Abstract
Background
Dogs with immune‐mediated disease often receive glucocorticoids with clopidogrel, but ulcerogenic effects of current protocols are unknown.
Hypothesis/Objectives
To compare gastrointestinal endoscopic findings among dogs administered clopidogrel, prednisone, and combination treatment.
Animals
Twenty‐four healthy research dogs.
Methods
Double‐blinded, placebo‐controlled randomized trial. Dogs received placebo, clopidogrel (2–3 mg/kg q24h), prednisone (2 mg/kg q24h), or prednisone with clopidogrel PO for 28 days. Attitude, food intake, vomiting, and fecal score were determined daily. Clinicopathologic testing was performed at baseline and on day 28. Gastrointestinal hemorrhages, erosions, and ulcers were numerated by 2 blinded investigators for endoscopies performed on days 0, 14, and 28, and endoscopic mucosal lesion scores were calculated. Results were compared using mixed model, split‐plot repeated measures ANOVAs and generalized estimating equation proportional odds models as appropriate. P < .05 was considered significant.
Results
Clinical signs of gastrointestinal bleeding were not noted. Endoscopic mucosal lesion scores differed significantly by group (F[3, 20] = 12.8, P < .001) and time (F[2, 40] = 8.3, P < .001). Posthoc analysis revealed higher lesion scores in the prednisone‐receiving groups (P ≤ .006 for each) and on day 14 (P ≤ .007 for each). Ulcers were identified in 4 dogs administered prednisone and 3 dogs administered prednisone/clopidogrel. Odds of having endoscopic mucosal lesion scores ≥4 were 7‐times higher for dogs in prednisone (95%CI 1.1, 43.0; P = .037) and prednisone‐clopidogrel (95%CI 1.1, 43.4; P = .037) groups than those in the placebo group.
Conclusions and Clinical Importance
Gastrointestinal bleeding and ulceration occur commonly in healthy dogs administered prednisone or prednisone/clopidogrel treatment, but not clopidogrel monotherapy. Though lesions are severe in many cases, they are not accompanied by clinical signs.
Keywords: antiplatelet, corticosteroid, gastrointestinal bleeding, glucocorticoid, thromboprophylaxis, ulcer
Abbreviations
- IMHA
immune‐mediated hemolytic anemia
- MCS
muscle condition score
1. INTRODUCTION
Thromboprophylaxis is standard of care for dogs with immune‐mediated hemolytic anemia (IMHA), because of a high incidence of thromboembolic disease in treated dogs.1, 2 Thromboprophylactic agents used in dogs include antiplatelet drugs and anticoagulant therapies.3, 4, 5 Advantages of antiplatelet drugs include widespread availability, limited cost, and the ability to achieve therapeutic effects with once daily oral administration—all of which could decrease the risk of owner noncompliance and degradation of the human‐animal bond during extended treatment. Antiplatelet effects of aspirin treatment are inconsistent at dosages ≤2 mg/kg q24h,6 and administration of aspirin at 2 mg/kg q24h with prednisone is associated with markedly higher odds of gastrointestinal bleeding compared to administration of prednisone or a placebo alone.7 Clopidogrel does not inhibit prostaglandin production but can induce gastritis, gastrointestinal hemorrhage, and ulcers in people.8, 9 Some studies8, 10 report an equivalent or increased amount of gastrointestinal bleeding in people receiving clopidogrel compared with aspirin, whereas others report a decreased amount of gastrointestinal disease.11, 12
The purpose of this randomized‐controlled double‐blinded study was to characterize clinical, clinicopathologic, and endoscopic changes in healthy dogs administered sustained placebo, clopidogrel (2–3 mg/kg q24h), prednisone (2 mg/kg q24h), or clopidogrel with prednisone. Our hypothesis was that sustained administration of clopidogrel, used singly or in combination with prednisone (2 mg/kg q24h), would induce gastrointestinal bleeding, and mucosal lesions would be more numerous for dogs that receive combination treatment than for dogs receiving prednisone alone.
2. MATERIALS AND METHODS
2.1. Study population
Sample size calculation was performed based on preliminary data demonstrating a 10 point increase in endoscopic mucosal lesion scores for dogs administered 2 mg/kg q24h prednisone for 28 days.13 Based on those results and assuming a SD of 4.9, enrollment of 6 dogs per group was calculated to have 85% power to find endoscopic scores of 5 and 15 significantly different with a significance of .05. Thus, 24 healthy dogs from the College's teaching and research colony were enrolled in the study. To avoid confounding from potential age‐related differences in gastrointestinal bleeding, healthy dogs were stratified by age before randomization to 1 of 4 groups using a random number sequence generator (https://www.random.org, accessed January 6, 2017). Because gastric biopsies taken from all dogs at the conclusion of baseline were positive for urease‐producing bacteria, stratification based on urease status was not necessary.
The study protocol was approved by the Institutional Animal Care and Use Committee at the University of Tennessee, College of Veterinary Medicine (protocol number 2335) in compliance with “The Guide for the Care and Use of Laboratory Animals” in laboratory animal facilities that are AAALAC certified and exceed NIH standards of care.
2.2. Treatment groups
Dogs were randomized to 1 of 4 treatment groups: (1) placebo, (2) clopidogrel (2‐3 mg/kg q24h) plus placebo, (3) prednisone (2 mg/kg q24h) plus placebo, and (4) clopidogrel plus prednisone. Dogs in the placebo group received 2 placebo capsules once daily, while dogs in groups 2 and 3 were administered 1 placebo capsule. Commercially available clopidogrel (Mylan Pharmaceuticals, Morgantown, West Virginia) and prednisone tablets (West‐Ward Pharmaceuticals Corp., Eatentown, New Jersey) were used. Lactose‐containing gelatin capsules (LetCo Medical, Decatur, Alabama) were assembled by the College's pharmacy for use as placebos. All treatments were administered in small meatballs once daily before feeding by an individual blinded to the individual treatments and groups.
2.3. Study periods
The study was broken into acclimation (days −13 to −7), baseline (days −6 to 0), and treatment (days 1–28) periods. During acclimation, dogs were administered fenbendazole (50 mg/kg q24h, PO, days −13 to −9) and ivermectin (200 μg/kg SQ once, day −13). Dogs also received imidacloprid and moxidectin (Advantage Multi for dogs, Bayer HealthCare, LLC, Shawnee Mission, Kansas), dosed per manufacturer's instructions, as part of routine colony prophylaxis.
