Abstract
It is well known that theta rhythms (3–8 Hz) are the fingerprint of hippocampus, and that neural activity accompanying encoding of words differs according to whether the items are later remembered or forgotten [“subsequent memory effect” (SME)]. Here, we tested the hypothesis that temporal synchronization of theta rhythms among hippocampus, amygdala, and neocortex is related to immediate memorization of repeated words. To address this issue, intracerebral electroencephalographic (EEG) activity was recorded in five subjects with drug‐resistant temporal lobe epilepsy (TLE), under presurgical monitoring routine. During the recording of the intracerebral EEG activity, the subjects performed a computerized version of Rey auditory verbal learning test (RAVLT), a popular test for the clinical evaluation of the immediate and delayed memory. They heard the same list of 15 common words for five times. Each time, immediately after listening the list, the subjects were required to repeat as many words as they could recall. Spectral coherence of the intracerebral EEG activity was computed in order to assess the temporal synchronization of the theta (about 3–8 Hz) rhythms among hippocampus, amygdala, and temporal‐occipital neocortex. We found that theta coherence values between amygdala and hippocampus, and between hippocampus and occipital‐temporal cortex, were higher in amplitude during successful than unsuccessful immediate recall. A control analysis showed that this was true also for a gamma band (40–45 Hz). Furthermore, these theta and gamma effects were not observed in an additional (control) subject with drug‐resistant TLE and a wide lesion to hippocampus. In conclusion, a successful immediate recall to the RAVLT was associated to the enhancement of temporal synchronization of the theta (gamma) rhythms within a cerebral network including hippocampus, amygdala, and temporal–occipital neocortex. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: theta rhythms, slow wave oscillations, spectral coherence, immediate memory, rey auditory verbal learning test, intracerebral electroencephalography (EEG)
INTRODUCTION
Theta rhythms (3–8 Hz) of mammalian hippocampal pyramidal neurons are involved in memory formation [Kirk and Mackay, 2003, Vertes et al., 2004]. They are produced by the rhythmical actions of (excitatory) cholinergic and (inhibitory) GABAergic “pacemaking” neurons of the medial septum and vertical limb of diagonal band nucleus in basal forebrain [MS/DBv; Vertes et al., 2004], which receive (excitatory) glutamatergic and serotoninergic inputs from supramammilary nucleus of hypothalamus, nucleus pontis oralis, and brainstem median raphe [Oddie et al., 1994; Vertes, 2005]. Theta rhythms in hippocampal and parahippocampal structures are completely suppressed by lesions of the MS/DBv affecting long‐term potentiation and learning/memory [Asaka et al., 2002; Leutgeb and Mizumori, 1999; Vertes, 2005]. Furthermore, theta rhythms increase in several regions of cerebral cortex during spatial learning/recall [Kahana et al., 1999] and encoding/retention of verbal material [Raghavachari et al., 2001, 2006; Sederberg et al., 2003]. Instead, theta rhythms in the amygdala, a structure of the medial temporal lobe (MTL), seem to be responsible for the facilitation of memory by emotional arousal and may promote plasticity in coactive structures of the temporal lobe [Paré, 2003].
Previous electroencephalographic (EEG) studies have shown that theta rhythms are involved in the phenomenon of “subsequent memory effect” (SME), namely they showed an increase of theta power during the encoding of the items that are subsequently recalled [Klimesch et al., 1996; Weiss et al., 2000]. However, the mentioned studies could not identify theta generators, as EEG was recorded from scalp electrodes. To overcome this issue, intracranial EEG recordings have been performed in epileptic patients performing word recall tasks during presurgical routine [Fell et al., 2001, 2003; Fernandez et al. 1999; Sederberg et al., 2003]. In general, a list of words was presented once and, after a delay, the subjects had to recall as many items as possible in any order. To prevent mental rehearsal of the words, immediately after list presentation, the subjects were required either to counting backwards [Fell et al. 2001, 2003] or to solve simple arithmetic problems [Sederberg et al. 2003]. In these conditions, subdural EEG recordings from cortical surface [Sederberg et al., 2003] have shown that successful encoding of words was associated with an increase of frontal and temporal theta power and a widely distributed increase of gamma power (30–100 Hz). Furthermore, intracerebral EEG recordings have indicated that successful encoding of words (i.e. SME) was related to increased temporal synchronization (spectral coherence) of gamma rhythms between hippocampus and rhinal‐paleocortex [Fell et al., 2001]. Such an increase was later shown to correlate with theta coherence between hippocampus and rhinal‐paleocortex [Fell et al., 2003]. No modulation of hippocampal theta power was observed in relation to the SME [Fell et al., 2003].
In this study, intracerebral EEG data were recorded in subjects with drug‐resistant temporal lobe epilepsy (TLE), in order to investigate the relationships between brain theta rhythms and immediate successful memorization of words. When compared with the mentioned field literature, this study is characterized by two novel aspects. First, the SME was evaluated by a popular test for the evaluation of immediate and delayed memory, namely Rey auditory verbal learning test [RAVLT; Rey, 1941]. The RAVLT is widely used in the clinical evaluation of memory in neurological and psychiatric patients, but neurophysiologic mechanisms at the basis of its performance are poorly known. In the RAVLT, subjects were repeatedly listening a list of words to be recalled with no parallel interfering demands. Second, we probed the working hypothesis that temporal synchronization due to the word presentation of the theta rhythms within a network including hippocampus, amygdala, and neocortex is related to successful immediate memorization of repeated words.
METHODS
Subjects
Five adult volunteers [three females, two male; three righthanded; mean of 47.5 years ± 5.9 standard error mean (SEM)] with TLE were included in the experimental group of this study. For descriptive purpose, we performed a control experiment on a sixth TLE patient (female; 46 years; righthanded) who had received a surgical removal of broad sectors of the right temporal cortex for a pharmacoresistent partial cryptogenic epilepsy about 5 years before the present experiment. Neuroradiological evaluation showed that there was a partial corpus callosum agenesia and that part of the right hippocampus, a small portion of ipsilateral amygdala, broad regions of the temporal neocortex, and secondary dilation of the T horn were spared. Quantitatively, we removed a portion of about 4 cm × 3 cm × 2 cm of the right temporal neocortex, a portion of about 1 cm × 1 cm × 1 cm of the right hippocampus, and a portion of about 3 cm × 2 cm × 1 cm of the right amygdala. All subjects gave their informed consent and were free to withdraw from the study at any time. The general procedures were approved by the local Institutional Ethics Committee (IRCCS Neuromed, Pozzilli, Italy) and were performed in accordance with the ethical standards laid down in the Declaration of Helsinki of 1964.
