Abstract
Posttraumatic stress disorder (PTSD) is a prevalent, debilitating, and difficult to treat psychiatric disorder. Very little is known of how PTSD affects neuroplasticity in the developing adolescent brain. Whereas multiple lines of research implicate amygdala‐centered network dysfunction in the pathophysiology of adult PTSD, no study has yet examined the functional architecture of amygdala subregional networks in adolescent PTSD. Using intrinsic functional connectivity analysis, we investigated functional connectivity of the basolateral (BLA) and centromedial (CMA) amygdala in 19 sexually abused adolescents with PTSD relative to 23 matched controls. Additionally, we examined whether altered amygdala subregional connectivity coincides with abnormal grey matter volume of the amygdaloid complex. Our analysis revealed abnormal amygdalar connectivity and morphology in adolescent PTSD patients. More specifically, PTSD patients showed diminished right BLA connectivity with a cluster including dorsal and ventral portions of the anterior cingulate and medial prefrontal cortices (p < 0.05, corrected). In contrast, PTSD patients showed increased left CMA connectivity with a cluster including the orbitofrontal and subcallosal cortices (p < 0.05, corrected). Critically, these connectivity changes coincided with diminished grey matter volume within BLA and CMA subnuclei (p < 0.05, corrected), with CMA connectivity shifts additionally relating to more severe symptoms of PTSD. These findings provide unique insights into how perturbations in major amygdalar circuits could hamper fear regulation and drive excessive acquisition and expression of fear in PTSD. As such, they represent an important step toward characterizing the neurocircuitry of adolescent PTSD, thereby informing the development of reliable biomarkers and potential therapeutic targets. Hum Brain Mapp 37:1120–1135, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: adolescents, amygdala, PTSD, intrinsic functional connectivity, grey matter
INTRODUCTION
Posttraumatic stress disorder (PTSD) is a debilitating and difficult to treat psychiatric disorder, characterized by a constellation of symptoms related to the experience of a traumatic event [Patel et al., 2012]. Adolescent PTSD is of particular concern, as PTSD is more chronic and highly prevalent at this crucial developmental stage, coupled with increased risk for psychopathology later in life [Ackerman et al., 1998; McLaughlin et al., 2013; Perry and Azad, 1999]. Critically, the perpetuating state of stress and anxiety in PTSD might disrupt neuroplasticity in the developing adolescent brain, thereby hampering normal development of cognitive and emotional functionalities [Davidson and McEwen, 2012; Lupien et al., 2009]. Yet, despite these concerns very little is known of how PTSD might affect brain network organization in adolescents. Whereas multiple lines of research suggest amygdala subregional defects in the pathophysiology of adult PTSD [Brown et al., 2014; Jovanovic and Ressler, 2010; Mahan and Ressler, 2012; Nicholson et al., 2015; Patel et al., 2012], no study has yet examined the functional architecture of amygdala subregional networks in adolescent PTSD. Knowledge on how major amygdalar circuits might be compromised in adolescent PTSD is crucial in gaining insight into the underlying pathophysiology, ultimately informing the development of reliable biomarkers and potential therapeutic targets.
The amygdala is a central component of brain's affective processing system [LeDoux, 2000; Pessoa and Adolphs, 2010], and neurocircuitry models of PTSD consistently emphasize its central role in PTSD symptomatology [Patel et al., 2012; Taghva et al., 2013]. Within these models, the amygdala is hyperresponsive to threat‐related stimuli and acts as an overactive fear generator, in which its projections to memory circuits drive fear of trauma‐related stimuli and context, while its projections to the brainstem, cerebellum, and hypothalamus drive excessive fear responses and hyperarousal [Cisler et al., 2014; Jovanovic and Ressler, 2010; Taghva et al., 2013]. Critically, the amygdala continues to drive excessive fear as medial prefrontal regions fail to downregulate amygdala hyperactivity [Jovanovic and Ressler, 2010; Taghva et al., 2013]. Within this amygdala‐centered circuitry, the basolateral (BLA) and centromedial (CMA) subnuclei of the amygdala play imperative and distinctive roles in fear processing, through their unique connectivity profiles with cortical and subcortical territories (see Supporting Information 1 for schematic overview). The BLA receives information from multiple brain systems and is a site of integration with cortical areas, including those that regulate fear [Jovanovic and Ressler, 2010; LeDoux, 2007; Sah et al., 2003]. It is heavily involved in the perception, evaluation, and memory formation of emotionally salient stimuli [Davis and Whalen, 2001; LeDoux, 2007; McGaugh et al., 1996]. The CMA, in contrast, receives mostly modulatory inputs from the BLA and orbitofrontal cortex, and is less heavily innervated by sensory and associative regions [Barbas et al., 2003; LeDoux, 2007; Sah et al., 2003]. It is the primary output site of the amygdala, and orchestrates fear responses via its projections to the brainstem, cerebellum, and hypothalamus [Davis and Whalen, 2001; LeDoux, 2007]. Despite the critical role of the amygdala in PTSD, very little is known of how BLA and CMA connectivity profiles may actually contribute to the pathophysiology of adolescent PTSD.
Most of our current knowledge about the distinctive functions and connectivity profiles of amygdala subregions stems from animal studies. However, recent advances in human neuroimaging have resulted in cytoarchitechtonic probability maps of the amygdaloid complex [Amunts et al., 2005], allowing to quantitatively map the unique connectivity profiles of amygdala subregions. Intrinsic functional connectivity (iFC) analysis, in particular, has proven a powerful method for delineating the functional architecture of intrinsically (i.e. spontaneously) coupled brain networks [Fox and Raichle, 2007]. As such, dissociable connectivity profiles of the BLA and CMA were recently demonstrated in healthy adults and adolescents [Gabard‐Durnam et al., 2014; Roy et al., 2009], consistent with earlier observations in rodents and primates [LeDoux, 2007; Sah et al., 2003]. These subregional connectivity profiles have been shown to reliably predict individual variations in anxiety in healthy adults [Li et al., 2012], and in typically developing children as young as age nine [Qin et al., 2014]. Moreover, widespread disruption of both BLA and CMA connectivity profiles has been demonstrated in adult and adolescent generalized anxiety disorder, suggesting impairments in both the experience and regulation of emotions [Etkin et al., 2009; Roy et al., 2013]. In parallel, disrupted BLA and CMA connectivity with regulatory prefrontal regions has been found in adult PTSD [Brown et al., 2014; Nicholson et al., 2015], suggesting a mechanism by which prefrontal regions fail to govern amygdala hyperactivity and reduce excessive fear. Of note, new evidence suggests that anxiety‐related alterations in amygdala subregional connectivity coincide with structural abnormalities within the BLA and CMA subnuclei [Etkin et al., 2009; Qin et al., 2014]. A coupling between brain structure and function might indeed be expected, as network communication and information processing depend heavily on structural properties of neurons (e.g., size, configuration, arrangement) [Zatorre et al., 2012]. As such, conjoint examination of amygdalar connectivity and morphology seems crucial for a deeper understanding of abnormal anxiety and its underlying pathophysiology.
