Abstract
Behavioral reactions to sensory stimuli during sleep are scarce despite preservation of sizeable cortical responses. To further understand such dissociation, we recorded intracortical field potentials to painful laser pulses in humans during waking and all‐night sleep. Recordings were obtained from the three cortical structures receiving 95% of the spinothalamic cortical input in primates, namely the parietal operculum, posterior insula, and mid‐anterior cingulate cortex. The dynamics of responses during sleep differed among cortical sites. In sleep Stage 2, evoked potential amplitudes were similarly attenuated relative to waking in all three cortical regions. During paradoxical, or rapid eye movements (REM), sleep, opercular and insular potentials remained stable in comparison with Stage 2, whereas the responses from mid‐anterior cingulate abated drastically, and decreasing below background noise in half of the subjects. Thus, while the lateral operculo‐insular system subserving sensory analysis of somatic stimuli remained active during paradoxical‐REM sleep, mid‐anterior cingulate processes related to orienting and avoidance behavior were suppressed. Dissociation between sensory and orienting‐motor networks might explain why nociceptive stimuli can be either neglected or incorporated into dreams without awakening the subject. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: pain, sleep, insula, suparsylvian opercular cortex, cingulated, intracerebral EEG, YAP laser
INTRODUCTION
The nature of the interaction between noxious somatic stimuli and brain activity during sleep remains mysterious [Kakigi et al., 2007]. Painful events can be incorporated into ongoing dreams [Maury, 1861; Raymond et al., 2002], and recent work using scalp‐recorded evoked potentials (EPs) has confirmed the persistence of sizeable cortical responses to nociceptive stimuli during all sleep stages, including paradoxical, or rapid eye movements (REM) sleep. It has also been established that noxious pulses at nociceptive threshold evoke arousal reactions in about 30% of cases [Bastuji et al., 2008; Lavigne et al., 2000], i.e., significantly more often than repetitive auditory stimuli at 70–80 decibels [Bastuji et al., 1995], or behaviorally significant stimuli such as the subject's own first name [Perrin et al., 1999].
The ascending nociceptive pathways (the spino‐thalamo‐cortical systems) are organized in such a way that a substantial portion of the spinal axons conveying nociceptive information projects to the brainstem reticular formation [reviews in Dostrowsky, 2006; Steriade, 1996; Willis and Westlund, 1997]. The excitatory output of the brainstem reticular formation on thalamic and cortical targets accounts for the strong arousing effect of pain stimuli. Given the hardwired arousing effect of noxious input, and even if the proportion of arousals is globally higher for pain than for other sensory systems, it remains surprising that (i) painful stimuli can be incorporated onto dreams, or even trigger them, without necessarily awakening the subject [Maury, 1861; Nielsen et al., 1993; Raymond et al., 2002] and (ii) up to 70% of stimuli at nociceptive threshold can evoke cortical EPs [Bastuji et al., 2008] and autonomic reactions [Chouchou et al., in press] without giving rise to electroencephalographic (EEG) or behavioral arousals. Such phenomena should imply some level of dissociation between cortical systems devoted to the sensory analysis of stimuli, allowing incorporation into ongoing cognitive activity such as dreams, and those systems determining the orienting reactions underlying arousal and withdrawal from the noxious input.
In primates, 95% of spinothalamic afferent input reaching the cortex activates, through direct thalamocortical connections, three separate cortical areas, namely the posterior granular insula (Brodman area, BA13), the suprasylvian parietal operculum (somatosensory area S2), and the mid‐anterior cingulate cortex (BA 24) [Dum et al., 2009]. In humans, these cortical areas form the core of the so‐called “Pain Matrix” (PM), or network of cortical structures that respond consistently to noxious mechanical or thermal stimuli [Apkarian et al., 2005; Frot and Mauguière, 1999; Garcia‐Larrea et al., 2003; Lenz et al., 1998; Peyron et al., 1999; Treede et al., 1999]. The lateral structures of the PM (posterior insula and suprasylvian operculum) are thought to subserve intensity coding and localization of pain inputs, while the medial PM system (anterior and mid‐cingulate cortex) is linked to the attentional (orienting and arousing) components of pain [Apkarian et al., 2005; Dum et al., 2009; Frot et al., 2008; Peyron et al., 2000; Vogt, 2005]. In this study, we used cortical point‐mapping in epileptic patients implanted with intracranial electrodes in these three crucial areas of the PM, so as to record the responses to nociceptive laser stimuli during both wakefulness and all‐night physiological sleep. Particular emphasis was put on recordings during paradoxical, or REM, sleep, to understand possible specific dissociations between cortical systems linked to withdrawal and arousal, and those sub‐serving the sensory analysis of painful stimuli that might underlie their incorporation into ongoing cognitive activity.
METHODS
Intracerebral Recording Procedure
The 11 patients included in this study suffered from partial refractory epilepsy. To delineate the extent of the cortical epileptogenic area and to plan a tailored surgical treatment, depth EEG recording electrodes were implanted according to the stereotactic technique of Talairach and Bancaud [ 1973]. Among other sites, all of these patients had electrodes chronically implanted in the posterior‐mid insula, in the supra‐sylvian operculum, and in the mid‐anterior cingulate for the recording of their seizures. The decision to explore these areas resulted from the observation during scalp video‐EEG recordings of ictal manifestations suggesting the possibility of seizures propagating to or originating from these regions [for a complete description of the rationale of electrode implantation, see Isnard et al., 2000, 2004]. This procedure aims at recording spontaneous seizures but also includes the functional mapping of potentially eloquent cortical areas using EPs recordings and cortical electrical stimulation [for a description of the stimulation procedure, see Mazzola et al., 2006; Ostrowsky et al., 2002]. In agreement with French regulations relative to invasive investigations with a direct individual benefit, patients were fully informed about electrode implantation, stereotactic EEG (SEEG), evoked potential recordings, and cortical stimulation procedures used to localize the epileptogenic cortical areas and gave their consent. The laser stimulation paradigm was submitted to, and approved by, the local Ethics Committee (CCPPRB Léon Bérard‐Lyon). The present experiments were supported by the French National Agency for Medical Research (INSERM). Patients were fully informed about the fact that night laser evoked potentials (LEP) recordings were not a part of the diagnostic procedure but were performed with research purposes, and gave their written informed consent.
Data from one patient were excluded because the exact position of two electrodes could not be ascertained due to movement induced during an epileptic seizure. In the remaining 10 patients (5 women, mean age 27 years, range: 18–51 years), LEP recordings were performed during one full night, at the end of the SEEG monitoring period, after 5–10 days of continuous SEEG monitoring, and in the patients' own recording room. At that time, any “first‐night” effect had faded away, and antiepileptic drugs had been tapered down so that all patients were under mono or bitherapy (carbamazepine, valproate, lamotrigine, levetiracetam, and pregabalin) with daily dosages at, or slightly under, the minimum of their usual therapeutic range.
