Abstract
The neural underpinnings of anorexia nervosa (AN) are poorly understood. Results from existing functional brain imaging studies using disorder‐relevant food‐ or body‐stimuli have been heterogeneous and may be biased due to varying compliance or strategies of the participants. In this study, resting state functional connectivity imaging was used. To explore the distributed nature and complexity of brain function we characterized network patterns in patients with acute AN. Thirty‐five unmedicated female acute AN patients and 35 closely matched healthy female participants underwent resting state functional magnetic resonance imaging. We used a network‐based statistic (NBS) approach [Zalesky et al., 2010a] to identify differences between groups by isolating a network of interconnected nodes with a deviant connectivity pattern. Group comparison revealed a subnetwork of connections with decreased connectivity including the amygdala, thalamus, fusiform gyrus, putamen and the posterior insula as the central hub in the patient group. Results were not driven by changes in intranodal or global connectivity. No network could be identified where AN patients had increased coupling. Given the known involvement of the identified thalamo‐insular subnetwork in interoception, decreased connectivity in AN patients in these nodes might reflect changes in the propagation of sensations that alert the organism to urgent homeostatic imbalances and pain‐processes that are known to be severely disturbed in AN and might explain the striking discrepancy between patient's actual and perceived internal body state. Hum Brain Mapp 36:1772–1781, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: fMRI, connectivity, network, posterior insula, thalamus
INTRODUCTION
Anorexia nervosa (AN) is a severe eating disorder that predominantly begins during early adolescence in females. Patients deprive themselves of food despite starvation, suffer severe body image distortions and often have a lack of insight about being ill. Mortality rates are among the highest in psychiatry [Steinhausen, 2002]. Despite high genetic heritability estimates [Kaye et al., 2009], our knowledge of the biological underpinnings of this devastating illness is very limited [Kaye et al., 2011, 2013a]. The use of modern noninvasive neuroimaging techniques may allow a deeper understanding of AN [Kidd and Steinglass, 2012].
However, compared to other neuropsychiatric disorders, neuroimaging research in AN has only recently gained momentum. Existing studies, which are often limited by small sample sizes, have focused on executive functioning [Kullmann et al., [Link]; Lock et al., 2011; Sato et al., 2013; Wierenga et al., 2014; Zastrow et al., 2009] or the processing of food/taste and other appetitive stimuli [Bischoff‐Grethe et al., 2013; Brooks et al., 2012; Holsen et al., 2014; Oberndorfer et al., 2013a; Wagner et al., 2007, 2008]. Results suggest that patients may have an altered sensitivity for sensory‐interoceptive and/or reward processes as well as an impaired awareness of homeostatic needs [Kaye et al., 2013b].
To date, only very few studies have tried to characterize functionally synchronized brain activity in AN. Functional connectivity magnetic resonance imaging (fcMRI) measures the temporal correlation of neural activity via the blood oxygen level‐dependent (BOLD) signal between different brain regions [Biswal et al., 1995]. Functional connectivity is typically determined while the participant is resting, since regions that are functionally related or coactivated during a cognitive task, tend to be temporally correlated at rest [Beckmann et al., 2005; Smith et al., 2009]. Groups of temporally correlated regions can be determined using independent component analysis (ICA) or seed based connectivity analysis [Cole et al., 2010] and are termed resting‐state networks. A major advantage of resting state compared to task‐based fMRI lies in it's lack of any domain bias, that is at the same time, a variety of functional networks with individual contribution to the complexity of the disorder can be observed. Moreover, potential bias due to patients' strategy to perform certain tasks can be limited by the minimum task requirement to lie still and not fall asleep.
In patients with AN, two groups of researchers [Cowdrey et al., 2014; Lee et al., 2014] reported increased functional connectivity between midline brain structures (dorsal anterior cingulate cortex (dACC), precuneus, retrosplenial cortex) and within the default mode network, respectively, when compared to healthy controls (HC). The latter finding is in line with results from an ICA‐based resting state study that we have published very recently [Boehm et al., 2014]. Another study [Favaro et al., 2012] found decreased connectivity within a ventral visual network in AN. Decreased connectivity in AN was also found for connections originating from the dorsal putamen [Favaro et al., 2014].
Instead of focusing on the connectivity of certain regions of interest (ROI) or components, recent studies have tried to model the brain as a complex network [Bassett et al., 2009; Bullmore and Sporns, 2009; Zalesky et al., 2010a], that is, as a graph whose nodes are interconnected by edges. The nodes commonly comprise a large number of structurally or functionally defined regions across the whole brain while the edges are the structural or functional connections between these regions [Bullmore and Bassett, 2011]. The advantage of such a mathematical framework is that it allows considering changes in the relationship between multiple regions in the context of whole‐brain networks [Zalesky et al., 2010a].
To determine differences in network connectivity patterns between patients with acute AN and pairwise matched female HC we used a network‐based statistic (NBS) approach [Zalesky et al., 2010a]. NBS identifies differences between groups by isolating interconnected nodes with a deviant connectivity pattern (subnetworks) and thus provides information about whole‐brain topology of complex networks. Previous studies in patients with major depressive disorder, schizophrenia and amyotrophic lateral sclerosis have used NBS to identify impaired network topology [Bai et al., 2012; Verstraete et al., 2011; Zalesky et al., 2011; Zhang et al., 2011].
METHODS
Participants
The sample consisted of 70 female volunteers: 35 acute AN (12–23 years old) and 35 pairwise matched, female HC (12–23 years old). AN participants were recruited from specialized eating disorder programs of a university child and adolescent psychiatry and psychosomatic medicine department and underwent magnetic resonance imaging (MRI) within 96 h after beginning behaviorally‐oriented nutritional rehabilitation programs. This sample overlaps with another recently published resting state study using a strictly hypothesis‐driven ICA approach [Boehm et al., 2014].
