Abstract
Cataract-associated gene discovery in human and animal models have informed on key aspects of human lens development, homeostasis and pathology. Additionally, in vitro models such as the culture of permanent human lens epithelium-derived cell lines (LECs) have also been utilized to understand the molecular biology of lens cells. However, these resources remain uncharacterized, specifically regarding their global gene expression and suitability to model lens cell biology. Therefore, we sought to molecularly characterize gene expression in the human LEC, SRA01/04, which is commonly used in lens studies. We first performed short tandem repeat (STR) analysis and validated SRA01/04 LEC for its human origin, as recommended by the eye research community. Next, we used Illumina HumanHT-12 v3.0 Expression BeadChip arrays to gain insights into the global gene expression profile of SRA01/04. Comparative analysis of SRA01/04 microarray data was performed using other resources such as the lens expression database iSyTE (integrated Systems Tool for Eye gene discovery), the cataract gene database Cat-Map and the published lens literature. This analysis showed that SRA01/04 significantly expresses >40% of the top iSyTE lens-enriched genes (313 out of 749) across different developmental stages. Further, SRA01/04 also significantly expresses ~53% (168 out of 318) of cataract-associated genes in Cat-Map. We also performed comparative gene expression analysis between SRA01/04 cells and the previously validated mouse LEC 21EM15. To gain insight into whether SRA01/04 reflects epithelial or fiber cell characteristics, we compared its gene expression profile to previously reported differentially expressed genes in isolated mouse lens epithelial and fiber cells. This analysis suggests that SRA01/04 has reduced expression of several fiber cell-enriched genes. In agreement with these findings, cell culture analysis demonstrates that SRA01/04 has reduced potential to initiate spontaneous lentoid body formation compared to 21EM15 cells. Next, to independently validate SRA01/04 microarray gene expression, we subjected several candidate genes to RT-PCR and RT-qPCR assays. This analysis demonstrates that SRA01/04 supports expression of many key gene associated with lens development and cataract, including CRYAB, CRYBB2, CRYGS, DKK3, EPHA2, ETV5, GJA1, HSPB1, INPPL1, ITGB1, PAX6, PVRL3, SFRP1, SPARC, TDRD7, and VIM, among others, and therefore can be relevant for understanding the mechanistic basis of these factors. At the same time, SRA01/04 cells do not exhibit robust expression of several genes known to be important to lens biology and cataract such as ALDH1A1, COL4A6, CP, CRYBA4, FOXE3, HMX1, HSF4, MAF, MEIS1, PITX3, PRX, SIX3, and TRPM3, among many others. Therefore, the present study offers a rich transcript-level resource for case-by-case evaluation of the potential advantages and limitations of SRA01/04 cells prior to their use in downstream investigations. In sum, these data show that the human LEC, SRA01/04, exhibits lens epithelial cell-like character reflected in the expression of several lens-enriched and cataract-associated genes, and therefore can be considered as a useful in vitro resource when combined with in vivo studies to gain insight into specific aspects of human lens epithelial cells.
Keywords: Human Lens Epithelial Cell line, SRA01/04, Microarrays, Gene expression, Lens, Cataract
1. Introduction
Various animal models such as mouse, chicken, xenopus and zebrafish have been used for understanding the biology of lens development (Cvekl and Zhang, 2017; Donner et al., 2006; Lachke and Maas, 2010). While these approaches are critical for testing in vivo the function of a potential cataract-associated gene, they can be limited by small amounts of tissue-material. Therefore, in vitro cell culture resources that may be amenable for biochemical functional assays can be considered for gaining mechanistic insights into the role of specific genes in the lens. However, it is necessary to first systematically characterize these in vitro cell culture resources in terms of the extent or limitations to which they can resemble properties of the lens tissue. Within the lens community, in vitro models of lens epithelial-derived cell lines (LECs) have been generated as resources for mechanistic investigation of lens biology. One commonly used human LEC is SRA01/04, a cell line originally derived from an infant patient who was treated for retinopathy. The SRA01/04 cell line was derived by transfecting lens cells with a plasmid vector carrying the large T antigen for Simian Virus 40 (Tag SV40) (Ibaraki et al., 1998). According to a recent PubMed search, >70 research publications have used SRA01/04 cells in their experiments. However, the SRA01/04 cell line is limitedly characterized. In the original report, SRA01/04 cells were shown to express CRYAA and CRYBB2 transcripts (Ibaraki et al., 1998). Further, in a later study, expression of miRNAs in SRA01/04 was characterized, which showed that SRA01/04 cells harbored miRNAs such as miR-124 and miR-31, among others (Tian et al., 2010). However, beyond these two examples, this commonly used human LEC has not been investigated with regards to lens-like character or expression of key lens genes.
In recent years, lack of validation and authentication for widely used cell lines has come under intense scrutiny. Indeed, as of 2013, all cell lines used in studies submitted to the three major eye journals – Investigative Ophthalmology & Visual Science, Experimental Eye Research, and Molecular Vision (Beebe, 2013; The Editors of Experimental Eye Research, 2013; The Editors of Molecular Vision, 2013) – need to be previously authenticated for species origin and validated for molecular identity. This shift to requirement of authentication and validation comes after the discovery that a widely used rat retinal ganglion cell line, RGC-5, was, in fact, of mouse origin and did not express retinal ganglion cell markers. Remarkably, RGC-5 matched the molecular identity of a mouse photoreceptor cell line, 661W (Krishnamoorthy et al., 2013). This is not an isolated problem limited to the eye community, as recently The American Type Culture Collection (ATCC) has discontinued the use and distribution of 29 human cell lines due to short tandem repeat (STR) profiling discrepancies (ATCC, 2016). Thus, it is necessary to undertake a systematic authentication and validation of the purported human LEC SRA01/04, as such knowledge will impact in vitro experimental design regarding the use of LECs in the eye research field.
Therefore, in the present study, we present a detailed validation and molecular characterization of gene expression of the LEC SRA01/04. Using STR profiling analysis, we validate that SRA01/04 cells are, indeed, of human origin. Through global mRNA expression analysis by microarrays followed by detailed comparative expression analysis, and independent RT-PCR and RT-qPCR validation, we demonstrate that SRA01/04 cells express a number of genes important for lens development, homeostasis and pathology. Moreover, these genes represent high-priority candidates in the lens that have been identified by the web-based bioinformatics tool iSyTE, which identifies genes with enriched expression in the lens, as well as the cataract-associated gene database Cat-Map. Further, we show broadly similar expression profiles of key genes between SRA01/04, previously validated mouse LEC 21EM15, and previously reported isolated mouse lens epithelium data. However, our analysis also shows that SRA0104 does not express several key lens and cataract genes, highlighting its limitations as well. Together, these data provide valuable gene expression information on SRA01/04 to the eye community that will allow them to assess the suitability of this LEC for studies which are focused on the specific factors whose expression is supported in these cells.
2. Materials and Methods
2.1. Cell Culture
SRA01/04 cells were obtained from Dr. Venkat Reddy who originally derived them from human lens epithelium (Ibaraki et al., 1998). 21EM15 cells, previously gifted to the Lachke Laboratory by Dr. John Reddan (Oakland University, Michigan) and HEK293T cells, generously provided by Dr. Donna Woulfe’s laboratory, were used for comparative analysis. All cell lines were cultured in Dulbecco’s Modification of Eagle’s Media (DMEM) with 4.5 g/L glucose, L-glutamine, and sodium pyruvate included (Corning Cellgro, Manassas VA). The culture media was supplemented with 10% Fetal Bovine Serum (Fisher Scientific, Pittsburg, PA) and 1% penicillin-streptomycin (GE Healthcare Life Sciences, Logan, UT). All cell lines were cultured in 100mm cell culture treated plates (Eppendorf) with 10mL of respective media. For the comparative analysis of spontaneous lentoid body formation in SRA01/04 and 21EM15, cells were cultured in 6-well plates. Images of cultured cells were taken using AMScope MU130 camera at 10X magnification. Lentoid bodies were counted in independently collected images from at least three biological replicates, and 2-level nested ANOVA was used to calculate significance. All cell lines were incubated at 37°C and 5% CO2.
2.2. Authentication of Human Origin of SRA01/04
Genomic DNA was extracted from cell lines using the Gentra Puregene Core Kit A (Qiagen, Venlo, Netherlands). Primers were based on human and mouse-specific short tandem repeats (STRs) as previously described (Almeida et al., 2014; Azari, Ahmadi et al., 2007) (Table 1). Polymerase Chain Reaction (PCR) amplification was performed with the Taq PCR Core Kit (Qiagen). For Human STR primers, 19μL ddH2O, 2.5μL 10x Coral Red Buffer, 0.75μL of 25mM MgCl2, 0.5μL 10μM; dNTP, 0.5μL each of 10mM forward and reverse primers, 0.25μL Taq polymerase, and 0.5μL of genomic DNA were used. For mouse STR primers, 20μL ddH2O, 2.5μL 10x Coral Red Buffer, 0.5μL 10μM; dNTP, 0.5μL each of 10mM forward and reverse primers, 0.5μL Taq polymerase, and 0.5μL of genomic DNA were used. The amplification program for the human STR primers was: 95°C for 3 min, followed by 35 cycles of 94°C for 30 sec, 64°C for 45 sec, 72°C for 45 sec, followed by 72°C for 10 minutes. The amplification program for the mouse STR primers was: 95°C for 3 min, followed by 30 cycles of 94°C for 30 sec, 60°C for 45 sec, 72°C for 45 sec, followed by 72°C for 10 minutes. PCR products were separated on a 1% agarose gel with ethidium bromide and imaged using a GelDoc-It 310 imager (UVP).
Table 1.
