Abstract
We investigated the role of the dopamine system [i.e., subcortical‐medial prefrontal cortex (mPFC) network] in dreaming, by studying patients with Parkinson's Disease (PD) as a model of altered dopaminergic transmission. Subcortical volumes and cortical thickness were extracted by 3T‐MR images of 27 PD patients and 27 age‐matched controls, who were asked to fill out a dream diary upon morning awakening for one week. PD patients do not substantially differ from healthy controls with respect to the sleep, dream, and neuroanatomical measures. Multivariate correlational analyses in PD patients show that dopamine agonist dosage is associated to qualitatively impoverished dreams, as expressed by lower bizarreness and lower emotional load values. Visual vividness (VV) of their dream reports positively correlates with volumes of both the amygdalae and with thickness of the left mPFC. Emotional load also positively correlates with hippocampal volume. Beside the replication of our previous finding on the role of subcortical nuclei in dreaming experience of healthy subjects, this represents the first evidence of a specific role of the amygdala‐mPFC dopaminergic network system in dream recall. The association in PD patients between higher dopamine agonist dosages and impoverished dream reports, however, and the significant correlations between VV and mesolimbic regions, however, provide an empirical support to the hypothesis that a dopamine network plays a key role in dream generation. The causal relation is however precluded by the intrinsic limitation of assuming the dopamine agonist dosage as a measure of the hypodopaminergic state in PD. Hum Brain Mapp 37:1136–1147, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: dopamine, mesolimbic dopaminergic system, amygdala, medial prefrontal cortex, hippocampus, dreaming, dream recall, sleep, MRI, VBM
INTRODUCTION
Generation and recall of dream experience is a largely unexplained phenomenon of human existence, and their neural mechanisms still remain mostly unknown. Due to the intrinsic difficulty in accessing directly to dream when it is generated, empirical research has been mainly devoted to the issue of dream recall [De Gennaro et al., 2012; Fagioli, 2002]. However, also the study of dream recall is characterized by specific methodological difficulties due to the lack of a stable correspondence between the physiological scenario in which dream is generated and the time in which dream mentation is collected [De Gennaro et al., 2012]. For these reasons, we have recently proposed to bypass some procedural problems in the study of neural correlates of dream recall and to focus the attention on the neuroanatomical differences associated to measures of dreaming [De Gennaro et al., 2011]. In fact, in the aforementioned study, we have correlated interindividual differences in qualitative and quantitative characteristics of dream mentation to gray matter (GM) volume and microstructural variations in the hippocampus and in the amygdala, as expressed by changes in cellular barriers that restrict the free diffusion of water molecules in tissues (i.e., mean diffusivity). We found a decreased microstructural integrity of the left amygdala, which was related to shorter dream reports and lower emotional load, while that of the right amygdala was related to lower bizarreness. Left amygdala volume was also associated to lower bizarreness. In our view, the relevance of this pioneering finding is twofold. However, it points to two deep GM structures, amygdala and hippocampus, which have been hypothesized having a crucial role respectively in the access and processing of the emotional [Hobson and Pace‐Schott, 2002; Maquet et al., 1996] and mnestic sources [Maquet et al., 1996; Nielsen and Stenstrom, 2005] of dreaming. However, they provide some support to the Reward Activation Model (RAM) for dreaming, according to which activation of the mesolimbic dopaminergic (ML‐DA) reward system during sleep contributes the emotional and motivational content of dreams [Perogamvros and Schwartz, 2012].
One major regulator of the ML‐DA system is the medial prefrontal cortex (mPFC), which makes direct and indirect connections to the hippocampus and amygdala [Patton et al., 2013]. A robust empirical evidence of a role of mPFC in dreaming comes from studies in patients with brain lesions [Solms, 1997], in patients who underwent prefrontal leukotomy [Solms, 2000], and in healthy subjects [Eichenlaub et al., 2014].
Therefore, independent—although few—findings point to a role of amygdala and hippocampus, on one hand, and of mPFC, on the other side, in the experience of dream recall. However, to the best of our knowledge, an empirical support to the idea that a deep GM nuclei‐mPFC network (presumably, the ML‐DA system) might play a key role in dream generation is still lacking.
For these reasons, here we use the brain structural approach [i.e., a high resolution T1‐weighted Magnetic Resonance Imaging (MRI) volumetric investigation] to evaluate the role of the two deep GM structures and mPFC in dream recall under the a priori hypothesis of their association in qualitative (and, secondarily, quantitative) aspects of dreams. To this aim, we have studied patients with Parkinson's Disease (PD) as a model of altered dopaminergic transmission. In fact, PD patients constitute a useful model for studying the neural substrates of dream recall. Even if PD involves multiple neuronal systems [Braak et al., 2003], it is histopathologically characterized by selective, progressive, and chronic degeneration of the nigrostriatal and mesocorticolimbic dopamine systems, and offers an opportunity to study the possible influence of these dopaminergic pathways on dreaming. Our hypothesis posits that the activation of the ML‐DA system during sleep contributes the emotional and motivational content of dreams, and that higher dosages of dopamine agonists (assuming them as a measure of the hypodopaminergic state) should be associated to impoverished dream reports.
MATERIALS AND METHODS
Participants
The study included 27 patients (21 men, 6 women; mean age ± standard error [SE] = 64.8 ± 1.6 years; age range = 46–79 years; education level: 11.0 ± 0.8 years) diagnosed with idiopathic PD according to the international guidelines [Hughes et al., 1992]. Participants were consecutively recruited at a university outpatient service for movement disorders (“Sapienza” University, Sant'Andrea Hospital, Rome, Italy) and were assessed at the Neuropsychiatry Laboratory of the I.R.C.C.S. Santa Lucia Foundation in Rome. All the included PD patients were under stable dopaminergic therapy excepting 3 who were at the very onset of the illness. Treatment informations (type of dopamine agonist, daily dosage, l‐dopa equivalents, time to maximum plasma concentration as well as receptor affinity) are reported in Table 1. All patients were at stage I or II of the disease, except 1 who was at stage III. Furthermore, they were not receiving deep brain stimulation and were not under continuous dopaminergic stimulation by subcutaneous apomorphine or intrajejunal l‐Dopa.
Table 1.
