Abstract
It is unknown to what extent briefly presented emotional words can be processed without awareness. By means of two independent functional magnetic resonance imaging studies, using either a block or an event‐related design, we investigated brain activation to very briefly presented threat related and neutral words during two backward masking conditions (with and without gap between target and mask). In both experiments, emotional words were perceived during the supraliminal “with gap” condition, but they were not recognized during the subliminal “without gap” condition, as indicated by signal detection theory analysis. Imaging results of both experiments showed increased activation of the amygdala, the medial prefrontal cortex and language‐processing cortical areas to negative versus neutral words during supraliminal but not subliminal conditions. These results suggest that even very briefly presented emotional words are capable of triggering increased cortical and subcortical processing; however, only when awareness of these stimuli is given. Hum Brain Mapp 36:655–665, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: amygdale, awareness, backward masking, emotion, fMRI, words
INTRODUCTION
Verbal emotional stimuli have been shown to activate the amygdala (Cunningham et al., 2004; Hamann and Mao, 2002; Herbert et al., 2009; Isenberg et al., 1999; Straube et al., 2011b), suggesting amygdala involvement in processing symbolic emotional stimuli. Besides the amygdala, emotional words have been shown to increase activation in several left hemispheric word‐processing areas, including inferior frontal gyrus (IFG, Kuchinke et al., 2005), fusiform gyrus (FG, Dehaene and Cohen, 2011; Luo et al., 2004), and angular gyrus (Hsu et al., 2012). Increased activation to emotional words was also found in areas involved in explicit emotional evaluation and memory such as the dorsomedial prefrontal cortex (DMPFC, Cato et al., 2004; Kensinger and Schacter, 2006; Straube et al., 2011b) and posterior cingulate gyrus (PCC, Jackson and Crosson, 2006; Maddock et al., 2003; Straube et al., 2004b). These findings indicate that emotional word processing is prioritized compared to neutral words across several stages of the information processing cascade.
At present, it is unclear to what extent the processing of emotional words can occur independently of conscious stimulus awareness. For pictorial stimuli, several neuroimaging studies found amygdalar activation to backward‐masked fearful or fear‐conditioned faces, which was interpreted to reflect automatic subcortical processing of threat (Liddell et al., 2005; Morris et al., 1998, 1999; Whalen et al., 1998; Williams et al., 2006b; but see Pessoa et al., 2006; Phillips et al., 2004; Straube et al., 2011a). However, more complex emotional pictures do not seem to be processed under backward masking conditions in healthy individuals (Hoffmann et al., 2012) and several previous backward masking studies have been associated with methodological and interpretative problems (for discussion see: Pessoa and Adolphs, 2010; Pessoa et al., 2006; Straube et al., 2010, 2011a).
Although subliminal processing of emotional pictorial stimuli has been repeatedly investigated with functional magnetic resonance imaging (fMRI), there are no comparable fMRI studies for emotional words. Naccache et al.(2005) investigated intracranial electroencephalography (EEG) to backward‐masked word stimuli in three epilepsy patients and reported differential amygdalar potentials to emotional versus neutral words. Remarkably, these potentials showed an unexpected late latency and clear variability in time course and polarity across patients. Furthermore, it remains unclear whether potential subliminal effects to emotional words can be identified in cortical areas. It has been suggested that processing of emotional connotation in words should be based on the orthographic, lexical, and semantic analysis of words in cortical areas (Naccache et al., 2005; Palazova et al., 2011).
In this study, we investigated neural correlates of subliminal and supraliminal processing of threat‐related words in healthy subjects using fMRI, two different designs and two independent samples. We utilized a block design as well as an event‐related design, the former to increase signal to noise ratio, the latter to analyze data on a trial‐by‐trial basis. To avoid confounding factors of different stimulus duration in subliminal and supraliminal conditions, words were always presented very briefly (17 ms) and only stimulus onset asynchrony (SOA) was varied. Furthermore, standard signal detection theory (SDT) methods were applied to ensure validity of the subliminal condition.
MATERIALS AND METHODS
Subjects
For each experiment, an independent sample of participants was recruited. Nineteen healthy, right‐handed volunteers participated in the first experiment. Three subjects were excluded because of awareness of masked stimuli (as described below). Furthermore, one subject was excluded from statistical analysis due to abnormal blood oxygenation level dependent (BOLD) signal in the amygdala regions of interest (ROI) (differing more than two standard deviations from the mean) resulting in a final sample size of 15 participants (9 females, mean age: mean = 24.8 years, SD = 4.0 years, range = 20–33 years). In the second experiment, 12 healthy, right‐handed subjects (9 females, mean age = 21.2 years, SD = 2.2 years, range = 19–27 years) participated. All participants had normal or corrected‐to‐normal vision and were native speakers of German. The experimental procedures were approved by the Ethics Committee of the University of Jena and all subjects provided informed consent to participate in the experiments.
Experiments and Stimuli
In both fMRI experiments, participants were exposed to visual target‐mask pairs, which aimed at manipulating awareness. Target stimuli consisted of 12 threat related and 12 neutral verbs (Experiment 1) and 20 threat related and 20 neutral verbs (Experiment 2). Word stimuli were matched for frequency of occurrence and number of syllables in German written language (COSMASII database, http://www.ids-mannheim.de/cosmas2). Negative words referred to actions that mostly harm other persons (e.g., to abuse, to murder). In contrast, neutral words referred to everyday life actions (e.g., to read, to water; for complete list see also Supporting Information). The mask was a pattern consisting of 12 rhombi arranged in a line.