To localize epileptogenic brain regions, all subjects underwent a comprehensive presurgical protocol described in details previously [Quarato et al., 2005], with the addition of an intracerebral EEG exploration. Aside from the chronic epilepsy, the clinical neurological examination was unremarkable (i.e. no sign of abnormality). Specifically, none of the subjects showed major overt cognitive deficits during the examination, as revealed by an interview about the general cognitive functions made by expert neurologists and psychologists of the Italian IRCCS Hospital Neuromed (G.D.G., P.P.Q., L.G.). All subjects fully understood experimenters' instructions and easily performed the experimental task. Table I reports demographic, clinical, neuropsychological, neuroradiological, and EEG data concerning all patients included in the study.
Table I.
Demographic, clinical, neuropsychological, neuroradiological and EEG data concerning all patients included in the study
| Gender | Age (years) | Duration of epilepsy (years) | Neuropsychological examination (episodic memory) | Brain MRl findings | EEG (irritative zone) | EEG (seizure onset zone) | |
|---|---|---|---|---|---|---|---|
| Subj 1 | M | 32 | 14 | Normal | rHS | rA, rH, rT pole | rH |
| Subj 2 | F | 50 | 32 | Normal | rT Iechemic encephalomacia | rNT, rH | rNT |
| Subj 3 | F | 47 | 27 | Normal | rHS | rA, rH | rH |
| Subj 4 | M | 46 | 23 | Normal | No pathological findings | r/IA, r/IH, r/IT pole | r/IH (I>r)) |
| Subj 5 | F | 46 | 21 | Normal | rHS | r/IH | rH |
| Subj 6 control | F | 47 | 24 | Visuospatial memory deficit | rHS (residual after anterior lobectomy) | rH (residual after anterior lobectomy) | rH (residual after anterior lobectomy) |
Abbreviations: m, male; f, female; r, right; l, left; A, amygdala; H, hippocampus; S, sclerosis; T, temporal; N, neocortex.
Experimental Task
The subjects performed a computerized version of the RAVLT [Novelli et al., 1986; Rey, 1941]. This test includes a list of 15 common words to be presented to each subject by headphones (listening/encoding phase). The words are read one‐by‐one with an interval between words of 2 s. Immediately after the presentation of the 15 words, the subjects are required to repeat back as many words as they could. In the repetition of the words, they have no constraint as presentation order and time. The encoding‐recall procedure is repeated five times. Twenty minutes after the end of the five presentations, the subjects are newly asked to repeat as many words as they could recall from the original list of words, in order to control the efficiency of long‐term memory processes.
Intracerebral EEG Exploration
To perform the intracerebral EEG exploration, subjects were surgically implanted with stick electrodes having recording contacts with a diameter of 1.0 mm and a length of 2.0 mm; along the stick, the electrode contacts were placed 2.0 mm apart (Ad‐Tech Medical Instrument Corporation; Racine, WI). These electrodes were longitudinally placed via occipital burr holes. Navigational views using three‐planar brain images were performed to correctly place the intracerebral EEG electrodes in the amygdala and hippocampus. The exact brain localization of the electrodes was determined on postimplantation computerized tomography (CT) scans through a semiautomated procedure based on a MATLAB routine (The Mathworks Inc., Natick). That routine fused the CT scans with preimplantation magnetic resonance images (MRIs) to define the position of the electrodes (Fig. 1) in relation to brain structures [Sebastiano et al., 2006]. For the purposes of this study, the intracerebral EEG exploration was performed in the right hippocampus and amygdala in two out of five experimental subjects (subjects no. 3 and 4), and in both right and left hippocampi and amygdala structures in the remaining subjects (subjects no. 1, 2, and 5) as a function of the clinical needs. For the control subject (subject no. 6), brain activity was recorded during the RAVLT administration from depth electrodes placed into the spared portion of right hippocampus. In all subjects, EEG activity of the neocortex was explored by the electrode contact at the tip of the stick electrodes, which was quite close to the cortical grey matter of occipital‐temporal neocortex (BA 38). Table II reports the total number of the electrode contacts in hippocampus and amygdala regions of interest.
Figure 1.
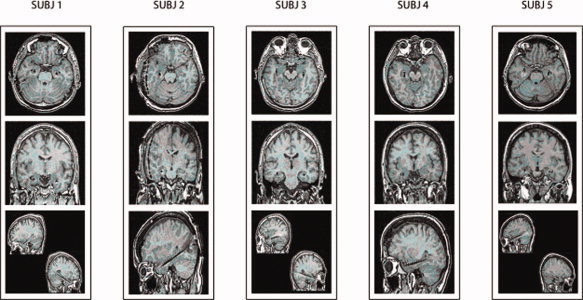
Magnetic resonance images of axial, coronal, and sagittal brain slices for the four subjects. These images show the location of the stick electrodes used for intracerebral electroencephalographic (EEG) recordings. The real volume of the electrode is about 10% of the displayed artifact. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table II.
Total number of the electrode contacts and their localization for the six patients recruited for this study
| Number of electrode contacts | |||
|---|---|---|---|
| Total | Amygdala | Hippocampus | |
| Subj 1 | 10 | 2 | 5 |
| Subj 2 | 10 | 2 | 1 |
| Subj 3 | 10 | 2 | 3 |
| Subj 4 | 12 | 2 | 4 |
| Subj 5 | 12 | 1 | 5 |
| Subj 6 control | 10 | 1 | |
While subjects were performing the RAVLT, the intracerebral EEG data were recorded using earlobes as an electrode reference, bandpass setting of 0.015 Hz–70 Hz, and sampling rate of 400 Hz. Eye movements and blinking were registered via an electro‐oculogram (EOG) derived by electrodes at the lateral canthus of the eyes. The intracerebral EEG segments contaminated by outgoing artifacts were rejected online.
Listening/Encoding‐Related Hippocampal Theta Power
The intracerebral EEG epochs with subtle ocular, muscular, and other types of artifacts were identified offline. A computerized procedure selected these EEG epochs using EEG and EOG signals as an input [Moretti et al. 2003]. The results of the procedure were then manually verified by two experimenters (F.V. and P.B.). The artifact‐free intracerebral EEG data were rereferenced to common average and segmented in single trials associated with the single words of the listening/encoding phase. Each intracerebral EEG single trial spanned from −2 s to +3 s from the onset of the word considered as a zerotime. For the subsequent data analysis, we selected only the electrode contacts that showed no pathological EEG activity throughout the investigation (e.g. pathological interictal activity or seizure onset) or were located out of lesional area, in line with previous reference field studies [Fell et al., 2003; Sederberg et al., 2003]. Specifically, they were localized in the hippocampus, amygdala, and close to temporo‐occipital neocortex (BA 38) of the right hemisphere in the experimental subjects nos. 3, 4, and 5, and of the left hemisphere in the experimental subjects nos. 1 and 2. Furthermore, for the control subject (no. 6), the electrode contacts were localized in the spared region of the right hippocampus and quite close to the temporal‐occipital neocortex (BA 38).
