Interleukin-27 (IL-27) is a heterodimeric cytokine composed of the subunits IL-27p28 and EBi3, and while the IL-27 heterodimer influences T cell activities, there is evidence that IL-27p28 can have EBi3-independent activities; however, their relevance to infection is unclear. Therefore, the studies presented here compared how IL-27p28 transgenics and IL-27p28−/− mice responded to the intracellular parasite Toxoplasma gondii.
Keywords: antibodies, T cell, IL-27, Toxoplasma, inflammation, antibody
ABSTRACT
Interleukin-27 (IL-27) is a heterodimeric cytokine composed of the subunits IL-27p28 and EBi3, and while the IL-27 heterodimer influences T cell activities, there is evidence that IL-27p28 can have EBi3-independent activities; however, their relevance to infection is unclear. Therefore, the studies presented here compared how IL-27p28 transgenics and IL-27p28−/− mice responded to the intracellular parasite Toxoplasma gondii. While the loss of IL-27p28 and its overexpression both result in increased susceptibility to T. gondii, the basis for this phenotype reveals distinct roles for IL-27p28. As a component of IL-27, IL-27p28 is critical to limit infection-induced T cell-mediated pathology, whereas the ectopic expression of IL-27p28 reduced the effector T cell population and had a major inhibitory effect on parasite-specific antibody titers and a failure to control parasite replication in the central nervous system. Indeed, transfer of immune serum to infected IL-27p28 transgenics resulted in reduced parasite burden and pathology. Thus, IL-27p28, independent of its role as a component of IL-27, can act as a negative regulator of humoral and cellular responses during toxoplasmosis.
INTRODUCTION
Several of the interleukin-6/12 (IL-6/12) family members are important for resistance to Toxoplasma gondii, and this is illustrated by the critical role of IL-12 in the development of cell-mediated immunity associated with the production of gamma interferon (IFN-γ) (1–3). IL-6 is also required for long-term control of T. gondii, but its protective function during toxoplasmosis is uncertain (4–6). IL-27 is a heterodimer composed of IL-27p28 and Epstein-Barr virus-induced gene 3 (EBi3), which is produced by monocytes, macrophages, and dendritic cells (DCs) in response to a variety of innate stimuli (7–9). During infection with T. gondii, IL-27 receptor (IL-27R) deficiency results in increased T cell responses, and these mice develop severe immune pathology associated with enhanced production of IFN-γ and reduced production of IL-10 (10–12). These suppressive effects of IL-27 are not restricted to toxoplasmosis, and similar immune hyperactivity is observed in IL-27R-deficient mice infected with a variety of other viral, bacterial, and parasitic organisms (11–15) or in IL-27p28−/− or EBi3−/− mice (16–19).
Consistent with its role as a member of the IL-6/12 family of cytokines, IL-27 signals through a heterodimeric receptor composed of IL-27Rα and the common cytokine receptor subunit gp130, which is also utilized by IL-6 (8, 20). In original models, it was proposed that IL-27p28 secretion would be dependent on EBi3 (8), but in mice IL-27p28 secretion is intact in the absence of EBi3 (21–23), which indicates that IL-27p28 has additional biological activities independent of its role as a component of IL-27. Indeed, IL-27p28 (which, like IL-6, is a 4 alpha helix bundle cytokine) can act as a low-affinity antagonist of the gp130 receptor and block the ability of IL-6 and IL-27 to signal (11, 23, 24). Alternatively, others have suggested that IL-27p28 alone or in combination with the soluble IL-6R can transduce a signal through gp130, although this activity appears 100- to 1,000-fold less efficient than that of the IL-27 heterodimer (25–27).
One approach to understanding the impact of IL-27p28 in the mouse system has been through the overexpression of individual subunits of this heterodimer to determine how they affect different disease states. Under normal circumstances, lymphocytes are largely negative for the individual IL-27 subunits, and in transgenic mice the Lck promoter has been utilized to drive expression of IL-27p28 or EBi3 by T and B cells (28). While these individual transgenic mice develop normally, the double transgenic mice lack Treg cells and display a scurfy-like disease associated with the ability of IL-27 to suppress IL-2 production (28, 29). The IL-27p28 transgenics have proven useful to understanding the impact of IL-27p28 on models of liver fibrosis and in experimental autoimmune encephalitis (EAE) and experimental autoimmune uveitis (EAU) (30–32). Additional studies on the IL-27p28 transgenics showed that in response to vaccination they had a major defect in the ability to form germinal center (GC) reactions and reduced antibody production (23), but whether infection can overcome this defect is unknown. Indeed, despite numerous reports that IL-27p28 is elevated during infection (17, 22, 33, 34), little is known about whether IL-27p28 (independent of its role in IL-27) impacts the immune response to a pathogen. The studies presented here compared the outcome of infection of the IL-27p28 transgenics with those of IL-27p28−/− mice, and while the loss of IL-27p28 results in the development of infection-induced pathology, its overexpression results in a modest reduction in the magnitude of the parasite-specific T cell response. However, the IL-27p28 transgenic mice showed increased susceptibility to chronic toxoplasmosis most closely associated with decreased GC formation and parasite-specific antibody titers and a reduced ability to control parasite replication in the central nervous system (CNS). Thus, IL-27p28, independent of its role as a component of IL-27, can act as a negative regulator of humoral and cellular responses during toxoplasmosis.
RESULTS
Differential functions of IL-27p28 during toxoplasmosis.
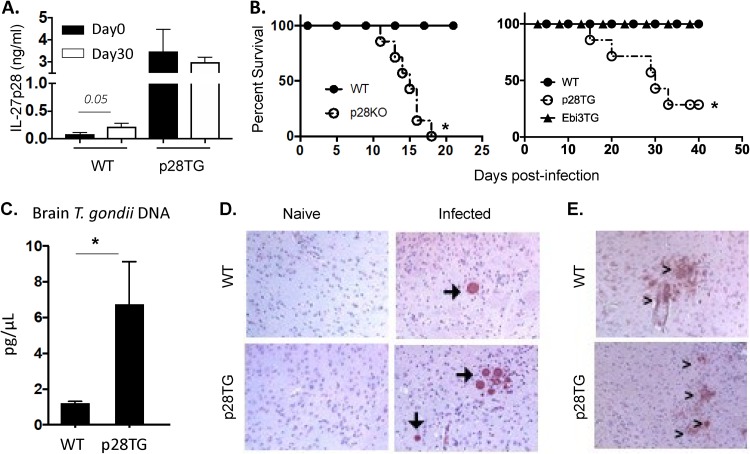
In wild-type (WT) mice, serum levels of IL-27p28 in uninfected mice are low but were elevated at day 30 postinfection, whereas in naive IL-27p28 transgenics, the constitutive levels of IL-27p28 were 10-fold higher than those of WT mice but were not altered after infection (Fig. 1A). Given the potential role of IL-27p28 as an antagonist of IL-6 and IL-27 signaling, studies were performed in which Lck-IL-27p28 and Lck-EBi3 transgenic mice (in which T and B cells overexpress these subunits) (28) were compared with IL-27p28−/− mice infected with T. gondii. As expected, WT mice survived infection but the IL-27p28−/− mice were acutely susceptible (Fig. 1B), associated with enhanced liver pathology and production of IFN-γ (see Fig. S1 in the supplemental material). Comparison of the different transgenic mouse strains revealed that, like the WT mice, the EBi3 transgenics survived the acute phase of infection and did not show any overt differences in susceptibility to the chronic phase of infection. In contrast, the IL-27p28 transgenic mice survived the acute phase of infection but displayed increased susceptibility to the chronic phase of this infection (Fig. 1B).