All dogs received ad libitum water and were fed a commercial kibble once daily in quantities sufficient to maintain ideal body condition. An observer not associated with the study performed twice daily observations throughout the study (days −13 to 28). The observer was blinded to the treatment groups, medications, and all study‐related findings. Attitude was characterized as normal or abnormal. Food intake was recorded to the nearest quartile consumed (0%, 25%, 50%, or 100%). The presence of vomiting, melena, or hematochezia was recorded, and feces were scored using a standard scale.14
2.4. Diagnostic testing
Dogs were confirmed to have negative fecal direct smears and fecal flotations (sugar and zinc sulfate) during the acclimation period (days −13 to −11). Clinicopathologic testing was performed at the conclusion of baseline and treatment by a commercial diagnostic laboratory (Antech Diagnostics, Fountain Valley, California). Testing included CBCs, serum biochemical profiles with lipase activity (PrecisionPSL™, Antech Diagnostics), urinalyses, and urine protein : creatinine ratios.
Dogs were anesthetized on days 0, 14, and 28 and positioned in left lateral recumbency for performance of gastrointestinal endoscopy. Dogs were premedicated with acepromazine (.02 mg/kg SQ) and butorphanol (.4 mg/kg SQ), had IV catheters placed, and then were induced using propofol (3–6 mg/kg IV to effect). After induction, dogs were intubated and isoflurane administered in oxygen was used to maintain general anesthesia. Crystalloid fluids were administered at a rate of 10 mL/kg/h IV. Heart rate, respiratory rate, pulse oximetry, end‐tidal carbon dioxide, systolic blood pressure (indirect Doppler flow technique), and temperature were monitored and recorded every 5 minutes throughout anesthesia.
A single individual (Jacqueline C. Whittemore), blinded to each dog's treatment group, performed endoscopic exploration using a standardized technique to avoid creation of iatrogenic lesions.15, 16 Briefly, continuous visual guidance was used to evaluate the upper esophageal sphincter through the duodenum. Within the stomach, the gastric body, antrum/pylorus, angularis incisura, and cardia were individually interrogated before pyloric intubation for duodenal evaluation. Endoscopic explorations were recorded using a digital capture system, with still images collected of the lower esophageal sphincter, gastric body, angularis incisura, antrum and pylorus, cardia, and duodenum. Supplementary images of focal abnormalities also were collected. Endoscopic evaluations were anonymized after data collection to blind investigators to dog, treatment group, and timepoint. Gastric biopsies were taken on day 0 and incubated in urease media to assess for the presence of potential Helicobacter spp.
2.5. Endoscopic scoring system
Two investigators (John Thomason, Jacqueline C. Whittemore) independently evaluated each endoscopic study and numerated mucosal lesions as per previous reports.7, 16, 17 Briefly, hemorrhages were defined as reddened areas with intact mucosa. Pinhead‐sized discontinuations in the mucosa were classified as punctate erosions, while larger breaches and those with detectable depth were classified as invasive erosions. Lesions with wide defects and craterous centers were classified as ulcers. Lesions were numerated by gastrointestinal region (esophagus, gastric body, pyloric antrum, angularis incisura, cardia, and duodenum). If >25 lesions were identified in a region, lesions were recorded as 26‐50, 51‐100, 100‐200, or >200 to avoid erroneous quantitation. For statistical analysis, scores >25 but ≤200 were entered according to the midpoint of the categorical range. Scores >200 were entered as 201 for statistical analysis.
Anonymized endoscopic evaluations were independently scored approximately 10 months after completion of data collection. After datasheets from the individual investigators were merged, the combined database was reviewed for areas of discordance. Each investigator independently reevaluated studies with discordant scores and corrected any self‐identified errors in scoring or data entry. The mean of lesion counts numerated by the 2 investigators was used for analyses. Total gastric mucosal endoscopic lesion scores were calculated using the Forsyth scoring system.17
2.6. Statistical and data analysis
Descriptive statistics were generated for relevant clinical, clinicopathologic, and endoscopic parameters. Results then were analyzed for normality using the Shapiro‐Wilk test and for the presence of outliers using box‐and‐whisker plots. For each study period, mean food intake, days of vomiting, and mean fecal score were determined. Clinicopathologic parameters evaluated were hematocrit and platelet count; albumin and BUN concentrations; BUN : creatinine and urine protein : creatinine ratios; activities of ALP, GGT, amylase, and lipase; and urine specific gravity.
Mixed model, split‐plot repeated measures ANOVAs that included fixed effects of treatment, time, and treatment‐by‐time interaction were used to compare clinical, clinicopathologic, and endoscopic data between treatment groups. The repeated measure of time was accounted for in a repeated statement. Dog nested within treatment group was included as a random effect. Fisher's least significant difference was performed for posthoc analyses. The Shapiro‐Wilk test of normality and QQ plots of the residuals were evaluated for each marker to confirm the assumption of normally distributed residuals had been met. Model assumptions regarding equality of variances were evaluated with Levene's test for equality of variances. Differences in marginal means were determined for markers with significant main effect or interaction terms. Nonnormally distributed data were logarithmically or rank‐transformed, as necessary, to meet underlying statistical assumptions. If logarithmic transformation was required, .05 was added to all values. The relative odds of having a total endoscopic mucosal lesion score ≥4 (e.g., >25 hemorrhages or punctate erosions, ≥1 invasive erosion, and/or ≥1 ulcer) was determined using a repeated measures generalized estimating equation proportional odds model with a binomial distribution and a logit link function. After data analysis revealed a lack of association between hemorrhages and group or timepoint (see Section 3), hemorrhages were excluded from lesion scores in order to increase the precision of the results. Commercial statistical software packages (MedCalc 15.8 MedCalc Software, Ostend, Belgium; SAS 9.4 release TS1M5, SAS Institute Inc., Cary, North Carolina) were used for all analyses. P < .05 was considered significant.
3. RESULTS
3.1. Study population
Baseline demographics for the 4 treatment groups are summarized in Table 1. There was no difference in body condition score among groups over time. All but 2 dogs had a muscle condition score (MCS) of 3 at all timepoints. One dog each in placebo and prednisone groups had a MCS of 3 at baseline but 2 on day 28. Attitude was categorized as normal on all days for all dogs. Food intake increased significantly over time (P = .01) but did not differ among groups. No dog developed vomiting, diarrhea, melena, or hematochezia during the study. Fecal score did not differ among groups over time.
Table 1.
