Abstract
In this study, an activation likelihood estimation (ALE) meta‐analysis was used to conduct a quantitative investigation of neuroimaging studies on divergent thinking. Based on the ALE results, the functional magnetic resonance imaging (fMRI) studies showed that distributed brain regions were more active under divergent thinking tasks (DTTs) than those under control tasks, but a large portion of the brain regions were deactivated. The ALE results indicated that the brain networks of the creative idea generation in DTTs may be composed of the lateral prefrontal cortex, posterior parietal cortex [such as the inferior parietal lobule (BA 40) and precuneus (BA 7)], anterior cingulate cortex (ACC) (BA 32), and several regions in the temporal cortex [such as the left middle temporal gyrus (BA 39), and left fusiform gyrus (BA 37)]. The left dorsolateral prefrontal cortex (BA 46) was related to selecting the loosely and remotely associated concepts and organizing them into creative ideas, whereas the ACC (BA 32) was related to observing and forming distant semantic associations in performing DTTs. The posterior parietal cortex may be involved in the semantic information related to the retrieval and buffering of the formed creative ideas, and several regions in the temporal cortex may be related to the stored long‐term memory. In addition, the ALE results of the structural studies showed that divergent thinking was related to the dopaminergic system (e.g., left caudate and claustrum). Based on the ALE results, both fMRI and structural MRI studies could uncover the neural basis of divergent thinking from different aspects (e.g., specific cognitive processing and stable individual difference of cognitive capability). Hum Brain Mapp 36:2703–2718, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: creativity, divergent thinking, activation likelihood estimation, MRI studies
INTRODUCTION
Creativity is the foundation of human civilizations, and all progress and innovation depend on the ability of humans to create new things. Thus, finding the mechanism of creativity in various fields, such as psychology, education, economics, and industry, is important [Fink et al., 2014]. Previous studies suggested that creative people are characterized by their ability to generate ideas by fluency (produce large quantities of ideas), flexibility (produce different ideas), and originality (produce novel outputs) [Fink et al., 2009; Guilford, 1950]. Motivated by Guilford's definition of creative people, experimental investigations on creativity are mainly facilitated by divergent thinking tasks (DTTs). DTTs comprise an important domain in elucidating the neural mechanisms of creative thinking. With the recent development of neuroimaging techniques, DTTs have been widely used in investigating the neural basis of creative thinking.
Experimental Tasks in Neuroimaging Studies on Divergent Thinking
Several DTTs, such as alternative uses test (AUT) [Guilford, 1967], creative story or sentence generation tasks [Howard‐Jones et al., 2005; Shah et al., 2011], creative metaphors [Benedek et al., 2014b; Mashal et al., 2007], match problems and remote associates test [Dietrich and Kanso, 2010], and Torrance test of creative thinking [Dietrich and Kanso, 2010; Fink et al., 2010], are widely used to investigate the neural basis of creativity [Dietrich and Kanso, 2010]. The match problems [Goel and Vartanian, 2005] and remote associates test [Jung‐Beeman et al., 2004; Kounios et al., 2004; Subramaniam et al., 2009] are used to investigate the neural basis of insight problem solving and mainly present one effective solution. Divergent thinking is often regarded as the ability to generate multiple original solutions to an open‐ended problem [Guilford, 1967]. In completing DTTs, subjects should simultaneously attend to numerous things (defocused attention) and form distant and unusual associations between different semantic concepts [Fink et al., 2009], or expand the concept of an object to generate novel and original ideas [Abraham et al., 2013]. In this study, the DTTs mainly refer to semantic divergent tasks, such as AUT, creative story generation, and novel metaphor task.
AUT
AUT is a widely used prototypical example of divergent thinking and well‐validated measure of creativity [Fink et al., 2010; Jung et al., 2010b; Kühn et al., 2014]. In classic AUT, subjects are instructed to generate alternative uses of conventional everyday objects, such as bricks or newspapers. Fluency, flexibility, and originality are investigated using AUT. To investigate the neural basis of divergent thinking, the uses of the objects test are considered control tasks (CTs), in which the subjects are asked to list as many common uses of a given object as possible [Kühn et al., 2014]. In addition, other studies used the object characteristics task (OCT) as CTs, in which the subjects are asked to name typical attributes of conventional objects [Fink et al., 2009, 2010].
Sentence or story generation task
In creative sentence or story generation task, the subjects are asked to generate a story or sentence based on a set of words [Bechtereva et al., 2004, 2000, Howard‐Jones, 2005]. Similar to AUT, to generate a creative sentence or story, semantic divergence is required to obtain contextual elements that are associated with a set of words, and find additional relationships or select combinations that contribute to the objective [Howard‐Jones, 2005].
Novel metaphor task
A metaphor is a figure of speech that describes a subject by asserting that it is, at some point of comparison, similar to an unrelated object. Benedek et al. [2014b] suggested that “metaphor comprehension involves forming an abstract connection between two concepts in semantic memory” and the connection is formed by extracting and relating similar properties of different concepts [Glucksberg, 2001, 2003]. For example, the metaphor “the close friends were a bag of toffees” involves the identification of the conceptual category “something that is sweet” extraction of related properties of close friends and toffee, and simultaneous inhibition of their unrelated properties. Therefore, semantic divergence, such as verbal fluency, is believed to be required for metaphor comprehension, as suggested by Beaty and Silvia [2013].
Based on the aforementioned discussion, AUT, creative story generation, and novel metaphor tasks are possibly open‐ended problems that require several common cognitive processes, such as semantic processing, defocused attention, as well as distant and unusual associations [e.g., Abraham et al., 2012; Fink et al., 2009, 2010, 2012; Howard‐Jones et al., 2005; Kleibeuker et al., 2013]. Therefore, discovering the brain basis of divergent thinking by integrating the functional magnetic resonance imaging (fMRI) results from these DTTs could be important and effective.
Neural Basis of Divergent Thinking
fMRI studies on divergent thinking
Prefrontal cortex is critical for creativity using DTTs [Abraham et al., 2012; Kleibeuker et al., 2013; Mashal et al., 2007]. Several studies have determined that the dorsolateral prefrontal cortex (DLPFC) and the ventrolateral prefrontal cortex (VLPFC) are involved in the comprehension of novel metaphors and generation of creative uses of common objects [Abraham et al., 2012; Mashal et al., 2007]. However, using AUT as experimental tasks, Kleibeuker et al. [2013] found that DLPFC (BA 6 or/and BA 9) is involved in generating creative ideas, whereas VLPFC is not. By comparing AU (completing AUT) and OC (completing OCT), Fink et al. [2009, 2010] discovered that the prefrontal cortex is not associated with generating creative uses [Fink et al., 2009]. However, the comparison of AU and fixation revealed that the left inferior frontal gyrus is involved in the creative idea generation in DTTs. In addition, several studies have indicated that the anterior cingulate cortex (ACC) is involved in completing DTTs [Abraham et al., 2012; Fink et al., 2009; Howard‐Jones et al., 2005; Kleibeuker et al., 2013].
Apart from the numerous studies that have emphasized the prominent role of the prefrontal cortex in divergent thinking, several studies have determined that a few regions of the parietal cortex, such as the left inferior parietal lobule (IPL) [Abraham et al., 2012; Benedek et al., 2014a; Fink et al., 2010], left supramarginal gyrus (SMG), and right angular gyrus [Fink et al., 2010], are involved in completing DTTs. In addition, activities in several regions of the temporal and occipital cortices, such as the left middle temporal gyrus (MTG) [Bechtereva et al., 2004; Fink et al., 2009; Mashal et al., 2007] and left fusiform gyrus (FG) [Abraham et al., 2012; Fink et al., 2012], are observed when participants perform DTTs.
Structural MRI studies on divergent thinking
Kanai and Rees [2011] suggested that “in the neuroscience of human behavior and cognition, interindividual differences are often treated as a source of ‘noise’ and therefore discarded through averaging data from a group of participants.” Creative individuals have been argued to possess several innate traits different from those of lower/noncreative individuals, such as defocused attention [Mendelsohn, 1976] and flat associative hierarchies [Mednick, 1962]. Moreover, structural differences may reveal a few innate traits or abilities of creative individuals [Fink et al., 2014]. Interindividual variability in divergent thinking has been recently predicted from the structures of gray [Fink et al., 2014; Jung et al., 2010b; Kühn et al., 2014] and white matter [Jung et al., 2010a; Takeuchi et al., 2010b] using structural MRI. Previous studies on brain structure determined that many regions in the posterior brain, such as the right cuneus [Jung et al., 2010b; Fink et al., 2014] and inferior parietal gyrus (IPG, BA 19) [Jung et al., 2010b], are associated with divergent thinking. Therefore, we speculated that these brain regions observed in the structural MRI studies on divergent thinking may be related to several innate traits of creative individuals.