FIG 1.
Impact of IL-27p28 on T. gondii infection. (A) Systemic level of IL-27p28 in naive and infected (day 30) C57BL/6 (WT) and IL-27p28 transgenic (p28TG) mice (n = 5 to 10, means ± standard errors of the means [SEM]). (B) Survival of B6 WT controls (n = 8 to 10), IL-27p28−/−(n = 7), Ebi3TG (n = 10), and IL-27p28TG (n = 10) mice infected intraperitoneally with T. gondii. (C) Parasite burden was measured by qPCR for parasite DNA at day 30 p.i. using brain tissue from WT and TG mice (n = 12 to 13, from 4 experiments, means ± SEM). (D) Immunohistochemistry sections of the brain from WT and TG mice on day 30 p.i. had large regions of high cyst burdens (arrows) in the brain compared to WT mice. Images are representative of 6 independent experiments. (E) iNOS staining (>) in brain tissue on day 30 p.i. Magnification, ×10. An asterisk indicates significant differences between the indicated groups (P < 0.05).
To understand the basis for the increased susceptibility of the IL-27p28 transgenic mice, studies were performed to assess parasite burden. At day 5 postinfection, fluorescent T. gondii was used to quantify the numbers of parasites in the circulation and revealed that the parasitemia in WT and IL-27p28 transgenic mice was comparable (Fig. S2). The use of cytospins of peritoneal exudate cells at day 10 postinfection demonstrated that parasites were absent from both sets of mice, indicating control at the site of infection was intact in the transgenic mice. Similarly, histological analysis of the lungs and liver at this time point did not reveal any overt differences in the parasite burden or levels of immune pathology in these experimental groups. By day 30, while these tissues from WT and transgenic mice had reduced inflammation and few parasites (data not shown), the CNS of WT mice was characterized by the presence of parasite cysts, mild encephalitis, and infiltration of inflammatory cells (Fig. 1D). In contrast, the IL-27p28 transgenics had increased levels of parasite DNA in the brain (Fig. 1C) and large numbers of cysts were readily apparent, and there were areas of necrosis associated with extensive areas of parasite replication (Fig. 1D). The ability of IFN-γ to activate macrophage production of inducible nitric oxide synthase (iNOS) is an important effector mechanism required to control T. gondii in the CNS (35), and immunohistochemistry for iNOS in the brains of infected WT mice revealed discrete areas of iNOS staining associated with areas of parasite replication (Fig. 1E). In the IL-27p28 transgenics, prominent iNOS staining was also detected, indicating that this arm of the effector response was not overtly compromised. Thus, while the IL-27p28-deficient mice infected with T. gondii die of immune-mediated disease (10, 12, 33), the IL-27p28 transgenics are capable of early control of T. gondii, but susceptibility is associated with elevated levels of parasite replication in the CNS.
Impact of IL-27p28 overexpression on T cell responses to T. gondii.
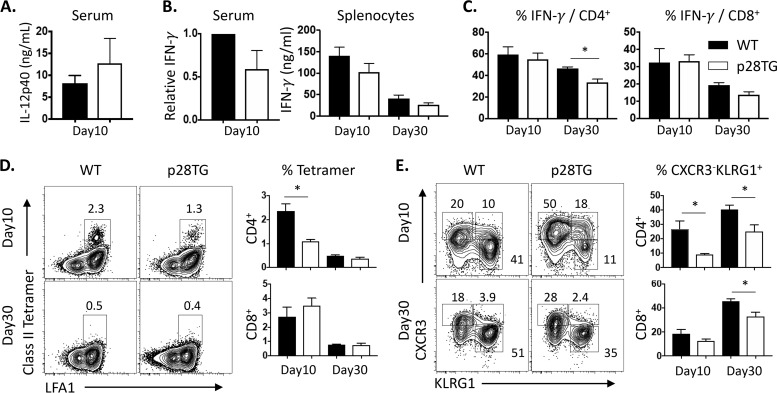
To determine the basis of the increased susceptibility of the IL-27p28 transgenic mice, the ability to produce IL-12 and IFN-γ and the quality of the T cell response in WT and transgenic mice were assessed during the acute (day 10) and chronic (day 30) stage of this infection. At day 10 postinfection, the WT and IL-27p28 transgenic mice had comparable circulating levels of IL-12p40 (Fig. 2A). However, while infection resulted in increased serum levels of IFN-γ, across five experiments this was decreased by 20 to 50% in the transgenic mice (Fig. 2B and Fig. S3A). However, at day 10 postinfection, when splenocytes from infected mice were cultured in the presence and absence of soluble Toxoplasma antigen (STag), the levels of IFN-γ produced by these mice were comparable (Fig. 2B). Similarly, at this time point the stimulation of splenocytes with phorbol myristate acetate-ionomycin (PMA-Iono) combined with intracellular staining for IFN-γ revealed the percentage of IFN-γ+ CD4+ and CD8+ T cell populations were increased in response to infection and were comparable in WT and transgenic mice. Without PMA-Iono, the low basal levels of IFN-γ produced by T cells from the spleen or peritoneal cavity were comparable, and these populations expressed high levels of T-bet (Fig. S3B and C). At day 30 postinfection, the levels of secretion of IFN-γ by splenocytes stimulated with STag were similar in WT and IL-27p28 transgenic mice, but in response to PMA-Iono there was a 15 to 20% reduction in the percentage of CD4+ T cells that produced IFN-γ, which was also apparent without stimulation (Fig. 2C and Fig. S3).
