Abstract
Ample evidence indicates that inhibitory control (IC), a key executive component referring to the ability to suppress cognitive or motor processes, relies on a right‐lateralized fronto‐basal brain network. However, whether and how IC can be improved with training and the underlying neuroplastic mechanisms remains largely unresolved. We used functional and structural magnetic resonance imaging to measure the effects of 2 weeks of training with a Go/NoGo task specifically designed to improve frontal top‐down IC mechanisms. The training‐induced behavioral improvements were accompanied by a decrease in neural activity to inhibition trials within the right pars opercularis and triangularis, and in the left pars orbitalis of the inferior frontal gyri. Analyses of changes in brain anatomy induced by the IC training revealed increases in grey matter volume in the right pars orbitalis and modulations of white matter microstructure in the right pars triangularis. The task‐specificity of the effects of training was confirmed by an absence of change in neural activity to a control working memory task. Our combined anatomical and functional findings indicate that differential patterns of functional and structural plasticity between and within inferior frontal gyri enhanced the speed of top‐down inhibition processes and in turn IC proficiency. The results suggest that training‐based interventions might help overcoming the anatomic and functional deficits of inferior frontal gyri manifesting in inhibition‐related clinical conditions. More generally, we demonstrate how multimodal neuroimaging investigations of training‐induced neuroplasticity enable revealing novel anatomo‐functional dissociations within frontal executive brain networks. Hum Brain Mapp 36:2527–2543, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: inhibitory control, plasticity, frontal, functional magnetic resonance imaging, voxel‐based morphometry, tract‐based spatial statistics
INTRODUCTION
Inhibitory control (IC) is a key component of executive functions referring to the ability to cancel cognitive or motor processes (Aron et al. 2004). An efficient suppression of unwanted responses allows maintaining well‐adapted goal‐directed behaviors in new or changing situations (Aron 2007; Dillon and Pizzagalli 2007).
Ample evidence indicates that IC relies on a cortico‐subcortical brain network including the posterior right inferior prefrontal gyrus (rIFG), the pre‐supplementary motor area (pre‐SMA) and the basal ganglia (BG) (Aron 2007, 2011; Garavan et al. 1999; Majid et al. 2012). However, while current literature provides a clear picture of the neural underpinnings of IC, whether and how this function can be improved by training remains largely unresolved. This lack of information on the effects of IC training on behavior and on the anatomo‐functional organization of the brain contrasts with the increasing number of studies that has focused on the effects of training in other executive functions (e.g., working memory: Klingberg 2010; or attention: Kramer et al. 1995; Green and Bavelier 2012; Slagter 2012). Beyond its conceptual importance, this question has a clinical relevance as deficits in IC represent a core component of several psychiatric disorders ranging from addiction (Harle et al. 2014; Monterosso et al. 2005) to attention deficit hyperactivity disorder (ADHD; e.g., Johnstone et al. 2012). Improving IC proficiency and the underlying brain networks with training may, thus, help the rehabilitation of inhibition‐related brain disorders.
Behaviorally, significant IC performance improvements have been reported after short‐term (50 min; Manuel et al. 2010; Manuel et al. 2013; Verbruggen and Logan 2008) or medium‐term training (10h over 3 weeks in Berkman et al. 2014; 7.5 h over 25 days in Johnstone et al. 2012; 5 h over 20 days in Thorell et al. 2009, see Spierer et al. 2013 for review).
To our knowledge, only four studies addressed the functional brain changes induced by IC training. Three of them showed that repeated associations between response inhibition and specific stimuli either cueing or triggering stopping led to an automatic triggering of inhibitory processes by the rIFG (Berkman et al. 2014; Lenartowicz et al. 2011) or parietal cortices in response to NoGo stimuli (Manuel et al. 2010). Manuel et al. (2013) further observed a reinforcement of top‐down controlled forms of IC mediated by a decrease in the response strength of a right fronto‐striatal network (including the inferior frontal gyrus (IFG)) to Stop signal after a short Stop‐Signal Task (SST) training.
These studies suggest that when the stimulus‐response (S‐R) mapping rules are held constant during training, automatic stimulus‐driven forms of IC develop and in turn support the behavioral IC improvement. Recent researches have indeed shown that after IC training on Go/NoGo tasks with constant S‐R mapping rules, NoGo stimuli eventually automatically triggered inhibition process via parietal cortices, which in turn minimized the contribution of top‐down IC processes during the task (Manuel et al. 2010). In contrast, top‐down controlled forms of inhibition are reinforced when the upcoming need for IC is not predictable and the S‐R mapping rules systematically vary in the task used to train IC. When the mapping of the NoGo stimuli with the inhibition goals systematically changes, the development of bottom‐up automatic forms of inhibition is prevented and top‐down frontal IC is repeatedly involved. In turn, frontal networks are modified (Manuel et al. 2013; Spierer et al. 2013). Consequently, as top‐down frontal control processes are largely domain‐general (Aron et al. 2014) and that Go/NoGo training with varying stimulus‐response mapping rules modify frontal structures, the effects of such training regimen should generalize to untrained inhibition tasks involving the same frontal control network.
Although the functional mechanisms underlying short training‐induced behavioral improvement in IC have been identified, the effects of long‐term training remain unresolved. Previous studies on IC plasticity involved indeed only training periods shorter than 10 days. In addition, recent evidence indicates that structural brain modifications might also be expected with executive function training (e.g., Thomas and Baker 2013 for review), but whether microstructural brain modifications may be induced by IC training in addition to functional changes has until now not been investigated. Current literature on training‐induced grey matter (GM) changes mostly suggests that behavioral improvements are supported by increases in GM density (e.g., Draganski et al. 2004; Gaser and Schlaug 2003; May 2011 for review; although see Granert et al. 2011 and Hanggi et al. 2010 for decreases of cortical density when training in the motor domain.). Training‐induced increases in the strength of white matter (WM) connectivity have been repeatedly observed within task‐relevant networks after training or in experts (e.g., Barnes and Finnerty 2010; Lee et al. 2010; Roberts et al. 2010).
To address these questions, we trained healthy volunteers on a Go/NoGo task 1h on a daily basis for 14 consecutive days. During training, stimulus‐response mapping rules were systematically varied to prevent the development of an automatized form of inhibition and reinforce top‐down frontal IC. Functional (hemodynamic responses) and structural changes (grey and WM microstructure) induced by the training were measured by recording magnetic resonance imaging (MRI) before and after the training. The task‐specificity of the effects of training was further controlled by recording fMRI responses during a 2‐back working memory task.
We hypothesize that our training regimen will modulate hemodynamic responses of the posterior parts of the right inferior frontal cortex to inhibition trials, these regions being central in IC (Aron et al. 2014). These functional changes should be accompanied by an increase in right IFG grey matter volume, and an increased fractional anisotropy (FA) within the same area.
MATERIALS AND METHODS
Participants
Twenty‐one healthy volunteers participated in this study. Three participants were excluded from the behavioral, BOLD and VBM analyses due to rapid head movements during scanning, technical problems and to missed responses in the Go/NoGo task greater than 2 standards deviations from the group mean. Another participant was excluded from the WM analysis due to movement‐related artefacts. He was replaced by a participant excluded from the behavioral, functional and GM analyses, who, however, showed sufficient quality of the diffusion tensor imaging (DTI) recording. Eighteen participants (10 female; mean age ± standard error of the mean (SEM): 25.1 ± 0.67 years; range: 22–32) were included in the analyses (with a swap between two participants for the white matter and grey matter analyses).
All participants were right handed according to the Edinburgh handedness inventory (Oldfield 1971) and had normal or corrected‐to‐normal vision. No participant had a history of neurological or psychiatric disease. Each participant provided written, informed consent to participate in the study. All procedures were approved by the local ethics committee.
Procedure and Task
The experiment was divided into three sessions: Pretraining; training; and post‐training, for a total duration of 16 days.
Pre‐ and post‐training sessions
During the pre‐ and post‐training MRI sessions, two functional MRI runs were recorded while the participants performed first an IC Go/NoGo task and then a working memory 2‐back task. A tapping condition was performed after the Go/NoGo and after the 2‐back task. DTI was recorded between the two functional runs and a structural MRI scan at the end of the session.
Go/NoGo task
In the Go/NoGo task, the stimuli were five consonants (S, T, M, H, X) and four vowels (A, E, I, O) presented in black on a white screen. Each trial started with the presentation of a centered black cross for a duration that randomly varied between 1,200 and 2,200 ms (100 ms steps). Immediately after the offset of the cross, one of the nine letters was presented in a pseudorandom fashion for 500 ms. Participants had a maximum of 1,700 ms to respond.
