Abstract
Biogenic volatile organic compounds (BVOCs) influence organism fitness by promoting stress resistance and regulating trophic interactions. Studies examining BVOC emissions have predominantly focussed on terrestrial ecosystems and atmospheric chemistry – surprisingly, highly productive marine ecosystems remain largely overlooked. Here we examined the volatilome (total BVOCs) of the microalgal endosymbionts of reef invertebrates, Symbiodiniaceae. We used GC-MS to characterise five species (Symbiodinium linucheae, Breviolum psygmophilum, Durusdinium trenchii, Effrenium voratum, Fugacium kawagutii) under steady-state growth. A diverse range of 32 BVOCs were detected (from 12 in D. trenchii to 27 in S. linucheae) with halogenated hydrocarbons, alkanes and esters the most common chemical functional groups. A thermal stress experiment on thermally-sensitive Cladocopium goreaui and thermally-tolerant D. trenchii significantly affected the volatilomes of both species. More BVOCs were detected in D. trenchii following thermal stress (32 °C), while fewer BVOCs were recorded in stressed C. goreaui. The onset of stress caused dramatic increases of dimethyl-disulfide (98.52%) in C. goreaui and nonanoic acid (99.85%) in D. trenchii. This first volatilome analysis of Symbiodiniaceae reveals that both species-specificity and environmental factors govern the composition of BVOC emissions among the Symbiodiniaceae, which potentially have, as yet unexplored, physiological and ecological importance in shaping coral reef community functioning.
Subject terms: Biogeochemistry, Biochemistry, Ocean sciences, Marine chemistry, Analytical biochemistry, Mass spectrometry
Introduction
Photosynthetic organisms, ranging from complex vascular plants to single celled microalgae, are major producers of biogenic volatile organic compounds (BVOCs), which can represent up to 10% of fixed carbon and play numerous physiological and ecological roles1,2. BVOCs are exceptionally diverse and highly reactive, with chemical lifetimes ranging from minutes (e.g. β-caryophyllene) to months (e.g. acetone)3, and can be synthesised as by-products of metabolic pathways4 or be produced to maintain metabolic homeostasis3. The roles played by these compounds are multifaceted, from protection against abiotic stress5,6 and pathogens7,8, to chemical signalling9–12.
Terrestrial tropical ecosystems are well recognised “hotspots” of global BVOC emissions13, but growing evidence also highlights the role of tropical marine ecosystems, such as coral reefs14,15 as major sources of BVOC emissions. Reef-building corals emit the highest recorded concentrations of the sulphur gas dimethyl sulfide (DMS; up to 18.7 µM reported in coral mucus)16,17, a compound involved in climate regulation18, coral stress response5,19 and functioning as an infochemical20,21. Furthermore, the presence of acetone and dichloromethane was recently identified in reef seawater samples, indicating BVOCs produced in coral reefs are likely to be a complex mixture22. In addition, cultures of the dinoflagellate endosymbionts of reef-building corals (Family: Symbiodiniaceae) can produce DMS19,23,24 and isoprene25. However, these observations are derived from targeted quantifications of specific BVOCs and likely represent only a small fraction of the compounds that Symbiodiniaceae can produce.
Emission of BVOCs from corals are thought to largely originate from Symbiodiniaceae due to the large quantities of carbon and metabolites they translocate26,27. Metabolic coupling between coral host and algal endosymbionts is critical for viable coral reef functioning – whereby the Symbiodiniaceae drive coral productivity, but can also govern susceptibility of their host to stressors by controlling the exchange of nutrients28. Recent studies have moved towards identifying the functional diversity amongst the Symbiodiniaceae to determine key traits of resistance to stress29,30, which is critical to understand their susceptibility to rising seawater temperatures. Whilst different metabolic traits and hence diagnostic metabolites appear central in governing this functional diversity31, the role of BVOCs remains largely unexplored.
The assortment of BVOCs produced by an organism has recently been termed the ‘volatilome’32,33. Assessments of volatilomes, or volatilomics, is widely used in medical research, where the approach has been used to diagnose patient health via bio-markers in exhaled breath34. More recently, volatilomics has also been used to quickly distinguish plant strains and to successfully monitor long-term plant health35, with this approach showing promise for the development of new non-invasive monitoring tools of taxonomic and functional diversity in aquatic ecosystems33. Indeed, volatilomics may be particularly useful in connecting ecological processes and biogeochemical cycles in the ocean, since emissions of BVOCs from photosynthetic organisms can have a strong influence on atmospheric chemistry by increasing local cloud albedo or the residence time of greenhouse gases36,37. Within this context, characterising the volatilome of highly productive, habitat forming marine organisms should enable more accurate modelling of the impact of tropical BVOCs on climate regulation2,38.
Here we provide the first characterisation of the volatilome of different Symbiodiniaceae species (spanning five genera) to determine whether and how volatile metabolite signatures are conserved across divergent taxa, and to identify volatiles that may be involved in previously overlooked physiological processes, ecosystem interactions and atmospheric cycling. In addition to this screening across-strains, we also examined the volatilomes of two Symbiodiniaceae strains with different thermal tolerance thresholds under conditions of heat stress, to investigate the extent of thermally-induced changes in the volatilome and identify BVOCs potentially involved in Symbiodiniaceae thermal tolerance.
Results
Initial screening experiment
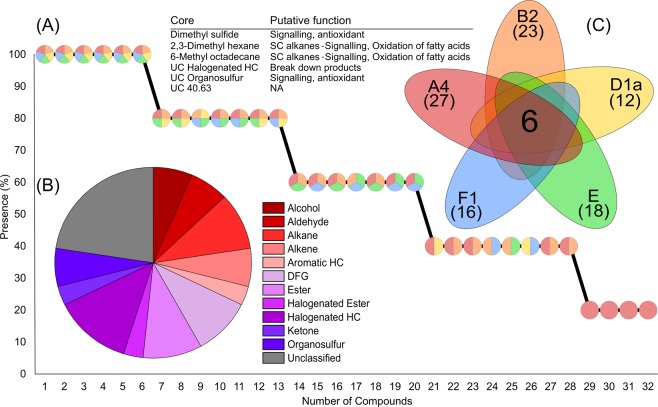
A total of 32 BVOCs were detected across Symbiodinium linucheae, Breviolum psygmophilum, Durusdinium trenchii, Effrenium voratum and Fugacium kawagutii, following blank subtraction and quality control (Table S1). Of these, 50% were successfully identified and a further 28% could be assigned to functional chemical groups. Six compounds were present in at least two out of three replicates in all species tested and defined as core components of the Symbiodiniaceae volatilome (Fig. 1A). These included DMS, 2,3-dimethyl hexane, 6-methyl octadecane and three compounds that could not be fully identified (including a halogenated hydrocarbon, an organosulfur compound and an unclassified compound (UC; UC40.63) (Fig. 1A). Excluding unclassified compounds (22.6%), halogenated hydrocarbon compounds constituted the largest functional group (12.9%), followed by alkanes and esters (both 9.7%) (Fig. 1B).
Figure 1.
