Abstract
Pancreatic invasive ductal adenocarcinoma (PDAC) is a representative intractable malignancy under the current cancer therapies, and is considered a scirrhous carcinoma because it develops dense stroma. Both PODXL1, a member of CD34 family molecules, and C5aR, a critical cell motility inducer, have gained recent attention, as their expression was reported to correlate with poor prognosis for patients with diverse origins including PDAC; however, previous studies reported independently on their respective biological significance. Here we demonstrate that PODXL1 is essential for metastasis of PDAC cells through its specific interaction with C5aR. In vitro assay demonstrated that PODXL1 bound to C5aR, which stabilized C5aR protein and recruited it to cancer cell plasma membranes to receive C5a, an inflammatory chemoattractant factor. PODXL1 knockout in PDAC cells abrogated their metastatic property in vivo, emulating the liver metastatic mouse model treated with anti-C5a neutralizing antibody. In molecular studies, PODXL1 triggered EMT on PDAC cells in response to stimulation by C5a, corroborating PODXL1 involvement in PDAC cellular invasive properties via specific interaction with the C5aR/C5a axis. Confirming the molecular assays, histological examination showed coexpression of PODXL1 and C5aR at the invasive front of primary cancer nests as well as in liver metastatic foci of PDAC both in the mouse metastasis model and patient tissues. Hence, the novel direct interaction between PODXL1 and the C5aR/C5a axis may provide a better integrated understanding of PDAC biological characteristics including its tumor microenvironment factors.
Abbreviations: PODXL1, Podocalyxin-like 1; PDAC, pancreatic invasive ductal adenocarcinoma; C5aR, Complement component 5a receptor 1 (C5aR1, CD88); CAF, cancer-associated fibroblast; EMT, epithelial-mesenchymal transition; iPS, induced pluripotent stem; ITGB1, Integrin β1; WT, wild type; KO, knockout; IHC, immunohistochemistry; IB, immunoblot; IP, immunoprecipitation; IF, immunofluorescence; HPNE, human immortalized pancreatic ductal epithelium; NHDF, normal human dermal fibroblast; MMP, matrix metalloproteinases; Ab, antibody
Introduction
Invasive ductal adenocarcinoma of the pancreas (PDAC) is the most common pancreatic malignancy and has been recently highlighted as a serious issue for cancer treatment, given persistent clinical intractability to known therapeutic countermeasures aimed at improving patient prognosis [1], [2]. PDAC shows unique histology, where proliferating cancer nests are surrounded by abundant collagenous stroma with cancer-associated fibroblasts (CAFs), inflammatory cells such as macrophages and lymphocytes, and scanty tumor blood vessels from neovascularization. These stromal components form a so called “tumor microenvironment” essential to cancer progression in vivo. Hence, tumor microenvironments are complex systems of various cellular components, observed to release diverse inflammatory chemokines that interact with cancer cells resulting in tumor growth, invasion and metastasis.
Podocalyxin-like 1 (PODXL1), is an anti-adhesive sialomucin, belonging to a member of the CD34 family harboring the extracellular domain and intracellular domain via the transmembrane portion. It is expressed by normal vascular endothelium, renal podocyte, and hematopoietic progenitors [3], [4], [5] and has been reported to be a stem cell marker including embryonic stem cells [6], [7]. Noticeably, there have been studies demonstrating correlation between PODXL1 expression and poor prognosis of patients with cancer of the diverse origins including colorectal cancer [8], gastric cancer [9], and pancreatic cancer [10]. Functionally, PODXL1 was reported to promote invadepodia formation and metastasis through Rac1/Cdc42/cortactin signaling in breast cancer cells [11], to induce collective migration of MCF-7 cells depending on activity of the actin scaffolding cytoplasmic protein ezrin [12]. Recently, it was revealed that PODXL1 was involved in efficient EMT extravasating via the Ezrin signaling on breast cancer and pancreatic cancer cells [13]. Hence, PODXL1 appears critically involved in causing invasion and metastasis of various cancers and is one of the most important regulators of human malignancies; however, the diverse biological roles of PODXL1 still require elucidation for generating future cancer therapeutics. On the other hand, multiple chemokines have been reported as produced in tumor microenvironment in response to inflammatory reaction and tumor immunity against cancer cells. Among them, C5a, a soluble complement which is related to inflammatory reaction and chemotactic response, has been reported to promote tumor development and progression including cellular motility and invasion, and its expression was related to poor prognosis in PDAC patients, on account of interaction with G-protein-coupled complement receptor, C5a receptor (C5aR; CD88) [14], [15], [16], [17]. Thus the C5a-C5aR interaction in cancer stroma has been recently highlighted as a key accelerator of cancer metastasis in tumors with diverse origins, such as breast, lung and gastric cancers [14], [15], [16], [17].
Here, we demonstrated the novel function of PODXL1 capture of a key metastatic regulator, C5aR, in the PDAC microenvironment, to stabilize and activate it as specific binding partner, triggering PDAC invasion and metastasis by accelerating tumor cell motility through activation of the C5aR/C5a axis.
Materials and methods
Cell culture and antibodies
Human PDAC lines, BxPC3 (ATCC: CRL-1687), AsPC-1 (ATCC: CRL-1682), MiaPaCa-2 (ATCC: CRM-CRL-1420), Panc-1 (ATCC: CRL-1469), an immortalized non-neopastic pancreatic ductal cell, HPNE (ATCC: CRL-4023), normal human dermal fibroblast, NHDF (ATCC: PCS-201-010) were grown in RPMI-1640 (Wako, Tokyo, Japan) supplemented with 10% of fetal bovine serum (FBS) in a 5% CO2 incubator. Authentication of each cell line was attached. Antibodies employed in this study were as follows. As a primary antibody, anti-PODXL1 (Sigma-Aldrich, Merk, St. Louis, MO), anti-Integrin β1 (Invitrogen, Thermo Fisher Scientific KK, Japan), anti-C5aR (CD88) (Hycult Biotech, Wayne, PA), anti-C5a (R&D Systems, Mineapollice, MN), anti-Myc-tag (Sigma-Aldrich), anti-FLAG-tag (Invitrogen, Thermo Fisher Scientific KK), anti-Tubulin (CST Japan, Tokyo, Japan), anti-E-Cadherin (Invitrogen, Thermo Fisher Scientific KK), anti-Snail (CST Japan), anti-Vimentin (CST Japan), anti-TRA-1-60 (CST Japan). As a secondary antibody, Alexa488-conjugated goat anti-mouse IgG(H + L), Alexa488-goat anti-mouse IgM, Alexa555-goat anti-mouse IgG(H + L), Alexa488-goat anti-rabbit IgG(H + L), Alexa555-goat anti-rabbit IgG(H + L), Alexa555-donkey anti-goat IgG(H + L), HRP-conjugated goat anti-mouse IgG(H + L), HRP-goat anti-rabbit IgG(H + L) (for all of these: Invitrogen, Thermo Fisher Scientific KK) were used for the immunohistochemistry, immunofluorescence and immunoblot assays.
Patients tissues, immunohistochemistry and immunofluorescence
Paraffin-embedded surgical specimens fixed with 10% buffered-formalin and made from the patient tissues of the pancreatic invasive ductal adenocarcinoma were obtained from the department of pathology, Niigata Cancer Center Hospital under approval by the ethics committees of Niigata University and Niigata Cancer Center. Ten cases of primary PDAC tissues (well differentiated; 3 cases, moderately differentiated; 4 case, poorly differentiated; 3 cases) and four cases of liver metastasis of the corresponding PDAC from the same individual among these ten patients were examined for expression for the proteins of the interest by immunohistochemistry and immunofluorescence, using anti-PODXL1, anti-C5aR, and anti-C5a, anti-TRA-1-60 antibodies. All specimens were autoclaved prior to incubation with the primary antibody (at 4 celcius for 12hours) for immunohistochemistry or immunofluorescence.
