Abstract
The Stroop interference task is a widely used paradigm to examine cognitive inhibition, which is a key component of goal‐directed behavior. With increasing age, reaction times in the Stroop interference task are usually slowed. However, to date it is still under debate if age‐related increases in reaction times are merely an artifact of general slowing. The current study was conducted to investigate the role of general slowing, as measured by Trail‐Making‐Test‐A, in age‐related alterations of Stroop interference. We applied Diffusion Tensor Imaging (DTI) to determine the topography of neuronal networks underlying Stroop interference under control of general slowing. On the behavioral level, linear regression analysis demonstrated that age accounted for significant variance on Stroop interference, whereas TMT‐A performance did not. Controlling for TMT‐A, DTI based white matter analyses demonstrated a strong association of Stroop interference with integrity measures of genu of corpus callosum, bilateral anterior corona radiata, and bilateral anterior limb of capsula interna. These pathways are associated with frontal brain regions by either connecting the bilateral dorsolateral prefrontal cortex or the anterior cingulate cortex with frontal and subcortical regions or by containing fibers which are part of cortico‐thalamic circuits that cross prefrontal regions. Importantly, results expand our knowledge of the neural basis of Stroop interference and emphasize the importance of white matter integrity of frontal pathways in the modulation of Stroop interference. Combining behavioral and DTI findings our results further suggest that cognitive inhibition, as measured by Stroop task, is a qualitatively distinct cognitive process that declines with age. Hum Brain Mapp 35:2448–2458, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: stroop interference, normal aging, general slowing, white matter integrity, DTI
INTRODUCTION
A central subcomponent of executive functioning describes the ability to suppress irrelevant information, restrain activation of an inappropriate prepotent response triggered by associated cues, and prevent access to irrelevant information [Bench et al., 1993; MacLeod, 2007]. This executive component, usually referred to as cognitive inhibition, is a key component of goal‐directed behavior and thus of daily functioning. A widely used paradigm to measure cognitive inhibition is the Stroop paradigm [Stroop, 1992]. It consists of color name words printed in both, congruent (e.g., green printed in green ink) and incongruent colors (e.g., green printed in blue ink). Individuals are asked to identify the color of a stimulus within both conditions. Response latency for incongruent trials is generally longer than response latency for congruent trials. This increase in latency on incongruent trials has been termed Stroop interference effect, which is thought to reflect cognitive inhibition.
With increasing age reaction times in the congruent condition of the Stroop task are usually slowed. While some studies demonstrated an increment of Stroop interference as well [for a brief review, see MacLeod, 1991; more recent studies: West and Alain, 2000; Davidson et al., 2003; Rush et al., 2006], others argue that the apparent age‐sensitivity of Stroop interference may merely be an artifact of a decline in information processing speed (respectively general slowing) and no evidence for a qualitatively different kind of processing that declines with age. A meta‐analysis by Verhaegen and De Meersman [1998] of 20 studies provides support for this view. This concept, known as the unspecific hypothesis of general slowing with increasing age, is not limited to inhibitory processes but is thought to apply to all cognitive processes [Salthouse, 1996]. To date, it is still under debate if age‐related decreases in cognitive inhibition, as measured by Stroop interference, are (at least in part) independent of general slowing.
On the behavioral level, up to now several studies have investigated the contribution of general slowing to age‐related increases in Stroop interference. In a study by Bugg et al. [2007] 284 subjects from the age of 20–89 years completed an abbreviated Stroop color‐naming task and an independent assessment of information processing speed. The authors demonstrated that age‐related increases in Stroop interference are partially attributable to general slowing. However, they emphasized that performance alterations are also attributed to cognitive inhibition as a task‐specific process. An older study by Salthouse and Meinz [1995] showed the same result. Further studies based on a Stroop‐task internal control of information processing speed have also consistently demonstrated an increment of Stroop interference with increasing age [e.g., Dulaney and Rogers, 1994; Houx et al., 1993; Spieler et al., 1996; West and Baylis, 1998; Troyer et al., 2006]. Besides this large amount of articles demonstrating an association between Stroop interference and age even after controlling for general slowing, some studies could not confirm this result [Graf et al., 1995; Zysset et al., 2007]. The authors of these works conclude that age effects in Stroop interference are based on age‐related general slowing primarily.
To determine whether the age‐related increase in Stroop interference is (at least in part) independent of general slowing, it is in addition to behavioral analyses required to know if Stroop interference relies on distinct neurobiological mechanisms. Several studies have emphasized the potential for white matter integrity damage to be an important factor in age‐associated cognitive decline [Madden et al., 2009]. In addition, distinct relations between white matter integrity characteristics and specific cognitive functions have been shown [Kennedy and Raz, 2009]. Against this background, white matter integrity qualifies as a good parameter to investigate and demarcate age‐sensitive cognitive processes, such as cognitive inhibition and information processing speed, on a neurobiological level. However, up to now no study was conducted to disentangle the relation between Stroop interference, information processing speed, and white matter integrity. The relationship between Stroop interference and white matter integrity has as yet mostly been examined in patients with schizophrenia [Takei et al. 2009; Yan et al., 2012] and other psychiatric disorders [e.g., Murphy et al., 2007; Li et al., 2010]. These studies mainly found frontal white matter brain regions to be associated with Stroop interference. Only a few studies investigated healthy subjects. In line with the patient studies, these works also emphasized white matter properties to be potential modulators of cognitive inhibition during Stroop task [Kennedy and Raz, 2009; Takeuchi et al., 2011]. Compared to Stroop interference, information processing speed and its relationship to integrity measures in healthy subjects has been extensively examined, whereby most studies demonstrated a strong association between both parameters in widespread frontal, temporal, parietal, and occipital white matter regions [e.g., Turken et al. 2008; Kennedy and Raz, 2009; Kochunov et al., 2010].
