Abstract
There is conflicting data regarding the ability of nitric oxide (NO) to promote or inhibit colorectal cancer cell proliferation. Furthermore, NO reacts rapidly with endogenous superoxide at a diffusion-controlled rate to give peroxynitrite (ONOO-), a strong oxidant and nitrating agent. The aim of this study was to assess the effects of exogenous NO and ONOO- on the proliferation of the colorectal cancer cell line Caco-2. NOR5 and SIN-1 were used as NO and ONOO- donors, respectively. Both NOR5 and SIN-1 inhibited the proliferation of the Caco-2 cells; however, the effect of NOR5 was slightly stronger than that of SIN-1. The results also indicated that NO plays a major role in the inhibition of SIN-1-induced proliferation of Caco-2 cells. The results of a terminal deoxynucleotidyl transferase dUTP nick end labeling assay, cell cycle analysis, and p21 protein expression measurement further indicated that NO induced S-G2/M phase arrest, but not apoptosis, in the Caco-2 cells. The results suggest that NO, rather than ONOO-, has the potential to repress the proliferation of Caco-2 cells by inducing S-G2/M cell cycle arrest.
Keywords: Nitric oxide, peroxynitrite, NOR5, SIN-1, colon cancer proliferation, reactive nitrogen species
Introduction
Colorectal cancer is a leading cause of death worldwide; it accounts for approximately 10% of all cancer-related deaths [1]. About 3-5% of colorectal cancers may be due to inherited genetic defects and up to 25% of cases may have some degree of familial association; however, the majority of colorectal cancers occur in a sporadic manner without a family history [2].
Nitric oxide synthase (NOS) has neuronal, inducible, and endothelial isoforms. It catalyzes the oxidation of L-arginine to generate nitric oxide (NO), which is a highly reactive radical that has several biological activities, including smooth muscle relaxation, inhibition of platelet aggregation, and neurotransmission [3]. NO, whether produced exogenously or endogenously, has been implicated in the pathogenesis of various human malignant tumors, including breast cancer [4], colorectal cancer [5], melanoma [6], and lung cancer [7]. However, it remains unclear whether NO functions as a pro-neoplastic or antineoplastic effector [8-11]. Furthermore, little information is available concerning peroxynitrite (ONOO-), which is produced by the reaction of NO with superoxide (O2 · -). ONOO- is a strong oxidant and nitrating agent that damages several molecules in cells, including DNA and proteins [12].
In the present study, the effects of exogenous NO and ONOO- on the proliferation of the colorectal cancer cell line Caco-2 were examined. NOR5 [13] and SIN-1 [14] were used as NO and ONOO- donors, respectively.
Materials and methods
Materials
(±)-N-[(E)-4-Ethyl-3-[(Z)-hydroxyimino]-6-methyl-5-nitro-3-heptenyl]-3-pyridinecarboxamide (NOR5), 3-(4-morpholinyl) sydnonimine hydrochloride (SIN-1), and ONOO- solution were supplied by Dojindo Laboratories (Kumamoto, Japan). Superoxide dismutase (SOD), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), propidium iodide (PI), In Situ Cell Death Detection Kit, 4’,6-diamidino-2-phenylindole (DAPI), fluorescein isothiocyanate (FITC), ribonuclease A (RNase A), and actinomycin D were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). A primary antibody against p21 protein and a secondary antibody (horseradish-peroxidase-linked antibody) were purchased from Cell Signaling Technology Japan, K.K. (Tokyo, Japan).