Electrode Implantation and Anatomical Localization of Recording Sites
The electrode implantation procedure was carried out using multiple contact electrodes introduced into the brain perpendicular to the midsagittal plane, according to the stereotactic technique of Talairach and Bancaud [ 1973]. Each electrode had 10–15 contacts, each of 2 mm length, separated by 1.5 mm, and could be left in place chronically up to 15 days. Coordinates of relevant targets were determined on the patient's brain magnetic resonance (MR) images according to previously described procedures [Frot and Mauguière, 1999, 2003; Ostrowsky et al., 2002]. Anatomical localization of the cortical electrode contacts was counterchecked using fusion of skull X‐ray after electrode implantation with the appropriate coronal MR slice of the patient's brain (MRIcro software) [Rorden and Brett, 2000]. Each contact and particularly those exhibiting the largest LEP amplitudes (Table I) were then localized in the Talairach space using their stereotactic coordinates: x for the lateral medial axis, with x = 0 being the coordinate of the sagittal interhemispheric plane; y for the rostrocaudal (anterior–posterior) axis, y = 0 being the coordinate of the vertical anterior commissure (VAC) plane, and z for the inferior–superior axis, z = 0 being the coordinate of the horizontal anterior commissure–posterior commissure (AC–PC) plane [Frot and Mauguière, 1999].
Table I.
Coordinates (atlas of Talairach and Tournoux) of contacts (in millimetres) where the maximal amplitudes of the C1–C2 components in referential mode were recorded
| Patients | Insular cortex | Opercular cortex | Cingular cortex | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | X | Y | Z | |
| P1 | 38 | 1 | 12 | 45 | 1 | 12 | 4 | 31 | 14 |
| P3 | 36 | −27 | 9 | 6 | 34 | 18 | |||
| P4 | 30 | −28 | 10 | ||||||
| P5 | 33 | −21 | 3 | 43 | 2 | 11 | −4 | 35 | 15 |
| P6 | 38 | −1 | −5 | 46 | −1 | 9 | −1 | 22 | 24 |
| P7 | 32 | −22 | 8 | 54 | −23 | 21 | −2 | 21 | 20 |
| P8 | 39 | −8 | 6 | 42 | −3 | 18 | 4 | 32 | 18 |
| P9 | 37 | −2 | −3 | 44 | −6 | 15 | |||
| P10 | 34 | −17 | 2 | 54 | −1 | 12 | |||
| P11 | 34 | −23 | 5 | 45 | 0 | 7 | |||
| Mean | 35.1 | −14.8 | 4.7 | 46.6 | −3.9 | 13.1 | 3.5 | 29.2 | 18.2 |
| SD | 3 | 11.2 | 5.5 | 4.7 | 8.1 | 4.6 | 1.8 | 6.1 | 3.6 |
Exploration of Suprasylvian Opercular, Insular, and Cingulate Cortices
The different sites of implantation are illustrated in Figure 1. The insula was explored in the 10 patients with the deepest contacts of 1–3 electrodes implanted in the suprasylvian opercular cortex or the first temporal gyrus. Thirty‐three electrode contacts explored the posterior insular cortex, distributed along the rostrocaudal axis, +1 mm rostral and −28 mm caudal to the VAC plane (y coordinates).
Figure 1.
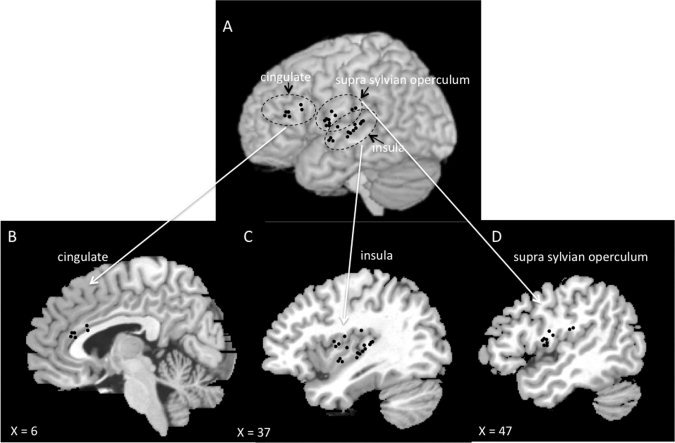
Anatomical sites of implantation of the 29 electrodes (black circles) used for recording. The sites of electrode entry in the brain convexity are shown in a lateral view of the normalized anatomical model of normal brain proposed by the McConnell Brain Imaging Center of the Montréal Neurological Institute (upper part of the figure). The intracerebral location of the six electrodes exploring the mid‐anterior cingulate is shown in frame B (sagittal view at Talairach's x = 6 mm), that of the 18 electrodes exploring the insular cortex in C (sagittal view at x = 37 mm) and that of the 10 electrodes exploring the supra sylvian opercular cortex in D (sagittal view at x = 47 mm).
The suprasylvian opercular cortex was explored in eight patients (26 electrode contacts). Six patients were implanted with a single opercular electrode, exploring either the pre‐ (five cases) or post‐central (one case) operculum. In the two other patients, both the frontal and the parietal opercular cortices were each implanted with 1 electrode, and yielded similar results. Twenty‐six contacts explored the suprasylvian opercular area, distributed along the rostrocaudal axis, +2 mm rostral and −23 mm caudal to the VAC plane (y coordinates).
The mid‐anterior cingulate was explored in six patients with 1 electrode. Data were collected using 12 electrode contacts, distributed along the rostrocaudal axis, between +38 mm and +21 mm rostral to the VAC plane (y coordinates).
Laser Stimulation Parameters
Radiant nociceptive heat pulses of 5 ms duration were delivered with a Nd:YAP‐laser (Yttrium Aluminium Perovskite; wavelength 1.34 μm; El.En.®). The laser beam was transmitted from the generator to the stimulating probe via an optical fiber of 10 m length (550 μm diameter with sub miniature version (SAV) A‐905 connector). Series of laser stimuli were delivered on the dorsum of the hand, contralateral to the hemispheric side of electrodes implantation. The intensity was kept stable for any given subject during the whole night, slightly above individual pain threshold (level 4 in a Likert‐type scale where 0 was “no sensation,” 8 = unbearable pain, and 4 was defined as “pricking, moderately painful”). Pain threshold was determined during wakefulness before sleep, using up to 10 stimulus trials, with ascending/descending intensity, by varying the laser energy and beam diameter (4–6 mm) and using a verbal numerical scale. Nociceptive threshold was obtained in all subjects with energy densities of 50–79 mJ/mm2. These pain threshold values were within the normal range of our laboratory and those reported by others using Nd:YAP lasers [Leandri et al., 2006; Perchet et al., 2008; Truini et al., 2007]. To avoid damaging the skin, stimulus blocks consisted of a maximum of 20 laser pulses and the heat spot was slightly shifted over the skin surface between two successive stimuli to avoid habituation and especially peripheral nociceptor fatigue [Schwarz et al., 2000]. Following preliminary work showing that delivering stimuli at short (<6 s) and constant intervals increased the probability of awakening [Bastuji et al., 2008], the interstimulus interval was pseudo‐randomly adjusted on line and varied between 10 and 25 s.