Baseline demographics of dogs stratified by age then randomized to receive placebo, clopidogrel with placebo, prednisone with placebo, or combination prednisone and clopidogrel for 28 days
| Placebo | Clopidogrel | Prednisone | Prednisone and clopidogrel | |
|---|---|---|---|---|
| Age (years) | 3.5 (2‐7) | 3.5 (2‐6) | 3.0 (2‐6) | 3.5 (2‐7) |
| Sex | ||||
|
2 | 3 | 3 | 1 |
|
2 | 1 | 2 | 3 |
|
2 | 2 | 1 | 2 |
| Breed | 4 beagles, 2 hounds | 4 beagles, 2 hounds | 3 beagles, 3 hounds | 4 beagles, 2 hounds |
| Weight (kg) | 16.2 ± 9.0 | 14.5 ± 6.5 | 16.3 ± 7.5 | 16.5 ± 5.4 |
| Body condition score | 5 (5‐7) | 6 (8‐8) | 6 (5‐7) | 5 (5‐8) |
| Muscle condition score | 3 (2‐3) | 3 (—) | 3 (2‐3) | 3 (—) |
Note: Age, body condition score, and muscle condition score are presented as median (range). Weight is presented as mean ± SD. — = not applicable.
3.2. Clinicopathologic data
Selected baseline and posttreatment clinicopathologic results are presented in Table 2. Hematocrit, MCV, MCHC, and platelet count were within the reference intervals (RI) at all timepoints for all but 2 dogs. One dog in the placebo group had a platelet count just below the reference interval (167 000 platelets/μL; RI 170 000‐400 000 platelets/μL) at baseline, but platelet clumping was noted on manual slide evaluation. Repeat automated and manual evaluation performed the next day was within normal limits. One dog in the prednisone/clopidogrel group had mild thrombocytopenia (136 000 platelets/μL) on day 28. This dog had >200 hemorrhages and >100 erosions noted in the stomach on the same day.
Table 2.
Selected clinicopathologic results for 24 healthy research dogs administered placebo, clopidogrel with placebo, prednisone with placebo, or combination prednisone and clopidogrel for 28 days
| RI | Placebo | Clopidogrel | Prednisone | Prednisone and clopidogrel | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 28 | Baseline | Day 28 | Baseline | Day 28 | Baseline | Day 28 | ||
| Amylase (IU/L) | 290‐1125 | 406α (336‐899) | 452β (154‐882) | 433α (381‐745) | 432β (337‐599) | 505α (337‐787) | 283β (220‐387) | 469α (379‐791) | 297β (207‐542) |
| Albumin (g/dL) | 2.7‐4.4 | 3.6B (3.4‐3.9) | 3.6B (3.2‐3.7) | 3.6B (3.2‐3.7) | 3.6B (3.2‐4.0) | 3.7AB (3.1‐3.8) | 3.9AB (3.3‐4.5) | 3.8A (3.4‐4.1) | 4.0A (3.7‐4.3) |
| ALP (IU/L) | 5‐131 | 27CD (21‐63) | 25CD (19‐49) | 34BC (30‐40) | 46AB (24‐194) | 31CD (22‐38) | 69A (49‐599) | 26D (11‐32) | 92A (46‐126) |
| GGT (IU/L) | 1‐12 | 5B,β (3‐6) | 6B,α (5‐7) | 6B,β (4‐6) | 5B,α (3‐8) | 6A,β (5‐11) | 9A,α (6‐35) | 7A,β (4‐9) | 10A,α (8‐10) |
| BUN (mg/dL) | 6‐31 | 17β (11‐18) | 22α (11‐24) | 15β (8‐24) | 18α (12‐31) | 14β (11‐41) | 23α (15‐44) | 14β (10‐43) | 17α (12‐38) |
| Cr (mg/dL) | .5‐1.6 | .6 (.6‐.8) | .7 (.6‐.9) | .7 (.6‐.9) | .8 (.7‐1.4) | .7 (.1‐2.0) | .6 (.5‐1.5) | .8 (.5‐2.1) | .6 (.5‐.9) |
| BUN : creatinine ratio | 4‐27 | 25BCD (14‐28) | 34AB (12‐40) | 22CD (11‐30) | 21BCD (17‐31) | 20D (16‐22) | 35A (25‐43) | 20D (14‐20) | 27ABC (20‐42) |
| Hematocrit (%) | 36‐60 | 51 (44‐60) | 52 (46‐56) | 49 (45‐55) | 49 (45‐58) | 52 (46‐55) | 51 (42‐53) | 50 (48‐54) | 50 (47‐55) |
| Lipase (U/L) | 24‐140 | 54BC (18‐80) | 51BC (22‐84) | 40BC (25‐151) | 44C (27‐70) | 55BC (36‐106) | 99A (34‐152) | 32C (17‐114) | 90AB (31‐124) |
| MCV (fL) | 58‐79 | 71 (70‐73) | 73 (69‐76) | 74 (73‐75) | 73 (71‐76) | 74 (69‐78) | 76 (69‐77) | 72 (69‐75) | 73 (70‐77) |
| MCHC (g/dL) | 30‐38 | 33 (31‐34) | 33 (32‐34) | 34 (31‐34) | 34 (34‐35) | 33 (31‐34) | 33 (32‐36) | 34 (32‐35) | 34 (32‐35) |
| Platelet count (×103/μL) | 170‐400 | 264 (167‐376) | 315 (199‐458) | 334 (202‐353) | 303 (185‐388) | 337 (239‐405) | 294 (249‐353) | 248 (194‐322) | 289 (136‐342) |
| Urine specific gravity | 1.015‐1.050 | 1.025 (1.018‐1.054) | 1.032 (1.004‐1.056) | 1.041 (1.003‐1.060) | 1.038 (1.003‐1.052) | 1.032 (1.024‐1.046) | 1.038 (1.021‐1.051) | 1.034 (1.015‐1.044) | 1.035 (1.013‐1.052) |
| Urine protein : creatinine ratio | <0.5 | 0.2β (.1‐1.0) | 0.2α (.1‐.5) | 0.1β (0‐.3) | 0.2α (0‐.4) | 0.1β (.1‐.2) | 0.2α (.1‐2.1) | 0.2β (0‐.4) | 0.9α (.1‐1.7) |
Note: Results are presented as median (range). RI = reference interval. Superscript letters highlight values with significant treatment‐by‐time, treatment, or time interactions. Values that do not share a common superscript letter differed significantly (P < .05) based on the main effect for treatment or the treatment‐by‐time interactions based on posthoc analysis. Values that do not share a superscript Greek letter differed significantly (P < .05) among time points independent of treatment based on posthoc analysis.
Biochemical analytes also were within reference intervals at both timepoints for the majority of dogs. Rank transformation was required before analysis of ALP and GGT activity, creatinine concentration, and urine protein : creatinine ratio.
Significant time effects and treatment‐by‐time interactions were noted for activities of ALP (P ≤ .001 for each) and lipase (P = .010 for each). Posthoc analysis confirmed these were because of significant increases over time in the prednisone‐receiving groups (P ≤ .008 for each). Similarly, significant treatment (P < .001) and time effects (P = .003) were identified for GGT activity because of increased activities in the prednisone‐receiving groups, although the treatment‐by‐time interactions were not significant. Amylase activity decreased significantly over time (P < .001). Although neither treatment nor treatment‐by‐time interactions were identified, visual review of the data suggested the overall decrease in amylase activity over time was because of decreased activities in the prednisone‐receiving groups.