Cell culture
The human colon cancer cell line Caco-2 was purchased from the European Collection of Cell Cultures (Salisbury, Wilts, UK) and cultured in Minimum Essential Medium (Life Technologies Corporation, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Nichirei Biosciences Inc., Tokyo, Japan) and 1% non-essential amino acids (Life Technologies Corporation, Carlsbad, CA, USA). The cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Cell viability assay
Cell viability was measured by an MTT assay as described previously [15,16]. Briefly, the cells were incubated with test reagents at a density of 2.5 × 104 cells/2 mL/9.5 cm2 well for 48 h. After incubation, the medium was removed and the cells were incubated with 1.1 mL of MTT solution (0.1 mL of 5 mg/mL MTT in 1 mL of medium) for 4 h. The product was eluted from the cells by the addition of 20% sodium dodecyl sulfate (SDS)/0.01 M HCl, after which absorbance was measured at 595 nm using an SH-1000Lab microplate reader (Corona Electric Co. Ltd, Ibaraki, Japan). Cell viability was calculated according to the following equation: cell viability (%) = (absorbance of experiment group/absorbance of control group) × 100.
Determination of DNA fragmentation in Caco-2 cells
DNA fragmentation in the nuclei of the Caco-2 cells, which is an indicator of apoptosis, was detected using In Situ Cell Death Detection Kit, fluorescein, and a confocal laser scanning microscope (LSM-510; Carl Zeiss Co., Ltd., Tokyo, Japan) at excitation and emission wavelengths of 495 and 530 nm, respectively [17]. Briefly, the cells were incubated at a density of 3.0 × 105 cells/0.8 cm2 on a Nunc™ Lab-Tek™ Chamber Slide (Thermo Fisher Scientific K.K., Tokyo, Japan) with the test reagents for 48 h. Blue coloring indicated cell nuclei stained by DAPI, whereas green coloring indicated TUNEL-positive nuclei. Clear light blue coloring (a mixture of blue and green) showed DNA fragmentation in nuclei.
Cell cycle analysis
Cell cycle analysis was performed by flow cytometry as reported previously [16,18]. Briefly, Caco-2 cells were incubated at a density of 1.0 × 106 cells/28 cm2 dish with the test reagents for 48 h and then collected by centrifugation (4°C, 200 × g, 5 min). The pellet obtained was fixed with 70% ethanol cooled at -20°C on ice for 30 min. Following fixation, the cells were incubated with 100 μg/mL RNase A at 37°C for 30 min. The cells were treated with PI (50 μg/mL) on ice in the dark for 30 min. The samples were filtered through a nylon mesh (35 µm) and examined using a FACS Aria III flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Measurement of p21 protein expression
The cells were treated with NOR 5 for 48 h, collected by centrifugation, and lysed. Cell lysates were subjected to SDS-polyacrylamide gel electrophoresis using a 7.5% polyacrylamide gel. Proteins were transferred onto polyvinylidene difluoride membranes by electroblotting, after which the membranes were incubated overnight in Tris buffered saline-Tween® 20 containing 3% skim milk and the respective primary antibody against p21 or β-actin. The membranes were then incubated with secondary antibody for 1 h, followed by chemiluminescent detection using ECL™ Prime Western Blotting Detection Reagent (GE Healthcare Japan Corporation, Tokyo, Japan).
Statistical analysis
Results have been presented as mean ± standard error of the mean (SEM). Data was compared between two groups using Student’s t-test. Differences in data among multiple groups were assessed by one-way analysis of variance followed by Scheffe’s multiple range test. Differences were considered statistically significant at P values < 0.05.
