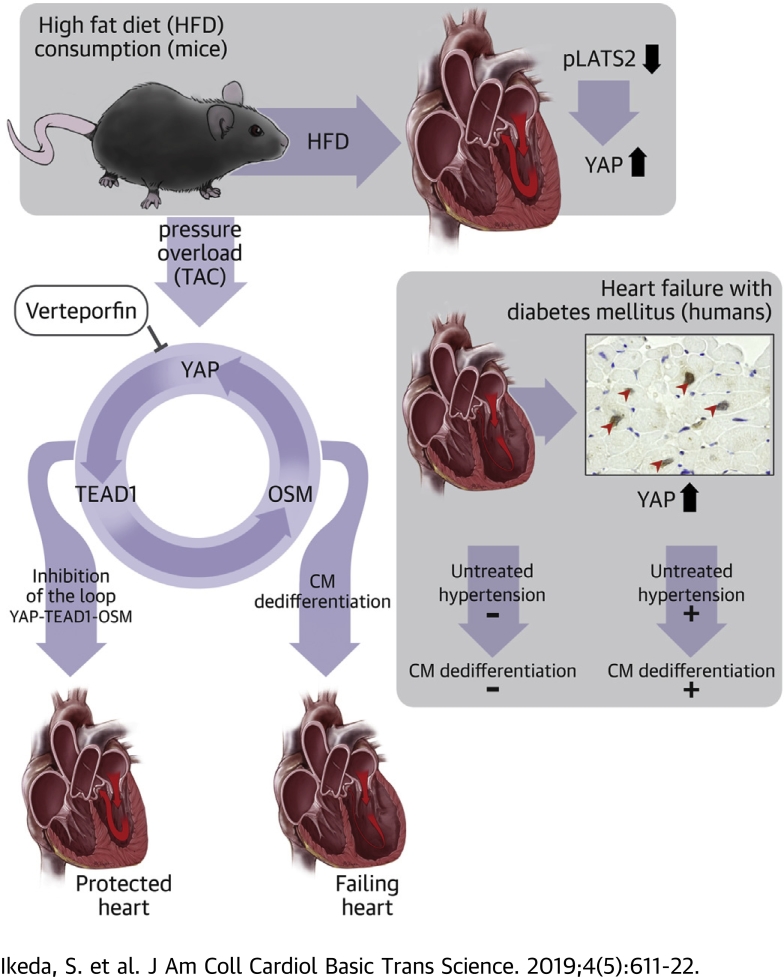
Visual Abstract
Key Words: diabetes, diabetic cardiomyopathy, Hippo pathway, pressure overload
Abbreviations and Acronyms: HF, heart failure; HFD, high-fat diet; Lats2, large tumor suppressor kinase 2; LV, left ventricular; Mst1, mammalian sterile 20-like 1; ND, normal diet; OSM, oncostatin M; PO, pressure overload; Runx1, runt-related transcription factor 1; TAC, transverse aortic constriction; TAZ, transcriptional coactivator with PDZ-binding motif; TEAD, transcriptional enhancer factor; YAP, Yes-associated protein
Highlights
-
•
YAP, a terminal effector of the Hippo signaling pathway, is activated in cardiomyocytes in response to high-fat diet consumption in mice and diabetes in patients.
-
•
Long-term activation of YAP in response to high-fat diet consumption is detrimental for the heart in the presence of pressure overload.
-
•
Detrimental effects of YAP during pressure overload are mediated through activation of a positive feedback loop, consisting of YAP, TEAD1, and OSM, and consequent dedifferentiation of cardiomyocytes.
-
•
Chemical inhibitors of YAP, TEAD1, or OSM may be effective in treating patients who have diabetes, high blood pressure, and metabolic syndrome to prevent heart failure syndromes.
Summary
Patients with diabetes are more prone to developing heart failure in the presence of high blood pressure than those without diabetes. Yes-associated protein (YAP), a key effector of the Hippo signaling pathway, is persistently activated in diabetic hearts, and YAP plays an essential role in mediating the exacerbation of heart failure in response to pressure overload in the hearts of mice fed a high-fat diet. YAP induced dedifferentiation of cardiomyocytes through activation of transcriptional enhancer factor 1 (TEAD1), a transcription factor. Thus, YAP and TEAD1 are promising therapeutic targets for diabetic patients with high blood pressure to prevent the development of heart failure.
Cardiovascular disease is a major cause of mortality in developed countries (1). Recently, the number of cases of cardiovascular disease associated with metabolic syndrome, such as obesity and diabetes mellitus, has been rapidly increasing worldwide (2). These patients often develop heart failure (HF) with either preserved left ventricular ejection fraction or reduced left ventricular ejection fraction, although metabolic derangements, such as insulin resistance, often favor development of HF with preserved left ventricular ejection fraction in obese patients with type 2 diabetes mellitus (3). Currently, the molecular mechanisms of cardiomyopathy associated with metabolic syndrome remain poorly understood, and thus, no specific treatment exists.
The Hippo signaling pathway is an evolutionarily conserved signaling pathway involved in organ size control, tissue regeneration, and tumorigenesis through regulation of apoptosis and cell proliferation (4). Major components of the Hippo pathway include upstream serine/threonine kinases, namely, Mst1/2 (mammalian sterile 20-like 1) and Lats1/2 (large tumor suppressor kinase 1 and 2), and downstream nuclear transcription factor cofactors YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif), where Mst1/2 and Lats1/2 negatively regulate nuclear levels of YAP and TAZ (4). YAP and TAZ bind to transcription factors, such as TEAD (transcriptional enhancer factor) and FoxO1 (forkhead box O1), thereby regulating a variety of cellular functions, including cell proliferation and cell survival (5). Activation of the Hippo pathway is intimately involved in the pathogenesis of heart disease, including ischemia/reperfusion injury 6, 7, cardiac remodeling, and HF 8, 9. YAP is also involved in regeneration of the postnatal heart after myocardial infarction 10, 11, 12.
