ABSTRACT
Background
Iron deficiency anemia during pregnancy is a significant public health problem in sub-Saharan Africa (SSA) and is associated with serious adverse health outcomes. Although it is recommended that all women receive iron supplementation during pregnancy, little research has been conducted to measure overall compliance with this recommendation or variation across SSA countries.
Objectives
To assess prevalence and sociodemographic-economic factors associated with adherence to iron supplementation among pregnant women in SSA.
Methods
This was a weighted population-based cross-sectional study of 148,528 pregnant women aged 15–49 y in 22 SSA countries that participated in the Demographic and Health Surveys (DHS) in 2013–2018 and measured iron supplementation during pregnancy. Adherence to iron supplementation was defined as using iron supplementation for ≥90 d during pregnancy of the most recent birth.
Results
The overall prevalence of adherence to ≥90 d of iron supplementation during pregnancy was 28.7%, ranging from 1.4% in Burundi to 73.0% in Senegal. Factors associated with adherence included receiving ≥4 antenatal care visits [adjusted Prevalence Ratio (aPR): 25.73; 95% CI: 22.36, 29.60] compared with no antenatal visits; secondary or higher education (aPR: 1.17; 95% CI: 1.14, 1.19) compared with no education; wealthy (aPR: 1.13; 95% CI: 1.10, 1.16) compared with poor; and older women aged 35–49 y (aPR: 1.07; 95% CI: 1.05, 1.10) compared with younger women aged 15–24 y.
Conclusions
Adherence to iron supplementation during pregnancy in SSA is low and varies substantially across countries and in relation to factors such as number of antenatal visits, education, and level of family wealth. These results underscore the need for increased efforts to improve the uptake of iron supplementation for pregnant women in SSA.
Keywords: adherence, sub-Saharan Africa, iron supplementation, antenatal care, women
Introduction
Globally, 2 billion people are affected by iron deficiency, with sub-Saharan Africa (SSA) carrying the greatest burden (1–3). The 2013 Lancet series on Maternal and Child Undernutrition reported that 38% of pregnant women aged 15–49 y were anemic and rates were higher in SSA at 56% compared with high-income countries at 22% (4). Iron demand increases by 3-fold during pregnancy (3, 5, 6). It is estimated that 1000 mg of iron daily is required for the mother and fetus throughout pregnancy, which may not be sustainable through regular diet alone, particularly in developing countries where a high burden of food insecurity exists (2, 7). Although there are a variety of factors that contribute to anemia during pregnancy in SSA, such as malaria, iron supplementation during pregnancy is recognized as a cost-effective and feasible method to help to prevent or ameliorate iron deficiency anemia and adverse pregnancy outcomes (8, 9). Because many women of reproductive age in SSA begin pregnancy with very little iron stores, daily iron supplementation has become a routine practice as part of antenatal care in anemia control and prevention (10–12). Lack of iron supplementation and poor adherence during pregnancy are associated with serious adverse health outcomes such as anemia, premature labor, maternal death, fetal death, low birth weight, postpartum hemorrhage, and poor mental development (13–15).
Poor adherence to iron supplementation constitutes a serious public health issue in SSA. Compared with high-income countries where adherence to iron supplementation during pregnancy ranges from 77% in Denmark (16) to 85% in Sweden (17), adherence has been reported to be as low as 22% in some SSA countries (18). Previous studies have used ≥90 d cut-off point to determine adherence to iron supplementation during pregnancy in SSA (19–23). However, many women in SSA countries do not adhere to iron supplementation during pregnancy due to a lack of knowledge about anemia, inadequate supply of tablets, poor utilization of antenatal care services, inability to pay for supplementation, misinformation about the benefit of supplementation, complaints about side effects, forgetfulness, and poor counseling (19, 21, 24–26). To tailor strategies to reduce the burden of iron deficiency-related adverse outcomes among pregnant women in SSA, it is important to understand regional and country-specific variation in the rate of adherence to iron supplementation. Such knowledge would guide prioritization of interventions to the most at-risk countries in SSA and to better understand potential reasons for low adherence. However, these estimates are lacking because most of the previous studies regarding adherence to iron supplementation have focused on only a few countries individually such as Ethiopia, Uganda, and Malawi (6, 19, 20, 22, 27–32). Therefore, we aimed to fill this gap in knowledge by conducting a large-scale study of adherence to iron supplementation during pregnancy in 22 SSA countries and associated sociodemographic-economic factors, primarily for purposes of program targeting, using the most recent Demographic and Health Surveys (DHS) data from 2013–2018.