Plasmid construction for a panel assay of the chemokine receptors
For the panel screening of chemokine receptors as a binding partner of PODXL1, the plasmids encoding, in total, forty human chemokine receptor were constructed, respectively. These were contransfected with human PODXL1 and subjected to immunoprecipitation assay. In detail, for the expressor plasmid construction, cDNAs of interest were subcloned into the pIDT-SMART (C-TSC) vector to obtain efficient gene expression in a transient manner [18]. The prepared cDNAs covering the entire ORF were as follows: human cDNAs encoding PODXL1 (transcript variant 1; GenBank NM_001018111) and chemokine receptors (C3aR, C5aR, CXCR1-6, CX3CR1, XCR1, CCR1-11, BLT1, 2, LTR1,2, EP1-4, DP1,2, FP, IP, TP, and LPAR1-6). PODXL1 was designed to express as a C-terminal 3xMyc-6xHis-tagged form. Chemokine receptors were all designed to express as C-terminal 3xFlag-6xHis-tagged forms.
Immunoblot and immunoprecipitation
All immunoblot analyses were performed with the cell lysates prepared with M-PER mammalian protein extraction reagent (Thermo Fisher Scientific) under standard conditions using each of the specific antibodies mentioned above. For the co-immunoprecipitation assay, HEK293T cells were transiently transfected with the PODXL1 expressor (PODXL1 with 3xMyc-6xHis-tagged at the C-terminal end). Chemokine receptors were all designed to express as C-terminal 3xFlag-6xHis-tagged forms. Combined with a series of chemokine receptor-expressors mentioned above using FuGENE-HD (Promega), respectively. Twenty-four hours post-cotransfection, cell pellets were harvested and lysed in M-PER buffer. The prepared lysates were then incubated with agarose beads conjugated with monoclonal anti-Myc tag antibody (MBL, Nagoya, Japan). The resultant immunoprecipitates were subjected to immunoblot probed with anti-Flag tag antibody. To examine direct binding between PODXL1 and C5aR, PODXL1 was purified from the cell lysate of the PODXL1-HA(x3)-His(x6)-tagged expressor-transfected HEK293T cells using anti-His-tag antibody-conjugated beads (#D291-8; anti-His-tag mAB-agarose beads; MBL Co. Ltd., Japan). C5aR protein was also isolated from the lysate of the C5aR-Flag(x3)-His(x6)-tagged expressor-transfected 293 T cells using anti-His-tag mAb beads. Each protein was mixed together, incubated for 30 min at RT, then the sample was precipitated using beads conjugated with anti-HA-tag antibody (#561-8; anti-HA-tag pAb-agarose beads; MBL Co. Ltd., Japan) for pull-down of PODXL1. The pull-down sample was subjected to SDS-PAGE, blotted onto membrane after fractioned, and immunoblotting with anti-HA antibody (#3724; HA-Tag C29F4; Cell Signaling Technology, Danvers, MA) or with anti-Flag antibody (#F1804; anti-Flag M2 mAb; Sigma Aldrich Japan, Tokyo, Japan) was performed, respectively. As a control, purified PODXL1-HA-His alone and PODXL1-HA-His incubated with heat-degraded C5aR-Flag-His protein were employed.
Generation of PODXL1−/− clones by the CRISPR/Cas9 system
PODXL1-knockout PDAC cell lines (for MIaPaCa-2, AsPC-1, and Panc-1) were generated using CRISPR/Cas9 system. Both plasmids, hCas9 (#41815 Addgene, Watertown, MA), and gRNA (guide RNA) Cloning Vector (#41824), were obtained from Addgene. The gRNA vector including PODXL1 target sequence (CGACACGATGCGCTGCGCGCtgg) located in part of the Exon 1 was prepared following the manufacturer’s instruction with “tgg” sequence as a Proto-spacer Adjacent Motif (PAM). The hCas9 and PODXL1 gRNA vector were cotransfected into cells using ViaFect™ Transfection Reagent (#E4981, Promega, Madison, WI). Twenty-four hours posttransfection, the cells were cultured with RPMI medium containing 500 μg/ml of Geneticin (#10131-35, Gibco, Thermo Fisher Scientific, Waltham, MA) for isolating the Geneticin-resistant clones. PODXL1-expression deficient clones from each PDAC line were confirmed by lack of PODXL1 protein, using immunoblot analysis with anti-PODXL1 antibody. Genetic mutation of PODXL1 in the knockout clone was also examined by genomic DNA sequencing of PCR-amplified product, using the specific primers for PODXL1-target sequence and the adjacent genomic DNA. The primers used for the sequencing were the following: Pod1.Ex1.check.Fw2: 5′-CAGCGGCAGGGAGGAAGAGC and Pod1.Ex1.check.Rv2 5′-GCGGTGCGGTCTCCCTTTTCTT.
Image based protein–protein interaction (PPI) analysis (Fluoppi assay)
The protein-protein interaction between PODXL1 and candidate proteins (EP4, CXCR2, C5AR, LTB4R, CTTN and CXCR1) was detected using the FluoPPI system (MBL, Nagoya, Japan), in which PPIs were visualized as fluorescent foci [19], [20]. PODXL1 was subcloned into a pAsh-MNL ver.2 plasmid to fuse with an Ash (homo oligomerized protein assembly helper) tag (PODXL1-Ash). Candidates were subcloned into a pMonti-Red-MNL plasmid to fuse with a Monti-Red (homo tetramer red fluorescent protein from Montipora sp.) tag (CTNN-red or C5aR-red). These plasmids of PODXL1-Ash and candidates-Red were co-transfected into HeLa cells using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions, respectively, and intracellular fluorescent foci with red color were observed by inverted fluorescence microscope (Olympus IX71, Olympus Co. Tokyo, Japan) and detected as an image using cellSens imaging software (Olympus Co.).
Invasion assay
Cells were starved by serum free medium for 24 h prior to invasion and migration assays. Cell invasion and migration assays were both evaluated by the Boyden chamber method using the filter inserts (pore size, 8 μm), pre-coated (for invasion assay) or not pre-coated (for migration assay) with Matrigel in 24-well plates (BD Biosciences, Franklin Lakes, NJ, USA). Each pancreatic cancer cell line (7.5 × 104 cells/insert for MIA PaCa-2, 2 × 105 cells/insert for AsPC-1, or 4 × 104 cells/insert for PANC-1) was seeded on the top chamber. The top chamber was filled with serum-free medium and the bottom chamber was filled with 10% FBS medium. The recombinant chemokines were then set in the bottom chamber at a final concentration of 25 ng/ml for CXCL8 (PeproTech, Rocky Hill, NJ, USA), and 0.5 μM for PGE2, 5 ng/mL for CX3CL1 (R&D Systems, Minneapolis, MN, USA) and C5a (ProSpec-Tany Technology Ltd, Ness Ziona, Israel). After incubation for 8 h in the case of PANC-1, or 48 h in the case of MIA PaCa-2 and AsPC-1cells, cells that passed through the filter and were attached to the lower surface of the filter were counted by staining with 0.01% crystal violet in 25% methanol. Cells attached to the lower surface of the filter membrane were then quantified by cell counting in five non-overlapping fields at × 100 magnification.
RNA interference assay using siRNA
Pre-designed siRNAs for human C5aR (ID D-005442-00-005) and control siRNA (ID D-001810-10-05) were purchased from Dharmacon (Lafayette, Colorado, USA). siRNAs (final concentration 50 nM) were transfected using Lipofectamin RNAiMAX reagent (Thermo Fisher Scientific). Forty-eight hours post-introduction of each siRNA, the cells were subjected to the invasion assay described above.
