Abstract
Cerebral activations involved in actual writing of a new story and the associated correlates with creative performance are still unexplored. To investigate the different aspects of the creative writing process, we used functional magnetic resonance imaging while 28 healthy participants performed a new paradigm related to creative writing: “brainstorming” (planning a story) and “creative writing” (writing a new and creative continuation of a given literary text), as well as an additional control paradigm of “reading” and “copying.” Individual verbal creativity was assessed with a verbal creativity test and creative performance with a qualitative rating of the creative products. “brainstorming” engaged cognitive, linguistic, and creative brain functions mainly represented in a parieto‐frontal‐temporal network, as well as writing preparation, and visual and imaginative processing. “creative writing” activated motor and visual brain areas for handwriting and additionally, cognitive and linguistic areas. Episodic memory retrieval, free‐associative and spontaneous cognition, and semantic integration were observed in a right lateralized activation pattern in bilateral hippocampi, bilateral temporal poles (BA 38), and bilateral posterior cingulate cortex in a “creative writing” minus “copying” comparison. A correlation analysis of “creative writing” minus “copying” with the creativity index revealed activation in the left inferior frontal gyrus (BA 45) and the left temporal pole (BA 38). Thus, verbal creativity during “creative writing” is associated with verbal and semantic memory as well as semantic integration. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: creativity, writing, temporal lobe
INTRODUCTION
Writing fascinating literature is one of the main artistic expressions of humans. However, actual writing of a creative story in a functional magnetic resonance imaging (fMRI) scanner has not yet been investigated. Currently, most creativity researchers agree that creativity “is the ability to produce work that is both novel (i.e., original, unexpected) and appropriate (i.e., useful, adaptive concerning task constraints)” [Sternberg, 1999]. Furthermore, a creative task should be heuristic (i.e., an open‐ended task and problem discovery is an important aspect), and a qualitative assessment of creativity should be grounded in domain‐specific judgments [Amabile, 1996].
In general, it can be assumed that everyone has a latent potential for producing creative artwork, considering creativity as cognitive processes [Ward, 1999]. With respect to professional writing in a literary context, creative writing comprises the critically reflected and professionally supervised acquisition of literary writing techniques [Ortheil, 2005]. Flower and Hayes [Flower, 1981] developed a model of the writing process that considered text composition as a cognitive process characterized by decisions, high hierarchical organization, and goal‐directed thinking. In their “cognitive process theory of writing,” the writing process consists of “planning,” “translating,” and “reviewing.” Flower and Hayes [ 1981] developed the “cognitive process theory of writing” from behavioral observations by protocol analysis about thinking aloud during the act of composing. The act of writing contains the task environment, the writer's long‐term memory and the writing processes. All these elements are characterized by a high hierarchical organization, and goal‐directed thinking. The writing process consists of “planning” by generating ideas from a long‐term‐memory retrieval, organizing the ideas into a meaningful structure and goal‐setting, “translating” by transcribing the ideas into a written text and applying the knowledge of language, and “reviewing” by evaluating and revising the written text.
Previous neuroscientific research on creative writing is sparse and thus little is known about its neural correlates. Some knowledge has been gathered on motor processes associated with writing [Katanoda et al., 2001]. However, the neural correlates of cognitive processes during writing are still unexplored. Current creativity studies can be categorized into divergent thinking, insight, and art performance; however, their results vary and creativity is apparently not associated with any particular brain area [Dietrich and Kanso, 2010]. In previous research, possible creativity‐relevant brain areas were mainly observed in frontal, temporal, and parietal brain regions [Heilman et al., 2003]. The prefrontal cortex (PFC) in particular seems to play a critical role for insight solutions and divergent thinking [Dietrich, 2004; Dietrich and Kanso, 2010]. Earlier studies about creative story generation associated verbal creativity predominantly with left parieto‐temporal regions (BAs 39/40), [Bechtereva et al., 2004] and divergent semantic processing with the right PFC (BAs 9/10) [Howard‐Jones et al., 2005]. A clinical lesion study [Troyer, 1998] on verbal fluency referred phonemic‐fluency associated with switching to the frontal lobe and semantic‐fluency associated with clustering to the temporal lobe.
To date, only one fMRI study [Howard‐Jones et al., 2005] and two positron emission tomography (PET) studies [Bechtereva et al., 2004; Bekhtereva et al., 2000] investigated creativity applying a story‐generation task. However, in all these studies, story generation was restricted to a mental process without physically performing the story writing itself. In contrast, we instructed participants to actually write down their individual continuation of a given literary text during fMRI scanning. Therefore, we applied an available neuroscientific fMRI scanner setting according to the contemporary methodologies of creativity [Fink et al., 2007]. The produced written text should serve as a control for brain activities during scanning, and as a real product of the scanning process enabling a qualitative assessment of the creative performance by the consensual assessment technique (CAT) according to Amabile [ 1996]. For this reason, we developed a paradigm based on a common practice and training method of creative writing (working creatively with a given literary text). Writing a continuation of a literary text is obviously a heuristic and open‐ended task [Amabile, 1996]. The requirements of the common definition of creativity [Sternberg, 1999] were met by our scanning task, including originality and novelty by composing an individual text version, and appropriateness by adapting to the given story beginning and its literary context. Thus, our task might comply better with real‐life creativity of literary writing.
Based on previous theoretical considerations, earlier neuroscientific findings on verbal creativity and story generation and behavioral observations on writing we hypothesized the following:
-
a
Actual and real‐life creative writing can be performed during fMRI‐scanning and should enable the identification of cognitive and creative processes by using a categorical design. Additionally, this offers a control for cerebral processes by producing a written text during scanning and enables an assessment of the performance during fMRI‐scanning.
-
b
The usage of our new paradigms based on the behavioral observations of Flower and Hayes [ 1981] should help to isolate different cerebral networks associated with processes involved in creative writing.
-
c
Subtraction analysis between the actual “creative writing” condition and the control condition “copying” (“creative writing ‐ copying”) should reveal the cognitive writing network, in particular the network of active writing a new own creative text version compared to copying one. We expected memory processes for long‐term memory retrieval referring probably to stored knowledge and generating ideas.
-
d
Creativity relevant neural functions can be identified by regression analysis of verbal creativity scores (CI) and blinded assessment of creativity of the written text. In accordance to previous imaging studies on verbal fluency [Troyer, 1998] and creative story generation [Howard‐Jones, 2005] the creativity relevant regions are proposed in frontal and temporal regions.
Altogether, the aim of our study was to develop a new fMRI paradigm to explore the cerebral network of creative writing processes. For that purpose, we followed the “cognitive process theory of writing” of Flower and Hayes [Flower and Haynes, 1981] to differentiate the processes related to creative writing. With respect to the “planning” and “translating” phases, we investigated brain activity during “brainstorming” and “creative writing” of a new continuation of a given literary text. “creative writing” was contrasted with the control condition “copying” a part of the text, to explore the cognitive and creative brain activity. By performing an additional correlation analysis for the creativity index (CI), Schoppe, 1975 of the participants and the ratings of the written text [CAT according to Amabile (Amabile, 1996)], we intended to isolate those areas that should predominantly indicate creativity during the writing process.
MATERIALS AND METHODS
Participants
Fourteen male and 14 female native German speakers (average age 24.0 ± 1.9 years), having no psychological or neurological disorders, participated in this study. All participants were strongly right‐handed (average score of the Edinburgh Handedness Test [Oldfield, 1971]: + 99.68 ± 1.67). All participants were inexperienced in creative writing and gave their written informed consent to the study, which was approved by the Ethics Committee of the Medical Faculty of the University of Greifswald.
