Abstract
As it is the case in brainstorming, each single idea a person generates to a specific problem may stimulate new ideas or solutions in others. In this fMRI study, we investigate the effects of cognitive stimulation via the exposure to other people's ideas on the originality of generated ideas. Participants are requested to generate alternative uses of conventional everyday objects subsequent to a short cognitive stimulation intervention in which they are exposed to other ideas, which were either common or highly original. In a control condition, meaningless pseudowords are shown. Results suggest that cognitive stimulation via common or moderately creative ideas was effective in improving creativity. At the neurophysiological level, temporo‐parietal brain regions (primarily right‐hemispheric) turned out to be particularly sensitive to cognitive stimulation, possibly indicating that cognitive stimulation via relevant memory cues results in a state of heightened focused attention to memory that facilitates efficient retrieval and recombination of existing knowledge. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: originality, creative cognition, cognitive stimulation, fMRI
INTRODUCTION
Creativity, commonly defined as the ability to produce work that is both novel (original, unique) and useful within a certain social context [e.g., Flaherty,2005; Stein,1953; Sternberg and Lubart,1996], has beneficial effects in a variety of areas of our everyday life. It appears to be crucial in culture, science and education just as in the economical or industrial domain. Even though the striking role of creativity in these areas appears to be out of debate, our understanding of this topic has long been grounded solely on anecdotal reports. In fact, unlike other mental ability constructs such as intelligence, creativity has long been viewed as a “difficult” trait and empirical studies on this topic were almost completely lacking. Guilford's seminal address at the American Psychological Association has brought about resurgence in this field of research. He specified several characteristics of creative people that can be measured by means of psychometric tests. Guilford [1950] refers to concepts such as ideational fluency, novelty, or the ability to think flexibly (i.e., the ability to produce different types of ideas) as being characteristic for creative people. Stimulated by Guilford's work, many creativity measures have been developed and empirically tested, which has in turn stimulated research activities in this nascent field.
Neuroscientific studies in the field of creativity aim at investigating the way the brain works when engaged in the performance of different creativity tasks. In this particular context, it appears to be worthy to note that relevant research in this field does not only investigate potential brain mechanisms underlying divergent thinking, but also focuses on insightful problem solving, visual imagery or performing arts, which may be seen as important facets of the complex construct of creativity as well. In the last decade, considerable progress has been achieved in this field. Recent fMRI studies reveal reliable evidence that frontal brain regions are critically involved in a variety of creativity‐related demands [for recent reviews see Arden et al.,2010; Dietrich and Kanso,2010]. Goel and Vartanian [2005], for instance, report evidence that classic creative problem solving tasks exhibited activation (relative to rather convergent baseline measurements) in the left dorsal lateral as well as in the right ventral lateral prefrontal cortex. Similarly, Carlsson et al. [2000] demonstrated that participants displayed a higher level of prefrontal brain activation when they were required to name as many different uses of bricks (i.e., Alternative Uses task, AU; known as a fairly good measure of creativity) as compared to the performance of the more intelligence‐related verbal fluency task (naming words that begin with a given letter). The particular role of prefrontal brain regions in creative cognition has been also confirmed by studies, which found that highly creative individuals exhibit stronger prefrontal brain activation during creative cognition than less creative people [e.g., Chávez‐Eakle et al.,2007; Gibson et al.,2009; see also Heilman et al.,2003].
Despite of the well‐documented involvement of frontal brain regions in creative cognition, there are also some recent studies, which emphasize the prominent role of posterior parietal brain regions in this mental ability domain [e.g., Bechtereva et al.,2004]. Howard‐Jones et al. [2005] had their participants generate creative and uncreative stories and observed that creative (as compared to uncreative) story generation was associated with stronger bilateral frontal activation and lower brain activity in the right inferior parietal lobe and similarly, Kowatari et al. [2009] report inverse correlations between creativity and brain activity in bilateral parietal brain regions while participants were designing new pens. In another creativity domain, Berkowitz and Ansari [2010] found that musicians deactivated the right temporo‐parietal junction (including the angular gyrus) during musical improvisation.
