Abstract
The astounding capacity for the human imagination to be engaged across a wide range of contexts is limitless and fundamental to our day‐to‐day experiences. Although processes of imagination are central to human psychological function, they rarely occupy center stage in academic discourse or empirical study within psychological and neuroscientific realms. The aim of this paper is to tackle this imbalance by drawing together the multitudinous facets of imagination within a common framework. The processes fall into one of five categories depending on whether they are characterized as involving perceptual/motor related mental imagery, intentionality or recollective processing, novel combinatorial or generative processing, exceptional phenomenology in the aesthetic response, or altered psychological states which range from commonplace to dysfunctional. These proposed categories are defined on the basis of theoretical ideas from philosophy as well as empirical evidence from neuroscience. By synthesizing the findings across these domains of imagination, this novel five‐part or quinquepartite classification of the human imagination aids in systematizing, and thereby abets, our understanding of the workings and neural foundations of the human imagination. It would serve as a blueprint to direct further advances in the field of imagination while also promoting crosstalk with reference to stimulus‐oriented facets of information processing. A biologically and ecologically valid psychology is one that seeks to explain fundamental aspects of human nature. Given the ubiquitous nature of the imaginative operations in our daily lives, there can be little doubt that these quintessential aspects of the mind should be central to the discussion. Hum Brain Mapp 37:4197–4211, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: imagination, brain networks, creativity, aesthetics, intentionality, imagery
INTRODUCTION
“All human accomplishment has this same origin, identically. Imagination is a force of nature.” — Saul Bellow, Henderson the Rain King
Ever since the inception of the approach, cognitive psychology (and subsequently cognitive neuroscience) has been dominated by the S–O–R model where the central idea is that stimuli within the environment are perceived by an organism who makes sense of this information and generates appropriate responses on the basis of the contingencies of the situation at hand and prior knowledge. Although the representational model in cognitive psychology is still the most influential in relating psychological function to brain function, the usefulness of this model is being increasingly questioned. Some call for an overhaul of its central assumptions while others seek to deemphasize or accentuate the focus of one or the other aspect of this neat cycle, which is typically instantiated in terms of information processing computations. The credo of predictive coding models [Clark, 2013; Grossberg, 2009], for instance, is that thought and action systems are characterized by the drive to predict effectively and efficiently. Embodied cognition advocates stress the central role of the body and its interaction with the information‐rich environment [Kiverstein and Miller, 2015; Wilson and Golonka, 2013], whereas the evolution within different aspects of the system that unfold over time are central to dynamical systems models [Beer, 2000; Gelfand and Engelhart, 2012]. These are certainly exciting times to be a cognitive psychologist or neuroscientist, particularly if one's principal focus is in the domains of perception and action.
There is, however, a glaring omission from these discussions that seek to characterize the overarching principles of the mind. An almost exclusive focus on the cycle of stimulus‐oriented thought and behavior has meant that the dynamics underlying spontaneous, stimulus‐independent and imaginative aspects of the mind, or indeed how they relate to the former, are largely overlooked [Christoff, 2012] or only discussed in a highly circumscribed manner. While such omissions are not due to any form of intentional snubbing but are a consequence of following the prevailing traditions and dogmas espoused within the discipline, the time has come to genuinely consider how our engagement in relatively tricky, sketchy, and esoteric realms of imagination fit with dominant views of how the mind works. A biologically and ecologically valid psychology can only be one that seeks to explain fundamental aspects of human nature, and there can be little doubt that imaginative aspects of the mind should be central to the discussion.
WHY DO WE NEED TO CONSIDER THE IMAGINATION AS A WHOLE?
A key criticism that is regularly leveled at experimental psychologists is the patent lack of ecological validity for most part; that laboratory‐based contexts do not accurately reflect the complex contingencies within the real world of the phenomenon that is being assessed. One can take this point much further though. What proportion of actual everyday psychological experience is being tested in empirical work? Just take the case of the kind of responses that are recorded as data and analyzed to evaluate psychological function. In day‐to‐day activities, responses are rarely required with an immediacy of seconds, and are, more often than not, non‐binary or qualitative. Response type and speed is also highly situation‐specific. For instance, when I receive a text message with the following instruction: “Call me when you get this!” my actions are not automatically prompted by the information within that sentence alone. How and when I choose to respond depends on a number of factors, such as who sent the message, my relationship with the person, prior experience, and resulting expectations, the physical context that I am in at the time, my current state of mind, and my personality disposition. The chosen outcome is informed by the dynamic and complex interplay between these factors.
The situation is further complicated when considering the fact that our minds are constantly occupied even when no response is required, and that the content of our contemplations is not necessarily related to the information that is presently coming through our senses. This is empirically supported by investigations of spontaneous cognition using retrospective thought sampling questionnaires. Within rest periods during an experiment where participants passively fixate on a centrally presented cross and there is neither a task to attend nor a response to prepare, participants report thinking about the stimuli they just encountered only 10% of the time. Instead, their minds engage in free and active internal mentation of the past, the future, non‐temporal aspects of the world, and so on [Andrews‐Hanna et al., 2010].
One of the critical questions to address is the type and extent of the overlap between imaginative and non‐imaginative aspects of perceptual, cognitive and behavioral function. How do neurocognitive models fare in explaining psychological function where the focus on S and R aspects of the S–O–R cycle is diminished? What are the keys to understanding the emergence of self‐propagating aspects of O? How does the essentially receptive–predictive cycle of the brain give rise to open‐ended imaginative thinking?
Such questions may raise a more fundamental issue of whether the disciplines of psychology and neuroscience should concern themselves with “non‐task specific” mental activities at all. And the answer would depend on the motivations of scientists in question. If the aim to understand the fundamental nature of human experience, then the answer would be in the affirmative, regardless of the substantial challenges involved in doing so. It is also worth noting that non‐task specific mental activities—in terms of spontaneous thought, stimulus‐independent thought, task‐unrelated thought, daydreaming or mind wandering—are already discussed within the psychological and neuroscientific literature as reflecting operations of imagination [e.g., Christoff, 2012; Giambra, 1995; Mason et al., 2007; Zedelius and Schooler, 2015]. This is paralleled by rather broad notions in the philosophical tradition about the processes of imagination, where “to imagine something is to form a particular sort of mental representation of that thing” [Gendler, 2013].
Indeed, much of the work on task‐unrelated mental activities is discussed with explicit reference to imagination relevant processes, such as imagery [e.g., TUIT: task‐unrelated imagery and thought in Giambra, 1995]. Others have pointed out the spontaneous and inward‐directed nature of task‐unrelated mental activities such that they occur when the “automatic activation of a personally relevant, but task‐unrelated, goal has temporarily drawn our attention away from the primary task” [Smallwood and Schooler, 2006]. Moreover, the fact that we now have abundant evidence to show that there is a substantial overlap between the neural networks and information processing mechanisms associated with such undirected or spontaneous facets of imagination and directed or deliberate facets of imagination [Schacter, 2012a; Smallwood et al., 2011; Stawarczyk and D'Argembeau, 2015] is indicative of the necessity to consider both sides of imagination in relation to one another.
The mere challenge of the enterprise should not be the reason to shy away from engaging with the topic of imagination head on. There are several examples of complex and central facets of the human experience (e.g., language, memory, consciousness, etc.) that have benefitted a great deal from having structured frameworks and classifications which aid us in getting our heads around the phenomenon in question. They are vital in being able to build and test hypotheses, and are hence necessary for progress to be made in any field. And indeed, often the findings show how the early ideas and frameworks fell short, and modifications or elaborations are made as a result (e.g., the Atkinson–Shiffrin memory model, Piaget's stages of cognitive development, etc.). It is, therefore, enormously useful to have appropriate terminology, a common understanding of the usage of that terminology and related concepts, and a structured framework to help comprehend the complexity. At present, there are several disparate ideas afloat on different aspects of imagination with very little crosstalk between the domains. The aim of this paper is to outline a theoretically and empirically informed novel framework that will help integrate these different strands in a meaningful manner with the hope that it will help promote seamless information flow, constructive discourse, and progress in the field.
CARVING IMAGINATION AT THE JOINTS: HINTS FROM PHILOSOPHY
Although the imagination has not figured prominently on the radar of psychologists and neuroscientists, the same cannot be said of other academic traditions. Philosophers have grappled with trying to understand the imagination for centuries, and there is a general consensus that the phenomenon is too broad to allow for a comprehensive definition or an exhaustive classification of its different facets [Gendler, 2013]. A nominal description of imagination from dictionaries of the English language is that it reflects the representation of conceptual content in the absence of external input. While this explanation may resonate with our folk notions of imagination, it is still quite unspecific. For instance, conceptual information in the form of rules can be actively maintained in working memory without being presently perceived through the senses.