Throughout the first experiment (block design), each target‐mask pair was presented during two backward‐masking conditions designed to establish subliminal and supraliminal target processing. On each trial, a target was presented for 17 ms, followed by a 200‐ms mask. In the subliminal condition, the mask onset immediately followed the target offset, whereas a 250‐ms blank screen was inserted between the target offset and mask onset in the supraliminal condition. Thus, the duration of target presentation was kept constant across conditions. After each target‐mask pair, a fixation cross was presented for 1,783 ms (subliminal condition), or 1,533 ms (supraliminal condition), resulting in an overall trial length of 2 s for each of both conditions (Fig. 1A–C). Extended pilot experiments ensured that the configuration of a 17‐ms lasting target stimulus followed by a 200 ms mask effectively prevented recognition of target words. Combining two experimental two‐level factors (i.e., target valence and awareness) yields four experimental conditions, which were presented within a block design. Each block consisted of 12 trials. The blocks were separated by a 14‐s fixation‐cross rest period. There were four blocks per experimental condition with four different versions of a pseudo‐randomized trial order. Altogether, there were 16 blocks, which were presented within a single run lasting about 10 min. Order of block category and masking condition was counterbalanced across subjects.
Figure 1.
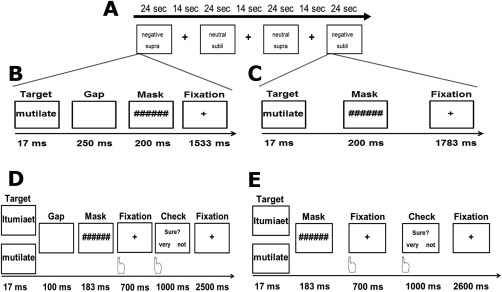
Experimental paradigm of Experiment 1 (Block design; A, B, C) and Experiment 2 (Event‐related design; D, E). In A, the structure of the Block design is displayed. In each trial in the blocks of Experiment 1, subjects were shown a negative/neutral word for 17 ms followed by a 200 ms lasting mask. The supraliminal condition (B) differed from the subliminal condition (C) due to a 250 ms long gap (blank screen) between target offset and mask onset. To obtain constant trial duration of 2 s a variable fixation cross was implemented. The trial structure of the event‐related design (Experiment 2) is shown in D and E. A negative/neutral word or a nonword was displayed for 17 ms in the supraliminal (D) and subliminal condition (E). In both conditions, a 183 ms lasting mask was displayed, whereas a 100 ms long gap was added to the supraliminal condition only. After displaying the target‐mask pair, the participants had to indicate by button press if they had seen a word. For this purpose, a 700 ms lasting gap picturing a fixation cross was set. Subsequently, a 1,000 ms lasting interval was defined in which the participants had time to rate their confidence (1 –not sure, 2 –sure). At the end of each trial, a variable fixation cross was applied to both conditions yielding a trial duration of 4500 ms.
The second experiment used an event‐related design, which provides less power than a block design but allows a trial‐by‐trial assessment of recognition of words and avoids anticipation effects. For every word, a string pattern was created by randomly mixing characters of every word to implement a nonword condition to allow the experimental task described below. According to the trial structure of the block design, the event‐related design likewise presented target‐mask pairs in supraliminal and subliminal conditions (Fig. 1D,E), using the same mask as in Experiment 1. The target, a negative or neutral word, or their adequate string pattern, was displayed for 17 ms in a pseudo‐randomized order. The manipulation of visibility was realized by a mask onset delay of 100 ms blank screen in the supraliminal condition and no delay in the subliminal condition. In comparison to the first experiment, the stimulus mask SOA was shortened in the second experiment to improve similarity between the subliminal and supraliminal conditions (see also Hoffmann et al., 2012). The duration of the mask was set to 183 ms. After displaying the target‐mask pair, the participants had to indicate by button press if they had seen a word during an interval of 700 ms where a fixation cross was shown. Subsequently, subjects had to rate the confidence of their decision (1–not sure vs. 2—sure; see Fig. 1) within a fixed interval of 1,000 ms. Between trials a fixation cross (2,500 ms supraliminal condition; 2,600 ms subliminal condition) was presented. SOA of presentations of words/strings was 4,500 ms. The whole experiment consisted of two runs, each with 160 trials (20 trials per condition: negative word, neutral word, and corresponding string patterns respectively presented with and without gap before the mask) yielding a duration of 24 min.
Stimuli were presented in both experiments using Presentation software (Version 9.81, Neurobehavioral Systems) and a liquid crystal display projector (TLP 710 E, Toshiba Corporation, Japan). The duration of stimulus presentation was confirmed using a photodiode (OPT101, Burr‐Brown, Tucson, AZ). DASYLab software (Datalog Gmbh, Germany) was used to record luminance changes at a sampling rate of 2 kHz. Resulting raw data were analyzed using Brain Vision Analyzer 2.0 (Brain Products Gmbh, Germany). For judgement required in the second experiment, subjects pressed one of two buttons of an optic fiber response box with either the index or middle finger of the right hand. Judgement data were recorded using Presentation software (Version 9.81, Neurobehavioral Systems).
After completion of the fMRI‐sessions and the behavioral discrimination experiment (see below), participants rated the words of each stimulus category using a 9‐point Likert scale to assess valence (1 = very unpleasant to 9 = very pleasant) and arousal (1 = not arousing to 9 = very arousing). Behavioral data were analyzed by means of repeated measures analysis of variance using Statistical Package for the Social Sciences (Version 16, SPSS, Chicago). A probability level of P < .05 was considered statistically significant. All data are expressed by mean ± standard error of means.
