Abstract
Background
Noise phobia is a common behavior problem in dogs for which there are limited treatment options.
Objective
To evaluate the efficacy and safety of imepitoin in comparison to placebo for the control of anxiety and fear associated with noise phobia in dogs.
Animals
Two hundred thirty‐eight client‐owned dogs with noise phobia were recruited in veterinary clinics.
Methods
This placebo‐controlled, randomized, double‐blinded, clinical trial used a predictable noise event as eliciting context, the traditional New Year's Eve fireworks in Germany and the Netherlands. Owners began treatment 2 days before the anticipated noise event with administration of either imepitoin 30 mg/kg body weight Q12h or placebo for 3 consecutive days. On New Year's Eve, owners noted their observations of their dog's fear and anxiety behavior at 1600, 2200, 0020, and 0100 hours and scored the overall treatment effect on the following day.
Results
In the 16‐item owner report of fear and anxiety signs, fear and anxiety behaviors were significantly reduced under imepitoin treatment compared to placebo (delta −6.1 scoring points; P < .0001). A significantly higher proportion of owners reported a good or excellent overall treatment effect in the imepitoin group compared to placebo (odds ratio 4.689; 95% CI, 2.79‐7.89; P < .0001).
Conclusion
Imepitoin effectively controls fear and anxiety associated with noise phobia in dogs.
Keywords: anxiolytic, clinical trial, firework, imepitoin, noise aversion, noise phobia, noise sensitivity
1. INTRODUCTION
Fear responses to noises are quite common in dogs with a prevalence of up to 49%,1 with a relevant proportion showing nongraded extreme responses to noises indicative of a phobia.2 Exposure to a phobic stimulus almost invariably provokes an immediate behavioral response with concomitant signs of autonomic arousal.2 A phobia in animals involves a marked, persistent, and excessive fear of clearly discernible circumscribed objects or situations. The term phobia is derived from human psychiatry, where it describes an irrational fear, and it is mainly used to create a qualitatively distinct category for the purposes of psychiatric diagnosis, but there is some debate over whether this term should be applied to animals. Although the terms “noise sensitivity,” “noise aversion,” or “fear response to noise” are used in the veterinary behavior literature, “noise phobia” is probably the most widely used term to describe the clinical complaint and is used in this article, defined as overly anxious reactions by dogs to specific sounds. This reaction is often stressful for the owners and a welfare issue for the affected dog. Diagnostically, it is useful to separate phobias from normal temporary responses which should be of less concern, but the point “at which a fear becomes a phobia is unknown”3; indeed, all data to date indicate that the behavioral response to an aversive event or situation is dimensional in nature and thus the problem exists as a spectrum which includes normality. Even if the paradigm used for conceptualizing problems in veterinary behavioral medicine places noise phobia within the context of a normal (albeit undesirable and potentially extreme) emotional response rather than an artificially constructed medical category, it does not negate the value of psychopharmacological interventions,4 which could protect the dogs' well‐being5 or enable behavioral management.6
Benzodiazepines, targeting GABAA (γ‐aminobutyric acid, type A) receptors, are commonly used to manage fear and anxiety‐related conditions. However, development of drug dependence, loss of efficacy (tolerance), and adverse drug effects limit their use.7 New approaches to improve the safety profile have been the focus of research for some time, looking mainly on GABAA subtype‐specific molecules or partial agonists.7 Imepitoin acts centrally as a partial agonist at the benzodiazepine‐binding site of the GABAA‐receptor with low affinity, potentiating neuronal inhibition resulting in anxiolytic and anticonvulsant effects.8 It is in veterinary use in many countries for the treatment of idiopathic epilepsy and is well tolerated.8 In standard rodent models, imepitoin has similar anxiolytic activities to benzodiazepines9 and a recent case series has suggested it might be useful for managing fear and anxiety‐related conditions in dogs.10 Therefore, the aim of this study was to determine the effectiveness and safety of imepitoin in comparison to placebo for reducing anxiety and fear associated with noise phobia in dogs.
2. METHODS
2.1. Study design
The study was conducted as a multicenter, randomized, double‐blinded, placebo‐controlled, clinical field trial according to Good Clinical Practice guidelines with client‐owned animals. The study design used a predictable noise event as the eliciting context: the traditional New Year's Eve fireworks in Germany and the Netherlands on December 31, 2016. All study procedures were timed around this event (Figure 1). Twenty‐one study sites participated in this study, in various geographic areas in Germany (17 sites) and the Netherlands (4 sites). Most practices were located in urban areas. In total, 251 dogs were included, and 238 were treated either with imepitoin or placebo in a ratio of approximately 1:1. During the evaluation phase of the study, all animals remained in their familiar surroundings. The 2 primary variables for efficacy evaluation were the owner assessment of overall treatment effect and a detailed owner assessment of behavioral responses.
Figure 1.
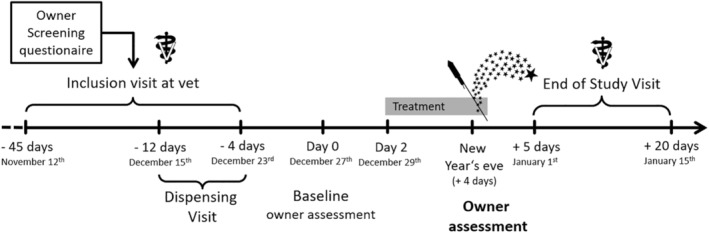
Study design and schedule of events
The conduct of the study was ethically approved before the start of the study by the relevant authorities in Germany and the Netherlands (Regierungspräsidium Karlsruhe, Niedersächsisches Landesamt für Verbraucherschutz und Lebbensmittelsicherheit, Landesamt für Natur Umwelt und Verbraucherschutz Nordrhein‐Westfalen, Landesamt für Soziales, Jugend und Versorgung Rheinland‐Pfalz, Minster van Economische Zaken).
