Abstract
Chemoresistance is one of the leading causes of cancer-related deaths in the United States. Triple negative breast cancer (TNBC), a subtype lacking the known breast cancer receptors used for targeted therapy, is reliant on chemotherapy as the standard of care. The Adenomatous Polyposis Coli (APC) tumor suppressor is mutated or hypermethylated in 70% of sporadic breast cancers with APC-deficient tumors resembling the TNBC subtype. Using mammary tumor cells from the ApcMin/+ mouse model crossed to the Polyoma middle T antigen (PyMT) transgenic model, we previously showed that APC loss decreased sensitivity to doxorubicin (DOX). Understanding the molecular basis for chemoresistance is essential for the advancement of novel therapeutic approaches to ultimately improve patient outcomes. Resistance can be caused via different methods, but here we focus on the DNA repair response with DOX treatment. We show that MMTV-PyMT;ApcMin/+ cells have decreased DNA damage following 24 hour DOX treatment compared to MMTV-PyMT;Apc+/+ cells. This decreased damage is first observed 24 hours post-treatment and continues throughout 24 hours of drug recovery. Activation of DNA damage response pathways (ATM, Chk1, and Chk2) are decreased at 24 hours DOX-treatment in MMTV-PyMT;ApcMin/+ cells compared to control cells, but show activation at earlier time points. Using inhibitors that target DNA damage repair kinases (ATM, ATR, and DNA-PK), we showed that ATM and DNA-PK inhibition increased DOX-induced apoptosis in the MMTV-PyMT;ApcMin/+ cells. In the current work, we demonstrated that APC loss imparts resistance through decreased DNA damage response, which can be attenuated through DNA repair inhibition, suggesting the potential clinical use of DNA repair inhibitions as combination therapy.
Abbreviations: APC, Adenomatous Polyposis Coli; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; Chk1, checkpoint kinase 1; Chk2, checkpoint kinase 2; CIS, cisplatin; CC3, cleaved caspase 3; DNA-PK, DNA-dependent protein kinase; DSBs, double stranded breaks; DOX, doxorubicin; ER, estrogen receptor; Etop, etoposide; H2AX, histone 2AX; HRR, homologous recombination repair; HER2, human epidermal growth factor; NHEJ, non-homologous end joining; PTX, paclitaxel; PR, progesterone receptor; PyMT, polyoma middle T antigen; STAT3, activation of signal transducer and activation of transcription 3; SSBs, single stranded breaks; TNBC, triple negative breast cancer; Topo IIα, topoisomerase IIα
Introduction
Breast cancer is one of the most commonly diagnosed cancers among US women. There are several subtypes of breast cancer, each with a unique molecular signature, prognosis, and treatment regimen [1]. Triple negative breast cancer (TNBC) is characterized by its lack of the three most frequently upregulated receptors in breast cancer: estrogen (ER), progesterone (PR), and human epidermal growth factor 2 (HER2) receptors [2]. As an aggressive subtype of breast cancer, TNBC has a poor prognosis despite medical advances [2], [3]. This is due to the limited targeted therapy options for TNBC; therefore, chemotherapeutic agents are the standard of care for these patients [3], [4]. While tumors initially respond well to chemotherapy, they often develop resistance, which is a major obstacle in cancer therapy and is one of the leading causes of cancer-related death [5], [6].
Previously, we have shown that the loss of the tumor suppressor Adenomatous Polyposis Coli (APC) imparts chemoresistance in breast cancer cells [7]. The APC tumor suppressor is lost in up to 70% of sporadic breast cancers, either through mutation or hypermethylation [8], [9], [10]. APC-deficient tumors, specifically with promoter methylation, were shown to correlate with ER and PR negative subtype of breast cancer, demonstrating that APC-deficient tumors have limited targeted therapy options, which could contribute to their poorer prognosis [9]. Understanding how APC loss affects response to chemoresistance is essential in improving patient outcome. Using the ApcMin/+ mouse model, with a nonsense mutation in one allele of Apc, we identified enhanced breast tumorigenesis in the presence of the Polyoma middle T antigen (PyMT) oncogene [11]. Using cells derived from this model, MMTV-PyMT;ApcMin/+ cells, we have demonstrated decreased sensitivity to doxorubicin (DOX) [7], [12]. We have previously shown that DOX resistance is mediated partially through activation of signal transducer and activator of transcription 3 (STAT3), but how APC loss is affecting other chemoresistant mechanisms still requires further investigation [12]. Understanding how APC loss can reduce sensitivity will be essential in identifying combination therapies to overcome this resistance.
Knowing how the chemotherapeutic agents work provides a first clue into potential mechanisms by which tumor cells evade death by chemotherapy. Many chemotherapeutic agents induce DNA damage to cause apoptosis; however, cancer cells have developed mechanisms to either repair damage or prevent damage from inducing apoptosis [13]. Based on the damage incurred, the cell activates distinct repair pathways, or if left unrepaired the damage can induce cell cycle arrest, promoting senescence or apoptosis [13], [14]. Damage can include inter- and intra-strand DNA crosslinks, base damages, altered bases, stalled replication forks, single stranded breaks (SSBs), or double stranded breaks (DSBs). Due to the lethal nature of DSBs, many cancer therapeutics work by inducing such damage. DOX, a topoisomerase II (topo II) inhibitor [15], is one of the most common single or combination therapies for women with TNBC [6], and works through induction of DSBs. DSB repair can be mediated through homologous recombination repair (HRR) or nonhomologous end joining (NHEJ). HRR requires a sister chromatid to be used as a template to minimize errors in the DNA, but this limits the occurrence of HRR, only taking place in S and G2 phases of the cell cycle. NHEJ does not have such limitations and occurs throughout the cell cycle. Both repair pathways are mediated by the repair serine/threonine kinases: ataxia telangiectasia mutated (ATM) or ataxia telangiectasia and Rad3 related (ATR), but only NHEJ activates DNA-dependent protein kinase (DNA-PK). Recent studies have shown that DNA damage repair pathways can be enhanced in cancer cells providing a survival advantage after chemotherapy [13]. For instance, cancer cells often contain mutations in repair pathway proteins, including ATM, and additionally inhibiting these repair kinases can restore sensitivity to irradiation [16]. Furthermore, DNA repair proteins are increasingly mutated as cancer progresses making these repair pathways ideal areas of therapeutic targets [17]. Herein, we investigate the impact of APC loss on DNA damage response after DOX treatment in breast cancer.