Methods
Data source and participants
For this study we included all SSA countries that participated in the DHS in the most recent 5-y period (2013 –2018) and measured iron supplementation during pregnancy. There were a total of 22 countries that had conducted a DHS survey in the years since 2013 and had asked about iron supplementation during pregnancy. These countries were Angola, Benin, Burundi, Chad, Congo Democratic Republic, Ethiopia, Gambia, Ghana, Kenya, Malawi, Mali, Namibia, Nigeria, Rwanda, Senegal, Sierra Leone, South Africa, Tanzania, Togo, Uganda, Zambia, and Zimbabwe. Each of these 22 countries contributed 1 survey. The DHS program is funded by the US Agency for International Development (USAID) and was implemented by ICF International (ICF). The DHS data are nationally representative surveys with >300 surveys conducted in over 90 developing countries around the world. The surveys were conducted using a multistage cluster sampling, stratified design to collect information about demographics and population health status, neonatal mortality, nutritional status, family planning, maternal and child health, malaria, and anemia in each country (33, 34). The year of administration of the relevant DHS survey for each country can be seen in Table 1.
TABLE 1.
Weighted sample size, adherence rate to iron supplementation, and multivariable-adjusted Prevalence Ratio by country and survey year (N = 148,528)
| Sample size, | Iron adherence ≥90 d, | Multivariable-adjusted analysis, | |||
|---|---|---|---|---|---|
| Countries | Most recent survey year | Na (%Nb) | Nc (%) | aPR (95% CI) | P value |
| Overall | 148,528 | 42,738 (28.7) | |||
| Angola | 2015–16 | 7293 (4.9) | 2528 (34.7) | 19.06 (15.86, 22.91) | <0.001 |
| Benin | 2017–18 | 6543 (4.4) | 3638 (55.6) | 40.18 (33.55, 48.12) | <0.001 |
| Burundi | 2016–17 | 8687 (5.9) | 124 (1.4) | ref. | |
| Chad | 2014–15 | 9100 (6.1) | 1090 (12.0) | 13.18 (10.92, 15.92) | <0.001 |
| Ethiopia | 2016 | 7286 (4.9) | 374 (5.1) | 6.81 (5.58, 8.31) | <0.001 |
| Gambia | 2013 | 3442 (2.3) | 1623 (47.2) | 28.42 (23.65, 34.16) | <0.001 |
| Ghana | 2014 | 3803 (2.6) | 2359 (62.0) | 32.51 (27.09, 39.01) | <0.001 |
| Kenya | 2014 | 6248 (4.2) | 487 (7.8) | 4.64 (3.80, 5.67) | <0.001 |
| Malawi | 2015–16 | 12,714 (8.6) | 4324 (34.0) | 24.59 (20.51, 29.48) | <0.001 |
| Mali | 2018 | 4923 (3.3) | 1624 (33.0) | 19.64 (16.30, 23.68) | <0.001 |
| Namibia | 2013 | 2124 (1.4) | 1146 (53.9) | 30.13 (25.07, 36.22) | <0.001 |
| Nigeria | 2013 | 16,580 (11.2) | 3808 (22.9) | 19.78 (16.49, 23.73) | <0.001 |
| RDC | 2013–14 | 9815 (6.6) | 487 (5.0) | 3.34 (2.73, 4.08) | <0.001 |
| Rwanda | 2014–15 | 5801 (3.9) | 205 (3.5) | 2.46 (1.97, 3.08) | <0.001 |
| Senegal | 2017 | 4695 (3.2) | 3417 (73.0) | 44.90 (37.44, 53.84) | <0.001 |
| Sierra Leone | 2013 | 5035 (3.4) | 2085 (41.4) | 22.63 (18.85, 27.18) | <0.001 |
| South Africa | 2016 | 2290 (1.5) | 1388 (60.6) | 35.84 (29.82, 43.08) | <0.001 |
| Tanzania | 2015–16 | 6198 (4.2) | 1347 (21.7) | 14.67 (12.19, 17.66) | <0.001 |
| Togo | 2013–14 | 4209 (2.8) | 1637 (38.9) | 27.67 (23.06, 33.21) | <0.001 |
| Uganda | 2016 | 9245 (6.2) | 2124 (22.9) | 14.73 (12.26, 17.71) | <0.001 |
| Zambia | 2013–14 | 7981 (5.4) | 5085 (63.7) | 43.30 (36.16, 51.84) | <0.001 |
| Zimbabwe | 2015 | 4516 (3.0) | 1838 (40.7) | 23.81 (19.82, 28.61) | <0.001 |
Na = weighted sample size of the combined dataset that is represented by that survey for each country.
Nb = the % of the combined dataset that is represented by that survey.
Nc = adherence to iron supplementation defined as taking iron tablets or syrup supplement for at ≥90 d during pregnancy of recent birth.
Model fully adjusted for country, number of antenatal care visits, pregnancy status, breastfeeding status, age, education status, marital status, wealth index status, place of residence, employment status, number of living children, household having a radio or television, having a prenatal nurse, and had a history of terminated pregnancy.
RDC = Democratic Republic of Congo. ref. = reference.
Ethical considerations
All the procedures and questionnaires for standard DHS surveys were reviewed and approved by the ICF Institutional Review Board (IRB) and the IRBs of the host countries. Each participant provided written and oral informed consent prior to the start of each survey and/or biomarker tests. Survey respondents were not coerced into participation (35) and all the data are Health Insurance Portability and Accountability Act (HIPAA) protected and deidentified. These ethical issues were handled by those who conducted the primary surveys and not the current authors of the manuscript.