Results
NOR5 spontaneously released NO at a steady rate in a neutral aqueous solution (half-life, about 20 h; 0.5 mM NOR5 in 0.1 M PBS, pH 7.4 at 37°C) [13]. SIN-1 spontaneously decomposes in the presence of molecular oxygen to generate NO and O2 · -, which react with each other to form ONOO- (rate constant k: 3.7 × 10-7 M-1s-1). Therefore, SIN-1 is a useful compound from which ONOO- can be efficiently generated [14]. Figure 1 shows that NOR5 and SIN-1 independently suppressed the growth of the Caco-2 cells in a dose-dependent manner after the cells were incubated with the respective compounds at concentrations of 0.1 to 0.5 mM for 48 h. At a concentration of 0.1 mM, NOR5 significantly inhibited cell growth, whereas SIN-1 did not elicit the same effect. Figure 2 shows the combined effects of SOD and SIN-1 on Caco-2 cell growth. SOD (0.5 and 5 U/mL) significantly augmented the inhibition of Caco-2 cell growth by 0.25 mM SIN-1; however, this was not the case for boiled SOD (B-SOD) even at a concentration of 5 U/mL. SOD (EC 1.15.1.1) is an enzyme that catalyzes the dismutation of O2 · - into hydrogen peroxide. It is believed that SOD diminishes O2 · - generation from SIN-1, which results in raised NO levels at the expense of ONOO- generation. Furthermore, as shown in Table 1, ONOO- did not have any significant effect on the Caco-2 cells at concentrations of 0.25 mM or less. These results indicate that NO, rather than ONOO-, potently suppresses the proliferation of Caco-2 cells.
Figure 1.
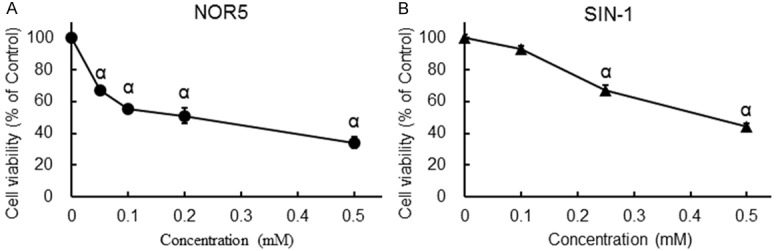
Effects of NOR5 (A) and SIN-1 (B) on the proliferation of Caco-2 cells. Caco-2 cells were treated with NOR5 or SIN-1 for 48 h. Cell proliferation was evaluated in an MTT assay. Data are expressed as mean ± SEM (n = 4-5). αIndicates P < 0.01, with data being significantly different from the corresponding value obtained in the absence of NOR5 or SIN-1.
Figure 2.
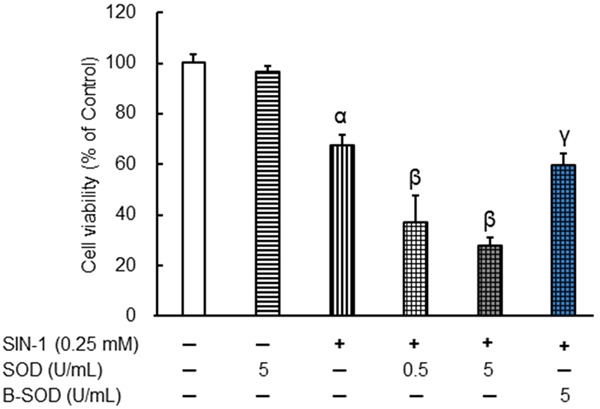
Combined effects of SOD and SIN-1 on the proliferation of Caco-2 cells. Caco-2 cells were treated with SOD and SIN-1 for 48 h. Cell proliferation was then investigated in an MTT assay. B-SODSOD (5 U/mL) was boiled at 100°C for 10 min. Data are expressed as mean ± SEM (n = 4-8). αIndicates P < 0.01, with data being significantly different from the corresponding value obtained in the absence of SOD or SIN-1. βIndicates P < 0.01, with data being significantly different from the corresponding value obtained in the presence of 0.25 mM SIN-1 alone. γIndicates P < 0.01, with data being significantly different from the corresponding value obtained in the presence of 0.25 mM SIN-1 plus 5 U/mL SOD.
Table 1.
Effects of ONOO- on the proliferation of Caco-2 cells
| Treatment | Concentration (mM) | Cell viability (% of control) |
|---|---|---|
| Control | 100.0 ± 10.2 | |
| ONOO- | 0.05 | 82.0 ± 3.8 |
| 0.1 | 91.2 ± 4.9 | |
| 0.25 | 81.8 ± 1.6 | |
| 0.5 | 59.4 ± 5.8α |
Caco-2 cells were treated with ONOO- for 48 h. Cell proliferation was evaluated by the MTT assay. Data are expressed as mean ± SEM (n = 4).
