Abstract
Functional imaging studies consistently find that emotional stimuli activate the posterior cingulate cortex, a region that appears to have memory‐related functions. However, prior imaging studies have not controlled for non‐emotional stimulus features that might activate this region by engaging memory processes unrelated to emotion. This study examined whether emotional words activated the posterior cingulate cortex when these potentially confounding factors were controlled. Sixty‐four pleasant and 64 unpleasant words were matched with neutral words on non‐emotional features known to influence memory. Eight subjects underwent block‐designed functional magnetic resonance imaging scans while evaluating the valence of these words. The posterior cingulate cortex was significantly activated bilaterally during both unpleasant and pleasant compared to neutral words. The strongest activation peak with both unpleasant and pleasant words was observed in the left subgenual cingulate cortex. Anteromedial orbital and left inferior and middle frontal cortices were also activated by both pleasant and unpleasant words. Right amygdala and auditory cortex were activated only by unpleasant words, while left frontal pole was activated only by pleasant words. The results show that activation of the posterior cingulate cortex by emotional stimuli cannot be attributed to the memory‐enhancing effects of non‐emotional stimulus features. The findings are consistent with the suggestion that this region may mediate interactions of emotional and memory‐related processes. The results also extend prior findings that evaluating emotional words consistently activates the subgenual cingulate cortex, and suggest a means of probing this region in patients with mood disorders. Hum. Brain Mapping 18:30–41, 2003. © 2002 Wiley‐Liss, Inc.
Keywords: emotion, affect, arousal, retrosplenial, subgenual, subcallosal, semantic, memory
INTRODUCTION
A more complete understanding of the neural bases of emotional influences on memory and cognition may facilitate new insights into the mechanisms of emotional disorders. We previously reported that the posterior cingulate cortex was strongly and consistently activated during the evaluation of threat‐related words [Maddock and Buonocore, 1997]. A subsequent meta‐analysis of functional imaging studies showed that the caudal part of the posterior cingulate cortex was the cortical region most consistently activated by emotional stimuli compared to nominally matched, emotionally neutral stimuli [Maddock, 1999]. However, there is considerable evidence that this region has functions related to episodic memory [Andreasen et al., 1995; Grasby et al., 1993; Henson et al., 1999; Maddock et al., 2001; Valenstein et al., 1987]. These contrasting observations could be reconciled by the hypothesis that the posterior cingulate cortex has a role in the modulation of memory by emotionally arousing stimuli [Maddock, 1999; Maddock and Buonocore, 1997]. Emotion can influence memory in a variety of ways [Reisberg and Heuer, 1995], but the most consistent finding is enhanced memory for emotional stimuli or events [Bradley et al., 1992; Cahill and McGaugh, 1998; Rubin and Friendly, 1986]. However, non‐emotional qualities of stimuli, such as imagery and familiarity, can also enhance post‐study memory performance, and previous functional imaging studies of emotional stimuli have not controlled for these factors. Vogt et al. [2000] have suggested that activation of the posterior cingulate cortex by emotional stimuli in functional imaging studies may be due to the confounding effects of non‐emotional influences on memory. To test this possibility, we created a set of 128 emotional words that were matched with a set of 128 neutral words on all non‐emotional qualities known to affect memory performance. By testing free recall for these words, we confirmed that the emotional words were consistently better recalled than the neutral words. Using functional magnetic resonance imaging (fMRI), we looked for brain regions more active when subjects evaluated the emotional words compared to the neutral words. A finding of posterior cingulate activation would be strong evidence against the alternative hypothesis that such activation results from non‐emotional influences on memory processing. In addition, behavioral studies have shown that positively and negatively valenced emotional stimuli have similar memory‐enhancing effects [Bradley et al., 1992; Doerksen and Shimamura, 2001]. To our knowledge, no prior fMRI or PET study has examined the brain responses to positively valenced words compared to neutral words. Thus, the current study tested whether both positively and negatively valenced emotional words activated the posterior cingulate cortex.
Previous studies have reported activation of the ventral anterior cingulate cortex, including the subgenual cortex, in response to emotionally salient words [Beauregard et al., 1997; Elliott et al., 2000; Tabert et al., 2001]. The subgenual cingulate cortex is strongly interconnected with the caudal posterior cingulate cortex [Van Hoesen et al., 1993], and is potentially significant in the pathophysiology of mood disorders. Structural MRI studies have shown significant volume reduction in this region in patients with familial mood disorders [Drevets et al., 1997; Hirayasu et al., 1999], which may result from decreased glial density [Ongur et al., 1998]. Drevets [2000] has suggested that the subgenual cingulate cortex has a role in the regulation of mood states, and that hypofunction of this region may confer vulnerability to mood disorders in some patients.
The amygdala has been consistently implicated in emotional processing, and recent evidence from human studies suggests that it participates in the enhancement of both perception of and memory for emotionally arousing stimuli [Adolphs et al., 1997; Anderson and Phelps, 2001; Cahill et al., 1995]. Amygdala activation in response to negatively valenced words has been observed in prior imaging studies [Isenberg et al., 1999; Strange et al., 2000; Tabert et al., 2001]. While animal studies demonstrate amygdala involvement in processing both negatively and positively valenced stimuli, human studies more often find evidence for amygdala involvement with negatively than positively valenced stimuli [Adolphs and Tranel, 2000; Everitt et al., 2000].
We examined the patterns of brain activation in eight normal subjects, evaluating the valence of unpleasant or pleasant emotional words compared to neutral words matched on factors known to influence memory performance. Stimulus selection was based on the ratings of 95 subjects using validated rating instruments. Enhanced post‐study memory for the emotional compared to neutral words was confirmed in 16 subjects. The study had three goals: 1) to determine if emotion‐related activation of the posterior cingulate cortex could be demonstrated when non‐emotional stimulus features known to influence memory performance were controlled; 2) to examine the brain responses during evaluation of both negatively and positively valenced emotional words; and 3) to determine if evaluating the valence of emotional compared to carefully matched neutral words reliably activated additional brain regions such as the subgenual cingulate cortex and the amygdala, which may function abnormally in emotional disorders.