Recording Procedures
After estimation of pain thresholds to laser, two separate runs of 10–15 stimulations were applied to the skin in the superficial radial nerve territory on the dorsum of the hand during pre‐sleep wakefulness, so as to obtain control responses. Then the patients were allowed to sleep at their own time. Before delivering any further laser stimulation, a minimum of 20 min of sleep was allowed from the first EEG signs of sleep onset. The identification of the different sleep stages (Stages 2–4, and REM sleep) was done on line by one of the investigators (HB) expert in sleep studies; this allowed to stimulate in each sleep stage, and to immediately discontinue the sequence if one stimulus awoke the sleeper. A second investigator entered the room and delivered nociceptive pulses transmitted through the optic fiber from the laser stimulator. The 10‐m optical fiber transited under the door separating the recording and sleeping areas, and allowed to stimulate conveniently any body part despite movements of the subjects during the night. Both the sleeping subject and the investigator wore eye protections. If any stimulus arouses the sleeper, the sequence was immediately discontinued. Runs of stimulations were delivered on the dorsum of the hand during the different sleep stages up to 6:00 AM.
Online EEG recordings (Micromed BrainQuick®, St. Etienne des Oullières, France) were obtained using a sampling frequency of 256 Hz and a bandpass filter of 0.03–100 Hz, both in bipolar and referential modes. The reference electrode was chosen for each patient on an implanted contact located in the skull. Blinks and saccades were recorded with 2 electro‐oculograms (EOG) electrodes placed on the supero‐ and infero‐lateral right canthus. EEG, EOG, and electrocardiogram (EKG) were recorded continuously during the night and were stored for offline analysis.
Data Analysis
Sleep stages were visually identified using more than 20 intracortical contacts with both bipolar and referential traces plus EOG. Sleep scoring of intracranial data was done according to the criteria of Rechtschaffen and Kales [ 1968] [see Magnin et al., 2004; Rey et al., 2007], so as to derive hypnograms based on 30‐s epochs and determine the vigilance state during which laser stimuli were delivered. More restricted criteria were used during the intervals immediately preceding and following each stimulus, in order to reject (a) cortical responses occurring during an arousal period, and (b) responses obtained following less than 1 min of continuous sleep.
Epoching of the EEG, selective averaging, and record analysis were performed offline using ASA® software (Advanced Neuro Technology (ANT), Eschende, The Netherlands). The continuous EEG was segmented into “epochs” beginning 100 ms before and ending 900 ms after the stimulus. A 100 ms prestimulus baseline correction was performed before averaging. Epochs presenting epileptic transient activities or artifacts were rejected from analysis, as were epochs recorded during transition phases between two sleep stages, or during an arousal period. Single‐stimulus LEPs were grouped separately as a function of the sleep stage during which they were recorded, and then averaged according to the different states of vigilance and contact positions. The LEP components, recorded in the three different structures, were identified as the most positive (or negative) peaks within a 160–420 ms latency window encompassing the corresponding waveform, determined from literature [Frot et al., 2008; Frot and Mauguière, 2003] and grand‐averages. The components were analyzed only when their amplitude during wakefulness exceeded by at least 3 SD the mean prestimulus baseline. Thus, a first negative component in the opercular and cingulate cortices [Frot et al., 2001, 2008] was not analyzed in this study because of its very small amplitude. The first component analyzed, peaking at approximately 200 ms, was positive in the opercular and cingulate cortices and negative or positive in the insular cortex: thus this component was not labeled according to its polarity but as Component 1 (C1) in order to facilitate the comparison between the three structures. In the same way, the second component, peaking at approximately 300 ms, was negative in the opercular and cingulate cortices and positive or negative in the insular cortex: this component was labeled Component 2 or C2.
In all figures, negative potentials at the intracortical recording site are represented upward. In the text, mean voltages and latencies are given ± 1 SE.
Statistical analyses of electrophysiological data were performed on averaged traces from each individual. Latencies of the two predominant components, C1 and C2, were considered. For amplitudes, statistical analysis performed either on peak‐to‐peak between C1 and C2 or from baseline to C1 and C2 amplitude values turned out to be similar so that data on peak‐to‐peak values only were reported. Latencies and amplitudes were measured on insular, opercular, and mid‐anterior cingulate contacts with higher amplitude in referential mode, for each subject and in each sleep stage. Data were then submitted to repeated‐measure analyses of variance (ANOVA). LEPs during slow wave sleep (SWS) were obtained in only three subjects and thus could be not included in the ANOVA. As each cortical structure was explored in a different number of subjects (insula: 10 patients, operculum: 8 patients, cingulate: 6 patients), three separate repeated‐measures one‐way ANOVAs were performed. Dependent variables were the latency and the amplitude of LEP components, and the within factor (state of vigilance) had three levels: waking, Stage 2, and REM sleep). The Geisser–Greenhouse (G‐G) correction was used to adjust degrees of freedom when needed. A significance level of P < 0.05 after G‐G correction was accepted as significant. Post‐hoc comparisons using confidence intervals were performed when ANOVA yielded significant results. Significance level of confidence intervals (CI) was set at 95%.
RESULTS
The 10 patients analyzed had electrodes chronically implanted in the posterior‐mid insula, 8 in the supra‐sylvan operculum, and 6 in the mid‐anterior cingulate cortex for the recording of their seizures (Fig. 1). We analyzed a total of 927 laser nociceptive stimuli, of which 748 (81%) applied during sleep. An average of 18 (10–30) stimuli per subject were delivered during wakefulness, 34 (10–74) during Stage 2 sleep, 6 (0–34) during SWS (not analyzed), and 35 (3–78) during paradoxical sleep.
Effects of Sleep Stages on LEP Latencies
During wakefulness, LEP latencies were comparable to those reported in previous LEP studies using intracortical electrodes [e.g., Frot et al., 2007, 2008]. ANOVA on LEP latencies showed a significant effect of sleep stage on the latency of component 1 (C1) in the insula [F(1,9) = 5.62; P = 0.019], the operculum [ANOVA; F(1,7) = 5.57; P = 0.031], but not in the cingulate [F(1,5) = 2.65; P = 0.121]. In the insula and operculum, the latency of C1 was significantly delayed in Stage 2 and REM sleep as compared to wakefulness (see Table II). There was no significant effect of sleep stage on the latency of component 2 (C2) in any structure (insula [F(1,9) = 1.85; P = 0.19]; operculum [F(1,7) = 1.21; P = 0.32]; cingulate [F(1,5) = 0.42; P = 0.572].