Instructions
Before scanning, participants received written instructions about the sequence of the tasks, the prohibition of head‐ and body movements except for necessary hand‐ and eye movements, the need of visual fixation of the presented cross during rest and the prohibition of oral communication except in case of an emergency.
Each of the tasks in the scanner was described in a highly standardized way. Participants were asked to carry out a task as long as it was presented to them. Furthermore, for “brainstorming” and “creative writing” participants were instructed to compose as creative stories as possible, with the restriction that stories should always be realistic and appropriate to the task. This explanation was derived from the most common definition of creativity of Sternberg and Lubart [ 1999]. Participants were allowed to write down a different story during “creative writing” as obtained during “brainstorming.”
Experimental Design
Participants lay supine on the scanner couch during task execution. The tasks were printed on sheets of paper (210 × 297 mm2), which were successively placed by an assistant on the inclined surface of a plastic desk positioned over the participants' hips (see Fig. 1). The assistant was standing beside the scanner during the fMRI investigation, changing the paper sheets between the different scanning tasks. Visual contact with the paper sheets for reading and writing was enabled by a double‐mirror system attached to the head coil. The participants' right upper arm was supported by cushions to restrict arm movements during writing, and the desk position was individually adjusted with respect to the arm length.
Figure 1.
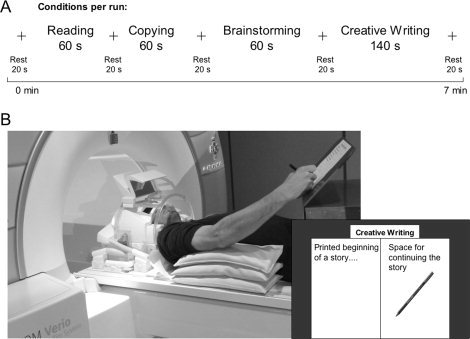
Scanning conditions and scanning environment. A: Conditions were presented in a uniform sequence. To ensure subjects' constant concentration during scanning, task duration was restricted to 60 s, while actual “creative writing” was performed for 140 s. Each condition was followed by a fixation cross lasting 20 s. B: Participant positioning during writing in the scanner. The space for writing included the whole paper sheet except the printed text beginning. Writing was performed with a conventional felt‐tip pen. Visual control was enabled by a double mirror system.
The presented texts A and B were passages of “The Loser,” written by Thomas Bernhard and selected by our coauthor from the University of Hildesheim. Text B is kept in the format of a newspaper report and is therefore highly different to the first text. The text and two examples for a highly creative continuation of the text are provided in the Supporting Information.
The experimental design included two blocks, each containing a different text, A or B, presented to each participant in a random order to prevent methodological artifacts. Each block included the four tasks in the following constant order:
-
1
Covert “reading” of the text (∼120 words) for 60 s.
-
2
“Copying” the first part of the given text (∼35 words) by writing with a felt‐tip pen for 60 s.
Both Tasks 1 and 2 served as control conditions. Participants were supposed to perform them continuously during the entire 60 s. In case they reached the end of the presented text before termination of this period, they had to start over. The constant performance during the activation period was controlled by the first author standing next to a participant in the scanner room.
-
3
“Brainstorming”: the first 30 words of the previous text were presented for 60 s, during which the participants thought about the presented text, generating ideas for their highly creative continuation without being allowed to actually write.
-
4
“Creative writing”: the actual writing of a new, creative continuation of the same given text for 140 s. The participants were instructed to physically write a new, individual, original but appropriate continuation of the given text. They were allowed to free themselves from the ideas obtained during “brainstorming” if they wished.
Between tasks, a fixation cross was presented for 20 s to assess baseline activities and to separate the various task‐dependent brain activities.
Behavior Evaluation
To assess the participants' behavior during scanning, a feedback questionnaire was given containing visual analogue scales (VAS) 10 cm in length. The participants were asked about the appropriateness of the setting, the concentration level during the scanning tasks, and the grade of parallelism between the stories during “brainstorming” and “creative writing.”
Creativity Evaluation
To assess participants' creativity, we applied two established methods: the CAT according to Amabile [ 1996], and a German verbal‐productive creativity test, the “Verbaler Kreativitäts‐Test” [Schoppe, 1975]; for further information see Supporting Information).
In accordance with the CAT [Amabile, 1996], all produced texts were typewritten and sent in a randomized order to five independent judges, who were generally familiar with the domain (German teachers). All judges rated the creativity of each text on a 10‐cm‐long VAS (from 0: not creative at all, to 10: extremely creative). The creativity score was calculated for every participant using the mean value over both texts A and B of the “creativity” criterion rating from all judges. To examine the interjudge reliability, we computed Cronbach's alpha using the Statistical Package for the Social Sciences (SPSS 19.0).
The verbal creativity test [Schoppe, 1975], yielding a creativity index (CI) (see Supporting Information), consisted of nine subtests analyzing the participants' verbal fluency and verbal production skills. Test evaluation was performed according to given instructions.
fMRI Data Acquisition
Data were acquired at a 3T Siemens Magnetom Verio (Siemens, Erlangen, Germany) with a 32‐channel head coil. For each block, A and B, 275 two‐dimensional echo‐planar images (EPI) were acquired with repetition time TR = 2,000 ms, echo time TE = 30 ms, flip angle α = 90°, and field‐of‐view (FOV) 192 × 192 mm2. Each volume consisted of 34 slices with a voxel size of 3 × 3 × 3 mm3 with a 1‐mm gap between them. The first two volumes of each session were discarded to allow for T1 equilibration effects. Thirty‐four phase and magnitude images were acquired in the same FOV by a gradient echo sequence with TR = 488 ms, TE(1) = 4.92 ms, TE(2) = 7.38 ms, and α = 60° to calculate a field map aiming at correcting geometric distortions in the EPI images. An anatomical T1‐weighted three‐dimensional magnetization prepared rapid gradient echo image was acquired for each participant. The total number of sagittal anatomical slices amounted to 176 (TR = 1,900 ms, TE = 2.52 ms, α = 90°, voxel size = 1 × 1 × 1 mm3).
Data Analysis
Data were analyzed using SPM5 (Wellcome Department of Cognitive Neuroscience, London, UK) running on Matlab version 7.4 (MathWorks; Natick, MA). Unwarping of geometrically distorted EPIs was performed in the phase encoding direction using the FieldMap Toolbox available for SPM5. Each individual scan was realigned to the first scan to correct for movement artifacts. The absolute realignment parameters x, y, z, pitch, roll and yaw were averaged over all participants for each condition, yielding the mean values of translational and rotational motion for a condition. The realigned and unwarped EPIs were coregistered to the T1‐weighted anatomical image. For normalization, the coregistered T‐1 image was segmented, normalized to the Montreal neurological institute (MNI) template, and the EPIs were resliced at 3 × 3 × 3 mm3. The resulting images were smoothed with a 9 × 9 × 9 mm3 [full‐width at half maximum (FWHM)] Gaussian Kernel filter to increase the signal‐to‐noise ratio. A temporal high‐pass filter (128 s) was applied to remove slow signal drifts. Movement parameters estimated during the realignment procedure were introduced as covariates into the model to control for variance due to head displacements. Individual statistical maps (fixed effect) of the main (“brainstorming” and “creative writing”) and control (“reading” and “copying”) conditions were evaluated for each participant using the general linear model. Corresponding contrast images of each participant were then entered into a random effect analysis at the second level of SPM5, which accounts for the variance between participants. One‐sample t tests were performed to evaluate significant activations per condition. A correlation analysis of verbal CIs with the imaging data was performed by calculating a simple regression. Spatial assignment of significant brain areas was conducted with the SPM Anatomy Toolbox Version 1.6 [Eickhoff et al., 2005] and, if regions were not defined by ANATOMY, using anatomical masks from the Automated Anatomical Labeling software [Tzourio‐Mazoyer et al., 2002]. Brain activations were superimposed on the MNI render brain and on the T1‐weighted Collins single‐participant brain. We reported significant brain activations above an intensity threshold of P < 0.001, uncorrected, and an extent voxel size threshold of 10 voxels for the main effects, and of 5 voxels for the comparison and correlation analyses.
