Abstract
Using MR‐based radial mapping, we assessed morphological alterations of the hippocampal dentate gyrus (DG) in patients with relapse‐onset multiple sclerosis (MS). We analyzed different stages of the disease and the association of DG alterations with hippocampal‐related cognitive functions. Using high‐resolution morphological imaging, hippocampal radial mapping analysis was performed in 28 relapsing‐remitting (RR), 34 secondary progressive, and 26 benign MS patients and 28 healthy controls (HC). Between‐groups differences of DG radial distance (from surface points to the central core of the hippocampus) and correlations with clinical, neuropsychological, and radiological measures were evaluated using surface‐based mesh modeling. Compared with HC, all MS clinical phenotypes revealed a larger radial distance of the DG, which was more marked on the left side. Radial distance enlargement was more pronounced in RRMS patients compared with the other disease clinical phenotypes and was inversely correlated to disease duration. Radial distance enlargement was correlated with higher T2 lesion volume and a better cognitive performance in RRMS and with a poor cognitive performance in secondary progressive and benign MS patients. Surface expansion of the DG might represent an inflammation‐induced neurogenic (reactive) process of the subgranular zone of the hippocampus primarily aimed at rescuing the functional competence of hippocampal circuitry. Hum Brain Mapp 36:4702–4713, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: multiple sclerosis, volumetric MRI, radial mapping, hippocampus, dentate gyrus, neurogenesis, inflammation, cognition
INTRODUCTION
Growing interest has been devoted, in the past few years, to the role of hippocampal injury for the development of spatial and episodic memory impairment in patients with multiple sclerosis (MS) [Dutta et al., 2013; Longoni et al., 2015; Roosendaal et al., 2010; Sicotte et al., 2008]. Anatomopathological substrates of hippocampal dysfunction in MS are likely to be diffuse demyelination and plaque formation [Geurts et al., 2007; Papadopoulos et al., 2009], neuroaxonal loss [Papadopoulos et al., 2009], and gliosis. Two recent pathological studies of the hippocampus in MS patients have also reported demyelination‐induced alterations in genic expression profiles, leading to abnormalities in hippocampal axonal traffic, synaptic plasticity and neurotransmitter homeostasis [Dutta et al., 2011, 2013].
The human dentate gyrus (DG) of the hippocampus, composed by three histologically distinct layers (the molecular layer, the granule cell layer, and the polymorphic layer), is an allocortex lamina which concavity envelops the Cornu Ammonis (CA) 4 subfield (also known as the hilus of the DG) [Duvernoy, 2005]. Consistently with its central location in the intrahippocampal circuitry, the DG plays an essential role in memory function: long‐term potentiation (LTP), a well‐characterized form of synaptic plasticity, is indeed activated in the DG by learning and memory tasks [Lynch, 2004]. Moreover, the acquisition of episodic memory during childhood has been linked to structural and molecular development of the hippocampus [Lavenex and Banta Lavenex, 2013]. Adult neurogenesis is probably another form of neural plasticity of the DG [Eriksson et al., 1998] that likely contributes to hippocampal‐dependent memory functions, with newly generated neurons critically enhancing accuracy of memory encoding [Clelland et al., 2009]. Within the subgranular zone of the DG, thousands of new granule cells are continuously generated throughout life [Eriksson et al., 1998], with 700 new neurons being added in each hippocampus per day in adult humans, corresponding to a daily exchange of 0.004% of the DG neurons [Spalding et al., 2013]. Noteworthy, in the light of the sophisticated interactions between the immune system and the neurogenic niche [Martino et al., 2011], inflammatory conditions of the CNS are able to modulate the homeostasis of neural stem/precursor cells (NPCs) and perturb some aspects of neurogenesis [Pluchino et al., 2008].
In this study, we hypothesized that, due to its exquisite composition, the DG of the hippocampus could undergo selected modifications of its volume in MS patients, which may differ according to the stage of the disease and which may result in differential functional competence of hippocampal circuitry. To test this hypothesis, we used surface‐based mesh modeling [Thompson et al., 2004], which has allowed a detailed, non‐invasive investigation of the regional shape alterations associated with several physiological [Luders et al., 2012] and pathological conditions [Apostolova et al., 2012; Gold et al., 2014; Narr et al., 2004; Thompson et al., 2004], to characterize local expansions or contractions of DG morphology across MS clinical phenotypes with a relapse‐onset disease and their correlation with neuropsychological performance. Differently from currently available methods for automated hippocampal segmentation, such as voxel‐based morphometry or FIRST, this technique allows exploring selective subregional hippocampal volume modifications in vivo.
METHODS
Subjects
We enrolled 88 (62 females, 26 males) right‐handed consecutive patients with MS (28 relapsing‐remitting [RR] MS, 34 secondary progressive [SP] MS, and 26 benign [B] MS, defined as an EDSS score ≤3.0 and a disease duration ≥15 years). To be included, patients had to be relapse and steroid‐free for at least one month and with stable disease‐related treatment for at least six months before MRI acquisition. Thirty‐seven patients (13 RRMS, 11 SPMS, and 13 BMS) were treated with interferon beta, 12 with glatiramer acetate (8 RRMS, 2 SPMS, and 2 BMS), and 12 with immunosuppressive treatments (1 RRMS and 11 SPMS).
Twenty‐eight right‐handed, age‐ and gender‐matched healthy controls (HC) (18 female, 10 males) with no previous history of neurological, psychiatric, or medical disorders, and a normal neurological exam were also enrolled.