Within the Stanford Encyclopedia of Philosophy, the definition given is that “to imagine something is to form a particular sort of mental representation of that thing” where imagining is seen as distinct from mental states such as perceiving, remembering, believing, desiring, anticipating, conceiving, and supposing [Gendler, 2013]. From the psychological and neuroscientific domains though, such distinctions do not appear to be tenable because, as will be explored in more detail subsequent sections, there is abundant evidence showing that remembering and conceiving are acts of imagination in that they impinge on specific declarative memory operations that involve construction or simulation [Buckner, 2010; Mullally and Maguire, 2013; Schacter et al., 2012].
In an attempt to provide an all‐inclusive yet pithy definition, Nigel J. T. Thomas stated that, “Imagination is what makes our sensory experience meaningful, enabling us to interpret and make sense of it, whether from a conventional perspective or from a fresh, original, individual one. It is what makes perception more than the mere physical stimulation of sense organs. It also produces mental imagery, visual and otherwise, which is what makes it possible for us to think outside the confines of our present perceptual reality, to consider memories of the past and possibilities for the future, and to weigh alternatives against one another. Thus, imagination makes possible all our thinking about what is, what has been, and, perhaps most important, what might be” [as cited in Manu, 2006]. This definition also has the problem of being very wide. However, its detail and comprehensiveness renders it to have utility in terms of serving as an anchor in the development of theoretical frameworks from which to understand imagination from psychological and neuroscientific perspectives as it taps many different realms of imagination [Abraham and Bubic, 2015]. The usefulness of this definition also lies in the fact that it highlights one of the central features of imagination—that this faculty allows us to contemplate matters beyond the immediate present.
Several taxonomies have been put forward to differentiate between aspects of imagination. These include spontaneous versus deliberate imagining, solitary versus social imagining, and sensory versus recreative versus creative imagining [Currie and Ravenscroft, 2002; Walton, 1990]. Each of these groupings emphasize specific distinguishing factors—the level of volition entailed in the directedness of the process, the involvement of a collective, and the perceptual or recollective or novel combinatorial nature of the process, respectively. Such dual and triple classifications, although non‐exhaustive, are also effective aids in the structuring and categorization of the complex mass of neuroscientific literature on imagination.
To date, the most comprehensive classification of imagination was undertaken by Leslie Stevenson who outlined 12 types or conceptions [Stevenson, 2003]. These include fundamental faculties, such as the ability to form mental images or conceive of anything at all, as well as thinking of something that is spatio‐temporally possible or real but not currently perceived. The ability to form beliefs, make non‐rational causal inferences, and conceive of some things as fictional while others are real is distinguished from the liability to believe something unreal to be real. Finally, the sensuous experience when appreciating works of art, beauty and expressions that reveal the true meaning of life, and the creation of such works to evoke such sensuous phenomenology are also given their due in this conceptualization. These conceptions are of a descriptive nature and their distinctions are not directly indicative of operationalized mechanisms. But, just as with the aforementioned categorizations, these more detailed characterizations, which I informally allocated above into three general groupings of sensory‐based, intentionality‐based, and phenomenology‐based distinctions, are also valuable as they allow us to determine the comprehensiveness of any information processing framework that is applied to understand the imagination.
As a final point in this section, it is worth noting that contemporary philosophical discourse on the imagination mainly centers around three domains [Gendler, 2013]: (a) the phenomenology and cognitive architecture of imagination, (b) aesthetics and imaginative engagement in fiction, and (c) how the ability to imagine and conceive shape possibility from the perspective of modal epistemology. Of these, the bulk of the investigations on the neuroscience of imagination can be said to address issues which are of relevance to the first domain (A)—correspondences between imagination and other mental states, understanding of self and others, representations of past and future events, and so on. Quite distinct from these is the focus on brain mechanisms underlying aesthetic engagement (B) or of hypothetical forms of reasoning (C), which have received substantially less focus within the neuroscience of imagination. This nexus between the philosophical and neuroscientific domains serves as a starting point for developing a common framework. I expand on these theoretical–empirical parallels within the framework by also incorporating the many aforementioned distinctions of how phenomenon relevant to the human imagination are construed (and investigated). This novel information processing framework of imagination will be outlined in the following section.
CARVING IMAGINATION AT THE JOINTS: HINTS FROM COGNITIVE NEUROSCIENCE
One commonality that is noticeable in the empirical literature on the diverse fields of imagination is that the classifications employed to indicate information processing distinctions when characterizing different processes of imagination are essentially the same as those used when describing non‐imaginative aspects of psychological function: top‐down versus bottom‐up, implicit versus explicit, intrinsic versus extrinsic, spontaneous versus deliberate, automatic versus controlled, global versus local. Nonetheless, although neuroscientific research is actively carried out in many different fields of imagination, there is little theoretical or empirical crosstalk between the domains. When there is dialogue between fields, it is mainly limited to contexts where a high degree of correspondence can be found across domains either in the resulting findings (e.g., engagement of similar brain regions) or in the theoretical rationale guiding the expectations (e.g., brain regions involved in perception overlap with those involved in imagination).
To help navigate through the major discoveries within neuroscience of imagination, a new framework will be rolled out over the next few sections. The many research domains will be allocated to five different categories based loosely on the philosophical ideas which were presented in the previous section regarding the classifications or types of imagination (Fig. 1), and their correspondences in terms of brain function (Fig. 2) which are described in detail below. These categories are labeled: (i) mental imagery‐based imagination, (ii) intentionality‐based imagination, (iii) novel combinatorial‐based imagination, (iv) phenomenology‐based imagination, and (v) altered states of imagination.
Figure 1.
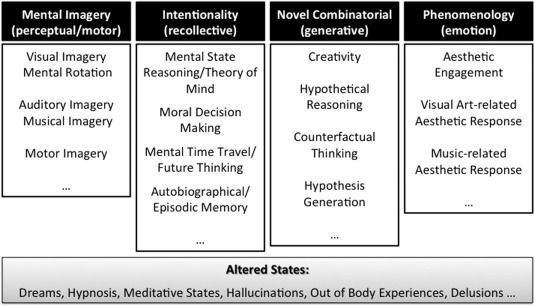
A schematic diagram of the five‐part or quinquepartite classification of operations relevant to the human imagination that have been categorized on the basis of similarities in their underlying putative neurocognitive mechanisms.
Figure 2.
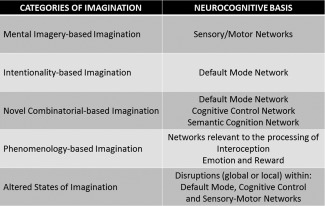
A generalized summary of the neurocognitive basis of each of the five categories of imagination.
Mental Imagery‐Based Imagination (Perceptual/Motor)
With regard to the kind of phenomena covered by the term imagination, much of the focus in the tradition of philosophy has been on the “quasi‐perceptual experience” of mental imagery [Thomas, 2014]. The founding theorists of modern day empirical psychology, such as William James and Gustav Fechner, who were steeped in traditions of philosophy, did give serious thought to understanding the difference between imagination and perception [James, 1891]. Among the many points of discourse were individual differences in imagination, types of mental images (visual, auditory, touch, motor), and how after‐images differ from imagination‐images.
In comparison to other aspects of imagination, the domain of mental imagery has received abundant attention in the post‐behaviorist era within psychology and neuroscience. Critical debates that have dominated this field, such as those concerning the format of mental representations [Pearson and Kosslyn, 2015; Pylyshyn, 2002], fuel much of the research impetus. One of the crucial issues has been to identify whether the brain regions that are involved in sensory perception or motion generation are also involved in the mental imagery of these states. Indeed considerable evidence lends support to this idea [Pearson et al., 2015]. For instance, perceiving or imagining single letters resulted in brain activity within early and late visual processing areas in the occipital and temporal lobes, indicating their involvement in both visual perception and visual imagery [Stokes et al., 2009]. Even among individuals who are fully blind from birth, mental imagery has been shown to activate the primary and secondary visual cortices [Lambert et al., 2004; Striem‐Amit et al., 2012].
Complementary findings of the overlap between perception and imagery have been also reported in the auditory domain for simple auditory features, music, language, and complex nonverbal sounds [Hubbard, 2010], as well as in the motor domain in the form of mental simulations of actions [Hétu et al., 2013]. For instance, both hearing or imagining complex nonverbal sounds led to activations in the secondary auditory cortex [Bunzeck et al., 2005]. There is also evidence for training‐specific effects on imagery. Musically trained participants outperform musically naïve counterparts on musical and nonmusical tasks of auditory imagery, but not visual imagery [Aleman et al., 2000]. Indeed, in a magnetoencephalography (MEG) study where musicians and non‐musicians imagined familiar melodies and then indicated whether a presented tone correctly continued the melody, incorrect tones led to an imagery mismatch negativity (MMN), which is a brain response indicating violation of an established rule, but only in musicians [Herholz et al., 2009].