RESULTS
Behavioral Results
Participants rated the situation of writing in the scanner as acceptable (appropriateness of the setting: 6.4 ± 2.3 credits). The participants tended to write a different version of text continuation during “creative writing” than what was invented during “brainstorming” [story parallels: 4.6 ± 2.3 (0 = different text; 10 = same text)]. Concentration during “brainstorming” and “creative writing” was rated as moderately high (average 7.5 ± 1.8), and both texts A and B did not differ with respect to concentration [average t < 1.23 (27); P > 0.23].
Evaluation of Creativity
The inter‐judge reliability of the CAT for the criterion “creativity” was satisfactory (Cronbach's alpha = 0.72). The verbal creativity test revealed an average CI of 107.14 ± 8.84 (range of our participants: 92–127 credits). The CI and CAT results were not associated (r = 0.27; n.s.).
Imaging Data
The averaged translational and rotational motion was 0.07 mm and 0.01 degrees for reading, 0.23 mm and 0.03 degrees for brainstorming and 0.39 mm and 0.05 degrees for creative writing. Thus movements were on average quite low, not exceeding half a millimetre. The fMRI results were calculated over both texts, since they did not differ with respect to participants' concentration during the performance.
Main Effects
“Reading” revealed a left lateralized brain network involving the typical neural systems for reading [Shaywitz and Shaywitz, 2008]: Broca's area (BA 44/45) and its homologue, parieto‐temporal regions [bilateral superior temporal sulci (STS), left Wernicke's area of BA 22/40), and the occipito‐temporal region (BA 19/37)]. Additional cognitive brain activity was observed bilaterally in the dorsolateral PFC (dlPFC, BA 9/46), anterior cingulum (BA 24/32), and the anterior insula (all with lateralization to the right hemisphere). Activations were additionally manifested in the motor system comprising the bilateral primary motor and sensorimotor cortices (both in the somatotopic representation of articulation), and the hand motor system located in the left precentral gyrus (BA 4). Furthermore, secondary motor areas [supplemental motor area (SMA) and premotor cortex (PMC)] were activated, as well as the left putamen, the thalami, the cerebellar hemispheres, and the vermis. The primary visual cortex was activated bilaterally (see Supporting Information Table I).
Table I.
Brain regions activated during “brainstorming”
| MNI‐coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | Brodmann area | t value | x | y | z | |
| Frontal | Left inferior frontal gyrus (p. triang.) | 44/45 | 10.53 | −42 | 21 | 3 |
| Left inferior frontal gyrus (p. oper.) | 44/45 | 7.54 | −48 | 15 | 9 | |
| Left inferior frontal gyrus (p. orb.) | 47 | 8.83 | −45 | 18 | −3 | |
| Right inferior frontal gyrus (p. triang.) | 44/45 | 4.21 | 51 | 21 | 24 | |
| Right inferior frontal gyrus (p. oper.) | 44/45 | 4.12 | 48 | 18 | 27 | |
| Right inferior frontal gyrus (p. orb.) | 47 | 7.50 | 45 | 24 | −9 | |
| Left anterior insula | 13 | 9.51 | −33 | 24 | 0 | |
| Right anterior insula | 13 | 7.59 | 39 | 24 | −6 | |
| Left inferior frontal gyrus/middle orbital gyrus | 10 | 4.27 | −45 | 45 | 0 | |
| Right inferior frontal gyrus/ middle orbital gyrus | 11 | 5.86 | 39 | 33 | −12 | |
| Left anterior cingulate cortex | 24/32 | 4.87 | −9 | 27 | 30 | |
| Left superior frontal gyrus/mPFC | 8 | 6.29 | −6 | 21 | 42 | |
| Right superior frontal gyrus/mPFC | 8 | 4.85 | 3 | 24 | 60 | |
| Left superior frontal gyrus/SMA | 6 | 9.36 | −3 | 9 | 60 | |
| Left middle frontal gyrus/dPMC | 6 | 9.69 | −42 | 0 | 51 | |
| Right middle frontal gyrus/dPMC | 6 | 4.01 | 48 | 15 | 51 | |
| Left middle frontal gyrus/dlPFC | 9/46 | 5.56 | −51 | 18 | 27 | |
| Parietal | Left supramarginal gyrus | 40 | 4.75 | −48 | −51 | 24 |
| Left angular gyrus | 39 | 4.57 | −45 | −51 | 21 | |
| Left inferior parietal lobule | 40 | 3.70 | −54 | −51 | 36 | |
| Temporal | Left medial temporal gyrus/STS | 7.54 | −48 | −39 | 6 | |
| Right medial temporal gyrus/STS | 6.35 | 51 | −30 | 0 | ||
| Left superior temporal gyrus | 21 | 6.56 | −57 | −39 | 9 | |
| Right superior temporal gyrus | 21 | 6.09 | 51 | −27 | 0 | |
| Left medial temporal gyrus | 21 | 6.02 | −57 | −9 | −6 | |
| Left temporal pole | 38 | 4.84 | −48 | 15 | −21 | |
| Occipital | Right occipital lobe | 17/18 | 10.21 | 21 | −90 | −3 |
| Left occipital lobe | 17/18 | 9.26 | −21 | −90 | −6 | |
| Left occipital gyrus | 19 | 5.55 | −24 | −84 | −18 | |
| Cerebellum | Right ant. cerebell hem. (Larsell H VI) | 5.57 | 36 | −66 | −21 | |
| Cerebellar vermis | 4.71 | 6 | −75 | −21 | ||
| Left post. cerebell hem. (Larsell H VII) | 4.62 | −39 | −63 | −30 | ||
“Copying” manifested its activation network predominantly in motor regions, including the primary motor cortex (BA 4, M1; the maximum located in the left hemispheric somatotopic representation of hand movements and additionally in bilateral articulation areas), the sensorimotor cortex (BA 1/2/3, S1; in the corresponding somatotopic representation of motor activity), the anterior part of the bilateral superior parietal lobule (BA 7), and secondary motor areas (BA 6, SMA, PMC), including the posterior end of the middle frontal gyrus [on the left representing Exner's Area (Roux et al., 2009)] and the frontal eye field, left thalamus, left putamen, and the cerebellum. Further activations were observed in visual and linguistic areas as the bilateral visual cortex, the bilateral occipito‐temporal cortex, and the frontal regions, including the bilateral dlPFC (BA 9/46), the left insula, and the right inferior frontal gyrus (IFG, BA 44; see Supporting Information Table II).
Table II.