Participants were instructed to reply as fast as possible by a button press with their right index finger each time a letter was shown on the screen, except for the letter “X”. When the letter “X” was presented, participants had to withhold their response. To increase response prepotency, the probability of trial occurrence in each block was of P = 0.3 for the No Go (“X”) and P = 0.7 for the Go. For the Go trials, the probability was equally distributed among the four other consonants (S, T, M, H) and the four vowels (A, E, I, O).
Each participant performed five blocks of 3 min, each including 80 trials. Twenty‐five seconds rest periods separated each block. After each rest period, the instructions were again presented to the participant for 3 s to inform them that a new block was going to start (Fig. 1).
Figure 1.
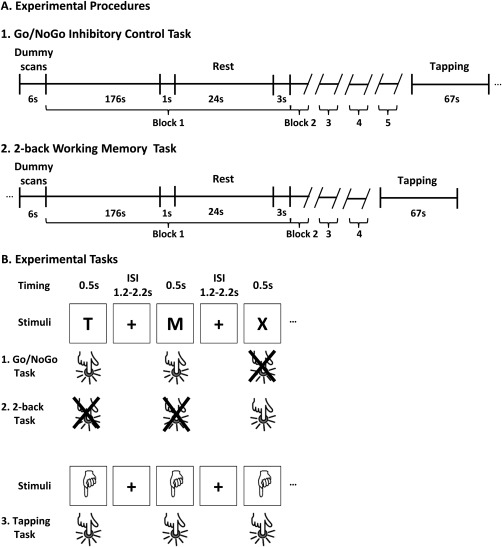
Functional MRI experimental procedures and tasks. A1 and A2: Experimental procedures. B: Experimental tasks. B1: In the inhibitory control (IC) Go/NoGo task, participants had to withhold their responses to a given letter (here, “X”) while responding as fast as possible to all other letters. B2: In the working memory 2‐back task, when the letter “X” was presented, participants were instructed to remember the penultimate letter and press a response button with their right index finger if this letter was a consonant and on another button if the letter was a vowel. B3: In the tapping task, participants had to respond to each picture of a hand. ISI: Inter stimulus interval.
Figure 2.
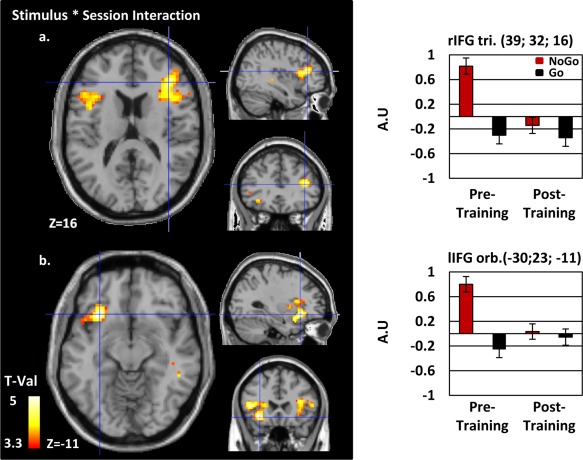
Functional neuroimaging results. The second level ANOVA contrasting responses to Go and NoGo trials for the pre‐ and post‐training sessions are presented on a normalized single‐subject brain in the MNI space. A significant reduction of BOLD activity after training in response to NoGo trials has been identified within a network involving the right (a) and left (b) inferior frontal gyri (rIFG, lIFG), (see Table 1 for additional results). Contrasts are represented at P < 0.001 uncorrected at the voxel level and FDR corrected at the cluster level (min cluster size = 100). Histograms on the right indicate the amplitude of the effect (Arbitrary Unit (AU)) for each conditions at clusters' local maxima. Tri: Triangularis; Orb.: Orbitalis. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Working memory task
In the working memory 2‐back task, the stimuli, number of trial per block, timing, and stimuli presentation parameters were the same as for the Go/NoGo task, except that each letter was presented with the same probability and the maximum response time was of 2,700 ms. When the letter “X” was presented, participants were instructed to remember the penultimate letter and press a response button with their right index finger if this letter was a consonant and on another button if the letter was a vowel. As the letters were presented randomly, it could be that the penultimate letter was also an “X”, so that the “X” was the cue and the target at the same time. Consequently, in the working memory task, possible responses could be five consonants (S, T, M, H, X) and four vowels (A, E, I, O). This unbalance could have biased the decision toward responding a consonant because the probability of being correct by (randomly) responding “consonant” would have been higher than by responding “vowel”. To prevent this bias, we added the vowel “U” to our set of stimuli so that each condition was equiprobable (Fig. 1). The task initially included five blocks, but after three participants we realized that it was too long and induced fatigue; the length of the task was, thus, reduced to four for the remaining participants (only the first four blocks were analyzed in the three first participants).
Tapping condition
The tapping condition consisted in the presentation of the picture of a hand for 500 ms 30 times on the screen over a period of 67 s, with the same intertrial interval as for the Go/NoGo and working memory tasks. Participants were instructed to press on the button with their right index finger each time the picture of a hand was presented. The tapping condition enabled isolating the motor brain activity related to button press, to eventually subtract it from the Go trial in the Go/NoGo task as well as from to the 2‐back task (see the MRI analyses section).
For the Go/NoGo, tapping and working memory tasks, stimulus delivery and response recording were controlled using E‐Prime 2.0 software (Psychology Software Tools, Sharpsburg, PA). The total duration for the Go/NoGo functional run was of 18 min 30 and 15 min for the 2‐back functional run. The total duration of an MRI session was about 45 min.
Training session
Participants were asked to practice on the Go/NoGo task on a computer of their choice in a quiet room for 1 h each day, every day for fourteen consecutive days. The Go/NoGo task was programmed on the java‐based Tatool open‐source programming framework (von Bastian et al. 2013). At the end of the pretraining MRI session, participants were provided with oral and written instructions on how to run the application on their computer. The stimuli presentation parameters were the same as for the MRI Go/NoGo task. During the training, the Go/NoGo task was composed of eight blocks of 150 trials, each separated by six 1‐min and one 3‐min self‐managed breaks. At the same time each day, the participants received via e‐mail a new executable module containing a new version of the paradigm: The task remained the same but the letter to which response had to be inhibited changed every day. The NoGo stimuli were modified each day according to a pseudorandomized order to control that there were no series of 2 or more days with the same NoGo stimuli. Participant's compliance and comprehension of the instructions were systematically checked by the experimenter after each session. The output of each training session was sent by the participant to the experimenter via e‐mail. The latter immediately checked that the right number of trials had been performed, and that the missed trials and false alarm rate was conform to our previous data on the topic (e.g., Manuel et al. 2010).
The delay between the first scan and the first training session varied between 0 and 10 days (mean ± SEM 3.8 ± 0.7). The post‐training MRI recording was always conducted the day after the last training session. The post‐training MRI session was conducted as the pretraining session.
MRI Acquisition and Processing
Data acquisition
For MRI data acquisition, we used a 3T MRI scanner (Discovery MR750; GE Healthcare, Waukesha, WI) with a 32‐channel standard head coil. Participants were positioned in the scanner with their head stabilized by sound‐attenuating memory foam to reduce head movements. Visual stimuli were presented on an LCD screen at 60 Hz with a resolution of 640 × 480 pixels (32″ NNL liquid crystal display (LCD) Monitor, NordicNeuroLab, Bergen, Norway), with visual angles of 1.34° (height) and 1.2° (length) on a screen located at the foot of the MRI bed visible for the participants via a mobile mirror system.
The structural T1‐weighted anatomical scans were acquired with coronal slice orientation. Acquisition parameters were: FSPGR BRAVO sequence, voxel size: 0.86 × 0.86 × 1 mm, matrix size: 256 × 256, Field of View (FOV): 22 cm, number of slices: 276, repetition time (TR) = 7300 ms, echo time (TE) = 2.8 ms, flip angle = 9°, parallel imaging acceleration factor of 1.5 with an intensity correction (SCIC).
Functional T2*‐weighted Echo Planar images (EPI; with a Gradient Echo sequence) were recorded using blood oxygenation level‐dependent contrast (BOLD, Kwong et al. 1992) to assess for local changes in brain activity. A total of 552 dynamic volumes were acquired during the first run (Go/NoGo task) and 447 during the second run (2‐back task). The tapping condition was performed during the 34 last volumes of each run. The EPI images were acquired with the following parameters: axial slice orientation, sequential ascending acquisitions, voxel size: 2.3 × 2.3 × 3 mm, acquired matrix size: 96 × 96, FOV: 22 cm, number of slices: 37, inter slice spacing = 0.2 mm, TR = 2,000 ms, TE = 30 ms, Flip angle = 85°, parallel imaging acceleration factor 2. Each session was preceded by 6 s of dummy scans to ensure a steady‐state magnetization of the tissues.
