Abstract
Unfortunately, the options for treating multidrug-resistant (MDR) Acinetobacter baumannii (A. baumannii) infections are extremely limited. Recently, fosfomycin and minocycline were newly introduced as a treatment option for MDR A. baumannii infection. Therefore, we investigated the efficacy of the combination of colistin with fosfomycin and minocycline, respectively, as therapeutic options in MDR A. baumannii pneumonia. We examined a carbapenem-resistant A. baumannii isolated from clinical specimens at Severance Hospital, Seoul, Korea. The effect of colistin with fosfomycin, and colistin with minocycline on the bacterial counts in lung tissue was investigated in a mouse model of pneumonia caused by MDR A. baumannii. In vivo, colistin with fosfomycin or minocycline significantly (p < 0.05) reduced the bacterial load in the lungs compared with the controls at 24 and 48 h. In the combination groups, the bacterial loads differed significantly (p < 0.05) from that with the more active antimicrobial alone. Moreover, the combination regimens of colistin with fosfomycin and colistin with minocycline showed bactericidal and synergistic effects compared with the more active antimicrobial alone at 24 and 48 h. This study demonstrated the synergistic effects of combination regimens of colistin with fosfomycin and minocycline, respectively, as therapeutic options in pneumonia caused by MDR A. baumannii.
Subject terms: Microbiology, Bacterial infection
Introduction
Acinetobacter baumannii is a well-documented, multidrug-resistant (MDR) nosocomial pathogen1. In the past, carbapenems have been recommended as the antibiotics of choice for treating A. baumannii. However, the increasing incidence of MDR strains has led to use of unconventional antibiotics, such as polymyxin, rifampicin, and tigecycline, for the treatment of MDR isolates2. Nevertheless, pneumonia caused by MDR A. baumannii infection has a high mortality rate3.
Colistin has been used increasingly for the treatment of MDR A. baumannii infection, despite its potential nephrotoxicity and neurotoxicity. It has shown excellent in vitro antibacterial effect against carbapenem-resistant A. baumannii4. However, low plasma concentrations, heteroresistance and rapid regrowth after colistin treatment have brought the efficacy of colistin monotherapy into question5.
Consequently, colistin-based combination treatments have been proposed to attain antibiotic synergy. A systematic review revealed that colistin-based combinations with several antibiotics exerted a synergistic effect against isolates of MDR A. baumannii and lowered the mortality rate in animal studies6–11.
Recently, minocycline and fosfomycin, two ‘old’ drugs, were newly introduced as treatment options for MDR A. baumannii infection. Minocycline had the second highest susceptibility rate (79.1%), followed by colistin in vitro12. Fosfomycin is active against Gram-positive and -negative bacteria13. Also, fosfomycin is an alternative agent for the treatment of MDR bacterial infections14,15.
However, to our literature search, little in vivo data are available on the use of colistin with minocycline or fosfomycin for the treatment of infection by MDR A. baumannii, especially pneumonia. Therefore, we investigated the in vivo efficacy of combinations of colistin with minocycline and fosfomycin, respectively, as therapeutic options in a mouse model of MDR A. baumannii pneumonia.
Results
Antibiotic susceptibility tests
Table 1 shows the minimum inhibitory concentrations (MICs) of piperacillin/tazobactam, ceftazidime, imipenem, amikacin, ciprofloxacin, tigecycline, colistin, minocycline, and fosfomycin of an A. baumannii isolate.
Table 1.
Antibiotic susceptibility of carbapenemase–producing A. baumannii.
| β-lactamase* | MIC(mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PIP/TAZ | CAZ | IMP | AMK | CIP | TIG | COL | MIN | FOS | |
| OXA-23 | >128 | >128 | 32 | >128 | >128 | 16 | 0.5 | 0.25 | >128 |
MIC, minimal inhibitory concentration; PIP/TAZ, piperacillin/tazobactam; CAZ, ceftazidime; IMP, imipenem; AMK, amikacin; CIP, ciprofloxacin; TIG, tigecycline; COL, colistin; MIN, minocycline; FOS, fosfomycin.
