epigenetic changes at adulthood. J Am Heart Assoc 6(4):e005796, PMID: 28400369, 10.1161/JAHA.117.005796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tentolouris N, Argyrakopoulou G, Katsilambros N. 2008. Perturbed autonomic nervous system function in metabolic syndrome. Neuromolecular Med 10(3):169–178, PMID: 18224460, 10.1007/s12017-008-8022-5. [DOI] [PubMed] [Google Scholar]
- Textor J, Hardt J, Knüppel S. 2011. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 22(5):745, PMID: 21811114, 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. 2016. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol 45(6):1887–1894, PMID: 28089956, 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. 2010. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141(2):122–131, PMID: 19910061, 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Thornburg KL. 2015. The programming of cardiovascular disease. J Dev Orig Health Dis 6(5):366–376, PMID: 26173733, 10.1017/S2040174415001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibu F, Hill J, Sharp H, Marshall K, Glover V, Pickles A. 2014. Evidence for sex differences in fetal programming of physiological stress reactivity in infancy. Dev Psychopathol 26(4 Pt 1):879–888, PMID: 24703466, 10.1017/S0954579414000194. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. 2002. The inflammatory reflex. Nature 420(6917):853–859, PMID: 12490958, 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. 1978. The infant’s response to entrapment between contradictory messages in face-to-face interaction. J Am Acad Child Psychiatry 17(1):1–13, PMID: 632477, 10.1016/S0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, de Kluizenaar Y, Pierik FH, Hofman A, van Ratingen SW, Zandveld PY, et al. . 2012. Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: the Generation R Study. Environ Health Perspect 120(5):746–751, PMID: 22306530, 10.1289/ehp.1104345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Ribas P, Pickles A, Tibu F, Sharp H, Hill J. 2017. Sex differences in the associations between vagal reactivity and oppositional defiant disorder symptoms. J Child Psychol Psychiatry 58(9):988–997, PMID: 28573761, 10.1111/jcpp.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Weigand LA, Bateman R, Griffioen K, Mendelowitz D, Mattson MP. 2014. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J Neurochem 129(4):573–580, PMID: 24475741, 10.1111/jnc.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm FH, Rattel JA, Wegerer M, Liedlgruber M, Schweighofer S, Kreibig SD, et al. . 2017. Attend or defend? Sex differences in behavioral, autonomic, and respiratory response patterns to emotion-eliciting films. Biol Psychol 130:30–40, PMID: 29054817, 10.1016/j.biopsycho.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Kimerling R. 1997. Gender issues in the assessment of posttraumatic stress disorder. In: Assessing Psychological Trauma and PTSD. Wilson JP, Keane TM, eds. New York, NY: Guilford Press, 192–238. [Google Scholar]
- Wu S, Deng F, Niu J, Huang Q, Liu Y, Guo X. 2011. Exposures to PM2.5 components and heart rate variability in taxi drivers around the Beijing 2008 Olympic Games. Sci Total Environ 409(13):2478–2485, PMID: 21492904, 10.1016/j.scitotenv.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Wu Y-C, Wang Y-J, Tseng G-F. 2011. Ascorbic acid and α-tocopherol supplement starting prenatally enhances the resistance of nucleus tractus solitarius neurons to hypobaric hypoxic challenge. Brain Struct Funct 216(2):105–122, PMID: 21287201, 10.1007/s00429-010-0300-y. [DOI] [PubMed] [Google Scholar]
- Yasuma F, Hayano J. 2004. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest 125(2):683–690, PMID: 14769752, 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]
- Ye Z, Lu X, Deng Y, Wang X, Zheng S, Ren H, et al. . 2018. In utero exposure to fine particulate matter causes hypertension due to impaired renal dopamine D1 receptor in offspring. Cell Physiol Biochem 46(1):148–159, PMID: 29614490, 10.1159/000488418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Kashima S, Higa Diez M, Kado Y, Sanada S, Doi H. 2016. Prenatal exposure to traffic-related air pollution and child behavioral development milestone delays in Japan. Epidemiology 27(1):57–65, PMID: 26247490, 10.1097/EDE.0000000000000361. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Gold DR, Stone PH, Suh HH, Schwartz J, Coull BA, et al. . 2010. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environ Health Perspect 118(3):324–330, PMID: 20064780, 10.1289/ehp.0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Fagan SE, Gao Y. 2017. Respiratory sinus arrhythmia activity predicts internalizing and externalizing behaviors in non-referred boys. Front Psychol 8:1496, PMID: 28955262, 10.3389/fpsyg.2017.01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.