Abstract
Creativity is commonly defined as the ability to produce something both novel and useful. Stimulating creativity has great significance for both individual success and social improvement. Although increasing creative capacity has been confirmed to be possible and effective at the behavioral level, few longitudinal studies have examined the extent to which the brain function and structure underlying creativity are plastic. A cognitive stimulation (20 sessions) method was used in the present study to train subjects and to explore the neuroplasticity induced by training. The behavioral results revealed that both the originality and the fluency of divergent thinking were significantly improved by training. Furthermore, functional changes induced by training were observed in the dorsal anterior cingulate cortex (dACC), dorsal lateral prefrontal cortex (DLPFC), and posterior brain regions. Moreover, the gray matter volume (GMV) was significantly increased in the dACC after divergent thinking training. These results suggest that the enhancement of creativity may rely not only on the posterior brain regions that are related to the fundamental cognitive processes of creativity (e.g., semantic processing, generating novel associations), but also on areas that are involved in top‐down cognitive control, such as the dACC and DLPFC. Hum Brain Mapp 37:3375–3387, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: divergent thinking, dACC, DLPFC, neural plasticity
INTRODUCTION
Creativity is commonly defined as the ability to produce something both novel and useful [Runco and Jaeger, 2012; Stein, 1953; Sternberg and Lubart, 1996]. As the fountainhead of the flowing water of human civilization, creativity is linked not only to social development but also to almost all areas of our everyday life [Dietrich and Kanso, 2010; Mumford, 2002]. The question of whether creativity can be developed with training has long attracted the attention of researchers. Various strategies and training paradigms have been used to stimulate creative thinking, such as imagery techniques, structuring group interactions, and providing incentives [Scott et al., 2004; Smith, 1998]. Among the different approaches that have been tried, cognitive stimulation, which involves exposure to the ideas of others, is one approach that has been shown to enhance creativity [Fink et al., 2010, 2012]. Other studies have tried to affect creativity by changing a person's internal state or external environment through meditation or simply walking at a natural pace [Ding et al., 2014; Oppezzo and Schwartz, 2014]. Reviews of the relevant research literature on creativity confirm the effectiveness (gains in performance) of creative cognition training [Ma, 2006; Scott et al., 2004].
Previous neuroimaging studies have explored the neural basis of creativity using a wide variety of tasks (such as divergent thinking tasks, insight problem solving, and artistic creation tasks) and measurement methods (such as task‐based fMRI, voxel‐based morphometry, resting state fMRI, and diffusion tensor imaging) [Abraham et al., 2012; Aziz‐Zadeh et al., 2013; Jung‐Beeman et al., 2004; Qiu et al., 2010; Saggar et al., 2015; Zhu et al., 2013]. These studies have revealed that creative thinking may be related to widespread brain areas rather than to a single area of the brain. Those brain areas were mainly located in the frontal cortex, cingulate cortex, and temporoparietal areas [Arden et al., 2010; Dietrich and Kanso, 2010; Sawyer, 2011]. Using activation likelihood estimation to ascertain the key regions of divergent thinking, our recent meta‐analysis found that the key regions were located in the lateral prefrontal cortex (LPFC), posterior parietal cortex (PPC), precuneus, anterior cingulate cortex (ACC), and several regions in the temporal cortex [Wu et al., 2015]. Recent network perspective tends to divide those brain areas into two brain networks: the default mode network (DMN) and the cognitive control network (CCN) [Beaty et al., 2016]. The DMN and the CCN likely correspond to the blind variation and the selective retention components of creative thinking, respectively [Campbell, 1960; Simonton, 1984]. The former network is related to creative thinking generation, while the latter network is related to top‐down cognitive control [Abraham, 2014; Jung et al., 2013; Mok, 2014].
Even though numerous studies have explored the neural mechanisms of creativity, the neural mechanisms underlying creative cognition training are still unknown. Prior cross‐sectional studies comparing expert and novice or high‐creative and low‐creative individuals may provide insights into experience‐related changes in the brain [Fink et al., 2009a; Gibson et al., 2009; Kowatari et al., 2009]. However, it is not possible to determine from cross‐sectional studies whether the observed functional and structural differences are due to pre‐existing differences or to the effects of practice or training.
Longitudinal studies revealing the brain plasticity induced by creativity training are still rare. Therefore, the specific structural and functional changes in the brain that are induced by creativity training are still unclear. Despite that, there is evidence that it is possible to reshape the brain through creativity training [Fink et al., 2006]. The participants, who were trained for about two weeks on various divergent thinking tasks, displayed comparatively higher synchronization of frontal EEG alpha activity, compared with pre‐training. A recent study by Fink et al. found that training of verbal creativity modulates brain activity in language and memory related regions, such as the left inferior parietal cortex (IPL) and the left middle temporal gyrus (MTG) [Fink et al., 2015].
Interestingly, even a short‐term intervention can change brain activity patterns with creative cognition tasks or in a resting state [Fink et al., 2010; Wei et al., 2014]. For example, Wei et al. [2014] found that resting‐state functional connectivity was altered after a short cognitive stimulation intervention. However, direct evidence from longitudinal investigations of functional and structural changes resulting from training is still lacking. Longitudinal studies that combine functional and structural imaging data may enhance our understanding of the neurophysiological effects of creative cognition training [Chen et al., 2007; Ilg et al., 2008; Supekar et al., 2013; Taubert et al., 2011].
The present study investigated the effects of a 20‐session, cognitive stimulation training intervention (one session each day), which involved exposing participants to the ideas of other people [Fink et al., 2010; Wei et al., 2014]. We employed divergent thinking tasks that are widely used to measure creativity [Fink and Benedek, 2014; Scott et al., 2004]. We proposed several hypotheses based on the findings from the previous studies [Ma, 2006; Scott et al., 2004]. First, we hypothesized that creative performance (originality and fluency of ideas) would be enhanced by training. Second, we hypothesized that brain activity changes would be observed in areas that are related to creative idea generation (e.g., the parietal lobe, precuneus and temporal lobe) and to cognitive control (e.g., the ACC and DLPFC) during divergent thinking tasks. Third, we hypothesized, based on the previous training studies using MRI [Bezzola et al., 2011; Olesen et al., 2004; Scholz et al., 2009; Zatorre et al., 2012], that specific training‐induced structural changes would be observed in these brain regions.