The BUN : creatinine ratio increased over time in the prednisone‐receiving groups, resulting in significant treatment‐by‐time (P = .030) and time (P < .001) associations. Although BUN concentration also increased significantly over time (P < .001), neither treatment group nor treatment‐by‐time interactions were noted. Creatinine concentration did not differ significantly over time or among treatment groups. Albumin concentration differed significantly by treatment group (P = .027), with higher concentrations in dogs receiving prednisone/clopidogrel combination treatment when compared to clopidogrel alone (P = .007) or placebo (P = .015). Urine specific gravity did not differ among treatment groups or over time. Urine protein : creatinine ratios, however, increased significantly over time (P < .001), with increases limited to prednisone‐receiving groups, although there was no significant treatment group or treatment‐by‐time interaction.
3.3. Endoscopy
Total anesthetic time was ≤30 minutes for each procedure, with the majority of procedures completed in ≤15 minutes. No anesthetic complications were noted, and indirect systolic blood pressure was ≥80 mmHg at all‐time points.
Gastric biopsies taken at baseline from all dogs were positive for urease‐producing bacteria.
Median (range) numbers for individual mucosal lesions in the stomach are summarized in Table 3. Ulceration only was identified on 7 studies, precluded quantitative analysis. Logarithmic transformation was required to meet assumptions for mixed model analysis of mucosal hemorrhages, punctate erosions, and invasive erosions.
Table 3.
Median (range) for gastric mucosal lesions identified on endoscopy for 24 healthy research dogs administered placebo, clopidogrel with placebo, prednisone with placebo, or combination prednisone and clopidogrel for 28 days
| Baseline | Day 14 | Day 28 | |
|---|---|---|---|
| Placebo | |||
| Hemorrhages | 24 (2‐57) | 1 (0‐13) | 2 (0‐38) |
| Punctate erosions | 1 (0‐4)D | 1 (0‐69)BCD | 0 (0‐5)CD |
| Invasive erosions | 0 (0‐1)B,χ | 0 (0‐203)B,α | 0 (0‐3)B,β |
| Ulcers** | 0 (0‐0) | 0 (0‐0) | 0 (0‐0) |
| Clopidogrel | |||
| Hemorrhages | 0 (0‐1) | 1 (0‐6) | 0 (0‐1) |
| Punctate erosions | 0 (0‐9)D | 3 (1‐24)ABC | 0 (0‐14)D |
| Invasive erosions | 0 (0‐1)B,χ | 1 (0‐22)B,α | 0 (0‐0)B,β |
| Ulcers** | 0 (0‐0) | 0 (0‐0) | 0 (0‐0) |
| Prednisone | |||
| Hemorrhages | 1 (0‐63) | 0 (0‐62) | 0 (0‐39) |
| Punctate erosions | 0 (0‐7)D | 49 (7‐228)A | 32 (8‐308)A |
| Invasive erosions | 0 (0‐4)A,χ | 27 (0‐168)A,α | 18 (0‐279)A,β |
| Ulcers** | 0 (0‐0) | 0 (0‐2) | 0 (0‐2) |
| Prednisone & Clopidogrel | |||
| Hemorrhages | 0 (0‐34) | 0 (0‐12) | 4 (0‐70) |
| Punctate erosions | 1 (0‐9)CD | 30 (1‐236)A | 19 (90‐110)A |
| Invasive erosions | 0 (0‐9)A,χ | 24 (0‐227)A,α | 15 (0‐40)A,β |
| Ulcers** | 0 (0‐0) | 1 (0‐6) | 0 (0‐0) |
Note: Values that do not share a common superscript letter differed significantly (P < .05) based on treatment‐by‐time or treatment interactions based on posthoc analysis. Values that do not share a Greek letter differed significantly (P < .05) among time points based on posthoc analysis. **Statistical comparisons were not performed because of limited occurrences based on posthoc analysis.
Total mucosal hemorrhages did not differ significantly by treatment, time, or treatment‐by‐time. Significant treatment‐by‐time (P = .011), treatment (P = .005), and time (P < .001) interactions were identified for gastric punctate erosions. Posthoc analysis revealed that differences were attributable to higher scores during treatment for dogs administered prednisone (P = .001) and prednisone/clopidogrel (P = .010) when compared to placebo on day 14. On day 28, differences were attributable to higher scores during treatment for dogs administered prednisone and prednisone/clopidogrel groups when compared to both placebo and clopidogrel (P ≤ .018 for each). Total invasive erosions increased significantly among treatment groups (P = .014) and over time (P < .001), because of higher scores for dogs in the prednisone‐receiving groups during treatment (P ≤ .024 for each). Invasive erosions were most numerous on day 14, although scores on day 28 also were significantly higher than at baseline. Both punctuate and invasive erosions were often marked and multifocal (Figure 1), although primarily concentrated in the antrum. Ulceration was noted in 7 dogs over the course of the study (Table 3, Figure 2). Ulcers were generally limited in size and depth. Four dogs in the prednisone group developed 1‐2 ulcers (day 14, 2 dogs; day 28, 2 dogs). Additionally, 3 dogs in the prednisone/clopidogrel group had ulcers identified on day 14 (2, 4, and 6 ulcers, respectively). Residual gastric contents were noted in the majority of dogs with gastrointestinal bleeding. One duodenal hemorrhage was noted on 1 endoscopic study each for 2 dogs.
Figure 1.
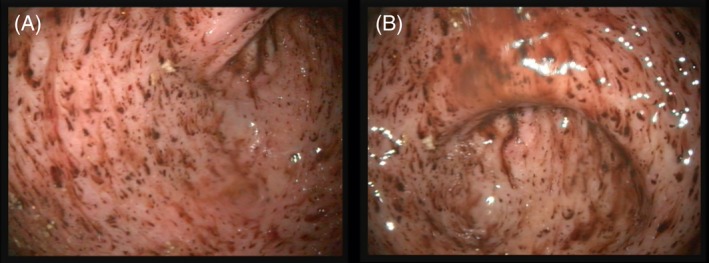
Diffuse punctate and invasive erosions spanning the body, incisura, and antrum of a healthy dog administered prednisone for 28 days
Figure 2.