Brain regions activated during “creative writing”
| MNI‐coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | Brodmann area | t value | x | y | z | |
| Frontal | Left precentral gyrus/M1 | 4 | 11.32 | −36 | −18 | 57 |
| Right precentral gyrus/M1 | 4 | 5.84 | 63 | −12 | 27 | |
| Left precentral gyrus/dPMC | 6 | 10.12 | −27 | −15 | 60 | |
| Right precentral gyrus/dPMC | 6 | 7.66 | 27 | −3 | 51 | |
| Left superior frontal gyrus/SMA | 6 | 9.20 | −3 | 0 | 66 | |
| Right superior frontal gyrus/SMA | 6 | 6.51 | 9 | 9 | 54 | |
| Left middle cingulate gyrus | 24/32 | 5.31 | −9 | 12 | 42 | |
| Right middle frontal gyrus | 9 | 8.81 | 51 | 6 | 33 | |
| Left middle frontal gyrus | 9 | 7.49 | −57 | 3 | 39 | |
| Right middle frontal gyrus | 9/46 | 4.37 | 33 | 45 | 36 | |
| Right inferior frontal gyrus (p. oper.) | 44 | 5.19 | 63 | 12 | 12 | |
| Left inferior frontal gyrus (p. oper.) | 44 | 4.54 | −54 | 12 | 3 | |
| Parietal | Left postcentral gyrus/S1 | 1/2/3 | 10.50 | −36 | −24 | 54 |
| Right postcentral gyrus/S1 | 1/2/3 | 5.96 | 42 | −33 | 45 | |
| Right superior parietal lobule | 7 | 10.17 | 21 | −60 | 60 | |
| Left superior parietal lobule | 7 | 7.66 | −24 | −54 | 57 | |
| Temporal | Left superior temporal sulcus/superior temporal gyrus | 22 | 4.80 | −57 | −42 | 12 |
| Occipito‐temporal | Right inferior temporal gyrus | 37 | 9.30 | 48 | −60 | −12 |
| Left inferior temporal gyrus | 37 | 8.14 | −45 | −72 | −3 | |
| Right occipital gyrus | 19 | 8.61 | 33 | −87 | 3 | |
| Left occipital gyrus | 19 | 8.38 | −39 | −81 | 3 | |
| Occipital | Right occipital lobe | 17/18 | 11.85 | 27 | −90 | 0 |
| Left occipital lobe | 17/18 | 9.88 | −21 | −93 | −3 | |
| Cerebellum | Right post. cerebell hem (Larsell H VIII) | 10.22 | 15 | −60 | −42 | |
| Right ant. cerebell. hem. (Larsell H IV‐V) | 9.67 | 18 | −51 | −18 | ||
| Cerebellar vermis | 8.18 | 6 | −63 | −33 | ||
| Left post. cerebell hem. (Larsell H VII) | 6.03 | −30 | −57 | −24 | ||
| Thalamus | Left thalamus | 5.74 | −12 | −18 | 12 | |
“Brainstorming” revealed a bilateral but more left‐focused network of activation (see Table I and Fig. 2). The strongest activation was observed in the left IFG (i.e., Broca's area BA 44/45, and BA 47), including the left anterior insula, left dlPFC (BA 9/46), and reaching to left lateral orbito‐frontal regions (BA 10). The right hemispheric brain activity was observed similarly in the right IFG (BA 44/45, and BA 47), including the right anterior insula and right orbital regions (BA 11). The dorsal PMC (dPMC, BA 6) was activated bilaterally. Further activity was observed in the left medial SMA (BA 6), medial PFC (BA 8), and the dorsal anterior cingulate cortex (dACC, BA 24/32). A broad left activation comprised the inferior parietal cortex (BA 39/40, including Wernicke's area), the superior temporal cortex (BA 22 and STS), and the temporal pole (BA 38). The right posterior STS showed a more circumscribed activation. Furthermore, bilateral visual cortical hemispheres (BAs 17/18/19) and the cerebellum (right anterior and left posterior cerebellar hemisphere and vermis) were activated.
Figure 2.
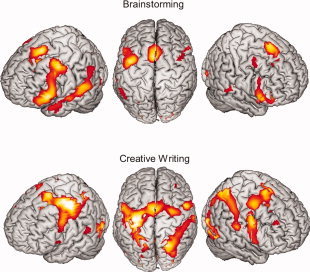
Overview of the cortical activation map for the main effects of the conditions “brainstorming” (top row) and “creative writing” (bottom row) projected on the segmented MNI‐brain thresholded with P < 0.001, uncorrected. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
“Creative writing” (see Table II and Fig. 2) activated predominantly motor‐associated areas including the primary motor cortex (BA 4, M1; located in the somatotopic area of the right hand and right articulation representation) with the corresponding somatosensory areas (BA 1/2/3, S1), as well as bilateral secondary motor areas (dPMC, SMA), the middle cingulum, bilateral superior parietal lobules (BA 7), the left thalamus, and cerebellar activity (mainly the right anterior lobe and vermis). The activation extended to bilateral occipital‐temporal regions (BAs 37 and BA 19) and to the bilateral visual cortex (BA 17/18). Furthermore, cerebellar activations were observed in the right anterior and both posterior lobes, as well in the vermis. In particular, the frontal activations were located in bilateral IFG (BAs 44), bilateral middle frontal gyrus (BA 9), and the right dlPFC (9/46), as well as temporal lobe activations in the posterior part of the superior temporal gyrus (BA 22/STS).
Differences Between the Contrasts of Creative Task Minus the Control Paradigm
“Creative writing” minus “copying” (“creative writing – copying”; see Table III, Fig. 3) focused the highest activation in the right medial temporal pole (BA 38) and the left temporal pole (BA 38). Activations were further located in the bilateral posterior cingulate cortex (BA 31) and the bilateral hippocampus. All regions showed lateralized activity to the right hemisphere.
Table III.
Brain regions for “creative writing ‐ copying”
| MNI‐coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | Brodmann area | t value | x | y | z | |
| Temporal | Right medial temporal pole | 38 | 7.68 | 45 | 12 | −27 |
| Left temporal pole | 38 | 5.73 | −42 | 3 | −27 | |
| Hippocampus | Right hippocampus | 4.76 | 18 | −18 | −15 | |
| Left hippocampus | 3.73 | −27 | −15 | −18 | ||
| Cingulum | Right posterior cingulate cortex | 31 | 5.69 | 12 | −48 | 36 |
| Left posterior cingulate cortex | 31 | 3.93 | −12 | −48 | 39 | |
Figure 3.
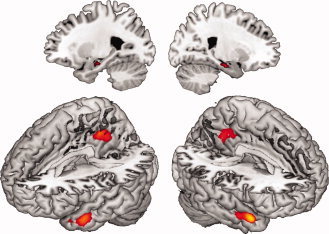
fMRI‐results of the contrast “creative writing ‐ copying,” P < 0,001, uncorrected. “Creative writing ‐ copying” revealed strongest activation in the right medial temporal pole. Further activation was located in the left temporal pole as well as bilateral hippocampi and bilateral posterior cingulate cortex. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Correlation Analysis of “Creative Writing – Copying” With CI and CAT Results
A positive correlation of “creative writing – copying” with the CI was observed in the left superior temporal gyrus reaching the temporal pole (BA 38, t = 3.78; −54, 0, −12) and the left IFG (BA 45, t = 3.69; −51, 21, 27; see Fig. 4).
Figure 4.