It has been proposed that either suppression of the upstream Hippo pathway components or stimulation of YAP can potentially be used as a therapeutic intervention to facilitate repair and regeneration of the heart after acute myocardial infarction 10, 11, 12; however, persistent inactivation of the Hippo pathway and consequent activation of YAP induces cardiac dysfunction in the presence of pressure overload (PO) through activation of cardiomyocyte dedifferentiation (13). This suggests that the function of YAP varies drastically depending on the type of stress. An important question remains as to whether persistent activation of YAP occurs in clinically relevant conditions and whether suppression of YAP improves such conditions.
YAP is up-regulated in liver cancer cells in response to high glucose (14) and in diabetic kidney epithelial cells (15). Patients with insulin resistance are more prone to developing hypertension, and the coexistence of diabetes and hypertension facilitates the development of HF 2, 16. We asked: 1) whether YAP promotes cardiac dysfunction in response to PO in mice fed a high-fat diet (HFD), a mouse model of type 2 diabetes mellitus; 2) whether the exacerbation of cardiomyopathy in HFD-fed mice in the presence of PO is accompanied by cardiomyocyte dedifferentiation; and 3) whether YAP is up-regulated in the human diabetic heart.
Methods
Mouse models
All animal experiments were conducted in accordance with protocols approved by the Rutgers University Animal Care and Use Committee. The background of all mice was C57BL/6J. Systemic TEAD1+/− mice have been described (13). Age- and sex-matched male littermate mice were used as controls. For HFD treatment, male mice were randomly divided into 2 groups and fed with either normal diet (ND) or HFD for 8 weeks (17). For verteporfin experiments, mice were randomly divided into 2 groups: verteporfin or DMSO control group. Twelve-week-old mice after sham operation or transverse aortic constriction (TAC) were injected intraperitoneally with verteporfin 100 mg/kg every other day for 10 days. To measure arterial pressure gradients, high-fidelity micromanometer catheters (1.4-F, Millar Instruments Inc., Houston, Texas) were used.
Transverse aortic constriction
The methods used to impose PO in mice have been described (13). Male mice at 12 weeks of age were randomly divided into 2 groups: PO with TAC or sham operation. We focused on male mice in this study because previous studies of YAP loss of function in the heart conducted in this laboratory also focused on male mice (13). Mice were anesthetized with pentobarbital sodium (60 mg/kg) and mechanically ventilated. The number of animals used is described in each figure legend. Successful application of TAC was confirmed by a transverse aortic velocity above 4 m/s, evaluated by Doppler echocardiography. Mice that died of HF were included in the survival analysis but were excluded from the assessment of cardiac function and histology. There were no unexpected adverse events during the procedure. All operations and analyses were performed in a blinded manner with regard to the genotype of mice.
Echocardiography
Mice were anesthetized using 12 μl/g body weight of 2.5% Avertin (Sigma-Aldrich, St. Louis, Missouri), and echocardiography was performed as described previously (13).
Human samples from explanted hearts
Samples for immunostaining
The study was approved by the Ethics Committee of Tohoku University Graduate School of Medicine. All patients provided written consent for the use of their heart tissues for research. Myocardial biopsy specimens were obtained from patients with HF in Tohoku University Hospital. Immunostaining was conducted using 66 consecutive observable biopsy specimens obtained from January 2016 to June 2017 (Supplemental Tables 1 and 2). Twenty-five patients were diagnosed with diabetes, and their average glycosylated hemoglobin level was 6.98 ± 0.68% (compared with 5.66 ± 0.32% in patients without diabetes; p < 0.001). Biopsy samples were fixed with 10% paraformaldehyde in phosphate-buffered saline (pH 7.4), paraffin embedded, and sectioned.
Samples for immunoblotting
The study was approved by the Ethics Committee of Taipei Veterans General Hospital, and all patients or their families expressed their willingness to participate through an informed consent form. The samples from the left atrial part of explanted hearts used in this study were obtained from patients who had received heart transplants and age-matched donors at the Taipei Veterans General Hospital. Patients with diabetes had their diabetes controlled with oral hypoglycemic agents. Immediately after tissue procurement, the samples for biochemical study were stored in liquid nitrogen and kept at −80°C.
Antibodies and reagents
The following primary antibodies were used: YAP (Cell Signaling, #14074, Cell Sugnaling Technology, Danvers, Massachusetts), phospho-YAP (Ser127) (Cell Signaling, #4911), LATS2 (Bethyl Laboratories, #A300-479A, Bethyl Laboratories, Montgomery, Texas), phospho-LATS2 (Thr1041) (Cell Signaling, #8654), atrial natriuretic peptide (Abcam, #ab180649, Abcam, Cambridge, United Kingdom), TEAD1 (Sigma-Aldrich, #AV39521), MYH7 (Sigma-Aldrich, # M8421), ACTA2 (Sigma-Aldrich, #A5228), OSM (Santa Cruz, #sc374039, Santa Cruz Biotechnology, Dallas, Texas), OSMR (Santa Cruz, #sc30011), cardiac troponin T antibody (Abcam, #ab33589), sarcomeric actinin (Abcam, #ab68167), CD45 (Abcam, #ab10558), CD68 (Abcam, #ab31630), Ly6G (Abcam, #ab25377), and α-tubulin (Sigma-Aldrich, #T6199). Immunoblot analyses and histological analyses were conducted as described previously (13).
