Abstract
Unilateral cochlear implant (CI) stimulation establishes hearing to children who are deaf but compromises bilateral auditory development if a second implant is not provided within ∼1.5 years. In this study we asked: 1) What are the cortical consequences of missing this early sensitive period once children reach adolescence? 2) What are the effects of unilateral deprivation on the pathways from the opposite ear? Cortical responses were recorded from 64‐cephalic electrodes within the first week of bilateral CI activation in 34 adolescents who had over 10 years of unilateral right CI experience and in 16 normal hearing peers. Cortical activation underlying the evoked peaks was localized to areas of the brain using beamformer imaging. The first CI evoked activity which was more strongly lateralized to the contralateral left hemisphere than normal, with abnormal recruitment of the left prefrontal cortex (involved in cognition/attention), left temporo‐parietal‐occipital junction (multi‐modal integration), and right precuneus (visual processing) region. CI stimulation in the opposite deprived ear evoked atypical cortical responses with abnormally large and widespread dipole activity across the cortex. Thus, using a unilateral CI to hear beyond the period of cortical maturation causes lasting asymmetries in the auditory system, requires recruitment of additional cortical areas to support hearing, and does little to protect the unstimulated pathways from effects of auditory deprivation. The persistence of this reorganization into maturity could signal a closing of a sensitive period for promoting auditory development on the deprived side. Hum Brain Mapp 37:135–152, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: bilateral cochlear implant, cortical maturation, single sided deaf, unilateral deaf, development, brain imaging/source localization, auditory cortex, hearing loss/deafness, adolescent, evoked related potential/evoked potential/electrophysiology
Abbreviations
- BEM
Boundary element model
- CI
Cochlear implant
- MNI
Montreal Neurologic Institute;
- MRI
Magnetic resonance imaging
- PBK
Phonemic Balanced Kindergarten;
- SD
Standard deviations
INTRODUCTION
Cochlear implants (CI) are surgically implanted auditory prostheses which provide hearing to individuals who are deaf. While CIs allow children to develop remarkable oral speech and language abilities, their hearing is significantly poorer than normal because the CI delivers only a crude representation of acoustic sounds and eliminates important cochlear processing. Prior to cochlear implantation, the absence of sound in early life leaves the auditory brain vulnerable to cross‐modal recruitment [Bavelier et al., 2006; Bavelier and Neville, 2002; Finney et al., 2001] by the visual [Lomber et al., 2010] and somatosensory systems [Lomber et al., 2010; Meredith and Lomber, 2011]. If hearing is not established during sensitive periods in development, this reorganization will impair hearing with a CI [Lee et al., 2001]. CIs have thus been provided to children within limited durations of bilateral deafness with aims to halt, and perhaps reverse, any such effects of deafness on the brain.
Traditionally, CIs have been provided to children in only one ear, which put them at risk for language delays and educational difficulties [Bess and Tharpe, 1984, 1986; Lieu et al., 2010]. While unilateral stimulation with an implant promotes auditory maturation in the brainstem [Gordon et al., 2006, 2013a; Thai‐Van et al., 2007], midbrain [Gordon et al., 2005] and cortex [Jiwani et al., 2013; Ponton and Eggermont, 2001; Sharma et al., 2005] of young children, depriving the opposite pathways of auditory input might leave the auditory system susceptible to deafness‐induced reorganization. Although we have already shown that delays of >1.5 years between the first and second implants distort bilateral auditory development [Gordon et al., 2011a, 2013a], it is also possible that there is an upper age after which it is too late to re‐establish function in the deprived pathways following long term unilateral CI use.
Graham and colleagues suggested that the mid‐teenage years could mark the end of a critical period for implanting the non‐implanted side in adolescents who used a unilateral CI to hear for most of their lives [Graham and Vickers, 2011; Graham et al., 2009]. Even in younger children, outcomes of bilateral implant decline as the delay between implants increases; speech comprehension in the second implanted ear is poorer [Gordon et al., 2011a; Gordon and Papsin, 2009; Graham and Vickers, 2011; Graham et al., 2009; Illg et al., 2013; Peters et al., 2007] and binaural hearing abilities are reduced [Chadha et al., 2011; Gordon et al., 2011a; Gordon and Papsin, 2009; Grieco‐Calub and Litovsky, 2010; Salloum et al., 2010; Van Deun et al., 2009] when the second implant is delayed. Moreover, the new implant may not be worn consistently in children implanted sequentially [Fitzgerald et al., 2013]. Poor performance of older children receiving a second implant may be attributed to increased dependency on the first and earlier implanted ear for hearing [Kral et al., 2013b], decreased motivation or emotional resistance to change in teenagers [Fitzpatrick and Irannejad, 2008], and/or decreased auditory plasticity with maturation [Lohmann and Kessels, 2014].
Limits in human auditory plasticity can be explored using electrophysiological measures. Unilateral CI stimulation/deprivation exceeding 1.5 years resulted in faster response latencies in the brainstem [Gordon et al., 2012] and abnormally strong activity driven by the first CI to both auditory cortices [Gordon et al., 2013b; Kral et al., 2013b], reflecting a strengthening of the immature auditory pathways from the more experienced implanted right ear. This was consistent with effects of unilateral deafness in an animal model of congenital deafness [Kral et al., 2013b] and might be explained by a disruption in the normal balance of inhibitory‐excitatory activity occurring with binaural hearing [Byrne and Byrne, 1993; Grothe et al., 2010; Kotak et al., 2005; Takesian et al., 2009]. Because the cortical changes observed in the children using unilateral CIs occurred over approximately the same time‐course of CI‐driven brainstem maturation (∼1.5 year) [Gordon et al., 2006], there appears to be an important early developmental period for bilateral input in the human auditory system [Gordon et al., 2013b].
Adolescence normally marks the maturation of the auditory cortex [Eggermont, 1988; Jiwani et al., 2013; Moore and Linthicum, 2007; Ponton et al., 2000] and could signal the end of another important developmental period. Much of the brain is maturing during this time. There is a slowing of white matter increase and a sharp decline in gray matter during adolescence [Lebel and Beaulieu, 2011] which likely reflects synaptic elimination [Blakemore, 2012; Giedd et al., 1999; Sowell et al., 2001] as existing connections in the mature brain are refined [Blakemore and Choudhury, 2006; Lohmann and Kessels, 2014] and as each cortical hemisphere becomes specialized [Davidson, 1984; Gotts et al., 2013; Le Grand et al., 2003; Rivera et al., 2005; Toga and Thompson, 2003; Zatorre and Belin, 2001]. Maturation driven by CI stimulation, particularly when only from one ear, could be very different from normal. Some children have been using unilateral CIs for many years and have developed age‐appropriate speech and language [Geers et al., 2008; Geers and Sedey, 2011] yet they still face challenges including increased effort/attention needed to hear [Gordon et al., 2013a; Hopyan‐Misakyan et al., 2009; Kronenberger et al., 2014; Lee et al., 2007; Pisoni et al., 2010; Powell, 1974; Steel et al., 2015]. Perhaps it is because of these challenges that adolescents using one CI seek a second implant for their long‐deprived ear. Whatever the motivation, this unique cohort provides a opportunity to stimulate the deaf ear for the first time and study the effects of long‐term unilateral implant stimulation in the adolescent brain, both in the experienced and deprived pathways.
In this study, we explored the underlying consequences of driving auditory maturation in the brainstem and cortex with CI stimulation from one ear. We hypothesized that maturation of the auditory cortex with over a decade of unilateral CI use drives lasting asymmetries in the auditory system but does not protect the opposite pathways from deprivation. Results supported these hypotheses suggesting that establishing binaural hearing may be challenging after unilaterally driven maturation of the auditory cortex.
MATERIALS AND METHODS
Participants
Thirty‐four adolescents who received one CI in their right ear by 3.2 ± 1.3 years of age after a limited period of bilateral auditory deprivation (1.8 ± 1.3 years) participated in this study. They had 12.4 ± 1.7 years of unilateral CI experience at the time of the test. All were successful implant users and had never received auditory stimulation in their opposite ear. Twenty‐one of these adolescents later received a second implant in their opposite‐left ear after 12.0 ± 2.1 years of unilateral deprivation on that side. Age at implantation on this second implanted side was 15.9 ± 2.0 years. Responses were recorded from the 21 bilateral CI recipients on the first day of activation of the second implant to study the long‐term cortical effects of single‐sided deafness on the deprived pathways in the mature auditory system. Etiology of deafness varied. All adolescents were implanted with a Cochlear Nucleus device from Cochlear Corporation Limited in both ears. All but two adolescents were right handed. Cortical responses evoked in CI users were compared with the same responses recorded from 16 adolescents with normal hearing (six boys: 10 girls) who were 15.9 ± 6.4 years and matched for chronological age and duration of hearing experience. All but three adolescents with normal hearing were right handed.
Recording Cortical Responses
Cortical responses were evoked by each ear/implant separately using 62‐cephalic electrodes and referenced to the right earlobe. In the CI group, responses were generated using biphasic electrical pulse trains of 250 pulses per second, lasting 36 ms and delivered by a single electrode (# 20) at the apex of the electrode array. These electrical pulse trains were presented at a rate of 1 Hz. Current levels were determined for each CI as in previous studies [e.g. Gordon et al., 2013b] by recording auditory brainstem responses at increasing intensities from the experienced and newly implanted sides separately. Brainstem responses were evoked by single biphasic pulses from electrode # 20 presented at 11 pulses per second and recorded using a NeuroScan‐4.3 system with a Synamps‐II amplifier from a mid‐line cephalic electrode referenced to the ipsilateral earlobe and filtered from 10 to 3000 Hz. Sweeps of ± 30–40 µV were rejected from the average. Current levels were increased within a comfortable range of intensities for the participant. The maximum levels at which wave eV amplitudes on each side were equal were used to evoke cortical responses. Levels measured with single pulses were reduced by 10 Current Units on each side so that pulse train stimulation would not exceed tolerance levels.
Cortical responses were evoked by 500 Hz tone‐bursts in children with normal hearing. This stimulus was chosen because this frequency was allocated to the same apical electrode (# 20) of the CI device used to evoke responses in all CI participants. The same stimulus duration (36 ms) and rate of presentation (1 Hz) was used as in the CI users. The tone was enveloped with a Tukey window over the first and last eights to minimize effects of high frequency onset and offset. Tone‐bursts were delivered at 40 dB above behavioural threshold and presented to the right and left ears separately using ER3‐14A insert earphones. Responses for all participants were recorded using the NeuroScan‐4.3 system with a Synamps‐II amplifier. They were sampled at a rate of 1,000 Hz and an online band‐pass filter between 0.15 to 100 Hz was applied. A minimum of 400 sweeps with at least two visually replicable cortical responses were obtained. Epochs which were greater than ±100 µV between the 100 and 800 ms latencies were rejected.