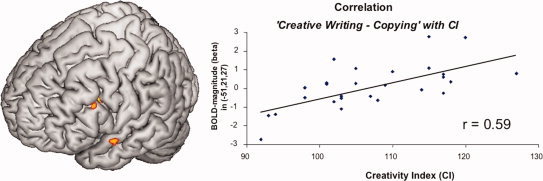
Positive Correlation between BOLD‐magnitude obtained during “creative writing ‐ copying” and the creativity index, P < 0,001, uncorrected. Predominantly activation in the Broca's area (BA 45) and the temporal pole showed positive associations with the creativity index. The positive correlation of BOLD‐magnitude and the CI are plotted on the right showing an r of 0.59. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Correlation analyses between the fMRI images of “creative writing – copying” and the CAT results did not show any significant results.
DISCUSSION
This study can be regarded as the first contribution to scientific literature that applies an actual writing paradigm with a creative story‐generation task as realistic as possible during fMRI‐scanning. For this purpose we investigated BOLD response during “brainstorming” and “creative writing” according to the writing model of Flower and Hayes [ 1981]. Our results provide a cerebral network during “brainstorming,” predominantly including language processing located in the bilateral IFG and left temporal activation, high hierarchical organization in the PFC, and cognitive decision processes in the dACC. “Creative writing” itself included activation patterns associated with handwriting, language processing, and cognitive areas. More specifically, the contrast “creative writing – copying” revealed areas associated with memory retrieval, semantic integration, free association, and spontaneous cognition. The findings on verbal creativity were located in a left fronto‐temporal network, consisting of the left IFG (BA 45) and the left temporal pole (BA 38).
Brainstorming
The strongest and most widespread activation of left frontal brain regions in “Brainstorming” (located in the left IFG, extending to the precentral gyrus, the SMA, and the medial left ACC) corresponds to the main brain activations in the generation of novel concepts and ideas in verbal creative tasks (e.g., “alternative uses” and “name invention”), [Fink et al., 2009].
The left lateralized frontal activations in the bilateral IFG (BAs 44/45 and 47) extending to the anterior insula are predominantly associated with language processing and verbal fluency. In particular, the left IFG (BAs 44/45) plays a key role in cognitive tasks demanding verbal information processing [Gernsbacher and Kaschak, 2003] and is critical for word retrieval and the conceptual system of speech production [Price, 2010]. Clinical lesion studies reported an association of damage to the frontal lobe (BAs 4, 6, 44) with letter fluency [Baldo et al., 2006], and dorsolateral damage with impaired switching during verbal fluency [Troyer et al., 1998]. Therefore, we suggest that the observed activation in these frontal linguistic areas during “brainstorming” might be associated with a flexible and divergent verbal thinking style quite similar to verbal fluency. Hence, they enable the verbal concept for composing the story.
The brain activities in the left dlPFC (BAs 9/46) and dACC (BAs 24/32) seem to be essential structures in “planning” a story, and are associated with higher cognitive control and initiation of writing. Suppression of irrelevant information and executive functions in internal goal‐directed behavior are common, well‐known functions of the PFC [Cohen et al., 2000]. The dlPFC is considered to be critical for creativity [Dietrich, 2004; Dietrich and Kanso, 2010] and even for creative story generation [Howard‐Jones et al., 2005], maintaining working memory, cognitive flexibility, and divergent thinking. The dACC is associated with cognitively demanding tasks and selection processes [Bush et al., 2000], and especially reward‐based decision making [Bush et al., 2002]. “Appropriateness,” however, is one of the criteria indicating creativity [Sternberg, 1999] and the dlPFC (BAs 9/46) and dACC (BAs 24/32) might enable the construction of an original and coherent story concept using idea selection and keeping a monitoring eye on the preparation phase.
The second largest activation pattern in “brainstorming” was revealed in the left parieto‐temporal regions (BAs 39/40, Wernicke's area, BA 22, STS) extending to anterior temporal regions (BA 38). In general, left parieto‐temporal regions are involved in fundamental language processing, and temporal lobe activations have been reported to be crucial for language and sentence comprehension, prelexical perception, and auditory processing of language [Price, 2010]. In earlier research, the left inferior parieto‐temporal regions (BAs 39/40) were considered to be crucial for solving verbal creative tasks by divergent and free‐associative thinking [Fink et al., 2009], for story generation from different semantic word sets [Bechtereva et al., 2004], and for insight strategy [Starchenko et al., 2003], thereby suggesting that the most important functions of these regions were related to the flexibility of thinking and involved in imagination and fantasy. Thus, our results agree with the importance of bilateral parietal (BAs 39/40) areas for creative verbal tasks.
Activity in the primary visual cortex might reflect a higher demand of visual processing compared to the resting period during linguistic stimuli processing [Gernsbacher and Kaschak, 2003]. Howard‐Jones et al. [ 2005] interpreted similar occipital activations by visual imagination of the story's scenario. In addition, motor areas were active [left SMA, left dPMC (both BAs 6), anterior cerebellar lobe, and the right vermis], although no visible motion was observed by the assistant standing at the left side of the participant during “brainstorming.” The dominant dPMC (BA 6) stores complex movement concepts, which are recruited during tasks like writing [Rijntjes et al., 1999]. The anterior cerebellar lobe integrates multisensory inputs of the cortex to a motor efference copy, enabling a continuous correction of complex hand movements [Bloedel, 1992]. Altogether, our findings support the assumption that “brainstorming” cannot be reduced to cognitive mechanisms and idea generation. Planning a story seems to comprise mental imagination that already includes the preparation for the following writing‐execution phase. This provides evidence for a more holistic and integrative brain activity model during the composition of a literary story.
In general, our “brainstorming” findings provide a brain network that obviously corresponds to the “planning” processes according to the “cognitive process theory of writing” of Flower and Hayes [Flower, 1981]. The “generating” element of “planning,” which also relates to the novelty and originality criteria of creativity according to Sternberg [ 1999], can be sustained by the observed corresponding brain activity in the frontal and parieto‐temporal network involving many creativity‐relevant processes, like divergent thinking, cognitive flexibility, and verbal fluency. “Organizing” and “goal setting” indicates a more critical and rational cognitive thinking style, which further constitutes the appropriateness creativity criterion. Therefore, the dlPFC and the dACC can be considered as the corresponding brain regions, since they are associated with insight, selection processing, and maintaining the management of several parallel tasks. Our results emphasize that literary story composition is based on very complex brain mechanisms, which are not localizable to any single brain area.
Creative Writing
The activation pattern during “creative writing” included areas previously associated with the execution of writing: the primary motor cortex, the PMC, the SMA (both BAs 6), the left superior parietal lobe (BA 7), and the right anterior cerebellar hemisphere [Katanoda et al., 2001]. Furthermore, “creative writing” revealed a strong activation in bilateral occipito‐temporal regions assignable to the posterior reading system [Shaywitz and Shaywitz, 2008] as well as in bilateral primary visual areas. As in “brainstorming,” activation in the occipital lobe might be associated not only with visual feedback but might also cause a visual imagery of the scenes planned to be written down [Howard‐Jones et al., 2005].
Otherwise, the cognitive writing processes of “creative writing” are assumed to include language processing (BAs 44, left BA 22/STS) as well as working memory and higher cognitive control in the dlPFC (bilateral BAs 9, right BA 9/46), and memory retrieval functions (bilateral temporal pole, hippocampi, and posterior cingulate cortex) in “creative writing – copying.” Obviously, the cognitive writing regions manifested a right hemisphere lateralization.