Hypotheses of this Study
Although previous reviews [Arden et al. 2010; Dietrich and Kanso, 2010] have summarized the brain activity patterns related to creativity, an integrated understanding of the information is difficult to obtain because of the diverse tasks used in creativity studies. Activation likelihood estimation (ALE) is a foci‐based meta‐analysis technique and can estimate the likelihood of brain activations across multiple studies [Eickhoff et al., 2009; Laird et al., 2005; Turkeltaub et al., 2002]. Thus, a significant convergence between activation foci from different experiments (contrast between experiment condition) may be observed using ALE [Herz et al., 2014; Krall et al., 2014].
Based on previous studies, we hypothesized that the brain networks of creative idea generation in DTTs may be composed of the lateral prefrontal cortices (LPFC), such as DLPFC and VLPFC [Abraham et al., 2012; Kleibeuker et al., 2013; Mashal et al., 2007], ACC [e.g., Abraham et al., 2012; Fink et al., 2009; Howard‐Jones et al., 2005; Kleibeuker et al., 2013], the posterior parietal regions [e.g., Fink et al., 2009, 2010, 2012], and the temporal and the occipital cortex [e.g., Bechtereva et al., 2004; Fink et al., 2009; Mashal et al., 2007].
First, numerous studies have demonstrated the involvement of semantic knowledge in generating creative ideas on divergent thinking [Fink et al., 2009; Howard‐Jones et al., 2005]. Distributed networks in the brain represent semantic knowledge [Broadbent, 1879; Lissauer and Jackson, 1988]. Previous studies [Demonet et al., 1992; Martin and Chao, 2001] also indicated that the semantic brain regions are distributed in the ventral and lateral temporal cortices (VTC and LTC, respectively), and the activation of these regions is affected by object categories [Martin and Chao, 2001]. Binder [2009] concluded that seven brain regions are associated with semantic processes, which refers to the cognitive act of accessing stored semantic knowledge about the world. These regions include the posterior IPL, LTC, VTC, dorsomedial prefrontal cortex, inferior frontal gyrus (IFG), ventromedial prefrontal cortex, and posterior cingulate gyrus (CG). Thus, several brain regions that are associated with semantic processing, such as the left IPL, left IFG, left VTC, and LTC, could be observed in the present ALE study.
Second, as Fink had explained, creative idea generation requires more internal cognitive demands, and the inhibition of irrelevant cognitive processes ensures that creative idea generation is not disturbed by irrelevant information [e.g., Fink et al., 2009, 2010, 2012]. Therefore, several brain regions associated with cognitive control and attentional networks, such as ACC [Botvinick et al., 2004; Kerns et al., 2004; Weissman et al., 2003], right DLPFC [Dietrich, 2004; Fox et al., 2006], and right posterior parietal regions [Posner and Petersen, 1989; Cabeza and Nyberg, 2000; Fink et al. 2009, 2010, 2012], could be observed in the present ALE study.
Third, based on previous structural studies [Fink et al., 2014; Jung et al., 2010a, 2010b; Takeuchi et al., 2010a, 2010b], we speculated that a few innate traits of creative individuals may be observed through ALE analysis of the structural studies in the divergent thinking.
METHOD
Selection of the Studies
We searched the PNAS, PLOS, MITPress, SAGE, Oxford Press Wiley, Elsevier Science, and Springer databases using the following keywords: creative, creativity, insight, innovation, drawing, music, art, divergent thinking, problem solving, fMRI, MRI, positron emission tomography (PET), neural correlations, and imaging.
A total of 34 published fMRI and PET studies on the neural basis of creativity were selected based on the following requirements: all the subjects in the study, which used MRI techniques, were healthy adults; the coordinates in each of the studies were in the standard Montreal Neurological Institute or Talairach space; and all the reported activation coordinates were based on the entire brain and obtained by comparing experimental tasks (i.e., DTTs) with CTs.
Based on the definition of divergent thinking [Guilford, 1950; Fink et al., 2009] and previous studies [Dietrich and Kanso, 2010; Fink et al., 2010; Howard‐Jones et al., 2005; Mashal et al., 2007], 17 published studies (10 fMRI and 7 structural studies; Table 1) were selected for the meta‐analysis of divergent thinking. Moreover, seven studies (Table 1), which included the results of DTTs > CTs and CTs > DTTs, were selected from the 11 fMRI studies for the meta‐analysis to obtain the brain images of deactivation under DTTs than CTs.
Table 1.
The MRI studies (fMRI and structural MRI studies) selected in ALE meta‐analysis of divergent thinking
| fMRI Studies | Scanner | Type of MRI | Sample | F/M | Age | DTTs | CTs | Time of DTT |
|---|---|---|---|---|---|---|---|---|
| Abraham et al. [2012] | 1.5‐T MRI | fMRI | 29 | 11/18 | 19–29 | AUT | OLT | 20 s |
| Benedek et al. [2014a]* | 3.0‐T MRI | fMRI | 35 | 24/11 | 18–29 | New ideas of AUT | Old ideas of AUT | 60 s |
| Benedek et al. [2014b] | 3.0‐T MRI | fMRI | 28 | 18/10 | 19–49 | NPT | LCT | 10 s |
| Fink et al [2009]* | 3.0‐T MRI | fMRI | 21 | 11/10 | 20–32 | AUT | OCTs | 20 s |
| Fink et al [2010]* | 3.0‐T MRI | fMRI | 31 | 18/13 | 19–29 | AUT | OCTs | 21 s |
| Fink et al [2011]* | 3.0‐T MRI | fMRI | 24 | 14/10 | 21–30 | AUT | AUT | 12 s |
| Howard‐Jones et al. [2005]* | 1.5‐T MRI | fMRI | 8 | 7/1 | 19–28 | CSG | UCSG | 10 s |
| Kleibeuker et al [2013]* | 3.0‐T MRI1.5‐T MRI | fMRI | adolescents:19 | 10/9 | 15–17 | AUT | OCTs | 15 s |
| adults:24 | 12/12 | 25–30 | ||||||
| Mashal et al [2007] | 3.0‐T MRI | fMRI | 15 | 7/8 | 21–31 | NMT | CMT | 15 s |
| Shah et al. [2011] | 1.5‐T MRI | fMRI | 28 | 14/14 | 24.0 ± 1.9 | CWT | Copying | 140 s |
| Structural studies | ||||||||
| Fink et al. [2014] | 3.0‐T MRI | Gray matter | 71 | 44/27 | 24.5 ± 44.9 | BIS; AUT | 2‐2.5 min for BIS; 2 min for AUT | |
| Jung et al. [2010b] | 3.0‐T MRI | Gray matter | 72 | 32/40 | 22.1±2.9 | 3 DTTs: Free condition of DFT; Four Line Condition of the DFT; AUT | 5 min for DFT; 4 min for FLC; 1 min for AUT | |
| Jung et al. [2010a] | 3.0‐T MRI | Whit matter | 61 | 28/33 | 23.7± 4.2 | 3 DTTs: Verbal and Drawing Creativity Tasks; AUT; generation of captions | 5 min for each task of the 3 DTTs | |
| Kühn et al. [2014] | 3.0‐T MRI | Gray matter | 21 | 16/5 | 21.3±1.85 | AUT | 2 min | |
| Takeuchi et al. [2010a] | 3.0‐T MRI | Gray matter | 55 | 13/42 | 21.7±1.44 | S‐A creativity test | 5 min for each task | |
| Takeuchi et al. [2010b] | 3.0‐T MRI | Whit matter | 55 | 13/42 | 21.7±1.44 | S‐A creativity test | 5 min for each task | |
| Zhu et al. [2013] | 3.0‐T MRI | Gray matter | 285 | 155/130 | F: 20.1 ± 1.2M: 19.68 ± 1.01 | Verbal TTCT | 45 min | |
F, female; M, male; DTTs, divergent thinking tasks; CTs, Control tasks.