There were 3 visits to the vet for the owner (Figure 1). At the inclusion visit, a physical examination, evaluation of eligibility‐based inclusion and exclusion criteria (see below), and blood sampling took place. The medication and the diary for the owner's observations were dispensed at a separate dispensing visit. A physical examination and the collection of the diary concluded the study at the end of the study visit.
2.2. Inclusion and exclusion criteria
All dogs enrolled in this study were client‐owned and lived in Germany or the Netherlands. The dog's owner or authorized agent signed an informed consent at the inclusion visit before study procedures. Dogs were recruited at veterinary practices during routine visits or after personal contact due to previous visits for this problem.
Dogs of any breed or mixed breed and of either sex that weighed greater than or equal to 2 kg were eligible to be screened. The diagnosis of noise phobia was based on ruling out medical causes through routine veterinary clinical examination alongside a history of signs of noise phobia with a score above 30 in the Lincoln Sound‐Sensitivity Scale (LSSS)11 and 5 confirmatory questions to exclude other anxiety problems
Dog displayed fear responses to specific, identifiable firework noises in previous year(s) without medical treatment.
The fear and anxiety response is reliably elicited upon exposure to loud, explosive noises such as fireworks, thunderstorms, or gunshots.
The fear and anxiety responses occur in the home (and might also occur when out).
Fear and anxiety responses occur while the owner or another familiar person is present.
The fear/anxiety is not generalized to the extent that the eliciting cues are too numerous to be identifiable and the dog rarely appears relaxed.
The cutoff point in the LSSS of 30 captures the essence of the definition of noise phobia used in here, that is, that it is an “excessive fear or anxiety of a sound”. Excessive can be considered to refer to either the severity of signs (ie, at least 2 signs with most intense form [score 5] every time the noise was heard [score 3]) or the number of signs (ie, all 16 signs occur frequently [score 2] in the lowest intensity [score 1]).
In addition, dogs had to be healthy or with a stable systemic disease, considered to be pain‐free for at least 2 weeks before inclusion (no painful condition or stable under pain management including medication) and have been with the current owner for at least 1 year. The owner had to agree that either he/she or another person familiar with the dog would be with the dog on New Year's Eve, and this person had observed a fear response in the dogs during previous fireworks.
Dogs suffering from generalized fears, as well as dogs on treatment with behavior‐modifying drugs in the previous 2 months were excluded. Sedatives were permitted for a single occasion and for purposes not related to behavior problems (eg, diazepam as a preanesthetic) up to 2 weeks before the start of the treatment. Known epilepsy or treatment with imepitoin during the last 5 months as well as dogs that had been treated to diminish signs of noise phobia in the past 12 months were excluded. Dogs with a history of, or concomitant disease, such as severely impaired hepatic function, severe renal or severe cardiovascular disorders were not included. Relevant family‐related risk factors affecting risk to individuals from the dog's behavior (eg, dogs that had bitten people in the past, toddlers in the home in the case of aggressive behavior toward people, aggression between dogs within home, or dogs that showed other aggressive tendencies of concern in the past) also led to exclusion.
An enrolled animal was removed from further participation in the study if the owner withdrew their consent, if the owner was noncompliant with the study procedures, if the temperament of the dog prohibited administration of the study drug, if there was a change in pain medication, if study medication was not administered, if the owner indicated that the dog was not exposed to firework noises on New Year's Eve, or if an adverse event or development of concomitant disease prohibited further participation. Treatment with anxiolytic, psychotropic, sedative, other behavior‐modifying drugs or homeopathic or natural remedies intended to reduce stress or anxiety was prohibited during the study.
The owners are provided with advice complementary to the treatment provided, to help to minimize the stress for the dog, and to avoid counterproductive measures by the owner that might bias outcome. These statements were not to punish the dog when scared, not to overly fuss or try to reassure the dog when scared, to ignore the noises as owner and to keep the dog in a safe and secure environment to avoid escaping. No other advice on environmental or behavior modification was provided.
2.3. Trial medication
Dogs received either imepitoin at a dose of approximately 30 mg/kg twice daily or visually identical placebo tablets. Dogs were administered the appropriate amount of tablets orally twice daily at approximately 12 hours intervals starting 2 days before the day of anticipated noise event. The first administration was in the morning of 29th December, and the last administration was in the evening of 31st December (ideally between 1800 and 2000 hours).
2.4. Assessment of fear and anxiety‐related behaviors
The primary efficacy assessment of the study aimed to show if there was superiority of imepitoin over the placebo. To assess this, 2 co‐primary efficacy variables were used.
The first primary efficacy variable was the owner's overall assessment of the treatment effect on the fear and anxiety behavior of the dog in response to fireworks on New Year's Eve, using a 5‐point ordinal score (Table 1). This assessment was done on January 1st, not earlier than 6:00 am This time point ensured a retrospective judgment for the whole duration of the noise event.
Table 1.