In vivo mouse liver metastasis model
1 × 106 cells of MiaPaCa-2, AsPC-1, or Panc-1 were injected into 6 week-old nude mouse spleen exteriorized through a left flank incision, respectively, followed by splenectomy 2 min later. The same number of the PODXL1−/− PDAC cells corresponding to each cell line were injected in the same way with the same timing. Approximately 30 days post-injection, the mice grew metastatic liver tumors derived from the injected PDAC cells, and they were then autopsied and compared for tumor formation pairwise with the mice implanted with PODXL1−/− cells under the stereo microscope. Total number of mice examined in each group was four (n = 4). Only in the case of Panc-1 splenic xenograft, number of mouse examined was two (n = 2). In the C5a blocking assay, each mouse with splenic injection of 1 × 106 of MiaPaCa-2 cells was treated with intravenously administrated 50 μg/mouse of anti-C5a neutralizing antibody on Day 0 (the day of cell-implant), Day 7, Day 14 (in total three times, and once every week), and autopsied and examined on Day 30 pairwise with mice (control group) treated with the same dose of goat-IgG. Totally, four mice (n = 4) were examined in each group. Excised tumors in all animal experiments were histologically examined with paraffin-embedded specimens by HE staining and immunohistochemistry using anti-human cytokeratin, CAM5.2 (BD Biosciences, San Jose, CA) to demonstrate human PDAC cells (MiaPaCa-2, AsPC-1, and Panc-1, respectively) origin.
Statistical analysis of metastatic tumors in mouse models
Based on macroscopic finding in combination with microscopic examination of the tissue specimens, number of liver metastasis was counted in each group of mouse splenically injected with MiaPaCa-2, AsPC-1 in every animal experiment. Average number of metastasis per liver in each case was statistically evaluated by t-test (shown as graph with P value).
Results
Characteristic expression of PODXL1 on human PDAC tissues
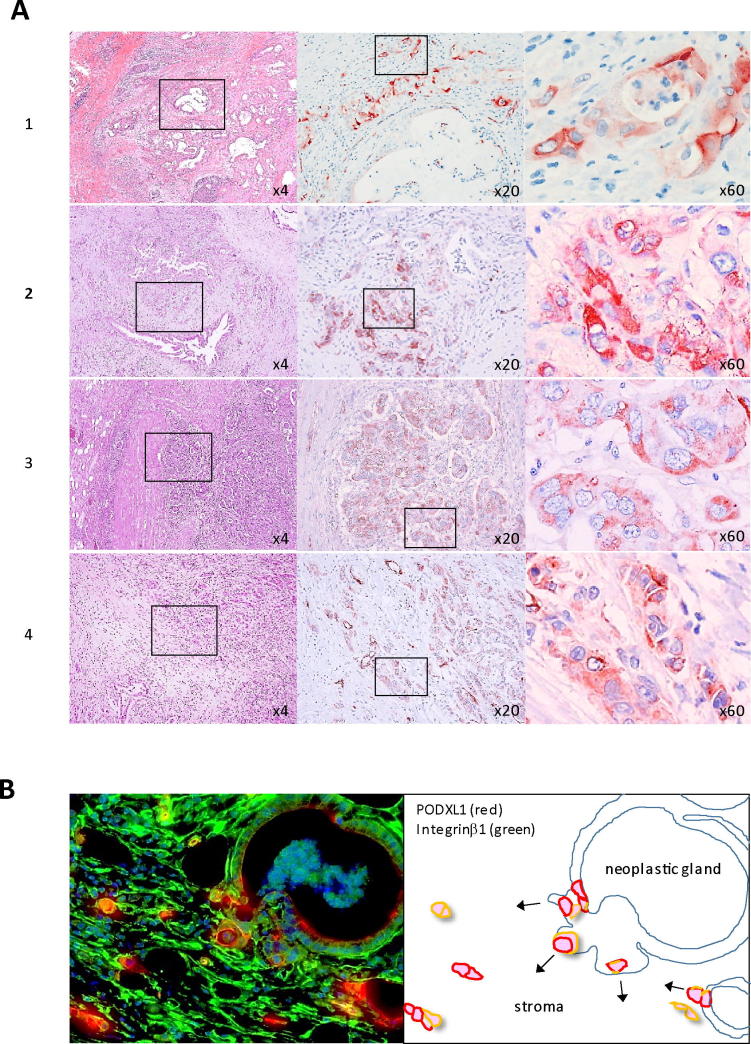
PODXL1 expression on PDAC tissues has been reported in previous studies that demonstrated PODXL1 preferentially expressed on the cancer nests in comparison with the non-neoplastic pancreatic acinus and duct, with the expression correlating to the patient’s poor prognosis [21]. Immunohistochemistry on representative primary PDAC patient tissues using anti-PODXL1 antibody revealed that strong membranous PODXL1 expression with or without cytoplasmic expression was observed mainly at the small collective cell forming-cancer nests at the invasive front of the PDAC tumor in examined cases (1; well differentiated type, 2,3; moderately differentiated type, 4; poorly differentiated type, respectively) (Figure 1A), but a small number of strong PODXL1-positive cancer cells were observed among the individual tumor glands adjacent to the small invasive nests (Supplementary Figure S1A). PODXL1 expression was not dependent on the differentiation type of PDAC but was detected in all types examined. It has been also reported that the glycosylated form of PODXL1 was recognized as TRA-1-60 antigen [22], an embryonic stem cell and iPS cell marker. TRA-1-60 expression was detected in similar patterns to that of PODXL1, where TRA-1-60 was strongly positive in small cancer nests at invasive foci in PDAC patient tissues under immunohistochemistry (Supplementary Figure S1B, upper panel) Immunofluorescence using anti-PODXL1 and anti-ITGB1 (Integrin β 1, CD29) antibodies highlighted the budding tumor cell from the neoplastic gland acquiring strong expression of PODXL1 as well as ITGB1, indicating PODXL1 might be required for epithelial-mesenchymal transition (EMT) of the PDAC cells (Figure 1B and Supplementary Figure S1B, lower panel). Accordingly, the budding single PDAC cell was also detected by immunofluorescence using TRA-1-60 antibody (Supplementary Figure S1B, lower panel). The robust expression of PODXL1 was also observed not only in PDAC but also various cancers, as for example, its expression on invasive nests of colorectal tubular adenocarcinomas (Supplementary Figure S1C).
Figure 1.
Expression of PODXL1 on human PDAC tissues from the patients. (A) IHC using anti-PODXL1 Ab on well differentiated type (1), moderately differentiated type (2, 3), and poorly differentiated type PDAC (4). Hatched box indicates the area for hyperview in each case (×4, ×20, ×60). (B) Double IF using anti-PODXL1 Ab (red) and ITGB1 (green) (left panel). Schematic representation of the PODXL1-expressing budding cancer cells from the tumor gland were highlighted.