MATERIALS AND METHODS
Experiment 1
Participants
All participants were right‐handed and met MRI safety criteria (e.g., no braces or metal implants), and none of them had a history of neurological or psychiatric illness (self‐reported, with no history of brain damage, schizophrenia, major depression, anxiety disorder, and insomnia). The study was approved by the Brain Imaging Center Institutional Review Board at the Southwest University, China. In accordance with the Declaration of Helsinki (1991), written informed consent was obtained from all participants. A total of 40 participants were recruited from Southwest University, China, and randomly assigned to the training group (TG) or control group (CG). One participant in the TG withdrew from the study after the pre‐test, one participant in the CG did not take the post‐test, and another participant in the TG did not obey the instructions for the post‐test in the scanner. Four participants in the TG and five participants in the CG were excluded from the analyses because of head motions greater than 3 mm maximum translation or 3° rotation during the fMRI scanning at the pre‐test or post‐test. Therefore, the final sample consisted of 14 participants in the TG (6 males and 8 females) and 14 participants in the CG (5 males 9 females) for the analysis. The two groups did not significantly differ in age or intelligence.
Procedure
First, participants in both the TG and the CG completed cognitive assessments at the pre‐test, which included a battery of divergent thinking tasks and a measure of intelligence. The first brain imaging data were recorded after those behavioral measures were completed. Then the TG participants were instructed to come to the laboratory every day to complete the 20‐session training procedure (one session per day). The participants in CG were untrained and needed not to come to the laboratory during these days. After the training procedure, the second brain imaging data and behavioral data were obtained for the TG and CG.
Assessment of general intelligence
All participants were tested individually on the Raven's Advanced Progressive Matrix (RAPM) [Raven, 1962], which is a recognized measure of intelligence that has a high degree of reliability and validity [Tang et al., 2012]. This measure consists of 36 nonverbal items; each item contains a 3 × 3 matrix with a missing piece to be completed. Participants were required to select the correct one out of eight alternatives. The current study used the total score of the test in the statistical analyses, in keeping with standard practice [Jaeggi et al., 2008; Takeuchi et al., 2010].
Tasks
DT tasks
Participants performed a battery of DT tasks before and after the training procedure, which were based on existing creativity tests [Benedek et al., 2006; Fink et al., 2006]. (i) Insight task (IT): participants were presented an unusual situation and asked to think of different causes for a given situation. (ii) Utopian situations task (UST): participants were instructed to imagine themselves in a given utopian situation and to generate original consequences of the situation. (iii) Product improvement task (PIT): participants were prompted to think about how to improve a product, such as a toy elephant, to make it more popular and interesting. (iv) Alternative uses task (AUT): participants attempted to think about unusual/original uses for everyday objects. The total duration of the four tasks was 24 minutes. Each task contained two items. For each item, participants had 3 minutes to write down their ideas. The items were divided into two parallel versions (version A and version B) to eliminate the potential effect of using the same items at the pre‐test and post‐test. One half of the participants used version A at the pre‐test and version B at the post‐test, while the other half used version B at the pre‐test and version A at the post‐test. The mean scores of these tasks were used to evaluate individuals’ divergent thinking ability.
All the generated ideas were rated for their originality by three trained raters who used a five‐point rating scale ranging from 1 (“not original”) to 5 (“highly original”). The fluency (the number of the ideas) and originality (average of the originality scores of the ideas) scores were obtained by averaging the raters’ scores. The raters displayed high internal consistency in their ratings (mean Cronbach alpha = 0.92). ANOVAs were conducted with group (TG and CG) and time period (pre‐ and post‐test) as factors.
fMRI task
An alternative uses task (AUT) and a control task (an object characteristics task, OCT) were used in the scanner during the pre‐test and post‐test. Each task had 12 items and each item was presented in separate blocks. The AUT required participants to think of as many original uses as possible for everyday objects in 20 s. The OCT task required participants to think of the typical characteristics of everyday objects within 20 s. After each item, participants had 4 s to press the buttons corresponding to the number of ideas they generated (four choices were provided: 0–1 ideas, 2–3 ideas, 4–5 ideas, and more than 5 ideas). There was a fixation point lasting 20 s between the items. The tasks were presented in a fixed sequence (Fixation—AUT1—Fixation—OCT1—Fixation—AUT2—Fixation—OCT2…). Participants were asked to write down the ideas they generated in the scanner after the scanning was completed. The responses of the participants were positively correlated (r = 0.81, P < 0.001) with the number of ideas they provided after scanning.
As was assessed in the DT tasks, all the generated ideas in the fMRI session were rated with a five‐point rating scale. The results displayed high internal consistency in the ratings (mean Cronbach's alpha = 0.90).
Cognitive stimulation training
The study's cognitive stimulation training was developed from the protocol described in Fink et al. [2010] and Wei et al. [2014]. First, an everyday object (e.g., “umbrella”) was presented and the participants were asked to generate as many novel and unusual uses as possible for the object in 3 minutes. Then, cognitive stimulation was provided by exposing them to external ideas for 1 minute (for each item there are three ideas, which were obtained in a preliminary experiment). Subsequently, participants had another 3 minutes to think of other novel and unusual uses for the same object. This procedure is known as an effective approach in group‐based brainstorming techniques [Dugosh et al., 2000] and its effectiveness has been confirmed by a previous research [Fink et al., 2010]. Participants in the TG were instructed to complete 20 sessions of training. They needed to complete four items during each session. Each training session took 28 minutes. The items used in the training sessions were different from those used during fMRI task.
Image acquisition
Images were acquired using a Siemens TRIO 3‐Tesla scanner. Participants lay supine with their heads snugly fixed with foam pads to minimize head movement, and were instructed to keep still. A total of 535 BOLD images were obtained using an Echo Planar Imaging (EPI) sequence with the following parameters: slices = 32; repetition time (TR)/echo time (TE) = 2000/30 ms; flip angle = 90°. FOV = 200 × 200 mm2; voxel size = 3.4 × 3.4 × 4 mm3; thickness = 3 mm; slice gap = 1mm. A magnetization‐prepared rapid gradient echo (MPRAGE) sequence was used to acquire high‐resolution T1‐weighted anatomical images (slices = 176; TR = 1,900 ms; TE = 2.52 ms; flip angle = 9°; inversion time = 900 ms; FOV= 256 × 256 mm2; voxel size = 1 × 1 × 1 mm3; thickness = 1.0 mm).
fMRI task analysis
Functional imaging data analyses were performed with SPM8 software (Wellcome Department of Imaging Neuroscience, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm). First, the functional data of each participant were motion‐corrected. Participants who exhibited a head motion of 3 mm maximum translation or 3.0° rotation were excluded from further analyses. Then, each participant's functional images were normalized to EPI templates based on the Montreal Neurological Institute space (resampling voxel size was 3 × 3 × 3 mm3). Spatial smoothing (8 mm FWHM Gaussian kernel) was conducted to increase signal to noise ratio.