FIG 2.
Impact of IL-27p28 on T cell and effector cytokine response in toxoplasmosis. (A) Serum IL-12p40 was measured by ELISA at day 10 p.i. (B) Relative IFN-γ levels in IL-27p28 transgenic mice were calculated by WT level (day 10, 1 to ∼10 ng/ml; day 30, ∼1 ng/ml). (Left) ELISA in serum was performed with means from 3 to 5 experiments. (Right) IFN-γ concentration was examined in culture supernatants of splenocytes stimulated with STag for 72 h. (C) IFN-γ+ frequency detected by intracellular staining of CD4+ and CD8+ T cells of splenocytes stimulated with PMA-ionomycin. (D) Use of tetramers to detect T. gondii-specific CD4+ and CD8+ T cells in the spleens of infected WT and IL-27p28 transgenic mice. (E) Expression of CXCR3 and KLRG1 by tetramer+ CD4+ and CD8+ T cells and the relative percentage of the CXCR3− KLRG1+ subset are provided for the indicated groups. Representative and combined data collected (means ± SEM, n = 7 to 9) from 2 to 3 independent experiments are shown. An asterisk indicates significant differences between the indicated groups (P < 0.05).
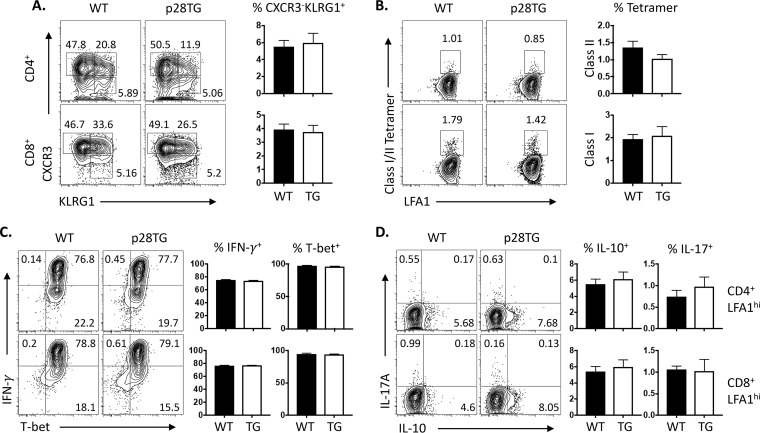
To better understand the impact of IL-27p28 on the T cell response, parasite-specific class I and II restricted tetramers (36) were combined with expression of CXCR3 and KLRG1 to characterize the relative proportion of the parasite-specific Tmem, Tint, or effector T cells (37, 38). In spleens, infection of WT and IL-27p28 transgenic mice resulted in a robust induction of parasite-specific CD4+ and CD8+ T cells, although the transgenic mice had a reduced total population of these CD4+ T cells (Fig. 2D). The most prominent difference with the CD4+ and CD8+ T cells from IL-27p28 transgenic mice was an increased percentage of the Tmem (CXCR3+ KLRG1−) population and a reduction in the CXCR3− KLRG1+ effector CD4+ and CD8+ T cells (Fig. 2E). However, when T cells from the brains of day 30 infected WT and IL-27p28 transgenics were assessed, there were no obvious differences in the phenotype of parasite-specific T cells based on expression of CXCR3 and KLRG1 (Fig. 3A and B), and the majority of the cells produced IFN-γ when stimulated with PMA-Iono and expressed high levels of T-bet (Fig. 3C). While these cells also could produce low levels of IL-10 and IL-17A (Fig. 3D), these responses were comparable in WT and IL-27p28 transgenic mice. Together, these results indicate that the expression of the IL-27p28 transgene does result in an alteration in the T cell response, but the ability to produce IFN-γ appeared largely intact and did not reduce the magnitude of the parasite-specific effector T cells present in the CNS.
FIG 3.
Effect of IL-27p28 overexpression on T cell responses in the brain during toxoplasmic encephalitis. (A) Expression of CXCR3 and KLRG1 by LFA1hi CD4+ and CD8+ T cells in brains and the relative percentages of the CXCR3− KLRG1+ subset are compared between WT and IL-27p28 mice. (B) Class II and class I tetramers to detect T. gondii-specific CD4+ and CD8+ T cells in the brains of infected WT and IL-27p28 transgenic mice. (C and D) Expression of IFN-γ and T-bet in LFA1hi CD4+ and CD8+ T cells (C) and their production of IL-10 and IL-17A (D). Representative flow plots are shown, while histograms represent the means (±SEM; n = 6 to 8) from 2 independent experiments.
Transgenic expression of IL-27p28 impacts humoral responses to T. gondii.