Similar evidence has been observed in recent fMRI studies on possible brain mechanisms underlying creative idea generation [Fink et al.,2009a,2010]. Idea generation is conceptualized as a cognitive process involving “both the retrieval of existing knowledge from memory and the combination of various aspects of existing knowledge into novel ideas” [Paulus and Brown,2007, p.252]. In our fMRI experiments, we presented everyday objects (such as “tin” or “umbrella”) and participants were instructed to generate as creative or original uses of the given objects, which had to be verbalized by the participants subsequent to a so‐called idea generation phase. The oral responses were recorded by the experimenter and rated with respect to their originality subsequent to the fMRI recording session. This task was contrasted to a more “convergent” task (i.e., Object Characteristics task, OC) requiring participants to name typical attributes of conventional objects (such as “shoes” or a “coat hook”). Perhaps the most important finding of our studies was that the generation of original ideas, in contrast to the production of typical object characteristics, was associated with more activation in the (anterior) supramarginal gyrus and stronger widespread deactivation in the inferior parietal cortex (around the angular gyri), especially in the right hemisphere.
In a more recent study of our laboratory [Fink et al.,2010] we moved a step further by addressing the research question as to how creative idea generation can be improved effectively by means of short‐term creativity interventions and whether any training effects are also reflected at the level of the brain. Participants were instructed to generate creative ideas to given verbal problems and in one experimental condition, they were cognitively stimulated via the exposure to ideas produced by other people. As it is the case in classic group‐based brainstorming techniques [Osborn,1957], each single idea or solution a person generates to a specific problem may stimulate new ideas or solutions in others. Relevant literature from the behavioral or cognitive creativity research tradition suggests that creative performance increases as a result of such idea sharing or idea exchange processes [Dugosh and Paulus,2005; Dugosh et al.,2000; Paulus and Brown,2007; Paulus and Nijstad,2003]. The findings of the Fink et al. [2010] study reveal performance increases because of the employed creativity interventions, which were also apparent at the level of the brain. The employed interventions recruit a complex and widespread neural network primarily involving posterior brain regions, which are known as important components of the neural network specialized for semantic information processing.
This fMRI study was designed to investigate the neurophysiological effects of cognitive stimulation on creative idea generation by stimulating participants with ideas of varying originality. Participants were requested to generate alternative uses of conventional everyday objects (AU task) subsequent to a short cognitive stimulation intervention in which they were confronted with ideas of other people, as they were obtained in a pre‐experimental pilot study. Similarly to Dugosh and Paulus [2005] we stimulated our participants by common or moderately creative (STIM common) and highly original ideas (STIM original). In a control condition, meaningless pseudowords were shown. In each experimental condition, participants had to respond as creatively and as originally as possible to the presented stimulus words. On the basis of existing behavioral research [e.g., Dugosh and Paulus,2005] we expect better performance when participants are cognitively stimulated via the exposure to other people's ideas (as opposed to the exposure to pseudowords). In addition, more importantly, these performance increases should be reflected in changes of functional patterns of brain activity. Based on the findings reported in Fink et al. [2009a,2010], we might assume temporo‐parietal brain regions (primarily in the right hemisphere) as being particularly sensitive to cognitive stimulation.
METHOD
Participants
Thirty‐two adult students (14 males and 18 females) participated in this fMRI study. Six participants had to be excluded from further analyses due to large movement artifacts during fMRI recording; two participants abandoned the fMRI scans because they felt claustrophobic. Only participants who moved less than 3 mm in any direction over the entire functional imaging session were included in the analysis. The final sample comprised 24 participants (10 males and 14 females) in the age range between 21 and 30 years (M = 24.9, SD = 2.9). All participants were healthy, right‐handed, normal/corrected‐to‐normal vision, gave written informed consent, and paid for their participation in the fMRI test session. The study was approved by the local ethics committee of the Medical University of Graz, Austria.
Materials and Procedure
This fMRI study was designed to investigate the effects of cognitive stimulation (via the exposure to other people's ideas) on creative idea generation and their neural correlates. Prior to the fMRI experiment, pilot studies were run to construct the stimulus materials. In these pre‐experimental pilot tests, we requested two female and two male participants, who did not take part in the fMRI experiments, to generate common and highly original uses of 68 given conventional everyday objects (such as “tin,” “pen,” or “umbrella”). Subsequently, the collected responses were evaluated by five raters with respect to their originality (intra class correlation ICC = 0.90) and, on the basis of this, categorized into common versus higher original responses by the experimenter (separately for each stimulus word). The final set of items consisted of 60 stimuli words (i.e., conventional everyday objects), for which two common and two highly original responses were available each.