In the first‐level analysis, each participant, each time period (pre‐ and post‐test), and each task type were modeled separately using the general linear model. The movement correction parameters were added as covariates of no interest. The blocks in which participants did not generate any answer were excluded from the analyses (1.41% of blocks were excluded). In light of the research questions and hypotheses, we performed a comparison to produce a “contrast image” for each participant: AUT versus OCT. A one‐sample t‐test was used for each contrast image to obtain the activity pattern for each time period. Regions reaching cluster‐level significance at P < 0.05, FEW corrected (following initial thresholding at P < 0.001, uncorrected) were reported.
Paired t‐tests were used to estimate the activity differences between the pre‐test and post‐test. For the whole brain analysis, brain regions that showed activity changes were reported with a voxel‐wise threshold of P < 0.001, cluster size greater than 10.
Small‐volume corrections (SVC) were performed across the regions of interest (ROIs). We defined the ROIs according to the prior neuroimaging studies about divergent thinking [Abraham et al., 2012; Beaty et al., 2016; Fink et al., 2009a, 2015; Jung et al., 2013; Wu et al., 2015]. Previous studies revealed that divergent thinking was closely related to some brain regions, including the ACC, DLPFC, precuneus, IPL and temporal lobule. The Wake Forest University (WFU) Pick Atlas [Maldjian et al., 2003] was used to define these areas: the bilateral ACC, bilateral precuneus, left and right DLPFC (BA45, BA46), left and right IPL, and left and right bilateral temporal lobule. The family‐wise error (FWE) method was used for multiple comparisons. The significance level was set at P < 0.05. Further ANOVAs were conducted in these significant regions with group (TG and CG) and time period (pre‐ and post‐test) as factors.
VBM analysis
The MR images were processed using SPM8 (Wellcome Department of Cognitive Neurology, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 7.8 (MathWorks Inc., Natick, MA). Each MR image was displayed in SPM8 to screen for artifacts and gross anatomical abnormalities. To attain better registration, the reorientation of the images was manually set to the anterior commissure. Segmentation of the images into gray matter (GM), white matter (WM), and cerebrospinal fluid was performed through the new segmentation toolbox in SPM8. Subsequently, we performed diffeomorphic anatomical registration through exponentiated lie (DARTEL) algebra in SPM8 for registration, normalization, and modulation [Ashburner, 2007]. To ensure conservation of regional differences in the absolute amounts of GM, the image intensity of each voxel was modulated by the Jacobian determinants. The registered images were transformed to Montreal Neurological Institute (MNI) space. Finally, the normalized modulated images (GM and WM images) were smoothed with an 8‐mm full‐width‐at‐half maximum Gaussian kernel to increase the signal‐to‐noise ratio.
Paired t‐tests were used to estimate GMV changes between the pre‐test and post‐test. On the whole brain analysis, the brain regions that showed GMV changes were reported with a voxel‐wise threshold of P < 0.001, cluster size greater than 100.
SVCs were performed across the ROIs defined by previous studies [Abraham et al., 2012; Beaty et al., 2016; Fink et al., 2009a, 2015; Jung et al., 2013; Wu et al., 2015]. The voxel‐wise threshold was set at P < 0.001. The Wake Forest University (WFU) Pick Atlas [Maldjian et al., 2003] was used to define these areas: the bilateral ACC, bilateral precuneus, left and right DLPFC (BA45, BA46), left and right IPL, and left and right temporal lobule. The FWE method was used for multiple comparisons. The significance threshold was set at P < 0.05. Further ANOVAs were conducted in these significant regions with group (TG and CG) and time period (pre‐ and post‐test) as factors.
Experiment 2
Given the small sample size of Experiment 1, Experiment 2, which ran at a separate time, was designed to confirm the observed training‐induced functional and structural brain changes. Because the CG in Experiment 1 did not yield significant functional or structural brain changes, Experiment 2 only included the TG. Other training studies have used a second experiment to confirm their findings [Olesen et al., 2004; Ventura‐Campos et al., 2013].
Participants
Fifteen new participants were recruited for this study. The inclusion and exclusion criteria were the same as those used in Experiment 1. The data of two participants were discarded because of head motion greater than 3 mm maximum translation or 3° rotation during the fMRI scanning at the pre‐test or post‐test. Following the same experimental procedure as conducted in the Experiment 1, the remaining 13 participants (mean age = 22.38 years; SD = 2.10; 5 males) underwent the pre‐ and post‐training behavioral measures and MRI scans and the training procedure. Participants and the experimenters had no information about the findings of Experiment 1.
Imaging data acquisition
MRI data were acquired using the same scanner with the same sequence. To control the potential head motion, the fMRI tasks were divided into two runs. Between the two runs, participants were told to keep their head still and the task would continue soon. A total of 542 BOLD images (271 volumes each run) were obtained. A magnetization‐prepared rapid gradient echo (MPRAGE) sequence was used to acquire high‐resolution T1‐weighted anatomical images.
Data analysis
The analyses followed the same procedure as those performed in Experiment 1. Paired t‐tests were used to estimate the differences between pre‐test and post‐test for the behavioral data and the MRI data (the functional activity changes and GMV changes). SVC were used for multiple comparisons.
RESULTS
Experiment 1
Behavioral data
The duration of the training session is 20–26 days (Mean = 22.29, SD = 1.90) (Figure 1). Participants in the TG completed one session each day. The behavioral data during the training sessions were shown in Figure 2.
Figure 1.
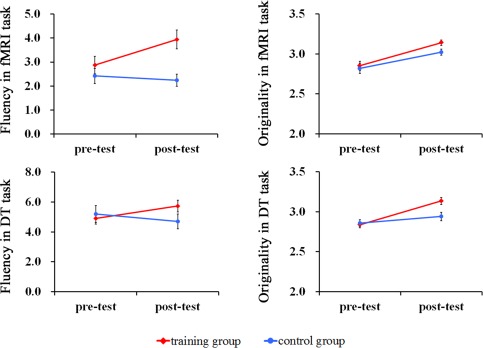
Cognitive stimulation training improved divergent thinking performance. Participants had significantly higher fluency and originality after training both in the fMRI tasks and in the DT tasks. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
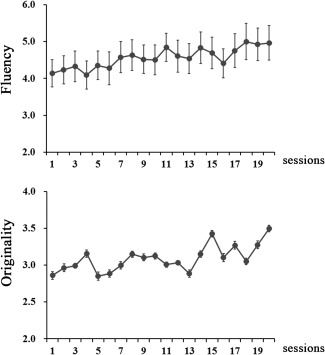
Behavioral data during the training sessions.