Real-time quantitative polymerase chain reaction
Primers used have been described previously (13). Quantitative real-time polymerase chain reaction was performed on the CFX 96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, California). The cycle threshold (Ct) value determined with CFX Manager Software version 2.0 (Bio-Rad) for all samples was normalized to Gapdh, and the relative fold change was computed by the comparative Ct (ΔΔCt) method.
Statistical analysis
Continuous data are presented as mean ± SEM or mean ± SD and visualized using bar graphs or box plots. In box plots, whiskers show minimum and maximum values, whereas bars represent the median and 25th and 75th percentiles. Sample sizes were determined using standard power analysis (statistical power ≥0.8, and α <0.05). Indicated sample size (in Figure legends) always refers to biological replicates (independent animals). No data were excluded from statistical analyses. Unless otherwise stated, statistical testing was performed with statistical analysis software (Excel Tokei 2015, Social Survey Research Information Co., Ltd., Tokyo, Japan). Student’s t-test (paired or unpaired, as appropriate) and analysis of variance followed by Tukey’s honest significant difference tests were used for comparisons between 2 or multiple groups, respectively. Survival curves were analyzed by Kaplan-Meier log-rank test. In Supplemental Figure 4, Student’s t-test and chi-square test were used to compare 2 quantitative variables. A p value of <0.05 was considered statistically significant.
Results
YAP was elevated in the hearts of HFD-fed mice
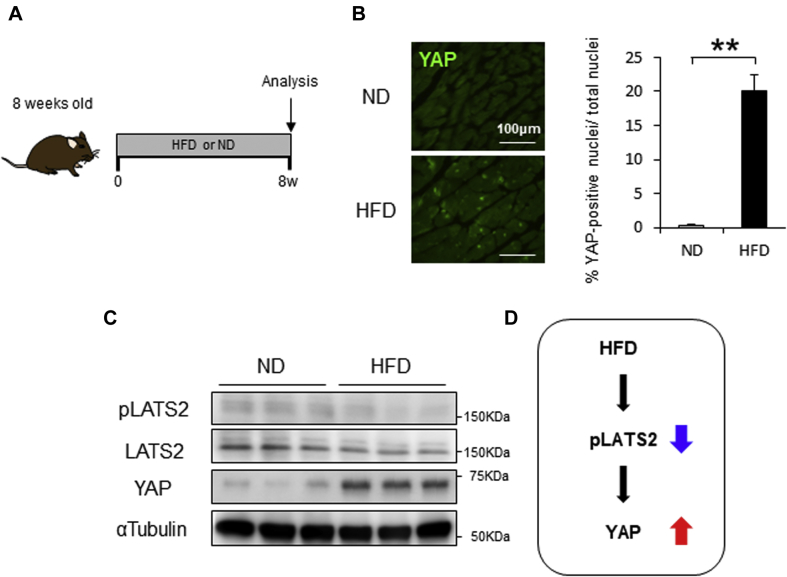
Consumption of a HFD by mice induces weight gain and insulin resistance, which in turn causes cardiac hypertrophy and diastolic dysfunction (17), mimicking diabetic cardiomyopathy in humans. HFD treatment for 8 weeks induced cardiac hypertrophy, as indicated by increases in heart weight/tibial length, cardiomyocyte cross-sectional area, and protein or mRNA expression of fetal-type genes, including ANP (atrial natriuretic peptide) and BNP (brain natriuretic peptide) (Figure 1A, Supplemental Figure 1). Immunostaining of heart sections showed that consumption of HFD significantly increased the number of cardiomyocytes with YAP-positive nuclei (Figure 1B). Immunoblot analyses confirmed that the level of total YAP protein was also increased in the hearts of mice fed HFD (Figure 1C). Furthermore, consumption of HFD for 8 weeks significantly down-regulated the level of phospho-Lats2, which suggests that this model mimics Hippo deficiency (Figure 1C, Supplemental Figure 2). Echocardiographic measurements (Supplemental Table 3) and hemodynamic measurements (Supplemental Table 4) indicated that consumption of HFD for 8 weeks did not induce systolic cardiac dysfunction, consistent with our previous results (17).
Figure 1.
HFD Induces Cardiac Activation of YAP in Mice
(A) Schematic representation of the experimental protocol. Mice were fed with either normal diet (ND) or high-fat diet (HFD) for 8 weeks. (B) Representative immunostaining of Yes-associated protein (YAP). Quantitative analysis is shown on the right (n = 6, each). (C) Representative gel pictures in the heart. (D) Effect of Hippo pathway by HFD feeding. All results are mean ± SEM. **p < 0.01 by analysis of variance.