All participants were diagnosed using the expert version of a semistructured research interview, the Structured Interview for Anorexia and Bulimia Nervosa for DSM‐IV (SIAB‐EX, [Fichter and Quadflieg, 1999], see also Supporting Information (SI) 1.2) and AN had to have a BMI below the 10th age percentile (if younger than 15.5 years) or a BMI below 17.5 kg/m2 (if older than 15.5 years) [Hebebrand et al., 2004; Kromeyer‐Hauschild, 2001] and no recent weight gain. Within the AN group, 32 (94.1%) of the patients were of the restrictive and 2 (5.9%) of the binge/purging subtype; 4 (11.4%) had comorbid psychiatric disorders (5.7% depressive disorders including dysthymia, 2.9% anxiety disorder, and 2.9% obsessive compulsive disorder). HC participants had to be of normal weight, eumenorrhoeic and without any history of psychiatric illness. We applied several additional exclusion criteria for each group (see Supporting Information 1.1) — most importantly a history of bulimia nervosa or “regular” binge eating, psychotropic medications within 4 weeks prior to the study, substance abuse and neurologic or medical conditions. Case‐control age‐matching was carried out using the Munkres algorithm [Munkres, 1957] resulting in a maximum difference of 0.9 years between the individuals within one pair.
This study was approved by the local Institutional Ethics Review Board, and all participants (and their guardians if underage) gave written informed consent.
Clinical Measures
To complement the information obtained with the clinical interviews, eating disorder‐specific psychopathology was assessed with the German version of the Eating Disorders Inventory (EDI‐2, [Paul, 2005]). Here we use a summary score representing “core” symptoms that was estimated by averaging across the following three subscales: “drive for thinness,” “body dissatisfaction,” and “bulimia.” Intelligence quotient (IQ) was assessed with a short version of the German adaption of the Wechsler Adult Intelligence Scale [von Aster et al., 2006] for participants aged 16 years and older or a short version of the German adaption of the Wechsler Intelligence Scale for Children [Petermann and Petermann, 2008] for participants aged 15 years or younger.
Data Acquisition
Images were acquired between 8 and 9 in the morning after an overnight fast using standard sequences with a 3 T MRI scanner (TRIO; Siemens, Erlangen, Germany) equipped with a standard head coil (Supporting Information 1.3 for details). During fMRI participants were instructed to lie still with closed eyes without falling asleep.
Preprocessing of Imaging Data
Functional and structural images were processed using SPM8 toolbox (http://www.fil.ion.ucl.ac.uk/spm/) within the Nipype framework (http://nipy.sourceforge.net/nipype/, [Gorgolewski et al., 2011]). The slice time corrected functional data were realigned and registered to their mean. The realigned files were coregistered to the subject's structural brain image. A DARTEL template was created using structural images from all subjects [Ashburner, 2007]. The EPI volumes were then normalized to MNI space using the DARTEL template and corresponding flow field. The resulting data were smoothed with an isotropic 8 mm FWHM Gaussian kernel.
We evaluated the quality of the fMRI data by manual inspection and using artifact detection tools [Whitfield‐Gabrieli et al., 2009]. Volumes that exceed an intensity threshold of three standard deviations (SD) or a threshold of 2 mm normalized movement in any direction were classified as outliers (motion‐outlier: AN: 0.31 ± 0.87 HC: 0.97 ± 2.67; intensity‐outlier: AN: 1.5 ± 2.37 HC: 2.38 ± 2.85); the two groups did not differ regarding numbers of motion‐ and intensity‐outliers (motion‐outlier: t(68)=1.38; P=0.17; intensity‐outlier: t(68)=1.38; P =0.17).
Using the DPARSF toolbox [Chao‐Gan and Yu‐Feng, 2010], implemented in MATLAB, temporal filtering between 0.01 and 0.08 Hz was applied. Then white matter and cerebrospinal fluid signal were regressed out. ROI were identified using a modified version of the AAL atlas [Tzourio‐Mazoyer et al., 2002] with 104 ROIs including a more fine‐grained parcellation in the superior frontal gyrus, insula, anterior cingulate cortex (ACC) and temporo‐parietal junction as described previously ([Borchardt et al., 2014; Lord et al., 2012], see also Supporting Information Table 1 for details). Mean time courses across voxels in each ROI were extracted and undirected correlation matrices were constructed by calculating Pearsons product moment correlation coefficients between each pair of time courses.
Table 1.
Basic demographic and clinical variables
| AN | HC | Test statistics | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | P | |
| Age | 16.10 | 2.56 | 16.16 | 2.64 | −0.09 | 68 | 0.927 |
| BMI | 14.78 | 1.26 | 20.81 | 2.72 | −11.91 | 68 | <0.001 |
| Minimal lifetime BMI | 14.42 | 1.28 | 19.87 | 2.35 | −11.98 | 66 | <0.001 |
| IQ | 111 | 11 | 112 | 10 | −0.36 | 68 | 0.720 |
| Parental SES | 3.77 | 0.81 | 4.34 | 0.80 | −2.97 | 68 | 0.004 |
| Handedness score | 0.51 | 2.06 | 1.77 | 3.60 | −1.79 | 68 | 0.077 |
| EDI‐2 core subscales | 71.34 | 20.46 | 50.15 | 16.76 | 4.62 | 68 | <0.001 |
| EDI‐2 Drive for thinness | 27.00 | 8.90 | 14.56 | 7.03 | 6.36 | 65 | <0.001 |
| EDI‐2 Body dissatisfaction | 33.27 | 10.02 | 24.94 | 9.51 | 3.52 | 66 | 0.001 |
| EDI‐2 Bulimia | 10.79 | 5.28 | 10.31 | 3.07 | 0.46 | 67 | 0.645 |
| Leptin (in ng/ml) | 1.32 | 1.56 | |||||
BMI and minimal lifetime BMI are displayed but statistical comparisons are based on BMI‐SDS values to ensure comparability across age (see Supporting Information 1.4). BMI, body mass index; EDI‐2, Eating disorder inventory, version 2; SCL‐90‐R GSI, Revised symptom checklist 90 global symptom score. IQ was assessed with a short version of the German adaption of the Wechsler Adult Intelligence Scale (von Aster et al., 2006) for participants aged 16 years and older or a short version of the German adaption of the Wechsler Intelligence Scale for Children (Petermann and Petermann, 2008) for participants aged 15 years or younger. Group differences were tested using with Student's t‐tests using SPSS v21.0 (SPSS, Chicago, Illinois).