Within 48 h of MRI acquisition, MS patients underwent a neurological evaluation with rating of the Expanded Disability Status Scale (EDSS) score and a neuropsychological assessment, designed to assess hippocampal verbal memory functions, which included the Paired Associate Word Learning [WL] Test [Novelli et al., 1986] and the Short Story (SS) Test [Novelli et al., 1986]. All tests were adapted to and validated in the Italian language. Referring to normative data [Novelli et al., 1986], individual raw scores were adjusted for age, sex and education.
Standard Protocol Approvals, Registrations, and Patient Consents
Approval was received from the ethical standards committee on human experimentation of San Raffaele Scientific Institute. Written informed consent was obtained from all subjects prior to study enrolment.
MRI Acquisition and Analysis
Using a 3.0 T scanner (Philips Medical Systems, Eindhoven, The Netherlands), the following sequences were obtained: (a) 3D T1‐weighted fast field echo (FFE) (TR/TE = 25/4.6 ms, flip angle = 30°, matrix size = 256 × 256, FOV = 230 × 230 mm2; 220 contiguous, axial slices with voxel size = 0.89 × 0.89 × 0.8 mm), and (b) dual‐echo turbo spin echo (TSE) (TR/TE = 2,599/16–80 ms, echo train length = 6, 44 contiguous 3‐mm thick axial slices with a matrix size = 256 × 256, FOV = 240 mm2).
All image preprocessing was performed by a single observer, experienced with hippocampal segmentation, blinded to subjects' identity.
T2‐hyperintense lesions were identified and T2 lesion volumes (LV) quantified using a local thresholding segmentation technique (Jim 5, Xinapse Systems Ltd., Northants, UK). After refilling of T1‐hypointense lesions [Chard et al., 2010], normalized brain (NBV), white matter (NWMV) and gray matter (NGMV) volumes were measured on three‐dimensional (3D) T1‐weighted images, using the Structural Imaging Evaluation of Normalized Atrophy (SIENAx) software.
Hippocampal Segmentation
Hippocampal segmentation was obtained for each subject from the 3D T1‐weighted images as previously described [Longoni et al., 2015]. After correction of intensity nonuniformity, images were aligned to the Montreal Neurological Institute (MNI) space and reformatted into the coronal plane. Then, manual tracing of the hippocampus was performed by an experienced neurologist unaware of the aims of the study according to a standardized protocol [Longoni et al., 2015] (Fig. 1). From contours, the volume of the traced structures was computed (LONI software) and retained for the subsequent steps of the analysis [Longoni et al., 2015].
Figure 1.
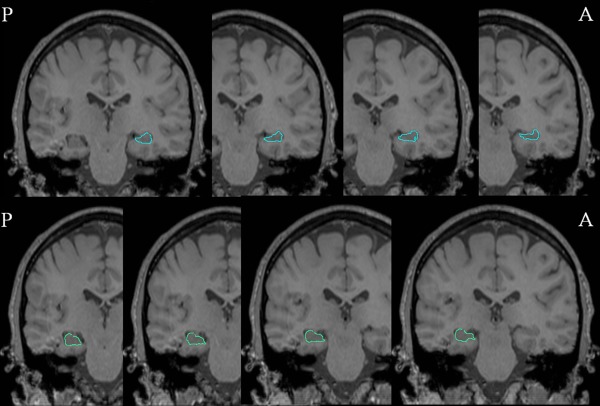
Radial mapping analysis: hippocampal segmentation. Coronal magnetic resonance imaging (MRI) slices from a healthy control in posterior‐anterior direction. Representative manual tracings of the right (light‐blue, top row) and left (light green, bottom row) hippocampus are shown.
Intra‐ and interobserver reproducibility of hippocampal segmentation has been tested in a previous study [Longoni et al., 2015], which showed an intraclass correlation coefficient (ICC) of 0.93 for intrarater and 0.91 for inter‐rater assessment.
Binarized hippocampal masks were transformed from the MNI to single subject's dual‐echo space and applied to T2 lesion masks to exclude all the extra‐hippocampal lesional voxels and calculate hippocampal T2 LV.
Surface‐Based Mesh Modeling
Hippocampal traces were converted to parametric surface meshes and a radial mapping technique was applied. This allowed to compare shape contractions and expansions of anatomical structures between populations (LONI software) [Thompson et al., 2004]. Briefly, manually derived contours were resampled in a common number of 100 × 150 surface vertices, which were made spatially uniform and homologous with each other [Narr et al., 2004]. From the parametric surface model of each individual hippocampus, a medial curve was derived along its anterior‐posterior axis, and the distance of each vertex from its medial core was calculated (“radial distance”). For each group of the study, an “average” hippocampus was obtained averaging the “radial distance” of each homologous vertex from all the subjects of the group. To check for possible biases caused by the position of the medial core, we derived the average medial core distances for the group of healthy controls and RRMS patients and plotted them in polar coordinates (rho, teta) allowing teta to span 360° with 150 equidistant steps and rho to be the radial distance at each vertex (Fig. 2).
Figure 2.
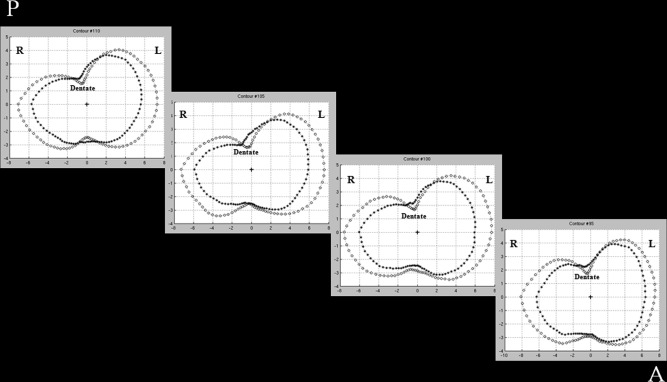
Plots of average core distance in HC and RRMS. Four sections of the left hippocampus where the average core distance (in mm) is plotted for healthy controls (HC) (circle) and relapsing‐remitting multiple sclerosis (RRMS) patients (asterisk). The position of the medial core is indicated for each section with a cross. L = left, R = right, A = anterior, P = posterior. See text for further details.