*There were no additional chromosomal beta-lactamase genes in this strain.
Time-kill test
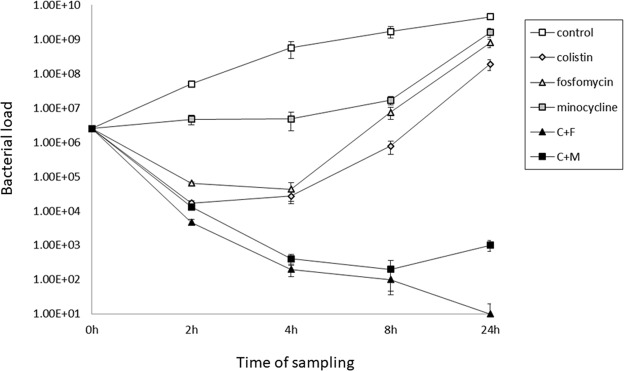
At 0.5 × MIC, combinations of colistin with fosfomycin and colistin with minocycline showed synergistic effects, with >2 log 10 reductions in CFU at 4 and 8 h. Moreover, this reduction in CFU was maintained at 24 h. The combination regimens showed synergistic effects with ≥2 log10 decreases compared with the monotherapy regimens. The time–kill curves are shown in Fig. 1.
Figure 1.
Time-kill curves. Colistin/fosfomycin and colistin/minocycline combinations showed bactericidal effects, with >3 log10 reductions in CFU at 4, 8, and 24 h. The combination regimens showed synergistic effects, with ≥2 log10 decreases in CFU compared with the monotherapy regimens (0.5 × MIC). (C: colistin; F: fosfomycin; M: minocycline).
Mouse model of MDR A. baumannii pneumonia
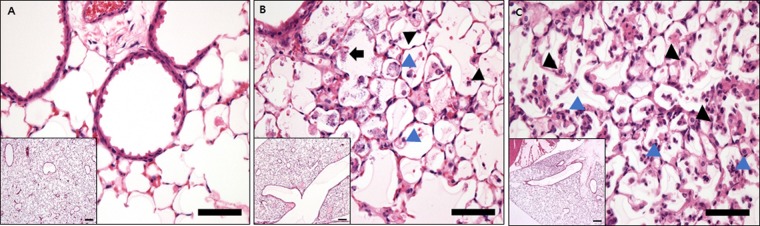
Figure 2 shows the lung pathology at 4, 24, and 48 h after nasal inoculation. Four hours after inoculation, no significant lesion was present in the alveoli, bronchioles, or bronchi, except for minimal edema in the alveolar cavity. At 24 h after nasal inoculation, moderate numbers of neutrophils and macrophages had infiltrated the alveoli, with moderate edema, and bacterial colonization was observed in the alveolar cavities. At 48 h after nasal inoculation, large numbers of macrophages and neutrophils had infiltrated the alveoli.
Figure 2.
Histopathology of the lung tissues of mice at 4 (A), 24 (B), and 48 h (C) after inoculation with A. baumannii (H&E; bar = 100 µm). (A) At 4 h after inoculation, no significant lesion was present in the alveoli, bronchioles, or bronchi, except for minimal edema in the alveolar cavity. (B) At 24 h after inoculation, moderate numbers of neutrophils (dark triangle) and macrophages (blue triangle) had infiltrated the alveoli; moderate edema was present, and bacterial colonization (dark arrow) was observed in the alveolar cavities. (C) At 48 h after inoculation, large numbers of neutrophils (dark triangle) and macrophages (blue triangle) had infiltrated the alveoli.