Network Based Statistics
NBS is a method for identifying a statistically significant cluster of connections revealing differences between two groups. Such a subnetwork is identified using a one‐sided t‐statistic, which may either reveal reduced (AN < HC) or increased (AN > HC) functional connectivity. NBS returns a single p‐value, which represents the likelihood that the subnetwork is due to a true effect in the data. This approach measures the entire cluster of returned connections, but does not identify the contribution of each component independently.
NBS is computed using the following steps, (1) Identify all ROI that are reduced in one specific group beyond a particular T value. (2) Select the largest contiguous cluster of these connections, and (3) validate the effect against surrogate data sets. To validate that the resulting subnetwork is not due to a random effect, group membership is resampled 5,000 times. The returned subnetwork is statistically significant at a family wise error (FWE) corrected value of P < 0.05. Although the network needs to be considered as a whole, the extent of the returned network can be varied using a network threshold parameter. Basically, this adjusts the extremity of deviation in a connection between groups required, before it is considered for inclusion in the NBS result. This technique has been described and validated in depth by [Zalesky et al., 2010a].
NBS results can depend on the underlying atlas used to parcellate the brain and how to obtain an optimal parcellation of the brain into meaningful regions remains an open issue [Sporns, 2011; Wig et al., 2011; Zalesky et al., 2010a, b]. To test the reproducibility of our findings we performed NBS using a variety of different parcellation templates comprising the original AAL template displaced by 1.5, 4.5, or 20 millimeters along a randomly generated vector for each region and evaluated the emerging changes at a t=4 (see also [Cocchi et al., 2012]). Any portion of a displaced region lying outside the brain volume was omitted. Effects of displacement were evaluated with four independent parcellation templates generated for each displacement magnitude.
Intranodal Homogeneity
To test, whether changes in interregional connectivity (such as identified with NBS) can be explained by differences regional connectivity [Zalesky et al., 2012] we estimated Kendall's coefficient concordance (KCC) for all voxels within each node implicated in the subnetwork (identified by NBS at t=4) as a measure of regional homogeneity (ReHo). The preprocessing steps were identical to the ones for NBS and individual KCC (with values from 0 to 1) were estimated using the DPARSFA software [Chao‐Gan and Yu‐Feng, 2010]. Group comparisons were performed using Student's t‐tests and associations between KCC of a specific node and inter‐regional connectivity (from the NBS analysis) for connections between this node and other nodes were estimated using Pearson correlations coefficients [Zalesky et al., 2012].
RESULTS
Sample Characteristics
There were no differences in age, IQ, or handedness score between the pairwise matched groups of AN and HC. However, as expected AN had a significantly lower BMI and lower minimal lifetime BMI. Furthermore AN had significantly higher eating disorder symptom scores as well as very low plasma leptin levels (normal range for young females is around 8.5 ± 0.9 ng/ml; Table 1).
Network Analysis
NBS was performed in both directions. That is, to look for a subnetwork in the AN cohort that is stronger than in HC and vice versa. When considering the direction HC > AN, a subnetwork of connections was found that survived FWE corrected statistical testing. This subnetwork survived FWE correction at multiple network thresholds between 2 and 4 with highly significant results (P < 0.027 or below for all tested network thresholds) with the strongest alterations forming a tightly interconnected subnetwork of 7 nodes (P = 0.017). The regions involved in this largely subcortical network were the amygdala (left), thalamus (left), fusiform gyrus (right), bilateral putamen and posterior insula (Fig. 1, Table 2). No network was found at any network threshold for the AN > HC, showing no consistent increase in functional connectivity in any network in AN patients.
Figure 1.
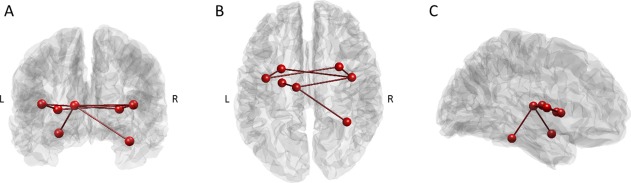
Subnetwork of nodes with reduced connectivity in patients with AN‐identified using NBS: back view (A), top view (B), and side view (C). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Connections identified by NBS at threshold = 4
| Region A | Region B |
|---|---|
| Amygdala Left | Thalamus Left |
| Fusiform Right | Thalamus Left |
| Putamen Left | Post Insula Left |
| Putamen Right | Post Insula Left |
| Putamen Left | Post Insula Right |
| Putamen Right | Post Insula Right |
| Thalamus Left | Post Insula Right |
All connections are undirected.
To ensure the reduced subnetwork in AN was not due to globally altered correlations of the BOLD time series between the various brain regions we estimated average correlation coefficients across all (modified) AAL nodes per participant similar to [Lord et al., 2012]. No group difference was observed (for HC mean=0.372, SD=0.123; for AN mean=0.321, SD=0.120; t=1.851, df=68, P =0.0685), however, when restricting this analysis to connections identified in the NBS, the mean correlation coefficients were significantly reduced in AN (for HC mean=0.127, SD=0.043; for AN mean=0.072, SD=0.051; t=14.950, df=68, P =5*10 e −6).