To assess local expansions or contractions affecting the DG, surface vertices of each right and left DG region were manually selected on the average shape obtained from the HC group, based on anatomical reference [Duvernoy, 2005]. This procedure yielded to the segmentation of the DG in about 900 vertices in each hemisphere (which is about 6% of each hippocampal surface). Radial distances calculated in DG vertices were then retained for statistical analysis.
Statistical Analysis
For clinical, demographic, and MRI volumetric variables, normality of data was explored using the Kolmogorov‐Smirnov Test. Between‐group differences were assessed using parametric and nonparametric tests as appropriate. Between‐group comparisons were defined a priori on the basis of clinical relevance. The following comparisons were assessed (in both directions): HC versus RRMS patients, RRMS versus SPMS patients, RRMS versus BMS patients, and SPMS versus BMS patients (SPSS Inc, Chicago, IL, release 20.0). Comparisons between BMS and SPMS patients versus HC are also reported for descriptive purposes.
An analysis of variance and Pearson correlation, controlling for age, were used to evaluate radial distance differences between groups at homologous surface locations and correlations with clinical (disease duration), neuropsychological and MRI (NWMV, NGMV, brain and hippocampal T2 LV) variables (LONI software). The resulting two‐tailed P and r values were mapped on the average 3D hippocampal shape obtained from each group; results were expressed as number of surface vertices in the DG showing a statistically significant difference (P < 0.05) in radial distance at between‐group comparisons, or a significant correlation with the variable of interest, as appropriate. False discovery rate (FDR) was used to correct for multiple comparisons.
RESULTS
Table 1 summarizes the main demographic, clinical, neuropsychological, and conventional MRI characteristics of the study groups as well as the results of the between‐group comparisons of these measures. As expected, BMS had a significantly longer disease duration than the other MS phenotypes (P < 0.001 for all the comparisons). EDSS was significantly higher in SPMS compared with RRMS and BMS patients (P < 0.001 for both the comparisons), and in RRMS compared with BMS patients (P = 0.02).
Table 1.
Main demographic, clinical and conventional MRI characteristics of multiple sclerosis (MS) patients (divided according to disease clinical phenotypes) and healthy controls
| HC (n = 28) | RRMS (n = 28) | SPMS (n = 34) | BMS (n = 26) | P | |
|---|---|---|---|---|---|
| Women/men | 18/10 | 20/8 | 24/10 | 18/8 | n.s. |
| Mean age (range) (yr) | 44.8 (25–69) | 39.7 (20–63) | 45.8 (24–67) | 44.0 (33–62) | n.s.* |
| Median EDSS (range) | – | 3.5 (1.0–4.5) | 6.0 (3.5–8.0) | 2.0 (1.0–3.0) | <0.001*** |
| Mean disease duration (range) [years] | – | 9.9 (0.1–22.7) | 13.9 (2.5–34.3) | 22.0 (15.1–33.3) | <0.001* |
| Mean education (range) (yr) | 12 (8–17) | 13 (8–17) | 12 (5–17) | 11 (8–17) | n.s.* |
| Word learning test mean score (SD) | – | 12.3 (2.8) | 11.4 (3.9) | 13.4 (3.4) | n.s.* |
| Short Story test mean score (SD) | – | 10.9 (4.1) | 10.9 (4.1) | 11.9 (3.8) | n.s.* |
| Mean brain T2 LV (range) (ml) | – | 11.6 (10.8) | 17.7 (14.6) | 12.5 (10) | 0.001* |
| Mean R hippocampal T2 LV (SD) (ml) | – | 0.004 (0.01) | 0.01 (0.05) | 0.005 (0.06) | n.s.*** |
| Mean L hippocampal T2 LV (SD) (ml) | – | 0.002 (0.05) | 0.009 (0.02) | 0.001 (0.01) | 0.03*** |
| Mean NGMV (SD) (ml) | 761 (75) | 665 (85) | 629 (104) | 640 (91) | <0.001** |
| Mean NWMV (SD) (ml) | 856 (71) | 864 (75) | 836 (104) | 887 (102) | n.s.** |
| Mean R NHV (SD) (ml) | 3.9 (0.5) | 3.5 (0.6) | 3.3 (0.5) | 3.3 (0.5) | <0.001** |
| Mean L NHV (SD) (ml) | 3.8 (0.5) | 3.5 (0.4) | 3.3 (0.6) | 3.2 (0.4) | 0.001** |
P values refer to: *ANOVA, Bonferroni corrected; **General Linear Model, age adjusted; ***Kruskal‐Wallis test.
HC = healthy controls; MS = multiple sclerosis; SD = standard deviation; n = number of subjects; RR = relapsing‐remitting; SP = secondary progressive; BMS = benign MS; EDSS = Expanded Disability Status Scale score; NBV = normalized brain volume; NGMV = normalized gray matter volume; NWMV = normalized white matter volume; LV = lesion volume; R = right; L = left; NHV = normalized hippocampal volume; n.s. = not significant.
Compared with HC, RRMS patients had lower NGMV (P = 0.001) and right hippocampal volume (P = 0.04). All the remaining a priori comparisons were not significant.