PO induced left ventricular dysfunction with cardiomyocyte de-differentiation in HFD-fed mice
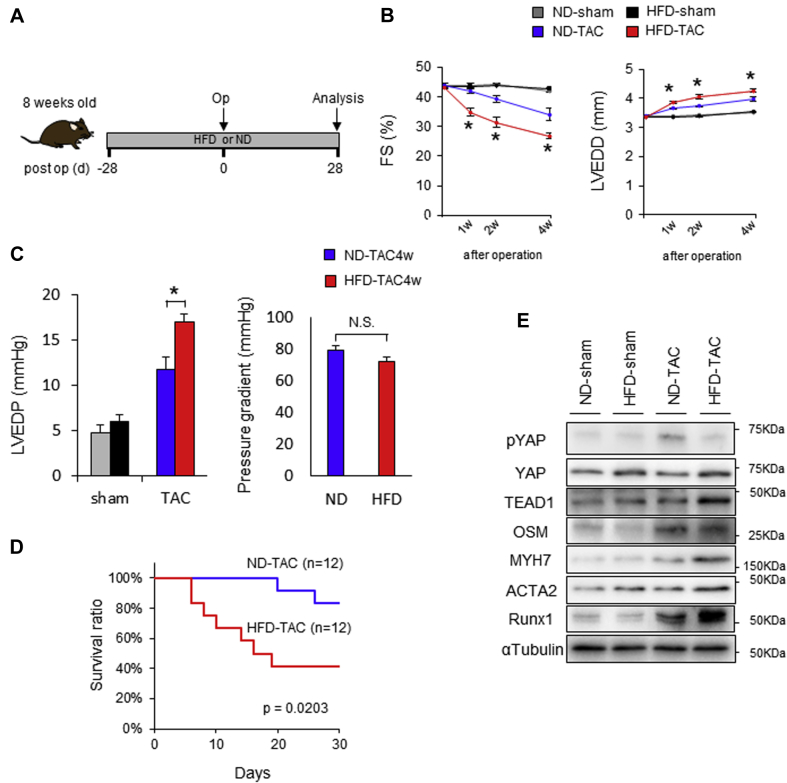
Superimposition of high blood pressure on metabolic syndrome or diabetes mellitus dramatically facilitates the development of HF in humans 2, 16, 18. We have shown previously that up-regulation of YAP alone in mice with a loss of Hippo function exacerbates PO-induced cardiac dysfunction through a YAP-TEAD1-oncostatin M (OSM)–dependent mechanism, which is accompanied by induction of cardiomyocyte dedifferentiation (13). We therefore evaluated whether PO-induced cardiac dysfunction is exacerbated in mice fed an HFD. To this end, we fed mice an HFD or ND for 4 weeks, subjected the mice to either TAC or sham operation while being fed the same diet, and then conducted analyses at 8 weeks (Figure 2A).
Figure 2.
Up-Regulation of YAP Contributes to the Development of Cardiac Dysfunction in Response to Pressure Overload in the Presence of HFD
(A) Schematic representation of the experimental protocol. In B–D, mice were fed with either ND or HFD for 8 weeks. Four weeks after the start of ND or HFD, mice were subjected to either sham operation or transverse aortic constriction (TAC) for 4 weeks (4w). (B) Time course of percent fractional shortening (%FS) and left ventricular end-diastolic dimension (LVEDD) evaluated with echocardiography (n = 6, each). (C) Left ventricular end-diastolic pressure (LVEDP) and pressure gradient, evaluated by hemodynamic measurements (n = 6, each). (D) Kaplan-Meier survival curves after TAC. (E) Representative gel pictures of YAP, TEAD1, OSM, MYH7, ACTA2, RUNX1, and α-tubulin in the heart (n = 6, each). All results are mean ± SEM. *p < 0.05 by analysis of variance. Abbreviations as in Figure 1.
TAC dramatically facilitated the progression of LV dysfunction, as indicated by decreases in fractional shortening and increases in LV end-diastolic dimension and death, in HFD-fed mice compared with mice that consumed an ND (Figures 2B to 2D). In HFD-fed mice, YAP was elevated with or without TAC, and expression of MYH7, ACTA2, and RUNX1, markers of cardiomyocyte dedifferentiation, was increased in the presence of TAC (Figure 2E, Supplemental Figure 3). Taken together, these observations suggest that dedifferentiation is facilitated by HFD consumption in the presence of PO, mimicking the cardiac phenotype of WW45cKO (homozygous knockout of WW45) mice in the presence of PO (13), in which both systolic dysfunction and cardiomyocyte dedifferentiation are exacerbated compared with in control mice.