Table II.
Quantitative analysis of LEPs in the different states of vigilance
| ANOVA | Mean difference | 95% Confidence intervals | P |
|---|---|---|---|
| LEP amplitude | |||
| Insula | |||
| (F(1,9) = 30.84; P < 0.001) | |||
| W–S2 | −77.35 μV | [−108.23 to −46.47] | 0.001 |
| W–PS | −72.40 μV | [−100.07 to −44.73] | 0.001 |
| S2–PS | 4.95 μV | [7.18 to 17.08] | 0.38 |
| Operculum | |||
| (F(1,7) = 22.25; P = 0.001) | |||
| W–S2 | −101.19 μV | [−154.17 to −48.20] | 0.003 |
| W–PS | −84.13 μV | [−111.60 to −56.65] | 0.001 |
| S2–PS | 17.06 μV | [−12.32 to 46.44] | 0.21 |
| Cingulate | |||
| (F(1,5) = 25.38; P = 0.001) | |||
| W–S2 | −35.67 μV | [−60.05 to −11.28] | 0.013 |
| W–PS | −51.83 μV | [−71.47 to −32.20] | 0.001 |
| S2–PS | −16.17 μV | [−27.06 to −5.27] | 0.012 |
| C1 latency | |||
| Insula | |||
| F(1,9) = 5.62; P = 0.019 | |||
| W–S2 | 20.10 ms | [0.34 to 39.86] | 0.047 |
| W–PS | 22.40 ms | [6.11 to 38.69] | 0.013 |
| S2–PS | 2.30 ms | [10.86 to −15.46] | 0.702 |
| Operculum | |||
| F(1,7) = 5.57; P = 0.031 | |||
| W–S2 | 23.50 ms | [1.99 to 45.02] | 0.036 |
| W–PS | 17.50 ms | [0.26 to 34.74] | 0.047 |
| S2–PS | −6.00 ms | [−17.76 to 5.76] | 0.267 |
Post hoc tests (confidence intervals) following significant ANOVA.
W: waking; S2: Stage 2; PS: paradoxical sleep.
Effects of Sleep Stages on LEP Amplitude
The peak‐to‐peak amplitudes of C1–C2 evoked by laser stimuli were significantly affected by sleep in the three cortical areas, insula [ANOVA; F(1,9) = 30.84; P < 0.001], operculum [ANOVA; F(1,7) = 22.25; P = 0.001], and cingulate [F(1,5) = 25.38; P = 0.001]. As compared to waking, LEP amplitude was significantly decreased during both Stage 2 and paradoxical‐REM sleep in each structure (see Table I and Fig. 2). Post‐hoc comparisons showed that LEP amplitudes remained unchanged between Stage 2 and REM sleep in the insular and the opercular cortices, while they were significantly decreased during REM sleep as compared to Stage 2 in the cingulate cortex. The mid‐cingulate region was the only area where C1–C2 amplitude presented a sizeable decrease between sleep Stage 2 and REM sleep, as illustrated in Figure 3 showing grand‐average LEPs and individual responses from a representative subject, respectively.
Figure 2.
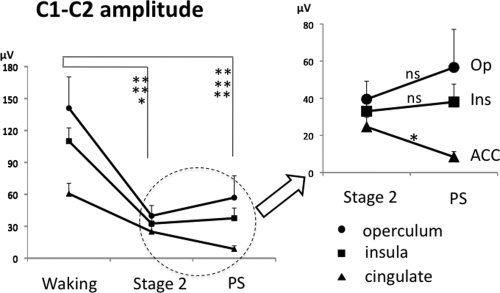
The statistical results of C1–C2 amplitudes in each condition and location are illustrated. Note the significant amplitude decrease in the cingulate cortex during paradoxical sleep as compared to stage 2.
Figure 3.
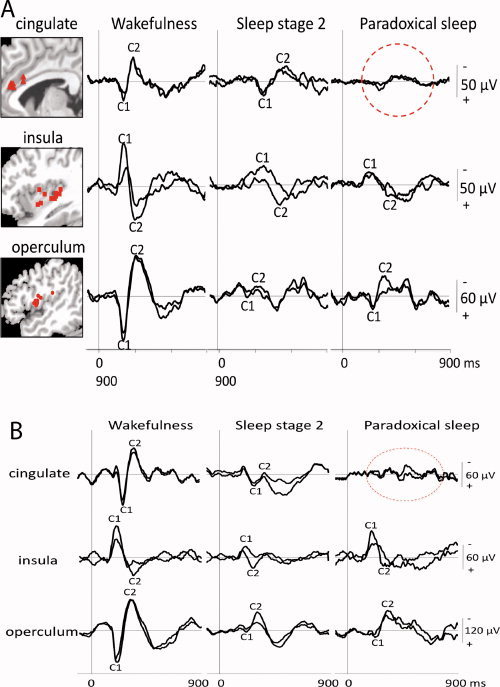
A: Grand average LEPs in referential recording mode during wakefulness, sleep stage 2, and paradoxical sleep in the operculum (bottom), the insula (middle), and the mid‐anterior cingulate (top). Traces recorded by the electrode contact yielding the largest amplitudes are superimposed on those from the adjacent contact. On the left part of the figure, for each structure, the Talairach's coordinates of the contacts where the maximal amplitudes of the C1–C2 components were recorded are indicated on mean sagittal MRIs (cingulate x = −1 to −6; insula x = −30 to −36; operculum x = −42 to −54). The precise location of all these contacts was verified by plotting them on the appropriate MRI slices of each patient. B: Average LEPs from mid‐anterior cingulate, insular and opercular cortices (referential recordings) in one representative patient during wakefulness, stage 2, and paradoxical sleep. Traces recorded by the electrode contact yielding the largest amplitudes are superimposed on those from the adjacent contact. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 4 illustrates the uneven distribution of LEP amplitude across structures during paradoxical (REM) sleep, with significant depression in mid‐cingulate and the opposite trend (with large scatter of values) in operculum and insula.
Figure 4.
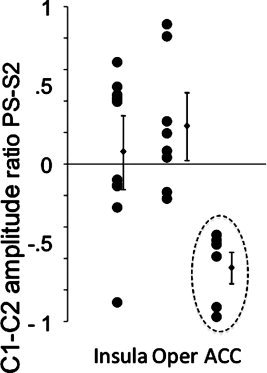
Ratio of peak‐to‐peak C1–C2 amplitudes in paradoxical sleep versus stage 2 [(PS‐S2)/S2] for the three cortical structures. The [(PS‐S2)/S2] ratio was more widely distributed and frequently positive for the insular and opercular cortices and clearly negative with a homogeneous distribution for the mid‐anterior cingulate cortex.
