Abstract
Studies on training/expertise‐related effects on human brain in context of neuroplasticity have revealed that plastic changes modulate not only task activations but also patterns and strength of internetworks and intranetworks functional connectivity in the resting state. Much has known about plastic changes in resting state on global level; however, how training/expertise‐related effect affects patterns of local spontaneous activity in resting brain remains elusive. We investigated the homogeneity of local blood oxygen level‐dependent fluctuations in the resting state using a regional homogeneity (ReHo) analysis among 16 acupuncturists and 16 matched nonacupuncturists (NA). To prove acupuncturists' expertise, we used a series of psychophysical tests. Our results demonstrated that, acupuncturists significantly outperformed NA in tactile‐motor and emotional regulation domain and the acupuncturist group showed increased coherence in local BOLD signal fluctuations in the left primary motor cortex (MI), the left primary somatosensory cortex (SI) and the left ventral medial prefrontal cortex/orbitofrontal cortex (VMPFC/OFC). Regression analysis displayed that, in the acupuncturists group, ReHo of VMPFC/OFC could predict behavioral outcomes, evidenced by negative correlation between unpleasantness ratings and ReHo of VMPFC/OFC and ReHo of SI and MI positively correlated with the duration of acupuncture practice. We suggest that expertise could modulate patterns of local resting state activity by increasing regional clustering strength, which is likely to contribute to advanced local information processing efficiency. Our study completes the understanding of neuroplasticity changes by adding the evidence of local resting state activity alterations, which is helpful for elucidating in what manner training effect extends beyond resting state. Hum Brain Mapp 35:1074–1084, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: expertise‐related functional plasticity, local modulation, regional homogeneity, acupuncturist, resting state functional MRI
INTRODUCTION
The human brain undergoes dynamic changes in response to experience/training throughout the life span. Recently, studies on training/expertise in the context of neuroplasticity have attracted much scientific attention. Previous conclusions have explicated differentiated functional response patterns to specific tasks under modulation of training effect (Erickson et al., 2007; Ilg et al., 2008; Kelly and Garavan, 2005; Koeneke et al., 2004; Tang et al., 2007; Thomas et al., 2009). In more advanced scenarios, extensive training normally leads to the mastery of a skill, namely expertise, which is demonstrated to have functional correlates in the brain of musicians (Kleber et al., 2010; Parsons et al., 2005), meditation experts (Brefczynski‐Lewis et al., 2007), esthetic experts (Kirk et al., 2009) and radiological experts (Harley et al., 2009). More recently, the academe have begun to realize that the training/expertise‐specific effect on functional originations may extend beyond task state to resting state.
In the absence of overt perceptual input and behavioral output, restful brain consists of spontaneous fluctuations in neuronal activity (Zhang and Raichle, 2010), as reflected in spontaneous activity of the blood oxygen level‐dependent (BOLD) functional magnetic resonance imaging (fMRI) signal, an indication of local neuronal activity (Lee et al., 2010). Spontaneous cortical activity has been proposed to play a pivotal role in maintaining the ongoing, internal representations (Lewis et al., 2009) which may be involved in the coding of expected sensory stimuli, prospective motor responses, and prior experience (Miall and Robertson, 2006; Raichle and Snyder, 2007), although its functional role is not well understood (Lewis et al., 2009). Studies have revealed that training/expertise‐related neuroplasticity changes can shape subsequent spontaneous activity by changing engagement of resting state networks or altering strength of internetworks or intranetworks functional connectivity within the resting brain (Albert et al., 2009; Lewis et al., 2009; Ma et al., 2011; Taubert et al., 2011). Nevertheless, a prominent phenomenal observation is that, concomitant with overt behavioral adaptation and conversancy in behavioral performance, aggregated subsystems/regions can perform specific function without perturbing the remainder of the system (Bassett et al., 2011), which may be accounted to increased local information processing efficiency (Brefczynski‐Lewis et al., 2007). Accordingly, we come to beg the question how spontaneous fMRI BOLD signals are modulated locally by training/expertise effects in the resting brain.
The ReHo method (Zang et al., 2004) has been developed to analyze the similarities or coherence of focal brain spontaneous low‐frequency (<0.08 Hz) BOLD signal fluctuations in voxelwise analysis across the whole brain. This method assumes that, within a functional cluster, the hemodynamic characteristics of every voxel would be similar or synchronous with those of each other, and such similarity could be changed or modulated by different conditions (Zang et al., 2004). The Kendall coefficient of concordance (KCC) (Kendall, 1990) was used to measure the similarity of the time series of one voxel with that of its nearest neighbors in a voxelwise manner. It has been successfully used to investigate the functional modulations in the resting state for pathological datasets (Yu et al., 2011ab) as well as in healthy subjects (Tian et al., 2011). On the other hand, we proposed a novel but robust model, namely acupuncturists. Acupuncturists serve as a pillar of physicians in China. Their professional skills were trained following standardized protocol in extended years, consolidated, and retained during clinical practice as a function of time and accumulative experience (Cheng, 2010). Basically, acupuncturists rely heavily on their tactile‐motor proficiency, which seems a unitary, even automatic process, but is substantially decomposable (Fig. 1). This skill possesses three interacting components: (1) exceptional tactile discrimination ability for the functioning digits which enable the acupuncture practitioners to distinguish subtle dynamic changes of manipulation sensation transmitted through fine needles due to a patient's constantly changing bodily response to each round of needling manipulation; (2) the ability of making precise and prompt adjustment in generating motor plans/commands for the following round of finger manipulation to achieve optimal clinical outcomes; (3) the execution of motor plans/commands into coordinated and fine finger movements. Meanwhile, perceiving the pain of others leads to empathy or personal distress, therefore emotion regulatory skills must operate in acupuncturists who inflict painful procedures in their daily practice with patients to prevent negative emotions from impairing their capacity of assistance (Cheng et al., 2007).
