Abstract
Recent developments in sensor technology and computational analysis methods enable new strategies to measure and interpret lung acoustic signals that originate internally, such as breathing or vocal sounds, or are externally introduced, such as in chest percussion or airway insonification. A better understanding of these sounds has resulted in new instrumentation that allows for highly accurate as well as portable options for measurement in the hospital, in the clinic, and even at home. This review outlines instrumentation for acoustic stimulation and measurement of the lungs. We first review the fundamentals of acoustic lung signals and the pathophysiology of the diseases that these signals are used to detect. Then, we focus on different methods of measuring and creating signals that have been used in recent research for pulmonary disease diagnosis. These new methods, combined with signal processing and modeling techniques, lead to a reduction in noise, and allow improved feature extraction and signal classification. We conclude by presenting the results of human subject studies taking advantage of both the instrumentation and signal processing tools to accurately diagnose common lung diseases. This paper emphasizes the active areas of research within modern lung acoustics, and encourages the standardization of future work in this field.
Index Terms: Acoustic, stethoscope, microphone, accelerometer, speaker, ultrasound, physiologic sensing, lung, pneumonia, COPD, asthma, machine learning, diagnosis
I. INTRODUCTION
The stethoscope has been developed and used for almost two centuries to allow physicians to evaluate the health of the lungs by listening to breath sounds using a technique called auscultation [1]. Other physical exam procedures, such as percussion, which involves tapping on the patient’s chest, and tactile fremitus, which involves assessing the way that vocal vibrations travel through the chest, have also remained core components of the respiratory physical exam [2; 3]. These methods are commonly employed in the preliminary diagnosis of lung pathologies due to their quick and inexpensive utility. Utilizing acoustic findings, trained physicians are able to differentiate between normal and pathological lungs in patients suffering from diseases such as pneumonia, pleural effusion, pneumothorax, chronic obstructive pulmonary disease (COPD), and asthma [2; 4; 5; 6; 7].
In spite of the clinical utility of the respiratory physical exam, these techniques suffer from subjectivity leading to interobserver variability in patient diagnoses [8; 9]. Further challenges include the training required to recognize the specific signals necessary to make a diagnosis. However, over the years, studies into the physics of the lungs have given us a holistic understanding of their response to acoustic stimuli, including factors such as how sound travels through the lung tissue (parenchyma) and the specific characteristics of the lung sound signals under healthy and pathologic conditions [10; 11; 12; 13].
This understanding has enabled acoustic methods to advance beyond the stethoscope to employ different hardware and software tools. This paper has been organized to quickly review basic sound types associated with the lung and provide an overview of the physics that govern sound transmission in the respiratory system (Sections II-IV). This is followed by presenting the diverse methods and procedures required for proper measurement of pulmonary acoustics for disease diagnosis, while focusing on presenting recent work in the field (Sections V-X). Recent advances include the use of microphone arrays [14; 15] and ultrasound [16; 17], as well as more so phisticated signal processing methods for processing and classification [18] such as wavelet analysis and support vector machines (SVM) [6].
Approaches rooted in these recent advances have been used for analysis of breath sounds, as well as other internal signals, such as vocal vibrations, together with the introduction of external stimuli into the lungs via the throat or chest. While thorough reviews of breath sound analysis have been conducted [19; 20; 21], to our knowledge a broader review of different acoustic analysis techniques for lung disease has not been performed. For this purpose, we review automatic classification methods that have been developed and tested in tandem with various acoustic signals both internal and external. This paper surveys current acoustic methods, including both measurement and actuation hardware; evaluates signal processing methods used to process and analyze measured sound; reviews selected human subject studies for validation of various methods; and outlines future challenges in the field.
II. PATHOPHYSIOLOGY AND ACOUSTICS OF COMMON LUNG DISEASES
Due to both infectious and non-infectious diseases, the lung can become abnormally occupied by air and fluid. Structural changes induced by disease cause alterations in acoustic transmission of frequencies through the thoracic cavity [22]. For the purposes of this review, we focus on pneumonia, pleural effusion, pneumothorax, chronic obstructive pulmonary disease (COPD), and asthma, as these diseases have pronounced acoustic findings and the majority of the research in the field has focused on these disorders. The diseases focused in this review can be grouped into two broad categories based on their pathophysiology: fluid and air accumulation. Pneumonia is the result of a lung infection which leads to inflammation and the accumulation of exudate (a protein-rich fluid) in the lungs [23]. The accumulation of this fluid provides the physiological basis for the acoustic physical exam findings of abnormal breath sounds and dullness to percussion [24]. The term consolidation refers to the area of lungs with fluid accumulation. Pleural effusion is similar to pneumonia in that it also results in the accumulation of fluid; however, the location of the fluid accumulation is in the cavity surrounding the lungs, called the pleura [25]. In addition to fluid, air can also abnormally occupy the chest cavity and lead to the collapse of the lungs in an acute disease called pneumothorax [26]. The presence of this air measurably changes the acoustic properties of the lungs [27]. Asthma and COPD are inflammatory disorders which affect the larger airways; this inflammation leads to the characteristic wheezes and other pathological breath sounds associated with these diseases [28; 29]. This inflammation also preferentially inhibits exhalation, leading to a trapping of air in the chest cavity, which may affect the resonance of the chest [30]. Unlike pneumothorax, which also results from air accumulation, asthma and COPD are chronic in nature.
III. Types of Acoustic Signals
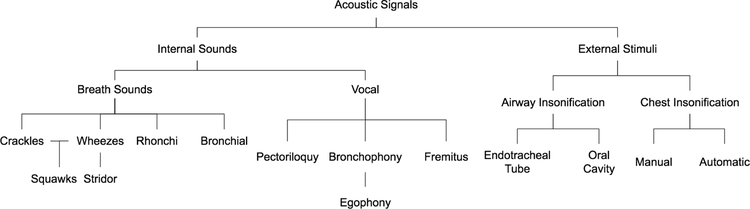
In this review, we break down the analysis of lung acoustics into two categories: analysis of “internal” signals, in particular, analysis of the sounds produced during breathing and from the vocal chords; and “external” signals, like those that result from chest percussion and airway insonification [2]. The types of acoustic signals are summarized in Fig. 1. A summary of the frequency ranges, signal types and analysis techniques for both internal and external signals is provided in Table I.
Figure 1:
Summary of the different types of acoustic signals for pulmonary disease diagnosis. This includes both internal sounds, such as breath sounds, and external stimuli, such as percussion.
Table I:
Sound Stimuli
| Sound | Frequency Ranges (Hz) | Signal Type | Analysis Methods |
|---|---|---|---|
| Breath sounds | 10–1200 Hz [3; 31; 32], 60–2000 Hz [33], 100–600 Hz [34], 37.5–1000 Hz [6; 19], 75–2000 Hz [35], 80–2000 Hz [36;37], 75–1000 Hz [38], 0–800 Hz [39] | Non-stationary | Meyer wavelet [5; 6], Autoregressive [36], Hilbert transform (envelopes) [5; 38], Fourier transform [32; 39; 39; 40; 41] |
| Vocal fremitus | 0–1000 Hz [42] | Non-stationary | Autoregressive [42] |
| Chest insonification | 80–1000 Hz [43; 44], 2–10 MHz [16; 17; 45; 46] | Stationary | Cross-correlation [44], Frequency response function [43; 47] |
| Airway insonification via mouth | 80–1000 Hz [15], 50–1600 Hz [48; 49], 75–2000 Hz [50], 200–2000 Hz [51], 50–2000 Hz [26], 0–20000 Hz [52], 3–35 Hz [53] | Stationary | FFT [15; 51; 53], FRF [48; 50], Convolution [51], Transit time analysis [50], Power analysis [50], Spectrogram [52] |
| Airway insonification via endotracheal tube | 20–1600 Hz [27; 54], 50–1600 Hz [26] | Stationary | PSD [26], FFT [27; 54] |
This table provides typical ranges for internal and external sounds useful for analyzing lung structure and/or function. FFT = Fast Fourier Transform, PSD = Power spectral density, FRF = Frequency response function
A. Internal: Breath sounds
Respiratory sounds occur as the result of air flowing through the lungs and are categorized as normal or abnormal (adventitious). Normal respiratory sounds are defined here as those that are in healthy airways by physiological unforced breathing. These sounds are generally subdivided into tracheobronchial and vesicular; the former originate in the trachea and larger bronchial airways, and the latter may originate in small branches of the airway tree further from the trachea or from other mechanisms at distal regions of the lung parenchyma [55]. Absence or deficiency of normal breath sounds or manifestation of adventitious sounds may be an indicator of pulmonary disease.
Adventitious respiratory sounds have been classified into several different types, depending on their spectral-temporal characteristics and their location [2; 3]. A variety of lung pathologies and injuries result in adventitious respiratory sounds and/or alter sound transmission pathways, with both spectrally and regionally differing effects that, if properly quantified, may provide additional information about the severity and location of the trauma or disease. We briefly review the different types of breath sounds and their spectral characteristics in this section, and refer the reader seeking more detailed explanations to the definitions and spectral characteristics set forward by the European Respiratory Society (ERS) [56].
1) Crackles: Crackles, also termed “crepitations” or “rales,” are short, discontinuous, and non-stationary sounds that can be detected at inspiratory and/or expiratory cycles [57]. Key features often extracted from crackles include their duration, wave form, and timing, which can be correlated to several pathologies, such as COPD, pneumonia, fibrosis, or bronchiectasis [28; 55; 57; 58].
2) Wheezes: Wheezes are adventitious continuous sounds that are typically heard at end of the inspiratory phase or in the early expiratory phase. They are often detected in subjects affected by obstructive diseases, in particular asthma and COPD [28; 58; 59; 60], as well as in sickle cell patients experiencing an acute pain crisis [57]. Their dominant spectral content is usually between 100 to 1000 Hz, with higher harmonics above 1 kHz.
3) Rhonchi: Rhonchi are musical low-pitched sounds characterized by a sinusoidal waveform [28]. The duration is generally higher than 100 ms and frequency content lower than 300 Hz. They are associated with abnormal airway collapsibility and the creation of breaches in fluid films [28]. Rhonchi can be considered indicators of airway lumen constriction associated with thickening of the mucosa, edema or bronchospasm (e.g. resulting from bronchitis and COPD) [28].
4) Stridor: Stridor, often considered a type of wheezing, refers to intense continuous monophonic sounds best heard over extra-thoracic airways. Stridor is usually considered an indicator of upper airway obstruction. Signal analysis reveals that stridor tends to have a sinusoidal waveform, with a fundamental frequency generally above 500 Hz [28; 61].
5) Squawks: Squawks are defined as a mixed sound since they are characterized by a crackle followed by a short musical component resembling a wheeze [28]. They are likely to occur in fibrotic pulmonary disorders. Acoustically, their waveform is similar to that of short wheezes, but they are often preceded by a crackle. Mean squawk duration is approximately 90–320 ms.
6) Bronchial: Bronchial breath sounds are blasting sounds, audible throughout inspiration and expiration, with a frequency range of 600–1000 Hz [21]. Although normal when heard over the neck, close to the bronchus, when bronchial sounds are heard over the lung fields it is usually due to a reduction in the amount of parenchyma through which the sounds pass resulting from disease [11]. Generally, they have a higher frequency content with respect to normal sounds; this is because consolidation causes a reduction of the low-pass filtering role of the alveolar region.
B. Internal: Vocal sounds
Vocal sounds can be viewed as internal to the body but are also an external stimulus to the airways and lungs. Vocal sounds consist of a fundamental frequency and several overtones that make up higher frequencies. In healthy patients, auscultation of vocal sounds often appear muffled due to poor transmission of sound in air-filled lungs, which filters out higher frequencies often with a peak at 130–250 Hz [42; 62]. Consolidation has been found to increase this transmission to 400 Hz [63]. Auscultation of vocal sounds has been used to exploit its frequency content and response, and is a valuable tool as certain vocal findings have been found to precede abnormal breath sounds [63]. Different methods of initiating vocal sounds have been explored by researchers, pairing different methods of actuation such as ultrasound or sound delivered via speakers with different types of sensors; however, as they are based on the same principles, it is often up to the physician to decide which method to use [11].
1) Pectoriloquy: Pectoriloquy refers to an abnormal increase in the clarity of whispered vocal sounds during auscultation due to the presence of lung consolidation, which increases the sound speed and thus lowers the damping of the vocal signal due to the efficiency of sound transmission through fluid. A study comparing different methods of diagnosing pneumonia, including auscultation of percussion, breath sounds, pectoriloquy, and bronchophony, showed that pectoriloquy had the lowest inter-rater agreement and was rarely detected by physicians [64; 65].
2) Bronchophony: Bronchophony applies the same principles of pectoriloquy, but is spoken instead of whispered. Egophony is a specific type of bronchophony. When a patient speaks the letter “E” it is normally heard on the chest wall as a muffled “E” in healthy patients, but in the presence of consolidation, it sounds like a spoken “A” [63]. “E” consists of a fundamental frequency between 100–400 Hz and overtones ranging from 2000–3500 Hz, while “A” consists of a higher fundamental frequency of up to 600 Hz [62]. Increased density in the parenchyma from pleural effusion or lung consolidation transmits the higher frequencies of “A” better than the lower frequencies of “E” with no significant transmission above 1000 Hz, and may stifle transmission from 100–300 Hz, causing the apparent “A” sound [62]. In several studies, egophony was found to have increased the likelihood of diagnosing pneumonia but had highly variable positive predictive values, from 20–56% [66].
3) Fremitus: For vocal fremitus, the input to the chest is the speech signal at the vocal chords and the output is the sound heard at the chest wall. To generate the speech in a standard way, the patient is asked to vocalize a constant sound, such as EEE [2]. The vibrations initiated by fremitus are decreased in the presence of conditions such as bronchial asthma, emphysema, obstruction, pleural effusion, and pneumothorax. Air trapping or accumulation decreases the transmission of lower frequency vibrations, while consolidation and inflammation increases transmission [67]. For these reasons, fremitus is often more useful in male patients, and has been observed to be ineffective in obese patients [63; 67]. Wipf et al. found that, along with pectoriloquy, vocal fremitus was rarely detected in patients with pneumonia[64]. Another study corroborates these findings, but suggests that it is difficult to calculate the reliability of fremitus because it occurs infrequently [65]. For pleural effusion, fremitus has been shown to have a high sensitivity (82%), specificity (86%), and negative predictive value (95%) for diagnosis, though the positive predictive value was low (56%) [68].
C. External: Airway insonification
The process of analyzing abnormalities in lung structure can also be done electronically, by introducing external sound through the oral cavity or the endotracheal tube [26; 27; 44; 48; 50; 54; 69]. Sounds introduced to the oral cavity have ranged from 50–2000 Hz [48; 50; 69], while sounds introduced through the endotracheal tube have ranged from 20–1600 Hz [27; 54]. A transfer function with the chest as the output has been used to characterize the structure and/or content of the parenchyma. By utilizing a fixed input signal, the specific frequencies of interest can be isolated and the differences between patients’ vocalization discussed above can be avoided [63; 67].