(A) Number of compounds detected across all Symbiodiniaceae isolates (present in at least 2 out of the 3 biological replicates), each pie-chart represents a compound and the colours denote which isolates the compound is present in. (B) The proportion of chemical functional groups across the 32 detected compounds from five Symbiodiniaceae species. (C) Venn style diagram85 showing the six core volatiles and their putative functions, as well as the number of compounds detected in each species (Symbiodinium linucheae (A4, red), Breviolum psygmophilum (B2, orange), Durusdinium trenchii (D1a, yellow), Effrenium voratum (E, green) and Fugacium kawagutii (F1, blue)). HC: hydrocarbon, DFG: diverse functional groups, SC: short chain, UC: unclassified (the number following UC indicates the retention time of the compound if the chemical functional group could not be determined).
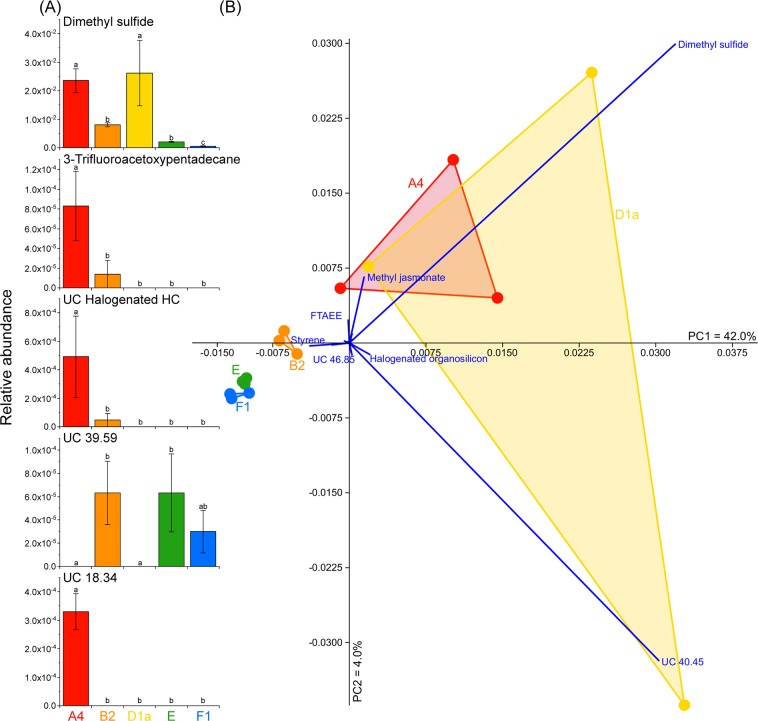
Four BVOCs were unique to S. linucheae, including 3-trifluoroacetoxypentadecane, squalene, an unknown halogenated hydrocarbon and a compound that could not be identified to functional chemical group (UC18.34). None of the volatilomes associated with any of the other Symbiodiniaceae strains comprised any unique BVOCs. The most BVOCs were detected in S. linucheae (27), followed by B. psygmophilum (23), E. voratum (18), F. kawagutii (16) and D. trenchii (12) (Fig. 1C). The abundance of five compounds differed significantly between species (one-way ANOVA; P < 0.01) – these included DMS, 3-trifluoroacetoxypentadecane, UC18.34, UC39.59 and an unclassified halogenated hydrocarbon (Fig. 2A). Principal component analysis (PCA) revealed tight groupings of B. psygmophilum, E. voratum and F. kawagutii volatilomes, while the S. linucheae and D. trenchii volatilomes overlapped, potentially due to higher variation between replicates (Fig. 2B). The variance in the principal component analysis was strongly influenced by DMS (loading score on PC1 axis = 0.72), UC40.45 (loading score on PC1 = 0.68), methyl jasmonate (loading score on PC1 = 0.03, loading score on PC2 = 0.15) and styrene (loading score on PC1 = −0.09) (Fig. 2B).
Figure 2.
(A) Normalised relative abundance (mean ± standard error) for the five compounds that differed significantly between species (ANOVA, P < 0.01, Metaboanalyst 4.084), significant differences are annotated on the plots. (B) Principal Component Analysis (PCA) of the volatilomes of Symbiodinium linucheae (A4, red), Breviolum psygmophilum (B2, orange), Durusdinium trenchii (D1a, yellow), Effrenium voratum (E, green) and Fugacium kawagutii (F1, blue). Bootstrap N = 1000. HC = Hydrocarbon, UC = Unclassified (the number following UC indicates the retention time of the compound if the chemical functional group could not be determined), FTAEE = 4-fluoro-3-trifluoromethylbenzoic acid eicosyl ester.
Thermal stress experiment
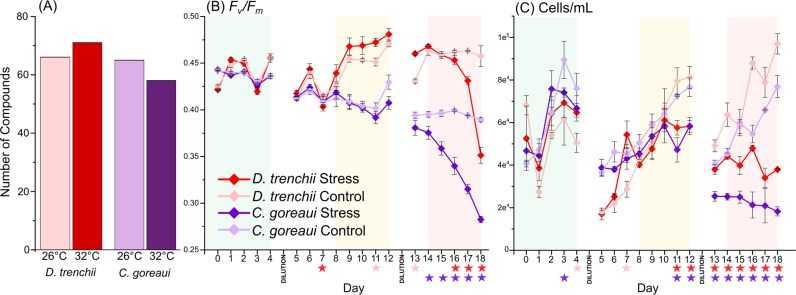
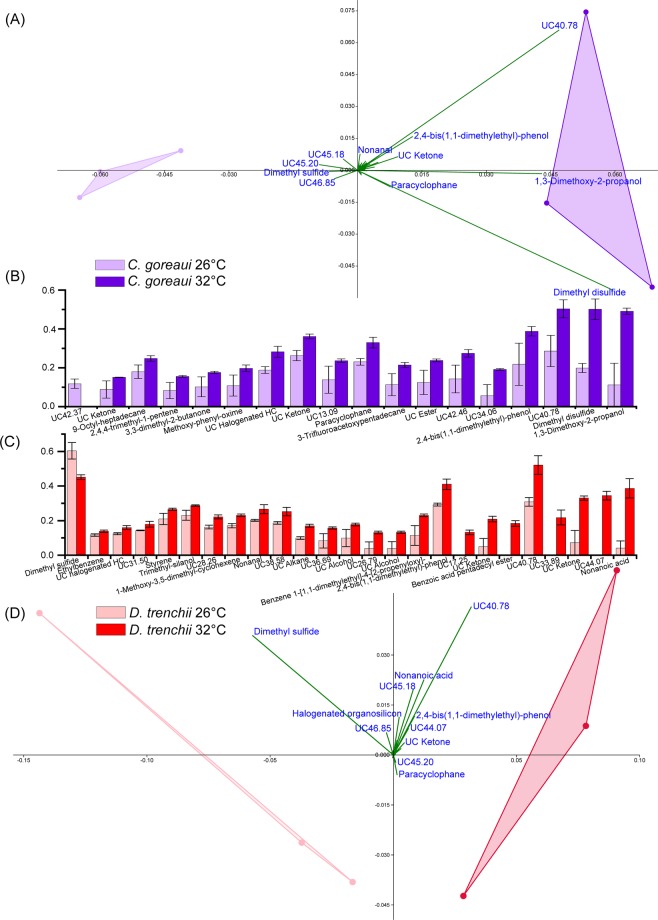
Both the heat-tolerant D. trenchii and heat-sensitive C. goreaui species exhibited a decrease in photochemical efficiency (Fv/Fm, dimensionless) and cell density during the thermal stress experiment, but the more thermally sensitive strain C. goreaui exhibited significant (Kruskal-Wallis, P < 0.05) declines in Fv/Fm two days earlier than D. trenchii (Fig. 3B,C). A total of 72 BVOCs were detected in this stress experiment after quality control, following thermal stress there was a significant shift in volatilome composition. The relative abundance of 18 compounds differed significantly between treatment and control in C. goreaui (P < 0.05; see Table S2 for full list of significant differences). This shift was also reflected in a decrease in the total number of compounds detected during stress (65 to 58; present in at least two out of three replicates; Fig. 3A). Conversely, more compounds were detected in D. trenchii during stress (66 to 71; Fig. 3A) and 25 BVOCs differed significantly (P < 0.05; Table S2) between treatments. Only four compounds (2,4-bis(1,1-dimethylethyl)-phenol, two UC ketones and UC40.78) that differed significantly between treatments were shared by both species during stress (Fig. 4B,C). DMS was recorded in lower amounts under thermal stress in D. trenchii and UC42.37 became undetectable in C. goreaui during thermal stress (Fig. 4B,C). All other significantly different compounds were measured in higher quantities during thermal stress (Fig. 4B,C). Of the compounds that differed significantly between control and heat stress treatment in C. goreaui, 1,3-dimethoxy-2-propanol and dimethyl disulfide (DMDS) showed the largest increase (94.94% and 98.52%, respectively) under stress (Fig. 4B). In D. trenchii, nonanoic acid and UC44.07 exhibited the largest increases (99.85% and 100%, respectively) in the heat stress treatment (Fig. 4C). Five compounds were only detected under thermal stress conditions: benzoic acid pentadecyl ester, UC44.07, UC33.89 and UC17.25 (Fig. 4C). PCA visualised the strong differentiation between the control and thermal stress volatilomes of C. goreaui (Fig. 4A), the principal components in this PCA were influenced by DMDS (PC1 loading = 0.66), UC40.78 (PC1 loading = 0.52) and 1,3-dimethoxy-2-propanol (PC1 loading = 0.48), which were all detected in higher levels during thermal stress (Fig. 4A). PCA also revealed clear distinction between control and stress treatments in D. trenchii, whereby the principal components in the D. trenchii PCA (Fig. 4D) were largely influenced by UC40.78 (PC1 loading = 0.46), nonanoic acid (PC1 loading = 0.18) and a stronger negative forcing from DMS (PC1 loading = −0.84).