Materials and methods
Cells and drug treatment
MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells were isolated as previously described from primary mouse mammary tumors [11] and grown in RPMI 1640 media supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1:5000 plasmocin (Invivogen). MMTV-PyMT;ApcMin/+ cells, expressing one truncated and one WT allele of APC, resemble the triple negative subtype of breast cancer cells (Supplemental Figure 1) [11]. Cells were passaged using 0.25% trypsin/EDTA and maintained at 37 °C with 5% CO2. Cells were plated and treated 24 hours later with each chemotherapeutic agent or solvent control: doxorubicin (500 nM, MP Biomedicals, LLC), etoposide (10 μM, Sigma), paclitaxel (2.5 μM, Sigma), cisplatin (16 μM, Sigma), VE-821 (10 μM, Selleckchem), KU-55955 (10 μM, Selleckchem), or NU-7441 (5 μM, Tocris). Drug treatments were performed for 24 hours unless otherwise indicated.
Immunoblot assays
Total protein lysates were isolated using a phosphatase inhibitor enhanced lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1% Triton-X, 0.5% NP-40, 50 mM NaF, 1 mM Na3VO4, 5 mM sodium pyrophosphate, 0.2 mM PMSF, 1x protease inhibitor cocktail (Fisher), and 1x phosphatase inhibitor cocktail 2 (Sigma)). Protein was loaded onto SDS-PAGE gels for electrophoresis and transferred to Immobilon-P membrane (Millipore). Membranes were blocked for 1 hour at room temperature in 5% non-fat dry milk in 1XTBS with 0.1% Tween (TBS-T). Anti-phospho H2AX (1:1000, Cell Signaling), anti-cleaved caspase 3 (1:1000, Cell Signaling), anti-phospho Chk1 Ser345 (1:500, Cell Signaling), anti-Total Chk1 (1:500, Abcam), anti-phospho Chk2 Thr68 (1:500, Abcam), anti-Total Chk2 (1:500, Abcam), anti-phospho ATM Ser1981 (1:500, GeneTex), anti-Total ATM (1:1000, Cell Signaling), anti-phospho ATR Thr1989 (1:1000, GeneTex), anti-Total ATR (1:1000, Cell Signaling), anti-HER2 (1:1000; Cell Signaling), anti-ER (1:1000, Santa Cruz), anti-PR (1:1000; Abcam) were used as primary antibodies. As loading controls, blots were probed for anti-β-Actin (1:25000, Sigma), anti-GAPDH (1:1000, Cell Signaling) or anti-Tubulin (1:1000, Sigma) for 1 hour at room temperature followed by 1 hour in secondary antibody. The phosphorylation of Chk1, Chk2, ATR, and ATM was normalized to total protein over GAPDH, Actin, or Tubulin. yH2AX and CC3 were normalized to Actin. Densitometry was performed using ImageJ Software.
Immunofluorescence
40,000 cells were plated on glass coverslips in a 12 well plate in triplicate and 24 hours later cells were treated as described above. After treatment, cells were fixed in 3.7% formaldehyde for 15 minutes and permeabilized in 0.3% Triton X-100 for 15 minutes. Antibodies were diluted in blocking buffer that consisted of 0.2% non-fat dry milk, 2% Bovine Serum Albumin and 0.3% Triton X-100 in phosphate buffered saline (PBS). Primary anti-phospho H2AX (1:650, Millipore) was applied to cells for 1 hour at 37 °C. Following washes in PBS, samples were incubated in goat-anti-rabbit Alexa Fluor 488 (1:1000, Life Technologies) and Alexa 555 conjugated Phalloidin (1:200, Life Technologies) to visualize F-actin. Slides were mounted with Fluoromount G with Hoescht (Sigma) to label cell nuclei. The percentage of positive cells was determined for each assay with at least 300 cells being counted per condition using the counting feature on an Evos Xl core microscope. Each assay was run in triplicate and repeated three times. Representative images were taken on Zeiss Axio A1 Microscope with an AxioCam MRc digital camera.
Alkaline comet assay
DNA damage was assessed using CometAssay ® Kit (Trevigen). Cells were resuspended in ice-cold 1X PBS to 1X105 cells/ml and mixed with molten LMAgarose at a volume ratio of 1:5. The cell/agarose mixture was spread evenly onto comet slides and then allowed to incubate at 4 °C in the dark for 30 minutes. The slides were then placed in cold lysis solution at 4 °C overnight. Slides were incubated in Alkaline Unwinding Solution pH > 13 (300 nM NaOH, 1 mM EDTA) for 1 hour at 4 °C in the dark, and then were electrophoresed at 1 Volt/cm at 300 mA for 45 minutes at 4 °C. Slides were washed twice with dH20 for 5 minutes, once with 70% ethanol for 5 minutes, and then dried at 37 °C. SYBR-Gold Staining Solution (1:10,000 in 10 mM Tris-HCL pH 7.5, 1 mM EDTA; Invitrogen) was used to stain the DNA for 30 minutes at room temperature in the dark. Images were captured using the Evos Xl core fluorescent microscope and at least 50 cells were analyzed for each slide using CometScore 2.0. Tail moment combines the amount of DNA in the tail with distance of migration.
Statistical analysis
All values are reported as mean ± SD. Data from multiple treatment groups were compared using a one-way ANOVA with a post-hoc Tukey’s t-test or a Kruskal-Wallis test.