Human and Mouse STR Primer Sequences
| Primer Set | F/R | Primer Sequence (5'--> 3') | Amplicon Size (bp) |
|---|---|---|---|
| Human STR Primers | |||
| CSF1PQ | Forward | AACCTGAGTCTGCCAAGGACTAGC | 291-331 |
| Reverse | TTCCACACACCACTGGCCATCTTC | ||
| D13S17 | Forward | ACAGAAGTCTGGGATGTGGA | 157-201 |
| Reverse | GCCCAAAAAGACAGACAGAA | ||
| D16S539 | Forward | GGGGGTCTAAGAGCTTGTAAAAAG | 264-304 |
| Reverse | GTTTGTGTGTGCATCTGTAAGCAT | ||
| D4S2408 | Forward | AATAAACTTCAACTTCAATTCATCC | 276 |
| Reverse | AGGTAAAGGCTCTTCTTGGC | ||
| D5S818 | Forward | GGTGATTTTCCTCTTTGGTATCC | 118-155 |
| Reverse | AGCCACAGTTTACAACATTTGTATCT | ||
| D7S820 | Forward | ATGTTGGTCAGGCTGACTATG | 211-251 |
| Reverse | GATTCCACATTTATCCTCATTGAC | ||
| THO1 | Forward | ATTCAAAGGGTATCTGGGCTCTGG | 171-215 |
| Reverse | GTGGGCTGAAAAGCTCCCGATTAT | ||
| VWA | Forward | GCCCTAGTGGATGATAAGAATAATCAGTATGTG | 123-181 |
| Reverse | GGACAGATGATAAATACATAGGATGGATGG | ||
| Mouse STR Primers | |||
| 12-1 | Forward | CAAAATTGTCATTGAACACATGTAA | 222-259 |
| Reverse | GCAATGGTCAAGAAATACTGAAGTACAA | ||
| 15-3 | Forward | TCTGGGCGTGTCTGTCATAA | 157-222 |
| Reverse | GTTCTCAGGGAGGAGTGTGCT | ||
| 18-3 | Forward | TCTTTCTCCTTTTGTGTCATGC | 281-313 |
| Reverse | GTCAAAGTTGGGGTTACAGAATG | ||
| 4-2 | Forward | AAGCTTCTCTGGCCATTTGA | 217-248 |
| Reverse | GTTCATAAACTTCAAGCAATGACA | ||
| 5-5 | Forward | CGTTTTACCTGGCTGACACA | 258-298 |
| Reverse | GATGCTTGCCTGTTCCTAGC | ||
| 6-4 | Forward | TTTGCAACAGCTCAGTTTCC | 276-311 |
| Reverse | GAATCGCTGGCAGATCTTAGG | ||
| 9-2 | Forward | GGATTGCCAAGAATTTGAGG | 318-360 |
| Reverse | GTCCATGAATCCAGACATTCC | ||
| X-1 | Forward | GGATGGATGGATGGATGAAA | 357-442 |
| Reverse | GAAGGTATATATCAAGATGGCATTATCA | ||
2.3. Microarray Expression Analysis
The microarray dataset for SRA01/04 gene expression was generated using the Illumina HumanHT-12 v3.0 Expression BeadChip. Data was analyzed in ‘R’ Statistical environment (http://www.r-project.org/index.html). Lumi package on Bioconductor (http://www.bioconductor.org/) was implemented for background correction, data normalized using ‘bjadjust’ and ‘Rank invariant’ methods. Probes with detection p-values ≤ 0.05 and expression intensities ≥100 were considered expressed and used for gene-level analysis. Expressed genes were then filtered for lens-enriched genes through iSyTE top genes for three developmental stages (embryonic day (E) 10.5, E11.5, and E12.5) and for cataract-associated genes through Cat-Map (http://cat-map.wustl.edu/). The purpose of this analysis was to evaluate whether genes relevant to lens development or cataract were expressed in SRA01/04. In the past we have demonstrated that E10.5 through E12.5 lens enriched expression datasets effectively identify majority of cataract-linked genes as well as lens development genes (Lachke et al., 2012), and therefore these datasets were used. Further, filtered genes identified by iSyTE and Cat-Map were used for Panther Gene Ontology analysis (http://geneontology.org/) for biological processes to identify enriched clusters. Significance was calculated using Fisher’s Exact test and Bonferroni corrected p-values. SRA01/04 gene expression was compared to previously reported microarray data on 21EM15 cells (Terrell et al., 2015) and isolated mouse lens epithelium and lens fiber cell (Nakahara et al., 2007). Because the microarrays are on different “platforms” (different microarray chips) and/or represent two different species (human versus mouse), to effectively compare gene expression, a signature set of genes was first identified for each cell type, and then used for comparative analysis as previously described (Terrell et al., 2015)
2.4. RT-PCR and RT-qPCR Analysis
Total RNA from SRA01/04 and HEK293T cell lines was isolated using RNAeasy Mini Kit (Qiagen) and was quantified using an ND-1000 nanodrop spectrophotometer (Thermo Scientific). cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, California) followed by semi-quantitative and real-time quantitative PCR (RT-PCR and RT-qPCR). For semi-quantitative RT-PCR, target gene regions were amplified using gene-specific primer sets (Table 2) and Taq PCR Core Kits (Qiagen) with 20.27μL ddH2O, 2.5μL 10x Coral Red Buffer, 0.5μL 10μM; dNTP, 0.5μL each of 10mM forward and reverse primers, 0.125μL Taq polymerase, and 0.5μL cDNA. Products were amplified with the program: 94°C for 2 minutes, followed by 35 cycles of 94°C for 15 sec, 58°C for 30 sec, 72°C for 30 sec, followed by 72°C for 7 minutes. Amplified products were separated on a 1% agarose gel with ethidium bromide and visualized using a GelDoc-It 210 imager (UVP). RT-qPCR was performed using a Power SYBR Green kit (Invitrogen Life Technology) and analyzed on a 7500 Fast PCR system (Applied Biosystems, Foster City, California). Three biological and three technical replicates were used for each cell line and primer set for RT-qPCR. Fold-change was calculated using the ΔΔCT-method using GAPDH as a house-keeping gene and statistical significance was determined using a two-level nested analysis of variance as described previously (Siddam et al., 2018).
Table 2.
Primers for RT-PCR and qRT-PCR assay
| Target | F/R | Primer Sequence (5' --> 3') | Amplicon Size (BP) |
|---|---|---|---|
| CRYAB | Forward | CCGCCTCTTTGACCAGTTCT | 154 |
| Reverse | CTTCTCCAGGCGCATCTCTG | ||
| CRYBB2 | Forward | AGGTTCTGTCCTAGTGCAGGC | 366 |
| Reverse | TAGTCTCCCTTCTCCAGCAGG | ||
| CRYGA | Forward | TCCTCATACCAGCTCGCACA | 176 |
| Reverse | CCCGGTAGTTGGGCATTTCA | ||
| CRYGS | Forward | GCTGTTCATCTGCCTAGTGGA | 191 |
| Reverse | CTGCCACGGTAGTTGGGTAG | ||
| DKK3 | Forward | ATACCATCCATGTGCACCGA | 241 |
| Reverse | TCTCCACAGCACTCACTGTC | ||
| EPHA2 | Forward | CTCACACACCCGTATGGCAA | 273 |
| Reverse | GAAGTTGGTGCCGTAGTCCA | ||
| ETV5 | Forward | ATGGTCCCAGGGAAATCTCG | 150 |
| Reverse | TGCTTCAGCTAACCAAGCCT | ||
| GJA1 | Forward | TCAAGGGCGTTAAGGATCGG | 181 |
| Reverse | CTGTCGCCAGTAACCAGCTT | ||
| HSPB1 | Forward | ACGCGGAAATACACGCTGC | 197 |
| Reverse | GCGGCAGTCTCATCGGATTT | ||
| INPPL1 | Forward | ACCGTAGCAAAGGAACAGCA | 99 |
| Reverse | TCTGTGGCTCCCCTGATCTT | ||
| ITGB1 | Forward | ACCGTAGCAAAGGAACAGCA | 99 |
| Reverse | TCTGTGGCTCCCCTGATCTT | ||
| MCM4 | Forward | ACTACCAGAGCGAGGAGCAG | 187 |
| Reverse | CGAGGGTATGCAGAAACCAT | ||
| PAX6 | Forward | AGTGAATCAGCTCGGTGGTG | 132 |
| Reverse | CCGTTGGACACCTGCAGAAT | ||
| PVRL3 | Forward | GTTACATTCCCGCTTGGAAA | 169 |
| Reverse | CCCAGTCAATATGTGCAACG | ||
| SFRP1 | Forward | GCGAGTACGACTACGTGAGC | 74 |
| Reverse | AGGTGGCTTGGTGTAGAAGC | ||
| SPARC | Forward | TTTCAGCCAGGAAGGCCAAA | 208 |
| Reverse | GCCTGTGAGATCCGACCATC | ||
| TDRD7 | Forward | GCAGACTTGTGGAAGCATCA | 248 |
| Reverse | GCCACAGGCACATACACATC | ||
| VIM | Forward | GGCGAGGAGAGCAGGATTTC | 95 |
| Reverse | TGGGTATCAACCAGAGGGAGT | ||
| PROX1 | Forward | TACGCACGTCAAGCCATCAA | 135 |
| Reverse | CAGGAATCTCTCTGGAACCTCA |
3: Results
3.1. Authentication of Human Origin
Before initiating detailed characterization of SRA01/04, we first sought to validate its human origin as recommended by the eye research community (Beebe, 2013; The Editors of Experimental Eye Research, 2013; The Editors of Molecular Vision, 2013). Therefore, we used human and mouse-specific primer sets designed to detect short tandem repeats (STRs) specific to the respective genomes as previously described (Azari et al., 2007; Almeida et al., 2014). As expected, SRA01/04 cells exhibit amplification of all human STR region-specific products (Fig. 1A). Further, amplification of human STR products in SRA01/04 cells resembles another validated human cell line, the human embryonic kidney cell line HEK293T (Dubridge et al., 1987; Lin et al., 2014). Importantly, while the mouse cell line 21EM15 shows amplification of the mouse STR region-specific products, these are absent in both SRA01/04 and HEK293T (Fig. 1B). These data confirm the human origin of the SRA01/04 cell line.
Fig. 1.