Figure 3.
Total number of compounds (present in at least two out of three replicates) detected in Durusdinium trenchii and Cladocopium goreaui control and stress treatments (A). Photosynthetic health (Fv/Fm) (B) and cell density (cell/mL) (C) of D. trenchii and C. goreaui throughout the thermal stress experiment. Significant differences (P < 0.05; Kruskal Wallis; IBM SPSS version 25) between treatments are indicated with a star, the star colour indicates the significantly decreased treatment. Shading on panels (B) and (C) indicates the time period the treatment cultures were held at 26 °C (green), 30 °C (yellow) and 32 °C (red), controls were held at 26 °C throughout, see Fig. S1 for full temperature profile. All error bars are standard error.
Figure 4.
Principal Component Analysis (PCA) of Cladocopium goreaui (A) (component 1 = 62.1% and component 2 = 29.6% of variance) and Durusdinium trenchii (D) (component 1 = 74.5% and component 2 = 17.7% of variance) during the thermal stress assay experiment (PAST; Bootstrap N = 1000). Fourth root normalised abundance of compounds that varied significantly between control (26 °C) and treatment (32 °C) (P < 0.05; Kruskal Wallis; IBM SPSS version 25) are shown for C. goreaui (B) and D. trenchii (C). All error bars are standard error. HC = Hydrocarbon, UC = Unclassified (the number following UC indicates the retention time of the compound if the functional group could not be determined).
Discussion
The multifaceted biological and ecological functions of BVOCs can influence ecosystem resilience2, and therefore understanding the roles of these compounds in shaping ‘healthy’ functioning of threatened ecosystems such as coral reefs is particularly important33. To contribute to an enhanced understanding of coral reef volatilomics, we performed the first characterisation of the volatilome of the coral endosymbionts Symbiodiniaceae, which revealed a substantial diversity of BVOC production. Our study screened species spanning a wide range of Symbiodiniaceae genera39, demonstrating that key species produce far more BVOCs than the iconic dimethyl sulfide. This diverse pool of volatiles included halogenated hydrocarbons, alkanes and esters among the most commonly detected compounds.
By screening five different species (spanning five genera), S. linuchae, B. psygmophilum, D. trenchii, E. voratum and F. kawagutii, we detected 32 different BVOCs. This diversity of BVOCs is consistent with a recent screening of three species of marine macroalgae (Ulva prolifera, Ulva linza and Monostroma nitidum), which identified 41 volatile compounds40. Similarly to our study, alkanes, alkenes, ketones, aldehydes, sulphur compounds, alcohols and esters were detected in the macroalgae studied40. However, unlike macroalgae, Symbiodiniaceae also produced halogenated hydrocarbons, and only benzaldehyde (known to be involved in signalling amongst insects41) was identified in both Symbiodiniaceae and macroalgae.
Of the 32 volatile compounds detected in this study, six were present in all five Symbiodiniaceae species and were therefore defined as core volatiles. Recognising the ubiquitous nature of certain volatiles is a first step in understanding the potential ecological relevance of compounds produced by this important family of microalgae. We defined DMS, 2,3-dimethyl hexane, 6-methyl octadecane, an UC halogenated hydrocarbon, UC organosulfur and UC40.63 as core volatiles. Two of these compounds, 2,3-dimethyl hexane and 6-methyl octadecane, are both short chain alkanes, which may have originated from the oxidation of fatty acids. The inclusion of DMS in the core set of volatiles produced by all Symbiodiniaceae genera examined here adds weight to the importance and ubiquitous nature of this compound in these organisms. However, it is notable that DMS relative abundance varied substantially between Symbiodiniaceae species, with significantly more DMS detected in S. linucheae and D. trenchii than all other species, while significantly less DMS was detected in F. kawagutii compared to all other species. Both S. linucheae and D. trenchii are known to be more heat resistant species29,30,42, potentially suggesting that the high amounts of DMS detected under steady state conditions positively influence their resilience, allowing these species to have a larger existing pool of antioxidants19,43.
In addition to DMS, differences between Symbiodiniaceae volatilomes were largely driven by the relative abundance of UC40.45, methyl jasmonate, styrene and 4-fluoro-3-trifluoromethylbenzoic acid eicosyl ester. Methyl jasmonate is ubiquitous in higher plants and is an important signalling molecule that regulates plant development and also plays a role in defence against biotic (e.g. herbivory) and abiotic (e.g. heat) stresses44. Methyl jasmonate was detected in all replicates of S. linucheae, and given previous observations in higher plants, this BVOC might be involved in the thermal resistance of this Symbiodiniaceae species. The function of the other two molecules, styrene and 4-fluoro-3-trifluoromethylbenzoic acid eicosyl ester, remain uncertain but styrene has previously been reported in higher plants45.