Localization of Cortical Evoked Peaks
The Time Restricted, Artefact and Coherence Source suppression (TRACS) linearly constrained minimum variance beamformer (adaptive spatial filter) [Wong and Gordon, 2009] was used to localize dipole activity underlying the evoked peaks in the cortex in response to stimulation from the experienced and naïve CIs, and right and left ears in normal hearing peers. Neuroanatomical data is unavailable for adolescents with long‐term CI experience because the internal portion of the CI device contains a magnet that renders it incompatible with magnetic resonance imaging (MRI). Dipole activity was thus reconstructed for each adolescent using age‐appropriate head model templates derived from the Montreal Neurologic Institute (MNI) MRI library. The Template‐O‐Matic toolbox was used to generate the age‐specific template head models and account for physiological changes occurring with maturation [Wilke et al., 2008]. Because all the participants in this study were adolescents, the same template head model was used.
A three‐layer boundary element model (BEM) mesh was constructed using the derived age‐appropriate head model templates. This was used to represent age‐appropriate head geometry and tissue conductivities of the brain, meninges, skull and scalp to account for spatial smearing of electric potentials at the surface of the head. A thin plate spline warp method [Darvas et al., 2006] was used to warp the 3‐layered triangular BEM mesh to the template MRI image of the human head and match the 3‐dimensional coordinates of the generic human scalp to the 10–20 electrode coordinate system used in the study [Acar and Makeig, 2013; Darvas et al., 2006]. The BEM model assumes that the head is a sphere of evenly distributed spaces (voxels) of 1 mm × 1 mm × 1 mm (3 mm3).
Source activity in the left hemisphere was assessed using region suppression of activity in the right hemisphere, and vice versa, as bilaterally correlated activity is known to pose a particular problem for source localization in auditory cortices [Dalal et al., 2006]. In adolescents using CIs, a time restricted artefact suppression algorithm was applied to suppress the implant‐generated electrical artefact, corresponding to the four largest singular vector values over the −80 to 10 ms latency range. This ensures that singular vectors representing up to 97% of the CI artefact are suppressed while maintaining responses beyond the stimulus [Wong and Gordon, 2009].
Peaks in the cortical response were visually identified at latencies corresponding to those previously reported from normal hearing peers and CI users [Gordon et al., 2008; Jiwani et al., 2013]. Peak latencies and amplitudes recorded at C z were marked for responses evoked by stimulation of the right and left ears in the normal hearing group and the experienced‐right and naïve‐left sides in the CI group (i.e., P 1, N 1 and P 2 for normal hearing responses and experienced CI stimulation; and N (ci) and P (ci) for responses evoked by stimulation of the naïve CI). Mean ± standard deviations (SD) are shown in Table 1 for each group.
Table 1.
Measures of response peaks at Cz electrode
| P 1 (Mean ± SD) | N 1/N (ci) (Mean ± SD) | P 2/P (ci) (Mean ± SD) | |
|---|---|---|---|
| Latency (ms) | |||
| Normal hearing right | 78.87 ± 9.20 | 108.83 ± 9.53 | 164.22 ± 17.94 |
| Normal hearing Left | 77.63 ± 4.46 | 111.64 ± 7.16 | 163.87 ± 19.19 |
| Experienced‐right CI | 79.59 ± 6.22 | 105.43 ± 9.57 | 162.20 ± 18.24 |
| Naïve‐left CI | – | 114.10 ± 8.34 | 184.58 ± 19.01 |
| Amplitude (µV) | |||
| Normal hearing right | 0.92 ± 1.46 | −1.56 ± 1.72 | 2.83 ± 1.96 |
| Normal hearing left | 1.06 ± 1.41 | −2.27 ± 1.95 | 2.61 ± 1.73 |
| Experienced‐right CI | 0.63 ± 2.92 | −1.92 ± 3.18 | 5.30 ± 3.61 |
| Naïve‐left CI | – | −12.27 ± 5.94 | 8.77 ± 5.86 |
Covariance was estimated over latency windows encompassing each peak of the cortical response for each individual [Van Veen et al., 1997]. A pseudo‐Z statistic was calculated relative to baseline activity in the pre‐stimulus interval (−200 to −80 ms) to normalize the signal‐to‐noise ratio of each voxel. Once sources were localized using this lead normalization process, the strength of source activity, measured in dipole moments (nAm), was computed for the peaks of the cortical waveform. A one‐tailed omnibus‐noise T‐test [Petersson et al., 1999] was used to calculate a statistical threshold pseudo‐Z value (P ≤ 0.0005) reflecting baseline brain activity. Only voxels with pseudo‐Z activity greater than this omnibus value were accepted and used in the analyses. Figure 1 provides an example of a response recorded from an adolescent with long‐term CI experience (Fig. 1A) with corresponding head topographies of potential distribution (Fig. 1B) and the activity underlying the mature peaks (P 1, N 1 and P 2) in 63,646 brain voxels (Fig. 1C). Source locations of the voxels with the strongest signal to noise ratio (pseudo‐Z value) in the red hotspots (in each temporal lobe) are marked in by the green cross hairs in the left and right hemispheres separately. The waveforms show the corresponding virtual channels of the marked voxel. As shown, the CI artefact has been suppressed leaving the peak dipole in the latency range of the P 1, N 1 or P 2 peak.
Figure 1.
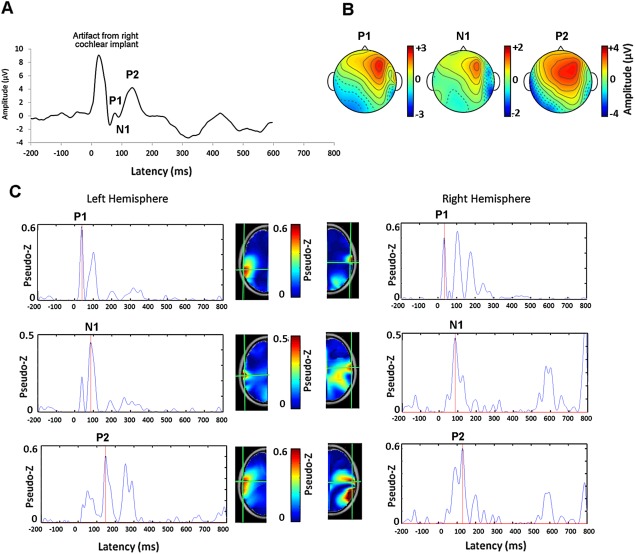
A. Example of a cortical evoked waveform at Cz recorded from one adolescent with 15.95 years of CI experience in the right ear indicates a mature response with peaks P 1, N 1 and P 2. The electrical artifact from the right CI preceding this response is clear. B. Head topographies of potential distribution for each peak (common averaged reference) of the mature cortical response are shown. C. The activity underlying the mature peaks in 63,646 brain voxels are shown relative to the noise floor using the pseudo‐Z. The virtual channel waveforms corresponding to the voxel with the strongest activity (red hotspots) are shown for the left and right hemispheres for each peak. Source locations are marked by the green cross hairs on the axial view of the age‐appropriate topographic head model derived from the MNI MRI Library. The virtual channel data reveals that the CI artifact has been suppressed in early latencies by the beamforming procedure and that the strongest dipole underlying each peak of the mature response occurs in areas of the left and right auditory cortices, in line with the latency of the cortical wave peaks.
Analyses of Dipole Activity
Dipole activity was visualized topographically on age‐appropriate MNI head model templates by plotting pseudo‐Z values in the 63,646 three‐dimensional voxels in brain space from highest (top) to lowest (bottom). It should be noted that significant dipole activity can exist in underlying layers. Mean beamformer brain images were created to assess areas of cortical activity which were consistently evoked by sound for each ear/implant. The dipole peak from the voxel with the strongest pseudo‐Z within the left and right auditory areas were measured from each participant. The location of the auditory cortex were identified within MNI coordinates (X = <−55, Y = >−35 to <−5, Z = >−10 to <20) mm in the left hemisphere and (X = >55, Y = >−35 to <−5, Z = >−10 to <20) mm in the right hemisphere in both the normal hearing and the CI group, consistent with other reports [Wong and Gordon, 2009; Gordon, Wong et al., 2010; Gordon, Wong et al., 2013b]. Two‐sided paired permutation tests were used to compare voxel‐by‐voxel dipole activity in 2,048 permutations [Blair and Karniski, 1993; Chau et al., 2004] to assess differences between the left and right hemispheres and permutation tests were also used to assess significant differences in activity for each voxel‐pair between each group. Although permutation tests provide strong control for family‐wise‐error rates [Groppe et al., 2011], further corrections were applied to account for the 62 recording channels used (Bonferroni correction P = 0.05/62 = 0.0008).
Repeated measures ANOVA with Bonferroni corrections were used to identify differences in peak dipole moment and latencies between activity evoked in each auditory cortex and in each group. These analyses were based on voxels with the strongest dipoles in the left and right auditory cortices for each cortical wavepeak in each individual. Percent cortical lateralization [(right hemisphere dipole – left hemisphere dipole)/(right hemisphere dipole + left hemisphere dipole) × 100] were calculated from these extracted peak dipoles.
Speech Perception Tests to Assess Outcomes with CIs
Functional outcomes with CIs were assessed using the age‐appropriate Phonemic Balanced Kindergarten (PBK) monosyllabic words open‐set speech perception test. This test assesses speech recognition abilities using a list of twenty‐five words. Words were presented at 0‐degree azimuth in a double‐walled sound‐proof booth, through a GSI‐61 Grason‐Stadler audiometer, using monitored‐live voice at 65 dB sound pressure level. Words were presented in quiet to the first and second implants separately and scored as a percentage of words repeated correctly. Given the focus on effects of unilateral stimulation/deprivation, testing occurred at initial stages of bilateral CI use (8.0 ± 2.1 months (range: 5.8–15.0 months). This was considered appropriate relative to the long duration of bilateral implant use required for binaural hearing to emerge even in children implanted at younger ages [Gordon et al., 2014]. Pearson correlations were used to assess associations between speech perception scores, duration of bilateral CI use, dipole moment and cortical lateralization.