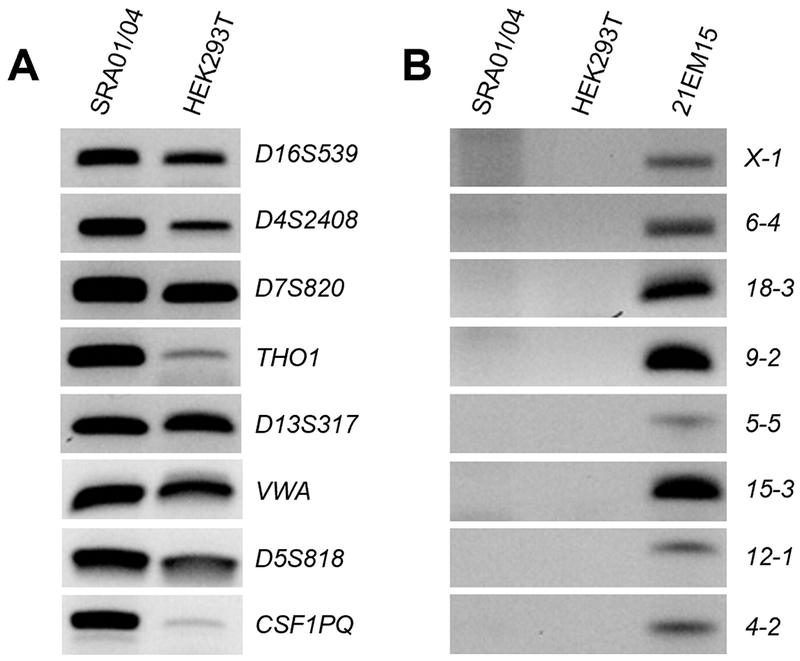
STR Analysis of SRA01/04 with Human and Mouse-Specific Primers. (A) Genomic DNA isolated from human lens epithelium-derived cell line SRA01/04 and human embryonic kidney cell line HEK293T was subjected to STR analysis by amplification of genomic regions of human DNA. Both cell lines exhibit amplification of human-specific STRs. (B) Genomic DNA extracted from SRA01/04, HEK293T, and the mouse LEC 21EM15 was subjected to STR analysis by mouse STR-specific primers. 21EM15 exhibits amplification of these mouse-specific STRs while neither human cell line show amplification of the same STRs.
3.2. SRA01/04 Microarray Gene Expression Analysis
While SRA01/04 has been utilized as an in vitro model for numerous lens epithelial cell studies, its global mRNA gene expression profile has not been defined. Therefore, we sought to characterize the expression profile of SRA01/04 cells by microarrays. Microarray analysis using the Illumina HumanHT-12 v3.0 Expression BeadChip platform was performed and processed based on a previously reported strategy (Anand et al., 2015). This involved background correction, normalization and transformation and data quality assessment (Fig. 2A). Quality control analysis was performed by Principal Component Analysis (PCA), which shows close clustering of the SRA01/04 replicates separate from non-lens cells such as the human kidney HEK293 cell line and human tracheal epithelial cells (hTECs) (Fig. 2B). This demonstrates the distinctive global mRNA gene expression profile of SRA01/04. While traditional microarray analysis identified 14,087 significantly expressed genes (p<0.05) with fluorescence expression intensities ranging from 12.0 to 29,597.0, based on our experience on reliably amplifying transcripts by RT-PCR, we focused on using a more stringent cut-off (p<0.05 and fluorescence expression intensity ≥100), which led to narrowing the profile to 8,328 expressed genes (Supplementary Table S1).
Fig. 2.
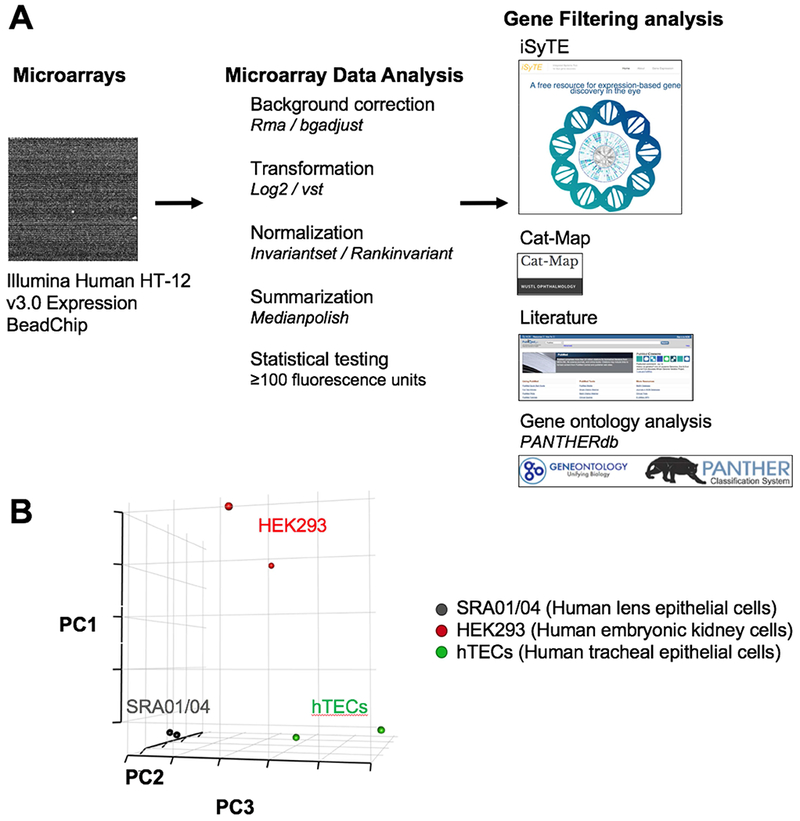
Microarray profiling of SRA01/04 LEC with Illumina HumanHT-12 v3.0 Expression BeadChip. (A) Microarray experimental design for SRA01/04 mRNA expression analysis. A flowchart of experimental design and processing strategy including background correction, normalization, transformation is given. The identified expressed genes were then subjected to downstream analysis using databases such as iSyTE and Cat-map, a review of the literature, and PANTHER gene ontology analysis. (B) Quality control was performed on the microarray dataset including Principal components analysis (PCA) which shows distinct clustering of SRA01/04 expression profile compared to non-lens cells, namely the human embryonic kidney cell line HEK293 and human tracheal epithelial cells (hTECs).
Next, to evaluate the lens-like character of SRA01/04, we analyzed the SRA01/04 microarray data set in the context of the bioinformatic tool iSyTE, which informs on the expression of key lens and cataract-associated genes. When compared to the top iSyTE identified genes exhibiting high lens-enriched expression at different lens stages (embryonic day (E)10.5, E11.5, and E12.5), ~42% (313 out of 749) are significantly expressed (p<0.05) in SRA01/04 with an fluorescence expression intensity units of ≥100 (Supplementary Table S2). In addition to analyzing the SRA01/04 gene expression to iSyTE identified genes, we compared the significantly expressed genes in SRA01/04 with genes listed in the web-based cataract-associated gene database Cat-Map (Shiels et al., 2010). Using an expression cut-off of ≥100 fluorescence expression intensity units, ~53% (168 out of 318) of the cataract-associated genes are significantly expressed (p<0.05) in SRA01/04 LEC (Supplementary Table S3). Further, we included other genes relevant to lens biology from the literature and integrating this information along with the above analysis of iSyTE and Cat-Map target genes, we present a list of 50 high-priority genes expressed in SRA01/04 cells (Table 3).
Table 3.
Microarray expression of high-priority lens and cataract genes in SRA01/04
| Gene | SRA 01/04 Expression | Association with Cataract and/or Function, Expression in the Lens | Citation |
|---|---|---|---|
| ALDH1A3 | 2207 | Mutation associated with human autosomal recessive anophthalmia and microphthalmia | (Semerci et al., 2014) |
| BCAR3 | 595 | Mouse mutants exhibit lens defects | (Kamaid and Giráldez, 2008; Near et al., 2009) |
| BTG1 | 1403 | Expressed in the lens | (Kamaid and Giráldez, 2008) |
| CELF1 | 141 | Mouse mutants exhibit lens fiber cell differentiation defects and cataract |
(Siddam et al., 2018) |
| CHMP4B | 1113 | Autosomal dominant progressive pediatric posterior subcapsular cataract | (Shiels et al., 2007) |
| COL18A1 | 1341 | Lens abnormalities; Col18a1:Hspg2 double mutant mouse models exhibit lens defects | (Menzel et al., 2004; Rossi et al., 2003) |
| COL4A1 | 4678 | Mouse mutants exhibit vacuolar cataract | (Van Agtmael et al., 2005) |
| COL4A5 | 1338 | Dominant X-linked congenital cataract | (Antignac et al., 1992) |
| CR1M1 | 440 | Mouse mutants exhibit smaller lenses | (Pennisi et al., 2007) |
| CRYAB | 269 | Autosomal dominant congenital cataract | (Berry et al., 2001) |
| CRYBB2 | 189 | Autosomal dominant progressive congenital Cataract | (Chen et al., 2013) |
| CRYGS | 104 | Autosomal dominant progressive cortical cataract | (Sun et al., 2005) |
| DHX32 | 850 | Mutation associated with human inherited retinal disease | (Astuti et al., 2018) |
| DKK3 | 2179 | Expressed in early mouse eye and lens development | (Ang et al., 2004) |
| EPHA2 | 207 | Autosomal dominant posterior polar cataract | (Shiels et al., 2008) |
| ERCC2 | 205 | Age related cortical and mixed cataract | (Unal et al., 2007; Padma et al., 2011) |
| ETV5 | 1297 | Nuclear effector of FGF signaling in mouse lens development | (Xie et al., 2016) |
| ETV6 | 363 | Expressed in lens development; regulated by Pax6 | (Cvekl et al., 2004) |
| FYCO1 | 274 | Autosomal recessive congenital cataracts | (Chen et al., 2011) |
| GALK1 | 1360 | Nuclear and age-related cataract | (Okano et al., 2001; Yasmeen et al., 2010) |
| GCNT2 | 160 | Autosomal recessive congenital cataracts | (Yu et al., 2001) |
| GJA1 | 1081 | Important for epithelial molecular transport and exchange; mutations associated cataract and other ocular defects | (Paznekas et al., 2003; Zampighi et al., 2000) |
| HMOX1 | 194 | Regulator of oxidative stress response in lens epithelium | (Rzymkiewicz et al., 2000) |
| HS2ST1 | 143 | Mutation results in eye defects | (Bullock et al., 1998) |
| HSPB1 | 7148 | Expressed in lens development | (Marvin et al., 2008) |
| INPPL1 | 1114 | Misregulation associated with keratoconus with cataract | (Hughes et al., 2011) |
| ITGA3 | 3556 | Itga3: Itga6 double null mouse exhibit lens defects | (De Arcangelis et al. , 1999) |
| ITGB1 | 11330 | Mouse mutants exhibit loss of lens epithelial cells | (Simirskii et al., 2007) |
| JAG1 | 179 | Mouse mutants exhibit lens fiber cell differentiation defects | (Le et al., 2012) |
| LAMA1 | 575 | Essential for lens development in zebrafish; mutation associated with lens extrusion defect | (Pathania, et al., 2014; Zinkevich et al., 2006) |
| LEPREL1 | 2361 | Nonsyndromic myopia and cataract | (Mordechai et al., 2011) |
| MAFG | 258 | Compound mutation with MafK results in cataract | (Agrawal et al., 2015) |
| MCM4 | 2241 | Expressed in lens epithelium | (Terrell et al., 2015) |
| MYH9 | 5046 | Cataract and other defects; mouse mutants exhibit lens defects | (Kunishima et al., 2001; Suzuki et al., 2013) |
| PAX6 | 355 | Congenital cataract, aniridia and corneal dystrophy; and induces ectopic lens | (Altmann et al., 1997; Glaser et al., 1994) |
| PVRL3 | 231 | Misregulation is associated with congenital cataract | (Weinstein et al., 1982) |
| PXDN | 1406 | Recessive congenital cataract | (Khan et al., 2011) |
| RAB3GAP1 | 1213 | Cataract and other phenotypes | (Handley et al., 2013) |
| RBM24 | 115 | Post-transcriptional regulator of Sox2 in lens development | Dash and Lachke, Unpublished Observations |
| RRAGA | 2433 | Nuclear and progressive posterior subcapsular cataract | (Chen et al., 2016) |
| SFRP1 | 1976 | Expressed in lens development | (Chen et al., 2004) |
| SMARCA4 | 2081 | Mutation causes lens fiber cell differentiation defects in mouse | (He et al., 2010) |
| SOX2 | 107 | Functions in mouse lens development; and mutations cause anophthalmia | (Donner et al., 2007) |
| SPARC | 2439 | Mouse mutants exhibit severe cataract and lens defects | (Fantes et al., 2003) |
| SPP1 | 135 | ECM component in postoperative capsular opacification, expressed by LECs on injury | (Saika et al., 2003) |
| STRA6 | 119 | Mutation causes anophthalmia | (Pasutto et al., 2007) |
| STX3 | 432 | Autosomal recessive congenital cataract | (Chograni et al., 2015) |
| SULF1 | 195 | Expressed in eye development | (Kalus et al., 2009) |
| TDRD7 | 689 | Deficiency causes cataract in human, mouse, and chicken | (Lachke et al., 2011; Tanaka et al., 2011) |
| VIM | 18429 | Heterozygous G596A mutation causes pulverulent cataract | (Muller et al., 2009) |
To gain insight into whether SRA01/04 cells exhibit functional patterns relevant to lens biology, we performed Panther Gene Ontology (GO) analysis for biological processes reflected in the list of SRA01/04 genes identified by iSyTE and Cat-Map analysis (n=451 genes expressed in SRA01/04 that are also identified in iSyTE and Cat-Map). This analysis identified several gene function clusters that include genes shown to be important for lens biology, such as “lens fiber cell development”, “epithelial cell development”, “lens morphogenesis in camera-type eye”, “camera-type eye morphogenesis”, and “developmental growth” (Table 4). Interestingly, each of these key clusters contains at least two keys genes that are also recognized among the 50 high-priority genes expressed in SRA01/04 cells (Table 3). This microarray analysis indicates that SRA01/04 cells exhibit high levels of expression of important genes associated with lens development, homeostasis and cataract.