Results
Decreased DNA damage in cells with low APC
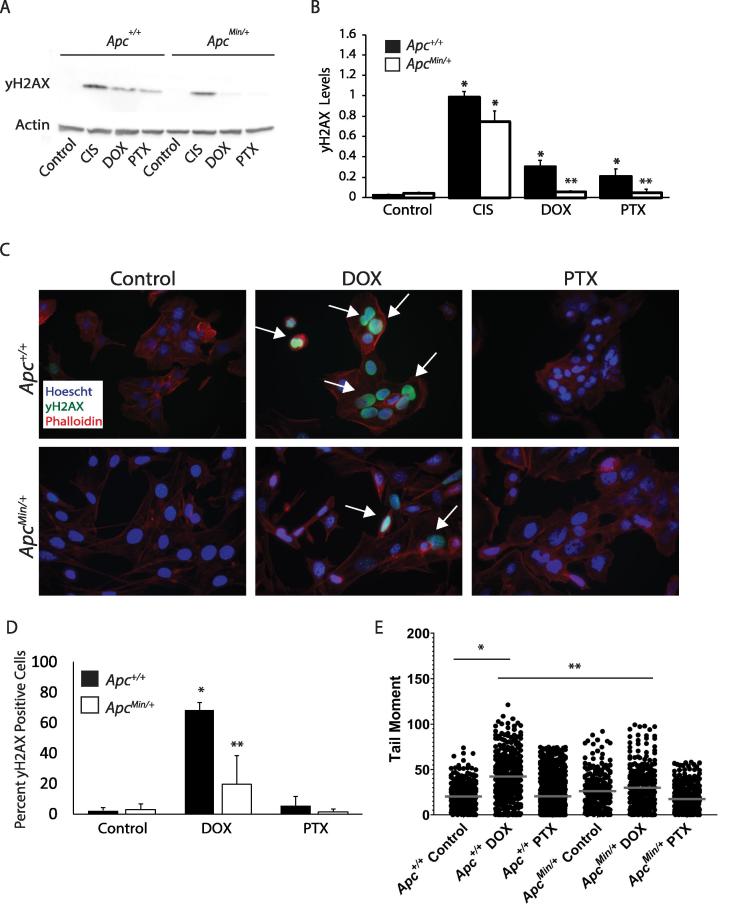
DOX causes DSBs in the DNA resulting in activation of two DNA repair pathways: NHEJ or HRR. One of the earliest events after DSBs is the phosphorylation of Ser139 on histone 2AX (H2AX), a known marker of DNA damage. The phosphorylated protein is then referred to as yH2AX. Following 24 hour treatment of chemotherapy drugs (DOX, PTX, or CIS), MMTV-PyMT;ApcMin/+ cells show decreased DNA damage signaling as measured by yH2AX. The decrease in yH2AX suggests decreased DNA damage is observed with APC loss following treatment with DOX or PTX (Figure 1A and B). This decreased DNA damage response was also seen by immunofluorescence where DOX treated MMTV-PyMT;ApcMin/+ cells showed a 40% decrease in the percent of yH2AX positive cells compared to MMTV-PyMT;Apc+/+ treated cells (Figure 1C and D). The alkaline comet assay was performed to determine whether the decreased yH2AX was due to decreased DNA damage following DOX treatment. Measurement of the tail moment showed that DOX treatment in the MMTV-PyMT;Apc+/+ cells resulted in an increased tail moment as expected. However, DOX-treated MMTV-PyMT;ApcMin/+ cells showed no change compared to the solvent control, and were significantly decreased compared to the DOX-treated control cells (Figure 1E). These data demonstrate that there is decreased DNA damage response following 24 hours of DOX treatment in MMTV-PyMT;ApcMin/+ cells compared to control. This decreased damage could be contributing to the DOX resistance we previously reported in APC-deficient cells [7].
Figure 1.
Decreased DOX-induced DNA damage in APC-deficient cells. A) Protein levels of phosphorylated H2AX (yH2AX), a marker of DNA damage, was measured in MMTV-PyMT;ApcMin/+ and MMTV-PyMT;Apc+/+ cells following 24 hr treatment of chemotherapeutic agents, cisplatin (CIS), doxorubicin (DOX), and paclitaxel (PTX). B) Quantification of western blots show that yH2AX was induced after DOX and PTX treatment in MMTV-PyMT;Apc+/+ cells, but not in MMTV-PyMT;ApcMin/+ cells. In contrast CIS treatment induced equal damage in both cell lines. C) Representative images of yH2AX immunofluorescence (IF) in DOX and PTX-treated MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. White arrows depict yH2AX positive cells, and the scale bar is 20 microns. D) Quantification of yH2AX IF images show an increased percentage of yH2AX in DOX-treated MMTV-PyMT;Apc+/+ cells, which is reduced in DOX treated MMTV-PyMT;ApcMin/+ cells. Each experiment was repeated 3 times, at least 150 cells were counted per condition in each experiment. E) MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells were treated with DOX or PTX for 24 hours and DNA damage was assessed by the comet assay. The tail moment was measured and showed that DOX-treated MMTV-PyMT;Apc+/+ cells have increased tail moment compared to solvent control. DOX-treated MMTV-PyMT;ApcMin/+ cells show no change compared to solvent control, and were significantly decreased compared to the DOX-treated MMTV-PyMT;Apc+/+ cells. The data are shown as means ± SD; *P < 0.05 comparing MMTV-PyMT;ApcMin/+ or MMTV-PyMT;Apc+/+ cells treated to solvent control and **P < 0.05 comparing MMTV-PyMT;ApcMin/+ to MMTV-PyMT;Apc+/+ cells.