In the subsequent fMRI experiment, participants worked on these stimulus words in three experimental conditions. (1) In the STIM original condition, the stimulus word appeared on the screen conjointly with two highly original example answers (as they were obtained in the pre‐experimental pilot test). (2) In the STIM common condition, the stimulus word was presented in combination with two common or moderately original example responses. Finally (3), in the control condition the stimulus word was accompanied by the presence of two meaningless words (pseudowords), which were of similar length than those in both STIM conditions. In each condition (STIM original, STIM moderate, and control), 20 stimulus words were presented, resulting in a total number of 60 trials.
Each trial started with the presentation of a fixation cross for a time‐period of 12 s. Then, as shown in Figure 1, the stimulus word appeared for 4 s, conjointly with two example answers, which were either highly original (STIM original), common or moderately original (STIM common), or meaningless words (control). In each condition, participants were instructed to respond as creatively and as originally as possible. After the presentation of the stimulus word, a white‐colored interrogation mark was displayed and remained on the screen for a time‐period of 12 s. During this time, participants had to think of possible original uses for the object denoted by the stimulus word. However, they were asked not to speak aloud. Then the interrogation mark changed its color from white into green and participants were asked to articulate their most original idea. For this, they had 3 s (see Fig. 1). The generated oral responses were recorded and later transcribed for further analyses. It should be noted that in five of the 24 participants technical problems did not allow the recording of the oral responses, leaving the responses of 19 participants for behavioral analyses (the fMRI data were analyzed for the total sample of 24 participants). At the end of each trial, participants were requested to evaluate the originality of their idea by pressing either the “creative” or “uncreative” button on the response console. The presentation of trials and the assignment of conditions were randomized. The total time of the task presentation was 36 min and the entire MRI session took about 45 min.
Figure 1.
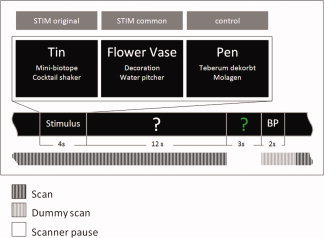
Overview of experimental design and measurement intervals. Each trial started with the presentation of a fixation cross (12 s), followed by the presentation of the stimulus word (i.e., conventional everyday objects such as “tin,” “flower vase,” or “pen” for a time period of 4 s, conjointly with two example answers, which were either highly original (STIM original), common or moderately original (STIM common), or meaningless words (control condition). In each condition, participants were instructed to respond as creatively and as originally as possible. Subsequently, a white‐colored interrogation mark appeared on the screen (for 12 s). During this so‐called idea generation interval participants had to think of possible responses to the given stimulus word and they were requested not to speak. Afterwards, the interrogation mark changed its color from white into green, signaling the participant to articulate his or her ideas (3 s). At the end of each trial, participants were requested to evaluate the originality of the idea they generated in this trial by pressing either the “creative” or “uncreative” button on the response console (3 s; button press, BP). Within the scan‐free time (Response) the oral responses were recorded and then transcribed by the experimenter for further analyses. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
MRI Data Acquisition
Imaging was performed on a 3.0‐T Tim Trio system (Siemens Medical Systems, Erlangen, Germany) using a 32‐channel head coil. BOLD‐sensitive T2*‐weighted functional images were acquired using a single shot gradient‐echo EPI pulse sequence (TR = 1,750 ms, TE = 25 ms, flip angle = 90°, slice thickness = 3 mm, matrix size 64 × 64, FOV = 192 mm, 30 slices per volume). To record the verbal response of the participants the scanner was interrupted for the 3 s interval reserved for the oral response that followed the idea generation interval of each trial. The first two volumes after each scanner pause were discarded to allow for T1 equilibration effects, resulting in 872 volumes. Field maps were created from a double echo gradient‐echo pulse sequence (31 slices, TE1 = 5.19 ms, TE2 = 7.65 ms, TR = 400 ms, slice gap = 0.9 mm, slice thickness = 3 mm, matrix size = 64 × 64, FOV = 192 mm). Visual stimuli were presented using the Software Presentation (Neurobehavioral Systems, Albany, CA).