Table 4.
Gene Ontology Analysis of iSyTE and Cat-Map Genes Expressed in SRA01/04
| GO Term | Sub-category | GO Reference Number | Number of genes in cluster | Genes | Bonferroni Corrected p-value |
|---|---|---|---|---|---|
| Lens Fiber Cell Development | 14 | 6 | EPHA2, TBC1D20, VIM, ABI2, WNT7B, TMOD1 | 0.0274 | |
| Epithelial Cell Development | 191 | 17 | FLNB, RILPL1, EPHA2, GJA1, GPX1, SPINT2, CDK6, TBC1D20, VIM, LAMB2, JAG1, PAX6, ABI2, WNT7B, COL18A1, TMOD1, BMP4 | 0.0297 | |
| Lens Morphogenesis in Camera-type Eye | 23 | 8 | BCAR3, EPHA2, TDRD7, TBC1D20, LCTL, PVRL3, ABI2, BMP4 | 0.00214 | |
| Camera-type Eye Morphogenesis | 114 | 16 | BCAR3, EPHA2, TDRD7, TBC1D20, ALDH1A3, LRP5, JAG1, PAX6, LCTL, ZHX2, PVRL3, ABI2, VEGFA, STRA6, BMP4, TFAP2A | 0.000218 | |
| Developmental Growth | 394 | 30 | ANXA1, TP53, GJA1, DHCR7, PLEKHA1, ZEB2, SMAD4, COL7A1, GPX1, AKAP13, AKIRIN1, SLIT3, BIN3, PTEN, LAMB2, ITGB1, SEMA5A, WNT7B, NBN, SOX2, SEMA3A, GSN, SFRP1, TNC, ENO3, SLIT2, STRA6, ERCC2, ATM, BMP4 | 0.000155 |
3.3. Comparative Analysis of SRA01/04 Expression with Established Datasets
Next, we sought to further interrogate the lens epithelial-like character of SRA01/04 cells. We approached this by comparing SRA01/04 gene expression with that of another established lens epithelium-derived cell line, the mouse LEC 21EM15 (Terrell et al., 2015), and to that of isolated mouse lens epithelial and fiber cells. Microarray expression values of key lens candidate genes for SRA01/04 and 21EM15 shows that they are broadly similar, with some key differences (Fig. 3A). While this comparative analysis has limitations due to species differences as well as due to technological differences between the microarray platforms, these data demonstrates similarities of expression for many important genes such as the high expression of CELF1, CHMP4B, CRIM1, CRYAB, EPHA2, FYCO1, GJA1, MAFG, PAX6, PDXN, SPARC and TDRD7 among others. Notable differences include higher expression in SRA01/04 of CRYBB2, ALDH1A3, COL14A1, LAMA1, RRAGA and STX3 compared to 21EM15 cells. Additionally, PROX1, MAF, MEIS1, ITGA6, SPP1 and other important lens genes exhibit lower expression in SRA01/04 cells compared to 21EM15 mouse LECs.
Fig. 3.
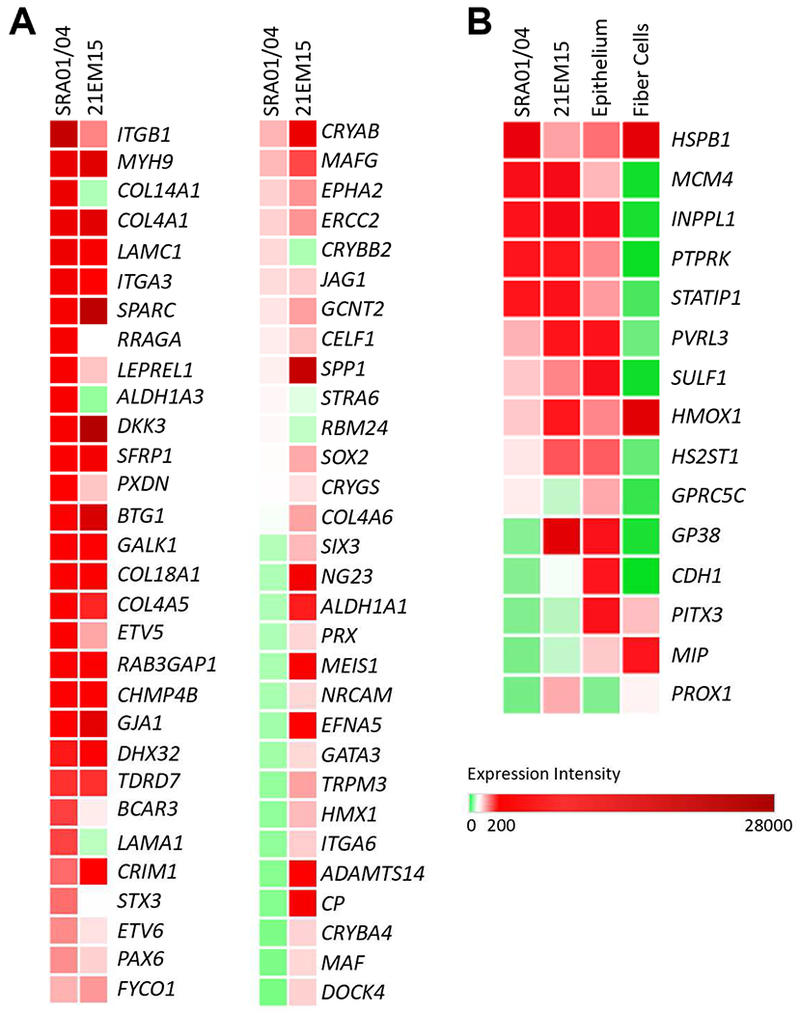
Comparative analysis of gene expression between SRA01/04, mouse LEC 21EM15 and isolated epithelial and lens fiber cells. (A) Comparative expression analysis of key lens genes in the LECs SRA01/04 and 21EM15. The expression profiles exhibit largely similar patterns with some notable differences. (B) Comparative expression analysis of key lens genes in SRA01/04, 21EM15, and previously reported isolated mouse lens epithelium and fiber cell data demonstrates the epithelial-like character of LECs.
Next, SRA01/04 gene expression was compared to previously reported isolated mouse lens fiber cell and epithelium microarrays from another group (Nakahara et al., 2007) (Fig. 3B). We selected genes that are conserved between mouse and human and show differential expression between lens fiber and epithelium. Largely, the expression patterns of these genes in SRA01/04, 21EM15 and isolated lens epithelial cells are similar, with some notable exceptions. Specifically, the expression profile of SRA01/04 recapitulates many of the key differences in gene expression between lens epithelial and fiber cells, such as high expression of the epithelial genes MCM4 and PTPRK, and low expression of the fiber cell-enriched gene MIP as well other key fiber cell genes PROX1 and MAF as mentioned above. Notably, this analysis shows that, unlike SRA01/04, the mouse LEC 21EM15 robustly expresses key fiber cell genes PROX1 and MAF and does not express the epithelial-cell enriched gene GPRC5C. This suggests differences in the potential of these two cell lines to initiate fiber cell-like expression. Indeed, 21EM15 cells spontaneously aggregate into 3D spheres, termed lentoid bodies (LBs), while SRA01/04 do not (Fig. 4A, B).
Fig. 4.