Indicates P < 0.05 when data is compared to that for the control cells.
In order to investigate whether NOR5-induced inhibition of Caco-2 cell proliferation is partially due to apoptosis, apoptotic cells were visualized in the TUNEL assay using a confocal laser scanning microscope (Figure 3). Blue coloring indicated nuclei stained by DAPI, whereas TUNEL-positive nuclei were green in color. Clear light blue coloring (a mixture of blue and green) was indicative of DNA fragmentation in the nuclei of apoptotic cells. Actinomycin D was used as the positive control in the study at a concentration of 0.05 mM. It caused DNA fragmentation in the nuclei, which is a hallmark of apoptotic cells [19]; however, NOR5 did not produce this effect in the apoptotic cells when it was used at 0.1 mM.
Figure 3.
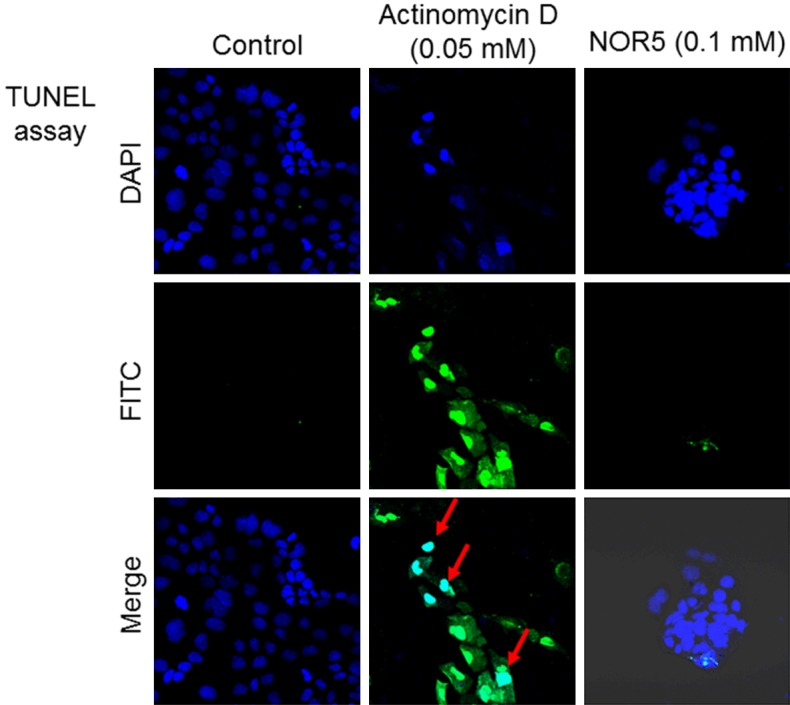
Fluorescence image of DNA fragmentation in Caco-2 cells treated with NOR5. Caco-2 cells were incubated with or without NOR5 for 48 h at 37°C, after which DNA fragmentation was monitored. The blue and green colors indicate DAPI-stained and TUNEL-positive nuclei, respectively. The clear light blue color (mixture of blue and green colors) shows DNA fragmentation in the nuclei. Data was collected from at least 10 random sections per sample. Each data is representative of the results from four experiments.
We also examined cell distribution in various cell cycle phases by flow cytometry to determine whether NOR5-induced cell growth inhibition involves cell cycle changes (Figure 4). Changes in the expression of p21, a cyclin-dependent kinase inhibitor that inhibits cell cycle arrest, were also measured [20] (Figure 5). The results showed cell accumulation in the S phase after the Caco-2 cells were treated with NOR5 (0.1 mM) for 48 h. This was associated with a decrease in the population of cells in the G0/G1 phase. NOR5 (0.1 mM) also significantly increased the expression level of p21 protein (Figure 5). This indicates that NOR5-induced inhibition of Caco-2 cell proliferation involves cell cycle arrest. These findings show that NOR5 induces S-G2/M phase arrest, but not apoptosis, in Caco-2 cells.