Behavioral Data Analysis
Originality of creative idea generation during the fMRI experiment was assessed by means of self‐ratings (within the scanner after each trial) and external ratings (outside the scanner by six independent raters). For the external ratings [cf. Amabile,1982], 3 females and 3 males were instructed to evaluate each single idea of a participant on a five‐point rating scale ranging from 1 (“highly original”) to 5 (“not original at all”). Subsequently, the ratings were averaged over all items of a condition, so that one originality measure was available for each condition and participant. Inter‐rater agreement was satisfactory (intra‐class correlation coefficients for the three conditions: STIM original: 0.80, STIM common: 0.74, and control: 0.79). For greater clarity, the scale of the originality ratings was inverted for all further analyses, with higher scores (maximum of 5) now indicating higher originality.
The self‐ratings and the external ratings, averaged for each participant and condition, were analyzed by means of ANOVAs for repeated measures with the within‐subjects factor condition (STIM original, STIM common, and control).
fMRI Data Analysis
Functional MRI data analysis was performed using SPM8 software (Wellcome Department of Imaging Neuroscience, London, UK). Preprocessing steps included fieldmap correction, motion correction, slice time acquisition correction, and spatial normalization into the standard space (Montreal Neurological Institute). Finally, the functional data were smoothed using a Gaussian filter of 10‐mm. A high‐pass filter with a cutoff frequency of 1/128 Hz was employed to remove low frequency drifts.
Each train of scans (for each trial, see Fig. 1) was treated as a single run. The onset of each stimulus presentation was convolved with the canonical form of the hemodynamic response function. Linear t‐contrasts were computed. The contrast images were entered into a random effects analysis (one‐sample t‐test). The following contrasts were analyzed: STIM common versus STIM original, STIM common versus control and STIM original versus control. All reported activations are significant at P < 0.0001 (uncorrected); only activation clusters exceeding a spatial extent threshold of 30 voxels are presented.
RESULTS
Behavioral Results
The three experimental conditions induced ideas of varying originality (quantified by means of external ratings), as it was reflected in a significant ANOVA effect of condition, F(2, 36) = 4.58, P < 0.05, η2 = 0.20. Post hoc comparisons by means of the Tukey‐HSD test reveal significant mean differences only between STIM common and the control condition (P < 0.05), indicating higher originality in STIM common (M = 3.08) than during the exposure to meaningless words (M = 2.94, see Fig. 2). A similar pattern of results was observed with respect to the self‐rated originality of ideas, but this effect failed to reach statistical significance in the ANOVA (P > 0.05).
Figure 2.

Externally rated originality of ideas generated during the experimental conditions (STIM original, STIM common, and control). Higher scores correspond to higher originality. * P < 0.05.
fMRI Results
Stimulating participants via the exposure to common ideas (STIM common)—which has proven to significantly enhance the originality of generated ideas—was associated with stronger activation (relative to control) in a left‐lateralized neural network involving middle temporal and superior frontal brain regions (see Fig. 3; see also Table I for the results of the comparisons between the experimental conditions). The reverse contrast (control > STIM common) revealed significant activation clusters in regions of the right inferior temporal gyrus, the superior parietal gyrus, and bilaterally in the precuneus (see Fig. 4).
Figure 3.
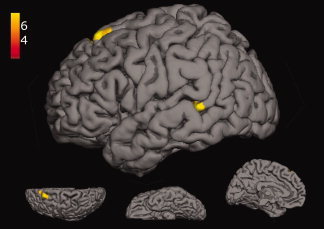
Significant activation clusters for the contrast between STIM common > control (yellow); lateral, superior, inferior, and medial view. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table I.