AUTs, Alternate Uses tasks; in which subjects were asked to generate unusual/original uses of conventional everyday objects; New ideas of AUT = the generated ideas were newly created; Old ideas of AUT = the generated creative ideas were retrieved from memory; OCTs, Object Characteristics Tasks; in which participants had to think of typical characteristics of conventional everyday objects; OLT, Object Location Task; in which a word of an object was presented with an location (e.g., Objects ‐> Office) and the participants were asked to generated a different object in the location; NPT, Novel Production Task; in which a short phrases relating a noun to an adjective in parentheses were presented and participants were asked to produce a creative metaphor that conveys the meaning of the adjective; LCT, literal control task; in which a short phrases relating a noun to an adjective in parentheses were presented and participants were asked to produce a synonym that conveys the meaning of the adjective; CSG, Creative Story generation; in which a 3‐words list was presented and the participants were asked to generate a creative story including these 3 words; UCSG, Uncreative Story generation; in which a 3‐words list was presented and the participants were asked to generate a uncreative story including these 3 words; NMT, Novel Metaphors Tasks; in which the participants were asked to indicate whither the two words presented were related metaphorically; CMT, Conventional Metaphors Tasks; DFT, Design Fluency Test; in the Free Condition of the DFT, subjects were instructed to draw as many unique designs; in For the Four Line condition of DFT, they were constrained in drawing designs composed of certain types of lines; Time of DTT = the period of idea generation; S‐A creativity test is similar to TTCT and has three problems: improve a product, find interesting and unusual uses for a certain object and list all the consequences should an improbable situation occur
The studies marked “*” including the contrasts of DTTs > CTs and CTs > DTTs.
ALE Technique
ALE is a common method to integrate neuroimaging results across studies [Laird et al., 2005; Turkeltaub et al., 2002]. In ALE, the significantly reported coordinates, namely, the active foci, were treated not as single points but as centers for 3D Gaussian probability distributions that reveal the spatial uncertainty associated with neuroimaging results [Spaniol et al., 2009; Turkeltaub et al., 2002]. Thereafter, the probability distributions of each experiment were combined into a modeled activation (MA) map using the recently proposed approach that prevents undue summation between foci [Turkeltaub et al., 2012]. The ALE scores were then calculated by considering the voxel‐wise union based on these individual MA maps. The likelihood of activation for each standard‐space voxel was calculated under a null distribution of spatial independence [Fitzgerald et al., 2008; Sabatinelli et al., 2011]. For statistical inference, the ALE results were assessed against a null distribution of random spatial association between experiments, which was conducted using a recently proposed analytical method [Eickhoff et al., 2012].
ALE Analysis of the Neuroimaging Studies
Meta‐analysis was performed using the revised version ([Eickhoff et al., 2009, 2012] of the ALE approach using GingerALE 2.1 software (http://brainmap.org/). In the ALE analysis of single datasets, COIs of fMRI studies on DTTs > CTs (17 contrast and 85 foci; Table 2) and CTs > DTTs (7 contrast and 30 foci; Table 3), as well as on structural studies (15 contrast and 67 foci; Table 4), were input separately. To obtain the optimal brain patterns of divergent thinking, the threshold of the results was set to a false discovery rate‐corrected P < 0.05 with a minimum volume of 100 mm3. The results were viewed using Mango software (http://ric.uthscsa.edu/mango/) and overlaid to a standard space using the Talairach file (Colin1.1.nii) (http://www.brainmap.org/ale). In the conjunction analysis, we compared the ALE results of functional studies and the ALE results of structural studies. Krall et al. (2014) argued that “the usage of peak coordinates might lead to mis‐ or over‐interpretation of the results in ALE analysis.” Thus, in this study, we reported the peak coordinates of the significant cluster and demonstrated the brain regions nearest the peak coordinates within ±5 mm.
Table 2.
The COIs of fMRI studies selected in ALE meta‐ analysis of divergent thinking (DTTs > CTs)
| Studies | Conditions | COI | Foci | Response | Ideas evaluation |
|---|---|---|---|---|---|
| Abraham et al. [2012] |
Divergent condition DivH: completing AUT DivL: completing Object‐Location task Control condition ConH: completing 2‐back working memory Task ConL: completing 1‐back working memory Task |
DivH > DivL (inclusive mask: DivH > ConH) | 15 | Button Presses: idea generation and response periods were not separated | The generated ideas not evaluated |
| Benedek et al. [2014a] |
OLD: generating idea retrieved from memory NEW: the newly created resulting idea |
NEW > OLD | 1 | Verbal Report: idea generation and response periods were not separated | The generated ideas evaluated according to both originality and appropriateness |
| Benedek et al. [2014b] |
METAPHOR: completing a NPT LITERAL: completing a LCT |
METAPHOR > LITERAL | 7 | Verbal Report: idea generation and response periods were separated | The generated ideas evaluated according to remoteness, novelty, and cleverness |
| Fink et al. [2009] |
AU: Completing AUT OC: Completing Object Characteristics Task |
AU > OC | 1 | Verbal Report: idea generation and response periods were not separated | the generated ideas evaluated according novelty and plausibility |
| Fink et al. [2010] |
AU and OC same with Fink et al. (2009) AUinc: reflecting on ideas or responses they gave during AUT AUstim: creative idea generation stimulated by external ideas |
AU > OC AU > AUinc AUinc > AU AUstim > AU |
1 2 1 3 |
Verbal Report: idea generation and response periods were separated | the generated ideas not evaluated |
| Fink et al. [2012] |
Orig: generating an unusual/original us of an objects stimulated by original usages generated by other people Comm: generating an unusual/original us of an objects stimulated by common usages generated by other people Contr: generating an unusual/original us of an objects stimulated by meaningless words |
Orig > Contr Orig > Comm |
1 3 |
Verbal Report: idea generation and response periods were separated | The generated ideas evaluated according to originality |
| Howard‐Jones et al. [2005] |
Creative Condition (CC): generate creative stories using word list Uncreative Condition (UC): generate uncreative stories using word list Related Condition (RC): the words in the word list being related each other Unrelated Condition (UC): the words in the word list not being related each other |
CC > UC CC > UC with UC > RC |
4 1 |
Verbal Report: idea generation and response periods were separated | the generated ideas evaluated according to originality |
| Kleibeuker et al. [2013] | AU & OC | AU > OC | 16 | Verbal Report: idea generation and response periods were separated | The generated ideas evaluated according to creativity |
| Mashal et al. [2007] |
NM: completing the NMT CM: completing the NMT UR: unrelated word pairs L: Literal expressions |
NM > CM NM > L NM > UR |
3 5 15 |
Silently decided whether the two words metaphorically related, literally related, or unrelated | The generated ideas not evaluated |
| Shah et al. [2011] | creative writing & copying | creative writing > copying | 6 | Physically writing idea generation and response periods were not separated | The generated story evaluated according to creativity |
Table 3.
The COIs of fMRI studies selected in ALE meta‐analysis of divergent thinking (CTs > CTTs)
| Studies | Conditions | CTs > DTTs | Foci |
|---|---|---|---|
| Benedek et al. [2014a] |
OLD: resulting idea retrieved from memory NEW: the newly created resulting idea |
OLD & NEW < 0 | 2 |
| Fink et al. [2009] |
AU: Completing AUT OC: Completing Object Characteristics Task |
OC > AU | 1 |
| Fink et al. [2010] |
AU and OC same with Fink et al. (2009) AUinc: reflecting on ideas or responses they gave during AUT AUstim: creative idea generation stimulated by external ideas |
OC > AU | 2 |
| Fink et al. [2012] |
Orig: object name presented with original answers of this object Comm: object name presented with common answers of this object Contr: object name presented with meaningless words |
Contr > Comm Contr > Orig |
2 2 |
| Howard‐Jones et al. [2005] |
Creative Condition (CC): indication to generate creative stories Uncreative Condition (UC): indication to generate uncreative stories Related Condition (RC): the words in the list related Unrelated Condition (URC): the words in the list not related |
UC > CC | 3 |
| Kleibeuker et al. [2013] | AU & OC | OC > AU | 18 |
Table 4.
The COIs of structural studies selected in ALE meta‐ analysis of divergent thinking
| Studies | Conditions | COI | Foci |
|---|---|---|---|
| Fink et al. [2014] | BIS fluency/flexibility | BIS fluency/flexibility positive association with gray matter density | 2 |
| AU originality | AU originality positive association with gray matter density | 2 | |
| BIS ∩ AU | 1 | ||
| Jung et al. [2010a] |
CCI: composite creativity index obtained by averaging the scores of four divergent thinking tasks FA: fractional anisotropy AD: Axial Diffusivity RD: Radial Diffusivity |
CCI correlated to FA | 2 |
| CCI correlated to RD | 3 | ||
| CCI correlated to AD | 2 | ||
| Jung et al. [2010b] | CCI: composite creativity index combined the scores of fluency, flexibility and uniqueness of AUT | negative correlation between cortical thickness and CCI | 5 |
| positive correlation between cortical thickness and CCI | 1 | ||
| Kühn et al. [2014] |
Average Creativity of AUT Cognitive Flexibility of AUT Average Uniqueness of AUT |
Positive Correlation between Gray Matter Volume and Cognitive Flexibility | 5 |
| Positive Correlation between Gray Matter Volume and Average Uniqueness | 5 | ||
| Positive Correlation between Gray Matter Volume and Average Creativity | 4 | ||
| Takeuchi et al. [2010a] |
S‐A creativity test rGMV: regional gray matter volume |
positive correlations between rGMV and S‐A creativity test score | 6 |
| Takeuchi et al. [2010b] |
S‐A creativity test FA |
correlations between FA values and the scores from the S‐A creativity test | 25 |
| Zhu et al. [2013] |
Verbal TTCT rGMV: regional gray matter volume rGWV: regional whit matter volume |
rGMV correlated with verbal creativity test score | 2 |
| rWMV correlated with verbal creativity test score | 2 |
RESULTS
Seventeen MRI publications of divergent thinking (10 for fMRI studies; 7 for structural MRI studies) with an average sample size of 27.27 ± 10.06 (153 females; 147 males) for fMRI studies and 88.57 ± 88.26 (301 females and 313 male) for structural MRI studies were included in the present ALE analysis. The ages of the subjects in the selected publications mainly ranged from 18 to 30.