No DG subregion was atrophic in MS patients, as a whole and according to disease clinical phenotype, compared with HC. The opposite comparison showed a larger radial distance of the DG in all MS clinical phenotypes when contrasted to HC, which was more pronounced for the left hippocampus (Table 2). These morphological alterations were especially evident (FDR < 5%) in the left hippocampus of RRMS (Fig. 3) and SPMS patients, while only a small cluster of larger radial distance in the left DG was found in BMS patients compared with HC (not surviving FDR correction). The comparison between disease clinical phenotypes showed that RRMS had a significant radial distance enlargement (FDR < 5%) of the DG compared with SPMS and BMS patients, whereas the opposite contrast showed only small clusters of larger radial distance in SPMS and BMS versus RRMS patients. Bilateral DG volumetric enlargement was found in SPMS versus BMS patients (FDR < 5% for the left and right hippocampus) (Table 2, Fig. 4).
Table 2.
Results from the between‐group comparisons of hippocampal dentate gyrus vertex analysis
| Right dentate gyrus | Left dentate gyrus | |||||
|---|---|---|---|---|---|---|
| No. vertices | Mean (SD) | Percentage change | No. vertices | Mean (SD) | Percentage change | |
| RRMS > HC | 56 | 2.66 (0.76) > 2.14 (0.78) | 24.5 | 148 | 2.40 (0.71) > 1.83 (0.65) | 31.1 |
| SPMS > HC | 18 | 2.47 (0.83) > 1.99 (0.69) | 24.2 | 164 | 2.67 (0.71) > 2.19 (0.70) | 21.5 |
| BMS > HC | – | – | 6 | 2.55 (0.65) > 2.15 (0.63) | 18.8 | |
| RRMS > SPMS | 18 | 2.66 (0.82) > 2.21 (0.69) | 19.9 | 63 | 2.75 (0.62) > 2.37 (0.67) | 16.1 |
| SPMS > RRMS | – | – | 38 | 3.15 (0.73) > 2.56 (0.70) | 23.4 | |
| RRMS > BMS | 58 | 2.84 (0.83) > 2.27 (0.84) | 25.0 | 184 | 3.01 (0.74) > 2.48 (0.70) | 21.5 |
| BMS > RRMS | – | – | 8 | 3.39 (0.85) > 2.79 (1.02) | 21.3 | |
| SPMS > BMS | 123 | 3.84 (0.89) > 3.21 (1.09) | 19.7 | 56 | 3.33 (0.80) > 2.82 (0.74) | 18.0 |
| BMS > SPMS | – | – | – | – | ||
The number of surface vertices out from the 900 of the dentate gyrus, showing a statistically significant difference in radial distance at between‐group comparisons (P < 0.05), radial distance means, SDs (in mm), and percentage of changes (i.e., relative difference between the means of the two groups being compared) for the significant vertices are provided.
HC = healthy controls; MS = multiple sclerosis; RR = relapsing‐remitting; SP = secondary progressive; BMS = benign MS; SD = standard deviation.
Figure 3.
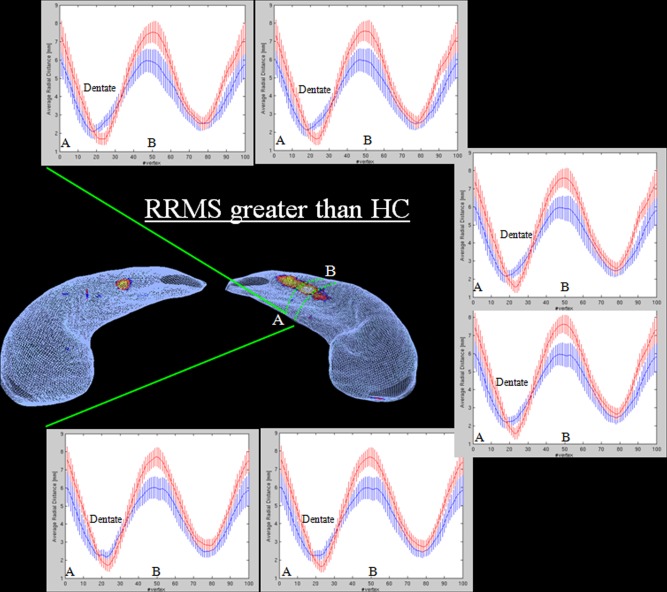
Radial mapping analysis: HC versus RRMS. Some representative contours extracted from the left hippocampal region included in dashed green lines. Contours were straightened and at each vertex, average and standard deviation of radial distances from relapsing‐remitting multiple sclerosis (RRMS) patients (blue) and healthy controls (HC) (red) were plotted. Letters A and B indicates the innermost and most lateral positions on the hippocampus.
Figure 4.
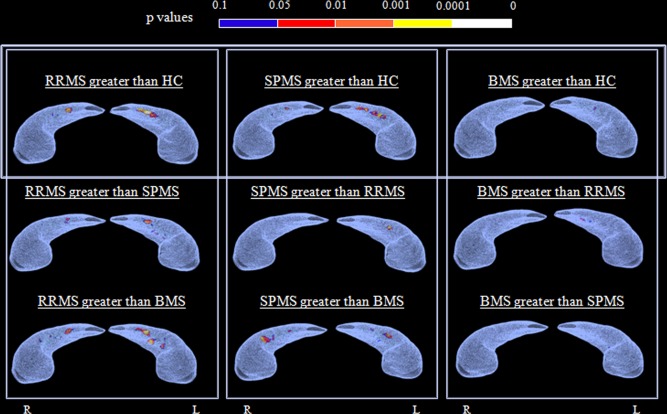
Radial mapping analysis: between‐group comparisons. The superior aspect of the average right and left hippocampus from the subjects included in each statistical comparison are shown. The color‐coded maps of P values show vertices of the dentate gyrus surface characterized by a statistically significant difference of radial distance at between‐group comparisons. Results are reported for P < 0.05. MS = multiple sclerosis; RR = relapsing‐remitting; SP = secondary progressive; BMS = benign MS; HC = healthy controls; R = right; L = left.