Effects on lung bacterial loads
Table 2 shows the lung bacterial loads in each group. Colistin, fosfomycin, and minocycline significantly (p < 0.05) reduced the bacterial loads in the lungs, compared with the controls, at 24 and 48 h. At 24 h after starting the antibiotic agents, fosfomycin and minocycline showed bactericidal effects, but colistin did not. However, colistin in combination with fosfomycin and minocycline, respectively, significantly (p < 0.05) reduced the bacterial load in the lungs compared with the controls at 24 and 48 h. In the combination groups, significant (p < 0.05) differences were noted in the bacterial loads compared with the more active antimicrobial alone. Moreover, the combination regimens all showed bactericidal and synergistic effects at 24 and 48 h compared with the more active antimicrobial alone. The combination of colistin with fosfomycin significantly (p < 0.05) reduced the bacterial load in the lungs at 48 h compared with colistin with minocycline.
Table 2.
Therapeutic effects on the lung bacterial loads at 24 and 48 h after starting the antibiotic agents.
| Antibiotic regimen | 24 h | 48 h |
|---|---|---|
| Control | 12.24 ± 0.44 | 12.99 ± 0.22 |
| COL | 9.65 ± 0.43a | 8.73 ± 0.34a |
| FOS | 7.68 ± 0.47a | 6.68 ± 1.02a |
| MIN | 9.26 ± 0.19a | 7.73 ± 1.33a |
| COL + FOS | 5.63 ± 0.26a,b | 3.46 ± 0.42a,b,c |
| COL + MIN | 6.07 ± 1.04a,b | 5.16 ± 0.83a,b |
COL, colistin; MIN, minocycline; FOS, fosfomycin.
The lung bacterial loads are expressed as the mean ± standard deviation of log10 CFU per gram of lung at 24 and 48 h.
aSignificant difference in bacterial load compared with the control group (p < 0.05).
bSignificant difference in bacterial load compared with the more active antibiotic alone (p < 0.05).
cSignificant difference in bacterial load compared with the other combination (p < 0.05).
Effects on survival
Table 3 shows the mortality rates of the mice. The mortality rate was 33.3% (4/12) in the untreated control group at 48 h. No significant difference in survival was observed among the control and antibiotic-treated groups.
Table 3.
Mortality rates in the control and antibiotic-treated groups.
| Antibiotic regimen | 24 h | 48 h |
|---|---|---|
| Control | 0/15 (0%) | 4/12 (33.3%) |
| COL | 0/15 (0%) | 0/12 (0%) |
| FOS | 0/15 (0%) | 0/12 (0%) |
| MIN | 0/15 (0%) | 0/12 (0%) |
| COL + FOS | 0/15 (0%) | 0/12 (0%) |
| COL + MIN | 0/15 (0%) | 0/12 (0%) |
COL, colistin; MIN, minocycline; FOS, fosfomycin.
Discussion
This study demonstrated the synergistic effects of regimens combining colistin with minocycline and fosfomycin, respectively, on pneumonia caused by MDR A. baumannii.
The treatment of A. baumannii infection is an important problem in the nosocomial setting16. In serious infections including pneumonia, initial therapy with an appropriate antibiotic is very important6. In addition to colistin, other antibiotic regimens have emerged, although none has been fully tested. Carbapenem with sulbactam showed a synergistic effect against in MDR A. baumannii strains6,7. Although the study population was heterogeneous and small and no control group was used, a clinical study of rifampicin in combination with colistin and imipenem, yielded promising results8,9. Tigecycline also showed good bacteriostatic activity against carbapenem-resistant A. baumannii in vitro6,10. However, the synergistic activity of many antibiotics in vitro does not correlate with that in vivo17. Nevertheless, some specific antibiotic combinations have shown increased in vivo efficacy against MDR isolates18.