This study identified multiple halogenated compounds however, only three were fully classified (1,2-dichloropropane, 3-trifluoroacetoxypentadecane & 4-fluoro-3-trifluoromethylbenzoic acid eicosyl ester). Halogenated compounds are of particular importance as they can degrade atmospheric ozone46, they are known to be produced naturally47 and have previously been reported from marine microalgae48. For example, three tropical microalgae (Amphora sp., Synechococcus sp. & Parachlorella sp.) can produce a range of iodinated and brominated compounds (methyl iodide, bromoform, dibromomethane, dibromochloromethane, and chloroform), with production shown to be species-specific and growth-phase dependent48, highlighting the importance of the physiological state of the cell for volatile emissions. Other examinations of a suite of common phytoplankton (Calcidiscus leptoporus, Emiliania huxleyi, Phaeodactylum tricornutum, Chaetoceros neogracilis and Dunaliella tertiolecta) demonstrated that all tested species emitted chloromethane, bromoform, bromomethane, chlorobenzene and dichlorobenzene49. Our understanding of the function of halogenated compounds remains in its infancy, with current hypotheses suggesting the tight coupling of their production with oxidative processes (potentially formed as side products during the breakdown of reactive oxygen species)50,51. Halogenated compounds are also thought to sometimes function as ‘infochemicals’, with studies on macroalgae demonstrating that bromoform supports the alga’s defence by functioning as an antimicrobial52–54. We observed that two halogenated compounds, including 3-trifluoroacetoxypentadecane and another unclassified halogenated BVOC, differed significantly between Symbiodiniaceae species and notably seem to co-occur (Correlation: 0.73, P Value = 0.002, Pearson R, Metaboanalyst4.055), with significantly higher levels of both compounds present in S. linucheae compared to all other species tested. 3-trifluoroacetoxypentadecane has previously been shown to have antimicrobial properties56, however, far more work is needed to accurately define the function of these halogenated compounds in Symbiodiniaceae.
This initial screening of a range of Symbiodiniaceae species has demonstrated the diversity and species-specific nature of the volatilome. Despite this diversity, a consistent core emerged, suggesting that some compounds have a conserved role across species. While appreciating these potential roles is important, most natural systems rarely remain in steady-state conditions for prolonged amounts of time. Currently, marine systems are experiencing an increase in the frequency and severity of harmful thermal stress events. How corals respond to heat-wave induced bleaching and mortality is often influenced by the thermal tolerance of their endosymbiotic algae (Symbiodiniaceae)28. A number of physiological traits appear to differentiate stress tolerant-versus-susceptible species (or genetic variants) of Symbiodiniaceae, including maintaining integrity of photosynthetic constituents30,57 and upregulation of pathways to ‘detoxify’ organelles58. However, the effect of thermal stress on the Symbiodiniaceae volatilome had not been explored until now.
With the onset of thermal stress, a larger number of BVOCs were detected in the thermally tolerant D. trenchii, while the thermally sensitive C. goreaui produced fewer compounds relative to control conditions. The ability to synthesise specific compounds under stress could be involved in the thermal tolerance of D. trenchii, as ‘de novo’ BVOC synthesis has been previously observed in higher plants during abiotic stresses4,6,59. During thermal stress, concomitantly with a decrease in cell health, a dramatic increase in nonanoic acid was recorded in D. trenchii. Nonanoic acid is a fatty acid, a group of compounds that can potentially result from increased cell membrane lysis60,61. Furthermore, significantly less DMS was detected in D. trenchii during stress, which was the only compound to significantly decrease in this species. Previous work targeting DMS production in other Cladocopium and Durusdinium Symbiodiniaceae strains also observed a decrease in DMS with the onset of heat stress19. Lower levels of DMS under thermal stress may either indicate that Symbiodiniaceae decrease their production, or that DMS degradation increases due to reactions with harmful molecules in response to thermal stress. Interestingly, in our study, we detected higher amounts of another sulphur compound, dimethyl disulfide (DMDS) in C. goreaui during stress. DMDS can be formed from the photo-oxidation of methanethiol62. These results indicate that we need to consider the full suite of volatile sulphur compounds to fully elucidate the role that these chemicals play in stress response and trophic interactions in coral reefs. An additional 17 compounds significantly increased during thermal stress in C. goreaui, with 1,3-dimethoxy-2-propanol, UC40.78 and DMDS the main drivers of the differentiation between treatments. Increases in 1,3-dimethoxy-2-propanol may have resulted from lipid peroxidation63,64. Whether these BVOCs are released prior to other visual (e.g. bleaching) or physiological30 stress indicators remains unknown and will be important to assess for the potential use of specific BVOCs to diagnose early stress responses.
The sheer quantity of detected and unidentified compounds that differed significantly across species and under heat stress highlights a critical need to robustly quantify and classify these BVOCs. However, lack of a comprehensive marine BVOC database severely limits our interpretation of volatilomic data, an issue that similarly limits progress for other metabolomic approaches33. Terrestrial BVOC studies have already identified ~30,000 volatile compounds to date65. The unidentifiable chemical diversity highlighted here teases at the potential unexplored roles of BVOCs in biological and ecological interactions in marine systems. These unidentified compounds should therefore not be discarded from future analyses as they may play key roles in stress response or could function as useful stress biomarkers that can be measured non-invasively. Volatile databases are continually improving and as such we have made available our mass spectra files (MSV000084436; MassIVE; 10.25345/C5RD5C) for future studies as more comprehensive databases become available.
Numerous BVOCs are known to induce the formation of secondary organic aerosols that enhance cloud formation and albedo (eg. DMS, benzaldehyde, toluene & styrene66,67), while other compounds can result in increased formation and residence time of crucial greenhouse gases such as ozone68. Marine BVOC emissions are often overlooked when it comes to global modelling of BVOC emissions, largely due to the lack of data from these ecosystems. However, marine ecosystems have a large influence on atmospheric chemistry. Tropical areas are known to have stronger convection forces that lead to greater transport of emitted BVOCs to the troposphere and stratosphere69. It is therefore essential to understand current tropical baseline emissions if we wish to accurately model future climate scenarios. Examining the Symbiodiniaceae volatilome is a key step towards understanding the prevalence and function of tropical marine BVOCs. However, further work is needed to clarify how free living Symbiodiniaceae BVOC production varies from endosymbiotic Symbiodiniaceae.
Here we demonstrate for the first time that volatile metabolites produced by Symbiodiniaceae are not only composed of a broad spectrum of BVOCs, but that their production can be influenced by stressful – suboptimal – conditions, suggesting that these overlooked BVOCs likely operate as key constituents regulating metabolic competency. We detected six BVOCs with putative signalling and antioxidant functions that were ubiquitous across the five Symbiodiniaceae genera investigated. Many of the BVOCs reported here are as yet uncharacterised, highlighting an urgent need to develop marine-specific annotation pipelines and to further identify new and abundant compounds. This is of particular relevance given that some of these compounds might play currently uncharacterised roles in resistance and survival of corals during thermal stress events. This work provides direction for future studies to start unravelling the complex functions of volatile metabolites in coral reefs. Corals are some of the most complex symbiotic metaorganisms and the many microbial partners they harbour are likely to contribute to their BVOC emissions.