Despite emerging evidence of anxiety‐related perturbations in major amygdalar circuits, almost nothing is known about the functional architecture of amygdala subregional networks in adolescent PTSD. Although there have been preliminary reports of amygdala‐prefrontal underconnectivity due to trauma in healthy and PTSD‐diagnosed teenagers [Nooner et al., 2013; Wolf and Herringa, 2015]. In fact, only two studies have examined amygdala subregional iFC in PTSD, though in adult patient communities [Brown et al., 2014; Nicholson et al., 2015], while conjoint examination of amygdalar connectivity and morphology has been lacking with regard to PTSD. We addressed this critical gap by uniquely examining BLA and CMA intrinsic functional connectivity in a sample of sexually abused adolescents with PTSD, relative to matched controls. Additionally, we employed voxel‐based morphometry to examine whether alterations in grey matter volume of amygdala subregions coincide with abnormalities in BLA and CMA connectivity. Multimodal imaging of the amygdaloid complex may provide complementary information and novel insights on PTSD pathophysiology, which otherwise would only be partially revealed by each modality separately. Based on earlier work implicating amygdala‐centered network dysfunction in abnormal fear processing and excessive fear responses [Barbas, 2007; Brown et al., 2014; Cisler et al., 2014; Etkin et al., 2009; Jovanovic and Ressler, 2010; LeDoux, 2007; Pessoa and Adolphs, 2010; Roy et al., 2013; Sah et al., 2003; Shin and Liberzon, 2010], we hypothesized adolescents with PTSD to show diminished BLA connectivity with regulatory prefrontal regions, such as the medial prefrontal and anterior cingulate cortices. Moreover, we hypothesized adolescents with PTSD to show increased CMA connectivity with regions involved in fear expression. These include regions that modulate CMA activity (e.g., orbitofrontal cortex), as well as regions involved in the actual execution of fear responses (e.g., brainstem and hypothalamus). Additionally, given that abnormal connectivity and morphology of amygdala subregions tend to accompany each other in anxious individuals [Etkin et al., 2009; Qin et al., 2014], we hypothesized that abnormal BLA and CMA connectivity would coincide with altered grey matter volume of amygdala subregions. Finally, we expected that abnormal amygdalar connectivity would relate to more PTSD symptoms of stress and anxiety.
METHOD
Participants
Nineteen sexually abused adolescents with a DSM‐IV diagnosis of PTSD (mean age = 16.16, SD = 1.79) and 23 age‐, sex‐, and IQ‐matched healthy controls with no history of significant psychotrauma (mean age = 15.52, SD = 1.78) were selected, as part of the EPISCA study (Emotional Pathways' Imaging Study in Clinical Adolescents). All PTSD patients had experienced repeated sexual abuse during their lifetime by one or more perpetrators in‐ or outside the family, and were referred for psychotherapy at an outpatient psychotrauma center. More details regarding participant inclusion are provided in Supporting Information 1.
Clinical Assessment
For all patients, after the clinical assessment by child and adolescent psychiatrists, DSM‐IV diagnoses of PTSD were further assessed by trained clinical psychologists based on the child and parent versions of the Anxiety Disorders Interview Schedule (ADIS) [Silverman and Albano, 2006], a diagnostic tool for obtaining DSM‐IV‐based classifications of anxiety disorders. Following this, additional clinical measures were used to assess the severity of PTSD and related internalizing symptoms. These included the Trauma Symptom Checklist for Children (TSCC) [Briere, 1996], the Adolescent Dissociative Experiences Scale (A‐DES) [Armstrong et al., 1997], and the Children's Depression Inventory (CDI) [Kovacs, 1992]. Though these questionnaires are not typically used for diagnostic purposes, clinical cut‐off scores have been suggested. For the TSCC a mean total score of ≥60 suggests acute and chronic posttraumatic symptomatology [Briere, 1996], with mean scores of ≥4.0 on the A‐DES suggesting pathological dissociation [Armstrong et al., 1997], and mean CDI scores of ≥16 indicating depressive symptomatology [Kovacs, 1992]. More detailed description of these questionnaires is provided in Supporting Information 1. The same measures were also applied for the control group, and control participants were excluded when they fulfilled the criteria for a DSM‐IV diagnosis or had (sub)clinical scores on clinical questionnaires.
Data Acquisition and Preprocessing
Resting‐state (RS) fMRI data were collected using a Philips 3 T Achieva MRI scanner (Philips Healthcare, Best, The Netherlands) with an 8‐channel SENSE (Sensitivity Encoding) head coil. Prior to scanning, all participants were accustomed to the scanning situation by lying in a dummy scanner and hearing scanner sounds. Participants were instructed to lie still with their eyes closed and not to fall asleep during the 6 min RS scan. More detail regarding data acquisition is provided in Supporting Information 1.
All data were preprocessed and analyzed using FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) version 5.0.1. Preprocessing consisted of (1) nonbrain‐tissue removal, (2) motion correction, (3) grand mean‐based intensity normalization of the entire 4‐D data set by a single scaling factor, (4) slice timing correction, (5) spatial smoothing with a 6 mm full width at half maximum Gaussian kernel, and (6) temporal bandpass filtering at 0.009 < f < 0.15 Hz, which improves BOLD signal estimation and produces connectivity patterns that relate most closely to task‐based activations [Biswal et al., 1995; Fox and Raichle, 2007; Fransson, 2006; Roy et al., 2009; Toro et al., 2008]. Finally, the RS data were registered to T1‐weighted anatomical images, and subsequently to the 2‐mm Montreal Neurological Institute (MNI) standard space image [Roy et al., 2013, 2009]. The maximum allowable mean displacement due to excessive head motion was set at 3 mm translation or 3° rotation in any direction. Additionally, to guard against the effects of in‐scanner micro‐motion on connectivity patterns we implemented motion‐censoring, also known as “scrubbing” [Power et al., 2012; Satterthwaite et al., 2013] (see Supporting Information 1 for details).
Region of Interest Definition
Using cytoarchitechtonic probabilistic maps of amygdala subnuclei provided in FSL's Juelich histological atlas [Amunts et al., 2005], BLA and CMA region of interest (ROI) masks were created in both hemispheres (see Supporting Information 1). These probability maps have been validated in pediatric postmortem studies [Kim et al., 2010], and have proven highly reliable and accurate in guiding amygdala parcellation in pediatric populations [Gabard‐Durnam et al., 2014; Qin et al., 2014, 2012; Roy et al., 2013]. In line with recent developmental studies [Qin et al., 2014, 2012], voxels were included in the ROI masks only if the probability of their assignment to the BLA or CMA was higher than any other nearby structures with greater than 40% likelihood. Each voxel was exclusively assigned to only one region, and overlapping voxels were assigned to the region that had the greatest probability [Qin et al., 2014, 2012]. These masks were used in subsequent iFC and structural analyses.
Functional Connectivity Analysis
Seed‐based whole brain iFC analysis was employed to reveal BLA and CMA connectivity [Fox and Raichle, 2007]. For each hemisphere, a general linear model was created that included individual participant's mean time series of both the BLA and CMA subnuclei as predictors [Roy et al., 2013, 2009]. Signal from the white matter and cerebrospinal fluid (see Supporting Information 1 for details), six motion parameters, and parameters obtained from the motion censoring procedure (see Supporting Information 1 for details) were temporally filtered and included in this model as covariates of no interest to correct for physiological and motion‐related noise. This resulted in individual subject‐level maps of all voxels uniquely exhibiting iFC with each amygdala subdivision, accounting for the relationships of the other subdivision [Roy et al., 2013]. Subject‐level iFC maps of the BLA and CMA were fed into a higher‐level mixed‐effects group analysis, with age, sex, and IQ (demeaned across groups) included as covariates of no interest. Resulting statistical maps were corrected for multiple comparisons using cluster‐based correction (p < 0.05, initial cluster forming threshold Z > 2.3). Additionally, similar to Qin et al. [2012], spatial correlations were computed between each participant's BLA and CMA connectivity maps to quantify the overall similarity between BLA and CMA target networks (see Supporting Information 1 for details). This provides important complementary information about the functional differentiation and segregation of BLA and CMA networks [Qin et al., 2012]. Previous studies have shown that abnormal anxiety not only impacts the connectivity strength of the BLA and CMA with their respective targets, but also shapes the functional differentiation between their connectivity profiles (i.e., increased overlap between BLA and CMA networks) [Etkin et al., 2009; Roy et al., 2013]. Finally, exploratory conjunction analyses examined whether PTSD may affect common connectivity patterns of BLA and CMA subregions (see Supporting Information 1 for details).