Next we assessed the extent of overlap between the significant HC > AN subnetwork identified with the NBS across the original and displaced parcellation templates to test the reproducibility of our results. Displacement of all nodes by 1.5 mm along randomly generated vectors revealed that the HC > AN subnetwork remained very stable (one new edge between fusiform and posterior insula in 75% of the models). Displacing by 4.5 mm resulted on average in 0.75 new nodes and 1.75 new edges while a displacement by 20 mm resulted on average in 2.75 new nodes and 3 new edges.
Finally, associations between connectivity and clinically relevant variables were explored. We extracted correlation coefficients from the original correlation matrices for each of the seven connections comprising the implicated subnetwork at t=4 (Amygdala Left ‐ Thalamus Left, Fusiform Right ‐ Thalamus Left, Putamen Left ‐ Post Insula Left, Putamen Right ‐ Post Insula Left, Putamen Left‐ Post Insula Right, Putamen Right ‐ Post Insula Right, Thalamus Left ‐ Post Insula Right) in each participant. We tested for associations with age, BMI‐SDS, the three EDI‐2 subscales (“drive for thinness,” “body dissatisfaction,” and “bulimia”) as well as the EDI‐2 core summary measure and the duration of the current AN episode (in months). Pearson correlation analyses were carried out independently in each participant group and did not yield any statistically significant results.
Intranodal Homogeneity
It has been suggested that changes in inter‐regional functional connectivity occur in the context of some global and/or local alterations in neural dynamics [Cocchi et al., 2012; Zalesky et al., 2012]. In our study, there were no significant differences in intranodal connectivity between AN and HC as measured by ReHo (KCC; Supporting Information Table 2). Next, we tested whether there are associations between KCC of a specific node and connectivity values (in the connectivity matrix underlying the NBS analysis) for connections between this node and other nodes (using Pearson correlations coefficients). Replicating [Zalesky et al., 2012] we found significant positive associations (low ‐ medium sized effects), that is, there was a robust link between regionally localized homogeneity and inter‐regional connectivity of the same brain region. However, this relationship was similar for both, AN and HC (see Supporting Information Table 3).
DISCUSSION
In a comparatively large sample of young unmedicated acute AN and pairwise matched HC we found a robust subnetwork of brain regions clustered around the posterior insula that showed a marked decrease in synchronized activity in the patient group. Of note, we only found a subnetwork with reduced connectivity estimates, while no subnetwork could be identified where AN patients had increased coupling.
This finding extends results from a relatively small number of previous resting state functional connectivity studies in AN using more conventional techniques. Favaro et al., reported decreased temporal coherence in a visual ventral network in AN using ICA and, using seed‐based analysis in a largely overlapping partially medicated adult sample, decreased functional connectivity of the putamen with the contralateral putamen, cingulate gyrus, amygdalae, and precentral gyrus [Favaro et al., 2012, 2014]. Using the dACC as an a priori seed region, Kim et al. [Kim et al., 2012] found decreased connectivity of this region with the DLPFC in medicated adult AN patients. However, two of the aforementioned studies also found increased coupling in AN – namely between dACC and retrosplenial cortex/precuneus [Kim et al., 2012] as well as in a somatosensory network [Favaro et al., 2012]. Furthermore, several studies have used ICA to target potential changes in the default mode network. While recovered AN participants were reported to show increased default mode network connectivity [Cowdrey et al., 2014], this was not confirmed in another study that investigated connectivity during a conditioned stimulus task [McFadden et al., 2014]. In parallel to the NBS approach described in this manuscript, we have also used ICA to target the default mode network and a few select other well‐characterized resting state networks in AN in a more hypothesis‐driven way [Boehm et al., 2014]. Interestingly, we found the anterior insula to be more strongly connected to the default mode network in patients with acute AN (and increased functional connectivity within the fronto‐parietal network).
These studies have all focused a priori on specific known resting state networks or considered connectivity from a few predetermined seed regions. Instead, NBS allowed us to isolate a set of interconnected regions (i.e., a subnetwork) based on information about whole‐brain functional organization without any a priori assumptions [Zalesky et al., 2010a] while applying very conservative measures to control for false positive effects due to multiple testing.
Hidden in the Sylvian fissure and interconnected with a large number of cortical areas the insula is a brain region thought to be implicated in a range of different functions including sensory perception (gustatory, olfactory, visual, auditory, and tactile inputs), the subsequent integration of exteroceptive and interoceptive information, cognition and emotion [Craig, 2009; Erberich et al., 2006; Kurth et al., 2010; Menon and Uddin, 2010]. The insula also has a remarkably high base rate of activation and considerable functional heterogeneity [Duncan and Owen, 2000; Nelson et al., 2010; Yarkoni et al., 2011]. Functional parcellation studies of the insula provide evidence for distinct subregions. While the ventroanterior and dorsoanterior regions are associated with chemosensory [Pritchard et al., 1999], socioemotional [Chang et al., 2011; Sanfey et al., 2003], and higher cognitive processing respectively [Dosenbach et al., 2006; Eckert et al., 2009], the posterior insula region is associated with pain and sensorimotor processing [Craig, 2002; Wager and Barrett, 2004]. According to a recent study the latter posterior region is functionally connected to supplementary motor area and somatosensory cortex as well as posterior temporal lobes right hippocampus, and rostral ACC [Chang et al., 2013; Deen et al., 2011].