Histogram of owner's rating of overall treatment effect on the anxiety behavior of dogs, assessed after the fireworks event
| Score | Description |
|---|---|
| Excellent Effect | The dog does not react to fireworks with anxious/fearful behavior at all |
| Good Effect | The dog's reactions are mild and it can calm down |
| Some Effect | The dog is reacting somewhat less/milder than in previous year(s) without treatment but it cannot calm down |
| No Effect | There is no reduction/change in the dog's reactions compared to previous year(s) without treatment |
| Worse Effect | The dog's reaction to fireworks is stronger than in previous year(s) without treatment |
The gray marked scores indicate an insufficient treatment response. This information was not disclosed to owners.
The second co‐primary efficacy variable was based on a detailed 16‐item owner questionnaire, reporting the dog's fear and anxiety signs. This questionnaire was modified from the LSSS11 by adopting the behaviors and their intensity scoring but not their frequency (as the current questionnaire was assessing response to a specific event, rather than over an extended period). After the model developed in the LSSS, the intensities of 16 behaviors during the 15 minutes before the assessment were classified on an ordinal scale from zero (not present) to 1 (small amount) through 5 (extensive amount) and added up to a total sum score (Table 2). The owners were asked to complete the score 4 days before New Year's Eve (ie, on December 27th) for baseline and at 1600 and 2200 hours on New Year's Eve, and at 0020 and 0100 hours on New Year's Day. In addition, they indicated whether fireworks were present during the last 20 minutes before each assessment.
Table 2.
Score of dog's fear and anxiety signs on a detailed 16‐item owner questionnaire
| Items (16) | Running around, drooling saliva, hiding, destructiveness, cowering, restlessness/pacing, aggressive behavior, “freezing to the spot” barking/whining/howling, panting, vomiting/defecating/urinating/diarrhea, owner‐seeking behavior, vigilance/scanning environment, bolts, shaking or trembling, and self‐harm |
| Score per item |
|
2.5. Statistics
Randomization was based on blocks of 2 using a pseudorandom number generator, so that the resulting assignment of medication numbers to the treatment group was both reproducible and nonpredictable. Each investigator received a set of medication numbers and assigned the medication numbers in ascending order to the included dogs.
The analysis of the co‐primary variables was performed on the Full Analysis Set (FAS). The FAS consisted of all dogs that were randomized to the treatment groups, received at least 1 dose of study medication, fulfilled major entry criteria, and completed at least the evaluations necessary for the analysis of the co‐primary efficacy variables. This population followed the intention‐to‐treat principle (Figure 2). Per protocol populations were defined, where dogs with major protocol deviations such as insufficient treatment compliance or administration of other behavior‐modifying drugs were excluded. For the first co‐primary variable, a generalized linear model with cumulative logit link was used to estimate and test the (cumulative) odds ratio of imepitoin versus placebo utilizing the GLIMMIX procedure of SAS (SAS Institute Inc, Cary, North Carolina). The model included treatment as a fixed effect and site and treatment‐by‐site as random effects. The second co‐primary variable was analyzed using a mixed model to estimate and test the difference in mean fear and anxiety behavior scores utilizing the MIXED procedure of SAS, with treatment, time point, and time point‐by‐treatment interaction as fixed effects, subject, site and site‐by‐treatment interaction as random effects, and baseline as covariate. Safety was analyzed for all dogs who received at least 1 dose of treatment (see Figure 2).
Figure 2.
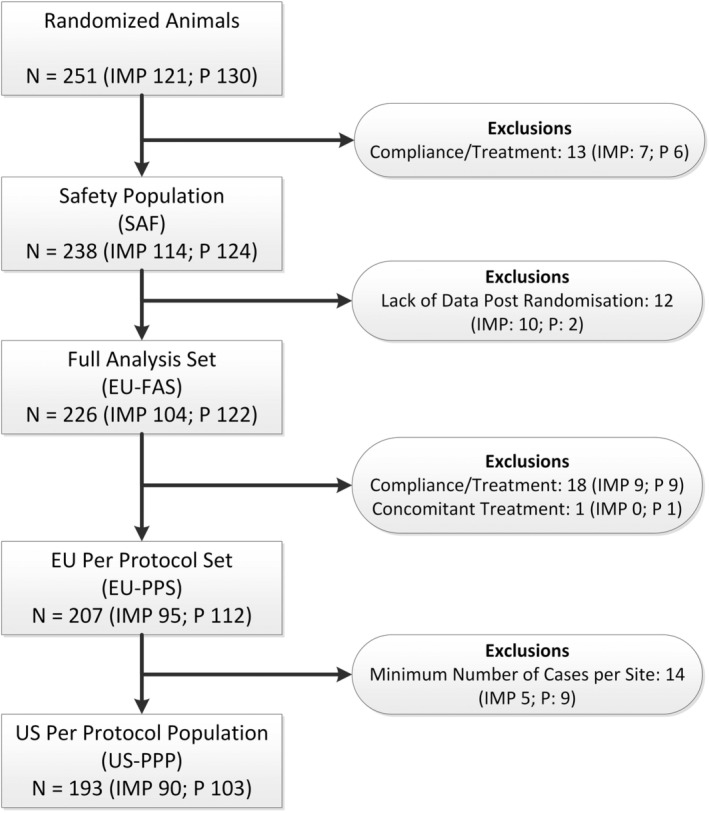
Flowchart of exclusions from the analyzed populations. From all dogs randomized into the study, dogs that received at least 1 dose of study medication were included in the safety analysis. Cases were excluded from primary efficacy population (Full Analysis Set, FAS) if there were not sufficient data to evaluate the end points (eg, termination of study before New Year's Eve). Excluding cases with protocol deviations formed additional per protocol sets (PPS). As per FDA guidance, also clinical sites with less than 4 evaluable cases had to be excluded, setting up the per protocol population for FDA (US‐PPP)
3. RESULTS
3.1. Demographics
The demographic data and baseline characteristics were well balanced between the 2 treatment groups in all populations (Table 3). More than half of the dogs were female (61% females; 39% males), and the majority of dogs (82%) were neutered or spayed. There was no obvious overrepresentation of any breed, and most dogs were of mixed breeds (39%).