Grey Matter Volume Analysis
To examine whether alterations in grey matter volume of amygdala subregions may coincide with abnormalities in BLA and CMA connectivity, optimized voxel‐based morphometry (VBM) was performed. Grey matter volume was analyzed using FSL's VBM tool [Douaud et al., 2007]. In brief, structural images were grey matter‐segmented, a study‐specific grey matter template was created, and all native‐space grey matter images were registered to this template. Finally, between groups voxelwise permutation‐based nonparametric testing of grey matter volume was restricted to the BLA and CMA subnuclei and corrected for multiple comparisons, using Threshold‐Free Cluster Enhancement (TFCE) with family‐wise error (FWE) correction (p < 0.05). Age, sex, and IQ (demeaned across groups) were included in the analyses as covariates to correct for their possible confounding effects. More details regarding the VBM analysis are provided in Supporting Information 1.
Functional Connectivity and Structure
To examine whether coinciding changes in amygdalar connectivity and structure are associated with each other, partial correlation analyses were performed. More specifically, patients’ mean grey matter volume and connectivity strength within regions of significant group differences were fed into a partial correlation model, adjusted for age, sex, and IQ.
Functional Connectivity and Symptom Severity
Partial correlation analyses in PTSD patients (adjusted for age, sex, and IQ) examined the association between subregional connectivity strength (i.e., mean Z values) within areas of significant group differences and PTSD symptoms of stress and anxiety (as measured with posttraumatic stress and anxiety subscales of the TSCC). For transparency, similar analyses in the PTSD group examined whether amygdala connectivity changes may also relate to depressive symptomatology (as measured with the CDI).
Structural Integrity and Symptom Severity
Though not a primary objective of this study, partial correlation analyses in PTSD patients (adjusted for age, sex, and IQ) examined the association between subregional volumetrics within areas of significant group differences and PTSD symptoms of stress and anxiety (i.e., TSCC). Likewise, similar analyses in the PTSD group examined whether volumetric changes within amygdala subregions may also relate to depressive symptoms (i.e., CDI).
PTSD Duration and Amygdalar Connectivity and Structure
As PTSD duration has been proposed to impact brain structure and function [Bremner, 2006], exploratory analyses examined the association between PTSD duration and amygdalar connectivity and structure. Specifically, partial correlation analyses in PTSD patients (adjusted for age, sex, and IQ) examined the association between PTSD duration and subregional connectivity and volumetrics within areas of significant group differences.
Effects of Comorbidity and Medication Use on Functional Connectivity
Similar to Roy et al. [2013] and Cullen et al. [2014], we performed post‐hoc analyses to examine the effects of comorbidity and medication use on iFC. Using mean Z values representing connectivity strength within regions of significant group differences, multivariate analyses of variance (MANOVA) were conducted to compare adolescents with PTSD (excluding either those with a comorbid disorder, those using medication, or both) to healthy adolescents. Additionally, we compared adolescent PTSD patients with a comorbid disorder to those without, while also comparing patients who were on medication to those who were not.
RESULTS
Sample Characteristics
As shown in Table 1, the matched groups did not differ with respect to age, sex, and IQ. Importantly, the groups also did not differ on measures of head movement during RS data acquisition. As expected, clinical measures revealed more PTSD, anxiety, and depressive symptoms in patients (Table 1). The patient group comprised 19 adolescents with a DSM‐IV diagnosis of PTSD, with mean illness duration of 5.23 years (SD = 3.58). Eight patients had a secondary comorbid disorder (depressive disorder N = 7, attention‐deficit hyperactivity disorder N = 1). Most patients were treatment‐naïve, with only three patients taking psychotropic medication. All PTSD patients had experienced serious and longstanding sexual abuse during their lifetime, including repeated or group rape, by one or more perpetrators in‐ or outside the family. In 77.8% of the cases, this was by another person than an attachment figure. Sexual abuse was reported to the police in 60.9% of the cases, child welfare was involved in 56.5% of the cases, while 17.4% had a child protection measure (family custody). None of the participating control adolescents had experienced significant psychotrauma, and were not involved with police, child welfare, or child protection.
Table 1.
Characteristics of adolescents with PTSD (patients) and healthy control participants (controls)
| Patients | Controls | |
|---|---|---|
| Characteristic | N = 19 | N = 23 |
| Age (years; mean ± SD) | 16.16 ± 1.79ns | 15.52 ± 1.78ns |
| Sex (N male/N female) | 2/17ns | 2/21ns |
| IQ (mean ± SD) | 98.74 ± 9.05ns | 103.96 ± 9.07ns |
| TSCC—Ab (mean ± SD) | 9.89 ± 5.77a | 4.26 ± 4.16a |
| TSCC—Sc (mean ± SD) | 13.0 ± 6.58a | 3.87 ± 3.95a |
| TSCC—Td (mean ± SD) | 50.93 ± 23.11a | 20.17 ± 17.19a |
| A‐DES (mean ± SD) | 2.60 ± 2.02a | 0.84 ± 0.90a |
| CDI (mean ± SD) | 16.10 ± 6.91a | 5.29 ± 5.43a |
| PTSD duration (years; mean ± SD) | 5.23 ± 3.58 | 0 |
| Current comorbidity (N) | ||
| ‐ Depressive disorder | 7 | 0 |
| ‐ ADHD | 1 | 0 |
| Medication use (N) | ||
| ‐ SSRI | 2 | 0 |
| ‐ Methylphenidate | 1 | 0 |
| Motion parameters | ||
| Translation (mm) | ||
| ‐ X | 0.003985 ± 0.0231ns | −0.001047 ± 0.0363ns |
| ‐ Y | −0.006069 ± 0.0655ns | −0.016853 ± 0.0416ns |
| ‐ Z | −0.019699 ± 0.1715ns | 0.013165 ± 0.0619ns |
| Rotation (°) | ||
| ‐ Pitch | 0.000547 ± 0.0013ns | −0.000397 ± 0.0021ns |
| ‐ Roll | 0.000063 ± 0.0005ns | 0.000002 ± 0.0081ns |
| ‐ Yaw | −0.000239 ± 0.0005ns | −0.000073 ± 0.0005ns |
Significant at p < 0.01.
ns Not significant at p < 0.05.
IQ, Intelligence Quotient; TSCC, Trauma Symptom Checklist for Children; A‐DES, Adolescent Dissociative Experiences Scale; CDI, Children's Depression Inventory; ADHD, Attention Deficit Hyperactivity Disorder; SSRI, Selective Serotonin Reuptake Inhibitors.
TSCC anxiety scale.
TSCC posttraumatic stress scale.
TSCC total score.
Note: Three patients did not complete the clinical questionnaires.
Functional Connectivity Analysis
Across groups, whole‐brain iFC analysis revealed dissociable BLA and CMA connectivity profiles with a distributed set of cortical and subcortical regions, consistent with established models of amygdaloid circuitry [LeDoux, 2007; Qin et al., 2012; Roy et al., 2013, 2009; Sah et al., 2003] (Fig. 1). Moreover, spatial similarity analysis of each participant's BLA and CMA connectivity maps revealed negative spatial correlations between BLA and CMA target networks (mean r = −0.23, p < 0.001), thus corroborating the distinctive and divergent connectivity profiles of the BLA and CMA in a quantitative manner (Fig. 1). As shown in Figure 2, direct group comparison of iFC revealed that PTSD patients had diminished right BLA connectivity with a cluster including dorsal and ventral portions of the anterior cingulate (ACC) and medial prefrontal (PFC) cortices (see Table S1 in Supporting Information 1 for more complete results). In contrast, PTSD patients showed increased left CMA connectivity with a cluster including the orbitofrontal and subcallosal cortices (Fig. 3) (see Table S2 in Supporting Information 1 for more complete results). Notably, group comparison of spatial similarity between BLA and CMA target networks revealed no significant group differences in the right (t(40) = −0.04, p = 0.97) or left (t(40) = 0.40, p = 0.69) hemisphere (Fig. 1). Thus, although the connectivity strength of the BLA and CMA with their respective targets is altered in adolescent PTSD, the functional differentiation and segregation of BLA and CMA networks is largely maintained.
Figure 1.