SUBJECTS AND METHODS
Subjects
Eight right‐handed subjects (6 women, 2 men; aged 24–45 years) were recruited by advertisement from the communities surrounding Davis, California, and gave informed consent to participate in this study. A brief medical screening interview was used to exclude subjects with any psychiatric or neurological illness or any condition or medication affecting neural or cerebrovascular function. Handedness was ascertained by a 17‐item questionnaire [Raczkowski and Kalat, 1974].
Validation of Stimuli
Neuroimaging studies using emotional words have typically compared them with neutral words matched only for length and frequency of usage. However, additional features of words may influence neural processing. Rubin [1980] factor‐analyzed 51 properties of words and identified six underlying factors, represented by length, imagery, familiarity, emotional valence (pleasant vs. unpleasant), emotional arousal (intensity), and ease of recall (in a free‐recall task). Ease of recall was found to be predicted by three of the other factors: imagery, frequency, and emotional arousal [Rubin and Friendly, 1986]. Thus, we created emotional and neutral word lists that were matched for imagery and frequency, as well as length and part of speech, but differed in ratings of emotional valence and emotional arousal. These latter two independent factors account for a majority of the variance in how human subjects describe emotional stimuli [Bradley and Lang, 1994]. Ninety‐five consenting subjects participated in rating 340 candidate nouns and adjectives (80 unpleasant, 80 pleasant, and 180 neutral nouns and adjectives) obtained from published studies [Rubin and Friendly, 1986] and our pilot work. Each word was rated by at least 20 subjects on three dimensions: emotional valence, emotional arousal, and imagery. The two emotional dimensions were rated with the 9‐point Self Assessment Maniken scales [Bradley and Lang, 1994]. Imagery was rated with a 9‐point Likert scale derived from Paivio et al. [1968] and validated in 75 consenting subjects rating 48 words from the set of Paivio et al. [1968] (r = .86). Familiarity of each word was approximated by the log of the frequency of usage [Kucera and Francis, 1967]. Length was quantified as number of syllables. From these data, we selected the 64 most unpleasant and most emotionally arousing words and 64 matched neutral words, and the 64 most pleasant and most emotionally arousing words and 64 matched neutral words (Table I). The words were then divided into two equivalent lists of 128 words (32 unpleasant, 32 pleasant, and 64 neutral). Sixteen consenting subjects then made an emotionality judgment (emotional or neutral) of 128 words from one of the two lists and their free recall of the words was tested after a 3‐min distracter task. Subjects recalled significantly more unpleasant and pleasant words than matched neutral words (4.2 vs. 1.8, t = 3.2, P < .006; 3.5 vs. 1.8, t = 3.7, P < .002, respectively). The unpleasant, pleasant, and neutral word sets include such words as mutilation, disaster, freedom, thrill, border, and capacity. (The complete list of stimuli is available upon request.)
Table I.
Characteristics of the 256 stimulus words†
| Word lists (n = 64 for each) | Valence | Arousal | Imagery | Frequency | Syllables | Number of nounsa |
|---|---|---|---|---|---|---|
| Unpleasant* | 7.4 (0.6) | 5.9 (0.7) | 5.9 (1.2) | 1.0 (0.6) | 2.5 (0.8) | 42 |
| Matched neutral | 4.9 (0.3) | 2.6 (0.7) | 5.7 (1.6) | 1.1 (0.6) | 2.3 (0.9) | 43 |
| Pleasant* | 2.5 (0.5) | 5.3 (0.8) | 5.4 (1.1) | 1.3 (0.6) | 2.5 (0.9) | 43 |
| Matched neutral | 4.9 (0.4) | 2.6 (0.8) | 5.0 (1.4) | 1.3 (0.6) | 2.4 (0.9) | 46 |
Values are expressed as mean (SD).
Valence, arousal, and imagery for each word were rated by at least 20 subjects (range = 20 to 75). Valence and Arousal were rated with the 9‐point Self Assessment Maniken scales [Bradley and Lang, 1994], with 9 referring to the most unpleasant or most arousing words. Imagery was rated on a 9‐point Likert scale with 9 referring to words with the most imagery. Frequency is the log of occurrences per million words [Kucera and Francis, 1967].
All words are either nouns or adjectives.
There were no significant differences between the unpleasant or the pleasant words and their matched neutral words on any variables except valence and arousal. There was no overlap in the valence or arousal ratings of the words on the emotional and neutral word lists. The unpleasant words had significantly higher arousal ratings than the pleasant words (t = 4.4, df = 126, P < .0001).
Procedures
For Task A (unpleasant and neutral words), subjects were instructed to make a silent judgment of whether each word referred to something emotionally unpleasant or emotionally neutral. For Task B (pleasant and neutral words), subjects were instructed to make a silent judgment of whether each word referred to something emotionally pleasant or emotionally neutral. Thus, throughout each scan, subjects maintained the mental set associated with evaluating the valence of words. However, the meanings of the words were emotional only during the unpleasant (or pleasant) blocks. No stimuli were presented during the first 32 seconds of each scan. Then, alternating blocks of emotional and neutral words were presented through headphones via a pneumatic audio system. Each block included eight words of the same type, presented one every 2 sec for a block duration of 16 sec. A total of 16 blocks (eight emotional, eight neutral) was presented. Order within scan (emotional or neutral first) was balanced across subjects. Six subjects performed Task A first, and two performed Task B first. Due to this imbalance in order and the significantly greater arousal ratings for the unpleasant than the pleasant words (Table I), no direct comparisons were made between pleasant and unpleasant words.
Imaging Protocol
Images were obtained with a 1.5 Tesla magnetic resonance imaging system (Signa Advantage; General Electric) running OS v. 5.7, with a local gradient coil insert (Medical Advances, Milwaukee, WI) to provide higher gradient performance (2 g/cm peak strength, 34 g/cm/msec max. slew rate). For each subject, a coronal high‐resolution Fast Spin Echo sequence was obtained for anatomical localization. Scan parameters were: TR, 3100; effective TE, 17 and 136; echo train, 8; matrix, 256 × 256; FOV, 22 cm; slice thickness, 6 mm; gap, 2 mm; slice range, posterior 96 mm to anterior 88 mm; 24 slices. Subsequently, a T2*‐weighted, gradient‐recalled echo planar imaging (EPI) sequence was tuned and shimmed for the functional scan. Parameters were: TR, 2000 msec; effective TE, 40 msec; FA, 90° matrix, 64 × 64; FOV, 22 cm; slice thickness, 6 mm; slice gap, 2 mm; voxel size, 3.44 by 3.44 by 6 mm; slice range, posterior 78 mm to anterior 64 mm, 18 slices; scan time, 288 sec. Activation was detected by the blood oxygenation level dependent contrast mechanism. Head motion was constrained by foam padding.
