Abstract
Meta‐analysis is an important tool for interpreting results of functional neuroimaging studies and is highly influential in predicting and testing new outcomes. Although traditional label‐based review can be used to search for agreement across multiple studies, a new function‐location meta‐analysis technique called activation likelihood estimation (ALE) offers great improvements over conventional methods. In ALE, reported foci are modeled as Gaussian functions and pooled to create a statistical whole‐brain image. ALE meta‐analysis and the label‐based review were used to investigate the Stroop task in normal subjects, a paradigm known for its effect of producing conflict and response inhibition due to subjects' tendency to perform word reading as opposed to color naming. Both methods yielded similar activation patterns that were dominated by response in the anterior cingulate and the inferior frontal gyrus. ALE showed greater involvement of the anterior cingulate as compared to that in the label‐based technique; however, this was likely due to the increased spatial level of distinction allowed with the ALE method. With ALE, further analysis of the anterior cingulate revealed evidence for somatotopic mapping within the rostral and caudal cingulate zones, an issue that has been the source of some conflict in previous reviews of the anterior cingulate cortex. Hum Brain Mapp 25:6–21, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: Stroop task, meta‐analysis, activation likelihood estimation, ALE, BrainMap, anterior cingulate, ACC
INTRODUCTION
Meta‐analysis, the post‐hoc combination of results from independent studies to estimate better a parameter of interest, is a tool that has been utilized for decades in many medical fields [Conn,1997; Mosteller and Colditz,1996; Petitti,1997]. As opposed to narrative reviews, meta‐analysis is a formal, statistical integration in which studies are collected, coded, and interpreted in an analytical and unbiased manner. In human functional brain mapping, authors have widely adopted the practice of reporting the brain locations of task‐induced activations as 3D (x, y, z) coordinates in stereotactic space. Due to this standard, meta‐analysis is emerging as a tool for identifying reliable patterns of activation wherein effects from multiple studies are combined to assess concordance and guide interpretation [Fox et al.,1998]. Traditional meta‐analyses merge nonsignificant results to test for significance in pooled data. In contrast, a new category of meta‐analysis exists in human brain mapping, termed function‐location meta‐analysis, which searches for locations of functional agreement among statistically significant effects. The primary goal of function‐location meta‐analysis is to determine consistent activity within the literature for certain paradigm classes or behavioral domains. Although the main objective is synthesizing large bodies of literature, it can also be useful in modeling expected activations in advance of an experiment, generating new hypotheses, or detecting outliers within results.
Meta‐analysis must be distinguished from literature review. The most common method of literature review is to construct a figure or table that summarizes the activation patterns of the studies under consideration. This can be done either by plotting foci on a standard brain or constructing tables that list coordinates of activation. This method is used widely for finding concordance among studies with similar experimental contrasts and is a well‐accepted technique [Barch et al.,2001; Becker et al.,1999; Buckner and Petersen,1996; Bush et al.,2000; Fiez et al.,1996; Owen,1997; Picard and Strick,1996]. A common modification of the literature review is label‐based, in which reported anatomical locations, or labels, are tallied and plotted [Ingham et al.,2003; Phan et al.,2002]. Labels can be derived directly from the publications (author labels) or through the use of a standard brain atlas (atlas labels). Despite their popularity, interpretation of these tables can be difficult. In addition, literature reviews typically are not quantitative and yield no formal estimate of probability.
Activation likelihood estimation (ALE) is a new method of quantitative, function‐location meta‐analysis, developed by Turkeltaub et al. [2002], that does not rely on the traditional tabular technique of establishing agreement across studies. In ALE, a set of studies dealing with a specific domain or paradigm in human brain mapping is collected and analyzed for concordance by modeling each reported focus of activation as the center of a Gaussian probability distribution. The 3D Gaussian distributions are then summed to create a whole‐brain statistical map that estimates the likelihood of activation for each voxel for that task as determined by the entire set of studies. In comparison with tabulation‐based meta‐analyses, the output of ALE is a simulated statistical parametric image (pseudo‐SPI) that allows for easier interpretation than do tabular literature reviews. Once the activation foci are selected from the literature, the ALE method is fully automated, quantifies the degree of agreement across studies, and uses significance thresholds to create statistically defensible conclusions [Chein et al.,2002; Turkeltaub et al.,2002].
The ALE approach differs from label‐based reviews in a fundamental way. In the voxel‐based ALE method (most automated), a group of coordinates are input, the spatial distribution of these coordinates is analyzed to search for concordance, and anatomical labels are applied to the resultant clusters as a last step. However, in a label‐based review the anatomical labels are applied first and then the clustering of labels is analyzed. In an atlas‐label review, coordinates are assigned by consulting an atlas, whereas author‐label reviews, the least automated technique described here, utilize the published labels assigned by authors.
Because agreement across studies is examined at the voxel level, ALE analyzes the task‐related activity with standardized location information. In contrast, label‐based reviews make use of generalized location information, as activation is often evaluated across large regions (e.g., entire gyri or even lobes). Incongruence in labeling or mislabeling by individual investigators reduces the tabular frequency for some labels and distributes these observations across nearby or possibly unrelated labels. As ALE is voxel‐based, it is unique in that it is not susceptible to errors caused by lack of spatial level distinction.
The primary objective of this study was to validate the ALE meta‐analysis method as compared to the more traditional label‐based methods to determine its relative strengths and weaknesses. This study was intended to extend the validation of ALE presented by Turkeltaub et al. [2002], which was relative to prospective functional magnetic resonance imaging (fMRI). By comparing ALE to label‐based reviews, we used the same coordinate‐based data, yet viewed in alternative ways. We hypothesized that the ALE meta‐analysis would return the expected results of concordance but that the greater level of spatial distinction allowed with ALE (relative to label‐based review) would provide new insight in converging activation results.
Two variations of the label‐based review and an ALE meta‐analysis of the Stroop color–word task [Stroop,1935] in normal subjects were carried out in this study. In the Stroop task, subjects view color names presented in various ink colors and are instructed to name the presented ink color. In the incongruent (Stroop interference) condition, color names are presented in nonmatching ink colors (e.g., the word “green” presented in red ink). The Stroop task is recognized universally as a standard in examining the neural substrates involved in attentional control because correct performance in color naming often competes with the tendency to execute the relatively automated function of word reading. During the incongruent condition, the two conflicting sources of color information cause an effect known as Stroop interference during which reaction times are prolonged due to the competing responses.
There is much discussion in the Stroop literature as to the trends of activation and this task is generally perceived as activating the anterior cingulate cortex, the prefrontal cortex, and parietal regions. In a brief literature author‐label review of 12 studies, Brown et al. [1999] determined that the two most common locations activated in response to performance of the Stroop task are the frontal cortex (10 of 13 experiments) and the anterior cingulate (8 of 13 experiments), but did not supply any further location information concerning these activations. The authors acknowledge in their review that “although most studies reported lateral or polar frontal lobe activation, the specific areas of frontal activation varied widely across studies.” The secondary objective of this study was to systematically identify regions of concordance in the Stroop task to understand more fully the network responsible for detection of conflict and response selection.
The Stroop studies may be parsed into two different groups based on use of a verbal response (overt or covert) or a manual (button press) response. Although the Stroop task is essentially a verbal task and it is reasonable to assume that some form of covert vocalization occurs during the manual Stroop, we hypothesized that the two response modalities would display different activation patterns due to a stronger emphasis on vocalization and articulation in the verbal as opposed to that in the manual Stroop task. Previous studies and reviews have examined the role that response modality plays in monitoring conflict in the anterior cingulate [Barch et al.,2001; Paus et al.,1993; Picard and Strick,1996] and found conflicting results. The final objective here was to study the patterns of convergence in verbal and manual Stroop to resolve the disagreement concerning the presence or absence of somatotopic mapping in the anterior cingulate cortex.