Within the motor domain, regions of the superior parietal lobule and premotor cortex have been shown to be engaged under conditions of executed reaching, observed reaching and imagined reaching [Filimon et al., 2007]. The pattern of motor imagery related brain engagement is also highly specific. For instance, the type of activation in relation to different effectors (arm, hand, mouth) along the premotor cortex corresponds to the somatotopic organization of the motor cortex for movement of those effectors [Wolfensteller et al., 2007]. Evidence also indicates that intentional facets of action are coded in the posterior parietal cortex as revealed by motor imagery of action goals in people who cannot actually move their limbs, such as in tetraplegic paralysis [Aflalo et al., 2015].
Other approaches that are informative in the context of mental imagery include investigations on cross‐modal facets of perception and imagery in terms of multisensory perception [Berger and Ehrsson, 2014] and sensory substitution in perception [Poirier et al., 2007]. Indeed, one attempt to differentiate modality‐specific from modality‐independent aspects of the imagery brain systems revealed that visual and auditory association cortices are engaged during mental imagery in a modality‐specific fashion whereas the modality‐independent “core” imagery network corresponds to the Default Mode Network (DMN) [Daselaar et al., 2010]. The DMN is comprised of brain areas that are strongly engaged under conditions of rest and spontaneous cognition [Andrews‐Hanna, 2012], and the significance of its role in imagination will be explored in more detail within the next subsection.
So the behavioral and neuroscientific evidence across sensory–motor domains supports the notion of a functional overlap between the neural substrates involved in perception/action and imagery of the same (Fig. 2). The literature also illustrates the inherent flexibility and plasticity within brain systems with regard to mental imagery as the engagement of brain regions is differentially modulated as a function of training (e.g., musicians) and mode of environmental sampling (e.g., congenital blindness).
Intentionality‐Based Imagination (Recollective/Social)
The neuroscientific approach to understanding psychological function faces abundant criticism from all quarters, with some questioning the very usefulness of this approach in delivering concrete answers about perception, cognition, emotion or action. For instance, one major criticism has been the inability of neuroimaging studies to deliver unanimous verdicts on competing theories that offer the best explanation for some facet of psychological function [Coltheart, 2006, 2013]. There is some push back that engages with such issues [Poldrack, 2006], but what is rarely reflected upon or given its due is how neuroimaging often allows us to discover commonalities in the underlying information processing mechanisms of aspects of psychological function that are not usually considered in relation to one another [Mather et al., 2013].
An outstanding example of this is in the case of processes of imagination such as autobiographical and episodic memory (e.g., reminiscing about my first day of primary school), episodic future thinking (e.g., imagining what my next birthday will be like), mental state reasoning or theory of mind (e.g., making inferences about what someone else is thinking about), self‐referential thinking (e.g., evaluating my own thoughts and behavior), and moral reasoning (e.g., gauging the permissibility of my own or someone else's action). What these operations have in common is that all of them engage core regions of the DMN, which include the medial prefrontal cortex (ventral and dorsal aspects), medial parietal cortex (retrosplenial and posterior cingulate cortices), anterior lateral temporal cortex, inferior parietal cortex (including the temporoparietal junction), and medial temporal lobe structures like the hippocampal formation [Andrews‐Hanna et al., 2014; Buckner et al., 2008; Mullally and Maguire, 2013; Schacter et al., 2012; Spreng et al., 2009].
The role of the DMN has been widely documented in association with literature that shows its consistent engagement during stimulus‐independent or spontaneous cognition, which automatically occurs under conditions of rest or low cognitive demand. Participants in fact report active internal mentation within such situations which take the form of thinking about their past, their future, and so on [Andrews‐Hanna et al., 2010]. So there is evidence of considerable overlap in the brain networks involved in diverse aspects of imagination, such as contemplating events that could unfold in one's future or evaluating another person's intention in a specific situation, regardless of whether these emerge as a result of spontaneous cognition or directed cognition.
In the early days of uncovering the functional profile associated with the DMN, another dominant idea about the role of the DMN was that it was engaged during “stimulus‐oriented thought” where the brain is primed toward the potential for encountering task relevant information [Gilbert et al., 2007]. No published study has directly tested for these competing alternatives within a single experiment, and other hypotheses have been proposed in the interim, such as, for instance, that the DMN is driven by significant revisions of cognitive context, regardless of whether the context is externally or internally focused [Crittenden et al., 2015].
The bulk of the studies reporting non‐task specific mental activities engaging the DMN interpret their findings in relation to the stimulus‐independent thought framework. But the adaptive nature of this internal mentation has also been given its due. The literature on prospection or future thinking, for instance, refers to imagining and simulating future scenarios and possibilities [Andrews‐Hanna et al., 2010; Bubić and Abraham, 2014; Buckner, 2010; Suddendorf and Corballis, 2007]. These would nonetheless be classified non‐task specific or stimulus independent because the “task” or rather the “possibility” being prepared for or being simulated is neither well‐defined nor directly related to the task at hand. Giambra [1995] held that task unrelated mentation was instantiated as directing thought “away from the current situation” which are nonetheless reflective of an individual's current concerns. Smallwood and Schooler [2006] in fact stated that “mind wandering can be seen as a goal‐driven process, albeit one that is not directed toward the primary task.”
Several proposals have been put forward to address the functional role and/or outline a common metric that can account for the involvement of the DMN (in whole or part) in this diverse array of mental operations. These include self‐projection [Buckner and Carroll, 2007], mental scene construction [Hassabis and Maguire, 2009], constructive simulation [Schacter, 2012b], proactive associative processing [Bar, 2007], and evaluative processing [Legrand and Ruby, 2009]. Interestingly, some evidence indicates that these may not be mutually exclusive hypotheses, and that varying functional roles may be differentially undertaken by distinct components of the same circuitry. For instance, Kurczek et al. [2015] reported that compared with healthy matched control participants, neurological patients with lesions of the medial prefrontal cortex showed impairments in self‐referential evaluation but not self‐projection, whereas patients with lesions of the medial temporal lobe showed the opposite pattern in that they were impaired in self‐projection but not self‐referential thinking [Kurczek et al., 2015].
When considering the commonalities between these different forms of imagination, it appears that the contexts that are evoked in each of these situations are distinctly “social” in that they involve having to make appraisals of, reason about, or evaluate actions and events that involve one or more entities. Although it may therefore seem reasonable to refer to this category of imagination as social, it might not be entirely accurate to do so. After all, as the landmark Heider and Simmel study as well as subsequent investigations on the attribution of causality and apparent behavior clearly demonstrate, we ascribe mental states and personality traits to non‐entities as well [Bloom and Veres, 1999; Heider and Simmel, 1944; Scholl and Tremoulet, 2000]. Such findings should force us to consider the larger question of what is “social” about social information if it cannot be fully defined in terms of entity‐hood, conspecifics and other biological features, or categories of social groups. For the present purpose of developing classifications of the operations of imagination, the notion of intentionality in relation to the “intentional stance” [Dennett, 1987] may provide a more viable and representative label for this category.
In this seminal work, Dennett [1987] distinguished between three stances that are in play (implicitly) in our minds as we evaluate events that come to pass around us. The physical stance is the one that is applied when the events in question can be explained by the actions of the physical forces in the world (e.g., A pencil that rolls off a table will fall down to the floor). This is distinguished from the design stance, which is applied when an occurrence can be accounted for in terms of the manner in which things are designed for a specific function (e.g., A thermostat automatically switches on and off to regulate the temperature in a room). For any happening that cannot be explained either in terms of the physical stance or the design stance, the intentional stance is automatically applied. Here the events are interpreted in a manner that is intentional or goal‐directed (e.g., If the reader has reached this point in the article, it must mean that s/he is interested in the ideas presented within. No purely physical phenomenon, such as wind, can account for the turning of the pages, and the pages were not designed to turn on their own).
So the operations of imagination discussed within this section (mental state reasoning, episodic future thinking, etc.) are classified into the intentionality‐based category of imagination as they trigger processing that is predominantly recollective in nature with a view to establishing the best possible explanation of a situation or event in question. This is brought about by means of spontaneous access to an extensive and diverse repertoire of relevant knowledge when processing such contexts. The best or most plausible explanation is the one that fits best with what is already known in terms of oneself and/or one's world‐view. The brain network that is most consistently implicated in intentionality‐based imagination is the DMN (Fig. 2). Indeed, a recent article even advocated that the DMN actually “primes the intentional stance” [Spunt et al., 2015].