Overview of significantly activated clusters (voxelwise P < 0.0001 uncorrected, k > 30) for the contrasts between the experimental conditions STIM original, STIM common, and control
| Contrast | MNI peak coordinate | k | t | Brain area |
|---|---|---|---|---|
| Comm > Contr | −54, −40, 1 | 33 | 5.696 | L mid temporal G |
| −15, 32, 58 | 50 | 5.490 | L sup frontal G | |
| Contr > Comm | 54, −58, −11 | 75 | 6.179 | R inf temporal G |
| 30, −58, 61 | 231 | 6.853 | R sup parietal G, R/L Precuneus | |
| Orig > Contr | −57 −55 13 | 475 | 7.848 | L mid/sup temporal G |
| Contr > Orig | 63, −31, 28 | 61 | 6.481 | R supramarginal G, R inf parietal G |
| −6, −76, 43 | 244 | 8.416 | L/R Precuneus, R sup parietal G | |
| Orig > Comm | −27, −13, −14 | 75 | 5.314 | L Hippocampus, L Parahippocampal G |
| −48, −64, −14 | 161 | 7.132 | L inf occipital G, L inf temporal G, L fusiform G | |
| −48, −61, 10 | 43 | 4.994 | L mid temporal G | |
| Comm > Orig | – | – | – | – |
G = gyrus; L = left hemisphere; R = right hemisphere; inf = inferior; sup = superior; mid = middle; Comm = STIM common; Contr = Control; Orig = STIM original.
Figure 4.
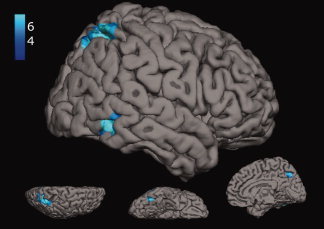
Significant activation clusters for the contrast between control > STIM common (blue); lateral, superior, inferior, and medial view. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Similarly, when participants were stimulated with highly original ideas, they exhibited comparatively strong activation (as contrasted to the control condition) in left‐hemispheric regions of the middle and superior temporal gyri. Moreover, the control condition, relative to STIM original, was accompanied with higher activation in the precuneus (bilaterally) and in several parietal brain regions of the right hemisphere such as the supramarginal gyrus and the superior parietal lobe (see Table I).
Finally, in contrasting both stimulation conditions (original vs. common) to each other, cognitive stimulation via the exposure to original (vs. common) ideas elicited stronger activation in the left‐hemispheric hippocampus and in the parahippocampal gyrus (see Table I). Significant activation clusters were also observed in a widespread left‐hemispheric network involving regions of the inferior temporal cortex, fusiform gyrus, mid temporal, and inferior occipital brain regions.
DISCUSSION
Analyses of performance data reveal that participants tend to be more creative when they are cognitively stimulated via the exposure to other people's ideas. Interestingly, this only applies for the stimulation with common or moderately original ideas. Dugosh and Paulus [2005] also report evidence that shared or common information may have a greater associative strength. According to the authors, common ideas are often accompanied by positive affective reactions and are (as opposed to unique information) more likely to be discussed and remembered, thereby increasing their associative strength [cf. Dugosh and Paulus,2005, p. 319). In a similar vein, Paulus and Brown [2007] refer to behavioral findings whereupon the exposure to other people's ideas may also have distracting or inhibiting effects on the generation of ideas [cf. also Nijstad and Stroebe,2006]. For instance, when a person is exposed to an idea to which she or he knows little about, or to an idea that has no relation to the semantic network of this person (as it was possibly the case in the STIM original condition), idea generation would be less effective. At the neurophysiological level, the exposure to original (as opposed to common) ideas was accompanied by hippocampal activation, along with activations in a complex and widespread left‐hemispheric network involving regions of the inferior temporal cortex, fusiform gyrus, mid temporal, and inferior occipital brain regions (see Table I). This result pattern may reflect the comparatively high complexity or demands of this condition, which was manifested in the recruitment of a broad neural network, specialized for memory and speech. The observed hippocampal activation associated with STIM original might possibly reflect the effortful attempts to generate ideas that can keep up with those presented in the stimulation phase. It appears to be less likely that the presented words exceeded the participants' vocabulary level or that the participants did not know how the example answers should relate to a stimulus word (the inter‐rater‐agreement of the stimulus material was high).
Stimulating individuals with common ideas had beneficial effects on creative idea generation. Relative to control, this condition was associated with stronger activation in a left‐lateralized neural network involving middle temporal and superior frontal brain areas and with lower activation in right‐hemispheric temporo‐parietal brain regions (see Figs. 3 and 4) and in the precuneus, bilaterally. We, however, did not observe specific neural correlates within limbic brain structures, which would have supported the assumption of positive affective reactions associated with common idea presentation at the neurophysiological level.