Figure 4.
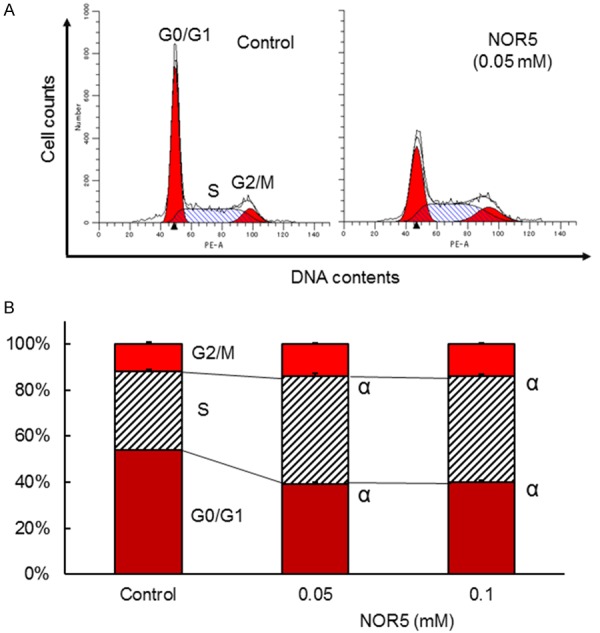
Effect of NOR5 on the distribution of Caco-2 cells in different phases of the cell cycle. Caco-2 cells were treated with NOR5 (0.05 or 0.1 mM) for 48 h. A. Representative flow cytometry charts. B. Effects of NOR5 (0.05 and 0.1 mM) on the percentages of Caco-2 cells in the G0/G1, S, and G2/M phases. Data are expressed as mean ± SEM (n = 4). αIndicates P < 0.01, with data being significantly different from the corresponding value obtained for the cells that were not exposed to NOR5.
Figure 5.
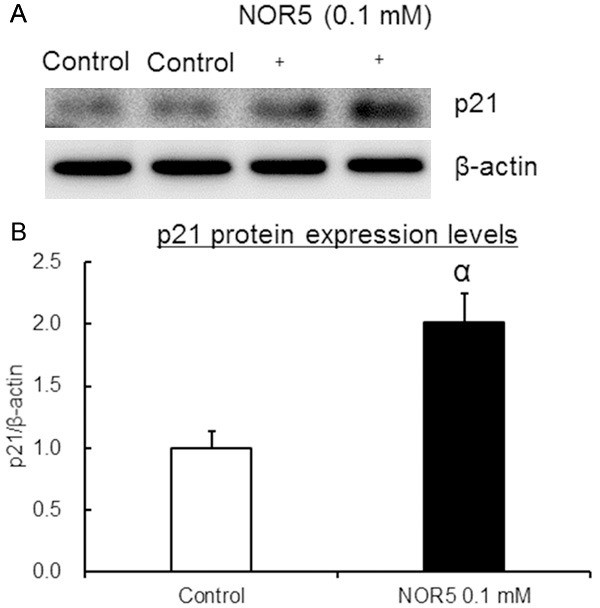
Changes in p21 protein expression in Caco-2 cells treated with NOR5. Caco-2 cells were treated with 0.1 mM NOR5 for 48 h. A. Representative detection bands for p21 and β-actin proteins obtained by western blotting. B. Calculated p21/β-actin ratio. Data are expressed as mean ± SEM (n = 8). αIndicates P < 0.01 when data is compared to that for the control cells.