As shown in Table 2, although the generated ideas were evaluated based on originality, appropriateness, novelty, creativity, or not evaluated, the instruction of each study almost required subjects to generate as many creative (original) ideas as possible. Thus, we thought that these brain functional patterns associated with DTs might be effective for ALE analysis by comparing the divergent thinking condition to the control condition.
ALE Results of Active Regions in DTTs
Table 4 and Figure 1 show the ALE results of fMRI studies on divergent thinking. Six clusters in the left hemisphere were more active under DTTs than those under CTs. The peak ALE values of the first cluster were located in IPL (BA 40) [the cluster coordinates are from (−62, −38, 28) to (−48, −28, 38)] and the main brain regions in the cluster included IPL (BA 40) and SMG (BA 40). The peak ALE values of the second cluster were located in the amygdala [the cluster coordinates are from (−30, −18, −18) to (−20, −8, −8)], and the main brain regions in the cluster included the hippocampus, amygdala, lateral globus pallidus, and parahippocampal (BA 28). The peak ALE values of the third cluster were located in the left FG (BA 37) [the cluster coordinates are from (−52, −64, −16) to (−42, −54, −2)], and the main brain regions in the cluster included FG (BA 37), the middle occipital gyrus (BA 19, BA 20, and BA 37), and the inferior temporal gyrus (BA 19 and BA 37). The peak ALE values of the fourth cluster were located in the middle frontal gyrus (MFG, BA 46) [the cluster coordinates are from (−48, 28, 12) to (−42, 36, 24)], and the main brain regions in the cluster included MFG (BA 46) and the inferior frontal gyrus (BA 46). The peak ALE values of the fifth cluster were located in the precentral gyrus (BA 6) [the cluster coordinates are from (−52, 0, 20) to (−44, 14, 26)], and the main brain regions in the cluster included MFG (BA 9) and the IFG (BA 9 and BA 44). The peak ALE values of the sixth cluster were located in the MTG (BA 39) [the cluster coordinates are from (−46, −74, 18) to (−44, −66, 24)], and the main brain regions in the cluster included MTG (BA 39).
Figure 1.
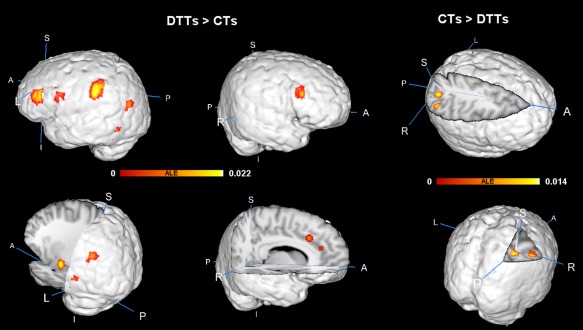
Brain regions showing significant activation likelihood across fMRI studies of DTTs versus CTs in ALE meta‐analysis (FDR‐corrected P < 0.05 with 100 mm3 cluster volume); ALE clusters were overlaid on the standard space using the Talairach file (Colin1.1.nii). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Three other clusters were located in the right hemisphere. The peak ALE values of the first cluster were located in the CG (BA 32) [the cluster coordinates are from (−4, 18, 26) to (10, 26, 36)], and the main brain regions in the cluster included CG (BA 32 and BA 24). The peak ALE values of the second cluster were observed in MFG (BA 46) [the cluster coordinates are from (46, 18, 20) to (50, 24, 26)], and the main brain regions in the cluster included MFG (BA 46) and IFG (BA 45). The peak ALE values of the third cluster were observed in ACC (BA 32) [the cluster coordinates are from (10, 32, 18) to (16, 38, 22)], and the main brain regions in the cluster included ACC (BA 32) and the medial frontal gyrus (BA 9).
ALE Results of Deactivated Regions Under DTTs
Table 4 and Figure 2 show the ALE results of fMRI studies on divergent thinking. Two clusters in the right hemisphere were more deactivated under DTTs than those under CTs. The peak ALE values of the first cluster were located in the precuneus (BA 19) [the cluster coordinates are from (26, −66, 40) to (34, −58, 46)], and the main brain regions in the cluster included the superior parietal lobule (SPL, BA 7), precuneus (BA 19), and inferior parietal lobule (IPL, BA 39). The peak ALE values of the second cluster were located in IPL (BA 40) [the cluster coordinates are from (44, −56, 36) to (52, −52, 44)], and the main brain regions in the cluster included IPL (BA 40).
Figure 2.
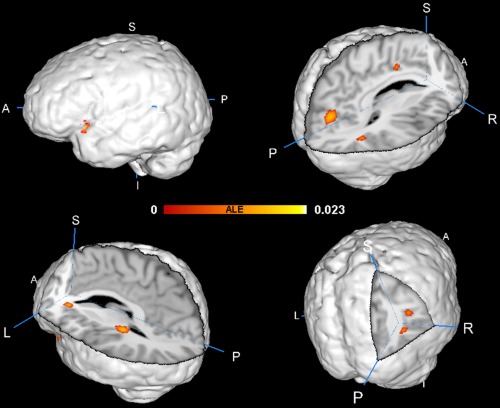
Brain regions showing significant activation likelihood across structural studies of DTTs in ALE meta‐analysis (FDR‐corrected P < 0.05 with 100 mm3 cluster volume); ALE clusters were overlaid on the standard space using the Talairach file (Colin1.1.nii). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
ALE Results of Structural Studies on Divergent Thinking
Table 5 and Figure 2 show the ALE results of structural studies. Three clusters were located in the left hemisphere. The peak ALE values of the first cluster were located in the insula of the prefrontal cortex (BA 13) [the cluster coordinates are from (−46, 6, −8) to (−36, 14, 2)], and the main brain regions in the cluster included the insula (BA 13), IFG (BA 47), and superior temporal gyrus (BA 38 and BA 22)]. The peak ALE values of the second cluster were located in the caudate tail [the cluster coordinates are from (−30, −38, 6) to (−22, −28, 14)], and the main brain regions in the cluster included the caudate tail and pulvinar. The peak ALE values of the third cluster were located in the claustrum [the cluster coordinates are from (−26, 22, 4) to (−18, 28, 10)], and the main brain regions in the cluster included the claustrum and caudate head.
Table 5.
The brain regions showing significant activation likelihood across fMRI studies of DTTs in ALE meta‐analysis (FDR‐corrected P < 0.05 with 100 mm3 cluster volume)
| Cluster | Volume (mm3) | Extrema value | x | y | z | Brain regions |
|---|---|---|---|---|---|---|
| DTTs > CC | ||||||
| 1 | 1064 | 0.022 | −56 | −32 | 34 | Left Inferior Parietal Lobule (BA 40) |
| 2 | 840 | 0.022 | −24 | −12 | −12 | Left Amygdala |
| 3 | 688 | 0.012 | 6 | 22 | 32 | Right Cingulate Cortex (BA 32) |
| 4 | 640 | 0.016 | −46 | −56 | −10 | Left Fusiform Gyrus (BA 37) |
| 5 | 640 | 0.016 | −46 | 32 | 18 | Left middle frontal gyrus (BA 46) |
| 6 | 264 | 0.012 | −48 | 2 | 26 | Left Precentral Gyrus (BA 6) |
| 7 | 248 | 0.010 | 48 | 22 | 22 | Right middle frontal gyrus (BA 46) |
| 8 | 184 | 0.010 | 48 | 22 | 22 | Right Anterior Cingulate (BA 32) |
| 9 | 168 | 0.010 | −46 | −68 | 20 | Left Middle Temporal Gyrus (BA 39) |
| CTs > DTTs | ||||||
| 1 | 328 | 0.016 | 30 | −62 | 42 | Right Precuneus (BA 19) |
| 2 | 296 | 0.015 | 46 | −54 | 42 | Right Inferior Parietal Lobule (BA 40) |
Coordinates of the local maxima in Talairach space are given
ALE clusters were overlaid to the standard space using the Talairach file (Colin1.1.nii).