Patients treatment
| Patients (N = 27) | Dopamine agonists (type) | Daily dosage | Daily dopamine agonists l‐dopa equivalents (mg) | Time to maximum plasma concentration Dopamine agonists (h) |
|---|---|---|---|---|
| N = 3 | No | n.a. | n.a. | n.a. |
| N = 2 | Ropinirole | 8 mg × 1/die; 2 mg × 1/die | 250 | 2 h |
| N = 2 | Ropinirole | 5 mg × 3/die | 375 | 2 h |
| N = 2 | Ropinirole | 8 mg × 2/die | 400 | 2 h |
| N = 2 | Ropinirole PR | 8 mg × 1/die | 200 | 6 h |
| N = 2 | Ropinirole PR | 8 mg × 1/die; 4 mg × 1/die | 300 | 6 h |
| N = 2 | Ropinirole PR | 8 mg × 2/die | 400 | 6 h |
| N = 2 | Rotigotine | 2 mg × 1/die | 100 | 12–18 h |
| N = 3 | Rotigotine | 8 mg × 1/die | 400 | 12–18 h |
| N = 4 | Pramipexole | 0,7 mg × 3/die | 300 | 1–3 h |
| N = 3 | Pramipexole ER | 1,05 mg × 1/die 0,26 mg × 1/die | 187,1 | 6–8 h |
n.a.: not applicable; h: hours; Ropinirole PR: ropinirole prolonged release; Pramipexole ER: pramipexole extended release.
Type of dopamine agonist, daily dosage, l‐dopa equivalents, time to maximum plasma concentration as well as receptor affinity for the 27 patients are reported.
Dopamine agonist affinity for specific dopamine receptors:
Ropinirole = is a nonergot dopamine agonist that exhibit highly specific affinity for the cerebral dopamine D3 receptor.
Rotigotine = is a nonergoline agonist of dopamine D1–D3 receptors with ∼15‐fold higher affinity for the D2 receptor than for the D1 receptor.
Pramipexole = is a full agonist at presynaptic and postsynaptic receptors of the dopamine D2 subfamily (which includes D2, D3, and D4 receptors), with highest affinity for the D3 subtype.
As controls, 27 healthy control (HC) subjects were recruited (16 men, 11 women; mean age ± SE = 60.5 ± 2.1 years; age range = 35–74 years; education level: 12.4 ± 0.7 years).
The two groups were not significantly different with respect to sociodemographic variables (gender: χ 2 = 10.05; P = 0.25; age: t52 = 1.61; P = 0.11; education level: t52 = 1.32; P = 0.19). Although not significantly different, age was appropriately controlled when it was susceptible to affect our analyses by considering it as a covariate.
Common inclusion criteria for PD and HC were (1) vision and hearing sufficient for compliance with the testing procedure; (2) Mini Mental State Examination (MMSE) score > 26 [Folstein et al., 1975]; (3) no dementia according to the Movement Disorder Society clinical diagnostic criteria [Emre et al., 2007] using an extensive neuropsychological battery; and (4) suitability for MRI scanning. Common exclusion criteria were (1) presence of major non stabilized medical illnesses (i.e., nonstabilized diabetes, obstructive pulmonary disease or asthma, hematologic/oncologic disorders, vitamin B12 or folate deficiency, pernicious anemia, clinically significant and unstable active gastrointestinal, renal, hepatic, endocrine or cardiovascular disorders and recently treated hypothyroidism); (2) known or suspected history of alcoholism, drug dependence and abuse, head trauma, and mental disorders (apart from mood or anxiety disorders) according to the DSM‐IV TR criteria [American Psychiatric Association, 2000]; (3) presence of vascular brain lesions, brain tumor and/or marked cortical and subcortical atrophy on MRI scan. In particular, the presence, severity, and location of vascular lesions were computed according to the semiautomated method recently published by our group [Iorio et al., 2013]; (4) presence of a REM Behavior Disorder (RBD) in their clinical history. The presence of sleep disorders, as indicated by a >5 score, was assessed by the 19‐item Italian version of the Pittsburgh Sleep Quality Index [Curcio et al., 2013].
Specific exclusion criteria for PD patients were the following: (1) history of neurological diseases other than idiopathic PD and (2) unclear history of chronic dopaminergic treatment responsiveness.
Patients and controls gave their informed written consent to participate in the study, which was approved by the Joint Ethics Committee of the I.R.C.C.S. Santa Lucia Foundation.
Study Design
All subjects who were considered eligible for the study were submitted to a neuropsychological examination and an MRI experimental protocol.
Cognitive evaluation
A trained psychologist, unaware of the study aims and design, conducted the cognitive evaluation of individuals eligible for the study. MMSE was used to obtain a global index of cognitive level, and the Mental Deterioration Battery [Carlesimo et al., 1996] was used to assess single cognitive domains.
Dream collection
Participants were requested to record at home their dreams after each morning awakening by dictating them into a hand‐held tape‐recorder. Subjects were instructed to give an as accurate as possible description of any aspect of the dream experienced and, when more than one dream was recalled, to specify when a distinct dream was going to be reported [Cohen, 1979]. The procedure was repeated for 7 consecutive days. Audio‐recording—instead of writing—was chosen since it provides a more accurate report of dream mentation and a higher compliance [Casagrande and Cortini, 2008].
After dream tape‐recording, a sleep diary was completed, in order to collect subjective estimates of the characteristics of the overall night‐time sleep (sleep latency, sleep duration, and possible occurrence of spontaneous arousal).
MRI protocol
All participants underwent the same imaging protocol, which included 3D T1‐weighted, T2‐weighted and FLAIR sequences using a 3T Allegra MR imager (Siemens, Erlangen, Germany) with a standard quadrature head coil. Whole‐brain T1‐weighted images were obtained in the sagittal plane using a modified driven equilibrium Fourier transform sequence (TE/TR = 2.4/7.92 ms, flip angle 15°, voxel size 1 × 1 × 1 mm3).