Discussion
NO is involved in diverse processes in numerous physiological and pathophysiological conditions. Previous studies have suggested that NO has a negative effect on the regulation of tumor cell behavior. Additionally, it has an antineoplastic effect in vivo and in vitro [4-11]. However, little information is available concerning the effects of ONOO- in addition to NO on cancer cell lines. In the present study, we investigated the possible roles of NO and ONOO- on the proliferation of the colorectal cancer cell line Caco-2 using NOR5 and SIN-1 as NO and ONOO- donors, respectively.
The typical characteristics of tumor cell proliferation are uncontrollable cell division and excessive cell growth. Therefore, inhibiting tumor cell proliferation is an important aspect of controlling tumorigenesis. We found that NOR5 and SIN-1 decreased the viability of the Caco-2 cells; however, NOR5 had a relatively stronger effect (Figure 1). Experiments on the combined effects of SIN-1 and SOD revealed that NO plays a major role in SIN-1-induced inhibition of Caco-2 cell proliferation (Figure 2 and Table 1).
Apoptosis, which is programmed cell death, plays a major role during developmental processes. However, it is circumvented during malignant transformation and tumor progression [21,22]. In eukaryotic cells, cell cycle checkpoints are regulatory pathways that control the order and timing of cell cycle transitions and ensure that critical events, such as DNA replication and chromosome segregation, are completed [23]. Thus, the induction of cell cycle arrest, in addition to apoptosis, appears to be a common mechanism underlying the cytotoxic effects of anticancer agents. In the present study, the TUNEL assay, cell cycle analysis, and p21 protein analysis (Figures 3, 4 and 5) indicated that NO induced S-G2/M phase arrest, but not apoptosis, in the Caco-2 cells. This confirms that NO inhibits DNA synthesis and induces a block at the S-G2/M boundary.
In conclusion, the present findings indicate that NO, rather than ONOO-, effectively suppresses proliferation and induces cell cycle arrest in Caco-2 cells. However, the detailed mechanisms underlying these effects of NO are unclear. Therefore, further investigations are required to identify the cellular targets of NO and the precise molecular mechanisms underlying the effects of NO in Caco-2 cells. These will provide data on the potential use of NO in preventing and treating colorectal cancer.
Disclosure of conflict of interest
None.
References
- 1.Stelzner F. Spontaneous change of malignancy of solid malignant tumors: statistical investigations of colorectal and pancreatic carcinoma. Chirurg. 2012;83:726–731. doi: 10.1007/s00104-011-2236-z. [DOI] [PubMed] [Google Scholar]
- 2.Link A, Balaguer F, Shen Y, Lozano JJ, Leung HC, Boland CR, Goel A. Curcumin modulates DNA methylation in colorectal cancer cells. PLoS One. 2013;8:e57709. doi: 10.1371/journal.pone.0057709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huerta S, Chilka S, Bonavida B. Nitric oxide donors: novel cancer therapeutics. Int J Oncol. 2008;33:909–927. [PubMed] [Google Scholar]
- 4.Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995;72:41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Ose SM, Klein S, Shields PG, Billiar TR, Harris CC. Frequent nitric oxide synthase-2 expression in human colon adenomas: Implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]
- 6.Ekmekcioglu S, Ellerhorst J, Smid CM, Prieto VG, Munsell M, Buzaid AC, Grimm EA. Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival. Clin Cancer Res. 2000;6:4768–4775. [PubMed] [Google Scholar]