Debriefing and Analysis of Detection Scores
In Experiment 1, participants were asked to describe any aspects of the stimuli presented after the scanning session. Thereafter, they were informed about the masking procedure. Subjects were shown exemplars of the masked target words and requested to indicate whether they had seen anything similar during scanning. Three subjects in the first experiment were excluded from statistical analysis because they reported having seen other stimuli besides the masks during the subliminal blocks. Furthermore, subjects of the first experiment participated in a behavioral discrimination experiment during which all target‐mask pairs were presented trial by trial under both masking conditions in random order. On each trial, subjects had to indicate by button press whether they had seen a negative word and to rate the confidence in their response on a three‐point scale (1 = not confident to 3 = very confident). As mentioned before, trial‐by‐trial responses were measured during the fMRI experiment in the event‐related design (Experiment 2). Only trials in which a word was detected, were analyzed in the supraliminal condition and only trials in which the word was not detected were analyzed in the subliminal condition.
Behavioral responses were analyzed with SDT methods (Macmillan and Creelman, 2005). Receiver operating characteristic (ROC) curves were obtained by plotting the hit rate against the false‐alarm rate for every confidence rating (three/two possible levels for hits and three /two for false alarms). For each subject, ROC curves were plotted for subliminally and supraliminally presented words, respectively, with the area under the ROC curve A′ representing their overall discrimination performance across several levels of the response criterion. Target recognition was considered being conscious when A′ values significantly exceeded 0.5, the value of A′ associated with chance performance; otherwise, recognition was considered to be unconscious (Macmillan and Creelman, 2005; Pessoa et al., 2005). A probability level of P < 0.05 was considered as statistically significant.
fMRI
In a 1.5‐Tesla magnetic resonance scanner (Magnetom Vision plus, Siemens, Erlangen, Germany), one run of 208 volumes (Experiment 1: block design) and two runs of 245 volumes (Experiment 2: event‐related design) were conducted using a T2*‐weighted echo‐planar sequence (TE = 50 ms, flip angle = 90°, matrix = 64 × 64, FOV = 192 mm, and TR = 2,980 ms). Each volume comprised 30 axial slices (thickness = 3 mm, gap = 1 mm, and in‐plane resolution = 3 × 3 mm). The first four volumes were discarded to ensure steady‐state tissue magnetization. Additionally, a high‐resolution T1‐weighted anatomical volume was recorded. Preprocessing and analysis of functional data was performed using the software BrainVoyager QX (Version 2.1, Brain Innovation, Maastricht, the Netherlands). All volumes were realigned to the first volume to minimize effects of head movements. Further data preprocessing comprised a correction for slice time errors, and spatial as well as temporal smoothing using Gaussian Kernels. Anatomical and functional images were coregistered and normalized to the Talairach space (Talairach et al., 1988).
Statistical analysis was performed by multiple linear regression of the signal time course at each voxel. The expected blood oxygen level‐dependent signal change for each event type (predictor) was modeled by a canonical hemodynamic response function. First, voxelwise statistical maps were generated and predictor estimates were computed for each individual. Then, predictor estimates were computed with group‐level t‐tests. Brain activation was analyzed within the amygdala, left FG, left angular gyrus, left IFG, DMPFC, and PCC which were defined a priori as ROI using Talairach daemon software (Lancaster et al., 2000) and according to our previous studies (e.g., Straube et al., 2004a, 2006, 2008). Statistical parametric maps resulting from voxelwise analysis were considered statistically significant for clusters that survived a correction for multiple comparisons. We used the approach as implemented in Brain Voyager (Goebel et al., 2006), which is based on a three dimensional extension of the randomization procedure described by Forman and colleagues (Forman et al., 1995). First, the voxel‐level threshold was set at P < .005 (uncorrected). Thresholded maps then were submitted to a correction for multiple comparisons based on the search space for each ROI. The correction criterion was based on the estimate of the map's spatial smoothness and on an iterative procedure (Monte Carlo simulation) for estimating cluster‐level false‐positive rates. After 1,000 iterations, the minimum cluster size threshold that yielded a cluster‐level false‐positive rate of 5% was applied to the statistical maps.
RESULTS
Experiment 1
Rating data
Analysis of postscanning valence and arousal ratings of words indicated that negative words were rated as more unpleasant (negative: 2.18 ± 0.78, neutral: 5.54 ± 0.83); t(14) = −10.93; P < 0.0001) and more arousing (negative: 6.46 ± 1.89, neutral: 1.54 ± 0.55; t(14) = 11.63; P < 0.0001) than neutral words.
Performance data
Analysis only includes subjects who did not recognize any aspect of the masked words. Therefore, three subjects were excluded from further analysis. In the masked condition, none of 15 subjects A′ values differed significantly from 0.5 (M A ′subli = 0.57; SD A ′subli = 0.09), whereas all 15 subjects did in the supraliminal condition (M A ′supra = 0.97; SD A ′subli = 0.05; see also Fig. 2 and Table 1). Furthermore, the A′ values of the supraliminal condition differed significantly from the values of the subliminal condition (Z = 4.76, P < 0.0001, Wilcoxon test).
Figure 2.