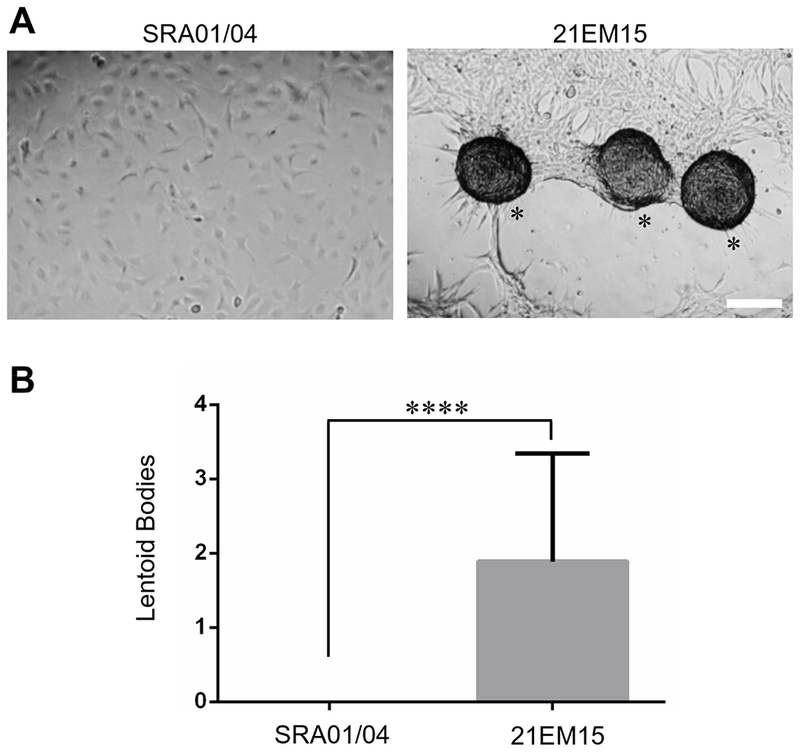
Comparison of lentoid body formation in SRA01/04 and 21EM15 cells. (A) Comparative analysis of SRA01/04 and 21EM15 cell culture demonstrates that SRA01/04 have reduced potential to spontaneously form lentoid bodies (LBs) as compared to 21EM15 (LBs are indicated by an asterisk). Scale bar = 100μm. (B) Quantification of spontaneous LBs formation in SRA01/04 and 21EM15. 21EM15 formed significantly more LBs (Average = 1.89) in a defined region compared to SRA01/04 (Average = 0) as determined by nested analysis of variance (ANOVA) (p-value = 0.000070).
3.4. RT-PCR Validates Expression of Key Genes in SRA01/04
Finally, we sought to independently validate expression of genes indicated by microarrays in the SRA01/04 LEC. We examined expression of several high-priority genes (Table 3) by RT-PCR assay. These candidate genes included crystallins CRYAB, CRYBB2, CRYGA, CRYGS as well as others such as DKK3, EPHA2, ETV5, MCM4, PAX6 and TDRD7 that exhibited enriched expression in lens development and/or are associated with ocular defects, including cataract. Another human-derived cell line, HEK293T kidney cells were used for comparison (Fig. 5). Further, several lens-enriched genes (CRYAB, DKK3, EPHA2, ETV5, ITGB1, SFRP1, VIM) were also independently validated for their enriched expression in SRA01/04 cells by real-time quantitative PCR (RT-qPCR) (Fig. 6). Additionally, we validated that the key lens fiber cell transcription factor PROX1 is not significantly expressed in the SRA01/04 LEC (Fig. 6). These data independently validate expression and enrichment of several key lens genes in SRA01/04 cells.
Fig. 5.
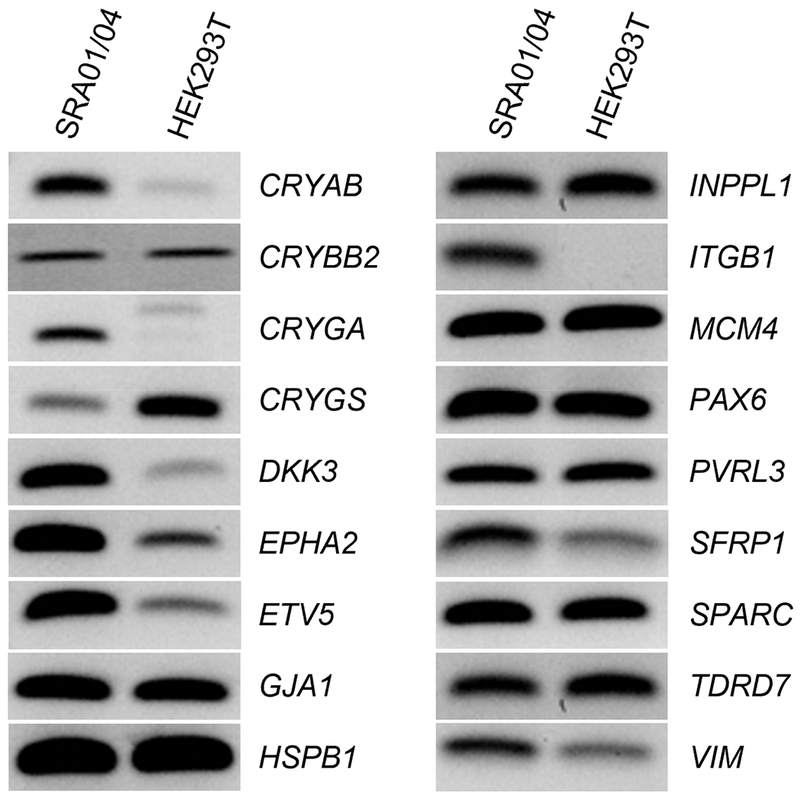
Semi-quantitative RT-PCR validation of key lens genes for SRA01/04 and Human Kidney HEK293T cell lines. RT-PCR validation of expression of several high-priority lens genes that were identified in SRA01/04 microarrays. The genes CRYAB, CRYBB2, CRYGA, CRYGS, DKK3, EPHA2, ETV5, GJA1, HSPB1, INPPL1, ITGB1, MCM4, PAX6, PVRL3, SFRP1, SPARC, TDRD7, and VIM exhibit robust expression in SRA01/04.
Fig. 6.
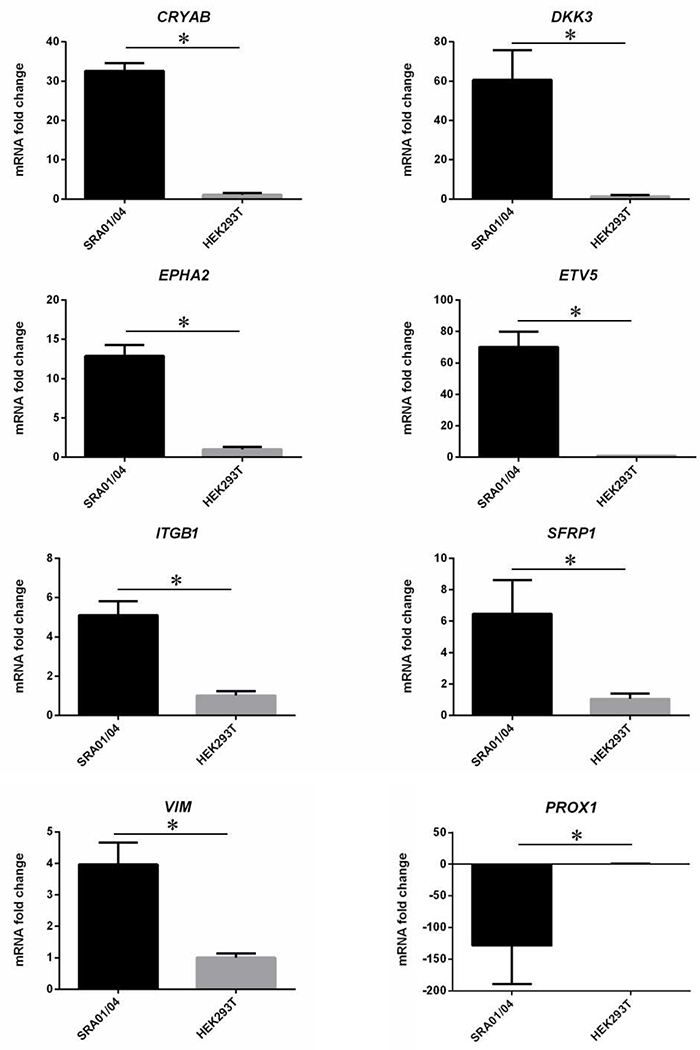
Quantitative real-time PCR of lens-enriched genes in SRA01/04 and HEK293T cell lines. Several robustly expressed lens genes (CRYAB, DKK3, EPHA2, ETV5, ITGB1, SFRP1, VIM) were subjected to RT-qPCR and were found to be significantly enriched in SRA01/04 cells. The lens fiber cell gene PROX1 was also examined by RT-qPCR analysis and was found to be significantly downregulated in SRA01/04 compared with HEK293T. RT-qPCR analysis was performed in biological triplicates and technical triplicates, ΔΔCT method used to calculate fold change, and nested ANOVA was used to determine significance. Asterisks denote a statistically significant difference (p<0.05).
4. Discussion
Here, we report the molecular characterization of gene expression of the human LEC SRA01/04. This cell line has been widely used as a reagent in lens studies (reported in at least 70 publications), but until now its human origin and lens epithelium character had not been thoroughly assessed. In this study, we performed STR analysis to first validate the human origin of SRA01/04 cells. Eight human-specific primers yielded positive amplification for the human-specific STR regions, while no amplification was observed for eight mouse region-specific primers, indicating that SRA01/04 are, indeed, of human origin.
Illumina HumanHT-12 v3.0 Expression BeadChip microarrays were used to characterize global mRNA expression patterns of SRA01/04. This was followed by a detailed comparative gene expression analysis between SRA01/04 microarrays and the lens using expression information in the iSyTE database in order to determine lens-expressed or lens-enriched expressed genes that are potentially also expressed in the cell line. Further, SRA01/04 gene expression was also compared to genes in the Cat-Map database, and to the previously characterized mouse LEC 21EM15 (Terrell et al., 2015), the human kidney cell line HEK293T (Dubridge et al., 1987; Lin et al., 2014), and to isolated lens epithelium and fiber cells.
iSyTE and Cat-Map based analysis of genes expressed in SRA01/04 show that many genes important for lens development and homeostasis, or those associated with cataract are expressed in this cell line, including key transcription factors such as PAX6, MAFG, and SOX2. Notably, some important fiber cell transcription factors, notably MAF and PROX1, are not significantly expressed (based on our stringent cut-off of expression intensity ≥100) in SRA01/04 cells, similar to the fiber cell factor MIP. Our analysis shows that SRA01/04 cells express several important cataract-associated genes including CRYAB, CRYBB2, CRYGS, FYCO1, GCNT2, GJA1, MAFG, MYH9, PAX6, PVRL3, PXDN, RAB3GAP1, TDRD7, and VIM. However, robust expression of the following key lens development or cataract associated genes was not observed in SRA01/04 cells: ALDH1A1, COL4A6, CP, CRYBA4, FOXE3, HMX1, HSF4, MAF, MEIS1, PITX3, PRX, SIX3, and TRPM3, among many others. Thus, this limitation of SRA01/04 cells not expressing several key factors important for lens development should be considered prior to their selection for specific downstream studies. The detailed microarray profile provided in this study (Supplementary Table S1) will serve to inform on the expression (or lack thereof) regarding genes of interest. However, we note that microarrays-based gene expression profiling is limited to optimal probe-binding kinetics and probe(s) design based on known gene sequences, and therefore may not be comprehensive and may include false negatives. Therefore, we suggest that researchers independently validate by RT-PCR or RT-qPCR the expression of their gene of interest, in addition to evaluation of the expression in the present microarray data, prior to initiating initiating detailed investigation. Further, the present analysis does not inform on the expression of key lens factors on the level of protein. Thus, in future, characterization of SRA01/04 and other cell lines should be performed by RNA-sequencing and proteome analyses.