DTI data was acquired using an echo planar imaging sequence (voxel size: 2 × 2 × 2 mm, acquired matrix size: 128 × 128, FOV: 26 cm, number of axial slices acquired: 60, interslice spacing gap = 0.2 mm, TR = 8,000 ms, TE = 83 ms, parallel imaging acceleration factor 2, number of acquired noncollinear directions = 30, with b‐value = 1,000 s/mm2; one b = 0 image was also acquired).
Functional MRI analysis
Pre‐ and post‐training MRI data were analyzed using the Statistical Parametric Mapping software (SPM8, the Welcome Trust Centre for Neuroimaging, Institute of Neurology, University College London, http://www.fil.ion.ucl.ac.uk/spm) running on Maltab 2012b (MathWorks, http://www.mathworks.com/, MA). The functional images were prepared following a preprocessing procedure for longitudinal designs. In a first step, the origin of the image was manually set on the anterior commissure for better registration. Volumes from the pre‐and the post training sessions were then jointly spatially realigned. The T1‐weighted images of the pre‐ and post‐sessions were also realigned. In a next step, the average of the two realigned T1 was coregistered to the mean of the fMRI image. Standard preprocessing procedures were applied to the functional images (Friston et al. 2007): acquisition delays corrected by slice timing procedure; spatial registration to the Montreal Neurological Institute (MNI) space with 3 × 3 × 3 mm3 voxel size using the spatial registration parameter estimates computed from the mean T1‐weighted images within the “unified segmentation” procedure (Ashburner and Friston 2005); and finally, smoothing with an isotropic 8‐mm full width at half‐maximum (FWHM) Gaussian kernel. The Artrepair toolbox was used on these images to detect presence of rapid movement between scans (one subject was excluded from BOLD analysis because more than 10% of scans showed rapid motion above 0.5 mm/TR for both sessions, see the “Participants” section).
Fixed effect analyses were performed on the preprocessed volumes (i.e., subject‐level analysis) using the general linear model applied on each voxel (Friston 1995; Worsley and Friston 1995). The Go/NoGo and 2‐back task were processed in two separate models, and the control tapping condition was present in each of them. Data for the first and second recording session were combined in each model. For the Go/NoGo task, each stimulus onset was modeled as a delta function, convolved with the hemodynamic response function (HRF; Model 1). Only correct Go and NoGo trials were considered in the analysis (misses and false alarms were modeled as condition of no interest). Stimuli in the 2‐back task (Model 2) and in the Tapping condition (models 1 and 2) were modeled as blocks convolved with the HRF independently of the accuracy of the response. Movement parameters were introduced as covariate of noninterest only in Model 1. Indeed, the inclusion of these parameters in Model 2 is not advisable as it is modeled as a block design (Johnstone et al. 2006). Time series from each voxels were high‐pass filtered with a 1/250 Hz threshold in both cases, to remove low frequency noise and signal drifts. In addition, an autoregressive function (AR(1)) was applied to correct for temporal correlations between neighboring voxels in the whole brain.
A second, group level analysis was performed separately for the Go/NoGo and the 2‐back tasks.
For the Go/NoGo task, the experimental conditions were modeled in a 2 × 2 flexible factorial model (Stimulus (NoGo; Go)* Session (Pre‐; Post‐training)). As there was a manual response in the Go but not in the NoGo condition, we subtracted the activation during the Go/NoGo tapping block from the responses to the Go trials by contrasting them (Go > tapping) to prevent motor activity from the button press to contaminate the results. This contrast, as well as the simple NoGo versus baseline contrast were computed as fixed effect analyses and then submitted to second level analyses. In the random effect analysis, the main effects of factors Session (Pre vs. Post) and Stimulus (NoGo vs. Go), as well as the interaction term were analyzed in both directions by t‐contrasts.
For the 2‐back task, the experimental condition was modeled in a paired T‐test model to compare brain activity between the two sessions. As for Go trials, the tapping block was subtracted from the 2‐back condition to prevent motor activity from the button press to contaminate the contrasts.
The significance threshold for the functional results was P < 0.001 at the voxel level, corrected for multiple comparison with an extended cluster threshold size of 100 contiguous voxels (p cluster < 0.05; false discovery rate (FDR) corrected for topological analysis (Chumbley and Friston 2009)).
An additional region of interest (ROI) analysis was performed over all participants to test for the task‐specificity of the effects of training by comparing changes in the IFG responses between the Go/NoGo and the 2‐back task using the Marsbar toolbox (http://marsbar.sourceforge.net/). Differences in brain activity between Pretraining (Nogo – Go) vrsus Post‐training (Nogo – Go) and Pretraining (2‐back – tapping) versus Post‐training (2‐back – tapping) was assessed with a two‐sample paired t‐test model within the AAL rIFG ROI (Tzourio‐Mazoyer et al. 2002). Results were extracted from the marsbar statistical table for this contrast.
The clusters' maxima were localized in the Montreal Neurological Institute and Hospital (MNI) space. The WFU pickAtlas software (Maldjian et al. 2003; Maldjian et al. 2004) was used to determine their anatomical locations based on the AAL atlas (Tzourio‐Mazoyer et al. 2002). The results are displayed according to the neurological convention.
Voxel‐based morphometry
Prior to automated processing all T1‐weighted images were visually inspected to detect artifacts or anatomical abnormalities. Voxel‐based morphometry (VBM; Ashburner and Friston 2000) was performed using the pairwise longitudinal registration toolbox, part of SPM12b (v5918, http://www.fil.ion.ucl.ac.uk/spm). This framework enables optimal data processing of longitudinal MRI data combining diffeomorphic and rigid‐body registration, as well as correcting for intensity inhomogeneity (Ashburner and Ridgway 2012). The computed Jacobian change map describes the rate of changes between time points, normalized by the time span inbetween. The mean T1 images of each participants stemming from the previous procedure were segmented in GM, WM and cerebrospinal fluid using the default segment procedure implemented in SPM12. This algorithm proposes an improved version of the “unified segmentation” method present in a previous version of SPM (Ashburner and Friston 2005; Streitburger et al. 2014). The GM and WM of the whole dataset were nonlinearly transformed to a customized template in standard MNI space using a diffeomorphic registration algorithm (DARTEL, Ashburner 2007). GM probability maps in the native space were multiplied by the Jacobian change rate map (computed during the pairwise longitudinal registration) for each of the participants, followed by a modulation to ensure that relative volumes were preserved after spatial normalization in MNI space using the flow field and templates previously generated by DARTEL (Good et al. 2001). Finally, the resulting images were submitted to a smoothing procedure with an 8 mm FWHM isotropic gaussian kernel to increase the signal to noise ratio.
After automated preprocessing all maps of between sessions differences were analyzed using random effect one‐sample T‐tests for significant grey matter volume (GMV) differences between pre‐ training and post‐training sessions. The search volume was restricted to the right and left inferior frontal gyri (rIFG and lIFG, respectively) defined by the AAL atlas (Tzourio‐Mazoyer et al. 2002).
We chose to analyze VBM and DTI data using a ROI‐based approach for the following reasons. First, ample literature points out the inferior frontal gyri as the key structures in IC (Aron et al., 2014 for review). Second, the whole‐brain functional results confirmed the critical role of the bilateral inferior frontal gyri in the training‐induced plasticity investigated in the present study. On this basis, and to increase our statistical power, we decided to restrict our analyses on the microstructural changes induced by the training to the bilateral IFG.
Separate voxel‐wise analyses (using one‐sample t‐test models) for rIFG and lIFG were performed with age, total intracranial volume and sex as confounding covariates. We used no grand mean scaling, no threshold masking, omitted global calculation, implicit and explicit masks on the predefined ROI. The significance threshold was set to p FWE < 0.05 corrected for multiple comparisons at the voxel level (FWE: family‐wise error rate corrected). In addition, the parameter estimates of the pre‐ versus post‐training GMV differences were extracted across participants in the local maxima in the rIFG and the corresponding voxel in the left hemisphere to statistically test whether the difference between the effects of training were different between the right and left IFG. In addition, to help disentangle the differential role of the left and right IFG in IC plasticity, this analysis enabled controlling for the regional specificity of the effects of training (Chumbley et al. 2010).