Novel Combinatorial‐Based Imagination (Counterfactual/Creative/Generative)
“A work of art is never complete, merely abandoned,” is a quote that is commonly attributed to Leonardo Da Vinci. The essence of what is conveyed in this statement hints at elements which are intrinsic to the processes of imagination that fall into this third category, such as novelty, open‐endedness, discovery, and generativity. When our powers of imagination are focused beyond the “what was” and “what is” and extends to the “what if” or “what might be,” the possibility space that we explore is considerably wider, and this is true across the domains of art, science, and commerce. What would happen to the world if the earth stopped rotating? How can watermelons, parsnips and mustard be combined to make a tasty meal? What new strategy could I devise for my team to be able to challenge our stronger competitors? These contexts are relatively open‐ended in one or more aspects of the problem solving/exploration process as they involve journeying within the possibility space to go beyond the status quo, and necessitate combining or evaluating existing knowledge in novel ways. Such situations therefore call for “novel combinatorial thinking” as they necessitate counterfactual reasoning, hypothesis generation, creativity, or hypothetical reasoning during problem solving/exploration.
When presented with a question or a problem to be resolved, the question exploration or problem solving process typically involves searching for ideas, solutions, strategies or plans. The process therefore requires getting from the problem (initial‐state) to the solution (goal‐state) via a specified course of action (operations‐state). Everyday problem solving, regardless of whether it involves the acquisition of new skills (e.g., learning to ice‐skate) or carrying out familiar actions (e.g., watering plants in a garden), is marked by well‐defined initial‐states and goal‐states as well as relatively logical and incremental courses of action in the operations‐state. In the case of novel‐combinatorial aspects of imagination though, one or more of these states during problem exploration is unknown or is relatively open‐ended, and involves more degrees of freedom.
Two examples from the domain of creativity can be taken to illustrate the specifics of such differences. Both tasks have clearly defined initial‐states but they differ greatly in terms of their goal‐states. When asked to invent as many uses as possible for a common object (e.g., newspaper), the problem‐solving scenario faced by a participant is open‐ended in terms of the goal‐state as the numerous potential uses/responses can be generated (divergent creativity task: Alternate Uses Task). In contrast, when asked to find a fourth word which forms a compound associate with three given words (e.g., nuclear/feud/album), the goal‐state is less open‐ended as the number of potential solutions/responses is limited (solution: family) (convergent creativity task: Remote Associates Test). But both are considered to be tasks that assess creative thinking because the possibility space being explored within the operations‐states is relatively open‐ended and necessitates non‐linear combinations of information to arrive at the solution by undergoing perspective shifts, changing mental sets, or overcoming functional fixedness [Abraham and Windmann, 2007].
A critical point to note at this juncture is that novelty in this context does not merge ex nihilo or from nothing. To borrow the words of Stein [1953], who is recognized as being the first to have articulated the currently accepted definition of creativity [Runco and Jaeger, 2012], it “… arises from a reintegration of already existing materials or knowledge, but when it is completed it contains elements that are new” [Stein, 1953]. This has parallels with the idea of novelty as arising from the blending or “bisociation” of two or more unrelated thought matrices to engender a new matrix of meaning [Koestler, 1969].
Operations that belong to the novel‐combinatorial category of imagination include creativity in problem solving and expression, divergent thinking, counterfactual reasoning, hypothesis generation, and hypothetical reasoning [Abraham and Bubic, 2015]. In stark contrast to the relatively consistent picture that emerges from investigations of intentionality‐based forms of imagination, the literature on novel combinatorial‐based imagination is far more heterogeneous and complicated. This is because it has received less attention from the empirical realm, and there is virtually no discourse between the sub‐domains. So it is exceedingly challenging to infer and present a comprehensive picture from the disparate findings in the literature. Nonetheless, a tentative case will be made for functional commonalities based on coherent patterns that emerge across the research areas within this category.
In the most general terms, creativity is defined as the capacity to generate responses that are both original (novel, unique, statistically rare) and appropriate (fitting, relevant, meaningful) to a particular end [Runco and Jaeger, 2012; Stein, 1953]. This definition of creativity applies to all categories of human endeavor regardless of whether it refers to creativity in service of problem solving, such as in scientific and applied domains, or creativity in service of expression, as in the fine and performing arts. The observed commonality across these spheres is that spontaneous and open‐ended production of ideas/responses (idea generation), creative or otherwise, leads to the engagement of parts of the DMN, particularly in the medial aspects of the prefrontal and frontopolar cortex. When constraints of ensuring relevance or appropriateness are applied to the generated responses (idea selection) in order to be deemed truly creative, semantic and cognitive control brain networks are activated, particularly lateral aspects of the prefrontal and frontopolar cortex [Abraham, 2014; Abraham et al., 2012; Beaty et al., 2015; Jung et al., 2013; Limb and Braun, 2008].
That patterns of brain engagement vary in terms of the degree to which the possibility space in the operations‐state is constrained can also be gleaned from other types of hypothetical thinking that call for novel‐combinatorial cognition, such as hypothesis generation and imagining fictional scenarios, as well as counterfactual and hypothetical reasoning. What seems to be the case is that when the possibility space that is being explored is, relatively speaking, more open‐ended (or less constrained), there is greater activity in medial prefrontal regions and other parts of the DMN. And, conversely, that when the possibility space that is being explored is, relatively speaking, less open‐ended (or more constrained), there is greater activity in lateral prefrontal regions and other parts of the semantic and cognitive control brain networks.
For instance, across verbal and non‐verbal domains, hypothesis generation (e.g., can a word be made out of the letters IKFEN?) commonly engages the ventral lateral prefrontal cortex [Goel and Vartanian, 2005; Vartanian and Goel, 2005]. Similar brain regions are selectively activated when evaluating whether scenarios containing fictional characters could occur in our reality as we know it (e.g., Is it possible to speak to Cinderella?) compared with those involving real entities (e.g., Is it possible to speak to George Bush?) [Abraham et al., 2008b]. The possibility space in such contexts is more constrained (or less open‐ended) as the degrees of freedom in what is being explored is narrowly limited to a few tangible options. Moreover, the goal‐states here involve reaching solutions or responses that can be deemed to be objectively correct.
In contrast, situations that call for counterfactual or hypothetical reasoning, which necessitate relatively wider and more open‐ended sampling and integration of information in the possibility space, consistently engage DMN regions such as the medial prefrontal cortex [Abraham et al., 2008a; Van Hoeck et al., 2013] and the hippocampus [Hassabis et al., 2007]. The mental operations targeted here include episodic counterfactual thinking (e.g., If I had left the office earlier, I would not have missed my train), semantic future thinking (e.g., Is it likely that Sydney will have a Disneyland in 50 years?), and imagining new visuospatial scenes. The goal‐states involve reaching solutions or responses that can only be deemed to be subjectively true or likely.
As the DMN is engaged in both novel combinatorial‐based and intentionality‐based imagination, its role needs further clarification. Both types of imagination entail the involuntary or spontaneous access to extensive and heterogeneous sources of knowledge with the goal of generating explanations/ideas/hypotheses in relation to a situation or event. The more open the possibility space or the wider the net that is cast to sample information needed to reach these explanations/ideas/hypotheses, the stronger the engagement of the DMN. The difference between the two appears to lie at the level of explanation/outcome. In keeping with the essentially receptive‐predictive cycle of human brain function [Bubic et al., 2010], the best possible explanation in intentionality‐based forms of imagination is one that fits best or offers the path of least resistance. The opposite is true in the case of novel‐combinatorial based imagination where the situation calls for either overriding the prepotent response or taking account of previously unconsidered perspectives—and this necessitates the added recruitment of non‐DMN networks.
So a complex interplay between the default mode, semantic cognition and cognitive control networks novel orchestrate novel combinatorial‐based facets of imagination (Fig. 2), and one of the metrics to consider when characterizing this system appears to be the degree of constraints/open‐endedness within different aspects of the problem solving/exploration process. This is a tentative hypothesis, but it is one that can be readily and systematically investigated.
Phenomenology‐Based Imagination (Aesthetic Engagement)
In stating that “the principle of true art is not to portray but to evoke,” the novelist Jerzy Kosinsky points to an essential feature that is common to all art forms—that a work of art is designed to elicit a specific response or set of responses. But what is curious about the elicited aesthetic response upon appreciating a work of art is that it is not a unidimensional reaction that one experiences. Instead, we undergo states of complex sensuous phenomenology that are subjective and cannot be fully explained by the sensory features of the object alone.
The focus within the psychology and neuroscience of aesthetics has largely been on the aesthetics of art, particularly visual art and music, although it is clear that aesthetic experiences also occur in non‐artifact based contexts [Palmer et al., 2013]. There are several ideas concerning the fundamentals of the aesthetic response, in terms of aesthetic experience, which is held to reflect an exceptional state of mind, as well as aesthetic preference, which is a judgment of beauty. These are regarded as relatively distinct components as art expertise significantly influences this latter more cognitive component of aesthetic appreciation, but not the affective experience of the same [Van Paasschen et al., 2015]. Within aesthetic experience itself, Marković [2012] distinguishes between three components: (a) aesthetic fascination as evidenced by high levels of arousal, absorption in attentional focus and a sense of loss of time, (b) aesthetic appraisal or cognitive engagement which allows one to transcend generic uses of meaning, and (c) aesthetic emotions which give rise to feelings of unity and connectedness with the object of aesthetic fascination and appraisal [Marković, 2012]. Aesthetic emotions are distinguished from non‐aesthetic or everyday emotions in that they are elicited when experiencing the aesthetic object, but do not serve utilitarian or homeostatic ends despite being associated with non‐action‐oriented bodily responses, such as pilorection and tears [Scherer, 2005].
