Abstract
The process of forming new associations between previously unrelated items of information, such as a name and a face, likely requires the integration of activity within multiple brain regions. The hippocampus and related structures in the medial temporal lobe are thought to be particularly critical in binding together items of information. We studied eight healthy young subjects with functional magnetic resonance imaging (fMRI) during the encoding of novel face‐name associations compared to viewing repeated face‐name pairs. A consistent pattern of activation was observed in the hippocampus, pulvinar nucleus of the thalamus, fusiform and dorsolateral prefrontal cortices across individual subjects. The location of the activation within the hippocampus was more anterior than previously reported in studies using similar novel vs. repeated paradigms with stimuli that did not specifically require relational processing among unrelated items. These data suggest that the process of forming new face‐name associations is supported by a distributed network of brain regions, and provide additional evidence for the essential role of the hippocampus in associative memory processes. Hum. Brain Mapping 14:129–139, 2001. © 2001 Wiley‐Liss, Inc.
Keywords: memory, hippocampus, magnetic resonance imaging, prefrontal cortex, human
INTRODUCTION
The ability to learn and remember associations between previously unrelated information is an essential aspect of episodic memory. Forming an association between a name and a face is a particularly difficult associative memory task, because names and faces are inherently unrelated. Despite the fact that face‐name association is a fundamental requirement of everyday memory, relatively little is known about the functional neuroanatomy subserving this process.
Given the critical role of the hippocampus and related structures in the medial temporal lobe (MTL) in episodic memory, these regions are particularly likely to be involved in forming a new face‐name association. The theory that the hippocampus is essential for “binding” together previously unrelated information [Eichenbaum, 1997] is supported by studies of amnestic patients with focal brain lesions [Chalfonte et al., 1996; Schacter and Church, 1995; Squire, 1992], animal models of amnesia [Squire and Zola‐Morgan, 1991] and animal electrophysiologic work [Bunsey and Eichenbaum, 1993; Suzuki and Eichenbaum, 2000; Wood et al., 1999]. In addition, in a meta‐review of PET and fMRI studies of memory processes, Schacter and Wagner [1999] noted that the studies yielding robust anterior MTL activations primarily utilized memory paradigms that required “relational” processing among stimuli. One of these studies [Henke et al., 1997] directly compared associative encoding of stimuli vs. non‐associative encoding of the same stimuli, and found hippocampal and parahippocampal activation only during the associative learning task.
In addition to the hippocampus, the encoding of novel face‐name associations likely requires the integration of several other brain regions, including the prefrontal and fusiform cortices. A large number of recent functional imaging studies have suggested that the dorsolateral prefrontal cortex is activated during memory tasks [D'Esposito et al., 1995; McCarthy et al., 1996; Rypma and D'Esposito, 1999; Wagner et al., 1998], and these regions would likely be involved in the process of associative encoding. In addition, both human lesion studies and functional imaging work have implicated a specific region in the right fusiform gyrus that is critical for the processing of human faces. [Damasio et al., 1990; Haxby et al., 1996; Kanwisher et al., 1997; Puce et al., 1995]. Analogous areas in the left fusiform and lingual gyri have demonstrated activation during verbal encoding tasks [Martin et al., 1996; Nobre et al., 1994].
We undertook this study, in healthy young subjects, to begin to examine the functional neuroanatomic networks subserving associative encoding. We chose to use unfamiliar faces with first names, which are inherently unrelated and thus require the formation of a novel association. Many of the above mentioned brain regions, specifically the hippocampal formation and the prefrontal cortices, have also been implicated in the detection of novelty. Previous studies have reported activation in posterior hippocampal and parahippocampal regions during the encoding of novel vs. repeated complex visual scenes [Rombouts et al., 1997; Stern et al., 1996]. To test the hypothesis that the encoding of novel associations would activate more anterior regions of the hippocampus, we chose to examine the encoding of novel face‐name associations compared to viewing repeated face‐name pairs, using functional MRI.
METHODS AND MATERIALS
Subjects
Eight right handed, English speaking healthy volunteers, age 22–32, were recruited through advertising at local universities. There were six females and two males. Subjects were screened for history of neurologic or psychiatric illness, and for any contraindication to MR imaging. None of the subjects were taking prescription or over‐the‐counter medications with central nervous system effects. All subjects were scanned during the morning hours. This study was approved by the Human Research Committee of the Massachusetts General Hospital. All subjects provided written informed consent.
Cognitive activation task
The task consisted of viewing faces unfamiliar to the study subjects paired with fictional first names, in a modified Novel vs. Repeated design [Stern et al., 1996]. Subjects were given explicit instructions to try to remember which name was associated with which face for later testing.
Stimuli
The face‐name stimuli consisted of a face shown on a black background with a fictional first name printed underneath the face, forming a face‐name pair. The faces were digital color photographs taken of individuals previously unknown to the subjects, with a digital camera (Fuji 800) with resolution of 924 × 1,096 mp. The faces were of individuals who varied in age (range 18–90) and ethnicity, with equal numbers of male and female faces. Popular first names were obtained from the public lists on the Internet of the most commonly used names for each decade from 1910 through 1990. First names were assigned to each face by the investigators.