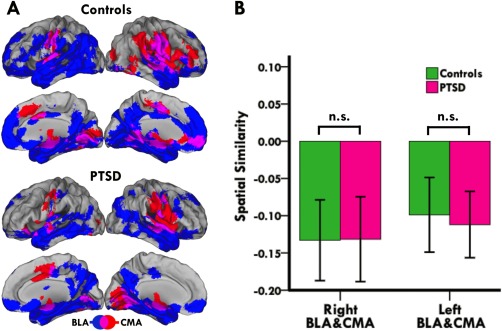
Dissociable connectivity profiles of BLA and CMA complexes. (A) Lateral and medial views of the BLA (blue) and CMA (red) target networks in controls and adolescent PTSD patients. Overlap between BLA and CMA networks is shown in purple. Images are in radiological convention (i.e., right is left and vice versa). (B) Bar graph showing spatial correlations between BLA and CMA networks in controls and patients. Positive values indicate similarities while negative values indicate differences between BLA and CMA networks. Group comparison of spatial similarities between BLA and CMA target networks revealed no significant group differences. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
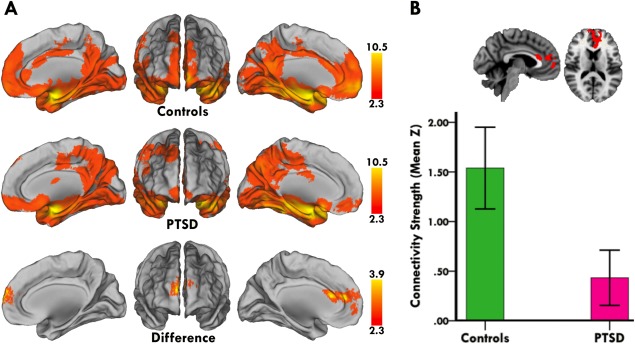
Abnormal right BLA connectivity in adolescent PTSD patients. (A) Medial and anterior views of right BLA connectivity in controls (first row) and adolescent PTSD patients (second row). Direct group comparison revealed that PTSD patients had diminished right BLA connectivity with a cluster including dorsal and ventral portions of the anterior cingulate and medial prefrontal cortices (third row). (B) Representative sagittal and axial slices of between group differences in right BLA connectivity, along with a bar graph showing lower mean connectivity strength (i.e., mean Z) in PTSD patients within the displayed cluster. Statistical maps are corrected for multiple comparisons at the cluster level (Z > 2.3, p < 0.05), and brain images are in radiological convention (i.e., right is left and vice versa). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
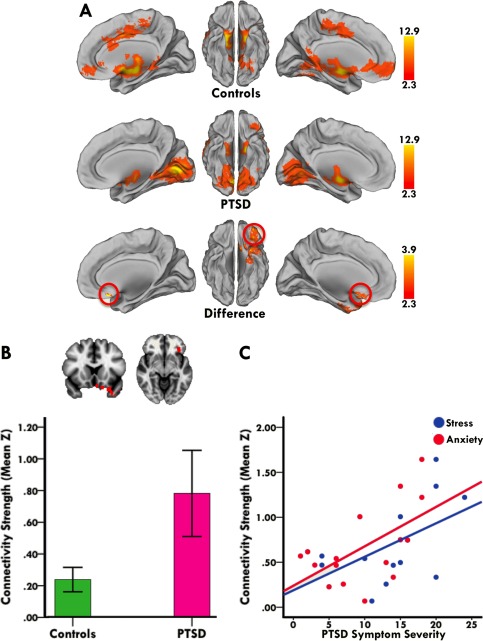
Abnormal left CMA connectivity in adolescent PTSD patients. (A) Medial and ventral views of left CMA connectivity in controls (first row) and adolescent PTSD patients (second row). Direct group comparison revealed that PTSD patients had increased left CMA connectivity with a cluster including the orbitofrontal and subcallosal cortices (third row). Red circles mark the effect site. (B) Representative coronal and axial slices of between group differences in left CMA connectivity, along with a bar graph showing higher mean connectivity strength (i.e., mean Z) in PTSD patients within the displayed cluster. Statistical maps are corrected for multiple comparisons at the cluster level (Z > 2.3, p < 0.05), and brain images are in radiological convention (i.e., right is left and vice versa). (C) Stronger CMA connectivity with the orbitofrontal/subcallosal region related to more symptoms of stress and anxiety in PTSD patients (r = 0.59 and 0.66; both p < 0.05). Individual patient's mean connectivity strength plotted against their symptom severity scores visualizes the direction of the association. PTSD‐related stress and anxiety was measured with the posttraumatic stress and anxiety subscales of the Trauma Symptom Checklist for Children (TSCC). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Grey Matter Volume Analysis
To examine whether alterations in grey matter volume of amygdala subregions may coincide with abnormalities in BLA and CMA connectivity, optimized VBM analysis was performed. As shown in Figure 4, our analysis revealed diminished grey matter volume in right BLA and CMA in PTSD patients (p < 0.05, TFCE and FWE‐corrected). Our analysis also revealed diminished grey matter volume in the left CMA in PTSD patients, albeit uncorrected for multiple comparisons (p < 0.05, TFCE‐corrected). Our structural analysis thus suggests that intra‐amygdalar abnormalities in grey matter volume coincide with amygdalar network dysfunction in adolescent PTSD.
Figure 4.
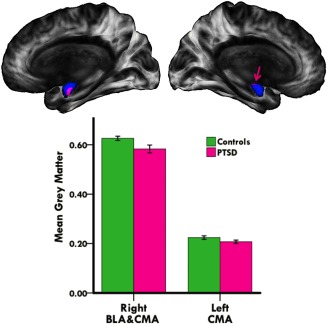
Abnormal grey matter volume of amygdala subregions in adolescent PTSD patients. Medial views of the right and left amygdaloid complex (blue), showing diminished grey matter volume of BLA (right hemisphere) and CMA (right and left hemispheres) subnuclei in adolescent PTSD patients (pink). Bar graph shows lower mean grey matter volume in PTSD patients within the displayed cluster. Right hemisphere results are corrected for multiple comparisons (p < 0.05, TFCE and FWE‐corrected), left hemisphere results are not (p < 0.05, TFCE‐corrected). Pink arrow marks the left CMA effect site. Brain images are in radiological convention (i.e., right is left and vice versa). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Functional Connectivity and Structure
To examine whether coinciding changes in amygdalar connectivity and structure are associated with each other, partial correlation analyses were performed. Although abnormal connectivity and morphology of amygdala subregions coincided in PTSD patients, we found no significant associations between them (p's > 0.05).
Functional Connectivity and Symptom Severity
Partial correlation analyses in patients examined the association between subregional connectivity strength within areas of significant group differences and PTSD symptoms of stress and anxiety. Our analyses revealed that stronger CMA connectivity with the orbitofrontal/subcallosal region related to more symptoms of stress and anxiety in PTSD patients (r = 0.59 and 0.66; both p < 0.05) (Fig. 3). Such association was not found for BLA‐medial prefrontal connectivity (p's > 0.05), which could be due to lack of statistical power and a possible ceiling effect in our relatively small sample of PTSD patients. In addition, no association was observed between amygdala subregional connectivity changes and depressive symptomatology in PTSD patients (p's > 0.05), suggesting that connectivity changes documented here might potentially be more characteristic of PTSD than depressive symptoms.
Structural Integrity and Symptom Severity
For transparency, partial correlation analyses in patients examined the association between subregional volumetrics within areas of significant group differences and PTSD symptoms of stress and anxiety. The analysis revealed no significant associations between structural integrity of amygdala subregions and PTSD symptomatology (p's > 0.05). Likewise, no associations were found between structural integrity of amygdala subregions and depressive symptoms (p's > 0.05).
PTSD Duration and Amygdalar Connectivity and Structure
Exploratory partial correlation analyses in PTSD patients examined the association between PTSD duration and subregional connectivity and volumetrics within areas of significant group differences. The analyses revealed that neither connectivity nor structure of the amygdaloid complex is associated with chronicity of PTSD symptoms (p's > 0.05). While this could relate to lack of statistical power and possibly a ceiling effect, it may also suggest that functional and structural changes we documented might be more representative of PTSD vulnerability and diagnosis rather than its duration.