DT tasks
Independent t‐tests showed that there was no significant difference between the two groups in either fluency or originality before training (all P > 0.05). The group × time period interaction was not significant for fluency. A significant main effect of time period was found for originality [F (1, 26) = 17.73; P < 0.05, η2 = 0.40] as well as group × time period interaction [F (1, 26) = 5.64; P < 0.05, η2 = 0.18]. The main effect of group was marginally significant [F (1, 26) = 4.12; P = 0.05, η2 = 0.14]. Subsequent t‐tests indicated the TG had significantly better scores at the post‐test than at the pre‐test (P < 0.05), while the CG's scores did not change significantly (Tables 1 and 2). More details about behavioral data were shown in supplementary materials.
Table 1.
Demographic and behavioral data
| Training group | Control group | |
|---|---|---|
| Age (years) | 19.43 ± 0.85 | 19.57 ± 0.65 |
| Raven's advanced progressive matrices score | 26.07 ± 4.76 | 24.79 ± 4.64 |
| Males/females | 6/8 | 5/9 |
Table 2.
Behavioral data before and after training
| Training group | Control group | |||
|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | |
| fMRI AUT | ||||
| Fluency | 2.88 ± 1.34 | 3.94 ± 1.46 | 2.42 ± 1.18 | 2.25 ± 0.94 |
| Originality | 2.85 ± 0.22 | 3.14 ± 0.12 | 2.82 ± 0.24 | 3.02 ± 0.14 |
| DT tasks | ||||
| Fluency | 4.91 ± 2.13 | 5.73 ± 1.83 | 5.20 ± 1.44 | 4.70 ± 1.45 |
| Originality | 2.84 ± 0.14 | 3.13 ± 0.17 | 2.86 ± 0.15 | 2.94 ± 0.19 |
fMRI task
Independent t‐tests found no significant differences in either fluency or originality (all P > 0.05) between the two groups before training. ANOVA revealed significant main effects of time period [F (1, 26) = 6.78; P < 0.05, η2 = 0.21], group [F (1, 26) = 5.98; P < 0.05, η2 = 0.19] and a significant group × time period interaction [F (1, 26) =13.27; P < 0.05, η2 = 0.34] for fluency. The post hoc t‐tests indicated that the TG generated more ideas at the post‐test than at the pre‐test (P < 0.05), while the number of the ideas generated by the CG did not change significantly. Only the main effect of time period was significant for originality [F (1, 26) = 33.38, P < 0.05, η2 = 0.56]. More details about behavioral data were shown in supplementary materials.
fMRI data
One sample t‐tests of the data for the AUT > OCT contrast revealed stronger pre‐test activity in the posterior brain areas, such as the bilateral cuneus and occipital areas (Table 3, Figure 3), while the opposite contrast (AUT < OCT) showed widespread activity in the bilateral temporoparietal areas (Table 3, Figure 3).
Table 3.
Divergent thinking task‐demand‐related activity at pretraining (AUT related to OCT)
| Brain areas | R/L | Maxima of cluster | t | Brodman areas | Cluster size (voxels) | ||
|---|---|---|---|---|---|---|---|
| AUT > OCT | |||||||
| Cuneus | R, L | 12 | −87 | 12 | 6.52 | 17, 18 | 210 |
| AUT < OCT | |||||||
| IPL/MTG/insula | L | −51 | −54 | 39 | 7.74 | 21, 22, 40, 48 | 2,194 |
| IPL/MTG | R | 45 | −48 | 42 | 8.02 | 21, 22, 40 | 2,347 |
| DLPFC | R | 33 | 54 | 6 | 5.61 | 9, 10, 46 | 704 |
| Insula/IFG | R | 63 | 12 | 3 | 4.53 | 48 | 312 |
| SMA | R, L | −9 | −9 | 57 | 4.93 | 6 | 194 |
Note: MTG, middle temporal gyrus; IFG, inferior frontal gyrus; DLPFC, dorsal lateral prefrontal cortex; IPL, inferior parietal lobule; SMA, supplementary motor area. Results were corrected by the cluster‐level FWE (voxel level uncorrected P < 0.001), corrected P < 0.05.
Figure 3.
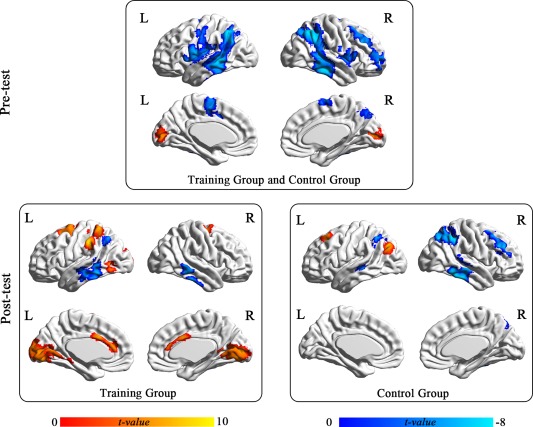
Activity patterns in the contrasts, AUT > OCT (red) and AUT < OCT (blue), before and after training. All effects were corrected by the cluster‐level FWE (voxel level uncorrected P <0.001), corrected P < 0.05. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The whole brain analysis revealed that the TG had increased activity after training in the bilateral dACC (cluster size = 12, MNI peak: 3 21 21, t = 5.51), left DLPFC (cluster size = 44, MNI peak: −27 27 24, t = 5.19), right DLPFC (cluster size = 24, MNI peak: 36 27 30, t = 5.86), left IPL/Postcentral Gyrus (cluster size = 43, MNI peak: −57 −30 45, t = 4.55), left IPL (cluster size = 27, MNI peak: −39 −48 48, t = 4.51), and right precuneus (cluster size = 11, MNI peak: 24 −66 48, t = 4.55), with a voxel‐wise threshold of P < 0.001 (cluster size > 10, uncorrected).
In ROI analysis, paired t‐tests of the TG revealed a significant post‐test increase in activity in the bilateral DLPFC, bilateral dACC, right precuneus, and left IPL (SVC, P corrected < 0.05), but no significant post‐test difference was observed in the CG (Table 4, Figure 4).
Table 4.