DISCUSSION
The changes of nociceptive responses during sleep differed between the operculo‐insular and the cingulate cortices. While opercular and insular responses to laser stimuli were attenuated to a similar level during all the sleep stages investigated, mid‐anterior cingulate potentials, which were only moderately attenuated during sleep Stage 2, abated drastically in paradoxical sleep, to the extent of decreasing below background noise in three subjects.
Functional Implications
The neuroanatomical basis subserving pain perception is known to involve two main subsystems, commonly labelled “lateral” and “medial” [Albe‐Fessard et al., 1985; Treede et al., 1999]. The lateral subsystem includes afferents from the lateral thalamus to SI and operculo‐insular cortices and is thought to encode intensity and localization of pain inputs [Apkarian et al., 2005; Garcia‐Larrea et al., 2010; Peyron et al., 2000]. The medial subsystem comprises projections to the mid‐anterior cingulate cortex from the medial thalamus [Baleydier and Mauguière, 1980; Dum et al., 2009; Treede et al., 1999], and is supposed to activate the motor, cognitive, and emotional pain components. Recent evidence in humans points to a simultaneous early activation of the operculo‐insular and mid‐cingulate cortices in response to noxious stimuli, suggesting that the processing of nociceptive information in the “medial” and “lateral” subsystems is, at least in its early phases, rather parallel than sequential [Frot et al., 2008]. The finding of short‐latency cingulate responses to pain suggests that the medial system is not exclusively devoted to slow affective processes [e.g., Price, 2000], but is also involved in fast reactions such as automatic orienting toward, and motor withdrawal from, pain stimuli. Indeed, although the thalamo‐cingulate projections have been often considered to subserve affective components of the pain experience [e.g. Kulkarni et al., 2005; Price, 2000; Rainville et al., 1997], recent evidence in humans and monkeys shows that the spinothalamic input to anterior cingulate cortex (ACC) concern mainly, if not exclusively, cingulate regions primary involved in motor control, orienting and attention for action [Dum et al., 2009; Frot et al., 2008; Vogt, 2005]. In this context, the selective depression of mid‐anterior cingulate responses observed during REM sleep is consistent with their role in motor withdrawal, as motor reactions to external sensory input are scarce during this vigilance stage [Morrison et al., 1995; Rechtschaffen et al., 1966; Sanford et al., 1992]. The fact that enhancement of mid‐cingulate responses can predict re‐emergence of motor reactions to pain in REM sleep further supports this interpretation [Mazza et al., 2010].
Mid‐anterior cingulate activity is likely to contribute significantly to frontal midline LEPs recorded on the scalp, as suggested by a number of convergent source localization studies [reviews Garcia‐Larrea et al., 2003; Kakigi et al., 2005]. The selective attenuation of frontal scalp LEPs in our previous study [Bastuji et al., 2008] and the suppression of intracranial cingulate LEP in the present one are likely to reflect the same phenomenon, namely a lack of mid‐frontal response to external inputs during REM sleep. This phenomenon has been reported in scalp responses to a variety of stimuli including auditory tones [Bastuji et al., 1995; Niiyama et al., 1994] and proper names [Perrin et al., 1999]. Since long date, the midline potentials are known to represent multimodal responses elicited by a variety of sensory stimuli [Bancaud et al., 1953; Davis et al., 1939; Iannetti et al., 2008]; their attenuation could therefore relate to a general state of frontal hyporeactivity to stimuli during REM sleep, of which the absence of responses to pain is only one particular example.
Possible Neurophysiological Substrate of the Observed Findings
While the homogeneous decrease of nociceptive cortical responses in sleep Stage 2 could be easily explained by the global disfacilitation of the cerebral activity that characterizes this stage, the specific and drastic reduction of the cingulate responses during paradoxical‐REM sleep remains puzzling, and may be due to multiple underlying mechanisms. A tonic suppression of ascending spinothalamic inputs has been suggested at the spinal cord level through presynaptic inhibition [Jones, 1993; Soja, 2007]. In our case, however, this would imply that such blockade predominates on nociceptive inputs to the medial cingulate cortex, relative to those projecting to lateral (sensory) targets, and such selectivity has not been demonstrated so far. In humans, the EEG activity is much more depressed at thalamic than at cortical levels during paradoxical‐REM sleep [Magnin et al., 2004; Rey et al., 2007]. Once more, while this phenomenon could account for a global decrease in cortical nociceptive responses during this stage, it cannot explain a specific reduction of the cingulate nociceptive response. What remains is the fact that cingulate and operculo‐insular cortices receive their spinothalamic input from different thalamic nuclei. The operculo‐insular cortices receive afferents essentially from lateral thalamic nuclei: ventral posterior group (VPL/VPM/VPI), posterior nuclei (Po) and posterior part of the ventral medial nucleus (VMpo) [Augustine, 1996; Craig et al., 1994; Freidman and Murray, 1986; Kobayashi et al., 2009; Montes et al., 2005; Stevens et al., 1993], whereas the cingulate cortex (Brodmann' area 24) receives direct projections from midline and intralaminar thalamic nuclei that are themselves the target of spinothalamic afferents [Baleydier and Mauguière, 1980; Hatanaka et al., 2003; Vogt, 2005]. The medial thalamic targets of the spinothalamic system are also supposed to participate in attention and arousal regulation during wakefulness [Frith and Friston, 1996; Portas et al., 1998] and phasic activation periods during paradoxical sleep [Mancia and Marini, 1995; Steriade et al., 1993; Wehrle et al., 2007]. Response attenuation in these thalamic regions could therefore contribute to the decrease of cortical cingulate responsiveness in REM sleep, but this hypothesis remains speculative pending direct thalamic recordings.
The Paradox of REM Sleep
PET‐scan studies have reported increased metabolism in the mid‐ and anterior cingulate during paradoxical sleep as compared to resting wakefulness [Braun et al., 1997; Maquet et al., 1996; Nofzinger et al., 1997], and this has been related to emotional features in dreams [Hobson and Pace‐Schott, 2002; Maquet et al. 2000]. Such results and the present electrophysiological data reflect different phenomena: taken together they suggest the existence of an increase in the cingulate energetic metabolism during REM sleep, most likely linked to the processing of ongoing mental (dream) activity, which is concurrent with a phasic inhibition of the orienting and withdrawal responses to external stimuli, even at nociceptive threshold. Such dissociation between ongoing activity and event‐related reactions corresponds indeed to the “paradoxical” nature of this sleep stage, where a rapid and activated EEG typically coexists with depressed responsiveness [Jouvet, 1965; Paré and Llinás, 1995]. Together with the relative preservation of sensory processing in opercular‐insular networks, this might explain why nociceptive (and other) stimuli can be incorporated into dreams during REM sleep without awakening the subject.