In the whole group of MS patients, larger radial distance of the DG was significantly correlated with a shorter disease duration and larger brain volumes (NWMV, NGMV) (Table 3).
Table 3.
Correlations between dentate gyrus (DG) volumetric modifications in multiple sclerosis (MS) patients and clinical, neuropsychological and conventional MRI measures
| Right dentate gyrus | Left dentate gyrus | ||||
|---|---|---|---|---|---|
| Group of patients | Variable of interest | No. vertices | r Value (range) | No. vertices | r Value (range) |
| All MS patients | Disease duration | 185 | −0.24 (−0.37 to −0.18) | 54 | −0.21 (−0.29 to −0.18) |
| NGMV | 162 | 0.24 (0.18 to 0.37) | 146 | 0.25 (0.18 to 0.34) | |
| NWMV | 40 | 0.23 (0.18 to 0.31) | 27 | 0.21 (0.18 to 0.25) | |
| RRMS | WL test | – | – | 11 | 0.50 (0.43 to 0.58) |
| SS test | – | – | 34 | 0.47 (0.42 to 0.57) | |
| Brain T2 LV | 4 | 0.42 (0.39 to 0.45) | 62 | 0.43 (0.37 to 0.56) | |
| Hippocampal T2 LV | 79 | 0.46 (0.38 to 0.60) | 34 | 0.44 (0.38 to 0.54) | |
| SPMS | WL test | 52 | −0.39 (−0.47 to −0.34) | 130 | −0.41 (−0.55 to −0.35) |
| SS test | – | – | 26 | −0.38 (−0.44 to −0.34) | |
| Brain T2 LV | 46 | 0.40 (0.34 to 0.47) | – | – | |
| Hippocampal T2 LV | 26 | 0.40 (0.34 to 0.48) | 44 | 0.41 (0.34 to 0.51) | |
| BMS | WL test | – | – | 12 | −0.43 (−0.52 to −0.40) |
For each correlation, the total number of surface vertices of the DG showing a significant correlation (P < 0.05) (Pearson correlation coefficient) with the variable of interest as well as the means and ranges of r values for the significant vertices are provided.
NGMV = normalized gray matter volume; NWMV = normalized white matter volume; LV = lesion volume; WL = word learning; SS = short story.
The association between DG radial distance modification versus neuropsychological performance and T2 LV showed a differential effect among disease clinical phenotypes (Table 3). In RRMS patients, left DG radial distance enlargement was significantly correlated with better performances at WL and SS tests. Bilateral DG radial distance enlargement was correlated with higher brain and hippocampal T2 LV (Fig. 5). In SPMS patients, DG radial distance enlargement was significantly correlated with worse performances at WL and SS tests as well as with higher brain and hippocampal T2 LV (Fig. 5).
Figure 5.
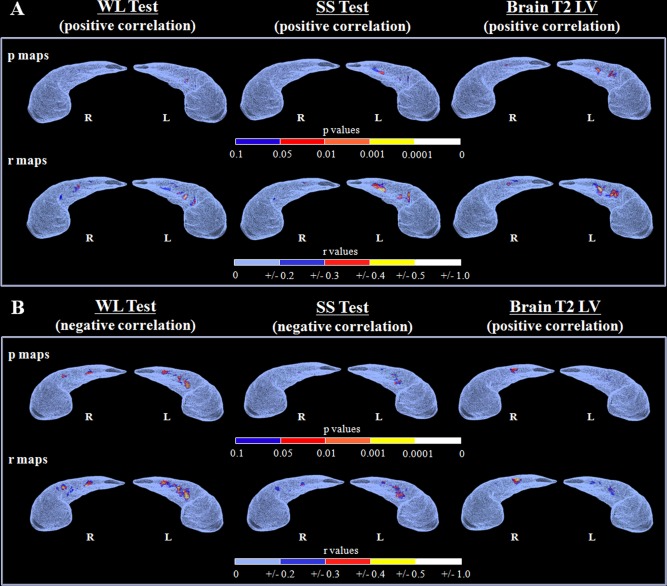
Radial mapping analysis: correlation analysis. The color‐coded maps show P and r values from the correlation analysis of the dentate gyrus surface (superior aspect) with word learning (WL) test, short story (SS) test and brain T2 lesion load for patients with relapsing remitting (A) and secondary progressive (B) MS. Results are reported for P < 0.05. R = right; L = left.
In BMS patients, left DG radial distance enlargement was significantly correlated with a worse performance at WL test.
DISCUSSION
By using surface‐based mesh modeling, we found a significant radial size enlargement of the DG in all MS groups (which was marginally significant for BMS patients), such an effect being more pronounced in the left hippocampus and in patients with RRMS.