Task conditions
The fMRI activation task consisted of three conditions (see Fig. 1):
Figure 1.
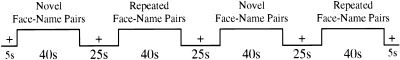
Diagram of “Block Design” fMRI paradigm, showing Novel Face‐Name Pairs, Repeated Face‐Name Pairs and Fixation (+) conditions. Each functional “Run” lasted for 4 min and 5 sec, and a total of six runs were shown to each subject.
-
1
Novel Face‐Name Pairs: Each face‐name pair was presented for 5 sec, followed by a brief (0.8 sec) white central fixation crosshair on a black background. Subjects viewed seven novel face‐name pairs during each Novel block.
-
2
Repeated Face‐Name Pairs: Subjects examined two repeated face‐name pairs (one male and one female). These two repeated face‐name pairs were first shown to the subject in a practice run, so that they were already familiar to the subjects at the beginning of the functional runs. As with the novel face‐name pairs, each repeated face‐name pair was shown for 5 sec, followed by a brief (0.8 sec) central fixation crosshair. The male and female face‐name pairs alternated throughout each Repeated block.
-
3
Visual Fixation: Subjects examined a white fixation cross (+) presented in the center of the visual field on a black background, to focus the subject's attention in the visual domain.
Six runs, each consisting of the format in Figure 1, were obtained on each subject in rapid succession. Before the scanning session and before each run, subjects were given explicit instructions to try to remember the name associated with each face. A total of 86 face‐name pairs were used over the course of the entire experiment (84 novel and two repeated). Each face‐name pair in the Novel condition was seen only once by each subject, whereas the two Repeated Face‐Name Pairs were each seen a total of 45 times over the course of the scanning session.
Visual stimuli were presented using a Macintosh Computer (Apple) connected to a Sharp 2000 color LCD projector. Images were projected through a collimating lens (Buhl Optical) onto a screen attached to the head coil during functional data acquisition.
Post‐scan testing
Immediately following the imaging session, subjects were tested for their memory of a subset of the face‐name pairs. Twelve faces were presented: six from the set of novel face‐name pairs, four distractor faces (not presented during the experiment), and the two faces from the repeated face‐name pairs presented throughout the scanning session. Subjects were asked to indicate if they had seen the face before with a yes/no answer, and if yes, to recall the name associated with the face.
Image acquisition
Structural and echo planar functional images were acquired on a 3.0 Tesla General Electric scanner with ANMR upgrade (Advanced NMR Systems, Wilmington, MA). The standard GE quadrature head coil was used. Subjects were positioned in the head coil with a cervical collar and foam padding to reduce head motion.
A sagittal localizer scan was first performed for positioning of the slice planes, followed by an automated shim procedure to improve B0 magnetic field inhomogeneity [Reese et al., 1995]. A spoiled gradient echo (SPGR) scan, consisting of 60 slices (resolution 1.6 × 1.6 × 2.8 mm), was obtained for registration with the functional data for anatomic localization.
Functional data were acquired using an asymmetric spin echo T2* weighted Blood Oxygen Level Dependent method (BOLD) with TR = 2,500 msec, TE = 40 msec, and Flip angle = 90. Voxel dimensions = 3.125 × 3.125 × 8.0 mm. Twenty slices (7 mm thick; 1 mm gap between slices) were acquired in a coronal orientation, perpendicular to the anterior commissure‐posterior commissure (AC‐PC) line. This orientation was chosen to maximize the in‐plane resolution within the hippocampus and reduce partial voluming between hippocampal and parahippocampal regions. Images were acquired beginning at the occipital pole. This acquisition protocol provided nearly whole brain coverage, but in some subjects excluded the most anterior extent of the frontal pole. Scanning time for each functional run was 4 min and 15 sec, consisting of 102 time points (four for T1 stabilization and 98 for functional data collection). A total of six functional runs per subject were acquired.
Image analysis
All functional data were motion‐corrected [Jiang et al., 1995] based upon Woods et al [1992]. The motion correction program results in the loss of the first and last slice, yielding 18 coronal slices for subsequent analysis. Runs showing greater than 2 mm of movement were excluded, resulting in an average of 5.1 usable runs per subject. The motion corrected runs were then averaged (timepoint to timepoint) to yield a single mean run of 98 timepoints for each of the 18 coronal slices.
To allow averaging across subjects, both the functional and structural image data were aligned in standard stereotaxic coordinate system [Talairach and Tournoux, 1988], using trilinear interpolation [Bush et al., 1996]. This transformation yielded 57 slices in the coronal orientation (voxel dimension, x,y,z: 3.13 × 3.13 × 3 mm).
Statistical maps, identifying voxels with significant positive task‐related signal changes, henceforth referred to as “activation” for individual subject data, were generated using a non‐parametric test, the Kolmogorov‐Smirnov (K‐S) statistic [Press et al., 1992].
Activation maps were generated in two ways: 1) the KS statistic was applied to the individual motion‐corrected data, before Talairach transformation, to examine activation in relation to individual subjects; and 2) the KS statistic was applied to both the individual and group averaged data after transformation into stereotaxic space.