PODXL1 is critically involved in metastasis of PDAC cells in vivo
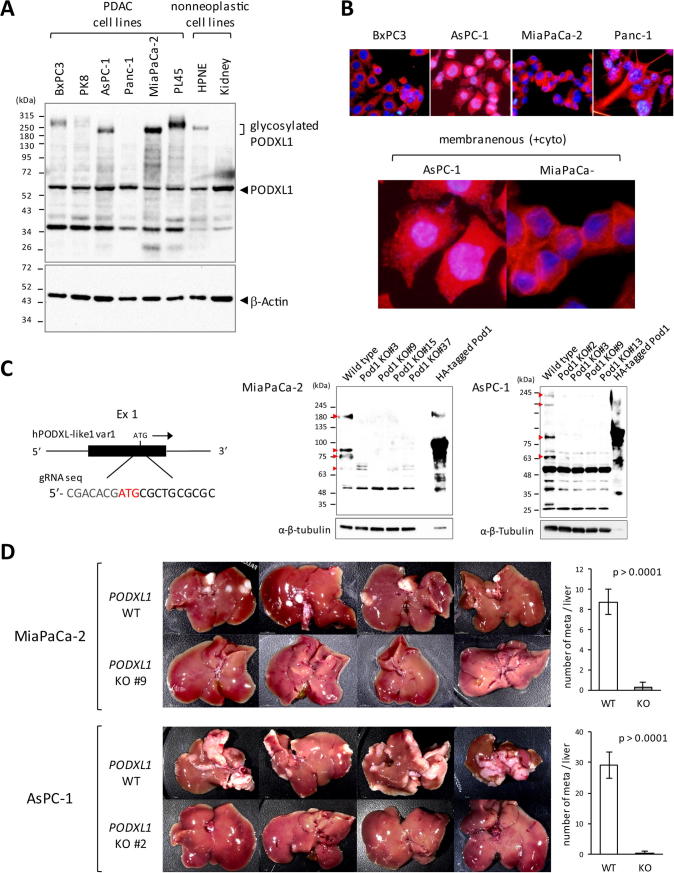
To elucidate the biological role of PODXL1 in PDAC cells in vivo, generation of PODXL1-targeted PDAC cell clones was achieved by using the CRISPR/Cas-9 genome editing system. First, endogenous expression of PODXL1 was examined in four different human PDAC lines established from different patients. As non-neoplastic counterparts, human immortalized pancreatic ductal epithelium (HPNE) and normal kidney epithelial cells were also examined for its expression. Consequently, all four PDAC lines showed strong expression of PODXL1 including the supershifted (hyperglycosylated) form (Figure 2A), and HPNE showed comparably weak PODXL1 expression with a similar pattern, but the glycosylated form was not detected in kidney cells (Figure 2A). These PDAC lines showed membranous expression of PODXL1 as well as its cytoplasmic expression (Figure 2B, upper panel), representing lamellipodia and plasma membrane localization in AsPC-1 and MiaPaCa-2 cells (Figure 2B, lower panel). Based on this finding, genome editing to knockout PODXL1, including variant 1 and 2, was performed using the CRISPR/Cas-9 system with the designed guideRNA (gRNA) recognizing the part of the exon 1 both on MiaPaCa-2 and AsPC-1, respectively (Figure 2C, left panel). Four respective PODXL1−/− (PODXL1-KO) clones were obtained both in MiaPaCa-2 (clone #3, 9, 15, 37) and AsPC-1 (clone #2, 3, 9, 13), all of which were confirmed as to specific impairment of PODXL1, utilizing immunoblots and genome sequencing (Figure 2C, right panel and Supplementary Figure S2A). Using these knockout (KO) clones, the murine pancreatic tumor model of liver metastases was established by hemispleen injection of the MiaPaCa-2 cells lacking PODXL1 expression (KO clone #9) and AsPC-1 PODXL1-KO cells (KO clone #2), and by using the wild type MiaPaCa-2 and AsPC-1 cells for comparison, respectively. After 30 days post-injection, the wild type MiaPaCa-2-implanted mice developed multiple metastatic foci in the liver, whereas no metastatic foci were observed in the mice implanted with the PODXL1-KO #9 clone (Figure 2D, upper panel). Similarly, loss of liver metastasis was observed in mice injected with the AsPC-1 PODXL1-KO #2 clone in contrast to the cases with the wild type cell injection (Figure 2D, lower panel). Number of the intrahepatic metastasis per liver between group of implanted PDAC cells bearing PODXL1 wild type and those of PODXL1-knockout (n = 4 in each group) was summarized in graph which showed statistical significance (Figure 2D). Similarly, loss of mesenteric lymph nodal metastasis was also observed in the mice treated with the KO clone of MiaPaCa-2 (n = 4 in each group, Supplementary Figure S2B), and the PODXL1-KO clone of Panc-1 PDAC cell showed the similar phenomenon (Supplementary Figure S2C). Histological examination of the intrahepatic and lymph nodal metastatic tumors demonstrated that metastatic tumors were derived from hemisplenically injected human PDAC cells in the mice treated with tumor cells bearing PODXL1 wild type, and no micrometastatic lesion was observed even microscopically in the liver of the mice injected with tumor cells harboring PODXL1-knockout (Supplementary Figure S3A, 3B, and 3C).
Figure 2.
Loss of liver metastasis in the PODXL1-deficient PDAC cells in vivo. (A) endogenous PODXL1 expression in six PDAC lines (BxPC3, PK-8, Panc-1, MiaPaCa-2 and PL45), HPNE and kidney epithelial cells as a non-neoplastic by IB using anti-PODXL1 Ab. Note the glycosylated form of PODXL1 showed as supershifted. (B) Expression of PODXL1 detected by IF. Hyperview of AsPC-1 and MiaPaCa-2 showed expression of PODXL1 at the surface membrane as well as at the cytoplasm. (C) Schematic representation of the guide RNA design for the knockout, recognizing a part of the exon 3 (black box) within the PODXL1 genome (left). Confirmation of the KO clones of MiaPaCa-2 (#3, #9, #15, #37) and AsPC-1(#2, #3, #9, #13) by IB using anti-PODXL1 Ab. The 293T lysate transfected with HA-tagged ful-length PODXL1 variant 1 was used as a positive control. α-β-tubulin indicated the IB using anti-total tubulin Ab. (D) Development of liver metastasis 30 days after splenic injection of the PODXL1-WT or the KO cells in Balb/c nu/nu mice (n = 4 in each group). Attached Graphs showed average number of liver metastasis/mouse in MiaPaCa-2-injected or AsPC-1-injected mice (n = 4/each WT or KO group, means and s.d. of quadruplicate were shown).
PODXL1 captures diverse chemokine receptors including C5aR
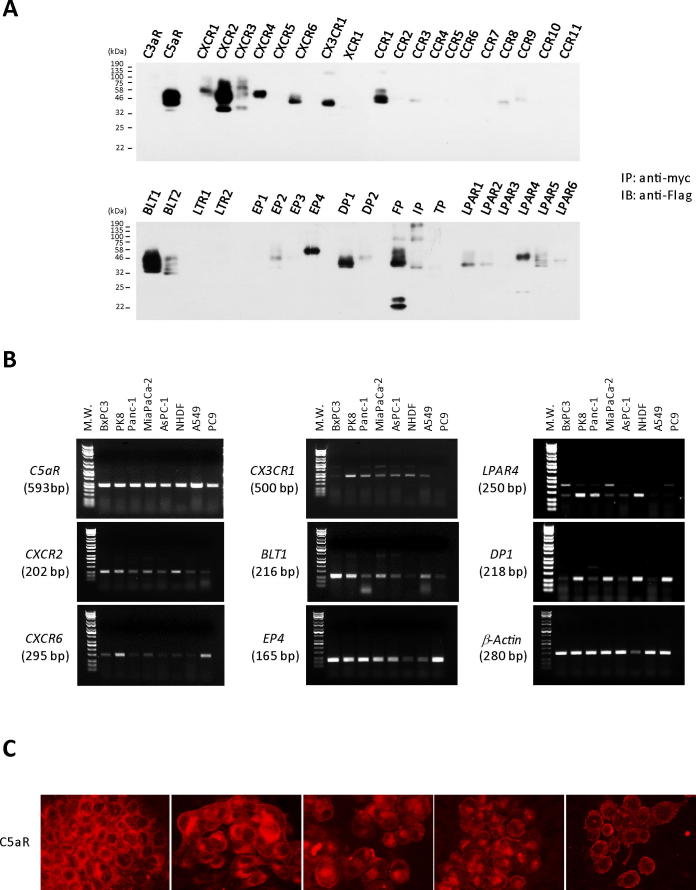
The next issue determined was how PODXL1 is critically involved in PDAC invasion/metastasis. Recently, the tumor microenvironment has received much attention because many current studies reveal its significant impact on cancer progression in terms of tumor growth, invasion and metastasis. The tumor microenvironment is a complex system comprised of fibrous stroma, tumor angiogenesis, tumor immunity and inflammatory reaction. Among these, we focused on chemokine receptors which affect the chemotactic cellular motility through interaction with soluble inflammatory chemotactic ligands in the tumor microenvironment. A panel screening for over forty chemokines was performed to find the specific association between PODXL1 and these molecules by employing immunoprecipitation. The results showed that PODXL1 bound multiple chemokine receptors such as C5aR (C5aR1), CXCR1, CXCR2, CXCR3, CXCR4, CXCR6, CX3CR1, CCR1, CCR3, CCR8, CCR9, BLT1, BLT2, EP2, EP4, DP1, DP2, FP, IP, LPAR1, LPAR2, LPAR4, LPAR5, and LPAR6 (Figure 3A). Among these, we further examined expression of selected chemokine genes semi-quantitatively by RT-PCR, which revealed enhanced coupling to PODXL1. Essentially all selected chemokines were more or less expressed on PDAC cells and C5aR receptor was the strongest and most consistently expressed on them (Figure 3B). Prominent membranous C5aR expression was detected on all five representative PDAC lines, BxPC3, PK8, Panc-1, MiaPaCa-2, and AsPC-1 (Figure 3C).
