Abstract
Background
The majority of children who present with their first episode of nephrotic syndrome achieve remission with corticosteroid therapy. Children who fail to respond to corticosteroids in the first episode of nephrotic syndrome (initial resistance) or develop resistance after one or more responses to corticosteroids (delayed resistance) may be treated with immunosuppressive agents including calcineurin inhibitors (CNI) (cyclosporin or tacrolimus) and with non‐immunosuppressive agents such as angiotensin‐converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB). However, response to these agents is limited so newer agents are being assessed for efficacy. This is an update of a review first published in 2004 and updated in 2006, 2010 and 2016.
Objectives
To evaluate the benefits and harms of different interventions used in children with idiopathic nephrotic syndrome, who do not achieve remission following four weeks or more of daily corticosteroid therapy.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies to 17 September 2019 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs were included if they compared different immunosuppressive agents or non‐immunosuppressive agents with placebo, prednisone or other agent given orally or parenterally in children aged three months to 18 years with steroid‐resistant nephrotic syndrome (SRNS). Studies, which enrolled children and adults but in which paediatric data could not be separated from adult data, were also included.
Data collection and analysis
Two authors independently searched the literature, determined study eligibility, assessed risk of bias and extracted data. For dichotomous outcomes, results were expressed as risk ratios (RR) and 95% confidence intervals (CI). For continuous outcomes, results were expressed as mean difference (MD) and 95% CI. Data were pooled using the random effects model. The certainty of the evidence was assessed using the GRADE approach.
Main results
Twenty‐five studies (1063 participants) were included. Fourteen studies were at low risk of bias for sequence generation and allocation concealment. Five and 19 studies were at low risk of performance and detection bias. Fourteen, 14 and 13 studies were at low risk of attrition bias, reporting bias and other bias respectively.
Cyclosporin compared with placebo or no treatment may increase the number of participants who achieve complete remission (4 studies, 74 participants: RR 3.50, 95% CI 1.09 to 11.20) or complete or partial remission (4 studies, 74 children: RR 3.15, 95% CI 1.04 to 9.57) by 6 months (low certainty evidence). It is uncertain whether cyclosporin increases the likelihood of worsening hypertension or reduces the likelihood of end‐stage kidney disease (very low certainty evidence).
CNI compared with IV cyclophosphamide (CPA) may increase the number of participants with complete or partial remission at 3 to 6 months (2 studies, 156 children: RR 1.98, 95% CI 1.25 to 3.13) (low certainty evidence) and probably reduces the number with treatment failure (non response, serious infection, persistently elevated creatinine (1 study, 124 participants: RR 0.32, 95% CI 0.18 to 0.58) (moderate certainty evidence) with little or no increase in serious infections (1 study, 131 participants: RR 0.49, 95% CI 0.16 to 1.56) (moderate certainty evidence).
Tacrolimus compared with cyclosporin may make little or no difference to the number who achieve complete or partial remission (2 studies, 58 participants: RR 1.05, 95% CI 0.87 to 1.25) (low certainty evidence) or in the number with worsening hypertension (2 studies, 58 participants: RR 0.41, 95% CI 0.08 to 2.15) (low certainty evidence).
Cyclosporin compared with mycophenolate mofetil (MMF) and dexamethasone probably makes little or no difference to the number who achieve complete or partial remission (1 study, 138 participants: RR 2.14, 95% CI 0.87 to 5.24) (moderate certainty evidence) and makes little or no difference to the number dying (1 study, 138 participants: RR 2.14, 95% CI 0.87 to 5.24) or with 50% reduction in glomerular filtration rate (GFR) (1 study, 138 participants: RR 2.29, 95% CI 0.46 to 11.41) (low certainty evidence).
Among children, who have achieved complete remission, tacrolimus compared with MMF may increase the number of children who maintain complete or partial response for 12 months (1 study, 60 children: RR 2.01, 95% CI 1.32 to 3.07) (low certainty evidence).
Oral CPA with prednisone compared with prednisone alone may make little or no difference to the number who achieve complete remission (2 studies, 84 children: RR 1.06, 95% CI 0.61 to 1.87) (low certainty evidence).
IV CPA compared with oral CPA (2 studies, 61 children: RR 1.58, 95% CI 0.65 to 3.85) and IV compared with oral CPA plus IV dexamethasone (1 study, 49 children: RR 1.13, 95% CI 0.65 to 1.96) may make little or no difference to the number who achieve complete remission (low certainty evidence).
It is uncertain whether rituximab and cyclosporin compared with cyclosporin increases the likelihood of remission because the certainty of the evidence is very low.
It is uncertain whether adalimumab or galactose compared with conservative therapy increases the likelihood of remission because the certainty of the evidence is very low.
Two studies reported that ACEi may reduce proteinuria in children with SRNS. One study reported that the dual angiotensin II and endothelin Type A receptor antagonist, sparsentan, may reduce proteinuria more effectively than the angiotensin receptor blocker, irbesartan.
Authors' conclusions
To date RCTs have demonstrated that CNIs may increase the likelihood of complete or partial remission compared with placebo/no treatment or CPA. For other regimens assessed, it remains uncertain whether the interventions alter outcomes because the certainty of the evidence is low. Further adequately powered, well designed RCTs are needed to evaluate other regimens for children with idiopathic SRNS. Since SRNS represents a spectrum of diseases, future studies should enrol children from better defined groups of patients with SRNS.
Plain language summary
Interventions for idiopathic steroid resistant nephrotic syndrome in children
What is the issue?
Nephrotic syndrome is a condition where the kidneys leak protein from the blood into the urine. Corticosteroids are used in the first instance to achieve remission. Other agents such as calcineurin inhibitors (cyclosporin, tacrolimus) or angiotensin‐converting enzyme inhibitors are required for those children do not respond to corticosteroids in their first episode of nephrotic syndrome (initial resistance) or who develop steroid resistance after one or more responses to corticosteroids (delayed resistance).
What did we do?
We searched Cochrane Kidney and Transplant's Specialised Register (up to 17 September 2019). Randomised controlled trials were included if they compared different immunosuppressive agents or non‐immunosuppressive agents with placebo, prednisone or other agent in children with steroid resistant nephrotic syndrome. Studies of new treatments were included as these included children as well as adults.
What did we find?
This review found that cyclosporin compared with placebo, no treatment or prednisone may increase the number of participants, in whom urine protein disappears (complete remission) or is markedly reduced (partial remission). Calcineurin inhibitors (cyclosporin, tacrolimus) also may increase the number of children, who achieve complete or partial remission compared with intravenous cyclophosphamide. There may be little or no benefit of other immunosuppressive agents studied so far. Angiotensin‐converting enzyme inhibitors may reduce the amount of protein in the urine.
Conclusions
Calcineurin inhibitors may increase the likelihood of complete or partial remission compared with placebo/no treatment or cyclophosphamide. However, the certainty of the evidence is low because the studies were small. It remains uncertain whether other interventions may alter outcomes due to few small studies. Larger and well‐designed randomised controlled trials are needed to evaluate other treatment combinations for children with steroid resistant nephrotic syndrome.
Summary of findings
Background
Description of the condition
Nephrotic syndrome is a condition in which the glomeruli of the kidney leak protein from the blood into the urine. It results in hypoproteinaemia and generalised oedema. Children with untreated nephrotic syndrome are at increased risk of bacterial infection, characteristically resulting in peritonitis, cellulitis or septicaemia, of thromboembolic phenomena and of protein calorie malnutrition with significant reductions in quality of life. Prospective studies of children with newly diagnosed idiopathic nephrotic syndrome identified through Paediatric Surveillance Units in the Netherlands, Australia and New Zealand reported incidences of idiopathic nephrotic syndrome of 1.12 to 1.9 per 100,000 children aged below 16 years (El Bakkali 2011; Sureshkumar 2014; Wong 2007). A literature review of studies from 1946 to 2014 found the average incidence of nephrotic syndrome from retrospective and prospective studies to be 4.7 (range 1.15 to 16.9) per 100,000 children (Chanchlani 2016). The proportion of children with steroid resistance disease varied between 2.1 to 27.3% (average 12.4%).
In clinical studies childhood nephrotic syndrome is classified into steroid‐sensitive nephrotic syndrome (SSNS), steroid‐resistant nephrotic syndrome (SRNS), congenital and infantile nephrotic syndrome (0 to 12 months) and nephrotic syndrome secondary to other diseases including Henoch Schönlein nephritis, systemic lupus erythematosus and hepatitis B nephropathy. Most children with primary nephrotic syndrome respond to corticosteroid therapy within four weeks. In those children who fail to respond to corticosteroids, kidney biopsy is performed to determine pathology. The majority of children with SRNS have focal segmental glomerulosclerosis (FSGS), mesangioproliferative glomerulonephritis (MesPGN) or minimal change disease (MCD). FSGS is a leading cause of end‐stage kidney disease (ESKD) in children. FSGS is a heterogeneous disease with some children having FSGS secondary to immunological factors, some children having FSGS secondary to mutations in the genes coding for podocyte proteins including podocin and nephrin and a few older children having FSGS secondary to hyperfiltration (reduced kidney mass, obesity, diabetes mellitus) (Deegens 2011). A study of 1783 unrelated families found that single gene mutations responsible for SRNS were identified in 29.5% families overall with mutations in 25.3% children aged 1 to 6 years, 17.8% in children aged 7 to 12 years and 10.8% in adolescents aged 13 to 18 years (Sadowski 2015). Few children with FSGS secondary to genetic mutations respond to immunosuppressive agents and in these children, nephrotic syndrome rarely recurs following kidney transplantation (Ding 2014). Children with SRNS may have corticosteroid resistant disease from initial presentation (Initial resistance) or may develop steroid resistance after one or more responses to corticosteroids (delayed resistance); children with delayed steroid resistance do not have disease causing gene mutations (Bierzynska 2017). About one third of children suffer recurrence of nephrotic syndrome following kidney transplantation. Recent data suggest that recurrence of disease post transplant is much more common in children with SRNS and delayed steroid resistance (Ding 2014). These data are consistent with an immunological cause of SRNS in these children.
Description of the intervention
Oral corticosteroids are the first‐line treatment for a child presenting with idiopathic nephrotic syndrome. For children who present with their first episode of nephrotic syndrome, about 90% will achieve remission with corticosteroid therapy (Koskimies 1982). Of those who respond, about 95% will have responded after four weeks of daily corticosteroid therapy and 98% will have responded after eight weeks of corticosteroid therapy (ISKDC 1981a).
Children who fail to respond to corticosteroids are treated with immunosuppressive agents such as calcineurin inhibitors (CNI) (cyclosporin, tacrolimus), cyclophosphamide (CPA), chlorambucil, mycophenolate mofetil (MMF), and the anti CD 20 monoclonal antibody, rituximab. Rates of complete and partial remission with CNI based on observational studies and individual groups in randomised controlled trials (RCTs) vary between 30% and 80% (Choudhry 2009; FSGS‐CT 2011; Niaudet 1994). Remission rates of up to 60% with combinations of intravenous (IV) methylprednisolone and CPA are reported in observational studies (Tune 1996) and of around 50% in individual treatment groups in RCTs (Gulati 2012; ISKDC 1974; ISKDC 1996). Failure to achieve complete or partial remission is associated with progression to ESKD (Gipson 2006). Other non‐immunosuppressive agents including angiotensin‐converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), and fish oil have also been used in SRNS.
How the intervention might work
Corticosteroids, immunosuppressive agents and monoclonal antibodies may act by suppressing production of plasma factors by T and B cells since immunological mechanisms are believed to be responsible for some cases of SRNS. Some immunosuppressive medications including dexamethasone, CNI, and rituximab may be effective in nonimmune causes of SRNS by directly targeting podocytes. ACEi and ARB reduce proteinuria and are aimed at reducing progressive glomerulosclerosis (Deegens 2011).
Why it is important to do this review
There is considerable diversity in the use of these agents with differences in treatment modes, combinations and dosage regimens. Optimal combinations with least toxicity remain to be determined. Despite the use of newer immunosuppressive agents, the response rate to therapy remains low. The aims of the update of this systematic review initially published in 2004 and updated in 2006, 2010 and 2016 were to identify new RCTs assessing the benefits and harms of interventions used to treat idiopathic SRNS in children and to incorporate them where appropriate in meta‐analyses to increase the evidence base available on the efficacy of treatment of SRNS in children.
Objectives
To evaluate the benefits and harms of different interventions used in children with idiopathic nephrotic syndrome, who do not achieve remission following four weeks or more of daily corticosteroid therapy.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs, in which different agents were used in the treatment of participants including children (aged three months to 18 years) with idiopathic SRNS, were included.
Types of participants
Inclusion criteria
Children aged three months to 18 years with SRNS (i.e. persistence of proteinuria > 3+ on dipstick, urinary protein‐creatinine ratio (UP/C) > 0.2 g/mmol (> 2 g/g) or > 40 mg/m2/h after four weeks or more of daily corticosteroid agent). Where a kidney biopsy was performed, only children with biopsy diagnoses of MCD, MesPGN, IgM nephropathy or FSGS were included. Children with initial steroid resistance and children with delayed steroid resistance were included. Children with disease‐causing genetic mutations associated with FSGS where kidney biopsy was not performed could also be included.
Where studies included adults and children were included and where paediatric data could not be separated, data of all participants in these studies were included in this review.
Exclusion criteria
Children with SSNS, children with congenital nephrotic syndrome and children with other kidney or systemic forms of nephrotic syndrome defined on kidney biopsy, clinical features or serology (e.g. post‐infectious glomerulonephritis, Henoch‐Schönlein nephritis, systemic lupus erythematosus, membranous glomerulopathy or mesangiocapillary glomerulonephritis) were excluded. Children with FSGS secondary to hyperfiltration (obesity, diabetes mellitus, reduced kidney mass) were excluded.
Types of interventions
All interventions were potentially eligible. Interventions considered were as follows.
IV corticosteroid agent versus oral corticosteroid agent, placebo or no intervention
Different doses and/or durations of IV corticosteroid agent
Non‐corticosteroid immunosuppressive agent (with or without concomitant use of corticosteroid agent) versus corticosteroid agent alone, placebo or no treatment
Two different non‐corticosteroid agents (with or without concomitant use of corticosteroid agent)
Different doses, durations and routes of administration of the same non‐corticosteroid agent (with or without concomitant use of corticosteroid agent)
Other non‐immunosuppressive agents such as ACEi or fish oil used with or without corticosteroid or non‐corticosteroid immunosuppressive agents.
Types of outcome measures
Primary outcomes
Number in complete remission during and following therapy (i.e. the child became oedema‐free and urine protein was < 1+ on dipstick, urinary UP/C < 0.02 g/mmol (< 0.2 g/g) or < 4 mg/m2/h for three or more consecutive days)
Number in partial remission with reduction in proteinuria (i.e. proteinuria < 2+, urinary UP/C < 0.2 g/mmol or < 40 mg/m2/h) and an increase in serum albumin levels
Number reaching ESKD.
Secondary outcomes
Changes in kidney function: serum creatinine (SCr); creatinine clearance (CrCl); estimated glomerular filtration rate (eGFR)
Adverse effects of therapy
Duration of remission or partial remission
Reduction in proteinuria.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 17 September 2019 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
No other resources were searched for this update because the scope of the Cochrane Kidney and Transplant Register of Studies covers the most likely sources of studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that were relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable. However, studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria. Disagreements were resolved in consultation with a third author.
Data extraction and management
Data extraction was carried out by the same authors independently using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Disagreements were resolved in consultation with a third author.
Assessment of risk of bias in included studies
Studies to be included were assessed independently by two authors without blinding to authorship or journal. Discrepancies were resolved by discussion with a third author.
The following items were assessed using the risk of bias assessment tool (Higgins 2011) (seeAppendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. remission or no remission) results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. protein excretion), the mean difference (MD) was to be used, or the standardised MD (SMD) if different scales were to be used.
Adverse events were reported in the text if they could not be included in meta‐analyses.
Unit of analysis issues
Data from cross‐over studies were included in the meta‐analyses if separate data for the first part of the study were available. Otherwise results of cross‐over studies were reported in the text only.
Dealing with missing data
Any further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review. We aimed to analyse available data in meta‐analyses using intention‐to‐treat (ITT) data. However, where ITT data were not provided, or additional information could not be obtained from authors, available published data were used in the analyses.
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I² values was as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a CI for I2) (Higgins 2011).
Assessment of reporting biases
The search strategy used aimed to reduce publication bias caused by lack of publication of studies with negative results. Where there were several publications on the same study, all reports were reviewed to ensure that all details of methods and results were included to reduce the risk of selective outcome reporting bias.
Data synthesis
Data was pooled using the random effects model but the fixed effects model was analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was planned to explore possible sources of heterogeneity (e.g. participants, treatments and study quality). Heterogeneity among participants could be related to age and renal pathology. Heterogeneity in treatments could be related to prior agent(s) used and the agent, dose and duration of therapy. However, there were insufficient studies of each intervention to allow subgroup analyses.
Sensitivity analysis
Sensitivity analysis was planned to determine the effect of removal of a single study on the results of a meta‐analysis when results of one study differed from other studies in the meta‐analysis. However, there were insufficient studies of each intervention to allow sensitivity analysis.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Complete remission
Partial remission
Complete or partial remission
Chronic kidney disease
Adverse events.
Results
Description of studies
Results of the search
For the initial 2004 version of the review, of the 1744 titles and abstracts screened, 10 studies were identified; one study was excluded so nine studies (10 reports) were included in the review (Bagga 2004; Chongviriyaphan 1999; Elhence 1994; Garin 1988; ISKDC 1970; ISKDC 1974; ISKDC 1996; Lieberman 1996; Ponticelli 1993a). An update in 2006 identified four additional studies of which two were included (Kleinknecht 1980; Yi 2006) so the 2006 update included 11 studies (13 reports). A second update in 2010 identified three additional studies and the full publication of one study previously available as an abstract (Yi 2006). Therefore 14 studies (18 reports) were included in the 2010 update; 494 children entered the studies and 449 were evaluated.
A further search to 2 March 2016 identified 21 new studies, of which five were included (FSGS‐CT 2011; Gulati 2012; Magnasco 2012; Sinha 2017; Wu 2015). The 2016 update included 19 studies (42 reports) comprising 820 children of whom 773 were evaluated (Figure 1). Although we were not able to obtain separate paediatric data from the authors, we chose to include FSGS‐CT 2011 because it was one of the largest studies looking at interventions for SRNS, 93 (67%) of participants were below 18 years of age and subgroup analyses by study authors showed no differences in outcomes between paediatric and adult participants. We also identified three ongoing studies. The first study evaluated the safety and efficacy of sparsentan (a dual endothelin receptor) in a phase 2 study compared with irbesartan (DUET 2017). The second study is evaluating the 12 month relapse free survival in children with SRNS treated with rituximab or tacrolimus (NCT02382575). The third study is evaluating ofatumumab compared with placebo in children with steroid‐ and CNI‐resistant nephrotic syndrome (NCT02394106).
1.

Flowchart of included and excluded studies
In 2019 we identified four new included studies (FONT I 2009; FONT II 2011; Shah 2017; Valverde 2010), In addition, results from the DUET 2017 study, which had been identified as an ongoing study in the 2016 update, were available. A review of previously excluded studies revealed an eligible study for inclusion (Bhaumik 2002). Four studies included both adult and paediatric participants and separate paediatric data were not available (Bhaumik 2002; DUET 2017; FONT I 2009; FONT II 2011). There were six additional reports of three already included studies (FSGS‐CT 2011; Gulati 2012; Sinha 2017), and one new report of an already excluded study. Two studies (NCT02382575; NCT02394106) listed as ongoing in the 2016 update are continuing. Of three additional ongoing studies, one is evaluating abatacept (Trachtman 2018), one is evaluating ACTH (NCT02972346), and one is evaluating sparsentan in a phase 3 study (DUPLEX 2018) in treatment resistant nephrotic syndrome. The 2019 update included 25 studies (69 reports) with five ongoing studies (Figure 1).
Included studies
The 25 included studies enrolled 1063 participants of which 1012 were evaluated. Study characteristics are shown in Characteristics of included studies.
Four studies compared cyclosporin with placebo, no treatment or methylprednisolone (84 enrolled/74 children and adults evaluated) (Bhaumik 2002; Garin 1988; Lieberman 1996; Ponticelli 1993a). Two studies (Garin 1988; Ponticelli 1993a) included children with MCD and FSGS, while two studies (Bhaumik 2002; Lieberman 1996) included only participants with FSGS. Three studies (Bhaumik 2002; Lieberman 1996; Ponticelli 1993a) included only participants with initial steroid resistance.
Two studies compared oral CNI with IV CPA. APN 2008 (32 children) compared oral cyclosporin with IV CPA in children with initial steroid resistance. Gulati 2012 (124/131 children evaluated) compared oral tacrolimus with IV CPA in children with initial and delayed steroid resistance. Both studies included children with MCD, FSGS and MesPGN.