D. External: Chest insonification
In addition to the vocal tract, sound can also be introduced directly to the chest, which can be done via percussion, electronic actuators, and ultrasound. Percussion is a term that refers to exciting a tissue with an impulsive force [22]. The input is a percussive stroke generated manually by a finger or automatically by an external tool; the output is the sound heard at the chest wall [22]. If the vibrations from the percussive stroke are less damped, they persist, known as a resonant or tympanic note. This occurs due to a significant acoustic mismatch between the gas-filled lungs and chest wall, causing reflections at the lung–chest wall interface, and thus longer vibrations. In contrast, flat percussive notes, which die out quickly, are caused by similar tissue beneath the chest wall, such as over the heart or due to the presence of fluid, which decreases the amount of acoustic mismatch [11].
In previous studies, dullness from percussion has been shown to be associated with increased likelihood of pneumonia, though its measurement is hindered by low inter-rater agreement [64; 65]. Auscultatory percussion, first described by Guarino, is a variation of direct percussion in which one side of the body is percussed and the sound assessed with a stethoscope at a distance or on the other side of the body [23]. Bohadana et al. expanded Guarino’s findings in a study of 98 patients with abnormal chest films; in the blinded study, direct percussion and auscultatory percussion were compared, demonstrating that both were about equally effective at detecting pleural effusions, but that neither technique could detect intrapulmonary masses less than 6 cm in diameter [70]. In a follow-up study, the same researchers recorded data from several sensors to create a sound map of the backs of three healthy subjects and four patients with large intrapulmonary lesions (from 6–10 cm), finding that scapulae play a large role in sound transmission for percussion of the sternum and that intrapulmonary lesions were not detectable [71]. This limitation in part arises from the low bandwidth of manual percussion sounds (less that 100 Hz), and in part due to the characteristic transmission of sound through bony structures as well as through the lung parenchyma.
Automated methods have attempted to address these limitations by expanding the bandwidth of the input signal to 1000 Hz and recording from areas that exclude the scapulae. Recent studies have introduced a frequency range of 50–1000 Hz at the chest to measure changes in velocity and arrival time, the transfer function, or reflections of sound waves [11; 43; 44; 47]. Apart from an impulsive signal, a controlled input signal such as a chirp can be input at the chest wall to analyze any abnormalities in its transmission through the chest structure [43; 47; 72].
In addition to audible range stimulation, ultrasound provides input into the chest of frequencies above 20 kHz, with most clinical ultrasound systems in the range of 2 to 10 MHz [16]. As the sound travels into the tissue, a reflection beam called an echo is formed; production and detection of echos is the basis for all diagnostic ultrasound systems [16]. If two adjacent tissues have different acoustic impedances, they will produce an echo. If the difference between the two tissues is very large, as is the case in the chest–lung interface, not enough ultrasound signal will remain to image beyond this interface, creating challenges for thoracic imaging [16]. Despite these challenges, several methods utilizing the artifacts of the ultrasound image can be used to great effect for pulmonary diagnosis, these methods are explored in Section V.E.
IV. PHYSICS OF THE HUMAN THORAX
The human thorax is comprised of four different types of materials with significantly different acoustic properties: hard tissue (bone), soft tissue (muscle, fat, etc.), air in the major conducting airways of the bronchial tree, and parenchymal tissue that is a heterogeneous mixture of soft tissue and air found in the alveolar sacs and smaller bronchioles. The characteristics of these different components affect how sound is transmitted through the thorax.
A. Absorption
Sound in the lumen of the lung airways experiences a frequency-dependent absorption into the airway walls and surrounding parenchymal tissue, in which high-frequency sounds propagate further within the airway branching structure, while low-frequency sounds tend to couple into the airway walls sooner [3; 11; 73]. However, due to the attenuation of higher frequency sounds in the surrounding parenchymal tissue, most of the signal energy of breath sounds recorded on the torso surface is concentrated at lower frequencies [3; 49].
Analysis of sound transmission in the chest cavity suggests that the chest, overall, acts as a low-pass filter, absorbing higher frequencies as sound travels through it [11; 35; 42; 48; 50]. This filtering effect is altered with the presence of different lung conditions, such as consolidation or fluid build-up, which on the one hand can create large acoustic impedance mismatches with healthy parenchymal tissue and air, but on the other hand acoustically couples better to surrounding soft tissue of the chest wall and inherently allows sound to propagate with less attenuation, as compared to healthy parenchymal tissue [43].
B. Resonance
Resonant frequencies are those frequencies at which acoustic waves are reflected back and forth due to interaction with boundaries or interfaces, leading to constructive interference and an amplified response. When a short (percussive) impulse, with broad frequency content, is applied to a system, the response at its resonant frequencies will persist longer than at other frequencies, particularly as damping (viscosity) in the system is reduced [74]. For the chest overall, this characteristic depends on several factors, including the size of the thorax. The first, or lowest, resonant frequency of the chest for men is around 125 Hz; for women it is slightly higher, at 150–175 Hz; for children it is 300–400 Hz [11]. For sound traveling in the lumen of the airways, the resonant frequencies are a strong function of the geometry and wall properties of the airways whereas direct chest stimulation will generally bypass these differences [75]. Resonances may also occur when pathologies create trapped air cavities below the torso surface, such as in the case of pneumoperitoneum [76; 77].
V. METHODS OF MEASUREMENT
The classic method of measuring lung sounds is with the stethoscope, which works by isolating the sounds from the vibrating chest wall and filtering out certain frequencies of interest mechanically. These sounds can be recorded for analysis by converting the acoustic pressure or vibrations of the chest–wall interface into electrical signals. In this section, we review the filtering characteristics of the stethoscope, discuss common types of sensors used to digitize this signal and their methods of transduction and end with a discussion of multi-point measurement systems. These sensors are discussed in the context of Computerized Respiratory Sound Analysis (CORSA) specification recommendations, which are based on a project of the European Respiratory Society [56].
A. The Stethoscope
Conventional stethoscopes rely on analog filtering and amplification of sound for interpretation by a trained professional [38]. Stethoscopes have two sides, a bell and a diaphragm, with different frequency characteristics. Bell chestpieces amplify sound below 112 Hz [78], demonstrating superior sound transmission at these frequencies compared to the diaphragm, which attenuates low frequencies [79]. Both bells and diaphragms have an important attenuation above 200 Hz, which limits the ability to discern sounds in this frequency range [78]. The sensitivity of the human ear ranges from 20–20,000 Hz [34; 79]. Despite the range of human hearing, because the ear follows a logarithmic sensitivity to frequency, greater changes are required at higher frequencies to discern them as different [38]. Electronic stethoscopes utilize a variety of sensors including condenser microphones and piezoelectric sensors in order to convert acoustic waves into electrical signals for filtering and processing [5; 27; 54]. Compared to the conventional version, electronic stethoscopes allow amplification of the acoustic signals, electronic removal of noise artifacts, and recording for playback and post-processing [79]. In spite of these advantages, electronic stethoscopes also suffer from excessive ambient noise, which is exacerbated by amplification; however, this is reduced with the implementation of low-pass filters from the bell and diaphragm [79]. Depending on the type of sensor utilized, and the analog and digital filters that are applied, the frequency range varies for these methods of measurement. The main strength in the electronic stethoscope is the integration of a familiar technology with advancements in electronics and signal processing (see Section VII). Low barriers of adoption for medical professionals can facilitate quicker clinical usage of lung acoustic diagnostics; however, its main weakness is site-specific dependencies with a single point of measurement.
B. Sensors
The vibrations of the chest–wall can be recorded using several different methods of transduction, such as condenser and piezoelectric transduction [56]. An ideal sensor should be small and lightweight, cost-efficient, produce reliable and sensitive measurements with little noise, and have reproducible frequency response [21]. Due to its flat frequency response, high SNR, and advancements in MEMS technology, we recommend condenser microphones for such an ideal sensor. CORSA provides standard considerations for picking a condenser microphone for respiratory recordings [80] A majority of recent studies reviewed in this article (see Table II) have also utilized condenser microphones.
Table II:
Comparison of acoustic methods for lung disorder diagnosis
| [Ref] | Sensor Type | (n) | Comparison | Results | |
|---|---|---|---|---|---|
| Pneumonia | |||||
| [4] | Contact microphone array | 20 | CXR | Study found that the system provided 70% sensitivity & 80% specificity in diagnosing patients with pneumonia without workup. | |
| [3] | Condenser microphone array | 5 | CXR | Built a spatial representation similar to CXR, with high active area in lower lung corresponding to lung consolidation. | |
| [111] | Ultrasound imaging | 58 | Fibroscopy or bacteriology | Evaluated the ability of ultrasound to differentiate between pneumonia and atelectasis. Dynamic air bronchograms were used to rule out atelectasis, with a specificity of 94% and sensitivity of 61%. | |
| [7] | Condenser microphone array | 122 | Physician diagnosis | 29% of symptomatic patients were found to have wheezes, 29% were found to have rhonchi, and 87% were found to have crackles. | |
| [72] | Condenser microphone | 13 | CXR | Encouraging results from proof-of-concept study with eight healthy subjects and five lobar pneumonia patients. | |
| Pleural Effusion | |||||
| [4] | Condenser microphone array | 20 | CXR | Pleural effusion was detected with a 95% sensitivity and 88% specificity. | |
| [87] | Condenser microphone array | 56 | CXR, Ultrasound | Agreement of diagnosis via clinical assessment compared to CXR was 80%, and with ultrasound was 75% | |
| Asthma | |||||
| [130] | Condenser microphone array | 31 | Spirometry | Found 81% and 74% accurate in differentiating between COPD and asthma; 85% overall accuracy when using quantitative data in addition to generated images | |
| [7] | Condenser microphone array | 51 | Physician diagnosis | 59% of symptomatic patients were found to have wheezes, 27% were found to have rhonchi, and 65% were found to have crackles. | |
| [158] | Contact microphone | 16 | Spirometry | An algorithm for wheeze peak detection and grouping to find episodes was used. The algorithm has a sensitivity of 100%, 87.8%, and 71% for high, middle, and low flow respectively. | |
| [36] | Contact microphone | 17 | Spirometry | Patients with bronchial asthma who had taken bronchodilator drugs showed a decrease in centroid frequency after taking the drugs during forced expiration | |
| [100] | IOS | 132 | Spirometry | While spirometry did not show significantly different results between asthmatic and healthy children, IOS indicated significant differences in resistance at 10 Hz and bronchodilator responses at 5 and 35 Hz. | |
| [102] | IOS | 41 | Spirometry | An ANN achieved a generalized classification accuracy of 94.44% in detecting airway obstruction from asthmatic children using their IOS patterns and age, weight, and height demographics. | |
| COPD | |||||
| [130] | Contact microphone array | 35 | Spirometry | 81% and 74% accurate in differentiating between COPD and asthma; 85% overall accuracy when using quantitative data in addition to generated images. | |
| [7] | Condenser microphone array | 94 | Physician diagnosis | 39% of symptomatic patients had wheezes, 26% had rhonchi, and 71% had crackles. | |
| [52] | Condenser microphone | 11 | - | Discriminated between “normal” smokers’ lungs and the presence of COPD in smokers by noting peak signal amplitudes in COPD patients were slightly larger at frequencies from 0–5 kHz. | |
| [159] | Condenser microphone array | 90 | Physician diagnosis | 11 parameters, including inspiratory/expiratory crackle rate and ratio of inspiration to expiration time, were found to be statistically significant in diagnosing COPD. | |
| [98] | IOS | 180 | Spirometry | Measured reactance, particularly at 5 Hz, was shown to be correlated with forced expiratory values and changes caused by airflow obstruction. | |
| [53] | IOS | 2609 | Spirometry, CT | IOS showed reduced impedance in COPD patients and could identify different subgroups of COPD patients based on severity. Data did not suggest IOS is an appropriate replacement for spirometry. | |
| [161] | Condenser microphone | 16 | Hospital visit | Promising results in predicting acute exacerbation COPD (AECOPD) were reported. | |
| Pneumothorax (PTX) | |||||
| [26] | Condenser microphone | 19 | Surgically induced | The occurence of PTX was associated with a drop in sound amplitude most noticeable in mid-frequency ranges (400–600Hz). Increasing the frequency further decreased amplitude due to increased tissue damping. | |
| [162] | Contact microphone array | 14 | CXR | Using dynamic breath sound distribution images generated from the VRI, diagnosing PTX had a sensitivity of 100% and specificity of 87%. | |
| [45] | Ultrasound | 43 | CT, CXR | On the basis of lung sliding in ultrasound, diagnosis of pneumothorax had 95.3% sensitivity and 91.1% sensitivity. | |
| Misc. | |||||
| Extravascular lung water | [46] | Ultrasound | 121 | CXR | Ultrasound was used to find echographic comets to determine extent of extravascular lung water; significant correlation was found between ultrasound and CXR methods (r = 0.78). |
| Consolidated lung | [106] | Ultrasound | 39 | CXR | Sonographic air bronchograms (92.30%), fluid bronchograms (92.30%), and superficial fluid alveolograms (100%) were observed. |
| Abnormal sounds | [139] | Condenser microphone | 20 | - | Using a stethoscope for recording and a condenser mic to upload lung sound data for processing, this study achieved 95% identification for normal lung sounds and 91.3% for abnormal sounds. |
| Pathology | [47] | Condenser microphone | 12 | Physician diagnosis | A classification accuracy of 91.7% was achieved by analyzing a chirp signal inserted into the patient chest using MFCC and KNN. |
| Smoking | [163] | Contact microphone array | 151 | CXR, Spirometry, Auscultation | Smokers showed a significant reduction of vibrational energy compared to nonsmokers, particularly with increase in pack-year of cigarette smoking. |
This table provides a comparison of different methods of acoustic detection for lung disorders. The lung disorder being detected is listed alongside the number of subjects in the study (n), the gold standard used for comparison and the major outcomes of the study. CXR = Chest x-ray, IOS = Impulse oscillometry system, VRI = Vibration response imaging, COPD = Chronic obstructive pulmonary disorder.
The two main types of microphones used for lung sound analysis are air-coupled and contact microphones. An air-coupled microphone converts changes in dynamic air pressure within the drum of a stethoscope created by the oscillatory movement of the diaphragm or bell pressed against the torso surface into electrical signals, while contact microphones convert mechanical stress caused by chest movements using piezoelectric transduction principles [18].