The current study was conducted to evaluate whether cognitive inhibition, as measured by the Stroop task, is a cognitive process that itself declines with age, or is just secondarily affected by age‐related general slowing. General slowing was assessed independently by the Trail‐Making‐Test A (TMT‐A), which is a popular and frequently applied neuropsychological measure of information processing speed [Salthouse et al., 2003; Jacobs et al., 2011; Katz et al., 2011]. Analyses included investigations on the behavioral level based on Stroop‐ and TMT‐A performances. Additionally, we analyzed the association between white matter integrity (as measured by Diffusion Tensor Imaging, DTI) and Stroop interference controlled for TMT‐A performances. We hypothesized that normal aging is associated with a decrease in Stroop‐ as well as TMT‐A performance. However, we expected that alterations in Stroop interference are at least in part independent of alterations in TMT‐A performance. Furthermore, we hypothesized that Stroop interference is associated with white matter integrity, even after controlling for TMT‐A performance. We expected the association between Stroop interference and white matter integrity to be focused on frontal white matter regions, as Diffusion‐Tensor‐Imaging (DTI) studies in psychiatric patients and several fMRI studies demonstrated significant associations between frontal brain areas and the performance of the Stroop incongruent condition [Murphy et al., 2007; Nee et al., 2007; Badzakova‐Trajkov et al., 2009; Li et al., 2010; Zoccatelli et al., 2010; Yan et al., 2012].
MATERIALS AND METHODS
Subjects
The data of 49 healthy adults were analyzed. The study group included 13 younger adults (age range: 22–37 years), 20 younger elderly (age range: 60–70 years), and 16 advanced elderly (age range: 71–85 years). All participants were recruited by advertisements posted in the University Medical Center Mainz and several public institutions and via newspaper announcement. The local Ethics Committee approved the study protocol, and all subjects provided written informed consent. Subjects were excluded if they had any psychiatric (e.g., depression, schizophrenia, alcohol abuse) or cognitive (e.g., dementia, mild cognitive impairment) illness, a history of brain damage, stroke or any central nervous system disorders, or if they were taking cognitive performance altering medications. All participants underwent DTI, an assessment of cognitive inhibition as measured by the Stroop task as well as an assessment of general slowing as measured by TMT‐A. DTI was conducted to analyze white matter fractional anisotropy (FA) and mean diffusivity (MD), which are different parameters to quantify white matter integrity [Basser and Pierpaoli, 1996; Beaulieu, 2002; Mori and Zhang, 2006].
Neuropsychological Materials
Cognitive inhibition
Cognitive inhibition was assessed using a computerized version of the Stroop task. Two strings of capital letters were presented beside each other in the center of a black screen (the anchor on the left, the target on the right). The anchor consisted of one of four color names (the German equivalents for “BLUE,” “GREEN,” “RED,” and “YELLOW”) presented in a neutral grey. The target consisted of either meaningless consonant strings (“QQQQ,” neutral condition) or the name of one of the four colors presented in one of these four colors. Participants were asked to indicate whether it matches or not by tapping a right and left response key, respectively. If the semantic meaning of the target was a color, this color could either match (congruent condition) or mismatch the semantic meaning of the anchor (incongruent condition). Participants started with two exercise blocks, the first comprising 24 neutral trials and the second comprising 24 trials of all types. Thereafter, they completed eight consecutive test blocks of 72 (plus four warm‐up) trials each, with an equal number of match and nonmatch trials. Between the stimulus presentation the screen remained blank for 400 ms. In case of an error, a gray “X” was presented beneath the stimuli for 300 ms. Stroop interference rates were computed as the difference of reaction times (in ms) in the incongruent and neutral match conditions of correct proceeded trials. This approach was used to adjust performances of the incongruent condition of the Stroop task for interference irrelevant, task‐specific processes like color‐perception.
Information processing speed
Information processing speed was assessed by the TMT‐A [Reitan, 1958]. The test requires an individual to draw lines sequentially connecting 25 encircled numbers distributed on a sheet of paper. Participants were instructed to complete the task as quickly and accurately as possible. In case of an error, participants were instructed to return to the circle where the error occurred and continue. The performance was rated according to response time.
MRI Data Acquisition
Brain imaging examinations were conducted in a Siemens 3T TrioTim MRI scanner (Siemens, Erlangen, Germany). Apart from the acquisition of routine T 1, PD/T 2 weighted, fluid attenuated inversion recovery (FLAIR) weighted and Time‐of‐Flight (TOF) sequences, a diffusion‐weighted, single‐shot, spin‐echo, echoplanar‐based sequence (30 directions; b = 1,000 s/mm2; matrix 128 × 128; section thickness 3 mm; voxel size 1.5 × 1.5 × 3 mm3; TR/TE 7,100 ms/102 ms) was applied. Diffusion‐weighted data were processed using FSL 4.1 (FMRIB Analysis Group, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl) and the following procedures: (i) motion and eddy current correction, (ii) adjusting Gradients accordingly by application of the rotational part of the resulting affine transformations, and (iii) removal of the skull and nonbrain tissue using Brain Extraction Tool [Smith, 2002]. To calculate FA‐ and MD maps, a single diffusion tensor was fitted to the data [Basser and Pierpaoli, 1996] using the toolkit CAMINO v. 2 (Microstructural Imaging Group, University College London, UK, http://web4.cs.ucl.ac.uk/research/medic/camino/pmwiki/pmwiki.php, 08‐31‐2012).