Figure 1.
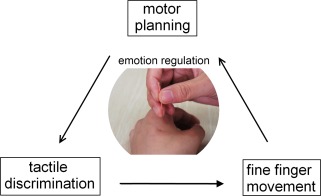
The schematic diagram of acupuncturist's tactile‐motor and emotion regulation proficiency. The patients' concurrent bodily response to each round of needling manipulation is transmitted to prohand through fine needles. The acupuncturist distinguishes the subtle difference between the actual tactile sensation and the expected one, which is followed by motor planning procedure. Then, the postural configuration from the motor plan is executed as acupuncturist's fine and coordinated finger movement over the needles. This feedback loop is repeated until the target response is obtained. At the same time, an emotion regulation mechanism must operate to prevent aversive emotions from affecting acupuncturists' subsequent behaviors. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com]
Therefore, in the current study, we adopted regional homogeneity (ReHo) approach and the expertise model of acupuncturists to assess how expertise affects local patterns of resting state activity. First, a series of psychophysical tasks were adopted to verify acupuncturists' behavioral proficiency in regards to tactile‐motor and emotion regulation ability. Second, taken the fact that resting state activity is modulated by prior learning experience (Albert et al., 2009), we expected to see changes in patterns of local brain activity in acupuncturists' group in regions responsible for tactile‐motor skills and higher order cognitive control ability. Third, we also conducted regression analysis between ReHo of regions showing significant group difference between groups vs. duration of acupuncture practice, as well as behavioral measurements.
Given the paucity of studies, we suggest that our study may reveal a novel connection between the neuroplasticity mechanism and resting state activity. We further suggest that elucidating the relationship between training and function coherence under restful state is important to determine in what manner training effects extend beyond training state.
MATERIALS AND METHODS
All research procedures were approved by the West China Hospital Subcommittee on Human Studies and were conducted in accordance with the Declaration of Helsinki.
Subjects
We investigated 32 healthy, right‐handed (Oldfield, 1971) adult volunteers divided into two groups of equivalent number of subjects. The experimental group, consisting of 16 licensed professional acupuncturists [8 males, mean age = 28 ± 1.6 years (mean ± standard deviation, SD)], was compared with a sample of 16 nonacupuncturists (NA), who were precisely age, gender, and level of education matched. All acupuncturists attended national medical schools, receiving 5 years training of basic acupuncture knowledge, passed the national exam, and became licensed professional acupuncture practitioner. The mean duration of acupuncture training and practice was 60 ± 15.5 (mean ± SD) months. On the other hand, the NA group were included in this study on condition that they had not attended any acupuncture lectures or the related fields, had no experience in acupuncture practice. Prescreening interviews were conducted so that the level of expertise in acupuncture and homogeneity in controls could be ensured. The subjects reported no past or current neurological, psychiatric, or neuropsychological problems, and did not take drugs or illegal medication before or during the study. Written informed consent was obtained after the experimental procedures were fully explained.
Behavioral Tasks
In an initial exploratory behavioral study, we abstracted three tests based on the core features of acupuncturists' training protocols and scenarios of daily clinical practice. In general, we examined their tactile‐motor proficiency of prohand and emotion regulation. One week before the scanning session, all the subjects participated in the following three behavioral tests. For all three tasks, the subjects were seated comfortably in a quiet room with minimal distraction from surroundings. After the same experimenter explained the experimental procedures and requirements of tasks to subjects, they were required to repeat the procedures and demands of tasks back to experimenter to ensure all details were explicitly comprehended. These tests did not begin until all of them understood what exactly they were asked to do.
Emotion Regulation Test
This task is designed to evaluate the subjects' emotion regulation ability following the revised paradigm from a previous publication (Cheng et al., 2007).
Before the task, participants filled out a series of self‐report dispositional measures, including the situational pain questionnaire (SPQ) that assess sensitivity to pain (Clark and Yang, 1983), the Emotional Contagion Scale (ECS) that measure the susceptibility to others' emotions (Doherty, 1997), and Interpersonal Reactivity Index (IRI) (Davis, 1994). During the task, all subjects were shown 120 visual stimuli (120 jpeg files) and these stimuli consisted of pictures of different body parts of both painful and neutral situations (Cheng et al., 2007; Lamm et al., 2011). Pictures were scenarios that are encountered in daily clinical practice. In half of the stimuli, the body parts were touched by a Q‐tip (nonpainful situations), and in the other half, they were pricked by an acupuncture needle (painful situations). All acupuncture sites were appropriately chosen with the assistance of an acupuncture physician of over 10 years' experience in acupuncture practice. The visual stimuli were delivered using E‐prime 2.0 (Psychology Software Tools). The sequence of images was randomized. Each image was displayed 4 s, and rating for unpleasantness lasts 5 s. Participants were asked to focus on the images shown on the screen and began to score only after the cue for scoring appeared. The screen for scoring read “How intense is the unpleasantness felt by you now?”. Participants responded on a 10‐point Likert scale ranging from 1 to 10, where 10 referred to the strongest unpleasantness possible, and 1 referred to the absence of unpleasantness.