The activation of the IFG (BA 44) and the posterior part of the left superior temporal region (BA 22 and STS) referred to language‐related processing [Price, 2010] and verbal creativity [Fink et al., 2009], again similar to “brainstorming.” The prominent activity in the dlPFC points to the importance of sustained attention and a high working memory load [Curtis and D'Esposito, 2003]. “Creative writing” might involve the dlPFC, keeping in mind several relevant pieces of information for working successfully with all ideas, strategies, and different parallel multimodal processes during writing. Moreover, the dlPFC has been suggested to supply essential brain processes for creativity [Dietrich, 2004]. Thus, our results suppose that these frontal regions are critical cognitive writing areas with respect to the high working memory load of the task itself, the required self‐critical attitude of the writer [Ortheil, 2005], and because of general connections to areas with stored domain‐specific knowledge, which is required for creative emergence [Heilman et al., 2003].
After subtracting motor processes (“creative writing – copying”), we observed a right lateralized activation network of predominantly memory retrieval functions, including temporal poles, hippocampi, and the posterior cingulate cortex, with a few foci located in the ventral precuneus. The hippocampus plays a key role in the memory system and thus refers to rigorous episodic memory retrieval during writing. In particular, the hippocampus was reported to be involved in word retrieval during speech production [Price, 2010] and, especially the left hippocampus, for word production in a spontaneous and free‐associative style [Price, 2010; Whitney et al., 2009]. Our “creative writing” task is comparable to verbal and episodic production in a free associative context. Memory retrieval is further associated with anterior temporal structures. The right temporal pole is involved in episodic, and the left temporal pole in semantic, memory retrieval [Olson et al., 2007]. Furthermore, in a complex language context, the bilateral anterior temporal regions are associated with sentence semantics [Price, 2010] and semantic integration processing [Binder et al., 2009; Jung‐Beeman, 2005]. Particularly, the right anterior temporal pole was associated with insight in solving verbal problems [Jung‐Beeman et al., 2004], which demands semantic integration. The key role of the temporal poles in the semantic systems might point to the important integration of the various contextual and verbal ideas, which must be transformed into a coherent concept for the story during creative writing. Taken together, by contrasting “creative writing” with “copying,” activations were observed in the bilateral temporal poles, which might account for semantic memory access and semantic integration processes additionally involved in inventing a new story instead of copying one.
Besides the temporal poles, the posterior cingulate cortex (PCC) subserves sentence comprehension [Price, 2010]. In particular, the PCC shows connections with the hippocampal system and is thus involved in episodic memory [Binder et al., 2009] as well as the conceptual processing of speech production [Price, 2010]. Furthermore, the PCC is involved in self‐referential integration [Northoff and Bermpohl, 2004] and has been described as a main structure of the brain's “default network” [Buckner et al., 2008], which is active during nonfocused attention and representing the awareness of the external environment. Within the “default network,” the PCC is also connected with the same memory structures (hippocampal system and temporal lobe), which emphasizes its role in episodic memory retrieval. Hence, the default network plays a key role in many mental processes (fantasy, imagination, thoughts), has been considered as our “inner mental life” [Buckner et al., 2008], and was even correlated with daydreaming [Mason et al., 2007]. Altogether, it provides the “spontaneous cognition” of humans [Buckner et al., 2008]. Obviously, the participants who are inexperienced in creative writing produce their individual text continuation during scanning by a kind of free‐associative writing on the paper, quite similarly to an unfocused attention task. Our cognitive writing results are in accordance with the observation on a free‐association style remarkable for poor writers by Flower and Hayes [ 1981].
In summary, “creative writing” can be understood as translating the conceptual and verbal ideas into a handwriting process after selection, semantic integration, and motor coordination. Corresponding to the “cognitive process theory of writing” of Flower and Hayes [ 1981], we revealed the importance of domain‐relevant knowledge for creative writing, including language processing, working memory functions, and predominantly, the memory system: episodic and semantic memory retrieval and the semantic integration in an original and coherent story concept. Furthermore, free association and spontaneous cognition are typical characteristics for actual story writing.
Correlation Analysis of “Creative Writing – Copying” With the CI and CAT Results
The correlation analysis of “creative writing – copying” with the CI demonstrated a positive association of activations in left fronto‐temporal areas with verbal creativity. Previous research on fronto‐temporal dementia has associated deficits in the fronto‐temporal region with an emergence of artistic creativity [Miller et al., 2000]. However, these authors [Miller et al., 2000] illustrated that patients with fronto‐temporal dementia might exhibit simply a kind of “pseudo‐creativity,” as they are rather less successful in common objective creativity tests [de Souza et al., 2010]. Findings of functional imaging studies in healthy participants are not completely comparable with reports in a lesion model, but our results underline a crucial involvement of these areas during “creative writing.”
The activation in the anterior part of the superior frontal gyrus, reaching the left temporal pole (BA 38), refers predominantly to the semantic system [Olson et al., 2007] and especially to semantic integration [Jung‐Beeman, 2005]. The second region of activation, a part of the left IFG (BA 45), was associated with word retrieval during speech production [Price 2010], lexical retrieval during verbal fluency [Heim et al., 2009], and verbal creative processing [Fink et al., 2009; Howard‐Jones et al., 2005; Mashal et al., 2007]. Finally, our results provide a lateralization on the language dominant hemisphere for verbal creativity. However, we are aware that these correlations do not highlight the “center of literary creativity,” because an objective and predominantly quantitative test may not at all be commensurate with complex literary story composition, and the CI assesses a personal trait rather than the actual creative performance inside the scanner. Nevertheless, fluency, flexibility, and efficient memory retrieval of stored semantic knowledge are common human abilities for a creative and cultural achievement such as writing literature. Thus, these areas provide important personal prerequisites for creative writing processes representing the access of verbal creativity located in the fronto‐temporal network. Finally, we could not identify activation in any regions positively associated with the CAT [Amabile, 1996], probably because the ratings did not reflect any creative quality associated with brain activation. However, since the CI, representing a personal trait vs. the CAT representing the personal performance during scanning, showed just as low an association, we cannot well deduce those abilities from verbal creativity to creative writing performance and are furthermore uncertain about qualitative creative brain correlates.
Taken together, “creative writing” involves various cognitive brain processes and brain areas representing verbal creativity. Nevertheless, individual literary creativity does not seem obviously localizable in any single brain area or assignable to any single cerebral function.
Discussion of Our Paradigm
Creativity is generally a poorly understood neuroscientific field of research, and to date, a coherent concept of its critical brain network does not exist because previous studies showed wide variation in their design and definition of creativity [Dietrich and Kanso, 2010]. Many studies [Chavez‐Eakle et al., 2007; de Souza et al., 2010; Fink et al., 2009] applied common objective creativity tests (i.e., Torrance Test of Creative Thinking), which separate creativity into single abilities. We agree that these abilities are important during creative writing, and of course, our correlation analysis of “creative writing – copying” with the CI revealed brain areas associated with them (i.e., divergent thinking, verbal fluency, cognitive flexibility, and the semantic system). Nevertheless, we doubt that the large spectrum of creative writing properties depends on a single mental ability and that such quantitative tests are adequate measurements, as differing from real‐life creativity. In contrast, we understand creativity during literary writing as a complex cognitive function in which additional selection, combination, and integration of an original and coherent story concept do play a central role, as emphasized by our results. For this reason, to assess an almost real‐life creative writing task, we applied a paradigm that can be classified into the established creativity task set of “story composition” [Bechtereva and Nagornova, 2007]. Earlier story‐generation studies [Bechtereva et al., 2004; Howard‐Jones et al., 2005] applied given word sets from different or the same semantic areas. In our study, a given beginning of a literary story was the reference for creativity, and we focused on investigating the entire process of creative writing. In contrast, Howard‐Jones et al. [ 2005] used story generation to examine divergent thinking processes, which might explain the differences between their results and ours. However, we assume that writing literature cannot be reduced to divergent thinking processes and that the creation of unusual, but often unrealistic, stories from the given word sets cannot be comparable with literary creativity.