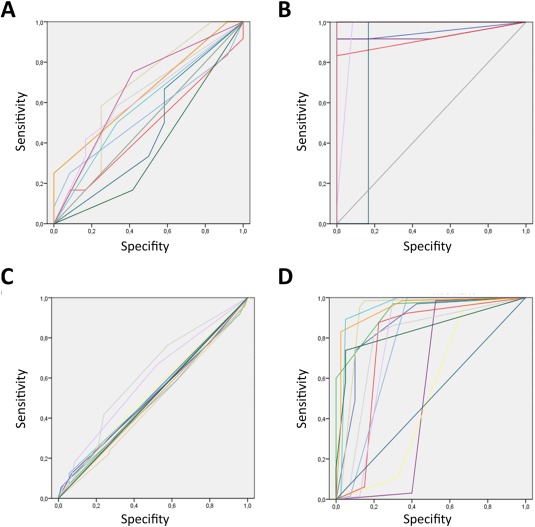
ROC curves of performance data. ROC curves of both experiments [Exp. 1 (A) subliminal and (B) supraliminal, Exp.2 (C) subliminal and (D) supraliminal] are displayed. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
Measures of sensitivity
| Exp. 1 | Exp. 2 | ||||
|---|---|---|---|---|---|
| Subject | A′ sub | A′ supra | Subject | A′ sub | A′ supra |
| 01 | 0.5 | 0.95c | 01 | 0.52 | 0.87c |
| 02 | 0.38 | 1.00c | 02 | 0.51 | 0.93c |
| 03 | 0.5 | 1.00c | 03 | 0.47 | 0.92c |
| 04 | 0.5 | 0.83b | 04 | 0.51 | 0.54 |
| 05 | 0.66 | 1.00c | 05 | 0.53 | 0.52 |
| 06 | 0.63 | 1.00c | 06 | 0.50 | 0.78c |
| 07 | 0.66 | 1.00c | 07 | 0.51 | 0.95c |
| 08 | 0.66 | 0.94c | 08 | 0.61a | 0.79c |
| 09 | 0.62 | 0.96c | 09 | 0.50 | 0.78c |
| 10 | 0.656 | 1.00c | 10 | 0.50 | 0.84c |
| 11 | 0.54 | 1.00c | 11 | 0.49 | 0.95c |
| 12 | 0.47 | 0.92c | 12 | 0.59 | 0.79c |
| 13 | 0.58 | 1.00c | |||
| 14 | 0.5 | 1.00c | |||
| 15 | 0.67 | 1.00c | |||
P < 0.05.
P < 0.01.
P < 0.005.
FMRI Data
Supraliminal condition
Increased activation to visible negative versus neutral words was found in the left (x, y, z: −21, −5, −12; t(14) = 5.85; cluster size: 1,296 mm3; P < 0.05; corrected for multiple comparisons) and right (x, y, z: 26, 0, −15; t(14) = 4.59; cluster size: 243 mm3; P < .05; corrected) amygdala. We also found differential activation in the left IFG (x, y, z: −47, 9, 22; t(14) = 7.38; cluster size: 432 mm3; x, y, z: −48, 26, 5; t(14) = 3.78; cluster size: 216 mm3; P < 0.05, corrected), the DMPFC (x, y, z: −3, 54, 37; t(14) = 6.64; cluster size: 7,074 mm3; P < 0.05; corrected), the PCC (x, y, z: −8, −45, 29; t(14) = 5.80; cluster size: 3,231 mm3; P < 0.05; corrected), the left FG (x, y, z: −38, −37, −13; t(14) = 4.30; cluster size: 504 mm3; P < 0.05; corrected), and the left angular gyrus (x, y, z: −61, −53, 21; t(14) = 5.40; cluster size: 3,456 mm3; P < 0.05; corrected). All ROI activations of the first experiment are also displayed in Figure 3.
Figure 3.
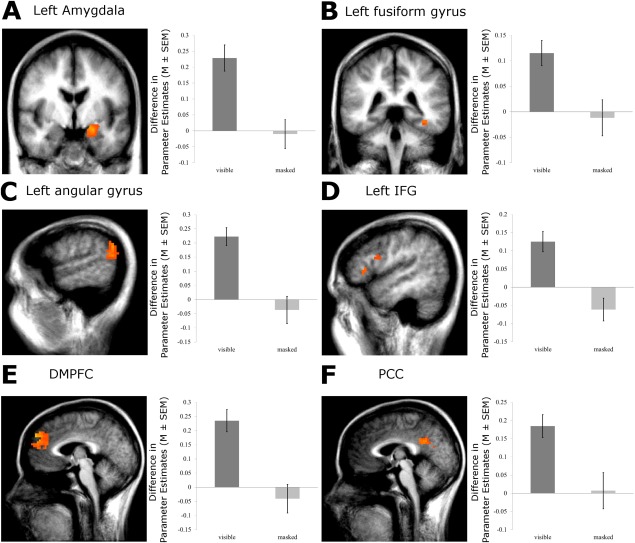
Experiment 1 Block design: ROI activation of the supraliminal and subliminal condition. There were stronger responses of the left amygdala, the left FG, the left IFG, the left angular gyrus, DMPFC, and PCC to negative versus neutral words. Statistical parametric maps of the ROI analysis are overlaid on an averaged T1 scan (radiological convention: left = right; A Amygdala, y = −5; B left FG, y = −37; C left angular gyrus, x = −57; D left IFG, x = −48; E DMPFC x =−3; and F PCC, x = −2). The plot shows the difference in mean parameter estimates of the clusters of the significant ROIs. In all ROIs, no differential activation was observed during the subliminal condition. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Subliminal condition
There was no significant activation in the ROIs during subliminal word presentation. Even when using an uncorrected threshold of P < 0.01, there were no effects (see also Fig. 3).