Further, comparative analysis of SRA01/04 expression patterns with that of isolated mouse lens epithelium and fiber cells demonstrates expression of epithelium-enriched genes, and lack of expression of key fiber cell genes, demonstrating some extent of lens epithelial-like character. Moreover, compared to the previously validated mouse LEC 21EM15, SRA01/04 does not express key fiber cell genes (e.g. MAF, PROX1, PRX), as discussed above. This is in agreement with the observation that 21EM15 cells spontaneously form lentoid bodies, while SRA01/04 cells do not exhibit this property. While it is currently not known if these 21EM15-derived lentoid bodies exhibit ectopic fiber cell differentiation, stem cell-derived transparent LBs are known to exhibit fiber cell-like gene expression (Fu et al., 2017; Qin et al., 2019; Yang et al., 2010). Nevertheless, this differential potential in forming LBs may reflect differences in intrinsic fiber cell differentiation potential between these two lens epithelium-derived cell lines. This data also serves to indicate that different lens cell lines, while commonly derived from the lens-epithelium, may still have varied intrinsic differentiation potential. Further, this comparison highlights that, despite broadly similar gene expression profiles, differential expression of a small number of key genes (e.g. MAF, PROX1, MIP) may underlie different properties of cell lines. It will be interesting in the future to further understand the molecular nature of these aggregates of 21EM15 lens epithelial cells. In addition, investigation into whether the potential of LECs, including 21EM15 and SRA01/04, to form lentoid bodies is influenced by exposure to signaling factors such as FGF will be insightful.
4.1. Conclusions
In summary, our data demonstrate that the human LEC SRA01/04 expresses several important genes that exhibit enriched expression in the lens and/or are associated with cataract. The global gene expression profile of SRA01/04 described here will be useful to the eye research community for examining if specific factors of interest and their downstream targets are indeed expressed in LECs, as a measure of suitability of these resources, before commencing their work to investigate the function of these factors. Notably, while the expression profile of SRA01/04 shows some similarities to isolated lens epithelium and to the previously characterized mouse LEC 21EM15, there are some important differences. These differences in gene expression may account for the different potential of these two cell lines, SRA01/04 and 21EM15, to form lentoid bodies. It is also important to note that while some gene expression similarities are observed between the two LECs and isolated lens epithelium, and that SRA01/04 does support expression of several genes relevant to lens biology and cataract, the stoichiometry of gene expression levels may not necessary be similar to that in cells of the in vivo lens tissue. Thus, it is recommended that any cell line based investigation is in conjunction with in vivo animal model studies or supported by primary cell culture.
In conclusion, we have authenticated the human origin and characterized the global gene expression of SRA01/04, which will allow the eye research community to have an informed assessment regarding the suitability of these cells as a reagent in ocular studies. This work will allow both retrospective evaluation of previously published work, and prospective assessment of future studies that use the SRA01/04 cell line. With the development of new eye gene discovery tools (Anand and Lachke, 2017; Budak et al., 2018; Kakrana et al., 2018; Lachke et al., 2012; Srivastava et al., 2017) and along with animal models, these cells will find increased utility in rapid mechanistic investigations of new genes linked to lens biology.
Supplementary Material
Highlights.
Validated that the lens epithelium-derived cell line SRA01/04 is of human origin
SRA01/04 expresses many iSyTE lens enriched- and Cat-Map cataract-associated genes
SRA01/04 mRNA expression profile shows similarities to isolated lens epithelium
The LECs SRA01/04 and 21EM15 differ in spontaneous lentoid body formation
SRA01/04 expresses PAX6, EPHA2, CRYAB, CRYBB2, SPARC, TDRD7 among other lens genes
Acknowledgments
This work was supported by the National Eye Institute of the National Institutes of Health under award number R01EY021505 [to S.L.]. B.A.T.W. received support from the Milton H. Stetson Memorial Summer Fellowship. J.R.B. and B.A.T.W. were supported by the Delaware Governor’s Bioscience Summer Fellowship. A.D. was supported by a Fight For Sight Summer Research Fellowship. The authors have no commercial interests. This work is dedicated to the memory of Dr. Venkat Reddy who originally derived the SRA01/04 cell line.
Grant support: Supported by National Institutes of Health (NIH) Grant R01 EY021505 to Dr. Salil A. Lachke
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal SA, Anand D, Siddam AD, Kakrana A, Dash S, Scheiblin DA, Dang CA, Terrell AM, Waters SM, Singh A, Motohashi H, Yamamoto M, Lachke SA, 2015. Compound mouse mutants of bZIP transcription factors Mafg and Mafk reveal a regulatory network of non-crystallin genes associated with cataract. Hum. Genet 134, 717–35. 10.1007/s00439-015-1554-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Hill C, Cole K, 2014. Mouse cell line authentication. Cytotechnology 66, 133–147. 10.1007/s10616-013-9545-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann CR, Chow RL, Lang RA, Hemmati-Brivanlou A, 1997. Lens Induction by Pax-6 in Xenopus laevis. Dev. Biol 185, 119–123. 10.1006/dbio.1997.8573 [DOI] [PubMed] [Google Scholar]
- Anand D, Agrawal SA, Siddam AD, Motohashi H, Yamamoto M, Lachke SA, 2015. An integrative approach to analyze microarray datasets for prioritization of genes relevant to lens biology and disease. Genomics Data 5, 223–227. 10.1016/j.gdata.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand D, Lachke SA, 2017. Systems biology of lens development: A paradigm for disease gene discovery in the eye. Exp. Eye Res 156, 22–33. 10.1016/j.exer.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang SJ, Stump RJW, Lovicu FJ, McAvoy JW, 2004. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr. Patterns 4, 289–295. 10.1016/j.modgep.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Antignac C, Zhou J, Sanak M, Cochat P, Roussel B, Deschênes G, Gros F, Knebelmann B, Hors-Cayla M-C, Tryggvason K, Gubler M-C, 1992. Alport syndrome and diffuse leiomyomatosis: Deletions in the 5’ end of the COL4A5 collagen gene. Kidney Int. 42, 1178–1183. https://doi.oig/10.1038/ki.1992.402 [DOI] [PubMed] [Google Scholar]
- Astuti GDN, Born LI Vanden, Khan MI, Hamel CP, Bocquet B, Manes G, Quinodoz M, Ali M, Toomes C, McKibbin M, El-Asrag ME, Haer-Wigman L, Inglehearn CF, Black GCM, Hoyng CB, Cremers FPM, Roosing S, 2018. Identification of Inherited Retinal Disease-Associated Genetic Variants in 11 Candidate Genes. Genes (Basel). 9, 21 10.3390/genes9010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATCC, 2016. Authentication Technology Applied at ATCC Reveals Misidentified Cell Lines [WWW Document]. Authentication Technol. Appl. ATCC Reveal. Misidentified Cell Lines; URL https://www.atcc.org/Products/Cells_and_Microorganisms/Cell_Lines/Misidentified_Cell_Lines.aspx (accessed 5.30.18). [Google Scholar]
- Azari S, Ahmadi N, Tehrani MJ, Shokri F, 2007. Profiling and authentication of human cell lines using short tandem repeat (STR) loci: Report from the National Cell Bank of Iran. Biologicals 35, 195–202. 10.1016/j.biologicals.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Beebe DC, 2013. The Use of Cell Lines to “Model” Ocular Tissues: Cautionary Tales. Investig. Opthalmology Vis. Sci 54, 5720 10.1167/iovs.13-12873 [DOI] [PubMed] [Google Scholar]
- Berry V, Francis P, Reddy MA, Collyer D, Vithana E, MacKay I, Dawson G, Carey AH, Moore A, Bhattacharya SS, Quinlan RA, 2001. Alpha-B Crystallin Gene (CRYAB) Mutation Causes Dominant Congenital Posterior Polar Cataract in Humans. Am. J. Hum. Genet 69, 1141–1145. 10.1086/324158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budak G, Dash S, Srivastava R, Lachke SA, Chandra Janga S, 2018. Express: A database of transcriptome profiles encompassing known and novel transcripts across multiple development stages in eye tissues. Exp. Eye Res 168, 57–68. 10.1016/j.exer.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock SL, Fletcher JM, Beddington RS, Wilson VA, 1998. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 12, 1894–1906. 10.1101/gad.12.12.1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-H, Huang C, Zhang B, Yin S, Liang J, Xu C, Huang Y, Cen L-P, Ng T-K, Zheng C, Zhang S, Chen H, Pang C-P, Zhang M, 2016. Mutations of RagA GTPase in mTORC1 Pathway Are Associated with Autosomal Dominant Cataracts. PLoS Genet. 12, e1006090 10.1371/journal.pgen.1006090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, Kantorow M, Pras E, Frydman M, Pras E, Riazuddin S, Riazuddin SA, Hejtmancik JF, 2011. Mutations in FYCO1 Cause Autosomal-Recessive Congenital Cataracts. Am. J. Hum. Genet 88, 827–838. 10.1016/j.ajhg.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Chen X, Hu Z, Lin H, Zhou F, Luo L, Zhang X, Zhong X, Yang Y, Wu C, Lin Z, Ye S, Liu Y, for the study group of, C., 2013. A Missense Mutation in CRYBB2 Leads to Progressive Congenital Membranous Cataract by Impacting the Solubility and Function of βB2-Crystallin. PLoS One 8, e81290 10.1371/journal.pone.0081290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stump RJW, Lovicu FJ, McAvoy JW, 2004. Expression of Frizzleds and secreted frizzled-related proteins (Sfrps) during mammalian lens development. Int. J. Dev. Biol 48, 867–877. 10.1387/ijdb.041882yc [DOI] [PubMed] [Google Scholar]