Decreased DNA damage over time
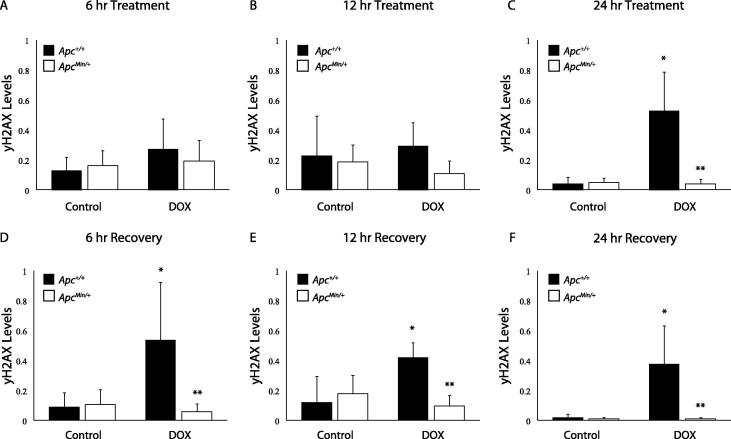
To identify if this decreased DNA damage after 24 hours of DOX treatment is due to reduced DNA damage response, we observed the dynamics of yH2AX formation and loss following treatment and recovery. Cells were treated with DOX for 6, 12, and 24 hours, with drug treatment being removed at 24 hours. Fresh media was given, and protein was collected at 6, 12, and 24 hours of recovery. In agreement with our initial DNA damage data (Figure 1), yH2AX was not differentially detected in the APC-deficient cells compared to controls until 24 hours of DOX treatment. This decrease in yH2AX levels continued throughout recovery (Figure 2A–F), demonstrating that the 24 hour timepoint is pivotal in understanding the mechanism behind decreased DNA damage signaling (Figure 2C). Cells were also treated with Etoposide (Etop), another topo II inhibitor, to determine whether the damage response is DOX-specific. APC status did not alter the response to Etop until 12 hours of recovery (Supplemental Figure 2E). Decreased DNA damage with APC loss is not specific to DOX treatment.
Figure 2.
Decreased DNA Damage Response after DOX treatment in APC-deficient cells. A-C) yH2AX protein levels were measured after 6, 12, or 24 hr treatment of DOX in MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. After 6 (A) and 12 (B) hrs, treatment with DOX resulted in no change in yH2AX in either cell line. C) After 24 hr treatment, DOX induced yH2AX in MMTV-PyMT;Apc+/+ cells, but not in MMTV-PyMT;ApcMin/+ cells. D–F) Following 24 hr treatment, drug was removed and fresh media was added to MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. Protein was collected at 6, 12, or 24 hr post-recovery. After 6 hr recovery (D), DOX induced yH2AX in MMTV-PyMT;Apc+/+ cells, but not in MMTV-PyMT;ApcMin/+ cells. E) After 12 hr recovery, DOX treated MMTV-PyMT;Apc+/+ cells had increased yH2AX protein levels. No DNA damage was measured in MMTV-PyMT;ApcMin/+ cells treated with DOX. F) DOX treated MMTV-PyMT;Apc+/+ cells exhibited increased yH2AX levels following 24 hr recovery, but no increase in yH2AX was observed in MMTV-PyMT;ApcMin/+ cells. *P < 0.05 comparing MMTV-PyMT;ApcMin/+ or MMTV-PyMT;Apc+/+ cells treated to solvent control and **P < 0.05 comparing MMTV-PyMT;ApcMin/+ to MMTV-PyMT;Apc+/+ cells.
Decreased DNA damage repair signaling
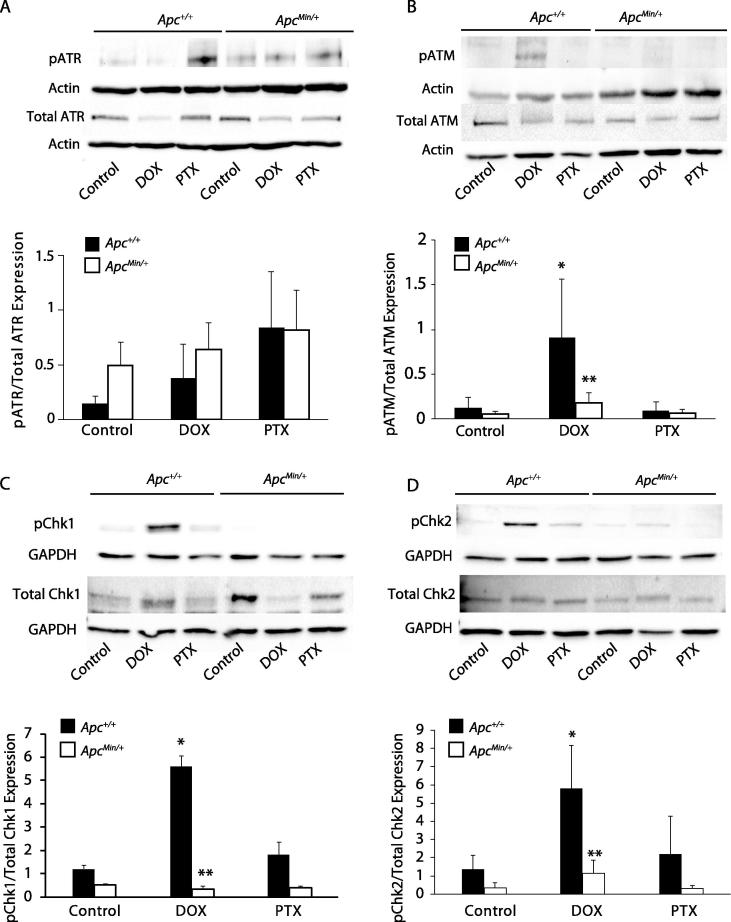
DSBs are repaired via HRR and NHEJ through the PI3K related kinases: ATM, ATR, and DNA-PK. These kinases are responsible for phosphorylating substrates for cell cycle arrest, checkpoint activation, and repair pathways [18]. We first wanted to see whether DOX treatment activated these kinases. Using the 24 hour treatment that we determined from measuring yH2AX dynamics, we measured activation of ATR via phosphorylation of ATR at Thr1989, and no change was observed with APC loss and treatment of either DOX or PTX (Figure 3A). Activation of ATM at 24 hrs post-treatment was measured via expression of phosphorylated ATM at Ser1981. DOX treatment increased ATM activation in MMTV-PyMT;Apc+/+ cells, but no change was observed in the APC-deficient cells (Figure 3B). PTX treatment caused no activation of ATM in either cell line (Figure 3B). To verify activation, we also assessed downstream targets of ATR and ATM, checkpoint kinase 1 (Chk1) and checkpoint kinase 2 (Chk2), respectively. In contrast to the results of pATR expression, Chk1 phosphorylation was observed in DOX-treated MMTV-PyMT;Apc+/+ cells but not in the MMTV-PyMT;ApcMin/+ cells (Figure 3C). In addition, phosphorylation of Chk2 was observed in MMTV-PyMT;Apc+/+ cells that were treated 24 hours with DOX, but was absent in DOX-treated MMTV-PyMT;ApcMin/+ cells (Figure 3D). PTX treatment resulted in no activation of Chk1 or Chk2 independent of APC (Figure 3C and D). MMTV-PyMT;ApcMin/+ cells show decreased DNA damage following 24 hours DOX treatment and decreased activation of DNA repair at this timepoint. The above studies have investigated a static time point of 24 hrs post treatment. While MMTV-PyMT;ApcMin/+ cells did not show ATM activation at 24 hours, a time course study of 6, 12, and 24 hrs DOX treatment showed ATM activation in MMTV-PyMT;ApcMin/+ cells at 12 hrs post-treatment (Supplemental Figure 3). Overall, there is reduced activation, and alteration of timing, of DSB repair response in the MMTV-PyMT;ApcMin/+ cells.