TBSS DTI analyses
DTI data were analyzed with the tract‐based spatial statistics approach (TBSS, Smith et al. 2006; Smith et al. 2007) using the FSL 5.0.4 software (FMRIB software library, http://www.fmrib.ox.ac.uk/fsl; Jenkinson et al. 2012). First, data were visually inspected for artifacts. Then, diffusion‐weighted images were affine‐aligned to the first b0 image to limit distortions due to head movements as well as eddy‐current using the eddy‐current correction tool of the FDT toolbox. Next, the diffusion tensor was fitted to the data with the DTIFIT tool to compute the FA diffusion index. An inspection of the FA data revealed a signal outside the 0–1 range for one of the participant previously integrated in fMRI and VBM analysis, indicating a bad signal to noise ratio. To avoid losing statistical power, his data were, thus, replaced by a participant who was previously excluded from the fMRI and VBM analyses due to excessive movement during the fMRI. His DTI data were not affected by large head movements. In the next steps, the FA data were processed with the same procedure for longitudinal TBSS study as in Engvig et al. (2012). The first step was to register the two time points of each participant on a common space to take account for residual variation due to multiple time‐points like the geometry variations following gradient calibration drift or changes in head position in the scanner. The FLIRT algorithm (Jenkinson and Smith 2001) was used to resample each time point to the halfspace between them to minimize registration bias toward one of the time‐points (Smith et al. 2001). The second step was to create an FA template image for each subject by averaging the two registered FA images: pre‐ and post‐training. These FA template images were nonlinearly transformed on the mean FA template provided by FSL (FMRIB58_FA) and then affine transformed on the standard MNI space. The resulting images were used to create the study‐specific mean FA image which was skeletonized with a threshold FA > 0.3 to generate the white‐matter tract skeleton representing tracts common to all subjects. The third step aimed at projecting each individual FA images of the pre and the post training sessions onto the reference skeleton generated during Step 2. Before Step 3, the pre and post training FA images were smoothed with a small gaussian kernel (sigma = 2) to further account for small registration errors between time points which may decrease the sensitivity to regional effects (Engvig et al. 2012; Madhyastha et al. 2014). Then, the identical nonlinear warp and skeleton projection as in Step 2 were applied to smoothed FA images. The utilization of an identical processing on a common template ensures that between‐sessions statistical difference could not be attributed to residual variation of processing between them.
To study the FA differences between the first and the second session, data from Step 3 were analyzed voxel‐wise using two‐sample paired t‐test statistics with permutation testing (randomize tool in FSL with 5,000 permutations; Nichols and Holmes 2002). As for the VBM analyses, this statistic was performed separately, voxel‐wise, within the rIFG and lIFG AAL ROIs (Tzourio‐Mazoyer et al. 2002). For this purpose, we created a mask by superposing the two ROI from the AAL atlas and the WM skeleton. Statistical inference was done based on the permuted P‐values, which included the threshold‐free cluster enhancement (TFCE) provided by FSL with a threshold of P < 0.05. The results were thickened to facilitate the visualization. In addition, the FA values across participant inside of local maxima of rIFG and its corresponding voxel in lIFG were extracted, to statistically test whether the differences between the effects of training were different between the right and left IFG with a paired t‐test.
RESULTS
Behavior
Participants completed the same Go/NoGo task during the pre‐ and post‐training sessions and each of the 14 days of training. The effects of training were assessed by comparing performance during the pretraining session and the post‐training fMRI session. Participants responded faster to Go trials after than before the training (mean±SEM: pretraining: 407.4 ± 9.1 ms post‐training: 380.1 ± 14.6 ms; t(17) = 2.5, P < 0.03; Dz = 0.6). There was no evidence for a change in false alarm rate (inaccurate responses to NoGo stimuli; mean±SEM: pretraining: 17.1 ± 2.5%; post‐training: 24.2 ± 5.0%; t(17) = −1.6, P = 0.1).
For the 2‐back working memory task, the rate of correct responses increased from the pre‐ to post‐training session (mean ± SEM: pretraining: 79.1 ± 4.2%; post‐training: 82.7 ± 2.2%; t(17) = −2.59, P < 0.03; Dz = 0.6).
Functional Magnetic Resonance Imaging
For the Go/NoGo task, whole‐brain data were analyzed with a 2*2 flexible factorial ANOVA design with factors Stimulus (NoGo; Go) and Session (Pre‐; Post‐training), the significance thresholds for all functional analyses were P < 0.001, min. cluster size = 100. There was a main effect of Session with stronger activity in the pre‐ than post‐training session in a right inferior and middle frontal network, extending to the bilateral inferior parieto‐temporal areas, also including the right angular gyrus and the left cerebellum (Supporting Information Fig. 2A). There was a main effect of stimulus with stronger activity to NoGo than Go trials in a large fronto‐parieto‐occipital bilateral network (Supporting Information Fig. 2B); and a stronger activity to Go than NoGo trials in the bilateral lingual gyri. Critically, there was a Stimulus * Session interaction within a bilateral fronto‐temporal network extending to the anterior insula. In the left and right inferior frontal gyri, the interaction was driven by a decrease in the neural activity to NoGo but not Go trials with training (mean ± SEM signal change; rIFG NoGo pretraining: 0.82 ± 0.13; rIFG NoGo post‐training −0.14 ± 0.13; rIFG Go pretraining: −0.3 ± 0.13; rIFG Go post‐training −0.34 ± 0.13; lIFG NoGo pretraining: 0.8 ± 0.12; lIFG NoGo post‐training 0.03 ± 0.12; lIFG Go pretraining: −0.26 ± 0.13; lIFG Go post‐training −0.05 ± 0.13; Fig. 2 and Table 1).
Table 1.
Locations of clusters local maxima for the functional MRI interaction
| Anatomical region | Vx | MNI coordinates | Z | P | ||
|---|---|---|---|---|---|---|
| A | ||||||
| Right inferior frontal pars triangularis | 272 | 39 | 32 | 16 | 4.30 | <0.001 |
| Right inferior frontal pars opercularis | 42 | 17 | 13 | 4.22 | <0.001 | |
| Right inferior frontal pars triangularis | 36 | 23 | 13 | 4.12 | <0.001 | |
| B | ||||||
| Left superior temporal | 623 | −39 | 8 | −26 | 4.82 | <0.001 |
| Left inferior frontal pars orbitalis | −30 | 23 | −11 | 4.68 | <0.001 | |
| Left insula | −33 | 14 | −17 | 4.49 | <0.001 | |
| C | ||||||
| Right superior temporal | 290 | 51 | −25 | 1 | 4.27 | <0.001 |
| Right temporal* | 48 | −43 | −5 | 4.26 | <0.001 | |
| Right middle temporal | 60 | −49 | 1 | 3.54 | <0.001 | |
Stereotaxic brain MNI coordinates for peak‐voxels of the functional interaction Session (Pre, Post) * Stimulus (NoGo, Go). Results are represented at P < 0.001 uncorrected at the voxel level and FDR corrected at the cluster level (min cluster size = 100). The table is organized by activation clusters with 3 local maxima more than 8mm apart. Vx = voxel size, Z = Z‐value, and P = P‐value of the voxel. An asterisk (*) indicates the nearest GM for this particular peak.
Whole‐brain analysis of the 2‐back task did not identify any differences in BOLD signal in a second level paired t‐test comparing brain activity between the pre‐ and the post‐training sessions (for the contrast 2‐back ‐ tapping). To further test for the specificity of the effects to the Go/NoGo (GNG) task, we selected the three clusters showing a change in activity during the GNG task before versus after the training and performed an ROI analysis on each of the clusters pre versus post training during the 2‐back task. None of the one‐tail t‐test reached significance (all P‐vals > 0.07), indicating that the change in neural activity induced by the training was specific to the GNG training.
The task‐specificity of the Go/NoGo training was statistically assessed by calculating the difference in brain activity before versus after the training between the Go/NoGo and the 2‐back task in a marsbar ROI model within the AAL rIFG. A two‐sample paired t‐test analysis (one‐tail) revealed that the effect of training was larger for the Go/NoGo than for the 2‐back task (mean ± SEM, GNG: 0.66 ± 0.19; NBACK: −0.19 ± 0.19; t(17) = −2.83, P = 0.006, Dz = 0.66).