Despite global and regional hippocampal volumetric reductions have been broadly observed in MS [Gold et al., 2010, 2014; Papadopoulos et al., 2009; Roosendaal et al., 2010; Sicotte et al., 2008] and non‐MS pathological conditions [Apostolova et al., 2012; Narr et al., 2004; Thompson et al., 2004], MRI evidences of hippocampal size enlargement are still relatively scanty. Using MRI‐based manual segmentation, an increase of bilateral hippocampal head has been recently detected in patients with Cushing's disease after resection of the secreting tumor [Toffanin et al., 2011]. In line with previous voxel‐based morphometry data [Holzel et al., 2011], a surface mesh model analysis [Luders et al., 2012] described a global volumetric increase of left hippocampus and of the left hippocampal head in long‐term meditators compared with controls. An abnormal shape enlargement of bilateral hippocampi has also been detected in offenders with psychopathy compared with controls [Boccardi et al., 2010]. Since MRI‐based measurements of hippocampal volume have been shown to correlate with anatomopathological neuronal counts [Dawe et al., 2011], the enlargement of the DG of our patients may reflect an increase in the cellular count at this level. This interpretation is apparently in contrast with the decrease of hippocampal cross‐sectional area reported by a pathologic MS study [Papadopoulos et al., 2009], which, however, did not specifically investigate the DG subregion. Additionally, the patients included in that study [Papadopoulos et al., 2009] were older (mean age: 61 vs. 44.8 years) and had longer disease duration (mean 27.8 vs. 14.1) than ours. Despite a 27 to 30% reduction of neuronal counts in CA1 and CA2‐CA3 subfields, no significant cellular loss and a neuronal shape change toward a more rounded shape were found in the hilus of the DG in MS patients compared with controls [Papadopoulos et al., 2009]. Such a relative sparing of the CA4 structures may partly rely on the scant proclivity for demyelinating lesion formation in such a region [Papadopoulos et al., 2009].
Current views support the notion that neurogenesis persists throughout the entire existence, being more prominent in the subventricular zone (SVZ) [Quinones‐Hinojosa et al., 2006] and the subgranular zone of the DG [Eriksson et al., 1998]. In humans, adult neurogenesis is thought to contribute to physiological regeneration and reparative processes after injury. New neurons in the adult DG have been implicated in learning and memory [Zhao et al., 2008], and the granule layer of the DG can increase or decrease in volume by a relatively large magnitude (5–20%) depending on rates of neurogenesis [Kohman and Rhodes, 2013]. Previous pathological studies from patients with stroke [Marti‐Fabregas et al., 2010] or Huntington's disease [Curtis et al., 2003] provided some evidence for a proliferative reaction of NPCs within the germinal niche that can then migrate to the injury site to promote the replacement of damaged cells. According to our present understanding, the DG enlargement we observed in RRMS and that was not detected in the more chronic phase of the disease (SPMS/BMS) might be interpreted as a reactivation of neurogenesis aimed at counteracting tissue damage and loss due to demyelination, neuroaxonal loss, and disturbed synaptic transmission. This process might represent a compensatory reactive neurogenic phenomenon aimed at reinstating the initially compromized hippocampal circuitry. This notion is strengthened by the results of the analysis of correlation with hippocampal verbal memory function. In RRMS patients, in whom DG morphological abnormalities were prominent, a direct correlation between the degree of DG enlargement and a high performance at neuropsychological tests was found, whereas the analysis of SPMS and BMS patients provided divergent results. Despite the impact of neurogenesis on hippocampal function has been recently challenged [Frankland et al., 2013], several experimental data have shown that reducing neurogenesis impairs subsequent hippocampal memory formation, while increasing neurogenesis facilitates hippocampal memory formation [Zhao et al., 2008]. As a consequence, we postulate that, independently of the underlying cellular substrate, the mechanisms leading to an expansion of the DG in the early, relapsing phase of MS might have a positive effect, which contributes to promote functional competence of the hippocampal circuitry. Modifications of these mechanisms with disease progression may finally impair hippocampal function.
Although a direct comparison between DG and SVZ NPCs is not possible, given the different role and fate of these cells [Martino et al., 2014], a 2‐3‐fold increase in cell density and proliferation was shown in the SVZ of postmortem MS brains compared with non‐neurological controls [Nait‐Oumesmar et al., 2007]. Another neuropathological study of subcortical chronic MS lesions detected a population of newborn neurons, functionally differentiated and integrated within local networks by synapse formation, which may have originated from NPCs in the adjacent SVZ [Chang et al., 2008].
In the absence of pathological assessment and without the application of sophisticated techniques which allow the study of cell turnover dynamics by birth dating cells, such as the quantification of nuclear bomb test‐derived 14C concentration [Spalding et al., 2013], we cannot completely rule out that factors different from an increased neuron formation may contribute to explain the larger DG radial size we found. By studying cell turnover dynamic of human postmortem hippocampi [Spalding et al., 2013], it has been shown that a large proportion of non‐neuronal cells are continuously exchanged, with an estimated annual turnover of these cells of 3.5% compared with 1.75% of hippocampal neuronal cells. Importantly and differently from neuronal cells, the turn‐over of these non‐neuronal cells declines with age. The notion that these non‐neuronal cells might account, at least in part, for the larger size of the DG we have found is also supported by a recent study which has applied the previous technique to postmortem human ischemic cortical tissue and found an increased turnover of non‐neuronal cells (glial cells, vascular cells, and other cell types involved in postischemic lesion consolidation) after ischemic stroke, which continued in the years after the event [Huttner et al., 2014].