1) Condenser microphones: Condenser microphones are a type of air-coupled microphone. Condenser microphones such as electret microphones utilize condenser transduction to detect changes in acoustic pressure that change the nominal capacitance values [18; 21; 81]. These microphones have a nearly flat frequency response over the audio range, leading to minimal distortion [21; 81]. However, they require acoustic coupling to the chest wall with an air cavity [59; 80]. Microelectromechanical systems (MEMS) microphones utilize condenser principles and provide a similar frequency range and SNR as compared to conventional condenser microphones while providing a smaller form factor [18; 82]. Due to their wide bandwidth, high sensitivity, established coupling methods, high SNR (according to CORSA recommendations), and low costs, condenser microphones are widely used [21; 59; 81].
2) Contact microphones: Contact microphones typically utilize piezoelectric transduction principles to create an output voltage proportional to the displacement of the sensor placed directly onto the skin without the use of an air chamber [80]. These sensors can be characterized by extremely high sensitivity (50 mV/Pa) and have the advantage of not picking up as much ambient noise as condenser microphones, but are conversely very sensitive to motion artifacts [80; 81]. A study found that loading effects due to the method of coupling from the transducer to the hand piece of the stethoscope is a significant noise source [83]. One design solution to this noise source is utilizing foam between the transducer and hand piece of the stethoscope; progressively softer foam results in reduction of physician hand noise but also lowers the sensitivity of the piezo sensor [83].
C. Multi-point measurements: Sensor array
Lung pathologies alter sound transmission pathways and have both spectral and regional effects that can benefit from simultaneous measurements at multiple points across the chest surface. Understanding the location of abnormal lung sounds can help identify areas of pathology as well as assessing the severity of the pathology based on its spatial distribution. To better assess location, simultaneous multi-sensor auscultation methods have been advanced to “map” sounds on the thoracic surface by several groups [34; 59; 84; 85; 86].
One multi-sensor system that has undergone considerable study into its efficacy has been branded as “vibration response imaging” (VRI). VRI involves creating a 2D representation of breath sounds using an array of electronic stethoscopes that pick up sounds from the chest using 18–40 piezo-acoustic sensors to create a gray scale image in real time that can dynamically track acoustic variation throughout the respiratory cycle [34]. The sensors in the array have a flat frequency response from 50–400 Hz [34]. This sensor array has been investigated for distinguishing between healthy subjects versus subjects with pleural effusion or pneumonia [4; 87;88], differences in lung sounds between asthmatic and healthy subjects [89], and differences between subjects with obstructive airway disease (OAD) and non-OAD subjects [58]. While this system has been applied to a variety of disorders, it remains bulky compared to systems with fewer sensors, as it relies on 40 different sensors coupled to the patient’s back via a low-suction vacuum. Another limitation is the fact that the signal is filtered from 150–250 Hz, which may reduce lung sound characteristics contained in frequency bands above 250 Hz [90].
While 2D visualization of breath sounds is an improvement over individual sensor recordings, 3D mapping of sound sources may provide further localization for diagnosis. Kompis et al. [3] attempted to form a three-dimensional (3D) acoustic image of the likely sound source location(s) by using multiple sensors and assuming “ray acoustic,” i.e., “incident field,” models for how sound propagated away from these sources. For future work, Kompis et al. noted that a useful imaging system for the human lung should: (a) be robust with respect to acoustic properties, especially speed of sound, which varies and is not precisely known; (b) provide 3D data sets and resulting images that are intuitively interpretable; and (c) be robust with respect to missing sensors or noisy data in individual sensors [3]. While sensor arrays are ideal for providing a 2D or 3D visualization of the lungs, as opposed to the stethoscope which can only provide one location at a time, current solutions remain bulky, and adoption in the clinic will be more difficult due to increased costs compared to point based measurements.
VI. METHODS OF ACTUATION FOR EXTERNAL SOURCES
Unlike internal sounds, which are non-stationary and not precisely repeatable signals [5], externally introduced sounds have the advantage of providing a fixed input signal generated using a variety of methods.
A. Actuators
Relatively few studies have investigated the use of fixed input signals for pulmonary diagnosis, leading to a lack of standardization for the transducers used to provide the input signal. Actuators rely on the same principles of transduction as sensors, such as electromotive and piezoelectric transduction but provide inverse functionality by converting an electrical signal into a mechanical signal. Several parameters are considered in choosing an actuator, such as bandwidth, displacement, force, power, size, and weight. The ideal actuator is a transducer that can generate a strong signal via displacement or force for a wide range of frequencies, without excessive power consumption [72].
1) Dynamic loudspeakers: Dynamic loudspeakers utilize electromagnetic induction to transduce an electrical signal into sound waves [91]. Surface exciters are a specific type of loudspeaker that do not have the cone or frame, so their vibrations are coupled to a surface instead of to the air. They are capable of producing a strong signal at low frequencies as low as 50 Hz, and can be made relatively small [72].
2) Electromechanical solenoids: Electromechanical solenoids consist of an electromagnet with a plunger, the plunger will move in or out depending on the direction of the current [92]. They are also capable of actuating strong low frequency signals but are bulky and require large amounts of current for operation, which reduces battery life [72].
3) Piezoelectric: Piezoelectric transducers have found use in generating and detecting ultrasound waves [93]; therefore, they are notable for their ability to generate large forces at high frequencies with a small size.
B. Audible sound: Chest input
Use of a fixed audible input signal into the chest is still an area of active research with fewer studies compared to breath sound analysis. Several recent studies have introduced sound through the chest to investigate how sound travels through the chest structure [43; 44; 72]. These studies utilized low frequency audio range transducers which produced signals generated within the range of 50–1000 Hz [43; 44; 72].
The first of these studies used an electromagnetic shaker (ET-132, Lab-works Inc., Costa Mesa, CA) to drive a periodic chirp signal with spectral content from 50–400 Hz into the sternum [43]. The upper limit of frequency was set by the signal-to-noise ratio (SNR) for the system, which was limited by the sensitivity of sensors available at the time of the study. Its goal was to investigate changes in acoustic signals during pneumothorax, which leads to air accumulation in the lungs and collapse of lung tissue. Simulations using finite element analysis (COMSOL) predicted changes in pneumothorax in the range of 100–200 Hz; initial human subject data comparing healthy recordings to two pneumothoracies of different severity demonstrated reduction in signal transmission at 120 Hz which tracked with severity. A later study utilized a compact shaker (Model 4810, Bruel & Kjaer) placed on the clavicle with a reference accelerometer attached to the shaker, and expanded the range to 80–1000 Hz, a range determined by the accelerometer’s sensitivity [44].
The most recent of these studies utilized a surface exciter to send a linear chirp from 50–1000 Hz into the chest at the sternum [72]. This study investigated changes in acoustic transmission during pneumonia, which leads to fluid accumulation in the chest. The study demonstrated reduction in signal transmission in the 200–400 Hz range when comparing healthy subjects to patients with lobar pneumonia. Another study using the same equipment expanded the frequency range to 50–1000 Hz and utilized a classification algorithm to classify subjects lungs as healthy or pathological with an accuracy of 92% [47].
These studies indicate potential for a fixed input signal in the range of 50–1000 Hz for pulmonary disease diagnosis; however, more studies with larger numbers of patients are needed to confirm these findings and establish a baseline for disease specific changes.
C. Audible sound: Oral cavity input
Several studies have investigated the introduction of sound through the mouth with external recording from the subject’s chest [44; 48; 50; 53; 69]. For mouth-to-chest wall transmission, sound propagation is primarily through the parenchyma, as determined by the consistency of sound transmission across different gases [50]. Studies have focused on both frequency based analysis of transmission, transit time analysis as well as a respiratory acoustic impedance approach to identify changes in lung pathology.
These studies produce an external sound using a signal generator which is sent directly into the patient’s mouth and may be broken up into high frequency analysis (50–2000 Hz) and low frequency analysis [48; 50; 69]. The frequency range of 5–20 kHz has also been investigated, and found to result in data variability between healthy subjects, motivating the use of data below 5 kHz for analysis [52]. Controlled inputs via the oral cavity control for the intersubject variability of breath sounds.
Low frequency analysis can be used to obtain the respiratory impedance by utilizing a pneumotacho-graph to measure flow combined with a loudspeaker, which generates low frequencies. Two approaches exist, each sending a different signal into the oral cavity: the forced oscillation technique (FOT) and impulse oscillometry system (IOS) [53; 94; 95].These techniques assess lung function by measuring acoustic impedance using sound waves inserted into the patients’ mouth. The FOT works by sending a sinusoidal signal of single frequencies into the mouth over a range of 3–35 Hz [94; 96], providing good time resolution with measures of respiratory impedance but taking longer because it only sends one frequency at a time [94]. In contrast, the IOS sends a square wave impulse containing frequencies ranging from 4–32 Hz. Though this results in inferior temporal resolution and can cause discomfort for patients at higher frequencies, it offers improved SNR and can show pressure-flow relationships using frequency analysis [94; 97].
Advantages of FOT/IOS include higher sensitivity in detecting peripheral airway obstruction, short duration, and a requirement for little patient cooperation compared to spirometry, which is difficult for children, the elderly, and physically or cognitively impaired patients [94; 96]. For these reasons, FOT/IOS have proven useful in human studies for diagnosing asthma and COPD [53; 95; 98; 99; 100]. Current challenges to FOT/IOS come from its relatively recent introduction to the clinical setting, making it difficult to compare results due to the lack of standardized, healthy values. There have also been mixed reports regarding the effect of demographics such as sex, age, and race on IOS measurements [96; 101], though a study using artificial neural networks (ANNs) to classify asthma in IOS patterns achieved an accuracy of 94% without including race as a parameter [96; 102].
D. Audible sound: Endotracheal input
Studies have also investigated introduction of sound directly into the lungs through an endotrachial tube [26; 27; 54]. Initial studies in a canine model suggested that a frequency range of 20–1600 Hz with uniform amplitude could be used distinguish between differences in lung structure due to pneumothorax [27; 54]. At frequencies below 100 Hz, the disease and control states were comparable; however, for higher frequencies from 300–1600 Hz clear attenuation occurred due to disease in all subjects. Specifically, for structural changes due to air accumulation in the lungs, the canine model demonstrated an important metric for measuring the impact on the frequency response. Since the spectrum contained a large number of values not well suited for diagnostic analysis, a ratio between the low (<220 Hz) and high (550–770 Hz) spectral energies was used to detect accumulation of air [27]
E. Ultrasound: Chest input
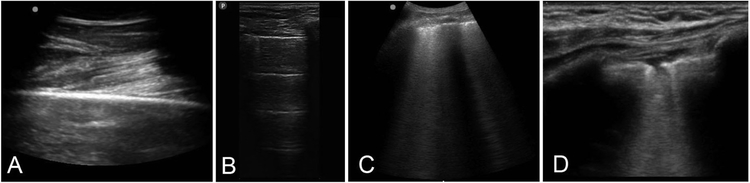
The transmission characteristics for ultrasound are similar to audible sound. Longitudinal waves are used in ultrasound, and the speed of ultrasound in soft tissue is about 1540 m/sec [11]. In ultrasound, the frequencies generated are high (1–15 MHz), which allow a short wavelength (1 to 0.1 mm) [16]. Therefore, it is suitable for detecting small differences in tissue boundaries which may not be picked up by an audible sound. Like audible sound, when the ultrasound wave encounters a boundary between two different media, some of the wave energy bounces back toward the source as an “echo” or reflection. The higher the difference in acoustic impedance between two objects, the higher the amount of reflection [103]. For example, at a soft tissue–bone boundary the ultrasound wave is highly reflected, and thus appears as a white (hyperechoic) line followed by a posterior acoustic shadow beyond the boundary (Fig 2a).
Figure 2:
(a) This soft tissue–bone boundary in which the ultrasound wave is highly reflected, and thus appears as a white (hyperechoic) line of the femur. (b) A-lines are static horizontal regularly-spaced hyperechoic lines that represent an aerated lung created by reverberation artifact. (c) B-lines are discrete, laser-like vertical projections arising from the pleural line and extending to the bottom of the screen without fading. (d) Shred (fractal) sign shows echo-poor areas of the pleura, represented as a non-smooth pleural line.
Lung ultrasound involves the interpretation of ultrasound artifacts instead of evaluation of the actual lung parenchyma. The soft tissue and pleural boundary has a high acoustic impedance mismatch, such that there is typically a reflection of 99.9% of ultrasound waves, rendering this interface virtually impenetrable to ultrasound [11; 16]. Since there is no ultrasound wave left to image beyond this boundary, performing an ultrasound of the lungs was initially thought to be impractical [104]; however, several studies have shown the usefulness of ultrasound in diagnosing several lung diseases through the identification of artifacts in the ultrasound signal [45]. There are a variety of static and dynamic artifacts that represent normal lung parenchyma.
As for electrocardiograms, a nomenclature has been created to describe and characterize these artifacts for communication. A-lines are static horizontal regularly-spaced hyperechoic lines that represent an aerated lung created by reverberation artifact (Fig 2b). A reverberation artifact is the bouncing of echo between pleural line and the ultrasound transducer. Likewise, lung sliding is a typical dynamic finding and represents the regular movement of the parietal pleura sliding along the visceral pleura. Lastly, Z-lines appear as bright beads with hyperechoic tails and are a common finding thought to be from microbubbles existing between the pleural layers [105].
There are also many ultrasound artifacts that represent pulmonary disease processes. In particular, comets, echo-graphic signs formed due to water thickened septa, as well as other static image irregularities combined with dynamic signs formed due to the gravity dependence of fluid in the lungs have proven to have diagnostic value [17; 46]. These signs hold particular weight when the alveolar air adjacent to the chest wall is replaced with fluid, allowing ultrasonic detection [106]. Various artifacts and syndromes associated with diseases are explored below.
Interstitial syndrome is a diffuse pulmonary process in which an ultrasound reverberation artifact, B-lines, represent fluid accumulation within pulmonary septa. B-lines are discrete, laser-like vertical projections arising from the pleural line and extending to the bottom of the screen without fading (Fig. 2c). Interstitial syndrome represents a variety of diseases such as pulmonary edema, interstitial pneumonia, acute respiratory distress syndrome, and pneumonitis.
The specific distribution of B-lines and the patient’s clinical presentation can help elucidate the cause of interstitial fluid [107]. For example, in patients with a moderate to high pretest probability for acute cardiogenic pulmonary edema, an ultrasound study showing B-lines can be used to strengthen a physician’s suspicion for pulmonary edema, while in patients with a low pretest probability of pulmonary edema, a negative ultrasound study can almost exclude the possibility of pulmonary edema [108].
Alveolar syndrome represents the loss of air within alveoli secondary to collapse or fluid accumulation (lung consolidation). Alveolar syndrome can be characterized by several ultrasonographic findings, including shred sign and tissue-like sign. Shred (fractal) sign shows echo-poor areas of the pleural, represented as a non-smooth pleural line (Fig 2d). Likewise, tissue-like sign, in which extensive consolidation will allow visualization of the underlying pulmonary tissue, is represented as a structure that appears like tissue. For these two findings, pulmonary ultrasound relies on the fact that 98% of consolidations reach the pleural surface and are thus viewable [109].
