Abstract
As a complex mental process, creativity requires the coordination of multiple brain regions. Previous pathological research on figural creativity has indicated that there is a mechanism by which the left side of the brain inhibits the activities of the right side of the brain during figural creative thinking, but this mechanism has not been directly demonstrated. In this study, we used functional magnetic resonance imaging (fMRI) to demonstrate the existence of this inhibitory mechanism in young adults (15 women, 11 men, mean age: 22 years) that were not artists. By making comparisons between brain activity during creative and uncreative tasks, we found increased activity in the left middle and inferior frontal lobe and strong decreases in activity in the right middle frontal lobe and the left inferior parietal lobe. As such, these data suggest that the left frontal lobe may inhibit the right hemisphere during figural creative thinking in normal people. Moreover, removal of this inhibition by practicing artistry or through specific damage to the left frontal lobe may facilitate the emergence of artistic creativity. Hum Brain Mapp 34:2724–2732, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: creativity, the left frontal lobe, functional magnetic resonance imaging, inhibitory mechanism
INTRODUCTION
Creativity is the ability to bring into being something that did not exist before. It is an important factor in the advancement of human civilization. Accordingly, it is of great value to explore the neural mechanisms that mediate this form of cognitive processing. Although researchers have attempted to address this issue using various techniques, and have made insightful findings [Fink et al., 2009; Jung et al., 2009a, 2010], to date, no consensus has been reached on how cortical areas interact or compete to achieve creativity. One assumption is that better interactions between cortical areas promote creativity. For example, using diffusion tensor imaging, Takeuchi et al. [2010] demonstrated that creativity is positively related to white matter strength in the bilateral prefrontal cortices, the body of the corpus callosum, and other areas. Interestingly, an alternative view speculates that relative isolation of specific cortical areas may lead them to function freely and thus facilitate the generation of novel and creative ideas. Pathological support from disconnected syndromes [Shenton et al., 2008] revealed that patients with bipolar disease and schizophrenia exhibited signs of high creativity [Strong et al., 2007]. Moreover, a study of normal subjects revealed that white matter integrity in the left frontal lobe was negatively related to creativity [Jung et al., 2010]. The fact that this body of work used a diverse set of creative domains may be one of reasons that have led to the discrepancies across these studies.
E.P. Torrance was a pioneer of creativity research that categorized creativity into figural, verbal and auditory creativity [Torrance, 1966]. In this study, we examined figural creativity, which has been considered to be a preferential function of the right hemisphere for several decades [Edwards, 1981]. Early beliefs regarding right hemispheric dominance in figural creativity were based on the left handedness of the great Renaissance artists Da Vinci, Michelangelo, and Raphael [Wright, 2007]. Decades ago, by studying split‐brain patients, Sperry [1974] surmised that the left hemisphere is specialized for language whereas the right hemisphere was specialized for vision, music, art, and other abilities. In recent years, the function of the right hemisphere in creativity was supported by brain imaging evidence [Carlsson et al., 2000]. Indeed, right hemispheric dominance in artists may be a genetic predisposition for creativity.
Interestingly, some brain diseases [Giles, 2004], especially left brain lesions [Seeley et al., 2008; Shamay‐Tsoory et al., 2011], seem to induce the sudden emergence of figural creativity. Fronto‐temporal lobe dementia (FTD) may be closely connected to the emergence of figural creativity [Miller et al., 2000, 1998; Miller and Hou, 2004]. A right brain lesion can cause inaccuracies in drawings, whereas a left brain lesion can enhance artistic creativity [Mendez, 2004]. A study of patients with Parkinson's disease showed that suppressive deep brain stimulation of the left hemisphere facilitated artistic production [Drago et al., 2009]. Similarly, Seeley et al. [2008] demonstrated increased figural creativity in one patient with severe degeneration of the left inferior frontal cortex. These intriguing findings suggest that left brain lesions may facilitate creative behaviors.
This paradoxical effect has been referred to as paradoxical functional facilitation (PFF) by Kapur [Kapur, 1996; Kapur et al., 2011]. Kapur proposed that PFF may be attributable to an inhibitory mechanism based on competition among different brain areas or compensation enhancement resulting from neural plasticity. Under the inhibitory mechanism, the right hemisphere's predominance in creative thinking may be inhibited by the left part of brain in normal people, but it could be disinhibited after damage to the left brain [Miller et al., 1996, 1998]. Under compensation enhancement, part of the right hemisphere may subsume the functions of damaged areas, causing more information flow in localized brain areas, and thus boosting the original right brain functions.