Our study revealed an asymmetry of the DG enlargement in MS patients, being the left DG larger than the right one. Imaging studies of normal individuals have reported subtle right larger than left hippocampal volumes [Narr et al., 2004], and a recent study which has assessed the trajectories of maturation of the hippocampus and its subfields has shown larger volume growth in the right than the left hippocampus and DG in childhood, with this asymmetry effect being more pronounced during adolescence [Krogsrud et al., 2014]. Based on this, in addition to a preponderance of the cellular reaction in the left DG, our results might be also explained by a more pronounced susceptibility to damage affecting the right hippocampus in the context of a more global reduction of cerebral asymmetries and an higher susceptibility to damage of the right hemisphere observed not only with aging, but also in many neurological diseases [Dolcos et al., 2002]. Support for the latter view might come from a previous study reporting a predominant volume loss of right hippocampus in MS patients [Roosendaal et al., 2010]. Intriguingly, in a previous work, we reported a shrinking of the hilus of the right DG in MS patients compared with controls, which was absent on the contralateral side [Longoni et al., 2015].
Due to the lack of anatomopathological support and the cross‐sectional nature of this study, any conclusion remains, however, speculative, and the exact neuronal mechanisms underlying our results are still to be established. The altered hippocampal surface may arise from biological mechanisms other than adult neurogenesis (e.g., gliosis, increase dendritic complexity of existing mature granule cells, increase angiogenesis, increase amount of extracellular fluid). Given its explorative nature, our work also suffers from a suboptimal detection of cortical and hippocampal lesions, given the lack of cortical‐directed sequences, such as double inversion recovery images. As a consequence, the contribution of hippocampal demyelination to DG modifications could have been underestimated. Other confounding factors on DG size, including physical exercise [Pereira et al., 2007] and depression [Gold et al., 2014] have not been specifically addressed by our study. From a methodological point of view, the position of the medial core could be biased depending on the particular distribution of changes at the boundaries. The effect of the deviation from a starlike topology would be the redistribution of the changes over symmetrical vertices and the consequent shift of the boundaries coming from the alignment of the medial cores. We checked for this by looking at the behavior at the opposite vertices and at boundaries as a whole, displaying average radial distances as contours at some relevant sections of the hippocampus, as shown in Figure 2. For some plots, in addition to a larger radial distance, there may be a shift of rotation, which may have exaggerated this effect. Clearly, the results strongly depend from the method we have chosen (the use of radial distance implies that the registration is obtained by aligning the centroids of each bidimensional contour). We cannot rule out that other methods, that use different criteria for registration, such as the minimization of mean square distance between vertices considering them as a whole, might provide different results. Another methodological issue is related to the use of a linear interpolation to resample the data as the first step of the preprocessing, which may have caused loss of spatial resolution. However, this was an essential step for the standardization of the manual segmentation process. Although the high reproducibility of the manual segmentation was assessed, we could not obtain an estimate of the uncertainty of the measurement. Future longitudinal studies are therefore warranted to confirm and deepen our findings. Despite the functional role of adult neurogenesis in CNS pathology is still debated, our results could promote an advancement in the understanding of disease pathobiology and a tool for monitoring the neuroprotective effects of disease‐modifying treatments.
ACKNOWLEDGMENTS
G.L., E.P., G.B., B.C., M.R., G.M., and A.F. have nothing to disclose. M.A.R. received speakers honoraria from Biogen Idec, Novartis, Genzyme, Sanofi‐Aventis, and Merk Serono and receives research support from the Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. G.C. has received consulting fees for participating on advisory boards from Novartis, Teva Pharmaceutical Ind. Ltd., Sanofi, Genzyme, Merck Serono, Bayer, Actelion, and honorarium for speaking activities for Novartis, Teva Pharmaceutical Ind. Ltd., Sanofi, Genzyme, Merck Serono, Bayer, Biogen, Serono Symposia International Foundation. M.F. serves on scientific advisory board for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Bayer Schering Pharma, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries; and receives research support from Bayer Schering Pharma, Biogen Idec, Merck Serono, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, and the Jacques and Gloria Gossweiler Foundation (Switzerland).
REFERENCES
- Apostolova L, Alves G, Hwang KS, Babakchanian S, Bronnick KS, Larsen JP, Thompson PM, Chou YY, Tysnes OB, Vefring HK, Beyer MK (2012): Hippocampal and ventricular changes in Parkinson's disease mild cognitive impairment. Neurobiol Aging 33:2113–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M, Ganzola R, Rossi R, Sabattoli F, Laakso MP, Repo‐Tiihonen E, Vaurio O, Kononen M, Aronen HJ, Thompson PM, GB Frisoni, J Tiihonen (2010): Abnormal hippocampal shape in offenders with psychopathy. Hum Brain Mapp 31:438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Smith MC, Yin X, Fox RJ, Staugaitis SM, Trapp BD (2008): Neurogenesis in the chronic lesions of multiple sclerosis. Brain 131:2366–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard DT, Jackson JS, Miller DH, Wheeler‐Kingshott CA (2010): Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J Magn Reson Imaging 32:223–228. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD,J, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009): A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, van Roon‐Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL (2003): Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci USA 100:9023–9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe RJ, Bennett DA, Schneider JA, Arfanakis K (2011): Neuropathologic correlates of hippocampal atrophy in the elderly: A clinical, pathologic, postmortem MRI study. PLoS One 6:e26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, Cabeza R (2002): Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev 26:819–825. [DOI] [PubMed] [Google Scholar]