Limitations of this Study
The population explored was composed by epileptic patients, whose cortical nociceptive processing might differ relative to that of non‐epileptic subjects. Notwithstanding such inevitable shortcoming, the phenomena we describe here were observed irrespective of the localization and extent of the epileptogenic focus (minute or extensive, temporo‐mesial, or neocortical). Furthermore, no difference was observed between patients and normal controls as regards sleep organization and pain thresholds, the latter being similar in the sides ipsilateral and contralateral to the epileptic focus, and comparable to those of control subjects [Perchet et al., 2008]. Antiepileptic drugs can modify the cortical activity, including during sleep, but their effects are widespread and have not been reported to act during one specific vigilance state, i.e., REM sleep [Duncan, 1997]. Some antiepileptic drugs, including pregabalin and oxcarbazepine, may attenuate acute nociceptive neuronal and behavioral responses in animal pain models [Tomic et al., 2010; You et al., 2009], and have analgesic effects on chronic neuropathic pain, but there is no clinical evidence of their effectiveness in clinical acute pain [e.g., Durkin et al., 2010; Wiffren et al., in press]. Moreover, carbamazepine has been reported to have no effect on laser evoked potential amplitude [Galeotti et al., 2005]. Furthermore, the nociceptive thresholds obtained in our patients were strictly comparable to those previously reported in age‐matched healthy subjects studied in our laboratory [Bastuji et al., 2008; Perchet et al., 2008].
Our study was based on acute near‐threshold thermal pain; the extent to which it may apply to tonic or chronic pain conditions remains therefore unclear and should be tested in further studies. Tonic pain is more powerful than phasic nociception to perturb sleep [Lavigne et al., 2004; Moldofsky, 2001], and it is likely that the cingulate attenuation observed in this study may be more easily overcome by painful stimuli of greater intensity and duration. It should be noted, however, that painful thermal stimuli up to 500 times longer than the laser pulses used herein yielded nocifensive reactions in only 2.5% of 351 stimuli applied during sleep [Lavigne et al., 2000], suggesting that a powerful inhibition of motor behavioral responses, perhaps mediated by the ACC, is indeed active in tonic conditions too.
Although stimulus energy remains constant along the experiment, there may have been minor variations of the energy actually delivered due to slight position changes of the laser pointer (inducing changes in the angle between the laser beam and the cutaneous surface) and/or small thermal skin changes during the night. However, neither changes in beam orientation, nor temperature skin changes is bound to one particular sleep stage [see Bastuji et al., 1988] and, thus, they cannot explain a dissociation of evoked responses between cortical regions, or between paradoxical sleep and sleep Stage 2.
Conclusions
The similar latency, but differential responsiveness, of operculo‐insular and mid‐cingulate regions during sleep suggests that these areas subserve parallel processes with different functional significance. Such dissociation between sensory and orienting‐motor networks might explain why nociceptive stimuli can be incorporated into dreams during paradoxical (REM) sleep without awakening the subject.
Acknowledgements
Authors are indebted to Drs. J. Isnard and P. Ryvlin for the opportunity to study their patients and to Dr. M. Guénot for stereotactic electrode implantations.
REFERENCES
- Albe‐Fessard D, Berkley KJ, Kruger L, Ralston HJ III, Willis WD Jr ( 1985): Diencephalic mechanisms of pain sensation. Brain Res 356: 217–296. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK ( 2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. [DOI] [PubMed] [Google Scholar]
- Augustine JR ( 1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguière F ( 1980): The duality of the cingulate gyrus in monkey‐neuroanatomical study and functional hypothesis. Brain 103: 525–554. [DOI] [PubMed] [Google Scholar]
- Bancaud J, Bloch V, Paillard J ( 1953): Contribution EEG à l'étude des potentiels évoqués chez l'Homme au niveau du vertex. Rev Neurol 89: 399–418. [PubMed] [Google Scholar]
- Bastuji H, Garcia‐Larrea L, Bertrand O, Mauguière F ( 1988): BAEP latency changes during nocturnal sleep are not correlated with sleep stages but with body temperature variations. Electroencephal Clin Neurophysiol 70: 9–15. [DOI] [PubMed] [Google Scholar]
- Bastuji H, Garcia‐Larrea L, Franc C, Mauguière F ( 1995): Brain processing of stimulus deviance during slow‐wave and paradoxical sleep: A study of human auditory evoked responses using the oddball paradigm. J Clin Neurophysiol 12: 155–167. [DOI] [PubMed] [Google Scholar]
- Bastuji H, Perchet C, Legrain V, Montes C, Garcia‐Larrea L ( 2008): Laser evoked responses to painful stimulation persist during sleep and predict subsequent arousals. Pain 137: 589–599 [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesensten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P ( 1997): Regional cerebral blood flow throughout the sleep–wake cycle. An H215O PET study. Brain 120: 1173–1197. [DOI] [PubMed] [Google Scholar]
- Chouchou F, Pichot V, Perchet C, Legrain V, Garcia‐Larrea L, Roche F, Bastuji H ( 2011): Autonomic pain responses during sleep: A study of heart rate variability. Eur J Pain 15: 554–560. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC, Zhang E‐T, Blomqvist A ( 1994): A thalamic nucleus specific for pain and temperature sensation. Nature 372: 770–773. [DOI] [PubMed] [Google Scholar]
- Davis H, Davis PA, Loomis AL, Harvey N, Hobart G ( 1939): Electrical reactions of the human brain to auditory stimulation during sleep. J Neurophysiol 2: 500–514. [Google Scholar]
- Dostrovsky JO ( 2006): Brainstem and thalamic relays In: Cervero F, Jensen TS, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier B.V; pp 127–139. [DOI] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL ( 2009): The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 29: 14223–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS ( 1997): Antiepileptic drugs and the electroencephalogram. Epilepsia 28: 259–266. [DOI] [PubMed] [Google Scholar]
- Durkin B, Page C, Glass P ( 2010): Pregabalin for the treatment of postsurgical pain. Expert Opin Pharmacother 11: 2751–2758. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Murray EA ( 1986): Thalamic connectivity of the second somatosensory area and neighboring somatosensory fields of the lateral sulcus of the Macaque. J Comp Neurol 252: 348–373. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ ( 1996): The role of the thalamus in ‘top down’ modulation of attention to sound. Neuroimage 4: 210–215. [DOI] [PubMed] [Google Scholar]