Although figural creativity researchers tend to refer to the inhibitory mechanism to explain pathological findings [Bogousslavsky, 2005; Mendez, 2004], thus far no one has directly proved its existence. Do the brains of normal people have this spontaneous left‐over‐right brain inhibitory mechanism during figural creative thinking? Further, it remains to be clarified whether the right hemisphere is inhibited by the left frontal lobe or the left posterior cortices. While some evidence favors a role for the left frontal lobe [Seeley et al., 2008] in inhibition, other reports illustrate that lesions in the left PC [Shamay‐Tsoory et al., 2011] or the left temporal lobe [Miller et al., 2000] are associated with elevated levels of originality. Therefore, a study in normal people that addresses these issues is warranted.
In this study, using functional magnetic resonance imaging (fMRI), we sought to investigate the left‐over‐right inhibitory mechanism during figural creative thinking in normal subjects who were not artists. To this end, we used the figural version of the Torrance Test of Creative Thinking (TTCT), a widely used psychometric test for creative behaviors [Kim, 2006], to evaluate visual imagination. The TTCT was developed by E. P. Torrance and is believed to reflect divergent thinking, a key element of creativity. The figural version of the TTCT requires the subjects to draw a novel picture based on simple line‐art images. To disassociate brain activities elicited by creative thinking from those elicited by normal (uncreative) thinking, we adopted two visual completion tasks. In one task, the subjects were encouraged to imagine a unique and originative picture based on the figure clue. In the other task, they were required to just construct a common pattern. Their drawings were assessed according to the TTCT's criteria to ensure creative thinking.
METHODS
Subjects
Twenty‐eight right‐handed (Edinburgh Handedness Inventory) volunteers (13 men, 15 women, 22 ± 1 years) from Sichuan University were recruited for the present study. All participants were students at the School of Medicine that did not have a history of neuropsychiatric disorders or brain damage (self‐report). None of the subjects had previously received professional training in art. Two of them were excluded because they forget several images they imagined in the scanner during the recall session, which they suggested may have been related to being tired. All of the participants completed informed consent forms before any experimental procedures were initiated, and they all had normal or corrected‐to‐normal vision.
Experimental Tasks
Visual clues were displayed on a white background, presented using the E‐prime 2.0 software package (http://www.pstnet.com/eprime.cfm), and projected on a screen behind the scanner. The subjects viewed the visual stimuli through a mirror attached to a head coil. Creative, uncreative, and fixation conditions were used in this paradigm. As shown in Figure 1, in the first picture (creative), the subjects had 24 s to use the clue to construct a novel and interesting image. In the second picture (uncreative), they had 12 s to figure out a common pattern, which did not have to be novel. In the cross picture (fixation), they were instructed to watch the cross without thinking anything (12 s). As soon as each condition ended, the subjects had 2 s to report whether they had completed the tasks by pressing a key. A black screen was used to indicate the start of a new loop. An experimental run consisted of 12 creative conditions, 12 uncreative conditions, and 12 fixation conditions. All subjects were trained using a demo program before the scans, and they confirmed that they fully understand the procedures. The subjects were asked to recall the images they produced after the scan.
Figure 1.
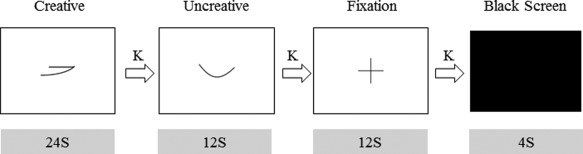
Experimental paradigm. The subjects were instructed to imagine novel and interesting pictures based on the given clues in the creative condition, to figure out a common pattern that did not have to be unique in the uncreative condition, and to look at the cross in the center of screen in the fixation condition. We presented 12 different pictures in both the creative and uncreative conditions. Creative thinking was assessed according to the TTCT criteria. (K, key press to report whether the tasks have been completed.)
Assessment of Creativity
All participants reported the productions they made during the MR scans after the scans were completed. The productions were judged according to the TTCT criteria. The original scoring system was based on fluency, originality, elaboration, abstractness of the titles, and resistance to premature closure. We did not ask the subjects to think about the details of the imagined pictures in the scanner because we wanted them to focus on the originality of their productions. Creating titles was performed after the scans. Therefore, we examined fluency, which indicates the meaningfulness of creations, originality, which describe the degree of novelty, and resistance to premature closure, which reflects the mind's openness. One psychologist who is familiar with the TTCTs scoring system was invited to judge the creations. Since our tasks were only part of the TTCT inventory, standard scores could not be converted. We used raw subscores to test the statistical differences between the two conditions using paired t‐tests.