Tactile Discrimination Ability Test
We opted spatial grating orientation (GO) discrimination task, which is validated as a dependable test of tactile function (Craig, 1999; Van Boven and Johnson, 1994). The spatial discrimination threshold (SDT) is an accurate measure of somatosensory perception and spatial acuity in humans (Hamm et al., 2006). SDT of the index finger and thumb of the prohand, that is, right hand in the current study, was assessed using “Johnson–Van Boven–Phillips domes” (Med‐Core, St. Louis). The fingers to be tested were immobilized through double‐sided tape affixed to the dorsal aspect of the finger and floor of the fixture to prevent exploratory movements. Grating domes were pressed manually onto to the palmar surface of the distal phalanx of target fingers of the dominant hand/prohand, using of a spring‐loaded apparatus with a spring‐loaded force of 1.5 ± 0.2 N, which was adapted following the fashion of a former study (Van Boven et al., 2005). Gratings were applied with the ridges oriented either along or across the long axis of the finger and subjects verbally reported the orientation of the grating as “along” or “across.” During this experiment, subjects were blindfolded and seated comfortably. The experimenter was auditorily cued to manual position 1 the domes for 1 s (Goldreich and Kanics, 2003) and release the grating when signaled by a computer‐driven timing mechanism (Zhang et al., 2005).
The subsequent experimental block on each finger consisted of 40 trials without feedback. Participants received a 15 s break after every 20 trials, a 1‐min break between fingers. Each trial consisted of two sequential stimulus presentations (interstimulus interval, 2 s) with gratings of identical groove width but differing 90° in orientation. In one presentation, the grooves were aligned parallel (vertical) to and in the other transverse (horizontal) to the long axis of the finger and stimulus order was chosen randomly. The criterion was determined as the accuracy of 75% or better (Hodzic et al., 2004; Van Boven and Johnson, 1994).
Fine Finger Movement Test
This task is designed to assess the degree of acupuncturists' fine‐finger movement skill following protocols that best mimic their daily clinical practice. In the standardized training protocol (Cheng, 2010), the rotation of needles is required to be fast and rhythmic to optimally elicit therapeutic effects. In this sense, all the participants were asked to rotate the needle as fast as they could in 30 s, while making every possible effort to keep each rotation angle between the interval of 90° and 180°. For this purpose, we designed a integrated system which enables online recording of subject's finger movement when subjects were needling. Acupuncture needles (2.5 inch in length and 0.25mm in diameter) were inserted 2 inch deep into pork. Among all available materials, we chose pork because the resistance delivered to the manipulating fingers could similarly mimic that of human body. Before the task, participants were seated before a table where our system was placed. No upper limb parts (e.g., palm, elbow, or arm) of the right side were allowed to touch the table. After participants tuned for their most comfortable needling position, the process was started. High‐speed camera (24 frames/s) recorded the whole process of manipulation. A marker equipped at the end of acupuncture needle was used to mark the starting position. An in‐house imaging processing program was used to calculate the rotation angle of each round. In later data analysis, the rounds of those whose rotation angle exceeded 180° or failed to reach 90° were not counted.
MRI Data Acquisition
Imaging data was collected using a 3T Siemens scanner (Allegra, Erlangen, Germany) at the Huaxi MR Research Center, West China Hospital of Sichuan University, Chengdu, China. A standard birdcage head coil was used, along with restraining foam pads to minimize head motion and to diminish scanner noise. After a localizer scan and conventional structural imaging, resting‐state functional images were obtained with an echo‐planar imaging (EPI) sequence (30 contiguous slices with a slice thickness of 5 mm; TR = 2,000 ms; TE = 30 ms; flip angle, 90°; field of view, 240 × 240mm2; data matrix, 64 × 64). During the 6‐min functional scan, subjects were instructed to keep their eyes closed, not to think about anything and stay awake during the entire session. After scanning, the subjects were asked whether they remained awake during the whole procedure. The structural images were used to exclude the possibility of clinical abnormalities by two expert radiologists.
MRI Data Preprocessing
None of the participants showed brain abnormalities on conventional MRI. Data preprocessing procedures were carried out using Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm) and Data Processing Assistant for Resting‐State fMRI (DPARSF) V2.0 Basic Edition (Yan and Zang, 2010). The first five volumes were discarded to eliminate nonequilibrium effects of magnetization and allow the participants to adapt to the EPI scanning environment. The images were corrected for the acquisition delay between slices, aligned to the first image of each session for motion correction and spatially normalized to the standard MNI template in SPM. No subjects had head motions exceeding 1 mm of movement or 1°of rotation in any direction. The linear trend of the time series was removed and band‐pass filtering (0.01 Hz < f < 0.08 Hz) was performed to reduce the influence of physiological noise (Biswal et al., 1995), such as the respiratory and cardiac rhythms. Individual ReHo maps were generated by assigning each voxel a value corresponding to the KCC of its time series with its nearest 26 neighboring voxels (Zang et al., 2004). Then a mask (made from the MNI template to assure matching with the normalization step) was used to remove nonbrain tissues and noise on the ReHo maps, and for standardization purposes the individual ReHo maps were divided by their own mean KCC within the mask (Wu et al., 2011; Yu et al., 2011aa). Finally, processed images were smoothed with an isotropic Gaussian kernel (full‐width at half‐maximum, 8 mm) (Zang et al., 2004).
Statistical Analysis
Statistical analysis was performed using SPM8. Voxel‐based comparison of whole brain ReHo maps was conducted. For the acupuncturist group and NA group, a one‐sample t‐test (P < 0.05, family‐wise error (FWE) correction throughout the whole brain) was performed to produce ReHo results across the subjects of each group. Then, a two‐sample t‐test (P < 0.05, FWE correction throughout the whole brain) was applied to compare the ReHo results between the acupuncturist group and NA group.
Regression Analysis
Regions where the acupuncture group showed significant differences over NA group in ReHo properties were determined as regions of interest (ROIs). The regression analysis was conducted among ROIs. First, we investigated whether these effects were a function of time; therefore, ReHo values of these regions were extracted, averaged, and regressed against the duration of the acupuncture practice. Second, we investigated how central representations of expertise corresponded to behavioral expertise. Accordingly, the ReHo values of ROIs were extracted, averaged, and regressed against behavioral scores. In total, 15 regression analyses were conducted.