Table 3.
Demographics and baseline characteristics per group (based on all dogs treated with at least 1 dose of treatment; safety population)
| Variable | Imepitoin (n = 114) | Placebo (n = 124) |
|---|---|---|
| Age, median (min‐max), y | 7.0 (1‐13) | 7.0 (1‐14) |
| Sex | ||
| Female, N (%) | 69 (60.5) | 76 (61.3) |
| Male, N (%) | 45 (39.5) | 48 (38.7) |
| Breeda | ||
| Mixed breed, N (%) | 44 (38.6) | 49 (39.5) |
| Other, N (%) | 42 (36.8) | 31 (25.0) |
| Body weight, mean ± SD, kg | 20.4 ± 12.3 | 20.0 ± 13.3 |
| Total Lincoln Sound Sensitivity Scale, median (min‐max) | 77.5 (32‐148) | 74.5 (32‐155) |
Only breeds >5% are shown. Other breed is a rare breed or a breed not recognized by the Federation Cynologique Internationale.
The proportions of dogs taking concomitant treatment were similar in both treatment groups (imepitoin 14%; placebo 18%). There was no specific treatment category predominantly represented, and 2 animals received prohibited drugs for nervous system disorders during the trial (psycholeptics: benzodiazepine; phenothiazine with aliphatic side‐chain). In line with hypothyroidism being the most frequently reported disease at baseline, thyroid preparations were the most commonly reported concomitant treatment (6%; imepitoin 4/104; placebo 9/122).
At inclusion, dogs presented with noise phobia defined here as “excessive fear or anxiety of a sound”, as indicated by a high score in the Lincoln Sound Sensitivity Scale in both groups (Table 3). In detail, 85% of dogs recruited showed at least 1 behavior at its most extreme (ie, score 5 in intensity), and the minimum number of signs shown by any dog recruited in this study was 4 (mode = 9 signs with score >0). The most common signs were shaking or trembling (95%), cowering (94%), and hiding (94%). Hiding is clearly indicative of the response interfering with the dog's ability to function normally in the home.
3.2. Treatment compliance
Compliance was generally high in both treatment groups, with 91 of 104 imepitoin‐treated dogs and 110 of 122 placebo‐treated dogs receiving all 6 administrations. Some owners continued administrations after 31st December (3 imepitoin, 4 placebo) due to ongoing occasional fireworks, so these dogs received 7 to 10 administrations in total. In 7 cases (5 imepitoin, 2 placebo), the investigator lowered the daily dose due to occurrence of ataxia.
3.3. Efficacy
Both co‐primary end points, the owner's overall assessment as well as their assessment of fear and anxiety behaviors, demonstrated efficacy for the imepitoin group at high thresholds of statistical significance.
The owner assessment of the overall treatment effect (recorded the day after the noise event, ie, January 1st) showed a significantly higher proportion of owners reporting a good or excellent treatment effect in the imepitoin group compared to placebo (Figure 3). A cumulative odds ratio of 4.7 (95% CI, 2.79‐7.89) with a P‐value <.0001 indicates that the odds for a favorable treatment assessment were 4.5 times higher for imepitoin compared to placebo. In the 16‐item modified LSSS owner questionnaire, fear and anxiety behaviors were significantly reduced for imepitoin versus placebo by a delta of −6.1 scoring points (P < .0001). This achievement must be compared to the average fear and anxiety increase from baseline in untreated (placebo) dogs, which reaches an amplitude of 17.97 = 24.88‐6.91 at the peak time point 0020 hours (amplitude = mean score at 0020 hours minus mean score at baseline; see Figure 4). Both treatment groups exhibited a characteristic progression of the fear and anxiety level towards midnight, which was followed by a gradual decline afterward (Figure 4). Fear and anxiety behaviors in the imepitoin treated dogs were reduced compared to placebo at each time point.
Figure 3.
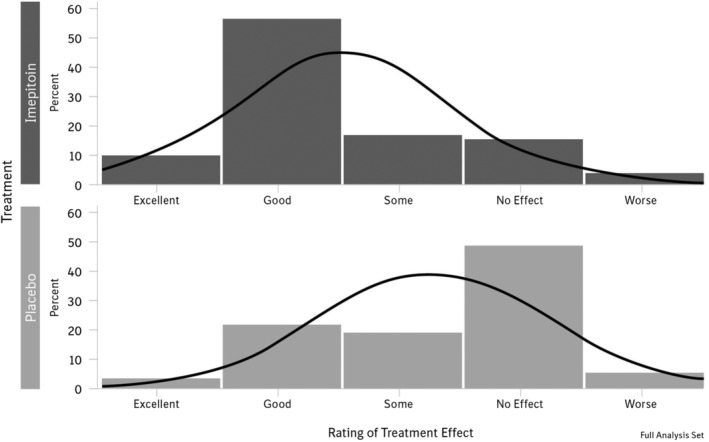
Histogram of owner's rating of overall treatment effect. There was a significantly higher proportion of owners reporting a good or excellent treatment effect in the imepitoin group compared to the placebo (P < .0001) with an odds ratio of 4.7 (95% confidence interval, 2.79‐7.89)
Figure 4.