fMRI Data Acquisition
Bold images were acquired using a Siemens 3.0T Trio scanner in the Huaxi MR Research Center (TR = 2,000 ms, TE = 30 ms, flip angle = 90°, 30 interleaved descending slices, voxel size=3.8 × 3.8 × 5.0 mm3). Anatomical reference images were acquired after the functional imaging and were collected using a 3D, GRE T1‐weighted sequence (TR = 1,900 ms, TE = 2.26 ms, flip angle = 9°, FOV = 256 × 256 mm2, voxel size = 1 × 1 × 1 mm3). The participants were instructed avoid moving their heads and to focus their eyes at the center of the screen during scanning. Swallowing was only allowed during presentation of a black screen. Ear plugs were used to reduce noise in the scanner. A total scan for one person took 40 min. A Vitamin E capsule was attached to the right forehead for orientation verification. Head motions were evaluated on an MR workstation as soon as the scans ended.
fMRI Data Analysis
The fMRI data were analyzed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm/). A total of 353 whole brain volumes were collected from each subject, with the first five volumes discarded. The remained images were corrected for slice timing with the middle slice as reference, realigned to remove head motion, normalized onto the echo planar imaging template image provided by SPM8, resampled with to a 3 × 3 × 3 mm3 voxel size, and then smoothed using an 8 mm full width at half maximum Gaussian kernel. The creative, uncreative, and fixation conditions were defined to construct the general linear model. A 1/128Hz high pass filter was applied to remove low frequency signal drifts.
We first explored the comparisons between creative–fixation, uncreative–fixation, and creative–uncreative. Then, to determine whether areas that were activated during the uncreative tasks were deactivated during the creative tasks, a brain mask was constructed from the activations of the uncreative tasks, using the SPM extension toolbox MarsBar (http://marsbar.sourceforge.net/). The mask was then used to constrain the analysis of the contrast uncreative–creative. Small Volume Correction (SVC, within 10 mm of the peak point) was applied to those areas of activation that did not survive a whole brain correction.
Region of Interest Analysis
Our primary interest was to examine whether the left prefrontal lobe or the posterior lobes inhibit the right hemisphere. However, group analyses showed no positive activations in the posterior lobes, even at very low thresholds. Thus, we only performed ROI analysis within the left (sphere, MNI, [−42 32 34], 10 mm) and right (sphere, MNI, [42 32 34], 10 mm) frontal lobes (Brodmann Area, BA9). Signal changes were computed in each condition for each subject, using MarsBar. Paired t‐tests were performed to examine the group signal differences between the creative and uncreative conditions.
RESULTS
Behavioral Results
Subjects generally got higher scores in the creative tasks. There isn't significant difference on the fluency index between the two conditions (P = 0.13). They got higher originality scores in the creative tasks (6.38 ± 2.53) than they did in the uncreative tasks (3.08 ± 0.69, P = 0.00).The scores on resistance to premature closure also showed statistical difference between the two conditions (creative: 13.69 ± 4.45; uncreative: 8.88 ± 2.70; P = 0.00). Hereby, we consider that our tasks had been properly completed during the scans.
Imaging Results
Both visual imagination tasks activated distributed brain areas, including the bilateral prefrontal lobes (PFC), temporal lobes, parietal lobes, occipital lobes, hippocampus, insula, and thalamus (P < 0.05, corrected, Fig. 2A). These are brain areas typically involved in figure construction.
Figure 2.
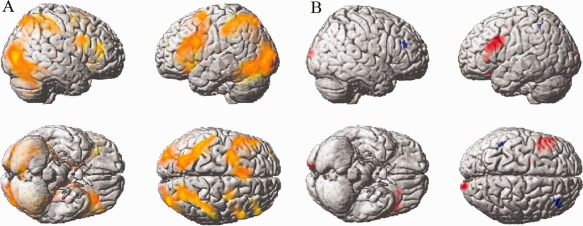
A: Both visual completion tasks activated similar brain regions (P < 0.05, corrected). Red: Creative > Fixation, Yellow: Uncreative > Fixation, Orange: Both. B: Different brain activations between the Creative and Uncreative conditions. Red indicates increased activity in the Creative condition, whereas blue indicates decreased activity in the Creative condition relative to the Uncreative condition.
One of our major interests was the comparison between the creative and uncreative conditions. In the creative–uncreative contrast (Fig. 2B), higher brain activities were found in the left middle prefrontal cortex (mPFC), the left inferior frontal gyrus (IFG), and the right middle occipital lobe (P < 0.05, SVC corrected, Table 1). As for the uncreative–creative contrast (masked with uncreative–fixation), we found robust clusters in the right middle frontal lobe (rmPFC) and the left inferior parietal cortex (P < 0.05, corrected).
Table 1.