Data Analysis
A Fourier transform‐based algorithm was used for reconstruction of the functional images and simultaneous removal of N/2 ghost artifacts [Buonocore and Gao, 1997]. Subsequent statistical analyses were performed with Medx software (Sensor Systems, Sterling, VA). After discarding the first 16 images, each functional scan was checked for motion by a weighted and thresholded center‐of‐intensity algorithm. Motion correction was performed with sinc interpolation for scans with displacement of 20 to 50% of a voxel width (0.69 mm to 1.72 mm). Scans would have been rejected if displacement exceeded 50% of a voxel width. Based on the signal detection analysis of Skudlarski et al. [1999], image data were high‐pass filtered to remove oscillations with frequencies less than 35% of the stimulus frequency (0.35 × 0. 03125 Hz = 0. 01094 Hz). This procedure removed oscillations with periods > 91 sec. For each scan, correlation coefficients were calculated between each voxel time series and a Poisson‐convolved, boxcar waveform and then converted to Z‐score maps. The number of activated voxels at each time lag (2–8 sec) was examined to determine empirically the optimal temporal offset, which was then applied to all voxels within each scan. The first image of each scan was spatially transformed onto a T2* EPI template conforming to the Talairach brain atlas (SPM95) using sinc interpolation, and resliced to a final voxel size of 3.4 × 3.4 × 3.4 mm. For each scan, these transformation parameters were used to spatially normalize the Z‐score map. Finally, normalized Z‐score maps for the eight subjects were combined into a group Z‐score map by dividing the sum of the eight Z‐scores by the square root of 8, at each voxel location (a fixed‐effects analysis). Voxels were considered significantly activated if they met threshold criteria for both peak and extent of activation. Activated voxels were required to have a Z value ≥ 3.73 (P ≤ 0. 0001) and to be contiguous with ≥ 5 voxels all having Z ≥ 2.57 (P ≤ 0.005) [Forman et al., 1995]. These criteria were used in a whole‐brain analysis to identify voxels significantly more activated during emotional than neutral words or during neutral than emotional words.
RESULTS
All subjects reported no difficulty hearing the words or judging their valence. Six of the 16 scans had motion greater than 20% of a voxel width and were corrected. None had motion greater than 50% of a voxel width. The optimal temporal offset ranged from 2 to 8 seconds (mean = 3.75 sec).
Table II and Figure 1 show that the posterior cingulate cortex was significantly more activated during the valence evaluation of unpleasant, emotionally arousing words than emotionally neutral words. The most statistically significant local maximum of activation was in the left subgenual anterior cingulate cortex. Other significantly activated regions included the anteromedial orbital cortex bilaterally, the right amygdala, right insula, right auditory cortex, left basal forebrain, left parahippocampal cortex, and left dorsolateral prefrontal cortices. The locations and Z‐scores of peak activation in these and additional activated regions are shown in Table II.
Table II.
Significant activations during the evaluation of unpleasant arousing words compared to neutral words*
| Brain regions (BA) | Number of voxels in cluster | Talairach coordinates of local maximuma | Z score of local maximum | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Midline cortical activations | |||||
| Anterior | |||||
| L. Subgenual anterior cingulate (24) | 31 | −7 | 24 | 0 | 6.74 |
| B. Orbital frontal (11, 10) | 9 | 0 | 55 | −14 | 3.86 |
| Posterior | |||||
| R. Posterior cingulate (30) | 10 | 14 | −38 | 7 | 4.92 |
| L. Precentral (4) | 7 | −4 | −34 | 62 | 4.33 |
| L. Posterior cingulate (31) | 14 | −7 | −21 | 38 | 4.21 |
| L. mid cingulate (24) | −7 | −7 | 41 | 3.75 | |
| L. Posterior cingulate (23) | 6 | −7 | −55 | 24 | 3.97 |
| Left hemisphere cortical activations | |||||
| L. Inferior frontal (45, 47) | 35 | −55 | 31 | 3 | 5.00 |
| L. Superior frontal (9) | 16 | −21 | 55 | 38 | 4.73 |
| L. Superior temporal sulcus (21, 22) | 22 | −48 | 0 | −17 | 4.65 |
| L. Inferior and middle temporal (20, 21) | 23 | −58 | −24 | −14 | 4.05 |
| L. Middle frontal (9) | 6 | −38 | 24 | 24 | 4.02 |
| L. Middle frontal (10) | 7 | −31 | 52 | 3 | 3.98 |
| Right hemisphere cortical activations | |||||
| R. Auditory cortex (41, 42) | 9 | 45 | −31 | 17 | 5.03 |
| R. Planum temporale and insula | 15 | 48 | −10 | 0 | 4.66 |
| R. Fusiform (20) | 6 | 38 | −17 | −28 | 4.37 |
| R. Inferior frontal operculum/Insula | 7 | 34 | 7 | 17 | 3.90 |
| R. Middle temporal (21) | 5 | 48 | 0 | −28 | 3.83 |
| Subcortical/cerebellar activations | |||||
| L. Tail of caudate | 28 | −17 | −28 | 21 | 5.83 |
| R. Thalamus | 7 | 21 | −21 | 3 | 5.29 |
| Midline cerebellum | 35 | 0 | −48 | −24 | 5.00 |
| L. Thalamus and septum | 83 | −10 | −7 | 7 | 4.66 |
| L. Parahippocampal | −17 | −45 | −3 | 4.28 | |
| L. Basal forebrain | −10 | −3 | −7 | 4.16 | |
| R. Caudate | 10 | 3 | 10 | 4.12 | |
| R. Cerebellum | 5 | 58 | −55 | −24 | 4.10 |
| L. Cerebellum | 28 | −24 | −58 | −21 | 4.05 |
| R. Amygdala | 11 | 24 | −7 | −7 | 3.82 |
Brain regions containing clusters significantly more activated during evaluation of unpleasant words than neutral words. A cluster is considered activated if it contains ≥ 5 contiguous suprathreshold voxels (voxel threshold = Z ≥ 2.57 (P ≤ 0.005), cluster threshold = P ≤ 0.001) and contains at least one local maximum voxel for which Z ≥ 3.73 (P ≤ 0. 0001). Additional regions with local maxima within the same cluster are indented following the strongest local maximum for that cluster. L = left, R = right, B = bilateral; BA = Brodmann's Area in the region of observed activation.