A cluster‐growing algorithm was used to identify the location (in Talairach coordinates) of the maximal activation within each cluster in the statistical maps [Bush et al., 1998]. To minimize the possibility of false positive results [Aguirre et al., 1998] only activations with clusters of greater than 5 voxels and P < 0.001 were considered significant for individual subject data. For the group data, activations with clusters greater than 10 contiguous voxels and P < 0. 00001 were considered significant. In addition, the percent change in BOLD signal intensity was calculated for regions showing significant activation [Manoach et al., 2000; Rauch et al., 2000] for correlation with the behavioral data.
RESULTS
Activation maps were generated for 3 contrasts: 1) Novel Face‐Name Pairs vs. Visual Fixation (Novel vs. Fixation), which primarily provided information regarding the encoding of complex visual face‐name stimuli compared to a low level visual fixation; 2) Repeated Face‐Name Pairs vs. Visual Fixation (Repeated vs. Fixation), which primarily provided information regarding the viewing of familiar complex visual stimuli compared to fixation; and 3) Novel Face‐Name Pairs vs. Repeated Face‐Name Pairs (Novel vs. Repeated), which held the visual complexity of the stimuli constant, and thus provided information regarding the encoding of novel face‐name associations compared to viewing well‐learned face‐name associations.
Table I presents the location, volume and significance levels for the activated regions for each of these comparisons. The most noteworthy patterns of activation will be discussed by anatomic region below.
Table I.
Summary of activations by contrast
| Region | Brodmann area | Talairach coordinates for max. voxel | Number of voxels | Significance | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Novel vs. Fixation | ||||||
| Striate | 17 | −9 | −81 | 6 | 924 | 4.1 × 10−13 |
| Fusiform | 19 | 43 | −66 | −21 | 1892 | 9.6 × 10−15 |
| 19 | −46 | −63 | −18 | 909 | 9.6 × 10−15 | |
| Anterior fusiform | 37 | 43 | −48 | −18 | 377 | 9.6 × 10−15 |
| Superior parietal | 7 | 25 | −60 | 40 | 68 | 5.2 × 10−8 |
| 7 | −25 | −54 | 46 | 25 | 2.3 × 10−9 | |
| Pulvinar | −15 | −33 | 3 | 479 | 6.5 × 10−14 | |
| 15 | −30 | 6 | 275 | 1.4 × 10−11 | ||
| Caudate | −9 | −6 | 12 | 15 | 2.2 × 10−7 | |
| Prefrontal | 6/8 | −40 | 6 | 34 | 419 | 6.5 × 10−14 |
| 44/45 | −46 | 18 | 15 | 212 | 1.4 × 10−11 | |
| 46 | −31 | 45 | 15 | 14 | 1.2 × 10−8 | |
| 46 | 43 | 27 | 21 | 348 | 4.6 × 10−13 | |
| Orbital frontal | 11 | 25 | 27 | −9 | 14 | 8.4 × 10−7 |
| Medial frontal | 9 | 0 | 48 | 37 | 110 | 4.3 × 10−8 |
| Repeated vs. Fixation | ||||||
| Fusiform | 19 | −34 | −87 | −3 | 464 | 1.6 × 10−11 |
| 19 | 12 | −84 | −3 | 701 | 1.4 × 10−11 | |
| 37 | −37 | −54 | −9 | 363 | 1.4 × 10−11 | |
| Extrastriate | 18 | 40 | −84 | 12 | 225 | 4.1 × 10−13 |
| 18 | 37 | −84 | −15 | 490 | 1.6 × 10−11 | |
| Novel vs. Repeated | ||||||
| Superior parietal | 7 | −25 | −57 | 46 | 223 | 1.3 × 10−9 |
| 7 | 31 | −66 | 28 | 190 | 3.0 × 10−8 | |
| Fusiform | 37/20 | −43 | −54 | 0 | 2153 | 1.8 × 10−13 |
| 37/20 | 37 | −57 | −9 | 1751 | 7.7 × 10−12 | |
| Pulvinar | −18 | −36 | 3 | 441 | 4.6 × 10−11 | |
| 12 | −33 | 6 | 152 | 1.3 × 10−9 | ||
| Hippocampus | 21 | −18 | −12 | 70 | 1.3 × 10−9 | |
| Superior temporal sulcus | 22 | −62 | 0 | 0 | 28 | 1.3 × 10−7 |
| Caudate | −15 | 3 | 12 | 211 | 3.0 × 10−8 | |
| Prefrontal | 6/8 | −21 | 12 | 53 | 479 | 3.0 × 10−8 |
| 8/9 | −12 | 39 | 46 | 147 | 1.3 × 10−9 | |
| 4 | 43 | 6 | 28 | 430 | 1.3 × 10−9 | |
| 45/46 | −43 | 18 | 18 | 885 | 1.8 × 10−13 | |
| 45/46 | 28 | 21 | 6 | 125 | 3.0 × 10−8 | |
| Orbital | 11 | 25 | 27 | −15 | 77 | 1.3 × 10−9 |
| 11 | −9 | 42 | −6 | 56 | 2.6 × 10−10 | |
| Medial frontal | 32 | −6 | 12 | 46 | 522 | 7.7 × 10−12 |
Hippocampal formation
The peak activation in the group data for the Novel vs. Repeated activation (P < 1 × 10−9, KS statistic) localized to the right anterior hippocampus (Talairach x,y,z coordinates of peak voxel: 21, −18, −12). Figure 2A shows the group averaged KS map superimposed on the group averaged T1 structural data in the coronal plane. Figure 2B shows the BOLD signal time course for the peak voxel within the hippocampus showing significant paradigm linked activation.
