Abstract
Astrocytes are critical for maintaining the homeostasis of the CNS. Increasing evidence suggests that a number of neurological and neuropsychiatric disorders, including chronic pain, may result from astrocyte ‘gliopathy’. Indeed, in recent years there has been substantial progress in our understanding of how astrocytes can regulate nociceptive synaptic transmission via neuronal-glial and glial-glial cell interactions, as well as the involvement of spinal and supraspinal astrocytes in the modulation of pain signaling and the maintenance of neuropathic pain. A role of astrocytes in the pathogenesis of chronic itch is also emerging. These developments suggest that targeting the specific pathways that are responsible for astrogliopathy may represent a novel approach to develop therapies for chronic pain and chronic itch.
Introduction
Astrocytes account for approximately 20–40% of all of the glial cells in the CNS. 1 Historically, they were thought principally to provide structural and nutrient support for neurons; however, it is now appreciated that they also play an active role in many critical neural processes2. Unlike other non-neuronal CNS cell types, such as microglia and oligodendrocytes, astrocytes are physically coupled to one another by gap junction protein complexes, which directly link the cytosol of adjoining cells to allow free exchange of ions and small cytosolic components3. Astrocytes can also be distinguished from other CNS glial cell types by their expression of glial fibrillary acidic protein (GFAP), which is present in all of their major branches and processes and dynamically changes during their transition to reactive states after insult or injury4,5 Importantly, each astrocyte occupies a non-overlapping territory or domain with the CNS6–8. Although the functional significance of this territorial organization is not fully understood, it appears to be disrupted during the transition of astrocytes to reactive states8. Additionally, a key discerning feature of astrocytes is their extensive contact with cerebral blood vessels, which allows them to modulate blood flow during neuronal activation7.
Astrocytes also have extensive contacts with interneuronal synapses; a single cortical astrocyte in the rodent brain can enwrap as many as 140,000 synapses and contact 4–6 neuronal soma and 300–600 neuronal dendrites9–12. It is important to note that astrocytes of different species vary considerably in their sizes and morphological complexity11,13. Furthermore, the human brain contains several subtypes of astrocytes that are not present in rodents11,13. The diameter of human cortical astrocytes is at twice as large as that of their rodent counterparts and can extend 10 times as many primary processes. Furthermore, a single human astrocyte may make contact with up to 2 million synapses 6,11. Interestingly, there is evidence to suggest that the increased morphological complexity of astrocytes that has occurred with primate phylogeny may subserve increased cognitive function 14.
Mounting evidence suggests that astrocytes are key regulators of CNS diseases — including neurodegenerative, neuropsychiatric and neurodevelopmental diseases and gliomas15–17— in which they drive pathogenesis in part through the regulation of neuroinflammation [G] 18–22.
Chronic pain, now recognized as a distinct disease that can be caused by many different etiologies, emerges as a result of activity-dependent synaptic changes that lead to the sensitization of the pain circuitry23. This sensitization is characterized by an increase in excitatory synaptic transmission and a loss of inhibitory synaptic transmission in pain circuits in the CNS (central sensitization)23,24. Importantly, there is increasing agreement that astrocyte-mediated neuroinflammation is a key mechanism underlying the maintenance of chronic pain 24,25.
This Review focuses on the major advances that have been made in our understanding of the role of astrocytes in the pathogenesis of neuropathic pain (BOX 1), a chronic pain condition in which the role of glia has been well-investigated. We will highlight the emerging literature supporting a role for astrocytes in the modulation of pain under homeostatic conditions (that is, in the absence of pathology) and discuss new evidence supporting the notion that astrocyte-mediated neuroinflammation is a central driver of neuropathic pain. We will highlight important differences in the distinct roles of microglia and astrocytes in the induction and maintenance of chronic neuropathic pain and describe how a crosstalk between astrocytes, neurons and microglia promotes pain pathogenesis in mammalian systems. We will also discuss the intersection of the pain and itch pathways and describe the emerging role of astrocytes in the pathogenesis of chronic itch conditions. Finally, we will consider how we can target astrogliopathy as a novel strategy to treat chronic pain and its related comorbidities.
BOX 1|. Chronic pain and neuropathic pain.
Whereas chronic pain is a general term that constitutes any persistent pain state, regardless of the underlying etiology, neuropathic pain was recently redefined by the International Association for the Study of Pain (IASP) to comprise pain resulting from lesions or disease of the somatosensory system, which may involve damage to the peripheral nerve as well as to the spinal cord and brain218. Clinical neuropathic pain is complex and chronic in nature, affecting 7–10% of the human population 219, and is associated with diabetic neuropathy, chemotherapy, major surgeries (such as amputation or thoracotomy), spinal cord injury, multiple sclerosis and stroke220. Neuropathic pain symptoms include hyperalgesia (increased painful response to noxious stimulation), allodynia (painful response to normally innocuous stimulation, such as light touch), and spontaneous pain (such as ‘burning’ pain). Of these, mechanical allodynia (tactile allodynia) and cold allodynia are cardinal features of neuropathic pain. Neuropathic pain has accompanying affective aspects and is also associated with depression, anxiety, and insomnia.
Commonly used animal models for neuropathic pain include the chronic constriction injury (CCI) model, the spinal nerve ligation (SNL) model, the partial sciatic nerve injury (PSNL) model and the spared nerve injury (SNI) model221–224. Increasing numbers of studies are using models of neuropathic pain with particular clinical relevance , such as chemotherapy-induced peripheral neuropathy (CIPN) models that use chemotherapy agents such as paclitaxel, oxaliplatin, bortezomib, and vincristine 82,225. Spinal cord injury is also frequently used to study central neuropathic pain, glial activation, and neuroinflammation63,112.
Mechanistically, neuropathic pain is a maladaptive response of the nervous system to damage and is driven by neural plasticity, including peripheral sensitization in primary sensory neurons and central sensitization in the spinal cord and brain23. Mounting evidence suggests that neuroinflammation, resulting from the activation of microglia and astrocytes, plays a critical role in the development and maintenance of neuropathic pain18,220,77,81,58,226−228.
Astrocytes in homeostatic conditions
In the absence of pathology, astrocytes provide metabolic support to neurons and contribute to the maintenance of physiological levels of extracellular potassium ions (K+), glutamate, and water 26–28 (FIG. 1). Astrocytes connect with one another to form intercellular gap junction communication routes via hemichannels [G] that directly link adjacent cells. This allows astrocytes to propagate calcium waves and to contribute to the spread of multiple long-distance signaling molecules29. Astrocytes also form a structural barrier around synapses, thereby ‘insulating’ them from glutamate spillover, while providing metabolic support30. In addition, their close contact with synapses enables astrocytes to regulate the local interstitial ionic and chemical environment during synaptic development and transmission 31,32. Indeed, astrocytes play an important role in the control of the formation, maturation, elimination and maintenance of synapses and support synaptic function through a variety of diffusible and contact-mediated signals16,33.
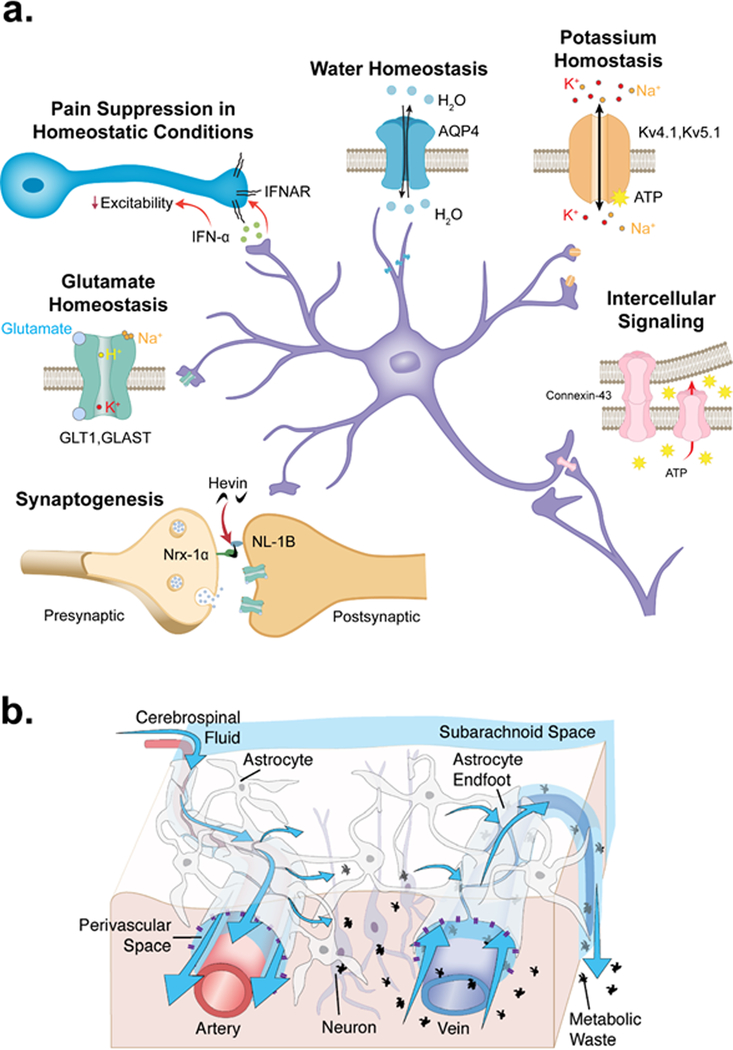
Fig. 1|. Homeostatic functions of astrocytes.
Summary of the various supportive and neuroprotective roles of astrocytes under physiological conditions. Astrocytes wrap around synapses to provide structural support and insulation for synapses and further regulate synaptogenesis via the expression of adhesion molecules such as hevin 235. Astrocytes express glutamate transporters, potassium channels and water channels, allowing them to contribute to the maintenance of glutamate homeostasis, potassium homeostasis and water homeostasis, respectively27. Astrocytes mediate long-range (intercellular) signaling via connexin-43-mediated gap-junction communication 29. Astrocytes in the spinal cord dorsal horn, the brain stem trigeminal nucleus and the sensory cortex (as well as in other regions) may also suppress physiological pain through the release of inhibitory molecules such as type-I interferons (IFN-Ι), which acts via its neuronal receptor, the interferon α/β receptor (IFNAR) 46. The glymphatic system utilizes the perivascular spaces formed by the vascular endfeet of astrocytes, which plaster around the vasculature. Cerebrospinal fluid (CSF) is driven through the periarterial space by arterial pulsations 41. The vascular endfeet of astrocytes are particularly enriched with AQP4 channels, allowing water flow which facilitates dispersal of cerebrospinal fluid (CSF) into the brain tissue alongside interstitial fluid (ISF). The mixture of CSF and ISF flows through the extracellular space (blue arrows indicate fluid flow) to perivenous spaces and cranial nerve tracts, permitting fluid and wastes to collect in lymphatic and cervical lymphatic vessels 41,212.
The growing understanding that astrocytes play an active role in regulating synaptic transmission in the CNS has led to the proposal that astrocytic processes are active components of synapses, exchanging signals with pre- and post-synaptic terminals to modulate or shape synaptic transmission34. Evidence for glutamate-dependent intracellular Ca2+ signaling in astrocytes, leading to the release of gliotransmitters that signal to nearby neurons (gliotransmission), was the foundation of the ‘tripartite synapse’ theory34; however, later work showed that the tripartite synapse, as classically conceived, may only exist transiently in the developing brain35. In mice, astrocytes downregulate Gq-coupled metabotropic glutamate receptors during development and in adult brains they fail to release Ca2+ from intracellular stores when exposed to glutamate35. Instead, brain-wide coordinated increases in the levels of Ca2+ in astrocytes in response to startle evoked by sound or air puffs have been documented, suggesting that astrocytes contribute to a general brain response rather than to synaptic activity in a specific circuit 36,37. Similar global Ca2+ increases evoked by locomotion are inhibited by α1 adrenergic receptor antagonists, suggesting that they are correlated with whole brain arousal 38. Notably, astrocytes can, in a Ca2+-dependent manner, modulate neural network activity through the active uptake of extracellular K+ 32,39, an important determinant of the resting membrane potential and excitability of neurons.
Under homeostatic conditions, astrocytes also form the structural basis for the glymphatic system (FIG. 1), a brain-wide fluid transport system that functions in the clearance of proteinaceous waste products, such as excess β-amyloid, tau, and α-synuclein, as well as metabolic byproducts 40,41.
Astrocytes in pain modulation
There is emerging evidence that astrocytes in the spinal cord dorsal horn (SDH) inhibit pain signaling under native physiological conditions in naïve animals. Astrocyte-derived ATP is hydrolyzed in the extracellular space to produce adenosine, which suppresses nociception via activation of adenosine A1 receptors expressed by sensory neurons 42,43. Interestingly, in a transgenic mouse model in which gliotransmission is attenuated through the glial-specific overexpression of a dominant negative SNARE domain (dnSNARE mice), there was a reduction in mechanical pain thresholds, suggesting that gliotransmission may modulate baseline mechanical nociception and inhibit pain under normal physiological conditons44. Later studies, however, have questioned the specificity of dnSNARE expression in astrocytes45. Spinal astrocytes also produce type-I interferons (IFN-Ι), anti-inflammatory cytokines [G] that inhibit spinal cord nociceptive synaptic transmission and pain by binding to Type-I IFN receptors on primary afferent presynaptic terminals in the SDH 46. Down-regulation of connexin-43 (CX43), the predominant gap-junction protein mediating intercellular communication between spinal astrocytes, in mouse astrocytes and satellite glial cells [G] also resulted in allodynia [G] in naïve animals, through the upregulation of interleukin-6 (IL-6)47,48. Collectively, these studies support the notion that astrocytes may serve to modulate pain responses in non-pathological homeostatic conditions (FIG. 1).