Of the many theories that have been put forward to explain the aesthetic response, some aim to account for specific components of the response (e.g., mere exposure effect, arousal dynamics, prototype theory and fluency theory), while others chalk out stages of information processing that underlie aesthetic appreciation more comprehensively (e.g., Shimamura's I‐SKE theory, Silvia's appraisal theory, Parson's cognitive developmental account, and Ognjenović's three stage theory) [Marković, 2012; Palmer et al., 2013]. For instance, the most influential theories that have been formulated to explain the neuroaesthetics of visual art and music are both multistage models where aesthetic experience is held to be orchestrated by a distributed set of neural networks devoted to different aspects of perceptual, cognitive and emotional information processing [Chatterjee and Vartanian, 2014; Leder and Nadal, 2014].
Two meta‐analyses have been carried out so far on the findings of functional neuroimaging studies that have investigated the brain correlates of the aesthetic response. The one brain structure that was commonly engaged in aesthetic appreciation—even across sensory modalities—was the anterior insula [Brown et al., 2011; Vartanian and Skov, 2014], a region which plays a key role in interoceptive awareness and in the processing of emotions [Barrett et al., 2007; Wiens, 2005; Zaki et al., 2012]. In fact, a recent study of the brain response during dynamic emotional experiences when hearing an audio narrative revealed the anterior insula to be the neural hub where interoceptive states of awareness are integrated with exteroceptive representations of emotional salience [Nguyen et al., 2016].
This association between aesthetic experience and interoceptive awareness is often extended within the literature on neuroaesthetics to interpret the findings as reflecting reward processing in the brain, particularly when there is accompanying brain activity within the basal ganglia. This is because features such as symmetry, harmony, fluency and good gestalt are conventionally associated with beauty and positive affect in aesthetic appreciation. Much evidence points to a general human preference for symmetrical or prototypical stimuli which has been ascribed to the fact that such forms are easier (or less cognitive demanding) to process than their counterparts [fluency: Reber et al., 2004]. Lower information processing costs also explain why stimuli that have been repeatedly seen before are evaluated as more pleasant [mere exposure effect: Zajonc, 1968].
Equating aesthetic appreciation solely with the experience of pleasure or “positive emotions” is, however, not without its problems. For one thing, there appears to be a general conflation of the concept of positive emotion with that of reward or positive reinforcement. It is also undeniable that aesthetic appraisal not only elicits positive emotions (e.g., pleasure) but also negative and other complex emotions (e.g., anger, confusion, regret, shame, and so on) [Silvia, 2009]. Moreover, there are innumerable examples of high art that one can readily call to mind where convention is thrown to the wind and characteristics like symmetry and fluency are purposefully obliterated to create a perceptual challenge. Such works are nonetheless also experienced to be deeply aesthetically satisfying. Indeed, the importance of negative affect in the aesthetic response is unmistakable as artists often design their works to violate predictions that have been built up.
The prediction error account of aesthetic appreciation does a handy job of accommodating seemingly counterintuitive processes that are play during aesthetic appreciation [Van de Cruys and Wagemans, 2011]. The central idea is that great works of art are designed such that expectations are generated and then destroyed at optimal points. The recipient of such works experiences ambiguity at first, but this feeling dissipates as meaning is derived by constructing a novel pattern, which comes about by forging new associations between the few conceptual hooks that have been provided. This process involves generating new meaning on a different level, and the success at piecing together this novel gestalt leads to a reduction in ambiguity and uncertainty, alongside a corresponding reward effect from having “solved the puzzle.” This has also been referred to as the “aesthetic aha” [Muth and Carbon, 2013]. Indeed, that aesthetic experiences are self‐rewarding as opposed to goal‐directed is considered to be one of its defining features [Apter, 1984]. Some neuropharmacological evidence to support this idea comes from a PET study of music listening where the nucleus accumbens, a brain structure which is central to the reward system, was strongly engaged during the experience of peak emotional responses to music accompanied by endogenous dopamine release in the striatum, which also occurred during peak emotional arousal [Salimpoor et al., 2011].
So the general conclusion from literature on aesthetic appreciation is that the brain regions corresponding to the information processing neural circuits that underlie interoception, emotion, and reward processing are involved in facilitating phenomenological aspects of aesthetic engagement (Fig. 2). And that the feeling of “reward” or positive reinforcement that accompanies aesthetic appreciation can occur through two different routes; (a) via resonance through ease of access, and (b) via success at the discovery of a novel perspective, association or insight. As a caveat, it is worth keeping in mind that the field of neuroaesthetics has largely focused on the neural response that accompanies the evaluation of classical aesthetic categories, that is, judgments of aesthetic works as being beautiful, sublime or pleasant. As other universal aesthetic categories [e.g., “interesting”—Ngai, 2012] have not received much focus, a question that is yet to be explored is how current neurocognitive models of the aesthetic response apply in the context of alternative categories.
Altered States of Imagination
This final category of imagination covers a range of heterogeneous states—some of which are functional or standard in that everyone must experience such states (dreaming) or can attempt to experience such states (hypnosis, meditation, use of psychedelic drugs), while others are decidedly dysfunctional or exceptional in that only a subset of the population undergo such experiences (e.g., hallucinations, delusions, confabulation, out‐of‐body experiences). Inferring consistent patterns from the diverse literature on these topics is tricky because such states are more challenging to investigate and are less well studied. Nonetheless, as these refer to altered states of imagination, it will be possible to draw on insights from the previously outlined domains of imagination to provide a useful context from which to better understand the information processing mechanisms underlying such states.
Dreaming, which is associated with stages of REM (rapid eye movement) sleep, is a state of imagination that each of us experiences regularly. Neuroimaging and EEG studies in this domain can be divided into those studying the brain correlates of dreaming relative to those investigating dream recall. Findings from the former in situ approach as well as the latter retrospective approach indicate that parts of the brain's DMN, such as the medial prefrontal, temporo‐parietal and hippocampal regions, which are involved in intentionality‐based imagination, are more strongly associated with dreaming while cognitive control regions, such as the lateral prefrontal cortex, are deactivated [De Gennaro et al., 2012; Fox et al., 2013; Hobson et al., 1998; Maquet, 2000; Maquet et al., 1996; Nir and Tononi, 2010].
In contrast, the opposite pattern of brain engagement occurs under conditions of hypnosis as these are accompanied by heightened activity in lateral prefrontal cognitive control regions alongside the deactivation of DMN regions [Deeley et al., 2012; Oakley and Halligan, 2009; Vuilleumier, 2014]. This dissociation between the neural correlates of dreaming and hypnosis is reflective of the fact that the former state is spontaneous and involuntarily elicited, whereas the latter state is deliberate and directed in nature. Meditative states, on the other hand, involve the interplay between spontaneous and deliberate components as both operations are integral to the process of meditation, and are differentially called upon depending on the type of meditative technique being applied [Brewer et al., 2011; Hasenkamp et al., 2012; Lutz et al., 2008; Manna et al., 2010; Tang et al., 2015].
The intake of psychedelics, such as lysergic acid diethylamide (LSD), psilocybin and ketamine, also give rise to temporary altered states of imagination and result in reduced connectivity between regions of the DMN, such as the medial prefrontal cortex and the posterior cingulate cortex. While the DMN is normally inversely coupled with “task positive” networks such as the executive or cognitive control network, the consumption of hallucinogens reverses this pattern such that there is heightened connectivity between the DMN and other task‐positive networks. Brain networks that orchestrate dissimilar functions become undifferentiated under such conditions leading to disorganized brain states and unconstrained cognition [Carhart‐Harris et al., 2014; Gallimore, 2015; Tagliazucchi et al., 2014; Vollenweider and Kometer, 2010].
Some have proposed that the psychedelic model of temporary brain disorganization can be taken as a model for systemic brain disorganization as seen in the case of psychosis which is often associated with phenomenon such as hallucinations and delusions [Carhart‐Harris et al., 2013; Corlett et al., 2007]. Hallucinations refer to the experience of perception in the absence of stimuli. They predominantly occur in the auditory and visual domains and are associated with corresponding sensory modality specific brain activity [Allen et al., 2008; Weiss and Heckers, 1999]. The brain basis of this phenomenon is held to result from reduced activity in brain networks that regulate top‐down control via cognitive monitoring and attentional inhibition [Ford et al., 2012; Hugdahl, 2015; Shine et al., 2014; Waters et al., 2014]. The issues of personal relevance and social significance have also been highlighted in association with hallucinations in psychosis, especially given the contributions of DMN brain regions [Bell, 2013; Waters et al., 2014]. One among these is the temporo‐parietal junction (TPJ). Lesions to this brain region leads to out‐of‐body experiences where the body is falsely perceived as being visuo‐spatially removed from its habitual location [Blanke and Arzy, 2005]. Indeed, focal stimulation of the TPJ can induce the experience of an illusory person who closely shadows the posture of the one being stimulated [Arzy et al., 2006].