Supra versus subliminal condition
The comparison of both visibility conditions revealed activation in the left (x, y, z: −21, −6, −14; t(14) = 6.46; cluster size: 972 mm3; P < 0.05; corrected) and right amygdala (x, y, z: 25, −1, −21; t(14) = 4.71; cluster size: 216 mm3; P < 0.05; corrected). Additionally, differential activation was also found in the left IFG (x, y, z: −45, 9, 20; t(14) = 6.42; cluster size: 909 mm3; P < 0.05; corrected), the DMPFC (x, y, z: 3, 46, 17; t(14) = 4.57; cluster size: 909 mm3, x, y, z: −5, 54, 33; t(14) = 5.15; cluster size: 993 mm3, P < 0.05; corrected), the PCC (x, y, z: −1, −26, 35; t(14) = 4.14; cluster size: 351 mm3; x, y, z: −3, −45, 25; t(14) = 5.85; cluster size: 999 mm3; x, y, z: 0, −22, 42; t(14) = 4.49; cluster size: 486 mm3; x, y, z: 4, −63, 23; t(14) = 4.81; cluster size: 649 mm3; P < 0.05; corrected), left FG (x, y, z: −30, −57, −16; t(14) = 5.75; cluster size: 450 mm3; P > 0.05; corrected), and left angular gyrus (x, y, z: −61, −56, 21; t(14) = 6.36; cluster size: 1,099 mm3; P < 0.05; corrected).
Experiment 2
Rating data
Analysis of postscanning valence and arousal ratings of words indicated that negative words were rated as more unpleasant (negative: 1.95 ± 0.49, neutral: 6.05 ± 0.67); t(11)= −24.19; P < 0.0001) and more arousing (negative: 7.28 ± 0.93, neutral: 2.10 ± 0.45; t(11) = 22.50; P < 0.0001) than neutral words.
Performance data
Only one of 12 subjects A′ values differed significantly from chance level in the subliminal condition (MA ′subli = 0.52; SDA ′subli = 0.04), whereas 10 of 12 subjects discriminated between word and nonword condition in the supraliminal condition (MA ′supra = 0.80; SDA ′subli = 0.14; see also Fig. 2 and Table 1). Furthermore, the A′values of the supraliminal condition differed significantly from the values of the subliminal condition (Z = 3.87, P < 0.0001, Wilcoxon test). Trial number of included trials due to the individuals ratings resulted in M = 34 (SD = 5.79) for the subliminal condition and M = 33.79 (SD = 5.27) for the supraliminal condition (t sub‐supra(23) = 0.11, n.s.).
FMRI Data
Supraliminal condition
The ROI‐analysis for the second experiment resulted in significantly activated clusters to negative versus neutral words in the left amygdala (x, y, z: −17, −4, −21; t(11) = 3.79; cluster size: 189 mm3; P < 0.05; corrected), the left IFG (x, y, z: −44, 28, 2; t(11) = 3.47; cluster size: 162 mm3; P < 0.05; corrected), the DMPFC (x, y, z: 2, 52, 38; t(11) = 6.73; cluster size: 1,836 mm3; x, y, z: −2, 50, 18; t(11) = 3.93; cluster size: 135 mm3; P < 0.05; corrected), the PCC (x, y, z: 5, −53, 21; t(11) = 5.80; cluster size: 702 mm3; P < 0.05; corrected). All ROI activations of the second experiment are displayed in Figure 4. There was a trend toward increased activation in left FG (x, y, z: −35, −45, −9; t(11) = 2.45) and left angular gyrus (x, y, z: −50, −56, 20; t(11) = 3.23) which, however, did not pass corrected statistical thresholds.
Figure 4.
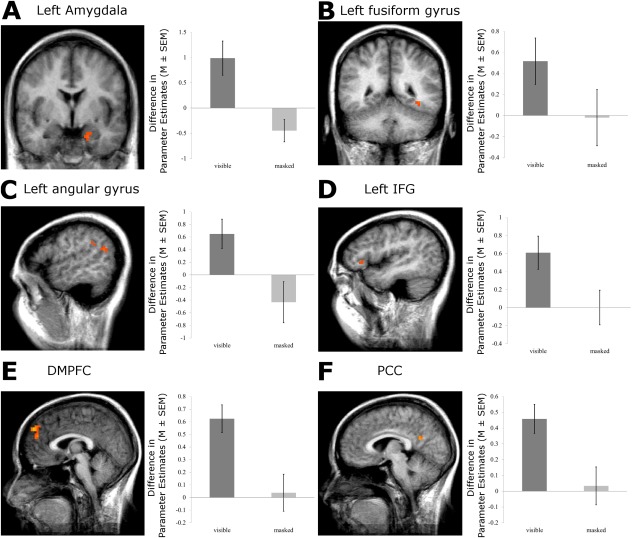
Experiment 2 Event‐related design: ROI activation of the supraliminal and subliminal condition. There were stronger responses of the left amygdala, left IFG, DMPFC, and PCC to negative versus neutral words. Statistical parametric maps of the ROI analysis are overlaid on an averaged T1 scan (radiological convention: left = right; A Amygdala, y = −4; D left IFG x = −44; E DMPFC, x = 2; and F PCC, x = 5). The plot shows the difference in mean parameter estimates of the clusters of the significant ROIs. In all ROIs, no differential activation was observed during the subliminal condition. Statistical maps of ROI‐Analysis that did not pass level of significance of P < 0.005 are displayed in B (Left FG, y = −45; P < 0.05) and C (Left angular gyrus, x = −50; P < 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Subliminal condition
There was no significant activation to unrecognized negative versus neutral words in the ROIs during the subliminal condition. Even when using an uncorrected threshold of P < 0.01, there were no effects (see also Fig. 4).