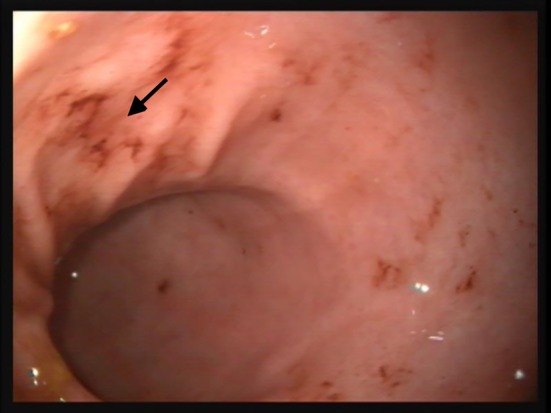
Multifocal erosions and ulceration (black arrow) in the antrum of a healthy dog administered prednisone and clopidogrel PO for 14 days
Total gastric endoscopic mucosal lesion scores are presented in Figure 3. Rank transformation of total gastric endoscopic mucosal lesion scores was necessary to meet model assumptions. Total gastric endoscopic mucosal lesion scores differed significantly by treatment group (F[3, 20] = 12.8, P < .001) and time (F[2, 40] = 8.3, P < .001), but not by group‐by‐time. Posthoc analysis revealed significantly higher lesion scores in the prednisone‐receiving groups (P ≤ .006 for each) and on day 14 (P ≤ .007 for each).
Figure 3.
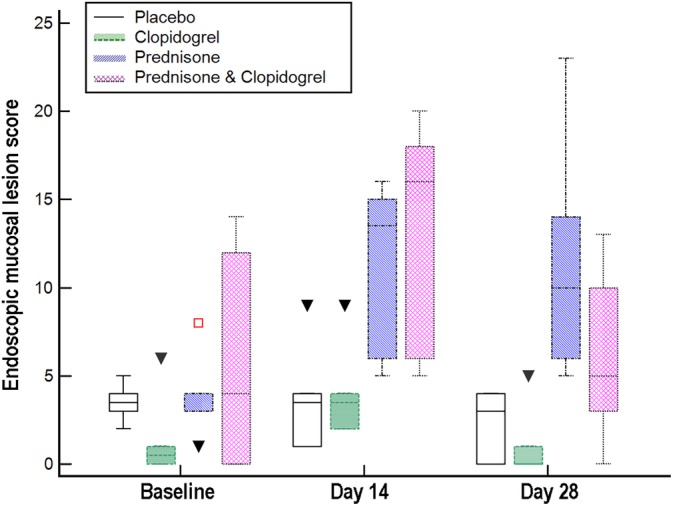
Box and whisker plots of total endoscopic mucosal lesion scores for 24 healthy dogs randomized to receive placebo, clopidogrel with placebo, prednisone with placebo, or prednisone and clopidogrel for 28 days. Open squares and closed triangles = outliers. Total gastric endoscopic mucosal lesion scores differed significantly by treatment group and time (P < .001 for each), but not by group‐by‐time. Posthoc analysis revealed significantly higher lesion scores in the prednisone‐receiving groups (P ≤ .006 for each) and on day 14 (P ≤ .007 for each)
Endoscopic mucosal lesion scores ≥4 were present on 33 studies, after hemorrhages were excluded from scoring, and differed significantly among treatment groups (P = .015) and over time (P = .018). Odds of having endoscopic mucosal lesion scores ≥4 were 7‐times higher for dogs in prednisone (95% CI 1.1, 43.0; P = .037) and prednisone‐clopidogrel (95% CI 1.1, 43.4; P = .037) groups than the placebo group. In contrast, dogs receiving clopidogrel were not more likely to have mucosal lesion scores ≥4 than dogs administered placebo.
4. DISCUSSION
Dogs administered prednisone alone or in combination with clopidogrel had significantly higher endoscopic mucosal lesion scores compared to other treatment groups and over time in this study. Punctate and invasive erosions differed significantly by treatment, time, and treatment‐by‐time (punctate erosions only), because of increased numbers of erosions in the prednisone‐receiving groups. Although erosions were most numerous on day 14, several dogs had new lesions identified on day 28. Ulceration was only identified in dogs administered prednisone (4) or combination prednisone/clopidogrel treatment (3). Lesions were predominantly located in the antrum, although diffuse disease was not uncommon. Increased gastrointestinal bleeding in dogs administered prednisone, with or without clopidogrel, resulted in 7 times higher odds of having an endoscopic mucosal lesion score ≥4 over time for these groups compared with placebo. Similar to results of prior studies of glucocorticoid‐ and aspirin‐induced bleeding,7, 16, 18, 19, 20 clinical signs were not identified. Notably, clopidogrel administration was not associated with the development of lesions or increased endoscopic lesion scores, and gastrointestinal bleeding did not significantly differ between dogs administered prednisone alone versus in combination with clopidogrel.
Sustained glucocorticoid excess causes gastrointestinal bleeding through inhibition of endogenous peroxidase, which causes free radical damage, decreased synthesis of prostaglandins, hyperacidity, and altered vascular permeability.21, 22, 23, 24, 25, 26, 27, 28, 29, 30 Consistent with prior reports,7, 19, 20, 31, 32 we identified a significant and marked increased in gastrointestinal bleeding in healthy dogs administered sustained glucocorticoid treatment. Results of this report conflict only with those of 1 study of sustained oral prednisone administration.16 The reason for disparity between that study and others, particularly this study and another 1 of similar design,7 is unknown. All 3 were randomized trials evaluating the gastrointestinal effects of prednisone, administered at the same dosage and for the same duration, with or without an antiplatelet medication in healthy research dogs positive on gastric biopsy for urease‐producing bacteria. Based on careful review of the methodology, the only identified difference was the use of younger study subjects (median age, 14 months; range, 12‐24 months) and compounded medications in the discordant study.16 Age was not identified as a covariate for bleeding in this or the other prior study,7 but a type 2 error cannot be ruled out given small sample sizes and the stratification of dogs by age before group randomization.
Clinicopathologic changes identified in this study were largely consistent those from 1 prior report.7 Specifically, prednisone administration significantly increased lipase, ALP, and GGT activity—resulting in significant treatment‐by‐time interactions (ALP and lipase) or treatment and time (GGT) interactions. Albumin concentrations were significantly higher for dogs in the prednisone‐receiving group, and urine protein : creatinine ratios increased significantly over time because of increases in the prednisone‐receiving groups. Consistent with 1 prior report,7 a significant treatment‐by‐time interaction was noted for BUN : creatinine ratios because of significantly increased ratios after treatment for dogs in the prednisone‐receiving groups. Some dogs in both the placebo and clopidogrel treatment groups had ratios above the reference interval, but results did not differ between baseline and treatment. Based on the latter finding, assessing for changes in the BUN : creatinine ratio after initiation of glucocorticoid treatment might provide better insight into the likelihood of gastrointestinal bleeding than evaluating an unpaired BUN : creatinine ratio.