Figure 3.
PODXL1 captures multiple chemokine receptors. (A) Panel screening of the forty chemokine receptors as a binding partner to PODXL1. Pull-down assay (IP) using anti-myc tag Ab for the lysates from the myc-tagged PODXL1-cotransfected 293T with each Flag-tagged chemokine receptor gene detected the specific binding partner to PODXL1. IB was performed by probing with anti-Flag Ab for the precipitates. (B) Expression of the each chemokine receptor that showed binding affinity to PODXL1 in Figure 3a was examined by RT-PCR among five PDAC lines, NHDF (fibroblast), A549 and PC9 (both lung adenocarcinoma lines). (C) IF using anti-C5aR Ab revealed membranous expression of C5aR on PDAC cells.
PODXL1 accelerates cellular motility by regulating functional localization and stabilizing C5aR, and triggers EMT via the C5aR/C5a axis on PDAC cells
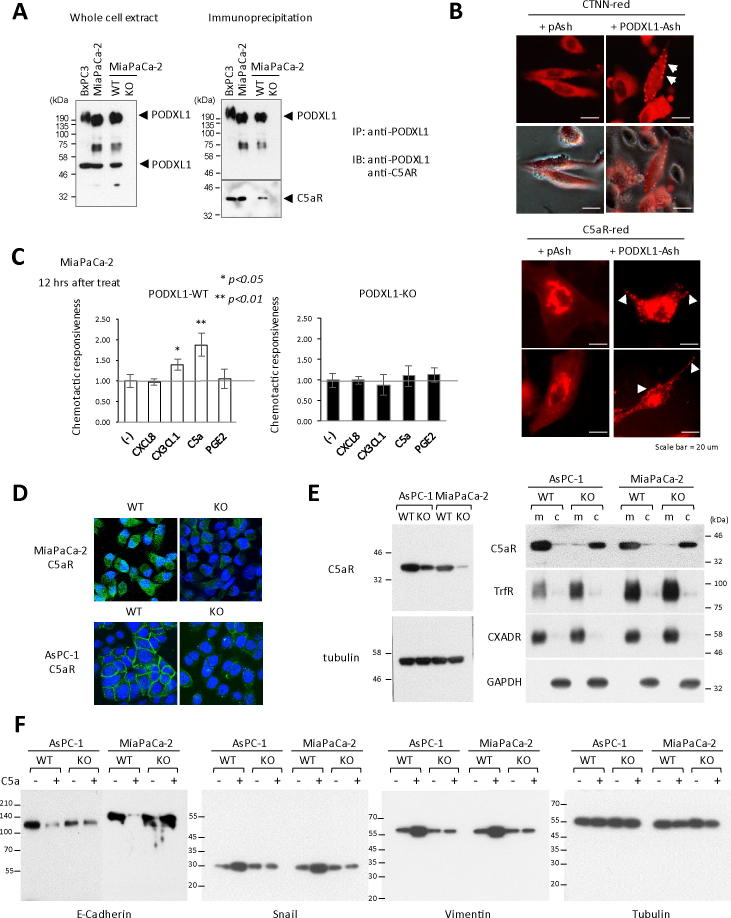
Based on these results, the biological role for protein coupling of PODXL1 and C5aR was investigated on PDAC cells bearing the wild type PODXL1, compared with the PODXL1-KO cells. Confirmed specific binding of PODXL1 to C5aR was observed only in wild type PODXL1 (PODXL1-WT) MIaPaCa-2 cells but not in the PODXL1-KO cells (Figure 4A); intracellular localization of C5aR was examined with or without PODXL1 as to whether or not this would be affected by the presence of PODXL1. In image based protein–protein interaction (PPI) analysis, the so called “Fluoppi” system, aggregated fluorescent dots appears only when two proteins of the interest directly binds to each other. Based on the result of Fluoppi analysis using HeLa cells, C5aR was successfully recruited to lamellipodia at the cell membrane on the PODXL1-cotransfected cells which was recognized as aggregated red dots, as in the case of Cortactin (CTNN)-PODXL1 cotransfected cells (Figure 4B and Supplementary Figure S4A). On the other hand, it was still retained at the cytoplasm without PODXL1 expression (only with pAsh) (Figure 4B). Other chemokine receptors, such as EP4 and CX3CR1 seen as candidate binding partner to PODXL1, also displayed changes in intracellular localization to their functional site in the presence of PODXL1 (Supplementary Figure S4B). Thus, PODXL1 appeared essential for targeting C5aR to its functional site by cellular assay. Direct coupling between PODXL1 and C5aR was also demonstrated by pull-down assay using anti-HA-conjugated beads to isolate binding partner of 3xHA-6xHis-tagged PODXL1 protein (Supplementary Figure S4C). Based on the immunoprecipitation assay results – establishing the diverse chemokine receptors as binding partner with PODXL1 – CXCL8 (ligand to CXCR2), CX3CL1 (ligand to CX3CR1), C5a (ligand to C5aR), and PEG2 (ligand to EP4), all of which showed augmented association, were incubated with MiaPaCa-2 cells and quantified for the increase in cellular motility, using transwell-chamber assay (Boyden chamber assay), between the PODXL1-WT and the PODXL1-KO cells. The data indicated that the ligand stimulation by C5a robustly increased PODXL1-WT MiaPaCa-2 cellular motility in comparison with that of C5a-stimulated PODXL1-KO clone (Figure 4C). This phenomenon was specific because the MiaPaCa-2 cells treated with C5aR siRNA markedly decreased the cellular motility even when incubating with C5a (Supplementary Figure S4A). The response to C5a seemed to be common to the other PDAC cells such as AsPC-1 and Panc-1 (Supplementary Figure S4B). We found that another feature of PODXL1-C5aR interaction was the pronounced attenuation of C5aR expression in the PODXL1-KO PDAC cells. In the immunofluorescence results, C5aR expression was much weaker in PODXL1-KO MiaPaCa-2 and AsPC-1 in contrast to the PODXL1-WT cells, and we made similar observations for PODXL1-deficient Panc-1 cells (Figure 4D and Supplementary Figure S6A). Total protein for C5aR was examined by immunoblot using anti-C5aR antibody, and its comparative reduction in the PODXL1-deficient cell was corroborated, using whole cell lysates, between the WT cells and the KO cells, in MiaPaCa-2 and AsPC-1, respectively (Figure 4E, left panel). Furthermore, immunoblot of the cellular fraction revealed that the majority of C5aR was detected in the membrane fraction of PODXL1-WT AsPC-1 and MiaPaCa-2 cells, respectively, while in the PODXL1-KO cells the majority of C5aR was contained in the cytosol fraction (Figure 4E, right panel). These results implied that lack of PODXL1 might impair the protein stability and the functional localization of C5aR in PDAC cells. Given the aggregate findings, we examined whether the PODXL1-C5aR interaction was involved in EMT of PDAC cells. Total cell lysates were prepared from each PDAC cell sample with or without C5a stimulation for 12 hours in the PODXL1-WT and the KO phenotype of AsPC-1 and MiaPaCa-2. The sample from the C5a-treated PODXL1-WT cells showed marked reduction of E-Cadherin in contrast to an increase in Snail and Vimentin, whereas the KO phenotype displayed no significant quantitative fluctuations of these proteins (Figure 4F). The PODXL1-C5aR/C5a axis was therefore demonstrated to be one of the critical pathways for triggering EMT of the PDAC cells.
Figure 4.