Supra versus subliminal condition
Contrasting visibility conditions, the left amygdala (x, y, z: −18, −4, −23; t(11) = 5.36; cluster size: 135 mm3; P < 0.05; corrected), left IFG (x, y, z: −45, 38, 6; t(11) = 3.59; cluster size: 162 mm3; P < 0.05; corrected), and the left DMPFC (x, y, z: −17, −47, 16; t(11) = 3.67; cluster size: 216 mm3; P < 0.05; corrected) were significantly activated. The obvious trends of activation differences in PCC (x, y, z: −3, −58, 28; t(11) = 3.64), left FG (x, y, z: −35, −45, −9; t(11) = 2.50) and left angular gyrus (x, y, z: −52, −56, 20; t(11) = 3.37) did not reach corrected statistical significance. Remarkably, as can be seen in Figures 3 and 4, the event‐related design revealed a very similar pattern of results compared to the first experiment. In Supporting Information, we provide further analysis showing the spatial overlap of results from both experiments.
DISCUSSION
In this study, we investigated brain responses to very briefly presented negative and neutral words applying two different paradigms. In both experiments, we showed that under supraliminal conditions briefly flashed negative words induced increased activation in cortical areas and the amygdala as compared to neutral words. These effects were absent under effective backward masking conditions suggesting that backward‐masking prevents increased cortical or subcortical processing of negative words.
Results for the amygdala in the supraliminal condition are in accordance with previous studies. The amygdala has been shown to be responsive to emotional words in several other studies (Hamann and Mao, 2002; Isenberg et al., 1999; Maddock et al., 2003; Straube et al., 2011b). In line with reported amygdala activation to several classes of emotional pictures (Hoffmann et al., 2012; Sabatinelli et al., 2005, 2011; Straube et al., 2008), the amygdala seems to be involved in the processing of the emotional relevance of stimuli independently of stimulus category.
Besides the amygdala, several left hemispheric cortical areas, involved in word processing, showed enhanced responses to negative versus neutral words, including the FG, the angular gyrus, and the IFG. The left FG, which includes the so‐called visual word form area, seems to be involved in increased processing of perceptual features of emotional words (Dehaene and Cohen, 2011; Luo et al., 2004). The left angular gyrus is involved in semantic processing and reading comprehension of words (Binder et al., 2009; Seghier and Price, 2013; Vigneau et al., 2006) suggesting enhanced semantic analysis of threat‐related words (Hsu et al., 2012). It should be noted that effects to threat‐related words were significant in the block design study but showed only a trend in the event‐related design in the FG and the angular gyrus, suggesting that our first experiment was more powerful to reveal activation of these areas to emotional words. Activation to negative versus neutral words in the left IFG was significant across designs and is in accordance with previous studies (Elliott et al., 2000; Maddock et al., 2003; Straube et al., 2004b). The activated part of the IFG include Brocas area, the main anterior language area, and our results indicate that even very briefly presented words strongly modulate activation in this area depending on the emotional relevance of words.
Moreover, we detected increased activation to negative words in the PCC and DMPFC. Activation of the PCC may be related to increased memory processing of emotional words (Maddock et al., 2003; Straube et al., 2004b). The DMPFC is involved in emotion–cognition interactions, such as evaluation of emotional words (Straube et al., 2011b). In this regard, several studies have shown increased DMPFC activation during emotional judgments concerning emotional stimuli in comparison to neutral ones (Lee and Siegle, 2012; Ochsner et al., 2002; Ochsner and Gross, 2005; Straube et al., 2004b).
In the effective backward masking condition, we did not observe effects to negative versus neutral words in any of the investigated brain regions. This contradicts the assumption of automatic processing of emotional words under backward masking conditions and is in contrast to findings in three epileptic patients implanted with electrodes in the amygdala (Naccache et al., 2005). This study reported relatively late differential potentials (> 800 ms) to emotional versus neutral words under backward masking conditions. However, the polarity and also the time point of this effect were very variable across patients, preventing firm conclusions. Furthermore, the reported late effects differed remarkably from results of scalp EEG studies, indicating emotion effects starting reliably about 200 ms after word onset in healthy participants (Keuper et al., 2013; Schacht and Sommer, 2009).
We suggest that our results may contribute to the debate on to which extend subliminally presented emotional stimuli are processed (Hoffmann et al., 2012; Lipka et al., 2011; Pessoa and Adolphs, 2010; Pessoa et al., 2006; Straube et al., 2010). Previous neuroimaging studies using pictorial stimuli and backward masking have shown opposing results stimuli with some studies reporting amygdala activation (Liddell et al., 2005; Morris et al., 1998, 1999; Whalen et al., 1998; Williams et al., 2006a, 2006b) while other studies did not (Hoffmann et al., 2012; Pessoa et al., 2006; Phillips et al., 2004; Straube et al., 2010). The lack of agreement between these studies might be explained at least partially with methodological differences like the application of signal detection methods to control visibility and the control of artifacts such as interactions between targets and masks (Hoffmann et al., 2012; Pessoa et al., 2005; Straube et al., 2010; Straube et al., 2011a).