Data Analysis
Dream reports
The tape‐recordings were transcribed verbatim into overall daily reports. An expert investigator, unaware of the study design, preliminarily identified the reports of distinct dreams and then pruned the report of each dream of the clauses not related to dream content (e.g., “I'm not sure, but I think …”) or repetitive of contents already encoded. Then two judges, also unaware of the study design, scored independently each dream report according to three 6‐point Likert rating scales of emotional intensity (emotional load, EL), bizarreness (B), and visual vividness (VV) [De Gennaro et al., 2003, 2010, 2011]. The rating scales ranged from 1 (“a very small extent”) to 6 (“a very great extent”). In other words, three distinct scores on 6‐point scales (EL, B, and VV) were attributed to each of the dreams the subjects reported. As in previous studies [De Gennaro et al., 2003; De Gennaro et al., 2011], these ratings were attributed by the two judges regardless of their emotional valence. Hence, EL scores rate the intensity of emotions, without any distinction between positive or negative affects. With respect to the scoring of bizarreness, the judges considered both (1) bizarre elements (improbable or impossible characters, metamorphoses, improbable, or impossible actions/inappropriate roles, improbable or impossible objects) and (2) script bizarreness (physically improbable or impossible plot, logically improbable or impossible plot, plot discontinuity, improbable or impossible settings). VV of each dream report was rated according to the following scores: (1) No image at all (only thinking of the object), (2) Very vague and dim, (3) Less vague, still dim, (4) Moderately clear and vivid, (5) Clear and reasonably vivid, (6) Perfectly clear and vivid as normal vision.
Training of the two judges was based on the database of dream report scores used in a previous study [De Gennaro et al., 2003]. Inter‐rater reliability for each rating scale was very high (K > 0.80), and cases of differences between the two judges were consensually solved.
Dream recall frequency (DRF) was computed as the average number of dreams reported by each subject per night, namely by dividing the total number of recalled dreams per 7 days. Moreover, the mean length of dream report per night was computed by dividing the total number of the words of all the pruned reports by the number of days (7 or less) where subjects were capable to recall one or more dreams (total word count, TWC: [Antrobus, 1983; Stickgold et al., 2001]).
In addition, the mean ratings of the perceptual and emotional characteristics of dreams (EL, B, and VV) were individually averaged as a function of the number of days where subjects were capable to recall one or more dreams. Hence, the dependent variables of the study were: DRF, TWC, EL, B, and VV.
MRI processing
The FreeSurfer imaging analysis suite (v5.1.0, http://surfer.nmr.mgh.harvard.edu/) was used for cortical reconstruction of the whole brain and deep GM structure volume extraction [Dale et al., 1999; Fischl and Dale, 2000]. With this software, the T1‐weighted images were registered to the Talairach space of each participant's brain with the skulls stripped. Images were then segmented into white matter (WM)/GM tissue based on local and neighboring intensities. The cortical surface of each hemisphere was inflated to an average spherical surface to locate both the pial surface and the WM/GM boundary. Preprocessed images were visually inspected before including into subsequent statistical analyses. Any topological defects were excluded from the subsequent analyses. Cortical thickness was measured based on the shortest distance between the pial surface and the GM/WM boundary at each point across the cortical mantle. The regional thickness value at each vertex for each participant, was mapped to the surface of an average spherical surface [Fischl et al., 1999] using automated parcellation in FreeSurfer [Fischl et al., 2004]. For each participant, volumes of the amygdala and the hippocampus of each hemisphere were extracted using automated procedures [Fischl et al., 2002]. In addition, cortical thickness of the mPFC was mapped, through the tkmedit tool of FreeSurfer, at the single‐subject level on the pial surface reconstruction, using the parcellation protocol provided in Ranta et al. [2009]. In particular, the inferior frontal sulcus was used to divide the PFC into superior and inferior areas through the entire PFC [Costafreda et al., 2006], and within the superior section of the PFC, medial portion corresponded to the medial PFC.
Statistical Analysis
Comparisons with healthy subjects
The two groups were compared by means of independent samples statistical analyses on four different sets of dependent variables: sleep measures, dream measures, volumetric measures of subcortical nuclei, and cortical thickness.
With respect to the neuroanatomical measures (i.e., volume of subcortical nuclei and cortical thickness), we preliminarily checked for a possible effect of age. To this aim, we have carried out both ANOVAs and analyses of covariance (ANCOVAs) with age as a covariate on the volumes of the brain ventricles since the CSF volumes are more sensitive to aging [i.e., Pfefferbaum et al., 2013]. ANOVAs and ANCOVAs results showed that age strongly affects between‐group differences (Table S1; Supporting Information). Most differences practically disappear after controlling for age. For this reason, we decided to carry our between‐group comparisons on the neuroanatomical measures by means of ANCOVAs. To correct for multiple comparisons, the Bonferroni correction was applied. Since it is too conservative in the case of correlated outcome variables, the alpha level was adjusted by taking into account the mean correlation between the dependent variables of each set of analyses [Perneger, 1998; Sankoh et al., 1997]. In the case of correlated outcome variables, a corrected alpha is required which is in between no correction at all and full Bonferroni correction. A mean correlation of zero gives a full Bonferroni adjustment, a mean correlation of one no adjustment at all, while for all the other values of the correlation the corrected alpha will be in between the two extremes [Perneger, 1998; Sankoh et al., 1997].
According to this procedure, we computed the mean correlation between the 18 dependent variables regarding volumes of subcortical nuclei (r = 0.43) and between the 48 dependent variables regarding measures of cortical thickness (r = 0.39). Consequently, the alpha level was adjusted to 0.0096 and to 0.0047, respectively.
Data on the correlations between dreams and neuroanatomical measures in the controls (i.e., without any information on the dopaminergic state) will be reported elsewhere as a part of a large ongoing replication and extension study of our previous investigation in healthy subjects [De Gennaro et al., 2011].
Analyses on PD patients
To assess the best MR‐derived anatomical predictors of dream recall, stepwise forward multiple regressions were performed using the measures of dream reports as dependent variables, and the neuroanatomical measures (mPFC thickness, amygdale, and hippocampus volume) and dopamine agonist dosage as independent variables (level of significance P ≤ 0.05). The dose of dopamine agonists was considered as an indirect measure of the dopaminergic state of the mesolimbic system.
Nevertheless, collinearity of interhemispheric measures and relatively small size of our PD sample forced us to perform a separate set of multiple regressions for left and right hemisphere. To this respect, it should be also considered that, except for DRF, measures of dream reports were characterized by a further reduction of sample size, since they had a value only when subjects actually report a dream (i.e., at least one dream/week).
Due to the limited sample size, age and gender were not included directly into the regression equation. Hence, their possible contribution in the relation between neuroanatomical and dream‐recall measures was evaluated by calculating the partial correlations between measures of dream reports and anatomical measures.
The multiple regressions that included the DRF as the dependent variable were carried out one the whole sample (N = 27). The multiple regressions that included TWC, EL, B, and VV as the dependent variables, were carried out by considering only the subjects who were successful in dream recall after one or more morning awakenings (N = 15).
RESULTS
Sociodemographic, clinical, and behavioral characteristics of the PD patients and HCs who participated in the study are summarized in Table 2.