RESULTS
Tone‐Bursts Preferentially Stimulate the Right Auditory Cortex in Adolescents With Normal Hearing
Cortical responses recorded at a midline‐cephalic location (Cz) with corresponding head topographies of potential distribution are shown in Figure 2A for the normal hearing group. Cortical responses with peaks P 1, N 1 and P 2, characteristic of the mature response were recorded in adolescents with normal hearing [Albrecht et al., 2000; Ponton et al., 2000; Wunderlich et al., 2006] when either the right (n = 13) or left ears (n = 12) were stimulated by 500 Hz tone‐bursts. The mean latencies and amplitudes for all the recorded responses appear in Table 1. The responses were similarly distributed over the scalp between the right and left ears. However, dipole moments underlying the mature cortical peaks were stronger in the right hemisphere. This is shown by the dark red hotspots in the right hemisphere mean brain plots of Figure 2B (axial view only) and by the larger right (red bars) than left cortex (blue bars) peak dipole magnitudes in Figure 2C. Dipoles in auditory cortex showed effects of side of stimulation [F (1,10) = 5.976, P = 0.035], hemisphere [F (1,10) = 8.018, P = 0.015] and an interaction of these factors [F (1,10) = 10.308, P = 0.009] but no differences between the different cortical peaks [F (2,20) = 8.018, P > 0.05]. This means that as mature cortical responses emerge, the right hemisphere preferentially responds to tone‐bursts, particularly when they are presented to the left ear.
Figure 2.
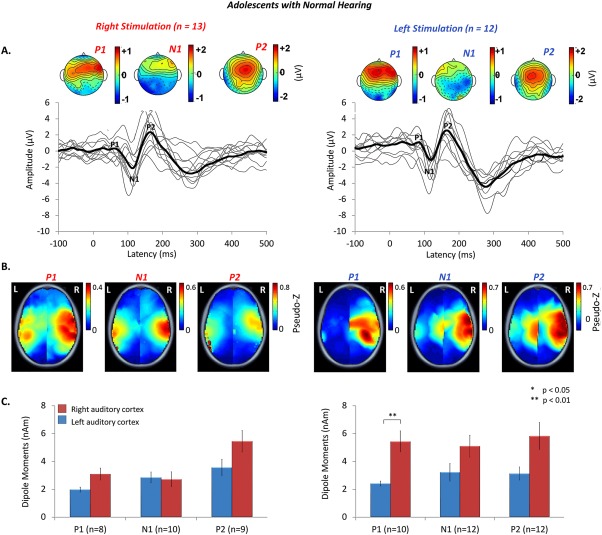
A. Auditory evoked cortical responses were stimulated from the right (n = 13) and left (n = 12) ears of adolescents with normal hearing, aged 15.9 ± 6.4 years. Individual responses from a midline cephalic electrode (C z) (thin grey lines), and grand mean responses (thick black lines) show three peaks (P 1, N 1, P 2), characteristic of mature responses. Topographic maps indicate similarly distributed scalp potentials (red = positive potentials, blue = negative potentials) for the mature peaks in both ears (common averaged reference). B. Mean peak dipole activity reveals stronger activity, shown by hotspots of dark red, in the right hemisphere, particularly for left ear stimulation. C. Mean [± 1 standard error (SE)] dipole moment strength is plotted for the left (blue bars) and right (red bars) auditory cortices for all cortical peaks. Consistent with the topographic plots in B. peak dipole activity is stronger in the right than left auditory cortex for stimulation of both ears, particularly for left ear stimulation.
Long Periods of Unilateral CI Use Drive Abnormal Patterns of Auditory Activity
Auditory stimulation with a CI for over 10 years appears to promote normal‐like maturation of cortical responses. The cortical waveform from electrode Cz, shown in Figure 3A sometimes contained stimulus artefact in early latencies from the short 36 ms CI pulse train. Because the stimulus was short, a polyphasic response was seen thereafter consisting of peaks with similar latencies to the normal hearing group (P 1: t (10)=−0.88, P > 0.05; N 1: t (10.18)=−1.3, P > 0.05; P 2: t (10.77) = 1.43, P > 0.05) and similar peak‐to‐peak amplitudes for the P 1‐N 1 complex [t (6.87) = 1.75, P > 0.05]. Nonetheless, differences from normal remained. The amplitude of peak P 2 was significantly larger in CI users [t (14.51) = 2.49, P < 0.05]. In the topological maps, a peripheral negativity corresponding to the implant on either side was seen and the current distribution of the N 1 revealed little clear negativity across the scalp (Fig. 3A). Furthermore, even though cortical activity evoked by the experienced CI was generated predominantly in areas of the temporal cortex, the auditory input in these adolescents activated the left and right auditory cortices differently from their normal hearing peers. Following artifact suppression and source localization with the TRACS beamformer, measured dipole activity underlying the mature cortical response was stronger in the left as compared to the right auditory cortex, as shown by the red hotspots in the brain images in Figure 3B (axial view only) and the extracted peak dipoles plotted in Figure 3C [F (2,24) = 10.978, P < 0.001; post hoc: P 1: t (26) = 2.4, P < 0.05, P 2: t (26) = 3.9, P < 0.01]. This reflects cortical activation in an opposite direction compared to the rightward hemispheric bias shown in Figure 2. There were no significant differences in these dipoles from normal across or within each of the three peaks [F (2,40) = 1.016, P > 0.05].
Figure 3.
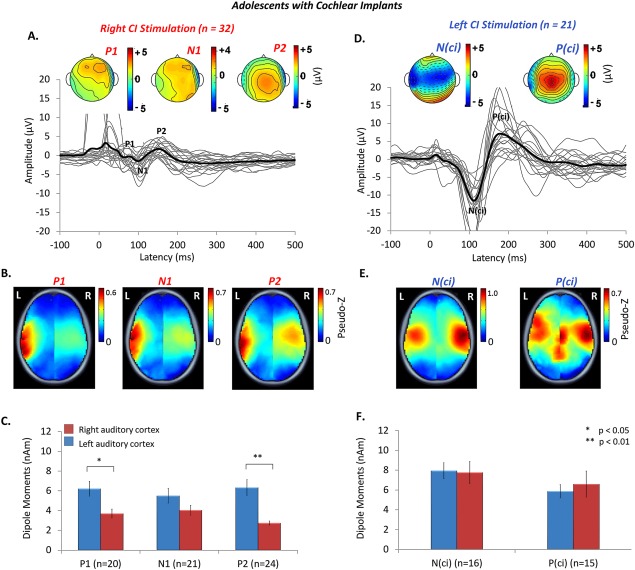
Auditory evoked cortical responses were stimulated from the experienced‐right (n = 32) and naïve‐left (n = 21) ears of adolescents with CIs, aged 15.9 ± 2.0 years. Cortical activity evoked by stimulation of the right ear after 12.4 ± 1.7 years of unilateral CI experience is shown in A. – C. Left ear had 12.0 ± 2.1 years of unilateral deprivation. Responses recorded within the first week of CI activation on that side are shown in D. – F. Individual responses from a midline cephalic electrode (Cz) (thin grey lines), and grand mean responses (thick black lines) show three peaks (P 1, N 1, P 2), characteristic of mature responses, evoked by stimulation of the experienced‐right CI in A., but an abnormal biphasic peaked response [N (ci), P (ci)] of abnormally large amplitudes was evoked by the naïve‐left side (n = 21), shown in D. Topographic maps (common averaged reference) indicate unusually similarly distributed scalp potentials for the three peaks of the mature response on the experienced side but a clearly opposite distribution for N (ci) (widely negative) than P (ci) (widely positive) from the naïve‐left side. B. A strong contralateral bias in the left hemisphere, shown by hotspots of dark red in the mean peak dipole activity plots, occurs with stimulation of the experienced‐right CI. By contrast, dipole activity evoked by stimulation of the naïve‐left ear in E. is symmetrically distributed in both hemispheres and is stronger compared to right ear dipoles for both peaks of the response. C. Mean (±1 SE) dipole moment strength is plotted for the left (blue bars) and right (red bars) auditory cortices for all cortical peaks on the experienced side. Consistent with the topographic plots in B., peak dipole activity is significantly stronger in the left than right auditory cortex for the mature peaks P 1 (P < 0.05) and P 2 (P < 0.01). F. The same responses are plotted for the naïve side and shows dipole activity consistent with the data in E.
Activity evoked by stimulation of the naïve pathways on the first day of activation of that left side was significantly different from the experienced side. As shown in Figure 3D, the morphology of these cortical waveforms was atypical and different from the response evoked by the experienced ear. At electrode C z, the waveforms were characterized by a large negative amplitude peak, labeled N (ci), followed by a large positive peak [P (ci)]. While the latency of these peaks were similar to that of N 1 and P 2 of the experienced side [N (ci)/N 1: t (11) = 1.85, P > 0.05; P (ci)/P 2: t (11) = 2.71, P > 0.05], the absolute amplitudes [N (ci)/N 1: t (11) = 3.45, P < 0.01; P (ci)/P 2: t (11) = 3.31, P < 0.01], and peak‐to‐peak amplitudes [N (ci)‐P (ci)/N 1‐P 2: t (11) = −3.37, P < 0.01] were significantly larger than responses from the experienced and normal hearing ears [F (3,70) = 5.4, P < 0.05]. Dipole activity was more widespread and symmetric across both auditory cortices [N (ci): t (20) = −0.56, P > 0.05; P (ci): t (20) = 1.6, P > 0.05] as shown in the brain plots in Figure 3E (axial view only). Dipole moments for both peaks were abnormally large in both hemispheres compared to the experienced side [F (1,42) = 29.589, P < 0.01] and both the right and left normal hearing ears [F (2,39) = 20.582, P < 0.01] (Figure 3F).
