Abstract
The KE family is a large three‐generational pedigree in which half of the members suffer from a verbal and orofacial dyspraxia in association with a point mutation in the FOXP2 gene. This report extends previous voxel‐based morphometric analyses of magnetic resonance imaging (MRI) scans (Watkins et al. [2002] Brain 125:465–478) using a bilateral conjunction analysis. This searches specifically for areas of grey matter density that differ bilaterally in the affected members compared with both matched controls and the unaffected family members. 3‐D T1‐weighted MRI datasets of 17 family members (10 affected, 7 unaffected) and matched controls were compared. The most significant findings were reduced grey matter density bilaterally in the caudate nucleus, the cerebellum, and the left and right inferior frontal gyrus in the affected members. In addition, increased grey matter density was found bilaterally in the planum temporale. These results confirm that a point mutation in FOXP2 is associated with several bilateral grey matter abnormalities in both motor and language related regions. The results also demonstrate the advantages of using a conjunction analysis when bilateral abnormalities are suspected. Hum. Brain Mapping 18:194–200, 2003. © 2003 Wiley‐Liss, Inc.
INTRODUCTION
We report on voxel‐based brain morphometry (VBM) in the KE family in which half of the members present with a pronounced speech and language disorder characterized as a verbal and orofacial dyspraxia [Vargha‐Khadem et al., 1995; Watkins et al., 2002b]. Candidate gene analysis has indicated that a point mutation in the FOXP2 gene is responsible for this disorder [Fisher et al., 1998; Lai et al., 2001].
Previous work using VBM analysis of magnetic resonance images has shown that there are subtle brain abnormalities in the affected family members [Vargha‐Khadem et al., 1998, Watkins et al., 1999, 2002a]. VBM is an automated procedure that compares grey matter density (i.e., proportion of tissue volume ascribed to grey matter) on a voxel‐by‐voxel basis between two groups using statistical parametric mapping (SPM) techniques [Ashburner and Friston, 2000]. The abnormalities found in the affected members were identified using a VBM contrast that detects regions of difference in grey matter density in each hemisphere separately [Ashburner and Friston, 2000] (see Fig. 1A). Results of this unilateral analysis revealed that there were regions of abnormally reduced or increased grey matter density in speech‐ and motor‐related areas [Watkins et al., 2002a]. Some of these regions, in particular the caudate nucleus, were abnormal in both hemispheres, whereas others, such as the inferior frontal gyrus and supplementary motor area, were abnormal only on the left. On the basis of these findings, it was concluded that the brain abnormality underlying the behavioural phenotype was in the frontostriatal system, parts of which were affected bilaterally.
Figure 1.
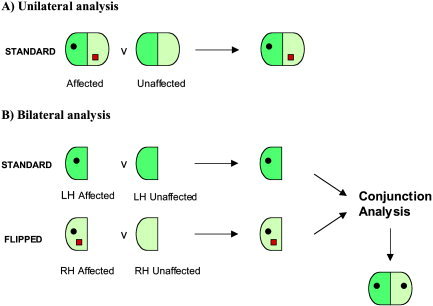
Illustration of unilateral (A) and bilateral (B) methods of VBM analysis. LH, left hemisphere; RH, right hemisphere.
Unilateral brain abnormalities of developmental origin, affecting the neural substrates of speech and language, rarely result in persistent and chronic dysphasia, and are likely to result in a much milder syndrome than if the same region is abnormal bilaterally [Landau and Kleffner 1957; Vargha‐Khadem et al., 1985]. This is presumably because of the capacity to reorganize function after early unilateral damage, provided a viable neural substrate is available intra‐ or inter‐hemispherically [Booth, 1999; Isaacs et al., 1996; Vicari et al., 2000]. When selective and chronic deficits are encountered, bilateral pathology of the systems subserving speech and language are then suspected [Vargha‐Khadem et al., 1985]. Therefore, in the case of the KE family, the severe and persistent phenotype of verbal and orofacial dyspraxia leads us to suspect bilateral pathology of these cortical and subcortical systems.
A recent development in the use of VBM allows the selective investigation of symmetrical bilateral abnormalities (see Fig. 1B). This is achieved by producing two statistical parametric maps (SPMs), one comparing two groups of standard images, and the other comparing the same two groups but with the images flipped across the midline from left to right. A conjunction analysis is then used to find regions of significant difference that are common to both SPMs [Salmond et al., 2000].