Table 2.
Demographic and clinical variables
| HC subjects (n = 27) | Patients with Parkinson disease (n = 27) | |
|---|---|---|
| Age in years (SE) | 60.5 ± 2.1 | 64.8 ± 1.6 |
| Education in years (SE) | 12.4 (0.7) | 11.0 (0.8) |
| Male/female | 16/11 | 21/6 |
| Unified PD Rating Scale‐III | 16.6 (2.2) | |
| Hoehn and Yahr scale | 1.63 (0.12) | |
| Disease duration in years (SE) | 3.74 (0.39) | |
| LEDD total (mg/day) | 248.1 (53) |
SE: standard error; LEDD: levodopa equivalent daily dose.
Values are listed as mean (SE).
Differences Between Groups
Sleep and dream measures
The results of ANCOVAs on sleep measures with age as a covariate did not indicate any significant difference between groups (Table 3). Confirming the previous results, the effect of the covariate was significant for most of the sleep measures (i.e., total sleep time, number of awakenings, wake after sleep onset, and sleepiness after final awakening). All these effects go in the direction of a worse sleep pattern as age progresses.
Table 3.
Comparisons of the sleep and dream measures between Parkinson Disease patients and HC
| Variables | Covariate (age) | PD | HC | F | d.f. | p |
|---|---|---|---|---|---|---|
| Sleep measures | ||||||
| Sleep latency | 1.12 (0.30) | 16.45 (2.40) | 13.88 (3.58) | 0.12 | 1,51 | 0.73 |
| Total Sleep Time | 7.66 (0.008) | 431.32 (14.7) | 407.11 (7.64) | 0.84 | 1,51 | 0.36 |
| Awakenings (#) | 5.41 (0.02) | 1.53 (0.21) | 1.06 (0.16) | 1.64 | 1,51 | 0.21 |
| Wake after sleep onset | 5.78 (0.02) | 31.65 (6.11) | 22.85 (3.83) | 0.61 | 1,51 | 0.44 |
| Sleepiness after awakening (range = 0–6) | 4.87 (0.03) | 2.94 (0.21) | 2.83 (0.19) | 0.62 | 1,51 | 0.44 |
| Dream measures | ||||||
| Dream recall rate (#) | 0.11 (0.73) | 0.32 (0.08) | 0.36 (0.07) | 0.15 | 1,51 | 0.70 |
| TWC | 0.02 (0.89) | 27.47 (8.11) | 94.25 (28.3) | 3.28 | 1,35 | 0.08 |
| Emotional load | 0.21 (0.65) | 1.67 (0.15) | 2.28 (0.25) | 3.58 | 1,35 | 0.07 |
| Vividness | 1.16 (0.29) | 2.44 (0.30) | 2.38 (0.27) | 0.02 | 1,35 | 0.87 |
| Bizarreness | 0.05 (0.83) | 1.70 (0.21) | 2.09 (0.24) | 1.28 | 1,35 | 0.26 |
Results of the analyses of covariance (ANCOVAs) on sleep and dream measures, comparing Parkinson Disease (PD) patients and HC and considering Age as a covariate. Mean values (and SEs) of each variable are also reported.
Differently, dream measures did not show any significant effect for the covariate or between‐group differences. Although not significant (0.05 > P < 0.10), it should be noted that dream reports of PD patients were shorter and less emotionally loaded. No subject or patient had a nightmare during the participation to the study.
Neuroanatomical measures
ANCOVAs on deep GM structure volumes did not show any significant difference between PD patients and HC. Covariate was indeed significant in most cases (Table S2; Supporting Information). In addition, the measures of cortical thickness did not differ between groups neither for the right (Table S3; Supporting Information) or the left hemisphere (Table S4; Supporting Information). At variance with the deep GM structure volumes, the effect of age (as a covariate) on the measures of cortical thickness was not significant.
Relations Between Dream and Neuroanatomical Measures in PD Patients
As previously described, multiple regressions were performed separately for the right and left hemisphere, using the measures of dream reports as dependent variables, and the neuroanatomical measures and dopamine agonist dosage as independent variables.
The upper panel of Table 4 reports the results for the left hemisphere. The multiple regression coefficient was significant only for VV (F 2,11 = 9.51, P = 0.004), accounting for a large percentage of the variance (Fig. 1). Volume of left amygdala (P = 0.002) and thickness of left mPFC (P = 0.04) significantly entered the regression equation. The semi‐partial correlations were r = 0.71 and r = 0.42, respectively. Although the multiple regression coefficient only approaches to significance (P < 0.10), it should be noted that dopamine agonist dosage (r = −0.52; P = 0.04) and the volume of left hippocampus (r = 0.51; P = 0.05) are significantly related to Emotional Load.
Table 4.
Relations between dream and neuroanatomical measures in PD patients
| Dependent variables | Variables in the equation | Beta | Partial correlation coefficients | Semipartial correlation coefficients | t | P‐level |
|---|---|---|---|---|---|---|
| Left hemisphere | ||||||
| DRF: multiple r = 0.29; F 1,24 = 2.21; P = 0.15 | DA | −0.29 | −0.29 | −0.29 | −1.49 | 0.15 |
| TWC: multiple r = 0.59; F 2,11 = 2.91; P = 0.09 | DA | −0.34 | −0.39 | −0.34 | −1.40 | 0.19 |
| LA | 0.47 | 0.50 | 0.47 | 1.92 | 0.08 | |
| EL: multiple r = 0.59; F 2,11 = 2.99; P = 0.09 | DA | −0.79 | −0.58 | −0.52 | −2.35 | 0.04 |
| LH | 0.71 | 0.54 | 0.51 | 2.12 | 0.05 | |
| B: multiple r = 0.44; F 1,12 = 2.83; P = 0.12 | DA | −0.44 | −0.44 | −0.44 | −1.58 | 0.12 |
| VV: multiple r = 0.80; F 2,11 = 9.51; P = 0.004 | LA | 0.71 | 0.76 | 0.71 | 3.87 | 0.002 |
| LmPFC | 0.42 | 0.57 | 0.42 | 2.32 | 0.04 | |
| Right hemisphere | ||||||
| DRF: multiple r = 0.29; F 1,24 = 2.21; P = 0.15 | DA | −0.29 | −0.29 | −0.29 | −1.49 | 0.15 |
| TWC: multiple r = 0.79; F 3,10 = 5.43; P = 0.02 | DA | −0.47 | −0.55 | −0.40 | −2.07 | 0.06 |
| RH | 0.44 | 0.50 | 0.36 | 1.82 | 0.09 | |
| RA | 0.41 | 0.50 | 0.35 | 1.82 | 0.09 | |
| EL: multiple r = 0.41; F 1,12 = 2.42; P = 0.14 | RA | 0.41 | 0.41 | 0.41 | 1.56 | 0.14 |
| B: multiple r = 0.74; F 3,10 = 2.84; P = 0.04 | DA | −0.54 | −0.42 | −0.31 | −2.17 | 0.05 |
| RH | 0.39 | 0.42 | 0.31 | 1.45 | 0.17 | |
| RA | 0.34 | 0.39 | 0.29 | 1.36 | 0.20 | |
| VV: multiple r = 0.67; F 2,11 = 4.41; P = 0.04 | RA | 0.64 | 0.65 | 0.64 | 2.84 | 0.01 |
| RmPFC | 0.28 | 0.35 | 0.28 | 1.24 | 0.24 | |
Results of the stepwise forward multiple regressions considering measures of dream reports (DRF, TWC, EL, B, and VV) as dependent variables, and hippocampus, amygdala, and medial prefrontal cortex anatomical measures (volume and cortical tickness, respectively). Variables entering the regression equation are reported. Their significant values are in bold when entering a significant multiple regression equation.