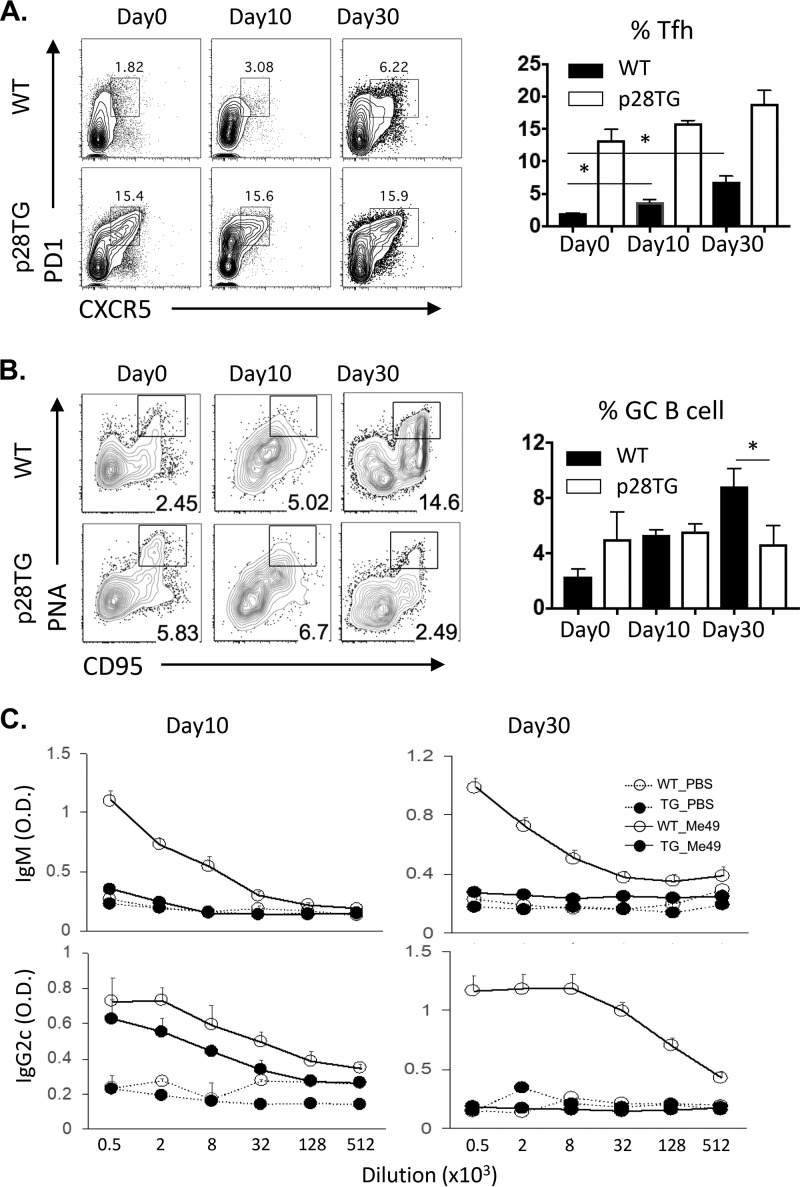
In murine models, antibodies have an important role in resistance to T. gondii (39, 40), and while overexpression of IL-27p28 antagonizes antibody production during vaccination with a T cell-dependent antigen (23), it was unclear if infection would overcome this defect. To assess the impact of IL-27p28 on the humoral response to T. gondii, an analysis of infection-induced Tfh cells (CXCR5+ PD1+) and germinal center B cells (PNA+ CD95+) was performed. By day 30 postinfection in the spleen, WT mice had a marked expansion of CXCR5+ PD1+ Tfh cells and an increased population of PNA+ CD95+ germinal center B cells (Fig. 4A and B). In naive IL-27p28 transgenic mice, there was a preexisting high basal level of CD4+ T cells that were CXCR5+ PD1+, but after infection this number did not significantly increase (Fig. 4A). Infection of WT mice resulted in an expansion of the PNA+ CD95+ germinal center B cells, whereas in the IL-27p28 transgenic mice there was a marked defect in the germinal center B cells at the chronic phase of infection. Consistent with this observation, in WT mice levels of total IgG, IgM, and IgA were increased 4- to 5-fold by infection and remained high during chronic infection. In the IL-27p28 mice these circulating antibodies were 50% lower before infection, and the levels were decreased ∼3-fold in response to infection (Table S1). Further analysis showed that WT mice generated high titers of toxoplasma-specific IgM and IgG2c, whereas in the IL-27p28 transgenic mice these levels were markedly reduced (Fig. 4C).
FIG 4.
IL-27p28TG mice have defective humoral responses following infection. (A and B) Kinetics of Tfh cells (PD1+, CXCR5+) (A) and GC B cells (PNA+ FAS+ cells) (B) are shown in WT and IL-27p28 transgenic mice after T. gondii infection. (C) Serum titers of parasite-specific IgM and IgG2c measured by ELISA after infection. Representative and combined data collected (n = 5 to 10, means ± SEM) from 2 to 3 independent experiments are shown. O.D., optical density. An asterisk indicates significant differences between the indicated groups (P < 0.05).
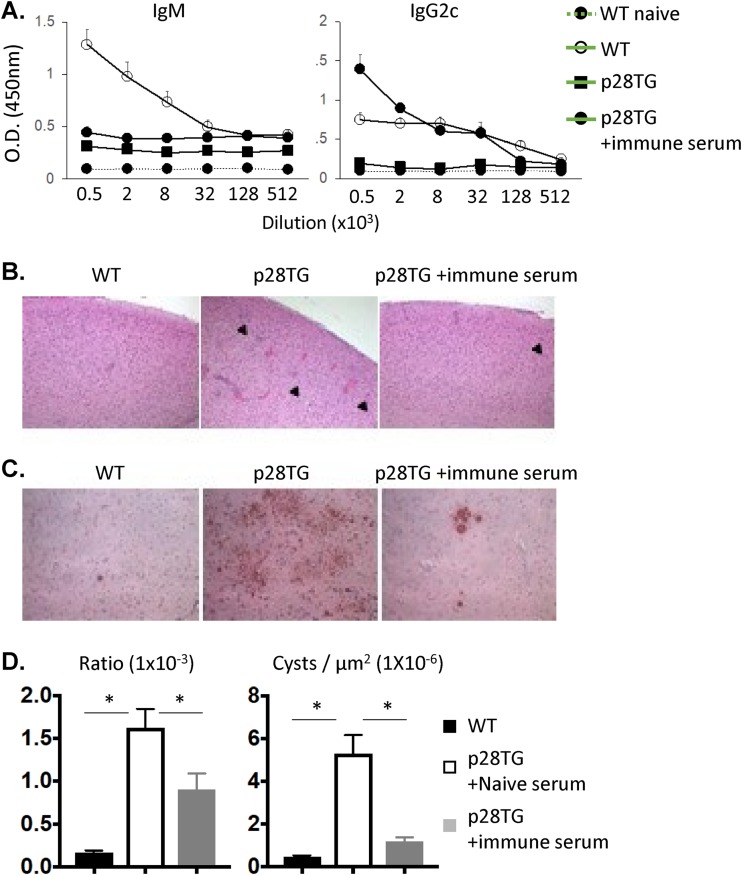
To determine if the reduced titers of parasite-specific antibodies in the IL-27p28 transgenic mice contributed to the increased parasite burden in the CNS, serum from uninfected or chronically infected WT mice was transferred into IL-27p28 transgenic mice starting at day 10 postinfection and every 4 days until day 26. Immunohistochemistry for immunoglobulins did allow the detection of IgG-positive cells in the brains of infected WT mice but was not able to detect extracellular IgG in the infected IL-27p28 transgenics (data not shown). However, the transfer of serum from infected mice led to a marked increase in the levels of parasite-specific IgG in blood (Fig. 5A) but did not change the phenotype or the number of the Toxoplasma-specific T cells, the Tfh populations, or the GC B cells (data not shown). Analysis of the pathology revealed that in the IL-27p28 transgenic mice that received the naive serum, the brains were characterized by large numbers of tissue cysts and extensive areas of parasite replication. In the mice that received immune serum, numbers of cysts and the area affected by replicating parasite were markedly reduced and the areas of extensive necrosis were absent (Fig. 5B to D). Together, these results demonstrate that during toxoplasmosis, although the IL-27p28 transgenic mice can generate parasite-specific CD4+ T cell responses that are typically required for IgM and IgG production (41), they have a major defect in the ability to produce pathogen-specific antibodies associated with a reduced ability to control T. gondii in the CNS.