MATERIALS AND METHODS
Literature Search and Selection
A comprehensive search for the body of literature investigating Stroop interference in normal subjects was carried out using an online citation indexing service (Medline) for studies with the keyword “Stroop” that were classified under the MeSH subject heading of Brain Mapping. Fifty‐four articles were returned. These search results were filtered to include only functional neuroimaging studies that published activation results as 3D coordinates (x, y, z) in stereotactic space. Filtering the results yielded 26 articles, which were entered into the BrainMap database (http://www.brainmap.org).
Data Coding and Correction
The 26 Stroop articles represented a total of 75 experiments, which were formed from the contrasts of 93 unique conditions. In the BrainMap database, a condition is defined as the behavioral state during which subjects are presented with a stimulus and given instructions as to the appropriate response. The contrasts that are generated when comparing brain activity across conditions are referred to as experiments, from which localized maxima of activation are extracted and reported via Talairach coordinates [Talairach and Tournoux,1988]. Details on the conditions, experiments, subject groups, and imaging modality were entered into BrainMap for the 26 Stroop articles. These 75 experiments reported 552 coordinate locations. Functional neuroimaging articles report coordinates that are spatially normalized relative to a variety of anatomical templates. The template of each article was noted and the coordinates were subsequently transformed to allow analysis relative to a single template. For example, coordinates published in Montreal Neurological Institute (MNI) space were transformed to the Talairach template according to the nonlinear Brett transformation [Brett,1999] included in the BrainMap environment.
Selection of Conditions and Contrasts
The BrainMap coding scheme provides criteria that guide the selection of experiments; therefore we utilized BrainMap to carry out further filtering of the studies at the experiment level. In the present meta‐analysis, we included only those studies that explored activations in the standard color‐word Stroop task. Several of the 75 experiments were eliminated as they included data acquired on non‐Stroop tasks such as the Flanker task and the Simon task [Derbyshire et al.,1998; Fan et al.,2003; Peterson et al.,2002]. This restriction also eliminated variations of the Stroop task such as the emotional Stroop [Isenberg et al.,1999; George et al.,1994; Whalen et al.,1998], the counting Stroop [Bantick et al.,2002; Bush et al.,1998], and other variations of the Stroop task [de Zubicaray et al.,2001; Milham et al.,2003]. Subject groups were limited to normal subjects [Milham et al.,2002; Yucel et al.,2002]. Deactivations were omitted. Coordinates corresponding to the analysis of the control subjects in Yucel et al. [2002] were omitted as they were results of individual subjects, rather than group‐mean data. These restrictions reduced the number of articles from 26 to 19 and the number of experiments from 70 to 27.
The final set of 27 experiments compared the incongruent condition to a control condition. In this group of Stroop studies, three control conditions were employed: congruent, neutral, and non‐lexical. In the congruent control condition, color names were displayed in matching ink colors (e.g., the word “green” presented in green ink). The neutral control utilized color‐neutral words, presented in various ink colors. The color‐neutral words were taken from categories such as animal names [Brown et al.,1999; Carter et al.,1995], articles of clothing [Mead et al.,2002], directions [Bench et al.,1993], or a random selection of words unrelated to color [Banich et al.,2000; Milham et al.,2001,2002; Taylor et al.,1997]. In the non‐lexical control condition, subjects named the color of non‐lexical stimuli such as crosses [Bench et al.,1993], squares [Brown et al.,1999], letters “XXXX” [Ruff et al.,2001; Steel et al.,2001], or pound signs “####” [George et al.,1994; Taylor et al.,1997]. The design of the various Stroop tasks was also differentiated by modality of response. In 6 studies, subjects were instructed to manually indicate the ink color by pressing one of three or four buttons, whereas 13 studies required subjects to verbally name the ink color either overtly or covertly. We elected not to include multiple contrasts from the same article so as not to bias the results of the meta‐analysis in favor of any subset of articles (i.e., those reporting the greatest number of contrasts). In those studies that included multiple experiments that met the selection criteria [Banich et al.,2000; Bench et al.,1993; Brown et al.,1999; Carter et al.,1995; Mead et al.,2002; Taylor et al.,1997], sets of coordinates were chosen for the Stroop task meta‐analysis by selecting the contrasts of the incongruent condition with the highest‐level control condition. That is, the experiments that contrasted the Stroop interference condition with the congruent condition were first selected for inclusion in the meta‐analysis, followed by those with the neutral condition, and lastly, those with the non‐lexical condition. The final collection of 19 color‐word Stroop studies included in the meta‐analysis consisted of 13 fMRI and 6 positron emission tomography (PET) studies (Table I).
Table I.
Data sources
| Author | n | Modality | Control | Response |
|---|---|---|---|---|
| Banich et al.,2000 | 14 | fMRI | Neutral | Manual |
| Bench et al.,1993 | 6 | PET | Neutral, nonlexical | Verbal |
| Brown et al.,1999 | 8 | fMRI | Neutral, nonlexical | Verbal |
| Carter et al.,1995 | 15 | PET | Congruent, neutral | Verbal |
| Carter et al.,2000 | 12 | fMRI | Congruent | Verbal |
| Derbyshire et al.,1998 | 6 | PET | Congruent | Verbal |
| Fan et al.,2003 | 12 | fMRI | Congruent | Manual |
| George et al.,1994 | 21 | PET | Nonlexical | Verbal |
| Leung et al.,2000 | 13 | fMRI | Congruent | Verbal |
| MacDonald et al.,2000 | 12 | fMRI | Congruent | Verbal |
| Mead et al.,2002 | 18 | fMRI | Congruent, neutral | Manual |
| Milham et al.,2001 | 16 | fMRI | Neutral | Manual |
| Milham et al.,2002 | 12 | fMRI | Congruent, neutral | Manual |
| Pardo et al.,1990 | 8 | PET | Congruent | Verbal |
| Peterson et al.,1999 | 34 | fMRI | Congruent | Verbal |
| Peterson et al.,2002 | 10 | fMRI | Congruent | Verbal |
| Ruff et al.,2001 | 12 | fMRI | Neutral | Manual |
| Steel et al.,2001 | 7 | fMRI | Nonlexical | Verbal |
| Taylor et al.,1997 | 12 | PET | Neutral, nonlexical | Verbal |
Nineteen publications were included in the Stroop meta‐analysis and literature review. All studies used a traditional color–word Stroop task in normal volunteers. All reported response locations in stereotactic coordinates. Studies differed in their control conditions and response modality (manual or verbal). The average number of subjects (n) in each study was 13.1 with a standard deviation of 6.4.
Label‐Based Reviews
Label‐based reviews of a particular domain of human functional brain mapping are often highly influential and well cited. To examine thoroughly the differences in results obtained with ALE meta‐analysis as compared to label‐based review, two different tabular reviews were carried out: an author‐label review that used author assigned anatomical labels (as‐published) and an atlas‐label review that utilized labels derived from the Talairach Daemon [Lancaster et al.,2000]. Anatomical labels of the regions of activations were collected directly from the publication or via the Talairach Daemon using the search for nearest gray matter. The number of times each label appeared was recorded, tallied, and plotted.