Recent reports show that ultrasound for pneumonia detection may be superior to chest radiograph; sensitivity and specificity for the diagnosis of pneumonia using ultrasound were 94% and 96% [110]. Lichtenstein et al. used a combination of static signs (irregular boundaries) and dynamic signs (absence of a sinusoidal irregularity) to diagnose alveolar consolidation, as in pneumonia, with a sensitivity of 90% and specificity of 98% when compared to CT [17]. Using visible air bronchograms on mechanically ventilated patients, Lichtenstein et al. also showed 94% specificity in distinguishing between pneumonia from atelectasis for mechanically ventilated patients who would have appeared the same by chest radiography [111].
Ultrasound evaluation of pleural effusion is an established technique with excellent test characteristics [112]. Fluid is an efficient conductor of ultrasound waves and can be visualized on evaluation [113]. Ultrasound is also helpful before thoracentesis, since it can reveal septations, estimate the size of effusion, and localize optimal needle placement. Similarly to alveolar consolidation, pleural effusion has also been diagnosed on ultrasound by using a combination of a static sign (sharp borders) and a dynamic sign (sinusoidal irregularity) which provided a sensitivity and specificity of 93% [17].
For pneumothorax, Lichtenstein et al. published an approach to evaluating pneumothorax with ultrasound by evaluating the artifact created as the visceral pleura slide against the parietal pleura [45]. A pneumothorax is represented as a loss of standard lung sliding seen in the healthy lung. A recently published review of eight high-quality studies found that overall, ultra-sound had a sensitivity of 90.0% and a specificity of98.2% for detecting pneumothorax [114].
Point-of-care ultrasound has been gaining traction within the medical community, and portable ultra-sound that is relatively inexpensive has facilitated this increased usage. While extensive studies have been done on pulmonary diagnosis using ultrasound, its use requires years of training to detect the artifacts associated with lung diseases. Furthermore, the presence of dynamic images in addition to static signs for disease detection make development of machine classifiers for disease more complex.
F. Audible Shear Waves: Chest input
While compression waves are useful in determining the effects of short range inter-molecular interactions and essential to high-resolution ultrasound imaging, acoustic shear waves can provide information about the shear elasticity and viscosity of a tissue [115; 116; 117]. Large changes in shear wave speed caused by diseases affecting the stiffness of the lungs and other tissues can provide diagnostic information [117]. Previous studies have used the modulated radiation force of ultrasound or a mechanical shaker to create a harmonic force in frequencies ranging from 100 to 500 Hz to detect viscoeleastic properties in a phantom and a pig lung using a laser doppler vibrometer or ultrasound for detection [118; 119; 120]. Future studies investigating the generation of surface waves on the lung through the intercostal spaces would be particularly interesting.
Acoustic shear wave motion throughout the lung parenchyma can be imaged using magnetic resonance (MR) elastography, a phase contrast MR imaging method [121]. Typically, an external vibration source applied to the chest is driven at frequencies on the order of 50 Hz. The shear wavelength and attenuation will be altered by changes in the lung mechanical properties. While MR imaging in general is particularly challenging in the lungs due to its heterogeneity and air content leading to lack of hydrogen (1H) atoms (abundant in soft tissue due to its water content), a few groups have had some success in applying MR elastography to the lung using both 1H [121; 122; 123] and polarized rare gas isotope (3He) MR imaging [124]. Measurable increases in lung stiffness have been observed in patients suffering from pulmonary fibrosis [125].
Shear wave motion can also be imaged via ultra-sound. In this method, surface wave propagation is induced by an electromagnetic shaker and detected by an ultrasound probe. Zhang et al. were able to show the feasibility of this method in differentiating between interstitial lung disease (ILD) and healthy patients, observing higher wave speed in ILD patients [126].
VII. SIGNAL PROCESSING
Analysis of sound transmission in the chest cavity suggests that the chest, overall, acts as a low-pass filter absorbing higher frequencies as sound travels through it [10; 11; 35; 42; 48; 50; 50; 62]. Spectral characteristics are affected by structural changes in the lungs caused by fluid or air accumulation, and can be studied and/or recognized using different techniques and classifiers in both the time and/or frequency domain.
A. Frequency range for analysis
Frequency analysis of lung sounds have shown to be a useful classifier for patients for diseases such as pneumonia, emphysema, pneumothorax, and asthma [6; 34; 36; 43; 127]. The frequency range considered physiologically important for nearly all heart and lung sounds is up to 2000 Hz [6; 78]. In various studies, frequency ranges of interest included 0–2000 Hz for breath sounds [3; 6; 19; 31; 33; 34; 82] or sound evaluated at the chest [15; 33]. For sound through the mouth, several studies have used a range of 50–1600 Hz [15; 48; 49; 51]. In general, frequencies above 100 Hz are used to eliminate significant noise from the heart and muscle, as well as 60 Hz electrical interference [128].
B. Frequency ranges for specific diseases
Pneumonia is often associated with crackles, which in one study was found to have an average frequency of 300 Hz [7; 21]. In a separate study, pneumonia was found to have a decrease in frequency response [72]. Another paper in line with these findings suggests that 300–600 Hz is important for pneumonia due to changes in bronchial breathing [128]. One review paper suggests a median frequency of 230 Hz for lung sounds [21].
For airway obstruction pathologies, such as asthma and COPD, the dominant relevant frequencies lie below 400 Hz [6]. The median frequency of lung sounds has been reported to be higher in asthmatic patients (239 Hz) than in healthy individuals (206 Hz) [33]; Schreur et al. found a lower median frequency of 165 Hz in allergen-induced asthma patients [129]. Another study found that asthmatic patients had wheezes and crackles with an average frequency of 300 Hz, while COPD patients had only crackles at the same average frequency, supporting the relevant frequency range below 400 Hz [7]. Patients with asthma also had a higher proportion of time spent wheezing at inspiration (10%) compared to COPD patients (1–2%) with average frequencies around 130–140 Hz[21]. Analysis strictly based on wheezes and crackles may not be a reliable method for diagnosing COPD, as a review paper found that the number of COPD crackles can vary per patient, but have an average lower inspiration frequency (233–311 Hz) than crackles in asthmatic patients (329 Hz) [21]. Pneumothorax has also been found to be associated with a significant drop in sound amplitude from 400–600 Hz [26].
C. Analysis methods
Several analysis methods are used for feature extraction from recordings of lung sounds. These analysis techniques are critical for classification by extracting distinctive features of sound such as wheezes, rhonchi, stridor, etc., which can be found in the time domain, frequency domain, or the time–frequency domain. We discuss frequently used methods, such as statistical analyses for time domain; the transfer function, Fourier transforms, and mel-frequency cepstrum analysis for the frequency domain; and wavelet analyses and the autoregressive (AR) model for the time-frequency domain.
1) Statistical analyses: Statistical methods, such as distribution features, higher-order statistics, and cross-correlation, have also been used to characterize lung sounds. While these methods are useful in both the time and frequency domain, they are especially important in the time domain, as the frequency domain often lends itself to other powerful analytical methods described below. Analysis in the time domain can also elicit unique information from non-periodic signals, such as crackles. Traditional frequency domain analyses assume stationarity that cannot be applied to such adventitious sounds and does not account for changes throughout the respiratory cycle [63].
Distribution features such as mean, median, mode, and variance can be useful in characterizing breath sounds and creating a mathematical model, such as wheezes which have biomodal distributions [63]. Breath sounds, however, are often more closely modeled as non-Gaussian, random processes, making distribution features less useful [21; 63]. Therefore, higher-order statistics, which measure deviations from the normal distribution, can be more useful. Higher order statistics including skewness and kurtosis, have also been utilized for breath sound analysis [21; 63]. Notably, kurtosis is a sensitive but not very specific measure; a few outliers can heavily influence its value. This characteristic has been used in detecting the presence of crackles [63].
Cross-correlation is another useful method for analysis in the time domain, and works by detecting similarities between two signals. This has been used in previous studies to analyze the time delay of sound traveling through the parenchyma with multi-sensor arrays [44; 84], which can facilitate understanding of sound wave speed/propagation through the lungs, including changes that relate to different diseases.
Calculating sound intensity has also been used to produce images; notably, application of multi-sensor arrays to calculate intensity has been shown to be useful in both localizing the signal and understanding its timing in the breathing cycle [3; 14; 38]. An envelope can be used to represent the acoustic energy for each sensor to provide an acoustic heat map of the lungs [90; 130]. A Hilbert transform can be utilized to calculate this envelope of the acoustic signals to provide a measure of sound intensity [38].
2) Discrete Fourier transform: The Fourier transform (FT) is the most commonly used spectral analysis technique to calculate the frequency content of a signal [20; 32; 39; 41; 130]. The discrete Fourier transform (DFT) is a type of Fourier transform that is used to analyze discretely sampled data, such as digitized lung sounds. The DFT takes a signal from the time domain to the frequency domain, but operates on an assumption of stationary data over a time period. The short-time Fourier transform (STFT) sacrifices frequency resolution in order to keep the time window short and more accurately track non-stationary signals [131]. The Fast Fourier transform (FFT) is an efficient algorithm used to calculate these Fourier transforms [132]. Different lung pathologies yield different sounds observed at the chest, such as wheezes and crackles, that contain different frequency ranges. Analysis of frequency range content and duration can be useful in distinguishing between diseases. Several studies have used different Fourier-related transforms to produce spectrograms or power spectral density (PSD) functions [39; 40; 133].
3) Frequency response function (FRF): The FRF, which can be calculated a few different ways, is essentially the ratio of the DFT of an output signal to the DFT of an input signal. It assumes the input signal is well-defined or measurable. Analysis using FRFs involves examining the amplitude or power of a signal that is transmitted from the input to the output within a range of frequencies. Several studies have utilized the FRF to understand the physics of the lungs, notably the ways in which sound is transmitted through the varying geometries in the parenchyma at different frequencies, and which frequencies become attenuated [2; 12; 15; 26; 50; 72]. Specifically, the FRF can change with different conditions, such as pneumonia, due to changes to the geometry of the lung, which cause it to transmit or attenuate sound differently [39]. Several studies have used the FRF to detect excess fluid in or around the lung [72; 134; 135; 136; 137] and pneumothorax [26].
4) Mel-frequency cepstrum: Similar to the DFT, mel-frequency cepstrum analysis is useful for feature extraction from audio signals. They closely follows the mel scale, which approximates the human hearing range [19]. This technique is commonly used in music information retrieval (MIR) to sort through different music types [138]. MFC is implemented using DFT coefficients that are filtered through the mel scale. The features extracted from MFC, outputting mel-frequency cepstrum coefficients (MFCC) that can then be fed into different classification schemes for automated lung sound analysis [19]. MFCC has been widely used in speech recognition and lung sound analysis in recent studies [19; 47; 55; 136; 139; 140; 141; 142; 143].
5) Wavelet and Hilbert–Huang transforms: Due to the non-stationary and non-periodic nature of breath sounds, the Fourier transform alone is insufficient to capture important time-domain characteristics of the signal [5; 144]. Using the wavelet transform (WT), partial time and frequency information can be utilized to help more accurately characterize the signal by means of windows of variable size [5; 131; 145]. Wavelet transforms focus on particular frequencies by applying successive high- and low-pass filters without the need to know a frequency range in advance [5]. Another method for modeling lung sounds this way is the Hilbert-Huang transform (HHT), used to extract oscillating components in the time-domain, which can be useful in determining characteristics of different lung sounds such as crackles [21], or denoising.
Several different wavelet transforms exist each with a different set of wavelet filters and characteristics [131; 146]. The Meyer wavelet filter, in particular, has been shown to be successful in characterizing COPD and asthma by enhancing the signal of interest and allowing the use of cross-correlation and thresholding for detection [5]. Furthermore, the use of wavelet-based features has been proved effective by the work of Kandaswamy et al., who reported a classification accuracy of 100% of the type of adventitious sounds for the training set using wavelet decomposition and ANNs [131]. Other studies have also shown the applicability of WT in analyzing lung sounds [145].
6) Autoregressive (AR) modeling: Autoregressive (AR) modeling, also known as linear predictive coding (LPC), is particularly useful for time-varying signals. By assuming that each sample can be represented by a linear combination of previous samples [55], the model yields an output dependent on the bases given by the previous samples, scaled by stochastic predictor coefficients. Intersubject and even intrasubject variability with respect to microphone and utterance can lead to poor predictor coefficients with high error, but a previous study suggests that the use of AR with a moving-average (ARMA) model minimizes this variability [42]. Studies have utilized the AR model to estimate the power spectral density function [36; 42], basic feature extraction [147; 148], and extraction with reduced computational complexity [149].
D. Classification algorithms
When used in tandem with pre-processing techniques, classification algorithms have proven useful for automatic detection of adventitious lung sounds and/or diagnosis of lung pathologies. In this section, we discuss the major classification algorithms that have been implemented with promising results.
1) Support vector machines (SVM): The support vector machine (SVM) classifier is a kernel-based supervised learning algorithm particularly useful for binary and/or non-linear classification. Training SVM classifiers involves building a model, mapping a decision boundary for each class, and defining a hyper-plane that separates the classes [19; 150]. Increasing the hyperplane margin, thus increasing the distance between the classes, helps improve classification accuracy. Only a few studies have implemented SVM for breath sound classification [150; 151]. Palaniappan et al. utilized SVM in order to classify healthy, pathological, and airway obstruction sounds, achieving a mean accuracy of 90.77% [19], but also showed that the k-nearest neighbors (KNN) algorithm was superior to SVM for generalization and classification [6]. Serbes et al. noted that improved pre-processing techniques boosted classification, with a maximum classification accuracy of crackles at 97.20% [146].
2) K-nearest neighbors (KNN): K-nearest neighbors is an instance-based classification algorithm that has been used in several studies to classify recorded signals into different abnormal lung sound types [37; 41; 47; 136; 139; 147; 148]. The algorithm is trained on a data set, compressing the data into representative cluster centers for each abnormal sound; newly recorded data is mapped onto the same space, and the algorithm finds the K nearest neighbors, or data points, as measured by Euclidean distance [139]. The majority cluster center represented by these neighbors corresponds to the class of the abnormal sound the data resembles. Rao et al. showed superior classification accuracy for pathology using KNN compared to Gaussian mixture models (GMM) and ANN [47]. Furthermore, for COPD specifically, using FOT measurements and utilizing KNN, SVM, and ANN showed consistently superior classification for KNN with an accuracy of 93–95% [152]. A study also comparing various pre-processing techniques in both KNN and SVM showed consistent superiority for KNN [136].