Two studies (Choudhry 2009 (41 children); Valverde 2010 (17 children)) compared oral cyclosporin with oral tacrolimus. Choudhry 2009 included children with initial or delayed steroid resistance and children with MCD, FSGS and MesPGN. Valverde 2010 did not report whether patients had initial or delayed steroid resistance, and did not state histological types.
FSGS‐CT 2011 (138 participants) compared cyclosporin with MMF and oral dexamethasone in children (93) and adults (45) with biopsy confirmed primary FSGS and initial steroid resistance. Separate paediatric data could not be obtained from the authors.
Wu 2015 (18/22 children evaluated) compared MMF, IV CPA or leflunomide in three groups already receiving prednisone and tacrolimus. The study included children with MCD, FSGS, MesPGN and IgM nephropathy. The authors did not state whether the children had initial or delayed steroid resistance.
Sinha 2017 (60 children) compared tacrolimus with MMF to maintain remission in children with initial or delayed steroid resistance, who had achieved remission with tacrolimus. The study included children with MCD and FSGS.
Two studies (84/93 children evaluated) compared oral CPA and prednisone with prednisone alone in children with initial steroid resistance (ISKDC 1974; ISKDC 1996). ISKDC 1974 included children with MCD, FSGS and MesPGN. ISKDC 1996 only included children with FSGS.
Three studies compared IV with oral CPA in children with initial or delayed steroid resistance (Elhence 1994; Mantan 2008; Shah 2017). In Mantan 2008 (49/52 children evaluated), IV dexamethasone was given to children in the oral CPA group. Elhence 1994 (11/13 children evaluated) only included children with MCD while Mantan 2008 and Shah 2017 (50 children) included children with MCD, FSGS and MesPGN.
Magnasco 2012 (31 children) compared rituximab and standard care (prednisolone and cyclosporin) with standard care alone in children with MCD, FSGS and unknown histology and with initial or delayed steroid resistance.
Kleinknecht 1980 (30 children) compared chlorambucil with indomethacin. This study did not report whether patients had initial or delayed steroid resistance. The study included children with MCD, FSGS and MesPGN.
ISKDC 1970 (31 children) compared azathioprine (AZA) and prednisone with placebo and prednisone in children with MCD, FSGS or MesPGN, who had initial steroid resistance.
Two studies evaluated ACEi. Bagga 2004 (25 children) compared different doses of the ACEi, enalapril in children with MCD, FSGS or MesPGN in a cross‐over study. Yi 2006 (45/57 children evaluated) compared the ACEi, fosinopril, and prednisone with prednisone alone. Both studies included children with initial and delayed steroid resistance.
DUET 2017 (96/109 adults and children evaluated for efficacy; all evaluated for adverse effects) compared the dual angiotensin II and endothelin type A receptor antagonist, sparsentan with the ARB, irbesartan in patients with primary FSGS.
Chongviriyaphan 1999 (5 children) compared fish oil with placebo in children with FSGS or MesPGN in a cross over study; the authors did not state whether the children had initial or delayed resistance.
FONT I 2009 (19/21 adults and children evaluated) compared adalimumab with rosiglitazone in participants with FSGS and initial steroid resistance.
FONT II 2011 (19/21 adults and children evaluated) compared adalimumab, galactose and conservative therapy in participants with therapy resistant primary FSGS.
Excluded studies
23 studies (39 reports) were excluded.
Adeniyi 1979 was excluded because 31/36 included children had nephrotic syndrome considered secondary to Plasmodium malariae.
Nine studies did not include children (Arora 2002; Koshikawa 1993; Kumar 2004a; Li 2006g; Ren 2011; Ren 2013; Saito 2014; Shibasaki 2004; Walker 1990).
Four studies did not include children with nephrotic syndrome (Kano 2003) or included children with an ineligible renal pathology (Buyukcelik 2002; Hari 2018; Saito 2017).
Two studies evaluated interventions in children with SSNS (Hiraoka 2000; Iyengar 2006).
Five studies evaluated interventions in both children with steroid‐resistant and steroid‐dependent disease and the results could not be separated (Jung 1990; Khemani 2016; Tejani 1988; Yi 2008; Zhao 2013a).
In one study, only children with SSNS were randomised; children with SRNS were not randomised (Ahn 2018).
One study was excluded because it was a single arm study (JPRN‐C000000007).
Risk of bias in included studies
2.
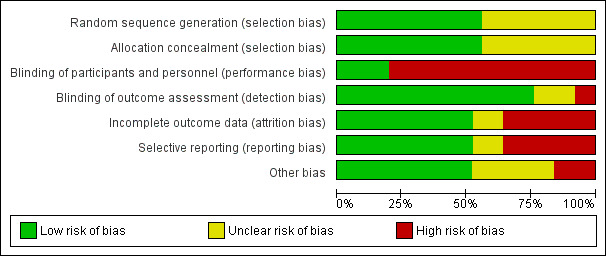
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
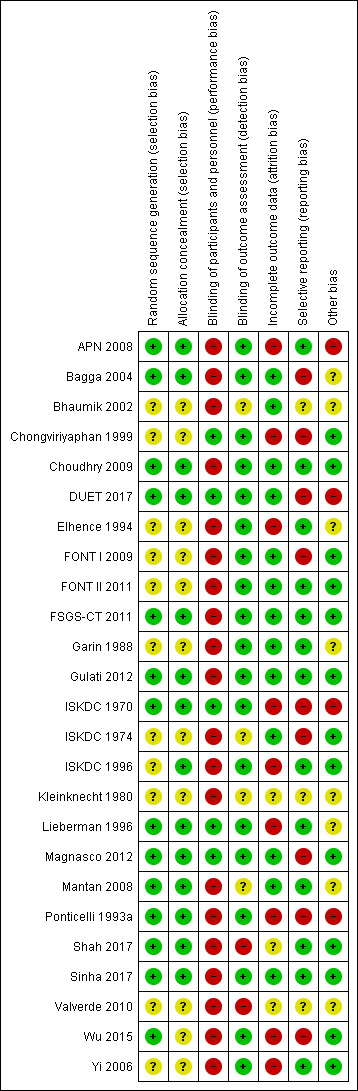
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation was satisfactory in 14 studies (APN 2008; Bagga 2004; Choudhry 2009; DUET 2017; FSGS‐CT 2011; Gulati 2012; ISKDC 1970; Lieberman 1996; Magnasco 2012; Mantan 2008; Ponticelli 1993a; Shah 2017; Sinha 2017; Wu 2015) and unclear in the remaining studies.
Allocation concealment was adequate in 14 studies (APN 2008; Bagga 2004; Choudhry 2009; DUET 2017; FSGS‐CT 2011; Gulati 2012; ISKDC 1970; ISKDC 1996; Lieberman 1996; Magnasco 2012; Mantan 2008; Ponticelli 1993a; Shah 2017; Sinha 2017) and unclear in the remaining studies.
Blinding
Five studies reported that care givers (families, research staff) were blinded to treatment groups (Chongviriyaphan 1999; DUET 2017; ISKDC 1970; Lieberman 1996; Magnasco 2012). In the remaining 20 studies, care givers were not blinded to treatment groups.
Nineteen studies were considered at low risk of detection bias as the outcome was laboratory‐based and unlikely to be influenced by blinding (APN 2008; Bagga 2004; Choudhry 2009; Elhence 1994; FONT I 2009; FONT II 2011; FSGS‐CT 2011; Garin 1988; ISKDC 1970; ISKDC 1996; Lieberman 1996; Ponticelli 1993a; Sinha 2017; Wu 2015; Yi 2006) or the outcome assessors were blinded to treatment groups (Chongviriyaphan 1999; DUET 2017; Gulati 2012; Magnasco 2012). In two studies (ISKDC 1974; Mantan 2008), outcome of proteinuria was measured on dipstick or in a laboratory and it was unclear in how many children the outcome was laboratory‐based. In two studies (Bhaumik 2002; Kleinknecht 1980), no information was provided on how the outcome was assessed. In two studies (Shah 2017; Valverde 2010) there was no blinding and outcome assessment could be influenced by lack of blinding.
Incomplete outcome data
Attrition bias was considered to be present if more than 10% of participants were excluded from analysis. Fourteen studies were considered to have provided complete outcome data (Bagga 2004; Bhaumik 2002; Choudhry 2009; DUET 2017; FONT I 2009; FONT II 2011; FSGS‐CT 2011; Garin 1988; Gulati 2012; ISKDC 1974; Magnasco 2012; Mantan 2008; Shah 2017; Sinha 2017). Nine studies did not provide complete outcome data. In the remaining two studies, available only as abstracts (Kleinknecht 1980; Valverde 2010), it was unclear whether complete outcome data was provided.
Selective reporting
Reporting bias was considered to be present if studies did not report on the number of patients with remission (complete or partial) and on adverse effects and if results of the primary outcome were not reported in a way that allowed inclusion of the data in meta‐analyses. Thirteen studies were considered to be free of selective reporting (APN 2008; Choudhry 2009; DUET 2017; Elhence 1994; FONT II 2011; FSGS‐CT 2011; Garin 1988; Gulati 2012; ISKDC 1996; Lieberman 1996; Mantan 2008; Shah 2017; Yi 2006). Nine studies were considered to have reported outcomes selectively or results for the primary outcome could not be included in meta‐analyses (Bagga 2004; Chongviriyaphan 1999; DUET 2017; FONT I 2009; ISKDC 1970; ISKDC 1974; Magnasco 2012; Ponticelli 1993a; Wu 2015). In the remaining three studies (Bhaumik 2002; Kleinknecht 1980; Valverde 2010), available only as abstracts, it was unclear whether there was selective reporting of outcomes.
Other potential sources of bias
Thirteen studies reported funding by university or government agencies or stated that they did not receive monetary support and were considered free of other potential sources of bias (Chongviriyaphan 1999; Choudhry 2009; FONT I 2009; FONT II 2011; FSGS‐CT 2011; Gulati 2012; ISKDC 1974; ISKDC 1996; Magnasco 2012; Shah 2017; Sinha 2017; Yi 2006; Wu 2015). Four studies reported funding from pharmaceutical companies and were considered at risk of potential bias (APN 2008; DUET 2017; ISKDC 1970; Ponticelli 1993a). Other potential sources of bias were unclear in the remaining eight studies as none reported on support.
The definition of steroid resistance varied between studies.
Nine studies defined steroid resistance as persistent proteinuria of > 4 mg/m2/h or UP/C > 1g/g after four weeks (FONT I 2009; FSGS‐CT 2011; Lieberman 1996; Wu 2015), five weeks (Kleinknecht 1980), six weeks (APN 2008) or eight weeks of daily prednisone (Bagga 2004; ISKDC 1970; ISKDC 1974). One study (FONT II 2011) defined steroid resistance as persistent proteinuria of > 4 mg/m2/h or UP/C > 1g/g “following a standard course of prednisone/prednisolone/methylprednisolone prescribed for FSGS therapy”.
Nine studies defined steroid resistance as persistent proteinuria > 40 mg/m2/h, > 2 g/g or above 1 g/m2/d after four weeks (Choudhry 2009; Gulati 2012; Mantan 2008; Shah 2017; Sinha 2017), five weeks (Ponticelli 1993a), eight weeks (Garin 1988; ISKDC 1996) or six months (Magnasco 2012) of prednisone.
Two studies defined steroid resistance as no response after eight weeks of prednisone (Bhaumik 2002; Yi 2006) but did not define the degree of proteinuria.
Four studies did not define steroid resistance (Chongviriyaphan 1999; DUET 2017; Elhence 1994; Valverde 2010).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Summary of findings for the main comparison. Cyclosporin versus placebo or no treatment for idiopathic steroid‐resistant nephrotic syndrome in children.
| Cyclosporin versus placebo/no treatment for idiopathic steroid‐resistant nephrotic syndrome in children | |||||
| Patient or population: idiopathic steroid‐resistant nephrotic syndrome in children Setting: paediatric nephrology services Intervention: cyclosporin Comparison: placebo/no treatment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo/no treatment | Risk with cyclosporin | ||||
| Complete remission: all renal pathologies | 57 per 1,000 | 200 per 1,000 (62 to 640) | RR 3.50 (1.09 to 11.20) | 74 (4) | ⊕⊕⊝⊝ LOW 1 2 |
| Complete remission: FSGS | 69 per 1,000 | 217 per 1,000 (67 to 702) | RR 3.14 (0.97 to 10.18) | 58 (3) | ⊕⊕⊝⊝ LOW 1 2 |
| Complete or partial remission: all renal pathologies | 229 per 1,000 | 720 per 1,000 (238 to 1,000) | RR 3.15 (1.04 to 9.57) | 74 (4) | ⊕⊕⊝⊝ LOW 1 2 |
| Complete or partial remission: FSGS | 333 per 1,000 | 887 per 1,000 (283 to 1,000) | RR 2.66 (0.85 to 8.31) | 49 (2) | ⊕⊕⊝⊝ LOW 1 2 |
| Adverse events: worsening of hypertension | 167 per 1,000 | 167 per 1,000 (28 to 997) | RR 1.00 (0.17 to 5.98) | 24 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Adverse events: infection | 429 per 1,000 | 300 per 1,000 (86 to 1,000) | RR 0.70 (0.20 to 2.51) | 17 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Adverse events: ESKD | 333 per 1,000 | 77 per 1,000 (10 to 597) | RR 0.23 (0.03 to 1.79) | 25 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; FSGS: focal segmental glomerulosclerosis; ESKD: end‐stage kidney disease | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Inadequate or unclear allocation concealment and sequence generation in two studies.
2 Small numbers of events and included patients in RCTs
Summary of findings 2. Calcineurin inhibitor versus IV cyclophosphamide for idiopathic steroid‐resistant nephrotic syndrome in children.
| Calcineurin inhibitor versus IV cyclophosphamide for idiopathic steroid‐resistant nephrotic syndrome in children | |||||
| Patient or population: idiopathic steroid‐resistant nephrotic syndrome in children Setting: paediatric nephrology services Intervention: calcineurin inhibitor (CNI) Comparison: IV cyclophosphamide (CPA) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with IV CPA | Risk with CNI | ||||
| Treatment response at 3 to 6 months: complete or partial remission | 397 per 1,000 | 787 per 1,000 (497 to 1,000) | RR 1.98 (1.25 to 3.13) | 156 (2) | ⊕⊕⊝⊝ LOW 1 2 |
| Treatment response at 3 to 6 months: complete remission | 128 per 1,000 | 440 per 1,000 (236 to 822) | RR 3.43 (1.84 to 6.41) | 156 (2) | ⊕⊕⊝⊝ LOW 1 2 |
| Treatment response at 3 to 6 months: partial remission | 269 per 1,000 | 452 per 1,000 (116 to 1,000) | RR 1.68 (0.43 to 6.56) | 156 (2) | ⊕⊝⊝⊝ VERY LOW 1 2 3 |
| Adverse events: treatment failure at 6 months (non response, serious infection, persistently elevated creatinine) | 541 per 1,000 | 173 per 1,000 (97 to 314) | RR 0.32 (0.18 to 0.58) | 124 (1) | ⊕⊕⊕⊝ MODERATE 2 |
| Adverse events: medications ceased due to adverse events | 154 per 1,000 | 31 per 1,000 (6 to 132) | RR 0.20 (0.04 to 0.86) | 131 (1) | ⊕⊕⊕⊝ MODERATE 2 |
| Adverse events: serious infections | 123 per 1,000 | 60 per 1,000 (20 to 192) | RR 0.49 (0.16 to 1.56) | 131 (1) | ⊕⊕⊕⊝ MODERATE 2 |
| Adverse events: death | 15 per 1,000 | 5 per 1,000 (0 to 122) | RR 0.33 (0.01 to 7.92) | 131 (1) | ⊕⊕⊝⊝ LOW 4 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 One study at high risk of attrition bias
2 Small numbers of patients included in studies
3 Significant heterogeneity between studies
4 Few events in singles study
Summary of findings 3. Tacrolimus versus cyclosporin for idiopathic steroid‐resistant nephrotic syndrome in children.
| Tacrolimus versus cyclosporin for idiopathic steroid‐resistant nephrotic syndrome in children | |||||
| Patient or population: idiopathic steroid‐resistant nephrotic syndrome in children Setting: Paediatric nephrology services Intervention: tacrolimus Comparison: cyclosporin | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with cyclosporin | Risk with tacrolimus | ||||
| Treatment response at 6 months: complete remission | 500 per 1,000 | 570 per 1,000 (320 to 1,000) | RR 1.14 (0.64 to 2.03) | 41 (1) | ⊕⊕⊝⊝ LOW 1 |
| Treatment response at 6 months: complete and partial remission | 750 per 1,000 | 428 per 1,000 (120 to 1,000) | RR 0.57 (0.16 to 2.08) | 41 (1) | ⊕⊕⊝⊝ LOW 1 |
| Treatment response at 12 months: complete remission | 500 per 1,000 | 400 per 1,000 (225 to 710) | RR 0.80 (0.45 to 1.42) | 58 (2) | ⊕⊕⊝⊝ LOW 2 |
| Treatment response at 12 months: complete and partial remission | 833 per 1,000 | 875 per 1,000 (725 to 1,000) | RR 1.05 (0.87 to 1.25) | 58 (2) | ⊕⊕⊝⊝ LOW 2 |
| Adverse events: persistent nephrotoxicity | 100 per 1,000 | 48 per 1,000 (5 to 485) | RR 0.48 (0.05 to 4.85) | 41 (1) | ⊕⊕⊝⊝ LOW 1 |
| Adverse events: worsening of hypertension | No events | No events | ‐ | 58 (2) | ⊕⊕⊝⊝ LOW 2 3 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Single small study
2 Two small studies with few events
3 Serious risk of bias issues in one included study
Summary of findings 4. Cyclosporin versus mycophenolate mofetil with pulse dexamethasone for idiopathic steroid‐resistant nephrotic syndrome in children.
| Cyclosporin versus mycophenolate mofetil with pulse dexamethasone for idiopathic steroid‐resistant nephrotic syndrome in children | |||||
| Patient or population: idiopathic steroid‐resistant nephrotic syndrome in children Setting: paediatric nephrology services Intervention: cyclosporin Comparison: mycophenolate mofetil with pulse dexamethasone (MMF + IV DEXA) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with MMF + IV DEXA | Risk with cyclosporin | ||||
| Treatment response at 52 weeks: complete remission (primary outcome 1, 2) | 91 per 1,000 | 195 per 1,000 (79 to 476) | RR 2.14 (0.87 to 5.24) | 138 (1) | ⊕⊕⊕⊝ MODERATE 1 |
| Treatment response at 52 weeks: partial remission (primary outcome 3) | 242 per 1,000 | 264 per 1,000 (148 to 468) | RR 1.09 (0.61 to 1.93) | 138 (1) | ⊕⊕⊕⊝ MODERATE 1 |
| Sustainable remission between 52 and 78 weeks: complete or partial remission (primary outcome 1, 2, 3) | 333 per 1,000 | 460 per 1,000 (300 to 700) | RR 1.38 (0.90 to 2.10) | 138 (1) | ⊕⊕⊕⊝ MODERATE 1 |
| CKD or death: death by 52 weeks | 30 per 1,000 | 5 per 1,000 (0 to 114) | RR 0.18 (0.01 to 3.75) | 138 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| CKD or death: 50% decline in GFR by 78 weeks | 30 per 1,000 | 69 per 1,000 (14 to 346) | RR 2.29 (0.46 to 11.41) | 138 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Adverse events (weeks 0 to 26): serious infection requiring hospitalisation | 106 per 1,000 | 69 per 1,000 (23 to 208) | RR 0.65 (0.22 to 1.96) | 138 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Adverse events (weeks 0 to 26): hypertension | 91 per 1,000 | 153 per 1,000 (60 to 390) | RR 1.68 (0.66 to 4.29) | 138 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Inadequate enrolment lead to uncertainty in results
2 Few events in study groups
Summary of findings 5. Tacrolimus versus mycophenolate mofetil to maintain remission for idiopathic steroid‐resistant nephrotic syndrome in children.
| Tacrolimus versus mycophenolate mofetil to maintain remission for idiopathic steroid‐resistant nephrotic syndrome in children | |||||
| Patient or population: idiopathic steroid‐resistant nephrotic syndrome in children Setting: paediatric nephrology services Intervention: tacrolimus to maintain remission Comparison: mycophenolate mofetil (MMF) to maintain remission | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with MMF to maintain remission | Risk with tacrolimus | ||||
| Number with complete or partial response at one year | 448 per 1,000 | 901 per 1,000 (592 to 1,000) | RR 2.01 (1.32 to 3.07) | 60 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Number with complete response at one year | 414 per 1,000 | 741 per 1,000 (459 to 1,000) | RR 1.79 (1.11 to 2.90) | 60 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Number with partial response at one year | 34 per 1,000 | 161 per 1,000 (20 to 1,000) | RR 4.68 (0.58 to 37.68) | 60 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Number with treatment failure by one year | 552 per 1,000 | 99 per 1,000 (33 to 298) | RR 0.18 (0.06 to 0.54) | 60 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Number with frequent relapses by one year | 345 per 1,000 | 97 per 1,000 (31 to 317) | RR 0.28 (0.09 to 0.92) | 60 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Number with steroid resistance by one year | 207 per 1,000 | 14 per 1,000 (0 to 254) | RR 0.07 (0.00 to 1.23) | 60 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Change in GFR | Change in GFR was 13 mL/min higher with tacrolimus (3.71 lower to 29.71 higher) compared to MMF | ‐ | 60 (1) | ⊕⊕⊝⊝ LOW 1 2 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Unclear how primary outcome of continuing remission was measured and whether it was blinded
2 Small study with few events
Summary of findings 6. Oral cyclophosphamide versus prednisone or placebo for idiopathic steroid‐resistant nephrotic syndrome in children.