Normalized lateralization values of the peak dipoles indicated that, while tonal stimuli lateralized to the right auditory cortex regardless of ear of stimulation in adolescents with normal hearing, long‐term right CI use promoted leftward lateralization in adolescents (Fig. 4). Despite developing mature‐like cortical responses with over a decade of CI experience, significantly strong left hemisphere lateralization was found for all peaks recorded in CI users [P 1: t (10) = −2.460, P = 0.034, N 1: t (14) = −2.260, P = 0.04, P 2: t (15) = −6.912, P < 0.001]. This pattern was significantly different from the responses of either ear in normal hearing peers [F (4,92) = 3.286, P = 0.015, post‐hoc: right stimulation: P 1: P = 0.03, P 2: P = 0.003; left stimulation: P 1: P = 0.002, N 1: P = 0.001, P 2: P < 0.001]. Dipole activity evoked by stimulation in the newly implanted ear was more symmetrical as shown by a reduction in lateralization for peaks N (ci) and P (ci) [N (ci): t (15) = −0.726, P = 0.479 P (ci) = 2.238: t (12), P = 0.816]. This was significantly different from the right lateralized responses stimulated by tones in normal left ears [F (1,7) = 6.674, P = 0.036, post‐hoc: N 1: P = 0.03, P 2: P = 0.006].
Figure 4.
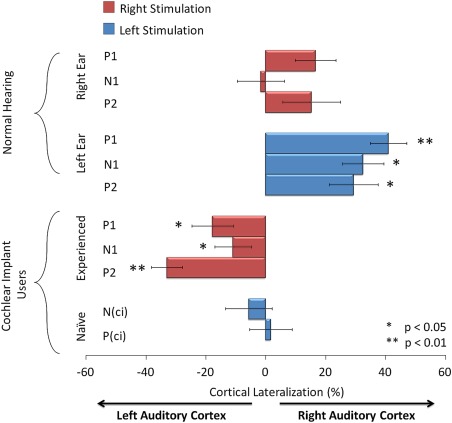
Mean (± 1 SE) percent cortical lateralization of peak dipoles was calculated using the formula: [(right hemisphere peak dipole moment – left hemisphere peak dipole moment)/(right hemisphere peak dipole moment + left hemisphere peak dipole moment) × 100]. Red bars indicate dipoles evoked from stimulation of the right ear/experienced CI and blue bars indicate activity from the left ear/naïve CI. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Permutation analyses were used to identify significant differences in activity between left and right auditory cortices in the normal hearing and CI groups. Unlike the peak dipole measures, which were specific to defined auditory areas, these analyses assessed whether every voxel in one hemisphere was significantly different from its corresponding voxel in the opposite hemisphere. This analysis revealed significant differences in activation between left and right temporo‐frontal and temporo‐parietal regions in both groups with unique patterns in the CI users relative to their normal hearing peers (Fig. 5A–D) (axial views only). Minimal differences in activity between the cortical hemispheres were evident when tone‐bursts were presented to normal right ears using this analysis (Fig. 5A). Significant differences were clearer in the auditory cortices as well as temporo‐frontal and temporo‐parietal regions for normal left ear stimulation (Fig. 5B). These differences were larger in the right (contralateral) than left cortical hemisphere. The CI group also showed strong contralateral temporo‐parietal activation from both ears underlying all response peaks (Fig. 5C). The ipsilateral temporo‐parietal junction was strongly activated in the later latency ranges (i.e P 2 for right experienced ear stimulation and P (ci) for naïve left stimulation) (Fig. 5C).
Figure 5.
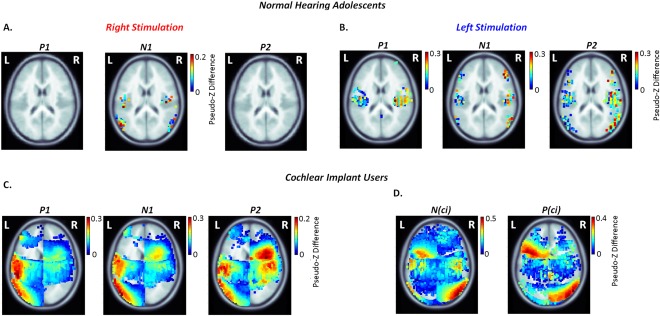
Cortical lateralization for each peak of the cortical waveform was assessed across 63,646 peak dipoles using permutation testing to compare activation of the left and right hemispheres. Significant absolute differences in dipole for right – left hemisphere (voxel‐wise permutation, P < 0.05, corrected for 62 recording channels; Bonferroni correction P = 0.05/62 = 0.0008) are plotted on topographic plots for stimulation of the right ear in A. and the left ear in B. for adolescents with normal hearing; and the right‐experienced CI in C. and naïve‐left side in D. in the implant cohort. Auditory activity lateralized towards the right hemisphere in normal hearing individuals, particularly in response to left ear stimulation, as shown by the dark red hotspots indicating greater inter‐hemispheric dipole differences. A significant shift in lateralization towards the contralateral left auditory cortex was observed in CI users with stimulation of the experienced‐right side. Cortical asymmetries towards the auditory cortices in either hemisphere were not clear when stimulated from the naïve side. Lateralization of secondary sources in the left temporo‐parietal junction and right temporo‐frontal cortex was present for the experienced‐right side, with stronger activity underlying P 2. This activity was reversed on the naïve‐left side and occurred with increased dipole strength in the right parietal‐temporal‐occipital association areas and left temporo‐frontal cortex.
Additional Cortical Areas Are Recruited by Cochlear Implant Stimulation Relative to Normal
We hypothesized that long‐term unilateral CI use would drive abnormal underlying cortical activity in the stimulated pathways compared to normal and leave the opposite deprived pathways vulnerable to significant change. To test this hypothesis, we compared dipoles in all 63,646 voxels between CI and normal hearing groups using permutation analyses. As shown in Figure 6A (axial view only), significantly larger dipoles were identified in the left frontal cortex underlying peak P 1, in a small left parietal area underlying N 1, and the right precuneus region underlying peak P 2 for the experienced CI side compared to responses from the right ear in the normal hearing group. This indicates that CI users recruit additional cortical areas to process sound. At the same time, depriving auditory input on the opposite side for over a decade led to significantly different activation in those pathways relative to responses from the normal hearing left ear. Dipole activity in the naïve‐left ear was significantly larger across most of the cortex, particularly for peak N (ci), compared to normal left ear evoked activity (Fig. 6B) (axial view only). This might indicate aberrant cortical organization as a result of long‐term deafness, in line with our hypothesis.
Figure 6.
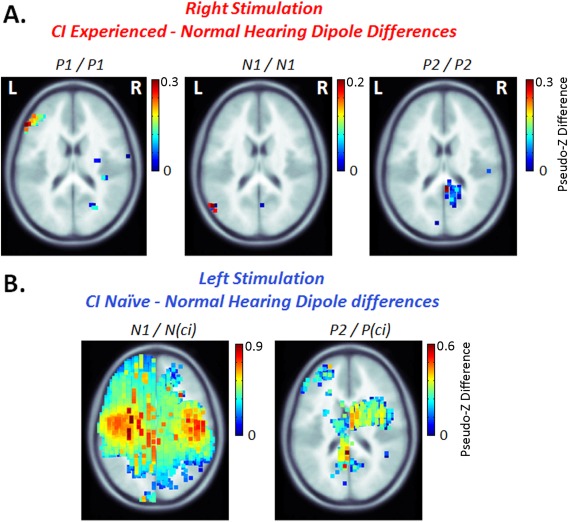
Permutation analyses were used to compare dipole activation between the group of CI users and the group of normal hearing peers. Significant absolute dipole differences between the two groups (voxel‐wise permutation, P < 0.05, corrected for 62 recording channels; Bonferroni correction P = 0.05/62 = 0.0008) are shown on topographic brain plots for experienced‐right CI – normal hearing right ear stimulation in A., and naïve‐left CI – normal hearing left ear stimulation in B. Dipole magnitudes underlying the mature cortical peaks were generally similar between adolescents with normal hearing and experienced CI users, but CI stimulation of the experienced side activated cortical regions in the left frontal cortex underlying peak P 1, a small left parietal area underlying N 1, and the right precuneus regions underlying P 2 which were not present in normal hearing peers, as shown by the dark red hotspots in A. By contrast, the naïve ear evoked abnormally large and diffuse cortical activity across the brain, with significantly stronger dipole activity compared to the left ear of the normal hearing cohort.
Abnormal Activity Evoked by the Naïve Side Predicts Poor Speech Perception Outcomes
We asked whether there was a relationship between cortical activity in CI users and their speech perception once they had used their bilateral CIs for at least 6 months. As shown in Figure 7A, speech perception performance with the experienced CI was high, with a mean ± 1 standard error score of 77.54 ± 2.88%. By contrast, this was significantly worse (14 ± 4.92%) when adolescents listened with their newly implanted ear alone (t (17) = 5.70, P < 0.01). All but 2 adolescents achieved less than 50% accuracy. There was no significant correlation between speech scores and duration of bilateral experience with either the experienced (R = −0.12, P = 0.65) or the newly implanted ear (R = 0.44, P = 0.08). An analysis of speech perception and dipole activity between both ears indicated a significant correlation between speech perception outcomes and left hemisphere lateralization for peak P 2 (R = −0.55, P = 0.02) (Fig. 7B). We hypothesized that this relationship reflects a supportive role of increased activity in the contralateral pathways towards the left cortex for speech perception, rather than reduced activity in the right auditory cortex. In support, a significant positive correlation between dipole strength and speech perception scores was found in the left auditory cortex (P 1: R = 0.48, P = 0.05, N 1: R = 0.51, P = 0.03, P 2: R = 0.53, P = 0.02) but not the right (P 1: R = −0.14, P = 0.62, N 1: R = 0.43, P = 0.06, P 2: R = 0.03, P = 0.92) for the experienced‐right CI. No significant correlations with speech outcomes were found for cortical activity evoked by the naïve CI. Further analyses confirmed that as the asymmetry in speech perception increased (better speech perception in the first implanted ear), the greater the asymmetry in left P 2 dipoles (larger left hemisphere dipoles when evoked by the first than second implanted ear) (R = 0.70, P = 0.004) and, consistently, the leftward cortical lateralization of P 2 increased (more leftward lateralization when stimulated by the first than second implant) (R = 0.56, P < 0.03). No other significant correlations were found (P > 0.05).
Figure 7.