Brain regions showing an activity change after training
| Group | Brain areas | R/L | Maxima of cluster | t | Brodman areas | Cluster size (voxels) | ||
|---|---|---|---|---|---|---|---|---|
| TG | ||||||||
| Post > pre | ||||||||
| DLPFC | L | −36 | 39 | 15 | 4.87 | 10, 46 | 28 | |
| IPL/PG | L | −57 | −30 | 45 | 4.55 | 40 | 28 | |
| IPL | L | −39 | −48 | 48 | 4.51 | 40 | 26 | |
| DLPFC | R | 36 | 27 | 30 | 5.86 | 9 | 13 | |
| dACC | R, L | 3 | 21 | 21 | 5.51 | 24, 32 | 11 | |
| Post < pre | None | |||||||
| CG | None | |||||||
Note: dACC, dorsal anterior cingulate gyrus; IPL, inferior parietal lobule; DLPFC, dorsal lateral prefrontal cortex; PG, postcentral gyrus; corrected for multiple comparisons (SVC, P corrected < 0.05).
Figure 4.
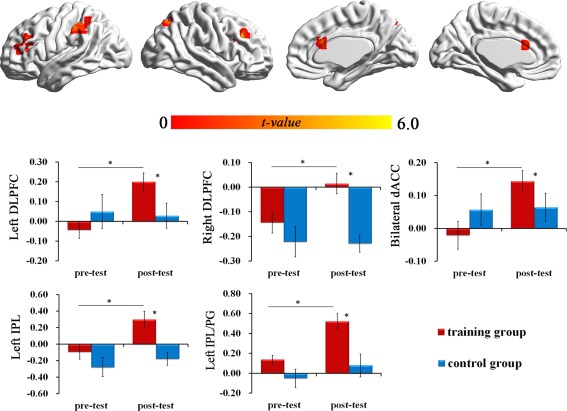
Increased activity (post‐test minus pre‐test) during the fMRI tasks. (A) Brain areas that showed increased activity in the TG. Significant levels for correction were set at P < 0.05, small volume corrected. (B) Bar charts displayed the average amount of activation or deactivation. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
ANOVA revealed significant group × time period interaction [F (1, 26) = 6.06; P < 0.05, η2 = 0.19] for the dACC. Significant group × time period interaction was also found in the right DLPFC [F (1, 26) = 4.62; P < 0.05, η2 = 0.15] and left DLPFC [F (1, 26) = 7.23; P < 0.05, η2 = 0.22]. Group × time period interaction in the left IPL/PG [F (1, 26) = 4.62; P < 0.05, η2 = 0.15] and left IPL [F (1, 26) = 4.86; P < 0.05, η2 = 0.16] were also significant.
The post hoc t tests indicated that the activity of these regions at post‐test were higher than at the pre‐test in the TG (P < 0.05), while the activity of these regions in the CG did not change significantly.
For the region of dACC, ANCOVA was performed using the GMV of the dACC (significant result from the VBM data) as a covariate. The results showed no significant group × time period interaction (P > 0.05) after adjusting the GMV of the dACC.
VBM data
The whole brain analysis revealed that the TG had increased GMV after training in the dACC (cluster size = 408, MNI peak: −3 29 19, t = 6.33), with a voxel‐wise threshold of P < 0.001 (cluster size > 100, uncorrected).
In the ROI analysis, paired t‐test of the TG revealed that the GMV of the dACC increased significantly after training (SVC, P corrected < 0.05, cluster size = 399, MNI peak: −3 29 19, Figure 4). No significant changes were observed in the CG over time.
ANOVA revealed significant group × time period interaction [F (1, 26) = 5.38; P < 0.05, η2 = 0.17] for the dACC. The post hoc t tests indicated that the GMV of the dACC at the post‐test were higher than at the pre‐test in the TG (P < 0.05), while the GMV of the dACC in the CG did not change significantly.
Experiment 2
As in Experiment 1, the behavioral results confirmed that performance improved with training. Paired t‐test revealed significant improvements in fluency both in the fMRI tasks (mean fluencypre = 2.44, SD = 1.10; mean fluencypost = 3.47, SD = 1.29; t = 3.58, P < 0.05) and in the DT tasks (mean fluencypre = 4.94, SD = 1.44; mean fluencypost = 5.61, SD = 1.77; t = 2.73, P < 0.05). Originality also improved significantly in the fMRI tasks (mean originalitypre = 2.58, SD = 0.20; mean originalitypost = 3.00, SD = 0.22; t = 6.53, P < 0.05) and DT tasks (mean originalitypre = 2.54, SD = 0.18; mean originalitypost = 2.63, SD = 0.16; t = 3.20, P < 0.05) (Figure 5).
Figure 5.
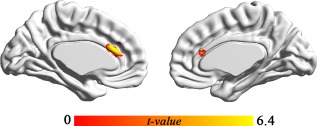
Structural changes. The gray matter volume increased in response to cognitive stimulation training. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Replicating the results of the fMRI tasks in Experiment 1, Experiment 2 found significantly higher post‐test activity in the dACC (P corrected < 0.05, cluster size = 25, MNI peak: 3 12 27, t = 5.28), left DLPFC (P corrected < 0.05, cluster size = 10, MNI peak: −39 36 9, t = 4.34) and IPL (P corrected < 0.05, cluster size = 52, MNI peak: −42 −57 57, t = 5.67). Experiment 2 also found that the GMV in the dACC was significantly increased after training (P corrected < 0.05, cluster size = 98, MNI peak: 5 29 22, t = 5.55). Moreover, the post‐test GMV of two cluster of the inferior temporal gyrus were significantly greater (P corrected < 0.05, cluster size = 583, MNI peak: −59 −18 −24, t = 7.74; cluster size = 172, MNI peak: −59 −18 −24, t = 6.83).
DISCUSSION
The present study examined whether divergent thinking training can improve creativity‐related performance and investigated the neural plasticity associated with such improvement. As expected, our behavioral results revealed that creative performance (originality and fluency) was effectively improved in the TG. Moreover, the results of Experiments 1 and 2 demonstrated there was significantly increased neural activity in the brain areas, such as the bilateral dACC, left DLPFC, and left IPL after training. Furthermore, the TG had an increased GMV in the dACC, indicating that divergent thinking training may lead to structural plasticity. This pattern of results demonstrates that creativity training can reshape both the function and structure of the brain. The implications for those regions that are reshaped by creativity training will be discussed as follows.
First, our results showed that training produced significant improvements at the behavioral level with respect to the fluency and originality of generated ideas. Specifically, fluency (the number of the ideas) and originality (the uniqueness of the ideas) in the TG were enhanced both in the fMRI tasks and in the DT tasks, while the performance of the CG exhibited no differences at the post‐test compared with the pre‐test. The results in the present study revealed that the increase of fluency seems to be stronger than originality. The different patterns of fluency and originality may suggest that participants tend to generate more ideas but they cannot ensure the quality of each idea. In general, consistent with the previous studies [Fink et al., 2012, 2015; Scott et al., 2004], the behavioral results revealed that the applied training program was effective in enhancing creativity performance.