Another relevant DMN region in this context is the ventral aspect of the medial prefrontal cortex and adjoining areas of the orbitofrontal cortex. Lesions to this region are associated with the phenomenon of confabulation or false memories, particularly of the spontaneous variety, which emerge unprovoked and the elaborations of which can reach fantastical proportions [Gilboa et al., 2006; Glowinski et al., 2008; Schnider, 2003]. Although its bears several similarities to the phenomenon of delusions, confabulations reflect “pseudo‐reminisces” whereas delusions are false beliefs that are held with conviction and associated with a high degree of fixation and preoccupation [Gilboa, 2010; Kopelman, 2010].
There is enormous heterogeneity associated with the presentation of delusions, which are dubbed polythematic when they occur across a range of unrelated topics, and monothematic when they revolve around a common theme [Coltheart et al., 2011]. Recent proposals about the brain basis of delusions have postulated that they are the result of severe disruptions to the receptive‐predictive cycle of the brain that are facilitated through frontostriatal loops. This manifests as “a failure to optimize uncertainty about sensory information” and leads to lower precision in prediction coding and prediction errors [Corlett et al., 2010; Picard and Friston, 2014]. Although there are some concerns about the extent to which this predictive error theory can comprehensively explain the exceedingly complex phenomenon of delusions [Griffiths et al., 2014; Sass and Byrom, 2015], it is a compelling framework that accounts for how such vivid distortions of belief inference are formed and maintained.
In summary, this last category of altered states of imagination is reflective of one of the twelve conceptualizations by Stevenson [2003], which was described as “the liability to think of something that the subject believes to be real, but which is not real.” In doing so, they essentially reflect deficient or erroneous “reality testing” [Gerrans, 2014] that comes about through local or global disruptions within the DMN, cognitive control and sensory‐motor brain networks.
CONCLUDING REMARKS
When outlining the categories of imagination in the previous sections, the operative word that I did not use before but do so now is “predominant.” These categories are not to be seen as mutually exclusive. Indeed it would be expected that most contexts of imagination would involve a dynamic interplay between processes belonging to the different categories. Both intentionality‐based and novel combinatorial‐based processes of imagination are necessarily re‐constructive in nature, but intentionality‐based or recollective processes of imagination will be more strongly drawn upon when the context involves piecing together information from existing knowledge in order to find the best explanation, expression or solution. In contrast, novel combinatorial processes of imagination will be more actively recruited when the context involves moving beyond existing knowledge to find new explanations, expressions or solutions. By the same token, mental imagery will be expected to accompany all other aspects of imagination to a lesser or greater degree depending on the demands of the context.
An overview of the current level of knowledge on the neuroscience of the human imagination has been provided in this paper. It should be readily apparent that while some aspects of imagination are relatively well studied (e.g., imagery), others are less so (e.g., altered states). It is therefore to be expected that the investigation of less explored fields will reveal several other factors as well as relations between factors that must be taken into account in order to suitably characterize the complex nature of the human imagination and its accompanying brain correlates.
This article represents a first attempt at synthesizing what is known thus far about the myriad facets of the human imagination across a variety of perspectives. It can be characterized as a product of marrying together the gist of dominant philosophical ideas on the imagination with paths of investigation and functional correspondences within neuroscience. In doing so, a theoretical framework using a five‐part or quinquepartite classification has been outlined which should serve to systematize, and thereby abet, our understanding of the workings of the human imagination from a neurocognitive standpoint (Figs. 1 and 2). Within this framework, processes relevant to the human imagination can be allocated to the categories of mental imagery‐based imagination, intentionality‐based imagination, novel combinatorial‐based imagination, phenomenology‐based imagination, and altered states of imagination.
This preliminary framework can be used to evaluate and integrate new developments and corresponding insights that stem from future investigations in this field as well as serve as a guide in inferring the fundamental principles by which the human imagination emerges in all its complexity. Without active discourse and dedicated empirical work involving an extensive cross‐disciplinary cohort of theoreticians and researchers, progress on understanding these rich and quintessential aspects of our daily mental life can only continue at the pace of a slow trickle. Scholarship on the human imagination has been delegated to waiting in the wings for far too long. It is time to bring it center stage.
ACKNOWLEDGMENTS
I am very grateful to Harry Ballan, Andreja Bubic and Kata Pauly‐Takacs for their feedback on this paper.
REFERENCES
- Abraham A (2014): Creative thinking as orchestrated by semantic processing vs. cognitive control brain networks. Front Human Neurosci 8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Bubic A (2015): Semantic memory as the root of imagination. Cogn Sci 6:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Windmann S (2007): Creative cognition: The diverse operations and the prospect of applying a cognitive neuroscience perspective. Methods (San Diego, Calif.) 42:38–48. [DOI] [PubMed] [Google Scholar]
- Abraham A, Schubotz RI, von Cramon DY (2008a): Thinking about the future versus the past in personal and non‐personal contexts. Brain Res 1233:106–119. [DOI] [PubMed] [Google Scholar]
- Abraham A, von Cramon DY, Schubotz RI (2008b): Meeting George Bush versus meeting Cinderella: The neural response when telling apart what is real from what is fictional in the context of our reality. J Cogn Neurosci 20:965–976. [DOI] [PubMed] [Google Scholar]
- Abraham A, Pieritz K, Thybusch K, Rutter B, Kröger S, Schweckendiek J, Stark R, Windmann S, Hermann C (2012): Creativity and the brain: Uncovering the neural signature of conceptual expansion. Neuropsychologia 50:1906–1917. [DOI] [PubMed] [Google Scholar]
- Aflalo T, Kellis S, Klaes C, Lee B, Shi Y, Pejsa K, Shanfield K, Hayes‐Jackson S, Aisen M, Heck C, Liu C, Andersen RA (2015): Neurophysiology. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science (New York, N.Y.) 348:906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A, Nieuwenstein MR, Böcker KB, de Haan EH (2000): Music training and mental imagery ability. Neuropsychologia 38:1664–1668. [DOI] [PubMed] [Google Scholar]
- Allen P, Larøi F, McGuire PK, Aleman A (2008): The hallucinating brain: A review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev 32:175–191. [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna JR (2012): The brain's default network and its adaptive role in internal mentation. Neuroscientist 18:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Huang C, Buckner RL (2010): Evidence for the default network's role in spontaneous cognition. J Neurophysiol 104:322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Smallwood J, Spreng RN (2014): The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter MJ (1984): Reversal theory, cognitive synergy and the arts In Crozier W. R. and Chapman A. J., editors. Advances in Psychology, Vol. 19 Amsterdam: North‐Holland: pp 411–426. [Google Scholar]
- Arzy S, Seeck M, Ortigue S, Spinelli L, Blanke O (2006): Induction of an illusory shadow person. Nature 443:287. [DOI] [PubMed] [Google Scholar]
- Bar M (2007): The proactive brain: Using analogies and associations to generate predictions. Trends Cogn Sci 11:280–289. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ (2007): The experience of emotion. Annu Rev Psychol 58:373–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Barry Kaufman S, Silvia PJ (2015): Default and executive network coupling supports creative idea production. Sci Rep 5:10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer RD (2000): Dynamical approaches to cognitive science. Trends Cogn Sci 4:91–99. [DOI] [PubMed] [Google Scholar]
- Bell V (2013): A community of one: Social cognition and auditory verbal hallucinations. PLoS Biol 11:e1001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger CC, Ehrsson HH (2014): The fusion of mental imagery and sensation in the temporal association cortex. J Neurosci 34:13684–13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Arzy S (2005): The out‐of‐body experience: Disturbed self‐processing at the temporo‐parietal junction. Neuroscientist 11:16–24. [DOI] [PubMed] [Google Scholar]
- Bloom P, Veres C (1999): The perceived intentionality of groups. Cognition 71:B1–B9. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H (2011): Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci U S A 108:20254–20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Gao X, Tisdelle L, Eickhoff SB, Liotti M (2011): Naturalizing aesthetics: Brain areas for aesthetic appraisal across sensory modalities. NeuroImage 58:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubić A, Abraham A (2014): Neurocognitive bases of future oriented cognition. Rev Psychol 21:3–15. [Google Scholar]