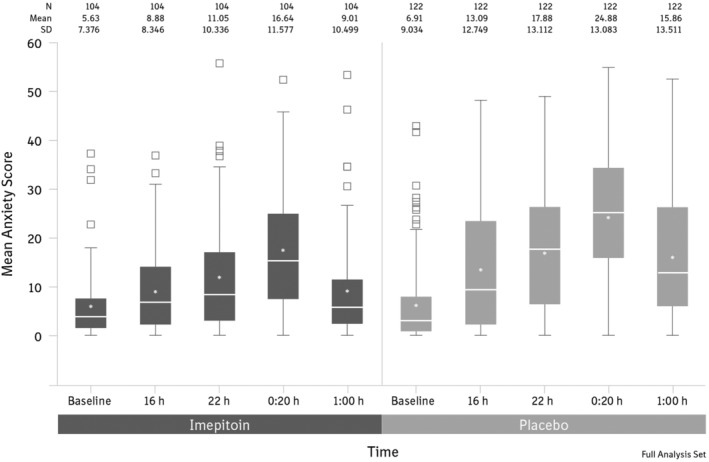
Evolution of anxiety score over time at baseline and on New Year's Eve. Anxiety behaviors were significantly reduced under imepitoin treatment compared to placebo (P < .0001)
To compare the efficacy at the highest intensity of fireworks, fear and anxiety scores during or immediately after the fireworks at time point 0020 hours were compared between treatment groups as a secondary variable. Significantly higher score values were recorded for the placebo group than for the imepitoin group (24.9 [SD 13.1] for placebo versus 16.6 [SD 11.6] for imepitoin; P < .0001). This statement holds true if the increase from the baseline score is considered instead. This is also verified by the data of the subfraction of animals who did no longer experience fireworks at the time point 0100 hours: In the absence of fireworks, the fear and anxiety level for both treatment groups broadly went down and the difference between the treatment groups disappeared (data not shown).
The time point when owner assessed that the dog could be left alone after midnight was analyzed as a secondary variable. Owners believed that they could leave their dog alone on average 162 minutes (i.e. ∼0240 hours; SD 133 minutes) and 203 minutes (i.e. ∼0322 hours; SD 210 minutes) after midnight in the imepitoin‐treated group and in the placebo group, respectively. The difference was not statistically significant. A notable proportion of owners in both treatment groups stated that, in principle, they would never leave their dog alone at New Year's Eve and so did not answer this question (15.4% in imepitoin group, 23% in placebo group). Therefore, this variable might be of limited reliability.
The robustness analyses based on the per protocol population confirmed all the above‐mentioned efficacy results (see Supporting Information).
3.4. Safety
Imepitoin was well tolerated with no serious adverse events recorded in this trial (a serious adverse event was defined as an unfavorable observation, which results in death or life‐threatening conditions, persistent or significant disability, abortion or birth defects or requires professional intervention). Reported adverse events were mostly mild and transient and included ataxia, increased appetite, lethargy, emesis, hyperactivity, and somnolence, all of which occurred at a higher frequency in the imepitoin‐treated group than in the placebo group (Table 4). All other adverse events occurred either in similar frequencies as in the placebo group or were reported only in very few cases (≤2.5%).
Table 4.
Incidence of adverse events (based on all dogs treated with at least 1 dose of treatment; safety population)
| Event | Imepitoin (n = 114) (%) | Placebo (n = 124) (%) |
|---|---|---|
| All adverse events | 55 (48) | 13 (10) |
| Ataxia | 40 (35) | 2 (2) |
| Increased appetite | 21 (18) | 1 (1) |
| Lethargy | 14 (12) | 2 (2) |
| Emesis | 10 (9) | 1 (1) |
| Hyperactivity | 7 (6) | 1 (1) |
| Somnolence | 4 (3) | … |
Events with >2.5% frequency are shown. Number of animals affected (at least 1 event).
Transient ataxia was the most commonly reported adverse event, affecting 35.1% (40/114) of the imepitoin‐treated cases, starting on the first day of treatment, mostly between 30 minutes and 4 hours after first drug administration. It resolved exponentially under continuous treatment, within a day in 51.2% and within 2 days in 24.4% of cases (Figure 5). Occurrence of ataxia was not overtly associated with specific demographic factors and did not appear to affect the perception of the treatment effect by the owner.
Figure 5.