To determine the hippocampal theta subbands, we preliminarily defined the frequency peak of the slow wave rhythms in the single subjects. In each subject, this frequency peak was the frequency bin showing the highest spectral power density in the 1–8 Hz range, calculated in the intracerebral EEG data from 1 s before (“baseline”) to 1 s after (“event”) the onset of the word presentation (frequency resolution of the power spectrum = 1 Hz). In line with the general theory by Klimesch [1999] suggesting the use of 3‐frequency bins subbands, two theta subbands of interest were considered: the slow wave rhythm 1 (SW1) ranged from the frequency peak to the frequency peak plus 2 Hz, and the slow wave rhythm 2 (SW2) ranged from the frequency peak plus 2 Hz to the frequency peak plus 4 Hz. For an individual frequency peak at 2 Hz, the theta subbands ranged 2–4 Hz for the SW1 and 4–6 Hz for the SW2. As the mean theta frequency peak was observed at 2.8 Hz, we selected only the individual theta frequencies above this peak to avoid including the delta band in the analysis. Of note, all subjects presented quite clear individual theta frequency peaks at hippocampus.
To compute changes of the theta power during the listening/encoding of the words, the well‐known procedure called event‐related desynchronization/synchronization (ERD/ERS) was followed [Pfurtscheller and Aranibar, 1979; Pfurtscheller and Lopes da Silva, 1999; Pfurtscheller and Neuper, 1994; Pfurtscheller et al., 1997]. In brief, the theta ERD (ERS) was defined as the percentage decrement (increment) of EEG‐band power in the period of 1 s after the onset of the word presentation (“event”) with reference to a “baseline” period from 1 s before to the word onset (“baseline”).
Listening/Encoding‐Related Coherence of Theta Rhythms
Spectral coherence is a normalized measure of the coupling between two signals at any given frequency [Halliday et al., 1995; Rappelsberger and Petsche, 1988]. The coherence values were calculated for each frequency bin by the following formula
which is the extension of the Pearson's correlation coefficient to complex number pairs. In this equation, f denotes the spectral estimate of two EEG signals x and y for a given frequency bin (λ). The numerator contains the crossspectrum for x and y (f xy), whereas the denominator contains the respective autospectra for x (f xx) and y (f yy). For each frequency bin (λ), the coherence value (Cohxy) is obtained by squaring the magnitude of the complex correlation coefficient R. This procedure returns a real number between 0 (no coherence) and 1 (maximal coherence). The statistical analysis of the EEG coherence values followed the procedure reported by Halliday et al. [1995].
For each subject, the mean coherence of the theta rhythms was separately computed for the group of intracerebral EEG single trials associated with successful immediate recall (“recalled” coherence) and for the group of intracerebral EEG single trials associated with unsuccessful immediate recall (“unrecalled” coherence). Specifically, four mean coherence values were obtained from each subject across the five listening repetitions of the encoding phase: the recalled coherence value for the baseline period (from 1 s before to the onset of the word presentation), the recalled value for the listening/encoding period (from the onset of the word presentation to 1 s later), the unrecalled coherence value for the baseline period, the unrecalled value for the listening/encoding period. Based on these values, the event‐related coherence (ERCoh) was separately evaluated for the recalled coherence and for the unrecalled coherence. Indeed, ERCoh is an index of EEG coherence changes related to an event, when spontaneous fluctuations of EEG coherence during baseline period are taken into account. Accounting for these baseline fluctuations is an important advantage with relatively small experimental groups, as it emphasizes the information content as revealed by absolute EEG coherence in the event period. Although this does not solve the intrinsic limitations of statistics on small data sets, statistically significant results may better emerge. Specifically, ERCoh was defined as the coherence at the listening/encoding period (from the onset of the word presentation to 1 s later) minus the coherence at the baseline period (from 1 s before to the onset of the word presentation). The positive (negative) values of the ERCoh denoted an increment (decrement) of the mean coherence during the listening/encoding period with reference to the baseline period.
Statistics
For the analysis of theta ERCoh, the hippocampal electrode contact chosen for each subject was that showing the highest listening‐related theta ERS across SW1 and SW2. The comparison across the five subjects of the theta ERCoh between unrecalled vs. recalled words was performed by ANOVA (P < 0.05). Mauchley's test evaluated the sphericity assumption. Correction of the degrees of freedom was made by Greenhouse‐Geisser procedure. Duncan test was used for posthoc comparisons (P < 0.05). Specifically, we performed three different 2‐way ANOVAs with the factor Recall performance (unrecalled words, recalled words; independent variable) and the factor Band (SW1, SW2). Each ANOVA separately evaluated the theta ERCoh between hippocampus and temporal‐occipital neocortex, between hippocampus and amygdala, and between amygdala and temporal‐occipital neocortex. The working hypothesis was that the encoding/listening‐related ERCoh is higher in magnitude for the recalled than unrecalled words.
Of note, because the RAVLT list is repeated five times, the majority of the unrecalled words might come from the initial presentations, whereas the majority of the recalled words might come from the final presentations. As a consequence, the recalled and unrecalled words might not only differ in the variable “recalled or not” but also in the relative position into the RAVLT list (namely, the recalled words might typically belong to the final presentations). We took into account this possible confound as follows. For each subject, the relative position of the recalled words into the RAVLT list was computed as follows. We associated a progressive number from 1 to 5 (weight) to each recalled word as a function of its position in the five repetitions of that word (when the recalled word was listened in the first presentation list, it took the number “1”; when it was listened in the second presentation, it took the number “2,” etc.). For a given subject, the mean of the weights relative to recalled words indexed the corresponding position in the five repetitions of the RAVLT list. The same procedure was followed for the unrecalled words. The means of the weights for the recalled and unrecalled words were separately used as covariates in the ANOVA analyses, in order to remove from the intracerebral EEG results the linear effects of the different position of the recalled and unrecalled words in the RAVLT list.