Activation Likelihood Estimation
ALE maps were created as described by Turkeltaub et al. [2002] using a full‐width half‐maximum (FWHM) of 10 mm. Statistical significance was determined using a permutation test of randomly generated foci. No assumptions were made concerning the distribution or spatial separation of these random foci. Five thousand permutations were computed using the same FWHM value and the same number of foci used in computing the ALE values. The test was corrected for multiple comparisons using the false discovery rate (FDR) method [Genovese et al.,2002; Laird et al.,2005]. All data processing was carried out using an in‐house Java version of ALE developed at the Research Imaging Center. Three different ALE maps were computed for all Stroop studies, Stroop studies that required an overt or covert verbal response, and Stroop studies that required a manual response. Whole‐brain maps of the ALE values were imported into AFNI [Cox,1996] and overlaid onto an anatomical template generated by spatially normalizing the International Consortium for Brain Mapping (ICBM) template to Talairach space [Kochunov et al.,2002].
RESULTS
Selected contrasts from the Stroop literature comparing the incongruent condition with a control condition yielded a total of 205 foci. These foci are viewed in Talairach space in the BrainMap database Java‐based application Search&View (Fig. 1). In this image, each color/symbol combination identifies a study within the BrainMap database, and the number displayed along with each focus refers to the experiment within the corresponding article. Pooling the results of 19 experiments onto a single brain resulted in a diffuse pattern of activation across all lobes, with some clustering evident in the frontal lobes.
Figure 1.
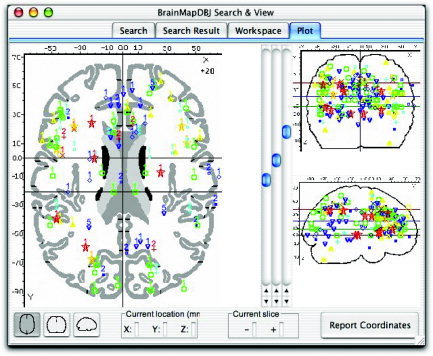
Stroop foci. Activation foci of the studies included in the Stroop meta‐analyses are seen here in Talairach space in the BrainMap Search&View environment. Selected contrasts within the 19 studies yielded a total of 205 foci. A diffuse pattern of activation is seen throughout the brain in the three orthogonal views, although some convergence is evident in the cingulate cortex (sagittal view). Each color/symbol combination identifies a study within the BrainMap database, and the number displayed along with each focus refers to the experiment within the corresponding article.
Label‐Based Reviews
Author‐label review
A review of author‐assigned labels was carried out in which each author label from the 205 coordinates was recorded, tallied, and plotted (Fig. 2). A standard nomenclature system was not employed for author labels across all 19 studies (Table II). Although many authors chose to report anatomical locations in terms of gyral anatomy (e.g., superior temporal gyrus), some preferred to use sulcal terms (e.g., superior temporal sulcus) or selected labels not indicative of any specific anatomical boundary (e.g., superior temporal, superior temporal region, or superior temporal cortex). To reduce the total number of labels, these various anatomical descriptors were merged (e.g., superior temporal). Some authors simply reported the lobe of activation, such as the parietal cortex [Taylor et al.,1997] and the occipital cortex [George et al.,1994; Peterson et al.,1999]. Labels that specified location only by lobe were not merged.
Figure 2.
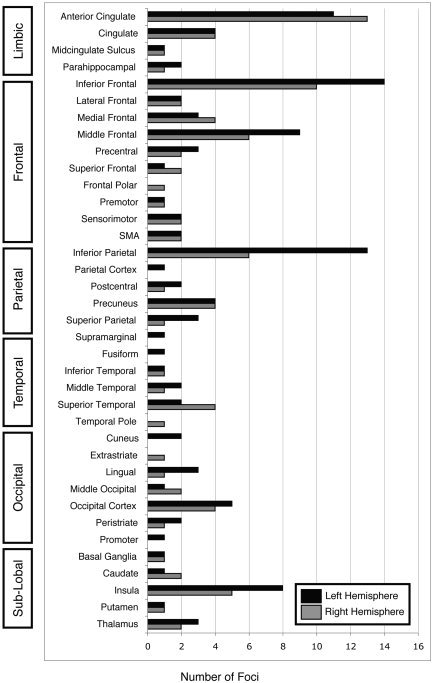
Author‐label review of the Stroop task. Author labels were recorded and tallied for all 205 foci in the Stroop articles. Labels were anatomical (“inferior frontal”), directional (“lateral frontal”), or functional (“sensorimotor”). Agreement was observed in the bilateral anterior cingulate, left inferior frontal, left inferior parietal, and left middle frontal regions.
Table II.
Categories of author nomenclature
| Author nomenclature | % of Foci |
|---|---|
| “Gyrus” | 35.6 |
| Sulcus | 2.4 |
| Lobule | 6.3 |
| Cortex | 11.7 |
| Lobe | 5.4 |
| Nuclei | 5.9 |
| None | 27.8 |
| Functional | 4.9 |
Many authors reported locations in gyral terms (73 foci), whereas sulcal labels were used for 5 foci. Thirteen foci were labeled “inferior” parietal lobule. Cortex descriptors (24 foci) included labels such as mesial frontal cortex and inferior parietal cortex, whereas lobe labels (11 foci) included parietal lobe. Twelve labels indicated nuclei (12 foci; i.e., thalamus, putamen, or caudate). No specific anatomical descriptor was indicated for 57 labels, such as middle frontal or inferior temporal. Ten labels were functional (i.e., premotor or supplementary motor area).
Anatomical labels were reported in all 19 articles. Some articles interspersed functional labels with anatomical ones in their results tables. For example, in Peterson et al. [1999], amid reports of activation in anatomical locations such as the middle temporal gyrus and inferior parietal cortex, activations were also listed in the sensorimotor cortex. The lack of standardization is evident by inspecting the bar graph of author‐assigned anatomical labels (Fig. 2). Although these trends in reporting anatomical labels are not inaccurate, they do present difficulties when attempting to include each focus in a review of author labels because this lack of consistency in naming schemes makes agreement difficult to assess and interpret.
The most frequently reported activation was in the left inferior frontal region (14 foci), followed by the left inferior parietal region (13 foci), and right and left anterior cingulate (13 and 11 foci, respectively). A high degree of agreement was found in the anterior cingulate gyrus (ACG)/cingulate region: authors reported 24 foci in the anterior cingulate (13 on the right; 11 on the left) and 8 foci in the cingulate gyrus (split equally across both hemispheres). Two additional foci were reported by George et al. [1994] in the right and left midcingulate sulcus.
Atlas‐label review
In the atlas‐label review of the Stroop studies, the 205 coordinates were assigned an anatomical label via the Talairach Daemon and tallied in bar graph format (Fig. 3). Activation was reported most frequently in the right and left inferior frontal gyrus (11 and 13 foci, respectively), right and left cingulate gyrus (9 and 11 foci, respectively), left middle frontal gyrus (11 foci), left inferior parietal lobule (11 foci), and the left precuneus (9 foci).
Figure 3.
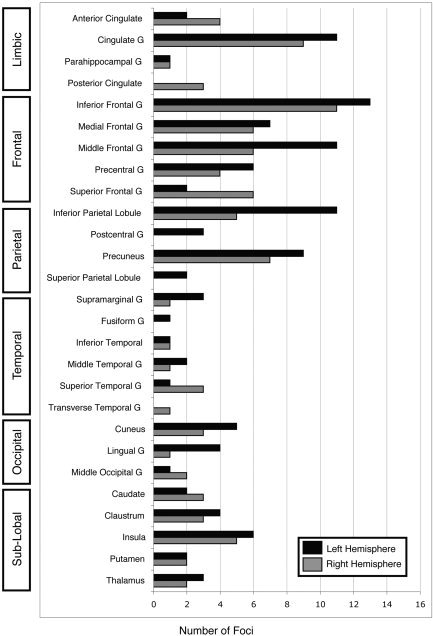
Atlas‐label review of the Stroop task. Using a standard nomenclature reduced the number of labels from 37 (authors) to 27 (atlas). The atlas‐label review found concordance in bilateral inferior frontal gyrus, bilateral cingulate gyrus, left middle frontal gyrus, left inferior parietal lobule, and left precuneus.