- Chograni M, Alkuraya FS, Ourteni I, Maazoul F, Lariani I, Chaabouni HB, 2015. Autosomal recessive congenital cataract, intellectual disability phenotype linked to STX3 in a consanguineous Tunisian family. Clin. Genet 88, 283–287. 10.1111/cge.12489 [DOI] [PubMed] [Google Scholar]
- Cvekl A, Yang Y, Chauhan BK, Cveklova K, 2004. Regulation of gene expression by Pax6 in ocular cells: a case of tissue-preferred expression of crystallins in lens. Int. J. Dev. Biol 48, 829–844. 10.1387/ijdb.041866ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Zhang X, 2017. Signaling and Gene Regulatory Networks in Mammalian Lens Development. Trends Genet. 33, 677–702. 10.1016/j.tig.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E, 1999. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development 126, 3957–3968. [DOI] [PubMed] [Google Scholar]
- Donner AL, Episkopou V, Maas RL, 2007. Sox2 and Pou2f1 interact to control lens and olfactory placode development. Dev. Biol 303, 784–799. 10.1016/j.ydbio.2006.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner AL, Lachke SA, Maas RL, 2006. Lens induction in vertebrates: Variations on a conserved theme of signaling events. Semin. Cell Dev. Biol 17, 676–685. 10.1016/j.semcdb.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Dubridge RB, Tang P, Hsia HANC, Leong P, Miller JH, Calos MP, 1987. Analysis of Mutation in Human Cells by Using an Epstein-Barr Virus Shuttle System. Mol. Cell. Biol 10.1128/MCB.7.1.379.Updated [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes J, Ragge NK, Lynch S-A, McGill NI, Collin JRO, Howard-Peebles PN, Hayward C, Vivian AJ, Williamson K, van Heyningen V, FitzPatrick DR, 2003. Mutations in SOX2 cause anophthalmia. Nat. Genet 33, 461–463. 10.1038/ng1120 [DOI] [PubMed] [Google Scholar]
- Fu Q, Qin Z, Jin X, Zhang L, Chen Z, He J, Yao K, 2017. Generation of Functional Lentoid Bodies From Human Induced Pluripotent Stem Cells Derived From Urinary Cells. Invest. Ophthalmol. Vis. Sci 58, 517–527. 10.1167/iovs.16-20504 [DOI] [PubMed] [Google Scholar]
- Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL, 1994. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat. Genet 7, 463–471. 10.1038/ng0894-463 [DOI] [PubMed] [Google Scholar]
- Güven M, Unal M, Batar B, Eroğlu E, Devarnoğlu K, Tamçelik N, Uçar D, Sarici A, 2007. Polymorphisms of DNA repair genes XRCC1 and XPD and risk of primary open angle glaucoma (POAG). Mol. Vis 13, 12. [PMC free article] [PubMed] [Google Scholar]
- Handley MT, Morris-Rosendahl DJ, Brown S, Macdonald F, Hardy C, Bem D, Carpanini SM, Borck G, Martorell L, Izzi C, Faravelli F, Accorsi P, Pinelli L, Basel-Vanagaite L, Peretz G, Abdel-Salam GMH, Zaki MS, Jansen A, Mowat D, Glass I, Stewart H, Mancini G, Lederer D, Roscioli T, Giuliano F, Plomp AS, Rolfs A, Graham JM, Seemanova E, Poo P, García-Cazorla A, Edery P, Jackson IJ, Maher ER, Aligianis IA, 2013. Mutation spectrum in RAB3GAP1, RAB3GAP2, and RAB18 and genotype-phenotype correlations in warburg micro syndrome and Martsolf syndrome. Hum. Mutat 34, 686–696. 10.1002/humu.22296 [DOI] [PubMed] [Google Scholar]
- He S, Pirity MK, Wang W-L, Wolf L, Chauhan BK, Cveklova K, Tamm ER, Ashery-Padan R, Metzger D, Nakai A, Chambon P, Zavadil J, Cvekl A, 2010. Chromatin remodeling enzyme Brg1 is required for mouse lens fiber cell terminal differentiation and its denucleation. Epigenetics Chromatin 3, 21 10.1186/1756-8935-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TV, Kumar PKR, Sutharzan S, Tsonis PA, Liang C, Robinson ML, 2014. Comparative transcriptome analysis of epithelial and fiber cells in newborn mouse lenses with RNA sequencing. Mol. Vis 20, 1491–1517. [PMC free article] [PubMed] [Google Scholar]
- Hughes AE, Bradley DT, Campbell M, Lechner J, Dash DP, Simpson DA, Willoughby CE, 2011. Mutation Altering the miR-184 Seed Region Causes Familial Keratoconus with Cataract. Am. J. Hum. Genet 89, 628–633. 10.1016/j.ajhg.2011.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaraki N, Chen S-C, Lin L-R, Okamoto H, Pipas JM, Reddy VN, 1998. Human Lens Epithelial Cell Line. Exp. Eye Res 67, 577–585. 10.1006/exer.1998.0551 [DOI] [PubMed] [Google Scholar]
- Kakrana A, Yang A, Anand D, Djordjevic D, Ramachandruni D, Singh A, Huang H, Ho JWK, Lachke SA, 2018. iSyTE 2.0: a database for expression-based gene discovery in the eye. Nucleic Acids Res. 46, D885 10.1093/nar/gkx837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus I, Salmen B, Viebahn C, von Figura K, Schmitz D, D’Hooge R, Dierks T, 2009. Differential involvement of the extracellular 6-O-endosulfatases Sulf1 and Sulf2 in brain development and neuronal and behavioural plasticity. J. Cell. Mol. Med 13, 4505–4521. 10.1111/j.1582-4934.2008.00558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaid A, Giráldez F, 2008. Btg1 and Btg2 gene expression during early chick development. Dev. Dyn. An Off. Publ. Am. Assoc. Anat 237, 2158–2169. 10.1002/dvdy.21616 [DOI] [PubMed] [Google Scholar]
- Khan K, Rudkin A, Parry DA, Burdon KP, McKibbin M, Logan CV, Abdelhamed ZIA, Muecke JS, Fernandez-Fuentes N, Laurie KJ, Shires M, Fogarty R, Carr IM, Poulter JA, Morgan JE, Mohamed MD, Jafri H, Raashid Y, Meng N, Piseth H, Toomes C, Casson RJ, Taylor GR, Hammerton M, Sheridan E, Johnson CA, Inglehearn CF, Craig JE, Ali M, 2011. Homozygous Mutations in PXDN Cause Congenital Cataract, Corneal Opacity, and Developmental Glaucoma. Am. J. Hum. Genet 89, 464–473. 10.1016/j.ajhg.20n.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Clark AF, Daudt D, Vishwanatha JK, Yorio T, 2013. A forensic path to RGC-5 cell line identification: lessons learned. Invest. Ophthalmol. Vis. Sci 54, 5712–5719. 10.1167/iovs.13-12085 [DOI] [PubMed] [Google Scholar]
- Kunishima S, Matsushita T, Kojima T, Amemiya N, Choi YM, Hosaka N, Inoue M, Jung Y, Mamiya S, Matsumoto K, Miyajima Y, Zhang G, Ruan C, Saito K, Song KS, Yoon HJ, Kamiya T, Saito H, 2001. Identification of six novel MYH9 mutations and genotype-phenotype relationships in autosomal dominant macrothrombocytopenia with leukocyte inclusions. J. Hum. Genet 46, 722–729. 10.1007/s100380170007 [DOI] [PubMed] [Google Scholar]
- Lachke SA, Alkuraya FS, Kneeland SC, Ohn T, Aboukhalil A, Howell GR, Saadi I, Cavallesco R, Yue Y, Tsai AC-H, Nair KS, Cosma MI, Smith RS, Hodges E, AlFadhli SM, Al-Hajeri A, Shamseldin HE, Behbehani A, Hannon GJ, Bulyk ML, Drack AV, Anderson PJ, John SWM, Maas RL, 2011. Mutations in the RNA Granule Component TDRD7 Cause Cataract and Glaucoma. Science. 331, 1571–1576. 10.1126/science.1195970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Ho JWK, Kryukov GV, O’Connell DJ, Aboukhalil A, Bulyk ML, Park PJ, Maas RL, 2012. iSyTE : Integrated Systems Tool for Eye Gene Discovery. Investig. Opthalmology Vis. Sci 53, 1617 10.1167/iovs.11-8839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Maas RL, 2010. Building the developmental oculome: Systems biology in vertebrate eye development and disease. Wiley Interdiscip. Rev. Syst. Biol. Med 2, 305–323. 10.1002/wsbm.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Conley KW, Mead TJ, Rowan S, Yutzey KE, Brown NL, 2012. Requirements for Jag1-Rbpj mediated Notch signaling during early mouse lens development. Dev. Dyn. An Off. Publ. Am. Assoc. Anat 241, 493–504. 10.1002/dvdy.23739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-C, Boone M, Meuris L, Lemmens I, Roy N Van, Soete A, Reumers J, Moisse M, Plaisance S, Drmanac R, Chen J, Speleman F, Lambrechts D, Peer Y Van de, Tavernier J, Callewaert N, 2014. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Commun 5, 4767 10.1038/ncomms5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin M, O’Rourke D, Kurihara T, Juliano CE, Harrison KL, Hutson LD, 2008. Developmental expression patterns of the zebrafish small heat shock proteins. Dev. Dyn. An Off. Publ. Am. Assoc. Anat 237, 454–463. 10.1002/dvdy.21414 [DOI] [PubMed] [Google Scholar]
- Menzel O, Bekkeheien RCJ, Reymond A, Fukai N, Boye E, Kosztolanyi G, Aftimos S, Deutsch S, Scott HS, Olsen BR, Antonarakis SE, Guipponi M, 2004. Knobloch syndrome: novel mutations in COL18A1, evidence for genetic heterogeneity, and a functionally impaired polymorphism in endostatin. Hum. Mutat 23, 77–84. 10.1002/humu.10284 [DOI] [PubMed] [Google Scholar]
- Mordechai S, Gradstein L, Pasanen A, Ofir R, El Amour K, Levy J, Belfair N, Lifshitz T, Joshua S, Narkis G, Elbedour K, Myllyharju J, Birk OS, 2011. High Myopia Caused by a Mutation in LEPREL1, Encoding Prolyl 3-Hydroxylase 2. Am. J. Hum. Genet 89, 438–445. 10.1016/j.ajhg.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Bhattacharya SS, Moore T, Prescott Q, Wedig T, Herrmann H, Magin TM, 2009. Dominant cataract formation in association with a vimentin assembly disrupting mutation. Hum. Mol. Genet 18, 1052–1057. 10.1093/hmg/ddn440 [DOI] [PubMed] [Google Scholar]