RESULTS
Behavioral Measurements
Emotion regulation test
The analyses of the dispositional measures revealed no differences between the two groups in terms of ECS, SPQ, and each subdomain of IRI scores (mean effects of the group). The detailed information was summarized in Table 1. The control group reported significantly higher unpleasantness intensity (P < 0.001) than the professional group. The results demonstrated that the acupuncturists had better emotional control ability than that of NA group.
Table 1.
Dispositional measurement of empathy and ratings of unpleasantness in the expert and the control groups
| Task | Experts (n = 16) | Controls (n = 16) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| ECS | 26.5 | 3.2 | 26.3 | 3.9 |
| SPQ | 5.5 | 0.5 | 5.4 | 0.8 |
| IRI(PT) | 18.5 | 3.2 | 17.9 | 3.5 |
| IRI(EC) | 21.2 | 3 | 20.0 | 3.2 |
| IRI(PD) | 13.1 | 4.3 | 13.2 | 4.8 |
| IRI(FS) | 17.5 | 4 | 17.8 | 4.1 |
| Unpleasantnessa | 2.7 | 0.7 | 6.3 | 1.0 |
The item that shows significant difference between groups (P < 0.001).
ECS, emotional contagion scale; SPQ, situational pain questionnaire; IRI, interpersonal reaction index; PT, perspective taking; EC, empathic concern; PD, personal distress; FS, fantasy; SD, standard deviation.
Tactile‐motor tests
Acupuncturists had significantly lower SDT than that of NA group for both right thumb (P < 0.05) and right index finger (P < 0.05). The detailed information was given in Table 2. The results demonstrated that the professionals outperformed the NA group in spatial acuity.
Table 2.
Psychophysical measure of tactile‐motor skills in the expert and the control groups
| Task | Experts (n = 16) | Controls (n = 16) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| NoRa | 78.7 | 6.9 | 47.9 | 7.7 |
| SDTb (index) | 0.94 | 0.23 | 1.11 | 0.18 |
| SDTb (thumb) | 1.16 | 0.18 | 1.25 | 0.11 |
The item that shows significant difference between groups (P < 0.001).
The item that shows significant difference between groups (P < 0.05).
NoR, number of rotations; SDT, spatial discrimination threshold; SD, standard deviation.
The results displayed that acupuncturists achieved significantly more rounds of needling when compared with NA (P < 0.001), as shown in Table 2. Our analysis showed that the control group was less capable of maintaining rotation angle steady in the prerequired range when attempting to deliver maximum number of rotations.
ReHo results
The ReHo results for the acupuncturist group and NA group as shown in Figure 2 (P < 0.05, FWE corrected). Main regions of the default mode network exhibited significantly higher ReHo values than other brain regions during resting state; in other words, these regions' KCC is larger than that of the rest of the brain, that is, the posterior cingulate, medial prefrontal and bilateral inferior parietal areas. The findings are in line with an early resting positron emission tomography (PET) study that revealed higher metabolism in these cerebral regions relative to the whole brain (Raichle et al., 2001).
Figure 2.
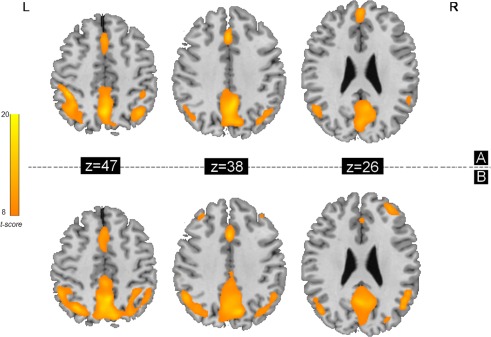
ReHo results shown as a Kendall's coefficient of concordance (KCC) map across the acupuncturist group (A) and NA group (B) under resting state (P < 0.05, FWE corrected). Main regions of the default mode network exhibited significantly higher ReHo values than other brain regions during resting state. L, left; R, right. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com]
As shown in Figures 3 and 4 and Table 3, the results of two‐sample t‐test (P < 0.05, FWE corrected) revealed that acupuncturists demonstrated significant increase in ReHo of the left ventral medal prefrontal cortex/orbitofrontal cortex (VMPFC/OFC) (Fig. 3) and the hand representation of primary motor area (MI), hand representation of primary somatosensory area (SI) contralateral to the prohand (Fig. 4). On the other hand, no brain regions with significantly decreased ReHo were found in acupuncturists. Our results indicate that ReHo can detect the changed local homogeneity of the BOLD signals.
Figure 3.
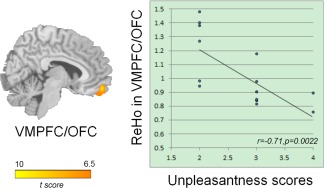
Results of ReHo group differences in cognitive control domain (P < 0.05, FWE corrected across the whole brain) and regression analysis between ReHo and unpleasantness (see Results section for details). The acupuncturist group showed increased ReHo in the left VMPFC/OFC. The images are displayed in sagittal view. Correlation analysis shows that the average ReHo values of the left VMPFC/OFC negatively correlated with ratings of unpleasantness, indicating ReHo can be used to predict behavioral outcomes. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com]
Figure 4.