FIG 5.
T. gondii-specific antibodies promote parasite control in infected IL-27p28 transgenic mice. IL-27p28 transgenic mice were treated with serum from uninfected or chronically infected WT mice starting at day 10 postinfection every 4 days. (A) Serum titers of parasite-specific IgM and IgG2c measured by ELISA. (B) H&E sections to examine histology and cyst burden (▶) of brains in the indicated groups at 36 days postinfection. (C) Immunohistochemistry for T. gondii. (D) The quantification and integration of the area of parasitic replication and cyst number in the brain are described in Materials and Methods. The data presented are collated (means ± SEM, n = 7 to 8) from 2 independent experiments.
DISCUSSION
Although IL-6 and IL-27 both utilize the gp130 receptor, these cytokines have distinct roles in resistance to T. gondii, and given the reported ability of IL-27p28 to antagonize their ability to signal through gp130, it was unclear whether overexpression of IL-27p28 during infection would impact either of these pathways. The studies presented here show that overexpression of IL-27p28 results in increased susceptibility to T. gondii and a major defect in the production of parasite-specific IgM and IgG titers that correlated with increased parasite burden in the CNS. The development of antibody responses during toxoplasmosis is an important process that is required for long-term resistance to this infection. Thus, the initial IgM response contributes to the restriction of parasite dissemination (42), while the maintenance of high titers of CD4+ T cell-dependent class-switched IgG is a hallmark of this persistent infection (41, 43–45). Furthermore, the B cell response is vital to protect the host from the CNS phase of infection (39, 40), but whether there is also a role for parasite-specific class-switched IgG that is produced locally within the CNS is unclear. The ability of immune serum to promote parasite control in these mice supports the idea that the reduced antibody responses in the IL-27p28 transgenic mice contribute to their increased susceptibility to toxoplasmic encephalitis. Given reports that IL-27p28 can antagonize gp130-mediated signaling, this phenotype is consistent with the observation that IL-6 is required to promote humoral responses required for control of T. gondii (46; J. S. Silver and C. A. Hunter, unpublished observations). Indeed, in other infectious systems IL-6 is prominently linked to the promotion of Tfh and B cell responses (47–49). Interestingly, the IL-27p28 transgenic mice had a high basal level of CD4+ T cells that express markers associated with Tfh cells, and the observation that this population did not expand during infection suggests that high basal levels of IL-27p28 interfere with Tfh homeostasis and thereby limit the ability to generate parasite-specific IgM and IgG titers.
There are multiple examples in diverse experimental settings that highlight the impact of signaling through gp130 on the magnitude of the T cell response that can be attributed to the effects of IL-6 or IL-27 (5, 23, 50–52). The lack of overt immune pathology observed in the IL-27p28 transgenic mice suggests that the ability of IL-27 to limit the inflammatory response is still operational despite the high levels of IL-27p28. However, while the IL-27p28 transgenics could generate significant numbers of parasite-specific CD4+ and CD8+ T cells, which appeared normal in the CNS, there was a reduced fraction of splenic parasite-specific effectors (CXCR3− KLRG1+), which suggests that IL-27p28 antagonizes the development of this effector population. There are systems where IL-27 has an important function in CD8+ T cell expansion (51, 53–55), and in mixed chimera settings during toxoplasmosis, those T cells that lack IL-27Rα are at a competitive disadvantage (56). Thus, while the alterations in parasite load and absence of antibody responses may have secondary impacts on the magnitude of the T cell response, the observation that IL-27p28 can antagonize the effects of IL-27 (23, 24, 31) may contribute to these altered T cell responses. However, it should be noted that reductions of this magnitude to the T cell response typically result in decreased pathology and increased resistance to toxoplasmic encephalitis (57, 58).
Recent reports have compared the secretion of IL-27p28 and EBi3 in murine systems and shown that IL-27p28 can be secreted as a monomer independently of EBi3, but EBi3 requires a partner for secretion (27; Matthias Feige, personal communication). This observation highlights that there is a major knowledge gap in how to interpret the relative ratio of IL-27p28 to the IL-27p28-EBi3 heterodimer during infection or inflammation and whether secreted IL-27p28 can partner with other cytokine receptor subunits or the high levels of soluble gp130, siIL-6Rα, and IL-27Rα present in the circulation and tissues (25, 51, 59, 60). Nevertheless, the finding that the overexpression of IL-27p28 is associated with reduced B cell responses during this infection is in accordance with the literature, which has implicated IL-27p28 alone as an antagonist of immune responses in the setting of vaccination (23), liver damage (61), fibrosis (32), sepsis (62), antitumor responses and graft rejection (24), EAU (30), and EAE (31). Regardless of whether IL-27p28 is a naturally occurring low-affinity antagonist of signaling through the gp130 receptor or secreted IL-27p28 can propagate low-level signals alone or in combination with other cytokine or cytokine receptor subunits (63), the data presented here support the idea that IL-27p28 can act as a prominent negative regulator of B cell responses in the setting of infection.
MATERIALS AND METHODS
Mice.
Female and male C57BL/6 mice were purchased (∼6 weeks old) from Taconic Biosciences and the Jackson Laboratory. The generation of IL-27p28 and EBi3-transgenic mice has been described previously (28), and IL-27p28−/− mice were generated by Lexicon Pharmaceuticals, Inc., and provided by Janssen. Mice were housed and bred in specific-pathogen-free (SPF) facilities in the Department of Pathobiology at the University of Pennsylvania in accordance with institutional guidelines. Cysts of the Me49 strain of T. gondii were collected from chronically infected CBA/ca mouse brain tissues. Host mice then were infected intraperitoneally (i.p.) with 20 cysts.
Cell staining and flow cytometry.
Cells from the indicated organs were prepared as described previously (37, 64) and were stained with LIVE/DEAD fixable aqua dead cell stain (L3457; Thermo Fisher) and antibodies specific for CD4 (GK1.5; 100447; BioLegend), CD8 (53-6.7; 562283; BD Biosciences), LFA1 (H155-78; 141008; BioLegend), KLRG1 (2F1; 11-583-82; eBiosciences), CXCR3 (CXCR3-173; 126516; BioLegend), CXCR5 (2G8; 551960; BD Biosciences), PNA (FL-1071; Vector Laboratories), CD95 (Jo2; 554258; BD Biosciences), PD-1 (29F.1A12; 135220; BioLegend), T-bet (4B10; 644810; BioLegend), IL-10 (JES5-16E3; 505022; BioLegend), IL-17A (TC11-18H10.1; 506904; BioLegend), and IFN-γ (XMG1.2; 505826; BioLegend). Major histocompatibility complex (MHC) class I (MHC I)-SVLAFRRL and MHC class II (MHC II)-AVEIHRPVPGTAPPSFSS tetramers were obtained as a gift from the National Institutes of Health Tetramer Core Facility.