- Dutta R, Chang A, Doud MK, Kidd GJ, Ribaudo MV, Young EA, Fox RJ, Staugaitis SM, Trapp BD (2011): Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol 69:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Chomyk AM, Chang A, Ribaudo MV, Deckard SA, Doud MK, Edberg DD, Bai B, Li M, Baranzini SE, RJ Fox, SM Staugaitis, WB Macklin, BD Trapp (2013): Hippocampal demyelination and memory dysfunction are associated with increased levels of the neuronal microRNA miR‐124 and reduced AMPA receptors. Ann Neurol 73:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM (2005): The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. Berlin: Springer. [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork‐Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998): Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Kohler S, Josselyn SA (2013): Hippocampal neurogenesis and forgetting. Trends Neurosci 36:497–503. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Bo L, Roosendaal SD, Hazes T, Daniels R, Barkhof F, Witter MP, Huitinga I, van der Valk P (2007): Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol 66:819–827. [DOI] [PubMed] [Google Scholar]
- Gold SM, Kern KC, O'Connor MF, Montag MJ, Kim A, Yoo YS, Giesser BS, Sicotte NL (2010): Smaller cornu ammonis 2‐3/dentate gyrus volumes and elevated cortisol in multiple sclerosis patients with depressive symptoms. Biol Psychiatry 68:553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, O'Connor MF, Gill R, Kern KC, Shi Y, Henry RG, Pelletier D, Mohr DC, Sicotte NL (2014): Detection of altered hippocampal morphology in multiple sclerosis‐associated depression using automated surface mesh modeling. Hum Brain Mapp 35:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW (2011): Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res 191:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner HB, Bergmann O, Salehpour M, Racz A, Tatarishvili J, Lindgren E, Csonka T, Csiba L, Hortobagyi T, Mehes G, E Englund, BW Solnestam, S Zdunek, C Scharenberg, L Ström, P Ståhl, B Sigurgeirsson, A Dahl, S Schwab, G Possnert, S Bernard, Z Kokaia, O Lindvall, J Lundeberg, J Frisén (2014): The age and genomic integrity of neurons after cortical stroke in humans. Nat Neurosci 17:801–803. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Rhodes JS (2013): Neurogenesis, inflammation and behavior. Brain Behav Immun 27:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsrud SK, Tamnes CK, Fjell AM, Amlien I, Grydeland H, Sulutvedt U, Due‐Tonnessen P, Bjornerud A, Solsnes AE, Haberg AK, J Skrane, KB Walhovd (2014): Development of hippocampal subfield volumes from 4 to 22 years. Hum Brain Mapp 35:5646–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P (2013): Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behav Brain Res 254:8–21. [DOI] [PubMed] [Google Scholar]
- Longoni G, Rocca MA, Pagani E, Riccitelli GC, Colombo B, Rodegher M, Falini A, Comi G, Filippi M (2015): Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct Funct 220:435–444. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Kurth F, Hong JY, Phillips OR, Wang Y, Gutman BA, Chou YY, Narr KL, Toga AW (2012): Global and regional alterations of hippocampal anatomy in long‐term meditation practitioners. Hum Brain Mapp 34:3369–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA (2004): Long‐term potentiation and memory. Physiol Rev 84:87–136. [DOI] [PubMed] [Google Scholar]
- Marti‐Fabregas J, Romaguera‐Ros M, Gomez‐Pinedo U, Martinez‐Ramirez S, Jimenez‐Xarrie E, Marin R, Marti‐Vilalta JL, Garcia‐Verdugo JM (2010): Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology 74:357–365. [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S, Bonfanti L, Schwartz M (2011): Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol Rev 91:1281–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G, Butti E, Bacigaluppi M (2014): Neurogenesis or non‐neurogenesis: that is the question. J Clin Invest 124:970–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nait‐Oumesmar B, Picard‐Riera N, Kerninon C, Decker L, Seilhean D, Hoglinger GU, Hirsch EC, Reynolds R, Baron‐Van Evercooren A (2007): Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proc Natl Acad Sci USA 104:4694–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM (2004): Regional specificity of hippocampal volume reductions in first‐episode schizophrenia. Neuroimage 21:1563–1575. [DOI] [PubMed] [Google Scholar]
- Novelli GLM, Papagano C, Vallar G, Capitani E, Cappa SF (1986): Tre test clinici di ricerca e di produzione lessicale. Taratura su soggetti normali. Arch Psicol Neurol Psichiatric 2:278–296. [Google Scholar]
- Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R (2009): Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol 19:238–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA (2007): An in vivo correlate of exercise‐induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 104:5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro‐Cervello C, Salani G, Porcheri C, Brambilla E, Cavasinni F, Bergamaschi A, JM Garcia‐Verdugo, G Comi, SJ Khoury, G Martino (2008): Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain 131:2564–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones‐Hinojosa A, Sanai N, Soriano‐Navarro M, Gonzalez‐Perez O, Mirzadeh Z, Gil‐Perotin S, Romero‐Rodriguez R, Berger MS, Garcia‐Verdugo JM, Alvarez‐Buylla A (2006): Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. J Comp Neurol 494:415–434. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Hulst HE, Vrenken H, Feenstra HE, Castelijns JA, Pouwels PJ, Barkhof F, Geurts JJ (2010): Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology 255:595–604. [DOI] [PubMed] [Google Scholar]
- Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, Bookheimer SY (2008): Regional hippocampal atrophy in multiple sclerosis. Brain 131:1134–1141. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, G Possnert, DC Mash, H Druid, J Frisén (2013): Dynamics of hippocampal neurogenesis in adult humans. Cell 153:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW (2004): Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage 22:1754–1766. [DOI] [PubMed] [Google Scholar]
- Toffanin T, Nifosi F, Follador H, Passamani A, Zonta F, Ferri G, Scanarini M, Amista P, Pigato G, Scaroni C, F Mantero, C Carollo, GI Perini (2011): Volumetric MRI analysis of hippocampal subregions in Cushing's disease: A model for glucocorticoid neural modulation. Eur Psychiatry 26:64–67. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH (2008): Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660. [DOI] [PubMed] [Google Scholar]