Assessment of adherence to iron supplementation (outcome)
Adherence to iron supplementation was the study outcome and was defined as using iron supplementation for ≥90 d during the pregnancy of the most recent birth. This was measured in the DHS data by the number of days when iron supplements (tablets or syrup) were taken as part of antenatal care. The adherence of ≥90 d threshold was chosen in accordance with previous studies (19–22).
Assessment of factors associated with iron supplementation adherence
We investigated the following factors to assess whether they were associated with adherence to iron supplementation: number of antenatal care visits, pregnancy status, breastfeeding status, age, education status, marital status, wealth index status, place of residence, employment status, number of living children, household owning a radio, household owning a television, having a prenatal nurse during the most recent pregnancy that led to a birth, and a history of terminated pregnancy (36). Previous studies reported that these sociodemographic-economic factors affect women's adherence to iron supplementation in SSA (19, 28, 29, 32, 37, 38). Based on previous literature, we recategorized the wealth index from 5 quintiles into 3 categories by combining poorest and poorer into one category (called “poor”); middle wealth level into the second category (called “middle”); and richer and richest into the third category (called “rich”), as previous researchers have done (39, 40). The age of the respondent at the time of the DHS interview was originally measured as a continuous variable and was categorized into 3 categories (15–24, 25–34, and 35–49 y). Number of antenatal care visits was grouped into 3 categories: none, 1–3 visits, and 4 or more visits.
Statistical analysis
We extracted iron supplementation information from the birth dataset for women who had a birth within the previous 5 y of the time of the survey for each country, and then linked this variable to each woman's health history data, using the DHS merging guideline. We then combined the files from the 22 countries into a single dataset. We conducted univariate analyses using frequency distributions for categorical variables to describe the characteristics of the study population. We calculated the prevalence of adherence to iron supplementation among pregnant women as the number of women who reported taking iron supplements for ≥90 d divided by the total number of women interviewed in that category and multiplied by 100. We performed the multivariable analysis using generalized estimating equations with independent correlation structure to identify factors associated with adherence to iron supplementation. To specify the use of the robust variance estimator for Poisson regression, we used the REPEATED statement (in SAS) (41). We applied sampling weights for the combined dataset for calculation of the descriptive statistics, but not for the regression analyses, as suggested by the DHS guidelines (33). We present descriptive results as adherence rates and the multivariable Poisson regression results as aPR with 95% CIs. We controlled for country in the aPR analyses and selected Burundi as the reference country because it was the country with the lowest rate of adherence to iron supplementation.
We conducted all analyses using SAS version 9.4 (SAS Institute) and R-3.4.3. We assessed statistical significance at P values <0.05.
Results
Sociodemographic characteristics of the respondents
A total of 148,528 women aged 15–49 y were included in this study. More than one-third of the study participants were aged 25–34 y (47.3%) and from poor households (43.0%). In addition, more than half of the survey respondents received ≥4 antenatal care visits in the most recent pregnancy resulting in a live birth (54.9%), resided in rural areas (69.3%), were married/living with a partner (86.9%), currently employed (66.6%), currently breastfeeding at the time of the DHS interview (52.0%), and had a prenatal nurse/midwife (52.0%) at the most recent birth. Approximately 40% of the women had primary education. Women with 1 or more living child aged <5 y at the time of the DHS interview constituted 72.4% of the survey respondents. A majority of the women had a radio in the household (53.0%) but most did not have a television (72.6%).
The adherence rate to iron supplementation
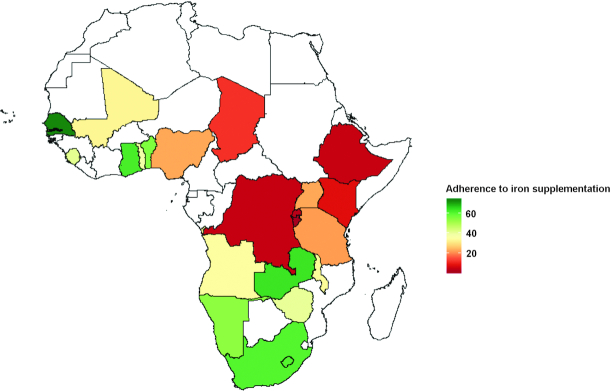
The overall adherence rate to iron supplementation across the 22 SSA countries was 28.7%, (95% CI: 28.5–28.9%), and ranged from (1.4%) in Burundi to (73.0%) in Senegal. Only 6 of the 22 countries we studied had adherence rates higher than 50% (Table 1 and Figure 1). The rate of respondents who took iron supplements during pregnancy for ≥90 d was highest among women with ≥4 antenatal care visits (40.3%), from a wealthy household (36.6%), living in urban areas (41.2%), and with at least a secondary education (41.0%). Furthermore, adherence to iron supplementation was more common among women who had access to a radio or television in the household (33.1% and 45.6%, respectively) compared with those without such items.
FIGURE 1.
Weighted adherence rate to iron supplementation (%) in pregnant women aged 15–49 y shaded by sub-Saharan countries in Africa. Countries shaded white were not included in the analysis.