Data Analyses
Before we analyzed the data we excluded all participants who demonstrated a percentage error rate above 2.5 standard deviations of the total group in the Stroop incongruent condition. Based on this criterion one participant was excluded. The final study group consisted of 49 subjects.
First, we analyzed the behavioral data. Nonparametric Spearman's rank correlation coefficients between age, Stroop interference rate as well as TMT‐A scores were calculated to confirm the frequently observed association between age and Stroop interference as well as age and information processing speed. As age was not distributed continuously, it was treated as a categorical variable (three categories: young adults, younger elderly, and advanced elderly). A multiple linear regression analysis was performed to examine the role of information processing speed in the association between age and Stroop interference and to control statistics for nuisance variables.
Subsequent to the behavioral analyses, we investigated whether Stroop interference is associated with white matter integrity even after controlling for TMT‐A. We applied a whole brain voxel‐wise correlation analysis between FA/MD data and Stroop interference using Tract‐Based Spatial Statistics [TBSS; Smith et al., 2006]. TBSS analyses included: (i) nonlinear registration to the FMRIB58_FA template of all subjects' FA data using FMRIB's nonlinear image registration tool [Rueckert et al., 1999], (ii) the creation and thinning of a mean FA image with a threshold 0.2 to obtain a mean FA skeleton that represented the centers of white matter trajectories, and (iii) projections of each subjects' aligned FA data onto the skeleton. To achieve skeletonized MD data the nonlinear warps and skeleton projection vectors of the FA images were applied to the MD data. Correlation analyses between FA/MD data and Stroop interference were performed using a linear regression model within the randomize tool, which tested the t value at each voxel against a null distribution that was obtained from 5,000 random permutations. To minimize the probability of false positive voxels and to confine the results to white matter pathways that demonstrated a strong association with Stroop interference, statistical significance was set at P < 0.025 corrected for multiple comparisons across voxels using the threshold‐free cluster‐enhancement option. Analyses were controlled for TMT‐A, age, gender, and years of education. In accordance with the behavioral analyses, age was included as a categorical variable, since age was not distributed continuously.
Although TBSS has many advantages compared to other analyses methods of diffusion data (e.g., accuracy of intersubject alignment) it is sensitive to outliers and limited in its ability to accurately construct the white matter skeleton in regions with a high number of crossing fibers [Smith et al., 2006; Herting et al., 2010]. To that end, we performed ROI‐based analyses outside the voxel‐wise framework subsequent to the whole brain TBSS analyses to support the TBSS results. ROIs were defined based on the JHU ICBM white matter label atlas, which contains hand‐segmented white matter parcellation maps [JHU ICBM‐DTI‐81; Mori et al., 2008]. We selected those labels of the atlas that corresponded to anatomical regions that showed significant correlations in voxel‐wise TBSS regression analyses. If only one side of a specific tract correlated with Stroop interference, we extracted both sides to examine possible lateralization specificities. Subsequently, we registered each subjects FA and MD image to the ICBM‐DTI‐81 template and calculated mean FA and MD values of the selected ROIs for each subject. Finally, we performed multiple linear regression analyses to assess the relationship between FA as well as MD values and Stroop interference rate by controlling for TMT‐A, age‐group, gender, and years of education. Furthermore, regression analyses served to compare FA/MD—covariate relationships.
RESULTS
Behavioral Analyses
The demographic‐ and behavioral characteristics of the study group are listed in Table 1. Correlation analyses demonstrated a strong association between age‐group and Stroop interference (r = 0.683, P < 0.001, spearman correlation coefficient) as well as age‐goup and TMT‐A scores (r = 0.702, P < 0.001, spearman correlation coefficient). Likewise, Stroop interference rate and TMT‐A score were strongly intercorrelated (r = 0.679, P < 0.001, spearman correlation coefficient). A subsequent multiple linear regression analysis with Stroop interference rate as dependent variable and age‐group, TMT‐A score, gender, and years of education as covariates showed an overall significant effect (F(1, 48) = 3.584, P = 0.013). The covariate age‐group was a significant predictor of Stroop interference rate (β = 0.453, P = 0.010). TMT‐A score (β = 0.003, P = 0.988), gender (β = 0.106, P = 0.450) and years of education (β = −0.048, P = 0.746) were not significant predictors.
Table 1.
Demographic, behavioral, and ROI‐based characteristics of the study group (N = 49)
| Younger adults | Younger elderly | Advanced elderly | |
|---|---|---|---|
| N | 13 | 20 | 16 |
| Age | 24.92 (3.88) | 62.85 (7.29) | 77.63 (4.29) |
| Education years | 13.31 (1.11) | 12.45 (3.27) | 12.38 (3.10) |
| Gender | |||
| Male | 6 (46%) | 11 (55%) | 4 (25%) |
| Female | 7 (54%) | 9 (45%) | 12 (75%) |
| TMT‐A (s) | 20.54 (4.10) | 33.30 (11.79) | 39.00 (9.57) |
| Stroop interference rate (ms) | 89.15 (57.48) | 334.25 (206.36) | 813.13 (900.38) |
| FA genu corpus callosum | 0.58 (0.03) | 0.54 (0.02) | 0.50 (0.03) |
| FA body corpus callosum | 0.54 (0.03) | 0.50 (0.03) | 0.46 (0.04) |
| FA anterior corona radiata right | 0.44 (0.04) | 0.40 (0.03) | 0.37 (0.03) |
| FA anterior corona radiata left | 0.44 (0.03) | 0.39 (0.03) | 0.36 (0.03) |
| FA anterior limb of internal capsule right | 0.53 (0.02) | 0.51 (0.02) | 0.48 (0.03) |
| FA anterior limb of internal capsule left | 0.53 (0.03) | 0.50 (0.03) | 0.48 (0.02) |
| MD genu Corpus Callosum | 0.84 (0.04) | 0.90 (0.04) | 0.97 (0.05) |
| MD body Corpus Callosum | 0.92 (0.05) | 1.00 (0.05) | 1.10 (0.06) |
| MD anterior corona radiata right | 0.74 (0.03) | 0.78 (0.05) | 0.83 (0.04) |
| MD anterior corona radiata left | 0.74 (0.03) | 0.78 (0.05) | 0.83 (0.04) |
| MD anterior limb of internal capsule right | 0.72 (0.03) | 0.74 (0.03) | 0.80 (0.06) |
| MD anterior limb of internal capsule left | 0.73 (0.02) | 0.74 (0.03) | 0.82 (0.06) |
Continuous variables are represented as mean (SD) and categorical variables as number (%).