BA, Brodmann area.
Four other clusters were located in the right hemisphere. The peak ALE values of the first cluster were located in the cuneus (BA 18) [the cluster coordinates are from (2, −78, 14) to (16, −68, 24)], and the main brain regions in the cluster included the cuneus (BA 17 and BA 18) and the precuneus (BA 31). The peak ALE values of the second cluster were located in the MTG (BA 39) [the cluster coordinates are from (42, −58, 20) to (46, −50, 28)], and the main brain regions in the cluster included MTG (BA 39) and STG (BA 39). The peak ALE values of the second cluster were located in CG (BA 24) [the cluster coordinates are from (6, −6, 36) to (16, −2, 40)], and the main brain regions in the cluster included CG (BA 24). The peak ALE values of the fourth cluster were located in the MTG and middle occipital gyrus (MOG, BA 19) [the cluster coordinates are from (36, −66, 8) to (40, −62, 14)], and the main brain regions in the cluster included MOG (BA 19) and MTG (BA 39 and BA 37) Table 6.
Table 6.
The brain regions showing significant activation likelihood across structural studies of DTTs in ALE meta‐analysis (FDR‐corrected P < 0.05 with 100 mm3 cluster volume);
| Cluster | Volume (mm3) | ALE value | x | y | z | Brain regions |
|---|---|---|---|---|---|---|
| 1 | 792 | 0.018 | 6 | −74 | 18 | Right Cuneus (BA 18) |
| 0.010 | 14 | −68 | 20 | Right Cuneus (BA 18) | ||
| 2 | 736 | 0.023 | −42 | 10 | −4 | Left Insula (BA 13) |
| 3 | 528 | 0.017 | −26 | −34 | 10 | Left Caudate Tail |
| 4 | 416 | 0.019 | −22 | 26 | 8 | Left Claustrum |
| 5 | 176 | 0.011 | 44 | −56 | 22 | Right Middle Temporal Gyrus (BA 39) |
| 0.011 | 44 | −52 | 26 | Right Superior Temporal Gyrus (BA 39) | ||
| 6 | 176 | 0.011 | 8 | −4 | 38 | Right Cingulate Gyrus (BA 24) |
| 0.011 | 14 | −4 | 38 | Right Cingulate Gyrus (BA 24) | ||
| 7 | 136 | 0.011 | 38 | −64 | 10 | Right Middle Occipital Gyrus (BA 19) |
ALE clusters were overlaid to the standard space using the Talairach file (Colin1.1.nii).
Coordinates of the local maxima in Talairach space are given.
BA, Brodmann area.
Furthermore, the results of the conjunction analysis indicated that no common region existed between the ALE results of DTTs > CTs and the results of the structural studies.
DISCUSSION
In this study, an ALE meta‐analysis was conducted to investigate the integrated brain patterns of divergent thinking. Based on our criteria, 17 studies (10 fMRI studies and 7 structural studies) on divergent thinking were selected. The ALE results of the fMRI studies showed that the bilateral DLPFC (BA 46), the right ACC (BA 32), the posterior parietal cortex (BA 7 and BA 40), and the left FG (BA 37) and MTG (BA 39) were involved in the creative idea generation. In addition, the ALE results of the structural studies indicated that several key brain regions associated with individual differences on divergent thinking, such as the right cuneus (BA 18), right MFG (BA 39), right SPG (BA 39), right MOG (BA 19), left caudate tail, and left claustrum, were mainly located in the right hemisphere. The implications of these key regions in divergent thinking are discussed in the following section.
Function of the Brain Regions in Divergent Thinking
Function of the frontal cortex in divergent thinking
Our meta‐analysis results showed that the bilateral DLPFC (BA 46), including MFG (BA 46) and IFG (BA 46), was more active under DTTs than that under CTs. Previous studies indicated that DLPFC is mainly related to the manipulation or observation of active information within working memory [Petrides, 1994; Owen et al., 1999; Carpenter et al., 2000]. In particular, Blumenfeld and Ranganath [2006, 2007] found that DLPFC is possibly involved in organization, such as the comparison or transformation of relationships among items that are active in the working memory [Wagner et al., 2001; Blumenfeld and Ranganath, 2006; Crone et al., 2006; Mohr et al., 2006] or chunking (organizing separate pieces of information into fewer units) [Bor et al., 2003, 2004; Miller et al., 1960]. Moreover, the lateral prefrontal cortex (DLPFC and VLPFC) had been suggested to have played important roles in the creative idea generation in DTTs, such as in semantic processing [Abraham et al., 2012; Fink et al., 2009], selective attention [Dietrich, 2004; Fink et al., 2009], cognitive flexibility (particularly switching between semantic categories or subcategories) [Hirshorn and Thompson‐Schill, 2006; Kleibeuker et al., 2013], sustained attention [Dietrich, 2004; Shah et al., 2011], and combining existing stored information [Dietrich, 2004; Kleibeuker et al., 2013]. Moreover, the lateral prefrontal cortex, such as the IFG, was sensitive to the influence of semantic distance or associative strength between concepts, such as weaker associative strength leading to a stronger BOLD response in these areas [Abraham et al., 2012; Bunge et al., 2005; Green et al., 2010]. Benedek et al. [2014a, b] further suggested that the “IFG involved in retrieving and selecting relevant remote associations, integration of loosely related semantic concepts, and eventually verbal elaboration of ideas.” Therefore, DLPFC (BA 46) may be involved in novel idea organization, such as organizing loosely or remotely related semantic concepts into creative ideas in working memory and retrieving and selecting relevant remote associations.
In addition, the right ACC (BA 32) was strongly activated under DTTs than that under CTs. The right ACC is related to numerous cognitive processes, such as monitoring conflict [Botvinick et al., 2004; Kerns et al., 2004; Weissman et al., 2003], generating creative ideas [Howard‐Jones et al., 2005], and evaluating [Ellamil et al., 2012]. Based on conflict monitoring theory [Botvinick et al., 2004; Kerns et al., 2004; Weissman et al. 2003], ACC is also related to the observation of competing responses, such as multiple associations or strategies involved in solving problems [Subramaniam et al., 2009]. Previous studies likewise indicated that ACC is involved in suppressing irrelevant thoughts [Anderson et al., 2004; Wyland et al., 2003] and the semantic processing of distant associations [Howard‐Jones et al., 2005; Seger et al., 2000]. Thus, our findings suggested that the right ACC (BA 32) may be involved in monitoring and forming distant semantic associations by suppressing irrelevant thoughts when performing DTTs.
Function of posterior parietal brain areas in divergent thinking
The ALE results showed that the left IPL (BA 40), including SMG (BA 40), was more active under DTTs than that under CTs. To a certain extent, many studies indicated that the left parietal regions in BA 40 play important roles in generating creative ideas [Abraham et al., 2012; Bechtereva et al., 2004; Fink et al., 2010]. In a recent study, Benedek et al. [2014a, b] suggested that the left IPL (BA 40) may be involved in episodic memory retrieval when generating new ideas. Other studies also indicated that IPL (BA 40) is related to the verbal generation of creative ideas [Pavlova and Romanenko, 1988; Bechtereva et al., 2004]. Binder et al. [2009] suggested that the posterior IPL is an important region for semantic processing. Moreover, the phonological loop store is related to the left posterior parietal cortices (BA 40), whereas the articulatory rehearsal process is related to the Broca's area (BA 6/44) [Baddeley, 2003; Jonides et al., 1996; Paulesu et al., 1993]. Therefore, we speculated that the stronger activation of the posterior parietal cortices (IPL, BA 40) may be related to the buffering of the relevant semantic information during divergent thinking.
In addition, the clusters exhibited peak values in the right precuneus (BA 19), including the right SPL (BA 7), and the right IPL (BA 40) demonstrated greater deactivation under DTTs than that under CTs. Posner and Petersen [1989] and Cabeza and Nyberg [2000] demonstrated the involvement of the right posterior parietal regions in attention. Cabeza et al. [2008] reported that the right posterior parietal regions are composed of the dorsal parietal cortex (DPC), including the intraparietal sulcus and SPL, and the ventral parietal cortex (VPC), including the supramarginal and angular gyri (also known as IPL). They argued that the DPC activity is related to preparatory top‐down attention (allocation of attentional resources to memory retrieval based on current goals), whereas the VPC activity reveals the capture of bottom‐up attention by the target (attention captured by the bottom‐up information that enters a working memory either from the senses or from long‐term memory) [Cabeza et al., 2008, 2011]. Therefore, as suggested by previous studies [Fink et al., 2009, 2010, 2012], the creative idea generation requires more internal processing demands, and the deactivation of the right SPL (BA 7) and IPL (BA 40) may be related to the inhibition of irrelevant cognitive processes (such as retrieval of prevalent, typical, or directly stimulus‐related information) under DTTs relative to CTs.