Independent factors associated with adherence to iron supplements
The country of residence was the factor most strongly associated with adherence to iron supplementation (Table 1). Countries with the highest rates of adherence to iron supplementation were Senegal, followed by Zambia, South Africa, Benin, Ghana, Gambia, Malawi, Namibia, Sierra Leone, Togo, and Zimbabwe. As one would expect, there was a dose-response relation between the number of antenatal care visits and adherence to iron supplementation. Women who received ≥4 antenatal care visits were 26 times more likely to adhere to iron supplementation than those who had no antenatal care visits aPR: 25.73 (95% CI: 22.36, 29.60). In addition, women's education level was an important factor associated with adherence to iron supplementation (Table 2). Adherence to iron supplementation during pregnancy was 17% higher among women with secondary/higher education aPR: 1.17 (95% CI: 1.14, 1.19) compared with uneducated women. Furthermore, a dose-response relation was evident between age and adherence to iron supplementation, where increasing age was positively associated with higher adherence to iron supplementation. Women aged 35–49 y were 7% more likely to adhere to iron supplementation compared with younger women, aged 15–24 y aPR: 1.07 (95% CI: 1.05, 1.10). Similarly, being wealthy was positively associated with a 13% higher rate of adherence to iron supplementation compared with being poor. Additionally, women who had a prenatal care nurse/midwife were 5% more likely to adhere to iron supplementation compared with those who did not. Possession of a television and a radio were each positively associated with adherence, with 4% and 3% higher rates of adherence to iron supplementation aPR: 1.04 (95% CI: 1.01, 1.05) and aPR: 1.03 (95% CI: 1.02, 1.05), respectively. Lastly, living in rural areas was negatively associated with adherence to iron supplementation aPR: 0.97 (95% CI: 0.95, 0.98).
TABLE 2.
Weighted sociodemographic characteristics of the sample, adherence rate, multivariable-adjusted Prevalence Ratio (N = 148,528)
| Sample size, | Iron adherence ≥90 d, | Multivariable-adjusted analysis, | ||
|---|---|---|---|---|
| Characteristic | N (%) | N (%) | aPR (95% CI) | P value |
| Age group, y | ||||
| 15–24 | 39,906 (26.9) | 11,187 (28.0) | ref. | |
| 25–34 | 70,276 (47.3) | 20,582 (29.3) | 1.05 (1.03, 1.07) | <0.001 |
| 35–49 | 38,347 (25.8) | 10,968 (28.6) | 1.07 (1.05, 1.10) | <0.001 |
| Number of antenatal care visits | ||||
| None | 18,238 (12.3) | 193 (1.1) | ref. | |
| 1 to 3 visits | 48,737 (32.8) | 9668 (19.8) | 15.77 (13.71, 18.15) | <0.001 |
| ≥4 visits | 81,554 (54.9) | 32,877 (40.3) | 25.73 (22.36, 29.60) | <0.001 |
| Wealth index status | ||||
| Poor | 63,879 (43.0) | 14,460 (22.6) | ref. | |
| Middle | 29,458 (19.8) | 8090 (27.5) | 1.03 (1.01, 1.06) | 0.002 |
| Rich | 55,192 (37.2) | 20,188 (36.6) | 1.13 (1.10, 1.16) | <0.001 |
| Place of residence | ||||
| Urban | 45,660 (30.7) | 18,821 (41.2) | ref. | |
| Rural | 102,868 (69.3) | 23,916 (23.3) | 0.97 (0.95, 0.98) | 0.001 |
| Education | ||||
| No education | 51,075 (34.4) | 11,482 (22.5) | ref. | |
| Primary | 56,171 (37.8) | 14,331 (25.5) | 1.08 (1.05, 1.10) | <0.001 |
| Secondary/higher | 41,275 (27.8) | 16,922 (41.0) | 1.17 (1.14, 1.19) | <0.001 |
| Marital status | ||||
| Never married | 8632 (5.8) | 3331 (38.6) | 1.01 (0.97, 1.04) | 0.61 |
| Married/living with partner | 129,095 (86.9) | 36,430 (28.2) | ref. | |
| Widowed/divorced/separated | 10,801 (7.3) | 2978 (27.6) | 1.00 (0.98, 1.04) | 0.51 |
| Employment status | ||||
| No | 49,491 (33.4) | 14,428 (29.2) | ref. | |
| Yes | 98,737 (66.6) | 28,239 (28.6) | 1.03 (1.02, 1.05) | <0.001 |
| Pregnancy status | ||||
| No | 131,601 (88.6) | 38,389 (29.2) | ref. | |
| Yes | 16,928 (11.4) | 4348 (25.7) | 1.02 (0.99, 1.05) | 0.09 |
| Breastfeeding status | ||||
| No | 71,337 (48.0) | 22,514 (31.6) | ref. | |
| Yes | 77,192 (52.0) | 20,224 (26.2) | 1.04 (1.02, 1.05) | <0.001 |
| Number of living children | ||||
| 1 to 4 | 107,469 (72.4) | 32,639 (30.4) | 1.04 (1.02, 1.06) | <0.001 |
| >4 | 41,059 (27.6) | 10,099 (24.6) | ref. | |
| Household has radio | ||||
| No | 69,749 (47.0) | 16,707 (23.9) | ref. | |
| Yes | 78,730 (53.0) | 26,018 (33.1) | 1.03 (1.02, 1.05) | <0.001 |
| Household has television | ||||
| No | 107,826 (72.6) | 24,211 (22.45) | ref. | |
| Yes | 40,623 (27.4) | 18,512 (45.6) | 1.04 (1.01, 1.05) | 0.004 |
| Had a history of terminated pregnancy | ||||
| No | 128,150 (86.3) | 36,338 (28.4) | ref. | |
| Yes | 20,342 (13.7) | 6394 (31.4) | 1.04 (1.02, 1.06) | <0.001 |
| Prenatal nurse/midwife | ||||
| No | 71,168 (48.0) | 17,713 (25.0) | ref. | |
| Yes | 77,229 (52.0) | 24,990 (32.4) | 1.05 (1.03, 1.07) | <0.001 |
Model fully adjusted for country, number of antenatal care visits, pregnancy status, breastfeeding status, age, education status, marital status, wealth index status, place of residence, employment status, number of living children, household having a radio or television, having a prenatal nurse, and had a history of terminated pregnancy. ref. = reference.