| Oral cyclophosphamide versus prednisone/placebo for idiopathic steroid‐resistant nephrotic syndrome in children | |||||
| Patient or population: idiopathic steroid‐resistant nephrotic syndrome in children Setting: paediatric nephrology services Intervention: oral cyclophosphamide (CPA) Comparison: prednisone/placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with prednisone/placebo | Risk with oral CPA | ||||
| Complete remission: all renal pathologies | 353 per 1,000 | 374 per 1,000 (215 to 660) | RR 1.06 (0.61 to 1.87) | 84 (2) | ⊕⊕⊝⊝ LOW 1 2 |
| Complete remission: FSGS | 250 per 1,000 | 253 per 1,000 (108 to 593) | RR 1.01 (0.43 to 2.37) | 63 (2) | ⊕⊕⊝⊝ LOW 1 2 |
| Complete or partial remission | 571 per 1,000 | 503 per 1,000 (303 to 829) | RR 0.88 (0.53 to 1.45) | 53 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Complete or partial remission: FSGS | 571 per 1,000 | 503 per 1,000 (303 to 829) | RR 0.88 (0.53 to 1.45) | 53 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Treatment failure | 360 per 1,000 | 572 per 1,000 (313 to 1,000) | RR 1.59 (0.87 to 2.88) | 60 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Adverse events: death (all causes) | 80 per 1,000 | 86 per 1,000 (15 to 476) | RR 1.07 (0.19 to 5.95) | 60 (1) | ⊕⊕⊝⊝ LOW 1 2 |
| Adverse events: hypertension with seizures | 40 per 1,000 | 28 per 1,000 (2 to 436) | RR 0.71 (0.05 to 10.89) | 60 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; FSGS: focal segmental glomerulosclerosis | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Serious risk of bias issues. Unclear sequence generation and allocation concealment. Attrition bias
2 Small number of included participants
Summary of findings 7. IV versus oral cyclophosphamide for idiopathic steroid‐resistant nephrotic syndrome in children.
| IV versus oral cyclophosphamide for idiopathic steroid‐resistant nephrotic syndrome in children | |||||
| Patient or population: idiopathic steroid‐resistant nephrotic syndrome in children Setting: paediatric nephrology services Intervention: IV cyclophosphamide (CPA) Comparison: oral CPA | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with oral CPA | Risk with IV CPA | ||||
| Complete remission | 414 per 1,000 | 654 per 1,000 (269 to 1,000) | RR 1.58 (0.65 to 3.85) | 61 (2) | ⊕⊕⊝⊝ LOW 1 2 |
| Partial remission | 80 per 1,000 | 80 per 1,000 (12 to 524) | RR 1.00 (0.15 to 6.55) | 50 (1) | ⊕⊕⊝⊝ LOW 3 |
| Continuing remission at one year | 160 per 1,000 | 120 per 1,000 (30 to 482) | RR 0.75 (0.19 to 3.01) | 50 (1) | ⊕⊕⊝⊝ LOW 2 3 |
| Adverse events: renal insufficiency | 120 per 1,000 | 40 per 1,000 (5 to 359) | RR 0.33 (0.04 to 2.99) | 50 (1) | ⊕⊕⊝⊝ LOW 2 3 |
| Adverse events: bacterial infection | 103 per 1,000 | 106 per 1,000 (10 to 1,000) | RR 1.02 (0.10 to 10.62) | 61 (2) | ⊕⊝⊝⊝ VERY LOW 1 2 3 |
| Adverse events: vomiting | 34 per 1,000 | 82 per 1,000 (12 to 558) | RR 2.38 (0.35 to 16.17) | 61 (2) | ⊕⊝⊝⊝ VERY LOW 1 2 3 |
| Adverse events: alopecia | 80 per 1,000 | 120 per 1,000 (22 to 658) | RR 1.50 (0.27 to 8.22) | 50 (1) | ⊕⊕⊝⊝ LOW 2 3 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 One study had unclear sequence generation and allocation concealment and significant attrition
2 Small numbers of enrolled patients
3 Small numbers of events
Summary of findings 8. IV cyclophosphamide versus oral cyclophosphamide plus IV dexamethasone for idiopathic steroid‐resistant nephrotic syndrome in children.
| IV cyclophosphamide versus oral cyclophosphamide plus IV dexamethasone for idiopathic steroid‐resistant nephrotic syndrome in children | |||||
| Patient or population: idiopathic steroid‐resistant nephrotic syndrome in children Setting: paediatric nephrology services Intervention: IV cyclophosphamide (CPA) Comparison: oral CPA plus IV dexamethasone (DEXA) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with oral CPA plus IV DEXA | Risk with IV CPA | ||||
| Treatment response at 6 months: complete remission | 478 per 1,000 | 540 per 1,000 (311 to 937) | RR 1.13 (0.65 to 1.96) | 49 (1) | ⊕⊕⊝⊝ LOW 1 |
| Treatment response at 6 months: partial remission | 87 per 1,000 | 77 per 1,000 (12 to 503) | RR 0.88 (0.14 to 5.79) | 49 (1) | ⊕⊕⊝⊝ LOW 1 |
| Treatment response at 6 months: complete or partial remission | 565 per 1,000 | 616 per 1,000 (384 to 983) | RR 1.09 (0.68 to 1.74) | 49 (1) | ⊕⊕⊝⊝ LOW 1 |
| Treatment response at 18 months: sustained remission/steroid‐sensitive relapses | 478 per 1,000 | 540 per 1,000 (311 to 937) | RR 1.13 (0.65 to 1.96) | 49 (1) | ⊕⊕⊝⊝ LOW 1 |
| Treatment response at 18 months: CKD | 43 per 1,000 | 38 per 1,000 (3 to 580) | RR 0.88 (0.06 to 13.35) | 49 (1) | ⊕⊕⊝⊝ LOW 1 |
| Adverse events: hypertension | 435 per 1,000 | 17 per 1,000 (0 to 296) | RR 0.04 (0.00 to 0.68) | 49 (1) | ⊕⊕⊝⊝ LOW 1 |
| Adverse events: bacterial infections | 348 per 1,000 | 230 per 1,000 (94 to 567) | RR 0.66 (0.27 to 1.63) | 49 (1) | ⊕⊕⊝⊝ LOW 1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; CKD: chronic kidney disease | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Single study with small number of enrolled participants
Summary of findings 9. Rituximab/cyclosporin/prednisolone versus cyclosporin/prednisolone for idiopathic steroid‐resistant nephrotic syndrome in children.
| Rituximab/cyclosporin/prednisolone compared to cyclosporin/prednisolone for idiopathic steroid‐resistant nephrotic syndrome in children | |||||
| Patient or population: idiopathic steroid‐resistant nephrotic syndrome in children Setting: paediatric nephrology services Intervention: rituximab/cyclosporin/prednisolone (RTX/CSA/PRED) Comparison: CSA/PRED | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with CSA/PRED | Risk with RTX/CSA/PRED | ||||
| Number with complete remission: complete remission in initial steroid resistance | 0 per 1,000 | 0 per 1,000 (0 to 0) | not estimable | 16 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Number with complete remission: complete remission in delayed steroid resistance | 375 per 1,000 | 428 per 1,000 (124 to 1,000) | RR 1.14 (0.33 to 3.94) | 15 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Number with complete remission: complete remission in all patients | 200 per 1,000 | 188 per 1,000 (44 to 788) | RR 0.94 (0.22 to 3.94) | 31 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Adverse events: bronchospasm/treatment discontinued | 0 per 1,000 | 0 per 1,000 (0 to 0) | RR 2.82 (0.12 to 64.39) | 31 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Adverse events: hypotension | 0 per 1,000 | 0 per 1,000 (0 to 0) | RR 2.82 (0.12 to 64.39) | 31 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Adverse events: skin rash | 0 per 1,000 | 0 per 1,000 (0 to 0) | RR 6.59 (0.37 to 117.77) | 31 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Adverse events: mild dyspnoea | 0 per 1,000 | 0 per 1,000 (0 to 0) | RR 4.71 (0.24 to 90.69) | 31 (1) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 High risk of attrition bias
2 Very small number of patients with few events
Cyclosporin versus placebo/prednisone
Remission by six months
Cyclosporin may increase the number of participants with SRNS who achieve complete remission compared with placebo or no treatment, irrespective of renal pathology (Analysis 1.1.1 (4 studies, 74 participants): RR 3.50, 95% CI 1.09 to 11.20; I2 = 0%) and in participants with FSGS (Analysis 1.1.2 (3 studies, 58 participants): RR 3.14, 95% CI 0.97 to 10.18; I2 = 0%) (low certainty evidence).
Cyclosporin may increase the number of participants achieving complete or partial remission irrespective of renal pathology (Analysis 1.2.1 (4 studies, 74 participants): RR 3.15, 95% CI 1.04 to 9.57; I2 = 60%) and in patients with FSGS (Analysis 1.2.2 (2 studies, 49 participants): RR 2.66, 95% CI 0.85 to 8.31; I2 = 70%) (low certainty evidence).
1.1. Analysis.
Comparison 1 Cyclosporin (CSA) versus placebo/no treatment, Outcome 1 Complete remission.
1.2. Analysis.
Comparison 1 Cyclosporin (CSA) versus placebo/no treatment, Outcome 2 Complete or partial remission.
Subgroup analysis, other than for renal pathology, was not possible because of small patient numbers.
Adverse events
It is uncertain whether cyclosporin increases the likelihood of worsening hypertension (Analysis 1.3.1), bacterial infections (Analysis 1.3.2), or reduces the likelihood of ESKD (Analysis 1.3.3) because the certainty of the evidence is very low.
1.3. Analysis.
Comparison 1 Cyclosporin (CSA) versus placebo/no treatment, Outcome 3 Adverse events.
The evidence was downgraded because of increased risk of bias and imprecision resulting from small study numbers with few events (Table 1),
Calcineurin inhibitors versus intravenous cyclophosphamide
Remission by three to six months
CNI compared with IV CPA may increase the number of children who achieve complete or partial remission (Analysis 2.1.1 (2 studies, 156 children): RR 1.98, 95% CI 1.25 to 3.13; I2 = 20%) or complete remission (Analysis 2.1.3 (2 studies, 156 children): RR 3.43, 95% CI 1.84 to 6.41; I2 = 0%) (low certainty evidence).
It is uncertain whether CNI compared with IV CPA increases the number of children with partial remission (Analysis 2.1.2 (2 studies, 156 children): RR 1.68, 95% CI 0.43 to 6.56; I2 = 71%) because the certainty of this evidence is very low.
Gulati 2012 reported the mean time to remission may be shorter with tacrolimus compared with IV CPA (Analysis 2.2 (1 study, 124 children): MD ‐1.00 months, 95% CI ‐1.60 to ‐0.40).
2.1. Analysis.
Comparison 2 Calcineurin inhibitor (CNI) versus IV cyclophosphamide (CPA), Outcome 1 Treatment response at 3 to 6 months.
2.2. Analysis.
Comparison 2 Calcineurin inhibitor (CNI) versus IV cyclophosphamide (CPA), Outcome 2 Mean time to remission.
Adverse effects
Gulati 2012 reported CNI compared with IV CPA probably reduces the number of children with treatment failure (non‐response at 6 months, > 1 episode of serious infection requiring hospitalisation or declining GFR) (Analysis 2.3.1 (1 study, 124 children): RR 0.32, 95% CI 0.18 to 0.58), the number with any serious adverse event (Analysis 2.3.2 (1 study, 131 children): RR 0.47, 95% CI 0.23 to 0.95), and the number who need to cease medications (Analysis 2.3.3 (1 study, 131 children): RR 0.20, 95% CI 0.04 to 0.86) (Moderate certainty evidence).
CNI compared with IV CPA may make little or no difference in the number with serious infections (Analysis 2.3.4 (1 study, 131 children): RR 0.49, 95% CI 0.16 to 1.56), the number of deaths (Analysis 2.3.5 (1 study, 131 children): RR 0.33, 95% CI 0.01 to 7.92) (low certainty evidence), or the number with persistent nephrotoxicity (Analysis 2.3.6 (1 study, 131 children): RR 4.93, 95% CI 0.24 to 100.65).
2.3. Analysis.
Comparison 2 Calcineurin inhibitor (CNI) versus IV cyclophosphamide (CPA), Outcome 3 Adverse events.
The certainty of the evidence was downgraded because of imprecision, heterogeneity between studies and risk of bias attributes (Table 2)
Tacrolimus versus cyclosporin
Remission by six and 12 months
At 6 months, Choudhry 2009 reported tacrolimus compared to cyclosporin may make little or no difference to the number of children who achieve complete remission (Analysis 3.1.1 (1 study, 41 children): RR 0.86, 95% CI 0.44 to 1.66), partial remission (Analysis 3.1.2 (1 study, 41 children): RR 1.43, 95% CI 0.62 to 3.28), or complete or partial remission (Analysis 3.1.3 (1 study, 41 children): RR 1.07, 95% CI 0.81 to 1.42) in children with initial or delayed steroid resistance (low certainty evidence).
At 12 months, tacrolimus compared to cyclosporin may make little or no difference to the number of children who achieve complete remission (Analysis 3.2.1 (2 studies, 58 children): RR 0.80, 95% CI 0.45 to 1.42; I2 = 0%), achieve partial remission (Analysis 3.2.2 (2 studies, 58 children): RR 1.53, 95% CI 0.92 to 2.56; I2 = 0%), or achieve complete or partial remission (Analysis 3.2.3 (2 studies, 58 children): RR 1.05, 95% CI 0.87 to 1.25; I2 = 0%) (low certainty evidence).
Choudhry 2009 reported tacrolimus compared with cyclosporin may reduce the number of children who relapse during treatment (Analysis 3.3 (1 study, 34 children): RR 0.22, 95% CI 0.06 to 0.90).
3.1. Analysis.
Comparison 3 Tacrolimus (TAC) versus cyclosporin (CSA), Outcome 1 Treatment response at 6 months.
3.2. Analysis.
Comparison 3 Tacrolimus (TAC) versus cyclosporin (CSA), Outcome 2 Treatment response at 12 months.
3.3. Analysis.
Comparison 3 Tacrolimus (TAC) versus cyclosporin (CSA), Outcome 3 Relapse following complete or partial remission.
Adverse events
Choudhry 2009 reported tacrolimus compared with cyclosporin may make little or no difference to change in GFR (Analysis 3.4 (1 study, 41 children): MD ‐0.70 mL/min, 95% CI ‐16.71 to 15.31).
Tacrolimus compared with cyclosporin may make little or no difference to the number with nephrotoxicity (Analysis 3.5.1; Analysis 3.5.2) or with worsening hypertension (Analysis 3.5.3) (low certainty evidence).
It is uncertain whether other reported adverse events differ between treatment groups (Analysis 3.5).
3.4. Analysis.
Comparison 3 Tacrolimus (TAC) versus cyclosporin (CSA), Outcome 4 Change in eGFR over 12 months.
3.5. Analysis.
Comparison 3 Tacrolimus (TAC) versus cyclosporin (CSA), Outcome 5 Adverse events.
The certainty of the evidence was downgraded because of imprecision resulting from small studies with few events (Table 4)
Cyclosporin versus mycophenolate mofetil plus pulse oral dexamethasone
Remission by 12 months
FSGS‐CT 2011 reported cyclosporin compared with MMF with oral dexamethasone probably makes little or no difference to the number achieving complete remission (Analysis 4.1.1 (1 study, 138 participants): RR 2.14, 95% CI 0.87 to 5.24), partial remission (Analysis 4.1.2 (1 study, 138 participants): RR 1.09, 95% CI 0.61 to 1.93), or complete or partial remission (Analysis 4.1.3 (1 study, 138 participants): RR 1.38, 95% CI 0.90 to 2.10) (moderate certainty evidence) at 12 months.
Cyclosporin compared with MMF with dexamethasone probably makes little or no difference to the numbers with complete (Analysis 4.2.1 (1 study, 138 participants): RR 1.38, 95% CI 0.41 to 4.66), partial (Analysis 4.2.2 (1 study, 138 participants): RR 1.05, 95% CI 0.56 to 1.98), or no sustainable remission of proteinuria between 52 and 78 weeks (Analysis 4.2.3 (1 study, 138 participants): RR 0.95, 95% CI 0.77 to 1.18) (moderate certainty evidence).
4.1. Analysis.
Comparison 4 Cyclosporin (CSA) versus mycophenolate mofetil (MMF) with pulse dexamethasone (DEXA), Outcome 1 Treatment response at 52 weeks.
4.2. Analysis.
Comparison 4 Cyclosporin (CSA) versus mycophenolate mofetil (MMF) with pulse dexamethasone (DEXA), Outcome 2 Sustainable remission between 52 and 78 weeks.
Adverse events
FSGS‐CT 2011 reported cyclosporin compared with MMF with dexamethasone may make little or no difference to the number dying (Analysis 4.3.1 (1 study, 138 participants): RR 2.14, 95% CI 0.87 to 5.24), developing a 50% decline in GFR (Analysis 4.3.2 (1 study, 138 participants): RR 2.29, 95% CI 0.46 to 11.41), or developing ESKD (Analysis 4.3.3 (one study, 138 participants): RR 4.58, 95% CI 0.55 to 38.22) (low certainty evidence). In this study, adverse effects were reported for 0 to 26 weeks as all participants were included up to that time.
Cyclosporin may make little or no difference to the number with serious infections requiring hospitalisation (Analysis 4.4.1 (1 study, 138 participants): RR 0.65, 95% CI 0.22 to 1.96), hypertension (Analysis 4.4.6 (1 study, 138 participants): RR 1.68, 95% CI 0.66 to 4.29) (low certainty evidence) or other adverse effects (Analysis 4.4).
4.3. Analysis.
Comparison 4 Cyclosporin (CSA) versus mycophenolate mofetil (MMF) with pulse dexamethasone (DEXA), Outcome 3 CKD or death.
4.4. Analysis.
Comparison 4 Cyclosporin (CSA) versus mycophenolate mofetil (MMF) with pulse dexamethasone (DEXA), Outcome 4 Adverse events (weeks 0 to 26).
The evidence was downgraded because of imprecision as the study did not recruit sufficient patients to exclude a difference between treatments and because of small numbers of adverse events.(Table 4).
Tacrolimus versus mycophenolate mofetil to maintain remission
Remission maintenance at 12 months
Sinha 2017 reported among children, who have achieved complete remission, tacrolimus compared with MMF may increase the number of children who maintain complete or partial response for 12 months (Analysis 5.1.1 (1 study, 60 participants): RR 2.01, 95% CI 1.32 to 3.07), complete response (Analysis 5.1.2 (1 study, 60 children): RR 1.79, 95% CI 1.11 to 2.90), or partial remission Analysis 5.1.3 (1 study, 60 participants): RR 4.68, 95% CI 0.58 to 37.68) (low certainty evidence).
Tacrolimus compared with MMF may reduce the number of children with treatment failure (Analysis 5.2.1 (1 study, 60 children): RR 0.18, 95% CI 0.06 to 0.54) and frequent relapses (Analysis 5.2.2 (1 study, 60 children): RR 0.28, 95% CI 0.09 to 0.92) but may make little or no difference to the number of children developing further steroid resistance (Analysis 5.2.3) (low certainty evidence).
Tacrolimus compared with MMF may make little or no difference to the relapse rate/year (Analysis 5.3 (1 study, 60 children): MD ‐0.12 number/year, 95% CI ‐0.56 to 0.32).
Tacrolimus compared with MMF may allow a lower mean prednisone dose to be used to maintain remission (Analysis 5.4 (1 study, 60 children): MD ‐0.20 mg/d, 95% CI ‐0.36 to ‐0.04).
5.1. Analysis.
Comparison 5 Tacrolimus (TAC) versus mycophenolate mofetil (MMF) to maintain remission, Outcome 1 Number with complete or partial response at one year.
5.2. Analysis.
Comparison 5 Tacrolimus (TAC) versus mycophenolate mofetil (MMF) to maintain remission, Outcome 2 Number with treatment failure by one year.
5.3. Analysis.
Comparison 5 Tacrolimus (TAC) versus mycophenolate mofetil (MMF) to maintain remission, Outcome 3 Relapses per year.