Highest local maximum in each region.
Figure 1.
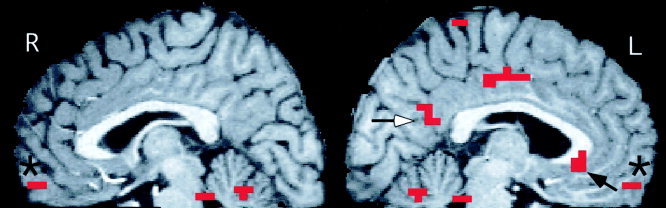
Areas of significantly greater activation in midline regions during the evaluation of unpleasant compared to matched neutral words. Shown are all significantly activated clusters (defined as a peak P ≤ 0. 0001 within a cluster of five voxels with P ≤ 0.005) across three adjacent sagital slices, centered at X = +3 (R) and X = −3 (L). Activations shown include the anteromedial orbital cortex bilaterally (*) and the left subgenual (black arrow) and caudal posterior cingulate (white arrow) cortices. Activated voxels arer superimposed on a high‐resolution MR image normalized into Talairach space.
Table III and Figure 2 show that the posterior cingulate cortex was significantly more activated during the valence evaluation of pleasant, emotionally arousing words than emotionally neutral words. The most statistically significant local maximum of activation was again in the left subgenual anterior cingulate cortex. Other significantly activated regions included the right anteromedial orbital, right medial frontal, right dorsal anterior cingulate, the left frontal polar, left temporal polar, and left dorsolateral prefrontal cortices. The locations and Z‐scores of peak activation in these and additional activated regions are shown in Table III.
Table III.
Significant activations during the evaluation of pleasant arousing words compared to neutral words*
| Brain regions (BA) | Number of voxels in cluster | Talairach coordinates of local maximuma | Z score of local maximum | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Midline Cortical Activations | |||||
| Anterior | |||||
| L. Subgenual anterior cingulate (32, 25) | 5 | −10 | 24 | −10 | 4.69 |
| R. Orbital frontal (11) | 11 | 7 | 62 | −14 | 4.43 |
| L. Frontal pole (10) | 5 | −3 | 58 | 10 | 4.21 |
| R. Medial frontal (6) | 8 | 3 | 10 | 62 | 4.16 |
| R. Anterior cingulate (32, 24) | 13 | 7 | 28 | 28 | 4.05 |
| Posterior | |||||
| B. Posterior cingulate (30, 23) | 9 | −3 | −48 | 7 | 4.30 |
| B. Posterior cingulate and precuneus (31, 7) | 13 | 0 | −38 | 41 | 3.81 |
| Left hemisphere activations | |||||
| L. Middle frontal (46, 9) | 15 | −34 | 38 | 21 | 4.44 |
| L. Inferior frontal (47, 45) | 19 | −55 | 31 | −3 | 4.08 |
| L. Temporal pole (38) | 8 | −34 | 10 | −21 | 3.99 |
| L. Middle frontal (8) | 8 | −48 | 17 | 45 | 3.92 |
| L. Planum temporale | 14 | −48 | −38 | 17 | 3.76 |
| Cerebellar/brain stem activations | |||||
| L. Medial cerebellum | 19 | −3 | −76 | −17 | 4.42 |
| B. Brain stem | 81 | 3 | −34 | −28 | 4.07 |
| Periaquaductal region | 0 | −31 | −10 | 3.90 | |
| R. Medial cerebellum | 14 | 14 | −62 | −21 | 3.74 |
Brain regions containing clusters significantly more activated during evaluation of unpleasant words than neutral words. A cluster is considered activated if it contains ≥ 5 contiguous suprathreshold voxels (voxel threshold = Z ≥ 2.57 (P ≤ 0.005), cluster threshold = P ≤ 0.001) and contains at least one local maximum voxel for which Z ≥ 3.73 (P ≤ 0. 0001). Additional regions with local maxima within the same cluster are indented following the strongest local maximum for that cluster. L = left; R = right; B = bilateral; BA = Brodmann's Area in the region of observed activation.
Highest local maximum in each region.
Figure 2.
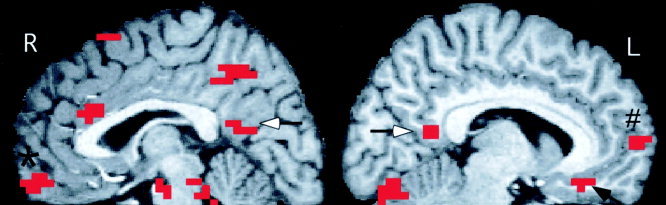
Areas of significantly greater activation in midline regions during the evaluation of pleasant compared to matched neural words. Shown are all significantly activated clusters (defined as a peak P ≤ 0. 0001 within a cluster of five voxels with P ≤ 0.005) on three adjacent sagital slices, centered at X = +3 (R) and X = −7 (L). Activations shown include the posterior cingulate cortex bilaterally (white arrows) the right anteromedial orbital cortex (*) and the left subgenual (black arrow) and the left subgenual (black arrow) and frontal polar (#) cortices. Activated voxels arer superimposed on a high‐resolution MR image normalized into Talairach space.
A few brain regions were significantly more activated during the evaluation of neutral words compared to unpleasant or pleasant words. The right putamen (local maximum at 28, 3, 3) left insula (local maximum at −41, −7, 14), and left postcentral cortex (local maximum at −58, −21, 24) were significantly more activated during neutral than unpleasant words. The right caudate (local maximum at 10, 21, 10), right lingual cortex (local maximum at 17, −52, 10), left brainstem (local maximum at −14, −24, −7) and left sublenticular region (local maximum at −17, −7, −7) were significantly more activated during neutral than pleasant words.