PODXL1 accelerates PDAC cellular motility by activating C5aR with triggering EMT. (A) IP assay demonstrated PODXL1 specifically bound to C5aR. Endogenous expression of PODXL1 in the PODXL1-WT and the KO MiaPaCa-2 cells (left). Precipitates by anti-PODXL1 Ab probed with anti-C5aR Ab showed PODXL1-C5aR binding not in the KO cells but in the WT cells (right). (B) Visualizing the alteration of C5aR intracellular localization with or without PODXL1 by Fluoppi assay. Cellular localization of C5aR was examined on HeLa cotransfected with PODXL1-Ash and C5aR-red compared with the cells transfected with C5aR-red alone (lower panel). Correct system behavior was confirmed by the PODXL1-Ash + CTTN-red cotransfected cells (CTTN is a known binding partner of PODXL1) as a positive control (upper panel). Arrows indicated membrane localization of fluorescent dots. (C) Loss of the cellular motility in the PODXL1-KO cells treated with C5a by invasion assay (Boyden chamber assay). Accelerated motility of the PODXL1-WT MiaPaCa-2 in response to C5a (left), loss of the response in the PODXL1-KO cells (right). (D) Endogenous expression of C5aR in the PODXL1-WT and the KO cells of MiaPaCa-2 or AsPC-1 by IF using confocal-laser scanning microscope. (E) Decreased expression of C5aR in the PODXL1-KO PDAC cells in comparison with that in the WT cells (left). Marked shift of intracellular localization of C5aR demonstrated by IB for the extracts between membrane fraction and cytosolic fraction from the PODXL1-WT and PODXL1-KO PDAC cells (right). Transferrin receptor and CXADR were shown as positive control for membrane extract. (F) EMT triggered with C5a stimulation in the PODXL1-WT PDAC cells. Decreased expression of E-Cadherin, upregulation of Snail and Vimentin in the WT cells only when stimulated with C5a by IB. Tubulin was shown as a control.
Significance of C5aR/C5a axis for the PDAC metastasis in the presence of PODXL1 in vivo
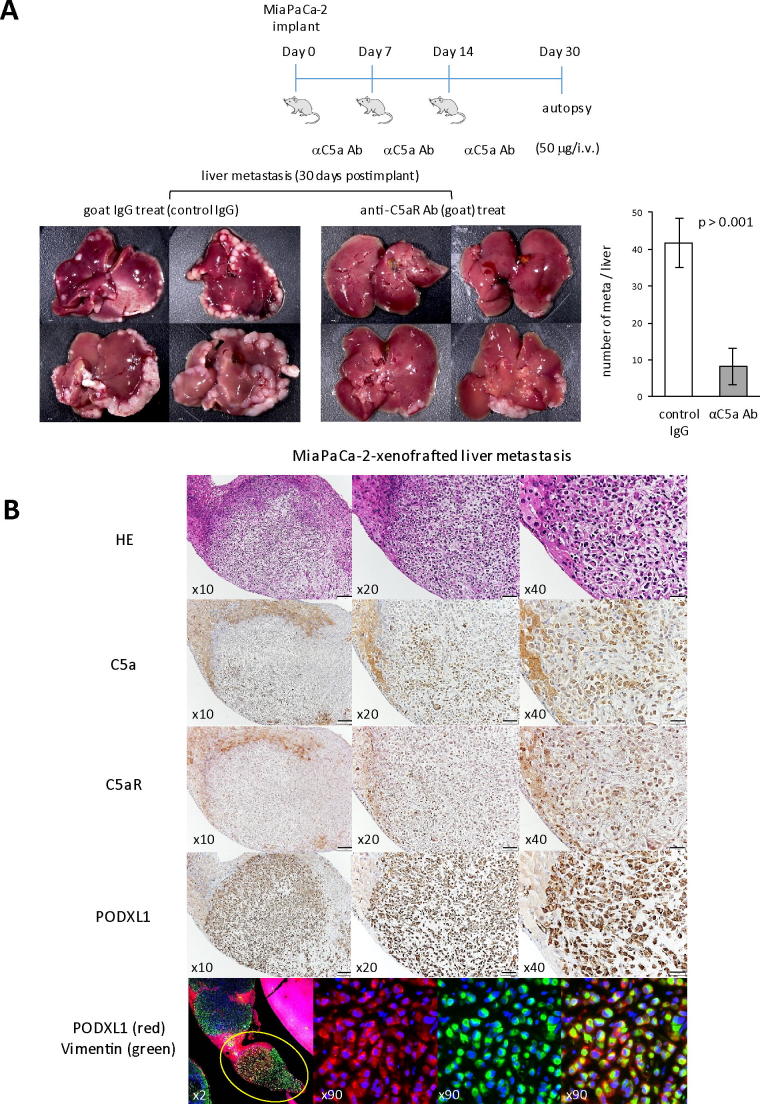
With the specific interaction of PODXL1 and C5aR in PDAC cells thus confirmed, the biological impact of C5aR/C5a signaling for promoting PDAC metastasis was examined using the experimental PDAC liver metastasis model in nude mice. Animals injected in the spleen with 1 × 106 of MiaPaCa-2 cells were either treated three times, in total, with anti-C5a neutralizing antibody (50 μg/every 7 days), or with the same dose of goat IgG as a control following the schedule shown in Figure 5A. Thirty days after splenic injection of MiaPaCa-2 cells, mice were freshly autopsied and examined for liver metastasis. Significant differences were thereby observed in number of metastatic tumor foci (representative histology for these metastatic tumors was shown in Supplementary Figure S6B) as shown in the stereoscopic image and the summarized graph, where the neutralizing antibody-treated mice were poorly showing in metastatic foci (Figure 5A). Thus, the activation of C5aR/C5a signal significantly contributed to liver metastasis formation of PDAC cells in vivo. Immunohistochemistry indicated that metastatic cancer cells were positive for C5a, C5aR, and PODXL1 (murine hepatocytes were also positive for C5a and C5aR), and immunofluorescence revealed the coexpression of PODXL1 and Vimentin as an EMT marker on cancer cells at the metastatic foci (Figure 5B).
Figure 5.
C5aR/C5a axis is essential to cause liver metastasis of MiaPaCa-2 in vivo. (A) Administration protocol of the C5a-neutralizing Ab (50 μg/mouse/time) to the mice splenically injected with MiaPaCa-2 (1 × 106 cells). The mice splenically injected with MiaPaCa-2 had in total three intravenous injections of the Ab, once per week, and were autopsied on the Day 30 (upper panel). Development of liver metastasis of implanted MiaPaCa-2 cells with or without C5a Ab-treatment (lower panel). Attached Graphs showed average number of liver metastasis/mouse in the mice treated with control IgG or those treated with anti-C5a Ab (n = 4/each group, means and s.d. of quadruplicate were shown). (B) IHC analyses of the metastatic focus of MiaPaCa-2 in the mouse model. Liver isolated from the mice without treatment by anti-C5a Ab shown above was subjected to IHC analysis detecting C5a, C5aR, and PODXL1 expression on metastatic focus. The bottom panel showed coexpression of PODXL1 (red) and Vimentin (green) on an individual cancer cell by double IF.