Thromboprophylaxis has become standard of care for dogs with IMHA.1, 2, 33 To decrease the risk of thromboembolic disease, thromboprophylaxis should be started when IMHA is diagnosed and continued until dogs are weaned off of prednisone.1 Treatment options include anticoagulants, which prevent fibrin formation and cross‐linkage, and antiplatelet drugs, which decrease platelet activation.1 Because venous thrombus formation depends more on fibrin than platelet activation and venous thromboembolism predominates in dogs with IMHA, anticoagulants are recommended as first‐line treatment for dogs with IMHA.1, 34 Unfortunately, anticoagulant therapies are expensive, require therapeutic drug monitoring, and/or require parenteral administration. Antiplatelet drugs commonly are used because of their limited cost, ability to achieve therapeutic effects with once daily oral administration, and widespread availability.7
Gastrointestinal bleeding is 1 of the top complications of thromboprophylactic treatment in people, although the underlying etiology of bleeding differs among therapeutic options.10, 35, 36 Aspirin causes gastric ulceration because of nonselective COX inhibition, increased intestinal permeability, and decreased recovery of barrier function after ischemic insult.17, 18, 37, 38 In contrast, gastrointestinal bleeding in patients administered clopidogrel is believed to result from decreased platelet‐mediated angiogenesis at sites of preexisting damage and decreased release of platelet‐derived growth factors involved in gastrointestinal mucosal healing.39, 40 Some studies8, 10 report an equivalent or increased amount of gastrointestinal disease in people receiving clopidogrel compared with aspirin, while others report a decreased amount of gastrointestinal disease.11, 12
Gastrointestinal bleeding occurs commonly in healthy dogs administered anti‐inflammatory dosages of aspirin.17, 18, 37 More recently, administration of aspirin at the currently recommended antiplatelet dosage (2 mg/kg q24h) was shown to induce gastrointestinal erosions and ulceration in healthy dogs.7 Lesions were most severe 14 days after initiation of treatment, but ongoing damage was identified on day 28. Further, coadministration of aspirin with prednisone more than doubled the odds of having an endoscopic mucosal lesion score ≥4 compared to receipt of prednisone alone.7 In contrast, clopidogrel administration was not associated with gastrointestinal bleeding in this study, and gastrointestinal bleeding scores did not differ between dogs administered prednisone alone versus combination prednisone/clopidogrel treatment. These findings support current recommendations to prioritize use of clopidogrel over aspirin for management of canine IMHA.1
Anticoagulants are believed to induce gastrointestinal bleeding through direct anticoagulant effects at sites of prior damage or sensitivity,41, 42 a similar mechanism of action to clopidogrel. However, bleeding risk varies dramatically among heparin and direct oral anticoagulants,41, 42, 43, 44, 45, 46 potentially suggesting additional etiopathogenic factors. In people, anticoagulants are associated with similar or higher rates of adverse events than antiplatelet drugs.35, 36, 47, 48 The incidence of gastrointestinal bleeding in dogs administered anticoagulants is unknown, and results of this study should not be extrapolated to their use.
Although biopsies were collected at baseline for determination of the presence of urease‐producing bacteria, we consider the impact of biopsy‐induced ulcers on the results of the study low. Neither lesion scores nor the number of punctate or invasive erosions differed significant among time points for dogs in the placebo group. The median numbers of punctate and invasive erosions identified in dogs in the prednisone‐receiving groups on days 14 and 28 were markedly greater than the number of biopsies taken. Lesions also were predominantly located in the antrum, inconsistent with the sampling protocol. Finally, new lesions were identified on day 28 in a number of dogs. These findings all suggest ongoing damage in dogs administered prednisone, versus delayed healing of prior biopsy sites, and are consistent with prednisone's previously described ulcerogenic effects.21, 22, 23, 24, 25, 26, 27, 28, 29, 30
This study had some additional limitations. Dogs enrolled in the study were all of moderate size with prednisone dosed on a milligrams per kilogram basis. Depending on whether a mass exponent of 0.67 or 0.71 is used for body surface area conversion,49 this resulted in administration of prednisone at a dose of 50.2 mg/m2 (range 44.9‐59.4) or 45.0 mg/m2 (range 37.8‐52.6), respectively. Results should be extrapolated with caution to very small or large dogs, which should be dosed on a mg/m2 basis with a maximum dose of 50–60 mg/m2/day.1 Although they ranged in age, dogs did not have underlying predisposing factors for gastrointestinal bleeding, such as anemia‐induced regional ischemia, concurrent diseases, or concurrent medications. Results might differ for dogs with IMHA or other diseases that are managed with concurrent glucocorticoid and thromboprophylactic treatment. Further, treatment was only administered for 28 days, while treatment for IMHA often lasts months. Both the number of gastrointestinal lesions and total lesion scores significantly decreased between 14 and 28 days. It is possible that prednisone‐induced gastrointestinal damage resolves with long‐term treatment, although this seems unlikely given identification of new lesions, including ulcers, in several dogs on day 28. The clinical relevance of these lesions currently is unclear. Although gastrointestinal lesions were marked in dogs in prednisone‐receiving groups, hematocrit did not significantly change over time and no dog developed anemia. Conversely, hematocrit significantly decreased in dogs administered steroids that developed gastrointestinal bleeding in 2 prior studies.7, 31 Quantitation of absolute reticulocyte counts might have added in detection of compensated blood loss, but it unfortunately was not performed. Similarly, fecal occult blood testing might have provided some insight into the minimum quantity of blood lost in dogs of this study, but it was not performed because of limitations in the accuracy of currently available tests.50 Although hypotension was not identified in any dog, continuous direct arterial blood pressure measurement was not performed. If hypotension occurred but was not recorded, it would have been transient because indirect measurements were collected every 5 minutes and, thus, unlikely to have meaningfully affected results of the study.