The upper and lower parts of the table report results of the multiple regressions for the left and right hemisphere, respectively.
DRF: dream recall frequency; TWC: total word count, EL: emotional load, B: bizarreness, VV: visual vividness; RH: right hippocampus, LH: left hippocampus, RA: right amygdala; LA: left amygdala, RmPFC: right medial prefrontal cortex; LmPFC: left medial prefrontal cortex; DA: dopamine agonists.
Figure 1.
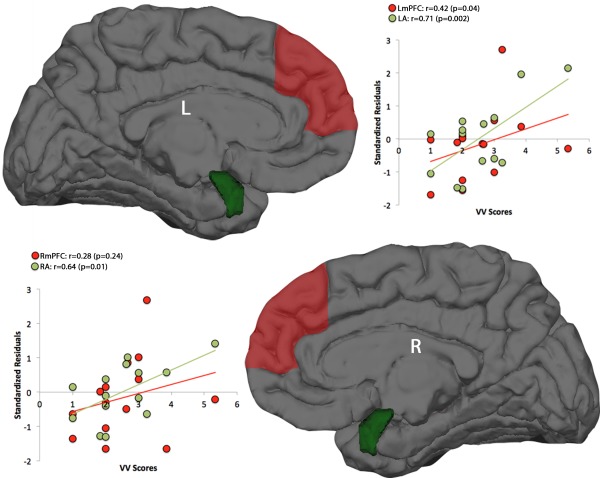
Scatterplots of the significant partial correlations between neuroanatomical measures and VV of dream recall. Upper panel: volumes of the left amygdala (LA) and thickness of the left medial prefrontal cortex (LmPFC) entering the regression equation (in ordinate expressed as standardized residuals) and VV scores (abscissa). Lower panel: volumes of the right amygdala (RA) and thickness of the right medial prefrontal cortex (RmPFC) entering the regression equation and VV scores. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The lower panel of Table 4 reports the results for the right hemisphere. The multiple regression coefficients were significant for TWC, Bizarreness, and VV. Dopamine agonists entered the regression equation significantly or close to significance for B (P = 0.05) and TWC (P = 0.06), respectively. Higher dosages were associated to lower values of TWC and B. Scores of VV (Fig. 1) had the significant contribution of the right amygdala, which showed a semipartial correlation r = 0.64 (P = 0.01). The right mPFC, although entering the regression equation (r = 0.28), was not significant.
As a final check, a possible contribution of age and gender in the relation between neuroanatomical and dream‐recall measures was evaluated by calculating the partial correlations between measures of dream reports and anatomical measures (Table S5; Supporting Information). Results of these further analyses entirely confirmed the results obtained without partialing out age and gender. All the variables significantly entering the regression equations were still significantly associated to dream measures when partialing out for age and gender. Similarly, a possible contribution of disease duration was also evaluated. In addition, in this case, this further variable did not significantly affect the whole pattern of results.
DISCUSSION
This study reports, for the first time, evidence that the mesolimbic dopamine system is involved in specific aspects of dream experience. These functional neuroanatomical correlates of dream recall have been identified in PD patients under a dopaminergic treatment, i.e. in patients with a presumably hypodopaminergic state. The main finding is that VV of dream reports is associated to both the amygdala volumes and the mPFC thickness. While the relation with amygdala involves both hemispheres, only left mPFC shows a significant association with some features of dream mentation. As a further finding, dopamine agonist dosage negatively correlates with some specific qualitative aspects of dream reports, mainly bizarreness and emotional load. In other words, higher dosages of DA agonists, assumed as an expression of a greater hypodopaminergic state, are associated to impoverished dreams. The left hippocampal volume is positively related to emotional load and, although not significantly entering the regression equation, the right hippocampal volume seems positively associated to bizarreness. These findings replicate what we have found in a larger sample of HC with respect to the right hippocampus, while the relation with the left hippocampus was not present in the previous study [De Gennaro et al., 2011].
Representativeness of Studying Dream Reports in PD Patients
Sleep structure of our PD patients, as evaluated by self‐rated measures, does not show any significant difference as compared to age‐matched HC. This confirms a polysomnographic evidence of a lack of significant differences in sleep architecture between early stage PD patients (1.9 years) and age‐matched HC [Diederich et al., 2013]. However, dream measures revealed only small differences, since we have found a not significant (P < 0.10) decrease in length of dream reports and in their emotional load.
Our results on deep GM structures and cortical differences of morphometric measures are quite straightforward. When controlling for age, any difference between PD patients and HC disappears. In fact, previous studies in non‐demented or early stage PD yielded contrasting results. While GM reduction has been reported by some studies mainly in frontal cortex, temporal lobe, or caudate nucleus [Burton et al., 2004; Negano‐Saito et al., 2005; Peran et al., 2010; Summerfield et al., 2005; Tinaz et al., 2011], others did not find significant changes in either cortical or subcortical GM [Feldmann et al., 2008; Messina et al., 2011; Price et al, 2004]. Since age was not partialed out in these comparisons, even in presence of two groups without significant mean differences, this may explain these inconsistent findings.