Focusing on each task separately, a significant difference was revealed pre versus post training for the GNG only (mean ± SEM, GNG PRE: 1.19 ± 0.18; GNG POST: 0.51 ± 0.13; PRE versus POST: t(17) = 3.48, P = 0.0014, Dz = 1; POST versus PRE: t(17) = −3.48, P = 0.99) and not for the NBACK (mean ± SEM, NBACK PRE: 0.098 ± 0.15; NBACK POST: 0.26 ± 0.16; PRE versus POST: t(17) = −1.03, P = 0.084; POST versus PRE: t(17) = 1.03, P = 0.15).
Structural Magnetic Resonance Imaging
Grey matter: Voxel‐based morphometry
Separated voxel‐wise analyses were conducted within the left and right IFG AAL ROI. The results show that GMV is higher in the rIFG after training (MNI xyz = 57 29 −7, p FWE < 0.05 voxel level corrected; Fig. 3).
To compare training‐induced GMV changes in the left versus the right IFG, we extracted the pre‐ versus post‐training GMV eigenvariates at the peak‐voxel of the right IFG and compared these values with those of the homotopic voxel of the left IFG with a paired t‐test. The change in GMV with training in the rIFG was higher than in the left IFG (mean ± SEM; rIFG 0.004 ± 0.0012; lIFG −0.0008 ± 0.0025 t(17) = 2.006, P = 0.06, Dz = 0.4).
Because the pre‐SMA has been repeatedly involved in IC, we performed a supplementary ROI analysis to test for GMV differences between the pre‐ and post‐training session in the left and right pre‐SMA: We did not find any evidence for an effect with a voxel‐wise one‐sample t‐test at our significance threshold of p FWE > 0.05.
White matter: DTI tract‐based spatial statistics
The same ROI‐based approach as for the VBM analyses was used for the TBSS analyses: Separated voxel‐wise analyses were conducted in the left and right IFG AAL ROI. The results show that the FA was higher within the rIFG ROI after than before training (MNIxyz = 41 33 7; pTFCE < 0.05, cluster level corrected; Fig. 4).
Figure 4.
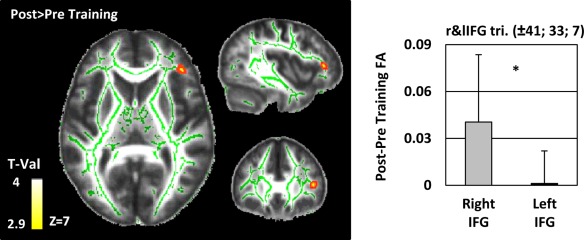
White matter tract‐based spatial statistics. Differences in fractional anisotropy (FA) between the pre‐ and post‐training sessions were estimated in the left and the right inferior frontal gyrus (IFG) as defined by the AAL atlas (Tzourio‐Mazoyer et al. 2002). Results are projected on the study‐specific mean FA image with a TFCE corrected threshold of P < 0.05. Results are thickened for visualization purpose. The study‐specific skeleton is displayed in green. The bargraph indicates the averaged values of the difference (Post ‐ Pre) for the right IFG and left IFG separately. As no FA differences were detected in the left IFG, we based our analyses on the homotopic right IFG peak‐voxel. The paired t‐test comparing the FA differences between the right and the left inferior frontal gyri revealed a larger increase in FA in the right than in the left IFG (asterisk: P = 0.001). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
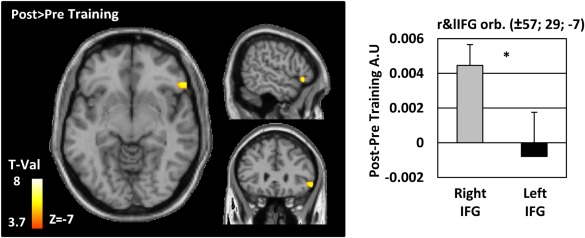
Grey matter voxel‐based morphometry results. Differences in grey matter volume (GMV) between the pre‐ and post‐training sessions were estimated in the left and the right inferior frontal gyrus (IFG) as defined by the AAL atlas (Tzourio‐Mazoyer et al. 2002). Results are projected on a normalized single‐subject brain in the MNI space with a FWE corrected threshold of P < 0.05 at the voxel level. Red‐Yellow voxels indicate GMV increase with training. The bargraph indicates the averaged values of the difference (Post‐Pre) for the right IFG and left IFG separately. As no volume differences were detected in the left IFG, we based our analyses on the homotopic right IFG peak‐voxel. A paired t‐test comparing the GMV differences between the right and the left inferior frontal gyri revealed a larger increase of GMV in the right but not the left IFG (asterisk: P = 0.001). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To compare changes in the left and right IFG, we proceeded in the same way as for the GM analysis: We extracted the pre‐ minus post‐training FA values at the peak‐voxel of the right IFG and compared these values with those of the homotopic voxel of the left IFG with a paired t‐test. The change in FA with training in the rIFG was higher than in the left IFG (mean ± SEM; rIFG 0.04 ± 0.01; lIFG 0.001 ± 0.005 t(17) = 3.955, P = 0.001, Dz = 0.9).
Figure 5 summarizes the main functional and structural results.
Figure 5.
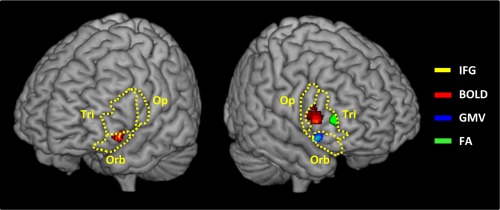
Schematic representation of the cluster maxima of the plastic modification in Blood oxygen level‐dependent signal (BOLD, red), grey matter volume (GMV, blue), and fractional anisotropy (FA, green). The left and right inferior frontal gyri (dashed yellow lines), pars opercularis (Op.), triangularis (Tri.), and orbitalis (Orb.) are represented. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Correlation analyses
There were no evidence for correlations between the functional and structural MRI training‐induced modifications, nor between these values and the changes in behavioral performance as indexed by response time (RT) of false alarm rate (all Pearson P‐values > 0.05).
DISCUSSION
Our results demonstrate the functional and structural brain plasticity induced by two weeks of IC training. The training improved Go/NoGo performance, and these behavioral changes were paralleled by a decrease in brain activity to inhibition trials within both the left and right inferior frontal gyri. Analyses of the brain anatomical changes induced by the training revealed an increase in GMV and in WM microstructure properties within the right inferior frontal gyrus.
Behaviorally, we showed that the training improved IC performance: response time to Go trials decreased while the rate of commission errors to NoGo trials remained stable. This specific pattern of result replicates previous literature on the effect of IC training and can be interpreted as reflecting an increase in the speed of inhibition processes (Benikos et al. 2013; Berkman et al. 2014; Manuel et al. 2010; Manuel et al. 2013; Verbruggen et al. 2012; White et al. 2014). Although an increase in IC proficiency would most intuitively be expected to manifest as a decrease in false alarm rate, current models suggest that a decrease in responses time to Go trials (with stable false alarm rate) can likewise index improved inhibitory control proficiency. Direct evidence for this assumption comes from White et al. (2014), who showed that Go response speed correlates with the activity of regions involved in IC (IFG, medial frontal gyrus and BG).
Functional magnetic resonance imaging (MRI) analyses revealed an interaction between the factors Session (Pre‐; Post‐training) and Stimulus type (Go; NoGo) within a bilateral inferior fronto‐temporal network. Our finding for a modification of the hemodynamic responses to NoGo but not to Go trials with training indicates that the Go/NoGo training selectively modified inhibition processes. The specificity of the effect of training to the trials involving inhibition rules out an explanation of our results in terms of mere test‐retest differences between the two MRI recordings. The task‐specificity of the effect of the Go/NoGo training was further confirmed by the lack of change in brain activity during the control working memory 2‐back task between the pre‐ and post‐IC training sessions (Thomas and Baker 2013).
IC improvements were accompanied by a decrease in left and right inferior frontal activity to NoGo trials. Converging functional and structural neuroimaging (Aron and Poldrack 2006; Aron et al. 2007; Forstmann et al. 2008; Garavan et al. 1999; Rubia et al. 2003) as well as clinical evidence (Aron et al. 2003b; Decary and Richer 1995; Floden and Stuss 2006; Picton et al. 2007) point out these regions as the core structures of the IC network and suggest that they act as an “active breaking” mechanism when response suppression is required (Aron et al. 2014).