3) Artificial neural networks (ANN): Neural networks have been used in several studies to automatically classify lung sounds, often yielding promising results [29; 32; 39; 102; 131; 133; 142; 145; 149; 150; 153]. The most common method for training ANNs is the backpropagation algorithm [102]. ANNs work by modeling complex relationships between inputs and outputs. The model is formed by an interconnected group of artificial neurons that learns by adjusting its connective weights using training data in order to more accurately represent these relationships with minimal error [149; 150]. Although ANNs are widely represented in the literature due to their ability to classify both linear and non-linear data with high accuracy, they require a large data set to avoid over-fitting [47; 151].
4) Gaussian mixture model (GMM): Gaussian mixture models (GMM) are widely used for speaker identification or verification [140]. Similarly to KNN, training data is used to obtain sound classes, which are represented by a GMM. Newly recorded data is mapped onto the same space and compared to each GMM, with the final classification based on the highest maximum likelihood (ML) criterion [140; 154]. Several studies have used GMMs to classify lung sounds [55; 140; 141; 154]. Bahoura et al. showed superior sensitivity and specificity using MFCC combined to GMM to classify normal versus wheeze sounds compared to autoregressive methods with KNN and wavelet transform with ANN [55]. Comparatively, Mayorga et al. showed promising accuracy for crackles due to its characteristic frequency peaks [154], which contributed to a more robust GMM due to higher variance and distinctive mean. However, wheezes, crackles, and asthma-related signals performed poorly due to less defined or complex signals that could be improved with a larger dataset including different measurement locations and age groups and/or focus on a specific frequency band.
5) Other methods: The use of several other classification algorithms for lung sound analysis, such as fuzzy logic and hybrid algorithms, has been documented in the literature. Fuzzy logic has been used to score the severity of certain pathologies such as asthma and COPD based on variables acquired by the physician and the patient, such as coughing frequency, forced expiratory volume, or frequency of missing school/work [155]. This system allows the combination of acoustic variables such as IOS measurments of acoustic impedance with clinical variables to reach a classification decision. Badnjevic et al. combined fuzzy logic with ANNs, an approach they call a “neuro-fuzzy” system to obtain 99.41% classification accuracy for asthma patients and 99.19% classification accuracy for COPD patients, showing that a combination of methods can yield better accuracy [29]. They furthered the ANNFL logic into an expert diagnostic system (EDS), yielding a sensitivity of 96% and specificity of 98% in a prospective system [156]. Barua et al. were also able to achieve a high classification accuracy using ANN, but recommended further development to extract expert rules to obtain a human understanding of the network’s knowledge. The authors suggested that obtaining these fuzzy logic decision rules could help create a more powerful hybrid neuro-fuzzy classifier, supporting the idea that hybrid algorithms yield superior classification [102]. In addition to superior classification, fuzzy-logic eliminates the black box nature inherent with ANNs, demystifying the logic behind its classifications, which is more user-friendly to doctors and upholds physician authority. Hybrid algorithms are a recent advance in classification methods and thus merit further study.
VIII. HUMAN SUBJECTS STUDIES
Respiratory diseases lead to structural lung changes that can be detected acoustically. The existence of such changes has spurred numerous studies utilizing both internal and external sounds to diagnose a variety of lung diseases. For the purposes of this review, we focus on pneumonia, asthma, COPD, pleural effusion and pneumothorax. We also include several miscellaneous studies with results that further elucidate different analysis methods. Table II summarizes human subject studies investigating acoustic changes in the diseases discussed in this review.
Acoustic detection of pneumonia and pleural ef-fusion represents an important clinical need due to the high cost and radiation exposure of the gold standard chest X-ray (CXR). For example, the cost of deploying an x-ray machine in Northern India is estimated at $51,500 plus $5,900 per year to operate [157]. Several studies utilizing both internal and external sounds have been conducted to aid in pneumonia diagnosis. Three studies utilized multiple microphones to provide a sound map of the chest similar to a chest x-ray [3; 4; 88]. The highest reported sensitivity and specificity for pneumonia using this method was 70% and 80%, respectively [4]. Furthermore, inter-observer agreement for this method was high, with 80% agreement on the presence of an abnormality and inter-rater agreement of 90% [88]. Pleural effusion was detected with higher accuracy, with sensitivity of 95%, specificity of 88%, and 80% agreement on diagnosis compared to CXR [4; 87].
Another important clinical need is the ability to distinguish between pneumonia and atelectasis (lung collapse), a distinction chest x-ray cannot provide. Ultrasound has been used to rule out atelectasis with a specificity of 94% and sensitivity of 61% [111]. Breath sounds have also been analyzed for pneumonia patients with standard electret microphones. In that study, 29% of pneumonia patients had wheezes, 29% had rhonchi and 87% had crackles [7]. The average frequency of the crackles which correlated most closely with diagnosis of pneumonia was found to be 300 Hz at inspiration and 285 Hz at expiration for pneumonia [7]. A more recent proof-of-concept study examining the use of external sound for pneumonia diagnosis utilized an external surface exciter with a linear chirp from 50–1000 Hz to detect pneumonia [72]. That study reported frequency changes between healthy and consolidated lung.
Discrimination between asthma and COPD also represents an important clinical problem, as current diagnosis is largely based on clinical observation. Quantifying the findings for these diseases provides a route for faster and more consistent diagnosis. The diagnosis of both asthma and COPD is based on signs of airway obstruction, causing COPD to be often misdiagnosed as asthma due to the overlapping symptoms [130]. One study, however, was able to differentiate between the two with 85% accuracy; its approach was based on dynamic images of lung activity acquired during a 12 second recording using an array of stethoscopes on the back [130].
Several studies on asthma have highlighted characteristics that could differentiate between the two disorders; one large study found that a majority of asthmatic patients had wheezes and crackles with an average frequency of 300 Hz, while a majority of COPD patients only had crackles with a similar average frequency [7]. An algorithm for wheeze peak detection was developed and tested on asthmatic patients with a sensitivity of 100%, 87.8%, and 71% for high, middle, and low flow rates, respectively, potentially allowing clinicians to diagnose asthma versus COPD based on the presence of wheezing episodes [158]. A study of asthmatic patients who had taken bronchodilator drugs showed a decreased in centroid frequency during forced expiration from 500 Hz to 200 Hz, suggesting that the frequency characteristics of breath sounds can be used to detect response to treatment [36].
Similarly, studies have focused on the various acoustic characteristics present in COPD patients. In a study of COPD patients with a history of smoking, these individuals were shown to have larger peak signal amplitudes from 0–5 kHz when compared with healthy subjects [52]. This suggests a frequency range of interest for COPD detection. Furthermore, another study evaluated eleven different parameters to diagnose COPD, such as lead and lag time of inspiration at different locations and ratio of inspira-tory to expiratory time; the authors concluded that all 11 parameters possessed differences between COPD and healthy patients that were statistically significant, suggesting that further study into these differences should be carried out [159].
The use of IOS, which measures lung function parameterized by airway acoustic input impedance, has also shown potential to differentiate asthma and COPD. In studies of COPD patients, IOS analyses showed changes in reactance are correlated with changes in airflow obstruction [98; 160], while another study using asthmatic children showed measurement of changes in resistance rather than reactance better indicated asthmatic patients [94; 100]. In spite of these promising results, a large 3-year clinical trial found that although IOS could differentiate COPD patients amongst healthy patients and determine the degree of severity of airflow limitation, measurement data from COPD patients was similar to that of healthy non-smokers. The authors concluded that IOS cannot yet replace spirometry but may instead prove valuable in distinguishing between different subgroups of COPD [53]. While IOS is a valuable tool, more portable acoustic methods of monitoring COPD patients for acute exacerbation, allowing patients to monitor their own health over time, have been demonstrated [161].
Similar to pneumonia, the current standard for diagnosing pneumothorax (PTX) includes radiography, which is both expensive and not readily available [27]. Several studies have examined changes in sound and frequency transmission due to the presence of air accumulation between the chest wall and lungs, which would affect how sound is transferred due to the existence of another interface. One study utilizing external stimuli found that a significant decrease in sound transmission and the power spectral density (PSD) function was evident using 400–600 Hz sound inserted at the mouth and measured over the chest [26]. In another study using an array of stethoscopes on the back, abnormalities of the pleural space were identified with a sensitivity of 100% and specificity of 87% [162]. Another study using ultrasound used the disappearance of lung sliding to represent the air pocket from PTX that causes the lung–wall interface to appear motionless [45]. This method had a sensitivity of 95.3% and specificity of 91.1%.
Several studies have investigated the broader question of how accurately lung pathology can be distinguished from healthy lung. Although this approach does not provide direct clinical benefit, it provides a foundation upon which future studies can build and demonstrates a variety of analysis methods and sensor technologies. Ultrasound has shown promise in prediction of consolidated lung, as well as for detecting fluid accumulation [46; 106]. One analytical method of interest is the use of MFCC coefficients combined with KNN to develop an automated method of extracting data from the frequency domain and classify the sounds into diseased and healthy categories.
Two recent studies utilizing this method demonstrated the use of this approach for breath sounds as well as external sounds. A 2015 study used a digital stethoscope to record lung sounds for classification of abnormal lung sounds achieving a classification accuracy of 91.3% [139]. A 2018 study extended this work to external sounds, using a surface exciter placed on the chest and a digital stethoscope placed on the back, achieving a classification accuracy of91.7% for distinguishing between healthy subjects and patients with lung pathology [47]. Finally, regular use of an array of stethoscopic measurements on the back has shown potential in preventative medicine, where it has been used to track the progression of lung disease in smokers. Changes in the intensity of breath sounds were correlated with pack-year history among smokers [163].
IX. CHALLENGES AND PROSPECTS: THE PATH TO STANDARDIZATION
Acoustic methods of pulmonary diagnosis are valuable indicators of respiratory health. Several factors have prevented clinical application of recent experimental advances in the field. In spite of decades of research in the area of breath sound analysis, standardized information for sensor usage, signal processing, nomenclature, and frequency or time information in the context of pathologies remains a challenge, arguably deterring the wider adoption of lung sound analysis in the clinic. Standardization of information with regards to the acoustic characteristics and physics of the lungs, especially with different pathologies, was undertaken by CORSA [56], but its recommendations still have not been widely adopted, and an updated standard is overdue [21]. Differing methods on what to sense (internal vs. external sounds) and how to analyze these signals, has made it difficult to begin translation of these methods into the clinic. Specifically, future work should focus on expanding the human subject studies in each disease to provide adequate samples for clinical application.
Translational challenges act as the main barrier between the different groups working on advancing acoustic analysis of the lungs. The creators of a device may not understand the different nomenclature or needs of physicians, and physicians may not have the engineering experience required to communicate sensor, processing, and/or classification specifications. As a result, research into both internal and external methods of acoustic analysis to diagnose the lungs has used a wide array of sensors, usually microphones or accelerometers, which have different specifications, yielding different measurements. With advances in transducer fabrication methods, such as using MEMS technology, and other sensor research, we converge to a description of the ideal sensor, one that is lightweight, reliable, and sensitive, which will help standardize our understanding of the characteristics of the lungs with respect to internal and external sounds.
In addition to translational challenges between clinicians and device engineers, those working on signal processing and classification techniques also need to be included in future collaborations. Despite the value of machine learning algorithms, it is important that they provide outputs that can be interpreted by a clinician rather than automatically outputting a diagnosis. Acoustic information can provide a digital biomarker for diagnosis or likelihood scores but should not be used to classify the patient without the context provided by a clinician and a thorough medical history.
Further research is needed to understand the clinical utility of analyzing external sound through the parenchyma instead of internal breath sounds. The analysis of sounds acquired through the parenchyma has the benefit of providing a controllable, well-characterized input signal that lends itself to application of new advances, including signal processing and classification schemes. The frequency ranges for the diseases discussed in this review are presented to provide a foundation for researchers in the field to develop such a system, consistent with CORSA, as well as other relevant research. Similarly, the use of an array of sensors is a notable method to improve SNR and provide localization information that invites further research. There are different external methods of sensor actuation with regard to location and system. The ideal actuator should be selected based on size (which influences portability, cost and comfort), intensity at frequencies of interest, and current draw, which affects battery life. The ideal system would also help with monitoring, as aforementioned; integration with the electronic health record (EHR) could contribute to online databases for pathologies, facilitating further research into changes in lungs parameters in response to disease.
X. LOOKING AHEAD: TELEMEDICINE AND MOBILE HEALTH
Outside the hospital, the capability of wirelessly transmitting and storing data is increasingly becoming a standard feature in medical devices [72; 161; 164]. This capability offers the potential to develop and advance the field of telemedicine, which will allow physicians to access live data on the acoustic biomarkers for their patients’ lung health and change the course of their care accordingly [161; 164]. This can be done through mobile solutions, such as apps, which are widely available. Certain digital stethoscopes have already provided this platform [165; 166; 167], but it has yet to be established for multi-sensor recording systems. Notably in the field of lung diagnosis, a telehealth system was prospectively studied by Gurbeta et al. for use in diagnosing patients with COPD and asthma based on neural network and fuzzy logic systems with promising results [168]. This system showed the potential of telemedicine and mobile health for improving quality of care in low-resource settings and less mobile patients. Integration of new technologies with these modern systems (EHR, mobile) is essential because it not only provides better means of monitoring, but also understanding patients with respect to differing demographics and conditions. Creation of such a database will facilitate development of better sensors and analytical techniques.
XI. CONCLUSION
Recent advances in acoustic monitoring for pulmonary diseases indicate the strong potential of these measurements to address a wide variety of clinical needs, in particular, providing quantitative metrics of lung health without the dangers and expense of radiography, and without the requirement for patient compliance associated with spirometry. In addition to these key advantages, acoustic monitoring also provides a route to telemedicine applications that can allow patients to be monitored outside of the clinic. Acoustic measurements can be taken with highly precise instrumentation, or with inexpensive sensors, making them well suited for hospital-grade diagnostics as well as home monitoring of chronic diseases. Despite the rich potential of acoustic monitoring, the physiological origins of the signals should be studied further with greater standardization and larger scale human studies to ensure maximum interpretability of these signals. Finally, an extensive open database, not just for breath sound recordings but also featuring responses to external stimuli, processing tools, and even microcontroller code, needs to be made available to truly expand the capability of researchers to investigate these phenomena, apply them to their own studies, and establish techniques that can facilitate their translation into the clinic.
Acknowledgments
The project is partially supported by the Pediatric Device Consortium at the University of California, San Francisco, an FDA grant program (award No. P50FD003793). A. Rao is supported by the Ruth L. Kirschstein Fellowship (F30), an NIH grant program (award No. 1F30HL140906). T.J. Royston is supported by pilot grant #2018–04 through the UIC NIH CTSA Award TR002003.
Contributor Information
Adam Rao, Email: adam.rao@ucsf.edu, Department of Bioengineering and Therapeutic Sciences, University of California, San Francisco, CA 94158 USA..