Summing up, the early stage PD patients selected for the current study do not differ from HC with respect to the sleep, dream and neuroanatomical measures. In our opinion, this should be considered an optimal (unbiased) base to assess the relation between neuroanatomical measures and dream recall under the assumption that dosage of dopamine agonists may be informative of the hypodopaminergic state of these patients.
The Involvement of the Mesolimbic System in Dream Recall
Our findings highlight the role of amygdala and mPFC in mediating specific aspects of dream reports, with also an association between the hypodopaminergic state and qualitative impoverishment of dream reports. With respect to the amygdala, the current finding should be considered a replication and an extension to PD of our study in HC [De Gennaro et al., 2011]. In that study, we found that a decreased microstructural integrity of the left amygdala was related to shorter dream reports and to lower emotional load, while that of the right amygdala was related to lower bizarreness. Left amygdala volume was also associated to lower bizarreness.
In mammals, the amygdala and mPFC are two key structures that play a key role in the acquisition, consolidation, and retrieval of fear memory. These two regions have extensive bidirectional connections and, in recent years, the neural circuits that mediate fear learning and fear extinction are beginning to be elucidated [Marek et al., 2013]. These regions track differential components of contextual fear, with amygdala being more involved in emotional processing and hippocampus in spatial one [Zelikowsky et al., 2014]. In our view, emotional (more than spatial) processing should explain the putative role of ML‐DA system in dreaming experience. Robust empirical evidence of a role of mPFC in dreaming comes from studies in patients with brain lesions. A damage of the mPFC, which includes the white matter surrounding the anterior horns of the lateral ventricles, is—although not invariably—associated to cessation of dreaming [Solms, 1997]. Consistently, a 70–90% of patients who underwent prefrontal leukotomy showed a (nearly) global cessation of dreaming [Solms, 2000]. In these patients, the ventromedial white matter containing dopaminergic projections to the frontal lobe were disconnected as a consequence of prefrontal leukotomy [Bradley et al., 1958]. Two types of “dreaming excess” have been also described [Solms, 1997]. Lesions in the anterior cingulate cortex and the basal forebrain are associated with increased frequency and vividness of dreams and their intrusion into waking life.
Recent measurement of regional cerebral blood flow (rCBF) using [15O]H2O positron emission tomography (PET) in healthy subjects, has shown that low dream‐recallers have lower rCBF in the mPFC during both REM sleep and wakefulness compared to high dream‐recallers [Eichenlaub et al., 2014]. It should be mentioned that single unit recordings in unanesthetized rats across the sleep–wake cycle show that dopamine neurons of the ventral tegmental area are activated during REM sleep, switching to a prominent bursting pattern [Dahan et al., 2007]. More directly, Léna et al. [2005] showed by intracerebral microdialysis in freely moving rats that dopamine concentrations are higher in mPFC during waking and REM sleep compared to slow‐wave sleep.
However, some basic aspects of dream recall (and generation) are probably subserved by different neural systems. Brain lesion studies [Solms, 1997, 2000] and PET study measurement in HCs [Eichenlaub et al., 2014] converge in pointing to the temporoparietal junction (TPJ) as a pivotal area for both the dreaming process and dream recall. The common interpretation on a possible role of TPJ in dreaming brings up a possible role in mediating sensorial aspects of dreams. In fact, what is still lacking is an integrated model of the specific role of the “posterior” system (i.e., TPJ) and the mesolimbic system (mainly, mPFC and amygdala) within the context of dream experience. Some authors [Domhoff, 2011; Pace‐Schott, 2007, 2011] have hypothesized that the neural substrate of dreaming could be a subsystem of the Default Mode Network. The recent PET study by Eichenlaub et al. [2014] has provided some empirical support to this view, although the findings of a crucial role of TPJ and mPFC in dream recall [Eichenlaub et al., 2014] and in wandering of the mind during wakefulness [Fox et al., 2013] can hardly be considered alone a final support to that hypothesis. The presents results provide some support to the RAM for dreaming, according to which the activation of the ML‐DA reward system during sleep contributes the emotional and motivational content of dreams [Perogamvros and Schwartz, 2012].
The Actual Role of Dopaminergic System
Our study was aimed at highlighting the role of ML‐DA system in dreaming by targeting our voxel‐based morphometric measures on mesolimbic regions, and assuming the dosage of dopamine agonists as a measure of the hypodopaminergic state The association between higher dosages and impoverished dream reports, however, and the significant correlations between VV and mesolimbic regions, however, are coherent with a dopaminergic interpretation.
At a first glance, our finding seems inconsistent with some reports of vivid dreams and intensified dreamlike mentation during the course of chronic levodopa treatment [Nausieda et al., 1982; Scharf et al., 1978]. In fact, this relation is quite far from being consolidated. As an example, McLaughlin et al. [2015] recently reported that a dopamine agonist (KB220Z) attenuates lucid nightmares in PTSD patients. Reviewing studies on this issue, we have found that most (if not all) studies reporting an increase of vivid dreams did not systematically evaluate characteristics of dream recall, but described clinic observations or reported percentage of patients showing “vivid dreams” as a symptom [e.g., Pinter et al., 1999; Virmani et al., 2015]. The same studies had detected the presence of RBD in their PD patients, and it is well‐known that vivid dreams and enacting behaviors are pathognomonic of this disorder. Actually, dream enacting behaviors have been found also in untreated PD [Pont‐Sunyer et al., 2015; Prudon et al., 2014; Yang et al., 2014]. Since most reports of more vivid dreams in PD patients seem being due to the presence of RBD, we have excluded patients with a history/diagnosis of RBD.
However, different results have been found when dream recall has been systematically evaluated by using a sleep and dream diary [Bugalho and Paiva, 2011; D′Agostino et al., 2010; Mariotti et al., 2015]. This underlines the importance to have a systematic measure of dreams. As based on intrinsically indirect measures, studies on dreaming should be based on a systematic collection and on an independent scoring/evaluation of the reports. In our opinion, some inconsistencies or discrepancies should be attributed to the methodological aspects of dream collection/analysis.
Limitations to the Study
Our study is based on a relatively small sample. Investigations on facets of dream reports obviously depend on the actual presence of dreams (i.e., at least one dream/week), that greatly affects sample size.
We are aware that current results are intrinsically correlative, without any relation of causality between dopamine agonist administration and dream characteristics. It should be also considered that the amygdala and mPFC are not uniquely related to the ML‐DA. Similarly, it should be considered that dopamine agonists also affect the nigrostriatal system without any possibility of disentangling these different actions. Furthermore, the assumption of dopamine agonist dosage as a measure of the hypodopaminergic state in PD also makes difficult any causal relation.