The training could have reduced the neural population engaged in inhibitory process triggered by the NoGo stimuli via the exclusion of task‐irrelevant neurons via synaptic pruning mechanisms (Johansen‐Berg 2012; Kelly et al. 2006; Logothetis et al. 2001; Poldrack 2000; Viswanathan and Freeman 2007; Zatorre et al. 2012). In turn, these effects would have led to a decrease in the hemodynamic response of the IFG. Such training‐induced increases in the specificity of task‐relevant networks would have sped up top‐down inhibition processes to eventually improve IC proficiency.
Concurring with our findings, decreases in BOLD responses within task‐relevant brain networks have been repeatedly reported after training of high‐order frontal executive tasks (planning: Beauchamp et al. 2003; working memory: Hempel et al. 2004; or switching: Jimura et al. 2014). However, current literature provides mixed evidence on the direction of the change of brain activity associated with IC behavioral improvements. So far, only four studies examined functional modifications induced by IC training, and three of them reported change in the right IFG activity. In accordance with our results, Manuel et al. (2013) reported that 1 h of SST training decreased the response strength of a right fronto‐striatal network (including the rIFG) to stop trials. In contrast, Lenartowicz et al. (2011) showed that after two 1‐h SST training sessions, the rIFG activity increased in response to stimuli previously associated with stopping. Finally, Berkman et al. (2014) reported no change in the response of the IC network to inhibition trials following ten 6‐min sessions of SST training, but only a decrease in the rIFG activity to the cues signaling a forthcoming need for IC.
These discrepancies might follow from the engagements of distinct neurocognitive mechanisms of IC functional plasticity depending on the parameters of the training task. In tasks with unvarying stimulus‐response mapping rules, automatic stimulus‐driven forms of IC have been shown to develop (Spierer et al. 2013 for review). Such mechanisms most likely took place in Berkman et al. (2014); Lenartowicz et al. (2011); Manuel et al. (2010) where no change in the rIFG response to NoGo stimuli was observed with training. In contrast to these studies, Manuel et al. (2013) and the present study used training tasks in which the associations between the NoGo stimuli and NoGo goals were systematically varied during the training. As a result, top‐down controlled forms of inhibition were repeatedly solicited and putatively reinforced during the training, which manifested as a decrease and not an increase in rIFG response to NoGo stimuli.
We further found that the training modified the activity of the left IFG to NoGo trials. This region has been advanced to regulate the efficiency of IC when task difficulty (Swick et al. 2008) or attentional demand increase (see also Hirose et al. 2012 for corroborating lesion data; Schel et al. 2014). The differential engagement of this structure between the beginning and the end of the training might, thus, reflect a reduction in task‐demand with learning.
Our finding for a modulation of temporal activity with IC training is more ambiguous. Temporal structures have been shown to interact with higher level prefrontal regions during IC tasks (Egner and Hirsch 2005) and to be modulated in inhibition‐related disorders (Solanto et al. 2009; Tamm et al. 2004). Modulations in auditory temporal areas might reflect changes in the processing of the Go and NoGo stimuli, from a direct, visual processing to a phonological encoding or auditory imagery of the sound of each letters. This strategic adjustment of stimuli processing might have facilitated the discrimination of the Go and NoGo stimuli and ultimately improved task performance. Subvocalizations when reading the letter stimuli could also explain the modification of the activity of the left IFG with training (Ferguson et al. 2014).
While functional changes manifested in both left and right IFG, we observed changes in GMV and in white matter FA only in the right IFG. Grey and/or white matter training‐induced structural plasticity has been so far reported in around twenty studies addressing the effects of 3 days to 3 years training regimens on various visuo‐motor or cognitive tasks (for review, Thomas and Baker 2013). However, to our knowledge our results constitute the first demonstration of micro‐structural changes associated with IC training.
Increase in GMV of the rIFG with training might have followed from neurogenesis (Tronel et al. 2010), gliogenesis, synaptogenesis and/or angiogenesis (May 2011 for review; Taubert et al. 2012; Zatorre et al. 2012). Further studies are, however, necessary to disentangle how these neurophysiological mechanisms might have supported the improvement in IC proficiency. The inferences from T1w imaging on brain anatomy are indeed at a coarse mesoscopic level and the nature of magnetic resonance (MR) contrast allows only for speculations regarding the underlying neurobiological processes. The association between increase in GMV the right IFG and increase in IC proficiency concurs with previous clinical evidence for negative correlations between rIFG grey matter volume and the strength of inhibition‐related symptomatology as drug craving or compulsions (Chamberlain et al. 2008a; Chamberlain et al. 2008b; Tabibnia et al. 2011), and suggests that the rIFG grey matter volume is a determinant of IC proficiency.
The increase in FA we observed in the rIFG between the pre‐ and post‐training session suggests that the Go/NoGo practice triggered changes in WM microstructure. Animal studies have suggested increased myelination as a possible mechanism underlying increases in FA (e.g. Demerens et al. 1996). Myelination processes have been observed to take place within the time range of our training session (Koenig et al. 1995) and associated with practice even in adulthood (Bengtsson et al. 2005; Mackey et al. 2012; Markham and Greenough 2004; Takeuchi et al. 2010). Consistent with our hypothesis for an improvement of the speed of IC with training, the increase in FA in the rIFG might reflect an enhancement of the communication pathway between the rIFG and the BG (Aron, 2011). Myelination levels indeed directly determine how fast action potentials spread along neural fibers (Bloom et al. 1988; Waxman 1980). Of note, other mechanisms including, for example, changes in axonal packing parameters or axons diameter also impact on FA and may have participated in the plastic changes we observed (Beaulieu 2002; Scholz et al. 2009).
As in our study, previous literature suggests that functional and structural training‐induced plasticity do not necessarily manifest over the same brain areas (Table 2). Ilg and colleagues (2008) for instance showed that healthy individuals, trained to practice on a mirror reading task 15 min a day for 2 weeks had a significant GMV increase in the right dorsolateral occipital cortex, accompanied by modifications of the functional activity within the same region but also extending to the superior parietal areas (see also Haier et al. 2009; Hegarty et al. 2012; Schmidt‐Wilcke et al. 2010 for corresponding findings). The relationship between training‐induced change in hemodynamic responses and grey and WM structures remains, however, largely unexplored. The colocalization of the hemodynamic, GMV and FA changes in the IFG suggests that the functional modifications, which likely took place before structural changes, may have triggered the microstructural plasticity. However, while both left and right IFG showed a corresponding decrease of functional change with training, only the right IFG showed associated GMV and FA changes. This dissociation between functional and structural plasticity could most parsimoniously be accounted for by quantitative differences in the involvement of the left and right IFG during the training: the functional engagement of the each IFG might have been sufficient to induce functional modifications within these areas, but only the right IFG would have been sufficiently solicited to trigger structural modifications.
Table 2.
Within‐IFG localizations of clusters local maxima for the functional and structural analyses (MNI coordinates)
| Pars orbitalis | Pars triangularis | Pars opercularis | ||||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| BOLD | (−30;23;−11) | (39;32;16) (36;23;13) | (42;17;13) | |||
| GMV | (57;29;−7) | |||||
| FA | (41;33;7) | |||||
MNI coordinates of within‐IFG localizations of cluster local maxima of the functional (BOLD), grey matter volume (GMV) and fractional anisotropy (FA) analyses. The three subregions of the inferior frontal gyrus are reported separately for the left and right hemisphere: left and right pars orbitalis, triangularis and opercularis. A partial overlap of the functional and structural data manifested in the rIFG pars triangularis (reduction of the BOLD signal and an increase of FA after training in this region).
Supporting this hypothesis, myriads of studies report a prominent role of the right IFG in IC (Aron et al. 2004, 2014 for reviews), with the left IFG playing an auxiliary role when task demands exceed the maximal engagement of the rIFG (Hirose et al. 2012). Accordingly, the lIFG would have been largely involved in the Go/NoGo task at the beginning of the IC training, but less when participants' IC proficiency improved. As a result, the left IGF manifested a decrease in activity with learning (as evident in the Session*Stimulus functional interaction), but no structural change.
While the BOLD, GMV and FA modifications took place within the IFG, they did not manifest in the same subregions of these gyri. This pattern suggests that in addition to their specific functional involvement during the training, the cytoarchitectonic specificities of the regions involved in trained task might have influenced the type of training‐induced plasticity they incurred.