Here we report on the use of this bilateral method of analysis to confirm previous findings, and to determine whether the left lateralized abnormalities that had been identified using the unilateral method were in fact bilateral. Moreover, a second conjunction analysis allowed us to differentiate between abnormalities common to the entire family vs. those specific to members with the FOXP2 mutation.
SUBJECTS AND METHODS
Subjects
This study involved the 17 family members and controls previously reported by Watkins et al. [2002a]. Ten affected family members were included (5 men, 5 women, mean age 28.1 years). Four were from the first and second generations, the remaining six from the third generation aged between 9 and 21 years. Seven unaffected members were also scanned (3 males, 4 females, age range 9–27 years; mean age 17.1 years). Seventeen normal controls were selected to match the family members on age and sex.
Data acquisition
All participants were scanned using a 1.5 T Siemens SP system with a T1‐weighted 3D MPRAGE sequence (sequence TR = 10 msec, TE = 4 msec, TI = 200 msec, flip angle = 12 degrees, matrix size = 256 × 256, field of view = 250 mm, partition thickness = 1.25 mm, 128 sagittal partitions, voxel size = 0.98 × 0.98 × 1.25 mm, acquisition time = 8.3 min).
Image processing
The 3D data sets were processed and analysed using SPM99 software (Wellcome Department of Imaging Neuroscience, UK). First, the images were spatially normalized to a T1 template (MNI). For the bilateral analysis, a symmetrical template was calculated from this T1 template, thus reducing asymmetries. The second stage segmented the images into grey matter, white matter, and cerebrospinal fluid compartments (again using a symmetric probabilistic template for the bilateral analysis). The grey matter partitions were then selected and duplicated for each data set, and then flipped in the transverse plane along the anterior‐–posterior axis [Salmond et al., 2000]. All images were smoothed using a 12‐mm FWHM isotropic Gaussian kernel. The voxel values thus provide an index of the amount of grey matter per unit volume under the smoothing kernel [Ashburner and Friston, 2000].
Data analysis
Statistical analysis was carried out in SPM99. The hypothesis for this analysis was that the affected members would have grey matter density abnormalities in regions involved in the speech–motor network.
In order to identify areas of significant abnormality specific to the affected members of the KE family and to identify areas of abnormality common to all the members of the KE family, two basic contrasts were used in interaction and conjunction analyses. These contrasts were: 1) Affected members vs. their controls; and 2) unaffected members vs. their controls.
Areas of abnormality specific to the affected members of the family
Unilateral analysis
An interaction analysis was carried out with the four groups (affected members, controls for the affected members, unaffected members, controls for the unaffected members) with the contrasts entered as −1 1 −1 1 (increased grey matter in the affected members) and −1 1 1 −1 (decreased grey matter in the affected members) in SPM99.
Bilateral analysis
A conjunction analysis was carried out with the interaction contrasts for the flipped and unflipped data as described in Salmond et al. [2000].
Areas of abnormality common to both affected and unaffected members of the KE family
Unilateral analysis
A conjunction analysis was carried out with the conjoined contrasts as (1–1 0 0; 0 0 1 −1) to look at increased grey matter density in both the affected and unaffected family members, and (−1 1 0 0; 0 0 −1 1) to look at decreased grey matter density in these family members.
Bilateral analysis
A two‐level conjunction was carried out with one level constraining the search to bilateral abnormalities [See Salmond et al., 2000] and the second level constraining the search to common family abnormalities.
Statistics
Findings of abnormalities in the affected family members, with respect to both unaffected members and controls, in the bilateral conjunction and the unilateral contrasts are reported at a significance level of P < 0.05 after correction for multiple comparisons, or at an uncorrected level of P < 0.001 if the region fell within the a priori hypothesis.
Regions of abnormality in grey matter density that were found to be common to both affected and unaffected members in comparison with controls are reported if they reached a corrected level of P < 0.05. As there was no a priori hypothesis, regions reaching an uncorrected level of significance only are not reported.
RESULTS
Unilateral method
Using a unilateral conjunction it was found that the affected family members have reduced grey matter density in several areas relative to unaffected members and controls, the most significant being the left caudate nucleus (see Table I). Less grey matter was also seen at closely homologous coordinates in the right caudate nucleus at a lower threshold. In addition, significant unilateral abnormalities were found in the left dorsal inferior frontal gyrus, the left precentral gyrus, and the right temporal pole (see Table I).
Table I.