Figure 3.
ATM-directed DNA damage repair in DOX treated cells. MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells were treated for 24 hrs with DOX and PTX. Protein was isolated and western blots were used to determine expression of DNA damage repair components. A) No change was observed in ATR phosphorylation (Thr1989) in either cell line following treatment. B) ATM activation was measured via phosphorylation of ATM (Ser1981). Increased phosphorylation of ATM was observed in DOX treated MMTV-PyMT;Apc+/+ cells, but not in DOX treated MMTV-PyMT;ApcMin/+ cells. C) DOX treated MMTV-PyMT;Apc+/+ cells expressed increased phosphorylation of Chk1 (Ser345), which is absent in MMTV-PyMT;ApcMin/+ cells. D) Increased phosphorylation of Chk2 (Thr68) was observed in DOX treated MMTV-PyMT;Apc+/+ cells, with no change observed in DOX treated MMTV-PyMT;ApcMin/+ cells. *P < 0.05 comparing MMTV-PyMT;ApcMin/+ or MMTV-PyMT;Apc+/+ cells treated to solvent control and **P < 0.05 comparing MMTV-PyMT;ApcMin/+ to MMTV-PyMT;Apc+/+ cells.
Inhibition of DNA repair pathways
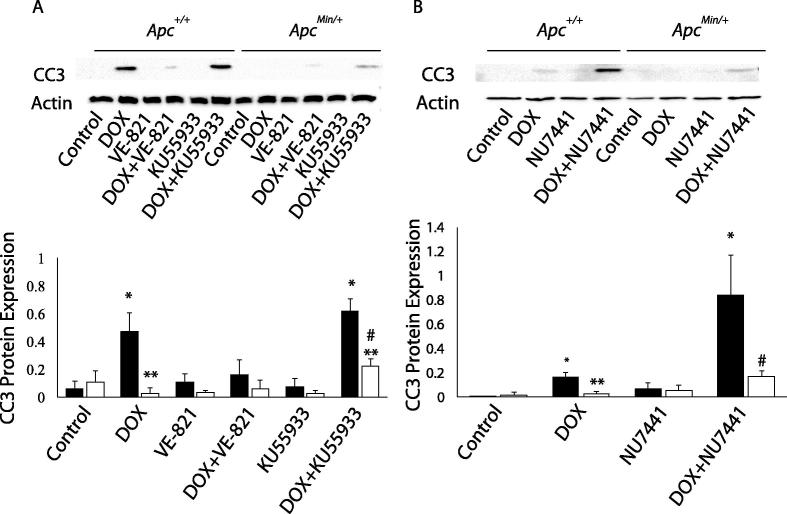
Due to reduced DNA damage response with APC loss, we investigated whether inhibiting DNA repair can prevent the APC-induced DOX resistance. DNA repair inhibitors, including those of ATR and ATM, have been shown to increase sensitivity to radiotherapy and DNA-damaging chemotherapy in cells that are DNA repair deficient [15], [19], [20], [21]. Therefore, we used inhibitors of ATR and ATM, VE-821 and KU55933, respectively, to increase DOX-induced apoptosis, as measured by cleaved caspase 3 (CC3), in MMTV-PyMT;ApcMin/+ cells compared to control cells. We observed that ATM inhibition via KU55933 treatment increased the DOX-induced apoptosis in MMTV-PyMT;ApcMin/+ cells compared to control cells (Figure 4A), while inhibition of ATR via VE-821 treatment did not. Since DOX induces DSBs, which can be repaired by HRR or NHEJ, and with NHEJ occurring throughout the cell cycle, we also inhibited the NHEJ-specific kinase DNA-PK using NU7441 [22]. NU7441 in combination with DOX increased DOX-mediated apoptosis in MMTV-PyMT;ApcMin/+ cells compared to single treatment with DOX in MMTV-PyMT;ApcMin/+ cells (Figure 4B). Combination treatment in MMTV-PyMT;Apc+/+ cells augmented DOX-mediated apoptosis demonstrating the importance of NHEJ in preserving cancer cells during DOX treatment.
Figure 4.