Spinal astrocytes in pathogenic pain
Several lines of evidence support a role of astrocytes in the pathogenesis of pain, especially neuropathic pain resulting from nerve injuries. First, it has been reported that there is a correlation between astrocyte hypertrophy in the SDH and pain hypersensitivity after peripheral nerve injury in mice and rats49. In addition, SDH astrogliosis – a reactive response of astrocytes that is characterized by morphological, molecular, and functional changes (BOX 2) —has been consistently observed following a variety of injuries leading to chronic pain (reviewed in REF. 9). For example, a robust induction of astrogliosis (indicated by increased GFAP expression) is induced in rodents by nerve trauma and spinal cord injury 49–52, chronic opioid exposure 53, intraplantar or intra-articular injection of complete Freund adjuvant (CFA) 54–57, bone 58 and skin cancer 59, chemotherapy, and HIV-induced neuropathy 60. Although insight into the reaction of human astrocytes to each of these pathologic states has been limited by the availability of tissue, analysis of post-mortem spinal cord tissue has demonstrated astrocyte activation in the SDH of HIV-positive patients61 and patients with longstanding complex regional pain syndrome62, indicating that astrogliosis also occurs in association with chronic pain in humans.
BOX 2|. Astrocyte heterogeneity and distinct functional states of astrocytes.
The emergence of high-throughput and affordable single cell sequencing technology has led to a deeper understanding of cellular diversity throughout the nervous system. Although work in this area is ongoing and rapidly evolving, at least 7 distinct, regionally-restricted subtypes of astrocytes have been identified in the brains of adult mice through single cell sequencing206,207 (see the figure, part a, top panel). Distinct subtypes of brainstem and spinal cord astrocytes, with unique structural, molecular, and functional properties have also been identified (see the figure, part a, bottom panel)229,230, indicating that this cellular diversity is a common feature throughout the CNS. Functional studies investigating these distinct populations are warranted; however, the molecular and cellular diversity among astrocytes may be important in conferring region-specific homeostatic functions. In the context of sensory neurobiology, understanding the regional diversity in critical regions such as the somatosensory cortex and thalamus, brainstem, and spinal dorsal horn are of key importance. In addition to regional heterogeneity, recent studies have begun to characterize phenotypic diversity among astrocytes in response to perturbations in homeostasis (see the figure, part b). For many decades, the phrase ‘astrocyte activation’ was used as the prevailing terminology to describe astrogliosis, which has been historically defined using relatively non-specific methods including upregulation of glial fibrillary acidic protein (GFAP) and changes in astrocyte morphology. It is becoming increasingly appreciated that several distinct states of activation exist, including a pro-inflammatory ‘A1’ state and a pro-regenerative ‘A2’ state 208. It is likely, however, that — under pathological conditions —astrocytes can exist in more than a simple binary state of activation, similar to recent evidence supporting a complex litany of microglia phenotypes in disease states231. The specific phenotype of reactive astrocytes in chronic pain conditions remains to be determined; however, based on the milieu of inflammatory cues produced by reactive astrocytes during the initiation and maintenance of chronic pain, it is likely that astrocytes undergo a transition to an A1-like state. It is possible that additional populations of astrocytes may also emerge with anti-inflammatory phenotypes, as has been demonstrated for microglia81. It has begun to be appreciated in the inflammation field that ‘anti-inflammation’ and ‘pro-resolution’ are not identical, with the latter involving the phagocytosis of macrophages and production of and response to specialized pro-resolving mediators (SPMs) such as resolvins and protectins232. Notably, SPMs have potent analgesic actions in animal models of inflammatory pain and neuropathic pain, acting to regulate the functions of immune cells, glial cells and neurons through specific GPCRs81. SPMs promote the resolution of inflammatory pain and neuropathic pain in part by inhibiting cytokine and chemokine release from astrocytes233,234. It will be of interest to examine whether astrocytes express SPM receptors and have a pro-resolving phenotype as ‘A3 astrocytes’. To fully understand the contribution of astrocytes in CNS disease pathogenesis, including chronic pain, future studies utilizing emerging technologies such as single cell transcriptomics, translatomics and mass cytometry are required to determine the specific phenotype of astrocytes (e.g. activation state) in chronic pain conditions, as well as in other disease states.
In mouse models of neuropathic pain, the time course of astrogliosis — which is induced early and is sustained for exceptionally long durations— points to a role for this reaction in both the acute to chronic pain transition and in the maintenance phase of pain. For example, in rats, GFAP upregulation remains prominent nine months after spinal cord injury, at which time the reactions of other cell types, such as microglia, have subsided 63. In further support of this idea, it has been shown that interfering with astrogliosis can modulate neuropathic pain. For example, inhibiting the increased proliferation of spinal astrocytes that is observed in the rodent SDH after nerve injury reduces neuropathic pain64. Other studies have shown that the inhibition of GFAP expression and astrogliosis after nerve injury is correlated with a reduction in neuropathic pain behaviors (reviewed in REF 4). That is, mice lacking Gfap exhibited neuropathic pain states with similar onset but a shortened duration after nerve injury compared to wildtype mice 65. Intrathecal (spinal) knockdown of GFAP expression with antisense oligonucleotide treatment in nerve injured animals also reduced neuropathic pain behaviors65. However, GFAP is also expressed by peripheral glial cells that are involved in pain regulation (such as satellite glial cells in the dorsal root ganglia (DRG) and Schwann cells in the sciatic nerve), making the cellular basis of this analgesic effect ambiguous4. Finally, spinal inhibition of signal transducers and activators of transcription 3 (STAT3)-mediated signaling, which drives the proliferation of astrocytes in the spinal cord after nerve injury, reduces the number of proliferating astrocytes with a corresponding recovery from neuropathic pain64.
In a second line of evidence linking astrocytes with pain, gain-of-function studies have shown that spinal astrocyte-derived mediators are sufficient to produce pain hypersensitivity. For example, it was shown that transgenic expression of the proinflammatory cytokine tumour necrosis factor (TNF) by astrocytes increases mechanical allodynia in a mouse neuropathy model66. Further, mice overexpressing C-C motif chemokine 2 (CCL2) in astrocytes display enhanced hyperalgesia [G] 67. Consistent with these findings, an intrathecal injection of TNF-treated astrocytes evokes persistent mechanical allodynia in mice through a mechanism involving the release of CCL2, demonstrating that adoptive transfer of activated astrocytes is sufficient to induce pain symptoms 68.
A third, and crucial, line of evidence linking astrocytes with pain is provided by the direct manipulation of astrocyte activity using pharmacological and optogenetic [G] approaches. Early studies utilizing astrocyte toxins, metabolic inhibitors that are preferentially taken up by astrocytes, or the astrocyte-enriched enzyme glutamine synthetase in rats suggested a probable role for astrocytes in pain4,69. Although none of these toxins is specific for astrocytes, their selectivity is improved when they are administered via a local intrathecal route that mainly targets spinal cord cells. To date, a large number of studies utilizing these compounds in a variety of acute and chronic pain models have observed attenuated pain behaviors, directly linking astrocytes with the induction and maintenance of inflammatory and neuropathic pain 9,56,69–75. A recent study demonstrated that transient optogenetic stimulation of spinal astrocytes could produce mechanical allodynia in naïve rats as rapidly as 1 hour post-stimulation, indicating that astrocyte activation is sufficient to drive the induction of pain 76. Future studies that use emergent technologies, such as inhibitory chemogenetics, to specifically target astrocytes or astrocyte activation are warranted to further strengthen this link. Nevertheless, we believe that the breadth of the current published literature clearly points to astrocytes as crucial drivers of pathological pain.
Astrocytes versus microglia in pain
Although this Review focuses on the role of astrocytes in chronic neuropathic pain, spinal microglia activation is also a key feature of several types of neuropathic pain (BOX 1). The contribution of microglia to the pathogenesis of chronic neuropathic pain is well-established and has been comprehensively discussed in several elegant reviews 4,22,75,77–81. However, important differences exist between spinal microgliosis and astrogliosis in the context of pain. Whereas astrocytes contribute to nearly all persistent pain conditions, microglia appear to regulate only certain types of pain conditions4. For example, whereas nerve injury results in the activation of both microglia and astrocytes, chemotherapy induced peripheral neuropathy (CIPN) in rats is associated with marked astrogliosis, but little or no microgliosis 82. Further, in the SDH of HIV-infected patients, chronic pain is associated with markers of astrogliosis but not microgliosis 61. In some conditions, such as in rodent bone cancer models of neuropathic pain, there are conflicting reports on whether microglia are activated, but the occurrence of reactive astrogliosis is not disputed4. Underscoring the selective contributions of microglia and astrocytes to different types of chronic pain, inhibition of microglial intracellular signaling pathways, such as the p38 MAP kinase pathway, reduces neuropathic pain symptoms in mice after nerve trauma but not in CIPN83,84. There are also important sex differences in the involvement of astrocytes versus microglia in chronic pain; whereas inhibitors that preferentially target astrocytes reduce neuropathic pain in both male and female mice, microglial inhibitors are only able to attenuate neuropathic pain in male mice in inflammatory pain and neuropathic pain models 84–86 (although the male-specific functions of microglia in pain pathogenesis may not apply to all the pain conditions87). Furthermore, a recent study shows that the loss of the voltage-gated sodium channel subtype Nav1.5 from astrocytes leads to worsened neuroinflammation and clinically-related symptoms in experimental autoimmune encephalomyelitis (EAE), a mouse model that is known to produce pain, in female but not male mice 88.
Both the time course and duration of gliosis following nerve injury also differs between microglia and astrocytes. Although the precise time course of disease varies depending on the individual study and injury model, microglial activation generally precedes and subsides prior to astrocyte activation. For example, following peripheral nerve injury in mice, spinal microglia rapidly proliferate and undergo morphological changes, reaching maximal levels within the first week following injury81. This reaction is transient and self-limiting, returning to baseline levels within 3 weeks. By contrast, astrogliosis is evident one week after nerve injury, and persists for many months 4,89. Studies using inhibitors that preferentially target astrocytes have demonstrated efficacy in attenuating both early and late-phases of neuropathic pain, whereas microglial inhibitors are effective primarily in the early phase90,91, thus correlating well with the kinetics of activation. For these reasons, microglia and astrocytes are posited to play distinct roles in the induction, maintenance and resolution of chronic pain, with microgliosis contributing to its onset and reactive astrocytes contributing to its later stages. This generalized model of neuropathic pain pathogenesis has been shown to be grossly accurate, although important differences emerge depending on the specific model investigated and the precise experimental definition of ‘gliosis’. Notably, in a bone cancer pain model in female rats, astrogliosis precedes microgliosis in the spinal cord and delayed microglia activation plays an active role in maintaining cancer pain87. Furthermore, optogenetic activation of astrocytes is sufficient to elicit microglial activation, suggesting that there are bilateral interactions between microglia and astrocytes in pain states.
Last, but not least, astrocytes and microglia may differ in their contribution to nociception more generally. Whereas astrocytes can modulate baseline pain sensitivity under the physiological conditions (as described above) a corresponding role of microglia has not yet been demonstrated.
Astrocyte signaling in chronic pain
Many cell types interact with nociceptive neurons and contribute to the pathogenesis of neuropathic pain through distinct signaling mechanisms, including Schwann cells, macrophages, keratinocytes, satellite glial cells, T-cells, oligodendrocytes, and microglia 21. Here, we focus on the contribution of astrocytes, and in particular, insights from recent studies into astrocytic intracellular signaling mechanisms and the actions of astrocyte-released neuromodulators after nerve injury (Table 1), revealing important roles for both neuron–astrocyte and microglia–astrocyte interactions in pain pathology.