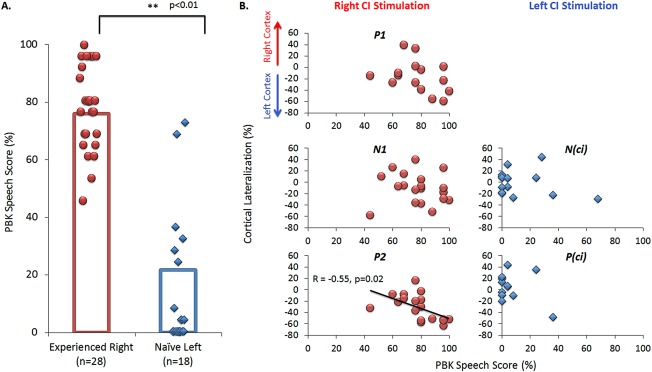
A. Speech perception outcomes measured from the experienced side (n = 28) (red) and naïve ear (n = 18) (blue) of CI users was assessed using the PBK speech test after 6 to 18 months of bilateral CI experience. Individual responses are indicated by the round symbols for the experienced‐right ear and diamonds for the naïve‐left ear, with mean performance shown by the bar graph. Performance on the experienced side was significantly better than the naïve side (P < 0.01). B. Analyses of cortical lateralization and performance on the PBK speech test indicated significant correlation between left hemisphere lateralization for peak P 2 (P < 0.05) and better speech scores on the experienced CI side. No significant correlations were found for dipole activity evoked by stimulation of the naïve side and speech perception. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
Data from this study suggest that hemispheric specialization to tone‐bursts in the right auditory hemisphere emerges with normal maturation of the auditory cortex. By contrast, adolescents who had over a decade of unilateral CI experience in the right ear developed cortical responses from the experienced ear which lateralized to the contralateral left auditory cortex despite developing mature normal‐like cortical peaks. Increased response in the left auditory cortex reflects abnormal strengthening from the unilaterally stimulated pathways and may support speech perception in CI users. On the other hand, bilateral cortical activation from the naïve‐left ear at initial stimulation coupled with poorer speech understanding in that newly implanted ear could reflect undefined and perhaps compromised auditory processing. Evidence of alternate auditory processing was supported by the stimulation of additional cortical areas in adolescents using CIs relative to their normal hearing peers, particularly when evoked by the newly implanted ear. These findings indicate that long‐term stimulation of the auditory system with a unilateral CI promotes an asymmetric mature auditory cortex while, at the same time, leaving pathways from the opposite ear unprotected from abnormal effects of deafness. Because sensitive periods in development typically end once a period of maturation is reached [Blakemore, 2012; Lenroot and Giedd, 2006; Lohmann and Kessels, 2014], it may be challenging to promote further changes in these adolescents from the newly implanted side.
Hemispheric Specialization Requires Normal Bilateral Hearing
Data in Figure 2 indicate a normally mature response to a 500 Hz tone‐burst with more activity in the right than left auditory cortex. Lateralization to the right hemisphere occurred for both left and right ear presentations (Figs. 4 and 5). A number of studies using electroencephalography (Picton et al., 1999; Hine and Debener, 2007; Hine et al., 2008), as measured here, as well as magnetoencepholography (Kanno et al., 1996; Pantev et al., 1998; Fujoika et al., 2003) have shown similar findings in adults. This specialization of the right auditory cortex for pure tones appears to have emerged in development as the same stimulation resulted in contralateral lateralization in younger children with normal hearing regardless of ear of presentation [Gordon et al., 2013b]. Lateralization of auditory evoked activity to the contralateral cortex in the immature brain is consistent with the anatomy of the auditory system; most ascending auditory fibers cross contralaterally in the brainstem [Glendenning et al., 1981; Nordeen et al., 1983; Ponton et al., 2001]. The aural preference in each cortical hemisphere for the contralateral ear of younger peers can also be explained by this anatomical arrangement [Kral, 2013; Kral et al., 2013a, 2013b].
Changes to cortical lateralization with age could occur as the structure and function of the brain becomes more specialized in each hemisphere. For example, cell columns become wider and more spaced with heavier axon myelination and increased inter‐connectivity in left auditory regions compared to right, providing specialized coding of temporal rather than spectral properties of sound [Penhune et al., 1996; Toga and Thompson, 2003; Zatorre and Belin, 2001; Zatorre et al., 2002]. The time course for many cortical changes are complete by late adolescence [Blakemore, 2012; Blakemore and Choudhury, 2006; Giedd et al., 1999; Giedd et al., 1996; Lebel and Beaulieu, 2011; Lenroot and Giedd, 2006; Lohmann and Kessels, 2014]. Auditory cortical responses are normally mature at this time [Eggermont and Ponton, 2003; Jiwani et al., 2013; Moore and Linthicum, 2007; Ponton et al., 2002; Ponton et al., 2000], corresponding with the emergence of better listening skills including perception of speech in noise and improved comprehension of degraded speech [Ponton et al., 2000].
Some of the same maturational processes likely occurred in adolescents using unilateral CIs, given that cortical peaks characteristic of adult responses were recorded in this group. The emergence of mature‐like cortical response peaks in implant users is consistent with previous reports [Jiwani et al., 2013] and linked to the development of superficial cortical layers through thalamo‐cortical and cortico‐cortical connections [Ponton and Eggermont, 2001]. Good speech perception outcomes with the first implant, plotted in Figure 7, coupled with mature cortical responses provide a generally positive impression of auditory development with long‐term CI stimulation. On the other hand, underlying dipole activity (Fig. 3) and cortical lateralization measures (Figs. 4 and 5) reveal abnormally strong dipoles of activity in the contralateral left auditory brain in response to stimulation of the experienced‐right implant. Thus, adolescents with right CIs did not develop the normal right brain hemispheric bias associated with non‐speech stimuli, despite having developed expected mature cortical waveforms.
Long Periods of Unilateral CI Use Strengthens Pathways From the Stimulated Ear
There are several reasons why adolescents using CIs may not have developed specialized auditory cortices similar to their normal hearing peers. They had been bilaterally deaf from early in life (typically from birth) and listened, often unilaterally, to a representation of sound missing much of its fine temporal information [Drennan and Rubinstein, 2008; Zeng et al., 2008].
It is possible that specialization has not yet emerged in these pathways, with contralateral afferent projections still dominating auditory input as in earlier development [Gordon et al., 2011b, 2013a]. Effects of unilateral auditory deprivation/stimulation appear to occur early and persist into maturation. In younger children with CIs, abnormal strengthening of activity in the contralateral pathways from the experienced‐right ear to the brainstem [Gordon et al., 2012] and cortex [Gordon et al., 2013b] was found when unilateral implant use exceeded 1.5 years. Stronger left than right hemisphere activation observed in Figures 3, 4, 5 are consistent with these findings, indicating that increased activity in the left auditory cortex from the right implanted ear continues into maturation with longer periods of unilateral CI use. There was also strengthening of pathways to the ipsilateral cortex from the unilaterally right stimulated ear in the immature cortex, as measured by a reversal of stimulus preference from the contralateral ear to the ipsilateral first implanted ear [Gordon et al., 2013b]. Interestingly, there were no significant increases in the ipsilateral dipole strength in that study relative to normal, revealing the power of the aural preference measure to detect plasticity in the ipsilateral cortex [Kral et al., 2013a, 2013b]. By contrast, the available data from adults with late onset unilateral deafness provide inconsistent EEG and MEG findings. Significant increases in responses recorded over the ipsilateral hemisphere were found in Ponton et al., 2001 and significant increases in dipoles located in both auditory cortices from EEG in Maslin et al., 2013. On the other hand, limited effects were found in several other studies [Vasama et al., 1995; Fujiki et al., 1998; Hine et al., 2008; Hanss et al., 2009]. One study of childhood unilateral deafness also showed minimal effects, perhaps for similar reasons [Vasama and Makela, 1994]. Although the aural preference could not be assessed in the present study because of the highly abnormal responses with very large underlying dipoles evoked in the newly implanted side, a significant increase in ipsilateral voxels in auditory areas was found underlying P 2 (Fig. 5C).
The clear finding of asymmetric strengthening of the contralateral pathways from the stimulated ear might be explained by increased excitability of auditory neurons from this side. Early unilateral auditory deprivation leads to a loss of cochlear nucleus neurons and reduced synaptic inhibition in pathways from the affected side [Blumenthal et al., 1999; Byrne and Byrne, 1993; Hashisaki and Rubel, 1989; Sanes and Kotak, 2011; Takesian et al., 2009], and enhanced excitatory glutamatergic conductance from the superior olive [Kotak and Sanes, 1996], to the inferior colliculus [Blumenthal et al., 1999; Byrne and Byrne, 1993; Kitzes, 1984; Lang et al., 2010; Popescu and Polley, 2010; Takesian et al., 2009] and primary auditory cortex of the stimulated pathways [Kotak et al., 2005; Kral et al., 2013a, 2013b; Moore et al., 2005; Popescu and Polley, 2010]. Importantly, effects are found only when the loss is present in early life [Blumenthal et al., 1999; Moore and Kowalchuk, 1988; Nordeen et al., 1983; Reale et al., 1987] and when it is unilateral [Moore, 1990]. Such deafness‐induced disruptions to the delicate balance of excitatory‐inhibitory inputs influence and shape the development of binaural hearing in the brainstem [Grothe et al., 2010] and neuronal circuits in the auditory cortex [King, 2010; Turrigiano and Nelson, 2004]. The coupling of excitatory‐inhibitory synaptic inputs in the auditory system is influenced by experience during development [King, 2010]. Maturation of inhibitory tuning occurs more slowly than that of excitation [Dorrn et al., 2010] and inhibitory synapses are more vulnerable to deafness‐induced perturbation [Sanes and Kotak, 2011]. Thus, the abnormal strengthening of pathways from the unilaterally stimulated CI ear could reflect a disrupted balance of excitatory‐inhibitory transmission during early development.
An alternate explanation for the abnormally increased dipoles in the left auditory cortex of CI users is the possibility of compensation for inadequate input from the unilateral CI. The left auditory cortex normally codes temporal information including speech. Although no speech sounds were provided to evoke the cortical responses in this study, speech perception in the experienced ear increased with stronger dipoles in the left auditory cortex (Fig. 7B]. This is consistent with a previous report showing better language outcomes in children with right CIs who lateralized auditory input in the contralateral left hemisphere [Chilosi et al., 2014]. Strong left cortical activity in individuals with normal hearing has been associated with better language skills [Kadis et al., 2011], more precise auditory processing [Schönwiesner et al., 2005; Zatorre and Belin, 2001; Zatorre et al., 2002] and increased reading and spelling skills [Abrams et al., 2006]. Enhanced activity in the left cortex of adolescents using CIs could thus mark plastic changes in the brain to compensate for the abnormal electrical input provided by the implant device and/or stimulation of the auditory pathways from only one side.