A similar pattern of findings was observed with respect to the STIM original versus control contrast, although behavioral analyses reveal no significant increases with respect to the originality of generated ideas. As shown in Table I, STIM original was accompanied (relative to the control condition) by stronger activation in left‐hemispheric regions of the mid and superior temporal gyri, along with lower activation in parietal brain regions of the right hemisphere.
Effects related to cognitive stimulation were most pronounced in left temporal brain regions (particularly in the middle temporal gyrus) and in posterior parietal regions of the right hemisphere. The middle temporal gyrus is believed to be part of the semantic system of the brain, responsible for storage and retrieval of semantic information [Binder et al.,2009]. Similarly, Jung‐Beeman [2005] highlights the role of the middle and superior temporal gyri in “semantic activation” (i.e., activating information related to an input word). Stimulating creative idea generation via the exposure to other people's ideas would certainly initiate cognitive processes such as activating and retrieving semantic information, which would explain the prominent role of temporal brain regions in both stimulation conditions. Stimulating participants via common or moderately creative ideas, which has turned out to be most effective in this study, additionally activated regions of the left superior frontal gyrus (relative to control). This brain region is reported to be critically involved in higher levels of working memory processing [e.g., Boisgueheneuc et al.,2006], which does not only include short‐term maintenance of relevant information but also mental processes such as monitoring and mental manipulation of this information.
Another important finding of this study was that cognitive stimulation (particularly STIM common) exhibits lower activation than the control condition in regions of the right superior parietal lobe. According to Cabeza et al. [2008], the dorsal parietal cortex (DPC), largely corresponding to the Brodmann area 7 (including among others the superior parietal lobule and the precuneus) is thought to be associated with the allocation of attentional resources to memory retrieval according to the goals of the rememberer, referred to as “top‐down attention” [Cabeza et al.,2008]. “Bottom‐up attention,” in contrast, is driven by incoming sensory information such as the capturing of attentional resources by relevant memory cues. Within the attention to memory (AtoM) framework proposed by Cabeza et al., the DPC is believed to support retrieval goals, while a more ventrally located attentional network of the parietal cortex [largely corresponding to Brodmann areas 39 and 40; cf. Cabeza et al.,2008] reflects “… attentional adjustments that are triggered by the products of ongoing MTL (medial temporal lobe) activity” (p. 620).
Creative idea generation following the presentation of meaningless words has been observed to be strongly associated with activity in the DPC in this study (see Fig. 4). In this condition (as opposed to both stimulation conditions), participants quasi worked “on their own” in thinking of possible ideas. This condition could be characterized as being more effortful and attention‐demanding, particularly in view of the fact that no relevant memory cues were available that could have been used by the participants in the subsequent generation of ideas, thereby requiring memory retrieval in a more top‐down fashion in terms of the AtoM model. This would explain why DPC activity was stronger in the control than in both stimulation conditions. In this particular context, it also appears interesting to note that cognitive stimulation via both common and original ideas was associated with activity in mid temporal brain regions (cf. Table I), which could—similarly to their presumed role in semantic activation [Jung‐Beeman,2005]—also hint at their possible involvement in initializing bottom‐up attention to memory [cf. Cabeza et al.,2008].
Right temporo‐parietal brain regions appear to have a particular role in creative cognition, especially when experimental tasks tap on the originality facet of creativity. In our previous fMRI experiments we observed evidence that the generation of original versus typical ideas was accompanied by lower brain activation in these brain regions [Fink et al.,2009a,2010]. This is supported by relevant literature in this field, which suggests that creative relative to uncreative story generation is associated with lower activity in the right inferior parietal lobe [Howard‐Jones et al.,2005]. Kowatari et al. [2009] found inverse correlations between creativity and brain activity in bilateral parietal brain regions while their participants were designing new pens and in a similar vein, Jung et al. [2010] report inverse relationships between measures of cortical thickness in right parietal regions and creativity, as it was measured by means of divergent thinking tasks. In another exciting study, Berkowitz and Ansari [2010] found that musicians (classically trained pianists) but not non‐musicians deactivated the right temporo‐parietal junction (rTPJ) during musical improvisation. In interpreting their findings, Berkowitz and Ansari [2010] refer to a framework by Corbetta et al. [2008] according to which the rTPJ is conceived as a part of a ventral attentional network of the brain involved in attending to environmental stimuli. Suppressed or attenuated activity in this region has been observed to occur to prevent reorienting attention to task‐irrelevant stimuli, which could interfere with task performance. In fact, deactivation of the rTPJ has been shown to correlate with successful task performance [for review see Corbetta et al.,2008]. Along these lines, we might speculate that the comparatively low activation in right temporo‐parietal brain regions (relative to control) could be also indicative of a more focused state of internal attention that is less likely disturbed by interfering, task‐irrelevant stimuli. This interpretation would be also supported by recent EEG studies in this field, which suggest increases in alpha activity, especially in right parietal brain regions during the generation of creative ideas [Fink et al.,2009a,b; Grabner et al.,2007]. Such increases in alpha activity are believed to reflect a state of heightened internal awareness that is less likely disturbed by interfering cognitive processes (such as bottom‐up stimulation via task‐irrelevant environmental stimuli), thereby facilitating the combination or the recombination of more distantly related information [see also von Stein and Sarnthein,2000].