The relative position into the RAVLT of the recalled and unrecalled words required a further control analysis. The rationale for this analysis is that the baseline period to assess the poststimulus theta coherence fell into a period of 1–2 s after the presentation of the previous word, and EEG activity following the word presentation might be different in the case that word is unrecalled or recalled. As a consequence, the EEG baseline period of a recalled word in the initial presentations is more likely to be preceded by unrecalled words, whereas the EEG baseline period of a recalled word in the final presentations is more likely to be preceded by recalled words. To control this possible confound, we performed 2‐way ANOVAs (P < 0.05) with the factor Recall performance (unrecalled words, recalled words; independent variable) and the factor Band (SW1, SW2). For the EEG power, three ANOVAs separately evaluated the baseline theta power in the hippocampus, amygdala, and temporal‐occipital neocortex. For the EEG spectral coherence, three ANOVAs separately evaluated the baseline theta coherence between hippocampus and temporal‐occipital neocortex, between hippocampus and amygdala, and between amygdala and temporal‐occipital neocortex. The working hypothesis was that these EEG variables did not differ in magnitude in the baseline period of the unrecalled vs. recalled words.
RESULTS
Performance to the RAVLT
In general, the amount of recalled words (out of the 15 encoded words) increased along the five listening repetitions of the RAVLT. At the group level, the mean ± SEM values of the recalled words were 5.8 ± 1.5, 8.6 ± 1.2, 9.8 ± 1.2, 10.4 ± 1.3, and 10.6 ± 1.6 from the first to the fifth listening repetition, respectively. In the later delay recall (15 min), the across subjects mean value of the recalled words was 11.6 ± 1.3, reflecting effective medium‐term memorization processes in the present patients (this does not exclude some possible slight memory deficits to specific neuropsychological testing). Individual performances to the RAVLT are reported in Table III.
Table III.
Individual performance as immediate and delayed recall to the Rey auditory verbal learning test (RAVTL)
| Immediate recall | Mean (±SE) | Delayed recall | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Subj 1 | 6 | 7 | 9 | 9 | 9 | 8 (±0.6) | 9 |
| Subj 2 | 10 | 12 | 11 | 12 | 13 | 11.6 (±0.5) | 12 |
| Subj 3 | 3 | 6 | 11 | 14 | 11 | 9.4 (±1.9) | 13 |
| Subj 4 | 3 | 6 | 6 | 7 | 6 | 5.6 (±0.7) | 9 |
| Subj 5 | 7 | 10 | 12 | 10 | 14 | 10.6 (±1.2) | 15 |
| Mean (±SE) | 5.8 (±1.5) | 6.6 (±1.2) | 9.8 (±1.2) | 10.4 (±1.3) | 10.6 (±1.6) | 11.6 (±1.3) | |
| Subj 6 control | 6 | 6 | 10 | 10 | 11 | 9 (±0.9) | 9 |
Hippocampal EEG Theta Power
Figure 2 shows the intracerebral EEG power density spectra at representative hippocampal electrode contacts for all subjects, that is the electrode contacts showing the maximal theta ERS. These spectra were calculated in the period ranging from 1 s before to 1 s after the onset of the 15 words presentation during the RAVLT administration. In all subjects, the power density spectra showed the individual frequency peak in the range of slow rhythms (2–5 Hz), as expected for hippocampal electrode contacts. The individual frequency peak of the slow wave rhythms was 3 Hz for the first subject; 2 Hz for the second, the third, and the fifth subject; and 5 Hz for the fourth subject. Table IV reports the frequency composition of the SW1 and SW2 subbands in all subjects. As control analyses, the ANOVAs showed no statistical difference in the baseline EEG power density (SW1, SW2) between the unrecalled and recalled words in the hippocampus (main effect Recall performance: F(1,4) = 0.086; P = 0.78), amygdala (main effect Recall performance: F(1,4) = 0.099; P = 0.76), and temporal‐occipital neocortex (main effect Recall performance: F(1,4) = 0.353; P = 0.58).
Figure 2.
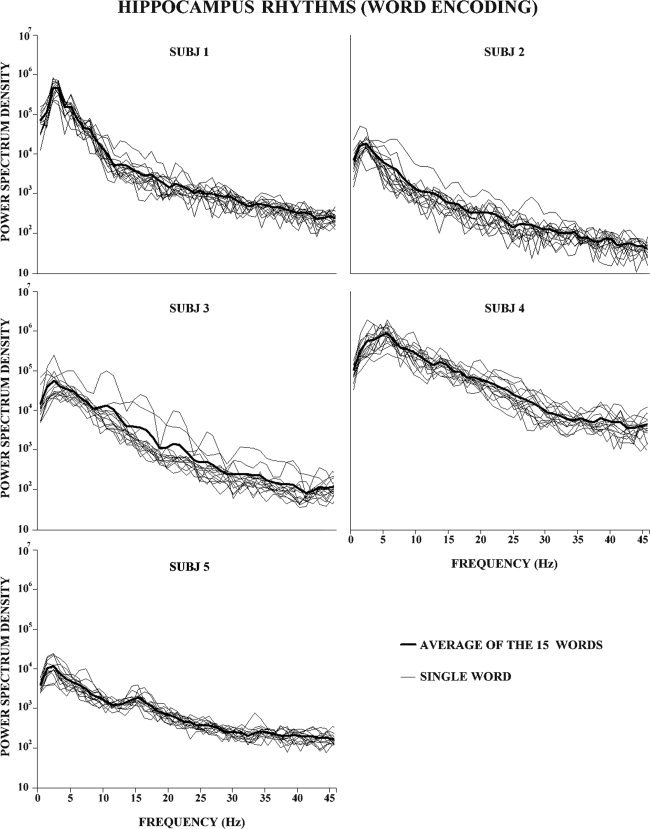
Intracerebral EEG rhythms (as revealed by power spectra) in the hippocampus during the encoding phase of RAVLT in the four subjects. The RAVLT included a list of 15 common words to be encoded/recalled five times. Each plot shows the intracerebral EEG power density spectra for the selected hippocampal electrode contact for each subject. Thin lines represent the average spectra across the five repetitions of each word of the RAVLT, whereas the thick line represents the mean across all 15 words and five encoding repetitions. See Methods section for further details.
Table IV.
Individual slow frequency peaks (iSWP) and frequency composition of the slow wave (SW) theta subbands in all subjects. See Methods section for further details
| ISWP (Hz) | SW1 (Hz) | SW2 (Hz) | |
|---|---|---|---|
| Subj 1 | 3 | 3–5 | 5–7 |
| Subj 2 | 2 | 2–4 | 4–6 |
| Subj 3 | 2 | 2–4 | 4–6 |
| Subj 4 | 5 | 5–7 | 7–9 |
| Subj 5 | 2 | 2–4 | 4–6 |
| Subj 6 control | 3 | 3–5 | 5–7 |
We also performed 2‐way ANOVAs with the factors Recall performance (unrecalled words, recalled words) and Band (SW1, SW2). Each ANOVA separately evaluated the theta ERD/ERS in hippocampus, amygdala, and temporal‐occipital neocortex. In line with previous evidence, [Fell et al., 2001], no statistically significant effect was observed in the hippocampus [Recall performance F(1,4) = 0.33; P = 0.59; Band F(1,4) = 0.27; P = 0.621; interaction F(1,4) = 0.49; P = 0.52]. The same was true in the amygdala [Recall performance F(1,4) = 1.23; P = 0.32; Band F(1,4) = 0.02; P = 0.87; interaction F(1,4) = 0.02; P = 0.88] and in the temporal‐occipital neocortex (Recall performance F(1,4) = 0.33; P = 0.59; Band F(1,4) = 0.27; P = 0.62; interaction F(1,4) = 0.49; P = 0.52).