Table 1:
Astrocyte-related signaling molecules and astrocyte-secreted neuromodulators in neuropathic pain.
| Mediator | Function | Pain model |
Species | Change post-Injury |
Mechanisms | Refs |
|---|---|---|---|---|---|---|
| Enzymes | ||||||
| pERK1/2 | MAP Kinase | SNL | Rat | ↑ | • Maintains mechanical allodynia | 98 |
| pJNK1 | MAP Kinase | SNL | Rat | ↑ | • Maintains mechanical allodynia | 52 |
| MMP2 | Type IV Collagenase / Protease | SNL | Mouse and rat | ↑ | • Maintains late phase of neuropathic pain • Activates IL-1β and ERK |
100 |
| TAK1 | MAP Kinase Kinase | SNL | Rat | ↑ | • Increases mechanical allodynia | 96 |
| tPA | Serine Protease | SNL | Rat | ↑ | • Increases mechanical allodynia | 101 |
| TRAF6 | E3 Ubiquitin Ligase | SNL | Mouse | ↑ | • Enhances mechanical allodynia • Increases JNK activation and CCL2 release |
131 |
| Mediators and Neuromodulators | ||||||
| CCL2 | Chemokine | SNL | Mouse | ↑ | • Enhances neuropathic pain • Activates CCR2 in neurons • Induces central sensitization |
144 |
| CXCL1 | Chemokine | CCI | Mouse | ↑ | • Maintains neuropathic pain • Activates CXCR2 in SDH neurons • Induces central sensitization |
91 |
| TNF-alpha | Cytokine | SNLT | Mouse | ↑ | • Leads to astrocyte activation and upregulation of chemokine expression | 66 |
| IL-1beta | Cytokine | SNL | Mouse and rat | ↑ | • Drives neuropathic pain and central sensitization | 100, 131 |
| bFGF (FGF-2) | Secreted Trophic Factor | SNL | Rat | ↑ | • Maintains late phase neuropathic pain • Activates JNK pathway and astrocytes |
72 |
| WNT3A | Secreted Signaling Protein | CCI | Mouse, Rat | ↑ | • Necessary for development and maintenance of pain • Induces astrocyte activation |
123 |
| TSP-4 | Adhesion Molecule | SNL | Rat | ↑ | • Increases excitatory presynaptic input • Promotes synaptogenesis |
238 |
| Transporters & Gap-Junction Proteins | ||||||
| Cx43 | Gap Junction and Hemi-channel Protein | CCI | Mouse, Rat | ↑ | • Upon conversion to hemichannel, leads to release of nociceptive mediators in extracellular space | 91 |
| GLT-1 | Glutamate Transporter | CCI | Rat | ↓ | • Downregulated in late stage of nerve injury • Increases extracellular glutamate levels |
103 |
| GLAST | Glutamate Transporter | CCI | Rat | ↓ | • Downregulated in late stage of nerve injury • increases extracellular glutamate levels |
103, 104 |
| Transcription Factors | ||||||
| NF-kB | Transcriptional Regulator | SNL | Mouse, Rat | ↑ | • Promotes neuropathic pain • Increases the expression of cytokines and chemokines |
130 |
| STAT3 | Transcriptional Regulator | SNL | Rat | ↑ | • Leads to proliferation and activation of astrocytes | 64 |
| Other Signaling Molecules | ||||||
| CXCR5 | Chemokine Receptor | SNL | Mouse | ↑ | • Activated by SDH neuron-derived CXCL13 • Drives neuropathic pain via astrocyte activation |
121 |
| IL-33 receptor/ ST2 | Cytokine Receptor | SNL | Mouse | ↑ | • Activates JAK2/STAT3 in SDH • Drives neuropathic pain via astrocyte activation |
127,128 |
Abbreviations: CCI, chronic construction injury; SNL, spinal nerve ligation; SNLT, spinal nerve ligation and transection
Kinase and protease pathways
The mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase 1 (ERK1) and ERK2, p38 MAPK (p38), c-Jun N-terminal kinase (JNK) and ERK5, show distinct activation patterns in SDH glial cells following nerve injury and play different roles in neuropathic pain induction and maintenance92,93. In rats, nerve injury results in increased phosphorylation of p38 (P-p38) in microglia, whereas increased phosphorylation of JNK (P-JNK) is observed in astrocytes52,94,95. Consistent with these findings, transforming growth factor-activated kinase-1 (TAK1), the upstream activator of JNK and c-Jun, and the downstream effectors of JNK are also activated in rat spinal astrocytes (but not microglia) by nerve injury 52,96. Of the three JNK isoforms (JNK1 1, JNK2 and JNK3), spinal astrocytes mainly express JNK155. Notably, intrathecal administration of the NMDA antagonist ketamine attenuated neuropathic pain by suppressing P-JNK in rat astrocytes97. Nerve injury also results in a dynamic activation of ERK in rat SDH glial cells: microglial phosphorylated ERK (p-ERK) is induced in the early-phase (the first week after nerve injury) but gradually reduces thereafter, whereas p-ERK is expressed in astrocytes during the late-phase following nerve injury 98, suggesting that p-ERK contributes to both the induction and maintenance of neuropathic pain.
Several proteases are also upregulated in spinal astrocytes after nerve injury. Matrix metalloproteases (MMPs) play an active role in neuroinflammation after brain injury in rodent models99. Interestingly, MMP9 and MMP2 differentially regulate microglial and astroglial activation and contribute to the induction and maintenance of neuropathic pain, respectively100. In rodents, spinal nerve ligation (SNL) induces a rapid and transient upregulation of MMP9 in DRG sensory neurons, leading to early activation of microglia and this is followed by a late but sustained MMP2 upregulation in spinal cord astrocytes, which regulates the late-phase of neuropathic pain through activation of IL-1β 100. Nerve injury further induces tissue type plasminogen activator (tPA) expression in rat spinal astrocytes to facilitate neuropathic pain 101. Tetramethylpyrazine (also known as ligustrazine) is a natural compound found in fermented soya and cocoa beans that exhibits potent anti-inflammatory activities in rats and has been shown to inhibit neuropathic pain by blocking astrocytic upregulation of MMP2 and P-JNK102.
Transporters and gap-junction proteins
The glutamate transporters GLT1 (also known as SLC1A2) and GLAST (also known as SLC1A3), which are highly (but not exclusively) expressed in astrocytes, modulate extracellular glutamate levels to maintain homeostasis of the CNS in normal physiological conditions (FIG. 1). Interestingly, nerve injury in rats elicits an initial upregulation of GLT1 and GLAST in the rat spinal cord, followed by a sustained downregulation of these proteins103,104. In rats, spinal administration of Riluzole, a positive glutamate transporter activity regulator, effectively attenuated neuropathic pain103, whereas inhibition of glutamate transporters increased spinal extracellular glutamate levels, leading to the development of spontaneous pain 105,106. More recently, a vector that selectively increases GLT1 expression in astrocytes when microinjected into the superficial SDH was developed and used to show that GLT1 overexpression in astrocytes reverses established neuropathic pain following spinal cord injury107.
Expression of the water channel aquaporin-4 (AQP4) is induced in spinal cord astrocytes after spinal cord injury in rats 51 and nerve injury in humans 108. The specific role of AQP4 in animal models of neuropathic pain remains to be determined. However, it has been hypothesized that AQP4 could be required for the maintenance of the homeostasis of the circuits mediating nociception, as mice lacking Aqp4 display reduced pain sensitivity (hypoalgesia) and dorsal horn sensitivity to noxious stimulation109. Notably, AQP4 autoantibodies are present in patients with neuromyelitis optica (NMO), a painful autoimmune disease involving spinal cord and nerve injury110.
One of the key properties of astrocytes is their formation of intercellular gap junction networks. CX43 is the predominant gap junction constituent expressed in astrocytes. CX43 hemichannels can release cytosolic compounds such as ATP or glutamate when open 111. In rodents, CX43 expression is upregulated in astrocytes following nerve lesion and spinal cord injury and inhibition of gap junction function by the non-selective inhibitor carbenoxolone (CBX) is effective in reversing mechanical allodynia in both mice and rats 91,112,113. Evidence also suggests that nerve injury may cause a functional switch from CX43-mediated gap-junctions to unopposed CX43 hemichannels, causing the extracellular release of key astrocytic mediators such as ATP, glutamate, and chemokines 91,114. Indeed, spinal cord injury-evoked ATP release and astrogliosis in the spinal cord are both diminished in Cx43−/− and Cx30−/− knockout mice, with a corresponding reduction in neuroinflammation115,116. Specific knockdown of CX43 expression via RNA interference (RNAi)47,91 also attenuated mechanical allodynia after nerve injury in both mice and rats. Selective CX43 blockade was also successfully achieved using the CX43 mimetic peptides Gap26 and Gap27, resulting in late-phase neuropathic pain relief in mice 91. Thus, persistent upregulation of CX43 is essential for maintaining neuropathic pain. Of note, CX43-mediated ATP release also serves as a mediator of intercellular astrocyte–microglial cell interactions: several ATP receptors, including P2X purinoreceptor 4 (P2RX4), P2RX7, and P2Y purinoreceptor 12 (P2RY12) are upregulated in spinal microglia and are critically involved in the pathogenesis of neuropathic pain78 (FIG. 2).
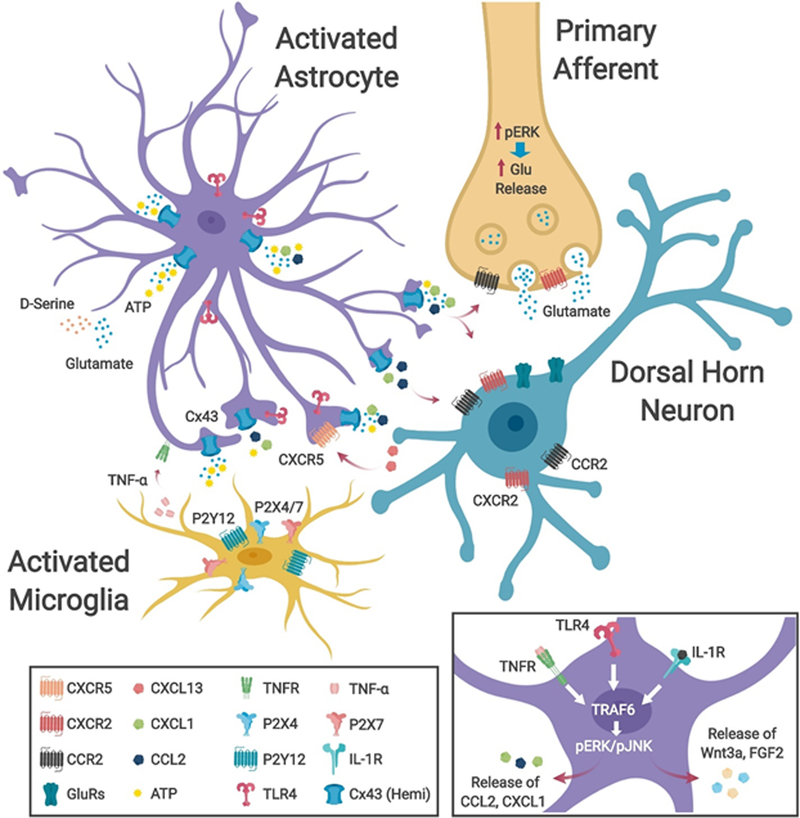
Fig. 2|. Astrocyte-neuron and astrocyte-microglial cell interactions in neuropathic pain.
Nerve injury-induces activation of astrocytes in the spinal dorsal horn (SDH), leading to astrocyte activation and astrogliosis. This involves a change in morphology, including a reduced complexity of astrocytic processes and a thickening of the main processes, the upregulation of glial fibrillary acidic protein (GFAP) and connexin 43 (CX43) and the activation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) signaling via phosphorylation (pERK, pJNK). The collective activation of these signaling pathways leads to the release of astroglial mediators, which alter neural excitability through modulation of excitatory and inhibitory synaptic transmission, leading to central sensitization and neuropathic pain and transition from acute to chronic pain4. Activation of astrocytes in neuropathic pain conditions results in upregulation of CX43 expression and a functional switch in CX43-mediated function from gap-junction communication to CX43 hemichannel-mediated paracrine signaling, resulting in increased release of proinflammatory chemokines (CXCL1 and CCL2), glutamate, and ATP. Spinal microglia express several receptors for ATP (including P2RX4, P2RX7 and P2RY12)78,81 and the activation of these microglial receptors induces the release of proinflammatory cytokines (including TNF and IL-1β), leading to sensitization of dorsal horn neurons. These cytokines also activate pJNK and pERK pathways in astrocytes, further increasing their production and release of proinflammatory chemokines. The release of astrocytic mediators (including CXCL1, CCL2 and IL-1β) promotes central sensitization via neuronal CXCR2, CCR2 and IL-1R receptors, which further stimulate ERK signaling in primary afferent central terminals and SDH neurons 91,144. Nerve injury-evoked release of CXCL13 from SDH neurons also activates astrocytes via the CXCR5 receptor to facilitate neuropathic pain121. Inset, activation of cytokine receptors (including TNFR and IL-1R) and toll-like receptor 4 (TLR4) in astrocytes by endogenous ligands and cytokines released from microglia and other astrocytes following nerve injury results in the upregulation of the transcriptional regulator TRAF6 and subsequent activation of pERK and pJNK pathways, leading to release of astroglial mediators, including chemokines, to facilitate neuropathic pain131. Astrocytes may also release growth factors including WNT3A and FGF2 to regulate neuropathic pain72,123.
The CX43 gap junction blocker CBX also inhibits another gap-junction mediator, pannexin-1 (PNX1), which is expressed in both astrocytes and microglia and itself modulates ATP release 117. In mouse spinal cord astrocytes, glucocorticoids regulate a diurnal exacerbation of mechanical allodynia after nerve injury during the late light phase through glucocorticoid-inducible kinase-1 (SGK-1) and PNX1-mediated ATP release 118. In addition, microglial PNX1-meidated release of IL-1β has been implicated in the mechanisms mechanical allodynia in an osteoarthritis model of joint pain 119, demonstrating roles for both astrocyte- and microglia-derived ATP in promoting neuropathic pain.
[H2] Glial mediators and neuromodulators
Peripheral nerve injury induces the expression of chemokines [G] such as CCL2 and CXC-chemokine ligand 1 (CXCL1) in mouse spinal cord astrocytes 59,91. In primary cultures of astrocytes, TNF rapidly induced expression of the pro-nociceptive chemokines CCL2, CXCL10, and CXCL1 through the upregulation of JNK, demonstrating cytokine–chemokine crosstalk in the pathogenesis of chronic pain59. Indeed, as discussed above, intrathecal injection of TNF-activated mouse astrocytes results in persistent mechanical allodynia via the release of CCL2 and CXCL1 in the SDH, whereas intrathecal neutralization of CCL2 and CXCL1 with neutralizing antibodies attenuates mechanical allodynia in murine models of neuropathic pain59,91. It has also been shown that there is an increase in astrocytic CCL2 expression in the medullary dorsal horn of the brain stem following trigeminal nerve injury in mice and that this contributes to trigeminal neuropathic pain120. Interestingly, CX43 also regulates chemokine release. Thus, CX43 blockade in astrocyte cultures and spinal cord slices inhibits the basal release of CCL2 and CXCL1, and further prevents the TNF-evoked release of these chemokines 91 (FIG. 2). CX43-mediated chemokine release may also involve other ATP-gated channels such as PNX-1 and P2RX7116. In addition to producing the chemokines CCL2 and CXCL1, mouse astrocytes also upregulate the chemokine receptor CXCR5 following SNL121. This study also demonstrated a marked reduction in the development of neuropathic pain symptoms such as mechanical allodynia and heat hyperalgesia in Cxcr5 knockout mice, as well as reduced reactive astrogliosis after SNL. Furthermore, it was demonstrated that spinal infusion of the CXCR5 ligand CXCL13, which is produced by SDH neurons, is sufficient to activate SDH astrocytes and elicit allodynia and hyperalgesia in wild type mice (but not Cxcr5 knockout mice)121.