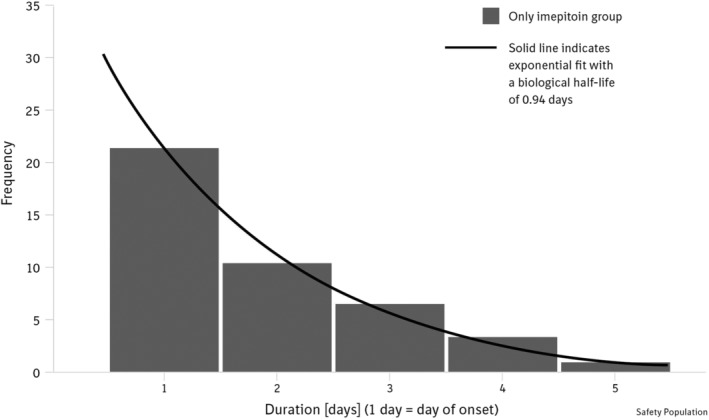
Ataxia was the most frequently reported adverse event in the imepitoin group. It was reported as occurring on the day of treatment initiation and resolving spontaneously under continuation of treatment in most cases on the same or the next day. Resolution decreased in an exponential fashion, with ~50% resolution within 24 hours after first drug administration. Data depicted as frequency of number of cases
4. DISCUSSION
Although noise phobia is most frequently associated with fireworks, it is more than just a seasonal or sporadic issue, as affected dogs often also react to a range of other noises such as thunder or gunshots.2, 12 Although some dogs with noise phobia respond only to certain sporadically occurring noises, others have phobias against many noises that may occur year round or to other, less salient, noises, such as traffic and TV noises.1 Noise phobia could also represent 1 of several expressions of a wider underlying anxiety problem.12 Accordingly, treatment approaches should be tailored to the individual dog's problem and might consist of short acute pharmaceutical interventions for occasional but predictably occurring events causing fear and anxiety or long‐term psychoactive medication either alone or in combination with a short‐acting anxiolytic when needed.12 In all cases, behavior treatment, preferably by a specialist, would be ideal in combination with psychoactive drugs to provide the best long‐term effect.2
Because there is strong evidence that dogs with noise phobia generalize fear of 1 type of loud sudden noise to others,1, 13 New Year fireworks provide a good standardization of the eliciting context for noise phobia more generally. Thus, this study provides evidence that imepitoin is effective in reducing signs of anxiety and fear associated with noise phobia in dogs.
Placebo‐controlled trials are considered the best evidence for anxiolytic treatments.14 Currently, only a third of owners with dogs suffering from fear responses to noises seek treatment advice,1 despite the fact that noise phobia has negative effects on both health and life span of dogs.15, 16
Regarding the dose regimen, we aimed to establish an efficacious blood concentration of imepitoin at the initiation of the noise event to preemptively limit the fear and anxiety experience. Because titration of the dose up to an efficacious level is not possible for sudden noise interventions, the drug was administered before New Year's Eve to ensure efficacy. The dose and dosing regimen of imepitoin in this study (approximately 30 mg/kg Q12h starting 2 days before the day of the anticipated event and continued through the day of the event) was chosen on the basis of our understanding of both the pharmacokinetic properties17 and previous studies10 to maximize the likely benefit at a minimum risk for the population, while recognizing that lower doses, shorter pretreatment times, or both might be sufficient in some dogs. For example, in a laboratory setting, a single dose of 20 mg imepitoin per kg body weight, administered 135 minutes before a noise event, has shown anxiolytic properties.9 This is consistent with the observations in this study, where a reduction in noise phobia response was still demonstrated in the few cases where the dose was lowered due to adverse events.
Anxiety and fear are difficult to measure, as they are subjective unpleasant feelings resulting in typical but varied behaviors. As dogs cannot express their feelings, investigations are typically limited to behavioral observations, which can be done best in familiar environments (ie, at home, in kennels or at work). The outcome in this clinical trial was based on rating scales that were previously established and commonly used in similar trials on anxiety problems.10, 18, 19 Although owner‐reported outcome delivers reliable and accurate descriptions of dogs' fearfulness, possible subjective and emotional inputs by owners are a risk for bias and needs to be controlled by a proper design of the questionnaires.20 It is clearly a limitation of such studies that questionnaires are difficult to design, and up to now there are no validated questionnaires. For example, in this study, we used a 5‐point questionnaire to assess the owners' impression on the overall treatment effect. To stimulate the owner to think how the dog would normally react to the fearful stimulus without treatment, categories with a clear reference to the expected and known reaction of the dog were linked to “previous year(s) without treatment”. Although this might help the owner to better understand the categories, it also introduces some inconsistency into the questionnaire. As treatment success was defined as reaching a meaningful benefit in the owner assessment of overall treatment effect and in the detailed owner assessment of behavioral responses using a modified version of the Lincoln Sound Sensitivity Scale, the results in the questionnaire appear reasonably robust.
The quality of life and well‐being of the dog are determined by the emotional and behavioral consequences of the fear and anxiety problem. In this study, the fear and anxiety scores at baseline were in the normative range in both treatment groups. Dogs receiving imepitoin treatment experienced an increase in fear and anxiety score at midnight during the highest intensity of firework exposure while showing clearly improved reactions at all time points compared to placebo. These might be considered acceptable “normal” fear responses that do not pose a practical threat to the animal's well‐being. Importantly, imepitoin‐treated dogs returned almost to their baseline score 1 hour after the highest intensity of fireworks (1‐hour time point). In comparison, placebo‐treated dogs showed increased fear and anxiety scores throughout New Year's Eve, including at the 1‐hour time point. Additionally, the majority of owners reported a good or excellent overall treatment effect in dogs treated with imepitoin, being in the same order of magnitude of owner‐reported treatment success for treatments like diazepam,21 dexmedetomidine19 or a combination of fluoxetine, diazepam, and behavior modification.22
In this study, ataxia was the most frequently reported adverse reaction. Drug‐induced ataxia is commonly associated with antiepileptics and benzodiazepines.23 Several studies provide evidence that certain subunits of the GABAA receptor are responsible for these effects and individual predisposition to transient ataxia is probably linked to certain point mutations.24, 25 In this study, there was a spontaneous resolution of ataxia with continuation of treatment, in most cases within 24 to 48 hours. Other less frequently reported mild and transient adverse events, such as increased appetite, lethargy, emesis, and hyperactivity, appear also to be related to imepitoin treatment. No withdrawal signs, drug tolerance, or serious adverse events were observed after this short treatment period, which were not to be expected based on previous data.8 The risks with imepitoin appear less than with benzodiazepines, which can cause, in addition to adverse events of sedation, ataxia, and increased appetite or agitation,21 a strong potential for drug dependency, development of tolerance, and exhibition of withdrawal signs with abrupt discontinuation.26 Another medication used for this condition, oromucosal dexmedetomidine is quite well tolerated in dogs at therapeutic doses with adverse events like sedation, vomiting, or urinary incontinence, but overdoses are associated with substantial risk to the animal in addition to the health risk for individuals administering the drug, necessitating the requirement for people to wear gloves administering the drug.27
A limitation of this study is the exclusion of dogs with a history of aggression problems. Although anxiety and fear are underlying causes in many cases of canine aggression problems and thus an anxiolytic treatment might be of benefit, these cases require a much more complex and individual approach preferably overseen by a specialized veterinarian. This is far beyond the standardized treatment scheme in this study, so further studies are required for this patient population.