Emily Huynh, Email: emily_huynh@berkeley.edu, Department of Bioengineering, University of California, Berkeley, CA 94720 USA.
Thomas J. Royston, Email: troyston@uic.edu, Department of Bioengineering, University of Illinois, Chicago, IL 60607-7022 USA.
Aaron Kornblith, Email: aaron.kornblith@ucsf.edu, Department of Emergency Medicine and Pediatrics, University of California, San Francisco, CA 94158 USA..
Shuvo Roy, Email: shuvo.roy@ucsf.edu, Department of Bioengineering and Therapeutic Sciences, University of California, San Francisco, CA 94158 USA..
REFERENCES
- [1].Laennec RTH and Forbes J, A Treatise on the Diseases of the Chest, and on Mediate Auscultation. Samuel S. and William Wood, 1838. [Google Scholar]
- [2].Cohen A, “Signal processing methods for upper airway and pulmonary dysfunction diagnosis,” IEEE Engineering in Medicine and Biology Magazine, vol. 9, no. 1, pp. 72–75, 1990. [DOI] [PubMed] [Google Scholar]
- [3].Kompis M, Pasterkamp H, and Wodicka GR, “Acoustic imaging of the human chest,” Chest, vol. 120, no. 4, pp. 1309–1321, 2001. [DOI] [PubMed] [Google Scholar]
- [4].Mor R, Kushnir I, Meyer J-J, Ekstein J, and Ben-Dov I, “Breath sound distribution images of patients with pneumonia and pleural effusion,” Respiratory care, vol. 52, no. 12, pp. 1753–1760, 2007. [PubMed] [Google Scholar]
- [5].Marshall A and Boussakta S, “Signal analysis of medical acoustic sounds with applications to chest medicine,” Journal of the Franklin Institute, vol. 344, no. 3–4, pp. 230–242, 2007. [Google Scholar]
- [6].Palaniappan R, Sundaraj K, and Sundaraj S, “A comparative study of the svm and k-nn machine learning algorithms for the diagnosis of respiratory pathologies using pulmonary acoustic signals,” BMC bioinformatics, vol. 15, no. 1, p. 223, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Murphy RL, “In defense of the stethoscope,” Respiratory Care, vol. 53, no. 3, pp. 355–369, 2008. [PubMed] [Google Scholar]
- [8].Loudon R and Murphy RL Jr, “Lung sounds,” American Review of Respiratory Disease, vol. 130, no. 4, pp. 663–673, 1984. [DOI] [PubMed] [Google Scholar]
- [9].Mangione S and Nieman LZ, “Pulmonary auscultatory skills during training in internal medicine and family practice,” American journal of respiratory and critical care medicine, vol. 159, no. 4, pp. 1119–1124, 1999. [DOI] [PubMed] [Google Scholar]
- [10].Forgacs P, “The functional basis of pulmonary sounds,” Chest, vol. 73, no. 3, pp. 399–405, 1978. [DOI] [PubMed] [Google Scholar]
- [11].Rice DA, “Transmission of lung sounds,” in Seminars in respiratory medicine, vol. 6, no. 03 Copyright© 1985 by Thieme Medical Publishers, Inc., 1985, pp. 166–170. [Google Scholar]
- [12].Wodicka GR, Stevens KN, Golub HL, Cravalho EG, and Shannon DC, “A model of acoustic transmission in the respiratory system,” IEEE Transactions on Biomedical Engineering, vol. 36, no. 9, pp. 925–934, 1989. [DOI] [PubMed] [Google Scholar]
- [13].Dai Z, Peng Y, Mansy HA, Sandler RH, and Royston TJ, “Comparison of poroviscoelastic models for sound and vibration in the lungs,” Journal of vibration and acoustics, vol. 136, no. 5, p. 050905, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dellinger RP, Parrillo JE, Kushnir A, Rossi M, and Kushnir I, “Dynamic visualization of lung sounds with a vibration response device: a case series,” Respiration, vol. 75, no. 1, pp. 60–72, 2008. [DOI] [PubMed] [Google Scholar]
- [15].Korenbaum V, Tagilâtsev A, Dâyachenko A, and Kostiv A, “Comparison of the characteristics of different types of acoustic sensors when recording respiratory noises on the surface of the human chest,” Acoustical Physics, vol. 59, no. 4, pp. 474–481, 2013. [Google Scholar]
- [16].Aldrich JE, “Basic physics of ultrasound imaging,” Critical care medicine, vol. 35, no. 5, pp. S131–S137, 2007. [DOI] [PubMed] [Google Scholar]
- [17].Lichtenstein DA, “Ultrasound in the management of thoracic disease,” Critical care medicine, vol. 35, no. 5, pp. S250–S261, 2007. [DOI] [PubMed] [Google Scholar]
- [18].Leng S, San Tan R, Chai KTC, Wang C, Ghista D, and Zhong L, “The electronic stethoscope,” Biomedical engineering online, vol. 14, no. 1, p. 66, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Palaniappan R and Sundaraj K, “Respiratory sound classification using cepstral features and support vector machine,” in Intelligent Computational Systems (RAICS), 2013 IEEE Recent Advances in. IEEE, 2013, pp. 132–136. [Google Scholar]
- [20].Gurung A, Scrafford CG, Tielsch JM, Levine OS, and Checkley W, “Computerized lung sound analysis as diagnostic aid for the detection of abnormal lung sounds: A systematic review and meta-analysis,” Respiratory medicine, vol. 105, no. 9, pp. 1396–1403, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Priftis KN, Hadjileontiadis LJ, and Everard ML, Breath Sounds: From Basic Science to Clinical Practice. Springer, 2018. [Google Scholar]
- [22].Yernault JC and Bohadana A, “Chest percussion,” European Respiratory Journal, vol. 8, no. 10, pp. 1756–1760, 1995. [DOI] [PubMed] [Google Scholar]
- [23].Guarino J, “Auscultatory percussion of the chest,” The Lancet, vol. 315, no. 8182, pp. 1332–1334, 1980. [DOI] [PubMed] [Google Scholar]
- [24].Metlay JP, Kapoor WN, and Fine MJ, “Does this patient have community-acquired pneumonia?: Diagnosing pneumonia by history and physical examination,” Journal of the American Medical Association, vol. 278, no. 17, pp. 1440–1445, 1997. [PubMed] [Google Scholar]
- [25].Porcel JM and Light RW, “Diagnostic approach to pleural effusion in adults,” Am Fam Physician, vol. 73, no. 7, pp. 1211–1220, 2006. [PubMed] [Google Scholar]
- [26].Mansy HA, Balk RA, Warren WH, Royston TJ,Dai Z, Peng Y, and Sandler RH, “Pneumothorax effects on pulmonary acoustic transmission,” Journal of applied Physiology, vol. 119, no. 3, pp. 250–257, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mansy H, Royston T, Balk R, and Sandler R, “Pneumothorax detection using pulmonary acoustic transmission measurements,” Medical and Biological Engineering and Computing, vol. 40, no. 5, pp. 520–525, 2002. [DOI] [PubMed] [Google Scholar]
- [28].Bohadana A, Izbicki G, and Kraman SS, “Fundamentals of lung auscultation,” New England Journal of Medicine, vol. 370, no. 8, pp. 744–751, 2014. [DOI] [PubMed] [Google Scholar]
- [29].Badnjevic A, Cifrek M, Koruga D, and Osmankovic D, “Neuro-fuzzy classification of asthma and chronic obstructive pulmonary disease,” BMC medical informatics and decision making, vol. 15, no. 3, p. S1, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Contoli M, Bousquet J, Fabbri L, Magnussen H, Rabe K,Siafakas N, Hamid Q, and Kraft M, “The small airways and distal lung compartment in asthma and copd: a time for reappraisal,” Allergy, vol. 65, no. 2, pp. 141–151, 2010. [DOI] [PubMed] [Google Scholar]
- [31].Pasterkamp H, Patel S, and Wodicka G, “Asymmetry of respiratory sounds and thoracic transmission,” Medical and Biological Engineering and Computing, vol. 35, no. 2, pp. 103–106, 1997. [DOI] [PubMed] [Google Scholar]
- [32].Forkheim KE, Scuse D, and Pasterkamp H, “A comparison of neural network models for wheeze detection,” in WESCANEX 95. Communications, Power, and Computing. Conference Proceedings., IEEE, vol. 1. IEEE, 1995, pp. 214–219. [Google Scholar]
- [33].Hu Y, Kim EG, Cao G, Liu S, and Xu Y, “Physiological acoustic sensing based on accelerometers: A survey for mobile healthcare,” Annals of biomedical engineering, vol. 42, no. 11, pp. 2264–2277, 2014. [DOI] [PubMed] [Google Scholar]
- [34].Becker HD, “Vibration response imaging–finally a real stethoscope,” Respiration, vol. 77, no. 2, pp. 236–239, 2009. [DOI] [PubMed] [Google Scholar]
- [35].Gavriely N, Palti Y, and Alroy G, “Spectral characteristics of normal breath sounds,” Journal of Applied Physiology, vol. 50, no. 2, pp. 307–314, 1981. [DOI] [PubMed] [Google Scholar]
- [36].Fiz JA, Jané R, Salvatella D, Izquierdo J, Lores L, Caminal P, and Morera J, “Analysis of tracheal sounds during forced exhalation in asthma patients and normal subjects: bronchodilator response effect,” Chest, vol. 116, no. 3, pp. 633–638, 1999. [DOI] [PubMed] [Google Scholar]
- [37].Kahya YP, Cini U, and Cerid O, “Real-time regional respiratory sound diagnosis instrument,” in Engineering in Medicine and Biology Society, 2003 Proceedings of the 25th Annual International Conference of the IEEE, vol. 4. IEEE, 2003, pp. 3098–3101. [Google Scholar]
- [38].Torres-Jimenez A, Charleston-Villalobos S, Gonzalez-Camarena R, Chi-Lem G, and Aljama-Corrales T, “Asymmetry in lung sound intensities detected by respiratory acoustic thoracic imaging (rathi) and clinical pulmonary auscultation,” in Engineering in Medicine and Biology Society, 2008. EMBS 2008. 30th Annual International Conference of the IEEE IEEE, 2008, pp. 4797–4800. [DOI] [PubMed] [Google Scholar]
- [39].Waitman LR, Clarkson KP, Barwise JA, and King PH, “Representation and classification of breath sounds recorded in an intensive care setting using neural networks,” Journal of clinical monitoring and computing, vol. 16, no. 2, pp. 95–105, 2000. [DOI] [PubMed] [Google Scholar]
- [40].Riella R, Nohama P, and Maia J, “Method for automatic detection of wheezing in lung sounds,” Brazilian Journal of Medical and Biological Research, vol. 42, no. 7, pp. 674–684, 2009. [DOI] [PubMed] [Google Scholar]
- [41].Oud M, Dooijes EH, and van der Zee JS, “Asthmatic airways obstruction assessment based on detailed analysis of respiratory sound spectra,” IEEE transactions on biomedical engineering, vol. 47, no. 11, pp. 1450–1455, 2000. [DOI] [PubMed] [Google Scholar]
- [42].Cohen A and Berstein A, “Acoustic transmission of the respiratory system using speech stimulation,” IEEE transactions on biomedical engineering, vol. 38, no. 2, pp. 126–132, 1991. [DOI] [PubMed] [Google Scholar]
- [43].Peng Y, Dai Z, Mansy HA, Sandler RH, Balk RA, and Royston TJ, “Sound transmission in the chest under surface excitation: an experimental and computational study with diagnostic applications,” Medical & biological engineering & computing, vol. 52, no. 8, pp. 695–706, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Korenbaum V and Shiriaev A, “Sound propagation through human lungs, under transmission sounding with acoustic signal of 80–1000 hz frequency band,” in Proceedings of Meetings on Acoustics 169ASA, vol. 23, no. 1 ASA, 2015, p. 020002. [Google Scholar]
- [45].Lichtenstein DA and Menu Y, “A bedside ultrasound sign ruling out pneumothorax in the critically iii: lung sliding,” Chest, vol. 108, no. 5, pp. 1345–1348, 1995. [DOI] [PubMed] [Google Scholar]
- [46].Jambrik Z, Monti S, Coppola V, Mottola G, Miniati M,Picano E et al. , “Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water,” American Journal of Cardiology, vol. 93, no. 10, pp. 1265–1270, 2004. [DOI] [PubMed] [Google Scholar]
- [47].Rao A, Chu S, Batlivala N, Zetumer S, and Roy S, “Improved detection of lung fluid with standardized acoustic stimulation of the chest,” IEEE journal of translational engineering in health and medicine, Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jones M and Thomas D, “Acoustic detection of physiological change within the thorax,” in Acoustic Sensing and Imaging, 1993., International Conference on. IET, 1993, pp. 201–205. [Google Scholar]
- [49].Wodicka G, Stevens K, Golub H, and Shannon D, “Spectral characteristics of sound transmission in the human respiratory system,” IEEE transactions on biomedical engineering, vol. 37, no. 12, pp. 1130–1135, 1990. [DOI] [PubMed] [Google Scholar]
- [50].Mahagnah M and Gavriely N, “Gas density does not affect pulmonary acoustic transmission in normal men,” Journal of Applied Physiology, vol. 78, no. 3, pp. 928–937, 1995. [DOI] [PubMed] [Google Scholar]
- [51].Korenbaum V, Tagilâtsev A, Kostiv A, Gorovoy S, Pochekutova I et al. , “Acoustic equipment for studying human respiratory sounds,” Instruments and Experimental Techniques, vol. 51, no. 2, pp. 296–303, 2008. [Google Scholar]
- [52].Goncharoff V, Jacobs J, and Cugell D, “Wideband acoustic transmission of human lungs,” Medical and Biological Engineering and Computing, vol. 27, no. 5, pp. 513–519, 1989. [DOI] [PubMed] [Google Scholar]
- [53].Crim C, Celli B, Edwards LD, Wouters E, Coxson HO,Tal-Singer R, and Calverley PM, “Respiratory system impedance with impulse oscillometry in healthy and copd subjects: Eclipse baseline results,” Respiratory medicine, vol. 105, no. 7, pp. 1069–1078, 2011. [DOI] [PubMed] [Google Scholar]
- [54].Royston T, Zhang X, Mansy H, and Sandler R, “Modeling sound transmission through the pulmonary system and chest with application to diagnosis of a collapsed lung,” The Journal of the Acoustical Society of America, vol. 111, no. 4, pp. 1931–1946, 2002. [DOI] [PubMed] [Google Scholar]
- [55].Bahoura M, “Pattern recognition methods applied to respiratory sounds classification into normal and wheeze classes,” Computers in biology and medicine, vol. 39, no. 9, pp. 824–843, 2009. [DOI] [PubMed] [Google Scholar]
- [56].Sovijärvi A, Vanderschoot J, and Earis J, Computerized Respiratory Sound Analysis (CORSA): Recommended Standards for Terms and Techniques: ERS Task Force Report. Munksgaard, 2000. [Google Scholar]