Recently, fosfomycin and minocycline were introduced as treatment options for the infection caused by MDR A. baumannii12,13. In our time–kill study, colistin with fosfomycin and minocycline, respectively, showed bactericidal and synergistic effects at 8 and 24 h. A previous study using time–kill tests documented synergistic and bactericidal effects of colistin and minocycline in 92% of the strains tested at 24 h2. In another study using the E-test, the fractional inhibitory concentration indices (FICIs) for combinations of polymyxin B and minocycline were generally ≤0.5 or >0.5–1.0, suggesting that polymyxin B and minocycline have a synergistic or additive effect19. In the same study, most FICIs for polymyxin B and fosfomycin were within the ranges of 0.5–1.0 and 1.0–4.0, suggesting that polymyxin B and fosfomycin exert an additive or independent effect19. In comparison, another study of combination therapy against A. baumannii found that colistin combined with fosfomycin was more effective than colistin monotherapy in 83.3% (24 h) and 66.7% (48 h) of MDR strains18.
We also found that colistin with minocycline and colistin with fosfomycin showed bactericidal and synergistic effects 24 and 48 h after nasal inoculation in vivo, in accordance with previous studies. Yang et al.20 reported that minocycline in combination with colistin had in vivo synergistic efficacy against MDR A. baumannii pneumonia. Bowers et al.21 showed that minocycline combined with polymyxin B further decreased the bacterial lung load at 24 h, compared with monotherapy. Sirijatuphat et al.22 reported that colistin with fosfomycin showed a synergistic effect against carbapenem-resistant A. baumannii. They also recently conducted a preliminary clinical study, which showed that patients with MDR A. baumannii infection given a combination of colistin and fosfomycin had significantly better microbiological responses with trends toward more favorable treatment outcomes and lower mortality compared with those treated with colistin alone23. However, because several types of infection, polymicrobial infections and concurrent antimicrobial agents were included in their study, it was heterogenous. Although our study was an in vivo animal study, not human study, it was more homogenous than previous study.
Interestingly, our in vivo results showed that the combination of colistin with fosfomycin significantly reduced the bacterial load in the lungs, compared with monotherapy and colistin with minocycline, at 48 h. Fosfomycin has a higher MIC against A. baumannii. For an antibiotic to be effective clinically, it must achieve concentrations in the interstitial fluid that exceed the MICs for the pathogens24,25. One study showed that fosfomycin achieved antimicrobially effective concentrations in infected lung tissue26. Also, without pharmacokinetics (PKs) of fosfomycin in this study, we used it every 4 h according to other study. These frequent injections of fosfomycin might attain more effective concentrations in infected lung tissue than minocycline. Moreover, in the combination therapy, fosfomycin was injected with colistin simultaneously, but minocycline was not. This difference explains the greater effectiveness of colistin with fosfomycin relative to colistin with minocycline at 48 h.
No significant difference in survival was observed among the control and antibiotic-treated groups. This finding might be due to the relatively short duration of follow-up in our study or the relatively low virulence of OXA-23 carbapenemase-producing A. baumannii. According to a previous report, OXA-23 carbapenemase-producing A. baumannii infection has a high mortality rate in intensive care unit patients27. Consequently, further evaluation of the mortality rate of OXA-23 carbapenemase-producing A. baumannii infection with a longer follow-up is needed.
Our study has some limitations. First, only one strain from a single center was used. However, we used an OXA-23 carbapenemase-producing A. baumannii isolated from clinical specimens obtained in our hospital. OXA-23 carbapenemase is the most common carbapenemase in South Korea28. Therefore, our study was meaningful in this regard. Second, we did not investigate the PKs of the drugs used in this study. However, we used dosages that have been used in other studies.
In conclusion, we investigated the in vivo efficacy of colistin in combination with minocycline and fosfomycin, as therapeutic options in a mouse model of MDR A. baumannii pneumonia. We demonstrated the in vivo synergistic effects of regimens combining colistin with minocycline and fosfomycin, on pneumonia caused by MDR A. baumannii. Large clinical trials are needed to clarify the role of regimens combining colistin with fosfomycin or minocycline in treating MDR A. baumannii pneumonia.