- Bubic A, von Cramon DY, Schubotz RI (2010): Prediction, cognition and the brain. Front Human Neurosci 4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL (2010): The role of the hippocampus in prediction and imagination. Annu Rev Psychol 61:27–48. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC (2007): Self‐projection and the brain. Trends Cogn Sci 11:49–57. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Wuestenberg T, Lutz K, Heinze HJ, Jancke L (2005): Scanning silence: Mental imagery of complex sounds. NeuroImage 26:1119–1127. [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Leech R, Erritzoe D, Williams TM, Stone JM, Evans J, DJ Sharp, A Feilding, RG Wise, Nutt DJ (2013): Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull 39:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, Chialvo DR, Nutt D (2014): The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Human Neurosci 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Vartanian O (2014): Neuroaesthetics. Trends Cogn Sci 18:370–375. [DOI] [PubMed] [Google Scholar]
- Christoff K (2012): Undirected thought: Neural determinants and correlates. Brain Res 1428:51–59. [DOI] [PubMed] [Google Scholar]
- Clark A (2013): Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci 36:181–204. [DOI] [PubMed] [Google Scholar]
- Coltheart M (2006): What has functional neuroimaging told us about the mind (so far)? Cortex 42:323–331. [DOI] [PubMed] [Google Scholar]
- Coltheart M (2013): How Can Functional Neuroimaging Inform Cognitive Theories? Perspect Psychol Sci 8:98–103. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Langdon R, McKay R (2011): Delusional belief. Annu Rev Psychol 62:271–298. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Honey GD, Fletcher PC (2007): From prediction error to psychosis: Ketamine as a pharmacological model of delusions. J Psychopharmacol (Oxford, England) 21:238–252. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Taylor JR, Wang XJ, Fletcher PC, Krystal JH (2010): Toward a neurobiology of delusions. Prog Neurobiol 92:345–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden BM, Mitchell DJ, Duncan J (2015): Recruitment of the default mode network during a demanding act of executive control. eLife 4:e06481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G, Ravenscroft I (2002): Recreative Minds: Imagination in Philosophy and Psychology. Oxford: Clarendon Press. [Google Scholar]
- Daselaar SM, Porat Y, Huijbers W, Pennartz CMA (2010): Modality‐specific and modality‐independent components of the human imagery system. NeuroImage 52:677–685. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Marzano C, Cipolli C, Ferrara M (2012): How we remember the stuff that dreams are made of: Neurobiological approaches to the brain mechanisms of dream recall. Behav Brain Res 226:592–596. [DOI] [PubMed] [Google Scholar]
- Deeley Q, Oakley DA, Toone B, Giampietro V, Brammer MJ, Williams SCR, Halligan PW (2012): Modulating the default mode network using hypnosis. Int J Clin Exp Hypn 60:206–228. [DOI] [PubMed] [Google Scholar]
- Dennett DC (1987): The Intentional Stance. Cambridge, Mass: MIT Press. [Google Scholar]
- Filimon F, Nelson JD, Hagler DJ, Sereno MI (2007): Human cortical representations for reaching: Mirror neurons for execution, observation, and imagery. NeuroImage 37:1315–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Dierks T, Fisher DJ, Herrmann CS, Hubl D, Kindler J, T Koenig, DH Mathalon, KM Spencer, W Strik, van Lutterveld R (2012): Neurophysiological studies of auditory verbal hallucinations. Schizophr Bull 38:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KCR, Nijeboer S, Solomonova E, Domhoff GW, Christoff K (2013): Dreaming as mind wandering: Evidence from functional neuroimaging and first‐person content reports. Front Human Neurosci 7:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore AR (2015): Restructuring consciousness –the psychedelic state in light of integrated information theory. Front Human Neurosci 9:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand LA, Engelhart S (2012): Dynamical systems theory in psychology: Assistance for the lay reader is required. Front Psychol 3:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler T (2013): Imagination In: Zalta EN, editor. The Stanford Encyclopedia of Philosophy (Fall 2013). Retrieved from http://plato.stanford.edu/archives/fall2013/entries/imagination/ [Google Scholar]
- Gerrans P (2014): Pathologies of hyperfamiliarity in dreams, delusions and déjà vu. Front Psychol 5:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambra LM (1995): A laboratory method for investigating influences on switching attention to task‐unrelated imagery and thought. Conscious Cogn 4:1–21. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW (2007): Comment on “Wandering minds: The default network and stimulus‐independent thought.” Science (New York, N.Y.) 317:43. [DOI] [PubMed] [Google Scholar]
- Gilboa A (2010): Strategic retrieval, confabulations, and delusions: Theory and data. Cogn Neuropsychiatry 15:145–180. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Alain C, Stuss DT, Melo B, Miller S, Moscovitch M (2006): Mechanisms of spontaneous confabulations: A strategic retrieval account. Brain: J Neurol 129:1399–1414. [DOI] [PubMed] [Google Scholar]
- Glowinski R, Payman V, Frencham K (2008): Confabulation: A spontaneous and fantastic review. Aust N Zeal J Psychiatry 42:932–940. [DOI] [PubMed] [Google Scholar]
- Goel V, Vartanian O (2005): Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set‐shift problems. Cerebr Cortex (New York, N.Y.: 1991) 15:1170–1177. [DOI] [PubMed] [Google Scholar]
- Griffiths O, Langdon R, Le Pelley ME, Coltheart M (2014): Delusions and prediction error: Re‐examining the behavioural evidence for disrupted error signalling in delusion formation. Cogn Neuropsychiatry 19:439–467. [DOI] [PubMed] [Google Scholar]
- Grossberg S (2009): Cortical and subcortical predictive dynamics and learning during perception, cognition, emotion and action. Philos Trans R Soc Lond Ser B, Biol Sci 364:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson‐Mendenhall CD, Duncan E, Barsalou LW (2012): Mind wandering and attention during focused meditation: A fine‐grained temporal analysis of fluctuating cognitive states. NeuroImage 59:750–760. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA (2009): The construction system of the brain. Philos Trans R Soc Lond Ser B, Biol Sci 364:1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA (2007): Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci U S A 104:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider F, Simmel M (1944): An experimental study of apparent behavior. Am J Psychol 57:243–259. [Google Scholar]
- Herholz SC, Lappe C, Knief A, Pantev C (2009): Imagery mismatch negativity in musicians. Ann N Y Acad Sci 1169:173–177. [DOI] [PubMed] [Google Scholar]
- Hétu S, Grégoire M, Saimpont A, Coll MP, Eugène F, Michon PE, Jackson PL (2013): The neural network of motor imagery: An ALE meta‐analysis. Neurosci Biobehav Rev 37:930–949. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Pace‐Schott EF, Stickgold R, Kahn D (1998): To dream or not to dream? Relevant data from new neuroimaging and electrophysiological studies. Curr Opin Neurobiol 8:239–244. [DOI] [PubMed] [Google Scholar]
- Hubbard TL (2010): Auditory imagery: Empirical findings. Psychol Bull 136:302–329. [DOI] [PubMed] [Google Scholar]
- Hugdahl K (2015): Auditory hallucinations: A review of the ERC “VOICE” project. World J Psychiatry 5:193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W (1891): The Principles of Psychology, Vol. 2 New York: Holt, Rinehart, and Winston. [Google Scholar]
- Jung RE, Mead BS, Carrasco J, Flores RA (2013): The structure of creative cognition in the human brain. Front Human Neurosci 7:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J Kiverstein, M Miller (2015): The embodied brain: towards a radical embodied cognitive neuroscience. Front Human Neurosci 9:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koestler A (1969): The Act of Creation. London: Hutchinson. [Google Scholar]
- Kopelman MD (2010): Varieties of confabulation and delusion. Cogn Neuropsychiatry 15:14–37. [DOI] [PubMed] [Google Scholar]
- Kurczek J, Wechsler E, Ahuja S, Jensen U, Cohen NJ, Tranel D, Duff M (2015): Differential contributions of hippocampus and medial prefrontal cortex to self‐projection and self‐referential processing. Neuropsychologia 73:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Sampaio E, Mauss Y, Scheiber C (2004): Blindness and brain plasticity: Contribution of mental imagery? An fMRI study. Brain Res Cogn Brain Res 20:1–11. [DOI] [PubMed] [Google Scholar]
- Leder H, Nadal M (2014): Ten years of a model of aesthetic appreciation and aesthetic judgments: The aesthetic episode – Developments and challenges in empirical aesthetics. Br J Psychol 105:443–464. [DOI] [PubMed] [Google Scholar]
- Legrand D, Ruby P (2009): What is self‐specific? Theoretical investigation and critical review of neuroimaging results. Psychol Rev 116:252–282. [DOI] [PubMed] [Google Scholar]
- Limb CJ, Braun AR (2008): Neural substrates of spontaneous musical performance: An FMRI study of jazz improvisation. PloS One 3:e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ (2008): Attention regulation and monitoring in meditation. Trends Cogn Sci 12:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna A, Raffone A, Perrucci MG, Nardo D, Ferretti A, Tartaro A, Londei A, Del Gratta C, Belardinelli MO, Romani GL (2010): Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res Bull 82:46–56. [DOI] [PubMed] [Google Scholar]