- [57].Siddiqui A and Ahmed S, “Pulmonary manifestations of sickle cell disease,” Postgraduate medical journal, vol. 79, no. 933, pp. 384–390, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang Z, Jean S, and Bartter T, “Lung sound analysis in the diagnosis of obstructive airway disease,” Respiration, vol. 77, no. 2, pp. 134–138, 2009. [DOI] [PubMed] [Google Scholar]
- [59].Pasterkamp H, Kraman SS, and Wodicka GR, “Respiratory sounds: advances beyond the stethoscope,” American journal of respiratory and critical care medicine, vol. 156, no. 3, pp. 974–987, 1997. [DOI] [PubMed] [Google Scholar]
- [60].Taplidou SA and Hadjileontiadis LJ, “Wheeze detection based on time-frequency analysis of breath sounds,” Computers in biology and medicine, vol. 37, no. 8, pp. 1073–1083, 2007. [DOI] [PubMed] [Google Scholar]
- [61].Reichert S, Gass R, Brandt C, and Andrès E, “Analysis of respiratory sounds: state of the art,” Clinical medicine. Circulatory, respiratory and pulmonary medicine, vol. 2, pp. CCRPM–S530, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sarkar M, Madabhavi I, Niranjan N, and Dogra M, “Auscultation of the respiratory system,” Annals of thoracic medicine, vol. 10, no. 3, p. 158, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gavriely N and Cugell DW, Breath sounds methodology. cRc PrEss, 1995. [Google Scholar]
- [64].Wipf JE, Lipsky BA, Hirschmann JV, Boyko EJ,Takasugi J, Peugeot RL, and Davis CL, “Diagnosing pneumonia by physical examination: relevant or relic?” Archives of Internal Medicine, vol. 159, no. 10, pp. 1082–1087, 1999. [DOI] [PubMed] [Google Scholar]
- [65].Spiteri M, Cook D, and Clarke S, “Reliability of eliciting physical signs in examination of the chest,” The Lancet, vol. 331, no. 8590, pp. 873–875, 1988. [DOI] [PubMed] [Google Scholar]
- [66].Metlay JP and Kapoor M, “Community-acquired pneumonia?” Jama, vol. 278, pp. 1440–1445, 1997. [PubMed] [Google Scholar]
- [67].Maitre B, Similowski T, and Derenne J, “Physical examination of the adult patient with respiratory diseases: inspection and palpation,” European Respiratory Journal, vol. 8, no. 9, pp. 1584–1593, 1995. [PubMed] [Google Scholar]
- [68].Kalantri S, Joshi R, Lokhande T, Singh A, Morgan M,Colford JM, and Pai M, “Accuracy and reliability of physical signs in the diagnosis of pleural effusion,” Respiratory medicine, vol. 101, no. 3, pp. 431–438, 2007. [DOI] [PubMed] [Google Scholar]
- [69].Wodicka G, DeFrain P, and Kraman S, “Bilateral asymmetry of respiratory acoustic transmission,” Medical and Biological Engineering and Computing, vol. 32, no. 5, pp. 489–494, 1994. [DOI] [PubMed] [Google Scholar]
- [70].Bohadana AB, Coimbra FT, and Santiago JR, “Detection of lung abnormalities by auscultatory percussion: a comparative study with conventional percussion,” Respiration, vol. 50, no. 3, pp. 218–225, 1986. [DOI] [PubMed] [Google Scholar]
- [71].Bohadana AB, Patel R, and Kraman SS, “Contour maps of auscultatory percussion in healthy subjects and patients with large intrapulmonary lesions,” Lung, vol. 167, no. 1, pp. 359–372, 1989. [DOI] [PubMed] [Google Scholar]
- [72].Rao A, Ruiz J, Bao C, and Roy S, “Tabla: A proof-of-concept auscultatory percussion device for low-cost pneumonia detection,” Sensors, vol. 18, no. 8, p. 2689, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wodicka G, Aguirre A, DeFrain P, and Shannon D, “Phase delay of pulmonary acoustic transmission from trachea to chest wall,” IEEE transactions on biomedical engineering, vol. 39, no. 10, pp. 1053–1059, 1992. [DOI] [PubMed] [Google Scholar]
- [74].Murray A and Neilson J, “Diagnostic percussion sounds: 1. a qualitative analysis,” Medical and biological engineering, vol. 13, no. 1, pp. 19–28, 1975. [DOI] [PubMed] [Google Scholar]
- [75].Peng Y, Dai Z, Mansy HA, Henry BM, Sandler RH,Balk RA, and Royston TJ, “Sound transmission in porcine thorax through airway insonification,” Medical & biological engineering & computing, vol. 54, no. 4, pp. 675–689, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mansy H, Royston T, and Sandler R, “Use of abdominal percussion for pneumoperitoneum detection,” Medical and Biological Engineering and Computing, vol. 40, no. 4, pp. 439–446, 2002. [DOI] [PubMed] [Google Scholar]
- [77].——, “Acoustic characteristics of air cavities at low audible frequencies with application to pneumoperitoneum detection,” Medical and Biological Engineering and Computing, vol. 39, no. 2, pp. 159–167, 2001. [DOI] [PubMed] [Google Scholar]
- [78].Abella M, Formolo J, and Penney DG, “Comparison of the acoustic properties of six popular stethoscopes,” The Journal of the Acoustical Society of America, vol. 91, no. 4, pp. 2224–2228, 1992. [DOI] [PubMed] [Google Scholar]
- [79].Goldberger JJ and Ng J, Practical signal and image processing in clinical cardiology. Springer, 2010. [Google Scholar]
- [80].Vannuccini L, Earis J, Helisto P, Cheetham B, Rossi M, Sovijarvi A, and Vanderschoot J, “Capturing and preprocessing of respiratory sounds,” European Respiratory Review, vol. 10, no. 77, pp. 616–620, 2000. [Google Scholar]
- [81].Zhdanov DS, Bureev AS, Khokhlova LA, Seleznev AI, and Zemlyakov IY, “Short review of devices for detection of human breath sounds and heart tones,” Biology and Medicine, vol. 6, no. 3, p. 1, 2014. [Google Scholar]
- [82].Mayat U, Qureshi F, Ahmed S, Athavale Y, and Krishnan S, “Towards a low-cost point-of-care screening platform for electronic auscultation of vital body sounds,” in Humanitarian Technology Conference (IHTC), 2017 IEEE Canada International IEEE, 2017, pp. 1–5. [Google Scholar]
- [83].Nelson G, Rajamani R, and Erdman A, “Vibro-acoustic model of a piezoelectric-based stethoscope for chest sound measurements,” Measurement Science and Technology, vol. 26, no. 9, p. 095701, 2015. [Google Scholar]
- [84].Bergstresser T, Ofengeim D, Vyshedskiy A, Shane J, and Murphy R, “Sound transmission in the lung as a function of lung volume,” Journal of Applied Physiology, vol. 93, no. 2, pp. 667–674, 2002. [DOI] [PubMed] [Google Scholar]
- [85].Charleston-Villalobos S, Cortés-Rubiano S, González-Camerena R, Chi-Lem G, and Aljama-Corrales T, “Respiratory acoustic thoracic imaging (rathi): assessing deterministic interpolation techniques,” Medical and Biological Engineering and Computing, vol. 42, no. 5, pp. 618–626, 2004. [DOI] [PubMed] [Google Scholar]
- [86].Henry B and Royston TJ, “Localization of adventitious respiratory sounds,” The Journal of the Acoustical Society of America, vol. 143, no. 3, pp. 1297–1307, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Anantham D, Herth FJ, Majid A, Michaud G, and Ernst A, “Vibration response imaging in the detection of pleural effusions: a feasibility study,” Respiration, vol. 77, no. 2, pp. 166–172, 2009. [DOI] [PubMed] [Google Scholar]
- [88].Bartziokas K, Daenas C, Preau S, Zygoulis P, Triantaris A, Kerenidi T, Makris D, Gourgoulianis KI, and Daniil Z, “Vibration response imaging: evaluation of rater agreement in healthy subjects and subjects with pneumonia,” BMC medical imaging, vol. 10, no. 1, p. 6, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wang Z, Bartter T, Baumman BM, Abouzgheib W, Chansky ME, and Jean S, “Asynchrony between left and right lungs in acute asthma,” Journal of Asthma, vol. 45, no. 7, pp. 575–578, 2008. [DOI] [PubMed] [Google Scholar]
- [90].Lev S, Glickman YA, Kagan I, Shapiro M, Moreh-Rahav O, Dahan D, Cohen J, Grinev M, and Singer P, “Computerized lung acoustic monitoring can help to differentiate between various chest radiographic densities in critically ill patients,” Respiration, vol. 80, no. 6, pp. 509–516, 2010. [DOI] [PubMed] [Google Scholar]
- [91].Talbot-Smith M, Audio engineer’s reference book. Focal Press, 2012. [Google Scholar]
- [92].Boldea I and Nasar SA, “Linear electric actuators and generators,” in Electric Machines and Drives Conference Record, 1997. IEEE International. IEEE, 1997, pp. MA1–1. [Google Scholar]
- [93].Redwood M, “Transient performance of a piezoelectric transducer,” The journal of the acoustical society of America, vol. 33, no. 4, pp. 527–536, 1961. [Google Scholar]
- [94].Brashier B and Salvi S, “Measuring lung function using sound waves: role of the forced oscillation technique and impulse oscillometry system,” Breathe, vol. 11, no. 1, p. 57, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ionescu CM and De Keyser R, “Relations between fractional-order model parameters and lung pathology in chronic obstructive pulmonary disease,” IEEE Transactions on Biomedical Engineering, vol. 56, no. 4, pp. 978–987, 2009. [DOI] [PubMed] [Google Scholar]
- [96].Smith H, Reinhold P, and Goldman M, “Forced oscillation technique and impulse oscillometry,” European Respiratory Monograph, vol. 31, p. 72, 2005. [Google Scholar]
- [97].Desiraju K and Agrawal A, “Impulse oscillometry: the state-of-art for lung function testing,” Lung India: official organ of Indian Chest Society, vol. 33, no. 4, p. 410, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gong S-G, Yang W-L, Zheng W, and Liu J-M, “Evaluation of respiratory impedance in patients with chronic obstructive pulmonary disease by an impulse oscillation system,” Molecular medicine reports, vol. 10, no. 5, pp. 2694–2700, 2014. [DOI] [PubMed] [Google Scholar]
- [99].Alfieri V, Aiello M, Pisi R, Tzani P, Mariani E, Marangio E, Olivieri D, Nicolini G, and Chetta A, “Small airway dysfunction is associated to excessive bronchoconstriction in asthmatic patients,” Respiratory research, vol. 15, no. 1, p. 86, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Song TW, Kim KW, Kim ES, Park J-W, Sohn MH, and Kim K-E, “Utility of impulse oscillometry in young children with asthma,” Pediatric Allergy and Immunology, vol. 19, no. 8, pp. 763–768, 2008. [DOI] [PubMed] [Google Scholar]
- [101].Komarow HD, Myles IA, Uzzaman A, and Metcalfe DD, “Impulse oscillometry in the evaluation of diseases of the airways in children,” Annals of Allergy, Asthma & Immunology, vol. 106, no. 3, pp. 191–199, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Barúa M, Nazeran H, Nava P, Diong B, and Goldman M, “Classification of impulse oscillometric patterns of lung function in asthmatic children using artificial neural networks,” in Engineering in Medicine and Biology Society, 2005. IEEE-EMBS 2005. 27th Annual International Conference of the IEEE, 2006, pp. 327–331. [DOI] [PubMed] [Google Scholar]
- [103].Brooks A, Connolly JA, and Chan O, Ultrasound in emergency care. John Wiley & Sons, 2008. [Google Scholar]
- [104].Desai S and Hansell D, “Lung imaging in the adult respiratory distress syndrome: current practice and new insights,” Intensive care medicine, vol. 23, no. 1, pp. 7–15, 1997. [DOI] [PubMed] [Google Scholar]
- [105].Irwin Z and Cook JO, “Advances in point-of-care thoracic ultrasound,” Emerg Med Clin North Am, vol. 34, no. 1, pp. 151–7, 2016. [DOI] [PubMed] [Google Scholar]
- [106].Targhetta R, Chavagneux R, Bourgeois J-M, Dauzat M,Balmes P, and Pourcelot L, “Sonographic approach to diagnosing pulmonary consolidation,” Journal of ultrasound in medicine, vol. 11, no. 12, pp. 667–672, 1992. [DOI] [PubMed] [Google Scholar]
- [107].Gargani L and Volpicelli G, “How i do it: lung ultrasound,” Cardiovascular ultrasound, vol. 12, no. 1, p. 25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Al Deeb M, Barbic S, Featherstone R, Dankoff J, and Barbic D, “Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis,” Academic Emergency Medicine, vol. 21, no. 8, pp. 843–852, 2014. [DOI] [PubMed] [Google Scholar]
- [109].Lichtenstein DA, Lascols N, Mezière G, and Gepner A, “Ultrasound diagnosis of alveolar consolidation in the critically ill,” Intensive care medicine, vol. 30, no. 2, pp. 276–281, 2004. [DOI] [PubMed] [Google Scholar]
- [110].Chavez MA, Shams N, Ellington LE, Naithani N,Gilman RH, Steinhoff MC, Santosham M, Black RE,Price C, Gross M et al. , “Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis,” Respiratory research, vol. 15, no. 1, p. 50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lichtenstein D, Mezière G, and Seitz J, “The dynamic air bronchogram: a lung ultrasound sign of alveolar consolidation ruling out atelectasis,” Chest, vol. 135, no. 6, pp. 1421–1425, 2009. [DOI] [PubMed] [Google Scholar]
- [112].Grimberg A, Shigueoka DC, Atallah ÁN, Ajzen S, and Iared W, “Diagnostic accuracy of sonography for pleural effusion: systematic review,” Sao Paulo Medical Journal, vol. 128, no. 2, pp. 90–95, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yang P-C, Luh K-T, Chang D-B, Wu H-D, Yu C-J, and Kuo S-H, “Value of sonography in determining the nature of pleural effusion: analysis of 320 cases.” AJR. American journal of roentgenology, vol. 159, no. 1, pp. 29–33, 1992. [DOI] [PubMed] [Google Scholar]
- [114].Alrajhi K, Woo MY, and Vaillancourt C, “Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis,” Chest, vol. 141, no. 3, pp. 703–708, 2012. [DOI] [PubMed] [Google Scholar]
- [115].Zhang X, Qiang B, Hubmayr RD, Urban MW, Kin-nick R, and Greenleaf JF, “Noninvasive ultrasound image guided surface wave method for measuring the wave speed and estimating the elasticity of lungs: A feasibility study,” Ultrasonics, vol. 51, no. 3, pp. 289–295, 2011. [DOI] [PubMed] [Google Scholar]