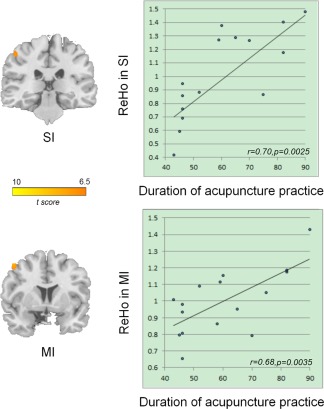
Results of ReHo group differences in tactile‐motor domain (P < 0.05, FWE corrected across the whole brain) and regression analysis between ReHo and duration of acupuncture practice (see Results section for details). The acupuncturist group showed increased ReHo in the hand representation of MI and hand representation of SI contralateral to the prohand. The images are displayed in the coronal view. Correlation analysis shows that the average ReHo values of the left MI, as well as left SI positively correlated with duration of the acupuncture practice. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com]
Table 3.
Significant ReHo differences (the acupuncturist group‐NA group) (P FWE < 0.05)
| Regions | Hemisphere | MNI coordinates (cluster maxima) | Voxels | t (cluster maxima) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| VMPFC/ OFC | L | −8 | 56 | −16 | 39 | 9.4 |
| MI | L | −29 | 5 | 59 | 19 | 8.3 |
| SI | L | −38 | −29 | 65 | 15 | 7.8 |
Regression Analysis
ReHo of the left VMPFC/OFC negatively correlated with the unpleasantness ratings in the acupuncturist group (r 1 = −0.71, p 1 = 0.0022; which when Bonferroni correcting for 15 tests was 0.0330, Fig. 3), after regressing out potential confounding variables of age, gender, and level of education in our analysis. No such correlations were found between sensorimotor outcomes and ReHo of sensorimotor regions.
ReHo of the left SI positively correlated with the duration of acupuncture practice (r 2 = 0.70, p 2 = 0.0025; which when Bonferroni correcting for 15 tests was 0.0375, Fig. 4), after regressing out potential confounding variables of age, gender, and level of education. The correlation between ReHo of MI and duration was r 3 = 0.68 with p 3 = 0.0035, which when Bonferroni corrected for 15 tests was 0.0525 (Fig. 4), after regressing out potential confounding variables of age, gender, and level of education. This indicated marginal correlation. No such significant correlation was found between ReHo of VMPFC/OFC and duration of acupuncture practice.
DISCUSSION
In the current study, we set out to explore how training/expertise effect modulates local pattern of resting state BOLD synchronizations using ReHo, an approach optimized for detecting local BOLD synchronization variations. For this purpose, we adopted a novel but robust expertise model, that is, acupuncturists (Fig. 1). Behavioral tests demonstrated that acupuncturists group had extraordinary and robust emotion regulation and tactile‐motor skills (Tables 1 and 2). Increased ReHo values were found in the bilateral VMPFC/OFC, the contralateral hand representation of left SI and MI (Figs.3 and 4). Interestingly, the unpleasantness intensity scores negatively correlated with ReHo value of VMPFC/OFC in acupuncturists group (Fig. 3). Additional analysis showed that ReHo values of SI and MI significantly correlated with the commencement of acupuncture practice (Fig. 4). The present findings add evidence to the perspective that experience‐dependent neuroplasticity can shape subsequent spontaneous activity within the resting brain. Our study furthers this understanding by demonstrating enhanced local spontaneous activity coherence in the resting brain, which is likely to be attributable to expertise.
ReHo Increases in the VMPFC/OFC for Skilled Acupuncturists and the Correlation between ReHo vs. Unpleasantness Ratings
It is interesting though expected that experts rated painful situations significantly less unpleasant than NA group. This is consistent with a former study (Cheng et al., 2007). The difference is not likely to be attributed to dispositional variables such as sensitivity to pain, empathy disposition, or emotion contagion because the two groups did not differ on these traits (Table 1). The acupuncturists must use certain optimized strategies to minimize the opportunities for negative emotions, which naturally arise in the process of acupuncture. With the regulatory mechanism, they are able to prevent the aversive emotional response from impairing their ability to heal or be of assistance. Therefore, we argue that this difference is probably induced by cognitive control mechanism, that is, emotional regulation, due to one's expertise and experience in medical practice (Cheng, 2010).
Our results displayed increased coherence of local BOLD signal fluctuations in the left VMPFC/OFC in skilled acupuncturists. This region is reported to be responsible for emotion regulation in a study involving acupuncturists (Cheng et al., 2007). A number of functional, behavioral, and lesion studies have indicated that the orbito‐frontal cortex is closely linked with emotion (Davidson et al., 2000). In particular, former analyses demonstrate that VMPFC/OFC is actively associated with suppressing or reappraising negative emotional stimuli and also involved in suppressing the influence of negative emotional stimuli on subsequent behavior (Quirk and Beer, 2006). Another support from the current study regarding the role of this region is negative correlation between unpleasantness scores, that is, the index of individual emotional regulation, and ReHo of the left VMPFC/OFC in acupuncturists. Altogether, we suggest that this region is likely to contribute to emotion regulation in acupuncturists. Critically, acquisition of cognitive skills produces long‐term plastic changes, showing differentiated BOLD patterns either under task (Kozasa et al., 2011) or at resting (Kilpatrick et al., 2011; Taylor et al., 2012). Recent evidence suggested that VMPFC/OFC is an important site showing plasticity in emotional regulation (Luders et al., 2009) and these representational changes may facilitate skill maintenance (DeFelipe, 2006). Studies using model of expertise reported less activated and narrowed areas of activation under task in experts compared with novices (Brefczynski‐Lewis et al., 2007). The shrink in representations probably derive from increased regional clustering and may reflect high efficiency of information transfer for low wiring costs due to neuroplasticity modulated by expertise in a specific task (Achard and Bullmore, 2007; Brefczynski‐Lewis, 2007). Considering the notion that the resting brain actively processes previous experiences (Miall and Robertson, 2006) and the fact that stimulus‐induced activity can modulate resting state activity (Northoff et al., 2010), we propose that increased coherence in regional BOLD fluctuations in the VMPFC/OFC found in our analysis may account for acupuncturists' emotional regulation ability as a reflection of plastic changes and can be explained by increased local clustering as a result of increment in regional information processing efficiency and the functional specialization within the region. Also, our findings also support the notion that local resting state activity is potentially useful in predicting behavioral outcomes (Tian et al., 2011).