EEG Theta Coherence
As control analyses, the ANOVAs showed no statistical difference in the baseline EEG spectral coherence (SW1, SW2) between the unrecalled and recalled words in the coupled regions of interest such as hippocampus/temporal‐occipital neocortex [main effect Recall performance: F(1,4) = 2.615; P = 0.18], hippocampus/amygdala [main effect Recall performance: F(1,4) = 0.32; P = 0.6), and amygdala/temporal‐occipital neocortex (main effect recall performance: F(1,4) = 3; P = 0.16].
Figure 3 illustrates, separately for each subject, the ERCoh values of the two theta subbands for recalled vs. unrecalled words relative to the functional couplings of hippocampus‐neocortex, hippocampus‐amygdala, and amygdala‐neocortex. Noteworthy, in all subjects the ERCoh values were higher for the recalled than for the unrecalled words with only four exceptions, which mainly regarded SW2 and functional coupling of amygdala (namely, Subject 1 amygdala‐neocortex, hippocampus‐amygdala, Subject 2 hippocampus‐amygdala, and Subject 4 amygdala‐neocortex). By averaging the ERCoh values of the five subjects, separately for recalled vs. unrecalled words and for the theta subbands of interest, this tendency became even clearer (see Fig. 4).
Figure 3.
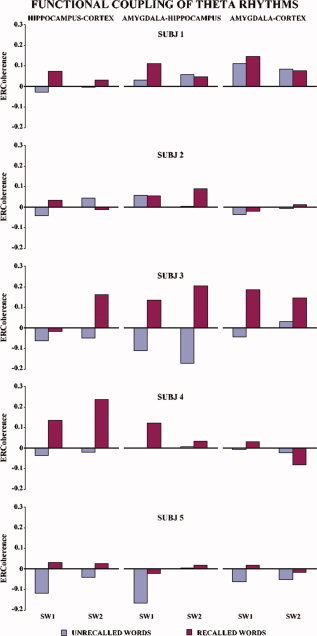
Temporal synchronization of intracerebral EEG rhythms during the encoding/listening of words of the RAVLT in the four subjects. Each bar represents the encoding‐related theta coherence (ERCoh) for the recalled (blue) vs. unrecalled (red) words for the two theta subbands (see methods for further details). The ERCoh values are higher for the recalled than for the unrecalled words with only three exceptions (Subject 1 amygdala‐neocortex, hippocampus‐amygdala and Subject 3 amygdala‐neocortex). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 4.
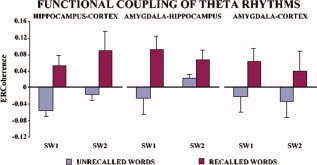
Average of encoding‐related theta coherence (ERCoh) values of the four subjects referred to the recalled (blue bars) vs. unrecalled (red bars) words for the two theta subbands (see Methods section for further details). The ERCoh values are always higher for successful recalled words. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The statistical analysis of the ERCoh showed the following statistically significant results. There was a main ANOVA effect of the factor Recall performance for the theta ERCoh between hippocampus and temporal‐occipital neocortex, revealing that the ERCoh was significantly higher for the recalled than unrecalled words [F(1,3) = 17.95; P < 0.024].
In the same direction, there was a main ANOVA effect of the factor Recall performance for the theta ERCoh between hippocampus and amygdala [F(1,3) = 10.03; P < 0.05]. Furthermore, a statistically significant interaction between the two factors [F(1,3) = 144.66; P < 0.0012] showed that the preponderance of the ERCoh values for the recalled words was especially evident at SW1 (P < 0.0005) when compared with SW2 (P < 0.05) frequencies.
Of note, no statistical effect was observed for the ERCoh between amygdala and temporal‐occipital neocortex [Recall performance: F(1,3) = 1.58; P = 0.29; Band: F(1,3) = 3.40; P = 0.16; interaction of the two factors: F(1,3) = 0.07; P < 0.80].
Control Analysis on Gamma Rhythms
As previous evidence [Aggleton and Brown, 1999; Canolty et al., 2006; Chrobak and Buzsáki, 1998; Eichenbaum et al., 1996; Fell et al., 2003; Fernández and Tendolkar, 2001] has shown the importance of gamma rhythms (around 40 Hz) during stimulus encoding, we performed a control analysis on gamma rhythms (40–45 Hz) along the network including hippocampus, amygdala, and temporal‐occipital neocortex.
Figure 5 illustrates individual gamma ERCoh values for the recalled and unrecalled words relative to the functional coupling of hippocampus‐neocortex, hippocampus‐amygdala, and amygdala‐neocortex. In general, the mean ERCoh values were higher in amplitude for the recalled than unrecalled words for the functional coupling of hippocampus‐neocortex, hippocampus‐amygdala, and amygdala‐neocortex. By averaging the ERCoh values of the five subjects, separately for recalled vs. unrecalled words and for the theta subbands of interest, this tendency became even clearer (see Fig. 6).
Figure 5.
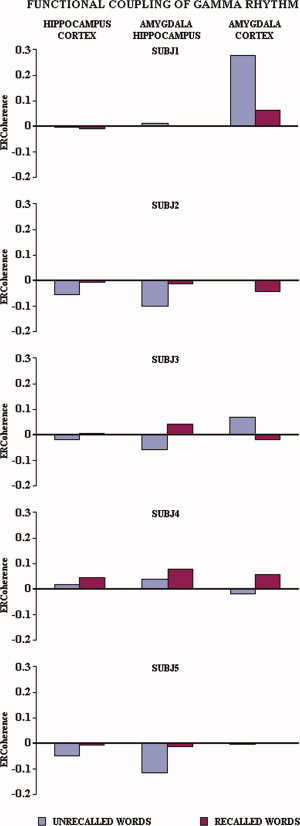
Temporal synchronization of intracerebral EEG rhythms during the encoding/listening of words of the RAVLT in the five experimental subjects. Each bar represents the encoding‐related coherence (ERCoh) for the recalled (blue) vs. unrecalled (red) words for the gamma band (see Methods section for further details). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 6.