Second, at the neurophysiological level, the role of the dACC in divergent thinking was particularly noteworthy, given both the functional activity differences and the gray matter changes between the pre‐ and post‐test. Significantly increased activity was observed in the dACC after training in the TG in the present study. This area is commonly thought to be related to response conflict monitoring, error detection and response selection [Holroyd et al., 2004; Mansouri et al., 2009]. Activation in the dACC has been widely observed in various divergent thinking tasks, providing evidence for the important role of this area in creative cognition [Abraham et al., 2012; Fink et al., 2009a; Howard‐Jones et al., 2005; Kleibeuker et al., 2013]. Activation of the ACC has also been observed in tasks that are not specific to divergent thinking tasks, such as insight problem‐solving, piano improvisation, visual art design and other creative tasks [Anderson et al., 2009; Aziz‐Zadeh et al., 2009; Berkowitz and Ansari, 2008; Darsaud et al., 2011; Kounios et al., 2006; Kowatari et al., 2009; Luo and Niki, 2003]. Considering the role of the dACC in response conflict monitoring, response errors, response selection [Holroyd et al., 2004; Mansouri et al., 2009], and its involvement in creative tasks, it seems that the dACC may contribute to a cognitive top‐down control mechanism that enhances the process of evaluation and exploration of generated ideas during creative thinking. Thus, increased activity in this area may be related to higher internal processing demands to evaluate the originality and appropriateness of ideas and to inhibit normally activated stereotypical thinking [Abraham et al., 2012; Mansouri et al., 2009]. In addition to the activity change, an increase in the GMV of the dACC was observed between the pre‐test and the post‐test. It is important to mention here that while the relationship between structure and function is complex, some researchers argue that structure informs and constrains function [Honey et al., 2010]. According to this view, the overlap between the results (observed functional and structural changes in the dACC) may give us some insights into the important role of the top‐down control mechanism in creative processes.
In addition, significantly increased post‐test activity was observed in the DLPFC during the fMRI creative tasks compared with the pretest. Previous studies have found that the DLPFC activity was always accompanied by activity in the ACC during creative tasks [Abraham et al., 2012; Howard‐Jones et al., 2005; Kleibeuker et al., 2013]. For example, Abraham et al. [2012] found the ACC and the DLPFC tend to be most responsive in conceptual expansion during difficult divergent thinking tasks compared with simple divergent thinking tasks. More importantly, they also found that these areas were more active during divergent thinking tasks compared with working memory tasks. Though the activation of the DLPFC also has been widely reported to be related to working memory [Curtis and D'Esposito, 2003], Abraham et al. [2012] proposed that this area also plays an important role in creative processes.
Studies that define different components of creativity may, to some extent, give us more direct and detailed evidence about the specific functions of these areas [Abraham, 2014; Ellamil et al., 2012]. For example, Ellamil et al. [2012], who divided creative processes into an idea generation process and an idea evaluation process, observed that activity in both the ACC and DLPFC increased during the evaluation (vs. generation) of creative thought. Furthermore, activity in these areas was positively related to how well participants were able to evaluate their outcomes. Theories of the regulation of cognition hold that the dACC is related to monitoring performance and detecting signals, while the DLPFC is more involved in the implementation of top‐down control [MacDonald et al., 2000]. Therefore, in our present study, we interpret the increased DLPFC activity as reflecting an enhancement of executive cognitive control involving integration, evaluation, and verification of relevant information, which may contribute to the generation of original ideas.
Posterior brain regions, such as the IPL also showed increased activity after training. This result is in agreement with several findings of brain activity during creative compared with non‐creative cognition. For instance, Aziz‐Zadeh et al. [2013], who had their participants complete a visuospatial divergent thinking task and a control task, observed that visuospatial divergent thinking (as compared with the control task) was associated with more activity in the posterior parietal cortex. Fink et al. [2010] compared a cognitive stimulation condition to a simple AU condition and found more activity in the middle temporal gyrus, angular gyrus, and the supramarginal gyrus. This region was discussed in terms of bottom‐up attention and automatically activated knowledge. Furthermore, MRI studies focusing on individual differences in creativity have confirmed the role of this region in creative processes. For example, Chávez‐Eakle et al. [2007] explored the relationship between brain cerebral blood flow (CBF) and verbal Torrance tests of creative thinking, and found that fluency was positively related to CBF in the inferior parietal lobule. Jung et al. [2010] observed negative correlations between divergent thinking ability and cortical thickness in the angular gyrus. Research using diffusion tensor imaging also reported a significant positive relationship between fractional anisotropy in regions of the bilateral temporo‐parietal junction and the right IPL and individual creativity (measured by divergent thinking tests). In general, the temporoparietal area is closely related to creative performance. Increased activity in this area may be related to the attention state during the generation of original ideas.
Furthermore, regions such as the dACC, DLPFC, and IPL, which show increased activity induced by training, are the key regions of large‐scale networks such as the cognitive control network (CCN) and the default mode network (DMN). Recent neuroscientific investigations tend to study creativity from the perspective of large‐scale networks [Abraham, 2014; Beaty et al., 2016; Jung et al., 2013; Mok, 2014]. Jung et al. [2013] reviewed structural studies on creativity and tried to understand the neural mechanisms underlying creativity through the features of the CCN and the DMN, as well as the relationship between these two networks. More specifically, it seems that the DMN is involved in the generation of original ideas and the CCN is involved in allocation of cognitive resources. Meanwhile, the salience network (SN) may modulate the interplay between these two networks. Previous studies have revealed that task‐based integration between the parietal lobe, dACC, and DLPFC is critical for guiding and supporting cognitive control according to goals and, potentially, arousal states [Cocchi et al., 2013, 2014; Dosenbach et al., 2007; Seeley et al., 2007]. Functional changes of the key regions may have an effect on these networks and the integration between these networks, which then affect creative behavioral performance.
This study has some limitations. One limitation is the small number of participants. Many participants were excluded because they withdrew from the study or had excessive head motion during scanning. Second, we used just two experiments to confirm the key regions that were sensitive to divergent thinking training. Although the longitudinal design we used can provide direct evidence for the neural basis of creativity training and its plasticity effects, studies with larger samples, different training approaches and active control group are needed in the future. Another limitation is the behavioral data during the fMRI tasks were collected after scanning. It may result in some bias of the behavioral scores in of fMRI task. In addition, this study did not investigate possible transfer effects of creativity training on other cognitive abilities, such as cognitive control or working memory. Further studies that examine transfer effects may give us insights into the causal explanation of creative and other cognitive abilities.