Function of the posterior temporal cortex in divergent thinking
Our results indicated that the posterior temporal cortex [left MTG (BA 39) and left FG (BA 37)] was related to divergent thinking. Objective concepts are suggested to be possibly represented by distributed networks in the brain [Broadbent, 1879; Lissauer and Jackson, 1988], and storage and retrieval partially depend on VTC and LTC [Gorno‐Tempini et al., 2004; Thompson‐Schill et al., 2005]. Moreover, Martin and Chao [2001] indicated that object naming and identifying are related to distributed brain regions, such as the left MTG and left FG. In addition, Knight and D'Esposito [2003] found that DLPFC is related to the maintenance operation or top‐down process that influences the individual external or internal milieu maintained by other posterior areas. In particular, the posterior cortical regions, such as the middle temporal cortex, are influenced by top‐down feedback information from the prefrontal cortex [Badre et al., 2005; Gold et al., 2006]. Therefore, the posterior temporal cortex [left MTG (BA 39) and left FG (BA 37)] and left DLPFC (BA 46) may be related to the generation of new ideas. We suggested that the posterior temporal cortex may be correlated with the activation of semantic items related to novel ideas, whereas the left DLPC (BA 46) may be involved in organizing these items into novel ideas.
Brain networks of divergent thinking based on ALE analyses of fMRI studies
As the parieto‐frontal integration theory [Jung and Haier, 2007] suggested, the brain network of the intelligence mainly includes the DLPFC (BAs 6, 9, 10, 45, 46, 47), IPL (BA 40 and BA 39), SPL (BA 7), and ACC (BA 32), as well as the regions within the temporal (BAs 21, 37) and occipital (BAs 18, 19) lobes. Based on our ALE results, we determined that the creative idea generation (DT) may have a few brain regions similar to intelligence, such as LPFC (BA 46), posterior parietal regions [IPL (BA 40) and SPL (BA 7)], ACC (BA 32), and posterior temporal cortex [MTG (BA 39) and FG (BA 37)]. Thus, we speculated that the brain networks of creative idea generation in DTTs may be also composed of these regions. Thereafter, we will discuss the brain networks associated with creativity based on the specific functions of each brain regions.
First, the left LPFC (BA 46), left IPL (BA 40), left MTG (BA 39), and left FG (BA 37) were associated with the semantic system [e.g., Binder et al., 2009; Gorno‐Tempini et al., 2004; Thompson‐Schill et al., 2005], which may be involved in creative idea generation. In particular, IFG (BA 46) was related to retrieving and selecting relevant remote association (e.g., Abraham et al., 2012; Benedek et al., 2014; Bunge et al., 2005; Green et al., 2010]; IPL (BA 40) may be involved in the buffering of the relevant semantic information in the working memory; and the left MTG (BA 39) and the left FG (BA 37) may be associated with the activated long‐term memory related to the creative idea generation. In addition, the left MFG (BA 46) may be related to organizing loosely or remotely related semantic concepts into creative ideas [e.g., Blumenfeld and Ranganath, 2006; Crone et al., 2006; Mohr et al., 2006; Wagner et al., 2001]. ACC (BA 32) is involved in monitoring and forming distant semantic associations by suppressing irrelevant thoughts [e.g., Anderson et al., 2004; Howard‐Jones et al., 2005; Seger et al., 2000; Wyland et al., 2003]. We speculated that the semantic system may play an important role in creative idea generation, including activating long‐term memory (left MTG), retrieving and selecting relevant remote association (IFG), organizing loosely or remotely related semantic concepts into creative ideas (left MFG), buffering of relevant semantic information (left IPL), and monitoring and forming distant semantic associations by suppressing irrelevant thoughts (right ACC).
Second, as Fink had explained, creative idea generation requires more internal cognitive demands, and the inhibition of irrelevant cognitive processes ensures that the creative idea generation is not disturbed by irrelevant information [e.g., Fink et al., 2009, 2010, 2012]. Therefore, we speculated that the deactivation of the right posterior parietal regions [IPL (BA 40) and SPL (BA 7)] may be associated with the inhibition of the irrelevant cognitive processes. Moreover, the posterior parietal regions (DPC and VPC) have direct anatomical connections with the prefrontal cortex, particularly DLPFC [Cavada and Goldman‐Rakic; 1989; Lewis and Essen, 2000; Petrides and Pandya, 1999]. MFG specifically contains intermixed neuronal populations connected with both DPC and VPC [Fox et al., 2006], and the activation of the right DPC and VPC is related to the spontaneous activity in the right MFG. Dietrich [2004] also argued that the right DLPFC is involved in the sustained attention, which is required by the processes involved in creative idea generation. Hence, our results indicated that the right frontal‐parieto brain network (e.g., right MFG and DLPFC‐right IPL and SPL) may be highly important for divergent thinking. In particular, we speculated that the right MFG (BA 46) may be related to focusing an individual's attention to the processes involved in creative idea generation, while interacting with the posterior parietal regions involved in the inhibition of the irrelevant cognitive processes.
Structural Studies on Divergent Thinking
Creative individuals had been argued to possess several innate traits different from the lower/noncreative individuals, such as defocused attention [Mendelsohn, 1976] and flat associative hierarchies [Mednick, 1962]. Moreover, DTTs are the widely used creative tests in the difference of creative individuals. We speculated that the structural studies of individual difference in divergent thinking may uncover several brain mechanisms of creativity.
First, as our ALE results indicated, numerous brain regions in TOP, such as the right cuneus (BA 18), right MTG (BA 39), and right MOG (BA 19), were related to the individuals’ differences in divergent thinking. Fink et al. [2014] showed that the gray matter density of the right cuneus (BA 18) is positively related to BIS fluency, as well as the flexibility and originality of AUT. Jung et al. [2010b] also demonstrated that the cortical thickness of the right cuneus (BA 18) and angular (BA 39) is negatively correlated with divergent thinking. The lower cortical thickness makes information flow more efficiently among the brain areas [Jung et al., 2010b], and higher gray matter density results in less energy use when the area is used for specific cognitive tasks [Haier et al., 2004]. In 2004, Dietrich argued that TOP may be devoted primarily to perception and long‐term memory. In addition, the visual cortices, the lingual gyrus, and the cuneus may be involved in the processing of the verbal stimuli in the performance of a word association task during visual mental imagery [Andreasen and Ramchandran; 2012; Kosslyn et al., 2001]. Therefore, we speculated that the individual difference of TOP may reveal that higher creative individuals have several innate traits in long‐term memory, perception, and mental imagery.
In addition, as shown in Table 4, the left caudate tail and left claustrum (the basal ganglia) were related to divergent thinking. The basal ganglia have been suggested to contain a high density of dopamine receptors, which are believed to be related to working memory [Goldman‐Rakic, 1994; McNab and Klingberg, 2008]. By disinhibiting the thalamocortical loops, the basal ganglia regulate the selective gating mechanism [Gruber et al., 2006]. Weinberger and Laruelle [2001] showed that a significant elevation in dopamine D2 receptors in the striatum is associated with schizophrenia. Moreover, previous studies suggested that schizophrenia is related to certain aspects of creativity, such as the lack of constraints and inhibition in their thinking, which resulted from hyperactive neurotransmitter dopamine signal transduction [Bleuler, 1978; Davis et al., 1990; Eysenck, 1993]. Takeuchi et al. [2010a] also suggested that “the increased regional gray matter volume in striatum (information filtering system) may be one of the common neural correlates of creativity and schizophrenia.” Thus, in this study, the significant relationship between divergent thinking and basal ganglia, such as the left caudate tail and left claustrum, may be related to the dopaminergic system, which may be associated with an inferior and irrelevant information filtering system (acquiring high scores in DTTs) that conversely facilitated the generation of creative ideas [Bleuler, 1978; Davis et al., 1990; Eysenck, 1993]. Thus, the innate trait of creative people may ensure them to attend to more things (objects and ideas) at the same time (defocused attention).