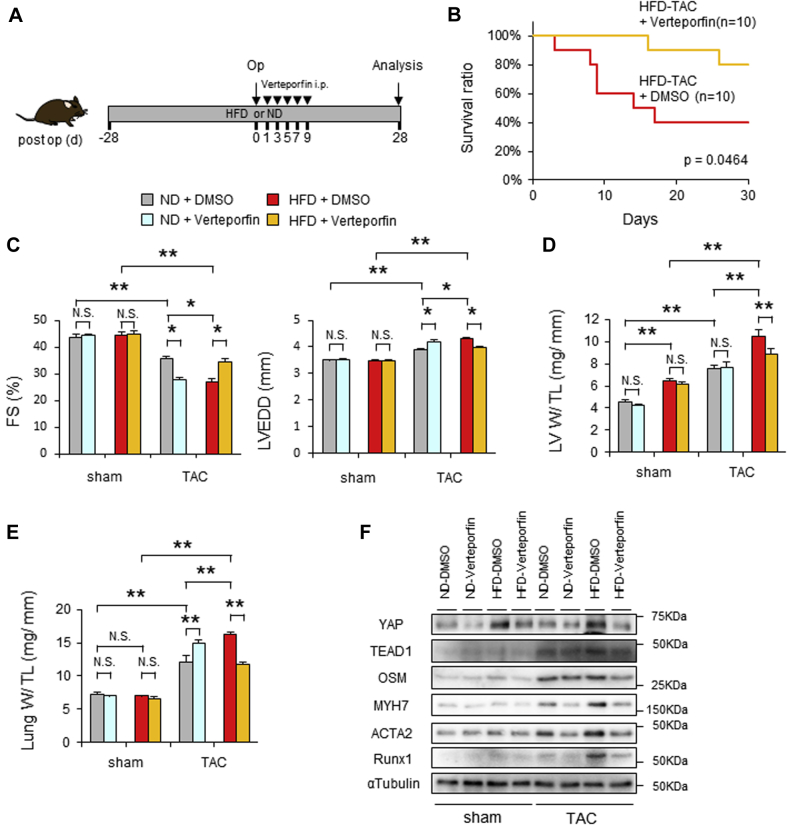
Verteporfin treatment improved cardiac function and survival rate of HFD-fed mice after PO
To evaluate the role of endogenous YAP in the development of cardiomyopathy in HFD-fed mice during PO, mice were treated with either verteporfin or vehicle (Figure 3A). Verteporfin is a benzoporphyrin derivative that is clinically available for photodynamic therapy. It disrupts the interaction between YAP and TEADs and is thus used as a specific inhibitor of YAP (13). We treated mice with verteporfin during the first 10 days after TAC. Verteporfin significantly improved the survival rate of HFD-fed mice after PO compared with vehicle treatment (Figure 3B). Furthermore, although verteporfin exacerbated TAC-induced LV dysfunction and dilation in ND-fed mice, as indicated by decreased fractional shortening, increased lung congestion, and increased LV end-diastolic dimension, it significantly normalized TAC-induced cardiac dysfunction and LV dilation (Figures 3C to 3E), as well as the increased expression of MYH7, ACTA2, and RUNX1 (Figure 3F, Supplemental Figure 4), in mice with HFD plus PO. These results suggest that YAP plays a crucial role in facilitating cardiac dysfunction in the presence of HFD consumption plus high blood pressure. Obesity induces low-grade systemic inflammation, which in turn promotes the development of cardiomyopathy (19). Thus, we evaluated the effect of verteporfin on inflammatory responses in the heart in the presence of HFD consumption with or without PO. Myocardial infiltration of CD45-positive cells (leukocytes), CD68-positive cells (macrophages), and Ly6G-positive cells (neutrophils) was enhanced by HFD plus PO, but the infiltration was significantly attenuated in the presence of verteporfin (Supplemental Figure 5).
Figure 3.
Verteporfin Improves Pressure Overload–Induced Cardiac Hypertrophy in the Presence of HFD
(A) Schematic representation of the experimental protocol. Mice were fed with either ND or HFD for 8 weeks. Four weeks after the start of ND or HFD, mice were subjected to either sham operation or TAC in the presence or absence of verteporfin for 4 weeks. (B) Kaplan-Meier survival curves for HFD-fed mice after TAC in the presence or absence of verteporfin. (C) %FS and LVEDD evaluated with echocardiography 4 weeks after TAC, with or without verteporfin treatment (n = 6, each). (D) Left ventricular weight to tibial length (LV W/ TL) ratio (n = 6, each). (E) Lung weight to tibial length (Lung W/ TL) ratio (n = 6, each). (F) Representative gel pictures of YAP, TEAD1, OSM, MYH7, ACTA2, RUNX1, and α-tubulin in the heart (n = 6, each). All results are mean ± SEM. *p < 0.05, **p < 0.01 by analysis of variance. i.p. = intraperitoneal; N.S. = not significant; Op = operation; post op = postoperative; other abbreviations as in Figures 1 and 2.
TEAD1 haploinsufficiency attenuates PO-induced cardiac dysfunction in HFD-fed mice
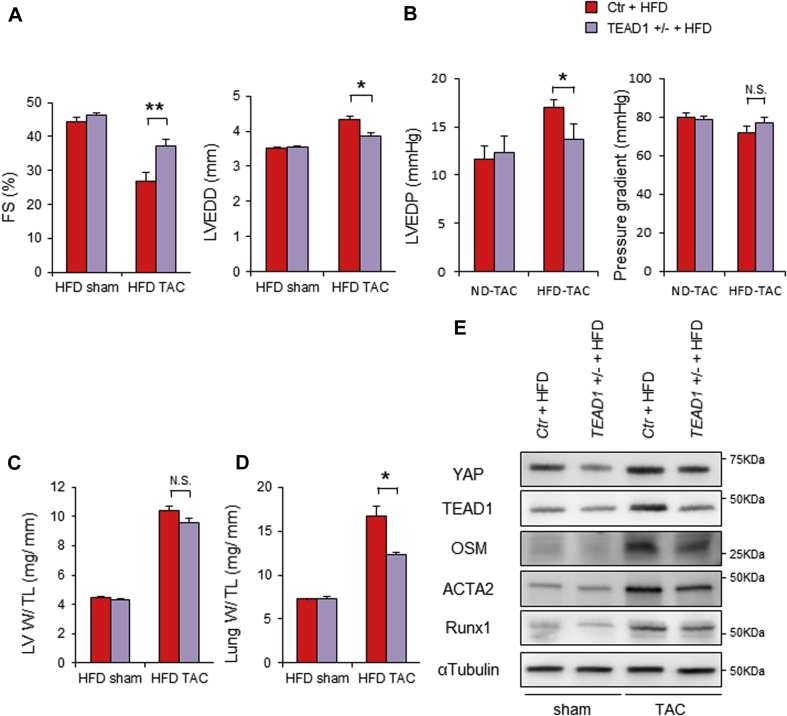
Because TEAD is a major transcription factor mediating the effect of YAP (5), we hypothesized that HFD-fed mice develop more severe HF in the presence of PO through a TEAD-dependent mechanism. To test this hypothesis, we investigated the effect of HFD consumption combined with TAC in TEAD1 heterozygous knockout mice (13). PO induced less severe LV dysfunction in HFD-fed TEAD1+/− mice than in HFD-fed control mice (Figures 4A to 4D, Supplemental Figures 6 and 7). The TAC-induced up-regulation of OSM and YAP in HFD-treated control hearts was attenuated in HFD-treated TEAD1+/− hearts, consistent with the notion that the YAP-TEAD1-OSM pathway forms a positive feedback mechanism (Figure 4E). HFD-fed TEAD1+/− mice exhibited lower expression of MYH7, ACTA2, and RUNX1, which indicates that PO-induced activation of TEAD1 in HFD-fed mice plays an important role in mediating cardiomyocyte dedifferentiation (Figure 4E).