Discussion
Our study indicated heterogeneity and disparities in adherence to iron supplementation across SSA countries. The overall prevalence of ≥90 d iron supplementation adherence during pregnancy was only 28.7%. Previous studies suggest that compliance with iron supplementation during pregnancy helps to prevent and control anemia and reduce neonatal mortality (20, 42). A study by Peña-Rosas and colleagues (2012) showed that prophylactic iron supplementation reduced the risk of maternal anemia at term by 70% and low birth weight by 16% compared with no iron supplementation (43). In addition, a study conducted by Habid and colleagues (2009) indicated that noncompliance with iron supplementation was associated with a 6-fold increase in the odds of having anemia during pregnancy (44). The low rate of adherence to iron supplementation that we found in this study is similar to findings from previous studies conducted in other SSA countries (19, 22, 28, 31, 32, 45). More importantly, our results indicate substantial variation in adherence to iron supplementation across these 22 countries, with Senegal having the highest rate of adherence. This finding is consistent with a study conducted by Seck and Jackson (2007) that reported an iron supplementation adherence rate of 69% in Senegal (46). The authors reported that counseling about the benefit of iron supplementation and free distribution of iron tablets led to a significantly increased rate of compliance among Senegalese pregnant women (46). National wealth and civil wars could also be contributing factors to lower rates. For example, the extremely low rate of iron supplementation (1.4%) observed in Burundi is concurrent with its stagnated poverty since the end of the civil war in 2005 with a current gross domestic product ranking at 169/205 in the world in 2018 (47). In contrast, Senegal, a stable country with strong economic development, has had iron supplementation programs for nearly 2 decades, resulting in the highest rate of iron adherence (46).
The association between increased age and adherence to iron supplementation during pregnancy could be explained by older women knowing the benefits of iron supplementation for preventing anemia or having experienced iron deficiency-related adverse outcomes. This observation is consistent with previous studies that found older women to be more compliant with iron supplementation than younger women (48–50). As one would expect, an increased number of antenatal care visits was strongly associated with higher adherence to iron supplementation. This finding aligns with previous studies that also found 4 or more antenatal care visits were positively associated with increased adherence to iron supplementation during pregnancy (51–53).
Increased education was positively associated with higher adherence to iron supplementation during pregnancy in this study. Educated women may be more informed about their health and adverse pregnancy outcomes compared with uneducated women. Furthermore, they may have more access to information about the benefits of iron supplementation through reading magazines or books than women who cannot read (19, 28, 37, 44, 54). We found that family wealth status was positively associated with adherence to iron supplementation during pregnancy. Wealthy women have stronger financial means to purchase iron tablets or syrup during pregnancy (19, 28, 37, 44, 54). The negative association between adherence to iron supplementation and living in rural areas could be due to disparities in access to iron supplementation despite having antenatal care (19). Interestingly, our study found that adherence to iron supplementation was significantly associated with ownership of a television and/or radio, indicating higher likelihood of exposure to mass media. This was not surprising and aligned with previous studies that found exposure to mass media was significantly associated with adherence to iron supplementation and could be useful in improving adherence (38, 55). Mass media is the most effective way to spread health messages in low-resource areas, such as SSA (56).
Study strengths and limitations
The strength of this study is the analysis of a nationally representative sample of women from 22 SSA countries to investigate factors associated with adherence to iron supplementation in these countries. As far as we are aware, this study is 1 of only a few studies using DHS data to examine adherence to iron supplementation across multiple SSA countries. This study adds to the body of literature on adherence to iron supplementation in SSA countries by investigating factors associated with adherence to iron supplementation in these countries. These findings will assist with improving iron deficiency anemia counseling practices and interventions in these countries and the region as a whole. However, the study has some limitations. First, the cross-sectional nature of the survey does not allow the determination of cause-effect relations. In addition, this study was limited to only 22 of the 48 countries in SSA. Despite these limitations, this study provides useful information regarding adherence to iron supplementation and associated factors among women of reproductive age.