Figure 2.
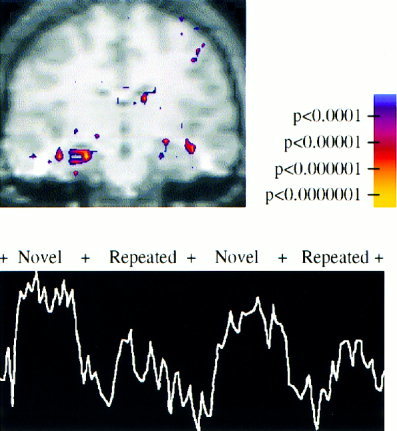
Activation map for group averaged data for the Novel vs. Repeated contrast is shown superimposed on the group averaged structural SPGR image (above), showing significant activation in the right anterior hippocampus (Talairach coordinates 21, −18, −12). Color scale indicating significance values is shown on the right. The MR signal from the voxel showing most significant activation (P < 1.3 × 10−9) within the right hippocampus (below).
Significant activation within the hippocampal formation (defined here as the hippocampus, subicular complex and the adjacent entorhinal cortex [Van Hoesen and Hyman, 1990]) was observed in all subjects for the Novel vs. Repeated contrast. The extent and location of the peak activation varied somewhat among the eight subjects (Fig. 3A). Five out of the eight subjects showed significant activation primarily in the right anterior hippocampus, two showed bilateral activation in the anterior and posterior hippocampal formation and one subject showed activation primarily in the left hippocampus and entorhinal cortex.
Figure 3.
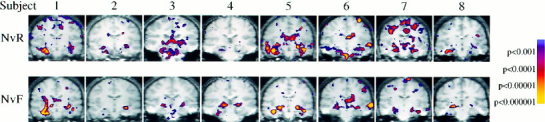
Activation maps for individual subjects shown superimposed on individual SPGR coronal images re‐oriented into standardized coordinate system. Images showing the greatest extent of significant activation (P < 0.001 at individual subject level) in medial temporal regions are shown for each subject for the Novel vs. Repeated (NvR, above) and the Novel vs. Fixation (NvF, below) contrasts. Although the extent and magnitude of activation varied, all subjects demonstrated significant activation within the hippocampal formation for the NvR contrast. The NvF contrast yielded a less consistent pattern of activation within the medial temporal lobe.
In the Novel vs. Fixation contrast, the activation pattern was much less consistent. Although some individual subjects showed significant activation within the hippocampal formation, several of the subjects demonstrated activation that was either superior or medial to the hippocampus (Fig. 3B). There was not a significant peak in the group data within the hippocampal formation or adjacent regions in the Novel vs. Fixation contrast.
No significant activation was seen in the hippocampal formation for the Repeated vs. Fixation comparison. A small region at the amygdala/anterior hippocampal border (12, −6, −15) showed a significant deactivation in the Repeated vs. Fixation contrast.
Prefrontal
Highly significant activations were found in bilateral prefrontal regions in both the Novel vs. Fixation and Novel vs. Repeated contrast in all subjects. No prefrontal activation was seen in the Repeated vs. Fixation contrast. There were 2 distinct regions of activation within the dorsolateral prefrontal cortex (Fig. 4).
Figure 4.
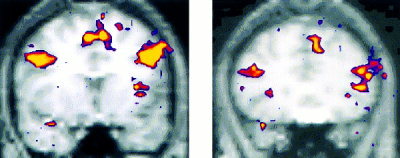
Activation map for group averaged data for the Novel vs. Repeated contrast showing significant activation in two locations within prefrontal cortex (shown on left) Brodmann areas 9/44, (Talairach coordinates for peak activated voxel x,y,z −43, 9, 31) and (shown on right) Brodmann areas 45/46 (28, 21, 6).
The first region of prefrontal activation was located primarily within Brodmann area (BA) 45, extending anteriorly into BA 46. The second prefrontal region was more superior and posterior primarily in BA 8/9, and extended into BA 6 posteriorly and BA 44 inferiorly. Both of these prefrontal regions were present bilaterally, but showed greater extent of activation on the left than the right in the Novel vs. Repeated comparison. Activation was also observed in orbital prefrontal regions bilaterally (BA 11) and in medial frontal regions (BA 8/9) in the Novel vs. Repeated contrast.
Fusiform gyri, extrastriate and striate cortex
Highly significant activations (P < 1 × 10−12) were observed bilaterally in the fusiform gyri and adjacent visual association cortices. The Novel vs. Fixation contrast yielded contiguous activation extending from the striate cortex (BA 17), posterior extrastriate and fusiform regions (BA 19), mid‐fusiform (BA 37) into the anterior fusiform (BA 20) and parahippocampal gyrus (BA 36) (Fig. 5).