Bilateral and unilateral conjunction analyses
| Region | Coordinates (mm) | Corrected P | Uncorrected P | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Unilateral conjunction analysis | |||||
| Affected members: less grey matter | |||||
| L head of caudate nucleus | −14 | −16 | 9 | <0.001 | |
| R head of caudate nucleus | 16 | 18 | 9 | 0.003 | |
| L dorsal inferior frontal gyrus | −42 | 38 | 14 | 0.001 | |
| L precentral gyrus | −52 | −2 | 26 | 0.001 | |
| R temporal pole | 22 | 10 | −36 | <0.001 | |
| Affected members: more grey matter | |||||
| L superior temporal gyrus | −44 | −36 | 12 | 0.004 | |
| R superior temporal gyrus | 58 | −32 | 9 | 0.001 | |
| L angular gyrus | −51 | −64 | 24 | <0.001 | |
| L cerebellum lobule VIIB | −42 | −51 | −45 | <0.001 | |
| Affected and unaffected: less grey matter | |||||
| L anterior cerebellum | −30 | −44 | −50 | 0.023 | |
| Affected and unaffected: more grey matter | |||||
| L inferior temporal lobe | −36 | −2 | −32 | 0.025 | |
| R inferior frontal sulcus | 52 | 21 | 34 | 0.025 | |
| Bilateral conjunction analysis | |||||
| Affected members: less grey matter | |||||
| Head of caudate nucleus | 15 | 16 | 10 | <0. 00001 | |
| Dorsal inferior frontal gyrus | 45 | 36 | 16 | <0. 0001 | |
| Precentral gyrus | 56 | −4 | 26 | 0.001 | |
| Ventral cerebellum lobule VIIB | 21 | −86 | −51 | 0.001 | |
| 34 | −78 | −57 | 0.001 | ||
| Ventral cerebellum lobule VIIIB | 21 | −50 | −64 | 0.001 | |
| 30 | −45 | −58 | 0.001 | ||
| 18 | −52 | −66 | 0.001 | ||
| 24 | −46 | −62 | 0.001 | ||
| Temporal pole | 26 | 15 | −40 | 0.001 | |
| Affected members: more grey matter | |||||
| Posterior superior temporal gyrus | 56 | −30 | 8 | <0.001 | |
| Angular gyrus | 48 | −62 | 27 | <0.001 | |
| Affected and unaffected: less grey matter | |||||
| Anterior calcarine sulcus | 21 | −56 | 8 | 0.012 | |
| Dorsal thalamus | 15 | −27 | 14 | 0.028 | |
| Affected and unaffected: more grey matter | |||||
| Inferior temporal lobe | 34 | −3 | −33 | 0.001 | |
| Frontal pole | 16 | 63 | −2 | 0.009 | |
Corrected P values included when they reach significance (P < 0.05).
There were also regions of increased grey matter density in the affected members, with indications of a bilateral abnormality in one area, the posterior superior temporal gyrus, whereas additional abnormalities in the angular gyrus and cerebellar lobule VIIB [Schmahmann et al., 1999] were found only in the left hemisphere (see Table I).
Bilateral model
The most significant region of reduced grey matter density bilaterally in the affected members was in the caudate nucleus (see Fig. 2), confirming the results of the unilateral conjunction. The bilateral conjunction analysis also showed that both the dorsal inferior frontal gyrus (corresponding to BA 45) [Tomaiuolo et al., 1999] and the precentral gyrus (lower primary motor cortex) were significantly abnormal in both hemispheres. These regions were only significant in the left hemisphere in the unilateral analysis (see Table I). In addition, a decrease in grey matter density was seen in the temporal pole region in the bilateral analysis, whereas with the unilateral model this area only reached significance in the right hemisphere.
Figure 2.
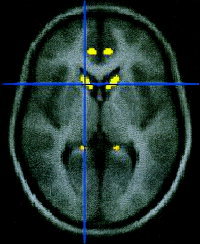
Result of the bilateral conjuction analysis. Less grey matter density in the caudate nuclei in the affected members. Left and right caudate nuclei, uncorrected P < 0.001 at coordinates ± 13 20 3.
A bilateral decrease in grey matter density was also seen in several regions of the ventral cerebellum (lobules VIIB and VIIIB) [Schmahmann et al., 1999], which were not seen in the unilateral conjunction analysis (see Table I).
An increase in grey matter density was found bilaterally in the posterior region of the superior temporal gyrus, which confirms that those regions of abnormality found with the unilateral model are symmetrical. The angular gyrus was also found to be abnormal bilaterally, whereas the unilateral conjunction suggested this was lateralized to the left hemisphere (see Table I).