The pars triangularis showed functional plastic as well as WM changes in the right hemisphere. In contrast, the right pars opercularis showed only functional changes. The pars orbitalis showed functional changes in the left hemisphere and GM change in the right hemisphere. Converging lesion (Aron et al. 2003a), functional neuroimaging (Levy and Wagner 2011) and diffusion evidence (Aron et al. 2007; Johansen‐Berg et al. 2004; Swann et al. 2012) suggest a prominent role of the posterior IFG subregions in IC (Aron et al. 2014 for review). In contrast, we showed associations between IC proficiency and grey and WM structure in the pars orbitalis and triangularis. This pattern suggests more complex dissociations as previously thought between the functional and structural roles of the subparts of the IFG in IC proficiency. In this regard, our pattern of results further demonstrates that parallel investigations of functional and structural training‐induced plasticity enable revealing novel dissociations between subregions within task‐relevant brain networks that the analyses at a single level would not have unveiled.
Our study, however, suffers several limitations. Most notably, further investigations are necessary to provide definitive evidence for a direct relationship between the training‐induced changes observed at the behavioral and at the brain levels. While the implementation of an active or passive control group would have helped ensuring that the differences we observed between the pre‐ and post‐training sessions were related to the IC training, the following results provide converging evidence that it was the case: i) At the functional level, there were no effects of training on the control working memory task but only on the trained Go/NoGo task: a liberal exploratory whole‐brain fMRI analysis comparing the pre‐ versus post‐training neural activity during the 2‐back task showed no evidence for an effect of training on working memory. In addition, we selected the three clusters showing a significant decrease of activation in the whole‐brain analysis on the Go/NoGo task and performed a ROI analysis on these clusters for the 2‐back task. None of them showed a change in activity between the pre‐ and post‐training 2‐back task; ii) Even within the Go/NoGo task, the effects of training were specific to the inhibition trials: there was a change of BOLD responses to NoGo but no to Go trials as indicated by an interaction between the factors Stimuli (Go; NoGo) and session (Pre‐; post‐training) driven by a decrease in the activity within the left and right IFG to Nogo trials and not to Go trials; iii) the effects of the training were specific to the left and right IFG, that are brain regions associated with IC by our main effect of Stimulus and by previous literature (Aron et al. 2014 for review). This pattern of result provides converging evidence that the observed effects indeed resulted from the Go/NoGo training. Another limitation of our study concerns whether the effect of training lasted after the end of the training. Our post‐training recording session took place right after the end of the training, leaving unresolved whether the same pattern persisted after weeks or months.
Furthermore, whether the behavioral improvements on the 2‐back task follow from a test‐retest effect or derive from the go/no training remains unresolved. Previous literature has indeed shown that inhibition is involved in working memory tasks (McNab et al. 2008; see Simmonds et al. 2008 for review), and reversely that working memory is supported by brain regions that were modified by our training regimen (Borst and Anderson, 2013; D'Esposito et al. 1998 for review). One could, thus, hypothesize that as there is a partial overlap of the brain regions underling both tasks, the effect of the Go/NoGo training might have transferred to the 2‐back. However, because i) there were functional changes during the GoNoGo task but not during the 2 back task; ii) the functional and structural changes manifested within IC brain areas; and iii) Go/NoGo but not working memory was trained, the observed structural changes are due the Go/NoGo training (with potentially also test‐retest effects), with the behavioral change in the 2 back task following only from familiarization with the task / test‐retest effects.
Collectively, our results reveal the patterns of functional and structural plastic reorganizations induced by 2 weeks of IC training. We confirmed current hypotheses on the mechanisms of training‐induced plasticity in IC by showing that inhibition processes triggered by NoGo stimuli can be enhanced by IC training with specifically designed Go/NoGo tasks: Top‐down IC structures are modified when stimulus‐response mapping rules are systematically varied during the training. We further showed that different patterns of training‐induced functional and structural plastic modifications took place within and between homotopic inferior frontal executive brain networks. These collective findings suggest that the IC training could help the rehabilitation of inhibition‐related brain disorders involving either structural or functional deficits in IC structures (Tabibnia et al. 2011). Further clinical studies are, however, required to test this hypothesis.
Supporting information
Supplementary Information
ACKNOWLEDGMENT
We thank Prof. Henri‐Marcel Hoogewoud for providing us access to the MRI of the Fribourg Hospital.
REFERENCES
- Aron AR (2007): The neural basis of inhibition in cognitive control. Neuroscientist 13:214–228. [DOI] [PubMed] [Google Scholar]
- Aron AR (2011): From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol Psychiatry 69:e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V (2007): Converging evidence for a fronto‐basal‐ganglia network for inhibitory control of action and cognition. J Neurosci 27:11860–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003a): Stop‐signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6:115–116. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA (2006): Cortical and subcortical contributions to Stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci 26:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2014): Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn Sci 18:177–185. [DOI] [PubMed] [Google Scholar]
- Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, Sahakian BJ, Robbins TW (2003b): Inhibition of subliminally primed responses is mediated by the caudate and thalamus: Evidence from functional MRI and Huntington's disease. Brain 126:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry—The methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Ridgway GR (2012): Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci 6:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SJ, Finnerty GT (2010): Sensory experience and cortical rewiring. Neuroscientist 16:186–198. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Dagher A, Aston JA, Doyon J (2003): Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. Neuroimage 20:1649–1660. [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15:435–455. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F (2005): Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8:1148–1150. [DOI] [PubMed] [Google Scholar]
- Benikos N, Johnstone SJ, Roodenrys SJ (2013): Short‐term training in the Go/Nogo task: Behavioural and neural changes depend on task demands. Int J Psychophysiol 87:301–312. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Kahn LE, Merchant JS (2014): Training‐induced changes in inhibitory control network activity. J Neurosci 34:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom FE, Lazerson A, Hofstadter L (1988): Brain, Mind and Behavior [Internet]. New York: Freeman. [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, Aitken M, Craig K, Owen AM, Bullmore ET, Robbins TW, Sahakian BJ (2008a): Orbitofrontal dysfunction in patients with obsessive‐compulsive disorder and their unaffected relatives. Science 321(5887):421–422. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies LA, Fineberg NA, Del Campo N, Suckling J, Craig K, Muller U, Robbins TW, Bullmore ET, Sahakian BJ (2008b): Grey matter abnormalities in trichotillomania: Morphometric magnetic resonance imaging study. Br J Psychiatry 193:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ (2009): False discovery rate revisited: FDR topological inference using Gaussian random fields. Neuroimage 44:62–70. [DOI] [PubMed] [Google Scholar]
- Chumbley J, Worsley K, Flandin G, Friston K (2010): Topological FDR for neuroimaging. Neuroimage 49:3057–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decary A, Richer F (1995): Response selection deficits in frontal excisions. Neuropsychologia 33:1243–1253. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C (1996): Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA 93:9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Pizzagalli DA (2007): Inhibition of action, thought, and emotion: A selective neurobiological review. Appl Prev Psychol 12:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004): Neuroplasticity: Changes in grey matter induced by training. Nature 427:311–312. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J (2005): The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage 24:539–547. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, Walhovd KB (2012): Memory training impacts short‐term changes in aging white matter: A longitudinal diffusion tensor imaging study. Hum Brain Mapp 33:2390–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MA, Nielsen JA, Anderson JS (2014): Altered resting functional connectivity of expressive language regions after speed reading training. J Clin Exp Neuropsychol 36:482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden D, Stuss DT (2006): Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci 18:1843–1849. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR (2008): Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: A model‐based approach. J Neurosci 28:9790–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Mattout J, Trujillo‐Barreto N, Ashburner J, Penny W (2007): Variational free energy and the Laplace approximation. Neuroimage 34:220–234. [DOI] [PubMed] [Google Scholar]
- Friston KJ (1995): Neuronal transients. Proc Biol Sci 261:401–405. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA (1999): Right hemispheric dominance of inhibitory control: An event‐related functional MRI study. Proc Natl Acad Sci USA 96:8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Schlaug G (2003): Gray matter differences between musicians and nonmusicians. Ann NY Acad Sci 999:514–517. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14:21–36. [DOI] [PubMed] [Google Scholar]