Conclusions
The findings from our study indicated a great heterogeneity in adherence to iron supplementation across SSA countries and in relation to women's demographic and personal characteristics. Receipt of antenatal care visits, being older, of higher socioeconomic status, higher education, having prenatal care via a nurse/midwife, and possession of a television and/or radio were independently and positively associated with adherence to iron supplementation. The low rate of adherence to iron supplementation in some of these SSA countries indicates the need for targeted interventions to prevent iron deficiency anemia in pregnancy and associated poor perinatal outcomes. As summarized by Seck and Jackson (2007), the following 3 evidence-based recommendations could potentially improve iron supplementation compliance by pregnant women. First, promote antenatal care visits and improve women's access to the supplements by providing free iron tablets or syrup during antenatal care to women with limited financial means. Second, provide intensive counseling to women during their childbearing years on the health benefits and possible side effects of iron supplementation. Third, women who acquire iron from the pharmacy should also receive appropriate counseling from the pharmacists regarding adherence to iron supplementation.
ACKNOWLEDGEMENTS
We thank the DHS program implemented by ICF for granting free access to the original data. The authors’ contributions were as follows— PS, KK, XG, PD, and DB: designed the research (project conception, development of overall research plan, and study oversight); XG, PD, PS, and DB: analyzed data or performed statistical analysis; PS, KK, MN, GL, XG, PD, and DB: wrote the paper (only authors who made a major contribution); and all authors read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: aPR, adjusted Prevalence Ratio; DHS, Demographic and Health Surveys; ICF, standard name for ICF International; IRB, Institutional Review Board; SSA, sub-Saharan Africa.
References
- 1. de Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. Geneva: World Health Organization; 2008. [Google Scholar]
- 2. Stoltzfus RJ, Dreyfuss ML.. Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. Washington DC: ILSI Press; 1998. [Google Scholar]
- 3. Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370(9586):511–20. [DOI] [PubMed] [Google Scholar]
- 4. Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Peña-Rosas JP, Bhutta ZA, Ezzati M; Group NIMS. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1(1):e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yakoob MY, Bhutta ZA.. Effect of routine iron supplementation with or without folic acid on anemia during pregnancy. BMC Public Health. 2011;11(3):S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haidar JA, Pobocik RS.. Iron deficiency anemia is not a rare problem among women of reproductive ages in Ethiopia: a community based cross sectional study. BMC Blood Disord. 2009;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner RE, Langkamp-Henken B, Littell RC, Lukowski MJ, Suarez MF. Comparing nutrient intake from food to the estimated average requirements shows middle- to upper-income pregnant women lack iron and possibly magnesium. J Am Diet Assoc. 2003;103(4):461–6. [DOI] [PubMed] [Google Scholar]
- 8. Titaley CR, Dibley MJ.. Factors associated with not using antenatal iron/folic acid supplements in Indonesia: the 2002/2003 and 2007 Indonesia Demographic and Health Survey. Asia Pac J Clin Nutr. 2015;24(1):162–76. [DOI] [PubMed] [Google Scholar]
- 9. Ogundipe O, Hoyo C, Østbye T, Oneko O, Manongi R, Terje Lie R, Kjersti Daltveit A. Factors associated with prenatal folic acid and iron supplementation among 21,889 pregnant women in Northern Tanzania: a cross-sectional hospital-based study. BMC Public Health. 2012;12:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMahon LP. Iron deficiency in pregnancy. Obstet Med. 2010;3(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thorand B, Schultink W, Gross R, Sastroamidjojo S, Wentzel S. Efficiency of the iron supplementation programme for pregnant women in Jeneponto, Sulawesi, Indonesia. Asia Pac J Clin Nutr. 1994;3(4):211–5. [PubMed] [Google Scholar]
- 12. Galloway R, Dusch E, Elder L, Achadi E, Grajeda R, Hurtado E, Favin M, Kanani S, Marsaban J, Meda N et al.. Women's perceptions of iron deficiency and anemia prevention and control in eight developing countries. Soc Sci Med. 2002;55(4):529–44. [DOI] [PubMed] [Google Scholar]
- 13. Abu-Ouf NM, Jan MM.. The impact of maternal iron deficiency and iron deficiency anemia on child's health. Saudi Med J. 2015;36(2):146–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115(2 Suppl):519–617. [DOI] [PubMed] [Google Scholar]
- 15. Gambling L, Danzeisen R, Fosset C, Andersen HS, Dunford S, Srai SK, HJ MC. Iron and copper interactions in development and the effect on pregnancy outcome. J Nutr. 2003;133(5 Suppl 1):1554s–6s. [DOI] [PubMed] [Google Scholar]