DISCUSSION
Evaluating the valence of both unpleasant and pleasant words was associated with significant activation of the posterior cingulate cortex bilaterally when compared to evaluating emotionally neutral words matched for imagery, frequency of usage, length, and part of speech. In functional imaging studies of both verbal and pictorial stimuli, this same caudal region of the posterior cingulate cortex is the brain area most consistently activated by emotionally salient compared to nominally matched neutral stimuli [Maddock, 1999]. The posterior cingulate cortex may have a greater role in emotional processing than had been assumed previously [Vogt et al., 1992; 2000]. This region receives strong afferent input from regions with functions related to emotion and social behavior, including the subgenual anterior cingulate cortex, the orbital frontal cortex, the dorsolateral prefrontal cortex (areas 9 and 46), and the superior temporal sulcus [Allison et al., 2000; Carmichael and Price, 1995; Goldman‐Rakic et al., 1984; Morris et al., 1999; Musil and Olsen 1993; Van Hoesen et al., 1993]. Increased activity in the caudal posterior cingulate cortex has been observed in emotional disorders, including schizophrenia [Andreasen et al., 1997; Haznedar et al., 1997] and major depression [Ho et al., 1996], and has been reported to correlate with severity of anxiety symptoms in major depression, obsessive–compulsive disorder, and social phobia [Bench et al., 1992; McGuire et al., 1994; Perani et al., 1995; Reiman, 1997]. Increased activity in the posterior cingulate cortex prior to treatment was significantly correlated with a positive response to surgical (cingulotomy) or medical (fluvoxamine) treatment for obsessive–compulsive disorder [Rauch et al., 2001].
Anatomical, clinical, and functional imaging studies indicate the posterior cingulate cortex has an important role in memory. It has strong, reciprocal connections with medial temporal lobe memory structures and the anterior and lateral thalamic nuclei [Bentovoglio et al., 1993; Suzuki and Amaral, 1994]. It is consistently activated during episodic memory retrieval [Andreasen et al., 1995; Cabeza and Nyberg, 2000; Grasby et al., 1993; Henson et al., 1999; Maddock et al., 2001]. It is significantly hypometabolic during the preclinical stage of Alzheimer's disease [Reiman et al., 1996], and lesions here are associated with amnesia [Gainotti et al., 1998; Valenstein et al., 1987].
We previously suggested that activation of the posterior cingulate cortex by emotional stimuli may reflect an interaction between emotion and memory, such as the enhancement of memory for emotional information [Maddock, 1999; Maddock and Buonocore, 1997]. However, the possibility that this activation resulted from the confounding effects of other memory‐enhancing stimulus features had not previously been excluded. In this study, all known non‐emotional, memory‐enhancing stimulus features were controlled. We conclude that the posterior cingulate cortex was activated specifically by the emotionality of the stimuli in this valence decision task.
Published behavioral studies and our free recall data show that enhanced memory for emotional stimuli is observed with both pleasant and unpleasant stimuli, indicating that this effect is associated with the arousal dimension of emotional stimuli and is independent of valence [Bradley et al., 1992; Doerksen and Shimamura, 2001; Hamann et al., 1999]. To our knowledge, no prior fMRI or PET study has compared the brain responses to pleasant words and matched neutral words. Our results show that activation of the posterior cingulate cortex by emotional words, like the memory‐enhancing effects of these stimuli, is associated with the emotional arousal ratings of the stimuli, irrespective of valence. Three prior functional imaging studies have shown that amygdala activity is associated with enhanced memory for emotional stimuli [Cahill et al., 1995; Canli et al., 2000; Hamann et al., 1999]. However, none of these studies included the posterior cingulate cortical region in their analyses; thus, they did not permit evaluation of its possible involvement in the network of brain regions mediating emotional enhancement of memory. We previously showed that the extent of posterior cingulate activation in an fMRI study comparing unpleasant and neutral words correlated significantly with the degree of recall advantage for the unpleasant words across subjects [Maddock and Buonocore, 1997]. However, a within‐subjects design would provide a more valid test of this relationship. Thus, an event‐related fMRI study targeting this region is needed to test the hypothesis that posterior cingulate cortex activation is associated with subsequently enhanced memory for emotional stimuli.
The most statistically significant local maximum of activation during evaluation of both unpleasant and pleasant words was observed in the left subgenual cingulate cortex (Tables I and II). An examination of susceptibility artifact in this region showed that the T2*‐weighted signal intensity at the locations of peak activation for unpleasant and pleasant words was adequate to permit detection of BOLD activation. For 15 of the 16 scans, signal at the peak coordinates ≥ 40% of the whole brain average. Mean signal values at the peak coordinates were 89% (SD 16%) and 51% (SD 10%) of whole brain averages, respectively, for unpleasant and pleasant words. Along with the results of earlier studies, our findings indicate that evaluating the affective meanings of emotional words is consistently associated with activation of the subgenual cingulate cortex in normal subjects. We found four prior functional imaging studies in which subjects attended to the affective meanings of emotional words. Three of these studies reported significant activation of the left anterior cingulate cortex ventral to the corpus callosum [Beauregard et al., 1997; Elliott et al., 2000; Tabert et al., 2001]. The fourth study used repeated rather than unique unpleasant words (eight repetitions) and did not observe subgenual activation [Maddock and Buonocore, 1997]. However, we have recently shown that repetition of emotional words is associated with significant habituation of the response in the subgenual cingulate cortex [Maddock et al., 2002]. Prior studies used either unpleasant words or sets of mixed valence words. This is the first study to show subgenual cingulate cortex activation with pleasant words and suggests that this region activates in response to emotionally arousing words irrespective of valence. In contrast to these results, four studies have used emotional and neutral words as stimuli in tasks that directed subjects' attention away from the emotional meanings of the words (e.g., the emotional Stroop task) [George et al., 1994; Isenberg et al., 1999; Strange et al., 2000; Whalen et al., 1998]. None of these studies observed activation of the subgenual cingulate cortex, suggesting that perceiving emotional words reliably activates this region only if attention is not directed away from their emotional meanings. Studies using nonverbal, emotional stimuli (facial expressions or arousing photographs) typically have not observed subgenual cingulate cortex activation [Lane et al., 1999; Maddock, 1999; Phillips et al., 1998; Taylor et al., 2000]. Emotional faces and pictures differ from emotional words by conveying affective meaning directly, rather than indirectly via language‐mediated processes. Appreciating emotionality in linguistic stimuli may involve a greater contribution from the subgenual cingulate cortex.