WNT proteins are a family of secreted signaling molecules that regulate various cellular processes, including neuroinflammation in disease states18,122. In mice and rats, nerve injury resulted in rapid and sustained upregulation of WNT3A and WNT5B, prototypical WNT ligands, and activation of signalling via the WNT–frizzled–β-catenin pathway in neurons and astrocytes in the SDH123,124. Spinal inhibition of WNT signaling pathways suppressed the development and maintenance of neuropathic pain in these animals and further blocked the nerve injury-induced astroglial reaction and neuroinflammation, supporting the notion that WNT signaling is an important mediator of neuropathic pain symptoms 123 (FIG. 2).
Recently, evidence has also implicated sphingolipids in astrocyte signaling and in the pathogenesis of neuropathic pain. Unbiased mass spectrometry-based metabolomics revealed significant changes in the ceramide catabolite N,N-dimethylspingosine (DMS) in the rat DRG and SDH following peripheral nerve injury. Furthermore, intrathecal administration of DMS in naive rats induced persistent mechanical allodynia that was associated with reactive astrogliosis 125. In addition, treatment of cultured astrocytes with DMS led to their release of CCL2 and IL-1β 125. CIPN is a dose-limiting factor of various chemotherapy drugs such as paclitaxel and bortezomib. A recent study showed that bortezomib administration in rats causes mechanical allodynia that correlates with increased biosynthesis of ceramide metabolites — including the sphingosine-1-phosphate receptor 1 (S1PR1) ligands S1P and dihydro-S1P — in the SDH. Interestingly, astrocyte-specific deletion of S1pr1 under the control of the Gfap promoter blocked the onset of neuropathic pain after administration of bortezomib 126.
[H2] Transcriptional regulators
Several transcription factors and regulators of gene expression have also been shown to be upregulated in spinal cord astrocytes in neuropathic pain. In addition to the c-Jun pathway 52, upregulation of members of the Janus kinase (JAK) family, leading to subsequent activation of the transcription factor STAT3 regulates astroglial proliferation and sustained neuropathic pain in rats following injury 64. Additionally, the IL-33 receptor interleukin-1 receptor-like 1 (IL1RL1, also known as ST2), contributes to neuropathic pain through glial activation127, possibly by acting as an upstream activator of the JAK2–STAT3 pathway in the SDH 128. NF-κB is another key transcription factor in the induction of the expression of inflammatory genes. Nerve injury in rats and mice upregulates NF-κB in spinal microglia in the induction phase (by day 3 after injury) 129 and in spinal astrocytes during the early maintenance phase (by day 7 after injury) 130, thus inducing and maintaining neuropathic pain symptoms. Additionally, TNF receptor-associated factor 6 (TRAF6) is critically involved in signal transduction by members of the TNF receptor and IL-1 receptor superfamily and in the regulation of gene transcription via NF-kB, and has been shown to play a role in the maintenance of neuropathic pain131. Spinal nerve injury in mice induces TRAF6 expression, primarily in astrocytes, by day 10 after injury, as well as in some microglia on day 3 after injury 131. TRAF6 regulates CCL2 expression in astrocyte cultures through the activation of JNK131. Moreover, spinal knockdown of murine TRAF6 expression partially reversed SNL-induced neuropathic pain and spinal CCL2 expression131, demonstrating an intersection between TRAF6 and the transcription of the genes encoding pro-inflammatory chemokines.
Toll-like receptors (TLRs) are key regulators of the NF-kB signaling pathway and of the subsequent synthesis of cytokines and chemokines in chronic pain132. TLR4, the best studied member of the TLR family, regulates neuropathic pain through its activation of microglia and astroglia in the SDH of mice and rats133. In addition, TLR4 is expressed by SDH astrocytes and contributes to the pathogenesis of CIPN-associated neuropathic pain in mice and rats 134. Spinal TLR4 was shown to regulate inflammatory and neuropathic pain in male but not female mice135, reminiscent of the reported gender difference in the role of microglia in neuropathic pain. Gender differences in pain sensitivity and analgesia are well-documented 136, and further studies are warranted to investigate gender-dimorphism of TLR4 in CIPN, as well as the role of other TLRs in chronic pain states between genders.
[H1] Astrocytes in central sensitization
Central sensitization is a form of synaptic plasticity in the spinal cord and brain that drives increased neuronal responsiveness in central pain pathways after priming by initial painful insults137–139. There is an increasing appreciation that neuroinflammation drives central sensitization during the development and maintenance of chronic pain24. In particular, neuroinflammation and central sensitization are responsible for wide-spread pain extending beyond the initial injury site (if such an injury site exists) in chronic pain conditions such as fibromyalgia and migraine24. Activation of NMDA receptors, phosphorylation of ERK and the loss of inhibitory input (disinhibition) in SDH neurons are key signatures of central sensitization138,140,141. Following painful insults, astrocyte-mediated neuroinflammation drives central sensitization via neuron–glial and glia–glial interactions (FIG. 2). Notably, pro-inflammatory cytokines and chemokines, produced by astrocytes and microglia, are important for the induction and maintenance of central sensitization.
As described above, chemokines serve as potent mediators for neuron–glial interactions in pathological pain142. Astrocyte-derived CCL2 and CXCL1 directly regulate central sensitization and pain via their neuronal receptors, CCR2 and CXCR1, respectively142. In mice, CCR2 is expressed by DRG and SDH neurons 143–145 and the exposure of spinal cord slices to CCL2 induces rapid P-ERK activation and ERK-dependent potentiation of NMDA currents in SDH neurons via the activation of CCR2 receptors144,145. CCL2 also produces dis-inhibition in spinal cord neurons by suppressing GABA-mediated transmission (FIG. 2) 146. In addition, CXCL1 induces ERK activation and neuronal plasticity in SDH neurons via CXCR2 receptors147. Nerve injury results in a long-term central sensitization, as reflected by persistent increases in excitatory postsynaptic currents (EPSCs) in the mouse spinal cord pain circuit 3 weeks after injury 91. Notably, both this late-phase spinal synaptic plasticity and central sensitization, as well as late-phase neuropathic pain are reversed by the CXCR2 antagonist SB225002 91. Conversely, neuron-derived CXCL13 drives astrocyte activation and neuropathic pain indirectly via CXCR5 expressed by SDH astrocytes 121. Thus, a neuron-to-glial CXCL13–CXCR5 signaling axis drives both neuropathic pain and astroglial activation.
IL-1β and TNF are two major pro-inflammatory cytokines that are critically important for the pathogenesis of pain148–150. IL-1β expression is induced in rat SDH astrocytes and microglia after nerve injury and in bone cancer 151. Caspase-1 cleaves and activates IL-1β, and thus serves as a key component of the inflammasome [G] during the pathogenesis of chronic pain152. MMP2 also plays a role in the cleavage and activation of IL-1β and astroglial activation in SDH, contributing to the maintenance of SNL-induced neuropathic pain in mice and rats100. IL-1β induces central sensitization in spinal pain circuits through multiple mechanisms, including presynaptic modulation of glutamate release153, post-synaptic regulation of NMDAR phosphorylation and function 56,151,153 and suppression of inhibitory synaptic transmission153. TNF is expressed by 90% of microglia and 40% of astrocytes in the SDH, but not by neurons154. Like IL-1β, TNF facilitates central sensitization via regulation of both excitatory and inhibitory synaptic transmission 153,155.
These glia-produced cytokines and chemokines are also key regulators of spinal cord long term potentiation (sLTP)156,157. sLTP is a special type of central sensitization but is not the only mechanism of central sensitization138. TNF signaling regulates sLTP via TNF receptor 1 (TNFR1) and TNFR2, which are present on both pre- and post-synaptic neuron terminals, to regulate neurotransmitter release and increase the activities of AMPA and NMDA receptors 158,159. IL-1β also regulates sLTP at both excitatory 160 and inhibitory synapses in SDH 161. Furthermore, treatment with a CCR2 antagonist inhibits sLTP, even when it is administered in the maintenance phase of neuropathic pain 145. Intriguingly, it has been demonstrated that astroglia-derived diffusible messengers such as D-serine can promote sLTP after traveling long distances through circulating cerebrospinal fluid (CSF) 162. Likewise, the transfer of spinal CSF from a donor animal displaying sLTP can induce sLTP in a naïve recipient animal 162.
[H1] Supraspinal astrocytes in chronic pain
Although most studies have focused on glial reactions to nerve injury in the spinal cord, a host of studies have observed astrocyte activation in the spinal trigeminal nucleus in the brain stem. Similar to the role of the spinal cord in the processing of peripheral nociception, this region has a role in the processing of pain in the trigeminal system, following trigeminal nerve injury 74 or masseter inflammation56. Activation of astrocytes in the spinal trigeminal nucleus has been shown to contribute to persistent orofacial pain56,74. Furthermore, administration of the astroglial toxin fluorocitrate can attenuate hyperalgesia following injury to the masseter or tooth pulp in rats 56,163 and can reduce facial pain following nerve injury in rats 74,164. The activation of astrocytes in the spinal trigeminal nucleus appears to share many features with spinal astrocytes: for example, astrocytic upregulation of GFAP, CX43, and IL-1β expression occurring following masseter injury in rats 56. Additionally, astrocytes in the spinal trigeminal nucleus express the purinergic receptor P2RX3, and pharmacologic inhibition of P2RX3 in rats can attenuate facial pain following chronic constriction injury of the trigeminal infraorbital nerve165. In human patients, post-mortem studies demonstrating pain-induced astrocyte activation in the trigeminal system are lacking; however, in one study of 17 patients with orofacial neuropathic pain, fMRI imaging revealed slow oscillatory activity through the ascending trigeminal pain pathway, which was absent in healthy control subjects and could not be evoked by an acute pain stimulus. This activity was proposed to be due to calcium waves propagated by activated astrocytes in patients with neuropathic pain 166, suggesting that astrocyte activation may contribute to pain pathogenesis in rodents and humans.
Evidence from animal models and human patients has also demonstrated that astrocyte activation occurs in higher brain regions associated with pain processing. Astrocyte activation was observed in the rostral ventromedial medulla of the brainstem following nerve injury via ligation of the infraorbital nerve and this activation modulated trigeminal neuropathic pain via TNF and IL-1β signaling in rats 167. Astrocyte activation in the ventrolateral periaqueductal grey, a key component of the descending pain modulation system, facilitates bone cancer pain in rats168. Additionally, astrocyte activation has been observed in other brain centers involved in pain processing, including parts of the anterior cingulate cortex following formalin injection, as well as the primary somatosensory (S1) cortex and thalamus following nerve injury in rats 169,170. A study using in vivo 2-photon calcium imaging investigated the activation of astrocytes in S1 in a mouse model of mirror-image pain, in which neuropathic pain occurs on the side contralateral to the injury site170. The authors observed enhanced calcium transients in S1 cortical astrocytes following contralateral nerve injury and the inhibition of astrocytes was able to attenuate synaptic remodeling and pain behaviors. Astrocytic release of thrombospondin 1 (TSP-1) is thought to be responsible for synaptic remodeling in the S1 cortex after injury170. In humans, imaging studies conducted on the brains of patients with chronic lower back pain have demonstrated glial activation in multiple brain regions, including the thalamus and somatosensory cortex 171, thus indicating possible astrocyte activation in higher brain regions in humans. Future studies aimed at identifying the role of activated astrocytes in higher brain regions are warranted and it will be of interest to determine whether there is also a concomitant neuronal activation. Recently, it was shown that optogenetic astrocyte activation evokes a BOLD fMRI response indicative of oxygen consumption but without modulation of neuronal activity in the brain172; however, the relevance of this finding to pain remains to be established.
[H1] Astrocytes in chronic itch
Pain and itch are distinct sensory modalities. Whereas pain leads to withdrawal responses following noxious stimulation, itch (pruritus) leads to scratching. Pain is also known to suppress itch, underlying the observation that the intrathecal application of analgesics such as morphine leads to excessive itching in rodents, primates, and humans 173–175. Itch is elicited by various skin pathologies, and it has long been known that local dermal immune cells such as histamine-releasing mast cells contribute to the pathogenesis of itch176. The last decade has seen tremendous progress in elucidating neuronal mechanisms of itch, including peripheral mechanisms such as itch receptors and itch transduction pathways, as well as the central mechanisms and neuronal pathways for the perception of itch 177,178. Further, the spinal cord neurocircuit responsible for the suppression of itch by pain has been identified177,179,180. For example, glutamate release from nociceptors via the vesicular glutamate transporter VGLUT2 is required to sense pain and suppress itch181. Notably, TLRs, which are widely expressed in immune cells, glial cells (microglia and astrocytes), and neurons have been implicated in itch182.
Whereas acute itch is associated with short-duration pruritic stimuli, chronic itch is an intractable symptom of inflammatory skin diseases and certain types of skin cancers 183,184. The mechanisms implicated in chronic itch include peripheral sensitization and central sensitization of the itch circuitry 185. One of the most striking differences between acute itch and chronic itch is the loss of inhibition — the compromise of the neural circuit by which pain suppresses itch in chronic itch conditions (FIG. 3) — that leads to a vicious cycle of itching and scratching186. In this condition, scratching paradoxically exacerbates itch sensation. Loss of inhibitory interneurons expressing the transcription factor Bhlhb5 resulted in chronic itch and severe skin lesion due to non-stop scratching in rodents, indicating that these neurons are responsible for the suppression of itch by pain 186.