ELISA for cytokine and immunoglobulins.
IL-27p28 was detected using the mouse IL-27p28/IL-30 Quantikine enzyme-linked immunosorbent assay (ELISA) kit (M2728; R&D Systems) according to the manufacturer’s protocol. For IFN-γ and IL-12p40 measurement, splenocytes (3 × 105 cells/well) were cultured for 72 h with soluble Toxoplasma antigen (STag; 12.5 ng/ml). Serum and supernatant from STag-stimulated splenocytes were collected, and capturing antibody (AN18 and C17.8) and biotinylated antibody (R4-6A2 and C15.6) of IFN-γ and IL-12p40 were used. Total serum IgG, IgA, or IgM was captured using unconjugated anti-mouse Ig (H+L; Southern Biotech) and detected using horseradish peroxidase (HRP)-conjugated goat anti-mouse IgA, IgG, or IgM (Southern Biotech), followed by TMB substrate treatment (BD Biosciences). For detection of parasite-specific antibodies, plates were coated with STag (12.5 ng/ml) and then blocked with 10% fetal bovine serum, and serially diluted serum was incubated overnight. IgM (SB73a) and IgG2c (polyclonal) then were detected using streptavidin-conjugated antibodies (Southern Biotech).
Reconstitution of parasite-specific antibodies.
Previous studies have established that the transfer of Toxoplasma-specific antibodies to uMT mice restores resistance to this infection (39, 65), and we have found that serum from infected uMT mice does not provide protection to B cell-deficient mice (Silver and Hunter, unpublished). Therefore, serum from naive mice or serum from WT mice chronically infected with the Me49 strain of T. gondii (6 to 8 weeks) was collected, and 0.2 ml of serum per mouse was transferred to IL-27p28 transgenic hosts at 10, 14, 18, 22, and 26 days postinfection.
Parasite quantification.
Brain and liver tissues (∼200 mg) were collected, and genomic DNA was isolated using DNeasy Blood & Tissue kits (Qiagen) according to the manufacturer’s manual. For measurement of parasite burden, the 35-fold repetitive T. gondii B1 gene (forward primer, 5′-TCCCCTCTGCTGGCGAAAAGT-3′; reverse primer, 5′-AGCGTTCGTGGTCAACTATCGATTG-3′) was amplified by real-time PCR with SYBR green PCR master mix (Applied Biosystems, Foster City, CA) in an AB7500 fast real-time PCR machine (Applied Biosystems, Foster City, CA) using previously published conditions (66). For the quantification of cyst number and area affected by parasitic replication, brain sections (7 μm) were scanned and analyzed by Aperio ImageScope (Leica). For cytospin count, single cells from the peritoneal cavity (1 × 105 cells/slide) were spun at 500 rpm for 5 min on glass slides. Cells were stained with Hemacolor Rapid Staining (Merck) and mounted with CytoSeal (Edmund Scientifics). Parasite dissemination in circulation was quantified with flow cytometry as previously reported (42). At day 5 postinfection, whole blood was drawn using a 22-gauge needle from vena cava and collected in 3.8% sodium citrate buffer. Free parasite was identified by size, and the expression of tdTomato and absolute numbers were calculated from the ratio to freshly isolated tachyzoites from cell culture.
Histology and immunohistochemistry.
Brain tissues were fixed in 10% buffered formalin, and 7-μm paraffin sections were prepared. Sections were stained with hematoxylin and eosin (H&E) or anti-iNOS (10 μg/ml) antibody (ab15323; Abcam). A biotinylated goat anti-rabbit antibody was used to visualize the iNOS expression, followed by Vectastain ABC reagent (Vector Laboratories, Burlingame, CA). As an HRP substrate that created a brown precipitate, DAB was added and then slides were counterstained by hematoxylin. Sections were mounted with Biomedia gel/mount (Electron Microscopy Sciences) and were visualized on an LSM-510 Meta confocal microscope (Zeiss).
Statistics.
An unpaired Student's t test, nonparametric Mann-Whitney U test, and log-rank test were used to determine the significance of differences; P values of less than 0.05 were considered significant.
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00455-19.
REFERENCES
- 1.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A 90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan IA, Matsuura T, Kasper LH. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun 62:1639–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yap G, Pesin M, Sher A. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol 165:628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 4.Jebbari H, Roberts CW, Ferguson DJ, Bluethmann H, Alexander J. 1998. A protective role for IL-6 during early infection with Toxoplasma gondii. Parasite Immunol 20:231–239. doi: 10.1046/j.1365-3024.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 5.Silver JS, Stumhofer JS, Passos S, Ernst M, Hunter CA. 2011. IL-6 mediates the susceptibility of glycoprotein 130 hypermorphs to Toxoplasma gondii. J Immunol 187:350–360. doi: 10.4049/jimmunol.1004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki Y, Rani S, Liesenfeld O, Kojima T, Lim S, Nguyen TA, Dalrymple SA, Murray R, Remington JS. 1997. Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect Immun 65:2339–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong WP, van Panhuys N, Chen J, Silver PB, Jittayasothorn Y, Mattapallil MJ, Germain RN, Caspi RR. 2015. NK-DC crosstalk controls the autopathogenic Th17 response through an innate IFN-gamma-IL-27 axis. J Exp Med 212:1739–1752. doi: 10.1084/jem.20141678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. 2002. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. 2011. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat Immunol 12:327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19:645–655. doi: 10.1016/S1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 11.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJM, O'Shea JJ, Hunter CA. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 8:1363. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 12.Hall Aisling OH, Beiting DP, Tato C, John B, Oldenhove G, Lombana Claudia G, Pritchard Gretchen H, Silver Jonathan S, Bouladoux N, Stumhofer Jason S, Harris Tajie H, Grainger J, Wojno Elia DT, Wagage S, Roos David S, Scott P, Turka Laurence A, Cherry S, Reiner Steven L, Cua D, Belkaid Y, Elloso MM, Hunter Christopher A. 2012. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. 2008. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol 180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 14.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. 2003. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity 19:657–667. doi: 10.1016/S1074-7613(03)00298-X. [DOI] [PubMed] [Google Scholar]