Figure 5.
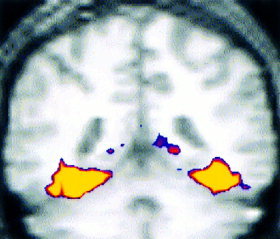
Activation map for group averaged data for Novel vs. Fixation contrast in the fusiform gyri (Brodmann areas 37/20, Talairach coordinates for peak activated voxel 43, −48, −18).
The Novel vs. Repeated comparison also yielded significant activation in bilateral fusiform gyri, extending anteriorly and medially into the parahippocampal gyrus (21, −33, −15), but not in striate and posterior extrastriate cortices. The Repeated vs. Fixation contrast showed significant activation only in more posterior fusiform regions (−37, −54, −9), extrastriate and striate cortices.
Basal ganglia and thalamus
Significant activation was observed in the caudate nucleus (left greater than right) and in the pulvinar nucleus of the thalamus bilaterally. The pulvinar activation was most prominent in the Novel vs. Fixation comparison, but was present to a lesser extent in the Novel vs. Repeated contrast.
Post‐scan testing
The mean performance on the facial recognition task was 94.1 ± 7.1% correct. Free recall for the name associated with the face was 58.3 ± 18.2% correct. All subjects correctly recalled the names associated with the faces of the repeated face‐name pairs. No information was collected as to the strategies employed by subjects.
To examine potential relationships between these memory test scores and degree of activation, we examined the correlations with the percent BOLD signal change in activated regions. There was a trend toward statistical significance between the percent correct on the facial recognition task and the percent BOLD signal change in the Novel vs. Repeated comparison in the right fusiform gyrus (R = 0.49, P < 0.07, Spearman's Rank). No other regions showed correlations approaching statistical significance.
DISCUSSION
These findings indicate that the encoding of novel face‐name associations is subserved by a distributed functional network of brain regions, including the hippocampal formation, dorsolateral prefrontal cortex, pulvinar nucleus of the thalamus, fusiform and adjacent areas of visual association cortex. The activation in these regions was highly significant and the pattern of activation was very consistent across individual subjects.
Although early attempts to activate the hippocampus with functional imaging experiments were disappointing, recent studies have increasingly demonstrated reliable activation in the hippocampus and related medial temporal lobe (MTL) structures during memory tasks (e.g., [Bookheimer et al., 2000; Brewer et al., 1998; Dolan and Fletcher, 1997; Gabrieli et al., 1997; Martin et al., 1997; Schacter et al., 1996; Stark and Squire, 2000; Stern et al., 1996; Strange et al., 1999; Wagner et al., 1998]). These imaging studies, however, have not created a consensus regarding the exact role of the hippocampal system in memory processes. Several explanatory models have been put forth (for review see [Cohen et al., 1999]). Two theories relevant to our paradigm, are the role of the hippocampus in novelty detection [Dolan and Fletcher, 1997; Strange et al., 1999; Tulving et al., 1996], and the role of the hippocampus in relational or associative memory processing [Dolan and Fletcher, 1997; Henke et al., 1997; Rombouts et al., 1997; Vandenberghe et al., 1996], each with supporting data from multiple functional imaging studies.
Our finding of consistent activation within the hippocampus is similar to the pattern of activation seen in the hippocampus with several other paradigms requiring associative memory processing [Dolan and Fletcher, 1997; Henke et al., 1997; Rombouts et al., 1997; Vandenberghe et al., 1996]. The activation in the anterior portion of the hippocampus was particularly striking, and is consistent with the hypothesis that anterior regions of the hippocampus may be essential for the encoding of associations between previously disparate items of information [Schacter and Wagner, 1999], acting to “bind” together the distributed representations of the information [Eichenbaum et al., 1996; Mishkin et al., 1998; Squire and Zola‐Morgan, 1991; Wallenstein et al., 1998].
As noted above, an alternative theory for the role of the hippocampus, is that the hippocampus may be primarily involved in novelty detection, which in turn, may facilitate encoding [Dolan and Fletcher, 1997; Strange et al., 1999; Tulving et al., 1996]. Recent event‐related fMRI studies, however, have suggested that activity during encoding in the hippocampus and related regions in the MTL predicted the success of retrieval among novel stimuli [Kirchhoff et al., 2000; Wagner et al., 1998], and thus is not merely involved in the detection of novel stimuli. Because this current study used a “block design” paradigm that compared the encoding of Novel face‐name associations to Repeated or highly overlearned face‐name associations, we cannot fully separate the contribution of novelty detection from associative encoding to the hippocampal activation. It is notable, however, that the location of the activation within the hippocampus in our study is more anterior (Y = −15 to −18) than most other reports using nearly identical Novel vs. Repeated paradigms with less overtly associative stimuli [Bates et al., 1997; Rombouts et al., 1999; Stern et al., 1996]. This finding suggests that, at the very least, the anterior hippocampus may be more responsive to novel associations than to novel stimuli that are not associative in nature.