Differential activations among the three conditions
| Cluster Size | Areas | Peak MNI coordinate | BA | |
|---|---|---|---|---|
| Creative>Uncreative (SVC, P < 0.05) | 16 | Left middle frontal gyrus | −24 26 −20 | 11,47 |
| Left inferior frontal gyrus | ||||
| 302 | Left middle frontal gyrus | −42 23 19 | 9,46 | |
| Left inferior frontal gyrus | ||||
| Left precentral gyrus | ||||
| 42 | Right middle occipital gyrus | 15 −100 13 | 18 | |
| Uncreative>Creativea (FWE, P < 0.05) | 15 | Right middle frontal gyrus | 39 38 25 | 10,46 |
| 6 | Left inferior parietal lobule | −45 −49 49 | 40 | |
| Uncreative>Fixation (FWE, P < 0.05) | 3,171(Right) 3,019(Left) | Left/right superior occipital gyrus | 33 −85 7 (Right) | 2,3,5,7,17,18, 19,37,39,40 |
| Left/right middle occipital gyrus | −51 −58 −14(Left) | |||
| Left/right inferior occipital gyrus | ||||
| Left/right lingual gyrus | ||||
| Left/right fusiform gyrus | ||||
| Left/right superior parietal lobule | ||||
| Left/right inferior parietal lobule | ||||
| Left/right supramarginal gyrus | ||||
| Left/right postcentral gyrus | ||||
| Left/right middle temporal gyrus | ||||
| Left/right inferior temporal gyrus | ||||
| Left/right calcarine gyrus | ||||
| 4,556 | Left/right caudate | −48 8 25 | 6,8,9,10,13,24,27, 32,38,44,45,46,47 | |
| Left/right inferior frontal gyrus | ||||
| Left/right middle frontal gyrus | ||||
| Left superior frontal gyrus | ||||
| Left/right hippocampus | ||||
| Left/right insula | ||||
| Left/right precentral gyrus | ||||
| Left/right thalamus |
A brain mask constructed from the result of uncreative–fixation was used to constrain this analysis.
SVC, small volume correction; FWE, family‐wise error rate correction.
ROI analysis revealed significant signal changes in the bilateral mPFC (BA9) when changing from uncreative tasks to creative thinking (Fig. 3). Specifically, the left mPFC exhibited elevated activations during creative thinking (paired t‐test, n = 26, P = 0.000), whereas the rmPFC showed decreased activity (paired t‐test, n = 26, P = 0.000).
Figure 3.
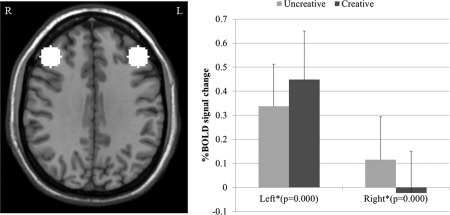
Signal changes from the Fixation to the imagination tasks in the left and right frontal lobes. The average intensity within the region of interest was computed for each subject and paired t‐tests were used to evaluate the signal difference between the Creative and Uncreative conditions.
DISCUSSION
This study was designed to investigate a possible brain inhibitory mechanism during figural creative thinking in subjects who were not artists. The results demonstrated that the left mPFC, the left IFG, and the right middle occipital lobe were significantly activated during creative tasks relative to uncreative tasks. Meanwhile, the right mPFC and the left inferior parietal lobe, which were involved in uncreative figure construction tasks, showed decreased activities in the creative condition. This brain activation pattern is in accordance with our hypotheses. Although the increase in the left frontal lobe and the decrease in the right frontal lobe may indicate left‐over‐right inhibitory control, other possibilities remain.
Activation and Deactivation in Frontal Lobes
We found that the left mPFC and IFG were more activated during creative tasks. The cluster in the left mPFC fell in BA9, which is a major area of the dorsolateral prefrontal cortex (dlPFC). Previous studies have suggested that the function of the left dlPFC is general executive control [MacDonald et al., 2000; Wood and Grafman, 2003]. Cognitive control is required when an input stimulus is ambiguous, or when there are possible conflicts among the different responses [Derrfuss et al., 2005]. As our tasks have all of these features, the activation of dlPFC is predictable. The left IFG is involved in semantic selection [Moss et al., 2005]. Although some early studies suggested that its role might be semantic retrieval [Demb et al., 1995], the findings of Thompson‐Schill indicated that this area was specifically involved in semantic selection and not retrieval [Thompson‐Schill et al., 1997]. This area is also important for language learning [Sakai et al., 2009], sentence comprehension [Friederici et al., 2003], and other processes. In the present study, the activation of the left IFG may reflect the involvement of verbal selection processing. Our subjects may have used a language‐thinking strategy to decide which product is more desirable.