5.4. Analysis.
Comparison 5 Tacrolimus (TAC) versus mycophenolate mofetil (MMF) to maintain remission, Outcome 4 Prednisone dose.
Adverse events
Sinha 2017 reported tacrolimus compared with MMF may make little or no change to GFR (Analysis 5.5 (1 study, 60 children): MD 13.00 mL/min, 95% CI ‐3.71 to 29.71) (low certainty evidence)
There may be little or no difference in serious adverse events (Analysis 5.6.1) and serious infections (Analysis 5.6.2) between tacrolimus versus MMF.
5.5. Analysis.
Comparison 5 Tacrolimus (TAC) versus mycophenolate mofetil (MMF) to maintain remission, Outcome 5 Change in GFR.
5.6. Analysis.
Comparison 5 Tacrolimus (TAC) versus mycophenolate mofetil (MMF) to maintain remission, Outcome 6 Adverse events.
The evidence was downgraded due to small numbers of included participants, with small number of events, and for unclear risk of detection bias.
Cyclophosphamide versus prednisone/placebo
Remission
CPA compared with prednisone/placebo may make little or no difference to the overall number of children (Analysis 6.1.1 (2 studies, 84 children): RR 1.06, 95% CI 0.61 to 1.87) or in those with FSGS (Analysis 6.1.2 (2 studies, 63 children): RR 1.01, 95% CI 0.43 to 2.37) who achieve complete remission (low certainty evidence).
ISKDC 1996 reported CPA compared with prednisone/placebo may make little or no difference to the number of children who achieved complete or partial remission between treatment groups (Analysis 6.2 (1 study, 53 children): RR 0.88, 95% CI 0.53 to 1.45) or to the number of children with treatment failure (increase in SCr by ≥ 30%, SCr > 4 mg/dL, dialysis, or transplant) (Analysis 6.3 (1 study, 60 children); RR 1.59, 95% CI 0.87 to 2.88) (low certainty evidence).
6.1. Analysis.
Comparison 6 Oral cyclophosphamide (CPA) versus prednisone/placebo, Outcome 1 Complete remission.
6.2. Analysis.
Comparison 6 Oral cyclophosphamide (CPA) versus prednisone/placebo, Outcome 2 Complete or partial remission.
6.3. Analysis.
Comparison 6 Oral cyclophosphamide (CPA) versus prednisone/placebo, Outcome 3 Treatment failure.
Adverse events
ISKDC 1996 reported CPA compared with prednisone/placebo may make little or no difference to the number of children who die (Analysis 6.4.1 (1 study, 60 children): RR 1.07, 95% CI 0.19 to 5.95) (low certainty evidence).
CPA compared with prednisone/placebo may make little or no difference to the number of children with hypertension with seizures (Analysis 6.4.1, (1 study, 60 children) RR 0.71, 95% CI 0.05, 10.89) (low certainty evidence).
There may be little or no difference in other adverse events between treatment groups (Analysis 6.4).
Adverse events in ISKDC 1974 were not reported separately for steroid‐sensitive and steroid‐resistant children so could not be included in the analyses.
6.4. Analysis.
Comparison 6 Oral cyclophosphamide (CPA) versus prednisone/placebo, Outcome 4 Adverse events.
The evidence was downgraded because of small studies with small event rates and risk of bias issues (Table 7).
Intravenous versus oral cyclophosphamide
Remission
IV CPA compared with oral CPA may make little or no difference to the number of children with SRNS who achieved complete remission (Analysis 7.1. (2 studies, 61 participants): RR 1.58, 95% CI 0.65 to 3.85), partial remission (Analysis 7.2 (1 study, 50 participants): RR 0.40, 95% CI 0.09 to 1.87), or continuing remission at one year (Analysis 7.3) (low certainty evidence).
It is uncertain whether IV CPA compared with oral CPA increases the time to remission (Analysis 7.4) or changes the duration of remission (Analysis 7.5).
7.1. Analysis.
Comparison 7 IV versus oral cyclophosphamide (CPA), Outcome 1 Complete remission.
7.2. Analysis.
Comparison 7 IV versus oral cyclophosphamide (CPA), Outcome 2 Partial remission.
7.3. Analysis.
Comparison 7 IV versus oral cyclophosphamide (CPA), Outcome 3 Continuing remission at one year.
7.4. Analysis.
Comparison 7 IV versus oral cyclophosphamide (CPA), Outcome 4 Time to remission.
7.5. Analysis.
Comparison 7 IV versus oral cyclophosphamide (CPA), Outcome 5 Mean duration of remission.
Adverse events
Shah 2017 reported IV CPA compared with oral CPA may make little or no difference to the likelihood of renal insufficiency (Analysis 7.6.1 (1 studies, 50 children): RR 0.33, 95% CI 0.04 to 2.99)
It is uncertain whether IV CPA compared with oral CPA makes any difference to the number with bacterial infections (Analysis 7.6.2 (2 studies, 61 children): RR 1.02, 95% CI 0.10 to 10.62) or to the number with vomiting (Analysis 7.6.3 (2 studies, 61 children): RR 2.38, 95% CI 0.35 to 16.17) (very low certainty evidence).
IV CPA compared with oral CPA may make little or no difference to the numbers with alopecia (Analysis 7.6.4 (1 studies, 50 children): RR 1.50, 95% CI 0.27 to 8.22) (low certainty evidence).
7.6. Analysis.
Comparison 7 IV versus oral cyclophosphamide (CPA), Outcome 6 Adverse events.
The evidence was downgraded because of few studies with small numbers of participants and events and for risk of bias issues (Table 7).
IV cyclophosphamide versus oral cyclophosphamide plus IV dexamethasone
Remission
Mantan 2008 reported IV CPA compared with oral CPA with dexamethasone may make little or no difference to the number of children with initial or delayed steroid resistance who achieve complete remission (Analysis 8.1.1 (1 study, 49 children): RR 1.13, 95% CI 0.65 to 1.96), partial remission (Analysis 8.1.2 (1 study, 49 children): RR 0.88, 95% CI 0.14 to 5.79), or complete or partial remission (Analysis 8.1.3 (1 study, 49 children): RR 1.09, 95% CI 0.68 to 1.74) after six months of treatment (low certainty evidence).
There may be little or no difference in the number of children with sustained remission or steroid‐sensitive relapses after 18 months of follow up (Analysis 8.2 (1 study, 49 children): RR 1.13, 95% CI 0.65 to 1.96) or in the number developing reduced kidney function (Analysis 8.2 (1 study, 49 children): RR 0.88, 95% CI 0.06 to 13.35) (low certainty evidence).
Among subgroups of initial SRNS (Analysis 8.3.1), late SRNS (Analysis 8.3.2), kidney pathology (Analysis 8.3.3; Analysis 8.3.4), IV CPA compared with oral CPA with dexamethasone may make little or no difference to the numbers achieving complete or partial remission.
8.1. Analysis.
Comparison 8 IV versus oral cyclophosphamide (CPA) plus IV dexamethasone (DEXA), Outcome 1 Treatment response at 6 months.
8.2. Analysis.
Comparison 8 IV versus oral cyclophosphamide (CPA) plus IV dexamethasone (DEXA), Outcome 2 Treatment response at 18 months.
8.3. Analysis.
Comparison 8 IV versus oral cyclophosphamide (CPA) plus IV dexamethasone (DEXA), Outcome 3 Complete or partial resistance in subgroups.
Adverse events
Mantan 2008 reported IV CPA compared with oral CPA may slightly reduce the likelihood of hypertension (Analysis 8.4.1 (1 study, 49 children): RR 0.04, 95% CI 0.00 to 0.68) but may make little or no difference to the number with bacterial infection (Analysis 8.4.7 (1 study, 49 children): RR 0.66, 95% CI 0.27 to 1.63) (low certainty evidence).
Except for hypokalaemia, which may be reduced with IV CPA compared with oral CPA, the other reported adverse events (cataracts/glaucoma, leucopenia, cushingoid features, cystitis, steroid encephalopathy, hair loss) may not differ between treatment groups (Analysis 8.4).
8.4. Analysis.
Comparison 8 IV versus oral cyclophosphamide (CPA) plus IV dexamethasone (DEXA), Outcome 4 Adverse events.
The evidence was downgraded because of a single study with small numbers of participants and events and for risk of bias (Table 8).
Rituximab/cyclosporin/prednisolone versus cyclosporin/prednisolone
It is uncertain whether rituximab compared with cyclosporin makes any difference in the percentage reduction in proteinuria at three months (‐12; 95% CI ‐73 to 110) between treatment groups overall or among children with initial SRNS (‐3; 95% CI ‐6.7 to 179) or among children with delayed steroid resistance (‐48; 95% CI ‐79 to 93) (Magnasco 2012).
It is uncertain whether rituximab compared with cyclosporin makes any difference to the number achieving remission at three months in children with initial steroid resistance (Analysis 9.1.1 (1 study, 15 children): RR not estimable), delayed steroid resistance (Analysis 9.1.2) (1 study, 15 children): RR 1.14 95% CI 0.33 to 3.94), or all children (Analysis 9.1.3) (1 study, 30 children): RR 0.94 95% CI 0.22 to 3.94) because the certainty of the evidence is very low. Remission was only seen in children with delayed steroid resistance (Analysis 9.1.2).
Comparing the two groups, there may be little or no difference between end of study creatinine (Analysis 9.2 (1 study, 31 participants): MD 0.00 mg/dL, 95% CI ‐0.23 to 0.23) and albumin levels (Analysis 9.3 (1 study, 31 participants): MD 0.25 g/L, 95% CI ‐0.22 to 0.72).
It is uncertain whether rituximab compared with cyclosporin makes any difference to the frequency of bronchospasm requiring treatment discontinuation, hypotension, skin rash, breathlessness or abdominal pain (Analysis 9.4).
9.1. Analysis.
Comparison 9 Rituximab/cyclosporin/prednisolone (RTX/CSA/Pred) versus CSA/Pred, Outcome 1 Number with complete remission.
9.2. Analysis.
Comparison 9 Rituximab/cyclosporin/prednisolone (RTX/CSA/Pred) versus CSA/Pred, Outcome 2 End of study creatinine.
9.3. Analysis.
Comparison 9 Rituximab/cyclosporin/prednisolone (RTX/CSA/Pred) versus CSA/Pred, Outcome 3 End of study serum albumin.
9.4. Analysis.
Comparison 9 Rituximab/cyclosporin/prednisolone (RTX/CSA/Pred) versus CSA/Pred, Outcome 4 Adverse events.
The evidence was downgraded because of a single small study with few events and a high risk of attrition bias (Table 9)
Chlorambucil versus indomethacin
Remission
It is uncertain whether chlorambucil compared with indomethacin increases the number who achieved complete remission (Analysis 10.1 (1 study, 30 children): RR 1.00, 95% CI 0.42 to 2.40) because of small participant numbers and high risk of bias (Kleinknecht 1980).
10.1. Analysis.
Comparison 10 Chlorambucil versus indomethacin, Outcome 1 Complete remission.
Adverse events
It is uncertain whether chlorambucil compared with indomethacin decreases the number developing ESKD (Analysis 10.2 (1 study, 30 children): RR 0.20, 95% CI 0.01 to 3.85) (Kleinknecht 1980).
10.2. Analysis.
Comparison 10 Chlorambucil versus indomethacin, Outcome 2 End‐stage kidney disease.
Triple therapy using different agents combined with tacrolimus and prednisone
Remission
It is uncertain whether MMF compared with CPA, leflunomide compared with MMF and leflunomide compared with CPA alters the outcome of remission in the short term or at 12 months (Analysis 11.1; Analysis 11.2) because of small participant numbers and high risk of bias for attrition and selection bias (Wu 2015).
11.1. Analysis.
Comparison 11 Triple therapy with cyclophosphamide (CPA), mycophenolate mofetil (MMF) or leflunomide (LEF), Outcome 1 Short‐term response (remission).
11.2. Analysis.
Comparison 11 Triple therapy with cyclophosphamide (CPA), mycophenolate mofetil (MMF) or leflunomide (LEF), Outcome 2 Long‐term response (remission at 12 months).
Adverse effects
These were not reported in sufficient detail to be included in meta‐analyses. The authors reported that adverse effects did not differ between groups.
Azathioprine versus placebo
Remission
ISKDC 1970 reported AZA compared with placebo may make little or no difference to the number of children who achieved complete remission (Analysis 12.1.1 (1 study, 31 children): RR 0.94, 95% CI 0.15 to 5.84) or complete or partial remission (Analysis 12.2.1 (1 study, 31 children): RR 0.94, 95% CI 0.28 to 3.09). The evidence was downgraded because of a small study with a high risk of attrition and reporting bias.
12.1. Analysis.
Comparison 12 Azathioprine (AZA) versus placebo, Outcome 1 Complete remission.
12.2. Analysis.
Comparison 12 Azathioprine (AZA) versus placebo, Outcome 2 Complete or partial remission.
Adverse events
Adverse events of AZA were not reported.
Adalimumab or galactose compared with conservative therapy
Response
It is uncertain whether adalimumab compared with conservative therapy (lisinopril, losartan and atorvastatin) makes any difference to the number of participants with therapy resistant FSGS who achieved a 50% reduction in proteinuria with stable GFR (Analysis 13.1.1 (1 study, 21 adults and children): RR 0.20, 95% CI 0.01 to 3.54). The evidence was downgraded because of small patient numbers and events (FONT II 2011).
13.1. Analysis.
Comparison 13 Adalimumab or galactose (ADA/GAL) versus conservative therapy, Outcome 1 Number with reduction in proteinuria & stable GFR.
Adverse events
Four serious adverse events were reported in the adalimumab group and three in the galactose group. The nature of these events was not reported.
Adalimumab compared with rosiglitazone
Response
With adalimumab, 4/10 participants with therapy resistant FSGS achieved a 50% reduction in proteinuria.
With rosiglitazone, 2/10 participants with therapy resistant FSGS achieved a 40% reduction in proteinuria.
Data were not compared in a meta‐analysis as the reported outcome measures differed between groups.
Adverse events
One (injection site reaction) of nine recorded adverse events was probably related to adalimumab.
Three (hives, penile swelling, dizziness) of 12 recorded adverse events were possibly related to rosiglitazone.
High versus low dose enalapril
Response
Low dose enalapril (0.2 mg/kg/d) reduced median urinary albumin/creatinine ratio from 3.9 (5th to 95th percentiles 1.9 to 11.6) to 2.3 (5th to 95th percentiles 0.8 to 5.2).
High dose enalapril (0.6 mg/kg/d) reduced median urinary albumin/creatinine ratio from 5.2 (5th to 95th percentiles 2.1 to 10.5) to 2.5 (5th to 95th percentiles 0.8 to 3.3).
No meta‐analyses of these data could be performed.
Adverse events
Serum creatinine and potassium levels were unchanged by enalapril.
Three children ceased enalapril because of a dry cough.
Fosinopril plus prednisone versus prednisone alone
Response
Yi 2006 reported fosinopril plus prednisone compared with prednisone alone may reduce the 24‐hour urinary protein excretion after four (Analysis 14.1.1 (1 study, 45 children): MD ‐1.27 g/d, 95% CI ‐1.62 to ‐0.92), eight (Analysis 14.1.2 (1 study, 45 children): MD ‐1.26 g/d, 95% CI ‐1.47 to ‐1.05), and 12 weeks of treatment (Analysis 14.1.3 (1 study, 45 children): MD ‐0.95 g/d, 95% CI ‐1.21 to ‐0.69).
Fosinopril plus prednisone compared with prednisone alone may reduce tubular proteins including retinol binding protein (Analysis 14.2.1 (1 study, 45 children): MD ‐0.21 mg/L, 95% CI ‐0.33 to ‐0.09) and beta‐2 microglobulin (Analysis 14.2.2 (1 study, 45 children): MD ‐0.17 mg/L, 95% CI ‐0.27 to ‐0.07).
Fosinopril plus prednisone compared with prednisone alone may make little or no difference to serum albumin at the end of treatment (Analysis 14.3 (1 study, 45 children): MD 1.20 g/L, 95% CI ‐6.58 to 8.98).
Fosinopril plus prednisone compared with prednisone alone may make little or no difference to systolic blood pressure (Analysis 14.4 (1 study, 45 children): MD ‐0.87 mm Hg, 95% CI ‐3.33 to 1.59) or serum potassium (Analysis 14.6 (1 study, 45 children): MD 0.20 mmol/L, 95% CI ‐0.34 to 0.74).
Fosinopril plus prednisone compared with prednisone alone may reduce creatinine clearance slightly (Analysis 14.5 (1 study, 45 children): MD ‐5.28 mL/min, 95% CI ‐9.66 to ‐0.90).
14.1. Analysis.
Comparison 14 Fosinopril plus prednisone versus prednisone alone, Outcome 1 Proteinuria.
14.2. Analysis.
Comparison 14 Fosinopril plus prednisone versus prednisone alone, Outcome 2 Tubular proteinuria.
14.3. Analysis.
Comparison 14 Fosinopril plus prednisone versus prednisone alone, Outcome 3 Serum albumin.
14.4. Analysis.
Comparison 14 Fosinopril plus prednisone versus prednisone alone, Outcome 4 Systolic blood pressure.
14.6. Analysis.
Comparison 14 Fosinopril plus prednisone versus prednisone alone, Outcome 6 Serum potassium.
14.5. Analysis.
Comparison 14 Fosinopril plus prednisone versus prednisone alone, Outcome 5 Creatinine clearance.
The evidence was downgraded because of small participant numbers and risk of bias issues.
Adverse events
Yi 2006 reported no participant developed cough, anaemia or allergic reactions.
Sparsentan versus irbesartan
Response
DUET 2017 reported sparsentan (all doses combined) compared with irbesartan may make little or no difference to the number of participants with reduction in proteinuria of > 40% and urinary protein creatinine ratio after eight weeks of treatment (Analysis 15.1 (1 study, 96 participants): RR 3.00, 95% CI 0.95 to 9.44).
There was a greater reduction in proteinuria with sparsentan (‐44.8%, 95% CI ‐52.7% to ‐35.7%) compared with irbesartan (‐18.5%, 95% CI ‐34.6% to 1.7%).
15.1. Analysis.
Comparison 15 Sparsentan versus irbesartan, Outcome 1 Partial remission (> 40% reduction in UP/C) at 8 weeks.
Adverse events
DUET 2017 reported sparsentan compared with irbesartan may make little or no difference to the number of participants with any treatment related adverse event (Analysis 15.2.1. (1 study, 109 participants) RR 1.21, 95% CI 0.73 to 2.01) or to the number of participants withdrawing from the study because of adverse effects (Analysis 15.2.2. (1 study, 109 participants) RR 0.99, 95% CI 0.09 to 10.52). Two patients discontinued sparsentan due to adverse effects (AKI, increased liver enzymes). One patient discontinued irbesartan due to hypoalbuminaemia.
Sparsentan compared with irbesartan may make little or no difference to the number of participants with hypotension (Analysis 15.2.3), peripheral oedema (Analysis 15.2.4) or hyperkalaemia (Analysis 15.2.5).
15.2. Analysis.
Comparison 15 Sparsentan versus irbesartan, Outcome 2 Adverse events.
The evidence was downgraded because of reporting bias and the short duration of treatment.
Tuna fish oil versus placebo
Response
It is uncertain whether fish oil compared with placebo makes any change to the degree of proteinuria or in creatinine clearance because the certainty of the evidence is very low (Chongviriyaphan 1999). The results from each part of the cross‐over study were combined so that the RR and 95% CI could not be calculated.
Adverse events
Adverse events were not reported.
The evidence was downgraded because of unclear sequence generation and allocation concealment, attrition and reporting bias.
Discussion
Summary of main results
In this update we have included 25 studies, enrolling 1063 participants of which 1012 were evaluated.
CNI (cyclosporin, tacrolimus) compared with placebo, no treatment, methylprednisolone (Analysis 1.1; Analysis 1.2) or IV CPA (Analysis 2.1) may increase the number of participants with SRNS who achieve complete and/or partial remission irrespective of renal pathology. There may be little or no difference in efficacy between cyclosporin and tacrolimus (Analysis 3.1; Analysis 3.2). Cyclosporin compared with MMF with dexamethasone probably makes little or no difference to the number achieving complete or partial remission (Analysis 4.1). In children, who have achieved remission, tacrolimus compared with MMF may increase the number of participants who maintain remission (Analysis 5.1). Limited information was available on adverse effects in all studies though serious adverse effects were more common with IV CPA (Analysis 2.3).
CPA compared with placebo or prednisone may make little or no difference to the number of children with complete or partial remission (Analysis 6.1; Analysis 6.2). In addition, IV CPA compared with oral CPA without (Analysis 7.1; Analysis 7.2) or with dexamethasone (Analysis 8.1) may make little or no difference to the number achieving complete or partial remission. Limited information on adverse effects was available.
Of newer agents, it is uncertain whether rituximab (compared with cyclosporin) (Analysis 9.1) and whether adalimumab and galactose (both compared with placebo) (Analysis 13.1) increase the number of participants who achieve complete or partial remission because of small numbers of included participants and few events.