Methods
Bacterial strains
We obtained five carbapenem-resistant A. baumannii, which have an OXA-23 carbapenemase, isolated from clinical specimens obtained at Severance Hospital, Seoul, South Korea. We selected the most virulent strain, which was isolated from a patient with pneumonia and bacteremia. The other strains were colonizers isolated from sputum specimens. A strain was considered resistant to carbapenems when the MIC against imipenem was ≥16 mg/L.
Antibiotic susceptibility tests
Antibiotic susceptibility tests were performed in duplicate using the agar dilution and broth microdilution methods according to the Clinical and Laboratory Standards Institute (CLSI)29. The MICs of piperacillin/tazobactam, ceftazidime, imipenem, amikacin, ciprofloxacin, minocycline, and fosfomycin were determined by the agar dilution method. The MICs of colistin were determined by the broth microdilution method. The MICs of fosfomycin were determined by the agar dilution method in cation-adjusted Mueller-Hinton medium, supplemented with 25 mg/L glucose-6-phosphate (Sigma-Aldrich., St. Louis, MO, USA). Because there are no CLSI interpretive criteria for fosfomycin against A. baumannii, the fosfomycin breakpoints for Escherichia coli were used according to the CLSI guidelines29. E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used for quality control of antimicrobial susceptibility testing.
Detection of carbapenemase genes by PCR
After DNA extraction multiplex PCR was performed to detect blaOXA genes (blaOXA-23-like, blaOXA-24-like, and blaOXA-58-like genes) and ISAba1-associated blaOXA-51-like gene30. Additionally, PCR was performed to detect blaOXA-182 gene31. We performed PCR to detect blaIMP-1-like, blaVIM-2-like, and blaSIM-1-like genes32. Sequence analysis was performed by a commercial laboratory (Macrogen, Seoul, South Korea).
Time–kill test
For both agents and their combinations, time–kill tests were performed using sub-inhibitory For both agents and their combinations, (0.5 × MIC)33. Ten-fold dilutions were inoculated onto Mueller–Hinton agar and colonies were counted at 0, 4, 8, and 24 h. Bactericidal activity was defined as a ≥3 log10 decrease compared with the initial inoculum. Synergy was defined as a ≥2 log10 decrease with the combination, compared with most active single agent34. Experiments were performed in triplicate on separate days. Results were read by two observers.
Mouse model of MDR A. baumannii pneumonia
The animal study was approved by the Institutional Animal Care and Use Committee of the Yonsei University College of Medicine (#2014-0275). All animal experimental protocols were performed in accordance with the relevant ethical guidelines and regulations. Immunocompetent, specific pathogen–free, 6-week-old female mice weighing 18–20 g (C57BL/6N) were used (Orient Bio, Seongnam, South Korea). Animals were rendered transiently neutropenic by injecting cyclophosphamide intraperitoneally (300 mg/kg body weight) in a volume of 0.2 mL 4 days before A. baumannii inoculation in the lung. The mice were anesthetized by intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. Then, 1.2 mL/kg of a 5 × 108 colony-forming units (CFU)/mL bacterial suspension was inoculated through the nose using a syringe35. After being kept in a vertical position for 4 min, the mice were maintained in a 30° decubitus position until regaining consciousness. Necropsy was performed on one mouse at 4, 24, and 48 h after the nasal inoculation. The entire lung was removed in a sterile fashion and evaluated pathologically, and the diagnosis of pneumonia was confirmed. Sterile lung specimens were fixed in 10% formalin and immersed in paraffin wax. Specimens were prepared in 5-μm cross sections and were examined under a light microscope after hematoxylin/eosin staining.
Study groups
The mice with pneumonia were randomized into six groups of 15 mice each five treatment groups and a control group. Colistin was administered to the first group, fosfomycin to the second, minocycline to the third, the colistin/fosfomycin combination to the fourth, and the colistin/minocycline combination to the fifth. No antibacterial agent was administered to the mice in the control group. After the experiment, the mice were euthanized by CO2 inhalation.