Comparison of author and atlas labels
Each author label was compared individually to the corresponding atlas label. We found that although 123 author labels agreed with the results of the Talairach Daemon search, 52 author labels did not. For example, a coordinate in the right inferior frontal gyrus was assigned the author label of “right temporal pole.” The remaining 30 labels were mismatches and included cases such as labeling a coordinate in the inferior parietal lobule as “parietal lobe” or the middle frontal gyrus as “lateral frontal cortex.” Although they affected the outcome of the author‐label review, these mismatches are not erroneous. Of 205 foci, 153 author labels thus agreed with their corresponding atlas labels and 52 did not.
The graphical results of the author‐label review and the atlas‐label review are similar but not identical, due to variations between authors as to the exact spatial extent of the neuroanatomical regions and to the lack of a standard nomenclature in anatomical labels. The author‐label review was based on 37 labels whereas the atlas‐label review used only 27. By using a wide range of naming styles, authors reduced the frequencies across the anatomical labels. For example, within the frontal lobe, “precentral gyrus” was seen more frequently within the atlas‐label review than in the author‐label review because some authors chose to name their coordinates in this region “sensorimotor.” This dilution of labels is the source for the differences seen in Figures 2 and 3, seen most clearly in the frontal lobe (5 atlas compared to 10 author labels).
According to both the author‐label review and the atlas‐label review, a high degree of agreement was seen in the ACG/cingulate region. The Talairach Daemon returned 26 foci in the anterior cingulate (4 foci in the right hemisphere; 2 foci in the left hemisphere) and cingulate gyrus (9 in the right; 11 in the left) (Table III). These foci were all labeled as cingulate or anterior cingulate coordinates by their respective authors and are plotted in Figure 4, in the sagittal view at x = 1 with the boundaries of the relevant Talairach regions. Most foci in Table III were assigned an atlas label of Brodmann area (BA) 32 of the cingulate gyrus (green region in Fig. 4). Of 34 cingulate foci in the author‐label review, most were classified as anterior cingulate: 24 anterior cingulate foci, 8 cingulate foci, and 2 midcingulate sulcus foci. Twenty‐six foci were confirmed by the Talairach Daemon to lie within the ACG/cingulate region. Eight foci were thus labeled as ACG/cingulate coordinates by authors but assigned labels from other regions by the Talairach Daemon (Table IV). Figure 5 allows for visual comparison between these eight foci and the nearby anterior cingulate and cingulate boundaries. In this figure, the coordinates are viewed at x = 1 mm (encompassing coordinates from x = −22 mm to x = +28 mm). Thus, while the George et al. [1994] coordinates appear to be located in the cingulate gyrus, they are actually located more laterally in the caudate.
Table III.
Agreement between atlas and author labels in the cingulate region
| Author label | Author | x | y | z | Atlas label (BA) |
|---|---|---|---|---|---|
| R cingulate | Steel | 12 | 36 | 15 | R anterior cingulate (32) |
| Steel | 3 | 42 | 9 | R anterior cingulate (32) | |
| L cingulate | Steel | −3 | 8 | 31 | L cingulate gyrus (24) |
| Steel | −9 | 14 | 31 | L cingulate gyrus (24 | |
| Steel | −3 | 36 | 26 | L anterior cingulate (32) | |
| R anterior cingulate | Banich | 6 | 18 | 40 | R cingulate gyrus (32) |
| Brown | 8 | 23 | 35 | R cingulate gyrus (32) | |
| Leung | 6 | 23 | 39 | R cingulate gyrus (32) | |
| MacDonald | 4 | 1 | 43 | R cingulate gyrus (24) | |
| Milham,2002 | 4 | 18 | 40 | R cingulate gyrus (32) | |
| Pardo | 9 | 18 | 31 | R cingulate gyrus (32) | |
| Pardo | 6 | 15 | 31 | R cingulate gyrus (32) | |
| Pardo | 15 | 24 | 29 | R cingulate gyrus (32) | |
| Peterson,2002 | 11 | 17 | 22 | R anterior cingulate (24) | |
| Peterson,1999 | 7 | 26 | 27 | R cingulate gyrus (32) | |
| Taylor | 3 | 35 | 18 | R anterior cingulate (32) | |
| L anterior cingulate | Brown | −4 | 14 | 35 | L cingulate gyrus (32) |
| Carter,2000 | 0 | 15 | 41 | Cingulate gyrus (32) | |
| Derbyshire | −2 | 14 | 40 | L cingulate gyrus (32) | |
| Derbyshire | 0 | 2 | 48 | Cingulate gyrus (24) | |
| George | −22 | 24 | 32 | L cingulate gyrus (32) | |
| Leung | −6 | 23 | 38 | L cingulate gyrus (32) | |
| Milham,2001 | 0 | 10 | 44 | Cingulate gyrus (32) | |
| Peterson,2002 | −10 | 15 | 24 | L cingulate gyrus (32) | |
| Peterson,1999 | −7 | 26 | 27 | L anterior cingulate (32) | |
| Peterson,1999 | −7 | 18 | 36 | L cingulate gyrus (32) |
Of 205 coordinates, 26 were assigned both an author and atlas label of cingulate gyrus or anterior cingulate gyrus. The coordinates listed here are plotted in Talairach space in Figure 4.
BA, Brodmann area.
Figure 4.
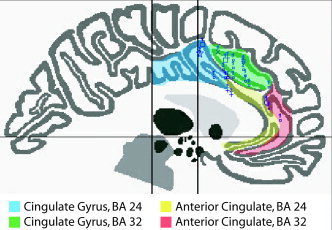
Foci in the anterior cingulate or cingulate gyrus. All 205 Stroop coordinates were input to the Talairach Daemon and assigned an anatomical label. Twenty‐six foci were labeled as anterior cingulate or cingulate gyrus (listed in Table III) and are seen here along with the boundaries of the corresponding Talairach regions, viewed at slice x = 1 mm (encompassing coordinates from x = −24 to +18 mm). Most foci were located in the anterior cingulate gyrus (BA 32; green shaded region above).
Table IV.
Disagreement between atlas and author labels in the cingulate region
| Author label | Author | x | y | z | Atlas label (BA) | Δ (mm) |
|---|---|---|---|---|---|---|
| R cingulate | Steel | 9 | −61 | 15 | R posterior cingulate (30) | 54 |
| Steel | 12 | −56 | 9 | R posterior cingulate (30) | 51 | |
| L cingulate | Fan | −4 | 38 | 30 | L medial frontal gyrus (9) | 6 |
| R anterior cingulate | Carter,1995 | 10 | 8 | 48 | R medial frontal gyrus (6) | 5 |
| Milham,2002 | 2 | 32 | 34 | R medial frontal gyrus (8) | 4 | |
| L anterior cingulate | Pardo | −12 | 42 | 21 | L medial frontal gyrus (9) | 9 |
| R midcingulate sulcus | George | 26 | −10 | 28 | R caudate | 20 |
| L midcingulate sulcus | George | −20 | 0 | 28 | L caudate | 17 |
Eight foci were assigned an author label in the cingulate region but assigned an atlas label from a different region by the Talairach Daemon. Most of these coordinates were located in the nearby medial frontal gyrus. The coordinates listed here are plotted in Talairach space in Figure 5. The approximate Euclidean distance from the coordinate to the closest point in the anterior cingulate or cingulate gyrus is indicated by (Δ).
BA, Brodmann area.
Figure 5.