For this purpose, the main critical aspect was to develop an available fMRI setting. According to Fink et al. [ 2007], such “paper‐and‐pencil tasks” inside an fMRI scanner are limited because of their task‐related motor artifacts and the impossibility of free‐hand writing inside the scanner. Nevertheless, we avoided performing our task in ways commonly used in the earlier story‐generation studies [Bechtereva et al., 2004; Howard‐Jones et al., 2005], since only a real product, such as the written text produced during “creative writing,” can serve as a control and evidence for participants' actual brain activities during the scanning task. We faced the reported limitations [Fink et al., 2007] by providing a scanning environment that enables real, creative performance of writing, as well as implementing controls for motor artifacts. Moreover, the texts produced during scanning could be used to assess the quality of creative performance by the CAT, applying domain‐specific judgments [Amabile, 1996]. We agree about the necessity of qualitative creativity measures, considering additionally the appropriateness of a creative product in contrast to the quantitative measures which are mainly constrained to the fluency of ideas [Fink et al., 2007].
To analyze the various creative thinking processes, we followed the common method of comparisons in creativity research [Bekhtereva and Nagornova, 2007; Fink et al., 2007]. Our results of “brainstorming ‒ reading” are in accordance with another story‐generation study [Bechtereva et al., 2004] applying a similar comparison. Additionally, we accomplished an analogous comparison (“creative writing – copying”) for both writing conditions. Obviously, “creative writing” allows a more personal and free‐associative thinking style, with the disadvantage of more inhomogeneity between the investigated participants. Nevertheless, our results do not disagree with contemporary creativity studies and are in accordance with earlier findings on cognitive writing processes assumed to be involved in creative writing.
Parallels of EEG Findings and fMRI Observations on the Prefrontal Role for Creative Processing
EEG studies reported increased frontal and frontoparietal alpha synchronization associated with creative task processing [e.g., Fink et al., 2009]. For other domains, such as composing a drawing, others found increased delta band synchronization and alpha band desynchronization in experts compared to nonexperts [Bhattacharya and Petsche, 2005]. Besides of the fact that areas positively associated with increased creativity during task solving in nonexperts might not be the same increasingly active in an expert group, there is evidence for the importance of the prefrontal cortex for creative information processing. Although distinctive neural processes are associated with creative abilities in different domains, the prefrontal cortex is functionally involved in processes generally necessary for creative cognition, since this area processes not only working memory, temporal integration and sustained attention, but also self‐reflected consciousness, cognitive flexibility and planning [Dietrich, 2004]. To date, it is not well understood, how these complex processes are represented in the prefrontal lobe. EEG studies revealed both increases and decreases in power and synchrony of different frequency bands depending on the nature of the task and the participants investigated [e.g., Petsche et al., 1997]. Additionally, there is an ongoing debate of how EEG‐Synchronization may be interpreted [e.g., Klimesch et al., 2007]. The only study comparing results of EEG and fMRI for the same paradigm of creative problem solving [Fink et al., 2009] reported high spatial overlap of synchronicity in the alpha band and fMRI activation in the prefrontal cortex of the left hemisphere. The authors interpreted their findings as an active inhibition of competing processes: the generation of novel, original ideas necessitates sematic selection “by selectively activating remote conceptual or semantic networks of the brain and inhibiting brain circuits that store similar semantic information” [Fink et al., 2009].
Limitations of the Study
Finally, there are several limitations of our study. We recruited healthy participants with an academic education and consequently a high‐level of intelligence. In general, a relationship was observed between the intelligence level and creativity [Jung et al., 2009]. All participants were further inexperienced in creative writing (except normal school education). Consequently, none of them could bring a well of domain‐specific knowledge or training in any writing techniques. No participants attained high CI scores (>130) in the creativity test [Schoppe, 1975], a level where high verbal creative performance might be expected. Our participants may have not displayed the exact prerequisites of creative writing. However, writing texts or stories is a common human ability, although the brain mechanisms might differ between inexperienced participants and trained ones.
To ensure participants' concentration during writing, the period for the single scanning tasks was quite small (1–2 min). This resulted in the criticism of insufficient scanning time for developing an entire creative story. The correlations between the CAT ratings (Amabile, 1996) and the CI, as well as the BOLD effects, failed significance.
Furthermore, we attempted to overcome the critical aspect of movement artifacts by a special arrangement of the scanning environment (e.g., special desk, double‐mirror system, and cushioning of arm and head). After scanning, we accomplished movement correction and remaining movement parameters were introduced as covariates. However, we cannot completely exclude the possibility that the BOLD response from the dominant motor and language performance might have interfered with the cognitive and creative brain processes, and even overshadowed the real creative moment. Finally, the complexity and the duration of our scanning tasks complicated the association of the observed brain areas with their functions during the creative writing process.
The length of the creative writing task was a compromise between the participants' need to express their ideas extensively and the time restrictions of an fMRI block design. Nearly 140 s was the minimum period judged necessary to write a new story, which some participants already considered as too short. During the creative writing task a person was developing ideas and writing them down continuously, presumably resulting in sustained neuronal activity throughout the whole task. This fact would result in superimposing BOLD curves, which can be approximated by a boxcar function over the entire task.
Additionally, we used the default high pass filter value of 128 s for all conditions, which might cut some responses from the longer lasting conditions. However, when comparing different high pass filters (128, 200, and 280 s) in two randomly selected participants, resulting fMRI‐maps were comparable while highest activated voxels differed within a range of one t value. Overall, a future study might investigate the same condition in several smaller time units.
CONCLUSION
In conclusion, by applying actual story writing in an fMRI‐scanner, we investigated the brain network during “creative writing.” We found that “brainstorming” involves fronto‐parieto‐temporal brain activity for generating novel and original ideas and composing the concept of the story. The observed premotor activity in “brainstorming” indicates the integrated preparation of the writing process. “Creative writing” combines handwriting processes and cognitive writing processes, which are predominantly associated with episodic memory, semantic integration, and a free associative and spontaneous cognitive text production. Verbal creativity involved in “creative writing” revealed a left fronto‐temporal network consisting of the left IFG (BA 45) and the left temporal pole (BA 38). Altogether, our results provide an overview on the brain network involved in creative writing processes by applying our new and successful real‐life creative writing paradigm with actual writing inside the scanner including acceptable and controlled methodologically restrictions. This study was a first step to investigate creative writing with neuroscientific methods and revealed restrictions and possibilities for future research.