- Granert O, Peller M, Gaser C, Groppa S, Hallett M, Knutzen A, Deuschl G, Zeuner KE, Siebner HR (2011): Manual activity shapes structure and function in contralateral human motor hand area. Neuroimage 54:32–41. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D (2012): Learning, attentional control, and action video games. Curr Biol 22:R197–R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Karama S, Leyba L, Jung RE (2009): MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual‐spatial task. BMC Res Notes 2:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanggi J, Koeneke S, Bezzola L, Jancke L (2010): Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Hum Brain Mapp 31:1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harle KM, Shenoy P, Stewart JL, Tapert SF, Yu AJ, Paulus MP (2014): Altered neural processing of the need to stop in young adults at risk for stimulant dependence. J Neurosci 34:4567–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty CE, Foland‐Ross LC, Narr KL, Townsend JD, Bookheimer SY, Thompson PM, Altshuler LL (2012): Anterior cingulate activation relates to local cortical thickness. Neuroreport 23:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel A, Giesel FL, Garcia Caraballo NM, Amann M, Meyer H, Wustenberg T, Essig M, Schroder J (2004): Plasticity of cortical activation related to working memory during training. Am J Psychiatry 161:745–747. [DOI] [PubMed] [Google Scholar]
- Hirose S, Chikazoe J, Watanabe T, Jimura K, Kunimatsu A, Abe O, Ohtomo K, Miyashita Y, Konishi S (2012): Efficiency of go/no‐go task performance implemented in the left hemisphere. J Neurosci 32:9059–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): Fsl. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jimura K, Cazalis F, Stover ER, Poldrack RA (2014): The neural basis of task switching changes with skill acquisition. Front Hum Neurosci 8:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H (2012): The future of functionally‐related structural change assessment. Neuroimage 62:1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM (2004): Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA 101:13335–13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SJ, Roodenrys S, Blackman R, Johnston E, Loveday K, Mantz S, Barratt MF (2012): Neurocognitive training for children with and without AD/HD. Atten Defic Hyperact Disord 4:11–23. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR (2006): Motion correction and the use of motion covariates in multiple‐subject fMRI analysis. Hum Brain Mapp 27:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Foxe JJ, Garavan H (2006): Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Arch Phys Med Rehabil 87:S20–S29. [DOI] [PubMed] [Google Scholar]
- Klingberg T (2010): Training and plasticity of working memory. Trends Cogn Sci 14:317–324. [DOI] [PubMed] [Google Scholar]
- Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, Jung‐Testas I, Robel P, Akwa Y, Baulieu EE (1995): Progesterone synthesis and myelin formation by Schwann cells. Science 268(5216):1500–1503. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Larish JF, Strayer DL (1995): Training for attentional control in dual task settings: A comparison of young and old adults. J Exp Psychol 1:50–76. [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng H‐M, Brady TJ, Rosen BR (1992): Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 89:5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Park JY, Jung WH, Kim HS, Oh JS, Choi CH, Jang JH, Kang DH, Kwon JS (2010): White matter neuroplastic changes in long‐term trained players of the game of "Baduk" (GO): A voxel‐based diffusion‐tensor imaging study. Neuroimage 52:9–19. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, Verbruggen F, Logan GD, Poldrack RA (2011): Inhibition‐related activation in the right inferior frontal gyrus in the absence of inhibitory cues. J Cogn Neurosci 23:3388–3399. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD (2011): Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Ann NY Acad Sci 1224:40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A (2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412:150–157. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Whitaker KJ, Bunge SA (2012): Experience‐dependent plasticity in white matter microstructure: Reasoning training alters structural connectivity. Front Neuroanat 6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhyastha T, Merillat S, Hirsiger S, Bezzola L, Liem F, Grabowski T, Jancke L (2014): Longitudinal reliability of tract‐based spatial statistics in diffusion tensor imaging. Hum Brain Mapp 35:4544–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid DS, Cai W, George JS, Verbruggen F, Aron AR (2012): Transcranial magnetic stimulation reveals dissociable mechanisms for global versus selective corticomotor suppression underlying the stopping of action. Cereb Cortex 22:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH (2004): Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21:450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Manuel AL, Bernasconi F, Spierer L (2013): Plastic modifications within inhibitory control networks induced by practicing a stop‐signal task: An electrical neuroimaging study. Cortex 49:1141–1147. [DOI] [PubMed] [Google Scholar]
- Manuel AL, Grivel J, Bernasconi F, Murray MM, Spierer L (2010): Brain dynamics underlying training‐induced improvement in suppressing inappropriate action. J Neurosci 30:13670–13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Greenough WT (2004): Experience‐driven brain plasticity: Beyond the synapse. Neuron Glia Biol 1:351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A (2011): Experience‐dependent structural plasticity in the adult human brain. Trends Cogn Sci 15:475–482. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED (2005): Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend 79:273–277. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S (2007): Effects of focal frontal lesions on response inhibition. Cereb Cortex 17:826–838. [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2000): Imaging brain plasticity: Conceptual and methodological issues–a theoretical review. Neuroimage 12:1–13. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Anderson EJ, Husain M (2010): Expert cognitive control and individual differences associated with frontal and parietal white matter microstructure. J Neurosci 30:17063–17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E (2003): Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 20:351–358. [DOI] [PubMed] [Google Scholar]
- Schel MA, Kuhn S, Brass M, Haggard P, Ridderinkhof KR, Crone EA (2014): Neural correlates of intentional and stimulus‐driven inhibition: A comparison. Front Hum Neurosci 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt‐Wilcke T, Rosengarth K, Luerding R, Bogdahn U, Greenlee MW (2010): Distinct patterns of functional and structural neuroplasticity associated with learning Morse code. Neuroimage 51:1234–1241. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen‐Berg H (2009): Training induces changes in white‐matter architecture. Nat Neurosci 12:1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA (2012): Conventional working memory training may not improve intelligence. Trends Cogn Sci 16:582–583. [DOI] [PubMed] [Google Scholar]
- Smith SM, De Stefano N, Jenkinson M, Matthews PM (2001): Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr 25:466–475. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM et al (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen‐Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ et al (2007): Acquisition and voxelwise analysis of multi‐subject diffusion data with tract‐based spatial statistics. Nat Protoc 2:499–503. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Schulz KP, Fan J, Tang CY, Newcorn JH (2009): Event‐related FMRI of inhibitory control in the predominantly inattentive and combined subtypes of ADHD. J Neuroimaging 19:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierer L, Chavan CF, Manuel AL (2013): Training‐induced behavioral and brain plasticity in inhibitory control. Front Hum Neurosci 7:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitburger DP, Pampel A, Krueger G, Lepsien J, Schroeter ML, Mueller K, Moller HE (2014): Impact of image acquisition on voxel‐based‐morphometry investigations of age‐related structural brain changes. Neuroimage 87:170–182. [DOI] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, Aron AR, Tandon N (2012): Roles for the pre‐supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. Neuroimage 59:2860–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU (2008): Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci 9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, Lee B, London ED (2011): Different forms of self‐control share a neurocognitive substrate. J Neurosci 31:4805–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R (2010): Training of working memory impacts structural connectivity. J Neurosci 30:3297–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL (2004): Event‐related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention‐deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 43:1430–1440. [DOI] [PubMed] [Google Scholar]
- Taubert M, Villringer A, Ragert P (2012): Learning‐related gray and white matter changes in humans: An update. Neuroscientist 18:320–325. [DOI] [PubMed] [Google Scholar]
- Thomas C, Baker CI (2013): Teaching an adult brain new tricks: A critical review of evidence for training‐dependent structural plasticity in humans. Neuroimage 73:225–236. [DOI] [PubMed] [Google Scholar]
- Thorell LB, Lindqvist S, Bergman Nutley S, Bohlin G, Klingberg T (2009): Training and transfer effects of executive functions in preschool children. Dev Sci 12:106–113. [DOI] [PubMed] [Google Scholar]
- Tronel S, Fabre A, Charrier V, Oliet SH, Gage FH, Abrous DN (2010): Spatial learning sculpts the dendritic arbor of adult‐born hippocampal neurons. Proc Natl Acad Sci USA 107:7963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Adams R, Chambers CD (2012): Proactive motor control reduces monetary risk taking in gambling. Psychol Sci 23:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD (2008): Automatic and controlled response inhibition: Associative learning in the go/no‐go and stop‐signal paradigms. J Exp Psychol Gen 137:649–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD (2007): Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci 10:1308–1312. [DOI] [PubMed] [Google Scholar]
- von Bastian CC, Locher A, Ruflin M (2013): Tatool: A Java‐based open‐source programming framework for psychological studies. Behav Res Methods 45:108–115. [DOI] [PubMed] [Google Scholar]
- Waxman SG (1980): Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 3:141–150. [DOI] [PubMed] [Google Scholar]
- White CN, Congdon E, Mumford JA, Karlsgodt KH, Sabb FW, Freimer NB, London ED, Cannon TD, Bilder RM, Poldrack RA (2014): Decomposing decision components in the stop‐signal task: A model‐based approach to individual differences in inhibitory control. J Cogn Neurosci 26:1601–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ (1995): Analysis of fMRI time‐series revisited—Again. Neuroimage 2:173–181. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen‐Berg H (2012): Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