No significant increases in peak dipoles were found from the experienced CI side relative to normal across the three peak latency ranges (P > 0.05) despite a significant increase in P 2 amplitude measured at electrode Cz as previously reported [Jiwani et al., 2013]. However, permutation analyses of each voxel in the P 2 latency range revealed significantly increased activity in the precuneus region (Fig. 6) and, in a within group analysis, increased activity in right ipsilateral auditory cortex relative to corresponding voxels in the opposite hemisphere (Fig. 5). Other areas were also recruited more strongly in CI users; larger contralateral activity in temporo‐parietal‐occipital areas across the three latency ranges (Fig. 5C), significantly increased activity in the left contralateral prefrontal cortex underlying P 1 (Fig. 6A) and a small area of left contralateral parietal activity underlying N 1 (Fig. 6A). The prefrontal and precuneus regions are areas involved in attention and effort [Fletcher et al., 1995; Kane and Engle, 2002] and the temporo‐parietal‐occipital junction integrates multimodal information [von Stein et al., 1999]. Overall, evidence of additional recruitment of these non‐auditory cortical areas suggests that even good CI users require increased cortical processing to support CI listening. Similar findings in successful CI users include increased activation of the left dorsolateral pre‐frontal, frontal, and parietal networks that include the precuneus region and are involved with higher cognitive functions [Giraud and Lee, 2007; Giraud et al., 2001; Lee and Winer, 2005]. It is clear that individuals compensate for CI listening by using increased multi‐sensory information [Doucet et al., 2006; Giraud and Lee, 2007; Giraud et al., 2001; Lee et al., 2007; Powell, 1974] and increased effort/attention [Gordon et al., 2006, 2013a; Hopyan‐Misakyan et al., 2009; Kronenberger et al., 2014; Pisoni et al., 2010]. This study provides further evidence that these networks are recruited in adolescents with CIs even in a passive response evoked by non‐speech stimulation from the ear they listened with for most of their lives. This suggests a developmental reorganization of normal cortical networks to support CI hearing.
Activity Evoked by Stimulation of the Newly Implanted Ear Is Abnormal
It is difficult to separate the effects of stimulation from one ear from the simultaneous effects of deprivation in the other ear in adolescents using unilateral CIs. We suggest, however, that leaving the opposite pathways deprived of input beyond the period of brainstem [Gordon et al., 2006, 2013a] and cortical [Jiwani et al., 2013] maturation further disrupts organization of activity in the auditory pathways. Cortical responses at initial stimulation of that side were atypical and dominated by an abnormally large biphasic waveform (Fig. 3), despite equal amplitudes of brainstem responses on each side. This may reflect cortical immaturity, as a similar response has been observed in normal hearing pre‐term infants [Wunderlich and Cone‐Wesson, 2006] and children with little auditory input due to GJB‐2 deafness [Gordon et al., 2011b]. This could also reflect abnormal cortical activity, as the same response was recorded from children with congenital deafness implanted late [Sharma et al., 2002] and in children who had poor speech perception with their implants [Gordon et al., 2008].
Cortical activity underlying the abnormal response in the newly implanted ear occurred with abnormally large peak dipole moments in auditory regions compared to the activity evoked by the opposite experience side and the normal hearing group (Fig. 3). Moreover, activity was more widespread (Fig. 6) with a loss of cortical lateralization (Fig. 5). Reduced cortical lateralization has been associated with poor auditory processing skills, decreased academic performance and increased difficulty with reading and spelling [Abrams et al., 2006]. Poor speech perception outcomes when adolescents were listening with the new implant after ∼8 months of experience (Fig. 7) is consistent with these reports, suggesting altered cortical processing in the deprived pathways. If changes are to occur with bilateral stimulation, considerably longer term follow‐up will be required as binaural hearing has been reported to take several years to emerge even in children implanted at younger ages [Gordon et al., 2014].
The long period of deprivation in this ear could have left it vulnerable to many structural and functional changes [Gordon et al., 2013b; Kral et al., 2013a, 2013b; Popescu and Polley, 2010], which allowed a reorganization of the auditory brain in favour of the hearing ear while reducing responsiveness of the deprived side to activate the cortex but not eliminating it [Kral et al., 2013a; Popescu and Polley, 2010]. Indeed, as shown in Figure 3, the deprived ear retains the ability to activate the cortex, albeit abnormally, even after over a decade unilateral deafness.
Similar cortical consequences of imbalanced input occur in the visual system, with prolonged monocular deprivation. Findings of reduced visual acuity from a deprived eye has been attributed to uneven competition between the two pathways [Blakemore, 1988; Hubel and Wiesel, 1970; Hubel et al., 1977; Le Vay et al., 1980; Lewis and Maurer, 2005], resulting in arrested [Antonini et al., 1999] or retracted [Antonini and Stryker, 1993; Antonini and Stryker, 1996] growth of axonal arbors in ocular dominance columns of the deprived eye [Hubel et al., 1977; Le Vay et al., 1980]. This in turn, resulted in jittered representation of visual input and a competitive disadvantage of this eye in the cortex [Antonini et al., 1999; Blakemore and Van Sluyters, 1974; Jeffrey et al., 2004]. In this study, the widespread dipole activity observed from the naïve CI, could similarly reflect undefined cortical activity across both hemispheres, and suggests that similar aberrant processes may underlie auditory cortical activity in these pathways. This might reflect an immaturity or disintegration of auditory areas in the cortex [Kral and Sharma, 2012] and would explain the inferior auditory outcome observed from this side (Fig. 7), thereby highlighting the deleterious effects of unilateral deprivation on the brain. The similarly large stimulation of contralateral temporo‐parietal‐occipital areas in response to this new implant may reflect recruitment of multi‐modal areas for auditory input.
CONCLUSION
Maturation of the auditory brain is an experience‐dependent process which promotes specialization of each cortical hemisphere in adolescents with normal hearing. By contrast, cortical organization in the auditory pathways differs from normal in adolescents who used a unilateral CI to hear for most of their lives. Present findings indicate that driving maturation of the auditory cortex with only one implant for over a decade leads to lasting asymmetries in the auditory system and leaves the deprived pathways unprotected from effects of unilateral auditory deprivation. Because sensitive periods typically end when maturation is reached, maturation of the cortex from the experienced CI may mark the closing of an important developmental period in the adolescent brain for gaining improved auditory function in the deprived pathways.
ACKNOWLEDGMENTS
The authors recognize the support and participation of adolescents and families who participated in this study. They thank Dr. Sam Doesburg for the helpful comments on the manuscript. They also thank Dr. Daniel D. E. Wong, Salim Dharssi, Marc Lalancette, Alexander Andrews, and Carmen McKnight for programming help and Parvaneh Abbasalipour for help with data collection.
Correction added on 26 October 2015, after first online publication.
REFERENCES
- Abrams DA, Nicol T, Zecker SG, Kraus N (2006): Auditory brainstem timing predicts cerebral asymmetry for speech. J Neurosci 26:11131–11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acar ZA, Makeig S (2013): Effects of forward model errors on EEG source localization. Brain Topography 26:378–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht R, Suchodoletz W, Uwer R (2000): The development of auditory evoked dipole source activity from childhood to adulthood. Clin Neurophysiol 111:2268–2276. [DOI] [PubMed] [Google Scholar]
- Antonini A, Fagiolini M, Stryker MP (1999): Anatomical correlates of functional plasticity in mouse visual cortex. J Neurosci 19:4388–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Stryker MP (1993): Rapid remodeling of axonal arbors in the visual cortex. Science 260:1819–1821. [DOI] [PubMed] [Google Scholar]
- Antonini A, Stryker MP (1996): Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. J Comp Neurol 369:64–82. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Dye MWG, Hauser PC (2006): Do deaf individuals see better? Trend Cognit Sci 10:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Neville HJ (2002): Cross‐modal plasticity: Where and how? Nat Rev Neurosci 3:443–452. [DOI] [PubMed] [Google Scholar]
- Bess FH, Tharpe AM (1984): Unilateral hearing impairment in children. Pediatrics 74:206–216. [PubMed] [Google Scholar]
- Bess FH, Tharpe AM (1986): An introduction to unilateral sensorineural hearing loss in children. Ear Hear 7:3 [DOI] [PubMed] [Google Scholar]
- Blair RC, Karniski W (1993): An alternative method for significance testing of waveform difference potentials. Psychophysiology 30:518–524. [DOI] [PubMed] [Google Scholar]
- Blakemore C (1988): The sensitive periods of the monkey visual cortex. In: Lennerstrand G, von Noorden GK, Campos E, editors. Strabismus and amblyopia: Experimental basis for advances in clinical management: 219–234. London: MacMillan Press (395).
- Blakemore SJ (2012): Imaging brain development: The adolescent brain. Neuroimage 61:397–406. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Van Sluyters RC (1974): Reversal of the physiological effects of monocular deprivation in kittens: Further evidence for a sensitive period. J Physiol 237:195–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S (2006): Development of the adolescent brain: Implications for executive function and social cognition. J Child Psychol Psychiatry 47:296–312. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M (1999): Effects of exercise training on older patients with major depression. Arch Intern Med 159:2349–2356. [DOI] [PubMed] [Google Scholar]
- Byrne A, Byrne D (1993): The effect of exercise on depression, anxiety and other mood states: A review. J Psychosom Res 37:565–574. [DOI] [PubMed] [Google Scholar]
- Chadha NK, Papsin BC, Jiwani S, Gordon KA (2011): Speech detection in noise and spatial unmasking in children with simultaneous versus sequential bilateral cochlear implants. Otol Neurotol 32:1057 [DOI] [PubMed] [Google Scholar]
- Chau W, McIntosh AR, Robinson SE, Schulz M, Pantev C (2004): Improving permutation test power for group analysis of spatially filtered MEG data. Neuroimage 23:983–996. [DOI] [PubMed] [Google Scholar]
- Chilosi AM, Comparini A, Cristofani P, Turi M, Berrettini S, Forli F, Orlandi G, Chiti A, Giannini N, Cipriani P (2014): Cerebral lateralization for language in deaf children with cochlear implantation. Brain Lang 129:1–6. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Sekihara K, Nagarajan SS (2006): Modified beamformers for coherent source region suppression. Biomed Eng IEEE Trans 53:1357–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas F, Ermer JJ, Mosher JC, Leahy RM (2006): Generic head models for atlas‐based EEG source analysis. Hum Brain Mapp 27:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R (1984): Affect, cognition, and hemispheric specialization. In: Izard CE, Kagan J, Zajoc R editors. Emotions Cognition and Behavior: 320–365. Cambridge University Press.