ReHo Increases in the SI and the MI for Skilled Acupuncturists and the Correlation between ReHo and Duration of Acupuncture Practice
The skilled acupuncturists of the current cross‐sectional study showed fine sensorimotor skill over NA group as shown in Table 2. Previous studies in humans and nonhuman primates establish that the chronic functional neuroplastic changes occur following extensive motor learning or following continued performance of complicated motor skills (Amunts et al., 1997; Sakata, 2005). Recent evidence further demonstrate that these plastic changes are even presentable in resting state (Xiong et al., 2009; Ma, 2011; Wolpert, 2011). On one hand, in motor learning theory, tactile information is constantly crucial to account for coding subsequent motor plan/calculating the outcome of motor execution (Wolpert et al., 2011) and for retention/execution of the acquired motor skill (Doyon et al., 2003). In particular, in our expertise model, the interaction with information form this sensory modality is even more necessary because it is indispensable in determining whether the optimal therapeutic effect is achieved or not in each round of manipulation. On the other hand, acupuncturists rely heavily on their fine motor skills in daily clinical practice. We would expect that these result in abundance in tactile and motor functions. Taken the fact that use is a major factor driving plasticity of cortical processing (Lissek et al., 2009) and the resting state is suggested to actively and selectively processes previous experiences (Miall and Robertson, 2006), it is reasonable to see plastic changes in regions responsible for the tactile‐motor skills in resting state. it is well established that the contralateral MI is engaged in the sequential finger movement control as suggested by former fMRI, PET, and electroencephalogram data (Frackowiak, 2004) and extensive activity shapes the functional representation in contralateral human motor hand area (Granert et al., 2011); SI plays an important role in the perception for the sense of touch (Frackowiak, 2004) and plastic changes in functional organizations are also implicated in this region in context of expertise together with motor learning (Hodzic et al., 2004; Wan and Schlaug, 2010). We argue that our results regarding hand representation of MI and SI may account for acupuncturists' tactile‐motor proficiency. Another rationale of this finding comes from Doyon and Ungerleider's theoretical model of motor learning (Doyon et al., 2003). After extended years of training and practice, the motor skill becomes automatic and at its retention state, during which skilled behavior is thought to require minimal cognitive resources, to be resistant to interference and the effects of time and to be readily executed after long delays without further practice on the task (Doyon and Benali, 2005). The motor cortical regions and parietal cortex are involved for this stage (Doyon et al., 2003).
The changes in brain activity levels in the sensory‐motor system are thought to follow Hebbian principle (Klintsova and Greenough, 1999), which describes a fundamental mechanism for synaptic plasticity in which an increase in synaptic efficacy is the outcome of one cell's repeated and persistent stimulation of another cell (Hebb, 1949). In other words, this refers to the notion that neurons that regularly “fire together, wire together.” Such increased synaptic connectivity may lead to increased local synchronization, as indicated by rs‐fMRI signal (Arthurs and Boniface, 2002; Giuliani et al., 2011), which is an indicator of neuronal activities (Lee et al., 2010). As a logical account, in our study, we observed increased local clustering of BOLD fluctuation in sensorimotor regions.
What's more, we detected positive correlations between ReHo of MI vs. duration of acupuncture practice and ReHo of SI vs. duration of acupuncture practice. The findings are consistent with the notion that use is a major factor driving plasticity of cortical processing (Lissek et al., 2009) and indicates that the these changes occur as a function of time. Whereas no relationship was detected between the duration of acupuncture practice and ReHo of left VMPFC/OFC. We proposed one possible explanation. It is likely that cognitive skill learning do not necessarily develop over the same time course with sensory‐motor skills (Draganski and May, 2008; Fields, 2011). Higher‐order cognitive function, such as emotion regulation, develops procedurally as acupuncturists interact with patients and are likely to be obtained on an individually variant basis. Therefore, the absence in correlation could probably be attributed to the difference in the individual's schedule of skill acquisition.
CONCLUSIONS
Resting state of human brain actively maintains ongoing representations that may be involved in the coding of expected sensory stimuli, prospective motor responses, and prior experience (Lewis et al., 2009). Our current study demonstrated that local coherence of rs‐fMRI signal increased in the expert group using a novel but robust model of expertise, acupuncturists. We suggested that expertise modulates local resting state BOLD fluctuations by changing regional clustering strength. Our study reveals a novel connection between neuroplasticity and subsequent local resting state activity, which may add a new dimension to our understanding on functional interpretation of resting brain activity.
Limitation and Future Directions
First, in a cross‐sectional study of this kind that involves a comparison between two rather disparate groups of individuals, it is not possible to definitively attribute the differences we observed exclusively to acupuncture training that characterizes the skilled acupuncturists. The correlations we found with duration of practice are more due to skill learning and plasticity; however, it will be necessary to conduct longitudinal studies within individuals to make stronger inferences about the impact of training per se. Second, given our relatively small sample size for fMRI experiment, the results and premise of the present study should be interpreted with caution until being replicated with larger samples. As a matter of fact, we are currently looking at a chance to conduct a further study with longitudinal design as well as increasing sample size.