The subgenual cingulate cortex has extensive connections with brainstem and limbic regions involved in emotional processing including the locus ceruleus, raphe nuclei, lateral hypothalamus, basal forebrain, and amygdala. It is also strongly connected with the posterior cingulate cortex and with cortical regions more generally recognized as being involved in emotional processing, including the anterodorsal cingulate cortex, the orbital and insular cortices, and the dorsolateral prefrontal cortex [Carmichael and Price, 1995; Drevets, 2000; Drevets et al., 1997]. Brain stimulation and lesion studies have implicated the subgenual cingulate cortex in the regulation of emotional, visceral, and neuroendocrine responses to emotionally salient stimuli [Drevets, 2000; Sullivan and Gratton, 1999]. Both structural and functional imaging studies have observed abnormalities in this region in patients with familial mood disorders. Drevets and colleagues [1997] demonstrated both decreased cerebral blood flow and decreased glucose metabolism in this region in patients with unipolar and bipolar mood disorders. Significant reductions in gray‐matter volume in the left subgenual region have been observed via MRI in patients with familial mood disorders [Drevets et al., 1997; Hirayasu et al., 1999]. A post mortem study of patients with familial mood disorders reported a significant reduction in the number and density of glia in the subgenual region [Ongur et al., 1998]. Resting metabolic activity in this region may also be associated with treatment response in patients with major depression [Mayberg et al., 2000; Wu et al., 1999]. Our findings suggest that functional imaging during the evaluation of emotionally salient words may have value in the assessment of subgenual cingulate function in patients with emotional disorders.
The anteromedial orbital prefrontal cortex was activated during the evaluation of both unpleasant and pleasant words. Activation of this region by both types of emotional words suggests that it results from emotional arousal in general and is not a valence‐specific effect. An examination of susceptibility artifact in this region showed that the T2*‐weighted signal intensity was adequate to permit detection of BOLD activation. For 15 of the 16 scans, signal at the peak coordinates ≥ 40% of the whole brain average. Mean signal values at the peak coordinates were 71% (SD 18%) and 58% (SD 25%) of whole brain average, respectively, for unpleasant and pleasant words. The location of activation appeared to correspond to the medial part of Brodmann's Area 11 (11m). Area 11m has strong connections with many limbic structures, including substantial reciprocal connections with the subgenual cingulate cortex and the basal nucleus of the amygdala [Carmichael and Price, 1995]. A distinguishing feature of area 11m is its unusually strong connections with the caudal part of the posterior cingulate cortex and with medial temporal lobe and thalamic memory regions [Carmichael and Price, 1995]. Co‐activation of area 11m and the caudal posterior cingulate cortex has often been observed during retrieval of emotionally salient autobiographical memories [Andreasen et al., 1995; Fischer et al., 1996; Maddock et al., 2001]. Activation in this region has also been associated with enhancement of subsequent memory for emotional films [Cahill et al., 1996] and with the retrieval of emotionally salient contextual information [Maratos et al., 2001]. The connectivity of this region and its frequent co‐activation with the posterior cingulate cortex suggest that it may be part of a functional circuit that contributes to some of the interactions between emotion and memory.
Activation of the infero‐lateral region of the left inferior frontal cortex was associated with evaluating the emotional meanings of both pleasant and unpleasant words compared to neutral words. Activation in this same region has previously been observed in response to emotionally salient words and names [Beauregard et al., 1997; Elliott et al., 2000; Maddock et al., 2001; Strange et al., 2000]. This activation may represent greater language‐mediated processing evoked by the emotional words. Activation of BA 9 on the left middle frontal cortex was also observed in association with both the pleasant and unpleasant words. Activation of this region has previously been associated with working memory and the retrieval of semantic information [Cabeza and Nyberg, 2000]. This region has strong, reciprocal connections with the caudal posterior cingulate cortex [Goldman‐Rakic et al., 1984; Morris et al., 1999] and is connected with area 11m, the entorhinal and parahippocampal cortices, and the amygdala [Amaral et al., 1992; Carmichael and Price, 1995; Suzuki and Amaral, 1994]. This activation may reflect affective and mnemonic processes associated with representing knowledge about the emotional meanings of words.
The left frontal pole was activated during the valence evaluation of pleasant words compared to matched neutral words. Activation in this region was not observed in the corresponding comparison for unpleasant words. Lane et al. [1999] reported left frontal polar activation while subjects viewed pleasant, but not unpleasant pictures, compared to neutral pictures. Shimoda and Robinson [1999] have demonstrated that patients with stroke damage in proximity to the left frontal pole are especially vulnerable to clinical depression during the acute post‐stroke period. Our finding of left frontal polar activation by pleasant words is consistent with the proposal of Davidson [1998] that this region may have a role specific to positively valenced emotional processes.
The right amygdala was activated during the valence evaluation of unpleasant words compared to matched neutral words. An examination of susceptibility artifact in this region showed that the T2*‐weighted signal intensity was adequate to permit detection of BOLD activation. For all eight scans, signal at the peak coordinates ≥ 70% of the whole brain average. Mean signal value at the peak coordinates was 90% (SD 12%) of the whole brain average. Prior studies have reported right, left, or bilateral amygdala activation in response to unpleasant words, but no consistent pattern of lateralized activation has emerged [Isenberg et al., 1999; Strange et al., 2000; Tabert et al., 2001]. The amygdala appears to have multiple roles in human affective processing, including enhanced perception of and enhanced memory for emotionally arousing stimuli. While this study does not isolate specific emotional processes, the pattern of findings is consistent with amygdala involvement in the emotional enhancement of both perception and memory. Co‐activation of the amygdala and the auditory cortex by the unpleasant words is consistent with amygdala involvement in emotional influences on perceptual processing of these auditory stimuli. Co‐activation of amygdala, parahippocampal cortex, and posterior cingulate cortex is consistent with amygdala involvement in emotional influences on memory processes. Significant amygdala activation was observed only with the unpleasant and not with the pleasant emotional words in this study. This is consistent with the suggestion that the amygdala responds to both positive and negative stimuli, but disproportionately to the latter [Adolphs and Tranel 2000; Everitt et al., 2000; Hamann et al., 1999]. It is important to note, however, that the design of this study precludes direct comparisons between the positive and negative stimuli. The unpleasant words had significantly higher arousal ratings than the pleasant words (Table I), and were presented before the pleasant words for most subjects. Significant activations that were observed only in response to the unpleasant words or the pleasant words may reflect these differences in arousal and/or order, rather than the difference in valence.