ACEi (Analysis 14.1) may reduce proteinuria in studies lasting for eight to 12 weeks but studies were too short to determine whether ACE inhibition provides long term reduction in proteinuria and protects against deterioration in kidney function. Also the dual endothelin receptor and angiotensin receptor blocker, sparsentan, compared with irbesartan may increase the number of participants with partial remission of proteinuria (Analysis 15.1), using a novel definition of > 40% proteinuria reduction and proteinuria ≤ 1.5 g/g (Troost 2018).
Overall completeness and applicability of evidence
Currently CNI, CPA and MMF are used to treat SRNS. Two studies have demonstrated that CNI are more effective than CPA with less toxicity. These data support the use of CNI in children with SRNS and suggest that CPA should not be used as first‐line treatment where CNI are available. Although there is moderate certainty evidence from a single study (FSGS‐CT 2011) that there is probably little or no difference in efficacy between cyclosporin and MMF with dexamethasone, we need further studies to evaluate the role of MMF as first‐line treatment. Rituximab is also used to treat children with SRNS, who are resistant to CNI. However, it is uncertain whether rituximab is of value in SRNS since only one small study (30 participants) with three months follow‐up has been published to date. Since a role for rituximab cannot be excluded, more RCTs of rituximab compared with CNI are justified. The efficacy of the new agents (adalimumab, galactose) is uncertain based on small studies (FONT I 2009; FONT II 2011), which were too small to draw any conclusions.
RCTs to date have been too small to determine any differences in response to immunosuppressive therapies in different pathologic subtypes and in initial compared to delayed steroid resistance. Most studies included a mix of histological subtypes; only seven studies (Bhaumik 2002; DUET 2017; FONT I 2009; FONT II 2011; FSGS‐CT 2011; ISKDC 1996; Lieberman 1996) recruited patients with FSGS alone. Non‐randomised studies (Ehrich 2007; Inaba 2016; Niaudet 1994) have suggested that children with MCD would be more likely to respond to treatment than children with FSGS. In contrast, the large Podonet study (Trautmann 2015) found no significant difference in remission rates after immunosuppressant therapy between MCD and FSGS.
Most studies have included patients with both initial and delayed steroid resistance. Subgroup analyses in studies which enrolled children with initial and delayed steroid resistance found no differences in efficacy between such patient groups (Gulati 2012; Mantan 2008; Sinha 2017). However, the subgroups involved small numbers of patients so a difference in efficacy of CNI between children with initial or delayed steroid resistance cannot be completely excluded. Observational studies (Ehrich 2007) suggested that the relative efficacies of treatment regimens differed between children with initial compared with delayed steroid resistance. Children with delayed steroid resistance have a higher incidence of recurrence post‐transplant (Ding 2014; Pelletier 2018), suggesting the possibility of a circulating factor pathogenesis, which may be more susceptible to immunosuppressive agents.
In this review, only two studies included information about genetic status (APN 2008; Choudhry 2009) but the data were not used to exclude children from studies. Data from the large PodoNet registry indicate that about a quarter of patients with SRNS (Trautmann 2015) may have an underlying genetic mutation. With the rapid increase in the number of genes identified in children with SRNS, it is likely that this proportion will rise. New studies should take into consideration genetic mutation status in the exclusion criteria as observational studies have shown that these are unlikely to respond to immunosuppressant therapy (Buscher 2010; Kemper 2018).
It remains unclear what therapeutic strategies should be used to maintain remission in patients who have achieved initial remission. We have only identified one study (Sinha 2017) which found that tacrolimus may be more effective than MMF in maintaining remission. An uncontrolled retrospective study of combinations of these agents (Gellermann 2012) suggest utility in adding MMF sequentially to CNI, with subsequent conversion to MMF monotherapy. Such observations of MMF in combination with CNI for maintenance immunotherapy need to be evaluated in an RCT.
There is a dearth of studies regarding new targeted treatments for SRNS. Only one small study has evaluated rituximab in childhood SRNS and found no clear benefit. The results of studies of rituximab, ofatumumab, abatacept and ACTH are awaited (NCT02382575; NCT02394106; NCT02972346; Trachtman 2018).
Although IV pulse methylprednisolone is often used in clinical practice in SRNS either alone or in combination, only one study identified for this review compared it with cyclosporin (Bhaumik 2002) and concluded that cyclosporin was superior in efficacy. In RCTs, dexamethasone was used in combination with MMF (FSGS‐CT 2011) and oral CPA (Mantan 2008) but it remains unclear whether dexamethasone contributed to the therapeutic response. There are case reports of the successful use of vincristine (Thalgahagoda 2017) in SRNS but this medication has not been evaluated in an RCT.
The current KDIGO guidelines (KDIGO 2012) recommend the use of ACEi or angiotensin receptor blockers to reduce proteinuria in SRNS patients. DUET 2017 found that sparsentan may be more effective in reducing proteinuria in patients with FSGS. A phase 3 study comparing sparsentan with irbesartan is underway (DUPLEX 2018).
We have not identified any RCTs evaluating any form of non‐pharmacologic strategies (e.g. plasmapheresis, LDL‐pheresis).
Quality of the evidence
Studies included in this systematic review were small, often of poor methodological quality and addressed several different therapeutic regimens, which limited the opportunities for meta‐analysis. Poor study quality can lead to overestimation of the efficacy of an intervention (Schulz 1995) and combining poor quality studies in meta‐analyses can thus overestimate the benefits of therapy (Moher 1998). Fourteen studies were at low risk for selection bias. Five studies were at low risk of performance bias although, since the majority of studies (19 studies) used a laboratory measurement of proteinuria for the primary outcome of remission, there was less risk of detection bias. Fourteen studies were considered to be free of attrition or of selective outcome bias. It is possible that attrition bias influenced the outcomes in the studies comparing cyclosporin with placebo/no treatment. In three of four studies included in the meta‐analysis comparing cyclosporin with placebo/no treatment, 10/59 (17%) randomised patients were excluded from analyses after randomisation. Studies with attrition bias and thus no intention‐to‐treat analysis can exaggerate the efficacy of the experimental treatment (Hollis 1999).
In many analyses there were no differences between the groups. However, the 95% CIs were often very wide, with the limits indicating the possibility of substantial benefit or substantial harm from the intervention(s) compared with the comparator(s). The results in many studies for some outcomes were therefore imprecise indicating that if these interventions were analysed in new studies, the results could change the estimates of benefits and harms considerably. Assessment by GRADE shown in the Summary of Findings Tables indicates that the certainty of evidence was generally low to very low for most comparisons due to increased risk of bias and imprecision. The only exception was the FSGS‐CT 2011, where the evidence was of moderate certainty for the primary outcomes (Table 4).
Potential biases in the review process
This review identified 25 studies of which four were available only as an abstract. Additional information was provided by the authors from two studies. The literature search undertaken and updated to 17 September 2019 is likely to have identified all relevant published studies including studies only available as abstracts. Since about 40% of study reports in the Cochrane Kidney and Transplant's Specialised Register have been identified by handsearching of conference proceedings, it remains possible that further studies of therapy for SRNS will be identified as conference proceedings from different congresses are searched. Recently abstracts presented at major conferences have become available via search engines particularly through EMBASE OVID SP.
Agreements and disagreements with other studies or reviews
The treatment of SRNS in children has been comprehensively reviewed by Chua 2009 and Colquitt 2007. Colquitt 2007 included nine RCTs (all included in this review), one controlled clinical trial (comparing six months with 18 months of IV methylprednisolone) and one prospective cohort study comparing IV methylprednisolone with IV dexamethasone. They concluded that while the available evidence suggested a beneficial effect of cyclosporin on remission rates and of CPA on time to remission, the strength of the conclusions was limited by the poor quality of included studies. Chua 2009 assessed observational studies, which evaluated complete or partial remission in 494 children treated with cyclosporin or tacrolimus, 192 treated with oral alkylating agents, 71 treated with IV CPA, and 204 treated with IV pulse corticosteroid with CPA or cyclosporin. Overall these observational studies indicated that one third to a half of patients with SRNS achieve complete remission when treated with cyclosporin or with CPA or with one of these agents combined with IV methylprednisolone. Recent analysis of data in the PodoNet registry confirms these outcomes (Trautmann 2017). RCTs indicate that patients treated with cyclosporin are more likely to achieve complete or partial remission when compared with placebo or no specific therapy or with IV CPA. Based on these studies, the KDIGO guidelines (KDIGO 2012) recommend that the initial treatment of children with SRNS should be with a CNI for a minimum of six months. Updated guidelines on glomerulonephritis including SRNS from KDIGO are expected soon.
Authors' conclusions
Implications for practice.
The update of this systematic review continues to highlight how few studies have addressed the efficacy of interventions for SRNS in children. The studies were generally small and of variable quality. Many studies did not provide data on the duration of remission, on kidney dysfunction including the number progressing to ESKD or on death although these are important patient‐centred outcomes. However, based on the included studies, CNI appear to be of benefit for children with SRNS while CPA is less effective and more toxic indicating that the initial treatment of SRNS should be with CNI if available. ACEi significantly reduce proteinuria in children with SRNS so they should be used in children with SRNS (Lombel 2013).
Implications for research.
Further studies are required to assess therapies in SRNS. In particular, further studies of MMF or rituximab compared with CNI are warranted including studies that assess the efficacy of different durations of CNI. These studies should be of sufficient duration to assess complete remission rates, relapse rates, kidney function and adverse events including episodes of acute kidney injury and to assess any differences in response between children with MCD or FSGS and between children with initial steroid resistance and those with delayed steroid resistance. In addition, studies should attempt to investigate the optimal dosing or blood concentrations of CNI or MMF required to achieve remission in children with SRNS. Children with genetic mutations resulting in SRNS rarely respond to therapy. Children entering RCTs should be screened for mutations before study entry and those with mutations should be excluded from studies of immunosuppressive agents because of the risks of toxic therapies in such children.
The responses of children with SRNS to current immunosuppressive agents are variable but in many studies fewer than 50% respond to any therapies. Therefore, different strategies are needed to treat SRNS in children without disease causing mutations but with steroid and CNI resistant disease. While newer agents (adalimumab, galactose) have been evaluated in small studies, no clear benefits or harms of these medications have been identified to date.
What's new
| Date | Event | Description |
|---|---|---|
| 9 October 2019 | New citation required but conclusions have not changed | New studies and interventions added |
| 9 October 2019 | New search has been performed | New search, new interventions included; types of participants extended to include "Children with disease‐causing genetic mutations associated with FSGS in whom a biopsy is not performed" |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 6 October 2016 | New citation required and conclusions have changed | Five new studies included, new interventions included |
| 6 October 2016 | New search has been performed | New search, summary of findings tables incorporated |
| 16 September 2014 | New search has been performed | Search strategies updated |
| 29 September 2010 | New citation required and conclusions have changed | Four new studies, new comparisons, risk of bias assessment replaces quality assessment and summary of findings tables included. |
| 9 October 2008 | Amended | Converted to new review format. |
Notes
2010: The risk of bias assessment tool has replaced the quality assessment checklist used in previous versions of this review.
2016: Summary of findings tables have been incorporated
2019: GRADE has been used to assess and report certainty in this update.
Acknowledgements
We are grateful to Dr Doaa Habashy who contributed to the original iterations of this review (Habashy 2001; Habashy 2003; Habashy 2004; Habashy 2006), contributing to the design, quality assessment, data collection, entry, analysis and interpretation, and writing.
We are grateful to Dr Sophia Wong who contributed to the 2016 update of this review.
This work was presented at the Annual Scientific Meeting of the Australian and New Zealand Society of Nephrology, Sydney, Australia, 2‐4 September, 2002 and published in abstract form in Nephrology in 2002. The authors would like to thank Dr Bagga and Dr Shah for providing additional study data. The authors wish to thank the Information Specialist of Cochrane Kidney and Transplant for her help with this study.
The authors are grateful to the following peer reviewers for their time and comments: William Wong (Department of Nephrology, Starship Children’s Hospital, Auckland, New Zealand), Frederick Kaskel MD, PhD (Chief Emeritus Nephrology), Kaye E Brathwaite MD (Children’s Hospital at Montefiore/Montefiore Medical Center, USA), Aditi Sinha (Assistant Professor, Division of Pediatric Nephrology, Department of Pediatrics, All India Institute of Medical Sciences, New Delhi, India).
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Cyclosporin (CSA) versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 All renal pathologies | 4 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [1.09, 11.20] |
| 1.2 FSGS | 3 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.97, 10.18] |
| 2 Complete or partial remission | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 All renal pathologies | 4 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [1.04, 9.57] |
| 2.2 FSGS | 2 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 2.66 [0.85, 8.31] |
| 3 Adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Worsening of hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 ESKD | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Calcineurin inhibitor (CNI) versus IV cyclophosphamide (CPA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment response at 3 to 6 months | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Complete or partial remission | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.25, 3.13] |
| 1.2 Partial remission | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.43, 6.56] |
| 1.3 Complete remission | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 3.43 [1.84, 6.41] |
| 2 Mean time to remission | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Treatment failure (non response, serious infection, persistently elevated creatinine) at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Any serious adverse effect | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Medications ceased due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Serious infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Persistent nephrotoxicity | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Tacrolimus (TAC) versus cyclosporin (CSA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment response at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Complete remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Complete and partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Treatment response at 12 months | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Complete remission | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.45, 1.42] |
| 2.2 Partial remission | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.92, 2.56] |
| 2.3 Complete and partial remission | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.25] |
| 3 Relapse following complete or partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Change in eGFR over 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Persistent nephrotoxicity | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.05, 4.85] |
| 5.2 Reversible nephrotoxicity | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.32, 1.41] |
| 5.3 Worsening of hypertension | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.08, 2.15] |
| 5.4 Headache | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [0.12, 66.44] |
| 5.5 Paraesthesia | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [0.12, 66.44] |
| 5.6 Hypertrichosis | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.38] |
| 5.7 Gingival hyperplasia | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.01, 0.56] |
| 5.8 Acne or skin infections | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.08, 1.74] |
| 5.9 Diarrhoea | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 5.71 [0.75, 43.36] |
| 5.10 Sepsis/pneumonia | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.06, 14.22] |
Comparison 4. Cyclosporin (CSA) versus mycophenolate mofetil (MMF) with pulse dexamethasone (DEXA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment response at 52 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Complete remission (primary outcome 1,2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Partial remission (primary outcome 3) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Complete or partial remission (primary outcome 1,2,3) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Sustainable remission between 52 and 78 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Complete remission (secondary outcome 1,2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Partial remission (secondary outcome 3) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 No sustainable remission (secondary outcome 4,5) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 CKD or death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Death by 52 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 50% decline in GFR by 78 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 ESKD by 78 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events (weeks 0 to 26) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Serious infection requiring hospitalisation | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Total Infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Total hospitalisations | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Gastrointestinal adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Neuropsychiatric conditions | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Tacrolimus (TAC) versus mycophenolate mofetil (MMF) to maintain remission.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with complete or partial response at one year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Complete or partial response | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Complete response | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Partial response | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number with treatment failure by one year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Treatment failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Frequent relapses | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Steroid resistance | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Relapses per year | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Prednisone dose | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Change in GFR | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 All serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Serious infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Hypovolaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Oral cyclophosphamide (CPA) versus prednisone/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 All renal pathologies | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.61, 1.87] |
| 1.2 FSGS | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.43, 2.37] |
| 2 Complete or partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 FSGS | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Treatment failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Death (all causes) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Hypertension with seizures | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Cystitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Bone marrow suppression | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. IV versus oral cyclophosphamide (CPA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.65, 3.85] |
| 2 Partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Continuing remission at one year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Time to remission | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Mean duration of remission | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Renal insufficiency | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.99] |
| 6.2 Bacterial infection | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.10, 10.62] |
| 6.3 Vomiting | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [0.35, 16.17] |
| 6.4 Alopecia | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.27, 8.22] |
Comparison 8. IV versus oral cyclophosphamide (CPA) plus IV dexamethasone (DEXA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment response at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Complete remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Complete or partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Treatment response at 18 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Sustained remission/steroid‐sensitive relapses | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 CKD | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Complete or partial resistance in subgroups | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Initial SRNS | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Late SRNS | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Minimal change disease | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 FSGS or MesPGN | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 cataract/glaucoma | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Cushingoid features | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Leucopenia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Cystitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Bacterial infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Hypokalaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Steroid encephalopathy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Hair loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Rituximab/cyclosporin/prednisolone (RTX/CSA/Pred) versus CSA/Pred.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with complete remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Complete remission in initial steroid resistance | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Complete remission in delayed steroid resistance | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Complete remission in all patients | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 End of study creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Initially resistant patients | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Delayed resistant patients | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 End of study serum albumin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Initially resistant patients | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Delayed resistant patients | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Abdominal pain | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Bronchospasm/treatment discontinued | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Hypotension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Skin rash | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Mild dyspnoea | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Chlorambucil versus indomethacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 End‐stage kidney disease | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 11. Triple therapy with cyclophosphamide (CPA), mycophenolate mofetil (MMF) or leflunomide (LEF).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term response (remission) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 MMF versus CPA | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 LEF versus MMF | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 LEF versus CPA | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Long‐term response (remission at 12 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 MMF versus CPA | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 LEF versus MMF | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 LEF versus CPA | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Azathioprine (AZA) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 All renal pathologies | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Complete or partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 All renal pathologies | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. Adalimumab or galactose (ADA/GAL) versus conservative therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with reduction in proteinuria & stable GFR | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Adalimumab versus conservative therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Galactose versus conservative therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Fosinopril plus prednisone versus prednisone alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proteinuria | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 After 4 weeks of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After 8 weeks of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 After 12 weeks of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Tubular proteinuria | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Retinol binding protein (mg/L) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Beta 2 microglobulin (mg/L) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serum albumin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Systolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Creatinine clearance | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 15. Sparsentan versus irbesartan.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Partial remission (> 40% reduction in UP/C) at 8 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Any treatment related adverse effect | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Serious adverse effect requiring study withdrawal | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Hypotension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Peripheral oedema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Hyperkalaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
APN 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | CSA group
CPA group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random lists, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Central allocation by study coordinator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or investigators; lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory measure of primary outcome unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up to 12 weeks, then non‐responders could be withdrawn to enter non‐responder protocol 5/15 CSA group withdrawn from 12 weeks onwards (4 treated with non‐responder protocol of high dose CSA) 14/17 CPA group withdrawn from 12 weeks onwards (7 treated with non‐responder protocol of pulse methylprednisolone) |
| Selective reporting (reporting bias) | Low risk | Complete or partial remission, adverse effects reported at 12 weeks |
| Other bias | High risk | Funded in part by a grant from Novartis Pharma |
Bagga 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | High dose enalapril
Low dose enalapril
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes opened by investigator, who did not manage the patients (information from author) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or investigators; lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory assessment of outcome unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised were included and completed the study (information from authors) |
| Selective reporting (reporting bias) | High risk | Outcomes reported (urinary albumin excretion, kidney function, adverse events) but no results could be included in meta‐analyses |
| Other bias | Unclear risk | Funding source not reported |
Bhaumik 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group A
Group B
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on how outcome was measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Abstract‐only publication |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Chongviriyaphan 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment
Control
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomised double‐blind placebo controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomised double‐blind placebo controlled study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Cross‐over study of 6 patients; I patient (17%) did not complete the study with no reason provided |
| Selective reporting (reporting bias) | High risk | Outcomes (urine protein excretion, CrCl) reported; no report of adverse effects |
| Other bias | Low risk | Study supported by Ramathibodi Research Grant No.25/1996, Mahidol University, Bangkok |
Choudhry 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | TAC group
CSA group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation list were generated off site by colleague not involved in the study |
| Allocation concealment (selection bias) | Low risk | Sealed opaque serially numbered envelopes opened at randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/investigators; lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was laboratory based and unlikely to be influenced by lack of blinding; blinding of outcome assessors, who assessed gum hypertrophy and hirsutism |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up |
| Selective reporting (reporting bias) | Low risk | Outcomes (complete remission, partial remission, relapse, adverse events) reported |
| Other bias | Low risk | Study medications only provided by Pancea Biotec, India |
DUET 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Sparsentan groups (dual endothelin and angiotensin inhibitor)
Irbestartan group (angiotensin inhibitor)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "At week 0, a computer‐generated randomization sequence, via an interactive Web response system, used to randomise patients (3:1) to receive sparsentan or irbesartan within sequential dose‐escalating, 20‐patient cohorts." Error in computer programme led to 2:1 randomisation |
| Allocation concealment (selection bias) | Low risk | At week 0, a computer‐generated randomization sequence, via an interactive Web response system, used to randomize patients (3:1) to receive sparsentan or irbesartan |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Investigators, participants, caregivers, and the study sponsor were blinded to treatment allocations until database extraction and unblinding at the completion of the 8‐week, double‐blind treatment period". Both medications were encapsulated in grey gelatin capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Investigators, participants, caregivers, and the study sponsor were blinded to treatment allocations until database extraction and unblinding at the completion of the 8‐week, double‐blind treatment period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported on 96/109 (88%) for efficacy; adverse effects reported for all 109 patients |
| Selective reporting (reporting bias) | High risk | Primary outcome was reduction in UP/Cr reported as geometric mean +/‐ 95% CI and could not be included in meta‐analysis. A secondary outcome defined by the authors of "FSGS partial remission end point (FPRE) (UP/C:≤ 1.5 g/g and > 40% reduction in proteinuria" and not pre‐specified in the protocol was included in meta‐analyses |
| Other bias | High risk | Trial organised and supported by Retrophin Inc. (San Diego, CA) |
Elhence 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | IV CPA group
Oral CPA group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/investigators; lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 15%; 2 from control group lost to follow‐up and excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Outcome (complete remission, non‐remission, adverse effects) reported |
| Other bias | Unclear risk | Funding source not reported |
FONT I 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Adalimumab group
Rosiglitazone group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% did not complete study; 1/11 did not complete rosiglitazone arm; 1/10 did not complete adalimumab arm |
| Selective reporting (reporting bias) | High risk | Expected outcomes reported but data could not be incorporated into meta‐analyses |
| Other bias | Low risk | This work was supported by grants from the NIH–NIDDK (5R21‐DK070341), and the GCRC program of the Division of Research Resources, NIH RR00046 (UNC) and NIH RR018535 (North Shore Long Island Jewish Health System) |
FONT II 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Adalimumab group
Galactose group
Control group
Co‐interventions in all participants
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes are laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in analyses |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | Funding from the National Institutes of Health—National Institute of Diabetes, Digestive, and Kidney Diseases, grant DK70341 (HT).Abbott Laboratories provided adalimumab for use in the project. Supported by NephCure Kidney International |
FSGS‐CT 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | DEXA/MMF group
CSA group
Co‐interventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedules using randomly permuted blocks of random sizes were prepared by the Data Coordinating centre stratified by eGFR, race |
| Allocation concealment (selection bias) | Low risk | Study investigators were blinded to randomised schedules |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study; lack of blinding could influence patient management differently between treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study investigators were blinded to results of interim analyses done for the Data and Safety Monitoring Board Laboratory values for primary outcomes and some secondary outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal participants were lost to follow up/did not attend assessments (< 1%); all patients included in outcome measurement |
| Selective reporting (reporting bias) | Low risk | All expected outcomes (remission, relapse, adverse effects) were reported |
| Other bias | Low risk | NIH funded |
Garin 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | CSA group
No treatment group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants/investigators not blinded; lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was laboratory outcome based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up and accounted for |
| Selective reporting (reporting bias) | Low risk | Complete/partial remission/adverse effects reported |
| Other bias | Unclear risk | Funding source not reported |
Gulati 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | TAC group
CPA group
Co‐interventions
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation with stratification, by initial or late resistance, was performed centrally by individuals not involved in trial implementation |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in opaque sealed envelopes The investigators were blinded to the randomisation schedules |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel delivering therapy were not blinded (one arm received tablets, one arm received injections) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessment is at low risk of bias as it was a laboratory measure and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Seven patients were lost to follow‐up (TAC (3), CPA (4)); this makes up 5% (7/131) and this number is unlikely to alter results; all included in safety analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes of interest (complete or partial remission, remission, adverse effects) have been reported |
| Other bias | Low risk | Study medications (tacrolimus and CPA) were provided by Panacea Biotec Study was supported by funding from the Indian Council of Medical Research |
ISKDC 1970.