DNA repair inhibition increases DOX-induced apoptosis. A) Following 24 hr treatment with single agents or combination of DOX with either VE-821 or KU55933, apoptosis was measured via cleaved caspase 3 (CC3). Treatment with VE-821 or KU55933 alone did not increase CC3 expression. Surprisingly, treatment with DOX and VE-821 decreased DOX-induced apoptosis in MMTV-PyMT;Apc+/+ cells. KU55933 treatment in combination with DOX-induced apoptosis in MMTV-PyMT;ApcMin/+ cells. B) CC3 expression was measured following 24 hr treatment of DOX, NU7441, or the combination in MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. NU7441 alone did not induce CC3 expression. The combination increased CC3 in MMTV-PyMT;Apc+/+ cells and increased the apoptotic response to DOX in MMTV-PyMT;ApcMin/+ cells. *P < 0.05 comparing MMTV-PyMT;ApcMin/+ or MMTV-PyMT;Apc+/+ cells treated to solvent control, **P < 0.05 comparing MMTV-PyMT;ApcMin/+ to MMTV-PyMT;Apc+/+ cells, and #P < 0.05 comparing the combination treatment versus the single agent (DOX).
Discussion
We previously demonstrated decreased sensitivity to DOX in MMTV-PyMT;ApcMin/+ cells compared to MMTV-PyMT;Apc+/+ cells [7], [12]. APC has a role in base excision repair, and cancer cells have been shown to have altered DNA repair pathways [23], [24]. However, it is unclear whether APC loss can enhance repair of the DSBs through NHEJ or HR repair. We investigated how loss of APC affected DNA repair response to DOX-induced DSBs. We show that repair to DOX-induced damage is mediated through activation of ATM/Chk1/Chk2, which is absent with APC loss. Targeting DSB repair pathways, HRR and NHEJ, showed promise at increasing DOX-induced apoptosis in MMTV-PyMT;ApcMin/+ cells.
Activation of ATM appears to play a more significant role in response to DOX treatment. DOX treatment activated ATM, but not ATR, in MMTV-PyMT;Apc+/+ cells, whereas MMTV-PyMT;ApcMin/+ cells showed reduced activation of ATM, but not ATR. While this suggests an importance of ATM activation in DOX response, activation of the downstream proteins of ATM and ATR, Chk2 and Chk1 respectively, both showed activation with DOX treatment in MMTV-PyMT;Apc+/+cells, which was absent with APC loss. Phosphorylation of Chk1 has been shown to be a better indicator of ATR activation, as opposed to the punitive marker, pATR [25]. While there is crosstalk between ATM and ATR kinase activity, the combination treatments of DOX with the ATM inhibitor (KU55933) or the ATR inhibitor (VE-821) on CC3 protein expression demonstrates the importance of ATM activation compared to ATR activation [26], [27]. Single treatment of KU55933 or VE-821 did not induce apoptosis in either cell line. In combination with DOX, only KU55933 showed a synergistic effect at inducing apoptosis specifically in the MMTV-PyMT;ApcMin/+ cells. This supports the activation of ATM being required for response to DOX treatment. The mechanism by which APC loss results in decreased phosphorylation of ATM/Chk1/Chk2 is unclear at this point.
Targeting NHEJ through inhibition of DNA-PK also increased DOX-induced apoptosis. Due to NHEJ not needing a sister chromatid present as a template for repair, NHEJ is more commonly used to repair DSBs by ligating both ends of the break [22], [28]. However, both NHEJ and HRR appear important in inducing DOX-mediated DNA repair with both ATM and DNA-PK inhibition re-sensitizing cells to DOX treatment. Future studies will investigate whether decreased damage is due to reduced intracellular DOX concentration, increased DNA repair efficiency, or decreased functionality of DOX.
Previously, we have shown that APC loss increased expression of the ATP-dependent efflux pump, multidrug resistance protein 1 (MDR1) through STAT3 activation [12]. MDR1 expression is induced with DOX exposure leading to resistance [29]. Therefore, it is possible that there is reduced intracellular DOX resulting in decreased DNA damage. STAT3 has also been shown to affect DNA repair, with low STAT3 decreasing ATM and ATR signaling through STAT3 transcriptional regulation of mediator of DNA damage checkpoint protein 1 (MDC1) [30]. This would suggest that enhanced STAT3 could be increasing ATM and ATR signaling resulting in enhanced repair. In addition, APC is known to bind to topoisomerase IIα (topo IIα), a target of DOX [31], [32]. DOX functions through intercalation into the DNA and inhibition of topo IIα, resulting in an accumulation of DSBs and induction of apoptosis. Whether this loss of interaction between APC and topo IIα affects the efficacy of DOX is unknown. Lack of APC could alter topo IIα activity, resulting in decreased DSBs with DOX exposure. Further study will investigate these three mechanisms that could be causing this decreased DNA damage at 24 hours in MMTV-PyMT;ApcMin/+ cells. The mechanism behind this reduced DNA damage response will be investigated to find how APC is imparting DOX resistance to access potential combination therapies to restore DOX sensitivity. Targeting the DNA repair pathways responsible for DSBs, NHEJ and HRR, can increase DOX sensitivity. ATM activation is critical in DOX-mediated repair, more so than ATR or DNA-PK, as demonstrated through inhibition of ATM showing the most therapeutic potential. In vivo mouse studies will be required to confirm this combination therapy.
Conclusion
Overall, we have shown that APC loss results in decreased DNA damage response to DOX. This decreased damage contributes to the reduced sensitivity to DOX that we previously have shown with APC loss [7], [12]. Targeting ATM and DNA-PK can increase DOX-induced apoptosis. This study suggests that targeting DNA repair pathways can be therapeutically advantageous in DOX-resistance breast cancer.
Acknowledgements
This research was supported by the American Cancer Society – Institutional Research Grant (JRP), ACS-Research Scholar Grant (JRP), the Indiana Clinical and Translational Sciences Institute, funded in part by grant #UL1 TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (JRP) (A. Shekhar, PI), Start-up funds from Indiana University School of Medicine – South Bend, and the Navari Family Foundation (JRP). CDS was supported by the Walther Cancer Foundation Interdisciplinary Interface Training Project. The authors would like to thank Jacqueline Kirsch for her technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2019.09.002.
Contributor Information
Casey D. Stefanski, Email: cstefans@nd.edu.
Kaitlyn Keffler, Email: kaitlyn.n.keffler.1@nd.edu.