Figure 4.
TEAD1 Deletion Attenuates Pressure Overload–Induced Cardiac Disfunction in the Presence of HFD
Control (Ctr) mice and TEAD1 heterozygous knockout (TEAD1+/−) mice were fed with either ND or HFD for 8 weeks. Four weeks after the start of ND or HFD, mice were subjected to either sham operation or TAC. (A) %FS and LVEDD evaluated with echocardiography 4 weeks after TAC (n = 6, each). (B) LVEDP and pressure gradient, evaluated by hemodynamic measurements (n = 6, each). (C) LV W/ TL ratio (n = 6, each). (D) Lung W/ TL ratio (n = 6, each). (E) Representative gel pictures of YAP, TEAD1, OSM, ACTA2, RUNX1, and α-tubulin. All results are expressed as mean ± SEM. *p < 0.05, **p < 0.01 by analysis of variance. Abbreviations as in Figures 1, 2, and 3.
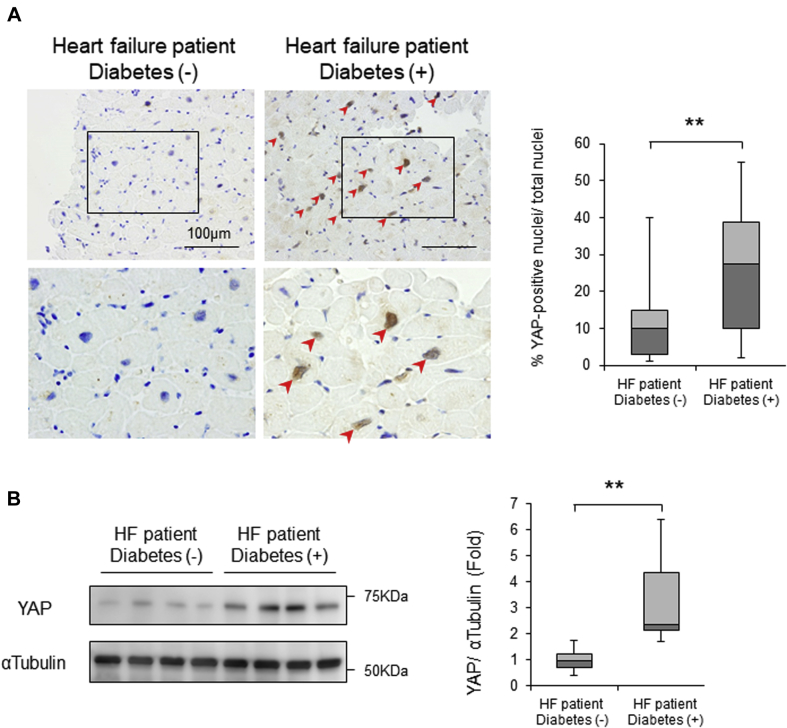
YAP expression was elevated in the hearts of patients with HF with diabetic cardiomyopathy
We investigated whether YAP is up-regulated in the hearts of HF patients with diabetes. Using myocardial biopsy specimens obtained from HF patients, immunostaining and immunoblot analyses showed that both nuclear expression of YAP in cardiomyocytes and the level of YAP protein expression in the heart were significantly greater in patients with HF with diabetes than in HF patients without diabetes (Figures 5A and 5B, Supplemental Figure 8). The extent of nuclear positivity of YAP, as indicated by the proportion of YAP-positive nuclei divided by total nuclei, was greater in patients with higher glycosylated hemoglobin, which suggests that the level of YAP may correlate with the severity of diabetes (Supplemental Figure 8B).
Figure 5.
Patients With HF With Diabetes Exhibit YAP Activation in Their Hearts
(A) Representative immunostaining of YAP in patients with heart failure (HF) with and without diabetes. Nuclear YAP accumulation is indicated by arrowheads. Quantitative analysis is shown on the right. (B) Representative gel pictures of YAP and α-tubulin in patients with HF with and without diabetes. Quantitative analysis is shown on the right (n = 7, each). **p < 0.01 by analysis of variance. The results are from 41 patients with HF without diabetes and 25 HF patients with diabetes. In box plots, whiskers show minimum and maximum values, while bars represent the median and 25th and 75th percentiles. YAP = Yes-associated protein. Abbreviations as in Figure 1.