TMT‐A: Trail‐Making‐Test part A; FA: fractional anisotropy; MD: mean diffusivity.
DTI Analyses
Whole brain voxel‐wise TBSS regression analyses demonstrated significant negative correlations between FA values and Stroop interference in genu and body of Corpus Callosum (CCgenu, CCbody) and left anterior corona radiata (CR). Furthermore, analyses demonstrated significant positive correlations between MD values and Stroop interference in CCgenu and CCbody, bilateral anterior CR, and left anterior limb of capsula interna (CI) (Fig. 1).
Figure 1.
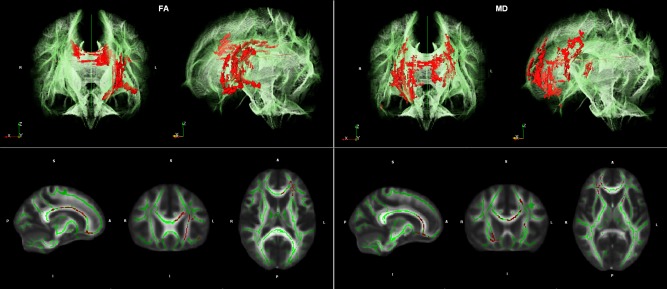
Illustration of results of the Tract‐based spatial statistic (TBSS) regression analyses. Regions with significant correlations (red) between fractional anisotropy (FA) measures and Stroop interference (left) as well as mean diffusivity (MD) measures and Stroop interference (right) are projected on cerebral white matter skeleton (green) in two different views (3D above, 2D below). TBSS results showed significant negative correlations (corrected P < 0.25) between FA values and Stroop interference in fronto‐parietal regions including the genu and body of Corpus Callosum (CC) and the left anterior corona radiata (left). Furthermore, TBSS analyses demonstrated significant positive correlations between MD values and Stroop interference in genu and body of CC, bilateral anterior corona radiata, and left anterior limb of capsula interna (right).
Results of the ROI‐based multiple linear regression analyses with FA/MD values as dependent variable and Stroop interference, TMT‐A score, age‐group, gender, and years of education as covariates are listed in Table 2. For scatterplots of integrity measures and Stroop interference scores see Figure 2. Mean ROI‐based FA and MD values of the study group are listed in Table 1. For scatterplots of age and Stroop interference as well as FA/MD values see Figures S3 and S4 in Supporting Information. ROI‐based analyses supported the significant negative association between FA of CCgenu as well as left anterior CR and Stroop interference that could be observed in the whole brain voxel‐wise analyses. Furthermore, a significant negative association could be observed in the right anterior CR. The association between FA of CCbody as well as bilateral anterior limb of CI and Stroop interference narrowly missed significance. ROI‐based regression analyses further supported the positive relationship between MD of CCgenu, bilateral anterior CR, as well as left anterior limb of CI and Stroop interference that could be demonstrated in the voxel‐wise analyses. In addition, a positive association between MD values and Stroop interference scores could be observed in the right anterior limb of CI. The relationship between MD of CCbody and Stroop interference missed significance. In summary, Stroop interference rate was significantly associated with the structural integrity of CCgenu, bilateral anterior CR, and bilateral anterior limb of CI (FA values demonstrated at least a tendency to significance). The importance of the body of the CC in Stroop interference demonstrated by the TBSS analyses could not be supported by the ROI‐based analyses.
Table 2.