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC (2010): Developmental sensory experience balances cortical excitation and inhibition. Nature 465:932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet M, Bergeron F, Lassonde M, Ferron P, Lepore F (2006): Cross‐modal reorganization and speech perception in cochlear implant users. Brain 129:3376–3383. [DOI] [PubMed] [Google Scholar]
- Drennan WR, Rubinstein JT (2008): Music perception in cochlear implant users and its relationship with psychophysical capabilities. J Rehabil Res Dev 45:779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ (1988): On the rate of maturation of sensory evoked potentials. Electroencephal Clin Neurophysiol 70:293–305. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW (2003): Auditory‐evoked potential studies of cortical maturation in normal hearing and implanted children: Correlations with changes in structure and speech perception. Acta Oto‐Laryngol 123:249–252. [DOI] [PubMed] [Google Scholar]
- Finney EM, Fine I, Dobkins KR (2001): Visual stimuli activate auditory cortex in the deaf. Nat Neurosci 4:1171–1174. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MB, Green JE, Fang Y, Waltzman SB (2013): Factors influencing consistent device use in pediatric recipients of bilateral cochlear implants. Cochlear Implant Int 14:257–265. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick MR, Irannejad S (2008): Adolescent readiness for change and the working alliance in counseling. J Counsel Dev 86:438–445. [Google Scholar]
- Fletcher P, Frith C, Baker S, Shallice T, Frackowiak R, Dolan R (1995): The mind's eye—precuneus activation in memory‐related imagery. NeuroImage 2:195–200. [DOI] [PubMed] [Google Scholar]
- Fujiki N, Naito Y, Nagamine T, Shiomi Y, Hirano S, Honjo I, Shibasaki H (1998): Influence of unilateral deafness on auditory evoked magnetic field. Neuroreport 9:3129–3133. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Ross B, Okamoto H, Takeshima Y, Kakigi R, Pantev C (2003): Tonotopic representation of missing fundamental complex sounds in the human auditory cortex. Eur J Neurosci 18:432–440. [DOI] [PubMed] [Google Scholar]
- Geers A, Tobey E, Moog J, Brenner C (2008): Long‐term outcomes of cochlear implantation in the preschool years: From elementary grades to high school. Int J Audiol 47:S21–S30. [DOI] [PubMed] [Google Scholar]
- Geers AE, Sedey AL (2011): Language and verbal reasoning skills in adolescents with 10 or more years of cochlear implant experience. Ear Hear 32:39S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999): Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2:861–863. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey B, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D (1996): Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb Cortex 6:551–559. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Lee HJ (2007): Predicting cochlear implant outcome from brain organisation in the deaf. Restorative Neurology and Neuroscience 25:381–390. [PubMed] [Google Scholar]
- Giraud AL, Price CJ, Graham JM, Truy E, Frackowiak RSJ (2001): Cross‐modal plasticity underpins language recovery after cochlear implantation. Neuron 30:657–664. [DOI] [PubMed] [Google Scholar]
- Glendenning K, Brusno‐Bechtold J, Thompson G, Masterton R (1981): Ascending auditory afferents to the nuclei of the lateral leminscus. J Comp Neurol 197:673–703. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Papsin BC (2009): Benefits of short interimplant delays in children receiving bilateral cochlear implants. Otol Neurotol 30:319 [DOI] [PubMed] [Google Scholar]
- Gordon KA, Papsin BC, Harrison RV (2005): Effects of cochlear implant use on the electrically evoked middle latency response in children. Hear Res 204:78–89. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Papsin BC, Harrison RV (2006): An evoked potential study of the developmental time course of the auditory nerve and brainstem in children using cochlear implants. Audiol Neurotol 11:7–23. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Tanaka S, Wong DD, Papsin BC (2008): Characterizing responses from auditory cortex in young people with several years of cochlear implant experience. Clin Neurophysiol 119:2347–2362. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Wong DD, Papsin BC (2010): Cortical function in children receiving bilateral cochlear implants simultaneously or after a period of interimplant delay. Otol Neurotol 31:1293–1299. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Jiwani S, Papsin BC (2011a): What is the optimal timing for bilateral cochlear implantation in children? Cochlear Implant Int 12:S8–S14. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Tanaka S, Wong DD, Stockley T, Ramsden JD, Brown T, Jewell S, Papsin BC (2011b): Multiple effects of childhood deafness on cortical activity in children receiving bilateral cochlear implants simultaneously. Clin Neurophysiol 122:823–833. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Salloum C, Toor GS, van Hoesel R, Papsin BC (2012): Binaural interactions develop in the auditory brainstem of children who are deaf: Effects of place and level of bilateral electrical stimulation. J Neurosci 32:4212–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KA, Jiwani S, Papsin BC (2013a): Benefits and detriments of unilateral cochlear implant use on bilateral auditory development in children who are deaf. Front Psychol 4:719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KA, Wong DD, Papsin BC (2013b): Bilateral input protects the cortex from unilaterally‐driven reorganization in children who are deaf. Brain 136:1609–1625. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Deighton MR, Abbasalipour P, Papsin BC (2014): Perception of binaural cues develops in children who are deaf through bilateral cochlear implantation. PloS One 9:e114841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Jo HJ, Wallace GL, Saad ZS, Cox RW, Martin A (2013): Two distinct forms of functional lateralization in the human brain. Proc Natl Acad Sci 110:E3435–E3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J, Vickers D (2011): Evidence of a ‘critical age'for sequential implantation of the second ear in congenitally deaf children. Cochlear Implant Int 12:S121–S123. [DOI] [PubMed] [Google Scholar]
- Graham J, Vickers D, Eyles J, Brinton J, Malky GA, Aleksy W, Martin J, Henderson L, Mawman D, Robinson P (2009): Bilateral sequential cochlear implantation in the congenitally deaf child: evidence to support the concept of a ‘critical age'after which the second ear is less likely to provide an adequate level of speech perception on its own. Cochlear Implant Int 10:119–141. [DOI] [PubMed] [Google Scholar]
- Grieco‐Calub TM, Litovsky RY (2010): Sound localization skills in children who use bilateral cochlear implants and in children with normal acoustic hearing. Ear Hear 31:645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe DM, Urbach TP, Kutas M (2011): Mass univariate analysis of event‐related brain potentials/fields I: A critical tutorial review. Psychophysiology 48:1711–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D (2010): Mechanisms of sound localization in mammals. Physiol Rev 90:983–1012. [DOI] [PubMed] [Google Scholar]
- Hanss J, Veuillet E, Adjout K, Besle J, Collet L, Thai‐Van H (2009): The effect of long‐term unilateral deafness on the activation pattern in the auditory cortices of French‐native speakers: influence of deafness side. BMC Neurosci 10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashisaki GT, Rubel EW (1989): Effects of unilateral cochlea removal on anteroventral cochlear nucleus neurons in developing gerbils. J Comp Neurol 283:465–473. [DOI] [PubMed] [Google Scholar]
- Hopyan‐Misakyan TM, Gordon KA, Dennis M, Papsin BC (2009): Recognition of affective speech prosody and facial affect in deaf children with unilateral right cochlear implants. Child Neuropsychol 15:136–146. [DOI] [PubMed] [Google Scholar]
- Hine J, Debener S (2007): Late auditory evoked potentials asymmetry revisited. Clin Neurophysiol 118:1274–1285. [DOI] [PubMed] [Google Scholar]
- Hine J, Thornton R, Davis A, Debener S (2008): Does long‐term unilateral deafness change auditory evoked potential asymmetries? Clin Neurophysiol 119:576–586. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN (1970): The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol 206:419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S (1977): Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond Ser B Biol Sci 377–409. [DOI] [PubMed] [Google Scholar]
- Illg A, Giourgas A, Kral A, Büchner A, Lesinski‐Schiedat A, Lenarz T (2013): Speech comprehension in children and adolescents after sequential bilateral cochlear implantation with long interimplant interval. Otol Neurotol 34:682–689. [DOI] [PubMed] [Google Scholar]
- Jeffrey BG, Wang YZ, Birch EE (2004): Altered global shape discrimination in deprivation amblyopia. Vision Res 44:167–177. [DOI] [PubMed] [Google Scholar]
- Jiwani S, Papsin BC, Gordon KA (2013): Central auditory development after long‐term cochlear implant use. Clin Neurophysiol 124:1868–1880. [DOI] [PubMed] [Google Scholar]
- Kadis DS, Pang EW, Mills T, Taylor MJ, McAndrews MP, Smith ML (2011): Characterizing the normal developmental trajectory of expressive language lateralization using magnetoencephalography. J Int Neuropsychol Soc 17:896 [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW (2002): The role of prefrontal cortex in working‐memory capacity, executive attention, and general fluid intelligence: An individual‐differences perspective. Psychonom Bull Rev 9:637–671. [DOI] [PubMed] [Google Scholar]
- Kanno A, Nakasato N, Fujita S, Seki K, Kawamura T, Ohtomo S, Fujiwara S, Yoshimoto T (1996): Right hemispheric dominance in the auditory evoked magnetic fields for pure‐tone stimuli. Electroencephalogr Clin Neurophysiol Suppl 47:129–132. [PubMed] [Google Scholar]
- Khosla D, Ponton CW, Eggermont JJ, Kwong B, Don M, Vasama JP (2003): Differential ear effects of profound unilateral deafness on the adult human central auditory system. J Assoc Res Otolaryngol 4:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ (2010): Auditory neuroscience: balancing excitation and inhibition during development. Curr Biol 20:R808–R810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzes L (1984): Some physiological consequences of neonatal cochlear destruction in the inferior colliculus of the gerbil, Meriones unguiculatus . Brain Res 306:171–178. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH (1996): Developmental influence of glycinergic transmission: Regulation of NMDA receptor‐mediated EPSPs. J Neurosci 16:1836–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH (2005): Hearing loss raises excitability in the auditory cortex. J Neurosci 25:3908–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A (2013): Auditory critical periods: A review from system's perspective. Neuroscience 247:117–133. [DOI] [PubMed] [Google Scholar]