While for several classes of unconsciously perceived pictorial threat stimuli a subcortical route to the amygdala has been proposed (Liddell et al., 2005; Morris et al., 1998, 1999; Whalen et al., 1998; Williams et al., 2006b; but see Hoffmann et al., 2012; Pessoa et al., 2006; Straube et al., 2011a) for most written verbal stimuli a complete subcortical processing of threat relevance seems to be rather unlikely. The classification of valence of a complex semantic stimulus requires at least some semantic analysis (Dehaene et al., 2005; Naccache et al., 2005; Schacht and Sommer, 2009). It has been suggested that subliminal word processing may cause bottom‐up activation in areas involved in orthographic, lexical, and semantic analysis. Nevertheless, further reverberation loops to build up synchrony with long‐distance loops are lacking during this process (Dehaene et al., 1998; Dehaene and Naccache, 2001). Conscious perception in contrast, requires sufficient stimulus strength and top‐down amplification via parieto‐frontal network activity which binds sensory processing in amplifying loops (Dehaene et al., 2006). Although we did observe increased responses in subcortical and cortical areas during conscious processing of emotional words, we found no evidence that increased emotional, perceptual, or lexico‐semantic processing of threat‐related words is preserved during effective backward masking of these words.
However, we do not exclude that effects of subliminal processing of emotional words might be detected in line with models described above when other experimental and/or analytical methods are used. In our studies, we used backward masking and strong criteria to distinguish subliminal and supraliminal emotional perception. Other methods such as the attentional blink or interocular suppression might result in other outcomes. However, while during the attentional blink semantic analysis seems to be preserved (Sergent et al., 2005; Vogel et al., 1998), it is inhibited for interocular suppression (Kang et al., 2011); therefore, even with this design a processing of emotional words seems to be unlikely. Furthermore, findings from ERP studies and functional imaging might differ. Due to its temporal resolution, event‐related potentials (ERPs) might resolve brain activation patterns which overlap in functional imaging (Straube et al., 2011a). Since scalp EEG studies show reliable relatively early effects to emotional versus neutral words (Kissler et al., 2007; Schacht and Sommer, 2009) future studies might investigate early ERPs to emotional words under aware and unaware conditions. However, until this issue has been investigated with suited methods, we believe it is the most parsimonious explanation at this stage of research that effective backward masking inhibits the differential processing of threat related versus neutral words in healthy individuals.
A limitation of our study might be underpowered samples in both experiments. In Supporting Information, we conducted an effect size analysis of unaware stimulus processing and compared the results with unaware word processing reported in the literature (see Supporting Information). We found small effect sizes for our experiments but effect directions mostly show the opposite direction than expected. This outcome, together with moderate effect sizes for the supraliminal condition, makes the existence of a nondetected true positive effect to unaware threat words rather unlikely. Nevertheless, we suggest that future studies should use bigger sample sizes.
CONCLUSIONS
Using two different paradigms and two independent samples, we revealed consistent cortical and subcortical brain activation to briefly presented negative words under ineffective backward‐masking conditions. Nevertheless, evidence of subliminal processing of emotional words was not found in either of the two experiments. For this reason, our results do not support the hypothesis that emotional connotation of words is processed during backward masking.
Supporting information
Supplementary Information
ACKNOWLEDGMENT
The authors would like to thank Claudia Heller, Christiane Fritz, and Andreas Sauer for their assistance with data acquisition and preprocessing as well as Dr. Andrea Liliana Clavijo McCormick and Carina Heitmann for their helpful suggestions on the manuscript.
Conflict of interest: The authors declare no competing financial interests.
REFERENCES
- Binder JR, Desai RH, Graves WW, Conant LL (2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato MA, Crosson B, Gökçay D, Soltysik D, Wierenga C, Gopinath K, Himes N, Belanger H, Bauer RM, Fischler IS, Gonzalez‐Rothi L, Briggs RW (2004): Processing words with emotional connotation: An FMRI study of time course and laterality in rostral frontal and retrosplenial cortices. J Cogn Neurosci 16:167–177. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK (2004): Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. J Cogn Neurosci 16:1717–1729. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L (2011): The unique role of the visual word form area in reading. Trends Cogn Sci 15:254–262. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L (2001): Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition 79:1–37. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Kerszberg M, Changeux JP (1998): A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci USA 95:14529–14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F (2005): The neural code for written words: A proposal. Trends Cogn Sci 9:335–341. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C (2006): Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci 10:204–211. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ (2000): Selective attention to emotional stimuli in a verbal go/no‐go task: An fMRI study. Neuroreport 11:1739–1744. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33:636–647. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E (2006): Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single‐subject to cortically aligned group general linear model analysis and self‐organizing group independent component analysis. Hum Brain Mapp 27:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Mao H (2002): Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport 13:15–19. [DOI] [PubMed] [Google Scholar]
- Herbert C, Ethofer T, Anders S, Junghofer M, Wildgruber D, Grodd W, Kissler J (2009): Amygdala activation during reading of emotional adjectives—an advantage for pleasant content. Soc Cogn Affect Neurosci 4:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Lipka J, Mothes‐Lasch M, Miltner WH, Straube T (2012): Awareness modulates responses of the amygdala and the visual cortex to highly arousing visual threat. Neuroimage 62:1439–1444. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Mickey BJ, Langenecker SA, Heitzeg MM, Love TM, Wang H, Kennedy SE, Peciña M, Shafir T, Hodgkinson CA, Enoch MA, Goldman D, Zubieta JK (2012): Variation in the corticotropin‐releasing hormone receptor 1 (CRHR1) gene influences fMRI signal responses during emotional stimulus processing. J Neurosci 32:3253–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattie B, Leon AC, Stern E (1999): Linguistic threat activates the human amygdala. Proc Natl Acad Sci USA 96:10456–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MA, Crosson B (2006): Emotional connotation of words: Role of emotion in distributed semantic systems. Prog Brain Res 156:205–216. [DOI] [PubMed] [Google Scholar]