| Methods |
|
|
| Participants |
|
|
| Interventions | AZA group
Placebo group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally derived table of random numbers |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Reports were sent to a co‐ordinator, who assigned treatment and distributed drugs identified by code numbers to pharmacists at each clinic" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants/investigators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of participants/investigators |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All patients followed up; 18% (7/38) excluded from analysis |
| Selective reporting (reporting bias) | High risk | Definition of partial remission not reported; no report of adverse effects |
| Other bias | High risk | Help with planning of study provided by employees of Wellcome Foundation and Burroughs Welcome |
ISKDC 1974.
| Methods |
|
|
| Participants |
|
|
| Interventions | CPA‐prednisone group
Prednisone group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/investigators; lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment by quantitative measurement of protein on overnight urine collection or semi‐quantitative based on urinalysis Unclear how many patients had laboratory assessment of outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up |
| Selective reporting (reporting bias) | High risk | Complete and partial remission reported but no definition for partial remission provided; adverse effects not reported specifically for steroid‐resistant patients |
| Other bias | Low risk | Support from NIH AM 14490‐93, National Kidney Foundation, Kidney Foundation of New York, John Rath Foundation |
ISKDC 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | CPA‐prednisone group
Prednisone group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/investigators; lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32/35 in treatment group and 21/25 in control group analysed for complete/partial remission and unclear why other patients not included. 11% excluded |
| Selective reporting (reporting bias) | Low risk | Outcomes of complete and partial remission, adverse events, kidney function included |
| Other bias | Low risk | Supported by NIH Grant 1 RO1 AM18234 and multiple other not for profit agencies in USA, UK, Netherlands |
Kleinknecht 1980.
| Methods |
|
|
| Participants |
|
|
| Interventions | Chlorambucil group
Indomethacin group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of investigators/participants; lack of blinding could influence management. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about how primary outcome was measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data only available from conference proceedings |
| Selective reporting (reporting bias) | Unclear risk | Complete remission (no definition provided), ESKD |
| Other bias | Unclear risk | Funding source not reported; data from conference proceedings |
Lieberman 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | CSA group
Placebo group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer generated list |
| Allocation concealment (selection bias) | Low risk | Central coordinator |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants/investigators; placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/16 excluded from cyclosporin group and 3/15 excluded from control group for noncompliance (2 each group, 1 unknown CSA group, 1 each group for rising Cr). In view of small numbers, results likely to influence results (23% excluded) |
| Selective reporting (reporting bias) | Low risk | Outcomes of complete or partial remission, adverse events, kidney function |
| Other bias | Unclear risk | Funding source not reported |
Magnasco 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | RTX group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permutated block randomisation with blocks of variable size |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by contacting the holder of the allocation schedule at central administration |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinical investigators, study nurses enrolling patients, and the statistician were not blinded to group assignment Study staff responsible for follow up were blinded so their management of patients would not be influenced by treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study staff responsible for facilitating follow‐up data measurements by contacting patient families by phone were kept blinded Also, as the outcome measured was a laboratory value, lack of blinding is unlikely to affect outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up All patients analysed; 1 patient from each group did not complete treatment due to adverse side effects |
| Selective reporting (reporting bias) | High risk | Data on partial remission not included Primary outcome (end study proteinuria) not provided in a form that can be included in meta‐analysis Adverse effects related to RTX were only reported |
| Other bias | Low risk | Supported by Italian Ministry of Health, the Renal Child Foundation, two other non‐Pharma related foundations |
Mantan 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | IV CPA group
Oral CPA + IV DEXA group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Stratified randomisation, in blocks of four, were done separately with computer‐generated numbers to allocate patients with initial and late steroid‐resistance randomly..." |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Allocation was concealed in sealed opaque envelopes, which were opened by an associate not involved in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/investigators; lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Primary outcome was serum albumin + urinary protein; urine protein measured either by urinalysis or UP/C. Unclear how many patients had laboratory measure of proteinuria |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/52 (6%) patients excluded after randomisation (IV CPA group (1); oral CPA + IV DEXA group (2)) for non‐compliance; unlikely to have influenced results |
| Selective reporting (reporting bias) | Low risk | Primary outcomes: number in complete or partial remission and adverse effects reported |
| Other bias | Unclear risk | Funding source not reported |
Ponticelli 1993a.
| Methods |
|
|
| Participants |
|
|
| Interventions | CSA group
No treatment group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table; stratified for adults/children |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes numbered in sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/investigators; lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory measure of primary outcome unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/20 (15%) children (all from no treatment group) lost to follow‐up and not included in results |
| Selective reporting (reporting bias) | High risk | No separate data available for adverse events in children |
| Other bias | High risk | Funded in part by Sandoz P.F, Milano, Italy |
Shah 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | IV CPA group
Oral CPA group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sequentially number sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and outcome assessment could be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not all patients who failed to achieve remission are accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | Authors state that they received no monetary assistance |
Sinha 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | TAC group
MMF group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permutated block randomisation; stratified for histology (FSGS or MCD) and type of remission (complete or partial) |
| Allocation concealment (selection bias) | Low risk | Allocation sequence, in a 1:1 ratio, was generated using Stata version 10.1 (StataCorp version 10, StataCorp College Station, TX) and sealed in opaque envelopes that were opened at randomisation by an investigator blinded to the randomisation schedule |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding could result in differences in management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Complete or partial remission was laboratory based, using UPCR (primary outcome); relapses were defined by dipstick |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up to 12 months of all but 1 patient (last analysis carried forwards) so data on all patients included in analyses |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | Study medications were provided by Panacea Biotec (India), which had no role in study development, implementation, or analysis. The study was in part supported by personnel from the Pediatric Renal Biology Program, funded by the Department of Biotechnology, Government of India. |
Valverde 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1
Group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be "a comparative, randomised clinical trial" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment and lack of blinding could influence outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether all treated patients were included |
| Selective reporting (reporting bias) | Unclear risk | Abstract‐only publication. Incomplete reporting of adverse effects |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Wu 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | MMF group
CPA group
LEF group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Simple randomization using a randomised digital table" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcome was laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four (18%) excluded from analysis for loss to follow up or other |
| Selective reporting (reporting bias) | High risk | Incomplete reporting of adverse events |
| Other bias | Low risk | Supported by National Natural Science Foundation of China and others |
Yi 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Fosinopril‐prednisone group
Prednisone group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Computer generated random numbers were used to randomly allocate patients ..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/investigators; lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory measurement of primary outcome unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/57 (21%) (fosinopril group (5); prednisone group (7)) lost to follow‐up and excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of study were reduction in proteinuria, CrCl; adverse effects reported |
| Other bias | Low risk | Ministry of Health Science Foundation of China (98‐1‐117) |
ACEi ‐ angiotensin converting enzyme inhibitors; ANC ‐ absolute neutrophil count; APN ‐ Arbeitgemeinschaft fur Padiatrische Nephrologie; ARB ‐ angiotensin receptor blocker; AZA ‐ azathioprine; BP ‐ blood pressure; CHF ‐ chronic heart failure; CNI ‐ calcineurin inhibitor; CPA ‐ cyclophosphamide; CSA ‐ cyclosporin; Cr ‐ creatinine; CrCl ‐ creatinine clearance; DBP ‐ diastolic blood pressure; DEXA ‐ dexamethasone; DM ‐ diabetes mellitus; eGFR ‐ estimated glomerular filtration rate; ESKD ‐ end‐stage kidney disease; FSGS ‐ focal segmental glomerulosclerosis; GFR ‐ glomerular filtration rate; GI ‐ gastrointestinal; GN ‐ glomerulonephritis; HCT ‐ haematocrit; HIV ‐ human immunodeficiency virus; HSP ‐ Henoch‐Schonlein purpura; INS ‐ idiopathic nephrotic syndrome; intermittent ‐ prednisone given on 3 consecutive days out of 7; IQR ‐ interquartile range; ISKDC ‐ International Study of Kidney Disease in Children; IV ‐ intravenous; LEF ‐ leflunomide; LFT ‐ liver function test; M/F ‐ male/female; MCD ‐ minimal change disease; MCGN ‐ mesangiocapillary glomerulonephritis; MesPGN ‐ mesangioproliferative glomerulonephritis; MI ‐ myocardial infarction; MMF ‐ mycophenolate mofetil; MNS ‐ membranous nephrotic syndrome; NSAIDs ‐ nonsteroidal anti‐inflammatory drugs; RCT ‐ randomised controlled trial; RTX ‐ rituximab; SBP ‐ systolic blood pressure; SC ‐ subcutaneous; SCr ‐ serum creatinine; SD ‐ standard deviation; SLE ‐ systemic lupus erythematosus; SRNS ‐ steroid‐resistant nephrotic syndrome; TAC ‐ tacrolimus; TB ‐ tuberculosis; UP/C ‐ urinary protein/urinary creatinine ratio; WCC ‐ white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adeniyi 1979 | Wrong population: children had nephrotic syndrome secondary to Plasmodium malariae (31/36) |
| Ahn 2018 | Wrong study design: children with SRNS were not randomised; only children with FRNS/SDNS were randomised |
| Arora 2002 | Wrong population: adult patients |
| Buyukcelik 2002 | Wrong population: study of gemfibrozil on lipid profiles in children with nephrotic syndrome; ineligible renal pathology as all except one had MPGN |
| Hari 2018 | Mixed population of children with nephrotic syndrome including MPGN, Membranous GN, FSGS and MCD |
| Hiraoka 2000 | Wrong population: SSNS patients |
| Iyengar 2006 | Wrong population: SSNS patients |
| JPRN‐C000000007 | Wrong study design: this study started on 1‐8‐2005 states it is a single arm study on the UMIN‐CTR Clinical Trial Registry. Number on Registry is UMIN‐CTR registry is C000000009 |
| Jung 1990 | Mixed population of steroid dependent and steroid resistant patients; unable to separate data |
| Kano 2003 | Wrong population: included patients did not have nephrotic syndrome but moderate proteinuria with normal serum albumin levels |
| Khemani 2016 | Mixed population: includes SDNS and SRNS patients and the data on these cannot be separated. No reply to email to chief investigator |
| Koshikawa 1993 | Wrong population: adult patients |
| Kumar 2004a | Wrong population: adults patients |
| Li 2006g | Wrong population: adult patients |
| Ren 2011 | Wrong population: adult patients |
| Ren 2013 | Wrong population: adult patients |
| Saito 2014 | Wrong population: adult patients |
| Saito 2017 | Wrong population: patients with membranous GN |
| Shibasaki 2004 | Wrong population: not clear if paediatric patients were included in study; includes patients with non MCD or FSGS pathology |
| Tejani 1988 | Mixed population: includes SSNS and SRNS patients and the results cannot be separated |
| Walker 1990 | Wrong population: adult patients |
| Yi 2008 | Probably not an RCT: no mention of "random" and group numbers unequal (87 vs 55). Includes largely steroid dependent patients and not steroid resistant patients |
| Zhao 2013a | Mixed population: includes both steroid‐resistant and steroid‐dependent patients and results cannot be separated |
FRNS ‐ frequently‐relapsing nephrotic syndrome; FSGS ‐ focal segmental glomerulosclerosis; GN ‐ glomerulonephritis; MCD ‐ minimal change disease; MPGN ‐ membranoproliferative glomerulonephritis; RCT ‐ randomised controlled trial; SDNS ‐ steroid‐dependent nephrotic syndrome; SRNS ‐ steroid‐resistant nephrotic syndrome; SSNS ‐ steroid‐sensitive nephrotic syndrome
Characteristics of ongoing studies [ordered by study ID]
DUPLEX 2018.
| Trial name or title | DUPLEX study |
| Methods | Open‐label RCT |
| Participants | Participants aged 8 to 75 years (US) or 18 to 75 years (outside US) with biopsy‐proven FSGS or MCD or FSGS with documented genetic mutation in podocyte protein Up/C ≥ 1.5 g/g at screening & eGFR > 30 mL/min/1.73 m2 |
| Interventions | Sparsentan 400 mg/day titrating to 800 mg/day Irbesartan 150 mg/day titrating to 300 mg/day |
| Outcomes | Slope of eGFR from week 6 to week 108 Proportion of patients achieving a Up/C ≤ 1.5 g/g and a > 40% reduction from baseline in Up/C at Week 36 |
| Starting date | April 3, 2018 |
| Contact information | Radko Komers, MD, PhD; medinfo@retrophin.com |
| Notes | NCT03493685. Estimated completion date is December 2022. Other name: 021FSGS16010 |
NCT02382575.
| Trial name or title | Efficacy and safety of rituximab to that of calcineurin inhibitors in children with steroid resistant nephrotic syndrome |
| Methods | Open‐label RCT |
| Participants | Children aged 3 to 16 years with SRNS (MCD, MesPGN or FSGS) |
| Interventions | Rituximab infusions weekly for 2 to 4 doses over up to 4 weeks compared with oral tacrolimus given until the child has achieved 6 months of relapse free survival |
| Outcomes | 12‐month relapse‐free survival in the ITT population; adverse effects |
| Starting date | March 2015; estimated enrolment 120 children |
| Contact information | Dr. Biswanath Basu, Nilratan Sircar Medical College, India (basuv3000@gmail.com) |
| Notes | Estimated study completion date March 2017 Other study numbers: PednephroRCT/PM/NRSMCH‐33, CTRI/2015/01/005364 |
NCT02394106.
| Trial name or title | Ofatumumab in children with steroid‐ and calcineurin‐inhibitor‐resistant nephrotic syndrome: a double‐blind randomised, controlled, superiority trial |
| Methods | RCT |
| Participants | Children aged 2 to 18 years with SRNS (MCD, MesPGN or FSGS) and resistance to CNI and MMF |
| Interventions | Single dose of IV Ofatumumab in normal saline versus placebo (normal saline alone); other immunosuppressive therapies will be withdrawn; all children with receive an ACEi |
| Outcomes | Complete or partial disease remission; adverse events |
| Starting date | March 2015; estimated enrolment 50 children |
| Contact information | Dr Gian Marco Ghiggeri, Istituto Giannina Gaslini, Italy (gmarcoghiggeri@ospedale‐gaslini.ge.it) |
| Notes | Estimated study completion date March 2018 |
NCT02972346.
| Trial name or title | Availability study of ACTH to treat children SRNS/SDNS |
| Methods | Open‐label parallel group RCT |
| Participants | 42 children aged 3 to 12 years with SDNS or SRNS & MCD |
| Interventions | Intervention: ACTH 0.4 U/kg/day (maximum 25 units) for three consecutive days every 4 weeks + routine treatment. Comparator: Routine treatment |
| Outcomes | 24 hr urinary protein excretion. Remission/relapse |
| Starting date | November 2016. Estimated completion date June 2019 |
| Contact information | Yufeng Li, Ph.D. mieuniversity@hotmail.com. Xinhua Hospital, Shanghai Jiao Tong University School of Medicine |
| Notes | Availability and Safety Study of ACTH to Treat Children with SRNS/SDNS |
Trachtman 2018.
| Trial name or title | A phase II randomised, placebo‐controlled, double‐blind, parallel arms with switchover, pilot study to evaluate the efficacy and safety of intravenous abatacept in treatment resistant nephrotic syndrome (focal segmental glomerulosclerosis/ minimal change disease) |
| Methods | Randomised placebo controlled trial (quadruple blind) |
| Participants | 90 patients aged ≥ 6 years with TRNS due to MCD or FSGS (Collapsing FSGS excluded), GFR ≥ 45 mL/min/1.73 m2. Patients stratified for age (< 18 and ≥ 18) and APOL1 risk status. Exclusions: Patients with recurrence of disease post transplant, secondary TRNS, DM, CHF, BMI > 40, recent or chronic infections |
| Interventions | 1. 16 week parallel arms comparing IV abatacept and placebo (normal saline) on days 1, 14, 28 and then every 28 days 2. 16 week cross‐over with placebo group receiving abatacept and abatacept group receiving placebo 3. 169 day abatacept extension with all receiving abatacept 4. Weight tiered dose of abatacept from 500 to 1000 mg. Children < 18 years weighing < 75 kg: 10 mg/kg/dose 4. Standard immunosuppression (CNI, MMF, prednisone) unchanged in 1 months, ACEi, ARB |
| Outcomes | 1. Difference in % of participants who achieve a renal response by 113 days (end of first 16 week parallel group study). Renal response defined as a ≥ 50% reduction in Up/C from baseline to day 113 with Up/C < 3g/g and eGFR > 90 mL/min/1.73 m2 (if below normal at baseline, remaining ≥ 75% of baseline. 2. Change in proteinuria, GFR, remission, quality of life (PROMIS), adverse events |
| Starting date | March 1, 2016. Estimated completion date June 2020 |
| Contact information | Anna Greka: agreka@bwh.harvard.edu |
| Notes | 27 study sites. NCT02592798. Sponsor: Bristol‐Myers Squibb |
ACEi ‐ angiotensin converting enzyme inhibitors; ARB ‐ angiotensin receptor blocker; BMI ‐ body mass index; CHF ‐ chronic heart failure; CNI ‐ calcineurin inhibitor; DM ‐ diabetes mellitus; FSGS ‐ focal segmental glomerulosclerosis; (e)GFR ‐ (estimated) glomerular filtration rate; MCD ‐ minimal change disease; MesPGN ‐ mesangioproliferative glomerulonephritis; MMF ‐ mycophenolate mofetil; SDNS ‐ steroid‐dependent nephrotic syndrome; SRNS ‐ steroid‐resistant nephrotic syndrome; TRNS ‐ treatment‐resistant nephrotic syndrome; Up/C ‐ urinary protein creatinine ratio
Differences between protocol and review
Summary of findings tables have been incorporated into the 2016 update.