Methods
Across strain screening of symbiodiniaceae volatilomes
Five isolates, each representing a distinct Symbiodiniaceae genus (Symbiodinium linucheae, Breviolum psygmophilum, Durusdinium trenchii, Effrenium voratum and Fugacium kawagutii; see Table 1), were maintained in exponential growth in a temperature controlled incubator (Labec; Marrickville, Australia) maintained at 25 °C ± 1.5 °C and under a light intensity of ca. 50 ± 5 μmol photons m−2 s−1 (HYDRA; Aquaillumination, Ames, Iowa) on a 12:12 light:dark cycle (as per Lawson et al.70). Cultures were grown in triplicate in 0.2 μm filtered artificial seawater71 with IMK medium (Diago, Japan) in sterile 250 mL Schott bottles. Growth and physiological condition of each culture was monitored daily in the five days prior to sampling with direct cell counts and Fast Repetition Rate fluorometry (FRRf; FastOcean, Chelsea Technologies Group, UK). The FRR fluorometer was programmed to deliver single turnover induction of photosystem II (PSII) (i.e. 100 × 1.1 µs flashlets spaced at 2.8 µs intervals) via a blue excitation LED (450 nm). Each acquisition recorded was the mean of 40 consecutive single turnover fluorescent transients, with intervals of 150 ms between acquisitions (detailed in Lawson et al.70). FRRf measurements were performed on 3 mL live culture, samples were first acclimated to low light (<5 µmol photons m−2 s−1) for ~15 minutes to relax non-photochemical quenching while simultaneously avoiding build-up of chlororespiration to ensure that only the maximum quantum yield of PSII (Fv/Fm) was assessed72. Cell counts were performed on live cultures using a Neubauer haemocytometer and 20x magnification on a Nikon Eclipse Ci-L compound microscope (Nikon Instruments; Melville, New York). In addition to this daily monitoring data, additional samples were taken on the day of BVOC sampling for FRRf, cell counts, and cell size. For cell size analysis, an aliquot of culture was loaded onto a Neubauer haemocytometer and using a Nikon Upright Fluorescence Microscope (Nikon Instruments; Melville, New York) a series of 48 images were taken for each sample. Using FIJI73,74 and White Balance software, these images were processed and the mean cell volume was recorded for each strain72. Values of Fv/Fm (dimensionless) varied on the day of sampling from 0.412 ± 0.016 (E. voratum) to 0.479 ± 0.002 (D. trenchii) (mean ± SE, n = 3; Fig. S2), a range expected across isolates growing in nutrient replete exponential growth30,72.
Table 1.
Symbiodiniaceae isolates examined in the screening experiment. Isolates were maintained in culture and no corals were directly handled in this study. Strains in bold were used for the thermal stress experiment, *indicates use in the screening experiment.
| ID | ITS2 Type | Origin | Host | Species |
|---|---|---|---|---|
| *RT379 | A4 | Bahamas | Plexaura homamalla (Coral) | Symbiodinium linucheae |
| *RT141 | B2 | Bermuda | Oculina diffusa (Coral) | Breviolum psygmophilum |
| 123SCF 058-04 | C1 | Magnetic Island (Pacific) | Acropora millepora (Coral) | Cladocopium goreaui |
| *amur-D-MI | D1a | Magnetic Island (Pacific) | A. muricata (Coral) | Durusdinium trenchii |
| *CCMP421 | E | Wellington, NZ | Free-living | Effrenium voratum |
| *f156 | F1 | Hawaii (Pacific) | Montipora veruccosa (Coral) | Fugacium kawagutii |
Symbiodiniaceae thermal assay experiment
Two Symbiodiniaceae strains characterised by different levels of thermal sensitivity, including the relatively heat sensitive SCF058-04 (Cladocopium goreaui) and heat tolerant amur-D-MI (Durusdinium trenchii)75–78, were subjected to a thermal stress experiment. These isolates were selected based on their differing thermal tolerance and their abundance on the Great Barrier Reef79,80. A control incubator (ARALAB; Sintra, Lisboa) was maintained at 26 °C ± 1.5 °C with a light intensity of ca. 100 ± 10 μmol photons m−2 s−1 (LEDs) on a 12:12 light:dark cycle. A parallel incubator (ARALAB; Sintra, Lisboa) was used for the heat treatment assay with identical settings as for the control, except that temperature was ramped from 26 °C to 30 °C over 4 days and then maintained at 30 °C for 4 days prior to resampling. The temperature was then ramped from 30 °C to 32 °C over 2 days and finally maintained at 32 °C for a further 4 days (Fig. S1). Six biological replicates of each culture were grown (n = 3 per each control and treatment). All cultures were monitored daily with FRRf and cell counts following the same procedures as used in the screening experiment. BVOC sampling was performed at the end of the stress period when the treatment cultures had been at 32 °C for 4 days. Additional samples were taken for cell imaging as per screening experiment on these BVOC sampling days.
BVOC sampling and volatilome retrieval
Two aliquots of 50 mL from each sample were each placed into a sterile 100 mL crimp cap vial to yield technical duplicate samples with 50 mL head space. Vials were capped and placed in a water bath under light intensity (cool white light, HYDRA; Aquaillumination, Iowa, USA) and temperature that matched their growth or treatment conditions. Samples were purged with instrument grade air (BOC Gases, Linde Group, Australia) for 30 minutes, whereby the purge outlet was passed over thermal desorption (TD) tubes (Tenax TA; Markes International Ltd, Llantrisant, UK; see Fig. S3), which were immediately capped post purge and stored at 4 °C until processing. All TD tubes were analysed within two weeks of sampling by desorbing samples with automated thermal desorption (ULTRA 2 & UNITY 2; Markes International Ltd, Llantrisant, UK) for 6 minutes at 300 °C and concentrated on a Tenax TA cold trap at −30 °C. This cold trap was then flash heated to 300 °C and the concentrated sample injected via a heated transfer line (150 °C) onto a 7890 A GC-MS (Agilent Technologies Pty Ltd, Melbourne) fitted with a BP1 capillary column (60 m × 0.32 mm, 1 µm film thickness; SGE Analytical Science Pty Ltd, Melbourne) at a flow rate of 2.3 mL/minute. Samples were run splitless to allow detection of trace compounds. To allow for complete desorption the GC oven was heated at 35 °C for 5 minutes then 4 °C min−1 to 160 °C then 20 °C min−1 to 300 °C for 10 minutes. The GC-MS was coupled to a mass-selective detector (Model 5975 C; Agilent Technologies Pty Ltd, Melbourne) that was set to a scanning range of 35–250 amu for the screening experiment and 35–300 amu for the stress experiment. The higher scanning range in the stress experiment was chosen as we examined only two species. The increased scanning range combined with the higher light levels likely led to the higher number of compounds detected in the stress experiment.