Based on Damasio's somatic marker hypothesis [Damasio, 1996] in which emotional states result from the fast and unconscious processing of exteroceptive and interoceptive sensory data – insular dysfunction, or the dysfunction of networks passing through the insula, has been repeatedly suggested as a core factor in AN etiology. More specifically it has been proposed that impaired integration of visual and body perception with feelings/emotions and the inhibition of higher cognitive processes may account for the striking symptomatology of AN [Nunn et al., 2008, 2011]. In line with that, a relatively large proportion of neuroimaging studies in AN have yielded differential neural responses in this region. Studies on food cue‐reactivity reported either insula hypo‐ [Gizewski et al., 2010; Holsen et al., 2012] or hyperactivity (Gizewski et al., 2010; Oberndorfer et al., 2013a) in patients with AN compared to HC. Two studies reported decreased insula responses to the oral application of sucrose as well as water in AN [Oberndorfer et al., 2013b; Wagner et al., 2008]. However, using a multimodal paradigm insula hyperactivation in AN was reported in response to aversive gustatory taste [Cowdrey et al., 2011]. Studies on body image processing in AN have yielded equally mixed results with either reduced [Sachdev et al., 2008] or increased [Friederich et al., 2010; Mohr et al., 2010] insula responses during the rating of thin self‐images or when contrasting the viewing of self versus nonself‐images. Our own ICA‐based study (see above, [Boehm et al., 2014]) and a recent pilot study (12 AN patients) combining reduced degree centrality (defined by the number of edges connecting to a node, i.e., brain voxel) and a techniques targeting changes in effective connectivity also provides evidence for altered resting state functional connectivity of the insula in AN [Kullmann et al., 2014]. Furthermore, changes in structural connectivity in AN as measured by diffusion tensor imaging are also in line with disturbances of brain networks involved in proprioception and the integration of visual information — processes that are essential for the representation of the body self‐image [Frieling et al., 2012; Via et al., 2014].
While most of the aforementioned functional studies have not differentiated between anterior and posterior insular cortex or not targeted an insular subregion, pain stimuli can be used to specifically probe the posterior insula, which is thought to function as the primary interoceptive cortex [Craig, 2002, 2011]. Two studies have used heat pain in patients with AN and found decreased posterior insula responses during stimulation [Bar et al., 2013; Strigo et al., 2013]. Since the insular cortex is well‐connected to the thalamus, amygdalae and basal ganglia [Augustine, 1996; Craig, 2011] reduced posterior insular responses are well in line with our own connectivity results and might lead to a relative insensitivity to pain, an established phenomenon in AN [de Zwaan et al., 1996; Lautenbacher et al., 1991; Raymond et al., 1999]. According to Craig, homeostatic afferent representations of the physiological condition of the body ascend from the spinal cord and brain stem via a pair of specific subnuclei in the thalamus to the posterior and midinsula [Craig, 2011]. Reduced connectivity in a subnetwork consisting of the thalamus, basal ganglia, amygdala, fusiform gyrus (including the fusiform body area) and the posterior insula (as the major hub) may thus reflect the altered calibration of signals such as pain, body size and hunger and subsequently contribute to abnormal cognitive‐affective and behavioral responses such as excessive physical activity. Within this framework, the finding from our recently published ICA‐based study in the same group of AN patients [Boehm et al., 2014] can be interpreted: Increased connectivity between the default‐mode network and the anterior insula, a subregion associated with socioemotional [Chang et al., 2011; Sanfey et al., 2003] and higher cognitive processing [Dosenbach et al., 2006; Eckert et al., 2009], may be a compensatory response to dysconnectivity in the afferent thalamus ‐ posterior insular network identified using NBS. However, more studies are needed to substantiate this hypothesis.
Finally, reduced interregional connectivity in AN could not be explained by local neural dynamics or abnormalities in global signal. There were no group differences in intranodal homogeneity of BOLD fluctuations. Further, although we could replicate previous findings showing that intranodal homogeneity of a ROI predicts connectivity between the very same node and other nodes across the brain [Zalesky et al., 2012]; this mechanism was the same for patients and HC. Likewise, there were no group differences in correlation coefficients averaged across all nodes of the modified AAL template [Lord et al., 2012]. Thus we are confident that dysconnectivity in a thalamo‐insular subnetwork is indeed a specific finding which may be related to the pathophysiology of AN even though we did not find any direct linear correlations between the degree of self‐reported symptoms and reduced connectivity in this network.
Limitations
Our study has to be seen in the light of the following limitations. First, properties of graph networks may be influenced by its density, chosen parcellation scheme, and the image preprocessing steps [Bassett et al., 2006; Braun et al., 2012; Liang et al., 2012; Zalesky et al., 2010b]. For these reasons, the current results may be restricted to the parameters and settings used (which were all common and comparable to similar studies). To address this issue, the regions of our modfied AAL template were displaced to introduce random variability in regional demarcations. The abnormal subnetwork in AN remained remarkably robust when applying small changes to the atlas template. However, with increasing spatial displacement of the atlas an increasing number of new nodes and edges were detected. Thus, our results seem to be both, robust to minor inaccuracies during realignment and registration but also specific to the original brain regions. Second, during resting‐state data acquisition participants were required to close their eyes which may lead to different levels of wakefulness. Since we did not monitor respiration or heart rate during scanning we cannot account fluctuations in wakefulness which may have influenced resting‐state dynamics [Tagliazucchi and Laufs, 2014]. Third, determining whether changed brain functioning is a consequence or a potential antecedent of pathologic eating behavior is one of the most difficult questions in the field of eating disorder research [Frank, 2013]. More resting state connectivity studies in long‐term recovered AN patients are needed to disentangle trait and state effects. However, strengths of the current study include the large sample size, the fact that all participants were young and unmedicated, the short duration of illness (reducing effects of chronic illness), the homogeneity of the sample (94% restrictive AN subtype) and the fact that time of scanning and food intake were experimentally controlled.
CONCLUSION
Taken together, results from this study suggest decreased connectivity in a thalamo‐insular subnetwork in patients with AN. This network is believed to be crucial for the propagation of sensations that alert the organism to urgent homeostatic imbalances and pain – a process that is known to be severely disturbed in AN. A lack of or erroneous interoceptive feedback might contribute to the striking discrepancy between their actual and perceived internal body state. Future studies are needed to test whether biofeedback and modern invasive or noninvasive brain stimulation techniques may potentially be of use to improve interoception by modulating activity and connectivity in the thalamo‐insular brain network.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors would like to express their gratitude to Laura Soltwedel, Benjamin Roschinski, Juliane Petermann, Franziska Neidel, Luisa Flohr, Eva Seeger, Lea Scheuvens, Juliane Hantke and Constanze Nicklisch for their assistance with participant recruitment and data collection and thank all participants for their time and cooperation.