Effects of Comorbidity and Medication Use on Functional Connectivity
Post‐hoc analyses examined the effects of comorbidity and medication use on group differences in iFC, per Roy et al. [2013] and Cullen et al. [2014]. Our analysis revealed that all group differences in iFC remained significant, while excluding patients with a secondary comorbid disorder (N = 8 excluded) (multivariate model: F(4,29) = 19.3, p < 0.001; right BLA: F(1,32) = 13.2, p < 0.001; left CMA: F(1,32) = 23.7, p < 0.001). Likewise, group differences in iFC remained significant, while excluding patients using medication (N=3 excluded) (multivariate model: F(4,34) = 21.78, p < 0.001; right BLA: F(1,37) = 19.8, p < 0.001; left CMA: F(1,37) = 15.4, p < 0.001). A final analysis excluding both patients with a secondary comorbid disorder and patients taking medication was not necessary, as only patients with a comorbid disorder were on medication. Additionally, we found no significant differences in iFC between PTSD patients with a comorbid disorder relative to those without (multivariate model: F(4,14) = 0.98, p = 0.45; right BLA: F(1,17) = 0.31, p = 0.87; left CMA: F(1,17) = 1.56, p = 0.23), or between patients who were on medication relative to those who were not (multivariate model: F(4,14) = 0.76, p = 0.57; right BLA: F(1,17) = 0.80, p = 0.38; left CMA: F(1,17) = 0.79, p = 0.38).
DISCUSSION
This study uniquely investigated the intrinsic functional architecture of amygdala‐centered networks in sexually abused adolescents with PTSD, relative to matched controls. We hypothesized altered BLA and CMA functional connectivity in PTSD patients, coupled with abnormalities in grey matter volume of amygdala subregions. In line with our hypotheses, we found evidence for altered BLA and CMA connectivity with regulatory prefrontal regions in PTSD patients. Critically, these connectivity changes coincided with diminished grey matter volume within BLA and CMA subnuclei, with CMA connectivity shifts additionally relating to more severe symptoms of PTSD. To our knowledge no previous study has characterized the emergence of such functional and structural abnormalities in severely traumatized adolescents. These findings therefore provide novel insights into how perturbations in major amygdalar circuits could hamper fear regulation and drive excessive acquisition and expression of fear in adolescent PTSD patients.
Amygdala Functional Connectivity
In line with neurocircuitry models of PTSD [Jovanovic and Ressler, 2010; Patel et al., 2012; Taghva et al., 2013], adolescent PTSD patients showed diminished right BLA connectivity with a cluster including dorsal and ventral portions of the anterior cingulate (ACC) and medial prefrontal (PFC) cortices. These interconnected medial prefrontal structures serve myriad functions, but they also play imperative and distinctive roles at various stages of fear processing through their unique relationships with the BLA and other brain regions [Etkin et al., 2011]. The dorsal ACC and adjacent dorsomedial PFC connect primarily to cognitive brain regions such as the lateral PFC, and are active in concert with the BLA during appraisal, acquisition, and cognitive regulation of fear [Chiba et al., 2001; Etkin et al., 2011; Vidal‐Gonzalez et al., 2006]. In contrast, the ventral ACC and adjacent ventromedial PFC connect directly and reciprocally to affective brain regions such as the BLA and hippocampus, and are mainly involved in automatic regulation and extinction of fear [Chiba et al., 2001; Etkin et al., 2011, 2006; Milad and Quirk, 2002; Myers‐Schulz and Koenigs, 2012; Vidal‐Gonzalez et al., 2006]. In fact, feed‐forward inhibitory projections from these ventral medial prefrontal areas to the BLA are deemed fundamental in regulating and extinguishing fear, as they compete with the fear pathway represented within the BLA‐to‐CMA microcircuit [Milad and Quirk, 2002; Peters et al., 2009; Vidal‐Gonzalez et al., 2006]. Noteworthy, this ventral inhibitory pathway also allows top–down governance of amygdala by dorsal ACC and dorsomedial PFC; regions that lack direct connections to the amygdaloid complex [Schiller and Delgado, 2010].
Diminished BLA connectivity with medial prefrontal areas as reported here could thus reflect a general dysfunction in fear processing, in which both the appraisal and acquisition of fear, as well as the effortful (i.e., dorsal) and automatic (i.e., ventral) regulation of fear are perturbed. This notion fits well with the clinical presentation of PTSD [Bradley et al., 2011; Ehring and Quack, 2010], and is supported by a wealth of data consistently linking abnormal experience and regulation of fear to disrupted functional integrity within the amygdala‐medial prefrontal circuit [Brown et al., 2014; Etkin et al., 2006; Etkin and Wager, 2007; Jin et al., 2013; Milad and Quirk, 2002; Myers‐Schulz and Koenigs, 2012; Shin and Liberzon, 2010; Sripada et al., 2012; Stevens et al., 2013; Vidal‐Gonzalez et al., 2006; Wolf and Herringa, 2015]. In fact, perturbed amygdala‐medial prefrontal coupling is deemed central to the pathophysiology of PTSD, as it promotes amygdala hyperactivity and diminished medial prefrontal control, thus increasing the propensity for excessive fear [Etkin and Wager, 2007; Jovanovic and Ressler, 2010]. As such, our finding provides novel evidence that disrupted BLA‐medial prefrontal connectivity might be a reliable neural marker of PTSD and a prominent feature of pediatric PTSD.
In line with recent work on the neurocircuitry of fear and anxiety [Milad and Rauch, 2007; Myers‐Schulz and Koenigs, 2012; Rempel‐Clower, 2007; Shin and Liberzon, 2010], adolescent PTSD patients also showed increased left CMA connectivity with a cluster including the orbitofrontal and subcallosal cortices. This increase in CMA connectivity was additionally related to more stress and anxiety symptoms in PTSD patients. The neighboring and highly interconnected orbitofrontal and subcallosal cortices serve numerous functions, but they also play a critical role in behavioral and physiological aspects of fear processing by modulating CMA activity [Barbas, 2007; Barbas et al., 2003; Hamani et al., 2011; Sah et al., 2003]. Such modulation of fear processing occurs mainly via orbitofrontal projections onto the CMA complex. Heavy projections from the posterior orbitofrontal cortex target the CMA via the intercalated masses, a subpopulation of amygdalar neurons sending feed‐forward inhibitory inputs to the CMA complex [Barbas, 2007]. The majority of CMA neurons are GABAergic [Pitkanen and Amaral, 1994; Saha et al., 2000], sending inhibitory projections to central autonomic structures such as the brainstem and hypothalamus [Barbas, 2007]. As such, orbitofrontal inhibition of the CMA via intercalated masses will thus result in disinhibition of central autonomic structures and produce a state of heightened vigilance and fear [Barbas, 2007].
We thus tentatively conclude that exaggerated CMA connectivity with orbitofrontal and subcallosal cortices could drive the perpetuating state of hypervigilance and fear often reported in patients with PTSD. In support of this notion, stronger CMA connectivity with the orbitofrontal/subcallosal region related to more stress and anxiety in our sample of adolescents with PTSD. Excessive fear and anxiety do indeed involve hyperactivity and hyperconnectivity within the amygdala‐orbitofrontal/subcallosal circuitry [Andreescu et al., 2014; Barbas and Zikopoulos, 2007; Gold et al., 2015; Holz et al., 2014; Milad and Rauch, 2007; Myers‐Schulz and Koenigs, 2012; Phillips et al., 2003; Sladky et al., 2015], and selective lesions within this circuitry significantly reduce the behavioral and physiological parameters of fear [Izquierdo et al., 2005; Kalin et al., 2007; Vasa et al., 2004]. However, our finding may also relate to augmented fear learning in PTSD, as recent data suggests a crucial role for the CMA not only in the expression but also the acquisition of fear. For instance, pretraining lesioning of the CMA in rodents impairs the acquisition of fear, while post‐training lesions of the CMA prevent the expression of leaned fear responses [Ciocchi et al., 2010; Wilensky et al., 2006; Zimmerman et al., 2007]. Moreover, animals with BLA lesions still demonstrate fear learning and this capacity is believed to be mediated by the CMA [Goosens and Maren, 2003]. In sum, our CMA findings document in humans what was previously established in animal studies, linking perturbations in the CMA‐orbitofrontal/subcallosal circuitry to abnormal acquisition and expression of fear.