- 16. Knudsen VK, Hansen HS, Ovesen L, Mikkelsen TB, Olsen SF. Iron supplement use among Danish pregnant women. Public Health Nutr. 2007;10(10):1104–10. [DOI] [PubMed] [Google Scholar]
- 17. Wulff M, Ekström E-C.. Iron supplementation during pregnancy in Sweden: to what extent is the national recommendation followed?. Acta Obstet Gynecol Scand. 2003;82(7):628–35. [DOI] [PubMed] [Google Scholar]
- 18. Ogundipe O, Hoyo C, Østbye T, Oneko O, Manongi R, Lie RT, Daltveit AK. Factors associated with prenatal folic acid and iron supplementation among 21,889 pregnant women in Northern Tanzania: a cross-sectional hospital-based study. BMC Public Health. 2012;12(1):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Titilayo A, Palamuleni ME, Omisakin O. Sociodemographic factors influencing adherence to antenatal iron supplementation recommendations among pregnant women in Malawi: analysis of data from the 2010 Malawi Demographic and Health Survey. Malawi Med J. 2016;28(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kassa ZY, Awraris T, Daba AK, Tenaw Z. Compliance with iron folic acid and associated factors among pregnant women through pill count in Hawassa city, South Ethiopia: a community based cross-sectional study. Reprod Health. 2019;16(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gebremedhin S, Samuel A, Mamo G, Moges T, Assefa T. Coverage, compliance and factors associated with utilization of iron supplementation during pregnancy in eight rural districts of Ethiopia: a cross-sectional study. BMC Public Health. 2014;14(1):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haile MT, Jeba AB, Hussen MA.. Compliance to prenatal iron and folic acid supplement and associated factors among women during pregnancy in South East Ethiopia: a cross-sectional study. J Nutr Health Food Eng. 2017;7(2):272–277, Doi: 10.15406/jnhfe.2017.07.00235 [DOI] [Google Scholar]
- 23. Assefa H, Abebe SM, Sisay M. Magnitude and factors associated with adherence to iron and folic acid supplementation among pregnant women in Aykel town, Northwest Ethiopia. BMC Pregnancy and Childbirth. 2019;19(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lacerte P, Pradipasen M, Temcharoen P, Imamee N, Vorapongsathorn T. Determinants of adherence to iron/folate supplementation during pregnancy in two provinces in Cambodia. Asia Pac J Public Health. 2011;23(3):315–23. [DOI] [PubMed] [Google Scholar]
- 25. Fieldler J, D'Agostino A, Sununtnasuk C, 2014. Nutrition technical brief: a rapid initial assessment of the distribution and consumption of iron-folic acid tablets through antenatal care in Ethiopia. Arlington, VA:USAID/Strengthening Partnerships, Results and Innovations in Nutrition Globally (SPRING) Project. [Google Scholar]
- 26. Gebre A, Mulugeta A, Etana B. Assessment of factors associated with adherence to iron-folic acid supplementation among urban and rural pregnant women in North Western zone of Tigray, Ethiopia: comparative study. Int J Nutr Food Sci. 2015;4(2):161–8. [Google Scholar]
- 27. Gebremariam AD, Tiruneh SA, Abate BA, Engidaw MT, Asnakew DT. Adherence to iron with folic acid supplementation and its associated factors among pregnant women attending antenatal care follow up at Debre Tabor General Hospital, Ethiopia, 2017. PLoS One. 2019;14(1):e0210086–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agegnehu G, Atenafu A, Dagne H, Dagnew B. Adherence to iron and folic acid supplement and its associated factors among antenatal care attendant mothers in Lay Armachiho health centers, Northwest, Ethiopia, 2017. Int J Reprod Med. 2019;2019:5863737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gebreamlak B, Dadi AF, Atnafu A. High adherence to iron/folic acid supplementation during pregnancy time among antenatal and postnatal care attendant mothers in governmental health centers in Akaki Kality Sub City, Addis Ababa, Ethiopia: hierarchical negative binomial Poisson regression. PLoS One. 2017;12(1):e0169415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Birhanu TM, Birarra MK, Mekonnen FA. Compliance to iron and folic acid supplementation in pregnancy, Northwest Ethiopia. BMC Research Notes. 2018;11(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arega Sadore A, Abebe Gebretsadik L, Aman Hussen M. Compliance with iron-folate supplement and associated factors among antenatal care attendant mothers in Misha District, South Ethiopia: community based cross-sectional study. J Environ Public Health. 2015;2015:781973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiwanuka TS, Ononge S, Kiondo P, Namusoke F. Adherence to iron supplements among women receiving antenatal care at Mulago National Referral Hospital, Uganda-cross-sectional study. BMC Research Notes. 2017;10(1):510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rutstein S, Rojas G. Guide to DHS statistics. Demographic and health surveys. Calverton, Maryland: ORCMacro; 2003. [Google Scholar]
- 34. Macro International Inc. Measure DHS: Demographic and Health Surveys, [Internet]. Available from: http://www.measuredhs.com/countries/browse_country.cfm?selected = 2, Accessed 13 May 2019.