The deactivated area, the rmPFC, is important for figural thinking. Compared with solving matching and analytic problems, figural thinking mainly induces activation in the rmPFC [Prabhakaran et al., 1997]. This area participates in mental rotation [Gauthier et al., 2002], object representation [Takahama et al., 2010], and other processes. Hypometabolism in this area can cause hallucinations in patients with Parkinson's disease [Sanchez‐Castaneda et al., 2010]. The rmPFC has also been frequently related to creativity [Kowatari et al., 2009]. Damage to this area may hamper creativity [Shamay‐Tsoory et al., 2011]. Although a recent review challenged this idea [Dietrich and Kanso, 2010], another meta‐analysis supported the importance of the right hemisphere for creative thinking [Mihov et al., 2010], regardless of the task design and creativity measure. Besides, studies on the domain of figural creativity are highly consistent [Bhattacharya and Petsche, 2005; Kowatari et al., 2009; Solso, 2001].
At least superficially, it seems that these activations and deactivations were just simple reflections of alterations between different ways of thinking. While the left part of brain has predominance in language thinking [Frost et al., 1999], the right part is more competent for figural thinking [Prabhakaran et al., 1997]. However, if the rmPFC was spontaneously involved in figure imagination even during uncreative tasks, it could certainly contribute to creative tasks. Although this area may be crucial for figural creativity, there is no immediately apparent reason for the deactivation of the rmPFC. A possible explanation here is that the dominance of the left hemisphere may lead to decreased activities in the right hemisphere.
The two brain hemispheres are anatomically connected via the corpus callosum and are typically in constant communication in normal subjects. However, they can work independently, as in patients with split brains [Gazzaniga, 1975]. In some aspects, they may cooperate to perceive emotions [Tamietto et al., 2006] and avoid false memories [Bergert, 2008]. In other aspects, they compete for dominance and inhibit the contralateral hemisphere [Daskalakis et al., 2002]. A remarkable finding by Sprague [1966] showed that cat's left superior colliculus inhibits the right hemisphere after the resections of the right visually responsive cortex. This inhibition can cause left visual loss that can be recovered by removing the left superior colliculus. A similar acoustic “Sprague effect” has also been found [Lomber et al., 2007]. Suppression on one side of the primary motor cortex (M1) can facilitate the contralateral M1, thus boosting functions of the ipsilateral hand movements [Kobayashi et al., 2009]. When one side predominates, the other is suppressed. It may also be understood as a dynamic balance.
Thus, in this study, the dominance of the left PFC in creative tasks may have led to the inhibition of the right PFC. However, it is important to note that the left hemisphere does not always predominate. A previous study suggested that artists mostly use the right PFC to deal with figures [Solso, 2001]. Their long‐term training facilitates the dominance of the right PFC, suppressing interference by the left PFC [Kowatari et al., 2009]. A similar process may occur for language, as language thinking is more frequently used in modern society and more people are left‐handed nowadays. It has been suggested that the left hemisphere's language predominance may interfere with the right hemisphere's specializations [Mendez, 2004].
This theory has found substantial support in past studies. A famous child savant called Nadia showed early extraordinary drawing skills, but showed artistic retrogression after she began to acquire verbal comprehension skills [Selfe, 1995]. A scientist called Anna showed a reverse pattern [Seeley et al., 2008]. It was found that her progressive dyslexia resulted in a sudden increase of figural creativity, which may be due to disinhibition. In comparison with dementia patients without musical or visual abilities, patients who displayed these abilities performed worse on verbal tasks [Miller et al., 2000]. A recent study showed that patients with semantic dementia performed better on visual search tasks than normal people [Viskontas et al., 2011]. Interestingly, Da Vinci and Picasso may have had dyslexia [Chakravarty, 2009]. These cases demonstrate that dynamic changes can occur in the relationships between figure ability and language ability.
Considering its positive activations, the left IFG is a possible source of this inhibitory control. The left IFG has been reported to have a role in inhibitory control [Swick and Ashley, 2008]. In this study, Swick and Ashley found that patients with damage to the left IFG exhibited greater error rates when performing Go/No‐Go tasks, a classic test for cognitive inhibition. Hamilton [Cris Hamilton and Martin, 2005] found loss of inhibitory control of language tasks in a patient with a left IFG lesion. Zhang and Li [2012] performed an ICA analysis to extract the independent components when subjects were doing response inhibition tasks. They found that the left IFG was linked to inhibitory control. Although the right IFG has also been related to response inhibition [Aron et al., 2003, 2004], some recent studies ascribed its role to attention [Hampshire et al., 2010; Zhang and Li, 2012]. Many suggest that different modules of inhibitory control may involve different brain areas [Chao et al., 2009; Ford et al., 2005]. In this study, the left IFG may have been activated because of its involvement in language thinking.