Familial abnormalities in grey matter density
A conjunction between comparisons of affected members and matched controls and unaffected members and matched controls revealed areas of abnormality that are common to both family groups. Only those surviving correction for multiple comparisons are reported. Reductions in grey matter density were found in several posterior regions, none of which were present in both the unilateral and bilateral models (see Table I). In terms of increased grey matter density, a region common to both affected and unaffected members was found in a region of the temporal lobe, adjacent to the temporal horn, in both the bilateral and unilateral models. Additional regions were found in the right prefrontal cortex (see Table I). None of these regions were in language‐related areas.
DISCUSSION
This study has confirmed that the most consistent morphometric finding in the affected KE family members is a bilateral reduction in grey matter density in the caudate nucleus. This is common to both unilateral and bilateral conjunction analyses, and confirms previous findings reported by Watkins et al. [2002a] using both VBM and volumetric measurements. The association of the caudate nucleus with motor planning and sequencing, and with cognitive function [Abdullaev et al., 1998; Jueptner et al., 1997] is suggestive of the role that this structural abnormality may play in the phenotype of the affected members [Watkins et al., 2002b].
The bilateral conjunction analysis also confirms that the regions of cortical abnormality that appeared to be bilateral using the standard unilateral model are symmetrically abnormal, for example, the posterior superior temporal gyri. This region has been implicated in speech perception and developmental language disorders. Scott et al. [2000] showed that the left superior temporal sulcus was specifically activated with the presentation of intelligible speech. In addition, studies have suggested that individuals with specific language impairment and developmental dyslexia have an abnormal asymmetry of the planum temporale, also in this area [Foster et al., 2002; Galaburda et al., 1985; Leonard et al., 2001]. Bilateral abnormalities were also confirmed in the cerebellum, a structure that has many connections to the prefrontal cortex, and has been highly implicated in motor and cognitive functions [Diamond, 2000; Jueptner and Weiller, 1998; Leiner et al., 1993; Rao et al., 1997].
In addition to confirming previous results, the bilateral conjunction also provided some new findings. In many of the regions where left lateralized abnormalities in cortical areas had been found using standard VBM contrasts, a symmetrical abnormality in both hemispheres was found using the bilateral model. These additional findings can be attributed to the greater specificity and selectivity of the bilateral model [Salmond et al., 2000]. This was the case for the inferior frontal gyrus region, the precentral gyrus, and the angular gyrus. These regions are also known to be highly involved in language, with BA 44/45 and the primary motor cortex relating particularly to speech production, paralleling the core features of impaired articulatory and orofacial sequencing in this phenotype [Lotze et al., 2000; Price, 2000; Watkins et al., 2002b]. The angular gyrus is also thought to be involved in language, specifically in semantic processing [Hart and Gordon, 1990; Price, 2000].
The bilateral nature of these cortical and subcortical abnormalities may explain the severe phenotype in the affected members of the KE family. Children with early focal brain lesions of the left hemisphere show a remarkable level of language proficiency with only subtle deficits, suggesting that speech and other language functions have reorganized to other brain regions [Isaacs et al., 1996; MacWinney et al., 2000; Vargha‐Khadem et al., 1985; Vicari et al., 2000]. In the case of bilateral abnormalities in these regions, reorganization would be compromised. The two reported cases with developmental bilateral perisylvian pathology support this conclusion, showing a failure to develop intelligible speech [Landau and Kleffner, 1957; Vargha‐Khadem et al., 1985].
In addition to selectively searching for bilateral abnormalities, the use of a conjunction analysis in this study has one further application. It allows regions of grey matter density that are common to both affected and unaffected family members, and different from controls, to be detected. In the case of a standard contrast between the affected group and normal controls, such abnormalities may be erroneously interpreted as being related to the specific genetic mutation in the affected family members. None of the regions found to be abnormal in both the affected and unaffected members were in language‐related areas.
In conclusion, this study highlights the striking nature of the bilateral abnormalities, particularly the reduction in grey matter density in the caudate nuclei that have been found in this autosomal dominant speech and language disorder.
Acknowledgements
We thank the members of the KE family and control subjects for their continued cooperation with our research programme. We also thank Frederique Liegeois for assistance with acquiring the data and, along with Torsten Baldeweg, for valuable input into this manuscript. Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from the Research and Development funding from the NHS Executive.