Results of ROI‐based multiple linear regression analyses
| Age group | Stroop interference | TMT‐A | Gender | Years of education | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | R 2 | |
| FA | |||||||||||
| Genu corpus callosum | −0.603 | <0.001 | −0.235 | 0.035 | −0.036 | 0.774 | −0.129 | 0.209 | −0.068 | 0.522 | 0.620 |
| Body corpus callosum | −0.604 | 0.001 | −0.235 | 0.052 | 0.047 | 0.732 | −0.103 | 0.354 | −0.004 | 0.974 | 0.551 |
| Anterior corona radiata right | −0.421 | 0.002 | −0.350 | 0.002 | −0.165 | 0.189 | −0.164 | 0.103 | −0.085 | 0.410 | 0.637 |
| Anterior corona radiata left | −0.533 | <0.001 | −0.315 | 0.007 | 0.018 | 0.890 | −0.155 | 0.141 | −0.097 | 0.374 | 0.600 |
| Anterior limb of internal capsule right | −0.524 | 0.001 | −0.214 | 0.075 | −0.084 | 0.542 | −0.170 | 0.128 | −0.068 | 0.552 | 0.553 |
| Anterior limb of internal capsule left | −0.439 | 0.007 | −0.253 | 0.055 | −0.022 | 0.882 | −0.225 | 0.067 | −0.043 | 0.730 | 0.467 |
| MD | |||||||||||
| Genu corpus callosum | 0.586 | <0.001 | 0.260 | 0.015 | 0.057 | 0.638 | 0.159 | 0.104 | 0.069 | 0.491 | 0.658 |
| Body corpus callosum | 0.753 | <0.001 | 0.157 | 0.107 | −0.080 | 0.437 | 0.074 | 0.407 | −0.125 | 0.182 | 0.706 |
| Anterior corona radiata right | 0.401 | 0.005 | 0.355 | 0.003 | 0.138 | 0.294 | 0.191 | 0.073 | 0.116 | 0.293 | 0.596 |
| Anterior corona radiata left | 0.453 | 0.003 | 0.297 | 0.017 | 0.086 | 0.540 | 0.182 | 0.108 | 0.130 | 0.269 | 0.540 |
| Anterior limb of internal capsule right | 0.448 | 0.006 | 0.339 | 0.012 | −0.019 | 0.901 | 0.062 | 0.610 | 0.006 | 0.963 | 0.465 |
| Anterior limb of internal capsule left | 0.500 | 0.001 | 0.317 | 0.013 | −0.014 | 0.921 | 0.126 | 0.275 | 0.086 | 0.473 | 0.519 |
Note: FA and MD values were set as dependent variable. Age group, Stroop interference rate, Trail‐Making‐Test‐A, gender, and years of education were set as covariates. FA: fractional anisotropy; MD: mean diffusivity; TMT‐A: Trail‐Making‐Test part A.
Figure 2.
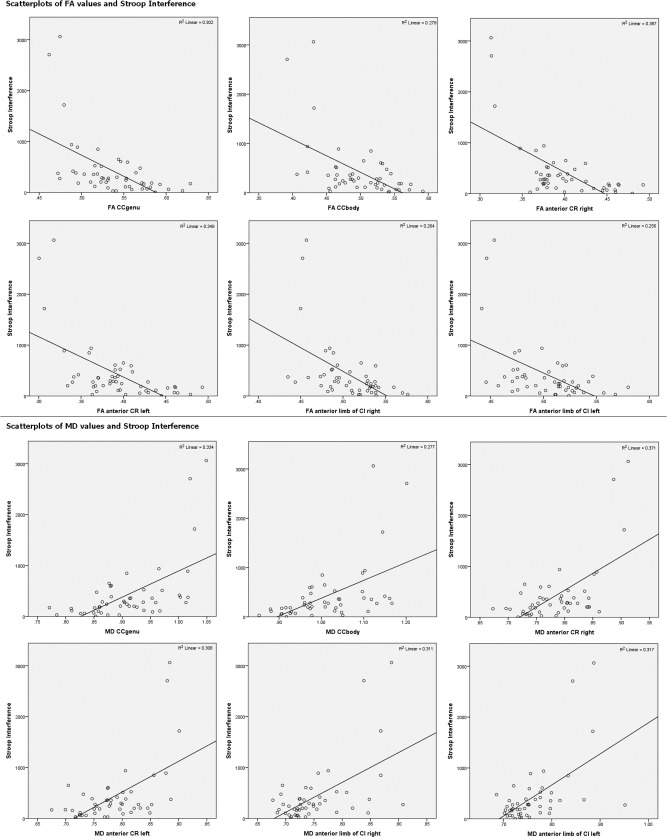
Scatterplots and R 2 values of the relationship between Stroop interference and FA/MD values.
In addition to the investigation of the relationship between integrity measures and Stroop interference controlled for nuisance variables, the ROI‐based multiple regression analyses were performed to compare FA/MD—covariate relationships. Regression analyses demonstrated that integrity measures of the CCgenu, bilateral anterior CR, and bilateral anterior limb of CI were not only related to Stroop interference but also age, suggesting that age‐related white matter degeneration might be functionally relevant for age‐related increases in Stroop interference (Table 2).
As a supplementary analysis, we investigated the relationship between reaction times in the neutral condition and white matter integrity to contrast and disentangle the neural foundation of the interference and neutral condition. According to the explorative investigation of the neural foundation of the interference condition, we performed a voxel‐wise TBSS regression analyses with integrity measures (FA, MD) as dependent variable and reaction times of the neutral condition, TMT‐A, age‐group, years of education, and gender as covariates. Analyses demonstrated no significant association between integrity measures and reaction times of the neutral condition of the Stroop task.
DISCUSSION
This study aimed to investigate whether an age‐related increase in cognitive inhibition, as measured by the Stroop task, is a distinct cognitive process that declines with age or merely reflects general slowing. To disentangle the effects of aging on cognitive inhibition from general slowing we applied analyses based on behavioral data. Furthermore, we analyzed the association between white matter integrity and Stroop interference under control of general slowing to consider the differentiability of Stroop interference from general slowing on a neurobiological level.
Behavioral Analyses
The behavioral data showed in accordance to previous findings a strong association between Stroop interference and age [e.g., West and Alain, 2000; Davidson et al., 2003; Rush et al., 2006]. Likewise, TMT‐A scores were strongly related to age. As Stroop interference and TMT‐A scores were also highly intercorrelated these simple correlation analyses support the assumption that an increase in Stroop interference merely reflects general slowing. However, when performing a linear regression analysis to control the influence of general slowing, the strength of association between age and Stroop interference decreased but remained significant. Furthermore, TMT‐A score was not a significant predictor of Stroop interference. Thus, in accordance to our hypothesis, results of the analyses of the behavioral data suggest that age‐related increases in Stroop interference are partially attributable to general slowing but are also to age‐related changes in task specific processes, such as cognitive inhibition. This result is consistent with a large number of studies that investigated the relationship between normal aging, Stroop interference, and general slowing based on behavioral data [Troyer et al., 2006; Dulaney and Rogers, 1994; Salthouse and Meinz, 1995; West and Baylis, 1998; Bugg et al., 2007; Houx et al., 1993; Spieler et al., 1996; Troyer et al., 2006].