Financial Disclosures: In the last two years, Dr. Roessner has received payment for consulting and writing activities from Lilly, Novartis, and Shire Pharmaceuticals, lecture honoraria from Lilly, Novartis, Shire Pharmaceuticals, and Medice Pharma, and support for research from Shire and Novartis. He has carried out (and is currently carrying out) clinical trials in cooperation with the Novartis, Shire, and Otsuka companies. Dr. Walter has received travel support and research awards or research support from AstraZeneca, Hexal, GlaxoSmithKline, and Janssen Research.
Conflict of interests: All other authors reported no biomedical financial interests or potential conflicts of interest.
REFERENCES
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Augustine JR (1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22:229–244. [DOI] [PubMed] [Google Scholar]
- Bai F, Shu N, Yuan Y, Shi Y, Yu H, Wu D, Wang J, Xia M, He Y, Zhang Z (2012): Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. J Neurosci 32:4307–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar KJ, Berger S, Schwier C, Wutzler U, Beissner F (2013): Insular dysfunction and descending pain inhibition in anorexia nervosa. Acta Psychiatr Scand 127:269–278. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Meyer‐Lindenberg A, Achard S, Duke T, Bullmore E (2006): Adaptive reconfiguration of fractal small‐world human brain functional networks. Proc Natl Acad Sci USA 103:19518–19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET, Meyer‐Lindenberg A, Apud JA, Weinberger DR, Coppola R (2009): Cognitive fitness of cost‐efficient brain functional networks. Proc Natl Acad Sci USA 106:11747–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff‐Grethe A, McCurdy D, Grenesko‐Stevens E, Zoe Irvine LE, Wagner A, Wendy Yau WY, Fennema‐Notestine C, Wierenga CE, Fudge JL, Delgado MR, McCurdy D, Grenesko‐Stevens E, Zoe Irvine LE, Wagner A, Wendy Yau WY, Fennema‐Notestine C, Wierenga CE, Fudge JL, Delgado MR, Kaye WH (2013): Altered brain response to reward and punishment in adolescents with Anorexia nervosa. Psychiatry Res 214:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Boehm I, Geisler D, King JA, Ritschel F, Seidel M, Deza Araujo Y, Petermann J, Lohmeier H, Weiss J, Walter M, et al. (2014): Increased resting state functional connectivity in the fronto‐parietal and default mode network in anorexia nervosa. Front Behav Neurosci 2;8:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt V, Krause AL, Starck T, Nissila J, Timonen M, Kiviniemi V, Walter M (2014): Graph theory reveals hyper‐functionality in visual cortices of Seasonal Affective Disorder patients. World J Biol Psychiatry 2:1–12. [DOI] [PubMed] [Google Scholar]
- Braun U, Plichta MM, Esslinger C, Sauer C, Haddad L, Grimm O, Mier D, Mohnke S, Heinz A, Erk S, et al. (2012): Test‐retest reliability of resting‐state connectivity network characteristics using fMRI and graph theoretical measures. Neuroimage 59:1404–1412. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, O'Daly O, Uher R, Friederich HC, Giampietro V, Brammer M, Williams SC, Schioth HB, Treasure J, Campbell IC (2012): Thinking about eating food activates visual cortex with reduced bilateral cerebellar activation in females with anorexia nervosa: an fMRI study. PLoS One 7:e34000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2009): Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS (2011): Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol 7:113–140. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Smith A, Dufwenberg M, Sanfey AG (2011): Triangulating the neural, psychological, and economic bases of guilt aversion. Neuron 70:560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG (2013): Decoding the role of the insula in human cognition: Functional parcellation and large‐scale reverse inference. Cereb Cortex 23:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao‐Gan Y, Yu‐Feng Z (2010): DPARSF: A MATLAB Toolbox for "Pipeline" data analysis of resting‐state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L, Bramati IE, Zalesky A, Furukawa E, Fontenelle LF, Moll J, Tripp G, Mattos P (2012): Altered functional brain connectivity in a non‐clinical sample of young adults with attention‐deficit/hyperactivity disorder. J Neurosci 32:17753–17761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF (2010): Advances and pitfalls in the analysis and interpretation of resting‐state FMRI data. Front Syst Neurosci 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowdrey FA, Park RJ, Harmer CJ, McCabe C (2011): Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psychiatry 70:736–743. [DOI] [PubMed] [Google Scholar]
- Cowdrey FA, Filippini N, Park RJ, Smith SM, McCabe C (2014): Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum Brain Mapp 35:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002): How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Craig AD (2011): Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci 1225:72–82. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1996): The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 351:1413–1420. [DOI] [PubMed] [Google Scholar]
- de Zwaan M, Biener D, Schneider C, Stacher G (1996): Relationship between thresholds to thermally and to mechanically induced pain in patients with eating disorders and healthy subjects. Pain 67:511–512. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA (2011): Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex 21:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE (2006): A core system for the implementation of task sets. Neuron 50:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM (2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23:475–483. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR (2009): At the heart of the ventral attention system: The right anterior insula. Hum Brain Mapp 30:2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erberich SG, Panigrahy A, Friedlich P, Seri I, Nelson MD, Gilles F. (2006): Somatosensory lateralization in the newborn brain. Neuroimage 29:155–161. [DOI] [PubMed] [Google Scholar]
- Favaro A, Santonastaso P, Manara R, Bosello R, Bommarito G, Tenconi E, Di Salle F (2012): Disruption of visuospatial and somatosensory functional connectivity in anorexia nervosa. Biol Psychiatry 72:864–870. [DOI] [PubMed] [Google Scholar]