- Frot M, Magnin M, Mauguiere F, Garcia‐Larrea L ( 2007): Human SII and Posterior Insula Differently Encode Thermal Laser Stimuli. Cerebral Cortex 17: 610–620. [DOI] [PubMed] [Google Scholar]
- Frot M, Mauguière F ( 1999): Timing and spatial distribution of somatosensory responses recorded in the upper bank of the sylvian fissure (SII area) in humans. Cereb Cortex 9: 854–863. [DOI] [PubMed] [Google Scholar]
- Frot M, Mauguière F ( 2003): Dual representation of pain in the operculoinsular cortex in humans. Brain 126: 1–13. [DOI] [PubMed] [Google Scholar]
- Frot M, Garcia‐Larrea L, Guénot M, Mauguière F ( 2001): Responses of the supra‐sylvian (SII) cortex in humans to painful and innocuous stimuli. A study using intra‐cerebral recordings. Pain 94: 65–73. [DOI] [PubMed] [Google Scholar]
- Frot M, Mauguière F, Magnin M, Garcia‐Larrea L ( 2008): Parallel processing of nociceptive A‐delta inputs in SII and midcingulate cortex in humans. J Neurosci 28: 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti F, Truini A Iannetti GD, Romaniello A, Biasiotta A, Mascia A, Virtuoso M, Cruccu G ( 2005): Laser evoked potentials and carbamazepine in epileptic patients. Neurophysiol Clin 35: 93–96. [DOI] [PubMed] [Google Scholar]
- Garcia‐Larrea L, Frot M, Valeriani M ( 2003): Brain generators of laser‐evoked potentials: From dipoles to functional significance. Neurophysiol Clin 33: 279–292. [DOI] [PubMed] [Google Scholar]
- Garcia‐Larrea L, Perchet C, Creac'h C, Convers P, Peyron R, Laurent B, Mauguière F, Magnin M ( 2010): Operculo‐insular pain (parasylvian pain): A distinct central pain syndrome. Brain 133: 2528–2539. [DOI] [PubMed] [Google Scholar]
- Hatanaka N, Tokunu H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Nambu A, Takada M ( 2003): Thalamocortical and intracortical connections of monkey cingulate motor areas. J Comp Neurol 462: 121–138. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Pace‐Schott EF ( 2002): The cognitive neuroscience of sleep: Neuronal systems, consciousness and learning. Nat Rev Neurosci 3: 679–693. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Hughes NP, Lee MC, Mouraux A ( 2008): Determinants of laser evoked EEG responses: Pain perception or stimulus saliency? J Neurophysiol 100: 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard J, Guénot M, Ostrowsky K, Sindou M, Mauguière F ( 2000): The role of the insular cortex in temporal lobe epilepsy. Ann Neurol 48: 614–623. [PubMed] [Google Scholar]
- Isnard J, Guénot M, Sindou M, Mauguière F ( 2004): Clinical manifestations of insular lobe seizures: A stereo‐electroencephalographic study. Epilepsia 45: 1079–1090. [DOI] [PubMed] [Google Scholar]
- Jones BE ( 1993): The organization of central cholinergic systems and their functional importance in sleep–waking states. Prog Brain Res 98: 61–71. [DOI] [PubMed] [Google Scholar]
- Jouvet M ( 1965): Paradoxical sleep. A study of its nature and mechanisms. Prog Brain Res 18: 20–62. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Inui K, Tamura Y ( 2005): Electrophysiological studies on human pain perception. Clin Neurophysiol 116: 743–763. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Wang X, Inui K, Qiu Y ( 2007): Modulation of pain‐related cortical activity by sleep and attention In: Lavigne G, Sessle BJ, Choinière M, Soja P, editors. Sleep and Pain. Seattle: IASO Press; pp 175–187. [Google Scholar]
- Kobayashi K, Winberry J, Liu CC, Treede RD, Lenz FA ( 2009): A painful cutaneous laser stimulus evokes responses from single neurons in the human thalamic principal somatic sensory nucleus ventral caudal (Vc). J Neurophysiol 101: 2210–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Youll P, Watson AQ, Derbyshire SWG, Frackowiak RSJ, Friston KJ, Jones AKP ( 2005): Attention to pain localization and unpleasantness discriminate the functions of the medial and lateral pain systems. Eur J Neurosci 21: 3133–3142. [DOI] [PubMed] [Google Scholar]
- Lavigne GJ, Zucconi M, Castronovo C, Manzini C ( 2000): Sleep arousal response to experimental thermal stimulation during sleep in human subjects free of pain and sleep problems. Pain 4: 283–290. [DOI] [PubMed] [Google Scholar]
- Lavigne G, Brousseau M, Kato T, Mayer P, Manzini C, Guitard F, Monplaisir J ( 2004): Experimental pain perception remains equally active over all sleep stages. Pain 110: 646–655. [DOI] [PubMed] [Google Scholar]
- Leandri M, Saturno M, Sadavecchia L, Ianetti G, Cruccu G, Truini A ( 2006): Measurement of skin temperature after infrared laser stimulation. Neurophysiol Clin 36: 207–218. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Rios M, Chau D, Krauss GL, Zirh TA, Lesser RP ( 1998): Painful stimuli evoke potentials recorded from the parasylvian cortex in humans. J Neurophysiol 80: 2077–2088. [DOI] [PubMed] [Google Scholar]
- Magnin M, Bastuji H, Garcia‐Larrea L, Mauguière F ( 2004): Human thalamic medial pulvinar nucleus is not activated during paradoxical sleep. Cereb Cortex 14: 858–862. [DOI] [PubMed] [Google Scholar]
- Mancia M, Marini G ( 1995): Orienting‐like reaction after ibotenic acid injections into the thalamic centre median nucleus in the cat. Arch Ital Biol 134: 65–80. [PubMed] [Google Scholar]
- Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G ( 1996): Functional neuroanatomy of human rapid‐eye‐movement sleep and dreaming. Nature 383: 163–166. [DOI] [PubMed] [Google Scholar]
- Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, Luxen A, Franck G, Van der Linden M, Smith C, Cleeremans A ( 2000): Experience‐dependent changes in cerebral activation during human REM sleep. Nat Neurosci 3: 831–836. [DOI] [PubMed] [Google Scholar]
- Maury A ( 1861): In Le sommeil et les rêves. Paris: Didier et Cie Libraires‐Editeurs; pp 133–134. [Google Scholar]
- Mazza S, Perchet C, Frot M, Magnin M, Garcia‐Larrea L, Bastuji H ( 2010): Behavioural and electrophysiological responses to painful stimuli during paradoxical sleep in the cingulate gyrus: A memory transfer from waking to sleep? Neurophysiol Clin 40: 173. [Google Scholar]
- Mazzola L, Isnard J, Mauguière F ( 2006): Somatosensory and pain responses to stimulation of the second somatosensory area (SII) in humans. A comparison with SI and insular responses. Cereb Cortex 16: 960–968. [DOI] [PubMed] [Google Scholar]
- Moldofski HK ( 2001): Disordered sleep in fibromyalgia and related myofascial pain conditions. Dent Clin North Am 45: 701–713. [PubMed] [Google Scholar]