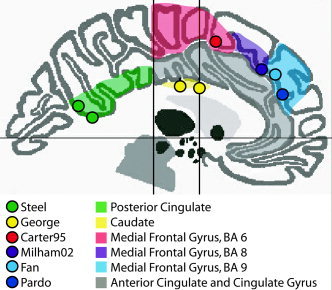
Foci mislabeled by authors. Eight coordinates were labeled as cingulate gyrus or anterior cingulate gyrus by the authors, but considered mislabeled by the Talairach Daemon (listed in Table IV). The coordinates are viewed at x = 1 mm (encompassing coordinates from x = −22 to +28 mm). The shaded region in which they are plotted corresponds to the anatomical label assigned by the Talairach Daemon.
Activation Likelihood Estimation
Pooled Stroop
The ALE meta‐analysis of Stroop studies revealed high ALE values in the limbic, frontal, and parietal lobes (Table V; Fig. 6A). Thirteen clusters were seen in the ACG (two clusters; one medial, one in the left hemisphere), bilateral frontal lobe (six clusters; four in the left hemisphere, two in the right hemisphere), left inferior parietal lobule (IPL), left precuneus, bilateral insula, and left supramarginal gyrus (P < 0.01). Extremely high ALE values were observed in the anterior cingulate gyrus (x = 1, y = 16, z = 38) with a cluster volume of 4,288 mm3. The clusters in the frontolateral cortex were identified as bilateral inferior frontal junction (IFJ), located between the precentral gyrus and inferior frontal gyrus. This region is known to be involved in cognitive control and is activated commonly in tasks such as the Stroop and the n‐back tasks [Derrfuss et al.,2004].
Table V.
ALE meta‐analysis of the Stroop color–word task*
| Anatomical region | BA | x | y | z | ALE (× 10−2) | Volume (mm3) |
|---|---|---|---|---|---|---|
| Anterior cingulate | 32 | 1 | 16 | 38 | 2.4 | 4,288 |
| Pooled | ||||||
| L inferior frontal junction | 6/9 | −44 | 5 | 33 | 1.9 | 1,680 |
| L inferior parietal lobule | 40 | −40 | −50 | 45 | 1.8 | 992 |
| L inferior frontal gyrus | 45 | −42 | 23 | 10 | 1.4 | 744 |
| L inferior frontal gyrus | 44 | −48 | 9 | 11 | 1.7 | 696 |
| L precuneus | 7 | −21 | −71 | 36 | 1.3 | 552 |
| R inferior frontal junction | 6/9 | 46 | 9 | 28 | 1.6 | 448 |
| L anterior cingulate gyrus | 32 | −3 | 38 | 25 | 1.4 | 360 |
| R insula | 13 | 36 | 12 | 7 | 1.2 | 312 |
| R superior frontal gyrus | 10 | 20 | 48 | 23 | 1.3 | 272 |
| L middle frontal gyrus | 9 | −42 | 30 | 31 | 1.3 | 200 |
| L supramarginal gyrus | 40 | −45 | −42 | 36 | 1.3 | 192 |
| L insula | 13 | −26 | 22 | 5 | 1.2 | 184 |
| Verbal | ||||||
| Anterior cingulate | 32 | 0 | 17 | 35 | 2.0 | 3,200 |
| L inferior frontal gyrus | 44 | −47 | 12 | 11 | 1.7 | 1152 |
| R superior frontal gyrus | 10 | 20 | 48 | 23 | 1.3 | 520 |
| L insula | 13 | −27 | 22 | 5 | 1.2 | 368 |
| R insula | 13 | 36 | 10 | 8 | 1.1 | 352 |
| R inferior frontal junction | 6/9 | 48 | 9 | 28 | 1.2 | 184 |
| L inferior parietal lobule | 40 | −40 | −52 | 44 | 1.2 | 168 |
| L inferior frontal junction | 6/9 | −46 | 8 | 35 | 1.1 | 112 |
| Manual | ||||||
| L inferior frontal junction | 6/9 | −43 | 4 | 35 | 1.2 | 2,136 |
| Anterior cingulate | 32 | 3 | 16 | 41 | 1.3 | 960 |
| L inferior parietal lobule | 40 | −47 | −40 | 47 | 1.2 | 792 |
| L middle frontal gyrus | 46 | −34 | 21 | 24 | 1.3 | 648 |
| L precuneus | 7 | −21 | −70 | 37 | 1.1 | 600 |
P < 0.01, all three meta‐analyses. In the pooled and verbal ALE meta‐analysis, the highest ALE values were found in the anterior cingulate gyrus. Meta‐analysis of the manual Stroop task showed strong results in the left inferior frontal junction. In the manual Stroop meta‐analysis, the peak observed in the anterior cingulate was much smaller than that seen in the verbal Stroop meta‐analysis. Each cluster listed above was observed with a peak P value of < 0.0002. Due to the method of statistical inference, it was not possible to assess the P value of the center of each cluster with any greater precision than P < 0.0002.
BA, Brodman area; ALE, activation likelihood estimation.
Figure 6.
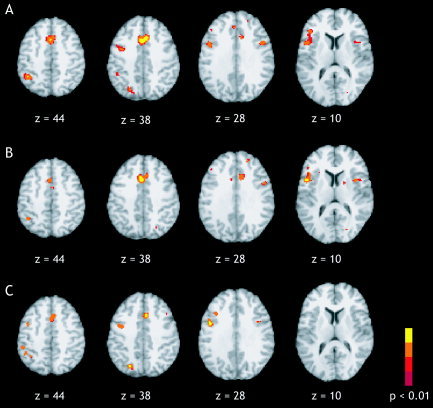
ALE maps for pooled, verbal, and manual Stroop (P < 0.01). A: In the pooled Stroop ALE meta‐analysis (19 studies, 205 foci) significant activation likelihood was seen in the anterior cingulate (z = 28, 38, 44). A lesser degree was seen in the left inferior frontal junction (z = 28, 38), the left inferior frontal gyrus (z = 10), and the left inferior parietal lobule (z = 38, 44). B: ALE meta‐analysis of verbal Stroop (13 studies, 153 foci) also revealed high ALE values in the anterior cingulate (z = 28, 38, 44) and left inferior frontal gyrus (z = 10). C: ALE meta‐analysis of manual Stroop (6 studies, 52 foci) resulted in clusters in the left inferior frontal junction (z = 28, 38, 44) and left inferior parietal lobule (z = 44).
Verbal Stroop
In the ALE meta‐analysis of Stroop tasks that required an overt or covert verbal response (13 studies with 153 coordinate sets), regions of high ALE values were identified in the left inferior frontal gyrus (IFG) near BA 44 and bilateral insula (P < 0.01), two regions commonly involved in articulation (Table V; Fig. 6B). The focus located in the ACG (x = 0, y = 17, z = 35) exhibited the highest activation likelihood and extended twice the volume of the second largest cluster (volume = 3,200 mm3). Smaller regions were found in the right superior frontal gyrus, bilateral IFJ, and left IPL.
Manual Stroop
In the Stroop literature, studies requiring a verbal response greatly outnumbered those requiring a manual (button press) response. Six studies with 52 coordinates were included in the manual Stroop meta‐analysis. Pooling these studies resulted in the determination of five clusters of significant ALE values in the left IFJ (BA 6/9), medial ACG, left IPL, left middle frontal gyrus, and left precuneus (P < 0.01; Table V, Fig. 6C). The left IFJ peak (x = −43, y = 4, z = 35) exhibited the highest ALE values with a volume of 2,136 mm3. The ACG cluster was much smaller in the manual Stroop results (volume = 960 mm3) than it was in the verbal Stroop results (volume = 3,200 mm3). Notably missing from ALE map of manual Stroop studies were the areas involved in speech production observed previously in the verbal Stroop results, namely left BA 44 and bilateral insula.