- Manu A (2006): The Imagination Challenge: Strategic Foresight and Innovation in the Global Economy. Berkeley: New Riders. [Google Scholar]
- Maquet P (2000): Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res 9:207–231. [DOI] [PubMed] [Google Scholar]
- Maquet P, Péters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G (1996): Functional neuroanatomy of human rapid‐eye‐movement sleep and dreaming. Nature 383:163–166. [DOI] [PubMed] [Google Scholar]
- Marković S (2012): Components of aesthetic experience: Aesthetic fascination, aesthetic appraisal, and aesthetic emotion. I‐Perception 3:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007): Wandering minds: The default network and stimulus‐independent thought. Science (New York, N.Y.) 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Cacioppo JT, Kanwisher N (2013): How fMRI can inform cognitive theories. Persp Psychol Sci 8:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA (2013): Memory, imagination, and predicting the future: A common brain mechanism? Neuroscientist 20:220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth C, Carbon CC (2013): The aesthetic aha: On the pleasure of having insights into Gestalt. Acta Psychol 144:25–30. [DOI] [PubMed] [Google Scholar]
- Ngai S (2012): Our Aesthetic Categories: Zany, Cute, Interesting. Cambridge, Mass: Harvard University Press. [Google Scholar]
- Nguyen VT, Breakspear M, Hu X, Guo CC (2016): The integration of the internal and external milieu in the insula during dynamic emotional experiences. NeuroImage 124:455–463. [DOI] [PubMed] [Google Scholar]
- Nir Y, Tononi G (2010): Dreaming and the brain: From phenomenology to neurophysiology. Trends Cogn Sci 14:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley DA, Halligan PW (2009): Hypnotic suggestion and cognitive neuroscience. Trends Cogn Sci 13:264–270. [DOI] [PubMed] [Google Scholar]
- Palmer SE, Schloss KB, Sammartino J (2013): Visual aesthetics and human preference. Annu Rev Psychol 64:77–107. [DOI] [PubMed] [Google Scholar]
- Pearson J, Kosslyn SM (2015): The heterogeneity of mental representation: Ending the imagery debate. Proc Natl Acad Sci U S A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J, Naselaris T, Holmes EA, Kosslyn SM (2015): Mental imagery: Functional mechanisms and clinical applications. Trends Cogn Sci 19:590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Friston K (2014): Predictions, perception, and a sense of self. Neurology 83:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier C, De Volder AG, Scheiber C (2007): What neuroimaging tells us about sensory substitution. Neurosci Biobehav Rev 31:1064–1070. [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2006): Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci 10:59–63. [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW (2002): Mental imagery: In search of a theory. Behav Brain Sci 25:157–182. 237. [DOI] [PubMed] [Google Scholar]
- Reber R, Schwarz N, Winkielman P (2004): Processing fluency and aesthetic pleasure: Is beauty in the perceiver's processing experience? Pers Soc Psychol Rev 8:364–382. [DOI] [PubMed] [Google Scholar]
- Runco MA, Jaeger GJ (2012): The standard definition of creativity. Creativ Res J 24:92–96. [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ (2011): Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci 14:257–262. [DOI] [PubMed] [Google Scholar]
- Sass L, Byrom G (2015): Phenomenological and neurocognitive perspectives on delusions: A critical overview. World Psychiatry 14:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL (2012a): Adaptive constructive processes and the future of memory. Am Psychologist 67:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL (2012b): Constructive memory: Past and future. Dialogues Clin Neurosci 14:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK (2012): The future of memory: Remembering, imagining, and the brain. Neuron 76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer KR (2005): What are emotions? And how can they be measured? Soc Sci Inform 44:695–729. [Google Scholar]
- Schnider A (2003): Spontaneous confabulation and the adaptation of thought to ongoing reality. Nat Rev Neurosci 4:662–671. [DOI] [PubMed] [Google Scholar]
- Scholl N, Tremoulet N (2000): Perceptual causality and animacy. Trends Cogn Sci 4:299–309. [DOI] [PubMed] [Google Scholar]
- Shine JM, O'Callaghan C, Halliday GM, Lewis SJG (2014): Tricks of the mind: Visual hallucinations as disorders of attention. Prog Neurobiol 116:58–65. [DOI] [PubMed] [Google Scholar]
- Silvia PJ (2009): Looking past pleasure: Anger, confusion, disgust, pride, surprise, and other unusual aesthetic emotions. Psychol Aesthet Creat Arts 3:48–51. [Google Scholar]
- Smallwood J, Schooler JW (2006): The restless mind. Psychol Bull 132:946–958. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW, Turk DJ, Cunningham SJ, Burns P, Macrae CN (2011): Self‐reflection and the temporal focus of the wandering mind. Conscious Cogn 20:1120–1126. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN (2009): The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta‐analysis. J Cogn Neurosci 21:489–510. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Meyer ML, Lieberman MD (2015): The default mode of human brain function primes the intentional stance. J Cogn Neurosci 27:1116–1124. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, D'Argembeau A (2015): Neural correlates of personal goal processing during episodic future thinking and mind‐wandering: An ALE meta‐analysis. Human Brain Mapp 36:2928–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MI (1953): Creativity and culture. J Psychol 36:311–322. [Google Scholar]
- Stevenson L (2003): Twelve conceptions of imagination. Br J Aesthet 43:238–259. [Google Scholar]
- Stokes M, Thompson R, Cusack R, Duncan J (2009): Top‐down activation of shape‐specific population codes in visual cortex during mental imagery. J Neurosci 29:1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striem‐Amit E, Cohen L, Dehaene S, Amedi A (2012): Reading with sounds: Sensory substitution selectively activates the visual word form area in the blind. Neuron 76:640–652. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC (2007): The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci 30:313–351. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Carhart‐Harris R, Leech R, Nutt D, Chialvo DR (2014): Enhanced repertoire of brain dynamical states during the psychedelic experience. Human Brain Mapp 35:5442–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Hölzel BK, Posner MI (2015): The neuroscience of mindfulness meditation. Nat Rev Neurosci 16:213–225. [DOI] [PubMed] [Google Scholar]
- Thomas NJT (2014): Mental imagery In: Zalta FN, editor. The Stanford Encyclopedia of Philosophy (Fall 2014). Retrieved from http://plato.stanford.edu/archives/fall2014/entries/mental-imagery/ [Google Scholar]
- Van de Cruys S, Wagemans J (2011): Putting reward in art: A tentative prediction error account of visual art. I‐Perception 2:1035–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoeck N, Ma N, Ampe L, Baetens K, Vandekerckhove M, Van Overwalle F (2013): Counterfactual thinking: An fMRI study on changing the past for a better future. Soc Cogn Affect Neurosci 8:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Paasschen J, Bacci F, Melcher DP (2015): The influence of art expertise and training on emotion and preference ratings for representational and abstract artworks. PloS One 10:e0134241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian O, Goel V (2005): Task constraints modulate activation in right ventral lateral prefrontal cortex. NeuroImage 27:927–933. [DOI] [PubMed] [Google Scholar]
- Vartanian O, Skov M (2014): Neural correlates of viewing paintings: Evidence from a quantitative meta‐analysis of functional magnetic resonance imaging data. Brain Cogn 87:52–56. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M (2010): The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat Rev Neurosci 11:642–651. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P (2014): Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin 44:323–337. [DOI] [PubMed] [Google Scholar]
- Walton KL (1990): Mimesis as Make‐Believe: On the Foundations of the Representational Arts. Cambridge, Mass: Harvard University Press. [Google Scholar]
- Waters F, Collerton D, Ffytche DH, Jardri R, Pins D, Dudley R …, Larøi F (2014): Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull 40:S233–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AP, Heckers S (1999): Neuroimaging of hallucinations: A review of the literature. Psychiatry Res 92:61–74. [DOI] [PubMed] [Google Scholar]
- Wiens S (2005): Interoception in emotional experience. Curr Opin Neurol 18:442–447. [DOI] [PubMed] [Google Scholar]
- Wilson AD, Golonka S (2013): Embodied cognition is not what you think it is. Cogn Sci 4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfensteller U, Schubotz RI, von Cramon DY (2007): Understanding non‐biological dynamics with your own premotor system. NeuroImage 36 Suppl 2:T33–T43. [DOI] [PubMed] [Google Scholar]
- Zajonc RB (1968): Attitudinal effects of mere exposure. J Pers Soc Psychol 9:1–27. 5667435 [Google Scholar]
- Zaki J, Davis JI, Ochsner KN (2012): Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage 62:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedelius CM, Schooler JW (2015): The richness of inner experience: Relating styles of daydreaming to creative processes. Front Psychol 6:2063. [DOI] [PMC free article] [PubMed] [Google Scholar]