CONCLUSION
In summary, the results of the present study showed the effects of creativity training on brain function and structure through short‐term divergent thinking training. Although not every brain area showed functional changes that corresponded to gray matter changes, our results offer insights into the neural plasticity with respect to both function and structure. It is encouraging to observe that neural plasticity can be achieved through training, not only by physical exercise (e.g., juggling, golf, or a sense of balance) or working memory, but also by complex cognitive abilities, such as creativity. Obviously, it is promising that human creativity capacities can be developed through well‐designed training programs, which may contribute to social development and human civilization.
Supporting information
Supporting Information
Jiangzhou Sun and Qunlin Chen contributed equally to this article.
The authors declare no conflict of interest.
REFERENCES
- Abraham A (2014): Creative thinking as orchestrated by semantic processing vs. cognitive control brain networks. Front Human Neurosci 8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Pieritz K, Thybusch K, Rutter B, Kroger S, Schweckendiek J, R Stark, S Windmann, Hermann C (2012): Creativity and the brain: Uncovering the neural signature of conceptual expansion. Neuropsychologia 50:1906–1917. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Anderson JF, Ferris JL, Fincham JM, Jung KJ (2009): Lateral inferior prefrontal cortex and anterior cingulate cortex are engaged at different stages in the solution of insight problems. Proc Natl Acad Sci 106:10799–10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden R, Chavez RS, Grazioplene R, Jung RE (2010): Neuroimaging creativity: A psychometric view. Behav Brain Res 214:143–156. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Aziz‐Zadeh L, Kaplan JT, Iacoboni M (2009): “Aha!”: The neural correlates of verbal insight solutions. Human Brain Mapp 30:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz‐Zadeh L, Liew SL, Dandekar F (2013): Exploring the neural correlates of visual creativity. Soc Cogn Affect Neurosci 8:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Silvia PJ, Schacter DL (2016): Creative cognition and brain network dynamics. Trends Cogn Sci 20:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Fink A, Neubauer AC (2006): Enhancement of ideational fluency by means of computer‐based training. Creativ Res J 18:317–328. [Google Scholar]
- Berkowitz AL, Ansari D (2008): Generation of novel motor sequences: The neural correlates of musical improvisation. Neuroimage 41:535–543. [DOI] [PubMed] [Google Scholar]
- Bezzola L, Merillat S, Gaser C, Jancke L (2011): Training‐induced neural plasticity in golf novices. J Neurosci 31:12444–12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DT (1960): Blind variation and selective retentions in creative thought as in other knowledge processes. Psychol Rev 67:380. [DOI] [PubMed] [Google Scholar]
- Chávez‐Eakle RA, Graff‐Guerrero A, García‐Reyna JC, Vaugier V, Cruz‐Fuentes C (2007): Cerebral blood flow associated with creative performance: A comparative study. Neuroimage 38:519–528. [DOI] [PubMed] [Google Scholar]
- Chen CH, Ridler K, Suckling J, Williams S, Fu CH, Merlo‐Pich E, Bullmore E (2007): Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry 62:407–414. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Zalesky A, Fornito A, Mattingley JB (2013): Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn Sci 17:493–501. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Halford GS, Zalesky A, Harding IH, Ramm BJ, Cutmore T, DHK Shum, Mattingley JB (2014): Complexity in relational processing predicts changes in functional brain network dynamics. Cerebr Cortex 24:2283–2296. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M (2003): Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7:415–423. [DOI] [PubMed] [Google Scholar]
- Darsaud A, Wagner U, Balteau E, Desseilles M, Sterpenich V, Vandewalle G, G Albouy, T Dang‐Vu, F Collette, Boly M, M Schabus, C Degueldre, A Luxen, P Maquet (2011): Neural precursors of delayed insight. J Cogn Neurosci 23:1900–1910. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R (2010): A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull 136:822–848. [DOI] [PubMed] [Google Scholar]
- Ding X, Tang YY, Cao C, Deng Y, Wang Y, Xin X, Posner MI (2014): Short‐term meditation modulates brain activity of insight evoked with solution cue. Social Cogn Affect Neurosci 10:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, MD Fox, AZ Snyder, JL Vincent, Raichle ME, BL Schlaggar, SE Petersen (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugosh KL, Paulus PB, Roland EJ, Yang HC (2000): Cognitive stimulation in brainstorming. J Person Social Psychol 79:722. [DOI] [PubMed] [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K (2012): Evaluative and generative modes of thought during the creative process. Neuroimage 59:1783–1794. [DOI] [PubMed] [Google Scholar]
- Fink A, Benedek M (2014): EEG alpha power and creative ideation. Neurosci Biobehav Rev 44:111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Benedek M, Neubauer AC (2006): Divergent thinking training is related to frontal electroencephalogram alpha synchronization. Eur J Neurosci 23:2241–2246. [DOI] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Benedek M, Reishofer G, Hauswirth V, Fally M, C Neuper, F Ebner, Neubauer AC (2009a): The creative brain: Investigation of brain activity during creative problem solving by means of EEG and FMRI. Hum Brain Mapp 30:734–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Graif B, Neubauer AC (2009b): Brain correlates underlying creative thinking: EEG alpha activity in professional vs. novice dancers. Neuroimage 46:854–862. [DOI] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Gebauer D, Reishofer G, Koschutnig K, Ebner F (2010): Enhancing creativity by means of cognitive stimulation: Evidence from an fMRI study. Neuroimage 52:1687–1695. [DOI] [PubMed] [Google Scholar]
- Fink A, Koschutnig K, Benedek M, Reishofer G, Ischebeck A, Weiss EM, Ebner F (2012): Stimulating creativity via the exposure to other people's ideas. Human Brain Mapp 33:2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Benedek M, Koschutnig K, Pirker E, Berger E, Meister S, AC Neubauer, I Papousek, Weiss EM (2015): Training of verbal creativity modulates brain activity in regions associated with language‐and memory‐related demands. Human Brain Mapp 36:4104–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C, Folley BS, Park S (2009): Enhanced divergent thinking and creativity in musicians: A behavioral and near‐infrared spectroscopy study. Brain Cogn 69:162–169. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MG, Cohen JD (2004): Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat Neurosci 7:497–498. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Thivierge JP, Sporns O (2010): Can structure predict function in the human brain? Neuroimage 52:766–776. [DOI] [PubMed] [Google Scholar]