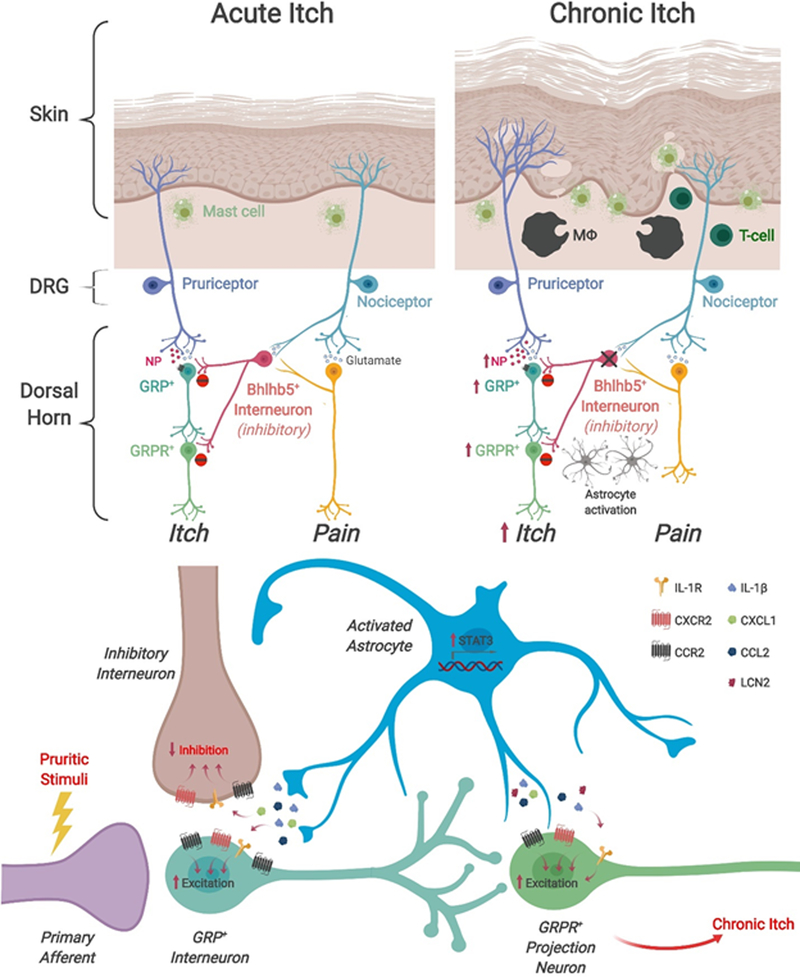
Fig. 3|. Propagation of chronic itch through disinhibition and astrocyte activation.
a| During acute itch conditions, pruritogenic stimuli activate peripheral pruriceptors (itch-sensing neurons), which subsequently activate excitatory gastrin-releasing peptide-expressing (GRP+) interneurons in the spinal dorsal horn through the release of neuropeptide B natriuretic polypeptide (NP)177,236. Activation of GRP+ neurons in turn activates gastrin-releasing peptide receptor-expressing (GRPR+) neurons which connect to projection neurons that project to higher order brain centers178. During homeostasis and in acute itch conditions, itch is gated by the pain circuitry, wherein activation of primary nociceptors by scratching activates BHLHB5-expressing (BHLHB5+) inhibitory interneurons186, which inactivate both GRP+ interneurons and GRPR+ projection neurons, thereby suppressing itch. Several pathological changes occur in chronic itch conditions. In the skin, chronic itch causes a hyperinnervation of the epidermis by peripheral pruriceptors, as well as increased numbers of mast cells and the presence of additional immune cell types such as macrophages and T-cells. A key feature of chronic itch conditions is the loss of pain-induced suppression of itch via the inhibition of inhibitory BHLHB5+ interneurons. As a consequence, scratching behaviors are able to go unchecked by the pain circuitry, further exacerbating the chronic itch condition. Importantly, chronic itch also induces activation of spinal astrocytes, which release neuroinflammatory cues that drive itch pathogenesis. b| Chronic itch-induced activation of astrocytes involves the activation of the transcription factor STAT3, which in turn induces the upregulation and release of lipocalin-2 (LCN2), which may activate GPR+ or GRPR+ neurons to promote chronic itch190. Chronic itch-induced activation of astrocytes may also involve the activation of MAP kinases that induce the upregulation of a milieu of proinflammatory cytokines and chemokines (including CXC-chemokine ligand 1 (CXCL1), C-C motif chemokine 2 (CCL2) and interleukin-1β (IL-1β)), which are proposed to act on presynaptic peripheral pruriceptors, postsynaptic GPR+ neurons, and GRPR+ neurons. The role of these pro-inflammatory cytokines/chemokines in chronic itch remains to be investigated. It is possible that the same inflammatory cytokines/chemokines regulate both chronic pain and chronic itch in distinct spinal circuits.
The study of itch is a newly emergent field and remains a work in progress. Given the current knowledge gaps, both in our understanding of the neurocircuitry of itch and the mechanisms underlying itch pathogenesis, the biology of itch continues to attract considerable attention. Although our understanding of the role of astrocytes in itch is still at an early stage187,188, the well-established role for astrocytes in neuropathic pain coupled with the intimate connection between the pain and itch circuitry implies a likely role for astrocytes in itch pathogenesis. Indeed, studies have emerged which suggest a key role of spinal astrocytes in the pathogenesis of chronic itch25,185,189. First, mouse models of atopic and contact dermatitis displayed sustained itch behaviors and long-term activation of astrocytes in SDH locations that topographically corresponded to the areas of the dermatome that were lesioned190,191. Intriguingly, it has been shown that scratching plays an essential role in spinal astrogliosis during itch, because the lesion-evoked astrogliosis in the SDH can be suppressed by preventing scratching of the itchy skin191. In support of the idea that astrocytes play a role in the pathogenesis of itch, intrathecal administration of the astroglial inhibitor L-alpha-aminoadipate reduced chronic itch after dry skin injury in mice191. Patients with cutaneous T-cell lymphoma (CTCL) suffer from severe chronic itch, and preliminary findings in a mouse model of CTCL 192, suggest that it is accompanied by persistent astrogliosis in the itch-affected SDH region (Q. Han and R-R. Ji, personal communication). Although these findings suggest that astrocytes are necessary for the production of chronic itch, it is unclear whether astrocytic activation is sufficient to evoke scratching. In sharp contrast to the findings in chronic itch, astrocytes do not appear to be involved in acute itch, as L-alpha aminoadipate failed to reduce scratching behavior induced by subcutaneous administration of chloroquine or a histamine releaser191
Our knowledge of the mechanisms underlying chronic pain and itch have revealed some striking similarities. TLR4, a key mediator of neuropathic pain, is induced in mouse spinal astrocytes in a chronic itch condition and is required for the development and maintenance of chronic itch191. Additionally, STAT3-dependent reactive astrogliosis in the SDH contributes to the pathogenesis of chronic itch: in mice, conditional disruption of astrocytic Stat3 or pharmacological inhibition of spinal STAT3 attenuated chronic itch 190. Mechanistically, STAT3-dependent upregulation of the innate immune factor lipocalin-2 (LCN2) was shown to be crucial for inducing chronic itch. As an astrocyte-secreted mediator, LCN2 exacerbated the pruritus induced by spinal injection of gastrin-releasing peptide (GRP) 190, which binds to its receptor GRPR to activate the itch circuitry in the spinal cord178 (FIG. 3). Additionally, a new study shows that interleukin-33 receptor (ST2) is upregulated in spinal cord astrocytes in chronic itch; and furthermore, spinal IL-33/ST2 signaling regulates chronic itch via JAK2-STAT3 cascade activation in spinal astrocytes 189.
Itch is also related to mechanical sensations. Alloknesis, a touch-elicited itch or mechanical itch, is mediated by central sensitization185,193 and a spinal cord inhibitory circuit194. Spinal astrocytes play an activate role in alloknesis. For example, dry skin injury resulted in robust alloknesis, which was suppressed by intrathecal injection of an astroglial inhibitor or a TLR4 antagonist191. As in chronic pain, proinflammatory cytokines and chemokines in the SDH also play a role in chronic itch and spinal inhibition of TNF reduced chronic pruritus195. Dry skin injury also led to an upregulation of CXCR3 and its ligand CXCL10 in the spinal cord and intrathecal injection of a CXCR3 antagonist or deletion of Cxcr3 alleviated chronic itch 196. These findings suggest that, in addition to the well-demonstrated peripheral actions of cytokines and chemokines in evoking pruritus in the skin197–199, proinflammatory cytokines and chemokines may drive chronic itch via astrocyte activation, neuroinflammation, disinhibition, and central sensitization. These astroglia-mediated mechanisms are common to chronic pain and chronic itch conditions (FIG. 3)185, further demonstrating the interconnected relationship of these two distinct sensory modalities.
This observed overlap between the mechanisms driving chronic pain and chronic itch prompts and important question: if the molecular mediators are shared between these two conditions, wherein do the differences lie? It is possible the same signaling pathways modulate both pain and itch in distinct locations and circuits. It is also possible that TLR4 and STAT3 regulate distinct downstream pathways in pain and itch circuits. Pain and itch may co-exist in some chronic conditions (such as ulcerated skin lesions) and this may be associated with a vicious cycle of ‘itch–scratch–itch’, further driving astrocyte activation. Future studies aimed at dissecting these distinct sensory modalities are needed, and should be focused both at the level of their neurocircuitry and on the astroglial mediators responsible for chronic pain and itch pathogenesis.
Novel therapeutic strategies
Safe and effective treatment of chronic pain is a challenge. A recent report states that more than 1.5 billion people worldwide suffer from chronic pain 200. In the USA, chronic pain conditions involving nerve injury and neurodegeneration affect 30% of the population, leading to an estimated cost of $600 billion for treatment and lost productivity201. Conventional pharmacological treatments such as opiates, antidepressants and anticonvulsants are only partially effective in managing chronic pain, frequently exacerbate pain conditions and associated comorbidities and are associated with significant side effects. For this reason, there is an urgent need for the development of safe, non-addictive, non-opioid based medications that also possess disease modifying properties.
Given the evidence supporting the notion that astrocytes play a key role in the induction and maintenance of persistent pain, it is important to consider how we can target astrogliopathy to treat chronic pain conditions. Eliminating astrocytes or blocking astrocyte function entirely using the astroglial toxins that are capable of attenuating neuropathic pain conditions in animal models is an untenable approach in human patients, given the well-established supportive and neuroprotective roles astrocytes play in organism health6. Additionally, developing glia-selective drugs for the treatment of neuropathic pain may prove difficult given the very limited repertoire of astrocyte-specific targets. Based on our present knowledge of astrocyte mediators of neuropathic pain, there are two possible strategies to be considered: targeting MAPK signaling pathways, hemichannels or purinergic receptors to suppress the release of glial mediators or targeting the downstream mediators released (at least in part) by astroglia, such as chemokine and cytokine signaling. It is noteworthy that more than 80% of patients with NMO experience pain including headache110. One key characteristic of NMO is the presence of serum AQP4 antibodies and the serum and CSF concentrations of IL-6, which induces AQP-4 antibody production by plasmablasts, are significantly elevated in patients with NMO. Studies have shown that prolonged treatment with tocilizumab, an FDA-approved anti-IL-6 antibody may be safe and effective in treating NMO202 as well as other neuropathic pain conditions203.
Given that astrogliopathy is a common feature of nearly all chronic pain pathologies, and that astroglial activation remains robust for the duration of the persistent pain condition, it is interesting to consider whether we could instead target the activation of astrocytes. In other words, can we block the transition of astrocytes to a pro-inflammatory reactive state, or favor their acquisition of a pro-resolving state, without affecting the normal homeostatic functions of astrocytes? In a sense, such a strategy would represent a movement towards an immunotherapeutic [G] approach to treating chronic pain. Although our present knowledge of the mechanisms underlying the activation of astrocytes is lacking, both in chronic pain and other CNS diseases, the identification of astrocyte activation checkpoints represents an intriguing future therapeutic strategy.
In addition to these approaches, several non-pharmacological treatments are currently under investigation in animal models, including cell-based therapies (such as bone marrow stem cells), exercise (such as prior voluntary wheel running) and neuromodulation (including spinal cord stimulation, DRG stimulation and acupuncture) and may offer ways to control chronic pain through the regulation of astroglial reactions and neuroinflammation 24,204,205.
[H1] Conclusions and future perspectives
As outlined above, chronic neuropathic pain can arise due to astrogliopathy, in which the normal capacity of astrocytes to maintain CNS homeostasis is disrupted. Astrogliopathy results in abnormal extracellular levels of glutamate, K+ and water, as well as the secretion of proinflammatory cytokines and chemokines due to CX43-mediated membrane leakage. These astrocyte-driven pathologies lead to neuronal hyperexcitability and neurotoxicity, neuroinflammation, and chronic pain. Importantly, astrocytes promote and maintain chronic pain conditions via neuron–glial and glia–glial interactions; astrocyte-produced mediators such as cytokines and chemokines are powerful neuromodulators regulating both excitatory and inhibitory synaptic transmission in the pain circuit, leading to central sensitization 153. Similar dysregulations of astrocyte function may also apply to chronic itch conditions. What is the path forward? What are the knowledge gaps that remain? In our opinion, future research should focus on several key areas of importance (FIG. 4).