The use of placebo in clinical trials for indications with an effective treatment is an area of some controversy, because applying placebo exposes dogs to potential distress.28 However, the use of placebo is the gold standard for ensuring accurate information on the real treatment benefit in the anticipated clinical use allowing an evidence‐based treatment decision. The use of a visually identical placebo within the exact same treatment scheme is particularly important in noise anxiety and fear problems as a high level of placebo response has been reported.18 Subjects were observed for a single event only and thus were not considered to be at risk of excessive harm. The use of dexmedetomidine oral gel as a comparator would have had methodological weaknesses as it requires additional handling and much more frequent dosing of the dogs, which would probably seriously reduce client compliance and necessitate a larger sample size. Consequently, the use of placebo is ethically justified in this study.
In conclusion, noise phobia is a common but under‐recognized problem of dogs that has relevant quality of life implications for the animal as well as its owner. Licensed treatment options are currently limited. In this study, we provide high‐quality evidence that imepitoin is effective for controlling the fear and anxiety associated with noise phobia in the majority of dogs. Imepitoin was well tolerated, and no serious adverse events were recorded. Even those owners who had dogs experiencing mild and generally transient adverse events such as ataxia considered treatment beneficial based on their willingness to continue treatment in most cases. Thus, imepitoin represents an apparently safe, simple, and effective option for the control of fear and anxiety associated with noise phobia in dogs.
CONFLICT OF INTEREST DECLARATION
This publication followed the GPP3 guidelines. O. Engle, R. Klee, B. Francke, and H‐W. Muller are employees of Boehringer Ingelheim Vetmedica GmbH, Germany, the marketing authorization holder of Pexion, containing imepitoin as active principle. O. Engle is holder of a patent related to imepitoin. D. Mills acted as consultant for Boehringer Ingelheim.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The conduct of the study was approved prior to study start by the relevant competent authorities in Germany and the Netherlands, as required by national legislation.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supporting Information.
ACKNOWLEDGMENT
The study was funded by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
The authors thank Jan Apelt (Essen, Germany), Jan Dolfing (Veldhoven, Netherlands), Joachim Dieter Fritz (Heilbronn, Germany), Tanja Golla (Düsseldorf, Germany), Marcus Hess (Haan, Germany), Patricia Kaulfuß (Mainz, Germany), Berthold Menzel (Recklinghausen, Germany), Frank Merkt (Esslingen, Germany), Paul Morssinkhof (Bergeijk, Netherlands), Ralf Nonnhoff (Hannover, Germany), Eva Schlensker (Köln, Germany), Friederike Schmidt (Peine, Germany), Susanne Schütterle (Alfter, Germany), Ralph M. Schuh (Mainz, Germany), Stefan Seifert (Bonn‐Beuel, Germany), Anja Sternberg (Hennef, Germany), Ralph‐Peter von Sturmberg (Köln‐Mülheim, Germany), Ischa Swartz (Amsterdam, Netherlands), Martin van der Weele (Oisterwijk, Netherlands), Gereon Winkler (Lohmar, Germany), and their teams for recruiting and assessing patients. In addition the authors thank Helge Bonaventura (Altenstadt, Germany) and Esther Herberich (Ingelheim, Germany) for the support in conducting the study.