- Howard‐Jones PA, Blakemore SJ, Samuel EA, Summers IR, Claxton G (2005): Semantic divergence and creative story generation: An fMRI investigation. Cogn Brain Res 25:240–250. [DOI] [PubMed] [Google Scholar]
- Ilg R, Wohlschlager AM, Gaser C, Liebau Y, Dauner R, Woller A, C Zimmer, J Zihl, Muhlau M (2008): Gray matter increase induced by practice correlates with task‐specific activation: A combined functional and morphometric magnetic resonance imaging study. J Neurosci 28:4210–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ (2008): Improving fluid intelligence with training on working memory. Proc Natl Acad Sci 105:6829–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung‐Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel‐Liu S, Greenblatt R, PJ Reber, Kounios J (2004): Neural activity when people solve verbal problems with insight. PLoS Biol 2:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Segall JM, Jeremy Bockholt H, Flores RA, Smith SM, Chavez RS, Haier RJ (2010): Neuroanatomy of creativity. Human Brain Mapp 31:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Mead BS, Carrasco J, Flores RA (2013): The structure of creative cognition in the human brain. Front Human Neurosci 7:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleibeuker SW, Koolschijn PCM, Jolles DD, De Dreu CK, Crone EA (2013): The neural coding of creative idea generation across adolescence and early adulthood. Front Human Neurosci 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounios J, Frymiare JL, Bowden EM, Fleck JI, Subramaniam K, Parrish TB, Jung‐Beeman M (2006): The prepared mind neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychol Sci 17:882–890. [DOI] [PubMed] [Google Scholar]
- Kowatari Y, Lee SH, Yamamura H, Nagamori Y, Levy P, Yamane S, Yamamoto M (2009): Neural networks involved in artistic creativity. Hum Brain Mapp 30:1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Niki K (2003): Function of hippocampus in “insight” of problem solving. Hippocampus 13:316–323. [DOI] [PubMed] [Google Scholar]
- Ma HH (2006): A synthetic analysis of the effectiveness of single components and packages in creativity training programs. Creativ Res J 18:435–446. [Google Scholar]
- MacDonald AW, 3rd , Cohen JD, Stenger VA, Carter CS (2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288:1835–1838. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ (2009): Conflict‐induced behavioural adjustment: A clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci 10:141–152. [DOI] [PubMed] [Google Scholar]
- Mok LW (2014): The interplay between spontaneous and controlled processing in creative cognition. Front Human Neurosci 8:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford MD (2002): Social innovation: Ten cases from Benjamin Franklin. Creativ Res J 14:253–266. [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T (2004): Increased prefrontal and parietal activity after training of working memory. Nat Neurosci 7:75–79. [DOI] [PubMed] [Google Scholar]
- Oppezzo M, Schwartz DL (2014): Give your ideas some legs: The positive effect of walking on creative thinking. J Exp Psychol Learn Mem Cogn 40:1142–1152. [DOI] [PubMed] [Google Scholar]
- Qiu J, Li H, Jou J, Liu J, Luo Y, Feng T, Z Wu, Zhang Q (2010): Neural correlates of the “Aha” experiences: Evidence from an fMRI study of insight problem solving. Cortex 46:397–403. [DOI] [PubMed] [Google Scholar]
- Raven JC (1962): Advanced Progressive Matrices: Sets I and II. London: HK Lewis. [Google Scholar]
- Runco MA, Jaeger GJ (2012): The standard definition of creativity. Creativ Res J 24:92–96. [Google Scholar]
- Saggar M, Quintin EM, Kienitz E, Bott NT, Sun Z, Hong WC, YH Chien, N Liu, RF Dougherty, Royalty A, G Hawthorne, AL Reiss (2015): Pictionary‐based fMRI paradigm to study the neural correlates of spontaneous improvisation and figural creativity. Sci Rep 5:10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer K (2011): The cognitive neuroscience of creativity: A critical review. Creativ Res J 23:137–154. [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen‐Berg H (2009): Training induces changes in white‐matter architecture. Nat Neurosci 12:1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G, Leritz LE, Mumford MD (2004): The effectiveness of creativity training: A quantitative review. Creativ Res J 16:361–388. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, AL Reiss, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonton DK (1984): Genius, Creativity, and Leadership: Historiometric Inquiries. Cambridge, MA: Harvard University Press. [Google Scholar]
- Smith GF (1998): Idea‐generation techniques: A formulary of active ingredients. J Creative Behav 32:107–134. [Google Scholar]
- Stein MI (1953): Creativity and culture. J Psychol 36:311–322. [Google Scholar]
- Sternberg RJ, Lubart TI (1996): Investing in creativity. Am Psychol 51:677. [Google Scholar]
- Supekar K, Swigart AG, Tenison C, Jolles DD, Rosenberg‐Lee M, Fuchs L, Menon V (2013): Neural predictors of individual differences in response to math tutoring in primary‐grade school children. Proc Natl Acad Sci 110:8230–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2010): White matter structures associated with creativity: Evidence from diffusion tensor imaging. Neuroimage 51:11–18. [DOI] [PubMed] [Google Scholar]
- Tang C, Li A, Huang H, Cheng X, Gao Y, Chen H, Q Huang, Y Luo, Y Xue, Zuo Q, L Cui (2012): Effects of lead pollution in SY River on children's intelligence. Life Sci J 9:458–464. [Google Scholar]
- Taubert M, Lohmann G, Margulies DS, Villringer A, Ragert P (2011): Long‐term effects of motor training on resting‐state networks and underlying brain structure. Neuroimage 57:1492–1498. [DOI] [PubMed] [Google Scholar]
- Ventura‐Campos N, Sanjuán A, González J, Palomar‐García MÁ, Rodríguez‐Pujadas A, Sebastián‐Gallés N, G Deco, Ávila C (2013): Spontaneous brain activity predicts learning ability of foreign sounds. J Neurosci 33:9295–9305. 23719798 [Google Scholar]
- Wei D, Yang J, Li W, Wang K, Zhang Q, Qiu J (2014): Increased resting functional connectivity of the medial prefrontal cortex in creativity by means of cognitive stimulation. Cortex 51:92–102. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang W, Tong D, Sun J, Chen Q, Wei D, Q Zhang, M Zhang, Qiu J (2015): A meta‐analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Human Brain Mapp 36:2703–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen‐Berg H (2012): Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Zhang Q, Qiu J (2013): Relating inter‐individual differences in verbal creative thinking to cerebral structures: An optimal voxel‐based morphometry study. PloS One 8:e79272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