Differences Between fMRI and Structural Studies
Results showed that fMRI and structural studies had no common regions, which may suggest that these studies uncovered different aspects of divergent thinking. The results of fMRI studies may reveal a few cognitive processes, such as semantic processing [e.g., Abraham et al., 2012; Bunge et al., 2005; Green et al., 2010], working memory [e.g., Fink et al., 2009], and cognitive control [Howard‐Jones et al., 2005; Subramaniam et al., 2009]. The structural studies of gray and white matter using structural MRI may reveal several interindividual variability in divergent thinking, such as information flowing among brain areas more efficiently [Jung et al., 2010b] and creative ability [Fink et al., 2014]. In addition, Kanai and Rees [2011] suggested that “in the neuroscience of human behavior and cognition, interindividual differences are often treated as a source of ‘noise’ and therefore discarded through averaging data from a group of participants.” This perspective may remind us that interindividual variability should be considered and multimodal MRI techniques should be used to investigate the comprehensive brain networks of divergent thinking in the future MRI studies.
LIMITATION AND FUTURE DIRECTIONS
In this study, an ALE meta‐analysis was conducted to quantitatively investigate neuroimaging studies on divergent thinking. The ALE results of the fMRI studies showed that the brain patterns of the creative idea generation in DTTs were composed of the prefrontal cortex (such as MFG, IFG, and ACC), posterior parietal cortex, and TOP. To a certain extent, these brain regions may be separated into two brain networks, namely, semantic (such as left IFG, left MFG, left IPL, and left MTG) and cognitive control systems (right ACC, right MFG, and right IPL). Basing on our ALE results of the structural studies, we determined that numerous brain regions may be related to a few innate traits of the creative idea generation in DTTs, such as the brain regions in the occipital cortex and the temporal cortex [cuneus (BA 18), right MTG (BA 39), and right MOG (BA 19)], as well as the brain regions in the dopamine system (left caudate tail and left claustrum). However, several limitations should be considered when interpreting our ALE results. First, the available number of publications included in these meta‐analyses is relatively small. Although these selected publications presented similar cognitive processes (such as semantic divergent thinking), the experiment paradigms (e.g., the duration of the creative idea generation, the type of the CTs), image acquisition techniques (such as 3‐T MRI and 1.5‐T MR), and the image analysis methods (such as the threshold of results: corrected or not, minimum cluster size) were not completely heterogeneous. Second, as the authors only presented results that they were interested in reporting (such as DTTs > CTs), several original data sets based on the meta‐analyses are not yet available. Thus, we could not determine whether the results are affected by unpublished data. Third, complex cognitive processes could be related to divergent thinking, such as working memory, cognitive control, semantic processing, and attention. However, our results could not conclusively uncover the integration of these cognitive processes on divergent thinking. Therefore, detailed experiments, advanced paradigms, and different MRI modalities should be used in future studies to determine the neural basis of divergent thinking.
REFERENCES
- Abraham A, Pieritz K, Thybusch K, Rutter B, Kröger S, Schweckendiek J, Stark R, Windmann S, Hermann C (2012): Creativity and the brain: uncovering the neural signature of conceptual expansion. Neuropsychologia 50:1906–1917. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JD (2004): Neural systems underlying the suppression of unwanted memories. Science 303:232–235. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Ramchandran K (2012): Creativity in art and science: are there two cultures? Dialogues Clin Neurosci 14:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden R, Chavez RS, Grazioplene R, Jung RE (2010): Neuroimaging creativity: A psychometric view. Behav Brain Res 214:143–156. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré‐Blagoev EJ, Insler RZ, Wagner AD (2005): Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 47:907–918. [DOI] [PubMed] [Google Scholar]
- Baddeley A (2003): Working memory: Looking back and looking forward. Nat Rev Neurosci 4:829–839. [DOI] [PubMed] [Google Scholar]
- Beaty RE, Silvia PJ (2013): Metaphorically speaking: Cognitive abilities and the production of figurative speech. Mem Cogn 41:255–267. [DOI] [PubMed] [Google Scholar]
- Bechtereva NP, Korotkov AD, Pakhomov S, Roudas MS, Starchenko MG, Medvedev SV (2004): PET study of brain maintenance of verbal creative activity. Int J Psychophysiol 53:11–20. [DOI] [PubMed] [Google Scholar]
- Bechtereva NP, Starchenko MG, Klyucharev VA, Vorobiev VA, Pakhomov SV, Medvedev SV (2000): The study of the brain's organization of creativity: 2. A positron emission tomography data. Hum Physiol 26:121–127. [Google Scholar]
- Benedek M, Jauk E, Fink A, Koschutnig K, Reishofer G, Ebner F, Neubauer AC (2014a): To create or to recall? Neural mechanisms underlying the generation of creative new ideas. Neuroimage 21:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Beaty R, Jauk E, Koschutnig K, Fink A, Silvia PJ, Dunst B, Neubauer AC (2014b): Creating metaphors: The neural basis of figurative language production. Neuroimage 15(90):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL (2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb. Cortex 19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C (2006): Dorsolateral prefrontal cortex promotes long‐term memory formation through its role in working memory organization. J Neurosci 26:916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler M (1978): The schizophrenic disorders: Long‐term patient and family studies. Yale University Press. [Google Scholar]
- Blumenfeld RS, Ranganath C (2007): Prefrontal cortex and long‐term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 13:280–291. [DOI] [PubMed] [Google Scholar]
- Bor D, Duncan J, Wiseman RJ, Owen AM (2003): Encoding strategies dissociate prefrontal activity from working memory demand. Neuron 37:361–367. [DOI] [PubMed] [Google Scholar]
- Bor D, Cumming N, Scott CE, Owen AM (2004): Prefrontal cortical involvement in verbal encoding strategies. Eur J Neurosci 19:3365–3370. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. (2004): Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci 8:539–546. [DOI] [PubMed] [Google Scholar]
- Broadbent WH (1879): A case of peculiar affecti0n of speech, with commentary. Brain 1:484–503. [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD (2005): Analogical reasoning and prefrontal cortex: Evidence for separable retrieval and integration mechanisms. Cerebral Cortex 15:239–249. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12:1–47. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M (2008): The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci 9:613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Mazuz YS, Stokes J, Kragel JE, Woldorff MG, Ciaramelli E, Olson IR, Moscovitch M (2011): Overlapping parietal activity in memory and perception: evidence for the Attention to Memory (AtoM) model. J Cogn Neurosci 11:3209–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Reichle ED (2000): Working memory and executive function: evidence from neuroimaging. Curr Opin Neurobiol 10:195–199. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman‐Rakic PS (1989): Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol 287:422–445. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA (2006): Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci 103:9315–9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Mansbach RS, Swerdlow NR, Campeau S, Braff DL, Geyer MA (1990): Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology 102:1–4. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R (1992): The anatomy of phonological and semantic processing in normal subjects. Brain 115:1753–1768. [DOI] [PubMed] [Google Scholar]
- Dietrich A (2004): The cognitive neuroscience of creativity. Psychon Bull Rev 11:1011–1026. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R (2010): A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull 136:822. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapping 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012): Activation likelihood estimation meta‐analysis revisited. Neuroimage 59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K (2012): Evaluative and generative modes of thought during the creative process. NeuroImage 59:1783–1794. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ (1993): Creativity and personality: Suggestions for a theory. Psychological Inquiry 4:147–178. [Google Scholar]
- Fink A, Grabner RH, Benedek M, Reishofer G, Hauswirth V, Fally M, Neuper C, Ebner F, Neubauer AC (2009): The creative brain: Investigation of brain activity during creative problem solving by means of EEG and fMRI. Hum Brain Mapp 30:734–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Gebauer D, Reishofer G, Koschutnig K, Ebner F (2010): Enhancing creativity by means of cognitive stimulation: Evidence from an fMRI study. Neuroimage 52:1687–1695. [DOI] [PubMed] [Google Scholar]
- Fink A, Koschutnig K, Benedek M, Reishofer G, Ischebeck A, Weiss EM, Ebner F (2012): Stimulating creativity via the exposure to other people's ideas. Hum Brain Mapp 33:2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Koschutnig K, Hutterer L, Steiner E, Benedek M, Weber B, Reishofer G, Papousek I, Weiss EM (2014): Gray matter density in relation to different facets of verbal creativity. Brain Struct Funct 219:1263–1269. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ (2008): A meta‐analytic study of changes in brain activation in depression. Hum Brain Mapp 29:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glucksberg S (2001): Understanding Figurative Language: From Metaphors to Idioms. New York, NY: Oxford University Press. [Google Scholar]