Discussion
Here we have demonstrated that YAP is activated in the heart in response to HFD consumption, where persistent activation of YAP exacerbates cardiac dysfunction in the presence of PO. Importantly, both pharmacological and genetic interventions to inhibit the YAP-TEAD pathway attenuate the PO-induced exacerbation of HF in the heart in the presence of HFD consumption.
YAP is activated in cardiomyocytes in the presence of metabolic syndrome/diabetes mellitus
HFD consumption by mice for 4 to 8 weeks induced activation of YAP in cardiomyocytes in vivo. Our results show that HFD consumption down-regulated the activity of Lats2, an immediate upstream kinase of YAP, and decreased Ser127 phosphorylation of YAP. HFD also inhibited the activity of Lats2 in a mouse model of nonalcoholic steatohepatitis (20). Thus, a core component of the Hippo pathway seems to be inactivated in response to diabetes. Previous studies have suggested that O-GlcNAcylation may also be involved in the regulation of YAP in diabetic conditions in other cell types (21). However, it remains unclear whether these mechanisms up-regulate YAP through modulation of Mst1 or Lats2. Both the nuclear localization of YAP in cardiomyocytes and the total YAP expression in the heart were also significantly up-regulated in patients with HF who had diabetes.
HFD consumption exacerbates PO-induced HF through the YAP-TEAD pathway
Activation of the upstream components of the Hippo pathway, including Mst1 and Lats2, generally promotes apoptosis and inhibits cell proliferation through suppression of YAP 4, 5. Mst1 inhibits autophagy, thereby causing cellular dysfunction because of deficient protein quality control and eventual cell death (9). Thus, excessive activation of the Hippo pathway during myocardial ischemia and reperfusion, hemodynamic overload, and HF is generally detrimental for the heart (5). Interestingly, however, inactivation of the Hippo pathway below physiological levels through homozygous down-regulation of WW45, a scaffolding protein of the Hippo pathway, actually facilitates HF in the presence of PO (13). However, conditions in which the Hippo pathway is inactivated or the level of YAP is chronically elevated in the heart remained to be elucidated. We propose that metabolic syndrome and diabetes may fall into this category. Importantly, high blood pressure in the presence of metabolic syndrome or diabetes mellitus facilitates the development of HF in humans 2, 16. Because inhibition of YAP attenuated the progression of PO-induced HF in WW45cKO mice and those that consumed HFD, pharmacological inhibition of YAP may be an appropriate intervention under clinical conditions in which YAP is chronically elevated in the presence of hypertension. Because inhibition of YAP suppressed PO-induced dedifferentiation of cardiomyocytes and inflammation in the heart in the presence of HFD, YAP may promote HF by stimulating cardiomyocyte dedifferentiation and inflammation in diabetic hearts in the presence of high blood pressure.
TEAD1 mediates PO-induced cardiac dysfunction in diabetic hearts
YAP is a transcription factor cofactor that promotes downstream transcription factor activity through direct interaction (5). Although modest up-regulation of YAP alone may not induce a prominent phenotype in the heart at baseline, the superimposition of PO up-regulates TEAD1 in the nucleus, which in turn promotes transcription of genes involved in cell proliferation and dedifferentiation in the presence of YAP. Dedifferentiated cardiac muscle may not produce sufficient contractility against PO (13). The results of our loss-of-function experiments clearly show that endogenous TEAD1 is involved in the development of PO-induced HF in diabetic hearts. Furthermore, verteporfin is known to disrupt the interaction between YAP and TEADs (13). Thus, we propose that both YAP and TEAD may be effective targets to prevent the development of HF in diabetic patients with hypertension.
YAP inhibitors as therapeutic options in cardiac disease
In summary, persistent activation of YAP promotes PO-induced HF in the presence of HFD consumption in mice, mimicking the condition of obesity, metabolic syndrome, and insulin resistance in patients. Despite the generally protective roles of YAP in the heart, suppression of YAP may effectively improve some cardiovascular conditions. It is therefore important to identify additional conditions in which YAP and TEAD1 are coactivated and test the efficacy of YAP inhibitors in these conditions.
Study limitations
The detailed molecular mechanisms by which diabetes inhibits LATS2 remain to be clarified.
We conducted this investigation with only male mice. Sex differences are noted in terms of responses to HFD consumption in mice (22). Whether high blood pressure exacerbates diabetic cardiomyopathy, and if so, whether the YAP-TEAD1 pathway is involved in the exacerbation in female mice as well remain to be elucidated. The HFD mouse model may not fully recapitulate type 2 diabetes. Thus, the role of the YAP-TEAD1 pathway should be confirmed in additional models of type 2 diabetes. Likewise, TAC may not faithfully mimic the condition of hypertension in humans, and multiple interventions that chronically impose PO on the LV need to be tested. Although our verteporfin treatment effectively inhibits the action of YAP-TEAD1 in vivo, the specificity of verteporfin should be further confirmed with cardiac-specific YAP knockout mice.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE 1: More than half of diabetic patients develop cardiac dysfunction, termed diabetic cardiomyopathy, a condition that is exacerbated in the presence of high blood pressure. The underlying molecular mechanism by which high blood pressure exacerbates cardiac dysfunction in diabetic patients is poorly understood.