Finally, we have not included in the exclusion criteria the consumption of any specific medication associated with nightmares [Thompson and Pierce, 1999]. However, our patients (and controls) did not report any nightmare across the whole study.
CONCLUSIONS
This study represents the first evidence of a specific role of ML‐DA system in dream recall. A natural advancement of our study will be the longitudinal evaluation of dream recall in de novo PD patients (i.e., before any treatment with dopamine agonists). A further advancement may be to target the dopaminergic system by PET or single‐photon emission computer tomography by using radiotracers, like [18F]9‐fluoropropyl‐(+)‐dihydrotetrabenazine (18F‐DTBZ; [18F]AV‐133) [Hsiao et al., 2014]. In such way, the direct measurement of dopaminergic degeneration could be associated to dream measures.
Supporting information
Supporting Information
ACKNOWLEDGMENT
The authors thank you Francesco Ernesto Pontieri, Associated Professor of Neurology of Sapienza University of Rome, who helped us in the patient recruitment. They also thank Ginevra Uguccioni for her help in collecting parts of data of PD patients. Conflict of interest: The authors declare no conflict of interest.
REFERENCES
- American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC: Task Force. [Google Scholar]
- Antrobus JS (1983): REM and NREM sleep reports: Comparison of word frequencies by cognitive classes. Psychophysiology 20:562–568. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, De Vos R, Jansen Steur E, Braak E (2003): Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24:197–211. [DOI] [PubMed] [Google Scholar]
- Bradley K, Dax E, Walshi K (1958): Modified leucotomy: Report of 100 cases. Med J Aust 1:133–138. [DOI] [PubMed] [Google Scholar]
- Bugalho P, Paiva T (2011): Dream features in the early stages of Parkinson's Disease. J Neural Transm 118:1613–1619. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT (2004): Cerebral atrophy in Parkinson's disease with and without dementia: A comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain 127:791–800. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Caltagirone C, Gainotti G (1996): The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36:378–384. [DOI] [PubMed] [Google Scholar]
- Casagrande M, Cortini P (2008): Spoken and written dream communication: Differences and methodological aspects. Conscious Cogn 17:145–158. [DOI] [PubMed] [Google Scholar]
- Cohen D. 1979. Sleep and dreaming: Origins, nature and functions. New York: Pergamon Press. [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS (2006): A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Hum Brain Mapp 27:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio G, Tempesta D, Scarlata S, Marzano C, Moroni F, Rossini PM, Ferrara M, De Gennaro L (2013): Validity of the Italian version of the Pittsburgh Sleep Quality Index (PSQI). Neurol Sci 34:511–519. [DOI] [PubMed] [Google Scholar]
- D'Agostino A, De Gaspari D, Antonini A, Kantzas I, Limosani I, Manzone ML, Schiavella M, Paganini L, Siri C, Rizzi P, Scarone S (2010): Cognitive Bizarreness in the Dream and Waking Mentation of Nonpsychotic Patients With Parkinson's Disease. J Neuropsychiatry Clin Neurosci 22:395–400. [DOI] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G (2007): Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology 32:1232–1241. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Cristiani R, Curcio G, Martiradonna V, Bertini M (2003): Alexithymia and dream recall upon spontaneous morning awakening. Psychosom Med 65:301–306. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Marzano C, Moroni F, Curcio G, Ferrara M, Cipolli C (2010): Recovery sleep after sleep deprivation almost completely abolishes dream recall. Behav Brain Res 206:293–298. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Cipolli C, Cherubini A, Assogna F, Cacciari C, Marzano C, Curcio G, Ferrara M, Caltagirone C, Spalletta G (2011): Amygdala and hippocampus volumetry and diffusivity in relation to dreaming. Hum Brain Mapp 32:1458–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Marzano C, Cipolli C, Ferrara M (2012): How we remember the stuff that dreams are made of: Neurobiological approaches to the brain mechanisms of dream recall. Behav Brain Res 226:592–596. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Rufra O, Pieri V, Hipp G, Vaillant M (2013): Lack of polysomnographic Non‐REM sleep changes in early Parkinson's disease. Mov Disord 28:1443–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domhoff GW (2011): The neural substrate for dreaming: is it a subsystem of the default network? Conscious Cogn 20:1163–1174. [DOI] [PubMed] [Google Scholar]
- Eichenlaub JB, Nicolas A, Daltrozzo J, Redouté J, Costes N, Ruby P (2014): Resting brain activity varies with dream recall frequency between subjects. Neuropsychopharmacology 39:1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007): Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 22:1689–1707. [DOI] [PubMed] [Google Scholar]
- Fagioli I (2002): Mental activity during sleep. Sleep Med Rev 6:307–320. [DOI] [PubMed] [Google Scholar]
- Feldmann A, Illes Z, Kosztolanyi P, Illes E, Mike A, Kover F, Balas I, Kovacs N, Nagy F (2008): Morphometric changes of gray matter in Parkinson's disease with depression: A voxel‐based morphometry study. Mov Disord 23:42–46. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM (1999): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Fox KC, Nijeboer S, Solomonova E, Domhoff GW, Christoff K (2013): Dreaming as mind wandering: Evidence from functional neuroimaging and first‐person content reports. Front Hum Neurosci 7:412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA, Pace‐Schott EF (2002): The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci 3:679–693. [DOI] [PubMed] [Google Scholar]
- Hsiao IT, Weng YH, Hsieh CJ, Lin WY, Wey SP, Kung MP, Yen TC, Lu CS, Lin KJ (2014): Correlation of Parkinson disease severity and 18F‐DTBZ positron emission tomography. JAMA Neurol 71:758–766. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992): Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio M, Spalletta G, Chiapponi C, Luccichenti G, Cacciari C, Orfei MD, Caltagirone C, Piras F (2013): White matter hyperintensities segmentation: A new semi‐automated method. Front Aging Neurosci 5:76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léna I, Parrot S, Deschaux O, Muffat‐Joly S, Sauvinet V, Renaud B, Suaud‐Chagny MF, Gottesmann C (2005): Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep–wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res 81:891–899. [DOI] [PubMed] [Google Scholar]