In spite of a moderate initial response rate, IMHA continues to have a high mortality rate.1 Immediate initiation of prednisone treatment remains the cornerstone of treatment.1 Our study confirmed prior findings that sustained oral prednisone administration is ulcerogenic in healthy dogs7 and significantly increases the odds of developing >25 punctate erosions, ≥1 invasive erosion, and/or ≥1 ulcer. Although the total number of lesions decrease between 14 and 28 days of administration, new lesions develop. Clinical signs of gastrointestinal bleeding do not occur, and clinicopathologic changes are minimal during the first month of administration. In contrast to aspirin antiplatelet treatment, clopidogrel does not induce gastrointestinal bleeding in dogs, and coadministration with prednisone does not increase the severity of gastric lesions or clinicopathologic changes. Further evaluation in dogs with naturally occurring hypercoagulability and immune‐mediated disease is necessary to confirm these findings and clarify their impact on disease management, thromboembolic risk, and long‐term survival.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study protocol was approved by the IACUC of the University of Tennessee, Knoxville (protocol number 2335) and performed in compliance with “The Guide for the Care and Use of Laboratory Animals” in laboratory animal facilities that are AAALAC certified and exceed NIH standards of care.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Whittemore JC, Mooney AP, Price JM, Thomason J. Clinical, clinicopathologic, and gastrointestinal changes from administration of clopidogrel, prednisone, or combination in healthy dogs: A double‐blind randomized trial. J Vet Intern Med. 2019;33:2618–2627. 10.1111/jvim.15630
REFERENCES
- 1. Swann JW, Garden OA, Fellman CL, et al. ACVIM consensus statement on the treatment of immune‐mediated hemolytic anemia in dogs. J Vet Intern Med. 2019;33:1141‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. deLaforcade A, Bacek L, Blais M‐C, et al. Consensus on the rational use of antithrombotics in veterinary critical care (CURATIVE): domain 1—defining populations at risk. J Vet Emerg Crit Care. 2019;29:37‐48. [DOI] [PubMed] [Google Scholar]
- 3. Weinkle TK, Center SA, Randolph JF, et al. Evaluation of prognostic factors, survival rates, and treatment protocols for immune‐mediated hemolytic anemia in dogs: 151 cases (1993–2002). J Am Vet Med Assoc. 2005;226:1869‐1880. [DOI] [PubMed] [Google Scholar]
- 4. Mellett AM, Nakamura RK, Bianco D. A prospective study of clopidogrel therapy in dogs with primary immune‐mediated hemolytic anemia. J Vet Intern Med. 2011;25:71‐75. [DOI] [PubMed] [Google Scholar]
- 5. Morassi A, Bianco D, Park E, et al. Evaluation of the safety and tolerability of rivaroxaban in dogs with presumed primary immune‐mediated hemolytic anemia. J Vet Emerg Crit Care. 2016;26:488‐494. [DOI] [PubMed] [Google Scholar]
- 6. McLewee N, Archer T, Wills R, et al. Effects of aspirin dose escalation on platelet function and urinary thromboxane and prostacyclin levels in normal. J Vet Pharmacol Ther. 2018;41:60‐67. [DOI] [PubMed] [Google Scholar]
- 7. Whittemore JC, Mooney AP, Price JM, et al. Clinical, clinicopathologic, and gastrointestinal changes from aspirin, prednisone, or combination therapy in healthy research dogs: a double‐blind randomized trial. J Vet Intern Med. 2019;33:1977‐1987. 10.1111/jvim.15577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai TJ, Lai KH, Hsu PI, et al. Upper gastrointestinal lesions in patients receiving clopidogrel anti‐platelet therapy. J Formos Med Assoc. 2012;111:705‐710. [DOI] [PubMed] [Google Scholar]
- 9. Hallas J, Dall M, Andries A, et al. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case‐control study. BMJ. 2006;333:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ibanez L, Vidal X, Vendrell L, et al. Upper gastrointestinal bleeding associated with antiplatelet drugs. Aliment Pharmacol Ther. 2006;23:235‐242. [DOI] [PubMed] [Google Scholar]
- 11. Harker LA, Boissel JP, Pilgrim AJ, et al. Comparative safety and tolerability of clopidogrel and aspirin: results from CAPRIE. CAPRIE steering committee and investigators. Clopidogrel versus aspirin in patients at risk of ischaemic events. Drug Saf. 1999;21:325‐335. [DOI] [PubMed] [Google Scholar]
- 12. Fork FT, Lafolie P, Toth E, et al. Gastroduodenal tolerance of 75 mg clopidogrel versus 325 mg aspirin in healthy volunteers. A gastroscopic study. Scand J Gastroenterol. 2000;35:464‐469. [DOI] [PubMed] [Google Scholar]
- 13. Whittemore JC, Mooney A, Mawby DI, Thomason J. Platelet function and endoscopic changes after clopidogrel, aspirin, prednisone, or combination therapy in dogs [abstract]. J Vet Intern Med. 2017;30:1282. [Google Scholar]
- 14. Greco DS. Diagnosis and dietary management of gastrointestinal disease; 2011. https://www.purinaveterinarydiets.com/media/1202/gi_quick_reference_guide.pdf
- 15. Wilson JE, Chandrasekharan NV, Westover KD, et al. Determination of expression of cyclooxygenase‐1 and ‐2 isozymes in canine tissues and their differential sensitivity to nonsteroidal anti‐inflammatory drugs. Am J Vet Res. 2004;65:810‐818. [DOI] [PubMed] [Google Scholar]
- 16. Heather Graham A, Leib MS. Effects of prednisone alone or prednisone with ultralow‐dose aspirin on the gastroduodenal mucosa of healthy dogs. J Vet Intern Med. 2009;23:482‐487. [DOI] [PubMed] [Google Scholar]
- 17. Forsyth SF, Guilford WG, Lawoko CR. Endoscopic evaluation of the gastroduodenal mucosa following non‐steroidal anti‐inflammatory drug administration in the dog. N Z Vet J. 1996;44:179‐181. [DOI] [PubMed] [Google Scholar]
- 18. Reimer ME, Johnston SA, Leib MS, et al. The gastroduodenal effects of buffered aspirin, carprofen, and etodolac in healthy dogs. J Vet Intern Med. 1999;13:472‐477. [DOI] [PubMed] [Google Scholar]
- 19. Rohrer CR, Hill RC, Fischer A, et al. Efficacy of misoprostol in prevention of gastric hemorrhage in dogs treated with high doses of methylprednisolone sodium succinate. Am J Vet Res. 1999;60:982‐985. [PubMed] [Google Scholar]
- 20. Rohrer CR, Fischer A, Fox LE, et al. Gastric hemorrhage in dogs given high doses of methylprednisolone sodium succinate. Am J Vet Res. 1999;60:977‐981. [PubMed] [Google Scholar]
- 21. Das D, Bandyopadhyay D, Bhattacharjee M, et al. Hydroxyl radical is the major causative factor in stress‐induced gastric ulceration. Free Radic Biol Med. 1997;23:8‐18. [DOI] [PubMed] [Google Scholar]