- 35. Mishra V, Vaessen M, Boerma JT, Arnold F, Way A, Barrere B, Cross A, Hong R, Sangha J. HIV testing in national population-based surveys: experience from the Demographic and Health Surveys. Bull World Health Organ. 2006;84(7):537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Macro International Inc. Measure DHS: Demographic and Health Surveys, [Internet]. Available from: https://dhsprogram.com/What-We-Do/Questionnaires.cfm, Accessed 13 May 2019.
- 37. Nisar YB, Dibley MJ, Mir AM. Factors associated with non-use of antenatal iron and folic acid supplements among Pakistani women: a cross sectional household survey. BMC Pregnancy and Childbirth. 2014;14(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Warvadekar K, Reddy JC, Sharma S, Dearden KA, Raut MK.. Socio-demographic and economic determinants of adherence to iron intake among pregnant women in selected low and lower middle income countries in Asia: insights from a cross-country analyses of global demographic and health surveys. Intl J Comm Med Public Health. 2018;5(4):1552–69. [Google Scholar]
- 39. Lunani LL, Abaasa A, Omosa-Manyonyi G. Prevalence and factors associated with contraceptive use among Kenyan women aged 15–49 years. AIDS Behav. 2018;22(Suppl 1):125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Titilayo A, Palamuleni ME, Olaoye-Oyesola JO, Owoeye OM. Religious perceptions and attitudes of men towards discontinuation of female genital cutting in Nigeria: evidence from the 2013 Nigeria Demographic and Health Survey. Afr J Reprod Health. 2018;22(1):20–8. [DOI] [PubMed] [Google Scholar]
- 41. Spiegelman D, Hertzmark E.. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 42. Titaley CR, Dibley MJ, Roberts CL, Agho K. Combined iron/folic acid supplements and malaria prophylaxis reduce neonatal mortality in 19 sub-Saharan African countries. Am J Clin Nutr. 2010;92(1):235–43. [DOI] [PubMed] [Google Scholar]
- 43. Pena-Rosas JP, De-Regil LM, Dowswell T, Viteri FE. Daily oral iron supplementation during pregnancy. The Cochrane Database Syst. Rev. 2012;12:Cd004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Habib F, Alabdin EH, Alenazy M, Nooh R. Compliance to iron supplementation during pregnancy. J Obstet Gynaecol. 2009;29(6):487–92. [DOI] [PubMed] [Google Scholar]
- 45. Sununtnasuk C, D'Agostino A, Fiedler JL. Iron+folic acid distribution and consumption through antenatal care: identifying barriers across countries. Public Health Nutr. 2016;19(4):732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seck BC, Jackson RT.. Determinants of compliance with iron supplementation among pregnant women in Senegal. Public Health Nutr. 2008;11(6):596–605. [DOI] [PubMed] [Google Scholar]
- 47. World Bank, Gross domestic product. 2018, [Internet]. Available from : http://databank.worldbank.org/data/download/GDP.pdf, Accessed 6 November 2019.
- 48. Taye B, Abeje G, Mekonen A. Factors associated with compliance of prenatal iron folate supplementation among women in Mecha district, Western Amhara: a cross-sectional study. Pan Afr Med J. 2015;20:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mithra P, Unnikrishnan B, Rekha T, Nithin K, Mohan K, Kulkarni V, Kulkarni V, Agarwal D. Compliance with iron-folic acid (IFA) therapy among pregnant women in an urban area of south India. Afr Health Sci. 2013;13(4):880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ogundipe O, Hoyo C, Ostbye T, Oneko O, Manongi R, Lie RT, Daltveit AK. Factors associated with prenatal folic acid and iron supplementation among 21,889 pregnant women in Northern Tanzania: a cross-sectional hospital-based study. BMC Public Health. 2012;12:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Getachew M, Abay M, Zelalem H, Gebremedhin T, Grum T, Bayray A. Magnitude and factors associated with adherence to iron-folic acid supplementation among pregnant women in Eritrean refugee camps, northern Ethiopia. BMC Pregnancy and Childbirth. 2018;18(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Molla T, Guadu T, Muhammad EA, Hunegnaw MT. Factors associated with adherence to iron folate supplementation among pregnant women in West Dembia district, northwest Ethiopia: a cross sectional study. BMC Research Notes. 2019;12(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tarekegn M, Wubshet M, Atenafu A, Derso T, Woretaw A. Antenatal care and mothers’ education improved iron-folic acid adherence at Denbiya district health centers, Northwest Ethiopia: using pills count method. Arch Public Health. 2019;77(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taye B, Abeje G, Mekonen A. Factors associated with compliance of prenatal iron folate supplementation among women in Mecha district, Western Amhara: a cross-sectional study. Pan Afr Med J. 2015;20:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chourasia A, Pandey CM, Awasthi A. Factors influencing the consumption of iron and folic acid supplementations in high focus states of India. Clin Epidemiol Global Health. 2017;5(4):180–4. [Google Scholar]
- 56. Ba DM, Ssentongo P, Agbese E, Kjerulff KH. Prevalence and predictors of contraceptive use among women of reproductive age in 17 sub-Saharan African countries: a large population-based study. Sexual & Reproductive Healthcare. 2019;21:26–32. [DOI] [PubMed] [Google Scholar]