- Favaro A, Tenconi E, Degortes D, Manara R, Santonastaso P (2014): Obstetric complications and striatum connectivity in AN. Int J Eat Disord 47:686–695. [DOI] [PubMed] [Google Scholar]
- Fichter M, Quadflieg N (1999). SIAB. Strukturiertes Inventar für Anorektische und Bulimische Essstörungen nach DSM‐IV und ICD‐10. Bern: Huber. [Google Scholar]
- Frank GK (2013): Altered brain reward circuits in eating disorders: Chicken or egg? Curr Psychiatry Rep 15:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich HC, Brooks S, Uher R, Campbell IC, Giampietro V, Brammer M, Williams SC, Herzog W, Treasure J (2010): Neural correlates of body dissatisfaction in anorexia nervosa. Neuropsychologia 48:2878–2885. [DOI] [PubMed] [Google Scholar]
- Frieling H, Fischer J, Wilhelm J, Engelhorn T, Bleich S, Hillemacher T, Dorfler A, Kornhuber J, de Zwaan M, Peschel T (2012): Microstructural abnormalities of the posterior thalamic radiation and the mediodorsal thalamic nuclei in females with anorexia nervosa—A voxel based diffusion tensor imaging (DTI) study. J Psychiatry Res 46:1237–1242. [DOI] [PubMed] [Google Scholar]
- Gizewski ER, Rosenberger C, de Greiff A, Moll A, Senf W, Wanke I, Forsting M, Herpertz S (2010): Influence of satiety and subjective valence rating on cerebral activation patterns in response to visual stimulation with high‐calorie stimuli among restrictive anorectic and control women. Neuropsychobiology 62:182–192. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS (2011): Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinf 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebebrand J, Casper R, Treasure J, Schweiger U (2004): The need to revise the diagnostic criteria for anorexia nervosa. J Neural Transm 111:827–840. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Lawson EA, Blum J, Ko E, Makris N, Fazeli PK, Klibanski A, Goldstein JM (2012): Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight‐restored women with anorexia nervosa. J Psychiatry Neurosci 37:322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Lawson EA, Christensen K, Klibanski A, Goldstein JM (2014): Abnormal relationships between the neural response to high‐ and low‐calorie foods and endogenous acylated ghrelin in women with active and weight‐recovered anorexia nervosa. Psychiatry Res 223:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M (2009): New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci 10:573–584. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wagner A, Fudge JL, Paulus M (2011): Neurocircuity of eating disorders. Curr Top Behav Neurosci 6:37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff‐Grethe A (2013a): Nothing tastes as good as skinny feels: The neurobiology of anorexia nervosa. Trends Neurosci 36:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Wagner A, Bischoff‐Grethe A (2013b): Does a shared neurobiology for foods and drugs of abuse contribute to extremes of food ingestion in anorexia and bulimia nervosa? Biol Psychiatry 73:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd A, Steinglass J (2012): What can cognitive neuroscience teach us about anorexia nervosa? Curr Psychiatry Rep 14:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KR, Ku J, Lee JH, Lee H, Jung YC (2012): Functional and effective connectivity of anterior insula in anorexia nervosa and bulimia nervosa. Neurosci Lett 521:152–157. [DOI] [PubMed] [Google Scholar]
- Kromeyer‐Hauschild K, Wabitsch M, Kunze D, Geller D, Geiss HC, Hesse V, Hippel A, von Jaeger U, Johnsen D, Korte W, Menner K, Müller G, Müller JM, Niemann‐Pilatus A, Remer T, Schaefer F, Wittchen H‐U, Zabransky S, Zellner K, Ziegler A, Hebebrand J (2001): Perzentile für den body mass index für das kindes‐ und jugendalter unter heranziehung verschiedener deutscher stichproben. Monatsschr Kinderheilkd 149:807–818. [Google Scholar]
- Kullmann S, Giel KE, Hu X, Bischoff SC, Teufel M, Thiel A, Zipfel S, Preissl H: Impaired inhibitory control in anorexia nervosa elicited by physical activity stimuli. Soc Cogn Affect Neurosci 9:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Giel KE, Teufel M, Thiel A, Zipfel S, Preissl H (2014): Aberrant network integrity of the inferior frontal cortex in women with anorexia nervosa. Neuroimage Clin 4:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB (2010): A link between the systems: Functional differentiation and integration within the human insula revealed by meta‐analysis. Brain Struct Funct 214:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenbacher S, Pauls AM, Strian F, Pirke KM, Krieg JC (1991): Pain sensitivity in anorexia nervosa and bulimia nervosa. Biol Psychiatry 29:1073–1078. [DOI] [PubMed] [Google Scholar]
- Lee S, Ran Kim K, Ku J, Lee JH, Namkoong K, Jung YC (2014): Resting‐state synchrony between anterior cingulate cortex and precuneus relates to body shape concern in anorexia nervosa and bulimia nervosa. Psychiatry Res 221:43–48. [DOI] [PubMed] [Google Scholar]
- Liang X, Wang J, Yan C, Shu N, Xu K, Gong G, He Y (2012): Effects of different correlation metrics and preprocessing factors on small‐world brain functional networks: A resting‐state functional MRI study. PLoS One 7:e32766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J, Garrett A, Beenhakker J, Reiss AL (2011): Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am J Psychiatry 168:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord A, Horn D, Breakspear M, Walter M (2012): Changes in community structure of resting state functional connectivity in unipolar depression. PLoS One 7:e41282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden KL, Tregellas JR, Shott ME, Frank GK (2014): Reduced salience and default mode network activity in women with anorexia nervosa. J Psychiatry Neurosci 39:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr HM, Zimmermann J, Roder C, Lenz C, Overbeck G, Grabhorn R (2010): Separating two components of body image in anorexia nervosa using fMRI. Psychol Med 40:1519–1529. [DOI] [PubMed] [Google Scholar]