It should be noted, however, that in apparent conflict with the above hypothesis, a recent study by Strange et al. [1999] suggested that anterior hippocampal activation was seen with novelty for perceptual characteristics whereas posterior hippocampal activation was seen with novelty for semantic exemplars. Activation in the right anterior hippocampus was also recently reported [Curtis et al., 2000] with object and spatial alternation tasks using repeated stimuli, which could be construed as evidence against the novelty hypothesis. Curtis et al. [2000] noted, however, that their tasks required “relational or contextual binding” that may have been novel to each stimulus trial. An alternative hypothesis for our result, as well as that of Curtis et al. [2000], is that the hippocampus might be involved in processing “interference” from previous memories of particular stimuli. Although the faces were unfamiliar to all subjects, the first names were common names that were likely previously associated with different faces in different contexts. Future experiments using event‐related techniques to compare encoding of novel face‐name associations with encoding of novel non‐associative stimuli may help to resolve the independent contributions of novelty and relational processing.
Although the activation in the hippocampus for the Novel vs. Repeated comparison was quite consistent across subjects, the apparent lack of activation in the hippocampus proper for the Novel vs. Fixation comparison remains somewhat puzzling. One potential explanation would be that the Repeated stimuli induced a priming effect with a reduction in activity below the fixation baseline. We did observe a small region showing deactivation in the Repeated vs. Fixation at the border of the amygdala and anterior hippocampus. This deactivation was quite circumscribed and was located anterior to the peak activation observed in the hippocampus for the Novel vs. Repeated contrast, and as such, is likely not the entire explanation for the apparent lack of activation in the Novel vs. Fixation contrast.
Although several subjects showed significant activation within the hippocampus proper for the Novel vs. Fixation comparison, many of the subjects demonstrated activation in regions superior to the hippocampus in this contrast. We did not observe a peak in this region in the group averaged data, likely because of the variability in the location of activation among individual subjects in the Novel vs. Fixation contrast. We initially thought this may have been a problem with misregistration or individual anatomic variation, but even within individual subjects, the peak activation for Novel vs. Fixation was frequently superior to the activation seen for the Novel vs. Repeated contrast. We have also observed this finding in a subsequent experiment using a similar paradigm with different subjects on a different magnet system [Sperling et al., 2001]. The anatomic substrate for the activation superior to the hippocampus remains unclear, and may represent the confluence of activation from smaller structures such as the tail of the caudate nucleus or the lateral geniculate with the hippocampus. Alternatively, this region could represent an anterior extension of pulvinar activation or a superior and posterior extension of the amygdala activation for the Novel vs. Fixation contrast, but the activation appeared to be distinct from these other regions in most individual subjects. It is also possible that this apparent activation is due to signal changes in draining veins running superior to the hippocampus. Future experiments using thinner slices and high resolution anatomic imaging will be useful to determine the exact origins of activation within this region.
Our findings regarding activation in the prefrontal cortex are consistent with a large number of recent functional imaging studies using encoding paradigms [D'Esposito et al., 1995; Wagner et al., 1998] and provide additional evidence for a critical role of the prefrontal cortex in the formation of new associations. The significance of the lateralization of prefrontal activation remains unclear. Our subjects showed robust activation in dorsolateral and inferior prefrontal regions bilaterally, but to a greater extent in the left hemisphere. Although some reports have suggested that the lateralization of activation in prefrontal regions is associated with encoding vs. retrieval requirements of the task [Tulving et al., 1994], others have suggested that prefrontal cortices show hemispheric specialization based on the content of the stimuli [Kelley et al., 1998]. Kelley et al. [1998] reported left prefrontal activation during word encoding, and right prefrontal activation during face encoding, and bilateral prefrontal activation for “namable objects.” Our finding of bilateral activation in these prefrontal regions is consistent with these results, as our task required encoding of both facial and verbal stimuli. The extent of activation, however, was greater on the left than the right, particularly in BA 8 and 45, perhaps suggesting a more extensive role for left prefrontal cortex in the encoding of associations between verbal and non‐verbal stimuli.
Interestingly, we observed the opposite lateralization in regions within the medial temporal lobe, with the right anterior hippocampus showing greater activation than the left. Several studies have shown content specific lateralization in the hippocampus and adjacent MTL regions, with faces typically activating right MTL regions [Kelley et al., 1998], and verbal material activated left MTL regions [Kirchhoff et al., 2000; Wagner et al., 1998]. Our findings are consistent with Haxby et al [Haxby et al., 1996] who reported activation in the left dorsolateral prefrontal cortex and the right hippocampus during encoding of novel faces.
Our findings also suggest that the fusiform gyrus, in addition to its role in facial processing [Damasio et al., 1990; Kanwisher et al., 1997], may also be involved in the encoding of novel face‐name stimuli. In our study, a large portion of the anterior fusiform gyri showed increased activity to Novel vs. Repeated face‐name pairs, suggesting that region may be directly involved in encoding facial stimuli. A recent PET study also suggested that the degree of activation in the fusiform correlates with successful encoding of face stimuli, above and beyond the visual processing of the face [Kuskowski and Pardo, 1999]. Other studies have suggested that anterior fusiform and more “downstream” anterior/inferior temporal regions may be differentially activated by famous vs. non‐famous faces [Ryan et al., 1998; Sergent et al., 1992; Tempini et al., 1998] suggesting that these areas of association cortex may be directly involved in recognizing whether a face is familiar.