- Glucksberg S (2003): The psycholinguistics of metaphor. Trends Cogn Sci 7:92–96. [DOI] [PubMed] [Google Scholar]
- Goel V, Vartanian O (2005): Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set‐shift problems. Cereb Cortex 15:1170–1177. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH (2006): Dissociation of automatic and strategic lexical‐semantics: Functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci 26:6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman‐Rakic PS (1994): Working memory dysfunction in schizophrenia. The Journal of neuropsychiatry and clinical neurosciences, 6:348–357. [DOI] [PubMed] [Google Scholar]
- Gorno‐Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL (2004): Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AE, Kraemer DJ, Fugelsang JA, Gray JR, Dunbar KN (2010): Semantic distance in analogical reasoning modulates frontopolar cortex activity. Cereb Cortex 20:70–76. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Dayan P, Gutkin BS, Solla SA (2006): Dopamine modulation in the basal ganglia locks the gate to working memory. Journal of computational neuroscience, 20:153–166. [DOI] [PubMed] [Google Scholar]
- Guilford JP (1950): Creativity. Am Psychol 5:444–454. [DOI] [PubMed] [Google Scholar]
- Guilford JP (1967): The nature of human intelligence. New York, NY: McGraw‐Hill. [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT (2004): Structural brain variation and general intelligence. NeuroImage 23:425–433. [DOI] [PubMed] [Google Scholar]
- Herz DM, Eickhoff SB, Løkkegaard A, Siebner HR (2014): Functional neuroimaging of motor control in Parkinson's disease: A meta‐analysis. Hum Brain Mapping 35:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson‐Schill SH (2006): Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal fluency. Neuropsychologica 44:2547–2557. [DOI] [PubMed] [Google Scholar]
- Howard‐Jones PA, Blakemore SJ, Samuel EA, Summers IR, Claxton G (2005): Semantic divergence and creative story generation: An fMRI investigation. Cogn Brain Res 25:240–250. [DOI] [PubMed] [Google Scholar]
- Hunt RR, Einstein GO (1981): Relational and item‐specific information in memory. J Verbal Learning Verbal Behav 20:497–514. [Google Scholar]
- Jonides J, Reuter‐Lorenz P, Smith EE, Awh E, Barnes L, Drain M, Glass J, Lauber E, Patalano A, Schumacher EH (1996): Verbal and spatial working memory. In: Medin D, editor. The Psychology of Learning and Motivation. pp 43–88. [Google Scholar]
- Jung‐Beeman M, Bowden EM, Jason Haberman J, Jennifer L, Frymiare JL, Arambel‐Liu S, Greenblatt R, Paul J, Reber PJ, Kounios J (2004): Neural activity when people solve verbal problems with insight. PLoS Biology 2: E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ (2007): The Parieto‐Frontal Integration Theory (P‐FIT) of intelligence: converging neuroimaging evidence. Behavioral and Brain Sciences 30:135–154. [DOI] [PubMed] [Google Scholar]
- Jung RE, Grazioplene R, Caprihan A, Chavez RS, Haier RG (2010a): White Matter Integrity, Creativity, and Psychopathology: Disentangling Constructs with Diffusion Tensor Imaging. PLoS ONE 5(3):e9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Segall JM (2010b): “Neuroanatomy of creativity.” Human brain mapping 31:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Rees G (2011): The structural basis of inter‐individual differences in human behaviour and cognition. Nat Rev Neurosci 12:231–242. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, et al. (2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303:1023–1026. [DOI] [PubMed] [Google Scholar]
- Kleibeuker SW, Koolschijn PC, Jolles DD, De Dreu CK, Crone EA (2013): The neural coding of creative idea generation across adolescence and early adulthood. Front Hum Neurosci 7:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, D'Esposito M (2003): Lateral prefrontal syndrome: A disorder of executive control. Neurological foundations of cognitive neuroscience, 259–279.
- Kosslyn SM, Ganis G, Thompson WL (2001): Neural foundations of imagery. Nat Rev Neurosci 2:635–642. [DOI] [PubMed] [Google Scholar]
- Kounios J, Frymiare JL, Bowden EM, Fleck JI, Subramaniam K, Parrish TB, Jung‐Beeman M (2006): The prepared mind neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychol Sci 17:882–890. [DOI] [PubMed] [Google Scholar]
- Krall SC, Rottschy C, Oberwelland E, Bzdok D, Fox PT, Eickhoff SB, Fink GR, Konrad K (2014): The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta‐analysis. Brain Struct Funct 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Ritter SM, Müller BC, Baaren RB, Brass M, Dijksterhuis A (2014): The importance of the default mode network in creativity—A structural MRI study. J Creative Behav 48:152–163. [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005): ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, van Essen DC (2000): Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol 428:112–137. [DOI] [PubMed] [Google Scholar]
- Lissauer H, Jackson M (1988): A case of visual agnosia with a contribution to theory. Cogn Neuropsychol 5:157–192. [Google Scholar]
- Martin A, Chao LL (2001): Semantic memory and the brain: Structure and processes. Curr Opin Neurobiol 11:194–201. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung‐Beeman M (2007): An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain Language 100:115–126. [DOI] [PubMed] [Google Scholar]
- F McNab, T Klingberg (2008): Prefrontal cortex and basal ganglia control access to working memory. Nature neuroscience 11:103–107. [DOI] [PubMed] [Google Scholar]
- Mednick SA (1962): The associative basis of the creative process. Psychol Rev 69:220–232. [DOI] [PubMed] [Google Scholar]
- Mendelsohn GA (1976): Associative and attentional processes in creative performance. J Pers 44:341–369. [Google Scholar]
- Mohr HM, Goebel R, Linden DE (2006): Content‐and task‐specific dissociations of frontal activity during maintenance and manipulation in visual working memory. J Neurosci 26:4465–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Herrod NJ, Menon DK, Clark JC, Downey SPMJ, Carpenter TA, Minhas PS, Turkheimer FE, Williams EJ, Robbins TW, Sahakian, BJ , Petrides, M , Pickard, JD (1999): Redefining the functional organization of working memory processes within human lateral prefrontal cortex. Eur J Neurosci 11:567–574. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ (1993): The neural correlates of the verbal component of working memory. Nature 362:342–345. [DOI] [PubMed] [Google Scholar]
- Pavlova LP, Romanenko AF (1988): The Systemic Approach to Psychophysiological Investigation of Human Brain. Leningrad: Nauka. [Google Scholar]
- Petrides M, Pandya DN (1994): Comparative architectonic analysis of the human and macaque frontal cortex Handbook of Neuropsychology. In: Boller F, Grafman J, editors, pp. 17–58, Elsevier. [Google Scholar]
- Petrides M, Pandya DN (1999): Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience, 11, 1011–1036. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, et al. (2011): Emotional perception: Meta‐analyses of face and natural scene processing. Neuroimage 54(3):2524–2533. [DOI] [PubMed] [Google Scholar]
- Seger CA, Desmond JE, Glover GH, Gabrieli JDE (2000): Functional magnetic resonance imaging evidence for right‐hemisphere involvement in processing of unusual semantic relationships, Neuropsychology 14:361–369. [DOI] [PubMed] [Google Scholar]
- Shah C, Erhard K, Ortheil HJ, Kaza E, Kessler C, Lotze M (2011): Neural correlates of creative writing: An fMRI study. Hum Brain Mapping 34(5):1088–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL (2009): Event‐related fMRI studies of episodic encoding and retrieval: Meta‐analyses using activation likelihood estimation. Neuropsychologia 47:1765–1779. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Kounios J, Parrish TB, Jung‐Beeman M. (2009): A brain mechanism for facilitation of insight by positive affect. J Cogn Neurosci 21:415–432. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y (2010a): “Regional gray matter volume of dopaminergic system associate with creativity: evidence from voxel‐based morphometry.” Neuroimage 51:578–585. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2010b): Cerebral blood flow during rest associates with general Intelligence and Creativity. NeuroImage 51:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Schill SL, Bedny M, Goldberg RF. (2005): The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol 15:219–224. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: method and validation. Neuroimage 16:765–780. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL (2001): Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage 14:1337–1347. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG (2003): Conflict monitoring in the human anterior cingulate during selective attention to global and local object features. NeuroImage 19:1361–1168. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Laruelle M (2001): Neurochemical and neuropharmacological imaging in schizophrenia. Neuropsychopharmacology: The fifth generation of progress, 833–856. [Google Scholar]
- Wyland CL, Kelley WM, Macrae CN, Gordon HL, Heatherton TF (2003): Neural correlates of thought suppression. Neuropsychologia 41:1863–1867. [DOI] [PubMed] [Google Scholar]
- Zhu F, Zhang Q, Qiu J (2013): Relating inter‐individual differences in verbal creative thinking to cerebral structures: an optimal voxel‐based morphometry study. PloS one 8:e79272. [DOI] [PMC free article] [PubMed] [Google Scholar]