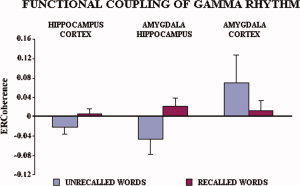
Average of encoding‐related gamma coherence (ERCoh) values of the five experimental subjects referred to the recalled (blue bars) vs. unrecalled (red bars) words for the gamma band (see Methods section for further details). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The statistical ANOVA design for the gamma ERCoh was similar to that used for the theta ERCoh. There was a main ANOVA effect of the factor Recall performance for the gamma ERCoh between hippocampus and temporal‐occipital neocortex, revealing that the gamma ERCoh was significantly higher in amplitude for the recalled than unrecalled words [F(1,4) = 8.09; P < 0.0466]. In the same direction, there was a main ANOVA effect of the factor Recall performance for the gamma ERCoh between hippocampus and amygdala [F(1,4) = 8.54; P < 0.0431]. In contrast, no ANOVA effect was observed for the gamma ERCoh between amygdala and temporal‐occipital neocortex [F(1,4) = 1.23; P = 0.3301].
Control Experiment
Our previous analysis showed that the successful recalled words of the Rey list are characterized by an increment of the theta (gamma) ERCoh (i.e. temporal synchronization) between the hippocampus and occipital‐temporal cortex, and between amygdala and hippocampus during the listening/encoding phase. If this increment of the theta (gamma) ERCoh depends on the integrity of the functional connectivity among neural populations of the hippocampus, amygdala, and temporo‐occipital neocortex, lesions within these areas should deeply affect this phenomenon. For illustrative purposes, we performed a control experiment on the sixth TLE subject who had received a surgical removal of broad sectors of the right temporal cortex (including hippocampus) about 5 years before this experiment (see Fig. 7).
Figure 7.
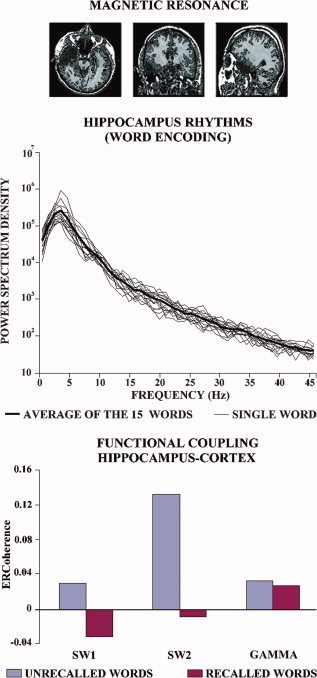
(Top) Magnetic resonance images of axial, coronal, and sagittal brain slices for a control TLE subject who had received resection of portions of right hippocampus, amygdala, and temporal neocortex due to pharmacologically resistant seizures. These images show the location of the stick electrodes used for intracerebral EEG recordings. The real volume of the electrode is about 10% of the displayed artifact. (middle) Intracerebral EEG power density spectrum at the hippocampal electrode contacts during the encoding phase of the RAVLT in the control TLE subject. Thin lines represent the average spectra across the five repetitions of each word of the RAVLT, while the tick line represents the mean across all 15 words and five encoding repetitions. (bottom) Temporal synchronization of the theta and gamma rhythms (ERCoh) between hippocampus and temporal‐occipital neocortex in the control subject during the encoding phase of the RAVLT. Each bar represents the encoding‐related theta ERCoh for the recalled (blue) vs. unrecalled (red) words. The highest ERCoh corresponds to unrecalled words. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The behavioral performance in the RAVLT was not different from that of the other subjects. In fact, the number of the recalled words along the five listening repetition were 6, 8, 10, 10, and 11, respectively. In the later delay recall, the number of the recalled words was 9. As shown in Figure 7, the intracerebral EEG power density spectrum at the electrode contacts placed into the spared hippocampus of this control subject is quite similar to those of the five experimental subjects with intact brain. In contrast to those subjects, the theta ERCoh between hippocampus and temporal‐occipital neocortex had a higher magnitude for unrecalled than recalled words, in line with the control hypothesis. The same was true even for the gamma ERCoh.
DISCUSSION
In this study, temporal synchronization (i.e. spectral coherence) of theta rhythms was explored in five TLE subjects along a brain network including hippocampus, amygdale, and temporal‐occipital neocortex, during the encoding of words. These words belonged to the RAVLT, a popular test that is widely used in the clinical evaluation of immediate and delayed memory in neurological and psychiatric patients [Novelli et al., 1986; Rey, 1941; namely, five presentations of the word list, each followed by the immediate repetition of the words]. We found that in all subjects event‐related theta coherence (ERCoh) values during the encoding phase between hippocampus and occipital‐temporal neocortex, and between amygdala and hippocampus had higher amplitude for the successful than unsuccessful immediate recall.
As the temporal synchronization (i.e. coherence) of EEG rhythms reflects cooperation between different brain regions during memory tasks [e.g. Sarnthein et al., 1998, Weiss and Rappelsberger, 2000], we propose that “SME” is related to a functional coupling (i.e. ERCoh) at theta frequencies of a cerebral network including hippocampus, amygdala, and temporal‐occipital neocortex. Specifically, this temporal synchronization at theta frequencies would enhance information transfer and processing between hippocampus and amygdala, and between hippocampus and temporal‐occipital neocortex in humans, in line with previous suggestions [Vertes, 2005]. In line with previous evidence showing the importance of gamma rhythms (around 40 Hz) during stimulus encoding [Aggleton and Brown, 1999; Canolty et al., 2006; Chrobak and Buzsáki, 1998; Eichenbaum et al., 1996; Fell et al., 2003; Fernández and Tendolkar, 2001], we performed a control analysis on gamma rhythms (40–45 Hz) along the network including hippocampus, amygdala, and temporal‐occipital neocortex. We found that the gamma functional coupling (i.e. ERCoh) between hippocampus and amygdala and between hippocampus and temporal‐occipital neocortex was higher in amplitude for the recalled than unrecalled words, showing a “SME”. However, it should be remarked that our procedure provides information about brain regions in which activity is correlated to successful encoding, but it does not enable us to evaluate if the temporal synchronization of theta (gamma) rhythms in the hippocampus, amygdala, and temporal‐occipital neocortical region of a hemisphere is crucial for encoding to occur. As a preliminary contribution to this issue, we performed a control experiment in a TLE subject who had received resection of portions of the right hippocampus, amygdala, and temporal cortex due to pharmacologically resistant seizures. In this subject, the hippocampal theta power was normal, but theta functional coupling (i.e. ERCoh) between spared hippocampus and temporal cortex was not related to successful immediate recall, in line with the idea that the temporal synchronization of the theta rhythms among hippocampus, amygdala, and temporal‐occipital neocortex is related to the functional integrity of the network. The same was true for the gamma functional coupling (i.e. ERCoh). However, the behavioral performance of the control subject was not different from those of the other volunteers, thus suggesting that other circuits including those of the spared hemisphere can support the immediate memory encoding of repeated words. If this control result were confirmed in a larger control group, we could explain some slight violations of the general rule that event‐related theta and gamma coherence in the hippocampus‐amygdala coupling, which was higher in amplitude for the recalled than unrecalled words. This might be due to some abnormality of the neural tissue in the regions of interest. Future studies should recruit several TLE patients with lesions in the hippocampus for a definitive evaluation of this explanation. At the present stage of the research, the control experiment in only one patient has obvious heuristic limitations and mere illustrative purposes.