Although the connectivity strength of the BLA and CMA with their respective targets was altered in adolescent PTSD patients, spatial similarity analysis revealed that BLA and CMA connectivity profiles in patients are essentially as segregated and divergent as in healthy controls. In other words, adolescent PTSD mainly affected the connectivity strength within BLA and CMA networks, while sparing the functional differentiation between them. A similar pattern was recently demonstrated in adult PTSD patients [Brown et al., 2014], which further supports this notion. Our findings thus confirm the distinctive and divergent connectivity profiles of the BLA and CMA [LeDoux, 2007; Qin et al., 2012; Roy et al., 2013, 2009; Sah et al., 2003], and suggest unique relationships between these amygdalar nuclei and regulatory prefrontal regions in the pathophysiology of adolescent PTSD. To our knowledge no previous study has characterized the emergence of such a pattern in severely traumatized adolescents. Converging lines of evidence suggest that experience‐dependent neuroplasticity in the developing adolescent brain plays a critical role in modulating the development of functional brain networks [Paus, 2005; Toga et al., 2006]. Moreover, animal data shows that stress and anxiety induce long‐lasting changes in amygdala connectivity and structure [Lupien et al., 2009]. We thus suggest that abnormal stress and anxiety early in life may cause the reconfiguration of amygdalar networks reported here and exacerbate vulnerability for stress‐related psychopathology.
It is worth mentioning that amygdalar connectivity changes we documented seemed somewhat lateralized, with PTSD patients exhibiting diminished right BLA and amplified left CMA coupling with regulatory frontal regions. The lateralization of amygdala function, especially of its different subregions, remains a topic of intense debate [Baas et al., 2004; Sergerie et al., 2008; Styliadis et al., 2014]. Some theories opine that right amygdala mediates relatively global and transient emotional responses, while its left counterpart seems to serve more specific and sustained forms of emotional responding [Baas et al., 2004; Sergerie et al., 2008]. Crucially, recent data suggests that right BLA encodes precise affective features (e.g., punishment), while left CMA seems to process general affective valence (e.g., good vs bad) [Styliadis et al., 2014]. One may thus speculate that the lateralized connectivity changes we seem to document could be indicative of a general dysfunction in affective processing among patients with PTSD. Although PTSD and anxiety seem to prompt lateralized changes in BLA and CMA connectivity [Brown et al., 2014; Nicholson et al., 2015; Roy et al., 2013], we do express our reservations on whether the apparent laterality of current and previous findings reflects genuine differences in affective processes. Clearly, additional studies are needed to further investigate the question of laterality in intrinsic connectivity of amygdala subregions in patients with PTSD.
Amygdala Structure
In conjunction with abnormal amygdalar connectivity, structural analysis revealed diminished grey matter volume of BLA and CMA subnuclei in adolescent PTSD patients. This corroborates previous reports on PTSD [Depue et al., 2014; Karl et al., 2006; Mollica et al., 2009; Morey et al., 2012; Rogers et al., 2009; Veer et al., 2015; Weems et al., 2013], and is in line with studies linking smaller amygdala volumes to enhanced fear conditioning and exaggerated stress reactivity [Gianaros et al., 2008; Hartley et al., 2011; Maroun et al., 2013; Yang et al., 2008], two prominent features of PTSD. For instance, strains of mice with relatively smaller amygdala volumes exhibit stronger fear conditioning and greater corticosterone responses to stress than mice with larger amygdala volumes [Yang et al., 2008]. Similarly, enhanced fear acquisition and stress reactivity, as measured by skin conductance and mean arterial pressure respectively, correlate with smaller amygdala volumes in humans [Gianaros et al., 2008; Hartley et al., 2011]. Our finding further links diminished amygdala volume to abnormal stress and anxiety, but also suggests that this intra‐amygdalar abnormality coincides with amygdalar network dysfunction in PTSD. Following this perspective, healthy individuals with genetic susceptibility for stress‐related psychopathology show disrupted amygdala‐prefrontal functional connectivity along with reduced amygdala volumetrics [Pezawas et al., 2005]. Additionally, highly anxious individuals show abnormal functional connectivity of amygdala subregions accompanied by changes in subregional volumetrics of the amygdaloid structure [Etkin et al., 2009; Qin et al., 2014].
Here we further implicate coinciding changes in amygdalar connectivity and structure in the pathophysiology of abnormal anxiety. Similar to previous reports though [Etkin et al., 2009; Pezawas et al., 2005; Qin et al., 2014], we found no significant correlation between these functional and structural alterations. While this could relate to lack of statistical power and a possible ceiling effect, it may also be suggestive of a complex structure–function relationship involving multiple moderating and mediating factors. The exact mechanism by which changes in structure may impact human brain function is still poorly understood. However, animal studies suggest that cellular changes in grey and white matter, including axon sprouting, dendritic arborization, and fiber organization, could impact network communication and information processing [Zatorre et al., 2012]. Future advances in imaging technology, and greater dialog between human neuroimaging and cellular/molecular neuroscience, could further our understanding of the complex interplay between brain structure and function.
Developmental data shows that abnormal stress and anxiety early in life cause greater dendritic arborization and increased synaptogenesis, leading to an initial increase in amygdala growth and activity [Davidson and McEwen, 2012; Tottenham and Sheridan, 2009; Vyas et al., 2006]. However, a prolonged period of stress‐induced increase in amygdala growth and activity promotes glucocorticoid receptor hypersensitivity and overexposure to stress hormones (i.e., neurotoxicity), eventually causing neural atrophy and even cell death [Hanson et al., 2015; Teicher et al., 2003; Tottenham and Sheridan, 2009; Yehuda, 2001]. Extrapolating from these data, we speculate that similar mechanisms could underlie reduced amygdala volumes observed in our sample of adolescent PTSD patients with a history of chronic exposure to stress. Indeed, the number of traumatic events, severity of exposure to trauma, and chronicity of PTSD all relate to smaller amygdala volumes in PTSD patients [Karl et al., 2006; Kuo et al., 2012; Mollica et al., 2009; Rogers et al., 2009; Veer et al., 2015], which further supports our hypothesized trajectory of amygdala atrophy. A similar trajectory has also been suggested in other stress‐related disorders such as depression, in which an initial increase in amygdala growth is followed by atrophy as depressive episodes become more severe and chronic [Lange and Irle, 2004; Sheline et al., 1998]. The fact that neither connectivity nor structure of the amygdala related to PTSD chronicity among our patients does not necessarily refute the proposed trajectory, as we lacked data on number of traumatic events and trauma exposure to fully test this assumption. Notwithstanding the possibility of low statistical power and/or ceiling effects, we cautiously suggest that amygdalar alterations we documented might be more representative of PTSD vulnerability and diagnosis rather than its duration. The findings could thus provide novel clues for how stress‐related alterations in the amygdaloid complex may contribute to PTSD pathophysiology in pediatric populations.