DTI Analyses
Importantly, analyses of white matter integrity demonstrated a strong association of integrity measures of CCgenu, bilateral anterior CR, and bilateral anterior limb of CI with Stroop interference. As these associations were adjusted for general slowing by independent measure, this result implies that these white matter regions specifically modulate Stroop interference and hence inhibitory control as a task‐specific process. Thus, in accordance to our hypothesis, we could delineate Stroop interference from general slowing on a neural level, based on white matter integrity measures. As a supplementary analysis we investigated the relationship between reaction times in the neutral condition and white matter integrity to contrast and disentangle the neural foundation of the interference and neutral condition. Neither FA values nor MD values were significantly associated with the Stroop neutral condition in the whole brain voxel‐wise TBSS analyses. As the neutral condition of the Stroop task is also regarded as a measure of information processing speed [Rodewald et al., 2011], the lack of a relationship between integrity measures and the Stroop neutral condition under control of TMT‐A (as another measure of information processing speed) might be explained by the similarity of those two tasks. Nevertheless, this result strengthens the specific importance of CCgenu, bilateral anterior CR, and bilateral anterior limb of CI in the modulation of Stroop interference, as these pathways were significantly related to the Stroop interference condition but not to the neutral condition.
Although the primary implication for the white matter analyses was to differentiate Stroop interference from general slowing on a neurobiological level, the results further add important information for a more detailed understanding of the neuronal basis of cognitive inhibition as measured by the Stroop task. To date, the role of white matter integrity in Stroop interference has almost exclusively been investigated in psychiatric patients [Murphy et al., 2007; Takei et al. 2009; Li et al., 2010; Yan et al., 2012]. These studies mainly found frontal white matter brain regions to be associated with Stroop interference. We could confirm these results in a study sample consisting of healthy adults. Our findings emphasize the importance for the structural integrity of genu of CC, bilateral anterior CR, and bilateral anterior limb of CI in the modulation of Stroop interference. The genu of the CC mainly connects ventral‐frontal regions and the bilateral dorsolateral prefrontal cortex (DLPFC) [Barbas and Pandya, 1984; Bloom and Hynd, 2005]. The anterior limb of the CI contains fibers of the anterior thalamic peduncle, which is part of cortico‐thalamic circuits that cross prefrontal regions [Nieuwenhuys et al., 2007]. The anterior corona radiata connects the ACC with other frontal and subcortical regions [Wakana et al., 2004; Mori et al., 2005]. As several functional activation studies demonstrated frontal brain regions (mainly dorsolateral prefrontal cortex, DLPFC; inferior frontal gyrus, iFG; and anterior cingulate cortex, ACC) to be important modulators of Stroop interference [e.g., Nee et al., 2007; Badzakova‐Trajkov et al., 2009; ZoccatelLi et al., 2010], these white matter pathways may facilitate neural transmission between these regions. Besides this study, only two further studies investigated the relation between white matter integrity and Stroop interference in healthy subjects [Sullivan et al., 2006; Kennedy and Raz, 2009]. Sullivan et al. [2006] performed a fiber‐tracking study based on 10 older adults, whereby analyses were restricted to the Corpus Callosum. In line with our results, the authors observed a significant relationship between Stroop word reading and regional segments of corpus callosal fiber properties. However, in contrast to our study, they did not find a significant association between Stroop interference and callosal fiber properties. This lack of significance may be based on the very small sample size in the study of Sullivan et al. A larger study group may have shown comparable results. Another study by Kennedy and Raz [2009] found a relationship between the FA of posterior white matter regions (parietal, splenium, and occipital) and Stroop interference. This discrepancy to our results may be based on variation in imaging and analysis methods. In contrast to our automatic whole‐brain analyses of 3T DTI data the authors investigated specific brain regions based on manually placed ROIs on 1.5T DTI data. It is also important to emphasize that Sullivan et al. [2006] and Kennedy and Raz [2009] investigated the relationship between Stroop interference and specific white matter regions without taking account of age‐related general slowing. Thus, the reported results may be confounded by age‐related alterations of information processing speed.
The applied ROI‐based multiple regression analyses demonstrated that integrity measures of genu of CC, bilateral anterior CR, and bilateral anterior limb of CI were not only related to Stroop interference but also to age. The association between white matter integrity of frontal pathways and age is in line with a vast literature on this subject [Sullivan and Pfefferbaum; Madden et al., 2012]. As both, Stroop interference and age, were associated with structural integrity measures of the aforementioned ROIs, integrity of these regions may mediate the relationship between age and Stroop interference. To understand the mechanism underlying this mediation, functional activation studies investigating cognitive inhibition during Stroop task (and related tasks) within normal aging may provide important information. Such investigations reveal that healthy elderly adults recruit a cortical network equivalent to that of young subjects but commonly, network structures are bilateral and stronger activated [Madden and Hoffman, 1997; Nielson et al., 2002; Mathis et al., 2009]. Further studies demonstrated that older adults recruit additional frontal areas compared to young adults [Nielson et al., 2002; Langenecker et al., 2004]. An approach to explain this hyperactivation in aging is made by the compensation‐related utilization of neural circuits hypothesis (CRUNCH) [Reuter‐Lorenz and Cappell, 2008] and the scaffolding theory of aging and cognition (STAC) [Park and Reuter‐Lorenz, 2009]. Both theories suggest that with increased task demands older adults engage additional brain regions bilaterally, to restrict cognitive decline due to age‐related neural degeneration, while young adults mostly recruit unilateral regions. However, a progressive decline of white matter integrity within the aging brain may impair additional activation or recruitment of frontal areas and thus counteract this compensatory mechanism with increasing age. Thus, disturbance in neural transmission combined with impairments in additional activation in old adults may explain the role of white matter integrity in age‐related decreases in cognitive inhibition during Stroop task.