The findings of this study agree and extend previous intracranial EEG evidence in epilepsy patients showing that theta coherence between hippocampus and rhinal paleocortex was associated with successful recall of words, whereas the relative theta power was not interrelated to the memory performance [Fell et al., 2003]. They are also in agreement with a bulk of previous investigations showing stringent relationships between the modulation of brain theta rhythms and memory formation [Basar et al., 2001; Bastiaansen and Hagoort, 2003; Klimesch et al., 1996; Mölle et al., 2002; Paller and Wagner, 2002; Sarnthein et al., 1998, Tesche and Karhu, 2000; Weiss and Rappelsberger, 2000; Weiss et al., 2000]. Of note, the present results did not show any “SME” for the event‐related theta power recorded from temporal‐occipital neocortex at odds with previous scalp EEG evidence [Klimesch et al., 1996; Weiss et al., 2000]. Indeed, there is a crucial methodological difference with those studies. In this study, we sampled the occipital‐temporal theta rhythms just from the unique intracerebral EEG electrode contact probing few millimetres of temporal‐occipital grey matter, whereas the mentioned scalp EEG evidence reflected mass neural synchronization of large cortical regions where summation mechanisms are emphasized.
Even though we cannot provide exhaustive explanation of the physiological mechanism at the basis of the our present results, it is likely that theta rhythms of amygdala and temporal‐occipital neocortex drive a large population of hippocampal neurons to activate NMDA type glutamate receptor‐channels (NMDA‐channels). When combined with the release of glutamate from different afferent fibers, hippocampal theta rhythms would cause the opening of NMDA‐channels improving memory and learning [Vertes et al., 2004]. In this framework, the temporal synchronization of the theta rhythms between hippocampus and amygdala, and between hippocampus and temporal‐occipital cortex, may enhance memory formation [Vertes, 2005]. It remains to further explore the relationships between the temporal synchronization of theta and gamma (around 40 Hz) rhythms during this process, for which previous investigations have been shown to be linked to successful memory performance [Aggleton and Brown, 1999; Canolty et al., 2006; Chrobak and Buzsáki, 1998; Eichenbaum et al., 1996; Fell et al., 2003; Fernández and Tendolkar, 2001].
Yet subdural and intracerebral EEG recordings in epilepsy patients during presurgical monitoring are an ideal neurophysiological technique having both high spatial and temporal resolutions, when data are collected from brain regions not showing pathological activity along experimental sessions. However, results of these recordings should be always evaluated taking into account the possibility that epilepsy might induce subtle alterations in remote intact neural circuits [Devinsky, 2005]. In this regard, recent magnetoencephalographic evidence in healthy subjects has found that successful recalls are associated with modulations of cortical oscillatory activity in the gamma and theta bands during encoding [Osipova et al., 2006].
In conclusion, we report for the first time that successful immediate recall of words encoded several times during the RAVLT is correlated with the temporal synchronization (coherence) of human neural activity at theta frequencies between hippocampus and amygdala, and between hippocampus and temporal‐occipital neocortex. Future research will clarify if memory performance in TLE subjects may be improved by training the temporal synchronization of these theta rhythms, using, for instance, EEG neurofeedback during computer aided memory tests [Hanslmayr et al., 2005]. With this approach, it might be possible to quantify the extent to which the phenomena we observed are relevant for successful recalls during the RAVLT.
REFERENCES
- Aggleton JP,Brown MW ( 1999): Episodic memory, amnesia, and the hippocampal‐anterior thalamic axis. Behav Brain Sci 22: 425–444, discussion 444–489. [PubMed] [Google Scholar]
- Asaka Y,Griffin AL,Berry SD ( 2002): Reversible septal inactivation disrupts hippocampal slow‐wave and unit activity and impairs trace conditioning in rabbits (Oryctolagus cuniculus). Behav Neurosci 116: 434–442. [PubMed] [Google Scholar]
- Basar E,Basar‐Eroglu C,Karakas S. Schurmann M ( 2001) Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 39: 241–248. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M,Hagoort P ( 2003): Event‐induced theta responses as a window on the dynamics of memory. Cortex 39: 967–992. [DOI] [PubMed] [Google Scholar]
- Canolty RT,Edwards E,Dalal SS,Soltani M,Nagarajan SS,Kirsch HE,Berger MS,Barbaro NM,Knight RT ( 2006): High gamma power is phase‐locked to theta oscillations in human neocortex. Science 313: 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ,Buzsáki G ( 1998): Operational dynamics in the hippocampal‐entorhinal axis. Neurosci Biobehav Rev 22: 303–310. [DOI] [PubMed] [Google Scholar]
- Devinsky O ( 2005): The myth of silent cortex and the morbidity of epileptogenic tissue: Implications for temporal lobectomy. Epilepsy Behav 7: 383–389. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H,Schoenbaum G,Young B,Bunsey M ( 1996): Functional organization of the hippocampal memory system. Proc Natl Acad Sci USA 93: 13500–13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J,Klaver P,Lehnertz K,Grunwald T,Schaller C,Elger CE,Fernandez G ( 2001): Human memory formation is accompanied by rhinal‐hippocampal coupling and decoupling. Nat Neurosci 4: 1259–1264. [DOI] [PubMed] [Google Scholar]
- Fell J,Klaver P,Elfadil H,Schaller C,Elger CE,Fernandez G ( 2003): Rhinal‐hippocampal theta coherence during declarative memory formation: Interaction with gamma synchronization? Eur J Neurosci 17: 1082–1088. [DOI] [PubMed] [Google Scholar]
- Fernández G,Tendolkar I ( 2001): Integrated brain activity in medial temporal and prefrontal areas predicts subsequent memory performance: Human declarative memory formation at the system level. Brain Res Bull 55: 1–9. [DOI] [PubMed] [Google Scholar]