Verbal Stroop vs. manual Stroop
One similarity between the verbal and manual Stroop meta‐analyses was the agreement across lobes. High ALE values were found in regions inside the limbic, frontal, and parietal lobes in both Stroop tasks. Although the ALE map for the verbal Stroop meta‐analysis included peaks in both the right and left hemispheres, manual Stroop results were limited to only the left hemisphere and medial ACG. Regional results indicate that both variations of the Stroop task rely on the anterior cingulate, left IFJ and left IPL, and that these regions are central to processing the Stroop effect.
To determine the extent of agreement between verbal and manual Stroop in terms of voxel‐wise agreement, a composite map of the ALE results for each response type was created. Three clusters were subsequently identified as involved in both verbal and manual Stroop with centers of mass at the anterior cingulate (x = 2, y = 16, z = 41), left IFJ (x = −44, y = 6, z = 34), and left IPL (x = −36, y = −52, z = 44) (P < 0.01). The ACG peak observed in the manual Stroop was not as robust as that in the verbal task, which was likely due in part to the smaller number of included studies. The center‐of‐mass of the manual Stroop cluster was located 6 mm superior to the verbal Stroop cluster; however, the two clusters were located in the same region of the anterior cingulate (BA 32), and the spatial extent of overlap can be seen in Figure 7 (P < 0.05). Regions of overlap in the left IFJ and the left IPL were smaller in extent than was the ACG overlap.
Figure 7.
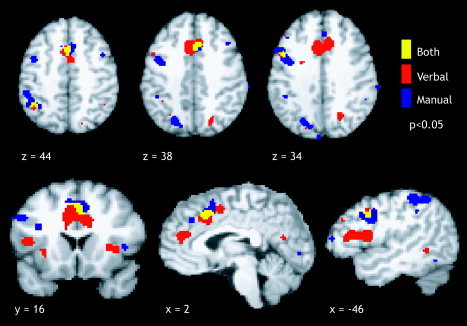
Composite maps for verbal and manual Stroop (P < 0.05). ALE meta‐analysis of the Stroop task with a verbal response (red), manual response (blue), and the overlap between the two response types (yellow) reveals a large region of overlap within the anterior cingulate gyrus, and two smaller regions of overlap in the left inferior frontal junction (x = −46, z = 34) and left inferior parietal lobule (z = 44). Our results suggest that these three regions are major components of a network for response conflict resolution in the Stroop task.
Once the ALE meta‐analysis for all Stroop studies was complete, the BrainMap database was searched to determine the foci of the original 205 that were located within an ROI that was defined by the extent of the ACG cluster from the pooled Stroop meta‐analysis. The bounding box of this ROI was obtained from the ALE map (P < 0.05; x = −12–16 mm, y = 0–32 mm, and z = 22–54 mm). Once the coordinates that fell within the bounding box were determined, they were inspected to verify which ones actually fell within the ACG cluster border. The bounding box search results can be seen in Figure 8, and the coordinates lying within the ACG border are listed in Table VI. From the original studies used in the meta‐analysis, 20 foci were located within the ACG ROI: 18 in the ACG/cingulate region, one in the medial frontal gyrus, and one in the right superior frontal gyrus. Of 26 ACG/cingulate gyrus foci, 18 were thus returned by backsampling from the ALE ROI to the articles in BrainMap.
Figure 8.
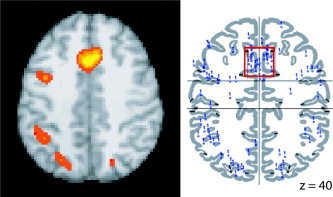
Backsampling the ALE anterior cingulate gyrus cluster. A region of interest (ROI) was drawn around the center of mass of the ALE cluster in the anterior cingulate for the pooled Stroop meta‐analysis (left; P < 0.05) and an ROI search in BrainMap Search&View determined that 20 foci from the original studies that were located within this boundaries of this anterior cingulate gyrus ROI (right; coordinates listed in Table VI).
Table VI.
Backsampling from ALE to original foci
| Author | x | y | z | TD label (BA) |
|---|---|---|---|---|
| Banich | 6 | 18 | 40 | R cingulate gyrus (32) |
| Brown | 8 | 23 | 35 | R cingulate gyrus (32) |
| Brown | −4 | 14 | 35 | L cingulate gyrus (32) |
| Carter,2000 | 0 | 15 | 41 | Cingulate gyrus (32) |
| Derbyshire | −2 | 14 | 40 | L cingulate gyrus (32) |
| Derbyshire | 0 | 2 | 48 | Cingulate gyrus (24) |
| Leung | 6 | 23 | 39 | R cingulate gyrus (32) |
| Leung | −6 | 23 | 38 | L cingulate gyrus (32) |
| MacDonald | 4 | 1 | 43 | R cingulate gyrus (24) |
| Milham,2001 | 0 | 10 | 44 | Cingulate gyrus (32) |
| Milham,2002 | 4 | 18 | 40 | R cingulate gyrus (32) |
| Milham,2002 | 4 | 10 | 54 | R superior frontal gyrus (6) |
| Milham,2002 | 2 | 32 | 34 | R medial frontal gyrus (6) |
| Pardo | 9 | 18 | 31 | R cingulate gyrus (32) |
| Pardo | 6 | 15 | 31 | R cingulate gyrus (32) |
| Peterson,1999 | 7 | 26 | 27 | R cingulate gyrus (32) |
| Peterson,1999 | −7 | 18 | 36 | L cingulate gyrus (32) |
| Steel | −3 | 8 | 31 | L cingulate gyrus (24) |
| Steel | −9 | 14 | 31 | L cingulate gyrus (24) |
Twenty foci from the studies used in the meta‐analysis were located within the ACG region of interest (ROI): 18 in the ACG/cingulate gyrus region, 1 in the medial frontal gyrus, and 1 in the right superior frontal gyrus. Of 26 ACG/cingulate gyrus foci, 18 were thus returned by backsampling from the ALE ROI to the studies in BrainMap. The remaining 8 ACG/cingulate gyrus foci were located nearby, just outside the boundaries of the ROI.
BA, Brodmann area.
Somatotopy in the cingulate motor area
We compared the ALE meta‐analyses for verbal and manual Stroop (P < 0.05) to investigate the issue of functional segregation within the cingulate motor area. High ALE values were found in regions extending along the length of the cingulate sulcus, rostral to the vertical plane passing through the anterior commissure (VCA), that clearly display multiple distinct areas for verbal and manual response types (Fig. 9). The motor region of the anterior cingulate is divided into the rostral cingulate zone (rCZ) located anterior to the VCA and superior to the corpus callosum, and the caudal cingulate zone (cCZ), which lies approximately posterior to the VCA. Furthermore, the rCZ is subdivided into an anterior division (rCZa) and a posterior division (rCZp). Two regions are seen in the rCZa: one large verbal area near the genu of the corpus callosum (x = 3, y = 41, z = 18) and one smaller manual area, posterior and superior to the verbal region (x = 2, y = 32, z = 34). Additionally, a verbal rCZp region (x = 1, y = 16, z = 36) is located inferior to a manual rCZp region (x = 3, y = 15, z = 43). The verbal rCZp cluster wraps up and around the manual rCZp cluster such that a portion of it lies in the cCZ, near the VCA. The manual region, in contrast, seems to extend toward the pre‐SMA and is located completely anterior to the VCA. Finally, we note that there are two regions of overlap between the verbal and manual response types: one large region in the rCZp (x = 2, y = 16, z = 41) and one smaller region in the rCZa (x = −3, y = 37, z = 25).
Figure 9.