Stephanie McClintock, Email: smcclint@nd.edu.
Lauren Milac, Email: lmilac@nd.edu.
Jenifer R. Prosperi, Email: jrprospe@iupui.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Receptor Status in APC-deficient cells. A) There was no HER2 amplification in either MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells as compared to the negative control of E0771 cells, a triple negative murine breast cancer cell line, and to MMTV-Neu tumor tissue lysate [33], [34]. B) MMTV-PyMT;ApcMin/+ cells show decreased ER expression compared to MMTV-PyMT;Apc+/+ cells, and comparable levels of ER expression to the negative E0771 cells. C) There was no PR expression in either MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells as compared to the negative control of E0771 cells. The uterine protein lysate was used as a positive control.
Decreased DNA Damage Response after DOX and Etop treatment in APC-deficient cells. A–C) After 6, 12, or 24 hr Etop treatment, yH2AX protein level was observed in MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. No change in yH2AX was observed in Etop treated MMTV-PyMT;ApcMin/+ cells compared to MMTV-PyMT;Apc+/+ cells throughout treatment. D-F) Following 24 hr treatment, drug was removed and fresh media was added to MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. Protein was collected at 6, 12, or 24 hr post-recovery. Following 6 hr recovery (D), Etop induced yH2AX in MMTV-PyMT;Apc+/+ cells, but not in MMTV-PyMT;ApcMin/+ cells. This decreased yH2AX in MMTV-PyMT;ApcMin/+ cells was observed throughout recovery. No DNA damage was measured in MMTV-PyMT;ApcMin/+ treated cells.
ATM activation after DOX treatment in MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. After 6, 12, or 24 hr DOX treatment, ATM activation was observed in MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. A) Representative western blots showing that ATM activation was seen in both MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells following DOX treatment but at different time points. B) MMTV-PyMT;Apc+/+ cells showed activation throughout the time course starting at 6 hrs and continuing up to 24 hrs. C) MMTV-PyMT;ApcMin/+ cells only showed activation at 12 hrs of treatment. *P < 0.05 comparing MMTV-PyMT;ApcMin/+ or MMTV-PyMT;Apc+/+ cells DOX treated to solvent control.
References
- 1.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. [DOI] [PMC free article] [PubMed]
- 2.Aysola K, Desai A, Welch C, Xu J, Qin Y, Reddy V, et al. Triple negative breast cancer – an overview. Hereditary Genet. 2013;2013(Suppl 2). [DOI] [PMC free article] [PubMed]
- 3.Mayer I.A., Abramson V.G., Lehmann B.D., Pietenpol J.A. New strategies for triple-negative breast cancer–deciphering the heterogeneity. Clin Cancer Res. 2014;20(4):782–790. doi: 10.1158/1078-0432.CCR-13-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovitt C.J., Shelper T.B., Avery V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer. 2018;18(1):41. doi: 10.1186/s12885-017-3953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8(35):59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Reilly E.A., Gubbins L., Sharma S., Tully R., Guang M.H., Weiner-Gorzel K. The fate of chemoresistance in triple negative breast cancer (TNBC) BBA Clin. 2015;3:257–275. doi: 10.1016/j.bbacli.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VanKlompenberg M.K., Bedalov C.O., Soto K.F., Prosperi J.R. APC selectively mediates response to chemotherapeutic agents in breast cancer. BMC Cancer. 2015;15:457. doi: 10.1186/s12885-015-1456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad C.P., Mirza S., Sharma G., Prashad R., DattaGupta S., Rath G. Epigenetic alterations of CDH1 and APC genes: relationship with activation of Wnt/beta-catenin pathway in invasive ductal carcinoma of breast. Life Sci. 2008;83(9–10):318–325. doi: 10.1016/j.lfs.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee N., Bhattacharya N., Alam N., Roy A., Roychoudhury S., Panda C.K. Subtype-specific alterations of the Wnt signaling pathway in breast cancer: clinical and prognostic significance. Cancer Sci. 2012;103(2):210–220. doi: 10.1111/j.1349-7006.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- 10.Van der Auwera I., Van Laere S.J., Van den Bosch S.M., Van den Eynden G.G., Trinh B.X., van Dam P.A. Aberrant methylation of the Adenomatous Polyposis Coli (APC) gene promoter is associated with the inflammatory breast cancer phenotype. Br J Cancer. 2008;99(10):1735–1742. doi: 10.1038/sj.bjc.6604705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prosperi J.R., Khramtsov A.I., Khramtsova G.F., Goss K.H. Apc mutation enhances PyMT-induced mammary tumorigenesis. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0029339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.VanKlompenberg M.K., Leyden E., Arnason A.H., Zhang J.T., Stefanski C.D., Prosperi J.R. APC loss in breast cancer leads to doxorubicin resistance via STAT3 activation. Oncotarget. 2017;8(61):102868–102879. doi: 10.18632/oncotarget.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torgovnick A., Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet. 2015;6 doi: 10.3389/fgene.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosoya N., Miyagawa K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014;105(4):370–388. doi: 10.1111/cas.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavande N.S., VanderVere-Carozza P.S., Hinshaw H.D., Jalal S.I., Sears C.R., Pawelczak K.S. DNA repair targeted therapy: the past or future of cancer treatment? Pharmacol Ther. 2016;160:65–83. doi: 10.1016/j.pharmthera.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber A.M., Ryan A.J. ATM and ATR as therapeutic targets in cancer. Pharmacol Ther. 2015;149:124–138. doi: 10.1016/j.pharmthera.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Kelley M.R., Logsdon D., Fishel M.L. Targeting DNA repair pathways for cancer treatment: what’s new? Future Oncol (London, England) 2014;10(7):1215–1237. doi: 10.2217/fon.14.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurz E.U., Lees-Miller S.P. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amst) 2004;3(8–9):889–900. doi: 10.1016/j.dnarep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Prevo R., Fokas E., Reaper P.M., Charlton P.A., Pollard J.R., McKenna W.G. The novel ATR inhibitor VE-821 increases sensitivity of pancreatic cancer cells to radiation and chemotherapy. Cancer Biol Ther. 2012;13(11):1072–1081. doi: 10.4161/cbt.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T., Shen Y., Chen Y., Hsieh J.-T., Kong Z. The ATM inhibitor KU55933 sensitizes radioresistant bladder cancer cells with DAB2IP gene defect. Int J Radiat Biol. 2015;91(4):368–378. doi: 10.3109/09553002.2015.1001531. [DOI] [PubMed] [Google Scholar]