- Fernandez G,Effern A,Grunwald T,Pezer N,Lehnertz K,Dumpelmann M,Van Roost D,Elger CE ( 1999): Real‐time tracking of memory formation in the human rhinal cortex and hippocampus. Science 285: 1582–1585. [DOI] [PubMed] [Google Scholar]
- Halliday DM,Rosenberg JR,Amjad AM,Breeze P,Conway BA,Farmer SF ( 1995): A framework for the analysis of mixed time series/point process data—Theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S,Sauseng P,Doppelmayr M,Schaubus M,Klimesch W ( 2005): Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Appl Psychophysiol Biofeedback 30: 1–10. [DOI] [PubMed] [Google Scholar]
- Kahana MJ,Caplan JB,Sekuler R,Madsen JR ( 1999): Using intracranial recordings to study thetaResponse to J. O'Keefe and N. Burgess (1999). Trends Cogn Sci 3: 406–407. [DOI] [PubMed] [Google Scholar]
- Kirk IJ,Mackay JC ( 2003): The role of theta‐range oscillations in synchronising and integrating activity in distributed mnemonic networks. Cortex 39: 993–1008. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Doppelmayr M,Russegger H,Pachinger T ( 1996): Theta band power in the human scalp EEG and the encoding of new information. Neuroreport 7: 1235–1240. [DOI] [PubMed] [Google Scholar]
- Klimesch W ( 1999): EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev 29: 169–195. [DOI] [PubMed] [Google Scholar]
- Leutgeb S,:Mizumori SJ ( 1999): Excitotoxic septal lesions result in spatial memory deficits and altered flexibility of hippocampal single‐unit representations. J Neurosci 19: 6661–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M,Marshall L,Fehm Hl,Born J ( 2002): EEG theta synchronization conjoined with alpha desynchronization indicate intentional encoding. Eur J Neurosci 15: 923–928. [DOI] [PubMed] [Google Scholar]
- Moretti DV,Babiloni F,Carducci F,Cincotti F,Remondini E,Rossini PM,Salinari S,Babiloni C ( 2003): Computerized processing of EEG‐EOG‐EMG artifacts for multi‐centric studies in EEG oscillations and event‐related potentials. Int J Psychophysiol 47: 199–216. [DOI] [PubMed] [Google Scholar]
- Novelli G,Papagno C,Capitani E,Laiacona M,Cappa SF,Vallar G ( 1986): Tre test clinici di memoria verbale a lungo termine. In: Taratura su soggetti normali. Arch Psicol Neurol Psichiat 47: 278–296. [Google Scholar]
- Oddie SD,Bland BH,Colom LV,Vertes RP ( 1994): The midline posterior hypothalamic region comprises a critical part of the ascending brainstem hippocampal synchronizing pathway. Hippocampus 4: 454–473. [DOI] [PubMed] [Google Scholar]
- Osipova D,Takashima A,Oostenveld R,Fernández G,Maris E,Jensen O ( 2006): Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci 26: 7523–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA,Wagner AD ( 2002): Observing the transformation of experience into memory. Trends Cogn Sci 6: 93–102. [DOI] [PubMed] [Google Scholar]
- Paré D ( 2003): Role of the basolateral amygdala in memory consolidation. Prog Neurobiol 70: 409–420. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Aranibar A ( 1979): Evaluation of event‐related desynchronization (ERD) preceding and following voluntary self‐paced movement. Electroencephalogr Clin Neurophysiol 46: 138–146. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Lopez da Silva F ( 1999) Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol 110: 1842–1857. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Neuper C ( 1994): Event‐related synchronization of mu rhythm in the EEG over the cortical hand area in man. Neurosci Lett 174: 93–96. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G,Neuper C,Flotzinger D,Pregenzer M ( 1997): EEG‐based discrimination between imagination of right and left hand movement. Electroencephalogr Clin Neurophysiol 103: 642–651. [DOI] [PubMed] [Google Scholar]
- Quarato PP,Di Gennaro G,Mascia A,Grammaldo LG,Meldolesi GN,Picardi A,Giampà T,Falco C,Sebastiano F,Onorati P,Manfredi M,Cantore G,Esposito V ( 2005): Temporal lobe epilepsy surgery: Different surgical strategies after a non‐invasive diagnostic protocol. J Neurol Neurosurg Psychiatry 76: 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S,Kahana MJ,Rizzuto DS,Caplan JB,Kirschen MP,Bourgeois B,Madsen JR ( 2001): Gating of human theta oscillations by a working memory task. J Neurosci 21: 3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S,Lisman JE,Tully M,Madsen JR,Bromfield EB,Kahana MJ ( 2006): Theta oscillations in human cortex during a working‐memory task: Evidence for local generators. J Neurophysiol 95: 1630–1638. [DOI] [PubMed] [Google Scholar]
- Rappelsberger P,Petsche H ( 1988): Probability mapping: Power and coherence analyses of cognitive processes. Brain Topogr Fall 1: 46–54. [DOI] [PubMed] [Google Scholar]
- Rey A ( 1941): Psychological examination of traumatic encephalopathy. Arch de Psychol 28: 286–340. [Google Scholar]
- Sarnthein J,Petsche H,Rappelsberger P,Shaw Gl,von Stein A ( 1998): Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA 95: 7092–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiano F,Di Gennaro G,Esposito V,Picardi A,Morace R,Sparano A,Mascia A,Colonnese C,Cantore G,Quarato PP ( 2006): A rapid and reliable procedure to localize subdural electrodes in presurgical evaluation of patients with drug‐resistant focal epilepsy. Clin Neurophysiol 117: 341–347. [DOI] [PubMed] [Google Scholar]
- Sederberg PB,Kahana MJ,Howard MW,Donner EJ,Madsen JR ( 2003): Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci 23: 10809–10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesche CD,Karhu J ( 2000): Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci USA 97: 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP ( 2005): Hippocampal theta rhythm: A tag for short‐term memory. Hippocampus 15: 923–935. [DOI] [PubMed] [Google Scholar]
- Vertes RP,Hoover WB,Viana Di Prisco G ( 2004): Theta rhythm of the hippocampus: Subcortical control and functional significance. Behav Cogn Neurosci Rev 3: 173–200. [DOI] [PubMed] [Google Scholar]
- Weiss S,Rappelsberger P ( 2000): Long‐range EEG synchronization during word encoding correlates with successful memory performance. Brain Res Cogn Brain Res 9: 299–312. [DOI] [PubMed] [Google Scholar]
- Weiss S,Müller HM,Rappelsberger P ( 2000): Theta synchronization predicts efficient memory encoding of concrete and abstract nouns. Neuroreport 11: 2357–2361. [DOI] [PubMed] [Google Scholar]