Treatment protocol
Treatments were initiated 4 h after nasal inoculation. The antibiotic agents were given by intraperitoneal injection at the following dosages: colistin, 20 mg/kg every 8 h36, fosfomycin, 100 mg/kg every 4 h37, and minocycline, 20 mg/kg every 12 h38. Colistin, fosfomycin, and minocycline were purchased from Sigma-Aldrich.
Effects on lung bacterial loads
Bacteria in the lungs of three mice were counted 24 and 48 h after starting the antibiotic administration. To eliminate any antibiotic carry-over effect, mice in the treatment groups were euthanized at least 3 h after the last antibiotic dose. For quantitative bacteriological studies, the lungs were removed, weighed, and homogenized in 1 mL of saline. Tenfold dilutions were made, and 100-μL aliquots were plated on tryptic soy agar with 5% sheep blood plates for 24 h at 37 °C. Colonies were counted for each dilution and each animal. Experiments were performed in triplicate. The results are expressed as means ± standard deviation (SD) log10 CFU per gram lung at 24 and 48 h, and differences between groups were calculated as follows: mean of the treated group – mean of the control group. Bactericidal activity was defined as a ≥3 log10 decrease compared with the initial inoculum. Synergy was defined as a ≥2 log10 decrease in killing by the combination compared with the most active single drug alone18.
Effects on survival
The survival rates of all mice at 24 and 48 h were recorded and compared among the treatment and control groups.
Statistical analysis
All bacterial counts are presented as the mean ± SD. Student’s t-test was used to analyze inter-group differences in the bacterial counts. To compare mortality between groups, Fisher’s exact test was used. In all tests, differences were considered to be statistically significant when the p-value was <0.05.
Acknowledgements
This study was supported by a faculty research grant from the Yonsei University College of Medicine (6-2017-0054).
Author contributions
Conception and design of study: N.S.K. and J.M.K. Acquisition of data: N.S.K., S.L., Y.L., H.C. and J.M.K. Data analysis and interpretation: N.S.K., S.L., Y.L., H.C. and J.M.K. Drafting of manuscript and critical revision: N.S.K., H.C., J.Y.A., S.J.J., S.J.S., J.Y.C., Y.H.C., J.Y., D.Y., Y.G.S. and J.M.K. Approval of final version of manuscript: N.S.K., S.L., Y.L., H.C., J.Y.A., S.J.J., S.J.S., J.Y.C., Y.H.C., J.Y., D.Y., Y.G.S. and J.M.K.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perez F, et al. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrobial agents and chemotherapy. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan TY, Ng LS, Tan E, Huang G. In vitro effect of minocycline and colistin combinations on imipenem-resistant Acinetobacter baumannii clinical isolates. The Journal of antimicrobial chemotherapy. 2007;60:421–423. doi: 10.1093/jac/dkm178. [DOI] [PubMed] [Google Scholar]
- 3.Li YJ, et al. Pneumonia caused by extensive drug-resistant Acinetobacter baumannii among hospitalized patients: genetic relationships, risk factors and mortality. BMC Infect Dis. 2017;17:371. doi: 10.1186/s12879-017-2471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montero A, et al. Antibiotic combinations for serious infections caused by carbapenem-resistant Acinetobacter baumannii in a mouse pneumonia model. The Journal of antimicrobial chemotherapy. 2004;54:1085–1091. doi: 10.1093/jac/dkh485. [DOI] [PubMed] [Google Scholar]
- 5.Lee HJ, et al. Synergistic activity of colistin and rifampin combination against multidrug-resistant Acinetobacter baumannii in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrobial agents and chemotherapy. 2013;57:3738–3745. doi: 10.1128/AAC.00703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song JY, et al. In vitro activities of carbapenem/sulbactam combination, colistin, colistin/rifampicin combination and tigecycline against carbapenem-resistant Acinetobacter baumannii. The Journal of antimicrobial chemotherapy. 2007;60:317–322. doi: 10.1093/jac/dkm136. [DOI] [PubMed] [Google Scholar]