REFERENCES
- Abdullaev YG, Bechtereva NP, Melnichuk KV (1998): Neuronal activity of human caudate nucleus and prefrontal cortex in cognitive tasks. Behav Brain Res 97: 159–177. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry: the methods. NeuroImage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Booth JR (1999): Functional organization of activation patterns in children: whole brain fMRI imaging during three different cognitive tasks. Prog. Neuro‐Psychopharmacol Biol Psychiat 23: 669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2000): Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev 71: 44–56. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Vargha‐Khadem F, Watkins KE, Monaco AP, Pembrey ME (1998): Localisation of a gene implicated in a severe speech and language disorder. Nat Genet 18: 168–170. [DOI] [PubMed] [Google Scholar]
- Foster LM, Hynd GW, Morgan AE, Hugdahl K (2002): Planum temporale asymmetry and ear advantage in dichotic listening in developmental dyslexia and attention‐deficit/hyperactivity disorder (ADHD). J Int Neuropsychol Soc 8: 22–36. [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N (1985): Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol 18: 222–233. [DOI] [PubMed] [Google Scholar]
- Hart‐ J Jr, Gordon B (1990): Delineation of single‐word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol 27: 226–231. [DOI] [PubMed] [Google Scholar]
- Isaacs E, Christie D, Vargha‐Khadem F, Mishkin M (1996): Effects of hemispheric side of injury, age at injury, and presence of seizure disorder on functional ear and hand assymetries in hemiplegic children. Neuropsychologia 34: 127–137. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE (1997): Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 77: 1325–1337. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C (1998): A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain 121: 1437–1449. [DOI] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha‐Khadem F, Monaco AP (2001): A forkhead‐domain gene is mutated in a severe speech and language disorder. Nature 413: 519–523. [DOI] [PubMed] [Google Scholar]
- Landau WM, Kleffner FR (1957): Syndrome of acquired aphasia with convulsive disorder in children. Neurology 10: 915–921. [DOI] [PubMed] [Google Scholar]
- Lotze M, Seggewies G, Erb M, Grodd W, Birbaumer N (2000): The representation of articulation in the primary sensorimotor cortex. Neuroreport 11: 2985–2989. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS (1993): Cognitive and language functions of the human cerebellum. Trends Neurosci 16: 444–447 [DOI] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzler J, Mohr CM, King WM, Freeman A (2001): Anatomical risk factors for phonological dyslexia. Cerebral Cortex 11: 148–157. [DOI] [PubMed] [Google Scholar]
- MacWhinney B, Feldman H, Sacco K, Valdés‐Pérez R (2000): Online measures of basic language skills in children with early focal brain lesions. Brain Lang 71: 400–431. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Doya K, Hikosaka O (2001): Parallel cortico‐basal ganglia mechanisms for acquisition and execution of visuomotor sequences: a computational approach. J Cogn Neurosci 13: 626–647. [DOI] [PubMed] [Google Scholar]
- Price C (2000): The anatomy of language: contributions from functional neuroimaging. J Anat 197: 335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR (1997): Distributed neural systems underlying the timing of movements. J Neurosci 17: 5528–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha‐Khadem F, Gadian DG, Friston KJ (2000): Detecting bilateral abnormalities with voxel based morphometry. Hum Brain Mapp 11: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M (1999): Three‐dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 10: 233–260. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJS (2000): Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123: 2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, Petrides M (1999): Morphology morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur J Neurosci 11: 3033–3046. [DOI] [PubMed] [Google Scholar]
- Vargha‐Khadem F, Watters GV, O'Gorman AM (1985): Development of speech and language following bilateral frontal lesions. Brain Lang 25: 167–183. [DOI] [PubMed] [Google Scholar]
- Vargha‐Khadem F, Watkins K, Alcock K, Fletcher P, Passingham R (1995): Praxic and nonverbal cognitive deficits in a large family with a genetically transmitted speech and language disorder. Proc Natl Acad Sci USA 92: 930–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha‐Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, Frackowiak RSJ, Friston KJ, Pembrey ME, Mishkin M, Gadian DG, Passingham RE (1998): Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci USA 95: 12695–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Albertoni A, Chilosi AM, Cipriani P, Cioni G, Bates E (2000): Plasticity and reorganization during language development in children with early brain injury. Cortex 36: 31–46. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Gadian DG, Vargha‐Khadem F (1999): Functional and structural brain abnormalities associated with a genetic disorder of speech and language. Am J Hum Genet 65: 1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Vargha‐Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, Frackowiak RSJ, Mishkin M, Gadian DG (2002a): MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain 125: 465–478. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Dronkers NF, Vargha‐Khadem F (2002b): Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain 125: 452–464. [DOI] [PubMed] [Google Scholar]