Posterior Cortices
Activities in the left parietal lobe were lower during the creative tasks relative to the uncreative tasks. The fronto‐parietal network is important for artistic creativities. A previous study has reported a negative correlation between figural creativity and activities in the parietal lobe [Kowatari et al., 2009]. They suggest that visuospatial processing may interfere with figural creativity. It has also been proposed that this area is deactivated to allow inhibition of attentional shifts toward task‐irrelevant stimuli during top‐down creative thinking [Berkowitz and Ansari, 2010].
The right occipital lobe (BA18) was activated during the creative tasks. Lying on the visual pathway, the occipital lobe is important for artistic creativity [Jung et al., 2010]. Petsche [1996] revealed using electroencephalography that acts of creative thinking were characterized by more coherent activity increases between occipital and frontopolar electrode sites. These activations suggest the presence of an early novel‐detection mechanism in the visual system or top‐down control of the construction of novel images.
Relation to Previous Studies: A General Scheme on Hemispheric Dominance and Figural Creativity
Previous studies have provided substantial support for this theory, although they all suggested disinhibition. Damage to the left frontal lobe may remove the source of inhibitory control, thus releasing the right hemisphere's potential in creative thinking [Miller et al., 1996; Seeley et al., 2008]. In professional artists, the long‐term training to make art may allow for right hemisphere dominance and even inhibition of the left hemisphere [Kowatari et al., 2009]. The findings of this study indicate the presence of this inhibitory mechanism in normal people. As shown in Figure 4, these data provide a general model of how brain organization impacts figural creativity. However, we wish to clarify that this model does not imply that anyone with left hemisphere damage can become an artist. Indeed, the model suggests that only those with artistic potentials mediated by the properties of the right hemisphere have this capacity.
Figure 4.
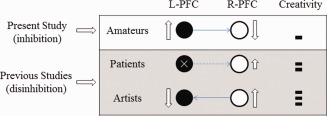
Inhibition and disinhibition of figural creativity. The data reported in the present study suggest that a left‐over‐right inhibitory mechanism exists in nonartists during figural creative thinking. Data presented in previous studies suggest that the release of inhibition can facilitate the emergence of creativity, as shown in patients who have suffered lesions in the left frontal lobe and in artists who have received long‐term artistic training in the use of the right hemisphere. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Limitations of the Study
In this study, we did not examine the subjects' intelligence. Some studies have indicated that there is a relationship between creativity and intelligence [Jung et al., 2009a; Takeuchi et al., 2011]. It is important to note, however, that a relationship between creativity and intelligence is debatable. In this regard, the strength of this relationship varies with different test methods for creativity and intelligence and different creativity domains. With the TTCT, Torrance found a minor correlation of r = 0.21 between verbal creativity and intelligence and a weak correlation between figural creativity and intelligence (r = 0.06) [Torrance, 1967]. Indeed, it is well known that artistic creativity can be achieved in subjects that are not particularly intelligent [Gordon, 2005]. Therefore, we consider that this relationship is very low. In addition, we demonstrated generated data that are consistent with the left‐over‐right inhibitory mechanism. However, a causal link cannot be established using the current experimental design and data analyses. It may be valuable to address this issue in future studies because it is not only important for creativity but also for the brain organization that underlies complex cognitive tasks.
CONCLUSIONS
In this study, we found a possible brain inhibitory mechanism in normal people that is engaged by figural creative tasks. Together with previous studies, this phenomenon has found support in various contexts. We suggest that this inhibitory control may be the result of hemispheric competition. As we do not yet know the mechanism underlying this inhibitory control, further studies are warranted.
These two authors contributed equally to this work.
REFERENCES
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003): Stop‐signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6:115–116. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177. [DOI] [PubMed] [Google Scholar]
- Bergert S (2008): The interhemispheric transfer of visual stimuli and its relation to functional cerebral asymmetries. Available at: http://www-brs.ub.ruhr-uni-bochum.de/netahtml/HSS/Diss/BergertSusanne/diss.pdf.