- Kral A, Sharma A (2012): Developmental neuroplasticity after cochlear implantation. Trend Neurosci 35:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Heid S, Hubka P, Tillein J (2013a): Unilateral hearing during development: Hemispheric specificity in plastic reorganizations. Front Syst Neurosci 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Hubka P, Heid S, Tillein J (2013b): Single‐sided deafness leads to unilateral aural preference within an early sensitive period. Brain 136:180–193. [DOI] [PubMed] [Google Scholar]
- Kronenberger WG, Colson BG, Henning SC, Pisoni DB (2014): Executive functioning and speech‐language skills following long‐term use of cochlear implants. J Deaf Stud Deaf Educ enu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Koegel LK, Ashbaugh K, Regester A, Ence W, Smith W (2010): Physical exercise and individuals with autism spectrum disorders: A systematic review. Res Autism Spectrum Disord 4:565–576. [Google Scholar]
- Le Grand R, Mondloch CJ, Maurer D, Brent HP (2003): Expert face processing requires visual input to the right hemisphere during infancy. Nat Neurosci 6:1108–1112. [DOI] [PubMed] [Google Scholar]
- Le Vay S, Wiesel TN, Hubel DH (1980): The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol 191:1–51. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C (2011): Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 31:10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA (2005): Principles governing auditory cortex connections. Cereb Cortex 15:1804–1814. [DOI] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Lee MC, Kim CS (2001): Deafness: Cross‐modal plasticity and cochlear implants. Nature 409:149–150. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Giraud AL, Kang E, Oh SH, Kang H, Kim CS, Lee DS (2007): Cortical activity at rest predicts cochlear implantation outcome. Cereb Cortex 17:909–917. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN (2006): Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30:718–729. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Maurer D (2005): Multiple sensitive periods in human visual development: Evidence from visually deprived children. Dev Psychobiol 46:163–183. [DOI] [PubMed] [Google Scholar]
- Lieu JE, Tye‐Murray N, Karzon RK, Piccirillo JF (2010): Unilateral hearing loss is associated with worse speech‐language scores in children. Pediatrics 125:e1348–e1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Kessels HW (2014): The developmental stages of synaptic plasticity. J Physiol 592:13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Meredith MA, Kral A (2010): Cross‐modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci 13:1421–1427. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Lomber SG (2011): Somatosensory and visual crossmodal plasticity in the anterior auditory field of early‐deaf cats. Hear Res 280:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR (1990): Auditory brainstem of the ferret: Bilateral cochlear lesions in infancy do not affect the number of neurons projecting from the cochlear nucleus to the inferior colliculus. Dev Brain Res 54:125–130. [DOI] [PubMed] [Google Scholar]
- Moore DR, Kowalchuk NE (1988): Auditory brainstem of the ferret: Effects of unilateral cochlear lesions on cochlear nucleus volume and projections to the inferior colliculus. J Comp Neurol 272:503–515. [DOI] [PubMed] [Google Scholar]
- Moore JK, Linthicum FHJ (2007): The human auditory system: A timeline of development. Int J Audiol 46:460–478. [DOI] [PubMed] [Google Scholar]
- Moore DR, Devlin JT, Raley J, Tunbridge E, Lanary K, Floyer‐Lea A, Narain C, Cohen I, Jezzard P, Burton MJ. (2005) Effects of Long Term Unilateral Hearing Loss on the Lateralization of fMRI Measured Activation in Human Auditory Cortex. Plasticity and Signal Representation in the Auditory System: Springer. pp 335–346.
- Nordeen K, Killackey H, Kitzes L (1983): Ascending auditory projections to the inferior colliculus in the adult gerbil, Meriones unguiculatus . J Comp Neurol 214:144–153. [DOI] [PubMed] [Google Scholar]
- Pantev C, Oostenveld R, Engelien A, Ross B, Roberts LE, Hoke M (1998): Increased auditory cortical representation in musicians. Nature 392:811–814. [DOI] [PubMed] [Google Scholar]
- Penhune V, Zatorre R, MacDonald J, Evans A (1996): Interhemispheric anatomical differences in human primary auditory cortex: Probabilistic mapping and volume measurement from magnetic resonance scans. Cereb Cortex 6:661–672. [DOI] [PubMed] [Google Scholar]
- Peters BR, Litovsky R, Parkinson A, Lake J (2007): Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otol Neurotol 28:649 [DOI] [PubMed] [Google Scholar]
- Petersson KM, Nichols TE, Poline JB, Holmes AP (1999): Statistical limitations in functional neuroimaging II. Signal detection and statistical inference. Philos Trans R Soc Lond B Biol Sci 354:1261–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Alain C, Woods DL, John MS, Scherg M, Valdes‐Sosa P, Bosch‐Bayard J, Trujillo NJ (1999): Intracerebral sources of human auditory‐evoked potentials. Audiol Neurootol 4:64–79. [DOI] [PubMed] [Google Scholar]
- Pisoni, DB , Conway CM, Kronenberger W, Henning S, Anaya E (2010): Executive function, cognitive control, and sequence learning in deaf children with cochlear implants. The Oxford Handbook of Deaf Studies Language and Education 2:439. [Google Scholar]
- Ponton C, Eggermont JJ, Khosla D, Kwong B, Don M (2002): Maturation of human central auditory system activity: Separating auditory evoked potentials by dipole source modeling. Clin Neurophysiol 113:407–420. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ (2001): Of kittens and kids: Altered cortical maturation following profound deafness and cochlear implant use. Audiol Neurotol 6:363–380. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M (2000): Maturation of human central auditory system activity: Evidence from multi‐channel evoked potentials. Clin Neurophysiol 111:220–236. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Vasama JP, Tremblay K, Khosla D, Kwong B, Don M (2001): Plasticity in the adult human central auditory system: Evidence from late‐onset profound unilateral deafness. Hear Res 154:32–44. [DOI] [PubMed] [Google Scholar]
- Popescu MV, Polley DB (2010): Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron 65:718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell RR (1974): Psychological effects of exercise therapy upon institutionalized geriatric mental patients. J Gerontol 29:157–161. [DOI] [PubMed] [Google Scholar]
- Reale RA, Brugge JF, Chan JC (1987): Maps of auditory cortex in cats reared after unilateral cochlear ablation in the neonatal period. Dev Brain Res 34:281–290. [DOI] [PubMed] [Google Scholar]
- Rivera S, Reiss A, Eckert M, Menon V (2005): Developmental changes in mental arithmetic: Evidence for increased functional specialization in the left inferior parietal cortex. Cereb Cortex 15:1779–1790. [DOI] [PubMed] [Google Scholar]
- Salloum CAM, Valero J, Wong DDE, Papsin BC, van Hoesel R, Gordon KA (2010): Lateralization of interimplant timing and level differences in children who use bilateral cochlear implants. Ear Hear 31:441 [DOI] [PubMed] [Google Scholar]
- Sanes DH, Kotak VC (2011): Developmental plasticity of auditory cortical inhibitory synapses. Hear Res 279:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönwiesner M, Rübsamen R, Von Cramon DY (2005): Hemispheric asymmetry for spectral and temporal processing in the human antero‐lateral auditory belt cortex. Eur J Neurosci 22:1521–1528. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ (2002): A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear Hear 23:532–539. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Kral A (2005): The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res 203:134–143. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW (2001): Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci 21:8819–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel MM, Papsin BC, Gordon KA (2015): Binaural fusion and listening effort in children who use bilateral cochlear implants: A psychoacoustic and pupillometric study. PloS One 10:e0117611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH (2009): Developmental hearing loss disrupts synaptic inhibition: Implications for auditory processing. Future Neurol 4:331–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai‐Van H, Cozma S, Boutitie F, Disant F, Truy E, Collet L (2007): The pattern of auditory brainstem response wave V maturation in cochlear‐implanted children. Clin Neurophysiol 118:676–689. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM (2003): Mapping brain asymmetry. Nat Rev Neurosci 4:37–48. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB (2004): Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5:97–107. [DOI] [PubMed] [Google Scholar]
- Vasama JP, Makela JP, Parkkonen L, Hari R (1994): Auditory cortical responses in humans with congenital unilateral conductive hearing loss. Hear Res 78:91–97. [DOI] [PubMed] [Google Scholar]
- Vasama JP, Makela JP, Pyykko I, Hari R (1995): Abrupt unilateral deafness modifies function of human auditory pathways. Neuroreport 6:961–964. [DOI] [PubMed] [Google Scholar]
- Vasama JP, Makela JP (1995): Auditory pathway plasticity in adult humans after unilateral idiopathic sudden sensorineural hearing loss. Hear Res 87:132–140. [DOI] [PubMed] [Google Scholar]
- Van Deun L, Van Wieringen A, Scherf F, Deggouj N, Desloovere C, Offeciers FE, Van de Heyning PH, Dhooge IJ, Wouters J (2009): Earlier intervention leads to better sound localization in children with bilateral cochlear implants. Audiol Neurootol 15:7–17. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, Van Drongelen W, Yuchtman M, Suzuki A (1997): Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. Biomed Eng IEEE Trans 44:867–880. [DOI] [PubMed] [Google Scholar]
- von Stein A, Rappelsberger P, Sarnthein J, Petsche H (1999): Synchronization between temporal and parietal cortex during multimodal object processing in man. Cereb Cortex 9:137–150. [DOI] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C (2008): Template‐O‐Matic: A toolbox for creating customized pediatric templates. NeuroImage 41:903–913. [DOI] [PubMed] [Google Scholar]
- Wong DDE, Gordon KA (2009): Beamformer suppression of cochlear implant artifacts in an electroencephalography dataset. Biomed Eng IEEE Trans 56:2851–2857. [DOI] [PubMed] [Google Scholar]
- Wong DDE, Gordon KA (2010): Hemispheric lateralization of cortical responses in children using bilateral cochlear implants. 33rd Midwinter Research Meeting of the Association for Research in Otolaryngology, Anaheim, CA.
- Wunderlich JL, Cone‐Wesson BK (2006): Maturation of CAEP in infants and children: A review. Hear Res 212:212–223. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone‐Wesson BK, Shepherd R (2006): Maturation of the cortical auditory evoked potential in infants and young children. Hear Res 212:185–202. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P (2001): Spectral and temporal processing in human auditory cortex. Cereb Cortex 11:946–953. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB (2002): Structure and function of auditory cortex: Music and speech. Trend Cogn Sci 6:37–46. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Rebscher S, Harrison W, Sun X, Feng H (2008): Cochlear implants: System design, integration, and evaluation. Biomed Eng IEEE Rev 1:115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]