The observed activity patterns of the precuneus, which is thought to be a part of the resting state brain network [see e.g., Cavanna and Trimble,2006], might also nicely fit into this picture. As the findings of this study suggest, both stimulation conditions were associated with weaker activation in this brain region relative to the control condition [see also Fink et al.,2010]. Raichle et al. [2001] specifically mention, “…the posterior cingulate cortex and adjacent precuneus can be posited as a tonically active region of the brain that may continuously gather information about the world around, and possibly within us” (p.681). Creative idea generation, particularly when participants are stimulated with other people's ideas, certainly requires states of heightened focused attention, implicating that such a broad information gathering activity needs to be temporarily suppressed [cf. Cavanna and Trimble,2006].
To sum up, the findings of this study suggest that cognitive stimulation via the exposure to common or moderately creative ideas was effective in improving creativity, and increases in performance were also reflected at the level of the brain. Relative to control, this condition was associated with stronger activation in a left‐lateralized neural network involving middle temporal and superior frontal brain areas and with lower activation in right‐hemispheric temporo‐parietal brain regions and in the precuneus, bilaterally. Accordingly, effective cognitive stimulation during creative idea generation appears to be associated with a complex neural network of brain regions, which are known as important components of cognitive processes such as attention, working memory and semantic information processing. The observed effects in right temporo‐parietal brain regions could be associated with the allocation of attentional resources to memory retrieval [cf. Cabeza et al.,2008] or with a state of heightened focused attention to memory that facilitates efficient retrieval and recombination of existing knowledge [cf. Corbetta et al.,2008]. Future research in this field will be challenged by the combined use of different neurophysiological measures/parameters (such as functional and structural characteristics of the brain) to learn more about the manifold ways creative cognition is manifested in our brains.
Acknowledgements
The authors wish to express their large gratitude to Franziska Voigt and Philipp Ludersdorfer for their valuable contributions to this research project.
REFERENCES
- Amabile T ( 1982): Social psychology of creativity: A consensual assessment technique. J Pers Soc Psychol 43: 997–1013. [Google Scholar]
- Arden R, Chavez RS, Grazioplene R, Jung RE ( 2010): Neuroimaging creativity: A psychometric view. Behav Brain Res 214: 143–156. [DOI] [PubMed] [Google Scholar]
- Bechtereva NP, Korotkov AD, Pakhomov SV, Roudas MS, Starchenko MG, Medvedev SV ( 2004): PET study of brain maintenance of verbal creative activity. Int J Psychophysiol 53: 11–20. [DOI] [PubMed] [Google Scholar]
- Berkowitz AL, Ansari D ( 2010): Expertise‐related deactivation of the right temporoparietal junction during musical improvisation. Neuroimage 49: 712–719. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL ( 2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19: 2767–2796. doi:10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B ( 2006): Functions of the left superior frontal gyrus in humans: A lesion study. Brain 129: 3315–3328. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M ( 2008): The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci 9: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson I, Wendt PE, Risberg J ( 2000): On the neurobiology of creativity. Differences in frontal activity between high and low creative subjects. Neuropsychologia 38: 873–885. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Chávez‐Eakle RA, Graff‐Guerrero A, García‐Reyna J, Vaugier V, Cruz‐Fuentes C ( 2007): Cerebral blood flow associated with creative performance: A comparative study. Neuroimage 38: 519–528. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL ( 2008): The reorienting system of the human brain: From environment to theory of mind. Neuron 58: 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Kanso R ( 2010): A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull 136: 822–848. [DOI] [PubMed] [Google Scholar]