- [116].Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, and Emelianov SY, “Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics,” Ultrasound in Medicine and Biology, vol. 24, no. 9, pp. 1419–1435, 1998. [DOI] [PubMed] [Google Scholar]
- [117].Sarvazyan AP, Urban MW, and Greenleaf JF, “Acoustic waves in medical imaging and diagnostics,” Ultrasound in Medicine and Biology, vol. 39, no. 7, pp. 1133–1146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Chen S, Fatemi M, and Greenleaf JF, “Quantifying elasticity and viscosity from measurement of shear wave speed dispersion,” The Journal of the Acoustical Society of America, vol. 115, no. 6, pp. 2781–2785, 2004. [DOI] [PubMed] [Google Scholar]
- [119].Zhang M, Nigwekar P, Castaneda B, Hoyt K, Joseph JV,di Sant’Agnese A, Messing EM, Strang JG, Rubens DJ, and Parker KJ, “Quantitative characterization of viscoelastic properties of human prostate correlated with histology,” Ultrasound in Medicine and Biology, vol. 34, no. 7, pp. 1033–1042, 2008. [DOI] [PubMed] [Google Scholar]
- [120].Zhang X, Kinnick R, and Greenleaf JF, “Viscoelasticity of lung tissue with surface wave method,” in Ultrasonics Symposium, 2008. IUS 2008. IEEE. IEEE, 2008, pp. 21–23. [Google Scholar]
- [121].Mariappan YK, Glaser KJ, Hubmayr RD, Manduca A,Ehman RL, and McGee KP, “Mr elastography of human lung parenchyma: technical development, theoretical modeling and in vivo validation,” Journal of Magnetic Resonance Imaging, vol. 33, no. 6, pp. 1351–1361, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Mariappan YK, Glaser KJ, Levin DL, Vassallo R,Hubmayr RD, Mottram C, Ehman RL, and McGee KP, “Estimation of the absolute shear stiffness of human lung parenchyma using 1h spin echo, echo planar mr elastography,” Journal of Magnetic Resonance Imaging, vol. 40, no. 5, pp. 1230–1237, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hirsch S, Posnansky O, Papazoglou S, Elgeti T, Braun J, and Sack I, “Measurement of vibration-induced volumetric strain in the human lung,” Magnetic resonance in medicine, vol. 69, no. 3, pp. 667–674, 2013. [DOI] [PubMed] [Google Scholar]
- [124].Goss B, McGee KP, Ehman E, Manduca A, and Ehman RL, “Magnetic resonance elastography of the lung: technical feasibility,” Magnetic resonance in medicine, vol. 56, no. 5, pp. 1060–1066, 2006. [DOI] [PubMed] [Google Scholar]
- [125].Marinelli JP, Levin DL, Vassallo R, Carter RE, Hubmayr RD, Ehman RL, and McGee KP, “Quantitative assessment of lung stiffness in patients with interstitial lung disease using mr elastography,” Journal of Magnetic Resonance Imaging, vol. 46, no. 2, pp. 365–374, 2017. [DOI] [PubMed] [Google Scholar]
- [126].Zhang X, Osborn T, and Kalra S, “A noninvasive ultra-sound elastography technique for measuring surface waves on the lung,” Ultrasonics, vol. 71, pp. 183–188, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Jané R, Salvatella D, Fiz J, and Morera J, “Spectral analysis of respiratory sounds to assess bronchodilator effect in asthmatic patients,” in Engineering in Medicine and Biology Society, 1998. Proceedings of the 20th Annual International Conference of the IEEE, vol. 6. IEEE, 1998, pp. 3203–3206. [Google Scholar]
- [128].Gross V, Dittmar A, Penzel T, Schuttler F, and Von Wichert P, “The relationship between normal lung sounds, age, and gender,” American journal of respiratory and critical care medicine, vol. 162, no. 3, pp. 905–909, 2000. [DOI] [PubMed] [Google Scholar]
- [129].Schreur H, Diamant Z, Vanderschoot J, Zwinderman AH, Dijkman JH, and Sterk PJ, “Lung sounds during allergen-induced asthmatic responses in patients with asthma.” American journal of respiratory and critical care medicine, vol. 153, no. 5, pp. 1474–1480, 1996. [DOI] [PubMed] [Google Scholar]
- [130].Guntupalli KK, Reddy RM, Loutfi RH, Alapat PM,Bandi VD, and Hanania NA, “Evaluation of obstructive lung disease with vibration response imaging,” Journal of Asthma, vol. 45, no. 10, pp. 923–930, 2008. [DOI] [PubMed] [Google Scholar]
- [131].Kandaswamy A, Kumar CS, Ramanathan RP, Jayaraman S, and Malmurugan N, “Neural classification of lung sounds using wavelet coefficients,” Computers in biology and medicine, vol. 34, no. 6, pp. 523–537, 2004. [DOI] [PubMed] [Google Scholar]
- [132].Cooley JW and Tukey JW, “An algorithm for the machine calculation of complex fourier series,” Mathematics of computation, vol. 19, no. 90, pp. 297–301, 1965. [Google Scholar]
- [133].Rietveld S, Oud M, and Dooijes EH, “Classification of asthmatic breath sounds: preliminary results of the classifying capacity of human examiners versus artificial neural networks,” Computers and Biomedical Research, vol. 32, no. 5, pp. 440–448, 1999. [DOI] [PubMed] [Google Scholar]
- [134].Donnerberg RL, Druzgalski CK, Hamlin RL, Davis GL, Campbell RM, and Rice DA, “Sound transfer function of the congested canine lung,” Respiratory Medicine, vol. 74, pp. 23–31, 1980. [PubMed] [Google Scholar]
- [135].Zaeim HM, Scheffer C, Blanckenberg M, and Dellimore K, “Evaluation of the use of frequency response in the diagnosis of pleural effusion on a phantom model of the human lungs,” in Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual International Conference of the IEEE IEEE, 2014, pp. 3418–3421. [DOI] [PubMed] [Google Scholar]
- [136].Yang F, Ser W, Yu J, Foo DC-G, Yeo DPS, Chia P-L, and Wong J, “Lung water detection using acoustic techniques,” in Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE IEEE, 2012, pp. 4258–4261. [DOI] [PubMed] [Google Scholar]
- [137].Mulligan K, Adler A, and Goubran R, “Detecting regional lung properties using audio transfer functions of the respiratory system,” in Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE IEEE, 2009, pp. 5697–5700. [DOI] [PubMed] [Google Scholar]
- [138].Bogdanov D, Wack N, Gómez E, Gulati S, Herrera P,Mayor O, Roma G, Salamon J, Zapata J, and Serra X, “Essentia: an open-source library for sound and music analysis,” in Proceedings of the 21st ACM international conference on Multimedia. ACM, 2013, pp. 855–858. [Google Scholar]
- [139].Chen C-H, Huang W-T, Tan T-H, Chang C-C, and Chang Y-J, “Using k-nearest neighbor classification to diagnose abnormal lung sounds,” Sensors, vol. 15, no. 6, pp. 13 132–13 158, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Bahoura M and Pelletier C, “Respiratory sounds classification using cepstral analysis and gaussian mixture models,” in Engineering in Medicine and Biology Society, 2004. IEMBS’04. 26th Annual International Conference of the IEEE, vol. 1. IEEE, 2004, pp. 9–12. [DOI] [PubMed] [Google Scholar]
- [141].Chien J-C, Wu H-D, Chong F-C, and Li C-I, “Wheeze detection using cepstral analysis in gaussian mixture models,” in Engineering in Medicine and Biology Society, 2007. EMBS 2007. 29th Annual International Conference of the IEEE IEEE, 2007, pp. 3168–3171. [DOI] [PubMed] [Google Scholar]
- [142].Aykanat M, Kılıç Ö, Kurt B, and Saryal S, “Classification of lung sounds using convolutional neural networks,” EURASIP Journal on Image and Video Processing, vol. 2017, no. 1, p. 65, 2017. [Google Scholar]
- [143].Aras S, Gangal A, and Bulbul Y, “Classification of healthy and pathologic lung sounds recorded with electronic auscultation,” in Signal Processing and Communications Applications Conference (SIU), 2015 23th IEEE, 2015, pp. 252–255. [Google Scholar]
- [144].Cohen L, Time-frequency analysis. Prentice hall, 1995, vol. 778. [Google Scholar]
- [145].Hashemi A, Arabalibiek H, and Agin K, “Classification of wheeze sounds using wavelets and neural networks,” in International Conference on Biomedical Engineering and Technology, vol. 11. IACSIT Press, 2011, pp. 127–131. [Google Scholar]
- [146].Serbes G, Sakar CO, Kahya YP, and Aydin N, “Feature extraction using time-frequency/scale analysis and ensemble of feature sets for crackle detection,” in Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE IEEE, 2011, pp. 3314–3317. [DOI] [PubMed] [Google Scholar]
- [147].Alsmadi SS and Kahya YP, “Online classification of lung sounds using dsp,” in Engineering in Medicine and Biology, 2002. 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society EMBS/BMES Conference, 2002. Proceedings of the Second Joint, vol. 2. IEEE, 2002, pp. 1771–1772. [Google Scholar]
- [148].Sankur B, Kahya YP, Güler EÇ, and Engin T, “Comparison of ar-based algorithms for respiratory sounds classification,” Computers in Biology and Medicine, vol. 24, no. 1, pp. 67–76, 1994. [DOI] [PubMed] [Google Scholar]
- [149].Folland R, Hines E, Dutta R, Boilot P, and Morgan D, “Comparison of neural network predictors in the classification of tracheal–bronchial breath sounds by respiratory auscultation,” Artificial intelligence in medicine, vol. 31, no. 3, pp. 211–220, 2004. [DOI] [PubMed] [Google Scholar]
- [150].Flietstra B, Markuzon N, Vyshedskiy A, and Murphy R, “Automated analysis of crackles in patients with interstitial pulmonary fibrosis,” Pulmonary medicine, vol. 2011, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Palaniappan R, Sundaraj K, and Ahamed NU, “Machine learning in lung sound analysis: a systematic review,” Bio-cybernetics and Biomedical Engineering, vol. 33, no. 3, pp. 129–135, 2013. [Google Scholar]
- [152].Amaral JL, Lopes AJ, Jansen JM, Faria AC, and Melo PL, “Machine learning algorithms and forced oscillation measurements applied to the automatic identification of chronic obstructive pulmonary disease,” Computer methods and programs in biomedicine, vol. 105, no. 3, pp. 183–193, 2012. [DOI] [PubMed] [Google Scholar]
- [153].Abbas A and Fahim A, “An automated computerized auscultation and diagnostic system for pulmonary diseases,” Journal of medical systems, vol. 34, no. 6, pp. 1149–1155, 2010. [DOI] [PubMed] [Google Scholar]
- [154].Mayorga P, Druzgalski C, Morelos R, Gonzalez O, and Vidales J, “Acoustics based assessment of respiratory diseases using gmm classification,” in Engineering in Medicine and Biology Society (EMBC), 2010 Annual International Conference of the IEEE IEEE, 2010, pp. 6312–6316. [DOI] [PubMed] [Google Scholar]
- [155].Zolnoori M, Zarandi MHF, Moin M, and Teimorian S, “Fuzzy rule-based expert system for assessment severity of asthma,” Journal of medical systems, vol. 36, no. 3, pp. 1707–1717, 2012. [DOI] [PubMed] [Google Scholar]
- [156].Badnjevic A, Gurbeta L, and Custovic E, “An expert diagnostic system to automatically identify asthma and chronic obstructive pulmonary disease in clinical settings,” Scientific reports, vol. 8, no. 1, p. 11645, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Maru DS-R, Schwarz R, Andrews J, Basu S, Sharma A, and Moore C, “Turning a blind eye: the mobilization of radiology services in resource-poor regions,” Globalization and health, vol. 6, no. 1, p. 18, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Homs-Corbera A, Fiz JA, Morera J, and Jané R, “Time-frequency detection and analysis of wheezes during forced exhalation,” IEEE Transactions on Biomedical Engineering, vol. 51, no. 1, pp. 182–186, 2004. [DOI] [PubMed] [Google Scholar]
- [159].Vyshedskiy A and Murphy R, “Acoustic biomarkers of chronic obstructive lung disease,” Research Ideas and Outcomes, vol. 2, p. e9173, 2016. [Google Scholar]
- [160].Kolsum U, Borrill Z, Roy K, Starkey C, Vestbo J,Houghton C, and Singh D, “Impulse oscillometry in copd: identification of measurements related to airway obstruction, airway conductance and lung volumes,” Respiratory medicine, vol. 103, no. 1, pp. 136–143, 2009. [DOI] [PubMed] [Google Scholar]
- [161].Fernandez-Granero MA, Sanchez-Morillo D, and Leon-Jimenez A, “Computerised analysis of telemonitored respiratory sounds for predicting acute exacerbations of copd,” Sensors, vol. 15, no. 10, pp. 26 978–26 996, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Blanco M, Mor R, Fraticelli A, Breen DP, and Dutau H, “Distribution of breath sound images in patients with pneumothoraces compared to healthy subjects,” Respiration, vol. 77, no. 2, pp. 173–178, 2009. [DOI] [PubMed] [Google Scholar]
- [163].Yigla M, Gat M, Meyer J-J, Friedman PJ, Maher TM, and Madison JM, “Vibration response imaging technology in healthy subjects,” American Journal of Roentgenology, vol. 191, no. 3, pp. 845–852, 2008. [DOI] [PubMed] [Google Scholar]
- [164].Lakhe A, Sodhi I, Warrier J, and Sinha V, “Development of digital stethoscope for telemedicine,” Journal of medical engineering & technology, vol. 40, no. 1, pp. 20–24, 2016. [DOI] [PubMed] [Google Scholar]
- [165].Devices Eko. (2017, Mar.), Eko Core Digital Stethoscope. [Online]. Available: https://ekodevices.com/
- [166].Littmann. (2017, Mar.), 3M Littmann Electronic Stethoscope. [Online]. Available: http://www.littmann.com/3M/enUS/littmannstethoscopes/products/~/3M-Littmann-Stethoscopes/Electronic-Stethoscopes
- [167].ThinkLabs. (2017, Mar.), ThinkLabs One Digital Stethoscope. [Online]. Available: http://www.thinklabs.com/
- [168].Gurbeta L, Badnjevic A, Maksimovic M, Omanovic-Miklicanin E, and Sejdic E, “A telehealth system for automated diagnosis of asthma and chronical obstructive pulmonary disease,” Journal of the American Medical Informatics Association, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]