Peaks were identified by manually comparing mass spectra against a commercial library (NIST08 library in NIST MS Search v.2.2 f; NIST, Gaithersburg, MD). Blank media samples were run in conjunction with all analysis; the average values for compounds present in the blanks were subtracted from all samples. Common contaminating ions (73, 84, 147, 149, 207 and 221 m/z) were removed using the Denoising function in OpenChrom81. Chromatograms were integrated in ChemStation (Agilent Technologies Pty Ltd, Melbourne) with an initial threshold of 14.5 and an initial peak width of 0.068. Files were processed using the MSeasyTkGUI package82 in R version 3.5.3 (R Development Core Team, 2015) and all putative compounds clustered. Any compound that did not occur in at least 4 of the 6 replicates (2 technical replicates per biological replicate) was considered contamination and removed. UC denotes an unclassified compound (the number following UC indicates the retention time of the compound if the functional group could not be determined).
Statistical analyses
A Principal Components Analysis (PCA; Bootstrap N = 1000) on data normalised to total cell volume was completed in the statistical package PAST83 to contrast the volatilomes between Symbiodiniaceae species (Screening experiment) and between temperatures (Stress experiment). Compounds were considered “core” components of the volatilome if they appeared in at least 2 out of 3 biological replicates in all species. To test for significant differences between species in the screening experiment, data were processed in MetaboAnalyst4.0, undergoing a generalised logarithm transformation and tested with a one-way ANOVA and Tukey’s HSD post hoc55,84. For treatments in the stress experiment, a Kruskal-Wallis test was used (IBM SPSS Statistics, version 25), as data did not meet the assumptions required for parametric tests.
Supplementary information
Acknowledgements
This research was supported by an Australian Government Research Training Program Scholarship awarded to C.A.L., J.B.R. was supported by Australian Research Council fellowship DE160100636 and D.J.S. by an ARC Discovery Grant (DP160100271). We thank Samantha Goyen, David Hughes, Graeme Poleweski, Scott Allchin, Stephanie Gardner and Axel Olander for their assistance transporting samples for analysis.
Author contributions
C.L., D.J.S., J.B.R., J.R.S. conceived and designed the project, C.L. performed the experiments, analysed the data and produced figures. M.P. provided technical and data analysis assistance. D.J.S. provided financial support. C.L. wrote the paper. All authors reviewed the manuscript.
Data availability
The datasets generated during the current study are available in the MassIVE database (https://massive.ucsd.edu) under accession numbers: MSV000084436.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-53552-0.
References
- 1.Shaw SL, Gantt B, Meskhidze N. Production and Emissions of Marine Isoprene and Monoterpenes: A Review. Adv. Meteorol. 2010;2010:1–24. doi: 10.1155/2010/408696. [DOI] [Google Scholar]
- 2.Peñuelas J, Llusià J. BVOCs: Plant defense against climate warming? Trends Plant Sci. 2003;8:105–109. doi: 10.1016/S1360-1385(03)00008-6. [DOI] [PubMed] [Google Scholar]
- 3.Kesselmeier J, Staudt M. Biogenic Volatile Organic Compunds (VOC): An Overview on Emission, Physiology and Ecology. J.Atmos.Chem. 1999;33:23–88. doi: 10.1023/A:1006127516791. [DOI] [Google Scholar]
- 4.Loreto F, Schnitzler JP. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Sunda W, Kieber DJ, Kiene RP, Huntsman S. An antioxidant function for DMSP and DMS in marine algae. Nature. 2002;418:317–20. doi: 10.1038/nature00851. [DOI] [PubMed] [Google Scholar]
- 6.Loreto F, Barta C, Brilli F, Nogues I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant, Cell Environ. 2006;29:1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- 7.Croft KPC, Jüttner F, Slusarenko AJ. Volatile Products of the Lipoxygenase Pathway Evolved from. Plant Physiol. 1993;101:13–24. doi: 10.1104/pp.101.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiojiri K, et al. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. 2006;103:16672–16676. doi: 10.1073/pnas.0607780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiestl FP. Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytol. 2015;206:571–577. doi: 10.1111/nph.13243. [DOI] [PubMed] [Google Scholar]
- 10.Loivamäki M, Mumm R, Dicke M, Schnitzler J-P. Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc. Natl. Acad. Sci. 2008;105:17430–17435. doi: 10.1073/pnas.0804488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Moraes CM, Mescher MC, Tumlinson JH. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 12.Laothawornkitkul J, et al. Isoprene emissions influence herbivore feeding decisions. Plant, Cell Environ. 2008;31:1410–1415. doi: 10.1111/j.1365-3040.2008.01849.x. [DOI] [PubMed] [Google Scholar]
- 13.Guenther A, et al. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature. Atmos. Chem. Phys. 2006;6:3181–3210. doi: 10.5194/acp-6-3181-2006. [DOI] [Google Scholar]
- 14.Exton DA, Mcgenity TJ, Steinke M, Smith DJ, Suggett DJ. Uncovering the volatile nature of tropical coastal marine ecosystems in a changing world. Glob. Chang. Biol. 2015;21:1383–1394. doi: 10.1111/gcb.12764. [DOI] [PubMed] [Google Scholar]
- 15.Jackson R, Gabric A, Cropp R. Effects of ocean warming and coral bleaching on aerosol emissions in the Great Barrier Reef, Australia. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-017-17765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broadbent AD, Jones GB. DMS and DMSP in mucus ropes, coral mucus, surface films and sediment pore waters from coral reefs in the Great Barrier Reef. Mar. Freshw. Res. 2004;55:849–855. doi: 10.1071/MF04114. [DOI] [Google Scholar]
- 17.Hopkins FE, Bell TG, Yang M, Suggett DJ, Steinke M. Air exposure of coral is a significant source of dimethylsulfide (DMS) to the atmosphere. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep36031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayers GP, Gras JL. Seasonal relationship between cloud condensation nuclei and aerosol methanesulphonate in marine air. Nature. 1991;354:56–58. doi: 10.1038/354056a0. [DOI] [Google Scholar]
- 19.Deschaseaux ESM, et al. Comparative response of DMS and DMSP concentrations in Symbiodinium clades C1 and D1 under thermal stress. J. Exp. Mar. Bio. Ecol. 2014;459:181–189. doi: 10.1016/j.jembe.2014.05.018. [DOI] [Google Scholar]
- 20.Seymour JR, Simo R, Ahmed T, Stocker R. Chemoattraction to dimethylsulfoniopropionate throughout the marine microbial food web. Science (80-.). 2010;329:342–346. doi: 10.1126/science.1188418. [DOI] [PubMed] [Google Scholar]
- 21.Nevitt GA, Veit RR, Kareiva P. Dimethyl sulphide as a foraging cue for Antarctic Procellariiform seabirds. Nature. 1995;376:680–682. doi: 10.1038/376680ao. [DOI] [Google Scholar]