Study Limitations and Strengths
The cross‐sectional nature of this study does not allow for firm conclusions regarding causality. Hence, we cannot fully ascertain whether amygdalar abnormalities reported in this study preceded or followed the onset of PTSD. Additionally, the small number of adolescent males in our sample may to some extent limit the generalizability of our results. Longitudinal research in larger samples with a more balanced male to female ratio could tackle these limitations. In line with other PTSD studies, some of our patients had a secondary comorbid depressive disorder, and this may potentially affect the specificity of our results. Such comorbidity, however, is deemed a typical element of clinical PTSD, and exclusion of these patients therefore would have resulted in a highly atypical sample lacking external validity [Morey et al., 2012; Shin and Liberzon, 2010; Zahn‐Waxler et al., 2000]. As such, we performed post‐hoc analyses but found no impact of comorbidity on amygdala iFC patterns. Most of our patients were treatment‐naïve, with only 3 patients taking psychotropic medication. While in potential this may marginally affect our findings, post‐hoc analyses showed no impact of medication use on amygdala iFC reported here. Importantly, leading researchers in the field of PTSD neuroimaging strongly argue against exclusion of medicated patients from studies as this may limit the generalizability of the findings [Lanius et al., 2010]. It should be noted that we did not include control participants with a history of trauma exposure resembling that of the PTSD patients. However, sexually abused adolescents without any symptomatology and not in need of any psychological help are often reluctant to participate in such studies, owing to feelings of shame and embarrassment associated with their trauma. We are therefore unable to fully ascertain whether trauma exposure itself, regardless of diagnostic status, is associated with amygdalar abnormalities reported here. Nonetheless, our findings merit attention as the first evidence for perturbed function and structure of amygdala subregions in traumatized adolescents with PTSD diagnosis. The findings provide an interesting focus for future research, which should further explore and validate amygdalar abnormalities reported here. Finally, physiological fluctuations (heart rate and respiration) were not recorded during resting‐state data acquisition. Although we applied temporal filtering and regressed out white matter and cerebrospinal fluid signal to eliminate physiological noise [Fox and Raichle, 2007; Hallquist et al., 2013; Windischberger et al., 2002], these physiological fluctuations may still have been a source of noise influencing our data.
Notwithstanding these limitations, our study has several strengths that increase the reliability of our findings. First, to allow more accurate group comparison of amygdalar networks, patients and healthy controls were matched for age, sex, and IQ, while including these variables in the analyses as covariates to account for their possible confounding effects. Second, we included a highly selected and homogenous patient group with regard to type of trauma and chronicity of PTSD, which further strengthens the reliability of our findings. Third, partitioning the amygdala into BLA and CMA complexes produced dissociable connectivity profiles that were consistent with established models of amygdaloid circuitry. As such, we were able to demonstrate anxiety‐related perturbations in amygdala subregional connectivity, consistent with what was previously established in rodents and primates. Finally, multimodal imaging of the amygdaloid complex in this study produced complementary and novel results, which otherwise would only be partially detected by each modality alone.
CONCLUSIONS
In summary, adolescent PTSD patients showed abnormal amygdala subregional connectivity with regulatory prefrontal regions, coupled with diminished grey matter volume of amygdala subregions. These findings provide unique insights into how perturbations in major amygdalar circuits could hamper fear regulation and drive excessive acquisition and expression of fear in PTSD patients. As such, they represent an important step toward characterizing the neurocircuitry of adolescent PTSD, thereby informing the development of reliable biomarkers and potential therapeutic targets. For a deeper understanding of PTSD pathophysiology, it would be important to examine whether functional and structural integrity of amygdala subregions predict susceptibility, chronicity, and treatment response in PTSD.
FINANCIAL DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors are extremely grateful to all participants involved in the study. The authors gratefully acknowledge the contribution of Paul H.F. Meens, Bianca G. van den Bulk, Mirjam A.W. Rinne‐Albers, Carien I. Gelderblom, Michele W.J.H. Huijberts, and also thank the participating centers: Leiden University Medical Center Departments of Psychiatry and Radiology, Leiden Institute for Brain and Cognition, Psychotrauma Center and Department of Child and Adolescent Psychiatry Rivierduinen, and the Child and Adolescent Psychotrauma Center in Haarlem.
REFERENCES
- Ackerman PT, Newton JE, McPherson WB, Jones JG, Dykman RA (1998): Prevalence of post traumatic stress disorder and other psychiatric diagnoses in three groups of abused children (sexual, physical, and both). Child Abuse Negl 22:759–774. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K (2005): Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 210:343–352. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L, Aizenstein H (2014): Emotion reactivity and regulation in late‐life generalized anxiety disorder: Functional connectivity at baseline and post‐treatment. Am J Geriatr Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JG, Putnam FW, Carlson EB, Libero DZ, Smith SR (1997): Development and validation of a measure of adolescent dissociation: The Adolescent Dissociative Experiences Scale. J Nerv Ment Dis 185:491–497. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS (2004): Lateralization of amygdala activation: A systematic review of functional neuroimaging studies. Brain Res Rev 45:96–103. [DOI] [PubMed] [Google Scholar]
- Barbas H (2007): Flow of information for emotions through temporal and orbitofrontal pathways. J Anat 211:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel‐Clower N, Ghashghaei T (2003): Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci 4:25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B (2007): The prefrontal cortex and flexible behavior. Neuroscientist 13:532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bradley B, DeFife JA, Guarnaccia C, Phifer J, Fani N, Ressler KJ, Westen D (2011): Emotion dysregulation and negative affect: Association with psychiatric symptoms. J Clin Psychiatry 72:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD (2006): Traumatic stress: Effects on the brain. Dialogues Clin Neurosci 8:445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J. (1996). Trauma Symptom Checklist for Children (TSCC). Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, McCarthy G, Morey RA (2014): Altered resting‐state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 39:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K (2001): Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata . Brain Res 888:83–101. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A (2010): Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468:277–282. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Steele JS, Lenow JK, Smitherman S, Everett B, Messias E, Kilts CD (2014): Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: An exploratory fMRI study. J Psychiatr Res 48:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes‐Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO (2014): Abnormal amygdala resting‐state functional connectivity in adolescent depression. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS (2012): Social influences on neuroplasticity: Stress and interventions to promote well‐being. Nat Neurosci 15:689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ (2001): The amygdala: Vigilance and emotion. Mol Psychiatry 6:13–34. [DOI] [PubMed] [Google Scholar]
- Depue BE, Olson‐Madden JH, Smolker HR, Rajamani M, Brenner LA, Banich MT (2014): Reduced amygdala volume is associated with deficits in inhibitory control: A voxel‐ and surface‐based morphometric analysis of comorbid PTSD/mild TBI. Biomed Res Int 2014:691505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen‐Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A (2007): Anatomically related grey and white matter abnormalities in adolescent‐onset schizophrenia. Brain 130:2375–2386. [DOI] [PubMed] [Google Scholar]
- Ehring T, Quack D (2010): Emotion regulation difficulties in trauma survivors: The role of trauma type and PTSD symptom severity. Behav Ther 41:587–598. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011): Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J (2006): Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51:871–882. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD (2009): Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 66:1361–1372. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007): Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fransson P (2006): How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44:2836–2845. [DOI] [PubMed] [Google Scholar]
- Gabard‐Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N (2014): The development of human amygdala functional connectivity at rest from 4 to 23years: A cross‐sectional study. Neuroimage 95C:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR (2008): Individual differences in stressor‐evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci 28:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Morey RA, McCarthy G (2015): Amygdala‐prefrontal cortex functional connectivity during threat‐induced anxiety and goal distraction. Biol Psychiatry 77:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S (2003): Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci 117:738–750. [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B (2013): The nuisance of nuisance regression: Spectral misspecification in a common approach to resting‐state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 82:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM (2011): The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry 69:301–308. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ (2015): Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol Psychiatry 77:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA (2011): Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb Cortex 21:1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz NE, Buchmann AF, Boecker R, Blomeyer D, Baumeister S, Wolf I, Rietschel M, Witt SH, Plichta MM, Meyer‐Lindenberg A, Banaschewski T, Brandeis D, Laucht M (2014): Role of FKBP5 in emotion processing: Results on amygdala activity, connectivity and volume. Brain Struct Funct. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA (2005): Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci 25:8534–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Qi R, Yin Y, Hu X, Duan L, Xu Q, Zhang Z, Zhong Y, Feng B, Xiang H, Gong Q, Liu Y, Lu G, Li L (2013): Abnormalities in whole‐brain functional connectivity observed in treatment‐naive post‐traumatic stress disorder patients following an earthquake. Psychol Med 1–10. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ (2010): How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry 167:648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ (2007): Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry 62:1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A (2006): A meta‐analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 30:1004–1031. [DOI] [PubMed] [Google Scholar]