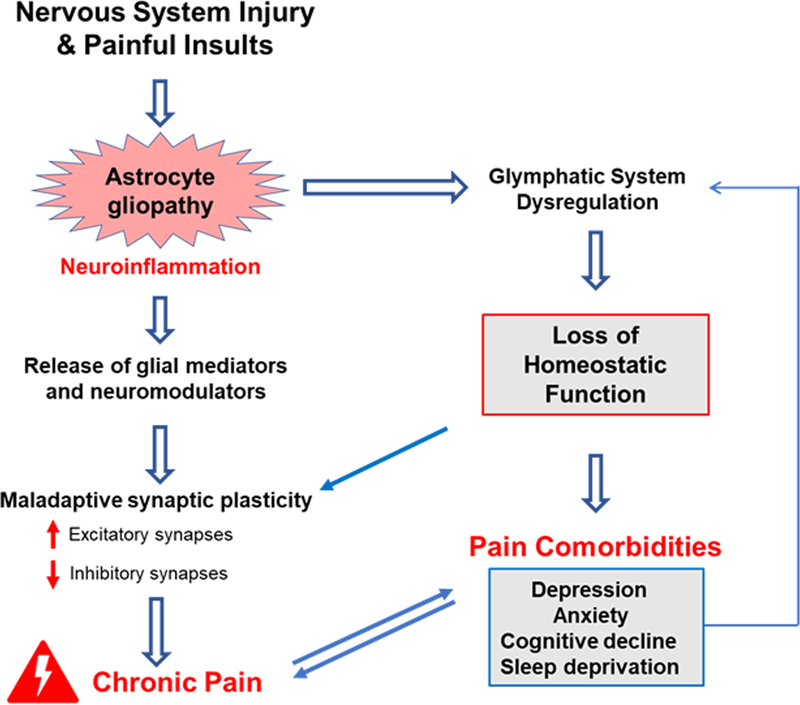
Fig. 4|. Future directions in the investigation of astrocytes in chronic pain.
Nervous system injuries (such as nerve injury, spinal cord injury or stroke) and painful insults (including arthritis or tumors) result in astrogliopathy, neuroinflammation, and chronic pain. To fully understand the contribution of astrocytes to chronic pain, we suggest several directions for future studies. The figure shows a schematic of the key mechanisms through which astrocytes have been proposed to influence chronic pain, each of which is a target for future investigation. Dashed arrows indicate hypotheses that remain to be tested. Research utilizing proteomics and lipidomics approaches is needed to identify the astroglial mediators produced in chronic pain conditions, as well as in physiological states. Chronic pain is chiefly an expression of maladaptive synaptic plasticity, which produces peripheral and central sensitization and thereby drives chronic pain23. During homeostasis, ongoing synaptic plasticity mediates the formation and breakdown of both excitatory and inhibitory synapses16; however, during chronic pain conditions, this balance is perturbed, leading to increased excitatory input and reduced inhibitory input. The synaptic mechanisms responsible for this phenomenon, as well as the identification of the precise astroglial synaptogenic factors that govern synapse assembly and pruning, require further investigation. It will also be of great interest to understand the connection between astrogliopathy and chronic pain co-morbidities, such as depression, anxiety, cognitive deficits, and sleep deprivation, as all these disorders involve neuroinflammation24. Sleep deprivation is not only a risk factor of clinical pain but also associated with increased pain sensitivity in mice 237. Importantly, sleep regulates the glymphatic system211, a brain-wide fluid transport system that clears proteinaceous waste 40. It is possible that glymphatic dysfunction caused by astrogliopathy would lead to a failure to clear these waste products and an accumulation of neuroinflammatory mediators, thereby driving chronic pain. Thus, studies aimed at addressing the role of glymphatic function (and dysfunction) in acute and chronic pain conditions remain an important future direction.
It is essential to consider the functional and regional diversity of astrocytes (BOX 2). Recent studies using whole nervous system single cell transcriptomics have revealed multiple distinct subpopulations of astrocytes based on their transcriptomes. One study, sequencing 690,000 cells taken from nine regions throughout the mouse brain, found numerous molecularly distinct populations of astrocytes 206. In another, sequencing over half a million cells throughout the nervous system of mice demonstrated the existence of at least seven molecularly distinct astrocyte subtypes, with region-specific distributions in the brain, and provided validation of the individual subtypes using distinct molecular markers207. Building upon this knowledge, understanding how distinct astroglial populations respond to chronic pain and other nervous system pathologies represents an important future research direction. In addition, it is crucial that we develop a more precise definition of ‘reactive astrocytes’ and ‘astrogliosis’, both in the context of neuropathic pain and more generally across CNS pathologies. The relatively recent demonstration of a pro-inflammatory ‘A1-like’ state and a pro-regenerative ‘A2-like’ reactive state of astrocytes 208 is likely to be just the tip of the iceberg. In analogy to the macrophage field, which began with a binary ‘M1’ and ‘M2’ classification and eventually evolved to include a broad spectrum of cellular phenotypes 209, omic-based approaches aimed at understanding the diverse phenotypic complexity of astrocytes will greatly enhance our knowledge and, in doing so, perhaps unveil new therapeutic targets. Given the persistent activation of astrocytes in many neuropathic pain conditions, these studies may identify distinct pro- and anti-inflammatory, as well as pro-resolving, astrocyte phenotypes that contribute to the maintenance and resolution of pain, as has been the case in microglia81 (BOX 2).
Although astrocyte-mediated synaptogenesis during development is well-studied, little is known about the contribution of astroglia-secreted molecules to chronic pain-associated synaptogenesis in spinal and brain circuits. Given that chronic pain is chiefly driven by central sensitization via synaptic plasticity, understanding how synaptogenic mediators are utilized in the context of persistent pain thus represents a critical area of future study. Interestingly, single-cell sequencing has identified a gene-expression module in astrocytes for synthesizing axonal and presynaptic components206.
Human astrocytes are larger than rodent astrocytes and have several distinct properties13,14. Investigating changes in human astroglia in chronic pain conditions through post mortem examination and real-time imaging of astroglial activation in disease will be an area of great importance in future research.
Last, but not least, we believe that the connection between astrogliopathy and chronic pain co-morbidities, such as depression, anxiety, sleep deprivation, and cognitive deficits (all known to involve neuroinflammation24), is an important area for future studies. Most patients with chronic pain have sleep disturbance and chronic fatigue210. The physiological relevance of sleep has been associated with the glymphatic system211, the recently identified brain-wide fluid transport system that clears proteinaceous waste and neurotoxins 40,211 through the perivascular space formed by the vascular endfeet of astrocytes. Multiple lines of experimental work have demonstrated the existence of the glymphatic system in experimental animals (reviewed in 212,213) and in the human brain 214,215 and it has been proposed that disruption of this system could exacerbate the intracerebral accumulation of neurotoxic protein metabolites and inflammatory mediators in the CSF (FIG. 4), which could contribute to chronic pain development and maintenance212. It is currently unknown whether glymphatic function modulates acute and chronic pain, but some correlative observations exist. For example, exercise and sleep are each independently associated with improved glymphatic circulation 211,216 and with attenuated measures of chronic pain. Likewise, the converse effect of pain on glymphatic function has yet to be determined. However, we predict that pain, especially chronic pain could suppress glymphatic circulation through the release of noradrenaline 217, as adrenergic activation is known to block glymphatic function211. Pain-associated astrogliosis could also induce a loss of perivascular AQP4 localization to suppress glymphatic circulation211.
Taken together, the evidence reviewed in this article suggests that targeting astrogliopathy, restoring the normal functions of astrocytes, or/and stimulating the regenerative and pro-resolving phenotype of astrocytes will help to alleviate chronic pain and associated comorbidities.
Acknowledgements
This work was supported by NIH grants DE17794 to R.R.J. and DE22743 to R.R.J. and M.N. M.N is also supported by the Lundbeck Foundation. C.R.D. is supported by the John J. Bonica Trainee Fellowship from the International Association for the Study of Pain and NIH T32 GM08600.
Glossary
- Neuroinflammation
A localized form of inflammation occurring in the peripheral nervous system and/or central nervous system
- Hemichannels
Assemblies composed of six connexin proteins, which form a pore that allows for the bidirectional flow of ions and signaling molecules.
- Cytokines
A large and diverse class of small (<30 kDa) proteins, glycoproteins, and peptides, which are secreted by cells and exert specific biological functions including pain modulations.
- Satellite glial cells
A cell type in the peripheral nervous system that is roughly equivalent in function to astrocytes in the CNS
- Nociceptor
A specialized neuron of the somatosensory nervous system that is capable of transducing nociceptive stimuli
- Analgesia
The absence of pain in response to stimulation that would normally be painful
- Allodynia
Pain in response to a stimulus that does not normally provoke pain. Mechanical allodynia is a cardinal feature of chronic pain.
- Hyperalgesia
Increased pain in response to a stimulus that ordinarily provokes pain
- Optogenetic
The use of light to activate or inhibit genetically-encoded ion channels expressed within a defined cell type of interest
- Chemokines
A specific family of immunomodulatory cytokines named for their ability to induce directed chemotaxis of immune effector cells
- Peripheral and/or central sensitization
Forms of neuronal and/or synaptic plasticity characterized by increased responsiveness of nociceptive neurons in the PNS or CNS to their normal input
- Inflammasome
A multimeric intracellular signaling complex responsible for the detection of pathogenic microorganisms and stress host-derived stressors leading to the activation of caspase-1 and the induction of inflammation
- Immunotherapeutic
A therapeutic agent used to treat a disease by activating or suppressing the immune system
- Glymphatic system
A waste clearance system in the CNS which utilizes a unique network of perivascular tunnels formed by astrocytes
Footnotes
Competing Interests
The authors declare that there are no competing interests.