- Berkowitz AL, Ansari D (2010): Expertise‐related deactivation of the right temporoparietal junction during musical improvisation. Neuroimage 49:712–719. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Petsche H (2005): Drawing on mind's canvas: Differences in cortical integration patterns between artists and non‐artists. Hum Brain Mapp 26:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogousslavsky J (2005): Artistic creativity, style and brain disorders. Eur Neurol 54:103–111. [DOI] [PubMed] [Google Scholar]
- Carlsson I, Wendt PE, Risberg J (2000): On the neurobiology of creativity. Differences in frontal activity between high and low creative subjects. Neuropsychologia 38:873–885. [DOI] [PubMed] [Google Scholar]
- Chakravarty A (2009): Artistic talent in dyslexia—A hypothesis. Med Hypotheses 73:569–571. [DOI] [PubMed] [Google Scholar]
- Chao H, Luo X, Chang J, Li C (2009): Activation of the pre‐supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time—An intra‐subject analysis. BMC Neurosci 10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cris Hamilton A, Martin RC (2005): Dissociations among tasks involving inhibition: A single‐case study. Cogn Affective Behav Neurosci 5:1–13. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R (2002): The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol 543:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE (1995): Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. J Neurosci 15:5870–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, Von Cramon DY (2005): Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Hum Brain Mapp 25:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Kanso R (2010): A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychological Bull 136:822. [DOI] [PubMed] [Google Scholar]
- Drago V, Foster PS, Skidmore FM, Heilman KM (2009): Creativity in Parkinson's disease as a function of right versus left hemibody onset. J Neurological Sci 276( 1‐2):179–183. [DOI] [PubMed] [Google Scholar]
- Edwards B (1981): Drawing on the right side of the brain. Plastic Reconstructive Surg 68:452. [Google Scholar]
- Fink A, Grabner RH, Benedek M, Reishofer G, Hauswirth V, Fally M, Neuper C, Ebner F, Neubauer AC (2009): The creative brain: investigation of brain activity during creative problem solving by means of EEG and FMRI. Hum Brain Mapp 30:734–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MRG, Everling S (2005): Neural processes associated with antisaccade task performance investigated with event‐related FMRI. J Neurophysiol 94:429. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Rüschemeyer SA, Hahne A, Fiebach CJ (2003): The role of left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cerebral Cortex 13:170. [DOI] [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW (1999): Language processing is strongly left lateralized in both sexes. Brain 122:199. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Hayward WG, Tarr MJ, Anderson AW, Skudlarski P, Gore JC (2002): BOLD activity during mental rotation and viewpoint‐dependent object recognition. Neuron 34:161–171. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. (1975): Review of the split brain. J Neurol 209:75–79. [DOI] [PubMed] [Google Scholar]
- Giles J (2004): Neuroscience: Change of mind. Nature 430:14–14. [DOI] [PubMed] [Google Scholar]
- Gordon N (2005): Unexpected development of artistic talents. Postgraduate Med J 81:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM (2010): The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage 50:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Gasparovic C, Chavez RS, Flores RA, Smith SM, Caprihan A, Yeo RA (2009a): Biochemical support for the “threshold” theory of creativity: A magnetic resonance spectroscopy study. J Neurosci 29:5319–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Segall JM, Jeremy Bockholt H, Flores RA, Smith SM, Chavez RS, Haier RJ (2010): Neuroanatomy of creativity. Hum Brain Mapp 31:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Grazioplene R, Caprihan A, Chavez RS, Haier RJ (2010): White matter integrity, creativity, and psychopathology: Disentangling constructs with diffusion tensor imaging. PLoS One 5:e9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N (1996): Paradoxical functional facilitation in brain‐behaviour research. Brain 119:1775. [DOI] [PubMed] [Google Scholar]
- Kapur N, Pascual‐Leone A, Ramachandran V (2011):The paradoxical brain: Cambridge Univ Pr. [Google Scholar]
- Kim KH (2006): Can we trust creativity tests? A review of the torrance tests of creative thinking (TTCT). Creativity Res J 18:3–14. [Google Scholar]
- Kobayashi M, Théoret H, Pascual‐Leone A (2009): Suppression of ipsilateral motor cortex facilitates motor skill learning. Eur J Neurosci 29:833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowatari Y, Hee Lee S, Yamamura H, Nagamori Y, Levy P, Yamane S, Yamamoto M (2009): Neural networks involved in artistic creativity. Hum Brain Mapp 30:1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S, Sprague JM (2007): Restoration of acoustic orienting into a cortically deaf hemifield by reversible deactivation of the contralesional superior colliculus: The acoustic “sprague effect”. J Neurophysiol 97:979. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288:1835. [DOI] [PubMed] [Google Scholar]
- Mendez MF (2004): Dementia as a window to the neurology of art. Med Hypotheses 63:1–7. [DOI] [PubMed] [Google Scholar]
- Mihov KM, Denzler M, Förster J (2010): Hemispheric specialization and creative thinking: A meta‐analytic review of lateralization of creativity. Brain Cogn 72:442–448. [DOI] [PubMed] [Google Scholar]