We did not observe significant correlations between the percent signal change in any region with the results of post‐scan memory testing, although as indicated above, there was a trend toward significance in the fusiform region. It should be noted that we only tested recognition of a small number of the novel face‐name pairs presented during the experiment and had a relatively small range of the percent correct responses on both memory measures, thus we likely did not have the data to perform optimal correlational analyses. Studies with event‐related techniques, focusing on successful vs. failed encoding of face‐name associations, should yield more information on brain‐behavioral correlations.
We chose to use a face‐name associative task, because faces and names are inherently unrelated, and it is widely acknowledged that proper names are particularly difficult to remember [Cohen, 1990]. It remains unclear whether the problem lies in learning (encoding) the name or recalling (retrieval) the name or in both processes. There are several theories as to why proper names might be particularly difficult to encode, as proper names are: 1) unique [Burton and Bruce, 1992], 2) arbitrary and often inherently meaningless [Cohen, 1990], and 3) difficult to “visualize” or “image,” which is a common mnemonic device to form associations [McCarty, 1980]. All of these properties make forming an “association” between a name and a face particularly troublesome [Cohen and Burke, 1993]. We hypothesized that this difficult paired‐associate learning task would engage the hippocampal system.
Our findings support the role of the anterior hippocampus in the encoding of novel associations, and suggest that there is a functional network of brain regions subserving associative memory for names and faces. The robust activation using the face‐name paradigm we observed in young subjects, suggest that this paradigm might prove useful in examining the alterations in the patterns of activation that may occur in healthy aging and in early Alzheimer disease.
Acknowledgements
We gratefully acknowledge the contribution of Terry Campbell for assistance with scan acquisition and Dr. Atif Zaheer for assistance with image processing. We thank the volunteers who participated in the study. This research was sponsored by grants from NINDS: K23‐NS02189 (RS), NIMH: NS60941 (DS), NIA: P01‐AG04953 (MA) and the Dana Foundation (MA).
REFERENCES
- Aguirre GK, Zarahn E, D'Esposito M (1998): A critique of the use of the Kolmogorov‐Smirnov (KS) statistic for the analysis of BOLD fMRI data. Magn Reson Med 39: 500–505. [DOI] [PubMed] [Google Scholar]
- Bates JF, Savage CR, Buckner RL, Schacter DL, Weisskoff R, Rosen B, Whalen PJ, Kennedy DN, Stern CE, Albert MS (1997): Abnormal activation of the hippocampal formation in mild Alzheimer disease during visual encoding. Soc Neurosci 23: 2169. [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak‐Vance MA, Mazziotta JC, Small GW (2000): Patterns of brain activation in people at risk for Alzheimer disease [see comments]. N Engl J Med 343: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD (1998): Making memories: brain activity that predicts how well visual experience will be remembered [see comments]. Science 281: 1185–1187. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H (1993): Critical role of the parahippocampal region for paired‐associate learning in rats. Behav Neurosci 107: 740–747. [DOI] [PubMed] [Google Scholar]
- Burton AM, Bruce V (1992): I recognize your face but I can't remember your name: a simple explanation? Br J Psychol 83: 45–60. [DOI] [PubMed] [Google Scholar]
- Bush G, Kennedy D, Jiang A, Talavage T (1996): An automated system of localization and characterization of functional MRI activations in four dimensions. Neuroimage 3: S55. [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998): The counting Stroop: an interference task specialized for functional neuroimaging—validation study with functional MRI. Hum Brain Mapp 6: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfonte BL, Verfaellie M, Johnson MK, Reiss L (1996): Spatial location memory in amnesia: binding item and location information under incidental and intentional encoding conditions. Memory 4: 591–614. [DOI] [PubMed] [Google Scholar]
- Cohen G (1990): Why is it difficult to put names to faces? Br J Psychol 81: 287–297. [Google Scholar]
- Cohen G, Burke DM (1993): Memory for proper names: a review. Memory 1: 249–263. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszaek T, Nash C (1999): Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus 9: 83–98. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Zald DH, Lee JT, Pardo JV (2000): Object and spatial alternation tasks with minimal delays activate the right anterior hippocampus proper in humans. Neuroreport 11: 2203–2207. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M (1995): The neural basis of the central executive system of working memory. Nature 378: 279–281. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H (1990): Face agnosia and the neural substrates of memory. Annu Rev Neurosci 13: 89–109. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PC (1997): Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature 388: 582–585. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (1997): How does the brain organize memories? Science 277: 333–335. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Schoenbaum G, Young B, Bunsey M (1996): Functional organization of the hippocampal memory system. Proc Natl Acad Sci USA 93: 13500–13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JDE, Brewer JB, Desmond JE, Glover GH (1997): Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 276: 264–266. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL (1996): Face encoding and recognition in the human brain. Proc Natl Acad Sci USA 93: 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG (1997): Human hippocampus establishes associations in memory. Hippocampus 7: 249–256. [DOI] [PubMed] [Google Scholar]