COMPETENCY IN MEDICAL KNOWLEDGE 2: The Hippo signaling pathway is an evolutionarily conserved signaling mechanism that controls organ size through regulation of apoptosis and cell proliferation. We found that YAP, the key nuclear transcription cofactor of the Hippo signaling pathway, and its downstream target, TEAD1, a transcription factor, are involved in the exacerbation of cardiac dysfunction in mice that consume a high-fat diet, a model of type 2 diabetes, in the presence of high blood pressure. The exacerbation of heart failure in diabetic hearts in the presence of high blood pressure was alleviated in the presence of a chemical inhibitor of YAP or genetic down-regulation of TEAD1. Although high blood pressure induced dedifferentiation of cardiomyocytes in diabetic hearts, this effect was alleviated in the presence of the YAP inhibitor or down-regulation of TEAD1. YAP is activated in human heart samples obtained from heart failure patients with a history of diabetes compared with those without a history of diabetes.
COMPETENCY IN MEDICAL KNOWLEDGE 3: Our study provides clinician scientists with novel insights regarding the molecular mechanism by which the presence of high blood pressure exacerbates the progression of cardiac dysfunction in patients with diabetes.
TRANSLATIONAL OUTLOOK 1: YAP and TEAD1 are critically involved in the development of heart failure in the diabetic heart in the presence of high blood pressure. Although YAP is generally protective against cardiac stress, it can be detrimental under some conditions. Our results clearly show that YAP can be an important therapeutic target in diabetic patients with high blood pressure.
TRANSLATIONAL OUTLOOK 2: Our study suggests that chemical inhibitors of YAP or TEAD1 may be effective in treating patients who have diabetes, high blood pressure, and obesity (metabolic syndrome) to prevent heart failure syndromes.
Acknowledgment
The authors thank Daniela Zablocki (Rutgers New Jersey Medical School) for assistance with the manuscript.
Footnotes
Dr. Sadoshima was supported in part by U.S. Public Health Service grants HL67724, HL91469, HL11233, HL132824, and AG23039, and by the Leducq Foundation Transatlantic Network of Excellence (15CBD04). Dr. Ikeda has been supported by a postdoctoral fellowship from the Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad and grants-in-aid for scientific research (18K15876). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Roger V.L. Epidemiology of heart failure. Circ Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrone-Filardi P., Paolillo S., Costanzo P., Savarese G., Trimarco B., Bonow R.O. The role of metabolic syndrome in heart failure. Eur Heart J. 2015;36:2630–2634. doi: 10.1093/eurheartj/ehv350. [DOI] [PubMed] [Google Scholar]
- 3.Seferovic P.M., Paulus W.J. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36:1718–1727. doi: 10.1093/eurheartj/ehv134. 1727a–c. [DOI] [PubMed] [Google Scholar]
- 4.Ma S., Meng Z., Chen R., Guan K.L. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- 5.Wackerhage H., Del Re D.P., Judson R.N., Sudol M., Sadoshima J. The Hippo signal transduction network in skeletal and cardiac muscle. Sci Signal. 2014;7:re4. doi: 10.1126/scisignal.2005096. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S., Yang G., Zablocki D. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest. 2003;111:1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Re D.P., Matsuda T., Zhai P. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol Cell. 2014;54:639–650. doi: 10.1016/j.molcel.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Re D.P., Yang Y., Nakano N. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J Biol Chem. 2013;288:3977–3988. doi: 10.1074/jbc.M112.436311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maejima Y., Kyoi S., Zhai P. Mst1 inhibits autophagy by promoting Beclin1-Bcl-2 interaction. Nat Med. 2013;19:1478–1488. doi: 10.1038/nm.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leach J.P., Heallen T., Zhang M. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature. 2017;550:260–264. doi: 10.1038/nature24045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Z., von Gise A., Zhou P. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115:354–363. doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xin M., Kim Y., Sutherland L.B. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda S., Mizushima W., Sciarretta S. Hippo deficiency leads to cardiac dysfunction accompanied by cardiomyocyte dedifferentiation during pressure overload. Circ Res. 2019;124:292–305. doi: 10.1161/CIRCRESAHA.118.314048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Qiao Y., Wu Q. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat Commun. 2017;8:15280. doi: 10.1038/ncomms15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J., Harris R.C. Interaction of the EGF receptor and the Hippo pathway in the diabetic kidney. J Am Soc Nephrol. 2016;27:1689–1700. doi: 10.1681/ASN.2015040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozkurt B., Aguilar D., Deswal A. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e535–e578. doi: 10.1161/CIR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura M., Liu T., Husain S. Glucogen synthase kinase-3α promotes fatty acid uptake and lipotoxic cardiomyopathy. Cell Metab. 2019;29:1119–1134.e12. doi: 10.1016/j.cmet.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page A., Dumesnil J.G., Clavel M.A. Metabolic syndrome is associated with more pronounced impairment of left ventricle geometry and function in patients with calcific aortic stenosis: a substudy of the ASTRONOMER (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin) J Am Coll Cardiol. 2010;55:1867–1874. doi: 10.1016/j.jacc.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 19.Frati G., Schirone L., Chimenti I. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc Res. 2017;113:378–388. doi: 10.1093/cvr/cvx011. [DOI] [PubMed] [Google Scholar]
- 20.Aylon Y., Gershoni A., Rotkopf R. The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes Dev. 2016;30:786–797. doi: 10.1101/gad.274167.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng C., Zhu Y., Zhang W. Regulation of the Hippo-YAP pathway by glucose sensor O-GlcNAcylation. Mol Cell. 2017;68:591–604.e5. doi: 10.1016/j.molcel.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Lainez N.M., Jonak C.R., Nair M.G. Diet-induced obesity elicits macrophage infiltration and reduction in spine density in the hypothalami of male but not female mice. Front Immunol. 2018;9:1992. doi: 10.3389/fimmu.2018.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.