Detection of C5aR, C5a and PODXL1 in human PDAC patient primary tissues and liver metastatisis
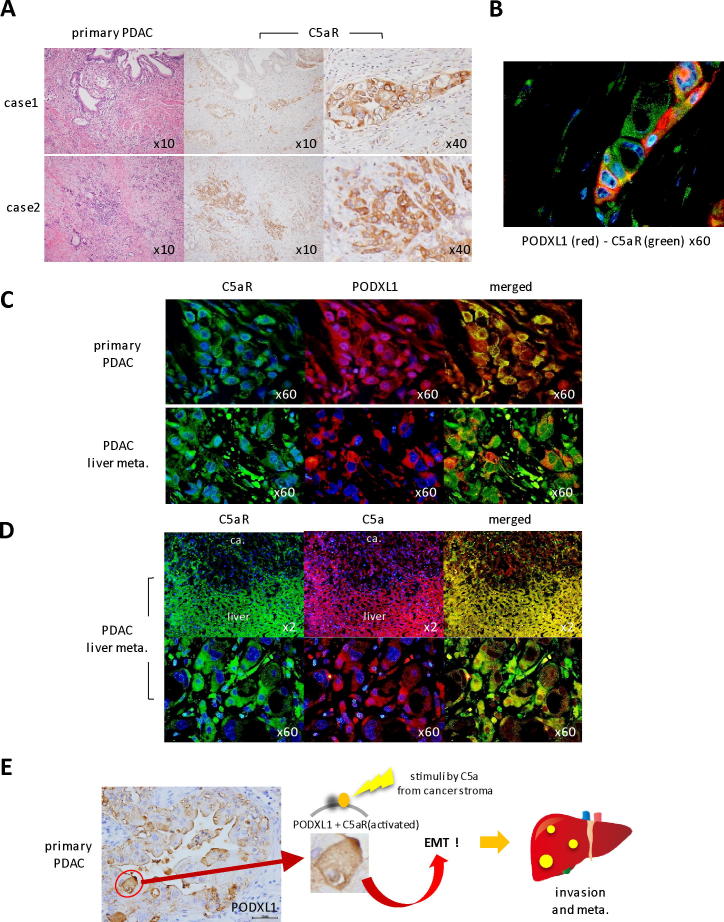
Since the interaction between PODXL1 and C5aR/C5a axis was established in the mouse PDAC-xenografted model, we then investigated the endogenous expression of these molecules in human patient tissues. Strong C5aR expression was detected on the invasive nests of primary PDAC tissues as well as PODXL1 expression (Figure 6A), and the merged image of double immunofluorescence detecting PODXL1 and C5aR displayed a few double positive cells inside the invasive cancer nest with membranous colocalization (Figure 6B). In liver metastasis from the same PDAC patient, C5aR and PODXL1 were consistently expressed on the same individual cancer cells as well as in the primary site (Figure 6C), and C5a, as a ligand to C5aR, was strongly positive both on non-neoplastic hepatocytes (Figure 6D, upper panel) and metastatic PDAC cells (Figure 6D, lower panel, hyperview). C5aR expression was also maintained at the lymph nodal metastasis of PDAC (Supplementary Figure S7).
Figure 6.
Endogenous expression of C5aR, PODXL1 and C5a on the PDAC patient tissues. (A) IHC staining of C5aR on the invasive nests of primary PDAC tissues (two cases of PDAC patients). (B) Double IF showed membranous colocalization of PODXL1 (red) and C5aR (green) on the cancer nest at the invasive front of human PDAC. (C) Coexpression of C5aR and PODXL1 on cancer cells of primary PDAC tissues and the liver metastasis of the same patient. (D) Coexpression of C5aR and C5a at the liver metastatic focus of the PDAC patient. Note that non-neoplastic hepatocytes are also positive for both. (E) Schematic representation of the specific interaction of PODXL1-C5aR/C5a axis in PDAC invasion/metastasis.
Discussion
In previous studies, expression of PODXL1 had been reported to reflect adverse prognosis for the patient with diverse cancer origins including breast, colorectal, gastric, and pancreatic adenocarcinoma [4], [23], [8], [9], [10]. Similarly, C5aR/C5a expression is known to correlate with clinical prognosis for various lineages of cancer [14], [16], [17]. Moreover, both PODXL1 signaling and C5aR/C5a signaling were proven molecularly to facilitate tumor cell invasion in vitro by activating specific kinases [13], [24], [25]. For example, PODXL1 triggers invasion by increased matrix metalloproteinases (MMP) 1 and 9 expression and increased activation of MAPK and PI3K activity through ezrin in breast cancer, and by elevating the soluble β-cat level/β-cat signaling through the p38 MAPK/GSK-3β pathway in glioblastoma [10], [12]. On the other hand, C5aR/C5a signaling was reported to raise the release of MMP from cancer cells – similarly to PODXL1 – to induce PI3K/AKT activation and inhibit p21/p-p21 expression, and even to exhibit immunomodulatory effects in facilitating cancer metastasis by suppressing effector CD8(+) and CD4(+) T-cell responses [25], [26], [27]. Collectively, this information implicates the presently studied molecules as crucially determinant for metastasis, and for resultant poor prognosis, in a wide range of tumors. However, these molecules have also been reported as being independently significant in their biology. In this study, we found the direct molecular connection between PODXL1 and C5aR/C5a axis by demonstrating that PODXL1 is considered to be one of the important binding partner with C5aR, which stabilizes C5aR and recruits it to its functional site (cell membrane) on PDAC cells. Namely, PODXL1 is considered to be essential to activation of C5aR, leading to acquisition of cellular motility imparting invasive and metastatic properties to PDAC cells (Figure 6E). This novel finding may provide a clear-cut explanation for the various earlier oncology studies on PODXL1 and C5aR/C5a, although further investigation is necessary for the detailed structure and function of the PODXL1-C5aR complex, and for the mechanism of recruitment of the complex to the plasma membrane. Our experiments also revealed the multifunctional aspects of PODXL1 appearing to allow capture of multiple chemokine/cytokine receptors induced in the tumor microenvironment. We suggest that PODXL1 plays a central role in progression of diverse cancers by activating the soluble factors in response to their unique tumor microenvironment. This notion is supported by earlier reports that the glycosylated form of PODXL1, TRA-1-60 antigen [22], [28], was utilized as an important cellular marker for iPS cells to confirm their full-reprogramming status [29], reflecting a wide action permitting multipotent differentiation in response to diverse stimuli.
In a recent trend, multiple oncology studies in tumor medicine have emphasized the importance of the expulsion of cancer stem cells, as they are strongly resistant to known anti-cancer treatments and hence are sources for recurring malignancy. With that in mind it remains unclear whether PODXL1-expressing tumors contain cancer stem-phenotype cells, but the tumors may yet correlate highly with relapse and progression specifically due to the diverse function of PODXL1/TRA-1-60 [30] in the microenvironment, as was demonstrated in this study. Our investigations, importantly, represent a framework for precision therapeutic control of the PODXL1-expressing cancer cell, so as to overcome intractable malignancies such as PDACs.
Conclusions
In this study, we found novel cross-talk between the critical two tumor regulators, PODXL1 and C5aR, in pancreatic cancer (PDAC) which significantly contribute PDAC invasion/metastasis. Through their specific interaction, PDAC acquired accelerated cellular motility and caused aggressive metastasis which was corroborated by in vivo assays of xenografted model using PODXL1-knockout PDAC cell in comparison with the wild type cell. Furthermore, PODXL1 may be multifunctional because it is capable of capturing various chemokine (cytokine) receptors, which suggests diverse role of PODXL1 in the tumor microenvironment.
Acknowledgments
Acknowledgements
We thank Dr. K. Homma and T. Kawasaki (Dept. of Pathology, Niigata Cancer Center Hospital) for kindly supporting pathological materials, also Ms. N. Sumi and Ms. A. Ageishi for technical assistance and preparation of manuscript.
Funding
This study was supported by Grant-in-Aid for Challenging Exploratory Research, Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP 16K15245 (E. Kondo).