- Montes C, Magnin M, Maarrawi J, Frot M, Convers P, Mauguière F, Garcia‐Larrea L ( 2005): Thalamic thermo‐algesic transmission: Ventral posterior (VP) complex versus VMpo in the light of a thalamic infarct with central pain. Pain 113: 223–232. [DOI] [PubMed] [Google Scholar]
- Morrison AR, Sanford LD, Ball WA, Mann G, Ross RJ ( 1995): Stimulus‐elicited behavior in rapid eye movement sleep without atonia. Behav Neurosci 109: 972–979. [DOI] [PubMed] [Google Scholar]
- Nielsen TA, McGregor DL, Zadra A, Ilnicki D, Ouellet L ( 1993): Pain in dreams. Sleep 16: 490–498. [PubMed] [Google Scholar]
- Niiyama Y, Fujiwara R, Satoh N, Hishikawa Y ( 1994): Endogenous components of event‐related potential appearing during NREM stage 1 and REM sleep in man. Int J Psychophysiol 17: 165–174. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Wiseman M, Kupfer DJ, Moore RY ( 1997): Forebrain activation in REM sleep: An FDG PET study. Brain Res 770: 192–201. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guénot M, Mauguière F ( 2002): Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cereb Cortex 12: 376–385. [DOI] [PubMed] [Google Scholar]
- Paré D, Llinás R ( 1995): Conscious and pre‐conscious processes as seen from the standpoint of sleep‐waking cycle neurophysiology. Neuropsychol 33: 1155–1168. [DOI] [PubMed] [Google Scholar]
- Perchet C, Godinho F, Mazza S, Frot M, Legrain V, Magnin M, Garcia‐Larrea L ( 2008): Evoked potentials to nociceptive stimuli delivered by CO2 or Nd:YAP lasers. Clin Neurophysiol 119: 2615–2622. [DOI] [PubMed] [Google Scholar]
- Perrin F, Garcia‐Larrea L, Mauguiere F, Bastuji H ( 1999): A differential brain response to the subject's own name persists during sleep. Clin Neurophysiol 110: 2153–2164. [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia‐Larrea L, Grégoire MC, Costes N, Convers F, Lavenne F, Mauguière F, Michel D, Laurent B ( 1999): Haemodynamic brain responses to acute pain in humans: Sensory and attentional networks. Brain 122: 1765–1779. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, García‐Larrea L ( 2000): Functional imaging of brain responses to pain. A review and meta‐analysis. Neurophysiol Clin 30: 263–288. [DOI] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD ( 1998): A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 18: 8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD ( 2000): Psychological and neural mechanisms of the affective dimension of pain. Science 288: 1769–1772. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC ( 1997): Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277: 968–971. [DOI] [PubMed] [Google Scholar]
- Raymond I, Nielsen TA, Lavigne G, Choinière M ( 2002): Incorporation of pain in dreams of hospitalized burn victims. Sleep 25: 765–770. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A ( 1968): A Manual of Standardized Terminology, Techniques and Scoring System For Sleep Stages of Human Subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute, University of California. [Google Scholar]
- Rechtschaffen A, Hauri P, Zeitlin M ( 1966): Auditory thresholds in REM and NREM sleep stages. Percept Mot Skills 22: 927–942. [DOI] [PubMed] [Google Scholar]
- Rey M, Bastuji H, Garcia‐Larrea L, Guillemant P, Mauguière F, Magnin M ( 2007): Human thalamic and cortical activities assessed by dimension of activation and spectral edge frequency during sleep wake cycles. Sleep 30: 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M ( 2000): Stereotaxic display of brain lesions. Behav Neurol 12: 191–200. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Morrison AR, Ball WA, Ross RJ, Mann GL ( 1992): Varying expression of alerting mechanisms in wakefulness and across sleep states. Electroenceph Clin Neurophysiol 82: 458–468. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Greffrath W, Büsselberg D, Treede R‐D ( 2000): Inactivation and tachyphylaxis of heat‐evoked inward currents in nociceptive primary sensory neurons of rats. J Physiol (Lond) 528: 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soja PJ ( 2007): Modulation of prethalamic sensory inflow during sleep versus wakefulness In: Lavigne G, Sessle BJ, Choinière M, Soja P, editors. Sleep and Pain. Seattle: IASO Press; pp 45–76. [Google Scholar]
- Steriade M ( 1996): Arousal: Revisiting the reticular activating system. Science 272: 225–226. [DOI] [PubMed] [Google Scholar]
- Steriade M, Curro Dossi R, Contreras D ( 1993): Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (approximately 40 Hz) spike‐bursts at approximately 1000 Hz during waking and rapid eye movement sleep. Neuroscience 56: 1–9. [DOI] [PubMed] [Google Scholar]
- Stevens RT, London SM, Apkarian AV ( 1993): Spinothalamocortical projections to the secondary somatosensory cortex (SII) in squirrel monkey. Brain Res 631: 241–246. [DOI] [PubMed] [Google Scholar]
- Talairach J, Bancaud J ( 1973): Stereotactic approach to epilepsy: Methodology of anatomo‐functional stereotaxic investigations. Prog Neurol Surg 5: 297–354. [Google Scholar]
- Tomić MA, Vucković SM, Stepanović‐Petrović RM, Ugresić ND, Prostran MS, Bosković B ( 2010): Synergic interactions between paracetamol and oxcarbazepine in somatic and visceral pain models in rodents. Anesth Analg 110: 1198–1205. [DOI] [PubMed] [Google Scholar]
- Treede RD, Kenshalo D, Gracely R, Jones A ( 1999): The cortical representation of pain. Pain 79: 105–111. [DOI] [PubMed] [Google Scholar]
- Truini A, Galeotti F, Cruccu G, Garcia‐Larrea L ( 2007): Inhibition of cortical responses to Aδ inputs by a preceding C‐related response. Testing the “First come, first served” hypothesis. Pain 131: 341–347. [DOI] [PubMed] [Google Scholar]
- Vogt BA ( 2005): Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6: 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle R, Kaufmann C, Wetter TC, Holsboer F, Auer DP, Pollmächer T, Czisch M ( 2007): Functional microstates within human REM sleep: First evidence from fMRI of a thalamocortical network specific for phasic REM periods. Eur J Neurosci 25: 863–871. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN ( 1997): Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 14: 2–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiffren PJ, Derry S, Moore RA ( 2011): Lamotrigine for acute and chronic pain. Cochrane Database Syst Rev 2: CD006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You HJ, Lei J, Arendt‐Nielsen L ( 2009): Selective inhibitory effects of pregabalin on peripheral C but not A‐delta fibres mediated nociception in intact and spinalized rats. Neuroscience 164: 1845–1853. [DOI] [PubMed] [Google Scholar]