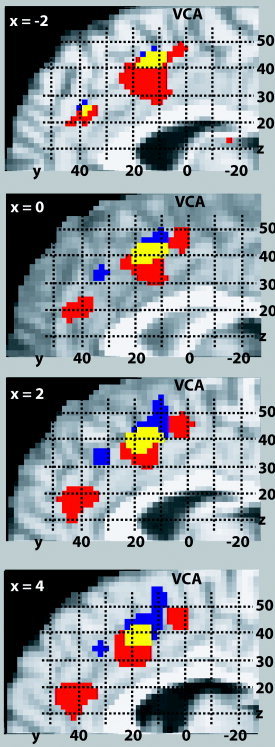
Somatotopy in the cingulate motor area. ALE meta‐analysis maps (P < 0.05) are shown for the Stroop task for verbal responses (red), manual responses (blue), and their overlap (yellow) at four slices in the sagittal orientation. A high probability for activation was found in regions that extend along the length of the cingulate sulcus, rostral to the vertical plane passing through the anterior commissure (VCA), that clearly display multiple distinct areas for verbal and manual response types.
DISCUSSION
We found agreement between the results of the author‐label review, the atlas‐label review, and the ALE method of meta‐analysis. Although we found some evidence that the lack of standard nomenclature caused the labels to be split too finely in the author‐label review, overall results of high agreement in the frontal lobe (specifically, the ACG and left inferior frontal gyrus) and left IPL are robust on both reviews and agree with the ALE results. The ALE results agree with the more conventional label‐based reviews; however, ALE offers the added benefits of being more quantitative and providing a measure of statistical reliability.
Label‐Based Reviews
Differences between the two label‐based reviews are due mainly to the lack of a standard nomenclature, which can be seen in the labels chosen by the authors that were split into parallel or redundant sets. Inaccuracies in author labeling can be classified into two categories: errors and mismatches. Errors occurred when the assigned label was simply wrong. Explanations for such mislabeling may include inexperience or insufficient attention to detail. Fifty‐two labels were classified as errors in the author‐label review. Mismatches present a more subtle type of mislabeling and occurred in 30 author labels. This mislabeling resulted when authors used a broad term when a more specific one would have worked just as well (e.g., parietal lobe instead of inferior parietal lobe) or when an alternative nomenclature was employed (e.g., motor cortex instead of precentral gyrus). The list of author labels used in the frontal lobe (Fig. 2) shows that the true agreement of activation locations is somewhat masked because the conventional anatomical terms such as inferior frontal gyrus, middle frontal gyrus, and precentral gyrus are mixed with alternative labels that are either directional (“lateral frontal” and “frontal polar”) or functional (“premotor” and “SMA”). This frontal lobe label example illustrates how labeling schemes are intrinsically problematic and often deliver ambiguous results.
At first glance, it seemed that there was a large discrepancy in results concerning concordance in the anterior cingulate and cingulate gyrus between the atlas‐label and author‐label reviews. After checking the author labels against the Talairach Daemon, eight incompatible cingulate labels were identified; four were in the medial frontal gyrus, within 10 mm of the cingulate gyrus (red, purple, and blue regions of Fig. 5). This would account for the greater concordance assessed in the medial frontal gyrus by Talairach Daemon as compared to author labels; however, these foci were located in a region that may be considered as cingulate gyrus within the bounds of intersubject variability. In the context of Stroop task performance, it may be accurate to label these foci as cingulate activation. Four other coordinates were categorized as errors (green and yellow regions of Fig. 5), as they were located in the posterior cingulate [Steel et al.,2001] and the caudate [George et al.,1994]. Technically, it was not incorrect for Steel et al. [2001] to label his foci as “cingulate” because these coordinates were located in the posterior cingulate; however, within the context of Stroop articles that focus on activation in the anterior cingulate cortex, this inexact label of “cingulate” is misleading and is therefore classified as an error. Cingulate concordance assessed by the atlas‐label review and the author‐label review thus is nearly identical, but only if consideration is allowed for the fact that the author label results were inflated toward homogeneity and the tendency to report activations in agreement with prior expected results. In addition, we note in Table III that there is no clear or well‐accepted distinction between the cingulate gyrus and the anterior cingulate gyrus among authors.
Our comparison of author and atlas labels revealed that author labels retained a discrepancy rate of 25% (52 of 205). Although this number is high, it is inflated in the same manner that the initial finding of disagreement in the cingulate cortex was inflated. In the ACG/cingulate gyrus region, eight foci seemed incorrect, but it was determined that four were close to the gyri boundaries and could be considered accurate within the limits of intersubject variability. By inspection, it was found that 38 of 52 foci with incongruent labels lay within reasonable distance to the boundaries of the authors' labels. Because most authors utilize high‐resolution MRI images when assigning anatomical labels and the Talairach Daemon labels are derived from a single atlas, a degree of discordance is expected when comparing author labels to atlas labels. We argue that of 205 foci, only 14 were truly inaccurate, and thus the author‐label review actually retained a discrepancy rate of 7% rather than 25%. Nevertheless, we recommend that all label‐based reviews should be carried out with an atlas, such as the Talairach Daemon, and an experienced neuroanatomist should verify the accuracy of each label.
Activation Likelihood Estimation
By eliminating the issues inherent in label‐based techniques and relying solely on coordinate data, we conclude that the ALE method is the preferred approach to searching for regions of agreement across multiple studies. Our ALE results of the verbal and manual Stroop color–word task were successful in identifying three regions of overlap: the anterior cingulate, left IFJ, and left IPL. These regions thus have been isolated as major components of the network for response conflict resolution in the Stroop task. In addition, by using the ALE method we were able to examine the cingulate motor area and find evidence for heterogeneity within this region based on response modality. As such, we conclude that ALE offers many benefits not possible with traditional label‐based reviews.
Interestingly, the cingulate gyrus response is much more prominent in the ALE meta‐analysis (Fig. 6) as compared to that in the label‐based reviews (Fig. 2 and 3). This is evidence of the effect of the higher level of spatial distinction allowed with the ALE method. Two things are responsible for this result. First, the ACG is a smaller region than are other regions seen in the ALE results, such as the inferior frontal gyrus. What seems like a stronger result in the IFG is actually smaller in comparison because the foci located in the IFG are spread out across a larger surface area than that in the cingulate cortex. Second, most foci reported in the frontal lobe are located on the lateral side of the brain, whereas the foci reported in the cingulate are located medially. Although label‐based reviews interpret the results separately for each hemisphere for the regions in the frontal lobe, they therefore should be evaluated collectively for both the right and left ACG and cingulate gyrus because most of these coordinate locations lie along the midline of the brain and thus should be treated effectively as a single unit.
Somatotopy in the cingulate motor area
Controversy exists concerning functional heterogeneity within the cingulate motor area. Despite differences in data and analysis method, there are striking similarities between areas of dissociation between verbal and manual responses in the Picard and Strick reviews [1996;2001], the Paus et al. [1993] study, and the ALE meta‐analysis presented here. Our ALE results agree with these three previous publications in concluding that somatotopic mapping exists in the ACG with multiple regions in the rCZ for hand and speech response types. In agreement with Paus et al. [1993], Paus [2001], and Picard and Strick [1996], two regions were seen in the rCZa: one large verbal area near the genu of the corpus callosum and one smaller manual area, posterior and superior to the verbal region. The manual region of the rCZp, in contrast, seems to extend toward the pre‐SMA and is located completely anterior to the VCA, a result that disagrees with the findings of Paus et al. [1993] and Picard and Strick [1996]. Our results agree with Picard and Strick [1996] and Paus et al. [1993] concerning the verbal regions in the rCZa and rCZp and localization of the hand areas in the rCZ in Picard and Strick [1996], but they are not consistent with idea that activations associated with manual responses are localized to cCZ.