Limitations
The study sample included younger adults, younger elderly, and advanced elderly. However, no middle aged subjects were included. Thus, age could not be treated as a continuous variable. Furthermore, the number of young adults (age range 22–37, n = 13) was considerably smaller than the number of older adults (age range 60–85, n = 36). Thus, the observed coherence between age and Stroop interference and the underlying mechanisms should be verified based on a lifespan design including a more homogeneous sample. Another limitation relates to our cross‐sectional study design. Cross‐sectional studies that investigate normal aging are potentially confounded by cohort differences and might overestimate age related differences, especially in cognitive decline. A longitudinal study lasting several years might more exactly display the evolution of cognitive decline and white matter integrity within healthy aging and is necessary to confirm the assumption of the functional relevance of age‐related WM degeneration in age‐related alterations of Stroop interference. A further limitation relates to the applied voxel‐wise TBSS analyses. TBSS is limited in its ability to accurately measure FA values in regions with a high number of crossing fibers. Thus, skeleton contiguity is not enforced at junctions [see Smith et al., 2006] making it difficult to interpret voxelwise statistics in these regions. Furthermore, TBSS is sensitive to outliers. However, to that end, follow‐up ROI based analyses were performed to support the TBSS results.
Conclusions
Analyses of the behavioral data demonstrated that normal aging is accompanied by a decrease in Stroop interference that is partially attributable to general slowing but also to age‐related changes in task‐specific processes such as inhibitory control. White matter integrity analyses demonstrated that Stroop interference relies on specific/distinct neural networks. Thus, our results suggest that cognitive inhibition, as measured by the Stroop task, is a qualitatively distinct kind of cognitive processing that declines with age and no mere artifact of general slowing. Importantly, our findings also expand the knowledge of the neural basis of Stroop interference and emphasize the importance of white matter integrity of frontal pathways in Stroop interference and age‐related performance alterations.
Supporting information
Supplementary Information Figure 1
Supplementary Information Figure 2
ACKNOWLEDGMENTS
This work is part of the doctoral thesis of the first author. We thank Christine Ploch and Lisa Zschutschke for their efforts with recruitment, training, and investigation of participants during the study. The authors have no conflicts of interest to declare.
REFERENCES
- Badzakova‐Trajkov G, Barnett KJ, Waldie KE, Kirk IJ (2009): An ERP investigation of the Stroop task: The role of the cingulate in attentional allocation and conflict resolution. Brain Res 1253:139–148. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya D (1984): Topography of commissural fibers of the prefrontal cortex in the rhesus monkey. Exp Brain Res 55:187–191. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (1996): Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson B 111(3):209–219. [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15:435–455. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RSJ, Dolan RJ (1993): Investigations of the functional anatomy of attention using the stroop test. Neuropsychologia 31:907–922. [DOI] [PubMed] [Google Scholar]
- Bloom JS, Hynd GW (2005): The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychol Rev 15:59–71. [DOI] [PubMed] [Google Scholar]
- Bugg JM, DeLosh EL, Davalos DB, Davis HP (2007): Age differences in Stroop interference: Contributions of general slowing and task‐specific deficits. Aging Neuropsychol Cogn 14:155–167. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Zacks RT, Williams CC (2003): Stroop interference, practice, and aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 10:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulaney CL, Rogers WA (1994): Mechanisms underlying reduction in Stroop interference with practice for young and old adults. J Exp Psychol Learn Memory Cogn 20:470–484. [DOI] [PubMed] [Google Scholar]
- Graf P, Uttl B, Tuokko H (1995): Color‐and picture‐word Stroop tests: Performance changes in old age. J Clin Exp Neuropsychol 17:390–415. [DOI] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ (2010): Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res 34:1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houx PJ, Jolles J, Vreeling FW (1993): Stroop interference: Aging effects assessed with the Stroop Color‐Word Test. Exp Aging Res 19:209–224. [DOI] [PubMed] [Google Scholar]
- Jacobs HIL, Leritz EC, Williams VJ, Van Boxtel MPJ, Elst W, Jolles J, Verhey FRJ, McGlinchey RE, Milberg WP, Salat DH (2011): Association between white matter microstructure, executive functions, and processing speed in older adults: The impact of vascular health. Hum Brain Mapp 34:77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LJ, Brown FC, Roth RM, Beers SR (2011): Processing Speed and Working Memory Performance in Those with Both ADHD and a Reading Disorder Compared with Those with ADHD Alone. Arch Clin Neuropsychol 26:425–433. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N (2009): Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia 47:916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Coyle T, Lancaster J, Robin D, Hardies J, Kochunov V, Bartzokis G, Stanley J, Royall D, Schlosser A (2010): Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage 49:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Nielson KA, Rao SM (2004): fMRI of healthy older adults during Stroop interference. Neuroimage 21:192–200. [DOI] [PubMed] [Google Scholar]
- Li Q, Sun J, Guo L, Zang Y, Feng Z, Huang X, Yang H, Lv Y, Huang M, Gong Q (2010): Increased fractional anisotropy in white matter of the right frontal region in children with attention‐deficit/hyperactivity disorder: A diffusion tensor imaging study. Neuroendocrinol Lett 31:747–753. [PubMed] [Google Scholar]
- MacLeod CM (1991): Half a century of research on the Stroop effect: An integrative review. Psychol Bull 109:163–203. [DOI] [PubMed] [Google Scholar]