- 7.Wolff M, Joly-Guillou ML, Farinotti R, Carbon C. In vivo efficacies of combinations of beta-lactams, beta-lactamase inhibitors, and rifampin against Acinetobacter baumannii in a mouse pneumonia model. Antimicrobial agents and chemotherapy. 1999;43:1406–1411. doi: 10.1128/AAC.43.6.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saballs M, et al. Rifampicin/imipenem combination in the treatment of carbapenem-resistant Acinetobacter baumannii infections. The Journal of antimicrobial chemotherapy. 2006;58:697–700. doi: 10.1093/jac/dkl274. [DOI] [PubMed] [Google Scholar]
- 9.Motaouakkil S, et al. Colistin and rifampicin in the treatment of nosocomial infections from multiresistant Acinetobacter baumannii. The Journal of infection. 2006;53:274–278. doi: 10.1016/j.jinf.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Peleg AY, Adams J, Paterson DL. Tigecycline Efflux as a Mechanism for Nonsusceptibility in Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:2065–2069. doi: 10.1128/AAC.01198-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrosillo N, Ioannidou E, Falagas ME. Colistin monotherapy vs. combination therapy: evidence from microbiological, animal and clinical studies. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2008;14:816–827. doi: 10.1111/j.1469-0691.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 12.Castanheira M, Mendes RE, Jones RN. Update on Acinetobacter species: mechanisms of antimicrobial resistance and contemporary in vitro activity of minocycline and other treatment options. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;59(Suppl 6):S367–373. doi: 10.1093/cid/ciu706. [DOI] [PubMed] [Google Scholar]
- 13.Silver Lynn L. Fosfomycin: Mechanism and Resistance. Cold Spring Harbor Perspectives in Medicine. 2017;7(2):a025262. doi: 10.1101/cshperspect.a025262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;46:1069–1077. doi: 10.1086/527442. [DOI] [PubMed] [Google Scholar]
- 15.Falagas ME, Kastoris AC, Karageorgopoulos DE, Rafailidis PI. Fosfomycin for the treatment of infections caused by multidrug-resistant non-fermenting Gram-negative bacilli: a systematic review of microbiological, animal and clinical studies. International journal of antimicrobial agents. 2009;34:111–120. doi: 10.1016/j.ijantimicag.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Murray CK, Hospenthal DR. Treatment of multidrug resistant Acinetobacter. Current opinion in infectious diseases. 2005;18:502–506. doi: 10.1097/01.qco.0000185985.64759.41. [DOI] [PubMed] [Google Scholar]
- 17.Paul M, et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. The Journal of antimicrobial chemotherapy. 2014;69:2305–2309. doi: 10.1093/jac/dku168. [DOI] [PubMed] [Google Scholar]
- 18.Fan B, Guan J, Wang X, Cong Y. Activity of Colistin in Combination with Meropenem, Tigecycline, Fosfomycin, Fusidic Acid, Rifampin or Sulbactam against Extensively Drug-Resistant Acinetobacter baumannii in a Murine Thigh-Infection Model. PLoS One. 2016;11:e0157757. doi: 10.1371/journal.pone.0157757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, et al. In vitro antibacterial activity of combinations of fosfomycin, minocycline and polymyxin B on pan-drug-resistant Acinetobacter baumannii. Experimental and therapeutic medicine. 2013;5:1737–1739. doi: 10.3892/etm.2013.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YS, et al. In Vivo and In Vitro Efficacy of Minocycline-Based Combination Therapy for Minocycline-Resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2016;60:4047–4054. doi: 10.1128/AAC.02994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowers DR, et al. Assessment of minocycline and polymyxin B combination against Acinetobacter baumannii. Antimicrobial agents and chemotherapy. 2015;59:2720–2725. doi: 10.1128/AAC.04110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tharavichitkul, P. S. C. et al. In vitro synergistic effects of a combination of fosfomycin and colistin against metallo-β-lactamase-producing Acinetobacter baumannii. Abstracts of the 31st annual meeting of the Infectious Disease Association of Thailand, Chonburi, Thailand (2005).