- Nakahara M, Nagasaka A, Koike M, Uchida K, Kawane K, Uchiyama Y, Nagata S, 2007. Degradation of nuclear DNA by DNase II-like acid DNase in cortical fiber cells of mouse eye lens. FEBS J. 274, 3055–3064. 10.1111/j.1742-46582007.05836.x. [DOI] [PubMed] [Google Scholar]
- Near RI, Smith RS, Toselli PA, Freddo TF, Bloom AB, Vanden Borre P, Seldin DC, Lerner A, 2009. Loss of AND-34/BCAR3 expression in mice results in rupture of the adult lens. Mol. Vis 15, 685. [PMC free article] [PubMed] [Google Scholar]
- Okano Y, Asada M, Fujimoto A, Ohtake A, Murayama K, Hsiao KJ, Choeh K, Yang Y, Cao Q, Reichardt JK, Niihira S, Imamura T, Yamano T, 2001. A genetic factor for age-related cataract: identification and characterization of a novel galactokinase variant, “Osaka,” in Asians. Am. J. Hum. Genet 68, 1036–1042. 10.1086/319512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padma G, Mamata M, Reddy KRK, Padma T, 2011. Polymorphisms in two DNA repair genes (XPD and XRCC1) – association with age related cataracts. Mol. Vis 17, 127–133. [PMC free article] [PubMed] [Google Scholar]
- Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nürnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernández-Martínez L, Keating S, Mortier G, Hennekam RCM, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nürnberg P, Reis A, Rauch A, 2007. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am. J. Hum. Genet 80, 550–560. 10.1086/512203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania M, Semina EV, Duncan MK, 2014. Lens extrusion from Laminin alpha 1 mutant zebrafish. ScientificWorldJournal. 2014, 524929 10.1155/2014/524929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW, 2003. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet 72, 408–418. 10.1086/346090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi DJ, Wilkinson L, Kolle G, Sohaskey ML, Gillinder K, Piper MJ, McAvoy JW, Lovicu FJ, Little MH, 2007. Crim1KST264/KST264 mice display a disruption of the Crim1 gene resulting in perinatal lethality with defects in multiple organ systems. Dev. Dyn. An Off. Publ. Am. Assoc. Anat 236, 502–511. 10.1002/dvdy.21015 [DOI] [PubMed] [Google Scholar]
- Qin Z, Zhang L, Lyu D, Li J, Tang Q, Yin H, Chen Z, Yao K, Fu Q, 2019. Opacification of lentoid bodies derived from human induced pluripotent stem cells is accelerated by hydrogen peroxide and involves protein aggregation. J. Cell Physiol 1–13. 10.1002/jcp.28943 [DOI] [PubMed] [Google Scholar]
- Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, Soininen R, 2003. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 22, 236–245. 10.1093/emboj/cdg019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzymkiewicz DM, Reddan JR, Andley UP, 2000. Induction of heme oxygenase-1 modulates cis-aconitase activity in lens epithelial cells. Biochem. Biophys. Res. Commun 270, 324–328. 10.1006/bbrc.2000.2408 [DOI] [PubMed] [Google Scholar]
- Saika S, Miyamoto T, Ishida I, Ohnishi Y, Ooshima A, 2003. Osteopontin: a component of matrix in capsular opacification and subcapsular cataract. Invest. Ophthalmol. Vis. Sci 44, 1622–1628. [DOI] [PubMed] [Google Scholar]
- Semerci CN, Kalay E, Yildirim C, Dinçer T, Olmez A, Toraman B, Koçyiğit A, Bulgu Y, Okur V, Satiroğlu-Tufan L, Akarsu NA, 2014. Novel splice-site and missense mutations in the ALDH1A3 gene underlying autosomal recessive anophthalmia/microphthalmia. Br. J. Ophthalmol 98, 832–840. 10.1136/bjophthalmol-2013-304058 [DOI] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Hejtmancik JF, 2010. Cat-Map: putting cataract on the map. Mol. Vis 16, 2007. [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HLS, Maraini G, Li A, Jiao X, Hejtmancik JF, 2008. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol. Vis 14, 2042. [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HLS, Yamada K, Yoshiura K, Niikawa N, Shim S, Hanson PI, 2007. CHMP4B, a Novel Gene for Autosomal Dominant Cataracts Linked to Chromosome 20q. Am. J. Hum. Genet 81, 596–606. 10.1086/519980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddam AD, Gautier-Courteille C, Perez-Campos L, Anand D, Kakrana A, Dang CA, Legagneux V, Méreau A, Viet J, Gross JM, Paillard L, Lachke SA, 2018. The RNA-binding protein Celf1 post-transcriptionally regulates p27Kip1 and Dnase2b to control fiber cell nuclear degradation in lens development. PLoS Genet. 14, e1007278 10.1371/journal.pgen.1007278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simirskii VN, Wang Y, Duncan MK, 2007. Conditional deletion of beta1-integrin from the developing lens leads to loss of the lens epithelial phenotype. Dev. Biol 306, 658–668. 10.1016/j.ydbio.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R, Budak G, Dash S, Lachke SA, Chandra Janga S, 2017. Transcriptome analysis of developing lens reveals abundance of novel transcripts and extensive splicing alterations. Sci. Rep 7, 1–17. 10.1038/s41598-017-10615-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Ma Z, Li Y, Liu B, Li Z, Ding X, Gao Y, Ma W, Tang X, Li X, Shen Y, 2005. Gamma-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. J. Med. Genet 42, 706–710. 10.1136/jmg.2004.028274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Kunishima S, Ikejiri M, Maruyama S, Sone M, Takagi A, Ikawa M, Okabe M, Kojima T, Saito H, Naoe T, Matsushita T, 2013. Establishment of mouse model of MYH9 disorders: heterozygous R702C mutation provokes macrothrombocytopenia with leukocyte inclusion bodies, renal glomerulosclerosis and hearing disability. PLoS One 8, e71187 10.1371/journal.pone.0071187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Hosokawa M, Vagin VV, Reuter M, Hayashi E, Mochizuki AL, Kitamura K, Yamanaka H, Kondoh G, Okawa K, Kuramochi-Miyagawa S, Nakano T, Sachidanandam R, Hannon GJ, Pillai RS, Nakatsuji N, Chuma S, 2011. Tudor domain containing 7 (Tdrd7) is essential for dynamic ribonucleoprotein (RNP) remodeling of chromatoid bodies during spermatogenesis. Proc. Natl. Acad. Sci. U. S. A 108, 10579–10584. 10.1073/pnas.1015447108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell AM, Anand D, Smith SF, Dang CA, Waters SM, Pathania M, Beebe DC, Lachke SA, 2015. Molecular characterization of mouse lens epithelial cell lines and their suitability to study RNA granules and cataract associated genes. Exp. Eye Res 131, 42–55. 10.1016/j.exer.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell AM, Anand D, Smith SF, Dang CA, Waters SM, Pathania M, Beebe DC, Lachke SA, 2015. Molecular characterization of mouse lens epithelial cell lines and their suitability to study RNA granules and cataract associated genes. Exp. Eye Res 131, 42–55. 10.1016/j.exer.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Editors of Experimental Eye Research, 2013. On the use of immortalized ocular cell lines in vision research: The unfortunate story of RGC-5. Exp. Eye Res 116, 433 10.1016/J.EXER.2013.08.002 [DOI] [PubMed] [Google Scholar]
- The Editors of Molecular Vision, 2013. On authentication of cell lines. Mol. Vis 19, 1848. [PMC free article] [PubMed] [Google Scholar]
- Tian L, Huang K, Duhadaway JB, Prendergast GC, Tian L, Huang K, Duhadaway JB, Prendergast GC, Tian L, Huang K, Duhadaway JB, Prendergast GC, 2010. Genomic Profiling of miRNAs in Two Human Lens Cell Lines Genomic Profiling of miRNAs in Two Human Lens Cell Lines. Curr. Eye Res 35, 812–818. 10.3109/02713683.2010.489182 [DOI] [PubMed] [Google Scholar]
- Van Agtmael T, Schlötzer-Schrehardt U, McKie L, Brownstein DG, Lee AW, Cross SH, Sado Y, Mullins JJ, Pöschl E, Jackson IJ, 2005. Dominant mutations of Co14a1 result in basement membrane defects which lead to anterior segment dysgenesis and glomerulopathy. Hum. Mol. Genet 14, 3161–3168. 10.1093/hmg/ddi348 [DOI] [PubMed] [Google Scholar]
- Weinstein BI, Schwartz J, Lonial H, Dominguez MO, Gordon GG, Hochstadt J, Southren DB, Dunn MW, Southren AL, 1982. Normal and conditionally transformed bovine lens epithelial cell lines containing alpha-and gamma-crystallin. Exp. Eye Res 34, 71–81. [DOI] [PubMed] [Google Scholar]
- Xie Q, McGreal R, Harris R, Gao CY, Liu W, Reneker LW, Musil LS, Cvekl A, 2016. Regulation of c-Maf and αA-Crystallin in Ocular Lens by Fibroblast Growth Factor Signaling. J. Biol. Chem 291, 3947–3958. 10.1074/jbc.M115.705103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yang Y, Brennan L, Bouhassira EE, Kantorow M, Cvekl A, 2010. Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions. FASEB J. 24, 3274–3283. 10.1096/fj.10-157255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmeen A, Riazuddin SA, Kaul H, Mohsin S, Khan M, Qazi ZA, Nasir IA, Zafar AU, Khan SN, Husnain T, Akram J, Hejtmancik JF, Riazuddin S, 2010. Autosomal recessive congenital cataract in consanguineous Pakistani families is associated with mutations in GALK1. Mol. Vis 16, 682. [PMC free article] [PubMed] [Google Scholar]
- Yu LC, Twu YC, Chang CY, Lin M, 2001. Molecular basis of the adult i phenotype and the gene responsible for the expression of the human blood group I antigen. Blood 98, 3840–3845. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Kreman M, 2000. Epithelial organization of the mammalian lens. Exp. Eye Res 71, 415–435. 10.1006/exer.2000.0895 [DOI] [PubMed] [Google Scholar]
- Zinkevich NS, Bosenko DV, Link BA, Semina EV, 2006. laminin alpha 1 gene is essential for normal lens development in zebrafish. BMC Dev. Biol 6, 13 10.1186/1471-213X-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