- 15.Liu FD, Kenngott EE, Schroter MF, Kuhl A, Jennrich S, Watzlawick R, Hoffmann U, Wolff T, Norley S, Scheffold A, Stumhofer JS, Saris CJ, Schwab JM, Hunter CA, Debes GF, Hamann A. 2014. Timed action of IL-27 protects from immunopathology while preserving defense in influenza. PLoS Pathog 10:e1004110. doi: 10.1371/journal.ppat.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung Y, Yamazaki T, Kim BS, Zhang Y, Reynolds JM, Martinez GJ, Chang SH, Lim H, Birkenbach M, Dong C. 2013. Epstein Barr virus-induced 3 (EBI3) together with IL-12 negatively regulates T helper 17-mediated immunity to Listeria monocytogenes infection. PLoS Pathog 9:e1003628. doi: 10.1371/journal.ppat.1003628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. 2006. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med 203:1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, Sellati R, Jiang H, Zhang S, Li H, Zhao J, Ting AT, Mayer L, Unkeless JC, Labadia ME, Hodge M, Li J, Xiong H. 2008. Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgamma t. Eur J Immunol 38:1204–1214. doi: 10.1002/eji.200838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Liang R, Luo W, Liu C, Wu X, Gao Y, Hao J, Cao G, Chen X, Wei J, Xia S, Li Z, Wen T, Wu Y, Zhou X, Wang P, Zhao L, Wu Z, Xiong S, Gao X, Gao X, Chen Y, Ge Q, Tian Z, Yin Z. 2013. High susceptibility to liver injury in IL-27 p28 conditional knockout mice involves intrinsic interferon-gamma dysregulation of CD4+ T cells. Hepatology 57:1620–1631. doi: 10.1002/hep.26166. [DOI] [PubMed] [Google Scholar]
- 20.Hunter CA, Kastelein R. 2012. Interleukin-27: balancing protective and pathological immunity. Immunity 37:960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanai K, Park AM, Yoshida H, Tsunoda I, Yoshie O. 2017. IL-35 suppresses lipopolysaccharide-induced airway eosinophilia in EBI3-deficient mice. J Immunol 198:119–127. doi: 10.4049/jimmunol.1600506. [DOI] [PubMed] [Google Scholar]
- 22.Kimura D, Miyakoda M, Kimura K, Honma K, Hara H, Yoshida H, Yui K. 2016. Interleukin-27-producing CD4(+) T cells regulate protective immunity during malaria parasite infection. Immunity 44:672–682. doi: 10.1016/j.immuni.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Stumhofer JS, Tait ED, Quinn WJ III, Hosken N, Spudy B, Goenka R, Fielding CA, O'Hara AC, Chen Y, Jones ML, Saris CJM, Rose-John S, Cua DJ, Jones SA, Elloso MM, Grötzinger J, Cancro MP, Levin SD, Hunter CA. 2010. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol 11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimozato O, Sato A, Kawamura K, Chiyo M, Ma G, Li Q, Tagawa M. 2009. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology 128:e816–e825. doi: 10.1111/j.1365-2567.2009.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crabe S, Guay-Giroux A, Tormo AJ, Duluc D, Lissilaa R, Guilhot F, Mavoungou-Bigouagou U, Lefouili F, Cognet I, Ferlin W, Elson G, Jeannin P, Gauchat JF. 2009. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J Immunol 183:7692–7702. doi: 10.4049/jimmunol.0901464. [DOI] [PubMed] [Google Scholar]
- 26.Garbers C, Spudy B, Aparicio-Siegmund S, Waetzig GH, Sommer J, Hölscher C, Rose-John S, Grötzinger J, Lorenzen I, Scheller J. 2013. An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 protein receptor homodimer. J Biol Chem 288:4346–4354. doi: 10.1074/jbc.M112.432955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller SI, Friedl A, Aschenbrenner I, Esser-von Bieren J, Zacharias M, Devergne O, Feige MJ. 2019. A folding switch regulates interleukin 27 biogenesis and secretion of its alpha-subunit as a cytokine. Proc Natl Acad Sci U S A 116:1585–1590. doi: 10.1073/pnas.1816698116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wojno EDT, Hosken N, Stumhofer JS, O'Hara AC, Mauldin E, Fang Q, Turka LA, Levin SD, Hunter CA. 2011. A role for IL-27 in limiting T regulatory cell populations. J Immunol 187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villarino AV, Stumhofer JS, Saris CJM, Kastelein RA, de Sauvage FJ, Hunter CA. 2006. IL-27 limits IL-2 production during Th1 differentiation. J Immunol 176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 30.Wang RX, Yu CR, Mahdi RM, Egwuagu CE. 2012. Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J Biol Chem 287:36012–36021. doi: 10.1074/jbc.M112.390625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong WP, Horai R, Mattapallil MJ, Silver PB, Chen J, Zhou R, Sergeev Y, Villasmil R, Chan CC, Caspi RR. 2014. IL-27p28 inhibits central nervous system autoimmunity by concurrently antagonizing Th1 and Th17 responses. J Autoimmun 50:12–22. doi: 10.1016/j.jaut.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitra A, Satelli A, Yan J, Xueqing X, Gagea M, Hunter CA, Mishra L, Li S. 2014. IL‐30 (IL27p28) attenuates liver fibrosis through inducing NKG2D‐rae1 interaction between NKT and activated hepatic stellate cells in mice. Hepatology 60:2027–2039. doi: 10.1002/hep.27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJM, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 34.Tong X, Chen S, Zheng H, Huang S, Lu F. 2018. Increased IL-27/IL-27R expression in association with the immunopathology of murine ocular toxoplasmosis. Parasitol Res 117:2255–2263. doi: 10.1007/s00436-018-5914-7. [DOI] [PubMed] [Google Scholar]
- 35.Scharton-Kersten TM, Yap G, Magram J, Sher A. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med 185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dupont CD, Christian DA, Selleck EM, Pepper M, Leney-Greene M, Harms Pritchard G, Koshy AA, Wagage S, Reuter MA, Sibley LD, Betts MR, Hunter CA. 2014. Parasite fate and involvement of infected cells in the induction of CD4+ and CD8+ T cell responses to Toxoplasma gondii. PLoS Pathog 10:e1004047. doi: 10.1371/journal.ppat.1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeLong JH, Hall AO, Konradt C, Coppock GM, Park J, Harms Pritchard G, Hunter CA. 2018. Cytokine- and TCR-mediated regulation of T cell expression of Ly6C and Sca-1. J Immunol 200:1761–1770. doi: 10.4049/jimmunol.1701154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu HH, Chan S-W, Gosling JP, Blanchard N, Tsitsiklis A, Lythe G, Shastri N, Molina-París C, Robey EA. 2016. Continuous effector CD8(+) T cell production in a controlled persistent infection is sustained by a proliferative intermediate population. Immunity 45:159–171. doi: 10.1016/j.immuni.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang H, Remington JS, Suzuki Y. 2000. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J Immunol 164:2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 40.Moretto MM, Hwang S, Khan IA. 2017. Downregulated IL-21 response and T follicular helper cell exhaustion correlate with compromised CD8 T cell immunity during chronic toxoplasmosis. Front Immunol 8:1436. doi: 10.3389/fimmu.2017.01436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glatman Zaretsky A, Silver JS, Siwicki M, Durham A, Ware CF, Hunter CA. 2012. Infection with Toxoplasma gondii alters lymphotoxin expression associated with changes in splenic architecture. Infect Immun 80:3602–3610. doi: 10.1128/IAI.00333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konradt C, Ueno N, Christian DA, Delong JH, Pritchard GH, Herz J, Bzik DJ, Koshy AA, McGavern DB, Lodoen MB, Hunter CA. 2016. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat Microbiol 1:16001. doi: 10.1038/nmicrobiol.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basavaraju A. 2016. Toxoplasmosis in HIV infection: an overview. Trop Parasitol 6:129–135. doi: 10.4103/2229-5070.190817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, Eibel H, Schlesier M, Peter HH. 2002. Severe deficiency of switched memory B cells (CD27+ IgM- IgD-) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood 99:1544–1551. doi: 10.1182/blood.V99.5.1544. [DOI] [PubMed] [Google Scholar]
- 45.Yates JL, Racine R, McBride KM, Winslow GM. 2013. T cell-dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. J Immunol 191:1240–1249. doi: 10.4049/jimmunol.1300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markine-Goriaynoff D, Nguyen TD, Bigaignon G, Van Snick J, Coutelier JP. 2001. Distinct requirements for IL-6 in polyclonal and specific Ig production induced by microorganisms. Int Immunol 13:1185–1192. doi: 10.1093/intimm/13.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. 2011. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One 6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kopf M, Herren S, Wiles MV, Pepys MB, Kosco-Vilbois MH. 1998. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med 188:1895–1906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeda K, Mehta H, Drevets DA, Coggeshall KM. 2010. IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production. Blood 115:4699–4706. doi: 10.1182/blood-2009-07-230631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schurmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. 2000. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med 6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 51.Harker JA, Dolgoter A, Zuniga EI. 2013. Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4(+) T cell responses and viral control during chronic infection. Immunity 39:548–559. doi: 10.1016/j.immuni.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holz K, Prinz M, Brendecke SM, Hölscher A, Deng F, Mitrücker H-W, Rose-John S, Hölscher C. 2018. Differing outcome of experimental autoimmune encephalitis in macrophage/neutrophil- and T cell-specific gp130-deficient mice. Front Immunol 9:836. doi: 10.3389/fimmu.2018.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Z, Liu JQ, Talebian F, Wu LC, Li S, Bai XF. 2013. IL-27 enhances the survival of tumor antigen-specific CD8+ T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur J Immunol 43:468–479. doi: 10.1002/eji.201242930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morishima N, Mizoguchi I, Okumura M, Chiba Y, Xu M, Shimizu M, Matsui M, Mizuguchi J, Yoshimoto T. 2010. A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J Biomed Biotechnol 2010:605483. doi: 10.1155/2010/605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. 2011. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol 41:47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- 56.Mayer KD, Mohrs K, Reiley W, Wittmer S, Kohlmeier JE, Pearl JE, Cooper AM, Johnson LL, Woodland DL, Mohrs M. 2008. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J Immunol 180:693–697. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- 57.Israelski DM, Araujo FG, Conley FK, Suzuki Y, Sharma S, Remington JS. 1989. Treatment with anti-L3T4 (CD4) monoclonal antibody reduces the inflammatory response in toxoplasmic encephalitis. J Immunol 142:954–958. [PubMed] [Google Scholar]
- 58.Reichmann G, Villegas EN, Craig L, Peach R, Hunter CA. 1999. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J Immunol 163:3354–3362. doi: 10.4049/jimmunol.169.2.937. [DOI] [PubMed] [Google Scholar]
- 59.Chehboun S, Labrecque-Carbonneau J, Pasquin S, Meliani Y, Meddah B, Ferlin W, Sharma M, Tormo A, Masson JF, Gauchat JF. 2017. Epstein-Barr virus-induced gene 3 (EBI3) can mediate IL-6 trans-signaling. J Biol Chem 292:6644–6656. doi: 10.1074/jbc.M116.762021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruiz-Riol M, Berdnik D, Llano A, Mothe B, Galvez C, Perez-Alvarez S, Oriol-Tordera B, Olvera A, Silva-Arrieta S, Meulbroek M, Pujol F, Coll J, Martinez-Picado J, Ganoza C, Sanchez J, Gomez G, Wyss-Coray T, Brander C. 2017. Identification of interleukin-27 (IL-27)/IL-27 receptor subunit alpha as a critical immune axis for in vivo HIV control. J Virol 91:e00441-17. doi: 10.1128/JVI.00441-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dibra D, Cutrera J, Xia X, Kallakury B, Mishra L, Li S. 2012. Interleukin-30: a novel antiinflammatory cytokine candidate for prevention and treatment of inflammatory cytokine-induced liver injury. Hepatology 55:1204–1214. doi: 10.1002/hep.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan J, Mitra A, Hu J, Cutrera JJ, Xia X, Doetschman T, Gagea M, Mishra L, Li S. 2016. Interleukin-30 (IL27p28) alleviates experimental sepsis by modulating cytokine profile in NKT cells. J Hepatol 64:1128–1136. doi: 10.1016/j.jhep.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tait Wojno ED, Hunter CA, Stumhofer JS. 2019. The immunobiology of the interleukin-12 family: room for discovery. Immunity 50:851–870. doi: 10.1016/j.immuni.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dupont CD, Pritchard GH, Hidano S, Christian DA, Wagage S, Muallem G, Wojno EDT, Hunter CA. 2015. Flt3 ligand is essential for survival and protective immune responses during toxoplasmosis. J Immunol 195:4369–4377. doi: 10.4049/jimmunol.1500690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sayles PC, Gibson GW, Johnson LL. 2000. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect Immun 68:1026–1033. doi: 10.1128/iai.68.3.1026-1033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. 2005. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol 165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.