- 23.Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrobial agents and chemotherapy. 2014;58:5598–5601. doi: 10.1128/AAC.02435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merrikin DJ, Briant J, Rolinson GN. Effect of protein binding on antibiotic activity in vivo. The Journal of antimicrobial chemotherapy. 1983;11:233–238. doi: 10.1093/jac/11.3.233. [DOI] [PubMed] [Google Scholar]
- 25.Frossard M, et al. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrobial agents and chemotherapy. 2000;44:2728–2732. doi: 10.1128/AAC.44.10.2728-2732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzi V, et al. Extracellular concentrations of fosfomycin in lung tissue of septic patients. The Journal of antimicrobial chemotherapy. 2010;65:995–998. doi: 10.1093/jac/dkq070. [DOI] [PubMed] [Google Scholar]
- 27.da Silva KE, et al. A high mortality rate associated with multidrug-resistant Acinetobacter baumannii ST79 and ST25 carrying OXA-23 in a Brazilian intensive care unit. PLoS One. 2018;13:e0209367. doi: 10.1371/journal.pone.0209367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee Y, et al. Carbapenem-non-susceptible Acinetobacter baumannii of sequence type 92 or its single-locus variants with a G428T substitution in zone 2 of the rpoB gene. The Journal of antimicrobial chemotherapy. 2011;66:66–72. doi: 10.1093/jac/dkq402. [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing 27th informational supplement. Approved standard M100-S27. Clinical and Laboratory Standards Institute, Wayne (2017).
- 30.Woodford N, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y, et al. Increasing prevalence of blaOXA-23-carrying Acinetobacter baumannii and the emergence of blaOXA-182-carrying Acinetobacter nosocomialis in Korea. Diagn Microbiol Infect Dis. 2013;77:160–163. doi: 10.1016/j.diagmicrobio.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Lee K, et al. Novel acquired metallo-beta-lactamase gene, bla(SIM-1), in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob Agents Chemother. 2005;49:4485–4491. doi: 10.1128/AAC.49.11.4485-4491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White RL, Burgess DS, Manduru M, Bosso JA. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrobial agents and chemotherapy. 1996;40:1914–1918. doi: 10.1128/AAC.40.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillai, S. K., Moellering, R. C. & Eliopoulos, G. M. Chapter 9: Antimicrobial combinations. In: Lorian, V. ed. Antibiotics in Laboratory Medicine. Philadelphia, PA: Lippincott Williams & Wilkins, 365–440 (2005).
- 35.Manepalli S, et al. Characterization of a cyclophosphamide-induced murine model of immunosuppression to study Acinetobacter baumannii pathogenesis. Journal of medical microbiology. 2013;62:1747–1754. doi: 10.1099/jmm.0.060004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pachon-Ibanez ME, et al. Efficacy of rifampin and its combinations with imipenem, sulbactam, and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrobial agents and chemotherapy. 2010;54:1165–1172. doi: 10.1128/AAC.00367-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lefort A, et al. Activity of fosfomycin alone or combined with cefoxitin in vitro and in vivo in a murine model of urinary tract infection due to Escherichia coli harbouring CTX-M-15-type extended-spectrum beta-lactamase. International journal of antimicrobial agents. 2014;43:366–369. doi: 10.1016/j.ijantimicag.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Ko WC, et al. In vitro and in vivo combinations of cefotaxime and minocycline against Aeromonas hydrophila. Antimicrobial agents and chemotherapy. 2001;45:1281–1283. doi: 10.1128/AAC.45.4.1281-1283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]