- Kang MS, Blake R, Woodman GF (2011): Semantic analysis does not occur in the absence of awareness induced by interocular suppression. J Neurosci 31:13535–13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL (2006): Processing emotional pictures and words: Effects of valence and arousal. Cogn Affect Behav Neurosci 6:110–126. [DOI] [PubMed] [Google Scholar]
- Keuper K, Zwitserlood P, Rehbein MA, Eden AS, Laeger I, Junghofer M, Zwanzger P, Dobel C (2013): Early prefrontal brain responses to the Hedonic quality of emotional words—a simultaneous EEG and MEG study. PLoS One 8:e70788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler J, Herbert C, Peyk P, Junghofer M (2007): Buzzwords: Early cortical responses to emotional words during reading. Psychol Sci 18:475–480. [DOI] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo ML, Conrad M, Herrmann M (2005): Incidental effects of emotional valence in single word processing: An fMRI study. Neuroimage 28:1022–1032. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ (2012): Common and distinct brain networks underlying explicit emotional evaluation: A meta‐analytic study. Soc Cogn Affect Neurosci 7:521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM (2005): A direct brainstem‐amygdala‐cortical ‘alarm’ system for subliminal signals of fear. Neuroimage 24:235–243. [DOI] [PubMed] [Google Scholar]
- Lipka J, Miltner WH, Straube T (2011): Vigilance for threat interacts with amygdala responses to subliminal threat cues in specific phobia. Biol Psychiatry 70:472–478. [DOI] [PubMed] [Google Scholar]
- Luo Q, Peng D, Jin Z, Xu D, Xiao L, Ding G (2004): Emotional valence of words modulates the subliminal repetition priming effect in the left fusiform gyrus: An event‐related fMRI study. Neuroimage 21:414–421. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD (2005): Detection Theory: A User's Guide, 2nd ed. Mahwah, NJ [u.a.]: Erlbaum. [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2003): Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ (1998): A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121:47–57. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ (1999): A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA 96:1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L, Gaillard R, Adam C, Hasboun D, Clemenceau S, Baulac M, Dehaene S, Cohen L (2005): A direct intracranial record of emotions evoked by subliminal words. Proc Natl Acad Sci USA 102:7713–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005): The cognitive control of emotion. Trends Cogn Sci 9:242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD (2002): Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14:1215–1229. [DOI] [PubMed] [Google Scholar]
- Palazova M, Mantwill K, Sommer W, Schacht A (2011): Are effects of emotion in single words non‐lexical? Evidence from event‐related brain potentials. Neuropsychologia 49:2766–2775. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R (2010): Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci 11:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Japee S, Ungerleider LG (2005): Visual awareness and the detection of fearful faces. Emotion 5:243–247. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Japee S, Sturman D, Ungerleider LG (2006): Target visibility and visual awareness modulate amygdala responses to fearful faces. Cereb Cortex 16:366–375. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SC, Morgan M, Young AW, Gray JA (2004): Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage 21:1484–1496. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ (2005): Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage 24:1265–1270. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J (2011): Emotional perception: Meta‐analyses of face and natural scene processing. Neuroimage 54:2524–2533. [DOI] [PubMed] [Google Scholar]
- Schacht A, Sommer W (2009): Emotions in word and face processing: Early and late cortical responses. Brain Cogn 69:538–550. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Price CJ (2013): Dissociating frontal regions that co‐lateralize with different ventral occipitotemporal regions during word processing. Brain Lang 126:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C, Baillet S, Dehaene S (2005): Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci 8:1391–1400. [DOI] [PubMed] [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH (2004a): Effect of task conditions on brain responses to threatening faces in social phobics: An event‐related functional magnetic resonance imaging study. Biol Psychiatry 56:921–930. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Glauer M, Miltner WH (2004b): Brain activation to phobia‐related words in phobic subjects. Neurosci Lett 372:204–208. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH (2006): Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry 59:162–170. [DOI] [PubMed] [Google Scholar]
- Straube T, Pohlack S, Mentzel HJ, Miltner WH (2008): Differential amygdala activation to negative and positive emotional pictures during an indirect task. Behav Brain Res 191:285–288. [DOI] [PubMed] [Google Scholar]
- Straube T, Dietrich C, Mothes‐Lasch M, Mentzel HJ, Miltner WH (2010): The volatility of the amygdala response to masked fearful eyes. Hum Brain Mapp 31:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Mothes‐Lasch M, Miltner WH (2011a): Neural mechanisms of the automatic processing of emotional information from faces and voices. Br J Psychol 102:830–848. [DOI] [PubMed] [Google Scholar]
- Straube T, Sauer A, Miltner WH (2011b): Brain activation during direct and indirect processing of positive and negative words. Behav Brain Res 222:66–72. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, Rayport M (1988): Co‐planar stereotaxic atlas of the human brain: 3‐dimensional proportional system: An approach to cerebral imaging. Stuttgart [u.a.]: Thieme. [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio‐Mazoyer N (2006): Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage 30:1414–1432. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ, Shapiro KL (1998): Electrophysiological evidence for a postperceptual locus of suppression during the attentional blink. J Exp Psychol Hum Percept Perform 24:1656–1674. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E (2006a): Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci 26:9264–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, Gordon E (2006b): Amygdala‐prefrontal dissociation of subliminal and supraliminal fear. Hum Brain Mapp 27:652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