- 7.Masri FA, Comhair SA, Koeck T, Xu W, Janocha A, Ghosh S, Dweik RA, Golish J, Kinter M, Stuehr DJ, Erzurum SC, Aulak KS. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med. 2005;172:597–605. doi: 10.1164/rccm.200411-1523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L, Wang Y, Tian DA, Yang J, Yang YZ. Decreased levels of nitric oxide production and nitric oxide synthase-2 expression are associated with the development and metastasis of hepatocellular carcinoma. Mol Med Rep. 2012;6:1261–1266. doi: 10.3892/mmr.2012.1096. [DOI] [PubMed] [Google Scholar]
- 9.Hussain SP, Trivers GE, Hofseth LJ, He P, Shaikh I, Mechanic LE, Doja S, Jiang W, Subleski J, Shorts L, Haines D, Laubach VE, Wiltrout RH, Djurickovic D, Harris CC. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res. 2004;64:6849–6853. doi: 10.1158/0008-5472.CAN-04-2201. [DOI] [PubMed] [Google Scholar]
- 10.Chao JI, Kuo PC, Hsu TS. Down-regulation of survivin in nitric oxide-induced cell growth inhibition and apoptosis of the human lung carcinoma cells. J Biol Chem. 2004;279:20267–20276. doi: 10.1074/jbc.M312381200. [DOI] [PubMed] [Google Scholar]
- 11.Sang J, Chen Y, Tao Y. Nitric oxide inhibits gastric cancer cell growth through the modulation of the Akt pathway. Mol Med Rep. 2011;4:1163–1167. doi: 10.3892/mmr.2011.535. [DOI] [PubMed] [Google Scholar]
- 12.Huie RE, Padmaja S. The reaction rate of nitric oxide with superoxide. Free Rad Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 13.Hirasawa Y, Sugimoto T, Fukuyama S, Kato Y, Takamatu H, Ohno M, Nishino S, Kato M, Maeda K, Seki J, Kita Y. Antianginal effects of FR144420, a novel slow nitric oxide-releasing agent. Eur J Pharmacol. 1996;303:55–59. doi: 10.1016/0014-2999(96)00105-7. [DOI] [PubMed] [Google Scholar]
- 14.Kankaanranta H, Knowles RG, Vuorinen P, Kosonen O, Holm P, Moilanen E. 3-Morpholino-sydnonimine-induced suppression of human neutrophil degranulation is not mediated by cyclic GMP, nitric oxide or peroxynitrite: inhibition of the increase in intracellular free calcium concentration by N-morpholino-iminoacetonitrile, a metabolite of 3-morpholino-sydnonimine. Mol Pharmacol. 1997;51:882–888. doi: 10.1124/mol.51.5.882. [DOI] [PubMed] [Google Scholar]
- 15.Sakuma S, Sumi H, Kohda T, Arakawa Y, Fujimoto Y. Effect of lipid peroxidation-derived products on the growth of human colorectal cancer cell line HT-29. J Clin Biochem Nutr. 2009;45:171–177. doi: 10.3164/jcbn.09-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohda T, Sakuma S, Abe M, Fujimoto Y. Monochloramine suppresses the proliferation of colorectal cancer cell line Caco-2 by both apoptosis and G2/M cell cycle arrest. Cell Biochem Funct. 2013;32:188–193. doi: 10.1002/cbf.2992. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto Y, Morinaga K, Abe M, Kitamura T, Sakuma S. Selenite induces oxidative DNA damage in primary rat hepatocyte cultures. Toxicol Lett. 2009;191:341–346. doi: 10.1016/j.toxlet.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Sakuma S, Abe M, Kohda T, Fujimoto Y. Hydrogen peroxide generated by xanthine/xanthine oxidase system represses the proliferation of colorectal cancer cell line Caco-2. J Clin Biochem Nutr. 2015;56:15–19. doi: 10.3164/jcbn.14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XF, Xiang L, Zhou Q, Carralot JP, Prunotto M, Niederfellner G, Pastan I. Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis. Proc Natl Acad Sci U S A. 2016;113:10666–10671. doi: 10.1073/pnas.1611481113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1 phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 22.Onishi Y, Ueha T, Kawamoto T, Hara H, Toda M, Harada R, Minoda M, Kurosaka M, Akisue T. Regulation of mitochondrial proliferation by PGC-1α induces cellular apoptosis in musculoskeletal malignancies. Sci Rep. 2014;4:3916. doi: 10.1038/srep03916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]