Supporting our conclusion for functional segregation within the ACG is the fact that we have chosen an advanced method of meta‐analysis that is more systematic than are traditional methods. ALE searches for regions of agreement and overlap across multiple studies with greater precision. In addition, this study includes additional data that was unavailable at the time of the previous studies and reviews. Our meta‐analysis data represents a cleaner subset of the literature as we have included only data acquired on the traditional Stroop task and have not confounded our study with multiple tasks involving various different types of processing.
Meta‐analysis can only answer the question that it was designed to address. A primary goal of this meta‐analysis was to determine locations of consistent activation within the Stroop task, and this question led to a discussion concerning the presence of somatotopy within the anterior cingulate. A deeper investigation of this topic would likely benefit from the inclusion of the large collection of data from alternate tasks that also are known to activate the ACG, namely verbal fluency, selective attention, Go/NoGo, and working memory. Further work thus will concentrate on expanding the paradigms included in this meta‐analysis with the goal of determining if the ACG is subdivided by response modality for a wider range of tasks. In addition, the questions concerning the strategic versus evaluative function [Carter et al.,2000] or the cognitive versus emotional divisions [Bush et al.,2000] of the ACG have gone unanswered here. Future paradigm driven or anatomically driven meta‐analyses may be designed with the goal of testing these issues.
Acknowledgements
We thank A. Uecker and P. Mickle Fox for their expertise in software programming and S. Farmer for her help in coding articles and checking author labels.
REFERENCES
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z‐P, Wright A, Shenker J, Magin R (2000): fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci 12: 988–1000. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I (2002): Imaging how attention modulates pain in humans using functional MRI. Brain 125: 310–319. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A (2001): Anterior cingulate cortex and response conflict: Effects of response modality and processing domain. Cereb Cortex 11: 837–848. [DOI] [PubMed] [Google Scholar]
- Becker JT, MacAndrew DK, Fiez JA (1999): A comment of the functional localization of the phonological storage subsystem of working memory. Brain Cogn 41: 27–38. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RSJ, Dolan RJ (1993): Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia 31: 907–922. [DOI] [PubMed] [Google Scholar]
- Brett M (1999): The MNI brain and the Talairach atlas, Cambridge Imagers. http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html
- Brown GG, Kindermann SS, Siegle GJ, Granholm E, Wong EC, Buxton RB (1999): Brain activation and pupil response during covert performance of the Stroop Color Word task. J Int Neuropsychol Soc 5: 308–319. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Petersen SE (1996): What does neuroimaging tell us about the role of prefrontal cortex in memory retrieval? Sem Neurosci 8: 47–55. [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998): The counting Stroop: an interference task specialized for functional neuroimaging—validation study with functional MRI. Hum Brain Mapp 6: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, MacDonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD (2000): Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 97: 1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD (1995): Interference and facilitation effects during selective attention: an HO PET study of Stroop task performance. Neuroimage 2: 264–272. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fissell K, Jacobs S, Fiez JA (2002): Functional heterogeneity within Broca's area during verbal working memory. Physiol Behav 77: 635–639. [DOI] [PubMed] [Google Scholar]
- Conn HO (1997): Interpretation of data from multiple trials: a critical review. J Intern Med 241: 177–183. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Wilson SJ, McMahon KL, Muthiah S (2001): The semantic interference effect in the picture‐word paradigm: an event‐related fMRI study employing overt responses. Hum Brain Mapp 14: 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW, Vogt BA, Jones AK (1998): Pain and Stroop interference tasks activate separate processing modules in anterior cingulate cortex. Exp Brain Res 118: 52–60. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY (2004): Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage 23: 604–612. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI (2003): Cognitive and brain consequences of conflict. Neuroimage 18: 42–57. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE (1996): A positron emission tomography study of the short‐term maintenance of verbal information. J Neurosci 16: 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Parson LM, Lancaster JL (1998): Beyond the single study: function/location meta‐analysis in cognitive neuroimaging. Curr Opin Neurobiol 8: 178–187. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Trimble MR, Horwitz B, Herscovitch P, Post RM (1994): Regional brain activity when selecting a response despite interference: an HO PET study of the Stroop and an Emotional Stroop. Hum Brain Mapp 1: 194–209. [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Ingham JC, Finn P, Fox PT (2003): Towards a functional neural systems model of developmental stuttering. J Fluency Disord 28: 297–318. [DOI] [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattie B, Leon AC (1999): Linguistic threat activates the human amygdala. Proc Natl Acad Sci USA 96: 10456–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Lancaster JL, Thompson P, Toga AW, Brewer P, Hardies J, Fox PT (2002): An optimized individual target brain in the Talairach coordinate system. Neuroimage 17: 922–927. [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uekcer AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005): ALE meta‐analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000): Automated Talairach labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC (2000): An event‐related functional MRI study of the Stroop Color Word Interference Task. Cereb Cortex 10: 552–560. [DOI] [PubMed] [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, Carter CS (2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Mead LA, Mayer AR, Bobholz JA, Woodley SJ, Cunningham JM, Hammeke TA, Rao SM (2002): J Int Neuropsychol Soc 8: 735–742. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ (2003): Practice‐related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage 18: 483–493. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF (2001): The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res 12: 467–473. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ (2002): Attentional control in the aging brain: Insights from an fMRI study of the Stroop task. Brain Cogn 49: 277–296. [DOI] [PubMed] [Google Scholar]
- Mosteller F, Colditz GA (1996): Understanding research synthesis (meta‐analysis): Annu Rev Public Health 17: 1–23. [DOI] [PubMed] [Google Scholar]
- Owen AM (1997): The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. Eur J Neurosci 9: 1329–1339. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME (1990): The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA 87: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T (2001): Primate anterior cingulate cortex: where motor control, drive, and cognition interface. Nat Rev Neurosci 2: 417–424. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E (1993): Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol 70: 453–469. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung H‐C, May J, Gore JC (2002): An event‐related fMRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res 13: 427–440. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC (1999): An fMRI study of Stroop Word‐Color Interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry 45: 1237–1258. [DOI] [PubMed] [Google Scholar]
- Petitti DB (1997): Meta‐analysis and endocrinology. Endocrinol Metab Clin North Am 26: 31–44. [DOI] [PubMed] [Google Scholar]
- Phan LK, Wager T, Taylor SF, Liberzon I (2002): Functional neuroanatomy of emotion: a meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (1996): Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6: 342–353. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (2001): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Woodward TS, Laurens KR, Liddle PF (2001): The role of the anterior cingulate cortex in conflict processing: evidence from Reverse Stroop Interference. Neuroimage 14: 1150–1158. [DOI] [PubMed] [Google Scholar]
- Steel C, Haworth EJ, Peters E, Hemsley DR, Sharma T, Gray JA, Pickering A, Gregory L, Simmons A, Bullmore ET, Williams SCR (2001): Neuroimaging correlates of negative priming. Neuroreport 12: 3619–3624. [DOI] [PubMed] [Google Scholar]
- Stroop JR (1935): Studies of interference in serial verbal reactions. J Exp Psychol 18: 643–662. [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA (1997): Isolation of specific interference processing in the Stroop Task: PET activation studies. Neuroimage 6: 81–92. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: method and validation. Neuroimage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL (1998): The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44: 1219–1228. [DOI] [PubMed] [Google Scholar]
- Yucel M, Pantelis C, Stuart GW, Wood SJ, Maruff P, Velakoulis D, Pipingas A, Crowe SF, Tochon‐Danguy HJ, Egan GF (2002): Anterior cingulate activation during Stroop task performance: a PET to MRI coregistration study of individual patients with schizophrenia. Am J Psychiatry 159 251–254. [DOI] [PubMed] [Google Scholar]