- 22. Filaretova L, Morozova O, Bagaeva T, et al. From gastroprotective to proulcerogenic action of glucocorticoids on the gastric mucosa. J Physiol Pharmacol. 2009;60(Suppl 7):79‐86. [PubMed] [Google Scholar]
- 23. Filaretova L, Podvigina T, Bagaeva T, et al. Dual action of glucocorticoid hormones on the gastric mucosa: how the gastroprotective action can be transformed to the ulcerogenic one. Inflammopharmacology. 2009;17:15‐22. [DOI] [PubMed] [Google Scholar]
- 24. Choquet A, Magous R, Bali JP. Gastric mucosal endogenous prostanoids are involved in the cellular regulation of acid secretion from isolated parietal cells. J Pharmacol Exp Ther. 1993;266:1306. [PubMed] [Google Scholar]
- 25. Bhattacharjee M, Chakraborty Y, Ganguly C, et al. Inhibition of gastric mucosal prostaglandin synthetase activity by mercaptomethylimidazole, an inducer of gastric acid secretion—plausible involvement of endogenous H2O2. Biochem Pharmacol. 1998;56:905‐913. [DOI] [PubMed] [Google Scholar]
- 26. Das D, Banerjee RK. Effect of stress on the antioxidant enzymes and gastric ulceration. Mol Cell Biochem. 1993;125:115‐125. [DOI] [PubMed] [Google Scholar]
- 27. Bandyopadhyay U, Bhattacharyya DK, Chatterjee R, et al. Localization of gastric peroxidase and its inhibition by mercaptomethylimidazole, an inducer of gastric acid secretion. Biochem J. 1992;284:305‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bandyopadhyay U, Chatterjee R, Chakraborty TK, et al. Activation of parietal cell by mercaptomethylimidazole: a novel inducer of gastric acid secretion. Biochem Pharmacol. 1997;54:241‐248. [DOI] [PubMed] [Google Scholar]
- 29. Bandyopadhyay U, Biswas K, Bandyopadhyay D, et al. Dexamethasone makes the gastric mucosa susceptible to ulceration by inhibiting prostaglandin synthetase and peroxidase—two important gastroprotective enzymes. Mol Cell Biochem. 1999;202:31‐36. [DOI] [PubMed] [Google Scholar]
- 30. Das D, De PK, Banerjee RK. Thiocyanate, a plausible physiological electron donor of gastric peroxidase. Biochem J. 1995;305:59‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sorjonen DC, Dillon AR, Powers RD, et al. Effects of dexamethasone and surgical hypotension on the stomach of dogs: clinical, endoscopic, and pathologic evaluations. Am J Vet Res. 1983;44:1233‐1237. [PubMed] [Google Scholar]
- 32. Tsukamoto A, Ohno K, Maeda S, et al. Effect of mosapride on prednisolone‐induced gastric mucosal injury and gastric‐emptying disorder in dog. J Vet Med Sci. 2012;74:1103‐1108. [DOI] [PubMed] [Google Scholar]
- 33. Goggs R, Blais M‐C, Brainard BM, et al. American College of Veterinary Emergency and Critical Care (ACVECC) consensus on the rational use of antithrombotics in veterinary critical care (CURATIVE) guidelines: small animal. J Vet Emerg Crit Care. 2019;29:12‐36. [DOI] [PubMed] [Google Scholar]
- 34. Goggs R, Bacek L, Bianco D, et al. Consensus on the rational use of antithrombotics in veterinary critical care (CURATIVE): domain 2—defining rational therapeutic usage. J Vet Emerg Crit Care. 2019;29:49‐59. [DOI] [PubMed] [Google Scholar]
- 35. Jameson SS, Baker PN, Deehan DJ, et al. Evidence‐base for aspirin as venous thromboembolic prophylaxis following joint replacement. Bone Joint Res. 2014;3:146‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nielen JTH, Dagnelie PC, Emans PJ, et al. Safety and efficacy of new oral anticoagulants and low‐molecular‐weight heparins compared with aspirin in patients undergoing total knee and hip replacements. Pharmacoepidemiol Drug Saf. 2016;25:1245‐1252. [DOI] [PubMed] [Google Scholar]
- 37. Ward DM, Leib MS, Johnston SA, et al. The effect of dosing interval on the efficacy of misoprostol in the prevention of aspirin‐induced gastric injury. J Vet Intern Med. 2003;17:282‐290. [DOI] [PubMed] [Google Scholar]
- 38. Little D, Jones SL, Blikslager AT. Cyclooxygenase (COX) inhibitors and the intestine. J Vet Intern Med. 2007;21:367‐377. [DOI] [PubMed] [Google Scholar]
- 39. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Circulation. 2010;122:2619‐2633. [DOI] [PubMed] [Google Scholar]
- 40. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Circulation. 2008;118:1894‐1909. [DOI] [PubMed] [Google Scholar]
- 41. Tao DL, Bien JY, DeLoughery TG, et al. Extended thromboprophylaxis with direct oral anticoagulants for medical patients: a systematic review and meta‐analysis. Blood. 2017;129:653. [DOI] [PubMed] [Google Scholar]
- 42. Tao D, Bien J, Shatzel J. A systematic review and meta‐analysis of randomized trials examining extended thromboprophylaxis with direct oral anticoagulants in hospitalized medical patients. Blood. 2016;128:1436. [DOI] [PubMed] [Google Scholar]
- 43. Vaduganathan M, Bhatt DL. Gastrointestinal bleeding with oral anticoagulation: understanding the scope of the problem. Clin Gastroenterol Hepatol. 2017;15:691‐693. [DOI] [PubMed] [Google Scholar]
- 44. Sengupta N, Marshall AL, Jones BA, et al. Rebleeding vs thromboembolism after hospitalization for gastrointestinal bleeding in patients on direct oral anticoagulants. Clin Gastroenterol Hepatol 2018;16:1893‐1900.e1892. [DOI] [PubMed] [Google Scholar]
- 45. Miller CS, Dorreen A, Martel M, et al. Risk of gastrointestinal bleeding in patients taking non–vitamin K antagonist oral anticoagulants: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2017;15:1674‐1683.e1673 [DOI] [PubMed] [Google Scholar]
- 46. Abraham NS, Noseworthy PA, Yao X, et al. Gastrointestinal safety of direct oral anticoagulants: a large population‐based study. Gastroenterology. 2017;152:1014‐1022.e1011. [DOI] [PubMed] [Google Scholar]
- 47. Mistry DA, Chandratreya A, Lee PYF. A systematic review on the use of aspirin in the prevention of deep vein thrombosis in major elective lower limb orthopedic surgery: an update from the past 3 years. Surg J. 2017;3:e191‐e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bien JY, Daughety MM, Tao DL, et al. The safety of aspirin vs. direct oral anticoagulants: a meta‐analysis of currently published clinical trials. Blood. 2017;130:3720. [Google Scholar]
- 49. Price GS, Frazier DL. Use of body surface area (BSA)‐based dosages to calculate chemotherapeutic drug dose in dogs: I. Potential problems with current BSA formulae. J Vet Intern Med. 1998;12:267‐271. [DOI] [PubMed] [Google Scholar]
- 50. Rice JE, Ihle SL. Effects of diet on fecal occult blood testing in healthy dogs. Can J Vet Res. 1994;58:134‐137. [PMC free article] [PubMed] [Google Scholar]