CONCLUSIONS
The results of this study help define the stimulus conditions associated with emotion‐related activation of the posterior and subgenual regions of the cingulate cortex, and suggest future studies to further elucidate the functional significance of these activations. The primary finding is that judging the valence of emotional words is associated with posterior cingulate cortex activation even when contrasted to neutral words that have been matched on all non‐emotional qualities known to influence memory for verbal stimuli. We conclude that this activation was due to the emotional salience of the stimuli and cannot be attributed to the memory‐enhancing effects of non‐emotional stimulus features. The findings also show that posterior cingulate cortex activation is observed with both positively and negatively valenced emotional stimuli. Activation of this region is thus not valence specific, but is associated with emotionally arousing stimuli in general. Further studies will be necessary to test the hypothesis that posterior cingulate cortex activation is specifically associated with emotion‐related memory enhancement. In addition, the findings confirm prior studies showing that evaluating the affective meanings of emotional words consistently activates the subgenual cingulate cortex and extends these observations to include both positively valenced and negatively valenced words. Functional imaging during valence decision tasks with emotional words may be an effective way to probe the functional integrity of these cingulate regions in patients with emotional disorders.
Acknowledgements
This research was supported by a Health System Research Award from the University of California Davis, and by a Faculty‐Alumni Research Development Award from the Department of Psychiatry, University of California Davis. We thank Patricia Foley and Victoria Cross for assistance with data collection.
REFERENCES
- Adolphs R, Tranel D. 2000. Emotion recognition and the human amygdala In Aggleton JP, editor. The Amygdala: a functional analysis. Oxford: Oxford University Press, p 587–630. [Google Scholar]
- Adolphs R, Cahill L, Schul R, Babinsky R. 1997. Impaired declarative memory for emotional stimuli following bilateral amygdala damage in humans. Learn Mem 4: 291–300. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. 2000. Social perception from visual cues: role of the STS region. Trends Cogn Sci 4: 267–278. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. 1992. Anatomical organization of the primate amygdaloid complex In: Aggleton JP, editor. The Amygdala: neurobiological aspects of emotion. New York: Wiley‐Liss, p 1–66. [Google Scholar]
- Anderson A, Phelps E. 2001. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 411: 305–309. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD. 1995. Remembering the past: Two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 152: 1576–1585. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL. 1997. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic‐naive patients. Lancet 349: 1730–1734. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Chertkow H, Bub D, Murtha S, Dixon R, Evans A. 1997. The neural substrate for concrete, abstract, and emotional word lexica: a positron emission tomography study. J Cogn Neurosci 9: 441–461. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG. 1992. The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol Med 22: 607–615. [DOI] [PubMed] [Google Scholar]
- Bentovoglio M, Kultas‐Ilinsky K, Ilinsky I. 1993. Limbic thalamus: structure, intrinsic organization, and connections In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser, p 71–122. [Google Scholar]
- Bradley MM, Lang PJ. 1994. Measuring emotion: the self assessment maniken and the semantic differential. J Behav Ther Exp Psychiatry 25: 49–59. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. 1992. Remembering pictures: pleasure and arousal in memory. J Exp Psychol Learn Mem Cogn 18: 379–390. [DOI] [PubMed] [Google Scholar]
- Buonocore MH, Gao L. 1997. Ghost artifact reduction for echo‐planar imaging using image phase correction. Mag Res Med 38: 89–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. 2000. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. 1998. Mechanisms of emotional arousal and lasting declarative memory. TINS 21: 294–299. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch H, McGaugh JL. 1995. The amygdala and emotional memory. Nature 377: 295–296. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. 1996. Amygdala activity at encoding correlated with long‐term free recall of emotional information. PNAS 93: 8016–8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JDE, Cahill L. 2000. Event‐related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci 20: RC99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. 1995. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 363: 615–641. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. 1998. Affective style and affective disorders: perspectives from affective neuroscience. Cogn Emot 12: 307–330. [Google Scholar]
- Doerksen S, Shimamura AP. 2001. Source memory enhancement for emotional words. Emotion 1: 5–11. [DOI] [PubMed] [Google Scholar]
- Drevets WC. 2000. Neuroimaging studies of mood disorders. Biol Psychiatry 48: 813–829. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JRJ, Todd RD, Reich T, Vannier M, Raichle ME. 1997. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386: 824–827. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. 2000. Selective attention to emotional stimuli in a verbal go/no‐go task: an fMRI study. Neuroreport 11: 1739–1744. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Hall J, Parkinson JA, Robbins TW. 2000. Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction In: Aggleton JP, editor. The Amygdala: a functional analysis. Oxford: Oxford University Press, p 353–390. [Google Scholar]
- Fischer H, Wik G, Fredikson M. 1996. Functional neuroanatomy of robbery re‐experience: affective memories studied with PET. Neuroreport 7: 2081–2086. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Mag Res Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Almonti S, Di Betta AM, Silveri MC. 1998. Retrograde amnesia in a patient with retrosplenial tumour. Neurocase 4: 519–526. [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Trimble MR, Horwitz B, Herscovitch P, Post RM. 1994. Regional brain activity when selecting a response despite interference: an H2O15 PET study of the Stroop and an emotional Stroop. Hum Brain Map 1: 194–209. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS, Selemon LD, Schwartz ML. 1984. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and the parahippocampal cortex in the rhesus monkey. Neuroscience 12: 719–743. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston KJ, Bench C, Frackowiak RSJ, Dolan RJ. 1993. Functional mapping of brain areas implicated in auditory‐verbal memory function. Brain 116: 1–20. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. 1999. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci 2: 289–293. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Luu C, Hazlett EA, Siegel JBV, Lohr J, Wu J, Haier RJ, Bunney J. 1997. Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry 154: 682–684. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. 1999. Recollection and familiarity in recognition memory: An event‐related functional magnetic resonance imaging study. J Neurosci 19: 3962–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun‐Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW. 1999. Subgenual cingulate cortex volume in first‐episode psychosis. Am J Psychiatry 156: 1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AP, Gillin JC, Buchsbaum MS, Wu JC, Abel L, Bunney WE, Jr. 1996. Brain glugose metabolism during non‐rapid eye movement sleep in major depression: a positron emission tomography study. Arch Gen Psychiatry 53: 645–652. [DOI] [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattie B, Leon AC, Stern E. 1999. Linguistic threat activates the human amygdala. PNAS 96: 10456–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera H, Francis WN. 1967. Computational analysis of present‐day American English. Providence, RI: Brown University Press. [Google Scholar]
- Lane RD, Chua PM, Dolan RJ. 1999. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia 37: 989–997. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. 1999. Retrosplenial cortex and emotion: New insights from functional imaging studies of the human brain. TINS 22: 310–316. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH. 1997. Activation of left posterior cingulate gyrus by the auditory presentation of threat‐related words: an FMRI study. Psychiatry Res Neuroimag 75: 1–14. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. 2001. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104: 667–676. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. 2002. Evaluating emotional words activates subgenual and posterior cingulate cortices. Presented at the Annual Meeting of Cognitive Neuroscience Society. April 16, 2002, San Francisco, CA.