- Maquet P, Péters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G (1996): Functional neuroanatomy of human rapid‐eye‐movement sleep and dreaming. Nature 383:163–166. [DOI] [PubMed] [Google Scholar]
- Marek R, Strobel C, Bredy TW, Sah P (2013): The amygdala and medial prefrontal cortex: Partners in the fear circuit. J Physiol 591:2381–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti P, Quaranta D, Di Giacopo R, Bentivoglio AR, Mazza M, Martini A, Canestri J, Della Marca G (2015): Rapid eye movement sleep behavior disorder: A window on the emotional world of Parkinson disease, a window on dreaming. Sleep 38:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Blum K, Oscar‐Berman M, Febo M, Agan G, Fratantonio JL, Simpatico T, Gold MS (2015): Putative dopamine agonist (KB220Z) attenuates lucid nightmares in PTSD patients: Role of enhanced brain reward functional connectivity and homeostasis redeeming joy. J Behav Addict 4:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina D, Cerasa A, Condino F, Arabia G, Novellino F, Nicoletti G, Salsone M, Morelli M, Lanza PL, Quattrone A (2011): Patterns of brain atrophy in Parkinson's disease, progressive supranuclear palsy and multiple system atrophy. Parkinsonism Relat Disord 17:172–176. [DOI] [PubMed] [Google Scholar]
- Nausieda PA, Weiner WJ, Kaplan LR, Weber S, Klawans HL (1982): Sleep disruption in the course of chronic levodopa therapy: An early feature of the levodopa psychosis. Clin Neuropharmacol 5:183–194. [DOI] [PubMed] [Google Scholar]
- Negano‐Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, Dagher A, Ito K (2005): Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 64:224–229. [DOI] [PubMed] [Google Scholar]
- Nielsen TA, Stenstrom P (2005): What are the memory sources of dreaming? Nature 437:1286–1289. [DOI] [PubMed] [Google Scholar]
- Pace‐Schott EF (2007): The frontal lobes and dreaming In: Barrett D, McNamara P, editors. The New Science of Dreaming. Westport, CT: Praeger, Greenwood Press; p 115–154. [Google Scholar]
- Pace‐Schott EF (2011): The neurobiology of dreaming, 5th ed. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier; p 563–575. [Google Scholar]
- Patton MH, Bizup BT, Grace AA (2013): The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J Neurosci 33:16865–16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peran P, Cherubini A, Assogna F, Piras F, Quattrocchi C, Peppe A, Celsis P, Rascol O, Démonet J‐F, Stefani A, Pierantozzi M, Pontieri FE, Caltagirone C, Spalletta G, Sabatini U (2010): Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain 133:3423–3433. [DOI] [PubMed] [Google Scholar]
- Perneger TV (1998): What is wrong with Bonferroni adjustments. BMJ 136:1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perogamvros L, Schwartz S (2012): The role of the reward system in sleep and dreaming. Neurosci Biobehav 36:1934–1951. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV (2013): Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas‐based parcellation of fMRI. Neuroimage 65:176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter MM, Pogarell O, Oertel WH (1999): Efficacy, safety, and tolerance of the non‐ergoline dopamine agonist pramipexole in the treatment of advanced Parkinson's disease: A double blind, placebo controlled, randomised, multicentre study. J Neurol Neurosurg Psychiatry 66:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont‐Sunyer C, Hotter A, Gaig C, Seppi K, Compta Y, Katzenschlager R, Mas N, Hofeneder D, Brücke T, Bayés A, Wenzel K, Infante J, Zach H, Pirker W, Posada IJ, Alvarez R, Ispierto L, De Fàbregues O, Callén A, Palasi A, Aguilar M, Marti MJ, Valldeoriola F, Salamero M, Poewe W Tolosa E (2015): The Onset of Nonmotor Symptoms in Parkinson's disease (The ONSET PD Study). Mov Disord 30:229–237. [DOI] [PubMed] [Google Scholar]
- Price S, Paviour D, Scahill R, Stevens J, Rossor M, Lees A, Fox N (2004): Voxel‐based morphometry detects patterns of atrophy that help differentiate progressive supranuclear palsy and Parkinson's disease. Neuroimage 23:663–669. [DOI] [PubMed] [Google Scholar]
- Prudon B, Duncan GW, Khoo TK, Yarnall AJ, Burn DJ, Anderson KN (2014): Primary sleep disorder prevalence in patients with newly diagnosed Parkinson's disease. Mov Disord 29:259–262. [DOI] [PubMed] [Google Scholar]
- Ranta ME, Crocetti D, Clauss JA, Kraut MA, Mostofsky SH, Kaufmann WE (2009): Manual MRI parcellation of the frontal lobe. Psychiatry Res 172:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD (1997): Some comments on frequently used multiple endpoint adjustments methods in clinical trials. Stat Med 16:2529–2542. [DOI] [PubMed] [Google Scholar]
- Scharf B, Moskowitz C, Lupton M, Klawans H (1978): Dream phenomena induced by chronic Levodopa therapy. J Neural Transm 43:143–151. [DOI] [PubMed] [Google Scholar]
- Solms M. 1997. The neuropsychology of dreams: A clinico‐anatomical study. Hillsdale: Erlbaum. [DOI] [PubMed] [Google Scholar]
- Solms M (2000): Dreaming and REM sleep are controlled by different brain mechanisms. Behav Brain Sci 23:843–850. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Maila A, Fosse R, Hobson JA (2001): Brain‐mind states: I. Longitudinal field study of sleep/wake factors influencing mentation report length. Sleep 24:171–179. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Junque C, Tolosa E, Salgado‐Pineda P, Gómez‐Ansón B, Marti MJ, Pastor P, Ramírez‐Ruíz B, Mercader J (2005): Structural brain changes in Parkinson disease with dementia: A voxel‐based morphometry study. Arch Neurol 62:281–285. [DOI] [PubMed] [Google Scholar]
- Thompson DF, Pierce DR (1999): Drug‐induced nightmares. Ann Pharmacother 33:93–98. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Courtney MG, Stern CE (2011): Focal cortical and subcortical atrophy in early Parkinson's disease. Mov Disord 26:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani T, Moskowitz CB, Vonsattel JP, Fahn S (2015): Clinicopathological characteristics of freezing of gait in autopsy‐confirmed Parkinson's disease. Mov Disord. doi: 10.1002/mds.26346. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Kim YE, Yun JY, Kim HJ, Jeon BS (2014): Identifying the clusters within nonmotor manifestations in early Parkinson's disease by using unsupervised cluster analysis. PloS One 9:e91906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS (2014): Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J Neurosci 34:8462–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