- Jiang A, Kennedy D, Baker J, Weisskoff R, Tootell R, Woods R, Benson R, Kwong K, Brady T, Rosen R, Belleveau J (1995): Motion detection and correction in functional MR imaging. Hum Brain Mapp 3: 1–12. [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997): The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE (1998): Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron 20: 927–936. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE (2000): Prefrontal‐temporal circuitry for episodic encoding and subsequent memory. J Neurosci 20: 6173–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuskowski MA, Pardo JV (1999): The role of the fusiform gyrus in successful encoding of face stimuli. Neuroimage 9: 599–610. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL (2000): Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 48: 99–109. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV (1996): Neural correlates of category‐specific knowledge. Nature 379: 649–652. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Weisberg J (1997): Modulation of human medial temporal lobe activity by form, meaning, and experience. Hippocampus 7: 587–593. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman‐Rakic P (1996): Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex 6: 600–611. [DOI] [PubMed] [Google Scholar]
- McCarty DL (1980): Investigation of a visual imagery mnemonic device for acquiring face—name associations. J Exp Psychol [Hum Learn] 6: 145–155. [PubMed] [Google Scholar]
- Mishkin M, Vargha‐Khadem F, Gadian DG (1998): Amnesia and the organization of the hippocampal system. Hippocampus 8: 212–216. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G (1994): Word recognition in the human inferior temporal lobe. Nature 372: 260–263. [DOI] [PubMed] [Google Scholar]
- Press W, Teukosky S, Vetterling W, Flannery B (1992): Numerical recipes in C: the art of scientific computing. Cambridge, England: Cambridge University Press. [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G (1995): Face‐sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol 74: 1192–1199. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK (2000): Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47: 769–776. [DOI] [PubMed] [Google Scholar]
- Reese TG, Davis TL, Weisskoff RM (1995): Automated shimming at 1.5 T using echo‐planar image frequency maps. J Magn Reson Imaging 5: 739–745. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Machielsen WC, Witter MP, Barkof F, Lindeboom J, Scheltens P (1997): Visual association encoding activates the medial temporal lobe: a functional magnetic resonance imaging study. Hippocampus 7: 594–601. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Scheltens P, Machielson WC, Barkhof F, Hoogenraad FG, Veltman DJ, Valk J, Witter MP (1999): Parametric fMRI analysis of visual encoding in the human medial temporal lobe. Hippocampus 9: 637–643. [DOI] [PubMed] [Google Scholar]
- Ryan J, Cohen NJ, Milham M, Wszalek T, Althoff R, Banich M, Webb A, Wright A, Kramer A, Liang ZP, Warren S, Magin R (1998): Effects of prior exposure to faces on the activity of visual processing regions of the brain: an fMRI study. Soc Neurosci Abstracts 23: 680. [Google Scholar]
- Rypma B, D'Esposito M (1999): The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci USA 96: 6558–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS (1996): Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci USA 93: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Church B (1995): Implicit memory in amnesic patients: when is auditory priming spared? J Int Neuropsychol Soc 1: 434–442. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD (1999): Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 9: 7–24. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B (1992): Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain 115: 15–36. [DOI] [PubMed] [Google Scholar]
- Sperling R, Greve D, Dale A, Killiany R, Rosen B, Holmes J, Rosas HD, Cocchiarella A, Firth P, Lange N, Routledge C, Albert M (2001): Effects of lorazepam and scopolamine on encoding face‐name associations: a pharmacologic fMRI trial. 7th Annual Meeting: Human Brain Mapping, Brighton, United Kingdom.
- Squire LR (1992): Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans [published erratum appears in Psychol Rev 1992 99:582]. Psychol Rev 99: 195–231. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola‐Morgan S (1991): The medial temporal lobe memory system. Science 253: 1380–1386. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR (2000): Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory [In Process Citation]. J Neurosci 20: 7776–7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR (1996): The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA 93: 8660–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ (1999): Segregating the functions of human hippocampus. Proc Natl Acad Sci USA 96: 4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Eichenbaum H (2000): The neurophysiology of memory. Ann NY Acad Sci 911: 175–191. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishing. [Google Scholar]
- Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS (1998): The neural systems sustaining face and proper‐name processing [In Process Citation]. Brain 121: 2103–2118. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S (1994): Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings [see comments]. Proc Natl Acad Sci USA 91: 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S (1996): Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex 6: 71–79. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Hyman BT (1990): Hippocampal formation: anatomy and the patterns of pathology in Alzheimer disease. Prog Brain Res 83: 445–457. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS (1996): Functional anatomy of a common semantic system for words and pictures [see comments]. Nature 383: 254–256. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge L, Desmond JE, Glover GH, Gabrieli JD (1998): Material‐specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport 9: 3711–3717. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL (1998): Building memories: remembering and forgetting of verbal experiences as predicted by brain activity [see comments]. Science 281: 1188–1191. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Eichenbaum H, Hasselmo ME (1998): The hippocampus as an associator of discontiguous events. Trends Neurosci 21: 317–323. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H (1999): The global record of memory in hippocampal neuronal activity [see comments]. Nature 397: 613–616. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC (1992): Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 16: 620–633. [DOI] [PubMed] [Google Scholar]