ACKNOWLEDGMENTS
This paper is supported by the Project for the National Key Basic Research and Development Program (973) under Grant Nos. 2011CB707700, 2012CB518501, the National Natural Science Foundation of China under Grant Nos. 81227901, 81271644, 30930112, 81000640, 81000641, 81101036, 81101108, 31200837 and the Fundamental Research Funds for the Central Universities. Also, we would like to thank Qin Ouyang, Qizhu Wu and Junran Zhang for technical assistance in conducting this research. Thanks should also be given to Lingmin Jin for helping designing and making stimuli for behavioral tasks.
REFERENCES
- Achard S, Bullmore E (2007): Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert NB, Robertson EM, Miall RC (2009): The resting human brain and motor learning. Curr Biol 19:1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Jäncke L, Steinmetz H, Schleicher A, Dabringhaus A, Zilles K (1997): Motor cortex and hand motor skills: Structural compliance in the human brain. Hum Brain Mapp 5:206–215. [DOI] [PubMed] [Google Scholar]
- Arthurs OJ, Boniface S (2002):How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci 2 5:27–31. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST (2011): Dynamic reconfiguration of human brain networks during learning. P Natl Acad Sci USA 108:7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Brefczynski‐Lewis J, Lutz A, Schaefer H, Levinson D, Davidson R (2007): Neural correlates of attentional expertise in long‐term meditation practitioners. P Natl Acad Sci USA 104:11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X (2010): Chinese acupuncture and moxibustion; Cheng X, ed. Beijing, Foreign Languages Press, pp 234–56. [Google Scholar]
- Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, Hung D, Decety J (2007): Expertise modulates the perception of pain in others. Curr Biol 17:1708–1713. [DOI] [PubMed] [Google Scholar]
- Clark WC, andYang JC (1983): Applications of sensory decision theory to problems in laboratory and clinical painIn Pain Measurements and Assessments, Melzack R., ed. New York:Raven Press, pp.15–25. [Google Scholar]
- Craig JC (1999): Grating orientation as a measure of tactile spatial acuity. Somatosens Mot Res 16:197–206. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH (2000): Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychol Bull 126:890. [DOI] [PubMed] [Google Scholar]
- Davis MH (1994): Empathy: A Social Psychological Approach Madison,Wisconsin:Westview Press, pp 167–95. [Google Scholar]
- DeFelipe J (2006): Brain plasticity and mental processes: Cajal again. Nat Rev Neurosci 7:811–817. [DOI] [PubMed] [Google Scholar]
- Doherty RW (1997): The emotional contagion scale: A measure of individual differences. J Nonverbal Behav 21:131–154. [Google Scholar]
- Doyon J, Benali H (2005): Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15:161–167. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG (2003): Distinct contribution of the cortico‐striatal and cortico‐cerebellar systems to motor skill learning. Neuropsychologia 41:252–262. [DOI] [PubMed] [Google Scholar]
- Draganski B, May A (2008): Training‐induced structural changes in the adult human brain. Behav Brain Res 192:137–142. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kim JS, Alvarado M, Kramer AF (2007): Training‐induced functional activation changes in dual‐task processing: An FMRI study. Cereb Cortex 17:192–204. [DOI] [PubMed] [Google Scholar]
- Fields RD (2011): Imaging learning: The search for a memory trace. Neuroscientist 17:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackowiak RSJ (2004): Human Brain Function. San Diego, California, Academic Press; pp 345–67. [Google Scholar]
- Giuliani NR, Drabant EM, Bhatnagar R, Gross JJ (2011): Emotion regulation and brain plasticity: Expressive suppression use predicts anterior insula volume. Neuroimage 58:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldreich D, Kanics IM (2003): Tactile acuity is enhanced in blindness. J Neurosci 23:3439–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granert O, Peller M, Gaser C, Groppa S, Hallett M, Knutzen A, Deuschl G, Zeuner KE, Siebner HR (2011): Manual activity shapes structure and function in contralateral human motor hand area. Neuroimage 54:32–41. [DOI] [PubMed] [Google Scholar]
- Hamm H, Naumann MK, Kowalski JW, Kütt S, Kozma C, Teale C (2006): Primary focal hyperhidrosis: Disease characteristics and functional impairment. Dermatology 212:343–353. [DOI] [PubMed] [Google Scholar]
- Harley EM, Pope WB, Villablanca JP, Mumford J, Suh R, Mazziotta JC, Enzmann D, Engel SA (2009): Engagement of fusiform cortex and disengagement of lateral occipital cortex in the acquisition of radiological expertise. Cereb Cortex 19:2746–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D (1949): The Organization of Behavior. New York:Wiley. [Google Scholar]
- Hodzic A, Veit R, Karim AA, Erb M, Godde B (2004): Improvement and decline in tactile discrimination behavior after cortical plasticity induced by passive tactile coactivation. J Neurosci 24:442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg R, Wohlschläger AM, Gaser C, Liebau Y, Dauner R, Wöller A, Zimmer C, Zihl J, Mühlau M (2008): Gray matter increase induced by practice correlates with task‐specific activation: A combined functional and morphometric magnetic resonance imaging study. J Neurosci 28:4210–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Garavan H (2005): Human functional neuroimaging of brain changes associated with practice. Cereb Cortex 15:1089–1102. [DOI] [PubMed] [Google Scholar]
- Kendall MaG, J.D (1990): Rank Correlation Methods. New York, Oxford University Press, pp 135–44. [Google Scholar]
- Kilpatrick LA, Suyenobu BY, Smith SR, Bueller JA, Goodman T, Creswell JD, Tillisch K, Mayer EA, Naliboff BD (2011): Impact of mindfulness‐based stress reduction training on intrinsic brain connectivity. Neuroimage 56:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U, Skov M, Christensen MS, Nygaard N (2009): Brain correlates of aesthetic expertise: A parametric fMRI study. Brain Cogn 69:306–315. [DOI] [PubMed] [Google Scholar]