Reference List
- 1.Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia 62, 1377–1391 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Ben Haim L. & Rowitch DH Functional diversity of astrocytes in neural circuit regulation. Nature reviews. Neuroscience 18, 31–41 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Giaume C. & McCarthy KD Control of gap-junctional communication in astrocytic networks. Trends Neurosci 19, 319–325 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Ji RR, Berta T. & Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain 154 S10–S28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathewson AJ & Berry M. Observations on the astrocyte response to a cerebral stab wound in adult rats. Brain research 327, 61–69 (1985). [DOI] [PubMed] [Google Scholar]
- 6.Kimelberg HK & Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 7, 338–353 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iadecola C. & Nedergaard M. Glial regulation of the cerebral microvasculature. Nat.Neurosci. 10, 1369–1376 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Oberheim NA, et al. Loss of astrocytic domain organization in the epileptic brain. J Neurosci 28, 3264–3276 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao YJ & Ji RR Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 7, 482–493 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bushong EA, Martone ME, Jones YZ & Ellisman MH Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22, 183–192 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberheim NA, et al. Uniquely hominid features of adult human astrocytes. J Neurosci 29, 3276–3287 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halassa MM, Fellin T, Takano H, Dong JH & Haydon PG Synaptic islands defined by the territory of a single astrocyte. J Neurosci 27, 6473–6477 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberheim NA, Wang X, Goldman S. & Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci 29, 547–553 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Han X, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laug D, Glasgow SM & Deneen B. A glial blueprint for gliomagenesis. Nat Rev Neurosci 19, 393–403 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke LE & Barres BA Emerging roles of astrocytes in neural circuit development. Nature reviews. Neuroscience 14, 311–321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molofsky AV, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 26, 891–907 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji RR, Xu ZZ & Gao YJ Emerging targets in neuroinflammation-driven chronic pain. Nat.Rev.Drug Discov. 13, 533–548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ransohoff RM How neuroinflammation contributes to neurodegeneration. Science 353, 777–783 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Heppner FL, Ransohoff RM & Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nature reviews. Neuroscience 16, 358–372 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Ji RR, Chamessian A. & Zhang YQ Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grace PM, Hutchinson MR, Maier SF & Watkins LR Pathological pain and the neuroimmune interface. Nat.Rev.Immunol. 14, 217–231 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costigan M, Scholz J. & Woolf CJ Neuropathic pain: a maladaptive response of the nervous system to damage. Annu.Rev.Neurosci. 32, 1–32 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji RR, Nackley A, Huh Y, Terrando N. & Maixner W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 129, 343–366 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuda M. Modulation of Pain and Itch by Spinal Glia. Neuroscience bulletin 34, 178–185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verkhratsky A, et al. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN.Neuro. 4 e0008. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simard M. & Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129, 877–896 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Cui Y, et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554, 323–327 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Cornell-Bell AH, Finkbeiner SM, Cooper MS & Smith SJ Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247, 470–473 (1990). [DOI] [PubMed] [Google Scholar]
- 30.Nedergaard M. & Verkhratsky A. Artifact versus reality--how astrocytes contribute to synaptic events. Glia 60, 1013–1023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djukic B, Casper KB, Philpot BD, Chin LS & McCarthy KD Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27, 11354–11365 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verkhratsky A. & Nedergaard M. Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond B Biol Sci 369, 20130595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eroglu C. & Barres BA Regulation of synaptic connectivity by glia. Nature 468, 223–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araque A, Parpura V, Sanzgiri RP & Haydon PG Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22, 208–215 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Sun W, et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding F, et al. alpha1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell calcium 54, 387–394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasan R, et al. Ca(2+) signaling in astrocytes from Ip3r2(−/−) mice in brain slices and during startle responses in vivo. Nature neuroscience 18, 708–717 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paukert M, et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F, et al. Astrocytes modulate neural network activity by Ca(2)(+)-dependent uptake of extracellular K(+). Sci.Signal. 5, ra26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iliff JJ, et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid beta. Sci Transl Med 4, 147ra111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedergaard M. Neuroscience. Garbage truck of the brain. Science 340, 1529–1530 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman N, et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat.Neurosci. 13, 883–888 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang JM, et al. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40, 971–982 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Foley JC, McIver SR & Haydon PG Gliotransmission modulates baseline mechanical nociception. Molecular pain 7, 93 (2011).This study, together with Fujita et al., 2014 and Liu et al., 2014, provides evidence for the existence of an astrocyte-mediated pain suppression system under homeostatic conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujita T, et al. Neuronal transgene expression in dominant-negative SNARE mice. J Neurosci 34, 16594–16604 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu CC, et al. Interferon alpha inhibits spinal cord synaptic and nociceptive transmission via neuronal-glial interactions. Scientific reports 6, 34356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohara PT, Vit JP, Bhargava A. & Jasmin L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J Neurophysiol. 100, 3064–3073 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morioka N, et al. Downregulation of spinal astrocytic connexin43 leads to upregulation of interleukin-6 and cyclooxygenase-2 and mechanical hypersensitivity in mice. Glia 66, 428–444 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Garrison CJ, Dougherty PM, Kajander KC & Carlton SM Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 565, 1–7 (1991).This study provides early evidence for a possible involvment of astrocytes in pain. [DOI] [PubMed] [Google Scholar]
- 50.Garrison CJ, Dougherty PM & Carlton SM GFAP expression in lumbar spinal cord of naive and neuropathic rats treated with MK-801. Exp.Neurol. 129, 237–243 (1994). [DOI] [PubMed] [Google Scholar]
- 51.Nesic O, et al. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J.Neurochem. 95, 998–1014 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Zhuang ZY, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J.Neurosci 26, 3551–3560 (2006).This study demonstrates that astrocytic JNK signaling contributes to the maintenance of nerve injury-induced neuropathic pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song P. & Zhao ZQ The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 39, 281–286 (2001).This study provides early evidence for an involvment of astrocytes in chronic morphine induced antinocieptive tolerance. [DOI] [PubMed] [Google Scholar]
- 54.Raghavendra V, Tanga FY & DeLeo JA Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur.J.Neurosci 20, 467–473 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Gao YJ, et al. The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition. Pain 148, 309–319 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo W, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J.Neurosci. 27, 6006–6018 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun S, et al. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain 129, 64–75 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Honore P, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 98, 585–598 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Gao YJ, et al. Selective inhibition of JNK with a peptide inhibitor attenuates pain hypersensitivity and tumor growth in a mouse skin cancer pain model. Exp.Neurol. 219, 146–155 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H, Yoon SY, Zhang H. & Dougherty PM Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J.Pain 13, 293–303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Y, Gelman BB, Lisinicchia JG & Tang SJ Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci 32, 10833–10840 (2012).This study provides evidence for the persistent activation of spinal cord astrocytes in a human chronic pain condition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Del Valle L, Schwartzman RJ & Alexander G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain, behavior, and immunity 23, 85–91 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Gwak YS, Kang J, Unabia GC & Hulsebosch CE Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp.Neurol. 234, 362–372 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuda M, et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain 134, 1127–39 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim DS, et al. Profiling of dynamically changed gene expression in dorsal root ganglia post peripheral nerve injury and a critical role of injury-induced glial fibrillary acidic protein in maintenance of pain behaviors [corrected]. Pain 143, 114–122 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeLeo JA, Rutkowski MD, Stalder AK & Campbell IL Transgenic expression of TNF by astrocytes increases mechanical allodynia in a mouse neuropathy model. Neuroreport 11, 599–602 (2000). [DOI] [PubMed] [Google Scholar]
- 67.Menetski J, et al. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience 149, 706–714 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Gao YJ, Zhang L. & Ji RR Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia 58, 1871–1880 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiang CY, Sessle BJ & Dostrovsky JO Role of Astrocytes in Pain. Neurochem.Res. (2012). [DOI] [PubMed] [Google Scholar]
- 70.Meller ST, Dykstra C, Grzybycki D, Murphy S. & Gebhart GF The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology 33, 1471–1478 (1994). [DOI] [PubMed] [Google Scholar]
- 71.Watkins LR, Martin D, Ulrich P, Tracey KJ & Maier SF Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain 71, 225–235 (1997). [DOI] [PubMed] [Google Scholar]
- 72.Ji RR, Kawasaki Y, Zhuang ZY, Wen YR & Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2, 259–269 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiang CY, et al. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J.Neurosci. 27, 9068–9076 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okada-Ogawa A, et al. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J.Neurosci. 29, 11161–11171 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ren K. & Dubner R. Interactions between the immune and nervous systems in pain. Nat.Med. 16, 1267–1276 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nam Y, et al. Reversible Induction of Pain Hypersensitivity following Optogenetic Stimulation of Spinal Astrocytes. Cell reports 17, 3049–3061 (2016).This study demonstrates that sterile astrocyte activation using optogenetic stimulation alone is sufficient to produce pain behaviors in naïve rats. [DOI] [PubMed] [Google Scholar]
- 77.Salter MW & Stevens B. Microglia emerge as central players in brain disease. Nature medicine 23, 1018–1027 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Inoue K. & Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nature reviews. Neuroscience 19, 138–152 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Scholz J. & Woolf CJ The neuropathic pain triad: neurons, immune cells and glia. Nat.Neurosci 10, 1361–1368 (2007). [DOI] [PubMed] [Google Scholar]
- 80.McMahon SB & Malcangio M. Current challenges in glia-pain biology. Neuron 64, 46–54 (2009). [DOI] [PubMed] [Google Scholar]
- 81.Chen G, Zhang YQ, Qadri YJ, Serhan CN & Ji RR Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 100, 1292–1311 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson CR, Zhang H. & Dougherty PM Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 274, 308–317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo X, et al. Intrathecal administration of antisense oligonucleotide against p38alpha but not p38beta MAP kinase isoform reduces neuropathic and postoperative pain and TLR4-induced pain in male mice. Brain, behavior, and immunity 72, 34–44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taves S, et al. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain, behavior, and immunity 55, 70–81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sorge RE, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat.Neurosci 18, 1081–1083 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen G, Luo X, Qadri MY, Berta T. & Ji RR Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neuroscience bulletin 34, 98–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Y, et al. Delayed Activation of Spinal Microglia Contributes to the Maintenance of Bone Cancer Pain in Female Wistar Rats via P2X7 Receptor and IL-18. J.Neurosci. 35, 7950–7963 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pappalardo LW, et al. Nav1.5 in astrocytes plays a sex-specific role in clinical outcomes in a mouse model of multiple sclerosis. Glia 66, 2174–2187 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Zhang J. & De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J.Neurochem. 97, 772–783 (2006). [DOI] [PubMed] [Google Scholar]
- 90.Raghavendra V, Tanga F. & DeLeo JA Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J.Pharmacol.Exp.Ther. 306, 624–630 (2003). [DOI] [PubMed] [Google Scholar]
- 91.Chen G, et al. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 137, 2193–2209 (2014).This study demonstrates that astrocytes upregulate Connexin-43 following nerve injury, driving release of CXCL1 which induces central sensitization in nociceptive neurons to maintain neuropathic pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Obata K. & Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 74, 2643–2653 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Ji RR, Gereau RW, Malcangio M. & Strichartz GR MAP kinase and pain. Brain Res.Rev. 60, 135–148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jin SX, Zhuang ZY, Woolf CJ & Ji RR p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J.Neurosci. 23, 4017–4022 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S. & Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia 45, 89–95 (2004). [DOI] [PubMed] [Google Scholar]
- 96.Katsura H, et al. Transforming growth factor-activated kinase 1 induced in spinal astrocytes contributes to mechanical hypersensitivity after nerve injury. Glia 56, 723–733 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Mei XP, et al. Inhibition of spinal astrocytic c-Jun N-terminal kinase (JNK) activation correlates with the analgesic effects of ketamine in neuropathic pain. Journal of neuroinflammation 8, 6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhuang ZY, Gerner P, Woolf CJ & Ji RR ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 114, 149–159 (2005). [DOI] [PubMed] [Google Scholar]
- 99.Ji RR, Xu ZZ, Wang X. & Lo EH Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol.Sci. 30, 336–340 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawasaki Y, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat.Med. 14, 331–336 (2008).This paper demonstrated that MMP-2 upregulation in spinal astrocytes after nerve injury contributes to neuropathic pain maintenance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kozai T, et al. Tissue type plasminogen activator induced in rat dorsal horn astrocytes contributes to mechanical hypersensitivity following dorsal root injury. Glia 55, 595–603 (2007). [DOI] [PubMed] [Google Scholar]
- 102.Jiang L, et al. Selective suppression of the JNK-MMP2/9 signal pathway by tetramethylpyrazine attenuates neuropathic pain in rats. Journal of neuroinflammation 14, 174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sung B, Lim G. & Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J.Neurosci. 23, 2899–2910 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xin WJ, Weng HR & Dougherty PM Plasticity in expression of the glutamate transporters GLT-1 and GLAST in spinal dorsal horn glial cells following partial sciatic nerve ligation. Mol.Pain 5, 15 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liaw WJ, et al. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain 115, 60–70 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Weng HR, Chen JH & Cata JP Inhibition of glutamate uptake in the spinal cord induces hyperalgesia and increased responses of spinal dorsal horn neurons to peripheral afferent stimulation. Neuroscience 138, 1351–1360 (2006). [DOI] [PubMed] [Google Scholar]
- 107.Falnikar A, Hala TJ, Poulsen DJ & Lepore AC GLT1 overexpression reverses established neuropathic pain-related behavior and attenuates chronic dorsal horn neuron activation following cervical spinal cord injury. Glia 64, 396–406 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tackley G, et al. Chronic neuropathic pain severity is determined by lesion level in aquaporin 4-antibody-positive myelitis. J Neurol Neurosurg Psychiatry 88, 165–169 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Bao F, Chen M, Zhang Y. & Zhao Z. Hypoalgesia in mice lacking aquaporin-4 water channels. Brain Res.Bull. 83, 298–303 (2010). [DOI] [PubMed] [Google Scholar]
- 110.Bradl M, et al. Pain in neuromyelitis optica--prevalence, pathogenesis and therapy. Nature reviews. Neurology 10, 529–536 (2014). [DOI] [PubMed] [Google Scholar]
- 111.Kang J, et al. Connexin 43 hemichannels are permeable to ATP. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 4702–4711 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen MJ, et al. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia 60, 1660–1670 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spataro LE, et al. Spinal gap junctions: potential involvement in pain facilitation. J.Pain 5, 392–405 (2004). [DOI] [PubMed] [Google Scholar]
- 114.Bennett MV, Contreras JE, Bukauskas FF & Saez JC New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 26, 610–617 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cronin M, Anderson PN, Cook JE, Green CR & Becker DL Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol.Cell Neurosci. 39, 152–160 (2008). [DOI] [PubMed] [Google Scholar]