- 22.Swan Hilton B., Crough Robert W., Vaattovaara Petri, Jones Graham B., Deschaseaux Elisabeth S. M., Eyre Bradley D., Miljevic Branka, Ristovski Zoran D. Dimethyl sulfide and other biogenic volatile organic compound emissions from branching coral and reef seawater: potential sources of secondary aerosol over the Great Barrier Reef. Journal of Atmospheric Chemistry. 2016;73(3):303–328. doi: 10.1007/s10874-016-9327-7. [DOI] [Google Scholar]
- 23.Broadbent AD, Jones GB, Jones RJ. DMSP in Corals and Benthic Algae from the Great Barrier Reef. Estuar. Coast. Shelf Sci. 2002;55:547–555. doi: 10.1006/ecss.2002.1021. [DOI] [Google Scholar]
- 24.Steinke M, Brading P, Kerrison P, Warner ME, Suggett DJ. Concentrations of dimethylsulfoniopropionate and dimethyl sulfide are strain-specific in symbiotic dinoflagellates (symbiodinium sp., dinophyceae) J. Phycol. 2011;47:775–783. doi: 10.1111/j.1529-8817.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 25.Exton DAA, Suggett DJJ, Mcgenity TJJ, Steinke M. Chlorophyll-normalized isoprene production in laboratory cultures of marine microalgae and implications for global models. Limnol. Oceanogr. 2013;58:1301–1311. doi: 10.4319/lo.2013.58.4.1301. [DOI] [Google Scholar]
- 26.Van Alstyne KL, Dominique VJ, Muller-Parker G. Is dimethylsulfoniopropionate (DMSP) produced by the symbionts or the host in an anemone-zooxanthella symbiosis? Coral Reefs. 2009;28:167–176. doi: 10.1007/s00338-008-0443-y. [DOI] [Google Scholar]
- 27.Burriesci MS, Raab TK, Pringle JR. Evidence that glucose is the major transferred metabolite in dinoflagellate-cnidarian symbiosis. J. Exp. Biol. 2012;215:3467–3477. doi: 10.1242/jeb.070946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suggett DJ, Warner ME, Leggat W. Symbiotic Dinoflagellate Functional Diversity Mediates Coral Survival under Ecological Crisis. Trends Ecol. Evol. 2017;32:735–745. doi: 10.1016/j.tree.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Díaz-Almeyda EM, et al. Intraspecific and interspecific variation in thermotolerance and photoacclimation in Symbiodinium dinoflagellates. Proc. R. Soc. B Biol. Sci. 2017;284:20171767. doi: 10.1098/rspb.2017.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goyen S, et al. A molecular physiology basis for functional diversity of hydrogen peroxide production amongst Symbiodinium spp. (Dinophyceae) Mar. Biol. 2017;164:1–12. doi: 10.1007/s00227-017-3073-5. [DOI] [Google Scholar]
- 31.Matthews Jennifer L., Oakley Clinton A., Lutz Adrian, Hillyer Katie E., Roessner Ute, Grossman Arthur R., Weis Virginia M., Davy Simon K. Partner switching and metabolic flux in a model cnidarian–dinoflagellate symbiosis. Proceedings of the Royal Society B: Biological Sciences. 2018;285(1892):20182336. doi: 10.1098/rspb.2018.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Alessandro, M. Assessing the importance of specific volatile organic compounds in multitrophic interactions. (Université de Neuchâtel, 2006).
- 33.Steinke, M., Randell, L., Dumbrell, A. J. & Saha, M. Volatile Biomarkers for Aquatic Ecological Research. Advances in Ecological Research 59 (Elsevier Ltd., 2018).
- 34.Amann Anton, Costello Ben de Lacy, Miekisch Wolfram, Schubert Jochen, Buszewski Bogusław, Pleil Joachim, Ratcliffe Norman, Risby Terence. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. Journal of Breath Research. 2014;8(3):034001. doi: 10.1088/1752-7155/8/3/034001. [DOI] [PubMed] [Google Scholar]
- 35.Jud W, Winkler JB, Niederbacher B, Niederbacher S, Schnitzler JP. Volatilomics: A non-invasive technique for screening plant phenotypic traits. Plant Methods. 2018;14:1–18. doi: 10.1186/s13007-018-0378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkinson, R. & Arey, J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review. Atmos. Environ. 37 (2003).
- 37.Atkinson R, Arey J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003;103:4605–4638. doi: 10.1021/cr0206420. [DOI] [PubMed] [Google Scholar]
- 38.Laothawornkitkul J, Taylor JE, Paul ND, Hewitt CN. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009;183:27–51. doi: 10.1111/j.1469-8137.2009.02859.x. [DOI] [PubMed] [Google Scholar]
- 39.LaJeunesse TC, et al. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 2018;28:2570–2580.e6. doi: 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto M, et al. Determination of Volatile Compounds in Four Commercial Samples of Japanese Green Algae Using Solid Phase Microextraction Gas Chromatography Mass Spectrometry. Sci. World J. 2014;2014:1–8. doi: 10.1155/2014/289780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang CY, Kim J, Kim H-H. Benzaldehyde Synergizes the Response of Female Xyleborinus saxesenii (Coleoptera: Curculionidae, Scolytinae) to Ethanol. J. Econ. Entomol. 2018;111:1691–1695. doi: 10.1093/jee/toy131. [DOI] [PubMed] [Google Scholar]
- 42.Swain TD, Chandler J, Backman V, Marcelino L. Consensus thermotolerance ranking for 110 Symbiodinium phylotypes: an exemplar utilization of a novel iterative partial-rank aggregation tool with broad application potential. Funct. Ecol. 2017;31:172–183. doi: 10.1111/1365-2435.12694. [DOI] [Google Scholar]
- 43.Jones G, King S. Dimethylsulphoniopropionate (DMSP) as an indicator of bleaching tolerance in scleractinian corals. J. Mar. Sci. Eng. 2015;3:444–465. doi: 10.3390/jmse3020444. [DOI] [Google Scholar]
- 44.Dar TA, Uddin M, Khan MMA, Hakeem KR, Jaleel H. Jasmonates counter plant stress: A review. Environ. Exp. Bot. 2015;115:49–57. doi: 10.1016/j.envexpbot.2015.02.010. [DOI] [Google Scholar]
- 45.Pagot Y, Belin J-M, Husson F, Spinnler H-E. Metabolism of phenylalanine and biosynthesis of styrene in Penicillium camemberti. J. Dairy Res. 2007;74:180–185. doi: 10.1017/S0022029906002251. [DOI] [PubMed] [Google Scholar]
- 46.Lim YK, Phang SM, Abdul Rahman N, Sturges WT, Malin G. Halocarbon emissions from marine phytoplankton and climate change. Int. J. Environ. Sci. Technol. 2017;14:1355–1370. doi: 10.1007/s13762-016-1219-5. [DOI] [Google Scholar]
- 47.Gribble GW. The diversity of naturally produced organohalogens. Chemosphere. 2003;52:289–297. doi: 10.1016/S0045-6535(03)00207-8. [DOI] [PubMed] [Google Scholar]
- 48.Lim YK, Phang SM, Sturges WT, Malin G, Rahman NBA. Emission of short-lived halocarbons by three common tropical marine microalgae during batch culture. J. Appl. Phycol. 2018;30:341–353. doi: 10.1007/s10811-017-1250-z. [DOI] [Google Scholar]
- 49.Colomb A, Yassaa N, Williams J, Peeken I, Lochte K. Screening volatile organic compounds (VOCs) emissions from five marine phytoplankton species by head space gas chromatography/mass spectrometry (HS-GC/MS) J. Environ. Monit. 2008;10:325–330. doi: 10.1039/b715312k. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen M, Collén J, Abrahamsson K, Ekdahl A. Production of halocarbons from seaweeds: an oxidative stress reaction? Sci. Mar. 1996;60:257–263. [Google Scholar]