Author contributions
E.K. conceived, designed, and supervised the study. K.S., H.I., S.M, W.S. and M.S. performed experiments. E.K. discussed the result with K.S., H.I., M.S. and wrote the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2019.09.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Neoptolemos J.P., Palmer D.H., Ghaneh P., Psarelli E.E., Valle J.W., Halloran C.M., Faluyi O., O'Reilly D.A., Cunningham D., Wadsley J. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos J.P., Kleeff J., Michl P., Costello E., Greenhalf W., Palmer D.H. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(16):333–348. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 3.Horvat R., Hovorka A., Dekan G., Poczewski H., Kerjaschki D. Endothelial cell membranes contain podocalyxin–the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol. 1986;102:484–491. doi: 10.1083/jcb.102.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somasiri A., Nielsen J.S., Makretsov N., McCoy M.L., Prentice L., Gilks C.B., Chia S.K., Gelmon K.A., Kershaw D.B., Huntsman D.G. Overexpression of the anti-adhesin podocalyxin is an independent predictor of breast cancer progression. Cancer Res. 2004;64:5068–5073. doi: 10.1158/0008-5472.CAN-04-0240. [DOI] [PubMed] [Google Scholar]
- 5.Doyonnas R., Nielsen J.S., Chelliah S., Drew E., Hara T., Miyajima A., McNagny K.M. Podocalyxin is a CD34-related marker of murine hematopoietic stem cells and embryonic erythroid cells. Blood. 2005;105:4170–4178. doi: 10.1182/blood-2004-10-4077. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya B., Miura T., Brandenberger R., Mejido J., Luo Y., Yang A.X., Joshi B.H., Ginis I., Thies R.S., Amit M. Gene expression in human embryonic stem cell lines: unique molecular signature. Blood. 2004;103:2956–2964. doi: 10.1182/blood-2003-09-3314. [DOI] [PubMed] [Google Scholar]
- 7.Richards M., Tan S.P., Tan J.H., Chan W.K., Bongso A. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2004;22:51–64. doi: 10.1634/stemcells.22-1-51. [DOI] [PubMed] [Google Scholar]
- 8.Kaprio T., Fermér C., Hagström J., Mustonen H., Böckelman C., Nilsson O., Haglund C. Podocalyxin is a marker of poor prognosis in colorectal cancer. BMC Cancer. 2014;14:493. doi: 10.1186/1471-2407-14-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laitinen A., Böckelman C., Hagström J., Kokkola A., Fermér C., Nilsson O., Haglund C. Podocalyxin as a prognostic marker in gastric cancer. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0145079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saukkonen K., Hagström J., Mustonen H., Juuti A., Nordling S., Fermér C., Nilsson O., Seppänen H., Haglund C. Podocalyxin is a marker of poor prognosis in pancreatic ductal adenocarcinoma. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin C.W., Sun M.S., Liao M.Y., Chung C.H., Chi Y.H., Chiou L.T., Yu J., Lou K.L., Wu H.C. Podocalyxin-like 1 promotes invadopodia formation and metastasis through activation of Rac1/Cdc42/cortactin signaling in breast cancer cells. Carcinogenesis. 2014;35:2425–2435. doi: 10.1093/carcin/bgu139. [DOI] [PubMed] [Google Scholar]
- 12.Sizemore S., Cicek M., Sizemore N., Ng K.P., Casey G. Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with ezrin. Cancer Res. 2007;67:6183–6191. doi: 10.1158/0008-5472.CAN-06-3575. [DOI] [PubMed] [Google Scholar]
- 13.Graves M.L., Cipollone J.A., Austin P., Bell E.M., Nielsen J.S., Gilks C.B., McNagny K.M., Roskelley C.D. The cell surface mucin podocalyxin regulates collective breast tumor budding. Breast Can Res. 2016;18:11. doi: 10.1186/s13058-015-0670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamura T., Yamamoto-Ibusuki M., Sueta A., Kubo T., Irie A., Kikuchi K., Kariu T., Iwase H. Influence of the C5a–C5a receptor system on breast cancer progression and patient prognosis. Breast Cancer. 2016;23:876–885. doi: 10.1007/s12282-015-0654-3. [DOI] [PubMed] [Google Scholar]
- 15.Kaida T., Nitta H., Kitano Y., Yamamura K., Arima K., Izumi D., Higashi T., Kurashige J., Imai K., Hayashi H. C5a receptor (CD88) promotes motility and invasiveness of gastric cancer by activating RhoA. Oncotarget. 2016;7:84798–84809. doi: 10.18632/oncotarget.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gua J., Dinga J.Y., Lu C.L., Lin Z.W., Chu Y.W., Zhao G.Y., Guo J., Ge D. Overexpression of CD88 predicts poor prognosis in non-small-cell lung cancer. Lung Cancer. 2013;81:259–265. doi: 10.1016/j.lungcan.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Wada Y., Maeda Y., Kubo T., Kikuchi K., Eto M., Imamura T. C5a receptor expression is associated with poor prognosis in urothelial cell carcinoma patients treated with radical cystectomy or nephroureterectomy. Oncol Lett. 2016;12:3995–4000. doi: 10.3892/ol.2016.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi M., Watanabe M., Kinoshita R., Kaku H., Ueki H., Futami J., Murata H., Inoue Y., Li S.A., Huang P. Dramatic increase in expression of a transgene by insertion of promoters downstream of the cargo gene. Mol Biotechnol. 2014;56:621–630. doi: 10.1007/s12033-014-9738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koyano F., Okatsu K., Kosako H., Tamura Y., Go E., Kimura M., Kimura Y., Tsuchiya H., Yoshihara H., Hirokawa T. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 20.Yamano K., Queliconi B.B., Koyano F., Saeki Y., Hirokawa T., Tanaka K., Matsuda N. Site-specific interaction mapping of phosphorylated ubiquitin to uncover parkin activation. J Biol Chem. 2015;290:25199–25211. doi: 10.1074/jbc.M115.671446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ney J.T., Zhou H., Sipos B., Büttner R., Chen X., Klöppel G., Gütgemann I. Podocalyxin-like protein 1 expression is useful to differentiate pancreatic ductal adenocarcinomas from adenocarcinomas of the biliary and gastrointestinal tracts. Hum Pathol. 2007;38:359–364. doi: 10.1016/j.humpath.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Schopperle W.M., DeWolf W.C. The TRA-1-60 and TRA-1-81 human pluripotent stem cell markers are expressed on podocalyxinin embryonal carcinoma. Stem Cells. 2007;25:723–730. doi: 10.1634/stemcells.2005-0597. [DOI] [PubMed] [Google Scholar]
- 23.Heby M., Elebro J., Nodin B., Jirström K., Eberhard J. Prognostic and predictive significance of podocalyxin-like protein expression in pancreatic and periampullary adenocarcinoma. BMC Clin Pathol. 2015;15:10. doi: 10.1186/s12907-015-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fröse J., Chen M.B., Hebron K.E., Reinhardt F., Hajal C., Zijlstra A., Kamm R.D., Weinberg R.A. Epithelial-mesenchymal transition induces podocalyxin to promote extravasation via ezrin signaling. Cell Rep. 2018;24:962–972. doi: 10.1016/j.celrep.2018.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vadrevu S.K., Chintala N.K., Sharma S.K., Sharma P., Cleveland C., Riediger L., Manne S., Fairlie D.P., Gorczyca W., Almanza O. Complement c5a receptor facilitates cancer metastasis by altering T-cell responses in the metastatic niche. Cancer Res. 2014;74:3454–3465. doi: 10.1158/0008-5472.CAN-14-0157. [DOI] [PubMed] [Google Scholar]
- 26.Nitta H., Wada Y., Kawano Y., Murakami Y., Irie A., Taniguchi K., Kikuchi K., Yamada G., Suzuki K., Honda J. Enhancement of human cancer cell motility and invasiveness by anaphylatoxin C5a via aberrantly expressed C5a receptor (CD88) Clin Cancer Res. 2013;19:2004–2013. doi: 10.1158/1078-0432.CCR-12-1204. [DOI] [PubMed] [Google Scholar]
- 27.Chen J., Li G.Q., Zhang L., Tang M., Gao X., Xu G.L., Wu Y.Z. Complement C5a/C5aR pathway potentiates the pathogenesis of gastric cancer by down-regulating p21 expression. Cancer Lett. 2018;412:30–36. doi: 10.1016/j.canlet.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Toyoda H., Nagai Y., Kojima A., Kinoshita-Toyoda A. Podocalyxin as a major pluripotent marker and novel keratan sulfate proteoglycan in human embryonic and induced pluripotent stem cells. Glycoconj J. 2017;34:817–823. doi: 10.1007/s10719-017-9801-8. [DOI] [PubMed] [Google Scholar]
- 29.Chan E.M., Ratanasirintrawoot S., Park I.H., Manos P.D., Loh Y.H., Huo H., Miller J.D., Hartung O., Rho J., Ince T.A. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- 30.Takata K., Saito K., Maruyama S., Miyata-Tanaka T., Iioka H., Okuda S., Ling Y., Karube K., Miki Y., Maeda Y. Identification of TRA-1-60-positive cells as a potent refractory population in follicular lymphomas. Cancer Sci. 2019;110:443–457. doi: 10.1111/cas.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.