Contributions of authors
Designing the review; EH, NW, JC
Undertaking review update: IL, EH, NW, JC
Coordinating the review; EH
Study selection, quality assessment, data collection; IL, EH, NW
Entering data into RevMan; IL, EH
Analysis of data; IL, EH
Interpretation of data; IL, EH, NW, JC
Writing the review; IL, EH, NW, JC
Providing general advice on the review; EH, NW, JC
Sources of support
Internal sources
No sources of support supplied
External sources
-
NHMRC, Australia.
Cochrane Kidney and Transplant is supported in part by NHMRC grants
Declarations of interest
Isaac Liu: none known
Narelle Willis: none known
Jonathan Craig: none known
Elisabeth Hodson: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
APN 2008 {published data only}
- Plank C, Kalb V, Hinkes B, Hildebrandt F, Gefeller O, Rascher W, et al. Cyclosporin A is superior to cyclophosphamide in children with steroid‐resistant nephrotic syndrome‐a randomized controlled multicentre trial by the Arbeitsgemeinschaft fur Padiatrische Nephrologie. Pediatric Nephrology 2008;23(9):1483‐93. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bagga 2004 {published data only}
- Bagga A, Mudigoudar BD, Hari P, Vasudev V. Enalapril dosage in steroid‐resistant nephrotic syndrome. Pediatric Nephrology 2004;19(1):45‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bagga A, Mudigoudar BD, Vasudev V, Hari P. Low (LD) versus high dose (HD) enalapril therapy in steroid resistant nephrotic syndrome (SRNS) [abstract]. 7th Asian Congress of Pediatric Nephrology; 2000 Nov 4‐6; Singapore. 2000. [CENTRAL: CN‐00583291]
Bhaumik 2002 {published data only}
- Bhaumik SK, Majumdar A, Barman SC. Comparison of pulse methyl prednisolone vs cyclosporin based therapy in steroid resistant focal segmental glomerulosclerosis [abstract]. Indian Journal of Nephrology 2002;12(4):190. [CENTRAL: CN‐00460392] [Google Scholar]
Chongviriyaphan 1999 {published data only}
- Chongviriyaphan N, Tapaneya‐Olarn C, Suthutvoravut U, Karnchanachumpol S, Chantraruksa V. Effects of tuna fish oil on hyperlipidemia and proteinuria in childhood nephrotic syndrome. Journal of the Medical Association of Thailand 1999;82 Suppl 1:S122‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Choudhry 2009 {published data only}
- Choudhry A, Bagga A, Menon S, Hari P. Randomized controlled trial (RCT) on efficacy and safety of cyclosporin (CyA) vs tacrolimus (Tac) in steroid resistant nephrotic syndrome (SRNS) [CRG030600042] [abstract no: 182 (OP)]. Pediatric Nephrology 2007;22(9):1480. [CENTRAL: CN‐00653722] [Google Scholar]
- Choudhry S, Bagga A, Hari P, Sharma S, Kalaivani M, Dinda A. Efficacy and safety of tacrolimus versus cyclosporine in children with steroid‐resistant nephrotic syndrome: a randomized controlled trial. American Journal of Kidney Diseases 2009;53(5):760‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DUET 2017 {published data only}
- Gesualdo L, Lieberman K, Tesar V, Srivastava T, Komers R. Antiproteinuric effect of sparsentan, a dual angiotensin II and endothelin type A receptor antagonist, in patients with primary focal segmental glomerulosclerosis (FSGS): a subgroup analysis of the DUET trial [abstract no: TO042]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii97. [EMBASE: 617290819] [Google Scholar]
- Hogan JJ, Derebail VK, Murphy E, Raguram PC, Pergola PE, Sanghani NS, et al. Long‐term effects of sparsentan, a dual angiotensin and endothelin receptor antagonist in primary focal segmental glomerulosclerosis (FSGS): interim 84‐week analysis of the DUET trial [abstract no: FR‐OR087]. Journal of the American Society of Nephrology 2018;29(Abstracts Suppl):61. [Google Scholar]
- Karol M, Pan‐Zhou XR, Tuller SE, Komers R. Sparsentan pharmacokinetics and pharmacodynamics as the basis of dose selection for primary focal segmental glomerular sclerosis (FSGS) [abstract no: SA‐PO635]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):843. [Google Scholar]
- Komers R, Gipson DS, Nelson P, Adler S, Srivastava T, Derebail VK, et al. Efficacy and safety of sparsentan compared with irbesartan in patients with primary focal segmental glomerulosclerosis: randomized, controlled trial design (DUET). Kidney International Reports 2017;2(4):654‐64. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar V, Trachtman H, Murphy E, Ferguson B, Komers R. No impact of newly initiated immunosuppressive therapy observed on long‐term antiproteinuric effect of sparsentan in focal segmental glomerulosclerosis: interim 84‐week analysis of the DUET trial [abstract no: SUN‐037]. Kidney International Reports 2019;4(7 Suppl):S168‐9. [EMBASE: 2002180135] [Google Scholar]
- Trachtman H, Nelson P, Adler S, Campbell KN, Chaudhuri A, Derebail VK, et al. DUET: A phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. Journal of the American Society of Nephrology 2018;29(11):2745‐54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H, Nelson PJ, Komers R. Efficacy and safety of sparsentan, a dual angiotensin II (Ang II) and endothelin (ET) type A receptor antagonist in patients with focal segmental glomerulosclerosis (FSGS): a phase 2 trial (DUET) [abstract no: HI‐OR06]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):2B. [Google Scholar]
- Trachtman H, Rychlik I, Haws R, Nester C, Fornoni A, Komers R. Newly administered immunosuppressive therapy (IST) has no impact on long‐term antiproteinuric effect of sparsentan (SPAR), a dual angiotensin and endothelin receptor antagonist, in patients with primary focal segmental glomerulosclerosis (FSGS): interim analysis of the DUET trial [abstract no: FP129]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i20. [EMBASE: 622606410] [Google Scholar]
- Trachtman H, Rychlik I, Haws RM, Nester CM, Fornoni A, Komers R. Long‐term effect of sparsentan (SPAR), a dual angiotensin and endothelin receptor antagonist, on proteinuria in patients with primary FSGS: interim analysis of the DUET trial [abstract no: FR‐OR026]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):44. [Google Scholar]
Elhence 1994 {published data only}
- Elhence R, Gulati S, Kher V, Gupta A, Sharma RK. Intravenous pulse cyclophosphamide ‐ a new regime for steroid‐resistant minimal change nephrotic syndrome. Pediatric Nephrology 1994;8(1):1‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gupta A, Elhence R, Kher V, Gulati S, Sharma RK. IV cyclophosphamide ‐ a new regimen for steroid nonresponsive minimal change disease [abstract]. 12th International Congress of Nephrology; 1993 Jun 13‐18; Jerusalem, Israel. 1993:84.
FONT I 2009 {published data only}
- Gassman JJ, Trachtman H, Gipson D, Friedman A, Greene T, Vento S, et al. Implementing a second randomized trial enrolling mid‐study treatment failures from the Focal Segmental Glomerular Sclerosis (FSGS) clinical trial [abstract no: 33]. Clinical Trials (London, England) 2007;4(4):382. [CENTRAL: CN‐00794067] [Google Scholar]
- Joy MS, Gipson DS, Dike M, Powell L, Thompson A, Vento S, et al. Phase I trial of rosiglitazone in FSGS: I. Report of the FONT Study Group. Clinical Journal of the American Society of Nephrology: CJASN 2009;4(1):39‐47. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy MS, Gipson DS, Powell L, MacHardy J, Jennette JC, Vento S, et al. Phase 1 trial of adalimumab in Focal Segmental Glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group. American Journal of Kidney Diseases 2010;55(1):50‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternant D, Paintaud G, Trachtman H, Gipson DS, Joy MS. A possible influence of age on absorption and elimination of adalimumab in focal segmental glomerulosclerosis (FSGS). European Journal of Clinical Pharmacology 2016;72(2):253‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Trachtman H, Gipson D, Vento S, Thompson A, Dike M. Novel therapies in resistant FSGS: preliminary results [abstract no: 879 (P)]. Pediatric Nephrology 2007;22(9):1629. [CENTRAL: CN‐00794725] [Google Scholar]
FONT II 2011 {published data only}
- Trachtman H, Vento S, Gipson D, Wickman L, Gassman J, Joy M, et al. Novel therapies for resistant focal segmental glomerulosclerosis (FONT) phase II clinical trial: study design. BMC Nephrology 2011;12:8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H, Vento S, Herreshoff E, Radeva M, Gassman J, Stein DT, et al. Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the FONT clinical trial group. BMC Nephrology 2015;16:111. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FSGS‐CT 2011 {published data only}
- D'Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, et al. Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(3):399‐406. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris M, Norwood V, Radeva M, Gassman JJ, Al‐Uzri A, Askenazi D, et al. Patient recruitment into a multicenter randomized clinical trial for kidney disease: report of the focal segmental glomerulosclerosis clinical trial (FSGS CT). Clinical & Translational Science 2013;6(1):13‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman J, Fine R, Gibson D, Greene T, Hogg R, Kaskel F, et al. A preliminary report of the focal segmental glomerulosclerosis clinical trial (FSGS‐CT) [abstract no: F‐PO1984]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):559A. [CENTRAL: CN‐00790759] [Google Scholar]
- Gassman JJ, Trachtman H, Gipson D, Friedman A, Greene T, Vento S, et al. Implementing a second randomized trial enrolling mid‐study treatment failures from the Focal Segmental Glomerular Sclerosis (FSGS) Clinical Trial [abstract no: 33]. Clinical Trials 2007;4(4):382. [CENTRAL: CN‐00767231] [Google Scholar]
- Gipson D, Radeva M, Fine R, Friedman A, Gassman J, Greene T, et al. Focal Segmental Glomerulosclerosis Clinical Trial (FSGS‐CT) study cohort [abstract no: TH‐PO165]. Journal of the American Society of Nephrology 2009;20(Abstracts issue):147‐8A. [CENTRAL: CN‐00793840] [Google Scholar]
- Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney International 2011;80(8):868‐78. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson DS, Trachtman H, Kaskel FJ, Radeva MK, Gassman J, Greene TH, et al. Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney International 2011;79(6):678‐85. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene T, Gassman J, Gipson D, Hogg R, Kaskel F, Moxey‐Mims M, et al. Multicenter randomized clinical trial of FSGS in children and young adults [abstract no: F‐PO1014]. Journal of the American Society of Nephrology 2005;16:558A. [CENTRAL: CN‐00794633] [Google Scholar]
- Hogg RJ, Friedman A, Greene T, Radeva M, Budisavljevic MN, Gassman J, et al. Renal function and proteinuria after successful immunosuppressive therapies in patients with FSGS. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(2):211‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JB, Winkler CA, Zhao X, Radeva MK, Gassman JJ, D'Agati VD, et al. Clinical features and histology of apolipoprotein L1‐associated nephropathy in the FSGS Clinical Trial. Journal of the American Journal of Nephrology 2015;26(6):1443‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JB, Zhao X, Winkler CA, Woroniecki R, Radeva M, Gassman JJ, et al. APOL1 variant‐associated FSGS is more aggressive but manifests similar response to cyclosporine and mycophenolate compared to others forms of primary FSGS [abstract no: FR‐OR050]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):41A. [Google Scholar]
- Middleton JP, Yorgin P, Gipson D, Radeva M, Fine R, Friedman A, et al. Prevalence of blood pressure (BP) control in the Focal Segmental Glomerulosclerosis Clinical Trial (FSGS‐CT) study cohort [abstract]. Journal of the American Society of Nephrology 2009;20(Abstracts issue):148A. [CENTRAL: CN‐00795224] [Google Scholar]
- Radeva M, McMahan J, Gassman J. Optimizing logistics under a three level administration system: coordination of the Focal Segmental Glomerular Sclerosis (FSGS) trial [abstract no: P63]. Clinical Trials (London, England) 2007;4(4):417‐8. [CENTRAL: CN‐00794883] [Google Scholar]
- Radeva M, Trachtman H, Fine R, Friedman A, Gassman J, Gipson D, et al. Measuring quality of life (QOL) in children and young adults in the focal segmental glomerulosclerosis clinical trial (FSGS‐CT) [abstract]. Clinical Trials 2010;7(4):471. [EMBASE: 70462847] [Google Scholar]
- Sethna CB, Boone V, Kwok J, Jun D, Trachtman H. Adiponectin in children and young adults with focal segmental glomerulosclerosis. Pediatric Nephrology 2015;30(11):1977‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Trachtman H, Fine R, Friedman A, Gassman J, Gipson D, Greene T, et al. Quality of life in children with focal segmental glomerulosclerosis (FSGS): baseline findings. Report of the FSGS‐Clinical Trial (CT) [abstract no: TH‐PO164]. Journal of the American Society of Nephrology 2009;20(Abstracts issue):147A. [CENTRAL: CN‐00795355] [Google Scholar]
- Woroniecki R, Du Z, Radeva M, Gassman JJ, Gipson DS, Trachtman H, et al. Osteopontin (OPN) and transforming growth factor beta 1 (TGFb1) in steroid resistant primary focal segmental glomerulosclerosis (FSGS), FSGS‐clinical trial (FSGS‐CT) experience [abstract no: SA‐PO390]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):727A. [Google Scholar]
Garin 1988 {published data only}
- Garin EH, Orak JK, Hiott KL, Sutherland SE. Cyclosporine therapy for steroid‐resistant nephrotic syndrome. A controlled study. American Journal of Diseases of Children 1988;142(9):985‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gulati 2012 {published data only}
- Gulati A, Bagga A, Nephrotic Syndrome Study Group. Multicenter randomized controlled trial comparing efficacy of tacrolimus to intravenous cyclophosphamide in children with steroid resistant nephrotic syndrome [abstract no: LB‐OR03]. Journal of the American Society of Nephrology 2011;22(Abstracts):1B. [CENTRAL: CN‐01657678] [Google Scholar]
- Gulati A, Hari P, Kanitkar M, Sharma J, Mantan M, Bagga A. Randomized, multicenter study on tacrolimus & alternate day (AD) prednisolone vs. intravenous (IV) cyclophosphamide & ad prednisolone for steroid resistant nephrotic syndrome (SRNS) [abstract no: 43]. Pediatric Nephrology 2010;25(9):1803. [EMBASE: 70438145] [Google Scholar]
- Gulati A, Sinha A, Gupta A, Kanitkar M, Sreenivas V, Sharma J, et al. Treatment with tacrolimus and prednisolone is preferable to intravenous cyclophosphamide as the initial therapy for children with steroid‐resistant nephrotic syndrome. Kidney International 2012;82(10):1130‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ISKDC 1970 {published data only}
- Abramowicz M, Barnett HL, Edelmann CM Jr, Greifer I, Kobayashi O, Arneil GC, et al. Controlled trial of azathioprine in children with nephrotic syndrome. A report for the international study of kidney disease in children. Lancet 1970;1(7654):959‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Arneil GC. International trial of azathioprine in nephrotic syndrome in childhood [abstract]. Nephron 1971;8:95. [CENTRAL: CN‐00602053] [Google Scholar]
ISKDC 1974 {published data only}
- Prospective, controlled trial of cyclophosphamide therapy in children with the nephrotic syndrome. Report of the International Study of Kidney Disease in Children. Lancet 1974;2(7878):423‐7. [MEDLINE: ] [PubMed] [Google Scholar]
ISKDC 1996 {published data only}
- Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr. Cyclophosphamide does not benefit patients with focal segmental glomerulosclerosis. A report of the International Study of Kidney Disease in Children. Pediatric Nephrology 1996;10(5):590‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kleinknecht 1980 {published data only}
- Kleinknecht C, Broyer M, Gubler MC, Palcoux JB. Irreversible renal failure after indomethacin in steroid‐resistant nephrosis. New England Journal of Medicine 1980;302(12):691. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kleinknecht C, Broyer M, Palcoux JB, Gubler MC. Irreversible renal failure (RF) after indomethacin in steroid resistant nephrosis (SRN) [abstract]. Kidney International 1979;16(1):96. [CENTRAL: CN‐00446116] [DOI] [PubMed] [Google Scholar]
Lieberman 1996 {published data only}
- Lieberman KV, Tejani A. A randomized double‐blind placebo‐controlled trial of cyclosporine in steroid‐resistant idiopathic focal segmental glomerulosclerosis in children. Journal of the American Society of Nephrology 1996;7(1):56‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tejani A, Lieberman K. A randomized placebo (PL) controlled double blind trial of cyclosporine (CSA) in steroid resistant (SR) idiopathic focal segmental glomerulosclerosis (FSGS) in children. A report of the New York, New Jersey Pediatric Nephrology Collaborative Study Group [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):289. [CENTRAL: CN‐00520391] [Google Scholar]
Magnasco 2012 {published data only}
- Magnasco A, Ravani P, Edefonti A, Murer L, Ghio L, Belingheri M, et al. Rituximab in children with resistant idiopathic nephrotic syndrome. Journal of the American Society of Nephrology 2012;23(6):1117‐24. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantan 2008 {published data only}
- Mantan M, Bagga A, Hari P. Efficacy of IV pulse cyclophosphamide (CP) vs IV steroids and oral CP for steroid resistant nephrotic syndrome [abstract no: OFC44]. Pediatric Nephrology 2004;19(9):C73. [CENTRAL: CN‐00653731] [DOI] [PubMed] [Google Scholar]
- Mantan M, Sriram CS, Hari P, Dinda A, Bagga A. Efficacy of intravenous pulse cyclophosphamide treatment versus combination of intravenous dexamethasone and oral cyclophosphamide treatment in steroid‐resistant nephrotic syndrome. Pediatric Nephrology 2008;23(9):1495‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ponticelli 1993a {published data only}
- Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, Rinaldi S, et al. A randomized trial of cyclosporine in steroid‐resistant idiopathic nephrotic syndrome. Kidney International 1993;43(6):1377‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shah 2017 {published data only}
- Ohri A, Phatarpekar A, Ali U, Tembekar Y. Randomized controlled trial of oral versus intravenous cyclophosphamide in idiopathic steroid resistant nephrotic syndrome [abstract no: 424]. Pediatric Nephrology 2010;25(9):1879. [EMBASE: 70438526] [Google Scholar]
- Shah KM, Ohri AJ, Ali US. A randomized controlled trial of intravenous versus oral cyclophosphamide in steroid‐resistant nephrotic syndrome in children. Indian Journal of Nephrology 2017;27(6):430‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinha 2017 {published data only}
- Bagga A, Gupta A, Dinda A, Hari P, Sinha A. Randomized controlled trial comparing efficacy of mycophenolate mofetil (MMF) & alternate day prednisone versus tacrolimus & prednisolone in maintaining remission in steroid resistant nephrotic syndrome (SRNS) [abstract no: P‐SUN200]. Pediatric Nephrology 2013;28(8):1600. [Google Scholar]
- Sinha A, Bagga A. Randomized trial on efficacy of mycophenolate mofetil versus tacrolimus in maintaining remission in children in steroid resistant nephrotic syndrome [abstract no: HI‐OR05]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):B2. [Google Scholar]
- Sinha A, Gupta A, Kalaivani M, Hari P, Dinda AK, Bagga A. Mycophenolate mofetil is inferior to tacrolimus in sustaining remission in children with idiopathic steroid‐resistant nephrotic syndrome. Kidney International 2017;92(1):248‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Valverde 2010 {published data only}
- Medeiros M, Fuentes Y, Valverde S, Hernandez AM. Superior results with tacrolimus than ciclosporine in children with steroid resistant nephrotic syndrome [abstract no: SA‐PO2854]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):774A. [Google Scholar]
- Valverde S, Hernandez AM, Velasquez L, Romero B, Mendoza A, Ramon G, et al. Efficacy of prednisone‐tacrolimus vs. prednisone‐cyclosporine in steroid‐resistant nephrotic syndrome [abstract no: 47]. Pediatric Nephrology 2010;25(9):1804. [EMBASE: 70438149] [Google Scholar]
Wu 2015 {published data only}
- Wu B, Mao J, Shen H, Fu H, Wang J, Liu A, et al. Triple immunosuppressive therapy in steroid‐resistant nephrotic syndrome children with tacrolimus resistance or tacrolimus sensitivity but frequently relapsing. Nephrology 2015;20(1):18‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yi 2006 {published data only}
- Li ZH, Yi ZW. Clinical study of fosinopril in children with steroid resistant nephrotic syndrome [abstract no: 2344]. 23rd International Congress of Paediatrics; 2001 Sep 9‐14; Beijing, China. 2001. [CENTRAL: CN‐00445802]
- Li ZH, Yi ZW. Clinical study of fosinopril in children with steroid resistant nephrotic syndrome [abstract no: P225]. Pediatric Nephrology 2001;16(8):C114. [Google Scholar]
- Yi Z, Li Z, Wu XC, He QN, Dang XQ, He XJ. Effect of fosinopril in children with steroid‐resistant idiopathic nephrotic syndrome. Pediatric Nephrology 2006;21(7):967‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adeniyi 1979 {published data only}
- Adeniyi A, Hendrickse RG, Soothill JF. A controlled trial of cyclophosphamide and azathioprine in Nigerian children with the nephrotic syndrome and poorly selective proteinuria. Archives of Disease in Childhood 1979;54(3):204‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahn 2018 {published data only}
- Ahn YH, Kang HG, Kim SH. Efficacy and safety of rituximab in children with refractory nephrotic syndrome: a multicenter clinical trial [abstract]. Kidney Research & Clinical Practice 2014;33(2):A1. [Google Scholar]
- Ahn YH, Kim SH, Han KH, Cho HY, Shin JI, Cho MH, et al. Efficacy and safety of rituximab in children with refractory nephrotic syndrome: a multicenter clinical trial [abstract no: 039]. Pediatric Nephrology 2013;28(8):1361. [EMBASE: 71126980] [Google Scholar]
- Ahn YH, Kim SH, Han KH, Choi HJ, Cho H, Lee JW, et al. Efficacy and safety of rituximab in childhood‐onset, difficult‐to‐treat nephrotic syndrome: a multicenter open‐label trial in Korea. Medicine 2018;97(46):e13157. [PUBMED: 30431588] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arora 2002 {published data only}
- Arora A, Ahlawat RS, Arora S, Arora N, Mandel AK. Randomised controlled study of enalapril in steroid resistant nephrotic syndrome. Indian Journal of Nephrology 2002;12(3):107‐12. [CENTRAL: CN‐00460300] [Google Scholar]
Buyukcelik 2002 {published data only}
- Buyukcelik M, Anarat A, Bayazit AK, Noyan A, Ozel A, Anarat R, et al. The effects of gemfibrozil on hyperlipidemia in children with persistent nephrotic syndrome. Turkish Journal of Pediatrics 2002;44(1):40‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Hari 2018 {published data only}
- Hari P, Khandelwal P, Satpathy A, Hari S, Thergaonkar R, Lakshmy R, et al. Effect of atorvastatin on dyslipidemia and carotid intima‐media thickness in children with refractory nephrotic syndrome: a randomized controlled trial. Pediatric Nephrology 2018;33(12):2299‐309. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hari P, Satpathy A, Thergaonkar R, Khandelwal P, Hari S, Lakshmy R, et al. Effect of atorvastatin on lipid profile and carotid intima media thickness (cIMT) in children with refractory nephrotic syndrome: a randomized double blind, placebo controlled trial [abstract no: FP‐S14‐3]. Pediatric Nephrology 2016;31(10):1737‐8. [EMBASE: 612479933] [DOI] [PubMed] [Google Scholar]
Hiraoka 2000 {published data only}
- Hiraoka M, Sudo M, West Japanese Cooperative Study of Kidney Disease in Children. Low versus standard dosage of prednisolone for initial treatment of idiopathic nephrotic syndrome in children [abstract no: A0445]. Journal of the American Society of Nephrology 1996;7(9):1335. [Google Scholar]
- Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, et al. Older boys benefit from higher initial prednisolone therapy for nephrotic syndrome. The West Japan Cooperative Study of Kidney Disease in Children. Kidney International 2000;58(3):1247‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, et al. Older boys benefit from intensive initial prednisolone therapy for nephrotic syndrome [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):103A. [CENTRAL: CN‐00550605] [Google Scholar]
Iyengar 2006 {published data only}
- Iyengar A, Damle H, Kulkarni C, Damle L, Phadke K. Steroid sparing effect of a herbal preparation in steroid‐dependent nephrotic syndrome. Pediatric Nephrology 2006;21(7):1031‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
JPRN‐C000000007 {published data only}
- Ito S. A combination of cyclosporine and prednisolone and a combination of methylprednisolone, cyclosporine and prednisolone for steroid‐resistant nephrotic syndrome in children: A randomized controlled study. https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000000010 (first received 1 August 2005).