- Kim JE, Lyoo IK, Estes AM, Renshaw PF, Shaw DW, Friedman SD, Kim DJ, Yoon SJ, Hwang J, Dager SR (2010): Laterobasal amygdalar enlargement in 6‐ to 7‐year‐old children with autism spectrum disorder. Arch Gen Psychiatry 67:1187–1197. [DOI] [PubMed] [Google Scholar]
- Kovacs M. (1992). The Children's Depression Inventory (CDI) manual. New York, NY: MultiHealth Systems. [Google Scholar]
- Kuo JR, Kaloupek DG, Woodward SH (2012): Amygdala volume in combat‐exposed veterans with and without posttraumatic stress disorder: A cross‐sectional study. Arch Gen Psychiatry 69:1080–1086. [DOI] [PubMed] [Google Scholar]
- Lange C, Irle E (2004): Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med 34:1059–1064. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Brewin CR, Bremner JD, Daniels JK, Friedman MJ, Liberzon I, McFarlane A, Schnurr PP, Shin L, Stein M, Vermetten E (2010): Does neuroimaging research examining the pathophysiology of posttraumatic stress disorder require medication‐free patients? J Psychiatry Neurosci 35:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (2007): The amygdala. Curr Biol 17:R868–R874. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000): Emotion circuits in the brain. Annu Rev Neurosci 23:155–184. [DOI] [PubMed] [Google Scholar]
- Li Y, Qin W, Jiang T, Zhang Y, Yu C (2012): Sex‐dependent correlations between the personality dimension of harm avoidance and the resting‐state functional connectivity of amygdala subregions. PLoS One 7:e35925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009): Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ (2012): Fear conditioning, synaptic plasticity and the amygdala: Implications for posttraumatic stress disorder. Trends Neurosci 35:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Ioannides PJ, Bergman KL, Kavushansky A, Holmes A, Wellman CL (2013): Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. Eur J Neurosci 38:2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B (1996): Involvement of the amygdala in memory storage: Interaction with other brain systems. Proc Natl Acad Sci U S A 93:13508–13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, Kessler RC (2013): Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry 52:815–830 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2002): Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL (2007): The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci 1121:546–561. [DOI] [PubMed] [Google Scholar]
- Mollica RF, Lyoo IK, Chernoff MC, Bui HX, Lavelle J, Yoon SJ, Kim JE, Renshaw PF (2009): Brain structural abnormalities and mental health sequelae in South Vietnamese ex‐political detainees who survived traumatic head injury and torture. Arch Gen Psychiatry 66:1221–1232. [DOI] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, Nasser JD, Wagner HR, McCarthy G, Mid‐Atlantic MW (2012): Amygdala volume changes in posttraumatic stress disorder in a large case‐controlled veterans group. Arch Gen Psychiatry 69:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers‐Schulz B, Koenigs M (2012): Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Mol Psychiatry 17:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Densmore M, Frewen PA, Theberge J, Neufeld RWJ, McKinnon MC, Lanius RA (2015): The dissociative subtype of posttraumatic stress disorder: Unique resting‐state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology 40:2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Mennes M, Brown S, Castellanos FX, Leventhal B, Milham MP, Colcombe SJ (2013): Relationship of trauma symptoms to amygdala‐based functional brain changes in adolescents. J Trauma Stress 26:784–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA (2012): Neurocircuitry models of posttraumatic stress disorder and beyond: A meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36:2130–2142. [DOI] [PubMed] [Google Scholar]
- Paus T (2005): Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci 9:60–68. [DOI] [PubMed] [Google Scholar]
- Perry BD, Azad I (1999): Posttraumatic stress disorders in children and adolescents. Curr Opin Pediatr 11:310–316. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R (2010): Emotion processing and the amygdala: From a 'low road' to 'many roads' of evaluating biological significance. Nat Rev Neurosci 11:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ (2009): Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer‐Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR (2005): 5‐HTTLPR polymorphism impacts human cingulate‐amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci 8:828–834. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R (2003): Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 54:515–528. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG (1994): The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: An immunohistochemical and in situ hybridization study. J Neurosci 14:2200–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V (2014): Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry 75:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Supekar K, Uddin LQ, Menon V (2012): Immature integration and segregation of emotion‐related brain circuitry in young children. Proc Natl Acad Sci U S A 109:7941–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel‐Clower NL (2007): Role of orbitofrontal cortex connections in emotion. Ann N Y Acad Sci 1121:72–86. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, Aoki S, Kato N, Kasai K (2009): Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post‐traumatic stress disorder. Psychiatry Res 174:210–216. [DOI] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, Benson B, Castellanos FX, Milham MP, Pine DS, Ernst M (2013): Intrinsic functional connectivity of amygdala‐based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 52:290–299 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP (2009): Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J (2003): The amygdaloid complex: Anatomy and physiology. Physiol Rev 83:803–834. [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TF, Henderson Z (2000): A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: A combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience 99:613–626. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH (2013): An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting‐state functional connectivity data. Neuroimage 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Delgado MR (2010): Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci 14:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL (2008): The role of the amygdala in emotional processing: A quantitative meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 32:811–830. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL (1998): Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport 9:2023–2028. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I (2010): The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35:169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W, Albano, A. (1996). The Anxiety Disorders Interview Schedule for DSM‐IV–Child and Parent Versions. San Antonio, TX: Raywind Publications. [Google Scholar]
- Sladky R, Hoflich A, Kublbock M, Kraus C, Baldinger P, Moser E, Lanzenberger R, Windischberger C (2015): Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for fMRI. Cereb Cortex 25:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I (2012): Altered resting‐state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci 37:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ (2013): Disrupted amygdala‐prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 47:1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styliadis C, Ioannides AA, Bamidis PD, Papadelis C (2014): Amygdala responses to valence and its interaction by arousal revealed by MEG. Int J Psychophysiol 93:121–133. [DOI] [PubMed] [Google Scholar]
- Taghva A, Oluigbo C, Corrigan J, Rezai AR (2013): Posttraumatic stress disorder: Neurocircuitry and implications for potential deep brain stimulation. Stereotact Funct Neurosurg 91:207–219. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM (2003): The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev 27:33–44. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER (2006): Mapping brain maturation. Trends Neurosci 29:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T (2008): Functional coactivation map of the human brain. Cereb Cortex 18:2553–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA (2009): A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front Hum Neurosci 3:68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa RA, Grados M, Slomine B, Herskovits EH, Thompson RE, Salorio C, Christensen J, Wursta C, Riddle MA, Gerring JP (2004): Neuroimaging correlates of anxiety after pediatric traumatic brain injury. Biol Psychiatry 55:208–216. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NYL, van Buchem MA, Spinhoven P, Elzinga BM, Rombouts SARB (2015): Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res Neuroimag 233:436–442. [DOI] [PubMed] [Google Scholar]
- Vidal‐Gonzalez I, Vidal‐Gonzalez B, Rauch SL, Quirk GJ (2006): Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem 13:728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S (2006): Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 143:387–393. [DOI] [PubMed] [Google Scholar]
- Weems CF, Scott BG, Russell JD, Reiss AL, Carrion VG (2013): Developmental variation in amygdala volumes among children with posttraumatic stress. Dev Neuropsychol 38:481–495. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE (2006): Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26:12387–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windischberger C, Langenberger H, Sycha T, Tschernko EM, Fuchsjager‐Mayerl G, Schmetterer L, Moser E (2002): On the origin of respiratory artifacts in BOLD‐EPI of the human brain. Magn Reson Imaging 20:575–582. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Herringa RJ (2015): Prefrontal‐amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology 41:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A (2008): Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology 33:2595–2604. [DOI] [PubMed] [Google Scholar]
- Yehuda R (2001): Biology of posttraumatic stress disorder. J Clin Psychiatry 62:41–46. [PubMed] [Google Scholar]
- Zahn‐Waxler C, Klimes‐Dougan B, Slattery MJ (2000): Internalizing problems of childhood and adolescence: Prospects, pitfalls, and progress in understanding the development of anxiety and depression. Dev Psychopathol 12:443–466. [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen‐Berg H (2012): Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Rabinak CA, McLachlan IG, Maren S (2007): The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem 14:634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