- Munkres J (1957): Algorithms for the assignment and transportation problems. J Soc Ind Appl Math 5:32–38. [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE (2010): Role of the anterior insula in task‐level control and focal attention. Brain Struct Funct 214:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn K, Frampton I, Gordon I, Lask B (2008): The fault is not in her parents but in her insula—A neurobiological hypothesis of anorexia nervosa. Eur Eat Disord Rev 16:355–360. [DOI] [PubMed] [Google Scholar]
- Nunn K, Frampton I, Fuglset TS, Torzsok‐Sonnevend M, Lask B (2011): Anorexia nervosa and the insula. Med Hypotheses 76:353–357. [DOI] [PubMed] [Google Scholar]
- Oberndorfer T, Simmons A, McCurdy D, Strigo I, Matthews S, Yang T, Irvine Z, Kaye W (2013a): Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Res 214:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberndorfer TA, Frank GK, Simmons AN, Wagner A, McCurdy D, Fudge JL, Yang TT, Paulus MP, Kaye WH (2013b): Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am J Psychiatry 170:1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul TT, A (2005). Eating Disorder Inventory‐2 (EDI‐2). Göttingen: Hogrefe; 48 p. [Google Scholar]
- Petermann F, Petermann U (2008): Hamburg wechsler intelligenztest für kinder IV (HAWIK‐IV). Bern Bern: Huber. [Google Scholar]
- Pritchard TC, Macaluso DA, Eslinger PJ (1999): Taste perception in patients with insular cortex lesions. Behav Neurosci 113:663–671. [PubMed] [Google Scholar]
- Raymond NC, Faris PL, Thuras PD, Eiken B, Howard LA, Hofbauer RD, Eckert ED (1999): Elevated pain threshold in anorexia nervosa subjects. Biol Psychiatry 45:1389–1392. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Mondraty N, Wen W, Gulliford K (2008): Brains of anorexia nervosa patients process self‐images differently from non‐self‐images: An fMRI study. Neuropsychologia 46:2161–2168. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD (2003): The neural basis of economic decision‐making in the Ultimatum Game. Science 300:1755–1758. [DOI] [PubMed] [Google Scholar]
- Sato Y, Saito N, Utsumi A, Aizawa E, Shoji T, Izumiyama M, Mushiake H, Hongo M, Fukudo S (2013): Neural basis of impaired cognitive flexibility in patients with anorexia nervosa. PLoS One 8:e61108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, et al. (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O (2011). Networks of the Brain. Cambridge: MIT. [Google Scholar]
- Steinhausen HC (2002): The outcome of anorexia nervosa in the 20th century. Am J Psychiatry 159:1284–1293. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Matthews SC, Simmons AN, Oberndorfer T, Klabunde M, Reinhardt LE, Kaye WH (2013): Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: Evidence of interoceptive dysregulation. Int J Eat Disord 46:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Laufs H (2014): Decoding wakefulness levels from typical fMRI resting‐state data reveals reliable drifts between wakefulness and sleep. Neuron 82:695–708. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Verstraete E, Veldink JH, Mandl RC, van den Berg LH, van den Heuvel MP (2011): Impaired structural motor connectome in amyotrophic lateral sclerosis. PLoS One 6:e24239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via E, Zalesky A, Sanchez I, Forcano L, Harrison BJ, Pujol J, Fernandez‐Aranda F, Menchon JM, Soriano‐Mas C, Cardoner N, et al. (2014): Disruption of brain white matter microstructure in women with anorexia nervosa. J Psychiatry Neurosci 39:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Aster M, Neubauer AC, Horn R (2006): WIE—Wechsler Intelligenztest für Erwachsene. Bern: Huber. [Google Scholar]
- Wager TD, Barrett LF (2004): From affect to control: functional specialization of the insula in motivation and regulation. PsycExtra. Retrieved from http://www.apa.org/psycextra/ on 24 December 2005. [Google Scholar]
- Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, Frank GK, Bailer UF, Fischer L, Nguyen V, et al. (2007): Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry 164:1842–1849. [DOI] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Mazurkewicz L, Fudge J, Frank GK, Putnam K, Bailer UF, Fischer L, Kaye WH (2008): Altered insula response to taste stimuli in individuals recovered from restricting‐type anorexia nervosa. Neuropsychopharmacology 33:513–523. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto‐Castanon A, LaViolette P, et al. (2009): Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first‐degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga C, Bischoff‐Grethe A, Melrose AJ, Grenesko‐Stevens E, Irvine Z, Wagner A, Simmons A, Matthews S, Yau WY, Fennema‐Notestine C, et al. (2014): Altered BOLD response during inhibitory and error processing in adolescents with anorexia nervosa. PLoS One 9:e92017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Schlaggar BL, Petersen SE (2011): Concepts and principles in the analysis of brain networks. Ann N Y Acad Sci 1224:126–146. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011): Large‐scale automated synthesis of human functional neuroimaging data. Nat Methods 8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET (2010a): Network‐based statistic: identifying differences in brain networks. Neuroimage 53:1197–1207. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Harding IH, Cocchi L, Yucel M, Pantelis C, Bullmore ET (2010b): Whole‐brain anatomical networks: Does the choice of nodes matter? Neuroimage 50:970–983. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, Egan GF, Pantelis C (2011): Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry 69:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Egan GF, Pantelis C, Bullmore ET (2012): The relationship between regional and inter‐regional functional connectivity deficits in schizophrenia. Hum Brain Mapp 33:2535–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastrow A, Kaiser S, Stippich C, Walther S, Herzog W, Tchanturia K, Belger A, Weisbrod M, Treasure J, Friederich HC (2009): Neural correlates of impaired cognitive‐behavioral flexibility in anorexia nervosa. Am J Psychiatry 166:608–616. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, Gong Q (2011): Disrupted brain connectivity networks in drug‐naive, first‐episode major depressive disorder. Biol Psychiatry 70:334–342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