- Kleber B, Veit R, Birbaumer N, Gruzelier J, Lotze M (2010): The brain of opera singers: Experience‐dependent changes in functional activation. Cereb Cortex 20:1144–1152. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Greenough WT (1999): Synaptic plasticity in cortical systems. Curr Opin Neurobiol 9:203–208. [DOI] [PubMed] [Google Scholar]
- Koeneke S, Lutz K, Wüstenberg T, Jäncke L (2004): Long‐term training affects cerebellar processing in skilled keyboard players. NeuroReport 15:1279. [DOI] [PubMed] [Google Scholar]
- Kozasa EH, Sato JR, Lacerda SS, Barreiros MAM, Radvany J, Russell TA, Sanches LG, Mello LEAM, Amaro EJr (2011): Meditation training increases brain efficiency in an attention task. Neuroimage 56:290–298. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T (2011): Meta‐analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54:2492–2502. [DOI] [PubMed] [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, Fenno LE, Ramakrishnan C, Deisseroth K (2010): Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465:788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M (2009): Learning sculpts the spontaneous activity of the resting human brain. P Natl Acad Sci USA 106:17558–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Wilimzig C, Stude P, Pleger B, Kalisch T, Maier C, Peters SA, Nicolas V, Tegenthoff M, Dinse HR (2009): Immobilization impairs tactile perception and shrinks somatosensory cortical maps. Curr Biol 19:837–842. [DOI] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C (2009): The underlying anatomical correlates of long‐term meditation: Larger hippocampal and frontal volumes of gray matter. Neuroimage 45:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Narayana S, Robin DA, Fox PT, Xiong J (2011): Changes occur in resting state network of motor system during 4 weeks of motor skill learning. Neuroimage 58:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Robertson EM (2006): Functional imaging:Is the resting brain resting? Curr Biol 16:R998–R1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Qin P, Nakao T (2010): Rest‐stimulus interaction in the brain: A review. Trends Neurosci 33:277–284. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Sergent J, Hodges DA, Fox PT (2005): The brain basis of piano performance. Neuropsychologia 43:199–215. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS (2006): Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Curr Opin Neurobiol 16:723–727. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. P Natl Acad Sci USA 98:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ (2007): A default mode of brain function: A brief history of an evolving idea. Neuroimage 37:1083–1090. [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Yu Q, Sui D, Rothbart MK, Fan M (2007): Short‐term meditation training improves attention and self‐regulation. P Natl Acad Sci USA 104:17152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Lohmann G, Margulies DS, Villringer A, Ragert P (2011): Long‐term effects of motor training on resting‐state networks and underlying brain structure. Neuroimage 57:1492–1498. [DOI] [PubMed] [Google Scholar]
- Taylor VA, Daneault V, Grant J, Scavone G, Breton E, Roffe‐Vidal S, Courtemanche J, Lavarenne AS, Marrelec G, Benali H (2012): Impact of meditation training on the default mode network during a restful state. Soc Cogn Affect Neurosci 1:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Marrett S, Saad ZS, Ruff DA, Martin A, Bandettini PA (2009): Functional but not structural changes associated with learning: An exploration of longitudinal Voxel‐Based Morphometry (VBM). Neuroimage 48:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Ren J, Zang Y (2011): Regional homogeneity of resting state fMRI signals predicts Stop signal task performanceNeuroimage 60:539–544. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Ingeholm JE, Beauchamp MS, Bikle PC, Ungerleider LG (2005): Tactile form and location processing in the human brain. P Natl Acad Sci USA 102:12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boven RW, Johnson KO (1994): A psychophysical study of the mechanisms of sensory recovery following nerve injury in humans. Brain 117:149. [DOI] [PubMed] [Google Scholar]
- Wan CY, Schlaug G (2010): Music making as a tool for promoting brain plasticity across the life span. Neuroscientist 16:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Diedrichsen J, Flanagan JR (2011): Principles of sensorimotor learning. Nat Rev Neurosci 12:739–751 [DOI] [PubMed] [Google Scholar]
- Wu QZ, Li DM, Kuang WH, Zhang TJ, Lui S, Huang XQ, Chan RCK, Kemp GJ, Gong QY (2011): Abnormal regional spontaneous neural activity in treatment‐refractory depression revealed by resting‐state fMRI. Hum Brain Mapp 32:1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Ma L, Wang B, Narayana S, Duff EP, Egan GF, Fox PT (2009): Long‐term motor training induced changes in regional cerebral blood flow in both task and resting states. Neuroimage 45:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Zang Y (2010): DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting‐state fMRI. Frontiers in systems neuroscience 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Yuan K, Zhao L, Dong M, Liu P, Wang G, Liu J, Sun J, Zhou G (2011a): Regional homogeneity abnormalities in patients with interictal migraine without aura: A resting‐state study. NMR Biomed 5:806–812. [DOI] [PubMed] [Google Scholar]
- Yu R, Zhao L, Tian J, Qin W, Wang W, Yuan K, Li Q, Lu L (2011b): Regional homogeneity changes in heavy male smokers: A resting‐state functional magnetic resonance imaging study. Addict Biol 10.1111/j.1369‐1600.2011.00359.x. [DOI] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L (2004): Regional homogeneity approach to fMRI data analysis. Neuroimage 22:394–400. [DOI] [PubMed] [Google Scholar]
- Zhang D, Raichle ME (2010): Disease and the brain's dark energy. Nat Rev Neurol 6:15–28. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mariola E, Stilla R, Stoesz M, Mao H, Hu X, Sathian K (2005): Tactile discrimination of grating orientation: fMRI activation patterns. Hum Brain Mapp 25:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]