Engel O, Müller HW, Klee R, Francke B, Mills DS. Effectiveness of imepitoin for the control of anxiety and fear associated with noise phobia in dogs. J Vet Intern Med. 2019;33:2675–2684. 10.1111/jvim.15608
Funding information Boehringer Ingelheim
REFERENCES
- 1. Blackwell EJ, Bradshaw JWS, Casey RA. Fear responses to noises in domestic dogs: prevalence, risk factors and co‐occurrence with other fear related behaviour. Appl Animal Behav Sci. 2013;145:15‐25. [Google Scholar]
- 2. Sherman BL, Mills DS. Canine anxieties and phobias: an update on separation anxiety and noise aversions. Vet Clin Small Anim Pract. 2008;38:1081‐1106. [DOI] [PubMed] [Google Scholar]
- 3. Overall KL. Clinical Behavioral Medicine for Small Animals. 1st ed St. Louis: Mosby; 1997:515. [Google Scholar]
- 4. Mills DS. Perspectives on assessing the emotional behavior of animals with behavior problems. Curr Opin Behav Sci. 2017;16:66‐72. [Google Scholar]
- 5. Karagiannis CI, Burman OH, Mills DS. Dogs with separation‐related problems show a "less pessimistic" cognitive bias during treatment with fluoxetine (reconcile) and a behaviour modification plan. BMC Vet Res. 2015;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mills DS. Medical paradigms for the study of problem behaviour: a critical review. Appl Anim Behav Sci. 2003;81:265‐277. [Google Scholar]
- 7. Skolnick P. Anxioselective anxiolytics: on a quest for the holy grail. Trends Pharmacol Sci. 2012;33:611‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rundfeldt C, Löscher W. The pharmacology of imepitoin: the first partial benzodiazepine receptor agonist developed for the treatment of epilepsy. CNS Drugs. 2014;28:29‐43. [DOI] [PubMed] [Google Scholar]
- 9. Engel O, Masic A, Landsberg G, et al. Imepitoin shows benzodiazepine‐like effects in models of anxiety. Front Pharmacol. 2018;9:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McPeake KJ, Mills DS. The use of imepitoin (Pexion) on fear and anxiety related problems in dogs ‐ a case series. BMC Vet Res. 2017;13:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mills DS, Braem Dube M, Zulch H. Stress and pheromonatherapy in small animal clinical behaviour. Vol 284 1st ed Chichester: Wiley‐Blackwell; 2013. [Google Scholar]
- 12. McPeake K, Affenzeller N, Mills D. Noise sensitivities in dogs: a new licensed treatment option. Vet Rec. 2017;180:353‐355. [DOI] [PubMed] [Google Scholar]
- 13. Storengen LM, Lingaas F. Noise sensitivity in 17 dog breeds: prevalence, breed risk andcorrelation with fear in other situations. Appl Anim Behav Sci. 2015;171:152‐160. [Google Scholar]
- 14. Schweizer E, Rickels K. Placebo response in generalized anxiety: its effect on the outcome of clinical trials. J Clin Psychiatry. 1997;58(Suppl 11):30‐38. [PubMed] [Google Scholar]
- 15. Dreschel NA. The effects of fear and anxiety on health and lifespan in pet dogs. Appl Anim Behav Sci. 2010;125:157‐162. [Google Scholar]
- 16. Siniscalchi M, McFarlane JR, Kauter KG, et al. Cortisol levels in hair reflect behavioural reactivity of dogs to acoustic stimuli. Res Vet Sci. 2013;94:49‐54. [DOI] [PubMed] [Google Scholar]
- 17. Rundfeldt C, Gasparic A, Wlaz P. Imepitoin as novel treatment option for canine idiopathic epilepsy: pharmacokinetics, distribution, and metabolism in dogs. J Vet Pharmacol Ther. 2014;37:421‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cracknell NR, Mills DS. A double‐blind placebo‐controlled study into the efficacy of a homeopathic remedy for fear of firework noises in the dog (Canis familiaris). Vet J. 2008;177:80‐88. [DOI] [PubMed] [Google Scholar]
- 19. Korpivaara M, Laapas K, Huhtinen M, Schöning B, Overall K. Dexmedetomidine oromucosal gel for noiseassociated acute anxiety and fear in dogs—a randomised, double‐blind, placebo‐controlled clinical study. Vet Rec. 2017;180:356. [DOI] [PubMed] [Google Scholar]
- 20. Tiira K, Lohi H. Reliability and validity of a questionnaire survey in canine anxiety research. Appl Anim Behav Sci. 2014;155:82‐92. [Google Scholar]
- 21. Herron ME, Shofer FS, Reisner IR. Retrospective evaluation of the effects of diazepam in dogs with anxiety‐related behavior problems. J Am Vet Med Assoc. 2008;233:1420‐1424. [DOI] [PubMed] [Google Scholar]
- 22. Ibanez M, Anzola B. Use of fluoxetine, diazepam, and behavior modification as therapy for treatment of anxiety‐related disorders in dogs. J Vet Behav. 2009;4:223‐229. [Google Scholar]
- 23. Van Gaalen J, Kerstens FG, Maas RPPWM, et al. Drug‐induced cerebellar ataxia: a systematic review. CNS Drugs. 2014;28:1139‐1153. [DOI] [PubMed] [Google Scholar]
- 24. Korpi ER, Kleingoor C, Kettenmann H, et al. Benzodiazepine‐induced motor impairment linked to point mutation in cerebellar GABAA receptor. Nat Review Drug Discov. 1993;361:356‐359. [DOI] [PubMed] [Google Scholar]
- 25. Milic M, Divljakovic J, Rallapalli S, et al. The role of a1 and a5 subunit‐containing GABAA receptors in motor impairment induced by benzodiazepines in rats. Behav Pharmacol. 2012;23:191‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loscher W, Honack D, Fassbender CP. Physical dependence on diazepam in the dog: precipitation of different abstinence syndromes by the benzodiazepine receptor antagonists Ro 15‐1788 and ZK 93426. Br J Pharmacol. 1989;97:843‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Food and Drug Administration (FDA) . Freedom of Information Summary: NADA 141–456 Sileo (dexmedetomidien oromucsal gel). In: Medicine CfV, ed. 2015.
- 28. Millum J, Grady C. The ethics of placebo‐controlled trials: methodological justifications. Contemp Clin Trials. 2013;36:510‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.