Jung 1990 {published data only}
- Jung A. Immunomodulatory treatment in children with steroid‐dependent/steroid‐resistant nephrotic syndrome [abstract no: 170]. European Journal of Clinical Investigation 1990;20(2 Pt II):A 33. [CENTRAL: CN‐00253605] [Google Scholar]
Kano 2003 {published data only}
- Kano K, Nishikura K, Yamada Y, Arisaka O. Effect of fluvastatin and dipyridamole on proteinuria and renal function in childhood IgA nephropathy with mild histological findings and moderate proteinuria. Clinical Nephrology 2003;60(2):85‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kano K, Nishikura K, Yamada Y, Arisaka O. Effect of fluvastatin and dipyridamole on proteinuria and renal function in childhood IgA nephropathy with mild histological findings and moderate proteinuria [abstract no: T756‐757]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):524‐5. [DOI] [PubMed] [Google Scholar]
- Kano K, Nishikura K, Yamada Y, Arisaka O. Effect of fluvastatin on proteinuria [abstract no: M‐PO20068]. Nephrology 2005;10(Suppl 1):A33. [CENTRAL: CN‐00774540] [Google Scholar]
- Kano K, Nishikura K, Yamada Y, Arisaka O. No effect of fluvastatin on the bone mineral density of children with minimal change glomerulonephritis and some focal mesangial cell proliferation, other than an ameliorating effect on their proteinuria. Clinical Nephrology 2005;63(2):74‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khemani 2016 {published data only}
- Khemani S, Moorani KN. Cyclosporine versus cyclophosphamide in childhood nephrotic syndrome. Journal of the Liaquat University of Medical & Health Sciences 2016;15(2):57‐62. [EMBASE: 611436094] [Google Scholar]
Koshikawa 1993 {published data only}
- Koshikawa S. Clinical evaluation of an immunosuppressive drug, mizoribine (HE‐69) on steroid resistant nephrotic syndrome ‐ a multicenter double‐blind comparison study with placebo. Jin to Toseki [Kidney and Dialysis] 1993;34:631‐50. [CENTRAL: CN‐00602134] [Google Scholar]
Kumar 2004a {published data only}
- Kumar NS, Mishra RN, Singh AK, Prakash J. Effect of ACE inhibitor and calcium channel blocker on proteinuria in subjects with steroid resistant idiopathic nephrotic syndrome [abstract no: CNO‐20]. Indian Journal of Nephrology 2001;11(3):110‐1. [CENTRAL: CN‐00461120] [Google Scholar]
- Kumar NS, Singh AK, Mishra RN, Prakash J. Comparative study of angiotensin converting enzyme inhibitor and calcium channel blocker in the treatment of steroid‐resistant idiopathic nephrotic syndrome. Journal of the Association of Physicians of India 2004;52:454‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Li 2006g {published data only}
- Li X, He Q, Lin W, Wang S, Chen J. Therapy of tacrolimus in steroid‐resistant minimal‐change nephrotic syndrome: a randomized controlled clinical trial [abstract no: F‐PO1105]. Journal of the American Society of Nephrology 2006;17(Abstracts):569A. [CENTRAL: CN‐01657685] [Google Scholar]
Ren 2011 {published data only}
- Ren HQ, Zhong YF, Li Y, Cai Q, Han SJ, Hao XK, et al. Low‐dose tacrolimus combined with tripterygium in treatment of steroid‐resistant nephrotic syndrome: a prospective randomized controlled trial. Academic Journal of Second Military Medical University 2011;32(12):1340‐5. [EMBASE: 2011698533] [Google Scholar]
Ren 2013 {published data only}
- Ren H, Shen P, Li X, Pan X, Zhang W, Chen N. Tacrolimus versus cyclophosphamide in steroid‐dependent or steroid‐resistant focal segmental glomerulosclerosis: a randomized controlled trial. American Journal of Nephrology 2013;37(1):84‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saito 2014 {published data only}
- Saito T, Iwano M, Matsumoto K, Mitarai T, Yokoyama H, Yorioka N, et al. Significance of combined cyclosporine‐prednisolone therapy and cyclosporine blood concentration monitoring for idiopathic membranous nephropathy with steroid‐resistant nephrotic syndrome: a randomized controlled multicenter trial. Clinical & Experimental Nephrology 2014;18(5):784‐94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Mitarai T, Yokoyama H, Yorioka N, Iwano M, Nishi S, et al. Effect of preprandial once‐a‐day administration of cyclosporine (CyA) for steroid resistant nephrotic syndrome (SRNS) [abstract no: SA‐PO997]. Journal of the American Society of Nephrology 2007;18(Abstracts):562A. [CENTRAL: CN‐01657682] [Google Scholar]
- Saito T, Watanabe M, Ogahara S, Shuto H, Kataoka H. Benefits of once‐a‐day preprandial administration and C2 monitoring for the cyclosporine (CyA) treatment of idiopathic membranous nephropathy (IMN) with steroid resistant nephrotic syndrome (SRNS) [abstract no: SA‐PO2293]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):634A. [Google Scholar]
- Saito T, Watanabe M, Ogahara S, Shuto H, Kataoka Y. Significance of blood concentration of cyclosporine at 2 hours after administration for the treatment of idiopathic membranous nephropathy with steroid resistant nephrotic syndrome [abstract no: SA285]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐01657683]
Saito 2017 {published data only}
- Saito T, Iwano M, Matsumoto K, Mitarai T, Yokoyama H, Yorioka N, et al. Mizoribine therapy combined with steroids and mizoribine blood concentration monitoring for idiopathic membranous nephropathy with steroid‐resistant nephrotic syndrome. Clinical & Experimental Nephrology 2017;21(6):961‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Watanabe M, Ogahara S, Shuto H, Kataoka Y. Effects of mizoribin combined with prednisolone for the treatment of idiopathic membranous nephropathy with steroid resistant nephrotic syndrome [abstract no: Su363]. NDT Plus 2010;3(Suppl 3):iii430‐1. [EMBASE: 70484583] [Google Scholar]
Shibasaki 2004 {published data only}
- Shibasaki T, Koyama A, Hishida A, Muso E, Osawa G, Yamabe H, et al. A randomized open‐label comparative study of conventional therapy versus mizoribine only therapy in patients with steroid‐resistant nephrotic syndrome (postmarketing survey). Clinical & Experimental Nephrology 2004;8(2):117‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tejani 1988 {published data only}
- Tejani A, Gonzalez R, Rajpoot D, Sharma R, Pomrantz A. A randomized trial of cyclosporine with low‐dose prednisone compared with high‐dose prednisone in nephrotic syndrome. Transplantation Proceedings 1988;20(3 Suppl 4):262‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Tejani A, Suthanthiran M, Pomrantz A. A randomized controlled trial of low‐dose prednisone and ciclosporin versus high‐dose prednisone in nephrotic syndrome of children. Nephron 1991;59(1):96‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tejani A, Suthanthiran M, Rajpoot D, Gonzalez R, Pomrantz A. Randomized trial of cylosporine and low dose prednisone (P&CS) vs conventional prednisone (P) therapy in recent onset (< 1 yr) nephrotic syndrome (NS) [abstract]. Kidney International 1989;35(1):213. [CENTRAL: CN‐01912553] [Google Scholar]
Walker 1990 {published data only}
- Walker RG, Kincaid‐Smith P. Cyclosporin a (Cy‐A) treatment in focal and segmental hyalinosis and sclerosis (FSHS): a controlled trial [abstract no: 17]. Kidney International 1989;36(6):1162. [CENTRAL: CN‐00509552] [Google Scholar]
- Walker RG, Kincaid‐Smith P. The effect of treatment of corticosteroid‐resistant idiopathic (primary) focal and segmental hyalinosis and sclerosis (focal glomerulosclerosis) with ciclosporin. Nephron 1990;54(2):117‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yi 2008 {published data only}
- Yi ZW, Wu XC, Xu H, Zhou LJ, Wu YB, Feng SP, et al. A prospective multicenter clinical control trial on treatment of refractory nephrotic syndrome with mycophenolate mofetil in children. Zhongguo Dangdai Erke Zazhi 2008;10(5):575‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Zhao 2013a {published data only}
- Zhao K, Li Q, Ma Q, Zhang L, Duan Y. Treatment with tacrolimus and prednisone is preferable to intravenous cyclophosphamide for children with difficult‐to‐treat nephrotic syndrome [abstract no: P‐SUN205]. Pediatric Nephrology 2013;28(8):1601‐2. [EMBASE: 71127684] [Google Scholar]
References to ongoing studies
DUPLEX 2018 {published data only}
- Komers R. Study of sparsentan in patients with primary focal segmental glomerulosclerosis (FSGS) (DUPLEX). www.clinicaltrials.gov/ct2/show/NCT03493685 (first received 10 April 2018).
NCT02382575 {published data only}
- Basu B. Efficacy and safety of rituximab to that of calcineurin inhibitors in children with steroid resistant nephrotic syndrome. www.clinicaltrials.gov/ct2/show/NCT02382575 2015; Vol. (first received 5 March 2015).
NCT02394106 {published data only}
- Ghiggeri GM. Ofatumumab in children with drug resistant idiopathic nephrotic syndrome. www.clinicaltrials.gov/ct2/show/NCT02394106 2015; Vol. (first received 20 March 2015).
NCT02972346 {published data only}
- Li Y. Availability study of ACTH to treat children SRNS/SDNS. www.clinicalTrials.gov/show/NCT02972346 (first received 23 November 2016). [Google Scholar]
Trachtman 2018 {published data only}
- Trachtman H, Gipson DS, Somers M, Spino C, Adler S, Holzman L, et al. Randomized clinical trial design to assess abatacept in resistant nephrotic syndrome. Kidney International Reports 2018;3(1):115‐21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bierzynska 2017
- Bierzynska A, McCarthy HJ, Soderquest K, Sen ES, Colby C, Ding WY, et al. Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney International 2017;91(4):937‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Buscher 2010
- Büscher AK, Kranz B, Büscher R, Hildebrandt F, Dworniczak B, Pennekamp P, et al. Immunosuppression and renal outcome in congenital and pediatric steroid‐resistant nephrotic syndrome. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(11):2075‐84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chanchlani 2016
- Chanchlani R, Parekh RS. Ethnic differences in childhood nephrotic syndrome. Frontiers in Pediatrics 2016;4:39. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chua 2009
- Chua A, Yorgin P. Steroid‐resistant nephrotic syndrome. In: Molony DA, Craig JC editor(s). Evidence‐Based Nephrology. Oxford: Wiley‐Blackwell, 2009:787‐805. [Google Scholar]
Colquitt 2007
- Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS. The clinical effectiveness and cost‐effectiveness of treatments for children with idiopathic steroid‐resistant nephrotic syndrome: a systematic review. Health Technology Assessment (Winchester, England) 2007;11(21):1‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deegens 2011
- Deegens JK, Wetzels JF. Immunosuppressive treatment of focal and segmental glomerulosclerosis: lessons from a randomized controlled trial. Kidney International 2011;80(8):798‐801. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ding 2014
- Ding WY, Koziell A, McCarthy HJ, Bierzynska A, Bhagavatula MK, Dudley JA, et al. Initial steroid sensitivity in children with steroid‐resistant nephrotic syndrome predicts post‐transplant recurrence. Journal of the American Society of Nephrology 2014;25(6):1342–8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehrich 2007
- Ehrich JH, Geerlings C, Zivicnjak M, Franke D, Geerlings H, Gellerman J. Steroid‐resistant idiopathic childhood nephrosis: overdiagnosed and undertreated. Nephrology Dialysis Transplantation 2007;22(8):2183‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
El Bakkali 2011
- Bakkali L, Rodrigues Pereira R, Kuik DJ, Ket JC, Wijk JA. Nephrotic syndrome in The Netherlands: a population‐based cohort study and a review of the literature. Pediatric Nephrology 2011;26(8):1241‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gellermann 2012
- Gellermann J, Ehrich JH, Querfeld U. Sequential maintenance therapy with cyclosporin A and mycophenolate mofetil for sustained remission of childhood steroid‐resistant nephrotic syndrome. Nephrology Dialysis Transplantation 2012;27(5):1970‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gipson 2006
- Gipson DS, Chin H, Presler TP, Jennette C, Ferris ME, Massengill S, et al. Differential risk of remission and ESRD in childhood FSGS. Pediatric Nephrology 2006;21(3):344‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
- Hollis S, Cambell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Inaba 2016
- Inaba A, Hamasaki Y, Ishikura K, Hamada R, Sakai T, Hataya H, et al. Long‐term outcome of idiopathic steroid‐resistant nephrotic syndrome in children. Pediatric Nephrology 2016;31(3):425‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ISKDC 1981a
- The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. Journal of Pediatrics 1981;98(4):561‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2012
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney International Supplements 2012;2:139‐274. [Google Scholar]
Kemper 2018
- Kemper MJ, Valentin L, Husen M. Difficult‐to‐treat idiopathic nephrotic syndrome: established drugs, open questions and future options. Pediatric Nephrology 2018;33(10):1641‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koskimies 1982
- Koskimies O, Vilska J, Rapola J, Hallman N. Long‐term outcome of primary nephrotic syndrome. Archives of Disease in Childhood 1982;57(7):544‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lombel 2013
- Lombel RM, Hodson EM, Gipson DS, Kidney Disease: Improving Global Outcomes. Treatment of steroid‐resistant nephrotic syndrome in children: new guidelines from KDIGO. Pediatric Nephrology 2013;28(3):409‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niaudet 1994
- Niaudet P. Treatment of childhood steroid‐resistant idiopathic nephrosis with a combination of cyclosporine and prednisone. French Society of Pediatric Nephrology. Journal of Pediatrics 1994;125(6 Pt 1):981‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pelletier 2018
- Pelletier JH, Kumar KR, Engen R, Bensimhon A, Varner JD, Rheault MN, et al. Recurrence of nephrotic syndrome following kidney transplantation is associated with initial native kidney biopsy findings [Erratum in: Pediatr Nephrol. 2019 Mar;34(3):539; PMID: 30443740]. Pediatric Nephrology 2018;33(10):1773‐80. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadowski 2015
- Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, et al. A single‐gene cause in 29.5% of cases of steroid‐resistant nephrotic syndrome. Journal of the American Society of Nephrology 2015;26(6):1279‐89. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sureshkumar 2014
- Sureshkumar P, Hodson EM, Willis NS, Barzi F, Craig JC. Predictors of remission and relapse in idiopathic nephrotic syndrome: a prospective cohort study. Pediatric Nephrology 2014;29(6):1039‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thalgahagoda 2017
- Thalgahagoda S, Abeyagunawardena S, Jayaweera H, Karunadasa UI, Abeyagunawardena AS. Pulsed vincristine therapy in steroid‐resistant nephrotic syndrome. BioMed Research International 2017;2017:1757940. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trautmann 2015
- Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, et al. Spectrum of steroid‐resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clinical Journal of The American Society of Nephrology: CJASN 2015;10(4):592‐600. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trautmann 2017
- Trautmann, A, Schnaidt S, Lipska‐Ziętkiewicz BS, Bodria M, Ozaltin F, et al. Long‐term outcome of steroid‐resistant nephrotic syndrome in children. Journal of the American Society of Nephrology 2017;28(10):3055–65. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Troost 2018
- Troost JP, Trachtman H, Nachman PH, Kretzler M, Spino C, Komers R, et al. An outcomes‐based definition of proteinuria remission in focal segmental glomerulosclerosis. Clinical Journal of the American Society of Nephrology: CJASN 2018;13(3):414‐21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tune 1996
- Tune BM, Lieberman E, Mendoza SA. Steroid‐resistant nephrotic focal segmental glomerulosclerosis: a treatable disease. Pediatric Nephrology 1996;10(6):772‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wong 2007
- Wong W. Idiopathic nephrotic syndrome in New Zealand children, demographic, clinical features, initial management and outcome after twelve‐month follow‐up: results of a three‐year national surveillance study. Journal of Paediatrics & Child Health 2007;43(5):337‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Habashy 2001
- Habashy D, Hodson E, Craig J. Interventions for idiopathic steroid‐resistant nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003594] [DOI] [PubMed] [Google Scholar]
Habashy 2003
- Habashy D, Hodson EM, Craig JC. Interventions for steroid‐resistant nephrotic syndrome: a systematic review. Pediatric Nephrology 2003;18(9):906‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Habashy 2004
- Habashy D, Hodson E, Craig J. Interventions for idiopathic steroid‐resistant nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003594.pub2] [DOI] [PubMed] [Google Scholar]
Habashy 2006
- Habashy D, Hodson E, Craig J. Interventions for idiopathic steroid‐resistant nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003594.pub3] [DOI] [PubMed] [Google Scholar]
Hodson 2011
- Hodson EM, Willis NS, Craig JC. Interventions for idiopathic steroid‐resistant nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD003594.pub4] [DOI] [PubMed] [Google Scholar]
Hodson 2016
- Hodson EM, Wong SC, Willis NS, Craig JC. Interventions for idiopathic steroid‐resistant nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD003594.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]