For a more precise investigation of the topic, the process of creative writing might be further subdivided and focused on single subprocesses to establish the neural correlates of specific neurocognitive processes. Therefore, future research will be necessary to understand the neuronal secret of writing excellent and absorbing literature.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information Table 1: Brain regions activated during ‘Reading’
REFERENCES
- Amabile TM ( 1996): Creativity in Context. Boulder, CO: Westview Press; pp 19–79. [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF ( 2006): Role of frontal versus temporal cortex in verbal fluency as revealed by voxel‐based lesion symptom mapping. J Int Neuropsychol Soc 12: 896–900. [DOI] [PubMed] [Google Scholar]
- Bechtereva NP, Nagornova ZV ( 2007): Changes in EEG coherence during tests for nonverbal (figurative) creativity. Fiziol Cheloveka 33: 5–13. [PubMed] [Google Scholar]
- Bechtereva NP, Starchenko MG, Kliucharev VA, Vorob'ev VA, Pakhomov SV, Medvedev SV ( 2000): Study of the brain organization of creativity. Part II. Positron emission tomography. Fiziol Cheloveka 26: 12–18. [PubMed] [Google Scholar]
- Bechtereva NP, Korotkov AD, Pakhomov SV, Roudas MS, Starchenko MG, Medvedev SV ( 2004): PET study of brain maintenance of verbal creative activity. Int J Psychophysiol 53: 11–20. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Petsche H ( 2005): Drawing on mind's canvas: Differences in cortical integration patterns between artists and non‐artists. Hum Brain Mapp 26: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL ( 2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19: 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloedel JR ( 1992): Functional heterogeneity with structural homogeneity: How does the cerebellum operate? Behav Brain Sci 5: 666–678. [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL. 2008. The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR ( 2002): Dorsal anterior cingulate cortex: A role in reward‐based decision making. Proc Natl Acad Sci USA 99: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez‐Eakle RA, Graff‐Guerrero A, Garcia‐Reyna JC, Vaugier V, Cruz‐Fuentes C ( 2007): Cerebral blood flow associated with creative performance: A comparative study. Neuroimage 38: 519–528. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS ( 2000): Anterior cingulate and prefrontal cortex: Who's in control? Nat Neurosci 3: 421–423. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M ( 2003): Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7: 415–423. [DOI] [PubMed] [Google Scholar]
- de Souza LC, Volle E, Bertoux M, Czernecki V, Funkiewiez A, Allali G, Leroy B, Sarazin M, Habert MO, Dubois B, Kas A, Levy R ( 2010): Poor creativity in frontotemporal dementia: A window into the neural bases of the creative mind. Neuropsychologia 48: 3733–3742. [DOI] [PubMed] [Google Scholar]
- Dietrich A ( 2004): The cognitive neuroscience of creativity. Psychon Bull Rev 11: 1011–1026. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R ( 2010): A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull 136: 822–848. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Fink A, Benedek M, Grabner RH, Staudt B, Neubauer AC ( 2007): Creativity meets neuroscience: Experimental tasks for the neuroscientific study of creative thinking. Methods 42: 68–76. [DOI] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Benedek M, Reishofer G, Hauswirth V, Fally M, Neuper C, Ebner F, Neubauer AC ( 2009): The creative brain: Investigation of brain activity during creative problem solving by means of EEG and FMRI. Hum Brain Mapp 30: 734–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower LS, Hayes JR ( 1981): A cognitive process theory of writing. Coll Compos Commun 32: 365–387. [Google Scholar]
- Gernsbacher MA, Kaschak MP ( 2003): Neuroimaging studies of language production and comprehension. Annu Rev Psychol 54: 91–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Nadeau SE, Beversdorf DO ( 2003): Creative innovation: Possible brain mechanisms. Neurocase 9: 369–379. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Amunts K ( 2009): Different roles of cytoarchitectonic BA 44 and BA 45 in phonological and semantic verbal fluency as revealed by dynamic causal modelling. Neuroimage 48: 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard‐Jones PA, Blakemore SJ, Samuel EA, Summers IR, Claxton G ( 2005): Semantic divergence and creative story generation: An fMRI investigation. Brain Res Cogn Brain Res 25: 240–250. [DOI] [PubMed] [Google Scholar]
- Jung RE, Gasparovic C, Chavez RS, Flores RA, Smith SM, Caprihan A, Yeo RA ( 2009): Biochemical support for the “threshold” theory of creativity: A magnetic resonance spectroscopy study. J Neurosci 29: 5319–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung‐Beeman M ( 2005): Bilateral brain processes for comprehending natural language. Trends Cogn Sci 9: 512–518. [DOI] [PubMed] [Google Scholar]
- Jung‐Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel‐Liu S, Greenblatt R, Reber PJ, Kounios J ( 2004): Neural activity when people solve verbal problems with insight. PLoS Biol 2: E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanoda K, Yoshikawa K, Sugishita M ( 2001): A functional MRI study on the neural substrates for writing. Hum Brain Mapp 13: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S ( 2007): EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Res Rev 53: 63–88. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung‐Beeman M ( 2007): An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain Lang 100: 115–126. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN ( 2007): Wandering minds: The default network and stimulus‐independent thought. Science 315: 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Boone K, Cummings JL, Read SL, Mishkin F ( 2000): Functional correlates of musical and visual ability in frontotemporal dementia. Br J Psychiatry 176: 458–463. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 8: 102–107. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y ( 2007): The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain 130 ( Part 7): 1718–1731. [DOI] [PubMed] [Google Scholar]
- Ortheil H‐J ( 2005): Creative writing In: Schütz E, Bittkow S, Oels D, Porombka S, Wegmann T, editors. Das BuchMarktBuch; Der Literaturbetrieb in Grundbegriffen. Reinbek bei Hamburg: Rowolt Taschenbuch Verlag; pp 100–103. [Google Scholar]
- Petsche H, Kaplan S, von Stein A, Filz O ( 1997): The possible meaning of the upper and lower alpha and frequency ranges for cognitive and creative tasks. Int J Psychophysiol 26: 77–97. [DOI] [PubMed] [Google Scholar]
- Price CJ ( 2010): The anatomy of language: A review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 1191: 62–88. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Dettmers C, Buchel C, Kiebel S, Frackowiak RS, Weiller C ( 1999): A blueprint for movement: Functional and anatomical representations in the human motor system. J Neurosci 19: 8043–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux FE, Dufor O, Giussani C, Wamain Y, Draper L, Longcamp M, Demonet JF ( 2009): The graphemic/motor frontal area Exner's area revisited. Ann Neurol 66: 537–545. [DOI] [PubMed] [Google Scholar]
- Schoppe K‐J, editor ( 1975): Verbaler Kreativitäts‐Test (V‐K‐T) [Verbal Creativity Test]. Göttingen: Hogrefe. [Google Scholar]
- Shaywitz SE, Shaywitz BA ( 2008): Paying attention to reading: The neurobiology of reading and dyslexia. Dev Psychopathol 20: 1329–1349. [DOI] [PubMed] [Google Scholar]
- Starchenko MG, Bekhtereva NP, Pakhomov SV, Medvedev SV ( 2003): Study of the brain organization of creative thinking. Fiziol Cheloveka 29: 151–152. [PubMed] [Google Scholar]
- Sternberg RJ, Lubart TI ( 1999): The concept of creativity, prospects, and paradigms In: Sternberg RJ, editor. Handbook of Creativity. Cambridge: Cambridge University Press; pp 3–15. [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss D ( 1998): Clustering and switching on verbal fluency: The effects of focal frontal‐ and temporal‐lobe lesions. Neuropsychologia 36: 499–504. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Ward TB, Smith SM, Finke RA ( 1999): Creative cognition In: Sternberg RJ, editor. Handbook of Creativity. Cambridge: Cambridge University Press; pp 189–212. [Google Scholar]
- Whitney C, Weis S, Krings T, Huber W, Grossman M, Kircher T ( 2009): Task‐dependent modulations of prefrontal and hippocampal activity during intrinsic word production. J Cogn Neurosci 21: 697–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information Table 1: Brain regions activated during ‘Reading’