- 116.Huang C, et al. Critical role of connexin 43 in secondary expansion of traumatic spinal cord injury. J.Neurosci. 32, 3333–3338 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garre JM, et al. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc.Natl.Acad.Sci.U.S.A 107, 22659–22664 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koyanagi S, et al. Glucocorticoid regulation of ATP release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nat Commun 7, 13102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mousseau M, et al. Microglial pannexin-1 channel activation is a spinal determinant of joint pain. Sci Adv 4, eaas9846 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang ZJ, et al. Chemokine CCL2 and its receptor CCR2 in the medullary dorsal horn are involved in trigeminal neuropathic pain. J Neuroinflammation. 9, 136 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang BC, et al. CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. The Journal of clinical investigation 126, 745–761 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yuan S, Shi Y. & Tang SJ Wnt signaling in the pathogenesis of multiple sclerosis-associated chronic pain. J Neuroimmune.Pharmacol. 7, 904–913 (2012). [DOI] [PubMed] [Google Scholar]
- 123.Zhang YK, et al. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin.Invest 123, 2268–2286 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu S, et al. Wnt/Ryk signaling contributes to neuropathic pain by regulating sensory neuron excitability and spinal synaptic plasticity in rats. Pain 156, 2572–2584 (2015). [DOI] [PubMed] [Google Scholar]
- 125.Patti GJ, et al. Metabolomics implicates altered sphingolipids in chronic pain of neuropathic origin. Nat Chem Biol 8, 232–234 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stockstill K, et al. Dysregulation of sphingolipid metabolism contributes to bortezomib-induced neuropathic pain. The Journal of experimental medicine 215, 1301–1313 (2018).This study demonstrates that chemotherapy-induced sphingolipid metabolites activate astrocytes to release pro-inflammatory mediators and drive neuropathic pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zarpelon AC, et al. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J 30, 54–65 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Liu S, et al. Spinal IL-33/ST2 Signaling Contributes to Neuropathic Pain via Neuronal CaMKII-CREB and Astroglial JAK2-STAT3 Cascades in Mice. Anesthesiology 123, 1154–1169 (2015). [DOI] [PubMed] [Google Scholar]
- 129.Suter MR, Wen YR, Decosterd I. & Ji RR Do glial cells control pain? Neuron Glia Biol. 3, 255–268 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miyoshi K, Obata K, Kondo T, Okamura H. & Noguchi K. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J.Neurosci. 28, 12775–12787 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu Y, et al. TRAF6 upregulation in spinal astrocytes maintains neuropathic pain by integrating TNF-alpha and IL-1beta signaling. Pain 155, 2618–2629 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu T, Gao YJ & Ji RR Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 28, 131–144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tanga FY, Nutile-McMenemy N. & DeLeo JA The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc.Natl.Acad.Sci.U.S.A 102, 5856–5861 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Y, et al. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain 15, 712–725 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sorge RE, et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J.Neurosci. 31, 15450–15454 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bartley EJ & Fillingim RB Sex differences in pain: a brief review of clinical and experimental findings. British journal of anaesthesia 111, 52–58 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Woolf CJ Evidence for a central component of post-injury pain hypersensitivity. Nature 306, 686–688 (1983). [DOI] [PubMed] [Google Scholar]
- 138.Ji RR, Kohno T, Moore KA & Woolf CJ Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26, 696–705 (2003). [DOI] [PubMed] [Google Scholar]
- 139.Woolf CJ Central sensitization: implications for the diagnosis and treatment of pain. Pain 152, S2–15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Woolf CJ & Salter MW Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 (2000). [DOI] [PubMed] [Google Scholar]
- 141.Latremoliere A. & Woolf CJ Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J.Pain 10, 895–926 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gao YJ & Ji RR Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol.Ther. 126, 56–68 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.White FA, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc.Natl.Acad.Sci.U.S.A 102, 14092–14097 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gao YJ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J.Neurosci. 29, 4096–4108 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xie RG, et al. Spinal CCL2 Promotes Central Sensitization, Long-Term Potentiation, and Inflammatory Pain via CCR2: Further Insights into Molecular, Synaptic, and Cellular Mechanisms. Neuroscience bulletin 34, 13–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gosselin RD, et al. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J.Neurochem. 95, 1023–1034 (2005). [DOI] [PubMed] [Google Scholar]
- 147.Zhang ZJ, Cao DL, Zhang X, Ji RR & Gao YJ Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 154, 2185–2197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sommer C, Schafers M, Marziniak M. & Toyka KV Etanercept reduces hyperalgesia in experimental painful neuropathy. J.Peripher.Nerv.Syst. 6, 67–72 (2001). [DOI] [PubMed] [Google Scholar]
- 149.Milligan ED & Watkins LR Pathological and protective roles of glia in chronic pain. Nat.Rev.Neurosci. 10, 23–36 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ren K. & Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res.Rev. 60, 57–64 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang RX, et al. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain 135, 232–239 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li WW, et al. The NALP1 inflammasome controls cytokine production and nociception in a rat fracture model of complex regional pain syndrome. Pain 147, 277–286 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kawasaki Y, Zhang L, Cheng JK & Ji RR Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J.Neurosci 28, 5189–5194 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Berta T, et al. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J Clin.Invest 124, 1173–1186 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang H, Nei H. & Dougherty PM A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J.Neurosci. 30, 12844–12855 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sandkuhler J. Learning and memory in pain pathways. Pain 88, 113–118 (2000). [DOI] [PubMed] [Google Scholar]
- 157.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 89, 707–758 (2009). [DOI] [PubMed] [Google Scholar]
- 158.Liu YL, et al. Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology 52, 708–715 (2007). [DOI] [PubMed] [Google Scholar]
- 159.Park CK, et al. Resolving TRPV1- and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci 31, 15072–15085 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gruber-Schoffnegger D, et al. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-alpha and IL-1beta is mediated by glial cells. J Neurosci 33, 6540–6551 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chirila AM, et al. Long-term potentiation of glycinergic synapses triggered by interleukin 1beta. Proc.Natl.Acad.Sci.U.S.A 111, 8263–8268 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kronschlager MT, et al. Gliogenic LTP spreads widely in nociceptive pathways. Science 354, 1144–1148 (2016).This study identified a diffusible form of LTP, induced by small, glia-derived mediators, which can be carried through the cerebrospinal fluid to distant synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xie YF, et al. Involvement of glia in central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn). Brain, behavior, and immunity 21, 634–641 (2007). [DOI] [PubMed] [Google Scholar]
- 164.Kobayashi A, et al. Mechanisms involved in extraterritorial facial pain following cervical spinal nerve injury in rats. Molecular pain 7, 12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mah W, et al. A role for the purinergic receptor P2X3 in astrocytes in the mechanism of craniofacial neuropathic pain. Scientific reports 7, 13627 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alshelh Z, et al. Chronic Neuropathic Pain: It’s about the Rhythm. J Neurosci 36, 1008–1018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wei F, Guo W, Zou S, Ren K. & Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J.Neurosci. 28, 10482–10495 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ni HD, et al. Astrocyte activation in the periaqueductal gray promotes descending facilitation to cancer-induced bone pain through the JNK MAPK signaling pathway. Molecular pain 15, 1744806919831909 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen FL, et al. Activation of astrocytes in the anterior cingulate cortex contributes to the affective component of pain in an inflammatory pain model. Brain Res.Bull. 87, 60–66 (2012). [DOI] [PubMed] [Google Scholar]
- 170.Kim SK, et al. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. The Journal of clinical investigation 126, 1983–1997 (2016).This paper provides evidence that cortical astrocytes are activated following nerve injury, driving local synaptic plasticity and neuropathic pain via astrocytic TSP-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Loggia ML, et al. Evidence for brain glial activation in chronic pain patients. Brain 138, 604–615 (2015).This neuroimaging study demonstrates that glial activation is observed in the pain-processing brain regions of patients with chronic lower back pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Takata N, et al. Optogenetic astrocyte activation evokes BOLD fMRI response with oxygen consumption without neuronal activity modulation. Glia 66, 2013–2023 (2018). [DOI] [PubMed] [Google Scholar]
- 173.LaMotte RH, Dong X. & Ringkamp M. Sensory neurons and circuits mediating itch. Nat.Rev.Neurosci 15, 19–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moser HR & Giesler GJ Jr. Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J Neurosci 33, 6093–6101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee H. & Ko MC Distinct functions of opioid-related peptides and gastrin-releasing peptide in regulating itch and pain in the spinal cord of primates. Sci.Rep. 5, 11676 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Steinhoff M, et al. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J.Invest Dermatol. 126, 1705–1718 (2006). [DOI] [PubMed] [Google Scholar]
- 177.Dong X. & Dong X. Peripheral and Central Mechanisms of Itch. Neuron 98, 482–494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sun YG & Chen ZF A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 (2007). [DOI] [PubMed] [Google Scholar]
- 179.Duan B, Cheng L. & Ma Q. Spinal Circuits Transmitting Mechanical Pain and Itch. Neuroscience bulletin 34, 186–193 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ross SE Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr.Opin.Neurobiol. 21, 880–887 (2011). [DOI] [PubMed] [Google Scholar]
- 181.Liu Y, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 68, 543–556 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu T. & Ji RR Toll-Like Receptors and Itch. in Itch: Mechanisms and Treatment (eds. Carstens E. & Akiyama T.) (Boca Raton (FL), 2014). [PubMed] [Google Scholar]
- 183.Yosipovitch G. & Bernhard JD Clinical practice. Chronic pruritus. N.Engl.J Med. 368, 1625–1634 (2013). [DOI] [PubMed] [Google Scholar]
- 184.Han Q, et al. miRNA-711 Binds and Activates TRPA1 Extracellularly to Evoke Acute and Chronic Pruritus. Neuron 99, 449–463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu T. & Ji RR New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch 465, 1671–1685(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ross SE, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65, 886–898 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Green D. & Dong X. Supporting itch: a new role for astrocytes in chronic itch. Nature medicine 21, 841–842 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tsuda M. Astrocytes in the spinal dorsal horn and chronic itch. Neurosci Res 126, 9–14 (2018). [DOI] [PubMed] [Google Scholar]
- 189.Du L. et al. Spinal IL-33/ST2 signaling mediates chronic itch in mice through the astrocytic JAK2-STAT3 cascade. Glia. 67, 1680–1693 (2019). [DOI] [PubMed] [Google Scholar]
- 190.Shiratori-Hayashi M, et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat.Med. 21, 927–931 (2015). [DOI] [PubMed] [Google Scholar]
- 191.Liu T, et al. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 157, 806–817 (2016).This study demonstrates that spinal astrocytes play an active role in regulating chronic itch but not acute itch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Han Q, et al. miRNA-711 Binds and Activates TRPA1 Extracellularly to Evoke Acute and Chronic Pruritus. Neuron 99, 449–463 e446 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Akiyama T, et al. A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain 156, 1240–1246 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bourane S, et al. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350, 550–554 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Miao X, et al. TNF-alpha/TNFR1 Signaling is Required for the Full Expression of Acute and Chronic Itch in Mice via Peripheral and Central Mechanisms. Neuroscience bulletin 34, 42–53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jing PB, et al. Chemokine Receptor CXCR3 in the Spinal Cord Contributes to Chronic Itch in Mice. Neuroscience bulletin 34, 54–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wilson SR, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155, 285–295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu B, et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proceedings of the National Academy of Sciences of the United States of America 113, E7572–E7579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Paus R, Schmelz M, Biro T. & Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J.Clin.Invest 116, 1174–1186 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Global Industry Analysts, Inc. Pain management — a global strategic business report (GIA, 2011). [Google Scholar]
- 201.Gereau R.W.t., et al. A pain research agenda for the 21st century. J Pain 15, 1203–1214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ringelstein M, et al. Long-term Therapy With Interleukin 6 Receptor Blockade in Highly Active Neuromyelitis Optica Spectrum Disorder. JAMA Neurol 72, 756–763 (2015). [DOI] [PubMed] [Google Scholar]
- 203.Sommer C. [Animal studies on neuropathic pain: the role of cytokines and cytokine receptors in pathogenesis and therapy]. Schmerz. 13, 315–323 (1999). [DOI] [PubMed] [Google Scholar]
- 204.Sato KL, Johanek LM, Sanada LS & Sluka KA Spinal cord stimulation reduces mechanical hyperalgesia and glial cell activation in animals with neuropathic pain. Anesth Analg 118, 464–472 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen G, Park CK, Xie RG & Ji RR Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J Clin.Invest 125, 3226–3240 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Saunders A, et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 174, 1015–1030 e1016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zeisel A, et al. Molecular Architecture of the Mouse Nervous System. Cell 174, 999–1014 e1022 (2018).This system-wide single cell RNA-sequencing study demonstrates the existence of at least seven unique populations of astrocytes in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).This study demonstrates that microglia, via the release of Il-1α, TNF, and C1q, promote the acquisition of a pro-inflammatory ‘A1’ astrocyte phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nahrendorf M. & Swirski FK Abandoning M1/M2 for a Network Model of Macrophage Function. Circ Res 119, 414–417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Smith MT & Haythornthwaite JA How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 8, 119–132 (2004). [DOI] [PubMed] [Google Scholar]
- 211.Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).This study demonstrates that enhanced glymphatic flow through channels formed by the perivascular endfeet of astrocytes drives removal of proteinaceous waste from the extracellular space during sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Plog BA & Nedergaard M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annual review of pathology 13, 379–394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Louveau A, et al. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. The Journal of clinical investigation 127, 3210–3219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ringstad G, Vatnehol SAS & Eide PK Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain : a journal of neurology 140, 2691–2705 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Myllyla T, et al. Assessment of the dynamics of human glymphatic system by near-infrared spectroscopy. Journal of biophotonics 11, e201700123 (2018). [DOI] [PubMed] [Google Scholar]
- 216.von Holstein-Rathlou S, Petersen NC & Nedergaard M. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neuroscience letters 662, 253–258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kimura M, et al. Impaired Pain-evoked Analgesia after Nerve Injury in Rats Reflects Altered Glutamate Regulation in the Locus Coeruleus. Anesthesiology 123, 899–908 (2015). [DOI] [PubMed] [Google Scholar]
- 218.Jensen TS, et al. A new definition of neuropathic pain. Pain 152, 2204–2205 (2011). [DOI] [PubMed] [Google Scholar]
- 219.van HO, Austin SK, Khan RA, Smith BH & Torrance N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 155, 654–662 (2013). [DOI] [PubMed] [Google Scholar]
- 220.Calvo M, Dawes JM & Bennett DL The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 11, 629–642 (2012). [DOI] [PubMed] [Google Scholar]
- 221.Bennett GJ & Xie YK A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33, 87–107 (1988). [DOI] [PubMed] [Google Scholar]
- 222.Kim SH & Chung JM An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50, 355–363 (1992). [DOI] [PubMed] [Google Scholar]
- 223.Seltzer Z, Dubner R. & Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43, 205–218 (1990). [DOI] [PubMed] [Google Scholar]
- 224.Decosterd I. & Woolf CJ Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158 (2000). [DOI] [PubMed] [Google Scholar]
- 225.Flatters SJ & Bennett GJ Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 109, 150–161 (2004). [DOI] [PubMed] [Google Scholar]
- 226.Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424, 778–783 (2003). [DOI] [PubMed] [Google Scholar]
- 227.Coull JA, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 (2005). [DOI] [PubMed] [Google Scholar]
- 228.Clark AK, et al. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J.Neurosci. 30, 573–582 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hochstim C, Deneen B, Lukaszewicz A, Zhou Q. & Anderson DJ Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133, 510–522 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tsai HH, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337, 358–362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hammond TR, et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 50, 253–271 e256 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bannenberg G. & Serhan CN Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim.Biophys.Acta 1801, 1260–1273 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Abdelmoaty S, et al. Spinal actions of lipoxin A4 and 17(R)-resolvin D1 attenuate inflammation-induced mechanical hypersensitivity and spinal TNF release. PloS one 8, e75543 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xu ZZ, et al. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Annals of neurology 74, 490–495 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Singh SK, et al. Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1alpha and NL1 via Hevin. Cell 164, 183–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mishra SK & Hoon MA The cells and circuitry for itch responses in mice. Science 340, 968–971 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Alexandre C, et al. Decreased alertness due to sleep loss increases pain sensitivity in mice. Nature medicine 23, 768–774 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kim DS, et al. Thrombospondin-4 Contributes to Spinal Sensitization and Neuropathic Pain States. J Neurosci 32, 8977–8987 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]