- Dugosh KL, Paulus PB ( 2005): Cognitive and social comparison processes in brain storming. J Exp Soc Psychol 41: 313–320. [Google Scholar]
- Dugosh KL, Paulus PB, Roland EJ, Yang H‐C ( 2000): Cognitive stimulation in brainstorming. J Pers Soc Psychol 79: 722–735. [DOI] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Benedek M, Reishofer G, Hauswirth V, Fally M, Neuper C, Ebner F, Neubauer AC ( 2009a): The creative brain: Investigation of brain activity during creative problem solving by means of EEG and fMRI. Hum Brain Mapp 30: 734–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Graif B, Neubauer AC ( 2009b): Brain correlates underlying creative thinking: EEG alpha activity in professional vs. novice dancers. Neuroimage 46: 854–862. [DOI] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Gebauer D, Reishofer G, Koschutnig K, Ebner F ( 2010): Enhancing creativity by means of cognitive stimulation: Evidence from an fMRI study. Neuroimage 52: 1687–1695. [DOI] [PubMed] [Google Scholar]
- Flaherty AW ( 2005): Frontotemporal and dopaminergic control of idea generation and creative drive. J Comp Neurol 493: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C, Folley BS, Park S ( 2009): Enhanced divergent thinking and creativity in musicians: A behavioral and near‐infrared spectroscopy study. Brain Cogn 69: 162–169. [DOI] [PubMed] [Google Scholar]
- Goel V, Vartanian O ( 2005): Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set‐shift problems. Cereb Cortex 15: 1170–1177. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Fink A, Neubauer AC ( 2007): Brain correlates of self‐rated originality of ideas: Evidence from event‐related power and phase‐locking changes in the EEG. Behav Neurosci 121: 224–230. [DOI] [PubMed] [Google Scholar]
- Guilford JP ( 1950): Creativity. Am Psychol 5: 444–454. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Nadeau SE, Beversdorf DO ( 2003): Creative innovation: Possible brain mechanisms. Neurocase 9: 369–379. [DOI] [PubMed] [Google Scholar]
- Howard‐Jones PA, Blakemore S‐J, Samuel EA, Summers IR, Claxton G ( 2005): Semantic divergence and creative story generation: An fMRI investigation. Brain Res Cogn Brain Res 25: 240–250. [DOI] [PubMed] [Google Scholar]
- Jung RE, Segall JM, Bockholt HJ, Flores RA, Smith SM, Chavez RS, Haier R ( 2010): Neuroanatomy of creativity. Hum Brain Mapp 31: 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung‐Beeman M ( 2005): Bilateral brain processes for comprehending natural language. Trends Cogn Sci 9: 512–518. [DOI] [PubMed] [Google Scholar]
- Kowatari Y, Lee SH, Yamamura H, Nagamori Y, Levy P, Yamane S, Yamamoto M ( 2009): Neural networks involved in artistic creativity. Hum Brain Mapp 30: 1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijstad B, Stroebe W ( 2006): How the group affects the mind: A cognitive model of idea generation in groups. Pers Soc Psychol Rev 10: 186–213. [DOI] [PubMed] [Google Scholar]
- Osborn AF ( 1957). Applied imagination, 1st ed. New York: Scribner's. [Google Scholar]
- Paulus PB, Brown VR ( 2007): Toward more creative and innovative group idea generation: A cognitive‐social‐motivational perspective of brainstorming. Soc Pers Psychol Compass 1: 248–265. [Google Scholar]
- Paulus PB, Nijstad BA ( 2003). Group creativity: Innovation through collaboration. Oxford: Oxford university press. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci U S A 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MI ( 1953): Creativity and Culture. J Psychol 36: 311–322. [Google Scholar]
- Sternberg RJ, Lubart TI ( 1996): Investing in creativity. Am Psychol 7: 677–688. [Google Scholar]
- Talairach J, Tournoux P ( 1988). Co‐planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical. [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single‐Subject Brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Von Stein A, Sarnthein J ( 2000): Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int J Psychophysiol 38: 301–313. [DOI] [PubMed] [Google Scholar]