- 51.Zuo Z. Why Algae Release Volatile Organic Compounds—The Emission and Roles. Front. Microbiol. 2019;10:1–7. doi: 10.3389/fmicb.2019.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohsawa N, Ogata Y, Okada N, Itoh N. Physiological function of bromoperoxidase in the red marine alga, Corallina pilulifera: Production of bromoform as an allelochemical and the simultaneous elimination of hydrogen peroxide. Phytochemistry. 2001;58:683–692. doi: 10.1016/S0031-9422(01)00259-X. [DOI] [PubMed] [Google Scholar]
- 53.Cabrita MT, Vale C, Rauter AP. Halogenated compounds from marine algae. Mar. Drugs. 2010;8:2301–2317. doi: 10.3390/md8082301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paul C, Pohnert G. Production and role of volatile halogenated compounds from marine algae. Nat. Prod. Rep. 2011;28:186–195. doi: 10.1039/C0NP00043D. [DOI] [PubMed] [Google Scholar]
- 55.Chong J, et al. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46:W486–W494. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hussein AO, Mohammed GJ, Hadi MY, Hameed IH. Phytochemical screening of methanolic dried galls extract of Quercus infectoria using gas chromatography-mass spectrometry (GC-MS) and Fourier transform-infrared (FT-IR) J. Pharmacogn. Phyther. 2016;8:49–59. doi: 10.5897/JPP2015.0368. [DOI] [Google Scholar]
- 57.Tchernov D, et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl. Acad. Sci. USA. 2004;101:13531–5. doi: 10.1073/pnas.0402907101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levin, R. et al. Sex, Scavengers, and Chaperones: Transcriptome Secrets of Divergeny Symbiodinium Thermal Tolerances. Mol. Biol. Evol. 1–30 (2016). [DOI] [PMC free article] [PubMed]
- 59.Beauchamp J, et al. Ozone induced emissions of biogenic VOC from tobacco: relationships between ozone uptake and emission of LOX products. Plant, Cell Environ. 2005;28:1334–1343. doi: 10.1111/j.1365-3040.2005.01383.x. [DOI] [Google Scholar]
- 60.Hillyer KE, Tumanov S, Villas-Bôas S, Davy SK. Metabolite profiling of symbiont and host during thermal stress and bleaching in a model cnidarian–dinoflagellate symbiosis. J. Exp. Biol. 2016;219:516–527. doi: 10.1242/jeb.128660. [DOI] [PubMed] [Google Scholar]
- 61.Yusupov M, et al. Effect of head group and lipid tail oxidation in the cell membrane revealed through integrated simulations and experiments. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-06412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bentley R, Chasteen TG. Environmental VOSCs - Formation and degradation of dimethyl sulfide, methanethiol and related materials. Chemosphere. 2004;55:291–317. doi: 10.1016/j.chemosphere.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 63.Farmer EE, Mueller MJ. ROS-Mediated Lipid Peroxidation and RES-Activated Signaling. Annu. Rev. Plant Biol. 2013;64:429–450. doi: 10.1146/annurev-arplant-050312-120132. [DOI] [PubMed] [Google Scholar]
- 64.Sousa BC, Pitt AR, Spickett CM. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic. Biol. Med. 2017;111:294–308. doi: 10.1016/j.freeradbiomed.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Peñuelas J, Llusia J. Plant VOC emissions: making use of the unavoidable. Trends Ecol. Evol. 2004;19:402–404. doi: 10.1016/j.tree.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Charlson RJ, Lovelock JE, Andreae MO, Warren SG. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature. 1987;326:655–661. doi: 10.1038/326655a0. [DOI] [Google Scholar]
- 67.Bruns EA, et al. Identification of significant precursor gases of secondary organic aerosols from residential wood combustion. Sci. Rep. 2016;6:1–9. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Na K, Moon KC, Yong PK. Source contribution to aromatic VOC concentration and ozone formation potential in the atmosphere of Seoul. Atmos. Environ. 2005;39:5517–5524. doi: 10.1016/j.atmosenv.2005.06.005. [DOI] [Google Scholar]
- 69.Randel WJ, Jensen EJ. Physical processes in the tropical tropopause layer and their roles in a changing climate. Nat. Geosci. 2013;6:169–176. doi: 10.1038/ngeo1733. [DOI] [Google Scholar]
- 70.Lawson CA, Raina JB, Kahlke T, Seymour JR, Suggett DJ. Defining the core microbiome of the symbiotic dinoflagellate, Symbiodinium. Environ. Microbiol. Rep. 2018;10:7–11. doi: 10.1111/1758-2229.12599. [DOI] [PubMed] [Google Scholar]
- 71.Berges JA, Franklin DJ, Harrison PJ. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001;37:1138–1145. doi: 10.1046/j.1529-8817.2001.01052.x. [DOI] [Google Scholar]
- 72.Suggett DJ, et al. Functional diversity of photobiological traits within the genus Symbiodinium appears to be governed by the interaction of cell size with cladal designation. New Phytol. 2015;208:370–381. doi: 10.1111/nph.13483. [DOI] [PubMed] [Google Scholar]
- 73.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rueden CT, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18:1–26. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rowan R. Coral bleaching: Thermal adaptation in reef coral symbionts. Nature. 2004;430:742–742. doi: 10.1038/430742a. [DOI] [PubMed] [Google Scholar]
- 76.Berkelmans R, van Oppen MJH. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. Biol. Sci. 2006;273:2305–12. doi: 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones AM, Berkelmans R, van Oppen MJH, Mieog JC, Sinclair W. A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc. Biol. Sci. 2008;275:1359–65. doi: 10.1098/rspb.2008.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stat M, Gates RD, Clade D. Symbiodinium in scleractinian corals: A “nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? J. Mar. Biol. 2011;2011:1–9. doi: 10.1155/2011/730715. [DOI] [Google Scholar]
- 79.Van Oppen MJH, Palstra FP, Piquet AMT, Miller DJ. Patterns of coral-dinoflagellate associations in Acropora: Significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proc. R. Soc. B Biol. Sci. 2001;268:1759–1767. doi: 10.1098/rspb.2001.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ulstrup KE, Van Oppen MJH. Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol. Ecol. 2003;12:3477–3484. doi: 10.1046/j.1365-294X.2003.01988.x. [DOI] [PubMed] [Google Scholar]
- 81.Wenig P, Odermatt J. OpenChrom: a cross-platform open source software for the mass spectrometric analysis of chromatographic data. BMC Bioinformatics. 2010;11:405. doi: 10.1186/1471-2105-11-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicolè F, et al. MSeasy: unsupervised and untargeted GC-MS data processing. Bioinformatics. 2012;28:2278–2280. doi: 10.1093/bioinformatics/bts427. [DOI] [PubMed] [Google Scholar]
- 83.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001;4:9. [Google Scholar]
- 84.Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37:W652–W660. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heberle, H., Meirelles, G. V., da Silva, F. R., Telles, G. P. & Minghim, R. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics16 (2015). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available in the MassIVE database (https://massive.ucsd.edu) under accession numbers: MSV000084436.