- Miller BL, Boone K, Cummings JL, Read SL, Mishkin F (2000): Functional correlates of musical and visual ability in frontotemporal dementia. Br J Psychiatry 176:458–463. [DOI] [PubMed] [Google Scholar]
- Miller BL, Hou CE (2004): Portraits of artists—Emergence of visual creativity in dementia. Arch Neurol 61:842–844. [DOI] [PubMed] [Google Scholar]
- Miller BL, Ponton M, Benson DF, Cummings JL, Mena I (1996): Enhanced artistic creativity with temporal lobe degeneration. Lancet 348:1744–1745. [DOI] [PubMed] [Google Scholar]
- Miller BL, Cummings J, Mishkin F, Boone K, Prince F, Ponton M, Cotman C (1998): Emergence of artistic talent in frontotemporal dementia. Neurology 51:978–982. [DOI] [PubMed] [Google Scholar]
- Moss H, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, Tyler L (2005): Selecting among competing alternatives: Selection and retrieval in the left inferior frontal gyrus. Cerebral Cortex 15:1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsche H (1996): Approaches to verbal, visual and musical creativity by EEG coherence analysis. Int J Psychophysiol 24( 1‐2):145–159. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JAL, Desmond JE, Glover GH, Gabrieli JDE (1997): Neural substrates of fluid reasoning: An fMRI study of neocortical activation during performance of the Raven's Progressive Matrices Test. Cogn Psychol 33:43–63. [DOI] [PubMed] [Google Scholar]
- Sakai KL, Nauchi A, Tatsuno Y, Hirano K, Muraishi Y, Kimura M, Bostwick M, Yusa N (2009): Distinct roles of left inferior frontal regions that explain individual differences in second language acquisition. Hum Brain Mapp 30:2440–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Castaneda C, Rene R, Ramirez‐Ruiz B, Campdelacreu J, Gascon J, Falcon C, Calopa M, Jauma S, Juncadella M, Junque C (2010): Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson's disease with dementia. Movement Disorders 25:615–622. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno‐Tempini ML, Foti D, Mackenzie IR, Miller BL (2008): Unravelling Bolero: Progressive aphasia, transmodal creativity and the right posterior neocortex. Brain 131( Part 1):39–49. [DOI] [PubMed] [Google Scholar]
- Selfe L (1995): Nadia reconsidered In: Golomb C, editor.The development of artistically gifted children: selected case studies.Hillsdale:Lawrence Erlbaum; p.197‐237. [Google Scholar]
- Shamay‐Tsoory S, Adler N, Aharon‐Peretz J, Perry D, Mayseless N (2011): The origins of originality: The neural bases of creative thinking and originality. Neuropsychologia 49:178–185. [DOI] [PubMed] [Google Scholar]
- Shenton M, Kawashima T, Nakamura M, Bouix S, Salisbury D, Westin C, McCarley R, Kubicki M (2008): Uncinate fasciculus and cingulum bundle findings in first episode schizophrenia and first episode bipolar disorder: A diffusion tensor imaging study. Eur Psychiatry 23:S43–S43. [Google Scholar]
- Solso RL (2001): Brain activities in a skilled versus a novice artist: An fMRI study. Leonardo 34:31–34. [Google Scholar]
- Sperry R (1974): Lateral specialization in the surgically separated hemispheres In: Worden F, editor.The Neurosciences: 3rd Study Program.New York:Rockefeller University Press; p5. [Google Scholar]
- Sprague JM (1966): Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science 153:1544. [DOI] [PubMed] [Google Scholar]
- Strong CM, Nowakowska C, Santosa CM, Wang PW, Kraemer HC, Ketter TA (2007): Temperament‐creativity relationships in mood disorder patients, healthy controls and highly creative individuals. J Affective Disorders 100( 1‐3):41–48. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V (2008): Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci 9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama S, Miyauchi S, Saiki J (2010): Neural basis for dynamic updating of object representation in visual working memory. Neuroimage 49:3394–3403. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R (2010): White matter structures associated with creativity: Evidence from diffusion tensor imaging. Neuroimage 51:11–18. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2011): Cerebral blood flow during rest associates with general intelligence and creativity. PLoS One 6:e25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M, Latini Corazzini L, de Gelder B, Geminiani G (2006): Functional asymmetry and interhemispheric cooperation in the perception of emotions from facial expressions. Exp Brain Res 171:389–404. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ (1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA 94:14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance E (1967): The Minnesota studies of creative behavior: National and international extensions. J Creat Behav 1:137–154. [Google Scholar]
- Torrance EP (1966): Nurture of creative talents. Theory Practice 5:167–173. [Google Scholar]
- Viskontas IV, Boxer AL, Fesenko J, Matlin A, Heuer HW, Miller JM, Bruce L (2011): Visual search patterns in semantic dementia show paradoxical facilitation of binding processes. Neuropsychologia 49:468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN, Grafman J (2003): Human prefrontal cortex: Processing and representational perspectives. Nat Revi Neurosci 4:139–147. [DOI] [PubMed] [Google Scholar]
- Wright E (2007):Left‐handed History of the World. New York: Barnes & Noble. p 88. [Google Scholar]
- Zhang S, Li CR (2012): Functional networks for cognitive control in a stop signal task: Independent component analysis. Hum Brain Mapp 33:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]