- Maratos EJ, Dolan RJ, Morris JS, Henson RNA, Rugg MD. 2001. Neural activity associated with episodic memory for emotional context. Neuropsychologia 39: 910–920. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva A, Mahurin RK, McGinnis S, Jerabek PA. 2000. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 48: 830–843. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RSJ, Dolan RJ. 1994. Functional anatomy of obsessive‐compulsive phenomena. Br J Psychiatry 164: 459–468. [DOI] [PubMed] [Google Scholar]
- Morris R, Pandya DN, Petrides M. 1999. Fiber system linking the mid‐dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J Comp Neurol 407: 183–192. [DOI] [PubMed] [Google Scholar]
- Musil SY, Olsen CR. 1993. The role of cat cingulate cortex in sensorimotor integration In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser, p 345–365. [Google Scholar]
- Ongur D, Drevets WC, Price JL. 1998. Glial reduction in the subgenual prefrontal cortex in mood disorders. PNAS 95: 13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivio A, Yuille JC, Madigan SA. 1968. Concreteness, imagery, and meaningfulness values for 925 nouns. J Exp Psychol 76: 1–25. [DOI] [PubMed] [Google Scholar]
- Perani D, Colombo C, Bressi S, Bonfanti A, Grassi F, Scarone S, Bellodi L, Smeraldi E, Fazio F. 1995. [18F] FDG PET study in obsessive‐compulsive disorder: a clinical/metabolic correlation study after treatment. Br J Psychiatry 166: 244–250. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Bullmore ET, Howard R, Woodruff PWR, Wright IC, Williams SCR, Simmons A, Andrew C, Brammer M, David AS. 1998. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Res Neuroimag 83: 127–138. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat JW. 1974. Reliability and validity of some handedness questionnaire items. Neuropsychologia 12: 43–47. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Dougherty DD, Cosgrove GR, Cassem EH, Alpert NM, Price BH, Nierenberg AA, Mayberg HS, Baer L, Jenike MA, Fischman AJ. 2001. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biol Psychiatry 50: 659–667. [DOI] [PubMed] [Google Scholar]
- Reiman E. 1997. The application of positron emission tomography to the study of normal and pathologic emotions. J Clin Psychiatry 58(Suppl 16): 4–12. [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. 1996. Preclinical evidence of Alzheimer's disease in persons homozygous for the E4 allele for apolipoprotein E. N Engl J Med 334: 752–758. [DOI] [PubMed] [Google Scholar]
- Reisberg D, Heuer F. 1995. Emotion's multiple effects on memory In: McGaugh JL, Weinberger NM, Lynch G, editors. Brain and memory: modulation and mediation of neuroplasticity. New York: Oxford University Press, p 84–92. [Google Scholar]
- Rubin DC. 1980. 51 properties of 125 words. J Verb Learn Verb Behav 19: 736–755. [Google Scholar]
- Rubin DC, Friendly, M . 1986. Predicting which words get recalled: measures of free recall, availability, goodness, emotionality, and pronunciability for 925 nouns. Mem Cogn 14: 79–94. [DOI] [PubMed] [Google Scholar]
- Shimoda K, Robinson RG. 1999. The relationship between poststroke depression and lesion location in long‐term follow‐up. Biol Psychiatry 45: 187–192. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Constable RT, Gore JC. 1999. ROC analysis of statistical methods used in functional MRI: Individual subjects. Neuroimage 9: 311–329. [DOI] [PubMed] [Google Scholar]
- Strange BA, Henson RNA, Friston KJ, Dolan RJ. 2000. Brain mechanisms for detecting perceptual, semantic and emotional deviance. Neuroimage 12: 425–433. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. 1999. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci 19: 2843–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. 1994. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol 350: 497–533. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Borod JC, Tang CY, Lange G, Wei TC, Johnson R, Nusbaum AO, Buchsbaum MS. 2001. Differential amygdala activation during emotional decision and recognition memory tasks using unpleasant words: an fMRI study. Neuropsychologia 39: 556–573. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. 2000. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia 38: 1415–1425. [DOI] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. 1987. Retrosplenial amnesia. Brain 110: 1631–1646. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Vogt BA. 1993. Connections of the monkey cingulate cortex In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser, p 345–365. [Google Scholar]
- Vogt BA, Finch DM, Olson CR. 1992. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 2: 435–443. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Abscher JR, Bush G. 2000. Human retrosplenial cortex: where is it and is it involved in emotion? TINS 23: 195–196. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. 1998. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44: 1219–1228. [DOI] [PubMed] [Google Scholar]
- Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, Najafi A, Klein E, Hazen K, Bunney WE. 1999. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry 156: 1149–1158. [DOI] [PubMed] [Google Scholar]