- MacLeod CM (2007): The concept of inhibition in cognition In: Gorfein DS, MacLeod CN, editors. Inhibition in Cognition. Washington: APA; pp 3–24. [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N, Song AW (2012): Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta 1822:386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW (2009): Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychol Rev 19:415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Hoffman JM (1997): Application of positron emission tomography to age‐related cognitive changes In: Krishman KRR, Doraiswamy PM, editors. Brain imaging in clinical psychiatry. New York: Dekker; pp 575–613. [Google Scholar]
- Mathis A, Schunck T, Erb G, Namer IJ, Luthringer R (2009): The effect of aging on the inhibitory function in middle‐aged subjects: A functional MRI study coupled with a color‐matched Stroop task. Int J Geriatr Psychiatry 24:1062–1071. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. (2008): Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PCM, Nagae‐Poetscher L (2005): MRI atlas of human white matter: Am Soc Neuroradiology. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J (2006): Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539. [DOI] [PubMed] [Google Scholar]
- Murphy CF, Gunning‐Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, Hrabe J, Kanellopoulos D, Shanmugham BR, Alexopoulos GS (2007): White‐matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry 61:1007–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J (2007): Interference resolution: Insights from a meta‐analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7:1–17. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H (2002): Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging 17:56–71. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C (2007): The Human Central Nervous System: A Synopsis and Atlas. 4th ed. New York: Springer-Verlag. [Google Scholar]
- Park DC, Reuter‐Lorenz P (2009): The adaptive brain: Aging and neurocognitive scaffolding. Ann Rev Psychol 60:173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM (1958): Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 8:271–276. [Google Scholar]
- Reuter‐Lorenz PA, Cappell KA (2008): Neurocognitive aging and the compensation hypothesis. Curr Direct Psychol Sci 17:177–182. [Google Scholar]
- Rodewald K, Rentrop M, Holt DV, Roesch‐Ely D, Backenstraß M, Funke J, Weisbrod M, Kaiser S (2011): Planning and problem‐solving training for patients with schizophrenia: A randomized controlled trial. BMC psychiatry 11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ (1999): Nonrigid registration using free‐form deformations: Application to breast MR images. IEEE Trans Med Imaging 18:712–721. [DOI] [PubMed] [Google Scholar]
- Rush BK, Barch DM, Braver TS (2006): Accounting for cognitive aging: Context processing, inhibition or processing speed? Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 13:588–610. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996): The processing‐speed theory of adult age differences in cognition. Psychol Rev 103:403–428. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE (2003): Executive functioning as a potential mediator of age‐related cognitive decline in normal adults. J Exp Psychol Gen 132:566–594. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Meinz EJ (1995): Aging, inhibition, working memory, and speed. J Gerontol Ser B Psychol Sci Soc Sci 50:297–306. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME (1996): Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer's type. J Exp Psychol Hum Percept Perform 22:461–479. [DOI] [PubMed] [Google Scholar]
- Stroop JR (1992): Studies of interference in serial verbal reactions. J Exp Psychol Gen 121:15–23. [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A (2006): Selective age‐related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex 16:1030–1039. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A (2006): Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30:749–761. [DOI] [PubMed] [Google Scholar]
- Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, Muroi M, Sasaki H, Aoki S, Kasai K (2009): Structural disruption of the dorsal cingulum bundle is associated with impaired Stroop performance in patients with schizophrenia. Schizophrenia Res 114:119–127. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R (2011): Regional gray and white matter volume associated with Stroop interference: Evidence from voxel‐based morphometry. Neuroimage 59:2899–2907. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Leach L, Strauss E (2006): Aging and response inhibition: Normative data for the Victoria Stroop Test. Aging Neuropsychol Cogn 13:20–35. [DOI] [PubMed] [Google Scholar]
- Turken AU, Whitfield‐Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JDE (2008): Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage 42:1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, De Meersman L (1998): Aging and the Stroop effect: A meta‐analysis. Psychol Aging 13:120–126. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PCM, Mori S (2004): Fiber Tract–based Atlas of Human White Matter Anatomy1. Radiology 230:77–87. [DOI] [PubMed] [Google Scholar]
- West R, Alain C (2000): Age‐related decline in inhibitory control contributes to the increased Stroop effect observed in older adults. Psychophysiology 37:179–189. [PubMed] [Google Scholar]
- West R, Baylis GC (1998): Effects of increased response dominance and contextual disintegration on the Stroop interference effect in older adults. Psychol Aging 13:206–217. [DOI] [PubMed] [Google Scholar]
- Yan H, Tian L, Yan J, Sun W, Liu Q, Zhang YB, Li XM, Zang YF, Zhang D (2012): Functional and anatomical connectivity abnormalities in cognitive division of anterior cingulate cortex in schizophrenia. PLOS One 7:e45659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccatelli G, Beltramello A, Alessandrini F, Pizzini FB, Tassinari G (2010): Word and position interference in Stroop tasks: A behavioral and fMRI study. Exp Brain Res 207:139–147. [DOI] [PubMed] [Google Scholar]
- Zysset S, Schroeter ML, Neumann J, Yves von Cramon D (2007): Stroop interference, hemodynamic response and aging: An event‐related fMRI study. Neurobiol Aging 28:937–946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Figure 1
Supplementary Information Figure 2