- 21.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seluanov A., Mao Z., Gorbunova V. Analysis of DNA double-strand break (DSB) repair in mammalian cells. J Vis Exp. 2010;43 doi: 10.3791/2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaiswal A.S., Narayan S. A novel function of adenomatous polyposis coli (APC) in regulating DNA repair. Cancer Lett. 2008;271(2):272–280. doi: 10.1016/j.canlet.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaiswal A.S., Balusu R., Armas M.L., Kundu C.N., Narayan S. Mechanism of adenomatous polyposis coli (APC)-mediated blockage of long-patch base excision repair. Biochemistry. 2006;45(51):15903–15914. doi: 10.1021/bi0607958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods D., Turchi J.J. Chemotherapy induced DNA damage response: convergence of drugs and pathways. Cancer Biol Ther. 2013;14(5):379–389. doi: 10.4161/cbt.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maréchal A., Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harbor Persp Biol. 2013;5(9) doi: 10.1101/cshperspect.a012716. a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuadrado M., Martinez-Pastor B., Murga M., Toledo L.I., Gutierrez-Martinez P., Lopez E. ATM regulates ATR chromatin loading in response to DNA double-strand breaks. J Exp Med. 2006;203(2):297. doi: 10.1084/jem.20051923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiruvella K.K., Liang Z., Wilson T.E. Repair of double-strand breaks by end joining. Cold Spring Harbor Persp Biol. 2013;5(5) doi: 10.1101/cshperspect.a012757. a012757-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabe Y., Konopleva M., Contractor R., Munsell M., Schober W.D., Jin L. Up-regulation of MDR1 and induction of doxorubicin resistance by histone deacetylase inhibitor depsipeptide (FK228) and ATRA in acute promyelocytic leukemia cells. Blood. 2006;107(4):1546–1554. doi: 10.1182/blood-2004-10-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry S.P., Townsend P.A., Knight R.A., Scarabelli T.M., Latchman D.S., Stephanou A. STAT3 modulates the DNA damage response pathway. Int J Exp Pathol. 2010;91(6):506–514. doi: 10.1111/j.1365-2613.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Azuma Y., Moore D., Osheroff N., Neufeld K.L. Interaction between tumor suppressor adenomatous polyposis coli and topoisomerase IIalpha: implication for the G2/M transition. Mol Biol Cell. 2008;19(10):4076–4085. doi: 10.1091/mbc.E07-12-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Coffey R.J., Osheroff N., Neufeld K.L. Topoisomerase IIalpha binding domains of adenomatous polyposis coli influence cell cycle progression and aneuploidy. PLoS One. 2010;5(4)::e9994-e. doi: 10.1371/journal.pone.0009994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnstone C.N., Smith Y.E., Cao Y., Burrows A.D., Cross R.S.N., Ling X. Functional and molecular characterisation of EO771.LMB tumours, a new C57BL/6-mouse-derived model of spontaneously metastatic mammary cancer. Dis Model Mech. 2015;8(3):237–251. doi: 10.1242/dmm.017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crosby E.J., Wei J., Yang X.Y., Lei G., Wang T., Liu C.-X. Complimentary mechanisms of dual checkpoint blockade expand unique T-cell repertoires and activate adaptive anti-tumor immunity in triple-negative breast tumors. OncoImmunology. 2018;7(5) doi: 10.1080/2162402X.2017.1421891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Receptor Status in APC-deficient cells. A) There was no HER2 amplification in either MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells as compared to the negative control of E0771 cells, a triple negative murine breast cancer cell line, and to MMTV-Neu tumor tissue lysate [33], [34]. B) MMTV-PyMT;ApcMin/+ cells show decreased ER expression compared to MMTV-PyMT;Apc+/+ cells, and comparable levels of ER expression to the negative E0771 cells. C) There was no PR expression in either MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells as compared to the negative control of E0771 cells. The uterine protein lysate was used as a positive control.
Decreased DNA Damage Response after DOX and Etop treatment in APC-deficient cells. A–C) After 6, 12, or 24 hr Etop treatment, yH2AX protein level was observed in MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. No change in yH2AX was observed in Etop treated MMTV-PyMT;ApcMin/+ cells compared to MMTV-PyMT;Apc+/+ cells throughout treatment. D-F) Following 24 hr treatment, drug was removed and fresh media was added to MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. Protein was collected at 6, 12, or 24 hr post-recovery. Following 6 hr recovery (D), Etop induced yH2AX in MMTV-PyMT;Apc+/+ cells, but not in MMTV-PyMT;ApcMin/+ cells. This decreased yH2AX in MMTV-PyMT;ApcMin/+ cells was observed throughout recovery. No DNA damage was measured in MMTV-PyMT;ApcMin/+ treated cells.
ATM activation after DOX treatment in MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. After 6, 12, or 24 hr DOX treatment, ATM activation was observed in MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells. A) Representative western blots showing that ATM activation was seen in both MMTV-PyMT;Apc+/+ and MMTV-PyMT;ApcMin/+ cells following DOX treatment but at different time points. B) MMTV-PyMT;Apc+/+ cells showed activation throughout the time course starting at 6 hrs and continuing up to 24 hrs. C) MMTV-PyMT;ApcMin/+ cells only showed activation at 12 hrs of treatment. *P < 0.05 comparing MMTV-PyMT;ApcMin/+ or MMTV-PyMT;Apc+/+ cells DOX treated to solvent control.